ABSTRACT
As one of four filament types, microtubules are a core component of the cytoskeleton and are essential for cell function. Yet how microtubules are nucleated from their building blocks, the αβ-tubulin heterodimer, has remained a fundamental open question since the discovery of tubulin 50 years ago. Recent structural studies have shed light on how γ-tubulin and the γ-tubulin complex proteins (GCPs) GCP2 to GCP6 form the γ-tubulin ring complex (γ-TuRC). In parallel, functional and single-molecule studies have informed on how the γ-TuRC nucleates microtubules in real time, how this process is regulated in the cell and how it compares to other modes of nucleation. Another recent surprise has been the identification of a second essential nucleation factor, which turns out to be the well-characterized microtubule polymerase XMAP215 (also known as CKAP5, a homolog of chTOG, Stu2 and Alp14). This discovery helps to explain why the observed nucleation activity of the γ-TuRC in vitro is relatively low. Taken together, research in recent years has afforded important insight into how microtubules are made in the cell and provides a basis for an exciting era in the cytoskeleton field.
KEY WORDS: Cytoskeleton, Microtubule, Microtubule nucleation
Summary: Research in recent years has afforded important insight into how microtubules are made in the cell and provides a basis for an exciting era in the cytoskeleton field.
Introduction
Microtubules (MTs) form a variety of cytoskeletal structures that are vital for the organization and function of the cell. MTs are polymers composed of αβ-tubulin heterodimers, which arrange into polar, ∼25 nm-wide, hollow tubes. In cells, MTs have stable, α-tubulin-exposed minus ends, whereas β-tubulin is exposed at the plus ends, which undergo dynamic instability (i.e. phases of repeated growth and shrinking; reviewed in Brouhard, 2015; Brouhard and Rice, 2018). Nucleation of MTs is the first step to generate the MT cytoskeleton. Therefore, where, when and how MTs are nucleated governs the resulting architecture of the cytoskeleton that supports cell function.
Although MTs can nucleate spontaneously in vitro from high concentrations of αβ-tubulin subunits, this process is energetically unfavorable and rarely occurs in the cell (Roostalu and Surrey, 2017; Voter and Erickson, 1984). Instead, MTs are nucleated from specific MT-organizing centers (MTOCs) within the cell, such as centrosomes, the Golgi, chromatin and pre-existing MTs (Lüders and Stearns, 2007; Petry and Vale, 2015). Three decades ago, γ-tubulin was identified as the universal MT nucleator required for MT nucleation at MTOCs (Oakley and Oakley, 1989). In recent years, a convergence of research has revealed the molecular architecture of γ-tubulin complexes, identified new nucleators and explained how MTs are nucleated in the cell to build the MT cytoskeleton. Here, we discuss these recent discoveries.
Composition and molecular architecture of γ-tubulin complexes
The key molecule γ-tubulin was originally identified in Aspergillus nidulans as a suppressor of a temperature-sensitive β-tubulin mutation and a member of the tubulin superfamily (Oakley and Oakley, 1989). In addition to their sequence similarity, the atomic structure of γ-tubulin shows a similar fold and conformation as α- and β-tubulin (Aldaz et al., 2005; Rice et al., 2008). In a cell, γ-tubulin is part of larger complexes, namely the 300 kDa γ-tubulin small complex (γ-TuSC), which contains γ-tubulin complex proteins (GCPs) GCP2 and GCP3 and forms the MT nucleator in the yeast Saccharomyces cerevisiae (where GCP2 and GCP3 are also known as Spc97 and Spc98, respectively; Oegema et al., 1999). In higher eukaryotes, the 2.2 MDa γ-tubulin ring complex (γ-TuRC) also contains the γ-TuSC in multiple copies, as well as GCP3 to GCP6 (Fava et al., 1999; Martin et al., 1998; Moritz et al., 1998; Murphy et al., 1998, 2001; Oegema et al., 1999; Zheng et al., 1995). Interestingly, upon high-salt treatment, the γ-TuRC dissociates into smaller components including the γ-TuSC, which has been studied extensively before more recent studies of the γ-TuRC (Fig. 1).
Fig. 1.
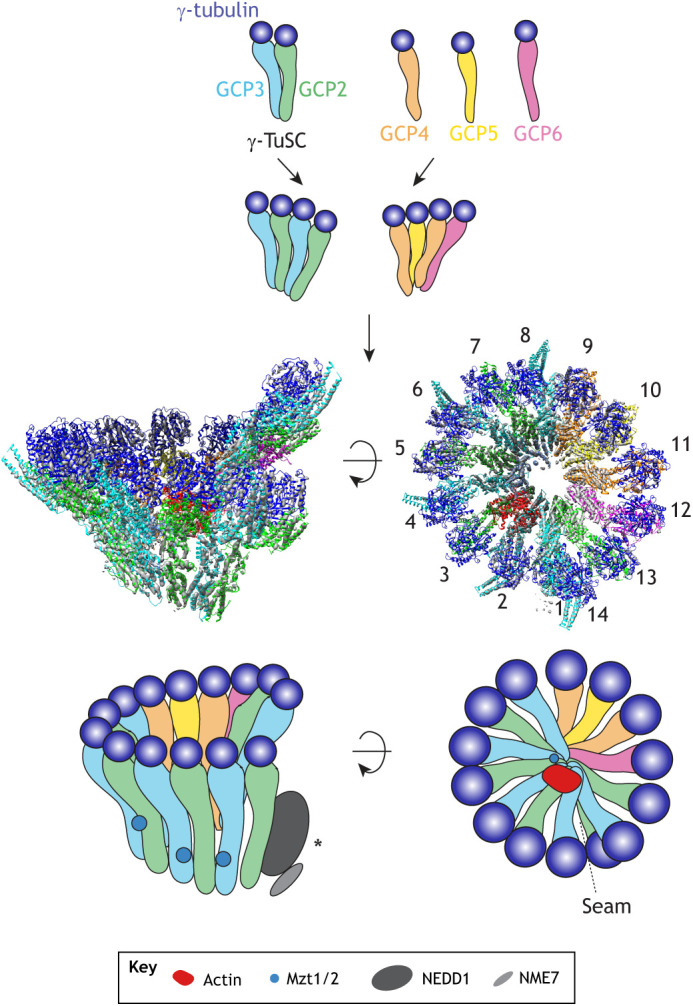
Molecular architecture of the γ-TuRC. γ-tubulin binds to GCP2–GCP3 and GCP4, GCP5 and GCP6 to form subcomplexes, which further associate with additional subunits, such as Mzt1 and Mzt2 (Mzt1/2), NEDD1, NME7 and actin-like protein, to form the γ-TuRC. The location of NME7 and NEDD1 in the γ-TuRC remains to be determined (*). Metazoan γ-TuRC forms an asymmetric helical structure, in which the distance between adjacent γ-tubulins is larger near the seam. An atomic model (PDB:6TF9) based on the cryo-EM reconstruction in Liu et al. (2020) is displayed alongside the schematized γ-TuRC.
Molecular architecture of the γ-TuSC and organism-specific differences in γ-tubulin complexes
The V-shaped γ-TuSC is composed of GCP2 and GCP3, which each have one γ-tubulin bound to their C terminus (Geissler et al., 1996; Knop et al., 1997; Kollman et al., 2008; Martin et al., 1998; Vinh et al., 2002) (Fig. 1). Interestingly, electron microscopy (EM) reconstruction of recombinant γ-TuSC has revealed that the two γ-tubulin copies are held at a distance and do not make protein–protein contact, while the N termini of GCP2 and GCP3 laterally associate (Kollman et al., 2008). This basic unit self-assembles in vitro into a helical structure capable of nucleating an MT (Farache et al., 2016; Kollman et al., 2008, 2010). In total, seven γ-TuSCs interact and expose the plus ends of 13 γ-tubulin molecules per turn. An attachment factor at the spindle pole body, Spc110 (the S. cerevisiae homolog of pericentrin), associates primarily with GCP3 on the outer base of the γ-TuSC and stabilizes the helical geometry (Kollman et al., 2010). Although the helical γ-TuSC assemblies share the 13-fold symmetry of an MT lattice, they exhibit an open conformation, in which the γ-tubulin molecules within each γ-TuSC do not make contacts (Kollman et al., 2010). This results in a mismatch with the MT lattice at positions where the tubulin dimers interact with each of their neighbors, leading to the proposal that γ-tubulin complexes change conformation to nucleate an MT.
Although most higher eukaryotes express GCP4, GCP5 and GCP6, these are non-essential in Schizosaccharomyces pombe and A. nidulans such that their deletion does not affect viability (Anders et al., 2006; Fava et al., 1999; Fujita et al., 2002; Gunawardane et al., 2000; Martin et al., 1998; Murphy et al., 1998, 2001; Oegema et al., 1999; Venkatram et al., 2004; Xiong and Oakley, 2009; Zhang et al., 2000; Zheng et al., 1995). In A. nidulans and Drosophila melanogaster, deletion of GCP4 to GCP6 results in loss of γ-TuRC assembly and localization from the spindle, or reduced chromosome-mediated nucleation (Goshima et al., 2007; Vérollet et al., 2006; Xiong and Oakley, 2009). Whereas the γ-TuSC is recruited to centrosomes in these organisms, and MT nucleation is observed either from γ-TuSCs or via γ-tubulin-independent pathways (Goshima et al., 2007; Vérollet et al., 2006; Xiong and Oakley, 2009), in human cells, loss of GCP4 to GCP6 results in loss of centrosomal γ-TuRC localization (Bahtz et al., 2012; Izumi et al., 2008). These differences in requirement for γ-TuRC subunits across various organisms suggest distinct regulation of γ-TuRC-mediated nucleation, potentially via localizing co-factors, or unique γ-tubulin-independent nucleation pathways across species.
Core components and molecular architecture of the γ-TuRC
Whereas early research assumed that the γ-TuSC forms the core ring and GCP4, GCP5 and GCP6 form the end of this cone-shaped structure, recent structural and biochemical studies have shown that GCP4 to GCP6 incorporate into the ring structure; they directly bind to γ-tubulin and interact laterally with other GCPs and the γ-TuSC (Fig. 1) (Consolati et al., 2020; Farache et al., 2016; Guillet et al., 2011; Haren et al., 2020; Liu et al., 2020; Wieczorek et al., 2020a). All GCPs share two short (100–200 amino acids) homologous regions, termed the GRIP1 and GRIP2 motifs (Gunawardane et al., 2000; Murphy et al., 2001), as well as non-homologous coiled-coiled domains. While the GRIP1 motif is involved in lateral contact surfaces between GCPs within γ-TuSCs, GRIP2 forms a part of the conserved γ-tubulin-binding interface (Guillet et al., 2011; Kollman et al., 2010).
To understand the metazoan γ-TuRC architecture and its detailed subunit arrangement, a high-resolution structure of the γ-TuRC was needed. This feat has been accomplished within the past year by independent studies that utilized single-particle cryo-EM to solve the structure of human (Consolati et al., 2020; Wieczorek et al., 2020a) and Xenopus laevis γ-TuRC (Liu et al., 2020) purified from endogenous sources, and recombinant γ-TuRCs reconstituted in insect (Wieczorek et al., 2021; Würtz et al., 2021) and mammalian (Zimmermann et al., 2020) expression systems (Fig. 1). Furthermore, the interaction between the core γ-TuRC subunits has been dissected recently (Haren et al., 2020). Altogether, several new insights into the molecular architecture of the γ-TuRC were obtained from these structural studies. First, the overall architecture of the metazoan γ-TuRC forms a cone-shaped structure where the base is formed by GCP2 to GCP6, while the plus ends of 13 γ-tubulins are positioned near the nucleation interface (Consolati et al., 2020; Liu et al., 2020; Wieczorek et al., 2020a). Similar to the first solved crystal structure of GCP4 (Guillet et al., 2011), all other GCPs also form elongated structures (Consolati et al., 2020; Liu et al., 2020; Wieczorek et al., 2020a). Second, the metazoan γ-TuRC adopts an asymmetric helical structure, where GCP4 to GCP6 are positioned together (at positions 9–12; Fig. 1) near the seam, while the remainder of the γ-TuRC is composed of GCP2 and GCP3 (Consolati et al., 2020; Liu et al., 2020; Wieczorek et al., 2020a). Furthermore, the positions of individual γ-tubulins within the γ-TuRC deviates from the symmetrical helical axis in both their radial and axial coordinates, and distances between adjacent γ-tubulins are larger near the seam close to two laterally-associated subcomplexes of GCP4–GCP5 and GCP4–GCP6 (Consolati et al., 2020; Liu et al., 2020; Wieczorek et al., 2020a). The diverse molecular architectures and sequences of the GCPs participate in joining the complex into its ring shape (Liu et al., 2020; Wieczorek et al., 2020a). Finally, a second, stable subcomplex consisting of GCP4–GCP5–GCP6 and γ-tubulin has recently been identified and can stimulate γ-TuRC assembly when added to γ-TuSCs (Haren et al., 2020), suggesting that this subcomplex could catalyze γ-TuRC assembly within the cytosol.
Additional protein components of γ-TuRC
In higher eukaryotes, the γ-TuRC also co-purifies with several additional proteins (Fig. 1), whose binding sites on the γ-TuRC and roles in MT nucleation are being elucidated (Liu et al., 2020; Oegema et al., 1999; Thawani et al., 2018; Wieczorek et al., 2020a; Zheng et al., 1995).
