Abstract
The metastatic process is arduous. Cancer cells must escape the confines of the primary tumor, make their way into and travel through the circulation, then survive and proliferate in unfavorable microenvironments. A key question is how cancer cells overcome these multiple barriers to orchestrate distant organ colonization. Accumulating evidence in human patients and animal models supports the hypothesis that clusters of tumor cells can complete the entire metastatic journey in a process referred to as collective metastasis. Here we highlight recent studies unraveling how multicellular coordination, via both physical and biochemical coupling of cells, induces cooperative properties advantageous for the completion of metastasis. We discuss conceptual challenges and unique mechanisms arising from collective dissemination that are distinct from single cell-based metastasis. Finally, we consider how the dissection of molecular transitions regulating collective metastasis could offer potential insight into cancer therapy.
Keywords: collective metastasis, polyclonal metastasis, CTC clusters, collective invasion, intercellular signaling, intercellular cooperation, nanolumina
INTRODUCTION
There are a number of reasons why it is difficult to directly observe key steps of the metastatic process in human patients: the location of most human tumors in internal organs deep inside the body, the long latency associated with the emergence of clinically evident metastatic disease (up to decades) (Pan et al., 2017), and the limited ability to detect microscopic metastases which already contain millions of cancer cells at the time of detection with current imaging technologies (Erdi, 2012). Nonetheless, snapshots obtained at the time of surgery, blood or tumor biopsies, and imaging provide valuable clues. These snapshots indicate a variety of forms of single or cluster-based dissemination across different cancer types. Importantly, experimental studies have shown that single versus multicellular disseminating tumor cells have remarkable divergences in phenotypes, migratory mechanisms, and success rates during metastasis. Multicellular organization has broad impacts on the capabilities of tumor cells at multiple points in the metastatic process, allowing them to migrate in cooperative and heterogeneous collectives, to better survive the stress of vascular circulation, to evade certain types of immune targeting, and to generate intercellular pro-proliferative signaling networks which drive overt metastasis formation (Figure 1). Though much remains to be uncovered, recent studies have elucidated multiple molecular mechanisms behind each of these features of collective metastasis and unveiled new avenues for development of anti-metastatic therapeutics.
Figure 1.
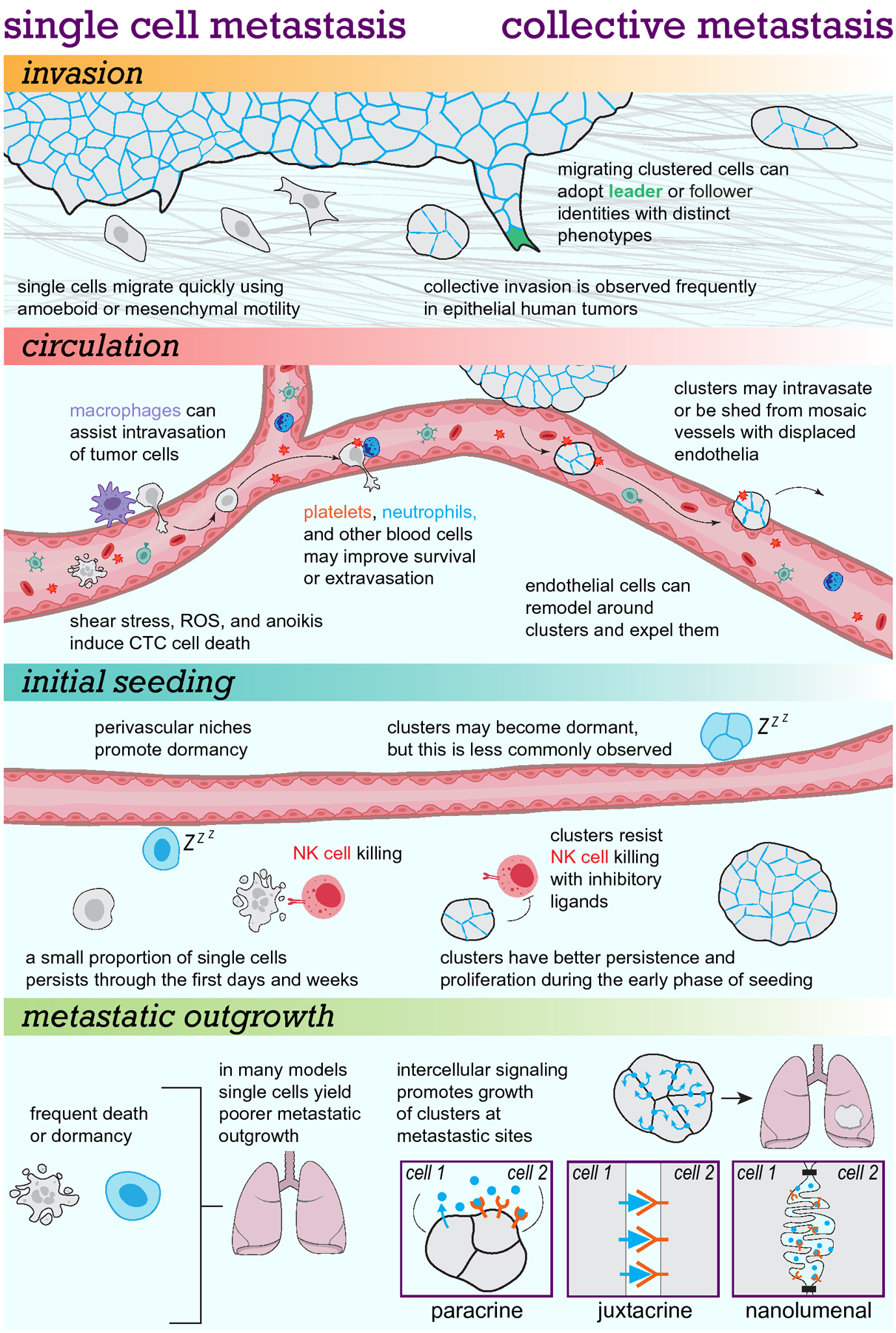
Our emerging understanding suggests key divergences between cluster-based collective metastasis and single cell-based mechanisms throughout the metastatic cascade.
Frequent observations of collective organization during local invasion by primary tumors
An early step in metastasis is local invasion of tumor cells into the surrounding tissues. Many normal epithelial tissues (and neoplastic derivatives such as carcinoma in situ) exist as multicellular collectives fenced behind a basement membrane. Invasive carcinoma is defined by the breach of basement membrane and migration of tumor cells into the surrounding tissue microenvironment and is associated with markedly higher rates of metastatic progression (Hu et al., 2008; Yu et al., 2011). Accordingly, the mechanisms of tumor invasion have been an area of intensive research for decades (Friedl and Alexander, 2011; Friedl et al., 1995; Lambert et al., 2017; Liotta and Kohn, 2001).
In human tumors, pathologists have long noted the presence of tumor “nests” adjacent to primary tumors. One such study in 1960 noted that the tissue bed surrounding tumors was often populated by groups of cells more frequently than single tumor cells, and speculated that these nests might be able to give rise to further tumor growth and dissemination (Leighton et al., 1960). The authors suggested that these aggregates were functioning as integrated units which worked cooperatively through supracellular organization and interactions with the microenvironment, not merely physical groupings of fully independent single cells. They went so far as to recommend development of “aggregate disrupting” agents to sensitize tumor nests to treatment.
Quantitative morphometric studies of invasion are challenging because they require either multiple parallel sections or thick reconstructions of tumor borders to determine if disseminated single cells are truly isolated. 3D reconstructions often reveal hidden connections between single cells and nearby tumor cells, revealing they are actually clusters, or between clusters and the main tumor body, revealing they are actually extensions from the primary tumor (Bronsert et al., 2014; Enderle-Ammour et al., 2017; Jensen et al., 2015; Kudo et al., 2013; Tian et al., 2020; Yoshizawa et al., 2020). In one such study, 3D reconstructions were used to directly quantify the presence of single and clustered tumor buds at tumor stromal borders in human pancreatic, colorectal, lung, and breast adenocarcinomas. After assessing over 5000 tumor buds and over 260,000 cancer cells, they did not observe any individual cells that were not connected to other tumor cells, indicating 100% of adjacent invaded tumor cells were part of collective units (Bronsert et al., 2014). Likewise, another study examined ductal and lobular human breast cancer samples to assess 3D morphology of peritumoral cancer cells (Khalil et al., 2017). Over 99% of invasive ductal carcinoma cells were in multicellular groups in the peritumoral area, and extent of collective invasion correlated with poorer prognosis.
Clusters can also be detected in the process of collectively invading into local lymphovascular channels, and this is frequently associated with poor prognosis. This feature, termed lymphovascular invasion (LVI), denotes the presence of tumor cells in peritumoral vessels or vessels within the tumor mass and frequently manifests as cohesive multicellular emboli (Mohammed et al., 2011). The presence of tumor emboli within lymphatic or blood vessels is correlated with poorer prognosis in pancreatic ductal adenocarcinoma (Takahashi et al., 2020), urothelial carcinoma (Cheng et al., 2009), sporadic colorectal cancer (Lim et al., 2010), and breast cancer (Hamy et al., 2018; Schoppmann et al., 2004). In inflammatory breast carcinoma, tumor emboli are particularly abundant in dermal lymphatics. These emboli strongly express the cell adhesion molecule E-cadherin, associate with the peau de l’orange phenotype observed clinically, and are prone to metastasize (Jolly et al., 2017; Robertson et al., 2010).
At the same time there are notable counterexamples in which invading tumor cells favor discohesion and single cell dissemination. For instance invasive lobular carcinomas, a breast cancer subtype accounting for 5–15% of cases (Weigelt et al., 2010), are associated with loss of function mutations in E-cadherin, single file morphology, and tendency toward individualization to rounded cancer cells (though collective organization of lobular carcinoma is reported in some studies) (Bruner and Derksen, 2018; Ciriello et al., 2015; Khalil et al., 2017). Another breast cancer subtype, metaplastic carcinoma, accounting for <5% of breast cancers (Weigelt et al., 2010), is associated with highly mesenchymal spindle cell morphology and gene expression indicative of epithelial-to-mesenchymal transitions (EMT) (Hennessy et al., 2009; McCart Reed et al., 2019; Taube et al., 2010). Taken together recent morphometric studies suggest a major, but importantly not universal, tendency toward multicellular organization in cancers derived from epithelial tissues.
Circulating tumor cell clusters vary greatly in their size and prevalence in blood
Locally invasive tumors often show increased propensity to metastasize to distant sites. The main routes of escape for tumor cells are drainage via blood vessels and via lymphatics. Since the first descriptions of circulating tumor cells (CTCs) in the blood (Ashworth, 1869), there has been extensive interest in enumerating and isolating rare circulating tumor cells, including circulating tumor cell clusters (CTC clusters). Technological developments in the last 15 years have greatly facilitated direct isolation and analysis of bona fide circulating tumor cells in patients (Aceto et al., 2015; Au et al., 2017; Ferreira et al., 2016; Giuliano et al., 2018; Pantel and Alix-Panabières, 2019). Studies of patient blood samples across the most common cancer types have since conclusively demonstrated that both single and clustered tumor cells are present in the vasculature (Aceto et al., 2014; Chang et al., 2016; Hou et al., 2012; Lee et al., 2017; Long et al., 2016b; Mu et al., 2015; Paoletti et al., 2015; Vona et al., 2004; Wang et al., 2017; Zhang et al., 2017; Zheng et al., 2017).
Overall, these studies indicate that CTCs occur at low concentrations in the peripheral blood. In particular, the number of CTCs appears highly variable per patient. In some cases, metastatic patients will have CTC counts in the 100s to 1000s per 7.5 mL blood draw (Hou et al., 2012; Jansson et al., 2016; Krebs et al., 2011). However, many other metastatic patients will have few or no detectable CTCs. In breast cancer, for example, multiple studies show that roughly 50% of metastatic patients will have fewer than 5 detectable CTCs per 7.5mL of blood (Cristofanilli et al., 2004; Cristofanilli et al., 2019; Larsson et al., 2018; Szczerba et al., 2019). But a number of factors affect CTC and CTC cluster detection rate. A typical tube of blood from a human cancer patient is approximately 7.5 to 10 mL, corresponding to an instantaneous sampling of less than 0.3% of the total blood volume. Thus, some patients with zero reported CTCs could represent false negatives due to insufficient sampling of the blood volume. To some extent, larger volume collection methods such as leukapheresis can overcome this sampling barrier, but at present these more invasive methods are not likely to be integrated into routine practice (Andree et al., 2018; Fehm et al., 2018; Fischer et al., 2013; Kim et al., 2019). Further, CTCs are undersampled temporally. CTC counts vary with cancer treatment, and can markedly drop with tumor shrinkage or rise with tumor progression (Crosbie et al., 2016; Nagrath et al., 2007; Yan et al., 2017; Yu et al., 2013). In addition, the vascular source has an important effect on CTC recovery. Compared with collection from the peripheral veins, blood collection from different draining venous and arterial beds can produce markedly different CTC counts, with increased collection of CTCs from draining veins proximal to the tumor (Buscail et al., 2019b; Crosbie et al., 2016; Kim et al., 2019; Nagrath et al., 2007; Reddy et al., 2016; Sun et al., 2018). In principle, the steady-state number of tumor cells in the blood could be reduced by features that shorten their half-lives in the blood. For instance, the greater hydrodynamic resistance and more rapid arrest of large CTC cluster microemboli compared to circulating single cells could decrease their accumulation and detection in the blood (Aceto et al., 2014; Au et al., 2016a; Gkountela et al., 2019). Ultimately, if these technical barriers for detecting CTCs can be overcome by improvements in technology, a clearer picture may emerge of the biological variation in CTC abundance within and between patients.
In this context, circulating tumor cell (CTC) clusters are even rarer than single CTCs, accounting for roughly 1 to 17% of detected CTCs in patients and CTC cluster detection varies greatly depending on the tumor type, stage, and CTC enumeration methodology (Amintas et al., 2020; Cho et al., 2012; Szczerba et al., 2019). The reported proportions of patients with detected CTC clusters range widely from 5 to 54% in breast cancer (Cho et al., 2012; Jansson et al., 2016; Larsson et al., 2018; Mu et al., 2015; Paoletti et al., 2015; Szczerba et al., 2019; Wang et al., 2017), 18 to 81% in pancreatic ductal adenocarcinoma (Amantini et al., 2019; Buscail et al., 2019a; Catenacci et al., 2015; Chang et al., 2016), and 26 to 50% in lung cancer (Hou et al., 2012; Manjunath et al., 2019; Murlidhar et al., 2017; Sawabata et al., 2020). The size of CTC clusters is also highly variable; clusters over 20 cells have been identified in the blood of patients across common cancer types, though they are more commonly reported as clumps of between 2 to 6 cells (Long et al., 2016b; Molnar et al., 2001; Sarioglu et al., 2015). Though large microemboli seem likely to occlude or arrest in capillaries, a recent study found that breast cancer CTC clusters as large as 20 cells can squeeze through 5 to 10 micron vessels in microfluidic chambers and zebrafish models (Au et al., 2016a). Importantly, accruing evidence suggests that patient populations in which these CTC clusters are detected often have greater rates of disease progression and poorer treatment response.
Circulating tumor cell clusters correlate with poorer clinical outcomes
Across many cancer types, CTC clusters are associated with worse clinical outcomes including disease progression and early mortality (Chang et al., 2016; Costa et al., 2020; Divella et al., 2014; Hou et al., 2012; Jansson et al., 2016; Kulasinghe et al., 2018; Larsson et al., 2018; Long et al., 2016b; Mu et al., 2015; Murlidhar et al., 2017; Okegawa et al., 2018; Paoletti et al., 2019; Sawabata et al., 2020; Wang et al., 2017; Zheng et al., 2017). In a number of these studies the presence of CTC clusters is an independent prognostic factor by Cox proportional hazards, yielding prognostic information beyond the presence of single CTCs alone (Table 1). CTC cluster counts also vary with clinical stage, with increasing detection often corresponding to metastatic progression. For example, a study in pancreatic cancer found that the mean number of detected CTC clusters per blood draw increased with disease progression from 0 to 9.2 to 15.2 to 71.2 through stages I-IV, respectively (Chang et al., 2016). In addition, studies have demonstrated that CTC cluster counts fluctuate in individual patients, often increasing with disease progression and decreasing with response to therapy (Larsson et al., 2018; Wang et al., 2017; Yu et al., 2013).
Table 1.
Clinical correlation of CTC clusters with poorer patient prognosis across common cancer types
| Citation | Cancer type | # of patients | OS HR (95% CI) | PFS HR (95% CI) | Were clusters an independent prognostic factor? |
|---|---|---|---|---|---|
| (Jansson et al., 2016) | Breasta | 50 | 7.0 (1.7 – 28.0) | 1.8 (0.5 – 6.5) | Yes |
| (Wang et al., 2017) | Breastb | 128 | 4.7 (1.9 – 11.6) | 3.0 (1.7 – 5.3) | Yes |
| (Larsson et al., 2018) | Breastb | 156 | 4.1 (2.0 – 8.3) | 2.6 (1.5 – 4.8) | Yes |
| (Paoletti et al., 2019) | Breastb | 549 | 15.1 (11.3 – 18.1) vs. 19.9 (17.1 – 21.8)† | NR | No |
| (Costa et al., 2020) | Breastb | 54 | 4.5 (1.6 – 12.8) | 4.0 (1.8 – 8.7) | Yes |
| (Divella et al., 2014) | Colorectal | 103 | 5.9 (2.9 – 86.2) | NR | Yes |
| (Zheng et al., 2017) | Gastricb | 86 | 4.5 (1.7 – 12.0) | 2.9 (1.2 – 6.8) | Yes |
| (Sawabata et al., 2020) | Lungb | 104 | 8.9 (2.4 – 32.9) | 4.4 (1.1 – 18.1) | Yes |
| (Hou et al., 2012) | Lung (SCLC)b | 97 | 2.9 (1.7 – 5.2) | 2.1 (1.2 – 3.5) | Yes |
| (Long et al., 2016a) | Melanomab | 128 | 5.1 (2.0 – 19.0) | NR | Yes |
| (Lee et al., 2017) | Ovarian | 54 | ns | ns | No* |
| (Chang et al., 2016) | PDACb | 63 | 8.2 (2.1 – 32.7)†† | 487 (12.4 – 12884.9)†† | Yes |
| (Okegawa et al., 2018) | Prostateb | 98 | 4.2 (2.4 – 5.6) | 4.4 (2.4 – 7.3) | Yes |
Summary of recent studies assessing the prognostic significance of circulating tumor cell clusters. OS = overall survival. PFS = progression free survival. HR = hazard ratio. CI = confidence interval. NR = not reported. ns = no significant difference.
longitudinal time-dependent analysis of CTC cluster presence.
baseline analysis of CTC cluster presence.
median survival in months (with 95% CI) of patients with vs. without detected CTC clusters.
analysis of patients with unfavorable CTC cluster counts (greater than the mean of all cases: >30 clusters/2 mL blood).
CTC-cluster positivity correlated with platinum resistance.
A potential confounding variable is that the frequency of single CTCs tends to co-vary with the frequency of CTC clusters. For example, retrospective analysis of samples collected in a large breast cancer clinical trial found that single and clustered CTC counts correlated such that CTC cluster count was not an independent prognostic factor when higher total CTC levels were taken into account (Paoletti et al., 2019). While more studies are indicated, at present the presence of clusters or an increase in their prevalence are concerning indicators of poor patient prognoses.
Increasing numbers of CTC clusters have also been postulated to directly cause patient morbidity even if they fail to generate distant metastases. CTC clusters can occlude vessels, as in pulmonary lymphangitic carcinomatosis when lung lymphatic vessels become obstructed and inflamed, in turn leading to respiratory distress (Klimek, 2019). Moreover, occlusion of vessels by tumor emboli in the brain can lead to cerebral infarction and has been speculated as a possible explanation for the preferential seeding of metastases in watershed regions, which are sites of narrowing of the vascular network to 50–150 μm arterioles (Delattre et al., 1988; Hwang et al., 1996). Thus, CTC clusters can have directly negative consequences on patient outcome in addition to seeding new secondary tumors.
