Abstract
In humans and mice, the first line of innate defense against inhaled pathogens and particles in the respiratory tract is airway mucus. The primary solid components of the mucus layer are the mucins MUC5AC and MUC5B, polymeric glycoproteins whose changes in abundance and structure can dramatically affect airway defense. Accordingly, MUC5AC/Muc5ac and MUC5B/Muc5b are tightly regulated at a transcriptional level by tissue-specific transcription factors in homeostasis and in response to injurious and inflammatory triggers. In addition to modulated levels of mucin gene transcription, translational and post-translational biosynthetic processes also exert significant influence upon mucin function. Mucins are massive macromolecules with numerous functional domains that contribute to their structural composition and biophysical properties. Single MUC5AC and MUC5B apoproteins have molecular masses of >400 kDa, and von Willebrand factor D-like as well as other cysteine-rich domain segments contribute to mucin polymerization and flexibility, thus increasing apoprotein length and complexity. Additional domains serve as sites for O-glycosylation, which increase further mucin mass several-fold. Glycosylation is a defining process for mucins that is specific with respect to additions of glycans to mucin apoprotein backbones, and glycan additions influence the physical properties of the mucins via structural modifications as well as charge interactions. Ultimately, through their tight regulation and complex assembly, airway mucins follow the biological rule of ‘form fits function’ in that their structural organization influences their role in lung homeostatic mechanisms.
Mucus: an essential first line of defense
Each day, respiratory tissues are exposed to billions of particles. Because inhaled particles include potentially infectious, injurious, and thus inflammatory stimuli, homeostatic mechanisms have evolved to reduce the inherent risks associated with their exposures in the lungs. The initial sites of particle exposure, the conducting airways, are lined by a barrier that constitutes the first line of innate defense. Defense is conferred by a mucus gel that traps particles and a ciliary escalator that transports mucus contents towards the oropharynx for ultimate elimination by expectoration or swallowing. This process is called mucociliary clearance (MCC) [1–3]. For MCC-mediated innate defense, two cell types are essential: ciliated cells and mucous cells [4–6].
In this review, we focus on mucous cells, and specifically, the mucin glycoproteins they produce, as critical mediators of the first line of innate defense. We use the term mucous cells to broadly define two mucin-producing cell types found in tracheobronchial submucosal glands (SMGs) and on airway surfaces. SMG mucous cells are interspersed among heterogeneous ciliated and secretory cell populations within glands and gland ducts in the large airways (trachea and bronchi) of humans and large mammals such as swine [7], but in mice these cells are present only in the trachea [8]. Surface mucous cells, often called goblet cells based on their chalice-shaped morphologies, are present on conducting airway surfaces, predominantly within the central tracheal and bronchial airways. Surface mucous cells are also present in small bronchiolar airways, but mucous cell numbers are lower in these peripheral air-spaces. Both mucous cell types produce secreted polymeric mucin glycoproteins that are released by regulated exocytosis and then become hydrated in the airway lumen to form the macromolecular matrix of the mucus gel [9–11].
Mucins are extremely large, heavily-glycosylated proteins with molecular masses ranging from 10 to 40 MDa. Over 20 different mucin genes are expressed in humans, each in a tissue-specific manner [12]. The lungs contain the membrane-bound mucins MUC1, MUC4, and MUC16, secreted mucin MUC7, and also the secreted polymeric mucins MUC5AC and MUC5B [13,14]. MUC5AC and MUC5B play dominant roles in the formation of viscoelastic mucus gels [15,16].
Mucin and mucus functions
Mucus is a complex mixture of salts, macromolecules, cells, and cellular debris that are contained in a hydrogel formed by polymeric mucins and water [17–21]. Mucus serves two important homeostatic functions in the lungs: innate airway defense and airway surface hydration. Mucus is critical for protection from inhaled particles and pathogens, as well as for the clearance of recruited leukocytes, dead cells, and endogenous debris [5]. Mucus also plays an important role in the maintenance of airway surface liquid equilibrium. In conjunction with the regulated transport of ions and water across membranes, mucus facilitates hydration of the underlying epithelium by modulating airway surface liquid osmotic gradients, thereby maintaining epithelial tonicity and responses to mechanical stress [22,23]. Hydration of mucus is also essential for maintaining the stability and function of cilia [24]. Accordingly, mucus dysfunction can result in, or worsen, disease states [2–4,25–28]. Maintaining healthy and effective mucociliary function thus requires tight regulation of the mucus gel.
Mucins are components of mucus whose regulated production and secretion are strictly controlled. Their co-ordinated regulation is tissue- and cell-specific, resulting in mucus gels whose physical properties are tailored to their tissue environment. Healthy airway mucus is comprised mainly of water (>95%), and mucins constitute over 80% of the mass of solid materials within the gel. Increasing the mass of mucins in mucus can have dramatic effects on gel transport. Accordingly, MUC5AC and MUC5B are tightly regulated at the levels of expression, polymerization, and glycosylation [29–31].
Transcriptional regulation of mucin expression
MUC5AC/Muc5ac and MUC5B/Muc5b (per standard rules of nomenclature, all letters are capitalized for the human gene; first letter capitalized with all following letters lowercase for mouse gene) are located on chromosome 11p15.5 in humans and 7F5 in mice [32–34]. Under normal conditions in human lungs, the MUC5AC gene is expressed in central airway tracheobronchial surface epithelial goblet cells at low levels, whereas MUC5B is primarily expressed in SMGs found throughout the central airways. MUC5B is also expressed in goblet cells in more peripheral bronchiolar airways, where glands are absent [35–39]. In mice, Muc5ac is hardly expressed at baseline, and Muc5b gene expression predominates within surface secretory cells [13,14,40,41]. Since mice lack submucosal glands in intrapulmonary airways, the patterns of gene expression for mouse airway mucins appear to resemble human bronchioles. MUC5AC/Muc5ac expression increases dramatically in lung diseases and in mouse models of lung disease. On the other hand, MUC5B/Muc5b expression remains relatively stable, or in some cases, its expression actually decreases [14,42–44].
With these patterns of gene expression, it is generally accepted that the two mucins serve separate functions: MUC5B is employed for MCC in regular maintenance of a healthy respiratory tract, and MUC5AC is primarily produced in response to acute respiratory inflammation for reasons that are not entirely clear. The consensus favoring a homeostatic role for MUC5B/Muc5b expression at baseline stems from findings that it is essential in the mouse respiratory tract to facilitate MCC and thus prevent infection [45,46]. On the other hand, induced expression of MUC5AC/Muc5ac in inflamed and diseased lungs is considered largely to be a detrimental factor, since it has been found to be overproduced in respiratory diseases such as asthma, where mucus obstruction plays an important role in pathophysiology [47]. To achieve these baseline and induced levels, mucin expression is broadly regulated by a network of transcription factors that are involved in secretory differentiation and inflammation signaling.
Mucin expression at baseline
In healthy lungs, baseline MUC5B/Muc5b gene expression is regulated by transcriptional mechanisms that are still poorly understood. The high levels of MUC5B/Muc5b transcripts expressed at baseline are linked to several gene regulatory programs that are tied to lung development and secretory epithelial cell lineage specification. Histological experiments and western blots demonstrate the prevalence of Muc5b in airway epithelium even in the absence of allergic response [41,48,49]. During embryonic lung development, the transcription factor Nkx2-1 represses Muc5b gene expression [50–53], whereas GATA-6 activates Muc5b expression [53,54]. Forkhead box a2 (Foxa2) is required for normal differentiation of the airway epithelium, and it also suppresses Muc5ac transcription, whereas selective deletion of Foxa2 results in increased Muc5ac mRNA and immunohistochemically detectable protein [55,56], suggesting that this key developmental transcription factor plays an important role in maintaing low levels of Muc5ac expression relative to abundant Muc5b transcripts.
Recently, it was revealed that the single-nucleotide polymorphism (SNP) rs35705950 in the promoter region of the human MUC5B gene strongly regulates its expression [39,57,58]. The presence of the rs35705950 minor allele enhances transcription of MUC5B, increasing mucin mRNA production, especially in the distal airways where SNP genotype correlates with MUC5B protein levels in the bronchioles in vivo and with promoter activity in vitro [3,39]. Importantly, rs35705950 strongly affects expression at baseline, with healthy subjects carrying either one or two copies of the minor allele expressing ~40-fold higher levels of MUC5B than major allele homozygotes [58]. The rs35705950 SNP is part of an active enhancer containing a FOXA2-binding site, suggesting that repression of MUC5B transcription by FOXA2 is lost when the gain-of-function SNP site is in an inactive state that was recently shown to potentially involve epigenetic control via methylation [59]. Since rs35705950 does regulate MUC5B expression under homeostatic conditions, it is important to note that the very high levels of expression imparted by rs35705950 may be detrimental in the long term. Chronic overexpression of MUC5B driven by rs35705950 is the single greatest risk factor for the development of idiopathic pulmonary fibrosis [60]. Paradoxically, though rs35705950 is a risk factor for developing the disease, patients who carry the minor allele variant actually live longer, on average, than their major allele-carrying counterparts [61]. Taken together, these data highlight the complexities underlying the regulation of the MUC5B gene and the long-term effects of MUC5B expression.
