Abstract
Burn injuries are a common form of traumatic injury that leads to significant morbidity and mortality worldwide. Burn injuries are characterized by inflammatory processes and alterations in numerous organ systems and functions. Recently, it has become apparent that the gastrointestinal bacterial microbiome is a key component of regulating the immune response and recovery from burn and can also contribute to significant detrimental sequelae after injury, such as sepsis and multiple organ failure. Microbial dysbiosis has been linked to multiple disease states, however, its role in exacerbating acute traumatic injuries, such as burn, are poorly understood. In this article, we review studies that document changes in the intestinal microbiome after burn injury, assess the implications in post-burn pathogenesis, and the potential for further discovery and research.
1. INTRODUCTION
Burn injuries are a subset of traumatic injuries to the skin that can be caused by a number of different insults. This includes thermal, chemical, radiation, electrical, friction, and cold burns (frostbite). The majority of burns are caused by thermal insults, including fire, hot objects, or hot liquids/scalding. As of 2016, an estimated 486,000 people in the United States received medical care related to a burn injury, with 40,000 people hospitalized due to their injury(1). Furthermore, incidents of burn injury are significantly higher in lower income countries with the WHO attributing up to 90% of the 11 million burn injuries world-wide in low income countries, and are a major cause of morbidity in the world(2).
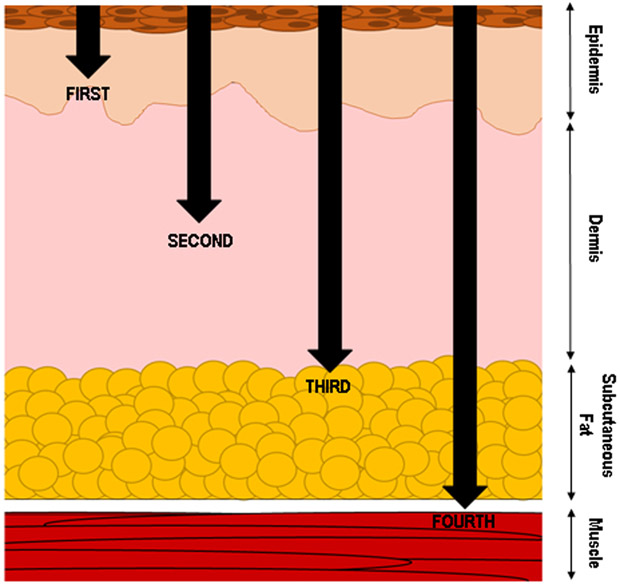
Burn injury severity is categorized by the depth and size of the wound as demonstrated in Figure 1. First degree burns have the injury limited to the epidermis, or outermost and superficial layer of the skin. Second degree or partial thickness burns are defined by the injury extending past the outermost layer into the dermis and often form blisters that are extremely painful. Depending on the depth of the partial thickness burn, surgery may be required. Third- and fourth-degree burns penetrate the full thickness of the dermis, with fourth degree burns causing deeper damage to underlying muscle or bone. Third- and fourth-degree burns are not painful due to destroyed nerve endings and require surgery and careful management of the burn area to prevent infection. Fourth-degree burns will often lead to the loss of the injured area. Along with the depth of the injury, the total surface area is an important component of determining burn injury severity. If the burn covers less than 10% of the total body surface area (TBSA), it is categorized as a minor burn. The classification for major burns is less well-defined, however, the following guidelines are often used to indicate a major burn: greater than 20% TBSA in adults, greater than 30% TBSA in children, and greater than 10% TBSA in elderly patients(3).
Figure 1. Categorization of burn wound injury.
In addition to wound size, the depth of tissue affected by burn injury contributes to the wound categorization, treatment and patient outcomes. Burn that are restricted to the epidermis are considered superficial and categorized as first-degree burns. Second-degree burns are partial thickness injuries that penetrate varying depths below the epidermis and into the dermis. Once the burn injury penetrates the entire dermal layer and begins to effect the subcutaneous fat layer, the injury is classified as a third-degree burn. These are considered full-thickness burns and result in the destruction of nerve endings which make the wound pain-free. However, burns of this depth require careful management and surgery to support healing and prevent wound infection. A burn that penetrates further and damages the underlying muscle, and even bone, are classified as fourth-degree burn. An injury of this severity often results in permanent damage to the tissue and possible amputation of the injured area.
2. RESPONSE TO BURN INJURY
The initial burn injury causes numerous detrimental effects not only to the immediate burn area but leads to a cascade of responses in the entire body that can lead to severe consequences such as shock and multiple organ failure. These systemic responses can cause serious detriment to the patient and their recovery. Immediately following injury, catecholamines, cortisone, and inflammatory cytokines like IL-6 and tumor necrosis factor (TNF) are released into systemic circulation. Burn injury usually leads to distributive shock, in which capillaries become leaky and fluid is lost to the extravascular space. This loss of fluid from the circulatory system results in edema, fluid accumulation in tissues, reduced cardiac output, and compromised delivery of oxygen to numerous bodily organs, including the gastrointestinal tract(4, 5). Furthermore, burns can be complicated by other injuries, such as inhalation injury which can compromise the airway. A small subset of burn injuries are also associated with other traumatic injuries, such as traumatic brain injury, soft tissue injury and/or fracture, or injuries to the thorax and/or abdomen(6, 7). Like with any trauma patient, airway, breathing, and circulation must be stabilized upon immediate arrival to the hospital.
Current standards in burn treatment require immediate fluid resuscitation to account for this hypovolemic state. Fluid requirements are calculated based on several different formulas; however, initial rates are dependent on the size of the burn area, the patient’s body weight, and eventual fluid output by the patient (urine volume)(8-10). The purpose of fluid resuscitation is to adequately perfuse and oxygenate organs to avoid complications such as renal failure(11, 12). A delicate balance must be maintained while resuscitating burn patients, as over-resuscitation combined with endothelial leakage observed can lead to “fluid creep” and severe consequences such as abdominal compartment syndrome, pulmonary edema, and decreased perfusion of the burn wound itself (9, 13).
The type of fluid used in resuscitation must also be considered: isotonic crystalloids, hypertonic solutions, colloids, and increasingly plasma is being used. Crystalloids, including Ringer’s lactate (RL) and normal saline, are readily available products and commonly used. However, over-resuscitation with RL has been associated with increased neutrophil activation after hemorrhage, and high volume administration of saline can lead to hyperchloremic acidosis(14, 15). Due to problems associated with over-resuscitation and edema, hypertonic solutions have also been used but require close monitoring due to risks of hypernatremia and subsequent renal failure(16). Recently, there has been an increased shift to using blood products, including plasma, as it is a physiologic fluid that may help prevent excess vascular leakage after burn injury(17). A rat model of burn injury demonstrated that addition of fresh frozen plasma (FFP) to resuscitation fluid helped diminish endothelial leakage(18). However, there are limited studies on the effect of the type of resuscitation fluids on the burn microbiome. One study using a swine burn model demonstrated that resuscitation volumes could influence the gut microbiota, such as a dose-dependent increase in Bacteroidetes and ameliorating growth of harmful Proteobacteria populations(19). A similar study used different resuscitation fluid paradigms in a swine model and found that while all groups experienced intestinal microbial dysbiosis following burn injury, limited-volume crystalloid (LV-Cr) resuscitation led to the most drastic dysbiosis and hepatocellular damage(20). However, further research needs to be done to fully understand the impact of burn resuscitation protocols on the microbiome.
While fluid resuscitation is paramount to burn treatment, it does not fully restore organ function. Burn patients still have numerous systemic abnormalities that must be closely monitored and treated. Inflammatory cytokine levels are consistently elevated in mouse models of burn injury for several days and a similar trajectory was observed in pediatric burn patients(21, 22). Finally, patients with severe burns enter a hypermetabolic state, and combined with systemic inflammation, are at risk for secondary infection, sepsis, and multiple organ failure.
Even after sufficient fluid resuscitation, patients still have experienced significant organ ischemia, and secondly, aggressive infusion of fluids leads to rapid re-introduction of oxygen to ischemic tissues, production of reactive oxygen species (ROS), and formation of free radicals. A major source of free radicals are neutrophils activated by damage associated molecular patterns (DAMPs) derived from injured tissue, leading to oxidative stress and injury of organs(23-26). Free radical-mediated injury after burn injury has been documented in numerous organs, including the lungs, liver, and the gastrointestinal tract(27-30). A number of studies have investigated antioxidants such as Vitamin C as a supplemental therapy to combat the severe oxidative stress encountered after a burn injury(31, 32).
Furthermore, this systemic inflammatory phase is characterized by release of inflammatory cytokines such as IL-1, IL-6, IL-18, and tumor necrosis factor (TNF), leading to further detrimental effects in numerous organ systems(33). The uncontrolled inflammatory response and release of cytokines can lead to systemic inflammatory response syndrome (SIRS), characterized by overactivation of the immune response(34). This uncontrolled activation of the inflammatory response leads to organ tissue damage, which exacerbates the injury itself. Under these circumstances, the inflammatory mediators produced by the host are causing more damage to organ systems rather than eliminating subsequent infections. Due to this, patients with severe burn injuries are also at risk to develop secondary infections, which can lead to sepsis and multiple organ failure. Several studies have implicated the gastrointestinal tract as a source of bacterial endotoxin products and infection due to its large reservoir of bacteria(35-38).
2.1. Gastrointestinal abnormalities following burn injury
Following a severe cutaneous burn injury, the gastrointestinal system is adversely impacted by the initial hypoxia, the subsequent free radical injury and inflammation, leading to deficits in gastrointestinal (GI) barrier integrity and immune function(39). The GI tract is host to the body’s largest reservoir of bacteria and maintaining homeostasis between the host and the microbiome is crucial. Numerous abnormalities in the GI system have been documented in burn patients, including increased gut permeability, decreased gut motility/transit, and increased gut bacteria translocation(40-46). Bacterial products and bacteria themselves have been detected in the mesenteric lymph node, liver, and lungs following injury(35, 38, 47). This infiltration of bacteria can exacerbate the tissue damage by recruiting more neutrophils who continue to release ROS and free radicals(28). Burn injury has been reported not only to harm the gastrointestinal organs but alters the microbial populations themselves. Animal models of burn injury and reports from patients have shown that bacterial diversity is diminished and beneficial species, such as Bifidobacterium, are decreased compared to healthy controls(36, 48, 49). Trauma patients also exhibit these changes within 72hrs, showing a reduction in Bacteroidales, Fusobacteriales and Verrucomicrobiales, accompanied by an increase in Clostridiales and Enterococcus bacteria(50). GI functional deficiencies can be complicated by treatments used for the burn injury, including opioid analgesics, which may inhibit gut transit and reduce motility(51). This slowed transit of digestion products can lead to increased bacterial overgrowth in the intestines and overgrowth of pathogenic bacteria, in particular Enterobacteriaceae(47, 52). Similar to analgesics, antibiotic usage is ubiquitous due to high rates of mortality associated with secondary infections in burn patients. However, its usage leads to disruption of the gastrointestinal microbiome and introduces another confounding factor in the management of burn patients(53-56). As expected, antibiotic usage is linked to a decrease in the diversity of the gut flora and can allow multi-drug resistant strains to flourish(57). Others have demonstrated that the microbiome plays a crucial role in numerous disease states, including in unexpected areas, such as the regulation of cardiorespiratory control and the strength of the immune response derived from vaccines(58, 59). Some studies have used manipulation of the microbiome to their advantage. In trauma patients, selective decontamination of the digestive tract (SDD), a prophylactic regimen of non-absorbable antibiotics with the aim to prevent nosocomial infections in critically ill patients, has shown to have some benefit in morbidity and mortality(60-62). However, there is still limited research in how the microbiome can affect the disease course of burn patients, as well as their potential as a therapeutic target or therapeutic that may aid the recovery of burn patients.
3. CONFOUNDING VARIABLES IN BURN INJURY
3.1. Alcohol Use
Most instances of burn injury do not occur in isolation and are complicated by factors such as the patient alcohol and/or drug usage. In nearly half of all burn injuries, patients are intoxicated at the time of admission following burn injury(63). Alcohol leads to lowered inhibition, loss of dexterity and balance, and leads to increased accidental injuries, including burn injury(64). It is well-established that alcohol intoxication at the time of burn injury leads to worse outcomes, including increased hospital stay, increased infection rates, and a higher rate of surgical procedures(65). Alcohol alone is a risk factor and one of the leading causes of morbidity and mortality worldwide(66). Furthermore, alcohol usage can disrupt the microbiome, leading to dysbiosis, as well as alter permeability of the intestinal barrier. Studies have shown increased levels of endotoxin in alcoholic patients compared to controls, as well as increased small intestinal bacterial overgrowth(67-69). Ethanol exposure also alters immune responses, such as decreasing NLRP3 activation and cytokine production(70-72). Taken together, alcohol exposure at the time of burn injury potentiates end organ damage, including the lungs, liver, and gastrointestinal tract(73-77).
