ABSTRACT
Ketone bodies have potential disease-modifying activity that represent a novel therapeutic approach for neurodegenerative diseases (NDD). The aim of this systematic review was to summarize and evaluate the evidence for the application of ketogenic therapies (dietary or exogenous ketogenic agents) for NDD and provide recommendations for future research. Eight databases were electronically searched for articles reporting on controlled trials (≥4 wk duration) that induced ketosis or elevated serum ketone concentrations in people with NDD. Of 4498 records identified, 17 articles met the inclusion criteria with a total of 979 participants including studies on mild cognitive impairment (MCI; n = 6), multiple sclerosis (n = 4), Alzheimer's disease (n = 5), Parkinson's disease (n = 1), and MCI secondary to Parkinson's disease (n = 1). Of 17 studies, 7 were randomized double-blind placebo-controlled trials. Most studies used dietary interventions (n = 9), followed by medium-chain triglycerides (n = 7) and a fasting protocol (n = 1). Generally, trials were 6 wk in duration and assessed cognition as the primary outcome. Studies were heterogeneous in type and severity of NDD, interventions used, and outcomes assessed. Overall, 3/17 studies carried a low risk of bias. Based on available evidence, exogenous ketogenic agents may be more feasible than dietary interventions in NDD from a compliance and adherence perspective; more research is required to confirm this. Recommendations for future research include improving exogenous formulations to reduce adverse effects, exploring interindividual factors affecting response-to-treatment, and establishing a “minimum required dose” for clinically meaningful improvements in disease-specific symptoms, such as cognition or motor function.
Keywords: ketones, neurodegenerative disease, cognition, neuroinflammation, systematic review
Introduction
Neurodegenerative diseases (NDD) are a major focus for research due to their prolific medical, social, and economic implications, and lack of effective therapies for their clinical management (1). Beyond neurodegeneration, several other pathophysiological commonalities exist across NDD [e.g. Alzheimer's disease (AD), Parkinson's disease (PD), multiple sclerosis (MS), amyotrophic lateral sclerosis] including protein aggregation and misfolding [e.g. amyloid-β (Aβ)], cerebral hypometabolism, neuroinflammation, excitotoxicity, oxidative stress, and mitochondrial dysfunction (2, 3). Under regular physiological conditions, the adult brain consumes ∼20% of basal metabolic energy, normally provided by the oxidation of glucose (100–120 g in 24 h) (4). Following neuronal injury, glucose uptake and utilization is significantly disrupted (cerebral hypometabolism) and is associated with mitochondrial dysfunction (5) and neuronal death (6). Deficiencies in glucose metabolism may precede clinical symptoms and act as a catalyst in pathophysiological cascades implicated in NDD (1). Therefore, therapies addressing metabolic dysfunction could be an important target for prevention and to delay disease progression.
Ketogenesis is an evolutionary survival mechanism whereby ketone bodies [acetoacetate (AcAc), β-hydroxybutyrate (BHB), and acetone (Ac)] are produced during periods of starvation, fasting, exercise, or reduced carbohydrate availability (7). In these conditions, the brain can adapt to the utilization of ketone bodies as substrates for energy production, providing ≤70% of cerebral energy requirements (8). Furthermore, they may attenuate cellular energy deficits seen in NDD by providing an alternative fuel source to glucose for the brain (9), reducing or mitigating cerebral hypometabolism, oxidative damage, and mitochondrial deficits (10). Preclinical evidence (both in vitro and in vivo) offers insight into the neuroprotective properties of ketogenic therapies (KT) (10–12), with several mechanisms relevant to NDD pathophysiology including: enhanced antioxidant mechanisms (8), increased mitochondrial respiration (13) and mitochondrial biogenesis in the hippocampus (14), increased Aβ clearance (15, 16), and protection against Aβ-induced neurotoxicity (17). Ketone bodies have also been shown to decrease proinflammatory cytokines (12, 18–20) and Aβ deposition (21), reduce glutamate toxicity (excitotoxicity) (18), and illicit beneficial downstream effects on learning and memory (22–24).
Human studies using therapeutic ketosis have predominantly assessed the ketogenic diet (KD) in adults and children with treatment-resistant refractory epilepsy, where reductions in seizure frequency and increases in seizure freedom have been reported (25–28). The classic KD consists of a 4:1 ratio of fats to protein and carbohydrates that physiologically mimics the fasting state (29). Although the classic KD is safe and generally well-tolerated, barriers to long-term adherence such as palatability issues, its restrictive nature, and side effect profile (30–32) has led to the exploration of dietary variations [e.g. modified Atkins diet; MAD (33, 34)] and the supplementation of exogenous ketogenic agents such as medium-chain triglycerides (MCTs) to promote the elevation of ketone bodies in the blood (12).
An emerging body of literature is now exploring the application of KT in NDD more broadly, however, relatively little is known of their efficacy from a clinical perspective. There is a timely need to better understand KT (dose, bioavailability, tolerability, safety, feasibility, and efficacy) as they pertain to NDD populations. As such, this systematic review aimed to evaluate the clinical evidence for the use of KT (dietary or exogenous ketogenic agents), describe their characteristics, assess their efficacy, safety, and feasibility, and provide recommendations for future research.
Methods
This systematic review was prospectively registered with PROSPERO international database of systematic reviews on 17 October, 2019 (#CRD42020156673), and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for transparent and comprehensive reporting of methodology and results (35).
Eligibility criteria
After an in-depth scoping search of existing literature to determine the breadth of scope of this review, the population, intervention, comparisons, and outcome (PICO) principles for systematic reviews were used to define the eligibility criteria (36).
Population: people with a NDD.
Intervention: ketosis; via exogenous supplementation of ketogenic agents, dietary modifications, or fasting.
Comparisons: controlled studies including randomized (parallel and crossover designs) and nonrandomized trials.
Outcome: disease-specific clinical outcomes.
Inclusion criteria included peer-reviewed studies that used an intervention that elevated serum ketone bodies in NDD (no serum concentration change cut-off was defined) and assessed clinically relevant outcomes. NDD was defined as diseases involving “progressive loss of structure or functioning of neurons, including the death of neurons” (37). Only controlled clinical trials were considered and these were limited to chronic interventions of ≥4 wk duration, to allow for clinically meaningful improvements (32). Exclusion criteria included reviews, editorials, commentaries, conference proceedings, preclinical studies (in vitro and in vivo), acute clinical studies (<4 wk), studies not published in English, or studies where the full text could not be retrieved.
Search strategy
Eight databases comprising EBSCOHost (including CINAHL, Medline, PsychINFO), Scopus (including PubMed, EMBASE), and Web of Science were electronically searched for articles from peer-reviewed journals from the databases’ date of inception up until 30 October, 2019. Database alerts were set up to notify the authors of any new publications up until article submission on 21 August, 2020. An example of the title and keyword search algorithm from Scopus are presented in Supplemental Table 1. The same search strategy and Medical Subject Headings (MeSH) terms were applied consistently across all databases, with minor adjustments to allow for variations in search symbols required for search queries. Reference lists of included studies (citation search) were also searched to identify any further eligible studies.
Data extraction
All articles from the systematic search across the 8 databases were downloaded to and sorted in EndNote version X9.2. Initially, article titles and abstracts were screened for eligibility and sorted accordingly. Full-text articles were retrieved if there was any uncertainty pertaining to an article's eligibility for inclusion. The primary reviewer (LSD) performed the initial screening of articles to determine their eligibility for inclusion. The shortlisted articles were screened by the second reviewer (GZS) to confirm they met the inclusion criteria and the final list was agreed on by both reviewers. Any discrepancies in eligibility were resolved through discussion between the 2 reviewers.
Data extraction was carried out on each eligible full-text article. Study characteristics were extracted including author, year of publication, study design, study population, number of participants, age (mean and SD), gender ratio, study location, recruitment strategy, screening tools, intervention, dosage, duration of trial, outcome measures, and findings.
The Cochrane Risk of Bias Tool 2.0 was used to assess risk of bias in randomized studies (38). This tool is appropriate for randomized, controlled trials and examines methodological bias in 5 domains: randomization, deviations from intended intervention, missing data, outcome measurement, and selection of reported result (38). Each study was assessed on methodological rigor and satisfactory reporting of criteria in the aforementioned domains. Every question can be answered with: no information (NI), yes (Y), possibly yes (PY), no (N), possibly no (PN), and not applicable (N/A). An algorithm is then used to assess the level of bias present in each domain (low risk, some risk, and high risk). For nonrandomized studies, the Risk of Bias In Non-Randomised Studies-of Interventions (ROBINS-I) tool was used (39). ROBINS-I considers 7 distinct domains where bias may be introduced and provides signalling questions to help reviewers make a risk of bias judgement for each domain, which carries forward to an overall evidence rating for each study (39).
Following this, the Grading of Recommendations Assessment, Development, and Education (GRADE) system was used to evaluate the certainty and quality of the evidence (40, 41). GRADE aims to provide evidence ratings in a transparent fashion to inform patient, clinician, and policy decision-making (42). Randomized trials lacking important limitations, as defined by the GRADE system, typically constitute high-quality evidence. In contrast, studies with high methodological limitations will result in the overall rating of the evidence being downgraded (41). Study design, consistency, directness, precision, and publication bias are considered, and evidence quality is given a rating of high, moderate, low, or very low (40).
Due to the heterogeneity present in the included studies (in NDD types and severity, interventions used, and outcome measures assessed) a quantitative analysis of the data in the form of a meta-analysis was not appropriate. Therefore, the characteristics of each study along with GRADE assessment were reported using a qualitative narrative synthesis approach (43).
Results
Study selection
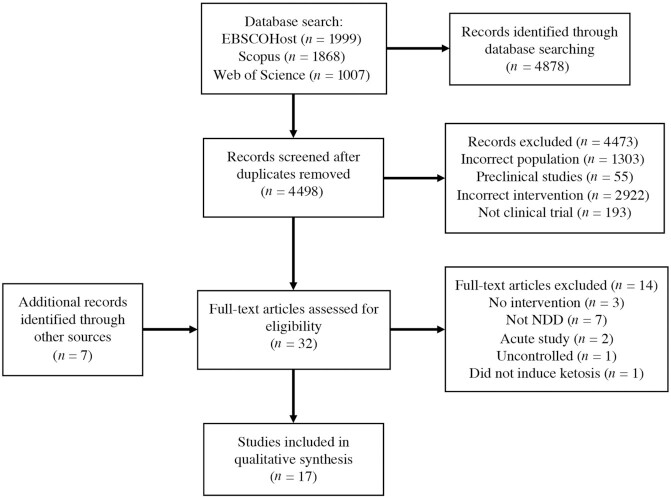
A total of 4878 records were identified, with 4498 remaining after duplicates were removed. After title and abstract screening, 29 full-text articles were retrieved (including 7 additional articles from various sources and database alerts), of which 17 met the inclusion criteria (44–61). Four articles (46, 47, 50, 52) reported results from 2 separate trials and the remaining 13 studies were unique. Figure 1 outlines the study search and selection process.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. NDD, neurodegenerative disease.
Study characteristics
Table 1 provides a detailed summary of the included studies. Sixteen studies used a randomized controlled design (44, 46–53, 55–61) and the remaining study was nonrandomized (54). Of the randomized trials, 7 were placebo-controlled (44, 46–49, 53, 55). Eight of the 9 dietary interventions used other dietary approaches; a high-carbohydrate diet (60, 61), a low-fat diet (56–58), the National Institute on Aging (NIA) diet (51), a modified Paleolithic diet (MPD) (59), or variations of a ketogenic diet (such as a fasting-mimicking diet) (50, 52) to serve as the control. Eleven studies were conducted in the USA, 2 in Germany, with the remaining 4 articles each from Canada, China, Iran, and New Zealand, respectively. The earliest study included was conducted in 2009 (46), with most recent articles published in 2020 (48, 57, 59).
