ABSTRACT
Results from observational studies indicate that whole grain (WG) intake is inversely associated with BMI and risk of weight gain. WG intake may influence energy balance and body composition through effects on appetite and energy intake. To evaluate the impact of WG food consumption on appetite and energy intake, a systematic review and meta-analysis was performed of results from randomized controlled trials (RCTs) assessing WG food consumption, appetite, and energy intake in adults. A search of PubMed, Scopus, and Food Science and Technology Abstracts yielded 36 RCTs measuring subjective appetite ratings after consuming WG foods compared with refined grain (RG) controls. Thirty-two of these studies reported AUCs for subjective appetite (hunger, fullness, satiety, desire to eat, or prospective consumption) and/or energy intake and were included in the meta-analysis. Pooled estimates from meta-analyses are expressed as standardized mean differences (SMDs). Compared with RG foods, intake of WG foods resulted in significant differences in AUCs for subjective hunger (SMD: −0.34; 95% CI: −0.46, −0.22; P < 0.001), fullness (SMD: 0.49; 95% CI: 0.31, 0.66; P < 0.001), satiety (SMD: 0.33; 95% CI: 0.18, 0.47; P < 0.001), and desire to eat (SMD: −0.33; 95% CI: −0.46, −0.20; P < 0.001). There were small, nonsignificant reductions in prospective consumption ratings (P = 0.08) and energy intake (P = 0.07) with WG intake compared with RG. These results support the view that consumption of WG foods, compared with RG foods, significantly impacts subjective appetite, and might partly explain the inverse associations between WG food intake and risk of overweight, obesity, and weight gain over time.
PROSPERO registration: CRD42020148217.
Keywords: whole grain, appetite, satiety, hunger, fullness, desire to eat, prospective food consumption, energy intake, meta-analysis, randomized controlled trials
Introduction
Whole grains (WGs) are intact, ground, cracked, or flaked grain kernels that contain all 3 anatomical components—endosperm, bran, and germ—in the same relative proportions as they exist in the intact kernel (1, 2). WG foods tend to be higher in fiber, B vitamins, iron, zinc, magnesium, and selenium compared with foods made predominantly with refined grains (RGs) (3). Accordingly, the 2015 Dietary Guidelines for Americans recommends at least half of daily grain intake to be from WGs and includes WG foods in every healthy eating pattern. However, most Americans continue to consume more RG foods (e.g., white bread, white rice) than WG foods (e.g., wholewheat bread, brown rice, oatmeal) (3).
Results from observational studies suggest that higher intake of WGs is associated with lower risk of weight gain and incident overweight or obesity (4–6), although, findings from short-term (≤16 wk), randomized controlled trials (RCTs) evaluating the effect of higher WG intake on body weight have been equivocal (4, 7). One of the potential mechanisms by which WGs could impact body weight over the long term is by suppressing appetite and, consequently, reducing energy intake. Many WG foods contain quantities of dietary fiber that have the potential to influence glucose metabolism (8–10), gastrointestinal transit (11), and gastrointestinal hormone secretions (12), all of which have the potential to impact appetite. Furthermore, fermentation of fiber and other phenolic compounds in WGs by gut microbes can create secondary metabolites, such as SCFAs, that could influence appetite and energy intake (13).
Appetite is typically measured with a series of questions relating to subjective sensations, such as hunger, fullness, satiety or satisfaction, desire to eat, and prospective consumption (14, 15). Visual analog scales (VAS) with an anchor term at each end of the scale have been validated as a method for assessing changes in appetite over time after food consumption (16). Satiety (How satisfied are you?) and fullness (“How full are you?”) are related concepts and often used interchangeably, depending on the preference of the investigator, but both questions have been validated (14, 16). Postprandial VAS scores often correlate with subsequent meal energy intake (15, 16). However, VAS scores alone can be unreliable as a proxy measure for subsequent energy intake, so, ideally, both subjective appetite sensations and energy intake should be measured (17).
Although many clinical trials have investigated the effect of WG consumption on subjective appetite or energy intake, due to mixed results and mostly small studies, the totality of evidence remains unclear. Therefore, the objective of this systematic review and meta-analysis was to evaluate the impact of consuming WGs, compared with RGs, on outcomes related to subjective appetite and energy intake in RCTs in adults. The primary outcome was hunger AUC and secondary outcomes were fullness AUC, desire to eat AUC, satiety AUC, prospective consumption AUC, and energy intake.
Methods
Literature searches
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for performing the systematic review and meta-analyses (18). A comprehensive literature search was conducted using the PubMed database, Scopus, and Food Science & Technology Abstracts, which covered studies published from 1946 through September 2019. The search was designed to identify publications of RCTs that examined WG intake from intact WGs (e.g., rye, oats, quinoa, brown rice, etc.) or foods made with WGs (e.g., breads, ready-to-eat breakfast cereals, etc.) and outcomes related to subjective appetite (hunger, fullness, satiety, desire to eat, prospective consumption), energy intake, gastric emptying, and appetite-related hormones (e.g., ghrelin, leptin). Full search term details are provided in Supplemental Table 1. Prior to the data analysis, in April 2020, the literature search was performed again in PubMed only to identify relevant studies published between the initial search and data analysis. No additional studies were identified.
Inclusion and exclusion criteria
Inclusion criteria consisted of RCTs conducted in adult humans (≥18 y of age), English language publications, WG foods (≥51% of grains being WG) (19, 20) as the main intervention compared with RG foods as a control, documented (or the ability to determine) quantitative intake of WG, and a measurement of subjective appetite, energy intake, appetite-related hormones, and/or gastric emptying time. Exclusion criteria included observational studies (cross-sectional, retrospective or prospective cohorts), case-control or single-arm studies with no control condition, studies in animals or in vitro, multicomponent interventions where the effect of WGs cannot be determined (e.g., intervention with WGs and additional fiber compared with RGs without added fiber), studies comparing different types of WGs without an RG control, studies on individual grain components (e.g., bran) or dietary supplements, interventions administered via tube feeding or enteral nutrition, studies in children (<18 y of age) or pregnant/lactating women, trials using medications or supplements known to influence appetite or gastric emptying, and studies in subjects with a chronic disease, with the exception of type 2 diabetes mellitus, obesity, or metabolic syndrome.
Screening and data extraction
Publications identified in each database using the search terms were combined and duplicates were removed. First-level screening of titles and abstracts was completed independently by a member of the research team (LMS) using Abstrackr (http://abstrackr.cebm.brown.edu/). Full texts of all publications identified as potentially eligible were obtained for further review. Publications that were unclear with respect to eligibility were resolved by discussion with the research team. Reference lists from eligible publications were reviewed to determine any additional studies for inclusion. Following the full-text review, PICO (population, intervention, comparator, and outcome) data were extracted from the eligible studies into a database independently by 1 reviewer (LMS) and verified for accuracy independently by a second reviewer (MLW). All discrepancies were resolved by discussion among the reviewers and referencing the original publication. Outcomes extracted from eligible studies included subjective appetite measures, energy intake (subsequent meal in acute studies and daily intake for chronic studies), appetite-related hormones, and gastric emptying.
In studies where outcomes were reported in bar graphs, Engauge Digitizer software version 4.1 (http://markummitchell.github.io/engauge-digitizer/) was used to estimate the means and SD or SEM in the graphs for inclusion in the database. If studies reported measuring subjective appetite or energy intake but did not report the data or variability, the corresponding author was contacted by e-mail to request the quantitative data. One author responded with additional data which was included in the data extraction. Two publications (21, 22) did not report SDs or SEMs and the authors did not respond to e-mail requests, therefore, the SDs for the outcomes were estimated as the maximum SD reported by other studies of the same duration.
For studies where the amount of WG in the final food was not documented, the recipes for test foods, or the label information for commercial products, was reviewed. For low-moisture foods (e.g., pasta, flakes) the percentage WG in the dry ingredients of the recipe was estimated as the percentage WG of the final food. For higher moisture foods (e.g., bread), the percentage WG in the dry ingredients of the recipe was estimated as the percentage WG of the final food after adjustment for the moisture content. If the moisture content of the final food was not provided in the publication, moisture content was estimated using Food Data Central from the USDA Agriculture Research Service (23).
Assessment of study quality
Risk of bias for each relevant outcome within a study was assessed independently by a member of the research team (LMS) with the Cochrane risk-of-bias tool for randomized trials (24). The quality of the evidence for each outcome was assessed through discussion among members of the research team using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method (25).
Statistical analysis
Meta-analyses were completed using MedCalc Statistical Software version 19.0.5 (MedCalc Software BVBA; https://medcalc.org; 2019). Subjective appetite measures were prespecified as the primary outcome, but because hunger was the most frequently measured outcome for subjective appetite, it was selected after the data extraction, but prior to completion of the meta-analysis, to be the primary outcome of the subjective appetite measures. The primary analysis for all subjective appetite measures used pooled SMD estimates (WG compared with RG control) and 95% CIs for AUCs of hunger, fullness, satiety, desire to eat, and prospective consumption. Although all studies used a VAS to measure appetite and calculate AUCs, some VAS scales differed in anchoring statements and length (i.e., not all used a 100-mm line). The use of SMDs allowed pooling of the results from studies with these different approaches. The primary analysis for energy intake measures used pooled SMD estimates (WG compared with RG control) and 95% CIs for caloric intake. Statistical significance for individual study and pooled SMDs was declared when the 95% CI did not include the null value of 0 (i.e., P value <0.05). Studies were weighted according to the inverse of the variance of each study's effect using random effects models. Random effects models were chosen for the primary analyses due to differences across studies in key design elements such as subject characteristics and length of test period. Fixed effects models were completed for hunger and other appetite measures in the main analysis (i.e., not for subgroups) as sensitivity analyses. Because results did not differ materially between random and fixed effects models, only results from the former are presented. The magnitude of effect sizes were interpreted as <0.40 = small, 0.40–0.70 = moderate, and >0.70 = large (26). Analyses were not completed for gastric emptying because only 3 studies with data were available and different methodologies were used for measuring gastric emptying rate and/or time (MRI, paracetamol, and ultrasound). Analyses for appetite-related hormones were not completed due to budgetary and time constraints.
Sensitivity analyses were completed for subjective appetite measurements to assess the degree to which varying time frames for determination of AUCs could have impacted the results. This was achieved by analyzing AUC measurements <180 min and ≥180 min separately. An additional sensitivity analysis on the subset of studies requiring calculation of the WG content was also completed.
