SUMMARY
Companies have recently begun to sell a new service to patients considering in vitro fertilization: embryo selection based on polygenic scores (ESPS). These scores represent individualized predictions of health and other outcomes derived from genomewide association studies in adults to partially predict these outcomes. This article includes a discussion of many factors that lower the predictive power of polygenic scores in the context of embryo selection and quantifies these effects for a variety of clinical and nonclinical traits. Also discussed are potential unintended consequences of ESPS (including selecting for adverse traits, altering population demographics, exacerbating inequalities in society, and devaluing certain traits). Recommendations for the responsible communication about ESPS by practitioners are provided, and a call for a society-wide conversation about this technology is made. (Funded by the National Institute on Aging and others.)
Most human traits — including height and body-mass index, cognitive and behavioral traits, and the risk of many diseases — are influenced by numerous differences in genetic variants. A polygenic score summarizes the combined effects of many genetic variants on a trait and imperfectly predicts an individual’s trait. Embryos produced through in vitro fertilization can now be tested to avoid genetic disorders (e.g., Tay–Sachs disease or cystic fibrosis) and to select for children who will share their parents’ traits (e.g., deafness or dwarfism). Embryos can also be tested to select for children with human leukocyte antigens that match those of a sick sibling, enabling more successful tissue or organ transplantation, or for children of a particular sex. Some companies — including Genomic Prediction (lifeview.com), Reprocare Genetics (reprocaregenetics.com), Orchid Health (orchidhealth.com), and MyOme (myome.com) — now offer embryo selection based on polygenic scores, or ESPS. Genomic Prediction currently offers ESPS to screen for type 1 and type 2 diabetes; breast, prostate, and testicular cancer; malignant melanoma; coronary artery disease; hypercholesterolemia; hypertension; and schizophrenia.1 As recently as December 2020, the company also advertised ESPS for idiopathic short stature and intellectual disability.2,3 Orchid Health offers ESPS to screen for several of the same conditions covered by Genomic Prediction as well as for inflammatory bowel disease and Alzheimer’s disease.4 Polygenic scores can also be used to screen embryos for nonclinical phenotypes. Indeed, in addition to offering ESPS for more than 25 common medical conditions, MyOme appears to be providing patient participants with embryo polygenic scores for education, household income, cognitive ability, and subjective well-being as part of a research protocol,5 and one of the founders of Genomic Prediction has speculated about some day offering ESPS in some countries to screen for above-average cognitive ability and skin color.3
Here we describe several nuances regarding the risks and expected gains associated with ESPS that may not be obvious to patients or clinicians. We provide recommendations for responsible communication about ESPS, urge the Federal Trade Commission (FTC) to oversee information disclosure, and, given the social risks of ESPS, call for a society-wide conversation as to whether ethical or regulatory frameworks should go beyond simply providing consumers with complete and accurate information.
A POTENTIALLY MISLEADING IMPRESSION
The emerging ESPS technology draws on polygenic scores produced in genomewide association studies. For several reasons, conclusions about polygenic scores based on these studies cannot simply be extrapolated to embryos. Although our arguments apply to ESPS for all traits, we begin here with a discussion of educational attainment.
The authors of a recent paper reported that according to current polygenic scoring for educational attainment, the prevalence of college completion is roughly 10% among persons in the lowest quintile and roughly 45 to 60% among those in the highest quintile.6 Drawing on these differences, advocates have encouraged patients and clinicians to imagine a scenario in which ESPS helps parents choose between two viable embryos: one with a polygenic score in the lowest quintile and one with a score in the highest quintile, with the latter appearing to be about five times more likely to complete college than the former.7
This scenario illustrates the ways in which framing the benefits of ESPS — even on the basis of an accurate portrayal of research on polygenic scores — can be misleading. First, the scenario is unlikely. The probability that parents have exactly two viable embryos, one in the top and one in the bottom quintile of polygenic scores, is less than 3% (see the Supplementary Appendix, available with the full text of this article at NEJM.org).
Second, the scenario invites the reader to assess the effectiveness of ESPS on the basis of the expected difference in the trait in a pair of embryos with extreme polygenic scores. This difference can be large even when the predictive power of the polygenic score is small. However, it is not the relevant measure for a potential customer. The relevant measure is the “expected gain” — that is, the expected difference in the trait (of the person the embryo will become) when choosing the embryo with the highest polygenic score as compared with an embryo selected at random (from the viable embryos), without the use of ESPS.8 We illustrate below how the use of this correct measure yields a more modest estimate of the effectiveness of ESPS.
Third, the observed differences in college completion discussed above are based on a sample of persons from different families, all of whom have European ancestries. In contrast, IVF embryos share the same biologic parents, and many potential ESPS customers will not have European ancestries; both factors reduce the expected gain.
Fourth, the relevant environmental context of the children of IVF customers will generally not be the same as that of the participants in the research yielding the polygenic score. Owing to these environmental differences, the expected gain from ESPS is smaller than what one might infer from the observed differences in rates of college completion.
