Abstract
Control of the host cell is crucial to the Apicomplexan parasite, Toxoplasma gondii, while it grows intracellularly. To achieve this goal, these single-celled eukaryotes export a series of effector proteins from organelles known as “dense granules” that interfere with normal cellular processes and responses to invasion. While some effectors are found attached to the outer surface of the parasitophorous vacuole (PV) in which Toxoplasma tachyzoites reside, others are found in the host cell’s cytoplasm and yet others make their way into the host nucleus, where they alter host transcription. Among the processes that are severely altered are innate immune responses, host cell cycle and association with host organelles. The ways in which these crucial processes are altered through the coordinated action of a large collection of effectors is as elegant as it is complex, and is the central focus of the following review; we also discuss recent advances in our understanding of how dense granule effector proteins are trafficked out of the PV.
Graphical Abstract
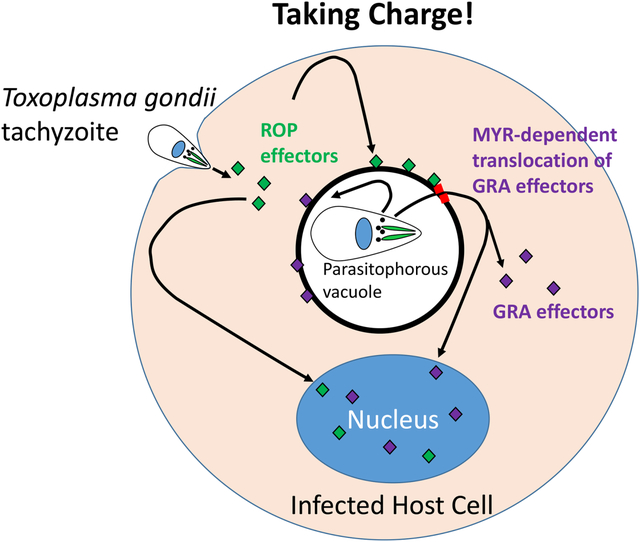
The single celled parasite, Toxoplasma gondii, deploys a series of parasitophorous vacuolar membrane (PVM)-transiting and PVM-attached effector proteins to control key host cellular processes, including innate immune responses, host cell cycle and association with host organelles. Here, we focus on advances in our understanding of how “GRA” effectors secreted from the parasite’s dense granules traffic to or beyond the PVM and the impacts they have once at their final destination.
Toxoplasma is a master manipulator that uses two secretory compartments to hijack many cellular functions
Toxoplasma gondii is an intracellular pathogen and its tachyzoite form, which is the rapidly growing, asexually reproducing stage found in the parasite’s intermediates hosts and the focus of this review, has the remarkable ability to invade and take control of a wide range of mammalian cells. This Apicomplexan species is extremely successful in being found worldwide and in a broad range of warm-blooded hosts. A critical element of its success is Toxoplasma’s ability to counter host defenses, attacking and hijacking evolutionarily conserved pathways common to most vertebrate cells. Residing inside a host cell enables these single-celled eukaryotes to accomplish such feats from within; it also provides the opportunity to steal nutrients and replicate in safety. Yet these advantages are not provided for free – most host cells contain mechanisms to detect and limit invaders – and because Toxoplasma replicates within a parasitophorous vacuole (PV), rather than existing free within the cytosol, all of these interactions must occur across one or more membranes. Toxoplasma meets these challenges by deploying a large repertoire of effectors (Table 1), parasite proteins that actively interact with the host cell to effect a change in the host cell’s function. To date, essentially all known Toxoplasma effectors are derived from two dedicated secretory organelles: rhoptries and dense granules (Fig. 1). Each of these organelles has unique kinetics of release during invasion and their contents subvert various host functions that would normally destroy or limit the growth of any less-well prepared pathogen.
Table 1.
Dense granule proteins that function as effector proteins and their described interaction with the host. Proteins in the upper section are MYR-dependent “PVM-transiting” and are translocated out of the parasitophorous vacuole to either the host nucleus or the host cytoplasm. Proteins in the lower section are MYR-independent, comprised of both “PVM-attached” effectors that mediate their function while anchored in the PVM by a transmembrane domain, usually with the relevant catalytic side facing the cytoplasm, or are associated with the membranous network located within the PV lumen.
| EFFECTOR | HOST CELL LOCATION | SUMMARY OF FUNCTION | |
|---|---|---|---|
| MYR-dependent | GRA16 | Nucleus | Binds HAUSP, PP2AB55, leads to c-Myc induction, p53 activation, cyclin A degradation, alterations in sterol pathways |
| GRA18 | Cytoplasm | Binds B-catenin degradation complex preventing B-catenin degradation, upregulates CCL17 and CCL22 | |
| GRA24 | Nucleus | Binds and triggers autophosphorylation of p38 MAPkinase, triggering cytokine production | |
| GRA28 | Nucleus | Upregulates CCL22 | |
| HCE1/TEEGR | Nucleus | Binds to E2F3/4, upregulates cell cycle genes, reduces chromatin access, limits cytokine production | |
| TgIST | Nucleus | Binds STAT1 and Mi2-NuRD complex silencing IGNg responses. | |
| TgNSM | Nucleus | Prevents necroptotic death | |
| MYR-independent | GRA7 | PVM Transmembrane | Binds to ROP18 and IRG6a to increase IRG polymerization |
| GRA12 | PV Lumen | Essential to IRG/GBP degradation | |
| GRA15 | PVM Transmembrane | Binds TRAF, Type II allele activates NFKB | |
| GRA60 | PVM Transmembrane | Associates with ROP18, aids in phosphorylating IRGs | |
| MAF1b | PVM Transmembrane | Mediates association of host mitochondria at PVM |
Figure 1.