Actin-like protein and the lumenal bridge
Structural studies have revealed surprising new components of the γ-TuRC: an α-helical protein density in the lumen (termed the lumenal bridge) (Fig. 1) and an actin-like protein within the core of the human (Consolati et al., 2020; Wieczorek et al., 2020a) and X. laevis γ-TuRC (Liu et al., 2020) structures. A recent study has assigned some of the protein density in the lumenal bridge to the accessory factor Mzt1 (also known as MOZART1; discussed below) (Wieczorek et al., 2020b) and replaced the actin-like protein with β-actin in γ-TuRCs reconstituted in insect cells (Wieczorek et al., 2021). Reconstituted γ-TuRCs lacking part of the lumenal density and actin-like protein form partial ring-shaped structures (Wieczorek et al., 2021), suggesting that these newly identified components support the structural integrity of the γ-TuRC.
Mzt1 and Mzt2
Mzt1 and Mzt2 (also known as MOZART2) – which in humans exists in two isoforms, Mzt2A and Mzt2B – recruit the γ-TuRC to centrosomes in both interphase and mitosis in human, yeast and plant cells (Dhani et al., 2013; Hutchins et al., 2010; Janski et al., 2008; Masuda et al., 2013; Nakamura et al., 2012; Teixidó-Travesa et al., 2010). Recent biochemical studies have demonstrated that Mzt1 forms modules by binding to the N termini of GCP3 and GCP6, forming a large fraction of the lumenal bridge of the γ-TuRC (Huang et al., 2020; Wieczorek et al., 2020b), while other Mzt–GCP modules may assemble on the outer perimeter of the γ-TuRC (Consolati et al., 2020; Huang et al., 2020; Wieczorek et al., 2020b). Furthermore, one Mzt2–GCP2 module interacts with a specific γ-TuRC nucleation activator (γ-TuNA) motif (Wieczorek et al., 2020b). It will be interesting to locate several other, currently unassigned Mzt–GCP modules in the γ-TuRC structures (Consolati et al., 2020; Wieczorek et al., 2020a,b). Recent functional data has provided further insight into how Mzt1 influences MT nucleation and its regulation within various cell types. Drosophila Mzt1 is dispensable for cell division but is specifically required to localize γ-TuRCs to basal bodies in spermatids (Tovey et al., 2018). Whereas purified yeast Mzt1 binds to and oligomerizes the γ-TuSC into nucleation-competent ring structures (Leong et al., 2019; Lin et al., 2016), in vivo experiments show that S. pombe Mzt1 regulates γ-TuRC localization in interphase but not in mitosis (Huang et al., 2020). Finally, recombinant γ-TuRCs reconstituted without Mzt1 are able to assemble into partial ring-shaped structures and nucleate MTs (Wieczorek et al., 2021). Taken together, these studies suggest that Mzt proteins may enable organelle-, cell type- and cell cycle-specific localization of the γ-TuRC to regulate MT nucleation in space, time and during cell differentiation.
NEDD1
The first identified γ-TuRC-binding factor was NEDD1 (also known as GCP-WD), a WD40 motif protein that binds to the γ-TuRC with its C-terminal domain but is not necessary for γ-TuRC assembly (Gunawardane et al., 2003; Haren et al., 2006; Liu and Wiese, 2008; Lüders et al., 2006; Ma et al., 2010; Manning et al., 2010; Vérollet et al., 2006; Zeng et al., 2009). NEDD1 homologs in plant, human and Drosophila S2 cells recruit the γ-TuRC during chromosome-dependent, as well as branching, MT nucleation (Chen et al., 2017a; Lüders et al., 2006; Uehara et al., 2009; Walia et al., 2014). In human cells, NEDD1 is required for recruiting the γ-TuRC to centrosomes (Haren et al., 2006; Lüders et al., 2006; Manning et al., 2010), but not in Drosophila or Xenopus (Liu and Wiese, 2008; Reschen et al., 2012; Vérollet et al., 2006). Despite these species-specific differences, NEDD1 is considered a factor that directly binds to the γ-TuRC, with its precise binding site yet to be determined.
NME7
NME7, a member of the NME family of enzymes and a kinase, co-purifies with the γ-TuRC (Choi et al., 2010; Hutchins et al., 2010; Liu et al., 2020; Teixidó-Travesa et al., 2010; Wieczorek et al., 2020a). NME7 localizes to centrosomes and the mitotic spindle, and reducing its expression leads to a minor reduction in MT nucleation from the centrosome (Liu et al., 2014a). However, the precise binding site of NME7 in the γ-TuRC and its role are yet to be identified.
Microtubule nucleation activity of γ-TuRC and its regulation
Purified γ-TuRC nucleates MTs in vitro from soluble αβ-tubulin dimers (Oegema et al., 1999; Zheng et al., 1995). Together with the structure of the γ-TuRC (Consolati et al., 2020; Kollman et al., 2010; Liu et al., 2020; Wieczorek et al., 2020a), this strongly supports a model of MT nucleation where the plus ends of γ-tubulins recruit and assemble αβ-tubulin into the MT lattice geometry (Fig. 2) (Keating and Borisy, 2000; Kollman et al., 2010; Moritz et al., 2000; Wiese and Zheng, 2000; Zheng et al., 1995). The nucleated MTs are found to have γ-TuRCs bound to their minus ends (Keating and Borisy, 2000; Zheng et al., 1995). Similarly, the γ-TuRC can bind to the end of a pre-formed, stable MT seed (Wiese and Zheng, 2000). While early works proposed an alternative model where γ-tubulins form a linear array in the γ-TuRC and constitute a protofilament within the MT lattice upon nucleation (Erickson, 2000), recent data heavily support γ-TuRC's function as a MT template (Consolati et al., 2020; Keating and Borisy, 2000; Kollman et al., 2010; Liu et al., 2020; Wieczorek et al., 2020a).
Fig. 2.
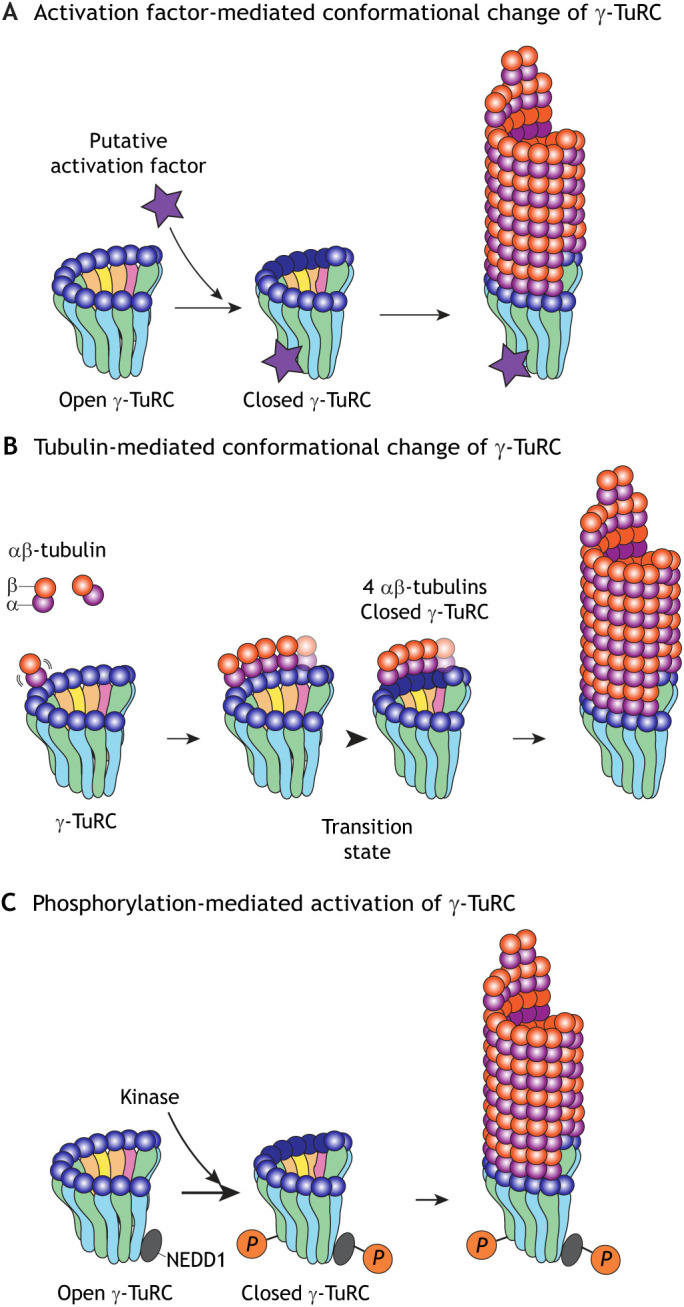
Models for the dynamics and transition state of microtubule nucleation from the γ-TuRC. (A) Activation model: a putative activation factor binds to the open pre-nucleation conformation of γ-TuRC and induces a change into a closed conformation. Closed γ-TuRC nucleates MTs similarly to blunt MT plus ends. (B) Tubulin model: a critical number of αβ-tubulin dimers assemble on the interface of an open pre-nucleation conformation of γ-TuRC. This transitions the γ-TuRC into a closed post-nucleation conformation that is driven by lateral αβ–αβ tubulin interactions. (C) Phosphorylation model: a kinase binds to and localizes the open pre-nucleation γ-TuRC to an MTOC, where it phosphorylates (P) specific γ-TuRC subunits to convert the γ-TuRC to a nucleation-competent complex. Phosphorylated γ-TuRCs are able to nucleate MTs efficiently.
Although structural studies have revealed the molecular architecture of the γ-TuRC, properties of MT nucleation by the γ-TuRC, such as the reaction kinetics and biochemical intermediates, remain important aspects to be defined. In the absence of a live-nucleation assay with the γ-TuRC, parallels between MT nucleation from the γ-TuRC and polymerization from a blunt MT plus end have been drawn, based on their similar geometry (Kollman et al., 2011; Wieczorek et al., 2015). Also, γ-TuRC-mediated MT nucleation has been compared to spontaneous MT nucleation. Whereas earlier studies have shown γ-TuRC-mediated nucleation to be more efficient than spontaneous nucleation (Oegema et al., 1999; Zheng et al., 1995), some recent findings have reported similar nucleation activity with and without γ-TuRCs (Choi et al., 2010; Kollman et al., 2010; Liu et al., 2014a). To resolve these questions, a quantitative single-molecule assay to study MT nucleation from the γ-TuRC has been developed (Consolati et al., 2020; Thawani et al., 2020). MT nucleation from the γ-TuRC occurs stochastically with a variable time delay until each γ-TuRC molecule nucleates an MT (Consolati et al., 2020; Thawani et al., 2020). The γ-TuRC also does not dissociate from the minus end of a nucleated MT (Consolati et al., 2020; Thawani et al., 2020). MT nucleation from X. laevis γ-TuRC requires a cooperative assembly of four αβ-tubulin dimers in the rate-limiting step (Thawani et al., 2020), whereas human γ-TuRC requires five (Wieczorek et al., 2021) or seven (Consolati et al., 2020) αβ-tubulin dimers. The differences in the critical number of tubulin dimers across studies could stem from a varied ratio between γ-tubulin subcomplexes and the intact γ-TuRC due to distinct purification schemes, or species-specific variation in γ-TuRC properties. In a side-by-side comparison, X. laevis γ-TuRC nucleated MTs more efficiently than the spontaneous MT nucleation, which instead requires the highly cooperative assembly of eight αβ-tubulin dimers in the rate-limiting step (Thawani et al., 2020). In contrast, MT ‘nucleation’ from the plus end of blunt MT seeds resembles MT polymerization, during which a single αβ-tubulin subunit is sufficient to overcome the kinetic barrier of lattice incorporation (Thawani et al., 2020). Interestingly, mutant γ-TuRC that consists of an incomplete ring of eight to ten γ-tubulins nucleates MTs equally efficiently as intact, wild-type γ-TuRC (Wieczorek et al., 2021). This raises further questions about the minimal γ-TuRC part required and the positioning of the first tubulin dimers for MT nucleation.
Structural mismatch between the γ-TuRC and the microtubule lattice
The cryo-EM structures of recombinant S. cerevisiae γ-TuSC oligomers (Kollman et al., 2010) and of human (Consolati et al., 2020; Wieczorek et al., 2020a) and X. laevis γ-TuRC (Liu et al., 2020) all show an open configuration such that γ-tubulins, particularly those bound to GCP3, are placed outward from the helical axis. Similarly, γ-tubulins bound to GCP4–GCP5–GCP6 near the seam are splayed and do not align with the helical axis (Consolati et al., 2020; Liu et al., 2020; Wieczorek et al., 2020a). As a result, native γ-TuRC displays an open conformation where γ-tubulins deviate from the perfect helical symmetry of an MT lattice. Furthermore, γ-tubulins across the seam have a shorter pitch than the three tubulin dimers in the MT lattice (Consolati et al., 2020; Liu et al., 2020; Wieczorek et al., 2020a).
