Abstract
Androgens regulate body composition and skeletal muscle mass in males, but the molecular mechanisms are not fully understood. Recently, we demonstrated that trenbolone (a potent synthetic testosterone analogue that is not a substrate for 5-alpha reductase or for aromatase) induces myotrophic effects in skeletal muscle without causing prostate enlargement, which is in contrast to the known prostate enlarging effects of testosterone. These previous results suggest that the 5α-reduction of testosterone is not required for myotrophic action. We now report differential gene expression in response to testosterone versus trenbolone in the highly androgen-sensitive levator ani/bulbocavernosus (LABC) muscle complex of the adult rat after 6 weeks of orchiectomy (ORX), using real time PCR. The ORX-induced expression of atrogenes (Muscle RING-finger protein-1 [MuRF1] and atrogin-1) was suppressed by both androgens, with trenbolone producing a greater suppression of atrogin-1 mRNA compared to testosterone. Both androgens elevated expression of anabolic genes (insulin-like growth factor-1 and mechano-growth factor) after ORX. ORX-induced increases in expression of glucocorticoid receptor (GR) mRNA were suppressed by trenbolone treatment, but not testosterone. In ORX animals, testosterone promoted WNT1-inducible-signaling pathway protein 2 (WISP-2) gene expression while trenbolone did not. Testosterone and trenbolone equally enhanced muscle regeneration as shown by increases in LABC mass and in protein expression of embryonic myosin by western blotting. In addition, testosterone increased WISP-2 protein levels. Together, these findings identify specific mechanisms by which testosterone and trenbolone may regulate skeletal muscle maintenance and growth.
Keywords: Androgen, Skeletal muscle, Hypogonadism, Hypertrophy, Atrophy
1. Introduction
Loss of muscle mass and bone mineral density (BMD) with aging predisposes individuals to increased risk of disability, falls, and dependency in the elderly [1,2]. Many factors contribute to this deleterious process, such as decreased physical activity, nutritional deficits, and reductions in anabolic stimuli, including growth hormone, insulin-like growth factor-1 (IGF-1), and testosterone [3]. In particular, the decline in testosterone that occurs with age may contribute to reduced muscle strength in men [4]. In addition, androgen deprivation has been shown to prevent muscle regeneration in aging mice [5]. As such, androgen therapy for older hypogonadal men holds promise for maintaining and increasing muscle mass [3], and for improving clinical outcomes and quality of life [6].
Recent research has focused on the safety and efficacy of testosterone replacement therapy [7] and of treatment with other androgens or selective androgen receptor modulators (SARMs) that are designed to prevent both sarcopenia and osteopenia [8]. Ideally, these treatments would produce beneficial responses in tissues of interest (e.g., muscle and bone), while avoiding androgenic activity in other tissues (e.g., prostate) [8]. In this regard, trenbolone (a highly potent non-5α-reducible and non-estrogenic synthetic testosterone analogue) holds great promise. The affinity of trenbolone for the human and rodent androgen receptor is 3-fold higher than that of testosterone and approximately equal to that of dihydrotestosterone (DHT) [8]; however, trenbolone produces less potent effects than testosterone in the prostate. As evidence, our laboratory has reported that low-dose trenbolone produces myotrophic action in muscle and prevents hypogonadism-induced bone loss in orchiectomized rats in a magnitude equal to that of supraphysiologic testosterone, without inducing prostate enlargement [9,10]. However, the mechanisms by which testosterone and trenbolone increase muscle mass remain poorly understood.
Skeletal muscle consists primarily of postmitotic fibers, which undergo constant remodeling and regeneration throughout the life span. The phosphoinositide 3-kinase (PI3K)-Akt1 pathway is considered a primary signaling pathway regulating muscle protein synthesis [11]. This pathway leads to the regulation of mTOR kinase, which is sufficient to induce muscle hypertrophy by enhancing integral components of the protein synthesis machinery [12]. IGF-1 is a key activator of the PI3K-Akt1 pathway [11]. The importance of local muscle-produced IGF-I in hypertrophy is emphasized by the normal growth seen in mice that lack the circulating ‘liver’ form of IGF-I [13], and by increased IGF-I mRNA levels in the overloaded muscles of surgically hypophysectomized rats [14]. In humans, testosterone deficiency is associated with reduced circulating IGF-1 [15] and intramuscular IGF-1 expression [16]. The ubiquitin–proteasome pathway increases protein breakdown during skeletal muscle atrophy. Two ubiquitin ligases, Muscle Ring Finger1 (MuRF1) and Muscle Atrophy F-box (MAFbx) also called Atrogin-1 [17,18], serve as markers of skeletal muscle atrophy under a multitude of catabolic perturbations, such as fasting, diabetes, cancer, renal failure, and experimental sepsis [17,19,20].
Testosterone induces expression of the myogenic regulatory factor MyoD and of myosin heavy chain, suggesting that testosterone may promote muscle regeneration [21]. Wingless/Int (Wnt) is a family of secreted glycoproteins that regulate cell proliferation and differentiation [22]. Testosterone activates Wnt signaling, which allows β-catenin to translocate to the nucleus where it activates transcription of Wnt regulated target genes and regulates the differentiation of satellite cells [23]. In adult skeletal muscle, Wnt signaling influences satellite cells, but the precise downstream effect is still under debate. A common target for both androgen and estrogen receptors, Wnt1-inducible signaling protein 2 (WISP2) has been demonstrated to be activated by both androgens and estrogens in cattle and sheep muscle [24].
The purpose of the current study was to examine the long-term effects of orchiectomy and androgen (testosterone or trenbolone) treatment on expression of anabolic and catabolic genes in an androgen sensitive muscle in mature male rats. Furthermore, we characterized the effects of both androgens on muscle regeneration by measuring the protein levels of embryonic myosin. In addition, we aimed to detect potential differences in mechanisms of action between testosterone and trenbolone in regulating muscle mass.
2. Materials and methods
2.1. Animal care
All experimental procedures conformed to the ILAR Guide to the Care and Use of Experimental Animals and were approved by the Institutional Animal Care and Use Committee at the Gainesville VA Medical Center. Barrier-raised and viral pathogen-free Fischer F344/Brown Norway male rats aged 10 months were obtained from Charles River Laboratories (Wilmington, MA). Animals were individually housed in an accredited animal facility at the Gainesville VA Medical Center under a 12 h light–12 h dark cycle. All rats underwent a one week acclimatization period prior to beginning experimental interventions. Rats were fed a diet of Purina rodent chow containing 3.3 kcal/g, distributed as 58.9% carbohydrate, 12.4% fat and 28.7% protein (No. 5001, Purina Mills, St. Louis MO) and tap water ad libitum.
2.2. Experimental design
Rats were divided into 4 groups (n = 10/group), including: sham surgery plus vehicle (SHAM), orchiectomy (ORX), ORX+(7.0 mg·week-1) testosterone-enanthate (TE) (ORX + TE), and ORX+(1.0 mg·week-1) trenbolone-enanthate (ORX + TREN). All treatment groups except SHAM group underwent bilateral orchiectomy and removal of epididymal fat under isoflurane anesthesia (5% induction, 1.5–2.5% maintenance), and received buprenorphine analgesia to reduce pain. A nutritional supplement (Jell-O cube with added protein and fat) (NIH protocol diet) was provided following the surgery for one week in order to minimize weight loss resulting from surgery. One week after the surgery, rats received weekly intramuscular (quadriceps) injections of vehicle (sesame oil) or androgen alternatively for five weeks. At day 42, rats were euthanized via intraperitoneal pentobarbital (120 mg/kg) injection. The levator ani/bulbocavernosus (LABC) muscle was removed, weighed, and snap frozen in liquid nitrogen and stored at −80 °C until further analysis. Previously, we have reported serum androgen levels, prostate weight, bone morphological and muscle adaptations, and hemoglobin and adipocytokine changes [25].
2.3. Hormone delivery
Testosterone-enanthate (Savient Pharmaceutical, East Brunswick, NJ), trenbolone-enanthate (Steraloids, Newport, RI), and vehicle were dissolved in sesame oil and administered (0.1 mL) once every seven days, under isoflurane anesthesia, into the quadriceps musculature. Injections were alternated between legs to reduce possible discomfort of repeated injections. Previous studies from our laboratory have reported that once-weekly supraphysiological (7.0 mg/week) TE administration [26–28] or once weekly TREN (1.0 mg/week) [10] administration successfully prevents skeletal muscle and bone loss in growing ORX rodents (aged 3 months).
