Abstract
Voltage-gated sodium channels (Navs) are critical determinants of cellular excitability. These ion channels exist as large heteromultimeric structures and their activity is tightly controlled. In neurons, the isoform Nav1.6 is highly enriched at the axon initial segment and nodes, making it critical for the initiation and propagation of neuronal impulses. Changes in Nav1.6 expression and function profoundly impact the input-output properties of neurons in normal and pathological conditions. While mutations in Nav1.6 may cause channel dysfunction, aberrant changes may also be the result of complex modes of regulation, including various protein-protein interactions and post-translational modifications, which can alter membrane excitability and neuronal firing properties. Despite decades of research, the complexities of Nav1.6 modulation in health and disease are still being determined. While some modulatory mechanisms have similar effects on other Nav isoforms, others are isoform-specific. Additionally, considerable progress has been made toward understanding how individual protein interactions and/or modifications affect Nav1.6 function. However, there is still more to be learned about how these different modes of modulation interact. Here, we examine the role of Nav1.6 in neuronal function and provide a thorough review of this channel’s complex regulatory mechanisms and how they may contribute to neuromodulation.
Keywords: voltage-gated sodium channel, action potential, axon initial segment, sodium currents, channelopathies, post-translational modifications, protein-protein interactions
1. Introduction
A well-functioning and healthy brain is dependent on the ability of neurons to integrate and relay impulses. These impulses are mediated by the activity of voltage-gated sodium channels (Navs) by controlling the initiation and propagation of electrical signals, which are fine-tuned by myriad signaling events to contribute as critical regulators of neuronal excitability [1].
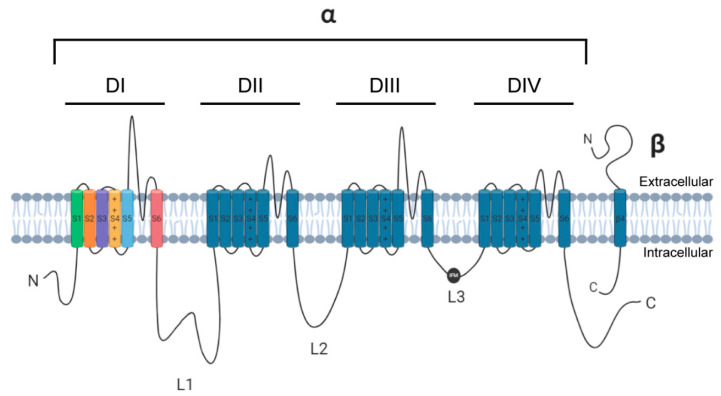
Navs exist as large complex heteromultimeric structures consisting of a pore-forming α subunit that may be covalently or non-covalently bound to auxiliary subunits, chief among these being β subunits (β1–4) (Figure 1) [2,3,4]. The Nav α subunit is comprised of a ~2000-amino acid polypeptide chain folded into a complex tertiary structure organized into four homologous transmembrane domains (DI-DIV), each containing six α-helical segments (S1–S6). The S1–S4 segments comprise the voltage sensing domain (VSD) which contains a number of positively charged lysine and arginine residues along the S4 helix that permit the channel to sense voltage changes across the membrane and is responsible for channel activation [5]. In proximity to the VSD are the S5–S6 segments that form the re-entrant P-loop and constitutes the ion-selective pore of the channel [6]. Linking the four domains of Nav α subunits are multiple intracellular loops (L1–L3) in addition to cytoplasmic N- and C-termini.
Figure 1.
Linear schematic of a voltage-gated sodium channel α subunit and an auxiliary β subunit. L3 depicts the IFM motif (black circle) for channel fast inactivation.
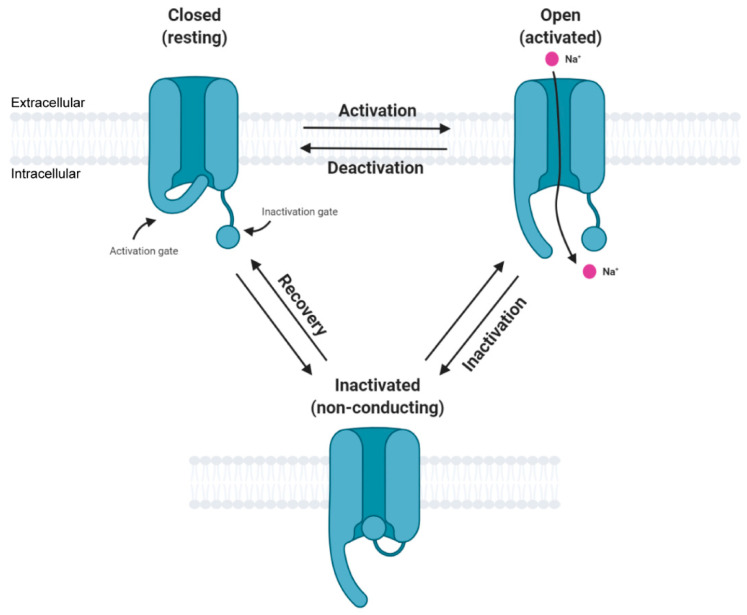
In general, the activation cycle for Navs features transitions between resting, activated, and inactivated states (Figure 2). Under resting (hyperpolarized) conditions, Navs are in their closed state and upon depolarization transition into an open, activated state that allows for sodium ion conductance, thus initiating depolarization, and corresponds to the upstroke of the action potential. Subsequently, the channel again transitions into an inactive state, thus allowing potassium and other conductances to contribute to the downstroke of the action potential. The third intracellular loop, L3, contains an inactivation particle consisting of hydrophobic residues (isoleucine-phenylalanine-methionine, IFM motif) that is largely responsible for channel fast inactivation [7,8,9,10]. Notably, Navs can undergo various post-translational modifications (PTMs) and binding interactions with other regulatory proteins that impact their structure, function, and trafficking [11,12,13].
Figure 2.
Simplified state transition model of voltage-gated sodium channels featuring closed, open, and inactivated states. This figure was created with BioRender.com.
To date, there are nine described voltage-gated sodium channel α subunit isoforms (Nav1.1–Nav1.9) with distinct functional and pharmacological characteristics and expression patterns [14]. Sequence alignments demonstrate that the sequence homology of mammalian Nav α subunits is quite high, sharing more than 50% homology in transmembrane and extracellular domains [15]. However, Navs display greater divergence within intracellular domains. Notably, the first intracellular loop (L1) varies in length between Nav isoforms and is often the target of extensive PTMs, including phosphorylation. The intracellularly accessible regions also contain additional targets for isoform-specific regulation by other PTMs and protein-protein interactions [11,16,17,18,19].
In the 40 years since Navs were first isolated, considerable progress has been made toward mapping the vast regulatory landscape of these ion channels. However there remains much we still do not understand about Nav regulation and its impact on cellular excitability, human physiology, and disease. In the brain, the voltage-gated sodium channel Nav1.6 is a critical driver in the initiation and propagation of action potentials in neurons. Consequently, aberrant alterations to Nav1.6 activity can have profound effects on input-output properties of neurons in healthy and diverse disease states. While mutations in Nav1.6 may cause aberrant channel activity (i.e., channelopathies), these changes may also be the result of extensive regulation by various signaling events impacting Nav1.6 activity and trafficking. In this review, we will provide an overview of Nav1.6 in neuronal function and a comprehensive road map into the nebulous landscape of Nav1.6 regulation and its impact on neuronal excitability.
2. Nav1.6 Overview
2.1. Discovery of Nav1.6
The voltage-gated sodium channel isoform Nav1.6 is encoded by the SCN8A gene and is a critical driver of action potential (AP) initiation and propagation in neurons. Nav1.6 was identified in the mid 1990’s by two separate groups almost a decade after the first cDNA clones of Navs were isolated [3,4,20,21]. Burgess et al. [21] identified the mouse Nav1.6 gene using positional cloning of the mouse neurological mutant for motor end-plate disease and found this channel to be highly expressed in the brain and spinal cord, but not in skeletal muscle or heart. In parallel, Schaller and colleagues [20] detected a novel sodium channel cDNA from rat brain using RT-PCR and were the first to report the full sequence of rat Nav1.6. Subsequently, the gene encoding for Nav1.6, SCN8A, was mapped to chromosome 12q13 in humans [22]. Additional investigation revealed reduced sodium currents and excitability in neuronal cultures of Scn8a null mice and suggested that Nav1.6 has a powerful impact in tuning APs that underlie neuronal excitability [12,23,24,25,26,27].
2.2. Nav1.6 Expression and Distribution
Distinct from the other Nav isoforms, Nav1.6 is broadly expressed in the nervous system. In the central nervous system (CNS), Nav1.6 is prominently expressed in a variety of excitatory and inhibitory neuronal cell types, such as hippocampal pyramidal and granule cells, retinal ganglion cells, cortical pyramidal neurons, motor neurons, and cerebellar Purkinje and granule cells where it canonically contributes to electrogenesis of excitable cells [20]. Surprisingly, Nav1.6 is also expressed in multiple glial cells within the CNS where it has been reported to play noncanonical roles in effector functions, such as phagocytosis, migration, proliferation, and secretion of chemokines/cytokines [12,20,28]. In the peripheral nervous system (PNS), Nav1.6 is expressed in a variety of ganglion cells, including dorsal root ganglion and trigeminal ganglion neurons where it is critical for peripheral sensory neuron transduction [29,30,31]. Additionally, Nav1.6 has also been detected in Schwann cells of the PNS, however its role in Schwann cells is not well understood [20,28]. Apart from the CNS and PNS, Nav1.6 is also expressed at a low level in cardiomyocytes [32,33] where it is thought to function as a Ca2+ cycling protein within t-tubules to impact Ca2+ dynamics via electrogenic Na+-Ca2+ exchange [33]. Intriguingly, Nav1.6 also exhibits high expression in various metastatic tumors, including cancers of the breast, prostate, lymph node, and cervix, and is believed to contribute toward cancer metastasis [34,35,36].
2.3. Nav1.6 Subcellular Localization in Neurons
Neurons are highly polarized cells and their architecture is defined by two prominent subcellular compartments: (1) somatodendritic, which receive and integrate neuronal synaptic inputs, and (2) axonal, which then process and transmit these inputs to postsynaptic targets [37]. A key determinant of this neuronal polarity is the unique subcellular localization of Nav1.6. This channel is highly concentrated at the axon initial segment (AIS) and at nodes of Ranvier, where it plays a critical role in the initiation and propagation of APs, respectively [38,39,40,41,42,43]. The AIS is a highly specialized membrane domain about 10–60 µM in length (depending on cell type) located at the proximal end of the axon and maintains neuronal polarity by functioning as a physiological and physical bridge between somatodendritic and axonal compartments. This region is characterized by a high density of ion channels, scaffolding proteins, kinases, and other critical proteins that orchestrate AP initiation [44,45,46,47,48,49]. Specifically, Nav1.6 is highly concentrated in the distal half of the mature AIS, whereas Nav1.2 is concentrated in the proximal half [43,50].
Interestingly, the localization of Nav1.6 at the AIS is developmentally controlled. Studies have shown that Nav1.2, but not Nav1.6, is clustered at the developing AISs and nodes of mice up through postnatal day 10, after which a developmental switch promotes the predominant expression of Nav1.6 in these subcellular compartments starting in the second postnatal week and into adulthood [51,52,53]. In mature AIS, Nav1.6 primarily controls orthodromic AP initiation in the distal AIS down the axon, while Nav1.2 contributes to antidromic backpropagation of APs into the soma and dendrites [43]. Although expression of Nav1.6 is predominantly localized to the AIS and nodes, the channel is also expressed in somatodendritic compartments, albeit to a lesser degree. Using a highly sensitive electron microscopic immunogold technique, Lorincz and Nusser [42] determined that Nav1.6 expression is approximately 35–80 times higher at the AIS than at the soma or proximal and distal dendrites. Indeed, patch-clamp, sodium imaging, and similar immunogold labeling techniques in pyramidal neurons have demonstrated a sodium conductance density as high as 2500–3000 pS/µm2 at the AIS [42,54] versus approximately 40 pS/µm2 in dendrites [55].
The ability of Nav1.6 to localize to the AIS and axonal nodes is dependent on protein-protein interactions with AnkyrinG (AnkG); a submembranous scaffolding protein and major structural orchestrator of the AIS and nodes [56]. Specifically, studies have shown that Nav1.6 contains the targeting motif |(V/A)P(I/L)AXXE(S/D)D| located in the second intracellular loop (L2) that allows channels to bind AnkG and concentrate Nav1.6 within these axonal compartments [57,58,59,60,61]. This targeting strategy is not unique to Nav1.6 and also localizes Nav1.2, voltage-gated potassium channels, cell adhesion molecules, and other regulatory proteins to the AIS [56,62,63,64]. To this end, Nav localization to the AIS may be sensitive to post-translational modulation. A previous study has shown that casein kinase II (CK2) may phosphorylate key serine residues within the AnkG binding motif of Nav1.2 and regulate insertion of Nav1.2 at the AIS in neurons [64,65]; however, this specific regulatory tripartite protein interaction has yet to be directly identified for Nav1.6 channels. However, Nav localization may be governed by additional mechanisms, as the localization of Nav1.6 to somatodendritic compartments does not appear to rely on AnkG binding [66].
The importance of Nav1.6 in neuronal excitability is underscored by Scn8a null mice that display significantly attenuated excitatory properties due to decreased surface membrane clustering of Nav1.6 at the AIS and nodes [49]. Although expression of Nav1.6 at the AIS and nodes is crucial for the initiation and propagation of signals down the axon, its expression within dendritic compartments also impacts synaptic transmission. Nav currents have been detected in numerous hippocampal and neocortical dendrites where they function to integrate synaptic inputs and contribute to local dendritic spike generation [67,68,69,70]. Patch-clamp experiments have also demonstrated that the axonal and dendritic Nav currents differ in their biophysical properties [71,72], which might suggest different Nav isoform expression at these subcellular compartments. However, several studies have detected Nav1.6 as the prominent dendritic Nav at postsynaptic membranes in cerebral and cerebellar cortices [38,42,73], indicating that the same Nav isoform may dominate in adult axons and dendrites. Thus, it is likely that the activity of Nav1.6 at the AIS/nodes and dendrites may be differentially regulated by other mechanisms, like post-translational modifications (PTMs) and protein-protein interactions [72]. Dendritic Nav1.6 activity has also been shown to contribute to the generation of dendritic spikes where it is thought to promote Ca2+ entry in spines, essentially acting as an AP booster at the synapse [73,74,75,76,77,78] to indirectly engage Ca2+ signaling machinery. Thus, Nav1.6 appears to be the predominant Nav localized to axonal and dendritic compartments, thereby providing exquisite control over input-output properties of neurons.
