Abstract
Osteoporosis is one of the major health issues associated with menopause-related estrogen deficiency. Various reports suggest that the hormonal changes related to menopausal transition may lead to the derangement of redox homeostasis and ultimately oxidative stress. Estrogen deficiency and oxidative stress may enhance the expression of genes involved in inflammation. All these factors may contribute, in synergy, to the development of postmenopausal osteoporosis. Previous studies suggest that estrogen may act as an antioxidant to protect the bone against oxidative stress, and as an anti-inflammatory agent in suppressing pro-inflammatory and pro-osteoclastic cytokines. Thus, the focus of the current review is to examine the relationship between estrogen deficiency, oxidative stress and inflammation, and the impacts of these phenomena on skeletal health in postmenopausal women.
Keywords: Inflammation, menopause, estrogen, osteoporosis, oxidative stress, review
1. INTRODUCTION
Progressive bone loss and skeletal fragility caused by osteoporosis is an emerging public health problem in a world experiencing a rapid increase in the elderly population. The primary risk factor for bone loss in midlife women is menopause [1, 2]. A high level of estrogen is present in women from the onset of menstrual periods during puberty until menopause, which marks the termination of reproductive age. Most estrogen is produced in the ovaries, released into the circulation and exert their effects on target tissues through endocrine signalling. After menopause, the circulating estrogen levels fall drastically when estrogen production from the ovaries ceases [3]. Approximately 50% of trabecular bone and 30% of cortical bone diminish during the course of a woman’s lifetime, of which half is lost during the first 10 years after menopause [4].
The pleiotropic effects of estrogen indicate that its deficiency will impact many signalling pathways in the body [5]. For example, metabolic changes due to menopause, like the accumulation of adipose tissue in the body, is associated with chronic low-grade inflammation and oxidative stress, leading to diseases like cancer [6]. The effects of menopause on oxidative stress and inflammation are also of particular interest in the field of osteoporosis because all of them are contributors to bone loss [7-9].
Therefore, this review aims to provide an overview of the relationship between estrogen deficiency, oxidative stress and inflammation and their combined effects on bone loss in women. The information obtained will be instrumental in planning preventive strategies against postmenopausal bone loss.
2. ESTROGENS AND ESTROGEN SIGNALLING PATHWAY
Estrogen is a group of steroid hormones governing the development of secondary female sex characteristics and regulating the female reproductive system. It also has other non-reproductive physiological roles. Estrogen is produced primarily in the ovaries through the stimulation of follicle-stimulating hormone, and in small amounts by the adrenal glands, breasts, adipose and liver. Endogenous estrogen is converted from androgens in women via a series of enzymatic reactions, which produce estrone (E1), estradiol (E2) and estriol (E3). In the ovary, androstenedione is produced from cholesterol and converted immediately into either E1 or testosterone. Aromatase, a cytochrome P450 enzyme in the endoplasmic reticulum of estrogen producing cells, then converts androstenedione and testosterone into E2. Estradiol is the potent and predominant estrogen present before the first period until the menopause. The strong potency of E2 is attributed to its high affinity towards estrogen receptors compared to other estrogen forms. Estrone is a weak estrogen found in women after menopause, which can be converted to E2 and vice versa. Estriol is the weakest estrogen produced in abundance during pregnancy and cannot be converted to E2 nor E1 [10].
Estrogen is a chemical messenger, which can travel through the circulatory system and interact with cells by binding to estrogen receptors. Estrogen signalling occurs via specific nuclear receptors by acting as ligand-activated transcription factors of two isomers of estrogen receptors (ERs), i.e. ER-alpha (ER-α) and ER-beta (ER-β) [11-14]. ER-α is found predominantly in the uterus, bone, adipose, liver, kidney, heart [12, 15, 16], whereas ER-β is found predominantly in the ovary, bladder, prostate, gastrointestinal tract, central nervous system and hematopoietic cells [12, 16]. Estrogen binds to its receptors in the nucleus, causing the receptor to dimerize and bind to estrogen response elements located in the promoters region of target genes. Subsequently, expression of these genes will be modulated, resulting in the biological actions of estrogen. In addition, ERs also regulate gene expression by influencing protein-protein interactions with other DNA-binding transcription factors in the nucleus. Estrogen also acts via nongenomic action through the activation of protein-kinase cascades via membrane-associated ERs [17].
3. Direct effects of estrogen on bone health
Estrogen exerts a strong influence on skeletal growth and homeostasis. During bone growth, estrogen is responsible for the proper closure of epiphyseal plates [18]. In bone, estrogens act directly via ERs on osteoblasts, osteocytes, osteoclasts, immune cells and other cells in maintaining bone mass [19-23]. Bone is being constantly remodelled via the actions of these bone cells [24]. Osteoblasts perform bone formation by laying down the new bone matrix and mineralize it, while osteoclasts break down the bone during bone resorption. The balance between both of these processes is crucial for sustaining bone mass and maintaining systemic skeletal homeostasis [25]. During puberty, estrogen increases bone mass through increasing number and activity of osteoblast, as well as decreasing osteoclast activity [26]. Estrogen also prevents apoptosis of osteocytes by preserving their autophagy function [27]. The inverse occurs during menopause, whereby the rate of bone resorption overwhelms bone formation, resulting in a decrease in bone mass [28].
Differential expression of ERs has been reported in osteoblasts and osteoclasts. In general, ERα mediates most actions of estrogen on bone cells. Activation of ER signalling pathway stimulates osteoblast differentiation and suppresses osteoclast activity [29]. Estrogen deficiency increases osteoclast formation and prolongs their lifespan. Estrogen deficiency activates the inflammatory cascades, leading to increase production of macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa-Β ligand (RANKL), which is an osteoprotegerin (OPG) ligand, by stromal-osteoblast lineage cells [30, 31]. The binding of RANKL to RANK receptors stimulates osteoclast differentiation and activity and prevents their apoptosis. The binding of M-CSF to its receptor also stimulates the proliferation and survival of osteoclast precursors and the mature osteoclasts [32]. OPG produced by the stromal-osteoblast lineage cells binds to RANKL and prevents the activation of the RANK-RANKL signalling pathway [33]. Estrogen is reported to increase both OPG and RANKL expression, but the OPG expression sustains for a longer period compared to RANKL, giving rise to a larger OPG/RANKL ratio [34-36]. ERα-knockout mice had less apoptotic osteoclasts than wildtype mice when estrogen was supplemented, suggesting that ERα is needed to upregulate the pro-apoptotic factor Fas ligands in osteoclasts [37]. Lower bone mass and strength developed in osteoblast-specific ERα-depleted mice, showing that estrogen acts on ERα in osteoblasts to achieve its skeletal protective effects [38].
The mechanical properties of bone are determined by its structural and material characteristics [39]. The deterioration of bone mass, macro and micro-architecture of the bone induced by estrogen deficiency will lead to a reduction of bone strength and increased risk of fracture. Ovariectomized (OVX) rats showed lower femoral or tibial bone volume, trabecular number (Tb.N), and trabecular thickness (Tb.Th) and higher structural model index and trabecular separation (Tb.Sp) compared to the sham group as evaluated by micro-computed tomography or bone structural histomorphometry analysis [40-46]. These degenerative changes result in increased porosity of the bone [47]. Similar changes were observed in OVX model of mice and rabbits [48, 49] The OVX rats also showed a significant reduction in density of the maxillary bone after 12 weeks, which could cause tooth loss [41].