EXPECTED GAIN
Sharing the same two biologic parents causes the expected gain from ESPS to be smaller for two reasons. First, because every embryo’s genome is a mixture of the biologic parents’ genomes, there is less variation in polygenic scores among the set of embryos produced by the same two biologic parents than among embryos produced by different pairs of biologic parents. (The relative amount of variation is even smaller when the biologic parents’ polygenic scores are correlated due to assortative mating.) With less variation, the expected gain will be smaller. Second, a sizeable portion of the predictive power of the polygenic score for persons from different families comes from “gene–environment correlation” — that is, persons with high polygenic scores are likely to be raised in family environments that promote educational attainment.9 For example, such persons are likely to have biologic parents with high polygenic scores, and those parents are likely to place a high value on and encourage higher educational attainment, having received higher education themselves. Gene–environment correlation inflates the predictive power of the polygenic score relative to what can be expected when selecting among any two embryos that share the same biologic parents, since each would be born into a similar environment. Reducing the predictive power of the polygenic score reduces the expected gain from ESPS.
Moreover, there exist interactions between genetic variants and environmental factors. These interactions may arise directly from features of the environment (e.g., availability of inexpensive high-calorie food) or from the environmental effects on gene expression (e.g., epigenetic mechanisms). Because of these interactions, the predictive power of a polygenic score is maximized when the person is from the same environment as the research participants from whom the polygenic scores were derived. But this will never be the case in ESPS. By the time that infants born today complete their schooling, they will be one or two generations younger than the research participants and will live in different environments.
Similarly, the expected gains associated with ESPS are lower when the biologic parents have an ancestral background that is different from that of the study sample used to create the polygenic score. Almost all human genetics research to date has been conducted with research participants of European ancestries. When polygenic scores constructed from such studies have been tested on participants who were not of European ancestries, their predictive power was much lower.10
Figure 1 shows the expected differences in educational attainment between the person with the highest polygenic score among 10 persons and a person selected randomly from this group. (See the Supplementary Appendix for a calculation of these numbers with the use of a theoretical framework based on that of Karavani et al.11) If we were to naively use the level of predictive power provided by between-family estimates, we would calculate the expected difference on the basis of the assumption that each person is from the population of research participants, each of whom is from a different family. There is a difference of 1.55 years of education between the person with the highest polygenic score among the 10 participants and a person selected at random from the group. That number falls to 1.36 years when we take into account the fact that the environmental context for children of parents who used IVF is likely to be somewhat different from that for children of parents who did not (assuming a genetic correlation of 0.87 on the basis of previous work6). However, the relevant calculation of expected gain associated with ESPS is also based on the assumption that selection was made from persons in the same family. This more accurate calculation of expected gain for embryos of European ancestry is 0.53 years. For embryos of admixed American ancestries, East Asian ancestries, or African ancestries, the expected gains are 0.40, 0.35, and 0.23 years, respectively.
Figure 1. Expected Difference in Educational Attainment between the Person with the Highest Polygenic Score and a Person Selected Randomly from a Group of 10 Persons.
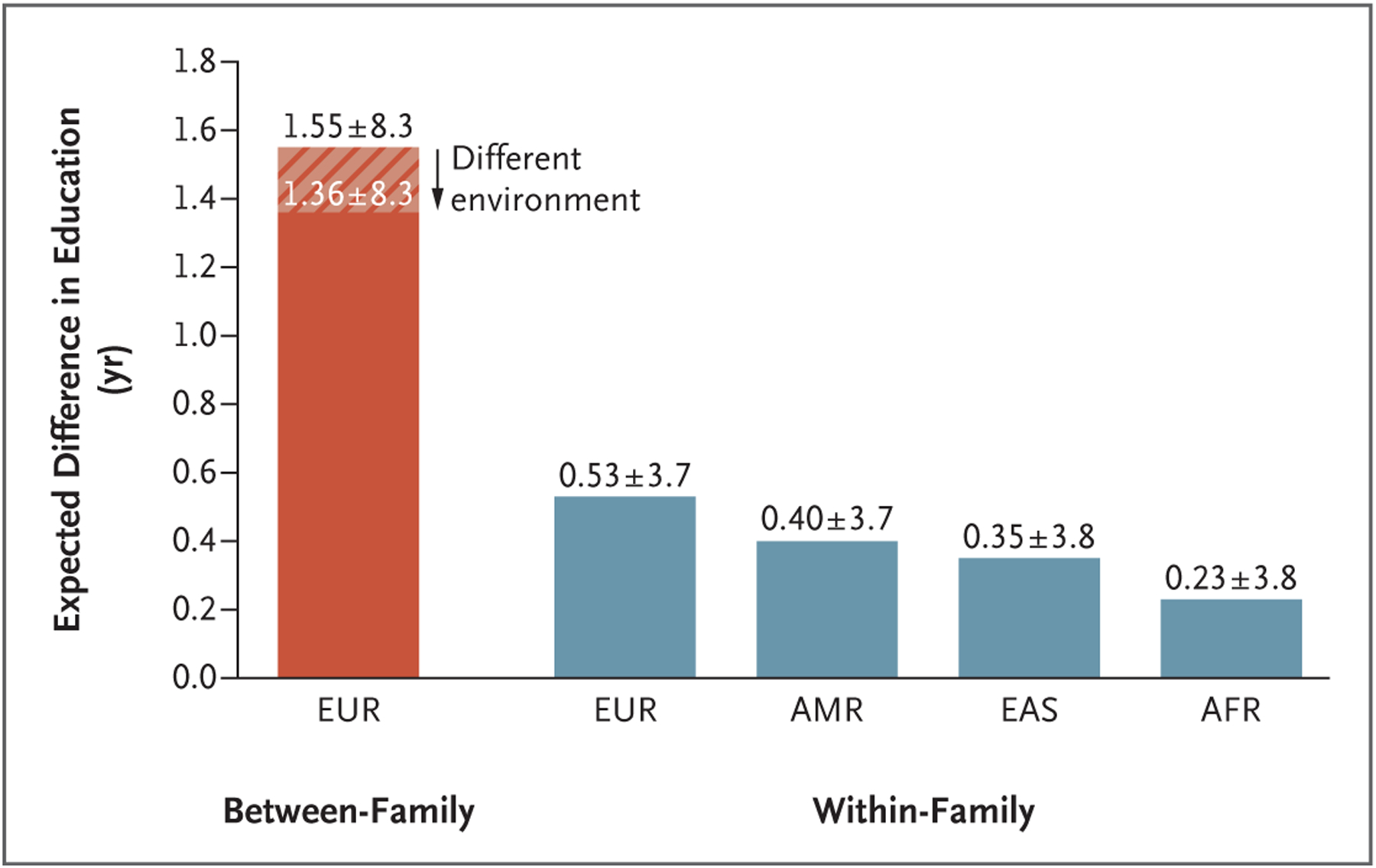
