Abstract
Intermediate filaments (IFs) formed by vimentin are less understood than their cytoskeletal partners, microtubules and F-actin, but the unique physical properties of IFs, especially their resistance to large deformations, initially suggest a mechanical function. Indeed, vimentin IFs help regulate cell mechanics and contractility, and in crowded 3D environments they protect the nucleus during cell migration. Recently, a multitude of studies, often using genetic or proteomic screenings show that vimentin has many non-mechanical functions within and outside of cells. These include signaling roles in wound healing, lipogenesis, sterol processing, and various functions related to extracellular and cell surface vimentin. Extracellular vimentin is implicated in marking circulating tumor cells, promoting neural repair, and mediating the invasion of host cells by viruses, including SARS-CoV, or bacteria such as Listeria and Streptococcus. These findings underscore the fundamental role of vimentin in not only cell mechanics but also a range of physiological functions.
Keywords: cytoskeleton, intermediate filaments, vimentin, nucleus, pathogenesis
Graphical Abstract
Vimentin forms a wickerwork network that provides mechanical protection of the nucleus and elasticity to the cytoskeleton. New work highlights vimentin’s life outside the cell. It is secreted by multiple cell types and can provide positive signals for wound healing and act as a cofactor for pathogen infection.
1. Introduction
Many studies of the intermediate filament (IF) system have focused on the mechanical properties of the networks formed by this cytoskeletal polymer. The non-cytoplasmic intermediate filaments, which compose structural materials such as nails and the slime secreted by hagfish [1] have evolved to perform specific mechanical tasks. This would initially suggest that the cytoplasmic IFs would as well have a predominantly mechanical role. Our attention in this review is focused on recent discoveries on both mechanical and non-mechanical roles of the protein vimentin and the filaments it forms (VIFs) in cell biology. Vimentin is expressed mainly in mesenchymal cells. Its basic structure is mostly in the form of αhelical coiled-coil dimers that assemble into 10 nm diameter filaments through a series of intermediate oligomers. Vimentin’s N-terminus is important for controlling filament assembly and both N- and C-terminal domains mediate many interactions with binding partners. The structure and assembly of VIFs are reviewed elsewhere [2]. Previous studies have shown significant contributions of vimentin to cell mechanics and contractility [3–5]. However, many studies also point to non-mechanical roles for this abundant protein, with cellular functions ranging from lipogenesis to regulating GTPase signaling and the transduction of signals from transmembrane receptors [6–10]. This brief summary highlights three distinct aspects of recent research related to vimentin and VIFs.
We will first focus on the expanding list of proteins, nucleic acids, and other molecules that bind vimentin, often with a change in their ability to transmit a signal or react with their ligands. In some respect this section is an update of earlier reviews that pointed to the importance of intermediate filaments in cell signaling and other non-mechanical functions [10,11].
We will then emphasize recent findings on how the mechanical properties of the vimentin network provides a function that cannot be fulfilled by either of the other two cytoskeletal elements, actin filaments and microtubules. We will discuss how VIFs create a cage around the nucleus, increasing the cell’s ability to withstand large strains during locomotion in confined 3D spaces without damage to the nucleus. This effect of the perinuclear VIF network is not evident in studies of cells on flat surfaces but leads to large effects on motility and nuclear shape in 3D culture systems.
Finally, we will discuss the rapidly emerging field of extracellular vimentin. Early studies suggested that the reaction between anti-vimentin antibodies and antigens on the external surfaces of cells might be due to cytoskeletal debris released from neighboring cells, or perhaps local and transient rupture of the plasma membrane [12]. However, it is now clear that cell surface vimentin appears in the absence of detectable cell damage and is important for infection by bacteria or viruses or signaling from one cell type to another [13].
2. Vimentin binds to diverse cellular targets
There is already a large catalog of proteins that link VIFs to F-actin and microtubules, and the physical properties of composite networks formed by multiple cytoskeletal network systems promote mechanical responses that cannot be achieved by any single network system. These topics have been recently reviewed in [14,15]. Here we summarize only a few of the most recently reported cytoskeletal ligands for vimentin, which illustrate how broadly vimentin can affect cell mechanics and other functions in ways that cannot be inferred simply from the mechanical properties of VIFs alone.
2.1. Novel cytoskeletal and focal adhesion links
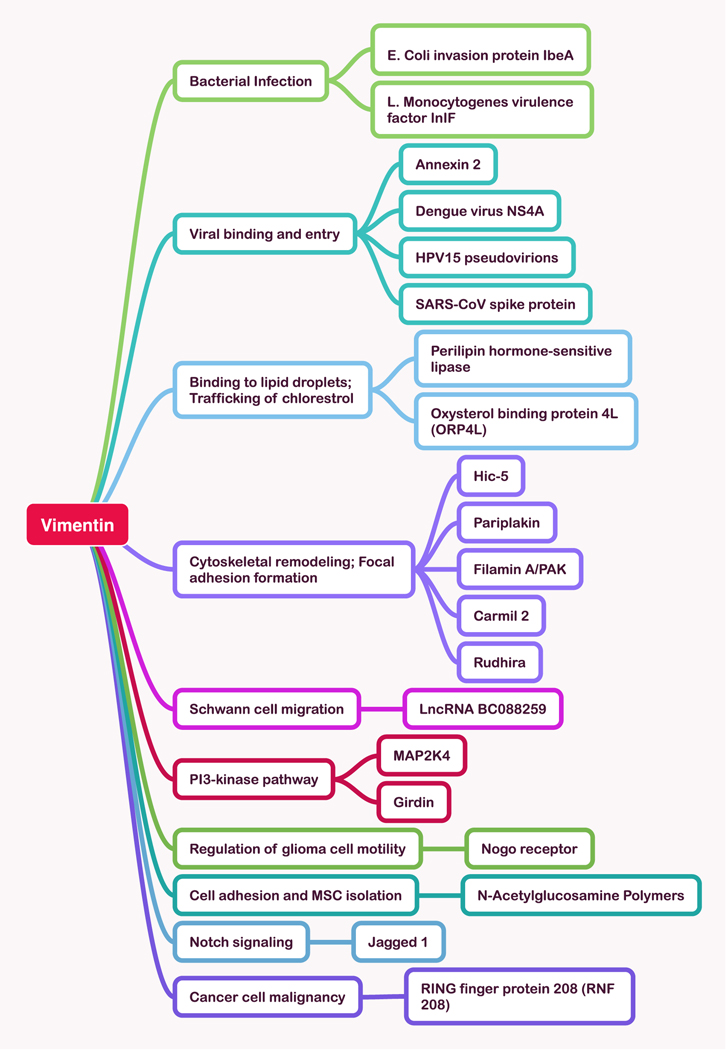
The most clearly established role of intracellular vimentin is to form a three-dimensional network as one of the three cytoskeletal systems. As this network system is increasingly studied, numerous new interacting proteins and regulators of VIFs are emerging. Here we summarize a few recent examples to illustrate the range of cytoskeletal structures that involve vimentin. In addition to the crosslinker plectin and multiple plus and minus end directed molecular motors [16] that link VIFs to microtubules, the protein Rudhira/Breast Carcinoma Amplified Sequence 3 (BCAS3) also links vimentin to microtubules and leads to MT stabilization, an effect that is essential for endothelial cell motility, focal adhesion dynamics, and angiogenesis [17]. Carmil, a protein that interacts with actin capping protein and hence affects actin dynamics also binds VIFs, suggesting a novel link between these two filament systems [18]. Another actin regulatory protein, girdin, that links AKT signaling to cytoskeletal dynamics was shown to bind vimentin by mass spectrometry and immunoprecipitation from pancreatic cancer cells [19]. Hic-5, a focal adhesion scaffold protein stabilizes the VIF network by modulation of RhoGTPases, and its ablation leads to the disassembly of VIF [20]. Filamin A, an actin crosslinker, directs the kinase PAK1 to vimentin, altering its assembly into VIFs [21]. A mechanical link from VIFs to the LINC complex mediates transmission of forces from the cell surface to the nucleus [22]. Linkage of VIFs to cortical actin by the crosslinker plectin, one of the first known integrators of the cytoskeleton, has now been seen to be essential for the cellular restructuring required for mitosis [23]. These and other examples of novel ligands for vimentin are summarized in Figure 1.
Figure 1:
Examples of newly reported ligands and targets of vimentin.
2.2. Regulation of lipid droplet formation.
Lipid droplets in adipocytes and other cell types have long been known to be surrounded by a network of VIFs [24]. This network is implicated in adrenal steroidogenesis [25] and its disruption inhibits lipid drop formation in 3T3L1 cells [26]. Proteomic analysis shows that vimentin is enriched in cholesterol-containing lipid droplets compared to triglycerol-based lipid droplets [27], and the binding of hormone sensitive lipase, the major neutral cholesterol esterase, to vimentin facilities delivery of free cholesterol to mitochondria for steroid hormone production [28]. Vimentin can bind directly to some phospholipids [29], and the binding to lipid droplets is proposed to be at least in part mediated by perilipin, which binds directly to the hydrophobic lipid droplet and has an acidic surface-exposed domain to which cationic arginine-rich sites in vimentin’s N-terminus can dock [30]. A mutation in human vimentin that disrupts VIFs leads to decreased perilipin and lipid accumulation in adipocytes, and is associated with progeroid syndrome [31]. Phosphorylation of the oxysterol binding protein 4L (ORP4L) leads to its binding to vimentin and facilitates cholesterol extraction from membranes, further implicating vimentin in lipid droplet formation and processing [32].
