Abstract
BACKGROUND & AIMS:
WAP four-disulfide core domain protein 2 (WFDC2), also known as human epididymis protein 4 (HE4), is a small secretory protein that is highly expressed in fibrosis and human cancers, particularly in the ovaries, lungs, and stomach. However, the role of WFDC2 in carcinogenesis is not fully understood. The present study aimed to investigate the role of WFDC2 in gastric carcinogenesis using preneoplastic metaplasia models.
METHODS:
Three spasmolytic polypeptide-expressing metaplasia (SPEM) models were established each in wild-type and Wfdc2-knockout mice, with DMP-777, L635, and high-dose tamoxifen, respectively. To reveal the functional role of WFDC2, we performed transcriptomic analysis with DMP-777-treated gastric corpus specimens.
RESULTS:
Wfdc2-knockout mice exhibited remarkable resistance against oxyntic atrophy, SPEM emergence, and accumulation of M2 type macrophages in all three SPEM models. Transcriptomic analysis revealed that Wfdc2 knockout prevented the upregulation of interleukin-33 (IL33) expression in the injured mucosal region of SPEM models. Notably, supplementation of rWFDC2 induced IL33 production and M2 macrophage polarization, and ultimately promoted SPEM development. Moreover, long-term treatment with rWFDC2 was able to induce SPEM development.
CONCLUSIONS:
WFDC2 expressed in response to gastric injury promotes SPEM through the upregulation of IL33 expression. These findings provide novel insights into the role of WFDC2 in gastric carcinogenesis.
Keywords: WFDC2, SPEM, M2 macrophage, Interleukin-33 (IL33)
Short Summary:
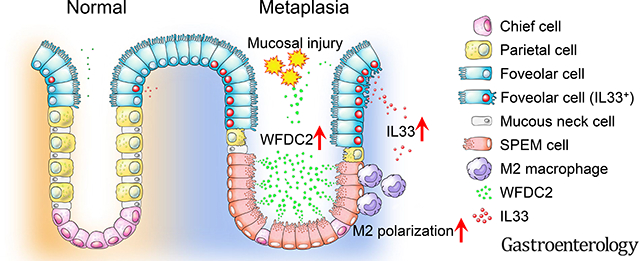
WFDC2 expression is upregulated in the tissue and gastric juice of preneoplastic metaplasia and gastric cancer. Wfdc2 knockout protected against spasmolytic polypeptide-expressing metaplasia (SPEM) in mice while the exogenous supplementation of rWFDC2 recovered SPEM development. rWFDC2 increased IL33 expression, which subsequently induced M2 macrophage polarization. Most importantly, long-term treatment of rWFDC2 could induce SPEM development alone, indicating the pivotal role of WFDC2 in early gastric carcinogenesis.
Graphical Abstract
INTRODUCTION
Gastric cancer is the third leading cause of cancer-related deaths worldwide.1 Despite an overall decrease in cancer mortality, patients with gastric cancer at an advanced stage still show poor prognosis. Although surgical resection is the standard treatment for curing gastric cancer, there is only a marginal chance of recovery for advanced stage patients with chemotherapy resistance.2 Thus, discovering molecular markers for early diagnosis and targeted therapy is crucial to improving the prognosis of gastric cancer.
Gastric carcinogenesis progresses through multiple stages, including oxyntic atrophy, preneoplastic metaplasia (spasmolytic polypeptide-expressing metaplasia [SPEM] and intestinal metaplasia [IM]), dysplasia, and cancer.3, 4 SPEM, which is considered a potential neoplastic precursor of IM,5 is characterized by spasmolytic polypeptide (TFF2) or Muc6/GSII expression in the basal region of the gland, comprising the chief cells after parietal cell loss.6–9 Preneoplastic SPEM lineage exhibits a distinct molecular signature, including the upregulation of CD44v9.10–12 There are several established methods to produce SPEM in mice.13 Pharmacological agents, DMP-777, L635, and high-dose tamoxifen, can lead to the development of acute SPEM with epithelial cell damage.14, 15 Interestingly, SPEM in such models does not readily progress to dysplasia in the absence of inflammatory responses, and gastritis and metaplasia often regress in immune-deficient mice.16 These findings indicate that an immune-related mechanism may play a key role in SPEM development.13
Gastritis induced by Helicobacter pylori (H. pylori) infection is considered a primary risk factor for human gastric cancer. H. pylori infection establishes an environment of robust and chronic inflammation that can induce carcinogenic driver mutations.1, 17 In mice, infection with H. pylori (SS1 strain) or H. felis mimics human H. pylori infection and leads to preneoplastic metaplasia with chronic inflammation.18 Indeed, H. felis infection has been found to induce a chronic SPEM condition accompanied by severe inflammation in mice7 that can further progress into dysplastic morphology.
Among the immune cells that infiltrate the mucosa in response to injury, alternatively activated (M2) macrophages appear to be critical in the progression to advanced SPEM, and depletion of macrophages has been found to attenuate metaplasia following parietal cell loss in SPEM models.19 We previously reported that the T-helper2 (Th2) cytokine interleukin (IL) 33 is a critical inflammatory mediator for SPEM progression and M2 macrophage polarization.20 IL33, secreted from gastric epithelial cells, functions as a stomach alarmin, initiating the activation of various immune cells through the ST2 receptor.21 Recently, our group found that IL33 induces the release of IL13 from ILC2 cells for the induction of metaplasia following acute oxyntic atrophy22. However, little is known about the regulation of IL33 in response to injury to the stomach and progression to SPEM.
WAP four-disulfide core domain protein 2 (WFDC2), also known as human epididymis protein 4 (HE4), is a small secretory protein highly expressed in human ovarian cancer, lung cancer, and fibrotic diseases.23–26 WFDC2 expression has been shown to be significantly associated with tumor progression and survival in breast and lung cancer patients.24, 27 Recent studies have further suggested that WFDC2 may play a role as a putative immune-modulator and host defender.25, 28–30 Nozaki et al.11 discovered that Wfdc2 is highly expressed in a DMP-777-induced SPEM model1111. Upregulated WFDC2 has also been confirmed in tissue samples of patients with preneoplastic metaplasia and gastric cancer.10, 31 However, the detailed role of WFDC2 in gastric carcinogenesis remains unknown.
Here, we induced acute SPEM models in Wfdc2-knockout and wild-type mice with DMP-777, L635, and high-dose tamoxifen treatments to elucidate the role of Wfdc2 in the development of preneoplastic metaplasia in the stomach. We further performed transcriptomic analysis to identify potential underlying mechanisms, particularly with respect to the interaction of WFDC2 with M2 macrophage polarization and IL33 secretion from epithelial cells. In addition, we administered exogenous rWFDC2 to the Wfdc2-knockout and wild-type mice to investigate its impact on the development of SPEM and to identify any potential regulatory mechanisms. Remarkably, Wfdc2 knockout elicited resistance against SPEM development, and rWFDC2 supplementation promoted SPEM through the upregulation of IL33. We believe that these findings may shed light on the role of WFDC2 in gastric carcinogenesis.
MATERIALS AND METHODS
Mice
Details on the generation of Wfdc2−/− mice are provided in the Supplementary Methods. Wfdc2+/+ (wild-type) and Wfdc2−/− (knockout) mice in a C57BL/6 background were bred under specific pathogen-free conditions. All animal experiments were conducted in accordance with Public Health Service Policies for the Humane Care and Use of Laboratory Animals and were approved by the IACUC, an AAALAC-accredited unit (#001071).
