Abstract
Background
The novel coronavirus (COVID-19) pandemic has affected humans worldwide and led to unprecedented stress and mortality. Detrimental effects of the pandemic on mental health, including risk of post-traumatic stress disorder (PTSD), have become an increasing concern. The identification of prospective neurobiological vulnerability markers for developing PTSD symptom during the pandemic is thus of high importance.
Methods
Before the COVID-19 outbreak (September 20, 2019–January 11, 2020), some healthy participants underwent resting-state functional connectivity MRI (rs-fcMRI) acquisition. We assessed the PTSD symptomology of these individuals during the peak of COVID-19 pandemic (February 21, 2020–February 28, 2020) in China. This pseudo-prospective cohort design allowed us to test whether the pre-pandemic neural connectome status could predict the risk of developing PTSD symptom during the pandemic.
Results
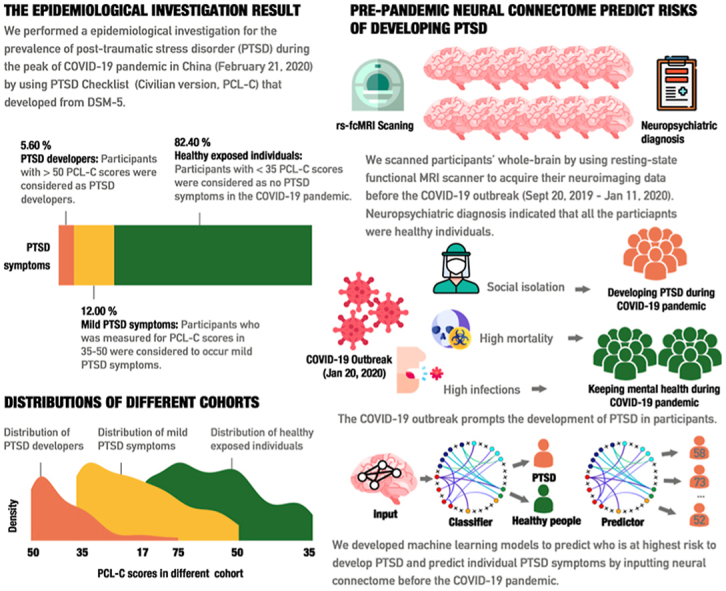
A total of 5.60% of participants (n = 42) were identified as being high-risk to develop PTSD symptom and 12.00% (n = 90) exhibited critical levels of PTSD symptoms during the COVID-19 pandemic. Pre-pandemic measures of functional connectivity (the neural connectome) prospectively classified those with heightened risk to develop PTSD symptom from matched controls (Accuracy = 76.19%, Sensitivity = 80.95%, Specificity = 71.43%). The trained classifier generalized to an independent sample. Continuous prediction models revealed that the same connectome could accurately predict the severity of PTSD symptoms within individuals (r2 = 0.31p<.0).
Conclusions
This study confirms COVID-19 break as a crucial stressor to bring risks developing PTSD symptom and demonstrates that brain functional markers can prospectively identify individuals at risk to develop PTSD symptom.
Keywords: COVID-19, Post-traumatic stress disorder, Deep learning, Prospective diagnosis
Graphical abstract
Highlights
-
•
COVID-19 pandemic brought out PTSD symptoms indeed.
-
•
The predated neural conncectome status can reliably predict vulnerability for developing the PTSD during COVID-19 pandemic.
-
•
The neural connectome preceding to COVID-19 pandemic can predict the severity of PTSD symptoms.
-
•
We provided a robust classifier to forewarn individuals at high risks of developing PTSD in the future.
1. Introduction
The outbreak of the novel 2019 coronavirus (COVID-19) pandemic started in January 2020 and has subsequently had an unprecedented impact on mental health. Despite identification of the viral causes of illness (respiratory syndrome coronavirus 2 (SARS-CoV-2)) (Paules et al., 2020), no effective antiviral treatments have been developed to control the symptoms of the disorder. Despite the development of efficient vaccines, the death toll and prevalence numbers for long term disabilities resulting from the virus remain high (Cao et al., 2020; Geleris et al., 2020; Holmes et al., 2020; Wang et al., 2020). A second global COVID-19 wave is further upending daily life around the world, including leading to strict lockdown policies in several European countries (see Dataset. S1).
Given the impact of the pandemic, concerns regarding the detrimental effects of the it are increasing. Of particular concern are psychiatric and psychological sequela of the pandemic. The high death toll of COVID-19, the risk of being infected and the limited measures to control the trajectory of the disorder contribute to increased stress and anxiety in the general population. Convergent evidence from initial studies demonstrated strongly increasing rates of stress-associated psychiatric disorders, including post-traumatic stress disorder (PTSD) and depression, as well as increasing suicide rates (Holmes et al., 2020) during the COVID-19 pandemic. As such, approaches that can help to identify individuals who are at an increased vulnerability to develop these mental disorders are urgently needed. The accurate identification of vulnerable individuals is also of utmost importance for the targeted development of preventive interventions and for identifying those in need of long-term treatment.
The COVID-19 pandemic poses a considerable threat to an unprecedented number of individuals, and thus is likely to lead to increased levels of PTSD symptoms, particularly in those with a pre-existing vulnerability. PTSD is a trauma-related psychiatric disorder that can develop after experiencing or witnessing a life-threatening event. Core symptoms include exaggerated anxiety and arousal as well as intrusive memories and avoidance which persist over months and cause substantial distress and functional impairments (Brewin et al., 2010; Hoge et al., 2004; Liberzon and Abelson, 2016; Mary et al., 2020; Thomas et al., 2010). Current neurobiological conceptualizations of PTSD suggest that trauma-induced dysregulation and preceding individual variations of specific brain systems could represent a crucial pathophysiologicical endpoint as well as vulnerability marker of PTSD (Yehuda, 2002). Evidence from animal models further promotes a biological vulnerability perspective such that variability in brain functional networks predicts the vulnerability to develop PTSD in rats following exposure to traumatic events (Dopfel et al., 2019). An understanding of the brain-based PTSD vulnerability markers in humans could therefore potentially contribute to the identification of individuals at highest risk to develop PTSD symptom during the COVID-19 pandemic.
Previous research on the neurobiological mechanism underlying PTSD mainly identified dysfunctions in five underlying domains and associated neural circuits which are implicated in fear learning, threat detection, emotion regulation/executive functions, contextual processing, and episodic prospection network (Shalev et al., 2017). Dysregulated fear learning has been consistently mapped to hyperactivity of a neural network, encompassing the amygdala, dorsal anterior cingulate gyrus (dACC), insula, and hippocampus (Andero et al., 2013; Shin et al., 2006). Threat detection and the detection of potentially dangerous stimuli has been mapped to the insula and dACC which represent core nodes of the brain salience network (SAN) (Seeley et al., 2007). PTSD has been closely linked to impaired emotional regulation, a regulatory function which critically relies on the integrity of frontoparietal network (FPN) and which is crucial for the fear learning (Anticevic et al., 2012; Niendam et al., 2012). On the symptomatic level hypoactivation of the FPN has been associated with biased attention to traumatic stimuli and intrusive memories (Ochsner et al., 2012; Philip et al., 2018). Finally, emerging studies also propose a neuropathological role of the episodic memory network (EMN) in PTSD (Pitman et al., 2012; Shin and Liberzon, 2010), such that a lack of functional integrity in the EM-associated hippocampal-prefrontal-thalamic circuitry has been associated with deficient threat/safety discrimination during fear generalization in patients with PTSD (Kheirbek et al., 2012). Recently, disrupted integrity of the ventral attention network (VAN) has also been identified as a potential biomarker of developing PTSD (Etkin et al., 2019). In sum, convergent evidence indicates that pathology of PTSD is accompanied by alterations in a neural connectome that involved into fear learning, threat detection, emotion regulation/executive functions, contextual processing, and also episodic prospection.
