Abstract
Background & Aim
Hepatitis B reactivation related to the use of immunosuppressive therapy remains a major cause of liver-related morbidity and mortality in hepatitis B endemic Asia-Pacific region. This clinical practice guidelines aim to assist clinicians in all disciplines involved in the use of immunosuppressive therapy to effectively prevent and manage hepatitis B reactivation.
Methods
All publications related to hepatitis B reactivation with the use of immunosuppressive therapy since 1975 were reviewed. Advice from key opinion leaders in member countries/administrative regions of Asian-Pacific Association for the study of the liver was collected and synchronized. Immunosuppressive therapy was risk-stratified according to its reported rate of hepatitis B reactivation.
Recommendations
We recommend the necessity to screen all patients for hepatitis B prior to the initiation of immunosuppressive therapy and to administer pre-emptive nucleos(t)ide analogues to those patients with a substantial risk of hepatitis and acute-on-chronic liver failure due to hepatitis B reactivation.
Keywords: APASL, Hepatitis B reactivation, Immunosuppressive therapy, Guideline
Introduction
More than four decades ago, based on serial measurement of hepatitis B virus (HBV) antigen and serum alanine aminotransferase (ALT) level, hepatitis due to HBV reactivation (HBVr) had been described in both hepatitis B surface antigen (HBsAg) positive and HBsAg negative but antibody (anti-HBs) positive patients with myeloproliferative and lymphoproliferative diseases treated with anti-tumor chemotherapy [1, 2]. In the 90s, prospective study showed that nearly half of the HBsAg-positive patients with malignant lymphoma treated with cytotoxic therapy, suffered from hepatitis due to HBVr [3]. With the subsequent use of more aggressive immunosuppressive therapy (IST) such as the conditioning regimens in allogeneic hematopoietic stem cell therapy, fatality due to HBVr in HBsAg positive patients with hematological malignancy became an increasingly important clinical problem [4, 5]. Hence, close monitoring of HBsAg-positive patients with malignancies receiving cytotoxic therapy was recommended [6]. Further progress in various medical disciplines with the use of more potent immunosuppressive therapy and targeted monoclonal therapy such as rituximab, a human/murine, chimeric anti-CD20 monoclonal antibody, fatal fulminant hepatic failure due to HBVr was noted even in those who had recovered from past HBV infection-HBsAg negative but hepatitis B core antibody (anti-HBc) positive with HBV DNA detectable only by sensitive nested PCR [7–13]. At the turn of the twenty-first century, with the availability of potent nucleos(t)ide analogues (NUCs), pre-emptive use of lamivudine and then entecavir and tenofovir were shown in randomized controlled trials to be highly effective in preventing HBVr and its liver-related morbidity and mortality in both HBsAg positive and HBsAg negative but anti-HBs and anti-HBc positive patients treated with cytotoxic chemotherapy [13–16].
Up till 2020, numerous clinical guidelines had been formulated aiming to reduce the occurrence of hepatitis due to HBVr in patients treated with IST [17–21]. Nevertheless, hepatitis due to HBVr leading to acute on chronic liver failure remains a major health threat in Asia-Pacific region, where HBV infection is endemic [22]. The major barriers appear to be related to the non-compliance of medical practitioners in other non-hepatology disciplines. This is further compounded by the recent rapid expansion on the use of new immunosuppressive agents such as tyrosine-kinase inhibitors (TKIs), [23–26] immune checkpoint inhibitors (ICIs) [27–29] used in the treatment of various cancers and tumor necrosis factor (TNF) antagonists [30–35] for many autoimmune diseases. Recently, in those chronic hepatitis C (CHC) patients coinfected with hepatitis B, HBVr has also been reported during and after treatment with direct-acting antiviral agents (DAAs) [36–48].
The deterring factors for the successful implementation of the guidelines include lack of attention to the prevalence of HBVr, unawareness of the ease of implementation of suitable preventive measures, miscalculations of the total costs to society and potentials for improvement in quality of care taking into consideration of the wide availability of potent generic NUCs at a very low cost and simple virological testing [49, 50]. We aim to develop a user-friendly clinical practise guideline for all related medical disciplines which will help to curtail the morbidity and mortality related to HBVr in subjects treated with IST, especially in HBV endemic Asia-Pacific region.
Methods
With the initiation by the steering committee of Asian-Pacific Association for the study of the liver (APASL), a panel of experts from 21 different administrative regions/countries in Asia-Pacific region was invited to form a working party which formulated this clinical practise guidance for HBVr in patients treated with IST. The panel includes not only hepatologists, but also oncologists, rheumatologists, transplant surgeons, nephrologists and interventional radiologists. The first working party meeting was conducted on 9th Nov 2019 in Boston during the American Association for the study of liver diseases (AASLD) annual meeting when the key questions related to HBVr were laid down, methods to develop the guidance were defined and drafting of recommendations was assigned. This guidance addresses the following questions: (1) What is the definition of HBVr? (2) Who should be screened? (3) What should be done for screening? (4) How should a patient planned for IST be managed and monitored? All panel members were required to disclose their relationships with industry during the guidelines formulation until accepted for publication by Hepatology International (official journal of APASL). The Chair (G Lau) and Co-Chairs (ML Yu, G Wong, A Thompson) of the guidelines committee must be free of any conflict of interest (COI) or other biases that could undermine the integrity or credibility of the work. The Chair, Co-Chairs and all panel members with relevant COI were required to declare the situation and recused themselves from any relevant discussions, voting, and drafting of recommendations. The Chair and Co-Chairs were responsible for writing up the guidelines with the support of all panel members. All recommendations were graded according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system [51]. The recommendations were presented at the 30th APASL hybrid annual meeting in Bangkok, Thailand (6th Feb 2021), with the comments incorporated.
Immunopathogenesis of hepatitis due to HBV reactivation
Hepatitis B virus (HBV) is a hepatotropic virus and after entry into hepatocytes, the HBV nucleocapsid containing partially double-stranded HBV DNA (dsDNA) enters the nucleus where the viral polymerase repairs dsDNA into full-length, covalently closed circular (cccDNA), the nuclear reservoir of HBV. Reverse transcription, viral replication, and encapsidation occur in the cytoplasm before either viral assembly and release or recycling of the nascent nucleocapsid into the nucleus to replenish the pool of cccDNA [52]. It is the persistence of these low levels of cccDNA in hepatocytes which are thought to explain the long-term risk of HBVr with potent IST that exists even in individuals who have cleared the HBV infection, with serological clearance of hepatitis B surface antigen (HBsAg) [53]. The clinical outcome of HBV infection is highly dependent on a complex interplay between the virus-specific host immune response involving cytotoxic HBV-specific CD8 T cell and natural killer (NK)/NK-T cell responses, cytokine-mediated non-cytolytic responses as well as B-cell-mediated humoral immunity [54]. In keeping with this, resolution of HBV infection with loss of HBsAg with or without the development of anti-HBs has been demonstrated to require CD4 helper T cells for the efficient development of virus-specific adaptive CD8 T cell responses and B-cell antibody production [55].
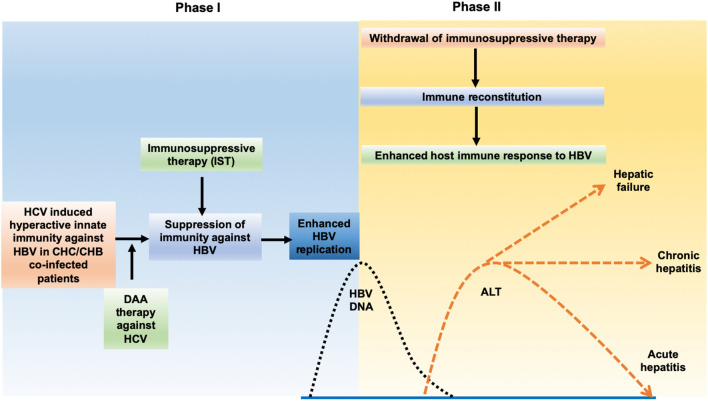
To date, the key biological pathways leading to the development or severity of clinically significant hepatitis due to HBVr are not well defined, other than by extrapolation from the mechanism of action of the etiological agent. There are also little data linking HBV sequence variation to risk or severity of HBV reactivation, and it should be assumed that all HBV genotypes and variants may be associated with reactivation. Nonetheless, based on serial measurements, HBV serological markers and liver function test, hepatitis due to HBVr have been identified as a 2-stage process. The initial phase is characterized by enhanced viral replication accompanied by markedly increase hepatic expression of viral antigen. It is postulated that this initial phase of HBVr occurs as a result of drug treatment that directly or indirectly inhibits the anti-HBV immune response targeted against HBV, with the highest risk associated with B cell depleting therapies [56, 57] and hematopoietic stem cell transplant (HSCT) [4–6]. HBVr has also been reported with the use of IST in solid organ transplantation, traditional chemotherapies including trans-arterial chemo-embolisation for hepatocellular carcinoma, [58] as well as the more recent tyrosine kinase inhibitors, [59] tumor necrosis factor antagonist [30–35] and proteasome inhibitor for the treatment of various malignancy and autoimmune diseases [60]. The HBV genome also contains a steroid-responsive element, and prolonged corticosteroid therapy has been associated with a moderate to high risk of HBVr [61]. HBVr has also been reported to occur indirectly in HCV and HDV co-infected patients as a result of antiviral therapy for HCV, or HDV respectively [62, 63]. This phenomenon reflects virus-virus interactions where the host immune response to one hepatitis virus inhibits replication of the other—normally HCV or HDV are dominant over HBV—and antiviral therapy for the dominant virus results in a secondary down-regulation of immune pathways that allow HBV replication to increase. The second phase occurred during immune reconstitution on withdrawal of the IST, [6] continuous rapid inhibition of HCV by direct-acting antiviral agents (DAAs) [40] or HIV by non-HBV active HAART therapy [64]. The immune response to the markedly enhanced hepatic expression of HBV antigen leads to liver injury, manifested as hepatitis, icteric hepatitis and fulminant acute-on-chronic hepatic failure (Fig. 1).
Fig. 1.
Pathogenesis of hepatitis due to hepatitis B virological reactivation (HBVr). Hepatitis due to HBVr is a two-phase process with an initial phase of enhanced HBV replication and hepatocyte expression of HBV antigen due to attenuation of host immunity against HBV. The use of steroid could further augment viral replication due to its effect on steroid-responsive elements in HBV. Attenuation of host immunity against HBV replication can also be related to removal of hyperactive innate immunity with DAA therapy against co-infected HCV. The second phase is characterized by immune reconstitution on withdrawal of immunosuppressive effect on HBVr due to withdrawal of the immunosuppressive therapy or continuous rapid suppression of HCV by DAAs. This will initiate the mounting of host immune response against heavily HBV antigen-laden hepatocyte, resulting in liver injury, manifested as elevation of serum ALT with mild hepatitis, icteric hepatitis, hepatic failure or even death
Incidence of HBVr treated with immunosuppressive agents
HBsAg positive patients (Table 1)
Table 1.