3.2. Advanced Age
Adults over the age of 65 are a growing percentage of the population(78). Within individuals of advanced age, there is increased incidence of chronic illnesses and a phenomenon known as “inflamm-aging,” which is a constant low-grade inflammatory state(79). Aging individuals have higher levels of TNFα and IL-6, but have diminished immune responses and are more susceptible to infections such as pneumonia(80, 81). Advanced age also leads to poor immune responses after burn, including delayed wound-healing, delayed inflammatory responses, and dysregulation of immune cells such as monocytes(82-85). While the elderly experience immune senescence with advanced age, the composition of their microbiome also changes, including greater proportion of Bacteroides and Clostridium groups(86-88). In a study using germfree mice, FMT were performed from young or old donor mice and transferred into young germfree mice. The mice inoculated with aged gut microbiota demonstrated increased intestinal inflammation and increased translocation of bacterial products from the gut lumen into systemic circulation, as well as immune cell activation(89). As for burn injuries, elderly patients consistently have poorer outcomes compared to younger patients, including increased morbidity, mortality, a delayed initial inflammatory response, followed by a prolonged hyperinflammation long after burn(84, 90). All of these aberrations in the aged population can compound in increased morbidity and mortality observed in elderly patients following burn injury, but the contribution of the microbiome is still poorly understood in the context of the elderly and acute injuries such as burn.
3.3. Sex
It is well-documented that there are differences in immune responses, leading to sexual disparities in numerous areas including the rate of autoimmune diseases and mortality after infection. Sex hormones are known to regulate immunity, with a general trend of females having a stronger immune response during reproductive age, including a more robust humoral response(91-93). Differences have also been observed in traumatic injuries(94-96). In a rat study of trauma/hemorrhagic shock (T/HS) and burn injury, females showed less lung and gut injury following both injury models compared to male mice. When the female rates were ovariectomized, this protection was lost(97). Neutrophil response to a T/HS or burn injury rat model also demonstrated sexual dimorphism. CD11b surface expression and respiratory burst activity was increased in male rats after injury compared to female rats. This effect was reduced with castration of male rats rats(98). In a study of trauma patients, there was a slight increase in survival for women under the age of 40 with an Injury Severity Score between 16 and 24. Women also had less infectious complications, however, they did have a higher rate of death with infection(99). Conversely, in an analysis of patients from the National Burn Repository, female patients had a higher incidence of death as a result of burn injury across all age groups compared to men(100). More studies are needed to elucidate the effect sex has on burn patient survival and recovery.
4. THE MICROBIOME
The microbiome constitutes a diverse and complex population of bacteria, fungi, viruses, and other microorganisms that colonizes a specific location of the body. Although there are several different microbiomes within the human body, including the skin and oral mucosa, this review will focus on the gastrointestinal microbiome. The GI or gut microbiota is the largest reservoir of bacterial species in the human body, with upwards of 1012 bacteria per gram of luminal contents(101, 102). In addition to large quantities of total bacteria, the human gut microbiota also exhibits sizable numbers of individual bacterial species. Although it was previously believed that around 1,000 bacterial species are present in the gut, recent studies indicate there may in fact be over 15,000 individual species of bacteria in the human gut microbiome(103-105).
The earliest studies into the microbiome were hampered by the inability to culture the majority of bacteria present in the gut. Scientists have since harnessed the power of high through-put sequencing to make advances in understanding the composition and function of the microbiome. A commonly used method to detect and assess bacterial species is 16S ribosomal RNA (rRNA) sequencing. The 16S rRNA gene is an evolutionarily conserved component of the ribosome consisting of both conserved and a hypervariable region. Researchers can differentiate between bacterial species by sequencing the hypervariable region of this gene. With the advent of next generation sequencing, newer studies are beginning to utilize whole-genome sequencing. These approaches allow scientists to detect strains of individual bacterial species and analyze pathogenic bacterial genes. Overall, these methods have expanded the studies able to be performed and shaped our understanding of the human microbiome in health and disease.
4.1. Normal Bacterial Composition
The human gut microbiome is a diverse community of bacterial species that varies widely between individuals. Bacterial community structure is thought to be influenced by individual genetics, diet, environmental factors and exposure to microbes(106). In addition, differences in both bacterial abundance and composition are apparent throughout the length of the gastrointestinal tract. However, the microbiome of the distal small intestine, cecum and large intestine share many commonalities(107). Throughout the years, this complexity has hampered the characterization of how a normal and healthy gut microbiome is structured. Nevertheless, scientists have identified several bacterial phyla and associated genera that are present across a number of intestinal microbiome studies. In general, the human intestinal microbiome is predominately composed of bacteria belonging to the Firmicutes, Actinobacteria and Bacteroidetes phylum(102). Core microbial genera belonging to the Firmicutes phyla include Ruminococcus, Faecalibacterium, Eubacterium, Clostridium and Roseburia(108). Pathogenic bacterial species, such as Enterobacteriaceae, are generally present at a very low abundance of <0.1% or less of the bacterial population(102). Recent studies looking at gut bacteria compositions across individuals have identified three general bacterial profiles, or enterotypes, that normal human gut microbiome samples cluster into(109, 110). However, studies are ongoing to determine what impact a person’s enterotype may have on disease outcomes and their diagnostic potential. In addition to the presence or absence of specific bacterial species, overall diversity of the gut microbiome is an important indicator of health. In the field of microbiome research, the diversity of bacteria found within a single sample is termed alpha diversity. This is determined by assessing the evenness (abundance of a species) and richness (number of different species present) of the microbiota. A healthy gut microbiome exhibits high diversity and studies indicate that individuals with lowered microbiome diversity are more associated with inflammatory phenotypes, obesity, and insulin resistance(111).
4.2. Functions of the Gut Microbiota and Microbial Metabolites
Studies involving germ-free mice, which lack a resident microbiome, reveal several crucial functions for the microbiota in regulating intestinal homeostasis. Compared to mice raised in normal conditions, germ-free mice exhibit severe defects in mucosal immunity. Additionally, they have been shown to have reduced intestinal motility and vascularity, deficient cytokine production and reduced epithelial cell turnover(112). Historically, the gut microbiome has been studied for its vital role in host metabolism. The resident bacteria of the gut are responsible for the fermentation of otherwise non-digestible complex carbohydrates and other xenobiotic compounds. The resulting bacterial metabolites provide a critical source of energy for cells throughout the body. Furthermore, these metabolites can enter the bloodstream where they have been shown to impact overall host metabolism, weight, and insulin sensitivity(113-115). Short-chain fatty acids are widely studied bacterial metabolites that include acetate, propionate, and butyrate. Acetate and propionate are primarily used by the liver and other peripheral tissues, where they are often converted into glucose or lipids. Butyrate is a major source of energy for the intestinal epithelium, with some studies indicating it may provide up to 80% of the energy source(116-118). A wide variety of bacteria belonging to the Bacteroidetes and Firmicutes phyla can produce different SCFAs. Bifidobacterium species are widely studied for their ability to produce the SCFA butyrate. Additional butyrate producing bacteria include Ruminococcaceae family members, such as Faecalibacterium prausnitzii, and various species of Roseburia within the family of Lachnospiraceae(119-121). In addition to regulating energy balance, SCFAs have been shown to promote intestinal barrier function via several mechanisms. Treatment of intestinal epithelial cells with SCFAs increases proliferation and tight junction expression and reduces epithelial permeability(122-124). Furthermore, SCFAs can modulate mucosal immune responses to suppress intestinal inflammation. For example, acetate has been shown to promote intestinal IgA production(125). In general, butyrate is considered an anti-inflammatory molecule that maintains immune homeostasis in the gut by promoting anti-inflammatory cytokines like IL-10 and maintenance of regulatory T cell function(126-128). Apart from regulating host metabolism and providing sources of energy, the bacteria that comprise the gut microbiota also regulate GI tract structure and integrity, modulate immune functions, and provide antimicrobial protection(102, 108). Therefore, maintaining a healthy gut microbiome is essential for the proper function of the gastrointestinal tract.
4.3. Health Implications of Microbial Dysbiosis
Although the bacterial populations of an individual vary over time, an important aspect of the human microbiome is its resilience and ability to recover from disturbances, such as antibiotic use(129). The inability of the gut microbiome to return to a healthy state is associated with dysfunctional gut health and a variety of diseases. Bacterial dysbiosis is described as a significant change in the intestinal microbiome, such as reduced diversity and increased abundance of pathogenic bacteria, that can result in adverse health effects(122). Many studies over the years have focused on the connection between bacterial dysbiosis and chronic inflammatory disorders of the gut. For example, gut bacterial dysbiosis is a common feature of patients with colorectal cancer and has been associated with chronic inflammation and colorectal cancer progression(130-132). The dysregulated immune responses central to inflammatory bowel disease (IBD) have been linked to dysbiosis of gut bacteria. Patients suffering from IBD display gut microbiomes that are distinct from healthy controls, with decreases in several beneficial bacteria and increases in Enterobacteriaceae, such as Proteobacteria, correlating with disease state(103, 133). Far less is understood about the impact of gut bacterial dysbiosis on more acute disorders, such as trauma and burn injury. Therefore, more research is needed to understand the contributions bacterial dysbiosis may have on the pathophysiology of severe burns.
5. IMPACT OF BURN INJURY ON GUT MICROBIOTA
It is clear from studies of inflammatory GI disorders, like IBD, that changes in the microbiome can significantly influence disease progression and outcomes. Although there are distinct differences between chronic disorders and acute trauma, like severe burn injury, both are impacted by intestinal inflammation and gut barrier disruption. Consequently, it is likely that bacterial dysbiosis would be prominent after severe burn injury and contribute to post-burn pathologies. This section will discuss changes in gut microbiota composition that occur after severe burn injury.
To begin to assess the microbiome’s impact on post-burn pathogenesis, early studies focused on detailing how the composition of the microbiome is altered after severe burn. Utilizing fecal samples from severe burn patients, Earley et al. showed clear evidence for bacterial dysbiosis following burn injury. The microbiome of control patients was dominated by Bacteroidaceae and Rimunococcaceae families, indicating healthy microbiome compositions. Both of these dominant bacterial groups were decreased in severe burn patient samples, which exhibited overall significantly different compositions compared to control samples by non-metric multidimensional analysis(47). Additionally, they observed a significant increase in the abundance of Enterobacteriaceae, a bacterial family that includes several pathogenic species. Indeed, the most abundant Enterobacteriaceae taxon (OTU) found in severe burn patients was attributed to E. coli O83:H1, a pathogenic and invasive strain of Escherichia coli(47). Other studies investigating compositional changes in the microbiome of severe burn patients reiterate reduced bacterial diversity accompanied by a decrease in beneficial bacteria like Bacteroidaceae and an increased presence of pathogenic Escherichia bacteria belonging to the Enterobactericeae family(48, 134). Escherichia coli is commonly associated with bacterial translocation and sepsis, which are known complications of severe burn injury(135). Therefore, increased abundance of Enterbactericeae, particularly strains of Escherichia coli, could provide a therapeutic target for reducing mortality associated with severe burns. As the abundance of bacterial species in the gut change following burn injury, there are likely accompanied by changes in the concentration of different bacterial metabolites. As discussed previously, SCFAs are widely studied bacterial metabolites that provide energy, modulate immune responses, and regulate gut barrier integrity. These beneficial metabolites are produced by several species among the Bacteroidaceae and Rimunococcaceae families, which have been shown to be reduced after severe burn injury(47, 48). In particular, studies show that after burn, patients exhibit decreases in the abundance of fecal Bifidobacterium, which is a major producer of butyrate(48). Additionally, investigators have assessed the concentrations of SCFAs in the stool samples of severe burn patients. One study following five patients with severe burns noted that the levels of SCFAs, including acetate, propionate, and butyrate, were reduced relative to normal levels. Although SCFA levels generally recovered as the patient did, levels of propionate and butyrate remained undetectable in the lone non-surviving patient(48). It is important to note that the few human studies described here utilize small patients sample sizes. Further research using patient samples is required to advance our understanding of how severe burn injury impacts the gut microbiome.
To reduce the impact of confounding factors present in patient studies, such as the complex diversity of the human microbiome and therapeutic interventions like antibiotics, several studies have examined the effect of severe burn injury on the microbiome in murine models. It should be noted that although both the mouse and human gut microbiomes are dominated by Bacteroidetes and Firmicutes phyla and share similarities in composition at higher taxonomic levels, including similar enterotype clustering, there are significant differences in composition at the bacterial species level(136, 137). In addition, human microbiome studies utilize stool samples while murine studies generally examine the bacterial content of the cecum. While there is significant overlap in the bacteria present throughout the lower GI tract (distal small intestines, cecum, and large intestine), the differences in bacterial composition based on location of sample collection can complicate analysis and is an important consideration. Although species differences may be present between humans and mice, similarities in bacterial families and dominant microbes indicate some conservation of the influence the microbiome has on intestinal function. Therefore, murine models represent a valuable tool that allow scientists to map broad shifts in microbial composition and examine the functional outcomes that may contribute to post-burn pathophysiology.