Establishment of Murine Models of SPEM
In the DMP-777 model, DMP-777 (350 mg kg−1) was fully dissolved in 0.5% methylcellulose (Sigma) with gentle agitation and orally inoculated. Mice were treated with DMP-777 for 7 or 14 days to develop SPEM. Following 14 days of DMP-777 treatment, a 14-day recovery phase was permitted after the cessation of treatment. In the L635 model, L635 (350 mg kg−1) was dissolved in deionized water and orally inoculated in the same manner as that in the DMP-777 model. Mice were treated with L635 daily for 3 days to develop SPEM. In the high-dose tamoxifen model, tamoxifen (250 mg kg−1; Sigma) was fully dissolved in corn oil (Sigma) with gentle agitation. The mice were intraperitoneally injected with tamoxifen for 3 consecutive days, followed by 3 days of cessation of treatment, to develop SPEM.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software v7.0. Statistical significance was determined using unpaired Student’s t-test (two-tailed) or one-way analysis of variance with Dunnett’s multiple comparison. Correlation analysis was performed using Pearson correlation coefficients (two-tailed). Kaplan–Meier survival analysis was conducted to compare gastric adenocarcinoma patient survival using the log-rank test. Data are presented as a mean ± SEM; p < 0.05 was considered statistically significant.
RESULTS
Upregulation of WFDC2 Expression in Preneoplastic Metaplasia in Humans and Mice
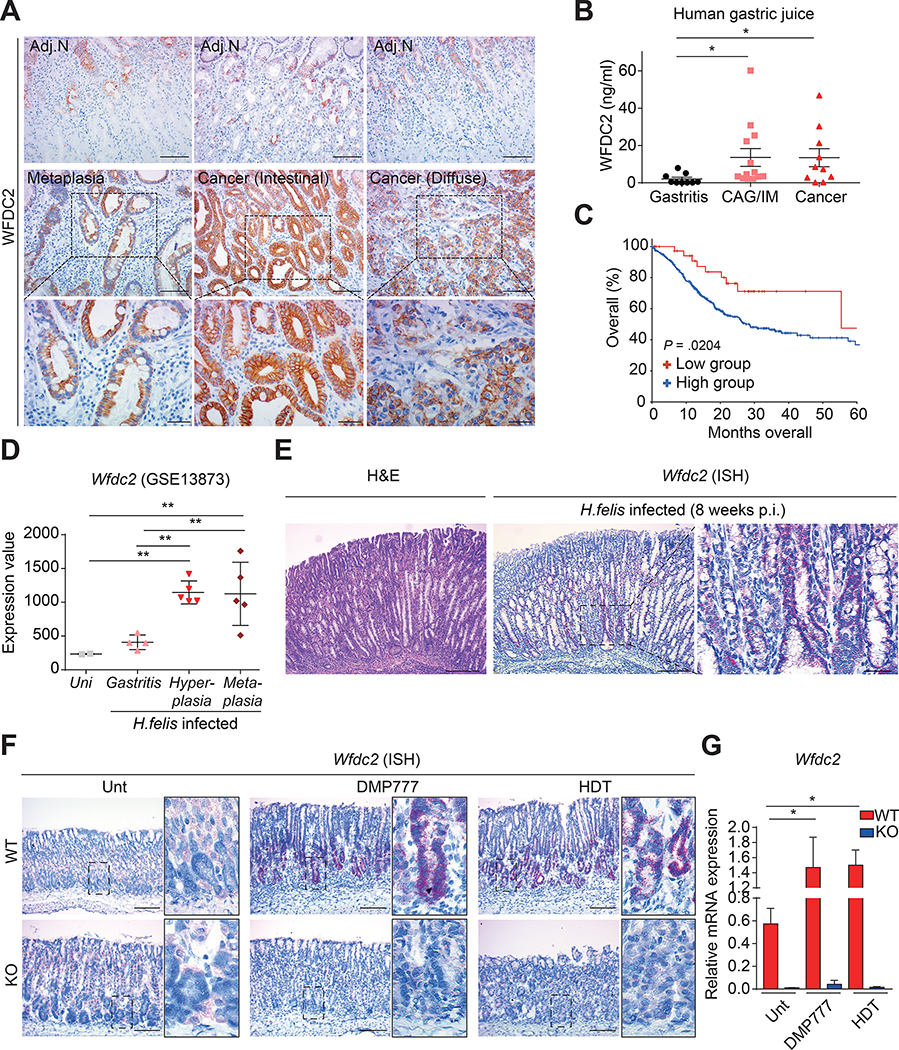
In line with a previous report,31 immunohistochemistry revealed a gradual increase in WFDC2-positive regions from preneoplastic metaplasia to gastric cancer, whereas adjacent normal regions showed minimal WFDC2 expression (Figure 1A and Supplementary Figure 1A). Remarkably, strong WFDC2 expression was detected on the cytosolic membrane of gastric cancer cells (Figure 1A), suggesting that WFDC2 may act as a signaling molecule.
Figure 1. WFDC2 expression in human and mice.
(A) Immunohistochemistry images for WFDC2 in human tissue. Top panels indicate adjacent normal (Adj.N) tissue regions to the metaplasia and gastric cancer. Scale bars, 100 μm (top and middle panels); 40 μm (bottom panels). (B) WFDC2 concentrations in gastric juice from patients with gastritis (n = 9), chronic atrophic gastritis/intestinal metaplasia (CAG/IM; n = 13), and gastric cancer (n = 10) (two-tailed unpaired Student’s t-test, *p < 0.05). (C) Kaplan–Meier survival graph for gastric adenocarcinoma patients. Statistical significance was assessed by the log-rank test (Low group WFDC2: expression z-score ≤−1.5). (D) Wfdc2 expression for H. felis-infected mice using GSE13873 (one-way analysis of variance with Dunnett’s multiple comparison, **p < 0.01). (E) Representative H&E and ISH images for the H. felis-infected mice. Scale bars, 200 μm (left and middle panels); 40 μm (right panel). (F) ISH images for Wfdc2. Scale bar, 100 μm. WT, wild-type; KO, knockout. (G) RT-qPCR for Wfdc2; the mRNA expression level was normalized by Gapdh (n = 4–5 per group, one-way analysis of variance with Dunnett’s multiple comparison, *p < 0.05). All data are presented as a mean ± SEM.
Of note, WFDC2 protein levels in gastric juice were greater in patients with chronic atrophic gastritis (CAG)/intestinal metaplasia (IM) and gastric cancer than in patients with gastritis (Figure 1B). The median overall survival of gastric cancer patients with lower WFDC2 expression was longer than that of patients with higher expression (55.43 months vs. 28.37 months) (Figure 1C), further substantiating a role for WFDC2 in gastric cancer progression and prognosis.
Public GEO data showed that the expression levels of Wfdc2 in hyperplasia or metaplasia specimens from H. felis-infected mice were higher than those of uninfected controls or weak gastritis samples (Figure 1D). In situ hybridization confirmed strong expression of Wfdc2 in metaplastic lesions that developed 8 weeks after H. felis infection (Figure 1E). Wfdc2 expression levels were also significantly increased in acute SPEM models induced with DMP-777 or high-dose tamoxifen, in which Wfdc2-expressing cells emerged from the base of the corpus glands (Figure 1F and G).
Wfdc2 Knockout Suppresses Oxyntic Atrophy in Drug-induced Metaplasia Models
Wfdc2 knockout created with exon 2 deletion was confirmed by in situ hybridization (Figure 1F) and reverse transcription-quantitative polymerase chain reaction (Figure 1G). Induction of SPEM with DMP-777, high-dose tamoxifen, and L63514, 15 caused epithelial cell damage and acute SPEM (Supplementary Figure 2A).
Macroscopically, the appearances of wild-type and knockout mice were not markedly different (Supplementary Figure 2B). Microscopically, however, wild-type mice treated with DMP-777 for 7 or 14 days developed abnormal gland structures with apparent epithelial cell damage, whereas knockout mice showed relatively intact gland structures (Figure 2A). DMP-777-induced increases in MKI67+ proliferating cells were significantly attenuated in the knockout mice (Figure 2B and Supplementary Figure 2C). Most of the proliferating cells in the mucosa were contained within the isthmus region in the DMP-777-treated knockout mice, but were found closer to the base of the gland in the DMP-777-treated wild-type mice (Figure 2C and Supplementary Figure 2C). These histological alterations recovered within 14 days after the cessation of DMP-777 treatment (Figure 2A–C and Supplementary Figure 2C).