The mineralizing activity of the bone in vivo can be measured by dynamic histomorphometry parameters. OVX rats are reported to have a lower double-labelled surface (sites of bone mineralization), bone formation rate and mineral appositional rate compared to the sham group [45, 50]. In bone cellular histomorphometry, lower osteoblast surface, osteoid surface and osteoid volume were observed in OVX rats, suggesting reduced osteoblast number and bone formation activity [41, 45, 51]. On the other hand, OVX rats experienced increased bone resorption as evidenced by increased osteoclast surface and eroded surface (sites of bone resorption) [41, 45, 51]. Alternatively, changes in bone remodelling activities were illustrated by circulating markers. Some studies reported increased bone resorption markers (like C-terminal cross-linking telopeptide type I collagen/CTX-1) and reduced bone formation markers (like alkaline phosphatase/ALP and osteocalcin) in OVX rats [41, 52]. However, examples of concurrent evaluation of both bone formation and resorption markers, suggestive of high bone turnover, are also common [53].
Ultimately, all of these structural and mineral degenerative changes result in a reduction in bone biomechanical strength in OVX rats. Reduced maximum/ultimate force to break the bone, elastic modulus and stress were observed in OVX rats compared to the sham group [40-49, 54].
Estrogen deficiency renders postmenopausal women vulnerable to osteoporosis [55]. Bone mineral density (BMD) by dual-energy X-ray absorptiometry (DXA) is considered the gold standard in determining osteoporosis [56]. Based on the World Health Organization (WHO), osteoporosis is defined by a BMD T-score ≤ -2.5. In multiple studies, DXA assessment revealed that osteoporosis prevalence was higher among the postmenopausal women compared to their men counterparts [57-60]. Decreased BMD was found to be significantly associated with age, menopause age and the year since menopause [57].
Mederle et al. reported that the decrease of E2 level in postmenopausal women was associated with a significant decrease in BMD at the lumbar spine and femoral neck [61]. Concurrent reduction of BMD and high bone turnover indicated by high circulating bone turnover markers, such as ALP, osteocalcin and CTX-1, are commonly observed among postmenopausal women [62]. Thompson et al. revealed that within 5 years of menopause, alveolar bone density loss was associated with elevated circulating levels of matrix metalloproteinase-2 (MMP-2) indicative of osteoclastic bone resorption [63]. Menopausal women were observed to have a higher level of bone resorption markers, like N-terminal propeptide of type I procollagen and CTX-l, and lower trabecular bone score, which correlated with skeletal microarchitecture deterioration [64].
4. estrogen deficiency and oxidative stress
Oxidative stress is defined as a disparity between the generation of reactive oxygen species (ROS) from various oxidation pathways and the antioxidant defence system in the body. The excess free radicals overwhelm the normal antioxidant capacity of the body, leading to damage of cellular macromolecules, such as enhanced lipid peroxidation, protein modification and DNA breakage, ultimately affecting cellular functioning [65].
Generally, ROS are short-lived but highly reactive chemical species containing oxygen [66]. The nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is a membrane-bound enzyme complex that generates ROS, including oxygen-derived free radicals like superoxide anion (O-) and the hydroxyl radical (H-), or non-radical molecules like hydrogen peroxide (H2O2) [67]. Free radicals react with oxygen to produce O-, which react with nitric oxide (NO) to produce peroxynitrite. The oxygen singlet also undergoes dismutation in a process catalysed by superoxide dismutase (SOD) to produce H2O2, which is detoxified into a water molecule by antioxidants enzymes such as catalase (CAT) and glutathione peroxidase (GSH-Px/GPX). GPX neutralizes H2O2 by taking hydrogens from two glutathione (GSH) molecules, thus forming two water molecules and one glutathione disulfide (GSSG). An increase in oxidative stress will cause intracellular GSSG accumulation and a decrease in GSH/GSSG ratio level [68, 69]. Therefore, the ratio of reduced GSH and oxidize GSSG is important in determining redox status and serves as useful indicators of oxidative stress markers. Glutathione-S-transferase (GST) catalyzes the conjugation of glutathione (GSH) to form endogenous and exogenous electrophilic compounds. GST plays a regulatory role in the mitogen-activated protein (MAP) kinase pathway involved in cellular survival and death signals [70-72].
In the culture of human bone marrow cells, H2O2 was shown to stimulate a significant increase in the formation and activity of osteoclast-like cells expressing tartrate-resistant acid phosphatase (TRAP). H2O2 also increased the expression of M-CSF, RANKL and RANKL/OPG ratio. Treatment with CAT significantly suppressed the formation of TRAP multinucleated cells as well as M-CSF and RANKL expression [73]. A study conducted by Lean et al. demonstrated that 17β-estradiol increased the expression of GPX in osteoclasts and antiestrogen ameliorated this effect. Overexpression of GPX in RAW 264.7 cells suppressed osteoclastic differentiation associated with inhibition of NFκB-activation mediated by RANKL [74]. In MLO-Y4 osteocyte-like cells, 17β-estradiol significantly increased the total glutathione S-transferase P expression and suppressed RANKL and sclerostin expression, RANKL release, and RANKL/OPG ratio [75]. Therefore, these studies demonstrated that estrogen can regulate the activity of bone cells by altering their redox status.
In animal studies, OVX rats exhibited a significant decrease in the activity of SOD, CAT, GPX, GST and GSH level, as well as an increase in malondialdehyde (MDA) level [76-78]. A previous study showed that estrogen deficiency compounded the effects of ageing on the oxidative status in rats, wherein LPO and NO level, as well as inducible NO synthase protein expression, were increased in the old rats compared to the young animals, and the changes were more prominent in the OVX group [79]. Mitochondria are a major source of ROS. Estrogen deficiency was shown to inhibit mitochondrial β-oxidation of fatty acid and increase ROS production. As evidence, the liver mitochondrial and peroxisomal H2O2 generation in OVX mice increased significantly, while the antioxidant enzyme activities decreased [80]. In OVX rats fed with high-fat diet, the expressions of antioxidant enzymes SOD and GPX were significantly suppressed whereas the expression of pro-oxidative enzyme NADPH oxidase was elevated compared to the control group. The resultant oxidative stress-activated mitogen-activated protein kinases (MAPK) pathway and upregulated ERK 1/2 and p38, leading to metabolic derangement of the rats in conjunction with high-fat diet [81]. Additionally, ovariectomy also increased the level of homocysteine (an indicator of cardiovascular disease), along with elevated oxidative stress markers MDA, oxidized-low density lipoprotein and GSSG levels in the rats [82]. A study by He et al. reported that lower BMD was associated with lower SOD and GPX levels and OPG expression in OVX rats compared to the control group. Ovariectomy also increased serum osteocalcin, ALP, MDA levels and RANKL expression in the rats [83]. Estrogen treatment was shown to normalize redox stress and preserve the bone health of the OVX rats [84]. In a study by Yang et al., the depleted antioxidant status due to ovariectomy was associated with declined autophagy and increased apoptosis of osteocytes. Treatment with estrogen was able to reverse these changes [85].
The link between oxidative stress and estrogen deficiency has been demonstrated by several human studies. Oxidative stress is hypothesized as one of the causes of physiological changes due to postmenopausal and ageing [86, 87]. Signorelli et al. reported that postmenopausal women (n=51, aged 52.1±1.3 years old) experienced a higher level of oxidative stress compared to fertile women (n=50, 32.5±1.1 years old), indicated by higher serum MDA, 4-hydroxynonenal and oxidized lipoprotein levels [7]. Another study also demonstrated that serum GSH levels decreased significantly while serum MDA and γ-glutamyltransferase levels increased significantly in the postmenopausal women group (n=16) compared to the premenopausal women group (n=17) [88]. Increased hydroperoxide (ROOH) level, a marker of lipid peroxidation, was negatively and independently associated with decreased BMD and increased bone resorption rate marked by CTX-1 levels in postmenopausal women [89]. Other studies showed that hormone replacement therapy (tibolone [90], oestradiol alone or in combination with medroxyprogesterone [91]) was able to reverse the decline in GSH, GPX and non-enzymatic antioxidants (alpha-tocopherol) while suppressing the level of lipid peroxidation markers in postmenopausal women [90, 91].