Subgroup analyses were performed on subjective appetite measures for type of WG, amount of WG consumed (less than or equal to the median, or greater than the median), feeding approach (matching available carbohydrates, matching calories and volume), and measurement timing (immediately after meal, subsequent meal). Similar subgroup analyses were performed for energy intake with the 1 difference of measurement timing (subsequent meal, third meal, or daily intake). Subgroup analyses were not possible for health status (e.g., type 2 diabetes), age, gender, or BMI due to an insufficient number of studies or combined reporting within studies (e.g., overweight and normal weight data combined) that did not allow for distinct subgroups.
Statistical heterogeneity was assessed using Cochran Q and the I2 statistic. An I2 value ≥40% was used to designate moderate or higher heterogeneity, in accordance with the recommendations in the Cochrane Handbook (27). The presence of publication bias was assessed visually by examining funnel plots measuring the SEM as a function of the SMD.
Results
A flow diagram summarizing the literature search process is shown in Figure 1. The scope of this review is limited to subjective appetite and energy intake. Therefore, of the 51 eligible articles included in the data extraction, 36 were included in the systematic review (13, 21, 22, 28–60) and 32 were included in the meta-analysis of subjective appetite and/or energy intake. The 4 studies excluded from the meta-analysis (57–60) reported measuring subjective appetite AUCs and/or energy intake, but did not show the data and authors did not respond to e-mail requests for the data.
FIGURE 1.
Flow diagram of literature search process for the effect of WGs on subjective appetite and energy intake in adults. FSTA, Food Science & Technology Abstracts; WG, whole grain.
Study characteristics are presented in Table 1. Thirty five (13, 21, 22, 28–47, 49–60) of the 36 studies were crossover in design, with only 1 parallel trial (48), and included data from 794 participants. Four studies included daily consumption of WGs or RGs for 3 to 8 wk (13, 48, 51, 60), whereas the remaining studies tested the response to acute intake of WGs and RGs. WG intake in longer-term feeding studies ranged from 48 to 145 g/d, and acute studies ranged from 40 g to 254 g. Subjective appetite was measured only in acute studies. The most common type of WG tested was rye in 15 publications (21, 30, 31, 34, 37, 38, 40–42, 44, 48, 49, 51, 55, 57), followed by wheat in 12 publications (22, 29, 32, 33, 36, 43, 48, 54, 55, 57, 58, 60). Other WGs tested included barley (39, 46, 54, 59), oats (28, 35, 45, 52), corn (50, 53), rice (47), buckwheat (56), and quinoa (56).
TABLE 1.
Summary of studies included in the systematic review and meta-analysis of WG intake and subjective appetite AUC and/or energy intake in adults1
| Study | Population | Health status | Study design | WG exposure2 | RG control exposure | Outcomes measured | Length of appetite testing | Effect of WG |
|---|---|---|---|---|---|---|---|---|
| Wolever et al. (28) | n = 40 | Healthy | Crossover | 40 g WG oats | Cream of rice in skim milk | Hunger | 180 min | NS hunger |
| 60% male | Acute appetite test | Fullness | NS fullness | |||||
| 39.2 ± 13.1 y | Matched available CHO | Desire to eat | NS desire to eat | |||||
| BMI: 26.5 ± 3.1 | Breakfast meal | Prospective consumption | NS prospective consumption | |||||
| Costabile et al. (29) | n = 14 | Healthy | Crossover | 117 g WG wheat in pasta | Wheat pasta | Hunger | 240 min | ↓Hunger |
| 50% male | Acute appetite test | Satiety | NS satiety | |||||
| 30 ± 2 y | Matched available CHO | Desire to eat | ↓Desire to eat | |||||
| BMI: 22 ± 1 | Breakfast meal | Energy intake | NS energy intake | |||||
| Energy intake at lunch | ||||||||
| Lee et al. (30) | n = 21 | Healthy | Crossover | 40 g WG rye in porridge | Wheat bread (55 g portion) | Hunger | 240 min | ↓Hunger (55 g) |
| 52% male | Acute appetite test | 55 g WG rye in porridge | Fullness | ↑Fullness (55 g) | ||||
| 38.6 ± 11.8 y | Matched calories | Desire to eat | NS desire to eat | |||||
| BMI: 24.9 ± 3.3 | Breakfast meal | |||||||
| Sandberg et al. (31) | n = 2148% male25.3 ± 3.9 yBMI: 22.7 ± 2.3 | Healthy | CrossoverSubsequent meal appetite testMatched available CHODinner mealEnergy intake at lunch | 134.5 g WG rye from rye flour bread133.5 g WG rye from flour/kernel blend bread | Wheat bread | HungerSatietyDesire to eatEnergy intake | 210 min | ↓Hunger↑Satiety (rye flour bread)↓Desire to eat (rye flour bread)NS energy intake |
| Cioffi et al. (32) | n = 16 | Healthy, overweight to obese | Crossover | 100 g WG wheat in pasta | Wheat pasta | Hunger | 240 min | NS hunger |
| 44% male | Acute appetite test | Fullness | ↑Fullness | |||||
| 44 ± 10 yBMI: 30.1 ± 2.8 | Matched calories and volumeLunch meal | SatietyProspective consumptionEnergy intake | ↑Satiety↓Prospective consumptionNS energy intake | |||||
| Cioffi et al. (33) | n = 8 | Healthy, normal weight to overweight | Crossover | 100 g WG wheat in pasta | Wheat pasta | Hunger | 240 min | NS hunger |
| 50% male | Acute appetite test | Fullness | NS fullness | |||||
| 39 ± 14 yBMI: 24.7 ± 2.7 | Matched calories and volumeLunch meal | SatietyProspective consumption | NS satietyNS prospective consumption | |||||
| Sandberg et al. (34) | n = 19 | Healthy | Crossover | 88.8 g WG rye from bread | Wheat bread | Hunger | 180 min | ↓Hunger |
| 45% male | Subsequent meal appetite test | Satiety | ↑Satiety | |||||
| 25.6 ± 3.5 y | Matched available CHO | Desire to eat | ↓Desire to eat | |||||
| BMI: 21.9 ± 1.9 | Dinner meal | |||||||
| Geliebter et al. (35) | n = 36 | Healthy | Crossover | 93.6 g WG from oatmeal | Corn flakes | Hunger | 180 min | ↓Hunger |
| 50% male | 50% normal weight | Acute appetite test | Fullness | ↑Fullness | ||||
| 26–31 y3 | 50% overweight | Matched calories and volumeBreakfast meal | ||||||
| BMI normal weight: 23 ± 2 males; 22 ± 2 females | ||||||||
| BMI overweight: 33 ± 3 males; 36 ± 7 females | ||||||||
| Gonzalez-Anton et al. (36) | n = 23 | Healthy | Crossover | 90.1 g WG from wheat bread | Wheat bread | Hunger | 180 min | NS hunger |
| 55% male | Normal weight to overweight | Acute appetite test | Fullness | NS fullness | ||||
| 26 ± 1 y | Matched available CHO | Satiety | NS satiety | |||||
| BMI: 23.8 ± 0.5 | Breakfast mealEnergy intake at lunch | Prospective consumption | NS prospective consumptionNS energy intake | |||||
| Energy intake | ||||||||
| Johansson et al. (37) | n = 23 | Healthy | Crossover | 60 g WG from rye crisp bread | Wheat crisp bread | Hunger | 240 min | ↓Hunger |
| 30% male | Normal weight to overweight | Acute appetite test | Fullness | ↑Fullness | ||||
| 60.1 ± 12.1 y | Matched calories | Desire to eat | NS desire to eat | |||||
| BMI: 23.8 ± 3.4 | Breakfast meal | |||||||
| Forsberg et al. (21) | n = 21 | Healthy | Crossover | 76 g WG from rye kernel crisp bread60.8 g WG from rye kernel crisp bread | Wheat bread (soft) | Hunger | 240 min | ↓Hunger |
| 47% male | Normal weight to overweight | Acute appetite test | Satiety | ↑Satiety (60.8 g) | ||||
| 39 ± 14 y | Matched calories | Desire to eat | ↓Desire to eat | |||||
| BMI: 23.3 ± 3 | Breakfast meal | Energy intake | ↓Energy intake (60.8 g) | |||||
| Energy intake at lunch | ||||||||
| Hartvigsen et al. (38) | n = 15 | Metabolic syndrome | Crossover | 90 g WG from rye kernel bread | Wheat bread | Hunger | 270 min | ↓Hunger |
| 47% male | Acute appetite test | Fullness | ↑Fullness | |||||
| 62.8 ± 4.2 y | Matched available CHO | Satiety | ↑Satiety | |||||
| BMI: 31.1 ± 3.2 | Breakfast mealEnergy intake at lunch | Prospective consumption | ↓Prospective consumption | |||||
| Energy intake | NS energy intake | |||||||
| Johansson et al. (39) | n = 19 | Healthy | Crossover | 96.8 g WG from boiled barley kernels | Wheat bread | Hunger | 120 min | NS hunger |
| 32% male24.2 ± 1.9 y | Subsequent meal appetite testMatched available CHO | SatietyDesire to eat | NS satietyNS desire to eat | |||||
| BMI: 22.3 ± 2 | Dinner meal | Energy intake | ↓Energy intake | |||||
| Energy intake at lunch | ||||||||
| Rosen et al. (40) | n = 10 | Healthy | Crossover | 98.5 g WG from rye bread | Endosperm rye breadWheat bread | Hunger | 120 min4 | ↓Hunger (porridge) |
| 50% male | Acute appetite test | 106.6 g WG from rye kernel porridge | Fullness | ↑Fullness (porridge) | ||||
| 26 ± 1.1 y | Matched available CHO | 97.1 g WG from wheat kernel porridge | Desire to eat | ↓Desire to eat (porridge) | ||||
| BMI: 22.6 ± 0.4 | Breakfast meal | Energy intake | ↓Energy intake (rye porridge) | |||||
| Energy intake at lunch | ||||||||
| Rosen et al. (41) | n = 20 | Healthy | Crossover | 84.7 g WG from commercial rye bread | Wheat bread | Hunger | 180 min | ↓Hunger (Evolo) |
| 50% male | Acute appetite test | 83.2 g WG from Amilo rye bread | Fullness | NS fullness | ||||
| 26.7 ± 0.9 y | Matched available CHO | 82 g WG from Evolo rye bread | Desire to eat | NS desire to eat | ||||
| BMI: 22.2 ± 0.39 | Breakfast meal | 82.7 g WG from Picasso rye bread | ||||||