2.4. Unique Biophysical Properties
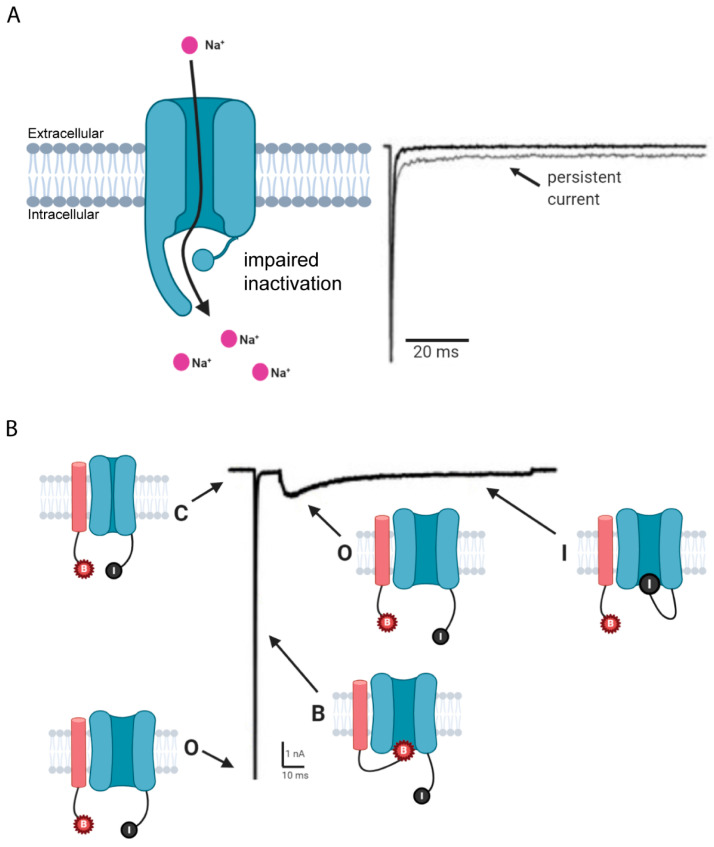
Nav1.6 displays unique biophysical properties that enable the channel to exert powerful tuning capabilities of neuronal signals. The first functional characterizations of Nav1.6 α subunits in heterologous cells revealed that Nav1.6 currents inactivated faster than other Nav isoforms and displayed distinct sodium currents, including persistent and resurgent currents [24,79,80]. While fast-inactivating transient sodium currents are traditionally described as producing the rising phase of the AP [81], Navs can also give rise to a noncanonical subtype of non-inactivating sodium currents termed persistent sodium current [24,29,82,83] (Figure 3A). In cerebral and cerebellar neurons, persistent current is predominantly generated by Nav1.6 and has been reported to be approximately five-fold higher than that generated by Nav1.2 [84]. Although these currents are typically small (0.5–2% of peak amplitude; [85]), when summated persistent sodium currents can amplify subthreshold neuronal inputs under physiological conditions [54,77]. Consequently, persistent sodium current has been shown by modeling and electrophysiology studies to lower the threshold for AP initiation and mediate repetitive AP firing in neurons [80,83,86]. Additionally, elevated persistent currents have been shown to increase the likelihood of premature firing in neurons [87] and can undergo extensive regulation by various protein-protein interactions and PTMs [11,88,89]. The physiological importance of persistent currents is highlighted by mutational studies that either decrease or increase Nav1.6 persistent current generation [79,80,87,90,91,92]. For example, while cerebellar Purkinje neurons isolated from Scn8a null mice display a 35% decrease in the transient sodium current, they display an even larger 70% reduction in the persistent current in addition to reduced repetitive firing capabilities compared to WT littermates [80]. Conversely, transgenic mice harboring mutations that increase persistent Nav1.6 sodium current exhibit neuronal hyperexcitability, spontaneous seizure activity, and even sudden unexplained death [87,91,92]. Thus, persistent currents generated by Nav1.6 can significantly impact the initiation and propagation of APs in synaptic transmission [87,90,93,94,95].
Figure 3.
Persistent and resurgent sodium currents. (A) Schematic of persistent sodium current traversing the channel due to incomplete, or impaired, inactivation. (B) Resurgent sodium current schematic of channel conformations that have undergone open channel block (B, blocking particle; I, inactivation particle). C, closed. O, open. B, block. I, inactivated. Figure was created with BioRender.com.
Nav1.6 also displays a unique resurgent current [96]; a distinct subtype of sodium current that is a voltage- and time-dependent property of Nav1.6 and occurs after depolarization at intermediate repolarizing potentials to elicit a small, transient current [97] (Figure 3B). Specifically, resurgent currents occur after depolarization and channel opening in which a subset of channels can undergo a blocked state that is faster than and distinct from traditional fast inactivation. While the endogenous blocking particle may vary between neuronal subtypes, β sodium channel subunits are postulated to be key orchestrators in the generation of resurgent current [88,98,99,100,101,102]. Upon repolarization, the blocking particle unbinds, subsequently allowing for a resurgence of transient sodium current through the pore [98]. First described in cerebellar Purkinje neurons [79,80], resurgent currents are thought to contribute to spontaneous firing and multi-peaked APs. In these studies, cultures from Scn8a null mice displayed dramatically reduced resurgent currents and attenuated repetitive AP firing in cerebellar Purkinje neurons. Modeling and electrophysiology studies also demonstrate the importance of resurgent currents in neuronal physiology [26,91,100,101,103], revealing that aberrant resurgent current generation by Nav1.6 contributes to altered neuronal excitability. Together, these reports suggest that Nav1.6 is largely responsible for the unique sodium currents necessary for repetitive AP firing in neurons.
Apart from the channel’s distinct sodium current properties, Nav1.6 α subunits are also known to exhibit fast activating and fast inactivating kinetics. Additionally, Nav1.6 is known to display a hyperpolarized shift in the voltage-dependence of activation compared to other neuronal Navs [24,29], indicating that Nav1.6 is activated earlier during depolarization. As previously mentioned, Nav1.6 is highly concentrated at the AIS in neurons and is thought to determine firing threshold [43,50]. In cultured hippocampal neurons of Scn8a null mice, there is a 5 mV depolarizing shift in the voltage-dependence of activation in addition to a 60% and 75% reduction in persistent and resurgent current [49]. Furthermore, neurons isolated from these mice appear to display an 8 mV depolarizing shift in the spike threshold, making the cells less excitable. Additional studies have demonstrated that the activation threshold in the distal AIS where Nav1.6 concentrates is hyperpolarized by approximately 12 mV compared to the proximal AIS near the soma (−55 mV distal, −43 mV proximal [43]), consistent with a role for Nav1.6 in lowering the threshold for AP initiation. In total, the unique biophysical characteristics and subcellular localization of Nav1.6 provide flexible and complex determinants for controlling neuronal excitability.
2.5. Pathophysiology
As a critical driver of APs in neurons, it is no surprise that dysfunction in Nav1.6 may lead to aberrant neuronal activity. Mutations in Nav1.6 are often associated with various neuropsychiatric disorders characterized by hyperexcitability, such as pain, epilepsy, and other neurodevelopmental disorders [14,27,92,104,105,106]. The role of Nav1.6 in human disease was first examined in patients displaying ataxia, dystonia, tremor, and intellectual disability, phenotypes that closely resembled the defects in Scn8a mutant mice [21,90,107,108]. However, it was not until 2012 that the first de novo mutation (N1768D) was discovered in Nav1.6 using whole genome sequencing of a child with severe early-onset epileptic encephalopathy, thus directly linking channel dysfunction to pathological phenotypes [87]. Notably, Nav channel dysfunction has been increasingly linked to pathogenic changes that contribute to seizure onset in epilepsy; a debilitating neurological disorder that affects approximately 1% of the world population [109]. Over 150 distinct mutations in the SCN8A gene have since been identified in patients with epilepsy and account for up to 1% of epilepsies [105]. Interestingly, the majority of Nav1.6 mutations that have been characterized display gain-of-function effects on channel biophysical properties, including premature activation, incomplete inactivation, and increased transient, persistent, and resurgent currents; characteristics that can contribute to hyperexcitability and increased neuronal activity [27,87,91,103,104,105,110,111]. However, loss-of-function mutations in Nav1.6 do exist and are thought to contribute to intellectual disability [27,107].
Unfortunately, a disproportionate number of SCN8A-associated epilepsies remain refractory to antiepileptic treatments [27,109]. Because of high sequence homology between Nav isoforms, designing Nav1.6-selective drugs remains a challenge. One of the first Nav1.6-selective inhibitors, XEN901, has been recently reported to inhibit Nav1.6 by binding to the channel’s voltage sensor, thus inhibiting its recovery from inactivation [112]. While XEN901 represents a promising Nav1.6-selective drug, this compound has only gone through Phase I clinical trials and is still in development [113]. Interestingly, several compounds exist that have been shown to selectively target pathological currents produced by Nav1.6. For instance, cannabidiol and GS967 (otherwise known as Prax330) have been shown to preferentially reduce aberrant persistent and resurgent currents over transient sodium currents, however these compounds do not appear to be selective for Nav1.6 and can target currents in other isoforms, like Nav1.2 [91,114,115,116]. More recently, anti-epileptic compound screens in zebrafish models of epilepsy revealed two novel blocking compounds, MV1312 and MV1369 [117]. Although MV1312 showed a 5–6 fold selectivity of Nav1.6 over Nav1.1–Nav1.7, this compound displays a comparable blocking affinity for Nav1.8, a major PNS isoform involved in pain sensation. Similarly, while M1369 also showed higher selectivity for Nav1.6, this compound also blocked Nav1.2. Thus, identifying alternative molecular determinants, such as those involved in isoform-specific Nav modulation, may provide promising mechanisms for targeting SCN8A-associated pathologies.
In addition to mutations in the SCN8A gene, non-genetic modifications in Nav1.6 expression and function may also contribute to excitability disorders, such as neuropathic pain [31,118,119], autism-spectrum disorders [106,120], ischemia [121], and stress-induced disorders [122,123] in addition to epilepsy [12,27,124]. Importantly, changes in Nav1.6 expression have been linked to non-genetic models of acquired epilepsy, in which seizures are induced by transient brain insult or chemoconvulsants [124,125]. Following seizure onset, Nav1.6 expression and persistent current have been reported to increase within hippocampal regions [124,125], whereas reduction in Nav1.6 activity has been shown to decrease seizure susceptibility [126,127,128], suggesting an early role for Nav1.6 in the development of seizures. Indeed, a recent study has also demonstrated that reducing the SCN8A transcript by 25–50% can delay seizure onset in SCN8A models of epilepsy [129], indicating that a general reduction in Nav1.6 activity may reduce seizure susceptibility. Notably, many of the pathological changes in Nav1.6 function and expression are significantly influenced by various intracellular mediators including second messengers, protein-protein interactions, and PTMs. Therefore, it is critical to understand the extensive regulatory landscape contributing to Nav1.6 modulation and how these processes may impact neuronal excitability.
3. Nav1.6 Regulation by Protein-Protein Interactions
Sodium channels, including Nav1.6, are subject to extensive regulation by various auxiliary proteins and second messengers. These regulatory processes are quite powerful, displaying developmental, spatial, and temporal specificity which can be mediated by many diverse stimuli and signaling pathways. Here we will highlight several protein-protein interactions by which Nav1.6 is regulated and how they contribute to neuronal function.
3.1. Sodium Channel β Subunits
Sodium channel β subunits (β1–β4) are small single-transmembrane auxiliary proteins that can function as cell-adhesion molecules and modulate Nav surface expression and function [130]. These subunits interact with Nav α subunits non-covalently (β1 and β3) and through covalent disulfide bonds (β2 and β4) [1]. Notably, several studies have implicated β subunit regulation of Nav1.6 in neuronal function. Studies of β1 null mice (Scn1b−/−) indicate that the interaction between β1 and Nav1.6 is important for Nav1.6 function at the AIS and for neurite outgrowth [131]. Nav1.6-expressing cerebellar neurons of β1 null mice also display striking reductions in resurgent sodium current [131]. Moreover, the β4 subunit has also been implicated in the generation of Nav1.6-mediated resurgent current in Purkinje and DRG neurons [99,101,132]. These reports suggest that the C-terminal portion of β subunits may act as an open channel blocker to mediate Nav1.6 resurgent current. Indeed, intracellular application of a peptide mimicking this sequence, amino acids 154–167 of the β4 subunit, has been shown to recapitulate resurgent currents in heterologous expression systems lacking endogenous open channel blockers [99,100]. Interestingly, the co-expression of Nav α subunits with the full-length β4 subunit is not sufficient to produce resurgent current in heterologous expression systems [84,132], indicating that other modulatory accessory proteins, or perhaps cellular background, over-ride this function. To this end, several studies have demonstrated that various PTMs on β subunits impact β subunit interactions with Nav α subunits. For instance, phosphorylation and palmitoylation have both been implicated in β subunit regulatory properties [133,134] and suggest a complex crosstalk between Nav auxiliary proteins and PTMs on Nav α subunit function.