5. ESTROGEN DEFICIENCY AND INFLAMMATION
Inflammation involves the coordinated action of many cell types and mediators in response to various harmful stimuli, such as damaged cells, pathogens, and toxicants, leading to the elimination of the insult and restoration of homeostasis [92]. Interleukins (ILs) are the most well-profiled inflammation markers in diseases. Some of the examples include IL-6, a proinflammatory cytokine produced in response to tissue injury [93], tumour necrosis factor-alpha (TNF-α), an important mediator of the inflammation [94] and interferon-gamma (IFN-γ), produced by activated T lymphocytes in response to inflammation [95]. Intracellular signalling pathways, including MAPK, NF-κB, Janus kinase (JAK)-signal transducer and activator of transcription (STAT) are involved in the regulation of inflammation in disease state [96, 97]. Toll-like receptors (TLR) mediated immune response through the induction of MMPs or inhibiting the expression of certain structural proteins. Animal studies showed that inhibition of TLR4 signalling is a pharmacological avenue for retarding the progression of osteoporosis [98].
In animals, T cells harvested from OVX mice produced insufficient TNF-α to induce RANKL-independent osteoclastic formation, but sufficient to increase osteoclastogenesis caused by M-CSF and RANKL through the engagement of TNF-α receptor p55 [99]. Reports showed that 17β–estradiol caused a 1.7 to 3.2-fold increase in osteoclast apoptotic proportion [100]. Incubation of normal human osteoblastic-like cells with 17β–oestradiol revealed a significant increase in tumour growth factor-beta (TGF-β) level after 24 hours [101]. The effects of TGF-β on osteoclasts suggest the involvement of estrogen in the direct inhibition of osteoclast resorption activity [102]. A study also showed that estrogen hastened the resolution of inflammation in RAW 264.7 cells through SOCS3 and STAT3 signalling pathways [103]. Thus, this could be one of the mechanisms estrogen prevents chronic inflammation in the body.
In vivo studies indicated that estrogen deficiency caused a significant increment in serum TNF-α and IL-6 levels in OVX animals compared to the sham group [104-106]. Ovariectomy also caused increased expression of cell adhesion molecules in blood vessels and circulating proinflammatory cytokines in rats [107, 108]. Since estrogen deprivation is associated with an increase in cytokine level, administration of estrogen is expected to decrease cytokine level. Estrogen administration significantly reduced IL-6, TNF-α and IL-1β expression while increasing IL-10 level in OVX rats [79]. OVX also enhanced inflammation at the visceral adipose tissue, as evidenced by reduced IL-10 level (an anti-inflammatory cytokine) and increased TNF-α level [109]. Estrogen replacement improved the inflammatory status of OVX rats by decreasing the TNF-α concentration level by 18% [109].
The pro-inflammatory effects of estrogen deficiency are mediated by multiple mechanisms. A study by Xu et al. showed that ovariectomy caused neuroinflammation by significantly increasing TLR-2 and TLR-4, active NF-κB, pro-IL-1β and pro-IL-18 level in the hippocampus of rats [110]. OVX and ER-α knockout mice demonstrated the deregulation of TLR2 signalling in the heart, resulting in a 5.7-fold increase in IL-6 and a 4.7-fold increase in phospho-Stat3 levels. This observation suggests an over-activation of the JAK/STAT3 pathway [111]. ArKO mice suffering from estrogen deficiency expressed significantly higher serum IL-6, TNF, MCP-1 and IFN-γ induced by LPS. These changes were significantly abrogated by the administration of selective agonists of ER-α [112]. TNF-overexpressing transgenic mice showed a dramatic loss of metaphyseal trabecular bone mass marked by significant decreases in both Tb.N and Tb.Th and cortical thickness bone compared to wild type (WT). These skeletal alternations corresponded to higher gene expression of TNF, IL-1β and RANKL in the transgenic mice compared to the WT [113]. In contrast, chronic E2 administration in OVX mice markedly increased the expression of IL-1β, IL-6 and IL-12p40 by lipopolysaccharides-stimulated resident peritoneal macrophages in vivo. This effect was attributed to inhibition of phosphoinositide 3-kinase (PI3K) pathway, which acts as a negative regulator to TLR4 signalling [114].
A large body of evidence suggests estrogen deficiency in postmenopausal women is related to an altered immune profile. Hot flash commonly experienced by postmenopausal women was associated with low-grade systemic inflammation indicated by a higher level of circulating IL-8 and TNF-α [115]. Modest weight gain among postmenopausal women was associated with a pro-inflammatory state indicated by increased intercellular adhesion molecule-1 (ICAM-1) and TNF-α [116]. Postmenopausal women free from any pro-inflammatory conditions had higher levels of IL-1, IL-6, and TNF-α compared to premenopausal women [117]. High levels of cytokines (IFN-α2, IFN-γ, IL-12p70, IL-33) and MCP-1 in apparently healthy postmenopausal women were associated with a decrease in hip BMD [118]. In vitro studies showed that TNF-α promotes RANKL-induced osteoclast formation through activation of PI3K/ protein kinase B (Akt) signalling, which ultimately contributes to bone loss in postmenopausal women who possessed an increased level of TNF-α [119].
CONCLUSION
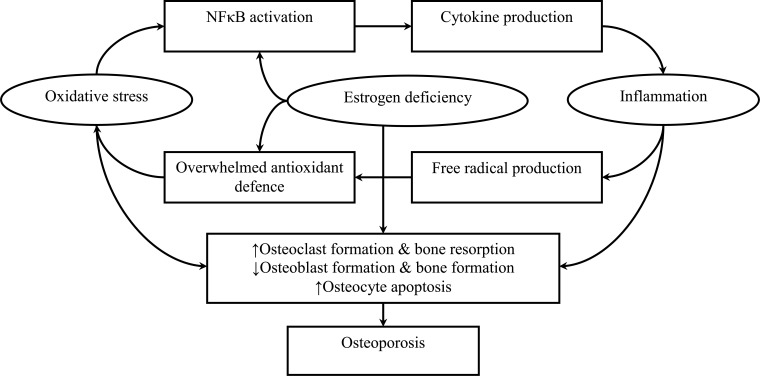
The current literature supports the multifaceted role of estrogen in preserving bone via direct binding to ERα primarily and ERβ, regulating redox status and inflammation. Estrogen deprivation due to menopause brings physiological challenges to women as the skeletal protective effects of estrogen are lost. The upswing in the ROS level and the release of pro-inflammatory cytokines adversely affect the survival and activity of osteoblasts but promote the formation and activity osteoclasts (Fig. 1). The corresponding unfavourable changes in bone remodelling skewing towards bone resorption, structural and mineral alternations are the main factors of postmenopausal osteoporosis. These mechanisms are potential avenues for interventions. For instance, phytoestrogens with antioxidants and anti-inflammatory activities may be used to prevent further bone loss of women, in conjunction with sufficient calcium and vitamin intake, as well as other lifestyle interventions. These approaches, however, should not supplant proper pharmacological agents for osteoporosis if the patients are at high risk of fracture. The use of hormone replacement therapy (HRT) is a rational approach to counter osteoporosis induced by estrogen deficiency [120, 121]. Estrogen replacement therapy is the Food and Drug Administration (FDA)-approved treatment to prevent osteoporosis in postmenopausal women. Studies showed that HRT can preserve BMD at all skeletal sites in postmenopausal women [122, 123]. The controversial Women's Health Initiative raised safety concerns of HRT, whereby an association between HRT and cardiovascular diseases and breast cancer was reported [124, 125]. Nonetheless, the data have been re-analysed and it was revealed that the HRT is effective and appropriate to prevent osteoporosis related-fracture [126, 127]. Even so, the choice of different approaches to rehabilitation and therapy still needs to be considered based on treatment feasibility, patient risk, and treatment cost-effectiveness.
