Abstract
T follicular helper (Tfh) cells cognately guide differentiation of antigen-primed B cells in secondary lymphoid tissues. “Tfh-like” populations not expressing the canonical Tfh cell transcription factor BCL6 have also been described, which can aid particular aspects of B cell differentiation. Tfh and Tfh-like cells are essential for protective and pathological humoral immunity. These CD4+ T cells that help B cells are polarized to produce diverse combinations of cytokines and chemokine receptors and can be grouped into distinct subsets that promote antibodies of different isotype, affinity, and duration, according to the nature of immune challenge. However, unified nomenclature to describe the distinct functional Tfh and Tfh-like cells does not exist. While explicitly acknowledging cellular plasticity, we propose categorizing these cell states into three groups based on phenotype and function, paired with their anatomical site of action.
T cells that help B cells
Adaptive immunity is characterized by the coordinated actions of T and B cells against a variety of pathogens, including viruses, bacteria, helminths, and fungi. While cellular immunity is mediated by cytotoxic CD8+ T lymphocytes and effector CD4+ T lymphocytes (T helper cells), antibodies produced by plasmablasts and plasma cells constitute the humoral arm of adaptive immune responses. Together, both arms complement each other’s role in fighting infections and in generating immunological memory, but they may also be dysregulated in patients suffering from cancer, allergy, immunodeficiency, or autoimmunity. Most long-lived highly specific antibody responses require help from CD4+ T cells. Originally, the CD4+ T helper type (Th)2 cell subset was thought to be responsible for directing antibody responses. Work from the past two decades has corrected and refined this model with the identification of T follicular helper (Tfh) cells --a subset of CD4+ T cells responsible for directing antibody production to a wide array of immune stimuli. However, unified nomenclature to describe functional Tfh and Tfh-like cells is currently lacking. Here, we propose a three-group nomenclature system to categorize these helper subsets based on phenotype, function, and anatomical localization.
Tfh cell differentiation and functional heterogeneity
Tfh cells are the primary T cells responsible for supporting B cell proliferation, survival, and differentiation in humans and mice. Their recognition as a functionally distinct T helper cell subset was facilitated by the identification of the chemokine receptor CXCR5 as a hallmark of B cell helper function, followed by the discovery of B cell lymphoma 6 (BCL6) as the key transcriptional regulator of Tfh cell differentiation [1–3]. Unlike other effector T helper cells, Tfh cells typically act in secondary lymphoid organs (SLOs), where they interact with B cells at multiple sites, including the T-B border inside B cell follicles and in germinal centers (GCs), to direct the isotype, specificity, and affinity of the B cell response through both contact-dependent signals and the production of cytokines (Box 1, Fig.1, Table 1).
Box 1. The nature of B cell help provided by Tfh cells depends on where they are located
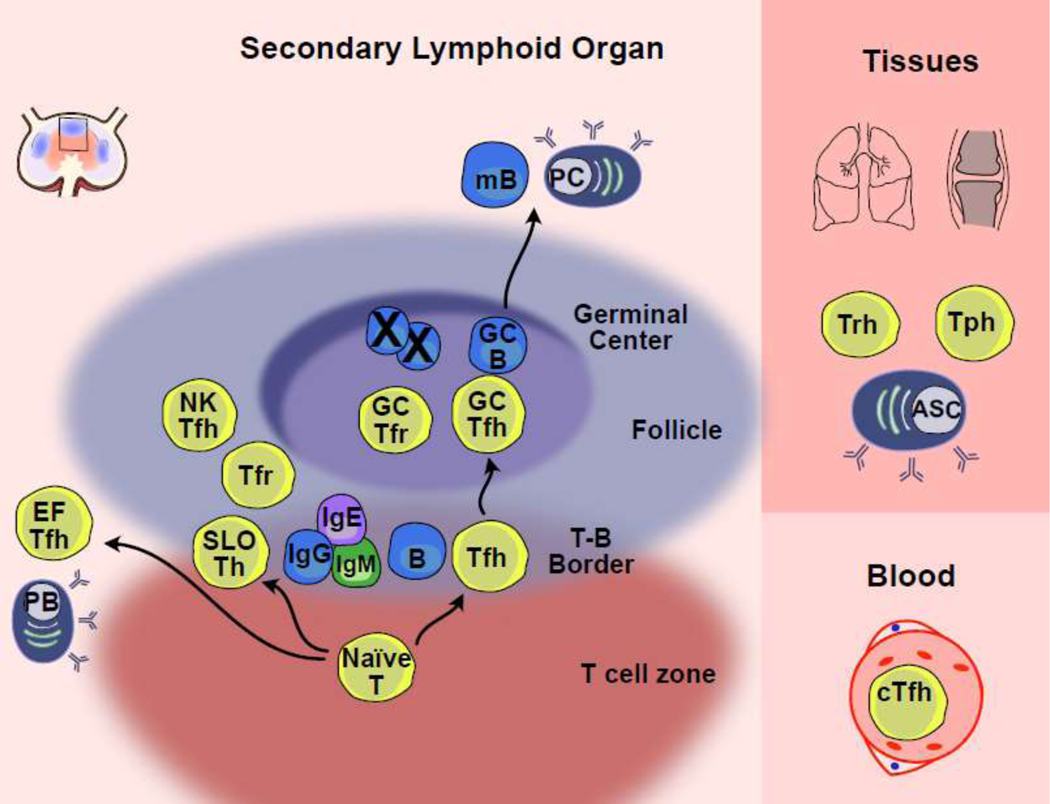
Tfh cells are named for their ability to provide help to the recirculating mature B cell pool, also known as follicular B cells, due to their migration between follicles of secondary lymphoid organs (SLO) (Fig. 1). Tfh cells can encounter recirculating follicular B cells at the borders between the T cell zones and the follicles (T-B border), in the B cell follicle, the interfollicular region, and in germinal centers (GC). During responses to protein antigens, the first cognate encounter between primed CXCR5+ CD4+ Tfh cells and B cells that have bound antigen typically occurs at the T-B border [83]. Here, Tfh and/or Tfh-like cells provide co-stimulatory signals, including CD40L and cytokines, to B cells, which initiate their differentiation and direct immunoglobulin (Ig) isotype switching [14, 84]. CD4+ T cells expressing intermediate amounts of CXCR5, immune checkpoint receptor programmed death-1 (PD-1), and BCL6, interacting with B cells at the T:B border have been called “pre-GC Tfh” cells, “pre-Tfh” cells, or “Tfh” cells [85]. We propose referring to them as Tfh cells, to emphasize their role as functional B cell helpers, in addition to being the precursors of GC-Tfh cells (Table 1).