2.3. Mechanisms of vimentin degradation.
The stability of vimentin depends to a great extent on its phosphorylation state, and at least some forms of soluble vimentin are a target for degradation. The ring finger protein 208 (RNF208), an estrogen-inducible E3 ligase, binds to vimentin that is phosphorylated at Ser39 and polyubiquitinates the Lys97 residue of vimentin, leading to its degradation. Downregulation of RNF208 in triple negative breast cancer is associated with greater malignancy and with increased vimentin stability [33]. Ubiquitination by gigaxonin, an E3-ligase targeting factor encoded by the giant axonal neuropathy (GAN) gene also leads to vimentin degradation, and mutations in GAN are associated with the accumulation of VIFs in GAN as well as other types of intermediate filaments in the nervous system [34]. VIFs are also subject to proteolysis by calpain during osmotic shock [35,36] and are substrates for some bacterial proteases [37]. VIFs are also cleaved by calpain during pyroptosis of inflammatory cells. The resulting disruption of the VIF network leads to fluidization of the cytoskeleton and softening of the cell [35].
2.4. Notch, NOGO, and PI 3-kinase signaling
Numerous signaling pathways interact with vimentin [7,8,10]. A study of shear stress effects on arterial remodeling show that shear stress increases phosphorylation of vimentin at serine 38, and this leads to binding of the Notch pathway component jagged 1 to vimentin. The resulting increase in Notch signaling is required for appropriate arterial remodeling [38]. Intracellular vimentin binds the NOGO receptor (NgR) which in mature form is trafficked to the plasma membrane of glioblastoma cells where it can inhibit their migration. When bound to vimentin in the cell interior, maturation of NgR is suppressed [39]. This finding may be related to the increased malignancy of glioblastomas expressing high levels of vimentin. Vimentin is also a ligand for mitogen-activated protein kinase kinase 4 (MAP2K4), and this interaction is reported to modulate the effect of MAP2K4 on phosphoinositide-3-kinase (PI3K)/AKT signaling, resulting in changes in breast cancer cell proliferation and migration [40].
2.5. Polysaccharides and nucleic acids
Proteins are not the only ligands for vimentin. On the cell surface vimentin can bind polymers containing N-acetylglucosamine (GlcNAc) [41]. This binding has been exploited to create improved methods for isolating mesenchymal stem cells with surface-exposed vimentin by use of GlcNAc-containing polymer-coated dishes [42]. Vimentin can itself be post-translationally modified by ligation of GlcNAc to serine [43], and this modification affects the assembly of vimentin into filament networks with subsequent effects on cell motility and pathogen invasion (see 4.5.1) [44]. Two non-coding RNA’s have also been identified as ligands of vimentin. The long non-coding RNA LncRNA BC088259 is upregulated after sciatic nerve injury and modulates the migration of Schwann cells. This effect on migration has been proposed to involve binding of LncRNA BC088259 to vimentin [45]. A different non-coding RNA, named down-regulated in its expression by hepatitis B virus X (dreh) is involved in glucose transport, and exogenous downregulation of dreh in 3T3-Li adipocytes increases glucose transport by increasing GLUT4 expression in the plasma membrane by a mechanism thought to involve vimentin [46]. This result supports the idea that vimentin is important for lipogenesis, as discussed in 2.2.
3. Perinuclear vimentin
3.1. What is a vimentin intermediate filament cage?
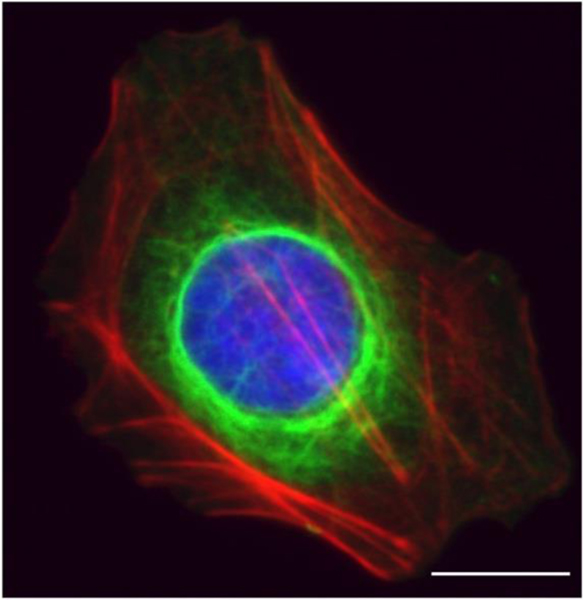
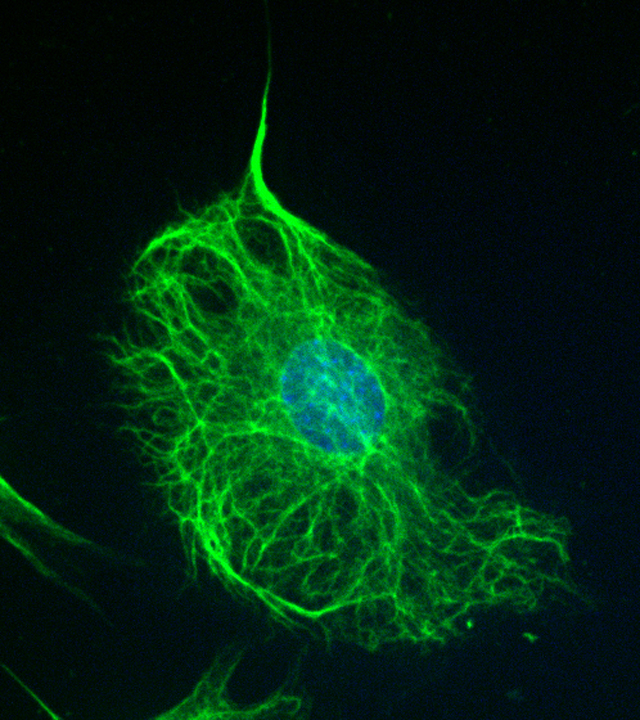
A robust feature of VIFs is their formation of a cage-like network that encircles the nucleus VIF with densely packed filaments (Figure 2) and radiates to the cell periphery [47]. This organization of VIFs depends on substrate stiffness. When cells are grown on soft substrates, the perinuclear vimentin cage is collapsed and localized closely around the nucleus. When cells grow on stiffer substrates, the spread area of the cell increases, and the VIF network extends more toward the cell periphery. These trends have been observed in multiple cell types, including human mesenchymal stem cells, endothelial cells, and mouse fibroblasts (3T3 cells) [47].
Figure 2:
Intermediate filament networks and the cell nucleus. The greater localization of VIFs juxtaposed to the nucleus is indicative of a distinct cage. Immunofluorescence image of a HUH7 cell marked for vimentin (green), actin (red), and DNA (blue). Scale bar is 15 μm.
There is evidence that VIFs establish indirect physical connections to the outer nuclear membrane through interactions with the linker of the nucleoskeleton and cytoskeleton (LINC) complex [48]. The LINC complex also connects to the nuclear lamina, a thin filamentous layer surrounding the nuclear periphery that is mainly composed of the type V IF proteins, the nuclear lamins [49,50]. While actin and microtubules also form indirect links to the nuclear envelope through the LINC complex, they do not form the cage-like structure around the nucleus, which seems to be unique to IFs. Recent high resolution studies [51] show that vimentin rings that form around the nucleus during initial cell spreading on a 2D surface do not appear to link with actin nor require tubulin. It is noteworthy that these latter VIF structures were originally identified as birefringent spheres with isotropic cores using polarized light microscopy. They form rapidly in live cells in response to substrate attachment and exhibit positive birefringence with respect to their circumferential axes which correlates with highly organized parallel arrays of VIFs, excluding both microtubules and [52].
Here we argue that the functional implications of the perinuclear cage are distinct from those of the cytoplasmic VIF network that extends into the cytoplasm, associates with focal adhesions, microtubules and forms links with the actin-rich cell cortex (5).
3.2. Separate roles for cytoplasmic versus perinuclear vimentin in cellular mechanics
3.2.1. VIF cytoplasmic network
There has been an emerging interest in the role of intermediate filaments in the mechanical properties of cells and their role in transmitting forces to the nuclear envelope. VIFs crosslink with actin and microtubules through plectin and other crosslinkers, creating the cytoskeletal links that connect the cell surface to the nucleus [23,53–55]. Vimentin is a major cargo for the microtubule motors kinesin and dynein, so motors may also be part of the bridging between VIFs and the microtubule network [56–59]. One of the first demonstrations of vimentin’s role in this process was done through the application of forces at the cell surface using micropipettes or manipulation of surface bound microbeads [60]. Pulling at the cell cortex distorts the cell nucleus and moves it in the direction of pull, indicating the direct transmission of forces through molecular connections between integrins, cytoskeletal filaments, and the nuclear envelope. Pulling on cells that lacked both microfilaments and microtubules still produced nuclear deformation, suggesting that the intermediate filament network alone is sufficient to transmit mechanical stress to the nucleus [60].
The transmission of forces through the cytoskeletal network of all three filament types depends on the mechanical properties of the network. Studies involving reconstituted cytoskeletal networks show that the mechanical properties of VIFs differ from those of actin and microtubules [61]. VIF networks are soft but have an extraordinary ability to stiffen when under strain and can withstand much larger strains without breaking as compared to actin and microtubules [61,62]. A number of studies have now shown that VIFs modify the mechanical properties of the cell itself, enhancing the cell elastic behavior [3–5,63], particularly under conditions of large cell strains [64,65] and in regions close to the perinuclear VIF network [66].
In the simplest physical picture, we can model the cytoskeleton as a spring with a spring constant k that is proportional to its stiffness. When a force F is applied to the boundary of the cell, it displaces the cell boundary a distance x to produce an equal and opposite force F, F = -kx, that is transmitted to the nucleus through the spring. For a soft cell, the nucleus feels little force as k is small whereas the larger k in a stiffer cell makes the nucleus feel a greater force. This simple picture suggests that the increased cell stiffness due to VIF facilitation of the transmission of forces applied at the cell boundary to the nucleus, allowing the nucleus to better ‘feel’ external forces. This also implies that it takes little force to displace the nucleus in a soft cell lacking VIF as compared to a stiffer cell that possesses a VIF network. A dramatic illustration of this point is the much easier isolation of nuclei from vimentin deficient cells with a micromanipulator as compared to their wild type counterparts that require depolymerization of F-actin for successful nucleus isolation [67].