“Between-family” indicates that each person was drawn from a different family, and “within-family” indicates that each person was drawn from the same family and shares the same two biologic parents. The hatch marks indicate the assumption that the distribution of family environments is the same as that in the genomewide association studies (GWAS) from which the polygenic score was constructed. The solid red and blue bars indicate the assumption that the distribution of family environments differs from the distribution in the GWAS from which the polygenic score was constructed, with a genetic correlation across environments equal to 0.87 (see Section 7 in the Supplementary Appendix). Ancestry groups (European [EUR], admixed American [AMR], East Asian [EAS], and African [AFR]) are defined in accordance with the groupings in the 1000 Genomes Project. The smaller expected differences among persons of non-EUR ancestries are due to the fact that the GWAS were conducted with the use of EUR samples. Plus–minus values represent the 95% prediction interval of the difference in educational attainment. Assumptions err on the side of increasing expected gains and narrowing prediction intervals; therefore, these values may be considered best-case-scenario estimates. Full details of all calculations are available in the Supplementary Appendix.
UNPREDICTABLE VARIATION
There are other reasons why ESPS may not meet customers’ expectations. One particularly relevant misconception is “genetic determinism”: potential customers may expect that ESPS guarantees the outcome they want.12 Furthermore, people tend to neglect variance,13 and polygenic scores do not capture all sources of genetic variation. Thus, as shown in Figure 1, the outcome would vary greatly around the numeric prediction. For example, for those with biologic parents who are of European ancestries, the 95% prediction interval of actual gain in score ranges from +4.2 years of education to −3.2 years.
UNINTENDED CONSEQUENCES
An additional risk is pleiotropy — the tendency of genetic variants to affect multiple phenotypes. In cases of pleiotropy,14 an embryo selected on the basis of a polygenic score for one trait may also have an unusually high (or low) polygenic score for other traits that parents do not intend to target. For example, if an embryo is selected on the basis of the polygenic score for educational attainment, the risk of bipolar disorder is increased by 16% from an absolute risk of 1.0% to 1.16% (see Section 4 in the Supplementary Appendix). The vast majority of relationships between genetic variants and traits are not yet known — and we will never know all of them. Furthermore, as polygenic scores improve and reproductive technology advances, increasing the expected gains of ESPS, the magnitude of its unintended consequences may also increase.
For the pleiotropic relationships that are known, the risks may be managed to some extent. In the example of educational attainment and bipolar disorder, embryos could be selected for having a high polygenic score for educational attainment and a low polygenic score for bipolar disorder. However, the risk of bipolar disorder would not be fully mitigated, because the polygenic score for bipolar disorder is not yet as predictive as that for educational attainment. Moreover, since selection in favor of one trait and against the other means that the embryo with the highest polygenic score for educational attainment cannot necessarily be selected, addressing the risks of pleiotropy would reduce the expected gain from ESPS.
CLINICAL OUTCOMES
The same issues apply to clinical outcomes. Even those who are proponents of the use of polygenic scores in the clinic acknowledge that we are only beginning to understand their utility among adults and that research is needed to establish both clinical and personal utility.15–17 Whatever the predictive power of various polygenic scores may turn out to be for the purpose of clinical decision making, it will be lower in the within-family ESPS context. The attenuation of predictive power within families is likely to be greatest for cognitive and behavioral traits such as educational attainment because the effects of the correlation between genetics and environment and of assortative mating are likely to be greater.18 As with educational attainment, predictions will be accompanied by a high degree of uncertainty, and as a result of pleiotropy, selection for a desirable phenotype may entail the unintentional selection for traits that are undesirable.
Table 1 shows the expected reduction in risk for certain clinical outcomes if parents make their selection on the basis of the best available polygenic score. These simulation results are, by and large, similar to evidence from sibling pairs.19,20 Here, we assume that the risk without ESPS corresponds to the lifetime risk in the general U.S. population. Similar to polygenic scores for educational attainment, most existing polygenic scores are derived from genomewide association studies of persons of European ancestries. As a result, the expected reductions in risk are smaller when the biologic parents of the embryos have non-European ancestries. When the risk of a clinical outcome is low, small reductions in absolute risk can correspond to large reductions in relative risk. Consider type 1 diabetes. Our simulations imply a relative reduction in risk of 35% for biologic parents with European ancestries, but the average lifetime risk for type 1 diabetes in the U.S. population is only 0.34%, implying a risk reduction of only 0.12 percentage points.
Table 1.
