SUMMARY
Parasitic neglected tropical diseases (NTDs) affect over one billion people worldwide, with individuals from communities in low-socioeconomic areas being most at risk and suffering the most. Disease management programs are hindered by the lack of infrastructure and resources for clinical sample collection, storage, and transport and a dearth of sensitive diagnostic methods that are inexpensive as well as accurate. Many diagnostic tests and tools have been developed for the parasitic NTDs, but the collection and storage of clinical samples for molecular and immunological diagnosis can be expensive due to storage, transport, and reagent costs, making these procedures untenable in most areas of endemicity. The application of membrane technology, which involves the use of specific membranes for either sample collection and storage or diagnostic procedures, can streamline this process, allowing for long-term sample storage at room temperature. Membrane technology can be used in serology-based diagnostic assays and for nucleic acid purification prior to molecular analysis. This facilitates the development of relatively simple and rapid procedures, although some of these methods, mainly due to costs, lack accessibility in low-socioeconomic regions of endemicity. New immunological procedures and nucleic acid storage, purification, and diagnostics protocols that are simple, rapid, accurate, and cost-effective must be developed as countries progress control efforts toward the elimination of the parasitic NTDs.
KEYWORDS: neglected tropical diseases, parasites, point-of-care diagnostics, rapid diagnostics, helminths, protozoa
INTRODUCTION
The disease burden due to parasitic infections is considerable, with over 1 billion people affected by neglected tropical diseases (NTDs) due to these pathogens (1, 2). Parasites causing NTDs include helminth, protozoan, and arthropod infections and predominantly afflict people in low-socioeconomic areas of the tropics and subtropics, although several also occur in more temperate regions (3, 4) (Table 1). These pathogens cost local economies billions of dollars each year due to medical treatment costs and loss of production in infected animals; nonetheless, they generally receive little public attention (1). Assessment of the years of life lost (YLL) and the years of life lived with disability (YLD) rather than mortality caused by various diseases led to the development of the term disability-adjusted life years (DALYs), allowing for a new way to illustrate the health impacts of NTDs (5, 6). Globally, NTDs caused by parasites result in the loss of over 25 million DALYs (5, 6). Management of these diseases involves treatment through chemotherapy, vector control (when applicable), improving hygiene and sanitation in areas of endemicity, education programs, and accurate diagnosis for surveillance (1, 7–9).
TABLE 1.
Parasitic NTDs and worldwide case numbersa
| Condition | Organism(s) | No. of cases worldwide |
|---|---|---|
| Protozoa | ||
| Human African trypanosomiasis (HAT) | Trypanosoma brucei | <1,000 |
| Chagas disease | Trypanosoma cruzi | 6–7 M |
| Cutaneous leishmaniasis | Leishmania tropica, Leishmania mexicana, Leishmania major | 30,000 |
| Mucocutaneous leishmaniasis | Leishmania braziliensis | |
| Visceral leishmaniasis | Leishmania donovani | |
| Filariasis | Wuchereria bancrofti, Brugia malayi, Brugia timori, Loa loa | 120 M |
| Helminths | ||
| Soil-transmitted helminthiasis (STH) | Ancylostoma duodenale, Necator americanus, Ascaris lumbrcoides, Trichuris trichiura, Strongyloides stercoralis | 1.5 B |
| Onchocerciasis | Onchocerca volvulus | 20.9 M |
| Drancunculiasis | Dracunculis medinensis | 54 |
| Echinococcosis | Echinococcus granulosus, Echinococcus multilocularis | 1 M |
| Taeniasis/cysticercosis | Taenia solium | 2.79 M |
| Fascioliasis | Fasciola hepatica, F. gigantica | 10,635 |
| Schistosomiasis | Schistosoma japonicum, Schistosoma mansoni, Schistosoma aematobium | 240 M |
| Arthropods | ||
| Scabies | Sarcoptes scabiei | 200 M |
While some success has been achieved in NTD control, for example the near elimination of Guinea worm (Dracunculus medinensis) infection in Asia and some African countries (10), and schistosomiasis (due to Schistosoma japonicum) in China (11), disease management programs exhibit some limitations. The majority of these infections occur in rural, low-socioeconomic communities with poor sanitation and limited road access (12–16), and these areas generally lack the infrastructure necessary for accurate parasite diagnosis such as reliable electricity, making diagnostic tests that require complicated equipment and an adequate power supply nonviable. This lack of resources also makes collection, storage, and transport of clinical samples (blood, serum, feces, urine, skin scrapings, and saliva) to in-country or external laboratories capable of undertaking the relevant diagnostic procedures difficult and costly (Table 2). Furthermore, the absence of reliable electricity makes refrigeration of samples before and during transport difficult if not impossible (17). Another factor affecting parasite control is climate change, which has significant effects on parasite distribution and control, causing changes in the environment and animals available for zoonosis (18). Increasing temperatures have caused an expansion of the tropical and subtropical zones, giving ideal soil conditions for soil-transmitted helminth (STH) survival, allowing increased vector distribution, and encouraging expansion of parasitic disease distribution to areas that previously had not been regions of endemicity (18–21). Globalization has caused in an increased risk of foodborne helminths due to immigration and global exportation and importation of food and live animals. Furthermore, urbanization has caused increased contact between wild animals and humans, allowing wildlife-maintained parasites to infect humans and domestic animals (22–24).
TABLE 2.
Clinically relevant laboratory-based diagnostic methods for parasitic NTDsa
| Parasite and condition | Parameter | Microscopy | Immunodiagnostics | DNA-based | Other/notes |
|---|---|---|---|---|---|
| Protozoa | |||||
| Human African trypanosomiasis | Sample type(s) | Blood smear, CSF, lymph, chancre | Serum, blood | Blood/serum | Positive serology requires confirmatory testing via visualization of parasites. T. rhodesiense has no available rapid test and requires lab-based serology testing. PCR may complement serology and parasite visualization if available. |
| Test name(s) | Direct or concentrated slide prep | Card agglutination, rapid lateral flow, ELISA, IFAT | PCR, qPCR | ||
| Reference(s) | 338 – 342 | 129, 343–345 | 346 – 348 | ||
| Chagas | Sample type(s) | Blood, buffy coat | Serum | Blood/serum, skin | PCR can be used for monitoring patients for reactivation risk; sensitive detection in acute disease. Parallel antigen-based tests are required to improve the accuracy of diagnosis. |
| Test name(s) | Direct blood smear or buffy coat | ELISA, IFA, TESA, IHA, Abbott TESA Chagas | PCR, qPCR | ||
| Reference(s) | 349 | 350 – 353 | 349, 353–358 | ||
| Cutaneous/mucocutaneous leishmaniasis | Sample type(s) | Skin/lesion biopsies | Skin/lesions | Parallel tests (histology, culture, PCR) for best diagnostic accuracy. RFLP-PCR distinguishes species. | |
| Test name(s) | Histology, NNN medium culture | PCR, qPCR, RFLP-PCR | |||
| Reference(s) | 359 – 362 | 361 – 364 | |||
| Visceral leishmaniasis | Sample type(s) | Bone marrow, whole blood, lymph node, spleen | Serum | Blood/serum | Bone marrow aspirate is the preferred specimen. Consider serology for suspected VL with negative or inconclusive histopathology, culture, and PCR. Immunocompromised individuals may have lower sensitivity to serological tests; PCR and culture are recommended. SMART Leish PCR has been developed by the U.S. Department of Defense and approved by the FDA but is not commercially available (365). |
| Test name(s) | Direct blood smear or buffy coat, histology, NNN media culture | ICT, IFA, ELISA, DAT | qPCR, PCR | ||
| Reference(s) | 359 – 361 | 189, 361, 366–369 | 361, 366, 370 | ||
| Helminths | |||||
| Filariasis | Sample type(s) | Blood | Serum | Midnight smears required for most species. However, W. bancrofti from Pacific islands and B. malayi in some Asian countries exhibit subperiodic periodicity and mf can always be found in peripheral blood (54, 371, 372). Negative antigen tests do not exclude infection or lymphatic damage in previous infection. | |
| Test name(s) | Thin/thick smears, concn (Knott’s, Nuclepore) | CFA, ELISA, BinaxNOW, Alere filariasis test strip | |||
| Reference(s) | 373 – 375 | 156, 209, 216, 373, 376–379 | |||
| Strongyloides | Sample type(s) | Sputum, stool, bowel biopsy | Serum | Stool, blood/serum | Larvae in stool and sputum. Clinical symptoms and epidemiological data key for initial evaluation. Microscopy often has low sensitivity; serology is recommended. A number of molecular diagnostics have been developed and are used in some diagnostic labs. |