Figure 2. Protective effect of Wfdc2 knockout (KO) against DMP-777 treatment.
(A) H&E images of the corpus from wild-type (WT) and KO mice during DMP-777 treatment. Scale bars, 100 μm. (B) MKI67+ proliferating cells in single glands quantified in WT and KO mice. Cell numbers were measured at the Top, Neck, and Base, each representing 1/3 of the total gland length (n = 5 per group, two-tailed unpaired Student’s t-test, ***p < 0.001) (C) Proliferating cell distributions in single glands. Each spot represents average loci of cells from the lowest gland base (0 μm) (two-tailed unpaired Student’s t-test, **p < 0.01, ***p < 0.001). (D) TEM images of parietal cells. Scale bars, 5000 nm. (E) ATP4A+ parietal cells in single glands quantified in WT and KO mice (n = 5 per group, two-tailed unpaired Student’s t-test, ***p < 0.001). (F) Blood gastrin concentrations in WT and KO mice (n = 3–4 per group, two-tailed unpaired Student’s t-test, ***p < 0.001). (G) MIST1+ chief cells in single glands quantified in WT and KO mice (n = 5 per group, two-tailed unpaired Student’s t-test, ***p < 0.001). (H) Average MUC5AC+ lengths measured from the top gland in WT and KO mice (n = 5 per group, two-tailed unpaired Student’s t-test). All data are presented as a mean ± SEM. Unt, Untreated; D7, 7 days of DMP-777 treatment; D14, 14 days of DMP-777 treatment; R14, 14 days of recovery.
Transmission electron microscopy (TEM) images revealed necrosis of parietal cells in the glands of the DMP-777-treated wild-type mice, whereas the cells remained relatively intact in Wfdc2-knockout mice (Figure 2D). Consistently, reductions in the number of ATP4A+ parietal cells were significantly alleviated (Figure 2E and Supplementary Figure 2D), and gastrin levels increased by DMP-777 were also significantly attenuated in the knockout mice (Figure 2F). However, there was no change in albumin levels, an indicator of protein synthesis and loss (Supplementary Figure 2E). Likewise, DMP-777-induced reductions of MIST1+ chief cells from the base of the gland were significantly suppressed in knockout mice (Figure 2G and Supplementary Figure 2D). However, hyperplasia of MUC5AC+ foveolar cells was observed in both wild-type and knockout mice (Figure 2H and Supplementary Figure 2D). All lineage alterations and elevation of gastrin levels due to DMP-777 recovered after 14 days of treatment cessation (Figure 2E–H and Supplementary Figure 2D). Similar to the DMP-777 model, L635- and high-dose tamoxifen-treated knockout mice also showed protection against SPEM-induced changes in proliferating cells (Supplementary Figure 3A, B, and E), corpus cell lineages (Supplementary Figure 3C, D, and F), and the ultrastructure of parietal cells (Supplementary Figure 4A and B).
Wfdc2 Knockout Inhibits SPEM and Preneoplastic Dysplasia Progression
SPEM is characterized by abundant expression of the spasmolytic polypeptide TFF2,7 as well as the downregulation of the zymogen granule maturation factor Mist1.32 As expected, we observed an increase in TFF2-expressing cells and loss of zymogen granules in mature chief cells upon DMP-777 treatment (Figure 3A and C). However, TFF2-expressing cells decreased significantly in Wfdc2-knockout mice (Figure 3A and B). TEM images demonstrated that normal chief cell morphology was preserved even after drug treatment, without loss of zymogen granules, in Wfdc2-knockout mice in all three models (Figure 3C, Supplementary Figure 4A and B).
Figure 3. Inhibition of SPEM in Wfdc2-knockout (KO) mice after DMP-777 treatment.
(A) Immunohistochemistry images for TFF2. Scale bars, 40 μm. (B) Quantification of TFF2+ cells in single glands (n = 5 per group, two-tailed unpaired Student’s t-test, ***p < 0.001). (C) TEM images of chief cells. Scale bars, 5000 nm. ZG, zymogen granule. (D) Immunofluorescence images for GSII, and GIF in wild-type (WT) and KO mice. Scale bars, 20 μm. (E) Comparison of SPEM cells during DMP-777 treatment. Each graph represents the number of positive cells in single glands (n = 5 per group, two-tailed unpaired Student’s t-test, **p < 0.01). (F) Immunofluorescence images for GSII, and MKI67. Scale bars, 20 μm. (G) Comparison of proliferative SPEM cells during DMP-777 treatment. Each graph represents the number of positive cells in single glands (n = 5 per group, two-tailed unpaired Student’s t-test, **p < 0.01, ***p < 0.001). (H) Immunofluorescence images for GSII, and CD44v9 of the Unt, DMP-777 (D7)-treated, and HDT-treated corpus. Scale bars, 100 μm. (I) RT-qPCR for Cftr and Gpx2 in the Unt, DMP-777 (D7)-treated, and HDT-treated corpus. mRNA expression levels were normalized by Gapdh (n = 5–7 per group, two-tailed unpaired Student’s t-test, *p < 0.05). All data are presented as a mean ± SEM. Unt, Untreated; D7, 7 days of DMP-777 treatment; D14, 14 days of DMP-777 treatment; R14, 14 days of recovery; HDT, high-dose tamoxifen.
Wild-type mice harbored a large number of SPEM cells co-stained with GSII and GIF from the base of the gland at day 7 of DMP-777 treatment, which turned to mostly GSII+ cells by day 14 (Figure 3D and E). However, GIF+ mature chief cells were retained at the base of the gland, and proliferative SPEM cells (GSII+ and MKI67+ cells) were significantly reduced in Wfdc2-knockout mice (Figure 3F and G). All SPEM features were reversed over the 14-day recovery phase without drug treatment, returning to untreated levels (Figure 3D–G).
After DMP-777 or tamoxifen treatment, basal cells in the corpus gland expressed CD44 variant 9 (CD44v9), a SPEM-specific marker, along with GSII in the wild-type, but not in the knockout, mice (Figure 3H). 51.7% of CD44v9-positive SPEM cells from wild-type mice expressed Wfdc2 (Supplementary Figure 4C). Furthermore, upregulation of SPEM-associated intestinal transcripts (Cftr, Gpx2)19 by DMP-777 or tamoxifen was attenuated in the knockout mice (Figure 3I).
We also examined the impact of Wfdc2 knockout on H. felis infection-induced preneoplastic dysplasia. After 11 months of H. felis infection, wild-type mice showed dysplastic and cystic changes, along with increased mucosal thickness and massive infiltration of inflammatory cells, all of which were not observed in pharmacological SPEM models (Supplementary Figure 5A). In addition, oxyntic atrophy and metaplasia were remarkable in wild-type mice chronically infected with H. felis. However, in knockout mice, histopathological changes were mild, and oxyntic atrophy and metaplastic cells were not observed.