TABLE 1.
Summary of study characteristics from included studies
| Study | Population | N | Age,1 y | Study location | Design | Intervention and dose | Study duration | Primary outcome | Results |
|---|---|---|---|---|---|---|---|---|---|
| Bock, M., et al. 2018 (52) | MS | 60 | 42.3 ± 12.5 | Germany | Randomized, controlled, 3-armed parallel, clinical pilot trial | Dietary; AKD, CR | 6 mo | MSQoL-54; eicosanoid gene expression | MSQoL-54 was inversely correlated with the expression of ALOX5 and COX1. Expression of ALOX5 in combined AKD/CR (KA) group was significantly reduced compared to control. Within-group comparison of KA group demonstrated the expression of COX1 and COX2 were also significantly impaired after the intervention |
| Brandt, J., et al. 2019 (51) | MCI - early AD | 14 | 71.3 ± 6.1 | USA | Randomized, parallel group controlled clinical trial | Dietary; MAD, NIAD | 12 wk | Cognition | Participants in the MAD group experienced improved Memory Composite Scores, although they were not statistically significant. Participants in the MAD group who were not adherent to the dietary protocol experienced a decline in memory |
| Choi, IY., et al. 2016 (50) | MS | 60 | 44.8 ± 11.1 | Germany | Randomized, 3-armed parallel, clinical pilot trial | Dietary; FMD, KD, control | 6 mo | Safety and feasibility; HRQoL | Administration of the FMD and KD in people with MS was safe and well tolerated. Positive effects of FMD or KD treatment were observed in RRMS participants in self-reported HRQoL measures including pain |
| Fortier, M., et al. 2019 (49) | MCI | 52 | 74.6 ± 6.4 | Canada | Randomized, placebo-controlled trial | MCT (liquid emulsion; 30 g in 250 mL lactose-free skim milk daily) | 6 mo | Brain ketone and glucose metabolism | Brain ketone metabolism, quantified with FDG-PET, increased by 230% for subjects in MCT group. Secondary measures of cognition improved within the MCT group at endpoint versus baseline. Language was the only cognitive domain that differed significantly between groups |
| Henderson, S., et al. 2009, 2011 (46, 47) | AD | 152 | 76.9 ± 8.3 | USA | Randomized, double-blind, placebo-controlled, parallel-group, multicenter trial | MCT (AC-1202; 30 g powdered sachet, equiv. to 10 g active AC-1202 daily) | 12 wk | Cognition | APOE-ε4(+) participants did not differ from placebo in the ADAS-Cog at any time point. APOE-ε4(–) participants performed significantly better than placebo in ADAS-Cog at day 45 and day 90. APOE-ε4 status appears to be a primary modulator of ketosis, as APOE-ε4(–) subjects had better response to treatment than APOE-ε4(+) |
| Henderson, S., et al. 2020 (48) | AD | 413 | 76.7 ± 6.5 | USA | Randomized, double-blind, placebo-controlled, parallel-group, multicenter trial | MCT (AC-1204); 40 g, equiv. to 20 g caprylic triglyceride daily) | 26 wk | Cognition | No significant effects were observed between groups in the ADAS-Cog or ADCS-CGIC scores at week 26 across treatment groups. A significant negative change was observed in the MMSE score from baseline; AC-1204 group performed worse than placebo. AC-1204 group reported significant improvements in QoL compared to placebo |
| Krikorian, R., et al. 2012 (61) | MCI | 23 | 69.4 ± 6 | USA | Randomized, controlled trial | Dietary; HC, LC | 6 wk | Cognition | LC diet improved secondary memory performance and paired associate learning task. Serum ketone body concentrations were associated with improved memory performance |
| Krikorian, R., et al. 2019 (60) | PD-MCI | 14 | 65.7 ± 5.8 | USA | Randomized, controlled, parallel-group trial | Dietary; HC, KD | 8 wk | Cognition | Nutritional ketosis enhanced cognitive performance in people with PD-MCI. A strong inverse association was observed between intervention-related weight reduction and memory enhancement in the KD group |
| Lee, J., et al. 2020 (59) | MS | 14 | 51.9 ± 10.1 | USA | Randomized, controlled parallel-group trial | Dietary; MPD, MKD, control | 12 wk | Feasibility, fatigue, QoL, cognition, physical function | Mean MFIS scores significantly decreased for the MPD group at endpoint. No differences in EDSS scores were observed within or between intervention groups. No changes observed between groups in the cognitive component of the PASAT, although the MPD group had significantly higher scores at endpoint compared to controls. The MKD group achieved maximal mean BHB concentrations of 1.48 ± 1.10 mmol/L, though these numbers declined by the 12-wk mark. Participants on the MKD experienced a decline in fasting glucose and insulin, suggesting improvements in glucose metabolism |
| Nagpal, R., et al. 2019 (58) | MCI | 17 | 64.6 ± 6.4 | USA | Randomized, double-blind, crossover, single-center pilot study | Dietary; MMKD, AHAD | 6 wk | AD biomarkers | Concentrations of Proteobacteria correlated positively with Aβ-42 and Aβ-40, whereas fecal propionate and butyrate correlated negatively with Aβ-42 in subjects with MCI. MMKD slightly reduced fecal lactate and acetate while increasing propionate and butyrate. AHAD increased acetate and propionate while reducing butyrate |
| Neth, B., et al. 2020 (57) | SCI-MCI | 20 | 64.2 ± 6.3 | USA | Randomized crossover design | Dietary; MMKD, AHAD | 6 wk | AD CSF biomarkers, cognition, cerebral blood flow, cerebral ketone uptake, metabolic parameters | Following MMKD, all participants saw an improvement in metabolic parameters (↓ HbA1c, glucose, insulin, triglycerides, and VLDL), an increase in cerebral perfusion and cerebral ketone body uptake along with improved memory performance assessed by FCSRT was also observed. An increase in CSF Aβ42 and decreased CSF tau was associated with MMKD. Following AHAD, no changes in metabolic parameters was noted, however, a reduction in CSF tau was observed |
| Phillips, M., et al. 2018 (56) | PD | 47 | 62.9 ± 7 | New Zealand | Randomized, controlled, parallel group, pilot trial | Dietary; KD, LFD | 8 wk | Motor and nonmotor function | MDS-UPDRS scores significantly decreased across groups. KD group scores decreased further in Part 1 (−4.58 ± 2.17 points, 41% improvement from BL scores) compared to LFD group (−0.99 ± 3.63 points, representing an 11% improvement). Largest between-group reductions were observed for urinary problems, pain, fatigue, daytime sleepiness, and cognitive impairment |
| Rebello, C., et al. 2015 (55) | MCI | 4 | 58–78 y | USA | Randomized, double-blind, placebo-controlled, parallel trial | MCT (Nestle™; 56 g daily, added to 6 ounces of Yoplait™ 99% fat-free fruit yogurt) | 24 wk | Cognition | MCT group (n = 1) increased ADAS-Cog score by 5 points. Second participant declined by 4 points. No effect on TMT or DST was observed. No statistical analysis performed due to small sample |
| Saadatnia, M., et al. 2009 (54) | MS | 80 | 29.9 ± 8.1 | USA | Prospective, parallel group, controlled trial | Fasting, control | 4 wk | Disease relapse | There were no significant changes in median number of MS attacks or in the EDSS in fasting group compared to controls 6 mo after intervention |
| Torosyan, N., et al. 2018 (44) | AD | 16 | 79.9 ± 9.2 | USA | Randomized, double-blind, placebo-controlled trial | MCT (caprylidene; 40 g daily) | 45 d | Regional cerebral blood flow | APOE-ε4(–) participants had significantly elevated rCBF in the left superior lateral temporal cortex by sVOI analysis after adopting a caprylidene diet for 45 d. Generally, greater increases in rCBF were observed with caprylidene therapy inAPOE-ε4(–) participants compared to APOE-ε4(+) counterparts |
| Xu, Q., et al. 2019 (53) | AD | 53 | 75.1 ± 7.5 | China | Randomized, double-blind, placebo-controlled trial | MCT (dosed TID; total daily dose 17.3 g) | 30 d | Cognition | Significant reductions in the ADAS-Cog-C (mean change –2.62 points from baseline) were observed in APOE-ε4(–) participants who received MCT treatment. All ADAS-Cog domains were significantly reduced after the intervention. After 30 d, concentrations of serum BHB and AcAc increased by 129% and 87%, respectively, in the MCT group. No significant changes were observed in ADL scores. Metabolomics revealed top 3 pathways implicated in MCT treatment were fatty acid biosynthesis, linoleic acid metabolism, and steroid hormone biosynthesis. A total of 15.2% of participants were considered “complete responders”; defined by a 7-point improvement on ADAS-Cog |
Values are mean ± SD unless otherwise specified. AD, Alzheimer's disease; ADAS-Cog, Alzheimer's Disease Assessment Scale-cognitive Subscale; ADCS-CGIC, Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change; ADL, activities of daily living; AHAD, American Heart Association Diet; AKD, adapted ketogenic diet; ALOX-5, arachidonate 5-lipoxygenase; APOE-ε4, apolipoprotein E allele ε4; BHB, β-hydroxybutyrate; BL, baseline; COX1, cyclooxygenase 1; COX2, cyclooxygenase 2; CR, calorie restriction; CSF, cerebrospinal fluid; DST, Digital Symbol Test; EDSS, Expanded Disability Status Scale; FCSRT, Free and Cued Selective Reminding Test; FDG-PET, Fluorodeoxyglucose Positron Emission Tomography; FMD, fasting mimicking diet; HbA1c, hemoglobin A1c; HC, high-carbohydrate; HRQoL, health-related quality of life; KA, ketogenic approach; KD, ketogenic diet; LC, low-carboydrate; LFD, low-fat diet; MAD, modified Atkin's diet; MCI, mild cognitive impairment; MCT, medium-chain triglycerides; MDS-UPDRS, Movement Disorders Society-Unified Parkinson Disease Rating Scale; MFIS, modified Fatigue Impact Scale; MKD, modified ketogenic diet; MMKD, modified Mediterranean ketogenic diet; MMSE, Mini-Mental State Examination; MPD, modified Paleolithic diet; MS, multiple sclerosis; MSQoL-54, Multiple Sclerosis Quality of Life-54 questionnaire; NDD, neurodegenerative disease; NIAD, National Institute on Aging Diet; PD, Parkinson's disease; PD-MCI, mild cognitive impairment due to Parkinson's disease; PASAT, Paced Auditory Serial Addition Test; QoL, quality of life; SCI, subjective cognitive impairment; rCBF, regional cerebral blood flow; RRMS, relapse-remitting multiple sclerosis; sVOI, standardized volumes of interest; TID, three times per day; TMT, Trail Making Test; VLDL, very-low-density lipoprotein; V-PAL, Verbal Paired Association Learning Test.
Study participants
The total sample size across all included studies [accounting for the 4 articles reporting on various outcomes from the same trials (46, 47, 50, 52)] was 979, with individual samples ranging from 4 to 413 participants. Studies included 548 females and 372 males; 2 studies (n = 30) did not specify the breakdown of male and female participants in their reporting (44, 60). The mean age (Mpooled ± SDpooled) of participants was 68.25 ± 7.34 y [although in 1 study only age range was reported (55), so this was excluded from this calculation].