As a result, it has been proposed that the transition of the γ-TuRC from an open conformation to a closed, MT lattice-like conformation occurs to reach nucleation competency (Kollman et al., 2015). As a proof-of-principle for a structure-based ‘activation’ model, a closed γ-TuSC conformation has been engineered by inducing artificial disulfide bonds between adjacent γ-tubulins, which leads to a closed γ-TuRC conformation with a geometry resembling the MT lattice, as observed using low-resolution EM (Kollman et al., 2015). Nevertheless, γ-tubulins in the induced ‘closed state’ are misaligned, as they adopt a curved conformation, which may be less compatible with MT nucleation and may explain why closed γ-TuRCs have only a two- to three-fold higher nucleation activity (Kollman et al., 2015). Therefore, clear evidence that demonstrates a conformational change in the γ-TuRC during MT nucleation remains lacking. This leaves open the possibility that activation co-factors may exist that could facilitate a favorable conformational change in the γ-TuRC and induce MT nucleation.
Localization of the γ-TuRC to MTOCs
Localization and activation of γ-TuRC to specific MTOCs at precise locations and times during the cell cycle regulate MT nucleation within the cell (Fig. 2A). Several candidates have been identified thus far to localize γ-TuRC to specific MTOCs, including the centrosomes, Golgi, nuclear envelope, mitochondria and cell cortex, as well as to existing MTs during branching MT nucleation. Some of these factors also contribute to the assembly of γ-tubulin complexes. Here, due to limited space, we only briefly discuss this aspect and refer the reader to other comprehensive reviews for in-depth discussion of γ-TuRC localization in the cell (Lin et al., 2015; Petry and Vale, 2015; Wu and Akhmanova, 2017).
In addition to factors that can localize the γ-TuRC to multiple MTOCs, such as NEDD1, several MTOC-specific proteins have been identified that recruit the γ-TuRC either via direct binding or indirectly. These include S. cerevisiae Spc110 and Spc72; S. pombe Pcp1 and Mto1; mammalian centrosome-specific CDK5RAP2 and its homologs Centrosomin in D. melanogaster and SPD-5 in Caenorhabditis elegans (Choi et al., 2010; Fong et al., 2008; O'Toole et al., 2012; Tovey et al., 2018; Wang et al., 2010; Zhang and Megraw, 2007); pericentrin (Lawo et al., 2012; Mennella et al., 2012; Wang et al., 2010; Woodruff et al., 2015; Zimmerman et al., 2004); CEP192 and its C. elegans homolog SPD-2 (Chinen et al., 2021; Gomez-Ferreria et al., 2007; Takahashi et al., 2002; Woodruff et al., 2015, 2017; Yang and Feldman, 2015; Zhu et al., 2008); the Golgi-specific MT organizers myomegalin, GM130 (also known as GOLGA2 in mammals), GMAP210 (also known as TRIP11) and AKAP450 (also known as AKAP9) (Chabin-Brion et al., 2001; Chen et al., 2017b; Efimov et al., 2007; Ori-McKenney et al., 2012; Ríos et al., 2004; Rivero et al., 2009; Roubin et al., 2013; Sanders et al., 2017; Tonucci et al., 2015; Wang et al., 2014; Wei et al., 2015; Wu et al., 2016); as well as the branching MT nucleation-specific hetero-octameric Augmin complex, TPX2 (Alfaro-Aco et al., 2019; Goshima et al., 2008; Hsia et al., 2014; Lawo et al., 2009; Liu et al., 2014b; Murata and Hasebe, 2007; Petry et al., 2011, 2013; Sánchez-Huertas et al., 2016; Song et al., 2018; Tariq et al., 2020; Uehara et al., 2009; Verma and Maresca, 2019) and EML3 (Luo et al., 2019). Several of these proteins, including CDK5RAP2, myomegalin, pericentrin and their homologs contain a 60-residue N-terminal centrosomin motif 1 (CM1) that is required for binding and recruitment of the γ-TuRC to MTOCs (Choi et al., 2010; Lin et al., 2014, 2016; Sawin et al., 2004; Zhang and Megraw, 2007). Although some of these factors have been studied in depth, in the future, it will be important to investigate how and where these localization factors bind to the γ-TuRC and how they influence its activity.
The activation model for regulation of γ-TuRC nucleation activity
Several candidates have been proposed to be activation factors for the γ-TuRC, including those involved in its localization, thereby constituting one pathway for regulating γ-TuRC nucleation activity (Fig. 2A).
CM1 motif-containing proteins CDK5RAP2, myomegalin and Spc110/Mto1
The most prominent candidate for a γ-TuRC activator is CDK5RAP2 and its yeast homologs Spc110 (in S. cerevisiae), Mto1 and Mto2 (Mto1/2; both in S. pombe), which are necessary for correct anchoring and arrangement of MTs at the centrosomes and at the Golgi (Choi et al., 2010; Fong et al., 2008). A γ-TuNA motif has been defined that resides within the conserved N-terminal CM1 domain of the CDK5RAP2 homologs and directly binds to the γ-TuRC (Choi et al., 2010; Consolati et al., 2020; Fong et al., 2008; Kollman et al., 2010; Lynch et al., 2014; Wieczorek et al., 2020a). Although it is clear that the CDK5RAP2 homologs and the γ-TuNA motif alone induce MT nucleation when overexpressed in cells (Choi et al., 2010; Cota et al., 2017; Lynch et al., 2014; Terada et al., 2003), Xenopus meiotic cytosol (Liu et al., 2020) or flies (Tovey et al., 2020), the direct effects of this motif on γ-TuRC activity has been found to be variable in purified systems. Indeed, whereas initial work demonstrated a 7.1-fold increase in γ-TuRC-mediated nucleation upon the addition of γ-TuNA (Choi et al., 2010), subsequent independent studies, using a similar setup, have reported a mere 1.7-fold increase (Liu et al., 2020) or no significant effect (Thawani et al., 2020). In contrast, the yeast homologs Spc110 and Mto1/2 likely function as activation factors by binding to γ-TuSCs and promoting their oligomerization (Leong et al., 2019; Lyon et al., 2016). Indeed, functional ring-shaped complexes resembling γ-TuRCs have been observed when the γ-TuSC was supplemented with Mto1/2 (Leong et al., 2019).
Furthermore, whether γ-TuNA or CM1 motifs influence the molecular structure of the γ-TuRC to execute its activator role has also been debated. The cryo-EM structure of the γ-TuRC, either purified in the presence of γ-TuNA motifs (Wieczorek et al., 2020a) or upon addition of γ-TuNA after purification (Liu et al., 2020), shows an open, not closed, conformation. In contrast, a recent publication has found significant structural differences between the cryo-EM structures of Spc110-bound yeast γ-TuRC and human γ-TuRCs, supporting a CM1-motif-driven structural rearrangement of the γ-TuRC (Brilot et al., 2021). Finally, similar to CDK5RAP2, the Golgi-specific MT nucleation factor myomegalin also contains a CM1 motif that binds and recruits the γ-TuRC to the Golgi (Chen et al., 2017b; Roubin et al., 2013; Wu et al., 2016). Nevertheless, further structural and reconstitution studies are needed to reveal the role of CM1-motif-containing proteins in activating, and modifying the conformation of, the γ-TuRC.
NME7
The γ-TuRC-binding kinase NME7 has also been proposed to be an activation factor (Liu et al., 2014a). However, the addition of NME7 to γ-TuRC nucleation assays results in only a small 2.5-fold (Liu et al., 2014a) or insignificant increase (Thawani et al., 2020) in nucleation. In addition, despite the presence of NME7 as a co-eluted component in several γ-TuRC purifications (Liu et al., 2020; Wieczorek et al., 2020a), the resulting EM structure shows an open γ-TuRC conformation. Therefore, whether NME7's kinase activity can indeed induce γ-TuRC activation remains an outstanding question.
TPX2
The Ran-GTP-regulated spindle assembly factor TPX2 is also proposed to function as an activation factor (Roostalu and Surrey, 2017; Tovey and Conduit, 2018). The MT nucleation activity of TPX2 is highly variable across species, in that D. melanogaster TPX2 (also known as MEI38) is not involved in MT nucleation in vivo, whereas human TPX2 prevents the disassembly of nascent MTs in vitro (Roostalu et al., 2015; Wieczorek et al., 2015). Recently, a bipartite γ-TuNA-like motif, potentially separated by a less conserved loop region, has been identified in X. laevis TPX2, which stimulates branching MT nucleation in meiotic cytosol (Alfaro-Aco et al., 2017). The addition of X. laevis TPX2 alone neither affects de novo γ-TuRC-mediated nucleation in Xenopus egg extracts when added at a 30-fold excess compared to its endogenous concentration (Alfaro-Aco et al., 2017), nor in an in vitro setup using purified proteins (Thawani et al., 2020). However, at higher concentrations, X. laevis TPX2 phase separates to promote γ-TuRC-independent MT assembly in vitro (King and Petry, 2020), similar to the reported role of human TPX2 in both γ-TuRC-dependent (Consolati et al., 2020) and -independent (Roostalu et al., 2015) nucleation. These differences in results could be due to the differences in concentration of TPX2 used across the studies or species-specific activity of the protein. In the future, side-by-side comparison of the activity of the various TPX2 homologs, as well as structural studies, will help to reveal whether and how TPX2 is involved in MT nucleation.
In summary, these putative activation factors clearly participate in robust γ-TuRC assembly or localization on MTOCs, but further investigation is necessary to unambiguously demonstrate whether they influence the conformation and nucleation activity of the γ-TuRC, which might be more complex in vivo.
The tubulin model – an alternative means of regulation of γ-TuRC-mediated nucleation
Since the γ-TuRC can nucleate MTs in their open conformation, we propose an alternative model where the assembly of αβ-tubulin dimers on the γ-TuRC ring itself can close the γ-tubulin ring and facilitate MT nucleation (Fig. 2B). Using a computational model, we have shown that the transition from an open to a closed γ-TuRC conformation can be driven by the assembly of αβ-tubulin on adjacent binding sites (Thawani et al., 2020). Specifically, four laterally associated αβ-tubulin dimers on the γ-TuRC comprise the rate-limiting transition state that closes the γ-TuRC. This computational model recapitulates the experimentally measured dynamics of MT nucleation by X. laevis γ-TuRCs (Thawani et al., 2020). It will be very interesting to know whether this will also be the case for γ-TuRCs from other species and to investigate how αβ-tubulin binding on distinct sites on the asymmetric γ-TuRC may influence its conformation by direct experimentation, such as visualizing intermediates in the nucleation pathway.
In this alternative model, the concentration and availability of αβ-tubulin at MTOCs could help to regulate the nucleation activity of the γ-TuRC in addition to affecting its localization and activation. Supporting this hypothesis, the translocation of tubulin into the nucleus of A. nidulans has been found to be concurrent with MT nucleation and spindle assembly (Ovechkina et al., 2003). Furthermore, MTOCs may specifically enrich for tubulin; for instance, C. elegans centrosomes contain 400 μM αβ-tubulin, as compared to 15 μM tubulin in the bulk cytosol (Baumgart et al., 2019), and sites of branching MT nucleation in Xenopus egg extracts are enriched with tubulin–TPX2 condensates (Alfaro-Aco et al., 2017, 2020; King and Petry, 2020). Also, XMAP215 (also known as CKAP5 in X. laevis; homolog of Stu2 in S. cerevisiae), a second bona fide MT nucleation factor that synergizes with the γ-TuRC, functions by binding to and directly recruiting αβ-tubulin on the γ-TuRC in S. cerevisiae and X. laevis (Gunzelmann et al., 2018; Thawani et al., 2018). It is also conceivable that the concentration of αβ-tubulin in the cytoplasm is reduced by factors such as stathmin proteins, which bind and sequester tubulin subunits, or MCAK (also known as KIF2C), which depolymerizes MTs (Andrews et al., 2004; Budde et al., 2001; Cassimeris, 2002). Indeed, we have shown that Xenopus Stathmin 1A and MCAK can inhibit the nucleation activity of γ-TuRC in vitro (Thawani et al., 2020).
Taken together, modulating the localization and availability of αβ-tubulin may be another mechanism that regulates nucleation activity of the γ-TuRC in a spatiotemporal manner, in addition to specifically localizing and activating the γ-TuRC (Fig. 2B). Nevertheless, this hypothesis remains a speculation and it will be exciting to investigate it in detail through direct experimentation. It will also be interesting to examine how the nucleotide bound to γ-tubulin may further regulate its conformation and, therefore, the MT nucleation activity of the γ-TuRC (Box 1). Finally, a cascade of posttranslational modification may further regulate γ-TuRC-mediated nucleation (Box 2; Fig. 2C).
Box 1. γ-tubulin conformation and GTP hydrolysis.
The crystal structure of γ-tubulin shows that its conformation matches the curved conformation of αβ-tubulin in solution, rather than the straight αβ-tubulin conformation found in the MT lattice (Aldaz et al., 2005; Rice et al., 2008). Similarly, γ-tubulin in yeast or metazoan γ-TuRCs also adopts a curved conformation (Consolati et al., 2020; Wieczorek et al., 2020a). In addition, purified γ-tubulin both in GTP- or GDP-bound states can self-assemble into the crystal lattice through lateral association (Aldaz et al., 2005; Rice et al., 2008). Thus, γ-tubulin behaves similarly to αβ-tubulin in that it is curved when in solution, but it remains to be determined whether αβ-tubulin adopts a straight conformation upon nucleating a MT lattice.