Against this background the present study capitalized on large-scale resting-state functional connectivity MRI (rs-fcMRI) data acquired before the COVID-19 pandemic (Sept 17, 2019–Jan 11, 2020) to predict risk of developing PTSD symptom during the peak of this pandemic in China (Feb 21, 2020). Briefly, 1211 participants were recruited and underwent brain rs-fcMRI scanning, and 750 of these participants were eligible (see Method and Materials). Two rounds of follow-up investigations were performed for this sample. In the first follow-up examination during the peak of the COVID-19 pandemic in China (Feb 21, 2020–Feb 28, 2020), levels of PTSD symptomatology were assessed with the PCL-C test and 42 participants were identified as high-risk population to develop PTSD symptom (PTSD+). This data allowed us to adopt a pseudo-prospective cohort design which aimed at determining prospective neurobiological markers for the propensity to development of PTSD symptom basing on the pre-pandemic neural connectome. To reach this goal, we selected 42 participants without PTSD symptomatology, but with matching demographic features and acquisition date to the PTSD + group, to serve as the healthy control group (PTSD-). Despite the large-scale sample, 84 participants were screened eligible for the current study. All the included participants underwent neuropsychiatric examinations, and showed no depression symptom, anxiety symptoms, insomnia, or recent experiences of negative life events prior to the COVID-19 outbreak (see Table 1).
Table 1.
Demographic information for samples. Hands represents participants' handedness, and OC indicates whether the participant is an only offspring in the family. In addition, FS describes whether the participant has foster parents, whilst SES refers to the economic status of participants. As to neuropsychiatric examinations, sub-scale of The State-Trait Anxiety Inventory (STAI), namely trait anxiety inventory (TAI) was used to check anxiety symptoms for participants. PCL-C (The PTSD Cheeklist-Civilian Version) was used to measure one's PTSD symptom. Self-rating depression scale (SDS) was adopted to test one's depression symptom. Adolescent Self-Rating Life Event Scale Checklist (ASLES) was utilized to investigate their recent negative life events. Positive and Negative Affect Scale (PANAS) was also conducted to acquire their emotional status at scanning date, with P for positive affect and N for negative affect. Pittsburg sleep quality index scale (PSQI) was used to identify whether one suffers from insomnia (see more details for neuropsychological examinations in Supplementary Materials). EI represents COVID-19 epidemic index (see Method and Materials section). Statistics are provided to show whether there were significant differences between the PTSD+ and PTSD-, using a non-parametric statistical model for quantifiable variables and a contingency table analysis for counted variables. BF indicates Bayesian factors of corresponding statistics (see Method and Materials for more details).
| PTSD + (main sample) |
PTSD- (main sample) |
BF10 | PTSD + (validation sample) |
PTSD- (validation sample) |
BF10 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| male | female | male | female | male | female | male | female | |||
| Gender | 18 | 24 | 16 | 26 | 0.45 | 9 | 7 | 6 | 10 | 0.91 |
| Age (S.D) | 20.20 (1.37) | 20.20 (1.32) | 20.00 (1.50) | 19.80 (1.36) | 0.55 | 20.10 (1.30) | 20.19 (0.91) | 19.69 (0.72) | 19.62 (0.89) | 0.75 |
| Races (%) | 66.67 (Han); 33.33 (others) | 73.80 (Han); 26.19 (others) | 0.01 | 68.75 (Han); 31.25 (Others) | 100.0 (Han); 0.00 (Others) | 2.11 | ||||
| OC (%) | 38.10 (OC); 61.90 (others) | 38.09 (OC); 61.90 (others) | 0.26 | 50.00 (OC); 50.00 (Others) | 43.75 (OC); 56.25 (Others) | 0.47 | ||||
| FS (%) | 90.47 (Parents); 9.52 (others) | 85.71 (Parents); 14.28 (others) | 0.03 | 87.50 (Parent); 12.50 (Other) | 87.50 (Parent); 12.50 (Other) | – | ||||
| SES (%) | 83.33 (Poor); 16.67 (others) | 71.42 (Poor); 25.88 (others) | 1.54 | 87.50 (Poor); 12.50 (Others) | 81.25 (Poor); 18.75 (Others) | 0.99 | ||||
| PCL-C | 56.07 ± 5.07 | 10.44 ± 3.22 | 5885* | 56.75 ± 10.04 | 40.25 ± 3.34 | 37* | ||||
| TAI | 38.62 ± 7.63 | 40.17 ± 7.05 | 0.34 | 40.44 ± 8.17 | 41.66 ± 6.71 | 0.23 | ||||
| SDS | 42.83 ± 6.34 | 42.53 ± 7.47 | 0.23 | 43.43 ± 5.21 | 41.87 ± 4.24 | 0.44 | ||||
| ASLES | 53.67 ± 12.71 | 51.26 ± 13.67 | 0.31 | 50.56 ± 10.41 | 51.73 ± 13.63 | 0.66 | ||||
| PANAS-P | 28.05 ± 6.06 | 29.21 ± 6.67 | 0.31 | 29.41 ± 6.89 | 30.01 ± 5.15 | 0.22 | ||||
| PANAS-N | 20.21 ± 6.25 | 18.17 ± 5.78 | .12 | 15.26 ± 5.03 | 15.10 ± 4.72 | 0.18 | ||||
| PSQI | 10.02 ± 2.91 | 9.98 ± 3.04 | .94 | 9.80 ± 3.41 | 9.01 ± 3.95 | 0.36 | ||||
| EI | 0.89 ± 1.14 | 0.92 ± 0.14 | .80 | 0.93 ± 0.56 | 0.89 ± 0.33 | 0.54 | ||||
During April 24, 2020 to May 1, 2020, a second follow-up investigation was collected, which identified additional 16 additional participants meeting the criteria for PTSD+. Likewise, 16 healthy participants matching demographic features and scanning dates were designated as the healthy control group, which was constituted as an independent test sample for validation. Participants in this independent sample were also screened for no depression symptoms, anxiety symptoms, insomnia, or recent experiences of negative life events before the COVID-19 outbreak (see Table 1).
Both support vector machine model (SVM) and ensemble machine learning algorithms were used for classifying individuals with high risk to develop PTSD symptom based on their pre-pandemic neural connectome. Further, given that the classifiers are promising for forwarning PTSD symptom, these classifiers trained in the current study have been provided in open access. The connectome measures were further used to predict continuous levels of PTSD symptom severity across individuals (see Fig. 1). Taken altogether, the current study aims to develop reliable neural models that allow the accurate identifications of high-risk individuals who will develop PTSD symptom, and predict the severity of PTSD symptoms within those individuals. Our goal is to provide a crucial forewarning system to identify individuals at an elevated risk to develop PTSD symptom – and validate this system using the COVID-19 pandemic as a testbed.
