Abstract
The major histocompatibility complex (MHC) class II transactivator (CIITA) is the master regulatory factor required for appropriate expression of class II MHC genes. Understanding the expression of CIITA is key to understanding the regulation of class II MHC genes. This report describes the independent regulation of two distinct CIITA promoters by cytokines with opposing functions, gamma interferon (IFN-γ) and transforming growth factor β (TGF-β). A functional analysis of deletion mutations of the upstream promoter (promoter III) identified an IFN-γ-responsive region located approximately 5 kb from the transcriptional start site. An in vivo DNase I hypersensitivity analysis detected a hypersensitive site in this area which supports the relevance of this region. When the downstream promoter (promoter IV) was studied by in vivo genomic footprinting, IFN-γ-induced changes at putative binding sites for STAT1, interferon regulatory factor 1 (IRF-1), and E-box proteins were seen. Gel shift and supershift analyses for IRF-1 confirmed the in vivo footprint results. The role of the IFN-γ-inducible transcription factor STAT1 was examined functionally. Although both promoters were controlled by STAT1, promoter-specific regulation was exhibited. The IFN-γ response of promoter III was completely dependent on STAT1 and not IRF-1, while promoter IV was partially activated by IRF-1 in the total absence of STAT1 expression. While both promoters were affected by TGF-β, activation of promoter III by IFN-γ was more severely diminished by TGF-β treatment. The differential control of CIITA promoters by TGF-β, IRF-1, and STAT1 may be important in refining regulation of class II MHC genes in different cell types and under different stimulatory conditions.
The class II major histocompatibility complex (MHC) molecules present antigenic peptides to CD4+ T cells through interactions with both the T-cell receptor and the CD4 molecule. Presentation of antigenic peptides by class II MHC molecules requires coexpression of (i) invariant chain (Ii), which not only binds to the antigen-binding cleft to prevent peptide binding in the endoplasmic reticulum but also targets class II MHC molecules to special cellular compartments where foreign peptides are loaded, and (ii) the enzymatic HLA-DM protein, which removes the Ii-derived peptide and facilitates loading of foreign peptides (9, 10, 13, 15, 31, 33, 48, 56). The class II MHC, Ii, and DM genes are controlled to different extents by the master transcriptional regulator, class II transactivator (CIITA) (3, 20).
CIITA was initially isolated by complementation cloning of RJ2.25, an in vitro mutagenized, class II MHC-defective B-cell line (51). CIITA not only restores class II MHC gene and antigen expression in RJ2.25 but also restores class II MHC expression in cells of the BLS-2 cell line (complementation group A), derived from patients suffering from the bare lymphocyte syndrome. Thus, this genetic defect in a subset of bare lymphocyte syndrome patients resides in the CIITA gene. Current evidence from our group shows that the BLS-2 defect, which involves deletion of a 72-bp CIITA exon, lies in the inability of the mutant CIITA to undergo nuclear translocation (7).
CIITA is a transcriptional coactivator that does not bind DNA yet exhibits a potent and specific effect on class II MHC gene transcription. The CIITA protein has domains normally associated with transcriptional activators such as acidic and proline-, serine-, and threonine-rich domains, and also contains an unusual and important consensus GTP-binding domain (5). Recent evidence shows that lack of CIITA results in a closed chromatin structure in the class II MHC promoter (44, 58). More importantly, reintroduction of CIITA into G3A, a mutagenized gamma interferon (IFN-γ)-unresponsive cell line that lacks CIITA expression, results in the opening and occupancy of previously closed class II MHC, Ii, and DM promoters (54, 58). The capacity of CIITA to open previously closed promoters appears to be restricted to IFN-γ-responsive cells and not to B cells. The biochemical mode by which CIITA functions is poorly understood, although one report shows that CIITA can interact with RFX5 in a yeast two-hybrid system (46). Others have shown interactions with Bob1, a B-cell factor, and with TAFII32, a subunit of the basal transcription factor TFIID (11, 12, 29). More recently, we have found that CIITA interacts with the coactivator CREB-binding protein (17). These multiple interactions may provide a model by which CIITA exerts its effects on gene transcription.
The expression of CIITA coincides with class II MHC gene expression, and this feature is distinct from other transcription factors that control the expression of class II MHC (28). These other transcription factors, primarily RFX and NF-Y, are ubiquitously expressed and cannot explain the restricted tissue and cell distribution of class II MHC. In contrast, the expression of CIITA is nearly identical to that of class II MHC genes. Two dominant regulators of class II MHC gene expression also control CIITA expression. IFN-γ upregulates while transforming growth factor beta (TGF-β) suppresses CIITA (4, 6, 24, 37, 52). The physiologic roles of IFN-γ and TGF-β in controlling class II MHC expression are well documented. For example, TGF-β−/− gene knockout mice develop severe autoimmune disease accompanied by the hyperexpression of class II MHC genes (14, 36, 49). In contrast, manifestations of autoimmunity are greatly diminished in mice that are both TGF-β−/− and I-A−/− (26), which suggests that many of TGF-β’s effects on the immune system are mediated via the suppression of class II MHC expression. Hence, an understanding of the regulation of CIITA is seminal in the definition of events that lie between the binding of IFN-γ and TGF-β to their respective receptors and the downstream activation or suppression of class II MHC genes.
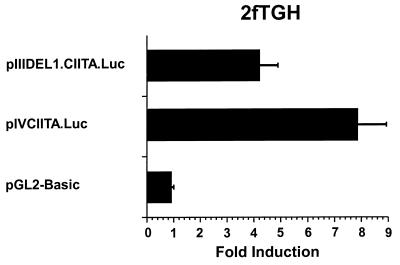
Previously, we and others have found that B-cell-specific expression of CIITA requires a small promoter region immediately upstream of the transcriptional start site (contained in pIIIDEL4.CIITA.Luc) (see Fig. 1) (25, 35, 41). Our study further indicated that IFN-γ induction of this promoter is possible in several different cell types and requires DNA sequences located at least 2.5 kb upstream of the start site. This IFN-γ-inducible region was shown to be functional both in the context of this CIITA promoter and when linked to a heterologous promoter.
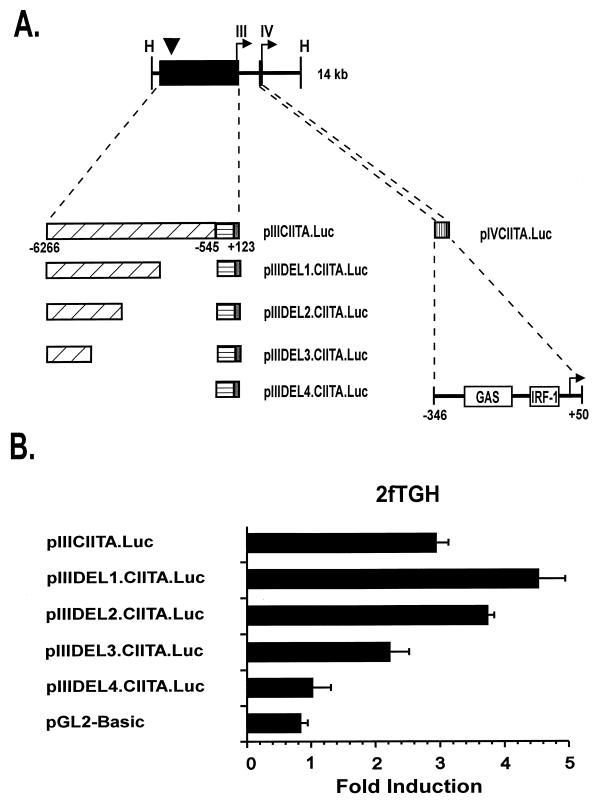
FIG. 1.
(A) Map of the 14-kb human CIITA gene fragment that contains both IFN-γ-inducible promoters of the CIITA gene. The locations of the transcriptional start sites of the upstream IFN-γ-responsive promoter (promoter III) and the downstream promoter (promoter IV) are shown by arrows. The location of the DNase I-hypersensitive site that lies approximately 6 kb upstream of the start site of promoter III is marked with an arrowhead. Locations of the DNA fragments used in reporter constructs described in this report are given (black boxes). Gray boxes indicate the 5′ untranslated regions in promoter III and IV constructs. A horizontally hatched box denotes the location of the 668-bp region previously shown to be required for constitutive basal promoter III activity in B cells (41). Regions that confer IFN-γ inducibility are indicated by diagonally and vertically hatched boxes for promoter III and promoter IV, respectively. Open boxes show the locations of the STAT1 (GAS) and IRF-1 consensus binding motifs in promoter IV. H, HindIII. (B) Deletion mutants that contain DNA sequences located distant to promoter III of CIITA confer IFN-γ inducibility in 2fTGH cells. Transient transfections of 2fTGH cells were performed by calcium phosphate coprecipitation. After 6 h, precipitates were removed and culture medium was added with or without 500 U of IFN-γ/ml. Cells were harvested for luciferase assays 14 h later. Luciferase activity was measured in RLU per microgram of protein. Fold induction after IFN-γ treatment was calculated by dividing the RLU of IFN-γ-treated samples by the RLU of untreated samples. Data shown are the averages of three independent experiments. Error bars represent standard errors of the means.