Interactions between Tfh cells and B cells further the differentiation of Tfh cells, as well as their migration into the GC to become “GC-Tfh” cells, where they mediate affinity-based selection of GC B cells (GC reaction), and the subsequent differentiation into memory B cells or long-lived antibody secreting plasma cells [86]. These GC-Tfh cells express the highest amounts of CXCR5, PD-1, and BCL6, and are typically located within the light zone of the GC. GC-Tfh cells are essential for GC function and maintenance [1, 3, 87]. Although the postulated functions are distinct between Tfh cells in supporting B cell activation and Ig class switching versus those of GC-Tfh cells in the selection of high affinity B cells, an accurate identification of these cell types without direct in vivo imaging is challenging. Of note, their presumed identification by flow cytometric analyses uses intermediate amounts of CXCR5 and PD-1 expressed on Tfh cells relative to high expression of these markers on GC-Tfh cells in mice and human SLOs [17].
In addition to acting in the follicles of SLOs, Tfh cells may also migrate to extrafollicular sites within SLOs, typically identified in splenic bridging channels (areas between the T zones and the red pulp). CD4+ T cells at these sites have been called “extrafollicular Tfh (EF-Tfh)” cells and have been observed in the context of autoimmunity [77]. Here, EF-Tfh cells support antibody secreting plasmablasts, although their role in other immune reactions remains to be defined.
The study of human Tfh cells is complicated by the limited experimental access to SLOs after natural infection or immunizations. Subsets of circulating CD4+CXCR5+ T cells with B cell helper functions have been shown to be phenotypically, functionally, and transcriptionally related to SLO Tfh cell subsets [5, 6, 69, 88, 89]. RNA and TCR sequencing of paired human blood and SLO Tfh cell subsets showed that CXCR5+PD-1+ circulating Tfh (cTfh) cells were transcriptionally related to SLO Tfh cells and shared TCR sequences, suggesting a common origin [90–93]. In mice, adoptive transfer of cTfh cells followed by immunization revealed that cTfh cells were rapidly recalled (as SLO Tfh cells), suggesting that cTfh lmight ikely act as memory cells [89].
Figure 1: Nomenclature used for CD4+ T cells that help B cells in different anatomic locations in mice and humans.
Left, Tfh and Tfh-like subsets are shown in regions of secondary lymphoid organs (SLOs) with particular functions depicted. Shown is a region of a lymph node, although similar structures exist in other SLOs. Extrafollicular T follicular helper (EFTfh); T follicular regulatory (Tfr); Natural killer Tfh (NKTfh); Secondary lymphoid organ Tfh (SLO Tfh); Plasmablast (PB); Antibody secreting cell (ASC); Germinal center B cell (GCB); Memory B (mB). Right, Tfh and related subsets in tissues and the blood with general anatomic distinctions and select functions indicated. Circulating Tfh (cTfh); T peripheral helper (Tph); T resident helper (Trh).
Table 1: A proposal of three groups of Tfh and Tfh-like subsets based on anatomical location of function.
BCL6-dependent indicates that Bcl6/BCL6 is required for development, even if it is no longer highly expressed. Secondary lymphoid organs (SLO) include lymph nodes, spleen, Peyer’s Patches, etc. cTfh, circulating Tfh; EF, extrafollicular; GC, germinal center; NKTfh, Natural killer Tfh; Tfh, T follicular helper; Tfr, T follicular regulatory; Th, T helper; Tph, T peripheral helper; Trh, T resident helper; PB, peripheral blood; CSR, class switch recombination. Grey text indicates the cell subsets that have not been well defined.
| Site | Tfh & Tfh-like cell subsets | BCL6-dependent | Group 1 | Group 2 | Group 3 | Primary Function |
|---|---|---|---|---|---|---|
| Secondary Lymphoid Organs | Tfh | YES | Tfh1 | Tfh2 | Tfh17 | Priming, differentiation & CSR of antigen-stimulated B cells |
| GC-Tfh | YES | GC-Tfh1 | GC-Tfh2 | GC-Tfh17 | GC B cell selection | |
| SLO-Th | NO | SLO-Th1 | SLO-Th2 | SLO-Th17 | Priming, differentiation & CSR of antigen-stimulated B cells | |
| EF-Tfh | ? | EF-Tfh1 | EF-Tfh2 | EF-Tfh17 | PB expansion in chronic inflammatory conditions | |
| NKTfh | YES | NKTfh1 | NKTfh2 | NKTfh17 | Boost B cell priming | |
| Tfr | YES | Tfr1 | Tfr2 | Tfr17 | Tfh regulation, GC B cell selection | |
| Blood | cTfh | YES | cTfh1 | cTfh2 | cTfh17 | Circulating memory reservoir |
| Non-lymphoid Tissues | Tph | ? | Tph1 | Tph2 | Tph17 | In situ B cell activation |
| Trh | YES | Trh1 | Trh2 | Trh17 | Reactivation of memory B cells in situ |
The isotype of the immunoglobulin (Ig) Fc region dictates multiple aspects of antibody function and differs based on the nature of the immune challenge. B cell isotype switching is regulated by specific cytokines, typically (but not exclusively) produced by Tfh cells. Thus, Tfh cells with distinct cytokine profiles are induced based on the nature of the immune stimulus [4]. These Tfh cells, in turn, promote the production of antibody isotypes differing in form and function; for example, in mice, IFNγ-driven IgG2 is induced during influenza virus infection and IL-4-driven IgE is induced against venoms [4]. Indeed, humans and mice possess Tfh cell subsets defined by the differential expression of chemokine receptors and the production of cytokines that parallel CD4+ T effector cell subsets [5–7]. For instance, Tfh cells can express the same lineage-defining transcription factors as their conventional T helper counterparts (e.g., TBET, GATA3, RORγT) [8]. In fact, tissue-homing effector CD4+ T cells as well as GC-homing Tfh cells have been proposed to represent a cellular continuum [7, 8]. Functional polarization of Tfh cells might be expected given that the stimulatory milieu directing CD4+ T effector cell polarization should likewise act on differentiating Tfh cells.
Tfh and Tfh-like cell heterogeneity exists but adequate nomenclature does not
It is important to distinguish different types of T cell -B cell interactions as they differentially impact the B cell populations and the antibodies induced during an immune response (Box 2). Note that effects on B cells and antibodies are not identical; for example, an effect on memory B cells may not be evident in the antibody response produced by plasmablasts during immunization. Both BCL6-dependent and -independent T cells shape antibody responses at different phases of the B cell response in response to diverse immune stimuli. For example, in mice, the early IgG1 B cell reaction to immunization includes the generation of both immunoglobulin class-switched memory B cells and short-lived, low affinity, antibody-secreting plasmablasts. Both responses require CD4+ T cells, but may differentially rely on Tfh cell help or GCs [9–11]. In contrast, B cell selection within the GC, and the ultimate production of long-lived, high affinity plasma cells/antibodies and highly mutated memory B cells, are both BCL6-and Tfh-dependent [9, 10]. Even this oversimplified scheme may differ in terms of the relevant T cell-B cell interactions depending on the anatomical site of induction. For example, in mice, highly specific IgA to food antigens can be produced in the gut in a Tfh cell-independent manner, distinguishing it from IgG1 and IgE [12].
