Abstract
Prevention of epilepsy is a great unmet need. Acute central nervous system (CNS) insults such as traumatic brain injury (TBI), cerebrovascular accidents (CVA), and CNS infections account for 15%−20% of all epilepsy. Following TBI and CVA, there is a latency of days to years before epilepsy develops. This allows treatment to prevent or modify postinjury epilepsy. No such treatment exists. In animal models of acquired epilepsy, a number of medications in clinical use for diverse indications have been shown to have antiepileptogenic or disease-modifying effects, including medications with excellent side effect profiles. These include atorvastatin, ceftriaxone, losartan, isoflurane, N-acetylcysteine, and the antiseizure medications levetiracetam, brivaracetam, topiramate, gabapentin, pregabalin, vigabatrin, and eslicarbazepine acetate. In addition, there are preclinical antiepileptogenic data for anakinra, rapamycin, fingolimod, and erythropoietin, although these medications have potential for more serious side effects. However, except for vigabatrin, there have been almost no translation studies to prevent or modify epilepsy using these potentially “repurposable” medications. We may be missing an opportunity to develop preventive treatment for epilepsy by not evaluating these medications clinically. One reason for the lack of translation studies is that the preclinical data for most of these medications are disparate in terms of types of injury, models within different injury type, dosing, injury–treatment initiation latencies, treatment duration, and epilepsy outcome evaluation mode and duration. This makes it difficult to compare the relative strength of antiepileptogenic evidence across the molecules, and difficult to determine which drug(s) would be the best to evaluate clinically. Furthermore, most preclinical antiepileptogenic studies lack information needed for translation, such as dose–blood level relationship, brain target engagement, and dose-response, and many use treatment parameters that cannot be applied clinically, for example, treatment initiation before or at the time of injury and dosing higher than tolerated human equivalent dosing. Here, we review animal and human antiepileptogenic evidence for these medications. We highlight the gaps in our knowledge for each molecule that need to be filled in order to consider clinical translation, and we suggest a platform of preclinical antiepileptogenesis evaluation of potentially repurposable molecules or their combinations going forward.
1 |. INTRODUCTION
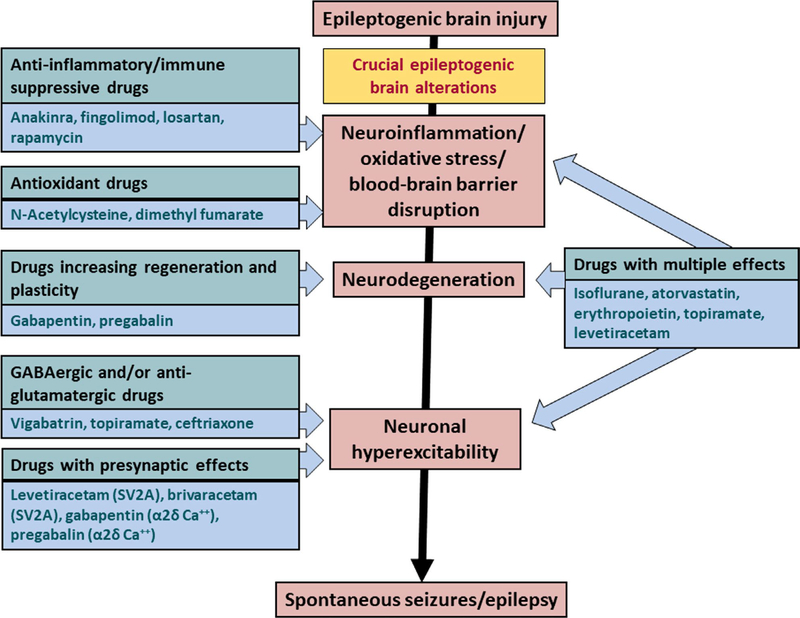
Prevention of epilepsy is a great unmet need. Acute central nervous system (CNS) insults such as traumatic brain injury (TBI), cerebrovascular accidents (CVA), and CNS infections account for approximately 15%−20% of all epilepsy in the developed world.1–3 Following TBI and CVA, there is a latency of days to years before epilepsy develops. This may allow intervention to prevent or modify postinjury epilepsy. However, no such treatment exists for patients at risk.1,4–6 In animal models of acquired epilepsy, a number of medications approved by the US Food and Drug Administration (FDA) and European Medicines Agency in clinical use for diverse indications have been reported to have an antiepileptogenic or disease-modifying effect, including medications with excellent side effect profiles.1,4,5 These include atorvastatin, ceftriaxone, losartan, isoflurane, N-acetylcysteine, and the antiseizure medications levetiracetam (LEV), brivaracetam (BRV), topiramate (TPM), gabapentin (GBP), pregabalin (PGB), vigabatrin, and eslicarbazepine acetate (ESL). In addition, there are preclinical antiepileptogenic data for anakinra, rapamycin, fingolimod, and erythropoietin,5 although these medications have potential for more serious side effects. These numerous drugs act on targets that are thought to be critically involved in epileptogenesis (Figure 1). However, there have been no new phase 3 preventive studies and only two phase 2 studies using such medications in the past 18 years.4,7 It is possible that we may be missing an opportunity to develop preventive treatment for epilepsy by not evaluating these “repurposable” medications clinically.
FIGURE 1.
Targets for antiepileptogenic and disease-modifying drug effects. Acute brain insults such as traumatic brain injury, stroke, status epilepticus, and encephalitis may induce a cascade of epileptogenic brain alterations. Some of these alterations may form targets for intervention as illustrated. GABAergic, γ-aminobutyric acidergic; SV2A, synaptic vesicle 2A
There are several reasons why preclinical studies of these medications have not been translated into clinical trials. The preclinical data concerning most of these medications are disparate in terms of types of injury, models within different injury types (eg, different models of status epilepticus [SE] and of TBI, with uncertain equivalency between models), dosing, injury to intervention initiation latencies, treatment duration, and outcome evaluation mode and duration. This makes it difficult to compare the relative strength of antiepileptogenic evidence across molecules, and difficult to determine which are the best to evaluate clinically. Furthermore, most studies lack information needed to design good clinical studies (eg, dose–blood level targets, dose-response, CNS levels) and use treatment parameters that cannot be applied clinically (treatment before or at the time of injury, dosing higher than tolerated human equivalent dosing). With the exception of LEV, none of these medications has been studied systematically with the view of translation into clinical studies. This has contributed to the fact that almost none of the preclinical projects have led to clinical translation.
The purpose of the present paper is to (1) review systematically animal and human antiepileptogenic evidence for approved, safe medications that have shown an antiepileptogenic or disease-modifying effect; (2) show where the gaps in our knowledge for each molecule are, to stimulate research to fill those gaps and allow better evaluation of which molecules should be taken into clinical studies; and (3) suggest a platform of preclinical antiepileptogenesis evaluation of potentially repurposable molecules going forward. In the context of this review, the term “antiepileptogenic” indicates a significant reduction in the incidence of epilepsy developing after brain injury, whereas “disease-modifying” indicates a reduction in the frequency, severity, or duration of spontaneous seizures, or a favorable effect on behavioral or cognitive comorbidities of acquired epilepsy.
2 |. LEVETIRACETAM
LEV, a synaptic vesicle 2A (SV2A) modulator, has a broad spectrum of antiepileptogenic or disease-modifying effects in kindling, genetic, and posttraumatic epilepsy (PTE) models, and has been suggested to have potential antiepileptogenic effects in two clinical studies, including one TBI/PTE phase 2 study.7 The antiepileptogenic/disease-modifying effects in animals occur generally in clinically applicable doses with clinically applicable plasma levels.
LEV dose-dependently protects against amygdala kindling, delaying8 or completely preventing9 full kindling and ameliorating severity of seizures in clinically applicable doses (13–54 mg/kg). It permanently suppresses kindling-induced afterdischarge, an indication of synchronized neuronal firing, which is a critical element of epileptogenesis.8,9 It has similar effects with similar doses in other kindling models, including corneal, audiogenic, and pentylenetetrazol (PTZ)-induced kindling.10–12
LEV also has strong antiepileptogenic effects in two genetic models of epilepsy. LEV treatment with 80 mg/kg/d for 3–6 weeks before the natural seizure onset prevents epilepsy in the “spontaneously epileptic rat,” and reduces incidence of epilepsy in the WAG/Rij model of absence epilepsy,13,14 the latter with the mean clinically relevant blood level of 39.9 μg/mL.
LEV does not prevent epilepsy after SE.15 However, it reduces its severity in several SE models and reduces neuronal excitability and synchronization of neuronal firing, two key electrophysiological features of epileptogenesis after a CNS insult. In the rat pilocarpine-induced SE (pilo-SE) model of temporal lobe epilepsy (TLE), this occurred dose-dependently with a bolus of 54 mg/kg after termination of SE followed by infusion of 50, 150, or 300 mg/kg/d for 21 days. The 300-mg/kg/d dose completely prevented these changes.16 Associated plasma LEV levels of 12.7 μg/mL (50 mg/kg/d) and 55.2 μg/mL (300 mg/kg/d) approximate trough and peak human plasma levels seen in a human phase 2 study of PTE prevention with LEV 55 mg/kg/d.17 In the mouse pilo-SE model, a single LEV dose of 500 mg/kg administered 30 minutes after SE cessation reduced frequency and severity of seizures 4 weeks later. This was associated with block of both cytotoxic and vasogenic edema, reduced blood-brain barrier (BBB) leakage at 3 hours-2 days after SE,18 and inhibition of increase in the expression of angiogenic factors, neovascularization, activation of microglia, and upregulation of proinflammatory cytokines.19 Chronic treatment (21 days after pilo-SE in rats) with LEV at clinically relevant exposures counteracted the increased amplitude of population spikes recorded in vivo in the dentate gyrus and reduced paired-pulse inhibition in the CA1, when compared to vehicle-treated rats. These effects were observed 3 days after cessation of LEV treatment and complete clearance of the drug from plasma, suggesting a longer-lasting neuronal plasticity and potential disease-modifying or antiepileptogenic effects.20
In a rat fluid percussion injury (FPI) TBI model of PTE in which seizures induced by FPI are exacerbated by subsequent administration of kainic acid 1–3 weeks after FPI, LEV (approximately 100 mg/kg/d) treatment for 1 week, started after FPI, reduced seizure severity and duration and neuronal degeneration in the hippocampal CA1 region.21,22 In the rat controlled cortical impact injury (CCI) PTE model, LEV (single dose, 150 mg/kg intraperitoneal [ip]) administered immediately after CCI inhibited the development of spontaneous epileptiform activity and stimulation-elicited hyperexcitability in neocortical slices from the injured area 2 weeks after injury; 14% of LEV-treated animals had spontaneous epileptiform activity compared with 75% of controls.23 In the undercut model of PTE, a cut through a cortical slice to remove cortical layers 1–3 and isolate slice layers III-VI and deep white matter induces hyperexcitability of the slices and epileptiform burst firing in response to external stimulation. Treatment of the slices with LEV (400 μmol/L) at 30–60 minutes after injury reduced the percentage of hyperexcitable slices from 50% to 25%, and increased seizure threshold almost twofold.24 In the iron-injection model, epilepsy develops after amygdalar/hippocampal injection of FeCl3, due to TBI-induced downregulation of glutamate transporters, which contributes to glutamatergic toxicity.25 Treatment with LEV 54 mg/kg/d for 14 days increased hippocampal levels of the glutamate transporters L-glutamate/L-aspartate transporter (GLAST), excitatory amino acid transporter-1, and γ-aminobutyric acid (GABA) transporter type 3.25
In addition, LEV positively impacts functional outcomes after TBI. In an Operation Brain Trauma Therapy (OBTT) project, a single intravenous LEV dose of 54 or 170 mg/kg 15 minutes after TBI in FPI and CCI models improved histological neuroprotection and cognitive function in both models, and motor function in the CCI model.26 The authors of the OBTT project described LEV as “the most promising drug tested thus far by OBTT, and the only drug to improve cognitive outcome in any model.” Similar improvement in motor and spatial learning occurred with LEV 50 mg/kg/d treatment for 10 days after penetrating ballistic brain injury.27
Thus, there is broad evidence of LEV’s antiepileptogenic and disease-modifying effects and some neuroprotective effect. The effects occur in the majority of the models with clinically relevant doses, and, in some models, with associated clinically applicable blood levels. This set of data is the most comprehensive of any of the drugs evaluated to date. However, there are no data on efficacy in in vivo PTE models with spontaneous seizures, and no systematic evaluation of response related to clinically applicable injury-treatment latencies.
Two human studies suggest antiepileptogenic effect of LEV. In one open-label phase 2 study, 1-month-long treatment of adults with TBI with high PTE risk with LEV 55 mg/kg/d starting ≤8 hours after injury showed a signal of PTE reduction 2 years after injury (10.9% [5/46] treated, compared with 20% [8/40], untreated, relative risk = 0.47, P = .18).7 In a Cleveland Clinic study of patients with refractory TLE treated surgically, patients treated with LEV peri-/postoperatively had greater seizure freedom at 5 years postsurgery than patients treated with any other antiseizure drug (ASD; 40% vs 57% seizure recurrence, risk ratio = 0.57, P = .003), even though they had more severe epilepsy preoperatively. The authors attributed this to an antiepileptogenic effect of LEV after surgery.28
Several lines of evidence indicate that SV2A is the primary site of antiepileptic (antiseizure) action for LEV.29 LEV’s antiepileptogenic effects are also likely to be mediated by this target, although other targets of LEV may contribute to the overall effect. Epileptogenesis is accelerated in kindling experiments with SV2A-deficient mice.30 Heterozygous SV2A mice, lacking approximately 50% of the protein, require much lower number of stimulations to reach fully kindled state in both corneal and amygdala kindling models.30 SV2A-targeted missense mutation also facilitates PTZ kindling in rats.31,32
Furthermore, SV2A expression is reduced in a rat post-SE model of epilepsy during both the latent period and chronic phase with spontaneous recurrent seizures (SRS)33 and is also reduced in the spontaneously epileptic rat.29,34 SV2A knockout animals develop severe epilepsy early in their ontogeny.29 The question arises how SV2A deficit leads to epileptogenesis, as the protein is present in both excitatory and inhibitory neurons under physiological conditions. Single-cell recordings in primary neuronal cultures from SV2A knockout animals indicate decreases in both excitatory and inhibitory postsynaptic currents.35 However, hippocampal slice recordings from SV2A knockout animals showed a more pronounced effect on inhibitory postsynaptic currents.35 Furthermore, SV2A missense mutation showed a preferential disruption of action potential-induced GABA, but not glutamate, release.31,32,36 Similarly, treatment of epileptic animals (lithium-pilocarpine model) with clinically relevant doses of LEV also had a differential effect on neurotransmitter levels measured by microdialysis in the hippocampus.37 LEV-treated epileptic rats had higher GABA levels in response to potassium-induced depolarization than vehicle-treated epileptic rats. There were no differences in glutamate levels.37 Collectively, these studies provide a converging body of evidence implicating SV2A in epileptogenesis through its effects on inhibitory GABAergic transmission. LEV treatment during epileptogenesis may be able to reverse these GABAergic deficits, which could manifest as an antiepileptogenic effect of the compound.
Other potential antiepileptogenic mechanisms of LEV include the antioxidative stress effect of free radical quenching,38,39 the anti-inflammatory effect of reduced production of interleukin (IL)-1β,18,19 and antiexcitotoxic effect with reduced release of intracellular calcium40 and upregulation of astrocytic glutamate transporters GLAST and glutamate transporter 1 (GLT-1).25
3 |. BRIVARACETAM
As outlined above, LEV has a unique anticonvulsant profile in animal models of seizures and epilepsy, due to its binding to SV2A.41 Testing of LEV analogs showed a striking correlation between their in vitro SV2A binding affinity and anticonvulsant activity in vivo.42 These observations indicated that SV2A is mediating the unique pharmacology of LEV and provided a screening tool, enabling the search for selective, high-affinity ligands for this novel mechanism. This led to the discovery of BRV,41 which is more selective for SV2A than LEV.
The potential involvement of SV2A in the epileptogenic process and BRV’s impact on it was further supported by a pharmacological study.43 The purpose was to explore whether BRV, a compound with better selectivity and 15- to 30-fold higher SV2A affinity than LEV and distinct SV2A interaction,44 would possess a superior activity against kindling acquisition.43 Corneal kindling of mice was conducted following 30-minute pretreatment with 10 times lower doses of BRV (0.21/6.8 mg/kg) than LEV (1.7–54 mg/kg), to generate comparable and therapeutically relevant plasma and brain levels with both compounds. Pretreatment with either BRV or LEV showed a similar, significant reduction in the incidence of generalized seizures. However, following cessation of drug treatment and a washout period, continued corneal stimulations revealed a persistent ~50% reduction in the incidence of generalized seizures in mice previously treated with the highest BRV dose of 6.8 mg/kg, with 0%−20% reduction with lower doses of 0.21–2.1 mg/kg. In contrast, all mice previously treated with LEV showed kindling development and returned to fully kindled state, similar to placebo-treated animals.43 Strong effects of BRV on kindling epileptogenesis were corroborated by another study using a rapid hippocampal kindling model in developing rats.45 Pretreatment with BRV 10 and 100 mg/kg of adult rats increased the number of stimulations required to engender generalized seizures by approximately 100% and 150%, respectively, and reduced the number of seizures by >50% for the 100-mg/g dose, although not for the 10-mg/kg dose. This was associated with a dose-dependent increase of ~40%−60% in the afterdischarge threshold at the 10- and 100-mg/kg doses. Corresponding blood levels for the 10- and 100-mg doses 30 minutes after BRV administration were approximately 7 and 100 μg/mL, respectively, and brain levels were approximately 2 and 60 μg/g of brain tissue, respectively. The effect was age-dependent, with less predictable effects for immature animals (Tables 1 and 2).
TABLE 1.
