Abstract
This study examined the effects of oral administration of water extract of chlorella (WEC) (100 mg/kg bodyweight) and phenethylamine (10 μg/kg bodyweight) on high-fat diet (HFD)-induced liver damage in mice. Phenethylamine significantly mitigated HFD-induced lipid oxidation (generation of malondialdehyde) and liver damage without markedly decreasing hepatic lipid accumulation. WEC exerted similar effects although with decreased efficacy. In addition, WEC and phenethylamine decreased the methylglyoxal levels and increased the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein levels in the liver. Methylglyoxal is generated from substrates of GAPDH, dihydroxyacetone phosphate and glyceraldehyde 3-phosphate. These facts indicate that methylglyoxal triggers oxidation of accumulated lipid, which generates malondialdehyde and consequently induces liver damage. Suppression of generation of toxic aldehydes by WEC and phenethylamine was also confirmed by maintaining hepatic cysteine, highly reactive to aldehydes. Thus, trace amounts of phenethylamine alleviate HFD-induced liver damage by regulating methylglyoxal via increase of GAPDH.
Subject terms: Nutrition, Obesity
Introduction
Chlorella pyrenoidosa, a freshwater unicellular green alga, and its water extract have a long history of usage as food supplements. Various animal studies and clinical trials have reported that C. pyrenoidosa exhibits biological activities, such as anti-dyslipidemic and immunomodulatory activities upon oral administration1,2. Previously, we had identified phenethylamine (PHA) in the hot water extract of C. pyrenoidosa (WEC) at 10 μg/g dry matter and demonstrated that low dose (60 ng/g of feed) of PHA extends the lifespan of superoxide dismutase-1 gene (Sod1) mutant adults of Drosophila melanogaster3. This suggested that treatment with a low dose of PHA ameliorates oxidative stress in D. melanogaster, as SOD-1 plays a substantial role in the antioxidant system.
Trace amounts of PHA, which is classified as a monoamine, are detected in various types of food, such as cheese, chocolate, wine, fermented soy paste, and soy sauce4–8. In mammals, PHA is generated from phenylalanine through enzymatic decarboxylation. The oral supplementation of PHA (10–60 mg/day) with selegiline (monoamine oxidase-B inhibitor; 10 mg/day) alleviated depression in humans9. The administration of high doses of PHA (25–75 mg/kg bodyweight) promoted psychomotor dysfunction and decrease in striatal biogenic amines in mice10. Previously, we had reported the biological activity of trace amounts of PHA in D. melanogaster. However, the biological activity of trace amounts of PHA has not been examined in mammals. This study aimed to elucidate the beneficial effects of trace amounts of PHA in mammals.
A previous animal study demonstrated that the administration of dried powder of C. pyrenoidosa ameliorated high-fat diet (HFD)-induced dyslipidemia in rats1. HFD promotes hepatic damage in rats by increases in lipid deposition and lipid peroxidation and decreases in antioxidant enzyme activities in the liver11,12, which have been reported to induce hepatitis, especially non-alcoholic fatty liver disease (NAFLD)13,14. There is an increased prevalence of NAFLD in both advanced and developing countries. The enhanced lipid deposition-induced oxidative stress in the liver contributes to the pathogenesis of NAFLD13,14, whereas the liver contains potent antioxidant enzyme systems, such as the superoxide dismutase (SOD) and glutathione peroxidase (GPX) systems15,16. The mechanism underlying increased oxidative stress in the fatty liver has not been elucidated. Recently, methylglyoxal was reported to increase intracellular oxidative stress17–19. Methylglyoxal can be generated from metabolites of glucose and fructose, such as dihydroxyacetone phosphate and glyceraldehyde 3-phosphate20, which are metabolized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH). In addition, methylglyoxal strongly reacts with the sulfhydryl, amino and guanidyl groups in amino acids and proteins21,22. Methylglyoxal-derived advanced glycation end products (AGEs), promotes the progression of steatosis to NAFLD and non-alcoholic steatohepatitis (NASH)23. Various studies have examined the inhibitory effect of food compounds on the formation of toxic AGEs using in vitro and in vivo system24,25. However, the administration of food compounds cannot effectively inhibit the generation of methylglyoxal.
This study aimed to examine the effects of trace amounts of PHA on HFD-induced oxidative stress and liver damage in mice. We found that PHA suppressed the peroxidation of lipids accumulated in the liver by decreasing the generation of methylglyoxal via increasing GAPDH in the liver.
Results
Effect of HFD feeding on mice
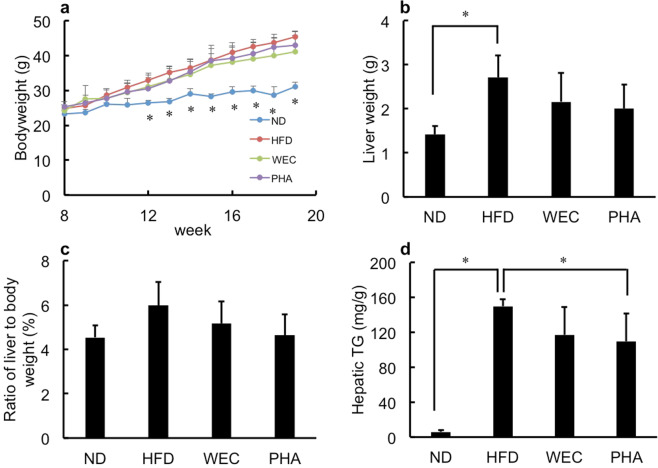
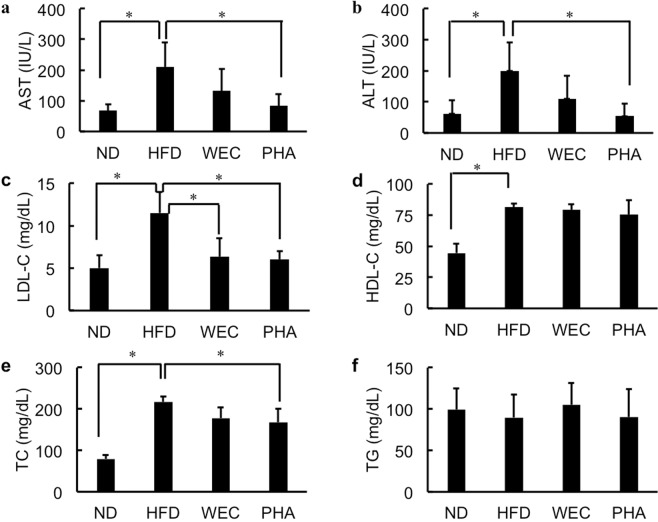
Induction of NAFLD by a high-fat diet in the C57BL/6 strain is widely used among mice26. As shown in Figs. 1 and 2, HFD feeding induced obesity, fatty liver, liver damage, and increase of plasma low-density lipoprotein-cholesterol (LDL-C). Furthermore, increased lipid peroxidation and decreased antioxidant enzyme activities (SOD and GPX) were observed (Fig. 3).