| 80.1 g WG from Vicello rye bread | ||||||||
| 82.8 g WG from Kaskelott rye bread | ||||||||
| Rosen et al. (42) | n = 14 | Healthy | Crossover | 118.2 g WG from D. Zlote rye bread | Wheat bread | Hunger | 180 min | ↓Hunger (Nikita, Rekrut) |
| 50% male | Acute appetite test | 125.7 g WG from H. Loire rye bread | Fullness | ↑Fullness | ||||
| 23.6 ± 0.5 y | Matched available CHO | 120.7 g WG from Nikita rye bread | Desire to eat | NS desire to eat | ||||
| BMI: 22 ± 0.5 | Breakfast meal | 122.2 g WG from Rekrut rye bread | ||||||
| 122.9 g WG from Amilo rye bread | ||||||||
| Kristensen et al. (43) | n = 16 | Healthy | Crossover | 83.6 g WG from wheat pasta | Wheat bread | Hunger | 180 min | NS hunger |
| 38% male | Acute appetite test | Wheat pasta | Fullness | NS fullness | ||||
| 24.1 ± 3.8 yBMI: 21.7 ± 2.2 | Matched available CHO and caloriesBreakfast mealEnergy intake at lunch | SatietyProspective consumptionEnergy intake | NS satietyNS prospective consumptionNS energy intake | |||||
| Solah et al. (22) | n = 22 | Healthy | Crossover | 53.1 g WG from boiled bulgur kernels | HA rice | Hunger | 240 min | NS hunger |
| Gender, age, BMI not reported | Acute appetite test | 53.1 g WG from steamed bulgur kernels | Fullness | NR fullness | ||||
| Matched calories and volume | 53.1 g WG from boiled Turkish bulgur kernels | Desire to eat | NR desire to eat | |||||
| Breakfast meal | Prospective consumption | NR prospective consumption | ||||||
| Rosen et al. (44) | n = 12 | Healthy | Crossover | 66.1 g WG from rye bread | Endosperm rye breadWheat breadEndosperm rye porridgeWheat porridge | Satiety | 180 min | NS satiety |
| 75% male | Acute appetite test | 51.1 g WG from rye porridge | ||||||
| 25.3 ± 0.8 y | Matched available CHO | |||||||
| BMI: 23.1 ± 0.6 | Breakfast meal | |||||||
| Hlebowicz et al. (45) | n = 12 | Healthy | Crossover | 42.5 g WG from oat flakes | Corn flakes | Satiety | 120 min | NS satiety |
| 50% male | Acute appetite test | |||||||
| 28 ± 4 y | Matched calories and volume | |||||||
| BMI: 22 ± 2 | Breakfast meal | |||||||
| Granfeldt et al. (46) | n = 10 | Healthy | Crossover | 89 g WG from hull-less barley kernel porridge76.2 g WG from hulled barley kernel porridge90.6 g WG from hull-less HA barley kernel porridge87.7 g WG from hulled HA barley kernel porridge | Wheat bread | Satiety | 180 min | ↑Satiety (hull-less porridge, hull-less HA porridge, hulled HA porridge) |
| 50% male | Acute appetite test | |||||||
| 34 ± 8 y | Matched available CHO | |||||||
| BMI: 21.2 ± 2 | Breakfast meal | |||||||
| 79.1 g WG from hulled barley flour porridge | ||||||||
| 90.3 g WG from hull-less HA barley flour porridge | ||||||||
| Hamad et al. (47) | n = 20 | Healthy and type 2 diabetes | Crossover | 70 g WG from long-grain brown rice | Long-grain white rice | Hunger | 120 min | NS all outcomes (for type 2 diabetes)NR hungerNR fullnessNR desire to eatNS prospective consumption |
| 40% male | Acute appetite test | Fullness | ||||||
| 25.4 ± 2 y male | Matched available CHO | Desire to eat | ||||||
| 24.7 ± 1.9 y female | Breakfast meal | Prospective consumption | ||||||
| BMI: 23.02 ± 1.62 male; 22.39 ± 1.32 female | ||||||||
| Roager et al. (13) | n = 50 | Overweight or obese, ≥1 of: impaired fasting glucose, dyslipidemia, hypertension | Crossover | Target ≥75 g WG/d from variety of WG foods | Target <10 g WG/d from variety of RG foods | Energy intake | NA | NS energy intake |
| 36% male | Daily consumption for 8 wk | |||||||
| 48.6 ± 11.1 y | Ad libitum intake | |||||||
| BMI: 28.9 ± 3.6 | Energy intake over the day | |||||||
| Suhr et al. (48) | n = 24/arm | Overweight to moderately obese | Parallel | No target intake | No target intake | Energy intake | NA | NS energy intake |
| WG rye: 46% male | Daily consumption for 6 wk | Mean intake WG rye: 124 ± 11.8 g/d | Mean intake WG: 5.4 ± 12.6 g/d | |||||
| 53 ± 8.9 y | Ad libitum intake | Mean intake WG wheat: 145 ± 12.1 g/d | ||||||
| BMI: 28 ± 1.9 | Energy intake over the day | |||||||
| WG wheat: 42% male; 48.2 ± 9.9 y; BMI: 27.7 ± 1.9 | ||||||||
| RG control: 50% male; 51.8 ± 9 y; BMI: 27.8 ± 2 | ||||||||
| Ibrugger et al. (49) | n = 12 | Healthy | Crossover | 90 g WG from rye kernel porridge | Wheat bread | Energy intake | NA | ↓Energy intake |
| 100% male25.6 ± 3.9 y | Matched available CHO and calories | |||||||
| BMI: 23.1 ± 1.2 | Dinner meal | |||||||
| Energy intake at lunch | ||||||||
| Luhovyy et al. (50) | n = 30 | Healthy | Crossover | 50 g WG from HA corn cookie | Wheat cookie | Energy intake | NA | NS energy intake |
| 100% male | Matched calories | |||||||
| 22.9 ± 0.6 y | Preload to lunch | |||||||
| BMI: 22.6 ± 0.3 | Energy intake at lunch | |||||||
| Isaksson et al. (51) | n = 24 | Healthy | Crossover | 55 g WG from rye porridge | Wheat bread | Energy intake | NA | NS energy intake |
| 21% male | Normal weight to moderately overweight | Daily consumption for 3 wk— breakfast meal onlyBreakfast meals Matched calories | ||||||
| 33 ± 13 yBMI: 23.4 ± 2.2 | ||||||||
| Energy intake over the day | ||||||||
| Zafar et al. (52) | n = 12 | Healthy | Crossover | 87 g WG from oatmeal | Wheat bread | Energy intake | NA | ↓Energy intake |
| 0% males | Matched available CHO | |||||||
| Age, BMI not reported | Breakfast meal | |||||||
| Energy intake at lunch | ||||||||
| Anderson et al. (53) | Study 1: | Healthy | Crossover | 50 g WG from HA corn in soup | HA corn starch in soup | Energy intake | NA | NS energy intake (30 min) |
| n = 17 | Matched calories and volume | ↓Energy intake (120 min) | ||||||
| 100% males | Preload to lunch | |||||||
| 20.2 ± 0.1 y | Energy intake at lunch (Study 1 = 30 min; Study 2 = 120 min) | |||||||
| BMI: 22.5 ± 0.3 | ||||||||
| Study 2: n = 16 | ||||||||
| 100% males | ||||||||
| 20.9 ± 0.3 y | ||||||||
| BMI: 22.5 ± 0.4 | ||||||||
| Schroeder et al. (54) | n = 50 | Healthy | Crossover | 84 g WG from barley hot cereal and snack mix78.5 g WG from wheat hot cereal and snack mix | Rice hot cereal | Hunger | 240 min | NS hunger |
| 26% male | Normal weight to obese | Acute appetite test | Fullness | NS fullness | ||||
| 31 ± 11 y | Matched calories and volume | Desire to eat | NS desire to eat | |||||
| BMI: 23 ± 3 | Breakfast meal and mid-morning snack | Prospective consumption | NS prospective consumption NS energy intake | |||||
| Energy intake at lunch | Energy intake | |||||||
| Isaksson et al. (55) | n = 22 | Healthy | Crossover | 162 g WG from rye porridge (breakfast) and wheat pasta (lunch)62 g WG from rye porridge (breakfast only) | Wheat bread (breakfast) and wheat pasta (lunch) | Energy intake | NS energy intake | |
| 36% male | Normal weight to moderately overweight | Matched calories | ||||||
| 40.7 ± 14.7 y | Breakfast and lunch meal | |||||||
| BMI: 23.2 ± 2.4 | Energy intake at dinner | |||||||
| Berti et al. (56) | Study 1: buckwheat | Healthy | Crossover | Study 1: 111.3 g WG from buckwheat pasta (large portion)55.7 g WG from buckwheat pasta (small portion)Study 2: 419.8 g WG from quinoa risotto (large portion)211.6 g WG from quinoa risotto (small portion) | Study 1: wheat pasta (large and small portions)Study 2: White rice (large and small portions) | Energy intake | NA | NS energy intake |
| n = 14 | Matched volume | |||||||
| 100% males | Mid-morning snack | |||||||
| 24 ± 2.6 y | Energy intake at lunch | |||||||
| BMI: 22.3 ± 2.7 | ||||||||
| Study 2: quinoa | ||||||||
| n = 12 | ||||||||
| 100% males | ||||||||
| 25.4 ± 2.2 y | ||||||||
| BMI: 23 ± 1.9 | ||||||||
| Breen et al. (57)5 | n = 10 | Type 2 diabetes | Crossover | 54.1 g WG from buttermilk wheat bread | Wheat bread | Hunger | 270 min | NS hunger |
| 60% male | Acute appetite test | 135.4 g WG from pumpernickel rye bread | Fullness | NS fullness | ||||
| 53.9 ± 5.5 y | Matched available CHO | Satiety | NS satiety | |||||
| BMI: 35.1 ± 7.5 | Breakfast meal | Prospective consumption | NS prospective consumption | |||||
| Holt et al. (58)5 | n = 10 | Healthy | Crossover | 79.8 g WG from wheat bread | Wheat bread | Hunger | 120 min | NR hunger |
| 30% males | Acute appetite test | Fullness | NR fullness | |||||
| 23.5 ± 6.2 y | Matched calories | |||||||
| BMI: 22.1 ± 1.3 | Breakfast Meal | |||||||
| Nilsson et al. (59)5 | n = 17 | Healthy | Crossover | 105.3 g WG from barley kernel bread | Wheat bread | Satiety | 180 min | ↑Satiety (high β-glucan barley bread) |
| 65% males | Subsequent meal appetite test | 124.4 g WG from cut barley kernel bread | ||||||
| 25.9 ± 3.2 y | Matched available CHO | 139.3 g WG from HA barley bread | ||||||
| BMI: 22.5 ± 2.1 | Dinner meal | 253.9 g WG from high β-glucan barley bread | ||||||
| 52.6 g WG from barley kernel bread | ||||||||
| Bodinham et al. (60)5 | n = 14 | Healthy | Crossover | 48 g WG from wheat bread | Wheat bread | Energy intake | NA | NS energy intake |
| 36% male | Daily consumption for 3 wk | |||||||
| 26 ± 1.4 y | Energy intake over the day | |||||||
| BMI: 21. 8 ± 0.8 |
CHO, carbohydrate; HA, high-amylose; NA, not applicable; NR, not reported; NS, not significant (P > 0.05); RG, refined grain; WG, whole grain.