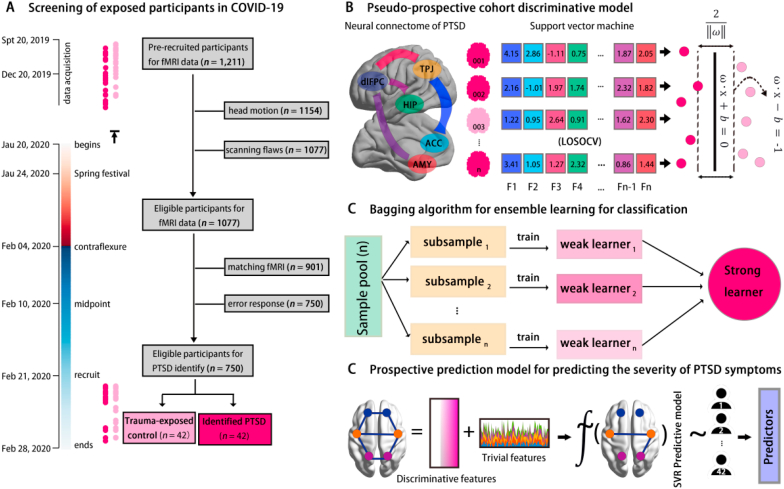
Fig. 1.
Workflow and framework of this study. Panel A illustrates the screening procedures of eligible participants for this study. The left bar denotes the timeline of the data acquisition in this study, with the “data acquisition” referring to the time duration of brain scanning, “begins” referring to the date of the beginning of the COVID-19 epidemic in mainland China, “recruit” for the beginning date of the follow-up data acquisition, and “ends” for the end date of follow-up data acquisition. The red bar refers to the timeline before the onset of the COVID-19 epidemic in mainland China, whilst the blue bar refers to the timeline after the onset of the COVID-19 epidemic in mainland China. Dots alongside of the timeline show the corresponding date of scanning (Top) and follow-up tests (Bottom) for PTSD+ (dark pink) and PTSD- (light pink). Panel B describes the support vector machine model (SVM) for the pseudo-prospective cohort design. The features of this model are the neural connectome of the PTSD network and the performance of the SVM that are obtained in leave-one-subject-out cross-validation (LOSOCV). Panel C depicts the model of ensemble learning by using Baggoing sampling. Panel D refers to the predictive framework of the pseudo-prospective cohort design. Discriminative features identified in the classification model were used as a feature for prediction of PTSD symptoms in the PTSD+. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2. Materials and methods
To achieve high reproducibility and transparency, “ProjectTemplate” package was drawn to record and trace all the data processes (see SM). This study has been approved by IRB of Southwest University (IRBSWU2002021).
2.1. Participants
42 participants (PTSD+, 24 females, Age: 20.20 ± 1.34, Range: 19–26) were identified as high-risk to develop PTSD symptom during the COVID-19 pandemic by using PTSD Checklist-Civilian Version (PCL-C), and 42 participants (PTSD-, 26 females, Age: 19.87 ± 1.40, Range: 18–26) without PTSD symptom were chosen as healthy controls by matching the scanning date and demographic features with PTSD+. All included participants were free from history of neuropsychiatric illness or intracranial/extracranial damage, and were not menstruating, pregnant, or breastfeeding. They were screened for no severe PTSD-related psychiatric conditions, such as depression, anxiety and sleep problems (see Fig. 1, Table 1 and SM).
2.2. PTSD + identifications
Given well psychometric properties, the PTSD Checklist-Civilian Version (PCL-C) was used in follow-up investigation. To identify PTSD symptoms of exposed participants in COVID-19 epidemic, the PCL-C was modified slightly to coincide with research goal (Conybeare et al., 2012; Ruggiero et al., 2003). Here, the item to describe stressful events was replaced to targeting event - namely COVID-19 epidemic (e.g., item 1: Disturbing recurring memories, thoughts, or images caused by the COVID-19 from it begun in China (i.e., Jan 23, 2020)). It showed satisfied psychometric properties of PCL-C in this study (Cronbach's ɑ = 0.911; McDonald's ω = 0.914; Gutman's λ = 0.928) (see SM) On the basis of diagnostic benchmark, PTSD + would be identified as of scores > 50 in PCL-C test (Conybeare et al., 2012).
2.3. Data acquisition and preprocessing of rs-fcMRI
The 8-min resting-state functional connectivity MRI (rs-fcMRI) scanning for each participant 264 was performed in SIMENS MAGNETOM PRISMA 3T scanner (Siemens Medical 265 Department, Erlangen, Germany) during the period: Sept 17, 2019–Jan 11, 2020. To reduce head-motion, we made use of foam padding. Participants were instructed to keep eyes open, and to take a rest without thinking during scanning. Parameters for scanning and preprocessing pipeline were in accordance with existing canonical studies and the criteria of the Human Connectome Project (HCP), which can be found in SM.
2.4. Statistical power analysis
To examine the statistical power, the sample size have been estimated by G*Power in the post-hoc analysis. In this vein, with random-effect (RE) parametric independent t-tests of effect sized d = 0.80 (high effect), error probabilities (α) = 0.05 and statistical power (1-β error probabilities) = 0.95 (Two-tails test), it showed that sample size of n = 42 for each group (df = 82, critical t = 1.989; noncentrality parameter = 3.667) attained the predefined criteria (Faul et al., 2009). Thus, the sample size of this study could reach adequate statistical power (see SM for full results).
2.5. Modeling COVID-19 epidemic index (EI)
The current study proposed to fit an epidemic index to quantify and evaluate the relative impact of the COVID-19 epidemic on specific individuals. Thus, we drew on shared Satellite Remote Sensing Image (S-RSI) data of Geographic Information System (GIS) and real-time COVID-19 epidemic map provided by Johns Hopkins University (JHG CSSE) (https://github.com/CSSEGISandData/COVID-19) to model the epidemic index (EI) for each participant.
This model included two free parameters - that is - the COVID-19 confirmed cases and geographical distance to the Wuhan (the epidemic-central area of COVID-19 pandemic in the China). To determine the first parameter, the cutoff point of the COVID-19 epidemic was calculated as a benchmark by using curvilinear function. Data for confirmed cases was derived from Johns Hopkins University (JHG CSSE), and was further confirmed by National Health Commission (NHC) 1259 and Center of Disease Control (CDC) of China (http://www.nhc.gov.cn/xcs/yqtb/) (see SI Methods). To determine the second parameter, we used S-RSI data from GIS to extract the distance between the exposed locations of each participant and Wuhan (N30°35′42.65″, E114°17′59.32″). The location of each participant was mapped into this model by retrieving geographical coordinates in the National Geomatic Center of China (NGCC, http://www.ngcc.cn/ngcc/). Once parameters have been determined, the conjunction function of logarithmic and hyperbolic equation was adopted to fit this model, with former function to fit the pattern of increasing confirmed cases and last function to fit the relationship of distance/confirmed cases and epidemic influences:
More details for EI can be found in SM Methods.
2.6. Identification of PTSD network
To build up PTSD network, both brain large-scale functional localized networks involving in PTSD were incorporated for selection of nodes. On the basis of previous canonical identifications, frontoparietal network (FPN), salience network (SAN), and ventral attention network (VAN) were included (Philip et al., 2018; Shalev et al., 2017), and functional episodic memories network (EMN, bilateral hippocampus, amygdala and mPFC) and fear learning network (FLN, bilateral insular, dACC, mPFC and amygdala) (Mary et al., 2020) were also included based on the importance of these networks in PTSD. Each node that derived from intrinsic networks was produced from Power atlas and was labeled by using BredDatabase platform provided by neuroinformatics group of DTU (https://neuro.imm.dtu.dk/service/). Thus, a small portion of nodes may share the same label but they locate in different brain space (see SM). More details for location of these nodes could be found in SM Extended Table 1.