2.4. Real-time PCR assessment of the gene expression
Real-time PCR was performed on the LABC muscle complex from a subset (n = 6) of animals from each group. Samples were chosen that best approximated the mean LABC mass of each group in order to obtain a representative sampling from each group. Total RNA was extracted from the LABC muscle with TRIzol Reagent (Invitrogen Life Technologies, Carlsbad, CA). The RNA was solubilized in nuclease-free H2O, incubated with DNase I (Invitrogen Life Technologies, Carlsbad, CA, USA) to remove any DNA present in the sample. Total RNA quantity was determined by absorbance at 260 nm using a NanoVue spectrophotometer (GE Healthcare, USA). For each sample, cDNA was synthesized from 1 microgram of RNA using a RETRO-script first-strand synthesis kit (Ambion, Austin, TX, USA). cDNA was used as a template for real-time PCR using the primers listed below and a 7300 real-time PCR system (Applied Biosystems, Foster City, CA, USA). TaqMan probe-based chemistry was used to detect PCR products, and quantification was performed using a relative standard curve. Primers were purchased from Life technologies: IGF-1 (Rn00710306_m1), mechano-growth Factor (MGF, Rn01503688_m1), androgen receptor (AR, Rn00560747_m1), atrogin-1 (Rn00591730_m1), MuRF-1 (Rn00590197_m1), glucocorticoid receptor (GR, Rn00561369_m1), WISP-2 (Rn00580932_m1), mTOR (Rn00571541_m1), and 18S (Hs99999901_s1). 18 s was used as an internal control and for normalization, as no differences were identified between any groups.
2.5. Western blots and proteomics
Muscle homogenates (n = 6/group) were subjected to western blot analysis as previously described [29,30]. LABC muscle was disrupted in RIPA buffer using a motorized mortar and pestle (DAKO). Protein concentration was determined (DC Protein Assay, Biorad) and aliquots of lysate containing equal amounts of protein (15–20 μg) were subjected to electrophoresis and transferred to nitro-cellulose (NuPage System, Invitrogen). Membranes were blocked for 1 h (TBS-Tween 20/0.05% milk). Primary antibody (rabbit anti-embryonic myosin 1:200, DSHB #F1.652; rabbit anti-cyclophilin A 1:2000, Santa Cruz #sc-133494; rabbit anti-WISP-2 1:200, Santa Cruz # sc-25442) was applied overnight at 4 °C. For embryonic myosin analysis, membranes were washed and incubated in secondary antibody (HRP-conjugated goat-anti-rabbit or -anti-mouse, 1:2000, Rockland) at room temperature for 1 h. Membranes were exposed to ECL detection (West Pico ECL, Pierce) and developed for 1–5 min. For proteomic analysis of bands following western blot, new gels were run and stained with Coomassie Blue Dye. Gels were destained (20% methanol:10% acetic acid) and submitted for analysis to the University of Florida Mass Spectrometry Core within the Interdisciplinary Center for Biotechnology Research (ICBR). Samples were excised from the destained gels, subjected to laboratory standard sample clean up, and then analyzed via HPLC mass spectrometry. The 95 kDa band of interest (embryonic myosin band) was sequenced by the University ICBR and found to have 100% sequence homology to myosin heavy chain. Sample protein sequencing results were analyzed using Scaffold software (http://www.proteomesoftware.com/). For western blot analysis, embryonic myosin expression was normalized to Cyclophilin A expression and a ratio of signal strength calculated using Image J software. For WISP-2 analysis, membranes were washed and incubated (light protected) in secondary antibody (LiCor goat anti rabbit IRDye 680-conjugated antibody, 1:10,000) at room temperature and exposed to the 700 nm filter on an Odyssey Imaging system. Image J software was employed for densitometric quantitation protein bands [29,30].
2.6. Data analysis
A one-way ANOVA was used for group comparisons. Post-hoc testing for ANOVAs was performed using Holm-Sidak post hoc test. A significance level of p < 0.05 was used for all comparisons. If the variances between groups were not equal (according to the Levene’s test), dependent variables were separately analyzed using the Kruskal–Wallis test, which is robust to outliers. When indicated, pair wise comparisons were further evaluated with the Mann–Whitney U test. All data were represented as mean ± standard error.
3. Results
3.1. Body weight and muscle mass
We have previously reported body weight and muscle mass data from this study [9]. Briefly, no significant differences in body weights were observed between groups. At sacrifice, the body weights were 444 ± 17 g (SHAM), 420 ± 13 g (ORX), 405 ± 11 g (ORX + TE), and 413 ± 8 g (ORX + TREN). LABC mass in ORX animals (0.68 ± 0.03 g) was 36% lower than SHAMs (1.07 ± 0.04 g) (p < 0.01). In contrast, LABC mass in ORX + TE (1.54 ± 0.03 g) and ORX + TREN animals (1.54 ± 0.04 g) was 44% higher than SHAMs (p < 0.01) and 126% higher than ORX animals (p < 0.01). No difference in LABC mass was present between ORX + TE and ORX + TREN animals (Table 1).
Table 1.
Body weight and LABC muscle mass.
| SHAM | ORX | ORX + TE | ORX + TREN | |
|---|---|---|---|---|
| Body weight (g) | 444 ± 17 | 420 ± 13 | 405 ± 11 | 413 ± 8 |
| LABC muscle weight (g) | 1.07 ± 0.04 | 0.68 ± 0.03a,c,d | 1.54 ± 0.03a,b | 1.54 ± 0.04 a,b |
Data are presented as mean ± SEM, n = 6/group.
Letters a–d indicate differences from respectively, labeled groups (a = vs. SHAM, b = vs. ORX, c = vs. ORX + TE, d = vs. ORX + TREN) at p < 0.01.
3.2. Muscle atrophy relevant genes expression
At 42 days after orchiectomy, MuRF1 and atrogin-1 mRNA expression in LABC muscle of ORX animals were 3-fold and 2-fold higher than SHAMs, respectively (Fig. 1, p < 0.05). TE and TREN prevented this increase, resulting in values not different from SHAMs. Furthermore, the reduction in atrogin-1 mRNA was greater following TREN treatment compared with TE treatment (p < 0.05).
Fig. 1.
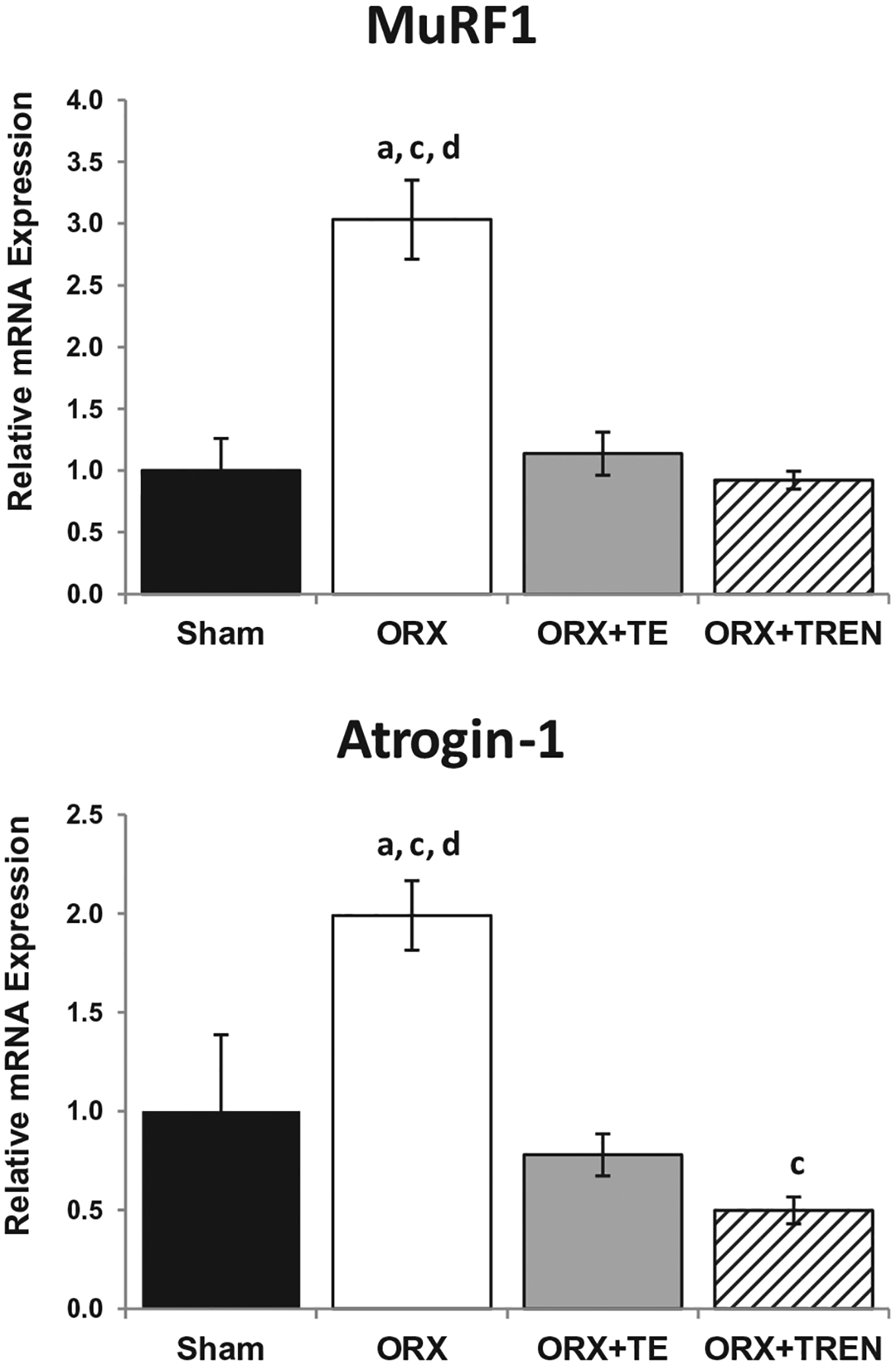
Atrogene (MuRF-1 and atrogin-1) mRNA expression in LABC muscle 42 days following Sham surgery or orchiectomy (ORX) alone or in combination with testosterone-enanthate (ORX + TE) or trenbolone-enanthate (ORX + TREN). Data are presented as mean ± SEM, n = 6/group. Letters a–d indicate differences from respectively, labeled groups (a = vs. SHAM, b = vs. ORX, c = vs. ORX + TE, d = vs. ORX + TREN) at p < 0.05.