Trials in mild cognitive impairment (MCI) were most common (6/17). MCI studies examined 130 participants (49, 51, 55, 58, 61), whilst studies in people with MS analyzed 154 participants (50, 52, 54, 59) and those in AD featured 634 participants (44, 46–48, 53). The remaining 2 studies assessed 47 participants with PD (56) and a further 14 participants with MCI secondary to PD (60).
Recruitment
Five studies advertised locally using flyers or radio advertisements (most commonly used approach) (46, 47, 56, 61) and others recruited via disease-specific medical centers or support groups (59), (54, 60). Six of the 17 included studies did not report on their recruitment strategy (44, 48, 49, 55, 57, 58) and 2 studies did not provide sufficient detail to understand how participants were recruited (50, 52). One study (51) utilized all the aforementioned strategies.
Intervention design
Interventions ranged in length from 30 d to 6 mo, with 6 wk (n = 4) being most common (44, 57, 58, 61). The majority of studies (n = 9) used variations of a KD to induce ketosis, followed by exogenous ketogenic supplementation in the form of MCTs (n = 7) and a fasting protocol (n = 1). None of the studies conducted any follow-up after the conclusion of the trial.
Compliance, withdrawals, and adverse events
Two studies (46, 51) reported compliance measures in satisfactory detail and performed statistical analyses on group differences in compliance. Almost half the articles (n = 8) outlined compliance strategies but did not report the outcomes, whilst other articles stated that participants were compliant with the protocol but did not report how compliance was measured or defined (n = 3). One study failed to report on compliance measures at all (54). Dietary studies often employed urinary or capillary BHB measurements for compliance and tended to report on baseline and endpoint values without analyzing adherence (50, 52, 56–61). For studies that adequately reported on compliance, protocol adherence using exogenous ketones was superior to that in dietary interventions (44, 61).
Most studies (11/17) reported on participant withdrawals and provided reasons in satisfactory detail (46,47, 49–53, 55, 56, 59, 60). Two studies did not report on withdrawals at all. Over 50% of included studies (n = 11) reported on adverse events (46–51, 53, 55–57, 60), the most common being gastrointestinal disturbance; including diarrhea, abdominal discomfort, and nausea; symptoms were transient and mild-to-moderate in nature. Adverse effects were more common with exogenous ketogenic agents than dietary interventions (46, 48, 49, 53, 55).
Overview of primary outcome measures
Due to variation in NDD included in this review, and the focus on disease-specific outcomes for clinical relevance, primary outcomes differed greatly between studies. Accordingly, primary outcomes are stratified by disease state and summarized below. Statistically significant changes in primary outcome measures due to ketogenic interventions in NDD are shown in Figure 2. Comparative measures of efficacy across disease states and intervention types were not possible, therefore, this figure should be interpreted by its 4 quadrants only and is not scaled from most effective to least effective. Studies that measured “cognition” using a neuropsychological testing battery (NTB) have been combined for illustrative purposes. Bubbles are placed randomly in their respective quadrants and are not representative of level of efficacy. Studies were deemed to have mixed effects if primary outcome measures demonstrated positive effects in some domains and no effect in others. Bubbles are color-coded based on their representative NDD; the number inside the bubble is the corresponding study citation and risk of bias is illustrated by border color (green = low risk of bias; orange = some risk of bias; red = high risk of bias).
FIGURE 2.
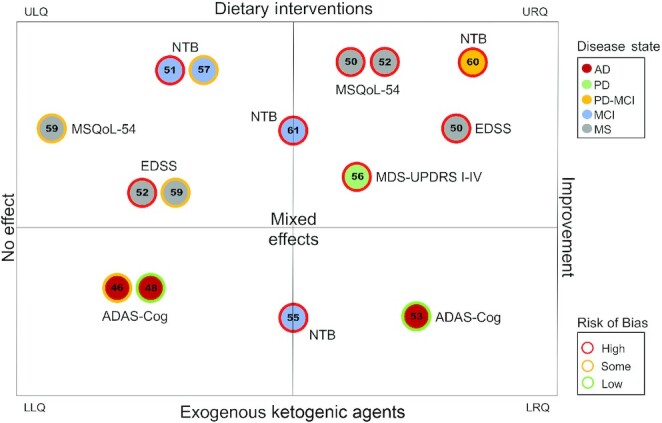
Evidence for clinical efficacy of ketogenic interventions. Upper left quadrant (ULQ): dietary interventions demonstrating no effect on primary outcomes. Upper right quadrant (URQ): dietary interventions demonstrating positive effects on primary outcome measures. Lower left quadrant (LLQ): interventions using exogenous ketogenic agents demonstrating no effect on primary outcomes. Lower right quadrant (LRQ): interventions using exogenous ketogenic agents demonstrating positive effects on primary outcome measures. Note: only outcome measures assessed by 2 or more studies are visualized here. Studies reporting on unique outcomes have not been included. AD, Alzheimer's disease; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive Subscale; EDSS, Expanded Disability Status Scale; MCI, mild cognitive impairment; MDS-UPDRS I-IV, Movement Disorders Society-Unified Parkinson Disease Rating Scale Parts I-IV; MS, multiple sclerosis; MSQoL-54, Multiple Sclerosis Quality of Life-54 questionnaire; NTB, neuropsychological test battery; PD, Parkinson's disease; PD-MCI, mild cognitive impairment secondary to Parkinson's disease
Mild Cognitive Impairment
Six studies investigating KT for MCI were included (49, 51, 55, 57, 58, 61); over half (n = 4) utilized a dietary intervention (51, 57, 58, 61) for a duration of 6 wk (57, 58, 61). Cognition was the predominant primary outcome measure assessed, with mixed effects observed (51, 55, 57, 61). Various NTBs were used to quantify cognitive scores. The Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) was administered across 2 studies; 1 (using MCTs for 24 wk) found appreciable improvements in word recall, word recognition, and remembering test instructions compared with placebo (55), whilst the other found no changes in cognition (57). A medium effect size was found in participants adhering to a low-carbohydrate diet compared with a high-carbohydrate diet using the Verbal Paired Association Learning test (V-PAL) (61), whilst no effects were observed in the Trail Making Test (55, 61) or Digit Symbol Test (55).
An individual study showed that increased cerebral ketone metabolism after MCT supplementation was associated with an improvement in secondary measures of cognition, with a significant between-group effect observed in the Boston Naming Test compared with placebo (49). Variations of the KD were found to favorably modulate several bacterial taxa in the microbiome (such as Firmicutes and Proteobacteria spp.), as well as SCFA production in the gut (57, 58). Fecal butyrate and propionate increased after a KD, whereas acetate and lactate decreased (58). The KD also modulated cerebrospinal fluid (CSF) biomarkers; CSF Aβ-42 increased, CSF tau decreased, and the CSF Aβ-42: tau ratio was also increased after treatment (58).
Alzheimer's Disease
Five studies reporting on 4 unique trials investigated the use of MCTs compared to placebo in people with mild to moderate AD (44, 46–48, 53). Trial duration was variable, ranging from 30 d (53) to 26 wk (48). The daily dose of MCTs ranged between 17.3 g and 40 g and caprylic acid (8:0) was the primary MCT used across interventions (44, 46–48). Cognition, measured using the ADAS-Cog, was the primary outcome of efficacy (3/5 studies) and mixed effects were observed (46, 48, 53). One trial found a significant reduction in ADAS-Cog scores (mean change of –2.62 points from baseline in 30 d using 17.3 g MCTs daily) compared with placebo, as well as significant improvements in each cognitive subdomain (53). A minimum 4-point reduction in ADAS-Cog within 6 mo is considered to be the minimum clinically important difference (MCID) (62). The second study found significant effects in treatment-compliant, APOE-ε4(–) participants only (46), and the third study found no significant effects, relative to placebo (48). Two studies used the Mini-Mental State Examination (MMSE); 1 reported no effect (46), whereas the second reported significant worsening in MMSE scores in the MCT group compared with placebo, relative to baseline values (48). An individual study found increased regional cerebral blood flow after 45 d of MCT treatment compared with placebo, however no effects were observed for participants carrying 1 or more copies of the APOE-ε4(+) allele (44).
Multiple Sclerosis
Four studies reporting on 3 unique trials (50, 52, 54, 59) used predominantly KD interventions (n = 3) (50, 52, 59) for a duration of 6 mo (50, 52). The majority (n = 3) of studies were in relapse-remitting MS (RRMS) (50, 52, 54) and 1 included all subtypes of MS (relapse-remitting, primary progressive, and secondary progressive) (59). No observable differences were noted amongst MS subtypes across the included studies. The Expanded Disability Status Scale (EDSS) (50, 52, 54, 59) and Multiple Sclerosis Quality of Life-54 questionnaire (MSQoL-54) (50,52,59) were most frequently used as primary outcome measures. One study reported a statistically significant difference in the EDSS after a KD for 6 mo compared with the control diet (50), a second reported a downward trend that did not reach statistical significance (52), whilst the remaining 2 studies reported no significant effect (54, 59). Using the MSQoL-54; 2 studies found a significant effect (particularly in physical and mental health composite scores) (50, 52), however, a third study saw no change (59). No effects were observed in the Modified Fatigue Impact Scale or Multiple Sclerosis Functional Composite (59). Adaptations of the KD significantly reduced the expression of proinflammatory enzymes (52), white blood cells, and lymphocytes compared with controls (standard diet) (50). Relative decreases in immune activity were positively correlated with improvements in the MSQoL-54 (50, 52).
Parkinson's Disease
Two studies used KD interventions 8 wk in duration in people with PD (56) and MCI secondary to PD (PD-MCI) (60). The Movement Disorders Society-Unified Parkinson Disease Rating Scale (MDS-UPDRS) was used across both studies; 1 found no significant effects (60), however, the second found that participants on the KD experienced a significantly larger decrease in Part 1 (nonmotor experiences of daily living) of the MDS-UPDRS compared with a low-fat diet (56). Other parts of the MDS-UPDRS improved, however, no significant differences were seen in Parts 2–4 (motor experiences of daily living; motor examination and motor complications, respectively) relative to the control diet.
Cognition was the primary outcome assessed in people with MCI secondary to PD. Significant improvements were found in the Controlled Oral Word Association test and the V-PAL, but not in the California Verbal Learning Test (60). Further, significant reductions were seen in body weight, waist circumference, fasting insulin, LDL cholesterol, and total cholesterol after a KD compared with baseline parameters (56, 60).