Because GTP-bound αβ-tubulin incorporates into a growing MT, it is conceivable that GTP binding to γ-tubulin also plays a role in MT nucleation. Indeed, mutations in the nucleotide binding site of γ-tubulin result in defects in MT nucleation and organization (Gombos et al., 2013; Jung et al., 2001; Wieczorek et al., 2021), but not in the assembly of the γ-TuSC (Wieczorek et al., 2015). These defects could be explained by the inability to assemble into the higher-order γ-TuRC. In addition, it is interesting to speculate that the nucleotide state of γ-tubulin could influence its propensity to bind the first ring of αβ-tubulin subunits on the nucleation interface, thereby influencing the nucleation activity of γ-TuRC.
Box 2. Phosphorylation of γ-TuRC core components regulates nucleation activity.
γ-tubulin itself is phosphorylated, which either destabilizes the γ-tubulin protein directly or influences organization of the resulting MT cytoskeleton (Keck et al., 2011; Lin et al., 2011; Vogel et al., 2001). In S. cerevisiae, casein kinase 1δ phosphorylates the γ-TuSC to correctly organize MTs (Peng et al., 2015). In higher eukaryotes, the mitotic kinases CDK1 (Oriolo et al., 2007) and PLK4 (Bahtz et al., 2012) phosphorylate GCP6, with the former resulting in reduced keratin binding by GCP6 and MT cytoskeleton remodeling (Oriolo et al., 2007). GSK3β phosphorylates GCP5 and negatively regulates the localization of γ-TuRC on centrosomes (Izumi et al., 2008). Additional phosphorylation sites in GCPs remain to be functionally characterized (Hegemann et al., 2011; Keck et al., 2011; Kettenbach et al., 2011; Lin et al., 2011; Santamaria et al., 2011). Furthermore, non-core γ-TuRC components are also phosphorylated; for instance, NEDD1 is phosphorylated by the mitotic kinases CDK1 and PLK1, which promotes its interaction with the Augmin complex (Haren et al., 2009; Johmura et al., 2011; Lüders et al., 2006; Santamaria et al., 2011; Uehara et al., 2009), and the NME7 kinase is autophosphorylated, which may promote its activity as a γ-TuRC activation factor (Liu et al., 2014a). In sum, the phosphorylation of γ-TuRC components affects the localization and stability of the complex.
The mitotic Aurora A kinase (AURKA) indirectly regulates γ-TuRC-mediated nucleation (Joukov and De Nicolo, 2018; Meunier and Vernos, 2016). In particular, the Ran-release factor TPX2 binds to Aurora A, which enables its phosphorylation-dependent activation and localization to spindle MTs (Bayliss et al., 2003; Eyers et al., 2003; Kufer et al., 2002; Tsai et al., 2003). The TPX2–Aurora A complex then recruits RHAMM (also known as HMMR), an MT-binding protein, and the γ-TuRC, and Aurora A phosphorylates NEDD1-bound γ-TuRC to promote MT nucleation around chromosomes (Groen et al., 2004; Pinyol et al., 2013; Scrofani et al., 2015). This cascade represents a third means to regulate γ-TuRC activity (Fig. 2C), in addition to the activation and tubulin models (Fig. 2A,B).
Discovery of new microtubule nucleation factors
Although γ-TuRC is the major MT nucleator in the cell, there is mounting evidence for other nucleation factors. First, several studies have reported that the activity of purified γ-TuRC is too low to account for the high nucleation activity observed in the cell (Choi et al., 2010; Kollman et al., 2010, 2015; Liu et al., 2014a). Second, MTs form in the absence of γ-tubulin, albeit in reduced numbers and with slower kinetics (Groen et al., 2009; Hannak et al., 2002; Rogers et al., 2008). Third, at least one other factor besides the γ-TuRC is necessary to restore MT nucleation from salt-stripped centrosomes (Moritz et al., 1998). Taken together, these findings highlight that a γ-TuRC-centered model of MT nucleation may be incomplete. In parallel, several other factors have been proposed to play a role in MT nucleation (Kollman et al., 2011; Roostalu and Surrey, 2017; Tovey and Conduit, 2018; Wiese and Zheng, 2006), as discussed below.
The XMAP215 family of microtubule nucleators
The well-characterized X. laevis MT polymerase XMAP215 has been proposed as a nucleation factor. XMAP215 and homologous polymerases, such as mammalian chTOG (also known as CKAP5), S. cerevisiae Stu2, S. pombe Alp14 and D. melanogaster Msps, contain N-terminal TOG domains that bind to αβ-tubulin and increase the speed of MT growth from the plus end up to 10-fold (Al-Bassam et al., 2006, 2012; Ayaz et al., 2012, 2014; Brouhard et al., 2008; Gard and Kirschner, 1987; Slep and Vale, 2007; Widlund et al., 2011). Whereas an initial study has shown that XMAP215 may be involved in centrosomal MT nucleation (Popov et al., 2002), this notion remains to be verified due to contradictory results obtained in subsequent studies (Reber et al., 2013) and challenges in differentiating MT polymerization and nucleation activities. XMAP215 also promotes spontaneous MT nucleation and outgrowth from pre-formed templates (Roostalu et al., 2015; Wieczorek et al., 2015), yet whether XMAP215 functions as a bona fide nucleator within the cell has been debated until recently.
Three recent studies have now demonstrated a role for XMAP215 homologs in MT nucleation (Fig. 3) (Flor-Parra et al., 2018; Gunzelmann et al., 2018; Thawani et al., 2018). XMAP215 and Alp14 are necessary for MT nucleation in Xenopus egg extracts (Thawani et al., 2018) and S. pombe (Flor-Parra et al., 2018), respectively. Here, the concentration of these proteins in the cytosol determines the rate and extent of MT nucleation, phenocopying the effect of γ-tubulin. Using purified proteins, XMAP215 synergizes with γ-TuRC (Thawani et al., 2018) and Stu2 synergizes with γ-TuSC (Gunzelmann et al., 2018) to nucleate MTs efficiently, whereas the individual components only exhibit a low nucleation activity (Gunzelmann et al., 2018; Thawani et al., 2018). While the N-terminal TOG domains are essential for the recruitment of αβ-tubulin, the C-terminal domain directly interacts with the γ-TuRC and/or γ-TuSC. The conserved C-terminal domain of XMAP215 binds to purified γ-tubulin (Thawani et al., 2018), and the MT-binding region of Stu2 also binds to the γ-TuSC (Gunzelmann et al., 2018) to promote efficient MT nucleation. Moreover, we have been able to directly visualize MT nucleation mediated by XMAP215 and/or the γ-TuRC using triple-color single-molecule microscopy (Thawani et al., 2020). Here, XMAP215 molecules first associate with the γ-TuRC to nucleate an MT and further polymerize the growing plus end processively (Thawani et al., 2020). Interestingly, a recent study highlights the possibility of additional binding sites for XMAP215 on the γ-TuRC, other than those in γ-tubulin, and discusses whether the activity of XMAP215 with γ-tubulin is additive or synergistic (King et al., 2020); this would be an interesting subject for further investigation. Notably, XMAP215 has a well-documented ability to promote spontaneous MT nucleation in vitro independent of the γ-TuRC (Gunzelmann et al., 2018; Roostalu et al., 2015; Wieczorek et al., 2015), and it will be interesting to examine to what extent this independent nucleation activity does generate the MT mass in vivo.
Fig. 3.
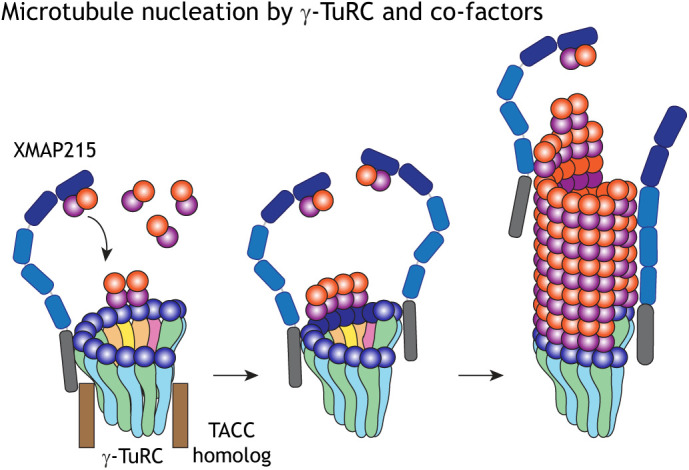
Microtubule nucleation by the γ-TuRC and the nucleation factor XMAP215. Schematic representation of how γ-TuRC and the XMAP215/Stu2 family of co-nucleators promote MT nucleation. XMAP215 binds to the γ-TuRC with its C terminus, possibly involving a TACC3 homolog, and promotes the arrival of αβ-tubulin dimers onto the γ-TuRC via its N-terminal domains. Thus, although the γ-TuRC independently can induce low levels of MT nucleation, its cooperation with XMAP215 promotes efficient MT nucleation.
Other putative nucleation factors
Several other nucleation factors have also been proposed, as discussed here.
TPX2
In vitro, human and X. laevis TPX2 promote spontaneous MT nucleation and assembly from pre-formed MT seeds, likely by recruiting tubulin and stabilizing αβ-tubulin oligomers in a manner similar to their effect on the MT lattice (King and Petry, 2020; Roostalu et al., 2015; Wieczorek et al., 2015; Zhang et al., 2017). However, these results have been questioned by research in Xenopus egg extract, where X. laevis TPX2 does not promote γ-TuRC-mediated nucleation directly, but instead requires Augmin and the γ-TuRC to specifically promote branching MT nucleation (Alfaro-Aco et al., 2017, 2019; King and Petry, 2020; Thawani et al., 2018, 2019, 2020). These differences may reflect a specific role of TPX2 in regulating large Xenopus meiotic spindles as compared to the smaller spindles in other cell types, or the use of different assay conditions. While it is well established that TPX2 activity is regulated by the cell cycle (Brunet et al., 2008; Gruss et al., 2001; Schatz et al., 2003), how it contributes to nucleation activity at distinct MTOCs, such as centrosomes, the Golgi and chromatin, across species remains to be clearly defined in a side-by-side comparison. Therefore, additional work that investigates the MT nucleation activity of TPX2 in the native cellular context is necessary to establish TPX2 as a bona fide MT nucleation factor.
TACC3
In yeast, the CM1 motif-containing TACC3 homologs Spc72 (in S. cerevisiae) and Alp7 (in S. pombe) have been shown to mediate interactions between the γ-TuSC and Stu2 and Alp14, respectively, allowing oligomerization of the γ-TuSC into functional complexes (Flor-Parra et al., 2018; Gunzelmann et al., 2018). However, the role of the higher eukaryotic ortholog TACC3 in γ-TuRC-mediated MT nucleation is yet to be dissected.
Phase-separating nucleation factors – BugZ
Several proteins have been shown to generate MTs in vitro. The spindle assembly factor BugZ (also known as ZNF207) enriches soluble αβ-tubulin into a condensed phase to promote spontaneous MT assembly (Jiang et al., 2015), similar to what has been proposed for TPX2 (King and Petry, 2020) and homologs of CDK5RAP2 and CEP192 in C. elegans (Woodruff et al., 2015, 2017). While this remains an exciting, yet heavily debated proposition (Raff, 2019; Rale et al., 2018), further work is necessary to examine the exact role of these proteins and, more broadly, phase separation in MT nucleation in vivo, specifically that mediated by the γ-TuRC.
Conclusions and outstanding questions
Decades of research have revealed how MTs are nucleated from the γ-TuRC and have illuminated γ-TuRC composition and molecular architecture, providing mechanistic insights and, most recently, identifying its cooperative nucleation with XMAP215. These findings represent a launching pad to investigate additional important outstanding questions.
First, how is the conformation of the γ-TuRC regulated during MT nucleation? Atomic snapshots of the nucleation reaction combined with single-molecule studies and simulations will ultimately help to reveal how MT nucleation occurs at the atomic level. Second, how is MT nucleation regulated in the cell? Thorough investigations of the extent to which MTOC-specific localizers and activators, posttranslational modifications and regulation of tubulin function to build a dynamic cytoskeleton, and the mechanisms involved, are yet to be performed. Third, recent advances suggest that additional bona fide MT nucleation factors exist, which function in either γ-TuRC-dependent or -independent pathways. It will be important to identify these factors and elucidate their mode of action. Finally, it will be important to further investigate how and to what extent γ-TuRC-independent means are deployed to generate MTs. These include the stabilization of MTs by CLASPs (Efimov et al., 2007), CAMSAP (Jiang et al., 2014) or doublecortin (DCX; Moores et al., 2004), or amplification of MT mass via MT severases (Vemu et al., 2018). Thus, exciting times await the cytoskeleton field, with the potential to fully explain how cellular structures are formed.
Acknowledgements
We thank Petry laboratory members Ray Alfaro-Aco and Michael Rale for discussions and Jens Lüders for comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
A.T. acknowledges support from a Princeton University Procter Honorific Fellowship. S.P. acknowledges support from the National Institutes of Health New Innovator Award (1DP2GM123493), the Pew Charitable Trusts Pew Scholars Program in the Biomedical Sciences (00027340) and the David and Lucile Packard Foundation (2014-40376). Deposited in PMC for release after 12 months.
