Abstract
In this Seminar, we highlight the main developments in the field of Alzheimer’s disease. The most recent data indicate that, by 2050, the prevalence of dementia will double in Europe and triple worldwide, and that estimate is 3 times higher when based on a biological (rather than clinical) definition of Alzheimer’s disease. The earliest phase of Alzheimer’s disease (cellular phase) happens in parallel with accumulating amyloid β, inducing the spread of tau pathology. The risk of Alzheimer’s disease is 60–80% dependent on heritable factors, with more than 40 Alzheimer’s disease-associated genetic risk loci already identified, of which the APOE alleles have the strongest association with the disease. Novel biomarkers include PET scans and plasma assays for amyloid β and phosphorylated tau, which show great promise for clinical and research use. Multidomain lifestyle-based prevention trials suggest cognitive benefits in participants with increased risk of dementia. Lifestyle factors do not directly affect Alzheimer’s disease pathology, but can still contribute to a positive outcome in individuals with Alzheimer’s disease. Promising pharmacological treatments are poised at advanced stages of clinical trials and include anti-amyloid β, anti-tau, and anti-inflammatory strategies.
Introduction
Alzheimer’s disease is the main cause of dementia and is quickly becoming one of the most expensive, lethal, and burdening diseases of this century.1 Since the Seminar published in 2016,2 important developments have taken place in the understanding of the underlying pathology, the recognition of multiple causative and protective genes, the identification of new blood-based and imaging biomarkers, and the first cautious signals of positive effects of disease-modifying treatments and lifestyle interventions. The aim of this new Seminar is to provide the reader with up to date insight into the field of Alzheimer’s disease.
Clinical signs and symptoms
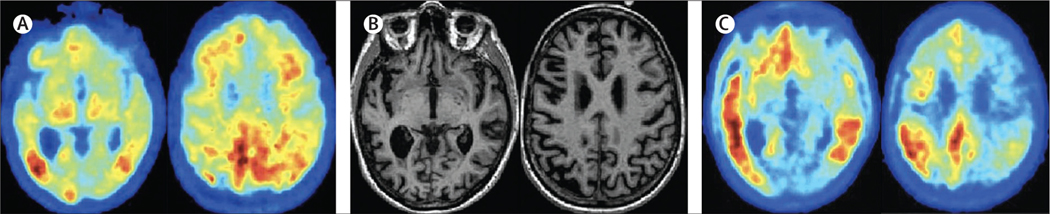
Three cases, in panel 1 (see also figure 1), illustrate the clinical spectrum of Alzheimer’s disease. Case A highlights Alzheimer’s disease that is determined genetically, as per the ongoing global initiatives of the Dominantly Inherited Alzheimer Network and Alzheimer Prevention Initiative and their associated clinical trials. Case B represents a language variant of Alzheimer’s disease, usually occurring at a younger age (under 70 years), illustrating the difficulty in recognising Alzheimer’s disease in those for whom memory problems are not the first and most prominent feature. Case C is a typical amnestic variant, more commonly seen in patients older than 70 years, illustrating the growing population affected by Alzheimer’s disease and dementia: older individuals often living alone, and increasingly dependent on others for care.
Search strategy and selection criteria
Between Dec 1, 2019, and Sept 1, 2020, we searched the Cochrane Library for articles published exclusively in English during 2010–15, PubMed for articles published during 2016–20, and Embase for articles published during 2016–20. We used the search term “Alzheimer’s disease” in combination with the following: “pathology”, “imaging”, “diagnosis”, “therapy”, “trials”, “epidemiology”, “CSF”, “genetics”, and “biomarkers”. We largely selected publications from the past 5 years, and especially focused on changes that occurred after the publication of the previous Seminar in 2016.1 We also searched the reference lists of articles identified by this search strategy and selected those that were judged relevant. Review articles and book chapters are cited to provide readers with references for more details than this Seminar can include.
Panel 1: Case vignettes
Mrs A, aged 42 years, a successful manager of an IT company, presents at the Alzheimer Centre Amsterdam because of self-perceived memory loss and loss of oversight and multitasking abilities. She recognises these complaints all too well because her mother had Alzheimer’s disease for 5 years, until her death at the age of 47 years. Two of her four brothers also had Alzheimer’s disease, and had been tested and found to be carriers of a PSEN1 mutation. Although she has not been tested herself, she always felt she would be a carrier and subsequently chose not to have children. She asked for a full evaluation because she wanted to have the option of participating in a clinical trial programme. Her Mini-Mental State Exam score was 27/30 and her Montreal Cognitive Assessment score was 24/30. Given her age, these scores suggest mild memory and executive disturbances, which were confirmed by neuropsychological testing. A brain MRI showed no abnormalities. Cerebrospinal fluid biomarker values were 750 pg/mL for amyloid β42, 335 pg/mL for tau, and 35 pg/mL for phosphorylated tau 181, all in the abnormal range. Serum neurofilament light chain value was 25 pg/mL, which is abnormal for her age, according to in-house defined reference curves. APOE status was ε3/ε4. All these biomarker values indicate the presence of Alzheimer’s disease pathology and onset in a clinically mildly affected patient. Genetic testing confirmed the presence of the same PSEN1 mutation carried by her brothers. She was informed about the diagnosis, followed up at 6 month intervals at the centre, and put on the list for a clinical trial within the Dominantly Inherited Alzheimer Network Trials Unit programme. She informed her colleagues at work and agreed to have regular meetings with the company’s physician.
Mr B, aged 62 years, is a high school teacher who presented to the neurologist with gradually progressive difficulty finding words and understanding sentences, and slight memory loss. He had visited another neurologist because of suspicion of a vascular event, but a brain MRI showed no abnormalities. On examination, his Mini-Mental State Exam score was 25/30 and the Montreal Cognitive Assessment score was 24/30, both within normal range for his age, with normal findings at routine neurological and laboratory investigations. Neuropsychological and detailed language assessment revealed a decrease in fluency, naming, and repetition of long sentences.
Review of the MRI showed slight asymmetry of the temporal lobes, with grade 2 hippocampal atrophy on the left side and grade 1 hippocampal atrophy on the right side, without any other abnormalities (figure 1B). Because of his young age, and his and his family’s desire to obtain a firm diagnosis to plan ahead and make proper adjustments to his working life, an amyloid-PET scan was done and showed diffuse cortical uptake of the ligand (figure 1A). As part of a research project, a tau-PET scan was done and showed left-temporal abnormal tau deposition (figure 1C). A diagnosis of logopenic variant of Alzheimer’s disease was made. Lifestyle advice was given and regular visits to a speech therapist were offered.2 Given the diagnosis and the perceived grim future, as well as the high demands of his job on his language skills, he decided to take sick leave from his job.
Mrs C, aged 78 years, lives independently on her own after being widowed 6 years ago. She was known to her general practitioner with controlled hypertension and moderate heart failure, for which she takes medication. Her son lives abroad and her daughter lives 100 km away. Both have demanding jobs and young children. During telephone and Skype calls, her children noticed increasing forgetfulness and one of the neighbours had recently informed the daughter that her mother mixed up the days, forgot to eat, and was not able to take good care of herself anymore. The daughter accompanied her mother to the Alzheimer Centre Amsterdam on referral by the general practitioner, who had initially dismissed the worries of the daughter. On examination by a geriatrician, she was found to be malnourished and underweight. The Mini-Mental State Exam score was 17/30 and a brief neuropsychological test battery showed scores below the norm for memory and executive function. Her score on the Amsterdam Instrumental Activities of Daily Living test3 was 58, indicating severe impairment. An MRI showed a medial temporal atrophy score of 2 bilaterally, and moderate to severe white matter changes (Fazekas score 2). A diagnosis of mild to moderate dementia due to Alzheimer’s disease with some vascular contribution was made, and a case manager was assigned to organise and supervise care to have her stay at home as long as possible. Vascular risk factors were checked and cholinesterase inhibitor therapy was started.
Panel 2: Fluid biomarker consortia relevant to the field of Alzheimer’s disease
Global Biomarker Standardization Consortium of the Alzheimer’s Association112
Aims to achieve consensus on the best ways to standardise and validate biomarker tests for use in global clinical practices.
Society for CSF Analysis and Clinical Neurochemistry113
Aims to exchange high-level international scientific experience, to facilitate the incorporation of cerebrospinal fluid diagnostics into clinical practice, and to give advice on the inclusion of cerebrospinal fluid analysis into clinical guidelines.
Foundation for the National Institutes of Health Biomarkers Consortium114
Aims to bring together the expertise and resources of various partners to rapidly identify, develop, and qualify potential high-impact biomarkers, particularly to enable improvements in drug development, clinical care, and regulatory decision making.
International Federation of Clinical Chemistry and Laboratory Medicine Working Group CSF Proteins115
Aims to develop certified reference material and reference methods for amyloid β42 or amyloid β40 and tau in cerebrospinal fluid.
Figure 1: Imaging findings of a case similar to patient B’s case in panel 1.
(A) Amyloid Pittsburgh compound B-PET scan showing amyloid deposition predominantly in the posterior cingulate region. (B) T1-weighted MRI images showing generalised cortical atrophy, left to right. (C) Tau-PET image using AV1451 tracer, showing left-sided inferotemporal lobe, parietal, and mild posterior cingulate deposition of tau. Image courtesy of Rik Ossenkoppele and Gil Rabinovici.
Diagnostic criteria: from clinical, to clinical and biological, to biological
The diagnosis of Alzheimer’s disease has gone from a purely pathological one, in the days of Alois Alzheimer (1864–1915) to a clinical, exclusionary approach in 1984. The clinical diagnosis was based on the criteria defined by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association,3 via a combined clinical and biological approach developed by the International Working Group4,5 and subsequent efforts by the National Institute on Aging and the Alzheimer’s Association working groups,6 incorporating biomarkers to make the categorisation of Alzheimer’s disease purely biological.7 Initially, the diagnosis of Alzheimer’s disease was restricted to the stage of dementia, a clinical syndrome characterised by substantial progressive cognitive impairment affecting several domains, or neurobehavioral symptoms of enough severity to cause evident functional impact on daily life. A person with dementia is no longer fully independent, and this loss of independence is the primary feature differentiating dementia from mild cognitive impairment.8
Given the developments in the biomarker field and the desire to make them usable in a diagnostic setting, Jack and colleagues8 grouped the biomarkers into A (amyloid), T (phosphorylated tau), and N (neurodegeneration, measured by total tau where applicable): the ATN frame work (appendix p 1). In this research framework, the diagnosis of Alzheimer’s disease is defined by the presence of amyloid β and phosphorylated tau. The presence of amyloid β (regardless of the presence of phosphorylated tau and neurodegeneration) is termed Alzheimer’s pathological change, basing the research diagnosis of Alzheimer’s disease on biomarker evidence only. Clinical stages can range from cognitively normal to mild cognitive impairment and dementia, stressing the continuum of Alzheimer’s disease, which spans a period of years. The ATN framework underpins the importance of amyloid β and tau as the defining characteristics of Alzheimer’s disease, consequently proposing that Alzheimer’s disease can be diagnosed by biomarkers only, and definitively distinguishing between the concepts of Alzheimer’s disease and dementia (figure 2).