Reported or calculated values.
Range of means provided because age reported by gender and weight status.
120 min measurement = AUC0–60min + AUC60–120min
Included in systematic review but data not available to include in meta-analysis.
WG intake and hunger
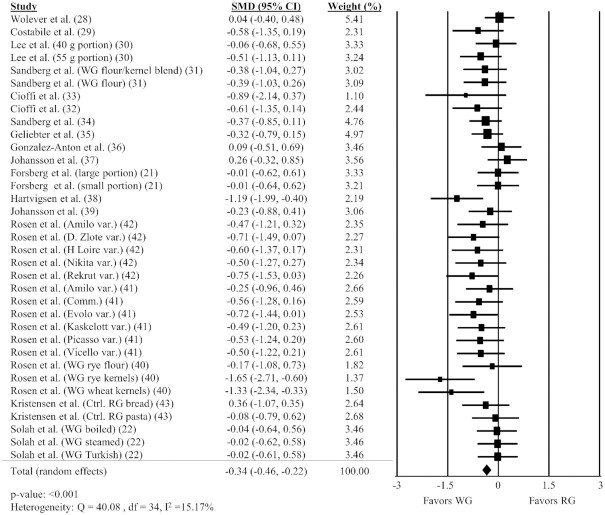
Overall, 35 comparisons reported in 18 different studies (21, 22, 28–43) were included in the analysis of the impact of WG on hunger AUC. Intake of WG foods resulted in significantly lower hunger AUC compared with RG foods (Figure 2, SMD: −0.34; 95% CI: −0.46, −0.22; P < 0.001) with no significant heterogeneity between studies (Q = 40.08, P = 0.22, I2 = 15.17%). A sensitivity analysis showed the timing of AUC measurement did not substantially impact the results, with the effect size differing slightly in studies <180 min or ≥180 min (Supplemental Table 2). A sensitivity analysis including the subset of studies requiring WG amounts to be calculated showed a slightly larger effect size than the analysis including all studies (SMD: −0.45); however, studies requiring calculation of WG content estimated slightly higher mean levels of WG intake (92.7 ± 5.3 g compared with 88.9 ± 4.4 g), which might contribute to this larger effect. Similar results were found for all other outcomes.
FIGURE 2.
Forest plot of the meta-analysis on the effect of WG intake on hunger in adults. Values are the standardized mean differences (SMDs) for hunger AUC between WG intake and RG intake (21, 22, 28–43). Comm., commercial; Ctrl., control; RG, refined grain; var., variety; WG, whole grain.
Findings from the subgroup analyses for hunger AUC are shown in Table 2. Hunger was lower in the WG group relative to the control when the test and control conditions were matched by available carbohydrate (P < 0.001). However, studies matched by calories or matched by calories and volume did not show a significant difference in hunger AUC between WG and RG controls.
TABLE 2.
Subgroup analyses for the effect of WGs on subjective appetite in adults1
| Outcome and subgroups | Number of comparisons/studies | Subjects (WG/control) | Effect estimate SMD (95% CI)2 | I 2 (%) | P value2 |
|---|---|---|---|---|---|
| Hunger AUC | |||||
| Type of WG | |||||
| Rye | 22/9 | 759 (378/381) | −0.42 (−0.57, −0.26) | 12.04 | <0.001 |
| Wheat | 10/7 | 330 (165/165) | −0.27 (−0.52, −0.02) | 22.14 | <0.031 |
| Amount of WG (median split) | |||||
| ≤88.8 g | 18/8 | 748 (374/374) | −0.20 (−0.34, −0.06) | 0.00 | 0.006 |
| >88.6 g | 17/10 | 531 (267/264) | −0.53 (−0.72, −0.35) | 16.07 | <0.001 |
| Feeding approach | |||||
| Matched available CHO | 24/11 | 821 (409/412) | −0.44 (−0.59, −0.30) | 10.65 | <0.001 |
| Matched calories | 7/4 | 276 (138/138) | −0.10 (−0.33, 0.14) | 0.00 | 0.421 |
| Matched calories and volume | 6/4 | 246 (123/123) | −0.22 (−0.47, 0.03) | 0.00 | 0.078 |
| Measurement timing | |||||
| Acute appetite test | 31/15 | 1096 (548/548) | −0.35 (−0.49, −0.21) | 24.80 | <0.001 |
| Subsequent meal appetite test | 4/3 | 183 (90/93) | −0.35 (−0.64, −0.06) | 0.00 | 0.019 |
| Fullness AUC | |||||
| Type of WG | |||||
| Rye | 17/6 | 531 (265/266) | 0.54 (0.32, 0.75) | 35.77 | <0.001 |
| Wheat | 6/5 | 170 (85/85) | 0.53 (0.12, 0.94) | 42.56 | 0.012 |
| Amount of WG (median split) | |||||
| ≤90.0 g | 13/6 | 496 (248/248) | 0.33 (0.12, 0.54) | 28.12 | 0.002 |
| >90.0 g | 12/6 | 357 (178/179) | 0.69 (0.43, 0.94) | 28.72 | <0.001 |
| Feeding approach | |||||
| Matched available CHO | 19/7 | 609 (304/305) | 0.57 (0.37, 0.77) | 34.09 | <0.001 |
| Matched calories | 5/3 | 194 (97/97) | 0.19 (−0.18, 0.57) | 43.81 | 0.315 |
| Satiety AUC | |||||
| Type of WG | |||||
| Rye | 10/5 | 351 (173/178) | 0.31 (0.07, 0.55) | 22.38 | 0.011 |
| Wheat | 6.5 | 178 (89/89) | 0.22 (−0.07, 0.51) | 0.00 | 0.141 |
| Barley | 7/2 | 156 (77/79) | 0.46 (0.15, 0.76) | 0.00 | 0.004 |
| Amount of WG (median split) | |||||
| ≤88.3 g | 12/5 | 323 (160/163) | 0.21 (−0.004, 0.42) | 0.00 | 0.055 |
| >88.3 g | 12/9 | 386 (191/195) | 0.42 (0.23, 0.63) | 0.00 | <0.001 |
| Feeding approach | |||||
| Matched available CHO | 19/9 | 561 (277/284) | 0.37 (0.21, 0.53) | 0.00 | <0.001 |
| Matched calories | 4/2 | 146 (73/73) | 0.08 (−0.24, 0.40) | 0.00 | 0.631 |
| Matched calories and volume | 3//3 | 66 (33/33) | 0.36 (−0.11, 0.84) | 0.00 | 0.132 |
| Measurement timing | |||||
| Acute appetite test | 20/10 | 526 (265/261) | 0.31 (0.14, 0.48) | 0.00 | <0.001 |
| Subsequent meal appetite test | 4/3 | 183 (90/93) | 0.38 (0.09, 0.67) | 0.00 | 0.011 |
| Desire to eat AUC | |||||
| Type of WG | |||||
| Rye | 21/8 | 727 (363/364) | −0.36 (−0.50, −0.21) | 0.00 | <0.001 |
| Amount of WG (median split) | |||||
| ≤88.8 g | 13/6 | 552 (276/276) | −0.23 (−0.40, −0.07) | 0.00 | 0.006 |
| >88.8 g | 13/5 | 341 (170/171) | −0.50 (−0.72, −0.28) | 6.56 | <0.001 |
| Feeding approach | |||||
| Matched available CHO | 20/8 | 681 (340/341) | −0.42 (−0.57, −0.27) | 0.00 | <0.001 |
| Matched calories | 5/3 | 212 (106/106) | −0.07 (−0.33, 0.20) | 0.00 | 0.623 |
| Measurement timing | |||||
| Acute appetite test | 21/8 | 711 (355/356) | −0.34 (−0.50, −0.19) | 14.12 | <0.001 |
| Subsequent meal appetite test | 4/3 | 182 (91/91) | −0.35 (−0.64, −0.06) | 0.00 | 0.018 |
| Prospective consumption AUC | |||||
| Type of WG | |||||
| Wheat | 5/4 | 150 (75/75) | −0.15 (−0.51, 0.21) | 19.32 | 0.406 |
| Amount of WG (median split) | |||||
| ≤86.8 g | 4/3 | 184 (92/92) | −0.17 (−0.46, 0.12) | 0.00 | 0.240 |
| >86.8 g | 4/4 | 116 (58/58) | −0.40 (−1.02, 0.23) | 62.96 | 0.213 |
| Feeding approach | |||||
| Matched avail CHO | 6/5 | 258 (129/129) | −0.21 (−0.53, 0.11) | 38.83 | 0.202 |
CHO, carbohydrate; RG, refined grain; SMD, standardized mean difference; WG, whole grain.
Effect estimates and P values from random effects models.
Three studies not included in the meta-analysis measured hunger AUC but did not report the data. All of these studies (47, 54, 57) did not show a significant effect of WG on hunger AUC compared with RG.