Fig. 1. The effects of WEC and PHA on bodyweight and liver wight.
Bodyweight change (a), liver weight (b), ratio of liver weight to bodyweight (c), and hepatic triglyceride levels (d) in the normal diet-fed (ND), high-fat diet-fed (HFD), Chlorella pyrenoidosa water extract-treated (WEC), and phenethylamine-treated (PHA) groups. The WEC and PHA groups were fed on HFD and administered with WEC (100 mg/kg bodyweight) and PHA (10 μg/kg bodyweight), respectively. Data are represented as mean ± standard deviation (n = 6). The means were compared using analysis of variance, followed by Dunnett’s test. *p < 0.05 vs. HFD group.
Fig. 2. The effects of WEC and PHA on plasma biochemical parameters.
Plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities (a, b) and the levels of low-density lipoprotein-cholesterol (LDL-C; c), high-density lipoprotein-cholesterol (HDL-C; d), total cholesterol (TC; e), and triglyceride (TG; f) in the normal diet-fed (ND), high-fat diet-fed (HFD), Chlorella pyrenoidosa water extract-treated (WEC), and phenethylamine-treated (PHA) groups. Data are represented as mean ± standard deviation (n = 6). The means were compared using analysis of variance, followed by Dunnett’s test. *p < 0.05 vs. HFD group.
Fig. 3. The effects of WEC and PHA on oxidative stress in liver.
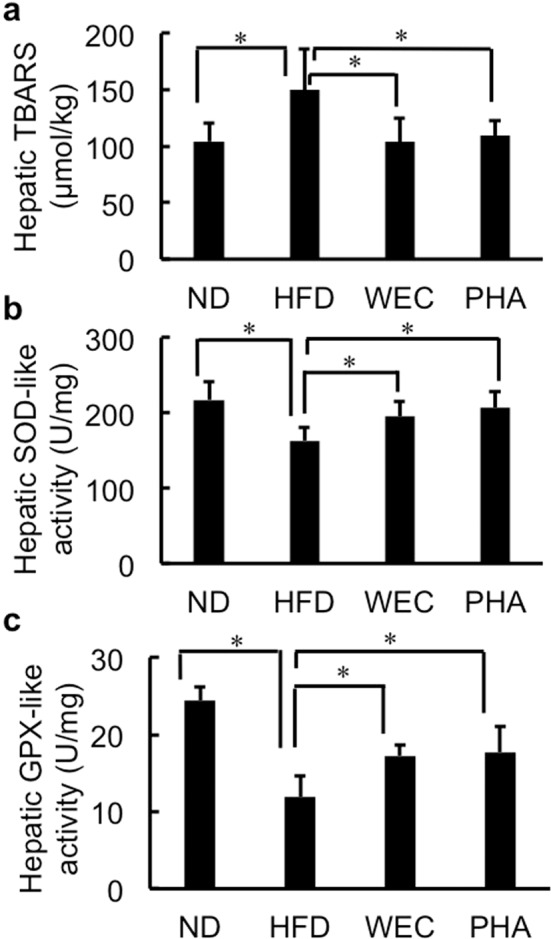
Thiobarbituric acid reactive substances (TBARS); (a) values (a), superoxide dismutase (SOD)-like activity (b), glutathione peroxidase (GPX)-like activity (c) in the liver of the normal diet-fed (ND), high-fat diet-fed (HFD), Chlorella pyrenoidosa water extract-treated (WEC), and phenethylamine-treated (PHA) groups. Data are represented as mean ± standard deviation (n = 6). The means were compared using analysis of variance, followed by Dunnett’s test. *p < 0.05 vs. HFD group.
Effects of WEC and PHA on bodyweight and liver weight
The total calorie intake was similar among the HFD, WEC, and PHA groups. However, the total calorie intake in the HFD, WEC, and PHA groups was higher than that in the normal diet (ND) group (Supplementary Fig. S1). The addition of WEC and PHA into drinking water did not affect water intake (Supplementary Fig. S1). The administration of WEC and PHA did not significantly affect bodyweight and liver weight of HFD-fed mice (Fig. 1a, b). The ratio of liver weight to bodyweight was the highest in the HFD group (Fig. 1c). However, the ratio of liver weight to bodyweight was not significantly different between all groups (including ND group). The ratio of liver weight to bodyweight in PHA groups was lower than that in the HFD group (p < 0.1; Fig. 1c).
Effects of WEC and PHA on liver and blood biochemical parameters
The administration of PHA significantly but slightly decreased liver TG (p < 0.05), while WEC did not significantly affect liver TG (Fig. 1d). PHA also significantly mitigated the HFD-induced enhanced plasma AST and ALT activities (p < 0.05; Fig. 2a, b). WEC showed similar tendency without statistic difference. WEC and PHA significantly decreased plasma LDL-C levels without affecting HDL-C levels (p < 0.05; Fig. 2c, d). In addition, PHA also significantly decreased TC (p < 0.05; Fig. 2e). As shown in Fig. 2f, the plasma TG levels were not significantly different between the HFD and other groups (ND, PHA, and WEC).
Effects of WEC and PHA on oxidative stress in the liver
The administration of WEC and PHA significantly mitigated the HFD-induced enhanced lipid peroxidation (p < 0.05; Fig. 3a) and significantly increased SOD-like and GPX-like activities in liver extract (p < 0.05; Fig. 3b, c). In contrast, low molecular weight compounds in the 75% ethanol-soluble fraction of the liver extract from all groups had negligible SOD-like activity (Supplementary Fig. S2).