Fig. (1).
The effects of estrogens deficiency on bone loss through oxidative stress and inflammation.
ACKNOWLEDGEMENTS
The authors thank The National University of Malaysia and the Ministry of Education, Malaysia, for funding the study via GUP-2017-012.
Funding Statement
This study was supported by The National University of Malaysia, Kuala Lampur, Malaysia (Grant No: GUP-2017- 012).
CONSENT FOR PUBLICATION
Not applicable.
Funding
This study was supported by The National University of Malaysia, Kuala Lampur, Malaysia (Grant No: GUP-2017-012).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Gallagher J.C., Goldgar D., Moy A. Total bone calcium in normal women: effect of age and menopause status. J. Bone Miner. Res. 1987;2(6):491–496. doi: 10.1002/jbmr.5650020605. [DOI] [PubMed] [Google Scholar]
- 2.Gauthier A., Kanis J.A., Jiang Y., Martin M., Compston J.E., Borgström F., Cooper C., McCloskey E.V. Epidemiological burden of postmenopausal osteoporosis in the UK from 2010 to 2021: estimations from a disease model. Arch. Osteoporos. 2011;6(1-2):179–188. doi: 10.1007/s11657-011-0063-y. [DOI] [PubMed] [Google Scholar]
- 3.Simpson E.R. Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol. 2003;86(3-5):225–230. doi: 10.1016/S0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 4.Riggs B.L., Melton L.J., III The prevention and treatment of osteoporosis. N. Engl. J. Med. 1992;327(9):620–627. doi: 10.1056/NEJM199208273270908. [DOI] [PubMed] [Google Scholar]
- 5.Vrtačnik P., Ostanek B., Mencej-Bedrač S., Marc J. The many faces of estrogen signaling. Biochem. Med. (Zagreb) 2014;24(3):329–342. doi: 10.11613/BM.2014.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madeddu C., Gramignano G., Floris C., Murenu G., Sollai G., Macciò A. Role of inflammation and oxidative stress in post-menopausal oestrogen-dependent breast cancer. J. Cell. Mol. Med. 2014;18(12):2519–2529. doi: 10.1111/jcmm.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Signorelli S.S., Neri S., Sciacchitano S., Pino L.D., Costa M.P., Marchese G., Celotta G., Cassibba N., Pennisi G., Caschetto S. Behaviour of some indicators of oxidative stress in postmenopausal and fertile women. Maturitas. 2006;53(1):77–82. doi: 10.1016/j.maturitas.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 8.McLean R.R. Proinflammatory cytokines and osteoporosis. Curr. Osteoporos. Rep. 2009;7(4):134–139. doi: 10.1007/s11914-009-0023-2. [DOI] [PubMed] [Google Scholar]
- 9.Cervellati C., Bonaccorsi G., Cremonini E., Bergamini C.M., Patella A., Castaldini C., Ferrazzini S., Capatti A., Picarelli V., Pansini F.S., Massari L. Bone mass density selectively correlates with serum markers of oxidative damage in post-menopausal women. Clin. Chem. Lab. Med. 2013;51(2):333–338. doi: 10.1515/cclm-2012-0095. [DOI] [PubMed] [Google Scholar]
- 10.Simpson E.R., Clyne C., Rubin G., Boon W.C., Robertson K., Britt K., Speed C., Jones M. Aromatase--a brief overview. Annu. Rev. Physiol. 2002;64(1):93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 11.Mauvais-Jarvis F., Clegg D.J., Hevener A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013;34(3):309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faulds M.H., Zhao C., Dahlman-Wright K., Gustafsson J-Å. The diversity of sex steroid action: Regulation of metabolism by estrogen signaling. J. Endocrinol. 2012;212(1):3–12. doi: 10.1530/JOE-11-0044. [DOI] [PubMed] [Google Scholar]
- 13.Green S., Kumar V., Krust A., Walter P., Chambon P. 1986. [DOI] [PubMed] [Google Scholar]
- 14.Kuiper G.G., Enmark E., Pelto-Huikko M., Nilsson S., Gustafsson J-A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stice J.P., Knowlton A.A. Estrogen, NFkappaB, and the heat shock response. Mol. Med. 2008;14(7-8):517–527. doi: 10.2119/2008-00026.Stice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuiper G.G., Carlsson B., Grandien K., Enmark E., Häggblad J., Nilsson S., Gustafsson J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 17.Björnström L., Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 2005;19(4):833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 18.Börjesson A.E., Lagerquist M.K., Windahl S.H., Ohlsson C. The role of estrogen receptor α in the regulation of bone and growth plate cartilage. Cell. Mol. Life Sci. 2013;70(21):4023–4037. doi: 10.1007/s00018-013-1317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bord S., Horner A., Beavan S., Compston J. Estrogen receptors α and β are differentially expressed in developing human bone. J. Clin. Endocrinol. Metab. 2001;86(5):2309–2314. doi: 10.1210/jc.86.5.2309. [DOI] [PubMed] [Google Scholar]
- 20.Braidman I.P., Hainey L., Batra G., Selby P.L., Saunders P.T., Hoyland J.A. Localization of estrogen receptor β protein expression in adult human bone. J. Bone Miner. Res. 2001;16(2):214–220. doi: 10.1359/jbmr.2001.16.2.214. [DOI] [PubMed] [Google Scholar]
- 21.Crusodé de Souza M., Sasso-Cerri E., Cerri P.S. Immunohistochemical detection of estrogen receptor beta in alveolar bone cells of estradiol-treated female rats: possible direct action of estrogen on osteoclast life span. J. Anat. 2009;215(6):673–681. doi: 10.1111/j.1469-7580.2009.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015;294(2):63–69. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Härkönen P.L., Väänänen H.K. Monocyte-macrophage system as a target for estrogen and selective estrogen receptor modulators. Ann. N. Y. Acad. Sci. 2006;1089(1):218–227. doi: 10.1196/annals.1386.045. [DOI] [PubMed] [Google Scholar]
- 24.Karsenty G., Wagner E.F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell. 2002;2(4):389–406. doi: 10.1016/S1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 25.Hadjidakis D.J., Androulakis I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006;1092:385–396. doi: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- 26.Väänänen H.K., Härkönen P.L. Estrogen and bone metabolism. Maturitas. 1996;(Suppl. 23):S65–S69. doi: 10.1016/0378-5122(96)01015-8. [DOI] [PubMed] [Google Scholar]
- 27.Florencio-Silva R., Sasso G.R.S., Sasso-Cerri E., Simões M.J., Cerri P.S. Effects of estrogen status in osteocyte autophagy and its relation to osteocyte viability in alveolar process of ovariectomized rats. Biomed. Pharmacother. 2018;98:406–415. doi: 10.1016/j.biopha.2017.12.089. [DOI] [PubMed] [Google Scholar]
- 28.Garnero P., Sornay-Rendu E., Chapuy M.C., Delmas P.D. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J. Bone Miner. Res. 1996;11(3):337–349. doi: 10.1002/jbmr.5650110307. [DOI] [PubMed] [Google Scholar]