Mucin overexpression in inflammation and disease
Selective induction of MUC5AC gene expression is characteristic of numerous respiratory and inflammatory disease states including asthma, cystic fibrosis, and chronic obstructive pulmonary disease, as well as transient infectious or injurious responses [14,16,49,62,63]. Accordingly, a large number of cytokine and growth factor signaling pathways have been shown to drive MUC5AC expression. In innate inflammation, tumor necrosis factor (TNF) activates mitogen-activated protein kinases ERK and P38, which in turn activate cyclic AMP-responsive element-binding protein (CREB) downstream. CREB binds to a cis region of the MUC5AC promoter, resulting in increased transcriptional activation [64,65]. Epidermal growth factor receptor (EGFR) signaling has also been shown to drive MUC5AC expression. EGFR-mediated MUC5AC transcriptional regulation may utilize some of the same signal transduction pathways implicated above for TNF [66–68].
EGFR signaling in mouse lung epithelial cells up-regulates Muc5ac via hypoxia inducible factor-1 (HIF-1)-binding site, which is conserved in mammalian MUC5AC orthologs and is present in the core promoter [14]. This effect of EGFR was also observed with the type 2 inflammatory cytokine IL-13 [42]. IL-13 and EGFR are drivers of mucin expression in classic type 2 inflammation settings such as allergy and asthma, and it has also been implicated in MUC5AC/Muc5ac responses to some viral infections [42,69–73]. Like EGFR, IL-13 also induces HIF-1 binding and Muc5ac promoter activation [14]. In addition to HIF-1, IL-13 has also been shown to induce Muc5ac expression via up-regulation of SAM-pointed domain-containing ETS transcription factor, which in turn inhibits Foxa2-mediated Muc5ac repression during inflammation [56,74]. This loss of repressor function, along with the induction of activating transcription factors, has been proposed as a mechanism for tightly controlling Muc5ac expression levels and localization [75,76].
Lastly, some core promoter elements include sites for specificity protein 1 (Sp1) and nuclear factor κ-B (NFκB), which are present in both the MUC5AC and MUC5B genes [77–81]. The extents to which these regulate baseline versus inducible expression are still being deciphered. Ultimately, while mucin expression levels are tightly regulated by transcription, the functions of those expressed are also significantly affected by protein biosynthesis and post-translational modifications.
Form fits function: airway mucin function depends on protein structure and formation
Structurally, mucins are huge macromolecules with apoprotein lengths ranging from 3000 to >5000 amino acids. MUC5AC and MUC5B monomers are ~500 nm long, and they form homopolymeric chains of up to 100s of μm in length [82,83]. Given the size of these sequences, it is no surprise that each mucin contains multiple functional domains that contribute to their structure and their function (Figure 1). The primary amino acid sequences of MUC5AC and MUC5B contain multiple cysteine-rich von Willebrand factor D (vWD)-like domains at their N-terminal and C-terminal regions. These vWD domains mediate mucin polymerization via disulfide bonding (described below) and thus contribute to the overall assembly and ultimate size of mature mucins [34,84–86]. In addition, MUC5AC and MUC5B have internal glycosylated domains, separated by short, cysteine-rich domain (CysD) segments, which demonstrate high intra- and interspecies conservation in structure, albeit with variation in their frequencies. This structural homology within CysDs suggests that those segments contribute to mucus gel formation [84,87,88]. This is supported by a recent functional study, showing that increasing the number of CysD residues in intestinal mucins increases the strength of the intestinal mucus layer and reduces bacterial infection in mice [89]. The glycosylated domains of mucins are rich in proline, threonine, and serine residues, and are thus referred to as ‘PTS’ domains. The frequencies and specific sequences of PTS domains vary across animal species, among mucins isoforms, and even between alleles of individual mucin genes, affording unique structural and functional modifications for specialization purposes [90–95].
Figure 1. MUC5AC/MUC5B structural domains and molecular interactions.
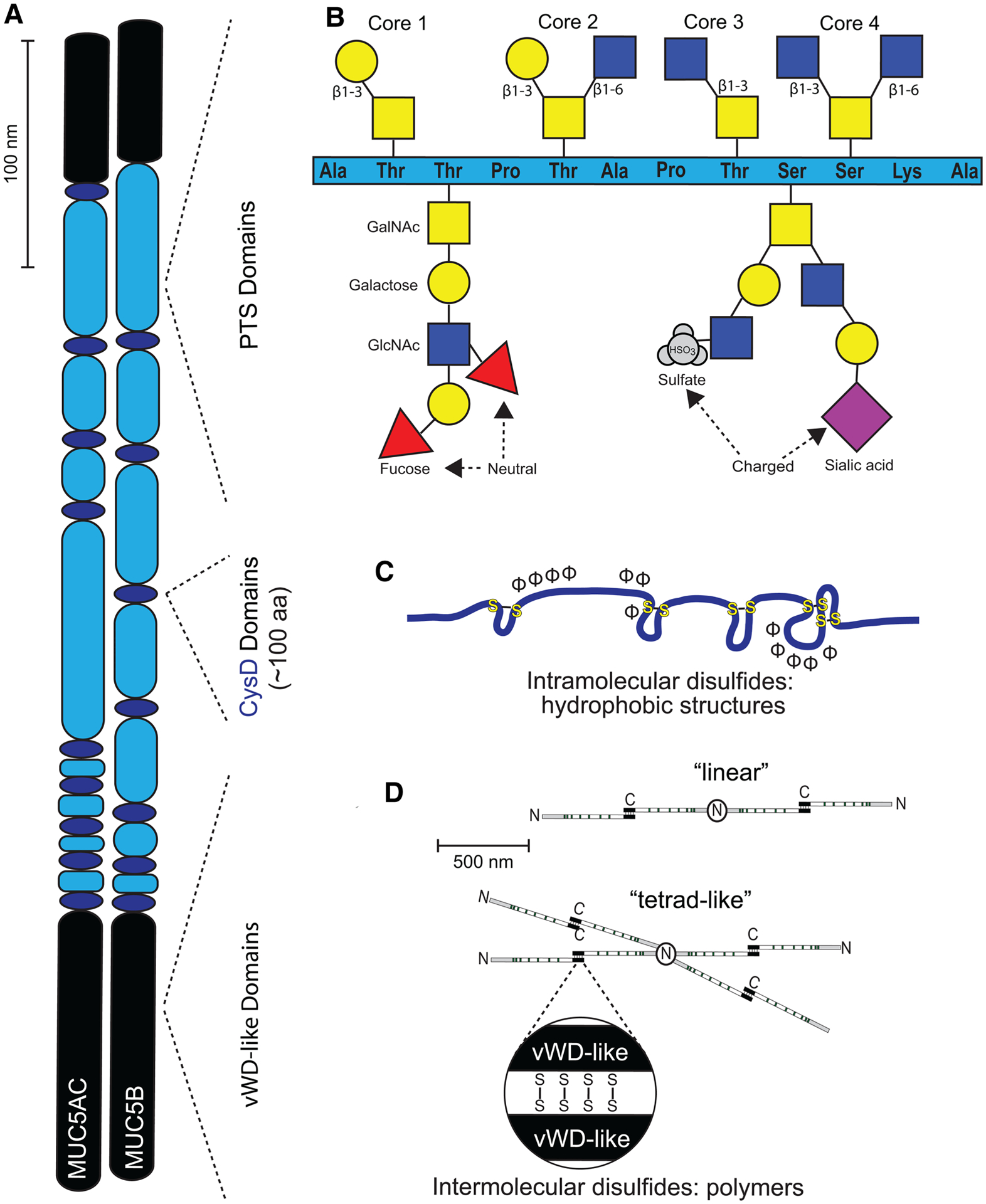
(A) MUC5AC and MUC5B apoproteins are lengthy constructs containing PTS domains (light blue ovals) interspersed with CysD (dark blue ovals), capped with vWD (black ovals). Each of these domains contributes toward overall structure, and thus function, of these mucins in a unique way. (B) PTS domains serve as sites for core glycosylation. GalNAc (yellow squares) is attached to serine and threonine residues for all four core structures. This is followed by β1–3 linkage of galactose (yellow circle) to form core 1, addition of both a β1–3 linkage of galactose and a β1–6 linkage of GlcNAc (blue squares), to form core 2, a β1–3 linkage of GlcNac to form core 3, or both a β1–3 linkage of GlcNAc and a β1–6 linkage of GlcNac to form core 4. These cores can be further elongated by the addition of extra sugars, including fucose (red triangles), or capped/terminated by SO4 (gray circles) or NeuNAc (purple diamonds). (C) CysD segments reinforce tertiary structure of mucins via intramolecular disulfide linkage. (D) vWD-like domains assist in assembly of homo-oligomers into linear- or tetrad-like structures via intermolecular disulfide linkage.
vWD domains
In one sense, mucins follow the classic model for protein assembly from primary through quaternary structuring (Figure 2). MUC5AC and MUC5B form three-dimensional conformations through intramolecular interactions at their globular ends, solidified with tertiary interactions between (and within) the molecular functional domains. These mucins also utilize disulfide intra- and intermolecular linkages to conduct quaternary protein assembly that results in complex homo-oligomers that begin with dimers attached via the cysteine-rich segments.