Box 2. Non-classical T helper cells for B cells
Multiple populations of Tfh-like cells exist in SLOs that affect aspects of the B cell response. For example, BCL6-expressing natural killer T cells (NKT; also known as NKTfh cells) have been shown to provide efficient help to alpha-GalCer-binding as well as non-cognate B cells through the production of IL-21 and IL-4 [94–96]. In some mouse models of vaccination, BCL6-independent CD4+ T cells can also provide help to B cells; these cells likely differ from classic effector Th cells by remaining in SLOs rather than rapidly emigrating to peripheral tissues to orchestrate cellular immunity [9, 13, 97]. However, their anatomic relationship and functional breadth has yet to be clarified. We propose here classifying them as SLO-Th to distinguish them from Th cells that exit SLOs (i.e., classic Th1, Th2, Th17) (Table 1). The Tfh cell regulatory counterpart, T follicular regulatory (Tfr) cells in mice, has been shown to typically derive from Foxp3+ precursors and function as a suppressive subset of regulatory T cells (Treg), while helping to shape the GC response [98].
In non-lymphoid tissues such as the joint synovium, a subset of PD-1hiCXCR5− cells has been described in autoimmune conditions e.g. rheumatoid arthritis; these cells have been designated T peripheral helper (Tph) cells in humans but their ontogeny and relationship to classic Tfh cells in SLOs remains unknown [78, 79]. Similar cells have been identified in the blood of patients with systemic lupus erythematosus (SLE) [76]. These populations are thought to support local production of autoimmune antibodies. Another population of BCL6-dependent CD4+ T cells residing outside of SLOs that help B cells are the recently described T resident helper (Trh) cells [99, 100]; these cells have promoted local B cell responses in the lungs of mice in models of virus re-infection. A similar population has been described in certain human tumors, with the production of CXCL13 promoting local memory B cell responses [101].
Even the different antibody isotypes produced by plasmablasts/plasma cells (e.g., IgG1 vs. IgG2 in mice) might be regulated by multiple types of T cells prior to the GC reaction (Box 1) [9, 10, 13, 14]. For instance, IL-4 and IL-21 production by mouse Tfh cells supports immunoglobulin isotype switching to IgG1 as well as plasma cell differentiation, whereas IFN-γ and IL-21 production can promote IgG2 and the differentiation of T-BET+CD11c+ atypical memory B cells, while IL-4 inhibits their formation [15]. Therefore, a flexible and inclusive nomenclature for the potentially distinct T cells that guide B cell differentiation (e.g., memory vs. plasmablast vs. plasma cell vs. atypical) and isotype switching is warranted.
Despite two decades of investigation, a unified terminology to describe different functional Tfh and Tfh-like cell states does not exist. As has been true for the innate lymphoid cell (ILC) and dendritic cell (DC) fields, when varied nomenclature is used for the same cell subset in different studies, questions arise about whether different functions of the same subset exist versus the existence of distinct subsets. For the ILC and DC fields, unified nomenclature has enabled cross-comparison in work performed by different groups [16]. As Tfh cells have been studied under a variety of immunological conditions, numerous states/subsets of Tfh cells have been identified, each with differential gene expression programs and functions. As pointed out a decade ago, it seems appropriate to use different nomenclatures while a field matures [17]. However, the nomenclature for the various Tfh and Tfh-like cell subsets has remained ambiguous, inconsistent, and fragmented. This calls for evolution of the nomenclature.
An inclusive, unified nomenclature for T cells that help B cells
Here, we provide a framework for unifying the various Tfh and Tfh-like cell populations that have been described across experimental systems by assigning them into three groups based on their phenotype and function (Fig. 2 and Table 1). This group assignment allows for comparison of T cells that act at different phases of the B cell response as well as for their distinct functional locations. While explicitly taking into account the high degree of cellular plasticity within the T helper and Tfh cell compartments, it is anticipated that this categorization can help the community to better align current efforts to improve our understanding of the complexities of T cells that help B cells.
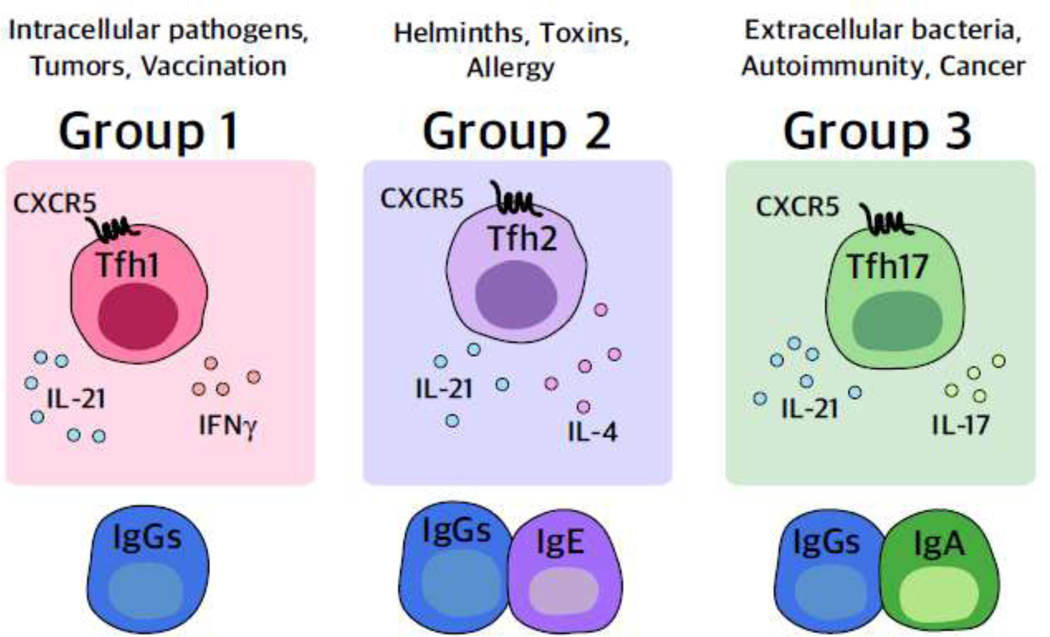
Key Figure, Figure 2: Proposed unified groups of Tfh and Tfh-like cells.
The schema depicts the proposed members of each group with one representative “functional” Tfh cell shown expressing the classic Tfh-defining chemokine receptor CXCR5. Signature cytokines along with the canonical cytokine IL-21 are shown but represent the group members without indicating a static state. For example, IL-21 may be produced during particular time windows rather than continuously and other cytokines such as IL-4 may be expressed by cells of all groups but at lower amounts than group 2. Each group encompasses Tfh and Tfh-like cells that can function at different sites within SLOs as well as outside of SLOs, with the central theme of the group being the similar transcription factor and cytokine expression patterns. Cooperation of the group members ultimately results in the production of the types of antibodies depicted at the bottom of the image, as well as humoral immunity to the types of challenges listed at the top.