3.2. Fibroblast Growth Factor Homologous Factors
Fibroblast growth factor homologous factors (FHF1-4 also known as FGF11-14) are a family of intracellular auxiliary proteins that, contrary to their FGF counterpart, are not secreted and do not directly stimulate FGF receptors [135,136,137]. While these signaling molecules have multiple interacting partners to modulate various cellular parameters [136,137,138], FHFs can also bind to the C-terminus of Nav channel α subunits and influence both current density and gating properties [66,139,140,141,142,143]. Each member of the FHF family has at least two splice variants (A and B) with distinct N-terminal sequences [144], and their interaction with Navs produce isoform-specific changes in channel function. For example, FHF4B, which contains a unique 69 amino acid N-terminus compared to other FHFs [144,145], suppresses Nav1.6 sodium currents and may regulate localization of the channel to the AIS in neurons [146,147], whereas FHF4A has no effect [146]. Several studies have also shown that FHF2A and FHF2B interactions with Nav1.6 differentially regulate channel activity. FHF2B has been shown to increase Nav1.6 current density, produce a depolarizing shift in channel availability, and positively regulate resurgent currents [102,148]. In contrast, FHF2A binding to Nav1.6 has been shown to negatively regulate resurgent current, enhance long-term inactivation, slow the kinetics of the recovery from inactivation, and produce an even larger depolarizing shift in availability in addition to increased current density [102,146,149].
Differential modulation of Nav1.6 resurgent currents by FHFs has been identified as a potential mechanism underlying nociception and pain. Painful sensations often arise from increased excitability of peripheral dorsal root ganglia (DRG) neurons which are known to express Nav1.6-mediated resurgent currents [96]. In DRG neurons isolated from animals with radicular pain, FHF2A expression has been shown to be acutely downregulated following inflammation, whereas FHF2B expression is upregulated [102]. Notably, enhanced expression of FHF2B in pain models has been shown to contribute to increased resurgent currents in DRG neurons and mediate hyperexcitability. Interestingly, application of a peptide that mimics the FHF2A long-term inactivation particle, which negatively regulates resurgent currents, was found to reduce hyperexcitability associated with pain [102]. Importantly, these studies demonstrate that FHF-Nav1.6 interactions dynamically contribute to altered neuronal excitability associated with nociception and pain.
3.3. Ca2+ and Calmodulin
Intracellular Ca2+ is a ubiquitous second messenger critical to many aspects of neuronal function. A rapid change in the internal Ca2+ concentration (from 50–100 nM up to ~20 µM) is coupled to neuronal depolarization and is central to synaptic transmission [150]. Detection of this Ca2+ concentration change depends on Ca2+-binding proteins capable of translating the signal. To this end, a predominant intracellular receptor for Ca2+ is calmodulin (CaM), a highly conserved Ca2+ sensor that provides complex opportunities to functionally modulate target proteins and provide feedback for membrane excitability. The refined ability for CaM to sense Ca2+ is reflected in its unique structure [151,152,153,154]. This ~17 kDa protein consists of two lobes, an N-terminal (N-lobe) and C-terminal (C-lobe) lobe, and are connected by a flexible linker. Each lobe has two Ca2+-binding EF-hands, which can coordinate binding of one Ca2+ ion for a total of four Ca2+ ions. Interestingly, the C-lobe of CaM binds Ca2+ with a six-time higher affinity than the N-lobe, thereby providing CaM with the ability to sense Ca2+ across a dynamic concentration range [155]. Moreover, CaM undergoes a conformational change following Ca2+ binding that can increase or decrease the affinity of CaM to its target protein [156,157], thus allowing CaM to display a wide range of binding and regulatory properties.
Interestingly, Ca2+ regulation of Navs was suspected soon after the primary amino acid sequence was determined, noting that the C-terminus of Navs contained features that resembled an EF-hand Ca2+ binding motif [158]. Subsequent yeast two hybrid screens using the Nav CTD as bait identified CaM as a binding partner [159], leading to the identification of two CaM binding motifs in the C-terminus of Navs: (1) an “IQ” motif ([I/L/V]QXXXRGXXX[R/K]) [160] and (2) a basic amphipathic α helix, both C-terminal to the EF-hand motif. The presence of both a potential Ca2+ binding site and CaM binding sites in the Nav CTD suggested that Nav α subunits may be sensitive to both Ca2+-dependent and –independent modes of regulation. However, the ability for Ca2+ to directly bind the EF-hand motif of Navs and modulate channel activity remains controversial [161,162,163,164]. Studies suggest that Ca2+-dependent regulation of channel activity instead occurs through associated CaM [164,165] and that the structural conformation of the EF-hand motif may dictate the binding mode of CaM to the nearby IQ motif [166]. Indeed, several studies have demonstrated that CaM is able to bind to the IQ motif and modulate current density and gating properties of various Nav isoforms in an isoform-dependent manner and revealed Ca2+-dependent and -independent modes of Nav regulation [161,167,168,169,170,171,172,173]. Notably, Nav1.6 displays a higher affinity toward Ca2+/CaM than apo-CaM (Ca2+-free) binding at the channel’s IQ motif (amino acids 1902–1912; [174]), suggesting that Nav1.6 may be differentially modulated by CaM depending on intracellular Ca2+. To this end, Ca2+/CaM binding has been shown to delay Nav1.6 channel inactivation by up to 50%, whereas apo-CaM binding enhances the rate of inactivation [168]. Incidentally, the Ca2+/CaM-dependent slowing of inactivation kinetics could potentially prolong AP duration by enhancing neurotransmitter release at the synapse, thus contributing to increased excitability. Furthermore, apo-CaM has also been shown to differentially modulate Nav1.6 sodium currents, revealing reduced transient and persistent currents with decreased and increased CaM binding, respectively [89,168]. These data reveal that Navs can be dynamically modulated via Ca2+-dependent and -independent mechanisms. Recent studies suggest that CaM also interacts with the N-terminal domain of Nav1.5, suggesting that multiple CaM binding domains may shape the Nav response to Ca2+ signaling [175]. Whether CaM binding to the channel may be regulated by PTMs or serve as an intermediate effector between Nav1.6 and downstream Ca2+/CaM-dependent targets, like the Ca2+/calmodulin-dependent protein kinase II (CaMKII), remains to be determined. Intriguingly, CaM interactions with the cardiac isoform Nav1.5 may be influenced by CaMKII phosphorylation of the channel. Specifically, CaM binding to Nav1.5 has been shown to decrease following CaMKII phosphorylation at S1938 and S1989 within the CTD of the channel [176]. This suggests that the temporal order of phosphorylation events on the cardiac isoform Nav1.5 could potentially act as a switch to specify regulation. However, such a complex mechanism for CaMKII-dependent regulation of CaM binding to Nav1.6 has not yet been identified.
4. Post-Translational Regulation of Nav1.6
In addition to being regulated by various protein-protein interactions, Nav1.6 is also extensively modulated by post-translational modifications (PTM). PTMs are protein modifications that occur after mRNA translation into a protein and are critical for protein maturation and function. These processes can be mediated by many diverse enzymes and signaling pathways, resulting in an attachment of a biochemical group (methylation, acetylation, phosphorylation), fatty acids (palmitoylation), polypeptide (ubiquitination, SUMOylation), or more complex molecules (glycosylation) that can produce either stable or reversible changes to a protein. Importantly, PTMs display precise coupling between known interaction sites of the modifying enzyme and a given amino acid sequence on the target/substrate protein, resulting in highly specific spatial and temporal control that allows neurons to fine tune the properties of a protein, like Nav1.6, depending on the cellular environment and contribute to the regulation of neuronal excitability.
4.1. Glycosylation
A common PTM of transmembrane proteins is glycosylation, which is the attachment of glycans (carbohydrate) to a protein. Early studies indicated that glycosylation of Navs, particularly Nav1.2, Nav1.4, Nav1.5, Nav1.6, and Nav1.7, is a crucial step for the biosynthesis, folding, and trafficking of sodium channels [177,178,179,180,181,182,183]. Nav gating properties can also be influenced by glycosylation, altering the voltage-dependence of activation and inactivation in addition to recovery kinetics [184,185,186,187]. Mice with a single amino acid deletion within DIVS6 of Nav1.6 (Ile1750del) exhibit defects in glycosylation due to alterations at an adjacent glycosylation site, resulting in chronic movement disorders due to reduced channel activity and defective localization at the AIS and nodes [183]. Therefore, glycosylation is an important modification influencing the subcellular localization of Nav1.6 and may contribute to alterations in neuronal excitability. Future studies will be useful to determine whether similar defects in glycosylation contribute toward pathogenic mechanisms associated with patient mutations.
4.2. Uniquitination
Ubiquitination is a powerful PTM for modulating trafficking and cell surface expression of Navs. Mediated by ubiquitin ligases, this process refers to the covalent addition of an ubiquitin protein, a ~8.5 kDa polypeptide of 76 amino acids, to the lysine residues of a targeted protein [188]. Proteins destined for internalization through this pathway are either degraded or recycled [189,190,191], and in some instances can alter protein function. Most Navs possess a PY motif (PPXY) usually found in the C-terminus and/or L1 of channels, with the exception of Nav1.4 and Nav1.9, which allow ubiquitin ligases to bind [192,193]. Nav1.6 contains multiple PY motifs and undergoes ubiquitin-dependent modulation. In mouse hippocampal neurons, p38 phosphorylation of Nav1.6 promotes Nedd4-induced ubiquitination and internalization of the channel [122,194]. Specifically, the ubiquitin ligase Nedd4-2 has been shown to interact with two PY motifs on Nav1.6; the Pro-Ser-Tyr1945 motif in the CTD and the Pro-Gly-Ser553-Pro motif in L1 of the channel [194]. Both motifs were found to be necessary for Nav1.6 modulation by p38, which is a mitogen activated protein kinase (MAPK) implicated in relaying stress responses [194,195]. Furthermore, abrogating Nedd4-2 interactions with Nav1.6 was found to block channel internalization and resulted in stress-mediated increases in Nav1.6 currents [194]. Together, these studies highlight a complex interaction between p38 MAPK phosphorylation and ubiquitination of Nav1.6 and suggest that crosstalk between these different PTMs may limit neuronal excitability in response to stress-induced stimuli.
4.3. Palmitoylation
S-palmitoylation is a reversible PTM that involves the addition of a 16-carbon palmitic fatty acid chain to the thiol group of an intracellular cysteine of the substrate protein through thioester linkage. Palmitoylation is known to dynamically regulate diverse proteins, impacting cell surface expression, trafficking, structural conformation, protein-protein interactions, and function [178,196,197]. Palmitoylation also plays crucial roles in ion channel regulation and is involved in various phases of the ion channel life cycle, including synthesis, maturation, trafficking, subcellular localization, and internalization [196]. The first characterization of S-palmitoylation of voltage-gated sodium channels identified this process to regulate the early stages of protein biosynthesis [178]. Recently, Nav1.6 was identified as a novel target for regulation by S-palmitoylation [198]. This study identified two palmitoylation sites (C1169, C1170) in L2 of the channel that appear to be responsible for modulating voltage-dependence of inactivation, and one site in the C-terminus (C1978) exclusive to Nav1.6 that enhances Nav1.6 current density [198]. Further characterization of these sites revealed a novel role of Nav1.6 palmitoylation in regulating neuronal excitability [198], showing that the ablation of C1169, C1170, and C1978 results in a substantial reduction in Nav1.6-mediated excitability of DRG neurons, indicating that targeting Nav1.6 palmitoylation may represent a potentially useful strategy to reduce neuronal excitability.
4.4. Phosphorylation
Phosphorylation is a crucial PTM that affects up to 30% of proteins in cells at any given time [199]. Catalyzed by protein kinases, this PTM is characterized by the reversible covalent addition of a negatively charged (−2) phosphate group onto a serine, threonine, or tyrosine residue of a target protein:
| MgATP1− + protein–O:H → protein–O:PO32− + MgADP + H+. |
Phosphorylation is perhaps the most extensively studied Nav PTM and has been shown to target multiple regions of sodium channels [11,16,17,18,19]. Nav phosphorylation is carried out by diverse kinases that can modulate various aspects of channel function. This kinase diversity represents multiple signaling pathways that enable Nav modulation in concert with other pathways, or distinctively by different second messengers, thus providing a trove of potential regulation of neuronal activity. For example, sodium channels from the CNS (Nav1.1 and Nav1.2), PNS (Nav1.7 and Nav1.8), cardiac tissue (Nav1.5), and skeletal muscle (Nav1.4) are modulated by the cAMP-dependent protein kinase PKA and/or PKC, which can be activated by Ca2+/lipid hydrolysis, producing differential effects on channel activity [13,200]. While PKC appears to consistently attenuate sodium currents across most isoforms [201,202,203,204,205,206], the effects of PKA phosphorylation are more diverse, resulting in attenuated tetrodotoxin-sensitive (TTX-S) sodium currents [202,207,208,209] while potentiating TTX-resistant (TTX-R) sodium currents [202,210,211,212], and producing shifts in voltage-dependent gating properties. The PKA phospho-sites S573 and S687, and the PKC phospho-site S576, for example, have been shown to contribute to the functional modulation of Nav1.2 sodium currents [206,208,213,214]. Interestingly, despite carrying homologous PKA and PKC phospho-sites, Nav1.6 appears to be largely resistant to modulation by these kinases in neurons [84], suggesting that Nav1.6 modulation may be targeted through a different signaling pathway.