The cell, of course, has means of generating its own forces. Vimentin is not the engine that drives the cell. That is the role of the contractile machinery of the cell, whose main components are the acto-myosin complex. However, there exist bidirectional interactions between the two networks. For instance, contractile actomyosin arcs mediate subcellular localization of the VIF network to the perinuclear region [68], and there is now significant evidence that VIFs assist in the transmission of the contractile actomyosin forces to the nucleus. When a cell adheres to a flat 2D substrate, the nucleus becomes more compressed over time as the cell spreading area increases [69,70]. One model for this behavior is that contractile forces generated by apical stress fibers push down on the nucleus, compressing it [70–72]. Several studies have now shown that disruption or deletion of the VIF network results in changes to nuclear shape [22,73], even when the F-actin network remains unchanged [74]. In the absence of VIF, the nucleus rounds up and is no longer compressed [69,74]. Using nuclear shape as a read-out for the forces applied to the nuclear surface, these studies indicate that VIFs facilitate nuclear deformation and assist the actomyosin network in pushing down on the nucleus. The exact mechanism by which VIFs apply forces to the nuclear envelope remains unclear, but it seems to require the LINC complex [22]. Another possibility is through vimentin’s crosslinks with actomyosin filaments where cutting these links by removing VIFs would decrease the force felt by the nucleus.
3.2.2. Vimentin perinuclear cage
New evidence shows that in addition to transmitting forces to the nucleus, VIFs can also resist the transfer of forces and reduce nuclear deformations. A clear demonstration of this was done using micropipette aspiration to directly apply pulling forces on adherent fibroblasts [75]. Cells transfected with siRNA to decrease vimentin expression had increased nuclear deformation from pulling forces. These results were reproduced using SW13 adrenal carcinoma clones that do not express vimentin. Interestingly, neither actin nor microtubules were required to resist local pulling forces, whereas lamin A was. The effects of vimentin did not require mechanical linkages to the nucleus, as was shown by altering the LINC complex protein KASH4. These results suggest that the VIF perinuclear cage confers stiffness to the nucleus.
Consistent with this picture are 3D confining cell motility experiments in wild type and vimentin-null mEFs [66,74]. We found that loss of vimentin increased cell motility through small pores, suggesting that the VIF network impedes confined motility. Cells lacking vimentin exhibited higher nuclear deformations after migrating through pores of 3 um diameter, which is a much smaller size than the effective diameter of an unstressed mEF nucleus (~10 um). Loss of vimentin also increased the rates of nuclear damage associated with confined motility, manifesting itself through enhanced nuclear blebs, nuclear envelope rupture, and DNA damage repair. These results reveal that the perinuclear VIF cage may have a distinct role in increasing the effective stiffness of the nuclear envelope.
Further studies are helping to paint a more complete picture of VIFs in 3D cell motility amongst varied cell lines. Recent studies indicate that VIFs also decrease confined cell motility in amoeboid cancer cells [76]. In dendritic cells vimentin is seen to enhance 3D movement [76] and provide mechanical resilience to protect the nucleus [77]. More studies are needed to determine how VIF network assembly and its interactions with the other cytoskeletal components help to establish different modes of confined cell motility.
Does vimentin facilitate or resist force transmission to the nucleus? We have laid out evidence for both cases. One plausible explanation that incorporates both effects is that the cytoplasmic VIF network and the perinuclear VIF cage might have separate roles in maintaining nuclear shape. The main mechanical function of the cytoplasmic VIFs might be to increase cytoskeletal stiffness and to assist with the transmission of forces to the nucleus. This is consistent with the decreased 3D motility rates of cells with VIFs [66,74,76]. On the other hand, cells containing a perinuclear VIF cage seems to serve as a protective structure that increases the effective stiffness of the nuclear envelope, as seen by reduced nuclear deformability in cells expressing vimentin [74,77]. In this way, VIFs not only enhance the cell stiffness but also the effective stiffness of the nuclear envelope. The cytoplasmic VIFs assist in transmitting forces to the nucleus, and the perinuclear cage resists them. How cells might regulate perinuclear versus cytoplasmic VIF assembly is unclear but could be in part established through connections with microtubules. Microtubules are required for moving VIFs away from the perinuclear region to the cell surface. When cells are treated with nocodazole which drives depolymerization of microtubules, VIFs retract to the nuclear surface with very few VIFs in the peripheral regions [56].
4. Vimentin appears on the surface of cells and is secreted by multiple cell types.
The presence of extracellular vimentin has been documented for at least 35 years, but a functional role for the surface exposure and the mechanisms by which vimentin is released from the cell interior are only recently beginning to be identified (Table 1). One early study of antibodies generated against surface-exposed antigens in malignant monocytes showed that these antibodies were specific to vimentin [12]. Since that time extracellular or cell surface vimentin has been documented in a wide array of settings in which it appears to play a role in both normal physiology and pathologic states, with a particular emphasis on circulating cancer cells and on infection by bacteria and viruses.
Table 1.
Sources of extracellular vimentin
| Cell type | Modification | Function | Reference |
|---|---|---|---|
| Neutrophil | Citrullinated | Released during apoptosis or NET formation Source of serum citrullinated vimentin commonly found in rheumatoid arthritis |
[80,110] |
| T-lymphocyte | Released on apoptosis | Binds phospholipase II to promote arachidonic acid | [81] |
| Monocyte/macrophage | Phosphorylated | Secreted after simulation by TNF-alpha or oxidized LDL | [83,95] |
| Astrocyte | Citrullinated; Packaged in exosome | Delivery to neuron to promote wound healing | [92–95] |
| Platelet | Altered fibrinolysis; Binding to von Willebrand factor | [82,85] | |
| Endothelial cells | Disulfide-dimerized | Increased platelet binding; decreased neutrophil binding. Ligand for CD44 and several pathogens | [13,85,87,104,116,118] |
| Senescent fibroblast | Malondialdehyde-ligated | Increased clearance by immune system | [79] |
| Cancer cells | Sometimes proteolyzed | Target for isolating circulating tumor cells, designing vaccines and enhanced chemotherapy | [21,33,94,97,100,103,136,140,141] |
4.1. Sources of extracellular vimentin.
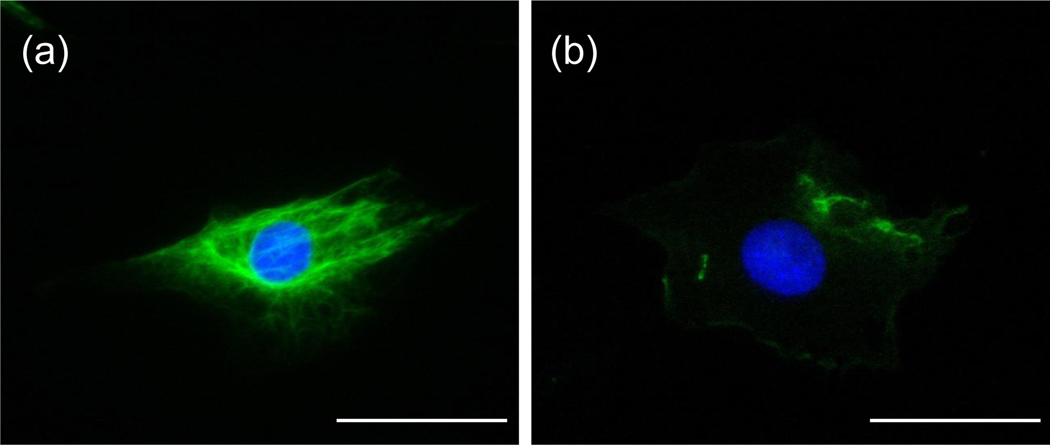
Extracellular vimentin can be generated by multiple mechanisms in addition to simple release of vimentin from necrotic cells or disrupted cell membranes, and autoantibodies to circulating, covalently modified vimentin are commonly found in inflammatory diseases [78]. Figure 3 shows the distinct patterns of intracellular and extracellular vimentin in cultured fibroblasts, a cell type that increases exposure of cell surface vimentin during senescence [79]. Neutrophils [80] and T lymphocytes [81] undergoing apoptosis release vimentin to their extracellular surface, where in the case of thymocytes, it recruits extracellular phospholipase II and is thought to promote production of arachidonic acid. Activated platelets and platelet-released micro-particles also expose vimentin on their surface, where vimentin binds vitronectin, which stabilizes plasminogen activator inhibitor I in its active form, suggesting a role for surface vimentin in fibrinolysis [82]. Macrophages are simulated by the cytokine TNF-alpha to secrete vimentin into the medium in a process that can be blocked by inhibition of protein kinase C [83]. Oxidized low-density lipoproteins can also stimulate macrophages to secrete vimentin, and extracellular vimentin can induce macrophages to release inflammatory cytokines. These effects might be related to the finding of increased serum levels of vimentin in patients with atherosclerotic coronary artery disease and in an Apo-E null mouse model of atherosclerosis, suggesting that serum vimentin might be a useful biomarker [84].
Figure 3:
Immunofluorescence images of (a) intracellular and (b) extracellular vimentin in mouse embryo fibroblasts. The cell on the left was treated with triton to permeabilize the cell membrane and the cell on the right was not. Vimentin is shown in green; DNA in blue. Scale bar is 50 μm.
Endothelial cells also secrete vimentin, and a subset of endothelial cells express it on their surface. Here it can interact with von Willebrand factor to form strings that mediate platelet adhesion in the vascular lumen and contribute to stroke pathology [85]. Extracellular vimentin can also bind P-selectin and lower the adhesivity of neutrophils to platelets and the endothelium [86]. A remarkable aspect of the vimentin secreted by endothelial cells is that it is a target of the PAL-E antibody, which has been used to detect blood capillaries and small veins [87]. Under non-reducing conditions, the antigen recognized by the PAL-E antibody has an apparent molecular weight of 120 kDa, or twice the size of vimentin, which drops to 55 kDa in reducing conditions, suggesting that this form of extracellular vimentin is composed of disulfide bonded dimers. The highly localized expression of this surface epitope suggests that vascular surface vimentin might be enriched at sites where circulating cells adhere most to the vessel surfaces [87].
