Abstract
Protein Kinase C-δ (PKCδ), regulates a broad group of biological functions and disease processes, including well-defined roles in immune function, cell survival and apoptosis. PKCδ primarily regulates apoptosis in normal tissues and non-transformed cells, and genetic disruption of the PRKCD gene in mice is protective in many diseases and tissue damage models. However pro-survival/pro-proliferative functions have also been described in some transformed cells and in mouse models of cancer. Recent evidence suggests that the contribution of PKCδ to specific cancers may depend in part on the oncogenic context of the tumor, consistent with its paradoxical role in cell survival and cell death. Here we will discuss what is currently known about biological functions of PKCδ and potential paradigms for PKCδ function in cancer. To further understand mechanisms of regulation by PKCδ, and to gain insight into the plasticity of PKCδ signaling, we have used functional proteomics to identify pathways that are dependent on PKCδ. Understanding how these distinct functions of PKCδ are regulated will be critical for the logical design of therapeutics to target this pathway.
Keywords: Protein kinase C, functional proteomics, signal transduction, cancer, apoptosis, therapeutics
1. Introduction
Post-translational modification of proteins by phosphorylation contributes to the regulation of most, if not all, cellular functions (Cohen, 2002). Indeed, up to 30% of proteins are known to be phosphorylated by serine/threonine, tyrosine, or histidine kinases, and dephosphorylated by phosphatases that recognize these specific residues (Cohen, 2000). Changes in phosphorylation status are typically rapid but transient, enabling dynamic responses to the cellular environment through the regulation of protein activity, stability, and subcellular localization. As many protein kinases and phosphatases are widely expressed, rapid activation/inactivation of signaling complexes and cascades may help determine the specificity of cellular responses to a diverse set of stimuli.
Protein kinase regulation by cyclic nucleotide second messengers was first described by Fischer and Krebs in 1968 (Walsh et al., 1968). The identification of Protein Kinase C (PKC)1 almost 10 years later expanded the paradigm to include Ca2+ and lipids as kinase regulators and led to the identification of membrane lipids as signaling molecules (Inoue et al., 1977; Takai et al., 1977). Further studies have identified 10 PKC isoforms that can be subdivided into families based on their differential dependency on lipid second messengers and calcium for activation (Newton, 2001). The development of isoform specific in vivo and in vitro tools has revealed unique roles for this kinase family in the regulation of the DNA damage response and repair, immune function, neural plasticity, cell migration, proliferation, survival, apoptosis and metabolism (Antal et al., 2015; Reyland, M.E. and Jones, D. N., 2016). Surprisingly, these studies have shown that a given PKC isoform can participate in many different cellular pathways, allowing dynamic “re-wiring” in response to changes in the cellular environment.
Given their contribution to a wide variety of signaling cascades and biological functions, it is likely that PKC isoforms play important roles in a variety of human diseases, particularly in the context of cancer. Here we will discuss what is currently known about biological functions of PKCδ with an emphasis on how dysregulation of PKCδ may contribute to cancer. We will also discuss potential targets of PKCδ that we have recently identified using a functional proteomics approach, and address prospects for targeting PKCδ in human disease.
2. Insights into biological functions of PKCδ from in vivo studies
Studies in PKCδ−/− mice reveal prominent roles for this isoform in regulation of proliferation, immune function and apoptosis (Reyland, M.E. and Jones, D. N., 2016), while in vitro tools such as si/shRNA have identified PKCδ-dependent regulation of signaling pathways that contribute to these biological endpoints (Reyland, M.E. and Jones, D. N., 2016). PKCδ−/− mice appear to develop normally, although some developmental programs such as mammary gland development may be delayed (Allen-Petersen et al., 2010) and fertility is reduced (Ma et al., 2015). However, with age PKCδ−/− mice show significant defects in immune function and develop a lupus-like autoimmune disease (Mecklenbrauker et al., 2002; Miyamoto et al., 2002). Mechanistically, this has been linked to a defect in the establishment of B-cell tolerance and impaired clonal deletion of autoreactive B cells (Banninger et al., 2011; Limnander et al., 2011; Mecklenbrauker et al., 2002; Miyamoto et al., 2002). Interestingly, the expression of a dominant negative form of PKCδ specifically in T cells can also induce a lupus-like autoimmune disease in mice (Gorelik et al., 2015), and in humans a similar phenotype has been described in a patient with a rare loss-of-function mutation in the PRKCD gene (Belot et al., 2013; Kuehn et al., 2013).
Many studies show that depletion of PKCδ can be protective in disease and tissue injury models in mice. This includes protection against diet-induced fatty liver disease (Greene et al., 2014), endotoxin induced lung injury (Chichger et al., 2012) and cytokine-stimulated death of pancreatic islet cells (Cantley et al., 2011). Inhibition of PKCδ also reduces amyloid-β levels and Alzheimer disease associated-phenotypes in mice (Du et al., 2018) and protects dopaminergic neurons in a Parkinson disease model (Gordon et al., 2012; Jin et al., 2014). In addition, conditional knock-out of PKCδ in osteoclasts increases bone mass (Cremasco et al., 2012; Li et al., 2020). Mechanistically, many of these findings may be related to the well-known function of PKCδ in the regulation of cell death, including findings from our lab that demonstrate PKCδ−/− mice are protected from ionizing radiation (IR)-induced apoptosis (Humphries et al., 2006).
While most studies suggest a pro-apoptotic function for PKCδ in normal tissues and non-transformed cells, PKCδ can clearly have pro-survival/pro-proliferative functions in some transformed cells and in mouse models of cancer. Indeed, depletion of PKCδ suppresses tumor formation in nearly all mouse models studied, providing firm evidence for a tumor-promoter function (Allen-Petersen et al., 2014; Mauro et al., 2010; Symonds et al., 2011). A more thorough understanding of the functional landscape of PKCδ is needed to shed light on the paradoxical and sometimes conflicting functions of PKCδ in vivo.
3. PKCδ and apoptosis
Dysregulation of apoptotic pathways contribute to tumor progression by increasing DNA instability and by promoting survival of tumor cells (Brown and Attardi, 2005; Zhivotovsky and Kroemer, 2004). Indeed, many anti-cancer therapeutics have been developed based on the principle of eliminating cancer cells through induction of apoptosis (Carneiro and El-Deiry, 2020; Jan and Chaudhry, 2019). Early studies from our lab and others established a requirement for PKCδ in response to a wide variety of cell damaging agents and under conditions where cell survival pathways are inhibited (Majumder et al., 2001; Matassa et al., 2001; Matassa et al., 2003). In vivo, salivary epithelial and smooth muscle cells isolated from PKCδ−/− mice are resistant to apoptotic stimuli, and the salivary gland and thymus gland in PKCδ−/− mice are protected from IR-induced damage (Allen-Petersen et al., 2010; Humphries et al., 2006; Leitges et al., 2001). A requirement for PKCδ for the induction of apoptosis by a wide variety of toxins suggests that it may be a global regulator of apoptosis. This perspective is supported by studies that show PKCδ depletion is protective in degenerative diseases where cell death contributes to pathogenesis, and in tissue injury models (Du et al., 2018; Gordon et al., 2012; Jin et al., 2014) (see section 2).
How PKCδ regulates apoptosis remains unclear, with evidence for both direct effects on the apoptotic machinery and indirect effects through integration with survival pathways and pathways that regulate cellular responses to damage. Mechanistic studies from our lab show that PKCδ is required for loss of mitochondrial membrane potential in response to cell toxins and for downstream events including activation of caspase and DNA fragmentation (Matassa et al., 2001; Reyland et al., 1999). Activation of PKCδ is also important for apoptosis induced by death receptors, including TRAIL and TNFα (Gonzalez-Guerrico and Kazanietz, 2005; Gordon et al., 2012; Lee et al., 2018; Xu et al., 2012). For instance, phorbol ester-induced apoptosis in LNCaP prostate cancer cells requires PKCδ for secretion of death receptor ligands and transduction of apoptotic signals downstream of death receptors (Gonzalez-Guerrico and Kazanietz, 2005). Similarly, PKCδ can regulate death receptor expression in response to endoplasmic reticulum stress to induce apoptosis (Goncalves et al., 2018; Liu, C. et al., 2019).
Activation of PKCδ during apoptosis is tightly regulated by mechanisms that orchestrate translocation of PKCδ to specific subcellular areas in response to apoptotic stimuli. This can include the plasma membrane and other subcellular organelles, most notably the nucleus (Reyland, M.E. and Jones, D. N., 2016; Yoshida, 2008). Our laboratory has defined critical events that mediate nuclear accumulation of PKCδ in response to genotoxic damage including tyrosine phosphorylation and caspase cleavage (Reyland, M.E. and Jones, D. N., 2016). PKCδ has been also shown to localize to the mitochondria in response to apoptotic stimuli such as oxidative stress and ischemia (Li et al., 1999; Majumder et al., 2001; Majumder et al., 2000; Murriel et al., 2004; Qi and Mochly-Rosen, 2008), and to the Golgi in response to ceramide (Kajimoto et al., 2004). Knowledge of how PKCδ is activated by apoptotic stimuli has been exploited therapeutically to suppress tissue injury. For instance, the Mochly-Rosen lab has shown that peptides that inhibit PKCδ translocation to the plasma membrane can be used prior to an experimentally induced ischemic event in mice to significantly reduce damage and decrease apoptosis (Bright et al., 2004; Bright et al., 2007; Churchill and Mochly-Rosen, 2007; Churchill et al., 2009). These findings have also been reported in ischemia reperfusion-induced lung injury, in which inhibition of PKCδ using siRNA or a PKCδ inhibitor peptide reduces damage and apoptotic cell death (Kim et al., 2016). Likewise, we and others have shown that agents that inhibit the activation of PKCδ protect against IR-induced damage in mice (Wie et al., 2017) and cytotoxin-induced renal cell injury (Pabla et al., 2011). Similarly, splice variants of PKCδ, PKCδIX and PKCδII, which are caspase-3 resistant, have been shown to inhibit apoptosis and/or promote cell survival (Apostolatos et al., 2012; Patel et al., 2006; Patel et al., 2013).
Regulation of apoptosis by PKCδ
Potential substrates of PKCδ in apoptosis include heat shock proteins, kinases and phosphatases, cell cycle regulators, transcription factors, apoptotic regulators, DNA damage response (DDR) and DNA repair proteins (Reyland, M.E. and Jones, D. N., 2016). PKCδ has been shown to target Bcl-2 proteins, providing evidence for a direct effect on apoptotic pathways. For instance, PKCδ can induce apoptosis by facilitating dephosphorylation of the proapoptotic protein Bad (Murriel et al., 2004), and through enhancing activation of Bax and Bak (Choi et al., 2006). Several studies suggest that PKCδ can also regulate protein stability or degradation. For instance, PKCδ directly targets and down-regulates the anti-apoptotic protein Mcl-1 to trigger apoptosis (Sitailo et al., 2006), while it upregulates TAp63 to induce apoptosis by increasing protein stability (Li et al., 2015). PKCδ has been shown to regulate mRNA 3’ end processing of the BIK protein to induce apoptosis through a mechanism that requires PKCδ association with the Star-PAP processing complex (Li et al., 2012). Additional mechanisms of regulation by PKCδ may include binding to and sequestering proteins that either inhibit or promote apoptosis such as Smac, an antagonist of inhibitor-of-apoptosis-proteins (IAPs) (Holmgren et al., 2016; Masoumi et al., 2012).
Evidence supports an emerging role for PKCδ in activation of the DNA-damage- response (DDR) and DNA repair pathways. PKCδ can function upstream of the DNA damage sensors, nuclear DNA-PK and Ataxia Telangiectasia Mutated (ATM), suggesting a role for PKCδ in direct regulation of the DDR (Arango et al., 2012; Bharti et al., 1998; Soriano-Carot et al., 2014). In these studies blocking PKCδ activity inhibits the phosphorylation of ATM and histone H2AX, two key regulators of the DDR (Arango et al., 2012; Li et al., 2004). Other studies support a role for PKCδ in mediating G1 arrest through induction of p21 (Liu et al., 2007; Nakagawa et al., 2005; Perletti et al., 2005; Ryer et al., 2005; Saha et al., 2014; Yoshida et al., 2006), in S phase arrest (Santiago-Walker et al., 2005), and in the maintenance of the G2/M DNA damage checkpoint (LaGory et al., 2010). Recently, Liu et al. has illustrated an important role for PKCδ in the development of the first cell stage of mouse embryos through the phosphorylation Cdc25b on Ser96 to promote G2/M transition (Liu, Y. et al., 2019). Taken together, these studies suggest that inhibition of the DDR, and subsequently DNA repair, may be a mechanism through which PKCδ promotes rapid cell death when appropriate.
4. PKCδ in Cancer
The ability of phorbol esters to promote tumors was described nearly 50 years ago (Baird and Boutwell, 1971). However, it was not until PKC was shown to be the major phorbol ester “receptor” in the cell that the contribution of PKC isoforms to human cancer was explored (Castagna et al., 1982; Kikkawa et al., 1983). Studies from the Newton lab indicate that functional genomic alterations of PKCδ are rare, and none have been definitively linked to cancer, supporting the paradigm that PKCδ is a cancer “supporter” and not a cancer “driver” (Antal et al., 2015). Thus, PKCδ activation, expression, localization and/or the oncogenic context may largely determine the function of this kinase in cancer. Data on expression of PKCδ in human tumors supports potential roles for PKCδ in both tumor suppression and tumor promotion. PKCδ expression is decreased in human squamous cell and bladder carcinomas, endometrial carcinoma, and colorectal cancer (D'Costa et al., 2006; Langzam et al., 2001; Reno et al., 2008; Su et al., 2020; Yadav et al., 2010), but increased in pancreatic cancer, breast cancer and non-small cell lung cancer (NSCLC) (Allen-Petersen et al., 2014; Evans et al., 2003; Symonds et al., 2011; Symonds et al., 2016). Our studies in K-ras mutant non-small cell lung cancer (NSCLC) cell lines suggest that PKCδ function may be dependent on oncogenic context and subcellular localization. PKCδ expression is increased in NSCLC cells that are dependent on K-ras for survival, and this correlates with increased nuclear abundance of PKCδ (Ohm et al., 2017). In these studies, NSCLC cells with high nuclear PKCδ are resistant to chemotherapy-induced apoptosis. Likewise, nuclear accumulation of PKCδ correlates with resistance to tyrosine kinase inhibitors (TKIs) (Lee et al., 2018).