Animal studies of epilepsy prevention using repurposable drugs
| Drug | Model (studies, n) |
Dose range, mg/kg/da | Human equivalent dose/d, 60-kg person, mgb | FDA-allowed max dose, mg/d | Time to treatment from injury | Treatment duration | Blood/ brain levelsc | Sufficient data for clinical translation?d | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kindling | SE | TBI in vivo | TBI in vitro | Genetic/other | ||||||||
| Levetiracetam | >5 | 5 | 3 | 1 | 2 | 7–700 | 68–6780 | 3000 | Pre-24 h post | 1 dose/3.5 mo | 1/0 | Yes |
| Brivaracetam | 2 | 0 | 0 | 0 | 0 | 0.21–6.8 (m),e 10–100 | 11–978 | 200 | Pre | 1–19 d | 0/0 | No |
| Losartan | 2 | 2 | 2f | 0 | 0 | 1–100 | 68–978 | 100 | Pre-40 min | 1 dose/28 d | 0/0 | No |
| Isoflurane | 0 | 2 | 0 | 0 | 0 | l%-2% | l%-2% | l%-3% | 1–24 h | 4 × 1 h, 1 d | 0/0 | No |
| Anakinra | 1 | 2 | 0 | 0 | 0 | 25–100 | 240–978 | 100 | Pre-3 h | 1–21 d | 0/0 | No |
| n-Acetylcysteine | 0 | 1 | 1 | 0 | 0 | 100–1000 | 978–9780 | 300 mg/kg/d | 0–1 h | 14–35 d | 0/0 | No |
| Atorvastatin/statinsg | 1 | 4 | 0 | 0 | 1 | 10–100g | 97–978 | 80 | Pre-3 h | 14–49 d | 0/0 | No |
| Ceftriaxone | 0 | 0 | 1 | 0 | 0 | 200 | 1.9 g | 4 g | 30 min | 7 d | 0/0 | No |
| Gabapentinoids | 0 | 2 | 0 | 3 | 1 | 100–400 G 50–100 P |
978–3870 486–978 |
3.6 g G 600 mg Pg | 1 h-7 d | 3–21 d | 0/0 | No |
| Topiramate | 2 | 2 | 0 | 0 | 0 | 10–200 | 978–1935 | 400 | During-1 h | 1 dose/15 d | 0/0 | No |
| Rapamycin | 0 | 2 | 1 | 0 | 0 | 3–6 rat 40 (m) | 28.8–198 | <40 | Pre-5 h after | 5–28 d | 0/0 | No |
| Vigabatrin | 2 | 2 | 0 | 0 | 1 | 75–1500 | 726–14 514 | 3g | Pre-2 d | 10 d-4 mo | 0/0 | No |
| Fingolimod | 0 | 2 | 0 | 0 | 1 | 1 rat 6 (m) | 9.6–28.8 | 0.5 mg | Pre-24 h after | 2–17 wk | 0/0 | No |
| Eslicarbazepine | 0 | 1 | 0 | 0 | 0 | 150–300 (m) | 732–1464 | 1200 mg | 9 d after | 6 wk | 1/1 | No |
Abbreviations: FDA, US Food and Drug Administration; G, gabapentin; m, mice; P, pregabalin; SE, status epilepticus; TBI, traumatic brain injury.
Doses are in mg/kg/d unless otherwise indicated.
Human equivalent dose in mg/kg was calculated using the formula described in Nair and Jacob,293 then applied to a 60-kg human.
0 = not done, 1 = done.
Minimal requirements for clinical translation: dose within human dosing range; treatment window > 1 hour for acute injury; plasma levels to target in humans.
All studies are in rats except where mice are indicated.
Models of blood-brain disruption and/or cortex exposure to albumin.
TABLE 2.
Human studies of epilepsy prevention potential using repurposable drugs and completed after 2007
| Drug | Condition | Study type | Outcome measure | Dose | Treatment latency after injury | Treatment duration | Evaluation time after injury (treatment end) | Effect | Effect size | Refs |
|---|---|---|---|---|---|---|---|---|---|---|
| Levetiracetam | PTE | Phase 2, OLa | % PTE | 55 mg/kg (max = 6000 mg/d) | 8 h | 30 d | 2 y (23 mo) | ↓ PTE | 45% | 7,17 |
| Post-TLE resection | Retrospective review | % epilepsy recurrence | NA | NA | NA | 5 y | ↓ epi recurrence | OR = 0.57 | 28 | |
| Atorvastatin | CVAb | Prospective cohort | % PSE | NA | <3 d | >3 d | 2.5 y | ↓ PSE | OR = 0.34 | 141 |
| Cardiovascular disease | Case-control | Hospitalization for epilepsy | NA | NA | Variable | Variable | OR = 0.65 | 142 | ||
| Statin treatment | Cross section | Epilepsy | NA | NA | Variable | Variable | OR = 0.64 | 143 | ||
| Vigabatrin | Epileptiform EEG | OLc | Seizure freedom | 100–150 mg/kg | At onset of EEG abnormality | Until 2 y | 2–8 y after 1st epileptiform EEG | 93%b | 255,256 |
Abbreviations: CVA, cerebrovascular accidents; EEG, electroencephalogram; NA, not available; OL, open label; OR, odds ratio; PSE, poststroke epilepsy; PTE, posttraumatic epilepsy; Refs, references; TLE, temporal lobe epilepsy.
OL compared with an observational arm with standard of care.
In patients with early poststroke seizures only.
Compared with a prospective cohort receiving standard of care.
Taken together, the rational design of BRV as a selective and optimized SV2A agent seems to confer it promising antiepileptogenic properties in the kindling models. This has triggered a significant interest in further determining BRV’s antiepileptogenic properties in other models of acquired epilepsy, including the FPI TBI model. Such studies are currently being performed.
4 |. LOSARTAN
Losartan is an FDA-approved angiotensin-type 1 receptor (AT1) antagonist used to treat hypertension. It has both neuroprotective and antiepileptogenic effects. The rationale for testing losartan as an antiepileptogenic drug stems from studies on the role of BBB in epileptogenesis46,47 which is mediated by albumin-induced transforming growth factor beta (TGFβ) signaling.48,49 The TGFβ family of proteins is a group of cytokines that play a role in intercellular communication and regulate cellular processes, including cell growth, migration, differentiation, apoptosis, inflammation, and expression of extracellular matrix proteins.50–52 There are seven known variants of TGFβ membrane receptor types (activinlike kinase [ALK] 1–7), of which two are expressed in the brain: ALK1 and ALK5. On ligand binding, ALK1 phosphorylates intracellular mediators Smad 1, 5, and 8, and ALK5 phosphorylates Smad 2 and 3. The downstream signaling involves interaction with other Smad proteins to form complexes that accumulate in the nucleus and promote transcriptional activity.50,51,53 Following injury and increase in BBB permeability, albumin diffuses into the brain and binds to ALK5 TGFβ receptors in astrocytes, leading to their activation and release of proinflammatory cytokines.49,54 Activated astrocytes further downregulate inward-rectifier potassium channel and glutamate transporter, resulting in reduced potassium and glutamate buffering, leading to neuronal hyperexcitability.55 In addition, astrocytic-mediated alterations in the extracellular matrix,56 excitatory synaptogenesis,57 and pathological plasticity contribute to the ongoing process of epileptogenesis.58
Losartan inhibits TGFβ signaling by preventing the phosphorylation of intracellular Smad proteins in animal models of chronic renal insufficiency, cardiomyopathy, and connective tissue disorders.59–61 Losartan prevents insult-related epilepsies by blocking the increase in phosphorphorylated Smad 2/3 levels following BBB disruption or direct exposure of the cerebral cortex to albumin.62 Perfusion of albumin over the cortex for 40 minutes, a model of BBB breakdown such as may occur after TBI or CVA, induces electrographic seizures that start on day 2 after perfusion and continue indefinitely. Coperfusion with a single dose of losartan 10 μmol/L reduced the incidence of epilepsy from 85% to 25%, and decreased epilepsy severity, reducing seizure frequency in epileptic animals from 6.1/wk to 0.2/wk, and average seizure duration from 13.7 to 5.4 seconds.62 In another experiment, losartan bolus of 100 mg/kg was administered systemically 40 minutes after albumin extravasation to mimic TBI, followed by 21-day-long treatment at therapeutic levels administered as 2 g/L drinking water/d. Electrocorticographic recording was started 7 days after the end of losartan treatment and lasted for 2 weeks. Losartan reduced incidence of epilepsy from 100% to 40% and reduced average seizure frequency in epileptic animals from 8 to 2.3/wk, without affecting seizure duration.62
Antiepileptogenic and disease-modifying effects of losartan have also been shown in other models. When administered to rats undergoing amygdala kindling, losartan (50 mg/kg ip 1 hour prior to each kindling stimulation over a period of 3 weeks of daily kindling) increased the threshold for stimuli-induced discharges and delayed the onset of seizures63 In another group, rats treated intracerebroventricularly with losartan at doses of 1 and 3 mg/kg/d required significantly more kindling stimulations to reach the fully kindled state than controls, and few animals did not reach the fully kindled state during the 21 days of daily kindling.63 In the post-SE kainate model of TLE, losartan (10 mg/kg/d) started 2 hours after SE onset and continued for 4 weeks delayed the development of epilepsy from 12 to 23 days, and reduced seizure frequency and duration, but not epilepsy incidence. In addition, losartan exerted neuroprotection selectively in the hippocampal CA1 region and positively affected epilepsy-provoked behavioral changes, including impulsivity, anxiety, and depression.64 Although treatment started during SE and may have reduced SE severity, this was not the case in other models in which SE was induced pharmacologically (Swissa and Friedman, personal communication). In the paraoxon model of SE-induced epileptogenesis, losartan (60 mg/kg ip daily for 3 days followed by 2 g/L treatment in the drinking water for an additional 18 days) reduced BBB breakdown 48 hours after SE and the incidence of delayed seizures from 59.1% to 5.56%.65 As angiotensin receptor expression is also upregulated in models of epilepsy, specifically in hypertensive rats,66 and facilitates neuroinflammation,67 it is still uncertain whether the protective effect of losartan is due to inhibition of angiotensin signaling, TGFβ signaling, or both.
Finally, losartan and other AT1 antagonists diminish neuroinflammation68–70 and were proposed as neuroprotective drugs in numerous in vitro studies,71,72 and in rodent models of stroke73,74 TBI,75–77 and neurodegeneration, including Alzheimer disease.78–80 Although there are no controlled clinical trials with AT1 antagonists as neuroprotective or antiepileptogenic agents, retrospective studies support the notion that AT1 antagonists provide relative neuroprotection compared to other antihypertensive drugs.81–86
These data suggest a significant antiepileptogenic and possibly neuroprotective potential for losartan and other AT1 receptor antagonists. At present, it is uncertain whether dosing humans with the equivalent doses used in animals is feasible, and there is no antiepileptogenic animal data on losartan blood levels to compare with clinically acceptable human levels. It is important to determine that effective antiepileptogenic doses/levels do not have the potential to cause hypotension, which could aggravate injury in patients with TBI or CVA. There are no dose-response antiepileptogenic data, and no injury–treatment latency data that would allow human study design, for example, of treatment initiation several hours after injury. These studies are needed to determine whether human studies are feasible.
5 |. ISOFLURANE
Isoflurane is a well-established anaesthetic. Its exact mechanism of anesthetic action has not been clearly delineated, but it likely binds to GABA, glutamate, and glycine receptors, with different effects on each receptor. Furthermore, isoflurane induces an epigenetic downregulation of brain-derived neurotrophic factor receptors and tyrosine receptor kinase B,87 the activation of which plays a role in epileptogenesis.88 The potentiating effect of isoflurane on GABAergic Cl− currents,89,90 inhibition of N-methyl-D-asparate (NMDA)-gated91 and voltage-dependent calcium currents,92 reduction of synaptic glutamate release,93 and its neuroprotective94 and anti-inflammatory95 properties make isoflurane an attractive antiepileptogenic drug. Short-duration treatments with isoflurane during or shortly after SE in the kainate or paraoxon models of epileptogenesis attenuated BBB breakdown, glial activation, and neuroinflammation, reduced neuronal damage, abolished epileptiform activity, and reduced the incidence of late epilepsy.96 The vasoprotective effect of isoflurane has also been shown in rodent models of stroke97–99 and in subarachnoid hemorrhage.100 Thus, preclinical data suggest isoflurane as a promising antiepileptogenic, vasoprotective, and neuroprotective agent in specific patient populations, including SE and severe traumatic or ischemic brain injuries that require general anesthesia. Timing and dose of isoflurane anaesthesia vary largely between studies, and the depth of isoflurane anaesthesia is not reported. Additional controlled preclinical studies are required with monitoring of the depth of anesthesia and using clinically relevant biomarkers before clinical studies can be designed.
6 |. ANAKINRA
IL-1 is a master cytokine driving both brain and systemic inflammation. It is encoded by IL1A and IL1B genes, generating two cytokines, IL-1α and IL-1β. These activate a ubiquitous cell surface receptor, IL-1 receptor type 1 (IL-1R1), which transduces the cellular signal upon agonist activation. Activation of the IL-1–IL-1R1 axis triggers a cascade of inflammatory molecules, including other cytokines and chemokines. IL-1 receptor antagonist (IL-1Ra) is the endogenous competitive antagonist of IL-1R1, which blocks the activity of both cytokines. More than 100-fold molar excess of IL-1Ra is needed to inhibit IL-1 activity.101 Anakinra (Kineret) is the human recombinant form of IL-1Ra, the first IL-1 targeting agent, introduced in 1993. Since then, anakinra has dominated therapies targeting IL-1 in autoinflammatory and autoimmune diseases with pathological cytokine involvement.101 Additional IL-1R1 blocking agents approved for medical use include the soluble decoy receptor (rilonacept) and canakinumab (Ilaris), the anti–IL-1β neutralizing monoclonal antibody. Other neutralizing or blocking antibodies, vaccine, and chimeric IL-1Ra–IL-1β combination are under clinical investigation.101 In the CNS, two randomized phase 2 studies in acute stroke102 and neurotrauma103 demonstrated that anakinra is safe, and improved neurological sequelae in stroke patients.
IL-1β drives the pathologic role of brain inflammation in epilepsy.104–112 Studies on surgically resected epileptogenic foci from drug-resistant forms of epilepsy with structural or infectious etiologies showed that the IL-1α–IL-1R1 axis is activated in neurons, glia, and endothelial cells of the BBB.104,107,113–115 Similar findings were reported in animal models of acute symptomatic seizures, febrile and nonfebrile SE, and models of acquired epilepsies,107,114 absence epilepsy, and progressive myoclonic epilepsy.116–118 Activation of this inflammatory signaling in animals is involved in epileptogenesis119–121 and contributes to seizure generation and recurrence,122 and to neurological comorbidities.123
Only three studies have addressed the role of anakinra in epileptogenesis. In one, anakinra (100 mg/kg ip, ie, total daily dose = 5 mg/rat, equivalent to human adult dose of 16 mg/kg)124 was coadministered with an investigational cyclooxygenase-2 (COX-2) antagonist (CAY 10404)125 at the time of Li+ pilocarpine–induced SE in 21-day-old rats, then daily for an additional 10 days. When compared with vehicle-treated rats, the drug combination reduced spontaneous seizures threefold over 3 weeks of video-electroencephalographic (EEG) recording, assessed 4 months post-SE. Single drugs alone were not tested in this model, and 1-day treatment with drug combination was not effective. In another study, anakinra was infused subcutaneously with osmotic minipumps for 7 days in adult rats (24 mg/rat/d; 70 mg/kg daily; equivalent to human adult dose of 11 mg/kg), starting 3 hours after SE induced by hippocampal electrical stimulation (male rats) or following Li+ pilocarpine (female rats).126 This was combined with daily ip injection of VX-765, which inhibits IL-1β biosynthesis by blocking caspase-1. The treatment resulted in pronounced (50%−90%) anti-inflammatory activity in the forebrain of electrical SE rats associated with neuroprotection, resulting in a rescue of the 25%−50% neuronal cell reduction induced by SE in the hippocampus and the cortex. A much less pronounced anti-inflammatory effect (20%−50%) and no neuroprotection were reported in Li+ pilocarpine rats. The frequency of spontaneous seizures was not modified during 2 weeks of EEG recordings 3 months post-SE. However, subsequent experiments in this SE model showed a progression in seizure frequency between 3 and 5 months after SE, and this was the outcome measure mostly affected by anti-inflammatory treatments that blocked such progression.120,121,127 VX-765 coadministered with Toll-like receptor 4 (TLR4) blocker cyanobacterial lipopolysaccharide (LPS)121 to mice with intra-amygdalar kainate-induced SE for 10 days starting at the time of spontaneous seizure onset reduced seizures by 90% and prevented their threefold progression, at 3 months post-SE. In the third study, anakinra (25 mg/kg ip, single bolus; human child equivalent dose is 6 mg/kg) injected 2 hours before rapid electrical hippocampal kindling in postnatal day 14 (P14) rats prevented the twofold increase in the number of stage 4 seizures and the threefold increase in their average duration induced by rat’s pre-exposure to systemic LPS.128
Experimental and clinical observations that anakinra reduces seizures support the utility of this drug for interrupting the vicious cycle of brain inflammation and sustained epileptic activity.104 Multicenter clinical studies are needed to establish efficacy, and to refine doses and treatment schedule as well as tolerance of anakinra in severe conditions such as febrile infection-related epilepsy109,112,129 and more broadly in pharmacoresistant epilepsies.110,111 For epilepsy prevention, animal studies are needed to test the effect of anakinra alone (doses and therapeutic window) on the onset, frequency, and progression of spontaneous seizures in adult and developmental models of acquired epilepsies.
7 |. N-ACETYLCYSTEINE
N-acetylcysteine (NAC), a clinically used antioxidant, is a precursor of reduced glutathione (GSH). Its tissue supplementation prevents GSH depletion during oxidative stress. NAC also provides sulfhydryl groups for direct scavenging of reactive oxygen free radicals (ROS). Epileptogenic injuries commonly lead to mitochondrial dysfunction and increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and xanthine oxidase activities, resulting in excessive generation of ROS (ie, oxidative stress) in brain tissue. Central and peripheral markers of oxidative stress are increased in drug-resistant epilepsy. There are critical points of intersection between oxidative stress and neuroinflammation that may be relevant for epilepsy and its comorbidities. In particular, ROS displace the binding of thioredoxin-interacting protein (TXNIP) to the antioxidant cytoplasmic protein thioredoxin.130 The free TXNIP, in turn, promotes NLRP3 inflammasome assembly and the consequent release of the ictogenic inflammatory molecules IL-1β and high-mobility group box 1 (HMGB1).130–132 ROS also induce a disulfide bond between C23 and C45 of HMGB1, generating the disulfide isoform, which has proinflammatory, neuromodulatory, and ictogenic activities by activating TLR4. Moreover, ROS modulate nuclear factor-κB (NFκB)-mediated transcriptional activation of various inflammatory genes133 and may provoke oxidative damage to proteins, lipids, and DNA. The available data support a vicious cycle that links ROS production and neuroinflammation to neuronal and glial dysfunction, thus representing a potential mechanism of epileptogenesis.120,127,134–137
Reduction of oxidative stress in animal models of epilepsy with small molecules reduces SE-induced neuronal damage and cognitive deficits. A recent hippocampal electrical SE study in rats used a combination of NAC and sulforaphane to increase both the acute endogenous antioxidant response and the longer-term antioxidant system through the transcriptional factor Nrf2, which induces the major antioxidant enzymes. These drugs when administered to rats during epileptogenesis (NAC 500 mg/kg twice daily for 1 week and sulforaphane 5 mg/kg once daily for 2 weeks ip, starting 1 hour post-SE) afforded neuroprotection by preventing the 40% drop in hilar interneurons and CA1 pyramidal cells. The treatment delayed the time of onset of epilepsy by 30%, achieved a 70% reduction in seizure frequency compared to controls, and resolved cognitive deficits.127 A similar therapeutic response occurred in kainate-induced SE models with the small molecule RTA 408, which removes the inhibitory control of the chaperon protein Keap1 on Nrf2, given alone137 or in combination with 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, a NADPH oxidase inhibitor,138 and with 1400W, a highly selective inhibitor of inducible nitric oxide synthase.136 These therapeutic effects outlasted the end of treatment by several weeks, suggesting disease-modifying effects. There is limited information on antioxidant drug effects in models of epilepsy induced by acute injuries other than SE. One notable study reported that chronic NAC treatment (100 mg/kg/d by intragastric gavage for 5 weeks postinjury), starting immediately after FPI in rats, normalized the FPI-induced reduction of seizure threshold to systemic PTZ (latency to myoclonus and time spent in tonic-clonic seizures) measured 5 weeks after the acute brain injury.139
Considering the evidence for oxidative stress in the brain of patients who died in SE or with chronic drug-resistant seizures,127 interventions with antioxidant drugs might improve the neurological sequelae after epileptogenic injuries or in progressive epilepsy. NAC is widely used clinically as a mucolytic agent and as an antidote to acetaminophen overdose. It has been used in clinical trials in cancer, cardiovascular disease, and a variety of neurological disorders. In human studies, NAC has been used at doses comparable with those effective in animal models of epileptogenesis. Extrapolating the human equivalent dose, effective NAC doses in rats correspond to 10 g/d for a 60-kg person. Intravenous infusion of 150 mg/kg NAC (9 g for a 60-kg person) in healthy individuals or Parkinson and Gaucher disease patients was well tolerated and increased brain GSH assessed by magnetic resonance spectroscopy. NAC up to 3.6 g/d for several weeks has been used in neurological and psychiatric disorders. Impaired redox homeostasis and oxidative damage were reported in a cystatin B deficiency mouse model of progressive myoclonus epilepsy (Unverricht–Lundborg disease).140 NAC treatment up to 6 g/d for several months in patients with Unverricht–Lundborg disease resulted in significant seizure improvement and was well tolerated.