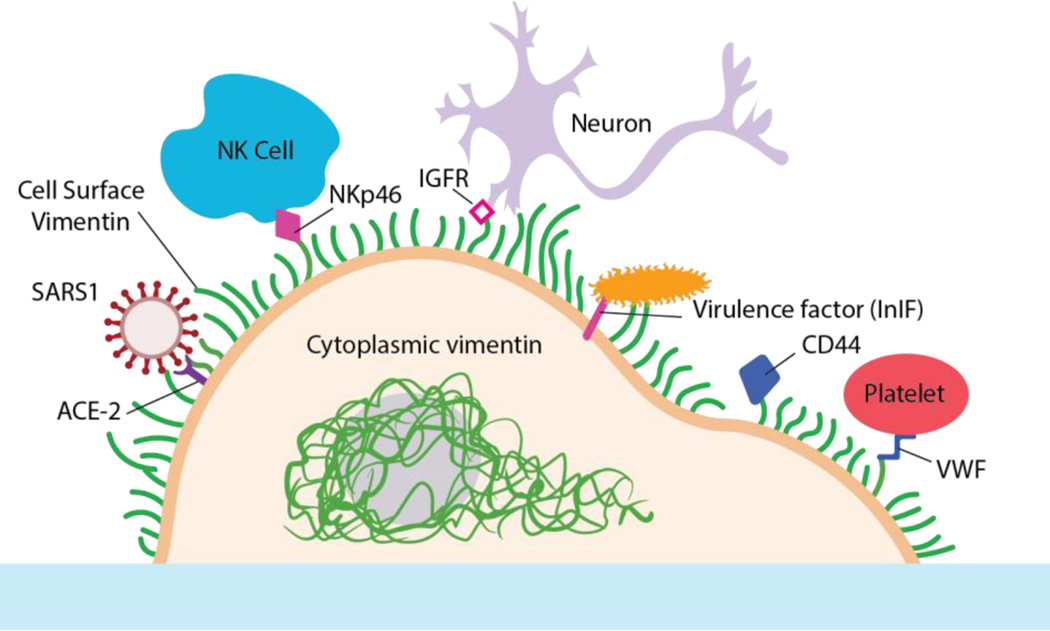
Secretion by both macrophages and endothelial cells requires post-translational modification of vimentin, and antibodies specific to cell surface vimentin can be generated [88,89]. An important finding is that cell surface vimentin appears to be mainly in the form of oligomers with 4 – 12 monomers, but is not filamentous, a difference that is likely to relate to the much higher affinity of the oligomers to lipid bilayers compared with VIFs [90]. Other mechanisms by which extracellular vimentin can be generated are illustrated in some of the examples listed below and summarized in Table 1. The functional aspects of extracellular vimentin, which have been identified, are illustrated in Figure 4.
Figure 4:
Examples of extracellular vimentin functions. Viruses are bound by specific surface proteins to vimentin to facilitate their delivery to the viral receptor that mediate entry. Bacterial virulence factors or adhesions bind vimentin directly to enable their entry into the host. Cell surface vimentin binds soluble CD44 and enhances its initiation of intracellular signals. Vimentin on the surface of activated monocytes binds the natural killer cell surface protein NKp46 to initiate cell killing. Extracellular vimentin binds IGFR to enhance neuronal repair.
4.2. Wound repair, regeneration, and senescence
One of the clearest beneficial effects of extracellular vimentin is seen in the mechanism by which mesenchymal cells at the edge of a wound in an ex vivo mock cataract surgery model transition to a myofibroblast phenotype. Following injury, vimentin is released into the extracellular space, where it binds to mesenchymal leader cells located at the wound edge and thereby supports the contractile cell phenotype that enables wound closure. In profibrotic environments, extracellular vimentin might contribute to deleteriously high conversion or maintenance of myofibroblasts [91]. Astrocytes, activated by injury, can produce extracellular vimentin in the form of exosomes that are delivered to neurons, which then can bind this protein on their surface even though they do not express it endogenously. Extracellular vimentin binds to neuronal insulin-like growth factor 1 receptor to promote axonal growth in vitro and improves recovering after spinal cord injury in mice [92,93]. Cell surface bound vimentin can promote entry of Clostridium botulinum C3 transferase (C3bot) and thereby might mediate the axonotrophic effects of C3bot after spinal cord injury [94]. Citrullinated vimentin has also been proposed as a marker for astrocyte activation [95]. Senescent human fibroblasts secrete a modified vimentin that is post-translationally modified on cysteine 328 by the oxidative adduct malondialdehyde (MDA) and MDA-modified vimentin plasma levels increase in a murine model of accelerated aging. This finding raises the possibility that innate immunity might recognize senescent cells by the presence of membrane-bound MDA-vimentin [79]. Extracellular vimentin can also have anti-inflammatory effects, as suggested by a study showing that extracellular vimentin blocked secretion of pro-inflammatory cytokines by dendritic cells through modulation of their response to lipopolysaccharide [96].
4.3. Vimentin on the surface of cancer cells
Several cancer cell types are associated with extracellular vimentin, and this finding has led to efforts to target it as a potential therapy. For example, normal human T lymphocytes express surface vimentin only after activation, but malignant Sezary lymphocytes express it constitutively [97]. Glioblastoma multiforme stem cells express surface vimentin, which promotes spheroid formation in vitro. Treatment of these cells with an anti-vimentin antibody leads to internalization of surface vimentin, lowered cell viability due to apoptosis, and diminished tumor growth in a mouse model [88,98]. Gastric cancer cells also express cell surface vimentin, and a magnetic bead isolation assay identified circulating tumor cells (CTCs) in peripheral blood of the majority of gastric cancer patients, with high vimentin positive CTCs correlating with poor prognosis [89]. Surface vimentin positive CTCs also have potential as predictors of relapse in postremission neuroblastoma [99]. Three different human prostate cell lines express vimentin that can be detected by monoclonal antibodies recognizing the coil one rod domain and the C-terminus of vimentin [100]. Anti-cell surface vimentin antibodies have also been used to detect stem-like hepatocellular carcinoma cells with enhanced metastatic potential [101]. Magnetic particles containing anti-vimentin antibodies have been used to isolate circulating cancer cells from the blood of mice with tumors generated by the human lung cancer cell line A549 [102] and macrophage-like CTCs from the blood of patients with gastrointestinal stromal tumors [103].
4.4. CD44 signaling
Soluble CD44, a hyaluronan (HA) ligand that is overexpressed during inflammation and in some cancers also binds vimentin at the surface of endothelial cells. The vimentin binding site on CD44 overlaps with its HA binding domain and targets the N-terminus of vimentin [104]. This interaction of CD44 and vimentin might relate to the finding that both CD44 and vimentin are increased on the surface of some cancer cell lines such as those from the prostate [100] and oral squamous cell carcinoma [105]. The interactions among CD44, HA, and vimentin might affect how circulating cancer cells or other cell types interact with the endothelium.
4.5. Intracellular and extracellular vimentin are involved in pathogen infection
Vimentin is increasingly found to affect invasion of cells by pathogens. Both bacteria and viruses bind cell surface vimentin. In some cases, this binding facilitates infection of the host cell by the pathogen, and in other cases binding to vimentin appears to compete with or otherwise inhibit binding of the pathogen to the receptor that enables its entry into the host cell. An enabling role of vimentin is consistent with numerous reports that vimentin null mice are relatively resistant to some forms of infection [106–109], but a direct relation is not clear since both extracellular and cytoskeletal vimentin are important elements in the innate immune response. A recent study showed that disassembly of the vimentin cytoskeleton is an essential part of the mechanism by which cells extrude DNA-containing neutrophil extracellular traps (NETs) [110] as defense against bacterial infection. This process requires the activity of protein arginine deiminase to convert arginine to citrulline on both histones and vimentin to disassemble chromatin and VIFs, suggesting that vimentin might also be released by this mechanism. Extracellular exposure of citrullinated autoantigens including vimentin is associated with NET formation in rheumatoid arthritis [111,112]. Whether extracellular vimentin can serve a protective role against pathogens is not yet clear, but there are numerous compelling examples of vimentin enhancing the infectivity of both bacteria and viruses and several demonstrations that antibodies or other ligands for vimentin can block infection.
4.5.1. Bacterial infection
Cytoskeletal VIFs are often remodeled during bacterial infection [37], and in some cases surface vimentin is a docking site for specific bacterial surface proteins. Many of these results are discussed in a recent review [113]. Here we cite a few more recent results showing the importance of vimentin for surface binding and intracellular invasion by bacteria.
VIFs are important for infection by Chlamydia trachomatis, and vimentin is a substrate for chlamydial protease-like activity factor [37]. VIF remodeling by Chlamydia requires vimentin glycosylation [44]. Cell surface vimentin is important for several bacterial infections, such as Listeria monocytogenes [114,115] and meningitic Escherichia coli [107,116]. Vimentin-dependent infection of human microvascular endothelial cells by Listeria monocytogenes increases as substrate stiffness increases [114]. The surface adhesion factor BspC of Streptococcus agalactiae, which causes meningitis, enables the bacterium to enter cerebral microvascular endothelial cells by binding their surface vimentin, as well as cytoskeletal vimentin. BspC is both necessary and sufficient to infect the microvascular endothelium and to induce neutrophil chemokine expression, and vim −/− mice are protected from infection by wild type bacteria [106]. Infection of monocytes by Mycobacterium tuberculosis also leads to increased expression of cell surface vimentin which is recognized by NKp46 located on the surface of natural killer cells [117].