- 29.Manolagas S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000;21(2):115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 30.Kousteni S., Chen J.R., Bellido T., Han L., Ali A.A., O’Brien C.A., Plotkin L., Fu Q., Mancino A.T., Wen Y., Vertino A.M., Powers C.C., Stewart S.A., Ebert R., Parfitt A.M., Weinstein R.S., Jilka R.L., Manolagas S.C. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298(5594):843–846. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- 31.Khosla S., Atkinson E.J., Dunstan C.R., O’Fallon W.M. Effect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly men. J. Clin. Endocrinol. Metab. 2002;87(4):1550–1554. doi: 10.1210/jcem.87.4.8397. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi N., Udagawa N., Akatsu T., Tanaka H., Isogai Y., Suda T. Deficiency of osteoclasts in osteopetrotic mice is due to a defect in the local microenvironment provided by osteoblastic cells. Endocrinology. 1991;128(4):1792–1796. doi: 10.1210/endo-128-4-1792. [DOI] [PubMed] [Google Scholar]
- 33.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 34.Hofbauer L.C., Khosla S., Dunstan C.R., Lacey D.L., Boyle W.J., Riggs B.L. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J. Bone Miner. Res. 2000;15(1):2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 35.Shevde N.K., Bendixen A.C., Dienger K.M., Pike J.W. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc. Natl. Acad. Sci. USA. 2000;97(14):7829–7834. doi: 10.1073/pnas.130200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bord S., Ireland D.C., Beavan S.R., Compston J.E. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone. 2003;32(2):136–141. doi: 10.1016/S8756-3282(02)00953-5. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura T., Imai Y., Matsumoto T., Sato S., Takeuchi K., Igarashi K., Harada Y., Azuma Y., Krust A., Yamamoto Y., Nishina H., Takeda S., Takayanagi H., Metzger D., Kanno J., Takaoka K., Martin T.J., Chambon P., Kato S. Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell. 2007;130(5):811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 38.Melville K.M., Kelly N.H., Khan S.A., Schimenti J.C., Ross F.P., Main R.P., van der Meulen M.C. Female mice lacking estrogen receptor-alpha in osteoblasts have compromised bone mass and strength. J. Bone Miner. Res. 2014;29(2):370–379. doi: 10.1002/jbmr.2082. [DOI] [PubMed] [Google Scholar]
- 39.Hart N.H., Nimphius S., Rantalainen T., Ireland A., Siafarikas A., Newton R.U. Mechanical basis of bone strength: Influence of bone material, bone structure and muscle action. J. Musculoskelet. Neuronal Interact. 2017;17(3):114–139. [PMC free article] [PubMed] [Google Scholar]
- 40.Deng Y-T., Kang W-B., Zhao J-N., Liu G., Zhao M-G. Osteoprotective effect of echinocystic acid, a triterpone component from eclipta prostrata, in ovariectomy-induced osteoporotic rats. PLoS One. 2015;10(8):e0136572–e0136572. doi: 10.1371/journal.pone.0136572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parvaneh K., Ebrahimi M., Sabran M.R., Karimi G., Hwei A.N., Abdul-Majeed S., Ahmad Z., Ibrahim Z., Jamaluddin R. Probiotics (Bifidobacterium longum) Increase Bone Mass Density and Upregulate Sparc and Bmp-2 Genes in Rats with Bone Loss Resulting from Ovariectomy. BioMed Res. Int. 2015;2015:897639. doi: 10.1155/2015/897639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernández-Murga M.L., Vinué Á., Caeiro J.R., Guede D., Tarín J.J., Andrés V., Cano A. Impact of estrogens on atherosclerosis and bone in the apolipoprotein E-deficient mouse model. Menopause. 2015;22(4):428–436. doi: 10.1097/GME.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 43.Du Z., Steck R., Doan N., Woodruff M.A., Ivanovski S., Xiao Y. Estrogen Deficiency-Associated Bone Loss in the Maxilla: A methodology to quantify the changes in the maxillary intra-radicular alveolar bone in an ovariectomized rat osteoporosis model. Tissue Eng. Part C Methods. 2015;21(5):458–466. doi: 10.1089/ten.tec.2014.0268. [DOI] [PubMed] [Google Scholar]
- 44.Zaid S.S.M., Sulaiman S.A., Othman N.H., Soelaiman I-N., Shuid A.N., Mohamad N., Muhamad N. Protective effects of Tualang honey on bone structure in experimental postmenopausal rats. Clinics (São Paulo) 2012;67(7):779–784. doi: 10.6061/clinics/2012(07)13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fathilah S.N., Nazrun Shuid A., Mohamed N., Muhammad N., Nirwana Soelaiman I. Labisia pumila protects the bone of estrogen-deficient rat model: A histomorphometric study. J. Ethnopharmacol. 2012;142(1):294–299. doi: 10.1016/j.jep.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 46.Mohamed N., Gwee Sian Khee S., Shuid A.N., Muhammad N., Suhaimi F., Othman F., Babji A.S., Soelaiman I.-N. 2012. [DOI] [PMC free article] [PubMed]
- 47.Liu Z., Liu L., Kang C., Xie Q., Zhang B., Li Y. Effects of estrogen deficiency on microstructural changes in rat alveolar bone proper and periodontal ligament. Mol. Med. Rep. 2015;12(3):3508–3514. doi: 10.3892/mmr.2015.3891. [DOI] [PubMed] [Google Scholar]
- 48.Ahmad N., Chillara R., Kushwaha P., Khedgikar V., Karvande A., Choudhary D., Adhikary S., Maurya R., Trivedi R. Evaluation of anti-osteoporotic activity of butanolic fraction from Passiflora foetida in ovariectomy-induced bone loss in mice. Biomed. Pharmacother. 2017;88:804–813. doi: 10.1016/j.biopha.2017.01.100. [DOI] [PubMed] [Google Scholar]
- 49.Wen X.X., Xu C., Wang F.Q., Feng Y.F., Zhao X., Yan Y.B., Lei W. Temporal changes of microarchitectural and mechanical parameters of cancellous bone in the osteoporotic rabbit. BioMed Res. Int. 2015;2015:263434. doi: 10.1155/2015/263434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aktifanus A.T., Shuid A.N., Rashid N.H.A., Ling T.H., Loong C.Y., Saat N.M., Muhammad N., Mohamed N., Soelaiman I.N. Comparison of the effects of tocotrienol and estrogen on the bone markers and dynamic changes in postmenopausal osteoporosis rat model. Asian J. Anim. Vet. Adv. 2012;7(3):225–234. doi: 10.3923/ajava.2012.225.234. [DOI] [Google Scholar]
- 51.Abdul-Majeed S., Mohamed N., Soelaiman I-N. Effects of tocotrienol and lovastatin combination on osteoblast and osteoclast activity in estrogen-deficient osteoporosis. Evid. Based Complement. Alternat. Med. 2012;2012:960742. doi: 10.1155/2012/960742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isomura H., Fujie K., Shibata K., Inoue N., Iizuka T., Takebe G., Takahashi K., Nishihira J., Izumi H., Sakamoto W. Bone metabolism and oxidative stress in postmenopausal rats with iron overload. Toxicology. 2004;197(2):93–100. doi: 10.1016/j.tox.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 53.Wheater G., Elshahaly M., Tuck S.P., Datta H.K., van Laar J.M. The clinical utility of bone marker measurements in osteoporosis. J. Transl. Med. 2013;11:201–201. doi: 10.1186/1479-5876-11-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu H., Li W., Jia S., Li B. Puerarin and zinc additively prevent mandibular bone loss through inhibiting osteoclastogenesis in ovariectomized rats. Histol. Histopathol. 2017;32(8):851–860. doi: 10.14670/HH-11-855. [DOI] [PubMed] [Google Scholar]