References
- Al-Bassam, J., van Breugel, M., Harrison, S. C. and Hyman, A. (2006). Stu2p binds tubulin and undergoes an open-to-closed conformational change. J. Cell Biol. 172, 1009-1022. 10.1083/jcb.200511010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bassam, J., Kim, H., Flor-Parra, I., Lal, N., Velji, H. and Chang, F. (2012). Fission yeast Alp14 is a dose-dependent plus end-tracking microtubule polymerase. Mol. Biol. Cell 23, 2878-2890. 10.1091/mbc.e12-03-0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldaz, H., Rice, L. M., Stearns, T. and Agard, D. A. (2005). Insights into microtubule nucleation from the crystal structure of human gamma-tubulin. Nature 435, 523-527. 10.1038/nature03586 [DOI] [PubMed] [Google Scholar]
- Alfaro-Aco, R., Thawani, A. and Petry, S. (2017). Structural analysis of the role of TPX2 in branching microtubule nucleation. J. Cell Biol. 216, 983-997. 10.1083/jcb.201607060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro-Aco, R., Thawani, A. and Petry, S. (2019). Biochemical reconstitution of branching microtubule nucleation. Cell Biology 39, 1784-1795. 10.1101/700047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro-Aco, R., Thawani, A. and Petry, S. (2020). Biochemical reconstitution of branching microtubule nucleation. Elife 9, e49797. 10.7554/eLife.49797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders, A., Lourenço, P. C. C. and Sawin, K. E. (2006). Noncore components of the fission yeast gamma-tubulin complex. Mol. Biol. Cell 17, 5075-5093. 10.1091/mbc.e05-11-1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews, P. D., Ovechkina, Y., Morrice, N., Wagenbach, M., Duncan, K., Wordeman, L. and Swedlow, J. R. (2004). Aurora B regulates MCAK at the mitotic centromere. Dev. Cell 6, 253-268. 10.1016/S1534-5807(04)00025-5 [DOI] [PubMed] [Google Scholar]
- Ayaz, P., Ye, X., Huddleston, P., Brautigam, C. A. and Rice, L. M. (2012). A TOG:αβ-tubulin complex structure reveals conformation-based mechanisms for a microtubule polymerase. Science 337, 857-860. 10.1126/science.1221698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz, P., Munyoki, S., Geyer, E. A., Piedra, F.-A., Vu, E. S., Bromberg, R., Otwinowski, Z., Grishin, N. V., Brautigam, C. A. and Rice, L. M. (2014). A tethered delivery mechanism explains the catalytic action of a microtubule polymerase. Elife 3, e03069. 10.7554/eLife.03069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahtz, R., Seidler, J., Arnold, M., Haselmann-Weiss, U., Antony, C., Lehmann, W. D. and Hoffmann, I. (2012). GCP6 is a substrate of Plk4 and required for centriole duplication. J. Cell. Sci. 125, 486-496. 10.1242/jcs.093930 [DOI] [PubMed] [Google Scholar]
- Baumgart, J., Kirchner, M., Redemann, S., Bond, A., Woodruff, J., Verbavatz, J.-M., Jülicher, F., Müller-Reichert, T., Hyman, A. A. and Brugués, J. (2019). Soluble tubulin is significantly enriched at mitotic centrosomes. J. Cell Biol. 218, 3977-3985. 10.1083/jcb.201902069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss, R., Sardon, T., Vernos, I. and Conti, E. (2003). Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol. Cell 12, 851-862. 10.1016/S1097-2765(03)00392-7 [DOI] [PubMed] [Google Scholar]
- Brilot, A. F., Lyon, A. S., Zelter, A., Viswanath, S., Maxwell, A., MacCoss, M. J., Muller, E. G., Sali, A., Davis, T. N. and Agard, D. A. (2021). CM1-driven assembly and activation of yeast γ-tubulin small complex underlies microtubule nucleation. Elife 10, e65168. 10.7554/eLife.65168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouhard, G. J. (2015). Dynamic instability 30 years later: complexities in microtubule growth and catastrophe. Mol. Biol. Cell 26, 1207-1210. 10.1091/mbc.E13-10-0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouhard, G. J. and Rice, L. M. (2018). Microtubule dynamics: an interplay of biochemistry and mechanics. Nat. Rev. Mol. Cell Biol. 19, 451-463. 10.1038/s41580-018-0009-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouhard, G. J., Stear, J. H., Noetzel, T. L., Al-Bassam, J., Kinoshita, K., Harrison, S. C., Howard, J. and Hyman, A. A. (2008). XMAP215 is a processive microtubule polymerase. Cell 132, 79-88. 10.1016/j.cell.2007.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet, S., Dumont, J., Lee, K. W., Kinoshita, K., Hikal, P., Gruss, O. J., Maro, B. and Verlhac, M.-H. (2008). Meiotic regulation of TPX2 protein levels governs cell cycle progression in mouse oocytes. PLoS ONE 3, e3338. 10.1371/journal.pone.0003338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde, P. P., Kumagai, A., Dunphy, W. G. and Heald, R. (2001). Regulation of Op18 during spindle assembly in Xenopus egg extracts. J. Cell Biol. 153, 149-158. 10.1083/jcb.153.1.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris, L. (2002). The oncoprotein 18/stathmin family of microtubule destabilizers. Curr. Opin. Cell Biol. 14, 18-24. 10.1016/S0955-0674(01)00289-7 [DOI] [PubMed] [Google Scholar]
- Chabin-Brion, K., Marceiller, J., Perez, F., Settegrana, C., Drechou, A., Durand, G. and Poüs, C. (2001). The Golgi complex is a microtubule-organizing organelle. Mol. Biol. Cell 12, 2047-2060. 10.1091/mbc.12.7.2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. W. C., Chen, Z. A., Rogala, K. B., Metz, J., Deane, C. M., Rappsilber, J. and Wakefield, J. G. (2017a). Cross-linking mass spectrometry identifies new interfaces of Augmin required to localise the γ-tubulin ring complex to the mitotic spindle. Biol. Open 6, 654-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. V., Buchwalter, R. A., Kao, L.-R. and Megraw, T. L. (2017b). A splice variant of centrosomin converts mitochondria to microtubule-organizing centers. Curr. Biol. 27, 1928-1940.e6. 10.1016/j.cub.2017.05.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinen, T., Yamazaki, K., Hashimoto, K., Fujii, K., Watanabe, K., Takeda, Y., Yamamoto, S., Nozaki, Y., Tsuchiya, Y., Takao, D.et al. (2021). Centriole and PCM cooperatively recruit CEP192 to spindle poles to promote bipolar spindle assembly. J. Cell Biol. 220, e202006085. 10.1083/jcb.202006085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, Y.-K., Liu, P., Sze, S. K., Dai, C. and Qi, R. Z. (2010). CDK5RAP2 stimulates microtubule nucleation by the gamma-tubulin ring complex. J. Cell Biol. 191, 1089-1095. 10.1083/jcb.201007030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consolati, T., Locke, J., Roostalu, J., Chen, Z. A., Gannon, J., Asthana, J., Lim, W. M., Martino, F., Cvetkovic, M. A., Rappsilber, J.et al. (2020). Microtubule nucleation properties of single human γTuRCs explained by their cryo-EM structure. Dev. Cell 53, 603-617.e8. 10.1016/j.devcel.2020.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota, R. R., Teixidó-Travesa, N., Ezquerra, A., Eibes, S., Lacasa, C., Roig, J. and Lüders, J. (2017). MZT1 regulates microtubule nucleation by linking γTuRC assembly to adapter-mediated targeting and activation. J. Cell. Sci. 130, 406-419. 10.1242/jcs.195321 [DOI] [PubMed] [Google Scholar]
- Dhani, D. K., Goult, B. T., George, G. M., Rogerson, D. T., Bitton, D. A., Miller, C. J., Schwabe, J. W. R. and Tanaka, K. (2013). Mzt1/Tam4, a fission yeast MOZART1 homologue, is an essential component of the γ-tubulin complex and directly interacts with GCP3(Alp6). Mol. Biol. Cell 24, 3337-3349. 10.1091/mbc.e13-05-0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimov, A., Kharitonov, A., Efimova, N., Loncarek, J., Miller, P. M., Andreyeva, N., Gleeson, P., Galjart, N., Maia, A. R. R., McLeod, I. X.et al. (2007). Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev. Cell 12, 917-930. 10.1016/j.devcel.2007.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, H. P. (2000). Gamma-tubulin nucleation: template or protofilament? Nat. Cell Biol. 2, E93-E96. 10.1038/35014084 [DOI] [PubMed] [Google Scholar]
- Eyers, P. A., Erikson, E., Chen, L. G. and Maller, J. L. (2003). A novel mechanism for activation of the protein kinase Aurora A. Curr. Biol. 13, 691-697. 10.1016/S0960-9822(03)00166-0 [DOI] [PubMed] [Google Scholar]
- Farache, D., Jauneau, A., Chemin, C., Chartrain, M., Rémy, M.-H., Merdes, A. and Haren, L. (2016). Functional analysis of γ-tubulin complex proteins indicates specific lateral association via their n-terminal domains. J. Biol. Chem. 291, 23112-23125. 10.1074/jbc.M116.744862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava, F., Raynaud-Messina, B., Leung-Tack, J., Mazzolini, L., Li, M., Guillemot, J. C., Cachot, D., Tollon, Y., Ferrara, P. and Wright, M. (1999). Human 76p: a new member of the gamma-tubulin-associated protein family. J. Cell Biol. 147, 857-868. 10.1083/jcb.147.4.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor-Parra, I., Iglesias-Romero, A. B. and Chang, F. (2018). The XMAP215 ortholog Alp14 promotes microtubule nucleation in fission yeast. Curr. Biol. 28, 1681-1691.e4. 10.1016/j.cub.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong, K.-W., Choi, Y.-K., Rattner, J. B. and Qi, R. Z. (2008). CDK5RAP2 is a pericentriolar protein that functions in centrosomal attachment of the gamma-tubulin ring complex. Mol. Biol. Cell 19, 115-125. 10.1091/mbc.e07-04-0371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, A., Vardy, L., Garcia, M. A. and Toda, T. (2002). A fourth component of the fission yeast gamma-tubulin complex, Alp16, is required for cytoplasmic microtubule integrity and becomes indispensable when gamma-tubulin function is compromised. Mol. Biol. Cell 13, 2360-2373. 10.1091/mbc.02-01-0603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard, D. L. and Kirschner, M. W. (1987). A microtubule-associated protein from Xenopus eggs that specifically promotes assembly at the plus-end. J. Cell Biol. 105, 2203-2215. 10.1083/jcb.105.5.2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler, S., Pereira, G., Spang, A., Knop, M., Souès, S., Kilmartin, J. and Schiebel, E. (1996). The spindle pole body component Spc98p interacts with the gamma-tubulin-like Tub4p of Saccharomyces cerevisiae at the sites of microtubule attachment. EMBO J. 15, 3899-3911. 10.1002/j.1460-2075.1996.tb00764.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombos, L., Neuner, A., Berynskyy, M., Fava, L. L., Wade, R. C., Sachse, C. and Schiebel, E. (2013). GTP regulates the microtubule nucleation activity of γ-tubulin. Nat. Cell Biol. 15, 1317-1327. 10.1038/ncb2863 [DOI] [PubMed] [Google Scholar]
- Gomez-Ferreria, M. A., Rath, U., Buster, D. W., Chanda, S. K., Caldwell, J. S., Rines, D. R. and Sharp, D. J. (2007). Human Cep192 is required for mitotic centrosome and spindle assembly. Curr. Biol. 17, 1960-1966. 10.1016/j.cub.2007.10.019 [DOI] [PubMed] [Google Scholar]
- Goshima, G., Wollman, R., Goodwin, S. S., Zhang, N., Scholey, J. M., Vale, R. D. and Stuurman, N. (2007). Genes required for mitotic spindle assembly in Drosophila S2 cells. Science 316, 417-421. 10.1126/science.1141314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima, G., Mayer, M., Zhang, N., Stuurman, N. and Vale, R. D. (2008). Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle. J. Cell Biol. 181, 421-429. 10.1083/jcb.200711053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen, A. C., Cameron, L. A., Coughlin, M., Miyamoto, D. T., Mitchison, T. J. and Ohi, R. (2004). XRHAMM functions in ran-dependent microtubule nucleation and pole formation during anastral spindle assembly. Curr. Biol. 14, 1801-1811. 10.1016/j.cub.2004.10.002 [DOI] [PubMed] [Google Scholar]
- Groen, A. C., Maresca, T. J., Gatlin, J. C., Salmon, E. D. and Mitchison, T. J. (2009). Functional overlap of microtubule assembly factors in chromatin-promoted spindle assembly. Mol. Biol. Cell 20, 2766-2773. 10.1091/mbc.e09-01-0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss, O. J., Carazo-Salas, R. E., Schatz, C. A., Guarguaglini, G., Kast, J., Wilm, M., Le Bot, N., Vernos, I., Karsenti, E. and Mattaj, I. W. (2001). Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell 104, 83-93. 10.1016/S0092-8674(01)00193-3 [DOI] [PubMed] [Google Scholar]