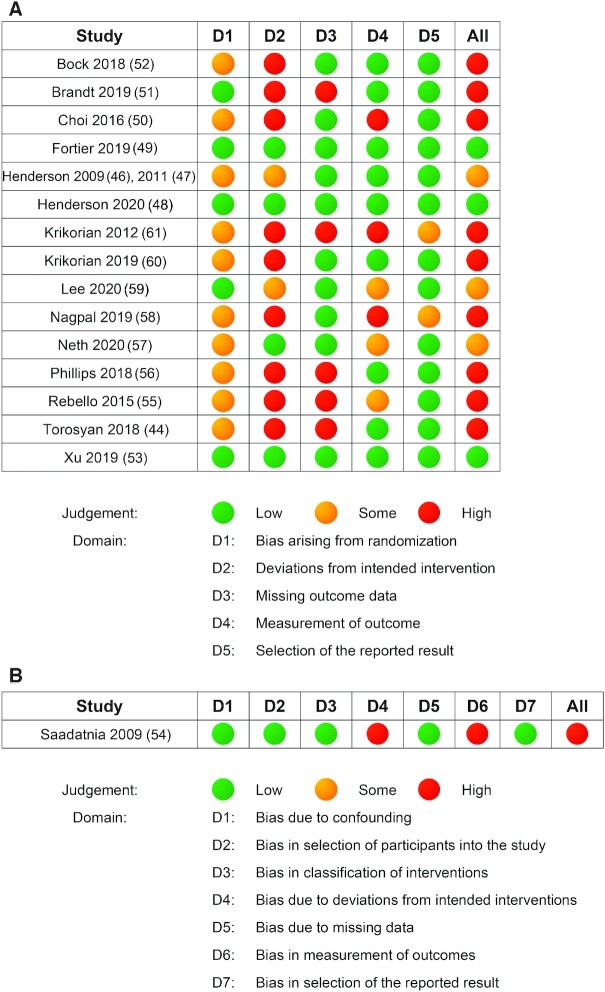
Risk of bias within and across studies
Figure 3 illustrates the risk of bias judgement for each domain (A: randomized studies; B: nonrandomized study). Three studies were judged to have low risk of bias overall (48, 49, 53), 5 were judged to have some concerns of bias (46, 47, 54, 57, 59), and the remaining 9 studies were deemed to be at high risk of bias (44, 50–52, 55, 56, 58, 60, 61). A total of 16 from 17 studies were randomized (44,46–53,55–61), with 5 studies providing sufficient detail of the randomization process and allocation concealment (48, 49, 51, 53, 59). Over half (n = 11) of the studies adequately accounted for missing data (46–50, 52–54, 57, 58, 60). Risk of bias in the domain of outcome measurement varied between the included studies. Although most studies (n = 10) were deemed to be at low risk of bias in this category (44, 46–49, 51–53, 56) a moderate to high risk of bias due to selective reporting of results was present in 2 studies (57, 59).
FIGURE 3.
Risk of bias assessments in alphabetical order of first authors’ names. Panel A: randomized studies using Cochrane's Risk of Bias tool. Panel B: nonrandomized studies using the ROBINS-I tool. Low (green): judged to be at low risk of bias for all domains. Some (yellow): judged to raise some concerns in ≥1 domain, but not to be at high risk of bias for any domain. High (red): judged to be at high risk of bias in ≥1 domain for this result OR the study is judged to have some concerns for multiple domains in a way that substantially lowers confidence in the result. ROBINS-I, Risk of Bias in Non-Randomised Studies-of Interventions.
GRADE assessment
Tables 2 , 3, 4, and 5 present the GRADE assessment conducted appraising KT in relation to each NDD across included studies. The summary of findings from the GRADE assessment are detailed in Table 6. Overall, the certainty of the evidence across all NDD was low to very low. Small sample sizes, methodological issues, and inconsistency in outcomes were the key issues identified across the evidence.
TABLE 2.
Should ketogenic therapies compared with control be used for people with Alzheimer's disease?
| GRADE domain | Judgement | Concerns about certainty domains |
|---|---|---|
| Methodological limitation of the studies | All trials in this sample (44, 46–48, 53) were judged to be at low risk of methodological bias in the assessed domains. There were some concerns in 2 studies (44, 47) about baseline differences between groups, however, we did not warrant this as serious enough to downgrade the level of evidence in this category | Not serious |
| Indirectness | Overall, the subsample of studies in AD was fairly well characterized. All studies were performed in people with mild to moderate AD. All studies used exogenous ketogenic agents in the form of MCTs, however, there are differences in fatty acid composition across MCT products which may affect the efficacy of the intervention. All studies used the ADAS-Cog as the validated scale to measure cognition (primary outcome) (46–48, 53), except for the trial by Torosyan (44) that measured regional cerebral flood flow. We judged the evidence to have no serious indirectness | Not serious |
| Imprecision | The total sample size across studies was 634, or 618 when considering the studies that all used the same primary outcome measure (ADAS-Cog). Based on Optimal Information Size (OIS), the sample exceeds the number of patients generated by a conventional sample size calculation for a single adequately powered trial. All studies were randomized and placebo-controlled, so the comparator was “no intervention.” Overall, 4/5 studies (44, 46–48) reported nonsignificant results when subgroup analyses are not considered | Not serious |
| Inconsistency | The direction and magnitude of effect varied across the different trials. Overall, the results showed either a small reduction in symptoms (i.e. an improvement in cognition) or no statistically significant change. When participants were stratified for APOE-ε4 status, APOE-ε4(–) participants experienced statistically significant improvements from baseline. One study (53) reported statistically significant reductions in the ADAS-Cog after 30 d treatment (52 participants), whereas other studies with larger samples (46–48) showed no statistically significant effects overall. In 1/5 studies (48), there was no change, and in some outcomes, participants worsened (MMSE, APOE-ε4(+) participants in ADAS-Cog). No significant between-group differences were observed. In 3/5 studies (44, 46, 48), there was no statistically significant change after the treatment overall (notwithstanding subgroup analysis). 1/5 study (small sample, short study duration, overall low ROB) (53) showed statistically significant improvements in cognition in the treatment group (MCT). We judged the evidence to be at serious risk of inconsistency | Serious |
| Publication bias | We did not suspect publication bias, due to the presence of both positive and negative trials in the evidence base. Furthermore, the systematic search strategy was comprehensive and covered an extensive number of databases | Not suspected |
AD, Alzheimer's disease; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive Subscale; APOE-ε4, apolipoprotein E allele ε4; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; MCT, medium-chain triglycerides; MMSE, Mini-Mental State Examination; ROB, risk of bias.
TABLE 3.
Should ketogenic therapies compared with control be used for people with mild cognitive impairment?
| GRADE domain | Judgement | Concerns about certainty domains |
|---|---|---|
| Methodological limitation of the studies | 1/5 trials (49) had low risk of bias across the 3 assessed domains and had the largest sample size (52 participants). Another study (57) did not clearly report on allocation concealment or sequence generation, which lowers confidence in the certainty of the evidence. The remaining 3 studies (55, 58, 61) were judged to have some or high risk of bias across methodological items assessed. Therefore, we have judged the trials to have very serious methodological limitations | Very serious |
| Indirectness | The patients, intervention, and comparators within included studies all provide direct evidence to the clinical question at hand. All studies included participants with a diagnosis of MCI. 4/6 studies measured cognition as the primary outcome, whilst 2 other individual studies measured brain ketone metabolism (49) and CSF AD biomarkers (58). Further, 4/6 studies implemented a dietary intervention (51, 57, 58, 61) whilst the remaining 2 trials (49, 55) used MCTs. The predominant primary outcome measure in this sample (cognition) was assessed using varying neuropsychological testing methods in different trials. We also note variation in the modified ketogenic diets adapted across the sample. We judged the trials to have no serious indirectness | Not serious |
| Imprecision | The total number of participants included in all trials was 130, which is concerning. Some studies reported small improvements in cognition using varying neuropsychological assessments (55, 61) and other trials reported “nonsignificant results” (51, 57) likely because of enrolling a small number of participants. We judged the evidence to have very serious imprecision | Very serious |
| Inconsistency | Overall, mixed results were consistently seen across studies which examined cognition as the primary outcome measure (4/6 studies) (51, 55, 57, 61). The direction and magnitude of effect varied across trials. One study reported an improvement in verbal learning memory (medium effect-size) (61) whilst another reported some improvements in specific testing outcomes within the ADAS-Cog, although this study had an extremely small sample and was deemed to be at high risk of bias (55). The remaining studies showed no significant effects on cognition, although effects were trending in the right direction. We judged the evidence to have serious inconsistency | Serious |
| Publication bias | We did not suspect publication bias, due to the presence of both positive and negative trials in the evidence base. Furthermore, the systematic search strategy was comprehensive and covered an extensive number of databases | Not suspected |
AD, Alzheimer's disease; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive Subscale; CSF, cerebrospinal fluid; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; MCI, mild cognitive impairment; MCT, medium-chain triglycerides.
TABLE 4.
Should ketogenic therapies compared with control be used for people with Parkinson's disease or MCI secondary to Parkinson's disease?
| GRADE domain | Judgement | Concerns about certainty domains |
|---|---|---|
| Methodological limitation of the studies | Both of the included studies in PD were judged to be at high risk of bias overall (56, 60). Although both studies reported on allocation concealment, there was no information available on random sequence generation, which lowered our certainty in the evidence. Further, in both studies participants and those delivering the intervention were aware of the participant's assigned intervention group. Therefore, we have judged the trials to have very serious methodological limitations | Very serious |
| Indirectness | The participants, interventions, and comparators all provide direct evidence to the clinical question. Both studies used a dietary intervention to induce ketosis and included participants with PD, however, 1 study specifically assessed participants with MCI secondary to PD (60). As such, different clinical outcomes were measured between trials. Krikorian used neuropsychological testing to evaluate cognitive changes (60), whilst Phillips measured motor function and activities of daily living using the MDS-UPDRS-I-IV (56). Krikorian also employed the MDS-UPDRS-I-IV to measure motor function, however, this was a secondary outcome in the study. We judged the evidence to have no serious indirectness | Not serious |
| Imprecision | The total number of participants across both trials was 61, which was concerning. Both trials reported mixed results in outcome measures assessed with significant and nonsignificant effects observed (56, 60). Due to the small sample size the precision and reliability of presented trials is low. We judged the evidence to be at very serious risk of imprecision | Very serious |
| Inconsistency | As primary outcome measures differed across studies, it was not possible to compare the consistency of magnitude of effects in included studies. Hence, we judged the evidence to be at very serious risk of inconsistency | Very serious |
| Publication bias | We did not suspect publication bias, due to the presence of both positive and negative trials in the evidence base. Furthermore, the systematic search strategy was comprehensive and covered an extensive number of databases | Not suspected |
GRADE, Grading of Recommendations, Assessment, Development and Evaluation; MCI, mild cognitive impairment; MDS-UPDRS I-IV, Movement Disorders Society-Unified Parkinson Disease Rating Scale Parts I-IV; PD, Parkinson's disease.
TABLE 5.
Should ketogenic therapies compared with control be used for people with multiple sclerosis?
| GRADE domain | Judgement | Concerns about certainty domains |
|---|---|---|
| Methodological limitation of the studies | Of the 4 studies included, 1/4 was judged to be at low risk of bias for allocation concealment and sequence generation (59). The remaining 3 studies had some concerns of bias in these domains. All included studies in this subsample (50, 52, 54, 59) were deemed to be a high risk of bias due to issues with blinding. We have judged the trials to have very serious methodological limitations | Very serious |
| Indirectness | The patients, intervention, and comparators in the studies mostly provide direct evidence to the clinical question at hand. All participants in the included trials had MS (3/4 studies were relapse-remitting only (50, 52, 54); and 1/4 included primary and secondary progressive as well as relapse-remitting types) (59). The interventions varied across the sample; 3/4 used a dietary intervention (50, 52, 59) and 1/4 used a fasting protocol (54). The types of outcome measures also varied across studies. We judged the evidence to have serious indirectness | Serious |
| Imprecision | The total number of participants included in trials in people with MS was 154, which was concerning. Please note 2/4 studies report on outcomes from the same study (50, 52). Small sample sizes and high drop-out rates in some studies significantly reduced data points for analysis which increases concerns about imprecision. 2/4 studies reported statistically significant findings (50, 52) whilst the remaining studies reported nonsignificant effects (54, 59). We judged the evidence to have very serious imprecision | Very serious |
| Inconsistency | Overall, results showed small improvements in clinical outcome measures (eicosanoid expression; MSQoL-54) (50, 52) or no change (MSQoL-54; disease relapse) (54, 59), however, variability in outcome measures renders the evidence inconsistent. Variation in the directionality of outcome effect was observed in 2/4 studies that measured MSQoL-54 and EDSS (50, 59). One study reported medium to large effect sizes in these scales (50) whilst the second reported nonsignificant effects (59). We judged the evidence to have very serious inconsistency | Very serious |
| Publication bias | We did not suspect publication bias, due to the presence of both positive and negative trials in the evidence base. Furthermore, the systematic search strategy was comprehensive and covered an extensive number of databases | Not suspected |
EDSS, Expanded Disability Status Scale; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; MS, multiple sclerosis; MSQoL-54, Multiple Sclerosis Quality of Life-54 questionnaire.
