Graphical abstract
Keywords: Epidemic pathogens, Nano/micro vehicles, High efficiency, Long-term, Cross-protection
Abbreviations: APCs, Antigen-presenting cells; aPVs, Acellular pertussis vaccines; AVA, Anthrax vaccine absorbed; BN-OMVs, Bovine serum albumin nanoparticles; CaPNs, Calcium phosphate nanoparticles; cCHP, Cholesteryl group-bearing pullulan; CFs, Colonization factors; CPS, Capsular polysaccharide; CPTEG:CPH, 1,8-bis(p-carboxyphenoxy)−3,6-dioxaoctane: 1,6-bis(p-carboxyphenoxy)hexane; CRKP, Carbapenem-resistant K. pneumoniae; CSP, Circumsporozoite protein; CS-TPP, Chitosan-tripolyphosphate; CTB, Cholera toxin B subunit; CTL, Cytotoxic T cell; Cu-Zn SOD, Cu-Zn superoxide dismutase; DC, Dendritic cells; ETEC, Enterotoxigenic E. coli; EVs, Extracellular vesicles; FHV, Flock house virus; FUC, Fucoidan; GEM, Gram-positive enhancer matrix; GM1, Monosialotetrahexosylganglioside; HBV, Hepatitis B virus; HEC, Hectorite; Hib, Haemophilus influenzae type b; HIV, Human immunodeficiency virus; HPV, Human papillomavirus; HR, Heptad repeat; HTCC, N-(2-hydroxy)-propyl-3-trimethyl ammonium chitosan chloride; HUS, Hemolytic–uremic syndrome; LDH, 1,8-bis(p-carboxyphenoxy)−3,6-dioxaoctane: Hydroxide; ICG, indocyanine green; LEPS, Liposomal encapsulation of polysaccharides; LOS, Lipooligosaccharide; LPS, Lipopolysaccharides; LTA, Heat-labile enterotoxin A subunit; LTB, Heat-labile enterotoxin B subunit; MN, Microneedle; MSNs, magnetic mesoporous silica nanoparticles; NF, Nanofibrous membrane; N-IpaD, N-terminal region of IpaD; NMVs, Nano-multilamellar lipid vesicles; NP/NCMP, Poly (glycerol adipate-co-ω-pentadecalactone) (PGA-co-PDL) polymeric nanoparticles (NPs) / L-leucine microcarriers (nanocomposite microparticles-ncmps); OMVs, Outer membrane vesicles; PA, Protective antigen; PAD4, Domain 4 of PA; PAPE, Pickering emulsion; PBAE, Poly (β-amino ester); PLA, Poly (lactic acid); PLG, Poly (lactid-co-glycolid); PDNVs, protoplast-derived nanovesicles; PLGA, Polylactic-co-glycolic acid; PM, Pulmonary; PS-GAMP, Pulmonary surfactant-biomimetic liposomes; RBD, Receptor binding domain; RV, Rabies virus; SBA-15, Santa Barbara Amorphous-15; SEM, Scanning electron microscopy; stx, Shiga toxin; STING, Stimulator of interferon genes; STxA, Shiga toxin A subunit; Tfh, T-follicular helper; TLR, Toll-like receptor; TMC, Trimethyl chitosan; TRM, Memory T cells; VLPs, Virus-like particles; wPV, whole-cell pertussis vaccine
Abstract
Prophylactic vaccines have evolved from traditional whole-cell vaccines to safer subunit vaccines. However, subunit vaccines still face problems, such as poor immunogenicity and low efficiency, while traditional adjuvants are usually unable to meet specific response needs. Advanced delivery vectors are important to overcome these barriers; they have favorable safety and effectiveness, tunable properties, precise location, and immunomodulatory capabilities. Nevertheless, there has been no systematic summary of the delivery systems to cover a wide range of infectious pathogens. We herein summarized and compared the delivery systems for major or epidemic infectious diseases caused by bacteria, viruses, fungi, and parasites. We also included the newly licensed vaccines (e.g., COVID-19 vaccines) and those close to licensure. Furthermore, we highlighted advanced delivery systems with high efficiency, cross-protection, or long-term protection against epidemic pathogens, and we put forward prospects and thoughts on the development of future prophylactic vaccines.
1. Introduction
Epidemic infections, such as cholera, malaria, and the sudden outbreak of COVID-19, cause global life threats and socioeconomic recession. One of the most devastating pandemics was the Black Death, an infection caused by Yersinia pestis, which killed over 75 million people in 1350. At present, the world is still in the midst of the COVID-19 pandemic, with 3,398,302 COVID-19-related deaths recorded as of May 19, 2021 (https://covid19.who.int/). Furthermore, antibiotic resistance leads to the ineffectiveness of traditional antibacterial treatments, and nearly half a million new cases of multidrug-resistant tuberculosis (MDR-TB) occur worldwide [1].
Among coping strategies for emerging infectious diseases, preventive vaccination is a crucial strategy [2] and the only one with wide and thorough effects on the public health. For example, since 2000, vaccination has reduced the reported incidence of measles by 83%, thereby preventing over 20 million deaths [3]. Vaccines work by training and utilizing the body’s immune system to recognize and fight the pathogens. However, the high infection rates, widespread transmission, or high fatal ratio of certain epidemic germs raised huge challenges for the design of prophylactic vaccines.
Vaccines have evolved from the live-attenuated or inactivated vaccines to the subunit/peptide vaccines. Regardless of the safety issues, the live-attenuated and inactivated vaccines, which are nanoparticles themselves [2], are ready to be captured and processed by antigen-presenting cells (APCs), including dendritic cells (DCs), macrophages, and B cells, resulting in an efficient immune response. In contrast, subunit vaccines or recombinant vaccines are characterized by improved safety, but much weaker immunogenicity. To break the bottleneck of previous vaccine types, advanced delivery systems (e.g., micro/nano delivery) exhibiting both high efficacy and safety are greatly required. Such systems will likely help to catalyze novel candidate vaccines toward clinical testing at an unprecedented speed. For example, lipid nanoparticles dictated the success of the mRNA vaccines in 2020, making an enormous contribution to control the spread of COVID-19 at a global level.
The advantages of vaccine delivery systems have been well documented in recent reviews. For example, Ding et al. [4] summarized the nanosystems with superior therapeutic or preventive effects, providing an important clue on maintenance of our well-being by exploiting the immunomodulatory property of nanomaterials. Other works also referred to using nanotechnology or materials science approaches with delivery systems [5], [6]. In addition, vaccine delivery systems focusing on tackling a particular infectious disease (e.g., COVID-19) [7] or cancer [8] were reviewed. Nevertheless, there has been no systematic summary of advanced delivery systems and design concepts for prophylactic vaccines for a wide range of epidemic infectious diseases. Taking into account the diverse species and pathogenic mechanisms of infection pathogens, the protection efficacy of different systems against the same infection has not been sufficiently compared. In addition, the requirements and priorities of the delivery systems for different infectious diseases or stages are also case by case. Obtaining such data would greatly support designing the optimal delivery vectors for specific infectious diseases.
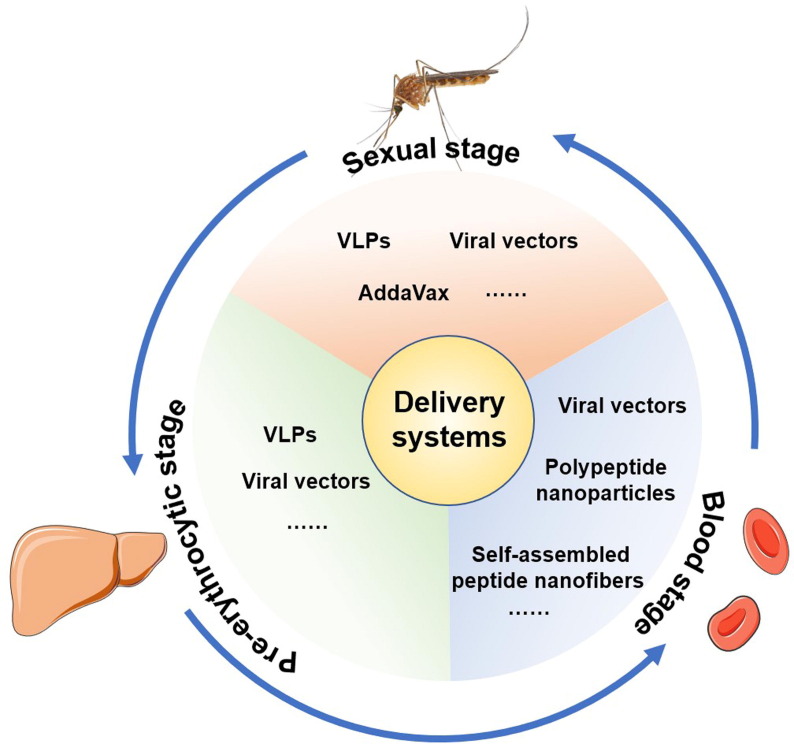
In this review, we provide an overview of the major or epidemic infectious diseases (pneumonia, diarrhea, candidiasis, malaria, and others) caused by bacteria, viruses, fungi, and parasites. We also list advanced delivery systems against different infectious diseases and compare their protective efficacy from different aspects. Moreover, we include the current vaccines and vaccine delivery systems that are either newly licensed (e.g., COVID-19 vaccines) or close to licensure. In particular, we highlight the advanced delivery systems with high efficiency, cross-protection, or long-term protection against epidemic pathogens.
2. Bacterial infectious diseases and advanced delivery systems
2.1. Respiratory infectious diseases and advanced delivery systems
Respiratory infections represent a serious health problem worldwide that mainly affects children, older people, and immunocompromised individuals. Pneumonia vaccines (e.g., 23-valent polysaccharide vaccines, 13-valent polysaccharide conjugate vaccines) have achieved great success worldwide. Nevertheless, the existence of a variety of serotypes (>95) of S. pneumoniae makes the capsular polysaccharide (CPS)-based vaccines unable to provide broad protection [9], while the cost of preparing vaccines containing all serotypes is very high. In addition, there may still be undetected serotypes [10]. In this case, effective delivery systems of proteinaceous antigens have been developed for the prevention of pneumonia. In addition to the delivery efficacy, a combined mucosal and systemic immune response is also appreciated for pneumonia vaccines [11], which not only requires novel delivery design but also a specific administration route (e.g., noninvasive mucosal routes). At present, there are a variety of delivery systems for pulmonary infectious diseases and they have shown good results (Table 1 ).
Table 1.
Effect of vaccines with delivery systems against pulmonary infectious diseases.
| Pathogenic bacteria | Delivery system | Antigen(s) | Adjuvants used | Route | Animal species | Protection after challenge | Ref. |
|---|---|---|---|---|---|---|---|
| S. pneumoniae | cCHP Nanogel | PspA | — | i.n. | Mice | 100% of animals surviving | [12] |
| Hybrid biological-biomaterial vector (PBAE and bacterial core) | PspA or PspAb | — | s.c. | Mice | 100% of animals surviving | [13] | |
| Liposomes | Polysaccharide (Serotypes 3) | — | i.n. | Mice | No results | [14] | |
| LEPS | PncO, GlpO, and polysaccharide (Serotypes 19F, 11A, and 35C) | — | s.c. | Mice | 100% of animals surviving | [15] | |
| Chitosan | PsaA | — | i.n. | Mice | 100% of animals surviving | [16] | |
| Chitosan | PsaA (DNA) | — | i.n. | Mice | Decreased bacterial colonization in nasopharynx | [17] | |
| Polyanhydride nanoparticles | PspA | — | s.c. | Mice | No results | [18] | |
| PLA microparticles | PspA | — | i.m. | Mice | No results | [19] | |
| NP/NCMP | PspA4Pro | — | PM | Mice | 67% of animals surviving | [20] | |
| L. lactis, L. casei, L. plantarum, and L. helveticus | PsaA (Surface)b | — | i.n. | Mice | Decreased bacterial colonization in the nasal mucosa | [21] | |
| L. casei | PspAb | — | i.n. | Mice | 33% of animals surviving | [22] | |
| L. casei | PspA5 (Cytoplasm)b or PspC (Cytoplasm)b | — | Mice | PspA5: 40% of animals surviving; PspC: 20% of animals surviving | [23] | ||
| L. casei | PspC (Surface or Cytoplasm)b | — | i.n. | Mice | Decreased bacterial colonization in the nasopharynx | [24] | |
| L. lactis | PspAb | — | i.n. | Mice | 40% of animals surviving | [25] | |
| L. lactis | PppAb | — | i.n. | Mice | 60% of adults and 70% of young mice surviving | [26] | |
| L. lactis (GEM) or live L. lactis | PppA or PppAb | — | i.n. or oral | Mice | Decreased bacterial number in the lungs and blood | [27] | |
| L. lactis (GEM) | IgA1p, PpmA, and SlrA | — | i.n. | Mice | Decreased bacterial number in the lungs, blood, and nose from trivalent vaccine and the divalent formulation containing SlrA and IgA1p | [28] | |
| VLP (Qβ) | TS3 and TS14 (chemically synthesized two kinds of capsular polysaccharides repeated units) | — | i.m. | Mice | TS14: 90% of animals surviving, compared with 66% of controls; TS3: 95% of animals surviving, compared with 40% of controls | [29] | |
| VLP (HBsAg) | Capsular polysaccharide 33F | — | s.c. | Mice | No results | [30] | |
| K. pneumoniae | OMVs | OMV components | — | i.p. | Mice | 100% of animals surviving | [31] |
| BN-OMVs | OMV components | — | s.c. | Mice | 100% of animals surviving | [32] | |
| Alginate microparticles | LPS of K. pneumoniae O1 serotype | — | i.m., i.t.a, or i.n. | Mice | Decreased bacterial loading in the lungs | [33] | |
| B. pertussis | OMVs | OMV components | — | i.p. or i.n.a | Mice | Decreased bacterial colonization in the lungs | [34] |
| OMVs | OMV components | — | s.c. | Mice | Decrease bacterial colonization in the lungs; slightly faster than that of wPV | ||
| OMVs | OMV components | — | PMa or s.c. | Mice | Decreased bacterial colonization in the lungs, trachea, and nose | [35] | |
| OMVs deriving from B. parapertussis | OMV components | — | i.p. | Mice | Cross-protection | [36] | |
| Lipid A-modified OMVs | OMV components | — | i.n. | Mice | Decreased bacterial counts in the lungs | [37] | |
| L. acid bacteria (GEM) | PTd, FHA, and PRN | — | i.p. or i.n.a | Mice | Decreased bacterial counts in the lungs and trachea (but not reaching statistical significance compared with antigen alone) | [38] | |
| PLGA nano/microparticle | PTd | — | s.c. | Mice | Decreased bacterial counts in the lungs | [39] | |
| PLG nano or microparticle | PTd and FHA | — | Oral, i.p.a, i.m.a, or s.c. | Mice | Decrease bacterial counts in the lungs | [40] | |
| Haemophilus influenzae type b (Hib) | Chitosan hydrogel (ViscoGel) | a commercial Hib conjugate vaccine (Act-Hib) | — | s.c. or i.m. | Mice | No results | [41] |
| VLP (HBsAg) | PRP polysaccharide | — | s.c. | Mice | No results | [42] | |
| Mycobacterium tuberculosis | Chitosan | Esat-6 three T cell epitopes (Esat-6/3e) and fms-like tyrosine kinase 3 ligand (FL) genes (DNA) | — | i.m. prime (Esat-6/3e-FL) and i.n. boost (Esat-6/3e) | Mice | Decreased bacterial counts in the lungs and spleens | [43] |
| Chitosan | Mycobacterium lipids | — | i.p. | Mice | No results | [44] | |
| Mycobacterium bovis | Liposome | Fusion of antigen 85b and Esat-6 | — | s.c. | Mice | Decreased bacterial counts in the lungs and spleen | [45] |
a. The better or the best route to achieve protection; b. Constructed in an expression vector; i.n., intranasal; s.c., subcutaneous; i.m., intramuscular; i.p., intraperitoneal; i.t., intratracheal; PM, pulmonary; —, without added adjuvant.
2.1.1. S. pneumoniae
S. pneumoniae is a global endemic pathogen causing a wide range of clinical diseases, such as pneumonia, meningitis, and sepsis, which frequently lead to death among children all over the world, especially in developing countries [46]. Colonization with S. pneumoniae in humans is universal [47]; however, it provides an opportunity for the remaining serotypes to establish residence and progress to virulence [48], [49]. In addition to direct infection, the bacteria usually exist in the form of biofilm, and some destructive events, such as viral infection, can prompt the release of a virulent subpopulation of bacteria to the lungs, blood, middle ear, and other parts of the body, causing the aforementioned diseases [50], [51]. Therefore, high levels of IgG antibodies produced by humoral immunity are very important for invasive infections, and antigen-specific sIgA antibodies are the key to prevention of S. pneumoniae colonization of the upper respiratory tract.
Various delivery vehicles (e.g., polymers, virus-like particles (VLPs), L. lactis, liposomes) have been used to deliver S. pneumoniae protein or glycan antigens, which showed a strong ability to prevent bacterial invasive infections and inhibit the colonization of the respiratory tract (Table 1). Among them, some vaccine formulations offered universal pneumococcal disease prevention. For example, Jones et al. proposed a vaccine platform through the liposomal encapsulation of polysaccharides (LEPS) technology. The completed LEPS vehicle (about 300 nm in size) was coupled with the PncO and GlpO protein antigens (identified through an antigen discovery and validation model that selectively targeted pneumococci virulence transition [52]) for the liposomal containment of polysaccharides (serotypes 19F, 11A, and 35C). Thus, this vaccine not only prevents the colonization of the most aggressive S. pneumoniae serotypes, but it also restricts virulence transition [15]. Since pneumonia vaccines are often used in children and older adults, the safety and immune activation ability of the delivery carriers need to be greater. Thus, we further focused on the latest several delivery systems that can produce the best protective effect.
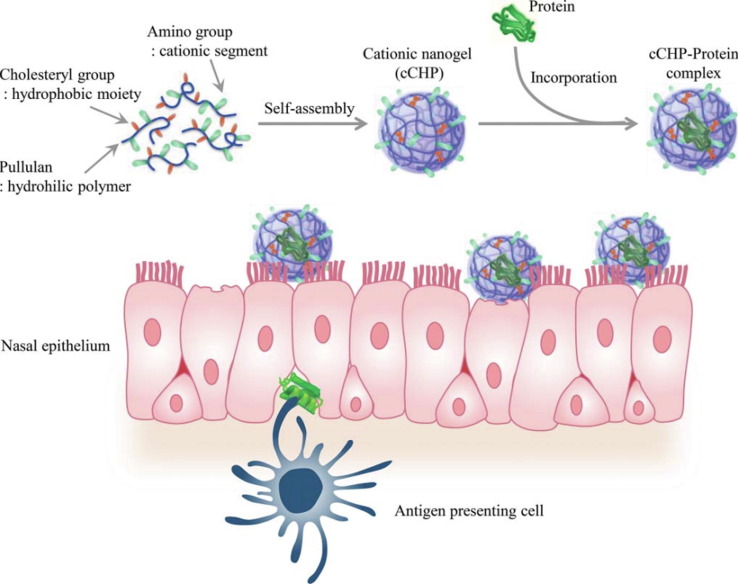
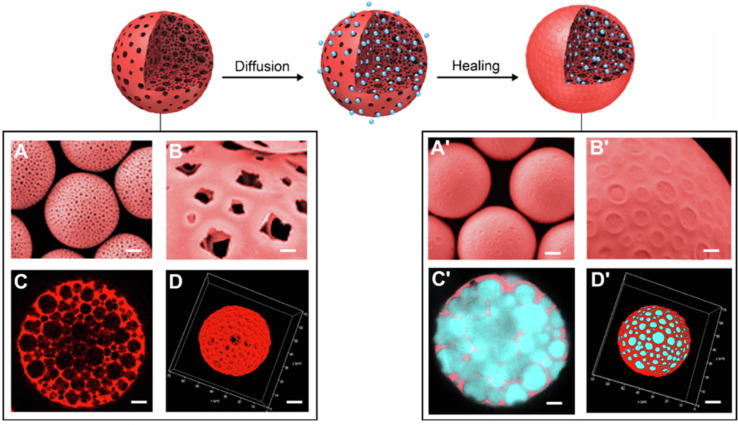
Nanogel It has been reported that a self-assembled nanosized hydrogel (nanogel) containing a cationic type of cholesteryl group-bearing pullulan (cCHP) can be used as a vaccine delivery system [53], [54], [55]. This nanogel could effectively transfer antigen to nasal epithelial cells and dendritic cells (DC) under the basement membrane and induce antigen-specific immune response as a non-adjuvanted vaccine (Fig. 1 ). After loading with a single protein antigen PspA, both specific IgG levels in the serum and bronchial fluids and IgA levels in the nasal fluid were significantly elevated in nasally administered mice [12], and all of the responses were involved in establishing protective immunity against pneumococci [56], [57], [58], [59]. Further, a study in rhesus macaques revealed that the cCHP-based pneumococcal vaccine induced significantly elevated PspA-specific IgG and IgA levels and kept them for a long time [60]. Moreover, positron emission tomography (PET) analysis combined with magnetic resonance imaging (MRI) has confirmed that the cCHP nanogel vaccine is not deposited in the olfactory bulbs and brains in macaques [60], suggesting that it is also safe to use as a nasal vaccine in humans.
Fig. 1.
Application of cCHP nanogel as nasal immune delivery system via nasal route[61]. cCHP is composed of a cholesteryl group-bearing pullulan (CHP) with a cationic amino group. cCHP nanogels can encapsulate proteins in the internal space through hydrophobic interactions and effectively retain them in the negatively charged nasal mucosa.
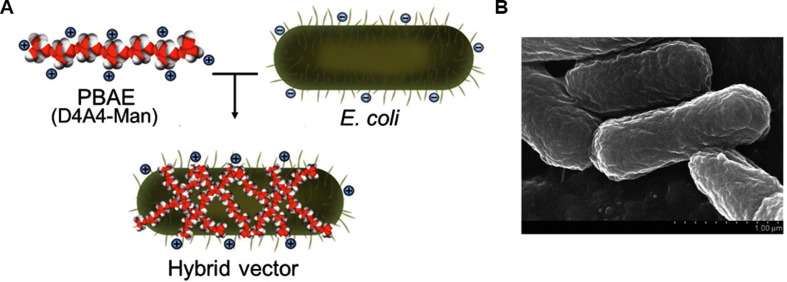
Hybrid biological-biomaterial vector Yi et al. have reported a combined delivery device named hybrid biological-biomaterial vector, which consists of a bacterial core electrostatically coated with a cationic polymer (a mannosylated poly (β-amino ester) (PBAE)) [62]. The biological portion of the vector is a bacterial cell (live or dead), containing natural adjuvant properties, and it is beneficial for passive targeting of phagocytic APCs (Fig. 2 ). The vector’s composition and the surface characteristics endowed by the mannosylated PBAE engage APC receptors and enhance the uptake upon vector administration. After subcutaneous injection of the vector expressing pneumonia PspA protein antigen in mice, a strong specific immune response providing a wide range of protection against 10 different clinical S. pneumoniae was induced. Moreover, the localization of PspA in the cytoplasm could provide a stronger immune protection effect than that in the periplasm or on the surface of bacteria [13]. As for the combination of biomaterial and biological components, each has its own antigen delivery characteristics and plays a role in the immune process. Moreover, such synergy is conducive for obtaining the best immune effects. In addition, E. coli can be further modified by genetic engineering to be more suitable for antigen delivery. Because the antigen is loaded with bacterial vectors, it provides a variety of possibilities for using different antigen locations and different loading forms, such as proteins and nucleic acids.
Fig. 2.
Construction of a hybrid biological-biomaterial vector[62]. (A) Schematic diagram of the hybrid biological-biomaterial vector preparation. (B) Scanning electron microscopy image of the vector.
2.1.2. Klebsiella pneumoniae
K. pneumoniae belongs to gram-negative family Enterobacteriaceae. It exists widely in the natural environment and acts as opportunistic pathogen, so serious infections may be induced in patients with severe infections and weakened immune system [63]. Klebsiella species remain the world’s most common nosocomial pathogens [64] and the main cause of hospital-acquired pneumonia resulting in high mortality rate. Currently, the biggest challenge of K. pneumoniae treatment is drug resistance, which makes the use of common antibiotics ineffective. Indeed, the extended-spectrum β-lactam-producing and carbapenem-resistant K. pneumoniae (CRKP) has been recognized by the World Health Organization as a critical public health threat [65].
Outer membrane vesicles (OMVs) A large number of gram-negative bacteria can naturally produce extracellular OMVs, which have significant advantages in vaccine development. Compared with other lipid nanoparticles, OMVs contain toll-like receptor (TLR) agonists, such as outer membrane proteins, lipoproteins and lipopolysaccharides (LPS), and a variety of immunogenic endogenous antigens. Generally, the diameter range of OMVs is from 50 nm to 250 nm, which is suitable for targeting and being phagocytized by APCs [66]. The use of OMVs has become a very promising strategy of vaccination. K. pneumoniae-derived OMVs can induce strong humoral and cellular immunity response, preventing bacteria-induced lethality in intraperitoneally immunized mice [31]. Although natural OMVs are considered potential vaccine candidates, they have some shortcomings. One is a wide size range of OMVs naturally secreted by bacteria, which may complicate the vaccine dynamics in vivo, and the low stability of natural OMVs also profoundly affects the vaccine effect. To solve these problems, Wu et al. described a core-shell structure to reinforce the OMVs by depositing the hollow-structured OMVs onto bovine serum albumin nanoparticles (BN-OMVs). The size of the BN-OMVs was mostly between 70 and 90 nm, and the immune response and protection against CRKP were significantly improved after subcutaneous vaccination [32].
Alginate microparticles Microcapsules have been developed as a delivery vector for mucosal vaccines because they could improve the uptake into APCs and sustain the release of antigenic material. As polymers, alginate microparticles are easy to obtain and inexpensive and have several advantages in vaccines. Unlike the formation of (lactide-co-glycolide) PLG particles, which require harsh denaturation conditions, the conditions for alginate microparticles formation are mild [67]. Moreover, the mucoadhesive properties of alginate microparticles may prolong the contact time with the absorptive epithelium and the mucosa-associated lymphoid tissue M cells, thus promoting the uptake of the coated antigen. When LPS of K. pneumoniae O1 was loaded in alginate microparticles, the particle size was less than 5 μm. Both effective systemic and mucosal immune responses were induced following nasal and inhalation administration, which protected rodents against lobar pneumonia [33]. Although LPS encapsulation with microspheres or liposomes can reduce its pyrogenic and toxic properties and induce protective polysaccharide antibody [68], the natural LPS can still cause severe inflammation. At present, many serotypes of K. pneumoniae have been identified, but only four of them (O1, O2, O3, and O5) can cause human diseases, indicating that O-polysaccharide-based vaccines could provide a high coverage. However, the O-polysaccharide has high specificity but low immunogenicity, which requires more efficient delivery systems.
