Abstract
Memory, one of the most vital aspects of the human brain, is necessary for the effective survival of an individual. ‘Memory’ can be defined in various ways but in an overall view, memory is the retention of the information that the brain grasps. Different factors are responsible for the disbalance in the brain’s hippocampus region and the acetylcholine level, which masters the memory and cognitive functions. Plants are a source of pharmacologically potent drug molecules of high efficacy. Recently herbal medicine has evolved rapidly, gaining great acceptance worldwide due to their natural origin and fewer side effects. In this review, the authors have discussed the mechanisms and pharmacological action of herbal bioactive compounds to boost memory. Moreover, this review presents an update of different herbs and natural products that could act as memory enhancers and how they can be potentially utilized in the near future for the treatment of severe brain disorders. In addition, the authors also discuss the differences in biological activity of the same herb and emphasize the requirement for a higher standardization in cultivation methods and plant processing. The demand for further studies evaluating the interactions of herbal drugs is mentioned.
Keywords: Cognition, Memory impairment, Nootropics, Memory enhancers, Herbal medicines, Phytochemicals
1. Introduction
1.1. Memory: Brief background
The human brain is the most complex organ in the body. One of its most impressive aspects is the ability to retain information, what is called memory (Christophel et al., 2017, Postle, 2016). Before this review work, we have to know the definition of what memory is. ‘Memory’ perhaps can be defined in various ways. An overall standard definition is: “Memory is the ability of an individual to record sensory stimuli, events, pieces of information etc. and retain them over short or long periods and recall the same whenever needed at a later date” (Zlotnik and Vansintjan, 2019). In short, memory is one of the most vital aspects for effective survival of human beings (Shiksharthi et al., 2011). But when we discuss memory, it is not only the retention of information, along with it we also consider some associated terms like- cognition, intelligence, attention, concentration, quality of life (QOL) etc. Intelligence is according to Ayurveda the combination of three capabilities of mind:
-
•
Acquisition: Capacity to grasp any information and to analyze it.
-
•
Retention: Retain the information inside the brain.
-
•
Recollection: Recall the information later (Rathee et al., 2008).
Based on the different mechanisms and types, memory can be classified into 3 types (Camina and Güell, 2017):
-
i)
Short-term memory (STM)–rapidly formed memory that is retained for a short time (minutes to hours).
-
ii)
Long-term memory (LTM)–a memory that is retained for long periods, e.g.-from hours to days, weeks, months, even years. (Rho et al., 2005).
-
iii)
Sensory Memory or Iconic Memory-the potential to remember temporarily the huge amounts of information that people experience every day
The up mentioned ‘retention’ and ‘recollection’ deal with STM and LTM respectively (Rathee et al., 2008).
1.2. Storing of memory inside the brain
When we are discussing memory, we have to know the mechanism by which various kinds of information are stored inside our brains. There is a region in our brain, named ‘Hippocampus’, located in the medial temporal lobe of the brain. This hippocampus is the center of all these memory and cognitive functions. At first, our brain cells grasp whatever we see, hear or do; then the hippocampus decides whether the information we grasped is important to store or not; if hippocampus decides to store the information, it retains inside our brains and we can memorize it, otherwise, the information gets deleted from our brain automatically (Shiksharthi et al., 2011, Kandel et al., 2014). This mechanism involves the interaction between various neurotransmitters in the brain (Shiksharthi et al., 2011, Sheffler and Pillarisetty, 2019). Acetylcholine (ACh) is the most important neurotransmitter involved in memory and cognitive functions apart from several neurodegenerative pathogenicities (Parle and Vasudevan, 2007, Akaike et al., 2018). One important enzyme involved in this mechanism is Acetylcholinesterase (AChE). Estimation of this enzyme’s activity is an important parameter to assess the central cholinergic function. Acetylcholinesterase (AChE) modulates proper levels of acetylcholine by breaking ACh into Choline (the building block of ACh). However, the excessive activity of AChE results in the deficiency of ACh, leading to memory impairment (Rathee et al., 2008, Shiksharthi et al., 2011).
1.3. Memory impairment
Poor memory, low retention power, difficulty to recall, lack of concentration, weak analyzing ability-these are very much common problems of the modern world. Besides some of the conditions, such as stress, ageing and emotions may lead to memory impairment, amnesia, dementia etc., and sometimes to some serious threats like Alzheimer’s disease (AD), Schizophrenia etc. (Gur and Gur, 2013, Hildebrandt, 2019). In AD, the function of the hippocampus is destroyed, so that the brain cannot manage to store different kinds of information, especially the new ones. Besides, the neurons start to get degenerated. This disease affects mostly the elderly population, ageing 65 years or more (Wortmann, 2015, Winblad et al., 2016, Alzheimer's Association, 2016). According to cholinergic hypothesis, memory impairs due to deficiency in the cholinergic function inside the brain. Cognitive dysfunction is correlated with this impaired cholinergic function (Dumas and Newhouse, 2011; Bohnen et al., 2018).
1.4. Memory enhancers
Functional foods, supplements and drugs that are able to improve memory, cognition intelligence and other mental functions are known as memory and cognitive enhancers (Lynch et al., 2014, Morè et al., 2020). There is another term that can be used to describe memory and cognitive enhancers: ‘Nootropics’ (Pieramico et al., 2014; Onaolapo et al., 2019).
Nootropics. The word ‘nootropic’ (Greek: noos-mind, tropein-to bend/turn or montor) was first coined in 1972 by Dr Corneliu E Giurgea. Nootropics are referred to as ‘smart drugs’ that act upon our brain cells. A nootropic is extremely non-toxic neuroprotective substance and can be used as memory and cognitive enhancer and in AD treatment (Colucci et al., 2012; Chaudhari et al., 2017). The functions of nootropics are: (a) Improving Acetylcholine level in the brain, (b) Improving O2 supply to the brain, (c) Supplying neurochemicals (e.g.-neurotransmitters, enzymes, hormones) to the brain (Jeon, 2015, Suliman et al., 2016, Crespo-Bujosa and Suárez Rodríguez, 2019). To improve memory and mood several nootropic agents are generally used such as Aniracetam, Oxiracetam, Pramiracetam, Piracetam and Choline esterase inhibitors like Donepezil, but the side effects of these agents (Talih and Ajaltouni, 2015, Zaami et al., 2020) have made their applicability limited.
1.5. Herbal remedies for impaired memory and cognition: Mechanisms and pharmacological actions
Herbal remedies are traditionally used all over the world to enhance poor memory and related ailments (Giampieri et al., 2014, Liao and Lin, 2012; Banerjee et al., 2021; Gregory et al., 2021). Several medicinal plants and their extracts have shown nootropic properties or memory-enhancing properties by suitable character of their medicinal components (Aguiar and Borowski, 2013, Onaolapo et al., 2019; Banerjee et al., 2021; Tandon et al., 2021). The increasing demand for herbal remedies worldwide is because herbal compounds have lesser or no side effects than any other chemical compound (Anand et al., 2019). Our very own Indian Ayurveda possesses a treasury of such medicinal plants that enhance memory, cognition and intelligence. These Ayurvedic herbs are now popular all over the world (Anand et al., 2019). Not only Indian Ayurveda, the Traditional Chinese Medicine (TCM), Japanese, Korean, African, American and European medicines also come with a huge number of medicinal plants helping in reversing memory impairment. The herbs acting upon brain cells are termed ‘nootropic herbs/drugs’ and their isolated compounds are called as ‘smart drugs’ or ‘cognitive enhancers’ or ‘brain booster’ (Hildt, 2013, Frati et al., 2015). According to Ayurveda, herbs that promote intelligence is known as ‘Medhya herbs/ Rasayana’ (Kulkarni et al., 2012, Reena et al., 2013) and includes 10 herbal drugs namely Jatamansi, Ashwagandha, Vacha, Jyotishmati, Shankhapushpi, Amalaki, Yashtimadhu, Kavach Beej, Bramhi, and Mandukparni (Lele, 2010). All these medicinal herbs increase the neurotransmitter level (especially acetylcholine) in the brain by inhibiting excess AChE activity and also improve blood circulation inside the brain thus providing sufficient supply of O2 to the brain cells (Colovic et al., 2013, Suliman et al., 2016). Some home remedies against poor memory are found in commonly known food plants, which we can have as oils and vegetables (Fernando et al., 2015, Hardman et al., 2016, Molz and Schröder, 2017). The World Health Organization (WHO) states that at present date 80% of the world populations are using herbal remedies for the improvement of health (Srivastava et al., 2019).
1.6. History of memory enhancers in Ayurveda and TCM
Ayurveda, the science of life is a long traditional alternative medicine system from the Indian subcontinent. This natural science is based on the fundamental laws of nature with therapeutic approach for treatment of innumerous human diseases. Aging represents a systematic involution of the human body and the brain is considered as the most vulnerable organ to this process. Therefore, to avoid this damage, the Rasayana therapy, a rejuvenation branch of Ayurvedic medicine was established. Various natural drugs that act as brain tonic, promote health of the brain, lead to the alleviation of behavioral disorders or improve memory impairment are utilized in Rasayana approach. Various memory enhancing and rejuvenating plants are used in Rasayana therapies, including: Acorus calamus, Bacopa monniera, Clitoria ternatea, Nardostachys jatamansi, Terminalia chebula and many others (Singh 2013).
Traditional Chinese medicine (TCM) is based on the balance between health and disease. According to theory of TCM, healthy body is the body in the balance, on the other hand disharmony is not the cause of disease. The disease is caused by life style, influence of pathogens and various negative effects. However, the balance can be restored by herbal treatment. For over thousands of years, Chinese people have accumulated experiences in treatment, diagnosis, and disease prevention to create a whole theoretical system of medicine therapy. Herbal medicines with memory enhancing properties are also encompassed in the TCM. Between the plants used in TCM for memory improvement belong: Huperzia serrata, Ginkgo biloba, Panax ginseng, Camellia sinensis and other (Yan et al., 2007). These and above-mentioned plants will be discussed in following sections.
2. List of herbs having memory and cognition-enhancing properties
2.1. Family Acoraceae
Acorus calamus L. / Sweet flag is a medicinal valuable semi-aquatic herb that grows throughout India, Central Asia, Siberia, Eastern Europe, Southern Russia, and could be found in gardens too. This perennial plant is not frost-tender and grows to a height of 1 m. It shows numerous therapeutic properties such as antiproliferative, antiulcerative, antibacterial, antioxidant, insecticidal, immunosuppressive, antifungal etc. (Pandit et al., 2011, Kumar et al., 2012a, Kumar et al., 2012b, Parki et al., 2017). A plethora of bioactive chemical constituents like β-asarone (40) (AChE inhibitor) (Mukherjee et al., 2007a), phenylpropanoid, saponins, lectins, flavonoids, sesquiterpene, alkaloids, α-asarone (67), phenols, monoterpene, and quinones, mucilages etc. are exhibited by this plant (Joshi, 2016). The rhizome of this plant ‘Acori Calami Rhizoma’, is mainly used to enhance/improve memory (May et al., 2016). Sweet flag is a very essential brain tonic as it shows a very short time results. Articles claiming this herb to cure AD are out (Nandakumar et al., 2013). Sweet flag strengthens the nervous system and increases the overall memory of the person. This is prescribed to patients who have epilepsy, amnesia, hysteria, insomnia, neurosis, remittent fevers, and melancholia (Huang et al., 2013, Sharma et al., 2014). The sweet flag contains essential oil in which 67 and 40 are present as the main components, which harbour anti-inflammatory properties to downregulate inflammatory cytokines (Shin et al., 2014, Lim et al., 2014).
2.2. Family Apiaceae
Centella asiatica (L.) Urban / Gotu kola (also known as Thankuni) is native to the wetlands in Asia and is widely scattered across the subtropical regions tropical geographical regions of India, Indonesia, Madagascar, China, Sri Lanka, and Nepal. This evergreen perennial plant is self-fertile, frost-tender, and grows to height of 20 cm. It is commonly used as a green leafy vegetable and as a medicinal herb in AM, Unani Medicine (UM), Traditional African Medicine (TAM) and TCM (Jahan et al., 2012). It contains a wide array of phytochemicals, which include flavonoids, tannins, triterpenoids (Asiatic acid, asiaticoside, madecassic acid, and madecassoside), glycosides, essential oils, alkaloids, and volatile fatty acids (Das, 2011). It effective enhances the blood circulation in different body parts including the brain. Moreover, it helps to protect the brain from damage and improves concentration. The brain is more receptive to information and the memory is enhanced (Mannangatti and Naidu, 2016). The usage of this herb is documented over the centuries in Indian medicine system for the treatment and prevention of several diseases and health-related ailments including cancer, wound healing, dementia, diabetes, skin problems, ulcers, for regenerating the brain and nerve cells, for combating ageing, asthma, (Singh et al., 2010, Sabaragamuwa et al., 2018) (Fig. 1). The positive results of Centella asiatica on the general ability, power of concentration and behaviour of mentally retarded children has been found in pharmacological and clinical trials. It is proposed that Centella asiatica and Bacopa monnieri have the similar medicinal value. A nervine tonic for treatment of various brain diseases or syrup to increase the memory of children are prepared from the whole plant. C. asiatica also inhibits scopolamine-induces memory impairment through the inhibition of AChE enzyme activity (Orhan et al., 2013). An animal study on mice model has shown asiaticoside as a neuroprotective agent and this effect could be linked with asiaticoside's anti-inflammatory activity by inhibition of the overactivated p38 MAPK pathway (Chen et al. 2014). In middle cerebral artery occlusion (MCAO) rats, the ethanolic extract of C. asiatica has demonstrated to be enhanced neurobehavioral activity, educed infarction volume along with preserved brain neuroanatomy, increased the status of antioxidants and decreased the levels of free radicals (Tabassum et al., 2013). In vitro experiment of water extract of this herb preserved/protected human neuroblastoma MC65 cell line and SH-SY5Y cells from β-amyloid toxicity (Soumyanath et al., 2012). Recently, in vitro examination of C. asiatica leaf extract significantly suppressed α-synuclein aggregation and prevented oligomer formation of aggregates that suggested the therapeutic potential of C. asiatica against Parkinson’s disease (PD) (Ruben et al., 2014).
Fig. 1.
Hallmarks of nootropic herbs.
2.3. Family Aquifoliaceae
Ilex paraguariensis (A.) St.-Hil. / Yerba mate is plant typically growing and processing in South America, especially in northern Argentina, Paraguay, Uruguay and southern Brazil (Bracesco et al., 2011). This evergreen tree/shrub growing up to height of 15 m has simple obovate-oblong leaves, 4-membered small green-white flowers and small red drupe fruits with diameter of 4–6 mm. It is traditionally used for a preparation of the beverage called mate, which possesses high nutritional values. I. paraguariensis extract contains polyphenols (chlorogenic acids), tannins, xanthines (caffeine, theophylline and theobromine), flavonoids (quercetin (80), kaempferol), purine alkaloids (methyl xanthines), saponins, and vitamins (A, C, B1, B12, E) (Bracesco et al., 2011). It’s entry into the body exhibit neuroprotective, diuretic, antimutagenic, anti-inflammatory, antioxidant, antidiabetic, anti-hyperglycemic, antidepressant, anticonvulsant, hypocholesterolaemic, antifungal, anti-obesity effects (Fernandes et al., 2017). The memory-enhancing activity of mate tea leaves are reported on different models of memory and learning (Prediger et al., 2008). The worthwhile effects of mate tea used for the enhancement of cognition, short- and long-term memory in animals were reported. It also has properties to treat dementia. More recently, several groups of researchers have demonstrated that I. paraguariensis derivatives are abundant with bioactive compounds which when enters CNS and produces significant therapeutic indices(Cittadini et al., 2019). Leaves aqueous extract of this medicinal herb has been shown to potentially inhibit scopolamine-induced memory loss in male Swiss mice (Santos et al., 2015).
2.4. Family Araliaceae
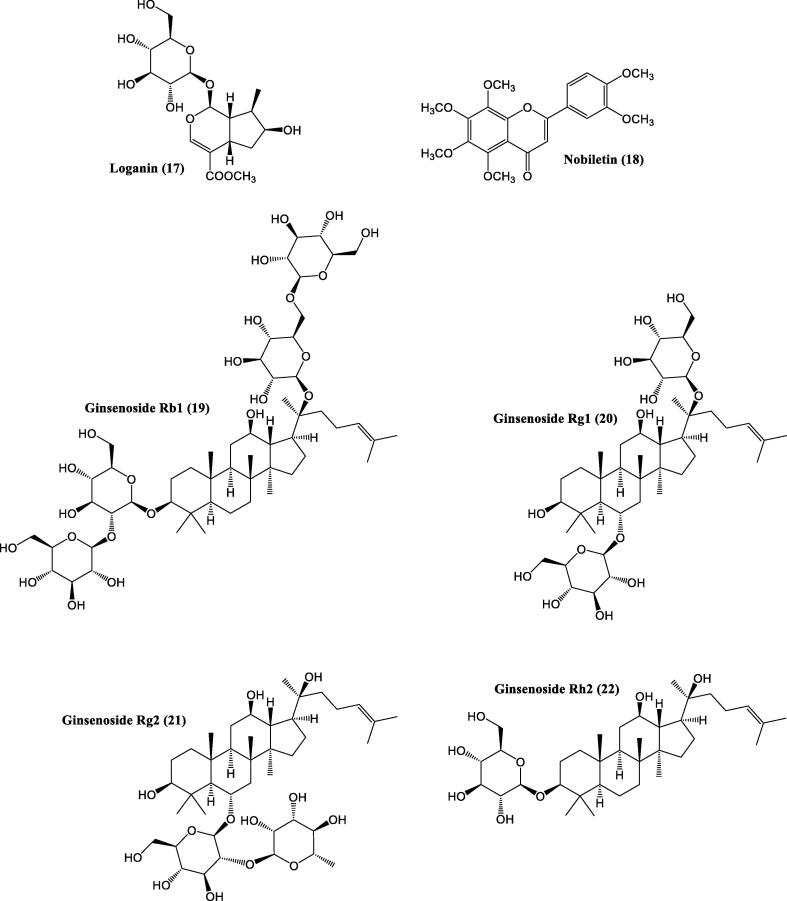
Panax ginseng C.A.Mey. / Ginseng grows only in the Northern Hemisphere, typically in cooler climates of North America and eastern Asia (northeast China, eastern Siberia, Korea and Bhutan). It is a perennial, hermaphrodite herb which grows to a height of 80 cm, has green-white small (3 mm) flowers and oval-shaped red drupe fruits, 6–7 mm long. This herb has a significant application in TCM for thousands of years. It is used mostly as a memory tonic and to improve memory and learning, especially in elderly people. It contains saponins that enhance memory in the scopolamine-induced learning impairment. It is proposed that the main active components in Ginseng roots, Ginsenosides (Rg1 (20), Rg2 (21), Rg3 (30), Rh2 (22) etc), induce in the central cholinergic nervous system the enhancement of choline uptake, which is important for learning and memory. In rats, the cyproheptadine-induced recognition deficits were improved by a component of ginseng saponin.
2.5. Family Asteraceae
Eclipta alba (L.) Hassk. / Bhringaraj grows commonly in moist places and called as a weed by the farmers all over the world. This annual herb growing to a height of 30–40 cm, has cylindrical roots and white flowers 6–8 mm in diameter. It is widely distributed in tropical areas worldwide e.g. in India, China, Bangladesh, Thailand, and Brazil. Wedelolactone, luteolin, ursolic acid, apigenin, eclalbasaponins, and oleanolic acid are some of the potential bioactive constituents of this plant. E. alba possesses multifunctional pharmacological properties and has proven beneficial effects in the treatment of various disorders including cancer, gastrointestinal disorders, malaria, fever, snake bite, cuts and wounds, skin disorders, arthritis, respiratory tract disorders (like asthma), inflammation, graying of hair and hair loss, liver disorders (like jaundice), microbial diseases, and spleen development (Jahan et al., 2014). Bhringaraj is traditionally used for its memory enhancing quality, and thus it is not suprising that several studies were conducted for this purpose. Dose of 100 and 200 mg/kg of the Bhringaraj leaves aqueous extract was administered to rats. Then transfer latency (TL), which represents a measure of acquisition and retrirval learning was evaluated on an elevated plus-maze test. The spatial habitual learning revealed a relevant advancement in retrieval memory of tested rodents (Banji et al., 2007, Bhaskar and Chintamaneni, 2014). Notably, the antiepilepsy activity of E. alba phytoconstituents (wedelolactone, β-amyrin, and luteolin) have been widely reported by several groups of researchers (Shaikh et al., 2012, Shaikh et al., 2013).
2.6. Family Caprifoliaceae
Nardostachys jatamansi (D.Don) DC. / Jatamansi is well-reputed Medhya herb that has a place of respect in the Indian Ayurveda. This small perennial flowering herb grows to a height of 10–50 cm and has bell-shaped pink flowers. It commonly grows in the Himalayas of Nepal, China and India. Ayurveda says the roots of Nardostachys jatamansi have anti-ischemic, antioxidant, anticonvulsant and neuroprotective activities. The ethanolic extract of Jatamansi at a dose of 200 mg/kg b.w. significantly lead to the improvement in learning and memory power in young mice. Moreover, the scopolamine (0.4 mg/kg, i.p.) and diazepam (1 mg/kg, i.p.)-induced amnesia was considerably reversed. It also reversed the natural ageing-induced amnesia in mice. From these results, it can be said that Jatamansi might prove to be a useful remedy for the treatment of dementia in elderly persons.
2.7. Family Celastraceae
Celastrus paniculatus Willd. / Jyotishmati (also known as Kangani and Malkangni) belongs to the genus of woody, climbing shrubs. The stems are up to 10 m long and twine into the surrounding vegetation. Pale brown bark is covered with small elongated lenticles. The leaves are simple, broad and oval with toothed margins. Jyotishmati, one of the most widely explored medicinal plant and well known Medhya Rasayana’ (nervine tonic) in Ayurvedic medicine (AM) (Malik et al., 2017). It is distributed in various geographical locations including Southeast Asian countries like Thailand, Sri Lanka, Nepal, Japan, Myanmar, China, India, Malaysia, Taiwan, Indonesia, Vietnam and Australia (Mishra 2011) at elevations up to 10–18 m height. The seeds and seeds oil is the main part of this plant that contains a plethora of phytochemicals including triterpenoid pristimerin, sesquiterpeniod polyalcohols esters (celapnin, polyalcohol A–D, malkanguniol, malkangunin), polyalcohol (paniculatusdiol, malangunin, malkanguniol and malkanginnol), alkaloids celastrine, paniculatin, calapagine, celapanine, calapanigine, sesquiterpenes, sterols (β-amyrin and β-sitosterol), saturated fatty acids (palmitic, stearic and lignoceric acid), unsaturated fatty acids (oleic, linoleic and linolenic), phenolic triterpenoids (paniculatadiol, celastrol), lipids etc. (Katekhaye et al., 2011). They have a bitter taste, an unpleasant odour and are regularly used as neuroprotective properties, cognitive disorders, and to sharpen/enhancing the memory and boosting intelligence (Bhanumathy et al., 2010a, Bhanumathy et al., 2010b, Arora and Pandey-Rai, 2014). Whole plant extract of C. paniculatus has demonstrated anti-epileptic function (Atigari et al., 2012). The seeds and the seed oil are commonly used as a brain tonic, in many central nervous system (CNS) disorders and as neuro-protective agent (Bhagya et al., 2016, Malik et al., 2017). The seed oil improved learning and memory whereas the levels of dopamine, noradrenaline and serotonin were decreased in the brain of rats. The oil of this plant significantly decreases AChE activity (Bhagya et al., 2016, Malik et al., 2017). In addition to neurological benefits, different plant parts of C. paniculatus has potentially shown to exhibit potent activity in wound healing, hypolipidemic, antioxidant, analgesic, anti-inflammatory, stomach ulcers, dyspepsia (Palle et al., 2018).