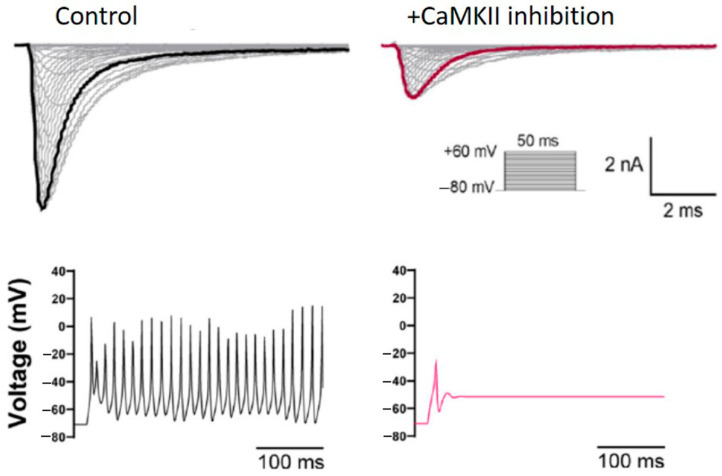
To this end, Nav1.6 has been recently identified as a target for modulation by CaMKII (Figure 4) [11]. CaMKII is a multifunctional Ser/Thr protein kinase highly concentrated in the brain and is implicated in the physiological and pathophysiological regulation of excitability [215]. Acute CaMKII inhibition has been shown to produce loss-of-function effects in Nav1.6 activity, including decreased transient and persistent Nav1.6 sodium currents in Purkinje neurons in addition to a depolarized shift in the voltage-dependence of activation in cells heterologously expressing Nav1.6. Further modeling the effects of CaMKII inhibition on Nav1.6 activity in Purkinje neurons has shown significantly reduced spontaneous and evoked excitability, suggesting that this mechanism may be important in regulating neuronal function [11]. Importantly, CaMKII modulation of Nav1.6 is mediated by phosphorylation of the channel at two distinct sites in the L1 region, including S561 and T642. This is consistent with previous reports identifying L1 as a hotspot for Nav PTMs and regulation [16,18,19,216,217,218]. Notably, the CaMKII-dependent phosphorylation sites S561 and T642 in Nav1.6 display homologous sites of regulation in other Nav isoforms (Figure 5). To date, Nav1.6 appears largely resistant to modulation by PKA [84]. While phosphorylation of S573 in Nav1.2 has been shown to mediate PKA-dependent reductions in Nav1.2 sodium currents [208], phosphorylation of S561 in Nav1.6 has been implicated in CaMKII-dependent modulation of the voltage-dependence of activation [11]. Moreover, CaMKII phosphorylation of Nav1.6 at T642 has been implicated in sodium current regulation, while CaMKII phosphorylation at the equivalent T594 site in Nav1.5 has been shown to regulate channel gating properties [11,18]. Together, these studies stress the intricacies underlying isoform-selectivity of CaMKII modulation and further highlight the diverse functional responses to phosphorylation of Navs at homologous sites by the same kinase or distinct signaling pathways. The possibility for CaMKII-dependent modulation of Nav1.6 is a fascinating nexus between a kinase implicated in synaptic plasticity and a channel critical for the initiation and propagation of APs. Additional studies investigating this relationship will be important to determine how this mechanism regulates neuronal excitability in physiology and disease.
Figure 4.
CaMKII modulates Nav1.6 activity and neuronal excitability. CaMKII inhibition reduces Nav1.6 sodium currents (top) and neuronal excitability (bottom) in simulated Purkinje neurons. This research was originally published in the Journal of Biological Chemistry [11], © the American Society for Biochemistry and Molecular Biology.
Figure 5.
Sequence alignment spanning homologous phosphorylation sites in Nav1.2, Nav1.5, and Nav1.6 in the L1 region between domains I and II. Blue represents PKA phosphorylation site. Yellow represents CaMKII phosphorylation site.
As discussed above, Nav1.6 is also modulated by p38 mitogen-activated protein kinase (MAPK). This kinase is classically linked to environmental stressors, including cell injury and hypoxia. Several TTX-S (Nav1.6 and Nav1.7) and TTX-R (Nav1.8 and Nav1.9) Navs can be subject to phosphorylation by these pathways and modulate aspects of their function and surface expression [119]. Phosphorylation of Nav1.6 by activated p38 occurs within L1, specifically at S553, which results in a reduction of Nav1.6 current [122]. As previously mentioned, p38 phosphorylation of Nav1.6 promotes Nedd4-induced ubiquitination of the channel to reduce Nav1.6 sodium current [194]. Two other major kinases included in the MAPK family are c-Jun N-terminal kinases (JNKs) and extracellular signal-regulated kinases (ERKs). Direct modulation of Nav1.6 by either of these kinases has yet to be identified; however, indirect modulation of Nav1.6 by JNK has been observed and is thought to contribute to Alzheimer’s disease (AD) pathogenesis [219]. In models of AD, the amyloid precursor protein (APP) has been shown to upregulate Nav1.6 expression and activity, which may contribute to membrane depolarization and increased spike frequency, thereby resulting in neuronal hyperexcitability [219,220,221,222]. The reciprocal has also been shown, whereby APP knockdown can reduce Nav1.6 expression and activity [222]. Interestingly, the ability of APP to modulate Nav1.6 sodium currents is mediated by activation of JNK, which in turn enables APP to upregulate Nav1.6 cell surface expression and enhance sodium current [219]. Together, these studies indicate that Nav1.6 modulation through MAPK pathways is complex and may be a critical player in pathophysiological neuronal excitability.
Several studies have also identified a role for glycogen synthase kinase-3 (GSK3) in regulating Nav1.6 activity. Beyond regulation of glycogen metabolism, this kinase plays important roles in the regulation of neuronal development and function, including synaptic plasticity and neuronal excitability [223,224,225]. A previous report demonstrated that pharmacological inhibition and genetic silencing of GSK3β produces loss-of-function effects on channel activity, resulting in decreased transient and persistent Nav1.6 sodium currents in addition to a leftward shift in channel availability [226]. In this work it was shown that GSK3β phosphorylates T1936 in the Nav1.6 CTD and that the interaction is important in regulating excitability of medium spiny neurons in the nucleus accumbens, implicating this mechanism in the dopamine reward pathway. A recent study suggests that FHF4 binding with the Nav1.6 CTD may be regulated by GSK3β phosphorylation of either FHF4, Nav1.6, or potentially both [227,228]. In particular, inhibiting GSK3β was found to decrease FHF4:Nav1.6 complex formation, which subsequently suppressed neuronal excitability and suggests that multiplexed signaling pathways are major determinants underlying Nav1.6 regulation and neuronal function [228,229,230].
5. Conclusions
Significant progress has been made toward understanding the intricate regulation of Nav1.6 in neuronal function, however the picture is far from complete. Navs undergo remarkably complex and extensive modes of regulation by many different auxiliary proteins and post-translational mechanisms, each of which are subject to regulation themselves by diverse signaling pathways. Although this review examined several aspects of Nav1.6 regulation, it is likely that Nav1.6 is sensitive to additional protein-protein interactions and PTMs that have yet to be identified. Furthermore, considerable crosstalk occurs between different modes of regulation, making it difficult to predict how a particular ensemble of modifications may impact channel properties and neuronal excitability. Overall, the studies reviewed here expand our current knowledge of Nav1.6 regulation and highlight important modulatory mechanisms mediating changes in neuronal excitability associated with health and disease.
Author Contributions
A.Z., A.H. and T.R.C. wrote, reviewed, and edited the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NIH/NINDS grants U54NS108874, R01NS053422, and R33DA041876. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Catterall W.A. Voltage-gated sodium channels at 60: Structure, function and pathophysiology. J. Physiol. 2012;590:2577–2589. doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catterall W.A. Structure and function of voltage-gated sodium channels at atomic resolution. Exp. Physiol. 2014;99:35–51. doi: 10.1113/expphysiol.2013.071969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noda M., Ikeda T., Suzuki H., Takeshima H., Takahashi T., Kuno M., Numa S. Expression of functional sodium channels from cloned cDNA. Nature. 1986;322:826–828. doi: 10.1038/322826a0. [DOI] [PubMed] [Google Scholar]
- 4.Noda M., Shimizu S., Tanabe T., Takai T., Kayano T., Ikeda T., Takahashi H., Nakayama H., Kanaoka Y., Minamino N., et al. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984;312:121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- 5.Stühmer W., Conti F., Suzuki H., Wang X.D., Noda M., Yahagi N., Kubo H., Numa S. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989;339:597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- 6.Noda M., Suzuki H., Numa S., Stühmer W. A single point mutation confers tetrodotoxin and saxitoxin insensitivity on the sodium channel II. FEBS Lett. 1989;259:213–216. doi: 10.1016/0014-5793(89)81531-5. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong C.M., Bezanilla F., Rojas E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J. Gen. Physiol. 1973;62:375–391. doi: 10.1085/jgp.62.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassilev P.M., Scheuer T., Catterall W.A. Identification of an intracellular peptide segment involved in sodium channel inactivation. Science. 1988;241:1658–1661. doi: 10.1126/science.2458625. [DOI] [PubMed] [Google Scholar]
- 9.Vassilev P., Scheuer T., Catterall W.A. Inhibition of inactivation of single sodium channels by a site-directed antibody. Proc. Nat. Acad. Sci. USA. 1989;86:8147–8151. doi: 10.1073/pnas.86.20.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West J.W., Patton D.E., Scheuer T., Wang Y., Goldin A.L., Catterall W.A. A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc. Nat. Acad. Sci. USA. 1992;89:10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zybura A.S., Baucum A.J., 2nd, Rush A.M., Cummins T.R., Hudmon A. CaMKII enhances voltage-gated sodium channel Nav1.6 activity and neuronal excitability. J. Biol. Chem. 2020;295:11845–11865. doi: 10.1074/jbc.RA120.014062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solé L., Tamkun M.M. Trafficking mechanisms underlying Na(v) channel subcellular localization in neurons. Channels. 2020;14:1–17. doi: 10.1080/19336950.2019.1700082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheuer T. Regulation of sodium channel activity by phosphorylation. Sem. Cell Dev. Biol. 2011;22:160–165. doi: 10.1016/j.semcdb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catterall W.A. Forty Years of Sodium Channels: Structure, Function, Pharmacology, and Epilepsy. Neurochem. Res. 2017;42:2495–2504. doi: 10.1007/s11064-017-2314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catterall W.A., Goldin A.L., Waxman S.G. International Union of Pharmacology. XLVII. Nomenclature and Structure-Function Relationships of Voltage-Gated Sodium Channels. Pharmacol. Rev. 2005;57:397. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 16.Berendt F.J., Park K.S., Trimmer J.S. Multisite phosphorylation of voltage-gated sodium channel alpha subunits from rat brain. J. Proteome Res. 2010;9:1976–1984. doi: 10.1021/pr901171q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerda O., Baek J.H., Trimmer J.S. Mining recent brain proteomic databases for ion channel phosphosite nuggets. J. Gen. Physiol. 2011;137:3–16. doi: 10.1085/jgp.201010555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashpole N.M., Herren A.W., Ginsburg K.S., Brogan J.D., Johnson D.E., Cummins T.R., Bers D.M., Hudmon A. Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel NaV1.5 gating by multiple phosphorylation sites. J. Biol. Chem. 2012;287:19856–19869. doi: 10.1074/jbc.M111.322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossie S., Catterall W.A. Phosphorylation of the alpha subunit of rat brain sodium channels by cAMP-dependent protein kinase at a new site containing Ser686 and Ser687. J. Biol. Chem. 1989;264:14220–14224. doi: 10.1016/S0021-9258(18)71666-9. [DOI] [PubMed] [Google Scholar]
- 20.Schaller K.L., Krzemien D.M., Yarowsky P.J., Krueger B.K., Caldwell J.H. A novel, abundant sodium channel expressed in neurons and glia. J. Neurosci. 1995;15:3231–3242. doi: 10.1523/JNEUROSCI.15-05-03231.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess D.L., Kohrman D.C., Galt J., Plummer N.W., Jones J.M., Spear B., Meisler M.H. Mutation of a new sodium channel gene, Scn8a, in the mouse mutant motor endplate disease. Nat. Genet. 1995;10:461–465. doi: 10.1038/ng0895-461. [DOI] [PubMed] [Google Scholar]
- 22.Plummer N.W., Galt J., Jones J.M., Burgess D.L., Sprunger L.K., Kohrman D.C., Meisler M.H. Exon organization, coding sequence, physical mapping, and polymorphic intragenic markers for the human neuronal sodium channel gene SCN8A. Genomics. 1998;54:287–296. doi: 10.1006/geno.1998.5550. [DOI] [PubMed] [Google Scholar]
- 23.García K.D., Sprunger L.K., Meisler M.H., Beam K.G. The sodium channel Scn8a is the major contributor to the postnatal developmental increase of sodium current density in spinal motoneurons. J. Neurosci. 1998;18:5234–5239. doi: 10.1523/JNEUROSCI.18-14-05234.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith M.R., Smith R.D., Plummer N.W., Meisler M.H., Goldin A.L. Functional Analysis of the Mouse Scn8a Sodium Channel. J. Neurosci. 1998;18:6093. doi: 10.1523/JNEUROSCI.18-16-06093.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin S.I., Khaliq Z.M., Aman T.K., Grieco T.M., Kearney J.A., Raman I.M., Meisler M.H. Impaired motor function in mice with cell-specific knockout of sodium channel Scn8a (NaV1.6) in cerebellar purkinje neurons and granule cells. J. Neurophysiol. 2006;96:785–793. doi: 10.1152/jn.01193.2005. [DOI] [PubMed] [Google Scholar]