- 55.Christenson E.S., Jiang X., Kagan R., Schnatz P. Osteoporosis management in post-menopausal women. Minerva Ginecol. 2012;64(3):181–194. [PubMed] [Google Scholar]
- 56.Cadarette S.M., McIsaac W.J., Hawker G.A., Jaakkimainen L., Culbert A., Zarifa G., Ola E., Jaglal S.B. The validity of decision rules for selecting women with primary osteoporosis for bone mineral density testing. Osteoporos. Int. 2004;15(5):361–366. doi: 10.1007/s00198-003-1552-7. [DOI] [PubMed] [Google Scholar]
- 57.Tian L., Yang R., Wei L., Liu J., Yang Y., Shao F., Ma W., Li T., Wang Y., Guo T. Prevalence of osteoporosis and related lifestyle and metabolic factors of postmenopausal women and elderly men: A cross-sectional study in Gansu province, Northwestern of China. Medicine (Baltimore) 2017;96(43):e8294–e8294. doi: 10.1097/MD.0000000000008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kazi M., Abullah F., Abbas Q., Bawany S. Assessment of Osteoporosis and Osteopenia amongst Menopausal Women of North Nazimabad, Karachi, Pakistan. Sindh University Research Journal-SURJ. 2019;51(01):147–150. doi: 10.26692/sujo/2019.01.26. [DOI] [Google Scholar]
- 59.Chan C.Y., Subramaniam S., Mohamed N., Ima-Nirwana S., Muhammad N., Fairus A., Ng P.Y., Jamil N.A., Abd Aziz N., Chin K-Y. Determinants of Bone Health Status in a Multi-Ethnic Population in Klang Valley, Malaysia. Int. J. Environ. Res. Public Health. 2020;17(2):E384. doi: 10.3390/ijerph17020384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Subramaniam S., Chan C-Y., Soelaiman I-N., Mohamed N., Muhammad N., Ahmad F., Abd Manaf M.R., Ng P-Y., Jamil N.A., Chin K-Y. Prevalence and Predictors of Osteoporosis Among the Chinese Population in Klang Valley, Malaysia. Appl. Sci. (Basel) 2019;9(9):1820. doi: 10.3390/app9091820. [DOI] [Google Scholar]
- 61.Mederle O.A., Balas M., Ioanoviciu S.D., Gurban C-V., Tudor A., Borza C. Correlations between bone turnover markers, serum magnesium and bone mass density in postmenopausal osteoporosis. Clin. Interv. Aging. 2018;13:1383–1389. doi: 10.2147/CIA.S170111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khanizadeh F., Rahmani A., Asadollahi K., Ahmadi M.R.H. Combination therapy of curcumin and alendronate modulates bone turnover markers and enhances bone mineral density in postmenopausal women with osteoporosis. Arch. Endocrinol. Metab. 2018;62(4):438–445. doi: 10.20945/2359-3997000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thompson D.M., Lee H.M., Stoner J.A., Golub L.M., Nummikoski P.V., Payne J.B. Loss of alveolar bone density in postmenopausal, osteopenic women is associated with circulating levels of gelatinases. J. Periodontal Res. 2019;54(5):525–532. doi: 10.1111/jre.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gutierrez-Buey G., Restituto P., Botella S., Monreal I., Colina I., Rodríguez-Fraile M., Calleja A., Varo N. Trabecular bone score and bone remodelling markers identify perimenopausal women at high risk of bone loss. Clin. Endocrinol. (Oxf.) 2019;91(3):391–399. doi: 10.1111/cen.14042. [DOI] [PubMed] [Google Scholar]
- 65.Gutteridge J.M.C., Halliwell B. Mini-Review: Oxidative stress, redox stress or redox success? Biochem. Biophys. Res. Commun. 2018;502(2):183–186. doi: 10.1016/j.bbrc.2018.05.045. [DOI] [PubMed] [Google Scholar]
- 66.Son Y., Kim S., Chung H-T. Methods. Enzymol. Vol. 528. Elsevier; 2013. pp. 27–48. [DOI] [PubMed] [Google Scholar]
- 67.Newsholme P., Cruzat V.F., Keane K.N., Carlessi R., de Bittencourt P.I.H. Jr Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016;473(24):4527–4550. doi: 10.1042/BCJ20160503C. [DOI] [PubMed] [Google Scholar]
- 68.Romagnoli C., Marcucci G., Favilli F., Zonefrati R., Mavilia C., Galli G., Tanini A., Iantomasi T., Brandi M.L., Vincenzini M.T. Role of GSH/GSSG redox couple in osteogenic activity and osteoclastogenic markers of human osteoblast-like SaOS-2 cells. FEBS J. 2013;280(3):867–879. doi: 10.1111/febs.12075. [DOI] [PubMed] [Google Scholar]
- 69.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016;1863(12):2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 71.Tew K.D., Townsend D.M. Glutathione-s-transferases as determinants of cell survival and death. Antioxid. Redox Signal. 2012;17(12):1728–1737. doi: 10.1089/ars.2012.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laborde E. Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ. 2010;17(9):1373–1380. doi: 10.1038/cdd.2010.80. [DOI] [PubMed] [Google Scholar]
- 73.Baek K.H., Oh K.W., Lee W.Y., Lee S.S., Kim M.K., Kwon H.S., Rhee E.J., Han J.H., Song K.H., Cha B.Y., Lee K.W., Kang M.I. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif. Tissue Int. 2010;87(3):226–235. doi: 10.1007/s00223-010-9393-9. [DOI] [PubMed] [Google Scholar]
- 74.Lean J.M., Jagger C.J., Kirstein B., Fuller K., Chambers T.J. Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology. 2005;146(2):728–735. doi: 10.1210/en.2004-1021. [DOI] [PubMed] [Google Scholar]
- 75.Domazetovic V., Fontani F., Marcucci G., Iantomasi T., Brandi M.L., Vincenzini M.T. Estrogen inhibits starvation-induced apoptosis in osteocytes by a redox-independent process involving association of JNK and glutathione S-transferase P1-1. FEBS Open Bio. 2017;7(5):705–718. doi: 10.1002/2211-5463.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El Wakf A.M., Hassan H.A., Gharib N.S. Osteoprotective effect of soybean and sesame oils in ovariectomized rats via estrogen-like mechanism. Cytotechnology. 2014;66(2):335–343. doi: 10.1007/s10616-013-9580-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Effendy N.M., Shuid A.N. Time and dose-dependent effects of Labisia pumila on bone oxidative status of postmenopausal osteoporosis rat model. Nutrients. 2014;6(8):3288–3302. doi: 10.3390/nu6083288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muthusami S., Gopalakrishnan V., Stanley J.A., Krishnamoorthy S., Ilangovan R., Gopalakrishnan V.K., Srinivasan N. Cissus quadrangularis prevented the ovariectomy induced oxidative stress in the femur of adult albino rats. Biomed. Pharmacother. 2016;81:416–423. doi: 10.1016/j.biopha.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 79.Kireev R.A., Tresguerres A.C., Garcia C., Borras C., Ariznavarreta C., Vara E., Vina J., Tresguerres J.A. Hormonal regulation of pro-inflammatory and lipid peroxidation processes in liver of old ovariectomized female rats. Biogerontology. 2010;11(2):229–243. doi: 10.1007/s10522-009-9242-2. [DOI] [PubMed] [Google Scholar]