Effects of WEC and PHA on hepatic methylglyoxal levels
The hepatic methylglyoxal levels were not significantly different between the HFD and ND groups. In contrast, the administration of WEC and PHA significantly decreased hepatic methylglyoxal levels. (p < 0.05; Fig. 4).
Fig. 4. The effects of WEC and PHA on hepatic methylglyoxal levels.
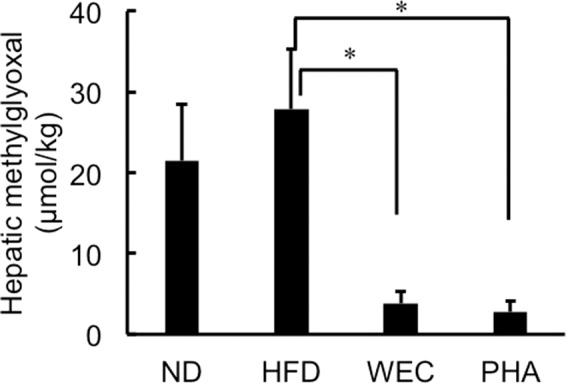
Hepatic methylglyoxal levels in the normal diet-fed (ND), high-fat diet-fed (HFD), Chlorella pyrenoidosa water extract-treated (WEC), and phenethylamine-treated (PHA) groups. Data are represented as mean ± standard deviation (n = 6). The means were compared using analysis of variance, followed by Dunnett’s test. *p < 0.05 vs. HFD group.
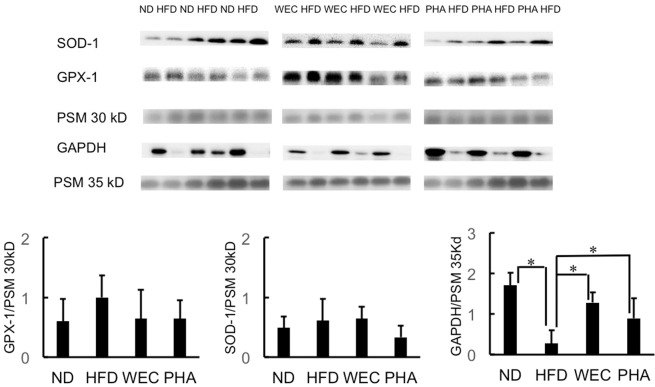
Effects of WEC and PHA on hepatic SOD1, GPX1, and GAPDH levels
In western blotting analysis, the housekeeping proteins, such as, GAPDH and β-actin, are used as loading controls. However, HFD feeding significantly decreased GAPDH levels (Fig. 5 and Supplementary Fig. S3) and tended to decrease β-actin (Supplementary Figs. S3 and S4). Among some mice in the HFD group, only a faint band of GAPDH was observed, whereas the pre-stained marker (35 kD) added to the sample exhibited similar band intensity. Thus, a pre-stained marker was used to normalize the expression levels of target proteins. As shown in Fig. 5, the SOD-1 and GPX-1 protein levels were not significantly different between the HFD and other groups (ND, PHA and WEC). On the other hand, the administration of WEC and PHA significantly increased GAPDH levels (p < 0.05; Fig. 5).
Fig. 5. The effects of WEC and PHA on hepatic protein levels.
Hepatic protein levels of SOD-1, GPX-1, and GAPDH in the normal diet-fed (ND), high-fat diet-fed (HFD), Chlorella pyrenoidosa water extract-treated (WEC), and phenethylamine-treated (PHA) groups. Data are represented as mean ± standard deviation (n = 6). The means were compared using analysis of variance, followed by Dunnett’s test. *p < 0.05 vs. HFD group. PSM, pre-stained marker.
Effect of WEC and PHA on hepatic cysteine levels
The hepatic cysteine levels were not significantly different before and after dithiothreitol (DTT) treatment in all groups (Supplementary Fig. S5), which indicated that most of the soluble cysteine in the liver exists in the reduced form. As shown in Fig. 6, the hepatic cysteine level in the ND group was significantly higher than those in the HFD group (p < 0.05). The administration of WEC and PHA significantly increased the hepatic cysteine levels in the mice fed on HFD to the hepatic levels observed in the ND group (p < 0.05).
Fig. 6. The effects of WEC and PHA on hepatic cysteine levels.
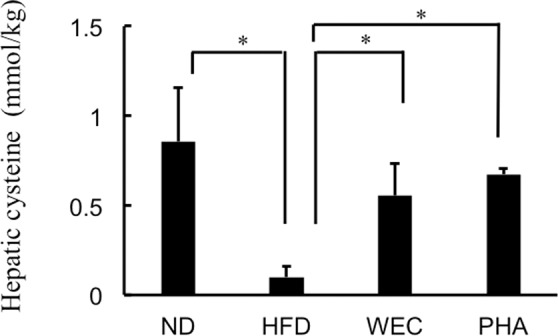
Hepatic levels of cysteine in the normal diet-fed (ND), high-fat diet-fed (HFD), Chlorella pyrenoidosa water extract-treated (WEC), and phenethylamine-treated (PHA) groups. Data are represented as mean ± standard deviation (n = 6). The means were compared using analysis of variance, followed by Dunnett’s test. *p < 0.05 vs. HFD group.
Discussion
PHA promotes reactive oxygen species production in the yeast and plant (tobacco) cell culture systems27,28. Additionally, the oral administration or injection of PHA (> 25 mg/kg bodyweight) resulted in impaired behaviors in rodents29,30. These findings indicate that PHA exerts adverse effects at high doses, which may be through the induction of enhanced oxidative stress. Previously, we had demonstrated that trace amounts of PHA (60 ng/g of feed) extended the life span of a Sod1 mutant adult of D. melanogaster3. However, the activity of such low dose of PHA has not been examined in mammals. In this study, the treatment dose of PHA for mice was determined based on the content of PHA in the diet of D. melanogaster (60 ng/g in feed) and daily food intake of mice (~4 g/20 g bodyweight/day), which corresponded to 12 μg/kg bodyweight. Thus, the HFD-fed mice were administered with PHA at a dose of 10 μg/kg bodyweight through drinking water in this study.
As summarized in Table 1, PHA significantly mitigated HFD-induced lipid peroxidation and liver damage without markedly decreasing hepatic lipid accumulation. It is worthy to note that PHA significantly increased hepatic GAPDH and cysteine levels and decresed methylglyoxal levels. WEC exerted similar effects with decreased efficacy.