2.7. SVM classification
2.7.1. Feature selection
The features for comprising the neural connectome of PTSD in classifiers were predefined as functional connectivity (FC) strengths. Further, the conjunctive scheme of parametric t-tests + LASSO was adopted to facilitate elimination of redundant features and obtaining discriminative features, the way retains model generalizability well (Bach, 2008). An independent sample t-test was launched initially to discern informative features roughly by comparing FC between two group at p < .05. Furthermore, L1-norm LASSO regression was carried out for more sophisticated filters at lambda = 0.01, which combined L-Lipschitz condition and Taylor's expansion to obtain optimal solutions (w) based on selecting most informative sparse features and regularizing trivial features to zero (C.-H. Zhang and Huang, 2008):
2.7.2. SVM model evaluation
An epsilon-insensitive support vector machine (EI-SVM) was deployed to classify PTSD+ and PTSD-. A Gaussian radial basis function kernel (RBF) was predefined, and C and γ parameters were optimized using the heuristic Particle Swarm Optimization (PSO) algorithm (Guyon et al., 2002). Feature engineering was done using the independent t-test and LASSO strategy described above, but only using the training data. To obviate potential risks of double dipping, the nested leave-one-subject-out cross-validation (LOSOCV) was exerted to evaluate model performance. Here, classifier training and testing were accomplished through two nested loops. The outer loop split data into training and test sets. The inner loop created multiple partitions of the training data in order to perform out-of-sample hyperparameter and feature selection. The trained weights associated with the best performing model instantiation were passed to the outer loop and applied to the test data (see SI Methods). In addition, the 10-fold CV was also used to validate the classifier's performance. These processes were implemented in LIBSVM v3.23 (Chang and Lin, 2011) and BrainNetClass (Version 1.1) toolbox (Zhou et al., 2020) (see Fig. 2B).
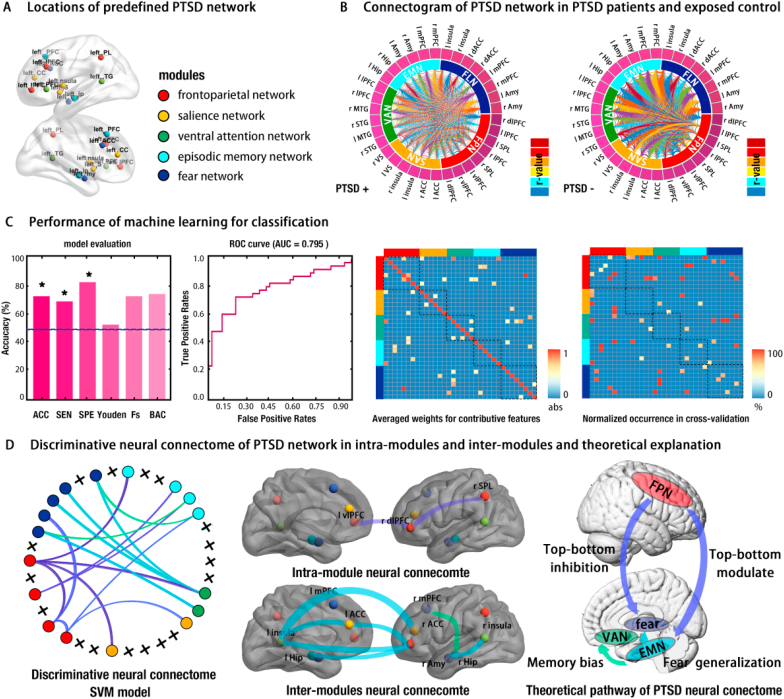
Fig. 2.
Pattern of neural connectome, performance of machine learning, and discriminative features for classification. Panel A illustrates the location nodes of the PTSD network we predefined beforehand. Panel B presents the connectogram of the fully-connected connectome for both PTSD+ (left) and PTSD- (right). Panel C provides the performance of the SVM and the corresponding ROC curve, with high values indicating good model performance (* = p < .05; ACC = accuracy; SEN = sensitivity; SPE = specificity; Youden = Youden index; Fs = F-score; BAC = balanced accuracy; AUC = area-under-curve). Right side of panel C shows the averaged weights matrix (34 nodes × 34 nodes, AWM) and normalized occurrence matrix (34 nodes × 34 nodes, NOM) for the contributive features respectively, with higher values of the elements (functional connectivity) in the matrix making a greater contribution to classification. Blocks alongside of the matrix indicate the corresponding sub-network (modules) of the PTSD network, with red for the frontoparietal network (FPN), orange for the salience network (SAN), green for the ventral attention network (VAN), blue for the episodic memory network (EMN), and dark blue for the fear network (fear). Panel D plots the refined neural connectome of the PTSD network with discriminative features (functional connectivity) and its theoretical explanation. The Final neural connectome of the PTSD network contains 17 nodes and 16 connections obtained from the conjunction of thresholding AWM (absolute weights > 0.8) and NOM (occurrence rates > 0.5). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.7.3. Cross-model validations of ensembles machine learning
Ensemble classifiers have been shown in some settings to achieve higher levels of performance with enhanced robustness (Pham et al., 2019). Thus, in addition to SVM model, the multilinear subspace learning of discriminant (mlSD) was adopted to classify PTSD+ and PTSD- individuals (Li et al., 2020). The mlSD was launched for tensor-to-vector projection, and obtained longest inter-label distance and shortest intra-label distance of mapping tensors for classification. Details for the algorithms and theories of mlSD could be found elsewhere (Lu et al., 2013).
3. Support vector regression (SVR) model
In addition to classifying individuals with high risk to develop PTSD symptom, the current study also aimed to predict the severity of PTSD symptom (quantified by PCL-C score) prospectively by using the neural connectome. To this end, instead of our SVM classifier, a support vector regression (SVR) model was trained to predict the PTSD symptom for participants who were identified as high-risk to develop PTSD symptom. Algorithms, parameters and processes involving in SVR were analogous to those described for the SVM classifications above (see SM for details).
4. Results
4.1. PTSD symptoms emerged in COVID-19 pandemic
To examine the prevalence of PTSD during the COVID-19 pandemic, 750 participants were investigated for their PTSD symptoms during Feb 21 - Feb 28, 2020. On the basis of diagnosis of PTSD Checklist (Civilian Version, PCL-C), 42 participants (5.60%) were identified as high risk to develop clinical PTSD symptom. Further, 90 participants (12.00%) were found to display critical PTSD symptoms, whilst remaining exposed participants lacked such symptoms. To test whether the neural connectome of the PTSD network could be used to prospectively classify high-risk participant to develop PTSD symptom in the COVID-19 pandemic, 42 matching participants who did not appear PTSD symptoms were designated as healthy controls. In brief, our findings suggested that the COVID-19 pandemic caused elevated levels of PTSD symptoms. More details can be found in SM.
4.2. Neural connectome accurately predicts PTSD conversion in COVID-19 pandemic
In line with our hypothesis, the neural connectome associating with PTSD symptom focused on FLN, SAN, FPN, EMN and VAN (see Introduction section) (see Fig. 2A and SM). For visual inspection of reconstructed neural connectome, group-averaged full-connected connectomes for both groups were illustrated in connectogram (see Fig. 2B). Results indicated the weaker local connections within FPN for PTSD + than for the PTSD- group. Weaker global connections were observed in PTSD + as well (see Fig. 2B).