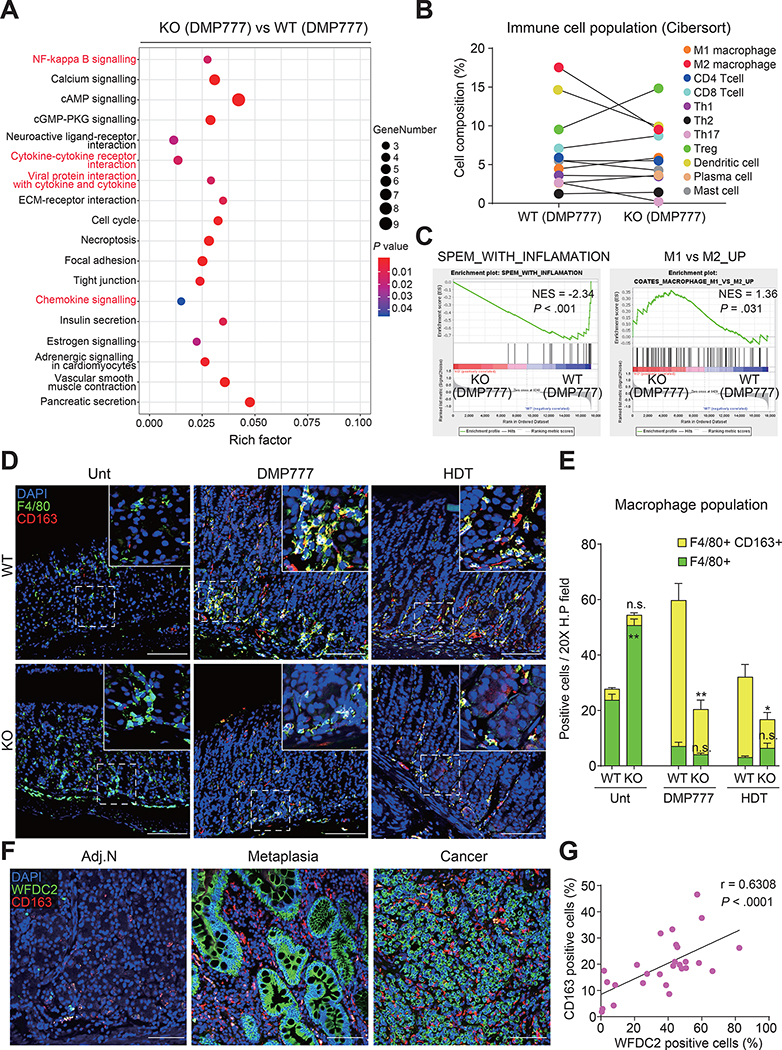
DMP-777-treated Wfdc2-knockout Mice Show Alterations in Immune-related Pathways and M2 Macrophages
Significant differences were found in the transcriptomes of fundus samples from DMP-777-treated wild-type and Wfdc2-knockout mice (Supplementary Figure 6A and B). KEGG pathway analysis revealed that immune-related pathways (nuclear factor-kappa B signaling, cytokine-cytokine receptor interaction, and chemokine signaling) were altered in Wfdc2-knockout mice (Figure 4A). CIBERSORT analysis showed that populations of M2 macrophages and dendritic cells were remarkably reduced in DMP-777-treated Wfdc2-knockout mice, whereas regulatory T cells were enriched (Figure 4B). GSEA showed that gene sets associated with the cell cycle, SPEM, and gastric cancer were negatively enriched in Wfdc2-knockout mice (Figure 4C, Supplementary Figure 6C and D), consistent with the alleviation of SPEM progression. Furthermore, a significant enrichment score was observed for gene sets distinguishing M2 and M1 macrophages (Figure 4C), indicating the alteration of M1/M2 polarization in Wfdc2-knockout mice.
Figure 4. Decrease in M2 macrophage accumulation in Wfdc2-knockout (KO) mice after DMP-777 treatment.
(A) Bubble plot showing selected top 18 terms of enriched KEGG pathways. P values were derived from Fisher’s exact test. (B) Computational comparison of average immune cell composition between DMP-777-treated wild-type (WT) and KO mice. The analysis was matched with a published signature file.51 (C) GSEA graphs for SPEM-related immune gene signatures comparing DMP-777-treated KO and WT mice. (D) Immunofluorescence images for F4/80, and CD163 of the Unt, DMP-777 (D7)-treated, and HDT-treated corpus. Scale bars, 100 μm. (E) Number of positives in a 20X high-power field of the Unt, DMP-777 (D7), and HDT groups. Data are presented as a mean ± SEM (n = 3 per group, two-tailed unpaired Student’s t-test, *p < 0.05, **p < 0.01). (F) Immunofluorescence images for WFDC2, and CD163 in adjacent normal (Adj.N), metaplasia, and cancer tissues from human patients. Scale bars, 100 μm. (G) Correlation between WFDC2-positive cells and CD163-positive cells in human tissue. Positive cells in human specimens (n = 27) were measured in a 20X high-power field and assessed by a two-tailed test using Pearson’s correlation coefficients. Unt, Untreated; D7, 7 days of DMP-777 treatment; HDT, high-dose tamoxifen.
Additionally, M2 macrophage (F4/80+ and CD163+ positive cells) populations were increased in the DMP-777- or tamoxifen-treated wild-type mice at the base of the gland where SPEM cells arose, which was significantly alleviated in Wfdc2-knockout mice (Figure 4D and E). Also, CD163+ cells significantly and gradually increased from metaplasia to gastric cancer in human patients (Figure 4F and Supplementary Figure 7A), which was positively correlated with the expansion of WFDC2+ cells in gastric lesions (Figure 4G, Supplementary Figure 7B).
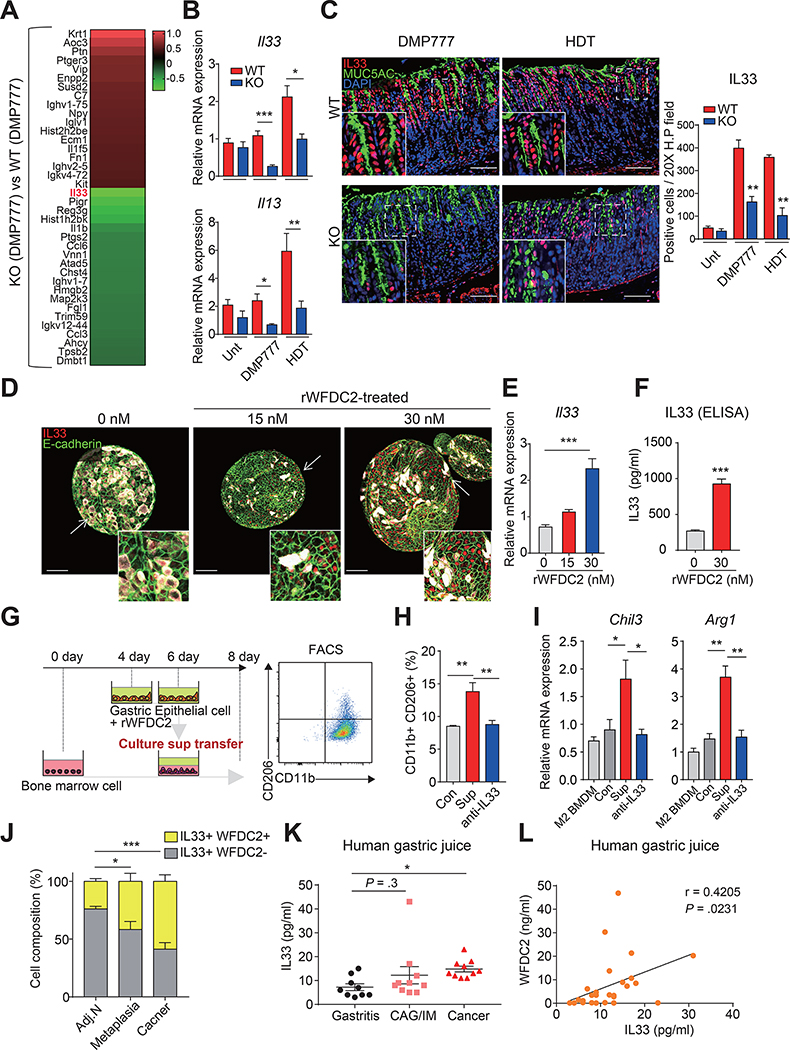
WFDC2 Promotes M2 Macrophage Polarization by Upregulating IL-33 Expression
Il33 was the most severely downregulated gene among genes associated with immune-related function in knockout mice (Figure 5A). IL33 secreted from epithelial cells can promote M2 macrophages polarization,21, 33 and a previous study showed that Il33-knockout mice fail to recruit M2 macrophages, thereby preventing SPEM development.20 Although acute injury by DMP-777 or high-dose tamoxifen promoted foveolar cell hyperplasia in both wild-type and Wfdc2-knockout mice, IL33 expression levels were markedly diminished in Wfdc2-knockout mice when compared with the wild-type mice (Figure 5B and C), and Il13 mRNA levels induced by IL33 were also significantly reduced (Figure 5B). Also, H. felis-infected wild-type mice showed elevated IL-33 expression in the pit region, as well as the lamina propria, where stromal and immune cells exist (Supplementary Figure 5B). However, in Wfdc2-knockout mice, IL-33 expression remained unchanged and contained within the mucosa.