In parallel, another laboratory used RNase protection analysis to show that the CIITA gene has multiple transcriptional start sites, a finding indicative of multiple promoters (35). These authors concluded that the human CIITA gene has four promoters designated by their location from 5′ (upstream) to 3′ (downstream) as promoter I, primarily expressed in dendritic cells; promoter II, expressed at insignificant levels and functionally not well understood; promoter III, primarily active in B cells; and promoter IV, expressed in response to IFN-γ (see Fig. 1A). A comparison of their and our published reports indicates that the promoter identified in our report corresponds to promoter III of their report. A more careful examination of their RNase protection data shows us that promoter III is also IFN-γ inducible in a number of different cell types, including endothelial cells and fibroblasts, although the inducibility of this promoter is weaker than that of promoter IV. Hence, there may exist two distinct IFN-γ-inducible regions that reside in promoters III and IV, respectively.
The purpose of this report is to resolve the issue of two distinct IFN-γ-inducible promoters for the CIITA gene and to perform a series of experiments comparing the inducibility of these promoters. This was achieved by in vivo analyses to detect changes in the chromatin or the binding of proteins to specific regions within each promoter. Upon verification of modifications in the chromatin by either in vivo DNase I hypersensitivity or genomic footprint analysis, the promoters were studied by gel shift analyses and/or in a luciferase reporter system. The promoters exhibited differential responses to the IFN-γ-induced transcription factors, STAT1 and IRF-1, as well as to TGF-β. The implications of two IFN-γ-responsive promoters with distinct patterns of response to cytokines and transcription factors may explain the complex pattern of class II MHC expression in different tissues and may have broad ramifications in tissue-specific immune responses, such as autoimmunity and transplantation responses.
MATERIALS AND METHODS
Cell lines.
2fTGH cells are derived from HT 1080 human fibrosarcoma cells that do not constitutively express class II MHC antigens but express high levels of these antigens after IFN-γ induction. U3A (generously provided by George Stark, Cleveland Clinic Foundation Research Institute, Cleveland, Ohio) is a STAT1-defective cell line derived from 2fTGH (39). U3A and 2fTGH cells were grown in Dulbecco’s modified Eagle’s medium (Gibco BRL) supplemented with 10% fetal calf serum, 2 mM l-glutamine, and penicillin and streptomycin (100 U/ml). Murine P19 embryonal carcinoma cells (CRL-1825; American Type Culture Collection) were grown in Alpha Eagle’s modified Eagle’s medium (Gibco BRL) supplemented as described above. U373-MG human glioblastoma multiforme cells were grown in McCoy’s 5A medium (Gibco BRL) supplemented as described above.
Constructs.
The isolation of clones containing the promoter regions of the human CIITA gene as well as the construction of pIIIDEL4.CIITA.Luc (previously called p668CIITA.Luc), pIIIDEL1.CIITA.Luc (previously p7000-2000CIITA.Luc), and pIIICIITA.Luc (previously p7000CIITA.Luc) has been described elsewhere (41). To obtain plasmids pIIIDEL2.CIITA.Luc and pIIIDEL3.CIITA.Luc, 2,181-bp XbaI-SphI (for pIIIDEL2.CIITA.Luc) and 1,068-bp XbaI-AccI (for pIIIDEL3.CIITA.Luc) fragments from the 5′ end of pIIICIITA.Luc were cloned into the KpnI site at the 5′ end of the CIITA sequences in pIIIDEL4.CIITA.Luc by filling in using the Klenow fragment and ligation of the blunted ends. CIITA promoter IV was generated by PCR using Taq polymerase and standard reaction conditions (Perkin-Elmer Cetus). Oligonucleotides 5′-TGAGTTGGAGAGAAACAGAG-3′ (sense) and 5′-CTGCTGGTGGCCTCTC-3′ (antisense) were used to amplify nucleotides −346 to +50 of the CIITA promoter IV sequence reported by Muhlethaler-Mottet et al. (35). Extensions (ACGTACAAGCTT) at the 5′ ends of the primers generated HindIII sites that were used to clone the amplified fragment into the HindIII site of pGL2-Basic (Promega) to create pIVCIITA.Luc. The template DNA for the PCR was a human CIITA genomic clone (clone 2) that has been previously described (41). Oligonucleotides 5′-CTCAGCGCTGCAGAAAGAActtagAAGGGAAAAAGAACTGCGGGGAG-3′ (sense; mutations shown in lowercase type) and 5′-TCGAAGTATTCCGCGTAC-3′ (antisense) were used in the PCR that was performed to create the IRF-1 site mutation in CIITA promoter IV. The template DNA for the PCR was the pIVCIITA.Luc plasmid. The primers were designed to amplify a region from the PstI site (underlined in the sense primer) in promoter IV to bp 248 in pGL2-Basic. The mutated amplified fragment was digested with PstI-XbaI and then substituted for the nonmutated PstI-XbaI fragment in pIVCIITA.Luc to create pmIRF.IVCIITA.Luc. Similarly, oligonucleotide 5′-CTGCAGAACCAGGCAG T TGGGATGCCACg gagtcTAAAGCACG TGG TGGCCACAG-3′ (sense; mutations shown in lowercase type) was used with the antisense primer described above to amplify a region from the BstXI site (underlined) in promoter IV to bp 248 in pGL2-Basic. The mutated amplified fragment was digested with BstXI-XbaI and then substituted for the nonmutated BstXI-XbaI fragment in pIVCIITA.Luc to create pmGAS.IVCIITA.Luc. Junctions of plasmids pIIIDEL2.CIITA.Luc and pIIIDEL3.CIITA.Luc and the inserts and junctions of pIVCIITA.Luc, pmIRF.IVCIITA.Luc, and pmGAS.IVCIITA.Luc have been confirmed by DNA sequencing. The pSVISGF2 plasmid, which contains the human IRF-1 cDNA, was generously provided by Richard Pine, New York University. To generate the IRF-1 expression plasmid, the human IRF-1 cDNA was removed from pSVISGF2 as an XbaI-HindIII fragment and subcloned into the XbaI-HindIII site of pcDNA3 (Promega).
Ligation-mediated PCR DNase I hypersensitivity mapping.
The DNase I hypersensitivity mapping technique used in this study uses ligation-mediated PCR optimized for amplification of long PCR fragments to detect blunt-ended DNase I-hypersensitive sites. One million cells were plated in 10-cm-diameter dishes and either left untreated or treated with IFN-γ for 5 h. Cells were permeabilized with 0.2% saponin in DNase I buffer (15 mM Tris [pH 7.4], 15 mM NaCl, 60 mM KCl, 3 mM MgCl2, 0.25 M sucrose, 1 mM dithiothreitol). The permeabilized cells were then treated with DNase I buffer containing 0.05% saponin and either 20 or 40 U of DNase I (Boehringer Mannheim) for 60 s. The buffer was removed from the dish, and the cells were lysed and digested by incubation for 3 h at 37°C in a buffer consisting of 0.15% sodium dodecyl sulfate, 10 mM Tris (pH 7.4), 10 mM EDTA, RNase A (100 μg/ml), proteinase K (400 μg/ml), and 1 mM dithiothreitol. Genomic DNA was then extracted with phenol-chloroform and was precipitated. Five μg of DNase I-digested genomic DNA or undigested DNA was ligated with the blunt-ended linker used in the ligation-mediated PCR protocol (57). One-fifth of the products of the ligation reaction was then amplified by PCR using Expand High Fidelity DNA polymerase (Boehringer Mannheim) according to the manufacturer’s recommended conditions. The initial extension time was 3 min. All PCRs used the linker primer 5′-GCGGTGACCCGGGAGATCTGAATTC-3′ in combination with a CIITA locus specific primer. For analysis of promoter IV, primer 104CIITA, 5′-GATTCCTACACAATGCGTTGCCTGGCTC-3′ (melting temperature [Tm] = 65°C), was used. For analysis of promoter III, primer CIITAint, 5′-CCTTTCGGTGCTGATACATGGTTC-3′ (Tm = 63°C), was used. One-fifth of the reaction mixture was loaded onto 1% agarose gels, and the fragments were separated by electrophoresis, transferred to a nylon membrane (Nytran; Schleicher and Schuell) and UV cross-linked. For detection of the amplification products, a probe was generated by using [α-32P]dCTP in PCRs using primers 104CIITA and CIITAint and the human CIITA genomic clone (mentioned above) as a template. The size of fragments was estimated by using both 1-kb and 100-bp markers (Gibco BRL), with correction for inclusion of the linker primer at the end of each fragment.