2.8. Family Combretaceae
Terminalia chebula Retz. / Haritaki is a deciduous tree, which grows in Southeastern Asia (India, China, Sri Lanka, Malaysia and Vietnam). The tree grows to a height of 30 m, the leaves are oval with opposite or alternate arrangement. The yellow flowers occur in terminal spikes or short panicles and emit unpleasant odor. The ovoid fruits are yellow to orange-brown. The ripe fruit of Terminalia chebula is valued due its beneficial health effects including memory and intellect enhancement. Further, it is believed that consumption of the fruit is efficient to prolong life, to improve eyesight and can delay ageing. To achieve the desired effects, consumption of one ripe fruit every morning is recommended.
2.9. Family Convolvulaceae
Convolvulus pluricaulis Choisy / Shankhpushpi (also known as Bindweed) is a well-reputed Medhya herb commonly found in southern India and Burma. This perennial herb has 10–40 cm long stems and small (5 mm) blue color flowers. In ancient times, it was a prominent memory-improving drug, a psychostimulant and tranquiliser in traditional Indian medicine (Shethiya and Mishra, 2010). A brain tonic is prepared from the whole plant and is used for memory and intellect improvements. Its consumption also claimed to prevent memory loss. The plant contains diverse phytochemicals including β-sitosterol, vitamin C or ascorbic acid (23), silane, decanoic acid, carbohydrates, vitamin E, valeric acid or pentanoic acid, cinnamic acid, squalene, linoleic acid, phthalic acid, gums, proteins, mucilages, several alkaloids (tropane), flavonoids (Kaempferol), and glycosides etc. as active chemicals that bring about its diverse beneficial pharmacological effects (Agarwa et al., 2014). Several groups of researches have shown that bioactive compounds of this herb displayed anti-ulcerogenic, neuroprotective, antimicrobial (anti-bacterial, antiviral), antioxidant, antianxiety, nootropic, anxiolytic, antiobsessive, anticonvulsant, antithyroid, hepatoprotective, antidepressant, hypotensive, anxiolytic, ameliorative property (Ravichandra et al., 2013; Rachitha et al., 2018; Anupama et al., 2019). In a preclinical study, the ethanolic extract of Convolvulus pluricaulis (in two doses- 100 mg and 200 mg per kg b.w.) reversed scopolamine-induced amnesia in rats (Nahata et al., 2008). Clinical studies of its polyherbal formulation justified its potential for the ancient claim of brain tonic. The memory enhancing and learning behavior properties of Shankphapushpi have become increasingly popular worldwide in recent years. Most recently, an aqueous extract of C. pluricaulis has demonstrated to the downregulated τ protein level, enhances locomotor deficits and increases longevity in human microtubule-associated protein tau (hMAPτ) induced Drosophila AD model (Anupama et al., 2019).
2.10. Family Cornaceae
Cornus officinalis Siebold & Zucc. / Japanese cornel is a deciduous tree widely spread/occurs in China, Japan and Korea. The tree grows to a height of 10 m, has a brown-gray bark and ovate leaves are 5–12 cm long. The flowers are bright yellow on bare stems in late winter or early spring. Fruits are red edible berries about 1.5 cm long. This plant is usually used as a food or due to its medicinal properties (Ma et al., 2014). A plethora of phytoconstituents is exhibited by C. officinalis including anthocyanins, flavonoids (quercetin), and phenolic acids (trans-cinnamic acid, benzoic acid), triterpenoids, anthocyanin, carbohydrates (Rudrapaul et al., 2015). Furthermore, this herb is known to harbour neuroprotective, antitumor, antidiabetic, immunomodulatory, cardioprotective, anti-amnestic, antimicrobial, antioxidant, anti-inflammatory, renal and hepatic protective, antiosteoporotic and insecticidal property (Czerwińska and Melzig, 2018). Cornel iridoid glycoside (CIG) is the main component in this herb, which has the ability to promote neurogenesis and to improve neurological function after ischemia in rats. Iridoids of C. officinalis also protect hippocampal cells that have suffered from glutamate (Jeong et al., 2012). Further assays in rats proved, that CIG treatment significantly improved the memory deficits seen in Fimbria-fornix transaction (FFT). It also reduced the loss of neurone in the mouse hippocampus (Zhao et al., 2010a, Zhao et al., 2010b). Many polyphenolic compounds obtained from C. officinalis fruits, e.g. tellimagrandin I, tellimagrandin II and isoterchebin, are important regulators of certain enzymes and downregulate total enzymes implicated in the development of AD neurodegeneration (Bhakta et al., 2017). The documented neuroprotective and anti-amnestic property of C. officinalis is attributed because of antiozidant and radical scavenging effects (Cooper and Ma, 2017, Huang et al., 2018).
2.11. Family Fabaceae
Desmodium gangeticum (L.) DC. / Shalparni is widely used in Ayurvedic medicine. This small shrub grows to a height of 1.2 m. The oblong leaves are simple with an alternative arrangement. The purple-white flowers have a bilateral symmetry. D. gangeticum has traditionally been used as astringent, anthelmintic, diuretic, laxative, antipyretic, and in the treatment and prevention of neurodegenerative diseases like dementia and mental disorders (Ma et al., 2011). Significant bioactive phytochemicals of this plant include flavonoids, alkaloids, terpenoids, phenolic compounds, and steroids. Furthermore, D. gangeticum has been documented to display immunomodulatory, antidiabetic, antioxidant, antimicrobial, anti-writhing, anti-inflammatory, renal-protective, antiulcer, hepatoprotective, antileishmanial, wound healing, and cardio-protective properties (Bhattacharjee et al., 2013). In laca mice, the alkaloid-rich fraction of D. gangeticum greatly reversed the scopolamine-induced amnesia at the dosage of 50 mg/kg (Mahajan et al., 2015). Salparni in mice significantly improves learning and memory abilities and reverses the scopolamine or natural ageing induced amnesia. It also decreases acetylcholinesterase activity in brain. Hence, D. gangeticum bears notable potential to its medicinal application for memory improving as well for the treatment of dementia and AD.
Glycyrrhiza glabra L. / Licorice is a legume that is native to Southern Russia and Asia, Mediterranean region and is broadly planted throughout the Middle East and Europe. It is a perennial plant which grows to a height of 1.2 m. The herb has a compound leaves about 7–15 cm long, purple to pale-blue flowers long about 0.8–1.2 cm and produces oblong pods with several seeds. This herb is known worldwide for its various curative properties. G. glabra contains diverse phytochemicals, which are of high medicinal value including flavonoids (liquiritin, liquiritigenin, rhamnoliquirilin, isoliquiritin, etc.), triterpenes (glycyrrhizin or glycyrrhizic acid, glycyrrhetinic acid monoglucuronide), isoflavonoids (Dehydroglyasperin C), saponin, tannins, glycosides etc. (Batiha et al., 2020; Han et al., 2020). Various parts of this plant have been used in the treatment of various health-related disorders such as roots in diabetes, flatulence and Graves’ disease, stem in tuberculosis, and leaves in wounds. Furthermore, this perennial herb has been widely acknowledged to treat various pathological conditions including epilepsy, stomach ulcers, jaundice, respiratory disorders, fever, hyperdipsia, hemorrhagic diseases, rheumatism, sexual debility, paralysis, skin diseases etc. Licorice, a root extract of G. glabra is generally used as a brain tonic and a brain re-vitalizer. This plant is termed in Ayurveda as ‘Medhya dravya' and it was commonly utilized to improve memory and intellect. The assays on mice confirmed, that aqueous extract of liquorice at the dose of 150 mg/kg significantly boosts memory and learning skills. Furthermore, diazepam (1 mg/kg i.p.) and scopolamine (0.4 mg/kg i.p.) induced amnesia was significantly reduced by liquorice extracts. Liquiritigenin (LIQ), a bioactive compound of G. glabra root has shown to inhibit glutamate-induced hippocampal neuronal cell death by downregulating ROS production, Ca2+ influx and lipid peroxidation, protected mitochondria from stress and MAPKs phosphorylation (p38) thereby LIO is attributed as a strong neuroprotective agent and could be a possibility to become a potent drug for AD and PD (Yang et al., 2013).
2.12. Family Ginkoaceae
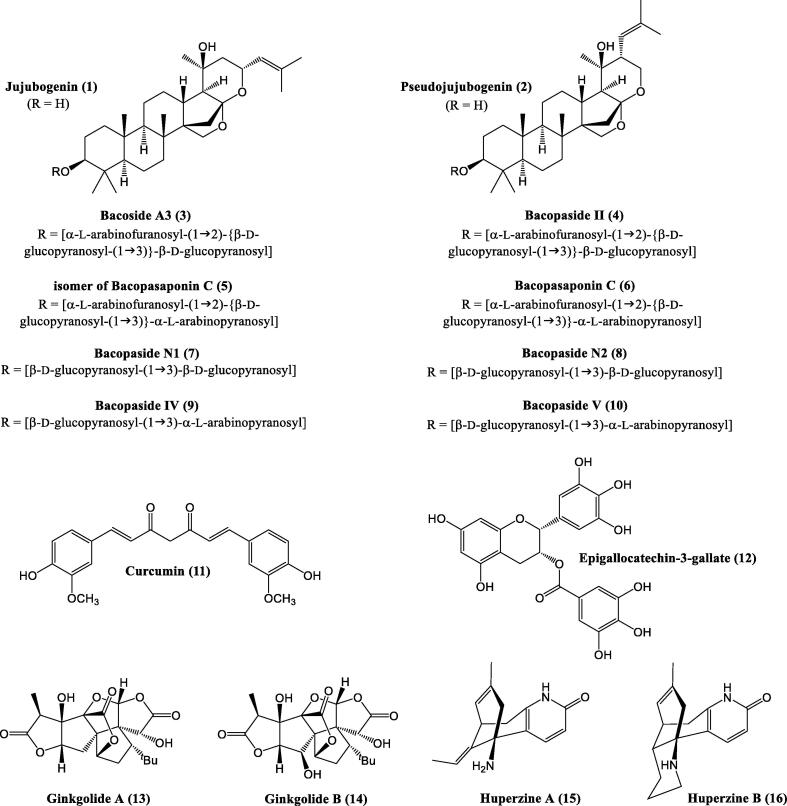
Ginkgo biloba L. / Ginkgo is a living fossil, native to China and Japan. It is a large deciduous tree growing up to a height of 35 m. The leaves are fan-shaped with two lobes, in antumn turn yellow and fall sometimes within a short period of time (1–15 days). Seeds are edible. EGb761, Ginkgo biloba leaves extract is famous globally for its beneficial pharmacological effect on neurotransmitter systems particularly in treating neurological disorders. EGb761 contain terpenoids (e.g., bilobalide, ginkgolides (28)) and ginkgolides A (13), B (14) (Fig. 2), C, J, and M, flavonoids (e.g. kaempferol, quercetin (80) and polymeric flavonoids) (Yuan et al., 2010). Among these, ginkgolides, a bioactive constituent of EGb761 has proven to interact with the cholinergic system and have neuroprotective and neural stem cells (NSCs) regenerative potential (Wang and Han, 2015, Ren et al., 2019). Ginkgo improves circulation of blood into the central nervous system and increases the distribution of nutrients and oxygen into the brain. It also eliminates free radicals in the body thereby improves memory and alertness. The ginkgo extract is prescribed for the treatment of amnesia and AD. Ginkgo is used as one of the important compounds in several herbal nerve tonics. Recently, this plant has demonstrated a positive effect on many neurological related health ailments including depression, psychosis, anxiety, and schizophrenia (Kumar et al., 2017). In clinical practice, Ginkgo biloba extract (GBE) has proven to treat cognitive disorders, memory impairment, AD and coronary heart disease and showed therapeutic effects at the biochemical and pharmacological levels (Vellas et al., 2012, Jahanshahi et al., 2012, Zhang et al., 2013). The extract is widely used to treat AD and cerebrovascular disease (CD) (Zhang et al., 2017). Notably, during cerebral ischemia (CI) bilobalide (BB) has proven to exhibit neuroprotective effects (Huang et al., 2012). Hence, From the molecular point of view, EGb761 display antioxidant activity and downregulating tau hyperphosphorylation in addition to the protection against Aβ-induced neurotoxicity and hence it could be a potential medication for treating AD.
Fig. 2.
Chemical structure of selected pro-cognitives and memory enhancers.
2.13. Family Hyperiaceae
Hypericum perforatum L. / St. John’s wort is native to subtropical and temperate geographical regions of Europe, western Asia, Middle-East India, northern Africa, Russia and China. This perennial self-fertile flowering plant grows up to a height of 1 m. It has woody stems near the base, the leaves are oblong, narrow, and about 1–2 cm long. The five petal flowers are about 2.5 cm wide and colored bright yellow. H. perforatum is approved for therapeutic intervention (Božin et al., 2013) and contains diverse phytochemicals such as flavonoids derivatives (rutin, amentoflavone, hyperoside, quercetin (80), biapigenin, isoquercitrin, kaempferol, etc.), phenolic compounds, acylated phloroglucinols (hyperforin and derivatives), and naphthodiantrones (hypericin and derivatives) (Russo et al., 2014; Oliveira et al., 2016). These phytochemicals confer an array of pharmacological properties such as antiviral, neuroprotective, antifungal, anti-ischemic, wound-healing, antibacterial, antioxidant, and antideprssant activity. In SH-SY5Y neuroblastoma cells, rutin prevents accumulation and cytotoxicity of β-amyloids, mitigates mitochondrial damage, ROS, oxidative stress, and reduces nitric oxide and proinflammatory cytokines production (Wang et al., 2012). It is an excellent tonic for brain damage and ameliorates spatial memory and learning behaviour against Aβ25–35-induced toxicity (Liu et al., 2013). It helps to regenerate the nervous system, specifically the myelin sheath that surrounds nerves. Possibly, the usage of Hypericum extract could be more beneficial for the treatment of depression associated with dementia than other antidepressants that may cause sedation.
2.14. Family Iridaceae
Crocus sativus L. / Saffron dried red–orange stigma (the only usable part of this plant) is well-known as a fruit colouring, flavoring agent, and exhibit beneficial biological/pharmacological effects. It is a flowering perennial plant which forms small brown compact corms that are flat on the base. The plant grows to a height of 10–25 cm. The purple flowers do not close during the night. The flowers consist of six petals, three stramens, and three stigmas. The red–orange stigmas are 2.5–3 cm long and using as a noble spice. Saffron is the world’s most expensive regularly used dietary spice (Moore et al., 2012). It is a native herb in Southwest Asia (Iran). The main bioactive chemical constituents are α-crocin (47) (C44H64O24), crocetin (70) (C20H24O4), picrocrocin (C16H26O7), safranal (81) (C10H14O), water-soluble carotenoids (crocins), vitamins (thiamine and riboflavin), carbohydrates, low concentration of monoterpene aldehydes and its glucosides (81 and picrocrocin), lipids, polypeptides, proteins, flavonoids (quercetin (80), kaempferol, isorhamnetin and anthocyanins e.g. petunidin, malvidin, and delphinidin), starch, amino acids, gums, and minerals (Goupy et al., 2013, Zeka et al., 2015). For centuries, saffron has been extensively used as anxiolytic activity, antitussive, antibacterial, antiinflammatery, hypolipidemic, antifungal, anti-diabetic, anticonvulsant, antityrosinase, anti-neuropathic pain, anticancer, antitussive, antioxidant, antiseptic, antidepressant, antinociceptive, anti-cancer, anti-tumor, liver and spleen enlargement prevention, lumbar pain remedy, chemoprotective, renal ischemia–reperfusion prevention, anti-genotoxic, an aphrodisiac, antispasmodic, tension relieving, expectorant, cardiovascular protective, antidote against poisoning, dysentery, cataract, measles, pre-eclampsia treatment, withdrawal syndrome inhibition, wound healing, and abscesses (Hosseinzadeh, 2014, Moshiri et al., 2015, Ghasemi et al., 2015). In addition to its historical value as a food additive, several studies recently indicated its potential use as neuroprotective (anti-Alzheimer, anti-Parkinson), learning and memory enhancer, anxiolytic and hypnotic, against cerebral ischemia, memory deficits and brain damage, against morphine dependency mitigation (Hosseinzadeh et al., 2012, Tashakori-Sabzevar et al., 2013). Moreover, enhancing disease in adjuvant-induced arthritis, minimizing oxidative damage to the kidney (Zamani et al., 2015). These compounds significantly reverse scopolamine-induced amnesia. A study reveals that the treatment of mild-to-moderate AD with Saffron is safe and effective in adults ageing 55 years or more (Akhondzadeh et al., 2010). Recently, a study on adult male Wistar rats administered with aqueous extract of saffron demonstrated that saffron significantly upregulated brain-derived neurotrophic factor (BDNF) (both transcriptomics and protein levels) and cyclic-AMP response element binding protein (CREB) (only protein levels) in the hippocampus which manifestate that saffron could be a potential lead molecule for AD treatment (Ghasemi et al., 2015).
2.15. Fasmily Lamiaceae
Melissa officinalis L. / Lemon balm is a perennial lemon-scented herb, which grows naturally in south-central Europe and the Mediterranean region. The herb is not frost tender and grows to a height of 70 cm. During summer, the white flowers appear. Although they are small and inconspicuous, honey bees love them. The tea prepared from this plant is used in TCM to calm nerves, for its spasmolytic effects (Kumar et al., 2013) and used in European medicine to improve senses and memory. It also strengthens brain cells and clears the head. Monoterpenes such as citral and citronellal are included in the essential oil of Melissa officinalis, which inhibits the AChE in a dose-dependent manner. Randomized, placebo-controlled, balanced-crossover and double blind studies confirmed influence of acute administered lemon balm on the modulation of mood and cognitive performance. It also shows a significant effect on people having severe dementia. Different studies show that Lemon balm has the potentials to manage the AD and to control agitation in AD patients (Kumar et al., 2013).
Ocimum tenuiflorum L. / Tulsi (also known as ‘holy basil’) is an aromatic plant belonging to the family Lamiaceae. This well-branched perennial herb grows up to a height of 1 m. The ovate, long and slightly toothed leaves are green or purple color. The small purplish orr white flowers are placed on terminal spikes. It is indigenous to the Indian Subcontinent and cultivated throughout the Southeast Asian tropics. Tulsi holds a place of respect since ancient times in India. The whole plant extract reverses the amnesia induced by scopolamine (0.4 mg/kg) and diazepam (1 mg/kg) in mice. It also reverses the memory deficits induced by ageing. The extract from Ocimum tenuiflorum decreases transfer latency and increases step-down latency in mice (in EPM test and PA paradigms as an exteroceptive behavioural model), in comparsion with control (treated with piracetam), scopolamine and age groups.
Salvia lavandulaefolia Vahl / Spanish sage is a perennial plant in family Lamiaceae. It is an evergreen shrub which grows to a height of 30 cm. The leaves are in opposite arrangement and contain essential oil with fragrance similar to rosemary. The small flowers are purplish and very attractive to bees. This small woody herb grows typically in rocky soil in the Mediterranean region (Spain and southern France). This herb become popular due its worthwile effects on depression, cerebral ischemia, memory disorders and anticholinesterase activity. The essential oil of Salvia contains 1,8-cineole, linalool, carvacrol and luteolin. Based on clinical data, the essential oil and extracts from Salvia lavandulaefolia suggested the potential for the therapy of AD and other memory-related disorders.
Salvia officinalis L. / Common sage is evergreen, perennial herb growing to a height of 60 cm. The oblong leaves are rugose, 6.5 cm long, and are green-gray color. The flowers are placed in spike and can be purple, pink, whire or red. The plant is native to the Mediterranean area, however due its popularity it is now cultivated in different places worldwide. It is one of our best-known herbs medicinally. It has a good reputation in British herbal encyclopaedias memory enhancing agents. The oil of Sage contains caryophyllene, camphor and borneol (54) etc. The sage proved the inhibition of acetylcholinesterase action. Furthur research exhibit that this herb may be useful in the treatment of mild-to-moderate AD.
Salvia rosmarinus Spenn. / Rosemary plant (synonym: Rosmarinus officinalis L.) in the family Lamiaceae, is an evergreen, perennial shrub growing to a height of 1.5 m. The plant is not frost tender, and has the linear leaves about 2–4 cm long, but only 2–5 cm wide. The leaves are green on the top and gray on the bottom. The small flowers are purple, deep blue, pink or white color. Rosemary plant originated in the Mediterranean area. Since ancient times, rosemary has been used for improvement and strengthening of the memory. In ancient Greece, it was considered as a mind stimulator. Even at the present date, students in Greece are burning the plant when they are studying for exams. Indeed, it affects as a stimulator of the blood flow into the brain and enhancer of mental alertness.
2.16. Family Magnoliaceae
Magnolia officinalis Rehder & Wilson / Magnolia tree grows naturally in China typically in mountains and valleys at altitudes of 300–1500 m. It is a deciduous tree growing up to a height of 20 m. The tree has a brown and thick bark, ovate enormous green leaves about 20–40 cm long and 10–20 cm wide, and fragrant creamy-white to butter-yellow large flowers about 10–14 cm in diameter. The bark of Magnolia officinalis (Magnoliaceae) is traditionally used in China as a medicine for the memory enhancement and for the treatment of neurosis, anxiety, stroke, dementia etc. Magnolia is able to inhibit scopolamine-induced memory impairment through the inhibition of AChE. The active constituent of Magnolia officinalis is 4-O-methylhonokiol, honokiol and magnolol (61) (Lee et al., 2011). The polyphenolic compounds 61 and honokiol are used in the treatment of fever, headache, neurosis, anxiety and stroke (Woodbury et al., 2013) since ancient times. In vivo experiments showed that honokiol was found to promote the level of acetylcholine in rat hippocampus. Both 61 and honokiol show AChE inhibitory property. The water maze and step-down avoidance tests have shown that Magnolia officinalis increasing the power of memory and learning skills.
2.17. Family Malvaceae
Theobroma cacao L. / Cacao tree in the family Malvaceae, is a small evergreen tropical tree, growing to a height of about 8 m. The large leaves about 40 cm long are simple, in alternative arrangement and periodically and replaced by new leaves. The small flowers are present at all times, however twice a year appear in abundance, their color can be white, yellow, reddish or pink. Flowers are clustered, odourless or foul-smelling and placed directly on the trunk and limbs. The fruits are elongated pods in various color from yellow to deep purple. The ovoid pod is up to 35 cm long, about 12 cm wide and contains 20–60 edible seeds, termed as beans. Cacao tree is native to Central and South America. Theobroma cacao contains different types of chemical compounds e.g. alkaloids (theobromine, theophylline and caffeine), glycosides, galactosides, tannins, polyphenols, triglycerides, coumarins, catechins, catechol, linoleic acids, rutin, vitexin etc. All these bring about its significant effect on the enhancement of memory.
2.18. Family Menispermaceae
Tinospora cordifolia (Willd.) Miers / Giloy is a deciduous climbing shrub. The simple heart-shaped leaves are in alternative arrangement. The species is dioecious, female flowers usually occur solitary, while male flowers are clustered. Red or orange ovoid fruits are clustered. The plant is native to the tropical regions (India, Sri Lanka and Myanmar). This multipurpose herb regenerates brain cells and the whole body. Memory enhancing properties of Tinospora cordifolia were observed on the memory and learning skills in normal and memory deficits animals. It increases the synthesis of Acetylcholine. The passive avoidance task and Hebb William maze proved its congnition enhancement property, when applied on normal and cognition deficits animals.