HBV reactivation and related complications among HBsAg-positive patients without NUC prophylaxis in Asia-Pacific region
| Authors (year) | Country/Region | Study design | HBVr case (n) | Total case (n) | HBVr rate | HBV-related hepatitis (n) | HBV-related hepatitis rate | HBV-related mortality rate |
|---|---|---|---|---|---|---|---|---|
| Hematopoetic stem cell transplantation | ||||||||
| Nakamoto (2014) [65] | Japan | OB | 2 | 2 | 100% | 2 | 100% | 0% |
| Lau (2002) [66] | HK | OB | 9 | 20 | 45% | 3 | 15% | NA |
| Cancer diseases | ||||||||
| Cytotoxic agents | ||||||||
| Lymphoma | ||||||||
| Lok (1991) [3] | HK | OB | 13 | 27 | 48% | 7 | 26% | 3.7% |
| Lau (2003) [14] | HK | RCT | 8 | 15 | 53% | 7 | 47% | 0.0% |
| Cheng (2003) [70] | Taiwan | OB steroid + | 18 | 25 | 72% | 15 | 60% | 4.0% |
| OB steroid- | 9 | 25 | 36% | 8 | 32% | 0.0% | ||
| Hsu (2008) [16] | Taiwan | RCT CHOP | 14 | 25 | 56% | 12 | 48% | 0.0% |
| Total | 62 | 117 | 53% | 49 | 42% | 0–4% | ||
| Hematologic malignancies | ||||||||
| Chen (2018) [71] | Taiwan | OB | 71 | 115 | 62% | NA | NA | NA |
| Breast Cancer | ||||||||
| Yeo (2004) [67] | HK | OB | 17 | 41 | 41% | NA | NA | |
| Long (2011) [104] | China | RCT | 6 | 21 | 29% | 0 | 0.0% | 0.0% |
| Kim (2007) [68] | Korea | OB + anthracycline | 23 | 111 | 21% | 23 | 21% | |
| Lee (2014) [73] | Korea | OB ± anthracycline | 13 | 92 | 14% | 6 | 6.5% | 0.0% |
| Total | 59 | 265 | 22% | 29 | 11% | 0–1% | ||
| Hepatocellular carcinoma | ||||||||
| TACE | ||||||||
| Jang (2006) [74] | Korea | RCT | 15 | 35 | 43% | 11 | 31% | 3.0% |
| Jang (2006) [75] | Korea | OB | 62 | 205 | 30% | 32 | 16% | 0.5% |
| Total | 77 | 240 | 32% | 43 | 18% | 0.5–3% | ||
| Systemic Chemotherapy | ||||||||
| Yeo (2004) [72] | HK | OB | 37 | 102 | 36% | 23 | 23% | 12% |
| Tyrosine kinase inhibitor | ||||||||
| Uhm (2018) [23] | Korea | OB-CML | 12 | 46 | 26% | NA | NA | 0.0% |
| Wang (2019) [24] | Taiwan | OB-CML | 5 | 13 | 38.5% | 3 | 23% | 0.0% |
| Yao (2019) [25] | Taiwan | OB-NSCLC-EGFRI | 16 | 171 | 9.4% | NA | NA | NA |
| Total | 33 | 230 | 14% | |||||
| Immune checkpoint inhibitors | ||||||||
| Pu (2020) [27] | Asia-Pacific | Review | 2 | 22 | 9.1% | 2 | 9.1% | 0% |
| Zhang (2019) [28] | China | OB | 5 | 29 | 17% | 4 | 14% | 0% |
| Lee (2020) [29] | Taiwan | OB | 1 | 6 | 17% | 1 | 17% | 0% |
| Total | 8 | 57 | 14% | 7 | 11.7% | 0% | ||
| Rheumatic disorders | ||||||||
| Lan (2011) [30] | Taiwan | OB (anti-TNF) | 5 | 8 | 63% | 5 | 63% | 0% |
| Tamori (2011) [31] | Japan | OB (anti-TNF) | 2 | 5 | 40% | NA | NA | 0% |
| Ryu (2012) [32] | Korea | OB (anti-TNF) | 4 | 29 | 14% | 2 | 6.9% | 0% |
| Tan (2012) [33] | China | OB (c-DMARD) | 2 | 23 | 9% | 0 | 0% | 0% |
| Lee (2013) [34] | Korea | Review | 14 | 74 | 19% | NA | NA | |
| Chen (2017) [35] | Taiwan | OB (c-DMARD) | 30 | 123 | 24% | NA | NA | |
| Total | 57 | 262 | 22% | 7 | 11.7% | 0% | ||
Data from Japan and Hong Kong have shown a 45–100% risk of HBVr and 15% hepatic failure in HBsAg positive patients receiving HSCT without antiviral prophylaxis [65, 66]. Two meta-analyses in Asia-Pacific region have shown > 30% risk of HBVr among HBsAg-positive lymphoma patients receiving rituximab-containing regimens [56, 57]. Among HBsAg-positive breast cancers patients receiving chemotherapy in Asia-Pacific region, the risks of HBVr and HBV-related hepatitis flare were around 22% (range 14–41%) and 11% (range 0–21%), respectively [67–69, 73]. The risk of HBVr among HBsAg-positive cancer patients receiving steroid-containing regimen was 26–72%, compared to 13–36% among those receiving non-steroid-containing regimens [70]. For patients with HBV-related HCC receiving transarterial chemo-embolization (TACE), data from South Korea observed a risk of 32% (30–43%) for HBVr and 18% (16–31%) for HBV-related hepatitis flare [74, 75]. Baseline HBV DNA levels > 2000 IU/ml, baseline cirrhosis and history of multiple-modality therapy for HCC were associated with a higher risk of HBVr [72, 74, 75].
Targeted therapies, monoclonal antibodies, biologics (Table 1)
TKIs are currently widely used as target therapy for lung cancers and chronic myeloid leukemia (CML). The HBVr risk was 26–38.5% among CML patients receiving imatinib from Taiwan and Korea studies [23, 24]. A recent Taiwanese study observed a moderate HBVr risk of 9.4% among patients with non-small cell lung cancers receiving epidermal growth factor receptor inhibitor [25].
ICIs are now approved for the treatment of various cancers. Case series studies from Taiwan and China showed that the risk of HBVr and its related hepatitis among HBsAg-positive cancer patients with ICIs therapy to be 14% (range 9.1–17%) and 11.7% (range 9.1–17%), respectively [27–29].
TNF- α inhibitors used for autoimmune diseases, such as rheumatic disorders and inflammatory bowel diseases, have been reported to be associated with HBVr risks between 14 and 63%, amid a relatively small case number in the published series [30–32]. The American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy also considered anti-TNF as moderate risk [17].
Disease-modifying antirheumatic drugs (bDMARDs/sDMARDs) had a HBVr risk of around 22% (range 9–63%) among HBsAg-positive patients [31–35]. HBVr, hepatitis flare-up and even fulminant hepatic failure had been observed in HBsAg-positive rheumatic patients receiving tocilizumab, an IL-6 receptor monoclonal antibody [76, 77]. Further studies are called upon in this field as anti- IL-6 was being used in the current COVID-19 pandemic in some parts of the world [78].
HBsAg (−)/anti-HBc (+) patients (Table 2)
Table 2.
HBV reactivation and related complications among HBsAg-negative patients without pre-emptive NUCs
| Authors (year) | Country/Region | Study design | HBVr case (n) | Total case (n) | HBVr rate | HBV-related hepatitis (n) | HBV-related hepatitis rate | HBV-related mortality rate |
|---|---|---|---|---|---|---|---|---|
| Hematopoetic stem cell transplantation | ||||||||
| Nakamoto (2014) [65] | Japan | OB | 6 | 83 | 7% | NA | NA | NA |
| Seto (2017) [79] | HK | OB | 13 | 62 | 21% | NA | NA | NA |
| Wu (2020) [80] | China | OB | 25 | 441 | 6% | NA | NA | NA |
| Nishikawa (2020) [81] | Japan | OB | 13 | 67 | 19% | NA | NA | NA |
| Total | 57 | 653 | 8.7% | |||||
| Lymphoma—anti-CD20-containing C/T | ||||||||
| Yeo (2009) [8] | HK | RCT | 5 | 21 | 24% | 5 | 24% | 4% |
| Matsue (2010) [9] | Japan | OB | 5 | 56 | 9% | 5 | 8.9% | 0% |
| Koo (2011) [105] | Singapore | OB | 2 | 62 | 3% | 2 | 3.2% | 1.6% |
| Huang (2013) [10] | Taiwan | RCT | 7 | 39 | 18% | 2 | 5.1% | 0% |
| Seto (2014) [11] | HK | OB | 19 | 63 | 30% | 0 | 0.0% | 0% |
| Hsu (2014) [12] | Taiwan | OB | 27 | 143 | 19% | 10 | 7.0% | 0% |
| Kusumoto (2019) [83] | Asia Pacific, Europe, Canada | OB | 25 | 232 | 10.8 | NA | NA | NA |
| Total | 65 | 384 | 16.9% | 24 | 6% | |||
| Lymphoma—non-rituximab C/T | ||||||||
| Lok (1991) [3] | HK | OB | 2 | 45 | 4% | 2 | 4.4% | 0% |
| Yeo (2009) [8] | HK | RCT | 0 | 25 | 0% | 0 | 0.0% | 0% |
| Total | 2 | 70 | 3% | 2 | 3% | |||
| Hematologic malignancies | ||||||||
| Chen (2018) [71] | Taiwan | OB (585 anti-HBc [+]) | 41 | 1676 | 2.4% | 36 | 2.1% | 0.06% |
| TKI | ||||||||
| Wang (2019) [24] | Taiwan | OB-CML (55% anti-HBc +) | 0 | 123 | 0.0% | 0 | 0% | 0.0% |
| Rheumatic disorders | ||||||||
| Lan (2011) [30] | Taiwan | OB (anti-TNF) | 1 | 70 | 1.4% | 1 | 1.4% | 0% |
| Tamori (2011) [31] | Japan | OB (anti-TNF) | 1 | 45 | 2.2% | 1 | 2.2% | 0% |
| Tan (2012) [33] | China | OB (c-DMARD) | 2 | 188 | 1.1% | 1 | 0.5% | 0% |
| Mori (2011) [106] | Japan | OB (anti-TNF) | 2 | 60 | 3.3% | 0 | 0.0% | 0% |
| Urata (2011) [107] | Japan | OB (anti-TNF) | 7 | 135 | 5.2% | NA | NA | 0% |
| Watanabe (2019) [108] | Japan | OB (c-DMARD) | 7 | 152 | 4.6% | |||
| Total | 20 | 650 | 3.1% | 3 | 0.8% | |||
Data from Asia-Pacific region observed 6%–29% risk of HBVr in resolved HBV patients receiving HSCT without antiviral prophylaxis [65, 79–81]. A meta-analysis showed that lymphoma patients receiving rituximab-containing regimens had significantly higher risk of HBVr than those receiving non-rituximab-containing regimens (10% vs 4%) [82]. Kusumoto et al. had shown that in the phase 3 GOYA and GALLIUM studies, there was no significant difference in the risk of HBV reactivation between obinutuzumab- and rituximab-based immunochemotherapy (p = 0.17) [83]. A meta-analysis of 328 solid tumor patients with resolved HBV infection from 3 studies showed a median HBVr risk of 3% (range 0.3–9%) [69]. Recently, a study in Hong Kong showed a one-year incidence of HBsAg seroreversion of 1.8% among patients with isolated anti-HBc seropositivity receiving steroid therapy [84]. All combinations of corticosteroids dosage and duration greater than 7 days increased the risk of hepatitis flare [84].