4.5.2. Viral infection
Both extracellular vimentin and intracellular VIFs have been identified as important mediators of multiple stages of virial infection, from initial cell invasion to intracellular viral replication and release. Cell surface vimentin is important for infection of cells by the coronaviruses SARS-CoV [118–120] and porcine reproductive and respiratory syndrome (PRRS) virus [121–124] and by enterovirus [125–127] which shares some common targets with coronavirus. One study found that annexin 2 binds vimentin, and only when both proteins are present can PRRS virus infect its host cell [121]. Surface vimentin is also involved in infection by Dengue virus [128,129] (which can be clinically similar to covid-19 [130]) and Japanese encephalitis virus [131]. The H9N2 subtype avian influenza virus binds vimentin during infection of Madin-Darby canine kidney (MDCK) cells. Incubation of these cells with an antibody against vimentin or downregulating vimentin expression with siRNA lowered the infection rate, whereas upregulating vimentin expression increased infectivity of MDCK cells [132]. Japanese encephalitis virus (JEV) also requires vimentin for infection of porcine kidney cells. Treatment with an anti-vimentin antibody blocked infection of these cells by the virus [131]. The Chandipura virus, which causes encephalitic complications in humans, colocalizes with vimentin on the surface of murine Neuro-2a cells, and its infection of these cells can be decreased either by prior incubation of the virus with purified vimentin or of the host cell with anti-vimentin antibodies [133].
With SARS-CoV, vimentin binds the viral spike protein and enhances its delivery to the receptor angiotensin-converting enzyme 2 [120]. SARS-CoV also upregulates cytoplasmic vimentin by the TGF-β pathway to promote a fibrotic response in lung cells [118], and other reports show that intracellular VIFs can also be important for viral infection and replication. Enterovirus requires reorganization of the VIF cytoskeleton for efficient replication and export [126]. The foot-and-mouth disease virus (FMDV) also requires the VIF network for optimal replication, and this requirement has been traced to the binding of the FMDV nonstructural protein 3A to vimentin [134].Cell surface vimentin is not necessarily a viral receptor or even an augmenting factor for viral entry. Studies of human papillomavirus (HPV) infection showed that HPV16 pseudovirions bound cell surface vimentin and that pretreatment of the virus with soluble vimentin lessened its ability to infect a host cell. In this setting vimentin did not act as a receptor but bound the viral proteins to dampen the initial steps of HPV16 infection [135].
4.6. Countermeasures to cancer and infection
An exciting aspect of work related to extracellular vimentin is the identification of soluble ligands with the potential to improve identification or removal of cancer cells, and to prevent vimentin-dependent routes of infection, especially with relation to coronaviruses. A number of approaches have used antibodies to extracellular vimentin in conjunction with antibodies to other cell surface markers to detect and isolate circulating cancer cells [89,102,103,136]. Both monoclonal anti-vimentin and a vimentin-specific DNA aptamer are being used to isolate circulating cancer cells or block the effect of vimentin [137]. Similar approaches are being applied to infection by SARS-CoV [120] SARS2-CoV [138] and other viruses [131]. Citrullinated vimentin in combination with other antigens has been tested in possible vaccines to stimulate CD4-mediated anti-tumor activity and showed some efficacy in a mouse model [139].
5. Conclusion.
The striking viscoelastic properties of VIF networks in vitro suggest cellular functions based on resistance to mechanical stress and regulation of cell shape. Indeed VIFs are essential for protection of the nucleus from potentially damaging forces and forms much of the cytoskeleton. However, many studies point to important non-mechanical functions. Vimentin, either in the form of small oligomers or VIFs, binds numerous proteins and nucleic acids, and functions to integrate with other cytoskeletal systems, regulate lipid metabolism and perform numerous other intracellular functions. In addition, the rapidly growing study of extracellular vimentin, especially in the regulated disassembly of VIF and the secretion of modified vimentin, reveals many new functions for the protein, from mediating pathogen invasion to promoting wound healing. Clearly there is much more to be learned about this abundant but understudied protein.
Acknowledgements.
This work was supported by grants NIH P01 GM096971 (AV, RDG, and PAJ) and NSF MCB 2032861 (AEG).
Abbreviations:
- AKT
Protein kinase B
- dreh
down-regulated in its expression by hepatitis B
- GlcNAc
N-acetylglucosamine
- IF
Intermediate filament
- LDL
low density lipoprotein
- LINC
linker of nucleoskeleton and cytoskeleton
- MDA
malondialdehyde
- VIF
vimentin intermediate filament
- TNF-alpha
tumor necrosis factor alpha
- IGFR
insulin like growth factor receptor
References
- 1.Koch EA, Spitzer RH, Pithawalla RB, Parry DA. 1994. An unusual intermediate filament subunit from the cytoskeletal biopolymer released extracellularly into seawater by the primitive hagfish (eptatretus stouti). J Cell Sci 107 ( Pt 11): 3133–44. [DOI] [PubMed] [Google Scholar]
- 2.Omary MB, Liem RKH. 2016. Intermediate filament proteins. Methods in enzymology: Science Direct. p 791. [Google Scholar]
- 3.Guo M, Ehrlicher AJ, Mahammad S, Fabich H, Jensen MH, Moore JR, Fredberg JJ, Goldman RD, Weitz DA. 2013. The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys J 105: 1562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vahabikashi A, Park CY, Perkumas K, Zhang Z, Deurloo EK, Wu H, Weitz DA, Stamer WD, Goldman RD, Fredberg JJ, Johnson M. 2019. Probe sensitivity to cortical versus intracellular cytoskeletal network stiffness. Biophys J 116: 518–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendez MG, Restle D, Janmey PA. 2014. Vimentin enhances cell elastic behavior and protects against compressive stress. Biophys J 107: 314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang L, Goldman RD. 2004. Intermediate filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol 5: 601–13. [DOI] [PubMed] [Google Scholar]
- 7.Helfand BT, Chou YH, Shumaker DK, Goldman RD. 2005. Intermediate filament proteins participate in signal transduction. Trends Cell Biol 15: 568–70. [DOI] [PubMed] [Google Scholar]
- 8.Ivaska J, Pallari HM, Nevo J, Eriksson JE. 2007. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res 313: 2050–62. [DOI] [PubMed] [Google Scholar]
- 9.Kidd ME, Shumaker DK, Ridge KM. 2014. The role of vimentin intermediate filaments in the progression of lung cancer. Am J Respir Cell Mol Biol 50: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paramio JM, Jorcano JL. 2002. Beyond structure: Do intermediate filaments modulate cell signalling? Bioessays 24: 836–44. [DOI] [PubMed] [Google Scholar]
- 11.Toivola DM, Tao GZ, Habtezion A, Liao J, Omary MB. 2005. Cellular integrity plus: Organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol 15: 608–17. [DOI] [PubMed] [Google Scholar]
- 12.Herrmann H, Aberer W, Majdic O, Schuler G, Wiche G. 1985. Monoclonal antibody to a 43 000 mr surface protein of a human leukaemia cell line (thp-1) crossreacts with the fibroblast intermediate filament protein vimentin. J Cell Sci 73: 87–103. [DOI] [PubMed] [Google Scholar]
- 13.Danielsson F, Peterson MK, Caldeira Araujo H, Lautenschlager F, Gad AKB. 2018. Vimentin diversity in health and disease. Cells 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battaglia RA, Delic S, Herrmann H, Snider NT. 2018. Vimentin on the move: New developments in cell migration. F1000Res 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowery J, Kuczmarski ER, Herrmann H, Goldman RD. 2015. Intermediate filaments play a pivotal role in regulating cell architecture and function. J Biol Chem 290: 17145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert A, Hookway C, Gelfand VI. 2016. Intermediate filament dynamics: What we can see now and why it matters. Bioessays 38: 232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi D, Inamdar MS. 2019. Rudhira/bcas3 couples microtubules and intermediate filaments to promote cell migration for angiogenic remodeling. Mol Biol Cell 30: 1437–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stark BC, Lanier MH, Cooper JA. 2017. Carmil family proteins as multidomain regulators of actin-based motility. Mol Biol Cell 28: 1713–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Chen H, Gao W, Wang S, Wu K, Lu C, Luo X, Li L, Yu C. 2020. Girdin interaction with vimentin induces emt and promotes the growth and metastasis of pancreatic ductal adenocarcinoma. Oncol Rep 44: 637–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vohnoutka RB, Gulvady AC, Goreczny G, Alpha K, Handelman SK, Sexton JZ, Turner CE. 2019. The focal adhesion scaffold protein hic-5 regulates vimentin organization in fibroblasts. Mol Biol Cell 30: 3037–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding I, Ostrowska-Podhorodecka Z, Lee W, Liu RSC, Carneiro K, Janmey PA, McCulloch CA. 2020. Cooperative roles of pak1 and filamin a in regulation of vimentin assembly and cell extension formation. Biochim Biophys Acta Mol Cell Res 1867: 118739. [DOI] [PubMed] [Google Scholar]
- 22.Tusamda Wakhloo N, Anders S, Badique F, Eichhorn M, Brigaud I, Petithory T, Vassaux M, Milan JL, Freund JN, Ruhe J, Davidson PM, Pieuchot L, Anselme K. 2020. Actomyosin, vimentin and linc complex pull on osteosarcoma nuclei to deform on micropillar topography. Biomaterials 234: 119746. [DOI] [PubMed] [Google Scholar]