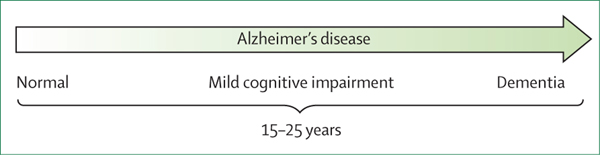
Figure 2: Alzheimer’s disease is a continuum.
The arrow points to the continuum of Alzheimer’s disease, stretching over a period of 15–25 years, in which Alzheimer’s disease pathology can be present without any symptoms via a stage of mild cognitive impairment leading up to overt dementia, illustrating that dementia is the end result of a long-time presence of Alzheimer’s disease pathology. Not every patient will necessarily follow this path by definition. Note: between normal and mild cognitive impairment, patients can experience subjective complaints, but not all complaints are early signs of dementia and the predictive value of having complaints for dementia is unknown.
Despite the critique that other key causes of dementia, in particular vascular disease, were omitted,9 the authors of the ATN framework argued that dementia has multiple underlying pathologies, of which Alzheimer’s disease is one, but Alzheimer’s disease is defined by the presence of amyloid β and tau (acknowledging that many other pathologies can also be present in these patients).10 The large number of ATN categories, combined with the fact that other pathologies are not evaluated in the scheme, makes the ATN approach not yet suitable for clinical practice.11 In addition, there are operational limitations to defining A, T, and N positivity or negativity, such as some biomarkers not having established cutoff points, and different biomarkers being combined in one category. Although the AT or ATN approach is the cornerstone of current trials of disease-modifying interventions in Alzheimer’s disease, clinical diagnosis still rests on the criteria set by the National Institute on Aging in 2011.6,12
The ATN framework clearly paves the way for a diagnosis before the stage of Alzheimer’s disease-associated dementia, and it makes individualised risk-profiling for patients with mild cognitive impairment feasible.13 However, a clinical encounter study evaluating doctor–patient communication in memory clinics showed that clinicians are reluctant to share specific prognostic information with patients with mild cognitive impairment.14 In the context of predementia diagnosis, subjective cognitive decline is even more challenging. A recent Personal View provides a clinical characterisation of subjective cognitive decline, and attempts to provide clinicians with guidance on how to deal with this decline (which might or might not be attributable to underlying Alzheimer’s disease).15 At a group level, ATN biomarkers clearly predict incident dementia in subjective cognitive decline, but individualised risk modelling remains challenging.16,17 In a Delphi study to identify topics most relevant to discuss in the diagnostic process, patients and caregivers indicated that they value precise and specific information, even when it does not provide complete certainty.18 Tools to support decision making and communication about Alzheimer’s disease diagnosis, such as ADappt,19 are urgently needed.
Epidemiology
Incidence and prevalence
In 2018, Alzheimer’s Disease International estimated a dementia prevalence of about 50 million people worldwide, projected to triple in 2050, with two-thirds living in low-income and middle-income countries.20 The most recent data estimate that dementia prevalence in Europe will double by 2050.1 Accumulating evidence suggests that the incidence of dementia is declining in high-income countries,21 although evidence for a decline in prevalence is less convincing.22
Mortality
The relatively stable prevalence despite decreasing incidence could be explained by a long disease duration, although studies on mortality do not support this notion. A US-based study evaluating survival after a dementia diagnosis in almost 60 000 individuals reported survival times of 3–4 years.23 In an European, memory clinic-based cohort, median survival time was 6 years after a diagnosis of Alzheimer’s disease dementia (median 6·2 years [range 6·0–6·5]).24 This estimate coincides with a multicentre study that provided estimates of duration not only of the dementia stage, but also of the prodromal (mild cognitive impairment) and of preclinical disease stage of Alzheimer’s disease.25 For an individual aged 70 years, duration estimates are 10 years for the preclinical stage, 4 years for the prodromal stage, and 6 years for the dementia stage of Alzheimer’s disease, totalling 20 years. A first attempt at estimating prevalence on the basis of a biological (rather than clinical) definition showed that, at the age of 85 years, the prevalence of biologically defined Alzheimer’s disease is 3 times higher than that of clinically defined Alzheimer’s disease.26
Risk factors for dementia and Alzheimer’s disease
The strongest risk factors for Alzheimer’s disease are advanced age (older than 65 years, although this is not a fixed definition) and carrying at least one APOE ε4 allele.27 Moreover, women are more likely to develop Alzheimer’s disease than are men, especially after the age of 80 years.20 Women are also more likely to have a higher tau load, despite having a similar amyloid β burden.28,29 In addition, cardiovascular risk factors and an unhealthy lifestyle have been associated with an increased risk of dementia. The Lancet Commission on Dementia Prevention estimated that 12 modifiable risk factors together account for roughly 40% of the worldwide risk of any type of dementia.30 These estimates illustrate that prevention by intervening on modifiable risk factors is of great relevance, even if most of the dementia burden cannot be prevented via a lifestyle-intervention approach. However, evidence suggests that vascular risk factors do not increase the risk of Alzheimer’s disease pathology as measured by cerebrospinal fluid biomarkers or PET.31–33 This evidence implies that lifestyle and vascular risk factors contribute to dementia, but not via the Alzheimer’s disease pathway.
Genetics
Causative and risk genes
Studies of twins showed that the risk of Alzheimer’s disease is 60–80% dependent on heritable factors.34 The common APOE ε4 allele explains a substantial part of, but does not completely account for the heritability of, Alzheimer’s disease.35,36 Large genome-wide association studies have been done to identify novel genetic variants in Alzheimer’s disease, the latest of which to date investigated about 150 000 people with Alzheimer’s disease and age-matched controls, and more than 300 000 people with a proxy-phenotype Alzheimer’s disease (parental history of Alzheimer’s disease) and controls (no parental history of Alzheimer’s disease), which increased the number of Alzheimer’s disease-associated risk alleles to more than 40.37 However, although the common APOE ε4 risk allele is associated with an estimated 3–4 times increased risk of Alzheimer’s disease across different genome-wide association studies, other Alzheimer’s disease risk alleles are associated with much smaller contributions to the total disease risk (odds ratio between 1·05 and 1·2; figure 3B).37
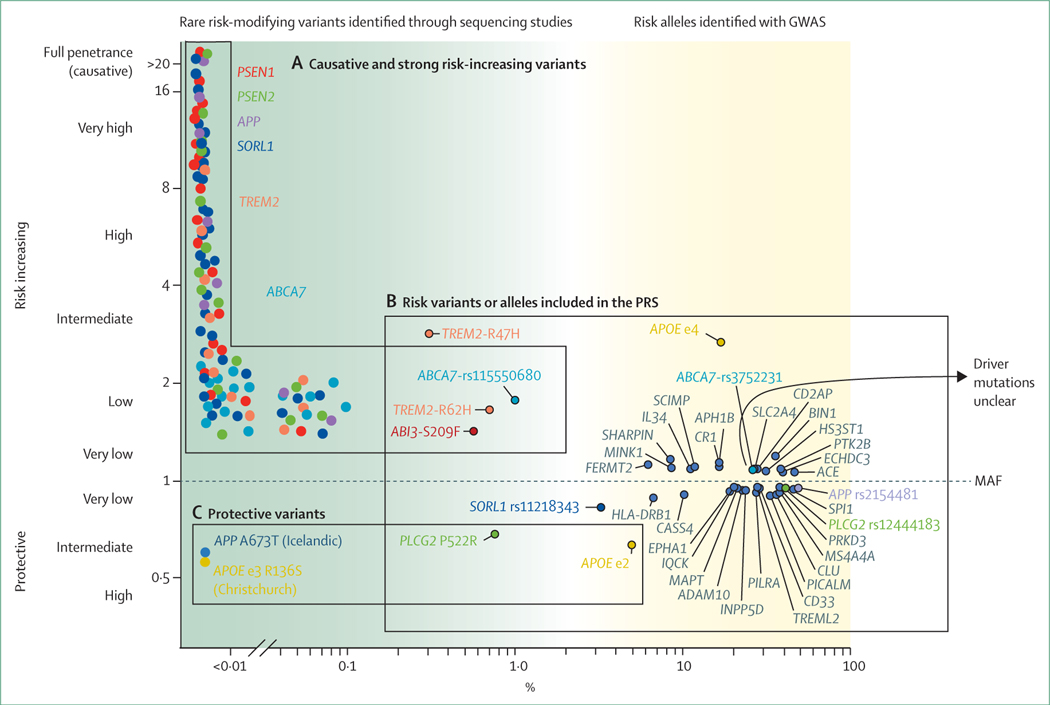
Figure 3: The genetic landscape of Alzheimer’s disease.
MAF (x-axis) is the frequency at which a non-reference (variant) allele occurs in the population. Variant carriers with OR=1 and non-carriers have the same odds of developing Alzheimer’s disease, variants with OR >1 are associated with an increased risk of Alzheimer’s disease, and variants with OR <1 are associated with a protective effect (y-axis). (A) Causative or strong risk increasing variants. A schematic representation of individual rare variants for which ORs cannot be estimated due to extreme variant rareness. Linkage studies in large pedigrees indicate that specific rare variants in PSEN1, PSEN2, and APP cause autosomal dominant Alzheimer’s disease, in some cases with age at onsets as early as 40 years old. Note that not all variants in these three genes give rise to autosomal dominant Alzheimer’s disease; some might be risk-modifiers or non-pathogenic. Further, evidence is accumulating that certain variants in the SORL1 gene are causative of Alzheimer’s disease before the age of 70 years. The Alzheimer’s disease-association of variants in the SORL1, ABCA7, and TREM2 genes was found in gene-based tests; carriers may come from small pedigrees with inheritance patterns of Alzheimer’s disease suggestive of autosomal dominant inheritance. (B) GWAS hits are common (by convention, MAF >1%) variants that represent risk alleles that occur with significantly different frequency in patients with Alzheimer’s disease and controls. Each variant is represented by the gene in which it occurs, or when the variant is non-coding, by the gene that maps closest to the variant (depicted in dark grey). (C) Protective variants are (very) rare variants suggested to confer resistance against age-associated or disease-associated risk factors of cognitive decline. GWAS=genome-wide association studies. MAF=minor allele frequency. OR=odds ratio. PRS=polygenic risk scores.
