Abstract
Previous studies regarding the zinc status in attention-deficit/hyperactivity disorder (ADHD) yielded inconsistent results. Thus, the present meta-analysis was aimed to estimate the association between hair and serum/plasma zinc levels and ADHD. Online databases of Medline, EMBASE, and Scopus were searched up to October 2020 with no limitation in time and language. Weighted mean differences (WMDs) of hair and serum/plasma zinc levels were calculated using a random-effects model. Overall, 22 articles with 1280 subjects with ADHD and 1200 controls were included. The pooled effect size indicated that serum/plasma zinc levels in subjects with ADHD were not statistically different than their controls (WMD = − 1.26 µmol/L; 95% CI − 3.72, 1.20). Interestingly, the exclusion of one study from the analysis showed that people with ADHD significantly have lower circulating levels of zinc compared to their controls (WMD: − 2.49 µmol/L; 95% CI − 4.29, − 0.69). Also, the pooled effect size indicated that hair zinc levels in cases with ADHD were not statistically different than their controls (WMD = − 24.19 μg/g; 95% CI − 61.80, 13.42). Present meta-analysis raises the possibility that subjects with ADHD are prone to have declined levels of zinc levels. Based on current findings, screening the zinc levels in subjects with ADHD could be reasonable. Further well-designed studies are needed to clarify the role of zinc in the etiology of ADHD.
Subject terms: Neuroscience, Neurological disorders, Malnutrition
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental disorder that affects approximately one in every ten children1. ADHD imposes a significant health and financial burden on patients, society, and the health care system2,3. Impulsivity decreased concentration and social interaction difficulties are the main manifestations of ADHD4.
The etiology of ADHD is multifactorial, including a contribution of genetic and environmental factors, perinatal risks, and pollutant exposure5,6. Several reports suggested the importance of vitamins and minerals in ADHD development and symptoms6,7. Both deficiency and excesses of minerals have been shown in relation to ADHD8. Among the trace elements zinc plays a crucial role in the etiology of ADHD 9. In ADHD, the dopaminergic and adrenergic systems are disrupted10. Zinc is involved in melatonin production which modulate the function of dopamine and facilitate dopamine signaling11.
Results of previous reports regarding the relationship between zinc levels and ADHD are inconsistent. Tippairote et al. in a case–control study, showed that higher hair zinc level was associated with greater ADHD symptoms and inattention12. On the other hand, Skalny et al. showed that lower levels of zinc and magnesium may significantly contribute to the severity of ADHD symptoms13. Also, several interventional studies suggested that zinc supplementation is effective in the improvement of ADHD symptoms14,15.
Previously, Luo et al. in a meta-analysis on 11 observational studies quantified the association between zinc levels and ADHD. The results showed no significant association between blood and hair zinc levels with ADHD16. A small sample size was one of the major limitations of this study and the large number of cases may increase the statistical power to clarify the relationship between zinc status and ADHD. Since that time, 11 additional studies have been published. Therefore, we conducted an updated meta-analysis, including more recent data, to provide quantitative estimates of the association between zinc status and ADHD.
Methods
The present study conducted based on the Preferred Reporting Items for Systematic Reviews and meta-analyses (PRISMA) statement17.
Search strategy
Electronic searches of PubMed, EMBASE, and Scopus were conducted up to October 2020 with no limitation in time and language. To search for titles, abstracts, and keywords of articles, a search was performed using “Zinc” OR “Trace Elements” OR “Trace Element*” AND “Attention Deficit Disorder with Hyperactivity” OR “ADHD”.
Study selection
After removing the duplicate studies, the title and abstract of the remaining studies were screened by two independent researchers (SMG and SEM). Finally, the full text of the relevant articles was reviewed and any discrepancy was resolved with the consensus of the researchers. All observational studies that examined peripheral levels of zinc (including blood and hair) between ADHD and control were included. We excluded trial and cohort studies, conference abstracts, letters, notes, editorials, reviews, or meta-analysis.
Data extraction
SMG and SEM extracted the required information from the included studies. Any disagreements were resolved by discussion or if necessary, by the third investigator (HM). Extracted information included: name of first author, publication year, country, sample size, mean age, body mass index, method of zinc assessment, criteria of ADHD diagnosis, the mean and corresponding standard deviation of zinc, and study design.
Quality assessment
Newcastle–Ottawa Scale (NOS) was used to evaluate the quality of the included studies. Articles with a total score of 0–4, 5–7, and 8–10 were considered as low, moderate and high quality, respectively.
Statistical analysis
To merge data, we used the random-effects model. In order to the calculation of effect size, the concentrations of zinc were converted to µmol/L. Heterogeneity of studies is determined using I2 and Chi-square test, high heterogeneity is determined by I2 above 50% and P value < 0.1. In order to determine the origin of heterogeneity, we used subgroup analysis and sensitivity analysis. Subgroup analysis was performed according to the type of samples (serum, plasma, blood), year of publication (≤ 2010, > 2010), method of zinc assessment (atomic absorption spectrophotometer, others), study design (case–control, cross-sectional), and sample size (< 100, > 100). Begg’s and Egger’s tests were used to examine the publication bias. STATA software (version 14)18 was used for statistical analysis. Statistically significance is confirmed with P value less than 0.05.