3.3. Muscle hypertrophy relevant genes expression
No difference in WISP-2 gene expression was present between SHAM, ORX, and ORX + TREN animals. Conversely, WISP-2 expression was 3-fold higher in the ORX + TE group compared with ORX and ORX + TREN animals and 7-fold higher than SHAMs (p < 0.05). mTOR gene expression was not different among groups (Fig. 2).
Fig. 2.
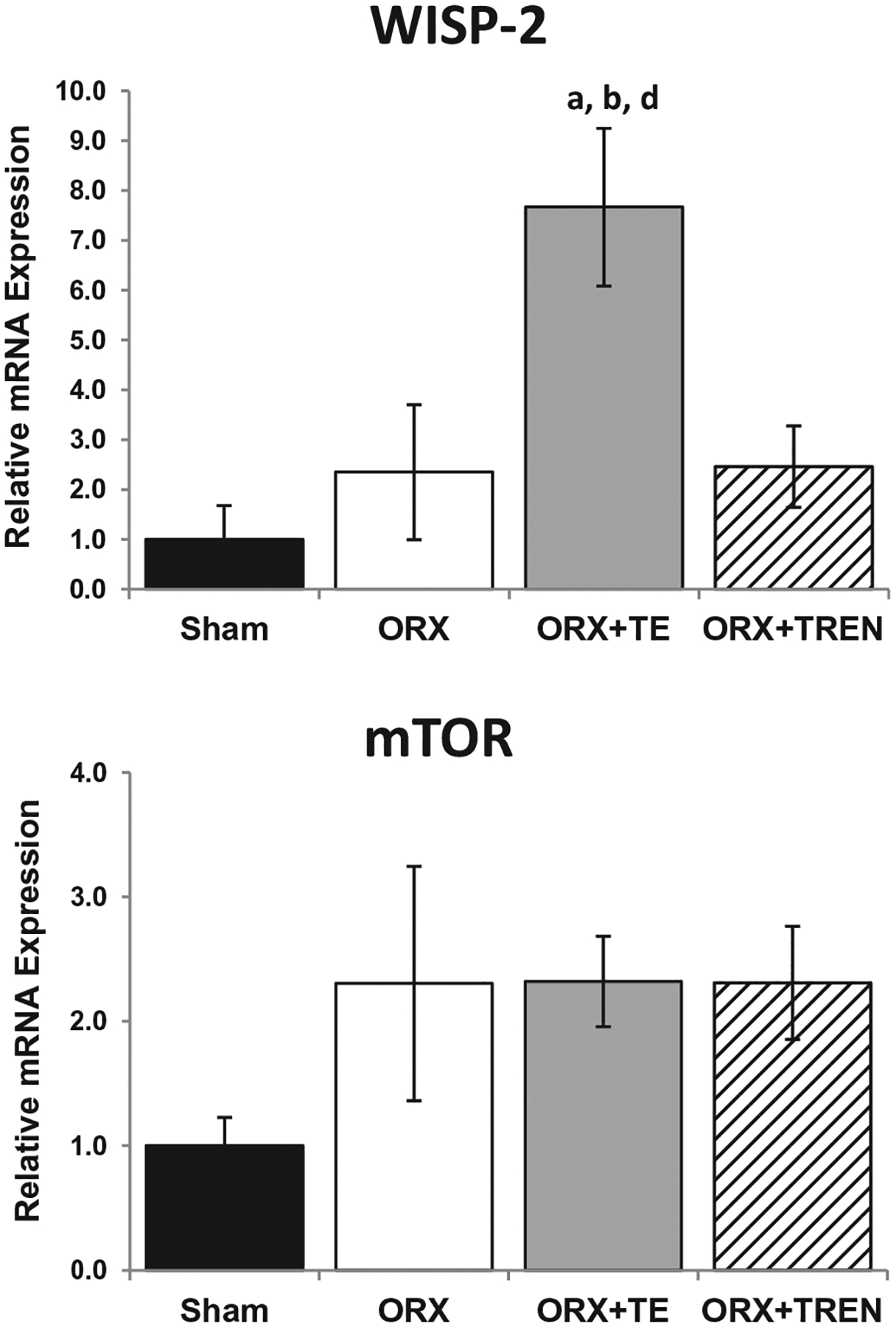
WISP-2 and mTOR mRNA expression in LABC muscle 42 days following Sham surgery or orchiectomy (ORX) alone or in combination with testosterone-enanthate (ORX + TE) or trenbolone-enanthate (ORX + TREN). Data are presented as mean ± SEM, n = 6/group. Letters a–d indicate differences from respectively, labeled groups (a = vs. SHAM, b = vs. ORX, c = vs. ORX + TE, d = vs. ORX + TREN) at p < 0.05.
3.4. Growth factor genes expression
ORX did not alter (total) IGF-1 mRNA expression in the LABC muscle compared with SHAMs. Conversely, (total) IGF-1 expression was ~5.0-fold higher in the LABC muscle of ORX + TE and ORX + TREN rats compared with ORX animals. MGF has been proposed to be a more potent form of IGF-I capable of promoting skeletal muscle hypertrophy and muscle cell proliferation [31]. No difference for MGF mRNA expression was present in the LABC muscle complex between ORX and SHAM animals. However, MGF mRNA expression was increased >5-fold in the LABC muscle of both ORX + TE (p = 0.015) and ORX + TREN (p = 0.039) animals compared with ORX group. Additionally, MGF mRNA was 4-fold higher in ORX + TE animals compared with SHAMs (p = 0.03) and a trend (p = 0.076) suggests 3.6-fold higher MGF mRNA in ORX + TREN animals compared with SHAMs (Fig. 3).
Fig. 3.