Evidence for PKCδ as a tumor promoter
Depletion of PKCδ suppresses tumor formation in nearly all mouse models studied, strongly supporting a tumor-promoter function for this kinase (Allen-Petersen et al., 2014; Mauro et al., 2010; Symonds et al., 2011). Furthermore, while PKCδ−/− mice have a reduced life span, presumably due to autoimmune disease, we have found no evidence of an increased rate of spontaneous tumors in PKCδ−/− mice up to 18 months of age (Reyland, et al. unpublished data). Our in vivo studies reveal that PKCδ functions as a tumor promoter in mouse models of mammary gland cancer, pancreatic cancer and lung cancer (Allen-Petersen et al., 2014; Mauro et al., 2010; Symonds et al., 2011). Using a urethane-induced lung cancer model in which nearly 100% of the tumors were determined to have K-ras mutations, we found that tumor incidence was dramatically reduced in urethane treated PKCδ−/− mice compared to PKCδ+/+ mice and further, the tumors in PKCδ−/− mice were significantly reduced in size (Symonds et al., 2011). In similar studies we showed that ErbB2/Her2neu induced mammary gland tumorigenesis is reduced in PKCδ−/− mice, and that increased expression of PKCδ correlated with poor prognosis in a subset of human breast cancer tumors (Allen-Petersen et al., 2014; McKiernan et al., 2008). Likewise, data from Mauro et al. shows that overexpression of PKCδ in PANC-1 cells results in decreased tumor latency in a mouse xenograft model (Mauro et al., 2010).
Maintenance of cancer stem cells may contribute to the tumor promoter phenotype associated with PKCδ. PKCδ supports the survival of cancer stem cells from multiple types of human tumors (Berardi et al., 2016; Chen et al., 2014), promotes c-Kit activation in colon cancer cells (Park et al., 2013) and helps to maintain the phenotype of tumor initiating cells through a cytokine-mediated autocrine positive feedback loop (Kim et al., 2015). Notably, the PKC inhibitor, sotrastaurin, can regulate stem cell properties in gastric cancer cells and inhibit metastasis and chemoresistance in gastric cancer (Yuan et al., 2019). Possibly linked to stem cell homeostasis, PKCδ has been shown to contribute to cellular senescence as part of a complex that represses hTERT transcription (Yamashita et al., 2016). Other studies indicate that expression of hTERT in adipose-derived stem cells is regulated by the PKCδ splice variant, PKCδVIII (Carter et al., 2013).
Additional mechanisms of tumor promotion by PKCδ may include regulation of cell migration and invasion (Allen-Petersen et al., 2014; Gan et al., 2012; Razorenova et al., 2011; Symonds et al., 2011; Zhao et al., 2009). Studies from the Cheresh lab demonstrate that integrin αVβ3 is increased during metastasis and promotes cancer stem cell survival and resistance to EGFR inhibitors (Seguin et al., 2014). Our lab and others have shown that PKCδ modulates integrin αVβ3 regulated survival pathways (Putnam et al., 2009; Symonds et al., 2016). In our studies, depletion of PKCδ resulted in decreased expression of integrin αVβ3, reduced ERK activation, and decreased anchorage independent growth (Symonds et al., 2016). Similar results have been shown in somatotropinomas and NSCLC cells, suggesting a potential mechanism for regulation of tumor progression and metastasis (Lei et al., 2018; Symonds et al., 2011).
A tumor promoter function for PKCδ seems incongruent with its central role in apoptosis. How then is the tumor promoter function of PKCδ specified in cancer cells? An interesting idea is that PKCδ function in cancer may be dictated by the specific oncogenic context. This has been explored largely in cells with activated K-ras, but the concept is also supported by studies that indicate a role for PKCδ in drug resistant K-ras NSCLC (Lee et al., 2018; Wilson et al., 2015). PKCδ has been validated as a synthetic lethal target in multiple cancers with aberrant activation of Ras signaling (Chen et al., 2011; Xia et al., 2009; Xia et al., 2007). Studies by Xia et al. show that PKCδ is required for the survival of cells engineered to overexpress activated K-ras, and that depletion of PKCδ in these cells induces apoptosis (Xia et al., 2009; Xia et al., 2007). We have explored this further using a panel of 17 NSCLC cells lines that represent two subpopulations of KRAS mutant NSCLC cells that differ in their functional dependence on K-ras (Collisson et al., 2011; Singh et al., 2009). Our studies show that functional dependency on K-ras is highly correlated with a pro-survival, pro-tumorigenic role for PKCδ, while in KRAS mutant lung cancers that are no longer functionally dependent on K-ras, PKCδ regulates the apoptotic response to chemotherapeutic agents (Ohm et al., 2017; Symonds et al., 2011). PKCδ functions downstream of K-ras in this model, as depletion of PKCδ does not suppress K-ras activation (Symonds et al., 2011). Our cumulative studies show that K-ras dependent NSCLC cells are dependent on PKCδ for many tumor associated phenotypes including in vivo tumor formation, cell migration and invasion, and anchorage independent growth (Ohm et al., 2017; Symonds et al., 2011; Symonds et al., 2016). Mechanistically, the requirement for PKCδ for the activation of extracellular-regulated-kinase (ERK) is a central theme that defines functional K-ras dependency in NSCLC cells. We have proposed that this is an example of “non-oncogene addiction” where cancer cells can become dependent on proteins that are non-essential for the survival of normal cells (Luo et al., 2009; Symonds et al., 2016). Taken together, these studies suggest that inhibition of PKCδ might be useful in certain subsets of cancer patients, particularly those who have developed resistance to TKIs and inhibitors that target Raf/MEK/ERK signaling. A variety of approaches to targeting PKCδ have been explored as discussed in section 7.
Evidence for PKCδ as a tumor suppressor
The pro-apoptotic role of PKCδ has led to the suggestion that it may act as a tumor suppressor (Reyland, M.E. and Jones, D. N., 2016). Support for this hypothesis is mostly based on in vitro studies that show suppression of proliferation by PKCδ in a variety of cancer cell lines. For instance, PKCδ can suppress proliferation of colon cancer cells (Hernández-Maqueda et al., 2013) through inhibition of Wnt signaling and hepatocellular carcinoma cells through regulation of Hedgehog signaling (Cai et al., 2009). Similarly, as discussed above, PKCδ can inhibit cell cycle progression and promote cell cycle arrest (LaGory et al., 2010; Nakagawa et al., 2005; Santiago-Walker et al., 2005). PKCδ regulation of apoptosis in prostate cancer cells through p38 MAPK is likewise tumor-suppressive (Fujii et al., 2000; Tanaka, Y. et al., 2003). While these in vitro studies are consistent with PKCδ functioning as a tumor suppressor, in vivo support for this hypothesis is limited. Early studies in transgenic mice that overexpress PKCδ showed resistance to chemically induced skin cancer (Reddig et al., 1999) but not UV radiation-induced skin cancer (Aziz et al., 2006). PKCδ may also be important for IR-induced inhibition of tumor growth downstream of PLOD3 (Baek et al., 2019). However, Cheng et al. have described a role for caspase-3/PKCδ/Akt/VEGF-A signaling in mediating repopulation of tumor cells following irradiation, which suggests PKCδ may function as a tumor promoter in this context (Cheng et al., 2019). Given the considerable in vitro data supporting pro-apoptotic and antiproliferative functions of PKCδ, a more thorough analysis of potential tumor suppressor functions in vivo is warranted. In particular, studies that address a tumor suppressor function for PKCδ in the context of a wider variety of genetic drivers are needed to fully understand the potential roles for PKCδ in tumorigenesis.
Perspective
While the preponderance of in vivo and in vitro studies indicate a tumor promoter function for PKCδ, these studies have limitations and also raise important questions. For instance, almost all studies that have examined a pro-tumorigenic role PKCδ have used models that are dependent on ErbB2/Her2 or K-ras signaling. These studies show that dependency on PKCδ is highly correlated with functional dependency on K-ras (Ohm et al., 2017; Symonds et al., 2011; Symonds et al., 2016) or on activated K-ras (Xia et al., 2009; Xia et al., 2007). Whether the tumor-promoter function of PKCδ can be extrapolated to other oncogenic contexts will need to be determined by examination of a wider group of cancers with different oncogenic drivers. Additional studies are also needed to tease out potential contributions of the pro-apoptotic function of PKCδ in cancer. For instance, our studies on K-ras mutant NSCLC cells do not address whether the PKCδ pro-apoptotic phenotype seen in some K-ras “independent” NSCLC cells is tumor-suppressive (Ohm et al., 2017; Symonds et al., 2011; Symonds et al., 2016). Such questions may be relevant to emerging roles for PKCδ in drug resistance (Lee et al., 2018; Wilson et al., 2015).
Finally, while evasion of apoptosis is a hallmark of cancer, based on the lack of compelling evidence that PKCδ can act as a tumor suppressor, it is possible that the pro-apoptotic function of PKCδ may not contribute significantly to tumorigenesis. Indeed, support for a pro-apoptotic function for PKCδ comes primarily from studies in non-cancer cells, including studies in PKCδ−/− mice which show a clear lymphoproliferative phenotype (Banninger et al., 2011; Limnander et al., 2011; Mecklenbrauker et al., 2002; Miyamoto et al., 2002). In these models, loss of PKCδ protects against tissue damage/injury in vivo, and clonal deletion of autoreactive B cells, at least in part through suppression of apoptosis (see section 2). PKCδ may have unique functions in cancer cells that support tumor progression. This raises interesting questions about the plasticity of signaling by PKCδ and the contribution of oncogenic context to re-wiring of PKCδ in some cancer cells. Understanding if PKCδ can function both as a tumor promoter and a tumor suppressor, and what determines PKCδ function in a particular cell and tissue context will be essential for advancing this kinase as a drug target in cancer.
6. Functional proteomic analyses reveals unique roles for PKCδ
Given the ubiquitous expression of PKCδ, and its contribution to many cellular processes, deciphering how its unique functions are specified is challenging. In some cases, manipulation of PKCδ expression and activity in cell lines has identified PKCδ-regulated signaling cascades that contribute to these biological endpoints (Li et al., 2020; Symonds et al., 2016; Wermuth et al., 2011). Several studies have approached this question using mRNA microarray analysis to determine changes in transcript abundance. For example, our lab has compared mRNA expression in K-ras mutant NSCLC cell lines with and without depletion of PKCδ (Symonds et al., 2016). KEGG analysis of this data showed that depletion of PKCδ decreased transcripts for proteins involved in cancer gene signaling, in particular the regulation of extracellular matrix and focal adhesion pathways (Symonds et al., 2016). In contrast, mRNA transcripts for proteins involved in metabolic processes such as glutathione metabolism, amino acid metabolism and nitrogen metabolism were increased (Symonds et al., 2016). Li et al. recently used RNA-seq to identify gene alterations in an inducible PKCδ knockout murine osteoclast model, and found sex-specific and PKCδ-dependent differences in collagen binding and peptidase regulation (Li et al., 2020). Contrary to our analysis, their studies showed that genes involved in focal adhesion and ECM–receptor interaction were upregulated, while many of the downregulated genes were involved in cell differentiation.
While gene expression profiling via high throughput analysis of RNA transcripts can be highly informative, transcript abundance does not necessarily correlate with protein expression (de Sousa Abreu et al., 2009; Vogel and Marcotte, 2012). Furthermore, pathway activation/inactivation is frequently regulated by post-translational modifications of proteins, rather than protein expression. Functional proteomic analysis enables profiling of changes in protein expression and post-translational modifications, such as phosphorylation, with the goal of identifying differentially regulated signal transduction pathways (Monti et al., 2019). Reverse Phase Protein Array (RPPA) uses a pre-specified set of antibodies to detect protein abundance in cell or tissue lysates (Petricoin et al., 2019). As the RPPA panel contains antibodies to many post-translationally modified proteins, dynamic changes in signaling pathways can also be detected. To explore PKCδ regulation of the functional proteome, we assayed protein abundance in biological triplicates of two sets of ParC5 cells that stably express unique PKCδ targeted shRNAs or their paired controls (shScr/shδ561 and shNT/shδ110). Analysis was done by the MD Anderson Functional Proteomics Core (www.mdanderson.org) using an RPPA platform consisting of antibodies to 371 proteins, including 100 antibodies that recognize proteins with specific post-translational modifications (the majority being anti-phosphorylation antibodies). ParC5 cells are a well-differentiated cell line derived from rat parotid acinar cells (Quissell et al., 1998). We have used these cells extensively to study DNA damage-induced apoptosis as they closely phenocopy physiologic models such as primary salivary acinar cells and mouse models of salivary gland function (Reyland, M.E. and Jones, D. N., 2016). In addition, analysis of changes in gene expression upon depletion of PKCδ in ParC5 cells (this manuscript) and several non-K-ras dependent NSCLC cell lines indicates that many common pathways are regulated (Symonds et al., 2016). Therefore, data from ParC5 cells may be generally extrapolatable to some subsets of cancer cells, particularly those that are not dependent on K-ras signaling (Ohm et al., 2017; Symonds et al., 2011; Symonds et al., 2016).