Table 1.
Summary for responses by administration of WEC and PHA on mice fed high fat diet.
| WEC | PHA | |
|---|---|---|
| Body weight | NS | NS |
| Liver TG | NS | slightly down |
| Plasma AST and ALT | NS | down |
| Plasma LDL-C | down | down |
| Plasma HDL-C | NS | NS |
| Hepatic TBARS | down | down |
| Hepatic SOD-like activity | up | up |
| Hepatic GPX-like activity | up | up |
| Hepatic SOD-1 level | NS | NS |
| Hepatic GPX-1 level | NS | NS |
| Hepatic GAPDH level | up | up |
| Hepatic methylglyoxal | down | down |
| Hepatic cysteine | up | up |
NS: no significant change (p > 0.05).
Some antioxidant foods or compounds, such as ascorbic acid (30 mg/kg bodyweight)31, onion oil (100 mg/kg bodyweight) and/or garlic tablets (500 mg/kg bodyweight)32, and green tea extract (1% w/w) in diet33, as well as epigallocatechin gallate (1 g/L via drinking water)34, can attenuate hepatic oxidative stress in rodents induced by HFD. However, the doses of antioxidants or antioxidant foods used in previous studies were far higher than that of PHA used in this study (10 μg/kg bodyweight). PHA did not significantly increase SOD-like activity of low molecular weight compounds in liver. This indicated that the beneficial effects of such low dose of PHA do not involve direct antioxidant activity but involve the modulation of the expression of endogenous proteins. Actually, antioxidant enzyme activities (SOD-like and GPX-like activities) were significantly increased in the PHA group. Unexpectedly, WEC and PHA did not significantly affect the hepatic SOD-1 and GPX-1 protein levels. On the other hand, HFD feeding significantly decreased GAPDH protein levels, which is consistent with the previous stidies35–37. WEC and PHA significantly increased the hepatic GAPDH protein levels.
GAPDH, a key glycolytic enzyme, can decrease the levels of glyceraldehyde 3-phosphate and its isomer (dihydroxyacetone phosphate), which are precursors of methylglyoxal20. Beisswenger et al. demonstrated that the methylglyoxal level in the human blood red cells was positively and negatively correlated with dihydroxyacetone phosphate levels and GAPDH activity, respectively38. Methylglyoxal, a highly reactive short-chain aldehyde, induces oxidative stress in the cells17–19. In addition, methylglyoxal reacts with the sulfhydryl group in free cysteine and cysteinyl residues of proteins21. In the present study, the hepatic methylglyoxal levels were not significantly different between the ND and HFD groups, while GAPDH level was significantly higher in ND group, which was possibly due to high carbohydrate loading in ND group. The hepatic lipid accumulation in the HFD group was significantly higher than that in the ND group. Methylglyoxal might trigger oxidation of excess amount of fat in the liver. This assumption was supported by the significant decrease of lipid oxidation (TBARS) in liver with decrease of methylglyoxal via administration of WEC and PHA. Fat-derived malondialdehyde can also react with sulfhydryl groups in cysteine39. Increased amount of hepatic level of malondialdehyde (~50 μmol/kg) was higher than amount of methylglyoxal in the HFD group. Thus, higher amounts of malondialdehyde in liver of HFD group decreased free cysteine level compared to ND group. Shortage of cysteine promoted the reaction of short-chain aldehydes, such as methylglyoxal and malondialdehyde, with the sulfhydryl groups of proteins, including SOD-1 and GPX-1 and decreases their activity without decreasing the protein levels. Based on the findings of this study, we proposed a hypothesis that HFD-induced decreased GAPDH levels can maintain high methylglyoxal level despite low carbohydrate loading, which triggers the oxidation of fat deposited in the liver and consequently increases fat-derived malondialdehyde. Furthermore, malondialdehyde and methylglyoxal denature antioxidant enzymes by reacting with the sulfhydryl groups and induce liver damage. In contrast, free cysteine can protect against liver damage by reacting with methylglyoxal and malondialdehyde. On the basis of these findings, we proposed a novel approach to alleviate NAFLD involves the suppression of methylglyoxal formation by increasing GAPDH, which maintains the anti-oxidation activity in the fatty liver. To the best of our knowledge, increase of GAPDH by food compound has not been reported. The dose of PHA used in this study (10 μg/kg bodyweight) can be obtained through the consumption of some fermented foods4–8. Thus, food habits can improve liver function through GAPDH-methylglyoxal pathway.
Some in vitro studies have suggested that mild oxidative stress and hypoxia up-regulate the expression of GAPDH40–42, whereas high oxidative stress aggregates and inactivates GAPDH and consequently induces the apoptosis of cells43,44. Hypoxia-induced factor (HIF) plays an important role in the up-regulation of GAPDH45. The HIF level is regulated by several enzymes. There are ongoing studies to examine the effect of PHA on the expression of proteins involved in the regulation of GAPDH levels, such as HIF.
The administration of WEC (100 mg/kg bodyweight) decreased the plasma LDL-C levels and hepatic lipid oxidation, which are consistent with the results of a previous study1. In addition, effects of WEC on hepatic GAPDH, methylglyoxal, and cysteine are similar to PHA as summarized in Table 1. The consumption of WEC at a dose of 100 mg/kg bodyweight can achieve a PHA dose of ~1 μg/kg bodyweight3. Furthermore, WEC contains 440 mg/100 g cysteine and cystine as half cystine, which is similar level in HFD (430 mg/100 g) (Table 2). However, average consumption of HFD per day (~4 g/20 g body weight) was 2000 times higher than the dose of WEC (2 mg/20 g). Therefore, effect of supplementation of cystine and cysteine from WEC on liver functions could be neglected. Hence, PHA in WEC may contribute to the beneficial activities as summarized in Table 1 including LDL-C-lowering activity of WEC. The LDL-C-lowering activity exhibited by a low dose of phytochemical compounds is an interesting finding. The mechanism involved in the LDL-lowering effect of trace amounts of PHA has not been elucidated.
Table 2.
Proximate composition and cystine content of animal diets (%).
| ND | HFD | |
|---|---|---|
| Water | 7.9 | 6.2 |
| Crude protein | 23.1 | 25.5 |
| Crude fat | 5.1 | 32 |
| Crude ash | 5.8 | 4.0 |
| Crude fiber | 2.8 | 2.9 |
| Carbohydrate | 55.3 | 29.4 |
| Cystine | 0.36 | 0.43 |
ND normal diet, HFD high-fat diet.