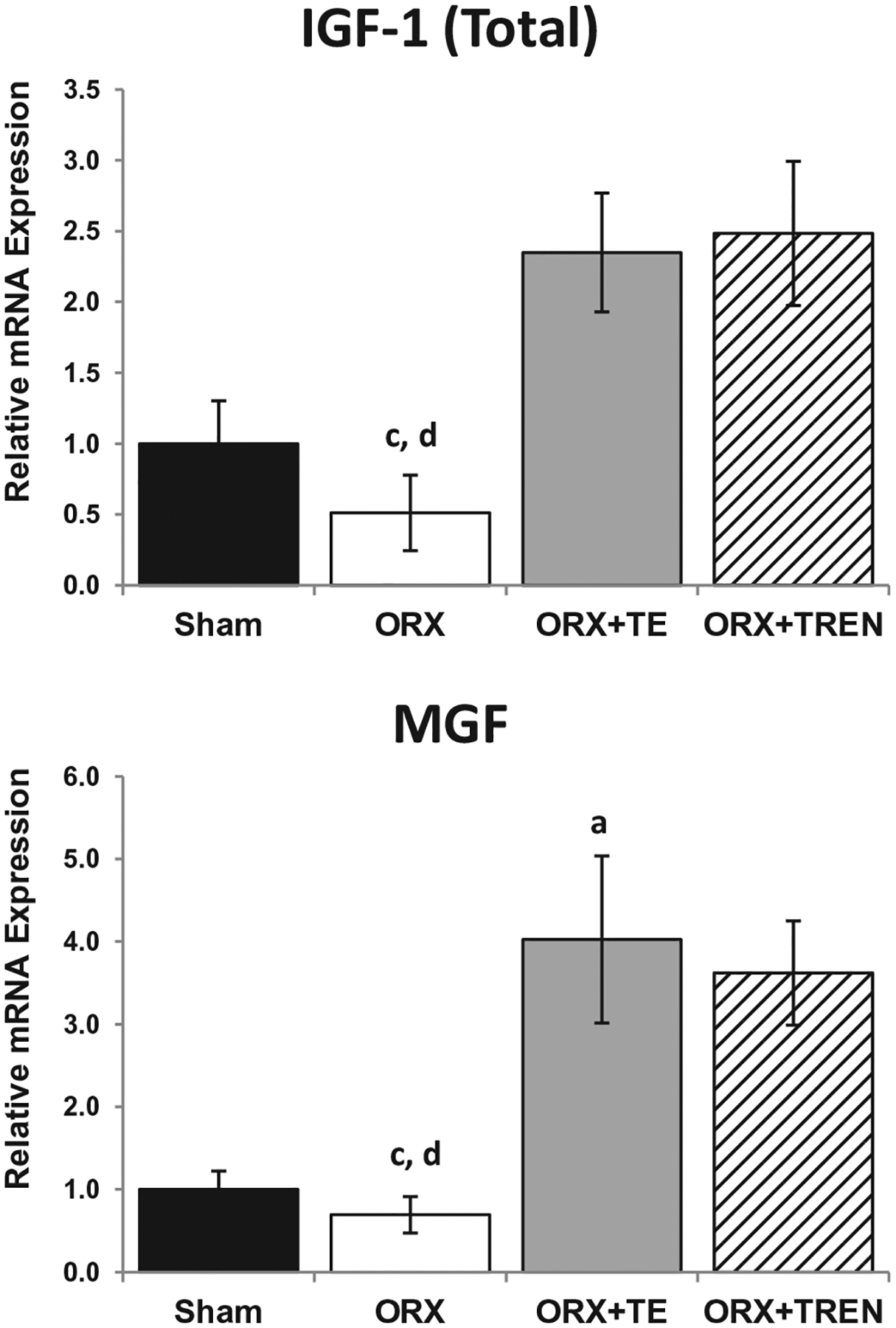
IGF-1 and MGF mRNA expression in LABC muscle 42 days following Sham surgery or orchiectomy (ORX) alone or in combination with testosterone-enanthate (ORX + TE) or trenbolone-enanthate (ORX + TREN). Data are presented as mean ± -SEM, n = 6/group. Letters a–d indicate differences from respectively, labeled groups (a = vs. SHAM, b = vs. ORX, c = vs. ORX + TE, d = vs. ORX + TREN) at p < 0.05.
3.5. Androgen receptor and glucocorticoid receptor gene expression
GR mRNA expression was 3.6-fold higher in ORX rats compared with SHAMs. TREN treatment prevented the ORX-induced increase in GR mRNA expression (p = 0.003) and a trend (p = 0.068) indicated that TE treatment may have also prevented this increase. GR expression was 50% lower in ORX + TREN animals compared with ORX + TE (p = 0.01). AR expression was not different amongst groups (Fig. 4).
Fig. 4.
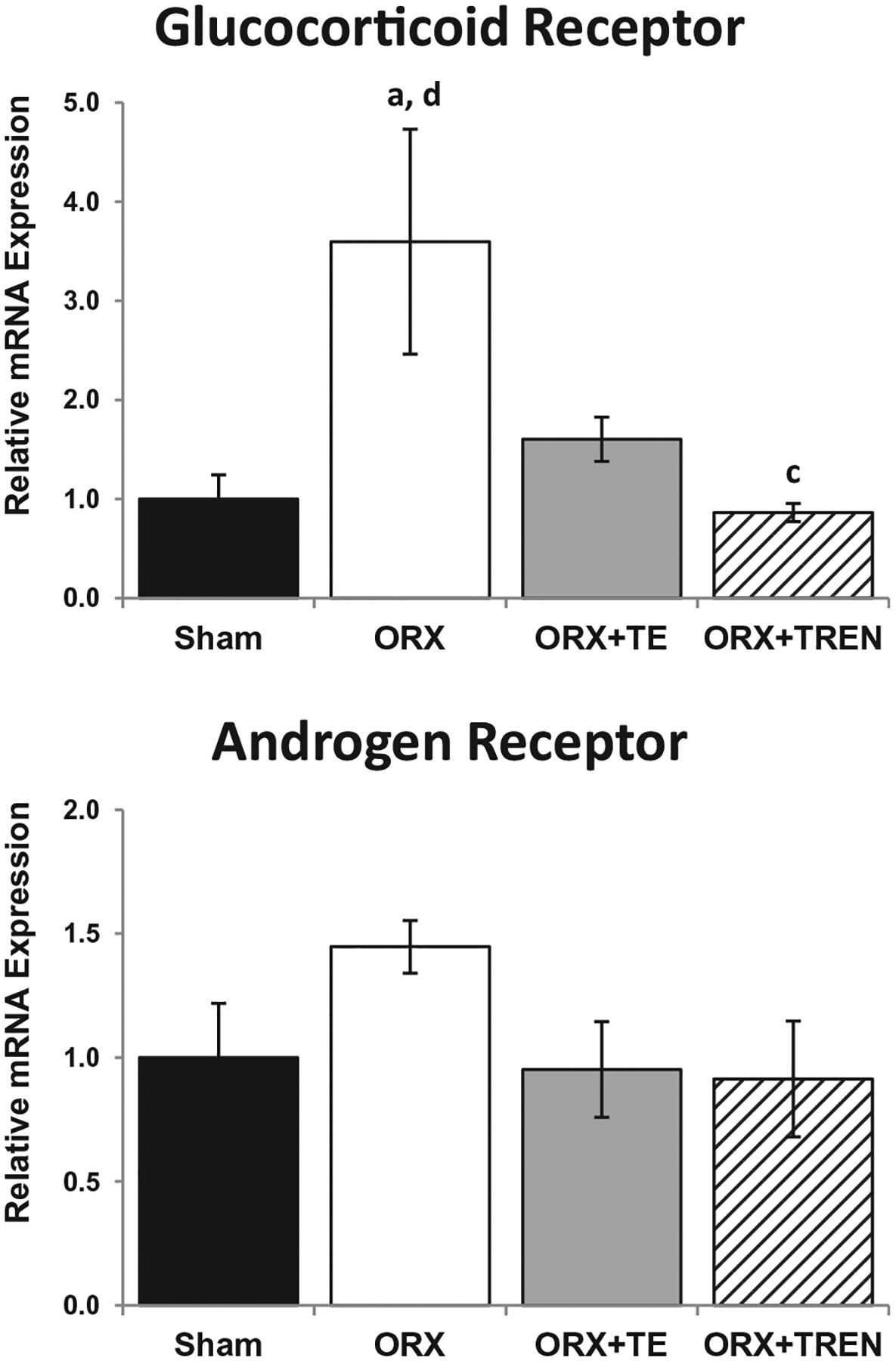
GR and AR mRNA expression in LABC muscle 42 days following Sham surgery or orchiectomy (ORX) alone or in combination with testosterone-enanthate (ORX + TE) or trenbolone-enanthate (ORX + TREN). Data are presented as mean ± -SEM, n = 6/group. Letters a–d indicate differences from respectively labeled groups (a = vs. SHAM, b = vs. ORX, c = vs. ORX + TE, d = vs. ORX + TREN) at p < 0.05.
3.6. Embryonic myosin and WISP-2 protein expression
In order to determine if LABC muscle was undergoing active growth we analyzed protein expression of embryonic myosin. Densitometric quantitation of bands (Fig. 5A and B) demonstrates low expression in SHAM and ORX animals, while those treated with TE and TREN exhibited a significantly greater amount of regenerative myosin expression (p < 0.001). While intact myosin heavy chain (MHC) is >220 kDa, we detected a 95 kDa band that likely represents the S1 region of MHC. To confirm the identity of the 95 kDa band, it was excised from gradient gels and subjected to HPLC/scaffold protein identification analysis (Fig. 5C). 100% sequence homology with several myosin family proteins was observed. WISP-2 protein was detected in all samples. There was no difference in sham or ORX thus they were treated as a single group. WISP-2 protein levels were increased only slightly in ORX + TREN animals. This increase was more pronounced in ORX + TE animals (p = 0.041, Fig. 6).