To probe whether the neural connectome status preceding COVID-19 outbreak could prospectively classify PTSD+ and PTSD- during the pandemic, the support vector machine (SVM) was trained by using leave-one-subject-out cross-validation (LOSOCV) for performance estimation. This machine learning model revealed a high accuracy of 76.19% (Sensitivity rate = 80.95%, Specificity rate = 71.43%, F-score = 75.00%, balance accuracy = 76.19 %, area under curve = 0.80) (see Fig. 2C). When 10-fold cross-validation was used to test classifier performance, it showed a similarly high accuracy of 70.00% (sensitivity = 69.05%, specificity = 70.95%, AUC = 0.74). A control analysis was conducted by selecting a new sample of matched controls, which showed similar results (accuracy = 75.00%, sensitivity = 73.81%, specificity = 76.19 %, AUC = 0.78). To validate the specificity of this neural connectome for classification, another control analysis was performed by selecting FCs of the visual network (VN) and auditory network (AN) as trained features, respectively. Results from this analysis demonstrated that the VN and AN connectomes cannot classify the PTSD + successfully (for VN, accuracy = 44.52%, sensitivity = 43.10%, specificity = 45.95 %, AUC = 0.41; for AN, accuracy = 52.38%, sensitivity = 47.62%, specificity = 57.14 %, AUC = 0.50), indicating that classifier results are specific to the neural connectome selected for PTSD symptoms.
In addition, an independent sample (16 PTSD+ and 16 PTSD-) was used to test the generalization capability for this trained classifier. These participants were derived from the same population, and were selected by the PCL-C screening in the second follow-up investigation (April 24, 2020 to May 1, 2020) (see SM). A high balance accuracy of 71.88% (Sensitivity rate = 75.00%, Specificity rate = 68.75%) was attained, showing a prospective identification of PTSD+ in the independent sample and indicating a well generalization capability.
Another major goal we pursued was to reveal how specific pathways (features) in neural connectome contributed to achieve the accurate classification. In this vein, the averaged weights matrix (AWM) describing absolute importance of each connection in neural connectome was examined, which captured 24 discriminative FCs at threshold of (│ω│> 0.5), such as rdlPFC-lSPL, rdlPFC-lACC, and lMTG-rAmy (see Fig. 3C and SM). To promote the performance of the classifier in generalization, a normalized occurrences matrix (NOM) quantifying relative contributions of each connection was further adopted, and revealed a convergent pattern (see Fig. 3C and SM). To determine the discriminative neural connectome pattern for classification, we performed a conjunction analysis to refine the PTSD neural connectome by mapping NOM into AWM. Results suggest that intra-connections within sub-modules play only minor roles in classification, whereas inter-connections between submodules play crucial roles for accurate classification (e.g., FPN-EMN, VAN-EMN, and VAN-FLN) (see Fig. 2D).
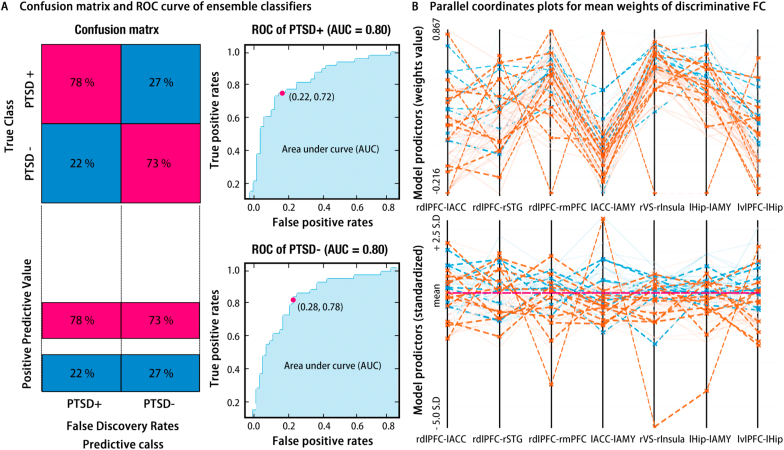
Fig. 3.
Results of ensemble classifiers of multilinear subspace learning of discriminant (mlSD). Left side of Panel A provides the confusion matrix for this ensemble classifier, and right side of panel A shows the corresponding ROC curves. Panel B illustrates the parallel coordinates plots for the classifier, with the top for the raw weights value and the bottom for the standardized weights. These plots indicate the importance and contributions of the features for classifier, with the orange line for PTSD+, the blue line for PTSD-, the solid line for correct classification, and the dashed line for incorrect classification. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To promote the robustness of classifier, the ensemble classifier of multilinear subspace learning of discriminant (mlSD) was implemented and revealed a robust performance for classification (Balanced accuracy = 73.8%, AUC = 0.80, see Fig. 3). Meanwhile, parallel coordinates analysis was drawn to determine contributive features (FCs), which revealed convergent results with SVM classifier (see SM). Likewise, the test to its generalization capability has been conducted by using this independent sample, and demonstrates a favorable generalization performance (Balanced accuracy = 68.75%, Sensitivity rate = 75.00%, Specificity rate = 62.50%).
To sum up, the neural connectome obtained prior to the COVID-19 pandemic can be used to accurately classify for participants who subsequently reported a PTSD symptomatology during the COVID-19 pandemic. Examining the most critical contributors to the classification accuracy revealed that the accuracy was largely depended on the interplay between top-bottom pathways (e.g., FPN-EMN, FPN-FLN and EMN-VAN) but not sole local communications (see Fig. 2D). Further, this provided a PTSD connectome more accurate to classify PTSD symptom from exposed control, and made detection of susceptible population for PTSD in COVID-19 more accessible. All trained classifiers and configurations are openly available at GitHub repository (https://github.com/Zhiyi-Chen-github/Classifier-PTSD-COVID-19) so as to promote estimations of risk of developing PTSD symptom during COVID-19 pandemic for other users.
4.3. Neural connectome predicts severity of PTSD symptom in COVID-19 pandemic
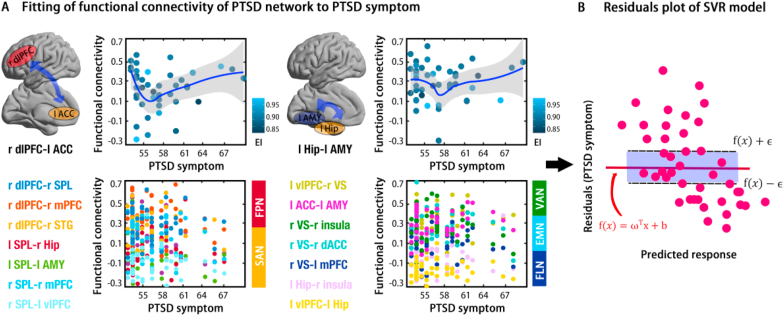
In addition to the categorical classification, we aimed to predict continuous levels of PTSD symptomatology in the 42 PTSD + individuals. Specifically, the support vector regression (SVR) model was used to investigate whether the neural connectome could predict severity of PTSD symptoms during COVID-19 pandemic, within afflicted individuals. Results of SVR model demonstrated a pronounced predictive role of this neural connectome to PTSD symptoms (R2 = 0.28, RMSE = 4.47; MAE = 3.67). To uncover which pathways (FCs) contributed to predictions, the top 10% contributive features for this prediction model were captured, including rdlPFC-lACC and lHip-lAMY (see Fig. 4A and B). Also, it was of practical interests to perform ensemble machine predictors boosting this performance. Bagged tress algorithm was adopted and demonstrated robust results for this prediction (R2 = 0.28, RMSE = 4.49; MAE = 3.35; see Fig. 4C).