Methods
Materials and reagents
WEC was prepared and supplied by Sun Chlorella (Kyoto, Japan). WEC contained ~10 μg/g PHA in dry matter and 4.4 mg/g half cystine (information from supplier). PHA, proteinase inhibitor cocktail, butylated hydroxytoluene (BHA), phosphate-buffered saline (PBS, pH 7.4), diethylenetriamine pentaacetic acid (DTPA), 1,1,3,3-tetraethoxypropane (TEP), thiobarbituric acid (TBA), 2,3-diaminonaphthalene (DAN), enhanced chemiluminescence (ECL) reagent, acetonitrile (high-performance liquid chromatography grade), and 4-vinylpyridine (4-VP) were purchased from Nacalai Tesque (Kyoto, Japan). Toronto Research Chemicals (Toronto, ON, Canada) provided 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AccQ). Heparin sodium was obtained from Nipro (Osaka, Japan). Cell lysis reagent was obtained from Sigma-Aldrich (St. Louis, MO, USA). Tween 20 was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Rabbit immunoglobulin G (IgG) against mouse SOD-1 and GPX-1 were obtained from Abcam (Cambridge, UK). Mouse anti-GAPDH and β-actin monoclonal antibodies, were obtained from Proteintech (Chicago, IL, USA) and Santa Cruz Biotechnology. Goat horseradish peroxidase (HRP)-conjugated rabbit and mouse IgGs were obtained from Cell Signaling Technology (Danvers, MA, USA). Stable isotope-labeled L-cysteine (L-cysteine-¹³C3,15N) was purchased from Taiyo Nippon Sanso (Tokyo, Japan).
Animal experiments
All animal experiments were performed according to the guidelines of the National Institutes of Health for the use of experimental animals. The experimental procedures were performed at the Louis Pasteur Center for Medical Research (Kyoto, Japan) and were approved by its Animal Care Committee (No. 20192).
Male C57BL/6 J mice (aged 7 weeks; bodyweight, 21–23 g) were purchased from Japan SLC (Shizuoka, Japan). The mice, which were housed in cages (three mice/cage), were maintained at 22 °C under a 12-h light/dark cycle with free access to rodent chow (Certified Diet MF, Oriental Yeast, Osaka, Japan) and tap water for 3 days. Subsequently, the mice were randomly divided into the following four groups (n = 6): ND group, fed on a standard diet; HFD group, fed on HFD; WEC group, fed on HFD and orally administered with WEC (100 mg/kg bodyweight) through drinking water; PHA group, fed on HFD and orally administered with PHA (10 μg/kg bodyweight) through drinking water. The proximate composition of animal diets is shown in Table 2. HFD (high-fat diet 32, CLEA Japan, Tokyo, Japan) comprises 32% crude fat with 60% of the calories derived from the fat. The amounts of WEC and PHA in drinking water were calculated based on the consumption of drinking water by mice in the previous 3 days. All mice received experimental diets for 12 weeks. The mice were then euthanized under isoflurane in the morning without fasting. The blood samples were collected from the inferior vena cava using a heparin-treated syringe. The plasma was separated by centrifuging the blood samples at 410 g for 5 min. The liver was excised and the blood in the liver was purged by infusing cold PBS into the portal vein. The plasma and liver samples were stored at −30 °C.
Biochemical analyses
The analysis of plasma AST and ALT activities and the plasma levels of TG, TC, HDL-C, and LDL-C contents were outsourced to Oriental Yeast (Osaka, Japan). To determine the TG levels in the liver, liver samples (20–30 mg) were homogenized in 200 μL of isopropanol using a BioMasher II (Nippi, Tokyo, Japan). The homogenates were centrifuged at 1100 × g for 10 min. The supernatant was subjected to a TG assay using the TG E-test Wako kit (Wako, Osaka, Japan).
TBARS assay
The working solutions of DTPA (2 mM) and BHA (10%) were prepared in 1 M sodium acetate buffer (pH 3.5) and methanol, respectively. To prepare the TBA solution, 0.1 g TBA was mixed with 5 mL of DTPA solution, 45 mL of hot distilled water, and 100 μL of BHA solution. The stock solution (2 mM) of TEP (a precursor of malondialdehyde) was prepared by adding 4.8 μL TEP to 10 mL of methanol. The standards (2–50 μM) were prepared from the stock solution of TEP. The liver tissues were homogenized in nine volumes (v/w) of 1.15% KCl solution. The homogenates were centrifuged at 2000 × g for 1 min. The supernatant or standard solution (25 μL) was mixed with TBA solution (100 μL), vortexed, and heated at 95 °C for 60 min. The reaction was terminated by cooling the reaction mixture on ice for 5 min. The reactants were centrifuged at 14,200 × g for 10 min and the absorbance at 515 nm of the supernatant was measured.
Evaluation of hepatic SOD-like and GPX-like activities
Liver samples were homogenized in five volumes (v/w) of 10 mM Tris-HCl buffer (pH 7.4) containing 0.25 M sucrose and 1 mM ethylenediaminetetraacetic acid (EDTA) using a BioMasher II. The homogenates were centrifuged at 10000 × g for 60 min and the total SOD-like activity in the supernatant was assayed using a WST-1 SOD assay kit (Dojindo, Kumamoto, Japan). To examine the SOD-like activity in the low molecular weight compounds in the extract, the liver tissues were homogenized with one volume (v/w) of PBS using a BioMasher II. The homogenate was mixed with six volumes (v/w) of ethanol. The resultant suspension was centrifuged at 14200 × g for 10 min. The SOD-like activity in the supernatant was assayed. The liver extract used for the TBARS assay was also used for assaying GPX-like activity. The GPX-like activity in the supernatant was assayed using a glutathione peroxidase activity colorimetric assay kit (BioVision, Milpitas, CA, USA).