Identifying Tfh cell group names by a “signature” cytokine would be inaccurate (e.g., there is likely to be more than one cytokine of functional importance in each group), and cytokine production is dynamic; indeed, combinations of cytokines may be present at different ratios over time, as has been shown for the production of IL-21 and IL-4 by murine Tfh cells [18]. Instead, we propose a group classification based on the combined cytokine/transcription factor/chemokine receptor phenotype and the functional characteristics of effector Tfh cells, while for ease of grouping, concomitantly acknowledging “signature” or essential cytokines (Fig. 2). Using parallel group names to the ones established for ILCs [16] and applicable to CD4+ T cell subsets [19] highlights a coordinated immune response (e.g., grouping CD4+ T effector, Tfh, ILC, antibody, etc.) to particular types of immune challenges (e.g., type 1 intracellular pathogens vs. type 2 helminth infections vs. type 3 extracellular pathogens).
Common features exist in the Tfh/Tfh-like cell subsets across the groups. For example, canonical Tfh cells in all groups express CXCR5, PD-1, ICOS, and BCL6. Another canonical feature of cells across the three groups is IL-21 production --a cytokine that is essential for multiple aspects of the B cell response, even if it is only transiently produced [20]. IL-4 is also considered a canonical Tfh cytokine but can be expressed at different levels by members in all groups with the highest expression in Group 2 and is therefore depicted with that group (Fig. 2).
Group 1 Tfh cells
Type 1 immunity controls intracellular pathogens and can contribute to anti-tumor immune responses as well driving autoimmune and inflammatory states [19]. Group 1 Tfh cells that arise during such conditions can produce IFN-γ, transiently express the transcription factor T-BET, and/or promote IgG subclasses that bind complement and FcRs. IFNγ-producing Tfh and Th1 cells have been shown to develop in mice simultaneously, with early IL-12 production promoting both fates through the upregulation of BCL6 and T-BET [21] – with the balance between these two transcription factors determining Tfh versus Th1 cell fates, respectively [22, 23]. Notably, IL-12 induces the highest proportion of Tfh-like IL-21-expressing cells from human naïve CD4+ T cells in vitro, a substantial proportion of which co-express IFNγ [24, 25]. IFNγ+, CXCR3+ or T-BET+ Tfh cells have been observed in mice and humans in vivo after viral infection (e.g., influenza), vaccination with squalene-based adjuvants, inactivated viral vaccines or adenoviral vectored vaccines, and in certain autoimmune states, including systemic lupus erythematosus (SLE) and juvenile dermatomyositis [26–32]. Ultimately, T-BET, which is essential for polarizing this Group 1 Tfh phenotype, must be silenced, and BCL6 expression is maintained for Tfh cell differentiation to proceed [26, 28, 30, 31].
Group 1 Tfh cells are associated with abundant IgG2 and moderate IgG1 production in mice [29, 31, 33]. Although human memory CXCR3+ Tfh cells produce IFNγ [6, 34], whether IFNγ can provide help to human B cells or direct immunoglobulin class switch recombination (CSR) is unclear [34, 35]. Indeed, human memory CXCR3+ Tfh cells are inefficient in inducing differentiation of human naïve B cells in vitro [5, 6]. However, following influenza virus booster vaccination, CXCR3+ Tfh cells dominate in peripheral blood, correlate with vaccine efficacy, and can activate human memory B cells to induce IgG production [27]. Therefore, this group of human circulating Tfh (cTfh) cells might only demonstrate functional activity if recently released from SLOs in the context of a recall immune response (rather than primary), and may use signals other than IFNγ to impact B cell function. Consistent with this, CXCR3+ Tfh cells in lymph nodes (LNs) from rhesus demonstrate IgG-inducing capabilities macaques after chronic simian immunodeficiency virus infection [36]. Additionally, a subset of Group 1 Tfh cells has been proposed to produce IL-10 during chronic viral infection with Clone13 lymphocytic choriomeningitis virus (LCMV) [37]. Different studies have referred to mouse and human Tfh cells in this group as “ex-T-bet Tfh”, “Tfh1”, “Tfh1-like”, “CXCR3+ Tfh” “IL10+IL21+ Tfh”, or Tfh-Th1-like” cells, which we now propose to collectively call Group 1 Tfh cells.
Group 2 Tfh cells
Type 2 immunity controls helminth infection and toxins but can also cause allergic reactions. Group 2 Tfh cells that arise during these conditions produce high amounts of IL-4, can express GATA3 or BATF, lack the chemokine receptors CXCR3 and CCR6, and are associated with the production of IgG1 and IgE in mice, and IgG4 and IgE in humans [38]. Using this definition, Group 2 Tfh cells have been observed in mice during allergic sensitization, helminth, protozoa, and Plasmodium sp. infections, following alum-adjuvanted protein-subunit vaccine administration, as well as in IgE-associated SLE [31, 38–45]. As IL-4 has also been considered a canonical Tfh cell cytokine that is expressed by Tfh cells during a wide variety of immune reactions and which might be possibly beneficial for GC B cell survival and differentiation [17], the distinction of IL-4-producing Group 2 Tfh cells from other Tfh groups is difficult without incorporating an associated antibody response.
What has become clear --primarily through mouse-based studies --is that Group 2 Tfh cells and Th2 cells are distinct in terms of location and function, with Th2 cells producing few cytokines in LNs and failing to participate in B cell responses, while conversely, Tfh cells play a limited role in type 2 tissue inflammation, as evidenced rom various allergy mouse models [38, 39, 42, 43, 46]. In vitro, such a clear functional demarcation is difficult to ascertain as a wide variety of T cells can sustain and guide B cell activation, presumably through the production of IL-21. Yet, as is likely true for all CD4+ T cell subsets, the relationship between Group 2 Tfh cells and Th2 cells is close, with interconversion between each subset being observed following Tfh2 or Th2 adoptive transfer into mice [47, 48].
In humans, cTfh cells that lack CXCR3 or CCR6 expression produce the highest amount of Type 2 cytokines IL-4, IL-5 and IL-13[5, 6, 44]. IL-4 producing Group 2 Tfh cells have been identified in human SLOs as being associated with IgG4-related disease, and circulating IL-4+IL13+ Tfh cells have been associated with IgE+ allergic states [38, 49].Thus, Tfh cells expressing IL-4 along with other cytokines such as IL-13, and preferentially inducing IgE and IgG1 (mouse) or IgG4 (human) antibodies, have been referred to as “Tfh2”, “Th2-Tfh”, “Th2-like Tfh”, “Tfh13” and “Tfh4” cells, which we now propose to collectively term Group 2 Tfh cells (Tfh2).
Group 3 Tfh cells
Type 3 immunity, epitomized by Th17 cells, is responsible for clearing fungal infections and extracellular bacteria, as well as regulating gut microbiota, but it also mediates the pathology underlying many autoimmune diseases, such as rheumatoid arthritis and SLE [50]. IL-17-expressing Tfh cells have been observed in humans and mice, and human CCR6+ Tfh cells from blood activate allogeneic B cells in vitro [5, 6]. However, as mentioned previously for other T cell subsets, it is difficult to know if such cellular interactions also occur in vivo.