2.19. Family Myristicaceae
Myristica fragrans Houtt. / Nutmeg tree is an evergreen tree which usually grows up to a height of 20 m. The dark green leaves are in alternative arrangement, about 5–15 cm long and 2–7 cm wide. The species is dioecious, the bell-shaped female and male flowers are borne on different plants. The female flowers are in small groups (1–3 flowers) and longer than the male flowers that are aggregated in larger goups (1–10 flowers). The fruit, similar in appearance to an apricot is a pendulous drupe with an edible pulp. Inside the fruit is shiny purple-brown seed about 2 cm long, covered with a red aril. Nugmet tree originated from Banda Islands, Indonesia. The n-hexane extract of M. fragrans seeds in three doses (5, 10 and 20 mg/kg p.o.) was administrated to mice with scopolamine (0.4 mg/kg i.p.) and diazepam (1 mg/kg i.p.) induced memory deficit. The passive-avoidance task and elevated plus-maze test were used to evaluate learning and memory parameters. A significant improvement in learning skills and memory power was recognized after 3 successive days of M. fragrans extract administration at a dose of 5 mg/kg p.o. to young and aged mice. Scopolamine and diazepam-induced memory impairment in mice was also reversed with the extract.
2.20. Family Orchidaceae
Gastrodia elata Blume is a perennial herb growing up to a height of 1 m. The unique botanical character of the herb is that the whole plant is chlorophyll-free. Except for florescence, the main live-cycle stages of the plant running underground. The vertical, leafless stems are yellowish and fringy golden color inflorescence is about 13–30 cm long. The ovoid rhizome is about 8–12 long. Gastrodia elata is found in North Korea, Siberia, Nepal, Bhutan, India, Japan (Hokkaido, Honshu, Shikoku, Kyushu), Taiwan as well as mainland China. For centuries, the herb is used to treat various disorders including epilepsy, spasm, headache, amnesia, dizziness, stoke etc. (Chinese Pharmacopoeia Commission, 2015). Rhizoma Gastrodiae (rhizome of G. elata) is the key part of this medicinal plant. Gastrodin (4-hydroxybenzyl alcohol-4-O-β-d-glucopyranoside) is considered as the most important bioactive constituents of Rhizoma Gastrodiae which exhibit high pharmacological intervention. A plethora of research has been investigated for gastrodin (C13H18O7), medicinal importance which has demonstrated remarkable beneficial bioactive activity including memory-improving, hypnotic, sedative, anti-epileptic, anti-vertigo, preventing osteonecrosis, analgesic, antidepressant, anxiolytic, anti-aging, lowering blood pressure etc. (Zhan et al., 2016). In the light of AD, gastrodin extract or its bioactive constituents has demonstrated to enhance learning and memory performance in an AD mouse model (Liu and Wang, 2012, Hu et al., 2014). In vitro investigation of gastrodin has shown to suppress intracellular and extracellular Aβ levels in a dose-dependent manner (Zhu et al., 2014) and further investigation indicated that the cause of this reduction could be associated with the mitigation of β and γ-secretase activities (Zhou et al., 2016).
2.21. Family Phyllanthaceae
Phyllanthus emblica L. / Amla (synonym: Emblica officinalis Gaertn.) is a tropical deciduous tree that grows up to a height of 18 m. It has green pinnately compound leaves with the greenish-yellow flowers. Edible globular fruits are greenish-yellow color. The tree grows in tropical Southeastern Asia, Southern China, Pakistan, and Bangladesh, particularly in central and southern India, Ceylon, Malaya and the Mascarene Islands. P. emblica contains diverse active constituents like different tannins, vitamin C, oils, polyphenols (gallic acid (75), punigluconin, ellagic acid (73), chebulinic acid, leutolin, apeigenin, quercetin (80), etc.), phyllemblin, amino acids, 3,6-di-O-galloyl-d-glucose, 1-O-galloyl-β-d-glucose, 3-ethylgallic acid (3-ethoxy-4,5-dihydroxybenzoic acid), 1,6-di-O-galloyl β-d-glucose, minerals, flavonoids (rutin and 80) etc (Variya et al., 2016, Yadav et al., 2017). Presence of vitamin-C, in the amla possesses beneficial calming effects on the memory improvement, cholesterol-lowering property and anticholinesterase activity. Amla shows positive results on memory enhancement in scopolamine (0.4 mg/kg, i.p.) and diazepam (1 mg/kg, i.p.) induced memory deficits. It also inhibits AChE activity (Reddy et al., 2010). A study in young and aged rats and mice model showed a dose-dependent improvement in memory scores after administration of Anwalachurna (50, 100, and 200 mg/kg, p.o.) (Parle and Vasudevan, 2007). Numerous studies have shown the neuropharmacological property of P. emblica in the treatment and prevention of dementia which is manifested by the multifunctional characteristic including anti-oxidant property, cholesterol-lowering property, anti-cholinesterase property, and potency to improve and reverse memory deficits (Ashwlayan and Singh, 2011, Perry and Howes, 2011, Golechha et al., 2012).
2.22. Family Plantaginaceae
Bacopa monnieri (L.) Pennel / Brahmi (also known as water hyssop) is a perennial, non-aromatic herb which grows to a height of 30 cm. The herb is frost tender, has oblong, succulent, thick leaves in alternative arrangement, that are edible. The small, white flowers have 4–5 petals. Brahmi is native to India and Australia (Aguiar and Borowski, 2013) and also grows throughout the United States and East Asia. Bacopa monnieri (BM) has a long history of use in the AM tradition in the treatment of several ailments, including epilepsy, learning enhancer, lack of concentration, mental illnesses, sedative, stroke, anxiety etc. (Srivastava et al., 2019). All of the herbs investigated for their memory-enhancing properties Brahmi is likely the most studied. The usage of Bacopa monnieri for memory enhancement can be dated back to 800 BCE in India (Rathee et al., 2008). In modern India, Brahmi is used as a tonic for school-going children to improve their mental capacity. The grasping power of the brain and the power to analyze grasped information are also increased by (Singh, 2013). Studies show that the major nootropic bioactive constituents in this herb are alkaloids (such as brahmine, nicotine (62), and herpestine), steroidal saponins (like; d-mannitol and hersaponin, bacopasides I-XII, bacopasaponins, bacosides A (42) and B, acid A, and monnierin), (Aguiar and Borowski, 2013, Le et al., 2015), which have beneficial influence on enhancement of memory and cognitive function. Bacopa monnieri exhibits neuroprotective, anti-oxidant, and hepatoprotective properties (Rastogi et al., 2012). These bioactive components have promising results in normal rats e.g. learning and memory were facilitated whereas the amnesic effect induced by electroshock, scopolamine and immobilization stress was inhibited. Furthermore, the activity of protein kinase C (PKC) in hippocamus was enhanced by Brahmi, which contributes to its nootropic action. Bacopa monnieri has also been documented to shown AChE inhibitory activity in patients with AD (Goswami et al., 2011). There have also been preliminary clinical studies suggesting significant improvement of neurocognitive function in humans after treatment with Bacopa monnieri (Peth-Nui et al., 2012, Downey et al., 2013, Neale et al., 2013). Brahmi contributes to soothing and relaxing of the brain cells and restore them to a regular functioning state in adults. Hence, all these findings demand to take further research to know the real mechanism of action regulation these pathways/processes.
2.23. Family Rutaceae
Murraya koenigii (L.) Sprengel / Curry tree in the family Rutaceae is an evergreen tree which grows to a height of 6 m. The odd-pinnate leaf usually consists of 11–21 green, ovate, leaflets about 2.5–5 cm long that are aromatic. The white, funnel-shaped flowers are small, arranged in clusters and have a sweet fragrance. Fruits are bluish-black, oblong drupes with a sweet, edible pulp and a single, large, non-edible seed. Curry tree grows in tropical to sub-tropical regions. This tree is native to Sri Lanka and India. Murraya koenigii leaves commonly known as ‘curry patta’, are used in Indian dishes as a very common food-additive. Reduction of brain cholinesterase activity as well as cholesterol-lowering effects were reported in diets added with curry leaves that may be attributed to the observed nootropic effect. Therefore, curry leaves can be investigated for use in the management of AD.
2.24. Family Solanaceae
Withania somnifera (L.) Dunal / Ashwagandha is a perennial, evergreen shrub in the family Solanaceae which grows up to a height of 1 m. The species is hermaphrodite and frost tender. The green leaves are elliptic about 10–12.5 cm long. The small, green flowers are bell-shaped. The fruit, enclosed in the calyx is a spherical, orange-red to red berry about 5–8 mm in diameter. It is usually cultivated in the drier regions of India, but found also in Nepal. Withania somnifera belongs to one of the most researched Ayurvedic herbs and its roots are one of the most remarkable ingredients in AM, similar to the status of Ginseng in Chinese therapies. Therefore, it is not surprising that Withania somnifera is often termed as the ‘Indian Ginseng’. The root extract from Ashwagandha (50, 100 and 200 mg/kg; p.o.) in mice led to improvement of retention of a passive avoidance task in a step-down paradigm. Ashwagandha further reversed disruption of acquisition and retention induced by scopolamine (0.3 mg/kg). Moreover, the amnesia produced by acute treatment with electroconvulsive shock (ECS) was attenuated by Ashwagandha immediately after training (Dhulley, 2001).
2.25. Family Theaceae
Camellia sinensis (L.) Kuntze / Green tea plant is an evergreen shrub which grows up to a height of 4 m. The tree has a rough, gray bark and strong taproot. The dark green leaves are oval, in alternative arrangement and about 5–10 cm long. The white flowers are quite fragrant, solitary or arranged in small groups. The flower is about 4 cm in diameter with 5–9 petals. Green tea plant is native to South, East and Southeast Asia. However, nowadays, it is cultivated across the subtropical and tropical regions of the world including Japan and Sri Lanka. Camellia sinensis plant produces three types of teas: non-fermented (white and green tea), partially fermented (oolong and red tea) and completely fermented (black tea) (Suzuki et al., 2016, Hayat et al., 2015) and interestingly its bioactive constituents greatly depend on the degree and ease of fermentation process. Tea is globally the second most consumed beverage after the water and its pharmacological properties are extensively documented. It is having potent neuroprotective, free radical scavenging, antioxidative, antioncogenic, hepatoprotective, anti-diabetic, antiviral, and chemopreventive properties because of its diverse chemical constituents including amino acids, aluminum, proteins, phenolic acids (chlorogenic acid, gallic acid (75) or trihydroxybenzoic acid, and caffeic acid), alkaloids (theophylline, caffeine, and theobromine), caffeine, flavonoids (quercetin (80), kaempferol, and myricetin) and polyphenols (catechins such as epigallocatechin-3-gallate (EGCG, 12), (−)-epicatechin gallate (ECG), (−)-epicatechin (EC, 49), and (–)-epigallocatechin (EGC)), carbohydrates, minerals, theanine, volatile organic compounds, trace elements, and fluoride (Mahmood et al., 2010, Legeay et al., 2015) that are beneficial in preventing a plethora of health related ailments including neurodegeneration, hypotension, vomiting, memory loss, skin cancer, lung cancer, and breast cancer, obesity, atherosclerosis, headaches, type 2 diabetes, stomach disorders, gastrointestinal cancer, ovarian cancer, PD, diarrhea, kidney stones (Shivashankara et al., 2014, Yang et al., 2014, Yang and Wang, 2016, Yokogoshi, 2017, Baliga et al., 2018). Since, green tree exhibits a plethora of bioactive molecules especially catechins, it is a well-known source of neuroprotection (Flôres et al., 2014, Schimidt et al., 2014, Fernando et al., 2017). It decreases the production of β-amyloid (Aβ) monomers, the major cause of AD, which forms proteins that are able to create the amyloid plaques in the brains of patients with AD (Polito et al., 2018). Several recent studies have found that teas (reg and green) as dietary supplements have significant potential to reduce/protect memory and learning deficits and hippocampus oxidative stress in an Alzheimer disease model and ischemia–reperfusion (IR) (Martins et al., 2017, Schimidt et al., 2017). The ability of green tea to stop the degeneration in neuronal cells results from the presence of EGCG in the brain, meaning that it can impart its antioxidative effects on the free radicals causing the brain damage. A mouse study in China shows that EGCG from green tea supports the formation of brain cells, which areimportant to memory and spatial learning (Weatherby, 2012). Hence, bioactive teas might be a suitable potent therapeutic candidate against neurodegenerative disorder (Chen et al., 2018) which demands an evaluation of these chemicals in a clinical setting. Furthermore, in clinical studies and various animal models, green tea intake has been shown to reduce cardiovascular disease by reducing total and LDL cholesterol, blood pressure and CVD mortality (Onakpoya et al., 2014, Yarmolinsky et al., 2015, Zhang et al., 2015).
2.26. Family Zingiberaceae
Curcuma longa L. / Turmeric is perennial, tropical, rhizomatous, herbaceous plant, which grows up to a height of 1.5 m. The leaves arise from the pseudostem, which is composed of long, interlocked, succulent leaf petioles. The simple leaves are long and can be simple green color or variegated. The flower spikes arise from the top of the pseudostem. The florets can be white, yellow, orange or pink in color, and the bracts can be also in various colors. The rhizome has a strong orange-yellow color, pepper-like aroma and bitter taste. Turmeric is a traditional Chinese herbal plant and is native to tropical Tamilnadu in Southeast India. Turmeric, obtained from the rhizome of C. longa is known as the golden spice and is widely considered a medicine for the treatment of a plethora of diseases comprising inflammations, arthritic, diabetic wound healing, anorexia, microbial infections, muscular disorders, cancer, hepatic disorders, diabetes, biliary disorders, sinusitis and cough (Li et al., 2012, Kuete, 2017). The rhizomes are the reservoir of diverse compounds out of which curcuminoids (25) (curcumin, 11), principal phenolic compound (Zhao et al., 2012) exhibit multifunction that is potent in the management of various diseases. Additionally, 11 also exhibited several pharmacological properties such as neuroprotective, hepatoprotective, cardiovascular protective, antidiabetic, anticoagulant, anti-inflammatory, antioxidant, antifungal, antiviral, antibacterial, antineoplastic, antifertility and immunostimulant activities in animals (Singh and Sharma, 2011). The pivotal potential of 11 has been well established in treating and preventing neurodegenerative disorders (Shl Kim, 2012, Villaflores et al., 2012). In wistar rats, curcuma oil has been documented to minimize inflammation of the endothelial cells in postmyocardial ischemia/ reperfusion (Manhas et al., 2014). Curcumin prevents the accumulation of Aβ or facilitates its disaggregation at low concentration levels (IC50 = 0.81–1 μM) (Fang et al., 2014). Curcumin has also been shown to protect against Aβ neurotoxicity by downregulating Aβ synthesis via inhibition of the presenilin 1 (PS1) expression and glycogen synthase kinase-3beta (GSK3β) expression (Caesar et al., 2012, Zhang et al., 2011). In SH-SY5Y neuroblastoma cells treated with 6-hydroxydopamine, 11 has been indicated to arbitrate neuroprotective activity by attenuating quinoprotein development, expression of p-p38 mitogen-activated protein kinases (MAPKs), and activation of caspase-3 (Meesarapee et al., 2014). Curcumin (11) substantially increased cognitive function in a streptozotocin (STZ) model of sporadic AD by restoring downregulated IGF-1 levels (Agrawal et al., 2010). Furthermore, another research found that 11 enhanced the neurotoxicity of 6-hydroxydopamine (6-OHDA) via its anti-inflammatory action and by restoring the expression of SOD-1 (Tripanichkul and Jaroensuppaperch, 2013). In mice, oral administrated aqueous extract of C. longa led to the inhibition of brain monoamine oxidase-A. 11, as the major bioactive compound from C. longa displays the neuroprotective properties against ethanol-induced brain injury. The 11 enhanced/increased locomotive function in a homocysteine rat model with PD (Mansouri et al., 2012). Concerning epilepsy, in the experimental seizure models, 11 has shown antiepileptic effects (Ahmad, 2013, Peng et al., 2012, Kiasalari et al., 2013) by its antioxidant activity (Choudhary et al., 2013, Noor et al., 2012). Furthermore, 11 is effective in preventing brain injury (cerebral stroke), in addition to antiepileptic effects (Lapchak, 2011, Liu et al., 2013, Yu et al., 2012, Zhao et al., 2010a, Zhao et al., 2010b). 11 has boosted candesartan's neuroprotective activity on brain ischemia via the repression of blood flow and oxidative stress (Awad, 2011). 11 pretreatment decreased infarction and brain lesions, and increased neurological function in rats following Traumatic Brain Injury (TBI) (Samini et al., 2013). In rats experiencing TBI, curcumin derivatives administration enhanced locomotive and cognitive functioning (Wu et al., 2011).
Zingiber officinale Roscoe / Ginger is a perennial, tropical, flowering plant, which grows up to a height of 1.5 m. The leaves are narrow and about 15–30 cm long. They are in alternative arrangement and arise from the pseudostem. Flowering heads are placed on short stems and the herb produces pale yellow, cone-shaped flowers. The rhizomes are irregular in shape with variable color from light bwown to dark yellow. Ginger was cultivated first in South Asia, but nowadays is also cultivated in the East Africa and the Caribbean region. Its rhizomes possess potent memory-enhancing properties. It improves memory power and the whole-body blood circulation including the supply of nutrients into the brain. Ginger significantly improved learning and memory by increasing whole-brain acetylcholinesterase inhibition activity in case of scopolamine-induced memory impairment. It is also reported to inhibit β-amyloid peptide accumulation. Gingerin, gingerol (56), shogaol and zingerone are the major active compounds in ginger.
3. Use of crude drugs from herbs in memory impairment
Artemisia asiatica Willd. (Asteraceae): a Korean tea plant. Methanolic extract of this plant was administrated to the beta-peptide infused rats. This β-peptide infusion caused toxicity in the PC12 cells in rats. Administration of Artemisia showed a significant result in reversing the toxicity in PC12 cells. A study demonstrated that an alkaloid of Artemisia appeared to be an acetylcholinesterase (AChE) inhibitor with a blocker of β-amyloid-induced neurotoxicity causing AD.
Celastrus paniculatus Willd. (Celastraceae): the aqueous fraction of C. paniculatus seeds was administrated to rats with sodium nitrite induced amnesia. The results show an increase in the brain’s acetylcholine (ACh) level by decreasing AChE activity, thus improving memory in amnesic models of mice (Bihaqi et al., 2011).
Corydalis ternata (Nakai) Nakai (Papaveraceae): a total methanolic extract of the tuber of Corydalis ternate exhibits considerable inhibition of acetylcholinesterase (AChE). Protopine, an isolated alkaloid from Corydalis showed a dose-dependent inhibitory effect on the acetylcholinesterase activity with IC50 = 50 μM. This inhibitory activity was specific reversible and competitive in the manner.
Foeniculum vulgare Mill. (Apiaceae): a whole plant methanolic extract significantly increases latency in step-down avoidance test as well as the inhibition of acetylcholinesterase in mice. Therefore, Foeniculum vulgare can be utilized in the therapy of cognitive disorders e.g. dementia and AD.
Gastrodia elata Blume (Orchidaceae): the methanolic extract of the rhizomes of Gastrodia elata in the passive avoidance task results in significant prolongation of the shortened step-through latency, induced by scopolamine. Similarly, the n-butanol and ethyl acetate fractions prepared from crude methanolic extract of Gastrodia elata, administered at the dose of 50.0 mg/kg for one week prolonged the scopolamine-induced step-through latency in rats. Moreover, the shortened step-through latency on the passive avoidance task induced by scopolamine was prolonged by gastrodin, as the product isolated from the n-butanol fraction and by p-hydroxybenzyl alcohol, obtained from the ethyl acetate fraction of the methanolic extract.
Glycyrrhiza glabra L. (Fabaceae): the aqueous extract of Glycyrrhiza glabra was administrated to one-month-old male albino Wistar rats with Diazepam-induced memory impairments. The experiment showed a significant increase in learning and memory in the rats and reversed the effect of Diazepam toxicity (Chakravarthi and Avadhani, 2013).
Ocimum tenuiflorum L. (Lamiaceae): a whole plant aqueous extract of dried Ocimum tenuiflorum were evaluated on the passive avoidance paradigm and elevated plus maze in mice. The step-down latency increasing and the transfer latency decreasing were evident in comparison to control group (treated with piracetam), scopolamine group and age group of mice. The results suggested possible application of Ocimum tenuiflorum in the therapy of cognitive disorders (e.g. AD and demetia).
Schisandra chinensis (Turcz.) Baill. (Schisandraceae): a deciduous climbing plant, which is native to Northern China and the Far Eastern Russia. The water fraction of this plant at the dose of 25 mg/kg after one-week administration on rats resulted in the prolongation of cycloheximide-induced step-through latency. Interestingly, the water fraction of Schisandra chinensis seems to be the major active fraction of this plant (Kopustinskiene and Bernatoniene, 2021; Szopa et al., 2017).
Scutellaria baicalensis Georgi (Lamiaceae): a flowering plant native to China, Korea and Siberia. The extracts of its roots are utilized in the traditional Korean medicine and are beneficial in treatment of the brain diseases. Further, it was reported that the orally administered extracts of this plant improved the memory impairment, which was induced by chronic lipopolysaccharide (LPS) infusion or by chronic cerebral hypoperfusion (Hwang et al., 2011).
4. Effective herbal formulations for memory enhancement
Abana is an Indian Ayurvedic polyherbal formulation, contains 16 well-known herbs as composition. It significantly reduces the activity of cholinesterase, which results to increase of acetylcholine level in the brain of aged and young mice. The ingredients of Abana express the antioxidant property, which reduces oxidative stress of the brain cells. Thus, Abana helps to reduce brain impairment and improves neuronal function. The influence of orally administered Abana on cognitive functions was monitored on groups of aged and young mice. Testing of memory was evaluated by using exteroceptive behavioral models (passive avoidance apparatus and elevated plus-maze test) as well as interoceptive behavioral models (amnesia induced by diazepam, scopolamine and ageing). Abana improves the memory score in dose-dependent manner of both aged and young mice. Even, Abana reverses the scopolamine- and diazepam-induced amnesia. Therefore, the polyherbal formulation Abana bears promising potential for the treatment of Alzheimer’s disease.
BR-16A (Mentat) is a herbal psychotropic preparation containing various indigenous plant extracts which are well-reputed in the AM. The compositional herbs are: Bacopa monnieri, Acorus calamus, Asparagus racemosus, Evolvulus alsinoides, Withania somnifera, Phyllanthus emblica and Triphala. Mentat on the elevated plus-maze test reduced transfer latency delay induced by scopolamine in mice. Moreover, evaluation of passive avoidance paradigm revealed that Mentat also reduces ECS-induced acute and chronic retrograde amnesia in rats. A combination of Mentat and a low dose of aniracetam (a well-known nootropic) in the passive avoidance task brings much better results in the reduction of mistakes. Clinical studies on different age groups with Mentat show improvement in memory quotient of tested subjects. In normal adults Mentat attenuates fluctuations of attention and increases memory span. In children with minimal brain damage or behavioural problems improves the ability to learn.
Brain-o-brain is capsule with a unique mixture of herbs like Shankhpushpi, Brahmi and Sweet flag to improve learning ability, memory power and naturally helps to avoid stress and depression. These herbal brain enhancer pills are made especially for all age groups to prevent or decrease forgetting. Indeed, regularly taken Brain-o-brain is effective to increase gasping power and natural ability of the brain. This fully natural remedy supports to overcome nervous exhaustion and mental fatigue.
Bramhi ghrita contains four Ayurvedic herbs including Brahmi (Bacopa monnieri) and cowmilk’s ghee. The memory and learning improvement effect of Brahmi ghrita applied in different doses (30, 50 and 100 mg/kg, p.o.) was tested in rodents. The elevated plus-maze test was used for evaluation of memory acquisition and retention in rats. Morris water maze test was used to interpret spatial memory in mice. The elevated plus-maze transfer latency and Morris water maze escape latency were significantly reduced after administration of Bramhi ghrita (50 and 100 mg/kg, p.o.).