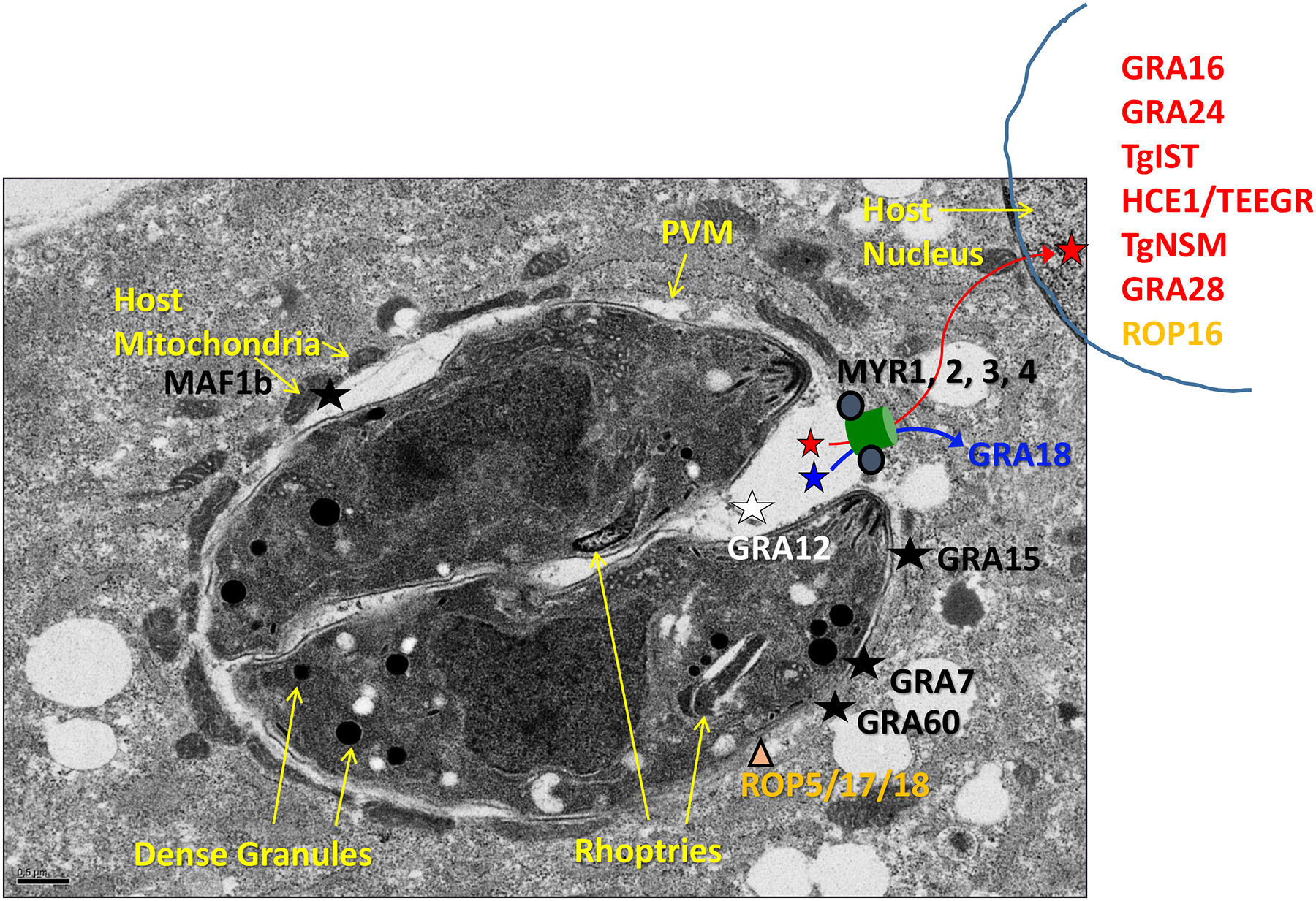
Toxoplasma dense granule effector proteins and their functional locations. After infection, Toxoplasma dense granules secrete a large number of proteins into the parasitophorous vacuole, some of which give the PV unique structure, others of which function in metabolism, and yet others of which are termed effector proteins and localize to various cellular compartments to interact with the host cell. Effectors that transit the PV and then exit via a MYR-dependent mechanism (conceptualized by a green channel but with an as-of-yet unknown structure and mechanism of operation) are then located in the host cytosol (blue) and the host nucleus (red). Effector proteins that contain a transmembrane domain are inserted into the PVM via a MYR-independent mechanism which remains unknown, and are indicated by black. One effector, GRA12, is indicated by white and remains within the PV, associating with the membranous network therein, where it nevertheless has an important impact on host cell functions. The rhoptry proteins ROP5/17/18 that sit on the PVM and interact with GRA effectors are also shown in orange; ROP17 also has a role in the export of PVM-transiting, MYR-dependent GRA effectors.
ROPs represent the first wave of attack
The first effector proteins to be released, the ROP proteins, are derived from the rhoptry organelles and are thought to be injected into the host cytoplasm as a single injection event at the earliest stages of invasion [1, 2]. This acts as a way to rapidly alter host signal transduction mechanisms, such as preventing apoptosis and subverting innate immune responses, e.g., through the action of a nuclear-localized tyrosine kinase, ROP16 [3, 4], which, depending on the allele present within a given strain, can phosphorylate host STAT3/6 in under a minute [5]. The strain-specificity of this effect is a common aspect of many of the most important effectors encoded within the Toxoplasma genome [6]. Indeed, extreme variation in both sequence and copy number has been a characteristic that has led to the designation of many Toxoplasma proteins as candidate effectors. Although the evolutionary pressures driving this variability between strains cannot be definitively known, many have speculated that the different strains may have evolved in ecosystems where one or more hosts are especially common and so the strains have evolved mechanisms to efficiently and specifically infect those hosts; when in a host that a given strain does not normally see, the results are unpredictable with consequences that can exacerbate or block the infection. For purposes of understanding what follows below, it is also important to know that Toxoplasma gondii has a clonal population structure with just a few very dominant clonotypes in Europe and North America. The three most common are referred to as Types I, II and III, respectively [7]. The hosts that each Type naturally co-evolved with are not known but all three are commonly seen in humans and their domestic livestock.
Many ROPs localize to the host-cytosolic surface of the PV where they can mitigate other effects of the immune response (Fig. 1). Examples of this class of effectors are the pseudokinase ROP5 and its serine/threonine kinase partners, ROP17/18 which again depending on the strain of parasite, can cooperate to phosphorylate and thereby inactivate host immunity-related GTPases (IRGs; [8–10]). Although these are crucial ways in which host defenses are rapidly and effectively subverted, this review is focused on the second wave of effectors to be introduced, the GRA proteins which emanate from the parasite’s dense granules. Much more on how rhoptry secretion is triggered and how ROP effectors operate can be found in the comprehensive article by Chaabene et al., in this same issue.
GRA effectors provide a second wave that can associate with or even transit across the PVM
Once the initial stages of invasion are complete, but possibly before the parasite has been fully engulfed within the parasitophorous vacuole membrane (PVM) that will ultimately surround it, dense granules secrete their contents (“GRAs”) into the nascent PV. These GRAs are ultimately found in various compartments [reviewed in [11] and [12]], including: 1) within the PV lumen, some being involved in the generation of an elaborate, nanotubular network; 2) within and on the PVM, including its host-cytosolic face, and in one case leading to the association of host mitochondria (see below); and 3) within the host cytosol and nucleus after being exported out of the PV. In addition to being secreted by intracellular parasites into the PV lumen, GRAs can be secreted by extracellular parasites, enabling the parasites to impact the extracellular milieu, thereby potentially impacting uninfected cells and general tissue functions [13, 14]. Lastly, and although the data for this is incomplete, there is some evidence that the dense granules may not be homogenous with respect to their protein cargo, possibly allowing the parasite a finer level of control in the packaging and timing of export of GRA effectors [12].
For many years, the PVM, once formed, was viewed as impermeable to parasite proteins present within the PV space (ROP effectors had been identified outside the PVM but they are believed to be directly injected during invasion, before PVM formation). In 2011, however, a GRA effector protein that modulates host NFκB activity, GRA15, was identified at the outer surface of the PVM, representing a new category of “PVM-attached” GRA effectors [15]. In 2013, the discovery of the first dense granule protein to exit the PV and be found within the host nucleus, GRA16, indicated that GRA effectors can also transit across the PVM as soluble entities and represent a second class of effector, “PVM-transiting” [16]. Immunofluorescence assays revealed that GRA16 traffics through the dense granules within the parasite, is secreted into the PV, where some accumulates and some is further trafficked across the PVM, ultimately reaching the host nucleus in significant (readily visible) amounts [17]. It was the combination of both a predicted export signal sequence and a nuclear-localization signal (NLS) that led Bougdour et al. to hypothesize that GRA16 could be a PVM-transiting effector and, since that time, similar logic has been used by several groups to identify additional such effectors, as detailed below.
Interestingly, in addition to these key characteristics, several of the PVM-transiting GRA effectors are subject to post-production proteolytic cleavage by the aspartyl protease 5 enzyme (ASP5) within the Golgi [18, 19]. The role of this cleavage is not known but it is required for GRA16’s efficient secretion and exit out of the PV (i.e., GRA16 versions with a mutated cleavage site that is not recognized by ASP5 are not seen within host nuclei [20]). PVM-transiting GRA effectors also tend to be not too large (under 800 amino acids in the primary translation product), have multiple repeated domains to interact with host proteins, and be predicted to have one or more intrinsically disordered domains. It is possible that this lack of structure allows these proteins to be transported out of the parasitophorous vacuole [20, 21].