8 |. ATORVASTATIN AND OTHER STATINS
Three clinical studies suggest possible antiepileptogenic/disease-modifying effect of statins. Statin treatment after CVA reduced the risk of poststroke early onset seizures, and reduced the risk of poststroke epilepsy in CVA patients with early poststroke seizures (odds ratio [OR] = 0.34) in a cohort of 1832 patients followed for a mean of 2.5 years.141 Treatment included atorvastatin ≥20 mg/d in 96% of cases, started within 3 days after stroke and continued for at least 3 days. It did not affect risk of poststroke epilepsy (PSE) overall, but the risk of PSE was low among patients without early poststroke seizures (4%), whereas it was high (31.7%) among patients with early poststroke seizures. In a cohort study of 150 555 older adults with cardiovascular disease treated with revascularization, there was a dose-dependent reduced risk for hospital admission with the diagnosis of epilepsy for current statin users (OR = 0.65) and for past statin users (OR = 0.72), with no benefit with non–statin cholesterol lowering drugs, beta blockers, and angiotensin-converting enzyme inhibitors. This suggests that statin use reduced either the risk of epilepsy or the risk of epilepsy severe enough to result in hospitalization.142 A retrospective cross-sectional study of risk factors for new onset geriatric epilepsy of veterans >66 years old including 1843 individuals with epilepsy and 1 023 376 individuals without epilepsy showed that statin prescription was associated with a lower likelihood of epilepsy (OR = 0.64).143
The preclinical evidence of the antiepileptogenic potential of atorvastatin or other statins is sparse. Studies have been done in PTZ,144 audiogenic,145 and other acute seizure models, with variable results, and with some suggestion that statins may impact the antiseizure efficacy of medications such as carbamazepine and valproate via either a pharmacodynamic or a pharmacokinetic effect,146 which could be one reason for the finding that statin use was associated with reduced risk of hospitalization for epilepsy. Statins have anti-inflammatory effects, reducing brain penetration by monocytes and lymphocytes, reducing production of IL-1β, tumor necrosis factor (TNF), interferon-γ, and IL-6, and increasing production of IL-10,147 have a free radical quenching effect,148 and impact neurosteroid synthesis, all mechanisms relevant to epileptogenesis. However, only a small number of preclinical studies have evaluated antiepileptogenesis of statins, with mixed results. Mice with pilocarpine-induced SE (Pi-SE) have increased susceptibility to PTZ-induced seizures. Treatment with atorvastatin after Pi-SE (10 or 100 mg/kg/d) for 14 days had no protective effect on PTZ seizure susceptibility at the end of the treatment, and no neuroprotective effect.149 The same treatment in a different group of animals improved depression and object recognition testing, dose-dependently decreased basal and SE-induced levels of IL-1β, IL-6, TNF, and INF-γ, and increased IL-10 levels in the hippocampus and cerebral cortex, indicating anti-inflammatory effects after an epileptogenic insult.150 Atorvastatin 20, 40, and 80 mg/kg/d administered during PTZ kindling for 7 weeks 1 hour before PTZ administration reduced severity of PTZ-kindled seizures, which outlasted atorvastatin treatment, suggesting a disease-modifying effect. It also reduced brain lipid peroxidation, increased glutathione levels, and prevented PTZ kindling-associated reduction in hippocampal GABA and dopamine and increase in glutamate levels.151
In the WAG/rij absence rats, atorvastatin treatment started at 45 days of age and continued for 17 weeks was ineffective at 5 mg/kg/d, whereas 10 mg/kg/d reduced the number of seizure discharges by 55% and cumulative duration of spike/wave discharges by 65% at 6 months, 1 month after treatment discontinuation, without affecting individual absence seizure duration. The effect was reduced to 44% and 41% reduction of the number and duration of discharges, respectively, at 10 mg/kg/d, 5 months after treatment discontinuation. Simvastatin 10 mg/kg/d and pravastatin 10 and 30 mg/kg/d had similar effects.152
In the rat electrically induced SE model, treatment with atorvastatin 10 mg/kg/d starting 7 days prior to SE and continued for 7 days after SE, with video-EEG monitoring for 6 weeks after SE, did not protect against SE, epileptogenesis, or BBB disruption in limbic brain regions (hippocampus, entorhinal cortex, piriform cortex) and did not reduce inflammation, neuronal death, or synaptic reorganization.153 In a rat kainate-SE model, simvastatin 1 mg/kg/d starting 0.5 hours after kainate-induced SE and lasting for 14 days resulted in reduction of EEG spikes at 6 months from 61.4/min to 31/min and reduced Racine score of spontaneous seizures from 3 to 2. This was associated with reduced lesion-induced expression of IL-1β and TNF at 3 days after kainate lesion, suppression of kainate-induced reactive astrocytosis 4–6 months after kainate lesion, and attenuation of loss of CA3 pyramidal neurons and dentate hilar interneurons, and reduced mossy fiber sprouting at 4–6 months.154 Finally, lovastatin 20-mg/kg/d treatment for 15 days starting 2 hours after pilo-induced SE onset decreased the levels of increased IL-1β, TNF, and IL-6 during the latent phase and of IL-1β and TNF during the chronic phase, and increased expression of the anti-inflammatory cytokine IL-10.155
No antiepileptogenic animal studies of statins done to date have shown disease prevention, only disease modification. There are no preclinical studies of antiepileptogenic effects of statins when administered at clinically relevant latency after injury, nor any studies after TBI or stroke. Given the promising clinical studies on statins, a back-translation to animal models would be important and could provide validation of preclinical models used in the search for epilepsy-preventing therapies.
9 |. CEFTRIAXONE
Glutamate is synthesized from glutamine in glutamatergic neurons, is released into the synaptic cleft, is actively removed from the synapse by membrane transporters primarily present on astrocytes,156,157 and is converted to glutamine by glutamine synthase and shuttled back to neurons.158 Increased posttraumatic neuronal glutamate exposure due to leakage from injured cells and deranged clearance mechanisms plays a major role in the pathophysiology of posttraumatic epileptogenesis.
Extracellular mechanisms for the enzymatic deactivation of glutamate have not been identified, and so the astrocytic glutamate transporters provide the only known endogenous mechanism for rapid glutamate clearance.159 GLT-1, the rodent analog of human excitatory amino acid transporter 2 (EAAT2), is responsible for almost all the glutamate clearance capacity in the rodent brain.160 Its absence contributes to reduced seizure threshold, increased lethal seizures, hippocampal neuronal degeneration, and vasogenic edema following trauma.161 Mutations involving genes coding for these transporters have been linked to the development of PTE following TBI in humans.162
Glutamate transport thus constitutes a powerful antiexcitotoxic mechanism in the brain. Rat TBI models show a large, transient posttraumatic decline in GLT-1 mRNA and protein levels and transporter function that starts within 24 hours of injury and normalizes by 6 weeks after injury.163–166 Possible explanations for these changes include increased endocytosis due to glutamate-induced clustering,167 caspase-3–mediated proteolysis of existing protein,168 and diminished de novo gene transcription.169,170
Excessive glutamate leakage from injured neurons together with transiently deranged synaptic glutamate clearance results in increased extracellular glutamate, which persists for days after trauma in humans as well as rodents.171,172 In addition to causing continuing excitotoxic injury, elevated extracellular glutamate also inhibits the gradient-driven system xc− antiporter, which takes up extracellular cysteine in exchange for glutamate.173,174 This exchange is the rate-limiting step in the synthesis of cysteine for eventual generation of the antioxidant GSH, and cells are thus rendered incapable of neutralizing ROS that are continuously generated by mitochondrial and cellular processes, eventually leading to cell death by oxidative stress.175,176
Post-TBI excitotoxicity and oxidative stress create an especially unfavorable milieu for the small cortical GABAergic inhibitory interneurons177,178 that restrain cortical circuits from excessive excitation despite their small numbers.179,180 Their high baseline energy makes them highly susceptible to metabolic stress after injury. Progressively worsening oxidative stress, coupled with the breakdown of the protective perineuronal net surrounding these cells, leads to their gradual loss, whereas excitatory neurons remain largely unaffected.181 Preferential loss of these inhibitory interneurons results in a progressive loss of GABAergic intracortical inhibition and may contribute to epileptogenesis.166,181
Ceftriaxone is a safe and commonly used β-lactam antibiotic approved by the FDA, with good BBB permeability and documented neuroprotective potential.182–186 It is also a potent stimulator of GLT-1 expression at CNS concentrations that are achievable in humans at doses used to treat bacterial meningitis (100 mg/kg/d, maximum dose = 4 g/d).187–189 Ceftriaxone increases EAAT2 expression via the NFκB signaling pathway.189 Marked upregulation of GLT-1 appears as early as 48 hours after the start of ceftriaxone treatment in both P9 rat spinal cord slice cultures and human fetal astrocytes, and persists for at least 1 week in vitro. In vivo ceftriaxone results in a threefold increase in GLT-1 protein expression in healthy rodent brains 5–7 days after start of treatment.187 The GLT-1 normalization after TBI may result from increase by ceftriaxone of the glutamate transporter gene SLC1A2 expression, which is reduced after TBI.166
In the only preclinical test of ceftriaxone’s antiepileptogenic capacity, treatment with 200 mg/kg/d (human equivalent dose for which is approximately 1.9 g/d for a 60-kg individual) for 1 week starting 30 minutes after TBI in rats restored GLT-1 expression in the lesioned cortex to near normal levels, reduced posttraumatic astroglial activation 7 days after TBI by 43%, and reduced seizure frequency 12 weeks after injury from 151 seizures/24 hours to 47, and seizure duration by 19%.164
Although GLT-1 upregulation does not seem to last long after cessation of treatment, ceftriaxone’s stabilization of GLT-1 levels in the acute and subacute posttraumatic period partially protects against delayed excitotoxic damage to the vulnerable GABAergic inhibitory interneuron system and preserves both intracortical inhibition (as measured by transcranial magnetic stimulation) and cortical Parvalbumin expression up to 6 weeks after TBI.166
Ceftriaxone-mediated restoration of GLT-1 expression and extracellular glutamate clearance also increases the activity of the system xc− antiporter, and ceftriaxone upregulates the expression of the antiporter directly via increased nuclear Nrf2 levels. These actions increase intracellular GSH and decrease oxidative stress.189
In addition to ceftriaxone, atypical β lactam compounds have increased GLT-1 expression and have protected against glutamate-mediated damage in preclinical studies. Sulbactam, a β lactam compound with little antimicrobial activity, prevented GLT-1 downregulation and delayed hippocampal neuronal death following global cerebral ischemia.190 Amoxicillin and amoxicillin/clavulanate both reverse ethanol-induced reductions in GLT-1 expression in the brains of alcohol-preferring rats, and decrease ethanol intake.191 Maslinic acid, a compound derived from a byproduct of olive oil extraction, increases GLT-1 expression, enhances glutamate clearance, prevents glutamate toxicity, and reduces infarct volumes.192,193
In conclusion, ceftriaxone restores posttraumatic downregulation of glutamate transport and enhances glutamate clearance in the acute and subacute periods after trauma, when glutamate toxicity is likely first to occur. The transient reduction of posttraumatic glutamate transport (weeks in rats) suggests the possibility of short treatment, which could be timed to target this transient mechanism. This would be clinically attractive. The PTE-protective effect shown so far has been mild and disease-modifying rather than antiepileptogenic. Studies with longer injury–treatment initiation latency, pharmacokinetics, and validation in other TBI and non-TBI models are needed to determine whether the effect is clinically translatable.
10 |. GABAPENTINOIDS AS REPURPOSED ANTIEPILEPTOGENIC DRUGS
GBP and PGB (gabapentinoids) were developed as drugs whose chemical backbones were derived from the structure of GABA; however, both are inactive at GABA receptors. Rather, both drugs bind with high affinity to subtypes of α2δ Ca++ channel subunits (α2δ−1>>α2δ2) encoded by CACNA2D1 and CACNA2D2.194–197 Gabapentinoids have antiepileptic effects, with a good safety profile.198 Rodent studies suggest antiepileptogenic or disease-modifying effects. Following injury or prolonged epileptiform activity, activated astrocytes release synaptogenic thrombospondins (TSPs) that bind to α2δ−1 receptors, contributing to excitatory synaptogenesis.199 Genetically induced increases200 and decreases201 in α2δ−1 expression are associated with enhanced or decreased epileptogenesis. Gabapentinoids interfere with binding of TSPs to α2δ−1 during development and after injury, resulting in a reduction of excitatory synaptogenesis.194,199,202 GBP also reduces glutamatergic signaling through effects on α2δ−1–NMDA receptor interactions203 and excitatory transmission that may, in part, account for antiepileptic and antiallodynic effects.
Early evidence that GBP might have antiepileptogenic effects was obtained in experiments on juvenile (P35) rats treated with GBP beginning 24 hours after kainic acid–induced SE.204 Mean plasma levels in rats 30 minutes after GBP 200 mg/kg were 90–187 μg/mL. Because of the short half-life of GBP in rodents, multiple doses per day were given in these204 and most other preclinical experiments. GBP at doses ranging from 200 mg/kg ip 2×/d for 30 days to 100 mg/kg 2×/d reduced hippocampal cell loss and the increases in glial fibrillary acidic protein that follow seizures. The incidence of spontaneous seizures was reduced in rats monitored for 5 days during continuous 60-day GBP treatment after kainate-induced SE, and there were positive behavioral effects. With these protocols, seizure reduction may have been due to anticonvulsant or antiepileptogenic mechanisms, or both. Similar protective effects and increased latency to seizures resulted from prolonged PGB treatment in the pilo-SE model.205 Brief (4–8 days) GBP treatment (400 mg/kg/d) beginning 1 day after SE reduced gliosis, inflammation, and hippocampal neuronal loss,206 increased seizure threshold, and reduced evoked seizure severity.207 GBP also has similar protective effects in brain ischemic injury,208 following direct cortical trauma,199,209 and with cortical freeze lesions.201,202,210 Progressive increases in excitatory connectivity and epileptogenesis, shown using laser scanning photostimulation of caged glutamate 2 weeks following neocortical trauma in the “undercut” model, are markedly reduced by even a brief 3-day GBP treatment after injury (100 mg/kg ip 3×/d).209 Recent results show long-lasting dose-dependent antiepileptogenic effects of PGB (50 or 100 mg/kg ip beginning at P7 ×3 weeks) in juvenile α2δ−1–overexpressing mice that persist for at least 3 months after the end of treatment (W. Zhang and D.A. Prince, unpublished data).
Additional behavioral experiments should be done to assess effects of gabapentinoids given early after injury on evolution of spontaneous seizures. Chronic recordings from hippocampus as well as neocortex should be done in such experiments to assess “subclinical” seizures that are not detectable with EEG and video monitoring alone. Furthermore, although increased threshold for parameters of evoked seizures or reduction of seizure frequency during treatment with gabapentinoids has been reported, data regarding antiepileptogenic effects on frequency of spontaneous, unprovoked seizures in a chronic model are lacking.
11 |. TOPIRAMATE
TPM, an ASD with multiple mechanisms of action, is widely used for the treatment of epilepsies. It is a broad-spectrum agent useful in both partial onset and generalized onset seizures.211 It has a combination of pharmacologic properties that include modulatory effects on Na+ channels, GABAA receptors, and glutamate receptors of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate type.212 Because of this combination of mechanism of action, TPM appeared to be an ideal candidate for antiepileptogenesis. Various studies have been performed in this respect.6 One of the first studies reported that administration of TPM after a pilocarpine-induced SE was effective in reducing the number of rats that developed epilepsy by >60% in comparison to controls.213 TPM was administered ip in different doses after 1 hour of SE and once daily for 4 additional days; intensive video-EEG monitoring was performed at 3–6 months after SE. The most promising antiepileptogenic effect was obtained at a dose of 30 mg/kg of TPM. Pharmacokinetic experiments with this dose in rodents resulted in an area under the plasma concentration-time curve (AUC) that is similar to the AUC obtained with 200 mg TPM in patients.214 A similar promising effect of TPM in the rat pilocarpine model was reported by Suchomelova et al215 for a dose of 10 mg/kg ip, although the effects of TPM in this study were at least in part due to disease modification rather than antiepileptogenesis, because TPM was given at 20, 40, or 70 minutes after SE onset. Interestingly, when using the same TPM dosing protocol with a higher dose (50 mg/kg), TPM did not prevent development of epilepsy at any time point tested,215 indicating that the use of adequate doses is important for any antiepileptogenic effect of this drug. However, several other studies with post-SE models of TLE did not confirm the antiepileptogenic effect of TPM reported by DeLorenzo et al,213 although a neuroprotective effect was determined in most studies.6 In some studies, TPM partially prevented the impairment of cognitive functions following SE, indicating a disease-modifying effect.216,217 Interestingly, similar to the findings of Suchomelova et al, this effect was dosedependent, with positive effects observed after 20 but not 100 mg/kg TPM.217 In the amygdala kindling model, TPM (100 or 200 mg/kg once daily) retarded kindling acquisition, indicating a disease-modifying effect.218–220 To our knowledge, there have been no antiepileptogenesis studies in TBI models. However, in a rat TBI model in which rats received TPM or vehicle at 30 minutes (30 mg/kg ip), and 8, 20, and 32 hours postinjury (30 mg/kg by mouth), TPM significantly improved composite neuroscores at 4 weeks postinjury and rotating pole performance at 1 and 4 weeks postinjury, suggesting a potentially beneficial effect on motor function following TBI.221 Plasma levels of TPM were not determined in any of the preclinical studies reviewed here. Overall, TPM might not be capable of preventing or modifying epilepsy when administered alone (but see the data in DeLorenzo et al213), but may add to the disease-modifying effects of other drugs reviewed here.