TABLE 6.
Summary of findings from the GRADE assessment
| Neurodegenerative disease | Outcome | Effect | Number of participants (studies) | Certainty in the evidence |
|---|---|---|---|---|
| Alzheimer's disease | Improvement in cognition | Most studies (3/5) showed no statistically significant effects of treatment | 634 (5 randomized controlled trials) | LOW1 certainty ⊕⊕OO |
| Mild cognitive impairment | Improvement in cognition | Studies which examined cognition showed small-medium improvements or no effect | 130 (6 randomized controlled trials) | VERY LOW2,3 certainty ⊕OOO |
| Parkinson's disease or MCI secondary to Parkinson's disease | Improvement in cognition and motor function | Both studies showed mixed results for measures of cognition and motor function | 61 (2 randomized controlled trials) | VERY LOW4,5 certainty ⊕OOO |
| Multiple sclerosis | Improvement in quality of life and disability | Most studies (3/5) showed no significant effect of treatment | 154 (3 randomized controlled trials; 1 nonrandomized prospective study) | VERY LOW6,7 certainty ⊕OOO |
Serious inconsistencies were observed across the evidence significantly lowering the certainty of the body of evidence. Therefore, we chose to downgrade certainty by 2 levels.
Very serious risk of bias across studies because of unclear or inadequate blinding, sequence generation, and allocation concealment.
Serious imprecision and inconsistency were considered together as there were small effects or “no effects” reported in studies.
Note. Indirectness was not downgraded because the physiological endpoints were comparable between studies despite differing interventions (dietary compared with exogenous ketogenic therapies).
Very serious risk of bias across studies because of unclear sequence generation and lack of blinding.
The evidence was rated to be of very low certainty due to serious or very serious concerns across 4/5 categories.
Very serious risk of bias across studies because of unclear sequence generation and lack of blinding.
The evidence was rated to be of very low certainty due to serious or very serious concerns across 4/5 categories.
Certainty of evidence key = VERY LOW, ⊕OOO; LOW, ⊕⊕OO; MODERATE, ⊕⊕⊕O; HIGH, ⊕⊕⊕⊕.
GRADE, Grading of Recommendations, Assessment, Development and Evaluation; MCI, mild cognitive impairment.
Discussion
This systematic review summarized and critically appraised interventional studies that used the induction of ketosis (either via exogenous supplementation with ketogenic agents, dietary modifications, or fasting) to treat NDD. Twelve (70.6%) of the included articles were published in the last 3 years, highlighting the emerging interest in this area of investigation. Based on the included studies, KT may confer a variety of benefits to people with NDD, potentially serving as a viable adjunctive treatment to conventional care. However, more robust large-scale clinical research is required to increase the certainty and reliability of the evidence. Overall, observed effects included improvements in measures of cognition (46, 53, 55, 60, 61), brain ketone utilization (49), regional cerebral blood flow (44), health-related quality of life (50), reductions in markers of inflammation (52), enhanced activities of daily living (56), metabolic parameters (55–57, 59, 60), and favorable effects on the gut microbiome (58). Each aim of this review (efficacy, safety and feasibility, recommendations for future research) is now addressed separately below.
Evidence for clinical utility of ketogenic interventions
As illustrated in Figure 2, available evidence for KT varies greatly between NDD populations, outcome measures, and intervention type, requiring further standardized investigation.
Mild Cognitive Impairment and Alzheimer's Disease
In people with MCI or mild to moderate AD, promising clinical evidence exists for the improvement of cognition in the domains of verbal memory (61) and word recognition and recall (55), however, no significant effects were observed in other domains such as language or praxis (55, 57). This suggests that KT may positively impact key brain regions responsible for memory and have a lesser impact on cognitive domains that tend to deteriorate later in the natural progression of neurodegeneration (63). Mechanisms underpinning cognitive improvements may be the product of neuroprotective mechanisms such as reductions in oxidative stress and inflammation, and improvements in synaptic transmission (10–12), or the bridging of cerebral energetic deficits (1). All included studies that measured BHB demonstrated increased concentrations (either in serum or urine) post-intervention. However, not all trials exceeded the accepted threshold for nutritional ketosis (≥ 0.5 mM), or more importantly, BHB concentrations that would be considered clinically significant in these populations (64). Several trials noted that improvements in memory were positively correlated with elevated serum BHB concentrations (46, 49, 53, 61). Ketone bodies can help to overcome cerebral hypometabolism by mitigating energy deficits caused by reduced glucose utilization, hence improving various facets of cognitive performance (1). Of note, 1 particular study (with low risk of bias) demonstrated significant improvements across all cognitive domains in 30 d as evidenced via the ADAS-Cog (53). These results are encouraging considering the magnitude of change in 30 d, however, should be interpreted with caution. Generally, improvements in the ADAS-Cog are associated with clinical improvements, although improvements do not always fully represent the spectrum of clinically important effects (65). In clinical trials for antidementia drugs, a 3–4 point reduction in the ADAS-Cog over 6 mo is considered to be clinically important (65, 66). This threshold has been challenged, with some studies suggesting the 4-point change carries no specific clinical relevance for patients (62, 65). Further research evaluating MCID in the context of the ADAS-Cog, along with patient-centered metrics are needed (66). The primary differentiating factor of this study was that MCTs were dosed 3 times daily (total daily MCT dose of 17.3 g), as opposed to once daily (44, 46, 48, 49, 55). Regular dosing throughout the day may allow for a more consistent supply of ketogenic substrates (particularly in the absence of a concurrent KD), increased tolerability, and reduced incidence of adverse effects (53). In contrast, when excluding subgroup analyses of compliance and genotype, 3 studies reported no significant effect on cognition post-intervention (46, 48, 51). The ketogenic potential of MCT formulations needs to be considered. Variation in MCT fatty acid composition can produce substantially different physiological effects, as noted in Henderson et al. (48). Pharmacokinetic studies may be valuable in further elucidating the ketogenic potential of differing exogenous formulations (67).
Additionally, ketogenic interventions in MCI and AD improved several peripheral metabolic markers implicated in the development and exacerbation of cognitive decline (68, 69). Across included studies, significant reductions in fasting glucose (55, 57), fasting insulin (55, 57), hemoglobin A1c (57), total triglycerides (57), and LDL (57) were reported. These studies are in line with others reporting improvements in insulin sensitivity, blood glucose concentrations, and lipemic control following a KD (70–73). Benefits to cognitive function may also be attributable to improvements in cardiovascular and metabolic risk factors (68, 69).
Consistently, across all studies, APOE-ε4(+) participants tended to respond poorly to treatment, compared with APOE-ε4(–) counterparts (44, 46, 47, 49, 51, 55, 58). APOE-ε4 status appears to strongly influence responsivity to KT in both acute (74) and chronic studies (44, 46, 49, 51, 55, 57–59, 61). KT appear most effective for APOE-ε4(–) individuals, hence this sample becoming the focus for more recent trials (48, 53). Differences in treatment outcomes between APOE-ε4(+) and APOE-ε4(–) may be attributable to many factors including advanced cerebral hypometabolism, mitochondrial enzyme dysfunction, and a greater degree of systemic inflammation. These factors may inhibit the efficient utilization of ketone bodies in APOE-ε4(+) individuals (44, 46, 48). Further, homozygous carriers of APOE-ε4 display disturbed fatty acid metabolism, which may be implicated in their poor responsivity to KT (75, 76).
The APOE-ε4 and sex interaction should be carefully considered in future studies given the increased risk and poorer outcomes in females compared with males (77–81).
Multiple Sclerosis
Health-related quality of life measures such as the MSQoL-54 and EDSS were most commonly used to quantify improvements in disease-specific outcomes resulting from KT, with mixed results (50, 52, 59). Significant improvements in both physical and mental health composite scores were observed, evidenced via MSQoL-54 (50). Currently, no MCID score is established for this tool, therefore, interpretation of clinically meaningful change is difficult (82). In studies that reported favorable outcomes in quality of life (50, 52), inverse associations were observed between expression of proinflammatory adipokines (ALOX5 and COX1) and the MSQoL-54 (52), suggesting benefits may be mediated by modulating inflammation. KD, fasting, and calorie restriction inhibit the production of key enzymes (e.g. COX1, COX2, ALOX5) required for the synthesis of proinflammatory eicosanoids implicated in the pathogenesis and exacerbation of MS (52, 83, 84). BHB may produce an anti-inflammatory effect by the inhibition of the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome, independent of starvation or calorie-restricted mechanisms such as autophagy (85). These effects demonstrate that KT may be able to modulate immune system hyperactivity, potentially slowing disease progression. However, not all studies were positive. One study found no significant effects across any quality of life or motor function outcomes in participants who adopted a KD compared with a modified Paleolithic-style diet, where several clinical disease markers showed significant improvements (59).
Parkinson's Disease
There is very limited clinical evidence available for the use of KT in people with PD. Significant improvements in nonmotor daily living experiences (Part 1 of MDS-UPDRS-I-IV) were observed in people with PD following a KD, however, no significant effects above and beyond that achieved with the control diet (low-fat diet) were observed in Parts 2–4 (56). Nonmotor symptoms such as fatigue, pain, urinary issues, and cognitive decline tend to be the more disabling factors of PD (86), and are least responsive to conventional pharmacological interventions such as L-dopa (87). The KD may be a viable adjunctive therapy alongside L-dopa, although more evidence is needed before this may be confidently implemented in clinical practice. It should be noted that some participants experienced transient exacerbated tremor or rigidity, which was intermittent in nature and most prominent in the KD group. The authors speculate that these effects may be secondary to significant increases in dietary fat which may have accelerated dopamine depletion or oxidative stress (56, 88). Although preliminary findings suggest the KD may be effective in improving measures of cognition (specifically, lexical access) in people with MCI secondary to PD, neither study found any benefit in motor functionality in people with PD (56, 60).
Safety, tolerability, and compliance
KT were found to be safe and generally well-tolerated. No serious intervention-related adverse events were reported. Interventions that administered an exogenous ketogenic agent (44, 46, 47, 49, 55) generally had better compliance and a more rigorous study design (due to use of placebo), although tolerability was an issue amongst some participants. Gastrointestinal disturbance, including nausea and diarrhea was commonly reported in these interventions. Gastrointestinal symptoms are likely to subside following an adequate adjustment period or may be mitigated with the development of improved formulations (67, 89). As evidenced through high noncompliance observed in some dietary interventions, KDs may not be a feasible approach to achieving therapeutic ketosis in NDD populations (51, 52). These findings are in agreeance with previous studies in adults adopting a KD, reporting a drop-out rate of 40–50% (90). Behavioral change is challenging in any population, particularly for clinical groups with reduced physical and/or cognitive function (91–94). Some dietary studies in this review enlisted the assistance of a “study partner” to support the participant with meal preparation and compliance with study protocols (51, 57, 59, 60). Caregiver burden resulting from participation in such protocols which ultimately led to numerous withdrawals from the trial, has been discussed elsewhere (95). In reviewed studies, the net benefit of enlisting a study partner is unclear as this was not adequately measured. Future studies could consider conducting measures of caregiver burden to better understand the impact and implications for future research (95). Consequently, it is imperative to examine whether 1) exogenous ketone supplementation can afford comparable clinical benefits to a KD or 2) whether administration of exogenous ketones in conjunction with a KD can mitigate potential deficits in efficacy occurring from nonadherence to the dietary protocol. A trial is currently underway evaluating the tolerability of an exogenous ketogenic agent (BHB) in conjunction with a KD, a low-glycemic index diet, or a standard diet in persons with Angelman Syndrome which may provide some preliminary insight into feasibility (96).