Figure 5. Novel function of WFDC2 as a regulator of interleukin-33 (IL33).
(A) Heatmap showing differentially expressed genes involved in immune-related Gene Ontology terms (GO: 0006955, GO: 0006954) in DMP-777-treated knockout (KO) mice compared to DMP-777-treated wild-type (WT) mice. (B) RT-qPCR for Il33 and Il13 in the Unt, DMP-777 (D7)-treated, and HDT-treated corpus; mRNA expression levels were normalized by Gapdh (n = 3–7 per group, two-tailed unpaired Student’s t-test, *p < 0.05, **p < 0.01, ***p < 0.001). (C) Immunofluorescence for MUC5AC, and IL33 in DMP-777 (D7) and HDT groups. Scale bar, 100 μm. Graph indicates the number of positives at epithelial regions in a 20X high-power field (n = 3 per group, two-tailed unpaired Student’s t-test, **p < 0.01). (D) Immunofluorescence for E-cadherin, MUC5AC and IL33 in rWFDC2-treated corpus organoids at 48 h after initial treatment. Scale bar, 100 μm. (E) RT-qPCR for Il33 in rWFDC2-treated corpus organoids; mRNA expression levels were normalized by Gapdh (n = 3–4 per each group, one-way analysis of variance with Dunnett’s multiple comparison, ***p < 0.001). (F) IL33 level in supernatants from gastric epithelial cells at 48 h after treatment with rWFDC2 (n = 3 per group, two-tailed unpaired Student’s t-test, **p < 0.01). (G–I) BMDMs were cultured for 8 days and supplemented with the supernatant from rWFDC2-treated gastric epithelium after 4 days of culture, and further analyzed with FACS. (H) FACS sorting for CD45+CD11b+CD206+ M2 macrophages and (I) RT-qPCR for M2 macrophage markers (Chil3 and Arg1). M2 BMDM indicates cells without supplement of supernatant as a normal control, while Con indicates cells supplemented with the supernatant of epithelial cells (+0 nM rWFDC2). Sup indicates cells supplemented with the supernatant of epithelial cells (+30 nM rWFDC2), and anti-IL33 indicates Sup (+30 nM rWFDC2) treated with anti-IL33 antibody. (n = 4 per group, one-way analysis of variance with Dunnett’s multiple comparison, *p < 0.05, **p < 0.01). (J) IL33-expressing cell composition (IL33+WFDC2-, IL33+WFDC2+) in a 20X high-power field of adjacent normal (Adj.N, n = 5), metaplasia (n = 8), and cancer (n = 8) tissues from human patients (two-tailed unpaired Student’s t-test, *p < 0.05 ***p < 0.001). (K) IL33 levels in human gastric juice from patients with gastritis (n = 9), chronic atrophic gastritis/intestinal metaplasia (CAG/IM, n = 10), and cancer (n = 10) (two-tailed unpaired Student’s t-test, *p < 0.05). (L) Correlation between IL33 and WFDC2 levels in human gastric juice (n = 29) assessed by a two-tailed test using Pearson’s correlation coefficients. All data are presented as a mean ± SEM. Unt, Untreated; D7, 7 days of DMP-777 treatment; HDT, high-dose tamoxifen.
Considering the previous transcriptomic and in vivo data, we postulated that WFDC2 could regulate IL33 expression in the gastric mucosa. Consistently, after treatment of fundus organoids with recombinant WFDC2 (rWFDC2), E-cadherin+ epithelial cells showed nuclear IL33 induction (Figure 5D). Furthermore, Il33 expression increased in a dose-dependent manner following rWFDC2 treatment (Figure 5E), whereas Muc5ac expression was not affected (Supplementary Figure 8A). IL33 secretion also significantly increased in the epithelial cells after rWFDC2 treatment (Figure 5F).
To examine whether rWFDC2-induced IL33 from gastric epithelial cells promotes M2 macrophage polarization, we cultured mouse bone marrow-derived macrophages (BMDMs) in vitro with epithelial cell supernatant conditioned with rWFDC2 (Figure 5G). Fluorescence-activated cell sorting (FACS) revealed a significant increase in the population of CD11b+/CD206+ M2 macrophages in BMDMs supplemented with the rWFDC2-conditioned media. However, this elevation of M2 macrophage polarization was significantly suppressed by IL33 neutralization with anti-IL33 (Figure 5H and Supplementary Figure 8B). The mRNA levels of M2 macrophage markers (Chil3, Arg1) also increased in supernatant-treated BMDMs, which was also attenuated by IL33 neutralization (Figure 5I). There was no change in M2 macrophage polarization with direct treatment of rWFDC2 to BMDMs (Supplementary Figure 8C and D), supporting that WFDC2 promotes M2 polarization indirectly through the induction of IL33 from gastric epithelial cells.
Similarly, cells co-expressing IL33 and WFDC2 gradually expanded from tissue samples of human patients with preneoplastic metaplasia to gastric cancer (Figure 5J and Supplementary Figure 9A), and IL33 levels in gastric juice from gastric cancer patients were significantly increased (Figure 5K). Indeed, there was a positive correlation between WFDC2 and IL33 levels in gastric juice samples (Figure 5L).
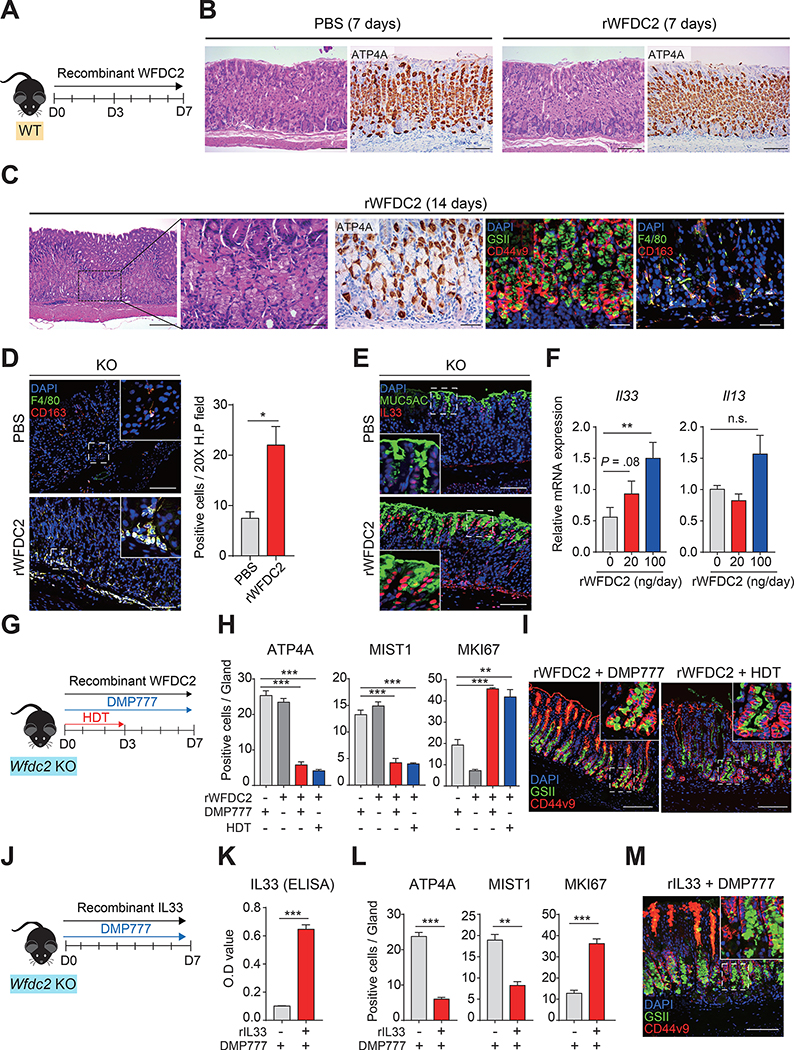
Infusion of rWFDC2 Leads to Spontaneous Metaplasia and Exacerbates Acute Injury-induced SPEM
Finally, we examined whether WFDC2 alone could promote metaplasia in the stomach in vivo. An osmotic pump was placed subcutaneously in wild-type mice to provide an equal dose of rWFDC2 for 7 days (Figure 6A). Similar to the effects of DMP-777 treated samples (Figure 5A), Il33 expression levels significantly increased in the rWFDC2-treated wild-type mice (Supplementary Figure 10A). Moreover, M2 macrophage polarization-related transcripts (Arg1, Anaxa1, Il33) and SPEM signatures were enhanced and positively enriched in the fundus samples of rWFDC2-treated wild-type mice (Supplementary Figure 10B and C). Infusion of rWFDC2 for 7 days did not result in histological changes, but significantly increased F4/80+/CD163+ M2 macrophages (Figure 6B and Supplementary Figure 10D). Strikingly, on day 14 of rWFDC2 infusion, spontaneous SPEM was induced with a significant increase in oxyntic atrophy (parietal cell loss) and accumulation of M2 macrophages without other pharmacological treatments (Figure 6C and Supplementary Figure 10E). Moreover, spontaneous SPEM appeared at the corpus/antrum border (Supplementary Figure 9B).