Several studies utilizing murine models of severe burn injury have shown changes in the gut microbiome after burn that mirror the bacterial dysbiosis found in human studies, including decreased abundance of dominant beneficial bacteria accompanied by an increase in the Enterobacteriaceae family, such as E. coli species(47, 49, 138). In addition, murine studies have shown that severe burn results in decreased SCFA levels, particularly butyrate, in cecal contents immediately following injury and for up to 7 days after injury(36, 139, 140). Accordingly, it has been found that the abundance of SCFA producing bacteria are reduced in murine models of severe burn injury. A study utilizing Sprague-Dawley rats and a 30% TBSA burn model showed a significant decrease in Clostridium IV and Clostridium XIV, which are both known butyrate producers(138). Furthermore, they found reduced levels of Lactobacillus, a commonly used probiotic, after burn injury(138). Studies show that the metabolite lactate, produced by Lactobacillus, is readily utilized by other bacteria to produce butyrate, and is therefore an important contributor to SCFA levels(141). These changes found in rats are consistent with mouse models of severe burn injury. A study published by Beckmann et al. revealed a reduction in Lactobacillaceae and Clostridiaceae bacterial families in mouse cecal contents after burn injury(49). Likewise, Feng et al. showed that Lactobacillus was a principal species, reduced after burn injury, that significantly correlated with SCFA levels(36). Interestingly, they also found that butyrate levels negatively correlated with the abundance of a prominent enteric pathogen, Escherichia-Shigella(36). This indicates that changes in the levels of SCFAs and the bacteria that produce them could potentially influence the abundance of pathogenic bacteria, thereby contributing to post-burn pathogenesis via multiple pathways.
6. THE GUT BARRIER, BACTERIAL TRANSLOCATION, AND BURN
The gastrointestinal tract is the largest mucosal surface in the body, responsible for regulating nutrient absorption while maintaining a barrier against environmental toxins and pathogens (Figure 2). The proximity of resident microbiota ensures that the barrier formed by intestinal epithelial cells is crucial for maintaining a homeostatic relationship between the host immune response and resident microbes. This intestinal epithelial barrier includes tight junction proteins that maintain close association of epithelial cells with each other, in addition to antimicrobial peptides (AMPs) and mucus which prevent bacterial overgrowth and invasion(142, 143). These components make intestinal epithelial cells the primary physical barrier that prevents enteric infections. Consequently, disruption of the intestinal barrier can dramatically impact health and is associated with a wide variety of gastrointestinal disorders(144). Nearly every aspect of the intestinal epithelial barrier is affected by severe burn. Studies show that burn injury reduces epithelial cell tight junction protein expression, disrupts tight junction localization, reduces mucus production, and inhibits the expression of AMPs(145, 146). Overall, these changes result in increased intestinal permeability(145, 147). Bacterial translocation resulting from intestinal barrier disruption is thought to contribute to severe consequences after burn injury, including sepsis and multiple organ failure(135). Indeed, bacteria and their products have been detected in the lung, liver, and mesenteric lymph nodes after severe burn injury(47, 148, 149). Further research is required to investigate the mechanisms by which bacterial translocation after severe burn contributes to mortality and the pathophysiology of systemic inflammation and organ dysfunction.
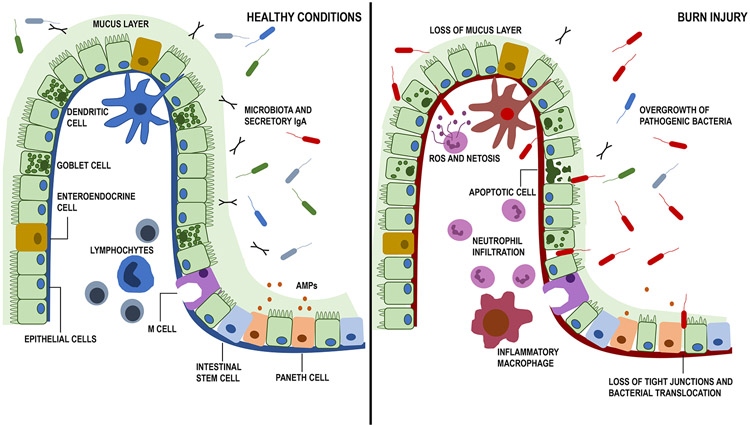
Figure 2. Representation of the intestinal barrier and gut microbiome under healthy and burn-injured conditions.
Under healthy conditions, the intestinal barrier is intact and composed of intestinal epithelial cells (IEC) that are tightly bound together by tight junction proteins. Goblet cells that produce mucus (light green) and enteroendocrine cells (yellow) are interspersed between IECs. In the intestinal crypt, there are intestinal stem cells (blue) that continuously replicate and regenerate IECs that are shed in the lumen. Paneth cells (orange) producing anti-microbial peptides (AMPs). M-cells (purple) are continuously testing luminal contents for uptake by any resident antigen presenting cells. Under the IEC barrier is the lamina propria, which contains a variety of immune cells, including dendritic cells, monocytes, and T/B cells (blue). Secretory IgA is continuously passed into the lumen to maintain homeostatic conditions with the microbiome. Under healthy conditions, there is a large diversity of intestinal microbiota with few pathogenic species. With burn injury (right panel), there is a loss of tight junction proteins and the mucus barrier, along with increased IEC apoptosis, leading to a leaky gut. Increased inflammatory cell infiltration by neutrophils and macrophages leads to production of reactive oxygen species (ROS) and further damage of the barrier, allowing for intestinal bacterial and bacterial products to translocate into systemic circulation. Furthermore, there is overgrowth of pathogenic bacterial species and loss of bacterial diversity in the lumen. This image was adapted from Hammer et. al, Alcohol Res. 2015;37(2):209-22.
Due to the close interactions between intestinal epithelial cells and the gut microbiome, the impact of gut bacteria on intestinal barrier function has been of recent interest. In particular, the contributions of both commensal and pathogenic bacteria to chronic disorders, like IBD and celiac disease, have been widely studied. Beneficial commensal bacteria have been shown to promote epithelial cell barrier function by reducing epithelial permeability and increasing tight junction protein expression(150-152). Additionally, bacterial metabolites including butyrate, acetate, and indole, can enhance the intestinal barrier(153-155). On the other hand, several pathogenic bacteria have been linked to gut barrier disruption and intestinal permeability(150). Certain invasive strains of Escherichia coli have been shown to target components of epithelial cell tight junctions to promote permeability(156). Other pathogenic bacteria that are known to disrupt epithelial tight junctions include Clostridium perfringens and Clostridium difficile(157, 158). Although the connection between the gut microbiome and intestinal barrier integrity has been established in other models of gastrointestinal inflammation, few studies have been conducted to discern the impact that changes in the microbiome have on gut barrier integrity following severe burn injury. It is likely that the increased abundance of pathogenic bacteria, like Enterobacteriaceae, could exacerbate intestinal barrier disruption after burn injury and promote the translocation of bacteria and bacterial endotoxins from the gut into the bloodstream. Further research in this area could provide new therapeutic targets to reduce the severity of sepsis, multiple organ failure and other critical complications of severe burn.
7. SYSTEMIC IMMUNE RESPONSE TO BURN
The initial immune response to burn injury is characterized by rapid activation of the innate immune system, including neutrophils, monocytes, and macrophages (Figure 3). These cells are able to recognize damage associated molecular patterns (DAMPs) and pathogen associated molecular patterns (PAMPs) through Toll-like receptors and NOD-like receptors(159-161). This activates the transcription factor NFκB, which regulates numerous pro-inflammatory downstream mediators, including TNF, IL-6, and IL-1β(162). When looking at genome-wide changes in leukocytes following burn injury, changes in gene expression were similar to those observed under severe trauma or endotoxemia(163). Furthermore, these changes in transcripts were observed as early as 4h after injury and persisted for weeks, indicating that traumatic injuries, including burn, lead to a rapid alteration in immune response followed by a chronic disruption in their activity
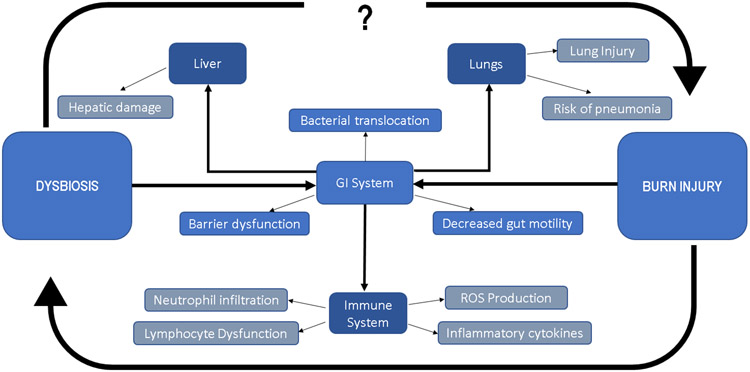
Figure 3. Representation of end organ injury and dysfunction following burn injury and microbial dysbiosis.
Both burn and dysbiosis are known to perturb the normal function of the gastrointestinal system. Factors altered in burn injury include barrier dysfunction, gut motility, and bacterial translocation into systemic circulation. This contributes to end organ dysfunction, including hepatic damage, lung injury, and pneumonia. Finally, burn injury and bacterial dysbiosis/translocation is linked to aberrant immune function, including neutrophil infiltration into various tissues, reactive oxygen species (ROS) and inflammatory cytokine production, and lymphocyte dysfunction. Burn injury itself is linked to dysbiosis, however, further work is needed to understand the contribution of microbial dysbiosis and its impact on deleterious outcomes for burn patients, as well as its impact on their recovery.
7.1. Long-term Disruptions in Immunity
While the initial response to burn injury is characterized by rapid onset of inflammation and a hypermetabolic state, the long-term consequences of burn injury are characterized by chronic dysfunction of the immune system. Compared to non-burn trauma patients, burn-injured patients displayed higher levels of inflammatory markers IL-6 and IL-18 in the plasma(164). A study following pediatric patients showed that markers of hypermetabolism (resting energy expenditure, body composition, metabolic markers) were elevated for up to three years post burn injury. Similarly, levels of catecholamines, cortisol, and acute phase proteins were elevated(165). A study of different types of skin trauma found that 84 days post-injury, burn injured mice had significantly elevated levels of IL-10 in the sera, accompanied by reduced numbers of lymphocytes, neutrophils, and eosinophils compared to animals receiving an excision injury(166) . In a study of sepsis patients, it was determined that higher IL-10 concentration in the sera correlated with increased mortality, likely due to immunosuppression(167). A similar elevation in IL-10 was observed in a prospective study of patients with systemic inflammatory response syndrome (SIRS)(168). Multiple studies have observed changes in serum cytokines between surviving and non-surviving burn patients and proposed prognostic indicators, including combinations of cytokines IL-6/IL-7/IL-10, and IL-1RA/IL-6/MCP-1(169, 170). Other cytokines, such as IL-27, have also been proposed as a diagnostic biomarker for sepsis or infection in critically ill patients(171-174).The cytokine milieu in burn patients is complex, and while numerous biomarkers have been identified for these critically ill patients, the profile of each patient is different and likely requires individualized care.
7.2. Neutrophils
There are numerous deficits in the pro-inflammatory response observed in several cell types following burn injury. For example, neutrophils are the first responders in many instances of injury, including burns. Studies have shown increased neutrophil populations in the blood, intestine, and lungs after burn injury(28, 29, 175). While key responders to the initial injury, neutrophils in burn patients have been reported to have reduced phagocytic and bacterial killing ability up to 28 days after burn injury(176). Furthermore, in an animal model of burn injury and secondary infection, neutrophils have been reported to exhibit reduced recruitment to the lungs after intranasal administration of lipopolysaccharide (LPS), as well as reduced NETosis or formation of neutrophil extracellular traps (NETs). NETs are a key component of neutrophil bactericidal activity to contain bacterial infection and infiltration(177). Similarly, directional migration speed of neutrophils was significantly reduced in neutrophils isolated from burn patients and correlated with the size and severity of the burn(178). Following the initial burn injury, neutrophil recruitment into the GI tract due to hypoxia and cellular damage compounds the injury through production of ROS, and studies have shown that neutrophils have delayed apoptosis after burn injury(179). This prolonged lifespan is problematic, as inflammation persists in organs and prevents resolution of the inflammatory process. Studies observing intestinal damage in Graft versus Host Disease (GVHD) have also identified neutrophils as a key player in intestinal damage once recruited and observed that eliminating their ability to produce ROS significantly improved tissue damage and reduced effector T cell recruitment(180). Paradoxically, while neutrophils have prolonged lifespans after burn injury, they are worse at eliminating repetitive infections from pathogens. A mouse model of cutaneous burn injury found that animals infected with Pseudomonas aeruginosa pneumonia were worse at clearing the bacteria when given two repeat infections. While they mounted a hyper-inflammatory response to the first infection, the repeated infection merely increased recruitment of neutrophils and macrophages to the lungs, without clearing the infection itself(181). Poor neutrophil bactericidal activity in response injury contributes to poor outcomes in burn patients and is likely exacerbated by documented shifts in the microbiome to overgrowth of pathogenic bacteria. However, the effect of bacterial changes on neutrophil function in the context of burn injury is not sufficiently understood or explored.