Absolute and Relative Reduction in Risks of Disease and Other Conditions with ESPS.*
| Condition | Lifetime Risk in United States (%) | Absolute Risk Reduction (Preferred)† | Relative Risk Reduction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EUR | AMR | EAS | AFR | EUR | AMR | EAS | AFR | ||
| percentage points (95% confidence interval) | percent (95% confidence interval) | ||||||||
| Type 1 diabetes | 0.34 | 0.12 (0.08–0.14) | 0.09 (0.07–0.13) | 0.09 (0.06–0.11) | 0.07 (0.05–0.07) | 35 (24–42) | 27 (21–38) | 25 (19–31) | 19 (14–21) |
| Type 2 diabetes | 35.3 | 5.5 (3.9–7.1) | 4.4 (3.2–5.6) | 3.9 (2.8–5.0) | 2.6 (1.9–3.3) | 16 (11–20) | 12 (9–16) | 11 (8–14) | 7 (5–9) |
| Breast cancer (women) | 12.9 | 1.9 (1.1–2.7) | 1.5 (0.92–2.2) | 1.3 (0.77–1.9) | 0.91 (0.52–1.3) | 15 (9–21) | 11 (7–17) | 10 (6–15) | 7 (4–10) |
| Prostate cancer | 12.1 | 4.0 (2.5–5.6) | 3.2 (2.0–4.5) | 2.9 (1.8–3.9) | 1.9 (1.2–2.7) | 33 (21–46) | 26 (17–37) | 24 (15–32) | 16 (10–22) |
| Malignant melanoma | 2.3 | 0.50 (0.44–0.55) | 0.40 (0.32–0.46) | 0.33 (0.29–0.42) | 0.23 (0.18–0.33) | 22 (19–24) | 18 (14–20) | 15 (13–19) | 10 (8–14) |
| Testicular cancer | 0.41 | 0.14 (0.11–0.15) | 0.10 (0.09–0.12) | 0.09 (0.08–0.12) | 0.07 (0.06–0.08) | 35 (26–37) | 25 (21–30) | 23 (19–28) | 17 (15–20) |
| Coronary artery disease | 6.7 | 1.1 (0.53–1.7) | 0.89 (0.39–1.4) | 0.79 (0.38–1.2) | 0.55 (0.27–0.79) | 17 (8–26) | 13 (6–21) | 12 (6–18) | 8 (4–12) |
| Hypercholesterolemia | 11.7 | 3.2 (3.1–3.3) | 2.5 (2.4–2.6) | 2.3 (2.2–2.3) | 1.5 (1.5–1.6) | 27 (26–28) | 22 (21–22) | 19 (19–20) | 13 (13–13) |
| Hypertension | 46.0 | 8.5 (8.3–8.6) | 6.6 (6.5–6.7) | 5.9 (5.9–6.1) | 4.0 (3.9–4.1) | 18 (18–19) | 14 (14–14) | 13 (13–13) | 9 (9–9) |
| Idiopathic short stature | 2.3 | 1.8 (1.7–1.8) | 1.5 (1.5–1.5) | 1.3 (1.3–1.4) | 0.95 (0.95–0.98) | 77 (76–78) | 65 (64–66) | 59 (59–60) | 42 (42–43) |
| Intellectual disability | 2.3 | 0.87 (0.78–0.90) | 0.67 (0.63–0.73) | 0.60 (0.56–0.67) | 0.41 (0.38–0.45) | 38 (34–40) | 30 (28–32) | 27 (28–32) | 18 (17–20) |
Shown are the absolute and relative reductions in risk for several clinical outcomes related to embryo selection based on polygenic scores (ESPS) in different ancestry groups. The assessments are based on simulated data and estimates of the within-family predictive power of current polygenic scores. The numbers in parentheses correspond to the 95% confidence interval of the estimate of risk reduction. Calculations correspond to a set of embryos for which the lifetime risk of the clinical outcome without ESPS was equal to the lifetime risk or prevalence of the condition in the U.S. population. Details of the simulations underlying these calculations are available in the Supplementary Appendix. AFR denotes African ancestries, AMR admixed American ancestries, EAS East Asian ancestries, and EUR European ancestries. Ancestry groups are defined in accordance with the groupings in the 1000 Genomes Project (see the Supplementary Appendix).
Absolute risk reduction should be a more salient feature of ESPS reports than relative risk aversion; see the box “Recommendations for Responsible Communication of Expected Gains from ESPS.”
In addition, the reduction in risk when using ESPS will depend on the level of risk for a given phenotype among the embryos that are not selected with ESPS. This risk may be higher or lower depending on factors such as family history, cultural differences, and discrimination. Figure 2 shows the expected effect of ESPS in three illustrative phenotypes with respect to relative and absolute risk reduction. The figure shows that the greatest reduction in absolute risk occurs when the risk without ESPS is 50%, but that reduction in relative risk is largest when the risk without ESPS is smallest.
Figure 2. Absolute and Relative Reductions in Risk for Hypertension, Type 2 Diabetes, and Coronary Artery Disease According to Ancestry.