Data of HBVr risk among HBsAg (-)/anti-HBc (+) patients receiving TKI or ICI are limited. A recent Taiwanese study observed that none of 123 CML patients with resolved HBV receiving TKI experienced HBVr [24]. Relating to TNF- inhibitors and DAMARDs, data from Asia-Pacific regions demonstrated a HBVr risk of around 3.1% (range 1.1–5.2%) [30–32]. A recent Taiwanese study reported one (1.6%) out of 64 rheumatic patients receiving tocilizumab experienced HBVr [76]. Further studies are needed in these areas.
HBVr among patients with HBV and HCV co-infections (Table 3)
Table 3.
Hepatitis B virus reactivation (HBVr) among patients with HBV and hepatitis C virus (HCV) co-infection or HCV infection with resolved HBV infection after direct-acting antiviral (DAA) treatment in Asia-Pacific region
| Authors (year) | Country/Region | Total patients (n) | Observation periods (months post-EOT) | Patients with HBVr [n (%)] | Patients with HBVr and hepatitis [n (%)] | Patients with HBVr and icteric hepatitis [n (%)] | Mortality in patients with HBVr [n (%)] | Pre-DAA HBV DNA (−) in patients with HBVr [n (%)] | Predictors |
|---|---|---|---|---|---|---|---|---|---|
| HBsAg-positive HBV/HCV co-infected patients | |||||||||
| Gane (2016) [37] | New Zealand | 8 | 3 | 6 (38) | 0/8 (0) | 0/8 (0) | 0/6 (0) | 3/6 (50) | NA |
| Doi (2017) [38] | Japan | 4 | 3 | 2 (50) | 0/4 (0) | 0/4 (0) | 0/2 (0) | 2/2 (100) | NA |
| Kawagishi (2017) [39] | Japan | 1 | 3 | 1 (100) | 0/1 (0) | 0/1 (0) | 0/1 (0) | 1/1 (100) | NA |
| Wang (2017) [40] | China | 10 | 3 | 3 (30) | 3/10 (30) | 1/10 (10) | 0/3 (0) | NA | NA |
| Tamori (2018) [41] | Japan | 22* | 3 | 3 (14) | 2/22 (9) | 0/0 (0) | 0/3 (0) | 3/3 (100) | NA |
| Liu (2018) [42] | Taiwan | 111 | 3 | 50 (45) | 5/111 (5) | 1/111 (1) | 0/50 (0) | 11/50 (22) | NA |
| Liu (2017) [44] | Taiwan | 12 | 3 | 2 (17) | 0/12 (0) | 0/12 (0) | 0/2 (0) | 1/2 (50) | NA |
| Lee (2018) [45] | Taiwan | 7 | 3 | 2 (29) | 1/7 (14) | 0/7 (0) | 0/2 (0) | 1/2 (50) | NA |
| Yeh (2020) [46] | Taiwan | 66* | 3–36 (mean 11) | 30 (45) | 6/66 (9) | 3/66 (5) | 2/30 (7)** | 15/30 (50) | BL ALT ≥ 80 U/L; HBsAg > 10 IU/ml |
| Total | 241 | 99 (41.1) | 17 (7.1) | 5 (2.1) | 2/99 (2.0) | 37/96 (38.5) | |||
| HBsAg-negative patients positive for anti-HBc antibody and/or anti-HBs antibody | |||||||||
| Doi (2017) [38] | Japan | 155 | 3 | 3 (1.9) | 0/155 (0) | 0/155 (0) | 0/3 (0) | 3/3 (100) | BL High ALT; Low Anti-HBs titer‡ |
| Kawagishi (2017) [39] | Japan | 84 | 1 | 4 (2.6) | 1/84 (1) | 0/84 (0) | 0/4 (0) | 4/4 (100) | BL Anti-HBs (-) or < 30 mIU/ml; EOT Anti-HBs (-) or < 12 mIU/ml‡ |
| Yeh (2017) [43] | Taiwan | 57 | 3 | 0 (0) | 0/57 (0) | 0/57 (0) | 0 (0) | NA | NA |
| Sulkowski (2016) [47] | Taiwan/Korea | 103 | 3 | 0 (0) | 0/103 (0) | 0/103 (0) | 0 (0) | NA | NA |
| Wang (2017) [40] | China | 124 | 3 | 0 (0) | 0/124 (0) | 0/124 (0) | 0 (0) | NA | NA |
| Tamori (2018) [41] | Japan | 765 | 3 | 1 (0.1) | 0/765 (0) | 0/765 (0) | 0 (0) | 1/1 (100) | NA |
| Ogawa (2018) [48] | Japan | 63 | 3 | 1 (2) | 0/63 (0) | 0/63 (0) | 0/1 (0) | 1/1 (100) | NA |
| Liu (2017) [44] | Taiwan | 81 | 3 | 0 (0) | 0/81 (0) | 0/81 (0) | 0 (0) | NA | NA |
| Lee (2018) [45] | Taiwan | 53 | 3 | 0 | (0) | 0/53 (0) | 0 (0) | NA | NA |
| Total | 1485 | 3 | 9 (0.6) | 1 (0.07) | 0 (0) | 0 (0) | 9/9 (100) | ||
HBVr, HBV DNA increases greater than 1 log10 IU/ml or HBV DNA reappearance
HBsAg hepatitis B surface antigen, anti-HBc anti-hepatitis B core antibody, anti-HBs anti-hepatitis B surface antibody, EOT end of treatment, n number
*Excluding patients with concomitant anti-HBV NUC therapy at initiation of DAA therapy
**Both patients had cirrhosis at baseline
‡Subjects of both positive and negative HBsAg analyzed together
The recent advance of DAAs has dramatically improved the treatment success of CHC infection, making HCV elimination possible in the near future [85]. The successful HCV eradication rate with DAAs is comparable between HCV mono-infected and HBV/HCV co- patients. However, coinfected patients are at risk of HBVr during and after DAA therapy and this occurs earlier and is clinically more significant than HBVr occurring with interferon-based therapy [37–47, 86–88]. Data from Asia-Pacific region demonstrated a risk of 41.1% (range 14–100%) for HBVr, 7.1% (range 0–30%) for HBV-related hepatitis flare, 2.1% (range 0–10%) for HBV-related icteric hepatitis, and 2% (range 0–7%) mortality (Table 3). The risk of HBVr was not associated with baseline HBV DNA levels. Patients with undetectable HBV DNA at baseline are still at risk of HBVr. In a recent study, baseline quantitative HBsAg (qHBsAg) titers were associated with HBVr. The 1-year cumulative incidence rate of HBVr was 42.5% in patients with baseline qHBsAg > 10 IU/ml, compared to 18.5% in those with baseline qHBsAg < 10 IU/ml [46].
Risk stratification (Table 4)
Table 4.
Risk stratification of HBV reactivation among HBsAg-positive patients and HBsAg-negative/anti-HBc-positive patients
| Risk level | HBV serology | |
|---|---|---|
| HBsAg( +) | HBsAg(-)/anti-HBc( +) | |
| High (> 10%) |
Anti-CD20 monoclonal antibodies: Rituximab, Ofatumumab, Obinutuzumab Steroid (high dose) ≥ 20 mg/day for ≥ 4 weeks Anti-TNF agents with higher potency: Adalimumab, Infliximab, Golimumab, Certolizumab Anthracyclines Hematopoietic stem cell transplantation (both allogeneic and autologous) DAA for HBV/HCV coinfection (high risk in meta-analysis and prospective study), except non-cirrhotics with HBsAg < 10 IU/ml Immune Checkpoint inhibitors (moderate to high risk): Anti-PD-1: nivolumab, pembrolizumab Anti-PD-L1: atezolizumab Anti-CTLA-4: ipilimumab Tyrosine kinase inhibitors (moderate-to-high): Imatinib, Nilotinib, Dasatinib, Erlotinib, Gefitinib, Osimertinib, Afatinib |
Anti-CD20 monoclonal antibodies: Rituximab, Ofatumumab, Obinutuzumab Allogeneic hematopoietic stem cell transplantation |
| Moderate (1–10%) |
Cytotoxic chemotherapy (except anthracyclines) Anti-TNF agents with lower potency: Etanercept Steroid (median dose): 10–20 mg/day for ≥ 4 weeks Proteasome inhibitor: Bortezomib Ustekinumab |
Anthracyclines Autologous hematopoietic stem cell transplantation Anti-TNF agents with higher potency: Adalimumab, Infliximab, Golimumab, Certolizumab Proteasome inhibitor: Bortezomib Ustekinumab |
| Low (< 1%) |
Methotrexate Azathioprine Steroid (low dose < 10 mg/day) DAA for HBV/HCV coinfection for non-cirrhotic patients with HBsAg < 10 IU/ml |
Cytotoxic chemotherapy (except anthracyclines) Steroid (high dose) ≥ 20 mg/day Anti-TNF agents with lower potency: Etanercept Tyrosine kinase inhibitors Imatinib, Nilotinib, Dasatinib DAA for HCV |
| Uncertain (More studies needed, no prophylaxis recommendation until further evidence) |
Abatacept Tocilizumab Ibrutinib Alemtuzumab Natalizumab Ocrelizumab Ibritumomab |
Immune Checkpoint inhibitors Anti-PD-1: nivolumab, pembrolizumab Anti-PD-L1: atezolizumab Anti-CTLA-4: ipilimumab |
There are three key risk factors associated with HBVr, [21, 89] namely: (1) Host factors: male sex, older age, presence of cirrhosis, and type of disease treated with IST, such as bone marrow transplant or solid-organ transplantation; (2) HBV virologic factors: HBsAg seropositivity, high baseline HBV DNA levels, HBeAg seropositivity, and absence of anti-HBs among patients with resolved HBV infection, or co-infection with HCV, HDV, or HIV; (3) Type and degree of IST: B-cell–depleting therapies, such as rituximab and ofatumumab, anthracycline derivatives, such as doxorubicin and epirubicin, medium (10–20 mg/day) or high dose (≥20 mg/day) prednisone therapy for ≥4 weeks, steroid-containing chemotherapy, and TNF- inhibitors, such as infliximab and etanercept.
A risk gradient for HBVr exists between people who are HBsAg-positive and people who are HBsAg-negative but anti-HBs positive [89–91]. The risk of HBVr is 5 to 8 times higher among those patients who are HBsAg-positive as compared to those who were HBsAg negative but anti-HBc positive. Among patients who are HBsAg-positive the best predictor of reactivation has been shown to be the level of HBV DNA at baseline [85]. The risk of HBVr in patients who are HBsAg negative and anti-HBc positive is lower, where high risk has been reported for patients being treated with B cell therapies or undergoing HSCT. In individuals who are HBsAg-negative and anti-HBc positive, the presence and titer of anti-HBs antibodies have been associated with some protection against HBVr [82, 92]. However data are limited, and at present, there is insufficient evidence to support the use of anti-HBs titres for clinical decision-making in this situation.