Transfections and luciferase assay.
Transient transfections of 2fTGH and U3A were performed by the calcium phosphate coprecipitation method (45). Cells were plated in six-well plates at a density of 6 × 104 cells/well and transfected 24 h later. Three micrograms of reporter construct or 3 μg of reporter construct in combination with 3 μg of negative control DNA (pcDNA3; Invitrogen), STAT1, or IRF-1 expression vector was added to each well, and the dishes were incubated at 37°C in 5% CO2. After 6 h, the precipitates were removed and the cells were rinsed twice with phosphate-buffered saline. Culture medium (2 ml per well) was added, and the plates were incubated for 12 h. During this period for experiments involving TGF-β treatment, the culture medium was supplemented with 10 ng of TGF-β1 (R & D Systems)/ml. After 12 h, culture medium was removed and replaced with fresh medium, with or without 500 U of recombinant human IFN-γ (Genentech)/ml. Cells were harvested for luciferase assays 14 h later. The STAT1 expression vector (generously provided by James Darnell, Jr., Rockefeller University, New York, N.Y.) has been previously described (19).
Transient transfections of P19 cells were performed by the calcium phosphate technique described above with the following modifications: cells were plated in 10-cm-diameter dishes at a density of 5 × 105 cells, each dish received 10 μg of reporter construct in combination with 10 μg of control DNA or the IRF-1 expression vector, culture medium (10 ml) was added, and cells were harvested for luciferase assays 14 h later.
Luciferase assays were performed with an LB 953 AutoLumat (EG&G Berthold) as previously described (2). The protein content of cell extracts was determined by the Bradford assay (1). Luciferase activity was measured as relative light units (RLU) per microgram of protein. Fold induction after treatments was calculated by dividing the luciferase activity of IFN-γ-treated samples by the RLU of untreated samples.
In vivo genomic footprinting.
Dimethyl sulfate (DMS) treatment of cells and genomic DNA preparation were performed as previously described (40). Cleaved genomic DNA was amplified by using a ligation-mediated PCR protocol (57, 59). Ten million cells were treated with 500 U of recombinant IFN-γ (Genzyme)/ml for 4, 8, or 24 h before DMS treatment and genomic DNA isolation. Three CIITA locus-specific primers were used to amplify cleaved fragments from the upper strand of CIITA promoter IV: CIITA4 up1, 5′-CTACCGCTGTTCCCCG-3′ (Tm= 61°C); CIITA4 up2, 5′-GCGGCAAGTCTGTGGCAGCTC-3′ (Tm = 65°C); and CIITA4 up3, 5′-GCGGCAAGTCTGTGGCAGCTCGTC-3′ (Tm = 68°C). The ligation-mediated PCR procedure was also performed for the lower strand, but no significant protections or enhancements were observed. The primers used for the lower strand were CIITA4 lo1, 5′-GGGCCTGGGACTCTC-3′ (Tm = 61°C); CIITA lo2, 5′-GGGCTGGCCACTGTGAGGAAC-3′ (Tm = 65°C); and CIITA lo3, 5′-GGCTGGCCACTGTGAGGAACCGACTG-3′ (Tm = 69°C).
Preparation of nuclear extracts and gel shift analyses.
Nuclear extracts were prepared by the method of Schreiber et al. from 2fTGH cells, uninduced and induced with 500 U of IFN-γ/ml for 14 h (47). The WT-IRF1/2CIITA oligonucleotide probe was as follows: 5′-CTGCAGAAAGAAAGTGAAAGGGAAAAAGAACT-3′. The additional oligonucleotide probes used as cold competitors were MT-IRF1/2CIITA, 5′-CTGCAGAAAGAActtagAAGGGAAAAAGAACT-3′, and IRF-E, 5′-CGGCCGCTTTCGATTTCGCTTTCCCCTAAATGGCTG-3′, an oligonucleotide that contains an inverted IRF-1-binding site (55). Antibodies were purchased from Santa Cruz Biotechnology. Gel shift analysis was performed as previously described (55). Briefly, 5 μg of nuclear extract and 2.5 × 10−2 pmol of annealed and 32P-labeled oligonucleotide probe (∼100,000 cpm) were incubated in a reaction mixture containing 50 mM KCl, 5 mM NaCl, 5% glycerol, 10 mM Tris (pH 7.9), 1.5 mM MgCl2, 1 mM dithiothreitol, and 2 μg of poly(dI)(dC) for 20 min at room temperature. Antibodies were incubated with nuclear extracts in the reaction mixture for 30 min on ice before the addition of the probe. Complexes were resolved by electrophoresis in 5% acrylamide-bisacrylamide (29:1) gels run in 0.5× Tris-buffered EDTA (TBE) at 4°C and 20 mA.
RESULTS
Functional analyses of deletion mutations of promoter III map an IFN-γ-responsive region to the distal end of this 7-kb region.
The intent of this report was to understand the functional differences of the two IFN-γ-responsive promoters (promoter III and promoter IV) of CIITA. This is important because different cell types may selectively utilize these two promoters, resulting in distinct patterns of immune stimulation in vivo. A previous report from our laboratory showed that pIIICIITA.Luc, which contains a large 7-kb region upstream of promoter III, included an IFN-γ-responsive region. To delineate this region, a series of internal deletion mutations (Fig. 1A) were produced. Three deletion constructs containing the most distal 3,563, 2,181, and 1,068 bp of DNA within the 7-kb fragment were linked to 668 bp of basal CIITA promoter III sequence and cloned into the luciferase reporter gene vector, pGL2-Basic. These plasmids were designated pIIIDEL1.CIITA.Luc, pIIIDEL2.CIITA.Luc, and pIIIDEL3.CIITA.Luc, respectively. As shown in Fig. 1B, all three constructs were IFN-γ inducible when transiently transfected into human 2fTGH fibroblasts, although the inducibility seen for plasmid pIIIDEL3.CIITA.Luc was reproducibly less. This maps the minimal IFN-γ-responsive region to the distal approximately 1 kb, although the additional sequences found in pIIIDEL2.CIITA.Luc augment the response. The pGL2-Basic vector plasmid and pIIIDEL4.CIITA.Luc, which contains the minimal B cell-promoter of CIITA promoter III, were used as negative controls. The full-length IFN-γ-inducible promoter III plasmid, pIIICIITA.Luc, which was previously shown to retain full IFN-γ inducibility, was used as a positive control.
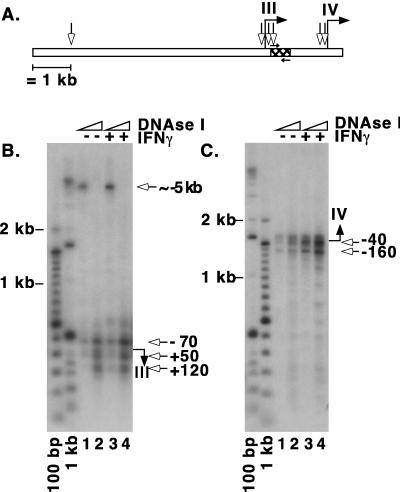
DNase I hypersensitivity analyses reveal sites located approximately 5 kb upstream of the transcriptional start site of promoter III and immediately adjacent to the start sites of both promoter III and promoter IV.
Another way to determine the functional importance of a DNA sequence is to examine for in vivo hallmarks of protein-DNA interactions as revealed either by DNase I hypersensitivity analysis or by genomic footprinting. Our group has previously reported that a 6-kb region upstream of the transcriptional start site of promoter III mediates the IFN-γ response (41). New data presented in Fig. 1B further showed that the most upstream 1 kb of DNA contains important regulatory sequences. The DNase I hypersensitivity method was used to indicate the locations of potential regulatory protein-DNA interactions, as this method can be used to scan large regions of DNA for structural changes in chromatin.
A modified DNase I hypersensitivity method using ligation-mediated PCR with optimization for amplification of long PCR fragments was established. The analysis was performed with U373-MG cells, one of several cell types for which we have previously shown the IFN-γ responsiveness of this region (41). Double-stranded breaks at hypersensitive sites were ligated to the blunt-ended linker commonly used in the in vivo genomic footprinting technique (57). Two CIITA locus-specific primers were used in combination with a linker-specific primer to amplify fragments ending with a hypersensitive site. The CIITA-specific primers were also used to generate a probe for Southern blot analysis of the amplified fragments (Fig. 2A). Amplification using an intron-specific primer allowed detection of hypersensitive sites 5′ of the promoter III start site. A DNase I-hypersensitive site was consistently observed approximately 5 kb upstream of this initiation site (Fig. 2B, lanes 1 and 3). This site was detectable before induction with IFN-γ, and upon induction a slight increase in sensitivity is observed. The location of the hypersensitive site correlates with the functional analysis shown in Fig. 1B, which shows IFN-γ inducibility residing in the furthest 1 kb of this 6-kb region upstream of promoter III. Upon using a higher concentration of DNase I, the 5-kb site disappeared and several hypersensitive sites were more clearly visible in the vicinity of the transcriptional start site (Fig. 2B, lanes 2 and 4). Promoter IV was analyzed by amplification in the downstream direction with a primer specific for sequences near the initiation site of promoter III (Fig. 2A). Hypersensitive sites were detected very close to the transcriptional start site for promoter IV (Fig. 2C). Again, hypersensitive sites were detectable before induction, but an increase in DNase I sensitivity was clearly observed after 5 h of IFN-γ treatment (compare lanes 1 and 2 to 3 and 4).