Based on the presence or absence of these risk alleles in the genome of an individual, a polygenic risk score can be calculated, which is currently able to distinguish between patients with Alzheimer’s disease and controls with 75–85% accuracy.38,39 Although the bulk of this accuracy can be ascribed to the APOE ε4 allele, the 40 or so other variants also collectively contribute substantially to Alzheimer’s disease risk.27 Functional annotation of these risk loci indicate that, next to amyloid β metabolism, the modulation of the immune response, cholesterol, lipid dysfunction, endocytosis, and vascular factors play a role in the development of Alzheimer’s disease.40–45 Next-generation sequencing techniques have shown rare protein-damaging variants in the SORL1,46 ABCA7,47 and TREM2 genes.48,49 These findings suggest that the intact protein products of these genes are essential in maintaining brain health (figure 3A).
Protective genes
The identification of risk-increasing genetic variants has fuelled the interest in the detection of protective genetic variants (figure 3C). Carriers of the protective APOE ε2 allele have an estimated 2 times decreased lifetime risk of Alzheimer’s disease compared with noncarriers,50 which translates into an exceptionally low likelihood of Alzheimer’s disease for homozygous APOE ε2 allele carriers.51 The discovery of the rare Ala673Thr Icelandic protective mutation of APP52 was associated with prolonged cognitive health. Similarly, compared with middle-aged individuals, a rare Pro522Arg amino acid change in the PLCG2 gene was associated with a near 2 times reduced risk of Alzheimer’s disease53 and other types of dementia, and with a 2·3 times increased chance of reaching 100 years in cognitive health.54,55 Genetic resilience was even reported in a person with a PSEN1 mutation who lived beyond the age of onset of symptoms common in her family, due to a homozygous rare protective variant in the APOE ε3 allele (Christchurch mutation).56 Variants in the klotho longevity gene were associated with a similar effect.57 Such protective genetic variants hold great promise in Alzheimer’s disease research, as they might pinpoint mechanistic processes protecting cognitive health.
Pathophysiology
Basic scientists designate the preclinical phase of Alzheimer’s disease as the cellular phase. Alterations in neurons, microglia, and astroglia drive the insidious progression of the disease before cognitive impairment is observed.58 Neuro-inflammation,59 alterations in the vessels,60,61 ageing,62 and dysfunction of the glymphatic system63 act upstream or in parallel to accumulating amyloid β in this cellular disease landscape. Amyloid β induces, via an unknown way, the spread of tau patho logy,64 which is associated with the appearance of necroptosis markers in neurons displaying granulovacuolar degeneration.65
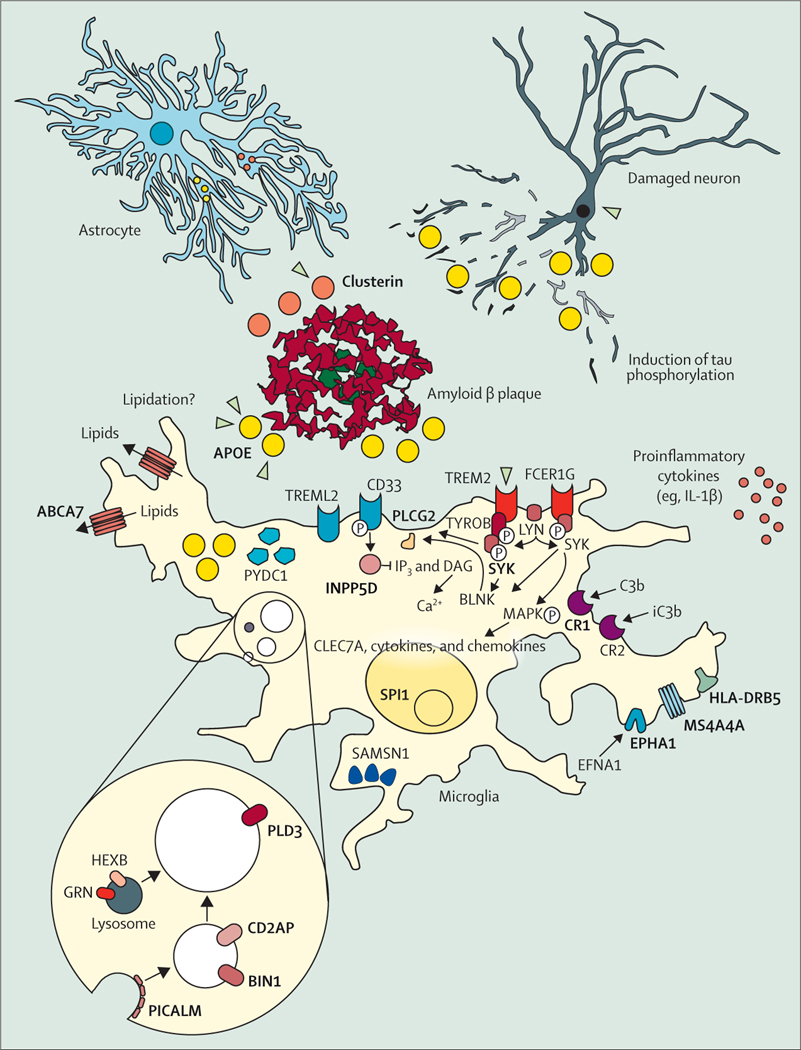
Single-cell transcriptome analysis has elucidated the microglia response.66 APOE and TREM2, two major Alzheimer’s disease risk genes, are important parts of this response.66–68 ApoE binds to amyloid β plaques,69 and the Alzheimer’s disease-associated genetic variants of TREM2 Arg47His, Arg62His, and Asp87Asn decrease binding of TREM2 to ApoE (figure 3).70 Several other proteins linked to genetic risk of Alzheimer’s disease, such as SHIP1, CD2AP, RIN3, BIN1, PLCG2, CASS4, and PTKB2 act presumably downstream of ApoE and TREM2 signal-modulating endocytosis, motility, and phagocytosis in microglia (figure 4). CD33 acts in opposition to TREM2,77 and MS4A4A modulates the secretion of soluble TREM2 protein.78 The fact that so many Alzheimer’s disease risk genes converge on microglial response pathways indicates their central role in the disease pathogenesis. However, further research is needed to elucidate whether the microglia response is to amyloid β plaques only,76 or that it also mediates toxicity induced by tau pathology79 or acts protectively against tau.80
Figure 4: The cellular phase of Alzheimer’s disease.
Although amyloid plaques (red, middle of the figure) and tau phosphorylation and tangles (neurons, top right corner) are still considered the defining features of Alzheimer’s disease, the focus of research has been widened from neurons to the response of other cell populations in the disease.71 The microglia-mediated inflammation, known for decades to be present in Alzheimer’s disease,72 has finally taken centre-stage in functional research on the pathogenesis of the disease. Many of the risk-genes protein products (bold and capitals) identified in Alzheimer’s disease (figure 3) are expressed and have functions in microglia. These genes become upregulated when microglia are exposed to amyloid plaques and many of the Alzheimer’s disease risk genes are enriched in the disease-associated microglia response that characterises this cell state.73–75 Other genes involved in this response and moderately positive in genome-wide association studies are indicated as well. Adapted from Sierksma et al,76 by permission of EMBO Molecular Medicine.
The contradictory effects of the microglia response partly reflect the limitations of mice models overexpressing tau for the study of Alzheimer’s disease. It is possible that strong transgenic tau overexpression79 induces an artificially strong neuroinflammatory response that is not seen in milder tau models.76,80 The use of mice models that do not overexpress tau,76 mouse-human chimeric mice,82,83 or new in-vitro models derived from human, induced pluripotent stem cells84 might help to explain the conflicting observations. Of note, all preclinical models are reductionistic in nature, implying that any conclusions towards therapeutic developments need to be made with caution.
Although cellular pathology has become central in the study of Alzheimer’s disease, great progress has also been made in understanding the preceding biochemical phase of the disease (in ATN terms, before A positivity [presence of amyloid β]). Thanks to cryo-electron microscopy, amyloid β85 and tau fibrils are now known in finer detail.86 Cryo-electron microscopy has also allowed full insight into how presenilins, the catalytic subunits of γ-secretases, interact with APP87 and Notch substrates.88 Complemented by functional studies on purified γ-secretase complexes, 71 it is now understood that clinical mutations in presenilins destabilise the γ-secretase–APP interactions, leading to premature release of longer, aggregation-prone amyloid β peptides. These insights support the development of new therapeutic approaches to tackle amyloid β in Alzheimer’s disease.
The role of amyloid β in the disease cascade needs to be reintegrated with concepts of resilience and susceptibility. To this end, the cellular responses of neurons, astroglia, microglia, pericytes, and endothelial cells, which are largely defined by the genetic makeup of a patient, will determine whether and how long a brain affected by amyloid pathology will continue to function normally.58,76 Once homoeostasis collapses, Alzheimer’s disease manifests itself clinically. Where and when tau influences this cellular phase is one of the most interesting questions for the field.
Apart from the core signature biochemical amyloid and tau pathology and the microglia response, which defines Alzheimer’s disease, it is clear that vasculature,89 the blood–brain barrier,89 the glymphatic63 and other clearance systems of the brain,61 the peripheral immune system,90 and potentially the gastrointestinal microbiome91 affect the clinical development of the disease. Vascular pathology also affects blood–brain barrier integrity.89,92 Leakage of the blood–brain barrier causes dementia independently from amyloid β and tau pathology, especially in APOE ε4 carriers.92
Biomarkers
The biological definition of Alzheimer’s disease is operationalised by the use of ATN biomarkers (appendix p 1).