CTC clusters are also found as heterotypic aggregates between tumor cells and platelets or immune cells encountered in the blood, but sometimes also with cells from the primary tumor microenvironment like fibroblasts and macrophages which have been carried along into the circulation (Duda et al., 2010; Jiang et al., 2017; Sarioglu et al., 2015). A recent study found that 8.6% of collected breast cancer CTCs were homotypic clusters but 3.4% were heterotypic white blood cell-CTC clusters, with the remaining 88% corresponding to single cell CTCs. In these heterotypic clusters roughly 25% of attached white blood cells were predicted to be T-cells. Of the remaining 75%, a large majority were neutrophils, which may have adhered to CTCs using VCAM1. Neutrophils conferred greater expression of cell-cycle genes and enhanced aggression in these circulating tumor cells (Szczerba et al., 2019). Neutrophils are also implicated in extravasation of tumor cells through their secretion of IL-8 which modulates endothelial barriers (Chen et al., 2018). These studies and others suggest that disrupting communication and aggregation between certain immune compartments and tumor cells in the circulation might benefit patients.
Platelets attached to CTCs can also play several important roles in promoting metastasis (Camerer et al., 2004; Haemmerle et al., 2017; Labelle et al., 2011). By coating CTCs, platelets can shield them from immune cells, from the physical stress of circulation, and inconveniently from some CTC detection methods when platelets mask tumor cell surface epitopes (Egan et al., 2014; Jiang et al., 2017). Cluster-platelet aggregation can also promote transendothelial migration and extravasation (Xiong et al., 2020). Moreover, platelet-derived TGFβ and NF-κB signaling can increase mesenchymal gene expression in CTCs and enhance metastasis (Labelle et al., 2011). These heterogeneous interactions with non-tumor blood cells can give CTCs a greater ability to survive in the bloodstream and generate new metastases.
Human studies and mouse models harbor evidence of metastases seeded by multiple cells
While clinical studies demonstrate that the presence of circulating clusters is often associated with poorer prognosis and metastatic progression, this does not provide direct evidence that metastases originate from circulating clusters of tumor cells. To answer this question, experimental models of metastasis are helpful to unambiguously trace the contribution of clusters to metastasis formation. Two such studies were recently carried out using breast cancer mouse models with primary tumors labeled with multiple fluorescent proteins to identify polyclonal (i.e. multi-color) metastases founded by multiple cells. By measuring the proportion of multi-color CTC clusters vs. single color individual CTCs, as well as identifying the fraction of metastases with multiple fluorescent tags (i.e. founded by multiple cells), the authors were able to back-calculate the metastatic potential of clusters vs. single cells in these systems. They found that CTC clusters were predicted to generate 50 to 97% of all metastases despite accounting for a small fraction of all CTCs (Aceto et al., 2014; Cheung et al., 2016). Thus, the metastatic potential of CTC clusters was predicted to be 20 to >50-fold higher than that of single cells in these models. In another study using a multi-color mouse model of pancreatic cancer, 80% of macrometastases to the diaphragm or peritoneum were seeded by multiple cells, despite only ~15% of CTCs circulating as clusters (Maddipati and Stanger, 2015). Studies in other models have similarly identified enhanced aggression of clusters and polyclonal metastasis formation in breast, colorectal, and ovarian cancer (Echeverria et al., 2018; Janiszewska et al., 2019; Kok et al., 2021; Liu et al., 2019; Lo et al., 2020; Mizukoshi et al., 2020; Naffar-Abu Amara et al., 2020) (Table 2). These experimental findings all point to greatly increased metastatic efficiency in circulating tumor cell clusters and demonstrate that, at least in some cancer models, they give rise to the majority of metastases despite their rarity (Cheung and Ewald, 2016).
Table 2.
Summary of experiments comparing the metastatic potential of single and clustered tumor cells.
| Citation | Model | Method | Findings |
|---|---|---|---|
| (Watanabe, 1954) | Mouse bronchogenic carcinoma | Jugular injection into mice | 92% take rate for clusters, 0% for single cells |
| (Fidler, 1973) | B16 mouse melanoma | Tail vein injection into mice | ~3-fold more lung metastases formed in cluster-injected mice after 2 weeks |
| (Liotta et al., 1976) | T-241 fibrosarcoma | Tail vein injection into mice | 13 to 25-fold more lung metastases formed in cluster-injected mice after 12 days |
| (Aceto et al., 2014) | MDA-MB-231-LM2 human breast cancer cell line | Orthotopic transplant into mice | ~50-fold more lung metastases formed by CTC clusters from tumor transplants relative to single CTCs |
| 4T1 mouse breast cancer cell line | Orthotopic transplant into mice | ~23-fold more lung metastases formed by CTC clusters from tumor transplants relative to single CTCs | |
| (Maddipati and Stanger, 2015) | KCPX mouse model of pancreatic cancer | Intraperitoneal injection into mice | >2-fold more metastases formed in cluster-injected mice after 3 weeks |
| KCPX mouse model of pancreatic cancer | Retro-orbital injection into mice | >15-fold more lung metastases formed in cluster-injected mice after 3 weeks | |
| KCPX mouse model of pancreatic cancer | Multi-color spontaneous mouse tumor model | 80% of large metastatic lesions to peritoneum and diaphragm arose from multiple cells | |
| (Cheung et al., 2016) | MMTV-PyMT mouse model of Luminal B breast cancer | Orthotopic transplant into mice | Estimated >97% of lung metastases were derived from clusters (95% CI: 74–100%) |
| MMTV-PyMT mouse model of Luminal B breast cancer | Tail vein injection into mice | >100-fold more lung metastases formed by cluster-injected mice after 3 weeks | |
| (Zajac et al., 2018) | Colorectal cancer PDX | Intraperitoneal injection | >20-fold higher tumor burden in cluster-injected mice after 40 days |
| (Allen et al., 2019) | B16F10 mouse melanoma cell line | Tail vein injection into mice | ~2-fold higher BLI signal of cluster-injected mice after 10 days |
| A375 human melanoma cell line | Tail vein injection into mice | ~3-fold higher BLI signal of cluster-injected mice after 10 days | |
| (Liu et al., 2019) | Breast cancer PDX | Orthotopic transplant into mice | 54% of lung metastases were polyclonal 6–8 weeks after transplant (based on 2-color fluorescence) |
| Breast cancer PDX | Tail vein injection into mice | >5-fold higher BLI signal of cluster-injected mice after 8 weeks | |
| (Lo et al., 2020) | 4T1 mouse breast cancer cell line | Tail vein injection into mice | ~8-fold higher BLI signal of cluster-injected Balb/c mice after 7 days vs. single cells |
| AT3 mouse breast cancer cell line | Tail vein injection into mice | ~500-fold higher BLI signal of cluster-injected C57BL/6 mice after 25 days vs. single cells | |
| (Wrenn et al., 2020) | MMTV-PyMT mouse model of Luminal B breast cancer | Tail vein injection into mice | 141 to 532-fold more lung metastases formed in cluster-injected mice after 3 weeks |
| (Wrenn et al., 2020) | MMTV-PyMT mouse model of Luminal B breast cancer | Intracardiac injection into mice | 7.6-fold more metastases to systemic organs in cluster injected mice after 6 weeks |
Summary of studies assessing the metastatic potential of single or clustered tumor cells in various mouse models of cancer.
In human tumors, DNA sequencing and phylogenetic analysis of metastases compared to primary tumors can reveal if metastases were clonally seeded by a single cell or instead seeded by multiple cell clones from the primary tumor (Birkbak and McGranahan, 2020; Gundem et al., 2015). Still, an important consideration when interpreting these findings is that polyclonal seeding could occur either by metastasis of multiclonal clusters or serial seeding of single cells. Conversely, seeding by monoclonal clusters or later clonal sweeps could result in monoclonal metastases despite a multicellular origin. Polyclonal metastases have been identified in prostate cancer (Gundem et al., 2015), lung cancer (Hu et al., 2020), colorectal cancer (Dang et al., 2020; Leung et al., 2017; Ulintz et al., 2018; Wei et al., 2017), ovarian cancer (McPherson et al., 2016), gastric cancer (Hirotsu et al., 2020), and intrahepatic cholangiocarcinoma (Dong et al., 2018). In rapid autopsy studies of metastatic breast cancer patients, 63 to 73% of patients had evidence of polyclonal metastasis (Siegel et al., 2018; Ullah et al., 2018). Further, this polyclonal organization can be observed from the first phases of neoplasia. In breast cancer, the invasion of genetically multiclonal clusters of cells is observed when ductal carcinoma in situ (DCIS) cells breach the mammary duct and escape into surrounding tissues (Casasent et al., 2018). Individual cells from DCIS regions (abnormal cells inside ducts) and invasive regions (outside ducts) were carefully collected using laser-capture microdissection followed by single cell sequencing. Tracking of clonal compositions of these areas indicated that many mutations were acquired at the DCIS stage, and that these multiclonal groups of DCIS cells co-migrated together as clusters to form invasive ductal carcinoma regions (Casasent et al., 2018). A similar study likewise individually microdissected regions of DCIS and invasive ductal carcinoma then subjected these samples to DNA sequencing. In 18 of 25 cases, sequencing indicated that invasive carcinomas were polyclonal, that is arising from multiple founding cells (Pareja et al., 2020). Genomic analysis has additionally demonstrated polyclonal seeding of lymph node metastases in colorectal and breast cancer (Ulintz et al., 2018; Ullah et al., 2018). These genetic findings further support a model of metastatic dissemination propagated by multiclonal groups of cells, rather than individual clones.
Still, these observational human studies cannot determine what proportion of polyclonal metastases were seeded simultaneously by clusters of cells, or serially by single cells. In mouse models of breast cancer, however, polyclonal metastases derived from cluster-based seeding are observed more frequently than metastases arising from serial seeding of individual cells. In three recent studies, tumor cells with different fluorescent tags were separately inoculated into the left and right mammary fat pads to generate two single-color tumors. If serial seeding of metastases were a frequent event, a high proportion of the metastases would be expected to be multi-color, that is derived from single cells from both the left and right tumor. However, when lung metastases were assessed, only 0 to 14% of them were two-color (Aceto et al., 2014; Cheung et al., 2016; Liu et al., 2019; Lo et al., 2020). These experiments indicate that the majority of polyclonal seeding in these models is not derived from serial seeding of cells but rather from cells which group together at the primary tumor site. While further and deeper sequencing of metastatic tumors will better elucidate seeding patterns, these experimental studies suggest that tumor cell clusters may be an important source for polyclonal metastases in human patients.
COOPERATIVE INTERACTIONS DURING COLLECTIVE CELL METASTASIS
Cooperation is the observation that individuals within a group coordinate their activities, resulting in collective benefit. In nature, cooperation is observed across biological time and length scales ranging from the population dynamics of T-cells (Polonsky et al., 2018) to hair follicle regrowth (Chen et al., 2015) to nest-site selection of honeybees (Seeley and Visscher, 2004). In each of these examples, communities of individuals use cooperation to their advantage to overcome obstacles, share information, or neutralize threats. For example during tissue development and wound repair, intercellular cooperation between local niche cells and stem cells maintain the correct balance of renewal and proliferation, a dialogue shaped by the physical topology of cells and their cell-cell contacts, extrinsic environmental signals like injury or inflammation, and bidirectional niche cell/stem cell signaling (Chen et al., 2015; Shyer et al., 2015; Xin et al., 2016). The field of microbiology serves as another instructive example; recent findings have upended the model that bacteria behave as “lone agents” and instead identified important cooperative behaviors like biofilm formation mediated by intercellular communication and quorum sensing (Ben-Jacob et al., 2012; Lambert et al., 2011; Papenfort and Bassler, 2016). Unlike stem cell/niche cell interactions with sender-receiver dynamics, in this instance all cells are competent to produce a quorum signal. When enough cells in the population express that signal its concentration passes a key threshold, inducing a community-level switch in phenotype. Tuning the degree of “self communication” vs. social or “neighbor communication” can generate emergent signaling circuits and population-level responses in natural or synthetic biological systems (Chen et al., 2015; Montaudouin et al., 2013; Youk and Lim, 2014). Further, in ecology cooperative interactions can be used to explain population dynamics that differ from logistic growth models. These divergences can be generated by “Allee effects” in which interactions amongst members of a population, such as cooperative feeding and shared contributions to defence, generate threshold effects whereby populations must reach an intermediate size before achieving maximum growth (Korolev et al., 2014).
In the field of cancer research, we are increasingly appreciating the degree to which cooperativity can promote disease progression (Tabassum and Polyak, 2015). Cancer cells are often associated with “selfish behavior” and uncontrolled growth arising from mutations releasing cells from the constraints of their original developmental programming (Archetti and Pienta, 2019). But the recognition that cancer cells maintain physical contact as clusters throughout the metastatic cascade suggests that intercellular cooperation might confer advantages during this process. Remarkably, recent findings demonstrate that the simple shift from single cells to clusters results in rapid and profound changes to cell state, accompanied by markedly greater likelihood of metastatic colonization. Here we review the multiple ways that multicellularity enables cooperative behaviors during the metastatic process and highlight the emerging molecular mechanisms supporting clusters’ heightened survival, outgrowth, and overall metastatic fitness.
Cellular specialization and intercellular communication in tumor cell collectives during invasion
Locally invading cancer cells face a multitude of challenges as they disperse from the primary tumor. Within a 3D context, these include pathfinding, coping with changing local environments, matrix remodeling, and metabolic demand (Yamada and Sixt, 2019). An individual invasive cell must acquire properties to overcome all these obstacles, often simultaneously, while retaining the capacity to later proliferate and expand into a secondary tumor. In clusters, these demands can be surmounted in part through cell specialization and intercellular communication.
A common motif across both normal collective migration and collective invasion by cancer cells is the emergence of distinct cellular states along the axis of migration; that is to say, there are “front” and “rear” cells within clusters which can have important differences in their phenotypes. At the extreme end of this spectrum, migrating clusters can arrange into single-file chains which is observed in melanoma and breast cancer invasion (Haeger et al., 2020; Khalil et al., 2017). Nonlinear nest-like groups of cells can also arrange themselves with one or more “leader” cells at the front-most edge of the cluster directing migration and remaining connected to several “followers” behind. Leaders, as their name implies, are usually thought to determine the direction of migration of the cluster. They can accomplish this by sensing the microenvironment through ECM-integrin signaling and responding to chemoattractants, and in turn can modify the path in front of them through traction forces or secretion of matrix metalloproteinases (Colak-Champollion et al., 2019; Haeger et al., 2014; Trepat et al., 2009). Follower cells, in turn, may assist leader cells via pro-survival signaling and maintaining the direction of migration, and can provide much of the actual traction force needed for movement (Konen et al., 2017; Trepat et al., 2009; Yamada and Sixt, 2019).
There are many different forms this leader-follower pattern can take depending on the biological context. During lateral line morphogenesis in zebrafish, multiple leader cells and followers within the migrating cluster maintain distinct but cooperative phenotypes through differential expression of chemokine and growth factor receptors (Aman and Piotrowski, 2008; Mishra et al., 2019). Another example during development is Drosophila border cell migration, in which non-motile polar cells activate JAK-STAT signaling in leading border cells to promote their motility (Mishra et al., 2019; Silver and Montell, 2001). In mammals, a prototypic example of this organization occurs during normal vascular sprouting; “tip” cells connected by cell-cell junctions to “stalk” cells lead multicellular cohorts of endothelial cells via VEGF chemotaxis (Gerhardt et al., 2003). Though the number of cells in each group and the molecular distinctions between them vary greatly, this leader-follower dichotomy is repeatedly observed across different species in both normal and disease contexts.
Functional experiments have confirmed that this leader-follower organization can be important for successful invasion and metastasis. A study in breast cancer found that basal cells expressing keratin-14 frequently led collectively invading strands in both mouse models and human tumor samples. Keratin-14 knockdown significantly reduced collective invasion and subsequent metastasis by clusters, indicating that disrupting the leader cell-associated gene expression of tumor cell clusters can greatly suppress their metastatic potential (Cheung et al., 2013). Another study found that laser ablation of leader cells interrupted forward invasion of collective strands in 3D culture (Zhang et al., 2019b). However, within 12 hours a new leader cell typically emerged and resumed invasion. The authors noted that new leader cells arose from follower cells and replaced existing leader cells even without laser ablation, suggesting that follower-leader states can dynamically interchange. Denser collagen matrices, which required greater energy consumption by leader cells, also hastened the emergence of new leader cells to replace the previous “tired” leaders (Zhang et al., 2019b). A number of other studies have likewise found that removal of leader cells or disruption of their function significantly impairs collective migration (Gao et al., 2017; Khalil et al., 2020; Kim et al., 2017; Yang et al., 2019). Many experimental techniques have been developed to further isolate and characterize the molecular properties of leader and follower cells. These approaches help identify leader vs. follower distinctions that confer disparate functions and facilitate cooperativity, including differences in transcription, metabolism, epigenetic modifications, senescence, and gene mutations (Commander et al., 2020; Kim et al., 2017; Konen et al., 2017; Summerbell et al., 2020; Zhang et al., 2019b; Zoeller et al., 2019). For example, after culturing cells transduced with the photoconvertible fluorophore Dendra2 in 3D ex vivo culture systems which promote collective invasion, photoconverted leader cells have been separated from non-leader cells by flow sorting for further phenotypic and genomic analyses (Konen et al., 2017). When specific markers for leader cells are known, such as Keratin-14 in breast and ovarian cancer, leader cells can instead be identified by differential expression through gene promoter-driven fluorescence expression or antibody-based methods (Bilandzic et al., 2019; Cheung et al., 2013; Cheung et al., 2016; Hwang et al., 2019b; Quan et al., 2020; Yang et al., 2019). Live imaging alternatively allows actively migrating leader cells to be analyzed in situ without disrupting their dynamic interactions with follower cells. Pairing live time-lapse microscopy with other techniques such as fluorescent metabolic indicators (Commander et al., 2020; Zhang et al., 2019b), traction force microscopy (Riahi et al., 2015; Trepat et al., 2009), organelle-specific dyes (Commander et al., 2020), and co-culture with non-tumor cells (Gaggioli et al., 2007; Hanley et al., 2020; Hwang et al., 2019a) can provide additional layers of information clarifying the role of leader cells during collective migration and invasion.
Similar experimental systems can also be used interrogate cooperative interactions between leader and follower cells to identify the signaling or communication that emerges when clustered cells segregate into these two identities. Cell mixing or co-culture experiments, leader or follower cell-specific gene knockdown, conditioned media treatment, and other techniques have been used to identify the molecular sources of leader-follower cooperative phenotypes. Experiments assessing isolated vs. mixed leader and follower cells have been particularly informative; one study found that mixing increasing proportions of purified leader cells with follower cells resulted in a dose-dependent increase in invasion. Leader-cell conditioned media could induce invasion of follower cells, supporting the hypothesis that secreted factors facilitate leader-follower communication (Konen et al., 2017). In lung cancer cells, VEGF was upregulated in leader cells, which in turn stimulated the motility of follower cells (Konen et al., 2017). These purified leader cells grew slowly compared to follower cells, but their growth was rescued by follower-conditioned media, suggesting both compartments generate important secreted signals. And, in thyroid cancer cells, CXCL12 secretion by leader cells increased the survival and anoikis-resistance of co-cultured cells (Kim et al., 2017). Communication between leaders and followers may also occur through modulation of existing soluble signaling molecule gradients. During melanoma migration, follower cells breakdown local LPA to form a chemotactic sink, generating an outward-facing chemotactic gradient that invasive cells follow (Muinonen-Martin et al., 2014). Thus follower-leader groups can participate in multiple modes of bidirectional signaling to modulate or support each other’s phenotypes.
In addition to secreted molecules, leader and follower cells can communicate directly at or through cell-cell junctions. One group recently demonstrated gap junction intercellular communication (GJIC) between leader and follower cells using fluorescence recovery after photobleaching. Leader and follower cells containing calcein dye were photobleached, but quickly recovered fluorescent calcein signal donated by neighboring cells indicating active intercellular transfer (Khalil et al., 2020). Cell-cell junctions can also facilitate juxtacrine signaling of receptors and ligands on adjacent cells. For instance, juxtacrine Notch1-Dll4 signaling between leader and follower cells can prevent the initiation of additional leader cells (Riahi et al., 2015). Cell-cell junction molecules themselves can communicate important information between leaders and followers. E-cadherin signaling between motile cells and polar cells during border cell migration communicates the direction of movement through positive feedback with Rac (Cai et al., 2014). As leader cells move forward, tension accumulates at cadherin junctions with follower cells. This tension can transduce a number of downstream signals, including relocalization of cytoskeletal proteins like merlin, ultimately increasing the migratory polarization of migrating cells (Das et al., 2015). In some instances, leader cells can also generate a peripheral actomyosin cable which prevents the initiation of new leader cells amongst their followers (Reffay et al., 2014). These findings affirm the concept that migrating clusters are often not just physically linked, but in many instances are communicating through mechanical or chemical signals downstream of cell-cell adhesion.