Based on the type and duration of IST and the status of HBV infection, the risk of HBVr was established to be low (<1%), moderate (1–10%) and high (>10%) (Table 4) [21, 90].
The rationale of pre-emptive use of nucleos(t)ide analogues
Soon after the global registration of lamivudine, the first randomized controlled trial to compare “early” pre-emptive (start lamivudine before or at the initiation of cytotoxic chemotherapy for lymphoma) to “deferred” treatment (lamivudine initiated only when HBVr was detected on monitoring) was conducted in Chinese with lymphoma. The rationale was based on the hypothesis that if one can inhibit the enhanced HBV replication during the initial phase of intense IST, then on immune-reconstitution during withdrawal of the IST, the amount of HBV antigen-laden hepatocytes as target for host immunity and hence the incidence of liver injury should be drastically reduced. This will be in contrast to the “deferred” approach as the immune response to HBV-antigen laden hepatocytes has already been initiated and indeed the markedly enhanced HBV replication has already been abating. In keeping with this, “early” pre-emptive approach is found to be superior to “deferred” use of lamivudine resulting in a marked reduction in the incidence of HBVr and hepatitis in HBsAg positive with lymphoma treated with intense cytotoxic IST [14]. Similarly, in a prospective, open-label cohort study on Chinese adults of HBV inactive carriers with concurrent IgAN (proteinuria ≥ 3.5 g/day), this pre-emptive “early” use of lamivudine was found to be highly effective in preventing HBVr and its related hepatitis [93]. Subsequent systemic review and meta-analysis, based on 14 studies showed that the relative risk for both HBVr and HBV-related hepatitis ranged from 0.00 to 0.21, favoring preemptive use of lamivudine. A significantly higher proportion of participants not treated with pre-emptive lamivudine suffered from a disruption of chemotherapy [94]. However, due to the low-resistant barrier of lamivudine, some patients might develop YMDD mutation and deter the effectiveness of such a pre-emptive approach. Hence, lamivudine was later replaced by high resistant barrier NUCs with adefovir, entecavir and tenofovir [13, 95, 96]. With a higher safety profile with long-term use of entecavir, tenofovir and lately TAF, their pre-emptive use to prevent HBVr in patients planned for IS therapy have been validated in randomized control trials (Table 5).
Table 5.
Randomized controlled trials supporting the benefit of pre-emptive antiviral therapy in preventing HBV reactivation
| Authors (year) | Conditions | Treatment | HBV status | Antivirals vs controls (n) | Antiviral duration | Definition of HBVr | Rate of HBVr | Hepatitis due to HBVr |
|---|---|---|---|---|---|---|---|---|
| Lau (2003) [14] | Lymphoma | CEOP, ABVD, CHOP, COPP | HBsAg + | 15 lamivudine vs 15 controls | 1 week prior– 6 weeks after | HBV DNA levels 10 × baseline | 0% (0/15) vs 53% 0% (8/15) p < 0.01 | 0% (0/15) vs 47% (7/15) p < 0.01 |
| Jang (2006) [15] | HCC | TACE | HBsAg + | 36 lamivudine vs 37 controls | Beginning–12 months after | HBV DNA levels 10 × baseline | 3% (1/36) vs 41% (15/37) p < 0.001 | 3% (1/36) vs 30% (11/37) p < 0.01 |
| Hsu (2008) [16] | Non-Hodgkin lymphoma | CHOP | HBsAg + | 26 lamivudine vs 25 controls | Beginning–2 months after | HBV DNA levels 10 × baseline | 12% (3/26) vs 56% (14/25) p < 0.01 | 8% (2/26) vs 48% (12/25) p < 0.01 |
| Huang (2013) [10] | CD20 + non-Hodgkin lymphoma | R-CHOP | HBsAg − anti-HBc + | 41 entecavir vs 39 controls | 1 week prior–3 months after |
HBV DNA level at 2,000 IU/ml Reverse HBsAg seroconversion |
2% (1/41) vs 18% (7/39) p < 0.05 | 2% (1/41) vs 3% (1/39) p = 1.0 |
| Huang (2014) [95] | Lymphoma | R-CHOP | HBsAg + | 61 entecavir vs 60 lamivudine | 1 week prior–6 months after | HBV DNA levels 10 × baseline or an absolute increase of 105 copies/ml or greater compared with the baseline | 7% (4/61) vs 60% (18/60) p < 0.01 | 0% (0/61) vs 13% (8/60) p < 0.01 |
| Ho (2015) [96] | CHB patients undergoing chemotherapy | Chemotherapy | HBsAg + | 35 lamivudine vs 35 adefovir dipivoxil | 1 week prior–6 months after | 1 log increase in HBV DNA levels higher than that of the preceding samples | 37% (13/35) vs 29% (10/35) p = 0.611* | 3% (1/35) vs 6% (2/35) p = 1.00 |
| Buti (2017) [13] | Hematologic malignancy | Rituximab-based regimens | HBsAg − anti-HBc + | 33 tenofovir disoproxilfumarate vs 28 controls | Beginning–18 months after | HBsAg and/or HBV DNA detection, or ≥ 1 log increase in HBV DNA levels from baseline | 0% (0/33) vs 11% (3/28) p = 0.091 | 0% (0/33) vs 4% (1/28) p= 0.274 |
All controls received antiviral therapy if reactivation occurred. *In HBsAg + and in HBsAg− anti-HBc + patients receiving cancer chemotherapy. + positive, − negative, ABVD adriamycin, bleomycin, vinblastine, dacarbazine, anti-HBc anti-hepatitis B core antibody IgG, CEOP cyclophosphamide, epirubicin, vincristine, prednisolone, CHOP cyclophosphamide, doxorubicin, vincristine, prednisolone, COPP cyclophosphamide, vincristine, procarbazine, prednisolone, HBsAg hepatitis B antigen, IU international unit, R-CHOP rituximab-CHOP, TACE transarterial chemoembolization with epirubicin and cisplatin
*The study showed that the rate of developing drug resistance mutations was comparable among the two groups [62% (8/13) vs 0% (0/10), p = 0.003]
Assessment by hepatologists for termination of NUC treatment
All patients who are planned for IST should have HBsAg, anti-HBs and anti-HBc tested at baseline (Fig. 2). The risk of IST for HBVr should be assessed (Table 4). For HBsAg-positive patients, serum HBV DNA, and possibly qHBsAg should be checked and monitored. Current data on other biomarkers, such as anti-HBs and anti-HBc titres, HBV core-related antigen (HBcrAg), ultra-sensitive HBsAg evaluation and HBV RNA, in the diagnosis and monitoring of HBV reactivation over the course of immunosuppressive treatments are not sufficient to be of any added practical clinical use [97]. Assessment of liver fibrosis, either invasively or non-invasively, should then be performed under the guidance of a hepatologist. All HBV treatment guidelines recommend patients with significant fibrosis (F2 or greater) should receive NUCs treatment, while close monitoring may be indicated for patients without significant fibrosis. Therefore, using liver fibrosis assessment to stratify preventive therapy in low to moderate risk patients is a logical approach. Various non-invasive assessments have been developed and adopted in some international management guidelines [98, 99]. Liver stiffness measurement (LSM) with transient elastography is most widely validated and is an accurate and reproducible method to predict advanced fibrosis or cirrhosis () in CHB patients [98, 99]. The key challenge of this tool is the confounding effect of alanine aminotransferase (ALT) level, such that decrease in LSM may only reflect ALT normalization. In HBVr patients, LSM should therefore be assessed after normalization of ALT levels to accurately diagnose the degree of fibrosis [98]. However, Jia et al. showed in a large cohort of Chinese patients with CHB, ALT levels up to five times the ULN did not significantly affect the diagnostic power of LSM [100]. Li et al. showed that patients with mildly elevated ALT levels had higher LSM cut-off values than patients with normal ALT levels on predicting F2-F4 (6.5 vs 6 kPa) and F4 (10.2 vs 7.8 kPa) [101]. Using cut-offs regardless of ALT levels, the diagnostic accuracy of LSM was 81% for F2-F4, and 89% for F4. Applying ALT-stratified cut-off values, the diagnostic accuracy of LSM was 82% for predicting F2-F4, and 86% for predicting F4 [101]. In regions where transient elastography is not readily accessible, serum test formulae based on common laboratory parameters have the advantages of high applicability. Examples include aspartate aminotransferase (AST) to platelet ratio index (APRI), Forns index and Fibrosis-4 (FIB-4) score [102].
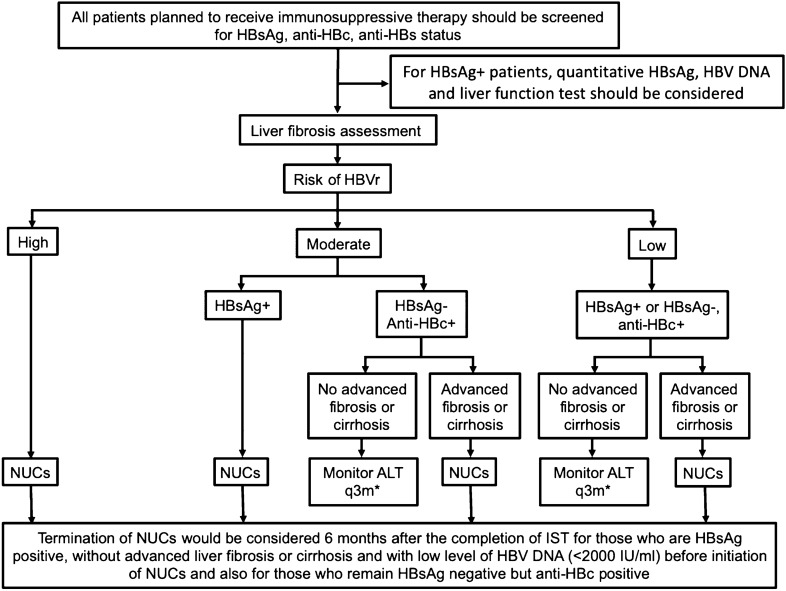
Fig. 2.