FIG. 2.
DNase I hypersensitivity analyses reveal hypersensitive sites in the proximal promoters of both promoters III and IV and an additional long-range hypersensitive site for promoter III. (A) Summary of hypersensitivity analysis and graphic representation of the CIITA genomic region showing the location of the primers and probe that were used for detection. The hatched region represents the probe used for Southern blot hybridization. The small arrows above (primer 104CIITA) and below (primer CIITAint) the hatched region indicate the locations of the primers used for the PCR. (B) Southern blot analysis of PCR-amplified products using primer CIITAint to detect promoter III-associated hypersensitive sites. The start site of the promoter is designated by a filled arrow to the right of the panel. Estimated positions of the hypersensitive sites are indicated with open arrows. Lanes 1 and 2 represent results for uninduced cells that were treated with 20 and 40 U of DNase I, respectively. Lanes 3 and 4 represent results for cells that were induced with IFN-γ for 5 h and treated with 20 and 40 U of DNase I, respectively. (C) Southern blot analysis of PCR-amplified products with primer 104CIITA to detect promoter IV-associated hypersensitive sites.
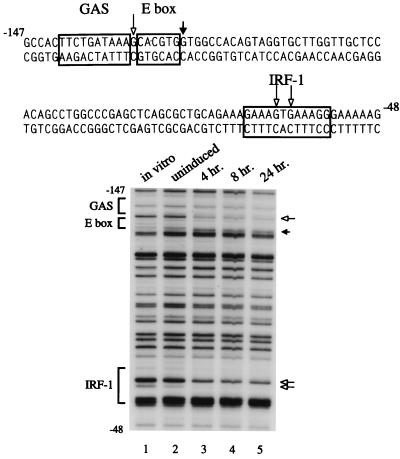
Genomic footprint analysis detects changes at putative STAT1, IRF-1, and E-box binding sites in promoter IV.
In contrast to the large span of endogenous DNA that is required to demonstrate IFN-γ inducibility for promoter III, the IFN-γ-responsive region of promoter IV consists of approximately 400 bp of DNA located downstream of promoter III (35). In addition, promoter IV uses a different transcriptional start site (Fig. 1A). In vivo genomic footprint analysis is the method of choice to define physiologically relevant sequences within such a relatively small region. Methylated genomic DNA was obtained from U373-MG cells that had either remained untreated or had been treated with IFN-γ for 4 to 24 h. IFN-γ treatment resulted in significant protections in promoter IV at three predominant sites: adjacent to a putative STAT1-binding site (GAS), within a putative IRF-1/IRF-2 site, and adjacent to an E-box motif (Fig. 3). Protection at the guanine residue that lies between the GAS and E-box sites was only present in IFN-γ-induced samples. These contacts were sustained even after 24 h of IFN-γ treatment. The only protections that were located directly over a putative transcription factor binding site were found in the IRF-1/IRF-2 site. There was a small amount of protection of this site before induction, but IFN-γ treatment resulted in a significant increase in the amount of protection. Analyses of methylated DNA obtained from another IFN-γ-responsive cell line, 2fTGH, showed similar protections, while analyses of DNA from human Raji B cells showed no protections, which is consistent with B cells using promoter III and not promoter IV (data not shown).
FIG. 3.
The in vivo footprint of CIITA promoter IV reveals protein-DNA contacts near the putative STAT1- and E-box-binding sites and within the IRF-1 site. The sequence of the promoter region is shown with the relevant cis-elements framed. Genomic footprints of the upper strand are shown in the lower panel. Lane 1 represents genomic DNA methylated in vitro to reveal the complete guanine ladder. Lanes 2 through 5 show the results of a time course of IFN-γ treatment using DNA from cells treated with DMS in culture. Open arrows indicate bases that are protected from modification. Filled arrows indicate bases for which modification is enhanced.
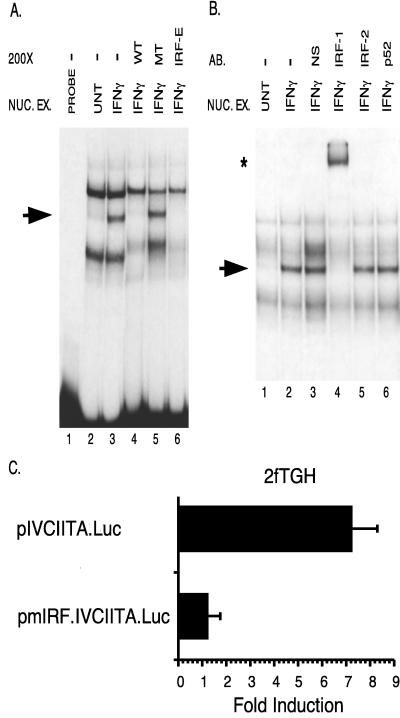
Gel shift and supershift analyses verify the binding of IRF-1 to its cognate binding site in promoter IV.
Gel shift and supershift analyses were performed to identify the proteins that interact with the in vitro-footprinted sequence. During the preparation of this manuscript, another group also demonstrated STAT1 binding to promoter IV in vitro (34). In the present study we examined actual binding of IRF-1 to the putative IRF-1/IRF-2-binding site. This is important because this site is also a potential IRF-2-binding site or might be recognized by yet another member of the increasingly large IRF family of DNA binding proteins (38). Furthermore, functional analyses revealed a more significant function for the IRF site than the STAT1-binding site (see below). An in vitro gel shift analysis was performed and demonstrated the formation of a complex on this site in response to IFN-γ treatment (Fig. 4A, compare lanes 2 and 3). Specific cold competitors consisting of either the wild-type IRF-binding site from promoter IV (lane 4) or a consensus IRF site (lane 6) eliminated the formation of this band, while a competitor with a mutated IRF-1/IRF-2-binding site did not (lane 5). Preincubation of the nuclear extracts with an anti-IRF-1 antibody resulted in a supershifted band, while preincubation with normal serum, isotype-matched antibodies against IRF-2, or the p52 NF-κB subunit did not (Fig. 4B, lane 4 versus lanes 3, 5, and 6). As shown in Fig. 4B, nuclear extracts were preincubated in the reaction mixture (with or without antibody) for 30 min. This preincubation led to a decrease and/or slight shift in the location of nonspecific bands versus the pattern seen in Fig. 4A, for which there was no preincubation. There are also slight differences in the nonspecific bands between lanes shown in Fig. 4B. The difference between lanes 2 and 3 probably reflects the difference in the protein content of the mixture in lane 3, which received added protein from the normal serum. Similarly, slight changes in nonspecific bands between lane 2 and lanes 4 to 6 probably reflect differences in protein content between the normal serum and the antibodies. In addition, a pmIRF.CIITA.Luc plasmid bearing a mutation of this IRF-1- binding site was not inducible by IFN-γ in transfection experiments performed in 2fTGH fibroblasts (Fig. 4C). Together, these results provide strong evidence that the site is required for induction and can indeed be recognized by IRF-1.
FIG. 4.
IRF-1 binds to the proximal IFN-γ-inducible promoter (promoter IV). (A) Gel shift analysis indicates that one protein complex is induced by IFN-γ (lane 2 versus lane 3) (arrow). Nuclear extracts were from 2fTGH cells induced with 500 U of IFN-γ/ml for 14 h (IFN-γ) and uninduced cells (UNT). The probe spans the IRF-1/IRF-2 site (Fig. 1A; Materials and Methods). Lane 1 contains probe only. Oligonucleotide competitors are designated in the top row and are used at 200-fold molar excess (200×). Abbreviations: WT, homologous CIITA IRF-1/IRF-2 competitor; MT, cold competitor with a mutated IRF-1/IRF-2 site; IRF-E, cold competitor with the IRF-1-binding site of the TAP1 gene. NUC. EX., nuclear extract. (B) Incubation with anti-IRF-1 induces a supershifted complex (star) and reduces the formation of the inducible complex (arrow). Antibodies are indicated at the top. Abbreviations: AB, antibody; NS, normal serum. (C) The IRF-1 site is required for induction of promoter IV by IFN-γ. Transient transfections of 2fTGH cells were performed by calcium phosphate coprecipitation using plasmids containing wild-type CIITA promoter IV (pIVCIITA.Luc) and promoter IV with a mutated IRF-1 site (pmIRF.IVCIITA.Luc). After 6 h, precipitates were removed and culture medium was added with or without 500 U of IFN-γ/ml. Cells were harvested for luciferase assays 14 h later. Fold induction after IFN-γ treatment was calculated by dividing the RLU of IFN-γ-treated samples by the RLU of untreated samples. Data shown are the averages of three independent treatment groups. Error bars represent standard errors of the means. This experiment has been repeated with similar results.