Imaging biomarkers
Established markers: MRI, 18fluorodeoxyglucose (18FDG)-PET, and amyloid-PET
The three best validated neuroimaging biomarkers for Alzheimer’s disease are medial temporal lobe atrophy on MRI and posterior cingulate and temporoparietal hypometabolism on 18FDG-PET as measures of neurodegeneration, and cortical amyloid β deposition on amyloid-PET imaging. A five-phase strategic roadmap showed that the three biomarkers have almost achieved analytical and clinical validity (phases 1 to 3), although evidence for their clinical utility (phases 4 and 5) is considered insufficient.93
Large prospective studies could provide answers regarding the clinical impact and utility of amyloid β imaging. The ABIDE study showed that amyloid β imaging improved diagnostic accuracy and confidence in a memory clinic setting with relatively young patients (under the age of 70 years).94 The IDEAS study, carried out in individuals aged 65 years and older, showed that amyloid-PET imaging affected clinical diagnosis and diagnostic confidence in about 60% of patients with mild cognitive impairment or dementia.95
Uncertainty regarding the order of tests hinders the widespread implementation of these imaging biomarkers. An interdisciplinary group of experts recently concluded that, although MRI is always recommended as the necessary first step after clinical evaluation, the decision on necessity and choice of the next biomarker test depends on the specific clinical profile and the individual diagnostic question.96 Amyloid-PET is most useful to rule out Alzheimer’s disease, whereas 18FDG-PET has value for the differential diagnosis of neurodegenerative diseases, prediction of short-term clinical outcome, and staging of extent and localisation of neurodegenerative processes. Such algorithms can also be used to support clinicians in the choice of whether or not to do an additional diagnostic test.96
Finally, consideration of regional (instead of global) cortical amyloid β deposition could allow detection of the earliest amyloid β stages (in temporobasal and frontomedial areas) with much higher sensitivity.73
Tau-PET
Tau-PET ligands allow the in-vivo characterisation of tau tracer retention, consistent with Braak stages.74 In contrast to amyloid β deposition, tau-PET binding topography correlates with cognitive deficits,97 is specific to the different Alzheimer’s disease clinical phenotypes,75 and is predictive of subsequent rates of cognitive decline98 and atrophy.99 Tau-PET is a powerful biomarker for differential diagnosis between Alzheimer’s disease-tauopathy and other neurodegenerative tauopathies.100 Finally, longitudinal tau-PET studies highlight the sensitivity of this technique to track the progression of the disease,101 and the spread of tau along brain networks, consistent with neuron-to-neuron propagation.102 Tau-PET also helps to better understand the role of tau and its interaction with amyloid β. Preliminary data suggest that amyloid β might both accelerate tau accumulation103 and allow the spread of tau outside of the medial temporal lobe.104
In May, 2020, the tau tracer flortaucipir was approved for clinical use by the US Food and Drug Administration. For tau-PET to enter clinical practice, methodological refinement is needed; for example, off-target and non-specific binding and analysis procedures are issues to be resolved.105,106 Second-generation tracers that seem to have better signal-to-noise ratio, less off-target binding, and lower non-specific binding than first-generation tracers have been developed.105,106
Other imaging modalities
Developments in PET ligands targeting SV2A imaging have opened new avenues to explore brain synaptic density.107 This progress is of particular interest in Alzheimer’s disease, with preliminary reports of decreased SV2A binding in the hippocampus in patients with mild cognitive impairment or Alzheimer’s disease.108 Further development of PET markers for neuroinflammation, α-synuclein, TDP43, and neurotransmitter systems are also eagerly awaited. Better use of multimodal neuroimaging is needed, including through the development of dual-phase amyloid-tau-PET imaging, hybrid PET-MR imaging, and artificial intelligence.
Fluid biomarkers
Amyloid β, phosphorylated tau, and neurodegeneration can also be ascertained via body fluid biomarkers (appendix p 1), greatly facilitated by the development of automated platforms for the analysis of amyloid β1–42, phosphorylated tau 181, and total tau. 109–111 Through extensive global collaboration (panel 2), reference methods and materials have been developed116 and assay outcomes between providers of cerebrospinal fluid biomarker assays for Alzheimer’s disease have been aligned.72 Standardised operating procedures for cerebrospinal fluid collection and analysis117,118 have also been developed, and a quality-control programme for monitoring consistency in analysis of the results has been firmly established.118,119 All these endeavors are directed at generating global, uniform cutoff points to define if a patient’s profile is Alzheimer’s disease-like.
Cerebrospinal fluid markers
Aside from the established cerebrospinal fluid biomarkers amyloid β1–42, amyloid β1–40, phosphorylated tau 181, and total tau, some new developments can be reported. Markers reflecting axonal damage and synaptic dysfunction are relevant in light of synaptic pathology being present early in the disease course, and of its relation with functional outcomes and cognitive decline. Several of these biomarkers are emerging (eg, neurogranin, SNAP25, synaptotagmins, and the neuronal calcium sensing protein VLP1).120–124 Of these, neurogranin seems the most promising, given its specificity for Alzheimer’s disease and its increase in early stages. YKL40 (CHI3L1), a microglia and astrocyte biomarker and a promising marker to monitor treatment effect, is especially increased in frontotemporal dementia and (to a lesser extent) in Alzheimer’s disease.122,125 Soluble TREM2 is interesting because of its previously mentioned link to genetics. Increases in serum concentrations of TREM2 are observed at a group level independently of the presence of the mutation, and concentrations appear to have a bimodal course along the Alzheimer’s disease spectrum.126
Some non-protein biomarkers are worth mentioning. Initial remarkable results on a plasma metabolomics profile127 were replicated,128–130 although with different profiles. An important issue for the metabolome is the absence of specificity to a disease process and the subtlety of changes.
Serum and plasma biomarkers
Ultrasensitive technologies enable the accurate measurement of CNS proteins in blood. A poignant example is neurofilament light, a major axonal cytoskeleton protein that is a cross-disease biomarker of neurode generation.131 Levels of neurofilament light are increased in blood similarly as in cerebrospinal fluid, making clinical implementation of this marker feasible. In the dementia spectrum, neurofilament light has particular promise in the diagnosis of frontotemporal dementia,132 which makes it potentially useful in monitoring treatment response.133–135
Also encouraging are the consistent and converging reports showing that reductions in plasma amyloid β concentrations in Alzheimer’s disease can be sensitively measured by immunoprecipitation combined with mass spectrometry, or microfluidics and other advanced technologies, such as Simoa, immunoreduction, and protein amide bond analysis.136–142 Results of current collaborative investigations will show which technology provides the best sensitivity for different purposes (eg, screening, stratification, and effect monitoring) and holds the strongest promise for implementation in high-throughput analysis, which is needed when drugs become available and prescreening and monitoring of amyloid β changes becomes relevant. As for phosphorylated tau, three recent papers show strong evidence of plasma phosphorylated tau 181 and 217 as diagnostic biomarkers for Alzheimer’s disease versus other dementias, and for identification of both amyloid β and phosphorylated tau pathology via PET.143–145
The exciting and rapid developments in plasma-based assays hold promise for prescreening in research (reducing the need for, and associated costs with, lumbar punctures and PET scans), and also, once properly validated, for diagnostic purposes in clinical practice.146
Treatment options
Non-pharmacological
Evidence for lifestyle changes
In 2019, WHO released the first guidelines for reduction of risk of cognitive decline and dementia.147 The guidelines acknowledge that, for some factors (eg, physical activity, diet, overweight or obesity, tobacco and alcohol use, hypertension, and diabetes), recommendations can be provided, although with different degrees of certainty. Some limitations in the current evidence include the scarcity of harmonisation (eg, exposure definition) and of long-term, randomised controlled trials, and little evidence from low-income and middle-income countries, where the prevalence of dementia is increasing rapidly.
The SPRINT-MIND trial reported that intensive blood pressure control (goal <120 mm Hg) is more effective in reducing the risk of cognitive impairment than standard blood pressure control (goal <140 mm Hg).148 These results further highlight the idea that what is good for the heart is good for the brain, although the question of the optimal therapeutic target remains, especially for individuals older than 70 years.
Multidomain interventions to prevent cognitive decline and dementia
Previous single-intervention failures stress the crucial need for a multimodal preventive approach that has been successful in the cardiovascular and diabetes prevention fields.149 The Finnish FINGER study was the first largescale, long-term, randomised controlled trial showing that a multidomain lifestyle-based intervention can reduce the risk of cognitive impairment among individuals at risk.150,151 FINGER combined healthy balanced nutrition, physical exercise, cognitive training and social activities, and vascular and metabolic risk management. The trial showed benefits on cognition, even in people with genetic susceptibility to Alzheimer’s disease. The French MAPT trial152 tested the association of a lifestyle intervention with omega-3 fatty acids supplements, and the Dutch PreDIVA trial153 focused on the pharmacological management of vascular and metabolic risk factors. Both trials were negative for the primary outcomes, although subgroup analyses suggested cognitive benefits in subpopulations of participants with increased risk of dementia. In a substudy using amyloid-PET in the MAPT trial,154 lifestyle intervention alone or in combination with omega-3 fatty acids was associated with improved primary cognitive outcome in people with positive amyloid β status. This finding highlights that even when lifestyle factors do not directly affect Alzheimer’s disease pathology, they can still contribute to a positive outcome in individuals with Alzheimer’s disease (appendix pp 2–4).
Future directions: from complexity to precision prevention
In 2020, more than 25 countries joined the World Wide FINGERS network, which aims to adapt, test, and optimise the FINGER model in different geographical, cultural, and economic settings. In many ongoing World Wide FINGERS trials, substudies are focusing on biomarkers (eg, the US-POINTER substudies with MRI, amyloid-PET and tau-PET), which will further clarify the role of various biomarkers and mechanisms among individuals at risk.155 The Multimodal Prevention Trial for Alzheimer’s Disease evaluates the feasibility of the FINGER multidomain lifestyle intervention in patients with prodromal Alzheimer’s disease. This trial is an example of potential future studies in which pharmacological and non-pharmacological preventive strategies can be tested in combination. The study is testing the feasibility of a multidomain intervention, combined with a medical food product that showed promising results after 2 years of treatment in a large randomised controlled trial in patients with prodromal Alzheimer’s disease,156 and sustained positive effects on clinical dementia rating and hippocampal volume after 3 years.157 This type of study is necessary to identify the prevention potential on an individual basis, ultimately enabling a future of personalised medicine for Alzheimer’s disease, in which multimodal interventions can be based on individually tailored combinations of lifestyle and drugs.