12 |. RAPAMYCIN, EVEROLIMUS, AND OTHER MAMMALIAN TARGET OF RAPAMYCIN INHIBITORS
The mammalian target of rapamycin (mTOR) is a ubiquitous protein kinase with multiple physiological functions, including regulation of cell growth, proliferation, and survival.222 In the brain, mTOR is also involved in numerous functions under physiological conditions that can affect neuronal signaling and excitability, such as shaping axonal and dendritic morphology, neurotransmitter receptor expression, and synaptic plasticity, and could thus potentially contribute to epileptogenesis under pathological conditions.222–225 In the disease tuberous sclerosis complex (TSC), an important genetic cause of epilepsy, hyperactivation of the mTOR pathway triggered by TSC gene mutations has been strongly implicated in promoting tumor growth, as well as contributing to epilepsy and other neurological symptoms.226 In mouse models of TSC, early treatment with rapamycin (3 mg/kg ip once daily until death) was suggested to have antiepileptogenic effects in preventing the development of epilepsy and the underlying molecular and histopathological mechanisms of epileptogenesis in presymptomatic mice.226,227 However, it is not clear whether this effect was due to an antiepileptogenic or disease-suppressing effect, because treatment with rapamycin continued until death, that is, there was no treatmentfree period to evaluate the effect after discontinuation of treatment. In a subsequent study, Rensing et al reported that intermittent dosing of rapamycin, with drug holidays of >3 weeks, maintains significant antiepileptogenic properties in mouse models of TSC.228
Although the therapeutic effects of mTOR inhibition may not come as a surprise in genetic disorders caused by mTOR hyperactivation, the widespread functions of mTOR suggest that mTOR pathway dysregulation could also be involved in epileptogenesis in other, more common types of epilepsy.229 Antiepileptogenic effects of rapamycin have been reported for different post-SE models of TLE.5,230 However, in most studies the effect of rapamycin on development of SRS was determined either during the period of rapamycin treatment or following relatively short periods of rapamycin withdrawal. Because rapamycin has been reported to exert an antiseizure effect on SRS231 and is eliminated extremely slowly from the brain following prolonged administration (with half-lives of ~300 hours in rodents232,233), most if not all of the reported effects of rapamycin on SRS development may be due to antiseizure rather than antiepileptogenic effects. When sufficiently long periods of withdrawal from rapamycin treatment were used, no effect on development of SRS was observed.234,235 However, in a PTE mouse model, in which rapamycin (6 mg/kg/d, ip) or vehicle solution was injected 1 hour after CCI and continued once daily for up to 4 weeks, a decrease in spontaneous seizure frequency and rate of developing PTE was observed during the entire 16-week continuous video-EEG monitoring session,236 indicating a true antiepileptogenic effect.
Overall, rapamycin and other mTOR inhibitors, including everolimus, might be interesting candidates for preventing epilepsy in genetic disorders of the mTOR pathway, and the TBI study of Guo et al236 suggests an antiepileptogenic effect in TBI-induced PTE, although these data need to be confirmed. One disadvantage of rapalogs such as rapamycin and everolimus is their poor brain penetration.233 Thus, novel mTOR inhibitors with improved brain penetration might be more suitable to evaluate whether mTOR inhibition in the brain has antiepileptogenic potential.233
13 |. VIGABATRIN
Vigabatrin is an inhibitor of GABA transaminase. It elevates GABA levels in brain, leading to seizure inhibition. It has been used as an ASD in Europe since 1980s, and in the USA since 2009. Vigabatrin has antiseizure effects in animal models such as the PTZ and maximal electroshock seizure tests.237,238 Preclinical evidence for an antiepileptogenic action of vigabatrin is more limited. Vigabatrin inhibits electrical kindling of the amygdala in rats, suggesting the possibility of an antiepileptogenic effect.239,240 However, it has no antiepileptogenic or neuroprotective effect following self-sustained SE induced by electrical stimulation of amygdala.241 In the rat kainate-induced SE model, vigabatrin reduces hippocampal neuronal death,242 but the neuroprotective effect is mild and limited to a subset of interneurons, with no effect on mossy fiber sprouting,243 and no known effect on SRS. It has similar hippocampal neuroprotective effects in the rat pilo-SE model, but again does not reduce the incidence of epilepsy.244 However vigabatrin, administered at 100 mg/kg/d daily between 1–5 months of age, decreased the frequency and cumulative duration of absence seizures assessed at 6 months in a genetically prone rat model of absence epilepsy.245
One specific etiology of epilepsy that deserves special attention for a potential disease-modifying effect of vigabatrin is TSC. TSC is a relatively common genetic cause of epilepsy associated with focal cortical malformations (cortical tubers) and mTOR pathway activation due to TSC1 or TSC2 gene mutations. Vigabatrin has strong, relatively selective efficacy for infantile spasms in TSC, eliminating spasms in about 95% of TSC patients,246 a much better efficacy than other treatments for spasms (eg, adrenocorticotropic hormone) or for other seizure types. Clinical observations suggest that vigabatrin may also improve cognitive status and developmental outcome in TSC, suggesting a potential for antiepileptogenic or disease-modifying properties in TSC. Vigabatrin reduces seizures in a TSC mouse model, although a preventative, antiepileptogenic effect was not tested. In addition to increasing brain GABA levels as expected, vigabatrin also decreased mTOR activity.247 As mTOR inhibitors, such as rapamycin, have potential disease-modifying effects in TSC, including preventive effects against epilepsy in TSC mouse models,227 modulation of the mTOR pathway could represent a mechanistic basis for a potential antiepileptogenic effect of vigabatrin in TSC.
Vigabatrin has been evaluated in several clinical studies of epilepsy prevention in TSC. Recent developments in early or even prenatal diagnosis of TSC have led to treatment of an increasing number of young infants with TSC before clinical seizures.248 In the study of Jóźwiak et al, 14 infants diagnosed with TSC by the end of the second month of age were followed with video-EEG until 24 months of age and treated with vigabatrin (100–150 mg/kg) if paroxysmal EEG activity appeared.249 Ten of 14 developed paroxysmal activity and were treated with vigabatrin before clinical seizure onset. Six of 10 eventually developed seizures, which were usually treatable with one drug. This outcome was compared with a control group of 31 young infants with TSC who were also followed prospectively until the end of the second year. Twenty-two of 31 (71%) developed epilepsy.249 Although there was no significant difference in the prevalence of epilepsy between the preventive and control groups, the percentage of seizure-free patients at 24 months was significantly higher in the preventively treated group: 13 of 14 (92.9%) versus 11 of 31 (35.4%; P = .004), or five of six (83.3%) vs two of 22 (9.1%; P = .0003) when considering only those who developed epilepsy.249 There was also a significant difference in the number of patients whose EEG normalized at 24 months on vigabatrin preventive versus standard treatment, eight of 10 (80%) versus two of 22 (9.1%; P = .0001). On longer follow-up at a median age of 8.8 years, it was possible to withdraw all ASDs in 55% of children in the preventive group, compared to 17% in the control group (P < .03).250
Currently, two major prospective, randomized trials are evaluating preventive use of vigabatrin in TSC: the EPISTOP project (ClinicalTrials.gov identifier NCT02098759),251 a large collaborative study funded by European Union within the Seventh Frame Program; and the PREVeNT trial (Preventing Epilepsy Using Vigabatrin in Infants With Tuberous Sclerosis Complex (ClinicalTrials.gov identifier NCT02849457).252 Both studies screen prospectively asymptomatic TSC patients with EEG and randomize patients with abnormal interictal EEGs to treatment with vigabatrin before versus after onset of seizures. In the EPISTOP study, infants treated preventively had lower risk of developing epilepsy. In those in whom epilepsy did develop, it did so significantly later than in the traditionally treated (after seizures) children (K. Kotulska, unpublished data). Results of the PREVeNT trial are not yet available.
In summary, results of preventative preclinical studies are mixed, but suggest that vigabatrin may have not only antiseizure but also antiepileptogenic properties, especially in some genetic epilepsies, such as TSC. Clinical studies are ongoing, but preliminary results seem to confirm a preventative effect of vigabatrin against epilepsy in TSC patients.
14 |. ESLICARBAZEPINE
ESL is approved as therapy for the treatment of partial onset seizures. ESL is a voltage-gated sodium channel (VGSC) blocker that competitively interacts with site 2 of the inactivated state of VGSC.253 It is chemically related to carbamazepine and oxcarbazepine, but has been specifically designed to reduce the production of toxic metabolites (such as epoxides), to avoid enantiomeric impurity, and to avoid the unnecessary production of enantiomers or diastereoisomers of metabolites and conjugates, without loss of pharmacological activity.253 In humans, ESL is a prodrug of eslicarbazepine, its major active metabolite, and the entity responsible for the pharmacological effect.
In an experimental pilocarpine mouse model of chronic epilepsy, EEG monitoring revealed that transient ESL treatment within the epileptogenic period (150–300 mg/kg for 6 weeks) resulted in a significant decrease in both the frequency and duration of epileptiform discharges at the chronic stage, that is, 8 weeks after the end of the treatment, indicating disease modification.254 Additionally, ESL treatment caused a significant decrease in mossy fiber sprouting into the inner molecular layer, attenuation of neuronal loss, and significantly less coordination impairment. Thus, transient ESL treatment attenuates the functional and morphological sequelae of SE and has a possible antiepileptogenic effect. The antiepileptogenic properties of ESL might be explained by the inhibition of the neuronal T-type Ca2+ channel Cav3.2, which plays a major role in epileptogenesis.255,256
The antiepileptogenic or disease-modifying effect of ESL treatment is currently being investigated in stroke patients at high risk for unprovoked seizures. Early prophylactic antiepileptogenic therapy with ESL in patients at high risk of developing poststroke seizures will be performed for 30 days. Although convincing data supporting this therapy duration are lacking, one previous trial in patients with nontraumatic, nonaneurysmatic spontaneous intracerebral hemorrhage (SICH) with another ASD had used the same time span. The early treatment may have had some neuroprotective effect on patients with SICH, and reduced early seizures.257,258
15 |. FINGOLIMOD
Fingolimod is a structural analog of sphingosine, a sphingolipid signaling molecule, which upon conversion to sphingosine 1-phosphate (S1P), can activate five G-protein–coupled receptors, termed S1P1–5. Similarly to sphingosine, fingolimod is phosphorylated to fingolimod-phosphate by sphingosine kinases, and acts as functional antagonist of S1P1 and S1P3–5 receptors. Fingolimod activation of S1P1 receptor results in receptor internalization and degradation. This mechanism underlies the inhibitory effect of fingolimod on lymphocyte egress from the thymus and peripheral lymphoid organs due to impaired S1P1 receptor sensing of S1P gradient. Based on this action, fingolimod (Gilenya, 0.5 mg daily orally) was approved in 2010 for treatment of relapsing-remitting multiple sclerosis (MS). The drug is well tolerated but may uncommonly cause liver problems (jaundice), infection, bradycardia, and rarely, leukoencephalopathy.259
Neuroprotective effects seen in animal models of MS were also reported in animal models of stroke and chronic neurodegenerative diseases.260 These effects, in analogy with MS, may also depend on fingolimod’s direct CNS actions mediated by S1P1 and S1P3 receptors expressed by astrocytes, thereby reducing neuroinflammation.
Fingolimod has therapeutic effects in models of epileptogenesis. Gao et al reported that fingolimod (1 mg/kg ip daily for 2 weeks starting 24 hours post-SE; corresponding to the human equivalent dose of 0.16 mg/kg) reduced by twofold the incidence and number of spontaneous seizures and their duration and generalization during 14 days of video monitoring in Li+ pilo-SE rats that developed epilepsy.261 This effect was associated with an average twofold reduction of activation of both microglia and astrocytes and expression of the ictogenic cytokines IL-1β and TNF-αtwofoldlesser extent in CA3 and hilus, and decreased by 25% mossy fiber sprouting. Fingolimod similarly reduced chronic seizures in mice exposed to suprahippocampal kainate-induced SE either when injected during epileptogenesis or in mice with established epilepsy.262 Fingolimod (6 mg/kg ip, corresponding to the human equivalent dose of 0.48 mg/kg) was administered in this model starting 1 hour after SE onset and for 14 days. Video-EEG monitoring was done during drug administration and after treatment for the following 2 weeks. In both time frames, the animals displayed a threefold decrease in seizure frequency. There was also a slight but significant reduction in stage 5 seizure duration. Additional therapeutic effects included neuroprotection in CA3 (threefold increase in neuronal density vs SE vehicles), two- to threefold reduced astrogliosis, and fourfold fewer T lymphocytes in the hippocampus, although these adaptive immunity cells were scarcely present in diseased tissue.262 Notably, Pitsch et al reported the upregulation of S1P1 and S1P3 receptors in the hippocampus of patients with pharmacoresistant TLE, providing evidence of drug target availability in human epilepsy. Supporting this, each dose of fingolimod at either 2 or 6 mg/kg administered sequentially for 1 week reduced spontaneous seizures in mice with established epilepsy by 50% and 90%, respectively.262
Fingolimod (1 mg/kg/d for 17 weeks in drinking water, followed by 1 or 5 months of drug washout) was also found to have antiepileptogenic and disease-modifying effects in WAG/Rij rats when treatment was started before absence seizures developed (P30 rats; 50% decrease in spike-and-wave discharge [SWD] number but no effect on average single SWD duration after 1 month of drug withdrawal). Antidepressantlike effects were also reported. However, these effects were transient and faded 5 months after drug washout. The drug showed a longer-lasting (up to 6 months) improvement of cognitive decline in this model.263 Finally, fingolimod reduced by half the 2.5-fold increase in seizure-induced P-glycoprotein expression measured 2 days after Li+ pilo-SE in the rat hippocampus (1 mg/kg ip at the time of SE induction, then daily for 2 days). This effect was associated with prevention of the threefold increase in hippocampal COX-2 and TNF and resulted in a twofold increased brain delivery of phenytoin, suggesting a potential value of fingolimod as adjuvant therapy for drug resistance to some ASDs.264
The anti-inflammatory and antioxidant effects of fingolimod (1 mg/kg ip) were associated with reduction of social deficits, cognitive impairments, neuronal loss, and neuroinflammation in a rat model of autism.265 Moreover, fingolimod (1 mg/kg ip, first injection at the time of injury) at 1, 24, and 48 hours postinjury improved neurological deficits (modified neurological severity score in vehicle: 5–8 vs fingolimod: 0.5–1 during 14 days postinjury) in mice exposed to neurotrauma by controlled cortical impact (velocity of 6 m/s and deformation depth of 1.5 mm). These effects were associated with twofold reduction in axonal damage, brain edema and microglia activation, normalization in the cortical levels of several inflammatory molecules, and prevention of T-cell brain extravasation assessed in the injured cortex. Anti-inflammatory Treg T cells and M2-type microglia and IL-10 were increased by more than twofold by fingolimod.266
These results support the antiepileptogenic and disease-modifying effects of fingolimod in animal models of SE. Investigations in additional models of acute brain injury (ie, TBI/PTE, CNS infection, stroke) are needed, as are studies of the best therapeutic window for drug intervention to maximize the drug’s therapeutic effects. Gender and postnatal development should also be considered, as all the available evidence relies upon the use of adult male rodents.
16 |. COMBINATION TREATMENT
Development and clinical manifestations of epilepsy are the consequence of pathologies of brain network dynamics and functional connectivity that may involve abnormal network pathways.1,267–270 The epileptic network is characterized by pathologic, seizure-generating “foci” embedded in a web of structural and functional connections, resulting in a complex relationship between foci and the surrounding network that drives seizure dynamics.268 Based on this concept, which also applies to other brain diseases, developing new therapies that act on individual drug targets may be less effective than multitargeted drugs or drug combinations, that is, “network pharmacology”.271–274
We recently proposed network pharmacology as a novel strategy for antiepileptogenesis, that is, drug administration during the latent period following an epileptogenic brain insult with the aim of preventing or modifying development of epilepsy.273,275 Rather than creating new multitargeted drugs, we suggested repurposing clinically established drugs to form rationally chosen drug cocktails for antiepileptogenesis. Surprisingly, only few previous studies have evaluated combination treatment for antiepileptogenesis.
More than 20 years ago, we reported that the anticonvulsant effect of 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(F)quinoxaline (NBQX), which acts on the AMPA receptor subtype of glutamate receptors, can be potentiated by extremely low doses (0.0001–0.1 mg/kg) of the NMDA receptor antagonist MK-801 (dizocilpine) in the amygdala kindling model. Similar supra-additive effects were seen when NBQX was combined with the competitive NMDA antagonist CGP39551 or the low-affinity, rapidly channel-blocking NMDA receptor antagonist memantine.276,277 Adverse effects were not potentiated by combining low doses of NMDA antagonists with NBQX. Based on these promising data, we recently combined NBQX with the clinically available NMDA receptor antagonist ifenprodil in the intrahippocampal kainate model of TLE, with the goal of preventing or modifying the development of epilepsy.278 When administered during the latent period following the kainate-induced SE, the combination did not prevent epilepsy but retarded granule cell dispersion, indicating a disease-modifying effect. Recently, the first AMPA antagonist, perampanel, was approved for treatment of epilepsy.279 Perampanel was shown to exert antiepileptogenic effects in different epilepsy models, including kindling and pilocarpine.45,280 In view of the finding that both glutamate receptor subtypes are critically involved in epileptogenesis,281 combinations of clinically available NMDA and AMPA antagonists are interesting candidates for antiepileptogenesis.
Another interesting example for combination treatment for antiepileptogenesis is the combination of phenobarbital with the diuretic bumetanide.282 Bumetanide inhibits the neuronal chloride cotransporter NKCC1 that acts as a chloride importer. A shift from hyperpolarizing to depolarizing GABA responses, which may lead to neuronal hyperexcitability, has been observed when expression of NKCC1 is higher than expression of the chloride exporter KCC2, as may occur in asphyxiated neonates with seizures and in the hippocampus of adults with TLE.283 Based on the hypothesis that increased expression of NKCC1 may be involved in the development of neuronal hyperexcitability during epileptogenesis in adults, we evaluated whether treatment with bumetanide (alone or in combination with phenobarbital) after pilo-SE alters the long-term consequences of the brain insult.282 None of the treatments prevented development of SRS, but the combined treatment counteracted the development of behavioral alterations associated with epilepsy, indicating a disease-modifying effect.282
Increasing evidence suggests an important role for inflammatory processes in epileptogenesis.1 Different inflammatory pathways, including IL-1 receptor/Toll-like receptor, COX-2, and TGF-β signaling, provide interesting targets for antiepileptogenic intervention. However, targeting each of these pathways alone may not be sufficient. Kwon et al combined an antagonist of IL-1 receptors (IL-1ra; anakinra), a COX-2 inhibitor (CAY 10404), and an antagonist of microglia activation of caspase-1 (minocycline) and tested them for neuroprotective and antiepileptogenic effects in an SE model in 2- to 3-week-old rats.125 None of these drugs was effective in attenuating neuronal damage or in limiting the development of spontaneous seizures when administered individually. When empiric binary combinations of these drugs were tried, the combined targeting of IL-1ra and COX-2 resulted in attenuation of acute brain injury, reduced the development of SRS, and limited the extent of mossy fiber sprouting. The authors concluded that “deployment of an empirically designed ‘drug cocktail’ targeting multiple inflammatory signaling pathways for a limited duration after an initial insult like SE may provide a practical approach to neuroprotection and antiepileptogenic therapy.” However, a subsequent study of combined anakinra and VX-765, a specific nonpeptide inhibitor of IL-1β cleavage and release, in two adult rat models of SE-induced epilepsy did not show any robust effect on epilepsy development but only neuroprotective effects.126
François et al examined whether the combination of two ASDs, TPM and diazepam, acting by different mechanisms, interferes with epileptogenesis in the lithium-pilocarpine model in rats.284 Although the treatment had partial neuroprotective effect in two areas critical for epileptogenesis, the hippocampus and ventral entorhinal cortex, it did not prevent epileptogenesis. The neuroprotective effect of combined treatment was more marked than the effect of treatment with TPM alone.284,285 Overall, it has yet to be demonstrated that epilepsy prevention is possible by rationally chosen combinations of repurposed drugs. However, the recent finding that the multitargeted anesthetic isoflurane prevents epilepsy in two rat models of acquired epilepsy96 indicates that drugs combining several relevant mechanisms may be another promising option for antiepileptogenesis.