Chyawanprash (Chy) is an Ayurvedic tonic, which contains almost 50 herbs and herbal extracts. One of its basic ingredients is Amla (Phyllanthus emblica) a rich natural source of vitamin C. Consumption of Chy is popular in Indian households. Animal studies confirmed significant influence of Chy in the memory impairment protection effect. Furthermore, administration of Chy (2% w/w) decreases thiobarbituric acid reactive substances (TBARS) in the brain and increases the levels of glutathione. Results indicate a decrease in the free radical generation and an increase in the protection from free radical. Thus, Chy could be an efficient remedy for the therapy of AD.
Memorin is comprised of 21 kinds of herbal extracts. The experimental studies of Memorin were made upon rats using passive avoidance learning paradigm in a shuttle box. Memorin at the dose of 200 mg/kg b.w./day was administered for a fortnight to animals. Administration of ECS produced significant retrograde amnesia in rats. Memorin was found to attenuate the ECS-induced amnesia. In clinical studies, when applied on human, Memorin was found to reverse poor memory, age-related dementia and mental fatigue.
Optimized-Sopung Sunkiwon (OSS) is a polyherbal formula comprising of six medicinal herbs from SopungSunkiwon. SopungSunkiwon is a traditional medicine usually predescribed for people suffering from neurodegenerative disorders. OSS significantly improves the memory functions via inhibition of AChE activity. Latency time in the passive avoidance test was significantly longer for mice treated with OSS compared with control group or group treated with scopolamine. Besides, mice treated with OSS exhibited increase of synaptic proteins that facilitate acetylcholine release and synaptic growth (e.g. PSD-95 and synaptophysin). These results show that OSS may act upon memory impairment and increase synaptophysin and PSD-95 in the brain (Choi et al., 2011).
Shimotsu-to literally mean ‘four substance decoction’. This TCM formula consists of following four crude herbal extracts: Japanese angelica root, peony root, rehmannia root and cnidium rhizome. Shimotsu-to have beneficial effects on spatial memory improvement in rats. Moreover, the study in rat models suggests importance of paeoniflorin (extracted form peony root) and tetramethylpyrazine (extracted from cnidium rhizome) as the enhancers of cognitive functions.
Yukmijihwang-tang derivatives is a Chinese polyherbal formulation used as memory or cognition enhancer. It consists of six diffrent herbal medicines. Chinese herbal textbook refers to YMJD as an anti-ageing prescription. Besides, it also helps to prevent memory and learning impairment in mice. Studies reported that YMJD in stressed rats could increase neurogenesis in the dentate gyrus (hippocampus) along with improvement of cognitive functions and memory retention.
5. Other advantageous compounds
Ascorbic acid (23) is found mainly in citrus fruits (e.g. lemon). Studies in aged mice have shown that 23 increased step-down latency and decreased transfer-latency, which indicated its ability to improve learning and memory. Moreover, memory impairment induced by diazepam and scopolamine was significantly reduced in young mice treated with 23.
Bacosides (24) (bacoside A (42) and bacoside B) are derived from the well-reputed Bacopa monnieri. However, recent study identified bacoside A (42) as the mixture of four different saponins, termed as: bacoside A3 (3), bacopaside II (4), bacopasaponin C (6) and isomer of bacopasaponin C (5) (Fig. 2). Similarly, bacoside B represents the mixture of saponins, which exact constitution is not clearly identified to date. To the best of our knowledge, bacoside B consists of four following saponins: bacopaside N1 (7), bacopaside N2 (8), bacopaside IV (9) and bacopaside V (10) (Fig. 2) (Deepak et al., 2013). In a clinical trial, 24 were administrated to elderly people (65 years or older) without a clinical sign of dementia in a double blind and placebo-controlled study (Table 4). The results showed significant enhancement in their auditory verbal learning and word recall. There are possibilities that the 24 can help to manage AD too (Kumar et al., 2012a, Kumar et al., 2012b).
Table 4.
Different phytochemicals for memory improvement.
| Sl no. | Chemical compound | Source plant | In vivo study model | Biological effect and target | Reference |
|---|---|---|---|---|---|
| 1 | l-Ascorbic acid (23) | Citrus fruits | Scopalamine and Diazepam-induced young animals, Aged mice |
|
(Parle and Dhingra, 2003) |
| 2 | Bacosides (24) | Bacopa monnieri | Elderly people (65 years or older) without clinical sign of dementia in double-blind, placebo-controlled clinical trial |
|
Kumar et al., 2012a, Kumar et al., 2012b |
| 3 | Curcuminoids (25) | Curcuma longa | Abeta-peptide infused rat models of AD |
|
Ahmed et al., 2010 |
| 4 | Dehydroevodiamine (26) | Evodia rutaecarpa | Rats with Scopalamine-induced memory deficit |
|
Park et al., 1996 |
| 5 | Dendrobium alkaloids (27) | Dendrobium moniliforme | Rats with LPS-induced memory deficit |
|
Li et al., 2010 |
| 6 | Epigallocatechin-3-gallate (EGCG, 12) | Camellia sinensis | Mice |
|
Weatherby, 2012 |
| 7 | Ginkgolides (28) | Ginkgo biloba | Patients with dementia of AD type and multi-infract dementia |
|
Kumar et al., 2012a, Kumar et al., 2012b |
| 8 | Ginsenosides (29):Rg1 (20), Rg2 (21), Rg3 (30), Rb1 (19), Rh2 (22), etc. | Panax ginseng | Healthy and young volunteers |
|
Kumar et al., 2012a, Kumar et al., 2012b |
| 9 | Huperzine A (15) | Huperzia serrata | Individuals with AD |
|
Kumar et al.,2012 |
| 10 | Loganin (17) | Cornus officinalis | Scopalamine-induced amnesic mice |
|
Lee K.Y et al., |
| 11 | Nobiletin (18) | Aurantii nobilis | Mice |
|
Yamakuni et al., 2010 |
| 12 | Phosphatidyl choline (31) |
|
Human |
|
Rathee et al., 2008 |
| 13 | Piperine (32) | Piper nigrum | Adult male Wistar rats |
|
Chonpathompikunlert et al., 2010 |
| 14 | Polygala saponins XXXII (PGS32, 33) | Polygala tenuifolia | Mice with scopalalmine-induced cognitive impairment |
|
Kumar et al., 2012a, Kumar et al., 2012b |
| 15 | Pseudocoptisin (34) | Corydalis tuber | Mice with scopolamine-induced memory impairrment |
|
Hung et al., 2008 |
| 16 | Oroxylin A (35) | Scutellaria bicalensis | Rats with 2VO-induced memory impairments |
|
Kim et al., 2006 |
| 17 | Sauroine (36) | Huperzia sqururus | Male Wistar rats |
|
Vallejo et al., 2009 |
| 18 | Sesquiterpene lactone (37) | Amberboa ramosa |
|
Ibrahim et al., 2013 |
Curcuminoids (25) represent major chemical components present in turmeric (Curcuma longa). Specifically, it is a mixture consisting of: curcumin (11), demethoxycurcumin (71) and bisdemethoxycurcumin (68) (Table 4). Effective on rats showing AD-like neuronal loss caused by a β-peptide infusion. 25 also increased PSD-95 and synaptophysin expression in the rat hippocampus.
Epigallocatechin-3-gallate (EGCG, 12) is the major catechin derivative found in green tea (Camellia sinensis). EGCG represents the most effective therapeutic component of green tea. EGCG possesses antioxidant properties and reduces lipid peroxidation in biological systems caused by free radicals (Kumar et al., 2012a, Kumar et al., 2012b). A study in rats revealed the ability of EGCG to increase neurogenesis in hippocampus. Moreover, dietary EGCG study in mice proved enhancement of spatial memory and object recognition (see Table 1, Table 2, Table 3, Table 5, Table 6).
Table 1.
List of herbs having memory and cognition enhancing properties.
| Sl. No. | Botanical name |
Genus Family |
Common name | Parts used | Active constituents | Reference |
|---|---|---|---|---|---|---|
| 1 | Acorus calamus |
Acorus Aracaceae |
Sweet flag | Rhizomes |
|
Shiksharthi et al., 2011 |
| 2 | Allium sativum |
Allium Amaryllidaceae |
Garlic | Bulb | (S)-allyl-l-cysteine | Semwal et al., 2014 |
| 3 | Amberboa ramosa |
Amberboa Asteraceae |
Sesquiterpene lactones:
|
Ibrahim et al., 2013 | ||
| 4 | Asparagus racemosus |
Asparagus Asparagaceae |
Shatavari | Roots | Triterpene saponins: Shatavarin I-IV | Shiksharthi et al., 2011 |
| 5 | Bacopa monnieri |
Bacopa Plantaginaceae |
Brahmi | Whole plant | Bacosides A and B | Shiksharthi et al., 2011 |
| 6 | Butea monosperma |
Butea Fabaceae |
Palash | Leaves | Malik et al., 2013 | |
| 7 | Camellia sinensis |
Camellia Theaceae |
Green tea | Leaves |
|
Shiksharthi et al., 2011; Compas, 2006 |
| 8 | Celastrus paniculatus |
Celastrus Celastraceae |
Jyotishmati | Seeds | Alkalloids: Celastrine, Paniculatin, Calapagone, Calapanigine etc | Shiksharthi et al., 2011 |
| 9 | Centella asiatica |
Centella Apiaceae |
Thankuni | Whole plant |
|
Shiksharthi et al., 2011 |
| 10 | Clitoria ternatea |
Clitoria Fabaceae |
Aparajita | Roots |
|
Shiksharthi et al., 2011 |
| 11 | Coeloglossum viride |
Coeloglossum Orchidaceae |
Frog orchid | Rhizomes | Martha, 2010 | |
| 12 | Convolvulus pluricaulis |
Convolvulus Convolvulaceae |
Shankh pushpin | Whole plant | Flavanoids | Shiksharthi et al., 2011 |
| 13 | Coriandrum sativum |
Coriandrum Apiaceae |
Corriander | Leaves and Seeds | Parle and Vasudevan, 2009 | |
| 14 | Cornus officinalis |
Cornus Cornaceae |
Japanese cornel | Fruits | Cornel iridoid glycosides (CIG):
|
Zhao et al., 2010a, Zhao et al., 2010b |
| 15 | Corydalis ternata |
Corydalis Papaveraceae |
Nakai | Tuber | Alkaloids:
|
Kim et al., 1999 |
| 16 | Crocus sativus |
Crocus Iridaceae |
Saffron | Whole plant |
|
Shiksharthi et al., 2011 |
| 17 | Curcuma longa |
Curcuma Zingiberaceae |
Turmeric | Rhizomes |
|
Shiksharthi et al., 2011 |
| 18 | Cyperus rotundus |
Cyperus Cyperaceae |
Java grass | Rhizomes | (Sunil et al., 2011; Kumar et al., 2012a, Kumar et al., 2012b) | |
| 19 | Dendrobium moniliforme |
Dendrobium Orchidaceae |
Sekkoku | Whole plant | ||
| 20 | Eclipta alba |
Eclipta Astearaceae |
Bhringaraj | Leaves |
|
Bhaskar and Chintamaneni, 2014 |
| 21 | Embelia ribes |
Embelia Primulaceae |
Black pepper | Roots | ||
| 22 | Euphrasia officinalis |
Euphrasia Orobanchaceae |
Powdered herb |
|
Sticher and Salama, 1981 | |
| 23 | Foeniculum vulgare |
Foeniculum Apiaceae |
Fennel | Whole plant | Joshi and Parle, 2006a, Joshi and Parle, 2006b, Joshi and Parle, 2006c | |
| 24 | Gastrodia elata |
Gastrodia Orchidaceae |
Gastrodia tuber | Root |
|
Shiksharthi et al., 2011 |
| 25 | Ginkgo biloba |
Ginkgo Ginkoaceae |
Ginkgo | Leaves |
|
Shiksharthi et al., 2011 |
| 26 | Glycyrrhiza glabra |
Glycyrrhiza Fabaceae |
Licorice | Roots | Glycyrrhizine | Shiksharthi et al., 2011 |
| 27 | Hibiscus asper |
Hibiscus Malvaceae |
Hibiscus | Leaves | Foyet et al., 2011 | |
| 28 | Huperzia serrata |
Huperzia Lycopodiaceae |
Club moss | Moss | Huperzine A and B | Jesky et al., 2011 |
| 29 | Hypericum perforatum |
Hypericum Hypericaceae |
St. John’s wort | Roots |
|
Shiksharthi et al., 2011 |
| 30 | Ilex paraguariensis |
Ilex Aquifoliaceae |
Mate tea | Leaves |
|
Shiksharthi et al., 2011 |
| 31 | Illicium verum |
Illicium Schisandraceae |
Star anise | Sesquiterpenoids: Veranisatins A, B, and C | ||
| 32 | Magnolia officinalis |
Magnolia Magnoliaceae |
Houpu Magnolia | Bark |
|
Reddy et al., 2010 |
| 33 | Marsilea minuta |
Marsilea Marsileaceae |
Marsilea | Leaves | Marsiline | Dwivedi et al., 2012 |
| 34 | Melissa officinalis |
Melissa Lamiaceae |
Lemon balm | Leaves | Rosmarinic acid | Awad et al., 2009 |
| 35 | Murraya koenigii |
Murraya Rutaceae |
Curry leaves | Leaves | Parle and Vasidevan, 2009 | |
| 36 | Myristica fragrans |
Myristica Myristicaceae |
Nutmeg | Seeds | Parle et al., 2004 | |
| 37 | Narcissus confuses |
Narcissus Amaryllidaceae |
Daffodil | Crude drug | Galanthamine | (Bastida and Viladomat, 2002) |
| 38 | Nardostachys jatamansi |
Nardostachys Caprifoliaceae |
Jatamansi | Whole plant |
|
Shiksharthi et al., 2011 |
| 39 | Nicotiana tabacum |
Nicotiana Solanaceae |
Tobacco plant | Leaves |
|
Dua et al., 2009 |
| 40 | Ocimum tenuiflorum |
Ocimum Lamiaceae |
Holy basil | Whole plant | Joshi and Parle, 2006a, Joshi and Parle, 2006b, Joshi and Parle, 2006c | |
| 41 | Panax ginseng |
Panax Arialaceae |
Ginseng | Roots |
|
Shiksharthi et al., 2011 |
| 42 | Phyllanthus emblica(synonym: Emblica officinalis) |
Phyllanthus Phyllanthaceae |
Amla | Fruit |
|
Shiksharthi et al., 2011 |
| 43 | Piper betle |
Piper Piperaceae |
Bittle leaf | Leaves | Arecoline | Shiksharthi et al., 2011 |
| 44 | Piper nigrum |
Piper Piperaceae |
Black pepper | Fruits | Piperine | Chonpathompikunlert et al., 2010 |
| 45 | Polygala tenuifolia |
Polygala Polyagalaceae |
Yuan zhi | Roots | Triterpenoid saponins | Kumar et al., 2013 |
| 46 | Polygonatum multiflorum |
Polygonatum Asperagaceae |
Solomon’s seal | Emodin | Lu et al., 2007 | |
| 47 | Prunus amygdalus |
Prunus Rosaceae |
Almond | Nuts |
|
Kumar and Seth |
| 48 | Pueraria tuberose |
Pueraria Fabaceae |
Vidarikand | Tubers | Flavanoids | Shiksharthi et al., 2011 |
| 49 | Punica granatum |
Punica Lythraceae |
Pomegaranate | Seeds |
|
Kumar et al., 2009 |
| 50 | Rehmannia glutinosa |
Rehmannia Orobanchaceae |
Sheng di huang | Roots |
|
Zhang et al., 2008 |
| 51 | Ricinus communis |
Ricinus Euphorbiaceae |
Castor oil plant | Beans | Ricinine | Dua et al., 2009 |
| 55 | Salvia lavandulaefolia |
Salvia Lamiaceae |
Spanish sage |
|
Reddy et al., 2010 | |
| 53 | Salvia officinalis |
Salvia Lamiaceae |
Common sage | Leaves |
|
Shiksharthi et al., 2011 |
| 54 | Salvia rosmarinus(synonym: Rosmarinus officinalis) |
Salvia Lamiaceae |
Rosemary | Leaves |
|
(Kosaka and Yokoi, 2003) |
| 55 | Schisandra chinensis |
Schisandra Schisandraceae |
Five flavor berries | (Hsieh et al., 1999) | ||
| 56 | Scutellaria baicalensis |
Scutellaria Lamiaceae |
Roots | Flavanoid: Oroxylin A | (Hwang et al., 2011) | |
| 57 | Sphaeranthus indicus |
Sphaeranthus Asteraceae |
Flowers | (Patel and Amin, 2012) | ||
| 58 | Solanum indicum |
Solanum Solanaceae |
Brinjal | Fruit | Gunawardena J | |
| 59 | Solanum melongana |
Solanum Solanaceae |
Eggplant | Fruit | Gunawardena J | |
| 60 | Taverniera cunefolia |
Taverniera Fabaceae |
Jamdhade and Surwase, 2013 | |||
| 61 | Terminalia chebula |
Terminalia Combretaceae |
Haritaki | Rhizomes | Chebulic acid | Shiksharthi et al., 2011 |
| 62 | Theobroma cacao |
Theobroma Malvaceae |
Cocoa | Seeds |
|
Adams et al., 2007 |
| 63 | Tinospora cordifolia |
Tinospora Menispermaceae |
Giloy | Whole plant |
|
Shiksharthi et al., 2011 |
| 64 | Uncaria tomentosa |
Uncaria Rubiaceae |
Bulbs | Alkaloids:
|
Heitzman et al., 2005 | |
| 65 | Vitis vinifera |
Vitis Vitaceae |
Grapes | Seeds | Resveretrol | Shiksharthi et al., 2011 |
| 66 | Withania somnifera |
Withania Solanaceae |
Ashwagandha | Roots |
|
Shiksharthi et al., 2011 |
| 67 | Zingiber officinale |
Zingiber Zingiberaceae |
Ginger | Rhizome |
|
Shiksharthi et al., 2011 |
Table 2.
Crude drugs to improve memory.
| Sl no. | Plant |
Genus Family |
Extracted in | Study model | Biological effects | Reference |
|---|---|---|---|---|---|---|
| 1 | Andrographis paniculata |
Andrographis Acanthaceae |
Methanol | Rats with streptozotocin and nicotinamide induced typeII diabetes |
|
Radhika et al., 2012 |
| 2 | Artemisia asiatica |
Artemisia Asteraceae |
Methanol | In vitro: Abeta-induced toxicity in rat PC12 cells |
|
Heo et al., 2000 |
| 3 | Butea frondosa |
Butea Fabaceae |
Hydro alcohol | Rats with scopolamine-induced amnesia |
|
Malik et al., 2012 |
| 4 | Celastrus paniculatus |
Celastrus Celastraceae |
Water | Rats with sodium nitrite induced amnesia |
|
Bhanumathy et al., 2010a, Bhanumathy et al., 2010b |
| 5 | Clitoria ternatea |
Clitoria Fabaceae |
Water | Young and adult rats | 1. Singificantly increased ACh content inrat hippocampus | Rai et al., 2002 |
| 6 | Convolvulus pluricaulis |
Convolvulus Convolvulaceae |
Water | Male Wistar rats with scopolamine induced neorutoxicity |
|
Bihaqi et al., 2011 |
| 7 | Cornus officinalis |
Cornus Cornaceae |
Methanol | HT22 hippocampal cell with glutamate toxicity | 1. Reversed glutamate toxicity in HT22 hippocampal cells | Jeong et al., 2011 |
| 8 | Corydalis ternate |
Corydalis Papaveraceae |
Methanol | Mice with scopolamine-induced memory deficit |
|
Kim et al., 1999 |
| 9 | Desmodium gangeticum |
Desmodium Fabaceae |
Water | Mice with scopolamine and ageing induced amnesia |
|
Joshi and Parle, 2006a, Joshi and Parle, 2006b, Joshi and Parle, 2006c |
| 10 | Eclipta alba |
Eclipta Asteraceae |
Distilled water | Mice | 1. Decreased transfer latency in EPM test | Banji et al., 2007 |
| 11 | Foeniculum vulgare |
Foeniculum Apiaceae |
Methanol | Mice |
|
Joshi and Parle, 2006a, Joshi and Parle, 2006b, Joshi and Parle, 2006c |
| 12 | Gastrodia elata |
Gastrodia Orchidaceae |
Methanol | Rats | 1. Increased shortened step through latency induced by scopolamine | Wu et al., 1996 |
| 13 | Glycyrrhiza glabra |
Glycyrrhiza Fabaceae |
Water | 1 month old male Wistar albino rats with Diazepam induced amnesia |
|
Chakravarthi and Avadhani, 2013 |
| 14 | Hibiscus asper |
Hibiscus Malvaceae |
Methanol | Male Wistar rats with 6-hydroxydopamine-lesion |
|
Foyet et al., 2011 |
| 15 | Hypericum perforatum |
Hypericum Hypericaceae |
Ethanol | Albino rats with scopolamine and sodium nitrite induced amnesia |
|
Kumar et al., 2000 |
| 16 | Marsilea minuta |
Marsilea Mercileaceae |
Ethanol | Rats with ECS treated and scopolamine induced amnesia |
|
Bhattamisra et al., 2012 |
| 17 | Nardostachys jatamansi |
Nardostachys Caprifoliaceae |
Ethanol | Young and aged mice with scopolamine and diazepam induced amnesia |
|
Joshi and Parle, 2006a, Joshi and Parle, 2006b, Joshi and Parle, 2006c |
| 18 | Nepeta menthoides |
Nepeta Lamiaceae |
Water | Rats with ECS induced amnesia | 1. Increased memory retention and retrieval | Sarahroodi et al., 2012 |
| 19 | Ocimum sanctum |
Ocimum Lamiaceae |
Water | Rats with scopolamine and ageing induced amnesia |
|
Joshi and Parle, 2006a, Joshi and Parle, 2006b, Joshi and Parle, 2006c |
| 20 | Schisandra chinensis |
Schisandra Schisandraceae |
Water | Rats | 1. Increased shortened step through latency induced by scopolamine | (Hsieh et al., 1999) |
| 21 | Scutellaria baicalensis |
Scutellaria Lamiaceae |
1. Rescued memory impairment caused by chronic LPS infusion | Hwang et al., 2011 | ||
| 22 | Sphaeranthus indicus |
Sphaeranthus Asteraceae |
Petrolium ether | Mice with scopolamine-induced memory impairment |
|
Patel and Amin, 2012 |
Table 3.
Different herbal formulations for memory enhancement.
| Sl no. | Hebal formuation | Components | Study model | Biological effects and targets | Reference |
|---|---|---|---|---|---|
| 1 | Abana |
|
In vivo-young and aged mice |
|
Parle and Vasudevan, 2007 |
| 2 | Brahmi-ghrita |
|
In vivo-mice |
|
Girish et al., 2004 |
| 3 | Brain-o-Brain |
|
In vivo-human (Chldren, adults and elderly people) |
|
|
| 4 | YMJD |
|
In vivo- stressed rats |
|
Rho et al., 2005 |
| 5 | Chyawanprash | 50 herbs employing Phyllanthus emblica | In vivo-mice with memory deficit |
|
Parle and Bansal, 2011 |
| 6 | Memofit |
|
In vivo- human |
|
|
| 7 | Memorin |
|
In vivo-rats with ECS-induced retrograde amnesia |
|
Vinekar et al., 1998 |
| 8 | Shimotsu-to |
|
In vivo-rats |
|
Watanabe, 1997 |
| 9 | OSS | In vivo- mice with scopalamine induced memory impairments |
|
Choi JG et al, 2011 | |
| 10 | Mentat |
|
In vivo- mice with ECS induced amnesia and Clinical trial on human (adults and children) |
|
Kulkarni and Verma,1992 |
| 11 | PMCMT |
|
In vivo- rats with ischemia-induced memory and cognitive impairment |
|
Yun et al., 2007 |
| 12 | PJBH |
|
|
Adams et al., 2007 |
Table 5.