Figure 2. Pathways of mucin biosynthesis.
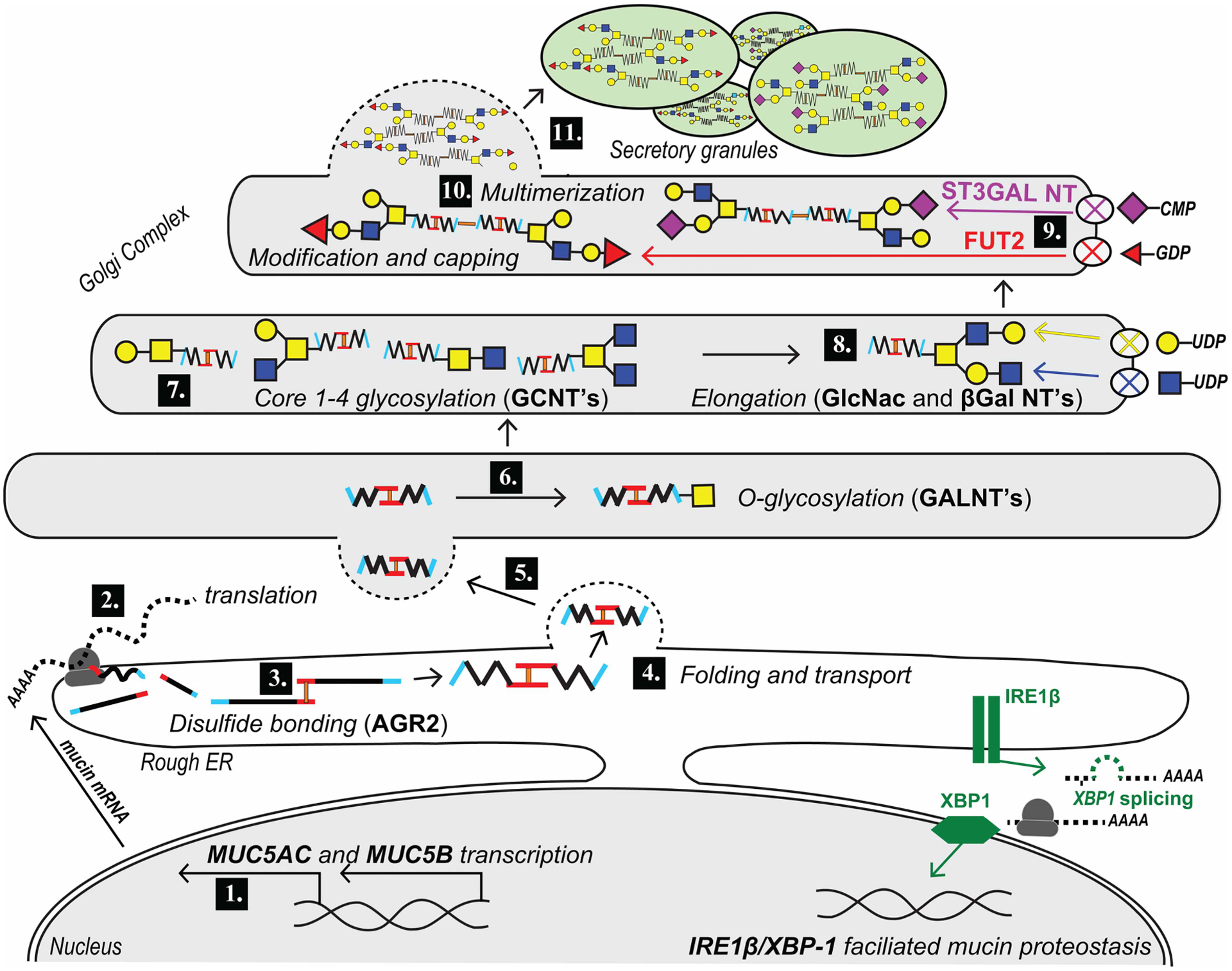
1. MUC5AC/MUC5B transcription in the nucleus is tightly regulated by specific transcription factors. 2. Co-translational import of mucin into the rough ER. 3. Assembly of mucin disulfide dimers is mediated by PDIs. 4 and 5. Mucin is folded and transported from ER to the Golgi complex under the guidance of chaperone and trafficking proteins. 6. In the cis Golgi, mucin is O-glycosylated by the addition of GalNAc (yellow squares) by GALNTs. 7. Mucin transits through the middle Golgi where additional core 1–4 structures are formed by the addition of GlcNAc (blue squares) and/or Gal (yellow circles) by CGNTs. 8. Sugars are extended by additional βGal and GlcNAc transferases (GalTs and GlcNAcTs). 9. Mucin glycosylation terminates in sialylation via α2,3-sialyltransferase or fucosylation via α1,2 fucosyltransferase enzymes ST3GAL3 and FUT2, respectively (fucose, red triangles; sialic acid, purple diamonds). 10. Mucin oligomeric subunits multimerize via N-terminal assembly. 11. Fully synthesized mucin is then transported from the trans-Golgi by vesicular trafficking to growing secretory granules, where it is stored for eventual release by regulated exocytosis.
Cysteine-mediated polymerization is essential for mucus viscoelasticity. These segments are stabilized by C-terminal-to-C-terminal disulfide linkages formed inside the endoplasmic reticulum (ER) via protein disulfide isomerase (PDI) enzymes [96]. Anterior gradient homolog (AGR)2, a disulfide isomerase-like protein, is essential for production of MUC2 in intestines, as well as MUC5AC and MUC5B overproduction in an asthma model [97,98]. Originally suggested as being involved in disulfide-mediated assembly, the role of AGR2 is now less clear [99]. AGR2 can be induced by XBP-1, a transcription factor, which in turn is activated by inositol-requiring enzyme (IRE)1β. IRE1β is specifically expressed in mouse and human respiratory mucosal cells and is required for mucin production [100].
The dimers formed in the ER are further connected via N-terminal-to-N-terminal linkage within the Golgi, thus establishing the mucin polymer in a manner similar to von Willebrand factor assembly [85,101–103]. It was recently observed that in the acidic, calcium-rich conditions of the Golgi, these disulfide bridges pull the D3 domains of MUC5B in close proximity to cross-link (non-covalently) into tetrad-like structures, which link in turn to form dense linear constructs for condensation of MUC5B in secretory granules [103,104]. Thus, MUC5AC and MUC5B proteins, which are already significant in size upon translation, become even larger as the result of disulfide-mediated polymerization in the ER and Golgi. In addition to this process of polymeric assembly, within the Golgi, MUC5AC and MUC5B become even more massive through glycosylation.
PTS domains
Glycosylation is the ultimate determining factor of mucin function, as these huge molecules are actually comprised 50–90% of carbohydrates [87]. Ultimately, glycosylation increases mucin size 3–5-fold [3,34], and glycans can dramatically affect the functions of mucins within the mucus gels that they form [12,105,106]. The composition of this dense mass of protein is highly variable between each mucin type, and because of its structural influences, greatly affects the function of individual mucins. The PTS tandem repeats in MUC5AC and MUC5B serve as the sites for this O-glycosylation, accommodating the addition of hundreds of O-glycans to each molecule [87].
Glycosylation
Inside the Golgi, O-glycosylation is initiated by the transfer of N-acetylgalactosamine (GalNAc) from UDP-GalNAc to serine and threonine amino acids within the mucin PTS domains by a polypeptide GalNAc-transferase (GALNT). In humans, 20 distinct GALNT family members have been identified with some GALNTs expressed ubiquitously, and others with restricted expression across tissue types. The addition of O-GalNAc to a serine/threonine amino acid in a PTS domain is affected by both the sequence of amino acids immediately flanking (−3 to +3) and longer-range interaction with neighboring glycosylated residues (6–17 residues away) [107,108]. This addition of GalNAc to the apomucin results in the formation of a ‘Tn’ antigen that functions as a scaffold for the further elaboration of mucin glycans [109]. Neutral sugar(s) can then be attached to O-GalNAc, which allows for the formation of the O-glycan ‘cores’ [12,110–112]. Core 1 structures are formed by the addition of galactose (Gal) β1–3 onto GalNAc-O-Ser/Thr, which is catalyzed by the glycosyltransferase C1GalT1 and which also requires the chaperone C1CALT1C1/Cosmc. The core 2 structure is formed by the addition of a second neutral sugar, N-acetylglucosamine (GlcNAc), β1–6 to the core 1 structure by core 2 synthases GCNT1/3. Core 3 structures are formed by the addition of a single GlcNAc in a β1–3 linkage to the GalNAc-O-Ser/Thr catalyzed by B3GNT6. Lastly, core 4 structures are formed by the further branching of a core 3 O-glycan via the addition of a second GlcNAc-linked β1–6 to the GalNAc-O-Ser/Thr catalyzed is GCNT3 [12,87,109]. These cores ultimately form a central glycosylation backbone off of which the rest of the glycan complexes are constructed.