| Test name(s) | agar plate culture, Baermann, sputum smear, histopathology | IFA, Western blotting | qPCR, LAMP | ||
| Reference(s) | 100, 380–387 | 385, 388, 389 | 390 – 392 | ||
| Soil-transmitted helminths | Sample type(s) | Stool | Stool | Multiplex PCR panels often combine STH with intestinal protozoan detection | |
| Test name(s) | Thick/thin smear, concn technique, agar plate | Multiplex PCR | |||
| Reference(s) | 100, 380, 393–395 | 396, 397 | |||
| Onchocerciasis | Sample type(s) | Skin | Serum | Antibody testing is not reliable; high cross-reactivity and not specific for current infection. Antigen testing has a higher sensitivity. Ultrasonography of nodules can also be used to identify adult worms (398). Skin snip PCR is not available clinically. | |
| Test name(s) | Skin snips, biopsy of onchercomas | ELISA, Ov-16 card test, ICT | |||
| Reference(s) | 399, 400 | 401 – 404 | |||
| Dracunculiasis | Clinical presentation; observation of adult female worm (405). | ||||
| Echinococcosis | Sample type(s) | Serum | Visualization of cysts (CT, MRI, ultrasonography), confirmed with serology or antigen testing. ELISA appears to be the most sensitive and specific of available assays. Antibody detection is more sensitive than antigen detection in diagnosing E. granulosus (406) | ||
| Test name(s) | Cyst aspirate or biopsy (if performed, risk of secondary seeding of infection) | IHA, ELISA, CIEP, RIA, TR-FLA, immunoblotting, latex agglutination, Western blotting | |||
| Reference(s) | 407, 408 | 406, 409–413 | |||
| Taeniasis/cysticerocosis | Sample type(s) | Stool | Serum | Plasma, CSF | Taeniasis can be diagnosed as eggs in feces via microscopy or PCR. Cysticercosis diagnosed from imaging (cysts) via CT and MRI, with confirmation via serology. Poor performance is identified with commercially available ELISA (414). MAT available in Europe, limited availability in the USA. qPCR may be useful in diagnosing subarachnoid NCC. |
| Test name(s) | Direct or concentrated slide prep | EITB, ELISA | qPCR | ||
| Reference(s) | 415 | 414, 416–418 | 419) | ||
| Fascioliasis | Sample type(s) | Stool, bile, duodenal aspirates | Serum | Serological diagnostics lack specificity, cross-react with other parasites. | |
| Test name(s) | IHA, IFA, ELISA, immunoblotting | ||||
| Reference(s) | 420, 421 | 166, 420, 422–425 | |||
| Schistosomiasis | Sample type(s) | Stool, urine (species dependent) | Serum | Serum, urine, feces | Microscopy can be used for species differentiation. In MGS, eggs may also be identified in ejaculate (426) and in vaginal swabs in women with FGS (427, 428). The majority of commercially available antibody tests are not species specific. |
| Test name(s) | Thin or thick smear, concentrated, urine filtrate, FLOTAC | ELISA, IHA, RIA, IFA, Western blotting, complement fixation | qPCR, PCR | ||
| Reference(s) | 44 – 47 | 12, 44, 168, 174, 429–431 | 12, 73, 432, 433 | ||
| Arthropod | |||||
| Scabies | Sample type(s) | Skin scrapings/biopsy | Clinical diagnosis based on itching, plaque, and location (interdigital spaces, skin folds). | ||
| Reference(s) | 434, 435 |
CIEP, countercurrent immunoelectrophoresis; CT, computed tomography; DAT, direct agglutination test; ELISA, enzyme-linked immunosorbent assay; EITB, enzyme-linked immunoelectrotransfer blot; FGS, female genital schistosomiasis; ICT, immunochromatographic test; IFA, immunofluorescent antibody test; IFAT, indirect fluorescent antibody test; IHA, indirect hemagglutination assay; mf, microfilariae; MGS, male genital schistosomiasis; MRI, magnetic resonance imaging; TR-FLA, time-resolved fluoroimmunoassay; TESA, trypomastigote excreted secreted antigens; RIA, radioimmunoassay.
Accurate diagnosis of NTDs is essential for the treatment and epidemiological monitoring of disease burdens. Mass drug administration (MDA) is the gold standard for large-scale treatment of parasitic diseases in areas of endemicity. However, due to a lack of education in many regions of endemicity, patients may not understand why they must take the drug, increasing mistrust and decreasing compliance and leading to low population coverage and little impact on overall prevalence (25). Furthermore, up to 80% of patients will experience transient side effects, causing these and others in the community to refuse taking part in future MDA due to apprehension about taking the drug (25, 26). Furthermore, since chemotherapy does not prevent reinfection, individuals can become reinfected almost immediately after treatment (26–28). Education programs, such as the Magic Glasses intervention for STHs, successfully tested in China, aim to change behavior and prevent infection by increasing knowledge about locally acquired parasite infections and correct hygiene practices, thereby decreasing reinfection rates after MDA (8, 29, 30). As such, community engagement is an essential component of improved MDA compliance (26, 31, 32).
Diagnostic tests for NTDs caused by parasites include laboratory-based methods, such as molecular and immunology-based assays. Samples are either sent to a central laboratory for analysis, or point-of-care (POC) testing is undertaken, where the assays are performed close to or near the patient. POC testing can result in decreased costs of diagnosis, improved efficiency, and optimized treatment regimens useful in resource-poor endemic countries with deficient infrastructure (33).
In 2015, the World Health Organization (WHO), The Foundation for Innovative Diagnostics (FIND), and the Centers for Disease Control and Prevention (CDC) defined criteria to be used as a benchmark for the use of POC diagnostic tests known as ASSURED (Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment-free, and Deliverable to end-users) applicable in resource-poor endemic regions (34). The acceptability of a suitable diagnostic test must conform to this six-step guide (Table 3). This method of selection has been successfully employed in the implementation of a number of diagnostic tests for malaria (35), but few accurate and accessible POC diagnostics and monitoring tests for the NTDs have been developed (36, 37). Recently, the ASSURED criteria have been reassessed and improved to give REASSURED (Real-time connectivity, Ease of collection, Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment free/simple and environmentally friendly, and Deliverable to end-user), aiming to improve disease control, strengthen health systems, and improve patient outcomes (38, 39) (Table 4).
TABLE 3.
WHO guidelines for evaluation, validation, and implementation of POC diagnostic testsa
| Step | Description |
|---|---|
| 1 | Define the purpose of the test (why, what, where, and who?) |
| 2 | Review the market |
| 3 | Review the regulatory approval by international and national bodies |
| 4 | Determine optimal diagnostic accuracy of test |
| 5 | Determine practical diagnostic accuracy of test |
| 6 | Monitor routine use of test |
Source: World Health Organization (34).
TABLE 4.
REASSURED criteria and definitions
| Acronym | Criterion | Definition |
|---|---|---|
| R | Real-time connectivity | Tests can be connected and/or a mobile phone or reader can be used to power the reaction and/or read test results to provide required data to decision-makers. |
| E | Ease of specimen collection | Tests should be designed to use specimens collected by noninvasive procedures. |
| A | Affordable | Tests must be affordable to the end-users and healthcare system. |
| S | Sensitive | Avoid false negatives. |
| S | Specific | Avoid false positives. |
| U | User-friendly | Simple procedure for testing: the test can be performed in a few steps that require minimal training. |
| R | Rapid and robust | Results must be available rapidly following patients first visit (about 15 min to 2 h); tests are able to survive supply chains without the need for specialized transport and storage conditions. |
| E | Equipment free or simple and environmentally friendly | Tests do not require special equipment or equipment can be operated by simple devices that are powered by solar or battery power (completed tests should be manufactured from recycled materials that require simple disposal). |
| D | Deliverable to end-user | Accessible to those who require tests the most |
The WHO has recently put out a call for expert advice on diagnostics targeting schistosomiasis and STH, highlighting issues with currently available diagnostics and looking toward the development of POC diagnostics, particularly for schistosomiasis. The focus is on easy interpretation by field workers and low cost (less expensive than two to three rounds of MDA), and any necessary equipment should be highly transportable and battery powered if electricity is required at all; the whole procedure should take less than a day between sample collection and interpretation of result and treatment decision (40).