Successful repurposing can be challenging, but it can provide unique and commercially viable business opportunities even in the limited time window of product protection. Smaller and larger companies look at opportunities for drug repurposing with different strategic and financial objectives, but in any case, the target profile and commercial objectives, clinical and regulatory submission plans, and business case must be well thought out to best ensure development and financial success.
17 |. INTELLECTUAL PROPERTY, EXCLUSIVITY, AND PRODUCT PROTECTION
Successful drug development for new chemical entities is very challenging and costly, and it takes many years to obtain a marketing license for commercialization.286 For the rather challenging CNS drug category, success rates have been in the low single-digit percentages. To successfully achieve a return on investment in the limited protected time window for commercialization, companies often pursue initially high drug pricing and an intense sales and marketing campaign that also has associated costs. Competition by generic drug entries ultimately brings prices down but also provides consumer value hurdles that new products must overcome. Drug repurposing offers a potentially attractive mechanism to lower the risks of drug development by marketing new indications for existing drugs. It has been suggested that repurposed drugs are more than twice as likely across all disease indications to make it to market compared to new drug compounds and at a substantially shorter development time and lower cost. However, major challenges for profiting from repurposed drugs include pricing and an adequate level and window of protection when a generic is available.
Optimally for a commercial developer, pharmaceutical products will have adequate protection for return on investment in the time following regulatory approval and obtaining a license for marketing authorization. It often takes several years after the product’s commercial launch to achieve “positive” return on the investment made to discover and develop the drug. Furthermore, when considering the capitalized investment across a pharmaceutical portfolio, it takes several more years to recover costs for programs that have failed.
Pharmaceutical product protection is principally obtained via patents granted by patent and trademark offices or via exclusivity granted through statutory provisions. Patents expire 20 years after filing and are a property right encompassing claims for innovative discoveries.287 For pharmaceutical products, the composition of matter (CoM) patent for a new chemical entity is claimed by chemical name or structure or both and provides the strongest intellectual property protection. Because these patent assignments are now recognized in most commercially important countries around the world, none but the patent holder or licensee can make, sell, or import the chemical for any use without infringement.
Pharmaceutical companies frequently explore new indications for repositioning or other mechanisms of product protection as “life cycle opportunities,” especially if CoM protection is lost. Protective life cycle strategies can also be considered for repurposing and include new formulations, new routes of administration, enantiomers, crystal polymorphs, combination products, and new uses.288 Other principal types of patents include a novel process (manufacturing), a unique and unanticipated formulation, or those that teach a new usage. Method of use (MoU) patents are commonly sought for new indications and can be a reasonable mechanism for product protection of repurposed drugs. Use infringement can result in litigation; however, in practice, legal action to identify and demonstrate infringement can be tedious, time-consuming, and costly.
Statutory data exclusivity is an attractive mechanism for product protection and can be very appropriate for repurposed drugs. There are different types of data exclusivity (“data protection”) and different durations of exclusivity, and the statutory provisions for exclusivity vary by country or region. Exclusivity blocks subsequent sponsors or competing drug developers from referencing their preclinical and clinical trial data, so that they may not be referenced in the regulatory filings to obtain marketing authorization for the same drug substance.
Periods of exclusivity begin at the time of data filing approval, and in the USA there are several types of data exclusivity relevant to epilepsy289: Orphan Drug Exclusivity (7 years) for diseases affecting <200 000 people; New Chemical Entity Exclusivity (5 years) and New Clinical Investigation Exclusivity (3 years, if the drug has previously been approved in another form), both requiring clinical data for the 505(b) (2) regulatory pathway; and Pediatric Exclusivity (6 months added to existing patents/exclusivity) for pediatric-related data. There are two 6-month periods of exclusivity that are relevant to generic Abbreviated New Drug Applications only: Patent Challenge and Competitive Generic Therapy. Antiepileptogenesis therapies might focus on specific, rare populations such as genetic epilepsies, which can fall into the orphan drug category. Although revenue returns might be limited in rare diseases, smaller companies can find orphan indications as an attractive business opportunity, especially for repurposed drugs, where investment, time for development, and competition might be considerably less.
Data exclusivity also exists for pharmaceuticals in Europe, and market exclusivity is a related form of additional protection.290 Based on the 2005 European Union Data Exclusivity Directive, this has been termed the “8 + 2 + 1” regime; it can include 8 years of data exclusivity, 2 years of marketing exclusivity, and a potential 1-year extension. A further 6-month exclusivity can be obtained for conducting pediatric studies.
There are recent examples of pharmaceutical companies employing a drug repurposing strategy in the CNS. Tecfidera (dimethyl fumarate), FDA approved for relapsing-remitting MS in 2013, was one of the most successful blockbuster drug launches in US history. Mixtures of fumarates have been used for decades for psoriasis and even as a consumer product to treat mold on furniture. Biogen successfully employed a strategy defining clinical efficacy and safety, dosing, and competitive pricing and commercialization. The company also pursued patent claims to pharmaceutical formulations and useful doses for treating MS that could provide a period of protection beyond data exclusivity. While resolving European patent challenges to secure additional levels of protection, Biogen delayed launch in Europe until the Committee for Medicinal Products for Human Use agreed that the active ingredient in Tecfidera should be classified as a New Active Substance, which provided a 10-year period of data and market exclusivity.
Another example is the case of ketamine for treatment-resistant depression. Ketamine, an NMDA receptor blocker, is approved for use as an anesthetic in the USA and has found numerous additional uses in clinical practice, particularly as an analgesic to reduce opiate use in the hospital setting, but also has been found to act effectively in the treatment of depression. Janssen Pharmaceutica repurposed ketamine’s more potent S-enantiomer, which has been approved as an intranasally delivered product for outpatient treatment-resistant depression. Product protection is likely to be achieved through data exclusivity and potentially through MoU patents. Use of the product in the outpatient clinic setting can substantially improve market access and uptake to improve successful commercialization in a limited time window of protection.
Successful repurposing can be challenging, but it can provide unique and commercially viable business opportunities even in the limited time window of product protection. Smaller and larger companies look at opportunities for drug repurposing with different strategic and financial objectives, but in any case, the target profile and commercial objectives, clinical and regulatory submission plans, and business case must be well thought out to best ensure development and financial success.
18 |. SUMMARY/CONCLUSION
This review shows that there is substantial evidence for antiepileptogenic and disease-modifying effects in animals of a number of approved drugs in models of acquired epilepsy, including several with excellent safety profiles. These drugs include diverse antiepileptogenic mechanisms of action, including reduction of glutamatergic toxicity (LEV, ceftriaxone), free radical scavenging (LEV, N-acetylcysteine, sulforaphane), blunting of the effect of BBB disruption (losartan), anti-inflammatory effects (LEV, losartan, anakinra, fingolimod, atorvastatin), modulation of neurogenesis, axonal and dendritic regeneration (rapamycin), modulation of excitatory synaptogenesis (gabapentinoids, rapamycin), modulation of postsynaptic excitatory transmission (TPM, perampanel), and modulation of presynaptic transmitter release (LEV, BRV)—a rich palette. However, with the exception of LEV and gabapentinoids, critical information is lacking on dose-response, human equivalent dosing, blood levels, and other pharmacokinetic parameters and on the effect of injury to treatment initiation latency on efficacy that would allow extrapolation from animal studies to human study design. Furthermore, most of the animal data pertain to SE or unorthodox TBI models such as cortical albumin exposure or undercut models. There are few data in standard in vivo PTE models such as FPI or CCI, and no data validating that antiepileptogenic effects in SE models also pertain to PTE models. These gaps limit translatability of the current animal data to human antiepileptogenic studies. The gaps can be filled with a systematic approach to animal studies of dose-response, pharmacokinetic, injury-treatment latency, and SE/PTE model comparisons. Fortunately, this approach has begun, for instance in recent work on combinatorial treatment of epilepsy prevention after SE.291 Furthermore, there is active effort to discover biomarkers of disease development (epileptogenesis) and surrogate efficacy biomarkers. Availability of such biomarkers would facilitate comparison of results across different models and translation to clinical preventive studies.292 Let us hope that the next few years will yield results that will lead to human epilepsy prevention studies—before the authors retire. It is interesting to note that some pharmaceutical companies currently plan epilepsy prevention trials based on promising preclinical data on clinically approved drugs, for instance with eslicarbazepine acetate for poststroke epilepsy.45,280 Furthermore, several startup companies (eg, OB Pharmaceuticals, Expesicor, LVM Biosciences) are currently developing novel innovative antiepileptogenic therapeutics. This renewed interest of both academia and industry in antiepileptogenesis suggests that the long hoped-for breakthrough may perhaps be on the horizon.
Key Points.
Epilepsy prevention is an unmet need, with prevention opportunity particularly after CNS injury; several approved drugs have antiepileptogenic effects in animals
These include atorvastatin, ceftriaxone, losartan, isoflurane, LEV, BRV, TPM, GBP, PGB, vigabatrin and others; we review their antiepileptogenic data
We highlight gaps in the data that have prevented clinical evaluation of these drugs, and that need to be filled for clinical translation
Except for LEV and GBP/PGB, critical information is lacking on dose-response, human dosing, pharmacokinetic parameters, and time-to-treatment impact
Most data are in SE or in rare TBI models, with few data in standard PTE models (FPI, CCI), and no data validating SE results in PTE models
ACKNOWLEDGMENTS
We thank Peter Abdow for technical assistance in preparation of the manuscript.
CONFLICT OF INTEREST
S.J. has received speaking and advisory board honoraria from Novartis, and is the recipient of the grant EPIMARKER from the Polish National Center for Research and Development (No. STRATEGMED3/306306/4/2016). P.K. has received research support from Eisai and Lundbeck. He serves on science/medical advisory boards for Alliance, Therma Neurosciences, and OB Pharmaceuticals. He has served as a paid consultant for Abbotts, Engage, SK Life Sciences, and UCB Pharma, has participated on advisory boards for Aquestive, Eisai, and UCB Pharma, and has received speaker honoraria from Aquestive, Eisai, Sunovion, and UCB Pharma. He is a cofounder, co-owner, and CEO of PrevEp. H.K. owns stock in UCB Pharma. M.K. has received honoraria from BIAL, Eisai, Novartis, and UCB. W.L. is a cofounder and co-owner of PrevEp. A.R. has relevant work funded by the National Institutes of Health National Institute of Neurological Disorders and Stroke (R01 NS088583). A.R. is also a founder and advisor to Neuromotion; serves on the scientific advisory board of EpiHunter, Gamify, and NeuroRex; and has received consulting fees or research support from Brainsway, Cavion, Neuroelectrics, Roche, Sage, and Takeda, outside the submitted work. R.T. is a past employee of and holds stock in Johnson & Johnson. He is CEO and sole employee of Amron Neuroscience. He is an independent director board member of AC Immune, Syndesi Therapeutics, and NeuroVision. He is on the scientific advisory board of or consults for Janssen, Neurop, and Kaerus Bioscience. The remaining authors have no conflicts of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Klein P, Dingledine R, Aronica E, et al. Commonalities in epileptogenic processes from different acute brain insults: do they translate? Epilepsia. 2018;59(1):37–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee PN, Filippi D, Allen HW. The descriptive epidemiology of epilepsy—a review. Epilepsy Res. 2009;85(1):31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34(3):453–68. [DOI] [PubMed] [Google Scholar]
- 4.Klein P, Tyrlikova I. Prevention of epilepsy: should we be avoiding clinical trials? Epilepsy Behav. 2017;72:188–94. [DOI] [PubMed] [Google Scholar]
- 5.Kaminski RM, Rogawski MA, Klitgaard H. The potential of antiseizure drugs and agents that act on novel molecular targets as antiepileptogenic treatments. Neurotherapeutics. 2014;11(2):385–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Löscher W, Brandt C. Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research. Pharmacol Rev. 2010;62(4):668–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein P, Herr D, Pearl PL, et al. Results of phase 2 safety and feasibility study of treatment with levetiracetam for prevention of posttraumatic epilepsy. Arch Neurol. 2012;69:1290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Löscher W, Hönack D, Rundfeldt C. Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy. J Pharmacol Exp Ther. 1998;284(2):474–9. [PubMed] [Google Scholar]
- 9.Stratton SC, Large CH, Cox B, Davies G, Hagan RM. Effects of lamotrigine and levetiracetam on seizure development in a rat amygdala kindling model. Epilepsy Res. 2003;53(1–2):95–106. [DOI] [PubMed] [Google Scholar]
- 10.Gower AJ, Noyer M, Verloes R, Gobert J, Wülfert E. ucb L059, a novel anti-convulsant drug: pharmacological profile in animals. Eur J Pharmacol. 1992;222(2–3):193–203. [DOI] [PubMed] [Google Scholar]
- 11.Matagne A, Klitgaard H. Validation of corneally kindled mice: a sensitive screening model for partial epilepsy in man. Epilepsy Res. 1998;31(1):59–71. [DOI] [PubMed] [Google Scholar]
- 12.Vinogradova LV, van Rijn CM. Anticonvulsive and antiepileptogenic effects of levetiracetam in the audiogenic kindling model. Epilepsia. 2008;49(7):1160–8. [DOI] [PubMed] [Google Scholar]
- 13.Yan H-D, Ji-qun C, Ishihara K, Nagayama T, Serikawa T, Sasa M. Separation of antiepileptogenic and antiseizure effects of levetiracetam in the spontaneously epileptic rat (SER). Epilepsia. 2005;46(8):1170–7. [DOI] [PubMed] [Google Scholar]
- 14.Russo E, Citraro R, Scicchitano F, et al. Effects of early long-term treatment with antiepileptic drugs on development of seizures and depressive-like behavior in a rat genetic absence epilepsy model. Epilepsia. 2011;52(7):1341–50. [DOI] [PubMed] [Google Scholar]
- 15.Brandt C, Glien M, Gastens AM, et al. Prophylactic treatment with levetiracetam after status epilepticus: lack of effect on epileptogenesis, neuronal damage, and behavioral alterations in rats. Neuropharmacology. 2007;53(2):207–21. [DOI] [PubMed] [Google Scholar]
- 16.Klitgaard H, Matagne A, Grimee R, Vanneste-goemaere J, Margineanu D-G. Electrophysiological, neurochemical and regional effects of levetiracetam in the rat pilocarpine model of temporal lobe epilepsy. Seizure. 2003;12(2):92–100. [DOI] [PubMed] [Google Scholar]
- 17.Klein P, Herr D, Pearl PL, et al. Results of phase II pharmacokinetic study of levetiracetam for prevention of post-traumatic epilepsy. Epilepsy Behav. 2012;24(4):457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh K, Inamine M, Oshima W, et al. Prevention of status epilepticus-induced brain edema and neuronal cell loss by repeated treatment with high-dose levetiracetam. Brain Res. 2015;1608:225–34. [DOI] [PubMed] [Google Scholar]
- 19.Itoh K, Ishihara Y, Komori R, et al. Levetiracetam treatment influences blood-brain barrier failure associated with angiogenesis and inflammatory responses in the acute phase of epileptogenesis in post-status epilepticus mice. Brain Res. 2016;1652:1–13. [DOI] [PubMed] [Google Scholar]
- 20.Margineanu D-G, Matagne A, Kaminski RM, Klitgaard H. Effects of chronic treatment with levetiracetam on hippocampal field responses after pilocarpine-induced status epilepticus in rats. Brain Res Bull. 2008;77:282–5. [DOI] [PubMed] [Google Scholar]
- 21.Chen YH, Huang EYK, Kuo TT, et al. Levetiracetam prophylaxis ameliorates seizure epileptogenesis after fluid percussion injury. Brain Res. 2016;1642:581–9. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y-H, Kuo T-T, Huang E-K, et al. Profound deficits in hippocampal synaptic plasticity after traumatic brain injury and seizure is ameliorated by prophylactic levetiracetam. Oncotarget. 2018;9(14):11515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling D, Yang L, Afroz S, et al. Levetiracetam prevents posttraumatic epileptogenesis in rat neocortex following controlled cortical impact injury. Paper presented at: American Epilepsy Society 2011 Annual Meeting; December 1–5, 2011; Baltimore, MD; Abstract 1.284. [Google Scholar]
- 24.Ling D, Yang LAH, Afroz S, et al. Levetiracetam and leupeptin prevent post-traumatic epileptogenesis in an in vitro model of neocortical injury. Epilepsia. 2009;50(S11):265.18717714 [Google Scholar]
- 25.Ueda Y, Doi T, Nagatomo K, Tokumaru J, Takaki M, Willmore LJ. Effect of levetiracetam on molecular regulation of hippocampal glutamate and GABA transporters in rats with chronic seizures induced by amygdalar FeCl3 injection. Brain Res. 2007;1151:55–61. [DOI] [PubMed] [Google Scholar]