WG intake and fullness
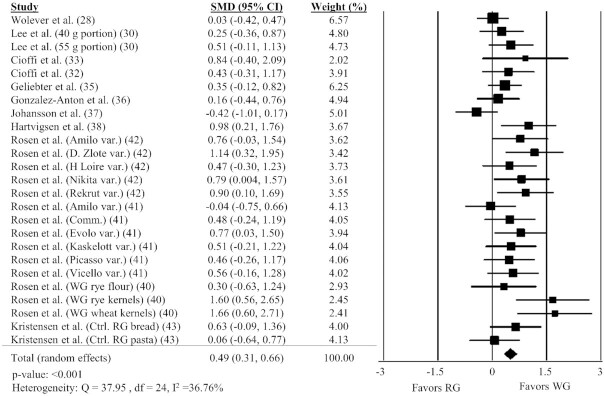
Twelve studies (28, 30, 32, 33, 35–38, 40–43) with 25 comparisons were included in the analysis of the effect of WG on fullness. Intake of WG foods resulted in significantly greater fullness AUC compared with RG foods (Figure 3; SMD: 0.49; 95% CI: 0.31, 0.66; P < 0.001) with moderate heterogeneity between studies (Q = 37.95, P = 0.035, I2 = 36.76%). A sensitivity analysis showed the timing of AUC measurement impacted the effect size, with studies measuring fullness at <180 min having a greater effect size than studies measuring at ≥180 min (Supplemental Table 2).
FIGURE 3.
Forest plot of the meta-analysis on the effect of WG intake on fullness in adults. Values are the standardized mean differences (SMDs) for fullness AUC between WG intake and RG intake (28, 30, 32, 33, 35, 36, 38–43). Comm., commercial; Ctrl., control; RG, refined grain; var., variety; WG, whole grain.
Subgroup analyses’ results for fullness AUC are shown in Table 2. There was a significant positive effect of WG on fullness in studies matched by available carbohydrate (P < 0.001), but not in studies that matched calories. There were insufficient studies matched by calories and volume to include as a subgroup. Subgroup analyses were not possible for acute compared with subsequent meal studies because all studies were acute.
Five studies (22, 47, 54, 57, 58) not included in the meta-analysis measured fullness AUC but did not report the data. Three studies (47, 54, 57) did not find a significant effect of WG on fullness AUC compared with RG. One of the studies only calculated fullness AUC to use in a satiety index (58) and thus did not statistically compare the AUC values between treatments. Solah et al. (22) did not report the statistical results for fullness AUC.
WG intake and satiety
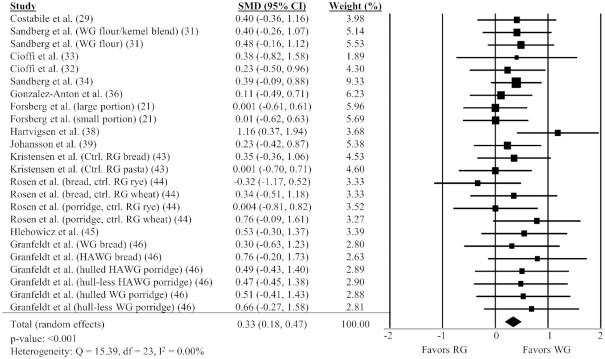
Thirteen studies (21, 29, 31–34, 36, 38, 39, 43–46) with 24 comparisons were included in the analysis of the effect of WG on satiety. There was a significant positive effect of WG on satiety AUC (Figure 4, SMD: 0.33; 95% CI: 0.18, 0.47; P < 0.001) with no significant heterogeneity between studies (Q = 15.40, P = 0.88, I2 = 0.00%). A sensitivity analysis showed the timing of AUC measurement did not impact the effect size although there were only 2 studies that measured satiety AUC for <180 min (Supplemental Table 2).
FIGURE 4.
Forest plot of the meta-analysis on the effect of WG intake on satiety in adults. Values are the standardized mean differences (SMDs) for satiety AUC between WG intake and RG intake (21, 29, 31–34, 36, 38, 39, 43–46). Ctrl., control; HAWG, high-amylose whole grain; RG, refined grain; var., variety; WG, whole grain.
Findings of the subgroup analyses for satiety AUC are shown in Table 2. There was a positive effect on satiety AUC with WG rye (P = 0.011) and WG barley (P = 0.004), but not WG wheat. There was also a significant positive effect of WG when tested at amounts greater than the median (88.25 g), but not less than or equal to the median. A significant positive effect of WG on satiety was determined in studies with test and control conditions matched by available carbohydrate (P < 0.001), but not when matched by calories or calories and volume.
Two studies (57, 59) not included in the meta-analysis measured satiety AUC but did not report the data. Breen et al. (57) did not find a significant effect of WG wheat bread or WG rye bread on satiety AUC compared with RG wheat bread in a study of subjects with type 2 diabetes. Nilsson et al. (59) found a high–β-glucan WG barley variety had a significantly positive effect on satiety AUC compared with RG wheat; however, other WG barley treatments were not significantly different from RG wheat.
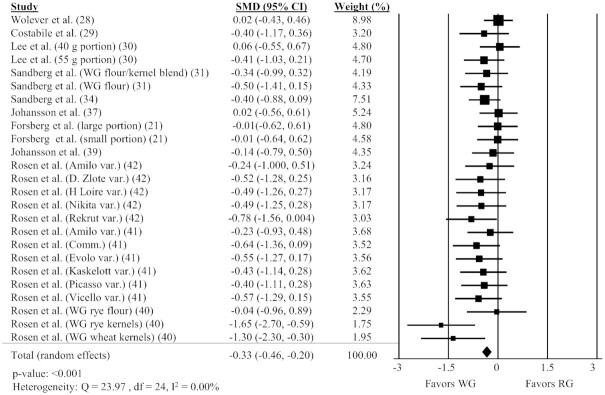
WG intake and desire to eat
Twenty-five comparisons reported in 11 different studies (21, 28–31, 34, 37, 39–42) were included in the analysis of the impact of WGs on desire to eat AUC. Intake of WG foods resulted in a significantly lower desire to eat AUC compared with RG foods (Figure 5; SMD: −0.33; 95% CI: −0.47, −0.20; P < 0.001) with no significant heterogeneity between studies (Q = 23.97, P = 0.46, I2 = 0.00%). A sensitivity analysis showed the timing of AUC measurement did not substantially impact the results, with the effect size differing slightly in studies <180 min or ≥180 min (Supplemental Table 2).
FIGURE 5.
Forest plot of the meta-analysis on the effect of WG intake on desire to eat in adults. Values are the standardized mean differences (SMDs) for desire to eat AUC between WG intake and RG intake (21, 28–31, 34, 37, 39–42). Comm., commercial; Ctrl., control; RG, refined grain; var., variety; WG, whole grain.
Results of subgroup analyses for desire to eat AUC are shown in Table 2. Desire to eat was lower in the WG group relative to the control when the test and control conditions matched by available carbohydrate (P < 0.001), but not when matched by calories.
Three studies (22, 47, 54) not included in the meta-analysis measured desire to eat AUC but did not report the data. Two studies (47, 54) did not find a significant effect of WGs on desire to eat AUC compared with RGs. The other study (22) did not report the statistical results for desire to eat AUC.
WG intake and prospective consumption
Eight comparisons reported in 7 different studies (28, 32, 33, 36, 38, 43, 47) were included in the analysis of the impact of WG on prospective consumption AUC. There was no effect of WG on prospective consumption AUC (Figure 6; SMD: −0.25; 95% CI: −0.52, 0.03) with no significant heterogeneity between studies (Q = 9.91, P = 0.19, I2 = 29.37%). Sensitivity analyses could not be performed because all studies measured prospective consumption at a time frame ≥180 min. There were no significant effects of WGs on prospective consumption AUC in any of the subgroups (Table 2).
FIGURE 6.
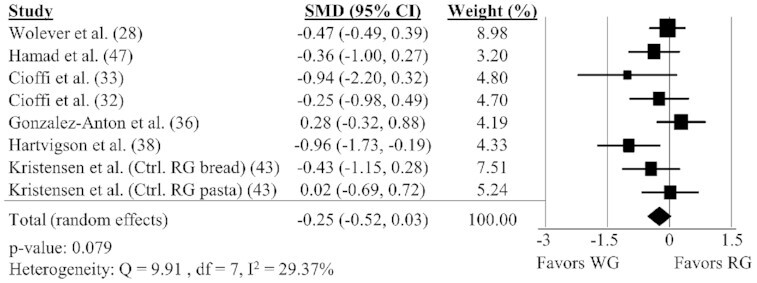
Forest plot of the meta-analysis on the effect of WG intake on prospective consumption in adults. Values are the standardized mean differences (SMDs) for prospective consumption AUC between WG intake and RG intake (32, 33, 36, 38, 43, 47, 61). Ctrl., control; RG, refined grain; var. variety; WG, whole grain.
Two studies (54, 57) not included in the meta-analysis measured prospective consumption AUC but did not report the data. One study (57) in subjects with type 2 diabetes did not find a significant effect of WG wheat bread or WG rye bread on prospective consumption AUC compared with RG wheat bread. The other study (54) found no significant difference in prospective consumption between WG barley or WG wheat and RG rice.
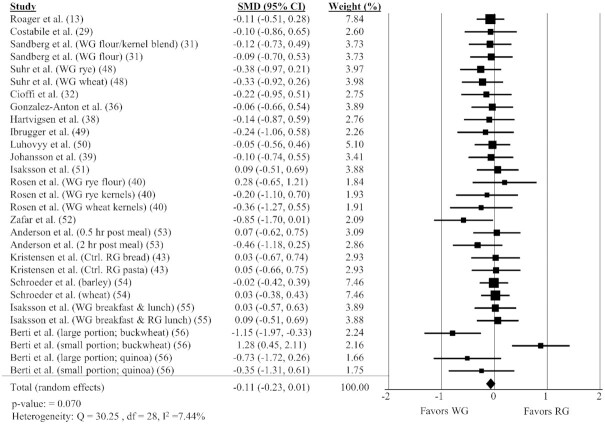
WG intake and energy intake
The impact of WGs on energy intake included 29 comparisons from 17 different studies (13, 29, 31, 32, 36, 38–40, 43, 48–56). There was a small, nonsignificant reduction in energy intake following WG consumption (Figure 7; SMD: −0.11; 95% CI: −0.23, 0.01; P = 0.070) and no significant heterogeneity between studies (Q = 30.25, P = 0.35, I2 = 7.44%). There was a significant effect of WGs on energy intake when the amount of WGs fed was >90.1 g (P = 0.006), the median amount among all studies, but this effect was not observed with amounts ≤90.1 g. No other subgroup analyses showed a significant effect on energy intake (Table 3).
FIGURE 7.
Forest plot of the meta-analysis on the effect of WG intake on energy intake in adults. Values are the standardized mean differences (SMDs) for caloric intake between WG intake and RG intake (13, 29, 31, 32, 36, 38–40, 43, 48–56). Ctrl., control; RG, refined grain; WG, whole grain.
TABLE 3.