- 26.Khaliq Z.M., Gouwens N.W., Raman I.M. The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: An experimental and modeling study. J. Neurosci. 2003;23:4899–4912. doi: 10.1523/JNEUROSCI.23-12-04899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Brien J.E., Meisler M.H. Sodium channel SCN8A (Nav1.6): Properties and de novo mutations in epileptic encephalopathy and intellectual disability. Front. Genet. 2013;4:213. doi: 10.3389/fgene.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pappalardo L.W., Black J.A., Waxman S.G. Sodium channels in astroglia and microglia. Glia. 2016;64:1628–1645. doi: 10.1002/glia.22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rush A.M., Dib-Hajj S.D., Waxman S.G. Electrophysiological properties of two axonal sodium channels, Nav1.2 and Nav1.6, expressed in mouse spinal sensory neurones. J. Physiol. 2005;564:803–815. doi: 10.1113/jphysiol.2005.083089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang R., Liu X., Wei L., Wang W., Zheng P., Yan X., Zhao Y., Liu L., Cao X. The modulation of the excitability of primary sensory neurons by Ca(2)(+)-CaM-CaMKII pathway. J. Neurosci. 2012;33:1083–1093. doi: 10.1007/s10072-011-0907-7. [DOI] [PubMed] [Google Scholar]
- 31.Chen L., Huang J., Zhao P., Persson A.K., Dib-Hajj F.B., Cheng X., Tan A., Waxman S.G., Dib-Hajj S.D. Conditional knockout of NaV1.6 in adult mice ameliorates neuropathic pain. Sci. Rep. 2018;8:3845. doi: 10.1038/s41598-018-22216-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noujaim S.F., Kaur K., Milstein M., Jones J.M., Furspan P., Jiang D., Auerbach D.S., Herron T., Meisler M.H., Jalife J. A null mutation of the neuronal sodium channel NaV1.6 disrupts action potential propagation and excitation-contraction coupling in the mouse heart. J. FASEB. 2012;26:63–72. doi: 10.1096/fj.10-179770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Struckman H.L., Baine S., Thomas J., Mezache L., Mykytyn K., Györke S., Radwański P.B., Veeraraghavan R. Super-Resolution Imaging Using a Novel High-Fidelity Antibody Reveals Close Association of the Neuronal Sodium Channel Na(V)1.6 with Ryanodine Receptors in Cardiac Muscle. Microsc. Microanal. 2020;26:157–165. doi: 10.1017/S1431927619015289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin S., Lv Y., Xu J., Mao X., Chen Z., Lu W. Over-expression of Nav1.6 channels is associated with lymph node metastases in colorectal cancer. World J. Surg. Oncol. 2019;17:175. doi: 10.1186/s12957-019-1715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao W., Zhang J., Körner H., Jiang Y., Ying S. The Emerging Role of Voltage-Gated Sodium Channels in Tumor Biology. Front. Oncol. 2019;9:124. doi: 10.3389/fonc.2019.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Charcas O., Espinosa A.M., Alfaro A., Herrera-Carrillo Z., Ramirez-Cordero B.E., Cortes-Reynosa P., Perez Salazar E., Berumen J., Gomora J.C. The invasiveness of human cervical cancer associated to the function of Na(V)1.6 channels is mediated by MMP-2 activity. Sci. Rep. 2018;8:12995. doi: 10.1038/s41598-018-31364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duménieu M., Oulé M., Kreutz M.R., Lopez-Rojas J. The Segregated Expression of Voltage-Gated Potassium and Sodium Channels in Neuronal Membranes: Functional Implications and Regulatory Mechanisms. Front. Cell Neurosci. 2017;11:115. doi: 10.3389/fncel.2017.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caldwell J.H., Schaller K.L., Lasher R.S., Peles E., Levinson S.R. Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites and synapses. Proc. Nat. Acad. Sci. USA. 2000;97:5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkins S.M., Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J. Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzoumaka E., Tischler A.C., Sangameswaran L., Eglen R.M., Hunter J.C., Novakovic S.D. Differential distribution of the tetrodotoxin-sensitive rPN4/NaCh6/Scn8a sodium channel in the nervous system. J. Neurosci. Res. 2000;60:37–44. doi: 10.1002/(SICI)1097-4547(20000401)60:1<37::AID-JNR4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 41.Osorio N., Alcaraz G., Padilla F., Couraud F., Delmas P., Crest M. Differential targeting and functional specialization of sodium channels in cultured cerebellar granule cells. J. Physiol. 2005;569:801–816. doi: 10.1113/jphysiol.2005.097022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorincz A., Nusser Z. Molecular identity of dendritic voltage-gated sodium channels. Science. 2010;328:906–909. doi: 10.1126/science.1187958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu W., Tian C., Li T., Yang M., Hou H., Shu Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat. Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- 44.Bender K.J., Trussell L.O. The Physiology of the Axon Initial Segment. Neurosci. Annu. Rev. 2012;35:249–265. doi: 10.1146/annurev-neuro-062111-150339. [DOI] [PubMed] [Google Scholar]
- 45.Kole M.H., Stuart G.J. Signal processing in the axon initial segment. Neuron. 2012;73:235–247. doi: 10.1016/j.neuron.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Grubb M.S., Shu Y., Kuba H., Rasband M.N., Wimmer V.C., Bender K.J. Short- and long-term plasticity at the axon initial segment. J. Neurosci. 2011;31:16049–16055. doi: 10.1523/JNEUROSCI.4064-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang C.Y., Rasband M.N. Axon initial segments: Structure, function, and disease. Ann. N. Y. Acad. Sci. 2018;1420:46–61. doi: 10.1111/nyas.13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leterrier C. The Axon Initial Segment: An Updated Viewpoint. J. Neurosci. 2018;38:2135. doi: 10.1523/JNEUROSCI.1922-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Royeck M., Horstmann M.T., Remy S., Reitze M., Yaari Y., Beck H. Role of axonal NaV1.6 sodium channels in action potential initiation of CA1 pyramidal neurons. J. Neurophysiol. 2008;100:2361–2380. doi: 10.1152/jn.90332.2008. [DOI] [PubMed] [Google Scholar]
- 50.Lorincz A., Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J. Neurosci. 2008;28:14329–14340. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaplan M.R., Cho M.H., Ullian E.M., Isom L.L., Levinson S.R., Barres B.A. Differential control of clustering of the sodium channels Na(v)1.2 and Na(v)1.6 at developing CNS nodes of Ranvier. Neuron. 2001;30:105–119. doi: 10.1016/S0896-6273(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 52.Van Wart A., Matthews G. Impaired firing and cell-specific compensation in neurons lacking Nav1.6 sodium channels. J. Neurosci. 2006;26:7172–7180. doi: 10.1523/JNEUROSCI.1101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akin E.J., Solé L., Dib-Hajj S.D., Waxman S.G., Tamkun M.M. Preferential targeting of Nav1.6 voltage-gated Na+ Channels to the axon initial segment during development. PLoS ONE. 2015;10:e0124397. doi: 10.1371/journal.pone.0124397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kole M.H., Stuart G.J. Is action potential threshold lowest in the axon? Nat. Neurosci. 2008;11:1253–1255. doi: 10.1038/nn.2203. [DOI] [PubMed] [Google Scholar]
- 55.Stuart G.J., Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- 56.Hedstrom K.L., Ogawa Y., Rasband M.N. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J. Cell Biol. 2008;183:635–640. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garrido J.J., Giraud P.C.E., Fernandes F., Moussif A., Fache M.P., Debanne D., Dargent B. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science. 2003;300:2091–2094. doi: 10.1126/science.1085167. [DOI] [PubMed] [Google Scholar]
- 58.Gasser A., Ho T.S., Cheng X., Chang K.J., Waxman S.G., Rasband M.N., Dib-Hajj S.D. An ankyrinG-binding motif is necessary and sufficient for targeting Nav1.6 sodium channels to axon initial segments and nodes of Ranvier. J. Neurosci. 2012;32:7232–7243. doi: 10.1523/JNEUROSCI.5434-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boiko T., Rasband M.N., Levinson S.R., Caldwell J.H., Mandel G., Trimmer J.S., Matthews G. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron. 2001;30:91–104. doi: 10.1016/S0896-6273(01)00265-3. [DOI] [PubMed] [Google Scholar]
- 60.Boiko T. Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J. Neurosci. 2003;23:2306–2313. doi: 10.1523/JNEUROSCI.23-06-02306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lemaillet G., Walker B., Lambert S. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J. Biol. Chem. 2003;278:27333–27339. doi: 10.1074/jbc.M303327200. [DOI] [PubMed] [Google Scholar]
- 62.Devaux J.J., Kleopa K.A., Cooper E.C., Scherer S.S. KCNQ2 is a nodal K+ channel. J. Neurosci. 2004;24:1236–1244. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan Z., Kao T., Horvath Z., Lemos J., Sul J.Y., Cranstoun S.D., Bennett V., Scherer S.S., Cooper E.C. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J. Neurosci. 2006;26:2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bréchet A., Fache M.P., Brachet A., Ferracci G., Baude A., Irondelle M., Pereira S., Leterrier C., Dargent B. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J. Cell Biol. 2008;183:1101–1114. doi: 10.1083/jcb.200805169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hien Y.E., Montersino A., Castets F., Leterrier C., Filhol O., Vacher H., Dargent B. CK2 accumulation at the axon initial segment depends on sodium channel Nav1. FEBS Lett. 2014;588:3403–3408. doi: 10.1016/j.febslet.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 66.Akin E.J., Tamkun M.M. Another piece to the intracellular FGF/Na+ channel puzzle. Proc. Nat. Aca. Sci. USA. 2016;113:5147–5149. doi: 10.1073/pnas.1604831113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goaillard J.-M., Moubarak E., Tapia M., Tell F. Diversity of Axonal and Dendritic Contributions to Neuronal Output. Front. Cell Neurosci. 2020;13:570. doi: 10.3389/fncel.2019.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gasparini S., Magee J.C. State-dependent dendritic computation in hippocampal CA1 pyramidal neurons. J. Neurosci. 2006;26:2088–2100. doi: 10.1523/JNEUROSCI.4428-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Larkum M.E., Waters J., Sakmann B., Helmchen F. Dendritic spikes in apical dendrites of neocortical layer 2/3 pyramidal neurons. J. Neurosci. 2007;27:8999–9008. doi: 10.1523/JNEUROSCI.1717-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamondi A., Acsády L., Buzsáki G. Dendritic spikes are enhanced by cooperative network activity in the intact hippocampus. J. Neurosci. 1998;18:3919–3928. doi: 10.1523/JNEUROSCI.18-10-03919.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colbert C.M., Magee J.C., Hoffman D.A., Johnston D. Slow recovery from inactivation of Na+ channels underlies the activity-dependent attenuation of dendritic action potentials in hippocampal CA1 pyramidal neurons. J. Neurosci. 1997;17:6512–6521. doi: 10.1523/JNEUROSCI.17-17-06512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gasparini S., Magee J.C. Phosphorylation-dependent differences in the activation properties of distal and proximal dendritic Na+ channels in rat CA1 hippocampal neurons. J. Physiol. 2002;541:665–672. doi: 10.1113/jphysiol.2002.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bywalez W.G., Patirniche D., Rupprecht V., Stemmler M., Herz A.V.M., Pálfi D., Rózsa B., Egger V. Local Postsynaptic Voltage-Gated Sodium Channel Activation in Dendritic Spines of Olfactory Bulb Granule Cells. Neuron. 2015;85:590–601. doi: 10.1016/j.neuron.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 74.Araya R., Nikolenko V., Eisenthal K.B., Yuste R. Sodium channels amplify spine potentials. Proc. Nat. Aca. Sci. USA. 2007;104:12347. doi: 10.1073/pnas.0705282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Engel D., Jonas P. Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron. 2005;45:405–417. doi: 10.1016/j.neuron.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 76.Leão R.M., Kushmerick C., Pinaud R., Renden R., Li G.-L., Taschenberger H., Spirou G., Levinson S.R., von Gersdorff H. Presynaptic Na+ channels: Locus, development, and recovery from inactivation at a high-fidelity synapse. J. Neurosci. 2005;25:3724–3738. doi: 10.1523/JNEUROSCI.3983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stuart G., Sakmann B. Amplification of EPSPs by axosomatic sodium channels in neocortical pyramidal neurons. Neuron. 1995;15:1065–1076. doi: 10.1016/0896-6273(95)90095-0. [DOI] [PubMed] [Google Scholar]
- 78.Golding N.L., Spruston N. Dendritic sodium spikes are variable triggers of axonal action potentials in hippocampal CA1 pyramidal neurons. Neuron. 1998;21:1189–1200. doi: 10.1016/S0896-6273(00)80635-2. [DOI] [PubMed] [Google Scholar]
- 79.Raman I.M., Bean B.P. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J. Neurosci. 1997;17:4517–4526. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raman I.M., Sprunger L.K., Meisler M.H., Bean B.P. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron. 1997;19:881–891. doi: 10.1016/S0896-6273(00)80969-1. [DOI] [PubMed] [Google Scholar]