Algorithm for the management of hepatitis B reactivation. All high-risk patients and moderate risk HBsAg + patients should be treated with pre-emptive NUCs irrespective of fibrosis status. All patients with advanced fibrosis or cirrhosis should be treated with NUCs irrespective risk stratifications. All HBsAg + patients should be treated with NUCs except for low-risk patients without advanced fibrosis or cirrhosis. Low-risk HBsAg + without advanced fibrosis or cirrhosis should be monitored with ALT testing every three months. Moderate and low risks HBsAg − anti-HBc + patients without advanced fibrosis or cirrhosis should be monitored with ALT testing every three months
Both HBsAg positive and HBsAg negative but anti-HBc positive patients treated with IST considered high risk should be initiated pre-emptive high-resistant barrier NUCs. For those with moderate risk, all HBsAg positive and those HBsAg negative but anti-HBc positive patients with advanced liver fibrosis or cirrhosis should be initiated pre-emptive high-resistant barrier NUCs. The preferred NUCs are entecavir, tenofovir or TAF. For those HBsAg negative and anti-HBc positive patients without advance fibrosis or cirrhosis, serum ALT should be monitored every 3 months. If elevated ALT > 2 × baseline detected at monitoring, HBsAg and HBV DNA should be performed and high-resistant barrier NUCs initiated if either test positive. For those with low-risk , pre-emptive NUCs should be initiated in both HBsAg positive and HBsAg negative but anti-HBc positive with advanced fibrosis or cirrhosis. Serum ALT should be monitored every 3 months in both HBsAg positive and HBsAg negative but anti-HBc positive patients with low-risk. The AASLD, AGA and EASL recommend antiviral treatment should be continued for at least 6 months after discontinuation of IST and at least 12 months for B cell-depleting agents. [17, 19, 20] Our panel recommended that under the guidance of a hepatologist, termination of NUCs would be considered 6 months after the completion of IST for HBsAg positive patients, without advanced liver fibrosis or cirrhosis and with low level of HBV DNA (< 2000 IU/ml) before initiation of NUCs. For those who remain HBsAg negative but anti-HBc positive, termination of NUCs should be considered 6 months after the completion of IST. HBV DNA monitoring-guided preemptive NUCs are effective for preventing HBV-related hepatitis in these patients [83]. Close monitoring every 3 months and prompt initiation of NUCs may be more cost-effective. In the future with more solid evidence, new biomarkers, such as HBV RNA, HBcrAg, may be helpful to decide when to terminate NUCs after completion of IST [103].
Comparison to previous guidelines
In the Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update, a section “Antiviral prophylaxis before immunosuppressive therapy or chemotherapy” was included to discuss this important topic [18]. Over the last few years, more data have been made available to allow better stratification of the risk of HBVr with various immunosuppressive agents, namely new biologics, targeted therapies, immunotherapies and anti-HCV direct-acting antiviral agents. The recommendations remain the same for HBsAg-positive patients to whom pre-emptive NUC therapy should be started. The main changes lay on the HBsAg-negative but anti-HBc positive patients, as in the 2015 guidelines it was mentioned that further studies are needed to compare the efficacy and cost-effectiveness of different preventive strategies (pre-emptive NUCs versus monitoring). Furthermore, HBsAg-negative, anti-HBc positive patients with undetectable serum HBV DNA and who receive IST regardless of anti-HBs status should be followed carefully by means of LFT with or without HBV DNA testing, and be treated with NUCs upon confirmation of HBVr. Liver fibrosis assessment was included in the algorithm as patients with advanced fibrosis and cirrhosis run a higher risk of mortality and morbidity with HBVr. Also, new data on HBVr with HBV/HCV coinfected patients treated with DAAs are included. The evolving data over the last few years have better elucidated the risk of HBVr in HBsAg-negative, anti-HBc positive patients. Hence, pre-emptive NUCs are now recommended for HBsAg-negative, anti-HBc positive patients in the moderate-risk and high-risk groups.
Recommendations
1. Definition
1.1. Reactivation of HBV
1.1.1. Exacerbation of chronic HBV infection (HBsAg +)
• ≥ 2 log increase in HBV DNA levels from baseline levels
[Grading: evidence—II-2, recommendation -1]
• Detection of HBV DNA with level > 100 IU/ml in a person with undetectable HBV DNA at baseline
[Grading: evidence—II-2, recommendation -1]
1.1.2. Reactivation of past HBV infection (HBsAg negative, anti-HBc positive) after the start of immunosuppressive therapy
• Reverse HBsAg seroconversion, HBsAg-negative becomes HBsAg-positive
[Grading: evidence—II-2, recommendation -1]
• Appearance of HBV DNA in absence of HBsAg, HBV DNA-undetectable becomes HBV DNA-detectable
[Grading: evidence—II-2, recommendation -1]
2. Who should be screened?
All patients planned to receive immunosuppressive therapy should be screened.
[Grading: evidence—II-2, recommendation -1]
3. What will be screened?
3.1 Screening should include HBsAg, anti-HBs and anti-HBc.
[Grading: evidence—II-2, recommendation -1]
3.2 For those HBsAg-positive patients, additional test for quantitative HBV DNA and HBsAg should be considered.
[Grading: evidence—II-2, recommendation -1]
3.3 All HBsAg positive and HBsAg negative but anti-HBc positive patients should have the degree of liver fibrosis assessed by a hepatologist. [Grading: evidence—III, recommendation -2]
4. Management
4.1 It is mandatory and life-saving to administer pre-emptive NUCs promptly to the followings:
• In high-risk group, all HBsAg positive or HBsAg negative but anti-HBc positive patients
[Grading: evidence—I, recommendation -1]
• In moderate-risk group, all HBsAg positive and those who are anti-HBc positive with advanced liver fibrosis or cirrhosis
[Grading: evidence—II-2, recommendation -1]
4.2 It is essential to administer pre-emptive NUCs to the following:
• In low-risk group, all HBsAg positive or HBsAg negative but anti-HBc positive with advanced liver fibrosis or cirrhosis [Grading: evidence - II-2, recommendation -1]
4.3 Preferred NUCs include entecavir, tenofovir and TAF
[Grading: evidence—I, recommendation -1]
4.4 Termination of NUCs should be considered 6 months after the completion of immunosuppressive therapy:
• For HBsAg-positive patients, without advanced liver fibrosis or cirrhosis
[Grading: evidence—II-2, recommendation -1]
• For HBsAg-positive patients with low level of HBV DNA (< 2000 IU/ml) before initiation of NUCs
[Grading: evidence—II-2, recommendation -1]
• For those who remain HBsAg negative but anti-HBc positive
[Grading: evidence—II-2, recommendation -1]
5.1 Liver function test* should be monitored every 12-weekly
5.1 In moderate-risk group, HBsAg negative but anti-HBc-positive patients with no advanced liver fibrosis or cirrhosis
[Grading: evidence—II-2, recommendation -1]
5.2 In low-risk group, HBsAg positive or HBsAg negative but anti-HBc positive with no advanced liver fibrosis or cirrhosis
[Grading: evidence—II-2, recommendation -2]
5.3 If ALT > 2 × baseline, check HBsAg, HBV DNA and treat with NUCs for HBsAg seroreversion and/or HBV DNA detectable
[Grading: evidence—II-2, recommendation -1]
*Liver function test includes ALT, AST, bilirubin, albumin, globulin
Conflict of Interest
Ming-Lung Yu received research grants from Abbott, BMS, Gilead and Merck and received fees for being a speaker/consultant from Abbvie, Abbott, BMS, Gilead, Merck, Ipsen and Roche. Grace Wong has served as an advisory committee member for Gilead Sciences and Janssen, as a speaker for Abbott, Abbvie, Bristol-Myers Squibb, Echosens, Furui, Gilead Sciences, Janssen and Roche, and received research grant from Gilead Sciences. Alexander Thompson has served as an advisory committee member for Gilead Sciences, Abbvie, Roche, BMS, Merck, Immunocore, Janssen, Assembly Biosciences, Arbutus, Eisai, Ipsen and Bayer, as a speaker for Gilead Sciences, Abbvie, Roche, BMS, and received research grant from Gilead Sciences, Merck, BMS, Abbvie. Jin-Lin Hou received grants and personal fees from Bristol-Myers Squibb during the conduct of the study, and grants and personal fees from Bristol-Myers Squibb, GlaxoSmithKline, and Novartis. Teerha Piratvisuth received fees for being a speaker and advisory board member from Bayer, BMS, Gilead Sciences, and Eisai and received research support from Fibrogen, Gilead Sciences, Janssen and Roche. Ji-Dong Jia received consultation and speaker fees from Bristol-Myers Squibb, Gilead, Merck Sharp and Dohme, Novartis, and Roche. Ann-Lii Cheng received consultant fees from Novartis, Merck Serono, Eisai, Merck Sharp and Dohme, ONXEO, Bayer, Bristol-Myers Squibb, and Ono Pharmaceutical. Tony Mok received fees for being a speaker, consultant, and advisory board member from AstraZeneca, Roche/Genentech, Lilly, Bristol Myers Squibb, Boehringer Ingelheim, Novartis, Merck Sharp & Dohme, Pfizer, Merck Serono, SFJ Pharmaceuticals Group, ACEA Biosciences, Vertex, Celgene, Ignyta, Fishawack Facilitate Ltd, Takeda, Janssen, Hutchison MediPharma, and received grants from AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, SFJ Pharmaceuticals Group, Roche, Merck Sharp & Dohme, Clovis Oncology, Bristol Myers Squibb, Xcovery. Diana A. Payawal has served as an advisory committee member for Mylan Pharmaceutical, as a speaker for Gilead Sciences, Mylan Pharmaceuticals, Echosense, Getz and Abbott. Tawesak Tanwandee received grants from Bristol-Myers Squibb and Merck. Masao Omata received fees for being a speaker, consultant, and advisory board member from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Otsuka, Astellas, Gilead Sciences, Chugai, Mitsubishi Tanabe, Kyorin, Merck Sharp and Dohme, Dainippon Sumitomo, Vertex Pharmaceuticals, Takeda, Merck Serono, and Zeria. George Lau, Hasmik Ghazinian, Masashi Mizokami, Gregory Cheng, Guo-Feng Chen, Zhen-Wen Liu, Oidov Baatarkhuu, Woon Leung Ng, Patrick Lau, Jer-Ming Chang, Saeed Hamid, A. Kadir Dokmeci, Rino A Gani, Diana A. Payawal, Pierce Chow, Joong-Won Park, Simone I Strasser, Rosmawaiti Mohamed, Khin Maung Win, Shiv Kumar Sari declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/25/2022
A Correction to this paper has been published: 10.1007/s12072-022-10301-2
Contributor Information
George Lau, Email: gkklau@hnhmgl.com, Email: gkklau@netvigator.com.
Ming-Lung Yu, Email: fish6069@gmail.com.