Results
Study selection
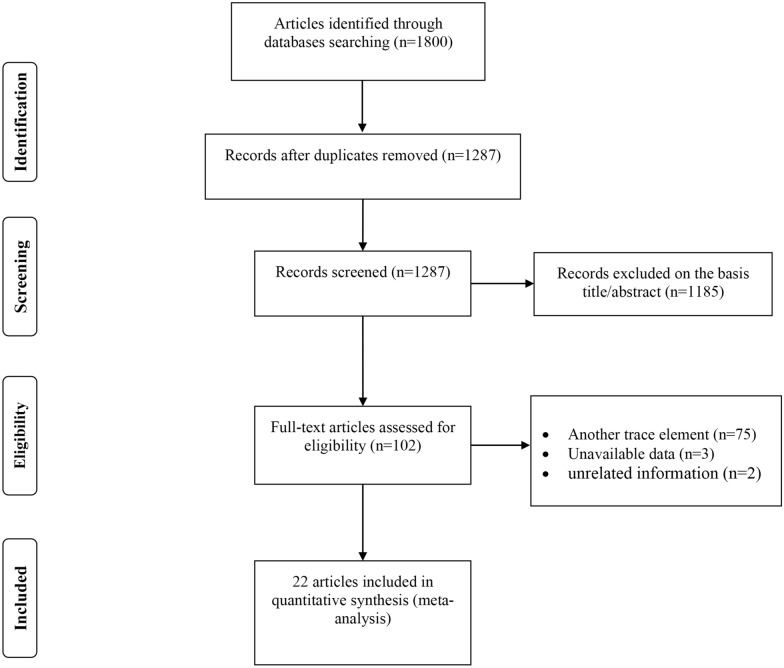
The study selection process is illustrated in Fig. 1. Among the 1800 studies found based on an electronic and manual search for all trace elements, 513 studies were duplicate. Screening the title and abstracts excluded 1185 documents. Among the remaining 102 articles 75 studies were related to other trace elements. Three studies were excluded due to unavailability of information regarding zinc levels19–21 and two studies were excluded due to lack of reporting information in case and control groups separately and reporting the level of zinc in hemoglobin22,23. Finally, 22 articles were included in this meta-analysis, 14 studies assessed circulating levels of zinc13,24–36 and 8 studies reported hair zinc levels12,37–43.
Figure 1.
PRISMA flowchart describing the study’s systematic literature search and study selection.
Characteristics of included studies
Characteristics of the included studies were summarized in Table 1. Included studies were conducted between 1990 and 2020, examining 1280 people with ADHD and 1200 controls. Except for one study24 in which both case and control groups had diabetes, other studies used healthy individuals and people with ADHD as control and case groups, respectively. Of the available studies, four were conducted in Egypt24,28,29,33, three in Russia13,37,38, two in Turkey32,36, two in the United States34,41, and others in Syria25, China26, Iran27, Slovakia30, Israel31, Saudi Arabia35, New Zealand39, Indonesia40, Thailand12, Korea42, and Georgia43. Twenty studies had case–control design and only two studies13,28 were conducted by cross-sectional design. According to the Newcastle–Ottawa scale, nine studies were assigned to moderate quality12,25,27,29,30,32,36,40,41, and the rest of them had high quality (Table 2).
Table 1.
Baseline characteristic of included studies.
| First author (year; location) | Study design | Sample | Criteria of ADHD | Population | Sample size | Matching | Mean age (years) | Method of assessment | Nos | |
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case\control | ||||||||
| Sakhr (2020; Egypt) | cc | Serum | DSM-IV | T1DM with ADHD | T1DM without ADHD | 20\40 | Age, sex |
Case:10.19 ± 2.34 Control:10.35 ± 3.29 |
Spectrophotometer | 8 |
| Hawari (2020; Syria) | cc | Serum | DSM-V | ADHD | Healthy | 29\30 | NR | NR | Spectrophotometer by colorimetric assay | 7 |
| Yang (2019; China) | cc | Serum | DSM-V | ADHD | Healthy | 419\395 | BMI z-score |
Case: 8.8 ± 2.1 Control: 8.9 ± 1.7 |
Atomic absorption spectrometry | 8 |
| Tinkov (2019; Russia) | cc | Serum | ICD-10 | ADHD | Healthy | 68\68 | Age, gender |
Case :6.4 ± 2.1 Control:6.4 ± 2.1 |
Inductively-coupled plasma mass spectrometry | 9 |
| Avval (2019; Iran) | cc | Serum | DSM-IV | ADHD | Healthy | 36\15 | Age, gender, family history of anemia |
Case:7.8 ± 2.12 Control:8.4 ± 3.11 |
Nr | 7 |
| Abdelnaby (2018; Egypt) | cs | Serum | DSM-IV | ADHD | Healthy | 25\25 | NR |
Case:4.00 ± 2.47 Control:5.66 ± 3.9 |
Routine kinetic and fixed-rate colorimetric methods | 6 |
| Elbaz (2017; Egypt) | cc | Serum | DSM-IV-R | ADHD | Healthy | 20\20 | Age, sex |
Case:7.74 ± 1.48 Control:7.40 ± 1.35 |
Nr | 7 |
| Viktorinova (2016; Slovakia) | cc | Plasma | ICD-10 | ADHD | Healthy | 58\50 | NR |
Case:9.4 ± 2.1 Control:8.9 ± 2.8 |
Atomic absorption spectrometry | 7 |
| Sandyk (1990; Israel) | cc | Serum | DSM-III-R | ADHD | Healthy | 43\28 | age |
Case:10.1 ± 2.4 Control:11.3 ± 3.2 |
Atomic absorption spectrophotometry | 8 |
| Bekaroglu (1996; Turkey) | cc | Serum | DSM-III-R | ADHD | Healthy | 48\45 | NR |
Case:9.2 ± 2 Control: 9.3 ± 2 |
Atomic absorption spectrophotometer | 7 |
| Mahmoud (2011; Egypt) | cc | Serum | DSM-IV | ADHD | Healthy | 58\25 | Age, sex, socioeconomic state |