- 23.Serres MP, Samwer M, Truong Quang BA, Lavoie G, Perera U, Görlich D, Charras G, Petronczki M, Roux PP, Paluch EK. 2020. F-actin interactome reveals vimentin as a key regulator of actin organization and cell mechanics in mitosis. Developmental Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franke WW, Hergt M, Grund C. 1987. Rearrangement of the vimentin cytoskeleton during adipose conversion - formation of an intermediate filament cage around lipid globules. Cell 49: 131–41. [DOI] [PubMed] [Google Scholar]
- 25.Almahbobi G, Hall PF. 1990. The role of intermediate filaments in adrenal steroidogenesis. J Cell Sci 97 ( Pt 4): 679–87. [DOI] [PubMed] [Google Scholar]
- 26.Lieber JG, Evans RM. 1996. Disruption of the vimentin intermediate filament system during adipose conversion of 3t3-l1 cells inhibits lipid droplet accumulation. J Cell Sci 109 ( Pt 13): 3047–58. [DOI] [PubMed] [Google Scholar]
- 27.Khor VK, Ahrends R, Lin Y, Shen WJ, Adams CM, Roseman AN, Cortez Y, Teruel MN, Azhar S, Kraemer FB. 2014. The proteome of cholesteryl-ester-enriched versus triacylglycerol-enriched lipid droplets. PLoS One 9: e105047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen WJ, Azhar S, Kraemer FB. 2016. Lipid droplets and steroidogenic cells. Exp Cell Res 340: 209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perides G, Harter C, Traub P. 1987. Electrostatic and hydrophobic interactions of the intermediate filament protein vimentin and its amino terminus with lipid bilayers. Journal of Biological Chemistry 262: 13742–9. [PubMed] [Google Scholar]
- 30.Heid H, Rickelt S, Zimbelmann R, Winter S, Schumacher H, Dorflinger Y, Kuhn C, Franke WW. 2014. On the formation of lipid droplets in human adipocytes: The organization of the perilipin-vimentin cortex. PLoS One 9: e90386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cogne B, Bouameur J-E, Hayot G, Latypova X, Pattabiraman S, Caillaud A, Si-Tayeb K, Besnard T, Kury S, Chariau C, Gaignerie A, David L, Bordure P, Kaganovich D, Bezieau S, Golzio C, Magin TM, Isidor B. 2020. A dominant vimentin variant causes a rare syndrome with premature aging. European Journal of Human Genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pietrangelo A, Ridgway ND. 2019. Phosphorylation of a serine/proline-rich motif in oxysterol binding protein-related protein 4l (orp4l) regulates cholesterol and vimentin binding. PLoS One 14: e0214768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pang K, Park J, Ahn SG, Lee J, Park Y, Ooshima A, Mizuno S, Yamashita S, Park KS, Lee SY, Jeong J, Ushijima T, Yang KM, Kim SJ. 2019. Rnf208, an estrogen-inducible e3 ligase, targets soluble vimentin to suppress metastasis in triple-negative breast cancers. Nat Commun 10: 5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahammad S, Murthy SN, Didonna A, Grin B, Israeli E, Perrot R, Bomont P, Julien JP, Kuczmarski E, Opal P, Goldman RD. 2013. Giant axonal neuropathy-associated gigaxonin mutations impair intermediate filament protein degradation. J Clin Invest 123: 1964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis MA, Fairgrieve MR, Den Hartigh A, Yakovenko O, Duvvuri B, Lood C, Thomas WE, Fink SL, Gale M Jr., 2019. Calpain drives pyroptotic vimentin cleavage, intermediate filament loss, and cell rupture that mediates immunostimulation. Proc Natl Acad Sci U S A 116: 5061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Gao W, Zhang Y, Cheng F, Eriksson JE, Etienne-Manneville S, Jiu Y. 2019. Engagement of vimentin intermediate filaments in hypotonic stress. J Cell Biochem 120: 13168–76. [DOI] [PubMed] [Google Scholar]
- 37.Dudiak BM, Maksimchuk KR, Bednar MM, Podracky CJ, Burg JM, Nguyen TM, Nwogbo FO, Valdivia RH, McCafferty DG. 2019. Insights into the autoproteolytic processing and catalytic mechanism of the chlamydia trachomatis virulence-associated protease cpaf. Biochemistry 58: 3527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Engeland NCA, Suarez Rodriguez F, Rivero-Muller A, Ristori T, Duran CL, Stassen O, Antfolk D, Driessen RCH, Ruohonen S, Ruohonen ST, Nuutinen S, Savontaus E, Loerakker S, Bayless KJ, Sjoqvist M, Bouten CVC, Eriksson JE, Sahlgren CM. 2019. Vimentin regulates notch signaling strength and arterial remodeling in response to hemodynamic stress. Sci Rep 9: 12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang YH, Han SR, Jeon H, Lee S, Lee J, Yoo SM, Park JB, Park MJ, Kim JT, Lee HG, Lee MS, Lee SH. 2019. Nogo receptor-vimentin interaction: A novel mechanism for the invasive activity of glioblastoma multiforme. Exp Mol Med 51: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S, Huang J, Zhang Y, Liu Y, Zuo S, Li R. 2019. Map2k4 interacts with vimentin to activate the pi3k/akt pathway and promotes breast cancer pathogenesis. Aging-Us 11: 10697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ise H, Kobayashi S, Goto M, Sato T, Kawakubo M, Takahashi M, Ikeda U, Akaike T. 2010. Vimentin and desmin possess glcnac-binding lectin-like properties on cell surfaces. Glycobiology 20: 843–64. [DOI] [PubMed] [Google Scholar]
- 42.Ise H, Matsunaga K, Shinohara M, Sakai Y. 2019. Improved isolation of mesenchymal stem cells based on interactions between n-acetylglucosamine-bearing polymers and cell-surface vimentin. Stem Cells International 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farach AM, Galileo DS. 2008. O-glcnac modification of radial glial vimentin filaments in the developing chick brain. Brain Cell Biol 36: 191–202. [DOI] [PubMed] [Google Scholar]
- 44.Tarbet HJ, Dolat L, Smith TJ, Condon BM, O’Brien ET 3rd, Valdivia RH, Boyce M. 2018. Site-specific glycosylation regulates the form and function of the intermediate filament cytoskeleton. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao C, Chen Y, Wang J, Qian T, Feng W, Chen Y, Mao S, Yu B. 2020. Lncrna bc088259 promotes schwann cell migration through vimentin following peripheral nerve injury. Glia 68: 670–9. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi N, Kimura AP, Ohmura K, Naito S, Yoshida M, Ieko M. 2020. Knockdown of long noncoding rna dreh facilitates cell surface glut4 expression and glucose uptake through the involvement of vimentin in 3t3-l1 adipocytes. Gene 735: 144404. [DOI] [PubMed] [Google Scholar]
- 47.Murray ME, Mendez MG, Janmey PA. 2014. Substrate stiffness regulates solubility of cellular vimentin. Mol Biol Cell 25: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ketema M, Kreft M, Secades P, Janssen H, Sonnenberg A. 2013. Nesprin-3 connects plectin and vimentin to the nuclear envelope of sertoli cells but is not required for sertoli cell function in spermatogenesis. Molecular biology of the cell 24: 2454–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burke B, Stewart CL. 2014. Functional architecture of the cell's nucleus in development, aging, and disease: Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- 50.Dechat T, Adam SA, Taimen P, Shimi T, Goldman RD. 2010. Nuclear lamins. Cold Spring Harbor Perspectives in Biology 2: a000547-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terriac E, Schutz S, Lautenschlager F. 2019. Vimentin intermediate filament rings deform the nucleus during the first steps of adhesion. Front Cell Dev Biol 7: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldman RD, Follett EA. 1970. Birefringent filamentous organelle in bhk-21 cells and its possible role in cell spreading and motility. Science 169: 286–8. [DOI] [PubMed] [Google Scholar]
- 53.Correia I, Chu D, Chou YH, Goldman RD, Matsudaira P. 1999. Integrating the actin and vimentin cytoskeletons. Adhesion-dependent formation of fimbrin-vimentin complexes in macrophages. J Cell Biol 146: 831–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esue O, Carson AA, Tseng Y, Wirtz D. 2006. A direct interaction between actin and vimentin filaments mediated by the tail domain of vimentin. J Biol Chem 281: 30393–9. [DOI] [PubMed] [Google Scholar]
- 55.Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. 2010. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol 189: 541–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prahlad V, Yoon M, Moir RD, Vale RD, Goldman RD. 1998. Rapid movements of vimentin on microtubule tracks: Kinesin-dependent assembly of intermediate filament networks. J Cell Biol 143: 159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gyoeva FK, Gelfand VI. 1991. Coalignment of vimentin intermediate filaments with microtubules depends on kinesin. Nature 353: 445–8. [DOI] [PubMed] [Google Scholar]
- 58.Hookway C, Ding L, Davidson MW, Rappoport JZ, Danuser G, Gelfand VI. 2015. Microtubule-dependent transport and dynamics of vimentin intermediate filaments. Mol Biol Cell 26: 1675–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gurland G, Gundersen GG. 1995. Stable, detyrosinated microtubules function to localize vimentin intermediate filaments in fibroblasts. J Cell Biol 131: 1275–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maniotis AJ, Chen CS, Ingber DE. 1997. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure tumors. Proc Nat Acad Sci 13: 1091–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janmey PA, Euteneuer U, Traub P, Schliwa M. 1991. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. Journal of Cell Biology 113: 155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Charrier EE, Janmey PA. 2016. Mechanical properties of intermediate filament proteins. Elsevier. p 35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber D, Merckling A, Langa F, Aumailley M, Delouvee A, Koteliansky V, Babinet C, Krieg T. 1998. Impaired mechanical stability, migration and contractile capacity in vimentin- deficient fibroblasts. J Cell Sci 111: 1897–07. [DOI] [PubMed] [Google Scholar]
- 64.Wang N, Stamenovic K. 2000. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. American Journal of Physiology - Cell Physiology 279: C188–C94. [DOI] [PubMed] [Google Scholar]