Changes in protein expression and post-translational modifications
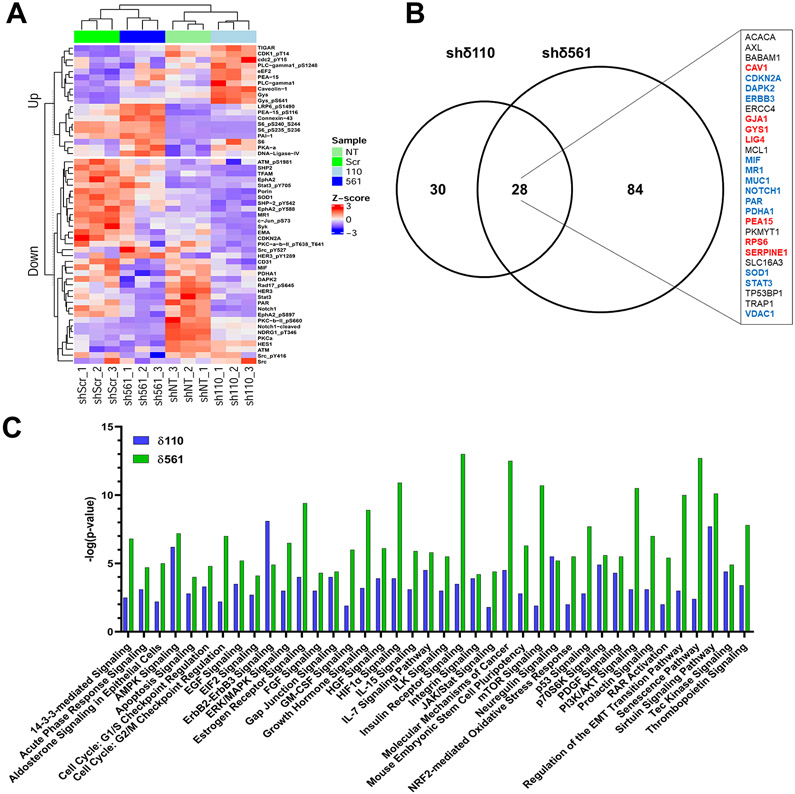
We first analyzed changes in the abundance of total proteins and post-translationally modified proteins using normalized values provided by the MD Anderson Functional Proteomics Core. Z-score transformation was then performed for the antibody signals found to be significantly altered (p<0.1) in both shRNA pairs (shScr versus shδ561 and shNT versus shδ110). Z-scores greater than 2 or less than 2 indicate significant upregulation or downregulation of the specific protein, respectively. The heatmap shown in Figure 1A depicts the Z-scores for the 54 proteins regulated in both pairs of PKCδ shRNA depleted cells, grouped into those that are up- or down-regulated. Antibodies that detect proteins with specific post-translational modifications revealed changes in the abundance of 21 phosphoproteins, with 8 increased and 13 decreased. The 19 proteins and protein modifications that were found to be up regulated include proteins that participate in processes previously shown to be regulated by PKCδ, including the DNA damage response, cell cycle checkpoint activation and DNA repair (e.g. TIGAR, CDK1 pT14/T15 and DNA ligase IV) and receptor signaling (e.g. PLC-gamma and PLC-gamma pS1248, LRP6 pS1490). Our analysis also suggest PKCδ regulation of other processes including glucose metabolism and nutrient sensing (e.g. S6 and phospho-S6, GYS1 and phospho-GYS1 and eEF2). Thirty-five proteins and protein modifications were shown to be down regulated upon depletion of PKCδ. This group includes proteins involved in the DNA damage response and cell cycle arrest (e.g. CDKN2A (p14/p16), RAD17 pS645, ATM and ATM pS1981 and DAPK2), proliferation and signaling pathway integration (e.g. Notch 1 cleaved, STAT3 and STAT3 pY705, Jun and Jun pS73) and mitochondrial function and the antioxidant response (e.g. SOD1, PDHA1, T-FAM). Down-regulated proteins also include receptor and non-receptor tyrosine kinases (e.g. EphA2/EphA2 pY588/S897, Her3/Her3 pY1289, Syk, Src/Src pY416/pY527) and tyrosine phosphatases (e.g. SHP2/SHP2 pY542). Finally, the downregulation of PKCα and PKCβ-II expression and phosphorylation suggests coordinated activation/expression of some PKC isoforms. Our lab has previously shown that PKCα is protective against PKCδ-induced apoptosis, offering a possible explanation for why PKCα may be reduced in the absence of PKCδ (Matassa et al., 2003).
Figure 1.
Protein abundance in biological triplicates of two sets of ParC5 cells that stably express unique PKCδ targeted shRNAs or their paired controls (shScr/shδ561 and shNT/shδ110) was analyzed in triplicate at the RPPA Core at MD Anderson Cancer Center (Houston, TX). A) Proteins with an antibody changed in the same direction (p<0.1) in both shRNA constructs were plotted as a heatmap using the ComplexHeatmap R package (Gu et al., 2016) following z-score transformation. B) Shown is the number of genes (p<0.05) regulated in each shRNA as well as those that are found in both shRNA cell lines. The inset shows those genes regulated in both shRNAs with increased expression (red), decreased expression (blue) or both increased and decreased expression (black). C) Each gene expression comparison (p<0.05) (shδ561 vs shScr and shδ110 vs shNT) was analyzed using Ingenuity Pathway Analysis software (QIAGEN, inc., Hilden, Germany) to identify biological networks and functional pathways. Shown are the top 33 pathways common to both shPKCδ knockdown pairs that were most significantly altered (p<0.005, [−log(p-value)>2.3]). Additionally, seven pathways that were very highly significant in one shRNA pair (p<0.00001, [−log(p-value)>5]) and strongly significant in the other (p<0.01, [−log(p-value)>1.9]) are included.
Remarkably, while many phosphorylation events appear to be regulated by PKCδ, the most dramatically altered post-translation modification observed in was total protein polyADP-ribosylation (PARylation). Protein PARylation was reduced 3.3-fold in shδ110 cells, and 2.1-fold in shδ561 cells relative to their controls. Protein PARylation is a reversible modification that is associated with cellular stress responses and is responsible for the regulation of chromatin organization, DNA repair, DNA replication, transcription as well as other processes (Schuhwerk et al., 2017; Wei and Yu, 2016). Based on our RPPA data, the decrease in PARylation observed is not a result of an increase in Poly(ADP-Ribose) Glycohydrolase, the enzyme responsible for removing PARylations (data not shown), or Poly(ADP-Ribose) polymerase.
The protein alterations identified by RPPA (Figure 1A) agree well with data from our lab and others that demonstrates a suppression of apoptosis with depletion or inhibition of PKCδ (Allen-Petersen et al., 2010; Humphries et al., 2008; Leitges et al., 2001). Importantly, our studies also suggest new targets of PKCδ that may contribute to the anti-apoptotic phenotype observed with PKCδ depletion. For example, we have shown that ERK activation by PKCδ is required for DNA damage-induced apoptosis (Ohm et al., 2019). Both phosphorylated and total astrocytic phosphoprotein PEA-15 (PEA-15) are increased with PKCδ depletion (Figure 1A). In its unphosphorylated state PEA-15 binds and sequesters ERK1/2 in the cytoplasm, inhibiting ERK nuclear translocation and activation of proliferative signaling (Greig and Nixon, 2014). Phosphorylation of PEA-15 on Ser116 results in the release and nuclear translocation of ERK1/2 coincidental with the recruitment of PEA-15 pS116 to the apoptotic death-initiation signaling complex (DISC) and inhibition of apoptosis (Formstecher et al., 2001; Renganathan et al., 2005). Similarly, our data shows increased expression of the p53 regulated protein, TIGAR (Figure 1A), which increases glucose metabolism via the pentose phosphate pathway. This was also a pathway identified in our previous analysis of NSCLC cells (Symonds et al., 2016). Activation of the pentose phosphate pathway by TIGAR has been shown to be anti-apoptotic by reducing oxidative stress and increasing DNA repair (Bensaad et al., 2006; Fico et al., 2004; Yu et al., 2015). Finally, Glycogen Synthase 1 (GYS1), another metabolism associated anti-apoptotic protein (Bhanot et al., 2015; Pelletier et al., 2012), and DNA Ligase IV, a component of the non-homologous end joining DNA repair pathway were also shown to be upregulated. It is worth remembering that these proteins and phosphoproteins are increased in cells depleted of PKCδ, and thus suggests that regulation by PKCδ must be both negative and indirect.
Ingenuity Pathway Analysis
To integrate specific changes in protein expression with changes in cell signaling pathways, we performed Ingenuity Pathway Analysis (IPA). Figure 1B shows the distribution of the 142 genes that were significantly (p<0.05) altered in the RPPA. From the RPPA, there were 30 and 84 uniquely altered genes in shδ110 and shδ561 compared to their controls, respectively, with 28 genes that were common between them. Because of the small number of genes that were significantly altered in both shPKCδ pairs (overlap in Venn diagram, Figure 1B), IPA was performed on significantly altered genes in each individual shRNA pair (shScr versus shδ561 and shNT versus shδ110). Figure 1C shows the top 33 pathways common to both shPKCδ knockdown pairs that were most significantly altered (p<0.005, [−log(p-value)>2.3]). Additionally, seven pathways that were very highly significant in one shRNA pair (p<0.00001, [−log(p-value)>5]) and strongly significant in the other (p<0.01, [−log(p-value)>1.9]) are also included.
Given the relatively small number of genes, we were not able to determine whether the identified pathways were up- and down-regulated. However, in some cases directionality can be inferred from the protein alterations identified in the RPPA (Figure 1A). Pathways identified by IPA validate a role for PKCδ in growth factor signaling, the DNA damage response and cell cycle regulation as well as apoptosis, the primary phenotype observed with PKCδ depletion. Importantly, the DNA damage checkpoints at both G1/S and G2M and the p53 signaling pathway were all found to be altered. PKCδ has been previously shown to be involved in G1/S progression, which was found to activate cell-cycle checkpoints and p53 (Santiago-Walker et al., 2005). In this regard, PKCδ regulation of G1/S progression has been shown to be pro-apoptotic (Santiago-Walker et al., 2005), which concurs with an anti-apoptotic phenotype with PKCδ depletion. The IPA analysis also shows an enrichment of energy/metabolism pathways, which included AMPK Signaling, Insulin Receptor Signaling, mTOR Signaling and Sirtuin Signaling. These pathways correlate well with previous studies as well as our unpublished data, where PKCδ has been shown to be involved in mitochondrial respiration (Mayr et al., 2004a; Mayr et al., 2004b), as well as our data that shows depletion of PKCδ alters amino acid metabolism and modulates the pentose phosphate pathway in NSCLC cells (Symonds et al., 2016). mTOR signaling is associated with increases in anabolism and cell proliferation and decreases in catabolism and apoptosis (Laplante and Sabatini, 2012). Increased phosphorylation of S6K, an mTOR substrate, suggests that mTOR signaling is increased, which concurs with the anti-apoptotic phenotype observed with PKCδ knockout. Sirtuin signaling opposes mTOR activity (Ghosh et al., 2010), thus based on the increase in mTOR activity, Sirtuin signaling is likely decreased, but this will need to be validated. Together, these changes suggest an increased proliferative state or decreased apoptosis. Ingenuity pathway analysis also demonstrates additional alterations in growth factor and proliferative signaling pathways that are impacted by the depletion of PKCδ, highlighting the important role of this kinase in cellular survival and the anti-apoptotic phenotype.
Our analyses provide confirmation of the numerous known PKCδ regulated cellular processes, but also suggest numerous opportunities for further investigation. For instance, it is noteworthy that while few gene expression changes overlap between the shRNA pairs, many of the same pathways identified by IPA are shared, suggesting that there are multiple ways to meet a common goal. This plasticity in signaling pathways may be similar to what has been identified in some cancer cells (Luo et al., 2009; Pagliarini et al., 2015; Quintanal-Villalonga et al., 2020). Overall, the protein expression and post-translational modifications observed in the RPPA and the pathway analysis in PKCδ depleted cells follow the general theme of upregulation of DNA damage response and a decrease in apoptosis. These analyses provide data for generation of new hypotheses regarding additional mechanisms that may drive pro-apoptotic and pro-proliferative phenotypes associated with PKCδ. For instance, our data shows that depletion of PKCδ activates ERK signaling via increased expression and phosphorylation of PEA-15 at S116 (Figure 1A). Likewise, upregulation of TIGAR is associated with protection from apoptosis (Bensaad et al., 2006). The identification of PKCδ as a regulator of these pathways may have implications for targeting drug resistant tumors that are reliant on PKCδ (Lee et al., 2018; Wilson et al., 2015).
7. Therapeutic Strategies for Targeting PKCδ
Given its role in regulating many aspects of cell signaling, numerous efforts have been made to target PKCδ activity. However, this is a challenging task, in part because the high degree of structural and sequence similarity between the different PKC isotypes results in a lack of specificity for a particular isotype. This is particularly true for reagents that target the catalytic domain, which often show inhibition of multiple kinases. Indeed, multiple compounds that were originally described as selective PKC inhibitors were in fact highly promiscuous and inhibited kinases or functioned via mechanisms that did not depend on kinase activity (Soltoff, 2007; Wu-Zhang and Newton, 2013). Unfortunately, a number of studies continue to use these compounds as selective PKC inhibitors.