2.1.3. Bordetella pertussis
Pertussis is a serious childhood respiratory disease, with the main feature of a persistent and paroxysmal cough, accompanied by a typical inhalation “whooping” sound. A typical pertussis infection usually lasts 3 months. Therefore, it is also called “hundred-day cough” [69]. The disease is caused by B. pertussis, which mainly spreads through aerosols and may settle in the respiratory tract, thereby damaging epithelial cells and impairing normal respiratory function. Although acellular pertussis vaccines (aPVs) containing multiple antigen components can provide wider coverage [70], there are still problems to be solved, such as poor immunization response and short-term protection. In addition, surveys in many countries have found that the incidence of pertussis is increasing, and there have even been pertussis outbreaks in many countries and regions in recent years [71], [72], [73]. This phenomenon is also known as the “Pertussis Resurgence” [74], [75]. Therefore, pertussis still poses a threat to the health of children, and a safer and more protective vaccine is needed.
Many delivery vehicles, such as OMVs, L. acid bacteria, and polymers have been developed against B. pertussis. Among them, OMVs have been most thoroughly studied. Many studies have demonstrated that OMVs derived from B. pertussis can protect mice from intranasal pertussis challenge through different immune routes, including intraperitoneal, subcutaneous, intranasal, and pulmonary (PM) [34], [35], [76]. The classical administration route of OMVs is subcutaneous or intraperitoneal, which does not cause an IgA reaction [77]. For respiratory diseases, mucosal immunity seems more important. By comparing the immune responses evoked by the PM and subcutaneous route, a distinct systemic and a stronger mucosal IgA and Th17 immune response were observed with PM immunization; the PM route also provided more effective inhibition of bacterial colonization in the respiratory tract [35]. Thus, mucosal immunization may be of great significance to improve protection against pertussis infection. Although the systemic proinflammatory cytokine responses have been greatly reduced compared with the whole-cell pertussis vaccine (wPV) [76], a further detoxification is necessary considering that native B. pertussis OMVs contain a lot of lipooligosaccharide (LOS), which could induce strong host inflammatory responses. Asensio et al. developed a recombinant B. pertussis strain expressing the lipid A-modifying enzyme, PagL, which hydrolyzes the ester bond at the 3 position of lipid A, resulting in tetra- instead of penta-acylated LOS. The OMVs derived from the recombinant strain retained protective capacity against intranasal challenge in intranasally immunized mice [37]. In addition, a cross-protection study found that OMVs derived from B. parapertussis, a close relative of B. pertussis that can also cause pertussis, could provide protection from both B. pertussis and B. parapertussis infections [36]. Further, long-term protection from pertussis OMV-based vaccine is based on the induction of lung-resident CD4+ memory T cells (TRM) [78], suggesting that effectiveness may cover the entire period of childhood.
Respiratory pathogenic bacterial infections involve colonization and invasion; therefore, mucosal immunity is considered an ideal choice for the prevention of respiratory diseases and blocking transmission because it involves both mucosal and humoral immune responses. Nasal mucosal immunity has the advantages of low response threshold, low antigen dosage, and no immune tolerance. However, under conventional conditions, antigens can only stay on the mucosal surface for a short time after entering the nasal cavity, and a small amount of proteolytic enzymes and mucus weaken the strength of the immune response. Therefore, the main requirement for a nasal mucosal immune delivery system is to enhance the adhesion and retention of antigens on the mucosal surface, enhance the immunogenicity of the antigens, and promote the effective uptake of antigens by DCs.
In addition, polysaccharide antigens have strong specificity, and the produced antibodies are protective. The polysaccharide conjugate vaccine for S. pneumoniae has achieved great success. However, the current chemical preparation method is a time-consuming and multistep process, which makes the vaccine expensive and difficult to popularize in developing countries. In contrast, the current biotechnology of coupled bacterial polysaccharide and carrier protein based on protein glycosylation modification has achieved partial success and is expected to overcome the limitations of polysaccharide conjugate vaccine. The use of VLP to couple a single polysaccharide repeat unit of S. pneumoniae can also produce a protective effect, suggesting that nanocarriers have advantages in stimulating polysaccharide immune responses. Therefore, the combination of a nanodelivery carrier and glycosylation system is expected to prepare a new, low-cost, high-efficiency pneumonia polysaccharide conjugate vaccine. Although a variety of probiotic carriers were used in the research of pneumococcal vaccines, the final immune effect was not optimal. It may be that, compared with the intestine, the respiratory environment is not suitable for their survival, colonization, presentation, and antigen release. In addition, about 80% of clinical isolates of K. pneumoniae belong to one of four serotypes (O1, O2, O3, and O5), so polysaccharide antigens can be used to prepare multivalent vaccines. However, the immunogenicity of OPS is generally low, especially for O2 polysaccharide repeat units composed of two simple galactoses, which makes it difficult for O2 antigen to stimulate an effective immune response using traditional vaccine strategies. Therefore, delivery systems for weak polysaccharide antigens need further development.
2.2. Intestinal infectious diseases and advanced delivery systems
Diarrhea, mainly manifested as watery and loose stools many times a day, is a major global health problem [79]. Bacterial diarrhea, specifically, is a more serious condition with severe symptoms. The most common microorganisms that cause bacterial diarrhea are Escherichia coli, Shigella, Salmonella, Yersinia, and Clostridium [80]. In bacterial diarrhea, pathogens attach to the epithelium, produce toxins, and increase intracellular cAMP or cGMP, ultimately accelerating the secretion process in the enterocytes [81]. Toxins may also induce the release of cytokines, leading to chemotaxis and eicosapentaenoic acid (prostaglandin) production, and aggravate the imbalance of water in the lumen. Excessive water and electrolytes in the intestinal cavity draw more fluid into the intestinal cavity, and the osmotic effect further worsens diarrhea [82]. Therefore, inhibiting bacterial colonization in the intestine can effectively prevent infection, and this ability often comes from IgA produced by mucosal immune response. It is an ideal way for antigens to directly act on the intestinal mucosal immune system to stimulate mucosal immune response. In addition, due to the higher safety of oral administration, some inorganic carriers have also been explored. However, unlike respiratory delivery, most vaccines need to overcome various difficulties to reach the intestinal tract, such as resistance to gastric acid and bile, ability to colonize the intestinal tract, and possible immune tolerance. The delivery systems for the prevention of bacterial diarrhea are summarized in Table 2 .
Table 2.
Effect of vaccines with delivery systems against intestinal infectious diseases.
| Pathogenic bacteria | Delivery system | Antigen(s) | Adjuvants used | Route | Animal species | Protection after challenge | Ref. |
|---|---|---|---|---|---|---|---|
| E. coli | L. reuteri | Fusion of ST and LTBb | — | Oral | Mice | Decreased gut/carcass weight (G/C) ratios | [83] |
| L. casei | F41 or K99 fimbriae (Surface)b | — | Oral or i.n. | Mice | Over 80% of animals surviving with a high dose, 9 weeks after the last immunization; Passive protection (F41 fimbriae): 90% of pups surviving, oral; 80% of pups surviving, i.n. | [84], [85], [86] | |
| L. casei | β-Intimin fragmentb | — | Oral or s.l. | Mice | Decreased bacterial recovery from feces | [87] | |
| L. casei | Fusion of K99, K88 fimbriae (Surface)b | fuse expressing LTB | Oral | Mice | Over 80% of animals surviving 3 weeks after the last immunization, and over 70% of animals surviving 9 weeks after the last immunization | [88] | |
| L. casei | FaeGb | co-expressing or fuse expressing mutated LTA and LTB | Oral | Mice | 100% of animals surviving | [89] | |
| L. plantarum | FaeG with DC-targeting peptideb | — | Oral | Mice | Inflammation of intestinal tissue prevented | [90] | |
| L. acidophilus | EspA and the Tir central domain (Secreted)b | — | Oral | Mice | 80% of animals surviving | [91] | |
| L. acidophilus | K99 (Surface)b | — | No results | Pigs | No results | [92] | |
| L. reuteri | PapG (Surface)b | — | No results | No results | [93] | ||
| Detoxified OMVs | OMV components | — | Eyedrop | Mice | 100% of animals surviving, compared with 20% of controls | [94] | |
| Chitosan + Eudragit L-100 | F4 fimbriae | — | Oral | Reduction in excretion of bacteria | [95] | ||
| Chitosan + Eudragit L-100 + OMVs | OMV components | — | Oral | Mice | No results | [96] | |
| PLGA | CS3, CS1, LTB, and chimeric CFA/I, CS2, CS3, and LTB | — | Oral, s.c., or i.p. | Mice | No results | [97], [98] | |
| PLG microspheres | CS6 | — | i.n. | Mice | No results | [99] | |
| Nano-multilamellar lipid vesicles (NMVs) | Stx2B | — | s.c. | Mice | 60% of animals surviving | [100] | |
| Oil-based VaxcineTM | Conjugation of O111 polysaccharide and EtxB | — | Oral | Rabbits and mice | No results | [101] | |
| LDH and HEC nanoparticles | IB | — | s.c. | Mice | No results | [102] | |
| SBA-15 | Int1b or O-polysaccharides | — | s.c. | Rabbits and mice | No results | [101], [103] | |
| Shigella | OMVs of six strains | — | Oral | Mice | Neonatal mice were 100% passively protected against S. flexneri 2a and S. flexneri 6, while the protective efficacies against S. dysenteriae 1, S. flexneri 3a, S. boydii 2, and S. sonnei were ~90% | [104] | |
| Detoxified OMVs | OMV components | Alhydrogel | i.n., i.d., s.c., i.p., or i.m. | Mice, rabbitsand human | No results | [105], [106], [107] | |
| OMVs or OMVs encapsulated in polyanhydride nanoparticles (OMV-NP) | OMV components | — | i.d., i.n., eyedrop, or oral | Mice | OMVs: 100% of animals surviving by nasal and ocular route, and no animal surviving by intradermal route; OMV-NP: 100% of animals surviving by the nasal, oral, and intradermal route | [108], [109] | |
| OMVs encapsulated in CS-TPP particles and Eudragit L-100 | OMV components | — | Oral or i.d. | Mice | passive immunity protection | [110] | |
| Self-assembled proteinaceous nanoparticles | O-polysaccharide | — | s.c. | Mice | 100% of animals surviving | [111] | |
| Chitosan | MxiH | — | i.n. | Mice | 60% of animals surviving, compared with 10% of controls | [112] | |
| Chitosan NF | N-IpaD | — | i.n. | Guinea pigs | 93.75% protective efficacy against ocular challenge in guinea pigs | [113] | |
| TMC nanoparticles | N-IpaD | — | Oral | Guinea pigs | 83.3% protection against ocular challenge in guinea pigs | [114] | |
| V. cholerae | OMVs | OMV components | — | Oral, i.n., or i.p. | Rabbits and mice | 60%–100% protective from watery diarrhea from different V. cholerae strains in rabbit; 100% protection against colonization with V. cholerae in neonatal mice from immunized dams | [115], [116] |
| L. casei or L. reuteri | CTB (Cytoplasm or secretory)b | — | No results | Mice | No results | [117] | |
| S. typhimurium | OMVs | OMV components | — | i.p. | Mice | Bacterial replication inhibited | [118] |
| S. typhi and paratyphi A | Bivalent OMVs | OMV components | — | Oral | Mice | 80% of animals surviving against S. typhi and 90% of animals surviving against S. paratyphi A | [119] |
| S. typhi | VLP (HBsAg) | Vi | — | s.c. | Mice | No results | [42] |
| S. enterica serovar Enteritidis | L. casei | FliC (Surface)b | — | Oral | Mice | Decreased bacterial counts in the spleen | [120] |
| S. enterica serovar Enteritidis | L. casei | FliC (Surface)b or fusion of FilC and SipC (Surface)b | — | No results | Mice | No results | [121] |
b. Constructed in an expression vector; i.n., intranasal; s.c., subcutaneous; i.m., intramuscular; i.p., intraperitoneal; i.d., intradermal; s.l., sublingual; —, without added adjuvant.
2.2.1. Pathogenic E. coli
Enterotoxigenic E. coli (ETEC) is the most common cause of diarrhea in humans [81]. It has become a major global food and waterborne pathogen involved in outbreaks of bloody diarrhea and hemolytic–uremic syndrome (HUS) worldwide [122]. Colonization factors (CFs), produced in ETEC, were considered as targets for vaccine production [123], [124]. Although about 25 different types of CFs have been identified [123], [125], naked CFs are not suitable for oral immunization because of their high sensitivity to the harsh environment of the gastrointestinal tract.
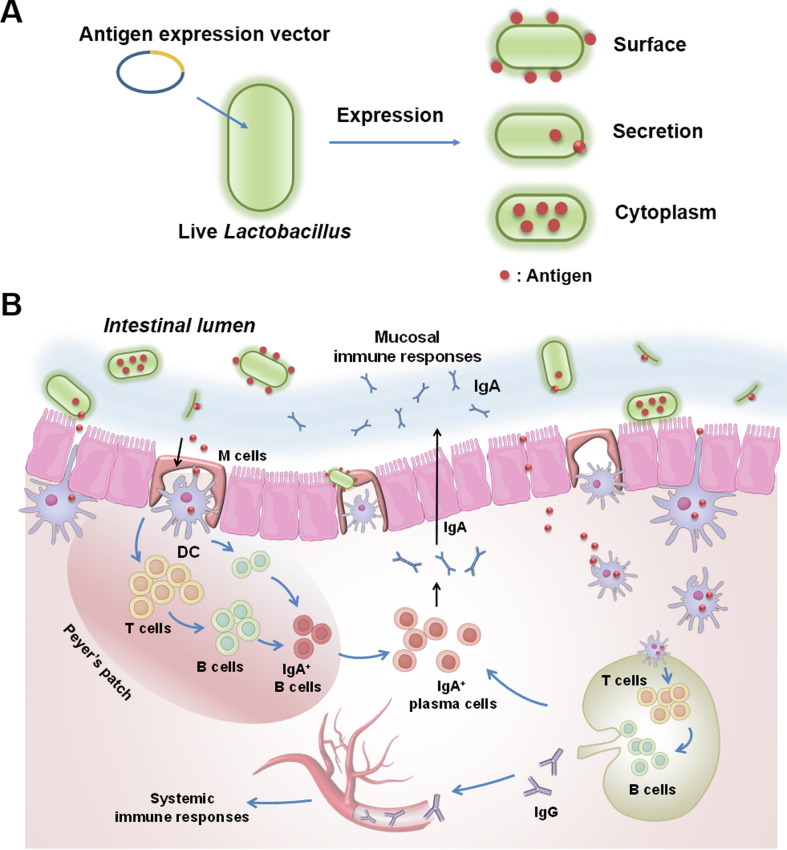
Lactic acid bacteria Lactic acid bacteria are a safe and efficient mucosal delivery carrier, which is widely used in the prevention of gastrointestinal infectious diseases. Compared with some other carriers, lactic acid bacteria are more favorable for oral administration because they can pass through the extreme environment of the stomach and colonize the intestine. Moreover, both strong antigen-specific systemic and mucosal immune responses are induced by lactic acid bacteria through mucosal immunity. In the design of E. coli vaccine, antigens could be located on the surface, in the cytoplasm, or be secretions of lactic acid bacteria (Fig. 3 ). At present, many lactic acid bacteria, such as L. reuteri, L. casei, L. plantarum, and L. plantarum, have been used to express a variety of antigens, but L. casei has been most studied [126]. For example, L. casei expressing F41 constructed by Liu et al. can stimulate strong systemic and local mucosal immune responses simultaneously after oral immunization, protecting mice from lethal challenge. It is worth noting that this immunization strategy can also stimulate long-term protection, considering that it can maintain more than 80% protection 9 weeks after the last immunization [84], [85]. Another study also revealed the passive protection effects of L. casei expressing F41 antigen considering that the orally or intranasally immunized dams provided 90% protection against lethal challenge to their offspring [86]. Furthermore, Yu et al. found that immunization with L. casei co-expression or fusion expression of FaeG antigen and fusion protein (including LTB and mutated LTA) can stimulate a stronger mucosal immune response and provide 100% protection [89]. Since the intestinal epithelium contains a large number of M cells, which can bind B5 toxins (such as LTB and CTB) through the GM1 receptor [127], B5 toxin proteins can be used as a mucosal adjuvant to further improve the mucosal immune response of the lactic acid bacteria-based delivery system.
Fig. 3.
Schematic diagram of construction of oral live lactic acid bacteria vector vaccine and its immune response. (A) Exogenous protein expression and localization in lactic acid bacteria. (B) Following oral vaccination, both systemic and local mucosal immune responses are induced simultaneously.
OMV OMVs have been widely studied for the use in vaccines. Intranasal immunization with OMVs from ETEC was shown to induce antibodies against various virulence proteins and inhibit bacterial colonization in the small intestine [128]. However, due to the existence of shiga toxin (STx) and LPS endotoxins, the OMVs produced from EHEC O157: H7 have intrinsic toxicity with the possibility to develop HUS; thus, the preparation of E. coli O157: H7 OMV vaccine needs to overcome its toxicity. Kim et al. generated attenuated OMVs through the mutation of msbB (encoding an acyltransferase that catalyzes the final myristoylation step during lipid A biosynthesis) and A subunit of STx [129], [130]; after immunizing mice by eyedrops and confirming the safety, both humoral response and mucosal (in tears, saliva, and feces) immune response were induced, providing enough protection from the challenge by the lethal HUS-causative agent (wild-type OMVs) [94]. Although there is relatively poor lymphoid tissue in the eyes and there are a few reports on immunization via eyedrop route, the connection of lacrimal duct and nasal cavity may result in immunoactivities in both nasal and ocular mucosa. Moreover, the homing of lymphocytes can distribute the effect of mucosal immune response on each mucosal surface of the body; therefore, the intestinal IgA can be successfully induced through other mucosal pathways. Considering that the response at the immune location is still the strongest, the oral route of OMVs should be further tried.
Compared with the application of OMVs-based vaccines in the prevention of respiratory diseases, OMVs delivery systems have more applications in intestinal diseases, and correspondingly, there are a series of successful modifications to OMVs. First, various methods can reduce the toxicity of OMV. The toxicity of OMVs can be reduced by deleting the lipid A modification gene msbB, htrB, or combined msbB and pagP [131], [132], [133], [134]. Another method to reduce the toxicity of OMVs is to pre-treat them with all-trans retinoic acid, active metabolite of vitamin A, which has both anti-inflammatory and mucosal adjuvant properties [135]. Then, through some modifications, the OMV yield can be improved. Choi et al. produced OmpC-enriched OMVs through overexpression of small RNAs, MicA, in S. typhimurium. The yield of OMVs was strongly increased and the OmpC-enriched OMVs promoted Th1- and Th17-type immune responses, providing full protection against lethal challenge by Salmonella [136]. Furthermore, the yield can also be improved through the deletion of tolR gene to disrupt the Tol-Pal system on the cell wall. After immunization through the subcutaneous route, the protective effects of OMVs in mice were not inferior compared with those of polysaccharide conjugate vaccines [137]. In addition, by further optimizing the form of OMVs, immune response and protection can be additionally improved, namely, OMVs encapsulated in polyanhydride nanoparticles (OMV-NPs) can induce Th1 immune responses [109], [138], which is more suitable for intracellular bacteria, such as S. flexneri. The OMV-NPs formulation even provided full protection on day 56 after nasal immunization in mice, while the protection with OMVs alone was only 40% [108]. Thus, OMVs, which are easier to produce, have a great potential to become a candidate product for the application of vaccines against intestinal infectious diseases.
Eudragit L-100/Chitosan Chitosan is a natural biodegradable polysaccharide derived from chitin, with good biocompatibility and adhesion properties. Although chitosan is capable of increasing transit time of antigens in the gastrointestinal tract and inducing mucosal immune response, its oral administration is limited by the low resistance to acidic pH [139], [140]. To solve this problem, Eudragit L-100, a coating material soluble at pH above 6.0, was used to protect the antigens from detrimental effects in the upper gastrointestinal tract. As reported, Eudragit L-100-based oral delivery vehicle loading ETEC F4 or F18 antigen could induce higher antigen-specific IgG1 and IgG2a antibody responses in serum and antigen-specific IgA in saliva compared with those with antigen immunization alone [141]. To further improve the immune response, a combined delivery system was built by coating Eudragit L-100 on the chitosan nanoparticles which loaded protein antigens or OMVs from ETEC. Oral immunization of the formulations in animals elicited antibody response and inhibited the ETEC colonization in small intestine [95], [96]. Thus, for some special delivery requirements, multiple delivery strategies can be used at the same time.
In addition to the vehicles described above, some other polymers, inorganic materials, and lipid or oil-based vehicles have been explored to develop E. coli vaccines (Table 2). Although strong immune response could be elicited in mice, most of the results lack the in vivo results about the protective effect, and therefore further confirmation is needed.
2.2.2. Shigella
Shigella species are gram-negative bacteria belonging to the Enterobacteriaceae family. According to the serological type, they are divided into four categories: S. dysenteriae, S. boydii, S. flexneri, and S. sonnei. Shigella spp. are the main bacterial causes of persistent epidemic diarrhea and result in almost 125 million diarrheal episodes and around 160,000 deaths annually [142]. Usually, a low-dose inoculum can cause the disease, resulting in aggressive watery or mucous/bloody diarrhea. However, there is currently no licensed vaccine against Shigella, and the continuous emergence of drug-resistant strains makes antibiotic treatment difficult [143].
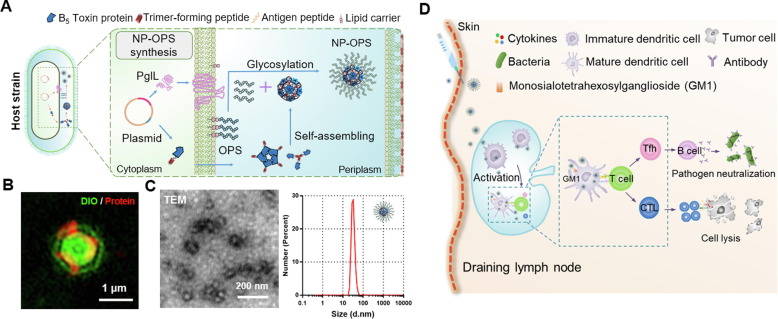
Self-assembled proteinaceous nanoparticles Recently, Pan et al. developed Nano-B5 platform to produce nanoscale bacterial polysaccharide conjugate vaccines in vivo. Proteinaceous nanoparticles were self-assembled via fusion of a natural adjuvant effective pentamer domain (bacterial B5 toxin) and an unnatural trimer domain. Then, O-polysaccharide of S. flexneri was conjugated to the nanoparticle through glycosylation system (Fig. 4 ) [111]. The nanovaccine can significantly slow down its dissipation at the injection site and enhance lymph node drainage. This delivery vector contains B5 toxin protein module such as CTB, which can bind to GM1 receptor on the surface of APCs, thus promoting antigen endocytosis and presentation [144]. After confirming the safety, subsequent subcutaneous immunization in mice indicated that both strong antigen-specific humoral and cellular immunity were induced, providing complete protection against challenge with 5 × half-lethal dose. In addition, the safety and high efficiency of the delivery carrier were further proven in the cynomolgus monkey model [111]. Polysaccharide-conjugated vaccines are considered the most successful form of bacterial vaccines [145]. However, the traditional chemical method is a time-consuming and costly process [146], so this biological method can solve this problem by one-step fermentation and one-step purification to obtain vaccine products [147], [148], [149], [150]. This study is also the first study to use a fully biosynthetic method to prepare a nanoscale polysaccharide-conjugated vaccine, leading to a great improvement in the immune effect.
Fig. 4.
Self-assembled proteinaceous nanoparticles for antigen delivery[111]. (A) Schematic diagram of protein self-assembly and conjugation with bacterial polysaccharide antigen through glycosylation system. (B) Super-resolution structured illumination microscopy images of nanoparticle-expressing bacteria. (C) Transmission electron microscopy images of the nanoparticles conjugating polysaccharide and their size distribution. (D) The vaccine can rapidly enter the draining lymph nodes and simultaneously stimulate strong humoral and cellular immune responses.
Chitosan-based delivery systems Chitosan particles are endowed with the properties to prevent encapsulated antigen degradation and extend antigen duration time on the mucosal surface [151]. As an alternative to the oral route for chitosan particles without an additional protection from acid degradation, direct nasal immunity may stimulate the systemic and intestinal IgA immune responses. A study showed that the loading of recombinant Shigella MxiH antigen into chitosan nanoparticles resulted in enhanced humoral and mucosal immune responses in intranasally immunized mice [112]. In addition, various chitosan-based delivery systems are available. For example, Jahantigh et al. described a chitosan nanofibrous membrane (NF) with a high surface area to volume ratio, which is more appropriate for nasal vaccine delivery. After loading N-terminal region of IpaD (N-IpaD) antigen of Shigella, the guinea pigs were intranasally administered, which resulted in induction of significant serum and mucosal antibody responses and protection [113]. Moreover, O-methylated free trimethyl chitosan (TMC) nanoparticles, which have both mucoadhesive properties and excellent absorption-enhancing effects, even at neutral pH over a wide pH range for oral delivery [152], or chitosan-tripolyphosphate (CS-TPP) nanoparticles coated with Eudragit L-100 [110], were developed to further improve the immune response and protective effect of oral or intranasal routes (Table 2). However, these immune effects do not seem to be as good as those of the two previous kinds of delivery systems; thus, further optimization of chitosan or combination with other systems should be explored to enhance immune response.
Similar to the respiratory tract infections, digestive tract infections often involve bacterial colonization and invasion. Therefore, mucosal administration is also the first option for immune route, and many delivery systems such as probiotics and OMVs have shown ideal effects in protective. However, unlike the respiratory tract, mucosal delivery in the digestive tract faces more difficulties that need to be overcome, including the acidic environment in the stomach, hydrolysis by gastrointestinal proteases, immune tolerance, and intestinal microbes. In addition, the colonization of gastrointestinal pathogens does not cause easy spreading of the pathogens from person to person. Therefore, the choice of mucosal immunity or other immunization pathways should be specifically weighed. In the prevention of digestive tract infectious diseases, although there are not many studies on non-mucosal immune delivery systems, some have shown great potential, such as the latest bacterial B5 toxin-based protein nanocarriers.
2.3. Other pathogenic bacterial diseases and advanced delivery systems
In addition to common pathogenic bacteria that cause respiratory tract infections and diarrhea, there are many bacteria that are highly pathogenic and have various infection methods and target organs, such as Bacillus anthracis, Staphylococcus aureus, and others. In addition, some pathogenic bacteria, such as Helicobacter pylori and Brucella, cause chronic infections that are easy to relapse or difficult to cure. For the prevention of diseases caused by these pathogenic bacteria, a series of efficient delivery systems have been developed (Table 3 ). This section mainly focuses on the delivery systems of some important pathogenic bacteria.
Table 3.