- 80.Oliveira M.C., Campos-Shimada L.B., Marçal-Natali M.R., Ishii-Iwamoto E.L., Salgueiro-Pagadigorria C.L. A Long-term Estrogen Deficiency in Ovariectomized Mice is Associated with Disturbances in Fatty Acid Oxidation and Oxidative Stress. Rev. Bras. Ginecol. Obstet. 2018;40(5):251–259. doi: 10.1055/s-0038-1666856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sankar P., Zachariah B., Vickneshwaran V., Jacob S.E., Sridhar M.G. Amelioration of oxidative stress and insulin resistance by soy isoflavones (from Glycine max) in ovariectomized Wistar rats fed with high fat diet: The molecular mechanisms. Exp. Gerontol. 2015;63:67–75. doi: 10.1016/j.exger.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 82.Shehata M., Kamel M.A. Protective effect of antioxidant adjuvant treatment with hormone replacement therapy against cardiovascular diseases in ovariectomized rats. Endocr. Regul. 2008;42(2-3):69–75. [PubMed] [Google Scholar]
- 83.He X.F., Zhang L., Zhang C.H., Zhao C.R., Li H., Zhang L.F., Tian G.F., Guo M.F., Dai Z., Sui F.G. Berberine alleviates oxidative stress in rats with osteoporosis through receptor activator of NF-kB/receptor activator of NF-kB ligand/osteoprotegerin (RANK/RANKL/OPG) pathway. Bosn. J. Basic Med. Sci. 2017;17(4):295–301. doi: 10.17305/bjbms.2017.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou X.J., Xia Y., Zhao Y.Y., Gu W.Q., Xiao X., Bai X.C., Liu J., Li M. Estradiol significantly increases the expression of antioxidant enzymes in osteoporotic rats and osteoblasts in vitro. Nan Fang Yi Ke Da Xue Xue Bao. 2018;38(4):402–408. doi: 10.3969/j.issn.1673-4254.2018.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang Y., Zheng X., Li B., Jiang S., Jiang L. Increased activity of osteocyte autophagy in ovariectomized rats and its correlation with oxidative stress status and bone loss. Biochem. Biophys. Res. Commun. 2014;451(1):86–92. doi: 10.1016/j.bbrc.2014.07.069. [DOI] [PubMed] [Google Scholar]
- 86.Giorgio M., Trinei M., Migliaccio E., Pelicci P.G. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007;8(9):722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 87.Russell S.J., Kahn C.R. Endocrine regulation of ageing. Nat. Rev. Mol. Cell Biol. 2007;8(9):681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- 88.Abdul-Rasheed O.F., Al-Shamma G.A., Zillo B.H. Serum γ-glutamyltransferase as oxidative stress marker in pre-and postmenopausal Iraqi women. Oman Med. J. 2010;25(4):286–288. doi: 10.5001/omj.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cervellati C., Bonaccorsi G., Cremonini E., Romani A., Fila E., Castaldini M.C., Ferrazzini S., Giganti M., Massari L. Oxidative stress and bone resorption interplay as a possible trigger for postmenopausal osteoporosis. BioMed Res. Int. 2014;2014:569563. doi: 10.1155/2014/569563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vural P., Akgül C., Canbaz M. Effects of menopause and tibolone on antioxidants in postmenopausal women. Ann. Clin. Biochem. 2005;42(Pt 3):220–223. doi: 10.1258/0004563053857941. [DOI] [PubMed] [Google Scholar]
- 91.Bednarek-Tupikowska G., Tworowska U., Jedrychowska I., Radomska B., Tupikowski K., Bidzinska-Speichert B., Milewicz A. Effects of oestradiol and oestroprogestin on erythrocyte antioxidative enzyme system activity in postmenopausal women. Clin. Endocrinol. (Oxf.) 2006;64(4):463–468. doi: 10.1111/j.1365-2265.2006.02494.x. [DOI] [PubMed] [Google Scholar]
- 92.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140(6):771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 93.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parameswaran N., Patial S. Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010;20(2):87–103. doi: 10.1615/CritRevEukarGeneExpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schroecksnadel K., Frick B., Winkler C., Fuchs D. Crucial role of interferon-gamma and stimulated macrophages in cardiovascular disease. Curr. Vasc. Pharmacol. 2006;4(3):205–213. doi: 10.2174/157016106777698379. [DOI] [PubMed] [Google Scholar]
- 96.Ambili R., Santhi W.S., Janam P., Nandakumar K., Pillai M.R. Expression of activated transcription factor nuclear factor-kappaB in periodontally diseased tissues. J. Periodontol. 2005;76(7):1148–1153. doi: 10.1902/jop.2005.76.7.1148. [DOI] [PubMed] [Google Scholar]
- 97.Garcia de Aquino S., Manzolli Leite F.R., Stach-Machado D.R., Francisco da Silva J.A., Spolidorio L.C., Rossa C., Jr Signaling pathways associated with the expression of inflammatory mediators activated during the course of two models of experimental periodontitis. Life Sci. 2009;84(21-22):745–754. doi: 10.1016/j.lfs.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 98.Vijayan V., Khandelwal M., Manglani K., Gupta S., Surolia A. Methionine down-regulates TLR4/MyD88/NF-κB signalling in osteoclast precursors to reduce bone loss during osteoporosis. Br. J. Pharmacol. 2014;171(1):107–121. doi: 10.1111/bph.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cenci S., Weitzmann M.N., Roggia C., Namba N., Novack D., Woodring J., Pacifici R. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J. Clin. Invest. 2000;106(10):1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hughes D.E., Dai A., Tiffee J.C., Li H.H., Mundy G.R., Boyce B.F. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat. Med. 1996;2(10):1132–1136. doi: 10.1038/nm1096-1132. [DOI] [PubMed] [Google Scholar]
- 101.Oursler M.J., Cortese C., Keeting P., Anderson M.A., Bonde S.K., Riggs B.L., Spelsberg T.C. Modulation of transforming growth factor-beta production in normal human osteoblast-like cells by 17 beta-estradiol and parathyroid hormone. Endocrinology. 1991;129(6):3313–3320. doi: 10.1210/endo-129-6-3313. [DOI] [PubMed] [Google Scholar]
- 102.Pfeilschifter J., Seyedin S.M., Mundy G.R. Transforming growth factor beta inhibits bone resorption in fetal rat long bone cultures. J. Clin. Invest. 1988;82(2):680–685. doi: 10.1172/JCI113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Villa A., Rizzi N., Vegeto E., Ciana P., Maggi A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci. Rep. 2015;5:15224. doi: 10.1038/srep15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Albayrak A., Uyanik M.H., Odabasoglu F., Halici Z., Uyanik A., Bayir Y., Albayrak F., Albayrak Y., Polat B., Suleyman H. The effects of diabetes and/or polymicrobial sepsis on the status of antioxidant enzymes and pro-inflammatory cytokines on heart, liver, and lung of ovariectomized rats. J. Surg. Res. 2011;169(1):67–75. doi: 10.1016/j.jss.2009.09.055. [DOI] [PubMed] [Google Scholar]
- 105.Delgobo M., Agnes J.P., Gonçalves R.M., Dos Santos V.W., Parisotto E.B., Zamoner A., Zanotto-Filho A. N-acetylcysteine and alpha-lipoic acid improve antioxidant defenses and decrease oxidative stress, inflammation and serum lipid levels in ovariectomized rats via estrogen-independent mechanisms. J. Nutr. Biochem. 2019;67:190–200. doi: 10.1016/j.jnutbio.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 106.Varga C., Veszelka M., Kupai K., Börzsei D., Deim Z., Szabó R., Török S., Priksz D., Gesztelyi R., Juhász B., Radák Z., Pósa A. The effects of exercise training and high triglyceride diet in an estrogen depleted rat model: The role of the heme oxygenase system and inflammatory processes in cardiovascular risk. J. Sports Sci. Med. 2018;17(4):580–588. [PMC free article] [PubMed] [Google Scholar]