Translocation of GRA effectors across the PVM is mediated by a novel complex
How the PVM-transiting effectors are trafficked out from the PV and across the PVM is not yet entirely clear. In the related Plasmodium parasites, a system for translocation across the PVM has been well characterized and is commonly referred to as PTEX for “Plasmodium translocon of exported proteins” [22]. As the name implies, PTEX includes a translocon, chaperone and other associated proteins [23]. As with GRA16, licensing of exported proteins for translocation appears dependent on cleavage by the Plasmodium equivalent of ASP5, albeit within the parasite’s endoplasmic reticulum rather than within the Golgi used by Toxoplasma. It seemed likely, therefore, that a machinery homologous to PTEX might be operating in Toxoplasma. Indeed Toxoplasma has homologues of several of the PTEX components, including the translocon itself. Upon further investigation, however, the two Toxoplasma homologues of PTEX that span the PVM, GRA17 and GRA23, function to form a channel for the import/export of small molecules under 2–3 kDa (ions, metabolites, etc.) across the PVM but they appear to play no role in the translocation of proteins across this barrier [24, 25].
Identification of Toxoplasma tachyzoite’s unique system of exporting proteins from the PV came from genetic studies aimed at identifying (a) novel effector(s). In brief, a 2013 observation that host cells infected by Toxoplasma but not Neospora tachyzoites upregulate host c-Myc [26] led Franco et al. to hypothesize that this effect was mediated by one or more effector proteins, and search for that effector by selecting for “Myr−“ mutants, i.e., mutants that are deficient in host Myc regulation [27]. Instead of finding the effector protein(s), however, this selection ultimately yielded four genes necessary for the export of seemingly all PVM-transiting GRA effectors [21, 27, 28]: MYR1, MYR2, MYR3 and the previously studied rhoptry protein kinase, ROP17. MYR1, MYR2 and MYR3 contain transmembrane domains and are thought to span the parasitophorous vacuolar membrane. Interestingly, MYR1 is cleaved by the same Golgi-resident aspartyl protease ASP5 that cleaves some of the GRA effector proteins [19] although the two pieces of MYR1 are kept in close apposition even after cleavage by one or more disulfide bonds [21]. Interestingly, while ASP5 function is necessary for translocation of GRAs out of the PV, its cleavage of MYR1 is not required as myr1 mutants with an altered ASP5-processing site show no apparent phenotype, including no effect on effector translocation [29].
Immunoprecipitation of MYR1-associating proteins identified at least three more components that associate with the MYR complex, all of which are required for GRA translocation across the PVM: the previously uncharacterized MYR4, a predicted protein phosphatase GRA44, and GRA45 [28, 30]. GRA45 was also identified in a CRISPR-screen for genes necessary for Toxoplasma tachyzoites to survive in macrophages. This latter work showed that GRA45 has a chaperone-like domain that is necessary for its role in moving GRAs across the PVM [31]. Thus, a total of 8 proteins (MYR1–4, GRA44/45, ROP17 and ASP5) are now known to all be required for this process. Interestingly, the enigma of why MYR1 is cleaved by ASP5 when ablating such cleavage has no measurable impact on effector translocation was extended by the finding that this is also true for MYR4, GRA44 and GRA45; all three are cleaved by ASP5 yet preventing cleavage by mutating the ASP5 target site had no measurable impact on their role in effector translocation for any of them [28]. While mutations disrupting ASP5 cleavage in each individual target had no measurable impact on translocation, it is possible that if the cleavage sites in all four proteins were simultaneously disrupted a cumulative effect would be observed. It is certainly hard to imagine that the cleavages serve no purpose and so the assays performed to date may just not have been sensitive enough.
The mechanism by which the MYR complex translocates proteins remains unknown, as does the energy source. However, the above work did give one clue to how this system might be regulated, as ROP17 was among the proteins necessary for the increased expression of host c-Myc. As mentioned above, this rhoptry protein kinase is injected early during invasion and coats the host-cytosolic surface of the PVM where it can phosphorylate and inactivate the host Immunity related GTPase (IRG) molecules that otherwise will destroy the PV [8]. ROP17’s kinase activity is also necessary for the translocation of GRA effectors out of the PV suggesting that it might be a way to activate the translocation system; i.e., only when the MYR proteins are secreted out of the parasites and integrated into the PVM are they accessible to phosphorylation by ROP17 [32]. This possibility is further supported by the fact that co-infecting a host cell with a wild type parasite rescues GRA translocation across the PVM enclosing a rop17 knockout mutant. Thus the activity of ROP17 is needed only outside the PVM, rather than some role within the parasite that might otherwise have been invoked.
PVM-transiting GRAs are crucial for growth in vivo but not in vitro
All evidence to date supports the role of MYR-dependent effectors playing a more substantial role during in vivo infection than in vitro infection. Loss of MYR-dependent translocation translates into a loss of parasite virulence and survival of the mouse, both in Type I and Type II parasites at doses that would normally be lethal on day 7 and 12, respectively [27]. On the other hand, initial in vitro Toxoplasma growth assays monitoring parasites per vacuole at 20 hours failed to discern a statistically significant difference in growth between myr knockout and wild type parasites, and it was only upon measuring plaque size 6 days post-infection that a statistically significant decrease in size was noted for the knockout’s plaques [21].
The more substantial impact of losing GRA translocation on in vivo vs. in vitro growth gives some clues as to the host functions that MYR-dependent effectors impact. At least six MYR-dependent effectors have been described to date: GRA16, GRA24, TgIST, HCE1/TEEGR, GRA18 and TgNSM, as well as a possible seventh, GRA28, as this protein also goes to the nucleus [33] and some of its known effects are MYR-dependent [34, 35], although its translocation has not been directly shown to rely on the MYR machinery. The roles of each of these GRA effectors are described further below; all but GRA18 are found in the host nucleus, and each has a profound impact on the host transcriptome. The totality of transcriptomic changes in the host cell during Toxoplasma infection can be broken into those responses that are MYR-dependent vs. those that are MYR-independent, the latter being due to the actions of the ROPs and MYR-independent, PVM-associating GRAs [29, 36]. The responses can be further subdivided into those that increase and those that decrease the RNA levels of various host genes. In some cases, certain transcriptional changes caused by MYR-dependent effectors have been observed to counter or mask the effects of MYR-independent effects. These countervailing effects have been described to maintain transcription of certain genes at the levels observed in uninfected cells [29].
In totality, MYR-dependent transcriptional changes are observed in about 2000 genes 6 hours after infection of host cells in vitro [29]. When analyzed by gene set enrichment analysis (GSEA), the most significantly upregulated gene sets are those that are involved in cell cycle regulation, and the most significantly downregulated are those that are involved in cytokine responses. To a lesser degree, but also significant, are those responses that involve host metabolism. Consistent with these findings are the functions ascribed to the various PVM-associating and PVM-transiting effector proteins identified to date. These will each be discussed below, under the broad category of the impacts each imparts.
GRA16 and HCE1/TEEGR impact pathways associated with the host cell cycle
Cells infected with Toxoplasma exhibit significant cell cycle alteration, and while the specifics of the alteration vary by cell type, it has generally been reported that Toxoplasma tachyzoites advance the cell into S phase [37] and arrest it in the G2 phase, prior to mitosis [14, 38]. Under certain circumstances this results in a binucleated phenotype as the infected cells fail to undergo proper cytokinesis during mitosis [39]. GRA16, the first identified PVM-transiting GRA, goes to the host nucleus where it directly impacts cell cycle-associated pathways. In particular, its ability to trigger the p53 pathway, which is associated with cell cycle arrest in response to DNA damage, has been identified to be a result of its N-terminal domain interacting with the host deubiquitinase, Herpesvirus-associated ubiquitin-specific protease (HAUSP) [17], leading to increased levels of Phosphatase and tensin homologue (PTEN) [40]. PTEN acts as a potent tumor suppressor by dephosphorylating phosphatidylinositol-3,4,5-trisphosphate, though it is reported to also have protein tyrosine phosphatase activity and the ability to function as a scaffold [41]. GRA16 has two independently functioning domains, however: the N-terminal domain prior to amino acid 237 is capable of binding to HAUSP while the C terminal domain is able to bind to PP2A-B55 molecule [17], the regulatory subunit that targets the PP2A phosphatase to c-Myc, among other targets. As such, GRA16 expression by Toxoplasma can induce significant amounts of the cell-cycle regulator c-Myc [42], most likely by preventing the proteolytic degradation of phosphorylated c-Myc. When GRA16 is over-expressed in human cells it has a pleiotropic effect, impacting pathways involved in glycolysis, metabolism, and also an impact on the cell cycle through reducing the levels of cyclin A [17]. This ability to degrade cyclin A may limit the amount of active CDK1 kinase which is necessary to progress into mitosis, though further experiments will be necessary to see if such a hypothesis bears out. Although studies have indicated that the N-terminal and C-terminal domains of GRA16 can function independently, there is no evidence that the two domains are ever separated and so their coordinated action is likely of some, as-yet-unknown, benefit to the parasite.