Effect of vaccines with delivery systems against different bacterial challenges.
| Pathogenic bacteria | Delivery system | Antigen(s) | Adjuvants used | Route | Animal species | Protection after challenge | Ref. |
|---|---|---|---|---|---|---|---|
| B. anthracis | Adenovirus | PAD4 | — | i.m. | Mice | 100% of animals surviving after single immunization | [153] |
| SFV | PA | — | s.c. | Mice | 100% of animals surviving | [154] | |
| FHV VLP | PA | — | s.c. | Rats | 100% of animals surviving after single immunization | [155] | |
| Bacteriophage T4 nanoparticle | PA | — | i.m. | Mice, rats, and rabbits | 100% of animals surviving | [156] | |
| Live influenza virus prime and killed RV vector or the vaccinia virus vector boost | PA | — | i.n. prime and i.m boost | Mice | No results | [157] | |
| TMV | PA232–247 and PA628–637 | — | i.p. | Mice | Almost no protection | [158] | |
| Liposomes | PA | Monophosphoryl lipid A | i.m. | Rabbits and rhesus macaques | 100% of animals surviving | [159], [160] | |
| Liposome-like vessels | PAD4 | Aluminium hydroxide | i.p. | Mice | 70% of animals surviving | [161] | |
| FUC-HTCC NPs | Anthrax vaccine AVA | — | i.p. | Mice | 100% of animals surviving | [162] | |
| Chitosan | PA | C48/80 | i.n. | Mice | No results | [163] | |
| Chitosan derivative TMC | PA | CpG or Poly I:C or not | s.c.a, i.m.a, or i.p. | Mice | 83.3% of animals surviving in s.c. or i.m. route | [164] | |
| CS-NH2 microparticles | PA | — | s.c. | Mice | No results | [165] | |
| Poly-l-lactide (PLLA) polylactide (PLA) microspheres | PA | — | i.m prime and either i.m. or i.n. boost | Mice | 100% of animals surviving | [166] | |
| Dendriplex PLGA nanoparticles | PA (DNA) | — | i.m. | Mice | High antibody titer but without neutralizing activity | [167] | |
| PLGA nanoparticles | PAD4 | — | i.p. | Mice | 11% of animals surviving after single immunization | [168] | |
| sucrose polymer Ficoll | PA | CpG-ODN | i.m. | Mice | 100% of animals surviving after single immunization | [169] | |
| Soybean oil-and-water nanoemulsion (NE) | PA | — | i.n. | Mice and guinea pigs | 100% of animals surviving | [170] | |
| L. acidophilus | PA(Surface)b | — | No results | No | No results | [171] | |
| L. casei | PAb | — | Oral or i.n. | Mice | No results | [172] | |
| L. acidophilus | PA with DC-targeting peptide (Secretory)b | — | Oral | Mice | 75% of animals surviving | [173] | |
| L. gasseri | PA with DC-targeting peptide (Secretory)b | — | Oral | Mice | 100% of animals surviving and 30% of animals surviving without DC-targeting peptide | [174], [175] | |
| S. enterica | PA, PAD1 and 4, and PAD4 | — | Oral | Mice | PA: 83% of animals surviving, PAD1 and 4: 25% of animals surviving, PAD4: no protection | [176] | |
| Neisseria meningitidis group B | E. coli OMV | Glycan antigens (Polysialic acid (PSA) and T antigen) | — | s.c. | Mice | 50% SBA was observed at over 100-fold dilutions of the serum | [177] |
| Brucella | SFV | Cu-Zn SOD | — | i.p. | Mice | 1.52 (3.07–1.55)c | [178] |
| IF3 | — | i.p. | Mice | 1.09 (6.96–5.87)c | [179] | ||
| Influenza virus | L7/L12 or Omp16 | — | i.n., eyedropa, or s.c. | Mice | The best: Omp: 16: 3.78 (4.54–0.76)c, eyedrop; Bivalent: 3.9 (4.54–0.64)c, eyedrop | [180] | |
| Influenza virus | Tetravalent vaccine formulation expressing Omp16, L7/L12, Omp19, and Cu-Zn SOD | — | i.n.a, eyedrop, or s.l. | Guinea pigs | The best: 2.8 (2.86–0.06)c, i.n. | [181] | |
| L. lactis | L7/L12 (Cytoplasm)b | — | Oral | Mice | 0.57 (7–6.43)c | [182] | |
| Cu-Zn SOD (secretory)b | — | Oral | Mice | 1.35 (7.1–5.75)c | [183] | ||
| L. lactis (Live or killed) | Omp31 (Cell Wall-Anchored)b | — | Oral or i.p. | Mice | No results | [184] | |
| Attenuated S. typhimurium | Fusion of L7/L12 and BLSb | — | Oral | Mice | Secretory expression: 1.55 (4.44–2.89)c; Intracellular expression: 1.32 (4.44–3.12)c | [185] | |
| L7/L12 | — | i.m. | Mice | About 1.7 (3.4–1.7)c | [186] | ||
| Ochrobactrum anthropi | Cu-Zn SOD | CpG | i.p. | Mice | 2.42 (5.30–2.88)c | [187] | |
| TMC | Omp19 | — | Orala or i.p. | Mice | The best: against B. abortus: 2.46 (6.3–3.84)c, oral; against B. melitensis: 2.38 (6.14–3.76)c, oral | [188] | |
| Mannosylated chitosan nanoparticles | FliC | — | s.c. | Mice | Against B. melitensis: 1.34 (5.67–4.33)c; against B. abortus: 1.22 (5.24–4.02)c | [189] | |
| escheriosome | L7/L12 | — | s.c. | Mice | 1.46 (4.58–2.93)c | [190] | |
| Cu-Zn SOD | IL-18 | s.c. | Mice | 1.5 (5.2–3.7)c | [191] | ||
| PLGA | L7/L12 | — | i.p. | Mice | 1.79 (5.94–4.15)c | [192] | |
| CaPNs | FliC, 7α-HSDH, BhuA and multi-epitopes (Poly B and poly T) | — | s.c. | Mice | The best: against B. melitensis: Poly B + T, 1.5 (5.77–4.27)c; against B. abortus: Poly B + T, 1.37 (5.29–3.92)c | [193] | |
| S. aureus | OMV (E. coli) | HlaH35L, SpAKKAA, FhuD2, Csa1A, and LukE | Alum | i.p. | Mice | 90% of animals surviving | [194] |
| PDNVs (E. coli) | SAcoagulase | — | i.p. | Mice | 100% of animals surviving | [195] | |
| extracellular vesicles (EVs) | HlaH35L, LukE and EVs components | — | s.c. | Mice | About 70% and 50% of animals surviving after challenging with two S. aureus isolates | [196] | |
| ICG-loaded MSNs | EVs | — | s.c. | Mice | Decreased bacterial loading in skin and organs | [197] | |
| PDNVs | PDNVs components | — | s.c. | Mice | No results | [198] | |
| L. lactis | ClfAb and FnbpAb | Freund’s adjuvant | Unknown | Rats | Less infected vegetations after challenging with S. aureus Newman in L. lactis ClfA-immunized animals; FnbpA did not have the same effect | [199] | |
| PilVax | B-cell epitope, D3, from FnbpA | — | i.n. | Mice | Decreased bacterial loading in intestine and nasal mucosa | [200] | |
| Cowpea mosaic virus | D2 domain of FnbpB | — | i.n. or oral | Mice | No results | [201] | |
| Live attenuated S. typhimurium | SaEsxAb and SaEsxBb | — | Oral | Mice | 22.2% and 44.4% of animals surviving after challenging with S. aureus USA 300 for SaEsxA and SaEsxB; no animals surviving after challenging with S. aureus Newman | [202] | |
| Red blood cell membrane-coated PLGA | Hla, α-toxin, PVL, and γ-toxin | — | s.c. | Mice | Decreased bacterial loading in skin, blood, and organs | [203], [204] | |
| PP7 (VLP) | AIP1S | — | i.m. | Mice | Inhibiting abscess area and dermonecrotic; Decreased bacterial loading at the site of infection | [205] | |
| PLGA | CNA19 | — | s.c. or i.n. | Mice | No results | [206], [207] | |
| PLGA | rSEA | — | i.p. | Mice | 100% of animals surviving | [208] | |
| Chitosan | rSEB | — | i.n. | No results | [209] | ||
| Chitosan | Ami | No results | No results | No results | [210] | ||
| Liposome | AdsA (mRNA) | — | i.m. or s.c. | Mice | No results | [211] | |
| H. pylori | L. lactis (GEM) | CUE | — | Oral | Mice | Decreased bacterial loading in gastric tissue | [212], [213] |
| L. acidophilus | Hp0410 | — | Oral | Mice | Decreased bacterial loading in gastric tissue | [214] | |
| L. plantarum | Urease Bsubunit (UreB) (Cytoplasm)b | — | Oral | Mice | Decreased bacterial loading in gastric tissue | [215] | |
| HP55/PLGA nanoparticles | Recombinant antigen CCF | — | Oral | Mice | Decreased bacterial loading in gastric tissue | [216] | |
| OMVs | OMV components | — | Oral | Mice | Decreased bacterial loading in gastric tissue | [217] | |
| liposomes | Fusion of the urease linear epitope (19 amino acid residues) and CTB | — | Oral | Mice | Decreased bacterial loading in gastric tissue | [218] | |
| Yersinia pestis | Bacteriophage T4 nanoparticles | Mutated capsular antigen F1 and low-calcium-response V antigen | — | i.m. | Rats | 100% of animals surviving | [155] |
| 20:80 CPTEG:CPH | Fusion of F1 and V antigens | Cyclic dinucleotides (CDNs) | s.c. | Mice | 100% of animals surviving at 14 days and 75% at 182 days after single immunization | [219] | |
| OMVs | OMV components | — | i.m. | Mice | 100% of animals surviving in subcutaneous challenge; 100% and 50% of animals surviving in intranasal challenge with a median and a high dose | [220] | |
| L. plantarum | LcrV (Surface)b | — | Oral | Mice | No results | [221] | |
a. The better or the best route to achieve protection; b. Constructed in an expression vector; c. Log10 units of protection, obtained by subtracting the mean log10CFU for the experimental group from the mean log10 CFU for the corresponding control group; i.n., intranasal; s.c., subcutaneous; i.m., intramuscular; i.p., intraperitoneal; s.l., sublingual; —, without added adjuvant.
2.3.1. B. anthracis
B. anthracis is the causative agent of anthrax, an acute zoonotic disease. Humans can be infected through direct contact with broken skin, contaminated meat, or inhalation [222]. The spores of B. anthracis have strong survival ability and are resistant to sunlight, high temperature, and disinfectants. However, the spores are easy to prepare at low cost, so they are regarded as potential biological weapons [223]. Vaccines are an effective means to prevent anthrax; however, traditional attenuated live vaccines or adsorbed vaccines have a series of problems, such as side effects, short duration of protective effect, and complicated immune procedures. For example, anthrax vaccine absorbed (AVA), the only USFDA-approved anthrax vaccine, needs to be administered six times within 18 months and enhanced once each year [224]. Therefore, the development of a safe and effective human vaccine is of great significance for the prevention and control of anthrax. So far, various delivery carriers (e.g., viral vectors, bacterial vectors, liposomes, and polymers) have been developed (Table 3). Because the main antigen of B. anthracis is protective antigen (PA) or its domain, it is easy to compare the effects of different delivery vehicles.
Viral vector Viral vector vaccines consist of a nonreplicating virus that contains certain genetic material from the pathogen that needs to be immunized. It seems to be an ideal vaccine delivery vector because of its natural viral structure, which can be well recognized by the immune system [225]. Replication-incompetent adenovirus expressing domain 4 of PA (PAD4) could induce a stronger humoral and cellular immune responses than AVA and provide full protection against lethal spore or B. anthracis strain challenge in a single injection in intramuscularly immunized mice [153]. Such potent protection was also reported in Semliki forest virus (SFV) vector loading PA through the challenge with the B. anthracis strain [154]. However, some other viral vector vaccines, such as influenza viruses and rabies virus (RV) expressing PA, were unable to induce anthrax toxin neutralization antibodies, although the antibody titers against PA were high [157], [226]. Interestingly, this situation could be solved by heterologous prime/boost immunization strategy, and the particularly effective program was an initial intranasal administration of a live influenza virus vector, followed by intramuscular boosting with either the killed RV vector or the vaccinia virus vector [157]. The mechanism of this phenomenon is still unclear and one possible explanation is that the combination of different heterologous vectors may affect B-cell affinity maturation and Ig gene high frequency mutation in germinal centers. In addition, some VLP (e.g. flock house virus (FHV) VLP and bacteriophage T4 nanoparticle) vaccine were also explored; they exhibited good protection against the challenge by anthrax lethal toxin in rats or inhalational anthrax in mice, rats, and rabbits [155], [156]. Although the viral vector delivery system can achieve 100% protection, some viruses such as adenovirus need to be further modified to avoid possible pre-existing immunity in humans [227].
Liposomes and polymers Other potential delivery vehicles have also been used to study anthrax vaccines. For example, PA encapsulated in liposomes containing monophosphoryl lipid A could induce full protection from lethal pulmonary challenge with B. anthracis spores in rabbit and monkey models [159], [160]. However, liposomes are prone to oxidative degradation, resulting in low stability and short shelf life when used as delivery systems [228]. This problem can be solved with liposome-like vessels prepared by nonionic surfactant [229]. These nonionic surfactant vesicles (NISV) are self-assembling lamellar structures; they resemble liposomes because they are biodegradable, nonimmunogenic, and capable of encapsulating biologically active cargo [161], [230] . Another example is a kind of chitosan nanoparticle. Liu et al. described chitosan-based microparticles (CS-NH2 MPs) with abundant amino groups. After loading PA, the formulation induced the complement system activation and enhanced antibody response [165]. Recently, Chuang et al. reported a positively or negatively charged fucoidan-quaternary chitosan nanoparticle by conjugate fucoidan (FUC) and a chitosan derivative (N-(2-hydroxy)-propyl-3-trimethyl ammonium chitosan chloride, HTCC). The surface charge was adjusted by varying the mass ratio of two polyelectrolytes. After combining FUC-HTCC nanoparticles with the approved anthrax vaccine AVA, the novel anthrax nanovaccine significantly increased the IgG antibody titers and provided complete protection compared with CpG plus AVA (75%) or AVA alone (50%) in mice exposed to anthrax lethal toxin challenge [162]. However, some other polymer particles (e.g., sucrose polymer, chitosan or its derivative, and others) need an addition of an adjuvant (e.g., CpG, mast cell activator compound 48/80 (C48/80), Poly I:C) to elicit an enhanced immune response [163], [164], [231]. Although mice immunized with these liposomes or polymer-based vaccines can achieve a high protection rate after challenge, the use of adjuvants reduces their competitiveness compared with viral vectors.
2.3.2. Brucella
Brucellosis is one of the five major zoonotic diseases in the world and seriously endangers public health. More than 500,000 new cases of brucellosis occur every year [232]. In the 21st century, brucellosis shows a rebound trend worldwide [233]. Brucella (the pathogen of brucellosis) is a kind of gram-negative, facultative intracellular proteus. It can be divided into more than 10 species. Among them, B. melitensis, B. abortus, and B. suis are the three most toxic species, which can infect both animals and humans. At present, there are no vaccines approved for human use. The animal vaccines against brucellosis are live attenuated vaccines, which still have potential serious safety risks in production and use [234].
Viral vectors Although DNA vaccines encoding B. abortus Cu-Zn superoxide dismutase (Cu-Zn SOD) or translation initiation factor 3 (IF3) could induce specific cellular immune response and provide protection against challenge with B. abortus virulent strain in mice [235], [236], high concentrations and repeated doses are needed to improve in vivo transfection efficiency. So far, some viral vectors (SFV vectors, and especially influenza viral vectors) have been developed, with high safety and immunogenicity demonstrated in various models (chicken, ferrets, rhesus macaques, and humans) [178], [179], [237], [238], [239]. Influenza viral vectors may be a promising candidate for human use because of the lack of pre-existing immunity in humans. After immunization with recombinant influenza A viruses of the subtypes H5N1 and H1N1 expressing Brucella protective antigen (ribosomal protein L7/L12 or Omp16), a strong cellular immune response and protective effect were induced, even comparable with those induced by a commercial B. abortus S19 vaccine [180]. Further, a tetravalent vaccine formulation (expressing Omp16, L7/L12, Omp19, and Cu-Zn SOD in recombinant influenza viral vector subtype H5N1 without an adjuvant) was developed to prevent human brucellosis [181], [240]. In addition, because Brucella can infect people of any age, the long-term protection of this viral vector vaccine also needs to be considered.
Bacterial vectorsL. lactis can be used as a delivery system by expressing B. abortus antigen in the cytoplasm, cell wall, or extracellularly [241]. However, the final protective effect may be related to the antigen type and its location. For example, oral administration of L. lactis expressing L7/L12 in the cytoplasm only provided partial protection in mice [182], while secretory expression of Cu-Zn SOD induced protective immunity similar to positive controls (immunized with B. abortus strain RB51) [183]. Studies on attenuated S. typhimurium vector indicated that secretory expressing Brucella L7/L12 or fusion antigen (fusing L7/L12 and lumazine synthase (BLS)) could induce stronger humoral and cellular immune responses and higher protection against Brucella infection than intracellular expressing vectors [185], [186]. Thus, construction of L. lactic-based vaccines with secretory expressing Brucella antigens may be a more suitable way to induce protective immunization. Because Brucella is an intracellular bacterium, cellular immunity is relatively more important and this response could be strengthened through the addition of CpG adjuvants [187]. It was found that mucosal immunization could also provide protection from mucosal infection by B. abortus [185]. Therefore, the choice of appropriate adjuvants and mucosal route are beneficial for inducing the protective response against B. abortus infection.
In addition, some other delivery systems, such as chitosan derivatives (TMC and mannosylated chitosan nanoparticles), liposomes, polymers, and inorganics were used for vaccine design [188], [189], [190], [191], [192], [193]. Although there have been few reports about these delivery vehicles, some of them have shown promising application prospects.
2.3.3. S. aureus
S. aureus is a pathogenic bacterium that causes a wide spectrum of human infections, inducing severe skin lesions, pneumonia, bacteremia, and meningitis. Overuse of antibiotic regimens has led to the emergence of resistance, which presents a great challenge to clinical treatment.
OMVs have been widely explored for the development of vaccine against S. aureus. For example, OMVs from modified E. coli were decorated with five S. aureus protective antigens by fusion expression with lipoprotein leader sequence. The concentration of antigens reached 5%–20% of total OMV proteins, and great protection was demonstrated in three mouse infection models (a sepsis model, a renal abscess model, and a skin infection model) [194]. Generally, although the removal of LPS reduced the toxicity of OMVs, there are still many toxic proteins on the outer membrane [242]. Kim et al. designed bacterial protoplast-derived nanovesicles (PDNVs) by depleting toxic outer membrane components of E. coli to improve the security and productivity of OMVs. This delivery platform could load different antigens. In immunized mice, strong antigen-specific humoral and cellular immune responses were induced, and full protection from lethal challenge with S. aureus was observed [195]. In addition, the OMVs of S. aureus itself were also used to prepare vaccines because they contain a variety of antigen components. Wang et al. prepared engineered extracellular vesicles (EVs) by expressing non-toxic HlaH35L and LukE in a S. aureus mutant strain. The animal experiment results indicated that the engineered EVs could induce stronger specific antibody response and protect mice in a lethal sepsis model. Further, an array of bacterial antigens, such as lipoproteins, exotoxins, and cytoplasmic proteins, could also be encapsulated as a vaccine platform [196]. Since S. aureus can invade and survive inside host cells, cellular immunity is also needed. By coating EVs on the surface of indocyanine green (ICG)-loaded magnetic mesoporous silica nanoparticles (MSNs), endocytosis EVs can escape from lysosomes through their breaking by heating ICG with laser irradiation, and improved CD8+ T cell responses were induced to prevent and treat S. aureus infection [197].
Generally speaking, there are two main strategies to prepare S. aureus vaccines using microbial secreted vesicles. One is to express S. aureus antigen in E. coli and the other is to load the antigen in secreted OMVs. As an engineering strain, E. coli is more convenient for gene operation, and it can be easily optimized. For the other, the EVs of S. aureus itself contain a large number of antigen components, so they may also be a potential candidate. However, the main problem to be solved includes the safety of EVs because EVs contain biologically active toxins and induce a strong inflammatory response [243], [244], [245].
For some pathogenic bacteria with various infection forms and target organs, a variety of advanced delivery systems have been developed; among them, biological vectors such as viral vectors and probiotic vectors have been widely used. For a B. anthracis vaccine, the antigen is relatively unique, so different delivery systems are more comparable. It has been found that the immune effect of viral vectors from animal experiments is better than that of probiotic vectors, which was also revealed in the Brucella vaccines studies. For this phenomenon, in addition to the carrier itself, the immunization method and immunization dose may also be important factors. Furthermore, due to the variety of bacteria, the same delivery system in different pathogenic bacteria vaccine research will provide references for the general application of an advanced delivery system.
3. Viral infection diseases and advanced delivery systems
Among infectious diseases, viral infections are one of the leading causes of mortality worldwide. They usually evolve to cause a global life threat and socioeconomic recession. Despite the huge challenges of the ongoing COVID-19 pandemic, the achieved progress on the drug treatment of other viruses (e.g., Ebola viruses, human immunodeficiency virus (HIV), and hepatitis viruses) is impressive. To overcome this hurdle, advanced vaccine delivery systems are more urgently required.
3.1. Major epidemic viral infections and prevention challenges
According to International Committee on Taxonomy of Viruses (ICTV), there are 36 classes, 168 families, and 6590 species of viruses (Virus Taxonomy: 2019 Release) [246]. Among the infectious viruses, an overwhelming majority of the viral infections lack the specific drugs, and vaccines have historically been the foremost effective means to save people’s lives. Recent epidemic virus diseases and major vaccine products or clinical candidates are listed in Table 4 .
Table 4.
The major virus-related diseases and vaccine development.
| Virus | Remarkable vaccines | Developer | Adjuvants used | Approval /Clinical | Vaccine type/Reference |
|---|---|---|---|---|---|
| HBV | Recombivax HB | Maurice Hilleman | Alum | 1986 | Subunit [247] |
| Influenza Virus | Inflexal® V | Crucell Berna Biotech | Virosomal | 1997 | Inactivated [248] |
| HPV | Gardasil®/Gardasil® 9 | Merck | Alum | 2006/2014 | Recombinant [249] |
| Cervarix®Cecolin | Glaxo Smith KlineInnovax | AS04Alum | 2007 2019 |
Recombinant [249] Recombinant |
|
| Ebola | Ervebo®VSV-EBOV-GP | Merck | — | 2019 | Attenuated [250], [251] |
| SARS-CoV-2 | mRNA-1273 | Moderna | Liposome | 2020 | mRNA vaccine [252] |
| AZD1222 | University Oxford/Astra Zeneca | — | 2020 | Adenovirus vector [253] | |
| Tozinameran(BNT162b2) | Pfizer & BioNTech | Liposome | 2020 | mRNA vaccine [254] | |
| BBIBP-CorV | Beijing Institute of Biological Products | Alum | 2021 | Inactivated [255] | |
| Ad5-nCoV | Beijing Institute of Biotechnology | — | 2021 | Adenovirus vector [256] | |
| HIV | ALVAC-HIV (vCP1521) prime, ALVAC-HIV /AIDSVAX gp120 boost | Thai Component and U. S. Army Medical Component | — | Phase II | RV306/NCT01931358 [257] |
| Noroviruses | GI.1/GII.4 Bivalent Virus like particle (VLP) Vaccine | TAKEDA benchmark | Alum | Phase II | NCT02153112 (Child); NCT02475278 (Adult) [258] |
| Norwalk VLP Vaccine | LigoCyte Pharmaceuticals | Chitosan/ MPL | Phase II | NCT00806962 [259] | |
| MERS | MVA-MERS-Sin escalating dose regimes | Marylyn Addo | — | Phase I | Attenuated [260] NCT03615911 |
3.1.1. Hepatitis B virus (HBV)
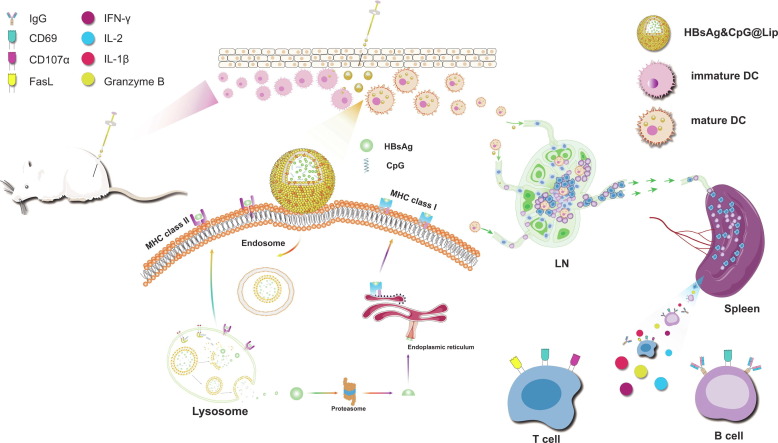
Globally, more than 290 million people are living with HBV [261]. HBV attacks the liver and accounts for more than 900,000 deaths per year. Under the worst-case scenario, there will be a projected 5.3 million additional chronic HBV infections among children born between 2020 and 2030 and one million additional HBV-related deaths among those children. The next-generation HBV vaccination is expected not only to prevent the virus infection, but also to accelerate the elimination of viral hepatitis or even cancer.
3.1.2. Influenza virus
During the 1918 influenza pandemic, 20–40 million deaths occurred in the second phase of the outbreak. Antigenic shifts and mutations of the genome between different species of influenza result in the high degree of variation, thereby enabling the emergence of novel influenza strains and drug resistance [262]. Every year, the WHO predicts the possible prevalent virus types and suggests the components for producing the influenza vaccines. However, the emergence of new strains continues to pose a public health threat. Therefore, an advanced vaccine delivery system with cross-protection efficacy would be very desirable.
3.1.3. Human papillomavirus (HPV)
HPV is the most common sexually transmitted infection, with the lifetime probability of acquiring HPV ranging between 85% for women and 91% for men. HPV vaccination is an effective primary prevention strategy to reduce HPV infections that can lead to cancer (e.g. cervical, vaginal, anal, and penile cancer) [263]. Although clinical trials of Gardasil and Cervarix have been extremely promising, these first-generation vaccines are not ideal vaccine candidates. Researchers are actively working on the development of other HPV vaccines or delivery systems that may be more effective against a broader range of HPV types (e.g., Gardasil 9-valent vaccine), cheaper (e.g., Cecolin), easier, or even therapeutic.
3.1.4. Ebola virus
Ebolavirus is a highly lethal viral pathogen that belongs to Filoviridae (filament-like viruses). It causes viral hemorrhagic fever. The 2014–2016 Ebola epidemic resulted in more than 28,000 cases and more than 11,000 deaths. Ervebo is the first vaccine licensed by FDA in 2019 for the prevention of Ebola virus disease [250], and over 218,000 doses have been administered to individuals, as of 2020. The vaccine is a recombinant live-attenuated Vesicular stomatitis virus (rVSV) in which the VSV-G envelope glycoprotein (GP) has been completely replaced by the Zaire ebolavirus GP (rVSVΔG-ZEBOV-GP). The data on immunogenicity demonstrate that anti-GP antibodies are generally detectable by 14 days after vaccination, with up to 100% seroconversion observed by 28 days after the dose [264]. Nevertheless, vaccine candidates with relatively short time to immunity after antigen challenge are still under development for immediate public health responses.