Multiple studies suggest a shared developmental program between Tfh cells and Th17 cells; both populations exhibit early IL-2 production but must avoid responding to IL-2 to differentiate [51]. Additionally, STAT3 signaling, induced by IL-6 and IL-21, is essential for the differentiation of human and murine Tfh and Th17 cells [5, 17, 52, 53]. Besides a putative shared ontogeny, human and murine Th17 and Tfh cells produce IL-21 and can secrete IL-17, although at differing concentrations [54]. Further, adoptive transfer of in vitro differentiated Th17 cells into wildtype mice can promote both GC formation and antibody production [55]. Whether this might be due to Tfh cell differentiation following transfer is not known, but mouse studies using genetic fate mapping have shown that Th17 cells in the gut can convert into Tfh cells [56]. This overlap in differentiation and plasticity between Tfh and effector CD4+ T cell populations is not unique to Group 3 cells; however, a ‘functionally’ distinct Th17-like Tfh cell population has been difficult to identify, as summarized below.
Cellular Th17 cell responses are essential ifor fighting fungal infections [19], yet only limited data exist supporting a role for antibody-mediated protection from fungal infections [57]. This suggests that a parallel Tfh-antibody program complementing the cellular immunity identified for Type 1 and Type 2 immune responses might not occur during all Type 3 responses. Further, in humans with mutations in IL-17 pathways, (i.e., RORC, IL17RA, IL17RC, IL17F, TRAF3IP2), antibody responses to infection or vaccination are not significantly impaired [58], and the frequency of IL-17+CCR6+ circulating Tfh cells in patients following vaccination or viral infection is inversely correlated with neutralizing antibody titers [59, 60]. Therefore, a clear role for Group 3 Tfh cells in pathogen protection remains unclear.
In the murine gut, Th17 (and Treg) conversion into Tfh cells has been postulated to regulate IgA production in response to microbiota [56, 61, 62] and numerous studies have associated increased frequencies of gut Tfh cells with IgA titers [63, 64]. However, loss of BCL6 in T cells (Bcl6fl/flCd4cre), thus ablating Tfh cell differentiation in mice, does not impair T cell-dependent IgA responses to bacteria or food antigens [12, 65, 66]. Further, IL-21 --which can be produced by both Tfh and Th17 cells, but not IL-17--promotes B cell IgA production [62, 67]. Therefore, a definitive role for Tfh17 cells in the induction of IgA in mice is lacking and little is known about the T cells that regulate IgA in humans [68]. We posit that Group 3 Tfh-like cell regulates this response (Fig. 2), but more work is needed to verify this association.
The strongest association of Group 3 Tfh cells with antibody induction comes from studies in mouse models and form patients with autoimmune conditions. For instance, the number of circulating Tfh cells is increased in systemic lupus erythematosus (SLE) patients compared with healthy controls and correlates with autoantibody titers [69]. IL-17-producing Tfh cells are present in the spleens of lupus-prone (BDX2, NZBxSWR) or autoantigen (self-DNA) immunized mice relative to non-autoimmune controls [70–72]. A direct impact of IL-17 on GC formation has been shown in lupus-prone (BDX2, Rc3h1san/san) or autoimmune arthritis-prone (K/BxN) mice, in which IL-17 neutralization or deficiency blocks the generation of both GCs and IgG autoantibodies relative to control mice [73–75]. However, whether IL-17 derives from Tfh, Th17, or another Tfh-like cell in these models has not been established. Multiple non-Tfh or Tfh-like cells are also observed in patients with autoimmune conditions such as lupus and rheumatoid arthritis or in mouse models of autoimmunity and have been found to associate with autoantibodies (Box 2). These include IL-21-or IL10-producing Th17 cells in the blood, T peripheral helper (Tph) cells in affected tissues, or extrafollicular Tfh cells in SLOs [76–79] (Box 2).
Therefore, the identity of the CD4+ T cell subsets that promote mucosal IgA production from B cells, as well as autoreactive IgGs remains unclear. Moreover, whether bona fide Tfh cells are necessary during Type 3 immune responses to pathogens remains untested. However, multiple studies have associated the presence of IL-17+ and/or CCR6+ Tfh cells with these humoral responses and have called them “Tfh17” and “Th17-like Tfh” cells[6, 17, 80]. If such cell types are found to regulate particular antibody classes, including IgA or autoreactive IgG, we posit they should be termed Group 3 Tfh or Group 3 helper cells, depending on their cellular identity.
Altogether, this new classification system aims to group CD4+ T cells that together, over time, and in different locations, help particular types of B cell responses with unique sets of cell surface and cytokine-mediated cues. The ensuing humoral responses generate antibodies with distinct effector mechanisms (e.g., complement activation versus FcR-mediated uptake versus myeloid cell degranulation, etc.) and anatomical spaces (e.g., mucosal surfaces, blood, fetus, etc.). Further, we argue that one advantage of this group-based nomenclature is that it allows flexibility in incorporating new subsets of Tfh and Th-like cells within each group.
Concluding Remarks
The framework proposed here is to be tested in a variety of animal models and human conditions to conclude whether such nomenclature, if adopted, can be useful to the field (see Outstanding Questions). It makes sense to adopt uniform and concise names for particular Tfh cell phenotypes, but by naming them, we do not mean to imply an immutable subset commitment. As previously argued, γδ, αβ and NKT cells are separate lineages that do not interconvert [81]. The same cannot be claimed for CD4+ T cell subsets (e.g., Th1, Th2, Tregs, etc.), nor Tfh cell subsets [7, 8]. These cell states are malleable to environmental cues [82]. For example, Tfh cells may produce different cytokines over the course of an immune reaction and may interconvert with other CD4+ T cell subsets; highly similar Tfh cells within a particular group may functionally differentiate from each other by distinct cytokine production (e.g., IL-13+IL-4+ Tfh cells would be a rare Group 2 Tfh cell only arising during allergen responses). Similarly, BCL6-independent T cells that harbor aspects of both Th effector and Tfh cells could regulate particular aspects of the B cell response. We propose that investigators identify the group being studied in their work, but then have the flexibility to name individual subsets within that group, using clear definitions according to the unique aspects of the cell being studied.
Outstanding questions
How plastic is the Tfh cell fate with other CD4+ T cell subsets in humans and mice? The extent of interconversion between different Tfh cell sub-groups and other T cell subsets has yet to be fully elucidated.
What is the nature of group 3 Tfh/Tfh-like cells in humans and mice? The identity of this subgroup of Tfh cells is not well defined and further work is required to determine the role of Tfh17 cells in type 3 Immunity.
What is the relative role of Tfh vs non-Tfh cells in class switching? Tfh cells and Bcl6-negative B cell helpers can direct class switching under different circumstances, yet the contribution of each T cell type to humoral immunity has not been well defined.
What are the specific molecular regulatory steps of Tfh cell differentiation and polarization? The molecular mechanisms that determine Tfh cell subgroup differentiation have not been fully defined; this is important knowledge for rational vaccine design that generates a B cell response of a desired type.
What cytokines in humans regulate class switching to different IgG subtypes? Cytokine dependent skewing of class switch recombination has been well defined in mice, but is less clear in humans, in particular for IFNγ.
What is the nature of the T cells that regulate antigen-specific IgA in the gut? IgA is one of the most abundant antibody isotypes in the body, and yet the nature of T cells that determine its production are not fully understood.