- 65.Gandikota MC, Pogoda K, van Oosten A, Engstrom TA, Patteson AE, Janmey PA, Schwarz JM. 2020. Loops versus lines and the compression stiffening of cells. Soft Matter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patteson AE, Pogoda K, Byfield FJ, Mandal K, Ostrowska-Podhorodecka Z, Charrier EE, Galie PA, Deptula P, Bucki R, McCulloch CA, Janmey PA. 2019. Loss of vimentin enhances cell motility through small confining spaces. Small 15: e1903180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stephens AD, Liu PZ, Banigan EJ, Almassalha LM, Backman V, Adam SA, Goldman RD, Marko JF. 2017. Chromatin histone modifications and rigidity affect nuclear morphology independent of lamins. Molecular biology of the cell 29: 220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiu Y, Lehtimaki J, Tojakander S, Cheng F, Jaalinoja H, Liu X, Varjosalo M, Eriksson JE, Lappalainen P. 2015. Bidirectional interplay between vimentin intermediate filaments and contractile actin stress fibers. Cell Reports 11: 1511–8. [DOI] [PubMed] [Google Scholar]
- 69.Li Y, Lovett D, Zhang Q, Neelam S, Kuchibhotla RA, Zhu R, Gundersen GG, Lele TP, Dickinson RB. 2015. Moving cell boundaries drive nuclear shaping during cell spreading. Biophysj 109: 670–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vishavkarma R, Raghavan S, Kuyyamudi C, Majumder A, Dhawan J, Pullarkat PA. 2014. Role of actin filaments in correlating nuclear shape and cell spreading. PLoS ONE 9: e107895–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alisafaei F, Jokhun DS, Shivashankar GV, Shenoy VB. 2019. Regulation of nuclear architecture, mechanics, and nucleocytoplasmic shuttling of epigenetic factors by cell geometric constraints. Proceedings of the National Academy of Sciences of the United States of America 116: 13200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khatau SB, Hale CM, Stewart-Hutchinson PJ, Patel MS, Stewart CL, Searson PC, Hodzic D, Wirtz D. 2009. A perinuclear actin cap regulates nuclear shape. Proceedings of the National Academy of Sciences 106: 19017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keeling MC, Flores LR, Dodhy AH, Murray ER, Gavara N. 2017. Actomyosin and vimentin cytoskeletal networks regulate nuclear shape, mechanics and chromatin organization. Sci Rep 7: 5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patteson AE, Vahabikashi A, Pogoda K, Adam SA, Mandal K, Kittisopikul M, Sivagurunathan S, Goldman A, Goldman RD, Janmey PA. 2019. Vimentin protects cells against nuclear rupture and DNA damage during migration. J Cell Biol 218: 4079–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neelam S, Chancellor TJ, Li Y, Nickerson JA, Roux KJ, Dickinson RB, Lele TP. 2015. Direct force probe reveals the mechanics of nuclear homeostasis in the mammalian cell. Proc Natl Acad Sci U S A 112: 5720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lavenus SB, Tudor SM, Ullo MF, Vosatka KW, Logue JS. 2020. A flexible network of vimentin intermediate filaments promotes the migration of amoeboid cancer cells through confined environments. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stankevicins L, Ecker N, Terriac E, Maiuri P, Schoppmeyer R, Vargas P, Lennon-Dumenil AM, Piel M, Qu B, Hoth M, Kruse K, Lautenschlager F. 2020. Deterministic actin waves as generators of cell polarization cues. Proc Natl Acad Sci U S A 117: 826–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Musaelyan A, Lapin S, Nazarov V, Tkachenko O, Gilburd B, Mazing A, Mikhailova L, Shoenfeld Y. 2018. Vimentin as antigenic target in autoimmunity: A comprehensive review. Autoimmun Rev 17: 926–34. [DOI] [PubMed] [Google Scholar]
- 79.Frescas D, Roux CM, Aygun-Sunar S, Gleiberman AS, Krasnov P, Kurnasov OV, Strom E, Virtuoso LP, Wrobel M, Osterman AL, Antoch MP, Mett V, Chernova OB, Gudkov AV. 2017. Senescent cells expose and secrete an oxidized form of membrane-bound vimentin as revealed by a natural polyreactive antibody. Proc Natl Acad Sci U S A 114: E1668–E77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moisan E, Girard D. 2006. Cell surface expression of intermediate filament proteins vimentin and lamin b1 in human neutrophil spontaneous apoptosis. J Leukoc Biol 79: 489–98. [DOI] [PubMed] [Google Scholar]
- 81.Boilard E, Bourgoin SG, Bernatchez C, Surette ME. 2003. Identification of an autoantigen on the surface of apoptotic human t cells as a new protein interacting with inflammatory group iia phospholipase a2. Blood 102: 2901–9. [DOI] [PubMed] [Google Scholar]
- 82.Podor TJ, Singh D, Chindemi P, Foulon DM, McKelvie R, Weitz JI, Austin R, Boudreau G, Davies R. 2002. Vimentin exposed on activated platelets and platelet microparticles localizes vitronectin and plasminogen activator inhibitor complexes on their surface. J Biol Chem 277: 7529–39. [DOI] [PubMed] [Google Scholar]
- 83.Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. 2003. Vimentin is secreted by activated macrophages. Nat Cell Biol 5: 59–63. [DOI] [PubMed] [Google Scholar]
- 84.Kim S, Cho W, Kim I, Lee SH, Oh GT, Park YM. 2020. Oxidized ldl induces vimentin secretion by macrophages and contributes to atherosclerotic inflammation. J Mol Med (Berl). [DOI] [PubMed] [Google Scholar]
- 85.Fasipe TA, Hong SH, Da Q, Valladolid C, Lahey MT, Richards LM, Dunn AK, Cruz MA, Marrelli SP. 2018. Extracellular vimentin/vwf (von willebrand factor) interaction contributes to vwf string formation and stroke pathology. Stroke 49: 2536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lam FW, Da Q, Guillory B, Cruz MA. 2018. Recombinant human vimentin binds to p-selectin and blocks neutrophil capture and rolling on platelets and endothelium. J Immunol 200: 1718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu B, deWaal RM, Mor-Vaknin N, Hibbard C, Markovitz DM, Kahn ML. 2004. The endothelial cell-specific antibody pal-e identifies a secreted form of vimentin in the blood vasculature. Mol Cell Biol 24: 9198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Noh H, Yan J, Hong S, Kong LY, Gabrusiewicz K, Xia X, Heimberger AB, Li S. 2016. Discovery of cell surface vimentin targeting mab for direct disruption of gbm tumor initiating cells. Oncotarget 7: 72021–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu M, Wang R, Sun X, Liu Y, Wang Z, Yan J, Kong X, Liang S, Liu Q, Zhao T, Ji X, Wang G, Wang F, Wang G, Chen L, Zhang Q, Lv W, Li H, Sun M. 2020. Prognostic significance of pd-l1 expression on cell-surface vimentin-positive circulating tumor cells in gastric cancer patients. Molecular Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hwang B, Ise H. 2020. Multimeric conformation of type iii intermediate filaments but not the filamentous conformation exhibits high affinity to lipid bilayers. Genes Cells. [DOI] [PubMed] [Google Scholar]
- 91.Walker JL, Bleaken BM, Romisher AR, Alnwibit AA, Menko AS. 2018. In wound repair vimentin mediates the transition of mesenchymal leader cells to a myofibroblast phenotype. Mol Biol Cell 29: 1555–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shigyo M, Kuboyama T, Sawai Y, Tada-Umezaki M, Tohda C. 2015. Extracellular vimentin interacts with insulin-like growth factor 1 receptor to promote axonal growth. Sci Rep 5: 12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shigyo M, Tohda C. 2016. Extracellular vimentin is a novel axonal growth facilitator for functional recovery in spinal cord-injured mice. Sci Rep 6: 28293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adolf A, Rohrbeck A, Munster-Wandowski A, Johansson M, Kuhn HG, Kopp MA, Brommer B, Schwab JM, Just I, Ahnert-Hilger G, Holtje M. 2019. Release of astroglial vimentin by extracellular vesicles: Modulation of binding and internalization of c3 transferase in astrocytes and neurons. Glia 67: 703–17. [DOI] [PubMed] [Google Scholar]
- 95.Jang B, Kim MJ, Lee YJ, Ishigami A, Kim YS, Choi EK. 2020. Vimentin citrullination probed by a novel monoclonal antibody serves as a specific indicator for reactive astrocytes in neurodegeneration. Neuropathol Appl Neurobiol. [DOI] [PubMed] [Google Scholar]
- 96.Yu MB, Guerra J, Firek A, Langridge WHR. 2018. Extracellular vimentin modulates human dendritic cell activation. Mol Immunol 104: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huet D, Bagot M, Loyaux D, Capdevielle J, Conraux L, Ferrara P, Bensussan A, Marie-Cardine A. 2006. Sc5 mab represents a unique tool for the detection of extracellular vimentin as a specific marker of sezary cells. J Immunol 176: 652–9. [DOI] [PubMed] [Google Scholar]
- 98.Noh H, Zhao Q, Yan J, Kong LY, Gabrusiewicz K, Hong S, Xia X, Heimberger AB, Li S. 2018. Cell surface vimentin-targeted monoclonal antibody 86c increases sensitivity to temozolomide in glioma stem cells. Cancer Lett 433: 176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Batth IS, Dao L, Satelli A, Mitra A, Yi S, Noh H, Li H, Brownlee Z, Zhou S, Bond J, Wang J, Gill J, Sholler GS, Li S. 2020. Cell surface vimentin-positive circulating tumor cell-based relapse prediction in a long-term longitudinal study of postremission neuroblastoma patients. International journal of cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Steinmetz NF, Maurer J, Sheng H, Bensussan A, Maricic I, Kumar V, Braciak TA. 2011. Two domains of vimentin are expressed on the surface of lymph node, bone and brain metastatic prostate cancer lines along with the putative stem cell marker proteins cd44 and cd133. Cancers (Basel) 3: 2870–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mitra A, Satelli A, Xia X, Cutrera J, Mishra L, Li S. 2015. Cell-surface vimentin: A mislocalized protein for isolating csvimentin(+)cd133(−) novel stem-like hepatocellular carcinoma cells expressing emt markers. International Journal of Cancer 137: 491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li G, Wang Y, Tan G. 2018. The construction of epcam/vimentin-plga/lipid immunomagnetic microspheres and the isolation of circulating tumor cells from lung cancer. Int J Clin Exp Pathol 11: 5561–70. [PMC free article] [PubMed] [Google Scholar]