3.1.5. SARS-CoV-2
The novel coronavirus SARS-CoV-2 causes coronavirus disease 2019 (COVID-19), which was declared a pandemic on March 11, 2020 by the WHO. Presently, COVID-19 affects most countries on our planet with the result of 163,869,893 confirmed cases, including 3,398,302 deaths, as of May 19, 2021 (https://covid19.who.int/). The pandemic has been considered the worst crisis since the World War II. Vital organs including the heart, liver, kidneys, gastrointestinal tract, and the central nervous system can be affected, causing multiple organ complications [7]. Fortunately, under global efforts, 15 COVID-19 vaccines have been developed at an unprecedented speed (as of May 7, 2021, the main examples can be seen in Table 4). The protection rates (>90%) of the first COVID-19 vaccines are exciting [265], and a reduction rate of 85% in BNT162b2 vaccine recipients has been newly reported [266].
3.1.6. HIV
The emergence of AIDS was first reported in 1981, followed by the identification of HIV as the cause of the disease. HIV/AIDS is now a global pandemic, and 32.7 million people have died from AIDS-related illnesses (as of 2019, according to the Global HIV & AIDS statistics-2020 fact sheet). Recently, an RV144 vaccine that contains canarypox vector ALVAC-HIV (vCP1521) prime and AIDSVAX®gp120 B/E protein boost advanced to phase III clinical trials in Thailand, which shed light on the recent progress. The protection rate was reported to be 31.2% [257]. However, several vaccine candidates (e.g., Env DNA-rAd5) failed to induce the antibodies with neutralization activity [267], and the first licensed vaccine is long expected.
3.1.7. Norovirus
Noroviruses are a major cause of acute gastroenteritis (AGE) around the world. They are transmitted through the fecal–oral route. This virus leads to 200,000 children deaths every year. Recently, the vaccine candidates have focused on the Norovirus VLP Vaccines. One of the most promising candidates is GI.1/GII.4 Bivalent VLP Vaccine with alum adjuvant in phase 2, from Takeda benchmark [258]. This formulation elicits robust immune responses, as Pan-IgG (>93%), IgA (>93%), and antigen-blocking antibodies (>83%) are already evident 8 days after vaccination. The other candidate is the intranasal Norwalk VLP Vaccine with chitosan/MPL adjuvants in phase 2 from Ligocytes [259]. IgG and IgA antibodies increased 4.8- and 9.1-fold, respectively, for the 100-μg dosage level. Although AGE is partially self-limited, it is also associated with a higher risk of severe or fatal consequences in vulnerable age and in the older persons with chronic diseases. In the near future, the development of norovirus vaccine should be expedited.
3.2. Delivery strategies against virus infection
Despite the broad medical impact from the vaccination, there are numerous globally devastating virus diseases without fully protective vaccines. As shown in Table 4, HIV vaccines are still restricted to the clinical trial phase. Even if the tested vaccine candidates help to produce antibodies, the protection (31.2%) may be inadequate. The poor efficacy is highly due to the lack of an efficient adjuvant/delivery system rather than the poor specificity of antigens. In this context, it is more important to develop a vaccine delivery system with high efficiency, long-term efficacy, or cross-protection. Efforts also include the material-based nano/microparticles, biological exosomes, or integrated vehicles, which are described in the section about prophylactic vaccines against bacterial diseases (2.1–2.3). In this section, we further highlighted the advanced vaccine delivery strategies that can meet the fundamental requirements for controlling the virus infections.
3.2.1. Advanced delivery strategy for robust/high protection efficiency
For the sudden outbreaks of virus infection (e.g., COVID-19 and Ebola), there is an urgent need to provide robust/high preventive vaccination. Although a few vaccines were licensed in emergency, these first-generation vaccines still need to achieve a higher protective efficacy in a timely manner to end the pandemic. To obtain a similar rapid protection but a much better safety, advanced micro/nano delivery systems with safer antigens (subunit or peptide antigens) are being explored. These vaccine delivery platforms involve synthetic particles, proteinaceous particles, rebuilt particulate adjuvant, and an integrated device.
i) Synthetic or proteinaceous micro/nano particles offer great utility in robust vaccine design. Ma’s group has designed a series of micro/nanoparticle as “Chassis” to mimic natural pathogen (shape, fluidity, or other properties), and assembled antigen and other components (e.g., adjuvant) on/in it to obtain a synthetic vaccine [8], [268]. For example, the polylactic-co-glycolic acid (PLGA) nanoparticles-stabilized Pickering emulsion (the core was liquid oil and the antigen was loaded in the nanoparticle gap among the oil surface) showed virus-mimicking deformability/mobility. In virtue of these advantages, the highest protection percentage of H1N1 vaccine was obtained with this chassis. The safety and efficacy were far better than those with commercial adjuvants (e.g., MF59). In another example, researchers developed a nanoparticle vaccine by covalently conjugating the self-assembled 24-mer ferritin (Ft) with the receptor binding domain (RBD) and/or heptad repeat (HR) subunits of SARS-CoV-2 S protein. The nanoparticle vaccination of rhesus macaques induced significantly higher neutralizing antibodies and T and B cell responses prior to boost immunization [269]. Since the components of Ft nanoparticles are from nature, this proteinaceous delivery system is of higher safety than the vaccines harboring artificial materials. However, the virus-mimicking system via the synthetic chassis (prepared of an FDA-approved material) also has translation merits, which facilitates the widespread vaccinations to curb the virus.
ii) Incorporating traditional vaccines adjuvants with advantageous physicochemical properties (e.g., size and mobility) is an intelligent manner to develop safe and efficient vaccines within a short time frame. As the most accessible adjuvant, alum is still the sole employed adjuvant in most countries. However, it tends to attach on the membrane rather than to enter the DCs, leading to the absence of intracellular process of the antigens, and thus limits T cell-mediated immunity. Xia et al. packed alum on the squalene/water interphase, forming an alum-stabilized Pickering emulsion (PAPE) [270]. “Inheriting” from alum and squalene, PAPE demonstrated a good biosafety profile. With the dense array of alum on the oil/water interphase, PAPE not only adsorbed large quantities of SARS-CoV-2 antigens, but it also harbored a higher affinity for DC uptake, which provoked the uptake and cross-presentation of the delivered antigens. Compared with alum-treated groups, more than six times higher antigen-specific antibody and three-fold more IFN-γ-secreting T cells were induced, indicating the potent humoral and cellular immune activations (Fig. 5 ). This work provided important insights towards a safe and efficient adjuvant platform for enhanced COVID-19 vaccines.
Fig. 5.
Particulate alum via Pickering emulsion as an enhanced COVID-19 vaccine adjuvant. (A) Schematic illustration of PAPE strategy. By “mirroring” clinically approved alum, PAPE “inherited” its acknowledged biosafety profile. (B) Efficient uptake of PAPE (red color in the left confocal images) and lysosomal escape (as indicated by the white arrow in the right penal) were acquired for the PAPE group. Scale bar = 10 µm. (C-D) Potent humoral and cellular response to SARS-CoV-2 RBD vaccinations. (C) Serum RBD-specific IgG titer. (D) ELISpot analysis of IFN-γ-spot-forming cells among splenocytes. Reprinted from [270], Copyright 2020, with permission from WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
iii) Novel administration route with integrated nanoparticles vaccines helped to catalyze vaccine candidates at a greater speed. Yang et al. described a method of Ebola vaccine using a DNA antigen coated on PLGA-polyl-lysine/poly-γ-glutamic acid (PLGA-PLL/γPGA) nanoparticle via a microneedle (MN) patch [271]. IgG1 subtype responses were significantly higher after immunization with nanoparticle (intramuscular injection) and still higher when administered by MN patch, while the MN patch showed the highest neutralizing activity, neutralizing over 50% of GP-pseudovirions. Chen et al. reported an influenza vaccination via sustained intradermal (ID) release of vaccines using implantable and patch-free chitosan MN. Chitosan MNs can be quickly and entirely implanted into the dermis to function as a depot and an immune-boosting agent for the extended release of vaccines and simultaneous activation of the immune system. The MN-induced immune-enhancing effect lasted for at least 16 weeks [272]. These integrated delivery systems enable precise vaccine administration by minimally trained personnel and even enhance the vaccine potency. In addition, the MN provides vaccine stability without refrigeration; it is expected to be manufactured at low cost to meet the needs of developing countries.
3.2.2. Advanced delivery strategy for long-term protection
Persistent protection is required against the virus with long incubation periods (e.g., 5–10 years for HIV infection) or resultant chronic diseases/cancer (e.g., HBV-induced liver cancer). These diseases even pose fatal challenge for the infectors that have access to anti-virus drugs. In this case, a vaccine with long-term prophylactic efficacy or a therapeutic effect was appreciated. Therefore, vaccine delivery strategies for protecting the antigen (protein or RNA) payload from environmental enzyme degradation or inducing cytotoxic T-cell activity against the infected cells are highly appreciated.
i) Advanced delivery systems (e.g., micro/nano formulation, hydrogel) are an important strategy for sustained antigen release. Protein/peptide/drugs are easily degraded by hydrolysis and enzymes in the human body, which results in their short half-life time and the need for frequent injections. Once loaded on micro/nano materials, physical protection or controllable release could be obtained with the material degradation for persistent outcome.
Lipids or polymers with good biocompatibility or degradability are attracting a great deal of interest among vaccine delivery systems. The success of the COVID-19 RNA vaccines from either Pfizer or Moderna is highly dependent on the novel lipid delivery system. These lipid nanoparticles substantially improved the stability of the mRNA and facilitated the RNA transfection efficiency in vivo [6]. Tian et al. fabricated a nanovector composed of peptide-based nanofibrous hydrogel, which was able to condense DNA and result in strong immune responses against HIV [273]. This nanovector was able to strongly activate both humoral and cellular immune responses in a balanced way, a result rarely reported in previous studies, which is crucial for HIV prevention and therapy. The nanofibrous structure of the hydrogel is critical for the dramatically improved immune responses compared with the existing materials.
A delivery system with the optimum size, structure, and properties could facilitate a long-acting antigen-specific response. Xi et al. reported a poly(lactide-co-glycolide) PLGA microcapsule-based formulation for high-performance vaccinations (Fig. 6 ) [274]. The special self-healing feature provides a mild and efficient paradigm for antigen microencapsulation. Although without traditional molecular adjuvant, these microcapsules could still create in situ beneficial immunization microenvironments at the vaccination site, wherein sustained antigen release, constant APC recruitment, and favorable acidic surrounding collaborated effectively. As a result, effective T cell response and prevention of postsurgical recurrence were acquired with various types of antigens. Wu et al. developed a thermal-sensitive hydrogel (a formulation of chitosan derivate and glycerophosphate) as intranasal H5N1 vaccine delivery system [275]. In terms of the thermal sensitivity and intensive positive charge, this hydrogel efficiently extended the residence time of antigen in the nasal cavity and promoted the central/effector TRM. Although a wave of efforts have spurred to improve the vaccine effect, these delivery systems were endowed with controllable attributes along with a persistent performance, which is promising to encapsulate other virus antigens and avoid frequent vaccination.
Fig. 6.
Strategy of using self-healing microcapsules to modulate immunization microenvironments for vaccination[274]. The corresponding characterizations of gigaporous microspheres and antigen-loaded microcapsules are displayed below: (A and A′) Scanning electron microscopy (SEM) images in a magnified view. Scale bars, 10 μm. (B and B′) SEM images in a local feature. Scale bars, 1 μm. (C and C′) Confocal images in two-dimensional (2D) cut view. Scale bars, 5 μm. (D and D′) 3D reconstruction. Scale bars, 10 μm.
ii) The enhanced antigen-specific cytotoxic T-cell (CTL) response is considered a promising strategy to prolong the vaccine efficacy. The sustained efficacy is possibly due to the repetitive antigenic surfaces that mimic influenza pathogenic structures, which activate the host immune system to fight against and finally cause the lysis of the pathogens. For example, Wang et al. fabricated double-layered protein nanoparticles via ethanol desolvation and chemical cross-linking of influenza matrix protein 2 ectodomain-neuraminidase (M2e-NA) recombinant proteins [276]. The layered M2e-NA nanoparticles induced a strong CTL response, contributing to long-lasting immune protection. In addition, extracellular vesicles (exosomes) released from CD8+ T cells contained antiviral membrane-bound factors that inhibited HIV-1 transcription in both acute and chronic infection models [277].
To achieve efficient CTL response, Ma’s group has developed various particulate strategies for enhanced cross-presentation [8], including positive charge, gas generating, ligand modification, and membrane fusion. For example, inspired by an important natural biological behavior, membrane fusion, Hu et al. constructed a pH-responsive nanocarrier (HBsAg&CpG@Lip) with a membrane fusion capacity for HBsAg intracellular delivery and subsequent processing by T cells (Fig. 7 ). This nanoparticle not only elicited a higher anti-HBsAg IgG and IgG2c/IgG1 ratio, but it also augmented the strong CTL response [278]. Lu et al. integrated a physiochemical merit (positive charge) and an immunopotentiator property (stimulator of interferon genes (STING)) in poly (lactic acid) (PLA) microparticles. Such a microparticle-based vaccine achieved 50% HBsAg seroconversion rate, reduced HBcAg in the liver, and also produced higher amount of memory T/B cells to confer protection in a sustained manner [279]. Although a much longer protection efficacy was not monitored, these studies opened alternative avenues for potent therapeutic vaccines, which is particularly important for the chronic infectors without efficient drugs.
Fig. 7.
Immunological mechanism of the HBsAg&CpG@Lip vaccination. With the assistance of the membrane fusion property, the antigen uptake/activation, cytosolic antigen release, and activation of lymph nodes were induced, promoting a prolonged and efficient humoral/cellular response. Reprinted from [278], Copyright (2020), with permission from Elsevier.
3.2.3. Advanced delivery strategy for cross-protection
Viruses are able to adapt to the changing environment, demanding a cross-protection immune response for the evolved multiple strains. Such a merit is particularly important when the virus mutates annually (e.g., Influenza virus) or raises the risk of coinfection with other pathogens (e.g., HPV). For example, antibodies produced from most influenza vaccines are strain-specific and display poor cross-reactivity with virus hemagglutinins of alternative subtypes [280]. In addition, cross-neutralizing titers, when noted, tend to be at least 100 times lower than type-specific titers and, therefore, cross-protection might be less durable than type-specific protection [281]. Recently, the strategy for cross-protection has mainly focused on incorporating a high conservative virus subdomain or eliciting the CD8+ T cell immunity or T-follicular helper (Tfh) cell immunity.
i) Utilizing the conservative virus subdomain is promising for achieving the cross-protection efficacy. Since HR is highly conservative in the coronaviruses evolution and harbors cross-reactive SARS-CoV-2 T-cell epitopes, the HR subdomain within S2 region of S protein is noticed. HR-containing nanoparticle vaccines were demonstrated to produce neutralizing antibodies against SARS-CoV, MERS-CoV, HCoV-229E, HCoVOC43, and RATG13 [269]. In addition, they induced higher percentages of Tfh and B cells within germinal centers, as well as IgG1 and IgG2b memory B cells. As B cell maturation relies on the coordination with Tfh, it is possible that CD4+ T epitopes within HR facilitate Tfh to recognize antigen-specific B cells with high affinity, thereby facilitating the maturation of plasma cells to cope with various virus subtypes.
Similar strategy is also meaningful for HPV prevention. As 70%–80% of cases of cervical cancer are caused by HPV-16 and HPV-18, these two types are potential candidates to achieve broader protection through cross-reactivity or expansion of the range of VLP types [282]. In this sense, the qualities of local in vivo concentration and potential effector cells (such as phagocytes or local memory B cells) also shape the cross-protection and longevity, which should be well tuned.
ii) Innovative delivery systems for CD8+ T cell-based cross-protection are another promising strategy that is being exploited. Chahal et al. developed an adjuvant-free mono-dispersed dendrimer nanoparticle (MDNP, ~100 nm) vaccine platform wherein antigens were encoded by multiple mRNA replicons. After a single immunization, it elicited vital CD8+ T and antibody responses that fully protected against lethal exposures to several deadly pathogens, including Ebola virus, H1N1 influenza, and Toxoplasma gondii [283]. This work may allow for rapid-response vaccines with broad efficacy, which could reduce the number and frequency of vaccinations and healthcare workers’ burden.
In another notable example, Wang et al. encapsulated a STING agonist with pulmonary surfactant-biomimetic liposomes (PS-GAMP) in an attempt to accelerate the breadth of nonreplicating influenza vaccines towards universality. This PS-biomimetic nanoparticle has generated wide-spectrum cross-protection against distant H1N1 and heterosubtypic H3N2, H5N1, and H7N9 viruses as early as 2 days after a single intranasal immunization. The adjuvant had potent effects on both primary and booster immune responses, raising serum Ag-specific IgG1 10-fold, IgG more than 100-fold, and IgG2c ~ 1000-fold compared with VN04 H5N1 vaccine alone. The cross-protection lasted for at least 6 months, concurrent with durable lung CD8+ resident TRM cells in mice. These CD8+ TRM cells, rather than circulating memory CD8+ T cells, contributed to the observed long-term protection as their function was not compromised by T cell egress inhibitor. That work strengthened the notion that even transient vaccine-activated innate immunity was sufficient to augment both humoral and cellular immune responses.
The intracellular delivery sites (lysosomal or non-lysosomal) and antigen processing (MHC I- or MHC II-mediated presentation) are crucial for cell-mediated response against most vaccine antigens, especially for viral infections and intracellular bacterial infections. The traditional process for exogenous antigens involves lysosomal degradation route and subsequent MHC II-medicated CD4+ T cell priming (humoral response). Some extracellular bacteria (e.g., S. pneumoniae) are not capable of entering the cells, which is indispensable to the intracellular delivery manner. To prevent such bacterial infections, a higher titer of antibodies that can neutralize the pathogens is needed. In this case, the aim of vaccine delivery systems mainly include eliciting efficient antibody response through a Th2-biased immune response mechanism. In contrast, to cope with the intracellular bacteria (e.g., S. typhi) or most viruses (e.g., SARS-CoV-2, influenza) that are lethal or variable, cellular response (CD8+ T priming) is particularly important for long-term or cross-protection. Therefore, the aforementioned cross-presentation delivery strategies (e.g., lysosomal escape and biomimetic membrane fusion) or delivery systems (e.g., pH-sensitive or positively charged synthetic particles/liposomes) should be designed to tune the intracellular fate (distribution and presentation) of exogenous antigens for CD8+ T priming.
4. Parasitic infectious diseases and advanced delivery systems
Parasitic infections are highly prevalent worldwide, and many parasitic infectious diseases (such as malaria, schistosomiasis, and African trypanosomiasis) seriously threaten human health, especially in poor countries and regions. However, most of these diseases have usually been neglected. Among them, malaria, caused by Plasmodium (including P. falciparum, P. vivax, P. malariae, P. ovale, and P. knowlesi), is associated with high morbidity and mortality worldwide. The most lethal is malaria caused by P. falciparum, which is mainly distributed in sub-Saharan Africa. Vaccines are important in the prevention of malaria; according to the life cycle of Plasmodium, there are three stages suitable for developing a potential malaria vaccine [284]. The first one is the pre-erythrocyte stage, which may involve antibody response to prevent sporozoites from invading hepatocytes [285], [286]. The second one is the blood-stage of the parasite. In this stage, red blood cell invasion could be controlled, resulting in fewer disease symptoms or asymptomatic infections [287], [288]. However, the immunogenicity of some high-conservation protein antigens is very low [289], [290], [291]. The third stage is the stage of sexual parasite forms or gametocytes. Vaccines designed for this stage are intended to block the spread of malaria.
Many malaria vaccine delivery systems have been developed (Fig. 8 ), and some of them have been tested in clinical trials, including two kinds of delivery vehicles (Table 5 ). For example, vaccine based on VLP is the most advanced malaria vaccine in the pre-erythrocyte stage to date. By fusing a recombinant protein of the P. falciparum circumsporozoite protein (CSP) [286], containing known B- and T-cell epitopes [292], to the hepatitis B surface antigen (HBsAg), the expressed fusion protein could self-assemble into VLPs (designated RTS) that display the antigens on their surface [293]. A phase III study of the candidate vaccine RTS,S/AS01, which was studied from 2009 to 2014 in children in seven sub-Saharan African countries, revealed that vaccine efficacy against clinical malaria over 12 months after 3 doses was 56% in the 5–17 month age group and 31% in infants at the age of 6–12 weeks [294]. Although the efficacy against clinical malaria decreased over time and vaccinated children suffered increased risk of clinical malaria compared with controls after 5 years [294], [295], malaria was prevented in young children who are usually at a high risk for severe malaria complications, and a booster dose could slow down the attenuation [296], [297]. Although these candidate vaccines are often unable to induce higher antibody titers, there is great hope to prevent malaria in countries with limited resources, especially in children. In addition, some other delivery carriers, such as self-assembling polypeptide [298], self-assembled nanofibers [299], or some novel adjuvants like AddaVax (a squalene-based oil-in-water nano-emulsion) [300], have also been developed, which may provide more choices for the development of a malaria vaccine.
Fig. 8.
Malaria parasite life cycle and delivery systems for vaccine development. The life cycle of malaria parasite contains three stages, including pre-erythrocyte stage, blood stage, and sexual stage. Many kinds of delivery systems have been explored for malaria vaccine design and have been developed to potential malaria vaccine candidates.
Table 5.
Current malaria vaccines with delivery systems in clinical trials.
| Vaccine candidate | Clinical trial registration number | Stage | Delivery system | Antigen(s) | Species and strain of malaria parasite | Adjuvants used |
|---|---|---|---|---|---|---|
| Pre-erythrocytic projects | ||||||
| RTS,S/AS01E | NCT00866619 | Phase III | VLP | Pf CSP (207–395)& HBsAg | P. falciparum | AS01E |
| RTS,S-AS01 fractional dose regimes | NCT02992119 | Phase II | VLP | Pf CSP (207–395)& HBsAg | P. falciparum | AS01B /AS01E |
| ChAd63/MVA ME-TRAP | NCT01635647 (VAC050) | Phase I/IIb | ChAd63, MVA | TRAP + ME epitopes (CS, LSA1, LSA3, STARP, EXP1, pb9) | P. falciparum | |
| ChAd63/MVA ME-TRAP +Matrix M™ | NCT01669512 (VAC048) | Phase I | ChAd63, MVA | TRAP + ME epitopes (CS, LSA1, LSA3, STARP, EXP1, pb9) | P. falciparum | Matrix M™ |
| CSVAC | NCT01450280 | Phase I | ChAd63, MVA | CS | P. falciparum | |
| R21/Matrix-M1 | NCT04704830 | Phase III | VLP | CSP less- HBsA | P. falciparum | Matrix-M1 |
| adjuv R21 (RTS,S-biosimilar) with ME-TRAP combined | NCT02905019 | Phase I/IIa | ChAd63, MVA | CSP less- HBsA + MeTRAPg | P. falciparum | Matrix-M1 |
| Blood stage projects | ||||||
| ChAd63 RH5 +/- MVA RH5 | NCT02181088 | Phase Ia | ChAd63, MVA | RH5 | P. falciparum | |
| ChAd63/MVA PvDBP | NCT01816113 | Phase Ia | ChAd63, MVA | PvDBP_RII | P. vivax | |
| Sexual stage projects | ||||||
| Pfs25 VLP | NCT02013687 | Phase I/IIa | VLP | Pfs25 | P. falciparum | Alhydrogel |
| ChAd63 Pfs25-IMX313/MVA Pfs25-IMX313 | NCT02532049 | Phase Ia | ChAd63, MVA | Pfs25 | P. falciparum | |
5. Fungal infectious diseases and advanced delivery systems
Currently, the incidence rate of fungal infections is increasing [301]. They often occur in individuals with weakened immune functions; some fungi can induce invasive fungal infections, with mortality rates of about 30%–40% [302]. Many kinds of fungal vaccines have been developed, but none were approved by FDA because patients with fungal disease generally have low immunity and are often unable to achieve effective response after immunization [303]. The Th1/Th17 profiles immunity can effectively prevent fungal infections [304].
Various delivery systems have been applied in antibacterial and viral vaccine studies, as described above. However, only a few vehicles have been used in antifungal vaccine design. For example, Chauhan et al. described an Escheriosomes-based delivery system to enhance the immune response to cytosolic antigens (cAg) of Candida albicans, an opportunistic human pathogen and the most common cause of fungal invasive infections [305]. When mice were immunized subcutaneously, a strong cellular immune response was induced, generating protective immunity (75% mice survived) against systemic C. albicans infection [306]. Furthermore, Carneiro et al. described another liposome, dioctadecildimethylammonium bromide: monoolein (DODAB:MO), consisting of DODAB and a stabilizer MO [307]. DODAB induced stronger cellular immune responses and has been used as a carrier in drug delivery studies [308], [309], [310]. By incorporating the cell wall surface proteins (CWSP) from C. albicans and subcutaneous immunization in mice, the DODAB:MO loaded with CWSP induced a strong specific Th1 immune response and protected 62.5% of mice from death of intravenous C. albicans infection [307]. De Bernardis et al. described a C. albicans vaccine (PEV7) by incorporating a truncated, recombinant aspartyl proteinase-2 of C. albicans into influenza virosomes, which were assembled in vitro from synthetic lipids and purified influenza virus envelope components. Following intravaginal route of immunization, this vaccine provided local, long-lasting mucosal immunity and protection against candida vaginitis [311].
Although there have been no further explorations of delivery systems against fungal infections, some promising vehicles, such as L. lactis particles and polymer micro/nanoparticles, could be tried because of their ability to induce a strong Th1/Th17 immune response.
6. Conclusions
Epidemic diseases have influenced the world throughout human civilization and even dictated the course of human history. Among treatment and prevention strategies, vaccination has played an irreplaceable role in saving people’s lives. Nevertheless, high infection rates, widespread transmission, or high fatal ratio of outbreaks raise huge challenges for vaccine design. In this review, we summarized the major devastating issues of various epidemic pathogens (bacteria, viruses, fungi, and parasites), the latest clinical vaccine candidates (e.g., COVID-19 vaccine licensure), and the recent developments in antigen delivery systems for preventing infectious diseases. We particularly emphasized the design of the delivery systems for robust/high protection, long-term protection, or/and cross-protection. The corresponding delivery systems involve synthetic micro/nanoparticles (e.g., polymer and lipid particles), biological particles (e.g., exosomes and viral vectors), and integrated devices (microneedle patch), with a great potential to break through the barriers of traditional vaccines and adjuvants. These delivery concepts are not only adapted to the antiviral vaccines but are also applicable for the vaccines against bacterial infections and other infectious diseases. Although there have been few studies on fungal and parasite vaccine delivery systems, some exciting results about vaccine delivery encourage further research on that matter.
These delivery materials, mainly including inorganic, polymers, liposomes, and protein carriers, have their own advantages for different types of diseases. Generally, the best immune route is often the one that is similar to the pathogen’s route in natural infection; hence, for intestinal and respiratory bacteria, the development of delivery vehicles suitable for mucosal routes is more attractive because they would stimulate both local and systemic immune responses. For example, for prevention of respiratory diseases by achieving effective respiratory tract mucosal immunity, materials with retention capacity and resistance to easy dilution and degradation are required. In this context, some advanced nanogels and chitosan have suitable characteristics endowing them with very good immune response effects. In contrast, to reach the intestinal mucosa, a vaccine needs to pass through the extreme stomach environment, so additional protection of the delivery vehicle is often required. Probiotic carriers have unique advantages in this respect and can colonize the intestines; in addition, the protective effect of probiotic-based vaccine delivery systems against digestive tract diseases seems to be better than that against respiratory infections, which may not only be related to the characteristics of antigens but also to the fact that probiotic carriers are more suitable for the intestinal mucosa. Furthermore, compared with other immunization methods, oral vaccines have a higher safety threshold. Therefore, OMVs (containing lipid A and other components) of some digestive tract bacteria can also be used as oral vaccines, and they have shown good results in animal models.