Fig. 4.
Performance of predictors for predicting PTSD symptoms using discriminative features of the neural connectome in a support vector regression model. Panel A provides the scatter plots for the top 10% contributive features of prediction (top) and others (bottom), with a dark blue line for fitting of the locally weighted regression and a shadow area for the 95% confidence interval (CI). EI refers to epidemic index (see Method and Materials). Panel B provides the residuals plot in the SVR model for PTSD+. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
All in all, the present findings suggest that the neural connectome status assessed before COVID-19 pandemic could classify categorical PTSD conversion risk during this pandemic, and could further predict the severity of PTSD within afflicted individuals. Trained symptom severity models have been provided openly for prediction of PTSD symptoms (https://github.com/Zhiyi-Chen-github/Classifier-PTSD-COVID-19).
5. Discussion
Concerns about the detrimental impact of the COVID-19 pandemic on mental health are increasing and have been strongly debated by the public health agencies worldwide. This study provided evidence that the majority of healthy young adults in China did not develop full clinical symptoms of PTSD during the COVID pandemic. However, a significant minority of individuals exhibited strongly increased PTSD symptoms during the pandemic and thus prospective identification of those at high risk and increased susceptibility of developing PTSD symptom during COVID-19 pandemic would be of tremendous importance to develop early intervention strategies. The major contributions of our study are threefold: (1) the epidemiological investigations during the peak of COVID-19 pandemic in mainland China (Feb 21, 2020–Feb 28, 2020) demonstrated that a considerable number of individuals reported severe levels of PTSD; (2) the first pseudo-prospective neuroimaging design to date could accurately classify participants at highest risk to develop PTSD symptom during the COVID-19 pandemic based on brain neural connectome data that was acquired before COVID-19 outbreak (Sept 20, 2019–Jan 11, 2020), demonstrating that the specific neural connectome may represent a robust biomarker for the vulnerability to develop PTSD symptoms; (3) prediction models were found to accurately predict individual levels of PTSD symptoms as well, which could allow for early identification of those at risk to develop the most severe forms of PTSD.
The results of our epidemiological survey confirmed emerging evidence for the negative impact of COVID-19 pandemic on mental health, with approx. 6% in the current sample fulfilling the initial criteria for PTSD. It is in accordance with existing literature that the prevalence of PTSD is 5–10 % in the general population (Kilpatrick et al., 2013; Yehuda et al., 2015). Thus, it is worthy to note that the COVID-19 pandemic brought about a certain stress to make individuals more vulnerable developing PTSD symptom. For instance, Liu et al. (2020) reported 7% prevalence rates of PTSD one month after the COVID-19 outbreak (Jan 30, 2020–Feb 8, 2020) in the most severely affected region in mainland China (e.g., Wuhan, Hubei) (N. Liu et al., 2020). Comparable rates were observed in other studies such that Sun et al. (2021) reported 4.6% (Jan 28, 2020–Feb 2, 2020), Torales et al. (2020) reported 7.0% and Jiang et al. (2020) reported 6.1% prevalence rates of PTSD (Jan 28, 2020) (Jiang et al., 2020; Torales, O'Higgins, Castaldelli-Maia and Ventriglio, 2020). In addition to China, analogous conclusions can be found for other areas, such as Ireland (Karatzias et al., 2020) and Spain (González-Sanguino et al., 2020). During the peak of the pandemic, several stress factors may have contributed to increasing stress exposure, including the threat of contagion and illness, the fear of supply shortage and may in turn have promoted the development of PTSD (Di Crosta et al., 2020; Jovanovic and Ressler, 2010), at least in vulnerable individuals. In addition, this risk may further be enhanced when these individuals experience social isolation and lack of social support during the period of strict lockdown and social distancing ( S. Liu et al., 2020). As such it is no wonder that substantial evidence points to the negative impact of COVID-19 pandemic on mental health, especially in the high risk to develop PTSD in the normal population.
In the current study, we found that some neural pathways within this connectome showed greater discriminability with respect to PTSD symptoms, such as the FPN-FLN connection and the FPN-EMN connection. Specifically, the stronger FPN-FLN connection shows high discriminative power in classifying PTSD+, and it thus provided insights into how emotional regulation works for top-down inhibition towards fear learning. Previous findings regarding the neural connections of PTSD have shown dysfunctional prefrontal-amygdala connections in PTSD patients (Jin et al., 2014; Stevens et al., 2013). Evidence derived from real-time neurofeedback training further pronounce that PTSD patients showed a clinical reliance of top-down emotional regulation deficits in PFC-amygdala connections (Nicholson et al., 2017). EMN was of crucial for contextual processing of stimuli information in PTSD patients (Shkreli et al., 2020). Generalizing threatening experiences to daily life in PTSD patients was related to the differential neural coding of safety and threat signals in the hippocampus and mPFC (Shalev et al., 2017). Thus, decoupling of FPN to EMN could reflect a deficient top-down modulations towards contextual information, and thus make individuals more vulnerable to produce PTSD symptom (Liberzon and Abelson, 2016).
Aside from top-down regulation, dysfunctional subcortical communication has been proposed as an important contributor to PTSD symptom. In line with this idea, our models relied heavily on contributions from EMN-FLN connections and FLN-VAN connection. A neurocircuit based model of PTSD encapsulated from quantifiable meta-analytic examinations identified dysregulations in inter-connections of widespread subcortical areas, especially in interaction of hubs of EMN and FLN (Hayes et al., 2012; Patel et al., 2012). As previously discussed, individual variances in EMN and FLN may represent the different ability for contextual processing of threatening stimuli and fear learning, this ability that may determine one's susceptibility in developing PTSD symptom. Taken together, these findings obtained in PTSD patients converge well with the identified circuits in the present study. Moreover it also suggest that individual variations in the brain connectome status of pathways engaged in fear learning, threat processing and salience, executive functions (emotional regulation), and contextual processing could represent susceptibility neuromarkers for individual variations in the risks to develop PTSD symptom during the COVID-19 pandemic.
The findings from the current study, though novel and important, should be considered in the context of several limitations and caveats. First, we built upon the machine-learning model to predict PTSD symptoms during the COVID-19 pandemic with moderate accuracy. However, the small sample size limits our ability to test its broader applicability and validity. To partly address this drawback, trained classifiers have been made accessible openly for re-training from future studies that recruit larger samples. Given these limitations, we would recommend users adopt this classifier as one tool for screening high-risk populations, but not a singular and decisive tool. In addition, a potential weakness of the study is the lack of pre-COVID PTSD symptom data. However, a strict psychopathological screening before study inclusion makes it unlikely that subjects with strong PTSD symptoms would have been included in the data acquisition. Most crucially, brain connectomic status may represent one vulnerability factor for developing PTSD symptom during the pandemic, but factors such as early life experience and genetics, among many others, may also contribute (Shalev et al., 2017). In addition, as limited by the lockdown policy, it was hard to investigate the prior stressful/traumatic events before this study. Also, the lacks of such information lets us hard to claim that the COVID-19 pandemic is the unique traumatic event to produce PTSD symptoms. Henceforth, conclusion of this study should be limited to that predated neural connectome status could encode risks of PTSD symptom in COVID-19 pandemic to some degree, but not decisive. Further, it should be in mind that the PCL-C test is not a golden criterion to diagnose PTSD patients. Likewise, lacking the exact information for the duration of PTSD symptoms still impedes to conclude that such PTSD symptoms are specific to COVID-19. Thus, we hope our findings could be validated by performing clinical assessments (e.g., the duration of enduring dysfunction) when the lockdown is dropped.