References
- 1.Wands JR, Chura CM, Roll FJ, Maddrey WC. Serial studies of hepatitis-associated antigen and antibody in patients receiving antitumor chemotherapy for myeloproliferative and lymphoproliferative disorders. Gastroenterology. 1975;68(1):105–112. [PubMed] [Google Scholar]
- 2.Hoofnagle JH, Dusheiko GM, Schafer DF, Jones EA, Micetich KC, Young RC, Costa J. Reactivation of chronic hepatitis B virus infection by cancer chemotherapy. Ann Intern Med. 1982;96(4):447–449. doi: 10.7326/0003-4819-96-4-447. [DOI] [PubMed] [Google Scholar]
- 3.Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100(1):182–188. doi: 10.1016/0016-5085(91)90599-g. [DOI] [PubMed] [Google Scholar]
- 4.Lau G, Liang R, Chiu EK, Lee CK, Lam SK. Hepatic events after bone marrow transplantation in patients with hepatitis B infection: a case controlled study. Bone Marrow Transplant. 1997;19(8):795–799. doi: 10.1038/sj.bmt.1700744. [DOI] [PubMed] [Google Scholar]
- 5.Lau G. Hepatitis B reactivation after chemotherapy: two decades of clinical research. Hepatol Int. 2008;2(2):152–162. doi: 10.1007/s12072-008-9056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang R, Lau GK, Kwong YL. Chemotherapy and bone marrow transplantation for cancer patients who are also chronic hepatitis B carriers: a review of the problem J. Clin Oncol. 1999;17(1):394–398. doi: 10.1200/JCO.1999.17.1.394. [DOI] [PubMed] [Google Scholar]
- 7.Hui CK, Cheung WW, Zhang HY, Au WY, Yueng YH, Leung AY, Leung N, Luk JM, Lie AK, Kwong YL, Liang R, Lau GK. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology. 2006;131(1):59–68. doi: 10.1053/j.gastro.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu MT, Chan HL, Hui EP, Lei KI, Mok TS, Chan PK. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27(4):605–611. doi: 10.1200/JCO.2008.18.0182. [DOI] [PubMed] [Google Scholar]
- 9.Matsue K, Kimura S, Takanashi Y, Iwama K, Fujiwara H, Yamakura M, Takeuchi M. Reactivation of hepatitis B virus after rituximab-containing treatment in patients with CD20-positive B-cell lymphoma. Cancer. 2010;116(20):4769–4776. doi: 10.1002/cncr.25253. [DOI] [PubMed] [Google Scholar]
- 10.Huang YH, Hsiao LT, Hong YC, Chiou TJ, Yu YB, Gau JP, Liu CY, Yang MH, Tzeng CH, Lee PC, Lin HC, Lee SD. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31(22):2765–2772. doi: 10.1200/JCO.2012.48.5938. [DOI] [PubMed] [Google Scholar]
- 11.Seto WK, Chan TS, Hwang YY, Wong DK, Fung J, Liu KS, Gill H, Lam YF, Lie AK, Lai CL, Kwong YL, Yuen MF. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol. 2014;32(33):3736–3743. doi: 10.1200/JCO.2014.56.7081. [DOI] [PubMed] [Google Scholar]
- 12.Hsu C, Tsou HH, Lin SJ, Wang MC, Yao M, Hwang WL, Kao WY, Chiu CF, Lin SF, Lin J, Chang CS, Tien HF, Liu TW, Chen PJ, Cheng AL, Taiwan Cooperative Oncology Group Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: a prospective study. Hepatology. 2014;59(6):2092–2100. doi: 10.1002/hep.26718. [DOI] [PubMed] [Google Scholar]
- 13.Buti M, Manzano ML, Morillas RM, García-Retortillo M, Martín L, Prieto M, Gutiérrez ML, Suárez E, Gómez Rubio M, López J, Castillo P, Rodríguez M, Zozaya JM, Simón MA, Morano LE, Calleja JL, Yébenes M, Esteban R. Randomized prospective study evaluating tenofovir disoproxil fumarate prophylaxis against hepatitis B virus reactivation in anti-HBc-positive patients with rituximab-based regimens to treat hematologic malignancies: the Preblin study. PLoS ONE. 2017;12(9):e0184550. doi: 10.1371/journal.pone.0184550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau G, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, Cheung M, Zhang HY, Lie A, Ngan R, Liang R. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125(6):1742–1749. doi: 10.1053/j.gastro.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Jang JW, Choi JY, Bae SH, Yoon SK, Chang UI, Kim CW, Cho SH, Han JY, Lee YS. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology. 2006;43(2):233–240. doi: 10.1002/hep.21024. [DOI] [PubMed] [Google Scholar]
- 16.Hsu C, Hsiung CA, Su IJ, Hwang WS, Wang MC, Lin SF, Lin TH, Hsiao HH, Young JH, Chang MC, Liao YM, Li CC, Wu HB, Tien HF, Chao TY, Liu TW, Cheng AL, Chen PJ. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin’s lymphoma: a randomized trial. Hepatology. 2008;47(3):844–853. doi: 10.1002/hep.22106. [DOI] [PubMed] [Google Scholar]
- 17.Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT, American Gastroenterological Association Institute American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148(1):215–219. doi: 10.1053/j.gastro.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 18.Sarin SK, Kumar M, Lau G, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152(6):1297–1309. doi: 10.1053/j.gastro.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, APASL ACLF Research Consortium (AARC) for APASL ACLF working Party et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13(4):353–390. doi: 10.1007/s12072-019-09946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uhm J, Kim S-H, Oh S, et al. High incidence of hepatitis B viral reactivation in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Blood. 2018;132(Supplement 1):3010. [Google Scholar]
- 24.Wang YH, Liang JD, Sheng WH, Tien FM, Chen CY, Tien HF. Hepatitis B reactivation during treatment of tyrosine kinase inhibitors-Experience in 142 adult patients with chronic myeloid leukemia. Leuk Res. 2019;81:95–97. doi: 10.1016/j.leukres.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Yao ZH, Liao WY, Ho CC, Chen KY, Shih JY, Chen JS, Lin ZZ, Lin CC, Yang JC, Yu CJ. Incidence of hepatitis B reactivation during epidermal growth factor receptor tyrosine kinase inhibitor treatment in non-small-cell lung cancer patients. Eur J Cancer. 2019;117:107–115. doi: 10.1016/j.ejca.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 26.Atteya A, Ahmad A, Daghstani D, Mushtaq K, Yassin MA. Evaluation of hepatitis B reactivation among patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. Cancer Control. 2020 doi: 10.1177/1073274820976594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pu D, Yin L, Zhou Y, Li W, Huang L, Cai L, Zhou Q. Safety and efficacy of immune checkpoint inhibitors in patients with HBV/HCV infection and advanced-stage cancer: a systematic review. Medicine (Baltimore) 2020;99(5):19013. doi: 10.1097/MD.0000000000019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Zhou Y, Chen C, Fang W, Cai X, Zhang X, Zhao M, Zhang B, Jiang W, Lin Z, Ma Y, Yang Y, Huang Y, Zhao H, Xu R, Hong S, Zhang L. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer. 2019;7(1):322. doi: 10.1186/s40425-019-0808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee PC, Chao Y, Chen MH, Lan KH, Lee IC, Hou MC, Huang YH. Risk of HBV reactivation in patients with immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J Immunother Cancer. 2020;8(2):e001072. doi: 10.1136/jitc-2020-001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lan JL, Chen YM, Hsieh TY, Chen YH, Hsieh CW, Chen DY, Yang SS. Kinetics of viral loads and risk of hepatitis B virus reactivation in hepatitis B core antibody-positive rheumatoid arthritis patients undergoing anti-tumour necrosis factor alpha therapy. Ann Rheum Dis. 2011;70(10):1719–1725. doi: 10.1136/ard.2010.148783. [DOI] [PubMed] [Google Scholar]
- 31.Tamori A, Koike T, Goto H, Wakitani S, Tada M, Morikawa H, Enomoto M, Inaba M, Nakatani T, Hino M, Kawada N. Prospective study of reactivation of hepatitis B virus in patients with rheumatoid arthritis who received immunosuppressive therapy: evaluation of both HBsAg-positive and HBsAg-negative cohorts. J Gastroenterol. 2011;46(4):556–564. doi: 10.1007/s00535-010-0367-5. [DOI] [PubMed] [Google Scholar]
- 32.Ryu HH, Lee EY, Shin K, Choi IA, Lee YJ, Yoo B, Park MC, Park YB, Bae SC, Yoo WH, Kim SI, Lee EB, Song YW. Hepatitis B virus reactivation in rheumatoid arthritis and ankylosing spondylitis patients treated with anti-TNFα agents: a retrospective analysis of 49 cases. Clin Rheumatol. 2012;31(6):931–936. doi: 10.1007/s10067-012-1960-1. [DOI] [PubMed] [Google Scholar]
- 33.Tan J, Zhou J, Zhao P, Wei J. Prospective study of HBV reactivation risk in rheumatoid arthritis patients who received conventional disease-modifying antirheumatic drugs. Clin Rheumatol. 2012;31(8):1169–1175. doi: 10.1007/s10067-012-1988-2. [DOI] [PubMed] [Google Scholar]
- 34.Lee YH, Bae SC, Song GG. Hepatitis B virus reactivation in HBsAg-positive patients with rheumatic diseases undergoing anti-tumor necrosis factor therapy or DMARDs. Int J Rheum Dis. 2013;16(5):527–531. doi: 10.1111/1756-185X.12154. [DOI] [PubMed] [Google Scholar]
- 35.Chen MH, Chen MH, Liu CY, Tsai CY, Huang DF, Lin HY, Lee MH, Huang YH. Hepatitis B virus reactivation in rheumatoid arthritis patients undergoing biologics treatment. J Infect Dis. 2017;215(4):566–573. doi: 10.1093/infdis/jiw606. [DOI] [PubMed] [Google Scholar]
- 36.Bersoff-Matcha SJ, Cao K, Jason M, Ajao A, Jones SC, Meyer T, Brinker A. Hepatitis B virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus: a review of cases reported to the U.S. food and drug administration adverse event reporting system. Ann Intern Med. 2017;166(11):792–798. doi: 10.7326/M17-0377. [DOI] [PubMed] [Google Scholar]
- 37.Gane EJ, Hyland RH, An D, Svarovskaia ES, Brainard D, McHutchison JG. Ledipasvir and sofosbuvir for HCV infection in patients coinfected with HBV. Antivir Ther. 2016;21:605–609. doi: 10.3851/IMP3066. [DOI] [PubMed] [Google Scholar]
- 38.Doi A, Sakamori R, Tahata Y, Urabe A, Morishita N, Yamada R, et al. Frequency of, and factors associated with, hepatitis B virus reactivation in hepatitis C patients treated with all-oral direct-acting antivirals: analysis of a Japanese prospective cohort. Hepatol Res. 2017;47:1438–1444. doi: 10.1111/hepr.12919. [DOI] [PubMed] [Google Scholar]
- 39.Kawagishi N, Suda G, Onozawa M, Kimura M, Maehara O, Ohara M, et al. Comparing the risk of hepatitis B virus reactivation between direct-acting antiviral therapies and interferon-based therapies for hepatitis C. J Viral Hepat. 2017;24:1098–1106. doi: 10.1111/jvh.12737. [DOI] [PubMed] [Google Scholar]