Determination of hepatic methylglyoxal levels
The methylglyoxal level was determined using the liquid chromatography-tandem mass spectrometry system (LC-MS/MS) after derivatization with DAN following the methods of Han et al. with minor modifications46. Liver samples were homogenized with one volume (v/w) of PBS using a BioMasher II. The homogenate was mixed with six volumes (v/w) of ethanol and the suspension was centrifuged at 14200 × g and 4 °C for 10 min. The supernatant (10 µL) was incubated with 50 μL of 0.1 % (w/v) DAN solution at 50 °C for 1 h. The reactants were mixed with 500 μL ethyl acetate and vortexed. The ethyl acetate layer (400 μL) was collected and evaporated. The residue was dissolved in a 30% aqueous acetonitrile solution. Aliquots of the solution were clarified by passing it through a Cosmonice filter W (Nacalai Tesque). DAN derivatives of methylglyoxal were quantified using an LC-MS/MS system equipped with an ODS-3 column in multiple reaction monitoring (MRM) mode. The MRM condition for the DAN derivatives were optimized using LabSolutions Version 5.65. A binary linear gradient, with 0.1% formic acid (solvent A) and 0.1% formic acid containing 80% acetonitrile (solvent B), was used at a flow rate of 0.2 mL/min. The gradient program was as follows: 0–15 min, 0–100% B; 15–20 min, 100% B; 20–20.1 min, 100–0% B; 20.1–30 min, 0% B. The column temperature was maintained at 40 °C.
Western blotting
The liver samples (~50 mg) were homogenized with 300 μL of cell lysis reagent containing 1% proteinase inhibitor using a BioMasher II. The homogenate was centrifuged at 12000 × g and 4 °C for 15 min. The protein concentration in the supernatant was measured using a BCA protein assay kit (Thermo Scientific, Rockford, IL, USA) and the concentration was adjusted to 10 μg/15 μL with the same buffer. All samples were mixed with the same volume of pre-stained marker (Prestained XL-Ladder, Integrale, Tokushima, Japan) to ensure the consistency of loading volume and transfer efficiency. The proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 12.5% gel. The resolved proteins were transferred to a polyvinylidene difluoride membrane (0.45 μm; GE Healthcare Life Sciences, Chicago, IL, USA) using a semi-dry blotting apparatus (WSE 4020, Atto, Tokyo, Japan). The band intensity of pre-stained markers was quantified using ImageJ 1.52a. The membranes were then blocked with 4% Block Ace solution (Megmilk Snow Brand, Sapporo, Japan) at room temperature (25 °C) for 30 min and washed five times with 50 mM Tris-HCl buffer (pH 7.5) containing 2.68 mM KCl, 137 mM NaCl, and 0.05% (v/v) Tween 20 (TBST) for 5 min at room temperature. Primary antibodies against SOD-1, GPX-1, GAPDH, and β-actin were diluted to 1:5000, 1:5000, 1:8000 and 1:1000 with 0.4% Block Ace solution containing 0.05% (v/v) Tween 20, respectively. After overnight incubation with the primary antibody, the membranes were washed with TBST for 5 min (5 times). HRP-conjugated secondary antibodies against rabbit or mouse IgG were diluted to 1:10000 and 1:12500 with 0.4% Block Ace containing 0.05% (v/v) Tween 20, respectively. The membranes were incubated with secondary antibodies for 1 h, followed by washing with TBST for 5 min (5 times) at room temperature. The membranes were soaked with ECL reagent for 1 min. Immunoreactive bands were detected using a Lumino Graph I (Atto). To correct loading volume and transfer efficiency, chemiluminescence intensity of each band was divided by the band intensity of pre-stained markers near the target protein (35 kDa for GAPDH and β-actin, 30 kD for SOD-1 and GPX-1). The ratio was used as band intensity. To compare band intensity of specific protein in different gels, the band intensity of each gel was normalized by the band intensity of common sample in HFD group, which was analyzed by all gels.
Determination of hepatic levels of reduced and oxidative forms of cysteine
The same sample used for the determination of methylglyoxal level was used for the determination of cysteine level. The supernatant (37.5 μL) was mixed with same volume of an internal standard (0.1 mM of L-cysteine-¹³C3,15N) and 22.5 μL of 75% ethanol or 2 mM DTT in 75% ethanol solution for 1 min to detect the levels of reduced cysteine and total cysteine, respectively. Next, the sample was incubated with 4-VP (2.5 μL) at 37 °C for 2 h. Ethyl acetate (500 μL) and distilled water (100 μL) were added to the reactants and vortexed. The water layer (50 μL) was collected and evaporated under vacuum. Sodium borate buffer (50 mM; pH 8.8; 80 μL) and 0.3% AccQ acetonitrile solution (20 μL) were added to the residue. The resultant solution was kept at 50 °C for 10 min. The reactant was clarified by passing it through a Cosmonice filter W. The resultant cysteine derivatives with 4-VP and AccQ were quantified using an LC-MS/MS system equipped with an ODS-3 column in MRM mode. The MRM conditions for the cysteine and cysteine-¹³C3,15N derivatives were optimized using LabSolutions Version 5.65. A binary linear gradient, with 0.1% formic acid (solvent A) and 0.1% formic acid containing 80% acetonitrile (solvent B), was used at a flow rate of 0.2 mL/min. The gradient program was as follows: 0–15 min, 0–50% B; 15–20 min, 50–100% B; 20–25 min, 100% B; 25–25.1 min, 100–0% B; 25.1–35 min, 0% B. The column temperature was maintained at 40 °C. The levels of cysteine in the extract were estimated using the following equation: [(peak area of cysteine derivative in the sample/peak area of the internal standard) × concentration of the internal standard].
Statistical analyses
The results are presented as mean ± standard deviation. The means was analyzed using one-way analysis of variance, followed by Dunnett’s test for multiple comparisons vs. HFD group. The differences were considered significant at p < 0.05 and to have a certain tendency at 0.05 <p < 0.1. GraphPad Prism 7 software (GraphPad Software, San Diego, CA, USA) was used for statistical analyses.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We would like to thank the Kyoto Louis Pasteur Center for Medical Research for allowing us to perform animal experiments.
Author contributions
K.S., M.F., and E.O. conceptualized the study; K.S. and Y.F.Z. designed the experiments; Y.F.Z. and A.M.-M. performed the experiments; Y.F.Z. prepared the draft manuscript; K.S. supervised the manuscript preparation.
Data availability
The data supporting the findings reported herein are available on reasonable request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41538-021-00105-3.