- Guillet, V., Knibiehler, M., Gregory-Pauron, L., Remy, M.-H., Chemin, C., Raynaud-Messina, B., Bon, C., Kollman, J. M., Agard, D. A., Merdes, A.et al. (2011). Crystal structure of γ-tubulin complex protein GCP4 provides insight into microtubule nucleation. Nat. Struct. Mol. Biol. 18, 915-919. 10.1038/nsmb.2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane, R. N., Martin, O. C., Cao, K., Zhang, L., Dej, K., Iwamatsu, A. and Zheng, Y. (2000). Characterization and reconstitution of Drosophila gamma-tubulin ring complex subunits. J. Cell Biol. 151, 1513-1524. 10.1083/jcb.151.7.1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardane, R. N., Martin, O. C. and Zheng, Y. (2003). Characterization of a new gammaTuRC subunit with WD repeats. Mol. Biol. Cell 14, 1017-1026. 10.1091/mbc.e02-01-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunzelmann, J., Rüthnick, D., Lin, T.-C., Zhang, W., Neuner, A., Jäkle, U. and Schiebel, E. (2018). The microtubule polymerase Stu2 promotes oligomerization of the γ-TuSC for cytoplasmic microtubule nucleation. Elife 7, e39932. 10.7554/eLife.39932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannak, E., Oegema, K., Kirkham, M., Gönczy, P., Habermann, B. and Hyman, A. A. (2002). The kinetically dominant assembly pathway for centrosomal asters in Caenorhabditis elegans is gamma-tubulin dependent. J. Cell Biol. 157, 591-602. 10.1083/jcb.200202047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haren, L., Remy, M.-H., Bazin, I., Callebaut, I., Wright, M. and Merdes, A. (2006). NEDD1-dependent recruitment of the gamma-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 172, 505-515. 10.1083/jcb.200510028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haren, L., Stearns, T. and Lüders, J. (2009). Plk1-dependent recruitment of gamma-tubulin complexes to mitotic centrosomes involves multiple PCM components. PLoS ONE 4, e5976. 10.1371/journal.pone.0005976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haren, L., Farache, D., Emorine, L. and Merdes, A. (2020). A stable core of GCPs 4, 5 and 6 promotes the assembly of γ-tubulin ring complexes. J. Cell. Sci. 133, jcs244368. 10.1242/jcs.244368 [DOI] [PubMed] [Google Scholar]
- Hegemann, B., Hutchins, J. R. A., Hudecz, O., Novatchkova, M., Rameseder, J., Sykora, M. M., Liu, S., Mazanek, M., Lénárt, P., Hériché, J.-K.et al. (2011). Systematic phosphorylation analysis of human mitotic protein complexes. Sci. Signal. 4, rs12. 10.1126/scisignal.2001993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia, K.-C., Wilson-Kubalek, E. M., Dottore, A., Hao, Q., Tsai, K.-L., Forth, S., Shimamoto, Y., Milligan, R. A. and Kapoor, T. M. (2014). Reconstitution of the augmin complex provides insights into its architecture and function. Nat. Cell Biol. 16, 852-863. 10.1038/ncb3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, T.-L., Wang, H.-J., Chang, Y.-C., Wang, S.-W. and Hsia, K.-C. (2020). Promiscuous binding of microprotein Mozart1 to γ-tubulin complex mediates specific subcellular targeting to control microtubule array formation. Cell Rep 31, 107836. 10.1016/j.celrep.2020.107836 [DOI] [PubMed] [Google Scholar]
- Hutchins, J. R. A., Toyoda, Y., Hegemann, B., Poser, I., Hériché, J.-K., Sykora, M. M., Augsburg, M., Hudecz, O., Buschhorn, B. A., Bulkescher, J.et al. (2010). Systematic analysis of human protein complexes identifies chromosome segregation proteins. Science 328, 593-599. 10.1126/science.1181348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi, N., Fumoto, K., Izumi, S. and Kikuchi, A. (2008). GSK-3beta regulates proper mitotic spindle formation in cooperation with a component of the gamma-tubulin ring complex, GCP5. J. Biol. Chem. 283, 12981-12991. 10.1074/jbc.M710282200 [DOI] [PubMed] [Google Scholar]
- Janski, N., Herzog, E. and Schmit, A.-C. (2008). Identification of a novel small Arabidopsis protein interacting with gamma-tubulin complex protein 3. Cell Biol. Int. 32, 546-548. 10.1016/j.cellbi.2007.11.006 [DOI] [PubMed] [Google Scholar]
- Jiang, K., Hua, S., Mohan, R., Grigoriev, I., Yau, K. W., Liu, Q., Katrukha, E. A., Altelaar, A. F. M., Heck, A. J. R., Hoogenraad, C. C.et al. (2014). Microtubule minus-end stabilization by polymerization-driven CAMSAP deposition. Dev. Cell 28, 295-309. 10.1016/j.devcel.2014.01.001 [DOI] [PubMed] [Google Scholar]
- Jiang, H., Wang, S., Huang, Y., He, X., Cui, H., Zhu, X. and Zheng, Y. (2015). Phase transition of spindle-associated protein regulate spindle apparatus assembly. Cell 163, 108-122. 10.1016/j.cell.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johmura, Y., Soung, N.-K., Park, J.-E., Yu, L.-R., Zhou, M., Bang, J. K., Kim, B.-Y., Veenstra, T. D., Erikson, R. L. and Lee, K. S. (2011). Regulation of microtubule-based microtubule nucleation by mammalian polo-like kinase 1. Proc. Natl. Acad. Sci. USA 108, 11446-11451. 10.1073/pnas.1106223108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov, V. and De Nicolo, A. (2018). Aurora-PLK1 cascades as key signaling modules in the regulation of mitosis. Sci. Signal. 11, eaar4195. 10.1126/scisignal.aar4195 [DOI] [PubMed] [Google Scholar]
- Jung, M. K., Prigozhina, N., Oakley, C. E., Nogales, E. and Oakley, B. R. (2001). Alanine-scanning mutagenesis of Aspergillus gamma-tubulin yields diverse and novel phenotypes. Mol. Biol. Cell 12, 2119-2136. 10.1091/mbc.12.7.2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating, T. J. and Borisy, G. G. (2000). Immunostructural evidence for the template mechanism of microtubule nucleation. Nat. Cell Biol. 2, 352-357. 10.1038/35014045 [DOI] [PubMed] [Google Scholar]
- Keck, J. M., Jones, M. H., Wong, C. C. L., Binkley, J., Chen, D., Jaspersen, S. L., Holinger, E. P., Xu, T., Niepel, M., Rout, M. P.et al. (2011). A cell cycle phosphoproteome of the yeast centrosome. Science 332, 1557-1561. 10.1126/science.1205193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenbach, A. N., Schweppe, D. K., Faherty, B. K., Pechenick, D., Pletnev, A. A. and Gerber, S. A. (2011). Quantitative phosphoproteomics identifies substrates and functional modules of Aurora and Polo-like kinase activities in mitotic cells. Sci. Signal. 4, rs5. 10.1126/scisignal.2001497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, M. R. and Petry, S. (2020). Phase separation of TPX2 enhances and spatially coordinates microtubule nucleation. Nat. Commun. 11, 270. 10.1038/s41467-019-14087-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, B. R., Moritz, M., Kim, H., Agard, D. A., Asbury, C. L. and Davis, T. N. (2020). XMAP215 and γ-tubulin additively promote microtubule nucleation in purified solutions. Mol. Biol. Cell 31, 2187-2194. 10.1091/mbc.E20-02-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop, M., Pereira, G., Geissler, S., Grein, K. and Schiebel, E. (1997). The spindle pole body component Spc97p interacts with the gamma-tubulin of Saccharomyces cerevisiae and functions in microtubule organization and spindle pole body duplication. EMBO J. 16, 1550-1564. 10.1093/emboj/16.7.1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollman, J. M., Zelter, A., Muller, E. G. D., Fox, B., Rice, L. M., Davis, T. N. and Agard, D. A. (2008). The structure of the gamma-tubulin small complex: implications of its architecture and flexibility for microtubule nucleation. Mol. Biol. Cell 19, 207-215. 10.1091/mbc.e07-09-0879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollman, J. M., Polka, J. K., Zelter, A., Davis, T. N. and Agard, D. A. (2010). Microtubule nucleating gamma-TuSC assembles structures with 13-fold microtubule-like symmetry. Nature 466, 879-882. 10.1038/nature09207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollman, J. M., Merdes, A., Mourey, L. and Agard, D. A. (2011). Microtubule nucleation by γ-tubulin complexes. Nat. Rev. Mol. Cell Biol. 12, 709-721. 10.1038/nrm3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollman, J. M., Greenberg, C. H., Li, S., Moritz, M., Zelter, A., Fong, K. K., Fernandez, J.-J., Sali, A., Kilmartin, J., Davis, T. N.et al. (2015). Ring closure activates yeast γTuRC for species-specific microtubule nucleation. Nat. Struct. Mol. Biol. 22, 132-137. 10.1038/nsmb.2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufer, T. A., Silljé, H. H. W., Körner, R., Gruss, O. J., Meraldi, P. and Nigg, E. A. (2002). Human TPX2 is required for targeting Aurora-A kinase to the spindle. J. Cell Biol. 158, 617-623. 10.1083/jcb.200204155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawo, S., Bashkurov, M., Mullin, M., Ferreria, M. G., Kittler, R., Habermann, B., Tagliaferro, A., Poser, I., Hutchins, J. R. A., Hegemann, B.et al. (2009). HAUS, the 8-subunit human augmin complex, regulates centrosome and spindle integrity. Curr. Biol. 19, 816-826. 10.1016/j.cub.2009.04.033 [DOI] [PubMed] [Google Scholar]
- Lawo, S., Hasegan, M., Gupta, G. D. and Pelletier, L. (2012). Subdiffraction imaging of centrosomes reveals higher-order organizational features of pericentriolar material. Nat. Cell Biol. 14, 1148-1158. 10.1038/ncb2591 [DOI] [PubMed] [Google Scholar]
- Leong, S. L., Lynch, E. M., Zou, J., Tay, Y. D., Borek, W. E., Tuijtel, M. W., Rappsilber, J. and Sawin, K. E. (2019). Reconstitution of microtubule nucleation in vitro reveals novel roles for Mzt1. Curr. Biol. 29, 2199-2207.e10. 10.1016/j.cub.2019.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, T., Gombos, L., Neuner, A., Sebastian, D., Olsen, J. V., Hrle, A., Benda, C. and Schiebel, E. (2011). Phosphorylation of the yeast γ-tubulin Tub4 regulates microtubule function. PLoS ONE 6, e19700. 10.1371/journal.pone.0019700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, T.-C., Neuner, A., Schlosser, Y. T., Scharf, A. N. D., Weber, L. and Schiebel, E. (2014). Cell-cycle dependent phosphorylation of yeast pericentrin regulates γ-TuSC-mediated microtubule nucleation. Elife 3, e02208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, T., Neuner, A. and Schiebel, E. (2015). Targeting of γ-tubulin complexes to microtubule organizing centers: conservation and divergence. Trends Cell Biol. 25, 296-307. 10.1016/j.tcb.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Lin, T.-C., Neuner, A., Flemming, D., Liu, P., Chinen, T., Jäkle, U., Arkowitz, R. and Schiebel, E. (2016). MOZART1 and γ-tubulin complex receptors are both required to turn γ-TuSC into an active microtubule nucleation template. J. Cell Biol. 215, 823-840. 10.1083/jcb.201606092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. and Wiese, C. (2008). Xenopus NEDD1 is required for microtubule organization in Xenopus egg extracts. J. Cell. Sci. 121, 578-589. 10.1242/jcs.018937 [DOI] [PubMed] [Google Scholar]
- Liu, P., Choi, Y.-K. and Qi, R. Z. (2014a). NME7 is a functional component of the γ-tubulin ring complex. Mol. Biol. Cell 25, 2017-2025. 10.1091/mbc.e13-06-0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, T., Tian, J., Wang, G., Yu, Y., Wang, C., Ma, Y., Zhang, X., Xia, G., Liu, B. and Kong, Z. (2014b). Augmin triggers microtubule-dependent microtubule nucleation in interphase plant cells. Curr. Biol. 24, 2708-2713. 10.1016/j.cub.2014.09.053 [DOI] [PubMed] [Google Scholar]
- Liu, P., Zupa, E., Neuner, A., Böhler, A., Loerke, J., Flemming, D., Ruppert, T., Rudack, T., Peter, C., Spahn, C.et al. (2020). Insights into the assembly and activation of the microtubule nucleator γ-TuRC. Nature 578, 467-471. 10.1038/s41586-019-1896-6 [DOI] [PubMed] [Google Scholar]
- Lüders, J. and Stearns, T. (2007). Microtubule-organizing centres: a re-evaluation. Nat. Rev. Mol. Cell Biol. 8, 161-167. 10.1038/nrm2100 [DOI] [PubMed] [Google Scholar]
- Lüders, J., Patel, U. K. and Stearns, T. (2006). GCP-WD is a gamma-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 8, 137-147. 10.1038/ncb1349 [DOI] [PubMed] [Google Scholar]