Though leader-follower arrangements have been identified in different forms of developmental collective migration, there are intriguing exceptions in which leader cells are not detected, or in which steering and pathfinding are actually driven by cells at the rear of the cluster (Colak-Champollion et al., 2019). Likewise, some cancers appear to collectively invade without leader-follower phenotypes. A recent study found that colorectal cancers can form large multicellular spheres with reversed (apical surface, basal core) polarity which migrate in an amoeboid-like manner without generating adhesive cellular protrusions or forming leader cells (Zajac et al., 2018). Another example is the normal development of mammary ducts, which is accomplished by the collective migration and bifurcation of multilayered bulb-like structures called terminal end buds (TEBs) which similarly lack protrusions and leader cells (Paine and Lewis, 2017). Developmental TEB migration shares common features with breast cancer invasion. In both cases multicellular groups of cells invade through the mammary stroma, secrete MMPs to facilitate migration, generate mixed luminal/basal cell populations, have reduced apical-basal polarization, and are assisted by local non-epithelial cells including fibroblasts and macrophages (McCaffrey et al., 2012; Paine and Lewis, 2017; Scheele et al., 2017; Wiseman et al., 2003). These studies highlight that collective migration can be carried out through various means of supracellular organization. But a unifying theme across disparate mechanisms of collective invasion and migration is that the diversity of phenotypic states within cell clusters can generate intercellular cooperativity and important pro-invasive features.
Still, yet another form of intercellular cooperation during invasion is through interaction with local non-tumor cells, extensively reviewed elsewhere (Binnewies et al., 2018; Egeblad et al., 2010; Hirata and Sahai, 2017; Lyssiotis and Kimmelman, 2017; Sahai et al., 2020). For instance, two-way paracrine signaling in which cancer cells secrete CSF1 and macrophages secrete EGF promotes breast cancer cell invasion (Patsialou et al., 2009). Macrophages can additionally contribute to invasion through breakdown or modification of the ECM (Finkernagel et al., 2016) or direct cytosolic transfer to tumor cells (Hanna et al., 2019; Roh-Johnson et al., 2017). Fibroblasts can promote collective invasion in several ways, including forming migration tracks within the ECM (Gaggioli et al., 2007), increasing expression of invasive and leader-cell associated genes (Hanley et al., 2020; Matsumura et al., 2019), and forming heterotypic N-cadherin/E-cadherin contacts with cancer cells which allow fibroblasts to promote and even lead collective invasion (Labernadie et al., 2017). Broadly, cooperative interactions amongst tumor/tumor or tumor/non-tumor cell collectives are increasingly appreciated to be critical mediators of invasion.
Cell-cell adhesion induces pro-survival signaling during early metastatic colonization
Cell-matrix attachment, namely through integrin-ECM interactions (Miranti and Brugge, 2002), is a fundamental regulator of epithelial cell survival (Miranti and Brugge, 2002; Taddei et al., 2012). Loss of integrin-ECM signaling results in downstream signals including the release of sequestered Bim protein, which can then translocate to the mitochondria and promote intrinsic apoptosis, or upregulation of Fas and Fas-L expression, which activate extrinsic apoptosis (Taddei et al., 2012). When cancer cells lose these signals in settings either without sufficient ECM, such as vascular or lymphatic channels, or without appropriate ECM, such as distant tissues with distinct and non-permissive matrix components, they become susceptible to programmed cell death (Celià-Terrassa and Kang, 2016; Piskounova et al., 2015; Valiente et al., 2014). But in certain contexts, cell-cell adhesion can override pro-apoptotic signals, preserving metastatic cell survival in these environments (Al Habyan et al., 2018; Kantak and Kramer, 1998; Liu et al., 2019; Zhao et al., 2010)
In some cases, cell-cell adhesion helps cells evade death by activating integrin signaling even when ECM is not present. In clustered carcinoma cells the cell-surface protein PVRL4, which can bind PVRL1 on adjacent cells (Pavlova et al., 2013), activates α6β4 integrin signaling. This in turn maintains expression of lipid repair enzyme GPX4, preventing lipid perodixation and subsequent cell death via ferroptosis (Brown et al., 2018). Clustering of integrins with other receptors at cell-cell contacts can also activate downstream pro-survival signaling. For example, integrins can interact directly with EGFR or its ligands at cell-cell contact sites, and activate downstream signaling (Nakamura et al., 1995; Yu et al., 2000). This ECM-independent induction of signaling is particularly important in fluid metastatic microenvironments, such as when ovarian cancer cells metastasize by shedding into the peritoneal fluid. Upregulation of E or N-cadherin and formation of multicellular aggregates protects ovarian cancer cells from anoikis in this liquid environment by activating PI3K and EGFR pathways (Hudson et al., 2008; Klymenko et al., 2017; Rayavarapu et al., 2015; Reddy et al., 2005). By either promoting integrin activation or bypassing it to activate downstream oncogenic signaling pathways, cell-cell adhesion provides an alternative route to pro-survival signaling when ECM contact is absent or non-permissive.
Oxidative stress is another major initiator of cell death during metastasis, and can greatly lower metastatic potential of tumor cells (Piskounova et al., 2015). Clustering helps cells mitigate reactive oxygen species (ROS) by several mechanisms. Clustering of detached cells can promote mitophagy, resulting in the clearance of damaged mitochondria and reduced ROS (Labuschagne et al., 2019). Recent studies in mouse models of breast cancer found that reducing expression of E-cadherin or p120-catenin increases local invasion, but ultimately reduces successful metastasis (Ilina et al., 2020; Kurley et al., 2020; Padmanaban et al., 2019) E-cadherin downregulated tumor cells had increased levels of oxidative stress and apoptosis and poorer overall metastasis formation. E-cadherin expression ultimately reduced ROS-promoting signals via modulation of TGFβ signaling, promoting survival and increasing metastatic colonization (Padmanaban et al., 2019). Though molecular mechanisms are still being uncovered, these studies suggest that cell-cell adhesion in tumor cell clusters can help circumvent major causes of apoptosis during metastasis such as ECM detachment and oxidative stress in multiple ways.
Multicellularity can modify certain tumor-immune cell interactions, promoting immune evasion
During dissemination and colonization, tumor cells can be targeted for destruction by immune cells patrolling tissues. Indeed immune escape is a critical step for successfully forming overt metastases (Mohme et al., 2017). Tumor cells can escape immune cells through many different mechanisms, making sensitizing tumors to the immune system challenging. These mechanisms include secreting cytokines or growth factors that can recruit tumor-promoting immune cells or inhibit the activity of anti-tumor immune cells; cancer cells can evade immune cells by avoiding antigen presentation; or tumor cells can co-opt nearby non-tumor cells, inducing them to become immunosuppressive (Beatty et al., 2015; Binnewies et al., 2018; Ennishi et al., 2019). Each of these heterotypic interactions, or combinations thereof, can help tumors escape one of the body’s most potent protections against metastasis (Binnewies et al., 2018).
It is still largely unknown whether single cells or clusters utilize distinct mechanisms of immune escape. Still, there are hints at cluster-specific mechanisms of immune evasion, particularly in regard to natural killer (NK) cells. NK cells play a key role in the targeting of metastases, and their infiltration into tumors often correlates with better patient prognoses (de Andrade et al., 2014; Malladi et al., 2016; Souza-Fonseca-Guimaraes et al., 2019). One recent study found that natural killer cells effectively killed single tumor cells, but not clusters (Lo et al., 2020). This depended partly on clusters’ ability to downregulate NK cell activating ligands, which include EMT promoting genes, and upregulate NK inhibitory ligands, which include cell-cell adhesion genes (Lo et al., 2020). In fact many cell-cell adhesion molecules function as NK cell inhibitory signals; classical E, N, and R-cadherins are ligands for the inhibitory KLRG1 receptor expressed on NK cells (Li et al., 2009). Downregulation of cell-cell adhesion, as in EMT, increases sensitivity to NK killing (Lo et al., 2020; López-Soto et al., 2013). This suggests that NK cells may be fundamentally better suited to kill aberrant post-EMT single cells rather than tumor cell clusters. However, a different study found that NK cells were able to specifically target and induce apoptosis in basal leader cells in collectively invading breast cancer strands, which could be exacerbated by antibody-dependent cell-mediated cytotoxicity. But after prolonged exposure to tumor cell clusters, NK cells were reprogrammed to a metastasis promoting state (Chan et al., 2020). Further studies are needed to assess how collectively invading clusters or micrometastases evade targeting by NK cells and shift them into more permissive cell states. Altogether, these studies suggest that the metastatic advantage of clusters may be modulated by the immune or microenvironmental milieu, an important area of future investigation.
In addition to NK cells, the pro and anti-tumorigenic attributes of macrophages, T-cells, neutrophils, and other immune populations are known to be major determinants of metastatic colonization (Binnewies et al., 2018; Kitamura et al., 2015). We do not yet understand if and how their response to single cells or multicellular clusters differs, though recent studies point at some potentially interesting lines of questioning. One such study used an unbiased shRNA screen to identify CD44, which can mediate breast cancer tumor cell cluster cell-cell adhesion (Liu et al., 2019), as a novel positive regulator of the inhibitory immune checkpoint gene PDL1 (Kong et al., 2020). Another class of cell-cell adhesion molecules identified in tumor cell clusters, nectins, also may promote immune evasion. Nectin-2 can bind to TIGIT expressed on T-cells, resulting in T-cell inhibition (Deuss et al., 2017; Yu et al., 2009). Circulating tumor cells are also vulnerable to immune attack while in transit in the bloodstream. Tumor cells may be able to evade circulating immune cells by multiple mechanisms including expression of PD-L1 (Mazel et al., 2015; Yue et al., 2018), upregulation of “don’t eat me” signals to avoid phagocytosis by macrophages (Baccelli et al., 2013; Baccelli et al., 2014; Steinert et al., 2014), or platelet coating and transfer of platelet-derived MHC I to tumor cells (Placke et al., 2012). But whether clusters and single cells utilize similar or distinct mechanisms of immune evasion in the blood remains unclear. Given the profound clinical impact of immune checkpoint blockade and chimeric antigen receptor T cells for many cancer types an important future direction is to develop a better understanding of how clustering impacts immune evasion.
Intercellular signaling promotes metastatic colonization and outgrowth by tumor cell clusters
The reproducible observations in many models that tumor cell clusters are intrinsically more proliferative and less apoptotic than single tumor cells suggests that cellular signaling pathways regulating those states are altered by clustering. Correspondingly, in normal cells cell-cell adhesion has long been known to strongly influence proliferation and survival (Benham-Pyle et al., 2015; Garcia et al., 2018; Livshits et al., 2012). Early findings suggested that single cells from certain tissues are apoptotic by default, unless rescued by the “social signaling” of adjacent cells – a mechanism which prevents lone cells from surviving in incorrect tissue locations (Raff, 1992). Other early studies in embryonic development noted “community effects”, in which direct interactions with neighboring cells were critical for promoting survival and differentiation (Gurdon, 1988). Using transplant experiments, researchers found that transplanting single cells early in development into different tissues could induce them to differentiate into the tissue present at that site. However, if cells were transplanted as a group they retained their original tissue type (Gurdon et al., 1993). Extensive studies since have shown that adhesion to neighboring cells, and the geometry of those adhesions, have profound effects on survival, proliferation, and differentiation of developing tissues (Gilmour et al., 2017; Xin et al., 2016).
Likewise, it has been speculated that cell-cell interaction is a prerequisite needed to achieve certain community-level effects and cooperative “decision making” in tumor cell communities (Ben-Jacob et al., 2012; Deisboeck and Couzin, 2009; Hickson et al., 2009; Jolly et al., 2018; Korolev et al., 2014). However, many of the specific mechanisms of intercellular signaling active in disseminated clusters, micrometastases, and overt metastases remain to be elucidated. But increasing evidence indicates that by disseminating as a cohesive group, tumor cells in clusters may be able to activate cell-cell signaling networks that promote metastasis.
Paracrine and interclonal signaling
Paracrine signaling between cells plays a key role in development in which morphogen gradients, chemoattractants, and other secreted molecules determine the placement and formation of tissues (Wartlick et al., 2011). These paracrine signals can operate over incredibly long distances, sometimes forming gradients across an entire organism. Alternatively, they can operate in a spatially restricted, short-range manner such as JAK-STAT signaling during border cell migration (Silver and Montell, 2001) or tethering of TGFβ to the extracellular matrix (Maeda et al., 2011). Though long-distance secretion and paracrine interactions can have an important role in cancer (Costa-Silva et al., 2015), short-range signal exchanges are also possible between adjacent tumor cells. Paracrine signaling molecules between nearby cells in this manner maintains high local signal concentrations and effective signaling induction (Müller and Schier, 2011). This can result in a minority of cells in the cluster shifting the phenotype of their neighbors. For example Twist1 and Snail1 expressed in EMT-high breast cancer cells can induce EMT gene expression and promote aggression in neighboring non-EMT cells though paracrine secretion and activation of Hedhehog signaling (Neelakantan et al., 2017). Thus the close spatial proximity of cells in a tumor cell cluster can facilitate a particularly rapid and spatially concentrated form of paracrine signaling.
Heterogeneity within clusters may result in producer-receiver dynamics in paracrine signaling circuits when subgroups within the cluster differentially express ligands and receptors. These kinds of interclonal interactions can result in emergent cooperative, neutral, or competitive dynamics between tumor cells (Kok et al., 2021; Martín-Pardillos et al., 2019; Marusyk et al., 2014; Tabassum and Polyak, 2015). Intercellular receptor-ligand interactions have been implicated in promoting primary or metastatic tumor cell cooperation and outgrowth via Wnt secretion (Cleary et al., 2014), cytokine production (Cleary et al., 2014; Janiszewska et al., 2019), and EGFR ligand exchange (Hobor et al., 2014; Naffar-Abu Amara et al., 2020; Wrenn et al., 2020b). A recent study using breast cancer xenograft models found that polyclonal mixtures of IL11 and FIGF secreting clones generated significantly greater metastatic growth than either clone alone (Janiszewska et al., 2019). Another study isolated clonal populations from an ovarian patient derived xenograft cell line. A multiclonal mixture generated significantly greater tumor burden after injection than 10 of 11 constituent clones – the only clone with an equivalent rate of growth had a particularly high degree of ERBB2 amplification which supported its anchorage-independent growth. However, alone that ERBB2-high clone could not generate solid peritoneal metastases unless exposed to the growth factor amphiregulin which was secreted by other clones (Naffar-Abu Amara et al., 2020). These findings highlight the powerful effects of beneficial interclonal interactions on metastatic outgrowth.
Direct signaling at cell-cell junctions
One emergent property of tumor cell clusters is that cells in such close proximity can signal directly to their adherent neighbors at sites of cell-cell contact (Toda et al., 2019). Perhaps the most obvious cluster-dependent signaling mechanism which this could enable is juxtacrine signaling, in which membrane-bound molecules on two apposing cells bind one another. Notch signaling, for example, is a well-described mechanism of juxtacrine signaling which can promote metastatic success (Boareto et al., 2016; Jackstadt et al., 2019). During normal development and homeostasis, membrane bound Notch ligands bind the Notch receptor, resulting in receptor cleavage and transport of the C-terminal domain of the receptor to the nucleus where it can alter cellular transcription (Siebel and Lendahl, 2017). The Notch pathway encompasses five different ligands and four different receptors, resulting in many potential combinations with specific signaling outputs (Meurette and Mehlen, 2018). In tumor cells, Notch ligands like JAG1 have been implicated in increased tumor cell growth and dissemination (Choi et al., 2008; Riahi et al., 2015) as well as lumen formation in colon cancer cells (Kawai et al., 2020). In triple negative breast cancer, Notch overexpression increased the proportion of K14+ to K14− cells nearly 3-fold by increasing rates of symmetric division (Granit et al., 2018). In another recent study using lung cancer cells, JAG1 was highly enriched in leader cells and anti-JAG1 antibody treatment reduced collective invasion. And in ovarian tumor cell clusters, juxtacrine interactions between JAG1 and Notch3 result in increased proliferation (Choi et al., 2008). Notch signaling can also occur through heterotypic interactions with cells in the TME (Biktasova et al., 2015; Lin et al., 2017) or modify the TME itself, including through downstream TFGβ signaling and neutrophil recruitment (Jackstadt et al., 2019). In addition to the Notch pathway, ligands from several other pathways known to promote growth or metastasis such as ERBB, Ephrin, Hedgehog, and integrin signaling can each function in a membrane-bound, juxtacrine manner (Friedl and Mayor, 2017; Lu et al., 2014; Pettigrew et al., 2014; Singh and Harris, 2005).
Adjacent tumor cells can also form gap junctions which permit the direct diffusion of signaling molecules between their cytosols (Hitomi et al., 2015). Intercellular communication through gap junctions has been shown to increase migration in prostate cancer cells (Zhang et al., 2015), to promote stemness in glioblastoma cells (Hitomi et al., 2015), to facilitate intercellular calcium transients in invasive glioma cells (Gritsenko et al., 2020; Osswald et al., 2015), to enhance EGF gradient sensing during collective migration (Ellison et al., 2016), and to promote anchorage-independent growth of breast cancer cells (Gava et al., 2018). Gap junction proteins can also form hemi-channels which modify metastatic behavior through signaling in the extracellular space. A recent study found that invading leader cells released adenosine into the extracellular space through connexin-43 hemichannels, and adenosine then activated Akt signaling through the adenosine receptor 1 (ADORA1) to promote collective invasion (Khalil et al., 2020). The unique ability of gap junctions to facilitate direct cytosol-to-cytosol transmission or rapid cytosol-to-extracellular space release makes them an intriguing target to disrupt tumor cell-cell communication (Aasen et al., 2016).
3D cell arrangment and morphology-dependent signaling
Development of 3D culture models has improved our ability to recapitulate the all-encompassing interactions of tumor cells with one another and their environment during ex vivo experiments (Shamir and Ewald, 2014; Simian and Bissell, 2017). In addition to allowing 3D cell-cell or cell-matrix adhesions to form, 3D culture also facilitates important changes in shape as tumor cells combine to form complex structures resembling spheres, cysts, strands, or buds (Jamieson et al., 2017; Padmanaban et al., 2020; Sachs et al., 2018; van de Wetering et al., 2015). In normal cells such shape changes can alter cellular functions greatly (Gilmour et al., 2017), as when lateral line cells form rosettes with a central lumen concentrating FGFs to regulate collective migration (Durdu et al., 2014), when gut epithelia buckle to form concentrated pockets of Shh signaling (Shyer et al., 2015), or when cell-cell contacts in embyros fracture which determines the first axis of symmetry (Dumortier et al., 2019). Given the critical role cell placement and shape plays in normal homeostasis and development, it seems likely that these features could similarly shape tumor biology and signaling during metastasis.
We recently described a form of pro-metastatic signaling in tumor cell clusters similarly dependent on their collective 3D architecture. We find that breast cancer tumor cell clusters form “nanolumina”, open intercellular spaces lined by microvilli-like structures and gated at either end by cell-cell junctions (Wrenn et al., 2020b). These intercellular cavities have been previously observed in normal and tumor mammary epithelia (Ewald et al., 2012; Mazzucchelli et al., 2019; Tarin, 1969), but not ascribed with major functional importance or signaling properties. We identified a critical function for nanolumina during primary and metastatic tumor outgrowth, during which they act as concentrated reservoirs of the growth factor epigen (Epgn), whose expression is induced upon clustering, which promotes tumor cell cluster proliferation. Cell-cell junctions restrict the permeability of nanolumina, preventing entrance of some molecules and egress of others. This creates a private signaling compartment where pro-growth signals can be maintained and exchanged between cells at high concentrations without diffusing into the local microenvironment. Therefore, the collective production and sensing of epigen by tumor cells in clusters represents a pro-growth signaling mechanism dependent on their 3D morphology and multicellular organization.