In addition to the above-mentioned mucosal immunity, for some invasive infectious diseases, delivery vehicles such as biomimetic carriers, liposomes, and proteinaceous particles have great potential because they can stimulate humoral immune responses stronger than those achieved from non-mucosal immune routes, thereby providing complete protection from the lethal challenges. However, many promising delivery systems are rarely further validated in other bacterial species, which also is a feature of bacterial research. The reason of this phenomenon may be the wide variety of infectious disease pathogens and the relatively scattered research in the prophylactic vaccine field. In addition, it is also due to the differences in the cultivation requirements and evaluation methods of different bacteria and restrictions on acquisition and permission.
According to the existing data, vaccines can stimulate effective immune protection soon after vaccination. However, only a few carriers (such as probiotics and polyanhydride particles) have been studied for long-term immune protection. Some marketed vaccines, such as acellular pertussis vaccines, face the problem of a reduction in the protective effect over years, so it is necessary to further explore whether different delivery systems could help to overcome this issue.
7. Challenges and future directions
Recent vaccine delivery systems have helped to catalyze a series of vaccine candidates. However, there are still some challenges in the delivery system design for prophylactic vaccines. The first one involves the vaccine delivery system against multidrug resistant opportunistic pathogens that may seriously threaten special populations, such as older individuals, immunosuppressed persons, or patients before surgery. Therefore, an optimal delivery system should meet some important additional requirements (such as higher safety, rapid onset with only one immunization needed, and adequate protection), and special animal models (e.g., older animals) should be used for evaluation. The second one is carrier-induced epitope suppression (CIES) by proteinaceous delivery carriers, such as self-assembled proteinaceous nanoparticles, VLP, and viral vectors. CIES has been a great concern because the same carrier may result in the dampening of the effect of subsequent vaccination. Although it has been shown that CIES could be overcome by high coupling densities, repeated injections, and/or higher doses, this problem has not yet been fully solved. The third one is the development of delivery systems that endow vaccines with therapeutic efficacy for chronic infections, such as hepatitis B, tuberculosis, and brucellosis. Therefore, a new balance between the Th1 and Th2 response needs to be established. Finally, the balance between the rational design for specific protection demands and the safety/feasibility requirements for clinical translation should not be overlooked. In response to the demand for targeted delivery, immune regulation, or specific population (e.g., children or older persons), complex design strategies (e.g., targeting peptides, adjuvants) are usually involved. When the delivery system includes multiple components, the difficulty of the quality control/process optimization and even the biosafety consideration is augmented, which decreases the chances for further application. In particular, the outbreak of epidemic disease unprecedentedly narrows the development period and requires delivery platforms that cater to fatal pathogens in a timely manner.
In the future, the resolution of these challenges is indispensable to the renewal of biomaterials, the intelligent design of delivery systems, the exploration of immunological mechanisms, and the assistance of innovative technology. First, the renewal of biomaterials with intelligent design, especially for the pathogen-mimicking systems (e.g., biologically derived exosomes or synthetic chassis), will help to prevent vaccine resistance or endow vaccines with the expected immunological response. An optimum delivery system (e.g., temperature-sensitive hydrogels or hollow porous MPs) can be applied to extend the administration route (e.g., nasal or inhalation vaccination) and functions at the initial site of pathogen invasion. In particular, by mimicking the physiochemical properties (e.g., mobility and deformability) or intracellular behavior (e.g., lysosomal escape pathway) of microorganisms, robust immune response or high CTL cross-protection is realized.
Second, to maximize protection and avoid ineffective responses, it is important to design individualized vaccine delivery systems depending on the pathogen’s characteristics (invasive or noninvasive; intracellular or extracellular) and the underlying immunological mechanisms. The delivery system can be designed to activate local innate responses that translate into the mucosal immunity (e.g., generation of secretory IgA or high-avidity CTL at mucosal sites). In addition, efficient delivery systems can be developed upon the novel theoretical mechanisms to address specific demands (e.g., a decrease of the pre-existing immunity of adenovirus vaccination, or eliciting the protection rate among older individuals).
Third, a combinatorial library of antigens, adjuvants, or delivery materials (e.g., lipids) is available, and the top candidate with specific merits can be screened promptly with the assistance of novel technology (e.g., nanotechnology and artificial intelligence). For example, the distribution route (e.g., cell membrane or acidic lysosome) and ultimate fate (e.g., enzyme degradation or ligand–receptor-based activation) of each component can be calculated and predicted. In this context, a delivery system can be designed for maximum immune efficiency in a coupled spatio-temporal manner. In addition, with the assistance of computers and artificial intelligence, more modules (even including special functions) will likely be designed to assemble a potentially enormous diversity of nanovaccine structures, which is expected to solve the problem of CIES of protein carrier vaccines.
Epidemic diseases have influenced the world throughout human civilization and even dictated the course of human history. Vaccine delivery strategies have been deeply studied in many fields, and their importance in the prevention of infectious diseases has become increasingly prominent. Although most of the advanced delivery systems are still in the laboratory research stage, several products have entered clinical trials or helped to catalyze a series of vaccine candidates, which should lead to advanced prophylactic vaccination with balanced safety and efficacy in the future. Due to the unparalleled need for vaccines globally, this field will continue to blossom in the prevention of infectious diseases.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This review was supported by the National Natural Science Foundation of China (No. 81930122, U20A20361, 32030062) and Beijing Municipal Natural Science Foundation (No. 2202056). We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
References
- 1.Kumar K., Abubakar I. Clinical implications of the global multidrug-resistant tuberculosis epidemic. Clin. Med. 2016;16:565–570. doi: 10.7861/clinmedicine.16-6-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin M.D., Shukla S., Chung Y.H., Beiss V., Chan S.K., Ortega-Rivera O.A., Wirth D.M., Chen A., Sack M., Pokorski J.K., Steinmetz N.F. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020;15:646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald N., Mohsni E., Al-Mazrou Y., Andrus J.K., Arora N., Elden S., Madrid M.-Y., Martin R., Mustafa A.M., Rees H., Salisbury D., Zhao Q., Jones I., Steffen C.A., Hombach J., O'Brien K.L., Cravioto A. Global vaccine action plan lessons learned I: Recommendations for the next decade. Vaccine. 2020;38:5364–5371. doi: 10.1016/j.vaccine.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng X., Xu W., Li Z., Song W., Ding J., Chen X. Immunomodulatory Nanosystems. Adv. Sci. 2019;6:1900101. doi: 10.1002/advs.201900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fries C.N., Curvino E.J., Chen J., Permar S.R., Fouda G.G., Collier J.H. Advances in nanomaterial vaccine strategies to address infectious diseases impacting global health. Nat. Nanotechnol. 2021;16:1–14. doi: 10.1038/s41565-020-0739-9. [DOI] [PubMed] [Google Scholar]
- 6.Tang Z., Kong N., Zhang X., Liu Y., Hu P., Mou S., Liljestrom P., Shi J., Tan W., Kim J.S., Cao Y., Langer R., Leong K.W., Farokhzad O.C., Tao W. A materials-science perspective on tackling COVID-19. Nat. Rev. Mater. 2020;5:847–860. doi: 10.1038/s41578-020-00247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan G., Madou M.J., Kalra S., Chopra V., Ghosh D., Martinez-Chapa S.O. Nanotechnology for COVID-19: Therapeutics and Vaccine Research. Acs Nano. 2020;14:7760–7782. doi: 10.1021/acsnano.0c04006. [DOI] [PubMed] [Google Scholar]
- 8.Xia Y., Song T., Hu Y., Ma G. Synthetic Particles for Cancer Vaccines: Connecting the Inherent Supply Chain. Acc. Chem. Res. 2020;53:2068–2080. doi: 10.1021/acs.accounts.0c00336. [DOI] [PubMed] [Google Scholar]
- 9.Rodgers G.L., Arguedas A., Cohen R., Dagan R. Global serotype distribution among Streptococcus pneumoniae isolates causing otitis media in children: potential implications for pneumococcal conjugate vaccines. Vaccine. 2009;27:3802–3810. doi: 10.1016/j.vaccine.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Mahoney R.T., Krattiger A., Clemens J.D., Curtiss R. 3rd, The introduction of new vaccines into developing countries. IV: Global Access Strategies. Vaccine. 2007;25:4003–4011. doi: 10.1016/j.vaccine.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 11.Holmgren J., Czerkinsky C. Mucosal immunity and vaccines. Nat. Med. 2005;11:S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 12.Kong I.G., Sato A., Yuki Y., Nochi T., Takahashi H., Sawada S., Mejima M., Kurokawa S., Okada K., Sato S., Briles D.E., Kunisawa J., Inoue Y., Yamamoto M., Akiyoshi K., Kiyono H. Nanogel-based PspA intranasal vaccine prevents invasive disease and nasal colonization by Streptococcus pneumoniae. Infect. Immun. 2013;81:1625–1634. doi: 10.1128/IAI.00240-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beitelshees M., Hill A., Li Y., Chen M., Ahmadi M.K., Smith R.J., Jr., Andreadis S.T., Rostami P., Jones C.H., Pfeifer B.A. Antigen delivery format variation and formulation stability through use of a hybrid vector. Vaccine X. 2019;1 doi: 10.1016/j.jvacx.2019.100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham E. Intranasal immunization with bacterial polysaccharide containing liposomes enhances antigen-specific pulmonary secretory antibody response. Vaccine. 1992;10:461–468. doi: 10.1016/0264-410x(92)90395-z. [DOI] [PubMed] [Google Scholar]
- 15.Jones C.H., Zhang G., Nayerhoda R., Beitelshees M., Hill A., Rostami P., Li Y., Davidson B.A., Knight P., 3rd, Pfeifer B.A. Comprehensive vaccine design for commensal disease progression. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1701797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J.H., Dai W.J., Chen B., Fan X.Y. Mucosal immunization with PsaA protein, using chitosan as a delivery system, increases protection against acute otitis media and invasive infection by Streptococcus pneumoniae. Scand. J. Immunol. 2015;81:177–185. doi: 10.1111/sji.12267. [DOI] [PubMed] [Google Scholar]
- 17.Xu J., Dai W., Wang Z., Chen B., Li Z., Fan X. Intranasal vaccination with chitosan-DNA nanoparticles expressing pneumococcal surface antigen a protects mice against nasopharyngeal colonization by Streptococcus pneumoniae. Clin. Vaccine Immunol. 2011;18:75–81. doi: 10.1128/CVI.00263-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haughney S.L., Petersen L.K., Schoofs A.D., Ramer-Tait A.E., King J.D., Briles D.E., Wannemuehler M.J., Narasimhan B. Retention of structure, antigenicity, and biological function of pneumococcal surface protein A (PspA) released from polyanhydride nanoparticles. Acta biomater. 2013;9:8262–8271. doi: 10.1016/j.actbio.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anish C., Upadhyay A.K., Sehgal D., Panda A.K. Influences of process and formulation parameters on powder flow properties and immunogenicity of spray dried polymer particles entrapping recombinant pneumococcal surface protein A. Int. J. Pharm. 2014;466:198–210. doi: 10.1016/j.ijpharm.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues T.C., Oliveira M.L.S., Soares-Schanoski A., Chavez-Rico S.L., Figueiredo D.B., Goncalves V.M., Ferreira D.M., Kunda N.K., Saleem I.Y., Miyaji E.N. Mucosal immunization with PspA (Pneumococcal surface protein A)-adsorbed nanoparticles targeting the lungs for protection against pneumococcal infection. PloS One. 2018;13 doi: 10.1371/journal.pone.0191692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira M.L., Areas A.P., Campos I.B., Monedero V., Perez-Martinez G., Miyaji E.N., Leite L.C., Aires K.A., Lee Ho P. Induction of systemic and mucosal immune response and decrease in Streptococcus pneumoniae colonization by nasal inoculation of mice with recombinant lactic acid bacteria expressing pneumococcal surface antigen A. Microbes Infect. 2006;8:1016–1024. doi: 10.1016/j.micinf.2005.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campos I.B., Darrieux M., Ferreira D.M., Miyaji E.N., Silva D.A., Areas A.P., Aires K.A., Leite L.C., Ho P.L., Oliveira M.L. Nasal immunization of mice with Lactobacillus casei expressing the Pneumococcal Surface Protein A: induction of antibodies, complement deposition and partial protection against Streptococcus pneumoniae challenge. Microbes Infect. 2008;10:481–488. doi: 10.1016/j.micinf.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira D.M., Darrieux M., Silva D.A., Leite L.C., Ferreira J.M., Jr., Ho P.L., Miyaji E.N., Oliveira M.L. Characterization of protective mucosal and systemic immune responses elicited by pneumococcal surface protein PspA and PspC nasal vaccines against a respiratory pneumococcal challenge in mice. Clin. Vaccine Immunol. 2009;16:636–645. doi: 10.1128/CVI.00395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernani Mde L., Ferreira P.C., Ferreira D.M., Miyaji E.N., Ho P.L., Oliveira M.L. Nasal immunization of mice with Lactobacillus casei expressing the pneumococcal surface protein C primes the immune system and decreases pneumococcal nasopharyngeal colonization in mice, FEMS Immunol. Med. Microbiol. 2011;62:263–272. doi: 10.1111/j.1574-695X.2011.00809.x. [DOI] [PubMed] [Google Scholar]
- 25.Hanniffy S.B., Carter A.T., Hitchin E., Wells J.M. Mucosal delivery of a pneumococcal vaccine using Lactococcus lactis affords protection against respiratory infection. J. Infect. Dis. 2007;195:185–193. doi: 10.1086/509807. [DOI] [PubMed] [Google Scholar]
- 26.Medina M., Villena J., Vintini E., Hebert E.M., Raya R., Alvarez S. Nasal immunization with Lactococcus lactis expressing the pneumococcal protective protein A induces protective immunity in mice. Infect. Immun. 2008;76:2696–2705. doi: 10.1128/IAI.00119-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vintini E., Villena J., Alvarez S., Medina M. Administration of a probiotic associated with nasal vaccination with inactivated Lactococcus lactis-PppA induces effective protection against pneumoccocal infection in young mice. Clin. Exp. Immunol. 2010;159:351–362. doi: 10.1111/j.1365-2249.2009.04056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Audouy S.A., van Selm S., van Roosmalen M.L., Post E., Kanninga R., Neef J., Estevao S., Nieuwenhuis E.E., Adrian P.V., Leenhouts K., Hermans P.W. Development of lactococcal GEM-based pneumococcal vaccines. Vaccine. 2007;25:2497–2506. doi: 10.1016/j.vaccine.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 29.Polonskaya Z., Deng S., Sarkar A., Kain L., Comellas-Aragones M., McKay C.S., Kaczanowska K., Holt M., McBride R., Palomo V., Self K.M., Taylor S., Irimia A., Mehta S.R., Dan J.M., Brigger M., Crotty S., Schoenberger S.P., Paulson J.C., Wilson I.A., Savage P.B., Finn M.G., Teyton L. T cells control the generation of nanomolar-affinity anti-glycan antibodies. J. Clin. Invest. 2017;127:1491–1504. doi: 10.1172/JCI91192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian W., Huang Z., Chen Y., Yang J., Wang L., Wu K., Chen M., Chen N., Duan Y., Shi J., Zhang Y., Li Q. Elicitation of integrated immunity in mice by a novel pneumococcal polysaccharide vaccine conjugated with HBV surface antigen. Sci. Rep. 2020;10:6470. doi: 10.1038/s41598-020-62185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee W.H., Choi H.I., Hong S.W., Kim K.S., Gho Y.S., Jeon S.G. Vaccination with Klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity. Exp. Mol. Med. 2015;47 doi: 10.1038/emm.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu G., Ji H., Guo X., Li Y., Ren T., Dong H., Liu J., Liu Y., Shi X., He B. Nanoparticle reinforced bacterial outer-membrane vesicles effectively prevent fatal infection of carbapenem-resistant Klebsiella pneumoniae. Nanomedicine. 2020;24 doi: 10.1016/j.nano.2019.102148. [DOI] [PubMed] [Google Scholar]
- 33.Jain R.R., Mehta M.R., Bannalikar A.R., Menon M.D. Alginate microparticles loaded with lipopolysaccharide subunit antigen for mucosal vaccination against Klebsiella pneumoniae. Biologicals. 2015;43:195–201. doi: 10.1016/j.biologicals.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Roberts R., Moreno G., Bottero D., Gaillard M.E., Fingermann M., Graieb A., Rumbo M., Hozbor D. Outer membrane vesicles as acellular vaccine against pertussis. Vaccine. 2008;26:4639–4646. doi: 10.1016/j.vaccine.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Raeven R.H.M., Brummelman J., Pennings J.L.A., van der Maas L., Helm K., Tilstra W., van der Ark A., Sloots A., van der Ley P., van Eden W., Jiskoot W., van Riet E., van Els C., Kersten G.F.A., Han W.G.H., Metz B. Molecular and cellular signatures underlying superior immunity against Bordetella pertussis upon pulmonary vaccination. Mucosal Immunol. 2018;11:1009. doi: 10.1038/mi.2017.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bottero D., Gaillard M.E., Errea A., Moreno G., Zurita E., Pianciola L., Rumbo M., Hozbor D. Outer membrane vesicles derived from Bordetella parapertussis as an acellular vaccine against Bordetella parapertussis and Bordetella pertussis infection. Vaccine. 2013;31:5262–5268. doi: 10.1016/j.vaccine.2013.08.059. [DOI] [PubMed] [Google Scholar]
- 37.Asensio C.J., Gaillard M.E., Moreno G., Bottero D., Zurita E., Rumbo M., van der Ley P., van der Ark A., Hozbor D. Outer membrane vesicles obtained from Bordetella pertussis Tohama expressing the lipid A deacylase PagL as a novel acellular vaccine candidate. Vaccine. 2011;29:1649–1656. doi: 10.1016/j.vaccine.2010.12.068. [DOI] [PubMed] [Google Scholar]
- 38.Shi W., Kou Y., Jiang H., Gao F., Kong W., Su W., Xu F., Jiang C. Novel intranasal pertussis vaccine based on bacterium-like particles as a mucosal adjuvant. Immunol. Lett. 2018;198:26–32. doi: 10.1016/j.imlet.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Li P., Asokanathan C., Liu F., Khaing K.K., Kmiec D., Wei X., Song B., Xing D., Kong D. PLGA nano/micro particles encapsulated with pertussis toxoid (PTd) enhances Th1/Th17 immune response in a murine model. Int. J. Pharm. 2016;513:183–190. doi: 10.1016/j.ijpharm.2016.08.059. [DOI] [PubMed] [Google Scholar]
- 40.Conway M.A., Madrigal-Estebas L., McClean S., Brayden D.J., Mills K.H. Protection against Bordetella pertussis infection following parenteral or oral immunization with antigens entrapped in biodegradable particles: effect of formulation and route of immunization on induction of Th1 and Th2 cells. Vaccine. 2001;19:1940–1950. doi: 10.1016/s0264-410x(00)00433-3. [DOI] [PubMed] [Google Scholar]
- 41.Neimert-Andersson T., Hallgren A.C., Andersson M., Langeback J., Zettergren L., Nilsen-Nygaard J., Draget K.I., van Hage M., Lindberg A., Gafvelin G., Gronlund H. Improved immune responses in mice using the novel chitosan adjuvant ViscoGel, with a Haemophilus influenzae type b glycoconjugate vaccine. Vaccine. 2011;29:8965–8973. doi: 10.1016/j.vaccine.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 42.An S.J., Woo J.S., Chae M.H., Kothari S., Carbis R. Preparation and testing of a Haemophilus influenzae Type b/Hepatitis B surface antigen conjugate vaccine. Vaccine. 2015;33:1614–1619. doi: 10.1016/j.vaccine.2015.01.061. [DOI] [PubMed] [Google Scholar]
- 43.Feng G., Jiang Q., Xia M., Lu Y., Qiu W., Zhao D., Lu L., Peng G., Wang Y. Enhanced immune response and protective effects of nano-chitosan-based DNA vaccine encoding T cell epitopes of Esat-6 and FL against Mycobacterium tuberculosis infection. PloS One. 2013;8 doi: 10.1371/journal.pone.0061135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Das I., Padhi A., Mukherjee S., Dash D.P., Kar S., Sonawane A. Biocompatible chitosan nanoparticles as an efficient delivery vehicle for Mycobacterium tuberculosis lipids to induce potent cytokines and antibody response through activation of gammadelta T cells in mice. Nanotechnology. 2017;28 doi: 10.1088/1361-6528/aa60fd. [DOI] [PubMed] [Google Scholar]
- 45.Kamath A.T., Rochat A.F., Christensen D., Agger E.M., Andersen P., Lambert P.H., Siegrist C.A. A liposome-based mycobacterial vaccine induces potent adult and neonatal multifunctional T cells through the exquisite targeting of dendritic cells. PloS One. 2009;4 doi: 10.1371/journal.pone.0005771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imai K., Petigara T., Kohn M.A., Nakashima K., Aoshima M., Shito A., Kanazu S. Risk of pneumococcal diseases in adults with underlying medical conditions: a retrospective, cohort study using two Japanese healthcare databases. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-018553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bogaert D., De Groot R., Hermans P.W. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 48.Richter S.S., Heilmann K.P., Dohrn C.L., Riahi F., Diekema D.J., Doern G.V. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999-2011(1.) Emerg. Infect. Dis. 2013;19:1074–1083. doi: 10.3201/eid1907.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinberger D.M., Malley R., Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378:1962–1973. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marks L.R., Davidson B.A., Knight P.R., Hakansson A.P. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. mBio. 2013;4:e00438–e513. doi: 10.1128/mBio.00438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marks L.R., Parameswaran G.I., Hakansson A.P. Pneumococcal interactions with epithelial cells are crucial for optimal biofilm formation and colonization in vitro and in vivo. Infect. Immun. 2012;80:2744–2760. doi: 10.1128/IAI.00488-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y., Hill A., Beitelshees M., Shao S., Lovell J.F., Davidson B.A., Knight P.R., 3rd, Hakansson A.P., Pfeifer B.A., Jones C.H. Directed vaccination against pneumococcal disease. Proc. Natl. Acad. Sci. U S A. 2016;113:6898–6903. doi: 10.1073/pnas.1603007113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ayame H., Morimoto N., Akiyoshi K. Self-assembled cationic nanogels for intracellular protein delivery. Bioconjug. Chem. 2008;19:882–890. doi: 10.1021/bc700422s. [DOI] [PubMed] [Google Scholar]
- 54.Nochi T., Yuki Y., Takahashi H., Sawada S., Mejima M., Kohda T., Harada N., Kong I.G., Sato A., Kataoka N., Tokuhara D., Kurokawa S., Takahashi Y., Tsukada H., Kozaki S., Akiyoshi K., Kiyono H. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat. Mater. 2010;9:572–578. doi: 10.1038/nmat2784. [DOI] [PubMed] [Google Scholar]
- 55.Yuki Y., Nochi T., Kong I.G., Takahashi H., Sawada S., Akiyoshi K., Kiyono H. Nanogel-based antigen-delivery system for nasal vaccines. Biotechnol. Genet. Eng. Rev. 2013;29:61–72. doi: 10.1080/02648725.2013.801226. [DOI] [PubMed] [Google Scholar]
- 56.Briles D.E., Hollingshead S.K., King J., Swift A., Braun P.A., Park M.K., Ferguson L.M., Nahm M.H., Nabors G.S. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 2000;182:1694–1701. doi: 10.1086/317602. [DOI] [PubMed] [Google Scholar]
- 57.Malley R., Trzcinski K., Srivastava A., Thompson C.M., Anderson P.W., Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. U S A. 2005;102:4848–4853. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu Y.J., Gross J., Bogaert D., Finn A., Bagrade L., Zhang Q., Kolls J.K., Srivastava A., Lundgren A., Forte S., Thompson C.M., Harney K.F., Anderson P.W., Lipsitch M., Malley R. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fukuyama Y., King J.D., Kataoka K., Kobayashi R., Gilbert R.S., Oishi K., Hollingshead S.K., Briles D.E., Fujihashi K. Secretory-IgA antibodies play an important role in the immunity to Streptococcus pneumoniae. J. Immunol. 2010;185:1755–1762. doi: 10.4049/jimmunol.1000831. [DOI] [PubMed] [Google Scholar]
- 60.Fukuyama Y., Yuki Y., Katakai Y., Harada N., Takahashi H., Takeda S., Mejima M., Joo S., Kurokawa S., Sawada S., Shibata H., Park E.J., Fujihashi K., Briles D.E., Yasutomi Y., Tsukada H., Akiyoshi K., Kiyono H. Nanogel-based pneumococcal surface protein A nasal vaccine induces microRNA-associated Th17 cell responses with neutralizing antibodies against Streptococcus pneumoniae in macaques. Mucosal Immunol. 2015;8:1144–1153. doi: 10.1038/mi.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakahashi-Ouchida R., Yuki Y., Kiyono H. Cationic pullulan nanogel as a safe and effective nasal vaccine delivery system for respiratory infectious diseases. Hum. Vacci. Immunother. 2018;14:2189–2193. doi: 10.1080/21645515.2018.1461298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y., Beitelshees M., Fang L., Hill A., Ahmadi M.K., Chen M., Davidson B.A., Knight P., 3rd, Smith R.J., Jr., Andreadis S.T., Hakansson A.P., Jones C.H., Pfeifer B.A. In situ pneumococcal vaccine production and delivery through a hybrid biological-biomaterial vector. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1600264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin R.M., Bachman M.A. Colonization, Infection, and the Accessory Genome of Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2018;8:4. doi: 10.3389/fcimb.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pendleton J.N., Gorman S.P., Gilmore B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti. Infect. Ther. 2013;11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 65.W.H. Organization, Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics WHO, 2017. https://www.who.int/zh/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed.