- Luo, J., Yang, B., Xin, G., Sun, M., Zhang, B., Guo, X., Jiang, Q. and Zhang, C. (2019). The microtubule-associated protein EML3 regulates mitotic spindle assembly by recruiting the Augmin complex to spindle microtubules. J. Biol. Chem. 294, 5643-5656. 10.1074/jbc.RA118.007164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, E. M., Groocock, L. M., Borek, W. E. and Sawin, K. E. (2014). Activation of the γ-tubulin complex by the Mto1/2 complex. Curr. Biol. 24, 896-903. 10.1016/j.cub.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon, A. S., Morin, G., Moritz, M., Yabut, K. C. B., Vojnar, T., Zelter, A., Muller, E., Davis, T. N. and Agard, D. A. (2016). Higher-order oligomerization of Spc110p drives γ-tubulin ring complex assembly. Mol. Biol. Cell 27, 2245-2258. 10.1091/mbc.E16-02-0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, W., Baumann, C. and Viveiros, M. M. (2010). NEDD1 is crucial for meiotic spindle stability and accurate chromosome segregation in mammalian oocytes. Dev. Biol. 339, 439-450. 10.1016/j.ydbio.2010.01.009 [DOI] [PubMed] [Google Scholar]
- Manning, J. A., Shalini, S., Risk, J. M., Day, C. L. and Kumar, S. (2010). A direct interaction with NEDD1 regulates gamma-tubulin recruitment to the centrosome. PLoS ONE 5, e9618. 10.1371/journal.pone.0009618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, O. C., Gunawardane, R. N., Iwamatsu, A. and Zheng, Y. (1998). Xgrip109: a gamma tubulin-associated protein with an essential role in gamma tubulin ring complex (gammaTuRC) assembly and centrosome function. J. Cell Biol. 141, 675-687. 10.1083/jcb.141.3.675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda, H., Mori, R., Yukawa, M. and Toda, T. (2013). Fission yeast MOZART1/Mzt1 is an essential γ-tubulin complex component required for complex recruitment to the microtubule organizing center, but not its assembly. Mol. Biol. Cell 24, 2894-2906. 10.1091/mbc.e13-05-0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella, V., Keszthelyi, B., McDonald, K. L., Chhun, B., Kan, F., Rogers, G. C., Huang, B. and Agard, D. A. (2012). Subdiffraction-resolution fluorescence microscopy reveals a domain of the centrosome critical for pericentriolar material organization. Nat. Cell Biol. 14, 1159-1168. 10.1038/ncb2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier, S. and Vernos, I. (2016). Acentrosomal microtubule assembly in mitosis: the where, when, and how. Trends Cell Biol. 26, 80-87. 10.1016/j.tcb.2015.09.001 [DOI] [PubMed] [Google Scholar]
- Moores, C. A., Perderiset, M., Francis, F., Chelly, J., Houdusse, A. and Milligan, R. A. (2004). Mechanism of microtubule stabilization by doublecortin. Mol. Cell 14, 833-839. 10.1016/j.molcel.2004.06.009 [DOI] [PubMed] [Google Scholar]
- Moritz, M., Zheng, Y., Alberts, B. M. and Oegema, K. (1998). Recruitment of the gamma-tubulin ring complex to Drosophila salt-stripped centrosome scaffolds. J. Cell Biol. 142, 775-786. 10.1083/jcb.142.3.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz, M., Braunfeld, M. B., Guénebaut, V., Heuser, J. and Agard, D. A. (2000). Structure of the gamma-tubulin ring complex: a template for microtubule nucleation. Nat. Cell Biol. 2, 365-370. 10.1038/35014058 [DOI] [PubMed] [Google Scholar]
- Murata, T. and Hasebe, M. (2007). Microtubule-dependent microtubule nucleation in plant cells. J. Plant Res. 120, 73-78. 10.1007/s10265-006-0054-z [DOI] [PubMed] [Google Scholar]
- Murphy, S. M., Urbani, L. and Stearns, T. (1998). The mammalian gamma-tubulin complex contains homologues of the yeast spindle pole body components spc97p and spc98p. J. Cell Biol. 141, 663-674. 10.1083/jcb.141.3.663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, S. M., Preble, A. M., Patel, U. K., O'Connell, K. L., Dias, D. P., Moritz, M., Agard, D., Stults, J. T. and Stearns, T. (2001). GCP5 and GCP6: two new members of the human gamma-tubulin complex. Mol. Biol. Cell 12, 3340-3352. 10.1091/mbc.12.11.3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, M., Yagi, N., Kato, T., Fujita, S., Kawashima, N., Ehrhardt, D. W. and Hashimoto, T. (2012). Arabidopsis GCP3-interacting protein 1/MOZART 1 is an integral component of the γ-tubulin-containing microtubule nucleating complex. Plant J. 71, 216-225. 10.1111/j.1365-313X.2012.04988.x [DOI] [PubMed] [Google Scholar]
- Oakley, C. E. and Oakley, B. R. (1989). Identification of gamma-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature 338, 662-664. 10.1038/338662a0 [DOI] [PubMed] [Google Scholar]
- Oegema, K., Wiese, C., Martin, O. C., Milligan, R. A., Iwamatsu, A., Mitchison, T. J. and Zheng, Y. (1999). Characterization of two related Drosophila gamma-tubulin complexes that differ in their ability to nucleate microtubules. J. Cell Biol. 144, 721-733. 10.1083/jcb.144.4.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori-McKenney, K. M., Jan, L. Y. and Jan, Y.-N. (2012). Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron 76, 921-930. 10.1016/j.neuron.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oriolo, A. S., Wald, F. A., Canessa, G. and Salas, P. J. I. (2007). GCP6 binds to intermediate filaments: a novel function of keratins in the organization of microtubules in epithelial cells. Mol. Biol. Cell 18, 781-794. 10.1091/mbc.e06-03-0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole, E., Greenan, G., Lange, K. I., Srayko, M. and Müller-Reichert, T. (2012). The role of γ-tubulin in centrosomal microtubule organization. PLoS ONE 7, e29795. 10.1371/journal.pone.0029795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovechkina, Y., Maddox, P., Oakley, C. E., Xiang, X., Osmani, S. A., Salmon, E. D. and Oakley, B. R. (2003). Spindle formation in Aspergillus is coupled to tubulin movement into the nucleus. Mol. Biol. Cell 14, 2192-2200. 10.1091/mbc.e02-10-0641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Y., Moritz, M., Han, X., Giddings, T. H., Lyon, A., Kollman, J., Winey, M., Yates, J., Agard, D. A., Drubin, D. G.et al. (2015). Interaction of CK1δ with γTuSC ensures proper microtubule assembly and spindle positioning. Mol. Biol. Cell 26, 2505-2518. 10.1091/mbc.E14-12-1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry, S. and Vale, R. D. (2015). Microtubule nucleation at the centrosome and beyond. Nat. Cell Biol. 17, 1089-1093. 10.1038/ncb3220 [DOI] [PubMed] [Google Scholar]
- Petry, S., Pugieux, C., Nédélec, F. J. and Vale, R. D. (2011). Augmin promotes meiotic spindle formation and bipolarity in Xenopus egg extracts. Proc. Natl. Acad. Sci. USA 108, 14473-14478. 10.1073/pnas.1110412108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry, S., Groen, A. C., Ishihara, K., Mitchison, T. J. and Vale, R. D. (2013). Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell 152, 768-777. 10.1016/j.cell.2012.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinyol, R., Scrofani, J. and Vernos, I. (2013). The role of NEDD1 phosphorylation by Aurora A in chromosomal microtubule nucleation and spindle function. Curr. Biol. 23, 143-149. 10.1016/j.cub.2012.11.046 [DOI] [PubMed] [Google Scholar]
- Popov, A. V., Severin, F. and Karsenti, E. (2002). XMAP215 is required for the microtubule-nucleating activity of centrosomes. Curr. Biol. 12, 1326-1330. 10.1016/S0960-9822(02)01033-3 [DOI] [PubMed] [Google Scholar]
- Raff, J. W. (2019). Phase separation and the centrosome: a fait accompli? Trends Cell Biol. 29, 612-622. 10.1016/j.tcb.2019.04.001 [DOI] [PubMed] [Google Scholar]
- Rale, M. J., Kadzik, R. S. and Petry, S. (2018). Phase Transitioning the Centrosome into a Microtubule Nucleator. Biochemistry 57, 30-37. 10.1021/acs.biochem.7b01064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber, S. B., Baumgart, J., Widlund, P. O., Pozniakovsky, A., Howard, J., Hyman, A. A. and Jülicher, F. (2013). XMAP215 activity sets spindle length by controlling the total mass of spindle microtubules. Nat. Cell Biol. 15, 1116-1122. 10.1038/ncb2834 [DOI] [PubMed] [Google Scholar]
- Reschen, R. F., Colombie, N., Wheatley, L., Dobbelaere, J., St Johnston, D., Ohkura, H. and Raff, J. W. (2012). Dgp71WD is required for the assembly of the acentrosomal Meiosis I spindle, and is not a general targeting factor for the γ-TuRC. Biol. Open 1, 422-429. 10.1242/bio.2012596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, L. M., Montabana, E. A. and Agard, D. A. (2008). The lattice as allosteric effector: structural studies of alphabeta- and gamma-tubulin clarify the role of GTP in microtubule assembly. Proc. Natl. Acad. Sci. USA 105, 5378-5383. 10.1073/pnas.0801155105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos, R. M., Sanchís, A., Tassin, A. M., Fedriani, C. and Bornens, M. (2004). GMAP-210 recruits gamma-tubulin complexes to cis-Golgi membranes and is required for Golgi ribbon formation. Cell 118, 323-335. 10.1016/j.cell.2004.07.012 [DOI] [PubMed] [Google Scholar]
- Rivero, S., Cardenas, J., Bornens, M. and Rios, R. M. (2009). Microtubule nucleation at the cis-side of the Golgi apparatus requires AKAP450 and GM130. EMBO J. 28, 1016-1028. 10.1038/emboj.2009.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, G. C., Rusan, N. M., Peifer, M. and Rogers, S. L. (2008). A multicomponent assembly pathway contributes to the formation of acentrosomal microtubule arrays in interphase Drosophila cells. Mol. Biol. Cell 19, 3163-3178. 10.1091/mbc.e07-10-1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roostalu, J. and Surrey, T. (2017). Microtubule nucleation: beyond the template. Nat. Rev. Mol. Cell Biol. 18, 702-710. 10.1038/nrm.2017.75 [DOI] [PubMed] [Google Scholar]
- Roostalu, J., Cade, N. I. and Surrey, T. (2015). Complementary activities of TPX2 and chTOG constitute an efficient importin-regulated microtubule nucleation module. Nat. Cell Biol. 17, 1422-1434. 10.1038/ncb3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubin, R., Acquaviva, C., Chevrier, V., Sedjaï, F., Zyss, D., Birnbaum, D. and Rosnet, O. (2013). Myomegalin is necessary for the formation of centrosomal and Golgi-derived microtubules. Biol. Open 2, 238-250. 10.1242/bio.20123392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Huertas, C., Freixo, F., Viais, R., Lacasa, C., Soriano, E. and Lüders, J. (2016). Non-centrosomal nucleation mediated by augmin organizes microtubules in post-mitotic neurons and controls axonal microtubule polarity. Nat. Commun. 7, 12187. 10.1038/ncomms12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, A. A. W. M., Chang, K., Zhu, X., Thoppil, R. J., Holmes, W. R. and Kaverina, I. (2017). Nonrandom γ-TuNA-dependent spatial pattern of microtubule nucleation at the Golgi. Mol. Biol. Cell 28, 3181-3192. 10.1091/mbc.e17-06-0425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria, A., Wang, B., Elowe, S., Malik, R., Zhang, F., Bauer, M., Schmidt, A., Silljé, H. H. W., Körner, R. and Nigg, E. A. (2011). The Plk1-dependent phosphoproteome of the early mitotic spindle. Mol. Cell. Proteomics 10, M110.004457. 10.1074/mcp.M110.004457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin, K. E., Lourenco, P. C. C. and Snaith, H. A. (2004). Microtubule nucleation at non-spindle pole body microtubule-organizing centers requires fission yeast centrosomin-related protein mod20p. Curr. Biol. 14, 763-775. 10.1016/j.cub.2004.03.042 [DOI] [PubMed] [Google Scholar]
- Schatz, C. A., Santarella, R., Hoenger, A., Karsenti, E., Mattaj, I. W., Gruss, O. J. and Carazo-Salas, R. E. (2003). Importin alpha-regulated nucleation of microtubules by TPX2. EMBO J. 22, 2060-2070. 10.1093/emboj/cdg195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrofani, J., Sardon, T., Meunier, S. and Vernos, I. (2015). Microtubule nucleation in mitosis by a RanGTP-dependent protein complex. Curr. Biol. 25, 131-140. 10.1016/j.cub.2014.11.025 [DOI] [PubMed] [Google Scholar]