An outstanding issue we anticipate but that will likely be resolved with further investigations is what to call the groups themselves. We named the groups for their representative Tfh subset (Fig. 2), while acknowledging that non-Tfh cells also participate in the response, and that these subsets are dynamic. As Group 1 Tfh is likely to be shortened to Tfh1 (and so on for the other groups) while still including non-Tfh cells, evolving nomenclature should be considered (e.g., Group 1 T-b cells (Tb1) or Group 1 T helper cells for B cells (Thb1), etc.). However, the important central theme raised in this opinion piece is that a variety of CD4+ T cell subsets can participate in a given humoral response, often with common patterns of cytokine production and transcription factor usage. Clearly, further defining and studying the interplay between these T cell subsets can reveal important mechanisms of B cell regulation and humoral immunity and represents a fruitful area of investigation.
Highlights
We propose a group 1, 2 and 3 classification system for CD4+ T cells that help B cells based on differential expression of signature cytokines, transcription factors (TF), and chemokine receptors, paralleling nomenclatures used for other lymphoid subsets.
This nomenclature is applicable to BCL6-dependent Tfh cells but also BCL6-independent Tfh-like cells that specifically help B cells and are functional in a variety of anatomical sites.
These three groups of Tfh/Tfh-like cells induce distinct types of B cell responses.
This concept does not exclude cellular plasticity, either between the three groups, or between Tfh cells and other effector CD4+ T cell types.
Acknowledgments
The Tfh cell field has numerous investigators that we could not include in this perspective due to journal limitations, but we thank them all for wonderful discussion on this topic over the years. Also, due to space and reference limitations we often referenced reviews rather than primary literature. We thank Hergen Spitz for helpful discussion on nomenclature proposals and Eisenbarth lab members and Gowthaman Uthaman for discussion and review of the manuscript. SCE is supported by R01AI136942, P01 HL132819, a gift from the Colton Center and a FAST Grant, George Mason Mercatus Center. MAL is supported by the Biotechnology and Biological Sciences Research Council (BBS/E/B/000C0427 and BBS/E/B/000C0428). DB is supported by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Emmy Noether Programme BA 5132/1-2 (252623821). DB is a member of Germany’s Excellence Strategy EXC2151 (390873048). JC is supported by R37 AR40072, R01 AR074545. NF is supported by Region Occitanie (#1901175), the European Regional Development Fund (MP0022856), INCA-12642, ANR-16-CE15-0019-02 and ANR-16-CE15-0002-02. CSM is supported by fellowships and grants from the Ministry of Health of the NSW government and NHMRC (GNT1138359) of Australia. SGT is supported by an NHMRC Leadership 3 Investigator Grant (1176665) and NHMRC program grant (1113904).
Glossary
- Atypical B cells
CD11c+T-BET+ B cells implicated in mouse and human host responses to vaccines, viral infections, autoimmunity, and immune aging
- BXD2
inbred mouse strain resulting from several crosses between C57BL/6J and DBA/2J strains; they display IL-17-producing T helper cells in GC and spontaneously develop autoimmune manifestations
- Complement
system of proteins, as an arm of the immune system that enhances the ability of antibodies and phagocytic cells to clear pathogens and damaged cells
- Circulating Tfh cells
CXCR5-expressing T helper cells found in the human and murine blood that acquire Tfh cell function in cases of antigen rechallenge
- Extrafollicular helper Tfh (EF-Tfh) cells
Tfh cells that localize in extrafollicular sites of SLOs whose function is to support antibody production by B cells
- Effector Tfh cells
Activated Tfh cells that control the outcome of antigen-primed B cells in the effector phase, as opposed to memory Tfh cells that are quiescent
- T follicular helper (Tfh) cells
CXCR5-expressing T helper cells controlled by the master regulator BCL6 that regulate the development of antibody-secreting cells and memory B cells in SLO
- GC-Tfh
Tfh cells that localize in GC where they control affinity-based selection of B cells
- K/BxN
mouse model resulting from the backcross of the TCR transgene KRN strain and the MHC class II molecule Ag7-expressing NOD strain. These mice develop spontaneous erosive arthritis. Serum transfer from these mice also causes arthritis
- Light zone
Part of the GC where GC-B cells are subjected to selection by GC-Tfh cells in the presence of follicular dendritic cells
- Foxp3
transcription factor that controls regulatory Treg differentiation
- FcRs
Fc receptors; surface receptors that allow the binding of the Fc region of antibodies attached to infected cells or invading pathogens. While FcRs are engaged, FcR-expressing cells are stimulated to perform antibody-mediated phagocytosis or antibody-dependent cell-mediated cytotoxicity
- Immunoglobulin (Ig) isotype switching (class switch recombination)
Rearrangement of the heavy chain locus of the Ig in antigen-primed B cells. Process regulated by specific cytokines that results in a different Ig isotype based on the nature of the immune challenge
- Memory CXCR3+ Tfh cells
Subset of circulating Tfh cells expressing the chemokine receptor CXCR3 and emerge after viral infection. Their potency to support B cells remains unknown
- Pre-GC Tfh ; Pre-Tfh
Early Tfh cells that localize at the T-B border after T cell priming; important as B cell helpers and precursors of GC-Tfh cells
- Red pulp
Area of the spleen engorged with blood where blood antigens and microorganisms are filtered. This area is separated from the splenic white pulp by the marginal zone
- Secondary lymphoid organs
include lymph nodes, Peyer’s Patches and the spleen. They contain naïve lymphocytes and correspond to the initiation sites of adaptive immune responses
- Splenic bridging channels
Areas in the spleen between the T zones and the red pulp where some extrafollicular plasma cells localize
- T-B border
Area of SLOs between the T cell zones and the B cell follicles where antigen-primed B cells and Tfh cells first encounter
- T peripheral helper (Tph) cells
found in non-lymphoid tissues of autoimmune patients ; PD-1hiCXCR5−BCL6low B cell helpers
- T follicular regulatory
GC suppressive cells that express conventional Tfh cell molecules as well as ones characteristic of Tregs
- T resident helper (Trh) cells
BCL6-dependent memory T helper cells that reside in tissue outside of SLOs; they become effector Tfh cells in case of antigen-rechallenge
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnston RJ, et al. , Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science, 2009. 325(5943): p. 1006–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nurieva RI, et al. , Bcl6 mediates the development of T follicular helper cells. Science, 2009. 325(5943): p. 1001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu D, et al. , The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity, 2009. 31(3): p. 457–68. [DOI] [PubMed] [Google Scholar]
- 4.Olatunde AC, Hale JS, and Lamb TJ, Cytokine-skewed Tfh cells: functional consequences for B cell help. Trends Immunol, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma CS, et al. , Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. Journal of Allergy and Clinical Immunology, 2015. 136(4): p. 993–1006.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita R, et al. , Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity, 2011. 34(1): p. 108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song W. and Craft J, T follicular helper cell heterogeneity: Time, space, and function. Immunol Rev, 2019. 288(1): p. 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu KT, et al. , Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity, 2011. 35(4): p. 622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaji T, et al. , Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. J Exp Med, 2012. 209(11): p. 2079–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toyama H, et al. , Memory B cells without somatic hypermutation are generated from Bcl6-deficient B cells. Immunity, 2002. 17(3): p. 329–39. [DOI] [PubMed] [Google Scholar]