Fig. 5.
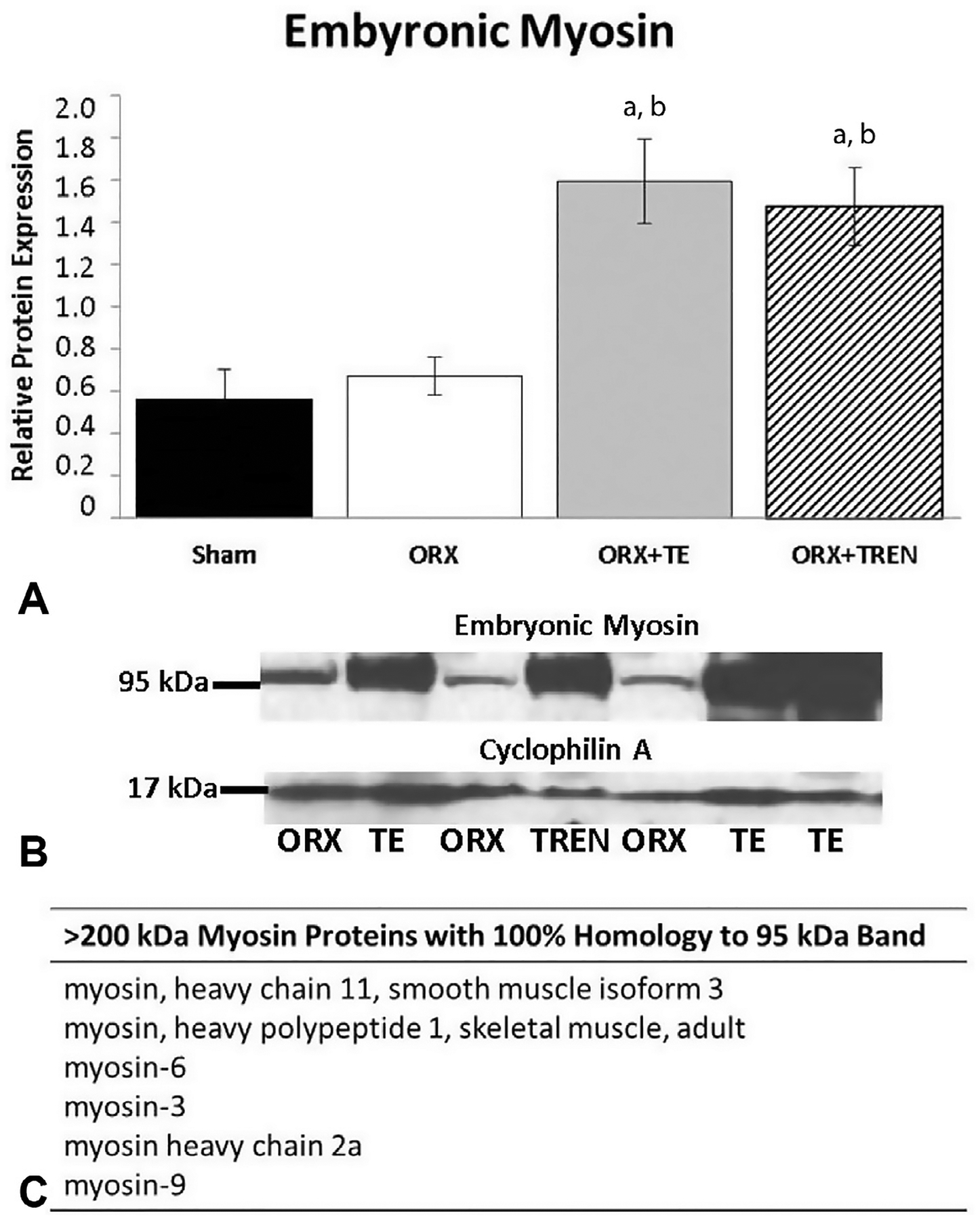
Embyonic myosin relative protein expression in LABC muscle 42 days following Sham surgery or orchiectomy (ORX) alone or in combination with testosterone-enanthate (ORX + TE) or trenbolone-enanthate (ORX + TREN). (A) Densitometric quantitation of relative protein expression. Letters a–d indicate differences from respectively labeled groups (a = vs. SHAM, b = vs. ORX, c = vs. ORX + TE, d = vs. ORX + TREN) at p < 0.05. (B) Representative western blot showing 95 kDa embryonic myosin band and 17 kDa cyclophylin A band. (C)>200 kDa myosin proteins that share 100% homology with the 95 kDa band. Data are presented as mean ± SEM, n = 6/group.
Fig. 6.
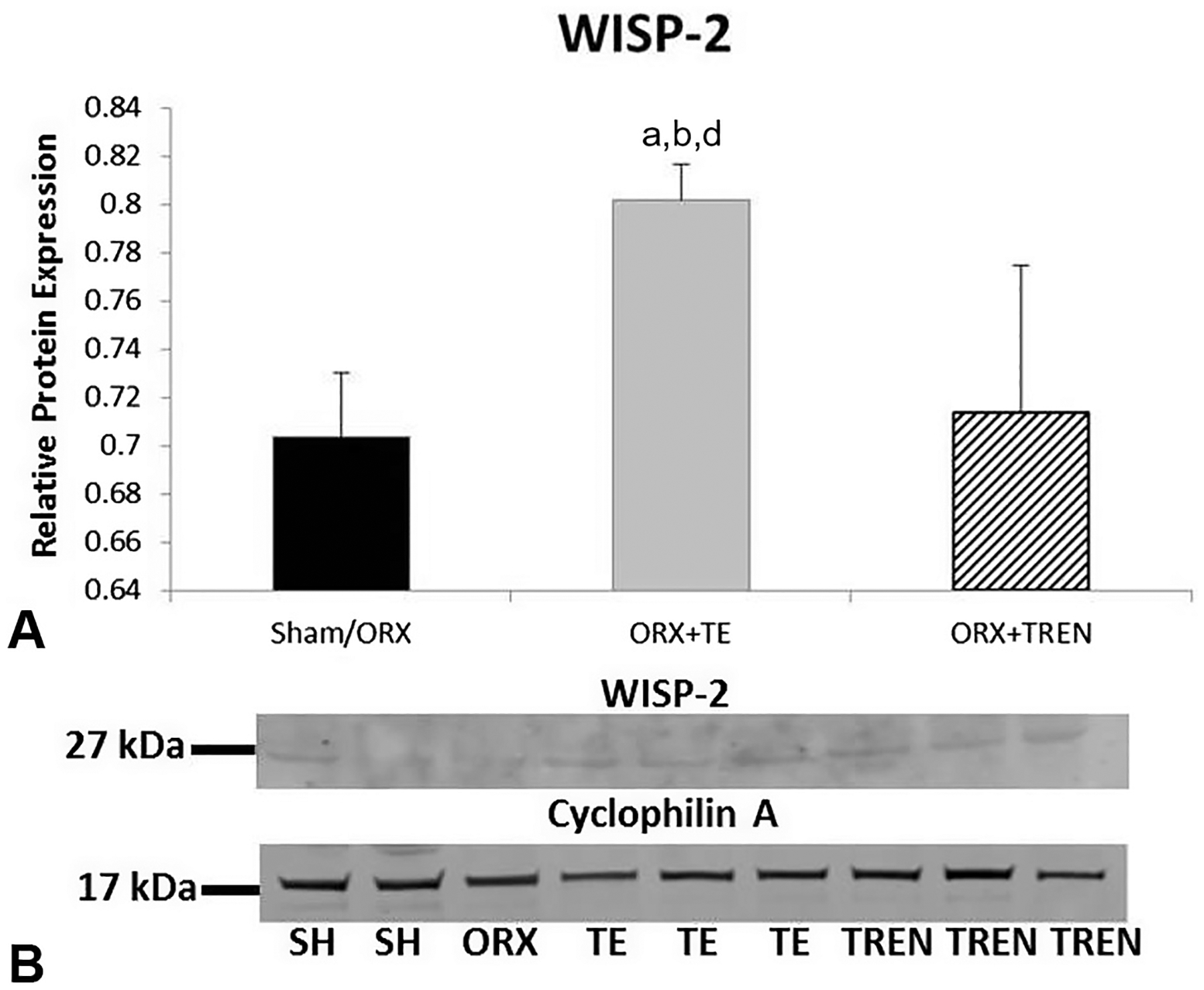
WISP relative protein expression in LABC muscle 42 days following Sham surgery or orchiectomy (ORX) alone or in combination with testosterone-enanthate (ORX + TE) or trenbolone-enanthate (ORX + TREN). (A) Densitometric quantitation of relative protein expression. Letters a–d indicate differences from respectively labeled groups (a = vs. SHAM, b = vs. ORX, c = vs. ORX + TE, d = vs. ORX + TREN) at p < 0.05. (B) Representative western blot showing 27 kDa WISP-2 band and 17 kDa cyclophylin A band. Data are presented as mean ± SEM, n = 6/group.