A comparison of the two promoters reveals that promoter IV is responsive to both STAT1 and IRF-1 but promoter III is not responsive to IRF-1.
Two predominant transcription factors which mediate the positive regulatory functions of IFN-γ are STAT1 and IRF-1. The STAT1 protein resides in the cytoplasm in a nonactive form. Upon IFN-γ binding, the chains of the IFN-γ receptor are cross-phosphorylated by JAK kinases. STAT1 is recruited, undergoes phosphorylation, homodimerizes, and translocates to the nucleus where it binds a consensus IFN-γ activation sequence (GAS) and induces gene transcription. Thus, activation of STAT1 by IFN-γ does not require de novo protein synthesis. In contrast, the transcription of IRF-1 is induced by IFN-γ treatment and requires both de novo transcription and protein synthesis. In fact, a GAS element is present in the promoter of the IRF-1 gene and STAT1 is a primary activator of IRF-1 transcription (43).
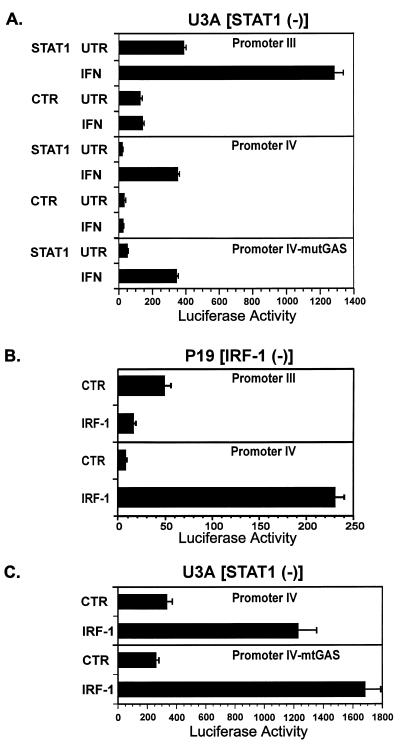
To more directly assess the involvement of IRF-1 and/or STAT1 in the regulation of CIITA promoters III and IV, mutant cell lines that selectively lack functional IRF-1 and STAT1 activities were used. U3A fibroblasts selectively lack STAT1 activity. The lack of functional STAT1 resulted in greatly reduced activation by IFN-γ of both promoter III (pIIIDEL1.CIITA.Luc) and promoter IV (pIVCIITA.Luc) (Fig. 5A, CTR data). Introduction of a vector constitutively expressing STAT1 and IFN-γ treatment resulted in a 20-fold induction of promoter IV and a 3.3-fold induction of promoter III by IFN-γ. Fold induction after IFN-γ treatment was calculated by dividing the luciferase activity of IFN-γ-treated samples by the activity of untreated samples. The induction of pIIIDEL1.CIITA.Luc by STAT1 has been previously reported by our laboratory (41). Interestingly, a promoter IV construct (pmGAS.IVCIITA.Luc) in which the GAS motif (TTCTGATAAA) was mutated to the sequence GGAGTCTAAA retained sevenfold inducibility by IFN-γ (Fig. 5A, mutGAS). This finding suggests that the GAS site per se may not be absolutely required for induction. Since the pmGAS.IVCIITA.Luc construct still retains the wild-type IRF-1 site, the requirement for STAT1 may be related at least partly to the reliance on STAT1 for induction of IRF-1 in these cells. An ∼50% decrease in induction by IFN-γ of the activity of pmGAS.IVCIITA.Luc vs pIVCIITA.Luc was also seen in 2fTGH cells (data not shown).
FIG. 5.
Regulation of the IFN-γ-inducible promoters by STAT1 and IRF-1. Transient transfections of U3A and P19 cells with the indicated plasmids (pIIIDEL1.CIITA.Luc for promoter III, pIVCIITA.Luc for promoter IV, pmGAS.IVCIITA.Luc for promoter IV-mtGAS, STAT1 for STAT1 expression plasmid, pcDNA3 for CTR, and IRF-1 for IRF-1 expression plasmid) were performed by calcium phosphate coprecipitation. (A) U3A is a STAT1-defective cell line derived from 2fTGH. Cultures were untreated (UNT) or treated with 500 U of human IFN-γ/ml (IFN) and harvested 14 h later. Luciferase activity was measured as RLU per microgram of protein. Error bars represent standard errors of the means. No IFN-γ induction was seen for control plasmids. These experiments have been repeated with similar results. (B) P19 is a murine embryonal carcinoma cell line that does not express IRF-1. Precipitates were removed after 6 h, and fresh culture medium was added. Cells were harvested for luciferase assays 24 h later. Luciferase activity was measured as RLU per microgram of protein. Results shown are the averages of three cultures per group. Error bars represent standard errors of the means. These experiments have been repeated with similar results. (C) U3A cells were cotransfected with the indicated plasmids. Cultures were untreated or treated with 500 U of human IFN-γ/ml and harvested 14 h later. Luciferase activity was measured as RLU per microgram of protein. Error bars represent standard errors of the means. No IFN-γ induction was seen for control or IRF-1 plasmids (data not shown). These experiments have been repeated with similar results.
To assess the involvement of IRF-1 in the control of the two CIITA promoters, these promoter-reporter constructs were transfected into the IRF-1-deficient embryonal carcinoma cell line P19 (Fig. 5B). The luciferase activity of the promoter III plasmid, pIIIDEL1.CIITA.Luc, was not enhanced when cotransfected with an IRF-1 expression vector into P19 cells. In fact it was consistently less than the activity seen in cells cotransfected with the pcDNA3 control vector (Fig. 5B). In contrast, cotransfection with IRF-1 resulted in a dramatic enhancement of the luciferase activity driven by promoter IV. This clearly indicates that promoter IV, unlike promoter III, requires IRF-1 for induction.
Although data presented in Fig. 5A shows that STAT1 plays an important role in the control of promoter IV, an extensive mutation of the STAT1-binding site lowered induction by only ∼50%. In light of the important role IRF-1 plays in activating promoter IV (Fig. 5B), a likely explanation is that the primary route by which STAT1 controls CIITA promoter IV is indirectly through activation of IRF-1 gene transcription. To further investigate the dependence of promoter IV for IRF-1, the promoter IV luciferase constructs were cotransfected with an the IRF-1 expression plasmid into U3A cells (Fig. 5C). The activity of the promoter IV construct was activated 3.6-fold by IRF-1 expression in these cells, which lack STAT1 expression. Additionally, the pmGAS.IVCIITA.Luc was responsive to IRF-1 expression (6.7-fold) (Fig. 5C, mtGAS), which supports the possibility that the STAT1 activation of IRF-1 is an important role of STAT1 in promoter IV activation.
The IFN-γ induction of CIITA promoter IV is greater than that of promoter III.
The extent of activation by IFN-γ for both promoters was examined by a comparison of reporter constructs containing either promoter III (pIIIDEL1.CIITA.Luc) or promoter IV (pIVCIITA.Luc) fused to the luciferase reporter gene. While both promoters contain IFN-γ-responsive sequences, the inducibility of promoter IV was consistently at least twofold greater than that of promoter III in the 2fTGH cells (Fig. 6). The pGL2-Basic plasmid is a promoterless negative control vector.
FIG. 6.
IFN-γ induction of the promoter IV is greater than that of promoter III. Transient transfections of 2fTGH cells were performed by calcium phosphate coprecipitation. After 6 h, precipitates were removed and culture medium was added with or without 500 U of IFN-γ/ml. Cells were harvested for luciferase assays 14 h later. Luciferase activity was measured as RLU per microgram of protein. Fold induction after IFN-γ treatment was calculated by dividing the RLU of IFN-γ-treated samples by the RLU of untreated samples. Data shown are the averages of four independent experiments. Error bars represent standard errors of the means.
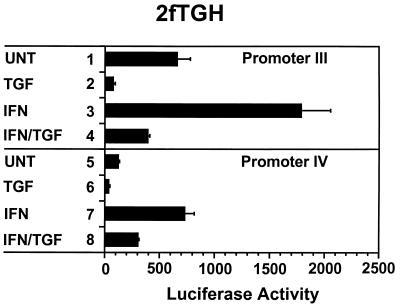
Both CIITA promoters are suppressed by TGF-β, but the suppression of promoter III is more complete.