Pharmacological
Cognitive enhancing treatments for Alzheimer’s disease
Approved treatments that encompass the standard of care for many patients with Alzheimer’s disease include cholinesterase inhibitors and the N-methyl-D-aspartate receptor antagonist memantine. No other symptomatic cognitive enhancing agent has been approved globally since the Seminar in 2016.1 Three programmes assessing the utility of 5-HT₆ receptor antagonists for cognitive improvement have shown that this pathway is not a viable therapeutic approach for cognition.158
Drugs to treat neuropsychiatric symptoms of Alzheimer’s disease
Progress is being made in developing psychotropic interventions specific for Alzheimer’s disease or for dementia. Pimavanserin is a 5-HT2A receptor inverse agonist that was assessed in a basket trial for dementia-related psychosis, which included patients with psychosis in the setting of Alzheimer’s disease, Parkinson’s disease with dementia, dementia with Lewy bodies, frontotemporal degeneration spectrum disorders, and vascular dementia.159 The trial was stopped early for success, and pimavanserin will be submitted to the US Food and Drug Administration as a therapy for dementia-related psychosis.
Agitation is a common problem in dementia, occurring in up to 70% of patients with Alzheimer’s disease in the course of their illness.160 Recent trials have been supportive of treatment with brexpiprazole (an atypical antipsychotic), citalopram (a selective serotonin reuptake inhibitor), and nabilone (a cannabinoid).161 These studies suggest that appropriate interventions can reduce agitation. Ongoing trials are assessing the efficacy of brexpiprazole, escitalopram, prazosin, dextromethorphan plus quinidine, and dextromethorphan plus bupropion for agitation related to Alzheimer’s disease.
Sleep and night-time behavioural disturbances disrupt the lives of patients and caregivers. A trial of suvorexant showed significant increases in total sleep time and decreased awakening after falling asleep. Suvorexant is a dual orexin antagonist approved for insomnia, and the authorised prescribing information now includes clinical trial and adverse event information regarding the use of the agent to treat insomnia in Alzheimer’s disease.162 Lemborexant, another dual orexin antagonist, is in a trial for irregular sleep-wake rhythm disorder in patients with Alzheimer’s disease.
Disease-modifying therapies for Alzheimer’s disease
Most of the Alzheimer’s disease drug-development pipeline is devoted to disease-modifying therapies (appendix pp 5–9).64,163 These agents are in secondary prevention trials in individuals with preclinical, prodromal or mild, or moderate-to-severe Alzheimer’s disease.
Amyloid β is the most common target of drug development programmes in phase 2 and phase 3. Growing evidence suggests that by removing amyloid β oligomers (soluble aggregates of amyloid β) and plaques (insoluble extracellular aggregates of fibrillar amyloid β) with monoclonal antibodies, disease progression can be slowed.164 Aducanumab, BAN2401, and gantenerumab all reduce amyloid β plaques.165 These agents also reduce phosphorylated tau, neurogranin, and neurofilament light in the cerebrospinal fluid; observations that suggest that removal of amyloid β is associated with downstream effects on tau pathology and neurodegeneration. In each case, ambiguities in the clinical trials remain to be resolved. No therapeutic agents have yet been approved by regulatory authorities, and phase 3 clinical trials (NCT04241068, NCT03443973, NCT04339413, NCT04592341, NCT03444870, NCT03887455, and NCT04468659) are ongoing. A recent phase 2 trial of donanemab suggests that this antibody directed against the pyroglutamate-modified form of amyloid β (an oligomer of amyloid β pE3 and amyloid β 42) has promise as an amyloid-targeted treatment.166 New phase 3 trials of donanemab (NCT04437511 and NCT04640077) have since been initiated.
Amyloid β vaccines are being tested in active immunotherapy trials and are a promising area for Alzheimer’s disease therapeutics. BACE1 and BACE2 inhibitors were a promising class of Alzheimer’s disease-modifying therapies that markedly reduce concentrations of cerebrospinal fluid amyloid β. Several of these trials have been stopped because of an acceleration of deterioration in cognition, elevated liver enzymes, or futility.167 Because many trials were stopped early on, it remains unclear whether longer treatments would have exerted beneficial effects. Further development of this class of agents is unlikely, unless major new insights into their safety and efficacy are achieved.
Tau biology is providing another repertoire of potentially important targets for disease-modifying therapies.168 Several monoclonal antibodies targeting different epitopes are in trials. The monoclonal antibodies are intended to engage extracellular tau as it spreads from cell to cell. Small molecules targeting tau aggregation and neurofibrillary tangle formation are being assessed. All these approaches come with potential side-effects and experts in the field should seriously think about risks to benefits and more complex trials, with better dose finding and measurements of therapeutic target engagement. Otherwise, it is probable that tau-targeted trials will end in premature futility analyses, with little additional learning as to why these trials are negative and what can be improved.
Neuroinflammation is recognised as a major component of the pathology of Alzheimer’s disease, contributing to disease progression and neurodegeneration. Oligomannate was approved in China in 2019, after a phase 3 trial conducted in the same country showed cognitive improvement.169 This agent is hypothesised to be efficacious, on the basis of non-clinical observations, in reducing brain inflammation in patients with Alzheimer’s disease through its effect on the gut microbiome, reducing dysbiosis, restoring normal gut bacterial composition, and decreasing peripheral inflammatory cell populations, which can contribute to central inflammation. A global trial (NCT04520412) is planned to determine the extent to which these effects can be reproduced in other populations.
Various other mechanisms are being targeted in Alzheimer’s disease drug development programmes (appendix pp 9–12). Infections are also hypothesised to contribute to Alzheimer’s disease onset or progression, and agents that target bacteria or viruses are in clinical trials for Alzheimer’s disease. Neuroprotection is essential for successful disease-modification, and some agents target neuroprotection directly through growth factors, mitochondrial function, or other mechanisms, in an effort to slow disease progression.
The Dominantly Inherited Alzheimer Network Treatment Unit is an adaptive prevention trial platform, assessing multiple agents simultaneously in individuals with autosomal dominant Alzheimer’s disease.170 A recent report presented at the 2020 Alzheimer’s and Parkinson’s Diseases Conference showed that, in a small sample of mutation carriers, neither solanezumab nor gantenerumab affected clinical outcomes compared with placebo.171 Gantenerumab, but not solanezumab, positively affected biomarker outcomes.171
An overview of the Alzheimer’s disease-modifying treatments pipeline shows that several agents have clinical or biomarker benefits, and confirmatory trials are being pursued. Some agents have been submitted to the US Food and Drug Administration or the European Medicines Agency for regulatory review. The development of improved trial designs, a larger repertoire of biomarkers reporting on a wider variety of cell processes, improved outcome measures, and better analytical approaches, along with improving insight into the biology of Alzheimer’s disease, support the optimism in the field that the emergence of important new therapies for Alzheimer’s disease might be imminent.
Conclusions
In the past 5 years, substantial progress has been made into understanding the pathophysiology and genetic basis of Alzheimer’s disease. The amyloid β cascade hypothesis has been modified by a more thorough understanding of the cellular, preclinical, phase of Alzheimer’s disease. Genetic studies have moved from pinpointing three causal genes and one risk gene to identifying a plethora of genes that can be put into a polygenic risk score for Alzheimer’s disease. The developments in biomarker diagnosis have led to a complete rethinking of how to label Alzheimer’s disease outside of and before clinical symptomatology, enabling the enrolment of patients in research in a much earlier phase of the disease, particularly now that blood biomarkers seem to be within reach. Further refinement of the diagnostic classification and pathological underpinnings of the disease will be made by molecular imaging, allowing visualisation of copathology and regional protein aggregation. Following these developments will be insights in risk reduction, primary and secondary prevention, non-pharmacological and pharmacological approaches, ultimately given in parallel and at a much earlier timepoint than has been trialled before. If the field keeps up this pace, very early identification and multimodal treatment of patients can become a reality.
Supplementary Material
Acknowledgments
Research at the Alzheimer Center Amsterdam is part of the neurodegeneration research programme of Amsterdam Neuroscience. The Alzheimer Centre Amsterdam is supported by Stichting Alzheimer Nederland and Stichting VUmc Fonds. The chair of Wiesje van der Flier is supported by the Pasman Stichting. MK and PS are recipients of the JPND-funded Euro-FINGERS project (#ZonMW 733051102).
Footnotes
Declaration of interests
PS reports research support from Alzheimer Nederland, Dioraphte, Stichting VUmc Fonds, and Stichting Alzheimer & Neuropsychiatrie; is a consultant for EIP Pharma, Vivoryon, Toyama Fuji Film, AC Immune, Axon Neuroscience, GemVax, Medavante, Novartis Cardiology, and Green Valley; is co-editor-in-chief of Alzheimer’s Research & Therapy; and is a part-time managing partner of the Life Science Partners Dementia Fund since Oct 1, 2020 (after acceptance of this Seminar). BDS reports research support from Janssen Pharmaceutica, European Research Council, various academic research sponsors, Methusalem Grant of the Flemish Government, Medical Research Council, Alzheimer Association (USA), Alzheimer Research UK, and Alzheimer Society (UK). BDS is a consultant for Eisai Pharmaceuticals and holds several patents (appendix p 13). MK reports research support from Academy of Finland, Swedish Research Council, Joint Program of Neurodegenerative Disorders, IMI, Knut and Alice Wallenberg Foundation, Center for Innovative Medicine, Stiftelsen Stockholms Sjukhem, Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, Alzheimer’s Research and Prevention Foundation, Alzheimerfonden, Hjärnfonden, Region Stockholm, and Leif Lundblad Foundation grants. MK is part of a guidelines development group in WHO, a Governance Committee member of the Global Council on Brain Health, and is on the advisory board for Combinostics, Roche, and Biogen. HH reports research support from ZonMw, NWO, EU-JPND, Alzheimer Nederland, and Stichting Dioraphte. GC reports research support from European Union Horizon 2020 programme, INSERM, Fondation d’entreprise MMA des Entrepreneurs du Futur, Fondation Alzheimer, Programme Hospitalier de Recherche Clinique, Région Normandie, Association France Alzheimer et maladies apparentées, and Fondation Vaincre Alzheimer. CET reports research support from European Commission (Marie Curie International Training Network, JPND), Health Holland, the Dutch Research Council (ZonMw), The Weston Brain Institute, and Alzheimer Nederland; and has the following industrial collaborations: collaboration contract with ADx Neurosciences, contract research or received grants from Probiodrug, AC Immune, Biogen-Esai, CogRx, Toyama, Janssen Prevention Center, Boehringer, AxonNeurosciences, Fujirebio, EIP farma, PeopleBio, and Roche, and is on the advisory board of Roche. JC has provided consultation to Acadia, Actinogen, AgeneBio, Alkahest, Alzheon, Annovis, Avanir, Axsome, Biogen, Cassava, Cerecin, Cerevel, Cognoptix, Cortexyme, EIP Pharma, Eisai, Foresight, GemVax, Green Valley, Grifols, Hisun, Karuna, Nutricia, Orion, Otsuka, ReMYND, Resverlogix, Roche, Samumed, Samus Therapeutics, Third Rock, Signant Health, Sunovion, Suven, and United Neuroscience pharmaceutical and assessment companies. JC receives support from Keep Memory Alive, COBRE grant #P20GM109025, TRC-PAD #R01AG053798, DIAGNOSE CTE #U01NS093334, and Nevada exploratory Alzheimer's Disease Research Center grant# P20 AG068053. JC owns the copyright of the Neuropsychiatric Inventory. WMVdF reports research support from ZonMw, NWO, EU-FP7, EU-JPND, Alzheimer Nederland, CardioVascular Onderzoek Nederland, Health~Holland, Topsector Life Sciences & Health, Stichting Dioraphte, Gieskes-Strijbis Fonds, Stichting Equilibrio, Pasman Stichting, Fujifilm, Boehringer Ingelheim, Life-MI, AVID, Janssen Stellar, Combinostics. WMVdF is chair of Pasman Stichting. WMVdF has performed contract research for Biogen and received speaker fees from Biogen and Roche.