In targeting PKC it is important to consider the specific role that the kinase plays in cellular signaling. Studies from the Newton lab suggest that a number of naturally occurring mutations of multiple PKC isotypes identified in cancer patients are loss of function and can promote tumorigenesis when tested in mouse models (Antal et al., 2015; Newton and Brognard, 2017). As a result, they suggest that, for cancer, therapeutic strategies should focus on rescuing rather than inhibiting PKC dependent signaling. However, as reviewed above, there is evidence that in some cancers PKCs promote tumorigenesis, and in these cases and in other diseases it may be more appropriate to inhibit PKC isotype function. Further, different isotypes of PKC can have opposing effects within the same tissue. For example, in ischemia reperfusion, PKCδ and PKCε have opposing effects. The pharmacological agents developed over the years to target PKC activity have been extensively reviewed elsewhere (Budas et al., 2007; Churchill et al., 2009; Cunningham et al., 2017; Das et al., 2016; Deka and Trivedi, 2019; Mackay and Twelves, 2007; Mochly-Rosen et al., 2012; Wu-Zhang and Newton, 2013). We have previously reviewed therapeutic approaches that target PKCδ (Reyland, M.E. and Jones, D. N., 2016). Here we highlight recent developments in targeting PKCδ for disease intervention.
Targeting the Regulatory Domains
The bryostatins are a family of macrolide lactones from the marine organism Bugula neritina (Pettit et al., 1970; Pettit et al., 1982). In common with phorbol esters, these compounds bind with high affinity to the C1 domains of PKC (de Vries et al., 1988; Kraft et al., 1986; Wender et al., 1988) and can lock PKC in an open conformation that ultimately leads to its down regulation (reviewed in (Antal et al., 2015; Newton, 2018; Newton and Brognard, 2017)). Recently it has been shown that bryostatins also target other C1 domain containing proteins including Munc13 family members (Blanco et al., 2019). Early on, it was recognized that while bryostatin-1 could target multiple isotypes of PKC to induce membrane translocation and down regulation, it showed differential effects on PKCδ. At low concentrations bryostatin-1 down-regulated PKCδ, similar to phorbol esters, but at high concentrations it protected against TPA induced down regulation (Szallasi et al., 1994a; Szallasi et al., 1994b). Additional studies showed that bryostatin-1 functions to inhibit PKCδ dependent apoptosis by preventing translocation of PKCδ to the plasma membrane, and the PKCδ dependent release of TNFα (Tanaka, Yuichi et al., 2003; von Burstin et al., 2010). These findings have been supported by further studies including with other bryostatin analogs that show differential effects on specific isotypes of PKC (Kedei et al., 2013; Kedei et al., 2011a; Kedei et al., 2011b). Therefore, there is a wealth of interest in discovering new bryostatin analogs for the manipulation of PKC activity in an isotype specific manner.
Bryostatin-1 showed significant efficacy against multiple cancer cell lines and early promise in clinical trials (Ajani et al., 2006; Barr et al., 2009; Ku et al., 2008; Propper et al., 1998; Zonder et al., 2001). However, adverse side effects or lack of significant outcomes has limited bryostatin development. Recently, bryostatin-1 has been investigated for use in chimeric antigen receptor T-cell (CART) therapy targeting CD22 in the treatment of leukemia and lymphoma (Ramakrishna et al., 2019). Resistance to CD22 CART therapies is associated with reduced antigen presentation on the tumor cell surface (Fry et al., 2018; Nguyen et al., 2016; Ramakrishna et al., 2019). Bryostatin-1 treatment of these resistant tumors leads to increased CD22 surface expression in both cells and ex vivo models of acute and chronic lymphocytic leukemias (ALL and CLL), and non-Hodgkin’s lymphoma (al-Katib et al., 1993a; al-Katib et al., 1993b; Biberacher et al., 2012; Ramakrishna et al., 2019) and also in vivo in human clinical trials (Varterasian et al., 1998; Varterasian et al., 2000). In CLL this appears to depend on the down regulation of PKCβ II (Biberacher et al., 2012). However, in ALL bryostatin may function through mechanisms that are related to broader changes in plasma membrane trafficking , as treatment with either enzastaurin targeting PKCβ or the pan PKC inhibitor, staurosporine did not upregulate CD22 levels (Ramakrishna et al., 2019). The development of bryostatins as potential therapeutic agents has been severely limited by their availability, and synthetic routes to bryostatins have been challenging. More efficient approaches to the synthesis of bryostatin-1 derivatives have recently been reported that make available large quantities for clinical studies and the exploration of novel derivatives with improved selectivity (Hardman et al., 2020; Wender et al., 2017).
Anthracycline antibiotics typified by doxorubicin are among the most effective and widely used anti-cancer compounds. These compounds generally bind to DNA and inhibit topoisomerase II (Gewirtz, 1999). Surprisingly, N-Benzyladriamycin-14-valerate (AD 198), a less toxic derivative of doxorubicin, was found to function by targeting PKCδ through its C1b domain to stimulate mitochondrial dependent apoptosis (Barrett et al., 2002; Roaten et al., 2002). Additionally, AD198 was found to protect against doxorubicin induced cardiac damage through activation of PKCε in cardiomyocytes (Cai et al., 2010). An improved derivative pivarubicin (AD 445) has recently been developed, which is more effective than doxorubicin in orthotopic mouse models of triple negative breast cancer, producing reduced growth rates and tumor regression in response to a single dose (Barrett et al., 2002; Roaten et al., 2002). Pivarubicin activity is dependent on activation of PKCδ and is also cardioprotective.
Ingenol mebutate (PEP005) is a phorbol ester derivative that received approval in the US and Europe for the treatment of actinic keratosis (Lebwohl et al., 2012), the precursor of squamous cell carcinoma (SCC), the second most common form of skin cancer. Multiple studies indicate that PEP005 acts primarily through its effects on PKCδ (Benhadji et al., 2008; Freiberger et al., 2015; Hampson et al., 2005; Serova et al., 2008). However, recent data suggests that patients treated with PEP005 actually show higher incidences of SCC, which has prompted its withdrawal from the European market (Mohd Mustapa et al., 2020). PEP005 was recently identified in high throughput screens to reverse the suppression of T-cell activity by inhibitory receptors (T-cell exhaustion) that are targeted in checkpoint inhibitor therapies e.g. against PD-1 or PD-L1 (Marro et al., 2019). This activity of PEP005 was dependent on PKC signaling, in this case most probably PKCθ, however the involvement of other PKC isotypes remains to be determined.
Targeting Kinase Activity
Uveal melanomas (UM) harboring mutations in the G proteins GNAQ or GNA11 (Chua et al., 2017; Van Raamsdonk et al., 2009; Van Raamsdonk et al., 2010) show hyperactivation of PKC-dependent MAPK signaling. These melanomas frequently overexpress the proteins mouse double minute (MDM)2 and MDMX12, which are inhibitors of p53, which itself is not mutated in UM (de Lange et al., 2012). Recent studies have shown that specifically depleting PKCδ inhibits UM growth and this is enhanced by reactivation of p53 dependent signaling by reducing/inhibiting MDM2/MDMX12-p53 interactions including by the use of nutlins (Carita et al., 2016; Heijkants et al., 2018), or by inhibition of mTORC1 downstream of p53 (Carita et al., 2016). Clinical trials with pan-PKC inhibitors in combination with inhibitors of MAPK signaling, showed promising effects but limiting toxicity (Carita et al., 2016). Clinical trials of the novel drug IDE196 (formerly known as LXS196), a pan PKC inhibitor with IC50 in the low nM to pM range that targets binding of ATP, showed early promise (Kapiteijn et al., 2019) for the treatment of metastatic UM, and follow up trials against other solid tumors harboring mutations in GNAQ or GNA11 or PKC-fusions are ongoing (NCT03947385).
PKCδ has recently been shown to play a central role in regulating the resistance of EGFR mutant NSCLC tumors to treatment with TKIs (Lee et al., 2018). TKI resistance in these tumors can occur through multiple mechanisms (Camidge et al., 2014; Minari et al., 2016), which appear to converge on the activity of PKCδ, as inhibition of PKCδ either pharmacologically or with shRNA restored gefitinib sensitivity. Co-treatment of tumors with EGFR inhibitors Osimertinib (AZD9291) and the PKC inhibitor sotrastaurin (AEB071) led to regression of tumor volume (Lee et al., 2018). In these tumors, PKCδ resistance requires nuclear localization as nuclear exclusion re-sensitizes cells to gefitinib treatment. We have previously shown that nuclear localization requires the activity of both importin-α and HSP90 to recognize a bipartite nuclear localization signal (Adwan et al., 2011; DeVries et al., 2002). The importance of this sequence was confirmed by Lee et al (Lee et al., 2018) and may help to explain the limited success of HSP90 inhibitors in treatment of NSCLC with EGFR mutants (Chatterjee et al., 2016). In a similar manner we demonstrated that K-ras-dependent NSCLC cells have increased nuclear localization of PKCδ and have reduced sensitivity to chemotherapeutic agents (Ohm et al., 2017), reinforcing the concept that co-inhibition of PKCδ represents a potential viable therapeutic avenue for treatment of NSCLC.
Inhibiting signaling pathways upstream of PKCδ activation
Head and neck cancer patients typically undergo radiation therapy and surgery, sometimes with additional chemotherapy (Gregoire et al., 2015). Although the goal of radiation treatment is to eradicate the tumor, most patients also suffer debilitating damage to the surrounding non-tumor tissues, which can impact their quality of life and in some cases, limit the duration of therapy (Jensen et al., 2019). As tyrosine phosphorylation plays an important role in activating the pro-apoptotic function of PKCδ in IR-treated tissues, we explored utilizing TKIs, including dasatinib and imatinib, with activity against c-Abl and/or c-Src for radioprotection of the salivary gland in vivo (Wie et al., 2014; Wie et al., 2017). We showed that TKIs could successfully inhibit PKCδ activation and provide robust and durable radioprotection of salivary gland function in a mouse head and neck IR model without impacting cancer therapy (Wie et al., 2017). Other studies have also supported this approach; Arany et al. have shown that depletion of PKCδ in the salivary gland using siRNA coupled nanoparticles can protect against IR-induced loss of salivary gland function (Arany et al., 2013; Arany et al., 2012), while Pabla et al. report that loss of PKCδ leads to reduced damage to the kidneys of mice treated with cisplatin for gastrointestinal cancer (Pabla et al., 2011). Together these studies provide a rationale for targeting pathways required for PKCδ activation for radio and chemo-protection of non-tumor tissues.
8. Conclusions
The ubiquitous expression of PKCδ suggests that it plays an essential role(s) in cell function. This is supported by numerous studies demonstrating a role for PKCδ in processes as diverse as apoptosis and tumorigenesis. With few examples of functional genetic alterations in PKCδ, regulation of the expression, subcellular localization or the activation may be the primary way PKCδ contributes to human diseases, including cancer. This suggests considerable plasticity in how PKCδ interfaces with cell signaling pathways. In the current studies we have expanded our knowledge of known PKCδ regulated pathways using functional proteomics. RPPA analysis of PKCδ depleted cells concurs with previous published data, demonstrating a role in MAPK/ERK signaling cascades, the regulation of apoptosis and the DNA damage response. In addition, it suggests a potential role for PKCδ in cellular energy and metabolism. Our studies provide new hypotheses regarding PKCδ function and suggest potential targets for therapeutic intervention in cancer.
Acknowledgements
We gratefully acknowledge the Bioinformatics Core at the University of Colorado Cancer Center, particularly the contribution of Dr. Andrew Goodspeed. We are also grateful for assistance by Ms. Olivia McGinn in performing the Ingenuity Pathway Analysis.
Funding
This work was supported by the National Institutes of Health [grant numbers R01DE015648, R01DE027517, T32CA190216, T32CA174648, F32DE029116].
Footnotes
Declaration of interest: none
Abbreviations: Protein Kinase C (PKC), Protein Kinase C-δ (PKCδ), Ionizing Radiation (IR), Non-small Cell Lung Cancer (NSCLC), Tyrosine Kinase Inhibitor (TKI), Epidermal Growth Factor Receptor (EGFR), Extra-cellular regulated kinase (ERK), Mitogen Activated Protein Kinase (MAPK), DNA-damage- response (DDR), Ataxia Telangiectasia Mutated (ATM), Reverse Phase Protein Array (RPPA), Ingenuity Pathway Analysis (IPA).