Case:8.3 ± 1.8 Control:8.6 ± 3.1 |
Color metric test without desproteinization | 9 |
| Antalis (2006; USA) | cc | Serum | CAARS | ADHD | Healthy | 12\12 | Gender, BMI, smoking |
Case:24.37 ± 2.3 Control:22.37 ± 2.4 |
Inductively coupled plasma spectrophotometry | 9 |
| Khan (2017; KSA) | cc | Plasma | DSM-IV | ADHD | Healthy | 41\41 | Age, gender | NR | Atomic absorption spectrophotometry | 9 |
| Yorbik (2008; Turkey) | cc | Plasma | DSM-IV | ADHD | Healthy | 28\24 | NR | NR | Atomic absorption spectrophotometry | 7 |
| Tinkov (2020; Russia) | cc | Hair | ICD-10 | ADHD | Healthy | 90\90 | Age, gender |
Case:5.47 ± 1.57 Control:5.47 ± 1.57 |
Inductively coupled plasma mass-spectrometry after microwave digestion | 9 |
| Skalny (2020; Russia) | cs | Hair | ICD-10 | ADHD | Healthy | 52\52 | Height-weight |
Case:5.15 ± 0.97 Control:5.13 ± 1.05 |
Inductively-coupled plasma mass spectrometry | 9 |
| Perham (2020; New Zealand) | cc | Hair | DSM-IV | ADHD | Healthy | 55\52 | Geographical locations, Socioeconomic backgrounds |
Case:9.78 ± 1.56 Control:10.08 ± 1.70 |
Mass spectrometry and temperature-controlled microwave digestion techniques | 9 |
| Setiawati (2019; Indonesia) | cc | Hair | CBRS | ADHD | Healthy | 23\21 | NR | NR | Atomic absorption spectrophotometry | 7 |
| Tippairote (2017; Thailand) | cc | Hair | DSM-V | ADHD | Healthy | 45\66 | NR |
Case:5.56 ± 1.34 Control:5.26 ± 1.29 |
Inductively coupled plasma mass spectrometry | 7 |
| Arnold (1990; USA) | cc | Hair | DSM-III | ADHD | Healthy | 18\7 | NR | NR | NR | 7 |
| Shin (2014; korea) | cc | Hair | DSM-IV | ADHD | Healthy | 41\42 | Age, gender |
Case:115.68 ± 35.67 Control:119.71 ± 34.97 |
Inductive coupled plasma-mass spectrometry | 8 |
| Tabatadze (2018; Georgia) | cc | Hair | DSM-V | ADHD | Healthy | 51\52 | NR | NR | Roentgen fluorescence spectrometer | 6 |
BMI body mass index, CC case–control, CS cross-sectional, Zn zinc, ADHD attention deficit hyperactivity disorder.
Table 2.
Quality assessments of included studies.
| Study | Case definition adequate | Representativeness of the cases | Selection of Controls | Definition of Controls | Comparability of cases and controls | Ascertainment of exposure | Same method of ascertainment | Non-response rate | Nos |
|---|---|---|---|---|---|---|---|---|---|
| Sakhr (2020; Egypt) | * | * | * | – | ** | * | * | * | 8 |
| Hawari (2020; Syria) | * | * | * | * | – | * | * | * | 7 |
| Yang (2019; China) | * | * | * | * | * | * | * | * | 8 |
| Tinkov (2019; Russia) | * | * | * | * | ** | * | * | * | 9 |
| Avval (2019; Iran) | * | * | – | * | ** | – | * | * | 7 |
| Abdelnaby (2018; Egypt) | * | * | – | * | – | * | * | * | 6 |
| Elbaz (2017; Egypt) | * | * | – | * | ** | – | * | * | 7 |
| Viktorinova (2016; Slovakia) | * | * | * | * | – | * | * | * | 7 |
| Sandyk (1990; Israel) | * | * | * | * | * | * | * | * | 8 |
| Bekaroglu (1996; Turkey) | * | * | * | * | – | * | * | * | 7 |
| Mahmoud (2011; Egypt) | * | * | * | * | ** | * | * | * | 9 |
| Antalis (2006; USA) | * | * | * | * | ** | * | * | * | 9 |
| Khan (2017; KSA) | * | * | * | * | ** | * | * | * | 9 |
| Yorbik (2008; Turkey) | * | * | * | * | – | * | * | * | 7 |
| Tinkov (2020; Russia) | * | * | * | * | ** | * | * | * | 9 |
| Skalny (2020; Russia) | * | * | * | * | ** | * | * | * | 9 |
| Perham (2020; NewZealand) | * | * | * | * | ** | * | * | * | 9 |
| Setiawati (2019; Indonesia) | * | * | * | * | – | * | * | * | 7 |
| Tippairote (2017; Thailand) | * | * | * | * | – | * | * | * | 7 |
| Arnold (1990; USA) | * | * | * | * | – | * | * | * | 7 |
| Shin (2014; South Korea) | * | * | – | * | ** | * | * | * | 8 |
| Tabatadze (2018; Georgia) | * | * | – | * | – | * | * | * | 6 |
NOS New-castle Ottawa Scale.