4. Discussion
Androgens are important regulators of body composition during postnatal development and aging. In addition, androgen treatment promotes muscle development and maintenance; although, the molecular basis of androgen-induced myotrophic effects remains largely unknown. In this study, we used the LABC muscle of the male rat, which is exquisitely sensitive to androgen status to identify and compare molecular/cellular responses to testosterone and trenbolone (a highly potent non-5α-reducible and non-estrogenic synthetic testosterone analogue) treatment. The LABC muscle is non-weight bearing and is therefore not influenced by reductions in quadrupedal ambulation that result from sex-steroid deficiency. LABC muscle expresses a higher density of androgen receptors than are found in other skeletal muscles [32]. We demonstrated that ORX resulted in chronic up-regulation of atrogenes (MuRF1 and atrogin-1) and that both androgens reversed the expression of these catabolic genes, with TREN treatment reducing atrogin-1 gene expression more so than TE. In addition, both TE and TREN increased anabolic (IGF-1 and MGF) gene expression in the LABC muscle. We also present the novel findings that the ORX-induced elevation in GR mRNA expression was suppressed by TREN treatment, while WISP-2 gene and protein expression was enhanced by TE, but not TREN. Further, we provide evidence that both TE and TREN promoted muscle growth by increasing embryonic myosin protein levels. There are two unique aspects to this study: (1) we employed a long-term treatment model to study the transcriptional program in skeletal muscle in a chronic time-frame, while most studies identify gene expression at the early stage of androgen administration and (2) to our knowledge, this is the first study directly comparing the androgen-induced myotrophic mechanisms between TE and TREN [8]. Our study provides insight into the role of the 5α-reductase enzyme in mediating the myotrophic effects of androgens.
To characterize the role of androgens in protein degradation, we focused on the E3 ubiquitin ligases MuRF1 and atrogin-1, which play central roles in muscle atrophy and have been designated as markers of skeletal muscle protein degradation [17,18]. Over the course of 7 days, castration has been shown to induce a larger increase in atrogin-1 and MuRF-1 gene expression than is observed with other skeletal atrophy models such as cachexia or fasting [33]. We expand on these results by demonstrating for the first time that elevated atrogin-1 and MuRF-1 mRNA levels were maintained 42 days after ORX and that chronic administration of either TE or TREN repressed expression of these two atrophic genes to the level of intact animals. Additionally, our study is the first to demonstrate that TREN treatment down-regulates atrogin-1 mRNA expression to a greater extent than TE following castration. This may be a result of trenbolone having a higher affinity for the androgen receptor than testosterone [8]. Although the catabolic roles of both MuRF1 and atrogin-1 are well established, changes in expression of each atrogene may differ by experimental model, which we speculate could indicate distinctions in their mechanisms of action. For example, atrophy was elicited in models of cirrhosis and burn, without changes of atrogin-1 mRNA expression [34,35]. Further, MuRF-1 −/− animals showed relative sparing of fiber size and tension following glucocorticoid myopathy, which was attributed to the preservation of the rate of protein synthesis [36]. MuRF-1 has been shown to mainly regulate myofibrillar proteins such as actin, myosin heavy chain, and myosin light chains 1; while atrogin-1 functions as a ligase for several regulatory proteins such as MyoD and eIF3f [37–39]. Clearly, suppression of ORX-induced elevations in MuRF-1 and atrogin-1 at least partially mediates the ability of androgens to preserve muscle mass; although, this may not explain the androgen-induced myotrophic actions we observed because MuRF-1 and atrogin-1 were only reduced to the level of intact SHAMs, while LABC muscle mass was increased >40% above SHAMs; suggesting that both anti-catabolic and anabolic processes are involved in androgen-induced muscle growth.
The Akt/mTOR signaling pathway is an acknowledged regulator of skeletal muscle mass. Activation of mTOR and its downstream signals is sufficient to induce muscle hypertrophy by enhancing integral components of the protein synthetic machinery [12]. Previously, testosterone has been shown to rapidly increase mTOR expression in myotubes [40]. In contrast, we report no difference between ORX and androgen treatment in the LABC muscle levels of mTOR mRNA gene expression after 42 days, which is consistent with some other studies [41,42]. Hourde et al. found that there is no change in Akt/mTOR signaling pathway after 3 months of castration or combined with androgen treatment [42]. The different findings in the literature could relate to the experimental design, such as the duration of treatment. For example, most previous studies demonstrate the effects of testosterone on mTOR gene expression at an early time point, while we examined these changes at a more chronic time point. As such, it appears that mTOR expression may be rapidly upregulated following androgen administration, yet remains only transiently elevated [43]. Finally, it could be possible that phosphorylation of mTOR is more responsive to androgen treatment. As evidence, White et al. reported in mice, that nandrolone increases phosphorylated mTOR protein concentrations after 4 weeks of treatment [44]. Certainly, more research is warranted concerning the long term effect of androgen in modulating mTOR activity in castrated animals.
One of the most notable findings from the current study is the TE-induced upregulation of WISP-2, which was first identified as being up-regulated in C57MG cells transformed by the Wnt-1 retrovirus [45]. The Wnt is a family of proteins that act as a molecular switch in the regulation of myogenesis [22]. It is reported that androgen regulates Wnt ligands signaling via the downstream effector β-catenin, as myogenic differentiation in mesenchymal multipotent cells is promoted by inducing testosterone and β-catenin interaction [46]. Upregulation of muscle β-catenin occurs during exercise and load-induced muscle hypertrophy [23], and it is required for muscle regeneration [46]. Furthermore, previous studies have demonstrated that WISP-2 is enhanced by estrogen, progesterone, and IGF-1 [47–49] and is upregulated in estrogen-induced growth in breast tumor cell line [50]. In our study, TE enhanced WISP-2 gene and protein expression while TREN did not. This difference may result from the ability of testosterone to undergo aromatization to estradiol, while trenbolone is non-estrogenic as a result of the 3-oxotriene structure of this androgen [8]. Interestingly, WISP2 is induced by testosterone in rat prostate epithelial cells indicating its role in prostate cancer development [51]; however, it remains unknown whether trenbolone treatment alone alters prostate WISP2 expression.
Both AR and GR signaling are known to influence skeletal muscle mass. In particular, androgen treatment increases skeletal muscle AR expression in humans and rats [52]. However, the upregulation of AR expression is mostly observed at an early stage, such as 60 min following testosterone treatment in skeletal muscle myotubes [43]. Interestingly, muscle AR mRNA was not different in trenbolone implanted steers compared to non-implanted control animals at days 7, 14, or 28 [53]. In the current study, we did not observe differences in AR expression 42 days after ORX or androgen treatment, perhaps because the elevation in AR expression occurs at an earlier time point or perhaps because an upregulation of AR expression is not required for androgen-induced myotrophic action. The extent to which the anabolic effects of androgens are mediated directly through the increased androgen receptor expression remains uncertain, since chemically-induced androgen suppression (via goserelin) [54] does not blunt increases in muscle strength and mass in response to strength training. Besides direct androgenic effects, androgens can indirectly promote muscle growth and regeneration by modulating GRs [55] or by stimulating GH/IGF-1 secretion [56]. The GR is an important modulator of skeletal muscle mass and acts by promoting muscle catabolism [57]. In this regard, anticatabolic mechanisms altered by testosterone may be associated with reductions in endogenous glucocorticoid activity in young growing rats [58]. In addition, it has been reported that GR activation is inhibited by IGF-1/AKT/mTOR signaling pathway and by stimulated expression of atrogenes, such as FOXO [59]. In our study, GR gene expression was increased after castration, and the upregulation of GR was prevented by androgen treatment, with TREN resulting in a greater reduction compared with TE. It has been shown that treatment of sheep with trenbolone also reduces muscle GR expression [60]. Our data indicate that GR expression within the LABC muscle is sensitive to supraphysiological TE and low-dose TREN while LABC AR expression is not, at least within the chronic time point observed in our study. These results suggest that androgens may at least partly maintain muscle mass via suppression of GR, through indirect regulation mechanisms, or via interactions between AR and GR, as others have reported [61,62]. In addition, the greater suppression of the GR by TREN warrants further investigations in order to determine whether trenbolone reverses GR-dependent catabolic effects more efficiently than testosterone.