- Slep, K. C. and Vale, R. D. (2007). Structural basis of microtubule plus end tracking by XMAP215, CLIP-170, and EB1. Mol. Cell 27, 976-991. 10.1016/j.molcel.2007.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, J.-G., King, M. R., Zhang, R., Kadzik, R. S., Thawani, A. and Petry, S. (2018). Mechanism of how augmin directly targets the γ-tubulin ring complex to microtubules. J. Cell Biol. 217, 2417-2428. 10.1083/jcb.201711090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, M., Yamagiwa, A., Nishimura, T., Mukai, H. and Ono, Y. (2002). Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex. Mol. Biol. Cell 13, 3235-3245. 10.1091/mbc.e02-02-0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq, A., Green, L., Jeynes, J. C. G., Soeller, C. and Wakefield, J. G. (2020). In vitro reconstitution of branching microtubule nucleation. Elife 9, e49769. 10.7554/eLife.49769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixidó-Travesa, N., Villén, J., Lacasa, C., Bertran, M. T., Archinti, M., Gygi, S. P., Caelles, C., Roig, J. and Lüders, J. (2010). The gammaTuRC revisited: a comparative analysis of interphase and mitotic human gammaTuRC redefines the set of core components and identifies the novel subunit GCP8. Mol. Biol. Cell 21, 3963-3972. 10.1091/mbc.e10-05-0408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada, Y., Uetake, Y. and Kuriyama, R. (2003). Interaction of Aurora-A and centrosomin at the microtubule-nucleating site in Drosophila and mammalian cells. J. Cell Biol. 162, 757-763. 10.1083/jcb.200305048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thawani, A., Kadzik, R. S. and Petry, S. (2018). XMAP215 is a microtubule nucleation factor that functions synergistically with the γ-tubulin ring complex. Nat. Cell Biol. 20, 575-585. 10.1038/s41556-018-0091-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thawani, A., Stone, H. A., Shaevitz, J. W. and Petry, S. (2019). Spatiotemporal organization of branched microtubule networks. Elife 8, e43890. 10.7554/eLife.43890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thawani, A., Rale, M. J., Coudray, N., Bhabha, G., Stone, H. A., Shaevitz, J. W. and Petry, S. (2020). The transition state and regulation of γ-TuRC-mediated microtubule nucleation revealed by single molecule microscopy. Elife 9, e54253. 10.7554/eLife.54253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonucci, F. M., Hidalgo, F., Ferretti, A., Almada, E., Favre, C., Goldenring, J. R., Kaverina, I., Kierbel, A. and Larocca, M. C. (2015). Centrosomal AKAP350 and CIP4 act in concert to define the polarized localization of the centrosome and Golgi in migratory cells. J. Cell. Sci. 128, 3277-3289. 10.1242/jcs.170878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey, C. A. and Conduit, P. T. (2018). Microtubule nucleation by γ-tubulin complexes and beyond. Essays Biochem. 62, 765-780. 10.1042/EBC20180028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey, C. A., Tubman, C. E., Hamrud, E., Zhu, Z., Dyas, A. E., Butterfield, A. N., Fyfe, A., Johnson, E. and Conduit, P. T. (2018). γ-TuRC Heterogeneity revealed by analysis of mozart1. Curr. Biol. 28, 2314-2323.e6. 10.1016/j.cub.2018.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey, C. A., Tsuji, C., Egerton, A., Bernard, F., Guichet, A., de la Roche, M. and Conduit, P. T. (2020). Auto-inhibition of Cnn binding to γ-TuRCs prevents ectopic microtubule nucleation and cell division defects. Cell Biol. 220, e202010020. 10.1083/jcb.202010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, M.-Y., Wiese, C., Cao, K., Martin, O., Donovan, P., Ruderman, J., Prigent, C. and Zheng, Y. (2003). A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat. Cell Biol. 5, 242-248. 10.1038/ncb936 [DOI] [PubMed] [Google Scholar]
- Uehara, R., Nozawa, R., Tomioka, A., Petry, S., Vale, R. D., Obuse, C. and Goshima, G. (2009). The augmin complex plays a critical role in spindle microtubule generation for mitotic progression and cytokinesis in human cells. Proc. Natl. Acad. Sci. USA 106, 6998-7003. 10.1073/pnas.0901587106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemu, A., Szczesna, E., Zehr, E. A., Spector, J. O., Grigorieff, N., Deaconescu, A. M. and Roll-Mecak, A. (2018). Severing enzymes amplify microtubule arrays through lattice GTP-tubulin incorporation. Science 361, eaau1504. 10.1126/science.aau1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatram, S., Tasto, J. J., Feoktistova, A., Jennings, J. L., Link, A. J. and Gould, K. L. (2004). Identification and characterization of two novel proteins affecting fission yeast gamma-tubulin complex function. Mol. Biol. Cell 15, 2287-2301. 10.1091/mbc.e03-10-0728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, V. and Maresca, T. J. (2019). Direct observation of branching MT nucleation in living animal cells. J. Cell Biol. 218, 2829-2840. 10.1083/jcb.201904114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vérollet, C., Colombié, N., Daubon, T., Bourbon, H.-M., Wright, M. and Raynaud-Messina, B. (2006). Drosophila melanogaster gamma-TuRC is dispensable for targeting gamma-tubulin to the centrosome and microtubule nucleation. J. Cell Biol. 172, 517-528. 10.1083/jcb.200511071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh, D. B. N., Kern, J. W., Hancock, W. O., Howard, J. and Davis, T. N. (2002). Reconstitution and characterization of budding yeast gamma-tubulin complex. Mol. Biol. Cell 13, 1144-1157. 10.1091/mbc.02-01-0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, J., Drapkin, B., Oomen, J., Beach, D., Bloom, K. and Snyder, M. (2001). Phosphorylation of gamma-tubulin regulates microtubule organization in budding yeast. Dev. Cell 1, 621-631. 10.1016/S1534-5807(01)00073-9 [DOI] [PubMed] [Google Scholar]
- Voter, W. A. and Erickson, H. P. (1984). The kinetics of microtubule assembly. Evidence for a two-stage nucleation mechanism. J. Biol. Chem. 259, 10430-10438. 10.1016/S0021-9258(18)90982-8 [DOI] [PubMed] [Google Scholar]
- Walia, A., Nakamura, M., Moss, D., Kirik, V., Hashimoto, T. and Ehrhardt, D. W. (2014). GCP-WD mediates γ-TuRC recruitment and the geometry of microtubule nucleation in interphase arrays of Arabidopsis. Curr. Biol. 24, 2548-2555. 10.1016/j.cub.2014.09.013 [DOI] [PubMed] [Google Scholar]
- Wang, Z., Wu, T., Shi, L., Zhang, L., Zheng, W., Qu, J. Y., Niu, R. and Qi, R. Z. (2010). Conserved motif of CDK5RAP2 mediates its localization to centrosomes and the Golgi complex. J. Biol. Chem. 285, 22658-22665. 10.1074/jbc.M110.105965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., Zhang, C. and Qi, R. Z. (2014). A newly identified myomegalin isoform functions in Golgi microtubule organization and ER-Golgi transport. J. Cell. Sci. 127, 4904-4917. 10.1242/jcs.155408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, J.-H., Zhang, Z. C., Wynn, R. M. and Seemann, J. (2015). GM130 regulates golgi-derived spindle assembly by activating TPX2 and capturing microtubules. Cell 162, 287-299. 10.1016/j.cell.2015.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlund, P. O., Stear, J. H., Pozniakovsky, A., Zanic, M., Reber, S., Brouhard, G. J., Hyman, A. A. and Howard, J. (2011). XMAP215 polymerase activity is built by combining multiple tubulin-binding TOG domains and a basic lattice-binding region. Proc. Natl. Acad. Sci. USA 108, 2741-2746. 10.1073/pnas.1016498108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek, M., Bechstedt, S., Chaaban, S. and Brouhard, G. J. (2015). Microtubule-associated proteins control the kinetics of microtubule nucleation. Nat. Cell Biol. 17, 907-916. 10.1038/ncb3188 [DOI] [PubMed] [Google Scholar]
- Wieczorek, M., Urnavicius, L., Ti, S.-C., Molloy, K. R., Chait, B. T. and Kapoor, T. M. (2020a). Asymmetric molecular architecture of the human γ-tubulin ring complex. Cell 180, 165-175.e16. 10.1016/j.cell.2019.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek, M., Huang, T.-L., Urnavicius, L., Hsia, K.-C. and Kapoor, T. M. (2020b). MZT proteins form multi-faceted structural modules in the γ-tubulin ring complex. Cell Rep 31, 107791. 10.1016/j.celrep.2020.107791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek, M., Ti, S.-C., Urnavicius, L., Molloy, K. R., Aher, A., Chait, B. T. and Kapoor, T. M. (2021). Biochemical reconstitutions reveal principles of human γ-TuRC assembly and function. J. Cell Biol. 220, e202009146. 10.1083/jcb.202009146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese, C. and Zheng, Y. (2000). A new function for the gamma-tubulin ring complex as a microtubule minus-end cap. Nat. Cell Biol. 2, 358-364. 10.1038/35014051 [DOI] [PubMed] [Google Scholar]
- Wiese, C. and Zheng, Y. (2006). Microtubule nucleation: gamma-tubulin and beyond. J. Cell. Sci. 119, 4143-4153. 10.1242/jcs.03226 [DOI] [PubMed] [Google Scholar]
- Woodruff, J. B., Wueseke, O., Viscardi, V., Mahamid, J., Ochoa, S. D., Bunkenborg, J., Widlund, P. O., Pozniakovsky, A., Zanin, E., Bahmanyar, S.et al. (2015). Centrosomes. Regulated assembly of a supramolecular centrosome scaffold in vitro. Science 348, 808-812. 10.1126/science.aaa3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff, J. B., Ferreira Gomes, B., Widlund, P. O., Mahamid, J., Honigmann, A. and Hyman, A. A. (2017). The centrosome is a selective condensate that nucleates microtubules by concentrating tubulin. Cell 169, 1066-1077.e10. 10.1016/j.cell.2017.05.028 [DOI] [PubMed] [Google Scholar]
- Wu, J. and Akhmanova, A. (2017). Microtubule-organizing centers. Annu. Rev. Cell Dev. Biol. 33, 51-75. 10.1146/annurev-cellbio-100616-060615 [DOI] [PubMed] [Google Scholar]
- Wu, J., de Heus, C., Liu, Q., Bouchet, B. P., Noordstra, I., Jiang, K., Hua, S., Martin, M., Yang, C., Grigoriev, I.et al. (2016). Molecular pathway of microtubule organization at the golgi apparatus. Dev. Cell 39, 44-60. 10.1016/j.devcel.2016.08.009 [DOI] [PubMed] [Google Scholar]
- Würtz, M., Böhler, A., Neuner, A., Zupa, E., Rohland, L., Liu, P., Vermeulen, B. J. A., Pfeffer, S., Eustermann, S. and Schiebel, E. (2021). Reconstitution of the recombinant human γ-tubulin ring complex. Open Biol. 11, 200325. 10.1098/rsob.200325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, Y. and Oakley, B. R. (2009). In vivo analysis of the functions of gamma-tubulin-complex proteins. J. Cell. Sci. 122, 4218-4227. 10.1242/jcs.059196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, R. and Feldman, J. L. (2015). SPD-2/CEP192 and CDK Are Limiting for Microtubule-Organizing Center Function at the Centrosome. Curr. Biol. 25, 1924-1931. 10.1016/j.cub.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Zeng, C. J. T., Lee, Y.-R. J. and Liu, B. (2009). The WD40 repeat protein NEDD1 functions in microtubule organization during cell division in Arabidopsis thaliana. Plant Cell 21, 1129-1140. 10.1105/tpc.109.065953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. and Megraw, T. L. (2007). Proper recruitment of gamma-tubulin and D-TACC/Msps to embryonic Drosophila centrosomes requires Centrosomin Motif 1. Mol. Biol. Cell 18, 4037-4049. 10.1091/mbc.e07-05-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L., Keating, T. J., Wilde, A., Borisy, G. G. and Zheng, Y. (2000). The role of Xgrip210 in gamma-tubulin ring complex assembly and centrosome recruitment. J. Cell Biol. 151, 1525-1536. 10.1083/jcb.151.7.1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, R., Roostalu, J., Surrey, T. and Nogales, E. (2017). Structural insight into TPX2-stimulated microtubule assembly. eLife 6, e30959. 10.7554/eLife.30959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y., Wong, M. L., Alberts, B. and Mitchison, T. (1995). Nucleation of microtubule assembly by a gamma-tubulin-containing ring complex. Nature 378, 578-583. 10.1038/378578a0 [DOI] [PubMed] [Google Scholar]
- Zhu, F., Lawo, S., Bird, A., Pinchev, D., Ralph, A., Richter, C., Müller-Reichert, T., Kittler, R., Hyman, A. A. and Pelletier, L. (2008). The mammalian SPD-2 ortholog Cep192 regulates centrosome biogenesis. Curr. Biol. 18, 136-141. 10.1016/j.cub.2007.12.055 [DOI] [PubMed] [Google Scholar]
- Zimmerman, W. C., Sillibourne, J., Rosa, J. and Doxsey, S. J. (2004). Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol. Biol. Cell 15, 3642-3657. 10.1091/mbc.e03-11-0796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, F., Serna, M., Ezquerra, A., Fernandez-Leiro, R., Llorca, O. and Luders, J. (2020). Assembly of the asymmetric human γ-tubulin ring complex by RUVBL1-RUVBL2 AAA ATPase. Sci. Adv. 6, eabe0894. 10.1126/sciadv.abe0894 [DOI] [PMC free article] [PubMed] [Google Scholar]