7.3. Macrophages
Similar to neutrophils, macrophages are activated by DAMPs and PAMPs in response to burn injury. After burn injury, macrophages produce copious pro-inflammatory cytokines, prostaglandins, and reactive nitrogen species(181-183). This hyperactive response by macrophages is thought to contribute to increased susceptibility of burn patients to sepsis due to the “two-hit” theory, in which an initial insult primes the patient’s immune response to be hyper-inflammatory, followed by a second insult (infection or sepsis). Following the second insult, the patient is unable to mount an appropriate immune response due to the initial hyperinflammatory state and succumbs to multiple organ failure(184). Again, dysbiosis in the burn microbiome to preferentially allow overgrowth of harmful species, such as the Enterobacteriaceae family, are likely to exacerbate the two-hit theory of burn injury and subsequent sepsis. Macrophage hyperactivity results in elevated TNF, IL-6, and nitric oxide (NO) levels following burn injury. In particular, excess NO production by macrophages isolated from burn injured mice suppressed proliferation of splenic T cells(185). While macrophages are hyperinflammatory in the short term, long-term effects of burn injury on hematopoiesis in the bone marrow lead to perturbed development of monocytes. As early as 48h after injury, monocyte progenitors isolated from bone marrow of burn and burn sepsis mice show impaired TNFα production following LPS stimulation(186). These unresponsive progenitors eventually replace the initial pool of hyperreactive macrophages, leading to the immunosuppression observed later in burn injury recovery.
7.4. Antigen Presenting Cells
Macrophages are only one type of antigen presenting cell (APC) that are crucial in initiating adaptive immune responses, including activation of T cells. APCs isolated from burn injured mice have a demonstrated weakness in activating the proliferation of naïve T cells(187). Dendritic cells (DCs) are another subset of professional antigen presenting cells and are an essential bridge between the innate and adaptive immune response. Dendritic cells isolated from burn-injured mice demonstrated a dampened response to TLR9 stimulation and had impaired T cell activation activity(188, 189). Burn injury also led to significant reduction of CD11c+ DCs in lymph nodes, along with upregulation of programmed death ligand 1 (PD-L1) expression on splenic DCs, which is a potent suppressor of T cell activation(190). PBMCs isolated from burn patients demonstrated that overexpression of transcription factor MafB correlated with the dendritic cell depletion observed in burn patients, and silencing MafB in an in vitro culture system restored myeloid dendritic cell differentiation(191). This long-term perturbance in APC development and differentiation contributes to post-burn pathogenesis. While trauma and burn injury is often described by its initial hyperinflammatory state, the long-term consequences of burn injury is characterized by immunosuppression and increased susceptibility to infection.
7.5. T Cells
In concert with deficiencies in the innate immune system, there are well-documented effects of burn injury on T cells and their ability to mount a response to infection. T cells maintain a balance of pro and anti-inflammatory activity in response to various stimuli. Naive T cells are activated by DCs through antigen presentation and antigen recognition by the T-cell receptor (TCR), along with co-stimulatory molecules such as CD28 and cytokines. Both CD4 and CD8 positive T cells have demonstrated perturbations following burn injury(192, 193). In a mouse model of burn injury, there was a significant decrease in the number of splenocytes following a 20% TBSA burn. This decrease in cellularity was persistent for 5 days after injury and a diminished proliferative response was observed in splenocytes(194, 195). Similarly, T cells isolated from burn injured mice demonstrated reduced IL-2 production and augmented interferon gamma (IFNγ) production(196). IL-2 is particularly important to stimulate proliferation of T cells, and a study of burn patients found that while all burn patients demonstrated a decrease in IL-2 production by peripheral blood mononuclear cells (PBMCs), surviving patients had a gradual recovery of IL-2 whereas non-survivors did not recover IL-2 production(197). In addition to decreased proliferation, T cell survival is reduced upon burn injury complicated with sepsis. Findings from a combined burn and sepsis model show significant reduction in T cell population of the mesenteric lymph node by apoptosis(198). T cells isolated from peripheral blood of burn injured patients did not show a significant change in the ratio of CD4 to CD8 T cells, however, there was a demonstrated increase in the release of cytokine IL-4 by day 5. This increase in IL-4 production was mainly by CD8 T cells(199). Regulatory T cells (Tregs) are another important subset of T-cells altered after traumatic injuries such as burn. Under normal conditions, Tregs maintain a careful balance by preventing overactivation of inflammatory mediators through suppression of T cell proliferation/activation and production of anti-inflammatory cytokines such as IL-10 and TGF-β. In a study of peripheral blood cells taken from patients, Tregs isolated from burn patients showed higher cell surface expression of key suppressive mediators such as CTLA-4. IL-10 and TGF-β production by Tregs was also increased in burn patients compared to healthy controls(200). Progressive immunosuppression is observed after trauma, and it is well-established that Tregs play a key role in depressing immune function, including reducing production of IFNγ by Th1 cells following injury(201). Tregs isolated lymph nodes draining the injured areas from burn injured animals are found to show markers of T cell receptor (TCR) activation after injury, including phosphorylation of ZAP70 and nuclear factor of activated T cells (NFAT)(202, 203). Increased Treg activity has also been noted in trauma patients and correlates with poor clinical outcomes(200, 201). Similarly, studies have shown that depletion of Tregs prior to injury restores the proliferative capacity of CD4 T cells following burn injury in mice(204). In addition, Th17 cells have been identified as crucial players after burn injury. At the injury site itself, Th17 cytokines such as IL-17 and IL-22 are elevated 3h post-injury(205). While IL-17 is considered inflammatory and can aid in the recruitment of neutrophils, IL-22 promotes integrity of mucosal barriers, such as in the lungs or GI tract. IL-22 acts to promote tight junction formation and proliferation of intestinal epithelial cells, which is integral to maintaining proper barrier function after burn injury and preventing multiple organ failure or sepsis from bacteria originating from the gut(205).
8. INFLAMMATORY RESPONSE IN THE GI TRACT
Some of the most dire consequences of burn injury are the result of remote organ injury, such as inflammation in the lungs, kidney, gastrointestinal system, and bone marrow(47, 186, 206-210). While systemic inflammatory responses account for some of the organ dysfunction observed, it is suggested that one of the main drivers of inflammation and perturbed immune responses following burn injury is the translocation of gut bacteria and/or bacterial products from the GI lumen to systemic circulation. The gut-origin hypothesis of sepsis, SIRS, and multiple organ dysfunction after burn injury states that breakdown of the gut barrier and hyperpermeability leads to the transport of toxic agents derived from gut bacteria into the portal circulation and mesenteric lymph(211). Toxic agents can include bacterial products like peptidoglycan or endotoxin, which can travel to distant organs to cause inflammation and organ failure. In support of this hypothesis, animal models of trauma have found that ligation of the mesenteric lymph duct can prevent acute lung and renal injury following intraperitoneal injection of LPS and models of hemorrhagic shock in rodents(212-214). Similarly, mice given prophylactic antibiotics prior to burn injury reduced hepatosteatosis and liver injury markers(215). However, further studies must be done on burn injury to connect microbial dysbiosis in the gut to end organ dysfunction.
The gut microbiome is a crucial regulator of the immune system under homeostatic conditions, and imbalances, such as during burn injury, can have serious consequences on the function of the immune system. As stated previously, it is well-established that a healthy gut microbiome is crucial to the development of a healthy and robust immune response. Studies on germ-free mice have shown that the microbiome is necessary for appropriate development of mucosal immunity. Similarly, peripheral lymphoid organs, such as mesenteric lymph nodes and Peyer’s patches are underdeveloped in germfree mice. Abnormal IgA and T cell responses are also observed(216, 217). A study of healthy volunteers found that the composition of their microbiome is directly correlated to their cytokine response as measured by stimulation of their peripheral blood mononuclear cells (PBMCs), indicating that differences in alpha or beta diversity correlate directly with their immune response(218). Other studies have indicated that dysbiosis can lead to altered response in specific immune cells. For example, mononuclear cells isolated from patients following antibiotic usage and diminished microbial diversity produced significantly decreased levels of TNFα following LPS stimulation(219). In a model of acute kidney injury following ischemia and reperfusion injury, supplementation of bacteria metabolites acetate, propionate, and butyrate reduced organ damage and inhibited the maturation of DCs, as well as inhibiting proliferation of T cells(220). In mouse models of sepsis, fecal microbial transplantation (FMT) from healthy littermates improved survival by 70%(221). In a similar study, mice receiving FMT from septic patients followed by cecal ligation and puncture (CLP) to induce sepsis had more severe liver damage compared to those receiving FMT from healthy donors(222). However, the contribution of the microbiome to immunological abnormalities following burn injury is not well-understood and lacking in research.
9. THERAPEUTIC POTENTIAL OF MANIPULATING THE GUT MICROBIOME
Over the years, manipulation of the microbiome has proven a promising therapeutic for trauma patients. The earliest studies of critically ill patients found that complications including pneumonia, urinary tract infections and sepsis were highly associated with enteric pathogens(223). Following this, studies were conducted to identify the benefit of antibiotic administration in critically ill patients. The resulting regiment of systemic and enteric antibiotics was proposed as selective digestive decontamination (SDD), which would go on to be studied in a variety of critically ill patient populations(223). A systematic review of studies involving the use of SDD and non-absorbable enteral antibiotics in severe burn patients showed reduced incidence of bloodstream Enterobacteriaceae, reduced incidence of pneumonia and overall improved survival of severe burn patients(224). However, widespread and long-term use of antibiotics can contribute to the propagation of antibiotic resistance and chronic disruption of the gut microbiota(55, 56). Further investigation into the complications associated with antibiotic use are required to delineate the impact their use may have on patients suffering from traumatic injuries.
More recent studies attempt to manipulate the microbiome using more targeted methods than general antibiotic use. As an alternative to removing bacterial populations from the gut, several researchers are studying the impact of fecal microbiota transplant and probiotic administration. FMT involves transfer of fecal matter from a healthy individual into the intestinal tract of another. This process attempts to cultivate a healthy microbiota in the patient that supports microbiome stabilization and gut function. After gaining increased prominence for its successful treatment of Clostridium difficile infections in hospitals, FMT is being assessed experimentally as a treatment for a variety of other gastrointestinal disorders(225). Probiotics utilize the specific administration of a few beneficial bacterial species to bolster commensal populations and promote healthy gut functions. Although many preliminary studies have been limited to mouse models, several indicate that FMT and probiotics are promising therapeutic options for severe burn patients(226). A recent study by Kuethe et al. utilized a mouse model of severe burn injury in which intestinal permeability and bacterial dysbiosis are evident at six days post burn. FMT was prepared from the cecal contents of control mice and administered via oral gavage twice daily on post-burn day two and three. They found that FMT restored mucosal integrity and reduced bacterial dysbiosis evaluated on post-burn day six(140). Their study indicates that FMT may be a viable therapeutic for severe burn injury, although further studies are required to assess its relevance in human patients.
Bifidobacterium are important butyrate producing bacteria that have been shown to promote gastrointestinal health in a variety of human studies, including alleviating symptoms of IBS and antibiotic associated diarrhea(227). As mentioned previously, Bifidobacterium are among the SCFA producing bacterial species that are significantly reduced in severe burn patients(48). Although there are currently no comprehensive human studies in the use of Bifidobacterium in severe burn patients, a mouse study by Wang et al. reveals that administration of Bifidobacteria after burn injury reduced intestinal mucosa damage and bacterial translocation(228). Utilizing a mouse model, Zhang et al. identified significantly reduced abundance of the butyrate producing bacteria Clostridium butyricum (C. butyricum) following burn injury, which correlated with reduced fecal butyrate and increased intestinal permeability. Oral administration of C. butyricum 24 hours after burn injury enhanced butyrate levels and reduced intestinal damage and permeability(229). In addition to the administration of butyrate producing bacteria, treatment of mice with sodium butyrate also protects against severe burn induced intestinal permeability and promotes the expression of intestinal epithelial cell tight junction proteins(146). Additionally, sodium butyrate treatment reduced acute lung injury and systemic inflammation after severe burn in a rat model(230). Therefore, the therapeutic targeting of butyrate production via probiotic administration appears to be a promising area of research in severe burn injury. However, it is important to note that not all bacterial strains and treatment regimens have been found to provide positive effects. In a human study of severe burn patients, prophylactic administration of Lactobacillus acidophilus and Lactobacillus rhamnosus was not associated with improved patient outcomes(231). Consequently, additional research is required to fully understand the potential of targeting the microbiome in therapeutics for burn injury and the mechanisms underlying the impact individual bacteria have on gut barrier function.