After core glycosylation, the further addition of Gals and GlcNAcs proceeds to lengthen core 1- and 3-anchored strands, and core 2 and 4 bi-antennary structures. In addition, cores 2 and 4 can become further branched leading to tri-antennary and tetra-antennary structures on mucins [113]. These chains are extended with highly variable combinations of glycans. Additionally, glycan chains may be modified by the addition of fucose by fucosyltransferases including FUT2 [109,114]. Ultimately, these elaborated structures terminate in either Gal or GlcNAc to form ‘uncapped’ glycan structures, or they can be ‘capped’ by sulfate (SO4) or sialic acid (NeuNac). In general, the addition of terminal sialic acid prevents further elaboration of the glycan chain [109,115–117]. The heterogeneity of glycan structures is what endows each mucin with unique physical properties. Sialic acid and sulfates, for example, confer negative charges to molecules, which introduce steric repulsion between glycosylated side chains that, in turn, confer rigidity to mucins [12,105,106,118]. In contrast, fucose can confer more neutrally charged attributes [12,87,105,106,119]. These can contribute to the viscous gel properties of mucins by regulating how many water molecules (or other charged ions) can interact with them.
CysD domains
Beyond glycosylation of the PTS domains, the CysD domains of mucins serve as sites that affect physical properties conferred by glycans. It has been suggested that the interruption of PTS domains in MUC5AC and MUC5B by CysD domains may confer flexibility within the molecules to allow for intermolecular interactions [87]. In addition, CysD’s also potentially serve as mannosylation sites, where a single α-mannose residue can be covalently added to the second carbon of tryptophan in WxxW motifs within CysDs. MUC5AC and MUC5B contain nine and seven potential mannosylation sites, respectively [12,88,120]. CysD mannosylation may be essential to the maturation of secreted mucins, as lack of mannosylation in MUC5AC and MUC5B arrests these proteins in the ER in a cell culture model of mucin synthesis [121]. While CysD mannosylation function is incompletely understood, it is thought to be related to intracellular trafficking, subcellular localization, and protein folding.
Transglutamination
One further potential post-translational modification to mucins is cross-linking via transglutamination. Transglutaminase 2 has been shown to mediate cross-linking of MUC2 via the second CysD domain as part of mucus formation [122,123]. Inhibition of this enzyme in a mouse model of asthma leads to decreased airway hyperresponsiveness, decreased number of inflammatory and goblet cells, and decreased expression of TNF, NFκB, and Muc5ac transcripts, indicating that transglutamination plays a key role in asthma-induced inflammation and mucin production [124]. In a somewhat conflicting study, overexpression of transglutaminase 1 in a mouse model of dry eye led to a decrease in goblet cells and Muc5ac [125]. Thus, there is still much to learn about transglutamination in mucin production and formation. It is possible that different transglutaminases serve unique functions in mucin regulation and structure, depending upon which tissues they are expressed in.
Summary and implications
For all their size and complexity, the synthesis of polymeric mucins is remarkably efficient. Polymeric mucins are translated and dimers are assembled within the endoplasmic reticulum in ~20 min [101,102]. Processing through the Golgi takes 90–120 min to yield a fully constructed mucin glycopolymer [102]. Upon completion, the mucins bud from the trans-Golgi and undergo homotypic fusion to form large secretory granules (SGs) that are calcium-rich vesicles and have low pHs [12,126]. Large SG’s mature can be stored for long periods of time, awaiting a secretory stimulus that mobilizes SGs towards apical plasma membrane docking sites where they fuse and release their contents via regulated exocytosis [40,127,128]. There is also emerging evidence that some mucins are secreted via small vesicles in a tonic fashion to maintain steady-state levels of secreted mucins in the mucus gel [41,129].
The formation of mucin glycopolymers is a complex and highly regulated process. Disulfide bonding and glycosylation are heterogeneous between different mucin types, tissues, and disease states versus homeostasis [12,130–132]. In healthy states, the production of hydrated and easily transported mucus is critical for maintaining airway homeostasis and MCC. In diseases such as asthma, the production of tenacious mucus plugs is clearly detrimental in the context of contemporary clinical settings. Emerging techniques have been utilized to produce glycopeptide polymers through direct polymerization of glycosylated monomers or post-polymerization glycosylation of reactive polypeptides [133]. These new synthetic routes not only begin to mimic the assembly process described on the cellular level, but also lead to polymers that recapitulate the structure and function of natural glycoproteins [134,135]. Delivery of glycopeptide polymers has immense potential to alleviate the effects of changes in natural mucus viscosity caused by disease or inflammation and help to return airways to homeostasis.
However, mucus overproduction in disease states may also reflect an evolutionarily guided mechanism for controlling the spread and transport of bacteria, viruses, and nematodes [136–140]. Recent data have demonstrated novel mechanisms through which mucins steer both innate and adaptive immune responses across many phases: mediating antigen presentation during early phases, driving inflammosuppression during persistent exposures during chronic phases, and inducing apoptosis of potentially toxic immune effector cells during late phases [113,141–144]. As data emerge in the field, it has become clear that mucus is more than just a sticky substance. Rather, it is a crucial component of an integrated system that is designed to maintain health and to do so in by employing diverse ways to promote health while limiting the detrimental effects of foreign and host-derived challenges.
Acknowledgements
We are grateful to our colleagues whose works are summarized in the present paper. We apologize that we were unable to comprehensively cover many important works on mucin expression, structure, and dynamics, particularly those that pertain to secretion mechanisms.
Funding
Support was provided by the U.S. National Institutes of Health [grants HL080396 and HL130938] (C.M.E.), HL007085 (A.L.S.), by the U.S. Department of Defense [grant PR160247] (C.M.E.), and by the American Thoracic Society Foundation (C.M.M.).
Abbreviations
- AGR2
anterior gradient homolog
- CGNT
core glycosyltransferase
- CREB
cAMP response element-binding protein
- CysD
cysteine-rich domain
- EGFR
epidermal growth factor receptor
- ER
endoplasmic reticulum
- Foxa2
forkhead box a2
- Gal
galactose
- GalNAc
N-acetylgalactosamine
- GALNT
GalNAc-transferase
- GalT
βGal transferase
- GlcNAc
N-acetylglucosamine
- GlcNAcT
GlcNAc transferase
- HIF-1
hypoxia inducible factor-1
- IRE1β
inositol-requiring enzyme (IRE)1β
- kDa
kilodalton
- MCC
mucociliary clearance
- NeuNAc
sialic acid
- NFκB
nuclear factor κ-B
- PDI
protein disulfide isomerase
- PTS
proline-, serine-, threonine-rich sequence
- SG
secretory granule
- SMG
submucosal gland
- SNP
single-nucleotide polymorphism
- SO4
sulfate
- TNF
tumor necrosis factor
- vWD
von Willebrand factor D
Footnotes
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Knowles MR and Boucher RC (2002) Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest 109, 571–577 10.1172/JCI15217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fahy JV and Dickey BF (2010) Airway mucus function and dysfunction. N. Engl. J. Med 363, 2233–2247 10.1056/NEJMra0910061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans CM, Fingerlin TE, Schwarz MI, Lynch D, Kurche J, Warg L et al. (2016) Idiopathic pulmonary fibrosis: a genetic disease that involves mucociliary dysfunction of the peripheral airways. Physiol. Rev 96, 1567–1591 10.1152/physrev.00004.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R and Boucher RC (2006) Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N. Engl. J. Med 354, 241–250 10.1056/NEJMoa043891 [DOI] [PubMed] [Google Scholar]