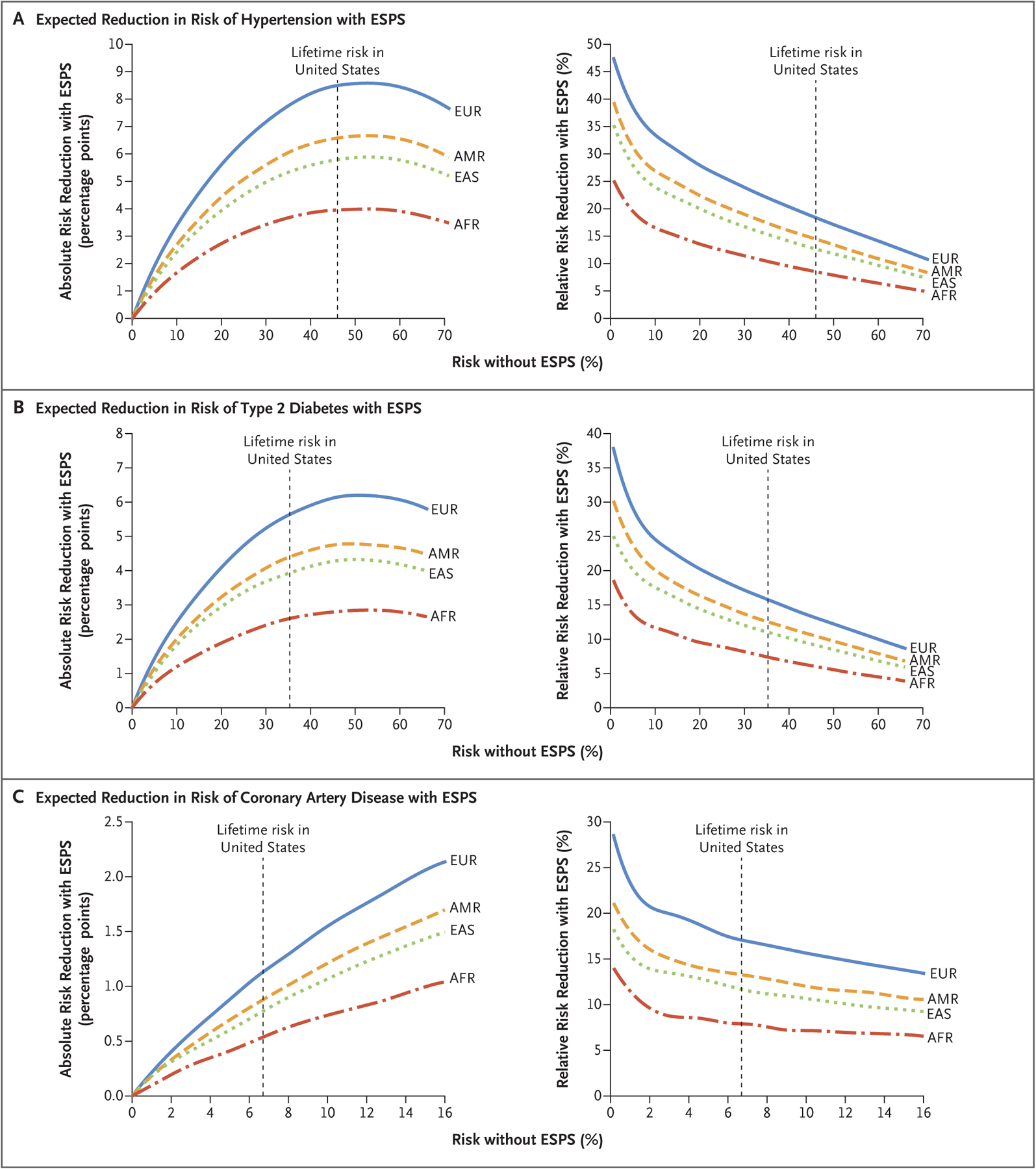
The calculations used to obtain the data provided are available in the Supplementary Appendix, where data on other clinical outcomes are also available. Risk without ESPS (embryo selection based on polygenic scores) may differ across embryos owing to family history or environmental conditions. Ancestry groups (EUR, AMR, EAS, and AFR) are defined in accordance with the groupings in the 1000 Genomes Project.
Finally, some phenotypes — including hypertension, hypercholesterolemia, and many (but not all) instances of intellectual disability — are defined using clinical cutoff points, which makes the phenotypes appear to be binary, when in fact they constitute the extreme end of a continuum. Consider idiopathic short stature, which has been defined as a height that is more than 2 standard deviations below the mean.21 A seemingly large risk reduction might not correspond to a very meaningful difference in height because ESPS might result in the selection of embryos that are just over the cutoff point. For instance, we calculate that ESPS can reduce the risk of having a child with idiopathic short stature by 1.8% as compared with 10 embryos selected at random. However, with ESPS the expected height of the eventual child would be increased by only 2.5 cm,11 an outcome that is unlikely to be practically meaningful and that in any case might surprise parents who believe they had successfully selected against short stature. Furthermore, in some instances, there is evidence that persons on the “unhealthy” side of a clinical threshold might later obtain health advantages because they would qualify for coverage of certain medical treatments, whereas their “healthy” counterparts would not.22
DISCUSSION
ESPS requires an urgent society-wide conversation. As the predictive power of polygenic scores increases and reproductive technology improves, the expected benefits of ESPS will continue to grow, whereas the clinical costs of IVF and hence ESPS will fall. Both forces may increase the market for ESPS and exacerbate its societal risks. Although ESPS, like other forms of embryo selection, has been largely used for purposes that some consider to be ethically appropriate — such as selection against genetic factors associated with morbidity or mortality — even selection along these dimensions raises issues of unequal access to technology, a concern that would probably exacerbate existing disparities in health owing to factors such as economic inequality, racism, and assortative mating. At least one company is already offering ESPS for nonclinical traits. Historical eugenic policies that sought to eliminate people deemed “feeble-minded” or otherwise socially “unfit” make embryo selection for educational attainment, income, intelligence, and related traits deeply concerning. Another very worrisome use of ESPS would be the selection of traits on the basis of social constructs of race, such as skin pigmentation, hair color, or facial features. Selection on the basis of such traits might reinforce racist conceptions of biologic superiority by signaling, either explicitly or implicitly, that certain traits carry value or stigma, possibly amplifying racial prejudice and discrimination.