Importantly, we found that targeting this intercellular structure can reduce metastatic outgrowth. Epigen suppression or treatment with IFNγ to induce nanolumenal paracellular permeability both significantly suppressed metastatic outgrowth, with Epgn knockdown reducing metastatic outgrowth of tumor cell clusters in the lungs by over 94%. Interestingly, dependence on epigen signaling and nanoluminal morphology varied amongst subtypes of breast cancer. We found that high epigen expression and nanolumina with restricted permeability were present in basal-like 2 triple negative breast cancers but not mesenchymal-like triple negative breast cancers. Basal-like 2 breast cancers have poor treatment response and a limited number of available therapies (Lehmann et al., 2011; Masuda et al., 2013; Wang et al., 2019). Reducing epigen expression or disrupting nanolumenal permeability reduced metastatic outgrowth in clusters generated from basal-like 2 cancer cells. These findings indicate that tumor cell clusters from specific subtypes of breast cancer, but not others, may rely on cooperative nanolumenal signaling generated by their 3D topology. We have much yet to learn about the role of nanolumina and nanolumenal trafficking of signaling molecules during metastasis. Further examination of these structures, including assessing their prevalence across other normal and malignant tissues, may generate important insights as to how multicellular morphology regulates signaling.
In summary, the heterogeneity, direct cell-cell contacts, and 3D arrangement of cells in a tumor cell clusters can each facilitate modified or novel mechanisms of intercellular signaling during metastatic colonization that are not achievable by single cells. In addition to improving our understanding of the means by which cells can metastasize, these emergent signaling mechanisms may represent potential therapeutic targets in patients with cluster-based dissemination.
UNRESOLVED QUESTIONS REGARDING SINGLE CELL VS. COLLECTIVE CELL METASTASIS
The field of collective metastasis remains an emerging area and many questions are still unanswered. Below we highlight a few questions we find particularly intriguing and discuss current perspectives on them based on recent findings.
How do bulky clusters enter and exit the bloodstream? Do they use the same mechanisms as single cells?
The exact details of tumor cell intravasation across different cancer types are still unclear, though the mechanisms used during metastasis are increasingly better understood (Bockhorn et al., 2007; Reymond et al., 2013). Cells may either approach blood vessels through random migration, or through active chemotaxis as in breast cancer when perivascular macrophages secrete EGF which attracts tumor cells (Roussos et al., 2011). To actually enter the circulation they must pass through surrounding basement membrane and past the tightly connected endothelial cells which form the vessel walls in a process of transendothelial migration known as diapedesis. Migration through these layers is difficult, but can be improved through different mechanisms; cells may secrete proteases like MT4MMP which can disrupt vessel integrity (Chabottaux et al., 2009), or squeeze through existing holes in the basement membrane (Baluk et al., 2003; Madsen and Sahai, 2010). Likewise local production of factors such as VEGF and TGFβ can weaken the endothelial barrier in mouse models of cancer, facilitating easier entry (Anderberg et al., 2013). Intravasation of tumor cells may also be assisted by other cells, particularly macrophages (Patsialou et al., 2009; Roh-Johnson et al., 2014). The collusion of perivascular macrophages, tumor cells, and cells expressing the actin regulatory MENA protein has been implicated in creating “doorways” through which tumor cells can pass into blood vessels (Karagiannis et al., 2017; Pignatelli et al., 2016). Thus, single tumor cells have a number of means by which to enter the circulation, with or without collaborating non-tumor cells.
However, the molecular and cellular events giving rise to multicellular tumor emboli are less clear. During diapedesis, cells squeeze through narrow openings ~3 μm wide between endothelial junctions (Baluk et al., 2003), a feat that seems difficult if not impossible for a 5-cell circulating cluster. An alternative hypothesis is that clusters may instead be shed directly into fragile adjacent or tumor-transecting blood vessels without the need for diapedesis (Bockhorn et al., 2007). Cluster shedding may also be facilitated by the formation of mosaic vessels, in which tumor cells displace endothelial cells and allow direct contact of the tumor mass with the bloodstream (Chang et al., 2000; Silvestri et al., 2020). Bypassing the need for transendothelial migration may protect tumor cells from the stress of migrating through ECM, pericytes, and endothelial cells, and instead allow tumor cell clusters immediate entry into the circulation despite their increased size.
Once in the circulation, tumor cells are surrounded by red blood cells and leukocytes and flowing at high speeds through vessels as large as the aorta and as small as <10 μm wide capillaries (Au et al., 2017; Au et al., 2016b). This environment generates substantial shear force which can increase cellular stress or even cause necrosis and cell fragmentation (Follain et al., 2020). Tumor cells must rapidly adapt to or exit this environment to move to the next phase of metastatic seeding (Follain et al., 2020; Gensbittel et al., 2021). In order to extravasate, tumor cells first slow down significantly either by generating adhesions with endothelial walls or by vessel occlusion (Follain et al., 2018; Kienast et al., 2010). In zebrafish models, which facilitate time lapse intravital imaging of cell circulation throughout an entire organism, tumor cells preferentially arrest in vessels with flow velocities below roughly 400–600 μm/second (Follain et al., 2018). There is also some evidence that clusters travel through vessels more slowly, facilitating longer interactions with endothelial cells (Choi et al., 2015; Patil et al., 2019). Some models suggest that clusters can also take advantage of different mechanisms of circulatory exit. Clusters may use endothelial remodeling to extravasate, in which endothelia enclose the arrested tumor cell cluster then expel it into the tissue (Allen et al., 2019; Follain et al., 2018). This mechanism was also observed to facilitate the extravasation of clusters of cardiac stem cells, hinting at a normal developmental role (Allen et al., 2017). CTC clusters were far more likely to use endothelial remodeling to extravasate than single cells in zebrafish models, and far more proliferative than single cells after extravasation (Allen et al., 2019). Despite intriguing differences in their means of exit, actual rates of extravasation between single cells and clusters appear similar (Allen et al., 2019). And while zebrafish provide an excellent model for live imaging of an intact circulatory system, further intravital observations of cluster entry and exit from the circulation in mammalian models will strengthen the human disease relevance of these models.
What is the relative efficiency of cluster-based and single-cell metastasis at each step of the metastatic cascade?
Multiple studies have used experimental metastasis assays to show that clustering of tumor cells increases their potential to generate distant metastases up to 500-fold more than equal numbers of single tumor cells (Table 2). But taking a step back, it is less obvious why clustering should provide increased efficiency compared with single tumor cells at earlier steps of metastasis. For example, one might predict that invasion of clusters is far less efficient simply because the small size of single cells allows them to navigate more restrictive environments (Mak et al., 2013; Wolf et al., 2013). In agreement, measured speeds of collective invasion are quite slow when compared to single cell migration; clusters often travel just 0.1–1 μm per minute (Friedl et al., 2012). But, while slower, migratory clusters may be better than single cells at following chemotactic cues. Clusters of mammary cells can sense gradients of EGF that are undetectable by single cells (Ellison et al., 2016) In glioma, inhibition of intercellular cooperation through downregulation of p120 cadherin impairs migratory persistence (Gritsenko et al., 2020). Lymphoid malignancies can also form multicellular aggregates that undergo faster and more directional chemotaxis as clusters (Malet-Engra et al., 2015). This increased sensitivity is partly due to the increased size of cell clusters, which allows them to sample a larger range of signal gradients. Clusters of cells could also accomplish directional migration by generating and sensing their own chemokine gradients, instead of relying solely on long-range signals (Donà et al., 2013). In principle, collective invasion could represent a balance between competing demands. Larger clusters may be slower and less able to negotiate dense environments, but they can generate cooperative intercellular signals.
Still, the relative rarity of CTC clusters compared to individual CTCs in patients indicates that, though collective organization is often heavily favored in the peritumoral area, individualized cells outnumber clustered cells once in the circulatory system. The increased barriers to intravasation by clusters mentioned above are one plausible contributor to this shift. Another reason for the low steady-state proportion of CTC clusters in the blood could be more rapid arrest. Although tumor cell clusters can traverse microfluidic vessels as small as capillaries (Au et al., 2016a) the measured half-life of CTC clusters in a mouse model of breast cancer was shorter than single cells, at 6–10 minutes vs. 25–30 minutes (Aceto et al., 2014). One breast cancer study found that the ratio of cells from tumor-draining vessels (local circulation) vs. heart puncture (systemic circulation) was over two-fold higher for CTC clusters than CTC single cells, suggesting enhanced rates of early arrest for tumor cell clusters (Szczerba et al., 2019). Still, clusters may be able to deform into single-file shapes that permit passage through narrow vessels and capillaries (Au et al., 2016b). Overall, these findings suggest that both single and clustered CTCs are cleared from the blood stream fairly rapidly, usually on the order of minutes to hours (Aceto et al., 2014; Meng et al., 2004; Sasportas and Gambhir, 2014).
But differences in metastatic success continue diverging considerably once cells have left the circulatory system. Prior studies, using mostly single tumor cells, have shown that the vast majority of cells are expected to die or enter dormancy within days of initial seeding (Chambers et al., 2002; Glaves et al., 1988; Luzzi et al., 1998; Yoshida et al., 1993). Recently, we observed that for every 1,000,000 single MMTV-PyMT breast cancer cells injected into mice, only 2.4 macrometastases formed after 3 weeks (Wrenn et al., 2020b). In contrast, for every 1,000,000 clustered cells, injected as small ~5–10 cell clusters, we observed over 1260 macrometastases. Closer examination at earlier time points revealed only 3% of the number of cells present in the lungs shortly after tail vein injection of single cells were detectable in the lungs 48 hours after injection. In contrast, in cluster-injected mice 30% of the number of arrested clusters present shortly after injection were present in the lungs 48 hours after injection. This ten-fold increase in early survival and persistence at metastatic sites could give clustered cells a major advantage over single tumor cells when seeding lung tissues.
During the final step of metastasis, colonization, disseminated tumor cells must not only survive but proliferate to establish overt metastases. Though it is possible for cells to begin proliferating shortly after seeding a metastatic site, some cancers are characterized by long latent periods in which metastatic cells remain viable, but dormant, after dispersal to other tissues (Carlson et al., 2019; Ghajar, 2015; Ghajar et al., 2013; Risson et al., 2020). Dormant single cells are detected more commonly than clusters, and the presence of cell-cell adhesion in fact can promote escape from dormancy (Ruppender et al., 2015). Degree of cell-cell adhesion may also regulate entrance into a proliferative state; in a recent study we found that breast cancer tumor cell clusters in 3D culture were largely growth arrested below a threshold size of ~10 cells, but above that size experienced rapid outgrowth (Wrenn et al., 2020a). As mentioned previously, a wide variety of cell-cell adhesion dependent mechanisms of signaling can feed into signaling pathways which regulate clusters’ proliferation. We identified the growth factor epigen as one such signal shared between clustered cells at metastatic sites. When Epgn was knocked down, injected clusters were equally as competent as control clusters to seed the lungs and persist for 3 weeks. However, Epgn knockdown reduced the outgrowth of those clusters in this metastatic environment over 15-fold. The signals regulating outgrowth in disseminated clusters and micrometastases are still mysterious, but our findings show that in some contexts intercellular signaling can be a major contributor to the massively increased outgrowth of metastasizing clusters vs. single cells.
What are the intersections between epithelial-mesenchymal transitions and collective metastasis?
The involvement of epithelial-to-mesenchymal transitions in tumor progression and metastasis has been the subject of intensive research for decades (for informative recent reviews see (Derynck and Weinberg, 2019; Pastushenko and Blanpain, 2019; Yang et al., 2020). Recent findings have shown that, in both normal and cancer contexts, cells can undergo incomplete, partial, or hybrid EMT (Jolly et al., 2019; Williams et al., 2019; Yang et al., 2020). Rather than being a binary on/off state, EMT is a continuum of transitions between fully epithelial and fully mesenchymal extremes (McFaline-Figueroa et al., 2019; Meyer-Schaller et al., 2019; Pastushenko and Blanpain, 2019). Importantly, partial or hybrid EMT states have been described in circulating tumor cell clusters (Sun et al., 2018; Zeinali et al., 2020), and the prevalence of hybrid EMT phenotypes can vary with disease progression and treatment (Chebouti et al., 2017; Yu et al., 2013). Single cell RNA sequencing of human head and neck squamous cell carcinomas also identify cells with partial EMT phenotypes in a subset of patient samples (Puram et al., 2017). Further, partial EMT can also be achieved by mechanisms independent of transcriptional repression such as by protein internalization of E-cadherin (Aiello et al., 2018).
In principle, clustered tumor cells with partial EMT phenotypes could maintain aspects of the epithelial program necessary for metastatic outgrowth (important for later steps of metastasis) alongside mesenchymal features, such as “loosening” of cell-cell contacts and enhanced motility (important for tumor dissemination and earlier steps of metastasis). But recent studies also suggest even greater complexity. For example, loss of the epithelial gene E-cadherin increases invasion but reduces systemic tumor dissemination, at least in some breast cancer models (Padmanaban et al., 2019). Further, in colon cancer PDX models, metastatic colonization of tumor cell clusters was reduced by knockdown of either E-cadherin or Zeb1, indicating a reliance on both epithelial and mesenchymal gene expression (Mizukoshi et al., 2020). These studies suggest further work is warranted to dissect functions of classically epithelial and mesenchymal genes at both earlier and later steps of metastasis. In addition, these observations support experimental and theoretical studies indicating that hybrid EMT state tumor cells could possess intrinsically more aggressive tumorigenic and metastatic behavior (George et al., 2017; Grosse-Wilde et al., 2015; Jolly et al., 2014; Kröger et al., 2019).
An alternative but not mutually exclusive mechanism is that EMT-high tumor cells cooperate with EMT-low tumor cells to promote metastasis. For example, clusters collected from malignant ascites in ovarian cancer patients demonstrate heterogeneity in EMT state and this heterogeneity can promote therapy resistance (Kan et al., 2020). Likewise, tumor cells with a more migratory, mesenchymal-like phenotype can facilitate the metastatic success of less invasive cells through various means including heterotypic cluster formation and paracrine signaling (Calbo et al., 2011; Campbell et al., 2020; Neelakantan et al., 2017; Tsuji et al., 2008). Together these studies support an important role not just for partial EMT, but for the cooperation of cells on different ends of the EMT spectrum to collectively accomplish invasion, survival, and outgrowth during metastasis. Further studies are needed to disentangle the molecular mechanisms connecting partial EMT, cooperativity, and metastatic potential.
How can cluster-based metastasis be interrupted therapeutically?
The unique properties of tumor cell clusters that promote metastasis could provide promising potential targets for clinical disruption. One such strategy would comprise treatments which block collective invasion by inhibiting leader cell activity. Leader cells may be targeted in multiple ways, such as suppressing leader-cell specific protein functions or metabolic states (Cheung et al., 2013; Commander et al., 2020; Khalil et al., 2019; Zhang et al., 2019a). Tumor cells with leader cell characteristics, e.g. basal keratin positive tumor cells, are also associated with micro-metastases at distant sites (Cheung et al., 2013; Lawson et al., 2015). Therapeutic targeting of leader cells could prevent invasion at the primary site, and possibly curb formation of metastases in distant organs, though this hypothesis remains to be tested rigorously.
A number of studies have also posited that killing or at least disaggregating CTC clusters in the circulation could benefit patients (Choi et al., 2015; Gkountela et al., 2019; Wei et al., 2018). Still, caveats to this strategy need to be carefully considered. Disaggregating CTC clusters could produce more potential seeds of metastasis, since they might be broken apart into viable single cells or simply smaller clusters. Additionally, CTC clusters are identified and held together by common cell-cell adhesion molecules such as E-cadherin (Cheung et al., 2016; Na et al., 2020; Padmanaban et al., 2019), Epcam (Allard et al., 2004), CD44 (Liu et al., 2019), desmosomal proteins (Aceto et al., 2014), or claudins (Li et al., 2019). Targeting any of these genes would be challenging given that normal cells expressing the same genes could also be impacted. But further study may reveal distinct properties of tumor cell-cell adhesions, such as specific activation states (Na et al., 2020), that allow them to be targeted with less collateral damage to normal epithelia.
Inhibiting the growth of micrometastases is also an important strategy to reduce metastasis-associated mortality. This is largely because metastasis outgrowth can generate fatal health outcomes, but also because for many patients their primary tumor may have already seeded micrometastases before diagnosis and treatment (Hu et al., 2019; Hüsemann et al., 2008; Janni et al., 2011; Tang et al., 2021). We might be able to harness the immune system to target disseminated clusters and micrometastases to prevent their expansion. The last decade has seen immense progress in cancer immunotherapy through checkpoint blockade therapies, CAR-T cells, and other personalized immunotherapies (Riley et al., 2019). It is plausible that the immune system could be modified to better target cluster-based metastasis, for example by ex vivo engineering of NK cells to more effectively kill clusters (Chan et al., 2020; Daher and Rezvani, 2018; Lo et al., 2020; Shimasaki et al., 2020). More detailed molecular insights into the activating and immunosuppressive signals generated by tumor cell clusters are needed first to develop these therapeutic approaches.
Another promising strategy may be to focus on the cooperation amongst cells that collectively promotes their metastatic potential, instead of targeting the individual cells themselves. For instance, therapeutics that block critical secreted paracrine molecules, disrupt juxtracrine interactions, or destroy nanolumina and other structures that facilitate intercellular communication may be effective. Resetting these clustered cell states to resemble those of individual cells may be able to mitigate the greatly increased metastatic efficiency that cells acquire after establishing cell-cell cohesion. A recent study found that interrupting integrin signaling generated by collectively invading sarcoma cells could enhance the efficacy of radiotherapy (Haeger et al., 2020). Alternatively, the highly interconnected nature of cancer cell collectives may itself generate therapeutic vulnerabilities. For instance, cell-cell contact can increase the potency of ionizing radiation or passage of toxic molecules when cells are electrically coupled by gap junctions (Calì et al., 2015; Fick et al., 1995). Disrupting cell-cell communication, or exploiting it to transmit anti-metastatic signals, may bring cells below molecular thresholds needed to acquire a highly proliferative, aggressive phenotype (Korolev et al., 2014). Importantly, as we have outlined above, intercellular cooperativity promotes metastasis throughout the entire metastatic cascade. Therefore multiple anti-collective therapies may be developed and used throughout the invasion, circulation, and colonization phases of metastasis. Still, It remains to be seen whether such strategies could be adapted to destroy collectively metastasizing cancers. But as more critical intercellular interactions are identified in cancer cell collectives, some may turn out to be fruitful clinical targets.
CONCLUSION
Here we have featured recent findings in the emerging field of collective metastasis regarding the manners in which single cell and cluster-based metastasis diverge. Already these findings suggest several key advantages generated specifically by cell-cell adhesion during metastasis. These emergent properties, dependent on the physical and biochemical coupling of tumor cells, occur throughout the metastatic cascade from beginning to end (Figure 1).
Tumor cells, particularly from epithelial-like cancers, are frequently organized as multicellular collectives during invasion, circulation, and metastatic seeding.
Clustered organization can dramatically enhance the likelihood of successful metastatic colonization in animal models and frequently correlates with poorer prognoses in human patients.
Intracluster heterogeneity during collective invasion generates leader-follower dynamics, potentially facilitating segregation of tasks and generating superior chemotaxis.
Cell-cell adhesion signaling can override pro-apoptotic cues, such as the loss of adequate integrin-ECM interactions, or prevent attack by natural killer cells.
Signaling mechanisms dependent on cell clustering such as short-range paracrine secretion, juxtacrine interactions, or nanolumina formation promote proliferation and metastatic colonization.
At present, a myriad of questions about the divergences between individual and collective metastasis remain unanswered. Overall tumor cell cluster biology remains in its infancy, but we anticipate that studies over the next several years will shed considerably more light on this process. Continually improving new technologies such as intravital imaging, single-cell sequencing, and CTC cluster isolation will be indispensable in unraveling these processes. As data accumulate and models of cluster-based dissemination in different cancer types crystallize, tractable therapeutic targets to disrupt collective metastasis and improve patient outcomes may reveal themselves.
FUNDING
The Department of Defense Breast Cancer Research Program (BCRP; W81XWH-18-1-0098), the NIH/NCI (R37CA234488), Susan G. Komen Foundation (Career Catalyst Research Grant), and the Burroughs Wellcome Fund Career Award for Medical Scientists.