Ketone measurement is key to understanding compliance and keto-adaptation. Different methods carry advantages and disadvantages relating to cost, ease of administration, accuracy, sensitivity, and participant burden (97). Capillary blood BHB measurement has the highest specificity and is most accurate in reflecting ketone concentrations compared with urinary or breath measurements (98). Ideally, studies should include regular measurements of blood ketone concentrations; optimally daily (56) or at a minimum weekly (56), to monitor protocol adherence and response to treatment. However, this may not always be feasible due to high costs and unwillingness of participants to endure regular finger-prick testing. Despite 0.5 mmol/L being a commonly accepted threshold for nutritional ketosis (99), serum BHB requirements for therapeutic indications (particularly in advanced stages of NDD) may be much higher. For example, in children with refractory epilepsy, serum BHB ≥4 mmol/L was associated with significantly greater reductions in seizure frequency compared to children with serum BHB <4 mmol/L (64). Combining ketone esters (KE) or ketone salts to MCT formulations may be an effective way to elevate blood ketones to higher concentrations for longer periods (100, 101), whilst simultaneously promoting endogenous ketone production. This would allow for a more sustained blood ketone profile which would be of benefit to NDD populations (102, 103). Combination formulations may also help to slow gastric emptying, potentially reducing adverse effects such as diarrhea (104). Furthermore, the fatty acid composition of MCT products should be carefully considered as this has a sizeable effect on ketogenic potential (105). Acute metabolic studies in healthy adults demonstrate octanoate (8:0) has a significantly greater dose-response effect on plasma ketones compared with decanoate (10:0) or dodecanoate (12:0) (106, 107). Total plasma ketones were 3-fold higher after 8:0 relative to 10:0, and 2-fold higher after 10:0 relative to 12:0 (106). Ketogenic potential is also moderated by meal composition and timing. A carbohydrate-containing breakfast did not reduce the absorption of medium-chain fatty acids (MCFA), however, the breakfast affected the subsequent conversion to ketones in the proceeding 3–4 h, potentially due to postprandial insulin secretion (106). MCT emulsification has been shown to increase plasma MCFA bioavailability 2–3 fold compared with nonemulsified MCTs, elevating plasma ketones 2–4-fold in a dose-dependent fashion. Gastrointestinal side effects decreased by ∼50% compared with nonemulsified MCTs (67). The formula was 60% 8:0 and 40% 10:0 (a common ratio in many commercially available products); a synergistic combination which may produce more sustained ketone body production (67, 108). For future interventions, administering emulsified MCTs without a meal may be desirable for maximizing resulting ketosis (106, 107). Exogenous KE can also induce ketosis in the absence of adhering to a KD (109) and are advantageous as they can directly elevate blood ketone concentrations, whilst simultaneously supplying ketogenic precursors (103). KE are powerful ketogenic agents; rapidly elevating blood BHB concentrations to higher concentrations for longer periods compared with MCTs (100). Human data using KE in clinical populations and long-term safety data is currently lacking and needs to be established (103).
Study quality and risk of bias
Dietary interventions (50–52, 56–61) had higher attrition, lower adherence, and more issues with risk of bias due to inherent aspects of study design (e.g. participants knowledge of intervention allocation). Various measures were implemented to increase compliance; such as weekly contact from the research team, nutritional education sessions, a “study partner” (usually a spouse or family member), and individualized meal plans tailored to the participants’ preferences, with varying degrees of success. Dietary behavior change is often difficult to navigate; particularly in a research setting (110). For many, a KD involves significant deviation from a “typical” diet and may be challenging for clinical populations to adopt, particularly those with mild to moderate cognitive decline where activities of daily living are affected (22).
Cochrane's risk of bias tool (38) was used to appraise randomized studies as it is considered the gold standard in the evaluation of study quality within randomized clinical trials (111). Dietary interventions naturally carry an inherent risk of bias as participants and members of the research team are aware of their assigned intervention and this is not accounted for in the Cochrane's risk of bias tool. Risk can be mitigated by blinding outcome assessors to group allocation; an approach implemented in 2 dietary studies (51, 56). Remaining studies did not report on outcome assessor blinding hence many dietary studies were judged to be at moderate to high risk of bias in this domain (50, 54, 55, 57–59, 61). Studies using exogenous ketogenic agents (44, 46, 48, 49, 53, 55) tended to have lower risk of bias due to a randomized, double-blind, placebo-controlled study design. Participant knowledge of their treatment in dietary interventions was the most prominent issue, coupled with nonadherence to the treatment protocol and a lack of analysis used to estimate the effect of adherence.
As KT are an emerging area of investigation, many studies were designed to be proof of concept trials. Small underpowered samples [7 studies had ≤20 participants (44, 51, 55, 57–60)] may limit the generalizability of results, however, this highlights the need for larger, adequately powered trials to build upon encouraging effects evidenced here. Issues with recruitment (51) and participant withdrawals after enrollment further contributed to small samples.
Summary of recommendations for future research
-
Compare ketogenic diets with exogenous ketogenic agents to ascertain which approach is superior considering:
feasibility
suitability for people with NDD
ability to produce clinically significant changes in disease-specific outcomes.
Determine the optimal daily dosing protocol required for exogenous ketones to maintain stable blood ketone concentrations.
Improve tolerability and reduce adverse effects related to the use of exogenous ketones using formulations containing both KE, KS, and MCT.
Conduct pharmacokinetic studies to inform development of formulations with maximal ketogenic potential.
Establish a “minimum required dose” for clinically significant improvements in disease-specific symptoms such as cognition or motor function.
Future research directions and recommendations
In the absence of effective treatments for NDD, investigating the therapeutic potential of ketone bodies is an important avenue which may lead to a novel disease-modifying agent for these conditions. This review has highlighted that participants were more compliant in interventions using supplementation of exogenous ketogenic agents compared with dietary interventions (44, 46, 47, 49, 55). Supplementation as opposed to dietary modifications may be the most feasible option to achieve nutritional ketosis and bridge cerebral energy deficits in populations with NDD. With regards to MCT treatment, due to disparities in total daily dose, composition of MCT supplements, and duration of intervention between studies, this prevents the ability to provide any general dosage recommendations at this time. Similarly, dietary approaches were highly variable, however, all included studies used a modified KD. The classic KD was not adopted in any instance. No single ketogenic dietary approach was found to be superior in terms of clinical outcomes, and all variations of a KD administered demonstrated an elevation in serum BHB relative to baseline (50–52, 56–61). Further work should be carried out to optimize exogenous ketogenic formulations to maximize their ketogenic potential, acceptability, and tolerability. In future, articles should provide clear detail on fatty acid composition of MCT formulations to allow comparative evaluations and ensure that ketone concentrations are closely monitored, not only for compliance but to inform clinical recommendations for product selection and dosing guidelines. Posology is another key area which requires further characterization in order to establish a “minimum required dose” applicable to disease-specific phenotypes. This will allow clinicians to provide a more personalized, evidence-based approach to treatment underpinned by clear guidelines resulting in optimal outcomes for their patients.
Strengths and limitations of this review
With the goal of clinical relevance in mind, this systematic review focused on controlled clinical studies that were chronic in nature to allow for meaningful changes to be observed. Primary outcomes related to improvement of disease state and/or quality of life in NDD were evaluated. A comprehensive list of databases (n = 8) were searched using MeSH terms, a wide scope, and an extensive keyword strategy in order to capture the totality of the literature currently available. Furthermore, conducting risk of bias and GRADE assessments served to highlight methodological strengths and weaknesses, along with the certainty of the evidence which will be valuable for optimizing study design in future trials.
Due to heterogeneity in disease state, study design, and primary outcome measures assessed, a meta-analysis was not feasible. Future reviews (with alternative aims) may consider focusing on 1 NDD (i.e. MCI), or intervention (KD or exogenous ketogenic agents) in order to assess efficacy in a quantitative manner once there is sufficient literature available. In addition, the variation in disease state (e.g. MS having a much younger age of onset than other NDD) meant there were significant differences in age between participants, which further confounds interpretation of the data.
Conclusions
This systematic review summarized and critically appraised 17 controlled trials investigating a ketogenic agent (dietary modification, fasting protocol, or exogenous supplement) to improve disease-related outcomes in people with NDD. Most studies focused on people with MCI, AD, or MS and used a ketogenic dietary intervention that was 6 wk long. Study outcomes were mixed across disease states and intervention type, with a high risk of bias present among included articles. Lack of reporting on randomization, allocation concealment, missing data, and adherence to trial protocol were the most common issues found. KT appear to be a promising tool in the clinical management of NDD, however, the current evidence is not sufficient to propose any formal clinical recommendations at this time. The authors anticipate that this review can be utilized effectively to highlight research gaps in the field and to inform prospective study design in order to enhance the quality of future research assessing the therapeutic potential of ketone bodies.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ contributions were as follows—LSD and GZS: conceptualized the review and formulated the systematic search strategy; LSD: conducted the preliminary literature review, database searches, data extraction, performed the risk of bias assessment, and drafted the manuscript; the eligibility of articles and discrepancies in the risk of bias assessment were discussed and resolved between LSD and GZS; GZS and CKL: supervised LSD and assisted with finalizing the manuscript; and all authors read and approved the final manuscript.
Notes
Author disclosures: GZS was supported by funding from a National Health and Medical Research Council (NHMRC)-Australian Research Council (ARC) Dementia Research Development Fellowship (APP1102532). As a medical research institute, NICM Health Research Institute receives research grants and donations from foundations, universities, government agencies, individuals, and industry. Sponsors and donors provide untied funding for work to advance the vision and mission of the Institute. The project that is the subject of this article was not undertaken as part of a contractual relation with any organization other than the funding declared above. NICM conducts clinical trials relevant to this topic area, for which further details can be provided on request. LSD, CKL, and GZS report no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
CKL and GZS are co-senior authors.
Abbreviations used: Aβ, amyloid-β; AD, Alzheimer's disease; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive Subscale; ALOX5, arachidonate 5-lipoxygenase; BHB, β-hydroxybutyrate; COX1, cyclooxygenase 1; CSF, cerebral spinal fluid; EDSS, Expanded Disability Status Scale; GRADE, Grading of Recommendations Assessment, Development, and Education; KD, ketogenic diet; KE, ketone esters; KT, ketogenic therapies; MCI, mild cognitive impairment; MCID, minimum clinically important difference; MCFA, medium-chain fatty acids; MCT, medium-chain triglyceride; MDS-UPDRS, Movement Disorders Society-Unified Parkinson Disease Rating Scale; MMSE, Mini-Mental State Examination; MS, multiple sclerosis; MSQoL-54, Multiple Sclerosis Quality of Life-54 questionnaire; NDD, neurodegenerative disease; NTB, neuropsychological testing battery; PD, Parkinson's disease; ROBINS-I, Risk of Bias in Non-Randomised Studies of Interventions; V-PAL, Verbal Paired Association Learning test; 8:0, caprylic acid; 10:0, decanoate; 12:0, dodecanoate.