Subgroup analyses for the effect of WGs on energy intake in adults1
| Outcome and subgroups | Number of comparisons/studies | Subjects (WG/control) | Effect estimate SMD (95% CI)2 | I 2 (%) | P value2 |
|---|---|---|---|---|---|
| Energy intake | |||||
| Type of WG | |||||
| Rye | 9/7 | 311 (156/155) | −0.09 (−0.30, 0.13) | 0.00 | 0.440 |
| Wheat | 8/7 | 326 (162/162) | −0.09 (−0.30, 0.12) | 0.00 | 0.407 |
| Other3 | 12/8 | 518 (259/259) | −0.17 (−0.45, 0.11) | 57.00 | 0.227 |
| Amount of WG (median split) | |||||
| ≤90.1 g | 15/11 | 660 (330/330) | −0.01 (−0.18, 0.16) | 16.94 | 0.928 |
| >90.1 g | 12/7 | 411 (207/204) | −0.27 (−0.46, −0.08) | 0.00 | 0.006 |
| Feeding approach | |||||
| Matched available CHO | 13/9 | 395 (197/198) | −0.13 (−0.32, 0.07) | 0.00 | 0.196 |
| Matched calories | 9/6 | 372 (188/184) | −0.08 (−0.28, 0.12) | 0.00 | 0.435 |
| Measurement timing | |||||
| Daily intake | 4/3 | 236 (120/116) | −0.17 (−0.42, 0.09) | 0.00 | 0.191 |
| Subsequent meal intake | 21/14 | 811 (405/406) | −0.10 (−0.26, 0.06) | 25.32 | 0.228 |
| Third meal intake4 | 3/2 | 108 (54/54) | −0.13 (−0.51, 0.24) | 0.00 | 0.478 |
CHO, carbohydrate; SMD, standardized mean difference; WG, whole grain.
Effect estimates and P values from random effects models.
Other category includes barley, buckwheat, corn, oat, and quinoa. There were not enough studies in individual grains to run a separate subgroup analysis.
Third meal refers to the next meal consumed after the subsequent meal (e.g., breakfast = test meal, lunch = subsequent meal, dinner = third meal).
Two studies (21, 60) not included in the meta-analysis measured energy intake but did not report the data. One study reported no significant effect of WGs compared with RGs on energy intake at a subsequent meal (60). Forsberg et al. (21) also reported no significant difference between WGs and RGs in energy intake at a subsequent meal with a large breakfast (∼600 kcal), but a significant effect of WGs on energy intake with a smaller breakfast (∼375 kcal).
Quality of evidence
The quality of evidence as assessed by GRADE criteria is summarized in Table 4. Overall, the evidence for subjective measures of appetite and energy intake was rated as moderate (low for prospective consumption). The evidence rating was downgraded due to concerns about risk of bias in the studies and possible publication bias. Sources of bias in the studies were typically inadequate description of randomization procedures or allocation concealment and inability to blind the treatments. There was also an indication of possible publication bias suggesting that smaller studies with results that did not confirm the main study hypothesis were not as likely to be published. Risk-of-bias assessment on the outcomes of individual studies and funnel plots for the main outcomes are shown in Supplemental Table 3 and Supplemental Figures 1–6.
TABLE 4.
Quality of evidence included in the systematic review and meta-analysis of WGs on subjective appetite measures and energy intake in adults, based on GRADE approach1
| Outcome | Risk of bias2 | Inconsistency3 | Indirectness | Imprecision | Publication bias4 | Decision5 |
|---|---|---|---|---|---|---|
| Hunger | Some concerns | Consistent | No serious indirectness | No serious imprecision | Possible | ⊕⊕⊕∅ Moderate |
| Fullness | Some concerns | Moderate | No serious indirectness | No serious imprecision | Possible | ⊕⊕⊕∅ Moderate |
| Satiety | Some concerns | Consistent | No serious indirectness | No serious imprecision | Undetected | ⊕⊕⊕∅ Moderate |
| Desire to eat | Some concerns | Consistent | No serious indirectness | No serious imprecision | Possible | ⊕⊕⊕∅ Moderate |
| Prospective consumption | Some concerns | Moderate | No serious indirectness | Moderate imprecision | Unable to determine6 | ⊕⊕∅∅ Low |
| Energy intake | Low | Consistent | No serious indirectness | Moderate imprecision | Possible | ⊕⊕⊕∅ Moderate |
GRADE, Grading of Recommendations Assessment, Development and Evaluation; WG, whole grain.
Ranked down primarily for inadequate description of allocation concealment and lack of blinding.
Based on I2 using thresholds in Cochrane Handbook for Systematic Reviews of Interventions, Version 6. The Cochrane Collaboration, 2019. Available at: https://training.cochrane.org/handbook/current.
Based on visual analysis of funnel plots.
Symbols are suggested representations of quality of evidence from GRADE Handbook (https://gdt.gradepro.org/app/handbook/handbook.html).
Only 8 studies and a minimum of 10 studies are generally needed to evaluate a funnel plot.
Discussion
The results of this meta-analysis of RCTs suggest that intake of WG foods reduces hunger and desire to eat and increases fullness and satiety compared with RG foods. There was no significant effect of WGs on energy intake at subsequent meals or across the day compared with RG foods from the subgroup analyses, although there was a small, nonsignificant reduction in energy intake in the main analysis when data were pooled (P = 0.07). To our knowledge, this is the first systematic review and meta-analysis to evaluate the effect of WG intake compared with RG intake on subjective appetite measures and energy intake. Several meta-analyses have evaluated the relation of WG intake to body weight, and observational data tend to support an inverse relation (4–6). However, RCTs with intervention periods of a few weeks up to a few months have shown mixed effects of WG intake on body weight change (4, 7, 13, 62, 63).
WG intake was associated with significant reductions in appetite ratings, with effects that were small to moderate in magnitude. There was also a small, nonsignificant reduction in energy intake that only became significant at high levels of WG intake (above the median level of 90.1 g). Taken together, these results suggest that WGs are able to reduce subjective appetite following a meal, but not enough to significantly impact acute energy intake at a subsequent meal or across the day. Also, considering the possible publication bias favoring studies that show beneficial effects of WGs on energy intake, it is likely that WGs have little impact on short-term energy intake, except perhaps at high levels of WG intake. Studies that assessed chronic consumption of WGs on energy intake were few and only 3 to 8 wk in duration (13, 48, 51). The longest RCT (13) did not find a significant impact of WGs on daily energy intake compared with an RG diet, but there was a significant change in body weight and body composition in the WG diet that correlated with a change in energy intake. Longer-term studies (>16 wk) might be able to determine whether small changes in energy intake associated with WG consumption can have cumulative long-term effects that explain differences in body weight reported in observational studies.
The diversity in studies allowed for several subgroup analyses. Firstly, the type of WG tested in the studies often varied, with WG rye and WG wheat being the most frequently tested and both significantly reducing hunger and desire to eat and increasing fullness compared with RGs. Interestingly, there were fewer studies on WGs such as oat and barley, which are rich in the viscous, fermentable fiber β-glucan and may impact appetite and energy intake differently from wheat and rye with primarily nonviscous and poorly fermentable fibers. Fermentation of fiber and other phenolic compounds in WGs by gut microbes can produce metabolites, such as SCFAs, that could influence appetite and energy intake beyond the subsequent meal (13). Furthermore, viscous fibers have been shown to slow gastric emptying and prolong the release of cholecystokinin in response to a fat-containing meal, possibly contributing to enhanced feelings of satiety (35, 64). The studies on oat and barley in this meta-analysis had mixed results regarding subjective appetite and energy intake so more studies in these WGs are needed.
Using a median split to create a dichotomy of “higher” and “lower” WG intake, the results suggest small effect sizes with lower levels of WGs and moderate effect sizes with higher levels of WGs. Of note, the quantities of WGs fed in most studies were relatively high, with medians in the range of 85 to 90 g. This is higher than typical consumption and recommendations for intake. Daily WG consumption in the United States is typically ≤16 g, and recommendations suggest 48 g/d (3, 65). Intake is somewhat higher in Europe, ranging from 23 to 36 g/d, but even populations in northern Europe with the highest intake (37–58 g/d) are still on the low end of the levels tested in these studies (65). Several studies included these high levels to achieve 50 g of available carbohydrate from the test food. One study fed >200 g WGs in a test food and noted it was necessary to achieve 50 g of available carbohydrate, but also acknowledged that >25% of the subjects were unable to finish consuming the entire test product (59). Thus, more studies are needed at lower, realistic levels of WGs recommended for a healthy diet.
The subgroup analyses also showed differences in appetite responses based on the feeding design. When interventions were matched for available carbohydrate, WGs were significantly different from RGs for hunger, fullness, and desire to eat. However, when studies were matched for calories or calories and volume (none were matched for volume alone), there was not a significant difference between WG and RG conditions. Because calories and volume both impact subjective appetite (66), this shows the importance of considering both factors in studies on food ingredients and appetite. WG foods have less available carbohydrates than RG foods due to their fiber content; therefore, studies that match available carbohydrates often fed a greater volume of food in the WG condition than the RG condition, which could contribute to greater feelings of fullness or lower feelings of hunger. Indeed, several of the investigators commented on the difference in portion size and calories potentially contributing to the findings (29, 36, 38, 42, 56). Although matching available carbohydrate might yield a more mechanistic understanding of the influence of glycemic response on appetite, it does not reflect typical consumption patterns or recommendations, which suggest substitutions, such as exchanging a slice of white bread for a slice of whole wheat bread. Substitution of foods containing WGs for similar foods made with RGs often results in increased dietary fiber intake and reduced energy density, both of which have been associated with lower daily energy consumption and less weight gain over time (66). Almost half (14 of 32) of studies in the current meta-analysis matched available carbohydrates and did not match conditions for calories or volume. Accordingly, there is a need for more studies matching calories and volume between WG and RG conditions to provide greater clarity regarding drivers of differences in indicators of appetite associated with WG food consumption.
Interestingly, studies that measured appetite at a subsequent meal fed ≥11 h after WG consumption showed significant effects on hunger, satiety, and desire to eat, similar in magnitude to differences observed in acute meal studies. The levels of WGs fed were also similar to acute meal studies, suggesting a potential long-term effect of WGs on appetite that could be mediated by slowing digestion or the fermentation of fibers from WGs in the colon. Nilsson et al. (59) showed that an evening meal with WGs significantly reduced the gastric emptying rate at a subsequent standardized breakfast meal, which could have contributed to the greater feelings of satiety after breakfast. Fermentation metabolites, such as SCFAs, have been shown to stimulate the production of appetite-related hormones, such as glucagon-like peptide-1 and peptide YY (67, 61, 68). A meta-analysis has shown glucagon-like peptide-1 to increase feelings of fullness and reduce energy intake in humans (69). These potential mechanisms by which WGs might impact long-term appetite and energy intake should be further investigated.