- 66.Mashburn-Warren L.M., Whiteley M. Special delivery: vesicle trafficking in prokaryotes. Mol. Microbiol. 2006;61:839–846. doi: 10.1111/j.1365-2958.2006.05272.x. [DOI] [PubMed] [Google Scholar]
- 67.Bowersock T.L., HogenEsch H., Suckow M., Guimond P., Martin S., Borie D., Torregrosa S., Park H., Park K. Oral vaccination of animals with antigens encapsulated in alginate microspheres. Vaccine. 1999;17:1804–1811. doi: 10.1016/s0264-410x(98)00437-x. [DOI] [PubMed] [Google Scholar]
- 68.Chhibber S., Wadhwa S., Yadav V. Protective role of liposome incorporated lipopolysaccharide antigen of Klebsiella pneumoniae in a rat model of lobar pneumonia. Jpn. J. Infect. Dis. 2004;57:150–155. [PubMed] [Google Scholar]
- 69.Zhang J.S., Wang H.M., Yao K.H., Liu Y., Lei Y.L., Deng J.K., Yang Y.H. Clinical characteristics, molecular epidemiology and antimicrobial susceptibility of pertussis among children in southern China. World J. Pediatr. 2020;16:185–192. doi: 10.1007/s12519-019-00308-5. [DOI] [PubMed] [Google Scholar]
- 70.van der Lee S., Hendrikx L.H., Sanders E.A.M., Berbers G.A.M., Buisman A.M. Whole-Cell or Acellular Pertussis Primary Immunizations in Infancy Determines Adolescent Cellular Immune Profiles. Front. Immunol. 2018;9:51. doi: 10.3389/fimmu.2018.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Belchior E., Guillot S., Poujol I., Thabuis A., Chouin L., Martel M., Delisle E., Six C., Guiso N., Levy-Bruhl D. Comparison of whole-cell versus acellular pertussis vaccine effectiveness in school clusters of pertussis, France. Med. Mal. Infect. 2013;50(2020):617–619. doi: 10.1016/j.medmal.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 72.Kuchar E., Karlikowska-Skwarnik M., Han S., Nitsch-Osuch A. Pertussis: History of the Disease and Current Prevention Failure. Adv. Exp. Med. Biol. 2016;934:77–82. doi: 10.1007/5584_2016_21. [DOI] [PubMed] [Google Scholar]
- 73.Kilgore P.E., Salim A.M., Zervos M.J., Schmitt H.J. Pertussis: Microbiology, Disease, Treatment, and Prevention. Clin. Microbiol. Rev. 2016;29:449–486. doi: 10.1128/CMR.00083-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fabricius G., Martin Aispuro P., Bergero P., Bottero D., Gabrielli M., Hozbor D. Pertussis epidemiology in Argentina: TRENDS after the introduction of maternal immunisation. Epidemiol. Infect. 2018;146:858–866. doi: 10.1017/S0950268818000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kapil P., Merkel T.J. Pertussis vaccines and protective immunity. Curr. Opin. Immunol. 2019;59:72–78. doi: 10.1016/j.coi.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raeven R.H., Brummelman J., Pennings J.L., van der Maas L., Tilstra W., Helm K., van Riet E., Jiskoot W., van Els C.A., Han W.G., Kersten G.F., Metz B. Bordetella pertussis outer membrane vesicle vaccine confers equal efficacy in mice with milder inflammatory responses compared to a whole-cell vaccine. Sci. Rep. 2016;6:38240. doi: 10.1038/srep38240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gestal M.C., Johnson H.M., Harvill E.T. Immunomodulation as a Novel Strategy for Prevention and Treatment of Bordetella spp. Infections. Front. Immunol. 2019;10:2869. doi: 10.3389/fimmu.2019.02869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zurita M.E., Wilk M.M., Carriquiriborde F., Bartel E., Moreno G., Misiak A., Mills K.H.G., Hozbor D. A Pertussis Outer Membrane Vesicle-Based Vaccine Induces Lung-Resident Memory CD4 T Cells and Protection Against Bordetella pertussis, Including Pertactin Deficient Strains. Front. Cell. Infect. Microbiol. 2019;9:125. doi: 10.3389/fcimb.2019.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Riddle M.S., DuPont H.L., Connor B.A. ACG Clinical Guideline: Diagnosis, Treatment, and Prevention of Acute Diarrheal Infections in Adults. Am. J. Gastroenterol. 2016;111:602–622. doi: 10.1038/ajg.2016.126. [DOI] [PubMed] [Google Scholar]
- 80.Akhondi H., Simonsen K.A. StatPearls; Treasure Island (FL): 2021. Bacterial Diarrhea. [PubMed] [Google Scholar]
- 81.Mirhoseini A., Amani J., Nazarian S. Review on pathogenicity mechanism of enterotoxigenic Escherichia coli and vaccines against it. Microb. Pathog. 2018;117:162–169. doi: 10.1016/j.micpath.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 82.Azer S.A., Tuma F. StatPearls; StatPearls, Treasure Island (FL): 2021. Infectious Colitis. [PubMed] [Google Scholar]
- 83.Wu C.M., Chung T.C. Mice protected by oral immunization with Lactobacillus reuteri secreting fusion protein of Escherichia coli enterotoxin subunit protein. FEMS Immunol. Med. Microbiol. 2007;50:354–365. doi: 10.1111/j.1574-695X.2007.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu J.K., Hou X.L., Wei C.H., Yu L.Y., He X.J., Wang G.H., Lee J.S., Kim C.J. Induction of immune responses in mice after oral immunization with recombinant Lactobacillus casei strains expressing enterotoxigenic Escherichia coli F41 fimbrial protein. Appl. Enviro. Microbiol. 2009;75:4491–4497. doi: 10.1128/AEM.02672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei C.H., Liu J.K., Hou X.L., Yu L.Y., Lee J.S., Kim C.J. Immunogenicity and protective efficacy of orally or intranasally administered recombinant Lactobacillus casei expressing ETEC K99. Vaccine. 2010;28:4113–4118. doi: 10.1016/j.vaccine.2009.05.088. [DOI] [PubMed] [Google Scholar]
- 86.Liu J.K., Wei C.H., Hou X.L., Yu L.Y. Passive protection of mice pups through oral or intranasal immunization of dams with recombinant Lactobacillus casei vaccine against ETEC F41. Res. Vet. Sci. 2014;96:283–287. doi: 10.1016/j.rvsc.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 87.Ferreira P.C., da Silva J.B., Piazza R.M., Eckmann L., Ho P.L., Oliveira M.L. Immunization of mice with Lactobacillus casei expressing a beta-intimin fragment reduces intestinal colonization by Citrobacter rodentium. Clin. Vaccine Immunol. 2011;18:1823–1833. doi: 10.1128/CVI.05262-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wen L.J., Hou X.L., Wang G.H., Yu L.Y., Wei X.M., Liu J.K., Liu Q., Wei C.H. Immunization with recombinant Lactobacillus casei strains producing K99, K88 fimbrial protein protects mice against enterotoxigenic Escherichia coli. Vaccine. 2012;30:3339–3349. doi: 10.1016/j.vaccine.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 89.Yu M., Qi R., Chen C., Yin J., Ma S., Shi W., Wu Y., Ge J., Jiang Y., Tang L., Xu Y., Li Y. Immunogenicity of recombinant Lactobacillus casei-expressing F4 (K88) fimbrial adhesin FaeG in conjunction with a heat-labile enterotoxin A (LTAK63) and heat-labile enterotoxin B (LTB) of enterotoxigenic Escherichia coli as an oral adjuvant in mice. J. Appl. Microbiol. 2017;122:506–515. doi: 10.1111/jam.13352. [DOI] [PubMed] [Google Scholar]
- 90.Yang G., Jiang Y., Tong P., Li C., Yang W., Hu J., Ye L., Gu W., Shi C., Shan B., Wang C. Alleviation of enterotoxigenic Escherichia coli challenge by recombinant Lactobacillus plantarum expressing a FaeG- and DC-targeting peptide fusion protein. Benef. Microbes. 2017;8:379–391. doi: 10.3920/BM2016.0116. [DOI] [PubMed] [Google Scholar]
- 91.Lin R., Zhang Y., Long B., Li Y., Wu Y., Duan S., Zhu B., Wu X., Fan H. Oral Immunization with Recombinant Lactobacillus acidophilus Expressing espA-Tir-M Confers Protection against Enterohemorrhagic Escherichia coli O157:H7 Challenge in Mice. Front. Microbiol. 2017;8:417. doi: 10.3389/fmicb.2017.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chu H., Kang S., Ha S., Cho K., Park S.M., Han K.H., Kang S.K., Lee H., Han S.H., Yun C.H., Choi Y. Lactobacillus acidophilus expressing recombinant K99 adhesive fimbriae has an inhibitory effect on adhesion of enterotoxigenic Escherichia coli. Microbiol. Immunol. 2005;49:941–948. doi: 10.1111/j.1348-0421.2005.tb03687.x. [DOI] [PubMed] [Google Scholar]
- 93.Ashrafi F., Fallah Mehrabadi J., Siadat S.D., Aghasadeghi M.R. Expression and Purification of the Uropathogenic Escherichia coli PapG Protein and its Surface Absorption on Lactobacillus reuteri: Implications for Surface Display System Vaccines. Jundishapur J. Microbiol. 2015;8:e25595. doi: 10.5812/jjm.25595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choi K.S., Kim S.H., Kim E.D., Lee S.H., Han S.J., Yoon S., Chang K.T., Seo K.Y. Protection from hemolytic uremic syndrome by eyedrop vaccination with modified enterohemorrhagic E. coli outer membrane vesicles. PloS One. 2014;9 doi: 10.1371/journal.pone.0100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khan M.S., Vishakante G.D. Development and evaluation of porous chitosan nanoparticles for treatment of enterotoxigenic Escherichia coil infection. J. Biomed. Nanotechnol. 2013;9:107–114. doi: 10.1166/jbn.2013.1471. [DOI] [PubMed] [Google Scholar]
- 96.Noroozi N., Gargari S.L.M., Nazarian S., Sarvary S., Adriani R.R. Immunogenicity of enterotoxigenic Escherichia coli outer membrane vesicles encapsulated in chitosan nanoparticles. Iran. J. Basic. Med. Sci. 2018;21:284–291. doi: 10.22038/ijbms.2018.25886.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nazarian S., Gargari S.L., Rasooli I., Hasannia S., Pirooznia N. A PLGA-encapsulated chimeric protein protects against adherence and toxicity of enterotoxigenic Escherichia coli. Microbiol. Res. 2014;169:205–212. doi: 10.1016/j.micres.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 98.Kordbacheh E., Nazarian S., Sadeghi D., Hajizadeh A. An LTB-entrapped protein in PLGA nanoparticles preserves against enterotoxin of enterotoxigenic Escherichia coli. Iran. J. Basic. Med. Sci. 2018;21:517–524. doi: 10.22038/IJBMS.2018.27017.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Byrd W., Cassels F.J. Intranasal immunization of BALB/c mice with enterotoxigenic Escherichia coli colonization factor CS6 encapsulated in biodegradable poly(DL-lactide-co-glycolide) microspheres. Vaccine. 2006;24:1359–1366. doi: 10.1016/j.vaccine.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 100.Rodrigues-Jesus M.J., Fotoran W.L., Cardoso R.M., Araki K., Wunderlich G., Ferreira L.C.S. Nano-multilamellar lipid vesicles (NMVs) enhance protective antibody responses against Shiga toxin (Stx2a) produced by enterohemorrhagic Escherichia coli strains (EHEC) Braz. J. Microbiol. 2019;50:67–77. doi: 10.1007/s42770-018-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Andrade G.R., New R.R., Sant'Anna O.A., Williams N.A., Alves R.C., Pimenta D.C., Vigerelli H., Melo B.S., Rocha L.B., Piazza R.M., Mendonca-Previato L., Domingos M.O. A universal polysaccharide conjugated vaccine against O111 E. coli. Hum. Vaccin. Immunother. 2014;10:2864–2874. doi: 10.4161/21645515.2014.972145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen W., Zhang B., Mahony T., Gu W., Rolfe B., Xu Z.P. Efficient and Durable Vaccine against Intimin beta of Diarrheagenic E. Coli Induced by Clay Nanoparticles. Small. 2016;12:1627–1639. doi: 10.1002/smll.201503359. [DOI] [PubMed] [Google Scholar]
- 103.Mercuri L.P., Carvalho L.V., Lima F.A., Quayle C., Fantini M.C., Tanaka G.S., Cabrera W.H., Furtado M.F., Tambourgi D.V., Matos Jdo R., Jaroniec M., Sant'Anna O.A. Ordered mesoporous silica SBA-15: a new effective adjuvant to induce antibody response. Small. 2006;2:254–256. doi: 10.1002/smll.200500274. [DOI] [PubMed] [Google Scholar]
- 104.Mitra S., Chakrabarti M.K., Koley H. Multi-serotype outer membrane vesicles of Shigellae confer passive protection to the neonatal mice against shigellosis. Vaccine. 2013;31:3163–3173. doi: 10.1016/j.vaccine.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 105.Gerke C., Colucci A.M., Giannelli C., Sanzone S., Vitali C.G., Sollai L., Rossi O., Martin L.B., Auerbach J., Di Cioccio V., Saul A. Production of a Shigella sonnei Vaccine Based on Generalized Modules for Membrane Antigens (GMMA), 1790GAHB. PloS One. 2015;10 doi: 10.1371/journal.pone.0134478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Raso M.M., Gasperini G., Alfini R., Schiavo F., Aruta M.G., Carducci M., Forgione M.C., Martini S., Cescutti P., Necchi F., Micoli F. GMMA and Glycoconjugate Approaches Compared in Mice for the Development of a Vaccine against Shigella flexneri Serotype 6. Vaccines. 2020;8:160. doi: 10.3390/vaccines8020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Obiero C.W., Ndiaye A.G.W., Scire A.S., Kaunyangi B.M., Marchetti E., Gone A.M., Schutte L.D., Riccucci D., Auerbach J., Saul A., Martin L.B., Bejon P., Njuguna P., Podda A. A Phase 2a Randomized Study to Evaluate the Safety and Immunogenicity of the 1790GAHB Generalized Modules for Membrane Antigen Vaccine against Shigella sonnei Administered Intramuscularly to Adults from a Shigellosis-Endemic Country. Front. Immunol. 2017;8:1884. doi: 10.3389/fimmu.2017.01884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Camacho A.I., Irache J.M., de Souza J., Sanchez-Gomez S., Gamazo C. Nanoparticle-based vaccine for mucosal protection against Shigella flexneri in mice. Vaccine. 2013;31:3288–3294. doi: 10.1016/j.vaccine.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 109.Camacho A.I., de Souza J., Sanchez-Gomez S., Pardo-Ros M., Irache J.M., Gamazo C. Mucosal immunization with Shigella flexneri outer membrane vesicles induced protection in mice. Vaccine. 2011;29:8222–8229. doi: 10.1016/j.vaccine.2011.08.121. [DOI] [PubMed] [Google Scholar]
- 110.Sarvary S., Gargari S.L.M., Nazarian S., Adriani R.R., Noroozi N. Immunogenicity of Shigella sonnei outer membrane vesicles extracted in different environmental conditions. Biologia. 2021:721–728. [Google Scholar]
- 111.Pan C., Wu J., Qing S., Zhang X., Zhang L., Yue H., Zeng M., Wang B., Yuan Z., Qiu Y., Ye H., Wang D., Liu X., Sun P., Liu B., Feng E., Gao X., Zhu L., Wei W., Ma G., Wang H. Biosynthesis of Self-Assembled Proteinaceous Nanoparticles for Vaccination. Adv. Mater. 2020;32 doi: 10.1002/adma.202002940. [DOI] [PubMed] [Google Scholar]
- 112.Gilavand F., Marzban A., Ebrahimipour G., Soleimani N., Goudarzi M. Designation of chitosan nano-vaccine based on MxiH antigen of Shigella flexneri with increased immunization capacity. Carbohydr. Polym. 2020;232 doi: 10.1016/j.carbpol.2019.115813. [DOI] [PubMed] [Google Scholar]
- 113.Jahantigh D., Saadati M., Ramandi M.F., Mousavi M., Zand A.M. Novel Intranasal Vaccine Delivery System by Chitosan Nanofibrous Membrane Containing N-Terminal Region of Ipad Antigen as a Nasal Shigellosis Vaccine, Studies in Guinea Pigs. J. Drug Delivery. Sci. Technol. 2014;24:33–39. [Google Scholar]
- 114.Akbari M.R., Saadati M., Honari H., Ghorbani H.M. IpaD-loaded N-trimethyl Chitosan Nanoparticles Can Efficiently Protect Guinea Pigs against Shigella flexneri. Iran. J. Immunol. 2019;16:212–224. doi: 10.22034/IJI.2019.80272. [DOI] [PubMed] [Google Scholar]
- 115.Roy N., Barman S., Ghosh A., Pal A., Chakraborty K., Das S.S., Saha D.R., Yamasaki S., Koley H. Immunogenicity and protective efficacy of Vibrio cholerae outer membrane vesicles in rabbit model. FEMS immunol. Med. Microbiol. 2010;60:18–27. doi: 10.1111/j.1574-695X.2010.00692.x. [DOI] [PubMed] [Google Scholar]
- 116.Schild S., Nelson E.J., Camilli A. Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect. Immun. 2008;76:4554–4563. doi: 10.1128/IAI.00532-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Okuno T., Kashige N., Satho T., Irie K., Hiramatsu Y., Sharmin T., Fukumitsu Y., Uyeda S., Yamada S., Harakuni T., Miyata T., Arakawa T., Imoto M., Toda A., Nakashima Y., Miake F. Expression and secretion of cholera toxin B subunit in lactobacilli. Biol. Pharm. Bull. 2013;36:952–958. doi: 10.1248/bpb.b12-01021. [DOI] [PubMed] [Google Scholar]
- 118.Alaniz R.C., Deatherage B.L., Lara J.C., Cookson B.T. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 2007;179:7692–7701. doi: 10.4049/jimmunol.179.11.7692. [DOI] [PubMed] [Google Scholar]
- 119.Howlader D.R., Koley H., Sinha R., Maiti S., Bhaumik U., Mukherjee P., Dutta S. Development of a novel S. Typhi and Paratyphi A outer membrane vesicles based bivalent vaccine against enteric fever. PloS One. 2018;13 doi: 10.1371/journal.pone.0203631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kajikawa A., Satoh E., Leer R.J., Yamamoto S., Igimi S. Intragastric immunization with recombinant Lactobacillus casei expressing flagellar antigen confers antibody-independent protective immunity against Salmonella enterica serovar Enteritidis. Vaccine. 2007;25:3599–3605. doi: 10.1016/j.vaccine.2007.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kajikawa A., Igimi S. Innate and acquired immune responses induced by recombinant Lactobacillus casei displaying flagellin-fusion antigen on the cell-surface. Vaccine. 2010;28:3409–3415. doi: 10.1016/j.vaccine.2010.02.077. [DOI] [PubMed] [Google Scholar]
- 122.Ameer M.A., Wasey A., Salen P. StatPearls; Treasure Island (FL): 2021. Escherichia Coli (E Coli 0157 H7) [PubMed] [Google Scholar]
- 123.Bourgeois A.L., Wierzba T.F., Walker R.I. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine. 2016;34:2880–2886. doi: 10.1016/j.vaccine.2016.02.076. [DOI] [PubMed] [Google Scholar]
- 124.Mansouri M., Mousavy S.J., Ehsaei Z., Nazarian S., Zali M.R., Moazzeni S.M. The codon-optimization of cfaE gene and evaluating its high expression capacity and conserved immunogenicity in Escherichia coli. Biologicals. 2013;41:169–175. doi: 10.1016/j.biologicals.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 125.Madhavan T.P., Sakellaris H. Colonization factors of enterotoxigenic Escherichia coli. Adv. Appl. Microbiol. 2015;90:155–197. doi: 10.1016/bs.aambs.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 126.LeCureux J.S., Dean G.A. Lactobacillus Mucosal Vaccine Vectors: Immune Responses against Bacterial and Viral Antigens. mSphere. 2018;3:e00061–e118. doi: 10.1128/mSphere.00061-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Beddoe T., Paton A.W., Le Nours J., Rossjohn J., Paton J.C. Structure, biological functions and applications of the AB5 toxins. Trends Biochem. Sci. 2010;35:411–418. doi: 10.1016/j.tibs.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roy K., Hamilton D.J., Munson G.P., Fleckenstein J.M. Outer membrane vesicles induce immune responses to virulence proteins and protect against colonization by enterotoxigenic Escherichia coli. Clin. Vaccine Immunol. 2011;18:1803–1808. doi: 10.1128/CVI.05217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim S.H., Kim K.S., Lee S.R., Kim E., Kim M.S., Lee E.Y., Gho Y.S., Kim J.W., Bishop R.E., Chang K.T. Structural modifications of outer membrane vesicles to refine them as vaccine delivery vehicles. Biochim. Biophys. Acta. 2009;1788:2150–2159. doi: 10.1016/j.bbamem.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim S.H., Lee S.R., Kim K.S., Ko A., Kim E., Kim Y.H., Chang K.T. Shiga toxin A subunit mutant of Escherichia coli O157:H7 releases outer membrane vesicles containing the B-pentameric complex. FEMS Immunol. Med. Microbiol. 2010;58:412–420. doi: 10.1111/j.1574-695X.2010.00654.x. [DOI] [PubMed] [Google Scholar]
- 131.Leitner D.R., Feichter S., Schild-Prufert K., Rechberger G.N., Reidl J., Schild S. Lipopolysaccharide modifications of a cholera vaccine candidate based on outer membrane vesicles reduce endotoxicity and reveal the major protective antigen. Infect. Immun. 2013;81:2379–2393. doi: 10.1128/IAI.01382-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee S.R., Kim S.H., Jeong K.J., Kim K.S., Kim Y.H., Kim S.J., Kim E., Kim J.W., Chang K.T. Multi-immunogenic outer membrane vesicles derived from an MsbB-deficient Salmonella enterica serovar typhimurium mutant. J. Microbiol. Biotechnol. 2009;19:1271–1279. [PubMed] [Google Scholar]
- 133.De Benedetto G., Alfini R., Cescutti P., Caboni M., Lanzilao L., Necchi F., Saul A., MacLennan C.A., Rondini S., Micoli F. Characterization of O-antigen delivered by Generalized Modules for Membrane Antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine. 2017;35:419–426. doi: 10.1016/j.vaccine.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 134.Rossi O., Caboni M., Negrea A., Necchi F., Alfini R., Micoli F., Saul A., MacLennan C.A., Rondini S., Gerke C. Toll-Like Receptor Activation by Generalized Modules for Membrane Antigens from Lipid A Mutants of Salmonella enterica Serovars Typhimurium and Enteritidis. Clin. Vaccine Immunol. 2016;23:304–314. doi: 10.1128/CVI.00023-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sinha R., Howlader D.R., Ta A., Mitra S., Das S., Koley H. Retinoic acid pre-treatment down regulates V. cholerae outer membrane vesicles induced acute inflammation and enhances mucosal immunity. Vaccine. 2017;35:3534–3547. doi: 10.1016/j.vaccine.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 136.Choi H.I., Kim M., Jeon J., Han J.K., Kim K.S. Overexpression of MicA induces production of OmpC-enriched outer membrane vesicles that protect against Salmonella challenge. Biochem. Biophys. Res. Commun. 2017;490:991–996. doi: 10.1016/j.bbrc.2017.06.152. [DOI] [PubMed] [Google Scholar]
- 137.Micoli F., Rondini S., Alfini R., Lanzilao L., Necchi F., Negrea A., Rossi O., Brandt C., Clare S., Mastroeni P., Rappuoli R., Saul A., MacLennan C.A. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc. Natl. Acad. Sci. U S A. 2018;115:10428–10433. doi: 10.1073/pnas.1807655115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tamayo I., Irache J.M., Mansilla C., Ochoa-Reparaz J., Lasarte J.J., Gamazo C. Poly(anhydride) nanoparticles act as active Th1 adjuvants through Toll-like receptor exploitation. Clin. Vaccine Immunol. 2010;17:1356–1362. doi: 10.1128/CVI.00164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Koide S.S. Chitin-chitosan: Properties, benefits and risks. Nutr. Res. 1998;18:1091–1101. [Google Scholar]
- 140.Ensign L.M., Cone R., Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012;64:557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee W.J., Cha S., Shin M., Jung M., Islam M.A., Cho C.S., Yoo H.S. Efficacy of thiolated eudragit microspheres as an oral vaccine delivery system to induce mucosal immunity against enterotoxigenic Escherichia coli in mice. Eur. J. Pharm. Biopharm. 2012;81:43–48. doi: 10.1016/j.ejpb.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 142.Baker S., The H.C. Recent insights into Shigella. Curr. Opin. Infect. Dis. 2018;31:449–454. doi: 10.1097/QCO.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kotloff K.L., Riddle M.S., Platts-Mills J.A., Pavlinac P., Zaidi A.K.M. Shigellosis. Lancet. 2018;391:801–812. doi: 10.1016/S0140-6736(17)33296-8. [DOI] [PubMed] [Google Scholar]
- 144.Stratmann T. Cholera Toxin Subunit B as Adjuvant–An Accelerator in Protective Immunity and a Break in Autoimmunity. Vaccines. 2015;3:579–596. doi: 10.3390/vaccines3030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Terra V.S., Mills D.C., Yates L.E., Abouelhadid S., Cuccui J., Wren B.W. Recent developments in bacterial protein glycan coupling technology and glycoconjugate vaccine design. J. Med. Microbiol. 2012;61:919–926. doi: 10.1099/jmm.0.039438-0. [DOI] [PubMed] [Google Scholar]
- 146.Jones C. Vaccines based on the cell surface carbohydrates of pathogenic bacteria. An. Acad. Bras. Cienc. 2005;77:293–324. doi: 10.1590/s0001-37652005000200009. [DOI] [PubMed] [Google Scholar]
- 147.Kay E., Cuccui J., Wren B.W. Recent advances in the production of recombinant glycoconjugate vaccines. NPJ vaccines. 2019;4:16. doi: 10.1038/s41541-019-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huang J., Pan C., Sun P., Feng E., Wu J., Zhu L., Wang H. Application of an O-Linked Glycosylation System in Yersinia enterocolitica Serotype O:9 to Generate a New Candidate Vaccine against Brucella abortus. Microorganisms. 2020;8:436. doi: 10.3390/microorganisms8030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sun P., Pan C., Zeng M., Liu B., Liang H., Wang D., Liu X., Wang B., Lyu Y., Wu J., Zhu L., Wang H. Design and production of conjugate vaccines against S. Paratyphi A using an O-linked glycosylation system in vivo. NPJ Vaccines. 2018;3:4. doi: 10.1038/s41541-017-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pan C., Sun P., Liu B., Liang H., Peng Z., Dong Y., Wang D., Liu X., Wang B., Zeng M., Wu J., Zhu L., Wang H. Biosynthesis of Conjugate Vaccines Using an O-Linked Glycosylation System. mBio. 2016;7:e00443–00416. doi: 10.1128/mBio.00443-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Koppolu B., Zaharoff D.A. The effect of antigen encapsulation in chitosan particles on uptake, activation and presentation by antigen presenting cells. Biomaterials. 2013;34:2359–2369. doi: 10.1016/j.biomaterials.2012.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Amidi M., Mastrobattista E., Jiskoot W., Hennink W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliv. Rev. 2010;62:59–82. doi: 10.1016/j.addr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 153.McConnell M.J., Hanna P.C., Imperiale M.J. Adenovirus-based prime-boost immunization for rapid vaccination against anthrax. Mol. Ther. 2007;15:203–210. doi: 10.1038/sj.mt.6300034. [DOI] [PubMed] [Google Scholar]