- 11.Takemori T, et al. , Generation of memory B cells inside and outside germinal centers. Eur J Immunol, 2014. 44(5): p. 1258–64. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, et al. , Divergent T follicular helper cell requirement for IgA and IgE production to peanut during allergic sensitization. Sci Immunol, 2020. 5(47). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyauchi K, et al. , Protective neutralizing influenza antibody response in the absence of T follicular helper cells. Nat Immunol, 2016. 17(12): p. 1447–1458. [DOI] [PubMed] [Google Scholar]
- 14.Roco JA, et al. , Class-Switch Recombination Occurs Infrequently in Germinal Centers. Immunity, 2019. 51(2): p. 337–350 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancro MP, Age-Associated B Cells. Annu Rev Immunol, 2020. 38: p. 315–340. [DOI] [PubMed] [Google Scholar]
- 16.Spits H, et al. , Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol, 2013. 13(2): p. 145–9. [DOI] [PubMed] [Google Scholar]
- 17.Crotty S, Follicular helper CD4 T cells (TFH). Annu Rev Immunol, 2011. 29: p. 621–63. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein JS, et al. , TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol, 2016. 17(10): p. 1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallusto F, Heterogeneity of Human CD4+ T Cells Against Microbes. Annual Review of Immunology, 2016. 34(1): p. 317–334. [DOI] [PubMed] [Google Scholar]
- 20.Tangye SG and Ma CS, Regulation of the germinal center and humoral immunity by interleukin-21. J Exp Med, 2020. 217(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakayamada S, et al. , Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity, 2011. 35(6): p. 919–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oestreich KJ, Huang AC, and Weinmann AS, The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. J Exp Med, 2011. 208(5): p. 1001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepper M, et al. , Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity, 2011. 35(4): p. 583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma CS, et al. , Early commitment of naïve human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol, 2009. 87(8): p. 590–600. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt N, et al. , Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity, 2009. 31(1): p. 158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alterauge D, et al. , Continued Bcl6 Expression Prevents the Transdifferentiation of Established Tfh Cells into Th1 Cells during Acute Viral Infection. Cell Rep, 2020. 33(1): p. 108232. [DOI] [PubMed] [Google Scholar]
- 27.Bentebibel SE, et al. , ICOS(+)PD-1(+)CXCR3(+) T follicular helper cells contribute to the generation of high-avidity antibodies following influenza vaccination. Sci Rep, 2016. 6: p. 26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang D, et al. , Transient T-bet expression functionally specifies a distinct T follicular helper subset. J Exp Med, 2018. 215(11): p. 2705–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lederer K, et al. , SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity, 2020. 53(6): p. 1281–1295 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell MD, et al. , IL-12 signaling drives the differentiation and function of a TH1-derived TFH1-like cell population. Sci Rep, 2019. 9(1): p. 13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinstein JS, et al. , STAT4 and T-bet control follicular helper T cell development in viral infections. J Exp Med, 2018. 215(1): p. 337–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva-Cayetano A, et al. , A booster dose enhances immunogenicity of the COVID-19 vaccine candidate ChAdOx1 nCoV-19 in aged mice. Med (N Y), 2021. 2(3): p. 243–262 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheikh AA, et al. , Context-Dependent Role for T-bet in T Follicular Helper Differentiation and Germinal Center Function following Viral Infection. Cell Rep, 2019. 28(7): p. 1758–1772 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Locci M, et al. , Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity, 2013. 39(4): p. 758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujieda S, Zhang K, and Saxon A, IL-4 plus CD40 monoclonal antibody induces human B cells gamma subclass-specific isotype switch: switching to gamma 1, gamma 3, and gamma 4, but not gamma 2. The Journal of Immunology, 1995. 155(5): p. 2318–2328. [PubMed] [Google Scholar]
- 36.Velu V, et al. , Induction of Th1-Biased T Follicular Helper (Tfh) Cells in Lymphoid Tissues during Chronic Simian Immunodeficiency Virus Infection Defines Functionally Distinct Germinal Center Tfh Cells. J Immunol, 2016. 197(5): p. 1832–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xin G, et al. , Single-cell RNA sequencing unveils an IL-10-producing helper subset that sustains humoral immunity during persistent infection. Nat Commun, 2018. 9(1): p. 5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gowthaman U, et al. , Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dolence JJ, et al. , Airway exposure initiates peanut allergy by involving the IL-1 pathway and T follicular helper cells in mice. J Allergy Clin Immunol, 2018. 142(4): p. 1144–1158 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim CJ, et al. , The Transcription Factor Ets1 Suppresses T Follicular Helper Type 2 Cell Differentiation to Halt the Onset of Systemic Lupus Erythematosus. Immunity, 2018. 49(6): p. 1034–1048 e8. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi T, et al. , Asthma-related environmental fungus, Alternaria, activates dendritic cells and produces potent Th2 adjuvant activity. J Immunol, 2009. 182(4): p. 2502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meli AP, et al. , T Follicular Helper Cell-Derived IL-4 Is Required for IgE Production during Intestinal Helminth Infection. J Immunol, 2017. [DOI] [PubMed] [Google Scholar]
- 43.Noble A. and Zhao J, Follicular helper T cells are responsible for IgE responses to Der p 1 following house dust mite sensitization in mice. Clin Exp Allergy, 2016. 46(8): p. 1075–82. [DOI] [PubMed] [Google Scholar]
- 44.Chan JA, et al. , Th2-like T Follicular Helper Cells Promote Functional Antibody Production during Plasmodium falciparum Infection. Cell Rep Med, 2020. 1(9): p. 100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fairfax KC, et al. , IL-4-secreting secondary T follicular helper (Tfh) cells arise from memory T cells, not persisting Tfh cells, through a B cell-dependent mechanism. J Immunol, 2015. 194(7): p. 2999–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reinhardt RL, Liang HE, and Locksley RM, Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol, 2009. 10(4): p. 385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ballesteros-Tato A, et al. , T Follicular Helper Cell Plasticity Shapes Pathogenic T Helper 2 Cell-Mediated Immunity to Inhaled House Dust Mite. Immunity, 2016. 44(2): p. 259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glatman Zaretsky A, et al. , T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med, 2009. 206(5): p. 991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maehara T, et al. , The expansion in lymphoid organs of IL-4(+) BATF(+) T follicular helper cells is linked to IgG4 class switching in vivo. Life Sci Alliance, 2018. 1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirota K, et al. , T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med, 2007. 204(1): p. 41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DiToro D, et al. , Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science, 2018. 361(6407). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suto A, et al. , Development and characterization of IL-21-producing CD4+ T cells. J Exp Med, 2008. 205(6): p. 1369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korn T, et al. , IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature, 2007. 448(7152): p. 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bauquet AT, et al. , The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol, 2009. 10(2): p. 167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitsdoerffer M, et al. , Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A, 2010. 107(32): p. 14292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirota K, et al. , Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat Immunol, 2013. 14(4): p. 372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doron I, et al. , Human gut mycobiota tune immunity via CARD9-dependent induction of anti-fungal IgG antibodies. Cell, 2021. 184(4): p. 1017–1031 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tangye SG, et al. , Human Inborn Errors of Immunity: 2019 Update on the Classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol, 2020. 40(1): p. 24–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huber JE, et al. , Dynamic changes in circulating T follicular helper cell composition predict neutralising antibody responses after yellow fever vaccination. Clin Transl Immunology, 2020. 9(5): p. e1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Juno JA, et al. , Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nature Medicine, 2020. 26(9): p. 1428–1434. [DOI] [PubMed] [Google Scholar]