References
- 1.Cherng JY, Shih MF. Preventing dyslipidemia by Chlorella pyrenoidosa in rats and hamsters after chronic high fat diet treatment. Life Sci. 2005;76:3001–3013. doi: 10.1016/j.lfs.2004.10.055. [DOI] [PubMed] [Google Scholar]
- 2.Otsuki T, Shimizu K, Iemitsu M, Kono I. Salivary secretory immunoglobulin a secretion increases after 4-weeks ingestion of chlorella-derived multicomponent supplement in humans: a randomized cross over study. Nutr. J. 2011;10:1–5. doi: 10.1186/1475-2891-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Y, et al. Phenethylamine in hot water extract of Chlorella pyrenoidosa expands lifespan of SOD1 mutant adults of Drosophila melanogaster at very low dose. J. Food Bioact. 2020;9:52–57. doi: 10.31665/JFB.2020.9218. [DOI] [Google Scholar]
- 4.Novella-Rodríguez S, Veciana-Nogués MT, Izquierdo-Pulido M, Vidal-Carou MC. Distribution of biogenic amines and polyamines in cheese. J. Food Sci. 2003;68:750–756. doi: 10.1111/j.1365-2621.2003.tb08236.x. [DOI] [Google Scholar]
- 5.Mayr MC, Schieberle P. Development of stable isotope dilution assays for the simultaneous quantitation of biogenic amines and polyamines in foods by LC-MS/ MS. J. Agric. Food Chem. 2012;60:3026–3032. doi: 10.1021/jf204900v. [DOI] [PubMed] [Google Scholar]
- 6.Landete JM, Ferrer S, Polo L, Pardo I. Biogenic amines in wines from three Spanish regions. J. Agric. Food Chem. 2005;53:1119–1124. doi: 10.1021/jf049340k. [DOI] [PubMed] [Google Scholar]
- 7.Cho TY, et al. Evaluation of biogenic amines in Korean commercial fermented foods. Korean. J. Food Sci. Technol. 2006;38:730–737. [Google Scholar]
- 8.Kim JH, Park HJ, Kim MJ, Ahn HJ, Byun MW. Survey of biogenic amine contents in commercial soy sauce. Korean J. Food Sci. Technol. 2003;35:325–328. [Google Scholar]
- 9.Sabelli H, Fink P, Fawcett J, Tom C. Sustained antidepressant effect of PEA replacement. J. Neuropsychiatry Clin. Neurosci. 1996;8:168–171. doi: 10.1176/jnp.8.2.168. [DOI] [PubMed] [Google Scholar]
- 10.Sengupta T, Mohanakumar KP. 2-phenylethylamine, a constituent of chocolate and wine, causes mitochondrial complex-I inhibition, generation of hydroxyl radicals and depletion of striatal biogenic amines leading to psycho-motor dysfunctions in Balb/c mice. Neurochem. Int. 2010;57:637–646. doi: 10.1016/j.neuint.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Rincon-Cervera MA, et al. Supplementation with antioxidant-rich extra virgin olive oil prevents hepatic oxidative stress and reduction of desaturation capacity in mice fed a high-fat diet: effects on fatty acid composition in liver and extrahepatic tissues. Nutrition. 2016;32:1254–1267. doi: 10.1016/j.nut.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Moradi R, et al. Effect of aqueous extract of Allium saralicum R.M. Fritsch on fatty liver induced by high-fat diet in Wistar rats. Comp. Clin. Pathol. 2019;28:1205–1211. doi: 10.1007/s00580-018-2834-y. [DOI] [Google Scholar]
- 13.Spahis S, Delvin E, Borys JM, Levy E. Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxid. Redox Signal. 2017;26:519–541. doi: 10.1089/ars.2016.6776. [DOI] [PubMed] [Google Scholar]
- 14.Farzanegi P, Dana A, Ebrahimpoor Z, Asadi M, Azarbayjani MA. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 2019;19:994–1003. doi: 10.1080/17461391.2019.1571114. [DOI] [PubMed] [Google Scholar]
- 15.Marklund SL. Extracellular superoxide dismutase and other superoxide dismutase isoenzymesin tissues from nine mammalian species. Biochem. J. 1984;222:649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brigelius-Flohe R, Maiorino M. Glutathione peroxidases. Biochim. Biophys. Acta, Gen. Sub. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Suh KS, et al. Deoxyactein protects pancreatic β-cells against methylglyoxal-induced oxidative cell damage by the upregulation of mitochondrial biogenesis. Int. J. Mol. Med. 2017;40:539–548. doi: 10.3892/ijmm.2017.3018. [DOI] [PubMed] [Google Scholar]
- 18.Cha, S. H., Hwang, Y., Heo, S. J. & Jun, H. S. Diphlorethohydroxycarmalol attenuates methylglyoxal-induced oxidative stress and advanced glycation end product formation in human kidney cells. Oxid. Med. Cell. Longev. 2018, 3654095 (2018). [DOI] [PMC free article] [PubMed]
- 19.Liu C, et al. Cytoprotective effects of a proprietary red maple leaf extract and its major polyphenol, ginnalin A, against hydrogen peroxide and methylglyoxal induced oxidative stress in human keratinocytes. Food Funct. 2020;11:5105–5114. doi: 10.1039/D0FO00359J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schalkwijk CG, Stehouwer C. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol. Rev. 2020;100:407–461. doi: 10.1152/physrev.00001.2019. [DOI] [PubMed] [Google Scholar]
- 21.Vasdev S, Stuckless J. Role of methylglyoxal in essential hypertension. Int. J. Angiol. 2010;19:e58–e65. doi: 10.1055/s-0031-1278375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henle T, Walter A, Haeßner R, Klostermeyer H. Detection and identification of a protein-bound imidazolone resulting from the reaction of arginine residues and methylglyoxal. Z. Lebensm. Unters. Forsch. 1994;199:55–58. doi: 10.1007/BF01192954. [DOI] [Google Scholar]
- 23.Patel R, et al. Effect of dietary advanced glycation end products on mouse liver. PLoS One. 2012;7:e35143. doi: 10.1371/journal.pone.0035143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu CH, Huang SM, Lin JA, Yen GC. Inhibition of advanced glycation endproduct formation by foodstuffs. Food Funct. 2011;2:224–234. doi: 10.1039/c1fo10026b. [DOI] [PubMed] [Google Scholar]