- 154.Wang H.C., An H.J., Yu Y.Z., Xu Q. Potentiation of anthrax vaccines using protective antigen-expressing viral replicon vectors. Immunol. Lett. 2015;163:206–213. doi: 10.1016/j.imlet.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 155.Manayani D.J., Thomas D., Dryden K.A., Reddy V., Siladi M.E., Marlett J.M., Rainey G.J., Pique M.E., Scobie H.M., Yeager M., Young J.A., Manchester M., Schneemann A. A viral nanoparticle with dual function as an anthrax antitoxin and vaccine. PLoS Pathog. 2007;3:1422–1431. doi: 10.1371/journal.ppat.0030142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tao P., Mahalingam M., Zhu J., Moayeri M., Sha J., Lawrence W.S., Leppla S.H., Chopra A.K., Rao V.B. A Bacteriophage T4 Nanoparticle-Based Dual Vaccine against Anthrax and Plague. mBio. 2018;9:e01926–18. doi: 10.1128/mBio.01926-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Langley W.A., Bradley K.C., Li Z.N., Smith M.E., Schnell M.J., Steinhauer D.A. Induction of neutralizing antibody responses to anthrax protective antigen by using influenza virus vectors: implications for disparate immune system priming pathways. J. Virol. 2010;84:8300–8307. doi: 10.1128/JVI.00183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McComb R.C., Ho C.L., Bradley K.A., Grill L.K., Martchenko M. Presentation of peptides from Bacillus anthracis protective antigen on Tobacco Mosaic Virus as an epitope targeted anthrax vaccine. Vaccine. 2015;33:6745–6751. doi: 10.1016/j.vaccine.2015.10.075. [DOI] [PubMed] [Google Scholar]
- 159.Peachman K.K., Li Q., Matyas G.R., Shivachandra S.B., Lovchik J., Lyons R.C., Alving C.R., Rao V.B., Rao M. Anthrax vaccine antigen-adjuvant formulations completely protect New Zealand white rabbits against challenge with Bacillus anthracis Ames strain spores. Clin. Vaccine Immunol. 2012;19:11–16. doi: 10.1128/CVI.05376-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rao M., Peachman K.K., Li Q., Matyas G.R., Shivachandra S.B., Borschel R., Morthole V.I., Fernandez-Prada C., Alving C.R., Rao V.B. Highly effective generic adjuvant systems for orphan or poverty-related vaccines. Vaccine. 2011;29:873–877. doi: 10.1016/j.vaccine.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gogoi H., Mani R., Malik A., Sehrawat P., Bhatnagar R. Co-Administration of Aluminium Hydroxide Nanoparticles and Protective Antigen Domain 4 Encapsulated Non-Ionic Surfactant Vesicles Show Enhanced Immune Response and Superior Protection against Anthrax. Vaccines. 2020;8:571. doi: 10.3390/vaccines8040571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chuang C.C., Tsai M.H., Yen H.J., Shyu H.F., Cheng K.M., Chen X.A., Chen C.C., Young J.J., Kau J.H. A fucoidan-quaternary chitosan nanoparticle adjuvant for anthrax vaccine as an alternative to CpG oligodeoxynucleotides. Carbohydr. Polym. 2020;229 doi: 10.1016/j.carbpol.2019.115403. [DOI] [PubMed] [Google Scholar]
- 163.Bento D., Staats H.F., Goncalves T., Borges O. Development of a novel adjuvanted nasal vaccine: C48/80 associated with chitosan nanoparticles as a path to enhance mucosal immunity. Eur. J. Pharm. Biopharm. 2015;93:149–164. doi: 10.1016/j.ejpb.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 164.Malik A., Gupta M., Mani R., Gogoi H., Bhatnagar R. Trimethyl Chitosan Nanoparticles Encapsulated Protective Antigen Protects the Mice Against Anthrax. Front. Immunol. 2018;9:562. doi: 10.3389/fimmu.2018.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu Y., Yin Y., Wang L., Zhang W., Chen X., Yang X., Xu J., Ma G. Engineering biomaterial-associated complement activation to improve vaccine efficacy. Biomacromolecules. 2013;14:3321–3328. doi: 10.1021/bm400930k. [DOI] [PubMed] [Google Scholar]
- 166.Flick-Smith H.C., Eyles J.E., Hebdon R., Waters E.L., Beedham R.J., Stagg T.J., Miller J., Alpar H.O., Baillie L.W., Williamson E.D. Mucosal or parenteral administration of microsphere-associated Bacillus anthracis protective antigen protects against anthrax infection in mice. Infect. Immun. 2002;70:2022–2028. doi: 10.1128/IAI.70.4.2022-2028.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ribeiro S., Rijpkema S.G., Durrani Z., Florence A.T. PLGA-dendron nanoparticles enhance immunogenicity but not lethal antibody production of a DNA vaccine against anthrax in mice. Int. J. Pharm. 2007;331:228–232. doi: 10.1016/j.ijpharm.2006.11.063. [DOI] [PubMed] [Google Scholar]
- 168.Manish M., Rahi A., Kaur M., Bhatnagar R., Singh S. A single-dose PLGA encapsulated protective antigen domain 4 nanoformulation protects mice against Bacillus anthracis spore challenge. PloS One. 2013;8 doi: 10.1371/journal.pone.0061885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kachura M.A., Hickle C., Kell S.A., Sathe A., Calacsan C., Kiwan R., Hall B., Milley R., Ott G., Coffman R.L., Kanzler H., Campbell J.D. A CpG-Ficoll Nanoparticle Adjuvant for Anthrax Protective Antigen Enhances Immunogenicity and Provides Single-Immunization Protection against Inhaled Anthrax in Monkeys. J. Immunol. 2016;196:284–297. doi: 10.4049/jimmunol.1501903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bielinska A.U., Janczak K.W., Landers J.J., Makidon P., Sower L.E., Peterson J.W., Baker J.R., Jr. Mucosal immunization with a novel nanoemulsion-based recombinant anthrax protective antigen vaccine protects against Bacillus anthracis spore challenge. Infect. Immun. 2007;75:4020–4029. doi: 10.1128/IAI.00070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.O'Flaherty S., Klaenhammer T.R. Multivalent Chromosomal Expression of the Clostridium botulinum Serotype A Neurotoxin Heavy-Chain Antigen and the Bacillus anthracis Protective Antigen in Lactobacillus acidophilus. Appl. Environ. Microbiol. 2016;82:6091–6101. doi: 10.1128/AEM.01533-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zegers N.D., Kluter E., van Der Stap H., van Dura E., van Dalen P., Shaw M., Baillie L. Expression of the protective antigen of Bacillus anthracis by Lactobacillus casei: towards the development of an oral vaccine against anthrax. J. Appl. Microbiol. 1999;87:309–314. doi: 10.1046/j.1365-2672.1999.00900.x. [DOI] [PubMed] [Google Scholar]
- 173.Mohamadzadeh M., Duong T., Sandwick S.J., Hoover T., Klaenhammer T.R. Dendritic cell targeting of Bacillus anthracis protective antigen expressed by Lactobacillus acidophilus protects mice from lethal challenge. Proc. Natl. Acad. Sci. U S A. 2009;106:4331–4336. doi: 10.1073/pnas.0900029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mohamadzadeh M., Durmaz E., Zadeh M., Pakanati K.C., Gramarossa M., Cohran V., Klaenhammer T.R. Targeted expression of anthrax protective antigen by Lactobacillus gasseri as an anthrax vaccine. Future Microbiol. 2010;5:1289–1296. doi: 10.2217/fmb.10.78. [DOI] [PubMed] [Google Scholar]
- 175.Kathania M., Zadeh M., Lightfoot Y.L., Roman R.M., Sahay B., Abbott J.R., Mohamadzadeh M. Colonic immune stimulation by targeted oral vaccine. PloS One. 2013;8 doi: 10.1371/journal.pone.0055143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Stokes M.G., Titball R.W., Neeson B.N., Galen J.E., Walker N.J., Stagg A.J., Jenner D.C., Thwaite J.E., Nataro J.P., Baillie L.W., Atkins H.S. Oral administration of a Salmonella enterica-based vaccine expressing Bacillus anthracis protective antigen confers protection against aerosolized B. anthracis. Infect. Immun. 2007;75:1827–1834. doi: 10.1128/IAI.01242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Valentine J.L., Chen L., Perregaux E.C., Weyant K.B., Rosenthal J.A., Heiss C., Azadi P., Fisher A.C., Putnam D., Moe G.R., Merritt J.H., DeLisa M.P. Immunization with Outer Membrane Vesicles Displaying Designer Glycotopes Yields Class-Switched, Glycan-Specific Antibodies. Cell Chem. Biol. 2016;23:655–665. doi: 10.1016/j.chembiol.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Onate A.A., Donoso G., Moraga-Cid G., Folch H., Cespedes S., Andrews E. An RNA vaccine based on recombinant Semliki Forest virus particles expressing the Cu, Zn superoxide dismutase protein of Brucella abortus induces protective immunity in BALB/c mice. Infect. Immun. 2005;73:3294–3300. doi: 10.1128/IAI.73.6.3294-3300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cabrera A., Saez D., Cespedes S., Andrews E., Onate A. Vaccination with recombinant Semliki Forest virus particles expressing translation initiation factor 3 of Brucella abortus induces protective immunity in BALB/c mice. Immunobiology. 2009;214:467–474. doi: 10.1016/j.imbio.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 180.Tabynov K., Sansyzbay A., Kydyrbayev Z., Yespembetov B., Ryskeldinova S., Zinina N., Assanzhanova N., Sultankulova K., Sandybayev N., Khairullin B., Kuznetsova I., Ferko B., Egorov A. Influenza viral vectors expressing the Brucella OMP16 or L7/L12 proteins as vaccines against B. abortus infection. Virol. J. 2014;11:69. doi: 10.1186/1743-422X-11-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bugybayeva D., Kydyrbayev Z., Zinina N., Assanzhanova N., Yespembetov B., Kozhamkulov Y., Zakarya K., Ryskeldinova S., Tabynov K. A new candidate vaccine for human brucellosis based on influenza viral vectors: a preliminary investigation for the development of an immunization schedule in a guinea pig model. Infect. Dis. Poverty. 2021;10:13. doi: 10.1186/s40249-021-00801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pontes D.S., Dorella F.A., Ribeiro L.A., Miyoshi A., Le Loir Y., Gruss A., Oliveira S.C., Langella P., Azevedo V. Induction of partial protection in mice after oral administration of Lactococcus lactis producing Brucella abortus L7/L12 antigen. J. Drug Target. 2003;11:489–493. doi: 10.1080/10611860410001670035. [DOI] [PubMed] [Google Scholar]
- 183.Saez D., Fernandez P., Rivera A., Andrews E., Onate A. Oral immunization of mice with recombinant Lactococcus lactis expressing Cu, Zn superoxide dismutase of Brucella abortus triggers protective immunity. Vaccine. 2012;30:1283–1290. doi: 10.1016/j.vaccine.2011.12.088. [DOI] [PubMed] [Google Scholar]
- 184.Shirdast H., Ebrahimzadeh F., Taromchi A.H., Mortazavi Y., Esmaeilzadeh A., Sekhavati M.H., Nedaei K., Mirabzadeh E. Recombinant Lactococcus Lactis Displaying Omp31 Antigen of Brucella melitensis Can Induce an Immunogenic Response in BALB/c Mice. Probiotics Antimicrob Proteins. 2021;13:80–89. doi: 10.1007/s12602-020-09684-1. [DOI] [PubMed] [Google Scholar]
- 185.Zhao Z., Li M., Luo D., Xing L., Wu S., Duan Y., Yang P., Wang X. Protection of mice from Brucella infection by immunization with attenuated Salmonella enterica serovar typhimurium expressing A L7/L12 and BLS fusion antigen of Brucella. Vaccine. 2009;27:5214–5219. doi: 10.1016/j.vaccine.2009.06.075. [DOI] [PubMed] [Google Scholar]
- 186.Senevirathne A., Hewawaduge C., Lee J.H. Live vaccine consisting of attenuated Salmonella secreting and delivering Brucella ribosomal protein L7/L12 induces humoral and cellular immune responses and protects mice against virulent Brucella abortus 544 challenge. Vet. Res. 2020;51:6. doi: 10.1186/s13567-020-0735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.He Y., Vemulapalli R., Schurig G.G. Recombinant Ochrobactrum anthropi expressing Brucella abortus Cu, Zn superoxide dismutase protects mice against B. abortus infection only after switching of immune responses to Th1 type. Infect. Immun. 2002;70:2535–2543. doi: 10.1128/IAI.70.5.2535-2543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Abkar M., Lotfi A.S., Amani J., Eskandari K., Ramandi M.F., Salimian J., Brujeni G.N., Alamian S., Kamali M., Koushki H. Survey of Omp19 immunogenicity against Brucella abortus and Brucella melitensis: influence of nanoparticulation versus traditional immunization. Vet. Res. Commun. 2015;39:217–228. doi: 10.1007/s11259-015-9645-2. [DOI] [PubMed] [Google Scholar]
- 189.Sadeghi Z., Fasihi-Ramandi M., Azizi M., Bouzari S. Mannosylated chitosan nanoparticles loaded with FliC antigen as a novel vaccine candidate against Brucella melitensis and Brucella abortus infection. J. Biotechnol. 2020;310:89–96. doi: 10.1016/j.jbiotec.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 190.Mallick A.I., Singha H., Khan S., Anwar T., Ansari M.A., Khalid R., Chaudhuri P., Owais M. Escheriosome-mediated delivery of recombinant ribosomal L7/L12 protein confers protection against murine brucellosis. Vaccine. 2007;25:7873–7884. doi: 10.1016/j.vaccine.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 191.Singha H., Mallick A.I., Jana C., Fatima N., Owais M., Chaudhuri P. Co-immunization with interlukin-18 enhances the protective efficacy of liposomes encapsulated recombinant Cu-Zn superoxide dismutase protein against Brucella abortus. Vaccine. 2011;29:4720–4727. doi: 10.1016/j.vaccine.2011.04.088. [DOI] [PubMed] [Google Scholar]
- 192.Singh D., Somani V.K., Aggarwal S., Bhatnagar R. PLGA (85:15) nanoparticle based delivery of rL7/L12 ribosomal protein in mice protects against Brucella abortus 544 infection: A promising alternate to traditional adjuvants. Mol. Immunol. 2015;68:272–279. doi: 10.1016/j.molimm.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 193.Sadeghi Z., Fasihi-Ramandi M., Bouzari S. Nanoparticle-Based Vaccines for Brucellosis: Calcium Phosphate Nanoparticles-Adsorbed Antigens Induce Cross Protective Response in Mice. Int. J. Nanomedicine. 2020;15:3877–3886. doi: 10.2147/IJN.S249942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Irene C., Fantappie L., Caproni E., Zerbini F., Anesi A., Tomasi M., Zanella I., Stupia S., Prete S., Valensin S., Konig E., Frattini L., Gagliardi A., Isaac S.J., Grandi A., Guella G., Grandi G. Bacterial outer membrane vesicles engineered with lipidated antigens as a platform for Staphylococcus aureus vaccine. Proc. Natl. Acad. Sci. U S A. 2019;116:21780–21788. doi: 10.1073/pnas.1905112116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kim O.Y., Choi S.J., Jang S.C., Park K.S., Kim S.R., Choi J.P., Lim J.H., Lee S.W., Park J., Di Vizio D., Lotvall J., Kim Y.K., Gho Y.S. Bacterial protoplast-derived nanovesicles as vaccine delivery system against bacterial infection. Nano Lett. 2015;15:266–274. doi: 10.1021/nl503508h. [DOI] [PubMed] [Google Scholar]
- 196.Wang X., Thompson C.D., Weidenmaier C., Lee J.C. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat. Commun. 2018;9:1379. doi: 10.1038/s41467-018-03847-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chen G., Bai Y., Li Z., Wang F., Fan X., Zhou X. Bacterial extracellular vesicle-coated multi-antigenic nanovaccines protect against drug-resistant Staphylococcus aureus infection by modulating antigen processing and presentation pathways. Theranostics. 2020;10:7131–7149. doi: 10.7150/thno.44564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fan X., Wang F., Zhou X., Chen B., Chen G. Size-Dependent Antibacterial Immunity of Staphylococcus aureus Protoplast-Derived Particulate Vaccines. Int. J. Nanomedicine. 2020;15:10321–10330. doi: 10.2147/IJN.S285895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Veloso T.R., Mancini S., Giddey M., Vouillamoz J., Que Y.A., Moreillon P., Entenza J.M. Vaccination against Staphylococcus aureus experimental endocarditis using recombinant Lactococcus lactis expressing ClfA or FnbpA. Vaccine. 2015;33:3512–3517. doi: 10.1016/j.vaccine.2015.05.060. [DOI] [PubMed] [Google Scholar]
- 200.Clow F., Peterken K., Pearson V., Proft T., Radcliff F.J. PilVax, a novel Lactococcus lactis-based mucosal vaccine platform, stimulates systemic and mucosal immune responses to Staphylococcus aureus. Immunol. Cell Biol. 2020;98:369–381. doi: 10.1111/imcb.12325. [DOI] [PubMed] [Google Scholar]
- 201.Brennan F.R., Bellaby T., Helliwell S.M., Jones T.D., Kamstrup S., Dalsgaard K., Flock J.I., Hamilton W.D. Chimeric plant virus particles administered nasally or orally induce systemic and mucosal immune responses in mice. J. Virol. 1999;73:930–938. doi: 10.1128/jvi.73.2.930-938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Xu C., Zhang B.Z., Lin Q., Deng J., Yu B., Arya S., Yuen K.Y., Huang J.D. Live attenuated Salmonella typhimurium vaccines delivering SaEsxA and SaEsxB via type III secretion system confer protection against Staphylococcus aureus infection. BMC Infect. Dis. 2018;18:195. doi: 10.1186/s12879-018-3104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang F., Fang R.H., Luk B.T., Hu C.J., Thamphiwatana S., Dehaini D., Angsantikul P., Kroll A.V., Pang Z., Gao W., Lu W., Zhang L. Nanoparticle-Based Antivirulence Vaccine for the Management of Methicillin-Resistant Staphylococcus aureus Skin Infection. Adv. Funct. Mater. 2016;26:1628–1635. doi: 10.1002/adfm.201505231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wei X., Gao J., Wang F., Ying M., Angsantikul P., Kroll A.V., Zhou J., Gao W., Lu W., Fang R.H., Zhang L. In Situ Capture of Bacterial Toxins for Antivirulence Vaccination. Adv. Mater. 2017;29:1701644. doi: 10.1002/adma.201701644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Daly S.M., Joyner J.A., Triplett K.D., Elmore B.O., Pokhrel S., Frietze K.M., Peabody D.S., Chackerian B., Hall P.R. VLP-based vaccine induces immune control of Staphylococcus aureus virulence regulation. Sci. Rep. 2017;7:637. doi: 10.1038/s41598-017-00753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Colonna C., Dorati R., Conti B., Caliceti P., Genta I. Sub-unit vaccine against S. aureus-mediated infections: set-up of nano-sized polymeric adjuvant. Int. J. Pharmaceut. 2013;452:390–401. doi: 10.1016/j.ijpharm.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 207.Genta I., Colonna C., Conti B., Caliceti P., Salmaso S., Speziale P., Pietrocola G., Chiesa E., Modena T., Dorati R. CNA-loaded PLGA nanoparticles improve humoral response againstS. aureus-mediated infections in a mouse model: subcutaneous vs. nasal administration strategy. J. Microencapsul. 2016;33:750–762. doi: 10.1080/02652048.2016.1260661. [DOI] [PubMed] [Google Scholar]
- 208.Chen L., Li S., Wang Z., Chang R., Su J., Han B. Protective effect of recombinant staphylococcal enterotoxin A entrapped in polylactic-co-glycolic acid microspheres against Staphylococcus aureus infection. Vet. Res. 2012;43:20. doi: 10.1186/1297-9716-43-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hosseini S.A., Nazarian S., Ebrahimi F., Hajizade A. Immunogenicity Evaluation of Recombinant Staphylococcus aureus Enterotoxin B (rSEB) and rSEB-loaded Chitosan Nanoparticles Following Nasal Administration. Iran. J. Allergy Asthma Immunol. 2020;19:159–171. doi: 10.18502/ijaai.v19i2.2767. [DOI] [PubMed] [Google Scholar]
- 210.Smitha K.T., Sreelakshmi M., Nisha N., Jayakumar R., Biswas R. Amidase encapsulated O-carboxymethyl chitosan nanoparticles for vaccine delivery. Int. J. Biol. Macromol. 2014;63:154–157. doi: 10.1016/j.ijbiomac.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 211.Arya S., Lin Q., Zhou N., Gao X., Huang J.D. Strong Immune Responses Induced by Direct Local Injections of Modified mRNA-Lipid Nanocomplexes. Mol. Ther. Nucleic Acids. 2020;19:1098–1109. doi: 10.1016/j.omtn.2019.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liu W., Tan Z., Xue J., Luo W., Song H., Lv X., Zheng T., Xi T., Xing Y. Therapeutic efficacy of oral immunization with a non-genetically modified Lactococcus lactis-based vaccine CUE-GEM induces local immunity against Helicobacter pylori infection. Appl. Microbiol. Biotechnol. 2016;100:6219–6229. doi: 10.1007/s00253-016-7333-y. [DOI] [PubMed] [Google Scholar]
- 213.Liu W., Tan Z., Liu H., Zeng Z., Luo S., Yang H., Zheng L., Xi T., Xing Y. Nongenetically modified Lactococcus lactis-adjuvanted vaccination enhanced innate immunity against Helicobacter pylori. Helicobacter. 2017;22 doi: 10.1111/hel.12426. [DOI] [PubMed] [Google Scholar]
- 214.Hongying F., Xianbo W., Fang Y., Yang B., Beiguo L. Oral immunization with recombinant Lactobacillus acidophilus expressing the adhesin Hp0410 of Helicobacter pylori induces mucosal and systemic immune responses. Clin. Vaccine Immunol. 2014;21:126–132. doi: 10.1128/CVI.00434-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Corthesy B., Boris S., Isler P., Grangette C., Mercenier A. Oral immunization of mice with lactic acid bacteria producing Helicobacter pylori urease B subunit partially protects against challenge with Helicobacter felis. J. Infect. Dis. 2005;192:1441–1449. doi: 10.1086/444425. [DOI] [PubMed] [Google Scholar]
- 216.Tan Z., Liu W., Liu H., Li C., Zhang Y., Meng X., Tang T., Xi T., Xing Y. Oral Helicobacter pylori vaccine-encapsulated acid-resistant HP55/PLGA nanoparticles promote immune protection. Eur. J. Pharm. Biopharm. 2017;111:33–43. doi: 10.1016/j.ejpb.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 217.Liu Q., Li X., Zhang Y., Song Z., Li R., Ruan H., Huang X. Orally-administered outer-membrane vesicles from Helicobacter pylori reduce H. pylori infection via Th2-biased immune responses in mice. Pathog. Dis. 2019;77:ftz050. doi: 10.1093/femspd/ftz050. [DOI] [PubMed] [Google Scholar]
- 218.Zhao W., Wu W., Xu X. Oral vaccination with liposome-encapsulated recombinant fusion peptide of urease B epitope and cholera toxin B subunit affords prophylactic and therapeutic effects against H. pylori infection in BALB/c mice. Vaccine. 2007;25:7664–7673. doi: 10.1016/j.vaccine.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 219.Wagner D.A., Kelly S.M., Petersen A.C., Peroutka-Bigus N., Darling R.J., Bellaire B.H., Wannemuehler M.J., Narasimhan B. Single-dose combination nanovaccine induces both rapid and long-lived protection against pneumonic plague. Acta Biomater. 2019;100:326–337. doi: 10.1016/j.actbio.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang X., Singh A.K., Zhang X., Sun W. Induction of Protective Antiplague Immune Responses by Self-Adjuvanting Bionanoparticles Derived from Engineered Yersinia pestis. Infect. Immun. 2020;88:e00081–20. doi: 10.1128/IAI.00081-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.del Rio B., Fuente J.L., Neves V., Dattwyler R., Seegers J.F., Gomes-Solecki M. Platform technology to deliver prophylactic molecules orally: an example using the Class A select agent Yersinia pestis. Vaccine. 2010;28:6714–6722. doi: 10.1016/j.vaccine.2010.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sweeney D.A., Hicks C.W., Cui X., Li Y., Eichacker P.Q. Anthrax infection. Am. J. Respir. Crit. Care Med. 2011;184:1333–1341. doi: 10.1164/rccm.201102-0209CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Darling R.G., Catlett C.L., Huebner K.D., Jarrett D.G. Threats in bioterrorism. I: CDC category A agents. Emerg. Med. Clin. North Am. 2002;20:273–309. doi: 10.1016/s0733-8627(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 224.Shi Z., Zeng M., Yang G., Siegel F., Cain L.J., van Kampen K.R., Elmets C.A., Tang D.C. Protection against tetanus by needle-free inoculation of adenovirus-vectored nasal and epicutaneous vaccines. J. Virol. 2001;75:11474–11482. doi: 10.1128/JVI.75.23.11474-11482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Peek L.J., Middaugh C.R., Berkland C. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 2008;60:915–928. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Smith M.E., Koser M., Xiao S., Siler C., McGettigan J.P., Calkins C., Pomerantz R.J., Dietzschold B., Schnell M.J. Rabies virus glycoprotein as a carrier for anthrax protective antigen. Virology. 2006;353:344–356. doi: 10.1016/j.virol.2006.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhang J., Tarbet E.B., Toro H., Tang D.C. Adenovirus-vectored drug-vaccine duo as a potential driver for conferring mass protection against infectious diseases. Expert Rev. Vaccines. 2011;10:1539–1552. doi: 10.1586/erv.11.141. [DOI] [PubMed] [Google Scholar]
- 228.Grit M., Crommelin D.J. Chemical stability of liposomes: implications for their physical stability. Chem. Phys. Lipids. 1993;64:3–18. doi: 10.1016/0009-3084(93)90053-6. [DOI] [PubMed] [Google Scholar]
- 229.Baillie A.J., Florence A.T., Hume L.R., Muirhead G.T., Rogerson A. The preparation and properties of niosomes–non-ionic surfactant vesicles. J. Pharm. Pharmacol. 1985;37:863–868. doi: 10.1111/j.2042-7158.1985.tb04990.x. [DOI] [PubMed] [Google Scholar]
- 230.Gogoi H., Mani R., Aggarwal S., Malik A., Munde M., Bhatnagar R. Crystalline and Amorphous Preparation of Aluminum Hydroxide Nanoparticles Enhances Protective Antigen Domain 4 Specific Immunogenicity and Provides Protection Against Anthrax. Int. J. Nanomedicine. 2020;15:239–252. doi: 10.2147/IJN.S219647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Milley B., Kiwan R., Ott G.S., Calacsan C., Kachura M., Campbell J.D., Kanzler H., Coffman R.L. Optimization, Production, and Characterization of a CpG-Oligonucleotide-Ficoll Conjugate Nanoparticle Adjuvant for Enhanced Immunogenicity of Anthrax Protective Antigen. Bioconjug. Chem. 2016;27:1293–1304. doi: 10.1021/acs.bioconjchem.6b00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Vishnu U.S., Sankarasubramanian J., Gunasekaran P., Rajendhran J. Identification of potential antigens from non-classically secreted proteins and designing novel multitope peptide vaccine candidate against Brucella melitensis through reverse vaccinology and immunoinformatics approach. Infect. Genet. Evol. 2017;55:151–158. doi: 10.1016/j.meegid.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 233.Olsen S.C., Palmer M.V. Advancement of knowledge of Brucella over the past 50 years. Vet. Pathol. 2014;51:1076–1089. doi: 10.1177/0300985814540545. [DOI] [PubMed] [Google Scholar]