In the previously mentioned transcriptomic studies of cells infected with myr1 knockout parasites, expression levels of genes related to the E2F family of transcription factors were significantly higher than in uninfected cells or those infected with wild type parasites [29]. In 2019, two reports identified a novel effector protein originating from the dense granules that functioned by directly interacting with E2F3 and E2F4 [43, 44], and that was responsible for the substantial upregulation of host cyclin E and CDK2, crucial regulators of entry into the S phase, in infected cells [43]. This new protein was dubbed both HCE1, because it was associated with substantial expression of host cyclin E and cyclin E-associated genes, and TEEGR for Toxoplasma E2F4-associated EZH2-inducing Gene Regulator [44]. HCE1/TEEGR has no predicted ASP5 cleavage site and exhibits no indication of ASP5-cleavage, yet its translocation out of the PV is ASP5-dependent. It has a nuclear localization signal (NLS), two large repeated domains of approximately 90 amino acids each, followed by a stretch of 24 amino acids repeated five times, and finally a C-terminal domain that is predicted to be disordered, a common characteristic of PVM-transiting GRA effectors. As predicted by its NLS and demonstrated binding to E2F transcription factors, HCE1/TEEGR localizes to the host nucleus. Interestingly, the related Apicomplexan Neospora caninum has a homolog of HCE1/TEEGR (BN104_015825) but it shares a low level of amino acid identity to the Toxoplasma version and appears to lack the latter’s biological functions.
Because the impact on cell cycle is intimately associated with changes in metabolism [45], such as steroid and lipid metabolism, it is possible that the selective advantage conferred by GRA16 and HCE1/TEEGR is creation of a more growth-permissive metabolic state (e.g., the G2 phase is when the host cell is itself growing and importing nutrients from the environment). Alternatively, the selective advantage conferred by HCE1/TEEGR could be a dampened immune response as a result of the chromatin condensation that occurs during G2.
GRA15 and HCE1/TEEGR impact NFκB, a critical node in the host immune response
Control of Toxoplasma relies crucially on the host’s anti-intracellular pathogen defenses, controlled by the IL-12/IFNγ cytokine axis that induces the Th1 branch of the immune system. It has long been known that IFNγ is central to the regulation of Toxoplasma in its ability to induce indoleamine dioxygenase (IDO) and degrade tryptophan, essential to Toxoplasma growth, as well as induce cytotoxic T cells and induce the host to express innate immune molecules that kill intracellular organisms [46]. In murine hosts, induction of the Th1 arm begins when dendritic cells, macrophages, neutrophils and inflammatory monocytes register infection by TLR11/12 binding to Toxoplasma’s profilin [47]. How profilin, which is an intracellular protein, is recognized by host TLR11/12 is not yet known but this interaction leads to the activation of NFκB, a transcription factor that promotes the production of IFNγ. Without IFNγ, normally nonlethal doses of Toxoplasma strain ME49 induce mortality beginning 6 days after infection [48]. Therefore, it is of no surprise that the parasite has evolved a substantial number of effector proteins to block the Th1/IL-12/IFNγ response in infected cells.
The NFκB family of transcription factors includes five components, p50, p52, RelA, RelB, and c-Rel. These cytoplasmic transcription factors form homo- and heterodimers to trigger transcription of immune genes related to combatting intracellular pathogens, and each combination of homo- or heterodimer can target different genes. Toxoplasma subverts NFκB signaling via a myriad of ways. First, Toxoplasma tachyzoites secrete GRA15, a PVM-associated effector whose N-terminal domain interacts with TRAF [49] to induce dimerization of various NFκB subunits and translocation to the nucleus. GRA15 from Type II, but not Types I and III strains, induces activation and translocation of RelA (p65) to the host nucleus, which promotes IL-12 and classical activation of infected macrophages [15, 50]. In human monocytes, this induction of NFκB can also lead to the production of substantial amounts of IL-1b [51].
To antagonize NFκB signaling, it is reported that HCE1/TEEGR binding of E2F3/4 leads to production of the chromatin remodeler EZH2. In switching on EZH2, the host chromatin is remodeled to be non-permissive to access by NFκB, effectively muting nuclear NFκB [44]. Thus, GRA15 and HCE1/TEEGR, at least, are impacting the same pathway in different ways. The possibility that additional effectors are intersecting this pathway seems likely, given its centrality to so much of how the immune system responds to infection.
GRA24 works through p38 MAPkinase to activate cytokine responses
Also involved in altering the IL-12/IFNγ cytokine axis is the effector GRA24. Similar to HCE1/TEEGR, this protein’s export depends on ASP5 processing but it, itself, shows no evidence of cleavage by the enzyme. GRA24 is an effector protein that has two identical kinase-interacting motifs (KIMs) capable of binding to the host’s p38 MAPkinase molecule and competing off the host regulatory elements suppressing the p38 response. In binding p38, GRA24 triggers autophosphorylation of the kinase leading to sustained kinase activity, bypassing the entire upstream activation [52]. This impacts host cells by altering early cytokines including IL-12 and MCP1.
GRA7, GRA12 and GRA60 help disarm a key host defense
In cases where Toxoplasma is entering a cell exposed to IFNγ, it must combat the downstream protein products of productive interferon signaling and it does this by undoing the chromatin modeling effects of IFNγ in infected cells, and finally eliminating the immunological effector proteins that are triggered by this cytokine. Direct killing of the parasite by protein products of interferon-regulated genes, including a number of immunity related GTPases (IRGs) in mice and GTP-binding proteins (GBPs) that coat and puncture the parasitophorous vacuole, is directly combated by Toxoplasma effector proteins. The ability of a Toxoplasma strain to combat these IRGs and GBPs is directly correlated with the strain’s virulence in mice suggesting a crucial role in this intermediate host, at least. As already mentioned, serine-threonine kinases from the rhoptries, ROP17 and ROP18, are injected into the host cell’s cytoplasm during invasion and coat the outside of the PVM along with a pseudokinase ROP5 that holds the IRG in proper conformation for ROP18 to phosphorylate [53]. Degradation of IRGs then occurs, neutralizing their impact. Several dense granule proteins are required for optimal IRG clearance: GRA7 increases the turnover of the IRG molecules by binding to a conserved domain of GBPs, promoting polymerization and ultimately rapid turnover via making substrates available for ROP18 [54]. GRA12, a protein associated with the nanotubular network that composes the PVM, is essential for the destruction of IRGs and GBPs, though how it does this has yet to be elucidated [55–57]. And finally, the recently discovered GRA60 is an intrinsically disordered protein that is found in the parasitophorous vacuole, inserts into the PVM with its N-terminal domain exposed to the host cytosol where it functions to neutralize IRGa6 and IRGb10 in concert with ROP18 [58]. These are all excellent examples of the cooperation between ROPS and GRAs in attacking host defenses.