- 81.Catterall W.A. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/S0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 82.Crill W.E. Persistent Sodium Current in Mammalian Central Neurons. Annu. Rev. Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- 83.Osorio N., Cathala L., Meisler M.H., Crest M., Magistretti J., Delmas P. Persistent Nav1.6 current at axon initial segments tunes spike timing of cerebellar granule cells. J. Physiol. 2010;588:651–670. doi: 10.1113/jphysiol.2010.183798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen Y., Yu F.H., Sharp E.M., Beacham D., Scheuer T., Catterall W.A. Functional properties and differential neuromodulation of Na(v)1.6 channels. Mol. Cell Neurosci. 2008;38:607–615. doi: 10.1016/j.mcn.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taddese A., Bean B.P. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron. 2002;33:587–600. doi: 10.1016/S0896-6273(02)00574-3. [DOI] [PubMed] [Google Scholar]
- 86.van Drongelen W., Lee H.C., Stevens R.L., Hereld M. Propagation of Seizure-like Activity in a Model of Neocortex. J. Clin. Neurophysiol. 2007;24:182–188. doi: 10.1097/WNP.0b013e318039b4de. [DOI] [PubMed] [Google Scholar]
- 87.Veeramah K.R., O’Brien J.E., Meisler M.H., Cheng X., Dib-Hajj S.D., Waxman S.G., Talwar D., Girirajan S., Eichler E.E., Restifo L.L., et al. De novo pathogenic SCN8A mutation identified by whole-genome sequencing of a family quartet affected by infantile epileptic encephalopathy and SUDEP. Am. J. Hum. Genet. 2012;90:502–510. doi: 10.1016/j.ajhg.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bant J.S., Raman I.M. Control of transient, resurgent, and persistent current by open-channel block by Na channel beta4 in cultured cerebellar granule neurons. Proc. Nat. Acad. Sci. USA. 2010;107:12357–12362. doi: 10.1073/pnas.1005633107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yan H., Wang C., Marx S.O., Pitt G.S. Calmodulin limits pathogenic Na+ channel persistent current. J. Gen. Physiol. 2017;149:277–293. doi: 10.1085/jgp.201611721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meisler M.H., Plummer N.W., Burgess D.L., Buchner D.A., Sprunger L.K. Allelic mutations of the sodium channel SCN8A reveal multiple cellular and physiological functions. Genetica. 2004;122:37–45. doi: 10.1007/s10709-004-1441-9. [DOI] [PubMed] [Google Scholar]
- 91.Patel R.R., Barbosa C., Brustovetsky T., Brustovetsky N., Cummins T.R. Aberrant epilepsy-associated mutant Nav1.6 sodium channel activity can be targeted with cannabidiol. Brain. 2016;139:2164–2181. doi: 10.1093/brain/aww129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lopez-Santiago L.F., Yuan Y., Wagnon J.L., Hull J.M., Frasier C.R., O’Malley H.A., Meisler M.H., Isom L.L. Neuronal hyperexcitability in a mouse model of SCN8A epileptic encephalopathy. Proc. Nat. Acad. Sci. USA. 2017;114:2383–2388. doi: 10.1073/pnas.1616821114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stafstrom C.E. Persistent sodium current and its role in epilepsy. Epilepsy Curr. 2007;7:15–22. doi: 10.1111/j.1535-7511.2007.00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xie W., Strong J.A., Ye L., Mao J.X., Zhang J.M. Knockdown of sodium channel NaV1.6 blocks mechanical pain and abnormal bursting activity of afferent neurons in inflamed sensory ganglia. Pain. 2013;154:1170–1180. doi: 10.1016/j.pain.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sittl R., Lampert A., Huth T., Schuy E.T., Link A.S., Fleckenstein J., Alzheimer C., Grafe P., Carr R.W. Anticancer drug oxaliplatin induces acute cooling-aggravated neuropathy via sodium channel subtype Na(V)1.6-resurgent and persistent current. Proc. Nat. Acad. Sci. USA. 2012;109:6704–6709. doi: 10.1073/pnas.1118058109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cummins T.R., Dib-Hajj S.D., Herzog R.I., Waxman S.G. Nav1.6 channels generate resurgent sodium currents in spinal sensory neurons. FEBS Lett. 2005;579:2166–2170. doi: 10.1016/j.febslet.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 97.Hille B. Ion Channels of Excitable Membranes. 3rd ed. Sinauer Associates, Inc.; Sunderland, MA, USA: 2001. [Google Scholar]
- 98.Raman I.M., Bean B.P. Inactivation and recovery of sodium currents in cerebellar Purkinje neurons: Evidence for two mechanisms. J. Biophys. 2001;80:729–737. doi: 10.1016/S0006-3495(01)76052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grieco T.M., Malhotra J.D., Chen C., Isom L.L., Raman I.M. Open-channel block by the cytoplasmic tail of sodium channel beta4 as a mechanism for resurgent sodium current. Neuron. 2005;45:233–244. doi: 10.1016/j.neuron.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 100.Patel R.R., Barbosa C., Xiao Y., Cummins T.R. Human Nav1.6 Channels Generate Larger Resurgent Currents than Human Nav1.1 Channels, but the Navβ4 Peptide Does Not Protect Either Isoform from Use-Dependent Reduction. PLoS ONE. 2015;10:e0133485. doi: 10.1371/journal.pone.0133485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barbosa C., Tan Z.Y., Wang R., Xie W., Strong J.A., Patel R.R., Vasko M.R., Zhang J.M., Cummins T.R. Navβ4 regulates fast resurgent sodium currents and excitability in sensory neurons. Mol. Pain. 2015;11:60. doi: 10.1186/s12990-015-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barbosa C., Xiao Y., Johnson A.J., Xie W., Strong J.A., Zhang J.M., Cummins T.R. FHF2 isoforms differentially regulate Nav1.6-mediated resurgent sodium currents in dorsal root ganglion neurons. Pflug. Arch. 2017;469:195–212. doi: 10.1007/s00424-016-1911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pan Y., Cummins T.R. Distinct functional alterations in SCN8A epilepsy mutant channels. J. Physiol. 2019;598:381–401. doi: 10.1113/JP278952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wagnon J.L., Bunton-Stasyshyn R.K., Meisler M.H. Chapter 10–Mutations of Sodium Channel SCN8A (Nav1.6) in Neurological Disease. In: Pitt G.S., editor. Ion Channels in Health and Disease. Academic Press; Cambridge, MA, USA: 2016. pp. 239–264. [DOI] [Google Scholar]
- 105.Meisler M.H., Helman G., Hammer M.F., Fureman B.E., Gaillard W.D., Goldin A.L., Hirose S., Ishii A., Kroner B.L., Lossin C., et al. SCN8A encephalopathy: Research progress and prospects. Epilepsia. 2016;57:1027–1035. doi: 10.1111/epi.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Butler K.M., da Silva C., Shafir Y., Weisfeld-Adams J.D., Alexander J.J., Hegde M., Escayg A. De novo and inherited SCN8A epilepsy mutations detected by gene panel analysis. Epilepsy Res. 2017;129:17–25. doi: 10.1016/j.eplepsyres.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Trudeau M.M., Dalton J.C., Day J.W., Ranum L.P., Meisler M.H. Heterozygosity for a protein truncation mutation of sodium channel SCN8A in a patient with cerebellar atrophy, ataxia, and mental retardation. J. Med. Genet. 2006;43:527–530. doi: 10.1136/jmg.2005.035667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sharkey L.M., Cheng X., Drews V., Buchner D.A., Jones J.M., Justice M.J., Waxman S.G., Dib-Hajj S.D., Meisler M.H. The ataxia3 Mutation in the N-Terminal Cytoplasmic Domain of Sodium Channel Nav1.6 Disrupts Intracellular Trafficking. J. Neurosci. 2009;29:2733. doi: 10.1523/JNEUROSCI.6026-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stafstrom C.E., Carmant L. Seizures and epilepsy: An overview for neuroscientists. Cold Spring Harb. Perspect. Med. 2015;5:a022426. doi: 10.1101/cshperspect.a022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Estacion M., O’Brien J.E., Conravey A., Hammer M.F., Waxman S.G., Dib-Hajj S.D., Meisler M.H. A novel de novo mutation of SCN8A (Nav1.6) with enhanced channel activation in a child with epileptic encephalopathy. Neurobiol. Dis. 2014;69:117–123. doi: 10.1016/j.nbd.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vaher U., Nõukas M., Nikopensius T., Kals M., Annilo T., Nelis M., Ounap K., Reimand T., Talvik I., Ilves P., et al. De novo SCN8A mutation identified by whole-exome sequencing in a boy with neonatal epileptic encephalopathy, multiple congenital anomalies, and movement disorders. J. Child Neurol. 2014;29:Np202–Np206. doi: 10.1177/0883073813511300. [DOI] [PubMed] [Google Scholar]
- 112.Bialer M., Johannessen S.I., Koepp M.J., Levy R.H., Perucca E., Tomson T., White H.S. Progress report on new antiepileptic drugs: A summary of the Fourteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XIV). I. Drugs in preclinical and early clinical development. Epilepsia. 2018;59:1811–1841. doi: 10.1111/epi.14557. [DOI] [PubMed] [Google Scholar]
- 113.Beatch G., Namdari R., Cadieux J., Kato H., Aycardi E. A Phase 1 Study to Assess the Safety, Tolerability and Pharmacokinetics of Two Formulations of a Novel Nav1.6 Sodium Channnel Blocker (XEN901) in Healthy Adult Subjects. (4757) Neurology. 2020;94:4757. [Google Scholar]
- 114.Wengert E.R., Saga A.U., Panchal P.S., Barker B.S., Patel M.K. Prax330 reduces persistent and resurgent sodium channel currents and neuronal hyperexcitability of subiculum neurons in a mouse model of SCN8A epileptic encephalopathy. Neuropharmacology. 2019;158:107699. doi: 10.1016/j.neuropharm.2019.107699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mason E.R., Cummins T.R. Differential Inhibition of Human Nav1.2 Resurgent and Persistent Sodium Currents by Cannabidiol and GS967. Int. J. Mol. Sci. 2020;21:2454. doi: 10.3390/ijms21072454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baker E.M., Thompson C.H., Hawkins N.A., Wagnon J.L., Wengert E.R., Patel M.K., George A.L., Jr., Meisler M.H., Kearney J.A. The novel sodium channel modulator GS-458967 (GS967) is an effective treatment in a mouse model of SCN8A encephalopathy. Epilepsia. 2018;59:1166–1176. doi: 10.1111/epi.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weuring W.J., Singh S., Volkers L., Rook M.B., van’t Slot R.H., Bosma M., Inserra M., Vetter I., Verhoeven-Duif N.M., Braun K.P.J., et al. NaV1.1 and NaV1.6 selective compounds reduce the behavior phenotype and epileptiform activity in a novel zebrafish model for Dravet Syndrome. PLoS ONE. 2020;15:e0219106. doi: 10.1371/journal.pone.0219106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xie W., Strong J.A., Zhang J.M. Local knockdown of the NaV1.6 sodium channel reduces pain behaviors, sensory neuron excitability, and sympathetic sprouting in rat models of neuropathic pain. Neuroscience. 2015;291:317–330. doi: 10.1016/j.neuroscience.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Laedermann C.J., Abriel H., Decosterd I. Post-translational modifications of voltage-gated sodium channels in chronic pain syndromes. Front. Pharmacol. 2015;6:263. doi: 10.3389/fphar.2015.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alshammari M.A., Khan M.R., Alasmari F., Alshehri A.O., Ali R., Boudjelal M., Alhosaini K.A., Niazy A.A., Alshammari T.K. Changes in the Fluorescence Tracking of NaV1.6 Protein Expression in a BTBR T+Itpr3tf/J Autistic Mouse Model. Neural Plast. 2019;2019:12. doi: 10.1155/2019/4893103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhu H., Lin W., Zhao Y., Wang Z., Lao W., Kuang P., Zhou H. Transient upregulation of Nav1.6 expression in the genu of corpus callosum following middle cerebral artery occlusion in the rats. Brain Res. Bull. 2017;132:20–27. doi: 10.1016/j.brainresbull.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 122.Wittmack E.K., Rush A.M., Hudmon A., Waxman S.G., Dib-Hajj S.D. Voltage-gated sodium channel Nav1.6 is modulated by p38 mitogen-activated protein kinase. J. Neurosci. 2005;25:6621–6630. doi: 10.1523/JNEUROSCI.0541-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu J.-X., Tong L., Hu L., Xia C.-M., Li M., Chen Q.-H., Chen F.-X., Du D.-S. Upregulation of Nav1.6 expression in the rostral ventrolateral medulla of stress-induced hypertensive rats. Hypertens. Res. 2018;41:1013–1022. doi: 10.1038/s41440-018-0105-6. [DOI] [PubMed] [Google Scholar]
- 124.Hargus N.J., Nigam A., Bertram E.H., 3rd, Patel M.K. Evidence for a role of Nav1.6 in facilitating increases in neuronal hyperexcitability during epileptogenesis. J. Neurophysiol. 2013;110:1144–1157. doi: 10.1152/jn.00383.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Blumenfeld H., Lampert A., Klein J.P., Mission J., Chen M.C., Rivera M., Dib-Hajj S., Brennan A.R., Hains B.C., Waxman S.G. Role of hippocampal sodium channel Nav1.6 in kindling epileptogenesis. Epilepsia. 2009;50:44–55. doi: 10.1111/j.1528-1167.2008.01710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martin M.S., Tang B., Papale L.A., Yu F.H., Catterall W.A., Escayg A. The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy of infancy. Hum. Mol. Genet. 2007;16:2892–2899. doi: 10.1093/hmg/ddm248. [DOI] [PubMed] [Google Scholar]