Contributor Information
Lauren S Dewsbury, NICM Health Research Institute, Western Sydney University, Penrith, New South Wales, Australia.
Chai K Lim, Department of Biomedical Sciences, Faculty of Medicine Health and Human Sciences, Macquarie University, Macquarie Park, New South Wales, Australia.
Genevieve Z Steiner, NICM Health Research Institute, Western Sydney University, Penrith, New South Wales, Australia; Translational Health Research Institute (THRI), Western Sydney University, Penrith, New South Wales, Australia.
References
- 1. Zilberter Y, Zilberter M. The vicious circle of hypometabolism in neurodegenerative diseases: ways and mechanisms of metabolic correction. J Neurosci Res. 2017;95:2217–35. [DOI] [PubMed] [Google Scholar]
- 2. Kiaei M. New hopes and challenges for treatment of neurodegenerative disorders: great opportunities for young neuroscientists. Basic Clin Neurosci. 2013;4:3–4. [PMC free article] [PubMed] [Google Scholar]
- 3. Lewerenz J, Maher P. Chronic glutamate toxicity in neurodegenerative diseases – what is the evidence?. Front Neurosci. 2015;9:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cahill GF, Herrera MG, Morgan AP, Soeldner JS, Steinke J, Levy PL, Reichard GA, Kipnis DM. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966;45:1751–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Błaszczyk JW. The emerging role of energy metabolism and neuroprotective strategies in Parkinson's disease. Front Aging Neurosci. 2018;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prins ML. Cerebral metabolic adaptation and ketone metabolism after brain injury. J Cereb Blood Flow Metab. 2008;28:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics access. Cell Metab. 2017;25:262–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins, Leukotrienes Essent Fatty Acids. 2004;70:309–19. [DOI] [PubMed] [Google Scholar]
- 9. Cahill GF. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. [DOI] [PubMed] [Google Scholar]
- 10. Paoli A, Bianco A, Damiani E, Bosco G. Ketogenic diet in neuromuscular and neurodegenerative diseases. Biomed Res Int. 2014;2014:474296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. White H, Venkatesh K, Venkatesh B. Systematic review of the use of ketones in the management of acute and chronic neurological disorders. J Neurol Neurosci. 2017;08:1–9. [Google Scholar]
- 12. Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006;17:431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giménez-Cassina A, Martínez-François JR, Fisher JK, Szlyk B, Polak K, Wiwczar J, Tanner GR, Lutas A, Yellen G, Danial NN. BAD-dependent regulation of fuel metabolism and K(ATP) channel activity confers resistance to epileptic seizures. Neuron. 2012;74(4):719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–35. [DOI] [PubMed] [Google Scholar]
- 15. Van Der Auwera I, Wera S, Van Leuven F, Henderson ST. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer's disease. Nutr Metab (Lond). 2005;2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J, Ho L, Qin W, Rocher AB, Seror I, Humala N, Maniar K, Dolios G, Wang R, Hof PRet al. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer's disease. FASEB J. 2005;19(6):659–61. [DOI] [PubMed] [Google Scholar]
- 17. Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL. D-b-Hydroxybutyrate protects neurons in models of Alzheimer's and Parkinson's disease. Proc Natl Acad Sci. 2000;97:5440–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JMet al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc Natl Acad Sci. 1997;94:4318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin Q, Ruuska SE, Shaw NS, Dong D, Noy N. Ligand selectivity of the peroxisome proliferator-activated receptor α. Biochemistry. 1999;38:185–90. [DOI] [PubMed] [Google Scholar]
- 20. Cullingford TE. The ketogenic diet; fatty acids, fatty acid-activated receptors and neurological disorders. Prostaglandins, Leukotrienes Essent Fatty Acids. 2004;70:253–64. [DOI] [PubMed] [Google Scholar]
- 21. Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE. Caloric restriction attenuates Aβ-deposition in Alzheimer transgenic models. Neurobiol Aging. 2005;26:995–1000. [DOI] [PubMed] [Google Scholar]
- 22. Lee M-T, Jang Y, Chang W-Y. How do impairments in cognitive functions affect activities of daily living functions in older adults?. PLoS One. 2019;14(6):e0218112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hashimoto M, Hossain S, Shimada T, Sugioka K, Yamasaki H, Fujii Y, Ishibashi Y, Oka JI, Shido O. Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer's disease model rats. J Neurochem. 2002;81:1084–91. [DOI] [PubMed] [Google Scholar]
- 24. Hashimoto M, Tanabe Y, Fujii Y, Kikuta T, Shibata H, Shido O. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid β infused rats. J Nutr. 2005;135:549–55. [DOI] [PubMed] [Google Scholar]
- 25. D'Andrea Meira I, Romão TT, Do Prado HJP, Krüger LT, Pires MEP, Da Conceição PO. Ketogenic diet and epilepsy: what we know so far. Front Neurosci. 2019;13:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kverneland M, Selmer KK, Nakken KO, Iversen PO, Taubøll E. A prospective study of the modified Atkins diet for adults with idiopathic generalized epilepsy. Epilepsy Behav. 2015;53:197–201. [DOI] [PubMed] [Google Scholar]
- 27. Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–6. [DOI] [PubMed] [Google Scholar]
- 28. Liu H, Yang Y, Wang Y, Tang H, Zhang F, Zhang Y, Zhao Y. Ketogenic diet for treatment of intractable epilepsy in adults: a meta-analysis of observational studies. Epilepsia Open. 2018;3:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leone A, De Amicis R, Lessa C, Tagliabue A, Trentani C, Ferraris C, Battezzati A, Veggiotti P, Foppiani A, Ravella Set al. Food and food products on the Italian market for ketogenic dietary treatment of neurological diseases. Nutrients. 2019;11:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sampath A, Kossoff EH, Furth SL, Pyzik PL, Vining EPG. Kidney stones and the ketogenic diet: risk factors and prevention. J Child Neurol. 2007;22:375–8. [DOI] [PubMed] [Google Scholar]
- 31. Furth SL, Casey JC, Pyzik PL, Neu AM, Docimo SG, Vining EPG, Freeman JM, Fivush BA. Risk factors for urolithiasis in children on the ketogenic diet. Pediatr Nephrol. 2000;15:125–8. [DOI] [PubMed] [Google Scholar]
- 32. Sherrier M, Li H. The impact of keto-adaptation on exercise performance and the role of metabolic-regulating cytokines. Am J Clin Nutr. 2019;110:562–73. [DOI] [PubMed] [Google Scholar]
- 33. El-Rashidy OF, Nassar MF, Abdel-Hamid IA, Shatla RH, Abdel-Hamid MH, Gabr SS, Mohamed SG, El-Sayed WS, Shaaban SY. Modified Atkins diet vs classic ketogenic formula in intractable epilepsy. Acta Neurol Scand. 2013;128:402–8. [DOI] [PubMed] [Google Scholar]
- 34. Miranda MJ, Turner Z, Magrath G. Alternative diets to the classical ketogenic diet – can we be more liberal?. Epilepsy Res. 2012;100:278–85. [DOI] [PubMed] [Google Scholar]
- 35. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eriksen MB, Frandsen TF. The impact of PICO as a search strategy tool on literature search quality: a systematic review. J Med Libr Assoc. 2018;106:420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. ICD Codes . ICD-10-CM Code G31.9 Degenerative Disease of Nervous System, Unspecified. [Internet]. 2020. [Accessed 2020 Nov 2]. Available from: https://icd.codes/icd10cm/G319. [Google Scholar]
- 38. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron Iet al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. GRADE: what is “quality of evidence” and why is it important to clinicians?. BMJ. 2008;336:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, Schiinemann HJ. GRADE: going from evidence to recommendations. BMJ. 2008;336:1049–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murad MH, Mustafa RA, Schünemann HJ, Sultan S, Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evid Based Med. 2017;22:85–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Torosyan N, Sethanandha C, Grill JD, Dilley ML, Lee J, Cummings JL, Ossinalde C, Silverman DH. Changes in regional cerebral blood flow associated with a 45 day course of the ketogenic agent, caprylidene, in patients with mild to moderate Alzheimer's disease: results of a randomized, double-blinded, pilot study. Exp Gerontol. 2018;111:118–21. [DOI] [PubMed] [Google Scholar]
- 45. Swidsinski A, Dörffel Y, Loening-Baucke V, Gille C, Göktas Ö, Reißhauer A, Neuhaus J, Weylandt KH, Guschin A, Bock M. Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet. Front Microbiol. 2017;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer's disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab. 2009;6:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Henderson ST, Poirier J. Pharmacogenetic analysis of the effects of polymorphisms in APOE, IDE and IL1B on a ketone body based therapeutic on cognition in mild to moderate Alzheimer's disease; a randomized, double-blind, placebo-controlled study. BMC Med Genet. 2011;12:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Henderson ST, Morimoto BH, Cummings JL, Farlow MR, Walker J. A placebo-controlled, parallel-group, randomized clinical trial of AC-1204 in mild-to-moderate Alzheimer's disease. J Alzheimer's Dis. 2020;75:547–57. [DOI] [PubMed] [Google Scholar]
- 49. Fortier M, Castellano CA, Croteau E, Langlois F, Bocti C, St-Pierre V, Vandenberghe C, Bernier M, Roy M, Descoteaux Met al. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimer's Dement. 2019;15:625–34. [DOI] [PubMed] [Google Scholar]
- 50. Choi IY, Piccio L, Childress P, Bollman B, Ghosh A, Brandhorst S, Suarez J, Michalsen A, Cross AH, Morgan TEet al. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. 2016;15:2136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brandt J, Buchholz A, Henry-Barron B, Vizthum D, Avramopoulos D, Cervenka MC. Preliminary report on the feasibility and efficacy of the modified Atkins diet for treatment of mild cognitive impairment and early Alzheimer's disease. J Alzheimer's Dis. 2019;68:969–81. [DOI] [PubMed] [Google Scholar]
- 52. Bock M, Karber M, Kuhn H. Ketogenic diets attenuate cyclooxygenase and lipoxygenase gene expression in multiple sclerosis. EBioMedicine. 2018;36:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu Q, Zhang Y, Zhang X, Liu L, Zhou B, Mo R, Li Y, Li H, Li F, Tao Yet al. Medium-chain triglycerides improved cognition and lipid metabolomics in mild to moderate Alzheimer's disease patients with APOE4−/−: a double-blind, randomized, placebo-controlled crossover trial. Clin Nutr. 2020;39(7):2092–105. [DOI] [PubMed] [Google Scholar]
- 54. Saadatnia M, Etemadifar M, Fatehi F, Ashtari F, Shaygannejad V, Chitsaz A, Maghzi AH. Short-term effects of prolonged fasting on multiple sclerosis. Eur Neurol. 2009;61:230–2. [DOI] [PubMed] [Google Scholar]