TgIST neutralizes STAT1 activity in the host cell nucleus
Signaling in response to IFNγ occurs when the IFNγ-receptor, composed of IFNGR1 and IFNGR2, is bound by its ligand, dimerizing the receptor and triggering autophosphorylation of the associated intracellular Janus-associated kinases (JAKs). In the classical pathway of JAK/STAT activation, these phosphorylate and activate signal-transducer-and-activator-of-transcription (STAT1) molecules associated with them. The STAT1 molecules dimerize following phosphorylation and reveal a nuclear localization signal, translocating to the nucleus and then binding to gamma-activated sites (GASs) in the promoters of ISGs (for review, see [59]). In the presence of Toxoplasma infection, STAT1 dimers are still found to be phosphorylated, translocated to the nucleus and bound to GAS, but the production of all STAT1-dependent GAS-regulated gene products is stopped [60, 61]. Toxoplasma accomplishes this by introducing a PVM-transiting dense granule effector protein named Inhibitor of STAT1 Transcriptional activity (TgIST) [62, 63]. TgIST binds independently to both phosphorylated STAT1 dimers and the nucleosome remodeling and deacetylase Mi2/NURD complex, bringing a chromosome remodeling repressive complex to the DNA loci normally targeted by STAT1 for activation. In doing so, it causes a tight repression of these loci and the shut-off of all STAT1-targeted genes, effectively blocking interferon signaling. As a result, Toxoplasma is able to use TgIST to shut off host transcription of ISGs, such as IDO [64]. Because STAT1 is a part of the homodimers that respond to type II interferon signaling from IFNγ as well as the STAT1/STAT2 heterodimers transducing the type I interferon signaling pathway, TgIST is also able to block interferon beta signaling [65].
As of the date of this publication, there are two bioRxiv submissions that suggest there is a deeper level of complexity to the story. Survival following interferon treatment by parasites lacking MYR1 was significantly lower than those with single deletions in TgIST, suggesting another PVM-transiting effector may be playing a role [66]. Separately, a newly identified effector protein encoded by TgGT1_235140 is purported to work in concert with TgIST to suppress necrosis related genes following interferon treatment [67], and our own unpublished work suggests that this protein is a MYR-dependent effector with suppressive effects based on transcriptomic work.
GRA18 and GRA28 subvert host cell recruitment to the site of infection
Recruiting the correct branch of the immune system to the location of pathogens is crucial for fighting an infection, and Toxoplasma can actively manipulate the host to recruit the wrong branch of the immune system. Activation of the Th2 branch of the immune system can actively suppress anti-parasite Th1 responses. Toxoplasma utilizes the effector protein GRA18 to turn on chemokine expression of Th2 chemokines [68]. In contrast to all other PVM-transiting effectors so far identified, GRA18 functions not in the host nucleus but from within the host cytoplasm. There, it forms a complex with other members of the beta-catenin destruction complex GSK3, PP2A-B56 and beta-catenin itself, thus preventing beta-catenin’s destruction. As a result, there is accumulation of beta-catenin which acts as a transcription factor in the nucleus, activating the WNT pathway. Expression of GRA18 alone in cells demonstrates its ability to upregulate cytokines associated with chemotaxis, including CCL17, CCL22 and CCL24. Both CCL17 and CCL22 utilize the CCR4 receptor, with the former reducing Treg expansion and promoting activation of Th1 and Th17 related cells in murine models [69]. CCL22, competing for the same receptor, attracts dendritic cells, NK cells, and antigen experienced T cells, particularly those with a Th2 phenotype [70]. CCL24 is well known to recruit eosinophils and basophils, also potentially amplifying a Th2 loop. Significant upregulation of CCL22 was observed in trophoblasts infected with Toxoplasma [34], and a recent bioRxiv paper suggests that this is caused by the dense granule protein GRA28 [35]. Originally described in 2016 through proximity labeling of proteins in the parasitophorous vacuole, GRA28 transits to the host nucleus [33]. GRA28 is substantially bigger than other known PVM-transiting effectors, being ~2800 amino acids compared to a range of ~400–800 amino acids for the majority, and yet the bioRxiv report argues that just the N terminal domain is necessary for the observed CCL22-induction phenotype. As all other nuclear-located dense granule proteins require MYR1 to exit the parasitophorous vacuole, it seems likely that GRA28 does as well but this has yet to be directly tested.
MAF1 is a PVM-associating effector that mediates association with host mitochondria
It has been observed that cells infected with Type I and Type III tachyzoites have their PVMs surrounded by closely apposed host mitochondria [71]. This remarkable phenotype is mediated by the product of a subset of genes within a tandemly repeated locus that is variable in sequence and gene number, depending on the strain. The particular version that mediates the mitochondrial association is termed mitochondrial-association factor 1b (MAF1b) [72, 73]. MAF1b possesses a predicted transmembrane domain which is presumed to be responsible for its association with the PVM. The close proximity to the host mitochondria potentially gives tachyzoites access to key metabolites and/or it might alter immune signals that originate from the surface of the mitochondria; there is also evidence that these PVM-associated mitochondria may also have a detrimental impact on growth by taking up essential fatty acids that the parasite has liberated by host autophagy [74]. The associating mitochondria may also be somewhat dysfunctional [75]. Regardless of which impact is most important, the data are clear that this association is of substantial importance in vivo during the acute and, possibly, chronic stages of the infection [76].
Conclusions, open questions, and enigmas
Many Toxoplasma GRA proteins are secreted effectors that act as crucial virulence factors to promote the parasites’ survival, growth and dissemination in their mammalian hosts. These proteins likely contribute significantly to defining Toxoplasma’s broad host range, including, in some instances, fine-tuning their infectivity to particular subsets of such hosts. Determining which intermediate host is the natural environment for a given strain of Toxoplasma is an almost impossible challenge, conceptually and logistically, but regardless, knowing how the different versions of effectors like GRA15 impact virulence in a wide range of mammals, including humans, is of great practical importance. Comparative studies with related genera, such as Hammondia and Neospora, may also provide crucial information as to the specific roles these rapidly evolving effectors play. Likewise, almost all the studies described here have been done with tachyzoites; how bradyzoites and the sexual stages, which are almost completely unexplored, do their own co-opting of host functions has yet to be investigated and will almost certainly yield many more, no less interesting mechanisms.
Identifying the entire spectrum of dense granule parasite effectors used by tachyzoites may also be on the near horizon, as recently developed techniques can be more comprehensive in their scope than the one-at-a-time candidate gene approach. For example, such new techniques can examine the whole genome under different conditions (e.g., CRISPR screens) or all Toxoplasma proteins exported to different compartments, including within the infected host cell (e.g., proximity labelling).
With an increasing number of effector proteins identified, it will be possible to examine their concerted interactions. A good paradigm for this is the involvement of multiple effector proteins in the tuning of the host NFκB and IFNγ responses, some acting as positive stimuli and some negative. But the impact of GRA effectors must be considered in conjunction with ROPs and other molecules that can impact the host cell. This interplay is perhaps epitomized by the interaction of ROP17 and the MYR machinery, and the cooperation between GRA7/12/60 working with ROP5/17/18 to neutralize IRGs attacking the PVM. As the complete inventory of all effectors is established and methods to dissect their impacts in concert are developed, many more such examples are almost certain to emerge.
In addition to the important biology yet to be done, there are many unanswered questions about the biochemistry of GRA effectors. For example, how the MYR system exports proteins is a complete mystery; neither the identity of the actual translocon, nor the role of the many components so far identified have been determined. Likewise, it is still unclear how the PVM-attached effectors (those with transmembrane domains) are inserted into this crucial membranous barrier between the parasite and host cell. It is tempting to speculate that there may also be other mechanisms of protein transport employed by the parasite, such as the linearly arranged “beads on a string” or “evacuoles” that appear to be vesicles that lead away from a given PV, in some cases reaching all the way to the host nucleus or another PV. The genesis and function(s) of these structures are not known and yet they contain a sampling of the GRA and ROP proteins comprising the PV and PVM. They may well be the result of vesiculation at the PVM and they could represent a novel way in which proteins are delivered to other compartments within the infected host cell.