Meta-analysis of mean blood zinc levels
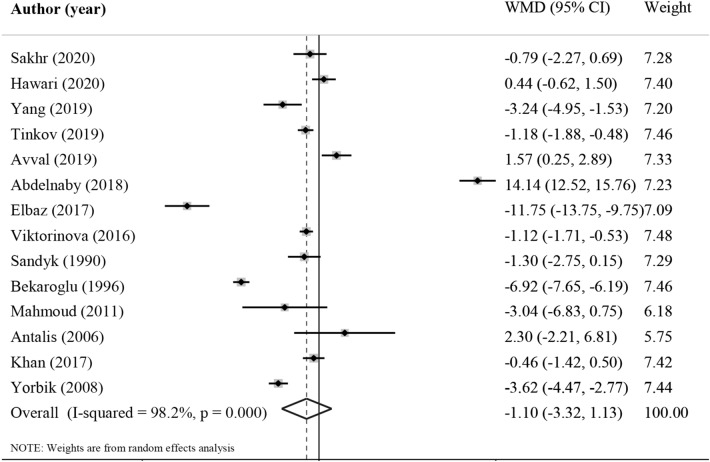
Fourteen studies assessed the association between blood zinc level and ADHD13,24–36, involving 902 cases and 818 controls. The pooled effect size indicated that serum/plasma zinc levels in subjects with ADHD were not statistically different than their controls (WMD = − 1.26 µmol/L; 95% CI − 3.72, 1.20, P = 0.31, Fig. 2). Interestingly, sensitivity analysis showed that exclusion of Abdelnaby's study28 from the analysis changed the overall effect size (WMD: − 2.49 µmol/L; 95% CI − 4.29, − 0.69). A significant heterogeneity was detected among studies (I2 = 98.2%, P < 0.001). Despite classification of the studies, no possible source of heterogeneity was found and the result remained non-significant in all categories (Table 3). No evidence of publication bias was observed among included studies (P = 0.87, Begg’s test and P = 0.45, Egger’s test).
Figure 2.
Forest plot for the association between serum zinc level and ADHD expressed as mean difference between case and control groups. The area of each square is proportional to the inverse of the variance of the WMD. Horizontal lines represent 95%Cis. Diamonds represent pooled estimates from random-effects analysis. WMD, weighted mean difference.
Table 3.
Subgroup analysis to assess the serum and hair zinc levels in subjects with ADHD.
| Sub grouped by | No. | WMD (95% CI) | P value | P-Heterogeneity | I2 (%) | P-between subgroup heterogeneity |
|---|---|---|---|---|---|---|
| Serum zinc | ||||||
| Sample type | ||||||
| Serum | 10 | − 0.67 (− 4.23, 2.90) | 0.714 | < 0.001 | 98.7 | |
| Plasma | 3 | − 1.73 (− 3.49, 0.02) | 0.053 | < 0.001 | 93.2 | 0.192 |
| Blood | 1 | − 3.24 (− 4.95, − 1.53) | < 0.001 | 0 | 0 | |
| Publication year | ||||||
| ≤ 2010 | 4 | − 2.92 (− 5.85, 0.02) | 0.052 | < 0.001 | 95.8 | < 0.001 |
| > 2010 | 10 | − 0.48 (− 3.05, 2.09) | 0.715 | < 0.001 | 98.1 | |
| Zinc assessment method | ||||||
| Atomic absorption spectrophotometer | 6 | − 2.79 (− 5.04, − 0.53) | 0.015 | < 0.001 | 97.3 | < 0.001 |
| Other | 8 | 0.23 (− 3.82, 4.28) | 0.912 | < 0.001 | 98.4 | |
| Sample size | ||||||
| ≤ 100 | 11 | − 0.88 (− 4.14, 2.39) | 0.598 | < 0.001 | 98.6 | 0.004 |
| > 100 | 3 | − 1.49 (− 2.32, − 0.67) | < 0.001 | 0.066 | 63.1 | |
| Study design | ||||||
| Case–control | 13 | − 2.31 (− 3.97, − 0.64) | 0.007 | < 0.001 | 96.7 | < 0.001 |
| Cross-sectional | 1 | 14.14 (12.52, 15.76) | < 0.001 | < 0.001 | 0 | |
| Hair zinc | ||||||
| Publication year | ||||||
| ≤ 2010 | 1 | − 65.10 (− 191.40, 61.20) | 0.312 | 0 | 0 | 0.237 |
| > 2010 | 7 | − 4.54 (− 38.76, 29.68) | 0.795 | < 0.001 | 98.2 | |
| Zinc assessment method | ||||||
| Atomic absorption spectrophotometer | 1 | 97.33 (33.73, 160.93) | 0.003 | 0 | 0 | 0.008 |
| Other | 7 | − 19.9 (− 54.14, 15.95) | 0.286 | < 0.001 | 98.2 | |
| Sample size | ||||||
| ≤ 100 | 5 | − 11.74 (− 49.82, 26.33) | 0.545 | < 0.001 | 98.8 | 0.712 |
| > 100 | 3 | − 2.78 (− 119.62, 114.07) | 0.963 | 0.003 | 82.8 | |
| Study design | ||||||
| Case–control | 7 | − 11.79 (− 49.59, 26.01) | 0.541 | < 0.001 | 98.3 | 0.544 |
| Cross-sectional | 1 | 17.57 (− 3.18, 38.32) | 0.097 | 0 | 0 | |
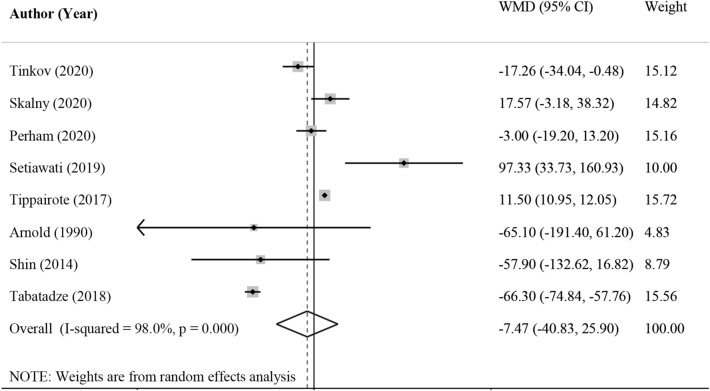
Meta-analysis of mean hair zinc levels
Eight studies reported sufficient data regarding hair zinc levels in ADHD and control subjects12,37–43, involving 375 cases and 382 controls. The pooled effect size indicated that hair zinc levels in cases with ADHD were not statistically different than their controls (WMD = − 24.19 μg/g; 95% CI − 61.80, 13.42, P = 0.20, Fig. 3). However, significant heterogeneity was detected across the studies (I2 = 98.1%, P < 0.001). Despite the different subgroup analysis, we could not detect the potential source of observed heterogeneity, as shown in Table 2. There was no evidence of publication bias among included studies (P = 0.62, Begg’s test and P = 0.16, Egger’s test).
Figure 3.