- 103.Li H, Meng QH, Noh H, Somaiah N, Torres KE, Xia X, Batth IS, Joseph CP, Liu M, Wang R, Li S. 2018. Cell-surface vimentin-positive macrophage-like circulating tumor cells as a novel biomarker of metastatic gastrointestinal stromal tumors. Oncoimmunology 7: e1420450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pall T, Pink A, Kasak L, Turkina M, Anderson W, Valkna A, Kogerman P. 2011. Soluble cd44 interacts with intermediate filament protein vimentin on endothelial cell surface. PLoS One 6: e29305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Irani S, Dehghan A. 2018. The expression and functional significance of vascular endothelial-cadherin, cd44, and vimentin in oral squamous cell carcinoma. J Int Soc Prev Community Dent 8: 110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deng L, Spencer BL, Holmes JA, Mu R, Rego S, Weston TA, Hu Y, Sanches GF, Yoon S, Park N, Nagao PE, Jenkinson HF, Thornton JA, Seo KS, Nobbs AH, Doran KS. 2019. The group b streptococcal surface antigen i/ii protein, bspc, interacts with host vimentin to promote adherence to brain endothelium and inflammation during the pathogenesis of meningitis. PLoS Pathog 15: e1007848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang SH, Chi F, Peng L, Bo T, Zhang B, Liu LQ, Wu X, Mor-Vaknin N, Markovitz DM, Cao H, Zhou YH. 2016. Vimentin, a novel nf-kappab regulator, is required for meningitic escherichia coli k1-induced pathogen invasion and pmn transmigration across the blood-brain barrier. PLoS One 11: e0162641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu W, Pante N. 2016. Vimentin plays a role in the release of the influenza a viral genome from endosomes. Virology 497: 41–52. [DOI] [PubMed] [Google Scholar]
- 109.Miller MS, Hertel L. 2009. Onset of human cytomegalovirus replication in fibroblasts requires the presence of an intact vimentin cytoskeleton. J Virol 83: 7015–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thiam HR, Wong SL, Qiu R, Kittisopikul M, Vahabikashi A, Goldman AE, Goldman RD, Wagner DD, Waterman CM. 2020. Netosis proceeds by cytoskeleton and endomembrane disassembly and pad4-mediated chromatin decondensation and nuclear envelope rupture. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Corsiero E, Bombardieri M, Carlotti E, Pratesi F, Robinson W, Migliorini P, Pitzalis C. 2016. Single cell cloning and recombinant monoclonal antibodies generation from ra synovial b cells reveal frequent targeting of citrullinated histones of nets. Ann Rheum Dis 75: 1866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen P, Fox DA, Pennathur S, Kaplan MJ. 2013. Nets are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 5: 178ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mak TN, Bruggemann H. 2016. Vimentin in bacterial infections. Cells 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bastounis EE, Yeh YT, Theriot JA. 2018. Matrix stiffness modulates infection of endothelial cells by listeria monocytogenes via expression of cell surface vimentin. Mol Biol Cell 29: 1571–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ghosh P, Halvorsen EM, Ammendolia DA, Mor-Vaknin N, O’Riordan MXD, Brumell JH, Markovitz DM, Higgins DE. 2018. Invasion of the brain by listeria monocytogenes is mediated by inlf and host cell vimentin. mBio 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zou Y, He L, Huang S-H. 2006. Identification of a surface protein on human brain microvascular endothelial cells as vimentin interacting with escherichia coli invasion protein ibea. Biochemical and Biophysical Research Communications 351: 625–30. [DOI] [PubMed] [Google Scholar]
- 117.Garg A, Barnes PF, Porgador A, Roy S, Wu S, Nanda JS, Griffith DE, Girard WM, Rawal N, Shetty S, Vankayalapati R. 2006. Vimentin expressed on mycobacterium tuberculosis-infected human monocytes is involved in binding to the nkp46 receptor. J Immunol 177: 6192–8. [DOI] [PubMed] [Google Scholar]
- 118.Li SW, Wang CY, Jou YJ, Yang TC, Huang SH, Wan L, Lin YJ, Lin CW. 2016. Sars coronavirus papain-like protease induces egr-1-dependent up-regulation of tgf-beta1 via ros/p38 mapk/stat3 pathway. Sci Rep 6: 25754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mossel EC, Wang J, Jeffers S, Edeen KE, Wang S, Cosgrove GP, Funk CJ, Manzer R, Miura TA, Pearson LD, Holmes KV, Mason RJ. 2008. Sars-cov replicates in primary human alveolar type ii cell cultures but not in type i-like cells. Virology 372: 127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu YT, Chien SC, Chen IY, Lai CT, Tsay YG, Chang SC, Chang MF. 2016. Surface vimentin is critical for the cell entry of sars-cov. J Biomed Sci 23: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chang XB, Yang YQ, Gao JC, Zhao K, Guo JC, Ye C, Jiang CG, Tian ZJ, Cai XH, Tong GZ, An TQ. 2018. Annexin a2 binds to vimentin and contributes to porcine reproductive and respiratory syndrome virus multiplication. Vet Res 49: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang WW, Zhang L, Ma XC, Gao JM, Xiao YH, Zhou EM. 2011. The role of vimentin during prrsv infection of marc-145 cells. Bing Du Xue Bao 27: 456–61. [PubMed] [Google Scholar]
- 123.Wang ZJ, Xu CM, Song ZB, Wang M, Liu QY, Jiang P, Li YF, Bai J, Wang XW. 2018. Vimentin modulates infectious porcine circovirus type 2 in pk-15 cells. Virus Res 243: 110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Q, Yoo D. 2015. Prrs virus receptors and their role for pathogenesis. Vet Microbiol 177: 229–41. [DOI] [PubMed] [Google Scholar]
- 125.Kobayashi K, Koike S. 2020. Cellular receptors for enterovirus a71. Journal of Biomedical Science 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Turkki P, Laajala M, Flodstrom-Tullberg M, Marjomaki V. 2020. Human enterovirus group b viruses rely on vimentin dynamics for efficient processing of viral nonstructural proteins. J Virol 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang W, Sun J, Wang N, Sun Z, Ma Q, Li J, Zhang M, Xu J. 2020. Enterovirus a71 capsid protein vp1 increases blood-brain barrier permeability and virus receptor vimentin on the brain endothelial cells. J Neurovirol 26: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Teo CS, Chu JJ. 2014. Cellular vimentin regulates construction of dengue virus replication complexes through interaction with ns4a protein. J Virol 88: 1897–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang J, Zou L, Yang Y, Yuan J, Hu Z, Liu H, Peng H, Shang W, Zhang X, Zhu J, Rao X. 2016. Superficial vimentin mediates denv-2 infection of vascular endothelial cells. Sci Rep 6: 38372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yan G, Lee CK, Lam LTM, Yan B, Chua YX, Lim AYN, Phang KF, Kew GS, Teng H, Ngai CH, Lin L, Foo RM, Pada S, Ng LC, Tambyah PA. 2020. Covert covid-19 and false-positive dengue serology in singapore. Lancet Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Das S, Ravi V, Desai A. 2011. Japanese encephalitis virus interacts with vimentin to facilitate its entry into porcine kidney cell line. Virus Res 160: 404–8. [DOI] [PubMed] [Google Scholar]
- 132.Yu YN, Zheng Y, Hao SS, Zhang Z, Cai JX, Zong MM, Feng XL, Liu QT. 2020. The molecular evolutionary characteristics of new isolated h9n2 aiv from east china and the function of vimentin on virus replication in mdck cells. Virology journal 17: 78–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kavathekar VK, Dhanavade MJ, Sonawane KD, Balakrishnan A. 2020. Role of cell surface vimentin in chandipura virus replication in neuro-2a cells. Virus Res 285: 198014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ma X, Ling Y, Li P, Sun P, Cao Y, Bai X, Li K, Fu Y, Zhang J, Li D, Bao H, Chen Y, Li Z, Wang Y, Lu Z, Liu Z. 2020. Cellular vimentin interacts with foot-and-mouth disease virus nonstructural protein 3a and negatively modulates viral replication. J Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schafer G, Graham LM, Lang DM, Blumenthal MJ, Bergant Marusic M, Katz AA. 2017. Vimentin modulates infectious internalization of human papillomavirus 16 pseudovirions. J Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Satelli A, Batth I, Brownlee Z, Mitra A, Zhou S, Noh H, Rojas CR, Li H, Meng QH, Li S. 2017. Emt circulating tumor cells detected by cell-surface vimentin are associated with prostate cancer progression. Oncotarget 8: 49329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zheng Y, Zhang J, Huang M, Wang T, Qu X, Wu L, Song J, Wang W, Song Y, Yang C. 2020. Selection of aptamers against vimentin for isolation and release of circulating tumor cells undergoing epithelial mesenchymal transition. Anal Chem 92: 5178–84. [DOI] [PubMed] [Google Scholar]
- 138.Carrick S. 2020. Nascent biotech has begun in vitro studies as a potential therapeutic agent against covid- 19. [Google Scholar]
- 139.Brentville VA, Metheringham RL, Daniels I, Atabani S, Symonds P, Cook KW, Vankemmelbeke M, Choudhury R, Vaghela P, Gijon M, Meiners G, Krebber WJ, Melief CJM, Durrant LG. 2020. Combination vaccine based on citrullinated vimentin and enolase peptides induces potent cd4-mediated anti-tumor responses. J Immunother Cancer 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu S, Liu L, Ye W, Ye D, Wang T, Guo W, Liao Y, Xu D, Song H, Zhang L, Zhu H, Deng J, Zhang Z. 2016. High vimentin expression associated with lymph node metastasis and predicated a poor prognosis in oral squamous cell carcinoma. Sci Rep 6: 38834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Du R-H, Liang L-R, Yang C-Q, Wang W, Cao T-Z, Li M, Guo G-Y, Du J, Zheng C-L, Zhu Q. 2020. Predictors of mortality for patients with covid-19 pneumonia caused by sars-cov-2: A prospective cohort study. European Respiratory Journal 55: in press. [DOI] [PMC free article] [PubMed] [Google Scholar]