References
- 1.Alzheimer Europe. Dementia in Europe Yearbook 2019: estimating the prevalence of dementia in Europe. 2020. https://www.alzheimereurope.org/content/download/195515/1457520/file/FINAL%2005707%20Alzheimer%20Europe%20yearbook%202019.pdf (accessed Jan 24, 2021).
- 2.Scheltens P, Blennow K, Breteler MM, et al. Alzheimer’s disease. Lancet 2016; 388: 505–17. [DOI] [PubMed] [Google Scholar]
- 3.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 2007; 6: 734–46. [DOI] [PubMed] [Google Scholar]
- 4.Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol 2010; 9: 1118–27. [DOI] [PubMed] [Google Scholar]
- 5.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34: 939–44. [DOI] [PubMed] [Google Scholar]
- 6.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7: 263–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knopman DS, Petersen RC, Jack CR Jr. A brief history of “Alzheimer disease”: multiple meanings separated by a common name. Neurology 2019; 92: 1053–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018; 14: 535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweeney MD, Montagne A, Sagare AP, et al. Vascular dysfunction—the disregarded partner of Alzheimer’s disease. Alzheimers Dement 2019; 15: 158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack CR Jr, Holtzman DM, Sperling R. Dementia is not synonymous with Alzheimer’s disease. Sci Transl Med 2019; 11: eaav0511. [DOI] [PubMed] [Google Scholar]
- 11.Altomare D, de Wilde A, Ossenkoppele R, et al. Applying the ATN scheme in a memory clinic population: the ABIDE project. Neurology 2019; 93: e1635–46. [DOI] [PubMed] [Google Scholar]
- 12.Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement (NY) 2019; 5: 272–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Maurik IS, Vos SJ, Bos I, et al. Biomarker-based prognosis for people with mild cognitive impairment (ABIDE): a modelling study. Lancet Neurol 2019; 18: 1034–44. [DOI] [PubMed] [Google Scholar]
- 14.Visser LNC, van Maurik IS, Bouwman FH, et al. Clinicians’ communication with patients receiving a mild cognitive impairment diagnosis: the ABIDE project. PLoS One 2020; 15: e0227282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol 2020; 19: 271–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Maurik IS, Slot RER, Verfaillie SCJ, et al. Personalized risk for clinical progression in cognitively normal subjects—the ABIDE project. Alzheimers Res Ther 2019; 11: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebenau JL, Timmers T, Wesselman LMP, et al. ATN classification and clinical progression in subjective cognitive decline: The SCIENCe project. Neurology 2020; 95: e46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fruijtier AD, Visser LNC, van Maurik IS, et al. ABIDE Delphi study: topics to discuss in diagnostic consultations in memory clinics. Alzheimers Res Ther 2019; 11: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Maurik IS, Visser LN, Pel-Littel RE, et al. Development and usability of ADappt: web-based tool to support clinicians, patients, and caregivers in the diagnosis of mild cognitive impairment and Alzheimer disease. JMIR Form Res 2019; 3: e13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alzheimer’s Disease International. World Alzheimer Report 2018. The state of the art of dementia research: new frontiers. September, 2018. https://www.alzint.org/u/WorldAlzheimerReport2018.pdf (accessed Sept 9, 2020).
- 21.Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time—current evidence. Nat Rev Neurol 2017; 13: 327–39. [DOI] [PubMed] [Google Scholar]
- 22.Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther 2016; 8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayeda ER, Glymour MM, Quesenberry CP, Johnson JK, Perez-Stable EJ, Whitmer RA. Survival after dementia diagnosis in five racial/ethnic groups. Alzheimers Dement 2017; 13: 761–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhodius-Meester HFM, Tijms BM, Lemstra AW, et al. Survival in memory clinic cohort is short, even in young-onset dementia. J Neurol Neurosurg Psychiatry 2019; 90: 726–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermunt L, Sikkes SAM, Hout AVD, et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’ s disease in relation to age, sex, and APOE genotype. Alzheimer Dementia 2019; 15: 888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jack CR Jr, Therneau TM, Weigand SD, et al. Prevalence of biologically vs clinically defined Alzheimer spectrum entities using the National Institute on Aging-Alzheimer’s Association research framework. JAMA Neurol 2019; 76: 1174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Lee SJ, Wolters FJ, Ikram MK, et al. The effect of APOE and other common genetic variants on the onset of Alzheimer’s disease and dementia: a community-based cohort study. Lancet Neurology 2018; 17: 434–44. [DOI] [PubMed] [Google Scholar]
- 28.Buckley RF, Mormino EC, Rabin JS, et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol 2019; 76: 542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mofrad RB, Tijms BM, Scheltens P, et al. Sex differences in CSF biomarkers vary by Alzheimer’s disease stage and APOE ε4 genotype. Neurology 2020; 95: e2378–88. [DOI] [PubMed] [Google Scholar]
- 30.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020; 396: 413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lane CA, Barnes J, Nicholas JM, et al. Associations between vascular risk across adulthood and brain pathology in late life: evidence from a British birth cohort. JAMA Neurol 2019: 77: 175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottesman RF, Mosley TH, Knopman DS, et al. Association of intracranial atherosclerotic disease with brain beta-amyloid deposition: secondary analysis of the ARIC study. JAMA Neurol 2019; 77: 350–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gustavsson AM, van Westen D, Stomrud E, Engstrom G, Nagga K, Hansson O. Midlife atherosclerosis and development of Alzheimer or vascular dementia. Ann Neurol 2020; 87: 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry 2006; 63: 168–74. [DOI] [PubMed] [Google Scholar]
- 35.Bellenguez C, Charbonnier C, Grenier-Boley B, et al. Contribution to Alzheimer’s disease risk of rare variants in TREM2, SORL1, and ABCA7 in 1779 cases and 1273 controls. Neurobiol Aging 2017; 59: e1–9. [DOI] [PubMed] [Google Scholar]
- 36.Paudel HK, Ridge PG, Mukherjee S, Crane PK, Kauwe JSK. Alzheimer’s disease: analyzing the missing heritability. PLoS One 2013; 8: e79771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jansen IE, Savage JE, Watanabe K, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat Genet 2019; 51: 404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Escott-Price V, Sims R, Bannister C, et al. Common polygenic variation enhances risk prediction for Alzheimer’s disease. Brain 2015; 138: 3673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Escott-Price V, Myers AJ, Huentelman M, Hardy J. Polygenic risk score analysis of pathologically confirmed Alzheimer disease. Ann Neurol 2017; 82: 311–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Marco LY, Venneri A, Farkas E, Evans PC, Marzo A, Frangi AF. Vascular dysfunction in the pathogenesis of Alzheimer’s disease—a review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol Dis 2015; 82: 593–606. [DOI] [PubMed] [Google Scholar]
- 41.Bennett RE, Robbins AB, Hu M, et al. Tau induces blood vessel abnormalities and angiogenesis-related gene expression in P301L transgenic mice and human Alzheimer’s disease. Proc Natl Acad Sci USA 2018; 115: E1289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verheijen J, Sleegers K. Understanding Alzheimer disease at the interface between genetics and transcriptomics. Trends Genet 2018; 34: 434–47. [DOI] [PubMed] [Google Scholar]
- 43.Naj AC, Schellenberg GD. Genomic variants, genes, and pathways of Alzheimer’s disease: an overview. Am J Med Genet B Neuropsychiatr Genet 2017; 174: 5–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardy J, Bogdanovic N, Winblad B, et al. Pathways to Alzheimer’s disease. J Intern Med 2014; 275: 296–303. [DOI] [PubMed] [Google Scholar]
- 45.Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet Med 2016; 18: 421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holstege H, van der Lee SJ, Hulsman M, et al. Characterization of pathogenic SORL1 genetic variants for association with Alzheimer’s disease: a clinical interpretation strategy. Eur J Hum Genet 2017; 25: 973–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuyvers E, De Roeck A, Van den Bossche T, et al. Mutations in ABCA7 in a Belgian cohort of Alzheimer’s disease patients: a targeted resequencing study. Lancet Neurol 2015; 14: 814–22. [DOI] [PubMed] [Google Scholar]
- 48.Jonsson T, Stefansson H, Steinberg S, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 2013; 368: 107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guerreiro R, Wojtas A, Bras J, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med 2013; 368: 117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Genin E, Hannequin D, Wallon D, et al. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry 2011; 16: 903–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reiman EM, Arboleda-Velasquez JF, Quiroz YT, et al. Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat Commun 2020; 11: 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jonsson T, Atwal JK, Steinberg S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 2012; 488: 96–99. [DOI] [PubMed] [Google Scholar]
- 53.Sims R, van der Lee SJ, Naj AC, et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat Genets 2017; 49: 1373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Lee SJ, Conway OJ, Jansen I, et al. A nonsynonymous mutation in PLCG2 reduces the risk of Alzheimer’s disease, dementia with Lewy bodies and frontotemporal dementia, and increases the likelihood of longevity. Acta Neuropathol 2019; 138: 237–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beker N, Sikkes SAM, Hulsman M, et al. Longitudinal maintenance of cognitive health in centenarians in the 100-plus study. JAMA Netw Open 2020; 3: e200094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arboleda-Velasquez JF, Lopera F, O’Hare M, et al. Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Nat Med 2019; 25: 1680–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belloy ME, Napolioni V, Han SS, Le Guen Y, Greicius MD, Alzheimer’s Disease Neuroimaging Initiative. Association of klothovs heterozygosity with risk of Alzheimer disease in individuals who carry APOE4. JAMA Neurol 2020; 77: 849–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Strooper B, Karran E. The cellular phase of Alzheimer’s disease. Cell 2016; 164: 603–15. [DOI] [PubMed] [Google Scholar]