Forest plot for the association between hair zinc level and ADHD expressed as mean difference between case and control groups. The area of each square is proportional to the inverse of the variance of the WMD. Horizontal lines represent 95%Cis. Diamonds represent pooled estimates from random-effects analysis. WMD, weighted mean difference. All statistical analyses were performed using Stata version 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP, www.stata.com).
Discussion
The present meta-analysis, including 22 studies and a total of 2428 people, showed that there was no statistically significant difference in serum/plasma and hair zinc levels between patients with ADHD and their controls. There was substantial heterogeneity among included studies. However, sensitivity analysis in studies examining the circulating zinc levels showed that excluding one study28 changed the overall effect. Circulating levels of zinc were significantly lower in subjects with ADHD compared to healthy controls after excluding Abdelnaby's study28.
Zinc deficiency is involved in a variety of neurological disorders including autism, seizures, depression, and anxiety disorders44. However, the exact mechanism of zinc in ADHD is still unclear. Dopamine is a neurotransmitter plays a crucial role in the pathophysiology of ADHD9. Previous studies reported that zinc is involved in the production of melatonin which could regulate dopamine levels and homeostasis45,46. Dysfunction in the dopamine transporter is another pathway that contributed to the etiology of ADHD9. Zinc binding to the dopamine receptors inhibits the dopamine re-uptake and increases the carrier-mediated dopamine efflux9,46. Also, zinc is an important cofactor for several enzymes in the brain involved in the neurotransmitters and prostaglandins production9.
Several studies have suggested the role of inflammation and oxidative stress in the pathogenesis of ADHD47,48. Although subjects with ADHD have normal levels of antioxidant capacity, their reaction to oxidative stress is impaired47. Elevated levels of pro-inflammatory cytokines could decrease the levels of zinc in patients with ADHD through the sequestration of zinc in the liver and spleen49. Zinc could exert anti-oxidative and anti-inflammatory properties through the protection of sulfhydryl groups of proteins from oxidation50. Zinc takes part in antioxidant enzyme production and acts as a cofactor of several enzymes50. Also, zinc modulates the chronic inflammatory status by reducing pro-inflammatory cytokines51. On the other hand, zinc supplementation showed beneficial effects in the alleviation of hyperactivity symptoms in zinc-deficient ADHD subjects52. Moreover, 150 mg/day zinc supplementation for 12 weeks led to a significant reduction in symptoms of hyperactivity, impulsivity, and impaired socialization in patients with ADHD53. Although, 30 mg/day zinc supplementation showed no significant effects on primary outcomes compared to the placebo, which might be due the low dosage of zinc54.
Lower levels of zinc in subjects with ADHD may be attributed to the dietary zinc intake or zinc absorption49. Also, zinc-wasting in the urine is another possible cause of low levels of zinc in children with ADHD55. It has been suggested that hyperactive children have increased levels of urinary zinc and reduce levels of plasma49.
Sensitivity analysis showed that the exclusion of Abdelnaby's study28 from the analysis changed the overall effect size. The pooled analysis without mentioned study showed significant lower levels of serum/plasma zinc in subjects with ADHD compared to their controls. Indeed, the mentioned study showed a significant higher levels of serum/plasma zinc in subjects with ADHD compared to the controls. This contradictory finding could be related to several factors e.g. different study design, small sample size, the different method in zinc measurement, and high risk of bias (NOS = 6).