Compound level in the natural sources.
| No. | Bioactive compound | Content | Source (part) | Reference |
|---|---|---|---|---|
| 1 | (S)-Allyl-l-cysteine (38) | 2.0–2.4 mg/100 g | Allium sativum (bulb) | Ryu and Kang, 2017 |
| 2 | Arecoline (39) | 1.5 ± 0.008% | Areca catechu (raw nuts) | Dutta et al., 2017 |
| 3 | β-Asarone (40) | 4.4% w/w | Acorus calamus (rhizome) | Hanson et al., 2005 |
| 4 | l-Ascorbic acid (23) | 34–38 mg/100 g | Phyllantuhus emblica (fruit) | Raghu et al., 2007 |
| 5 | Bacopaside I (41) | 0.29–1.36% | Bacopa monnieri (whole plant) | Christopher et al., 2017 |
| 6 | Bacoside A (42) | 1.44–5.40% | Bacopa monnieri (whole plant) | Christopher et al., 2017 |
| 7 | Baicalin (43) Baicalein (44) |
8.1–15.6% 0.2–1.2% |
Scutellaria baicalensis (roots) | Zheljazkov et al., 2007 |
| 8 | Carnosic acid (45) | 18.2–35.1 mg/g | Salvia rosmarinus (leaves) | Hidalgo et al., 1998 |
| 9 | Chebulic acid (46) | 7.99 g/kg | Terminalia chebula (dry fruit) | Pfundstein et al., 2010 |
| 10 | α-Crocin (47) | >10% | Crocus sativus (dry saffron's mass) | Shahdadi et al., 2016 |
| 11 | Curcumin (11) | 3–5% | Curcuma longa (rhizome) | Guimarães et al., 2020 |
| 12 | Emodin (48) | 156.37 ± 0.179 μg/g | Polygonum multiflorum (dried tuberous root) | Yang et al., 2018 |
| 13 | (-)-Epicatechin (EC, 49) | 270–1235 mg/100 g | Theobroma cacao (beans) | Othman et al., 2010 |
| 14 | Epigalactocatechin-3-gallate (EGCG, 12) | 7380 mg/100 g | Camellia sinensis (dried leaves) | Sharifi-Rad et al., 2020 |
| 15 | Essential oil - α-Thujone (50) - β-Thujone (51) - α-Pinene (52) - β-Pinene (53) - Borneol (54) |
0.93% − 26.68% − 5.35% − 3.58% − 5.44% − 3.69% |
|
Damyanova et al., 2016 |
| 16 | Flavonoids (55) (total content) | 14.30 mg/g | Ginkgo biloba (fresh leaves) | Shi et al., 2012 |
| 17 | Gingerol (56) | 75.25 ± 2.03 mg/100 g | Zingiber officinale (fresh rhizome) | Saensouk and Chumroenphat, 2018 |
| 18 | Ginsenoside Rg1 (20) Ginsenoside Re (57) Ginsenoside Rb1 (19) Ginsenoside Rc (58) Ginsenoside Rb2 (59) Ginsenoside Rd (60) |
3.3 mg/g 2.0 mg/g 5.8 mg/g 1.7 mg/g 2.3 mg/g 0.4 mg/g |
Panax ginseng (roots) | Lee et al., 2015 |
| 19 | Huperzine A (15) | 182.55 ± 0.18 μg/g | Huperzia serrata (moss) | Ma et al., 2005 |
| 20 | Loganin (17) | 10.4 μg/mg | Cornus officinalis (dried fruit) | Ahn et al., 2017 |
| 21 | Magnolol (61) | 2–10% | Magnolia officinalis (bark) | Patocka et al., 2006 |
| 22 | Nicotine (62) | 0.3–3.5% | Nicotiana tabacum (dried leaves) | Fatica et al., 2019 |
| 23 | Piperine (32) | 2–7.4% | Piper nigrum (fruits) | Gorgani et al., 2017 |
| 24 | Resveratrol (63) | 50–100 μg/g | Vitis vinifera (grape skin) | Singh et al., 2015 |
| 25 | Ricinine (64) | 0.13–0.26% | Ricinus communis (fresh leaves) | Burgess et al., 1988 |
| 26 | Rosmarinic acid (65) | 3.67–8.07% | Melissa officinalis (leaves) | Kittler et al., 2018 |
| 27 | Tannic acid (66) | 0.16–0.77 mg/g | Clitoria ternatea (raw mature seeds) | Al-Snafi, 2016 |
| 28 | Tannic acid (66) | 4.1 g/100 g | Punica granatum (dry seeds) | S. Mohammed, 2008 |
Table 6.
Summarized biological assays of active compounds.
| No. | Compound | Effective concentration/dose | Biological activity | Reference |
|---|---|---|---|---|
| 1 | α-Asarone (67) | IC50 = 46.38 ± 2.69 μM | In vitro AChE inhibitory potential | Mukherjee et al., 2007b |
| 2 | β-Asarone (40) | IC50 = 3.33 ± 0.02 μM | In vitro AChE inhibitory potential | Mukherjee et al., 2007b |
| 3 | Bisdemethoxycurcumin (68) | IC50 = 16.84 μM | In vitro inhibition of AChE | Ahmed and Gilani, 2009 |
| 4 | (+)-Catechin (69) | 1–10 μM | Attenuation of hippocampal cell death | Bastianetto et al., 2000 |
| 5 | Crocetin (70) | IC50 = 96.33 ± 0.11 μM | Inhibition of AChE | Geromichalos et al., 2012 |
| 6 | Curcumin (11) | IC50 = 67.69 μM | In vitro inhibition of AChE | Ahmed and Gilani, 2009 |
| 7 | Curcumin (11) | IC50 = 0.81–1 μM | Inhibition of Aβ aggregation or its disaggregation promotion | Yang et al., 2005 |
| 8 | Curcuminoids (25) | IC50 = 19.67 μM | In vitro inhibition of AChE | Ahmed and Gilani, 2009 |
| 9 | Demethoxycurcumin (71) | IC50 = 33.14 μM | In vitro inhibition of AChE | Ahmed and Gilani, 2009 |
| 10 | Dimethylcrocetin (72) | IC50 = 107.1 ± 1.9 μM | Inhibition of AChE | Geromichalos et al., 2012 |
| 11 | Ellagic acid (73) | IC50 = 45.63 μM | Inhibition of AChE | G and De, 2011 |
| 12 | Epigalactocatechin-3-gallate (EGCG, 12) | Daily oral dose of 9 mg/kg of EGCG given for 12 months | Improvement of visual recognition memory | de la Torre et al., 2016 |
| 13 | Galanthamine (74) | IC50 = 1.995 μM | Inhibition of AChE (rat cortex) | Tang and Han, 1999 |
| 14 | Gallic acid (75) | IC50 = 5.85 μM | Inhibition of AChE | G and De, 2011 |
| 15 | Ginsenoside Rg5 (76) | IC50 = 18.4 μM | Inhibition of AChE | Kim et al., 2013 |
| 16 | Ginesnoside Rh3 (77) | IC50 = 10.2 μM | Inhibition of AChE | Kim et al., 2013 |
| 17 | Huperzine A (15) | IC50 = 0.082 μM | Inhibition of AChE (rat cortex) | Tang and Han, 1999 |
| 18 | Loganin (17) | IC50 = 0.33 ± 0.05 μM | Inhibition of AChE | Bhakta et al., 2016 |
| 19 | Physostigmine (78) | IC50 = 1.42 μM | Inhibition of AChE | G and De, 2011 |
| 20 | Protopine (79) | IC50 = 50 μM | Inhibition of AChE | Kim et al., 1999 |
| 21 | Quercetin (80) | 5–25 μM | Attenuation of hippocampal cell death | Bastianetto et al., 2000 |
| 22 | Resveratrol (63) | 5–25 μM | Attenuation of hippocampal cell death | Bastianetto et al., 2000 |
| 23 | Safranal (81) | IC50 = 21.09 ± 0.17 μM | Inhibition of AChE | Geromichalos et al., 2012 |
Ginkgolides (28), a kind of terpene lactones, are derived from the living fossil tree Ginkgo biloba. Extract from Ginkgo biloba enhances the rate of acetylcholine turnover. Further, the stimulation of ligands binding to muscarinic receptors in the hippocampus was observed after application of the extract. It improves dementia and cognitive deficits. The extract also proves significant antioxidant activity via reduction of free radicals in animals. In a study, patients with dementia of AD type and multi-infract dementia were administrated with 28 supplement. The results showed improvement in their task-assessing speed for processing ability (Kumar et al., 2012a, Kumar et al., 2012b).
Ginsenosides are extracted from the plant Panax ginseng. There is about 30 major members of ginsenosides, including Rb1 (19) and Rg1 (20) (Fig. 2), as the most active compounds. Other active ingredients are Rg2 (20), Rg3 (30) etc. Both major active ginsenosides 20 and 19 can increase the neuroplasticity in efficacy and structure. A study in normal adult mice proved that small molecular drug 20 is able to improve proliferation and differentiation of neural progenitor cells in hippocampus. Moreover, it also improved a global ischemia model in gerbils. Further beneficial effects of 20 include following results: enhancement of the deteriorated immune system, improvement of motor and behavior response caused by age-related alterations, promoting activity of the hippocampal neuronal function in aged rats and partial protection against the glutamate-induced excitotoxicity. The structure of 20 consists of panaxatriol and two sugar moieties, while the structure of 19 is composed of panaxatriol with four sugar moieties. Another study revealed potential of ginsenoside Rh2 (22) to reverse suppresion of long-term potentiation induced by scopolamine in the CA1 hippocampal region (Kumar et al., 2012a, Kumar et al., 2012b). Standardized ginseng extract (G115) significantly improved retention, improves learning, greatly increased the locomotor activity of mice.
Loganin (17) was used in oral treatment of mice in passive avoidance test with Cornus officinalis fruits (100 mg/kg b.w.) as one of its major constituent (1 and 2 mg/kg b.w.). Loganin (Fig. 2) considerably reversed memory deficits induced by scopolamine. Further, orally treated mice with 17 in the Morris water maze test significantly improved memory deficits induced by scopolamine, which showed the formation of long-term and/or short-term spatial memory. Moreover, considerable inhibition of AChE activity was observed after administration of 17 (2 mg/kg b.w.) (Lee et al., 2009).
Nobiletin (18) is derived from herb Aurantii nobilis, a component of TCM. Nobiletin (Fig. 2) administered daily within four months avoided to the memory impairment in fear conditioning. Immunohistochemical analysis in transgenic mice shows hippocampal decrease of the Aβ deposi (Yamakuni et al., 2010).
Pseudocoptisine (34) is a hexacyclic quaternary alkaloid found in tubers of Corydalis turtschaninovii, which showed a dose-dependent inhibitory activity of AChE with IC50 = 12.8 μM. The oral administration of 34 at the dose of 2.0 mg/kg in mice reversed the cognitive impairment in passive avoidance test and decreased the escape latencies in training trials. According to these results, it is assumed that 34 can be a useful remedy for treatment of cognitive impairment (Huang et al., 2008).
6. Quality and safety of herbal drugs
Phytochemical profile of plants is significantly affected by the environment. In the same species, levels of bioactive constituents can vary according to geographical location, weather condition, soil composition and other aspects. Even on the same location, the chemical composition in plants varies from year to year due to local weather divergency (temperature, drought, flood, etc.). The constituent levels in wild plants are in general more variable. These negative effects in commercial plants can be reduced by standardization of cultivation techniques. Indeed, plants that originate from characterized and uniform genetic source and that grown under controlled conditions contain more stable levels of bioactive compounds.
The concentration of nootropics depends on the mechanism of plant processing. The whole plant, essential oil, roots, leaves, fruits, etc. contain various levels of desired compounds, therefore it is important to use parts of plant with higher concentration of drugs. Diverse extraction methods produce mixture of substances in various levels. Solvents, temperature, time of extraction, etc. significantly affect composition of the final mixture. In addition, some of the natural products containing bioactive compounds are prepared without standardization, therefore the composition of the products may vary between producers and even batches. Detailed standardization in plant processing is needed for a higher quality of final products.
An application of the same nootropic may result in various biological effects. The major advantage of herbal drugs is presence of several active or supporting compounds compared to a single-component drugs. This fact represents a unique challenge for the identification of active constituents and further evaluation of their bioactivity. It is further important to detect and separate any potential hazardous compounds. Inadequate separation techniques may reduce the levels of active compound and lead to reduced bioactivity. Further, the application of nootropics plays a crucial role in the bioavailability. Natural products are applied in different forms such as tablets, capsules, dried plant, raw herb, tinctures (alcoholic extracts), tisanes (hot water extracts) and others. This also impacts on the biological activity of drug.
Another interesting point to consider represents assumptions and expectations of customers in extraordinary effects of natural products. The customers usually believe that natural products on the markets are high-quality products only. However, even if the herb has been reported to have a remarkable bioactivity, there can be a significant difference in the levels of active constituents. Moreover, it is generally believed that natural products are inherently safe without adverse effects and without any chance of overdosing. Although natural products may also have adverse effects and interactions type herb-herb or herb-drug are possible. Therefore, further studies are needed for a deeper insight into the interactions of nootropics (Wachtel-Galor and Benzie 2011).
7. Conclusions and future directions
From this study, it is clear that plants, in the forms of herb foods and spices, play a vital role in enhancing poor memory (Eufoliye et al., 2012). Various medicinal plants are used worldwide (i.e. in Indian Ayurveda, TCM, Korean medicine, African medicine, American medicine etc.) and they have shown significant memory and cognition improving activity on animal models. These medicinal plants contain a variety of phytochemicals that are proven to improve cognition, intelligence, attention and concentration by maintaining the proper level of neurotransmitter Acetylcholine (ACh) inside the brain by providing a controlled activity of the enzyme Acetylcholinesterase (AChE). The different kinds of poly-herbal formulations also work well on the central nervous system. Besides preclinical studies, some clinical trials are also being made which have also given positive results. As these medicinal plants show significant results on animal models of amnesia, dementia and AD-type dementia, it can be said that there is a possibility that these herbs can be used for the treatment of some serious brain disorders in humans (e.g. AD) in near future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
A.D. was supported by “Faculty Research and Professional Development Fund” (FRPDF) from Govt. of West Bengal and Presidency University and DBT-BUILDER from Department of Biotechnology, Govt. of India.
Funding
The author 'PO' would also like to acknowledge the funding received from UHK VT2019-2021.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Patrik Oleksak, Email: patrik.oleksak@uhk.cz.
Abhijit Dey, Email: abhijit.dbs@presiuniv.ac.in.
References
- Adams M., Gmunder F., Hamburger M. Plants traditionally used in age related brain disorders- A survey on ethnobotanical literature. J. Ethnopharmacol. 2007;113:363–381. doi: 10.1016/j.jep.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Agarwa P., Sharma B., Fatima A., Jain S.K. An update on Ayurvedic herb Convolvulus pluricaulis Choisy. Asian Pacific journal of tropical biomedicine. 2014;4(3):245–252. doi: 10.1016/S2221-1691(14)60240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal R., Mishra B., Tyagi E., Nath C., Shukla R. Effect of curcumin on brain insulin receptors and memory functions in STZ (ICV) induced dementia model of rat. Pharmacol. Res. 2010;61(3):247–252. doi: 10.1016/j.phrs.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Aguiar S., Borowski T. Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res. 2013;16(4):313–326. doi: 10.1089/rej.2013.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M. Protective effects of curcumin against lithium–pilocarpine induced status epilepticus, cognitive dysfunction and oxidative stress in young rats. Saudi journal of biological sciences. 2013;20(2):155–162. doi: 10.1016/j.sjbs.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed T., Gilani A.H. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol. Biochem. Behav. 2009;91:554–559. doi: 10.1016/j.pbb.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Ahmed T., Enam S.A., Gilani A.H. Curcuminoids enhance memory in an amyloid-infused rat model of Alzheimer’s disease. Neuroscience. 2010;16:1296–1306. doi: 10.1016/j.neuroscience.2010.05.078. [DOI] [PubMed] [Google Scholar]
- Ahn J.H., Mo E.J., Jo Y.H., Kim S.B., Hwang B.Y., Lee M.K. Variation of loganin content in Cornus officinalis fruits at different extraction conditions and maturation stages. Biosci Biotechnol Biochem. 2017;81:1973–1977. doi: 10.1080/09168451.2017.1361807. [DOI] [PubMed] [Google Scholar]
- Akaike A., Shimohama S., Misu Y. Springer; Singapore: 2018. Nicotinic Acetylcholine Receptor Signaling in Neuroprotection. [PubMed] [Google Scholar]
- Akhondzadeh S., Sabet M.S., Haricharan M.H., Togha M., Cheraghmakani H., Razeghi S., Hejazi S.S., Yousefi M.H., Alimardani R., Jamshidi A., Rezazadeh S.A., Yousefi A., Zare F., Moradi A., Vossoughi A. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology. 2010;207:637–643. doi: 10.1007/s00213-009-1706-1. [DOI] [PubMed] [Google Scholar]
- Al-Snafi A. Pharmacological importance of Clitoria ternatea – A review. IOSR Journal of Pharmacy. 2016;6:68–83. [Google Scholar]
- Alzheimer's Association. (2016). 2016 Alzheimer's disease facts and figures. Alzheimer's & Dementia, 12(4), 459-509. [DOI] [PubMed]
- Arora N, Pandey-Rai S (2014) GC–MS analysis of the essential oil of Celastrus paniculatus
- Anand U, Jacobo-Herrera N, Altemimi A, Lakhssassi N. A comprehensive review on medicinal plants as antimicrobial therapeutics: potential avenues of biocompatible drug discovery. Metabolites. 2019;9(11):258. doi: 10.3390/metabo9110258. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anupama K.P., Shilpa O., Antony A., Tilagul S.K., Hunasanahally G.P. Convolvulus pluricaulis (Shankhapushpi) ameliorates human microtubule-associated protein tau (hMAPτ) induced neurotoxicity in Alzheimer’s disease Drosophila model. Journal of Chemical Neuroanatomy. 2019;95:115–122. doi: 10.1016/j.jchemneu.2017.10.002. In this issue. [DOI] [PubMed] [Google Scholar]
- Ashwlayan V.D., Singh R. Reversal effect of Phyllanthus Emblica (Euphorbiaceae) Rasayana on memory deficits in mice. Int J Appl Pharm. 2011;3:10–15. [Google Scholar]
- Atigari D.V., Gundamaraju R., Sabbithi S., Chaitanya K.B., Ramesh C. Evaluation of antiepileptic activity of methanolic extract of Celastrus paniculatus willd whole plant in rodents. Int J Pharm Phytopharmacol Res. 2012;2(1):20–25. [Google Scholar]
- Awad A.S. Effect of combined treatment with curcumin and candesartan on ischemic brain damage in mice. Journal of Stroke and Cerebrovascular Diseases. 2011;20(6):541–548. doi: 10.1016/j.jstrokecerebrovasdis.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Awad R., Muhammad A., Durst T., Trudeau V.L., Arnason J.T. Bioassay‐guided fractionation of lemon balm (Melissa officinalis L.) using an in vitro measure of GABA transaminase activity. Phytotherapy Research. 2009;23(8):1075–1081. doi: 10.1002/ptr.2712. [DOI] [PubMed] [Google Scholar]
- Baliga M.S., Shivashankara A.R., Simon P., Rao S., Palatty P.L. Polyphenols: Mechanisms of Action in Human Health and Disease. Academic Press; 2018. Hepatoprotective Effects of Green Tea and Its Polyphenols: A Revisit; pp. 415–420. [Google Scholar]
- Banerjee S., Anand U., Ghosh S., Ray D., Ray P., Nandy S., Deshmukh G.D., Tripathi V., Dey A. Bacosides from Bacopa monnieri extract: An overview of the effects on neurological disorders. Phytotherapy Research. 2021 doi: 10.1002/ptr.7203. [DOI] [PubMed] [Google Scholar]
- Banji O., Banji D., Annamalai A.R., Manavalan R. Investigation on the effect of Eclipta alba on animal models of learning and memory. Indian J. Physiol. Pharmacol. 2007;51:274–278. [PubMed] [Google Scholar]
- Bastianetto S., Zheng W.-H., Quirion R. Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br. J. Pharmacol. 2000;131:711–720. doi: 10.1038/sj.bjp.0703626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastida J., Viladomat F. Alkaloids of Narcissus. In: Hanks G., editor. Medicinal and Aromatic Plants — Industrial Profiles. Narcissus and Daffodil. The Genus Narcissus. Vol. 21. Taylor and Francis; London: 2002. pp. 141–213. [Google Scholar]
- Batiha G.E.S., Beshbishy A.M., El-Mleeh A., Abdel-Daim M.M., Devkota H.P. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules. 2020;10(3):352. doi: 10.3390/biom10030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagya V., Christofer T., Shankaranarayana Rao B.S. Neuroprotective effect of Celastrus paniculatus on chronic stress-induced cognitive impairment. Indian Journal of Pharmacology. 2016;48(6):687–693. doi: 10.4103/0253-7613.194853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakta H.K., Park C.H., Yokozawa T., Min B.-S., Jung H.A., Choi J.S. Kinetics and molecular docking studies of loganin, morroniside and 7-O-galloyl-d-sedoheptulose derived from Corni fructus as cholinesterase and β-secretase 1 inhibitors. Arch Pharm Res. 2016;39:794–805. doi: 10.1007/s12272-016-0745-5. [DOI] [PubMed] [Google Scholar]
- Bhakta H.K., Park C.H., Yokozawa T., Tanaka T., Jung H.A., Choi J.S. Potential anti-cholinesterase and beta-site amyloid precursor protein cleaving enzyme 1 inhibitory activities of cornuside and gallotannins from Cornus officinalis fruits. Arch. Pharm. Res. 2017;40:836–853. doi: 10.1007/s12272-017-0924-z. [DOI] [PubMed] [Google Scholar]
- Bhanumathy M., Chandrasekar S.B., Chandur U., Somasundaram T. Phyto-pharmacology of Celastrus paniculatus: an Overview. International Journal of Pharmaceutical Sciences and Drug Research. 2010;2(3):176–181. [Google Scholar]
- Bhanumathy M., Harish M.S., Shivaprasad H.N., Sushma G. Nootropic activity of Celastrus paniculatus seeds. Pharm. Biol. 2010;48:324–327. doi: 10.3109/13880200903127391. [DOI] [PubMed] [Google Scholar]
- Bhaskar M., Chintamaneni M. Withania somnifera and Eclipta alba ameliorate oxidative stress induced mitochondrial dysfunction in an animal model of Alzheimer’s disease. The American Journal of Phytomedicine and Clinical Therapeutics. 2014;2:140–152. [Google Scholar]
- Bhattacharjee A., Shashidhara S.C., Saha S. Phytochemical and ethnopharmacological profile of Desmodium gangeticum (L.) DC.: A review. Int. J. Biomed. Res. 2013;4:507–515. [Google Scholar]
- Bhattamisra S.K., Singh P.N., Singh S.K. Effect of standardized extract of Marsilea minuta on learning and memory performance in rat amnesic models. Pharm. Biol. 2012;50:766–772. doi: 10.3109/13880209.2011.632421. [DOI] [PubMed] [Google Scholar]