- 234.Conde-Alvarez R., Arce-Gorvel V., Gil-Ramirez Y., Iriarte M., Grillo M.J., Gorvel J.P., Moriyon I. Lipopolysaccharide as a target for brucellosis vaccine design. Microb. Pathog. 2013;58:29–34. doi: 10.1016/j.micpath.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 235.Onate A.A., Cespedes S., Cabrera A., Rivers R., Gonzalez A., Munoz C., Folch H., Andrews E. A DNA vaccine encoding Cu, Zn superoxide dismutase of Brucella abortus induces protective immunity in BALB/c mice. Infect. Immun. 2003;71:4857–4861. doi: 10.1128/IAI.71.9.4857-4861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Munoz-Montesino C., Andrews E., Rivers R., Gonzalez-Smith A., Moraga-Cid G., Folch H., Cespedes S., Onate A.A. Intraspleen delivery of a DNA vaccine coding for superoxide dismutase (SOD) of Brucella abortus induces SOD-specific CD4+ and CD8+ T cells. Infect. Immun. 2004;72:2081–2087. doi: 10.1128/IAI.72.4.2081-2087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hien T.T., de Jong M., Farrar J. Avian influenza–a challenge to global health care structures. N. Engl. J. Med. 2004;351:2363–2365. doi: 10.1056/NEJMp048267. [DOI] [PubMed] [Google Scholar]
- 238.Romanova J., Krenn B.M., Wolschek M., Ferko B., Romanovskaja-Romanko E., Morokutti A., Shurygina A.P., Nakowitsch S., Ruthsatz T., Kiefmann B., Konig U., Bergmann M., Sachet M., Balasingam S., Mann A., Oxford J., Slais M., Kiselev O., Muster T., Egorov A. Preclinical evaluation of a replication-deficient intranasal DeltaNS1 H5N1 influenza vaccine. PloS One. 2009;4 doi: 10.1371/journal.pone.0005984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wacheck V., Egorov A., Groiss F., Pfeiffer A., Fuereder T., Hoeflmayer D., Kundi M., Popow-Kraupp T., Redlberger-Fritz M., Mueller C.A., Cinatl J., Michaelis M., Geiler J., Bergmann M., Romanova J., Roethl E., Morokutti A., Wolschek M., Ferko B., Seipelt J., Dick-Gudenus R., Muster T. A novel type of influenza vaccine: safety and immunogenicity of replication-deficient influenza virus created by deletion of the interferon antagonist NS1. J. Infect. Dis. 2010;201:354–362. doi: 10.1086/649428. [DOI] [PubMed] [Google Scholar]
- 240.Bugybayeva D., Ryskeldinova S., Zinina N., Sarmykova M., Assanzhanova N., Kydyrbayev Z., Tabynov K. Development of Human Vectored Brucellosis Vaccine Formulation: Assessment of Safety and Protectiveness of Influenza Viral Vectors Expressing Brucella Immunodominant Proteins in Mice and Guinea Pigs. Biomed. Res. Int. 2020;2020:1438928. doi: 10.1155/2020/1438928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ribeiro L.A., Azevedo V., Le Loir Y., Oliveira S.C., Dieye Y., Piard J.C., Gruss A., Langella P. Production and targeting of the Brucella abortus antigen L7/L12 in Lactococcus lactis: a first step towards food-grade live vaccines against brucellosis. Appl. Environ. Microbiol. 2002;68:910–916. doi: 10.1128/AEM.68.2.910-916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Park K.S., Choi K.H., Kim Y.S., Hong B.S., Kim O.Y., Kim J.H., Yoon C.M., Koh G.Y., Kim Y.K., Gho Y.S. Outer membrane vesicles derived from Escherichia coli induce systemic inflammatory response syndrome. PloS One. 2010;5 doi: 10.1371/journal.pone.0011334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Brown L., Wolf J.M., Prados-Rosales R., Casadevall A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015;13:620–630. doi: 10.1038/nrmicro3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hong S.W., Choi E.B., Min T.K., Kim J.H., Kim M.H., Jeon S.G., Lee B.J., Gho Y.S., Jee Y.K., Pyun B.Y., Kim Y.K. An important role of alpha-hemolysin in extracellular vesicles on the development of atopic dermatitis induced by Staphylococcus aureus. PloS One. 2014;9 doi: 10.1371/journal.pone.0100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Jun S.H., Lee J.H., Kim S.I., Choi C.W., Park T.I., Jung H.R., Cho J.W., Kim S.H., Lee J.C. Staphylococcus aureus-derived membrane vesicles exacerbate skin inflammation in atopic dermatitis. Clin. Exp. Allergy. 2017;47:85–96. doi: 10.1111/cea.12851. [DOI] [PubMed] [Google Scholar]
- 246.Walker P.J., Siddell S.G., Lefkowitz E.J., Mushegian A.R., Adriaenssens E.M., Dempsey D.M., Dutilh B.E., Harrach B., Harrison R.L., Hendrickson R.C., Junglen S., Knowles N.J., Kropinski A.M., Krupovic M., Kuhn J.H., Nibert M., Orton R.J., Rubino L., Sabanadzovic S., Simmonds P., Smith D.B., Varsani A., Zerbini F.M., Davison A.J. Changes to virus taxonomy and the Statutes ratified by the International Committee on Taxonomy of Viruses. Arch. Virol. 2020;165(2020):2737–2748. doi: 10.1007/s00705-020-04752-x. [DOI] [PubMed] [Google Scholar]
- 247.Tulchinsky T.H. Case Studies in Public Health ed. Academic Press; 2018. Chapter 19 - Maurice Hilleman: Creator of Vaccines That Changed the World. [Google Scholar]
- 248.Herzog C., Hartmann K., Kunzi V., Kursteiner O., Mischler R., Lazar H., Gluck R. Eleven years of Inflexal (R) V-a virosomal adjuvanted influenza vaccine. Vaccine. 2009;27:4381–4387. doi: 10.1016/j.vaccine.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 249.Malik H., Khan F.H., Ahsan H. Human papillomavirus: current status and issues of vaccination. Arch. Virol. 2014;159:199–205. doi: 10.1007/s00705-013-1827-z. [DOI] [PubMed] [Google Scholar]
- 250.Irvine D.J., Aung A., Silva M. Controlling timing and location in vaccines. Adv. Drug Del. Rev. 2020;158:91–115. doi: 10.1016/j.addr.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Marzi A., Robertson S.J., Haddock E., Feldmann F., Hanley P.W., Scott D.P., Strong J.E., Kobinger G., Best S.M., Feldmann H. EBOLA VACCINE. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science. 2015;349:739–742. doi: 10.1126/science.aab3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T., C.S. Group Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Clutterbuck E.A., Collins A.M., Cutland C.L., Darton T.C., Dheda K., Dold C., Duncan C.J.A., Emary K.R.W., Ewer K.J., Flaxman A., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Galiza E., Goodman A.L., Green C.M., Green C.A., Greenland M., Hill C., Hill H.C., Hirsch I., Izu A., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Libri V., Lillie P.J., Marchevsky N.G., Marshall R.P., Mendes A.V.A., Milan E.P., Minassian A.M., McGregor A., Mujadidi Y.F., Nana A., Padayachee S.D., Phillips D.J., Pittella A., Plested E., Pollock K.M., Ramasamy M.N., Ritchie A.J., Robinson H., Schwarzbold A.V., Smith A., Song R., Snape M.D., Sprinz E., Sutherland R.K., Thomson E.C., Torok M.E., Toshner M., Turner D.P.J., Vekemans J., Villafana T.L., White T., Williams C.J., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., Oxford C.V.T.G. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G.P., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Tureci O., Nell H., Schaefer A., Unal S., Tresnan D.B., Mather S., Dormitzer P.R., Sahin U., Jansen K.U., Gruber W.C., Grp C.C.T. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Xia S.L., Zhang Y.T., Wang Y.X., Wang H., Yang Y.K., Gao G.F., Tan W.J., Wu G.Z., Xu M., Lou Z.Y., Huang W.J., Xu W.B., Huang B.Y., Wang H.J., Wang W., Zhang W., Li N., Xie Z.Q., Ding L., You W.Y., Zhao Y.X., Yang X.Q., Liu Y., Wang Q., Huang L.L., Yang Y.L., Xu G.X., Luo B.J., Wang W.L., Liu P.P., Guo W.S., Yang X.M. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wu S., Zhong G., Zhang J., Shuai L., Zhang Z., Wen Z., Wang B., Zhao Z., Song X., Chen Y., Liu R., Fu L., Zhang J., Guo Q., Wang C., Yang Y., Fang T., Lv P., Wang J., Xu J., Li J., Yu C., Hou L., Bu Z., Chen W. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020;11:4081. doi: 10.1038/s41467-020-17972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Rerks-Ngarm S., Pitisuttithum P., Nitayaphan S., Kaewkungwal J., Chiu J., Paris R., Premsri N., Namwat C., de Souza M., Adams E., Benenson M., Gurunathan S., Tartaglia J., McNeil J.G., Francis D.P., Stablein D., Birx D.L., Chunsuttiwat S., Khamboonruang C., Thongcharoen P., Robb M.L., Michael N.L., Kunasol P., Kim J.H., Investigators M.-T. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N. Engl. J. Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 258.Atmar R.L., Cramer J.P., Baehner F., Han C., Borkowski A., Mendelman P.M. An Exploratory Study of the Salivary Immunoglobulin A Responses to 1 Dose of a Norovirus Virus-Like Particle Candidate Vaccine in Healthy Adults. J. Infect. Dis. 2019;219:410–414. doi: 10.1093/infdis/jiy529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.El-Kamary S.S., Pasetti M.F., Mendelman P.M., Frey S.E., Bernstein D.I., Treanor J.J., Ferreira J., Chen W.H., Sublett R., Richardson C., Bargatze R.F., Sztein M.B., Tacket C.O. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J. Infect. Dis. 2010;202:1649–1658. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Koch T., Dahlke C., Fathi A., Kupke A., Krahling V., Okba N.M.A., Halwe S., Rohde C., Eickmann M., Volz A., Hesterkamp T., Jambrecina A., Borregaard S., Ly M.L., Zinser M.E., Bartels E., Poetsch J.S.H., Neumann R., Fux R., Schmiedel S., Lohse A.W., Haagmans B.L., Sutter G., Becker S., Addo M.M. Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: an open-label, phase 1 trial. Lancet Infect. Dis. 2020;20:827–838. doi: 10.1016/S1473-3099(20)30248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Cooke G.S., Andrieux-Meyer I., Applegate T.L., Atun R., Burry J.R., Cheinquer H., Dusheiko G., Feld J.J., Gore C., Griswold M.G., Hamid S., Hellard M.E., Hou J., Howell J., Jia J., Kravchenko N., Lazarus J.V., Lemoine M., Lesi O.A., Maistat L., McMahon B.J., Razavi H., Roberts T.R., Simmons B., Sonderup M.W., Spearman C.W., Taylor B.E., Thomas D.L., Waked I., Ward J.W., Wiktor S.Z. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2019;4:135–184. doi: 10.1016/S2468-1253(18)30270-X. [DOI] [PubMed] [Google Scholar]
- 262.Singh L., Kruger H.G., Maguire G.E.M., Govender T., Parboosing R. The role of nanotechnology in the treatment of viral infections. Ther. Adv. Infect. Dis. 2017;4:105–131. doi: 10.1177/2049936117713593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Peterson C.E., Silva A., Holt H.K., Balanean A., Goben A.H., Dykens J.A. Barriers and facilitators to HPV vaccine uptake among US rural populations: a scoping review. Cancer Causes Control. 2020;31:801–814. doi: 10.1007/s10552-020-01323-y. [DOI] [PubMed] [Google Scholar]
- 264.Coller B.G., Blue J., Das R., Dubey S., Finelli L., Gupta S., Helmond F., Grant-Klein R.J., Liu K., Simon J., Troth S., VanRheenen S., Waterbury J., Wivel A., Wolf J., Heppner D.G., Kemp T., Nichols R., Monath T.P. Clinical development of a recombinant Ebola vaccine in the midst of an unprecedented epidemic. Vaccine. 2017;35:4465–4469. doi: 10.1016/j.vaccine.2017.05.097. [DOI] [PubMed] [Google Scholar]
- 265.Prubeta B.M. Current State of the First COVID-19 Vaccines. Vaccines. 2021;9:30. doi: 10.3390/vaccines9010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Amit S., Regev-Yochay G., Afek A., Kreiss Y., Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397:875–877. doi: 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Williams W.B., Liao H.-X., Moody M.A., Kepler T.B., Alam S.M., Gao F., Wiehe K., Trama A.M., Jones K., Zhang R., Song H., Marshall D.J., Whitesides J.F., Sawatzki K., Hua A., Liu P., Tay M.Z., Seaton K.E., Shen X., Foulger A., Lloyd K.E., Parks R., Pollara J., Ferrari G., Yu J., Vandergrift N., Montefiori D.C., Sobieszczyk M.E., Hammer S., Karuna S., Gilbert P., Grove D., Grunenberg N., McElrath M.J., Mascola J.R., Koup R.A., Corey L., Nabel G.J., Morgan C., Churchyard G., Maenza J., Keefer M., Graham B.S., Baden L.R., Tomaras G.D., Haynes B.F. Diversion of HIV-1 vaccine-induced immunity by gp41-microbiota cross-reactive antibodies. Science. 2015;349:aab1253. doi: 10.1126/science.aab1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ma G.H., Yue H. Advances in Uniform Polymer Microspheres and Microcapsules: Preparation and Biomedical Applications. Chinese J. Chem. 2020;38:911–923. [Google Scholar]
- 269.Ma X.C., Zou F., Yu F., Li R., Yuan Y.C., Zhang Y.W., Zhang X.T., Deng J.Y., Chen T., Song Z., Qiao Y.D., Zhan Y.K., Liu J., Zhang J.S., Zhang X., Peng Z.L., Li Y.Z., Lin Y.T., Liang L.T., Wang G.W., Chen Y.S., Chen Q.E., Pan T., He X., Zhang H. Nanoparticle Vaccines Based on the Receptor Binding Domain (RBD) and Heptad Repeat (HR) of SARS-CoV-2 Elicit Robust Protective Immune Responses. Immunity. 2020;53:1315–1330.e9. doi: 10.1016/j.immuni.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Peng S., Cao F.Q., Xia Y.F., Gao X.D., Dai L.P., Yan J.H., Ma G.H. Particulate Alum via Pickering Emulsion for an Enhanced COVID-19 Vaccine Adjuvant. Adv. Mater. 2020;32 doi: 10.1002/adma.202004210. [DOI] [PubMed] [Google Scholar]
- 271.Yang H.-W., Ye L., Guo X.D., Yang C., Compans R.W., Prausnitz M.R. Ebola Vaccination Using a DNA Vaccine Coated on PLGA-PLL/gamma PGA Nanoparticles Administered Using a Microneedle Patch. Adv. Healthc. Mater. 2017;6:1600750. doi: 10.1002/adhm.201600750. [DOI] [PubMed] [Google Scholar]
- 272.Chen Y., Lai K., Chiu Y., Wu Y., Shiau A., Chen M. Implantable microneedles with an immune-boosting function for effective intradermal influenza vaccination. Acta Biomater. 2019;97:230–238. doi: 10.1016/j.actbio.2019.07.048. [DOI] [PubMed] [Google Scholar]
- 273.Tian Y., Wang H., Liu Y., Mao L., Chen W., Zhu Z., Liu W., Zheng W., Zhao Y., Kong D., Yang Z., Zhang W., Shao Y., Jiang X. A Peptide-Based Nanofibrous Hydrogel as a Promising DNA Nanovector for Optimizing the Efficacy of HIV Vaccine. Nano Lett. 2014;14:1439–1445. doi: 10.1021/nl404560v. [DOI] [PubMed] [Google Scholar]
- 274.Xi X., Ye T., Wang S., Na X., Wang J., Qing S., Gao X., Wang C., Li F., Wei W., Ma G. Self-healing microcapsules synergetically modulate immunization microenvironments for potent cancer vaccination. Sci. Adv. 2020;6:eaay7735. doi: 10.1126/sciadv.aay7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wu Y., Wei W., Zhou M., Wang Y., Wu J., Ma G., Su Z. Thermal-sensitive hydrogel as adjuvant-free vaccine delivery system for H5N1 intranasal immunization. Biomaterials. 2012;33:2351–2360. doi: 10.1016/j.biomaterials.2011.11.068. [DOI] [PubMed] [Google Scholar]
- 276.Wang Y., Deng L., Gonzalez G.X., Luthra L., Dong C.H., Ma Y., Zou J., Kang S.M., Wang B.Z. Double-Layered M2e-NA Protein Nanoparticle Immunization Induces Broad Cross-Protection against Different Influenza Viruses in Mice. Adv. Healthc. Mater. 2020;9 doi: 10.1002/adhm.201901176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Hu G., Yang L., Cai Y., Niu F., Mezzacappa F., Callen S., Fox H.S., Buch S. Emerging roles of extracellular vesicles in neurodegenerative disorders: focus on HIV-associated neurological complications. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2016.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hu F., Yue H., Lu T., Ma G. Cytosolic delivery of HBsAg and enhanced cellular immunity by pH-responsive liposome. J. Control. Release. 2020;324:460–470. doi: 10.1016/j.jconrel.2020.05.042. [DOI] [PubMed] [Google Scholar]
- 279.Lu T., Hu F., Yue H., Yang T., Ma G. The incorporation of cationic property and immunopotentiator in poly (lactic acid) microparticles promoted the immune response against chronic hepatitis B. J. Control. Release. 2020;321:576–588. doi: 10.1016/j.jconrel.2020.02.039. [DOI] [PubMed] [Google Scholar]
- 280.de Vries R.D., Rimmelzwaan G.F. Viral vector-based influenza vaccines. Hum. Vacci. Immunother. 2016;12:2881–2901. doi: 10.1080/21645515.2016.1210729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Schiller J.T., Mueller M. Next generation prophylactic human papillomavirus vaccines. Lancet Oncol. 2015;16:e217–E225. doi: 10.1016/S1470-2045(14)71179-9. [DOI] [PubMed] [Google Scholar]
- 282.Roden R.B.S., Stern P.L. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat. Rev. Cancer. 2018;18:240–254. doi: 10.1038/nrc.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Chahal J.S., Khan O.F., Cooper C.L., McPartlan J.S., Tsosie J.K., Tilley L.D., Sidik S.M., Lourido S., Langer R., Bavari S., Ploegh H.L., Anderson D.G. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. U S A. 2016;113:e4133–4142. doi: 10.1073/pnas.1600299113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Frimpong A., Kusi K.A., Ofori M.F., Ndifon W. Novel Strategies for Malaria Vaccine Design. Front. Immunol. 2018;9:2769. doi: 10.3389/fimmu.2018.02769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Roestenberg M., McCall M., Hopman J., Wiersma J., Luty A.J., van Gemert G.J., van de Vegte-Bolmer M., van Schaijk B., Teelen K., Arens T., Spaarman L., de Mast Q., Roeffen W., Snounou G., Renia L., van der Ven A., Hermsen C.C., Sauerwein R. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 2009;361:468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 286.Nussenzweig V., Nussenzweig R.S. Rationale for the development of an engineered sporozoite malaria vaccine. Adv. Immunol. 1989;45:283–334. doi: 10.1016/s0065-2776(08)60695-1. [DOI] [PubMed] [Google Scholar]
- 287.Cohen S., Mc G.I., Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 288.Baruch D.I., Gormely J.A., Ma C., Howard R.J., Pasloske B.L. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. U S A. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Baum J., Chen L., Healer J., Lopaticki S., Boyle M., Triglia T., Ehlgen F., Ralph S.A., Beeson J.G., Cowman A.F. Reticulocyte-binding protein homologue 5 - an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int. J. Parasitol. 2009;39:371–380. doi: 10.1016/j.ijpara.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 290.Rodriguez M., Lustigman S., Montero E., Oksov Y., Lobo C.A. PfRH5: a novel reticulocyte-binding family homolog of plasmodium falciparum that binds to the erythrocyte, and an investigation of its receptor. PloS One. 2008;3 doi: 10.1371/journal.pone.0003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Partey F.D., Castberg F.C., Sarbah E.W., Silk S.E., Awandare G.A., Draper S.J., Opoku N., Kweku M., Ofori M.F., Hviid L., Barfod L. Kinetics of antibody responses to PfRH5-complex antigens in Ghanaian children with Plasmodium falciparum malaria. PloS One. 2018;13 doi: 10.1371/journal.pone.0198371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kumar K.A., Sano G., Boscardin S., Nussenzweig R.S., Nussenzweig M.C., Zavala F., Nussenzweig V. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature. 2006;444:937–940. doi: 10.1038/nature05361. [DOI] [PubMed] [Google Scholar]
- 293.Cohen J., Nussenzweig V., Nussenzweig R., Vekemans J., Leach A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum. Vaccin. 2010;6:90–96. doi: 10.4161/hv.6.1.9677. [DOI] [PubMed] [Google Scholar]
- 294.Rts S.C.T.P. Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Olotu A., Fegan G., Wambua J., Nyangweso G., Leach A., Lievens M., Kaslow D.C., Njuguna P., Marsh K., Bejon P. Seven-Year Efficacy of RTS, S/AS01 Malaria Vaccine among Young African Children. N. Engl. J. Med. 2016;374:2519–2529. doi: 10.1056/NEJMoa1515257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Otieno L., Oneko M., Otieno W., Abuodha J., Owino E., Odero C., Mendoza Y.G., Andagalu B., Awino N., Ivinson K., Heerwegh D., Otsyula N., Oziemkowska M., Usuf E.A., Otieno A., Otieno K., Leboulleux D., Leach A., Oyieko J., Slutsker L., Lievens M., Cowden J., Lapierre D., Kariuki S., Ogutu B., Vekemans J., Hamel M.J. Safety and immunogenicity of RTS, S/AS01 malaria vaccine in infants and children with WHO stage 1 or 2 HIV disease: a randomised, double-blind, controlled trial. Lancet Infect. Dis. 2016;16:1134–1144. doi: 10.1016/S1473-3099(16)30161-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Sanchez L., Vidal M., Jairoce C., Aguilar R., Ubillos I., Cuamba I., Nhabomba A.J., Williams N.A., Diez-Padrisa N., Cavanagh D., Angov E., Coppel R.L., Gaur D., Beeson J.G., Dutta S., Aide P., Campo J.J., Moncunill G., Dobano C. Antibody responses to the RTS, S/AS01E vaccine and Plasmodium falciparum antigens after a booster dose within the phase 3 trial in Mozambique. NPJ Vaccines. 2020;5:46. doi: 10.1038/s41541-020-0192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Kaba S.A., Brando C., Guo Q., Mittelholzer C., Raman S., Tropel D., Aebi U., Burkhard P., Lanar D.E. A nonadjuvanted polypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria. J. Immunol. 2009;183:7268–7277. doi: 10.4049/jimmunol.0901957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Rudra J.S., Mishra S., Chong A.S., Mitchell R.A., Nardin E.H., Nussenzweig V., Collier J.H. Self-assembled peptide nanofibers raising durable antibody responses against a malaria epitope. Biomaterials. 2012;33:6476–6484. doi: 10.1016/j.biomaterials.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Luo K., Gordy J.T., Zavala F., Markham R.B. A chemokine-fusion vaccine targeting immature dendritic cells elicits elevated antibody responses to malaria sporozoites in infant macaques. Sci. Rep. 2021;11:1220. doi: 10.1038/s41598-020-79427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Richardson M., Lass-Florl C. Changing epidemiology of systemic fungal infections. Clin. Microbiol. Infect. 2008;14:5–24. doi: 10.1111/j.1469-0691.2008.01978.x. [DOI] [PubMed] [Google Scholar]
- 302.Barchiesi F., Orsetti E., Osimani P., Catassi C., Santelli F., Manso E. Factors related to outcome of bloodstream infections due to Candida parapsilosis complex. BMC Infect. Dis. 2016;16:387. doi: 10.1186/s12879-016-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Nami S., Mohammadi R., Vakili M., Khezripour K., Mirzaei H., Morovati H. Fungal vaccines, mechanism of actions and immunology: A comprehensive review. Biomed. Pharmacother. 2019;109:333–344. doi: 10.1016/j.biopha.2018.10.075. [DOI] [PubMed] [Google Scholar]
- 304.Lin L., Ibrahim A.S., Xu X., Farber J.M., Avanesian V., Baquir B., Fu Y., French S.W., Edwards J.E., Jr., Spellberg B. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Correia A., Lermann U., Teixeira L., Cerca F., Botelho S., da Costa R.M., Sampaio P., Gartner F., Morschhauser J., Vilanova M., Pais C. Limited role of secreted aspartyl proteinases Sap1 to Sap6 in Candida albicans virulence and host immune response in murine hematogenously disseminated candidiasis. Infect. Immun. 2010;78:4839–4849. doi: 10.1128/IAI.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Chauhan A., Swaleha Z., Ahmad N., Farazuddin M., Vasco A., Abida M., Mohammad O. Escheriosome mediated cytosolic delivery of Candida albicans cytosolic proteins induces enhanced cytotoxic T lymphocyte response and protective immunity. Vaccine. 2011;29:5424–5433. doi: 10.1016/j.vaccine.2011.05.066. [DOI] [PubMed] [Google Scholar]
- 307.Carneiro C., Correia A., Lima T., Vilanova M., Pais C., Gomes A.C., Real Oliveira M., Sampaio P. Protective effect of antigen delivery using monoolein-based liposomes in experimental hematogenously disseminated candidiasis. Acta Biomater. 2016;39:133–145. doi: 10.1016/j.actbio.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 308.Lincopan N., Espindola N.M., Vaz A.J., da Costa M.H., Faquim-Mauro E., Carmona-Ribeiro A.M. Novel immunoadjuvants based on cationic lipid: Preparation, characterization and activity in vivo. Vaccine. 2009;27:5760–5771. doi: 10.1016/j.vaccine.2009.07.066. [DOI] [PubMed] [Google Scholar]
- 309.Hussain M.J., Wilkinson A., Bramwell V.W., Christensen D., Perrie Y. Th1 immune responses can be modulated by varying dimethyldioctadecylammonium and distearoyl-sn-glycero-3-phosphocholine content in liposomal adjuvants. J. Pharm. Pharmacol. 2014;66:358–366. doi: 10.1111/jphp.12173. [DOI] [PubMed] [Google Scholar]
- 310.Oliveira A.C., Martens T.F., Raemdonck K., Adati R.D., Feitosa E., Botelho C., Gomes A.C., Braeckmans K., Real Oliveira M.E. Dioctadecyldimethylammonium:monoolein nanocarriers for efficient in vitro gene silencing. ACS Appl. Mater. Interfaces. 2014;6:6977–6989. doi: 10.1021/am500793y. [DOI] [PubMed] [Google Scholar]
- 311.De Bernardis F., Amacker M., Arancia S., Sandini S., Gremion C., Zurbriggen R., Moser C., Cassone A. A virosomal vaccine against candidal vaginitis: immunogenicity, efficacy and safety profile in animal models. Vaccine. 2012;30:4490–4498. doi: 10.1016/j.vaccine.2012.04.069. [DOI] [PubMed] [Google Scholar]