- 26.Browning M, Shear DA, Bramlett HM, et al. Levetiracetam treatment in traumatic brain injury: operation brain trauma therapy. J Neurotrauma. 2016;33(6):581–94. [DOI] [PubMed] [Google Scholar]
- 27.Caudle KL, Lu X-C, Mountney A, Shear DA, Tortella FC. Neuroprotection and anti-seizure effects of levetiracetam in a rat model of penetrating ballistic-like brain injury. Restor Neurol Neurosci. 2016;34(2):257–70. [DOI] [PubMed] [Google Scholar]
- 28.Jehi LE, Irwin AI, Kayyali H, Vadera S, Bingaman W, Najm I. Levetiracetam may favorably affect seizure outcome after temporal lobectomy. Epilepsia. 2012;53(6):979–86. [DOI] [PubMed] [Google Scholar]
- 29.Löscher W, Gillard M, Sands ZA, Kaminski RM, Klitgaard H. Synaptic vesicle glycoprotein 2A ligands in the treatment of epilepsy and beyond. CNS Drugs. 2016;30(11):1055–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaminski R, Gillard M, Leclerq K, et al. Proepileptic phenotype of SV2 deficient mice is associated with reduced anticonvulsant efficacy of levetiracetam. Epilepsia. 2009;50(7):1729–40. [DOI] [PubMed] [Google Scholar]
- 31.Tokudome K, Okumura T, Shimizu S, et al. Synaptic vesicle glycoprotein 2A (SV2A) regulates kindling epileptogenesis via GABAergic neurotransmission. Sci Rep. 2016;6(1):27420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokudome K, Okumura T, Terada R, et al. A missense mutation of the gene encoding synaptic vesicle glycoprotein 2A (SV2A) confers seizure susceptibility by disrupting amygdalar synaptic GABA release. Front Pharmacol. 2016;7:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Vliet EA, Aronica E, Redeker S, Boer K, Gorter JA. Decreased expression of synaptic vesicle protein 2A, the binding site for levetiracetam, during epileptogenesis and chronic epilepsy. Epilepsia. 2009;50(3):422–33. [DOI] [PubMed] [Google Scholar]
- 34.Hanaya R, Hosoyama H, Sugata S, et al. Low distribution of synaptic vesicle protein 2A and synaptotagimin-1 in the cerebral cortex and hippocampus of spontaneously epileptic rats exhibiting both tonic convulsion and absence seizure. Neuroscience. 2012;221:12–20. [DOI] [PubMed] [Google Scholar]
- 35.Bartholome O, Van den Ackerveken P, Sánchez Gil J, et al. Puzzling out synaptic vesicle 2 family members functions. Front Mol Neurosci. 2017;10:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohno Y, Tokudome K. Therapeutic role of synaptic vesicle glycoprotein 2A (SV2A) in modulating epileptogenesis. Neurol Disord Drug Targets. 2017;16:463–71. [DOI] [PubMed] [Google Scholar]
- 37.Pichardo Macías LA, Ramírez Mendiola BA, Contreras García IJ, et al. Effect of levetiracetam on extracellular amino acid levels in the dorsal hippocampus of rats with temporal lobe epilepsy. Epilepsy Res. 2018;140:111–9. [DOI] [PubMed] [Google Scholar]
- 38.Oliveira AA, Almeida JPC, Freitas RM, et al. Effects of levetiracetam in lipid peroxidation level, nitrite-nitrate formation and antioxidant enzymatic activity in mice brain after pilocarpine-induced seizures. Cell Mol Neurobiol. 2007;27(3): 395–406. [DOI] [PubMed] [Google Scholar]
- 39.Mazhar F, Malhi SM, Simjee SU. Comparative studies on the effects of clinically used anticonvulsants on the oxidative stress biomarkers in pentylenetetrazole-induced kindling model of epileptogenesis in mice. J Basic Clin Physiol Pharmacol. 2017;28(1):31–42. [DOI] [PubMed] [Google Scholar]
- 40.Nagarkatti N, Deshpande LS, DeLorenzo RJ. Levetiracetam inhibits both ryanodine and IP3 receptor activated calcium induced calcium release in hippocampal neurons in culture. Neurosci Lett. 2008;436(3):289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klitgaard H, Matagne A, Nicolas J-M, et al. Brivaracetam: rationale for discovery and preclinical profile of a selective SV2A ligand for epilepsy treatment. Epilepsia. 2016;57(4):538–48. [DOI] [PubMed] [Google Scholar]
- 42.Noyer M, Gillard M, Matagne A, Hénichart J-P, Wülfert E. The novel antiepileptic drug levetiracetam (ucb LO59) appears to act via a specific binding site in CNS membranes. Eur J Pharmacol. 1995;286:137–46. [DOI] [PubMed] [Google Scholar]
- 43.Matagne A, Margineanu D-G, Kenda B, Michel P, Klitgaard H. Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A. Br J Pharmacol. 2008;154:1662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood M, Gillard M. Evidence for a differential interaction of brivaracetam and levetiracetam with the synaptic vesicle 2A protein. Epilepsia. 2017;58:255–62. [DOI] [PubMed] [Google Scholar]
- 45.Dupuis N, Matagne A, Staelens L, et al. Anti-ictogenic and antiepileptogenic properties of brivaracetam in immature and mature rats. Epilepsia. 2015;56(5):800–5. [DOI] [PubMed] [Google Scholar]
- 46.Seiffert E, Dreier JP, Ivens S, et al. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24(36):7829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomkins O, Friedman O, Ivens S, et al. Blood–brain barrier disruption results in delayed functional and structural alterations in the rat neocortex. Neurobiol Dis. 2007;25(2):367–77. [DOI] [PubMed] [Google Scholar]
- 48.Ivens S, Kaufer D, Flores LP, et al. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130(Pt 2):535–47. [DOI] [PubMed] [Google Scholar]
- 49.Cacheaux LP, Ivens S, David Y, et al. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. J Neurosci. 2009;29(28):8927–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. [DOI] [PubMed] [Google Scholar]
- 51.Massagué J How cells read TGF-β signals. Nat Rev Mol Cell Biol. 2000;1(3):169–78. [DOI] [PubMed] [Google Scholar]
- 52.Massague J TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13(10):616–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horbelt D, Denkis A, Knaus P. A portrait of transforming growth factor β superfamily signalling: background matters. Int J Biochem Cell Biol. 2012;44:469–74. [DOI] [PubMed] [Google Scholar]
- 54.Frigerio F, Frasca A, Weissberg I, et al. Long-lasting pro-ictogenic effects induced in vivo by rat brain exposure to serum albumin in the absence of concomitant pathology. Epilepsia. 2012;53(11):1887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.David Y, Cacheaux LP, Ivens S, et al. Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis? J Neurosci. 2009;29(34):10588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim SY, Senatorov VV, Morrissey CS, et al. TGFβ signaling is associated with changes in inflammatory gene expression and perineuronal net degradation around inhibitory neurons following various neurological insults. Sci Rep. 2017;7(1):7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weissberg I, Wood L, Kamintsky L, et al. Albumin induces excitatory synaptogenesis through astrocytic TGF-β/ALK5 signaling in a model of acquired epilepsy following blood-brain barrier dysfunction. Neurobiol Dis. 2015;78:115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salar S, Lapilover E, Müller J, et al. Synaptic plasticity in area CA1 of rat hippocampal slices following intraventricular application of albumin. Neurobiol Dis. 2016;91:155–65. [DOI] [PubMed] [Google Scholar]
- 59.Lim D-S, Lutucuta S, Bachireddy P, et al. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation. 2001;103(6):789–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lavoie P, Robitaille G, Agharazii M, Ledbetter S, Lebel M, Larivière R. Neutralization of transforming growth factor-beta attenuates hypertension and prevents renal injury in uremic rats. J Hypertens. 2005;23(10):1895–903. [DOI] [PubMed] [Google Scholar]
- 61.Cohn RD, van Erp C, Habashi JP, et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13(2):204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bar-Klein G, Cacheaux LP, Kamintsky L, et al. Losartan prevents acquired epilepsy via TGF-β signaling suppression. Ann Neurol. 2014;75(6):864–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nozaki T, Ura H, Takumi I, Kobayashi S, Maru E, Morita A. The angiotensin II type I receptor antagonist losartan retards amygdala kindling-induced epileptogenesis. Brain Res. 2018;1694:121–8. [DOI] [PubMed] [Google Scholar]
- 64.Tchekalarova JD, Ivanova NM, Pechlivanova DM, et al. Antiepileptogenic and neuroprotective effects of losartan in kainate model of temporal lobe epilepsy. Pharmacol Biochem Behav. 2014;127:27–36. [DOI] [PubMed] [Google Scholar]
- 65.Bar-Klein G, Lublinsky S, Kamintsky L, et al. Imaging blood-brain barrier dysfunction as a biomarker for epileptogenesis. Brain. 2017;140(6):1692–705. [DOI] [PubMed] [Google Scholar]
- 66.Pechlivanova DM, Stoynev AG, Tchekalarova JD. The effects of chronic losartan pretreatment on restraint stress-induced changes in motor activity, nociception and pentylenetetrazol generalized seizures in rats. Folia Med. 2011;53(2):69–73. [DOI] [PubMed] [Google Scholar]
- 67.Sun H, Wu HQ, Yu X, et al. Angiotensin II and its receptor in activated microglia enhanced neuronal loss and cognitive impairment following pilocarpine-induced status epilepticus. Mol Cell Neurosci. 2015;65:58–67. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe K, Taniguchi M, Miyoshi M, et al. Effects of central injection of angiotensin-converting-enzyme inhibitor and angiotensin type 1 receptor antagonist on the brain NF-kappaB and AP-1 activities of rats given LPS. Peptides. 2006;27(6):1538–46. [DOI] [PubMed] [Google Scholar]
- 69.Benicky J, Sánchez-Lemus E, Honda M, et al. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology. 2011;36(4):857–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Min L-J, Mogi M, Shudou M, et al. Peroxisome prolifera-tor-activated receptor-γ activation with angiotensin II type 1 receptor blockade is pivotal for the prevention of blood-brain barrier impairment and cognitive decline in type 2 diabetic mice. Hypertension. 2012;59(5):1079–88. [DOI] [PubMed] [Google Scholar]
- 71.Bull ND, Johnson TV, Welsapar G, DeKorver NW, Tomarev SI, Martin KR. Use of an adult rat retinal explant model for screening of potential retinal ganglion cell neuroprotective therapies. Invest Ophthalmol Vis Sci. 2011;52(6):3309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J, Pang T, Hafko R, Benicky J, Sanchez-Lemus E, Saavedra JM. Telmisartan ameliorates glutamate-induced neurotoxicity: roles of AT(1) receptor blockade and PPARγ activation. Neuropharmacology. 2014;79:249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu H, Liu X, Wei X, et al. Losartan, an angiotensin II type 1 receptor blocker, ameliorates cerebral ischemia-reperfusion injury via PI3K/Akt-mediated eNOS phosphorylation. Brain Res Bull. 2012;89(1–2):65–70. [DOI] [PubMed] [Google Scholar]
- 74.Smeda JS, Daneshtalab N. The effects of poststroke captopril and losartan treatment on cerebral blood flow autoregulation in SHRsp with hemorrhagic stroke. J Cereb Blood Flow Metab. 2011;31(2):476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villapol S, Yaszemski AK, Logan TT, Sánchez-Lemus E, Saavedra JM, Symes AJ. Candesartan, an angiotensin II AT1-receptor blocker and PPAR-γ agonist, reduces lesion volume and improves motor and memory function after traumatic brain injury in mice. Neuropsychopharmacology. 2012;37(13):2817–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Villapol S, Saavedra JM. Neuroprotective effects of angiotensin receptor blockers. Am J Hypertens. 2015;28(3):289–99. [DOI] [PubMed] [Google Scholar]
- 77.Timaru-Kast R, Wyschkon S, Luh C, et al. Delayed inhibition of angiotensin II receptor type 1 reduces secondary brain damage and improves functional recovery after experimental brain trauma. Crit Care Med. 2012;40(3):935–44. [DOI] [PubMed] [Google Scholar]
- 78.Ongali B, Nicolakakis N, Tong X-K, et al. Angiotensin II type 1 receptor blocker losartan prevents and rescues cerebrovascular, neuropathological and cognitive deficits in an Alzheimer’s disease model. Neurobiol Dis. 2014;68:126–36. [DOI] [PubMed] [Google Scholar]
- 79.Sabharwal R, Chapleau MW. Autonomic, locomotor and cardiac abnormalities in a mouse model of muscular dystrophy: targeting the renin-angiotensin system. Exp Physiol. 2014;99(4):627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu D, Shi J, Zhang Y, et al. Central angiotensin II stimulation promotes β amyloid production in Sprague Dawley rats. PLoS ONE. 2011;6(1):e16037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Antoniou T, Camacho X, Yao Z, Gomes T, Juurlink DN, Mamdani MM. Comparative effectiveness of angiotensin-receptor blockers for preventing macrovascular disease in patients with diabetes: a population-based cohort study. CMAJ. 2013;185(12):1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Devereux RB, Dahlöf Björn, Gerdts E, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the losartan intervention for endpoint reduction in hypertension (LIFE) trial. Circulation. 2004;110(11):1456–62. [DOI] [PubMed] [Google Scholar]
- 83.Wachtell K, Lehto M, Gerdts E, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention for End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol. 2005;45(5):712–9. [DOI] [PubMed] [Google Scholar]
- 84.Shiraishi J, Sawada T, Koide M, Yamada H, Matsubara H. Cardiocerebrovascular protective effects of valsartan in high-risk hypertensive patients with coronary artery disease (from the Kyoto Heart Study). Am J Cardiol. 2012;109(9):1308–14. [DOI] [PubMed] [Google Scholar]
- 85.Papademetriou V, Farsang C, Elmfeldt D, et al. Stroke prevention with the angiotensin II type 1-receptor blocker candesartan in elderly patients with isolated systolic hypertension: the Study on Cognition and Prognosis in the Elderly (SCOPE). J Am Coll Cardiol. 2004;44(6):1175–80. [DOI] [PubMed] [Google Scholar]
- 86.Lithell H, Hansson L, Skoog I, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21(5):875–86. [DOI] [PubMed] [Google Scholar]
- 87.MJ, Dong L, Jia M, et al. Epigenetic enhancement of brain-derived neurotrophic factor signaling pathway improves cognitive impairments induced by isoflurane exposure in aged rats. Mol Neurobiol. 2014;50(3):937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu G, Gu B, He X-P, et al. Transient inhibition of TrkB kinase after status epilepticus prevents development of temporal lobe epilepsy. Neuron. 2013;79(1):31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jones MV, Brooks PA, Harrison NL. Enhancement of gam-ma-aminobutyric acid-activated Cl- currents in cultured rat hippocampal neurones by three volatile anaesthetics. J Physiol. 1992;449:279–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brohan J, Goudra BG. The role of GABA receptor agonists in anesthesia and sedation. CNS Drugs. 2017;31(10):845–56. [DOI] [PubMed] [Google Scholar]
- 91.Ming Z, Knapp DJ, Mueller RA, Breese GR, Criswell HE. Differential modulation of GABA- and NMDA-gated currents by ethanol and isoflurane in cultured rat cerebral cortical neurons. Brain Res. 2001;920(1–2):117–24. [DOI] [PubMed] [Google Scholar]
- 92.Buljubasic N, Rusch NJ, Marijic J, Kampine JP, Bosnjak ZJ. Effects of halothane and isoflurane on calcium and potassium channel currents in canine coronary arterial cells. Anesthesiology. 1992;76(6):990–8. [DOI] [PubMed] [Google Scholar]
- 93.Larsen M, Valo E, Berg-Johnsen J, Langmoen I. Isoflurane reduces synaptic glutamate release without changing cytosolic free calcium in isolated nerve terminals. Eur J Anaesthesiol. 1998;15(2):224–9. [PubMed] [Google Scholar]
- 94.Matchett GA, Allard MW, Martin RD, Zhang JH. Neuroprotective effect of volatile anesthetic agents: molecular mechanisms. Neurol Res. 2009;31(2):128–34. [DOI] [PubMed] [Google Scholar]
- 95.Altay O, Suzuki H, Hasegawa YU, Ostrowski RP, Tang J, Zhang JH. Isoflurane on brain inflammation. Neurobiol Dis. 2014;62:365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bar-Klein G, Klee R, Brandt C, et al. Isoflurane prevents acquired epilepsy in rat models of temporal lobe epilepsy. Ann Neurol. 2016;80(6):896–908. [DOI] [PubMed] [Google Scholar]
- 97.Chi OZ, Mellender SJ, Kiss GK, Liu X, Weiss HR. Blood-brain barrier disruption was less under isoflurane than pentobarbital anesthesia via a PI3K/Akt pathway in early cerebral ischemia. Brain Res Bull. 2017;131:1–6. [DOI] [PubMed] [Google Scholar]
- 98.Liu Y-C, Lee Y-D, Wang H-L, et al. Anesthesia-induced hypothermia attenuates early-phase blood-brain barrier disruption but not infarct volume following cerebral ischemia. PLoS ONE. 2017;12(1):e0170682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bezerra FJ, Brown M, Alarcon W, et al. Rate and extent of leakage of a magnetic resonance contrast agent tend to be lower under isoflurane anesthesia in comparison to halothane in a rat model of embolic stroke. Neurol Res. 2014;36(9):847–50. [DOI] [PubMed] [Google Scholar]
- 100.Altay O, Suzuki H, Hasegawa YU, et al. Isoflurane attenuates blood-brain barrier disruption in ipsilateral hemisphere after subarachnoid hemorrhage in mice. Stroke. 2012;43(9):2513–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Emsley H, Smith C, Georgiou R, et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76(10):1366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Helmy A, Guilfoyle MR, Carpenter KLH, Pickard JD, Menon DK, Hutchinson PJ. Recombinant human interleukin-1 receptor antagonist in severe traumatic brain injury: a phase II randomized control trial. J Cereb Blood Flow Metab. 2014;34(5):845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vezzani A, Balosso S, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. 2019;15:457–72. [DOI] [PubMed] [Google Scholar]
- 105.Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflammation. 2018;15(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Paudel YN, Shaikh MF, Shah S, Kumari Y, Othman I. Role of inflammation in epilepsy and neurobehavioral comorbidities: implication for therapy. Eur J Pharmacol. 2018;837:145–55. [DOI] [PubMed] [Google Scholar]
- 107.van Vliet EA, Aronica E, Vezzani A, Ravizza T. Review: Neuroinflammatory pathways as treatment targets and biomarker candidates in epilepsy: emerging evidence from preclinical and clinical studies. Neuropathol Appl Neurobiol. 2018;44(1):91–111. [DOI] [PubMed] [Google Scholar]
- 108.Vezzani A, Fujinami RS, White HS, et al. Infections, inflammation and epilepsy. Acta Neuropathol. 2016;131(2):211–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kenney-Jung DL, Vezzani A, Kahoud RJ, et al. Febrile in-fection-related epilepsy syndrome treated with anakinra. Ann Neurol. 2016;80(6):939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jyonouchi H, Geng L. Intractable epilepsy (IE) and responses to anakinra, a human recombinant il-1 receptor agonist (IL-1ra): case reports. J Clin Cell Immunol. 2016;7(5):456–60. [Google Scholar]