- 5.Ma J, Rubin BK and Voynow JA (2017) Mucins, mucus, and goblet cells. Chest Epub ahead of print 10.1016/j.chest.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 6.Bustamante-Marin XM and Ostrowski LE (2017) Cilia and mucociliary clearance. Cold Spring Harb. Perspect. Biol 9, a028241 10.1101/cshperspect.a028241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ermund A, Meiss LN, Rodriguez-Pineiro AM, Bähr A, Nilsson HE, Trillo-Muyo S et al. (2017) The normal trachea is cleaned by MUC5B mucin bundles from the submucosal glands coated with the MUC5AC mucin. Biochem. Biophys. Res. Commun 492, 331–337 10.1016/j.bbrc.2017.08.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borthwick DW, West JD, Keighren MA, Flockhart JH, Innes BA and Dorin JR (1999) Murine submucosal glands are clonally derived and show a cystic fibrosis gene-dependent distribution pattern. Am. J. Respir. Cell Mol. Biol 20, 1181–1189 10.1165/ajrcmb.20.6.3475 [DOI] [PubMed] [Google Scholar]
- 9.Jeffery PK (1983) Morphologic features of airway surface epithelial cells and glands. Am. Rev. Respir. Dis 128(2 Pt 2), S14–S20 10.1164/arrd.1983.128.2P2.S14 [DOI] [PubMed] [Google Scholar]
- 10.Widdicombe JH and Wine JJ (2015) Airway gland structure and function. Physiol. Rev 95, 1241–1319 10.1152/physrev.00039.2014 [DOI] [PubMed] [Google Scholar]
- 11.Verdugo P, Aitken M, Langley L and Villalon MJ (1987) Molecular mechanism of product storage and release in mucin secretion. II. The role of extracellular Ca++. Biorheology 24, 625–633 10.3233/BIR-1987-24615 [DOI] [PubMed] [Google Scholar]
- 12.Corfield AP (2015) Mucins: a biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta, Gen. Subj 1850, 236–252 10.1016/j.bbagen.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 13.Roy MG, Rahmani M, Hernandez JR, Alexander SN, Ehre C, Ho SB et al. (2011) Mucin production during prenatal and postnatal murine lung development. Am. J. Respir. Cell Mol. Biol 44, 755–760 10.1165/rcmb.2010-0020RC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young HWJ, Williams OW, Chandra D, Bellinghausen LK, Pérez G, Suárez A et al. (2007) Central role of Muc5ac expression in mucous metaplasia and Its regulation by conserved 5′ elements. Am. J. Respir. Cell Mol. Biol 37, 273–290 10.1165/rcmb.2005-0460OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill DB, Vasquez PA, Mellnik J, McKinley SA, Vose A, Mu F et al. (2014) A biophysical basis for mucus solids concentration as a candidate biomarker for airways disease. PLoS ONE 9, e87681 10.1371/journal.pone.0087681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirkham S, Sheehan JK, Knight D, Richardson PS and Thornton DJ (2002) Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem. J 361(Pt 3), 537–546 10.1042/bj3610537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deneuville E, Perrot-Minot C, Pennaforte F, Roussey M, Zahm J-M, Clavel C et al. (1997) Revisited physicochemical and transport properties of respiratory mucus in genotyped cystic fibrosis patients. Am. J. Respir. Crit. Care Med 156, 166–172 10.1164/ajrccm.156.1.9606123 [DOI] [PubMed] [Google Scholar]
- 18.Casado B, Pannell LK, Iadarola P and Baraniuk JN (2005) Identification of human nasal mucous proteins using proteomics. Proteomics 5, 2949–2959 10.1002/pmic.200401172 [DOI] [PubMed] [Google Scholar]
- 19.Tomkiewicz RP, App EM, Zayas JG, Ramirez O, Church N, Boucher RC et al. (1993) Amiloride inhalation therapy in cystic fibrosis. Influence on ion content, hydration, and rheology of sputum. Am. Rev. Respir. Dis 148(4 Pt 1), 1002–1007 10.1164/ajrccm/148.4_Pt_1.1002 [DOI] [PubMed] [Google Scholar]
- 20.Cavaliere F, Masieri S, Vagnoni S, Proietti R and Magalini SI (1989) Airway secretion electrolytes: reflection of water and salt states of the body. Crit. Care Med 17, 891–894 10.1097/00003246-198909000-00010 [DOI] [PubMed] [Google Scholar]
- 21.Bhaskar KR, O’Sullivan DD, Seltzer J, Rossing TH, Drazen JM and Reid LM (1985) Density gradient study of bronchial mucus aspirates from healthy volunteers (smokers and nonsmokers) and from patients with tracheostomy. Exp. Lung Res 9, 289–308 10.3109/01902148509057529 [DOI] [PubMed] [Google Scholar]
- 22.Tarran R, Button B and Boucher RC (2006) Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu. Rev. Physiol 68, 543–561 10.1146/annurev.physiol.68.072304.112754 [DOI] [PubMed] [Google Scholar]
- 23.Anderson WH, Coakley RD, Button B, Henderson AG, Zeman KL, Alexis NE et al. (2015) The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am. J. Respir. Crit. Care Med 192, 182–190 10.1164/rccm.201412-2230OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Button B, Cai L-H, Ehre C, Kesimer M, Hill DB, Sheehan JK et al. (2012) A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337, 937–941 10.1126/science.1223012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boucher RC (2007) Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu. Rev. Med 58, 157–170 10.1146/annurev.med.58.071905.105316 [DOI] [PubMed] [Google Scholar]
- 26.O’Riordan TG, Zwang J and Smaldone GC (1992) Mucociliary clearance in adult asthma. Am. Rev. Respir. Dis 146, 598–603 10.1164/ajrccm/146.3.598 [DOI] [PubMed] [Google Scholar]
- 27.Smaldone GC (2001) Deposition and clearance: unique problems in the proximal airways and oral cavity in the young and elderly. Respir. Physiol 128, 33–38 10.1016/S0034-5687(01)00262-6 [DOI] [PubMed] [Google Scholar]
- 28.Svartengren M, Falk R and Philipson K (2005) Long-term clearance from small airways decreases with age. Eur. Respir. J 26, 609–615 10.1183/09031936.05.00002105 [DOI] [PubMed] [Google Scholar]
- 29.Williams OW, Sharafkhaneh A, Kim V, Dickey BF and Evans CM (2006) Airway mucus: from production to secretion. Am. J. Respir. Cell Mol. Biol 34, 527–536 10.1165/rcmb.2005-0436SF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bansil R and Turner BS (2018) The biology of mucus: composition, synthesis and organization. Adv. Drug Deliv. Rev 124, 3–15 10.1016/j.addr.2017.09.023 [DOI] [PubMed] [Google Scholar]
- 31.France MM and Turner JR (2017) The mucosal barrier at a glance. J. Cell Sci 130, 307–314 10.1242/jcs.193482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pigny P, Guyonnet-Duperat V, Hill AS, Pratt WS, Galiegue-Zouitina S, d’Hooge MC et al. (1996) Human mucin genes assigned to 11p15.5: identification and organization of a cluster of genes. Genomics 38, 340–352 10.1006/geno.1996.0637 [DOI] [PubMed] [Google Scholar]
- 33.Escande F, Porchet N, Aubert J-P and Buisine M-P (2002) The mouse Muc5b mucin gene: cDNA and genomic structures, chromosomal localization and expression. Biochem. J 363(Pt 3), 589–598 10.1042/bj3630589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thornton DJ, Rousseau K and McGuckin MA (2008) Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol 70, 459–486 10.1146/annurev.physiol.70.113006.100702 [DOI] [PubMed] [Google Scholar]
- 35.Hovenberg HW, Davies JR and Carlstedt I (1996) Different mucins are produced by the surface epithelium and the submucosa in human trachea: identification of MUC5AC as a major mucin from the goblet cells. Biochem. J 318(Pt 1), 319–324 10.1042/bj3180319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma P, Dudus L, Nielsen PA, Clausen H, Yankaskas JR, Hollingsworth MA et al. (1998) MUC5B and MUC7 are differentially expressed in mucous and serous cells of submucosal glands in human bronchial airways. Am. J. Respir. Cell Mol. Biol 19, 30–37 10.1165/ajrcmb.19.1.3054 [DOI] [PubMed] [Google Scholar]
- 37.Groneberg DA, Eynott PR, Oates T, Lim S, Wu R, Carlstedt I et al. (2002) Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respir. Med 96, 81–86 10.1053/rmed.2001.1221 [DOI] [PubMed] [Google Scholar]
- 38.Seibold MA, Smith RW, Urbanek C, Groshong SD, Cosgrove GP, Brown KK et al. (2013) The idiopathic pulmonary fibrosis honeycomb cyst contains a mucocilary pseudostratified epithelium. PLoS ONE 8, e58658 10.1371/journal.pone.0058658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakano Y, Yang IV, Walts AD, Watson AM, Helling BA, Fletcher AA et al. (2016) MUC5B promoter variant rs35705950 affects MUC5B expression in the distal airways in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med 193, 464–466 10.1164/rccm.201509-1872LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR et al. (2004) Mucin is produced by Clara cells in the proximal airways of antigen-challenged mice. Am. J. Respir. Cell Mol. Biol 31, 382–394 10.1165/rcmb.2004-0060OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y, Ehre C, Abdullah LH, Sheehan JK, Roy M, Evans CM et al. (2008) Munc13–2−/− baseline secretion defect reveals source of oligomeric mucins in mouse airways. J. Physiol 586, 1977–1992 10.1113/jphysiol.2007.149310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, Paquet AC et al. (2007) IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am. J. Respir. Cell Mol. Biol 36, 244–253 10.1165/rcmb.2006-0180OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Innes AL, Woodruff PG, Ferrando RE, Donnelly S, Dolganov GM, Lazarus SC et al. (2006) Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest 130, 1102–1108 10.1378/chest.130.4.1102 [DOI] [PubMed] [Google Scholar]