Suppression of CIITA transcription by TGF-β is an important route by which class II MHC hyperexpression is prevented in physiologic conditions (24, 37). We tested the effect of TGF-β on the reporter constructs bearing promoter III (pIIIDEL1.CIITA.Luc) or promoter IV (pIVCIITA.Luc) sequences. As shown in Fig. 7 (lanes 3 and 4), the promoter III construct was inducible by IFN-γ and this induction was almost completely suppressed by TGF-β. While promoter IV was also inducible by IFN-γ, TGF-β lowered the luciferase activity of pIVCIITA.Luc by only 50% (lanes 7 and 8). Treatment with TGF-β alone lowered the basal activity of both promoter III (41) and promoter IV, which indicates that TGF-β may interfere with promoter activity even in the absence of IFN-γ (compare lanes 1 and 2 and lanes 5 and 6). The higher basal activity seen in this experiment for the promoter III plasmid versus the promoter IV plasmid was not a consistent finding, but the more intense suppression of promoter III by TGF-β was a consistent finding.
FIG. 7.
TGF-β suppresses both promoters, but suppression of promoter III is more pronounced. Transient transfections of 2fTGH cells were performed by calcium phosphate coprecipitation. After 6 h, precipitates were removed and culture medium was added with (TGF) or without (UNT) 10 ng of TGF-β/ml. After 12 h, culture medium was changed to medium with (IFN) or without (UNT) 500 U of IFN-γ/ml. Cells were harvested for luciferase assays 14 h later. Luciferase activity was measured as RLU per microgram of protein. Data shown are the averages of three cultures per group. Error bars represent standard errors of the means. These experiments have been repeated with similar results.
DISCUSSION
The induction of genes by the type I and II IFNs has been extensively studied and remains a foundation for our understanding of other cytokine pathways. Through elegant biochemistry, somatic mutagenesis, and gene complementation, many of the molecular mediators have been defined. For the type II IFN pathway, it is well known that IFN-γ binds to its cognate receptor, resulting in receptor phosphorylation and the docking of the STAT1 protein. STAT1 is in turn phosphorylated, and this modification leads to the formation of a homodimer (8) (Fig. 8). The homodimer is then translocated into the nucleus to bind DNA promoter elements that activate gene expression. On another level, the IRF-1 transcription factor has a STAT1-binding site in its promoter and is dependent on STAT1 for its transcriptional activation (43). Thus, STAT1 and IRF-1 are two prominent transcriptional mediators of the IFN-γ pathway.
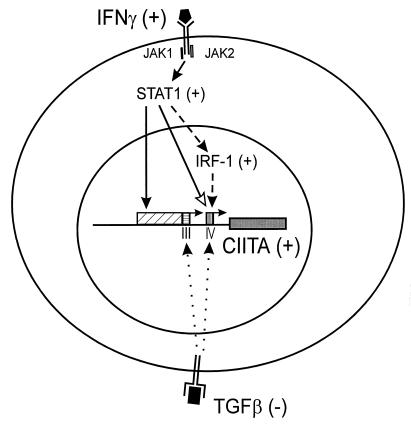
FIG. 8.
Model of the regulation of class II MHC genes by IFN-γ. B-cell constitutive expression of CIITA is mediated by the proximal 5′-flanking sequences of promoter III (horizontally hatched box). In contrast, induction of this promoter by IFN-γ is mediated by distal upstream sequences (diagonally hatched box). Binding of IFN-γ to its receptor activates JAK kinases which results in phosphorylation of STAT1. It is likely that STAT1 activation is accompanied by activation of the CIITA promoter directly via STAT1 binding to sequences in this region (black arrow). In addition, STAT1 activation induces transcription of CIITA promoter IV both by binding directly to sequences in this promoter (open arrow) and by inducing the transcription of IRF-1 that is also required for promoter activation (dashed arrow). CIITA then activates transcription of the class II MHC, Ii, and DM genes through common sequences found in the promoter regions of these genes. Binding of TGF-β to its receptor interferes with CIITA gene expression by attenuating the basal activity of both CIITA promoters. It is likely that suppression by TGF-β occurs by a mechanism that is distinct from the pathway of induction by IFN-γ.
Although many of the players in the IFN-γ pathway have been defined, one major unresolved issue in the pathway of IFN-γ-induced gene activation is the induction of class II MHC genes. We and others have shown more than a decade ago that the promoter sequences that mediate IFN-γ induction of class II MHC genes contain the W, X, and Y motifs (16, 28, 53). None of these motifs resemble any other IFN-γ consensus sequences that have been described. Using somatic genetics, two other groups have identified cell lines that are selectively defective in the IFN-γ induction of class II MHC genes but not other IFN-γ-responsive genes (27, 30). Together, these data indicated that the induction of class II MHC genes requires molecular mediators that are unique to this pathway. On the other hand, the analysis of in vitro-generated cell lines indicated that the lack of STAT1 and IRF-1 leads to the lack of and lowering of class II MHC expression, respectively. These studies clearly demonstrated that these classical molecules, identified in the IFN-γ induction pathway, are critical for the induction of class II MHC genes by IFN-γ (18, 32, 50). Therefore, class II MHC gene induction must rely on the presence of STAT1 and IRF-1, yet its own IFN-γ-responsive promoter sequences do not contain apparent STAT1 or IRF-1 binding sites.
The identification of the CIITA molecular provided the crucial missing link in the elucidation of this pathway. CIITA per se is induced by IFN-γ treatment (4, 6, 52), and its expression is greatly reduced in STAT1−/− gene knockout mice (32) and decreased in IRF-1−/− gene knockout mice (18). How STAT1 and IRF-1 control the CIITA promoter is one of the important questions addressed by the present report. Data presented here show that the answer is complex. The CIITA gene has two IFN-γ-responsive promoters: one is contiguous with the B-cell promoter (promoter III); a second uses a different transcriptional start site and lies downstream of the B-cell promoter (promoter IV) (Fig. 8). The IFN-γ responsiveness of the former is the weaker of the two when assayed in vitro, although it is clearly responsive to the addition of either IFN-γ or STAT1 but not IRF-1. It is contained in a region that is located as far as 6 kb away from the B-cell transcriptional start site. In contrast, promoter IV yields a stronger IFN-γ response in vitro, is well contained, responds to the addition of IFN-γ, and requires STAT1 and IRF-1 for optimal gene expression. The two promoters show differences in the vigor of their response to IFN-γ in vitro, but it should be noted that many important distally located promoter regions were not initially recognized until transgenic mice were used in their analysis. In contrast to in vitro analyses, such distal sequences are frequently critical for gene expression in vivo. It will be of interest to determine the respective roles of these two promoters in vivo.
The differential strengths of these two promoters may also be related to their dependence on the STAT1 and IRF-1 factors. While both STAT1 and IRF-1 activate promoter IV, promoter III is only activated by STAT1 (Fig. 8). A cooperatively between STAT1 and IRF-1 may be the reason for the enhanced response of promoter IV to IFN-γ. Interestingly, an examination of sequence of the IFN-γ-responsive region of promoter III has revealed two potential GAS sites (data not shown). The activation of STAT1 is an immediate response and does not require protein synthesis, while the activation of IRF-1 is a secondary response that requires protein synthesis and prior activation of other molecules, including STAT1. It has been shown that STAT1 mediates a faster IFN-γ response, while IRF-1 mediates a slower one. The availability of two promoters with differential responses to IRF-1 but similar responses to STAT1 may provide a mechanism to allow the fine tuning of class II MHC gene induction in different tissues. Prolonged expression of class II MHC in tissues that might be prone to autoimmune recognition would not be beneficial to the host, and these might be prevented if the tissue favors the use of promoter III and not IV. On the other hand, sustained class II MHC induction might be preferential in the elimination of pathogens, and both promoters might then be utilized. It is of interest that a careful examination of a previous report indicates to us that IFN-γ induces promoter III in some cells but not others (35). It is important to determine the differential usage of promoters III and IV by cells in various physiologic states that lead to heightened class II MHC expression, such as autoimmune disorders and immune activation by pathogenic or allogenic foreign antigens.
During the preparation of this manuscript, another group reported the induction of promoter IV (34). Their and our reports are in agreement as to the induction of promoter IV by IFN-γ. Gel shift and supershift analyses in this report additionally show the binding of IRF-1 to the CIITA promoter and the critical role of IRF-1 in activating promoter IV. In vivo footprint analyses shown in this report lend further credence to the physiologic importance of the IRF-1 target site of promoter IV in intact cells. The involvement of both of these factors was further shown in the present report by the use of IRF-1- and STAT1-negative cell lines. Our study significantly extends the former findings by demonstrating that although the IFN-γ-induced response can be mediated by both promoters III and IV, the two show differential dependency on STAT1 and IRF-1 (Fig. 8).