- 107.Li P., Liu H., Sun P., Wang X., Wang C., Wang L., Wang T. Chronic vagus nerve stimulation attenuates vascular endothelial impairments and reduces the inflammatory profile via inhibition of the NF-κB signaling pathway in ovariectomized rats. Exp. Gerontol. 2016;74:43–55. doi: 10.1016/j.exger.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 108.Abu-Taha M., Rius C., Hermenegildo C., Noguera I., Cerda-Nicolas J.M., Issekutz A.C., Jose P.J., Cortijo J., Morcillo E.J., Sanz M.J. Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J. Immunol. 2009;183(2):1393–1402. doi: 10.4049/jimmunol.0803157. [DOI] [PubMed] [Google Scholar]
- 109.Rodrigues M.F.C., Ferreira F.C., Silva-Magosso N.S., Barbosa M.R., Souza M.V.C., Domingos M.M., Canevazzi G.H.R., Stotzer U.S., Peviani S.M., de Lira F.S., Selistre de Araújo H.S., Perez S.E.A. Effects of resistance training and estrogen replacement on adipose tissue inflammation in ovariectomized rats. Appl. Physiol. Nutr. Metab. 2017;42(6):605–612. doi: 10.1139/apnm-2016-0443. [DOI] [PubMed] [Google Scholar]
- 110.Xu Y., Sheng H., Bao Q., Wang Y., Lu J., Ni X. NLRP3 inflammasome activation mediates estrogen deficiency-induced depression- and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav. Immun. 2016;56:175–186. doi: 10.1016/j.bbi.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 111.Cordeau P., Jr, Lalancette-Hébert M., Weng Y.C., Kriz J. Estrogen receptors alpha mediates postischemic inflammation in chronically estrogen-deprived mice. Neurobiol. Aging. 2016;40:50–60. doi: 10.1016/j.neurobiolaging.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 112.Yang Y.H., Ngo D., Jones M., Simpson E., Fritzemeier K.H., Morand E.F. Endogenous estrogen regulation of inflammatory arthritis and cytokine expression in male mice, predominantly via estrogen receptor alpha. Arthritis Rheum. 2010;62(4):1017–1025. doi: 10.1002/art.27330. [DOI] [PubMed] [Google Scholar]
- 113.Moon N., Effiong L., Song L., Gardner T.R., Soung D.Y. Tart cherry prevents bone loss through inhibition of RANKL in TNF-overexpressing mice. Nutrients. 2018;11(1):63. doi: 10.3390/nu11010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Calippe B., Douin-Echinard V., Laffargue M., Laurell H., Rana-Poussine V., Pipy B., Guéry J.C., Bayard F., Arnal J.F., Gourdy P. Chronic estradiol administration in vivo promotes the proinflammatory response of macrophages to TLR4 activation: involvement of the phosphatidylinositol 3-kinase pathway. J. Immunol. 2008;180(12):7980–7988. doi: 10.4049/jimmunol.180.12.7980. [DOI] [PubMed] [Google Scholar]
- 115.Huang W.Y., Hsin I.L., Chen D.R., Chang C.C., Kor C.T., Chen T.Y., Wu H.M. Circulating interleukin-8 and tumor necrosis factor-alpha are associated with hot flashes in healthy postmenopausal women. 2017. [DOI] [PMC free article] [PubMed]
- 116.Cronin B.E., Allsopp P.J., Slevin M.M., Magee P.J., McCaffrey T.A., Livingstone M.B.E., Strain J.J., McSorley E.M. The effect of weight change over a 2-year period on inflammatory status in postmenopausal women. Eur. J. Clin. Nutr. 2018;72(3):388–393. doi: 10.1038/s41430-017-0014-9. [DOI] [PubMed] [Google Scholar]
- 117.Zhang J., Wang H., Yang S., Wang X. Comparison of lipid profiles and inflammation in pre- and post-menopausal women with cerebral infarction and the role of atorvastatin in such populations. Lipids Health Dis. 2018;17(1):20. doi: 10.1186/s12944-018-0669-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ilesanmi-Oyelere B.L., Schollum L., Kuhn-Sherlock B., McConnell M., Mros S., Coad J., Roy N.C., Kruger M.C. Inflammatory markers and bone health in postmenopausal women: A cross-sectional overview. Immun. Ageing. 2019;16:15–15. doi: 10.1186/s12979-019-0155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zha L., He L., Liang Y., Qin H., Yu B., Chang L., Xue L. TNF-α contributes to postmenopausal osteoporosis by synergistically promoting RANKL-induced osteoclast formation. Biomed. Pharmacother. 2018;102:369–374. doi: 10.1016/j.biopha.2018.03.080. [DOI] [PubMed] [Google Scholar]
- 120.Wells G., Tugwell P., Shea B., Guyatt G., Peterson J., Zytaruk N., Robinson V., Henry D., O’Connell D., Cranney A. Osteoporosis methodology group and the osteoporosis research advisory group. Meta-analyses of therapies for postmenopausal osteoporosis. V. Meta-analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal women. Endocr. Rev. 2002;23(4):529–539. doi: 10.1210/er.2001-5002. [DOI] [PubMed] [Google Scholar]
- 121.Torgerson D.J., Bell-Syer S.E. Hormone replacement therapy and prevention of nonvertebral fractures: A meta-analysis of randomized trials. JAMA. 2001;285(22):2891–2897. doi: 10.1001/jama.285.22.2891. [DOI] [PubMed] [Google Scholar]
- 122.Cauley J.A., Robbins J., Chen Z., Cummings S.R., Jackson R.D., LaCroix A.Z., LeBoff M., Lewis C.E., McGowan J., Neuner J., Pettinger M., Stefanick M.L., Wactawski-Wende J., Watts N.B., Women’s Health Initiative Investigators Effects of estrogen plus progestin on risk of fracture and bone mineral density: The Women’s Health Initiative randomized trial. JAMA. 2003;290(13):1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 123.Greenspan S.L., Beck T.J., Resnick N.M., Bhattacharya R., Parker R.A. Effect of hormone replacement, alendronate, or combination therapy on hip structural geometry: A 3-year, double-blind, placebo-controlled clinical trial. J. Bone Miner. Res. 2005;20(9):1525–1532. doi: 10.1359/JBMR.050508. [DOI] [PubMed] [Google Scholar]
- 124.Beral V., Million Women Study Collaborators Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–427. doi: 10.1016/S0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 125.Rossouw J.E., Anderson G.L., Prentice R.L., LaCroix A.Z., Kooperberg C., Stefanick M.L., Jackson R.D., Beresford S.A., Howard B.V., Johnson K.C., Kotchen J.M., Ockene J., Writing Group for the Women’s Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 126.de Villiers T.J., Pines A., Panay N., Gambacciani M., Archer D.F., Baber R.J., Davis S.R., Gompel A.A., Henderson V.W., Langer R., Lobo R.A., Plu-Bureau G., Sturdee D.W. International menopause society. Updated 2013 international menopause society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric. 2013;16(3):316–337. doi: 10.3109/13697137.2013.795683. [DOI] [PubMed] [Google Scholar]
- 127.de Villiers T.J., Gass M.L., Haines C.J., Hall J.E., Lobo R.A., Pierroz D.D., Rees M. Global consensus statement on menopausal hormone therapy. Climacteric. 2013;16(2):203–204. doi: 10.3109/13697137.2013.771520. [DOI] [PubMed] [Google Scholar]