- 44.Lachowicz-Scroggins ME, Yuan S, Kerr SC, Dunican EM, Yu M, Carrington SD et al. (2016) Abnormalities in MUC5AC and MUC5B protein in airway mucus in asthma. Am. J. Respir. Crit. Care Med 194, 1296–1299 10.1164/rccm.201603-0526LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM et al. (2014) Muc5b is required for airway defence. Nature 505, 412–416 10.1038/nature12807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Livraghi-Butrico A, Grubb BR, Wilkinson KJ, Volmer AS, Burns KA, Evans CM et al. (2017) Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease. Mucosal Immunol. 10, 829 10.1038/mi.2017.29 [DOI] [PubMed] [Google Scholar]
- 47.Evans CM, Raclawska DS, Ttofali F, Liptzin DR, Fletcher AA, Harper DN et al. (2015) The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat. Commun 6, 559 10.1038/ncomms7281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen LP, Omoluabi O, Parra S, Frieske JM, Clement C, Mmar-Aouchiche Z et al. (2008) Chronic exposure to β-blockers attenuates inflammation and mucin content in a murine asthma model. Am. J. Respir. Cell Mol. Biol 38, 256–262 10.1165/rcmb.2007-0279RC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ordoñez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM et al. (2001) Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am. J. Respir. Crit. Care Med 163, 517–523 10.1164/ajrccm.163.2.2004039 [DOI] [PubMed] [Google Scholar]
- 50.DeFelice M, Silberschmidt D, DiLauro R, Xu Y, Wert SE, Weaver TE et al. (2003) TTF-1 phosphorylation is required for peripheral lung morphogenesis, perinatal survival, and tissue-specific gene expression. J. Biol. Chem 278, 35574–35583 10.1074/jbc.M304885200 [DOI] [PubMed] [Google Scholar]
- 51.Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM et al. (1996) The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 10, 60–69 10.1101/gad.10.1.60 [DOI] [PubMed] [Google Scholar]
- 52.Maeda Y, Chen G, Xu Y, Haitchi HM, Du L, Keiser AR et al. (2011) Airway epithelial transcription factor NK2 homeobox 1 inhibits mucous cell metaplasia and Th2 inflammation. Am. J. Respir. Crit. Care Med 184, 421–429 10.1164/rccm.201101-0106OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jonckheere N, Velghe A, Ducourouble M-P, Copin M-C, Renes IB and Van Seuningen I (2011) The mouse Muc5b mucin gene is transcriptionally regulated by thyroid transcription factor-1 (TTF-1) and GATA-6 transcription factors. FEBS J. 278, 282–294 10.1111/j.1742-4658.2010.07945.x [DOI] [PubMed] [Google Scholar]
- 54.Yang H, Lu MM, Zhang L, Whitsett JA and Morrisey EE (2002) GATA6 regulates differentiation of distal lung epithelium. Development 129, 2233–2246 [DOI] [PubMed] [Google Scholar]
- 55.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW and Bry K (2005) Interleukin-1β causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am. J. Respir. Cell Mol. Biol 32, 311–318 10.1165/rcmb.2004-0309OC [DOI] [PubMed] [Google Scholar]
- 56.Wan H, Kaestner KH, Ang SL, Ikegami M, Finkelman FD, Stahlman MT et al. (2004) Foxa2 regulates alveolarization and goblet cell hyperplasia. Development 131, 953–964 10.1242/dev.00966 [DOI] [PubMed] [Google Scholar]
- 57.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC et al. (2013) MUC5B promoter polymorphism and interstitial lung abnormalities. N. Engl. J. Med 368, 2192–2200 10.1056/NEJMoa1216076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE et al. (2011) A common MUC5B promoter polymorphism and pulmonary fibrosis. N. Engl. J. Med 364, 1503–1512 10.1056/NEJMoa1013660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Helling BA, Gerber AN, Kadiyala V, Sasse SK, Pedersen BS, Sparks L et al. (2017) Regulation of MUC5B expression in idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol 57, 91–99 10.1165/rcmb.2017-0046OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okamoto T, Mathai SK, Hennessy CE, Hancock LA, Walts AD, Stefanski AL et al. (2018) The relationship between complement C3 expression and the MUC5B genotype in pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol Epub ahead of print 10.1152/ajplung.00395.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peljto AL, Zhang Y, Fingerlin TE, Ma S-F, Garcia JGN, Richards TJ et al. (2013) Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA 309, 2232–2239 10.1001/jama.2013.5827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koeppen M, McNamee EN, Brodsky KS, Aherne CM, Faigle M, Downey GP et al. (2013) Detrimental role of the airway mucin Muc5ac during ventilator-induced lung injury. Mucosal Immunol. 6, 762–775 10.1038/mi.2012.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Currier MG, Lee S, Stobart CC, Hotard AL, Villenave R, Meng J et al. (2016) EGFR interacts with the fusion protein of respiratory syncytial virus strain 2–20 and mediates infection and mucin expression. PLoS Pathog. 12, e1005622 10.1371/journal.ppat.1005622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song KS, Lee WJ, Chung KC, Koo JS, Yang EJ, Choi JY et al. (2003) Interleukin-1β and tumor necrosis factor-α induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases-MSK1-CREB activation in human airway epithelial cells. J. Biol. Chem 278, 23243–23250 10.1074/jbc.M300096200 [DOI] [PubMed] [Google Scholar]
- 65.Kim S-W, Hong JS, Ryu S-H, Chung W-C, Yoon J-H and Koo JS (2007) Regulation of mucin gene expression by CREB via a nonclassical retinoic acid signaling pathway. Mol. Cell. Biol 27, 6933–6947 10.1128/MCB.02385-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takeyama K, Dabbagh K, Lee H-M, Agusti C, Lausier JA, Ueki IF et al. (1999) Epidermal growth factor system regulates mucin production in airways. Proc. Natl Acad. Sci. U.S.A 96, 3081–3086 10.1073/pnas.96.6.3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF and Nadel JA (2000) Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J. Immunol 164, 1546–1552 10.4049/jimmunol.164.3.1546 [DOI] [PubMed] [Google Scholar]
- 68.Perrais M, Pigny P, Copin MC, Aubert JP and Van SI (2002) Induction of MUC2 and MUC5AC mucins by factors of the epidermal growth factor (EGF) family is mediated by EGF receptor/Ras/Raf/extracellular signal-regulated kinase cascade and Sp1. J. Biol. Chem 277, 32258–32267 10.1074/jbc.M204862200 [DOI] [PubMed] [Google Scholar]
- 69.Han M, Hong JY, Jaipalli S, Rajput C, Lei J, Hinde JL et al. (2017) IFN-γ blocks development of an asthma phenotype in rhinovirus-infected baby mice by inhibiting type 2 innate lymphoid cells. Am. J. Respir. Cell Mol. Biol 56, 242–251 10.1165/rcmb.2016-0056OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hewson CA, Haas JJ, Bartlett NW, Message SD, Laza-Stanca V, Kebadze T et al. (2010) Rhinovirus induces MUC5AC in a human infection model and in vitro via NF-κB and EGFR pathways. Eur. Respir. J 36, 1425–1435 10.1183/09031936.00026910 [DOI] [PubMed] [Google Scholar]
- 71.Barbier D, Garcia-Verdugo I, Pothlichet J, Khazen R, Descamps D, Rousseau K et al. (2012) Influenza A induces the major secreted airway mucin MUC5AC in a protease–EGFR–extracellular regulated kinase–Sp1–dependent pathway. Am. J. Respir. Cell Mol. Biol 47, 149–157 10.1165/rcmb.2011-0405OC [DOI] [PubMed] [Google Scholar]
- 72.Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH et al. (2008) Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat. Med 14, 633–640 10.1038/nm1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hashimoto K, Graham BS, Ho SB, Adler KB, Collins RD, Olson SJ et al. (2004) Respiratory syncytial virus in allergic lung inflammation increases Muc5ac and gob-5. Am. J. Respir. Crit. Care Med 170, 306–312 10.1164/rccm.200301-030OC [DOI] [PubMed] [Google Scholar]