The present study has some limitations that should be acknowledged. We observed a significant heterogeneity among included studies that could affect the generalizability of results. However, our attempts to detect the potential source of heterogeneity through different subgroup analysis were unsuccessful. The observed heterogeneity in the present meta-analysis could be related to several factors including demographic and clinical differences, BMI, study design, adjusted models for statistical analysis, risk of bias, and methods for assessing zinc levels. Small sample sizes of individual studies are another limitation of the present study. Almost all of the included studies except one26 were performed on less than 200 participants. Moreover, included studies did not evaluate the dietary intake of zinc in study participants which could affect the results because that amount of zinc intake is related to the serum zinc concentration56. Also, many factors could affect hair zinc levels57, which should be taken into the interpretation of results.
Conclusion
Present meta-analysis raises the possibility that subjects with ADHD are prone to have declined levels of zinc levels. Based on current findings screening the zinc levels at the beginning of the diagnosis in subjects with ADHD could be reasonable. Further well-designed studies are needed to clarify the role of zinc in the etiology of ADHD.
Author contributions
S.M.G. and S.E.M. and F.A. wrote the main manuscript text and H.M. supervised the research. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ezpeleta L, De La Osa N, Doménech JM. Prevalence of DSM-IV disorders, comorbidity and impairment in 3-year-old Spanish preschoolers. Soc. Psychiatry Psychiatr. Epidemiol. 2014;49:145–155. doi: 10.1007/s00127-013-0683-1. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R, Goldenberg M, Perry R, Ishak WW. The quality of life of adults with attention deficit hyperactivity disorder: A systematic review. Innov. Clin. Neurosci. 2012;9:10. [PMC free article] [PubMed] [Google Scholar]
- 3.Danckaerts M, et al. The quality of life of children with attention deficit/hyperactivity disorder: A systematic review. Eur. Child Adolesc. Psychiatry. 2010;19:83–105. doi: 10.1007/s00787-009-0046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma A, Couture J. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD) Ann. Pharmacother. 2014;48:209–225. doi: 10.1177/1060028013510699. [DOI] [PubMed] [Google Scholar]
- 5.Kim J-W. Environmental risk factors for attention deficit hyperactivity disorder and implications for clinical practice. J. Korean Acad. Child Adolesc. Psychiatry. 2011;22:10–15. doi: 10.5765/JKACAP.2011.22.1.010. [DOI] [Google Scholar]
- 6.Nigg JT, Lewis K, Edinger T, Falk M. Meta-analysis of attention-deficit/hyperactivity disorder or attention-deficit/hyperactivity disorder symptoms, restriction diet, and synthetic food color additives. J. Am. Acad. Child Adolesc. Psychiatr. 2012;51:86–97. doi: 10.1016/j.jaac.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinn N. Nutritional and dietary influences on attention deficit hyperactivity disorder. Nutr. Rev. 2008;66:558–568. doi: 10.1111/j.1753-4887.2008.00107.x. [DOI] [PubMed] [Google Scholar]
- 8.Hariri, M. & Azadbakht, L. Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: a systematic review on the recent literature. International journal of preventive medicine 6, 1–10 (2015). [DOI] [PMC free article] [PubMed]
- 9.Lepping P, Huber M. Role of zinc in the pathogenesis of attention-deficit hyperactivity disorder. CNS Drugs. 2010;24:721–728. doi: 10.2165/11537610-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Kasperek K, Feinendegen L, Lombeck I, Bremer H. Serum zinc concentration during childhood. Eur. J. Pediatr. 1977;126:199–202. doi: 10.1007/BF00477045. [DOI] [PubMed] [Google Scholar]
- 11.Arnold LE, et al. Serum zinc correlates with parent-and teacher-rated inattention in children with attention-deficit/hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 2005;15:628–636. doi: 10.1089/cap.2005.15.628. [DOI] [PubMed] [Google Scholar]
- 12.Tippairote T, Temviriyanukul P, Benjapong W, Trachootham D. Hair zinc and severity of symptoms are increased in children with attention deficit and hyperactivity disorder: A hair multi-element profile study. Biol. Trace Elem. Res. 2017;179:185–194. doi: 10.1007/s12011-017-0978-2. [DOI] [PubMed] [Google Scholar]
- 13.Skalny AV, et al. Serum zinc, copper, zinc-to-copper ratio, and other essential elements and minerals in children with attention deficit/hyperactivity disorder (ADHD) J. Trace Elem. Med. Biol. 2020;58:126445. doi: 10.1016/j.jtemb.2019.126445. [DOI] [PubMed] [Google Scholar]
- 14.Salehi B, Mohammadbeigi A, Sheykholeslam H, Moshiri E, Dorreh F. Omega-3 and Zinc supplementation as complementary therapies in children with attention-deficit/hyperactivity disorder. J. Res. Pharm. Pract. 2016;5:22. doi: 10.4103/2279-042X.176561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghanizadeh A, Berk M. Zinc for treating of children and adolescents with attention-deficit hyperactivity disorder: A systematic review of randomized controlled clinical trials. Eur. J. Clin. Nutr. 2013;67:122–124. doi: 10.1038/ejcn.2012.177. [DOI] [PubMed] [Google Scholar]
- 16.Luo J, Mo Y, Liu M. Blood and hair zinc levels in children with attention deficit hyperactivity disorder: A meta-analysis. Asian J. Psychiatry. 2020;47:101805. doi: 10.1016/j.ajp.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.College Station, T. S. L. StataCorp. 2015. Stata Statistical Software: Release 14. (2015).