Footnotes
DECLARATIONS
EDW and KJC are inventors on a pending patent application related to collective metastasis by tumor cell clusters
CITATIONS
- Aasen T, Mesnil M, Naus CC, Lampe PD, and Laird DW (2016). Gap junctions and cancer: communicating for 50 years. Nature Reviews Cancer 16, 775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al. (2014). Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aceto N, Toner M, Maheswaran S, and Haber DA (2015). En Route to Metastasis: Circulating Tumor Cell Clusters and Epithelial-to-Mesenchymal Transition. Trends Cancer 1, 44–52. [DOI] [PubMed] [Google Scholar]
- Aiello NM, Maddipati R, Norgard RJ, Balli D, Li J, Yuan S, Yamazoe T, Black T, Sahmoud A, Furth EE, et al. (2018). EMT Subtype Influences Epithelial Plasticity and Mode of Cell Migration. Developmental cell 45, 681–695.e684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Habyan S, Kalos C, Szymborski J, and McCaffrey L (2018). Multicellular detachment generates metastatic spheroids during intra-abdominal dissemination in epithelial ovarian cancer. Oncogene 37, 5127–5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibhr JW, and Terstappen LWMM (2004). Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas but not in Healthy Subjects or Patients With Nonmalignant Diseases. Clinical Cancer Research 10, 6897–6904. [DOI] [PubMed] [Google Scholar]
- Allen TA, Asad D, Amu E, Hensley MT, Cores J, Vandergriff A, Tang J, Dinh P-U, Shen D, and Qiao L (2019). Circulating tumor cells exit circulation while maintaining multicellularity, augmenting metastatic potential. Journal of Cell Science 132, jcs231563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TA, Gracieux D, Talib M, Tokarz DA, Hensley MT, Cores J, Vandergriff A, Tang J, de Andrade JB, and Dinh PU (2017). Angiopellosis as an alternative mechanism of cell extravasation. Stem Cells 35, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman A, and Piotrowski T (2008). Wnt/beta-catenin and Fgf signaling control collective cell migration by restricting chemokine receptor expression. Developmental cell 15, 749–761. [DOI] [PubMed] [Google Scholar]
- Amantini C, Morelli MB, Nabissi M, Piva F, Marinelli O, Maggi F, Bianchi F, Bittoni A, Berardi R, Giampieri R, et al. (2019). Expression Profiling of Circulating Tumor Cells in Pancreatic Ductal Adenocarcinoma Patients: Biomarkers Predicting Overall Survival. Front Oncol 9, 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amintas S, Bedel A, Moreau-Gaudry F, Boutin J, Buscail L, Merlio JP, Vendrely V, Dabernat S, and Buscail E (2020). Circulating Tumor Cell Clusters: United We Stand Divided We Fall. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderberg C, Cunha SI, Zhai Z, Cortez E, Pardali E, Johnson JR, Franco M, Páez-Ribes M, Cordiner R, and Fuxe J (2013). Deficiency for endoglin in tumor vasculature weakens the endothelial barrier to metastatic dissemination. Journal of Experimental Medicine 210, 563–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andree KC, Mentink A, Zeune LL, Terstappen L, Stoecklein NH, Neves RP, Driemel C, Lampignano R, Yang L, Neubauer H, et al. (2018). Toward a real liquid biopsy in metastatic breast and prostate cancer: Diagnostic LeukApheresis increases CTC yields in a European prospective multicenter study (CTCTrap). Int J Cancer 143, 2584–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archetti M, and Pienta KJ (2019). Cooperation among cancer cells: applying game theory to cancer. Nature Reviews Cancer 19, 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth T (1869). A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J 14, 146–149. [Google Scholar]
- Au SH, Edd J, Haber DA, Maheswaran S, Stott SL, and Toner M (2017). Clusters of Circulating Tumor Cells: a Biophysical and Technological Perspective. Curr Opin Biomed Eng 3, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au SH, Storey BD, Moore JC, Tang Q, Chen Y-L, Javaid S, Sarioglu AF, Sullivan R, Madden MW, O’Keefe R, et al. (2016a). Clusters of circulating tumor cells traverse capillary-sized vessels. Proceedings of the National Academy of Sciences 113, 4947–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au SH, Storey BD, Moore JC, Tang Q, Chen YL, Javaid S, Sarioglu AF, Sullivan R, Madden MW, O’Keefe R, et al. (2016b). Clusters of circulating tumor cells traverse capillary-sized vessels. Proc Natl Acad Sci U S A 113, 4947–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bäuerle T, Wallwiener M, et al. (2013). Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nature biotechnology 31, 539–544. [DOI] [PubMed] [Google Scholar]
- Baccelli I, Stenzinger A, Vogel V, Pfitzner BM, Klein C, Wallwiener M, Scharpff M, Saini M, Holland-Letz T, Sinn HP, et al. (2014). Co-expression of MET and CD47 is a novel prognosticator for survival of luminal breast cancer patients. Oncotarget 5, 8147–8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Morikawa S, Haskell A, Mancuso M, and McDonald DM (2003). Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. The American journal of pathology 163, 1801–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, Lee JW, Clendenin C, Gladney WL, Knoblock DM, and Guirnalda PD (2015). Exclusion of T cells from pancreatic carcinomas in mice is regulated by Ly6Clow F4/80+ extratumoral macrophages. Gastroenterology 149, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Jacob E, Coffey DS, and Levine H (2012). Bacterial survival strategies suggest rethinking cancer cooperativity. Trends Microbiol 20, 403–410. [DOI] [PubMed] [Google Scholar]
- Benham-Pyle BW, Pruitt BL, and Nelson WJ (2015). Mechanical strain induces E-cadherin–dependent Yap1 and β-catenin activation to drive cell cycle entry. Science (New York, NY) 348, 1024–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biktasova AK, Dudimah DF, Uzhachenko RV, Park K, Akhter A, Arasada RR, Evans JV, Novitskiy SV, Tchekneva EE, Carbone DP, et al. (2015). Multivalent Forms of the Notch Ligand DLL-1 Enhance Antitumor T-cell Immunity in Lung Cancer and Improve Efficacy of EGFR-Targeted Therapy. Cancer research 75, 4728–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilandzic M, Rainczuk A, Green E, Fairweather N, Jobling TW, Plebanski M, and Stephens AN (2019). Keratin-14 (KRT14) positive leader cells mediate mesothelial clearance and invasion by ovarian cancer cells. Cancers 11, 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, et al. (2018). Understanding the tumor immune microenvironment (TIME) for effective therapy. Nature Medicine 24, 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkbak NJ, and McGranahan N (2020). Cancer Genome Evolutionary Trajectories in Metastasis. Cancer cell 37, 8–19. [DOI] [PubMed] [Google Scholar]
- Boareto M, Jolly MK, Goldman A, Pietilä M, Mani SA, Sengupta S, Ben-Jacob E, Levine H, and Onuchic JN (2016). Notch-Jagged signalling can give rise to clusters of cells exhibiting a hybrid epithelial/mesenchymal phenotype. J R Soc Interface 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockhorn M, Jain RK, and Munn LL (2007). Active versus passive mechanisms in metastasis: do cancer cells crawl into vessels, or are they pushed? Lancet Oncol 8, 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronsert P, Enderle-Ammour K, Bader M, Timme S, Kuehs M, Csanadi A, Kayser G, Kohler I, Bausch D, Hoeppner J, et al. (2014). Cancer cell invasion and EMT marker expression: a three-dimensional study of the human cancer-host interface. J Pathol 234, 410–422. [DOI] [PubMed] [Google Scholar]
- Brown CW, Amante JJ, and Mercurio AM (2018). Cell clustering mediated by the adhesion protein PVRL4 is necessary for alpha6beta4 integrin-promoted ferroptosis resistance in matrix-detached cells. The Journal of biological chemistry 293, 12741–12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner HC, and Derksen PWB (2018). Loss of E-Cadherin-Dependent Cell-Cell Adhesion and the Development and Progression of Cancer. Cold Spring Harb Perspect Biol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscail E, Alix-Panabières C, Quincy P, Cauvin T, Chauvet A, Degrandi O, Caumont C, Verdon S, Lamrissi I, Moranvillier I, et al. (2019a). High Clinical Value of Liquid Biopsy to Detect Circulating Tumor Cells and Tumor Exosomes in Pancreatic Ductal Adenocarcinoma Patients Eligible for Up-Front Surgery. Cancers (Basel) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscail E, Chiche L, Laurent C, Vendrely V, Denost Q, Denis J, Thumerel M, Lacorte JM, Bedel A, and Moreau-Gaudry F (2019b). Tumor-proximal liquid biopsy to improve diagnostic and prognostic performances of circulating tumor cells. Molecular oncology 13, 1811–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Chen SC, Prasad M, He L, Wang X, Choesmel-Cadamuro V, Sawyer JK, Danuser G, and Montell DJ (2014). Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell 157, 1146–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbo J, van Montfort E, Proost N, van Drunen E, Beverloo HB, Meuwissen R, and Berns A (2011). A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer cell 19, 244–256. [DOI] [PubMed] [Google Scholar]
- Calì B, Ceolin S, Ceriani F, Bortolozzi M, Agnellini AH, Zorzi V, Predonzani A, Bronte V, Molon B, and Mammano F (2015). Critical role of gap junction communication, calcium and nitric oxide signaling in bystander responses to focal photodynamic injury. Oncotarget 6, 10161–10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, and Coughlin SR (2004). Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood 104, 397–401. [DOI] [PubMed] [Google Scholar]
- Campbell NR, Rao A, Zhang M, Baron M, Heilmann S, Deforet M, Kenny C, Ferretti L, Huang T-H, Garg M, et al. (2020). Cell state diversity promotes metastasis through heterotypic cluster formation in melanoma. bioRxiv, 2020.2008.2024.265140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson P, Dasgupta A, Grzelak CA, Kim J, Barrett A, Coleman IM, Shor RE, Goddard ET, Dai J, Schweitzer EM, et al. (2019). Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nature cell biology 21, 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casasent AK, Schalck A, Gao R, Sei E, Long A, Pangburn W, Casasent T, Meric-Bernstam F, Edgerton ME, and Navin NE (2018). Multiclonal Invasion in Breast Tumors Identified by Topographic Single Cell Sequencing. Cell 172, 205–217.e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catenacci DV, Chapman CG, Xu P, Koons A, Konda VJ, Siddiqui UD, and Waxman I (2015). Acquisition of Portal Venous Circulating Tumor Cells From Patients With Pancreaticobiliary Cancers by Endoscopic Ultrasound. Gastroenterology 149, 1794–1803.e1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celià-Terrassa T, and Kang Y (2016). Distinctive properties of metastasis-initiating cells. Genes Dev 30, 892–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabottaux V, Ricaud S, Host L, Blacher S, Paye A, Thiry M, Garofalakis A, Pestourie C, Gombert K, and Bruyere F (2009). Membrane-type 4 matrix metalloproteinase (MT4-MMP) induces lung metastasis by alteration of primary breast tumour vascular architecture. Journal of cellular and molecular medicine 13, 4002–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, and MacDonald IC (2002). Dissemination and growth of cancer cells in metastatic sites. Nature Reviews Cancer 2, 563–572. [DOI] [PubMed] [Google Scholar]
- Chan IS, Knútsdóttir H, Ramakrishnan G, Padmanaban V, Warrier M, Ramirez JC, Dunworth M, Zhang H, Jaffee EM, Bader JS, et al. (2020). Cancer cells educate natural killer cells to a metastasis-promoting cell state. The Journal of cell biology 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC, Chang YT, Chen JY, Jeng YM, Yang CY, Tien YW, Yang SH, Chen HL, Liang TY, Wang CF, et al. (2016). Clinical Significance of Circulating Tumor Microemboli as a Prognostic Marker in Patients with Pancreatic Ductal Adenocarcinoma. Clin Chem 62, 505–513. [DOI] [PubMed] [Google Scholar]
- Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, and Munn LL (2000). Mosaic blood vessels in tumors: Frequency of cancer cells in contact with flowing blood. Proceedings of the National Academy of Sciences 97, 14608–14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebouti I, Kasimir-Bauer S, Buderath P, Wimberger P, Hauch S, Kimmig R, and Kuhlmann JD (2017). EMT-like circulating tumor cells in ovarian cancer patients are enriched by platinum-based chemotherapy. Oncotarget 8, 48820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Wang L, Plikus MV, Jiang TX, Murray PJ, Ramos R, Guerrero-Juarez CF, Hughes MW, Lee OK, Shi S, et al. (2015). Organ-level quorum sensing directs regeneration in hair stem cell populations. Cell 161, 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MB, Hajal C, Benjamin DC, Yu C, Azizgolshani H, Hynes RO, and Kamm RD (2018). Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. Proceedings of the National Academy of Sciences 115, 7022–7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Montironi R, Davidson DD, and Lopez-Beltran A (2009). Staging and reporting of urothelial carcinoma of the urinary bladder. Modern Pathology 22, S70–S95. [DOI] [PubMed] [Google Scholar]
- Cheung KJ, and Ewald AJ (2016). A collective route to metastasis: Seeding by tumor cell clusters. Science 352, 167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KJ, Gabrielson E, Werb Z, and Ewald AJ (2013). Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 155, 1639–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN, Gorin MA, Verdone JE, Pienta KJ, Bader JS, et al. (2016). Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci U S A 113, E854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho EH, Wendel M, Luttgen M, Yoshioka C, Marrinucci D, Lazar D, Schram E, Nieva J, Bazhenova L, Morgan A, et al. (2012). Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Phys Biol 9, 016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J-H, Park JT, Davidson B, Morin PJ, Shih I-M, and Wang T-L (2008). Jagged-1 and Notch3 Juxtacrine Loop Regulates Ovarian Tumor Growth and Adhesion. Cancer research 68, 5716–5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, Kim JK, Yang YJ, Kim P, Yoon K-H, and Yun SH (2015). Urokinase Exerts Antimetastatic Effects by Dissociating Clusters of Circulating Tumor Cells. Cancer research 75, 4474–4482. [DOI] [PubMed] [Google Scholar]
- Ciriello G, Gatza Michael L., Beck Andrew H., Wilkerson Matthew D., Rhie Suhn K., Pastore A, Zhang H, McLellan M, Yau C, Kandoth C, et al. (2015). Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell 163, 506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary AS, Leonard TL, Gestl SA, and Gunther EJ (2014). Tumour cell heterogeneity maintained by cooperating subclones in Wnt-driven mammary cancers. Nature 508, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colak-Champollion T, Lan L, Jadhav AR, Yamaguchi N, Venkiteswaran G, Patel H, Cammer M, Meier-Schellersheim M, and Knaut H (2019). Cadherin-Mediated Cell Coupling Coordinates Chemokine Sensing across Collectively Migrating Cells. Current Biology 29, 2570–2579.e2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commander R, Wei C, Sharma A, Mouw JK, Burton LJ, Summerbell E, Mahboubi D, Peterson RJ, Konen J, Zhou W, et al. (2020). Subpopulation targeting of pyruvate dehydrogenase and GLUT1 decouples metabolic heterogeneity during collective cancer cell invasion. Nature communications 11, 1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et al. (2015). Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature cell biology 17, 816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa C, Muinelo-Romay L, Cebey-López V, Pereira-Veiga T, Martínez-Pena I, Abreu M, Abalo A, Lago-Lestón RM, Abuín C, Palacios P, et al. (2020). Analysis of a Real-World Cohort of Metastatic Breast Cancer Patients Shows Circulating Tumor Cell Clusters (CTC-clusters) as Predictors of Patient Outcomes. Cancers 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LWMM, et al. (2004). Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. New England Journal of Medicine 351, 781–791. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Pierga J-Y, Reuben J, Rademaker A, Davis AA, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado R, Mavroudis D, et al. (2019). The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper. Critical Reviews in Oncology/Hematology 134, 39–45. [DOI] [PubMed] [Google Scholar]
- Crosbie PA, Shah R, Krysiak P, Zhou C, Morris K, Tugwood J, Booton R, Blackhall F, and Dive C (2016). Circulating Tumor Cells Detected in the Tumor-Draining Pulmonary Vein Are Associated with Disease Recurrence after Surgical Resection of NSCLC. J Thorac Oncol 11, 1793–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher M, and Rezvani K (2018). Next generation natural killer cells for cancer immunotherapy: the promise of genetic engineering. Curr Opin Immunol 51, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang HX, Krasnick BA, White BS, Grossman JG, Strand MS, Zhang J, Cabanski CR, Miller CA, Fulton RS, Goedegebuure SP, et al. (2020). The clonal evolution of metastatic colorectal cancer. Science Advances 6, eaay9691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Safferling K, Rausch S, Grabe N, Boehm H, and Spatz JP (2015). A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nature cell biology 17, 276–287. [DOI] [PubMed] [Google Scholar]
- de Andrade LF, Ngiow SF, Stannard K, Rusakiewicz S, Kalimutho M, Khanna KK, Tey S-K, Takeda K, Zitvogel L, and Martinet L (2014). Natural killer cells are essential for the ability of BRAF inhibitors to control BRAFV600E-mutant metastatic melanoma. Cancer research 74, 7298–7308. [DOI] [PubMed] [Google Scholar]
- Deisboeck TS, and Couzin ID (2009). Collective behavior in cancer cell populations. Bioessays 31, 190–197. [DOI] [PubMed] [Google Scholar]
- Delattre JY, Krol G, Thaler HT, and Posner JB (1988). Distribution of brain metastases. Arch Neurol 45, 741–744. [DOI] [PubMed] [Google Scholar]
- Derynck R, and Weinberg RA (2019). EMT and Cancer: More Than Meets the Eye. Dev Cell 49, 313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuss FA, Gully BS, Rossjohn J, and Berry R (2017). Recognition of nectin-2 by the natural killer cell receptor T cell immunoglobulin and ITIM domain (TIGIT). Journal of Biological Chemistry 292, 11413–11422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divella R, Daniele A, Abbate I, Bellizzi A, Savino E, Simone G, Giannone G, Giuliani F, Fazio V, and Gadaleta-Caldarola G (2014). The presence of clustered circulating tumor cells (CTCs) and circulating cytokines define an aggressive phenotype in metastatic colorectal cancer. Cancer Causes & Control 25, 1531–1541. [DOI] [PubMed] [Google Scholar]
- Donà E, Barry JD, Valentin G, Quirin C, Khmelinskii A, Kunze A, Durdu S, Newton LR, Fernandez-Minan A, Huber W, et al. (2013). Directional tissue migration through a self-generated chemokine gradient. Nature 503, 285–289. [DOI] [PubMed] [Google Scholar]
- Dong LQ, Shi Y, Ma LJ, Yang LX, Wang XY, Zhang S, Wang ZC, Duan M, Zhang Z, Liu LZ, et al. (2018). Spatial and temporal clonal evolution of intrahepatic cholangiocarcinoma. J Hepatol 69, 89–98. [DOI] [PubMed] [Google Scholar]
- Duda DG, Duyverman AMMJ, Kohno M, Snuderl M, Steller EJA, Fukumura D, and Jain RK (2010). Malignant cells facilitate lung metastasis by bringing their own soil. Proceedings of the National Academy of Sciences 107, 21677–21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumortier JG, Le Verge-Serandour M, Tortorelli AF, Mielke A, de Plater L, Turlier H, and Maître JL (2019). Hydraulic fracturing and active coarsening position the lumen of the mouse blastocyst. Science (New York, NY) 365, 465–468. [DOI] [PubMed] [Google Scholar]
- Durdu S, Iskar M, Revenu C, Schieber N, Kunze A, Bork P, Schwab Y, and Gilmour D (2014). Luminal signalling links cell communication to tissue architecture during organogenesis. Nature 515, 120–124. [DOI] [PubMed] [Google Scholar]