- Bihaqi S.W., Singh A.P., Tiwari M. In vivo investigation of the neuroprotective property of Convolvulus pluricaulis in scopolamine-induced cognitive impairments in Wistar rats. Indian Journal of Pharmacology. 2011;43:520–525. doi: 10.4103/0253-7613.84958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen N.I., Grothe M.J., Ray N.J., Müller M.L., Teipel S.J. Recent advances in cholinergic imaging and cognitive decline—revisiting the cholinergic hypothesis of dementia. Current geriatrics reports. 2018;7(1):1–11. doi: 10.1007/s13670-018-0234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracesco N, Sanchez A.G., Contreras V, Menini T, Gugliucci A. Recent advances on Ilex paraguariensis research: minireview. Journal of Ethnopharmacology. 2011;136(3):378–384. doi: 10.1016/j.jep.2010.06.032. [DOI] [PubMed] [Google Scholar]
- Burgess E.P.J., Koha E.M.W.T., Hutchins R.F.N., Douglas L. Toxicity of leaves from the castor oil plant, Ricinus communis L. (Euphorbiaceae), to adult grass grub, Costelytra zealandica (White) (Coleoptera: Scarabaeidae) New Zealand Journal of Experimental Agriculture. 1988;16:63–66. doi: 10.1080/03015521.1988.10425615. [DOI] [Google Scholar]
- Caesar I., Jonson M., Nilsson K.P.R., Thor S., Hammarström P. Curcumin promotes A-beta fibrillation and reduces neurotoxicity in transgenic Drosophila. PLoS ONE. 2012;7(2) doi: 10.1371/journal.pone.0031424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camina E., Güell F. The neuroanatomical, neurophysiological and psychological basis of memory: Current models and their origins. Front. Pharmacol. 2017;8:438. doi: 10.3389/fphar.2017.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthi K.K., Avadhani R. Beneficial effect of aqueous root extract of Glycyrrhiza glabra on learning and memory using different behavioral models: An experimental study. Journal of Natural Science, Biology and Medicine. 2013;4:420–425. doi: 10.4103/0976-9668.117025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari K.S., Tiwari N.R., Tiwari R.R., Sharma R.S. Neurocognitive effect of nootropic drug Brahmi (Bacopa monnieri) in Alzheimer's disease. Annals of neurosciences. 2017;24(2):111–122. doi: 10.1159/000475900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Yin Z.J., Jiang C. Asiaticoside attenuates memory impairment induced by transient cerebral ischemia-reperfusion in mice through anti-inflammatory mechanism. Pharmacol Biochem Behav. 2014;122:7–15. doi: 10.1016/j.pbb.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Chen S.Q., Wang Z.S., Ma Y.X., Zhang W., Lu J.L., Liang Y.R., Zheng X.Q. Neuroprotective effects and mechanisms of tea bioactive components in neurodegenerative diseases. Molecules. 2018;23(3):512. doi: 10.3390/molecules23030512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Pharmacopoeia Commission . Chinese Medical Science and Technology Press; Beijing: 2015. Pharmacopoeia of the People's Republic of China. [Google Scholar]
- Choi J.G., Yang W.M., Kang T.H., Oh M.S. Effects of optimized-SopungSunkiwon on memory impairment and enhancement. Neurosci. Lett. 2011;491:93–98. doi: 10.1016/j.neulet.2010.12.058. [DOI] [PubMed] [Google Scholar]
- Chonpathompikunlert P., Wattanathorn J., Muchimapura S. Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit like condition of Alzheimer's disease. Food Chem. Toxicol. 2010;48:798–802. doi: 10.1016/j.fct.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Choudhary K.M., Mishra A., Poroikov V.V., Goel R.K. Ameliorative effect of Curcumin on seizure severity, depression like behavior, learning and memory deficit in post-pentylenetetrazole-kindled mice. Eur. J. Pharmacol. 2013;704(1–3):33–40. doi: 10.1016/j.ejphar.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Christophel T.B., Klink P.C., Spitzer B., Roelfsema P.R., Haynes J.D. The distributed nature of working memory. Trends in cognitive sciences. 2017;21(2):111–124. doi: 10.1016/j.tics.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Christopher C., Johnson A.J., Mathew P.J., Baby S. Elite genotypes of Bacopa monnieri, with high contents of Bacoside A and Bacopaside I, from southern Western Ghats in India. Ind. Crops Prod. 2017;98:76–81. doi: 10.1016/j.indcrop.2017.01.018. [DOI] [Google Scholar]
- Cittadini M.C., Repossi G., Albrecht C., Di Paola Naranjo R., Miranda A.R., de Pascual‐Teresa S., Soria E.A. Effects of bioavailable phenolic compounds from Ilex paraguariensis on the brain of mice with lung adenocarcinoma. Phytotherapy Research. 2019;33(4):1142–1149. doi: 10.1002/ptr.6308. [DOI] [PubMed] [Google Scholar]
- Colovic M.B., Krstic D.Z., Lazarevic-Pasti T.D., Bondzic A.M., Vasic V.M. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr. Neuropharmacol. 2013;11(3):315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci L., Bosco M., Ziello A.R., Rea R., Amenta F., Fasanaro A.M. Effectiveness of nootropic drugs with cholinergic activity in treatment of cognitive deficit: a review. Journal of experimental pharmacology. 2012;4:163. doi: 10.2147/JEP.S35326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E.L., Ma M.J. Alzheimer disease: clues from traditional and complementary medicine. J. Tradit. Complement. Med. 2017;7:380–385. doi: 10.1016/j.jtcme.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Bujosa, H. B., & Suárez Rodríguez, R. L. (2019). Nootropics: Phytochemicals with Neuroprotective and Neurocognitive Enhancing Properties. http://dx.doi.org/10.15584/ejcem.2019.3.9
- Czerwińska M.E., Melzig M.F. Cornus mas and Cornus Officinalis—Analogies and Differences of Two Medicinal Plants Traditionally Used. Front. Pharmacol. 2018;9:894. doi: 10.3389/fphar.2018.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damyanova S., Mollova S., Stoyanova A., Gubenia O. Chemical composition of Salvia officinalis l. essential oil from Bulgaria. Ukrainian Food Journal. 2016;5:695–700. doi: 10.24263/2304-974X-2016-5-4-8. [DOI] [Google Scholar]
- Das A.J. Review on nutritional, medicinal and pharmacological properties of Centella asiatica (Indian pennywort) J Biol Active Prod Nat. 2011;1(4):216–228. [Google Scholar]
- de la Torre R., de Sola S., Hernandez G., Farré M., Pujol J., Rodriguez J., Espadaler J.M., Langohr K., Cuenca-Royo A., Principe A., Xicota L., Janel N., Catuara-Solarz S., Sanchez-Benavides G., Bléhaut H., Dueñas-Espín I., del Hoyo L., Benejam B., Blanco-Hinojo L., Videla S., Fitó M., Delabar J.M., Dierssen M. Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with Down’s syndrome (TESDAD): a double-blind, randomised, placebo-controlled, phase 2 trial. The Lancet Neurology. 2016;15:801–810. doi: 10.1016/S1474-4422(16)30034-5. [DOI] [PubMed] [Google Scholar]
- Dhuley J.N. Nootropic-like effect of ashwagandha (Withania somnifera L.) in mice. Phytother. Res. 2001;15:524–528. doi: 10.1002/ptr.874. [DOI] [PubMed] [Google Scholar]
- Downey L.A., Kean J., Nemeh F., Lau A., Poll A., Gregory R., Zangara A. An acute, double-blind, placebo-controlled crossover study of 320 mg and 640 mg doses of a special extract of Bacopa monnieri (CDRI 08) on sustained cognitive performance. Phytother. Res. 2013;27(9):1407–1413. doi: 10.1002/ptr.4864. [DOI] [PubMed] [Google Scholar]
- Dua J.S., Prasad D.N., Tripathi A.C., Gupta R. Role of traditional medicine in neuropsychopharmacology. Asian J. Pharm. Clin. Res. 2009;2:72–76. [Google Scholar]
- Dumas J.A., Newhouse P.A. The cholinergic hypothesis of cognitive aging revisited again: cholinergic functional compensation. Pharmacol. Biochem. Behav. 2011;99(2):254–261. doi: 10.1016/j.pbb.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D., Ramanna C., Kamath V. Estimation of arecoline content of various forms of areca nut preparations by high-pressure thin-layer chromatography. Journal of Advanced Clinical & Research Insights. 2017;4:31–37. doi: 10.15713/ins.jcri.153. [DOI] [Google Scholar]
- Dwivedi P., Singh R., Malik M.T., Jawaid T. A traditional approach to herbal nootropic agents: an overview. International Journal of Pharmaceutical Sciences and Research. 2012;3:630–636. [Google Scholar]
- Elufioye T.O., Oladele A.T., Cyril-Olutayo C.L., Agbedahunsi J.M., Adesanya S.A. Ethnomedicinal study and screening of plants used for memory enhancement and antiaging in Sagamu, Nigeria. European Journal of Medicinal Plants. 2012;2:262–275. [Google Scholar]
- Fang L., Gou S., Liu X., Cao F., Cheng L. Design, synthesis and anti-Alzheimer properties of dimethylaminomethyl-substituted curcumin derivatives. Bioorg. Med. Chem. Lett. 2014;24(1):40–43. doi: 10.1016/j.bmcl.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Fatica A., Di Lucia F., Marino S., Alvino A., Zuin M., De Feijter H., Brandt B., Tommasini S., Fantuz F., Salimei E. Study on analytical characteristics of Nicotiana tabacum L., cv. Solaris biomass for potential uses in nutrition and biomethane production. Sci. Rep. 2019;9:16828. doi: 10.1038/s41598-019-53237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando W.M.A.D.B., Martins I.J., Goozee K.G., Brennan C.S., Jayasena V., Martins R.N. The role of dietary coconut for the prevention and treatment of Alzheimer's disease: potential mechanisms of action. Br. J. Nutr. 2015;114(1):1–14. doi: 10.1017/S0007114515001452. [DOI] [PubMed] [Google Scholar]
- Fernando W., Somaratne G., Goozee K.G., Williams S., Singh H., Martins R.N. Diabetes and Alzheimer’s disease: can tea phytochemicals play a role in prevention? J. Alzheimers Dis. 2017;59(2):481–501. doi: 10.3233/JAD-161200. [DOI] [PubMed] [Google Scholar]
- Flôres M.F., Martins A., Schimidt H.L., Santos F.W., Izquierdo I., Mello-Carpes P.B., Carpes F.P. Effects of green tea and physical exercise on memory impairments associated with aging. Neurochem. Int. 2014;78:53–60. doi: 10.1016/j.neuint.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Foyet H.S., Hritcu L., Ciobica A., Stefan M., Kamtchouing P., Cojocaru D. Methanolic extract of Hibiscus asper leaves improves spatial memory deficits in the 6-hydroxydopamine-lesion rodent model of Parkinson's disease. J. Ethnopharmacol. 2011;133:773–779. doi: 10.1016/j.jep.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Frati, P., Kyriakou, C., Del Rio, A., Marinelli, E., Montanari Vergallo, G., Zaami, S., & P Busardo, F. 2015. Smart drugs and synthetic androgens for cognitive and physical enhancement: revolving doors of cosmetic neurology. Current neuropharmacology, 13(1), 5-11. [DOI] [PMC free article] [PubMed]
- G, N., De, B. 2011. Acetylcholinesterase inhibitory activity of Terminalia chebula, Terminalia bellerica and Emblica officinalis and some phenolic compounds. International Journal of Pharmacy and Pharmaceutical Sciences, 3, 121–124.
- Geromichalos G.D., Lamari F.N., Papandreou M.A., Trafalis D.T., Margarity M., Papageorgiou A., Sinakos Z. Saffron as a source of novel acetylcholinesterase inhibitors: molecular docking and in vitro enzymatic studies. J Agric Food Chem. 2012;60:6131–6138. doi: 10.1021/jf300589c. [DOI] [PubMed] [Google Scholar]
- Ghasemi T., Abnous K., Vahdati F., Mehri S., Razavi B.M., Hosseinzadeh H. Antidepressant effect of Crocus sativus aqueous extract and its effect on CREB, BDNF, and VGF transcript and protein levels in rat hippocampus. Drug research. 2015;65(07):337–343. doi: 10.1055/s-0034-1371876. [DOI] [PubMed] [Google Scholar]
- Giampieri F., Alvarez-Suarez J.M., Battino M. Strawberry and human health: Effects beyond antioxidant activity. J. Agric. Food. Chem. 2014;62(18):3867–3876. doi: 10.1021/jf405455n. [DOI] [PubMed] [Google Scholar]
- Golechha M., Bhatia J., Singh Arya D. Studies on effects of Emblica officinalis (Amla) on oxidative stress and cholinergic function in scopolamine induced amnesia in mice. J. Environ. Biol. 2012;33(1):95. [PubMed] [Google Scholar]
- Gorgani L., Mohammadi M., Najafpour G.D., Nikzad M. Piperine—The Bioactive Compound of Black Pepper: From Isolation to Medicinal Formulations. Compr. Rev. Food Sci. Food Saf. 2017;16:124–140. doi: 10.1111/1541-4337.12246. [DOI] [PubMed] [Google Scholar]
- Goswami S., Saoji A., Kumar N., Thawani V., Tiwari M.T. Effect of Bacopa monnieri on cognitive functions in Alzheimer’s disease patients. Int J Collaborat Res Int Med Public Health. 2011;3:285–293. [Google Scholar]
- Goupy P., Vian M.A., Chemat F., Caris-Veyrat C. Identification and quantification of flavonols, anthocyanins and lutein diesters in tepals of Crocus sativus by ultra-performance liquid chromatography coupled to diode array and ion trap mass spectrometry detections. Ind. Crops Prod. 2013;44:496–510. [Google Scholar]
- Gregory J., Vengalasetti Y.V., Bredesen D.E., Rao R.V. Neuroprotective herbs for the management of Alzheimer’s disease. Biomoleculaes. 2021;11(4):543. doi: 10.3390/biom11040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães A.F., Vinhas A.C.A., Gomes A.F., Souza L.H., Krepsky P.B., Guimarães A.F., Vinhas A.C.A., Gomes A.F., Souza L.H., Krepsky P.B. Essential oil of Curcuma longa L. rhizomes chemical composition, yield variation and stability. Quim. Nova. 2020;43:909–913. doi: 10.21577/0100-4042.20170547. [DOI] [Google Scholar]
- Gur R.C., Gur R.E. Memory in health and in schizophrenia. Dialogues in clinical neuroscience. 2013;15(4):399. doi: 10.31887/DCNS.2013.15.4/rgur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.B., Wu Y., Wang S., Yi L., Qiu R., Zhou H., Wan X., Xu X.Z., Zhou H.L., Wu Y., Hu Y.L. Chemical constituents and chemotaxonomic study of Glycyrrhiza glabra L. Biochemical Systematics and Ecology. 2020;92 doi: 10.1016/j.bse.2020.104130. [DOI] [Google Scholar]
- Hanson K.M., Gayton-Ely M., Holland L.A., Zehr P.S., Söderberg B.C.G. Rapid assessment of beta-asarone content of Acorus calamus by micellar electrokinetic capillary chromatography. Electrophoresis. 2005;26:943–946. doi: 10.1002/elps.200410165. [DOI] [PubMed] [Google Scholar]
- Hardman R.J., Kennedy G., Macpherson H., Scholey A.B., Pipingas A. Adherence to a Mediterranean-style diet and effects on cognition in adults: a qualitative evaluation and systematic review of longitudinal and prospective trials. Frontiers in nutrition. 2016;3:22. doi: 10.3389/fnut.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat K., Iqbal H., Malik U., Bilal U., Mushtaq S. Tea and its consumption: benefits and risks. Crit. Rev. Food Sci. Nutr. 2015;55(7):939–954. doi: 10.1080/10408398.2012.678949. [DOI] [PubMed] [Google Scholar]
- Heitzman M.E., Neto C.C., Winiarz E., Vaisberg A.J., Hammond G.B. Ethnobotany, phytochemistry and pharmacology of Uncaria (Rubiaceae). Phytochemistry. 2005;66(1):5–29. doi: 10.1016/j.phytochem.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Heo H.J., Yang H.C., Cho H.Y., Hong B., Lim S.T., Park H.J., Kim K.H., Kim H.K., Shin D.H. Inhibitory effect of Artemisia asiatica alkaloids on acetylcholinesterase activity from rat PC12 cells. Mol. Cells. 2000;10:253–262. [PubMed] [Google Scholar]
- Hidalgo P.J., Ubera J.L., Tena M.T., Valcárcel M. Determination of the Carnosic Acid Content in Wild and Cultivated Rosmarinus officinalis. J. Agric. Food Chem. 1998;46:2624–2627. doi: 10.1021/jf970974j. [DOI] [Google Scholar]
- Hildebrandt H. Elsevier Science & Technology; 2019. Cognitive Neuropsychological Rehabilitation of Memory: Assessment and Treatment of Memory Disorders. [Google Scholar]
- Hildt E. Springer; Dordrecht: 2013. Cognitive enhancement–A critical look at the recent debate. In Cognitive enhancement; pp. 1–14. [Google Scholar]
- Hosseinzadeh H. Saffron: a herbal medicine of third millennium. Jundishapur journal of natural pharmaceutical products. 2014;9(1):1. doi: 10.17795/jjnpp-16700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh H., Sadeghnia H.R., Ghaeni F.A., Motamedshariaty V.S., Mohajeri S.A. Effects of saffron (Crocus sativus L.) and its active constituent, crocin, on recognition and spatial memory after chronic cerebral hypoperfusion in rats. Phytother. Res. 2012;26(3):381–386. doi: 10.1002/ptr.3566. [DOI] [PubMed] [Google Scholar]
- Hsieh M.T., Tsai M.L., Peng W.H., Wu C.R. Effects of Fructus schizandrae on cycloheximide‐induced amnesia in rats. Phytotherapy Research. 1999;13(3):256–257. doi: 10.1002/(SICI)1099-1573(199905)13:3<256::AID-PTR435>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hu Y., Li C., Shen W. Gastrodin alleviates memory deficits and reduces neuropathology in a mouse model of Alzheimer's disease. Neuropathology. 2014;34(4):370–377. doi: 10.1111/neup.12115. [DOI] [PubMed] [Google Scholar]
- Huang C., Li W.G., Zhang X.B., Wang L., Xu T.L., Wu D., Li Y. Alpha-asarone from Acorus gramineus alleviates epilepsy by modulating A-type GABA receptors. Neuropharmacology. 2013;65:1–11. doi: 10.1016/j.neuropharm.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Huang J., Zhang Y., Dong L., Gao Q., Yin L., Quan H. Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis Sieb. et Zucc. J. Ethnopharmacol. 2018;213:280–301. doi: 10.1016/j.jep.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Huang M., Qian Y., Guan T., Huang L., Tang X., Li Y. Different neuroprotective responses of Ginkgolide B and bilobalide, the two Ginkgo components, in ischemic rats with hyperglycemia. Eur. J. Pharmacol. 2012;677(1–3):71–76. doi: 10.1016/j.ejphar.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Hung T.M., Ngoc T.M., Youn U.J., Min B.S., Na M., Thoung P.T., Bae K. Anti-amnestic activity of pseudocoptisine from Corydalis Tuber. Biol. Pharm. Bull. 2008;31:159–162. doi: 10.1248/bpb.31.159. [DOI] [PubMed] [Google Scholar]
- Hwang Y.K., Jinhua M., Choi B.R., Cui C.A., Jeon W.K., Kim H., Kim H.Y., Han S.H., Han J.S. Effects of Scutellaria baicalensis on chronic cerebral hypoperfusion-induced memory impairments and chronic lipopolysaccharide infusion-induced memory impairments. J. Ethnopharmacol. 2011;137:681–689. doi: 10.1016/j.jep.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Ibrahim, M., Farooq, T., Hussain, A., Hussain, N, Gulzar, T., Hussain, I., Akash, M.S., & Rehmani, F.S. 2013. Acetyl and butyryl cholinesterase inhibitory sesquiterpene lactones from Amberboa ramosa. Chemistry Central Journal, 7, 116. Ind Crop Prod 61:345–351. [DOI] [PMC free article] [PubMed]
- Jahan, R., Al-Nahain, A., Majumder, S., & Rahmatullah, M. (2014). Ethnopharmacological significance of Eclipta alba (L.) hassk. (Asteraceae). International scholarly research notices, 2014. [DOI] [PMC free article] [PubMed]
- Jahan R., Hossain S., Seraj S., Nasrin D., Khatun Z., Das P.R., Rahmatullah M. Centella asiatica (L.) Urb.: Ethnomedicinal uses and their scientific validations. American-Eurasian. Journal of Sustainable Agriculture. 2012;6(4):261–270. [Google Scholar]
- Jahanshahi M., Nikmahzar E., Yadollahi N., Ramazani K. Protective effects of Ginkgo biloba extract (EGB 761) on astrocytes of rat hippocampus after exposure with scopolamine. Anatomy & cell biology. 2012;45(2):92–96. doi: 10.5115/acb.2012.45.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamdhade V.C., Surwase B.S. Memory enhancing activity of Taverniera cuneifolia (roth) arn: a substitute for commercial liquorice. International Journal of Pharma & Bio Sciences. 2013;4:277–285. [Google Scholar]
- Jeon, J. P. (2015). Nootropics for Healthy Individuals. https://digitalrepository.trincoll.edu/cgi/viewcontent.cgi?article=1060&context=fypapers
- Jeong E.J., Kim T.B., Yang H., Kang S.Y., Kim S.Y., Sung S.H. Neuroprotective iridoid glycosides from Cornus officinalis fruits against glutamate-induced toxicity in HT22 hippocampal cells. Phytomedicine. 2012;19:317–321. doi: 10.1016/j.phymed.2011.08.068. [DOI] [PubMed] [Google Scholar]
- Jesky R., Hailong C. Are herbal compounds the next frontier for alleviating learning and memory impairments? An integrative look at memory, dementia and the promising therapeutics of traditional Chinese medicines. Phytother. Res. 2011;25:1105–1118. doi: 10.1002/ptr.3388. [DOI] [PubMed] [Google Scholar]
- Joshi H., Parle M. Antiamnesic effects of Desmodium gangeticum in mice. Yakugaku Zasshi. 2006;126:795–804. doi: 10.1248/yakushi.126.795. [DOI] [PubMed] [Google Scholar]
- Joshi H., Parle M. Evaluation of nootropic potential of Ocimum sanctum Linn. in mice. Indian J. Exp. Biol. 2006;44:133–136. [PubMed] [Google Scholar]
- Joshi H., Parle M. Nardostachys jatamansi Improves Learning and Memory in Mice. J. Med. Food. 2006;9:113–118. doi: 10.1089/jmf.2006.9.113. [DOI] [PubMed] [Google Scholar]
- Joshi R.K. Acorus calamus Linn.: phytoconstituents and bactericidal property. World J. Microbiol. Biotechnol. 2016;32(10):164. doi: 10.1007/s11274-016-2124-2. [DOI] [PubMed] [Google Scholar]
- Kandel E.R., Dudai Y., Mayford M.R. The molecular and systems biology of memory. Cell. 2014;157(1):163–186. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Katekhaye S., Duggal S., Singh A.P. An inside preview of nutritional and pharmacological profile of Celastrus paniculatus. Int J Recent Adv Pharm Res. 2011;1:19–24. [Google Scholar]
- Kiasalari Z., Roghani M., Khalili M., Rahmati B., Baluchnejadmojarad T. Antiepileptogenic effect of curcumin on kainate-induced model of temporal lobe epilepsy. Pharm. Biol. 2013;51(12):1572–1578. doi: 10.3109/13880209.2013.803128. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Jeon S.J., Son K.H., Jun J.W., Lee S., Yoon B.H., Choi J.W., Cheong J.H., Ko K.H., Ryu J.H. Effect of the flavonoid, oroxylin A, on transient cerebral hypoperfusion-induced memory impairment in mice. Pharmacology Biochemistry and Behavior. 2006;85:658–668. doi: 10.1016/j.pbb.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Kim E.-J., Jung I.-H., Van Le T.K., Jeong J.-J., Kim N.-J., Kim D.-H. Ginsenosides Rg5 and Rh3 protect scopolamine-induced memory deficits in mice. J Ethnopharmacol. 2013;146:294–299. doi: 10.1016/j.jep.2012.12.047. [DOI] [PubMed] [Google Scholar]
- Kim S.R., Hwang S.Y., Jang Y.P., Park M.J., Markelonis G.J., Oh T.H., Kim Y.C. Protopine from Corydalis ternata has anticholinesterase and antiamnesic activities. Planta Med. 1999;65:218–221. doi: 10.1055/s-1999-13983. [DOI] [PubMed] [Google Scholar]
- Kittler J., Krüger H., Ulrich D., Zeiger B., Schütze W., Böttcher Ch., Krähmer A., Gudi G., Kästner U., Heuberger H., Marthe F. Content and composition of essential oil and content of rosmarinic acid in lemon balm and balm genotypes (Melissa officinalis) Genet Resour Crop Evol. 2018;65:1517–1527. doi: 10.1007/s10722-018-0635-4. [DOI] [Google Scholar]
- Kuete, V. (2017). Medicinal spices and vegetables from Africa.