In contrast to the IFN-γ pathway, the induction or inhibition of genes by TGF-β is not well understood. Several early mediators of this pathway have been well studied, and it is generally accepted that the Smad2, Smad3, and Smad4 proteins are involved, as all three molecules are required for the physiologic function of TGF-β (22). More recently, two proteins termed FAST-1 and FAST-2 that also participate in this pathway have been identified (23, 60). Although it is currently a focus of much research, the exact mechanism(s) of TGF-β-induced gene expression is not yet well defined. Some studies have shown that TGF-β can activate AP-1-containing sequences (21); however, the role of AP-1 in the expression of genes that are biologically activated by TGF-β is less well established.
Even less understood is the process by which TGF-β suppresses gene expression, and the study of suppression of CIITA transcription by TGF-β could provide important insight into this. The impetus for understanding the suppression of class II MHC gene expression by TGF-β is strong, attributed to the physiologic importance of this suppression. The study of TGFβ−/− gene knockout mice demonstrated that the primary phenotype of these mice is the existence of hyperactivated T-cell responses. To determine if such responses are due to a lack of class II MHC downregulation due to the absence of TGF-β, TGF-β−/− I-A−/− double knockout mice were produced and found not to exhibit the hyperactivated T-cell state (26). This finding provides strong evidence that an important biologic role of TGF-β is to reduce the level of class II MHC expression. Uncontrolled elevation of class II MHC expression in the absence of TGF-β leads to T-cell activation and pathologic sequelae.
In light of the important physiologic context of class II MHC gene suppression by TGF-β, the present study shows that its negative regulation of class II MHC occurs through the suppression of both CIITA promoters III and IV (Fig. 8). The suppression of promoter III is more complete than that of promoter IV, which likely explains the divergent pattern of TGF-β suppression of class II MHC found in different cell types. Presumably, cells that preferentially use promoter III would be more susceptible to TGF-β suppression. Preliminary data indicates that the basal 668-bp region within promoter III is sufficient to mediate the suppression of basal activity by TGF-β (42). With this finding, the small region in each of these two promoters that mediates TGF-β suppression is well defined, such that detailed mutagenesis is feasible, and should provide important insights toward understanding how TGF-β suppresses class II MHC gene expression. Importantly, this provides a unique model for understanding how TGF-β suppresses genes that are physiologically relevant.
In conclusion, this report provides a comprehensive analysis of how two crucial cytokines control the expression of the CIITA gene. These data provide important information linking the binding of cytokines to receptors at cell surfaces to the induction or suppression of CIITA, leading ultimately to the alteration of class II MHC gene expression. Both the activator, IFN-γ, as well as the repressor, TGF-β, can alter the promoter activity of CIITA. In turn, alterations in CIITA expression control the expression of class II MHC molecules, as well as other molecules important in class II MHC antigen presentation. The IFN-γ pathway utilized for CIITA promoter activation is complex and may reflect the nature of class II MHC gene regulation in different tissues and under different physiologic conditions. The TGF-β-mediated suppression of class II MHC may be utilized to dissect the poorly understood process of TGF-β-mediated gene repression.
ACKNOWLEDGMENTS
This work was supported by grants AI029564, AI41751, AI41580, and NS34190 to J.P.-Y.T. J.F.P. is a Postdoctoral Fellow of the National Multiple Sclerosis Society (grant FG-1173-A-1).
REFERENCES
- 1.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 2.Brasier A R, Tate J E, Habener J F. Optimized use of the firefly luciferase assay as a reporter gene in mammalian cell lines. BioTechniques. 1989;7:1116–1122. [PubMed] [Google Scholar]
- 3.Chang C-H, Guerder S, Hong S-C, van Ewijk W, Flavell R A. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity. 1996;4:167–178. doi: 10.1016/s1074-7613(00)80681-0. [DOI] [PubMed] [Google Scholar]
- 4.Chang C H, Fontes J D, Peterlin M, Flavell R A. Class II transactivator (CIITA) is sufficient for the inducible expression of major histocompatibility complex class II genes. J Exp Med. 1994;180:1367–1374. doi: 10.1084/jem.180.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin K-C, Li G X, Ting J P-Y. Importance of acidic, proline/serine/threonine-rich, and GTP-binding regions in the major histocompatibility complex class II transactivator: generation of transdominant-negative mutants. Proc Natl Acad Sci USA. 1997;94:2501–2506. doi: 10.1073/pnas.94.6.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin K-C, Mao C, Skinner C, Riley J L, Wright K L, Moreno C S, Stark G R, Boss J M, Ting J P-Y. Molecular analysis of G1B and G3A IFN-γ mutants reveals that defects in CIITA or RFX result in defective class II MHC and Ii gene induction. Immunity. 1994;1:687–697. doi: 10.1016/1074-7613(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 7.Cressman, D. E., K.-C. Chin, D. J. Taxman, and J. P.-Y. Ting. Submitted for publication.
- 8.Darnell J E., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 9.Denzin L K, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II αβ dimers and facilitates peptide loading. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 10.Fling S P, Arp B, Pious D. HLA-DMA and -DMB genes are both required for MHC class II/peptide complex formation in antigen-presenting cells. Nature. 1994;368:554–558. doi: 10.1038/368554a0. [DOI] [PubMed] [Google Scholar]
- 11.Fontes J D, Jabrane-Ferrat N, Toth C R, Peterlin B M. Binding and cooperative interactions between two B cell-specific transcriptional coactivators. J Exp Med. 1996;183:2517–2521. doi: 10.1084/jem.183.6.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontes J D, Jiang B, Peterlin B M. The class II trans-activator CIITA interacts with the TBP-associated factor TAFII32. Nucleic Acids Res. 1997;25:2522–2528. doi: 10.1093/nar/25.12.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fremont D H, Hendrickson W A, Marrack P, Kappler J. Structures of an MHC class II molecule with covalently bound single peptides. Science. 1996;272:1001–1004. doi: 10.1126/science.272.5264.1001. [DOI] [PubMed] [Google Scholar]
- 14.Geiser A G, Letterio J J, Kulkarni A B, Karlsson S, Roberts A B, Sporn M B. Transforming growth factor beta 1 (TGF-β1) controls expression of major histocompatibility genes in the postnatal mouse: aberrant histocompatibility antigen expression in the pathogenesis of the TGF-β1 null mouse phenotype. Proc Natl Acad Sci USA. 1993;90:9944–9948. doi: 10.1073/pnas.90.21.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh P, Amaya M, Mellins E, Wiley D C. The structure of an intermediate in class II MHC maturation: CLIP bound to HLA-DR3. Nature. 1995;378:457–462. doi: 10.1038/378457a0. [DOI] [PubMed] [Google Scholar]
- 16.Glimcher L H, Kara C J. Sequences and factors: a guide to MHC class-II transcription. Annu Rev Immunol. 1992;10:13–49. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]
- 17.Harton, J. Personal communication.
- 18.Hobart M, Ramassar V, Goes N, Urmson J, Halloran P F. IFN regulatory factor-1 plays a central role in the regulation of the expression of class I and II MHC genes in vivo. J Immunol. 1997;158:4260–4269. [PubMed] [Google Scholar]
- 19.Improta T, Schindler C, Horvath C M, Kerr I M, Stark G R, Darnell J E., Jr Transcription factor ISGF-3 formation requires phosphorylated Stat91 protein, but Stat113 protein is phosphorylated independently of Stat91 protein. Proc Natl Acad Sci USA. 1994;91:4776–4780. doi: 10.1073/pnas.91.11.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh-Lindstrom, Y., J. F. Piskurich, Y. Wang, J. Platt, B. H. Koller, and J. P.-Y. Ting. Submitted for publication.