- 111.DeSena AD, Do T, Schulert GS. Systemic autoinflammation with intractable epilepsy managed with interleukin-1 blockade. J Neuroinflammation. 2018;15(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dilena R, Mauri E, Aronica E, et al. Therapeutic effect of anakinra in the relapsing chronic phase of febrile infection–related epilepsy syndrome. Epilepsia Open. 2019;4(2):344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aronica E, Crino PB. Inflammation in epilepsy: clinical observations. Epilepsia. 2011;52(Suppl 3):26–32. [DOI] [PubMed] [Google Scholar]
- 114.Vezzani A, Aronica E, Mazarati A, Pittman QJ. Epilepsy and brain inflammation. Exp Neurol. 2013;244:11–21. [DOI] [PubMed] [Google Scholar]
- 115.Ravizza T, Balosso S, Vezzani A. Inflammation and prevention of epileptogenesis. Neurosci Lett. 2011;497(3):223–30. [DOI] [PubMed] [Google Scholar]
- 116.Akin D, Ravizza T, Maroso M, et al. IL-1β is induced in reactive astrocytes in the somatosensory cortex of rats with genetic absence epilepsy at the onset of spike-and-wave discharges, and contributes to their occurrence. Neurobiol Dis. 2011;44(3):259–69. [DOI] [PubMed] [Google Scholar]
- 117.Kovács Z, Czurkó A, Kékesi KA, Juhász G. Intracerebroventricularly administered lipopolysaccharide enhances spike-wave discharges in freely moving WAG/Rij rats. Brain Res Bull. 2011;85(6):410–6. [DOI] [PubMed] [Google Scholar]
- 118.Okuneva O, Li Z, Körber I, et al. Brain inflammation is accompanied by peripheral inflammation in Cstb −/− mice, a model for progressive myoclonus epilepsy. J Neuroinflammation. 2016;13(1):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dube CM, Ravizza T, Hamamura M, et al. Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J Neurosci. 2010;30(22):7484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Terrone G, Balosso S, Pauletti A, Ravizza T, Vezzani A. Inflammation and reactive oxygen species as disease modifiers in epilepsy. Neuropharmacology. 2019. 10.1016/j.neuropharm.2019.107742 [DOI] [PubMed] [Google Scholar]
- 121.Iori V, Iyer AM, Ravizza T, et al. Blockade of the IL-1R1/TLR4 pathway mediates disease-modification therapeutic effects in a model of acquired epilepsy. Neurobiol Dis. 2017;99:12–23. [DOI] [PubMed] [Google Scholar]
- 122.Vezzani A, Maroso M, Balosso S, Sanchez M-A, Bartfai T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun. 2011;25(7):1281–9. [DOI] [PubMed] [Google Scholar]
- 123.Mazarati AM, Lewis ML, Pittman QJ. Neurobehavioral comorbidities of epilepsy: role of inflammation. Epilepsia. 2017;58(Suppl 3):48–56. [DOI] [PubMed] [Google Scholar]
- 124.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–61. [DOI] [PubMed] [Google Scholar]
- 125.Kwon YS, Pineda E, Auvin S, Shin D, Mazarati A, Sankar R. Neuroprotective and antiepileptogenic effects of combination of anti-inflammatory drugs in the immature brain. J Neuroinflammation. 2013;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Noe FM, Polascheck N, Frigerio F, et al. Pharmacological blockade of IL-1β/IL-1 receptor type 1 axis during epileptogenesis provides neuroprotection in two rat models of temporal lobe epilepsy. Neurobiol Dis. 2013;59:183–93. [DOI] [PubMed] [Google Scholar]
- 127.Pauletti A, Terrone G, Shekh-Ahmad T, et al. Targeting oxidative stress improves disease outcomes in a rat model of acquired epilepsy. Brain. 2019;142(7):e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Auvin S, Shin D, Mazarati A, Sankar R. Inflammation induced by LPS enhances epileptogenesis in immature rat and may be partially reversed by IL1RA. Epilepsia. 2010;51(Suppl 3):34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sa M, Singh R, Pujar S, et al. Centromedian thalamic nuclei deep brain stimulation and anakinra treatment for FIRES—two different outcomes. Eur J Paediatr Neurol. 2019;23(5):749–54. [DOI] [PubMed] [Google Scholar]
- 130.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxininteracting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–40. [DOI] [PubMed] [Google Scholar]
- 131.Rubartelli A, Gattorno M, Netea MG, Dinarello CA. Interplay between redox status and inflammasome activation. Trends Immunol. 2011;32:559–66. [DOI] [PubMed] [Google Scholar]
- 132.Lu B, Wang H, Andersson U, Tracey KJ. Regulation of HMGB1 release by inflammasomes. Protein Cell. 2013;4(3):163–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L. The role of oxidative stress during inflammatory processes. Biol Chem. 2014;395(2):203–30. [DOI] [PubMed] [Google Scholar]
- 134.Rowley S, Patel M. Mitochondrial involvement of oxidative stress in temporal lobe epilepsy. Free Radic Biol Med. 2013;62:121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pearson JN, Rowley S, Liang L-P, White AM, Day BJ, Patel M. Reactive oxygen species mediate cognitive deficits in experimental temporal lobe epilepsy. Neurobiol Dis. 2015;82:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Puttachary S, Sharma S, Verma S, et al. 1400W, a highly selective inducible nitric oxide synthase inhibitor is a potential disease modifier in the rat kainate model of temporal lobe epilepsy. Neurobiol Dis. 2016;93:184–200. [DOI] [PubMed] [Google Scholar]
- 137.Shekh-Ahmad T, Eckel R, Dayalan Naidu S, et al. KEAP1 inhibition is neuroprotective and suppresses the development of epilepsy. Brain. 2018;141(5):1390–403. [DOI] [PubMed] [Google Scholar]
- 138.Shekh-Ahmad T, Lieb A, Kovac S, et al. Combination antioxidant therapy prevents epileptogenesis and modifies chronic epilepsy. Redox Biol. 2019;26:101278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Silva LFA, Hoffmann MS, Rambo LM, et al. The involvement of Na+, K+-ATPase activity and free radical generation in the susceptibility to pentylenetetrazol-induced seizures after experimental traumatic brain injury. J Neurol Sci. 2011;308(1–2):35–40. [DOI] [PubMed] [Google Scholar]
- 140.Lehtinen MK, Tegelberg S, Schipper H, et al. Cystatin B deficiency sensitizes neurons to oxidative stress in progressive myoclonus epilepsy, EPM1. J Neurosci. 2009;29(18):5910–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guo J, Guo J, Li J, et al. Statin treatment reduces the risk of poststroke seizures. Neurology. 2015;85(8):701–7. [DOI] [PubMed] [Google Scholar]
- 142.Etminan M, Samii A, Brophy JM. Statin use and risk of epilepsy: a nested case-control study. Neurology. 2010;75(17):1496–500. [DOI] [PubMed] [Google Scholar]
- 143.Pugh MJV, Knoefel JE, Mortensen EM, Amuan ME, Berlowitz DR, Van Cott AC. New-onset epilepsy risk factors in older veterans. J Am Geriatr Soc. 2009;57(2):237–42. [DOI] [PubMed] [Google Scholar]
- 144.Funck VR, de Oliveira CV, Pereira LM, et al. Differential effects of atorvastatin treatment and withdrawal on pentylenetetrazol-induced seizures. Epilepsia. 2011;52:2094–104. [DOI] [PubMed] [Google Scholar]
- 145.Osterweil E, Chuang S-C, Chubykin A, et al. Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model of fragile X syndrome. Neuron. 2013;77(2):243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Scicchitano F, Constanti A, Citraro R, De Sarro G, Russo E Statins and epilepsy: preclinical studies, clinical trials and statin-anticonvulsant drug interactions. Curr Drug Targets. 2015;16(7):747–56. [DOI] [PubMed] [Google Scholar]
- 147.Clarke RM, Lyons A, O’Connell F, et al. A pivotal role for interleukin-4 in atorvastatin-associated neuroprotection in rat brain. J Biol Chem. 2008;283:1808–17. [DOI] [PubMed] [Google Scholar]
- 148.Ifergan I, Wosik K, Cayrol R, et al. Statins reduce human blood-brain barrier permeability and restrict leukocyte migration: relevance to multiple sclerosis. Ann Neurol. 2006;60:45–55. [DOI] [PubMed] [Google Scholar]
- 149.Oliveira CVD, Zorzi VN, Fighera MR, Royes LFF, Furian AF, Oliveira MS. Subtle improvement of seizure susceptibility by atorvastatin treatment during epileptogenesis. Pharmacol Rep. 2018;70(2):364–71. [DOI] [PubMed] [Google Scholar]
- 150.Oliveira CVD, Grigoletto J, Canzian JM, et al. Effect of atorvastatin on behavioral alterations and neuroinflammation during epileptogenesis. Epilepsy Behav. 2018;78:109–17. [DOI] [PubMed] [Google Scholar]
- 151.Sehar N, Agarwal NB, Vohora D, Raisuddin S. Atorvastatin prevents development of kindling by modulating hippocampal levels of dopamine, glutamate, and GABA in mice. Epilepsy Behav. 2015;42:48–53. [DOI] [PubMed] [Google Scholar]
- 152.Citraro R, Chimirri S, Aiello R, et al. Protective effects of some statins on epileptogenesis and depressive-like behavior in WAG/Rij rats, a genetic animal model of absence epilepsy. Epilepsia. 2014;55(8):1284–91. [DOI] [PubMed] [Google Scholar]
- 153.van Vliet EA, Holtman L, Aronica E, Schmitz LJM, Wadman WJ, Gorter JA. Atorvastatin treatment during epileptogenesis in a rat model for temporal lobe epilepsy. Epilepsia. 2011;52:1319–30. [DOI] [PubMed] [Google Scholar]
- 154.Xie C, Sun J, Qiao W, et al. Administration of simvastatin after kainic acid-induced status epilepticus restrains chronic temporal lobe epilepsy. PLoS ONE. 2011;6:e24966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gouveia T, Scorza FA, Iha HA, et al. Lovastatin decreases the synthesis of inflammatory mediators during epileptogenesis in the hippocampus of rats submitted to pilocarpine-induced epilepsy. Epilepsy Behav. 2014;36:68–73. [DOI] [PubMed] [Google Scholar]
- 156.Furness DN, Dehnes Y, Akhtar AQ, et al. A quantitative assessment of glutamate uptake into hippocampal synaptic terminals and astrocytes: new insights into a neuronal role for excitatory amino acid transporter 2 (EAAT2). Neuroscience. 2008;157(1):80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Melone M, Bellesi M, Conti F. Synaptic localization of GLT-1a in the rat somatic sensory cortex. Glia. 2009;57(1):108–17. [DOI] [PubMed] [Google Scholar]
- 158.Walls AB, Waagepetersen HS, Bak LK, Schousboe A, Sonnewald U. The glutamine–glutamate/GABA cycle: function, regional differences in glutamate and GABA production and effects of interference with GABA metabolism. Neurochem Res. 2014;40(2):402–9. [DOI] [PubMed] [Google Scholar]
- 159.Danbolt NC. Glutamate uptake. Prog Neurogibol. 2001;65(1):1–105. [DOI] [PubMed] [Google Scholar]
- 160.Lehre KP, Danbolt NC. The number of glutamate transport subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18(21):8751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tanaka K, Watase K, Manabe T, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276(5319):1699–702. [DOI] [PubMed] [Google Scholar]
- 162.Kumar RG, Breslin KB, Ritter AC, Conley YP, Wagner AK. Variability with astroglial glutamate transport genetics is associated with increased risk for post-traumatic seizures. J Neurotrauma. 2018;36(2):230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Raghavendra Rao VL, Başkaya MK, Doğan A, Rothstein JD, Dempsey RJ. Traumatic brain injury down-regulates glial glutamate transporter (GLT-1 and GLAST) proteins in rat brain. J Neurochem. 1998;70(5):2020–7. [DOI] [PubMed] [Google Scholar]
- 164.Goodrich GS, Kabakov AY, Hameed MQ, Dhamne SC, Rosenberg PA, Rotenberg A. Ceftriaxone treatment after traumatic brain injury restores expression of the glutamate transporter, GLT-1, reduces regional gliosis, and reduces post-traumatic seizures in the rat. J Neurotrauma. 2013;30(16):1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dorsett CR, McGuire JL, Niedzielko TL, et al. Traumatic brain injury induces alterations in cortical glutamate uptake without a reduction in glutamate transporter-1 protein expression. J Neurotrauma. 2017;34(1):220–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hameed MQ, Hsieh T-H, Morales-Quezada L, et al. Ceftriaxone treatment preserves cortical inhibitory interneuron function via transient salvage of GLT-1 in a rat traumatic brain injury model. Cereb Cortex. 2019;29(11):4506–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nakagawa T, Otsubo Y, Yatani Y, Shirakawa H, Kaneko S. Mechanisms of substrate transport-induced clustering of a glial glutamate transporter GLT-1 in astroglial-neuronal cultures. Eur J Neurosci. 2008;28(9):1719–30. [DOI] [PubMed] [Google Scholar]
- 168.Boston-Howes W, Gibb SL, Williams EO, Pasinelli P, Brown RH, Trotti D. Caspase-3 cleaves and inactivates the glutamate transporter EAAT2. J Biol Chem. 2006;281(20):14076–84. [DOI] [PubMed] [Google Scholar]
- 169.Rao VLR, Dogan A, Todd KG, et al. Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J Neurosci. 2001;21(6):1876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Maragakis NJ, Rothstein JD. Glutamate transporters: animal models to neurologic disease. Neurobiol Dis. 2004;15(3):461–73. [DOI] [PubMed] [Google Scholar]
- 171.Yamamoto T, Rossi S, Stiefel M, et al. CSF and ECF glutamate concentrations in head injured patients. Acta Neurochir Suppl. 1999;75:17–9. [DOI] [PubMed] [Google Scholar]
- 172.Zhuang Z, Shen Z, Chen Y, et al. Mapping the changes of glutamate using glutamate chemical exchange saturation transfer (GluCEST) technique in a traumatic brain injury model: a longitudinal pilot study. ACS Chem Neurosci. 2019;10(1):649–57. [DOI] [PubMed] [Google Scholar]
- 173.Murphy TH, Schnaar RL, Coyle JT. Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cystine uptake. FASEB J. 1990;4(6):1624–33. [PubMed] [Google Scholar]
- 174.Bridges RJ, Natale NR, Patel SA. System Xc− cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol. 2012;165(1):20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lewerenz J, Klein M, Methner A. Cooperative action of glutamate transporters and cystine/glutamate antiporter system Xc- protects from oxidative glutamate toxicity. J Neurochem. 2006;98(3):916–25. [DOI] [PubMed] [Google Scholar]
- 176.Tan S, Schubert D, Maher P. Oxytosis: a novel form of pro-grammed cell death. Curr Top Med Chem. 2001;1(6):497–506. [DOI] [PubMed] [Google Scholar]
- 177.Rudy B, Fishell G, Lee SH, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71(1):45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kelsom C, Lu W. Development and specification of GABAergic cortical interneurons. Cell Biosci. 2013;3(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cammarota M, Losi G, Chiavegato A, Zonta M, Carmignoto G. Fast spiking interneuron control of seizure propagation in a cortical slice model of focal epilepsy. J Physiol. 2013;591(4):807–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hsieh T-H, Lee HHC, Hameed MQ, Pascual-Leone A, Hensch TK, Rotenberg A. Trajectory of parvalbumin cell impairment and loss of cortical inhibition in traumatic brain injury. Cereb Cortex. 2017;27(12):5509–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cui C, Cui Y, Gao J, et al. Neuroprotective effect of ceftriaxone in a rat model of traumatic brain injury. Neurol Sci. 2014;35(5):695–700. [DOI] [PubMed] [Google Scholar]
- 183.Jagadapillai R, Mellen NM, Sachleben LR, Gozal E. Ceftriaxone preserves glutamate transporters and prevents intermittent hypoxia-induced vulnerability to brain excitotoxic injury. PLoS ONE. 2014;9(7):e100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zumkehr J, Rodriguez-Ortiz CJ, Cheng D, et al. Ceftriaxone ameliorates tau pathology and cognitive decline via restoration of glial glutamate transporter in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2015;36(7):2260–71. [DOI] [PubMed] [Google Scholar]
- 185.Krzyżanowska W, Pomierny B, Bystrowska B, et al. Ceftriaxone- and N-acetylcysteine-induced brain tolerance to ischemia: influence on glutamate levels in focal cerebral ischemia. PLoS ONE. 2017;12(10):e0186243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tikhonova MA, Ho S-C, Akopyan AA, et al. Neuroprotective effects of ceftriaxone treatment on cognitive and neuronal deficits in a rat model of accelerated senescence. Behav Brain Res. 2017;330:8–16. [DOI] [PubMed] [Google Scholar]
- 187.Rothstein JD, Patel S, Regan MR, et al. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–7. [DOI] [PubMed] [Google Scholar]
- 188.Lee S-G, Su Z-Z, Emdad L, et al. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. 2008;283(19):13116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lewerenz J, Albrecht P, Tien MLT,, et al. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. J Neurochem. 2009;111(2):332–43. [DOI] [PubMed] [Google Scholar]
- 190.Cui X, Li LI, Hu Y-Y, Ren S, Zhang M, Li W-B. Sulbactam plays neuronal protective effect against brain ischemia via upregulating GLT1 in rats. Mol Neurobiol. 2015;51(3):1322–33. [DOI] [PubMed] [Google Scholar]
- 191.Goodwani S, Rao P, Bell RL, Sari Y. Amoxicillin and amoxicillin/clavulanate reduce ethanol intake and increase GLT-1 expression as well as AKT phosphorylation in mesocorticolimbic regions. Brain Res. 2015;1622:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Guan T, Qian Y, Tang X, et al. Maslinic acid, a natural inhibitor of glycogen phosphorylase, reduces cerebral ischemic injury in hyperglycemic rats by GLT-1 up-regulation. J Neurosci Res. 2011;89(11):1829–39. [DOI] [PubMed] [Google Scholar]
- 193.Qian Y, Guan T, Tang X, et al. Astrocytic glutamate transporter-dependent neuroprotection against glutamate toxicity: an in vitro study of maslinic acid. Eur J Pharmacol. 2011;651(1–3):59–65. [DOI] [PubMed] [Google Scholar]
- 194.Eroglu Ç, Allen NJ, Susman MW, et al. Gabapentin receptor alph-a2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dolphin AC. Calcium channel alpha2-gamma subunits in epilepsy and as targets for antiepileptic drugs. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, eds. Jasper’s Basic Mechanisms of the Epilepsies. 4th ed. Bethesda, MD: National Center for Biotechnology Information; 2012. [PubMed] [Google Scholar]
- 196.Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel α2-δ (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res. 2007;73:137–50. [DOI] [PubMed] [Google Scholar]
- 197.Stahl SM, Porreca F, Taylor CP, Cheung R, Thorpe AJ, Clair A. The diverse therapeutic actions of pregabalin: is a single mechanism responsible for several pharmacological activities? Trends Pharmacol Sci. 2013;34:332–9. [DOI] [PubMed] [Google Scholar]
- 198.Morrell MJ, McLean MJ, Willmore LJ, et al. Efficacy of gabapentin as adjunctive therapy in a large, multicenter study. Seizure. 2000;9:241–8. [DOI] [PubMed] [Google Scholar]
- 199.Li H, Graber KD, Jin S, McDonald W, Barres BA, Prince DA. Gabapentin decreases epileptiform discharges in a chronic model of neocortical trauma. Neurobiol Dis. 2012;48(3): 429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Faria LC, Gu F, Parada I, Barres B, Luo ZD, Prince DA. Epileptiform activity and behavioral arrests in mice overexpressing the calcium channel subunit α2δ−1. Neurobiol Dis. 2017;102:70–80. [DOI] [PubMed] [Google Scholar]