6. Conclusions
COVID-19 pandemic has disrupted almost all aspects of life, and led to acute psychiatric outcomes and widespread mental health problems. This is the first study using pseudo-prospective cohort design to test whether the COVID-19 pandemic leads to PTSD symptoms and, if so, whether they can be predicted by the neural connectome status preceding COVID-19 outbreak. Here we provided evidence that the COVID-19 pandemic led to PTSD in about 6% of the population. Drawing upon ensemble machine learning, neural connectome status prior to the COVID-19 pandemic (Sept 20, 2019–Jan 11, 2020) was found to predict the acute PTSD symptoms during the COVID-19 pandemic. This work could form the basis for development of practical and accurate neuromarkers that can inform the identification of individuals who are susceptible to developing PTSD symptom. Predictors of machine learning further enabled the prediction of PTSD symptoms by using predated neural connectome status. Thus, another potential implication might be to provide a tool to forewarn the high-risk crowds of developing PTSD symptom in COVID-19 pandemic, and this may facilitate to ease the burden of psychiatric staff and public mental health authorities. Lastly, because pre-pandemic neural connectome status was found to be a robust risk factor for mental health in the current study, it suggests that the brain neural systems health is likely to be vulnerable for disruptions in the COVID-19 pandemic as well.
Data availability
D Data and scripts have been open accessed in Open framework science (OSF) (10.17605/OSF.IO/D8QCU).
Declarations
Ethics approval and consent to participation.
This study was approved by the Institutional Review Board (IRB) of the Southwest University (CHINA).
Consent for publication
Not applicable.
Availability of data and material
All the analytic data and scripts have been open accessed in Open framework science (OSF) (10.17605/OSF.IO/D8QCU). All the codes supporting this study are available in Github (https://github.com/Zhiyi-Chen-github/Classifier-PTSD-COVID-19). The neuroimaging data that support the findings of this study are available on request from the corresponding author TY. The data are not publicly available due to local policy and laws promulgated from National Health Commission (NHC) of the People's Republic of China.
Funding
This study was supported by the National Natural Science Foundation of China (31571128; 31971026; 31800959), Scientific innovation projects for postgraduates in Chongqing (CYB21082) and the Fundamental Research Funds for the Central Universities in China (SWU1509392, SWU1809357; SWU118091).
CRediT authorship contribution statement
Zhiyi Chen: Conceptualization, Methodology, Software, Writing – original draft, and, Visualization. Pan Feng: Writing – original draft, Formal analysis, and, Validation. Benjamin Becker: Writing – review & editing, Methodology, or, Validation. Matthew R. Nassar: Writing – review & editing, Methodology, or, Validation. Fuschia Sirois: Writing – review & editing, Methodology, or, Validation. Bernhard Hommel: Writing – review & editing, Methodology, or, Validation. Chenyan Zhang: Writing – review & editing, Methodology, or, Validation. Qinghua He: Project administration, Writing – review & editing, or, Funding acquisition. Jiang Qiu: Project administration, Writing – review & editing, or, Funding acquisition. Li He: Validation, and, Investigation. Xu Lei: Project administration, Writing – review & editing, or, Funding acquisition. Hong Chen: Project administration, Writing – review & editing, or, Funding acquisition. Tingyong Feng: Conceptualization, Supervision, Project administration, and, Funding acquisition.
Declaration of competing interest
All the authors claimed no potential conflicts of interests.
Acknowledgments
We sincerely appreciate the Brain-Behavioral-Project team (School of Psychology, Southwest University, China) for great contributions to recruit these data and exert the follow-up investigations.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100378.
List of abbreviations
- COVID-19
Corona Virus Disease 2019
- dACC
Dorsal anterior cingulate
- dlPFC
Dorsolaterial prefrontal cortex
- EMN
Episodic memory network
- FPN
Frontoparietal network
- FLN
Fear-learning network
- PTSD
Post-traumatic Stress Disorder
- SAN
Salience network
- SVM
Support vector machine
- S- RSI
Satellite remote sensing images
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Andero R., Jovanovic T., Chen Y.T., Salahuddin H., Cameron M.D., Bannister T.D.…Binder E.B. Amygdala-dependent fear is regulated by Oprl1 in mice and humans with PTSD. Sci. Transl. Med. 2013;5(188) doi: 10.1126/scitranslmed.3005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Cole M.W., Murray J.D., Corlett P.R., Wang X.-J., Krystal J.H. The role of default network deactivation in cognition and disease. Trends Cognit. Sci. 2012;16(12):584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach F.R. Consistency of the group lasso and multiple kernel learning. J. Mach. Learn. Res. 2008;9(Jun):1179–1225. [Google Scholar]
- Brewin C.R., Gregory J.D., Lipton M., Burgess N. Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychol. Rev. 2010;117(1):210. doi: 10.1037/a0018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y.…Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.-C., Lin C.-J. LIBSVM: a library for support vector machines. ACM transactions on intelligent systems and technology (TIST) 2011;2(3):1–27. [Google Scholar]
- Conybeare D., Behar E., Solomon A., Newman M.G., Borkovec T. The PTSD Checklist—civilian Version: reliability, validity, and factor structure in a nonclinical sample. J. Clin. Psychol. 2012;68(6):699–713. doi: 10.1002/jclp.21845. [DOI] [PubMed] [Google Scholar]
- Di Crosta A., Palumbo R., Marchetti D., Ceccato I., La Malva P., Maiella R. Individual differences, economic stability, and fear of contagion as risk factors for PTSD symptoms in the COVID-19 emergency. Front. Psychol. 2020;11:567367. doi: 10.3389/fpsyg.2020.567367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopfel D., Perez P.D., Verbitsky A., Bravorivera H., Ma Y., Quirk G.J., Zhang N. Individual variability in behavior and functional networks predicts vulnerability using an animal model of PTSD. Nat. Commun. 2019;10(1):2372. doi: 10.1038/s41467-019-09926-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Maronkatz A., Wu W., Fonzo G., Huemer J., Vertes P.E.…Keller C.J. Using fMRI connectivity to define a treatment-resistant form of post-traumatic stress disorder. Sci. Transl. Med. 2019;11(486) doi: 10.1126/scitranslmed.aal3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Buchner A., Lang A.-G. Statistical power analyses using G* Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Sanguino C., Ausín B., Castellanos M., Saiz J., López-Gómez A., Ugidos C., Muñoz M. Mental health consequences during the initial stage of the 2020 Coronavirus pandemic (COVID-19) in Spain. Brain Behav. Immun. 2020;87:172–176. doi: 10.1016/j.bbi.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon I., Weston J., Barnhill S., Vapnik V. Gene selection for cancer classification using support vector machines. Mach. Learn. 2002;46(1–3):389–422. [Google Scholar]