10. FUTURE DIRECTIONS AND PERSPECTIVES
Altogether, there is much that is still unknown about the contribution of the microbiome and microbial changes in the context of burn injury. Recent studies are elucidating the changes observed in the gastrointestinal microbial population in acute traumatic injuries, however, their effect on the subsequent immune response and recovery from burn injury are still understudied. Although dysbiosis, overgrowth of potentially harmful bacteria, breakdown in the intestinal barrier, and aberrant immune responses have been documented following burn injury, the causal relationship between these components requires further investigation and study. Current studies indicate that the reservoir of gastrointestinal microbes is a contributor to end organ damage across the body, including the lungs, liver, and kidneys. Recent work in inflammatory disease states have suggested that pre- and probiotics may be an avenue for further exploration in treating acute trauma patients. Similarly, beneficial bacterial SCFAs may be another area of potential therapeutic development. Studies of the microbiome in the context of acute trauma, such as burn injury, are likely to provide a rich source of potential therapeutic targets or agents. In addition, more studies should be conducted to understand mechanistically how the microbiome contributes to immune responses and dysfunction after burn injury.
Acknowledgments
The authors acknowledge the support from the National Institutes of Health R01 AA015731, R01 GM128242, and T32 AA013527; F30 DK123929.
Footnotes
Conflict of Interest Disclosure
The authors have no conflicts of interest to declare
References
- 1.Association AB: Burn Incidence and Treatment in the United States: 2016. Burn Incidence Fact Sheet. [Google Scholar]
- 2.WHO: WHO ∣ Burns. Who, 2018. [Google Scholar]
- 3.Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S: Burn injury. Nat Rev Dis Prim 6(1):11, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rae L, Fidler P, Gibran N: The Physiologic Basis of Burn Shock and the Need for Aggressive Fluid Resuscitation. Crit Care Clin 32(4):491–505, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Lorente JA, Ezpeleta A, Esteban A, Gordo F, de la Cal MA, Díaz C, Arévalo JM, Tejedor C, Pascual T: Systemic hemodynamics, gastric intramucosal Pco2 changes, and outcome in critically ill burn patients. Crit Care Med 28(6):1728–1735, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Santaniello JM, Luchette FA, Esposito TJ, Gunawan H, Reed RL, Davis KA, Gamelli RL: Ten Year Experience of Burn, Trauma, and Combined Burn/Trauma Injuries Comparing Outcomes. J Trauma Inj Infect Crit Care 57(4):696–701, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Grigorian A, Nahmias J, Schubl S, Gabriel V, Bernal N, Joe V: Rising mortality in patients with combined burn and trauma. Burns 44(8):1989–1996, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Pham TN, Cancio LC, Gibran NS: American Burn Association Practice Guidelines Burn Shock Resuscitation. J Burn Care Res 29(1):257–266, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Cancio LC, Salinas J, Kramer GC: Protocolized Resuscitation of Burn Patients. Crit Care Clin 32(4):599–610, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Stander M, Wallis LA: The Emergency Management and Treatment of Severe Burns. Emerg Med Int, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tejiram S, Romanowski KS, Palmieri TL: Initial management of severe burn injury. Curr Opin Crit Care 25(6):647–652, 2019. [DOI] [PubMed] [Google Scholar]
- 12.Clark AT, Li X, Kulangara R, Adams-Huet B, Huen SC, Madni TD, Imran JB, Phelan HA, Arnoldo BD, Moe OW et al. : A Cohort Study From the Parkland Burn Intensive Care Unit. J Burn Care Res 40(1):72–78, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pruitt BA: Protection from Excessive Resuscitation: “Pushing the Pendulum Back.” J Trauma Inj Infect Crit Care 49(3):567–568, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Todd SR, Malinoski D, Muller PJ, Schreiber MA: Lactated Ringer's is Superior to Normal Saline in the Resuscitation of Uncontrolled Hemorrhagic Shock. J Trauma Inj Infect Crit Care 62(3):636–639, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Rhee P, Burris D, Kaufmann C, Pikoulis M, Austin B, Ling G, Harviel D, Waxman K: Lactated Ringer’s Solution Resuscitation Causes Neutrophil Activation after Hemorrhagic Shock. J Trauma Inj Infect Crit Care 44(2):313–319, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Haberal M, Abali AE, Karakayali H: Fluid management in major burn injuries. Indian J Plast Surg 43(3):29, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurney JM, Kozar RA, Cancio LC: Plasma for burn shock resuscitation: is it time to go back to the future? Transfusion 59(S2):1578–1586, 2019. [DOI] [PubMed] [Google Scholar]
- 18.Vigiola Cruz M, Carney BC, Luker JN, Monger KW, Vazquez JS, Moffatt LT, Johnson LS, Shupp JW: Plasma Ameliorates Endothelial Dysfunction in Burn Injury. J Surg Res 233:459–466, 2019. [DOI] [PubMed] [Google Scholar]
- 19.McIntyre MK, Winkler CJ, Gómez BI, Lapierre JP, Little JS, Dubick MA, Nicholson SE, Burmeister DM: The Effect of Burn Resuscitation Volumes on the Gut Microbiome in a Swine Model. Shock, 2020. [DOI] [PubMed] [Google Scholar]
- 20.Muraoka WT, Granados JC, Gomez BI, Nicholson SE, Chung KK, Shupp JW, Bynum JA, Dubick MA, Burmeister DM: Burn resuscitation strategy influences the gut microbiota-liver axis in swine. Sci Rep 10(1):15655, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finnerty CC, Przkora R, Herndon DN, Jeschke MG: Cytokine expression profile over time in burned mice. Cytokine 45(1):20–25, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DMM, Rocha AMC, Jeschke MG: Cytokine expression profile over time in severely burned pediatric patients. Shock 26(1):13–19, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Horton JW: Free radicals and lipid peroxidation mediated injury in burn trauma: the role of antioxidant therapy. Toxicology 189(1–2):75–88, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Hatherill JR, Till GO, Bruner LH, Ward PA: Thermal injury, intravascular hemolysis, and toxic oxygen products. J Clin Invest 78(3):629–636, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward PA, till GO: Pathophysiologic Events Related to Thermal Injury of Skin. J Trauma Inj Infect Crit Care 30:75–79, 1990. [DOI] [PubMed] [Google Scholar]
- 26.Till GO, Guilds LS, Mahrougui M, Friedl HP, Trentz O, Ward PA: Role of xanthine oxidase in thermal injury of skin. Am J Pathol 135(1):195–202, 1989. [PMC free article] [PubMed] [Google Scholar]
- 27.Kabasakal L, Şener G, Çetinel Ş, Contuk G, Gedik N, Yeğen BÇ: Burn-induced oxidative injury of the gut is ameliorated by the leukotriene receptor blocker montelukast. Prostaglandins, Leukot Essent Fat Acids 72(6):431–440, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Akhtar S, Li X, Chaudry IH, Choudhry MA: Neutrophil chemokines and their role in IL-18-mediated increase in neutrophil O 2 – production and intestinal edema following alcohol intoxication and burn injury. Am J Physiol Liver Physiol 297(2):G340–G347, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Kovacs EJ, Schwacha MG, Chaudry IH, Choudhry MA: Acute alcohol intoxication increases interleukin-18-mediated neutrophil infiltration and lung inflammation following burn injury in rats. Am J Physiol Cell Mol Physiol 292(5):L1193–L1201, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Curtis BJ, Shults JA, Boe DM, Ramirez L, Kovacs EJ: Mesenchymal stem cell treatment attenuates liver and lung inflammation after ethanol intoxication and burn injury. Alcohol 80:139–148, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rehou S, Shahrokhi S, Natanson R, Stanojcic M, Jeschke MG: Antioxidant and Trace Element Supplementation Reduce the Inflammatory Response in Critically Ill Burn Patients. J Burn Care Res 39(1):1–9, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizzo JA, Rowan MP, Driscoll IR, Chung KK, Friedman BC: Vitamin C in Burn Resuscitation. Crit Care Clin 32(4):539–546, 2016. [DOI] [PubMed] [Google Scholar]
- 33.Sood RF, Gibran NS, Arnoldo BD, Gamelli RL, Herndon DN, Tompkins RG: Early leukocyte gene expression associated with age, burn size, and inhalation injury in severely burned adults. J Trauma Acute Care Surg 80(2):250–257, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM et al. : The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315(8):801, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deitch EA, Berg R: Bacterial Translocation from the Gut. J Burn Care Rehabil 8(6):475–482, 1987. [PubMed] [Google Scholar]
- 36.Feng Y, Huang Y, Wang Y, Wang P, Wang F: Severe burn injury alters intestinal microbiota composition and impairs intestinal barrier in mice. Burn trauma 7:20, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao Y-M, Yu Y, Sheng (C.Y. Sheng) Z-Y, Tian H-M, Wang Y-P, Lu L-R, Yu Y: Role of gut-derived endotoxaemia and bacterial translocation in rats after thermal injury: effects of selective decontamination of the digestive tract. Burns 21(8):580–585, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Magnotti LJ: Gut-Derived Mesenteric Lymph. Arch Surg 134(12):1333, 1999. [PubMed] [Google Scholar]
- 39.Herndon DN, Zeigler ST: Bacterial translocation after thermal injury. Crit Care Med 21(Supplement):S50–54, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Huang H-H, Lee Y, Chen C-Y: Effects of burns on gut motor and mucosa functions. Neuropeptides 72:47–57, 2018. [DOI] [PubMed] [Google Scholar]
- 41.Oliveira HM, Sallam HS, Espana-Tenorio J, Chinkes D, Chung DH, Chen JDZ, Herndon DN: Gastric and small bowel ileus after severe burn in rats: The effect of cyclooxygenase-2 inhibitors. Burns 35(8):1180–1184, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y, Jiang Z, Sun Y, Wang X, Ma E, Wilmore D: The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns: a randomized, double-blind, controlled clinical trial. J Parenter Enter Nutr 27(4):241–245, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Sallam HS, Oliveira HM, Gan HT, Herndon DN, Chen JDZ: Ghrelin improves burn-induced delayed gastrointestinal transit in rats. Am J Physiol Integr Comp Physiol 292(1):R253–R257, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Ryan CM, Yarmush ML, Burke JF, Tompkins RG: Increased gut permeability early after burns correlates with the extent of burn injury. Crit Care Med 20(11):1508–1512, 1992. [DOI] [PubMed] [Google Scholar]
- 45.Baron P, Traber LD, Traber DL, Nguyen T, Hollyoak M, Heggers JP, Herndon DN: Gut Failure and Translocation Following Burn and Sepsis. J Surg Res 57(1):197–204, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Mosier MJ, Pham TN, Klein MB, Gibran NS, Arnoldo BD, Gamelli RL, Tompkins RG, Herndon DN: Early Enteral Nutrition in Burns: Compliance With Guidelines and Associated Outcomes in a Multicenter Study. J Burn Care Res 32(1):104–109, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Earley ZM, Akhtar S, Green SJ, Naqib A, Khan O, Cannon AR, Hammer AM, Morris NL, Li X, Eberhardt JM et al. : Burn Injury Alters the Intestinal Microbiome and Increases Gut Permeability and Bacterial Translocation. PLoS One 10(7):e0129996, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimizu K, Ogura H, Asahara T, Nomoto K, Matsushima A, Hayakawa K, Ikegawa H, Tasaki O, Kuwagata Y, Shimazu T: Gut microbiota and environment in patients with major burns – A preliminary report. Burns 41(3):e28–e33, 2015. [DOI] [PubMed] [Google Scholar]
- 49.Beckmann N, Pugh AM, Caldwell CC: Burn injury alters the intestinal microbiome’s taxonomic composition and functional gene expression. PLoS One 13(10):e0205307, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howard BM, Kornblith LZ, Christie SA, Conroy AS, Nelson MF, Campion EM, Callcut RA, Calfee CS, Lamere BJ, Fadrosh DW et al. : Characterizing the gut microbiome in trauma: significant changes in microbial diversity occur early after severe injury. Trauma Surg Acute Care Open 2(1):e000108, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma A, Jamal MM: Opioid Induced Bowel Disease: a Twenty-first Century Physicians’ Dilemma. Curr Gastroenterol Rep 15(7):334, 2013. [DOI] [PubMed] [Google Scholar]
- 52.Stein K, Hieggelke L, Schneiker B, Lysson M, Stoffels B, Nuding S, Wehkamp J, Kikhney J, Moter A, Kalff JC et al. : Intestinal manipulation affects mucosal antimicrobial defense in a mouse model of postoperative ileus. PLoS One 13(4):e0195516, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krishnan P, Frew Q, Green A, Martin R, Dziewulski P: Cause of death and correlation with autopsy findings in burns patients. Burns 39(4):583–588, 2013. [DOI] [PubMed] [Google Scholar]