- 59.Venegas C, Kumar S, Franklin BS, et al. Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer’s disease. Nature 2017; 552: 355–61. [DOI] [PubMed] [Google Scholar]
- 60.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 2018; 14: 133–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Da Mesquita S, Louveau A, Vaccari A, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018; 560: 185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu T, Aron L, Zullo J, et al. REST and stress resistance in ageing and Alzheimer’s disease. Nature 2014; 507: 448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plog BA, Nedergaard M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol 2018; 13: 379–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell 2019; 179: 312–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koper MJ, Van Schoor E, Ospitalieri S, et al. Necrosome complex detected in granulovacuolar degeneration is associated with neuronal loss in Alzheimer’s disease. Acta Neuropathol 2020; 139: 463–84. [DOI] [PubMed] [Google Scholar]
- 66.Keren-Shaul H, Spinrad A, Weiner A, et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 2017; 169: 1276–90. [DOI] [PubMed] [Google Scholar]
- 67.Parhizkar S, Arzberger T, Brendel M, et al. Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat Neurosci 2019; 22: 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sala Frigerio C, Wolfs L, Fattorelli N, et al. The major risk factors for Alzheimer’s disease: age, sex, and genes modulate the microglia response to Aβ plaques. Cell Rep 2019; 27: 1293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer’s disease. J Cell Biol 2017; 217: 459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yeh FL, Wang Y, Tom I, Gonzalez LC, Sheng M. TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron 2016; 91: 328–40. [DOI] [PubMed] [Google Scholar]
- 71.Szaruga M, Munteanu B, Lismont S, et al. Alzheimer’s-causing mutations shift Aβ length by destabilizing γ-secretase-Aβn interactions. Cell 2017; 170: 443–56. [DOI] [PubMed] [Google Scholar]
- 72.Kuhlmann J, Andreasson U, Pannee J, et al. CSF Abeta1–42— an excellent but complicated Alzheimer’s biomarker—a route to standardisation. Clin Chim Acta 2017; 467: 27–33. [DOI] [PubMed] [Google Scholar]
- 73.Grothe MJ, Barthel H, Sepulcre J, et al. In vivo staging of regional amyloid deposition. Neurology 2017; 89: 2031–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cho H, Choi JY, Hwang MS, et al. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol 2016; 80: 247–58. [DOI] [PubMed] [Google Scholar]
- 75.Ossenkoppele R, Schonhaut DR, Baker SL, et al. Tau, amyloid, and hypometabolism in a patient with posterior cortical atrophy. Ann Neurol 2015; 77: 338–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sierksma A, Lu A, Mancuso R, et al. Novel Alzheimer risk genes determine the microglia response to amyloid-beta but not to TAU pathology. EMBO Mol Med 2020; 12: e10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Griciuc A, Patel S, Federico AN, et al. TREM2 acts downstream of CD33 in modulating microglial pathology in Alzheimer’s disease. Neuron 2019; 103: 820–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deming Y, Filipello F, Cignarella F, et al. The MS4A gene cluster is a key modulator of soluble TREM2 and Alzheimer’s disease risk. Sci Transl Med 2019; 11: eaau2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leyns CEG, Ulrich JD, Finn MB, et al. TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc Natl Acad Sci USA 2017; 114: 11524–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leyns CEG, Gratuze M, Narasimhan S, et al. TREM2 function impedes tau seeding in neuritic plaques. Nat Neurosci 2019; 22: 1217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sasaguri H, Nilsson P, Hashimoto S, et al. APP mouse models for Alzheimer’s disease preclinical studies. EMBO J 2017; 36: 2473–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mancuso R, Van Den Daele J, Fattorelli N, et al. Stem-cell-derived human microglia transplanted in mouse brain to study human disease. Nat Neurosci 2019; 22: 2111–16. [DOI] [PubMed] [Google Scholar]
- 83.Hasselmann J, Coburn MA, England W, et al. Development of a chimeric model to study and manipulate human microglia in vivo. Neuron 2019; 103: 1016–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park J, Wetzel I, Marriott I, et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat Neurosci 2018; 21: 941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gremer L, Schölzel D, Schenk C, et al. Fibril structure of amyloid-beta (1–42) by cryo-electron microscopy. Science 2017; 358: 116–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fitzpatrick AWP, Falcon B, He S, et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 2017; 547: 185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou R, Yang G, Guo X, Zhou Q, Lei J, Shi Y. Recognition of the amyloid precursor protein by human g-secretase. Science 2019; 363: eaaw0930. [DOI] [PubMed] [Google Scholar]
- 88.Yang G, Zhou R, Zhou Q, et al. Structural basis of Notch recognition by human γ-secretase. Nature 2019; 565: 192–97. [DOI] [PubMed] [Google Scholar]
- 89.Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 2019; 25: 270–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gate D, Saligrama N, Leventhal O, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 2020; 577: 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dodiya HB, Frith M, Sidebottom A, et al. Synergistic depletion of gut microbial consortia, but not individual antibiotics, reduces amyloidosis in APPPS1–21 Alzheimer’s transgenic mice. Sci Rep 2020; 10: 8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Montagne A, Nation DA, Sagare AP, et al. APOE4 leads to bloodbrain barrier dysfunction predicting cognitive decline. Nature 2020; 581: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frisoni GB, Boccardi M, Barkhof F, et al. Strategic roadmap for an early diagnosis of Alzheimer’s disease based on biomarkers. Lancet Neurol 2017; 16: 661–76. [DOI] [PubMed] [Google Scholar]
- 94.de Wilde A, van der Flier WM, Pelkmans W, et al. Association of amyloid positron emission tomography with changes in diagnosis and patient treatment in an unselected memory clinic cohort: the ABIDE project. JAMA Neurol 2018; 75: 1062–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rabinovici GD, Gatsonis C, Apgar C, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among medicare beneficiaries with mild cognitive impairment or dementia. JAMA 2019; 321: 1286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rhodius-Meester HFM, van Maurik IS, Koikkalainen J, et al. Selection of memory clinic patients for CSF biomarker assessment can be restricted to a quarter of cases by using computerized decision support, without compromising diagnostic accuracy. PLoS One 2020; 15: e0226784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aschenbrenner AJ, Gordon BA, Benzinger TLS, Morris JC, Hassenstab JJ. Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer disease. Neurology 2018; 91: e859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brier MR, Gordon B, Friedrichsen K, et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med 2016; 8: 338ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.La Joie R, Visani AV, Baker SL, et al. Prospective longitudinal atrophy in Alzheimer’s disease correlates with the intensity and topography of baseline tau-PET. Sci Transl Med 2020; 12: eaau5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ossenkoppele R, Rabinovici GD, Smith R, et al. Discriminative accuracy of [18F]flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA 2018; 320: 1151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harrison TM, La Joie R, Maass A, et al. Longitudinal tau accumulation and atrophy in aging and Alzheimer disease. Ann Neurol 2019; 85: 229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Franzmeier N, Neitzel J, Rubinski A, et al. Functional brain architecture is associated with the rate of tau accumulation in Alzheimer’s disease. Nat Commun 2020; 11: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jack CR Jr, Wiste HJ, Schwarz CG, et al. Longitudinal tau PET in ageing and Alzheimer’s disease. Brain 2018; 141: 1517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang L, Benzinger TL, Su Y, et al. Evaluation of tau imaging in staging Alzheimer disease and revealing interactions between beta-amyloid and tauopathy. JAMA Neurol 2016; 73: 1070–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Villemagne VL, Dore V, Burnham SC, Masters CL, Rowe CC. Imaging tau and amyloid-beta proteinopathies in Alzheimer disease and other conditions. Nat Rev Neurol 2018; 14: 225–36. [DOI] [PubMed] [Google Scholar]
- 106.Leuzy A, Chiotis K, Lemoine L, et al. Tau PET imaging in neurodegenerative tauopathies-still a challenge. Mol Psychiatry 2019; 24: 1112–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cai Z, Li S, Matuskey D, Nabulsi N, Huang Y. PET imaging of synaptic density: a new tool for investigation of neuropsychiatric diseases. Neurosci Lett 2019; 691: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen MK, Mecca AP, Naganawa M, et al. Assessing synaptic density in Alzheimer disease with synaptic vesicle glycoprotein 2a positron emission tomographic imaging. JAMA Neurol 2018; 75: 1215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blennow K, Shaw LM, Stomrud E, et al. Predicting clinical decline and conversion to Alzheimer’s disease or dementia using novel Elecsys Aβ(1–42), pTau and tTau CSF immunoassays. Sci Rep 2019; 9: 19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer’s disease concord with amyloid-beta PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement 2018; 14: 1470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaplow J, Vandijck M, Gray J, et al. Concordance of Lumipulse cerebrospinal fluid t-tau/Abeta42 ratio with amyloid PET status. Alzheimers Dement 2020; 16: 144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Global Biomarker Standardization Consortium. https://www.alz.org/research/for_researchers/partnerships/gbsc (accessed Sept 9, 2020).
- 113.Society for CSF Analysis and Clinical Neurochemistry. https://h001.ssl-redirect.de/www.neurochem.info (accessed Sept 1, 2020).
- 114.FNIH Biomarkers Consortium. https://fnih.org/what-we-do/biomarkers-consortium (accessed Sept 1, 2020).
- 115.International Federation of Clinical Chemistry and Laboratory Medicine Working Group CSF Proteins. https://www.ifcc.org/ifccscientific-division/sd-working-groups/csf-proteins-wg-csf (accessed Sept 1, 2020).