In this review, we provide an overview of the challenges posed by currently available collection, storage and diagnostic procedures for parasitic NTDs and emphasize how revolutionary membrane technologies can be used to streamline these processes. Specifically, we focus on (i) a description of the commonly used procedures used for clinical sample collection, storage, and an appraisal of the diagnostic methods available; (ii) the application of membrane technology for clinical sample collection, storage, and transport; and (iii) the uses of membrane technology for clinical sample preparation, including nucleic acid purification and antigen/antibody detection.
OVERVIEW OF CURRENT DIAGNOSTIC METHODS
Microscopy-Based Procedures
Accurate diagnosis of a parasitic infection involves the use of laboratory techniques, clinical history, geographic location, and travel history (28, 41). The current gold standard of diagnosis involves microscopic identification of parasitic stages in clinical samples, usually blood, urine, or feces (28, 41, 42). Low-cost and accessible microscopy-based procedures in areas of endemicity include the Kato-Katz thick-smear method (KK), the formalin-ether concentration technique (FECT), and urine filtration (for Schistosoma haematobium) (26–28, 43–47) (Table 2). However, these methods can be time-consuming and require skilled laboratory scientists that must pass regular competency testing to identify parasite eggs, larvae, and cysts (42). Furthermore, good-quality microscopes with light sources, thus requiring electricity, are necessary for parasite detection and also include correct objectives, including oil immersion objectives, calibration, and regular maintenance, making them problematic in resource-poor regions (42). Microscopy-based procedures also lack sensitivity and specificity due to the difficulty in identifying parasites to the species level, even with strict morphological criteria to follow (28, 48–50).
Another important factor affecting microscopic diagnosis is timing, since the parasite may not be identifiable if investigation is undertaken too early or too late during infection. For example, during Schistosoma spp. infections, diagnosis by microscopic identification of parasite eggs in fecal samples is not possible until the infection becomes patent, when eggs are being produced, 4 to 8 weeks postinfection (51, 52). Another example is lymphatic filariasis, where microfilariae may not be present in the patients with overt clinical symptoms or exhibit periodicity (53, 54). Damage to lymphatic systems which results in elephantiasis may occur years before the physical manifestation of edema, decreasing the utility of microscopy as a diagnostic in late infections or chronic diseases where antigen testing is preferred (54) (Table 2).
Immunodiagnostics
Serological assays use organism-specific antigens or antibodies to diagnose parasitic diseases. Antigens consist of shed parasite-derived products that are detectable in the blood, serum, urine, saliva, and vaginal fluids of infected individuals (12, 55–59), whereas antibodies are specific responses by the host to the invading parasite and can be primarily detected in blood and sera. Antibody detection can have issues with cross-reactivity, and interpretation can be difficult in some later flow assays; since the parasites are present long after the infection has cleared, however, these methods can be useful in areas of low endemicity or chronic infections, where parasitemia may be low in infected individuals (12, 60) (Table 2). The majority of RDTs are based on antibody detection.
Serological methods include the indirect hemagglutination test, direct or indirect immunofluorescent antibody tests (DFAs or IFAs), complement fixation (CF) tests, rapid diagnostics tests (RDTs), such as immunochromatographic antigen detection and the enzyme-linked immunosorbent assay (ELISA) (61–64) (Table 2). ELISAs use 96-well plates that have been coated to allow for protein binding (65–67). The wells are coated with the antigen of interest or primary antibody before being washed and blocked, using agents such as bovine serum albumen, ovalbumin, and other animal proteins to prevent nonspecific protein binding (65–67). The samples are then incubated with an enzyme-conjugated antibody (primary and/or secondary), followed by the addition of a substrate, such as horseradish peroxidase or alkaline phosphatase, which initiates a visible color change within 30 min (65–67). Replacing the plastic wells used for ELISA with a nitrocellulose membrane simplified the method significantly, giving rise to the Dot-ELISA (68, 69). This change also reduced cost the associated with the traditional ELISA (68, 69).
Most available POC tests are immunology based due to their accessibility and relative simplicity for use in the field and have been widely used in the past for diagnosis and control program monitoring (Tables 2 and 5). More details, as well as examples for RDT immunodiagnostics, are presented below in “RDT Immunodiagnostics.”
TABLE 5.
Advantages and disadvantages of different protocols used in the diagnosis of parasitic NTDsa
| Diagnostic test/sample collection tool | Advantages | Disadvantages |
|---|---|---|
| Immunoassaysb | ||
| ELISA | High sensitivity and specificity, high throughput, simple, rapid | Lab-based technique, requires specialized technicians, high reagent costs. |
| Dot-ELISA | Simple, rapid, results easy to interpret visually, require small sample volumes, can be performed external to a lab. | High reagent cost, samples must be stored below 4°C, sensitivity, specificity dependent on parasitemia. |
| LFIA | Rapid, simple, higher sensitivity and specificity than ELISA, can be interpreted visually, require small sample volumes | Samples must be stored below 4°C, sensitivity and specificity depend on parasitemia, cross-reactivity issues, some problems with false negatives/positives and trace bands. |
| Schistosomiasis POC-CCA cassette-based test | Antigen detection. Commercially available POC detection of S. mansoni and S. haematobium | Significant issues with detecting false positives, difficult test interpretation, cross reactivity noted with other helminth species. |
| Molecular | ||
| Whatman | Samples stable at room temp for 10 yrs, small sample volumes required, simple sample collection, low-cost transport. | Complex processing requirements, no research on use of Fusion 5 in parasitic NTD diagnosis. |
| Fusion 5 | Increased wicking speed, increased efficiency of DNA capture; effective for DNA extraction from blood, pig mucin, saliva, buccal swabs, and cigarette buds. | High cost, requires large sample volumes, only studied on saliva and blood, no research on use of AOMs for parasitic NTD diagnosis. |
| DNA dipstick | Low cost, can be combined with LAMP assay. Simple workflow. | Not tested on clinical samples, only tested on S. japonicum parasite material and infected snails. |
| AOM | Rigid membrane, increased wicking speed, high sensitivity for diagnosis of bacterial and viral infections | Complex processing requirements, no research on use of Fusion 5 in parasitic NTD diagnosis. |
| Djinni Chip | Low cost, LAMP assay on the chip. Simple workflow. | Not yet used for parasite NTD diagnosis. Contamination in a “mock field” trial. |
| SPS and microfluidic chip | SPS separates plasma for blood without electricity. LAMP occurs on the chip. | Has only been performed in laboratories and on blood spiked with S. mansoni DNA |
ELISA, enzyme-linked immunosorbent assay; LFIA, lateral flow immunodiagnostic assays; AOM, aluminum oxide membrane; SPS, superhydrophobic plasma separator.
In antibody-based ELISAs, it can be difficult to distinguish between past and present infections.
Molecular-Based Diagnostics
Nucleic acid amplification tests (NAATs) exhibit high sensitivity and specificity in areas with both high and low intensity parasite infections and involve a number of PCR (PCR)-based methods, including conventional PCR, direct-PCR, real-time PCR (qPCR), droplet digital PCR, and loop-mediated isothermal amplification (LAMP) (Table 2) (70–72). NAATs have many advantages compared to other diagnostic methods, including increased sensitivity, specificity, and flexibility of testing (58, 71–73). One of the main factors preventing widespread use of NAATs as POC diagnostics is the cost of reagents equipment, the need for electricity, the requirement for a unidirectional workflow to minimize contamination, and the cost of the methods required to purify nucleic acids from clinical samples (74, 75). Recently, however, new molecular-based machines, including the Djinni chip (which we discuss below in “DNA-Based RDT”) and GeneXpert (76, 77). GeneXpert is a module which runs and reads Xpert cartridges for various tests, including tuberculosis (TB) in sputum samples (78) and, more recently, COVID-19, for which emergency FDA approval has been granted (79). The GeneXpert units have been placed in diagnostic laboratories across Africa, and thus their presence could be leveraged for the molecular diagnosis of endemic NTDs, including parasitic diseases.