The strengths of the current systematic review and meta-analysis include a comprehensive search of 3 databases to ensure broad coverage of the literature and the inclusion of a number of subgroup and sensitivity analyses that have helped identify hypotheses for additional research and gaps in the available evidence. However, the systematic review and meta-analysis was also limited by poor reporting of the amount of WGs in the studies, which resulted in the exclusion of several studies and 17 of 36 studies requiring calculations to estimate WG content based on recipes. Additional studies were excluded that measured subjective appetite but did not calculate the AUC. The present analysis also only included studies that provided WG foods where ≥51% of the grain was WG. There was also an indication of possible publication bias suggesting that smaller studies with results that did not confirm the main study hypothesis were not as likely to be published. Finally, there were no studies in participants with type 2 diabetes that met the inclusion criteria and provided data for the meta-analysis. Furthermore, only 1 study in the meta-analysis was completed in participants with metabolic syndrome (38). Thus, more investigation is needed in these populations to determine whether effects of WG consumption on appetite differs between groups with and without metabolic dysregulation.
In summary, the results from this systematic review and meta-analysis show that consumption of WG foods, compared with RG foods, modestly but significantly reduced hunger and desire to eat and increased fullness and satiety, but showed only a small, nonsignificant reduction in energy intake at the next meal or across the day. Thus, although it is plausible that effects of WG consumption on appetite and subsequent energy intake contribute to the associations of greater WG intake with lower risks for weight gain and overweight or obesity reported in observational studies, additional research, especially with longer feeding periods, will be needed before firm conclusions can be drawn in this regard. More studies are warranted to further clarify the effects of different WG types and of consumption in amounts consistent with current recommendations. Particular attention should be paid to the research design to ensure calories and volume are matched because these factors can greatly influence appetite and subsequent energy intake.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—LMS, YZ, KK, and KCM: contributed to the conception and design of the systematic review and meta-analysis; LMS: reviewed the publications, extracted the data, and conducted the risk of bias assessment; MLW: verified the accuracy of the extraction and analyzed the data; LMS and KCM: interpreted the data, performed the GRADE assessment, and prepared the manuscript; and all authors: read and approved the final manuscript.
Notes
This research was funded by Bell Institute of Nutrition, General Mills, Inc.
Author disclosures: KCM, MLW, and LMS are employees of Midwest Biomedical Research, which has received research funding from General Mills, Inc., Kellogg Company, and the Quaker division of PepsiCo. KK and YZ are employees of General Mills, Inc. The funding sponsor provided comments on early aspects of the study design. Interim analyses and the final data were shared with the sponsor prior to publication, but the final decision for all aspects of study conduct and manuscript content is that of the authors alone.
Supplemental Tables 1–3 and Supplemental Figures 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: GRADE, Grading of Recommendations Assessment, Development and Evaluation; RCT, randomized controlled trial; RG, refined grain; SMD, standardized mean difference; VAS, visual analog scale; WG, whole grain.
Contributor Information
Lisa M Sanders, Midwest Biomedical Research, Addison, IL, USA.
Yong Zhu, Bell Institute of Nutrition, General Mills, Inc., Minneapolis, MN, USA.
Meredith L Wilcox, Midwest Biomedical Research, Addison, IL, USA.
Katie Koecher, Bell Institute of Nutrition, General Mills, Inc., Minneapolis, MN, USA.
Kevin C Maki, Midwest Biomedical Research, Addison, IL, USA; Indiana University, Department of Applied Health Science, School of Public Health, Bloomington, IN, USA.
References
- 1. Van Der Kamp JW, Poutanen K, Seal CJ, Richardson DP. The HEALTHGRAIN definition of ‘whole grain’. Food Nutr Res. 2014;58(1):22100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Association of Cereal Chemists International . Whole grain definition. Cereal Foods World; 1999;45:79. [Google Scholar]
- 3. US Department of Health and Human Services and US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th ed. USDA; 2015. [Google Scholar]
- 4. Maki KC, Palacios OM, Koecher K, Sawicki CM, Livingston KA, Bell M, Nelson Cortes H, McKeown NM. The relationship between whole grain intake and body weight: results of meta-analyses of observational studies and randomized controlled trials. Nutrients. 2019;11(6):1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schlesinger S, Neuenschwander M, Schwedhelm C, Hoffmann G, Bechthold A, Boeing H, Schwingshackl L. Food groups and risk of overweight, obesity, and weight gain: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2019;10(2):205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giacco R, Della Pepa G, Luongo D, Riccardi G. Whole grain intake in relation to body weight: from epidemiological evidence to clinical trials. Nutr Metab Cardiovasc Dis. 2011;21(12):901–8. [DOI] [PubMed] [Google Scholar]
- 7. Pol K, Christensen R, Bartels EM, Raben A, Tetens I, Kristensen M. Whole grain and body weight changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr. 2013;98(4):872–84. [DOI] [PubMed] [Google Scholar]
- 8. Musa-Veloso K, Poon T, Harkness LS, O'Shea M, Chu Y. The effects of whole-grain compared with refined wheat, rice, and rye on the postprandial blood glucose response: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2018;108(4):759–74. [DOI] [PubMed] [Google Scholar]
- 9. Marventano S, Vetrani C, Vitale M, Godos J, Riccardi G, Grosso G. Whole grain intake and glycaemic control in healthy subjects: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017;9(7):769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tosh SM. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur J Clin Nutr. 2013;67(4):310–7. [DOI] [PubMed] [Google Scholar]
- 11. Müller M, Canfora EE, Blaak EE. Gastrointestinal transit time, glucose homeostasis and metabolic health: modulation by dietary fibers. Nutrients. 2018;10(3):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lafond DW, Greaves KA, Maki KC, Leidy HJ, Romsos DR. Effects of two dietary fibers as part of ready-to-eat cereal (RTEC) breakfasts on perceived appetite and gut hormones in overweight women. Nutrients. 2015;7(2):1245–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roager HM, Vogt JK, Kristensen M, Hansen LBS, Ibrugger S, Maerkedahl RB, Bahl MI, Lind MV, Nielsen RL, Frokiaer Het al. . Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut. 2019;68(1):83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blundell J, De Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, Mela D, Salah S, Schuring E, Van Der Knaap H. Appetite control: methodological aspects of the evaluation of foods. Obes Rev. 2010;11(3):251–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stubbs RJ, Hughes DA, Johnstone AM, Rowley E, Reid C, Elia M, Stratton R, Delargy H, King N, Blundell J. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr. 2000;84(4):405–15. [DOI] [PubMed] [Google Scholar]
- 16. Flint A, Raben A, Blundell J, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes. 2000;24(1):38–48. [DOI] [PubMed] [Google Scholar]
- 17. Mattes R. Hunger ratings are not a valid proxy measure of reported food intake in humans. Appetite. 1990;15(2):103–13. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. US Food and Drug Administration . Health claim notification for whole grain foods with moderate fat content. [Internet]. [cited 2019 Oct 30]. Available from: https://www.fda.gov/food/food-labeling-nutrition/health-claim-notification-whole-grain-foods-moderate-fat-content. [Google Scholar]
- 20. USDA Food Safety Inspection Service . Food Safety and Inspection Service guideline on whole grain statements on the labeling of meat and poultry products. [Internet]. [cited 2019 Oct 30]. Available from: https://www.fsis.usda.gov/wps/wcm/connect/6ea06856-e04d-46d7-befd-5b9287c55640/Guideline-Whole-Grain-Statements-Labeling.pdf?MOD=AJPERES. [Google Scholar]
- 21. Forsberg T, Aman P, Landberg R. Effects of whole grain rye crisp bread for breakfast on appetite and energy intake in a subsequent meal: two randomised controlled trails with different amounts of test foods and breakfast energy content. Nutr J. 2014;13:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Solah V, Fenton H, Kerr D, Crosbie G, Siryani S. Measurement of satiety of wheat-based bulgur by intervention and sensory evaluation. Cereal Foods World. 2007;52(1):15–9. [Google Scholar]
- 23. US Department of Agriculture Agricultural Research Service . FoodData Central homepage. [Internet]. [cited 2020 Jan 16 ]. Available from: fdc.nal.usda.gov. [Google Scholar]
- 24. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SMet al. . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 25. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, Akl EA, Guyatt GH, on behalf of the Cochrane GRADEing Methods Group. Chapter 15: Interpreting results and drawing conclusions. In: Cochrane handbook for systematic reviews of interventions [Internet]. Cochrane Training;. 2019. p.; 403–31.. [cited 2020 May 5]. Available from: https://training.cochrane.org/handbook/current/chapter-15. [Google Scholar]
- 27. Deeks J, Higgins J, Altmann D. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch Veditors. Cochrane handbook for systematic reviews of interventions. [Internet]. Cochrane Training; 2019. [cited 2020 May 5]. Available from: https://training.cochrane.org/handbook/current/chapter-10. [Google Scholar]