- Echeverria GV, Powell E, Seth S, Ge Z, Carugo A, Bristow C, Peoples M, Robinson F, Qiu H, Shao J, et al. (2018). High-resolution clonal mapping of multi-organ metastasis in triple negative breast cancer. Nature communications 9, 5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan K, Cooke N, and Kenny D (2014). Living in shear: platelets protect cancer cells from shear induced damage. Clinical & experimental metastasis 31, 697–704. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Nakasone ES, and Werb Z (2010). Tumors as organs: complex tissues that interface with the entire organism. Developmental cell 18, 884–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison D, Mugler A, Brennan MD, Lee SH, Huebner RJ, Shamir ER, Woo LA, Kim J, Amar P, Nemenman I, et al. (2016). Cell–cell communication enhances the capacity of cell ensembles to sense shallow gradients during morphogenesis. Proceedings of the National Academy of Sciences 113, E679–E688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enderle-Ammour K, Bader M, Ahrens TD, Franke K, Timme S, Csanadi A, Hoeppner J, Kulemann B, Maurer J, Reiss P, et al. (2017). Form follows function: Morphological and immunohistological insights into epithelial-mesenchymal transition characteristics of tumor buds. Tumour Biol 39, 1010428317705501. [DOI] [PubMed] [Google Scholar]
- Ennishi D, Takata K, Béguelin W, Duns G, Mottok A, Farinha P, Bashashati A, Saberi S, Boyle M, and Meissner B (2019). Molecular and genetic characterization of MHC deficiency identifies EZH2 as therapeutic target for enhancing immune recognition. Cancer discovery 9, 546–563. [DOI] [PubMed] [Google Scholar]
- Erdi YE (2012). Limits of Tumor Detectability in Nuclear Medicine and PET. Mol Imaging Radionucl Ther 21, 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald AJ, Huebner RJ, Palsdottir H, Lee JK, Perez MJ, Jorgens DM, Tauscher AN, Cheung KJ, Werb Z, and Auer M (2012). Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium. J Cell Sci 125, 2638–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehm TN, Meier-Stiegen F, Driemel C, Jäger B, Reinhardt F, Naskou J, Franken A, Neubauer H, Neves RP, and van Dalum G (2018). Diagnostic leukapheresis for CTC analysis in breast cancer patients: CTC frequency, clinical experiences and recommendations for standardized reporting. Cytometry Part A 93, 1213–1219. [DOI] [PubMed] [Google Scholar]
- Ferreira MM, Ramani VC, and Jeffrey SS (2016). Circulating tumor cell technologies. Molecular oncology 10, 374–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick J, Barker FG 2nd, Dazin P, Westphale EM, Beyer EC, and Israel MA (1995). The extent of heterocellular communication mediated by gap junctions is predictive of bystander tumor cytotoxicity in vitro. Proceedings of the National Academy of Sciences of the United States of America 92, 11071–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler IJ (1973). The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer 9, 223–227. [DOI] [PubMed] [Google Scholar]
- Finkernagel F, Reinartz S, Lieber S, Adhikary T, Wortmann A, Hoffmann N, Bieringer T, Nist A, Stiewe T, Jansen JM, et al. (2016). The transcriptional signature of human ovarian carcinoma macrophages is associated with extracellular matrix reorganization. Oncotarget 7, 75339–75352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JC, Niederacher D, Topp SA, Honisch E, Schumacher S, Schmitz N, Föhrding LZ, Vay C, Hoffmann I, and Kasprowicz NS (2013). Diagnostic leukapheresis enables reliable detection of circulating tumor cells of nonmetastatic cancer patients. Proceedings of the National Academy of Sciences 110, 16580–16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follain G, Herrmann D, Harlepp S, Hyenne V, Osmani N, Warren SC, Timpson P, and Goetz JG (2020). Fluids and their mechanics in tumour transit: shaping metastasis. Nature Reviews Cancer 20, 107–124. [DOI] [PubMed] [Google Scholar]
- Follain G, Osmani N, Azevedo AS, Allio G, Mercier L, Karreman MA, Solecki G, Garcia Leòn MJ, Lefebvre O, Fekonja N, et al. (2018). Hemodynamic Forces Tune the Arrest, Adhesion, and Extravasation of Circulating Tumor Cells. Developmental cell 45, 33–52.e12. [DOI] [PubMed] [Google Scholar]
- Friedl P, and Alexander S (2011). Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147, 992–1009. [DOI] [PubMed] [Google Scholar]
- Friedl P, Locker J, Sahai E, and Segall JE (2012). Classifying collective cancer cell invasion. Nature cell biology 14, 777–783. [DOI] [PubMed] [Google Scholar]
- Friedl P, and Mayor R (2017). Tuning Collective Cell Migration by Cell-Cell Junction Regulation. Cold Spring Harb Perspect Biol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Noble PB, Walton PA, Laird DW, Chauvin PJ, Tabah RJ, Black M, and Zänker KS (1995). Migration of coordinated cell clusters in mesenchymal and epithelial cancer explants in vitro. Cancer research 55, 4557–4560. [PubMed] [Google Scholar]
- Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, and Sahai E (2007). Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nature cell biology 9, 1392–1400. [DOI] [PubMed] [Google Scholar]
- Gao XL, Wu JS, Cao MX, Gao SY, Cen X, Jiang YP, Wang SS, Tang YJ, Chen QM, Liang XH, et al. (2017). Cytokeratin-14 contributes to collective invasion of salivary adenoid cystic carcinoma. PLoS One 12, e0171341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MA, Nelson WJ, and Chavez N (2018). Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harbor perspectives in biology 10, a029181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gava F, Rigal L, Mondesert O, Pesce E, Ducommun B, and Lobjois V (2018). Gap junctions contribute to anchorage-independent clustering of breast cancer cells. BMC Cancer 18, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensbittel V, Kräter M, Harlepp S, Busnelli I, Guck J, and Goetz JG (2021). Mechanical Adaptability of Tumor Cells in Metastasis. Developmental cell 56, 164–179. [DOI] [PubMed] [Google Scholar]
- George JT, Jolly MK, Xu S, Somarelli JA, and Levine H (2017). Survival Outcomes in Cancer Patients Predicted by a Partial EMT Gene Expression Scoring Metric. Cancer research 77, 6415–6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, and Shima D (2003). VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. The Journal of cell biology 161, 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar CM (2015). Metastasis prevention by targeting the dormant niche. Nature reviews Cancer 15, 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DYR, et al. (2013). The perivascular niche regulates breast tumour dormancy. Nature cell biology 15, 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour D, Rembold M, and Leptin M (2017). From morphogen to morphogenesis and back. Nature 541, 311–320. [DOI] [PubMed] [Google Scholar]
- Giuliano M, Shaikh A, Lo HC, Arpino G, De Placido S, Zhang XH, Cristofanilli M, Schiff R, and Trivedi MV (2018). Perspective on Circulating Tumor Cell Clusters: Why It Takes a Village to Metastasize. Cancer Res 78, 845–852. [DOI] [PubMed] [Google Scholar]
- Gkountela S, Castro-Giner F, Szczerba BM, Vetter M, Landin J, Scherrer R, Krol I, Scheidmann MC, Beisel C, Stirnimann CU, et al. (2019). Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 176, 98–112.e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaves D, Huben RP, and Weiss L (1988). Haematogenous dissemination of cells from human renal adenocarcinomas. Br J Cancer 57, 32–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit RZ, Masury H, Condiotti R, Fixler Y, Gabai Y, Glikman T, Dalin S, Winter E, Nevo Y, Carmon E, et al. (2018). Regulation of Cellular Heterogeneity and Rates of Symmetric and Asymmetric Divisions in Triple-Negative Breast Cancer. Cell reports 24, 3237–3250. [DOI] [PubMed] [Google Scholar]
- Gritsenko PG, Atlasy N, Dieteren CEJ, Navis AC, Venhuizen JH, Veelken C, Schubert D, Acker-Palmer A, Westerman BA, Wurdinger T, et al. (2020). p120-catenin-dependent collective brain infiltration by glioma cell networks. Nat Cell Biol 22, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse-Wilde A, Fouquier d’Hérouël A, McIntosh E, Ertaylan G, Skupin A, Kuestner RE, del Sol A, Walters KA, and Huang S (2015). Stemness of the hybrid Epithelial/Mesenchymal State in Breast Cancer and Its Association with Poor Survival. PLoS One 10, e0126522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JMC, Papaemmanuil E, Brewer DS, Kallio HML, Högnäs G, Annala M, et al. (2015). The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon JB (1988). A community effect in animal development. Nature 336, 772–774. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Tiller E, Roberts J, and Kato K (1993). A community effect in muscle development. Curr Biol 3, 1–11. [DOI] [PubMed] [Google Scholar]
- Haeger A, Alexander S, Vullings M, Kaiser FMP, Veelken C, Flucke U, Koehl GE, Hirschberg M, Flentje M, Hoffman RM, et al. (2020). Collective cancer invasion forms an integrin-dependent radioresistant niche. J Exp Med 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeger A, Krause M, Wolf K, and Friedl P (2014). Cell jamming: Collective invasion of mesenchymal tumor cells imposed by tissue confinement. Biochimica et Biophysica Acta (BBA) - General Subjects 1840, 2386–2395. [DOI] [PubMed] [Google Scholar]
- Haemmerle M, Taylor ML, Gutschner T, Pradeep S, Cho MS, Sheng J, Lyons YM, Nagaraja AS, Dood RL, and Wen Y (2017). Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nature communications 8, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamy AS, Lam GT, Laas E, Darrigues L, Balezeau T, Guerin J, Livartowski A, Sadacca B, Pierga JY, Vincent-Salomon A, et al. (2018). Lymphovascular invasion after neoadjuvant chemotherapy is strongly associated with poor prognosis in breast carcinoma. Breast Cancer Res Treat 169, 295–304. [DOI] [PubMed] [Google Scholar]
- Hanley CJ, Henriet E, Sirka OK, Thomas GJ, and Ewald AJ (2020). Tumor-Resident Stromal Cells Promote Breast Cancer Invasion through Regulation of the Basal Phenotype. Molecular Cancer Research 18, 1615–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna SJ, McCoy-Simandle K, Leung E, Genna A, Condeelis J, and Cox D (2019). Tunneling nanotubes, a novel mode of tumor cell–macrophage communication in tumor cell invasion. Journal of cell science 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy BT, Gonzalez-Angulo A-M, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee J-S, Fridlyand J, Sahin A, Agarwal R, and Joy C (2009). Characterization of a naturally occurring breast cancer subset enriched in epithelial-tomesenchymal transition and stem cell characteristics. Cancer research 69, 4116–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson J, Diane Yamada S, Berger J, Alverdy J, O’Keefe J, Bassler B, and Rinker-Schaeffer C (2009). Societal interactions in ovarian cancer metastasis: a quorum-sensing hypothesis. Clin Exp Metastasis 26, 67–76. [DOI] [PubMed] [Google Scholar]
- Hirata E, and Sahai E (2017). Tumor Microenvironment and Differential Responses to Therapy. Cold Spring Harb Perspect Med 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotsu Y, Hada M, Amemiya K, Oyama T, Mochizuki H, and Omata M (2020). Multi-regional sequencing reveals clonal and polyclonal seeding from primary tumor to metastases in advanced gastric cancer. J Gastroenterol 55, 553–564. [DOI] [PubMed] [Google Scholar]
- Hitomi M, Deleyrolle Loic P., Mulkearns-Hubert Erin E., Jarrar A, Li M, Sinyuk M, Otvos B, Brunet S, Flavahan William A., Hubert Christopher G., et al. (2015). Differential Connexin Function Enhances Self-Renewal in Glioblastoma. Cell reports 11, 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobor S, Van Emburgh BO, Crowley E, Misale S, Di Nicolantonio F, and Bardelli A (2014). TGFα and amphiregulin paracrine network promotes resistance to EGFR blockade in colorectal cancer cells. Clin Cancer Res 20, 6429–6438. [DOI] [PubMed] [Google Scholar]
- Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, Priest LJ, Greystoke A, Zhou C, Morris K, et al. (2012). Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 30, 525–532. [DOI] [PubMed] [Google Scholar]
- Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, Richardson A, Violette S, Nikolskaya T, and Nikolsky Y (2008). Regulation of in situ to invasive breast carcinoma transition. Cancer cell 13, 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Ding J, Ma Z, Sun R, Seoane JA, Scott Shaffer J, Suarez CJ, Berghoff AS, Cremolini C, Falcone A, et al. (2019). Quantitative evidence for early metastatic seeding in colorectal cancer. Nature Genetics 51, 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Li Z, Ma Z, and Curtis C (2020). Multi-cancer analysis of clonality and the timing of systemic spread in paired primary tumors and metastases. Nat Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson LG, Zeineldin R, and Stack MS (2008). Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clinical & experimental metastasis 25, 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, and Riethmüller G (2008). Systemic spread is an early step in breast cancer. Cancer cell 13, 58–68. [DOI] [PubMed] [Google Scholar]
- Hwang HJ, Oh M-S, Lee DW, and Kuh H-J (2019a). Multiplex quantitative analysis of stroma-mediated cancer cell invasion, matrix remodeling, and drug response in a 3D co-culture model of pancreatic tumor spheroids and stellate cells. Journal of Experimental & Clinical Cancer Research 38, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang PY, Brenot A, King AC, Longmore GD, and George SC (2019b). Randomly Distributed K14(+) Breast Tumor Cells Polarize to the Leading Edge and Guide Collective Migration in Response to Chemical and Mechanical Environmental Cues. Cancer research 79, 1899–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang TL, Close TP, Grego JM, Brannon WL, and Gonzales F (1996). Predilection of brain metastasis in gray and white matter junction and vascular border zones. Cancer 77, 1551–1555. [DOI] [PubMed] [Google Scholar]
- Ilina O, Gritsenko PG, Syga S, Lippoldt J, La Porta CAM, Chepizhko O, Grosser S, Vullings M, Bakker G-J, Starruß J, et al. (2020). Cell–cell adhesion and 3D matrix confinement determine jamming transitions in breast cancer invasion. Nature cell biology 22, 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackstadt R, van Hooff SR, Leach JD, Cortes-Lavaud X, Lohuis JO, Ridgway RA, Wouters VM, Roper J, Kendall TJ, Roxburgh CS, et al. (2019). Epithelial NOTCH Signaling Rewires the Tumor Microenvironment of Colorectal Cancer to Drive Poor-Prognosis Subtypes and Metastasis. Cancer cell 36, 319–336.e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson PR, Dekkers JF, Rios AC, Fu NY, Lindeman GJ, and Visvader JE (2017). Derivation of a robust mouse mammary organoid system for studying tissue dynamics. Development 144, 1065–1071. [DOI] [PubMed] [Google Scholar]
- Janiszewska M, Tabassum DP, Castaño Z, Cristea S, Yamamoto KN, Kingston NL, Murphy KC, Shu S, Harper NW, Del Alcazar CG, et al. (2019). Subclonal cooperation drives metastasis by modulating local and systemic immune microenvironments. Nature Cell Biology 21, 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janni W, Vogl FD, Wiedswang G, Synnestvedt M, Fehm T, Jückstock J, Borgen E, Rack B, Braun S, Sommer H, et al. (2011). Persistence of Disseminated Tumor Cells in the Bone Marrow of Breast Cancer Patients Predicts Increased Risk for Relapse—A European Pooled Analysis. Clinical Cancer Research 17, 2967–2976. [DOI] [PubMed] [Google Scholar]
- Jansson S, Bendahl P-O, Larsson A-M, Aaltonen KE, and Rydén L (2016). Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer 16, 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen DH, Reibel J, Mackenzie IC, and Dabelsteen E (2015). Single cell migration in oral squamous cell carcinoma - possible evidence of epithelial-mesenchymal transition in vivo. J Oral Pathol Med 44, 674–679. [DOI] [PubMed] [Google Scholar]
- Jiang X, Wong KHK, Khankhel AH, Zeinali M, Reategui E, Phillips MJ, Luo X, Aceto N, Fachin F, Hoang AN, et al. (2017). Microfluidic isolation of platelet-covered circulating tumor cells. Lab Chip 17, 3498–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly MK, Boareto M, Debeb BG, Aceto N, Farach-Carson MC, Woodward WA, and Levine H (2017). Inflammatory breast cancer: a model for investigating cluster-based dissemination. NPJ breast cancer 3, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly MK, Huang B, Lu M, Mani SA, Levine H, and Ben-Jacob E (2014). Towards elucidating the connection between epithelial-mesenchymal transitions and stemness. J R Soc Interface 11, 20140962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly MK, Kulkarni P, Weninger K, Orban J, and Levine H (2018). Phenotypic Plasticity, Bet-Hedging, and Androgen Independence in Prostate Cancer: Role of Non-Genetic Heterogeneity. Frontiers in Oncology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly MK, Somarelli JA, Sheth M, Biddle A, Tripathi SC, Armstrong AJ, Hanash SM, Bapat SA, Rangarajan A, and Levine H (2019). Hybrid epithelial/mesenchymal phenotypes promote metastasis and therapy resistance across carcinomas. Pharmacology & Therapeutics 194, 161–184. [DOI] [PubMed] [Google Scholar]
- Kan T, Wang W, Ip PP, Zhou S, Wong AS, Wang X, and Yang M (2020). Single-cell EMT-related transcriptional analysis revealed intra-cluster heterogeneity of tumor cell clusters in epithelial ovarian cancer ascites. Oncogene 39, 4227–4240. [DOI] [PubMed] [Google Scholar]
- Kantak SS, and Kramer RH (1998). E-cadherin regulates anchorage-independent growth and survival in oral squamous cell carcinoma cells. The Journal of biological chemistry 273, 16953–16961. [DOI] [PubMed] [Google Scholar]
- Karagiannis GS, Pastoriza JM, Wang Y, Harney AS, Entenberg D, Pignatelli J, Sharma VP, Xue EA, Cheng E, D’Alfonso TM, et al. (2017). Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S, Yamazaki M, Shibuya K, Yamazaki M, Fujii E, Nakano K, and Suzuki M (2020). Three-dimensional culture models mimic colon cancer heterogeneity induced by different microenvironments. Scientific Reports 10, 3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A, Ilina O, Vasaturo A, Venhuizen J-H, Vullings M, Venhuizen V, Bilos A, Figdor C, Span P, and Friedl P (2019). Leader cell activity and collective invasion by an autocrine nucleotide loop through connexin-43 hemichannels and ADORA1 (bioRxiv). [DOI] [PMC free article] [PubMed]
- Khalil AA, Ilina O, Gritsenko PG, Bult P, Span PN, and Friedl P (2017). Collective invasion in ductal and lobular breast cancer associates with distant metastasis. Clinical & Experimental Metastasis 34, 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil AA, Ilina O, Vasaturo A, Venhuizen JH, Vullings M, Venhuizen V, Bilos A, Figdor CG, Span PN, and Friedl P (2020). Collective invasion induced by an autocrine purinergic loop through connexin-43 hemichannels. The Journal of cell biology 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WEF, Goldbrunner R, Herms J, and Winkler F (2010). Real-time imaging reveals the single steps of brain metastasis formation. Nature Medicine 16, 116–122. [DOI] [PubMed] [Google Scholar]
- Kim TH, Wang Y, Oliver CR, Thamm DH, Cooling L, Paoletti C, Smith KJ, Nagrath S, and Hayes DF (2019). A temporary indwelling intravascular aphaeretic system for in vivo enrichment of circulating tumor cells. Nature communications 10, 1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Choi YW, Lee J, Soh EY, Kim J-H, and Park TJ (2017). Senescent tumor cells lead the collective invasion in thyroid cancer. Nature communications 8, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Qian B-Z, and Pollard JW (2015). Immune cell promotion of metastasis. Nature Reviews Immunology 15, 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimek M (2019). Pulmonary lymphangitis carcinomatosis: systematic review and meta-analysis of case reports, 1970–2018. Postgrad Med 131, 309–318. [DOI] [PubMed] [Google Scholar]
- Klymenko Y, Johnson J, Bos B, Lombard R, Campbell L, Loughran E, and Stack MS (2017). Heterogeneous Cadherin Expression and Multicellular Aggregate Dynamics in Ovarian Cancer Dissemination. Neoplasia 19, 549–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok SY, Oshima H, Takahashi K, Nakayama M, Murakami K, Ueda HR, Miyazono K, and Oshima M (2021). Malignant subclone drives metastasis of genetically and phenotypically heterogenous cell clusters through fibrotic niche generation. Nature communications 12, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konen J, Summerbell E, Dwivedi B, Galior K, Hou Y, Rusnak L, Chen A, Saltz J, Zhou W, Boise LH, et al. (2017). Image-guided genomics of phenotypically heterogeneous populations reveals vascular signalling during symbiotic collective cancer invasion. Nature communications 8, 15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong T, Ahn R, Yang K, Zhu X, Fu Z, Morin G, Bramley R, Cliffe NC, Xue Y, Kuasne H, et al. (2020). CD44 Promotes PD-L1 Expression and Its Tumor-Intrinsic Function in Breast and Lung Cancers. Cancer research 80, 444–457. [DOI] [PubMed] [Google Scholar]
- Korolev KS, Xavier JB, and Gore J (2014). Turning ecology and evolution against cancer. Nat Rev Cancer 14, 371–380. [DOI] [PubMed] [Google Scholar]
- Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G, et al. (2011). Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 29, 1556–1563. [DOI] [PubMed] [Google Scholar]
- Kröger C, Afeyan A, Mraz J, Eaton EN, Reinhardt F, Khodor YL, Thiru P, Bierie B, Ye X, Burge CB, et al. (2019). Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proceedings of the National Academy of Sciences 116, 7353–7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Shimazu Y, Yagishita H, Izumo T, Soeno Y, Sato K, Taya Y, and Aoba T (2013). Three-dimensional reconstruction of oral tongue squamous cell carcinoma at invasion front. Int J Dent 2013, 482765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulasinghe A, Schmidt H, Perry C, Whitfield B, Kenny L, Nelson C, Warkiani ME, and Punyadeera C (2018). A collective route to head and neck cancer metastasis. Scientific reports 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurley SJ, Tischler V, Bierie B, Novitskiy SV, Noske A, Varga Z, Zürrer-Härdi U, Brandt S, Carnahan RH, Cook RS, et al. (2020). A Requirement for p120-catenin in the metastasis of invasive ductal breast cancer. J Cell Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M, Begum S, and Hynes RO (2011). Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer cell 20, 576–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labernadie A, Kato T, Brugues A, Serra-Picamal X, Derzsi S, Arwert E, Weston A, Gonzalez-Tarrago V, Elosegui-Artola A, Albertazzi L, et al. (2017). A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nature cell biology 19, 224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne CF, Cheung EC, Blagih J, Domart MC, and Vousden KH (2019). Cell Clustering Promotes a Metabolic Switch that Supports Metastatic Colonization. Cell Metab 30, 720–734.e725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AW, Pattabiraman DR, and Weinberg RA (2017). Emerging Biological Principles of Metastasis. Cell 168, 670–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G, Estévez-Salmeron L, Oh S, Liao D, Emerson BM, Tlsty TD, and Austin RH (2011). An analogy between the evolution of drug resistance in bacterial communities and malignant tissues. Nature reviews Cancer 11, 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson A-M, Jansson S, Bendahl P-O, Levin Tykjaer Jörgensen C, Loman N, Graffman C, Lundgren L, Aaltonen K, and Rydén L (2018). Longitudinal enumeration and cluster evaluation of circulating tumor cells improve prognostication for patients with newly diagnosed metastatic breast cancer in a prospective observational trial. Breast Cancer Research 20, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson DA, Bhakta NR, Kessenbrock K, Prummel KD, Yu Y, Takai K, Zhou A, Eyob H, Balakrishnan S, Wang C-Y, et al. (2015). Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 526, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Kim EJ, Cho Y, Kim S, Chung HH, Park NH, and Song YS (2017). Predictive value of circulating tumor cells (CTCs) captured by microfluidic device in patients with epithelial ovarian cancer. Gynecol Oncol 145, 361–365. [DOI] [PubMed] [Google Scholar]
- Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, and Pietenpol JA (2011). Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121, 2750–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton J, Kalla RL, Turner JM Jr., and Fennell RH Jr. (1960). Pathogenesis of tumor invasion. II. Aggregate replication. Cancer research 20, 575–586. [PubMed] [Google Scholar]
- Leung ML, Davis A, Gao R, Casasent A, Wang Y, Sei E, Vilar E, Maru D, Kopetz S, and Navin NE (2017). Single-cell DNA sequencing reveals a late-dissemination model in metastatic colorectal cancer. Genome Res 27, 1287–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-F, Chen J-Y, Ho Y-H, Hsu W-H, Wu L-C, Lan H-Y, Hsu DS-S, Tai S-K, Chang Y-C, and Yang M-H (2019). Snail-induced claudin-11 prompts collective migration for tumour progression. Nature cell biology 21, 251–262. [DOI] [PubMed] [Google Scholar]
- Li Y, Hofmann M, Wang Q, Teng L, Chlewicki LK, Pircher H, and Mariuzza RA (2009). Structure of natural killer cell receptor KLRG1 bound to E-cadherin reveals basis for MHC-independent missing self recognition. Immunity 31, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SB, Yu CS, Jang SJ, Kim TW, Kim JH, and Kim JC (2010). Prognostic significance of lymphovascular invasion in sporadic colorectal cancer. Dis Colon Rectum 53, 377–384. [DOI] [PubMed] [Google Scholar]
- Lin S, Negulescu A, Bulusu S, Gibert B, Delcros J-G, Ducarouge B, Rama N, Gadot N, Treilleux I, and Saintigny P (2017). Non-canonical NOTCH3 signalling limits tumour angiogenesis. Nature communications 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta LA, and Kohn EC (2001). The microenvironment of the tumour–host interface. Nature 411, 375–379. [DOI] [PubMed] [Google Scholar]
- Liotta LA, Saidel MG, and Kleinerman J (1976). The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res 36, 889–894. [PubMed] [Google Scholar]
- Liu X, Taftaf R, Kawaguchi M, Chang YF, Chen W, Entenberg D, Zhang Y, Gerratana L, Huang S, Patel DB, et al. (2019). Homophilic CD44 Interactions Mediate Tumor Cell Aggregation and Polyclonal Metastasis in Patient-Derived Breast Cancer Models. Cancer Discov 9, 96–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livshits G, Kobielak A, and Fuchs E (2012). Governing epidermal homeostasis by coupling cell-cell adhesion to integrin and growth factor signaling, proliferation, and apoptosis. Proceedings of the National Academy of Sciences of the United States of America 109, 4886–4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HC, Xu Z, Kim IS, Pingel B, Aguirre S, Kodali S, Liu J, Zhang W, Muscarella AM, Hein SM, et al. (2020). Resistance to natural killer cell immunosurveillance confers a selective advantage to polyclonal metastasis. Nature Cancer 1, 709–722. [DOI] [PubMed] [Google Scholar]
- Long E, Ilie M, Bence C, Butori C, Selva E, Lalvee S, Bonnetaud C, Poissonnet G, Lacour JP, Bahadoran P, et al. (2016a). High expression of TRF2, SOX10, and CD10 in circulating tumor microemboli detected in metastatic melanoma patients. A potential impact for the assessment of disease aggressiveness. Cancer Med 5, 1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E, Ilie M, Bence C, Butori C, Selva E, Lalvée S, Bonnetaud C, Poissonnet G, Lacour JP, Bahadoran P, et al. (2016b). High expression of TRF2, SOX10, and CD10 in circulating tumor microemboli detected in metastatic melanoma patients. A potential impact for the assessment of disease aggressiveness. Cancer Med 5, 1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Soto A, Huergo-Zapico L, Galván JA, Rodrigo L, de Herreros AG, Astudillo A, and Gonzalez S (2013). Epithelial–Mesenchymal Transition Induces an Antitumor Immune Response Mediated by NKG2D Receptor. The Journal of Immunology 190, 4408–4419. [DOI] [PubMed] [Google Scholar]
- Lu H, Clauser KR, Tam WL, Fröse J, Ye X, Eaton EN, Reinhardt F, Donnenberg VS, Bhargava R, Carr SA, et al. (2014). A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nature cell biology 16, 1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, and Groom AC (1998). Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. The American journal of pathology 153, 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssiotis CA, and Kimmelman AC (2017). Metabolic Interactions in the Tumor Microenvironment. Trends in cell biology 27, 863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddipati R, and Stanger BZ (2015). Pancreatic Cancer Metastases Harbor Evidence of Polyclonality. Cancer Discov 5, 1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen CD, and Sahai E (2010). Cancer dissemination--lessons from leukocytes. Developmental cell 19, 13–26. [DOI] [PubMed] [Google Scholar]
- Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, Sasaki T, Stavnezer E, Iannotti J, Schweitzer R, et al. (2011). Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr Biol 21, 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak M, Reinhart-King CA, and Erickson D (2013). Elucidating mechanical transition effects of invading cancer cells with a subnucleus-scaled microfluidic serial dimensional modulation device. Lab Chip 13, 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malet-Engra G, Yu W, Oldani A, Rey-Barroso J, Gov, Nir S, Scita G, and Dupré L (2015). Collective Cell Motility Promotes Chemotactic Prowess and Resistance to Chemorepulsion. Current Biology 25, 242–250. [DOI] [PubMed] [Google Scholar]
- Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, de Stanchina E, and Massagué J (2016). Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell 165, 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath Y, Upparahalli SV, Suvilesh KN, Avella DM, Kimchi ET, Staveley-O’Carroll KF, Li G, and Kaifi JT (2019). Circulating tumor cell clusters are a potential biomarker for detection of non-small cell lung cancer. Lung Cancer 134, 147–150. [DOI] [PubMed] [Google Scholar]
- Martín-Pardillos A, Valls Chiva Á, Bande Vargas G, Hurtado Blanco P, Piñeiro Cid R, Guijarro PJ, Hümmer S, Bejar Serrano E, Rodriguez-Casanova A, Diaz-Lagares Á, et al. (2019). The role of clonal communication and heterogeneity in breast cancer. BMC Cancer 19, 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusyk A, Tabassum DP, Altrock PM, Almendro V, Michor F, and Polyak K (2014). Non-cell-autonomous driving of tumour growth supports sub-clonal heterogeneity. Nature 514, 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, Lehmann BD, Pietenpol JA, Hortobagyi GN, et al. (2013). Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res 19, 5533–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y, Ito Y, Mezawa Y, Sulidan K, Daigo Y, Hiraga T, Mogushi K, Wali N, Suzuki H, Itoh T, et al. (2019). Stromal fibroblasts induce metastatic tumor cell clusters via epithelial-mesenchymal plasticity. Life Sci Alliance 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel M, Jacot W, Pantel K, Bartkowiak K, Topart D, Cayrefourcq L, Rossille D, Maudelonde T, Fest T, and Alix-Panabières C (2015). Frequent expression of PD-L1 on circulating breast cancer cells. Molecular oncology 9, 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli S, Piccotti F, Allevi R, Truffi M, Sorrentino L, Russo L, Agozzino M, Signati L, Bonizzi A, Villani L, et al. (2019). Establishment and Morphological Characterization of Patient-Derived Organoids from Breast Cancer. Biological Procedures Online 21, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey Luke M., Montalbano J, Mihai C, and Macara Ian G. (2012). Loss of the Par3 Polarity Protein Promotes Breast Tumorigenesis and Metastasis. Cancer cell 22, 601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCart Reed AE, Kalaw E, Nones K, Bettington M, Lim M, Bennett J, Johnstone K, Kutasovic JR, Saunus JM, and Kazakoff S (2019). Phenotypic and molecular dissection of metaplastic breast cancer and the prognostic implications. The Journal of pathology 247, 214–227. [DOI] [PubMed] [Google Scholar]
- McFaline-Figueroa JL, Hill AJ, Qiu X, Jackson D, Shendure J, and Trapnell C (2019). A pooled single-cell genetic screen identifies regulatory checkpoints in the continuum of the epithelial-to-mesenchymal transition. Nature Genetics 51, 1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson A, Roth A, Laks E, Masud T, Bashashati A, Zhang AW, Ha G, Biele J, Yap D, Wan A, et al. (2016). Divergent modes of clonal spread and intraperitoneal mixing in high-grade serous ovarian cancer. Nature Genetics 48, 758–767. [DOI] [PubMed] [Google Scholar]
- Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, Beitsch PD, Leitch M, Hoover S, Euhus D, et al. (2004). Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res 10, 8152–8162. [DOI] [PubMed] [Google Scholar]
- Meurette O, and Mehlen P (2018). Notch Signaling in the Tumor Microenvironment. Cancer cell 34, 536–548. [DOI] [PubMed] [Google Scholar]
- Meyer-Schaller N, Cardner M, Diepenbruck M, Saxena M, Tiede S, Lüönd F, Ivanek R, Beerenwinkel N, and Christofori G (2019). A Hierarchical Regulatory Landscape during the Multiple Stages of EMT. Developmental cell 48, 539–553.e536. [DOI] [PubMed] [Google Scholar]
- Miranti CK, and Brugge JS (2002). Sensing the environment: a historical perspective on integrin signal transduction. Nature cell biology 4, E83–E90. [DOI] [PubMed] [Google Scholar]
- Mishra AK, Campanale JP, Mondo JA, and Montell DJ (2019). Cell interactions in collective cell migration. Development (Cambridge, England) 146, dev172056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukoshi K, Okazawa Y, Haeno H, Koyama Y, Sulidan K, Komiyama H, Saeki H, Ohtsuji N, Ito Y, Kojima Y, et al. (2020). Metastatic seeding of human colon cancer cell clusters expressing the hybrid epithelial/mesenchymal state. Int J Cancer 146, 2547–2562. [DOI] [PubMed] [Google Scholar]
- Mohammed RAA, Martin SG, Mahmmod AM, Macmillan RD, Green AR, Paish EC, and Ellis IO (2011). Objective assessment of lymphatic and blood vascular invasion in lymph node-negative breast carcinoma: findings from a large case series with long-term follow-up. The Journal of Pathology 223, 358–365. [DOI] [PubMed] [Google Scholar]
- Mohme M, Riethdorf S, and Pantel K (2017). Circulating and disseminated tumour cells — mechanisms of immune surveillance and escape. Nature Reviews Clinical Oncology 14, 155–167. [DOI] [PubMed] [Google Scholar]
- Molnar B, Ladanyi A, Tanko L, Sréter L, and Tulassay Z (2001). Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin Cancer Res 7, 4080–4085. [PubMed] [Google Scholar]
- Montaudouin C, Anson M, Hao Y, Duncker SV, Fernandez T, Gaudin E, Ehrenstein M, Kerr WG, Colle J-H, and Bruhns P (2013). Quorum sensing contributes to activated IgM-secreting B cell homeostasis. The Journal of Immunology 190, 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Z, Wang C, Ye Z, Austin L, Civan J, Hyslop T, Palazzo JP, Jaslow R, Li B, Myers RE, et al. (2015). Prospective assessment of the prognostic value of circulating tumor cells and their clusters in patients with advanced-stage breast cancer. Breast Cancer Res Treat 154, 563–571. [DOI] [PubMed] [Google Scholar]
- Muinonen-Martin AJ, Susanto O, Zhang Q, Smethurst E, Faller WJ, Veltman DM, Kalna G, Lindsay C, Bennett DC, and Sansom OJ (2014). Melanoma cells break down LPA to establish local gradients that drive chemotactic dispersal. PLoS biol 12, e1001966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P, and Schier AF (2011). Extracellular movement of signaling molecules. Developmental cell 21, 145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murlidhar V, Reddy RM, Fouladdel S, Zhao L, Ishikawa MK, Grabauskiene S, Zhang Z, Lin J, Chang AC, Carrott P, et al. (2017). Poor Prognosis Indicated by Venous Circulating Tumor Cell Clusters in Early-Stage Lung Cancers. Cancer research 77, 5194–5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na TY, Schecterson L, Mendonsa AM, and Gumbiner BM (2020). The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proceedings of the National Academy of Sciences of the United States of America 117, 5931–5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naffar-Abu Amara S, Kuiken HJ, Selfors LM, Butler T, Leung ML, Leung CT, Kuhn EP, Kolarova T, Hage C, Ganesh K, et al. (2020). Transient commensal clonal interactions can drive tumor metastasis. Nature communications 11, 5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, et al. (2007). Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450, 1235–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Iwamoto R, and Mekada E (1995). Membrane-anchored heparin-binding EGF-like growth factor (HB-EGF) and diphtheria toxin receptor-associated protein (DRAP27)/CD9 form a complex with integrin alpha 3 beta 1 at cell-cell contact sites. Journal of Cell Biology 129, 1691–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan D, Zhou H, Oliphant MUJ, Zhang X, Simon LM, Henke DM, Shaw CA, Wu M-F, Hilsenbeck SG, White LD, et al. (2017). EMT cells increase breast cancer metastasis via paracrine GLI activation in neighbouring tumour cells. Nature communications 8, 15773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okegawa T, Ninomiya N, Masuda K, Nakamura Y, Tambo M, and Nutahara K (2018). AR-V7 in circulating tumor cells cluster as a predictive biomarker of abiraterone acetate and enzalutamide treatment in castration-resistant prostate cancer patients. Prostate 78, 576–582. [DOI] [PubMed] [Google Scholar]
- Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, Weil S, Horstmann H, Wiestler B, Syed M, et al. (2015). Brain tumour cells interconnect to a functional and resistant network. Nature 528, 93–98. [DOI] [PubMed] [Google Scholar]
- Padmanaban V, Grasset EM, Neumann NM, Fraser AK, Henriet E, Matsui W, Tran PT, Cheung KJ, Georgess D, and Ewald AJ (2020). Organotypic culture assays for murine and human primary and metastatic-site tumors. Nat Protoc 15, 2413–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanaban V, Krol I, Suhail Y, Szczerba BM, Aceto N, Bader JS, and Ewald AJ (2019). E-cadherin is required for metastasis in multiple models of breast cancer. Nature 573, 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine IS, and Lewis MT (2017). The Terminal End Bud: the Little Engine that Could. J Mammary Gland Biol Neoplasia 22, 93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, and Dowsett M (2017). 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. New England Journal of Medicine 377, 1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantel K, and Alix-Panabières C (2019). Liquid biopsy and minimal residual disease— latest advances and implications for cure. Nature Reviews Clinical Oncology 16, 409–424. [DOI] [PubMed] [Google Scholar]
- Paoletti C, Li Y, Muñiz MC, Kidwell KM, Aung K, Thomas DG, Brown ME, Abramson VG, Irvin WJ Jr., Lin NU, et al. (2015). Significance of Circulating Tumor Cells in Metastatic Triple-Negative Breast Cancer Patients within a Randomized, Phase II Trial: TBCRC 019. Clin Cancer Res 21, 2771–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti C, Miao J, Dolce EM, Darga EP, Repollet MI, Doyle GV, Gralow JR, Hortobagyi GN, Smerage JB, Barlow WE, et al. (2019). Circulating Tumor Cell Clusters in Patients with Metastatic Breast Cancer: a SWOG S0500 Translational Medicine Study. Clin Cancer Res 25, 6089–6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenfort K, and Bassler BL (2016). Quorum sensing signal-response systems in Gram-negative bacteria. Nature reviews Microbiology 14, 576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareja F, Brown DN, Lee JY, Da Cruz Paula A, Selenica P, Bi R, Geyer FC, Gazzo A, da Silva EM, Vahdatinia M, et al. (2020). Whole-Exome Sequencing Analysis of the Progression from Non–Low-Grade Ductal Carcinoma In Situ to Invasive Ductal Carcinoma. Clinical Cancer Research 26, 3682–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastushenko I, and Blanpain C (2019). EMT Transition States during Tumor Progression and Metastasis. Trends in cell biology 29, 212–226. [DOI] [PubMed] [Google Scholar]
- Patil R, Tan X, Bartosik P, Detappe A, Runnels JM, Ghobrial I, Lin CP, and Niedre M (2019). Fluorescence monitoring of rare circulating tumor cell and cluster dissemination in a multiple myeloma xenograft model in vivo. J Biomed Opt 24, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsialou A, Wyckoff J, Wang Y, Goswami S, Stanley ER, and Condeelis JS (2009). Invasion of Human Breast Cancer Cells In vivo Requires Both Paracrine and Autocrine Loops Involving the Colony-Stimulating Factor-1 Receptor. Cancer research 69, 9498–9506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova NN, Pallasch C, Elia AEH, Braun CJ, Westbrook TF, Hemann M, and Elledge SJ (2013). A role for PVRL4-driven cell-cell interactions in tumorigenesis. eLife 2, e00358–e00358.23682311 [Google Scholar]
- Pettigrew CA, Asp E, and Emerson CP Jr. (2014). A new role for Hedgehogs in juxtacrine signaling. Mech Dev 131, 137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli J, Bravo-Cordero JJ, Roh-Johnson M, Gandhi SJ, Wang Y, Chen X, Eddy RJ, Xue A, Singer RH, Hodgson L, et al. (2016). Macrophage-dependent tumor cell transendothelial migration is mediated by Notch1/Mena(INV)-initiated invadopodium formation. Sci Rep 6, 37874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ, and Morrison SJ (2015). Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placke T, Örgel M, Schaller M, Jung G, Rammensee H-G, Kopp H-G, and Salih HR (2012). Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer research 72, 440–448. [DOI] [PubMed] [Google Scholar]