- 54.Alp E, Coruh A, Gunay GK, Yontar Y, Doganay M: Risk Factors for Nosocomial Infection and Mortality in Burn Patients. J Burn Care Res 33(3):379–385, 2012. [DOI] [PubMed] [Google Scholar]
- 55.Feng Y, Huang Y, Wang Y, Wang P, Song H, Wang F: Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS One 14(6):e0218384, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deitch EA, Maejima K, Berg R: Effect of Oral Antibiotics and Bacterial Overgrowth on the Translocation of the GI Tract Microflora in Burned Rats. J Trauma Inj Infect Crit Care 25(5):385–392, 1985. [DOI] [PubMed] [Google Scholar]
- 57.Xu L, Surathu A, Raplee I, Chockalingam A, Stewart S, Walker L, Sacks L, Patel V, Li Z, Rouse R: The effect of antibiotics on the gut microbiome: a metagenomics analysis of microbial shift and gut antibiotic resistance in antibiotic treated mice. BMC Genomics 21(1):263, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hagan T, Cortese M, Rouphael N, Boudreau C, Linde C, Maddur MS, Das J, Wang H, Guthmiller J, Zheng N-Y et al. : Antibiotics-Driven Gut Microbiome Perturbation Alters Immunity to Vaccines in Humans. Cell 178(6):1313–1328.e13, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Connor KM, Lucking EF, Golubeva AV, Strain CR, Fouhy F, Cenit MC, Dhaliwal P, Bastiaanssen TFS, Burns DP, Stanton C et al. : Manipulation of gut microbiota blunts the ventilatory response to hypercapnia in adult rats. EBioMedicine 44:618–638, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de La Cal MA, Cerdá E, García-Hierro P, van Saene HKF, Gómez-Santos D, Negro E, Lorente JA: Survival Benefit in Critically Ill Burned Patients Receiving Selective Decontamination of the Digestive Tract. Ann Surg 241(3):424–430, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J, Zhu L, Xu M, Han J, Bai X, Yang X, Zhu H, Xu J, Zhang X, Gong Y et al. : Selective decontamination of the digestive tract ameliorates severe burn-induced insulin resistance in rats. Burns 41(5):1076–1085, 2015. [DOI] [PubMed] [Google Scholar]
- 62.Aboelatta YA, Abd-Elsalam AM, Omar AH, Abdelaal MM, Farid AM: Selective digestive decontamination (SDD) as a tool in the management of bacterial translocation following major burns. Ann Burns Fire Disasters 26(4):182–8, 2013. [PMC free article] [PubMed] [Google Scholar]
- 63.Hadjizacharia P, O’Keeffe T, Plurad DS, Green DJ, Brown CVR, Chan LS, Demetriades D, Rhee P: Alcohol exposure and outcomes in trauma patients. Eur J Trauma Emerg Surg 37(2):169–175, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith G, Branas C, Miller T: Fatal Nontraffic Injuries Involving Alcohol: A Metaanalysis. Ann Emerg Med 33(6):659–668, 1999. [PubMed] [Google Scholar]
- 65.Silver GM, Albright JM, Schermer CR, Halerz M, Conrad P, Ackerman PD, Lau L, Emanuele MA, Kovacs EJ, Gamelli RL: Adverse clinical outcomes associated with elevated blood alcohol levels at the time of burn injury. J Burn Care Res, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mokdad AH: Actual Causes of Death in the United States, 2000. JAMA 291(10):1238, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Bode JC, Bode C, Heidelbach R, Dürr HK, Martini GA: Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology 31(1):30–4, 1984. [PubMed] [Google Scholar]
- 68.Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A: Colonic microbiome is altered in alcoholism. Am J Physiol Liver Physiol 302(9):G966–G978, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P: Intestinal Dysbiosis: A Possible Mechanism of Alcohol-Induced Endotoxemia and Alcoholic Steatohepatitis in Rats. Alcohol Clin Exp Res 33(10):1836–1846, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor AN, Tio DL, Heng NS, Yirmiya R: Alcohol Consumption Attenuates Febrile Responses to Lipopolysaccharide and Interleukin-1beta in Male Rats. Alcohol Clin Exp Res 26(1):44–52, 2002. [PubMed] [Google Scholar]
- 71.Hoyt LR, Ather JL, Randall MJ, DePuccio DP, Landry CC, Wewers MD, Gavrilin MA, Poynter ME: Ethanol and Other Short-Chain Alcohols Inhibit NLRP3 Inflammasome Activation through Protein Tyrosine Phosphatase Stimulation. J Immunol 197(4):1322–1334, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doremus-Fitzwater TL, Gano A, Paniccia JE, Deak T: Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiol Behav 148:131–44, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen MM, O’Halloran EB, Shults JA, Kovacs EJ: Kupffer Cell p38 Mitogen-Activated Protein Kinase Signaling Drives Postburn Hepatic Damage and Pulmonary Inflammation When Alcohol Intoxication Precedes Burn Injury. Crit Care Med 44(10):e973–e979, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen MM, Palmer JL, Ippolito JA, Curtis BJ, Choudhry MA, Kovacs EJ: Intoxication by Intraperitoneal Injection or Oral Gavage Equally Potentiates Postburn Organ Damage and Inflammation. Mediators Inflamm 2013:1–10, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rendon JL, Li X, Akhtar S, Choudhry MA: Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury. Shock, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hammer AM, Morris NL, Cannon AR, Khan OM, Gagnon RC, Movtchan NV, van Langeveld I, Li X, Gao B, Choudhry MA: Interleukin-22 Prevents Microbial Dysbiosis and Promotes Intestinal Barrier Regeneration Following Acute Injury. Shock, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shults JA, Curtis BJ, Chen MM, O’Halloran EB, Ramirez L, Kovacs EJ: Impaired respiratory function and heightened pulmonary inflammation in episodic binge ethanol intoxication and burn injury. Alcohol 49(7):713–720, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ortman JM, Velkoff V a., Hogan H: An aging nation: The older population in the United States. Econ Stat Adm US Dep Commer, 2014. [Google Scholar]
- 79.Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G: Inflamm-aging: An Evolutionary Perspective on Immunosenescence. Ann N Y Acad Sci 908(1):244–254, 2006. [DOI] [PubMed] [Google Scholar]
- 80.Singh T, Newman AB: Inflammatory markers in population studies of aging. Ageing Res Rev 10(3):319–329, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boyd AR, Orihuela CJ: Dysregulated inflammation as a risk factor for pneumonia in the elderly. Aging Dis 2(6):487–500, 2011. [PMC free article] [PubMed] [Google Scholar]
- 82.Hearps AC, Martin GE, Angelovich TA, Cheng W-J, Maisa A, Landay AL, Jaworowski A, Crowe SM: Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 11(5):867–875, 2012. [DOI] [PubMed] [Google Scholar]
- 83.Brubaker AL, Rendon JL, Ramirez L, Choudhry MA, Kovacs EJ: Reduced Neutrophil Chemotaxis and Infiltration Contributes to Delayed Resolution of Cutaneous Wound Infection with Advanced Age. J Immunol 190(4):1746–1757, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stanojcic M, Chen P, Xiu F, Jeschke MG: Impaired Immune Response in Elderly Burn Patients. Ann Surg 264(1):195–202, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gould L, Abadir P, Brem H, Carter M, Conner-Kerr T, Davidson J, DiPietro L, Falanga V, Fife C, Gardner S et al. : Chronic wound repair and healing in older adults: Current status and future research. Wound Repair Regen 23(1):1–13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G et al. : Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci 108(Supplement_1):4586–4591, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao J, Abe F, Osawa R: Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol 16(1):90, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wheatley EG, Curtis BJ, Hulsebus HJ, Boe DM, Najarro K, Ir D, Robertson CE, Choudhry MA, Frank DN, Kovacs EJ: Advanced Age Impairs Intestinal Antimicrobial Peptide Response and Worsens Fecal Microbiome Dysbiosis Following Burn Injury in Mice. SHOCK 53(1):71–77, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fransen F, van Beek AA, Borghuis T, Aidy S El, Hugenholtz F, van der Gaast-de Jongh C, Savelkoul HFJ, De Jonge MI, Boekschoten MV, Smidt H et al. : Aged Gut Microbiota Contributes to Systemical Inflammaging after Transfer to Germ-Free Mice. Front Immunol 8:1385, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jeschke MG, Pinto R, Costford SR, Amini-Nik S: Threshold age and burn size associated with poor outcomes in the elderly after burn injury. Burns 42(2):276–281, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Verthelyi D: Sex hormones as immunomodulators in health and disease. Int Immunopharmacol 1(6):983–993, 2001. [DOI] [PubMed] [Google Scholar]
- 92.Bird MD, Karavitis J, Kovacs EJ: Sex differences and estrogen modulation of the cellular immune response after injury. Cell Immunol 252(1–2):57–67, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lockshin MD: Sex differences in autoimmune disease. Lupus 15(11):753–756, 2006. [DOI] [PubMed] [Google Scholar]
- 94.Bösch F, Angele MK, Chaudry IH: Gender differences in trauma, shock and sepsis. Mil Med Res 5(1):35, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Angele MK, Pratschke S, Hubbard WJ, Chaudry IH: Gender differences in sepsis. Virulence 5(1):12–19, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Choudhry MA, Schwacha MG, Hubbard WJ, Kerby JD, Rue LW, Bland KI, Chaudry IH: Gender differences in acute response to trauma-hemorrhage. Shock 24(Supplement 1):101–106, 2005. [DOI] [PubMed] [Google Scholar]
- 97.Ananthakrishnan P, Cohen DB, Xu DZ, Lu Q, Feketeova E, Deitch EA: Sex hormones modulate distant organ injury in both a trauma/hemorrhagic shock model and a burn model. Surgery 137(1):56–65, 2005. [DOI] [PubMed] [Google Scholar]
- 98.Deitch EA, Ananthakrishnan P, Cohen DB, Xu DZ, Feketeova E, Hauser CJ: Neutrophil activation is modulated by sex hormones after trauma-hemorrhagic shock and burn injuries. Am J Physiol Circ Physiol 291(3):H1456–H1465, 2006. [DOI] [PubMed] [Google Scholar]
- 99.Croce MA, Fabian TC, Malhotra AK, Bee TK, Miller PR: Does Gender Difference Influence Outcome? J Trauma Inj Infect Crit Care 53(5):889–894, 2002. [DOI] [PubMed] [Google Scholar]
- 100.Kerby JD, McGwin G, George RL, Cross JA, Chaudry IH, Rue LW: Sex Differences in Mortality After Burn Injury: Results of Analysis of the National Burn Repository of the American Burn Association. J Burn Care Res 27(4):452–456, 2006. [DOI] [PubMed] [Google Scholar]
- 101.Berg RD: The indigenous gastrointestinal microflora. Trends Microbiol 4(11):430–435, 1996. [DOI] [PubMed] [Google Scholar]
- 102.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D: Role of the normal gut microbiota. World J Gastroenterol 21(29):8787–8803, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR: Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 104(34):13780–13785, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA: Diversity of the human intestinal microbial flora. Science (80- ) 308(5728):1635–1638, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ley RE, Turnbaugh PJ, Klein S, Gordon JI: Microbial ecology: human gut microbes associated with obesity. Nature 444(7122):1022–1023, 2006. [DOI] [PubMed] [Google Scholar]
- 106.Consortium HMP: Structure, function and diversity of the healthy human microbiome. Nature 486(7402):207–214, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vasapolli R, Schütte K, Schulz C, Vital M, Schomburg D, Pieper DH, Vilchez-Vargas R, Malfertheiner P: Analysis of Transcriptionally Active Bacteria Throughout the Gastrointestinal Tract of Healthy Individuals. Gastroenterology 157(4):1081–1092.e3, 2019. [DOI] [PubMed] [Google Scholar]
- 108.Ruan W, Engevik MA, Spinler JK, Versalovic J: Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration. Dig Dis Sci 65(3):695–705, 2020. [DOI] [PubMed] [Google Scholar]
- 109.Cox MJ, Cookson WO, Moffatt MF: Sequencing the human microbiome in health and disease. Hum Mol Genet 22(R1):R88–94, 2013. [DOI] [PubMed] [Google Scholar]
- 110.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM et al. : Enterotypes of the human gut microbiome. Nature 473(7346):174–180, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S et al. : Richness of human gut microbiome correlates with metabolic markers. Nature 500(7464):541–546, 2013. [DOI] [PubMed] [Google Scholar]
- 112.Shanahan F: The host-microbe interface within the gut. Best Pr Res Clin Gastroenterol 16(6):915–931, 2002. [DOI] [PubMed] [Google Scholar]
- 113.Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G et al. : Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535(7612):376–381, 2016. [DOI] [PubMed] [Google Scholar]