- Kopustinskiene D.M., Bernatoniene J. Antioxidant Effects of Schisandra chinensis Fruits and Their Active Constituents. Antioxidants (Basel) 2021;10(4):620. doi: 10.3390/antiox10040620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K., Yokoi T. Carnosic acid, a component of rosemary (Rosmarinus officinalis L.), promotes synthesis of nerve growth factor in T98G human glioblastoma cells. Biological & Pharmaceutical Bulletin. 2003;26(11):1620–1622. doi: 10.1248/bpb.26.1620. [DOI] [PubMed] [Google Scholar]
- Kulkarni R., Girish K.J., Kumar A. Nootropic herbs (Medhya Rasayana) in Ayurveda: an update. Pharmacogn. Rev. 2012;6(12):147. doi: 10.4103/0973-7847.99949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Singh, S., E Barreto, G., Aliev, G., & Echeverria, V. 2017. Ginkgo biloba as an alternative medicine in the treatment of anxiety in dementia and other psychiatric disorders. Current drug metabolism, 18(2), 112-119. [DOI] [PubMed]
- Kumar H., Kim B.W., Song S.Y., Kim J.S., Kim I.S., Kwon Y.S., Choi D.K. Cognitive enhancing effects of alpha asarone in amnesic mice by influencing cholinergic and antioxidant defense mechanisms. Biosci. Biotechnol. Biochem. 2012;76(8):1518–1522. doi: 10.1271/bbb.120247. [DOI] [PubMed] [Google Scholar]
- Kumar H., More S.V., Han S.D., Choi J.Y., Choi D.K. Promising therapeutics with natural bioactive compounds for improving learning and memory- a review of randomized trials. Molecules. 2012;17:10503–10539. doi: 10.3390/molecules170910503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H., Song S.Y., More S.V., Kang S.M., Kim B.W., Kim I.S., Choi D.K. Traditional Korean East Asian medicines and herbal formulations for cognitive impairment. Molecules. 2013;18:14670–14693. doi: 10.3390/molecules181214670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Maheshwari K., Singh V. Protective effects of Punica granatum seeds extract against aging and scopolamine induced cognitive impairments in mice. Afr. J. Tradit. Complement. Altern. Med. 2009;6:49–56. doi: 10.4314/ajtcam.v6i1.57073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Singh P.M., Murugnandam A.V., Bhattacharya S.K. Effect of Indian Hypericum perforatum Linn on animal models of cognitive dysfunction. J. Ethnopharmacol. 2000;72:119–128. doi: 10.1016/s0378-8741(00)00216-6. [DOI] [PubMed] [Google Scholar]
- Lapchak P.A. Neuroprotective and neurotrophic curcuminoids to treat stroke: a translational perspective. Expert Opin. Invest. Drugs. 2011;20(1):13–22. doi: 10.1517/13543784.2011.542410. [DOI] [PubMed] [Google Scholar]
- Le X.T., Pham H.T.N., Van Nguyen T., Nguyen K.M., Tanaka K., Fujiwara H., Matsumoto K. Protective effects of Bacopa monnieri on ischemia-induced cognitive deficits in mice: the possible contribution of bacopaside I and underlying mechanism. J. Ethnopharmacol. 2015;164:37–45. doi: 10.1016/j.jep.2015.01.041. [DOI] [PubMed] [Google Scholar]
- Lee S.M., Bae B.-S., Park H.-W., Ahn N.-G., Cho B.-G., Cho Y.-L., Kwak Y.-S. Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J. Ginseng Res. 2015;39:384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Lee Y.M., Lee C.K., Jung J.K., Han S.B., Hong J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011;130(2):157–176. doi: 10.1016/j.pharmthera.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Lee Y.K., Yuk D.Y., Ilkin T. Protective effect of the ethanol extracts of Magnolia officinalis and 4-0-methylhonokiol on scopolamine-induced memory impairment and the inhibition of acetylcholinesterase activity. Nat. Med. 2009;63:274–282. doi: 10.1007/s11418-009-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legeay S., Rodier M., Fillon L., Faure S., Clere N. Epigallocatechin gallate: a review of its beneficial properties to prevent metabolic syndrome. Nutrients. 2015;7(7):5443–5468. doi: 10.3390/nu7075230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lele R.D. Four new approaches for validation of Ayurvedic herbal drugs. International journal of Ayurveda research. 2010;1(3):136. doi: 10.4103/0974-7788.72483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Zhang Y., Su T., Feng L., Long Y., Chen Z. Silica-coated flexible liposomes as a nanohybrid delivery system for enhanced oral bioavailability of curcumin. Int. J. Nanomed. 2012;7:5995. doi: 10.2147/IJN.S38043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C.H., Lin J.Y. Purification, partial characterization and anti-inflammatory characteristics of lotus (Nelumbo nucifera Gaertn) plumule polysaccharides. Food Chem. 2012;135(3):1818–1827. doi: 10.1016/j.foodchem.2012.06.063. [DOI] [PubMed] [Google Scholar]
- Lim H.W., Kumar H., Kim B.W., More S.V., Kim I.W., Park J.I., Choi D.K. β-Asarone (cis-2, 4, 5-trimethoxy-1-allyl phenyl), attenuates pro-inflammatory mediators by inhibiting NF-κB signaling and the JNK pathway in LPS activated BV-2 microglia cells. Food Chem. Toxicol. 2014;72:265–272. doi: 10.1016/j.fct.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Liu X., Wang M.Y. Improving effects of gastrodin on learning and memory in model rats with Alzheimer disease induced by intra-hippocampal Aβ1-40 injection. Chin. J. Clin. Pharmacol. Ther. 2012;17:408–411. [Google Scholar]
- Liu, Z. J., Liu, W., Liu, L., Xiao, C., Wang, Y., & Jiao, J. S. 2013. Curcumin protects neuron against cerebral ischemia-induced inflammation through improving PPAR-gamma function. Evidence-Based Complementary and Alternative Medicine, 2013. [DOI] [PMC free article] [PubMed]
- Lu M.C., Hsieh M.T., Wu C.R., Cheng H.Y., Hsieh C.C., Lin Y.T., Peng W.H. Ameliorating effect of emodin, a constitute of Polygonatum multiflorum, on cycloheximide-induced impairment of memory consolidation in rats. J. Ethnopharmacol. 2007;112:552–556. doi: 10.1016/j.jep.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Lynch G., Cox C.D., Gall C.M. Pharmacological enhancement of memory or cognition in normal subjects. Front. Syst. Neurosci. 2014;8:90. doi: 10.3389/fnsys.2014.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Wang K.J., Cheng C.S., Yan G.Q., Lu W.L., Ge J.F., Li N. Bioactive compounds from Cornus officinalis fruits and their effects on diabetic nephropathy. J. Ethnopharmacol. 2014;153(3):840–845. doi: 10.1016/j.jep.2014.03.051. [DOI] [PubMed] [Google Scholar]
- Ma X., Tan C., Zhu D., Gang D.R. Is There a Better Source of Huperzine A than Huperzia serrata? Huperzine A Content of Huperziaceae Species in China. J. Agric. Food Chem. 2005;53:1393–1398. doi: 10.1021/jf048193n. [DOI] [PubMed] [Google Scholar]
- Ma X., Zheng C., Hu C., Rahman K., Qin L. The genus Desmodium (Fabaceae)-traditional uses in Chinese medicine, phytochemistry and pharmacology. J. Ethnopharmacol. 2011;138(2):314–332. doi: 10.1016/j.jep.2011.09.053. [DOI] [PubMed] [Google Scholar]
- Mahajan K., Kumar D., Kumar S. Antiamnesic activity of extracts and fraction of Desmodium gangeticum. JPTRM. 2015;3:67–77. [Google Scholar]
- Mahmood T., Akhtar N., Khan B.A. The morphology, characteristics, and medicinal properties of Camellia sinensis’ tea. J Med Plants Res. 2010;4(19):2028–2033. [Google Scholar]
- Malik J., Karan M., Dogra R. Ameliorating effect of Celastrus paniculatus standardized extract and its fractions on 3-nitropropionic acid induced neuronal damage in rats: Possible antioxidant mechanism. Pharm. Biol. 2017;55(1):980–990. doi: 10.1080/13880209.2017.1285945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik J., Kumar M., Deshmukh R., Kumar P. Ameliorating effect of lyophilized extract of Butea frondosa leaves on scopolamine-induced amnesia in rats. Pharm. Biol. 2013;51:233–239. doi: 10.3109/13880209.2012.717229. [DOI] [PubMed] [Google Scholar]
- Manhas A., Khanna V., Prakash P., Goyal D., Malasoni R., Naqvi A., Jagavelu K. Curcuma oil reduces endothelial cell-mediated inflammation in postmyocardial ischemia/reperfusion in rats. J. Cardiovasc. Pharmacol. 2014;64(3):228–236. doi: 10.1097/FJC.0000000000000110. [DOI] [PubMed] [Google Scholar]
- Mannangatti P., Naidu K.N. The Benefits of Natural Products for Neurodegenerative Diseases. Springer; Cham: 2016. Indian herbs for the treatment of neurodegenerative disease; pp. 323–336. [DOI] [PubMed] [Google Scholar]
- Mansouri Z., Sabetkasaei M., Moradi F., Masoudnia F., Ataie A. Curcumin has neuroprotection effect on homocysteine rat model of Parkinson. J. Mol. Neurosci. 2012;47(2):234–242. doi: 10.1007/s12031-012-9727-3. [DOI] [PubMed] [Google Scholar]
- Martins A., Schimidt H.L., Garcia A., Altermann C.D.C., Santos F.W., Carpes F.P., Mello-Carpes P.B. Supplementation with different teas from Camellia sinensis prevents memory deficits and hippocampus oxidative stress in ischemia-reperfusion. Neurochem. Int. 2017;108:287–295. doi: 10.1016/j.neuint.2017.04.019. [DOI] [PubMed] [Google Scholar]
- May B.H., Feng M., Zhou I.W., Chang S.Y., Lu S.C., Zhang A.L., Xue C.C. Memory impairment, dementia, and Alzheimer's disease in classical and contemporary traditional Chinese medicine. The Journal of Alternative and Complementary Medicine. 2016;22(9):695–705. doi: 10.1089/acm.2016.0070. [DOI] [PubMed] [Google Scholar]
- Meesarapee B., Thampithak A., Jaisin Y., Sanvarinda P., Suksamrarn A., Tuchinda P., Sanvarinda Y. Curcumin I mediates neuroprotective effect through attenuation of quinoprotein formation, p-p38 MAPK expression, and caspase-3 activation in 6-hydroxydopamine treated SH-SY5Y cells. Phytother. Res. 2014;28(4):611–616. doi: 10.1002/ptr.5036. [DOI] [PubMed] [Google Scholar]
- Mishra M. Wild harvesting and management of some medicinal plants in the natural forest of Central India. Indian J Fundam Appl Life Sci. 2011;1(2):90–97. [Google Scholar]
- Molz P., Schröder N. Potential therapeutic effects of lipoic acid on memory deficits related to aging and neurodegeneration. Front. Pharmacol. 2017;8:849. doi: 10.3389/fphar.2017.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.C., Spink J., Lipp M. Development and application of a database of food ingredient fraud and economically motivated adulteration from 1980 to 2010. J. Food Sci. 2012;77(4):R118–R126. doi: 10.1111/j.1750-3841.2012.02657.x. [DOI] [PubMed] [Google Scholar]
- Morè L., Lauterborn J.C., Papaleo F., Brambilla R. Enhancing cognition through pharmacological and environmental interventions: Examples from preclinical models of neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2020;110:28–45. doi: 10.1016/j.neubiorev.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Moshiri M., Vahabzadeh M., Hosseinzadeh H. Clinical applications of saffron (Crocus sativus) and its constituents: a review. Drug research. 2015;65(06):287–295. doi: 10.1055/s-0034-1375681. [DOI] [PubMed] [Google Scholar]
- Mukherjee P.K., Kumar V., Mal M., Houghton P.J. Acorus calamus.: SCIENTIFIC validation of Ayurvedic tradition from natural resources. Pharm. Biol. 2007;45(8):651–666. [Google Scholar]
- Mukherjee P., Venkatesan K., Mal M., Houghton P. In vitro Acetylcholinesterase Inhibitory Activity of the Essential Oil from Acorus calamus and its Main Constituents. Planta Med. 2007;73:283–285. doi: 10.1055/s-2007-967114. [DOI] [PubMed] [Google Scholar]
- Nahata A., Patil U.K., Dixit V.K. Effect of Convulvulus pluricaulis Choisy. on learning behaviour and memory enhancement activity in rodents. Nat. Prod. Res. 2008;22:1472–1482. doi: 10.1080/14786410802214199. [DOI] [PubMed] [Google Scholar]
- Nandakumar S., Menon S., Shailajan S. A rapid HPLC-ESI-MS/MS method for determination of β-asarone, a potential anti-epileptic agent, in plasma after oral administration of Acorus calamus extract to rats. Biomed. Chromatogr. 2013;27(3):318–326. doi: 10.1002/bmc.2794. [DOI] [PubMed] [Google Scholar]
- Neale C., Camfield D., Reay J., Stough C., Scholey A. Cognitive effects of two nutraceuticals G inseng and B acopa benchmarked against modafinil: a review and comparison of effect sizes. Br. J. Clin. Pharmacol. 2013;75(3):728–737. doi: 10.1111/bcp.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor N.A., Ezz H.S.A., Faraag A.R., Khadrawy Y.A. Evaluation of the antiepileptic effect of curcumin and Nigella sativa oil in the pilocarpine model of epilepsy in comparison with valproate. Epilepsy Behav. 2012;24(2):199–206. doi: 10.1016/j.yebeh.2012.03.026. [DOI] [PubMed] [Google Scholar]
- Oliveira A.I., Pinho C., Sarmento B., Dias A.C.P. Neuroprotective activity of Hypericum perforatum and its major components. Frontiers in Plant Science. 2016;7:1004. doi: 10.3389/fpls.2016.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onakpoya I., Spencer E., Heneghan C., Thompson M. The effect of green tea on blood pressure and lipid profile: a systematic review and meta-analysis of randomized clinical trials. Nutrition, Metabolism and Cardiovascular Diseases. 2014;24(8):823–836. doi: 10.1016/j.numecd.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Onaolapo A.Y., Obelawo A.Y., Onaolapo O.J. Brain Ageing, Cognition and Diet: A Review of the Emerging Roles of Food-Based Nootropics in Mitigating Age-related Memory Decline. Current aging science. 2019;12(1):2–14. doi: 10.2174/1874609812666190311160754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orhan I.E., Atasu E., Senol F.S., Ozturk N., Demirci B., Das K., Sekeroglu N. Comparative studies on Turkish and Indian Centella asiatica (L.) Urban (gotu kola) samples for their enzyme inhibitory and antioxidant effects and phytochemical characterization. Ind. Crops Prod. 2013;47:316–322. [Google Scholar]
- Othman, A., Jalil, A., Jalil, M., Kong, K.W., A, I., Abd, N., Ghani, Adenan, I. 2010. Epicatechin content and antioxidant capacity of cocoa beans from four different countries. Afr J Biotechnol, 9. https://doi.org/10.5897/AJB09.1219
- Palle S., Kanakalatha A., Kavitha C.N. Gastroprotective and antiulcer effects of Celastrus paniculatus seed oil against several gastric ulcer models in rats. Journal of Dietary Supplements. 2018;15(4):373–385. doi: 10.1080/19390211.2017.1349231. [DOI] [PubMed] [Google Scholar]
- Pandit S., Mukherjee P.K., Ponnusankar S., Venkatesh M., Srikanth N. Metabolism mediated interaction of α-asarone and Acorus calamus with CYP3A4 and CYP2D6. Fitoterapia. 2011;82(3):369–374. doi: 10.1016/j.fitote.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Park C.H., Kim S.H., Choi W., Lee Y.J., Kim J.S., Kang S.S., Suh Y.H. Novel anticholinesterase and antiamnesic activities of dehydroevodiamine, a constituent of Evodia rutaecarpa. Planta Med. 1996;62:405–409. doi: 10.1055/s-2006-957926. [DOI] [PubMed] [Google Scholar]
- Parki A., Chaubey P., Prakash O., Kumar R., Pant A.K. Seasonal variation in essential oil compositions and antioxidant properties of Acorus calamus L. accessions. Medicines. 2017;4(4):81. doi: 10.3390/medicines4040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parle M., Bansal N. Antiamnesic activity of an ayurvedic formulation chyawanprash in mice. EvidenceBased Complementary and Alternative Medicine. 2011;2011:1–9. doi: 10.1093/ecam/neq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parle M., Vasudevan M. Memory enhancing activity of Abana: an Indian Ayurvedic Poly-Herbal formulation. J. Health Sci. 2007;53:43–52. [Google Scholar]
- Parle M., Vasudevan M. Memory-enhancing activity of Coriamdrum sativum in rats. Pharmacologyonline. 2009;2:827–839. [Google Scholar]
- Parle M., Dhingra D. Ascorbic acid: a promising memory-enhancer in mice. Journal of Pharmacological Sciences. 2003;93(2):129–135. doi: 10.1254/jphs.93.129. [DOI] [PubMed] [Google Scholar]
- Parle M., Dhingra D., Kulkarni S.K. Improvement of mouse memory by Myristica fragrans seeds. J. Med. Food. 2004;7:157–161. doi: 10.1089/1096620041224193. [DOI] [PubMed] [Google Scholar]
- Patocka, J., Jakl, J., Strunecka, A. 2006. Expectations of biologically active compounds of the genus Magnolia in biomedicine. Journal of Applied Biomedicine, 4. https://doi.org/10.32725/jab.2006.019
- Patel M.B., Amin D. Sphaeranthus indicus flower derived constituents exhibits synergistic effect against acetylcholinesterase and possess potential antiamnestic activity. Journal of Complementary & Integrative Medicine. 2012;9 doi: 10.1515/1553-3840.1618. [DOI] [PubMed] [Google Scholar]
- Peng D.U., Tang H.Y., Xin L.I., Lin H.J., Peng W.F., Yu M.A., Xin W.A.N.G. Anticonvulsive and antioxidant effects of curcumin on pilocarpine-induced seizures in rats. Chin. Med. J. 2012;125(11):1975–1979. [PubMed] [Google Scholar]
- Perry E., Howes M.J.R. Medicinal plants and dementia therapy: herbal hopes for brain aging? CNS Neurosci. Ther. 2011;17(6):683–698. doi: 10.1111/j.1755-5949.2010.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peth-Nui, T., Wattanathorn, J., Muchimapura, S., Tong-Un, T., Piyavhatkul, N., Rangseekajee, P., ... & Vittaya-areekul, S. 2012. Effects of 12-week Bacopa monnieri consumption on attention, cognitive processing, working memory, and functions of both cholinergic and monoaminergic systems in healthy elderly volunteers. Evidence-Based Complementary and Alternative Medicine, 2012. [DOI] [PMC free article] [PubMed]
- Pfundstein B., El Desouky S.K., Hull W.E., Haubner R., Erben G., Owen R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): characterization, quantitation and determination of antioxidant capacities. Phytochemistry. 2010;71:1132–1148. doi: 10.1016/j.phytochem.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Pieramico V., Esposito R., Cesinaro S., Frazzini V., Sensi S. Effects of non-pharmacological or pharmacological interventions on cognition and brain plasticity of aging individuals. Front. Syst. Neurosci. 2014;8:153. doi: 10.3389/fnsys.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito C.A., Cai Z.Y., Shi Y.L., Li X.M., Yang R., Shi M., Ye J.H. association of tea consumption with risk of Alzheimer’s disease and anti-beta-amyloid effects of tea. Nutrients. 2018;10(5):655. doi: 10.3390/nu10050655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle B.R. How does the brain keep information “in mind”? Current directions in psychological science. 2016;25(3):151–156. doi: 10.1177/0963721416643063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prediger R.D., Fernandes M.S., Rial D, Wopereis S, Pereira V.S., Bosse T.S>, Da Silva C.B., Carradore R.S., Machado M.S., Cechinel-Filho V., Costa-Campos L. Effects of acute administration of the hydroalcoholic extract of mate tea leaves (Ilex paraguariensis) in animal models of learning and memory. Journal of Ethnopharmacology. 2008;120(3):465–473. doi: 10.1016/j.jep.2008.09.018. In this issue. [DOI] [PubMed] [Google Scholar]
- Rachitha P., Krupashree K., Jayashree G.V., Kandikattu H.K., Amruta N., Gopalan N., Rao M.K., Khanum F. Chemical composition, antioxidant potential, macromolecule damage and neuroprotective activity of Convolvulus pluricaulis. Journal of Traditional and Complementary Medicine. 2018;8(4):483–496. doi: 10.1016/j.jtcme.2017.11.002. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhika P., Annapurna A., Rao S.N. Immunostimulant, cerebroprotective & nootropic activities of Andrographis paniculata leaves extract in normal & type 2 diabetic rats. Indian Journal of Medicinal Research. 2012;135:636–641. [PMC free article] [PubMed] [Google Scholar]
- Raghu V., Platel K., Srinivasan K. Comparison of ascorbic acid content of Emblica officinalis fruits determined by different analytical methods. J. Food Compos. Anal. 2007;20:529–533. doi: 10.1016/j.jfca.2007.02.006. [DOI] [Google Scholar]
- Rastogi M., Ojha R., Prabu P.C., Devi D.P., Agrawal A., Dubey G.P. Prevention of age-associated neurodegeneration and promotion of healthy brain ageing in female Wistar rats by long term use of bacosides. Biogerontology. 2012;13:183–195. doi: 10.1007/s10522-011-9367-y. [DOI] [PubMed] [Google Scholar]
- Rathee P., Chaudhary H., Rathee S., Rathee D. Phcog rev.: review article natural memory boosters. Pharmacogn. Rev. 2008;2:249–256. [Google Scholar]
- Ravichandra V.D., Ramesh C., Sridhar K.A. Hepatoprotective potentials of aqueous extract of Convolvulus pluricaulis against thioacetamide induced liver damage in rats. Biomedicine & Aging Pathology. 2013;3(3):131–135. [Google Scholar]
- Reddy K.Y., Lakshmi S.M., Kumar A.S. Review on effect of natural memory enhancing drugs on dementia. International Journal of Phytopharmacology. 2010;1:1–7. [Google Scholar]
- Reena K., Abhimanyu K., Kumar K.N.S. Formulation and standardization of Medhya Rasayana–A novel Ayurvedic compound nootropic drug. Pharmacognosy Journal. 2013;5(2):72–76. [Google Scholar]
- Ren C., Ji Y.Q., Liu H., Wang Z., Wang J.H., Zhang C.Y., Yin P.Y. Effects of Ginkgo biloba extract EGb761 on neural differentiation of stem cells offer new hope for neurological disease treatment. Neural Regener. Res. 2019;14(7):1152. doi: 10.4103/1673-5374.251191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho S., Kang M., Choi B., Sim D., Lee J., Lee E., Cho C., Oh J.W., Park S., Ko S., Shin M., Hong M., Bae H. Effects of yukmijihwang-tang derivatives (YMJD), a memory enhancing herbal extract, on the gene-expression profile in the rat hippocampus. Biol. Pharm. Bull. 2005;28:87–93. doi: 10.1248/bpb.28.87. [DOI] [PubMed] [Google Scholar]