- Hayes J.P., Hayes S.M., Mikedis A.M. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol. Mood Anxiety Disord. 2012;2(1):9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge C.W., Castro C.A., Messer S.C., McGurk D., Cotting D.I., Koffman R.L. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N. Engl. J. Med. 2004;351(1):13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- Holmes E.A., O'Connor R.C., Perry V.H., Tracey I., Wessely S., Arseneault L., Ballard C., Christensen H., Cohen Silver R., Everall I., Ford T., John A., Kabir T., King K., Madan I., Michie S., Przybylski A.K., Shafran R., Sweeney A., Worthman C.M.…Bullmore E. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet. Psychiatr. 2020;7(6):547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H.-j., Nan J., Lv Z.-y., Yang J. Psychological impacts of the COVID-19 epidemic on Chinese people: exposure, post-traumatic stress symptom, and emotion regulation. Asian Pacific J. Trop. Med. 2020;8(7):252–259. [Google Scholar]
- Jin C., Qi R., Yin Y., Hu X., Duan L., Xu Q. Abnormalities in whole-brain functional connectivity observed in treatment-naive post-traumatic stress disorder patients following an earthquake. Psychol. Med. 2014;44(9):1927–1936. doi: 10.1017/S003329171300250X. [DOI] [PubMed] [Google Scholar]
- Jovanovic T., Ressler K.J. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am. J. Psychiatr. 2010;167(6):648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatzias T., Shevlin M., Murphy J., McBride O. Posttraumatic stress symptoms and associated comorbidity during the COVID-19 pandemic in Ireland. A Population-Based Study. 2020;33(4):365–370. doi: 10.1002/jts.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek M.A., Klemenhagen K.C., Sahay A., Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat. Neurosci. 2012;15(12):1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D.G., Resnick H.S., Milanak M.E., Miller M.W., Keyes K.M., Friedman M.J. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J. Trauma Stress. 2013;26(5):537–547. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Lai L., Cui S. On the adversarial robustness of subspace learning. IEEE Trans. Signal Process. 2020;68:1470–1483. [Google Scholar]
- Liberzon I., Abelson J.L. Context processing and the neurobiology of post-traumatic stress disorder. Neuron. 2016;92(1):14–30. doi: 10.1016/j.neuron.2016.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Zhang F., Wei C., Jia Y., Shang Z., Sun L. Prevalence and predictors of PTSS during COVID-19 outbreak in China hardest-hit areas: gender differences matter. Psychiatr. Res. 2020;287:112921. doi: 10.1016/j.psychres.2020.112921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Yang L., Zhang C., Xiang Y.-T., Liu Z., Hu S., Zhang B. Online mental health services in China during the COVID-19 outbreak. The Lancet Psychiatry. 2020;7(4):e17–e18. doi: 10.1016/S2215-0366(20)30077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Plataniotis K.N., Venetsanopoulos A. CRC press; 2013. Multilinear Subspace Learning: Dimensionality Reduction of Multidimensional Data. [Google Scholar]
- Mary A., Dayan J., Leone G., Postel C., Fraisse F., Malle C. Resilience after trauma: the role of memory suppression. Science. 2020;367(6479) doi: 10.1126/science.aay8477. [DOI] [PubMed] [Google Scholar]
- Nicholson A.A., Rabellino D., Densmore M., Frewen P.A., Paret C., Kluetsch R. The neurobiology of emotion regulation in posttraumatic stress disorder: amygdala downregulation via real‐time fMRI neurofeedback. Hum. Brain Mapp. 2017;38(1):541–560. doi: 10.1002/hbm.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam T.A., Laird A.R., Ray K.L., Dean Y.M., Glahn D.C., Carter C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognit. Affect Behav. Neurosci. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012;1251:E1. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Spreng R.N., Shin L.M., Girard T.A. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2012;36(9):2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. Jama. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- Pham B.T., Prakash I., Singh S.K., Shirzadi A., Shahabi H., Bui D.T. Landslide susceptibility modeling using reduced error pruning trees and different ensemble techniques: hybrid machine learning approaches. Catena. 2019;175:203–218. [Google Scholar]
- Philip N.S., Barredo J., van’t Wout-Frank M., Tyrka A.R., Price L.H., Carpenter L.L. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol. Psychiatr. 2018;83(3):263–272. doi: 10.1016/j.biopsych.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012;13(11):769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero K.J., Del Ben K., Scotti J.R., Rabalais A.E. Psychometric properties of the PTSD checklist—civilian version. J. Trauma Stress. 2003;16(5):495–502. doi: 10.1023/A:1025714729117. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev A., Liberzon I., Marmar C. Post-traumatic stress disorder. N. Engl. J. Med. 2017;376(25):2459–2469. doi: 10.1056/NEJMra1612499. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L.M., Rauch S.L., Pitman R.K. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann. N. Y. Acad. Sci. 2006;1071(1):67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shkreli L., Woud M.L., Ramsbottom R., Rupietta A.E., Waldhauser G.T., Kumsta R., Reinecke A. Angiotensin involvement in trauma processing—exploring candidate neurocognitive mechanisms of preventing post-traumatic stress symptoms. Neuropsychopharmacology. 2020;45(3):507–514. doi: 10.1038/s41386-019-0553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J.S., Jovanovic T., Fani N., Ely T.D., Glover E.M., Bradley B., Ressler K.J. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J. Psychiatr. Res. 2013;47(10):1469–1478. doi: 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Sun Z., Wu L., Zhu Z., Zhang F., Shang Z., Jia Y., Gu J., Zhou Y., Wang Y., Liu N., Liu W. Prevalence and risk factors for acute posttraumatic stress disorder during the COVID-19 outbreak. J. Affect. Disord. 2021;283:123–129. doi: 10.1016/j.jad.2021.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J.L., Wilk J.E., Riviere L.A., McGurk D., Castro C.A., Hoge C.W. Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq. Arch. Gen. Psychiatr. 2010;67(6):614–623. doi: 10.1001/archgenpsychiatry.2010.54. [DOI] [PubMed] [Google Scholar]
- Torales J., O'Higgins M., Castaldelli-Maia J.M., Ventriglio A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int. J. Soc. Psychiatr. 2020 doi: 10.1177/0020764020915212. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/s0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Post-traumatic stress disorder. N. Engl. J. Med. 2002;346(2):108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Hoge C.W., McFarlane A.C., Vermetten E., Lanius R.A., Nievergelt C.M. Post-traumatic stress disorder. Nat Rev Dis Primers. 2015;1:15057. doi: 10.1038/nrdp.2015.57. [DOI] [PubMed] [Google Scholar]
- Zhang C.-H., Huang J. The sparsity and bias of the lasso selection in high-dimensional linear regression. Ann. Stat. 2008;36(4):1567–1594. [Google Scholar]
- Zhou Z., Chen X., Zhang Y., Hu D., Qiao L., Yu R., Yap P.T., Pan G., Zhang H., Shen D. A toolbox for brain network construction and classification (BrainNetClass) Hum. Brain Mapp. 2020;41(10):2808–2826. doi: 10.1002/hbm.24979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
D Data and scripts have been open accessed in Open framework science (OSF) (10.17605/OSF.IO/D8QCU).
All the analytic data and scripts have been open accessed in Open framework science (OSF) (10.17605/OSF.IO/D8QCU). All the codes supporting this study are available in Github (https://github.com/Zhiyi-Chen-github/Classifier-PTSD-COVID-19). The neuroimaging data that support the findings of this study are available on request from the corresponding author TY. The data are not publicly available due to local policy and laws promulgated from National Health Commission (NHC) of the People's Republic of China.