- 21.Kerr L D, Miller D B, Matrisian L M. TGF-β1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell. 1990;61:267–278. doi: 10.1016/0092-8674(90)90807-q. [DOI] [PubMed] [Google Scholar]
- 22.Kretzschmar M, Massague J. SMADs: mediators and regulators of TGF-β signaling. Curr Opin Genet Dev. 1998;8:103–111. doi: 10.1016/s0959-437x(98)80069-5. [DOI] [PubMed] [Google Scholar]
- 23.Labbe E, Silvestri C, Hoodless P A, Wrana J L, Attisano L. Smad2 and Smad3 positively and negatively regulate TGFβ-dependent transcription through the forkhead DNA-binding protein FAST2. Mol Cell. 1998;2:109–120. doi: 10.1016/s1097-2765(00)80119-7. [DOI] [PubMed] [Google Scholar]
- 24.Lee Y J, Han Y, Lu H T, Nguyen V, Qin H, Howe P H, Hocevar B A, Boss J M, Ransohoff R M, Benveniste E N. TGF-β suppresses IFN-γ induction of class II MHC gene expression by inhibiting class II transactivator messenger RNA expression. J Immunol. 1997;158:2065–2075. [PubMed] [Google Scholar]
- 25.Lennon A M, Ottone C, Rigaud G, Deaven L L, Longmire J, Fellous M, Bono R, Alcaide-Loridan C. Isolation of a B-cell-specific promoter for the human class II transactivator. Immunogenetics. 1997;45:266–273. doi: 10.1007/s002510050202. [DOI] [PubMed] [Google Scholar]
- 26.Letterio J J, Geiser A G, Kulkarni A B, Dang H, Kong L, Nakabayashi T, Mackall C L, Gress R E, Roberts A B. Autoimmunity associated with TGF-β1-deficiency in mice is dependent on MHC class II antigen expression. J Clin Investig. 1996;98:2109–2119. doi: 10.1172/JCI119017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loh J E, Chang C H, Fodor W L, Flavell R A. Dissection of the interferon gamma-MHC class II signal transduction pathway reveals that type I and type II interferon systems share common signaling component(s) EMBO J. 1992;11:1351–1363. doi: 10.1002/j.1460-2075.1992.tb05180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mach B, Steimle V, Martinez-Soria E, Reith W. Regulation of MHC class II genes: lessons from a disease. Annu Rev Immunol. 1996;14:301–331. doi: 10.1146/annurev.immunol.14.1.301. [DOI] [PubMed] [Google Scholar]
- 29.Mahanta S K, Scholl T, Yang F C, Strominger J L. Transactivation by CIITA, the type II bare lymphocyte syndrome-associated factor, requires participation of multiple regions of the TATA box binding protein. Proc Natl Acad Sci USA. 1997;94:6324–6329. doi: 10.1073/pnas.94.12.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao C, Davies D, Kerr I M, Stark G R. Mutant human cells defective in induction of major histocompatibility complex class II genes by interferon gamma. Proc Natl Acad Sci USA. 1993;90:2880–2884. doi: 10.1073/pnas.90.7.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin W D, Hicks G G, Mendiratta S K, Leva H I, Ruley H E, Van Kaer L. H2-mutant mice are defective in the peptide loading of class II molecules, antigen presentation, and T cell repertoire selection. Cell. 1996;84:543–550. doi: 10.1016/s0092-8674(00)81030-2. [DOI] [PubMed] [Google Scholar]
- 32.Meraz M A, White J M, Sheehan K C F, Bach E A, Rodig S J, Dighe A S, Kaplan D H, Riley J K, Greenlund A C, Campbell D, Carver-Moore K, DuBois R N, Clark R, Aguet M, Schreiber R D. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 33.Miyazaki T, Wolf P, Tourne S, Waltzinger C, Dierich A, Barois N, Ploegh H, Benoist C, Mathis D. Mice lacking H2-M complexes, enigmatic elements of the MHC class II peptide-loading pathway. Cell. 1996;84:531–541. doi: 10.1016/s0092-8674(00)81029-6. [DOI] [PubMed] [Google Scholar]
- 34.Muhlethaler-Mottet A, Di Berardino W, Otten L A, Mach B. Activation of the MHC class II transactivator CIITA by interferon-gamma requires cooperative interaction between Stat1 and USF-1. Immunity. 1998;8:157–166. doi: 10.1016/s1074-7613(00)80468-9. [DOI] [PubMed] [Google Scholar]
- 35.Muhlethaler-Mottet A, Otten L A, Steimle V, Mach B. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO J. 1997;16:2851–2860. doi: 10.1093/emboj/16.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakabayashi T, Letterio J J, Geiser A G, Kong L, Ogawa N, Zhao W, Koike T, Fernandes G, Dang H, Talal N. Up-regulation of cytokine mRNA, adhesion molecule proteins, and MHC class II proteins in salivary glands of TGF-β1 knockout mice: MHC class II is a factor in the pathogenesis of TGF-β1 knockout mice. J Immunol. 1997;158:5527–5535. [PubMed] [Google Scholar]
- 37.Nandan D, Reiner N E. TGF-β attenuates the class II transactivator and reveals an accessory pathway of IFN-γ action. J Immunol. 1997;158:1095–1101. [PubMed] [Google Scholar]
- 38.Nguyen H, Hiscott J, Pitha P M. The growing family of interferon regulatory factors. Cytokine Growth Factor Rev. 1997;8:293–312. doi: 10.1016/s1359-6101(97)00019-1. [DOI] [PubMed] [Google Scholar]
- 39.Pellegrini S, John J, Shearer M, Kerr I M, Stark G R. Use of a selectable marker regulated by alpha interferon to obtain mutations in the signaling pathway. Mol Cell Biol. 1989;9:4605–4612. doi: 10.1128/mcb.9.11.4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfeifer G P, Tanguay R L, Steigerwald S D, Riggs A D. In vivo footprint and methylation analysis by PCR-aided genomic sequencing: comparison of active and inactive X chromosome DNA at the CpG island and promoter of human PGK-1. Genes Dev. 1990;4:1277–1287. doi: 10.1101/gad.4.8.1277. [DOI] [PubMed] [Google Scholar]
- 41.Piskurich J F, Wang Y, Linhoff M W, White L C, Ting J P. Identification of distinct regions of 5′ flanking DNA that mediate constitutive, IFN-γ, STAT1, and TGF-β-regulated expression of the class II transactivator gene. J Immunol. 1998;160:233–240. [PubMed] [Google Scholar]
- 42.Piskurich, J. F. Unpublished data.
- 43.Rein T, Muller M, Zorbas H. In vivo footprinting of the IRF-1 promoter: inducible occupation of a GAS element next to a persistent structural alteration of the DNA. Nucleic Acids Res. 1994;22:3033–3037. doi: 10.1093/nar/22.15.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rigaud G, Barbaro A D, Nicolis M, Cestari T, Ramarli D, Riviera A-P, Accolla R S. Induction of CIITA and modification of in vivo HLA-DRA promoter occupancy in normal thymic epithelial cells treated with IFN-γ. J Immunol. 1996;156:4254–4258. [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 46.Scholl T, Mahanta S K, Strominger J L. Specific complex formation between the type II bare lymphocyte syndrome-associated transactivators CIITA and RFX5. Proc Natl Acad Sci USA. 1997;94:6330–6334. doi: 10.1073/pnas.94.12.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schreiber E, Matthias P, Muller M M, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherman M A, Weber D A, Jensen P E. DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity. 1995;3:197–205. doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 49.Shull M M, Ormsby I, Kier A B, Pawlowski S, Diebold R J, Yin M, Allen R, Sidman C, Proetzel G, Calvin D. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sims T N, Goes N B, Ramassar V, Urmson J, Halloran P F. In vivo class II transactivator expression in mice is induced by a non-interferon-gamma mechanism in response to local injury. Transplantation. 1997;64:1657–1664. doi: 10.1097/00007890-199712270-00005. [DOI] [PubMed] [Google Scholar]
- 51.Steimle V, Otten L A, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–146. [PubMed] [Google Scholar]
- 52.Steimle V, Siegrist C A, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. 1994;265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 53.Ting J P-Y, Baldwin A S. Regulation of MHC gene expression. Curr Opin Immunol. 1993;5:8–16. doi: 10.1016/0952-7915(93)90074-3. [DOI] [PubMed] [Google Scholar]
- 54.Ting J P-Y, Wright K L, Chin K-C, Brickey W J, Li G. The DMB promoter: delineation, in vivo footprint, trans-activation and transdominant-suppression. J Immunol. 1997;159:5457–5462. [PubMed] [Google Scholar]
- 55.White L C, Wright K L, Felix N J, Ruffner H, Reis L F, Pine R, Ting J P-Y. Regulation of LMP2 and TAP1 genes by IRF-1 explains the paucity of CD8+ T cells in IRF-1−/− mice. Immunity. 1996;5:365–376. doi: 10.1016/s1074-7613(00)80262-9. [DOI] [PubMed] [Google Scholar]
- 56.Wilson I A. Another twist to MHC-peptide recognition. Science. 1996;272:973–974. doi: 10.1126/science.272.5264.973. [DOI] [PubMed] [Google Scholar]
- 57.Mueller P R, Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989;246:780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- 58.Wright K L, Chin K-C, Linhoff M, Skinner C, Brown J A, Boss J M, Stark G R, Ting J P-Y. CIITA stimulation of transcription factor binding to MHC class II and associated promoters in vivo. Proc Natl Acad Sci USA. 1998;95:6267–6272. doi: 10.1073/pnas.95.11.6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright K L, Ting J P. In vivo footprint analysis of the HLA-DRA gene promoter: cell-specific interaction at the octamer site and up-regulation of X box binding by interferon gamma. Proc Natl Acad Sci USA. 1992;89:7601–7605. doi: 10.1073/pnas.89.16.7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou S, Zawel L, Lengauer C, Kinzler K W, Vogelstein B. Characterization of human FAST-1, a TGFβ and activin signal transducer. Mol Cell. 1998;2:121–127. doi: 10.1016/s1097-2765(00)80120-3. [DOI] [PubMed] [Google Scholar]