- 114.Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG: Minireview: Gut microbiota: the neglected endocrine organ. Mol Endocrinol 28(8):1221–1238, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI: Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3(4):213–223, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bergman EN: Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev 70(2):567–590, 1990. [DOI] [PubMed] [Google Scholar]
- 117.Roediger WE: Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 83(2):424–429, 1982. [PubMed] [Google Scholar]
- 118.Macfarlane GT, Macfarlane S: Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J Clin Gastroenterol 45 Suppl:S120–7, 2011. [DOI] [PubMed] [Google Scholar]
- 119.Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L: Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front Microbiol 7:979, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Louis P, Flint HJ: Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 294(1):1–8, 2009. [DOI] [PubMed] [Google Scholar]
- 121.Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA: Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol 10:277, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barko PC, McMichael MA, Swanson KS, Williams DA: The Gastrointestinal Microbiome: A Review. J Vet Intern Med 32(1):9–25, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peng L, Li ZR, Green RS, Holzman IR, Lin J: Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr 139(9):1619–1625, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sakata T: Stimulatory effect of short-chain fatty acids on epithelial cell proliferation in the rat intestine: a possible explanation for trophic effects of fermentable fibre, gut microbes and luminal trophic factors. Br J Nutr 58(1):95–103, 1987. [DOI] [PubMed] [Google Scholar]
- 125.Wu W, Sun M, Chen F, Cao AT, Liu H, Zhao Y, Huang X, Xiao Y, Yao S, Zhao Q et al. : Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol 10(4):946–956, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T et al. : Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504(7480):446–450, 2013. [DOI] [PubMed] [Google Scholar]
- 127.Kim CH, Park J, Kim M: Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw 14(6):277–288, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, Chen F, Xiao Y, Zhao Y, Yao S et al. : Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun 9(1):3555, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R: Diversity, stability and resilience of the human gut microbiota. Nature 489(7415):220–230, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dai Z, Zhang J, Wu Q, Chen J, Liu J, Wang L, Chen C, Xu J, Zhang H, Shi C et al. : The role of microbiota in the development of colorectal cancer. Int J Cancer 145(8):2032–2041, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gagnière J, Raisch J, Veziant J, Barnich N, Bonnet R, Buc E, Bringer MA, Pezet D, Bonnet M: Gut microbiota imbalance and colorectal cancer. World J Gastroenterol 22(2):501–518, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sánchez-Alcoholado L, Ramos-Molina B, Otero A, Laborda-Illanes A, Ordóñez R, Medina JA, Gómez-Millán J, Queipo-Ortuño MI: The Role of the Gut Microbiome in Colorectal Cancer Development and Therapy Response. Cancers (Basel) 12(6):1406, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Halfvarson J, Brislawn CJ, Lamendella R, Vázquez-Baeza Y, Walters WA, Bramer LM, D’Amato M, Bonfiglio F, McDonald D, Gonzalez A et al. : Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol 2:17004, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang X, Yang J, Tian F, Zhang L, Lei Q, Jiang T, Zhou J, Yuan S, Wang J, Feng Z et al. : Gut microbiota trajectory in patients with severe burn: A time series study. J Crit Care 42:310–316, 2017. [DOI] [PubMed] [Google Scholar]
- 135.MacFie J, O’Boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P: Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut 45(2):223–228, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hugenholtz F, de Vos WM: Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci 75(1):149–160, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nagpal R, Wang S, Solberg Woods LC, Seshie O, Chung ST, Shively CA, Register TC, Craft S, McClain DA, Yadav H: Comparative Microbiome Signatures and Short-Chain Fatty Acids in Mouse, Rat, Non-human Primate, and Human Feces. Front Microbiol 9:2897, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang G, Sun K, Yin S, Jiang B, Chen Y, Gong Y, Yang Z, Chen J, Yuan Z, Peng Y: Burn Injury Leads to Increase in Relative Abundance of Opportunistic Pathogens in the Rat Gastrointestinal Microbiome. Front Microbiol 8:1237, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rice TC, Armocida SM, Kuethe JW, Midura EF, Jain A, Hildeman DA, Healy DP, Gulbins E, Caldwell CC: Burn injury influences the T cell homeostasis in a butyrate-acid sphingomyelinase dependent manner. Cell Immunol 313:25–31, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kuethe JW, Armocida SM, Midura EF, Rice TC, Hildeman DA, Healy DP, Caldwell CC: Fecal Microbiota Transplant Restores Mucosal Integrity in a Murine Model of Burn Injury. Shock 45(6):647–652, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Muñoz-Tamayo R, Laroche B, Walter E, Doré J, Duncan SH, Flint HJ, Leclerc M: Kinetic modelling of lactate utilization and butyrate production by key human colonic bacterial species. FEMS Microbiol Ecol 76(3):615–624, 2011. [DOI] [PubMed] [Google Scholar]
- 142.Capaldo CT, Powell DN, Kalman D: Layered defense: how mucus and tight junctions seal the intestinal barrier. J Mol Med 95(9):927–934, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chelakkot C, Ghim J, Ryu SH: Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med 50(8):103, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Van Meerveld BG, Verne GN: Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 24(6):503–512, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.He W, Wang Y, Wang P, Wang F: Intestinal barrier dysfunction in severe burn injury. Burn Trauma 7:24, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liang JB, Wang P, Feng YH, Huang YL, Wang FJ, Ren H: Effects of sodium butyrate on intestinal barrier of severe scald mice and the related mechanism. Zhonghua Shao Shang Za Zhi 36(1):48–53, 2020. [DOI] [PubMed] [Google Scholar]
- 147.Deitch EA: Intestinal permeability is increased in burn patients shortly after injury. Br J Surg 77(5):587–592, 1990. [PubMed] [Google Scholar]
- 148.Jones WG, Minei JP, Barber AE, Rayburn JL, Fahey TJ, Shires GT: Bacterial translocation and intestinal atrophy after thermal injury and burn wound sepsis. Ann Surg 211(4):399–405, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gianotti L, Alexander JW, Pyles T, James L, Babcock GF: Relationship between extent of burn injury and magnitude of microbial translocation from the intestine. J Burn Care Rehabil 14(3):336–342, 1993. [DOI] [PubMed] [Google Scholar]
- 150.Martens EC, Neumann M, Desai MS: Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol 16(8):457–470, 2018. [DOI] [PubMed] [Google Scholar]
- 151.Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL: Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol 295(5):G1025–34, 2008. [DOI] [PubMed] [Google Scholar]
- 152.Anderson RC, Cookson AL, McNabb WC, Park Z, McCann MJ, Kelly WJ, Roy NC: Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol 10(1):316, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bansal T, Alaniz RC, Wood TK, Jayaraman A: The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci U S A 107(1):228–233, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T et al. : Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469(7331):543–547, 2011. [DOI] [PubMed] [Google Scholar]
- 155.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ: Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27(2):104–119, 2008. [DOI] [PubMed] [Google Scholar]
- 156.Shawki A, McCole DF: Mechanisms of Intestinal Epithelial Barrier Dysfunction by Adherent-Invasive. Cell Mol Gastroenterol Hepatol 3(1):41–50, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA: Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun 69(3):1329–1336, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Saitoh Y, Suzuki H, Tani K, Nishikawa K, Irie K, Ogura Y, Tamura A, Tsukita S, Fujiyoshi Y: Tight junctions. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science (80- ) 347(6223):775–778, 2015. [DOI] [PubMed] [Google Scholar]
- 159.Ogura H, Tanaka H, Koh T, Hashiguchi N, Kuwagata Y, Hosotsubo H, Shimazu T, Sugimoto H: Priming, Second-Hit Priming, and Apoptosis in Leukocytes from Trauma Patients. J Trauma Inj Infect Crit Care 46(5):774–783, 1999. [DOI] [PubMed] [Google Scholar]
- 160.Manson J, Thiemermann C, Brohi K: Trauma alarmins as activators of damage-induced inflammation. Br J Surg 99(S1):12–20, 2012. [DOI] [PubMed] [Google Scholar]
- 161.Paterson HM, Murphy TJ, Purcell EJ, Shelley O, Kriynovich SJ, Lien E, Mannick JA, Lederer JA: Injury Primes the Innate Immune System for Enhanced Toll-Like Receptor Reactivity. J Immunol 171(3):1473–1483, 2003. [DOI] [PubMed] [Google Scholar]
- 162.Osuka A, Ogura H, Ueyama M, Shimazu T, Lederer JA: Immune response to traumatic injury: harmony and discordance of immune system homeostasis. Acute Med Surg 1(2):63–69, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP et al. : A genomic storm in critically injured humans. J Exp Med 208(13):2581–2590, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mace JE, Park MS, Mora AG, Chung KK, Martini W, White CE, Holcomb JB, Merrill GA, Dubick MA, Wolf SE et al. : Differential expression of the immunoinflammatory response in trauma patients: Burn vs. non-burn. Burns 38(4):599–606, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, Suman OE, Mlcak RP, Herndon DN: Long-Term Persistance of the Pathophysiologic Response to Severe Burn Injury. PLoS One 6(7):e21245, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Valvis SM, Waithman J, Wood FM, Fear MW, Fear VS: The Immune Response to Skin Trauma Is Dependent on the Etiology of Injury in a Mouse Model of Burn and Excision. J Invest Dermatol 135(8):2119–2128, 2015. [DOI] [PubMed] [Google Scholar]
- 167.Li X, Xu Z, Pang X, Huang Y, Yang B, Yang Y, Chen K, Liu X, Mao P, Li Y: Interleukin-10/lymphocyte ratio predicts mortality in severe septic patients. PLoS One 12(6):e0179050, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Matera G, Puccio R, Giancotti A, Quirino A, Pulicari M, Zicca E, Caroleo S, Renzulli A, Liberto M, Focà A: Impact of interleukin-10, soluble CD25 and interferon-γ on the prognosis and early diagnosis of bacteremic systemic inflammatory response syndrome: a prospective observational study. Crit Care 17(2):R64, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gauglitz GG, Finnerty CC, Herndon DN, Mlcak RP, Jeschke MG: Are serum cytokines early predictors for the outcome of burn patients with inhalation injuries who do not survive? Crit Care 12(3):R81, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hur J, Yang HT, Chun W, Kim J-H, Shin S-H, Kang HJ, Kim HS: Inflammatory Cytokines and Their Prognostic Ability in Cases of Major Burn Injury. Ann Lab Med 35(1):105–110, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wong HR, Liu KD, Kangelaris KN, Lahni P, Calfee CS: Performance of interleukin-27 as a sepsis diagnostic biomarker in critically ill adults. J Crit Care 29(5):718–722, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wong HR, Lindsell CJ, Lahni P, Hart KW, Gibot S: Interleukin 27 as a Sepsis Diagnostic Biomarker in Critically Ill Adults. Shock 40(5):382–386, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hanna WJ, Berrens Z, Langner T, Lahni P, Wong HR: Interleukin-27: a novel biomarker in predicting bacterial infection among the critically ill. Crit Care 19(1):378, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Finnerty CC, Herndon DN, Chinkes DL, Jeschke MG: Serum cytokine differences in severely burned children with and without sepsis. Shock 27(1):4–9, 2007. [DOI] [PubMed] [Google Scholar]