Overall, then, the interplay of the extensive and complex repertoire of GRA and ROP effectors whose export differs in timing and mechanism allows for a level of finesse in control that we have only started to unravel. Exciting times await!
Acknowledgements
We are grateful to Dr. Lena Pernas for use of her EM image and all our colleagues for their helpful input to this review. The work described here from our laboratory was funded by NIH RO1 AI021423 and AI129529. The authors declare no financial or other conflict of interest.
References
- 1.Kemp LE, Yamamoto M, Soldati-Favre D. Subversion of host cellular functions by the apicomplexan parasites. FEMS Microbiol Rev. 2013;37(4):607–31. doi: 10.1111/1574-6976.12013. [DOI] [PubMed] [Google Scholar]
- 2.Boothroyd JC, Dubremetz JF. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat Rev Microbiol. 2008;6(1):79–88. doi: 10.1038/nrmicro1800. [DOI] [PubMed] [Google Scholar]
- 3.Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445(7125):324–7. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto M, Standley DM, Takashima S, Saiga H, Okuyama M, Kayama H, Kubo E, Ito H, Takaura M, Matsuda T, Soldati-Favre D, Takeda K. A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J Exp Med. 2009;206(12):2747–60. doi: 10.1084/jem.20091703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong YC, Reese ML, Boothroyd JC. Toxoplasma rhoptry protein 16 (ROP16) subverts host function by direct tyrosine phosphorylation of STAT6. J Biol Chem. 2010;285(37):28731–40. doi: 10.1074/jbc.M110.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukhopadhyay D, Arranz-Solis D, Saeij JPJ. Influence of the Host and Parasite Strain on the Immune Response During Toxoplasma Infection. Front Cell Infect Microbiol. 2020;10:580425. doi: 10.3389/fcimb.2020.580425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172(6):1561–6. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 8.Etheridge RD, Alaganan A, Tang K, Lou HJ, Turk BE, Sibley LD. The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host Microbe. 2014;15(5):537–50. doi: 10.1016/j.chom.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murillo-Leon M, Muller UB, Zimmermann I, Singh S, Widdershooven P, Campos C, Alvarez C, Konen-Waisman S, Lukes N, Ruzsics Z, Howard JC, Schwemmle M, Steinfeldt T. Molecular mechanism for the control of virulent Toxoplasma gondii infections in wild-derived mice. Nat Commun. 2019;10(1):1233. doi: 10.1038/s41467-019-09200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunn JP, Feng CG, Sher A, Howard JC. The immunity-related GTPases in mammals: a fast-evolving cell-autonomous resistance system against intracellular pathogens. Mamm Genome. 2011;22(1–2):43–54. doi: 10.1007/s00335-010-9293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clough B, Frickel EM. The Toxoplasma Parasitophorous Vacuole: An Evolving Host-Parasite Frontier. Trends Parasitol. 2017;33(6):473–88. doi: 10.1016/j.pt.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Mercier C, Cesbron-Delauw MF. Toxoplasma secretory granules: one population or more? Trends Parasitol. 2015;31(11):604. doi: 10.1016/j.pt.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Leroux LP, Dasanayake D, Rommereim LM, Fox BA, Bzik DJ, Jardim A, Dzierszinski FS. Secreted Toxoplasma gondii molecules interfere with expression of MHC-II in interferon gamma-activated macrophages. Int J Parasitol. 2015;45(5):319–32. doi: 10.1016/j.ijpara.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Wong ZS, Sokol-Borrelli SL, Olias P, Dubey JP, Boyle JP. Head-to-head comparisons of Toxoplasma gondii and its near relative Hammondia hammondi reveal dramatic differences in the host response and effectors with species-specific functions. PLoS Pathog. 2020;16(6):e1008528. doi: 10.1371/journal.ppat.1008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KD, Saeij JP. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med. 2011;208(1):195–212. doi: 10.1084/jem.20100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bougdour A, Tardieux I, Hakimi MA. Toxoplasma exports dense granule proteins beyond the vacuole to the host cell nucleus and rewires the host genome expression. Cell Microbiol. 2014;16(3):334–43. doi: 10.1111/cmi.12255. [DOI] [PubMed] [Google Scholar]
- 17.Bougdour A, Durandau E, Brenier-Pinchart MP, Ortet P, Barakat M, Kieffer S, Curt-Varesano A, Curt-Bertini RL, Bastien O, Coute Y, Pelloux H, Hakimi MA. Host cell subversion by Toxoplasma GRA16, an exported dense granule protein that targets the host cell nucleus and alters gene expression. Cell Host Microbe. 2013;13(4):489–500. doi: 10.1016/j.chom.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Hammoudi PM, Jacot D, Mueller C, Di Cristina M, Dogga SK, Marq JB, Romano J, Tosetti N, Dubrot J, Emre Y, Lunghi M, Coppens I, Yamamoto M, Sojka D, Pino P, Soldati-Favre D. Fundamental Roles of the Golgi-Associated Toxoplasma Aspartyl Protease, ASP5, at the Host-Parasite Interface. PLoS Pathog. 2015;11(10):e1005211. doi: 10.1371/journal.ppat.1005211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coffey MJ, Sleebs BE, Uboldi AD, Garnham A, Franco M, Marino ND, Panas MW, Ferguson DJ, Enciso M, O’Neill MT, Lopaticki S, Stewart RJ, Dewson G, Smyth GK, Smith BJ, Masters SL, Boothroyd JC, Boddey JA, Tonkin CJ. An aspartyl protease defines a novel pathway for export of Toxoplasma proteins into the host cell. Elife. 2015;4. doi: 10.7554/eLife.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curt-Varesano A, Braun L, Ranquet C, Hakimi MA, Bougdour A. The aspartyl protease TgASP5 mediates the export of the Toxoplasma GRA16 and GRA24 effectors into host cells. Cell Microbiol. 2016;18(2):151–67. doi: 10.1111/cmi.12498. [DOI] [PubMed] [Google Scholar]
- 21.Marino ND, Panas MW, Franco M, Theisen TC, Naor A, Rastogi S, Buchholz KR, Lorenzi HA, Boothroyd JC. Identification of a novel protein complex essential for effector translocation across the parasitophorous vacuole membrane of Toxoplasma gondii. PLoS Pathog. 2018;14(1):e1006828. doi: 10.1371/journal.ppat.1006828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho CM, Beck JR, Lai M, Cui Y, Goldberg DE, Egea PF, Zhou ZH. Malaria parasite translocon structure and mechanism of effector export. Nature. 2018;561(7721):70–5. doi: 10.1038/s41586-018-0469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews KM, Pitman EL, de Koning-Ward TF. Illuminating how malaria parasites export proteins into host erythrocytes. Cell Microbiol. 2019;21(4):e13009. doi: 10.1111/cmi.13009. [DOI] [PubMed] [Google Scholar]