- 116.Pannee J, Gobom J, Shaw LM, et al. Round robin test on quantification of amyloid-beta 1–42 in cerebrospinal fluid by mass spectrometry. Alzheimers Dement 2016; 12: 55–59. [DOI] [PubMed] [Google Scholar]
- 117.Engelborghs S, Niemantsverdriet E, Struyfs H, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement (Amst) 2017; 8: 111–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hok AHYS, Willemse EAJ, Teunissen CE, Del Campo M. Guidelines for CSF processing and biobanking: impact on the identification and development of optimal CSF protein biomarkers. Methods Mol Biol 2019; 2044: 27–50. [DOI] [PubMed] [Google Scholar]
- 119.Mattsson N, Andreasson U, Persson S, et al. CSF biomarker variability in the Alzheimer’s Association quality control program. Alzheimers Dement 2013; 9: 251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Galasko D, Xiao M, Xu D, et al. Synaptic biomarkers in CSF aid in diagnosis, correlate with cognition and predict progression in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement (N Y) 2019; 5: 871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hoglund K, Schussler N, Kvartsberg H, et al. Cerebrospinal fluid neurogranin in an inducible mouse model of neurodegeneration: a translatable marker of synaptic degeneration. Neurobiol Dis 2020; 134: 104645. [DOI] [PubMed] [Google Scholar]
- 122.Janelidze S, Hertze J, Zetterberg H, et al. Cerebrospinal fluid neurogranin and YKL-40 as biomarkers of Alzheimer’s disease. Ann Clin Transl Neurol 2016; 3: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Duits FH, Brinkmalm G, Teunissen CE, et al. Synaptic proteins in CSF as potential novel biomarkers for prognosis in prodromal Alzheimer’s disease. Alzheimers Res Ther 2018; 10: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schindler SE, Li Y, Todd KW, et al. Emerging cerebrospinal fluid biomarkers in autosomal dominant Alzheimer’s disease. Alzheimers Dement 2019; 15: 655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Teunissen CE, Elias N, Koel-Simmelink MJ, et al. Novel diagnostic cerebrospinal fluid biomarkers for pathologic subtypes of frontotemporal dementia identified by proteomics. Alzheimers Dement (Amst) 2016; 2: 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suarez-Calvet M, Capell A, Araque Caballero MA, et al. CSF progranulin increases in the course of Alzheimer’s disease and is associated with sTREM2, neurodegeneration and cognitive decline. EMBO Mol Med 2018; 10: e9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mapstone M, Cheema AK, Fiandaca MS, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med 2014; 20: 415–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.de Leeuw FA, Peeters CFW, Kester MI, et al. Blood-based metabolic signatures in Alzheimer’s disease. Alzheimers Dement (Amst) 2017; 8: 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stamate D, Kim M, Proitsi P, et al. A metabolite-based machine learning approach to diagnose Alzheimer-type dementia in blood: results from the European Medical Information Framework for Alzheimer disease biomarker discovery cohort. Alzheimers Dement (N Y) 2019; 5: 933–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van der Lee SJ, Teunissen CE, Pool R, et al. Circulating metabolites and general cognitive ability and dementia: evidence from 11 cohort studies. Alzheimers Dement 2018; 14: 707–22. [DOI] [PubMed] [Google Scholar]
- 131.Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol 2019; 76: 1035–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Al Shweiki MR, Steinacker P, Oeckl P, et al. Neurofilament light chain as a blood biomarker to differentiate psychiatric disorders from behavioural variant frontotemporal dementia. J Psychiatr Res 2019; 113: 137–40. [DOI] [PubMed] [Google Scholar]
- 133.Olsson B, Alberg L, Cullen NC, et al. NFL is a marker of treatment response in children with SMA treated with nusinersen. J Neurol 2019; 266: 2129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018; 141: 2382–91. [DOI] [PubMed] [Google Scholar]
- 135.Sormani MP, Haering DA, Kropshofer H, et al. Blood neurofilament light as a potential endpoint in phase 2 studies in MS. Ann Clin Transl Neurol 2019; 6: 1081–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Janelidze S, Stomrud E, Palmqvist S, et al. Plasma beta-amyloid in Alzheimer’s disease and vascular disease. Sci Rep 2016; 6: 26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ovod V, Ramsey KN, Mawuenyega KG, et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement 2017; 13: 841–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schindler SE, Bollinger JG, Ovod V, et al. High-precision plasma beta-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019; 93: e1647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Verberk IMW, Slot RE, Verfaillie SCJ, et al. Plasma amyloid as prescreener for the earliest Alzheimer pathological changes. Ann Neurol 2018; 84: 648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Palmqvist S, Janelidze S, Stomrud E, et al. Performance of fully automated plasma assays as screening tests for Alzheimer disease-related beta-amyloid status. JAMA Neurol 2019; 76: 1060–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nabers A, Perna L, Lange J, et al. Amyloid blood biomarker detects Alzheimer’s disease. EMBO Mol Med 2018; 10: e8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Teunissen CE, Chiu MJ, Yang CC, et al. Plasma amyloid-beta (Abeta42) correlates with cerebrospinal fluid abeta42 in Alzheimer’s disease. J Alzheimers Dis 2018; 62: 1857–63. [DOI] [PubMed] [Google Scholar]
- 143.Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med 2020; 26: 387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Janelidze S, Mattsson N, Palmqvist S, et al. Plasma p-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med 2020; 26: 379–86. [DOI] [PubMed] [Google Scholar]
- 145.Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA 2020; 324: 772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mattke S, Cho SK, Bittner T, Hlavka J, Hanson M. Blood-based biomarkers for Alzheimer’s pathology and the diagnostic process for a disease-modifying treatment: projecting the impact on the cost and wait times. Alzheimer Dement 2020; 12: e12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.WHO. Risk reduction of cognitive decline and dementia: WHO guidelines. 2019. Geneva: World Health Organization. https://www.who.int/mental_health/neurology/dementia/guidelines_risk_reduction/en/ (accessed Sept 1, 2020). [PubMed] [Google Scholar]
- 148.SPRINT MIND Investigators for the SPRINT Research Group, Williamson JD, Pajewski NM, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 2019; 321: 553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol 2018; 14: 653–66. [DOI] [PubMed] [Google Scholar]
- 150.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385: 2255–63. [DOI] [PubMed] [Google Scholar]
- 151.Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol 2018; 75: 462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Andrieu S, Guyonnet S, Coley N, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol 2017; 16: 377–89. [DOI] [PubMed] [Google Scholar]
- 153.Moll van Charante EP, Richard E, Eurelings LS, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet 2016; 388: 797–805. [DOI] [PubMed] [Google Scholar]
- 154.Delrieu J, Payoux P, Carrie I, et al. Multidomain intervention and/ or omega-3 in nondemented elderly subjects according to amyloid status. Alzheimers Dement 2019; 15: 1392–401. [DOI] [PubMed] [Google Scholar]
- 155.Kivipelto M, Mangialasche F, Snyder HM, et al. World-Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement 2020; 16: 1078–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Soininen H, Solomon A, Visser PJ, et al. 24-month intervention with a specific multinutrient in people with prodromal Alzheimer’s disease (LipiDiDiet): a randomised, double-blind, controlled trial. Lancet Neurol 2017; 16: 965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Soininen H, Solomon A, Visser PJ, et al. 36-month LipiDiDiet multinutrient clinical trial in prodromal Alzheimer’s disease. Alzheimer Dement 2020; published online Sept 13. 10.1002/alz.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Atri A, Frolich L, Ballard C, et al. Effect of idalopirdine as adjunct to cholinesterase inhibitors on change in cognition in patients with Alzheimer disease: three randomized clinical trials. JAMA 2018; 319: 130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cummings J, Ballard C, Tariot P, et al. Pimavanserin: potential treatment for dementia-related psychosis. J Prev Alzheimers Dis 2018; 5: 253–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Halpern R, Seare J, Tong J, Hartry A, Olaoye A, Aigbogun MS. Using electronic health records to estimate the prevalence of agitation in Alzheimer disease/dementia. Int J Geriatr Psychiatry 2019; 34: 420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu CS, Ruthirakuhan M, Chau SA, Herrmann N, Carvalho AF, Lanctot KL. Pharmacological management of agitation and aggression in Alzheimer’s disease: a review of current and novel treatments. Curr Alzheimer Res 2016; 13: 1134–44. [DOI] [PubMed] [Google Scholar]
- 162.Janto K, Prichard JR, Pusalavidyasagar S. An update on dual orexin receptor antagonists and their potential role in insomnia therapeutics. J Clin Sleep Med 2018; 14: 1399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cummings JC, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer’s disease drug development pipeline: 2020. Alzheimer Dement 2020; 6: e12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sevigny J, Chiao P, Bussiere T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016; 537: 50–56. [DOI] [PubMed] [Google Scholar]
- 165.van Dyck CH. Anti-amyloid-β monoclonal antibodies for Alzheimer’s disease: pitfalls and promise. Bio Psychiatry 2017; 83: 311–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lilly. News release: Lilly’s donanemab slows clinical decline of Alzheimer’s disease in positive phase 2 trial. Jan 11, 2021. https://investor.lilly.com/news-releases/news-release-details/lillysdonanemab-slows-clinical-decline-alzheimersdisease#:~:text=11%2C%202021%20%2FPRNewswire%2F%20%2D%2D,results%20from%20Eli%20Lilly%20 and (accessed Jan 24, 2021)
- 167.Egan MF, Kost J, Voss T, et al. Randomized trial of verubecestat for prodromal Alzheimer’s disease. N Engl J Med 2019; 380: 1408–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cummings J, Blennow K, Johnson K, et al. Anti-tau trials for Alzheimer’s disease: a report from the EU/US/CTAD Task Force. J Prev Alzheimers Dis 2019; 6: 157–63. [DOI] [PubMed] [Google Scholar]
- 169.Wang X, Sun G, Feng T, et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acidsshaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res 2019; 29: 787–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bateman RJ, Benzinger TL, Berry S, et al. The DIAN-TU Next Generation Alzheimer’s prevention trial: adaptive design and disease progression model. Alzheimers Dement 2017; 13: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Alzforum. In DIAN-TU, Gantenerumab Brings Down Tau. By a Lot. Open Extension Planned. April10, 2020. https://www.alzforum.org/news/conference-coverage/dian-tu-gantenerumab-bringsdown-tau-lot-open-extension-planned (accessed Jan 26, 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.