- 25.Wang WX, et al. Inhibiting the formation of advanced glycation end-products by three stilbenes and the identification of their adducts. Food Chem. 2019;295:10–15. doi: 10.1016/j.foodchem.2019.02.137. [DOI] [PubMed] [Google Scholar]
- 26.Aydos LR, et al. Nonalcoholic fatty liver disease induced by high-fat diet in C57bl/6 models. Nutrients. 2019;11:3067. doi: 10.3390/nu11123067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips JA, Macreadie IG. Inhibition of respiration in yeast by 2-phenylethylamine. Curr. Bioact. Compd. 2018;14:67–69. doi: 10.2174/1573407213666161207115458. [DOI] [Google Scholar]
- 28.Kawano T, et al. Phenylethylamine-induced generation of reactive oxygen species and ascorbate free radicals in tobacco suspension culture: mechanism for oxidative burst mediating Ca2+ influx. Plant Cell Physiol. 2000;41:1259–1266. doi: 10.1093/pcp/pcd053. [DOI] [PubMed] [Google Scholar]
- 29.Dourish CT. Behavioural effects of acute and chronic β-phenylethylamine administration in the rat: Evidence for the involvement of 5-hydroxytryptamine. Neuropharmacology. 1981;20:1067–1072. doi: 10.1016/0028-3908(81)90098-8. [DOI] [PubMed] [Google Scholar]
- 30.Dourish CT. A pharmacological analysis of the hyperactivity syndrome induced by β-phenylethylamine in the mouse. Br. J. Pharmacol. 1982;77:129–139. doi: 10.1111/j.1476-5381.1982.tb09278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira CPMS. Vitamin C and vitamin E in prevention of nonalcoholic fatty liver disease (NAFLD) in choline deficient diet fed rats. Nutr. J. 2003;2:1–5. doi: 10.1186/1475-2891-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Din SH, Sabra AN, Hammam OA, Ebeid FA, El-Lakkany NM. Pharmacological and antioxidant actions of garlic and/or onion in non-alcoholic fatty liver disease (NAFLD) in rats. J. Egypt. Soc. Parasitol. 2014;44:295–308. doi: 10.12816/0006468. [DOI] [PubMed] [Google Scholar]
- 33.Park HJ, et al. Green tea extract attenuates hepatic steatosis by decreasing adipose lipogenesis and enhancing hepatic antioxidant defenses in ob/ob mice. J. Nutr. Biochem. 2011;22:393–400. doi: 10.1016/j.jnutbio.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Kuzu N, et al. Epigallocatechin gallate attenuates experimental non-alcoholic steatohepatitis induced by high fat diet. J. Gastroenterol. Hepatol. 2008;23:465–470. doi: 10.1111/j.1440-1746.2007.05052.x. [DOI] [PubMed] [Google Scholar]
- 35.Bruce KD, Sihota KK, Byrne CD, Cagampang FR. The housekeeping gene YWHAZ remains stable in a model of developmentally primed nonalcoholic fatty liver disease. Liver Int. 2012;32:1315–1321. doi: 10.1111/j.1478-3231.2012.02813.x. [DOI] [PubMed] [Google Scholar]
- 36.Araujo LCC, Bordin S, Carvalho CRO. Reference gene and protein expression levels in two different NAFLD mouse models. Gastroenterol. Res. Pract. 2020;2020:1–7. doi: 10.1155/2020/1093235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan X, et al. High-fat diet alters the expression of reference genes in male mice. Front. Nutr. 2020;7:589771. doi: 10.3389/fnut.2020.589771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beisswenger P. Glyceraldehyde-3-phosphate dehydrogenase activity as an independent modifier of methylglyoxal levels in diabetes. Biochim. Biophys. Acta, Mol. Basis Dis. 2002;1637:98–106. doi: 10.1016/S09254439(02)00219-3. [DOI] [PubMed] [Google Scholar]
- 39.Siu GM, Draper HH. Metabolism of malonaldehyde in vivo and in vitro. Lipids. 1982;17:349–355. doi: 10.1007/BF02535193. [DOI] [PubMed] [Google Scholar]
- 40.Feldhaus LM, Liedtke AJ. mRNA expression of glycolytic enzymes and glucose transporter proteins in ischemic myocardium with and without reperfusion. J. Mol. Cell. Cardiol. 1998;30:2475–2485. doi: 10.1006/jmcc.1998.0810. [DOI] [PubMed] [Google Scholar]
- 41.Ito Y, Pagano PJ, Tornheim K, Brecher P, Cohen RA. Oxidative stress increases glyceraldehyde-3-phosphate dehydrogenase mRNA levels in isolated rabbit aorta. Am. J. Physiol. 1996;270:H81–H87. doi: 10.1152/ajpheart.1996.270.1.H81. [DOI] [PubMed] [Google Scholar]
- 42.Zhong H, Simons JW. Direct comparison of GAPDH, β-actin, cyclophilin, and 28S rRNA as internal standards for quantifying RNA levels under hypoxia. Biochem. Biophys. Res. Commun. 1999;259:523–526. doi: 10.1006/bbrc.1999.0815. [DOI] [PubMed] [Google Scholar]
- 43.Nakajima H, et al. The active site cysteine of the proapoptotic protein glyceraldehyde-3-phosphate dehydrogenase is essential in oxidative stress-induced aggregation and cell death. J. Biol. Chem. 2007;282:26562–26574. doi: 10.1074/jbc.M704199200. [DOI] [PubMed] [Google Scholar]
- 44.Cumming RC, Schubert D. Amyloid-beta induces disulfide bonding and aggregation of GAPDH in Alzheimer’s disease. FASEB J. 2005;19:2060–2062. doi: 10.1096/fj.05-4195fje. [DOI] [PubMed] [Google Scholar]
- 45.Camacho-Jiménez L, et al. Regulation of glyceraldehyde-3-phosphate dehydrogenase by hypoxia inducible factor 1 in the white shrimp during hypoxia and reoxygenation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019;235:56–65. doi: 10.1016/j.cbpa.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Han Y, et al. Plasma methylglyoxal and glyoxal are elevated and related to early membrane alteration in young, complication-free patients with type 1 diabetes. Mol. Cell. Biochem. 2007;305:123–131. doi: 10.1007/s11010-007-9535-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings reported herein are available on reasonable request from the corresponding author.