References
- Adwan TS, Ohm AM, Jones DN, Humphries MJ, Reyland ME, 2011. Regulated binding of importin-alpha to protein kinase Cdelta in response to apoptotic signals facilitates nuclear import. J Biol Chem 286(41), 35716–35724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajani JA, Jiang Y, Faust J, Chang BB, Ho L, Yao JC, Rousey S, Dakhil S, Cherny RC, Craig C, Bleyer A, 2006. A multi-center phase II study of sequential paclitaxel and bryostatin-1 (NSC 339555) in patients with untreated, advanced gastric or gastroesophageal junction adenocarcinoma. Invest New Drugs 24(4), 353–357. [DOI] [PubMed] [Google Scholar]
- al-Katib A, Mohammad RM, Dan M, Hussein ME, Akhtar A, Pettit GR, Sensenbrenner LL, 1993a. Bryostatin 1-induced hairy cell features on chronic lymphocytic leukemia cells in vitro. Exp Hematol 21(1), 61–65. [PubMed] [Google Scholar]
- al-Katib A, Mohammad RM, Khan K, Dan ME, Pettit GR, Sensenbrenner LL, 1993b. Bryostatin 1-induced modulation of the acute lymphoblastic leukemia cell line Reh. J Immunother Emphasis Tumor Immunol 14(1), 33–42. [DOI] [PubMed] [Google Scholar]
- Allen-Petersen BL, Carter CJ, Ohm AM, Reyland ME, 2014. Protein kinase Cdelta is required for ErbB2-driven mammary gland tumorigenesis and negatively correlates with prognosis in human breast cancer. Oncogene 33(10), 1306–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen-Petersen BL, Miller MR, Neville MC, Anderson SM, Nakayama KI, Reyland ME, 2010. Loss of protein kinase C delta alters mammary gland development and apoptosis. Cell Death Dis 1, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal CE, Hudson AM, Kang E, Zanca C, Wirth C, Stephenson NL, Trotter EW, Gallegos LL, Miller CJ, Furnari FB, Hunter T, Brognard J, Newton AC, 2015. Cancer-associated protein kinase C mutations reveal kinase's role as tumor suppressor. Cell 160(3), 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolatos A, Song S, Acosta S, Peart M, Watson JE, Bickford P, Cooper DR, Patel NA, 2012. Insulin promotes neuronal survival via the alternatively spliced protein kinase CdeltaII isoform. J Biol Chem 287(12), 9299–9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango D, Parihar A, Villamena FA, Wang L, Freitas MA, Grotewold E, Doseff AI, 2012. Apigenin induces DNA damage through the PKCdelta-dependent activation of ATM and H2AX causing down-regulation of genes involved in cell cycle control and DNA repair. Biochem Pharmacol 84(12), 1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany S, Benoit DS, Dewhurst S, Ovitt CE, 2013. Nanoparticle-mediated gene silencing confers radioprotection to salivary glands in vivo. Mol Ther 21(6), 1182–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany S, Xu Q, Hernady E, Benoit DS, Dewhurst S, Ovitt CE, 2012. Pro-apoptotic gene knockdown mediated by nanocomplexed siRNA reduces radiation damage in primary salivary gland cultures. J Cell Biochem 113(6), 1955–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz MH, Wheeler DL, Bhamb B, Verma AK, 2006. Protein kinase C delta overexpressing transgenic mice are resistant to chemically but not to UV radiation-induced development of squamous cell carcinomas: a possible link to specific cytokines and cyclooxygenase-2. Cancer Res 66(2), 713–722. [DOI] [PubMed] [Google Scholar]
- Baek JH, Yun HS, Kwon GT, Lee J, Kim JY, Jo Y, Cho JM, Lee CW, Song JY, Ahn J, Kim JS, Kim EH, Hwang SG, 2019. PLOD3 suppression exerts an anti-tumor effect on human lung cancer cells by modulating the PKC-delta signaling pathway. Cell Death Dis 10(3), 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird WM, Boutwell RK, 1971. Tumor-promoting activity of phorbol and four diesters of phorbol in mouse skin. Cancer Res 31(8), 1074–1079. [PubMed] [Google Scholar]
- Banninger GP, Cha S, Said MS, Pauley KM, Carter CJ, Onate M, Pauley BA, Anderson SM, Reyland ME, 2011. Loss of PKCdelta results in characteristics of Sjogren's syndrome including salivary gland dysfunction. Oral Dis 17(6), 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr PM, Lazarus HM, Cooper BW, Schluchter MD, Panneerselvam A, Jacobberger JW, Hsu JW, Janakiraman N, Simic A, Dowlati A, Remick SC, 2009. Phase II study of bryostatin 1 and vincristine for aggressive non-Hodgkin lymphoma relapsing after an autologous stem cell transplant. Am J Hematol 84(8), 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CM, Lewis FL, Roaten JB, Sweatman TW, Israel M, Cleveland JL, Lothstein L, 2002. Novel extranuclear-targeted anthracyclines override the antiapoptotic functions of Bcl-2 and target protein kinase C pathways to induce apoptosis. Molecular cancer therapeutics 1(7), 469–481. [PubMed] [Google Scholar]
- Belot A, Kasher PR, Trotter EW, Foray AP, Debaud AL, Rice GI, Szynkiewicz M, Zabot MT, Rouvet I, Bhaskar SS, Daly SB, Dickerson JE, Mayer J, O'Sullivan J, Juillard L, Urquhart JE, Fawdar S, Marusiak AA, Stephenson N, Waszkowycz B, M WB, Biesecker LG, G CMB, Rene C, Eliaou JF, Fabien N, Ranchin B, Cochat P, Gaffney PM, Rozenberg F, Lebon P, Malcus C, Crow YJ, Brognard J, Bonnefoy N, 2013. Protein kinase cdelta deficiency causes mendelian systemic lupus erythematosus with B cell-defective apoptosis and hyperproliferation. Arthritis Rheum 65(8), 2161–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhadji KA, Serova M, Ghoul A, Cvitkovic E, Le Tourneau C, Ogbourne SM, Lokiec F, Calvo F, Hammel P, Faivre S, Raymond E, 2008. Antiproliferative activity of PEP005, a novel ingenol angelate that modulates PKC functions, alone and in combination with cytotoxic agents in human colon cancer cells. Br J Cancer 99(11), 1808–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH, 2006. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126(1), 107–120. [DOI] [PubMed] [Google Scholar]
- Berardi DE, Flumian C, Rodriguez CE, Bessone MI, Cirigliano SM, Joffé ED, Fiszman GL, Urtreger AJ, Todaro LB, 2016. PKCδ Inhibition Impairs Mammary Cancer Proliferative Capacity But Selects Cancer Stem Cells, Involving Autophagy. J Cell Biochem 117(3), 730–740. [DOI] [PubMed] [Google Scholar]
- Bhanot H, Reddy MM, Nonami A, Weisberg EL, Bonal D, Kirschmeier PT, Salgia S, Podar K, Galinsky I, Chowdary TK, Neuberg D, Tonon G, Stone RM, Asara J, Griffin JD, Sattler M, 2015. Pathological glycogenesis through glycogen synthase 1 and suppression of excessive AMP kinase activity in myeloid leukemia cells. Leukemia 29(7), 1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti A, Kraeft SK, Gounder M, Pandey P, Jin S, Yuan ZM, Lees-Miller SP, Weichselbaum R, Weaver D, Chen LB, Kufe D, Kharbanda S, 1998. Inactivation of DNA-dependent protein kinase by protein kinase Cdelta: implications for apoptosis. Mol Cell Biol 18(11), 6719–6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberacher V, Decker T, Oelsner M, Wagner M, Bogner C, Schmidt B, Kreitman RJ, Peschel C, Pastan I, Meyer Zum Buschenfelde C, Ringshausen I, 2012. The cytotoxicity of anti-CD22 immunotoxin is enhanced by bryostatin 1 in B-cell lymphomas through CD22 upregulation and PKC-betaII depletion. Haematologica 97(5), 771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco FA, Czikora A, Kedei N, You Y, Mitchell GA, Pany S, Ghosh A, Blumberg PM, Das J, 2019. Munc13 Is a Molecular Target of Bryostatin 1. Biochemistry 58(27), 3016–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright R, Raval AP, Dembner JM, Perez-Pinzon MA, Steinberg GK, Yenari MA, Mochly-Rosen D, 2004. Protein kinase C delta mediates cerebral reperfusion injury in vivo. J Neurosci 24(31), 6880–6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright R, Steinberg GK, Mochly-Rosen D, 2007. DeltaPKC mediates microcerebrovascular dysfunction in acute ischemia and in chronic hypertensive stress in vivo. Brain Res 1144, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Attardi LD, 2005. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer 5(3), 231–237. [DOI] [PubMed] [Google Scholar]
- Budas GR, Koyanagi T, Churchill EN, Mochly-Rosen D, 2007. Competitive inhibitors and allosteric activators of protein kinase C isoenzymes: a personal account and progress report on transferring academic discoveries to the clinic. Biochem Soc Trans 35(Pt 5), 1021–1026. [DOI] [PubMed] [Google Scholar]
- Cai C, Lothstein L, Morrison RR, Hofmann PA, 2010. Protection from doxorubicin-induced cardiomyopathy using the modified anthracycline N-benzyladriamycin-14-valerate (AD 198). J Pharmacol Exp Ther 335(1), 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Li J, Gao T, Xie J, Evers BM, 2009. Protein kinase Cdelta negatively regulates hedgehog signaling by inhibition of Gli1 activity. J Biol Chem 284(4), 2150–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camidge DR, Pao W, Sequist LV, 2014. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 11(8), 473–481. [DOI] [PubMed] [Google Scholar]
- Cantley J, Boslem E, Laybutt DR, Cordery DV, Pearson G, Carpenter L, Leitges M, Biden TJ, 2011. Deletion of protein kinase Cdelta in mice modulates stability of inflammatory genes and protects against cytokine-stimulated beta cell death in vitro and in vivo. Diabetologia 54(2), 380–389. [DOI] [PubMed] [Google Scholar]
- Carita G, Frisch-Dit-Leitz E, Dahmani A, Raymondie C, Cassoux N, Piperno-Neumann S, Némati F, Laurent C, De Koning L, Halilovic E, 2016. Dual inhibition of protein kinase C and p53-MDM2 or PKC and mTORC1 are novel efficient therapeutic approaches for uveal melanoma. Oncotarget 7(23), 33542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro BA, El-Deiry WS, 2020. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol 17(7), 395–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter G, Apostolatos A, Patel R, Mathur A, Cooper D, Murr M, Patel NA, 2013. Dysregulated Alternative Splicing Pattern of PKCdelta during Differentiation of Human Preadipocytes Represents Distinct Differences between Lean and Obese Adipocytes. ISRN Obes 2013, 161345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y, 1982. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem 257(13), 7847–7851. [PubMed] [Google Scholar]
- Chatterjee S, Bhattacharya S, Socinski MA, Burns TF, 2016. HSP90 inhibitors in lung cancer: promise still unfulfilled. Clin Adv Hematol Oncol 14(5), 346–356. [PubMed] [Google Scholar]
- Chen Z, Forman LW, Miller KA, English B, Takashima A, Bohacek RA, Williams RM, Faller DV, 2011. Protein kinase Cdelta inactivation inhibits cellular proliferation and decreases survival in human neuroendocrine tumors. Endocr Relat Cancer 18(6), 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Forman LW, Williams RM, Faller DV, 2014. Protein kinase C-δ inactivation inhibits the proliferation and survival of cancer stem cells in culture and in vivo. BMC Cancer 14, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, He S, Wang M, Zhou L, Zhang Z, Feng X, Yu Y, Ma J, Dai C, Zhang S, Sun L, Gong Y, Wang Y, Zhao M, Luo Y, Liu X, Tian L, Li C, Huang Q, 2019. The Caspase-3/PKCδ/Akt/VEGF-A Signaling Pathway Mediates Tumor Repopulation during Radiotherapy. Clin Cancer Res 25(12), 3732–3743. [DOI] [PubMed] [Google Scholar]
- Chichger H, Grinnell KL, Casserly B, Chung CS, Braza J, Lomas-Neira J, Ayala A, Rounds S, Klinger JR, Harrington EO, 2012. Genetic disruption of protein kinase Cdelta reduces endotoxin-induced lung injury. Am J Physiol Lung Cell Mol Physiol 303(10), L880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Kim MJ, Kang CM, Bae S, Cho CK, Soh JW, Kim JH, Kang S, Chung HY, Lee YS, Lee SJ, 2006. Activation of Bak and Bax through c-abl-protein kinase Cdelta-p38 MAPK signaling in response to ionizing radiation in human non-small cell lung cancer cells. J Biol Chem 281(11), 7049–7059. [DOI] [PubMed] [Google Scholar]
- Chua V, Lapadula D, Randolph C, Benovic JL, Wedegaertner PB, Aplin AE, 2017. Dysregulated GPCR Signaling and Therapeutic Options in Uveal Melanoma. Mol Cancer Res 15(5), 501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill EN, Mochly-Rosen D, 2007. The roles of PKCdelta and epsilon isoenzymes in the regulation of myocardial ischaemia/reperfusion injury. Biochem Soc Trans 35(Pt 5), 1040–1042. [DOI] [PubMed] [Google Scholar]
- Churchill EN, Qvit N, Mochly-Rosen D, 2009. Rationally designed peptide regulators of protein kinase C. Trends Endocrinol Metab 20(1), 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, 2000. The regulation of protein function by multisite phosphorylation--a 25 year update. Trends Biochem Sci 25(12), 596–601. [DOI] [PubMed] [Google Scholar]
- Cohen P, 2002. The origins of protein phosphorylation. Nat Cell Biol 4(5), E127–130. [DOI] [PubMed] [Google Scholar]
- Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, Cooc J, Weinkle J, Kim GE, Jakkula L, Feiler HS, Ko AH, Olshen AB, Danenberg KL, Tempero MA, Spellman PT, Hanahan D, Gray JW, 2011. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med 17(4), 500–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremasco V, Decker CE, Stumpo D, Blackshear PJ, Nakayama KI, Nakayama K, Lupu TS, Graham DB, Novack DV, Faccio R, 2012. Protein kinase C-delta deficiency perturbs bone homeostasis by selective uncoupling of cathepsin K secretion and ruffled border formation in osteoclasts. J Bone Miner Res 27(12), 2452–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AD, Qvit N, Mochly-Rosen D, 2017. Peptides and peptidomimetics as regulators of protein-protein interactions. Curr Opin Struct Biol 44, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Costa AM, Robinson JK, Maududi T, Chaturvedi V, Nickoloff BJ, Denning MF, 2006. The proapoptotic tumor suppressor protein kinase C-delta is lost in human squamous cell carcinomas. Oncogene 25(3), 378–386. [DOI] [PubMed] [Google Scholar]
- Das J, Ramani R, Suraju MO, 2016. Polyphenol compounds and PKC signaling. BBA - General Subjects 1860(10), 2107–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange J, Teunisse AF, Vries MV, Lodder K, Lam S, Luyten GP, Bernal F, Jager MJ, Jochemsen AG, 2012. High levels of Hdmx promote cell growth in a subset of uveal melanomas. Am J Cancer Res 2(5), 492–507. [PMC free article] [PubMed] [Google Scholar]
- de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C, 2009. Global signatures of protein and mRNA expression levels. Mol Biosyst 5(12), 1512–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries DJ, Herald CL, Pettit GR, Blumberg PM, 1988. Demonstration of sub-nanomolar affinity of bryostatin 1 for the phorbol ester receptor in rat brain. Biochem Pharmacol 37(21), 4069–4073. [DOI] [PubMed] [Google Scholar]
- Deka SJ, Trivedi V, 2019. Potentials of PKC in Cancer Progression and Anticancer Drug Development. Curr Drug Discov Technol 16(2), 135–147. [DOI] [PubMed] [Google Scholar]
- DeVries TA, Neville MC, Reyland ME, 2002. Nuclear import of PKCdelta is required for apoptosis: identification of a novel nuclear import sequence. EMBO J 21(22), 6050–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Zhao Y, Li C, Zheng Q, Tian J, Li Z, Huang TY, Zhang W, Xu H, 2018. Inhibition of PKCdelta reduces amyloid-beta levels and reverses Alzheimer disease phenotypes. J Exp Med 215(6), 1665–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JD, Cornford PA, Dodson A, Neoptolemos JP, Foster CS, 2003. Expression patterns of protein kinase C isoenzymes are characteristically modulated in chronic pancreatitis and pancreatic cancer. Am J Clin Pathol 119(3), 392–402. [DOI] [PubMed] [Google Scholar]
- Fico A, Paglialunga F, Cigliano L, Abrescia P, Verde P, Martini G, Iaccarino I, Filosa S, 2004. Glucose-6-phosphate dehydrogenase plays a crucial role in protection from redox-stress-induced apoptosis. Cell Death Differ 11(8), 823–831. [DOI] [PubMed] [Google Scholar]
- Formstecher E, Ramos JW, Fauquet M, Calderwood DA, Hsieh JC, Canton B, Nguyen XT, Barnier JV, Camonis J, Ginsberg MH, Chneiweiss H, 2001. PEA-15 mediates cytoplasmic sequestration of ERK MAP kinase. Dev Cell 1(2), 239–250. [DOI] [PubMed] [Google Scholar]
- Freiberger SN, Cheng PF, Iotzova-Weiss G, Neu J, Liu Q, Dziunycz P, Zibert JR, Dummer R, Skak K, Levesque MP, Hofbauer GF, 2015. Ingenol Mebutate Signals via PKC/MEK/ERK in Keratinocytes and Induces Interleukin Decoy Receptors IL1R2 and IL13RA2. Mol Cancer Ther 14(9), 2132–2142. [DOI] [PubMed] [Google Scholar]
- Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, Shalabi H, Fountaine TJ, Shern JF, Majzner RG, Stroncek DF, Sabatino M, Feng Y, Dimitrov DS, Zhang L, Nguyen S, Qin HY, Dropulic B, Lee DW, Mackall CL, 2018. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nature Medicine 24(1), 20-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Garcia-Bermejo ML, Bernabo JL, Caamano J, Ohba M, Kuroki T, Li L, Yuspa SH, Kazanietz MG, 2000. Involvement of protein kinase C delta (PKCdelta) in phorbol ester-induced apoptosis in LNCaP prostate cancer cells. Lack of proteolytic cleavage of PKCdelta. J Biol Chem 275(11), 7574–7582. [DOI] [PubMed] [Google Scholar]
- Gan X, Wang J, Wang C, Sommer E, Kozasa T, Srinivasula S, Alessi D, Offermanns S, Simon MI, Wu D, 2012. PRR5L degradation promotes mTORC2-mediated PKC-δ phosphorylation and cell migration downstream of Gα12. Nat Cell Biol 14(7), 686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz DA, 1999. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 57(7), 727–741. [DOI] [PubMed] [Google Scholar]
- Ghosh HS, McBurney M, Robbins PD, 2010. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One 5(2), e9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves GL, Costa-Pessoa JM, Thieme K, Lins BB, Oliveira-Souza M, 2018. Intracellular albumin overload elicits endoplasmic reticulum stress and PKC-delta/p38 MAPK pathway activation to induce podocyte apoptosis. Sci Rep 8(1), 18012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Guerrico AM, Kazanietz MG, 2005. Phorbol ester-induced apoptosis in prostate cancer cells via autocrine activation of the extrinsic apoptotic cascade: a key role for protein kinase C delta. J Biol Chem 280(47), 38982–38991. [DOI] [PubMed] [Google Scholar]
- Gordon R, Anantharam V, Kanthasamy AG, Kanthasamy A, 2012. Proteolytic activation of proapoptotic kinase protein kinase Cdelta by tumor necrosis factor alpha death receptor signaling in dopaminergic neurons during neuroinflammation. J Neuroinflammation 9, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik G, Sawalha AH, Patel D, Johnson K, Richardson B, 2015. T cell PKCdelta kinase inactivation induces lupus-like autoimmunity in mice. Clin Immunol 158(2), 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene MW, Burrington CM, Lynch DT, Davenport SK, Johnson AK, Horsman MJ, Chowdhry S, Zhang J, Sparks JD, Tirrell PC, 2014. Lipid metabolism, oxidative stress and cell death are regulated by PKC delta in a dietary model of nonalcoholic steatohepatitis. PLoS One 9(1), e85848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire V, Langendijk JA, Nuyts S, 2015. Advances in Radiotherapy for Head and Neck Cancer. J Clin Oncol 33(29), 3277–3284. [DOI] [PubMed] [Google Scholar]
- Greig FH, Nixon GF, 2014. Phosphoprotein enriched in astrocytes (PEA)-15: a potential therapeutic target in multiple disease states. Pharmacol Ther 143(3), 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Eils R, Schlesner M, 2016. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32(18), 2847–2849. [DOI] [PubMed] [Google Scholar]
- Hampson P, Chahal H, Khanim F, Hayden R, Mulder A, Assi LK, Bunce CM, Lord JM, 2005. PEP005, a selective small-molecule activator of protein kinase C, has potent antileukemic activity mediated via the delta isoform of PKC. Blood 106(4), 1362–1368. [DOI] [PubMed] [Google Scholar]
- Hardman C, Ho S, Shimizu A, Luu-Nguyen Q, Sloane JL, Soliman MSA, Marsden MD, Zack JA, Wender PA, 2020. Synthesis and evaluation of designed PKC modulators for enhanced cancer immunotherapy. Nature Communications, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijkants RC, Nieveen M, Hart K.C.t., Teunisse AFAS, Jochemsen AG, 2018. Targeting MDMX and PKCδ to improve current uveal melanoma therapeutic strategies. Oncogenesis 7(3), 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Maqueda JG, Luna-Ulloa LB, Santoyo-Ramos P, Castañeda-Patlán MC, Robles-Flores M, 2013. Protein Kinase C Delta Negatively Modulates Canonical Wnt Pathway and Cell Proliferation in Colon Tumor Cell Lines. PLoS ONE 8(3), e58540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren C, Cornmark L, Lonne GK, Masoumi KC, Larsson C, 2016. Molecular characterization of protein kinase C delta (PKCdelta)-Smac interactions. BMC Biochem 17(1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MJ, Limesand KH, Schneider JC, Nakayama KI, Anderson SM, Reyland ME, 2006. Suppression of apoptosis in the protein kinase Cdelta null mouse in vivo. J Biol Chem 281(14), 9728–9737. [DOI] [PubMed] [Google Scholar]
- Humphries MJ, Ohm AM, Schaack J, Adwan TS, Reyland ME, 2008. Tyrosine phosphorylation regulates nuclear translocation of PKCdelta. Oncogene 27(21), 3045–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Kishimoto A, Takai Y, Nishizuka Y, 1977. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem 252(21), 7610–7616. [PubMed] [Google Scholar]
- Jan R, Chaudhry GE, 2019. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv Pharm Bull 9(2), 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SB, Vissink A, Limesand KH, Reyland ME, 2019. Salivary Gland Hypofunction and Xerostomia in Head and Neck Radiation Patients. J Natl Cancer Inst Monogr 2019(53). [DOI] [PubMed] [Google Scholar]
- Jin H, Kanthasamy A, Harischandra DS, Kondru N, Ghosh A, Panicker N, Anantharam V, Rana A, Kanthasamy AG, 2014. Histone hyperacetylation up-regulates protein kinase Cdelta in dopaminergic neurons to induce cell death: relevance to epigenetic mechanisms of neurodegeneration in Parkinson disease. J Biol Chem 289(50), 34743–34767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto T, Shirai Y, Sakai N, Yamamoto T, Matsuzaki H, Kikkawa U, Saito N, 2004. Ceramide-induced apoptosis by translocation, phosphorylation, and activation of protein kinase Cdelta in the Golgi complex. J Biol Chem 279(13), 12668–12676. [DOI] [PubMed] [Google Scholar]
- Kapiteijn E, Carlino M, Boni V, Loirat D, Speetjens F, Park J, Calvo E, Carvajal R, Nyakas M, Gonzalez-Maffe J, Zhu X, Thiruvamoor R, Yerramilli-Rao P, Piperno-Neumann S, 2019. A Phase I trial of LXS196, a novel PKC inhibitor for metastatic uveal melanoma (abstract). Cancer Research 79(Suppl. 13), CT068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedei N, Lewin NE, Geczy T, Selezneva J, Braun DC, Chen J, Herrmann MA, Heldman MR, Lim L, Mannan P, Garfield SH, Poudel YB, Cummins TJ, Rudra A, Blumberg PM, Keck GE, 2013. Biological profile of the less lipophilic and synthetically more accessible bryostatin 7 closely resembles that of bryostatin 1. ACS Chem Biol 8(4), 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedei N, Lubart E, Lewin NE, Telek A, Lim L, Mannan P, Garfield SH, Kraft MB, Keck GE, Kolusheva S, Jelinek R, Blumberg PM, 2011a. Some phorbol esters might partially resemble bryostatin 1 in their actions on LNCaP prostate cancer cells and U937 leukemia cells. Chembiochem 12(8), 1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedei N, Telek A, Czap A, Lubart ES, Czifra G, Yang D, Chen J, Morrison T, Goldsmith PK, Lim L, Mannan P, Garfield SH, Kraft MB, Li W, Keck GE, Blumberg PM, 2011b. The synthetic bryostatin analog Merle 23 dissects distinct mechanisms of bryostatin activity in the LNCaP human prostate cancer cell line. Biochem. Pharmacol 81(11), 1296–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U, Takai Y, Tanaka Y, Miyake R, Nishizuka Y, 1983. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem 258(19), 11442–11445. [PubMed] [Google Scholar]
- Kim H, Zhao J, Zhang Q, Wang Y, Lee D, Bai X, Turrell L, Chen M, Gao W, Keshavjee S, Liu M, 2016. deltaV1-1 Reduces Pulmonary Ischemia Reperfusion-Induced Lung Injury by Inhibiting Necrosis and Mitochondrial Localization of PKCdelta and p53. Am J Transplant 16(1), 83–98. [DOI] [PubMed] [Google Scholar]
- Kim RK, Suh Y, Hwang E, Yoo KC, Choi KS, An S, Hwang SG, Kim IG, Kim MJ, Lee HJ, Lee SJ, 2015. PKCδ maintains phenotypes of tumor initiating cells through cytokine-mediated autocrine loop with positive feedback. Oncogene 34(46), 5749–5759. [DOI] [PubMed] [Google Scholar]
- Kraft AS, Smith JB, Berkow RL, 1986. Bryostatin, an activator of the calcium phospholipid-dependent protein kinase, blocks phorbol ester-induced differentiation of human promyelocytic leukemia cells HL-60. Proc Natl Acad Sci U S A 83(5), 1334–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku GY, Ilson DH, Schwartz LH, Capanu M, O'Reilly E, Shah MA, Kelsen DP, Schwartz GK, 2008. Phase II trial of sequential paclitaxel and 1 h infusion of bryostatin-1 in patients with advanced esophageal cancer. Cancer Chemother Pharmacol 62(5), 875–880. [DOI] [PubMed] [Google Scholar]
- Kuehn HS, Niemela JE, Rangel-Santos A, Zhang M, Pittaluga S, Stoddard JL, Hussey AA, Evbuomwan MO, Priel DA, Kuhns DB, Park CL, Fleisher TA, Uzel G, Oliveira JB, 2013. Loss-of-function of the protein kinase C delta (PKCdelta) causes a B-cell lymphoproliferative syndrome in humans. Blood 121(16), 3117–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGory EL, Sitailo LA, Denning MF, 2010. The protein kinase Cdelta catalytic fragment is critical for maintenance of the G2/M DNA damage checkpoint. J Biol Chem 285(3), 1879–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langzam L, Koren R, Gal R, Kugel V, Paz A, Farkas A, Sampson SR, 2001. Patterns of protein kinase C isoenzyme expression in transitional cell carcinoma of bladder. Relation to degree of malignancy. Am J Clin Pathol 116(3), 377–385. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM, 2012. mTOR signaling in growth control and disease. Cell 149(2), 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebwohl M, Swanson N, Anderson LL, Melgaard A, Xu Z, Berman B, 2012. Ingenol mebutate gel for actinic keratosis. N Engl J Med 366(11), 1010–1019. [DOI] [PubMed] [Google Scholar]