- 28. Wolever TM, Jones PJ, Jenkins AL, Mollard RC, Wang H, Johnston A, Johnson J, Chu Y. Glycaemic and insulinaemic impact of oats soaked overnight in milk vs. cream of rice with and without sugar, nuts, and seeds: a randomized, controlled trial. Eur J Clin Nutr. 2019;73(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Costabile G, Griffo E, Cipriano P, Vetrani C, Vitale M, Mamone G, Rivellese AA, Riccardi G, Giacco R. Subjective satiety and plasma PYY concentration after wholemeal pasta. Appetite. 2018;125:172–81. [DOI] [PubMed] [Google Scholar]
- 30. Lee I, Shi L, Webb DL, Hellstrom PM, Riserus U, Landberg R. Effects of whole-grain rye porridge with added inulin and wheat gluten on appetite, gut fermentation and postprandial glucose metabolism: a randomised, cross-over, breakfast study. Br J Nutr. 2016;116(12):2139–49. [DOI] [PubMed] [Google Scholar]
- 31. Sandberg JC, Bjorck IME, Nilsson AC. Effects of whole grain rye, with and without resistant starch type 2 supplementation, on glucose tolerance, gut hormones, inflammation and appetite regulation in an 11–14.5 hour perspective; a randomized controlled study in healthy subjects. Nutr J. 2017;16(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cioffi I, Ibrugger S, Bache J, Thomassen MT, Contaldo F, Pasanisi F, Kristensen M. Effects on satiation, satiety and food intake of wholegrain and refined grain pasta. Appetite. 2016;107:152–8. [DOI] [PubMed] [Google Scholar]
- 33. Cioffi I, Santarpia L, Vaccaro A, Iacone R, Labruna G, Marra M, Contaldo F, Kristensen M, Pasanisi F. Whole-grain pasta reduces appetite and meal-induced thermogenesis acutely: a pilot study. Appl Physiol Nutr Metab. 2016;41(3):277–83. [DOI] [PubMed] [Google Scholar]
- 34. Sandberg JC, Bjorck IM, Nilsson AC. Rye-based evening meals favorably affected glucose regulation and appetite variables at the following breakfast; a randomized controlled study in healthy subjects. PLoS One. 2016;11(3):e0151985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Geliebter A, Grillot CL, Aviram-Friedman R, Haq S, Yahav E, Hashim SA. Effects of oatmeal and corn flakes cereal breakfasts on satiety, gastric emptying, glucose, and appetite-related hormones. Ann Nutr Metab. 2015;66(2–3):93–103. [DOI] [PubMed] [Google Scholar]
- 36. Gonzalez-Anton C, Rico MC, Sanchez-Rodriguez E, Ruiz-Lopez MD, Gil A, Mesa MD. Glycemic responses, appetite ratings and gastrointestinal hormone responses of most common breads consumed in Spain. A randomized control trial in healthy humans. Nutrients. 2015;7(6):4033–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johansson DP, Lee I, Riserus U, Langton M, Landberg R. Effects of unfermented and fermented whole grain rye crisp breads served as part of a standardized breakfast, on appetite and postprandial glucose and insulin responses: a randomized cross-over trial. PLoS One. 2015;10(3):e0122241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hartvigsen ML, Gregersen S, Laerke HN, Holst JJ, Bach Knudsen KE, Hermansen K. Effects of concentrated arabinoxylan and beta-glucan compared with refined wheat and whole grain rye on glucose and appetite in subjects with the metabolic syndrome: a randomized study. Eur J Clin Nutr. 2014;68(1):84–90. [DOI] [PubMed] [Google Scholar]
- 39. Johansson EV, Nilsson AC, Ostman EM, Bjorck IM. Effects of indigestible carbohydrates in barley on glucose metabolism, appetite and voluntary food intake over 16 h in healthy adults. Nutr J. 2013;12:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosen LA, Ostman EM, Bjorck IM. Effects of cereal breakfasts on postprandial glucose, appetite regulation and voluntary energy intake at a subsequent standardized lunch; focusing on rye products. Nutr J. 2011;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosen LA, Ostman EM, Bjorck IM. Postprandial glycemia, insulinemia, and satiety responses in healthy subjects after whole grain rye bread made from different rye varieties. 2. J Agric Food Chem. 2011;59(22):12149–54. [DOI] [PubMed] [Google Scholar]
- 42. Rosen LA, Ostman EM, Shewry PR, Ward JL, Andersson AA, Piironen V, Lampi AM, Rakszegi M, Bedo Z, Bjorck IM. Postprandial glycemia, insulinemia, and satiety responses in healthy subjects after whole grain rye bread made from different rye varieties. 1. J Agric Food Chem. 2011;59(22):12139–48. [DOI] [PubMed] [Google Scholar]
- 43. Kristensen M, Jensen MG, Riboldi G, Petronio M, Bugel S, Toubro S, Tetens I, Astrup A. Wholegrain vs. refined wheat bread and pasta. Effect on postprandial glycemia, appetite, and subsequent ad libitum energy intake in young healthy adults. Appetite. 2010;54(1):163–9. [DOI] [PubMed] [Google Scholar]
- 44. Rosen LA, Silva LO, Andersson UK, Holm C, Ostman EM, Bjorck IM. Endosperm and whole grain rye breads are characterized by low post-prandial insulin response and a beneficial blood glucose profile. Nutr J. 2009;8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hlebowicz J, Wickenberg J, Fahlstrom R, Bjorgell O, Almer LO, Darwiche G. Effect of commercial breakfast fibre cereals compared with corn flakes on postprandial blood glucose, gastric emptying and satiety in healthy subjects: a randomized blinded crossover trial. Nutr J. 2007;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Granfeldt Y, Liljeberg H, Drews A, Newman R, Björck I. Glucose and insulin responses to barley products: influence of food structure and amylose-amylopectin ratio. Am J Clin Nutr. 1994;59(5):1075–82. [DOI] [PubMed] [Google Scholar]
- 47. Hamad S, Zafar TA, Sidhu J. Parboiled rice metabolism differs in healthy and diabetic individuals with similar improvement in glycemic response. Nutrition. 2018;47:43–9. [DOI] [PubMed] [Google Scholar]
- 48. Suhr J, Vuholm S, Iversen KN, Landberg R, Kristensen M. Wholegrain rye, but not wholegrain wheat, lowers body weight and fat mass compared with refined wheat: a 6-week randomized study. Eur J Clin Nutr. 2017;71(8):959–67. [DOI] [PubMed] [Google Scholar]
- 49. Ibrugger S, Vigsnaes LK, Blennow A, Skuflic D, Raben A, Lauritzen L, Kristensen M. Second meal effect on appetite and fermentation of wholegrain rye foods. Appetite. 2014;80:248–56. [DOI] [PubMed] [Google Scholar]
- 50. Luhovyy BL, Mollard RC, Yurchenko S, Nunez MF, Berengut S, Liu TT, Smith CE, Pelkman CL, Anderson GH. The effects of whole grain high-amylose maize flour as a source of resistant starch on blood glucose, satiety, and food intake in young men. J Food Sci. 2014;79(12):H2550–6. [DOI] [PubMed] [Google Scholar]
- 51. Isaksson H, Tillander I, Andersson R, Olsson J, Fredriksson H, Webb DL, Aman P. Whole grain rye breakfast – sustained satiety during three weeks of regular consumption. Physiol Behav. 2012;105(3):877–84. [DOI] [PubMed] [Google Scholar]
- 52. Zafar TA, Kabir Y, Ghazaii C. Low glycemic index foods suppress glycemic responses, appetite and food intake in young Kuwaiti females. Kuwait J Sci Eng. 2011;38(1A):111–23. [Google Scholar]
- 53. Anderson GH, Cho CE, Akhavan T, Mollard RC, Luhovyy BL, Finocchiaro ET. Relation between estimates of cornstarch digestibility by the Englyst in vitro method and glycemic response, subjective appetite, and short-term food intake in young men. Am J Clin Nutr. 2010;91(4):932–9. [DOI] [PubMed] [Google Scholar]
- 54. Schroeder N, Gallaher DD, Arndt EA, Marquart L. Influence of whole grain barley, whole grain wheat, and refined rice-based foods on short-term satiety and energy intake. Appetite. 2009;53(3):363–9. [DOI] [PubMed] [Google Scholar]
- 55. Isaksson H, Sundberg B, Aman P, Fredriksson H, Olsson J. Whole grain rye porridge breakfast improves satiety compared to refined wheat bread breakfast. Food Nutr Res. 2008;52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Berti C, Riso P, Brusamolino A, Porrini M. Effect on appetite control of minor cereal and pseudocereal products. Br J Nutr. 2005;94(5):850–8. [DOI] [PubMed] [Google Scholar]
- 57. Breen C, Ryan M, Gibney MJ, Corrigan M, O'Shea D. Glycemic, insulinemic, and appetite responses of patients with type 2 diabetes to commonly consumed breads. Diabetes Educ. 2013;39(3):376–86. [DOI] [PubMed] [Google Scholar]
- 58. Holt SHA, Brand-Miller JC, Stitt PA. The effects of equal-energy portions of different breads on blood glucose levels, feelings of fullness and subsequent food intake. J Am Diet Assoc. 2001;101(7):767–73. [DOI] [PubMed] [Google Scholar]
- 59. Nilsson AC, Ostman EM, Holst JJ, Bjorck IM. Including indigestible carbohydrates in the evening meal of healthy subjects improves glucose tolerance, lowers inflammatory markers, and increases satiety after a subsequent standardized breakfast. J Nutr. 2008;138(4):732–9. [DOI] [PubMed] [Google Scholar]
- 60. Bodinham CL, Hitchen KL, Youngman PJ, Frost GS, Robertson MD. Short-term effects of whole-grain wheat on appetite and food intake in healthy adults: a pilot study. Br J Nutr. 2011;106(3):327–30. [DOI] [PubMed] [Google Scholar]
- 61. Tarini J, Wolever TM. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab. 2010;35(1):9–16. [DOI] [PubMed] [Google Scholar]
- 62. Sadeghi O, Sadeghian M, Rahmani S, Maleki V, Larijani B, Esmaillzadeh A. Whole-grain consumption does not affect obesity measures: an updated systematic review and meta-analysis of randomized clinical trials. Adv Nutr. 2020;11(2):280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393(10170):434–45. [DOI] [PubMed] [Google Scholar]
- 64. Burton-Freeman B, Davis PA, Schneeman BO. Plasma cholecystokinin is associated with subjective measures of satiety in women. Am J Clin Nutr. 2002;76(3):659–67. [DOI] [PubMed] [Google Scholar]
- 65. Seal CJ, Nugent AP, Tee ES, Thielecke F. Whole-grain dietary recommendations: the need for a unified global approach. Br J Nutr. 2016;115(11):2031–8. [DOI] [PubMed] [Google Scholar]
- 66. Gerstein DE, Woodward-Lopez G, Evans AE, Kelsey K, Drewnowski A. Clarifying concepts about macronutrients’ effects on satiation and satiety. J Am Diet Assoc. 2004;104(7):1151–3. [DOI] [PubMed] [Google Scholar]
- 67. Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, De Backer F, Neyrinck AM, Delzenne NM. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90(5):1236–43. [DOI] [PubMed] [Google Scholar]
- 68. Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein–coupled receptor FFAR2. Diabetes. 2012;61(2):364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Verdich C, Flint A, Gutzwiller J-P, Naslund E, Beglinger C, Hellstrom P, Long S, Morgan L, Holst J, Astrup A. A meta-analysis of the effect of glucagon-like peptide-1 (7-36) amide on ad libitum energy intake in humans. J Clin Endo Metab. 2001;86(9):4382–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.