A disadvantage of NAATs is that the sensitivity and specificity of some methods can be decreased for certain parasite species and sample types. For example, during chronic Chagas disease, where the causative parasite, Trypanosoma spp., is not circulating in the blood, NAATs are unable to reliably detect infection (80). In these cases, serological tests are necessary to confirm infection; indeed, parallel antigen tests are suggested for improved diagnostic accuracy for Chagas disease (80) (Table 2). However, PCR can be useful in monitoring for reactivation of disease (Table 2). The three most commonly used nucleic acid purification methods are: (i) organic methods using phenol-chloroform; (ii) inorganic methods based on binding nucleic acids to silica/Chelex substrates in the presence of high-salt solutions; and (iii) solid-phase extraction methods, which act by binding DNA to paramagnetic or silica beads (81–84). A series of wash and centrifugation steps remove contaminants that may be present in the sample before the final elution step (81, 82, 85, 86). These methods are often laborious, involving complicated reaction steps and necessitating trained technicians and electrical equipment, and expensive. Thus, these methods are problematic for incorporation in POC diagnosis in resource-poor areas of endemicity (87, 88).
Clinical Samples and Parasite Locations
Parasites that cause NTDs have differing life cycles and infect different bodily tissues and fluids that can vary depending on the life cycle stage and infection status (acute versus chronic), and some, such as lymphatic filariasis, experience periodicity in peripheral blood; this affects the type of clinical sample harboring parasite material that can be utilized for diagnostic purposes (57, 89, 90). Parasites that invade the circulatory system as adults or larval stages directly release waste products, surface materials, eggs, and DNA into the circulatory system, which can be detected subsequently in host bodily fluids (57). For example, sloughed-off tegument from adults and schistosomula larvae and eggs, and DNA, including cell-free DNA, have been successfully detected in the feces, blood, serum, urine, saliva, and vaginal fluids of mammalian hosts harboring Schistosoma blood flukes in the blood vessels surrounding the gut (S. mansoni and S. japonicum) or the bladder (S. haematobium) (12, 57, 58, 70).
Intestinal helminths release eggs, immature and adult life cycle stages, waste products, surface material, and DNA directly into the gastrointestinal tract, which can be detected in fecal samples. Material from intestinal worm infections can also be detected in blood and serum, urine, and saliva (12, 91, 92). This may be due to mucosal changes leading to “leaky gut” syndrome caused by some parasites, such as Strongyloides stercoralis (65, 93), or due to migrating larval stages such as is the case for hookworm species, S. stercoralis and Ascaris lumbricoides, which all enter the bloodstream at various stages of their life cycle (94–97). A. lumbricoides and hookworm also undergo tracheal migration, which accounts for parasite material detectable in saliva (96, 97). The main gastrointestinal parasites are the soil-transmitted helminths (STHs), which include hookworms (Ancylostoma duodenale, Necator americanus), whipworms (Trichuris trichiura), and roundworms (A. lumbricoides) and are estimated to infect over 1.5 billion people globally (98, 99). These parasites reside in the gastrointestinal tract, releasing eggs into the external environment in the feces, which develop into infectious larvae or mature eggs, ready to continue the life cycle (99, 100). DNA, antigens and whole parasite stages have been successfully detected in feces, blood, and serum of hosts infected with STHs (100).
The quality and quantity of parasite material in clinical samples are integral for precise diagnosis of NTDs (101–103). Accurate parasite diagnostics for human and animal hosts are key to ensuring both effective treatment and in surveillance, thereby helping to reduce rebound infections in areas where treatment is curtailed and where less-sensitive diagnostic tools may have been applied so as to confirm that parasite control measures leading to elimination have been successful (8, 12, 104). Key to this are the collection, storage, and transport of clinical samples to designated laboratories for NTD diagnosis.
Storage and Transport of Clinical Samples
Clinical samples that can be used in parasite diagnosis are feces, urine, skin scrapings, blood and sera, and saliva; each of these present different issues for collection, storage, and processing. Subjects can collect saliva, urine, and stool samples themselves when provided with appropriate containers, whereas trained professionals are required to collect blood, sera, and skin scrapings, thereby increasing labor costs (105, 106). After collection, these samples must be stored correctly, often by refrigeration or freezing, and rapidly transported to a laboratory for analysis.
The cost of storage and transport depends on the sample (Table 1). Blood and serum must be stored in individual EDTA tubes, which themselves have a high cost, but are also expensive in terms of transport and shipping due to their weight (107). Fecal storage containers themselves can be lower in cost, but samples must undergo fixation, which increases costs. Which fixatives are used depends on whether it is a clinical setting or a field setting and on the downstream application—molecular, microscopy, or immunodiagnostics. Fixation often takes place in 10% formalin, 80% ethanol, polyvinyl-alcohol (PVA) solutions, sodium acetate acetic acid and formalin, zinc sulfite-polyvinyl alcohol, or fresh, with costs ranging from $2 to $5 (AUD) per sample in addition to the transport costs (108–110). Some of these fixative methods can impact the method of detection; for instance, killing trophozoites or larvae mean that they are no longer motile and cannot be seen on a wet mount, but the dead parasites can be stained and viewed as a fixed mount. Other fixatives impact DNA integrity, and thus downstream detection needs to be considered. A clinical setting, where samples will be sent to a lab for testing quite quickly, will not have the same requirements as in the field, where times between defecation and fixing the sample can vary and long distances between field collection and processing in a laboratory can be quite lengthy and often without refrigeration (109, 111, 112). Urine and saliva collection containers are also low cost but must be refrigerated or frozen until used in diagnostic processing (113, 114). Saliva samples can also be fixed in PVA and 10% formalin and stored at room temperature in specially designed collection kits, again resulting in increased cost (113).
Parasite NTDs primarily occur in remote communities located in predominantly lower socioeconomic regions of the tropics and subtropics (12–16). Accordingly, collected samples may remain at room temperature for prolonged periods before transport to a testing facility (101–103). Furthermore, they are often exposed to high temperatures during transportation, which can result in the disintegration of protozoan trophozoites and cysts, as well as increased lysing of some helminth eggs (hookworm) and the disintegration of larvae (Strongyloides) (101–103). Consequently, storage and transportation procedures must be effective, accessible, and affordable.
Whatman FTA cards: sample collection and storage.
Whatman Flinders Technology Associates (FTA) matrix cards (Fig. 1) consist of cellulose and contain a proprietary mix of chemicals that lyse cells on contact and physically entrap and stabilize DNA (105, 115). This occurs when the long DNA molecules become entangled with fibers of the filter paper, while other cell debris and contaminants are washed through the paper (116). This protects nucleic acids in most clinical samples, including blood, tissues, and buccal scrapes, from degradation by oxidation, ultraviolet light, and fungi at room temperature for up to 10 years (117–121).
FIG 1.
Whatman FTA classic card. Sample pads contain cellulose matrix for DNA sample capture.
The compact nature of Whatman filter paper technology permits their transport via standard mail, while still maintaining DNA stability, which allows for cost-effective and reliable storage and transport (122). Furthermore, this technology can also help streamline sample collection. For example, a small finger prick is all that is necessary to obtain a blood sample, instead of collection by venipuncture, which requires the presence of a trained professional (105). FTA cards can be stored at the collection location at room temperature as long as required instead of the immediate transportation to a reference laboratory or the immediate refrigeration that is normally required for analysis of clinical samples (117–120). Whatman FTA cards have been utilized to store DNA from forensic, bacterial, and viral samples, cytological materials, and parasites (115, 123–127). Parasite entrapment has mainly focused on protozoa, including Plasmodium, Trypanosoma, Leishmania, and Babesia (Table 2), with studies successfully utilizing FTA cards for short- and long-term storage of clinical samples containing parasite DNA.