- 19.Yousef S, et al. Attention deficit hyperactivity disorder and environmental toxic metal exposure in the United Arab Emirates. J. Trop. Pediatr. 2011;57:457–460. doi: 10.1093/tropej/fmq121. [DOI] [PubMed] [Google Scholar]
- 20.Ode A, et al. Manganese and selenium concentrations in umbilical cord serum and attention deficit hyperactivity disorder in childhood. Environ. Res. 2015;137:373–381. doi: 10.1016/j.envres.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Islam K, et al. A study on association of iron deficiency with attention deficit hyperactivity disorder in a tertiary care center. Indian J. Psychiatry. 2018;60:131. doi: 10.4103/psychiatry.IndianJPsychiatry_197_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo A. Decreased serum Cu/Zn SOD associated with high copper in children with attention deficit hyperactivity disorder (ADHD) J. Cent. Nerv. Syst. Dis. 2010;2:S4553. doi: 10.4137/JCNSD.S4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nigg JT, Elmore AL, Natarajan N, Friderici KH, Nikolas MA. Variation in an iron metabolism gene moderates the association between blood lead levels and attention-deficit/hyperactivity disorder in children. Psychol. Sci. 2016;27:257–269. doi: 10.1177/0956797615618365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakhr HM, Hassan MH, Desoky T. Possible associations of disturbed neurometals and ammonia with glycaemic control in type 1 diabetic children with attention deficit hyperactivity disorder. Biol. Trace Elem. Res. 2020;198:68–76. doi: 10.1007/s12011-020-02063-5. [DOI] [PubMed] [Google Scholar]
- 25.Hawari I, Eskandar MB, Alzeer S. The role of lead, manganese, and zinc in autism spectrum disorders (ASDs) and attention-deficient hyperactivity disorder (ADHD): A case-control study on Syrian children affected by the Syrian crisis. Biol. Trace Elem. Res. 2020;197:107–114. doi: 10.1007/s12011-020-02146-3. [DOI] [PubMed] [Google Scholar]
- 26.Yang R, et al. Blood levels of trace elements in children with attention-deficit hyperactivity disorder: Results from a case-control study. Biol. Trace Elem. Res. 2019;187:376–382. doi: 10.1007/s12011-018-1408-9. [DOI] [PubMed] [Google Scholar]
- 27.Zahedi Avval, F., Soltanifar, A., Moharreri, F., Kamrani, M. & Mohamadi Rad, M. M. Assessment of serum levels of iron and zinc in children with ADHD compared to healthy controls: A case-control study. Iranian Journal of Psychiatry and Behavioral Sciences 13, 125–136 (2019).
- 28.Abd El Naby, S. A. & Naguib, Y. M. Sociodemographic, electrophysiological, and biochemical profiles in children with attention deficit hyperactivity disorder and/or epilepsy. Behav. Neurol.2018, 1–12 (2018). [DOI] [PMC free article] [PubMed]
- 29.Elbaz F, Zahra S, Hanafy H. Magnesium, zinc and copper estimation in children with attention deficit hyperactivity disorder (ADHD) Egypt. J. Med. Hum. Genet. 2017;18:153–163. doi: 10.1016/j.ejmhg.2016.04.009. [DOI] [Google Scholar]
- 30.Viktorinova A, et al. Changed plasma levels of zinc and copper to zinc ratio and their possible associations with parent-and teacher-rated symptoms in children with attention-deficit hyperactivity disorder. Biol. Trace Elem. Res. 2016;169:1–7. doi: 10.1007/s12011-015-0395-3. [DOI] [PubMed] [Google Scholar]
- 31.Sandyk R. Zinc deficiency in attention-deficit hyperactivity disorder. Int. J. Neurosci. 1990;52:239–241. doi: 10.3109/00207459009000526. [DOI] [PubMed] [Google Scholar]
- 32.Bekaroǧlu M, et al. Relationships between serum free fatty acids and zinc, and attention deficit hyperactivity disorder: A research note. J. Child Psychol. Psychiatry. 1996;37:225–227. doi: 10.1111/j.1469-7610.1996.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 33.Mahmoud MM, El-Mazary A-AM, Maher RM, Saber MM. Zinc, ferritin, magnesium and copper in a group of Egyptian children with attention deficit hyperactivity disorder. Ital. J. Pediatr. 2011;37:60. doi: 10.1186/1824-7288-37-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antalis CJ, et al. Omega-3 fatty acid status in attention-deficit/hyperactivity disorder. Prostaglandins Leukot. Essent. Fatty Acids. 2006;75:299–308. doi: 10.1016/j.plefa.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Khan SA. Levels of zinc, magnesium and iron in children with attention deficit hyperactivity disorder. Electron. J. Biol. 2017;13:183–187. [Google Scholar]
- 36.Yorbik O, et al. Potential effects of zinc on information processing in boys with attention deficit hyperactivity disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32:662–667. doi: 10.1016/j.pnpbp.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Tinkov AA, et al. ICP-MS assessment of hair essential trace elements and minerals in Russian preschool and primary school children with attention-deficit/hyperactivity disorder (ADHD) Biol. Trace Elem. Res. 2019;196:400–409. doi: 10.1007/s12011-019-01947-5. [DOI] [PubMed] [Google Scholar]