Figure 6. Exacerbation of SPEM in Wfdc2-knockout (KO) mice by infusion of rWFDC2.
(A) Schematic image showing infusion of rWFDC2 (100 ng/day) in wild-type (WT) mice by an osmotic pump. (B) H&E and immunohistochemistry images of ATP4A. Scale bar, 100 μm. (C) H&E, immunohistochemistry, and immunofluorescence images after 14 days of rWFDC2 (100 ng/day) infusion in WT mice. Scale bars, 100 μm (left panel); 40 μm (other panels). (D) Immunofluorescence images for F4/80, and CD163 after 7 days of rWFDC2 infusion in KO mice. Scale bar, 100 μm. The graph represents the number of positive cells in a 20X high-power field of control (PBS) and rWFDC2-infused groups (n = 3 per group, two-tailed unpaired Student’s t-test, *p < 0.05). (E) Immunofluorescence images for MUC5AC and IL33 after 7 days of rWFDC2 infusion in KO mice. Scale bar, 100 μm. (F) RT-qPCR for Il33 and Il13 in rWFDC2-infused KO mice; mRNA expression levels were normalized by Gapdh (n = 4 per group, one-way analysis of variance with Dunnett’s multiple comparison, **p < 0.01). (G) Schematic image showing SPEM induction in rWFDC2-infused KO mice (100 ng/day). (H) Number of positive cells in single glands with (+) or without (–) rWFDC2, DMP-777, and HDT (n = 3 per group, two-tailed unpaired Student’s t-test, **p < 0.01, ***p < 0.001). (I) Immunofluorescence images for GSII, and CD44v9 in rWFDC2-infused (+ DMP-777 or + HDT) KO mice. Scale bar, 100 μm. (J) Schematic image showing SPEM induction in rIL33-infused KO mice (0.0125 mg kg−1day−1). (K) IL33 concentration in the blood and (L) the number of positive cells in single glands with (+) or without (–) rIL33 and DMP-777 (n = 3–4 per group, two-tailed unpaired Student’s t-test, **p < 0.01, ***p < 0.001). (M) Immunofluorescence images for GSII, and CD44v9 in rIL33-infused (+ DMP-777) KO mice. Scale bar, 100 μm. All data are presented as a mean ± SEM. HDT, high-dose tamoxifen.
Likewise, the Wfdc2-knockout mice infused with rWFDC2 showed significant accumulation of F4/80+/CD163+ M2 macrophages (Figure 6D) and increased IL33 expression (Figure 6E and F). Il13 expression was also increased, although statistical significance was not achieved (p-value = 0.1) (Figure 6F). Remarkably, in the Wfdc2-knockout mice, rWFDC2 and DMP-777 combination treatment significantly diminished ATP4A+ parietal cells and MIST1+ chief cells, and expanded MKI67+ proliferating cells when compared with either rWFDC2 or DMP-777 single treatment (Figure 6H and Supplementary Figure 10F). Additionally, CD44v9+/GSII+ SPEM cells were observed at the base of the gland (Figure 6I).
To elucidate whether rWFDC2-induced SPEM was IL33-dependent, we continuously infused recombinant IL33 (rIL33) with DMP-777 in Wfdc2-knockout mice (Figure 6J and K), which resulted in SPEM phenotypes, including reductions in parietal cells and chief cells, expansion of proliferating cells, and development of SPEM cells (Figure 6L and M and Supplementary Figure 10G), supporting the key role of IL33 in WFDC2-promoted SPEM. In addition, the transcript levels of Chil3 and Th2 cytokines significantly increased (Supplementary Figure 10H).
DISCUSSION
Several studies have explored the role of WFDC2 as a candidate biomarker for various types of neoplasia, including lung cancer, ovarian cancer, and breast cancer. Patients with high WFDC2 expression typically show poor prognosis, supporting the pivotal role of WFDC2 in cancer progression and malignancy.24, 27 A recent study showed that WFDC2 is also involved in kidney fibrosis and inflammatory bowel disease.26, 29 Despite its implications in numerous diseases, the role of WFDC2 in gastric carcinogenesis has remained obscure. We previously reported that WFDC2 expression is upregulated in the gastric preneoplasia of SPEM and IM patients and in human gastric cancer tissues.11, 31 In the present study, we further identified strong WFDC2 staining in the cytosolic membrane of gastric cancer tissue. Previous studies have suggested that WFDC2 and its family members could regulate signaling pathways, such as NF-kappaB, AKT, and EGFR pathways,28, 34, 35 implicating WFDC2 as a putative ligand that binds to certain signaling receptors. We found that higher WFDC2 expression was also significantly associated with the poor prognosis of gastric cancer patients. Although a previous report showed that serum WFDC2 levels were not altered in gastric cancer patients,24 we found increased WFDC2 levels in the gastric juice samples of metaplasia and gastric cancer patients, indicating that WFDC2 can be a biomarker for gastric cancer.
Gastric carcinogenesis evolves through multiple stages, including oxyntic atrophy, SPEM, IM, dysplasia, and eventually gastric cancer. Oxyntic atrophy is the primary event during gastric carcinogenesis and leads to the emergence of metaplastic lineages that are susceptible to neoplastic transformation under chronic inflammation. Conventional SPEM induction methods using DMP-777, L635, high-dose tamoxifen, and Helicobacter species infection create a state of oxyntic atrophy. In previous studies, treatment with omeprazole, which inhibits the proton pump of parietal cells, ameliorated oxyntic atrophy, metaplasia, foveolar cell hyperplasia, and increases in proliferating cells,15, 36 indicating that the maintenance of parietal cell populations and their normal physiological functions is important in preventing gastric carcinogenesis.