- Rudrapaul P., Kyriakopoulos A.M., De U.C., Zoumpourlis V., Dinda B. New flavonoids from the fruits of Cornus mas. Cornaceae. Phytochem. Lett. 2015;11:292–295. [Google Scholar]
- Russo E., Scicchitano F., Whalley B.J., Mazzitello C., Ciriaco M., Esposito S., Patanè M., Upton R., Pugliese M., Chimirri S., Mammì M., Palleria C., De Sarro G. Hypericum perforatum: pharmacokinetic, mechanism of action, tolerability, and clinical drug–drug interactions. Phytotherapy Research. 2014;28(5):643–655. doi: 10.1002/ptr.5050. [DOI] [PubMed] [Google Scholar]
- Ryu J.H., Kang D. Physicochemical Properties, Biological Activity, Health Benefits, and General Limitations of Aged Black Garlic: A Review. Molecules. 2017;22:919. doi: 10.3390/molecules22060919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S. Mohammed, H., 2008. Inhibitory effect of tannic acid extracted from grape seeds and pomegranate peels on some microorganisms. Mesopotamia Journal of Agriculture, 36, 12–18. https://doi.org/10.33899/magrj.2008.26578
- Sabaragamuwa R., Perera C.O., Fedrizzi B. Centella asiatica (Gotu kola) as a neuroprotectant and its potential role in healthy ageing. Trends Food Sci. Technol. 2018;79:88–97. [Google Scholar]
- Saensouk S., Chumroenphat T. by different drying metthods. International Congress: Botanical research in Tropical Asia. Zingiberaceae; Project: 2018. 6-gingerol content of ginger (Zingiber officinale Roscoe) [Google Scholar]
- Samini F., Samarghandian S., Borji A., Mohammadi G. Curcumin pretreatment attenuates brain lesion size and improves neurological function following traumatic brain injury in the rat. Pharmacol. Biochem. Behav. 2013;110:238–244. doi: 10.1016/j.pbb.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Santos E.C.S., Bicca M.A., Blum-Silva C.H., Costa A.P.R., Dos Santos A.A., Schenkel E.P., Farina M., Reginatto F.H., De Lima T.C.M. Anxiolytic-like, stimulant and neuroprotective effects of Ilex paraguariensis extracts in mice. Neuroscience. 2015;292:13–21. doi: 10.1016/j.neuroscience.2015.02.004. In this issue. [DOI] [PubMed] [Google Scholar]
- Sarahroodi S., Jafari-Najafi R., Nasri S., Rohampour K., Maleki-Jamshid A., Esmaeili S. Effects of Nepeta menthoides aqueous extract on retention and retrieval of memory in mice. Pak. J. Biol. Sci. 2012;15:1085–1089. doi: 10.3923/pjbs.2012.1085.1089. [DOI] [PubMed] [Google Scholar]
- Schimidt H.L., Garcia A., Martins A., Mello-Carpes P.B., Carpes F.P. Green tea supplementation produces better neuroprotective effects than red and black tea in Alzheimer-like rat model. Food Res. Int. 2017;100:442–448. doi: 10.1016/j.foodres.2017.07.026. [DOI] [PubMed] [Google Scholar]
- Schimidt H.L., Vieira A., Altermann C., Martins A., Sosa P., Santos F.W., Carpes F.P. Memory deficits and oxidative stress in cerebral ischemia–reperfusion: Neuroprotective role of physical exercise and green tea supplementation. Neurobiol. Learn. Mem. 2014;114:242–250. doi: 10.1016/j.nlm.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Semwal P., Kapoor T., Anthwal P., Sati B., Thapliyal A. Herbal extract as potential modulator and drug for synaptic pasticity and neurodegenerative disorders. International Journal of Pharmaceutical Sciences Review and Research. 2014;25:69–79. [Google Scholar]
- Shahdadi H., Barati F., Bahador R.S., Eteghadi A. Clinical applications of saffron (Crocus sativus) and its constituents: A literature review. Der Pharmacia Lettre. 2016;8:205–209. [Google Scholar]
- Shaikh M.F., Sancheti J., Sathaye S. Phytochemical and pharmacological investigations of Eclipta alba (Linn.) Hassak leaves for antiepileptic activity. International. Journal of Pharmacy and Pharmaceutical Sciences. 2012;4(4):319–323. [Google Scholar]
- Shaikh M.F., Sancheti J., Sathaye S. Effect of Eclipta alba on acute seizure models: a GABAA-mediated effect. Indian journal of pharmaceutical sciences. 2013;75(3):380. doi: 10.4103/0250-474X.117432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad M., Pezzani R., Redaelli M., Zorzan M., Imran M., Ahmed Khalil A., Salehi B., Sharopov F., Cho W.C., Sharifi-Rad J. Preclinical Activities of Epigallocatechin Gallate in Signaling Pathways in Cancer. Molecules. 2020;25(3):467. doi: 10.3390/molecules25030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V., Singh I., Chaudhary P. Acorus calamus (The Healing Plant): a review on its medicinal potential, micropropagation and conservation. Nat. Prod. Res. 2014;28(18):1454–1466. doi: 10.1080/14786419.2014.915827. [DOI] [PubMed] [Google Scholar]
- Sheffler Z.M., Pillarisetty L.S. StatPearls [Internet] StatPearls Publishing; 2019. Physiology, Neurotransmitters. [Google Scholar]
- Shethiya N.K., Mishra S.H. Review on ethnomedicinal uses and phytopharmacology of memory boosting herb Convolvulus pluricaulis Choisy. Australian Journal of Medical Herbalism. 2010;22:19–25. [Google Scholar]
- Shi J., Zou X., Zhao J., Mel H., Wang K., Wang X., Chen H. Determination of total flavonoids content in fresh Ginkgo biloba leaf with different colors using near infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012;94:271–276. doi: 10.1016/j.saa.2012.03.078. [DOI] [PubMed] [Google Scholar]
- Shiksharthi A.R., Mittal S., Ramana J. Systemic review of herbals as potential memory enhancers. International Journal of Research in Pharmaceutical and Biomedical Sciences. 2011;3:918–925. [Google Scholar]
- Shin J.W., Cheong Y.J., Koo Y.M., Kim S., Noh C.K., Son Y.H., Sohn N.W. α-Asarone ameliorates memory deficit in lipopolysaccharide-treated mice via suppression of pro-inflammatory cytokines and microglial activation. Biomolecules & therapeutics. 2014;22(1):17. doi: 10.4062/biomolther.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivashankara A.R., Kumar A., Ravi R., Simon P., Rai P., Francis A., Baliga M.S. Hepatoprotective Effects of Green Tea and its Polyphenols: Preclinical Observations. Academic Press; 2014. pp. 715–721. [Google Scholar]
- SHL Kim, D., Y Kim, J., & Han, Y. (2012). Curcuminoids in neurodegenerative diseases. Recent patents on CNS drug discovery, 7(3), 184-204. [DOI] [PubMed]
- Singh C.K., Liu X., Ahmad N. Resveratrol, in its natural combination in whole grape, for health promotion and disease management. Ann N Y Acad Sci. 2015;1348:150–160. doi: 10.1111/nyas.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H.K. Brain Enhancing Ingredients from Āyurvedic Medicine: Quintessential Example of Bacopa monniera, a Narrative Review. Nutrients. 2013;5:478–497. doi: 10.3390/nu5020478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Sharma P. Hepatoprotective effect of curcumin on lindane-induced oxidative stress in male wistar rats. Toxicology international. 2011;18(2):124. doi: 10.4103/0971-6580.84264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Gautam A, Sharma A et al. 2010 Centella asiatica (L.): a plant with immense medicinal potential but threatened. Int J Pharm Sci. 4(2):Article 003. ISSN 0976-044X.
- Soumyanath A, Zhong YP, Henson E. Centella asiatica extract improves behavioral deficits in a mouse model of Alzheimer’s disease: investigation of a possible mechanism of action. Int J Alzheimers Dis. 2012. Article id: 381974. [DOI] [PMC free article] [PubMed]
- Srivastava A., Srivastava P., Pandey A., Khanna V.K., Pant A.B. New Look to Phytomedicine. Academic Press; 2019. Phytomedicine: A Potential Alternative Medicine in Controlling Neurological Disorders; pp. 625–655. [Google Scholar]
- Sticher O., Salama O. Glycosides of Euphrasia species. Part 1. Euphroside, a newiridoid glucoside from Euphrasia salisburgensis Hoppe. Helv. Chim. Acta. 1981;6:78–81. [Google Scholar]
- Suliman, N. A., Taib, M., Norma, C., Moklas, M., Aris, M., Adenan, M. I., ... & Basir, R. 2016. Establishing natural nootropics: recent molecular enhancement influenced by natural nootropic. Evidence-Based Complementary and Alternative Medicine, 2016. [DOI] [PMC free article] [PubMed]
- Sunil A.G., Kesavanarayanan K.S., Kalaivani P., Sathiya S., Ranju V., Priya R.J., Pramila B., Paul F.D.S., Venkhatesh J., Babu C.S. Total oligomeric flavonoids of Cyperus rotundus ameliorates neurological deficits, excitotoxicity and behavioral alterations induced by cerebral ischemic–reperfusion injury in rats. Brain Research Bulletin. 2011;84(6):394–405. doi: 10.1016/j.brainresbull.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Miyoshi N., Hayakawa S., Imai S., Isemura M., Nakamura Y. Health benefits of tea consumption. Humana Press; Cham: 2016. pp. 49–67. [Google Scholar]
- Szopa A., Ekiert R., Ekiert H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: a review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochemistry Reviews. 2017;16(2):195–218. doi: 10.1007/s11101-016-9470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabassum R., Vaibhav K., Shrivastava P. Centella asiatica attenuates the neurobehavioral, neurochemical and histological changes in transient focal middle cerebral artery occlusion rats. Neurol Sci. 2013;34(6):925–933. doi: 10.1007/s10072-012-1163-1. [DOI] [PubMed] [Google Scholar]
- Talih F., Ajaltouni J. Probable Nootropicinduced Psychiatric Adverse Effects: A Series of Four Cases. Innovations in clinical neuroscience. 2015;12(11–12):21. [PMC free article] [PubMed] [Google Scholar]
- Tandon B., Anand U., Alex B.K., Kaur P., Nandy S., Shekhawat M.S., Sanyal R., Pandey D.K., Koshy E.P., Dey A. Statistical optimization of in vitro callus induction of wild and cultivated varieties of Mucuna pruriens L. (DC.) using response surface methodology and assessment of L-Dopa biosynthesis. Industrial Crops Products. 2021;169 doi: 10.1016/j.indcrop.2021.113626. [DOI] [Google Scholar]
- Tang X.C., Han Y.F. Pharmacological Profile of Huperzine A, a Novel Acetylcholinesterase Inhibitor from Chinese Herb. CNS Drug Rev. 1999;5:281–300. doi: 10.1111/j.1527-3458.1999.tb00105.x. [DOI] [Google Scholar]
- Tashakori-Sabzevar F., Hosseinzadeh H., Sadat Motamedshariaty V., Reza Movassaghi A., Ahmad Mohajeri S. Crocetin attenuates spatial learning dysfunction and hippocampal injury in a model of vascular dementia. Current neurovascular research. 2013;10(4):325–334. doi: 10.2174/15672026113109990032. [DOI] [PubMed] [Google Scholar]
- Tripanichkul W., Jaroensuppaperch E.O. Ameliorating effects of curcumin on 6-OHDA-induced dopaminergic denervation, glial response, and SOD1 reduction in the striatum of hemiparkinsonian mice. Eur Rev Med Pharmacol Sci. 2013;17(10):1360–1368. [PubMed] [Google Scholar]
- Vallejo M.G., Ortega M.G., Cabrera J.L., Carlini V.P., Barioglio S.R., Almiron R.S., Ramirez O.A., Agnese A.M. Sauroine, an alkaloid from Huperzia saururus with activity in wistar rats in electrophysiological and behavioral assays related to memory retention. Journal of National Products. 2009;72:156–158. doi: 10.1021/np800151v. [DOI] [PubMed] [Google Scholar]
- Variya B.C., Bakrania A.K., Patel S.S. Emblica officinalis (Amla): A review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol. Res. 2016;111:180–200. doi: 10.1016/j.phrs.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Vellas B., Coley N., Ousset P.J., Berrut G., Dartigues J.F., Dubois B., Touchon J. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer's disease (GuidAge): a randomised placebo-controlled trial. The Lancet Neurology. 2012;11(10):851–859. doi: 10.1016/S1474-4422(12)70206-5. [DOI] [PubMed] [Google Scholar]
- Villaflores O.B., Chen Y.J., Chen C.P., Yeh J.M., Wu T.Y. Curcuminoids and resveratrol as anti-Alzheimer agents. Taiwanese Journal of Obstetrics and Gynecology. 2012;51(4):515–525. doi: 10.1016/j.tjog.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Vinekar A.S., Andrade C., Sriprada V.T., George J., Joseph T., Chandra J.S. Attenuation of ECS-induced retrograde amnesia by using an herbal formulation. The Journal of ECT. 1998;14:83–88. [PubMed] [Google Scholar]
- Wachtel-Galor S., Benzie I.F.F. Herbal Medicine: An Introduction to Its History, Usage, Regulation, Current Trends, and Research Needs. In: Benzie I.F.F., Wachtel-Galor S., editors. Herbal Medicine: Biomolecular and Clinical Aspects. CRC Press/Taylor & Francis; Boca Raton (FL): 2011. [PubMed] [Google Scholar]
- Wang C., Han Z. Ginkgo biloba extract enhances differentiation and performance of neural stem cells in mouse cochlea. Cell. Mol. Neurobiol. 2015;35(6):861–869. doi: 10.1007/s10571-015-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, H. 1997. Candidates for cognitive enhancer extracted from medicinal plants: paeoniflorin and tetramethylpyrazine. Behavioural Brain Research, 83, 135-41. Willd. seeds and antioxidant, anti-inflammatory study of its various solvent extracts. [DOI] [PubMed]
- Wang S.W., Wang Y.J., Su Y.J., Zhou W.W., Yang S.G., Zhang R., Zhao M., Li Y.N., Zhang Z.P., Zhan D.W., Liu R.T. Rutin inhibits β-amyloid aggregation and cytotoxicity, attenuates oxidative stress, and decreases the production of nitric oxide and proinflammatory cytokines. Neurotoxicology. 2012;33(3):482–490. doi: 10.1016/j.neuro.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Winblad B., Amouyel P., Andrieu S., Ballard C., Brayne C., Brodaty H., Fratiglioni L. Defeating Alzheimer's disease and other dementias: a priority for European science and society. The Lancet Neurology. 2016;15(5):455–532. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]
- Woodbury A., Yu S.P., Wei L., Garcia P. Neuro-modulating effects of honokiol: a review. Frontiers in Neurology. 2013;4:130. doi: 10.3389/fneur.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortmann M. World Alzheimer report 2014: Dementia and risk reduction. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2015;11(7):P837. [Google Scholar]
- Wu A., Ying Z., Schubert D., Gomez-Pinilla F. Brain and spinal cord interaction: a dietary curcumin derivative counteracts locomotor and cognitive deficits after brain trauma. Neurorehabilitation and Neural Repair. 2011;25(4):332–342. doi: 10.1177/1545968310397706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S.S., Singh M.K., Singh P.K., Kumar V. Traditional knowledge to clinical trials: a review on therapeutic actions of Emblica officinalis. Biomed. Pharmacother. 2017;93:1292–1302. doi: 10.1016/j.biopha.2017.07.065. [DOI] [PubMed] [Google Scholar]
- Yamakuni T., Nakajima A., Ohizumi Y. Preventive action of nobiletin, a constituent of AURANTII NOBILIS PERICARPIUM with anti-dementia activity, against amyloid-beta peptide-induced neurotoxicity expression and memory impairment. Yakugaku ZasshiJournal of the Pharmaceutical Society of Japan. 2010;130:517–520. doi: 10.1248/yakushi.130.517. [DOI] [PubMed] [Google Scholar]
- Yan H., Li L., Tang X.C. Treating senile dementia with traditional Chinese medicine. Clin Interv Aging. 2007;2:201–208. [PMC free article] [PubMed] [Google Scholar]
- Yang E.J., Park G.H., Song K.S. Neuroprotective effects of liquiritigenin isolated from licorice roots on glutamate-induced apoptosis in hippocampal neuronal cells. Neurotoxicology. 2013;39:114–123. doi: 10.1016/j.neuro.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Yang C.S., Wang H. Cancer preventive activities of tea catechins. Molecules. 2016;21(12):1679. doi: 10.3390/molecules21121679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.S., Chen G., Wu Q. Recent scientific studies of a traditional Chinese medicine, tea, on prevention of chronic diseases. Journal of traditional and complementary medicine. 2014;4(1):17–23. doi: 10.4103/2225-4110.124326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Lim G.P., Begum A.N., Ubeda O.J., Simmons M.R., Ambegaokar S.S., Chen P.P., Kayed R., Glabe C.G., Frautschy S.A., Cole G.M. Curcumin Inhibits Formation of Amyloid β Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid in Vivo*. J. Biol. Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- Yang, H.J., Kim, M.J., Kang, H.J., Lee1, H.Y., Park1, Y.M., Lee1, Y.H., Kang1, Y.G., Hwa1, G.P., Kang2, Y.S., Jung2, Y.M., Lee3, N.K., Park4, K.-H. 2018. Immunomodulating Properties of Polygonum multiflorum Extracts on Cyclophosphamide-induced Immunosuppression Model. Indian Journal of Pharmaceutical Sciences, 80, 749–755. https://doi.org/10.4172/pharmaceutical-sciences.1000416
- Yarmolinsky J., Gon G., Edwards P. Effect of tea on blood pressure for secondary prevention of cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2015;73(4):236–246. doi: 10.1093/nutrit/nuv001. [DOI] [PubMed] [Google Scholar]
- Yokogoshi, H. 2017. 22 Green Tea in the Protection against Neurodegeneration. Health Benefits of Green Tea: An Evidence-based Approach, 185.
- Yu L., Yi J., Ye G., Zheng Y., Song Z., Yang Y., Bao Q. Effects of curcumin on levels of nitric oxide synthase and AQP-4 in a rat model of hypoxia–ischemic brain damage. Brain Res. 2012;1475:88–95. doi: 10.1016/j.brainres.2012.07.055. [DOI] [PubMed] [Google Scholar]
- Yuan F., Yu R., Yin Y., Shen J., Dong Q., Zhong L., Song L. Structure characterization and antioxidant activity of a novel polysaccharide isolated from Ginkgo biloba. Int. J. Biol. Macromol. 2010;46(4):436–439. doi: 10.1016/j.ijbiomac.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Yun Y.J., Lee B., Hahm D.H., Kang S.K., Han S.M., Lee H.J., Pyun K.H., Shim I. Neuroprotective effect of palmul-chongmyeong-tang on ischemia-induced learning and memory deficits in the rat. Biol. Pharm. Bull. 2007;30:337–342. doi: 10.1248/bpb.30.337. [DOI] [PubMed] [Google Scholar]
- Zaami S., Rinaldi R., Bersani G., Del Rio A., Ciallella C., Marinelli E. Nootropics use in the workplace: psychiatric and ethical aftermath towards the new frontier of bioengineering. Eur. Rev. Med. Pharmacol. Sci. 2020;24(4):2129–2139. doi: 10.26355/eurrev_202002_20393. [DOI] [PubMed] [Google Scholar]
- Zamani Taghizadeh Rabe, S., Sahebari, M., Mahmoudi, Z., Hosseinzadeh, H., Haghmorad, D., Tabasi, N., ... & Mahmoudi, M. 2015. Inhibitory effect of Crocus sativus L. ethanol extract on adjuvant-induced arthritis. Food and agricultural immunology, 26(2), 170-180.
- Zeka K., Ruparelia K.C., Continenza M.A., Stagos D., Vegliò F., Arroo R.R. Petals of Crocus sativus L. as a potential source of the antioxidants crocin and kaempferol. Fitoterapia. 2015;107:128–134. doi: 10.1016/j.fitote.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Zhan H.D., Zhou H.Y., Sui Y.P., Du X.L., Wang W.H., Dai L. The rhizome of Gastrodia elata Blume-An ethnopharmacological review. J. Ethnopharmacol. 2016;189:361–385. doi: 10.1016/j.jep.2016.06.057. [DOI] [PubMed] [Google Scholar]
- Zhang C.Y., Chen R., Wang F., Ren C., Zhang P., Li Q., Liu C.F. EGb-761 attenuates the anti-proliferative activity of fluoride via DDK1 in PC-12 cells. Neurochem. Res. 2017;42(2):606–614. doi: 10.1007/s11064-016-2115-6. [DOI] [PubMed] [Google Scholar]
- Zhang, C., Qin, Y. Y., Wei, X., Yu, F. F., Zhou, Y. H., & He, J. 2015. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. [DOI] [PubMed]
- Zhang C., Ren C., Chen H., Geng R., Fan H., Zhao H., Geng D. The analog of Ginkgo biloba extract 761 is a protective factor of cognitive impairment induced by chronic fluorosis. Biol. Trace Elem. Res. 2013;153(1–3):229–236. doi: 10.1007/s12011-013-9645-4. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang H., Si L., Li Y. Curcumin mediates presenilin-1 activity to reduce β-amyloid production in a model of Alzheimer’s disease. Pharmacol. Rep. 2011;63(5):1101–1108. doi: 10.1016/s1734-1140(11)70629-6. [DOI] [PubMed] [Google Scholar]
- Zhao J., Yu S., Zheng W., Feng G., Luo G., Wang L., Zhao Y. Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochem. Res. 2010;35(3):374–379. doi: 10.1007/s11064-009-0065-y. [DOI] [PubMed] [Google Scholar]
- Zhao L.H., Ding Y.X., Zhang L., Li L. Cornel iridoid glycoside improves memory ability & promotes neuronal survival in fimbria-fornix transected rats. European journal of Phermacology. 2010;647:68–74. doi: 10.1016/j.ejphar.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Zhao X., Xu Y., Zhao Q., Chen C.R., Liu A.M., Huang Z.L. Curcumin exerts antinociceptive effects in a mouse model of neuropathic pain: descending monoamine system and opioid receptors are differentially involved. Neuropharmacology. 2012;62(2):843–854. doi: 10.1016/j.neuropharm.2011.08.050. [DOI] [PubMed] [Google Scholar]
- Zheljazkov V.D., Cantrell C.L., Ebelhar M.W., Coker C., Evans W.B. Quality Assessment and Yield of Baikal Skullcap (Scutellaria baicalensis) Grown at Multiple Locations. HortScience. 2007;42:1183–1187. doi: 10.21273/HORTSCI.42.5.1183. [DOI] [Google Scholar]
- Zhou N.N., Zhu R., Zhao X.M., Liang P. Effect and mechanism of traditional Chinese herbs against Abeta expression in brain tissues of mice with Alzheimer's disease. Chin. J. Path. 2016;45:780–785. doi: 10.3760/cma.j.issn.0529-5807.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Zhu R., Huang T.X., Zhao X.M., Zhang J.M., Liang P. Screening of 10 types of Chinese herbal compounds inhibiting Abeta and their possible related mechanism in vitro. Acta Pharm. Sin. 2014;49:800–806. [PubMed] [Google Scholar]
- Zlotnik G., Vansintjan A. Memory: An extended definition. Front. Psychol. 2019;10 doi: 10.3389/fpsyg.2019.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]