- 61.Tsuji M, et al. , Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science, 2009. 323(5920): p. 1488–92. [DOI] [PubMed] [Google Scholar]
- 62.Cao AT, et al. , Interleukin (IL)-21 promotes intestinal IgA response to microbiota. Mucosal Immunol, 2015. 8(5): p. 1072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Proietti M, et al. , ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in Peyer’s patches to promote host-microbiota mutualism. Immunity, 2014. 41(5): p. 789–801. [DOI] [PubMed] [Google Scholar]
- 64.Kubinak JL, et al. , MyD88 signaling in T cells directs IgA-mediated control of the microbiota to promote health. Cell Host Microbe, 2015. 17(2): p. 153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bai X, et al. , T Follicular Helper Cells Regulate Humoral Response for Host Protection against Intestinal Citrobacter rodentium Infection. J Immunol, 2020. 204(10): p. 2754–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bunker JJ, et al. , Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. Immunity, 2015. 43(3): p. 541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang X, et al. , IL-21 Promotes Intestinal Memory IgA Responses. J Immunol, 2020. 205(7): p. 1944–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dullaers M, et al. , A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity, 2009. 30(1): p. 120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simpson N, et al. , Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum, 2010. 62(1): p. 234–44. [DOI] [PubMed] [Google Scholar]
- 70.Ding Y, et al. , IL-17RA is essential for optimal localization of follicular Th cells in the germinal center light zone to promote autoantibody-producing B cells. J Immunol, 2013. 191(4): p. 1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu HY, Quintana FJ, and Weiner HL, Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+ CD25-LAP+ regulatory T cell and is associated with down-regulation of IL-17+ CD4+ ICOS+ CXCR5+ follicular helper T cells. J Immunol, 2008. 181(9): p. 6038–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wen Z, et al. , RORgammat licenses the differentiation and function of a unique subset of Tfh cells in response to immunogenic self-DNA in systemic lupus erythematosus. Arthritis Rheumatol, 2021. [DOI] [PubMed] [Google Scholar]
- 73.Lee KC, et al. , Intestinal iNKT cells migrate to liver and contribute to hepatocyte apoptosis during alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol, 2019. 316(5): p. G585–G597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsu HC, et al. , Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol, 2008. 9(2): p. 166–75. [DOI] [PubMed] [Google Scholar]
- 75.Wu HJ, et al. , Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity, 2010. 32(6): p. 815–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caielli S, et al. , A CD4(+) T cell population expanded in lupus blood provides B cell help through interleukin-10 and succinate. Nat Med, 2019. 25(1): p. 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Odegard JM, et al. , ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med, 2008. 205(12): p. 2873–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rao DA, et al. , Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature, 2017. 542(7639): p. 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sakuragi T, et al. , Autoreactivity of Peripheral Helper T Cells in the Joints of Rheumatoid Arthritis. J Immunol, 2021. 206(9): p. 2045–2051. [DOI] [PubMed] [Google Scholar]
- 80.Wichner K, et al. , Dysregulated development of IL-17-and IL-21-expressing follicular helper T cells and increased germinal center formation in the absence of RORgammat. FASEB J, 2016. 30(2): p. 761–74. [DOI] [PubMed] [Google Scholar]
- 81.O’Shea JJ and Paul WE, Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science, 2010. 327(5969): p. 1098–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kiner E, et al. , Gut CD4+ T cell phenotypes are a continuum molded by microbes, not by TH archetypes. Nature Immunology, 2021. 22(2): p. 216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kerfoot SM, et al. , Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity, 2011. 34(6): p. 947–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toellner KM, et al. , T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J Exp Med, 1998. 187(8): p. 1193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McHeyzer-Williams LJ, et al. , Follicular helper T cells as cognate regulators of B cell immunity. Curr Opin Immunol, 2009. 21(3): p. 266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vinuesa CG, et al. , Follicular Helper T Cells. Annu Rev Immunol, 2016. 34: p. 335–68. [DOI] [PubMed] [Google Scholar]
- 87.Liu X, et al. , Bcl6 expression specifies the T follicular helper cell program in vivo. J Exp Med, 2012. 209(10): p. 1841–52, S1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bentebibel SE, et al. , Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med, 2013. 5(176): p. 176ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.He J, et al. , Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity, 2013. 39(4): p. 770–81. [DOI] [PubMed] [Google Scholar]
- 90.Brenna E, et al. , CD4(+) T Follicular Helper Cells in Human Tonsils and Blood Are Clonally Convergent but Divergent from Non-Tfh CD4(+) Cells. Cell Rep, 2020. 30(1): p. 137–152 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heit A, et al. , Vaccination establishes clonal relatives of germinal center T cells in the blood of humans. J Exp Med, 2017. 214(7): p. 2139–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hill DL, et al. , The adjuvant GLA-SE promotes human Tfh cell expansion and emergence of public TCRbeta clonotypes. J Exp Med, 2019. 216(8): p. 1857–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vella LA, et al. , T follicular helper cells in human efferent lymph retain lymphoid characteristics. J Clin Invest, 2019. 129(8): p. 3185–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gaya M, et al. , Initiation of Antiviral B Cell Immunity Relies on Innate Signals from Spatially Positioned NKT Cells. Cell, 2018. 172(3): p. 517–533 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.King IL, et al. , Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol, 2011. 13(1): p. 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chang PP, et al. , Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol, 2011. 13(1): p. 35–43. [DOI] [PubMed] [Google Scholar]
- 97.Kotov JA and Jenkins MK, Cutting Edge: T Cell-Dependent Plasmablasts Form in the Absence of Single Differentiated CD4(+) T Cell Subsets. J Immunol, 2019. 202(2): p. 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stebegg M, et al. , Regulation of the Germinal Center Response. Frontiers in Immunology, 2018. 9(2469). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Son YM, et al. , Tissue-resident CD4(+) T helper cells assist the development of protective respiratory B and CD8(+) T cell memory responses. Sci Immunol, 2021. 6(55). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Swarnalekha N, et al. , T resident helper cells promote humoral responses in the lung. Sci Immunol, 2021. 6(55). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gu-Trantien C, et al. , CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight, 2017. 2(11). [DOI] [PMC free article] [PubMed] [Google Scholar]