- Lee PC, Fang YF, Yamaguchi H, Wang WJ, Chen TC, Hong X, Ke B, Xia W, Wei Y, Zha Z, Wang Y, Kuo HP, Wang CW, Tu CY, Chen CH, Huang WC, Chiang SF, Nie L, Hou J, Chen CT, Huo L, Yang WH, Deng R, Nakai K, Hsu YH, Chang SS, Chiu TJ, Tang J, Zhang R, Wang L, Fang B, Chen T, Wong KK, Hsu JL, Hung MC, 2018. Targeting PKCdelta as a Therapeutic Strategy against Heterogeneous Mechanisms of EGFR Inhibitor Resistance in EGFR-Mutant Lung Cancer. Cancer Cell 34(6), 954–969 e954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z, Wang J, Sun W, Chen X, Jiao W, Zhang H, Lei T, Li F, 2018. PKCδ reveals a tumor promoter function by promoting cell proliferation and migration in somatotropinomas. Int J Clin Exp Pathol 11(1), 208–215. [PMC free article] [PubMed] [Google Scholar]
- Leitges M, Mayr M, Braun U, Mayr U, Li C, Pfister G, Ghaffari-Tabrizi N, Baier G, Hu Y, Xu Q, 2001. Exacerbated vein graft arteriosclerosis in protein kinase Cdelta-null mice. J Clin Invest 108(10), 1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Wang X, Rasheed N, Hu Y, Boast S, Ishii T, Nakayama K, Nakayama KI, Goff SP, 2004. Distinct roles of c-Abl and Atm in oxidative stress response are mediated by protein kinase C delta. Genes Dev 18(15), 1824–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Li C, Wu M, Chen Q, Wang Q, Ren J, Zhang Y, 2015. PKCdelta stabilizes TAp63 to promote cell apoptosis. FEBS Lett 589(16), 2094–2099. [DOI] [PubMed] [Google Scholar]
- Li L, Lorenzo PS, Bogi K, Blumberg PM, Yuspa SH, 1999. Protein kinase Cdelta targets mitochondria, alters mitochondrial membrane potential, and induces apoptosis in normal and neoplastic keratinocytes when overexpressed by an adenoviral vector. Mol Cell Biol 19(12), 8547–8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, He T, Wu D, Zhang L, Chen R, Liu B, Yuan J, Tickner J, Qin A, Xu J, Rong L, 2020. Conditional Knockout of PKC-delta in Osteoclasts Favors Bone Mass Accrual in Males Due to Decreased Osteoclast Function. Front Cell Dev Biol 8, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Laishram RS, Ji Z, Barlow CA, Tian B, Anderson RA, 2012. Star-PAP control of BIK expression and apoptosis is regulated by nuclear PIPKIalpha and PKCdelta signaling. Mol Cell 45(1), 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limnander A, Depeille P, Freedman TS, Liou J, Leitges M, Kurosaki T, Roose JP, Weiss A, 2011. STIM1, PKC-delta and RasGRP set a threshold for proapoptotic Erk signaling during B cell development. Nat Immunol 12(5), 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li H, Zheng H, Zhai M, Lu F, Dong S, Fang T, Zhang W, 2019. CaSR activates PKCdelta to induce cardiomyocyte apoptosis via ER stressassociated apoptotic pathways during ischemia/reperfusion. Int J Mol Med 44(3), 1117–1126. [DOI] [PubMed] [Google Scholar]
- Liu H, Lu ZG, Miki Y, Yoshida K, 2007. Protein kinase C delta induces transcription of the TP53 tumor suppressor gene by controlling death-promoting factor Btf in the apoptotic response to DNA damage. Mol Cell Biol 27(24), 8480–8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Deng X, Wu D, Jin M, Yu B, 2019. PKCdelta promotes fertilization of mouse embryos in early development via the Cdc25B signaling pathway. Exp Ther Med 18(5), 3281–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Solimini NL, Elledge SJ, 2009. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136(5), 823–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Baumann C, Viveiros MM, 2015. Lack of protein kinase C-delta (PKCdelta) disrupts fertilization and embryonic development. Mol Reprod Dev. [DOI] [PubMed] [Google Scholar]
- Mackay HJ, Twelves CJ, 2007. Targeting the protein kinase C family: are we there yet? Nat Rev Cancer 7(7), 554–562. [DOI] [PubMed] [Google Scholar]
- Majumder PK, Mishra NC, Sun X, Bharti A, Kharbanda S, Saxena S, Kufe D, 2001. Targeting of protein kinase C delta to mitochondria in the oxidative stress response. Cell Growth Differ 12(9), 465–470. [PubMed] [Google Scholar]
- Majumder PK, Pandey P, Sun X, Cheng K, Datta R, Saxena S, Kharbanda S, Kufe D, 2000. Mitochondrial translocation of protein kinase C delta in phorbol ester-induced cytochrome c release and apoptosis. J Biol Chem 275(29), 21793–21796. [DOI] [PubMed] [Google Scholar]
- Marro BS, Zak J, Zavareh RB, Teijaro JR, Lairson LL, Oldstone MBA, 2019. Discovery of Small Molecules for the Reversal of T Cell Exhaustion. Cell Rep 29(10), 3293–3302 e3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoumi KC, Cornmark L, Lonne GK, Hellman U, Larsson C, 2012. Identification of a novel protein kinase Cdelta-Smac complex that dissociates during paclitaxel-induced cell death. FEBS Lett 586(8), 1166–1172. [DOI] [PubMed] [Google Scholar]
- Matassa AA, Carpenter L, Biden TJ, Humphries MJ, Reyland ME, 2001. PKCdelta is required for mitochondrial-dependent apoptosis in salivary epithelial cells. J Biol Chem 276(32), 29719–29728. [DOI] [PubMed] [Google Scholar]
- Matassa AA, Kalkofen RL, Carpenter L, Biden TJ, Reyland ME, 2003. Inhibition of PKCalpha induces a PKCdelta-dependent apoptotic program in salivary epithelial cells. Cell Death Differ 10(3), 269–277. [DOI] [PubMed] [Google Scholar]
- Mauro LV, Grossoni VC, Urtreger AJ, Yang C, Colombo LL, Morandi A, Pallotta MG, Kazanietz MG, Bal de Kier Joffe ED, Puricelli LL, 2010. PKC Delta (PKCdelta) promotes tumoral progression of human ductal pancreatic cancer. Pancreas 39(1), e31–41. [DOI] [PubMed] [Google Scholar]
- Mayr M, Chung YL, Mayr U, McGregor E, Troy H, Baier G, Leitges M, Dunn MJ, Griffiths JR, Xu Q, 2004a. Loss of PKC-delta alters cardiac metabolism. Am J Physiol Heart Circ Physiol 287(2), H937–945. [DOI] [PubMed] [Google Scholar]
- Mayr M, Siow R, Chung YL, Mayr U, Griffiths JR, Xu Q, 2004b. Proteomic and metabolomic analysis of vascular smooth muscle cells: role of PKCdelta. Circ Res 94(10), e87–96. [DOI] [PubMed] [Google Scholar]
- McKiernan E, O'Brien K, Grebenchtchikov N, Geurts-Moespot A, Sieuwerts AM, Martens JW, Magdolen V, Evoy D, McDermott E, Crown J, Sweep FC, Duffy MJ, 2008. Protein kinase Cdelta expression in breast cancer as measured by real-time PCR, western blotting and ELISA. Br J Cancer 99(10), 1644–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklenbrauker I, Saijo K, Zheng NY, Leitges M, Tarakhovsky A, 2002. Protein kinase Cdelta controls self-antigen-induced B-cell tolerance. Nature 416(6883), 860–865. [DOI] [PubMed] [Google Scholar]
- Minari R, Bordi P, Tiseo M, 2016. Third-generation epidermal growth factor receptor-tyrosine kinase inhibitors in T790M-positive non-small cell lung cancer: review on emerged mechanisms of resistance. Transl Lung Cancer Res 5(6), 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S, Nakayama KI, 2002. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature 416(6883), 865–869. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D, Das K, Grimes KV, 2012. Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov 11(12), 937–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd Mustapa MF, de Berker D, McGregor JM, Exton LS, Hughes BR, Levell NJ, 2020. Suspension of marketing authorization for ingenol mebutate. Br J Dermatol. [DOI] [PubMed] [Google Scholar]
- Monti C, Zilocchi M, Colugnat I, Alberio T, 2019. Proteomics turns functional. J Proteomics 198, 36–44. [DOI] [PubMed] [Google Scholar]
- Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D, 2004. Protein kinase Cdelta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J Biol Chem 279(46), 47985–47991. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Oliva JL, Kothapalli D, Fournier A, Assoian RK, Kazanietz MG, 2005. Phorbol ester-induced G1 phase arrest selectively mediated by protein kinase Cdelta-dependent induction of p21. J Biol Chem 280(40), 33926–33934. [DOI] [PubMed] [Google Scholar]
- Newton AC, 2001. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev 101(8), 2353–2364. [DOI] [PubMed] [Google Scholar]
- Newton AC, 2018. Protein kinase C: perfectly balanced. Critical Reviews in Biochemistry and Molecular Biology 0(0), 208–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC, Brognard J, 2017. Reversing the Paradigm: Protein Kinase C as a Tumor Suppressor. Trends Pharmacol Sci 38(5), 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen S, Majzner RG, Chien C, Qin H, Fry T, 2016. Determination of the effect of target site density on the efficacy of CD22 chimeric antigen receptor t-cell therapy to treat acute lymphoblastic leukemia. Journal of Clinical Oncology 34(15_suppl), 10536–10536. [Google Scholar]
- Ohm AM, Affandi T, Reyland ME, 2019. EGF receptor and PKCdelta kinase activate DNA damage-induced pro-survival and pro-apoptotic signaling via biphasic activation of ERK and MSK1 kinases. J Biol Chem 294(12), 4488–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohm AM, Tan AC, Heasley LE, Reyland ME, 2017. Co-dependency of PKCdelta and K-Ras: inverse association with cytotoxic drug sensitivity in KRAS mutant lung cancer. Oncogene 36(30), 4370–4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabla N, Dong G, Jiang M, Huang S, Kumar MV, Messing RO, Dong Z, 2011. Inhibition of PKCdelta reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J Clin Invest 121(7), 2709–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini R, Shao W, Sellers WR, 2015. Oncogene addiction: pathways of therapeutic response, resistance, and road maps toward a cure. EMBO Rep 16(3), 280–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Kim WK, Song M, Park M, Kim H, Nam HJ, Baek SH, Kim H, 2013. Protein kinase C-delta-mediated recycling of active KIT in colon cancer. Clin Cancer Res 19(18), 4961–4971. [DOI] [PubMed] [Google Scholar]
- Patel NA, Song SS, Cooper DR, 2006. PKCdelta alternatively spliced isoforms modulate cellular apoptosis in retinoic acid-induced differentiation of human NT2 cells and mouse embryonic stem cells. Gene Expr 13(2), 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Apostolatos A, Carter G, Ajmo J, Gali M, Cooper DR, You M, Bisht KS, Patel NA, 2013. Protein kinase C delta (PKCdelta) splice variants modulate apoptosis pathway in 3T3L1 cells during adipogenesis: identification of PKCdeltaII inhibitor. J Biol Chem 288(37), 26834–26846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J, Bellot G, Gounon P, Lacas-Gervais S, Pouyssegur J, Mazure NM, 2012. Glycogen Synthesis is Induced in Hypoxia by the Hypoxia-Inducible Factor and Promotes Cancer Cell Survival. Front Oncol 2, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perletti G, Marras E, Dondi D, Osti D, Congiu T, Ferrarese R, de Eguileor M, Tashjian AH Jr., 2005. p21(Waf1/Cip1) and p53 are downstream effectors of protein kinase C delta in tumor suppression and differentiation in human colon cancer cells. Int J Cancer 113(1), 42–53. [DOI] [PubMed] [Google Scholar]
- Petricoin E 3rd, Wulfkuhle J, Howard M, Pierobon M, Espina V, Luchini A, Liotta LA, 2019. RPPA: Origins, Transition to a Validated Clinical Research Tool, and Next Generations of the Technology. Adv Exp Med Biol 1188, 1–19. [DOI] [PubMed] [Google Scholar]
- Pettit GR, Day JF, Hartwell JL, Wood HB, 1970. Antineoplastic components of marine animals. Nature 227(5261), 962–963. [DOI] [PubMed] [Google Scholar]
- Pettit GR, Herald CL, Doubek DL, Herald DL, Arnold E, Clardy J, 1982. AntiNeoplastic Agents .86. Isolation and Structure of Bryostatin-1. J Am Chem Soc 104(24), 6846–6848. [Google Scholar]
- Propper DJ, Macaulay V, O'Byrne KJ, Braybrooke JP, Wilner SM, Ganesan TS, Talbot DC, Harris AL, 1998. A phase II study of bryostatin 1 in metastatic malignant melanoma. Br J Cancer 78(10), 1337–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam AJ, Schulz VV, Freiter EM, Bill HM, Miranti CK, 2009. Src, PKCalpha, and PKCdelta are required for alphavbeta3 integrin-mediated metastatic melanoma invasion. Cell Commun Signal 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Mochly-Rosen D, 2008. The PKCdelta -Abl complex communicates ER stress to the mitochondria - an essential step in subsequent apoptosis. J Cell Sci 121(Pt 6), 804–813. [DOI] [PubMed] [Google Scholar]
- Quintanal-Villalonga A, Chan JM, Yu HA, Pe'er D, Sawyers CL, Sen T, Rudin CM, 2020. Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat Rev Clin Oncol 17(6), 360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quissell DO, Barzen KA, Redman RS, Camden JM, Turner JT, 1998. Development and characterization of SV40 immortalized rat parotid acinar cell lines. In Vitro Cell Dev Biol Anim 34(1), 58–67. [DOI] [PubMed] [Google Scholar]