Thus, it is noteworthy that Wfdc2 knockout suppressed oxyntic atrophy in all SPEM models established in the present study. Moreover, proliferating cells and chief cell loss, which are distinct signatures of metaplasia, were also alleviated in Wfdc2-knockout mice. Elevated gastrin levels in the DMP-777 model were also suppressed, although albumin levels (a diagnostic factor for Menetrier disease remained unchanged, indicating that Wfdc2 knockout did not cause other stomach lesions.37 However, further investigation is needed to identify why foveolar cell hyperplasia was maintained in Wfdc2-knockout mice despite the lower levels of gastrin. The parietal cells of Wfdc2-knockout mice also showed mild damage and a shrunken morphology, although cell populations were still preserved and necrosis was rarely observed. A previous report suggested that parietal cell loss is not a prerequisite for gastric cancer development.38 Research has also shown that targeted apoptosis of parietal cells by diphtheria toxin does not induce SPEM, which is attributable to a lack of an immune response, in contrast to other SPEM models. These results imply that parietal cell damage does not persist and progress toward a severe phenotype in the absence of inflammation. Indeed, Ifng and Il17a, which are known to directly act on parietal cells and lead to epithelial cell death39, 40 have been found to be downregulated in Wfdc2-knockout mice (Supplementary Figure 11B). Previous studies have revealed that IL-33 promotes the production of IFN-γ IL-17A in injury and infection states 41, 42, suggesting that blockade of IL-33-induced production of IFN-γ and IL-17A in Wfdc2-knockout mice may explain the lack of parietal cell loss in pharmacologic models of gastric injury.
Following parietal cell loss, spasmolytic polypeptide (TFF2)-expressing cells emerged from the basal region where GIF+ chief cells normally exist.7 While the origin of SPEM cells is controversial, these metaplastic cells express distinct molecular signatures, including co-expression of GSII and GIF, intestinal transcript expression, and CD44v9+ expression.10, 12, 19 After immediate damage by DMP777 (D2), Wfdc2 was dominantly expressed in a subset of chief cells. Then, the major Wfdc2-producing cells became CD44v9+ SPEM cells that originated from the chief cells (Supplementary Figure 11A). The enrichment and proliferation of SPEM cells in our SPEM induction models were significantly diminished in Wfdc2-knockout mice. Our GSEA data also suggested that gene sets known to be associated with gastric cancer or SPEM were downregulated in Wfdc2-knockout mice.43, 44
Although recent reports have proposed that SPEM is associated with a gastric repair mechanism,44, 45 several lines of evidence indicate that SPEM progresses to an advanced phenotype predisposed to neoplasia when combined with inflammation.5, 18 We previously revealed that M2 macrophages are critical accelerators of the progress to advanced SPEM and that depletion of macrophages prohibits SPEM after parietal cell loss.19 Other groups have also shown that M2 macrophages could control CD44v9 expression and promote gastric cancer.45, 46 However, the triggering mechanism of M2 macrophage recruitment during SPEM formation remains obscure. In line with previous observations, we discovered that immune-related pathways and immune cell populations, especially M2 macrophages, were altered in the DMP-777-treated corpus and that enriched CD163+ M2 macrophage populations were significantly reduced in Wfdc2-knockout mice. Furthermore, the number of M2 type macrophages was substantially increased in the metaplasia and cancer regions of human samples and was significantly correlated with the number of WFDC2-positive cells, indicating that WFDC2 may be related to regulation of M2 macrophage populations. Indeed, WFDC2 indirectly contributed to M2 macrophage polarization by provoking the release of IL33, an epithelial cell-derived cytokine.
It is difficult to determine whether the Th1 or Th2 response is responsible for SPEM development, since both Th1- and Th2-related cytokines are elevated in gastritis or the SPEM environment.6, 47, 48 Recent studies have reported that various cytokines affect the development of metaplasia.20, 47 Among the significantly different gene sets related to inflammation in DMP-777-treated mice or rWFDC2-infused mice, we postulated IL33 as a potential target regulated by WFDC2. Unlike other cytokines that are normally secreted from immune cells, IL33 is constitutively expressed in gastric epithelial cells, especially foveolar cells, and can be increased in response to injury, functioning as a stomach alarmin and governing the Th2 immune response through its receptor ST2, which can subsequently promote M2 macrophage polarization.20, 21, 33 Moreover, IL33 is associated with gastric cancer prognosis and progression.20, 49 Here, we showed that Wfdc2-knockout mice exhibit significantly reduced IL33 expression levels, along with the downregulation of IL13, a Th2 cytokine. Conversely, treatment with rWFDC2 increased IL33 expression levels in gastric epithelial cells and indirectly promoted M2 macrophage polarization through WFDC2-induced IL33 secretion. Consistently, in vivo infusion of rWFDC2 in Wfdc2-knockout mice recovered IL33 expression and aggravated DMP-777- or tamoxifen-induced SPEM with severe oxyntic atrophy and hyperproliferation. Thus, we considered that the effect of Wfdc2 knockout on the prevention of SPEM is dependent on IL33, which was confirmed by the infusion of rIL33. A recent study indicated that IL33 induces intestinal goblet cell hyperplasia,50 supporting our finding that IL33 levels are positively associated with WFDC2 for the development of IM, which is characterized by the emergence of goblet cells.
Highlighting the role of WFDC2 in gastric carcinogenesis, our results collectively demonstrated that WFDC2 promotes preneoplastic metaplasia through the upregulation of IL33 in response to gastric injury.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT:
WFDC2 expression is upregulated in the tissue of preneoplastic metaplasia and gastric cancer but its role remains unelucidated. We created Wfdc2 knockout mice and investigated the role of WFDC2 in the development of gastric cancer and its underlying mechanism.
NEW FINDINGS:
Gastric juice of gastric cancer patients was enriched with WFDC2. Wfdc2 knockout protected mice against the development of the preneoplastic lesion, spasmolytic polypeptide-expressing metaplasia (SPEM). rWFDC2 increased IL33 expression, which subsequently induced M2 macrophage polarization. Most importantly, long-term treatment of rWFDC2 could induce SPEM alone, highlighting the pivotal role of WFDC2 in early stage gastric cancer development.
LIMITATIONS:
This study was performed in mice and studies are needed in humans.
IMPACT:
WFDC2 may be an excellent biomarker for the detection of early gastric cancer and provide a novel target for gastric cancer treatment.
Acknowledgments
Grant support: This project was supported by the Korea Mouse Phenotyping Project (NRF-2016M3A9D5A01952416), by the Ministry of Food and Drug Safety (14182MFDS978), and by the Brain Korea 21 PLUS Project for Medical Science at Yonsei University. KML is supported by a grant from the National Research Foundation (NRF) funded by the Ministry of Science and ICT (MSIT) (2018R1A5A2025286). JRG is supported by grants from a Department of Veterans Affairs Merit Review Award IBX000930 and NIH R01 DK101332.
Abbreviations used in this paper.