- 40.Wang C, Ji D, Chen J, Shao Q, Li B, Liu J, et al. Hepatitis due to reactivation of hepatitis B virus in endemic areas among patients with hepatitis C treated with direct-acting antiviral agents. Clin Gastroenterol Hepatol. 2017;15:132–136. doi: 10.1016/j.cgh.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 41.Tamori A, Abiru S, Enomoto H, Kioka K, Korenaga M, Tani J, et al. Low incidence of hepatitis B virus reactivation and subsequent hepatitis in patients with chronic hepatitis C receiving direct-acting antiviral therapy. J Viral Hepat. 2018;25:608–611. doi: 10.1111/jvh.12840. [DOI] [PubMed] [Google Scholar]
- 42.Liu CJ, Chuang WL, Sheen IS, Wang HY, Chen CY, Tseng KC, et al. Efficacy of Ledipasvir and Sofosbuvir treatment of HCV infection in patients coinfected with HBV. Gastroenterology. 2018;154:989–997. doi: 10.1053/j.gastro.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 43.Yeh ML, Huang CF, Hsieh MH, Ko YM, Chen KY, Liu TW, et al. Reactivation of hepatitis B in patients of chronic hepatitis C with hepatitis B virus infection treated with direct acting antivirals. J Gastroenterol Hepatol. 2017;32:1754–1762. doi: 10.1111/jgh.13771. [DOI] [PubMed] [Google Scholar]
- 44.Liu CH, Liu CJ, Su TH, et al. Hepatitis B virus reactivation in patients receiving interferon-free direct-acting antiviral agents for chronic hepatitis C virus infection. Open Forum Infect Dis. 2017 doi: 10.1093/ofid/ofx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SW, Lee TY, Yang SS, Peng YC, Yeh HZ, Chang CS. Prevalence of hepatitis B reactivation among Chinese individuals with chronic hepatitis C treated with pan-oral direct-acting antivirals. Gastroenterol Res. 2018;11(2):124–129. doi: 10.14740/gr971w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeh ML, Huang CF, Huang CI, et al. Hepatitis B-related outcomes following direct-acting antiviral therapy in Taiwanese patients with chronic HBV/HCV co-infection. J Hepatol. 2020;73(1):62–71. doi: 10.1016/j.jhep.2020.01.027. [DOI] [PubMed] [Google Scholar]
- 47.Sulkowski MS, Chuang WL, Kao JH, et al. No evidence of reactivation of hepatitis B virus among patients treated with Ledipasvir-Sofosbuvir for hepatitis C virus infection. Clin Infect Dis. 2016;63(9):1202–1204. doi: 10.1093/cid/ciw507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogawa E, Furusyo N, Murata M, Toyoda K, Hayashi T, Ura K. Potential risk of HBV reactivation in patients with resolved HBV infection undergoing direct-acting antiviral treatment for HCV. Liver Int. 2018;38:76–83. doi: 10.1111/liv.13496. [DOI] [PubMed] [Google Scholar]
- 49.Toy M, Hutton DW, So SK. Cost-effectiveness and cost thresholds of generic and brand drugs in a national chronic hepatitis B treatment program in China. PLoS ONE. 2015;10(11):e0139876. doi: 10.1371/journal.pone.0139876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao Y, Thompson AJ, Howell J. Point-of-care tests for hepatitis b: an overview. Cells. 2020;9(10):2233. doi: 10.3390/cells9102233.PMID:33023265;PMCID:PMC7650625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsukuda S, Watashi K. Hepatitis B virus biology and life cycle. Antiviral Res. 2020;182:104925. doi: 10.1016/j.antiviral.2020.104925. [DOI] [PubMed] [Google Scholar]
- 53.Hui CK, Bowden S, Luk JM, Fong DYT, Zhang HY, Jackson K, Au WY, Lie A, Chim CS, Kwong YL, Liang R, Locarnini S, Lau G. Intrahepatic hepatitis B virus covalently closed circular DNA predicts post-chemotherapy Hepatitis B reactivation. Blood. 2005;15:2616–2617. doi: 10.1182/blood-2004-09-3402. [DOI] [PubMed] [Google Scholar]
- 54.Lang J, Neumann-Haefelin C, Thimme R. Immunological cure of HBV infection. Hepatol Int. 2019;13(2):113–124. doi: 10.1007/s12072-018-9912-8. [DOI] [PubMed] [Google Scholar]
- 55.Lau G, Suri D, Liang R, Chokshi S, Thomas MG, Nanji A, Yuen ST, Williams R, Naoumov NV. Resolution of chronic hepatitis B and Anti-HBs seroconversion in man by adoptive transfer of immunity to hepatitis B core antigen. Gastroenterology. 2002;122:614–624. doi: 10.1053/gast.2002.31887. [DOI] [PubMed] [Google Scholar]
- 56.Evens AM, Jovanovic BD, Su YC, Raisch DW, Ganger D, Belknap SM, Dai MS, Chiu BC, Fintel B, Cheng Y, Chuang SS, Lee MY, Chen TY, Lin SF, Kuo CY. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann Oncol. 2011;22(5):1170–1180. doi: 10.1093/annonc/mdq583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong HJ, Ni LN, Sheng GF, et al. Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximab-chemotherapy: a meta-analysis. J Clin Virol. 2013;57(3):209–214. doi: 10.1016/j.jcv.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 58.Jang JW, Choi JY, Bae SH, Kim CW, Yoon SK, Cho SH, Yang JM, Ahn BM, Lee CD, Lee YS, Chung KW, Sun HS. Transarterial chemo-lipiodolization can reactivate hepatitis B virus replication in patients with hepatocellular carcinoma. J Hepatol. 2004;41(3):427–435. doi: 10.1016/j.jhep.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 59.Huang L, Jiang S, Shi Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001–2020) J Hematol Oncol. 2020;13(1):143. doi: 10.1186/s13045-020-00977-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J, Huang B, Li Y, Zheng D, Zhou Z, Liu J. Hepatitis B virus reactivation in patients with multiple myeloma receiving bortezomib-containing regimens followed by autologous stem cell transplant. Leuk Lymphoma. 2015;56(6):1710–1717. doi: 10.3109/10428194.2014.941833. [DOI] [PubMed] [Google Scholar]
- 61.Tur-Kaspa R, Shaul Y, Moore DD, Burk RD, Okret S, Poellinger L, Shafritz DA. The glucocorticoid receptor recognizes a specific nucleotide sequence in hepatitis B virus DNA causing increased activity of the HBV enhancer. Virology. 1988;167(2):630–633. [PubMed] [Google Scholar]
- 62.Cheng X, Uchida T, Xia Y, Umarova R, Liu CJ, Chen PJ, Gaggar A, Suri V, Mücke MM, Vermehren J, Zeuzem S, Teraoka Y, Osawa M, Aikata H, Tsuji K, Mori N, Hige S, Karino Y, Imamura M, Chayama K, Liang TJ. Diminished hepatic IFN response following HCV clearance triggers HBV reactivation in coinfection. J Clin Invest. 2020;130(6):3205–3220. doi: 10.1172/JCI135616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koh C, Canini L, Dahari H, Zhao X, Uprichard SL, Haynes-Williams V, Winters MA, Subramanya G, Cooper SL, Pinto P, Wolff EF, Bishop R, Ai Thanda Han M, Cotler SJ, Kleiner DE, Keskin O, Idilman R, Yurdaydin C, Glenn JS, Heller T. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis. 2015;15(10):1167–1174. doi: 10.1016/S1473-3099(15)00074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dore GJ, Soriano V, Rockstroh J, Kupfer B, Tedaldi E, Peters L, Neuhaus J, Puoti M, Klein MB, Mocroft A, Clotet B, Lundgren JD, SMART INSIGHT study group Frequent hepatitis B virus rebound among HIV-hepatitis B virus-coinfected patients following antiretroviral therapy interruption. AIDS. 2010;24(6):857–865. doi: 10.1097/QAD.0b013e328334bddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakamoto S, Kanda T, Nakaseko C, Sakaida E, Ohwada C, Takeuchi M, Takeda Y, Mimura N, Iseki T, Wu S, Arai M, Imazeki F, Saito K, Shirasawa H, Yokosuka O. Reactivation of hepatitis B virus in hematopoietic stem cell transplant recipients in Japan: efficacy of nucleos(t)ide analogues for prevention and treatment. Int J Mol Sci. 2014;15(11):21455–21467. doi: 10.3390/ijms151121455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lau GK, He ML, Fong DY, Bartholomeusz A, Au WY, Lie AK, Locarnini S, Liang R. Preemptive use of lamivudine reduces hepatitis B exacerbation after allogeneic hematopoietic cell transplantation. Hepatology. 2002;36(3):702–709. doi: 10.1053/jhep.2002.35068. [DOI] [PubMed] [Google Scholar]
- 67.Yeo W, Chan PK, Ho WM, et al. Lamivudine for the prevention of hepatitis B virus reactivation in hepatitis B s-antigen seropositive cancer patients undergoing cytotoxic chemotherapy. J Clin Oncol. 2004;22(5):927–934. doi: 10.1200/JCO.2004.05.161. [DOI] [PubMed] [Google Scholar]
- 68.Kim MK, Ahn JH, Kim SB, et al. Hepatitis B reactivation during adjuvant anthracycline-based chemotherapy in patients with breast cancer: a single institution’s experience. Korean J Intern Med. 2007;22(4):237–243. doi: 10.3904/kjim.2007.22.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paul S, Saxena A, Terrin N, Viveiros K, Balk EM, Wong JB. Hepatitis B virus reactivation and prophylaxis during solid tumor chemotherapy: a systematic review and meta-analysis. Ann Intern Med. 2016;164(1):30–40. doi: 10.7326/M15-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng AL, Hsiung CA, Su IJ, et al. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology. 2003;37(6):1320–1328. doi: 10.1053/jhep.2003.50220. [DOI] [PubMed] [Google Scholar]
- 71.Chen CY, Tien FM, Cheng A, Huang SY, Chou WC, Yao M, Tang JL, Tien HF, Sheng WH. Hepatitis B reactivation among 1962 patients with hematological malignancy in Taiwan. BMC Gastroenterol. 2018;18(1):6. doi: 10.1186/s12876-017-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yeo W, Lam KC, Zee B, et al. Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy. Ann Oncol. 2004;15(11):1661–1666. doi: 10.1093/annonc/mdh430. [DOI] [PubMed] [Google Scholar]
- 73.Lee HJ, Kim DY, Keam B, et al. Lamivudine prophylaxis for hepatitis B virus carrier patients with breast cancer during adjuvant chemotherapy. Breast Cancer. 2014;21(4):387–393. doi: 10.1007/s12282-012-0417-3. [DOI] [PubMed] [Google Scholar]
- 74.Jang JW, Choi JY, Bae SH, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemolipiodolization. Hepatology. 2006;43(2):233–240. doi: 10.1002/hep.21024. [DOI] [PubMed] [Google Scholar]
- 75.Jang JW, Kwon JH, You CR, et al. Risk of HBV reactivation according to viral status and treatment intensity in patients with hepatocellular carcinoma. Antivir Ther. 2011;16(7):969–977. doi: 10.3851/IMP1840. [DOI] [PubMed] [Google Scholar]