Legal regulation of both human reproductive decisions and “laboratory-developed tests,” including IVF “add-ons,” such as preimplantation genetic testing for aneuploidy, is highly complex, as are the related ethical concerns.23–28 In the United States, there is a strong legal (indeed, constitutional) and ethical tradition of viewing reproductive decisions — whether or not to have children, with whom, and how — as matters of private individual choice. Yet the aggregation of many individual reproductive decisions over successive generations can have profound societal consequences, such as altering population demographics,29 exacerbating inequalities, and devaluing certain traits.
However, the fact that there are legal and cultural challenges involved in prohibiting ESPS in the near term does not mean that no legal tools exist to curb misunderstanding and misuse of this emerging technology. Companies offering ESPS are legally (and ethically) required to avoid misrepresentations or omissions that are likely to mislead consumers and are material to the decision to use a service. The FTC Act (15 U.S.C. §§ 45, 52–55) gives the FTC the authority to prohibit unfair or deceptive acts or practices as well as the dissemination of misleading claims about services. The FTC should help establish what counts as adequate evidence to support claims about the expected gains of ESPS and what counts as adequate information disclosure in this context, as it did in the 1990s and 2000s in curbing the practice of IVF clinics that provided misleading rates of successful pregnancies.30
In the meantime, professional medical societies should develop policies and guidance in this space, and companies themselves should demonstrate that the information they provide to customers is complete, accurate, and well understood before they offer ESPS services. This is a tall order. Predicting a child’s probable characteristics with the use of embryo screening is scientifically complex. Any one of the issues discussed in this article would be difficult to communicate accurately — even to other scientists and clinicians; collectively, these issues constitute a formidable challenge for ESPS companies, which must ensure that their customers understand what they are doing. Some of the existing literature on the effective communication of risk and uncertainty31 (see box) offers initial recommendations for responsibly communicating the expected gains of ESPS to diverse consumers. Because decisions regarding the use of ESPS may be made long before a formal informed-consent process takes place,44 companies offering ESPS must be scrupulous regarding the information communicated in blogs, websites, advertising materials, and media statements.
We have focused on how the gains associated with ESPS may not be as great as expected. Yet some might perceive that even those gains that can be provided are worth the risk. For instance, the expected gain of 0.23 to 0.53 years of education would have a greater effect than many environmental interventions that have been implemented.45 It seems plausible that some patients who have undergone IVF will find ESPS attractive — more so as the expected gains increase. These gains should be assessed in the context of risks. ESPS might be most attractive to those already undergoing both IVF and preimplantation genetic diagnosis (PGD) for other reasons. However, persons contemplating IVF, PGD, or both for the purposes of ESPS should weigh the risks and uncertainties of these technologies to women and their future children.46,47
Unless and until ESPS is more robustly regulated, companies and clinicians who insist on offering this unproved, societally risky service should channel any access to ESPS through research protocols, at no cost to patient participants, in order to generate much-needed evidence about the effects of this experimental technology that can be used to inform policy. However, we emphasize that evidence regarding both the clinical risks and the expected gains associated with ESPS represents only one contribution to the ethical calculus.
Supplementary Material
Recommendations for Responsible Communication of Expected Gains from ESPS.
Emphasize absolute, not relative, risk reduction.
Patients have reported greater intention to accept interventions,32 and health care professionals have reported greater willingness to purchase,33 prescribe,34,35 and view interventions as therapeutically effective,35,36 when the benefits are presented in terms of relative rather than absolute risk reduction. Given this consistent trend in the literature,37,38 absolute risk reduction should be the most salient measure of expected gain in tables, figures, and other materials.39,40 Relative risk reduction associated with embryo selection based on polygenic scores (ESPS) should never be presented in isolation.41
Provide phenotype-specific estimates of expected gains.
In the phenotypes we assessed, expected gains from ESPS differed widely — from an absolute risk reduction of 0.12% to 8.5% and a relative risk reduction of 15% to 80% in persons of European ancestries. Companies should provide expected estimates of gain for each phenotype for which screening is offered as well as for the screening of multiple phenotypes at once. Expected gains from select phenotypes should not be offered as examples from which consumers and clinicians might improperly generalize.41 Further, consumers should be aware that “expected gains” for phenotypes that are defined by clinical cutoff points may not be practically meaningful.
Provide ancestry-specific estimates of expected gains.
Currently, ESPS is not nearly as effective for consumers with non-European ancestries. Both the expected gains for each ancestral group and the uncertain gains for those of multiple ancestries should be prominently acknowledged, in plain language. Technical statements buried in fine print, such as “in demographics different from the Caucasian training set, sensitivity will be reduced,”42 are inadequate.
Provide risk-specific estimates of expected gains.
Expected gains will differ depending on the lifetime risk of the phenotype in the embryo “population.” This risk, in turn, will depend on family history and on the environment in which the resulting child is expected to be reared.
Emphasize that expected gains (and risks) are uncertain.