- Ramakrishna S, Highfill SL, Walsh Z, Nguyen SM, Lei H, Shern JF, Qin H, Kraft IL, Stetler-Stevenson M, Yuan CM, Hwang JD, Feng Y, Zhu Z, Dimitrov D, Shah NN, Fry TJ, 2019. Modulation of Target Antigen Density Improves CAR T-cell Functionality and Persistence. Clin Cancer Res 25(17), 5329–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razorenova OV, Finger EC, Colavitti R, Chernikova SB, Boiko AD, Chan CK, Krieg A, Bedogni B, LaGory E, Weissman IL, Broome-Powell M, Giaccia AJ, 2011. VHL loss in renal cell carcinoma leads to up-regulation of CUB domain-containing protein 1 to stimulate PKC{delta}-driven migration. Proc Natl Acad Sci U S A 108(5), 1931–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddig PJ, Dreckschmidt NE, Ahrens H, Simsiman R, Tseng CP, Zou J, Oberley TD, Verma AK, 1999. Transgenic mice overexpressing protein kinase Cdelta in the epidermis are resistant to skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res 59(22), 5710–5718. [PubMed] [Google Scholar]
- Renganathan H, Vaidyanathan H, Knapinska A, Ramos JW, 2005. Phosphorylation of PEA-15 switches its binding specificity from ERK/MAPK to FADD. Biochem J 390(Pt 3), 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reno EM, Haughian JM, Dimitrova IK, Jackson TA, Shroyer KR, Bradford AP, 2008. Analysis of protein kinase C delta (PKC delta) expression in endometrial tumors. Hum Pathol 39(1), 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyland ME, Anderson SM, Matassa AA, Barzen KA, Quissell DO, 1999. Protein kinase C delta is essential for etoposide-induced apoptosis in salivary gland acinar cells. J Biol Chem 274(27), 19115–19123. [DOI] [PubMed] [Google Scholar]
- Reyland ME, Jones DN, 2016. Multifunctional roles of PKCdelta: Opportunities for targeted therapy in human disease. Pharmacol Ther 165, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyland ME, Jones DN, 2016. Multifunctional roles of PKCdelta: Opportunities for targeted therapy in human disease. Pharmacology & Therapeutics In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roaten JB, Kazanietz MG, Caloca MJ, Bertics PJ, Lothstein L, Parrill AL, Israel M, Sweatman TW, 2002. Interaction of the novel anthracycline antitumor agent N-benzyladriamycin-14-valerate with the C1-regulatory domain of protein kinase C: structural requirements, isoform specificity, and correlation with drug cytotoxicity. Molecular cancer therapeutics 1(7), 483–492. [PubMed] [Google Scholar]
- Ryer EJ, Sakakibara K, Wang C, Sarkar D, Fisher PB, Faries PL, Kent KC, Liu B, 2005. Protein kinase C delta induces apoptosis of vascular smooth muscle cells through induction of the tumor suppressor p53 by both p38-dependent and p38-independent mechanisms. J Biol Chem 280(42), 35310–35317. [DOI] [PubMed] [Google Scholar]
- Saha K, Adhikary G, Kanade SR, Rorke EA, Eckert RL, 2014. p38delta regulates p53 to control p21Cip1 expression in human epidermal keratinocytes. J Biol Chem 289(16), 11443–11453. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Santiago-Walker AE, Fikaris AJ, Kao GD, Brown EJ, Kazanietz MG, Meinkoth JL, 2005. Protein kinase C delta stimulates apoptosis by initiating G1 phase cell cycle progression and S phase arrest. J Biol Chem 280(37), 32107–32114. [DOI] [PubMed] [Google Scholar]
- Schuhwerk H, Bruhn C, Siniuk K, Min W, Erener S, Grigaravicius P, Kruger A, Ferrari E, Zubel T, Lazaro D, Monajembashi S, Kiesow K, Kroll T, Burkle A, Mangerich A, Hottiger M, Wang ZQ, 2017. Kinetics of poly(ADP-ribosyl)ation, but not PARP1 itself, determines the cell fate in response to DNA damage in vitro and in vivo. Nucleic Acids Res 45(19), 11174–11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin L, Kato S, Franovic A, Camargo MF, Lesperance J, Elliott KC, Yebra M, Mielgo A, Lowy AM, Husain H, Cascone T, Diao L, Wang J, Wistuba II, Heymach JV, Lippman SM, Desgrosellier JS, Anand S, Weis SM, Cheresh DA, 2014. An integrin beta(3)-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat Cell Biol 16(5), 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serova M, Ghoul A, Benhadji KA, Faivre S, Le Tourneau C, Cvitkovic E, Lokiec F, Lord J, Ogbourne SM, Calvo F, Raymond E, 2008. Effects of protein kinase C modulation by PEP005, a novel ingenol angelate, on mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling in cancer cells. Mol Cancer Ther 7(4), 915–922. [DOI] [PubMed] [Google Scholar]
- Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, Settleman J, 2009. A gene expression signature associated with "K-Ras addiction" reveals regulators of EMT and tumor cell survival. Cancer Cell 15(6), 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitailo LA, Tibudan SS, Denning MF, 2006. The protein kinase C delta catalytic fragment targets Mcl-1 for degradation to trigger apoptosis. J Biol Chem 281(40), 29703–29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff SP, 2007. Rottlerin: an inappropriate and ineffective inhibitor of PKCdelta. Trends Pharmacol Sci 28(9), 453–458. [DOI] [PubMed] [Google Scholar]
- Soriano-Carot M, Quilis I, Bano MC, Igual JC, 2014. Protein kinase C controls activation of the DNA integrity checkpoint. Nucleic Acids Res 42(11), 7084–7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CM, Weng YS, Kuan LY, Chen JH, Hsu FT, 2020. Suppression of PKCdelta/NF-kappaB Signaling and Apoptosis Induction through Extrinsic/Intrinsic Pathways Are Associated Magnolol-Inhibited Tumor Progression in Colorectal Cancer In Vitro and In Vivo. Int J Mol Sci 21(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds JM, Ohm AM, Carter CJ, Heasley LE, Boyle TA, Franklin WA, Reyland ME, 2011. Protein kinase C delta is a downstream effector of oncogenic K-ras in lung tumors. Cancer Res 71(6), 2087–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds JM, Ohm AM, Tan AC, Reyland ME, 2016. PKCdelta regulates integrin alphaVbeta3 expression and transformed growth of K-ras dependent lung cancer cells. Oncotarget 7(14), 17905–17919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi Z, Denning MF, Smith CB, Dlugosz AA, Yuspa SH, Pettit GR, Blumberg PM, 1994a. Bryostatin 1 protects protein kinase C-delta from down-regulation in mouse keratinocytes in parallel with its inhibition of phorbol ester-induced differentiation. Mol Pharmacol 46(5), 840–850. [PubMed] [Google Scholar]
- Szallasi Z, Smith CB, Pettit GR, Blumberg PM, 1994b. Differential regulation of protein kinase C isozymes by bryostatin 1 and phorbol 12-myristate 13-acetate in NIH 3T3 fibroblasts. J. Biol. Chem 269(3), 2118–2124. [PubMed] [Google Scholar]
- Takai Y, Kishimoto A, Inoue M, Nishizuka Y, 1977. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem 252(21), 7603–7609. [PubMed] [Google Scholar]
- Tanaka Y, Gavrielides MV, Mitsuuchi Y, Fujii T, Kazanietz MG, 2003. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. J Biol Chem 278(36), 33753–33762. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Gavrielides MV, Mitsuuchi Y, Fujii T, Kazanietz MG, 2003. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. The Journal of biological chemistry 278(36), 33753–33762. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O'Brien JM, Simpson EM, Barsh GS, Bastian BC, 2009. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457(7229), 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green G, Bouvier N, Sozen MM, Baimukanova G, Roy R, Heguy A, Dolgalev I, Khanin R, Busam K, Speicher MR, O'Brien J, Bastian BC, 2010. Mutations in GNA11 in uveal melanoma. N Engl J Med 363(23), 2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varterasian ML, Mohammad RM, Eilender DS, Hulburd K, Rodriguez DH, Pemberton PA, Pluda JM, Dan MD, Pettit GR, Chen BD, Al-Katib AM, 1998. Phase I study of bryostatin 1 in patients with relapsed non-Hodgkin's lymphoma and chronic lymphocytic leukemia. J Clin Oncol 16(1), 56–62. [DOI] [PubMed] [Google Scholar]
- Varterasian ML, Mohammad RM, Shurafa MS, Hulburd K, Pemberton PA, Rodriguez DH, Spadoni V, Eilender DS, Murgo A, Wall N, Dan M, Al-Katib AM, 2000. Phase II trial of bryostatin 1 in patients with relapsed low-grade non-Hodgkin's lymphoma and chronic lymphocytic leukemia. Clin Cancer Res 6(3), 825–828. [PubMed] [Google Scholar]
- Vogel C, Marcotte EM, 2012. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13(4), 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Burstin VA, Xiao L, Kazanietz MG, 2010. Bryostatin 1 Inhibits Phorbol Ester-Induced Apoptosis in Prostate Cancer Cells by Differentially Modulating Protein Kinase C (PKC) Translocation and Preventing PKC -Mediated Release of Tumor Necrosis Factor- Mol. Pharmacol. 78(3), 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DA, Perkins JP, Krebs EG, 1968. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem 243(13), 3763–3765. [PubMed] [Google Scholar]
- Wei H, Yu X, 2016. Functions of PARylation in DNA Damage Repair Pathways. Genomics Proteomics Bioinformatics 14(3), 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wender PA, Cribbs CM, Koehler KF, Sharkey NA, Herald CL, Kamano Y, Pettit GR, Blumberg PM, 1988. Modeling of the bryostatins to the phorbol ester pharmacophore on protein kinase C. Proc Natl Acad Sci U S A 85(19), 7197–7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wender PA, Hardman CT, Ho S, Jeffreys MS, Maclaren JK, Quiroz RV, Ryckbosch SM, Shimizu AJ, Sloane JL, Stevens MC, 2017. Scalable synthesis of bryostatin 1 and analogs, adjuvant leads against latent HIV. Science 358(6360), 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermuth PJ, Addya S, Jimenez SA, 2011. Effect of protein kinase C delta (PKC-delta) inhibition on the transcriptome of normal and systemic sclerosis human dermal fibroblasts in vitro. PLoS One 6(11), e27110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wie SM, Adwan TS, DeGregori J, Anderson SM, Reyland ME, 2014. Inhibiting tyrosine phosphorylation of protein kinase Cdelta (PKCdelta) protects the salivary gland from radiation damage. J Biol Chem 289(15), 10900–10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wie SM, Wellberg E, Karam SD, Reyland ME, 2017. Tyrosine Kinase Inhibitors Protect the Salivary Gland from Radiation Damage by Inhibiting Activation of Protein Kinase C-delta. Mol Cancer Ther 16(9), 1989–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FH, Johannessen CM, Piccioni F, Tamayo P, Kim JW, Van Allen EM, Corsello SM, Capelletti M, Calles A, Butaney M, Sharifnia T, Gabriel SB, Mesirov JP, Hahn WC, Engelman JA, Meyerson M, Root DE, Janne PA, Garraway LA, 2015. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell 27(3), 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Zhang AX, Newton AC, 2013. Protein kinase C pharmacology: refining the toolbox. The Biochemical journal 452(2), 195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Chen Z, Forman LW, Faller DV, 2009. PKCdelta survival signaling in cells containing an activated p21Ras protein requires PDK1. Cell Signal 21(4), 502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Forman LW, Faller DV, 2007. Protein kinase C delta is required for survival of cells expressing activated p21RAS. J Biol Chem 282(18), 13199–13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Su L, Liu X, 2012. PKCdelta regulates death receptor 5 expression induced by PS-341 through ATF4-ATF3/CHOP axis in human lung cancer cells. Mol Cancer Ther 11(10), 2174–2182. [DOI] [PubMed] [Google Scholar]
- Yadav V, Yanez NC, Fenton SE, Denning MF, 2010. Loss of protein kinase C delta gene expression in human squamous cell carcinomas: a laser capture microdissection study. Am J Pathol 176(3), 1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S, Fujii K, Zhao C, Takagi H, Katakura Y, 2016. Involvement of the NFX1-repressor complex in PKC-δ-induced repression of hTERT transcription. J Biochem 160(5), 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, 2008. Nuclear trafficking of pro-apoptotic kinases in response to DNA damage. Trends Mol Med 14(7), 305–313. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Liu H, Miki Y, 2006. Protein kinase C delta regulates Ser46 phosphorylation of p53 tumor suppressor in the apoptotic response to DNA damage. J Biol Chem 281(9), 5734–5740. [DOI] [PubMed] [Google Scholar]
- Yu HP, Xie JM, Li B, Sun YH, Gao QG, Ding ZH, Wu HR, Qin ZH, 2015. TIGAR regulates DNA damage and repair through pentosephosphate pathway and Cdk5-ATM pathway. Sci Rep 5, 9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Yangmei Z, Rongrong S, Xiaowu L, Youwei Z, Sun S, 2019. Sotrastaurin attenuates the stemness of gastric cancer cells by targeting PKCδ. Biomed Pharmacother 117, 109165. [DOI] [PubMed] [Google Scholar]
- Zhao CT, Li K, Li JT, Zheng W, Liang XJ, Geng AQ, Li N, Yuan XB, 2009. PKCdelta regulates cortical radial migration by stabilizing the Cdk5 activator p35. Proc Natl Acad Sci U S A 106(50), 21353–21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivotovsky B, Kroemer G, 2004. Apoptosis and genomic instability. Nat Rev Mol Cell Biol 5(9), 752–762. [DOI] [PubMed] [Google Scholar]
- Zonder JA, Shields AF, Zalupski M, Chaplen R, Heilbrun LK, Arlauskas P, Philip PA, 2001. A phase II trial of bryostatin 1 in the treatment of metastatic colorectal cancer. Clin Cancer Res 7(1), 38–42. [PubMed] [Google Scholar]