- 201.Lau LA, Noubary F, Wang D, Dulla CG. α2δ−1 signaling drives cell death, synaptogenesis, circuit reorganization, and gabapentin-mediated neuroprotection in a model of insult-induced cortical malformation. eNeuro. 2017;4(5):ENEURO.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Andresen L, Hampton D, Taylor-Weiner A, et al. Gabapentin attenuates hyperexcitability in the freeze-lesion model of developmental cortical malformation. Neurobiol Dis. 2014;71: 305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen J, Li L, Chen S-R, et al. The α2δ−1-NMDA receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep. 2018;22:2307–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cilio MR, Bolanos AR, Liu Z, et al. Anticonvulsant action and long-term effects of gabapentin in the immature brain. Neuropharmacology. 2001;40:139–47. [DOI] [PubMed] [Google Scholar]
- 205.André V, Rigoulot M-A, Koning E, Ferrandon A, Nehlig A. Long-term pregabalin treatment protects basal cortices and delays the occurrence of spontaneous seizures in the lithium-pilocarpine model in the rat. Epilepsia. 2003;44:893–903. [DOI] [PubMed] [Google Scholar]
- 206.Rossi AR, Angelo MF, Villarreal A, Lukin J, Ramos AJ. Gabapentin administration reduces reactive gliosis and neurodegeneration after pilocarpine-induced status epilepticus. PLoS ONE. 2013;8:e78516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rossi A, Murta V, Auzmendi J, Ramos A. Early gabapentin treatment during the latency period increases convulsive threshold, reduces microglial activation and macrophage infiltration in the lithium-pilocarpine model of epilepsy. Pharmaceuticals. 2017;10(4):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Traa BS, Mulholland JD, Kadam SD, Johnston MV, Comi AM. Gabapentin neuroprotection and seizure suppression in immature mouse brain ischemia. Pediatr Res. 2008;64:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Takahashi DK, Jin S, Prince DA. Gabapentin prevents progressive increases in excitatory connectivity and epileptogenesis following neocortical trauma. Cereb Cortex. 2018;28:2725–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kim TY, Niimi K, Takahashi E. Analysis of the protective effects of the α 2/δ subunit of voltage-gated Ca 2+ channels in brain injury. Brain Res. 2017;1655:138–44. [DOI] [PubMed] [Google Scholar]
- 211.Faught E Topiramate in the treatment of partial and generalized epilepsy. Neuropsychiatr Dis Treat. 2007;3:811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553–64. [DOI] [PubMed] [Google Scholar]
- 213.DeLorenzo RJ, Morris A, Blair R, Wallace M, Razvi B. Topiramate is both neuroprotective and antiepileptogenic in the pilocarpine model of status epilepticus. Epilepsia. 2002;43(Suppl. 7):15. [Google Scholar]
- 214.Schidlitzki A, Bascuñana P, Srivastava PK, et al. Proof-of-concept that network pharmacology is effective to modify development of acquired temporal lobe epilepsy. Neurobiol Dis. 2020;134:104664. 10.1016/j.nbd.2019 [DOI] [PubMed] [Google Scholar]
- 215.Suchomelova L, Baldwin RA, Kubova H, Thompson KW, Sankar R, Wasterlain CG. Treatment of experimental status epilepticus in immature rats: dissociation between anticonvulsant and antiepileptogenic effects. Pediatr Res. 2006;59:237–43. [DOI] [PubMed] [Google Scholar]
- 216.Cha BH, Silveira DC, Liu X, Hu Y, Holmes GL. Effect of topiramate following recurrent and prolonged seizures during early development. Epilepsy Res. 2002;51:217–32. [DOI] [PubMed] [Google Scholar]
- 217.Frisch C, Kudin AP, Elger CE, Kunz WS, Helmstaedter C. Amelioration of water maze performance deficits by topiramate applied during pilocarpine-induced status epilepticus is negatively dose-dependent. Epilepsy Res. 2007;73:173–80. [DOI] [PubMed] [Google Scholar]
- 218.Amano K, Hamada K, Yagi K, Seino M. Antiepileptic effects of topiramate on amygdaloid kindling in rats. Epilepsy Res. 1998;31:123–8. [DOI] [PubMed] [Google Scholar]
- 219.Mazarati A, Shin D, Auvin S, Sankar R. Age-dependent effects of topiramate on the acquisition and the retention of rapid kindling. Epilepsia. 2007;48:765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sankar R, Auvin S, Kwon YS, Pineda E, Shin D, Mazarati A. Evaluation of development-specific targets for antiepileptogenic therapy using rapid kindling. Epilepsia. 2010;51(Suppl 3):39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hoover RC, Motta M, Davis J, et al. Differential effects of the anticonvulsant topiramate on neurobehavioral and histological outcomes following traumatic brain injury in rats. J Neurotrauma. 2004;21:501–12. [DOI] [PubMed] [Google Scholar]
- 222.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wong M Mammalian target of rapamycin (mTOR) pathways in neurological diseases. Biomed J. 2013;36:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Crino PB. The mTOR signalling cascade: paving new roads to cure neurological disease. Nat Rev Neurol. 2016;12:379–92. [DOI] [PubMed] [Google Scholar]
- 225.Switon K, Kotulska K, Janusz-Kaminska A, Zmorzynska J, Jaworski J. Molecular neurobiology of mTOR. Neuroscience. 2016;341:112–53. [DOI] [PubMed] [Google Scholar]
- 226.Wong M Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: from tuberous sclerosis to common acquired epilepsies. Epilepsia. 2010;51:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zeng L-H, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rensing N, Han L, Wong M. Intermittent dosing of rapamycin maintains antiepileptogenic effects in a mouse model of tuberous sclerosis complex. Epilepsia. 2015;56(7):1088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Before VA, Unfolds E. Finding the epileptogenesis switch. Nat Med. 2012;18:1626–7. [DOI] [PubMed] [Google Scholar]
- 230.Galanopoulou AS, Gorter JA, Cepeda C. Finding a better drug for epilepsy: the mTOR pathway as an antiepileptogenic target. Epilepsia. 2012;53(7):1119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Huang X, Zhang H, Yang J, et al. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40:193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Abs E, Goorden SMI, Schreiber J, et al. TORC1-dependent epilepsy caused by acute biallelic Tsc1 deletion in adult mice. Ann Neurol. 2013;74:569–79. [DOI] [PubMed] [Google Scholar]
- 233.Brandt C, Hillmann P, Noack A, et al. The novel, catalytic mTORC1/2 inhibitor PQR620 and the PI3K/mTORC1/2 inhibitor PQR530 effectively cross the blood-brain barrier and increase seizure threshold in a mouse model of chronic epilepsy. Neuropharmacology. 2018;140:107–20. [DOI] [PubMed] [Google Scholar]
- 234.Shima A, Nitta N, Suzuki F, Laharie A-M, Nozaki K, Depaulis A. Activation of mTOR signaling pathway is secondary to neuronal excitability in a mouse model of mesio-temporal lobe epilepsy. Eur J Neurosci. 2015;41:976–88. [DOI] [PubMed] [Google Scholar]
- 235.Drion CM, Borm LE, Kooijman L, et al. Effects of rapamycin and curcumin treatment on the development of epilepsy after electrically induced status epilepticus in rats. Epilepsia. 2016;57(5):688–97. [DOI] [PubMed] [Google Scholar]
- 236.Guo D, Zeng L, Brody DL, Wong M. Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS ONE. 2013;8(5):e64078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Luszczki JJ, Czuczwar SJ. Isobolographic characterization of interactions between vigabatrin and tiagabine in two experimental models of epilepsy. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):529–38. [DOI] [PubMed] [Google Scholar]
- 238.Haugvicová R, Kubová H, Mareš P. Does vigabatrin possess an anticonvulsant action against pentylenetetrazol-induced seizures in developing rats? Physiol Res. 2002;51(4):363–70. [PubMed] [Google Scholar]
- 239.Shin C, Rigsbee LC, McNamara JO. Anti-seizure and antiepileptogenic effect of γ-vinyl γ-aminobutyric acid in amygdaloid kindling. Brain Res. 1986;398(2):370–4. [DOI] [PubMed] [Google Scholar]
- 240.Schwabe K, Ebert U, Löscher W. Bilateral microinjections of vigabatrin in the central piriform cortex retard amygdala kindling in rats. Neuroscience. 2004;129(2):425–9. [DOI] [PubMed] [Google Scholar]
- 241.Halonen T, Nissinen J, Pitkänen A. Chronic elevation of brain GABA levels beginning two days after status epilepticus does not prevent epileptogenesis in rats. Neuropharmacology. 2001;40(4):536–50. [DOI] [PubMed] [Google Scholar]
- 242.Halonen T, Miettinen R, Toppinen A, Tuunanen J, Kotti T, Riekkinen PJ. Vigabatrin protects against kainic acid-induced neuronal damage in the rat hippocampus. Neurosci Lett. 1995;195(1):13–6. [DOI] [PubMed] [Google Scholar]
- 243.Pitkänen A, Nissinen J, Jolkkonen E, Tuunanen J, Halonen T. Effects of vigabatrin treatment on status epilepticus-induced neuronal damage and mossy fiber sprouting in the rat hippocampus. Epilepsy Res. 1999;33(1):67–85. [DOI] [PubMed] [Google Scholar]
- 244.André V, Ferrandon A, Marescaux C, Nehlig A. Vigabatrin protects against hippocampal damage but is not antiepileptogenic in the lithium-pilocarpine model of temporal lobe epilepsy. Epilepsy Res. 2001;47(1–2):99–117. [DOI] [PubMed] [Google Scholar]
- 245.Russo E, Citraro R, Scicchitano F, et al. Vigabatrin has antiepileptogenic and antidepressant effects in an animal model of epilepsy and depression comorbidity. Behav Brain Res. 2011;225(1):373–6. [DOI] [PubMed] [Google Scholar]
- 246.Hancock E, Osborne JP. Vigabatrin in the treatment of infantile spasms in tuberous sclerosis: literature review. J Child Neurol. 1999;14(2):71–4. [DOI] [PubMed] [Google Scholar]
- 247.Zhang BO, McDaniel SS, Rensing NR, Wong M. Vigabatrin inhibits seizures and mTOR pathway activation in a mouse model of tuberous sclerosis complex. PLoS ONE. 2013;8(2):e57445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Słowińska M, Jóźwiak S, Peron A, et al. Early diagnosis of tuberous sclerosis complex: a race against time. How to make the diagnosis before seizures? Orphanet J Rare Dis. 2018;13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Jóźwiak S, Kotulska K, Domańska-Pakieła D, et al. Antiepileptic treatment before the onset of seizures reduces epilepsy severity and risk of mental retardation in infants with tuberous sclerosis complex. Eur J Paediatr Neurol. 2011;15:424–31. [DOI] [PubMed] [Google Scholar]
- 250.Jozwiak S, Słowińska M, Borkowska J, et al. Preventive antiepileptic treatment in tuberous sclerosis complex: long-term, prospective trial. Pediatr Neurol. 2019;101:18–25. [DOI] [PubMed] [Google Scholar]
- 251.Jozwiak S. Long-term, prospective study evaluating clinical and molecular biomarkers of epileptogenesis in a genetic model of epilepsy—tuberous sclerosis complex (EPISTOP). 2014. [cited 2020 Feb 18]. Available at: https://clinicaltrials.gov/ct2/show/NCT02098759?term=NCT02098759&rank=1.
- 252.Bebin M Preventing epilepsy using vigabatrin in infants with tuberous sclerosis complex. 2016. [cited 2020 Feb 18]. Available at: https://clinicaltrials.gov/ct2/show/NCT02849457?term=NCT02849457&rank=1.
- 253.Benes J, Parada A, Figueiredo AA, et al. Anticonvulsant and sodium channel-blocking properties of novel 10,11-dihydro-5H-dibenz[b, f]azepine-5-carboxamide derivatives. J Med Chem. 1999;42(14):2582–7. [DOI] [PubMed] [Google Scholar]
- 254.Doeser A, Dickhof G, Reitze M, et al. Targeting pharmacoresistant epilepsy and epileptogenesis with a dual-purpose antiepileptic drug. Brain. 2015;138(Pt 2):371–8. [DOI] [PubMed] [Google Scholar]
- 255.Becker AJ, Pitsch J, Sochivko D, et al. Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J Neurosci. 2008;28(49):13341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Su H, Sochivko D, Becker A, et al. Upregulation of a T-type Ca2+ channel causes a long-lasting modification of neuronal firing mode after status epilepticus. J Neurosci. 2002;22(9):3645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Gilad R, Boaz M, Dabby R, Sadeh M, Lampl Y. Are post intracerebral hemorrhage seizures prevented by anti-epileptic treatment? Epilepsy Res. 2011;95(3):227–31. [DOI] [PubMed] [Google Scholar]
- 258.Sykes L, Wood E, Kwan J. Antiepileptic drugs for the primary and secondary prevention of seizures after stroke. Cochrane Database Syst Rev. 2014;1:CD005398. [DOI] [PubMed] [Google Scholar]
- 259.Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010;33(2):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Brunkhorst R, Vutukuri R, Pfeilschifter W. Fingolimod for the treatment of neurological diseases—state of play and future perspectives. Front Cell Neurosci. 2014;8:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gao F, Liu Y, Li X, Wang Y, Wei D, Jiang W. Fingolimod (FTY720) inhibits neuroinflammation and attenuates spontaneous convulsions in lithium-pilocarpine induced status epilepticus in rat model. Pharmacol Biochem Behav. 2012;103(2): 187–96. [DOI] [PubMed] [Google Scholar]
- 262.Pitsch J, Kuehn JC, Gnatkovsky V, et al. Anti-epileptogenic and anti-convulsive effects of fingolimod in experimental temporal lobe epilepsy. Mol Neurobiol. 2019;56(3):1825–40. [DOI] [PubMed] [Google Scholar]
- 263.Leo A, Citraro R, Amodio N, et al. Fingolimod exerts only temporary antiepileptogenic effects but longer-lasting positive effects on behavior in the WAG/Rij rat absence epilepsy model. Neurotherapeutics. 2017;14(4):1134–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Gao F, Gao Y, Meng F, Yang C, Fu J, Li Y. The sphingosine 1-phosphate analogue FTY720 alleviates seizure-induced overexpression of P-glycoprotein in rat hippocampus. Basic Clin Pharmacol Toxicol. 2018;123(1):14–20. [DOI] [PubMed] [Google Scholar]
- 265.Wu H, Wang X, Gao J, et al. Fingolimod (FTY720) attenuates social deficits, learning and memory impairments, neuronal loss and neuroinflammation in the rat model of autism. Life Sci. 2017;173:43–54. [DOI] [PubMed] [Google Scholar]
- 266.Gao C, Qian YU, Huang J, et al. A three-day consecutive fingolimod administration improves neurological functions and modulates multiple immune responses of CCI mice. Mol Neurobiol. 2017;54(10):8348–60. [DOI] [PubMed] [Google Scholar]
- 267.Kramer MA, Cash SS. Epilepsy as a disorder of cortical network organization. Neuroscientist. 2012;18:360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Holmes MD, Tucker DM. Identifying the epileptic network. Front Neurol. 2013;4:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Khambhati AN, Davis KA, Oommen BS, et al. Dynamic network drivers of seizure generation, propagation and termination in human neocortical epilepsy. PLoS Comput Biol. 2015;11:e1004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Smith EH, Schevon CA. Toward a mechanistic understanding of epileptic networks. Curr Neurol Neurosci Rep. 2016;16:97. [DOI] [PubMed] [Google Scholar]
- 271.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–90. [DOI] [PubMed] [Google Scholar]
- 272.Ainsworth C Networking for new drugs. Nat Med. 2011;17:1166–8. [DOI] [PubMed] [Google Scholar]
- 273.Löscher W, Klitgaard H, Twyman RE, Schmidt D. New avenues for anti-epileptic drug discovery and development. Nat Rev Drug Discov. 2013;12:757–76. [DOI] [PubMed] [Google Scholar]
- 274.Boezio B, Audouze K, Ducrot P, Taboureau O. Network-based approaches in pharmacology. Mol Inform. 2017;36(10). 10.1002/minf.201700048 [DOI] [PubMed] [Google Scholar]
- 275.White HS, Löscher W. Searching for the ideal antiepileptogenic agent in experimental models: single treatment versus combinatorial treatment strategies. Neurotherapeutics. 2014;11: 373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Löscher W, Rundfeldt C, Hönack D. Low doses of NMDA receptor antagonists synergistically increase the anticonvulsant effect of the AMPA receptor antagonist NBQX in the kindling model of epilepsy. Eur J Neurosci. 1993;5:1545–50. [DOI] [PubMed] [Google Scholar]
- 277.Löscher W, Hönack D. Over-additive anticonvulsant effect of memantine and NBQX in kindled rats. Eur J Pharmacol. 1994;259:R3–5. [DOI] [PubMed] [Google Scholar]
- 278.Schidlitzki A, Twele F, Klee R, et al. A combination of NMDA and AMPA receptor antagonists retards granule cell dispersion and epileptogenesis in a model of acquired epilepsy. Sci Rep. 2017;7:12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Löscher W, Schmidt D. Epilepsy: perampanel—new promise for refractory epilepsy? Nat Rev Neurol. 2012;8:661–2. [DOI] [PubMed] [Google Scholar]
- 280.Mohammad H, Sekar S, Wei Z, Moien-Afshari F, Taghibiglou C. Perampanel but not amantadine prevents behavioral alterations and epileptogenesis in pilocarpine rat model of status epilepticus. Mol Neurobiol. 2019;56(4):2508–23. [DOI] [PubMed] [Google Scholar]
- 281.Rogawski MA, Donevan SD. AMPA receptors in epilepsy and as targets for antiepileptic drugs. Adv Neurol. 1999;79:947–63. [PubMed] [Google Scholar]
- 282.Brandt C, Nozadze M, Heuchert N, Rattka M, Loscher W. Disease-modifying effects of phenobarbital and the NKCC1 inhibitor bumetanide in the pilocarpine model of temporal lobe epilepsy. J Neurosci. 2010;30(25):8602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci. 2014;15:637–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.François J, Koning E, Ferrandon A, Nehlig A. The combination of topiramate and diazepam is partially neuroprotective in the hippocampus but not antiepileptogenic in the lithium-pilocarpine model of temporal lobe epilepsy. Epilepsy Res. 2006;72:147–63. [DOI] [PubMed] [Google Scholar]
- 285.Rigoulot MA, Koning E, Ferrandon A, Nehlig A. Neuroprotective properties of topiramate in the lithium-pilocarpine model of epilepsy. J Pharmacol Exp Ther. 2004;308:787–95. [DOI] [PubMed] [Google Scholar]
- 286.Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve RD productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9:203–14. [DOI] [PubMed] [Google Scholar]
- 287.United States Patent and Trademark Office. General information concerning patents. October 2015. [cited 2020 Feb 18]. Available at: https://www.uspto.gov/patents-getting-started/general-information-concerning-patents.
- 288.Gupta H, Kumar S, Roy SK, Gaud RS. Patent protection strategies. J Pharm Bioallied Sci. 2010;2(1):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.US Food and Drug Administration. Development & approval process | drugs. [cited 2020 Feb 18]. Available at: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/default.htm.
- 290.European Commission, Enterprise and Industry Directorate-General. Guidance on elements required to support the significant clinical benefit in comparison with existing therapies of a new therapeutic indication in order to benefit from an extended (11-year) marketing protection period. November 2007. [cited 2020 Feb 18]. Available at: https://ec.europa.eu/health//sites/health/files/files/eudralex/vol-2/c/guideline_14-11-2007_en.pdf.
- 291.Löscher W The holy grail of epilepsy prevention: preclinical approaches to antiepileptogenic treatments. Neuropharmacology. 2019. 10.1016/j.neuropharm.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 292.Vespa PM, Shrestha V, Abend N, et al. The epilepsy bioinformatics study for anti-epileptogenic therapy (EpiBioS4Rx) clinical biomarker: study design and protocol. Neurobiol Dis. 2019;123:110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and humans. J Basic Clin Pharm. 2016;7:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]