- 175.Li X, Schwacha MG, Chaudry IH, Choudhry MA: Acute alcohol intoxication potentiates neutrophil-mediated intestinal tissue damage after burn injury. Shock 29(3):377–83, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dunn JLM, Kartchner LB, Gast K, Sessions M, Hunter RA, Thurlow L, Richardson A, Schoenfisch M, Cairns BA, Maile R: Mammalian target of rapamycin regulates a hyperresponsive state in pulmonary neutrophils late after burn injury. J Leukoc Biol 103(5):909–918, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sakuma M, Khan MAS, Yasuhara S, Martyn JA, Palaniyar N: Mechanism of pulmonary immunosuppression: extrapulmonary burn injury suppresses bacterial endotoxin–induced pulmonary neutrophil recruitment and neutrophil extracellular trap (NET) formation. FASEB J 33(12):13602–13616, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Butler KL, Ambravaneswaran V, Agrawal N, Bilodeau M, Toner M, Tompkins RG, Fagan S, Irimia D: Burn Injury Reduces Neutrophil Directional Migration Speed in Microfluidic Devices. PLoS One 5(7):e11921, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Akhtar S, Li X, Kovacs EJ, Gamelli RL, Choudhry MA: Interleukin-18 Delays Neutrophil Apoptosis following Alcohol Intoxication and Burn Injury. Mol Med 17(1–2):88–94, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schwab L, Goroncy L, Palaniyandi S, Gautam S, Triantafyllopoulou A, Mocsai A, Reichardt W, Karlsson FJ, Radhakrishnan SV, Hanke K et al. : Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nat Med 20(6):648–654, 2014. [DOI] [PubMed] [Google Scholar]
- 181.Molloy RG, O’Riordain M, Holzheimer R, Nestor M, Collins K, Mannick JA, Rodrick ML: Mechanism of increased tumor necrosis factor production after thermal injury. Altered sensitivity to PGE2 and immunomodulation with indomethacin. J Immunol 151(4):2142–9, 1993. [PubMed] [Google Scholar]
- 182.Fukushima R, Alexander JW, Wu JZ, Mao JX, Szczur K, Stephens AM, Ogle JD, Ogle CK: Time course of production of cytokines and prostaglandin E2 by macrophages isolated after thermal injury and bacterial translocation. Circ Shock 42(3):154–62, 1994. [PubMed] [Google Scholar]
- 183.Schwacha MG, Somers SD: Thermal injury-induced immunosuppression in mice: the role of macrophage-derived reactive nitrogen intermediates. J Leukoc Biol 63(1):51–58, 1998. [DOI] [PubMed] [Google Scholar]
- 184.Deitch EA: Multiple Organ Failure Pathophysiology and Potential Future Therapy. Ann Surg 216(2):117–134, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Luo G, Peng D, Zheng J, Chen X, Wu J, Elster E, Tadaki D: The role of NO in macrophage dysfunction at early stage after burn injury. Burns 31(2):138–144, 2005. [DOI] [PubMed] [Google Scholar]
- 186.Muthu K, He LK, Melstrom K, Szilagyi A, Gamelli RL, Shankar R: Perturbed Bone Marrow Monocyte Development Following Burn Injury and Sepsis Promote Hyporesponsive Monocytes. J Burn Care Res 29(1):12–21, 2008. [DOI] [PubMed] [Google Scholar]
- 187.Kupper TS, Green DR, Durum SK, Baker CC: Defective antigen presentation to a cloned T helper cell by macrophages from burned mice can be restored with interleukin-1. Surgery 98(2):199–206, 1985. [PubMed] [Google Scholar]
- 188.Shen H, de Almeida PE, Kang KH, Yao P, Chan CW: Burn Injury Triggered Dysfunction in Dendritic Cell Response to TLR9 Activation and Resulted in Skewed T Cell Functions. PLoS One 7(11):e50238, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fazal N: OX62+OX6+OX35+ rat dendritic cells are unable to prime CD4+ T cells for an effective immune response following acute burn injury. Results Immunol 3:64–72, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Patil NK, Bohannon JK, Luan L, Guo Y, Fensterheim B, Hernandez A, Wang J, Sherwood ER: Flt3 Ligand Treatment Attenuates T Cell Dysfunction and Improves Survival in a Murine Model of Burn Wound Sepsis. Shock 47(1):40–51, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Williams KN, Szilagyi A, He L-K, Conrad P, Halerz M, Gamelli RL, Shankar R, Muthumalaiappan K: Dendritic Cell Depletion in Burn Patients Is Regulated by MafB Expression. J Burn Care Res 33(6):747–758, 2012. [DOI] [PubMed] [Google Scholar]
- 192.Patenaude J, D’Elia M, Hamelin C, Garrel D, Bernier J: Burn injury induces a change in T cell homeostasis affecting preferentially CD4 + T cells. J Leukoc Biol 77(2):141–150, 2005. [DOI] [PubMed] [Google Scholar]
- 193.Duan X, Yarmush D, Leeder A, Yarmush ML, Mitchell RN: Burn-induced immunosuppression: attenuated T cell signaling independent of IFN-γ- and nitric oxide-mediated pathways. J Leukoc Biol 83(2):305–313, 2008. [DOI] [PubMed] [Google Scholar]
- 194.Choudhry MA, Messingham KAN, Namak S, Colantoni A, Fontanilla CV, Duffner LA, Sayeed MM, Kovacs EJ: Ethanol exacerbates T cell dysfunction after thermal injury. Alcohol 21(3):239–243, 2000. [DOI] [PubMed] [Google Scholar]
- 195.Choudhry MA, Ahmad S, Thompson KD, Sayeed MM: T-lymphocyte Ca2+ signalling and proliferative responses during sepsis. Shock 1(4):267–272, 1994. [DOI] [PubMed] [Google Scholar]
- 196.Kavanagh EG, Kelly JL, Lyons A, Soberg CC, Mannick JA, Lederer JA: Burn injury primes naive CD4+ T cells for an augmented T-helper 1 response. Surgery 124(2):269–277, 1998. [PubMed] [Google Scholar]
- 197.Teodorczyk-Injeyan JA, Sparkes BG, Mills GB, Peters WJ, Falk RE: Impairment of T cell activation in burn patients: a possible mechanism of thermal injury-induced immunosuppression. Clin Exp Immunol 65(3):570–81, 1986. [PMC free article] [PubMed] [Google Scholar]
- 198.Fazal N, Al-Ghoul WM: Thermal injury-plus-sepsis contributes to a substantial deletion of intestinal mesenteric lymph node CD4 T cell via apoptosis. Int J Biol Sci 3(6):393–401, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zedler S, Bone RC, Baue AE, Donnersmarck GH v., Faist E: T-cell reactivity and its predictive role in immunosuppression after burns. Crit Care Med 27(1):66–72, 1999. [DOI] [PubMed] [Google Scholar]
- 200.Huang L, Yao Y, Dong N, Yu Y, He L, Sheng Z: Association between regulatory T cell activity and sepsis and outcome of severely burned patients: a prospective, observational study. Crit Care 14(1):R3, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.MacConmara MP, Maung AA, Fujimi S, McKenna AM, Delisle A, Lapchak PH, Rogers S, Lederer JA, Mannick JA: Increased CD4+ CD25+ T Regulatory Cell Activity in Trauma Patients Depresses Protective Th1 Immunity. Trans . Meet Am Surg Assoc 124:179–188, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hanschen M, Tajima G, O’Leary F, Ikeda K, Lederer JA: Injury Induces Early Activation of T-Cell Receptor Signaling Pathways in CD4+ Regulatory T Cells. Shock 35(3):252–257, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Choudhry MA, Mao H, Haque F, Khan M, Fazal N, Sayeed MM: Role of NFAT and AP-1 in PGE2-Mediated T Cell Suppression in Burn Injury. Shock 18(3):212–216, 2002. [DOI] [PubMed] [Google Scholar]
- 204.MacConmara MP, Tajima G, O'Leary F, Delisle AJ, McKenna AM, Stallwood CG, Mannick JA, Lederer JA: Regulatory T cells suppress antigen-driven CD4 T cell reactivity following injury. J Leukoc Biol 89(1):137–147, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sasaki JR, Zhang Q, Schwacha MG: Burn induces a Th-17 inflammatory response at the injury site. Burns 37(4):646–651, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Davis CS, Albright JM, Carter SR, Ramirez L, Kim H, Gamelli RL, Kovacs EJ: Early Pulmonary Immune Hyporesponsiveness Is Associated With Mortality After Burn and Smoke Inhalation Injury. J Burn Care Res 33(1):26–35, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mosier MJ, Pham TN, Klein MB, Gibran NS, Arnoldo BD, Gamelli RL, Tompkins RG, Herndon DN: Early Acute Kidney Injury Predicts Progressive Renal Dysfunction and Higher Mortality in Severely Burned Adults. J Burn Care Res 31(1):83–92, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Davis CS, Janus SE, Mosier MJ, Carter SR, Gibbs JT, Ramirez L, Gamelli RL, Kovacs EJ: Inhalation Injury Severity and Systemic Immune Perturbations in Burned Adults. Ann Surg 257(6):1137–1146, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Epstein MD: Increased Gut Permeability Following Burn Trauma. Arch Surg 126(2):198, 1991. [DOI] [PubMed] [Google Scholar]
- 210.Cohen MJ, Carroll C, He L-K, Muthu K, Gamelli RL, Jones SB, Shankar R: Severity of Burn Injury and Sepsis Determines the Cytokine Responses of Bone Marrow Progenitor-Derived Macrophages. J Trauma Inj Infect Crit Care 62(4):858–867, 2007. [DOI] [PubMed] [Google Scholar]
- 211.Deitch EA: Gut-origin sepsis: Evolution of a concept. Surg 10(6):350–356, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sambol JT, Xu D-Z, Adams CA, Magnotti LJ, Deitch EA: Mesenteric lymph duct ligation provides long term protection against hemorrhagic shock-induced lung injury. Shock 14(3):416–420, 2000. [PubMed] [Google Scholar]
- 213.Niu C-Y, Zhao Z-G, Ye Y-L, Hou Y-L, Zhang Y-P: Mesenteric lymph duct ligation against renal injury in rats after hemorrhagic shock. Ren Fail 32(5):584–591, 2010. [DOI] [PubMed] [Google Scholar]
- 214.Watkins AC, Caputo FJ, Badami C, Barlos D, Xu DZ, Lu Q, Feketeova E, Deitch EA: Mesenteric Lymph Duct Ligation Attenuates Lung Injury and Neutrophil Activation After Intraperitoneal Injection of Endotoxin in Rats. J Trauma Inj Infect Crit Care 64(1):126–130, 2008. [DOI] [PubMed] [Google Scholar]
- 215.Chen MM, Zahs A, Brown MM, Ramirez L, Turner JR, Choudhry MA, Kovacs EJ: An alteration of the gut-liver axis drives pulmonary inflammation after intoxication and burn injury in mice. Am J Physiol Liver Physiol 307(7):G711–G718, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bauer H, Horowitz RE, Levenson SM, Popper H: The response of the lymphatic tissue to the microbial flora. Studies on germfree mice. Am J Pathol 42:471–83, 1963. [PMC free article] [PubMed] [Google Scholar]
- 217.Sommer F, Bäckhed F: The gut microbiota — masters of host development and physiology. Nat Rev Microbiol 11(4):227–238, 2013. [DOI] [PubMed] [Google Scholar]
- 218.Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, Jansen T, Jacobs L, Bonder MJ, Kurilshikov A et al. : Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell, 2016. [DOI] [PubMed] [Google Scholar]
- 219.Lankelma JM, Belzer C, Hoogendijk AJ, de Vos AF, de Vos WM, van der Poll T, Wiersinga JW: Antibiotic-Induced Gut Microbiota Disruption Decreases TNF-α Release by Mononuclear Cells in Healthy Adults. Clin Transl Gastroenterol 7(8):e186, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJF, de Almeida DC, Bassi EJ, Moraes-Vieira PM, Hiyane MI, Rodas ACD et al. : Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. J Am Soc Nephrol 26(8):1877–1888, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kim SM, DeFazio JR, Hyoju SK, Sangani K, Keskey R, Krezalek MA, Khodarev NN, Sangwan N, Christley S, Harris KG et al. : Fecal microbiota transplant rescues mice from human pathogen mediated sepsis by restoring systemic immunity. Nat Commun 11(1):2354, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu Z, Li N, Fang H, Chen X, Guo Y, Gong S, Niu M, Zhou H, Jiang Y, Chang P et al. : Enteric dysbiosis is associated with sepsis in patients. FASEB J 33(11):12299–12310, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Stoutenbeek CP, Van Saene HK, Miranda DR, Zandstra DF: A new technique of infection prevention in the intensive care unit by selective decontamination of the digestive tract. Acta Anaesthesiol Belg 34(3):209–221, 1983. [PubMed] [Google Scholar]
- 224.Rubio-Regidor M, Martín-Pellicer A, Silvestri L, van Saene HKF, Lorente JA, de la Cal MA: Digestive decontamination in burn patients: A systematic review of randomized clinical trials and observational studies. Burns 44(1):16–23, 2018. [DOI] [PubMed] [Google Scholar]
- 225.Ooijevaar RE, Terveer EM, Verspaget HW, Kuijper EJ, Keller JJ: Clinical Application and Potential of Fecal Microbiota Transplantation. Annu Rev Med 70:335–351, 2019. [DOI] [PubMed] [Google Scholar]
- 226.Corcione S, Lupia T, De Rosa FG, (ESCMID) H and MISG (ESGHAMI) of the ES of CM and ID: Microbiome in the setting of burn patients: implications for infections and clinical outcomes. Burn Trauma 8:tkaa033, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tojo R, Suárez A, Clemente MG, de los Reyes-Gavilán CG, Margolles A, Gueimonde M, Ruas-Madiedo P: Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol 20(41):15163–15176, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang Z, Xiao G, Yao Y, Guo S, Lu K, Sheng Z: The role of bifidobacteria in gut barrier function after thermal injury in rats. J Trauma 61(3):650–657, 2006. [DOI] [PubMed] [Google Scholar]
- 229.Zhang D, Zhu C, Fang Z, Zhang H, Yang J, Tao K, Hu D, Han J, Yang X: Remodeling gut microbiota by Clostridium butyricum (C.butyricum) attenuates intestinal injury in burned mice. Burns S0305-4179(19):30641–30642, 2020. [DOI] [PubMed] [Google Scholar]
- 230.Liang X, Wang RS, Wang F, Liu S, Guo F, Sun L, Wang YJ, Sun YX, Chen XL: Sodium butyrate protects against severe burn-induced remote acute lung injury in rats. PLoS One 8(7):e68786, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fleming D, Jiang Y, Opoku K, Alhaj Saleh A, Larumbe-Zabala E, Kesey JE, Griswold JA, Dissanaike S: Prophylactic Probiotics in Burn Patients: Risk versus Reward. J Burn Care Res 40(6):953–960, 2019. [DOI] [PubMed] [Google Scholar]