- 55. Rebello CJ, Keller JN, Liu AG, Johnson WD, Greenway FL. Pilot feasibility and safety study examining the effect of medium chain triglyceride supplementation in subjects with mild cognitive impairment: a randomized controlled trial. BBA Clin. 2015;3:123–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Phillips MCL, Murtagh DKJ, Gilbertson LJ, Asztely FJS, Lynch CDP. Low-fat versus ketogenic diet in Parkinson's disease: a pilot randomized controlled trial. Mov Disord. 2018;33:1306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Neth BJ, Mintz A, Whitlow C, Jung Y, Solingapuram Sai K, Register TC, Kellar D, Lockhart SN, Hoscheidt S, Maldjian Jet al. Modified ketogenic diet is associated with improved cerebrospinal fluid biomarker profile, cerebral perfusion, and cerebral ketone body uptake in older adults at risk for Alzheimer's disease: a pilot study. Neurobiol Aging. 2020;86:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nagpal R, Neth BJ, Wang S, Craft S, Yadav H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer's disease markers in subjects with mild cognitive impairment. EBioMedicine. 2019;47:529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee JE, Titcomb TJ, Bisht B, Rubenstein LM, Louison R, Wahls TL. A modified MCT-based ketogenic diet increases plasma β-hydroxybutyrate but has less effect on fatigue and quality of life in people with multiple sclerosis compared to a modified paleolithic diet: a waitlist-controlled, randomized pilot study. J Am Coll Nutr. 2020;0:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Krikorian R, Shidler MD, Summer SS, Sullivan PG, Duker AP, Isaacson RS, Espay AJ. Nutritional ketosis for mild cognitive impairment in Parkinson's disease: a controlled pilot trial. Clin Park Relat Disord. 2019;1:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging. 2012;33:425.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rockwood K, Fay S, Gorman M. The ADAS-cog and clinically meaningful change in the VISTA clinical trial of galantamine for Alzheimer's disease. Int J Geriat Psychiatry. 2010;25:191–201. [DOI] [PubMed] [Google Scholar]
- 63. Kueper JK, Speechley M, Montero-Odasso M. The Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog): modifications and responsiveness in pre-dementia populations. A narrative review. JAD. 2018;63:423–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gilbert DL, Pyzik PL, Freeman JM. The ketogenic diet: seizure control correlates better with serum β-hydroxybutyrate than with urine ketones. J Child Neurol. 2000;15:787–90. [DOI] [PubMed] [Google Scholar]
- 65. Rockwood K, Fay S, Gorman M, Carver D, Graham JE. The clinical meaningfulness of ADAS-Cog changes in Alzheimer's disease patients treated with donepezil in an open-label trial. BMC Neurol. 2007;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schrag A, Schott JM. What is the clinically relevant change on the ADAS-Cog?. J Neurol Neurosurg Psychiatry. 2012;83:171–3. [DOI] [PubMed] [Google Scholar]
- 67. Courchesne-Loyer A, Lowry CM, St-Pierre V, Vandenberghe C, Fortier M, Castellano CA, Wagner JR, Cunnane SC. Emulsification increases the acute ketogenic effect and bioavailability of medium-chain triglycerides in humans. Curr Dev Nutr. 2017;1:e000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Leritz EC, McGlinchey RE, Kellison I, Rudolph JL, Milberg WP. Cardiovascular disease risk factors and cognition in the elderly. Curr Cardiovasc Risk Rep. 2011;5:407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Grodstein F. Cardiovascular risk factors and cognitive function. Alzheimer's & Dementia. 2007;3:S16. [DOI] [PubMed] [Google Scholar]
- 70. Yancy WS, Foy M, Chalecki AM, Vernon MC, Westman EC. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hussain TA, Mathew TC, Dashti AA, Asfar S, Al-Zaid N, Dashti HM. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition. 2012;28:1016–21. [DOI] [PubMed] [Google Scholar]
- 72. Hu T, Mills KT, Yao L, Demanelis K, Eloustaz M, Yancy WS, Kelly TN, He J, Bazzano LA. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol. 2012;176:S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dashti HM, Mathew TC, Khadada M, Al-Mousawi M, Talib H, Asfar SK, Behbahani AI, Al-Zaid NS. Beneficial effects of ketogenic diet in obese diabetic subjects. Mol Cell Biochem. 2007;302:249–56. [DOI] [PubMed] [Google Scholar]
- 74. Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, Hyde K, Chapman D, Craft S. Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25:311–4. [DOI] [PubMed] [Google Scholar]
- 75. Chouinard-Watkins R, Plourde M. Fatty acid metabolism in carriers of apolipoprotein e epsilon 4 allele: is it contributing to higher risk of cognitive decline and coronary heart disease?. Nutrients. 2014;6:4452–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Plourde M, Vohl MC, Vandal M, Couture P, Lemieux S, Cunnane SC. Plasma n–3 fatty acid response to an n–3 fatty acid supplement is modulated by apoE 4 but not by the common PPAR-L162V polymorphism in men. Br J Nutr. 2009;102:1121–4. [DOI] [PubMed] [Google Scholar]
- 77. Duarte AI, Santos MS, Oliveira CR, Moreira PI. Brain insulin signalling, glucose metabolism and females’ reproductive aging: a dangerous triad in Alzheimer's disease. Neuropharmacology. 2018;136:223–42. [DOI] [PubMed] [Google Scholar]
- 78. Rahman A, Jackson H, Hristov H, Isaacson RS, Saif N, Shetty T, Etingin O, Henchcliffe C, Brinton RD, Mosconi L. Sex and gender driven modifiers of Alzheimer's: the role for estrogenic control across age, race, medical, and lifestyle risks. Front Aging Neurosci. 2019;11:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Scheyer O, Rahman A, Hristov H, Berkowitz C, Isaacson RS, Diaz Brinton R, Mosconi L. Female sex and Alzheimer's risk: the menopause connection. J Prev Alzheimer's Dis. 2018;5(4):225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mosconi L, Rahman A, Diaz I, Wu X, Scheyer O, Hristov HW, Vallabhajosula S, Isaacson RS, de Leon MJ, Brinton RD. Increased Alzheimer's risk during the menopause transition: a 3-year longitudinal brain imaging study. PLoS One. 2018;13:e0207885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Brinton RD. Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer's disease. Adv Drug Deliv Rev. 2008;60:1504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jongen PJ. Health-related quality of life in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs. 2017;31:585–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nathan J, Kale DK, Naik VD, Thakker F, Bailur S. Dietary therapy in secondary progressive multiple sclerosis: a case report. Cureus. 2019;11(8):e5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brenton JN, Banwell B, Bergqvist AGC, Lehner-Gulotta D, Gampper L, Leytham E, Coleman R, Goldman MD. Pilot study of a ketogenic diet in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm. 2019;6(4):e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hinnell C, Hurt CS, Landau S, Brown RG, Samuel M, Burn DJ, Wilson KC, Hindle JV. Nonmotor versus motor symptoms: how much do they matter to health status in Parkinson's disease?. Mov Disord. 2012;27:236–41. [DOI] [PubMed] [Google Scholar]
- 87. Sethi K. Levodopa unresponsive symptoms in Parkinson disease. Mov Disord. 2008;23:521–33. [DOI] [PubMed] [Google Scholar]
- 88. Milder JB, Liang L-P, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Harvey C, Schofield GM, Williden M, McQuillan JA. The effect of medium chain triglycerides on time to nutritional ketosis and symptoms of keto-induction in healthy adults: a randomised controlled clinical trial. J Nutr Metab. 2018;2018:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Klein P, Tyrlikova I, Mathews GC. Dietary treatment in adults with refractory epilepsy: a review. Neurology. 2014;83:1978–85. [DOI] [PubMed] [Google Scholar]
- 91. Forster SE, Jones L, Saxton JM, Flower DJ, Foulds G, Powers HJ, Parker SG, Pockley AG, Williams EA. Recruiting older people to a randomised controlled dietary intervention trial – how hard can it be?. BMC Med Res Methodol. 2010;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Crichton GE, Howe PRC, Buckley JD, Coates AM, Murphy KJ, Bryan J. Long-term dietary intervention trials: critical issues and challenges. Trials. 2012;13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Fabricatore AN, Wadden TA, Moore RH, Butryn ML, Gravallese EA, Erondu NE, Heymsfield SB, Nguyen AM. Attrition from randomized controlled trials of pharmacological weight loss agents: a systematic review and analysis: obesity management. Obes Rev. 2009;10:333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Middleton KR, Anton SD, Perri MG. Long-term adherence to health behavior change access. Am J Lifestyle Med. 2013;7:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’ s disease. Alzheimer's Dement Transl Res Clin Interv. 2018;4:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Herber DL, Weeber EJ, D'Agostino DP, Duis J. Evaluation of the safety and tolerability of a nutritional Formulation in patients with ANgelman Syndrome (FANS): study protocol for a randomized controlled trial. Trials. 2020;21:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dhatariya K. Blood ketones: measurement, interpretation, limitations, and utility in the management of diabetic ketoacidosis. Rev Diabet Stud. 2016;13:217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Arora S, Henderson SO, Long T, Menchine M. Diagnostic accuracy of point-of-care testing for diabetic ketoacidosis at emergency-department triage: β-hydroxybutyrate versus the urine dipstick. Diabetes Care. 2011;34:852–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Harvey CJDC, Schofield GM, Williden M. The use of nutritional supplements to induce ketosis and reduce symptoms associated with keto-induction: a narrative review. PeerJ. 2018;6:e4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Stubbs BJ, Cox PJ, Evans RD, Santer P, Miller JJ, Faull OK, Magor-Elliott S, Hiyama S, Stirling M, Clarke K. On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kesl SL, Poff AM, Ward NP, Fiorelli TN, Ari C, Van Putten AJ, Sherwood JW, Arnold P, D'Agostino DP. Effects of exogenous ketone supplementation on blood ketone, glucose, triglyceride, and lipoprotein levels in Sprague-Dawley rats. Nutr Metab (Lond). 2016;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ari C, Koutnik AP, DeBlasi J, Landon C, Rogers CQ, Vallas J, Bharwani S, Puchowicz M, Bederman I, Diamond DMet al. Delaying latency to hyperbaric oxygen-induced CNS oxygen toxicity seizures by combinations of exogenous ketone supplements. Physiol Rep. 2019;7:e13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Poff AM, Rho JM, D'Agostino DP. Ketone administration for seizure disorders: history and rationale for ketone esters and metabolic alternatives. Front Neurosci. 2019;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. D'Agostino D, Arnold P, Kesl S. Compositions and Methods for Producing Elevated and Sustained Ketosis. International Patent WO2014153416A1, 2015. [Google Scholar]
- 105. Vandenberghe C, St-Pierre V, Fortier M, Castellano CA, Cuenoud B, Cunnane SC. Medium chain triglycerides modulate the ketogenic effect of a metabolic switch. Front Nutr. 2020;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. St-Pierre V, Vandenberghe C, Lowry CM, Fortier M, Castellano CA, Wagner R, Cunnane SC. Plasma ketone and medium chain fatty acid response in humans consuming different medium chain triglycerides during a metabolic study day. Front Nutr. 2019;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Vandenberghe C, St-Pierre V, Pierotti T, Fortier M, Castellano CA, Cunnane SC. Tricaprylin alone increases plasma ketone response more than coconut oil or other medium-chain triglycerides: an acute crossover study in healthy adults. Curr Dev Nutr. 2017;1:e000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sonnay S, Chakrabarti A, Thevenet J, Wiederkehr A, Christinat N, Masoodi M. Differential metabolism of medium-chain fatty acids in differentiated human-induced pluripotent stem cell-derived astrocytes. Front Physiol. 2019;10:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Brunengraber H. Potential of ketone body esters for parenteral and oral nutrition. Nutrition. 1997;13:233–5. [DOI] [PubMed] [Google Scholar]
- 110. Browne S, Minozzi S, Bellisario C, Sweeney MR, Susta D. Effectiveness of interventions aimed at improving dietary behaviours among people at higher risk of or with chronic non-communicable diseases: an overview of systematic reviews. Eur J Clin Nutr. 2019;73:9–23. [DOI] [PubMed] [Google Scholar]
- 111. Jørgensen L, Paludan-Müller AS, Laursen DRT, Savović J, Boutron I, Sterne JAC, Higgins JPT, Hróbjartsson A. Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Syst Rev. 2016;5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.