As discussed above, testosterone may promote skeletal muscle hypertrophy via indirect mechanisms. One possibility is that androgen treatment enhances production of growth factors, such as IGF-1. Testosterone administration increases both circulating and local concentrations of IGF-1 within human skeletal muscle [56,63]. In this regard, IGF-1 stimulates satellite cell activity and is implicated as one of the primary regulators of muscle regeneration. Stimulation of muscle cells with IGF-1 is sufficient to induce hypertrophy and protect against muscle damage [64,65]. Our current data is consistent with the literature in showing that IGF-1 transcription is greatly enhanced by TE or TREN administration [53,56,63]. We also demonstrate that the androgen-induced changes in MGF mRNA expression were greater than that of IGF-1; however, the degree to which IGF-1/MGF influence muscle growth in our study remains unknown. Interestingly, there may be a ceiling effect of IGF-1/MGF-induced muscle gains where greater expression does not produce more muscle growth [66], perhaps explaining why identical muscle growth appears in animals administered graded doses of TREN [10].
In summary, our results indicate that TE and TREN modulate mass of a particularly androgen-sensitive muscle in skeletally mature rats. The observation that catabolic/anabolic alterations involving atrogin-1 and MuRF-1 down-regulation, IGF-1 and MGF induction, and inhibition of the GR after androgen treatment suggests that multiple mechanisms may mediate androgen-induced myotrophic action. Our data suggest that long-term androgen administration may promote muscle hypertrophy by increasing muscle protein synthesis mediated via IGF-1/MGF and inhibiting protein degradation. These data may help to elucidate the signaling cascades mediated by androgens and may aid in the development of effective and safe anabolic therapies. Further work should examine the time course in which these and other androgens alter anabolic and catabolic gene expression in various skeletal muscles and other androgen sensitive tissues.
Acknowledgements
This work was supported by VA Merit Award to SEB and a VA Career Development Award-2 to J.F.Y. F.Y. is supported by Paralyzed Veterans of America (PVA) Fellowship.
References
- [1].Clark DJ, Fielding RA. Neuromuscular contributions to age-related weakness. J Gerontol A Biol Sci Med Sci 2012;67:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hicks GE, Shardell M, Alley DE, Miller RR, Bandinelli S, Guralnik J, et al. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2012;67:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Borst SE. Interventions for sarcopenia and muscle weakness in older people. Age Ageing 2004;33:548–55. [DOI] [PubMed] [Google Scholar]
- [4].Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab 1996;81:4358–65. [DOI] [PubMed] [Google Scholar]
- [5].Serra C, Tangherlini F, Rudy S, Lee D, Toraldo G, Sandor NL, et al. Testosterone improves the regeneration of old and young mouse skeletal muscle. J Gerontol A Biol Sci Med Sci 2013;68:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bhasin S, Calof OM, Storer TW, Lee ML, Mazer NA, Jasuja R, et al. Drug insight: testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Rev Endocrinol 2006;2:146–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yarrow JF, McCoy SC, Borst SE. Tissue selectivity and potential clinical applications of trenbolone (17β-hydroxyestra-4, 9, 11-trien-3-one): a potent anabolic steroid with reduced androgenic and estrogenic activity. Steroids 2010;75:377–89. [DOI] [PubMed] [Google Scholar]
- [9].McCoy SC, Yarrow JF, Conover CF, Borsa PA, Tillman MD, Conrad BP, et al. 17β-Hydroxyestra-4, 9, 11-trien-3-one (Trenbolone) preserves bone mineral density in skeletally mature orchiectomized rats without prostate enlargement. Bone 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yarrow JF, Conover CF, McCoy SC, Lipinska JA, Santillana CA, Hance JM, et al. 17β-Hydroxyestra-4, 9, 11-trien-3-one (trenbolone) exhibits tissue selective anabolic activity: effects on muscle, bone, adiposity, hemoglobin, and prostate. Am J Physiol-Endocrinol Metab 2011;300. E650–E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 2005;37:1974–84. [DOI] [PubMed] [Google Scholar]
- [12].Sartorelli V, Fulco M. Molecular and cellular determinants of skeletal muscle atrophy and hypertrophy. Sci STKE 2004. 2004:re11. [DOI] [PubMed] [Google Scholar]
- [13].Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci USA 1999;96:7088–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Adams GR, Haddad F. The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol 1996;81:2509–16. [DOI] [PubMed] [Google Scholar]
- [15].Grinspoon S, Corcoran C, Lee K, Burrows B, Hubbard J, Katznelson L, et al. Loss of lean body and muscle mass correlates with androgen levels in hypogonadal men with acquired immunodeficiency syndrome and wasting. J Clin Endocrinol Metab 1996;81:4051–8. [DOI] [PubMed] [Google Scholar]
- [16].Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M, et al. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab 1998;83:1886–92. [DOI] [PubMed] [Google Scholar]
- [17].Bodine SC, Latres E, Baumhueter S, Lai VK-M, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Sci Signal 2001;294:1704. [DOI] [PubMed] [Google Scholar]
- [18].Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 2001;98:14440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wray CJ, Mammen J, Hershko DD, Hasselgren P-O. Sepsis upregulates the gene expression of multiple ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol 2003;35:698–705. [DOI] [PubMed] [Google Scholar]
- [20].Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 2004;18:39–51. [DOI] [PubMed] [Google Scholar]
- [21].Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 2003;144:5081–8. [DOI] [PubMed] [Google Scholar]
- [22].Clevers H Wnt/β-catenin signaling in development and disease. Cell 2006;127:469–80. [DOI] [PubMed] [Google Scholar]
- [23].Armstrong DD, Esser KA. Wnt/β-catenin signaling activates growth-control genes during overload-induced skeletal muscle hypertrophy. Am J Physiol-Cell Physiol 2005;289:C853–9. [DOI] [PubMed] [Google Scholar]
- [24].Kongsuwan K, Knox M, Allingham P, Pearson R, Dalrymple B. The effect of combination treatment with trenbolone acetate and estradiol-17β on skeletal muscle expression and plasma concentrations of oxytocin in sheep. Domest Anim Endocrinol 2012;43:67–73. [DOI] [PubMed] [Google Scholar]
- [25].Yarrow JF, Beggs LA, Conover CF, McCoy SC, Beck DT, Borst SE. Influence of androgens on circulating adiponectin in male and female rodents. PLoS ONE 2012;7:e47315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yarrow JF, Conover CF, Purandare AV, Bhakta AM, Zheng N, Conrad B, et al. Supraphysiological testosterone enanthate administration prevents bone loss and augments bone strength in gonadectomized male and female rats. Am J Physiol 2008;295:E1213–22. [DOI] [PubMed] [Google Scholar]
- [27].Borst SE, Conover CF, Carter CS, Gregory CM, Marzetti E, Leeuwenburgh C, et al. Anabolic effects of testosterone are preserved during inhibition of 5alpha-reductase. Am J Physiol 2007;293:E507–14. [DOI] [PubMed] [Google Scholar]
- [28].Borst SE, Lee JH, Conover CF. Inhibition of 5alpha-reductase blocks prostate effects of testosterone without blocking anabolic effects. Am J Physiol 2005;288:E222–7. [DOI] [PubMed] [Google Scholar]
- [29].Ross HH, Fillmore HL. Identification of a novel human MT5-MMP transcript variant in multipotent NT2 cells. FEBS Lett 2007;581:5923–8. [DOI] [PubMed] [Google Scholar]
- [30].Ross HH, Levkoff LH, Marshall GP 2nd, Caldeira M, Steindler DA, Reynolds BA, et al. Bromodeoxyuridine induces senescence in neural stem and progenitor cells. Stem Cells 2008;26:3218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yang S, Alnaqeeb M, Simpson H, Goldspink G. Cloning and characterization of an IGF-1 isoform expressed in skeletal muscle subjected to stretch. J Muscle Res Cell Motil 1996;17:487–95. [DOI] [PubMed] [Google Scholar]