- 24.Gold DA, Kaplan AD, Lis A, Bett GC, Rosowski EE, Cirelli KM, Bougdour A, Sidik SM, Beck JR, Lourido S, Egea PF, Bradley PJ, Hakimi MA, Rasmusson RL, Saeij JP. The Toxoplasma Dense Granule Proteins GRA17 and GRA23 Mediate the Movement of Small Molecules between the Host and the Parasitophorous Vacuole. Cell Host Microbe. 2015;17(5):642–52. doi: 10.1016/j.chom.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwab JC, Beckers CJ, Joiner KA. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc Natl Acad Sci U S A. 1994;91(2):509–13. doi: 10.1073/pnas.91.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franco M, Shastri AJ, Boothroyd JC. Infection by Toxoplasma gondii specifically induces host c-Myc and the genes this pivotal transcription factor regulates. Eukaryot Cell. 2014;13(4):483–93. doi: 10.1128/EC.00316-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franco M, Panas MW, Marino ND, Lee MC, Buchholz KR, Kelly FD, Bednarski JJ, Sleckman BP, Pourmand N, Boothroyd JC. A Novel Secreted Protein, MYR1, Is Central to Toxoplasma’s Manipulation of Host Cells. mBio. 2016;7(1):e02231–15. doi: 10.1128/mBio.02231-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cygan AM, Theisen TC, Mendoza AG, Marino ND, Panas MW, Boothroyd JC. Coimmunoprecipitation with MYR1 Identifies Three Additional Proteins within the Toxoplasma gondii Parasitophorous Vacuole Required for Translocation of Dense Granule Effectors into Host Cells. mSphere. 2020;5(1). doi: 10.1128/mSphere.00858-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naor A, Panas MW, Marino N, Coffey MJ, Tonkin CJ, Boothroyd JC. MYR1-Dependent Effectors Are the Major Drivers of a Host Cell’s Early Response to Toxoplasma, Including Counteracting MYR1-Independent Effects. mBio. 2018;9(2). doi: 10.1128/mBio.02401-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blakely WJ, Holmes MJ, Arrizabalaga G. The Secreted Acid Phosphatase Domain-Containing GRA44 from Toxoplasma gondii Is Required for c-Myc Induction in Infected Cells. mSphere. 2020;5(1). doi: 10.1128/mSphere.00877-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Sangare LO, Paredes-Santos TC, Hassan MA, Krishnamurthy S, Furuta AM, Markus BM, Lourido S, Saeij JPJ. Genome-wide screens identify Toxoplasma gondii determinants of parasite fitness in IFNgamma-activated murine macrophages. Nat Commun. 2020;11(1):5258. doi: 10.1038/s41467-020-18991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panas MW, Ferrel A, Naor A, Tenborg E, Lorenzi HA, Boothroyd JC. Translocation of Dense Granule Effectors across the Parasitophorous Vacuole Membrane in Toxoplasma-Infected Cells Requires the Activity of ROP17, a Rhoptry Protein Kinase. mSphere. 2019;4(4). doi: 10.1128/mSphere.00276-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nadipuram SM, Kim EW, Vashisht AA, Lin AH, Bell HN, Coppens I, Wohlschlegel JA, Bradley PJ. In Vivo Biotinylation of the Toxoplasma Parasitophorous Vacuole Reveals Novel Dense Granule Proteins Important for Parasite Growth and Pathogenesis. mBio. 2016;7(4). doi: 10.1128/mBio.00808-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ander SE, Rudzki EN, Arora N, Sadovsky Y, Coyne CB, Boyle JP. Human Placental Syncytiotrophoblasts Restrict Toxoplasma gondii Attachment and Replication and Respond to Infection by Producing Immunomodulatory Chemokines. mBio. 2018;9(1). doi: 10.1128/mBio.01678-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudzki EN, Ander SE, Coombs RS, Alrubaye HI, Cabo LF, Blank ML, Gutierrez-Melo N, Dubey JP, Coyne CB, Boyle JP. Toxoplasma gondii GRA28 is required for specific induction of the regulatory chemokine CCL22 in human and mouse cells. BIORXIV preprint. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rastogi S, Xue Y, Quake SR, Boothroyd JC. Differential Impacts on Host Transcription by ROP and GRA Effectors from the Intracellular Parasite Toxoplasma gondii. mBio. 2020;11(3). doi: 10.1128/mBio.00182-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molestina RE, El-Guendy N, Sinai AP. Infection with Toxoplasma gondii results in dysregulation of the host cell cycle. Cell Microbiol. 2008;10(5):1153–65. doi: 10.1111/j.1462-5822.2008.01117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brunet J, Pfaff AW, Abidi A, Unoki M, Nakamura Y, Guinard M, Klein JP, Candolfi E, Mousli M. Toxoplasma gondii exploits UHRF1 and induces host cell cycle arrest at G2 to enable its proliferation. Cell Microbiol. 2008;10(4):908–20. doi: 10.1111/j.1462-5822.2007.01093.x. [DOI] [PubMed] [Google Scholar]
- 39.Velasquez ZD, Conejeros I, Larrazabal C, Kerner K, Hermosilla C, Taubert A. Toxoplasma gondii-induced host cellular cell cycle dysregulation is linked to chromosome missegregation and cytokinesis failure in primary endothelial host cells. Sci Rep. 2019;9(1):12496. doi: 10.1038/s41598-019-48961-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SG, Seo SH, Shin JH, Yang JP, Lee SH, Shin EH. Increase in the nuclear localization of PTEN by the Toxoplasma GRA16 protein and subsequent induction of p53-dependent apoptosis and anticancer effect. J Cell Mol Med. 2019;23(5):3234–45. doi: 10.1111/jcmm.14207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee YR, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol. 2018;19(9):547–62. doi: 10.1038/s41580-018-0015-0. [DOI] [PubMed] [Google Scholar]
- 42.Panas MW, Boothroyd JC. Toxoplasma Uses GRA16 To Upregulate Host c-Myc. mSphere. 2020;5(3). doi: 10.1128/mSphere.00402-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panas MW, Naor A, Cygan AM, Boothroyd JC. Toxoplasma Controls Host Cyclin E Expression through the Use of a Novel MYR1-Dependent Effector Protein, HCE1. mBio. 2019;10(2). doi: 10.1128/mBio.00674-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braun L, Brenier-Pinchart MP, Hammoudi PM, Cannella D, Kieffer-Jaquinod S, Vollaire J, Josserand V, Touquet B, Coute Y, Tardieux I, Bougdour A, Hakimi MA. The Toxoplasma effector TEEGR promotes parasite persistence by modulating NF-kappaB signalling via EZH2. Nat Microbiol. 2019;4(7):1208–20. doi: 10.1038/s41564-019-0431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez-Alvarez M, Zhang Q, Finger F, Wakelam MJ, Bakal C. Cell cycle progression is an essential regulatory component of phospholipid metabolism and membrane homeostasis. Open Biol. 2015;5(9):150093. doi: 10.1098/rsob.150093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sturge CR, Yarovinsky F. Complex immune cell interplay in the gamma interferon response during Toxoplasma gondii infection. Infect Immun. 2014;82(8):3090–7. doi: 10.1128/IAI.01722-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plattner F, Yarovinsky F, Romero S, Didry D, Carlier MF, Sher A, Soldati-Favre D. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe. 2008;3(2):77–87. doi: 10.1016/j.chom.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240(4851):516–8. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 49.Sangare LO, Yang N, Konstantinou EK, Lu D, Mukhopadhyay D, Young LH, Saeij JPJ. Toxoplasma GRA15 Activates the NF-kappaB Pathway through Interactions with TNF Receptor-Associated Factors. mBio. 2019;10(4). doi: 10.1128/mBio.00808-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie Y, Wen H, Yan K, Wang S, Wang X, Chen J, Li Y, Xu Y, Zhong Z, Shen J, Chu D. Toxoplasma gondii GRA15II effector-induced M1 cells ameliorate liver fibrosis in mice infected with Schistosomiasis japonica. Cell Mol Immunol. 2018;15(2):120–34. doi: 10.1038/cmi.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gov L, Karimzadeh A, Ueno N, Lodoen MB. Human innate immunity to Toxoplasma gondii is mediated by host caspase-1 and ASC and parasite GRA15. mBio. 2013;4(4). doi: 10.1128/mBio.00255-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pellegrini E, Palencia A, Braun L, Kapp U, Bougdour A, Belrhali H, Bowler MW, Hakimi MA. Structural Basis for the Subversion of MAP Kinase Signaling by an Intrinsically Disordered Parasite Secreted Agonist. Structure. 