- 127.Makinson C.D., Tanaka B.S., Lamar T., Goldin A.L., Escayg A. Role of the hippocampus in Nav1.6 (Scn8a) mediated seizure resistance. Neurobiol. Dis. 2014;68:16–25. doi: 10.1016/j.nbd.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wong J.C., Makinson C.D., Lamar T., Cheng Q., Wingard J.C., Terwilliger E.F., Escayg A. Selective targeting of Scn8a prevents seizure development in a mouse model of mesial temporal lobe epilepsy. Sci. Rep. 2018;8:126. doi: 10.1038/s41598-017-17786-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lenk G.M., Jafar-Nejad P., Hill S.F., Huffman L.D., Smolen C.E., Wagnon J.L., Petit H., Yu W., Ziobro J., Bhatia K., et al. Scn8a Antisense Oligonucleotide Is Protective in Mouse Models of SCN8A Encephalopathy and Dravet Syndrome. Ann. Neurol. 2020;87:339–346. doi: 10.1002/ana.25676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.O’Malley H.A., Isom L.L. Sodium channel β subunits: Emerging targets in channelopathies. Annu. Rev. Physiol. 2015;77:481–504. doi: 10.1146/annurev-physiol-021014-071846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brackenbury W.J., Calhoun J.D., Chen C., Miyazaki H., Nukina N., Oyama F., Ranscht B., Isom L.L. Functional reciprocity between Na+ channel Nav1.6 and beta1 subunits in the coordinated regulation of excitability and neurite outgrowth. Proc. Nat. Acad. Sci. USA. 2010;107:2283–2288. doi: 10.1073/pnas.0909434107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aman T.K., Grieco-Calub T.M., Chen C., Rusconi R., Slat E.A., Isom L.L., Raman I.M. Regulation of persistent Na current by interactions between beta subunits of voltage-gated Na channels. J. Neurosci. 2009;29:2027–2042. doi: 10.1523/JNEUROSCI.4531-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grieco T.M., Afshari F.S., Raman I.M. A role for phosphorylation in the maintenance of resurgent sodium current in cerebellar purkinje neurons. J. Neurosci. 2002;22:3100–3107. doi: 10.1523/JNEUROSCI.22-08-03100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bouza A.A., Philippe J.M., Edokobi N., Pinsky A.M., Offord J., Calhoun J.D., Lopez-Florán M., Lopez-Santiago L.F., Jenkins P.M., Isom L.L. Sodium channel β1 subunits are post-translationally modified by tyrosine phosphorylation, S-palmitoylation, and regulated intramembrane proteolysis. J. Biol. Chem. 2020;295:10380–10393. doi: 10.1074/jbc.RA120.013978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Olsen S.K., Garbi M., Zampieri N., Eliseenkova A.V., Ornitz D.M., Goldfarb M., Mohammadi M. Fibroblast growth factor (FGF) homologous factors share structural but not functional homology with FGFs. J. Biol. Chem. 2003;278:34226–34236. doi: 10.1074/jbc.M303183200. [DOI] [PubMed] [Google Scholar]
- 136.Schoorlemmer J., Goldfarb M. Fibroblast growth factor homologous factors are intracellular signaling proteins. Curr. Biol. 2001;11:793–797. doi: 10.1016/S0960-9822(01)00232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schoorlemmer J., Goldfarb M. Fibroblast growth factor homologous factors and the islet brain-2 scaffold protein regulate activation of a stress-activated protein kinase. J. Biol. Chem. 2002;277:49111–49119. doi: 10.1074/jbc.M205520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pablo J.L., Pitt G.S. Fibroblast Growth Factor Homologous Factors: New Roles in Neuronal Health and Disease. Neuroscientist. 2016;22:19–25. doi: 10.1177/1073858414562217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dib-Hajj S.D., Waxman S.G. Isoform-specific and pan-channel partners regulate trafficking and plasma membrane stability; and alter sodium channel gating properties. Neurosci. Let. 2010;486:84–91. doi: 10.1016/j.neulet.2010.08.077. [DOI] [PubMed] [Google Scholar]
- 140.Goetz R., Dover K., Laezza F., Shtraizent N., Huang X., Tchetchik D., Eliseenkova A.V., Xu C.F., Neubert T.A., Ornitz D.M., et al. Crystal structure of a fibroblast growth factor homologous factor (FHF) defines a conserved surface on FHFs for binding and modulation of voltage-gated sodium channels. J. Biol. Chem. 2009;284:17883–17896. doi: 10.1074/jbc.M109.001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu C., Dib-Hajj S.D., Waxman S.G. Fibroblast growth factor homologous factor 1B binds to the C terminus of the tetrodotoxin-resistant sodium channel rNav1.9a (NaN) J. Biol. Chem. 2001;276:18925–18933. doi: 10.1074/jbc.M101606200. [DOI] [PubMed] [Google Scholar]
- 142.Wang C., Wang C., Hoch E.G., Pitt G.S. Identification of novel interaction sites that determine specificity between fibroblast growth factor homologous factors and voltage-gated sodium channels. J. Biol. Chem. 2011;286:24253–24263. doi: 10.1074/jbc.M111.245803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang C., Chung B.C., Yan H., Lee S.Y., Pitt G.S. Crystal structure of the ternary complex of a NaV C-terminal domain, a fibroblast growth factor homologous factor, and calmodulin. Structure. 2012;20:1167–1176. doi: 10.1016/j.str.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Munoz-Sanjuan I., Smallwood P.M., Nathans J. Isoform diversity among fibroblast growth factor homologous factors is generated by alternative promoter usage and differential splicing. J. Biol. Chem. 2000;275:2589–2597. doi: 10.1074/jbc.275.4.2589. [DOI] [PubMed] [Google Scholar]
- 145.Wang Q., McEwen D.G., Ornitz D.M. Subcellular and developmental expression of alternatively spliced forms of fibroblast growth factor 14. Mech. Dev. 2000;90:283–287. doi: 10.1016/S0925-4773(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 146.Laezza F., Lampert A., Kozel M.A., Gerber B.R., Rush A.M., Nerbonne J.M., Waxman S.G., Dib-Hajj S.D., Ornitz D.M. FGF14 N-terminal splice variants differentially modulate Nav1.2 and Nav1.6-encoded sodium channels. Mol. Cell Neurosci. 2009;42:90–101. doi: 10.1016/j.mcn.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xiao M., Bosch M.K., Nerbonne J.M., Ornitz D.M. FGF14 localization and organization of the axon initial segment. Mol. Cell Neurosci. 2013;56:393–403. doi: 10.1016/j.mcn.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wittmack E.K., Rush A.M., Craner M.J., Goldfarb M., Waxman S.G., Dib-Hajj S.D. Fibroblast Growth Factor Homologous Factor 2B: Association with Nav1.6 and Selective Colocalization at Nodes of Ranvier of Dorsal Root Axons. J. Neurosci. 2004;24:6765. doi: 10.1523/JNEUROSCI.1628-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rush A.M., Wittmack E.K., Tyrrell L., Black J.A., Dib-Hajj S.D., Waxman S.G. Differential modulation of sodium channel Nav1.6 by two members of the fibroblast growth factor homologous factor 2 subfamily. Euro J. Neurosci. 2006;23:2551–2562. doi: 10.1111/j.1460-9568.2006.04789.x. [DOI] [PubMed] [Google Scholar]
- 150.Xia Z., Storm D.R. The role of calmodulin as a signal integrator for synaptic plasticity. Nat. Rev. Neurosci. 2005;6:267–276. doi: 10.1038/nrn1647. [DOI] [PubMed] [Google Scholar]
- 151.Babu Y.S., Sack J.S., Greenhough T.J., Bugg C.E., Means A.R., Cook W.J. Three-dimensional structure of calmodulin. Nature. 1985;315:37–40. doi: 10.1038/315037a0. [DOI] [PubMed] [Google Scholar]
- 152.Kretsinger R.H., Rudnick S.E., Weissman L.J. Crystal structure of calmodulin. J. Inorg. Biochem. 1986;28:289–302. doi: 10.1016/0162-0134(86)80093-9. [DOI] [PubMed] [Google Scholar]
- 153.Chattopadhyaya R., Meador W.E., Means A.R., Quiocho F.A. Calmodulin structure refined at 1.7 Å resolution. J. Mol. Biol. 1992;228:1177–1192. doi: 10.1016/0022-2836(92)90324-D. [DOI] [PubMed] [Google Scholar]
- 154.Zhang M., Tanaka T., Ikura M. Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nat. Struct. Biol. 1995;2:758–767. doi: 10.1038/nsb0995-758. [DOI] [PubMed] [Google Scholar]
- 155.Linse S., Helmersson A., Forsén S. Calcium binding to calmodulin and its globular domains. J. Biol. Chem. 1991;266:8050–8054. doi: 10.1016/S0021-9258(18)92938-8. [DOI] [PubMed] [Google Scholar]
- 156.O’Neil K.T., DeGrado W.F. How calmodulin binds its targets: Sequence independent recognition of amphiphilic alpha-helices. Trend Biochem. Sci. 1990;15:59–64. doi: 10.1016/0968-0004(90)90177-D. [DOI] [PubMed] [Google Scholar]
- 157.Tidow H., Nissen P. Structural diversity of calmodulin binding to its target sites. J. FEBS. 2013;280:5551–5565. doi: 10.1111/febs.12296. [DOI] [PubMed] [Google Scholar]
- 158.Babitch J. Channel hands. Nature. 1990;346:321–322. doi: 10.1038/346321b0. [DOI] [PubMed] [Google Scholar]
- 159.Mori M., Konno T., Ozawa T., Murata M., Imoto K., Nagayama K. Novel Interaction of the Voltage-Dependent Sodium Channel (VDSC) with Calmodulin: Does VDSC Acquire Calmodulin-Mediated Ca2+-Sensitivity? Biochemistry. 2000;39:1316–1323. doi: 10.1021/bi9912600. [DOI] [PubMed] [Google Scholar]
- 160.Bähler M., Rhoads A. Calmodulin signaling via the IQ motif. FEBS Lett. 2002;513:107–113. doi: 10.1016/S0014-5793(01)03239-2. [DOI] [PubMed] [Google Scholar]
- 161.Shah V.N., Chagot B., Chazin W.J. Calcium-Dependent Regulation of Ion Channels. Calcium Bind. Proteins. 2006;1:203–212. [PMC free article] [PubMed] [Google Scholar]
- 162.Shah V.N., Wingo T.L., Weiss K.L., Williams C.K., Balser J.R., Chazin W.J. Calcium-dependent regulation of the voltage-gated sodium channel hH1: Intrinsic and extrinsic sensors use a common molecular switch. Proc. Natl. Acad. Sci. USA. 2006;103:3592–3597. doi: 10.1073/pnas.0507397103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wingo T.L., Shah V.N., Anderson M.E., Lybrand T.P., Chazin W.J., Balser J.R. An EF-hand in the sodium channel couples intracellular calcium to cardiac excitability. Nat. Struct. Mol. Biol. 2004;11:219–225. doi: 10.1038/nsmb737. [DOI] [PubMed] [Google Scholar]
- 164.Kim J., Ghosh S., Liu H., Tateyama M., Kass R.S., Pitt G.S. Calmodulin mediates Ca2+ sensitivity of sodium channels. J. Biol. Chem. 2004;279:45004–45012. doi: 10.1074/jbc.M407286200. [DOI] [PubMed] [Google Scholar]
- 165.Miloushev V.Z., Levine J.A., Arbing M.A., Hunt J.F., Pitt G.S., Palmer A.G., 3rd Solution structure of the NaV1.2 C-terminal EF-hand domain. J. Biol. Chem. 2009;284:6446–6454. doi: 10.1074/jbc.M807401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gardill B.R., Rivera-Acevedo R.E., Tung C.C., Van Petegem F. Crystal structures of Ca(2+)-calmodulin bound to Na(V) C-terminal regions suggest role for EF-hand domain in binding and inactivation. Proc. Nat. Acad. Sci. USA. 2019;116:10763–10772. doi: 10.1073/pnas.1818618116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gaudioso C. Calmodulin and calcium differentially regulate the neuronal Nav1.1 voltage-dependent sodium channel. Biochem. Biophys. Res. Commun. 2011;411:329–334. doi: 10.1016/j.bbrc.2011.06.142. [DOI] [PubMed] [Google Scholar]
- 168.Herzog R.I., Liu C., Waxman S.G., Cummins T.R. Calmodulin binds to the C terminus of sodium channels Nav1.4 and Nav1.6 and differentially modulates their functional properties. J. Neurosci. 2003;23:8261–8270. doi: 10.1523/JNEUROSCI.23-23-08261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sarhan M.F., Tung C.C., Van Petegem F., Ahern C.A. Crystallographic basis for calcium regulation of sodium channels. Proc. Nat. Acad. Sci. USA. 2012;109:3558–3563. doi: 10.1073/pnas.1114748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Deschenes I. Isoform-specific modulation of voltage-gated Na(+) channels by calmodulin. Circ. Res. 2002;90:e49–e57. doi: 10.1161/01.RES.0000012502.92751.E6. [DOI] [PubMed] [Google Scholar]
- 171.Tan H.L. A calcium sensor in the sodium channel modulates cardiac excitability. Nature. 2002;415:442–447. doi: 10.1038/415442a. [DOI] [PubMed] [Google Scholar]
- 172.Young K.A., Caldwell J.H. Modulation of skeletal and cardiac voltage-gated sodium channels by calmodulin. J. Physiol. 2005;565:349–370. doi: 10.1113/jphysiol.2004.081422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Biswas S. Calmodulin regulation of Nav1.4 current: Role of binding to the carboxyl terminus. J. Gen. Physiol. 2008;131:197–209. doi: 10.1085/jgp.200709863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Reddy Chichili V.P., Xiao Y., Seetharaman J., Cummins T.R., Sivaraman J. Structural basis for the modulation of the neuronal voltage-gated sodium channel NaV1.6 by calmodulin. Sci. Rep. 2013;3:2435. doi: 10.1038/srep02435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang Z., Vermij S.H., Sottas V., Shestak A., Ross-Kaschitza D., Zaklyazminskaya E.V., Hudmon A., Pitt G.S., Rougier J.-S., Abriel H. Calmodulin binds to the N-terminal domain of the cardiac sodium channel Na(v)1.5. Channels. 2020;14:268–286. doi: 10.1080/19336950.2020.1805999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Burel S., Coyan F.C., Lorenzini M., Meyer M.R., Lichti C.F., Brown J.H., Loussouarn G., Charpentier F., Nerbonne J.M., Townsend R.R., et al. C-terminal phosphorylation of NaV1.5 impairs FGF13-dependent regulation of channel inactivation. J. Biol. Chem. 2017;292:17431–17448. doi: 10.1074/jbc.M117.787788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Waechter C.J., Schmidt J.W., Catterall W.A. Glycosylation is required for maintenance of functional sodium channels in neuroblastoma cells. J. Biol. Chem. 1983;258:5117–5123. doi: 10.1016/S0021-9258(18)32546-8. [DOI] [PubMed] [Google Scholar]
- 178.Schmidt J.W., Catterall W.A. Palmitylation, sulfation, and glycosylation of the alpha subunit of the sodium channel. Role of post-translational modifications in channel assembly. J. Biol. Chem. 1987;262:13713–13723. doi: 10.1016/S0021-9258(19)76485-0. [DOI] [PubMed] [Google Scholar]
- 179.Ednie A.R., Bennett E.S. Modulation of voltage-gated ion channels by sialylation. Compr. Physiol. 2012;2:1269–1301. doi: 10.1002/cphy.c110044. [DOI] [PubMed] [Google Scholar]