- Polonsky M, Rimer J, Kern-Perets A, Zaretsky I, Miller S, Bornstein C, David E, Kopelman NM, Stelzer G, Porat Z, et al. (2018). Induction of CD4 T cell memory by local cellular collectivity. Science (New York, NY) 360. [DOI] [PubMed] [Google Scholar]
- Puram SV, Tirosh I, Parikh AS, Patel AP, Yizhak K, Gillespie S, Rodman C, Luo CL, Mroz EA, Emerick KS, et al. (2017). Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 171, 1611–1624.e1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan Q, Wang X, Lu C, Ma W, Wang Y, Xia G, Wang C, and Yang G (2020). Cancer stem-like cells with hybrid epithelial/mesenchymal phenotype leading the collective invasion. Cancer Sci 111, 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC (1992). Social controls on cell survival and cell death. Nature 356, 397–400. [DOI] [PubMed] [Google Scholar]
- Rayavarapu RR, Heiden B, Pagani N, Shaw MM, Shuff S, Zhang S, and Schafer ZT (2015). The role of multicellular aggregation in the survival of ErbB2-positive breast cancer cells during extracellular matrix detachment. The Journal of biological chemistry 290, 8722–8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Liu L, Ren C, Lindgren P, Boman K, Shen Y, Lundin E, Ottander U, Rytinki M, and Liu K (2005). Formation of E-cadherin-mediated cell-cell adhesion activates AKT and mitogen activated protein kinase via phosphatidylinositol 3 kinase and ligand-independent activation of epidermal growth factor receptor in ovarian cancer cells. Molecular Endocrinology 19, 2564–2578. [DOI] [PubMed] [Google Scholar]
- Reddy RM, Murlidhar V, Zhao L, Grabauskiene S, Zhang Z, Ramnath N, Lin J, Chang AC, Carrott P, Lynch W, et al. (2016). Pulmonary venous blood sampling significantly increases the yield of circulating tumor cells in early-stage lung cancer. The Journal of Thoracic and Cardiovascular Surgery 151, 852–858. [DOI] [PubMed] [Google Scholar]
- Reffay M, Parrini MC, Cochet-Escartin O, Ladoux B, Buguin A, Coscoy S, Amblard F, Camonis J, and Silberzan P (2014). Interplay of RhoA and mechanical forces in collective cell migration driven by leader cells. Nature cell biology 16, 217–223. [DOI] [PubMed] [Google Scholar]
- Reymond N, d’Água BB, and Ridley AJ (2013). Crossing the endothelial barrier during metastasis. Nature Reviews Cancer 13, 858–870. [DOI] [PubMed] [Google Scholar]
- Riahi R, Sun J, Wang S, Long M, Zhang DD, and Wong PK (2015). Notch1-Dll4 signalling and mechanical force regulate leader cell formation during collective cell migration. Nature communications 6, 6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RS, June CH, Langer R, and Mitchell MJ (2019). Delivery technologies for cancer immunotherapy. Nature reviews Drug discovery 18, 175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risson E, Nobre AR, Maguer-Satta V, and Aguirre-Ghiso JA (2020). The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nature Cancer 1, 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson FM, Bondy M, Yang W, Yamauchi H, Wiggins S, Kamrudin S, Krishnamurthy S, Le-Petross H, Bidaut L, and Player AN (2010). Inflammatory breast cancer: the disease, the biology, the treatment. CA: a cancer journal for clinicians 60, 351–375. [DOI] [PubMed] [Google Scholar]
- Roh-Johnson M, Bravo-Cordero JJ, Patsialou A, Sharma VP, Guo P, Liu H, Hodgson L, and Condeelis J (2014). Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene 33, 4203–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh-Johnson M, Shah AN, Stonick JA, Poudel KR, Kargl J, Yang GH, di Martino J, Hernandez RE, Gast CE, Zarour LR, et al. (2017). Macrophage-Dependent Cytoplasmic Transfer during Melanoma Invasion In Vivo. Developmental cell 43, 549–562.e546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos ET, Balsamo M, Alford SK, Wyckoff JB, Gligorijevic B, Wang Y, Pozzuto M, Stobezki R, Goswami S, Segall JE, et al. (2011). Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J Cell Sci 124, 2120–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppender N, Larson S, Lakely B, Kollath L, Brown L, Coleman I, Coleman R, Nguyen H, Nelson PS, Corey E, et al. (2015). Cellular Adhesion Promotes Prostate Cancer Cells Escape from Dormancy. PLoS One 10, e0130565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H, et al. (2018). A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 172, 373–386.e310. [DOI] [PubMed] [Google Scholar]
- Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, et al. (2020). A framework for advancing our understanding of cancer-associated fibroblasts. Nature reviews Cancer 20, 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarioglu AF, Aceto N, Kojic N, Donaldson MC, Zeinali M, Hamza B, Engstrom A, Zhu H, Sundaresan TK, Miyamoto DT, et al. (2015). A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat Methods 12, 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasportas LS, and Gambhir SS (2014). Imaging Circulating Tumor Cells in Freely Moving Awake Small Animals Using a Miniaturized Intravital Microscope. PLOS ONE 9, e86759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawabata N, Susaki Y, Nakamura T, Kawaguchi T, Yasukawa M, and Taniguchi S (2020). Cluster circulating tumor cells in surgical cases of lung cancer. General Thoracic and Cardiovascular Surgery 68, 975–983. [DOI] [PubMed] [Google Scholar]
- Scheele CLGJ, Hannezo E, Muraro MJ, Zomer A, Langedijk NSM, van Oudenaarden A, Simons BD, and van Rheenen J (2017). Identity and dynamics of mammary stem cells during branching morphogenesis. Nature 542, 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppmann SF, Bayer G, Aumayr K, Taucher S, Geleff S, Rudas M, Kubista E, Hausmaninger H, Samonigg H, Gnant M, et al. (2004). Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg 240, 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley TD, and Visscher PK (2004). Quorum sensing during nest-site selection by honeybee swarms. Behavioral Ecology and Sociobiology 56, 594–601. [Google Scholar]
- Shamir ER, and Ewald AJ (2014). Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nature reviews Molecular cell biology 15, 647–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimasaki N, Jain A, and Campana D (2020). NK cells for cancer immunotherapy. Nature Reviews Drug Discovery 19, 200–218. [DOI] [PubMed] [Google Scholar]
- Shyer AE, Huycke TR, Lee C, Mahadevan L, and Tabin CJ (2015). Bending gradients: how the intestinal stem cell gets its home. Cell 161, 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebel C, and Lendahl U (2017). Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol Rev 97, 1235–1294. [DOI] [PubMed] [Google Scholar]
- Siegel MB, He X, Hoadley KA, Hoyle A, Pearce JB, Garrett AL, Kumar S, Moylan VJ, Brady CM, Van Swearingen AE, et al. (2018). Integrated RNA and DNA sequencing reveals early drivers of metastatic breast cancer. J Clin Invest 128, 1371–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver DL, and Montell DJ (2001). Paracrine Signaling through the JAK/STAT Pathway Activates Invasive Behavior of Ovarian Epithelial Cells in Drosophila. Cell 107, 831–841. [DOI] [PubMed] [Google Scholar]
- Silvestri VL, Henriet E, Linville RM, Wong AD, Searson PC, and Ewald AJ (2020). A tissue-engineered 3D microvessel model reveals the dynamics of mosaic vessel formation in breast cancer. Cancer Research, canres.1564.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simian M, and Bissell MJ (2017). Organoids: A historical perspective of thinking in three dimensions. J Cell Biol 216, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AB, and Harris RC (2005). Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cellular Signalling 17, 1183–1193. [DOI] [PubMed] [Google Scholar]
- Souza-Fonseca-Guimaraes F, Cursons J, and Huntington ND (2019). The Emergence of Natural Killer Cells as a Major Target in Cancer Immunotherapy. Trends in immunology 40, 142–158. [DOI] [PubMed] [Google Scholar]
- Steinert G, Schölch S, Niemietz T, Iwata N, García SA, Behrens B, Voigt A, Kloor M, Benner A, Bork U, et al. (2014). Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer research 74, 1694–1704. [DOI] [PubMed] [Google Scholar]
- Summerbell ER, Mouw JK, Bell JSK, Knippler CM, Pedro B, Arnst JL, Khatib TO, Commander R, Barwick BG, Konen J, et al. (2020). Epigenetically heterogeneous tumor cells direct collective invasion through filopodia-driven fibronectin micropatterning. Science Advances 6, eaaz6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y-F, Guo W, Xu Y, Shi Y-H, Gong Z-J, Ji Y, Du M, Zhang X, Hu B, Huang A, et al. (2018). Circulating Tumor Cells from Different Vascular Sites Exhibit Spatial Heterogeneity in Epithelial and Mesenchymal Composition and Distinct Clinical Significance in Hepatocellular Carcinoma. Clinical Cancer Research 24, 547–559. [DOI] [PubMed] [Google Scholar]
- Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, et al. (2019). Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566, 553–557. [DOI] [PubMed] [Google Scholar]
- Tabassum DP, and Polyak K (2015). Tumorigenesis: it takes a village. Nature reviews Cancer 15, 473–483. [DOI] [PubMed] [Google Scholar]
- Taddei ML, Giannoni E, Fiaschi T, and Chiarugi P (2012). Anoikis: an emerging hallmark in health and diseases. The Journal of Pathology 226, 380–393. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Katsuta E, Yan L, Tokumaru Y, Katz MHG, and Takabe K (2020). Transcriptomic Profile of Lymphovascular Invasion, a Known Risk Factor of Pancreatic Ductal Adenocarcinoma Metastasis. Cancers (Basel) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W-F, Wu M, Bao H, Xu Y, Lin J-S, Liang Y, Zhang Y, Chu X-P, Qiu Z-B, Su J, et al. (2021). Timing and Origins of Local and Distant Metastases in Lung Cancer. Journal of Thoracic Oncology. [DOI] [PubMed] [Google Scholar]
- Tarin D (1969). Fine structure of murine mammary tumours: the relationship between epithelium and connective tissue in neoplasms induced by various agents. British Journal of Cancer 23, 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, et al. (2010). Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proceedings of the National Academy of Sciences 107, 15449–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Qian B, Zhang S, Guo R, Zhang H, Jeannon JP, Jin R, Feng X, Zhan Y, Liu J, et al. (2020). Three-dimensional reconstruction of laryngeal cancer with whole organ serial immunohistochemical sections. Sci Rep 10, 18962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda S, Frankel NW, and Lim WA (2019). Engineering cell–cell communication networks: programming multicellular behaviors. Current Opinion in Chemical Biology 52, 31–38. [DOI] [PubMed] [Google Scholar]
- Trepat X, Wasserman MR, Angelini TE, Millet E, Weitz DA, Butler JP, and Fredberg JJ (2009). Physical forces during collective cell migration. Nature Physics 5, 426–430. [Google Scholar]
- Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, and Hu GF (2008). Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer research 68, 10377–10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulintz PJ, Greenson JK, Wu R, Fearon ER, and Hardiman KM (2018). Lymph Node Metastases in Colon Cancer Are Polyclonal. Clin Cancer Res 24, 2214–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah I, Karthik GM, Alkodsi A, Kjällquist U, Stålhammar G, Lövrot J, Martinez NF, Lagergren J, Hautaniemi S, Hartman J, et al. (2018). Evolutionary history of metastatic breast cancer reveals minimal seeding from axillary lymph nodes. J Clin Invest 128, 1355–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente M, Obenauf Anna C., Jin X, Chen Q, Zhang Xiang H.F., Lee Derek J., Chaft Jamie E., Kris Mark G., Huse Jason T., Brogi E, et al. (2014). Serpins Promote Cancer Cell Survival and Vascular Co-Option in Brain Metastasis. Cell 156, 1002–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J, Taylor-Weiner A, Kester L, et al. (2015). Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vona G, Estepa L, Béroud C, Damotte D, Capron F, Nalpas B, Mineur A, Franco D, Lacour B, Pol S, et al. (2004). Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology 39, 792–797. [DOI] [PubMed] [Google Scholar]
- Wang C, Mu Z, Chervoneva I, Austin L, Ye Z, Rossi G, Palazzo JP, Sun C, Abu-Khalaf M, Myers RE, et al. (2017). Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res Treat 161, 83–94. [DOI] [PubMed] [Google Scholar]
- Wang D-Y, Jiang Z, Ben-David Y, Woodgett JR, and Zacksenhaus E (2019). Molecular stratification within triple-negative breast cancer subtypes. Scientific Reports 9, 19107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartlick O, Mumcu P, Jülicher F, and Gonzalez-Gaitan M (2011). Understanding morphogenetic growth control — lessons from flies. Nature Reviews Molecular Cell Biology 12, 594–604. [DOI] [PubMed] [Google Scholar]
- Watanabe S (1954). The metastasizability of tumor cells. Cancer 7, 215–223. [DOI] [PubMed] [Google Scholar]
- Wei Q, Ye Z, Zhong X, Li L, Wang C, Myers RE, Palazzo JP, Fortuna D, Yan A, Waldman SA, et al. (2017). Multiregion whole-exome sequencing of matched primary and metastatic tumors revealed genomic heterogeneity and suggested polyclonal seeding in colorectal cancer metastasis. Ann Oncol 28, 2135–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R. r., Sun D. n., Yang H, Yan J, Zhang X, Zheng X. l., Fu X. h., Geng M. y., Huang X, and Ding J (2018). CTC clusters induced by heparanase enhance breast cancer metastasis. Acta Pharmacologica Sinica 39, 1326–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt B, Geyer FC, and Reis-Filho JS (2010). Histological types of breast cancer: how special are they? Molecular oncology 4, 192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ED, Gao D, Redfern A, and Thompson EW (2019). Controversies around epithelial–mesenchymal plasticity in cancer metastasis. Nature Reviews Cancer 19, 716–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, and Werb Z (2003). Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. The Journal of cell biology 162, 1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Te Lindert M, Krause M, Alexander S, Te Riet J, Willis AL, Hoffman RM, Figdor CG, Weiss SJ, and Friedl P (2013). Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. Journal of Cell Biology 201, 1069–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrenn ED, Moore BM, Greenwood E, McBirney M, and Cheung KJ (2020a). Optimal, Large-Scale Propagation of Mouse Mammary Tumor Organoids. J Mammary Gland Biol Neoplasia, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrenn ED, Yamamoto A, Moore BM, Huang Y, McBirney M, Thomas AJ, Greenwood E, Rabena YF, Rahbar H, Partridge SC, et al. (2020b). Regulation of Collective Metastasis by Nanolumenal Signaling. Cell 183, 395–410 e319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin T, Greco V, and Myung P (2016). Hardwiring Stem Cell Communication through Tissue Structure. Cell 164, 1212–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong G, Chen J, Zhang G, Wang S, Kawasaki K, Zhu J, Zhang Y, Nagata K, Li Z, Zhou BP, et al. (2020). Hsp47 promotes cancer metastasis by enhancing collagen-dependent cancer cell-platelet interaction. Proceedings of the National Academy of Sciences 117, 3748–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, and Sixt M (2019). Mechanisms of 3D cell migration. Nature Reviews Molecular Cell Biology 20, 738–752. [DOI] [PubMed] [Google Scholar]
- Yan WT, Cui X, Chen Q, Li YF, Cui YH, Wang Y, and Jiang J (2017). Circulating tumor cell status monitors the treatment responses in breast cancer patients: a meta-analysis. Sci Rep 7, 43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Cao M, Liu Y, He Y, Du Y, Zhang G, and Gao F (2019). Inducible formation of leader cells driven by CD44 switching gives rise to collective invasion and metastases in luminal breast carcinomas. Oncogene 38, 7113–7132. [DOI] [PubMed] [Google Scholar]
- Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori G, et al. (2020). Guidelines and definitions for research on epithelial–mesenchymal transition. Nature Reviews Molecular Cell Biology 21, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Fujikawa T, Tanabe A, and Sakurai K (1993). Quantitative analysis of distribution and fate of human lung cancer emboli labeled with 125I-5-iodo-2’-deoxyuridine in nude mice. Surg Today 23, 979–983. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T, Hong S-M, Jung D, Noë M, Kiemen A, Wu P-H, Wirtz D, Hruban RH, Wood LD, and Oshima K (2020). Three-dimensional analysis of extrahepatic cholangiocarcinoma and tumor budding. The Journal of Pathology 251, e5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youk H, and Lim WA (2014). Secreting and sensing the same molecule allows cells to achieve versatile social behaviors. Science (New York, NY) 343, 1242782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K-D, Wu L-M, Liu G-Y, Wu J, Di G-H, Shen Z-Z, and Shao Z-M (2011). Different Distribution of Breast Cancer Subtypes in Breast Ductal Carcinoma in situ (DCIS), DCIS with Microinvasion, and DCIS with Invasion Component. Annals of Surgical Oncology 18, 1342–1348. [DOI] [PubMed] [Google Scholar]
- Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al. (2013). Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science (New York, NY) 339, 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Harden K, C Gonzalez L, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, et al. (2009). The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nature Immunology 10, 48–57. [DOI] [PubMed] [Google Scholar]
- Yu X, Miyamoto S, and Mekada E (2000). Integrin alpha 2 beta 1-dependent EGF receptor activation at cell-cell contact sites. Journal of Cell Science 113, 2139–2147. [DOI] [PubMed] [Google Scholar]
- Yue C, Jiang Y, Li P, Wang Y, Xue J, Li N, Li D, Wang R, Dang Y, and Hu Z (2018). Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy. Oncoimmunology 7, e1438111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac O, Raingeaud J, Libanje F, Lefebvre C, Sabino D, Martins I, Roy P, Benatar C, Canet-Jourdan C, Azorin P, et al. (2018). Tumour spheres with inverted polarity drive the formation of peritoneal metastases in patients with hypermethylated colorectal carcinomas. Nature cell biology 20, 296–306. [DOI] [PubMed] [Google Scholar]
- Zeinali M, Lee M, Nadhan A, Mathur A, Hedman C, Lin E, Harouaka R, Wicha MS, Zhao L, Palanisamy N, et al. (2020). High-Throughput Label-Free Isolation of Heterogeneous Circulating Tumor Cells and CTC Clusters from Non-Small-Cell Lung Cancer Patients. Cancers 12, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Hitomi M, Bar-Shain N, Dalimov Z, Ellis L, Velpula KK, Fraizer GC, Gourdie RG, and Lathia JD (2015). Connexin 43 expression is associated with increased malignancy in prostate cancer cell lines and functions to promote migration. Oncotarget 6, 11640–11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhao L, Zhou P, Ma H, Huang F, Jin M, Dai X, Zheng X, Huang S, and Zhang T (2017). Circulating tumor microemboli (CTM) and vimentin+ circulating tumor cells (CTCs) detected by a size-based platform predict worse prognosis in advanced colorectal cancer patients during chemotherapy. Cancer Cell Int 17, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Goliwas KF, Wang W, Taufalele PV, Bordeleau F, and Reinhart-King CA (2019a). Energetic regulation of coordinated leader-follower dynamics during collective invasion of breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Goliwas KF, Wang W, Taufalele PV, Bordeleau F, and Reinhart-King CA (2019b). Energetic regulation of coordinated leader-follower dynamics during collective invasion of breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America 116, 7867–7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Barclay M, Hilkens J, Guo X, Barrow H, Rhodes JM, and Yu L-G (2010). Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Molecular Cancer 9, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Fan L, Zhou P, Ma H, Huang S, Yu D, Zhao L, Yang S, Liu J, Huang A, et al. (2017). Detection of Circulating Tumor Cells and Circulating Tumor Microemboli in Gastric Cancer. Transl Oncol 10, 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller EL, Pedro B, Konen J, Dwivedi B, Rupji M, Sundararaman N, Wang L, Horton JR, Zhong C, Barwick BG, et al. (2019). Genetic heterogeneity within collective invasion packs drives leader and follower cell phenotypes. Journal of Cell Science 132, jcs231514. [DOI] [PMC free article] [PubMed] [Google Scholar]