2017;25(1):16–26. doi: 10.1016/j.str.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reese ML, Shah N, Boothroyd JC. The Toxoplasma pseudokinase ROP5 is an allosteric inhibitor of the immunity-related GTPases. J Biol Chem. 2014;289(40):27849–58. doi: 10.1074/jbc.M114.567057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alaganan A, Fentress SJ, Tang K, Wang Q, Sibley LD. Toxoplasma GRA7 effector increases turnover of immunity-related GTPases and contributes to acute virulence in the mouse. Proc Natl Acad Sci U S A. 2014;111(3):1126–31. doi: 10.1073/pnas.1313501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fox BA, Guevara RB, Rommereim LM, Falla A, Bellini V, Petre G, Rak C, Cantillana V, Dubremetz JF, Cesbron-Delauw MF, Taylor GA, Mercier C, Bzik DJ. Toxoplasma gondii Parasitophorous Vacuole Membrane-Associated Dense Granule Proteins Orchestrate Chronic Infection and GRA12 Underpins Resistance to Host Gamma Interferon. mBio. 2019;10(4). doi: 10.1128/mBio.00589-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michelin A, Bittame A, Bordat Y, Travier L, Mercier C, Dubremetz JF, Lebrun M. GRA12, a Toxoplasma dense granule protein associated with the intravacuolar membranous nanotubular network. Int J Parasitol. 2009;39(3):299–306. doi: 10.1016/j.ijpara.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 57.Wang JL, Bai MJ, Elsheikha HM, Liang QL, Li TT, Cao XZ, Zhu XQ. Novel roles of dense granule protein 12 (GRA12) in Toxoplasma gondii infection. FASEB J. 2020;34(2):3165–78. doi: 10.1096/fj.201901416RR. [DOI] [PubMed] [Google Scholar]
- 58.Nyonda MA, Hammoudi PM, Ye S, Maire J, Marq JB, Yamamoto M, Soldati-Favre D. Toxoplasma gondii GRA60 is an effector protein that modulates host cell autonomous immunity and contributes to virulence. Cell Microbiol. 2020:e13278. doi: 10.1111/cmi.13278. [DOI] [PubMed] [Google Scholar]
- 59.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–86. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 60.Rosowski EE, Nguyen QP, Camejo A, Spooner E, Saeij JP. Toxoplasma gondii Inhibits gamma interferon (IFN-gamma)- and IFN-beta-induced host cell STAT1 transcriptional activity by increasing the association of STAT1 with DNA. Infect Immun. 2014;82(2):706–19. doi: 10.1128/IAI.01291-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider AG, Abi Abdallah DS, Butcher BA, Denkers EY. Toxoplasma gondii triggers phosphorylation and nuclear translocation of dendritic cell STAT1 while simultaneously blocking IFNgamma-induced STAT1 transcriptional activity. PLoS One. 2013;8(3):e60215. doi: 10.1371/journal.pone.0060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gay G, Braun L, Brenier-Pinchart MP, Vollaire J, Josserand V, Bertini RL, Varesano A, Touquet B, De Bock PJ, Coute Y, Tardieux I, Bougdour A, Hakimi MA. Toxoplasma gondii TgIST co-opts host chromatin repressors dampening STAT1-dependent gene regulation and IFN-gamma-mediated host defenses. J Exp Med. 2016;213(9):1779–98. doi: 10.1084/jem.20160340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olias P, Etheridge RD, Zhang Y, Holtzman MJ, Sibley LD. Toxoplasma Effector Recruits the Mi-2/NuRD Complex to Repress STAT1 Transcription and Block IFN-gamma-Dependent Gene Expression. Cell Host Microbe. 2016;20(1):72–82. doi: 10.1016/j.chom.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bando H, Sakaguchi N, Lee Y, Pradipta A, Ma JS, Tanaka S, Lai DH, Liu J, Lun ZR, Nishikawa Y, Sasai M, Yamamoto M. Toxoplasma Effector TgIST Targets Host IDO1 to Antagonize the IFN-gamma-Induced Anti-parasitic Response in Human Cells. Front Immunol. 2018;9:2073. doi: 10.3389/fimmu.2018.02073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matta SK, Olias P, Huang Z, Wang Q, Park E, Yokoyama WM, Sibley LD. Toxoplasma gondii effector TgIST blocks type I interferon signaling to promote infection. Proc Natl Acad Sci U S A. 2019;116(35):17480–91. doi: 10.1073/pnas.1904637116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seizova S, A G, M C, L W, K R, C T. Toxoplasma gondii bradyzoites induce transcriptional changes to host cells and prevent IFNγ-mediated cell death. BIORXIV preprint. 2019. [Google Scholar]
- 67.A R, LD S. Toxoplasma gondii secreted effectors co-opt host repressor complexes to inhibit necroptosis. BIORXIV preprint. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He H, Brenier-Pinchart MP, Braun L, Kraut A, Touquet B, Coute Y, Tardieux I, Hakimi MA, Bougdour A. Characterization of a Toxoplasma effector uncovers an alternative GSK3/beta-catenin-regulatory pathway of inflammation. Elife. 2018;7. doi: 10.7554/eLife.39887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heiseke AF, Faul AC, Lehr HA, Forster I, Schmid RM, Krug AB, Reindl W. CCL17 promotes intestinal inflammation in mice and counteracts regulatory T cell-mediated protection from colitis. Gastroenterology. 2012;142(2):335–45. doi: 10.1053/j.gastro.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 70.Scheu S, Ali S, Ruland C, Arolt V, Alferink J. The C-C Chemokines CCL17 and CCL22 and Their Receptor CCR4 in CNS Autoimmunity. Int J Mol Sci. 2017;18(11). doi: 10.3390/ijms18112306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Melo EJ, de Carvalho TU, de Souza W. Penetration of Toxoplasma gondii into host cells induces changes in the distribution of the mitochondria and the endoplasmic reticulum. Cell Struct Funct. 1992;17(5):311–7. doi: 10.1247/csf.17.311. [DOI] [PubMed] [Google Scholar]
- 72.Pernas L, Adomako-Ankomah Y, Shastri AJ, Ewald SE, Treeck M, Boyle JP, Boothroyd JC. Toxoplasma effector MAF1 mediates recruitment of host mitochondria and impacts the host response. PLoS Biol. 2014;12(4):e1001845. doi: 10.1371/journal.pbio.1001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blank ML, Parker ML, Ramaswamy R, Powell CJ, English ED, Adomako-Ankomah Y, Pernas LF, Workman SD, Boothroyd JC, Boulanger MJ, Boyle JP. A Toxoplasma gondii locus required for the direct manipulation of host mitochondria has maintained multiple ancestral functions. Mol Microbiol. 2018;108(5):519–35. doi: 10.1111/mmi.13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pernas L, Bean C, Boothroyd JC, Scorrano L. Mitochondria Restrict Growth of the Intracellular Parasite Toxoplasma gondii by Limiting Its Uptake of Fatty Acids. Cell Metab. 2018;27(4):886–97 e4. doi: 10.1016/j.cmet.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 75.Syn G, Anderson D, Blackwell JM, Jamieson SE. Toxoplasma gondii Infection Is Associated with Mitochondrial Dysfunction in-Vitro. Front Cell Infect Microbiol. 2017;7:512. doi: 10.3389/fcimb.2017.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.English ED, Boyle JP. Impact of Engineered Expression of Mitochondrial Association Factor 1b on Toxoplasma gondii Infection and the Host Response in a Mouse Model. mSphere. 2018;3(5). doi: 10.1128/mSphere.00471-18. [DOI] [PMC free article] [PubMed] [Google Scholar]