RAPID DIAGNOSTIC TESTS
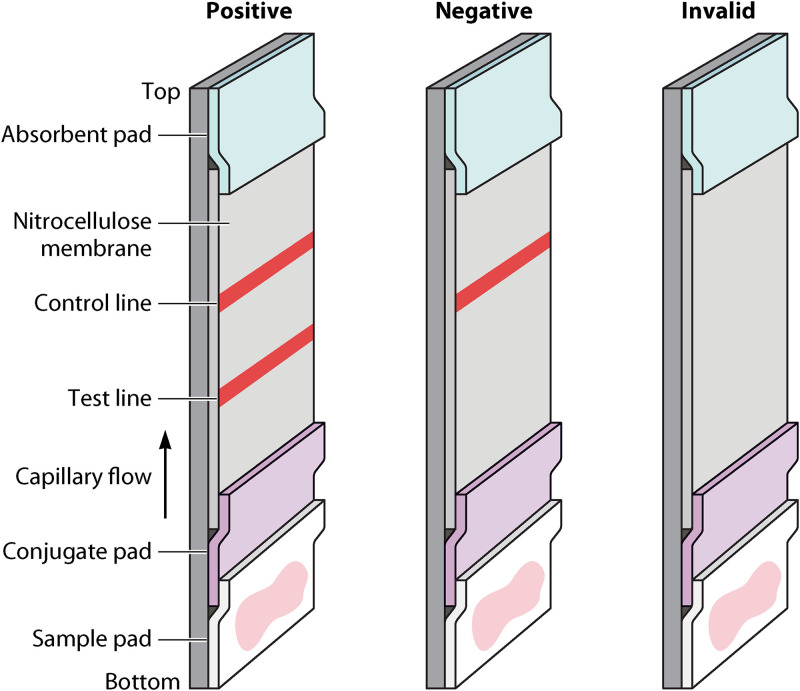
Rapid diagnostic tests (RDTs) are compact and affordable POC diagnostic tools that give rapid results. RDTs of varying efficacy have been developed for all NTDs except for scabies, STH, and dracunculiasis (128–147). Many available RDTs for NTDs utilize immunochromatography to detect circulating parasite-specific antigens (148) (Table 2). RDTs can come in dipstick or cassette form and can give results in approximately 20 min (149). RDTs that are based on immunochromatographic methods and utilize nitrocellulose membrane, which is a porous membrane with a high affinity for the hydrophobic binding of proteins (150, 151). The nitrocellulose allows wicking when the clinical sample is added, resulting in the formation of a single-colored control line for a negative result or two-colored lines (one the control line) for a positive result (152). Absence of the control line invalidates the test, and it must be repeated. These lines appear due the binding of parasite antigens present in the clinical sample to specific antibodies present on the nitrocellulose, which will be bound to gold nanoparticles, dye-loaded polymeric beads, fluorescent beads, magnetic nanoparticles, or carbon-based nanoparticles (153–158).
Molecular based RDTs are rarer; however, there are a few in early development stages that utilize LAMP in conjunction with a rapid DNA extraction step (76, 77, 159–162). These methods to date have also included the use of nitrocellulose membranes for DNA capture, with one molecular based RDT, the DjinniChip (76, 77), giving a similar read out to immunochromatographic diagnostics, with a test and control line appearing as the reaction completes, while others rely on fluorescence, turbidity, or other visual changes in the reaction mix to determine positivity.
RDT Immunodiagnostics
Dot-ELISA.
The ELISA is a solid-phase enzyme immunoassay that acts by the complexing of antibodies and antigens with substrates to give qualitative results that are visible to the naked eye (66, 67, 163). There are four types of ELISA: direct ELISA, indirect ELISA, sandwich ELISA, and competitive ELISA (163). Direct ELISA involves the direct binding of enzyme-linked antibodies to the antigen of interest (163) Indirect, sandwich, and competitive ELISAs work in multiple processes, involving the action of a primary antibody and a secondary enzyme-linked antibody (163).
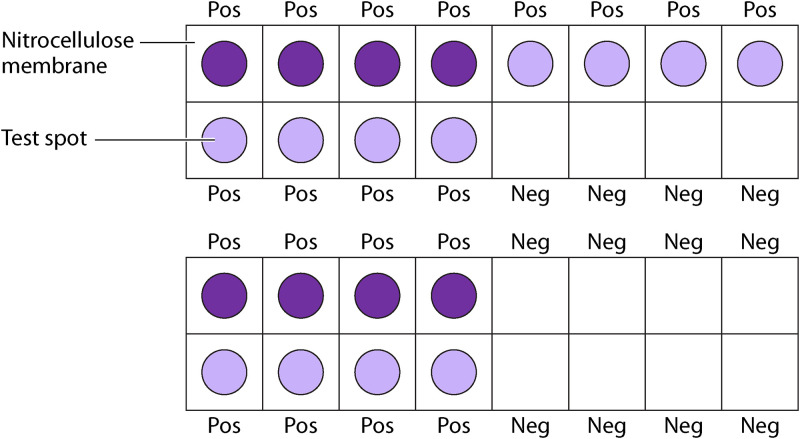
Dot-ELISA (Fig. 2) utilizes a sandwich ELISA, capturing the antigen of interest between a primary and secondary antibody (164). To begin the process, the appropriately labeled primary antibody is incubated on pieces of nitrocellulose paper and allowed to dry. The nitrocellulose is then incubated with blocking solution for 15 min to prevent nonspecific protein binding (150, 151). Next, the nitrocellulose is incubated with the clinical test sample for 30 min, followed by incubation with a conjugated antibody (69, 150). Finally, the nitrocellulose is incubated with an enzyme substrate for 30 min, which reacts with the enzyme and allows for the formation of a colored dot if parasite antigens are present (69, 150). A positive clinical test sample is observed as a color change on the paper, allowing direct visualization of the result (150, 151). The use of nitrocellulose reduces the nonspecific binding of proteins usually observed with plastic plates, increases the sensitivity and specificity, and requires substantially less parasite reagent and clinical test sample than the standard ELISA (150, 151). However, the stability of many of these antibodies can decrease significantly if not stored below 4°C (165).
FIG 2.
Diagram showing Dot-ELISA diagnostics, showing interpretation of positive and negative samples.
The Dot-ELISA has simplified the ELISA procedure and made it more accessible for POC diagnosis, giving sensitive results without the requirement of electrically powered equipment (69, 166). It is also rapid, can be completed at room temperature, and gives results that can be readily interpreted visually (151). However, due to the requirement for costly reagents and the high cost of storage to maintain sample viability, this assay remains generally inaccessible in resource poor regions (Table 4) (165). Nevertheless, to date, this method has been used, with a sensitivity and specificity similar to that of traditional ELISA, for the diagnosis of many parasitic diseases including: malaria, trypanosomiasis, leishmaniasis, toxoplasmosis, theileriosis, schistosomiasis, echinococcosis, fascioliasis, toxocariasis, taeniasis, and trichinosis (69, 150, 167–176) (Table 1).
Dot-ELISA dipsticks.
Nitrocellulose-based dipsticks were first developed to improve the accessibility of the Dot-ELISA (177). Dipsticks are prepared before going to the field by cutting nitrocellulose paper into small strips impregnated with a specific antigen or antibody for the parasite species to be identified to give test and control lines and glued to acetate strips. In the field, the strips are incubated with patient serum for 7 min, washed, and incubated with a conjugated antibody for 7 min before incubation with a specific enzyme substrate for a further 2 min (153, 154). Positive results are observed as the appearance of control and test bands in the test cell, which occurs due to a sandwich immunoassay in the presence of the targeted biomarker (153, 154). Nitrocellulose paper can also act as a blood separator, allowing for the separation of serum from blood cells by capillary action. The separated serum then travels up the strip, and the used blood separator can be removed and discarded, allowing for POC testing using whole blood samples (156–158). The BinaxNOW malaria assay and Diamed OptiMAL are RDTs that can successfully diagnose Plasmodium falciparum and P. vivax infections—and likely P. ovale, P. malariae, and P. knowlesi infections as well; however, sufficient testing has not been performed to determine the sensitivity and specificity of these species (178–180). Furthermore, RDTs cannot detect low parasitemia, leading to false negatives (181) (Table 4). Nitrocellulose-based dipsticks have since been improved to allow for higher sensitivity, specificity, and applicability for POC diagnostics, giving rise to lateral-flow immunochromatographic assays (LFIAs).