- 38.Skalny AV, et al. Hair trace element concentrations in autism spectrum disorder (ASD) and attention deficit/hyperactivity disorder (ADHD) J. Trace Elem. Med. Biol. 2020;61:126539. doi: 10.1016/j.jtemb.2020.126539. [DOI] [PubMed] [Google Scholar]
- 39.Perham JC, Shaikh NI, Lee A, Darling KA, Rucklidge JJ. Toward ‘element balance’in ADHD: An exploratory case control study employing hair analysis. Nutr. Neurosci. 2020 doi: 10.1080/1028415X.2019.1707395. [DOI] [PubMed] [Google Scholar]
- 40.Setiawati Y, Mukono H, Wahyuhadi J, Warsiki E. The influence of lead (Pb), zinc (Zn), ratio lead (Pb) to zinc (Zn) in attention deficit hyperactivity disorder (ADHD) Indian J. Public Health Res. Dev. 2019;10:1497–1502. doi: 10.5958/0976-5506.2019.02112.0. [DOI] [Google Scholar]
- 41.Arnold LE, Votolato NA, Kleykamp D, Baker GB, Bornstein RA. Does hair zinc predict amphetamine improvement of ADD/hyperactivity? Int. J. Neurosci. 1990;50:103–107. doi: 10.3109/00207459008987161. [DOI] [PubMed] [Google Scholar]
- 42.Shin D-W, Kim E-J, Oh K-S, Shin Y-C, Lim S-W. The relationship between hair zinc and lead levels and clinical features of attention-deficit hyperactivity disorder. J. Korean Acad. Child Adolesc. Psychiatry. 2014;25:28–36. doi: 10.5765/jkacap.2014.25.1.28. [DOI] [Google Scholar]
- 43.Tabatadze T, Kherkheulidze M, Kandelaki E, Kavlashvili N, Ivanashvili T. Attention deficit hyperactivity disorder and hair heavy metal and essential trace element concentrations. Is there a link? Georgian Med. News. 2018;284:88–92. [PubMed] [Google Scholar]
- 44.Vela G, et al. Zinc in gut–brain interaction in autism and neurological disorders. Neural Plast. 2015;2015:1–15. doi: 10.1155/2015/972791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rink L. Zinc and the immune system. Proc. Nutr. Soc. 2000;59:541–552. doi: 10.1017/S0029665100000781. [DOI] [PubMed] [Google Scholar]
- 46.Chen M-D, Lin P-Y, Sheu WH-H. Zinc coadministration attenuates melatonin’s effect on nitric oxide production in mice. Biol. Trace Elem. Res. 1999;69:261–268. doi: 10.1007/BF02783878. [DOI] [PubMed] [Google Scholar]
- 47.Joseph N, Zhang-James Y, Perl A, Faraone SV. Oxidative stress and ADHD: A meta-analysis. J. Atten. Disord. 2015;19:915–924. doi: 10.1177/1087054713510354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leffa DT, Torres IL, Rohde LA. A review on the role of inflammation in attention-deficit/hyperactivity disorder. NeuroImmunoModulation. 2018;25:328–333. doi: 10.1159/000489635. [DOI] [PubMed] [Google Scholar]
- 49.Villagomez A, Ramtekkar U. Iron, magnesium, vitamin D, and zinc deficiencies in children presenting with symptoms of attention-deficit/hyperactivity disorder. Children. 2014;1:261–279. doi: 10.3390/children1030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee, S. R. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid. Med. Cell. Longev.2018, 1–11 (2018). [DOI] [PMC free article] [PubMed]
- 51.Olechnowicz J, Tinkov A, Skalny A, Suliburska J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018;68:19–31. doi: 10.1007/s12576-017-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dodig-Curković K, Dovhanj J, Curković M, Dodig-Radić J, Degmecić D. The role of zinc in the treatment of hyperactivity disorder in children. CActa Med. Croat. Cas. Hravatske Akad. Med. Znan. 2009;63:307–313. [PubMed] [Google Scholar]
- 53.Bilici M, et al. Double-blind, placebo-controlled study of zinc sulfate in the treatment of attention deficit hyperactivity disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2004;28:181–190. doi: 10.1016/j.pnpbp.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 54.Arnold LE, et al. Zinc for attention-deficit/hyperactivity disorder: placebo-controlled double-blind pilot trial alone and combined with amphetamine. J. Child Adolesc. Psychopharmacol. 2011;21:1–19. doi: 10.1089/cap.2010.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ward NI. Assessment of chemical factors in relation to child hyperactivity. J. Nutr. Environ. Med. 1997;7:333–342. doi: 10.1080/13590849762466. [DOI] [Google Scholar]
- 56.Moran VH, et al. The relationship between zinc intake and serum/plasma zinc concentration in children: A systematic review and dose-response meta-analysis. Nutrients. 2012;4:841–858. doi: 10.3390/nu4080841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tippairote, T. & Trachootham, D. Zinc Status in Hair Samples and Common Neurodevelopmental Disorders. J. Neurol. Neuromed.2, 1–10 (2017).