- 76.Kuo MH, Tseng CW, Lu MC, Tung CH, Tseng KC, Huang KY, Lee CH, Lai NS. Risk of hepatitis B virus reactivation in rheumatoid arthritis patients undergoing tocilizumab-containing treatment. Dig Dis Sci. 2021;2:1–9. doi: 10.1007/s10620-020-06725-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sonneveld MJ, Murad SD, van der Eijk AA, de Man RA. Fulminant liver failure due to hepatitis B reactivation during treatment with tocilizumab. ACG Case Rep J. 2019;6(12):e00243. doi: 10.14309/crj.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.APASL Covid-19 Task Force. Lau G, Sharma M. Clinical practice guidance for hepatology and liver transplant providers during the COVID-19 pandemic: APASL expert panel consensus recommendations. Hepatol Int. 2020;14(4):415–428. doi: 10.1007/s12072-020-10054-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seto WK, Chan TS, Hwang YY, Wong DK, Fung J, Liu KS, Gill H, Lam YF, Lau EHY, Cheung KS, Lie AKW, Lai CL, Kwong YL, Yuen MF. Hepatitis B reactivation in occult viral carriers undergoing hematopoietic stem cell transplantation: a prospective study. Hepatology. 2017;65(5):1451–1461. doi: 10.1002/hep.29022. [DOI] [PubMed] [Google Scholar]
- 80.Wu T, Wu N, Ma YX, Wu J, Gao Y, Pan XB. Role of hepatitis B antibody in predicting reactivation of resolved hepatitis B virus infection in leukemia patients. Antiviral Res. 2020;177:104765. doi: 10.1016/j.antiviral.2020.104765. [DOI] [PubMed] [Google Scholar]
- 81.Nishikawa K, Kimura K, Kanda Y, Sugiyama M, Kakihana K, Doki N, Ohashi K, Bae SK, Takahashi K, Ishihara Y, Mizuno I, Onishi Y, Onozawa M, Onizuka M, Yamamoto M, Ishikawa T, Inoue K, Kusumoto S, Hashino S, Saito H, Kanto T, Sakamaki H, Mizokami M. A prospective trial of vaccine to prevent hepatitis B virus reactivation after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020;55(7):1388–1398. doi: 10.1038/s41409-020-0833-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paul S, Dickstein A, Saxena A, Terrin N, Viveiros K, Balk EM, Wong JB. Role of surface antibody in hepatitis B reactivation in patients with resolved infection and hematologic malignancy: a meta-analysis. Hepatology. 2017;66(2):379–388. doi: 10.1002/hep.29082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kusumoto S, Arcaini L, Hong X, Jin J, Kim WS, Kwong YL, Peters MG, Tanaka Y, Zelenetz AD, Kuriki H, Fingerle-Rowson G, Nielsen T, Ueda E, Piper-Lepoutre H, Sellam G, Tobinai K. Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood. 2019;133(2):137–146. doi: 10.1182/blood-2018-04-848044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wong GL, Wong VW, Yuen BW, Tse YK, Yip TC, Luk HW, Lui GC, Chan HL. Risk of hepatitis B surface antigen seroreversion after corticosteroid treatment in patients with previous hepatitis B virus exposure. J Hepatol. 2020;72(1):57–66. doi: 10.1016/j.jhep.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 85.Lau GK, Leung YH, Fong DY, Au WY, Kwong YL, Lie A, Hou JL, Wen YM, Nanj A, Liang R. High hepatitis B virus (HBV) DNA viral load as the most important risk factor for HBV reactivation in patients positive for HBV surface antigen undergoing autologous hematopoietic cell transplantation. Blood. 2002;99(7):2324–2330. doi: 10.1182/blood.v99.7.2324. [DOI] [PubMed] [Google Scholar]
- 86.Kanda T, Lau GKK, Wei L, Moriyama M, Yu ML, Chuang WL, Ibrahim A, Lesmana CRA, Sollano J, Kumar M, Jindal A, Sharma BC, Hamid SS, Kadir Dokmeci A, Mamun-Al-Mahtab, McCaughan GW, Wasim J, Crawford DHG, Kao JH, Ooka Y, Yokosuka O, Sarin SK, Omata M. APASL HCV guidelines of virus-eradicated patients by DAA on how to monitor HCC occurrence and HBV reactivation. Hepatol Int. 2019;13(6):649–661. doi: 10.1007/s12072-019-09988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu ML, Chen PJ, Dai CY, Hu TH, Huang CF, Huang YH, Hung CH, Lin CY, Liu CH, Liu CJ, Peng CY, Lin HC, Kao JH, Chuang WL. 2020 Taiwan consensus statement on the management of hepatitis C: part (II) special populations. J Formos Med Assoc. 2020;119(7):1135–1157. doi: 10.1016/j.jfma.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 88.Chen G, Wang C, Chen J, Ji D, Wang Y, Wu V, Karlberg J, Lau G. Hepatitis B reactivation in hepatitis B and C coinfected patients treated with antiviral agents: a systematic review and meta-analysis. Hepatology. 2017;66(1):13–26. doi: 10.1002/hep.29109. [DOI] [PubMed] [Google Scholar]
- 89.Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148(1):221–244. doi: 10.1053/j.gastro.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 90.Mallet V, van Bömmel F, Doerig C, Pischke S, Hermine O, Locasciulli A, Cordonnier C, Berg T, Moradpour D, Wedemeyer H, Ljungman P, ECIL-5 Management of viral hepatitis in patients with haematological malignancy and in patients undergoing haemopoietic stem cell transplantation: recommendations of the 5th European Conference on Infections in Leukaemia (ECIL-5) Lancet Infect Dis. 2016;16(5):606–617. doi: 10.1016/S1473-3099(16)00118-3. [DOI] [PubMed] [Google Scholar]
- 91.Doyle J, Raggatt M, Slavin M, McLachlan SA, Strasser SI, Sasadeusz JJ, Howell J, Hajkowicz K, Nandurkar H, Johnston A, Bak N, Thompson AJ. Hepatitis B management during immunosuppression for haematological and solid organ malignancies: an Australian consensus statement. Med J Aust. 2019;210(10):462–468. doi: 10.5694/mja2.50160. [DOI] [PubMed] [Google Scholar]
- 92.Pei SN, Ma MC, Wang MC, Kuo CY, Rau KM, Su CY, Chen CH. Analysis of hepatitis B surface antibody titers in B cell lymphoma patients after rituximab therapy. Ann Hematol. 2012;91(7):1007–1012. doi: 10.1007/s00277-012-1405-6. [DOI] [PubMed] [Google Scholar]
- 93.Fang J, Li W, Tan Z, Li D. Comparison of prednisolone and lamivudine combined therapy with prednisolone monotherapy on carriers of hepatitis B virus with IgA nephropathy: a prospective cohort study. Int Urol Nephrol. 2014;46(1):49–56. doi: 10.1007/s11255-013-0480-5. [DOI] [PubMed] [Google Scholar]
- 94.Loomba R, Rowley A, Wesley R, Liang TJ, Hoofnagle JH, Pucino F, Csako G. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148(7):519–528. doi: 10.7326/0003-4819-148-7-200804010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang H, Li X, Zhu J, Ye S, Zhang H, Wang W, Wu X, Peng J, Xu B, Lin Y, Cao Y, Li H, Lin S, Liu Q, Lin T. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: a randomized clinical trial. JAMA. 2014;312(23):2521–2530. doi: 10.1001/jama.2014.15704. [DOI] [PubMed] [Google Scholar]
- 96.Ho EY, Yau T, Rousseau F, Heathcote EJ, Lau GK. Preemptive adefovir versus lamivudine for prevention of hepatitis B reactivation in chronic hepatitis B patients undergoing chemotherapy. Hepatol Int. 2015;9(2):224–230. doi: 10.1007/s12072-015-9612-6. [DOI] [PubMed] [Google Scholar]
- 97.Svicher V, Salpini R, Malagnino V, Piermatteo L, Alkhatib M, Cerva C, Sarmati L. New markers in monitoring the reactivation of hepatitis B virus infection in immunocompromised hosts. Viruses. 2019;11(9):783. doi: 10.3390/v11090783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wong GL. Non-invasive assessments for liver fibrosis: the crystal ball we long for. J Gastroenterol Hepatol. 2018;33:1009–1015. doi: 10.1111/jgh.14103. [DOI] [PubMed] [Google Scholar]
- 99.Loomba R, Adams LA. Advances in non-invasive assessment of hepatic fibrosis. Gut. 2020;69(7):1343–1352. doi: 10.1136/gutjnl-2018-317593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jia J, Hou J, Ding H, Chen G, Xie Q, Wang Y, Zeng M, Zhao J, Wang T, Hu X, Schuppan D. Transient elastography compared to serum markers to predict liver fibrosis in a cohort of Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2015;30(4):756–762. doi: 10.1111/jgh.12840. [DOI] [PubMed] [Google Scholar]
- 101.Li Q, Chen L, Zhou Y. Diagnostic accuracy of liver stiffness measurement in chronic hepatitis B patients with normal or mildly elevated alanine transaminase levels. Sci Rep. 2018;8(1):5224. doi: 10.1038/s41598-018-23646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Agbim U, Asrani SK. Non-invasive assessment of liver fibrosis and prognosis: an update on serum and elastography markers. Expert Rev Gastroenterol Hepatol. 2019;13(4):361–374. doi: 10.1080/17474124.2019.1579641. [DOI] [PubMed] [Google Scholar]
- 103.Kaewdech A, Tangkijvanich P, Sripongpun P, Witeerungrot T, Jandee S, Tanaka Y, Piratvisuth T. Hepatitis B surface antigen, core-related antigen and HBV RNA: Predicting clinical relapse after NA therapy discontinuation. Liver Int. 2020;40(12):2961–2971. doi: 10.1111/liv.14606. [DOI] [PubMed] [Google Scholar]
- 104.Long M, Jia W, Li S, Jin L, Wu J, Rao N, Feng H, Chen K, Deng H, Liu F, Su F, Song E. A single-center, prospective and randomized controlled study: can the prophylactic use of lamivudine prevent hepatitis B virus reactivation in hepatitis B s-antigen seropositive breast cancer patients during chemotherapy? Breast Cancer Res Treat. 2011;127(3):705–712. doi: 10.1007/s10549-011-1455-9. [DOI] [PubMed] [Google Scholar]
- 105.Koo YX, Tay M, Teh YE, Teng D, Tan DS, Tan IB, Tai DW, Quek R, Tao M, Lim ST. Risk of hepatitis B virus (HBV) reactivation in hepatitis B surface antigen negative/hepatitis B core antibody positive patients receiving rituximab-containing combination chemotherapy without routine antiviral prophylaxis. Ann Hematol. 2011;90(10):1219–1223. doi: 10.1007/s00277-011-1241-0. [DOI] [PubMed] [Google Scholar]
- 106.Mori S. Past hepatitis B virus infection in rheumatoid arthritis patients receiving biological and/or nonbiological disease-modifying antirheumatic drugs. Mod Rheumatol. 2011;21(6):621–627. doi: 10.1007/s10165-011-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Urata Y, Uesato R, Tanaka D, Kowatari K, Nitobe T, Nakamura Y, Motomura S. Prevalence of reactivation of hepatitis B virus replication in rheumatoid arthritis patients. Mod Rheumatol. 2011;21(1):16–23. doi: 10.1007/s10165-010-0337-z. [DOI] [PubMed] [Google Scholar]
- 108.Watanabe T, Fukae J, Fukaya S, Sawamukai N, Isobe M, Matsuhashi M, Shimizu M, Akikawa K, Tanimura K, Atsumi T, Koike T. Incidence and risk factors for reactivation from resolved hepatitis B virus in rheumatoid arthritis patients treated with biological disease-modifying antirheumatic drugs. Int J Rheum Dis. 2019;22(4):574–582. doi: 10.1111/1756-185X.13401. [DOI] [PubMed] [Google Scholar]