Companies should make clear that ESPS predictions have very wide prediction intervals that sometimes cross zero and that pleiotropy presents both risks and uncertainties regarding the other traits that do or might correlate with those the parent is selecting.
Avoid exaggerating the benefits of screening additional embryos.
Claims such as “the more sibling embryos you have to choose from, the greater the relative reduction in risk”41 are misleading. Even for cases in which the expected gains of ESPS increase significantly with each additional embryo for the first five embryos, the incremental gains will be smaller with each of the next five additional embryos and will slow dramatically thereafter.11 This caution will be especially important if progress in stem-cell technologies makes it possible to create sperm or egg cells from a person’s blood or skin cells, yielding many more embryos, noninvasively, than is possible today.43,44
Acknowledgments
Supported by grants from the National Institute on Aging (R01AG042568-04 and R24AG065184) to Drs. Benjamin, Cesarini, Laibson, Meyer, Turley, and Visscher; a grant from Open Philanthropy (010623-00001) to Drs. Benjamin, Cesarini, Meyer, and Turley; a grant from the Ragnar Söderberg Foundation (E42/15) to Drs. Benjamin and Cesarini; funds from the Pershing Square Fund for Research on the Foundations of Human Behavior to Dr. Laibson; funds from the Stanley Family Foundation to Drs. Martin, Neale, and Hyman; a grant from the National Institute of Mental Health (K99MH117229) to Dr. Martin; a grant from the National Institute on Aging (R00AG062787-03) to Dr. Turley; grants from the Robert Wood Johnson Foundation (76565) and from the Russell Sage Foundation and the JPB Foundation (1903-13498) to Dr. Meyer; and grants from the National Health and Medical Research Council (1113400) and the Australian Research Council (FL180100072) to Dr. Visscher.
We thank Elise Robinson, Carl Shulman, and Alex Young for helpful comments and discussions and Grant Goldman and Daniel Rosica for research assistance.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Genome prediction traits offerings. April15, 2021. (https://www.scribd.com/document/503075196/Genome-Prediction-Traits-Offerings-4-15-21).
- 2.Expanded preimplantation genomic testing. Genomic Prediction, 2020. (https://genomicprediction.com/).
- 3.LeMieux J Polygenic risk scores and genomic prediction: Q&A with Stephen Hsu. GEN News. April1, 2019. (https://www.genengnews.com/insights/polygenic-risk-scores-and-genomic-prediction-qa-with-stephen-hsu/3). [Google Scholar]
- 4.Orchid Health 2021. (https://www.orchidhealth.com/).
- 5.Study summary for participants. MyOme, Inc. (https://web.archive.org/web/20201118195803/https://myome.com/report/report-15135/). [Google Scholar]
- 6.Lee JJ, Wedow R, Okbay A, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet 2018;50:1112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu S Genomic prediction: a hypothetical (embryo selection). Information Processing. July25, 2018. (https://infoproc.blogspot.com/2018/07/genomic-prediction-hypothetical-embryo.html).
- 8.Lencz T, Backenroth D, Green A, Weissbrod O, Zuk O, Carmi S. Utility of polygenic embryo screening for disease depends on the selection strategy. November26, 2020. (https://www.biorxiv.org/content/10.1101/2020.11.05.370478v2).preprint. [DOI] [PMC free article] [PubMed]
- 9.Kong A, Thorleifsson G, Frigge ML, et al. The nature of nurture: effects of parental genotypes. Science 2018;359:424–8. [DOI] [PubMed] [Google Scholar]
- 10.Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet 2019;51:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karavani E, Zuk O, Zeevi D, et al. Screening human embryos for polygenic traits has limited utility. Cell 2019;179(6):1424–1435.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dar-Nimrod I, Heine SJ. Genetic essentialism: on the deceptive determinism of DNA. Psychol Bull 2011;137:800–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vivalt E, Coville A. How do policy-makers update their beliefs? April23, 2021. (https://evavivalt.com/wp-content/uploads/How-Do-Policymakers-Update.pdf).