- 180.Baycin-Hizal D., Gottschalk A., Jacobson E., Mai S., Wolozny D., Zhang H., Krag S.S., Betenbaugh M.J. Physiologic and pathophysiologic consequences of altered sialylation and glycosylation on ion channel function. Biochem. Biophys. Res. Commun. 2014;453:243–253. doi: 10.1016/j.bbrc.2014.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lazniewska J., Weiss N. The "sweet" side of ion channels. Rev. Physiol. 2014;167:67–114. doi: 10.1007/112_2014_20. [DOI] [PubMed] [Google Scholar]
- 182.Mercier A., Clément R., Harnois T., Bourmeyster N., Bois P., Chatelier A. Nav1.5 channels can reach the plasma membrane through distinct N-glycosylation states. Biochim. Biophys. Acta. 2015;1850:1215–1223. doi: 10.1016/j.bbagen.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 183.Jones J.M., Dionne L., Dell’Orco J., Parent R., Krueger J.N., Cheng X., Dib-Hajj S.D., Bunton-Stasyshyn R.K., Sharkey L.M., Dowling J.J., et al. Single amino acid deletion in transmembrane segment D4S6 of sodium channel Scn8a (Nav1.6) in a mouse mutant with a chronic movement disorder. Neurobiol. Dis. 2016;89:36–45. doi: 10.1016/j.nbd.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Recio-Pinto E., Thornhill W.B., Duch D.S., Levinson S.R., Urban B.W. Neuraminidase treatment modifies the function of electroplax sodium channels in planar lipid bilayers. Neuron. 1990;5:675–684. doi: 10.1016/0896-6273(90)90221-Z. [DOI] [PubMed] [Google Scholar]
- 185.Bennett E., Urcan M.S., Tinkle S.S., Koszowski A.G., Levinson S.R. Contribution of sialic acid to the voltage dependence of sodium channel gating. A possible electrostatic mechanism. J. Gen. Physiol. 1997;109:327–343. doi: 10.1085/jgp.109.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang Y., Hartmann H.A., Satin J. Glycosylation Influences Voltage-Dependent Gating of Cardiac and Skeletal Muscle Sodium Channels. J. Membr. Biol. 1999;171:195–207. doi: 10.1007/s002329900571. [DOI] [PubMed] [Google Scholar]
- 187.Tyrrell L., Renganathan M., Dib-Hajj S.D., Waxman S.G. Glycosylation Alters Steady-State Inactivation of Sodium Channel Nav1.9/NaN in Dorsal Root Ganglion Neurons and Is Developmentally Regulated. J. Neurosci. 2001;21:9629. doi: 10.1523/JNEUROSCI.21-24-09629.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pickart C.M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 189.Abriel H., Staub O. Ubiquitylation of Ion Channels. Physiology. 2005;20:398–407. doi: 10.1152/physiol.00033.2005. [DOI] [PubMed] [Google Scholar]
- 190.Shih S.C., Sloper-Mould K.E., Hicke L. Monoubiquitin carries a novel internalization signal that is appended to activated receptors. J. EMBO. 2000;19:187–198. doi: 10.1093/emboj/19.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ciechanover A. Proteolysis: From the lysosome to ubiquitin and the proteasome. Nat. Rev. Mol. Cell Biol. 2005;6:79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 192.Fotia A.B., Ekberg J., Adams D.J., Cook D.I., Poronnik P., Kumar S. Regulation of neuronal voltage-gated sodium channels by the ubiquitin-protein ligases Nedd4 and Nedd4-2. J. Biol. Chem. 2004;279:28930–28935. doi: 10.1074/jbc.M402820200. [DOI] [PubMed] [Google Scholar]
- 193.Rougier J.S., van Bemmelen M.X., Bruce M.C., Jespersen T., Gavillet B., Apothéloz F., Cordonier S., Staub O., Rotin D., Abriel H. Molecular determinants of voltage-gated sodium channel regulation by the Nedd4/Nedd4-like proteins. American journal of physiology. Cell Physiol. 2005;288:C692–C701. doi: 10.1152/ajpcell.00460.2004. [DOI] [PubMed] [Google Scholar]
- 194.Gasser A., Cheng X., Gilmore E.S., Tyrrell L., Waxman S.G., Dib-Hajj S.D. Two Nedd4-binding motifs underlie modulation of sodium channel Nav1.6 by p38 MAPK. J. Biol. Chem. 2010;285:26149–26161. doi: 10.1074/jbc.M109.098681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Obata T., Brown G.E., Yaffe M.B. MAP kinase pathways activated by stress: The p38 MAPK pathway. Crit. Care. Med. 2000;28:N67–N77. doi: 10.1097/00003246-200004001-00008. [DOI] [PubMed] [Google Scholar]
- 196.Shipston M.J. Ion channel regulation by protein palmitoylation. J. Biol. Chem. 2011;286:8709–8716. doi: 10.1074/jbc.R110.210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shipston M.J. Ion channel regulation by protein S-acylation. J. Gen. Physiol. 2014;143:659–678. doi: 10.1085/jgp.201411176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pan Y., Xiao Y., Pei Z., Cummins T.R. S-palmitoylation of the sodium channel Nav1.6 regulates its activity and neuronal excitability. J. Biol. Chem. 2020 doi: 10.1074/jbc.RA119.012423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ardito F., Giuliani M., Perrone D., Troiano G., Lo Muzio L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review) Int. J. Mol. Med. 2017;40:271–280. doi: 10.3892/ijmm.2017.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cantrell A.R., Catterall W.A. Neuromodulation of Na+ channels: An unexpected form of cellular plasticity. Nat. Rev. Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- 201.Numann R., Catterall W.A., Scheuer T. Functional modulation of brain sodium channels by protein kinase C phosphorylation. Science. 1991;254:115–118. doi: 10.1126/science.1656525. [DOI] [PubMed] [Google Scholar]
- 202.Vijayaragavan K., Boutjdir M., Chahine M. Modulation of Nav1.7 and Nav1.8 peripheral nerve sodium channels by protein kinase A and protein kinase C. J. Neurophysiol. 2004;91:1556–1569. doi: 10.1152/jn.00676.2003. [DOI] [PubMed] [Google Scholar]
- 203.Numann R., Hauschka S.D., Catterall W.A., Scheuer T. Modulation of skeletal muscle sodium channels in a satellite cell line by protein kinase C. J. Neurosci. 1994;14:4226–4236. doi: 10.1523/JNEUROSCI.14-07-04226.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bendahhou S., Cummins T.R., Potts J.F., Tong J., Agnew W.S. Serine-1321-independent regulation of the mu 1 adult skeletal muscle Na+ channel by protein kinase C. Proc. Nat. Acad. Sci. USA. 1995;92:12003–12007. doi: 10.1073/pnas.92.26.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Qu Y., Rogers J., Tanada T., Scheuer T., Catterall W.A. Modulation of cardiac Na+ channels expressed in a mammalian cell line and in ventricular myocytes by protein kinase C. Proc. Natl. Acad. Sci. USA. 1994;91:3289–3293. doi: 10.1073/pnas.91.8.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cantrell A.R., Tibbs V.C., Yu F.H., Murphy B.J., Sharp E.M., Qu Y., Catterall W.A., Scheuer T. Molecular mechanism of convergent regulation of brain Na(+) channels by protein kinase C and protein kinase A anchored to AKAP-15. Mol. Cell Neurosci. 2002;21:63–80. doi: 10.1006/mcne.2002.1162. [DOI] [PubMed] [Google Scholar]
- 207.Li M., West J.W., Lai Y., Scheuer T., Catterall W.A. Functional modulation of brain sodium channels by cAMP-dependent phosphorylation. Neuron. 1992;8:1151–1159. doi: 10.1016/0896-6273(92)90135-Z. [DOI] [PubMed] [Google Scholar]
- 208.Smith R.D., Goldin A.L. Phosphorylation at a single site in the rat brain sodium channel is necessary and sufficient for current reduction by protein kinase A. J. Neurosci. 1997;17:6086–6093. doi: 10.1523/JNEUROSCI.17-16-06086.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Smith R.D., Goldin A.L. Functional analysis of the rat I sodium channel in xenopus oocytes. J. Neurosci. 1998;18:811–820. doi: 10.1523/JNEUROSCI.18-03-00811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ono K., Fozzard H.A., Hanck D.A. Mechanism of cAMP-dependent modulation of cardiac sodium channel current kinetics. Circ. Res. 1993;72:807–815. doi: 10.1161/01.RES.72.4.807. [DOI] [PubMed] [Google Scholar]
- 211.Matsuda J.J., Lee H., Shibata E.F. Enhancement of rabbit cardiac sodium channels by beta-adrenergic stimulation. Circ. Res. 1992;70:199–207. doi: 10.1161/01.RES.70.1.199. [DOI] [PubMed] [Google Scholar]
- 212.Fitzgerald E.M., Okuse K., Wood J.N., Dolphin A.C., Moss S.J. cAMP-dependent phosphorylation of the tetrodotoxin-resistant voltage-dependent sodium channel SNS. J. Physiol. 1999;516 Pt 2:433–446. doi: 10.1111/j.1469-7793.1999.0433v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Murphy B.J., Rossie S., De Jongh K.S., Catterall W.A. Identification of the sites of selective phosphorylation and dephosphorylation of the rat brain Na+ channel alpha subunit by cAMP-dependent protein kinase and phosphoprotein phosphatases. J. Biol. Chem. 1993;268:27355–27362. doi: 10.1016/S0021-9258(19)74257-4. [DOI] [PubMed] [Google Scholar]
- 214.Cantrell A.R., Smith R.D., Goldin A.L., Scheuer T., Catterall W.A. Dopaminergic modulation of sodium current in hippocampal neurons via cAMP-dependent phosphorylation of specific sites in the sodium channel alpha subunit. J. Neurosci. 1997;17:7330–7338. doi: 10.1523/JNEUROSCI.17-19-07330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bayer K.U., Schulman H. CaM Kinase: Still Inspiring at 40. Neuron. 2019;103:380–394. doi: 10.1016/j.neuron.2019.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Herren A.W., Weber D.M., Rigor R.R., Margulies K.B., Phinney B.S., Bers D.M. CaMKII Phosphorylation of Na(V)1.5: Novel in Vitro Sites Identified by Mass Spectrometry and Reduced S516 Phosphorylation in Human Heart Failure. J. Proteome Res. 2015;14:2298–2311. doi: 10.1021/acs.jproteome.5b00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Herren A.W., Bers D.M., Grandi E. Post-translational modifications of the cardiac Na channel: Contribution of CaMKII-dependent phosphorylation to acquired arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 2013;305:H431–H445. doi: 10.1152/ajpheart.00306.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Baek J.H., Cerda O., Trimmer J.S. Mass spectrometry-based phosphoproteomics reveals multisite phosphorylation on mammalian brain voltage-gated sodium and potassium channels. Semin. Cell Dev. Biol. 2011;22:153–159. doi: 10.1016/j.semcdb.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li S., Wang X., Ma Q.-H., Yang W.-l., Zhang X.-G., Dawe G.S., Xiao Z.-C. Amyloid precursor protein modulates Nav1.6 sodium channel currents through a Go-coupled JNK pathway. Sci. Rep. 2016;6:39320. doi: 10.1038/srep39320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ciccone R., Franco C., Piccialli I., Boscia F., Casamassa A., de Rosa V., Cepparulo P., Cataldi M., Annunziato L., Pannaccione A. Amyloid β-Induced Upregulation of Na(v)1.6 Underlies Neuronal Hyperactivity in Tg2576 Alzheimer’s Disease Mouse Model. Sci. Rep. 2019;9:13592. doi: 10.1038/s41598-019-50018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang X., Zhang X.G., Zhou T.T., Li N., Jang C.Y., Xiao Z.C., Ma Q.H., Li S. Elevated Neuronal Excitability Due to Modulation of the Voltage-Gated Sodium Channel Nav1.6 by Abeta1-42. Front. Neurosci. 2016;10:94. doi: 10.3389/fnins.2016.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu C., Tan F.C., Xiao Z.C., Dawe G.S. Amyloid precursor protein enhances Nav1.6 sodium channel cell surface expression. J. Biol. Chem. 2015;290:12048–12057. doi: 10.1074/jbc.M114.617092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kim W.-Y., Snider W. Functions of GSK-3 Signaling in Development of the Nervous System. Front. Mol. Neurosci. 2011;4:44. doi: 10.3389/fnmol.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Jaworski T. Control of neuronal excitability by GSK-3beta: Epilepsy and beyond. Biochim. Biophys. Acta. 2020;1867:118745. doi: 10.1016/j.bbamcr.2020.118745. [DOI] [PubMed] [Google Scholar]
- 225.Jaworski T., Banach-Kasper E., Gralec K. GSK-3β at the Intersection of Neuronal Plasticity and Neurodegeneration. Neural. Plast. 2019;2019:4209475. doi: 10.1155/2019/4209475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Scala F., Nenov M.N., Crofton E.J., Singh A.K., Folorunso O., Zhang Y., Chesson B.C., Wildburger N.C., James T.F., Alshammari M.A., et al. Environmental Enrichment and Social Isolation Mediate Neuroplasticity of Medium Spiny Neurons through the GSK3 Pathway. Cell Rep. 2018;23:555–567. doi: 10.1016/j.celrep.2018.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wadsworth P.A., Folorunso O., Nguyen N., Singh A.K., D’Amico D., Powell R.T., Brunell D., Allen J., Stephan C., Laezza F. High-throughput screening against protein:protein interaction interfaces reveals anti-cancer therapeutics as potent modulators of the voltage-gated Na+ channel complex. Sci. Rep. 2019;9:16890. doi: 10.1038/s41598-019-53110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hsu W.-C., Nenov M.N., Shavkunov A., Panova N., Zhan M., Laezza F. Identifying a kinase network regulating FGF14:Nav1.6 complex assembly using split-luciferase complementation. PLoS ONE. 2015;10:e0117246. doi: 10.1371/journal.pone.0117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ali S.R., Liu Z., Nenov M.N., Folorunso O., Singh A., Scala F., Chen H., James T.F., Alshammari M., Panova-Elektronova N.I., et al. Functional Modulation of Voltage-Gated Sodium Channels by a FGF14-Based Peptidomimetic. ACS Chem. Neurosci. 2018;9:976–987. doi: 10.1021/acschemneuro.7b00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ali S.R., Singh A.K., Laezza F. Identification of Amino Acid Residues in Fibroblast Growth Factor 14 (FGF14) Required for Structure-Function Interactions with Voltage-gated Sodium Channel Nav1.6. J. Biol. Chem. 2016;291:11268–11284. doi: 10.1074/jbc.M115.703868. [DOI] [PMC free article] [PubMed] [Google Scholar]