- 74.Park K-S, Korfhagen TR, Bruno MD, Kitzmiller JA, Wan H, Wert SE et al. (2007) SPDEF regulates goblet cell hyperplasia in the airway epithelium. J. Clin. Invest 117, 978–988 10.1172/JCI29176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tompkins DH, Besnard V, Lange AW, Wert SE, Keiser AR, Smith AN et al. (2009) Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells. PLoS ONE 4, e8248 10.1371/journal.pone.0008248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y et al. (2009) SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Invest 119, 2914–2924 10.1172/JCI39731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Di YP, Zhao J and Harper R (2012) Cigarette smoke induces MUC5AC protein expression through the activation of Sp1. J. Biol. Chem 287, 27948–27958 10.1074/jbc.M111.334375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hewson CA, Edbrooke MR and Johnston SL (2004) PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGF-α, Ras/Raf, MEK, ERK and Sp1-dependent mechanisms. J. Mol. Biol 344, 683–695 10.1016/j.jmb.2004.09.059 [DOI] [PubMed] [Google Scholar]
- 79.Thai P, Loukoianov A, Wachi S and Wu R (2008) Regulation of airway mucin gene expression. Annu. Rev. Physiol 70, 405–429 10.1146/annurev.physiol.70.113006.100441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fujisawa T, Chang MM-J, Velichko S, Thai P, Hung L-Y, Huang F et al. (2011) NF-κB mediates IL-1β- and IL-17A-induced MUC5B expression in airway epithelial cells. Am. J. Respir. Cell Mol. Biol 45, 246–252 10.1165/rcmb.2009-0313OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song S-Y, Jung EC, Bae CH, Choi YS and Kim Y-D (2014) Visfatin induces MUC8 and MUC5B expression via p38 MAPK/ROS/NF-κB in human airway epithelial cells. J. Biomed. Sci 21, 49 10.1186/1423-0127-21-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sheehan JK, Howard M, Richardson PS, Longwill T and Thornton DJ (1999) Physical characterization of a low-charge glycoform of the MUC5B mucin comprising the gel-phase of an asthmatic respiratory mucous plug. Biochem. J 338(Pt 2), 507–513 10.1042/bj3380507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sheehan JK, Brazeau C, Kutay S, Pigeon H, Kirkham S, Howard M et al. (2000) Physical characterization of the MUC5AC mucin: a highly oligomeric glycoprotein whether isolated from cell culture or in vivo from respiratory mucous secretions. Biochem. J 347(Pt 1), 37–44 10.1042/bj3470037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bäckström M, Ambort D, Thomsson E, Johansson ME and Hansson GC (2013) Increased understanding of the biochemistry and biosynthesis of MUC2 and other gel-forming mucins through the recombinant expression of their protein domains. Mol. Biotechnol 54, 250–256 10.1007/s12033-012-9562-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wickström C and Carlstedt I (2001) N-terminal cleavage of the salivary MUC5B mucin. Analogy with the Van Willebrand propolypeptide? J. Biol. Chem 276, 47116–47121 10.1074/jbc.M106593200 [DOI] [PubMed] [Google Scholar]
- 86.Raynal BD, Hardingham TE, Sheehan JK and Thornton DJ (2003) Calcium-dependent protein interactions in MUC5B provide reversible cross-links in salivary mucus. J. Biol. Chem 278, 28703–28710 10.1074/jbc.M304632200 [DOI] [PubMed] [Google Scholar]
- 87.Rose MC and Voynow JA (2006) Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev 86, 245–278 10.1152/physrev.00010.2005 [DOI] [PubMed] [Google Scholar]
- 88.Ambort D, van der Post S, Johansson ME, Mackenzie J, Thomsson E, Krengel U et al. (2011) Function of the CysD domain of the gel-forming MUC2 mucin. Biochem. J 436, 61–70 10.1042/BJ20102066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gouyer V, Dubuquoy L, Robbe-Masselot C, Neut C, Singer E, Plet S et al. (2015) Delivery of a mucin domain enriched in cysteine residues strengthens the intestinal mucous barrier. Sci. Rep 5, 9577 10.1038/srep09577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo X, Pace RG, Stonebraker JR, Commander CW, Dang AT, Drumm ML et al. (2011) Mucin variable number tandem repeat polymorphisms and severity of cystic fibrosis lung disease: significant association with MUC5AC. PLoS ONE 6, e25452 10.1371/journal.pone.0025452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo X, Zheng S, Dang H, Pace RG, Stonebraker JR, Jones CD et al. (2014) Genome reference and sequence variation in the large repetitive central exon of human MUC5AC. Am. J. Respir. Cell Mol. Biol 50, 223–232 10.1165/rcmb.2013-0235OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lakshmanan I, Ponnusamy MP, Macha MA, Haridas D, Majhi PD, Kaur S et al. (2015) Mucins in lung cancer: diagnostic, prognostic, and therapeutic implications. J. Thorac. Oncol 10, 19–27 10.1097/JTO.0000000000000404 [DOI] [PubMed] [Google Scholar]
- 93.Lang T, Hansson GC and Samuelsson T (2007) Gel-forming mucins appeared early in metazoan evolution. Proc. Natl Acad. Sci. U.S.A 104, 16209–16214 10.1073/pnas.0705984104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leal J, Smyth HDC and Ghosh D (2017) Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm 532, 555–572 10.1016/j.ijpharm.2017.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fowler J, Vinall L and Swallow D (2001) Polymorphism of the human mucgenes. Front. Biosci 6, D1207–D1215 10.2741/Fowler [DOI] [PubMed] [Google Scholar]
- 96.Asker N, Axelsson MA, Olofsson S-O and Hansson GC (1998) Human MUC5AC mucin dimerizes in the rough endoplasmic reticulum, similarly to the MUC2 mucin. Biochem. J 335(Pt 2), 381–387 10.1042/bj3350381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park S-W, Zhen G, Verhaeghe C, Nakagami Y, Nguyenvu LT, Barczak AJ et al. (2009) The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc. Natl Acad. Sci. U.S.A 106, 6950–6955 10.1073/pnas.0808722106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schroeder BW, Verhaeghe C, Park S-W, Nguyenvu LT, Huang X, Zhen G et al. (2012) AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am. J. Respir. Cell Mol. Biol 47, 178–185 10.1165/rcmb.2011-0421OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bergström JH, Berg KA, Rodríguez-Piñeiro AM, Stecher B, Johansson ME and Hansson GC (2014) AGR2, an endoplasmic reticulum protein, is secreted into the gastrointestinal mucus. PLoS ONE 9, e104186 10.1371/journal.pone.0104186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martino MB, Jones L, Brighton B, Ehre C, Abdulah L, Davis CW et al. (2013) The ER stress transducer IRE1β is required for airway epithelial mucin production. Mucosal Immunol. 6, 639–654 10.1038/mi.2012.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ridley C, Kirkham S, Williamson SJ, Davis CW, Woodman P and Thornton DJ (2016) Biosynthesis of the polymeric gel-forming mucin MUC5B. Am. J. Physiol. Lung Cell. Mol. Physiol 310, L993–L1002 10.1152/ajplung.00046.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sheehan JK, Kirkham S, Howard M, Woodman P, Kutay S, Brazeau C et al. (2004) Identification of molecular intermediates in the assembly pathway of the MUC5AC mucin. J. Biol. Chem 279, 15698–15705 10.1074/jbc.M313241200 [DOI] [PubMed] [Google Scholar]
- 103.Trillo-Muyo S, Nilsson HE, Recktenwald CV, Ermund A, Ridley C, Meiss LN et al. (2018) Granule-stored MUC5B mucins are packed by the non-covalent formation of N-terminal head-to-head tetramers. J. Biol. Chem 293, 5746–5754 10.1074/jbc.RA117.001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ridley C, Kouvatsos N, Raynal BD, Howard M, Collins RF, Desseyn J-L et al. (2014) Assembly of the respiratory mucin MUC5B: a new model for a gel-forming mucin. J. Biol. Chem 289, 16409–16420 10.1074/jbc.M114.566679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Majima Y, Harada T, Shimizu T, Takeuchi K, Sakakura Y, Yasuoka S et al. (1999) Effect of biochemical components on rheologic properties of nasal mucus in chronic sinusitis. Am. J. Respir. Crit. Care Med 160, 421–426 10.1164/ajrccm.160.2.9805117 [DOI] [PubMed] [Google Scholar]
- 106.Esther CR J, Hill DB, Button B, Shi S, Jania C, Duncan EA et al. (2017) Sialic acid-to-urea ratio as a measure of airway surface hydration. Am. J. Physiol. Lung Cell. Mol. Physiol 312, L398–L404 10.1152/ajplung.00398.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Revoredo L, Wang S, Bennett EP, Clausen H, Moremen KW, Jarvis DL et al. (2016) Mucin-type O-glycosylation is controlled by short- and long-range glycopeptide substrate recognition that varies among members of the polypeptide GalNAc transferase family. Glycobiology 26, 360–376 10.1093/glycob/cwv108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gerken TA, Jamison O, Perrine CL, Collette JC, Moinova H, Ravi L et al. (2011) Emerging paradigms for the initiation of mucin-type protein O-glycosylation by the polypeptide GalNAc transferase family of glycosyltransferases. J. Biol. Chem 286, 14493–14507 10.1074/jbc.M111.218701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA and Tabak LA (2012) Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology 22, 736–756 10.1093/glycob/cwr182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gerken TA, Raman J, Fritz TA and Jamison O (2006) Identification of common and unique peptide substrate preferences for the UDP-GalNAc: polypeptide α-N-acetylgalactosaminyltransferases T1 and T2 derived from oriented random peptide substrates. J. Biol. Chem 281, 32403–32416 10.1074/jbc.M605149200 [DOI] [PubMed] [Google Scholar]