- 14.Watanabe K, Stringer S, Frei O, et al. A global overview of pleiotropy and genetic architecture in complex traits. Nat Genet 2019;51:1339–48. [DOI] [PubMed] [Google Scholar]
- 15.Lambert SA, Abraham G, Inouye M. Towards clinical utility of polygenic risk scores. Hum Mol Genet 2019;28(R2):R133–R142. [DOI] [PubMed] [Google Scholar]
- 16.Lewis CM, Vassos E. Polygenic risk scores: from research tools to clinical instruments. Genome Med 2020;12:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torkamani A, Wineinger NE, Topol EJ. The personal and clinical utility of polygenic risk scores. Nat Rev Genet 2018;19:581–90. [DOI] [PubMed] [Google Scholar]
- 18.Selzam S, Ritchie SJ, Pingault J-B, Reynolds CA, O’Reilly PF, Plomin R. Comparing within- and between-family polygenic score prediction. Am J Hum Genet 2019;105:351–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treff NR, Eccles J, Lello L, et al. Utility and first clinical application of screening embryos for polygenic disease risk reduction. Front Endocrinol (Lausanne) 2019;10:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Treff NR, Marin D, Lello L, Hsu S, Tellier LCAM. Preimplantation genetic testing: preimplantation genetic testing for polygenic disease risk. Reproduction 2020;160:A13–A17. [DOI] [PubMed] [Google Scholar]
- 21.Bonioli E, Tarò M, Rosa CL, et al. Heterozygous mutations of growth hormone receptor gene in children with idiopathic short stature. Growth Horm IGF Res 2005;15:405–10. [DOI] [PubMed] [Google Scholar]
- 22.Almond D, Doyle JJ Jr, Kowalski AE, Williams H. Estimating marginal returns to medical care: evidence from at-risk newborns. Q J Econ 2010;125:591–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson J, Malpas P, Hammarberg K, et al. Do à la carte menus serve infertility patients? The ethics and regulation of in vitro fertility add-ons. Fertil Steril 2019;112:973–7. [DOI] [PubMed] [Google Scholar]
- 24.Pergament D, Ilijic K. The legal past, present and future of prenatal genetic testing: professional liability and other legal challenges affecting patient access to services. J Clin Med 2014;3:1437–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayefsky MJ. Comparative preimplantation genetic diagnosis policy in Europe and the USA and its implications for reproductive tourism. Reprod Biomed Soc Online 2016;3:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayefsky M Who should regulate preimplantation genetic diagnosis in the United States? AMA J Ethics 2018;20(12):E1160–E1167. [DOI] [PubMed] [Google Scholar]
- 27.Bayefsky MJ, Berkman BE. Implementing expanded prenatal genetic testing: should parents have access to any and all fetal genetic information? Am J Bioeth 2021January18(Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lou H Eugenics then and now: constitutional limits on the use of reproductive screening technologies. Hastings Constit Law Q 2015;42:393–414 (https://repository.uchastings.edu/hastings_constitutional_law_quaterly/vol42/iss2/4/). [Google Scholar]
- 29.Bu Z, Chen Z-J, Huang G, et al. Live birth sex ratio after in vitro fertilization and embryo transfer in China — an analysis of 121,247 babies from 18 centers. PLoS One 2014;9(11):e113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz MA. Federal Trade Commission staff concerns with assisted reproductive technology advertising. Fertil Steril 1995;64:10–2. [PubMed] [Google Scholar]
- 31.van der Bles AM, van der Linden S, Freeman ALJ, et al. Communicating uncertainty about facts, numbers and science. R Soc Open Sci 2019;6:181870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malenka DJ, Baron JA, Johansen S, Wahrenberger JW, Ross JM. The framing effect of relative and absolute risk. J Gen Intern Med 1993;8:543–8. [DOI] [PubMed] [Google Scholar]
- 33.Fahey T, Griffiths S, Peters TJ. Evidence based purchasing: understanding results of clinical trials and systematic reviews. BMJ 1995;311:1056–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bobbio M, Demichelis B, Giustetto G. Completeness of reporting trial results: effect on physicians’ willingness to prescribe. Lancet 1994;343:1209–11. [DOI] [PubMed] [Google Scholar]
- 35.Bucher HC, Weinbacher M, Gyr K. Influence of method of reporting study results on decision of physicians to prescribe drugs to lower cholesterol concentration. BMJ 1994;309:761–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naylor CD, Chen E, Strauss B. Measured enthusiasm: does the method of reporting trial results alter perceptions of therapeutic effectiveness? Ann Intern Med 1992;117:916–21. [DOI] [PubMed] [Google Scholar]
- 37.Covey J A meta-analysis of the effects of presenting treatment benefits in different formats. Med Decis Making 2007;27:638–54. [DOI] [PubMed] [Google Scholar]
- 38.Edwards A, Elwyn G, Covey J, Matthews E, Pill R. Presenting risk information — a review of the effects of “framing” and other manipulations on patient outcomes. J Health Commun 2001;6:61–82. [DOI] [PubMed] [Google Scholar]
- 39.Gigerenzer G, Gaissmaier W, Kurz-Milcke E, Schwartz LM, Woloshin S. Helping doctors and patients make sense of health statistics. Psychol Sci Public Interest 2007;8:53–96. [DOI] [PubMed] [Google Scholar]
- 40.Fischhoff B, Brewer NT, Downs JS, eds. Communicating risks and benefits: an evidence-based user’s guide. Silver Spring, MD: Department of Health and Human Services, Food and Drug Administration, 2011. [Google Scholar]
- 41.An introduction to relative risk reduction, or RRR. Genomic Prediction. December23, 2019. (https://gpclaboratory.com/blog/1).
- 42.Regalado A Here is some of the description and disclaimer on the polygenic embryo test. Twitter (@antonioregalado). November 8, 2019. (https://twitter.com/antonioregalado/status/1192899142537949184).
- 43.Mathews DJH, Donovan PJ, Harris J, Lovell-Badge R, Savulescu J, Faden R. Pluripotent stem cell-derived gametes: truth and (potential) consequences. Cell Stem Cell 2009;5:11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kraft SA, Porter KM, Duenas DM, et al. Assessing parent decisions about child participation in a behavioral health intervention study and utility of informed consent forms. JAMA Netw Open 2020;3(7):e209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meghir C, Palme M. Educational reform, ability, and family background. Am Econ Rev 2005;95:414–24. [Google Scholar]
- 46.Jiang Z, Wang Y, Lin J, Xu J, Ding G, Huang H. Genetic and epigenetic risks of assisted reproduction. Best Pract Res Clin Obstet Gynaecol 2017;44:90–104. [DOI] [PubMed] [Google Scholar]
- 47.Zacchini F, Arena R, Abramik A, Ptak GE. Embryo biopsy and development: the known and the unknown. Reproduction 2017;154(5):R143–R148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