Lateral-flow immunochromatographic assays.
LFIAs are promising POC diagnostic devices due to their simplicity, low cost, robustness, and ease of implementation in remote regions of endemicity (182). The inclusion of nanotechnology in LFIAs has significantly improved disease detection by spectroscopic, optical, fluorescent, and electrochemical readouts, allowing for improved sensitivity and lower costs (152). Dipstick-based LFIAs, also known as test strips (Fig. 3), utilize chromatographic methods to separate different sample components based on longitudinal or transverse flow through a carrier and immunochemical reactions to detect positive infections; the immunoreagents are immobilized on the carrier through which the sample fluid flows (183). A sample is dropped on the base of the strip, usually made of nitrocellulose or glass fibers, and eluted using a small amount of buffer that contains the immunoreagent. This dye-labeled complex is present on the nitrocellulose strip in a small test line, along with a control line (184). The control line contains antibodies specific for the indicator-labeled antibody complex and will appear in all tests, including negative results, due to a color change upon binding of the antibody complex. Test lines are embedded areas of target indicator-labeled antibodies and change color upon binding of target antigens present in a sample (178). Immunochromatography is rapid, stable at temperatures up to 40°C, and reagent and energy conservative, and hundreds of samples can be processed simultaneously, making it ideal for POC diagnosis and epidemiological monitoring (154). Furthermore, capture antibodies can be placed onto the membrane in advance and then blocked, dried, and stored for up to 3 months at 4°C, while maintaining stability for use (185). LFIAs have been studied extensively for the diagnosis of malaria (P. falciparum and P. vivax primarily), visceral leishmaniasis, filariasis, trichomoniasis, toxoplasmosis, piroplasmosis, amoebiasis, cryptosporidiosis, giardiasis, trypanosomiasis, schistosomiasis, taeniasis, fasciolosis, trichinellosis, and echinococcosis (154, 156–158, 178–180, 186–213) (Table 1). Currently, however, there are only a small number of approved LFIAs for POC diagnosis of malaria, visceral leishmaniasis, giardiasis, amoebiasis, cryptosporidiosis, and schistosomiasis (74).
FIG 3.
Diagram of a nitrocellulose based lateral flow assay showing interpretations for positive, negative, and invalid results.
Nitrocellulose-based LFIAs appear to be a promising tool for POC diagnosis of parasitic diseases, with research demonstrating that LFIAs exhibit higher sensitivity and specificity than other immunodiagnostic methods—such as ELISA, immunofluorescence antibody tests (IFATs), and direct antiglobulin tests (DATs)—and microscopy-based diagnostic methods (210, 214). However, as shown with studies on malaria, the sensitivity and specificity of LFIAs depend on infection intensity (195, 203, 214) (Table 4). Low parasitemia (<250 parasites/μl) fails to reach the threshold of detection and can result in false negatives. False negatives can also be seen in individuals with very high levels of parasitemia, the prozone effect, due to excess antigens binding to the detecting antibody, leaving no epitopes available for capture or the test band antibody, which results in no band occurring for the test band (215). LFIAs cannot quantify infection burdens and, like other immunodiagnostic tests, generally fail to detect low parasitemia or low intensity infections; however, due to their relative simplicity, they could be used by untrained technicians in areas of endemicity where laboratories are not accessible or are poorly equipped (203, 206). However, despite ease of use, the interpretation of results can cause difficulty due to trace bands or multiple bands (216). In parasite-endemic areas, low levels of parasitemia may lead to asymptomatic infections, and thus a positive RDT with fever may indicate a plasmodium infection but not the cause of the fever (215); thus, sole reliance on RDT diagnostics can be problematic. Instead, RDTs can be one of several diagnostics, along with clinical symptoms and patient history, to determine patient status which is suggested for a number of parasitic NTDs (215) (Table 2). In malaria, changes to parasite antigens may also result in false negatives, such as variations in Pf-HRP2 or pLDH in Plasmodium falciparum (215, 217).
The timing of reading a lateral flow assay can also affect interpretation. A lateral flow assay for Chagas disease, PATH-Lemos, had optimal sensitivity and specificity (99.5 and 96.8%) after 20 min, which decreased slightly at 25 min (98.9 and 94.0%) (131). Longer times were not tested, but it might be expected to drop, and thus it may be critical for this, and other POC immunoassays, to read results at the right time. In addition to these issues, incorrect and length of storage can affect test reliability. While the inclusion of nanotechnology in immunoassays has greatly improved the rapidity and usability of LFIAs, there are several issues that are often overlooked. This includes nonspecific absorption of protein and gold nanoparticles, which can have serious effects on readability and sensitivity and result in high rates of false positives and negatives (152). Furthermore, LFIAs exhibit problems with cross-reactivity when blood samples are used instead of serum, causing decreased sensitivity (Table 4). This is due to the presence of cellular enzymes in whole blood or of fibrinogen and paraproteins in unclotted plasma, which prevents increased viscosity and hinders accurate sample pipetting (74).
The LFIA used for diagnosis of echinococcosis (VIRapid HYDATIDOSIS; Vircell, Granada, Spain) (serum) exhibits cross-reactivity when Taenia solium coinfections occur (218), while a dipstick LFIA for Schistosoma japonicum diagnosis (serum) exhibits cross-reactivity with Clonorchis, Paragonimus, or Angiostrongylus cantanensis coinfections (219, 220). An LFIA developed for Wucheria bancrofti infection detection (serum) exhibited high specificity and sensitivity, with little cross-reactivity observed in patients with Onchocera, Loa Loa, or Strongyloides infections (221), further suggesting that LFIAs may function more optimally when used on serum samples, which requires a centrifuge, thereby increasing costs (194, 195, 206). However, an LFIA developed for O. volvulus detection was shown to work as well with blood as with sera (222). In addition, a superhydrophobic plasma separator and microfluidic chip (162), which we discuss in more detail below, can separate serum from blood without electricity and may in future be combined with LFIAs to produce an optimal RDT for POC diagnostics in areas of endemicity. In clinical settings in developed nations, LFIAs may be used as part of a diagnostic process that often requires parallel testing with different diagnostics to improve sensitivity (Table 2).
Schistosomiasis POC-CCA cassette-based test.
The POC-CCA cassette assay is the only commercially available (Rapid Medical Diagnostics) POC for schistosomiasis detection, detecting S. mansoni primarily, although can detect other schistosome species, S. haematobium and S. japonicum, with less sensitivity (223). While treatment is the same for all species, species determination can be important when considering disease and damage caused by infection, for example, risk of bladder cancer with S. haematobium infection (224). The cassette uses urine to determine infection. There have been two iterations of this cassette, with an earlier version requiring a buffer addition, with the latest no longer requiring it. However, a high rate of false positives, low sensitivity or low reactivity in individuals not excreting eggs, and interpretation of trace results are barriers to successful implementation of this diagnostic (225). The test is also much more sensitive in detecting S. mansoni infections compared to S. haematobium, and there may be issues with use in areas with only S. haematobium present, or where the two schistosomes are coendemic. This obviously limits its potential use, particularly in Africa where both species are present, often in the same geographical setting. The presence of hybrid schistosome species in Africa may also be confounding factors for test accuracy (226–229). There has also been cross-reactivity identified with other helminth parasites (230). Interpretation of trace results remains a major stumbling block for replacement of KK with the POC-CCA (225, 231, 232). As part of the REASSURED (Tables 2 and 3) criteria that POCs should meet, usability and interpretation are key for field personnel with minimal training, as well as avoiding false negatives and positives (40).