- [32].Yarrow JF, McCoy SC, Borst SE. Intracrine and myotrophic roles of 5α reductase and androgens: a review. Med Sci Sports Exerc 2012;44:818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pires-Oliveira M, Maragno ALG, Parreiras-e-Silva LT, Chiavegatti T, Gomes MD, Godinho RO. Testosterone represses ubiquitin ligases atrogin-1 and Murf-1 expression in an androgen-sensitive rat skeletal muscle in vivo. J Appl Physiol 2010;108:266–73. [DOI] [PubMed] [Google Scholar]
- [34].Fang C-H, Li B-G, James JH, King J-K, Evenson AR, Warden GD, et al. Protein breakdown in muscle from burned rats is blocked by insulin-like growth factor I and glycogen synthase kinase-3β inhibitors. Endocrinology 2005;146:3141–9. [DOI] [PubMed] [Google Scholar]
- [35].Merli M, Giusto M, Molfino A, Bonetto A, Rossi M, Corradini SG, et al. MuRF-1 and p-GSK3β expression in muscle atrophy of cirrhosis. Liver Int 2013. [DOI] [PubMed] [Google Scholar]
- [36].Baehr LM, Furlow JD, Bodine SC. Muscle sparing in muscle RING finger 1 null mice: response to synthetic glucocorticoids. J Physiol 2011;589:4759–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, et al. The E3 ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab 2007;6:376–85. [DOI] [PubMed] [Google Scholar]
- [38].Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, et al. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol 2009;185:1083–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Polge C, Heng A-E, Jarzaguet M, Ventadour S, Claustre A, Combaret L, et al. Muscle actin is polyubiquitinylated in vitro and in vivo and targeted for breakdown by the E3 ligase MuRF1. FASEB J 2011;25:3790–802. [DOI] [PubMed] [Google Scholar]
- [40].Sasai N, Agata N, Inoue-Miyazu M, Kawakami K, Kobayashi K, Sokabe M, et al. Involvement of PI3K/Akt/TOR pathway in stretch-induced hypertrophy of myotubes. Muscle Nerve 2010;41:100–6. [DOI] [PubMed] [Google Scholar]
- [41].Haren M, Siddiqui A, Armbrecht H, Kevorkian R, Kim M, Haas M, et al. Testosterone modulates gene expression pathways regulating nutrient accumulation, glucose metabolism and protein turnover in mouse skeletal muscle. Int J Androl 2011;34:55–68. [DOI] [PubMed] [Google Scholar]
- [42].Hourde C, Jagerschmidt C, Clément-Lacroix P, Vignaud A, Ammann P, Butler-Browne G, et al. Androgen replacement therapy improves function in male rat muscles independently of hypertrophy and activation of the Akt/mTOR pathway. Acta Physiol 2009;195:471–82. [DOI] [PubMed] [Google Scholar]
- [43].Basualto-Alarcón C, Jorquera G, Altamirano F, Jaimovich E, Estrada M. Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy. Med Sci Sports Exerc 2013. [DOI] [PubMed] [Google Scholar]
- [44].White JP, Gao S, Puppa MJ, Sato S, Welle SL, Carson JA. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cell Endocrinol 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, et al. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci 1998;95:14717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Armstrong DD, Wong VL, Esser KA. Expression of β-catenin is necessary for physiological growth of adult skeletal muscle. Am J Physiol-Cell Physiol 2006;291:C185–8. [DOI] [PubMed] [Google Scholar]
- [47].Inadera H, Hashimoto S-i, Dong H-Y, Suzuki T, Nagai S, Yamashita T. WISP-2 as a novel estrogen-responsive gene in human breast cancer cells. Biochem Biophys Res Commun 2000;275:108–14. [DOI] [PubMed] [Google Scholar]
- [48].Banerjee S, Sengupta K, Saxena NK, Dhar K, Banerjee SK. Epidermal growth factor induces WISP-2/CCN5 expression in estrogen receptor-α-positive breast tumor cells through multiple molecular cross-talks. Mol Cancer Res 2005;3:151–62. [DOI] [PubMed] [Google Scholar]
- [49].Dhar G, Mehta S, Banerjee S, Gardner A, McCarty BM, Mathur SC, et al. Loss of WISP-2/CCN5 signaling in human pancreatic cancer: a potential mechanism for epithelial-mesenchymal-transition. Cancer Lett 2007;254:63–70. [DOI] [PubMed] [Google Scholar]
- [50].Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001;410:50–6. [DOI] [PubMed] [Google Scholar]
- [51].Asirvatham A, Schmidt M, Gao B, Chaudhary J. Androgens regulate the immune/inflammatory response and cell survival pathways in rat ventral prostate epithelial cells. Endocrinology 2006;147:257–71. [DOI] [PubMed] [Google Scholar]
- [52].Sar M, Lubahn DB, French FS, Wilson EM. Immunohistochemical localization of the androgen receptor in rat and human tissues. Endocrinology 1990;127:3180–6. [DOI] [PubMed] [Google Scholar]
- [53].Pampusch M, White M, Hathaway M, Baxa T, Chung K, Parr S, et al. Effects of implants of trenbolone acetate, estradiol, or both, on muscle insulin-like growth factor-I, insulin-like growth factor-I receptor, estrogen receptor-α, and androgen receptor messenger ribonucleic acid levels in feedlot steers. J Anim Sci 2008;86:3418–23. [DOI] [PubMed] [Google Scholar]
- [54].Kvorning T, Andersen M, Brixen K, Schjerling P, Suetta C, Madsen K. Suppression of testosterone does not blunt mRNA expression of myoD, myogenin, IGF, myostatin or androgen receptor post strength training in humans. J Physiol 2007;578:579–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Carson JA, Lee WJ, McClung J, Hand GA. Steroid receptor concentration in aged rat hindlimb muscle: effect of anabolic steroid administration. J Appl Physiol 2002;93:242–50. [DOI] [PubMed] [Google Scholar]
- [56].Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol-Endocrinol Metab 2002;282:E601–7. [DOI] [PubMed] [Google Scholar]
- [57].Zhao W, Qin W, Pan J, Wu Y, Bauman WA, Cardozo C. Dependence of dexamethasone-induced Akt/FOXO1 signaling, upregulation of MAFbx, and protein catabolism upon the glucocorticoid receptor. Biochem Biophys Res Commun 2009;378:668–72. [DOI] [PubMed] [Google Scholar]
- [58].Santidrian S, Moreyra M, Munro H, Young V. Effect of testosterone on the rate of myofibrillar protein breakdown in castrated and adrenalectomized male rats measured by the urinary excretion of 3-methylhistidine. Metabolism 1982;31:1200–5. [DOI] [PubMed] [Google Scholar]
- [59].Waddell DS, Baehr LM, van den Brandt J, Johnsen SA, Reichardt HM, Furlow JD, et al. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol-Endocrinol Metab 2008;295:E785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sharpe P, Buttery P, Haynes N. The effect of manipulating growth in sheep by diet or anabolic agents on plasma cortisol and muscle glucocorticoid receptors. Br J Nutr 1986;56:289–304. [DOI] [PubMed] [Google Scholar]
- [61].Van Balkom RH, Dekhuijzen PR, Folgering HT, Veerkamp JH, Van Moerkerk HT, Fransen JA, et al. Anabolic steroids in part reverse glucocorticoid-induced alterations in rat diaphragm. J Appl Physiol 1998;84:1492–9. [DOI] [PubMed] [Google Scholar]
- [62].Yen PM, Liu Y, Palvimo JJ, Trifiro M, Whang J, Pinsky L, et al. Mutant and wild-type androgen receptors exhibit cross-talk on androgen-, glucocorticoid-, and progesterone-mediated transcription. Mol Endocrinol 1997;11:162–71. [DOI] [PubMed] [Google Scholar]
- [63].Sheffield-Moore M. Androgens and the control of skeletal muscle protein synthesis. Ann Med 2000;32:181–6. [DOI] [PubMed] [Google Scholar]
- [64].Stevens-Lapsley JE, Ye F, Liu M, Borst SE, Conover C, Yarasheski KE, et al. Impact of viral-mediated IGF-I gene transfer on skeletal muscle following cast immobilization. Am J Physiol-Endocrinol Metab 2010;299:E730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ye F, Mathur S, Liu M, Borst SE, Walter GA, Sweeney HL, et al. Overexpression of IGF-1 attenuates skeletal muscle damage and accelerates muscle regeneration and functional recovery after disuse. Exp Physiol 2013;98: 1038–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Barton ER. Viral expression of insulin-like growth factor-I isoforms promotes different responses in skeletal muscle. J Appl Physiol 2006;100:1778–84. [DOI] [PubMed] [Google Scholar]