- BMDM
bone marrow-derived macrophage
- CAG
chronic atrophic gastritis
- GEO
Gene Expression Omnibus
- GSEA
gene set enrichment analysis
- IL
interleukin
- IM
intestinal metaplasia
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- SPEM
spasmolytic polypeptide-expressing metaplasia
- rWFDC2
recombinant WFDC2
- rIL33
recombinant IL33
- TEM
transmission electron microscopy
- Th2
T helper cell 2
- WFDC2
WAP four-disulfide core domain protein 2
Footnotes
Disclosures: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunt RH, Camilleri M, Crowe SE, et al. The stomach in health and disease. Gut 2015;64:1650–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi WJ, Gao JB. Molecular mechanisms of chemoresistance in gastric cancer. World J Gastrointest Oncol 2016;8:673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenring JR, Nam KT. Oxyntic atrophy, metaplasia, and gastric cancer. Prog Mol Biol Transl Sci 2010;96:117–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correa P A human model of gastric carcinogenesis. Cancer Res 1988;48:3554–60. [PubMed] [Google Scholar]
- 5.Yoshizawa N, Takenaka Y, Yamaguchi H, et al. Emergence of spasmolytic polypeptide-expressing metaplasia in Mongolian gerbils infected with Helicobacter pylori. Lab Invest 2007;87:1265–76. [DOI] [PubMed] [Google Scholar]
- 6.Nam KT, Lee HJ, Sousa JF, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 2010;139:2028–2037 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TC, Goldenring JR, Dangler C, et al. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology 1998;114:675–89. [DOI] [PubMed] [Google Scholar]
- 8.Radyk MD, Burclaff J, Willet SG, et al. Metaplastic Cells in the Stomach Arise, Independently of Stem Cells, via Dedifferentiation or Transdifferentiation of Chief Cells. Gastroenterology 2018;154:839–843 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burclaff J, Willet SG, Saenz JB, et al. Proliferation and Differentiation of Gastric Mucous Neck and Chief Cells During Homeostasis and Injury-induced Metaplasia. Gastroenterology 2020;158:598–609 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weis VG, Sousa JF, LaFleur BJ, et al. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages identifies markers of metaplastic progression. Gut 2013;62:1270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nozaki K, Ogawa M, Williams JA, et al. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology 2008;134:511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wada T, Ishimoto T, Seishima R, et al. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer Sci 2013;104:1323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen CP, Mills JC, Goldenring JR. Murine Models of Gastric Corpus Preneoplasia. Cell Mol Gastroenterol Hepatol 2017;3:11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldenring JR, Ray GS, Coffey RJ, et al. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology 2000;118:1080–93. [DOI] [PubMed] [Google Scholar]
- 15.Huh WJ, Khurana SS, Geahlen JH, et al. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 2012;142:21–24 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eaton KA, Ringler SR, Danon SJ. Murine splenocytes induce severe gastritis and delayed-type hypersensitivity and suppress bacterial colonization in Helicobacter pylori-infected SCID mice. Infect Immun 1999;67:4594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sepulveda AR. Helicobacter, Inflammation, and Gastric Cancer. Curr Pathobiol Rep 2013;1:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology 2005;128:1567–78. [DOI] [PubMed] [Google Scholar]
- 19.Petersen CP, Weis VG, Nam KT, et al. Macrophages promote progression of spasmolytic polypeptide-expressing metaplasia after acute loss of parietal cells. Gastroenterology 2014;146:1727–38 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen CP, Meyer AR, De Salvo C, et al. A signalling cascade of IL-33 to IL-13 regulates metaplasia in the mouse stomach. Gut 2018;67:805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buzzelli JN, Chalinor HV, Pavlic DI, et al. IL33 Is a Stomach Alarmin That Initiates a Skewed Th2 Response to Injury and Infection. Cell Mol Gastroenterol Hepatol 2015;1:203–221 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer AR, Engevik AC, Madorsky T, et al. Group 2 innate lymphoid cells coordinate damage response in the stomach. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Dowdy S, Tipton T, et al. HE4 as a biomarker for ovarian and endometrial cancer management. Expert Rev Mol Diagn 2009;9:555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwahori K, Suzuki H, Kishi Y, et al. Serum HE4 as a diagnostic and prognostic marker for lung cancer. Tumour Biol 2012;33:1141–9. [DOI] [PubMed] [Google Scholar]
- 25.Nagy B Jr., Nagy B, Fila L, et al. Human Epididymis Protein 4: A Novel Serum Inflammatory Biomarker in Cystic Fibrosis. Chest 2016;150:661–72. [DOI] [PubMed] [Google Scholar]
- 26.LeBleu VS, Teng Y, O’Connell JT, et al. Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis. Nat Med 2013;19:227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamei M, Yamashita S, Tokuishi K, et al. HE4 expression can be associated with lymph node metastases and disease-free survival in breast cancer. Anticancer Res 2010;30:4779–83. [PubMed] [Google Scholar]
- 28.Bouchard D, Morisset D, Bourbonnais Y, et al. Proteins with whey-acidic-protein motifs and cancer. Lancet Oncol 2006;7:167–74. [DOI] [PubMed] [Google Scholar]
- 29.Parikh K, Antanaviciute A, Fawkner-Corbett D, et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature 2019;567:49–55. [DOI] [PubMed] [Google Scholar]
- 30.James NE, Emerson JB, Borgstadt AD, et al. The biomarker HE4 (WFDC2) promotes a pro-angiogenic and immunosuppressive tumor microenvironment via regulation of STAT3 target genes. Sci Rep 2020;10:8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Neal RL, Nam KT, LaFleur BJ, et al. Human epididymis protein 4 is up-regulated in gastric and pancreatic adenocarcinomas. Hum Pathol 2013;44:734–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mills JC, Goldenring JR. Metaplasia in the Stomach Arises From Gastric Chief Cells. Cell Mol Gastroenterol Hepatol 2017;4:85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurowska-Stolarska M, Stolarski B, Kewin P, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol 2009;183:6469–77. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Huang L, Wang S, et al. WFDC2 contributes to epithelial-mesenchymal transition (EMT) by activating AKT signaling pathway and regulating MMP-2 expression. Cancer Manag Res 2019;11:2415–2424. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Xiong Y, Yuan L, Chen S, et al. WFDC2 suppresses prostate cancer metastasis by modulating EGFR signaling inactivation. Cell Death Dis 2020;11:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogawa M, Nomura S, Car BD, et al. Omeprazole treatment ameliorates oxyntic atrophy induced by DMP-777. Dig Dis Sci 2006;51:431–9. [DOI] [PubMed] [Google Scholar]
- 37.Nomura S, Yamaguchi H, Ogawa M, et al. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 2005;288:G362–75. [DOI] [PubMed] [Google Scholar]
- 38.Burclaff J, Osaki LH, Liu D, et al. Targeted Apoptosis of Parietal Cells Is Insufficient to Induce Metaplasia in Stomach. Gastroenterology 2017;152:762–766 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bockerstett KA, Osaki LH, Petersen CP, et al. Interleukin-17A Promotes Parietal Cell Atrophy by Inducing Apoptosis. Cell Mol Gastroenterol Hepatol 2018;5:678–690 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osaki LH, Bockerstett KA, Wong CF, et al. Interferon-gamma directly induces gastric epithelial cell death and is required for progression to metaplasia. J Pathol 2019;247:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferhat M, Robin A, Giraud S, et al. Endogenous IL-33 Contributes to Kidney Ischemia-Reperfusion Injury as an Alarmin. J Am Soc Nephrol 2018;29:1272–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang Y, Jie Z, Hou L, et al. IL-33 promotes innate IFN-gamma production and modulates dendritic cell response in LCMV-induced hepatitis in mice. Eur J Immunol 2015;45:3052–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calcagno DQ, Leal MF, Assumpcao PP, et al. MYC and gastric adenocarcinoma carcinogenesis. World J Gastroenterol 2008;14:5962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engevik AC, Feng R, Choi E, et al. The Development of Spasmolytic Polypeptide/TFF2-Expressing Metaplasia (SPEM) During Gastric Repair Is Absent in the Aged Stomach. Cell Mol Gastroenterol Hepatol 2016;2:605–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertaux-Skeirik N, Wunderlich M, Teal E, et al. CD44 variant isoform 9 emerges in response to injury and contributes to the regeneration of the gastric epithelium. J Pathol 2017;242:463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y, Zhang S, Wang Q, et al. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J Hematol Oncol 2017;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Syu LJ, El-Zaatari M, Eaton KA, et al. Transgenic expression of interferon-gamma in mouse stomach leads to inflammation, metaplasia, and dysplasia. Am J Pathol 2012;181:2114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nam KT, Lee HJ, Mok H, et al. Amphiregulin-deficient mice develop spasmolytic polypeptide expressing metaplasia and intestinal metaplasia. Gastroenterology 2009;136:1288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eissmann MF, Dijkstra C, Jarnicki A, et al. IL-33-mediated mast cell activation promotes gastric cancer through macrophage mobilization. Nat Commun 2019;10:2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waddell A, Vallance JE, Hummel A, et al. IL-33 Induces Murine Intestinal Goblet Cell Differentiation Indirectly via Innate Lymphoid Cell IL-13 Secretion. J Immunol 2019;202:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Z, Huang A, Sun J, et al. Inference of immune cell composition on the expression profiles of mouse tissue. Sci Rep 2017;7:40508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.