- 111.Gerken TA, Zhang J, Levine J and Elhammer A (2002) Mucin core O-glycosylation is modulated by neighboring residue glycosylation status. Kinetic modeling of the site-specific glycosylation of the apo-porcine submaxillary mucin tandem repeat by UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases T1 and T2. J. Biol. Chem 277, 49850–49862 10.1074/jbc.M205851200 [DOI] [PubMed] [Google Scholar]
- 112.Lo-Guidice JM, Wieruszeski JM, Lemoine J, Verbert A, Roussel P and Lamblin G (1994) Sialylation and sulfation of the carbohydrate chains in respiratory mucins from a patient with cystic fibrosis. J. Biol. Chem 269, 18794–18813 [PubMed] [Google Scholar]
- 113.Kiwamoto T, Katoh T, Evans CM, Janssen WJ, Brummet ME, Hudson SA et al. (2015) Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J. Allergy Clin. Immunol 135, 1329–1340.e1-9 10.1016/j.jaci.2014.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ma B, Simala-Grant JL and Taylor DE (2006) Fucosylation in prokaryotes and eukaryotes. Glycobiology 16, 158R–184R 10.1093/glycob/cwl040 [DOI] [PubMed] [Google Scholar]
- 115.Degroote S, Ducourouble M-P, Roussel P and Lamblin G (1999) Sequential biosynthesis of sulfated and/or sialylated Lewis x determinants by transferases of the human bronchial mucosa. Glycobiology 9, 1199–1211 10.1093/glycob/9.11.1199 [DOI] [PubMed] [Google Scholar]
- 116.Brockhausen I (2003) Sulphotransferases acting on mucin-type oligosaccharides. Biochem. Soc. Trans 31, 318–325 10.1042/bst0310318 [DOI] [PubMed] [Google Scholar]
- 117.Groux-Degroote S, Krzewinski-Recchi M-A, Cazet A, Vincent A, Lehoux S, Lafitte J-J et al. (2008) IL-6 and IL-8 increase the expression of glycosyltransferases and sulfotransferases involved in the biosynthesis of sialylated and/or sulfated Lewisx epitopes in the human bronchial mucosa. Biochem. J 410, 213–223 10.1042/BJ20070958 [DOI] [PubMed] [Google Scholar]
- 118.Shogren R, Gerken TA and Jentoft N (1989) Role of glycosylation on the conformation and chain dimensions of O-linked glycoproteins: light-scattering studies of ovine submaxillary mucin. Biochemistry 28, 5525–5536 10.1021/bi00439a029 [DOI] [PubMed] [Google Scholar]
- 119.Cone RA (2009) Barrier properties of mucus. Adv. Drug. Deliv. Rev 61, 75–85 10.1016/j.addr.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 120.Hofsteenge J, Muller DR, de Beer T, Loffler A, Richter WJ and Vliegenthart JF (1994) New type of linkage between a carbohydrate and a protein: C-glycosylation of a specific tryptophan residue in human RNase Us. Biochemistry 33, 13524–13530 10.1021/bi00250a003 [DOI] [PubMed] [Google Scholar]
- 121.Perez-Vilar J, Randell SH and Boucher RC (2004) C-Mannosylation of MUC5AC and MUC5B Cys subdomains. Glycobiology 14, 325–337 10.1093/glycob/cwh041 [DOI] [PubMed] [Google Scholar]
- 122.Bradway SD, Bergey EJ, Scannapieco FA, Ramasubbu N, Zawacki S and Levine MJ (1992) Formation of salivary-mucosal pellicle: the role of transglutaminase. Biochem. J 284(Pt 2), 557–564 10.1042/bj2840557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Recktenwald CV and Hansson GC (2016) The reduction-insensitive bonds of the MUC2 mucin are isopeptide bonds. J. Biol. Chem 291, 13580–13590 10.1074/jbc.M116.726406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim DY, Park BS, Hong GU, Lee BJ, Park JW, Kim SY et al. (2011) Anti-inflammatory effects of the R2 peptide, an inhibitor of transglutaminase 2, in a mouse model of allergic asthma, induced by ovalbumin. Br. J. Pharmacol 162, 210–225 10.1111/j.1476-5381.2010.01033.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Corrales RM, de Paiva CS, Li D-Q, Farley WJ, Henriksson JT, Bergmanson JP et al. (2011) Entrapment of conjunctival goblet cells by desiccation-induced cornification. Invest. Ophthalmol. Vis. Sci 52, 3492–3499 10.1167/iovs.10-5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ambort D, Johansson ME, Gustafsson JK, Nilsson HE, Ermund A, Johansson BR et al. (2012) Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc. Natl Acad. Sci. U.S.A 109, 5645–5650 10.1073/pnas.1120269109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Davis CW, Dowell ML, Lethem M and Van Scott M (1992) Goblet cell degranulation in isolated canine tracheal epithelium: response to exogenous ATP, ADP, and adenosine. Am. J. Physiol 262(5 Pt 1), C1313–C1323 10.1152/ajpcell.1992.262.5.C1313 [DOI] [PubMed] [Google Scholar]
- 128.Adler KB, Tuvim MJ and Dickey BF (2013) Regulated mucin secretion from airway epithelial cells. Front. Endocrinol 4, 129 10.3389/fendo.2013.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu Y, Abdullah LH, Doyle SP, Nguyen K, Ribeiro CM, Vasquez PA et al. (2015) Baseline goblet cell mucin secretion in the airways exceeds stimulated secretion over extended time periods, and is sensitive to shear stress and intracellular mucin stores. PLoS ONE 10, e0127267 10.1371/journal.pone.0127267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mantle M, Stewart G, Zayas G and King M (1990) The disulphide-bond content and rheological properties of intestinal mucins from normal subjects and patients with cystic fibrosis. Biochem. J 266, 597–604 [PMC free article] [PubMed] [Google Scholar]
- 131.Schulz BL, Sloane AJ, Robinson LJ, Prasad SS, Lindner RA, Robinson M et al. (2007) Glycosylation of sputum mucins is altered in cystic fibrosis patients. Glycobiology 17, 698–712 10.1093/glycob/cwm036 [DOI] [PubMed] [Google Scholar]
- 132.Venkatakrishnan V, Thaysen-Andersen M, Chen SC, Nevalainen H and Packer NH (2015) Cystic fibrosis and bacterial colonization define the sputum N-glycosylation phenotype. Glycobiology 25, 88–100 10.1093/glycob/cwu092 [DOI] [PubMed] [Google Scholar]
- 133.Tachibana Y, Matsubara N, Nakajima F, Tsuda T, Tsuda S, Monde K et al. (2002) Efficient and versatile synthesis of mucin-like glycoprotein mimics. Tetrahedron 58, 10213–10224 10.1016/S0040-4020(02)01359-5 [DOI] [Google Scholar]
- 134.Krannig K-S and Schlaad H (2014) Emerging bioinspired polymers: glycopolypeptides. Soft Matter 10, 4228–4235 10.1039/c4sm00352g [DOI] [PubMed] [Google Scholar]
- 135.Song Z, Han Z, Lv S, Chen C, Chen L, Yin L et al. (2017) Synthetic polypeptides: from polymer design to supramolecular assembly and biomedical application. Chem. Soc. Rev 46, 6570–6599 10.1039/C7CS00460E [DOI] [PubMed] [Google Scholar]
- 136.Lai SK, Wang Y-Y, Hida K, Cone R and Hanes J (2010) Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc. Natl Acad. Sci. U.S.A 107, 598–603 10.1073/pnas.0911748107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jin C, Kenny DT, Skoog EC, Padra M, Adamczyk B, Vitizeva V et al. (2017) Structural diversity of human gastric mucin glycans. Mol. Cell. Proteomics 16, 743–758 10.1074/mcp.M117.067983 [DOI] [PubMed] [Google Scholar]
- 138.Skoog EC, Padra M, Åberg A, Gideonsson P, Obi I, Quintana-Hayashi MP et al. (2017) Baba dependent binding of Helicobacter pylori to human gastric mucins cause aggregation that inhibits proliferation and is regulated via ArsS. Sci. Rep 7, 40656 10.1038/srep40656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L et al. (2011) Muc5ac: a critical component mediating the rejection of enteric nematodes. J. Exp. Med 208, 893–900 10.1084/jem.20102057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hasnain SZ, Wang H, Ghia JE, Haq N, Deng Y, Velcich A et al. (2010) Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology 138, 1763–1771 10.1053/j.gastro.2010.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA et al. (2012) Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483, 345–349 10.1038/nature10863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Knoop KA, McDonald KG, McCrate S, McDole JR and Newberry RD (2015) Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol. 8, 198–210 10.1038/mi.2014.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K et al. (2013) Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342, 447–453 10.1126/science.1237910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Janssen WJ, Stefanski AL, Bochner BS and Evans CM (2016) Control of lung defence by mucins and macrophages: ancient defence mechanisms with modern functions. Eur. Respir. J 48, 1201–1214 10.1183/13993003.00120-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]