The WHO is currently seeking expert feedback for target product profiles (TPPs) in concordance with the new 2021-2030 roadmap where diagnostics are highlighted as a crucial target. Within these TPPs the POC-CCA test, previously recommended by the WHO for control programs, has been highlighted as problematic due to low sensitivity in low-prevalence areas, with high false positivity in areas where prevalence is <10% (40). Manufacturing issues were also highlighted that resulted in variable performance in different product lots, including very high false-positive rates. A recent study in Brazil tested the POC-CCA RDT in an area where schistosomiasis was not endemic (233). All study participants were negative by microscopic methods (KK and helmintix); however, 37.9% were positive by POC_CCA, giving a specificity of 62.1% (233). Furthermore, there were differences in test results in fresh urine compared to the same urine samples stored at –20°C for 1 year, as per the manufacturer’s instructions. The reported difference was more than could be accounted for different batches of test were used. We highlight here specificity issues with the test that need to be addressed. There is an ongoing clinical trial into the sensitivity and specificity of a POC-CCA for S. japonicum that is due to finish in early 2021 and may shed more light on the sensitivity and specificity of the POC-CCA (234).
DNA-Based RDT
Whatman FTA cards: DNA-based diagnostics.
Standard filter papers or qualitative filter papers differ in content from Whatman FTA cards since they do not contain the proprietary chemicals for DNA capture and storage (119). Standard filter paper technologies have been used widely for genetic and protein analyses; however, improvements in whole-genome amplification, single-nucleotide polymorphism analyses, and DNA extraction methods are required (235–244). Studies utilizing dried blood spots (DBS) from newborn screening found that samples stored below –20°C for up to 20 years maintained viability equal to that of fresh DBS; however, DNA recovery declined in samples stored at room temperature for over 10 years, particularly for amplification of larger DNA fragments (236, 243). Serological detection of parasite antigen eluted from DBS on conventional Whatman filter paper has been successfully applied in the diagnosis of strongyloidiasis and lymphatic filariasis, but samples still require storage in a 4°C refrigerator at collection locations and at –20°C until analysis (245–247). This means that while filter papers can be useful for large-scale sampling (248), diagnostics, and rapid DNA purification and extraction, they cannot maintain DNA stability at room temperature as efficiently as Whatman FTA cards (119, 161, 249, 250). As such, samples stored on filter papers would either require storage at –20°C during transport, or they would need to be transported and processed rapidly at room temperature.
Limited research has been undertaken to determine the efficacy of Whatman FTA cards for storing parasite DNA; however, parasite samples stored on FTA cards have been used successfully in PCR and LAMP assays for diagnosis, genotyping, and population screening (102, 105, 251–261) (Table 1). Recent studies utilizing qPCR and direct PCR for detecting Dirofilaria repens microfilariae in blood stored on FTA cards suggest that loss of parasite DNA during extraction may be an issue due to the heterogeneous distribution of parasites and the lower volumes of blood used in spots compared to that of normal whole-blood samples used for conventional DNA extraction (262–264). However, these studies also found that although in low quantity, sufficient DNA could be recovered from FTA cards for conventional PCR analysis (264, 265). A study of different preservative methods for hookworm (Necator americanus)-spiked fecal samples suggested that FTA cards maintain DNA stability for long periods at 32°C compared to other storage methods, including 5% potassium dichromate, 90% ethanol, Formalternate, PAXgene, RNAlater, two-step desiccation ethanol-silica gel, and storage at –20°C (101). Despite the relatively high cost (AUD $2.20/sample) of FTA cards (101, 266), this initial outlay is compensated by savings in transport and storage costs (101, 266). By combining FTA cards with a comparatively low-cost diagnostic method, such as LAMP or direct PCR, diagnosis could become straightforward and accessible in remote, resource-poor areas (267–272).
DNA dipstick.
The DNA dipstick is a rapid method for DNA extraction and is made from Whatman filter paper number 1 that is dipped in paraplast, leaving a small section for DNA binding (159, 161). The dipstick was initially developed for extracting DNA from plants, and plant pathogens but has a universal application, and the method could be performed by lay persons in the field since the workflow is quite simple and, when combined with a LAMP assay, it is a potential low-cost POC diagnostic. The sample DNA is being extracted from is placed in a tube with ball bearings, shaken vigorously, and then the DNA dipstick is inserted three times into the lysate. The DNA dipstick is the dipped three times into a tube containing wash buffer and then three times into the tube containing LAMP reagents. The LAMP is run, and the result is visualized by a color change or flocculation. To date, the dipstick has been tested on S. japonicum parasite material (adult worms and eggs) and infected snails (273). The method needs to be tested on clinical samples to determine whether the method would be able to extract—and then detect with a subsequent LAMP assay—parasite DNA from clinical samples.
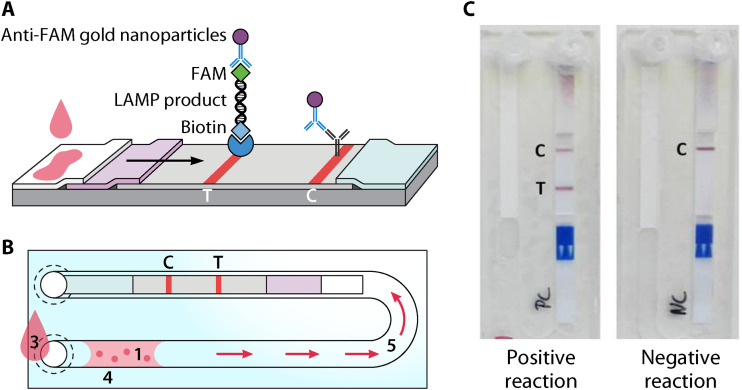
DjinniChip.
The DjinniChip (Fig. 4) is a novel LAMP-based molecular diagnostic assay that has been used for the diagnosis, surveillance, and control of trachoma, caused by Chlamydia trachomatis (274). The chips are assembled by injection-molding cyclic olefin copolymer, and the required LAMP reagents are freeze-dried into the channel (274). A lateral-flow test strip (LFS) is then placed into the chip, and the bottom of the chip is sealed. The clinical sample is added to the chip, and the sample extract is driven by capillary action into the reaction channel, reconstituting the LAMP reagents, and the chip is further sealed (274). After a 35-min incubation at 65°C, the sample is then on placed on ice blocks to stop reactions, and chromatographic buffer is used to manually drive the amplified reaction toward the LFS, giving a visible result within 10 min due to the use of gold nanoparticles attached to anti-fluorescein-antibodies (274). The DjinniChip is low cost (<$2 [US] per test), is rapid, has a total test time of 50 min, requires only a portable heat block that can be powered with a car battery, and can be completed by untrained personnel, making it accessible in resource-poor regions (274). The requirement for ice, which was transported in a cool box in a “mock field” trial, will limit some usage in very remote areas (76, 77).
FIG 4.
Schematic principle of the DjinniChip. (A) LAMP product on lateral flow strip. (B) Components and processes. The DjinniChip contains lyophilized reagents (1) and a lateral flow test strip (2). A liquid sample (3) is loaded onto the chip through its inlet. The liquid sample reconstitutes the reagents (4). Chlamydia trachomatis DNA is amplified at 65°C. The reaction mix is pumped gently with a syringe to the lateral flow test (5). The appearance of the control band (labeled “C”) indicates the correct function of the lateral flow test. The appearance of the test band (labeled “T”) indicates the presence of C. trachomatis in the sample. (C) Detection on DjinniChips. (Reproduced from reference 77.)
The DjinniChip has yet to be used for parasite diagnosis, but given the low price, simplicity, ease of assembly, lack of requirement for expensive laboratory equipment and trained personnel, rapid results visible to the naked eye, and stability in many environmental conditions, this chip provides an exciting opportunity for rapid accessible POC diagnosis of parasitic NTDs. However, the DjinniChip still requires further optimization to prevent contamination, which occurs due to the manual handling steps, to the improve the sensitivity and specificity of the test. A “mock field” trial on spiked samples resulted in a higher number of false positives (76, 77). Contamination in field settings will be an ongoing issue for all RTDs. At least some training may be required on proper aseptic techniques for those using the tests in field situations.
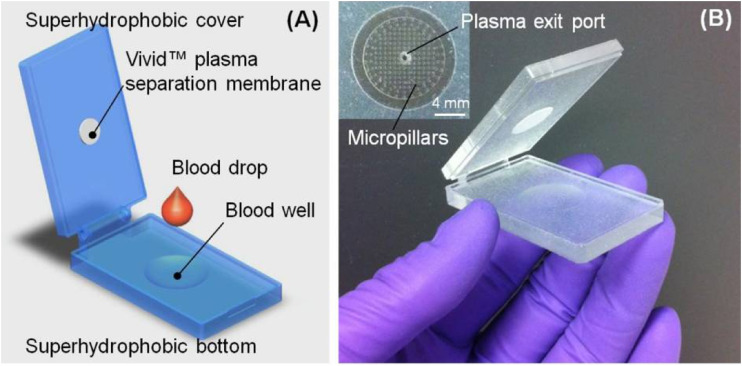
Superhydrophobic plasma separator and microfluidic chip.
The superhydrophobic plasma separator (SPS) was developed to separate plasma from whole blood without electricity, making it ideal as part of an RDT (Fig. 5) (162). Whole blood often inhibits downstream applications such as PCR or LAMP, necessitating the removal of the blood cellular components or the separation of the blood by centrifugation with only the plasma section retained. In this case, the SPS was combined with a LAMP assay performed on a microfluidic chip with plasma separated from whole blood by the SPS (162, 275). Then, 200 μl of whole blood was spiked with S. mansoni DNA with a recovery rate of 84.5% compared to the more traditional method of centrifugation for separation of plasma from whole blood. As the amount of S. mansoni DNA increased, so also did the recovery rate, although not significantly, and the detection limit was identified as 0.5 fg of S. mansoni DNA (162).
FIG 5.
Diagram (A) and images (B) of the superhydrophobic separator. (Reproduced from reference 162 with permission of the Royal Society of Chemistry.)
The SPS is made up of a hinged slide coated in hydrophobic substrate, with the lower slide containing a small well for the blood to be placed, and a small membrane on the upper slide. After whole blood is placed in the well, the slides are closed together, forming a smear. The SPS relies on natural sedimentation, with the heavier red and white blood cells moving down toward the bottom of the well, leaving the top portion clearer and containing primarily plasma. The plasma is filtered through the membrane for further cleaning of the sample. Once the plasma was separated, it was then removed and tested for S. mansoni using a real-time LAMP on a microfluidic chip (275).
This method has not been tested under field conditions or on “real-world”’ samples and may require further optimization for the field. Any schistosome DNA detected in plasma will be cell-free DNA and therefore present in small amounts. This method only uses 200 μl of whole blood, and a minimum concentration of cell free schistosome DNA is required for detection. It would also be difficult to produce large numbers of the SPS device for field testing since it is currently produced by three-dimensional printing, nor is it clear whether the SPS or microfluidic chips could be re-used, which may increase costs. However, the SPS is a promising method that can also be used in conjunction with immunodiagnostics where plasma separation may also be beneficial.
Fusion 5.
Fusion 5 technology is a silica-based filter (GE Healthcare, USA) designed to replace the solid materials that are currently used in lateral flow immunoassays and allows the manufacture of lateral-flow tests on a single sample (116). This technology reduces test times and costs and is naturally hydrophilic (116). Standard test strips are made of many different components laid over each other with an overlap, requiring the correct pressure between each layer to allow the sample to flow efficiently from one component to the next (116). Fusion 5 consists of glass fibers containing a plastic binder, which increases its mechanical strength, maintains hydrophilicity, and allows fast wicking speed, giving quicker test times (116). Fusion 5 technology has been shown to exhibit increased nucleic acid capture efficiency rates compared to that of other Whatman cellulose-based filters (116). These attributes allow Fusion 5 to have many functions, including a blood separator, a sample pad, a reaction substrate, and a conjugate release membrane (116).
In 2009, a nucleic acid extraction method was designed, called filtration isolation of nucleic acids (FINA), utilizing Fusion 5 technology as the nucleic acid capture membrane (276). Briefly, a Fusion 5 disk was sandwiched between 707 blotter paper and Parafilm, with a hole in the center for the disk where a clinical blood sample for testing was applied. This was then washed and placed directly in a PCR tube for amplification (276). Using this procedure, HIV proviral DNA was successfully extracted and provided 98.8% sensitivity and 100% specificity in all qPCR experiments compared to viral load data obtained through reverse transcription-PCR (276, 277). Importantly, dried blood modules stored at 37°C for up to 5 weeks maintained DNA stability of samples (276). Lysing the red blood cells with Triton-X and attaching the disk to the side of the PCR tube further optimized the method, yielding 100% sensitivity and specificity (278). These methods produced a low-cost device for DNA extraction; however, they are only applicable to blood samples. In 2015, a study created a novel paper-based micro-fluid device using Fusion 5 as the sample capture filter. For this method, the sample was loaded onto the capture filter and washed three times with water. NaOH, HCl, and water were then aspirated through the filter, which was then taken for PCR verification (116). This device was able to purify genomic DNA from more diverse clinical samples, including whole blood, dried blood spots, buccal swabs, saliva, and cigarette butts with high efficiency (116).
Fusion 5 has also been used as part of a simple paper microfluidic device for sample preparation and nucleic acid extraction. In this “microfluidic origami,” a stack of flat polymer sheets is combined in a way that folding activates the DNA purification process (279). Fusion 5 membrane, cut to specific measurements by lasers, was used as the DNA capture filter with this device (279). The DNA capture filter was placed on the origami device, and a number of folding steps preceded placement of the sample on the filter, which was then washed, dried, and eluted using a low concentration of salt solution (279). This study successfully purified Escherichia coli DNA from pig mucin for conventional PCR amplification (279). The use of Fusion 5 technology for nucleic acid purification is still in its infancy; however, this technology presents an exciting opportunity for the development of simple and cost-effective purification of parasite genomic DNA, including parasite cell-free DNA that might be present particularly in blood, saliva, and urine, combined with isothermal amplification.
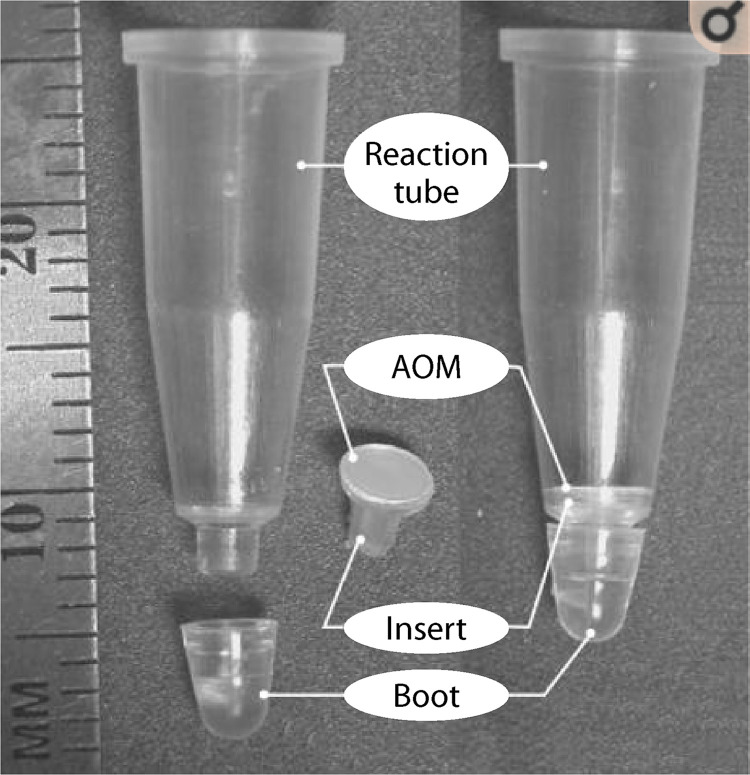
Aluminum oxide membrane.
Aluminum oxide membranes (AOMs) form through the anodization of aluminum in acid electrolytes resulting in a semiordered parallel porous oxide membrane with a closed base (280, 281). The anodizing voltage and choice of electrolyte mediates AOM pore size, ultimately giving a rigid membrane that has 50% porosity, which allows a high rate of liquid flow (282). This has allowed AOMs to have many functions, including the filtration of biological materials (e.g., blood and bovine serum albumin), as a matrix for artificial tissue growth and cell culture, and as a template for nanodevice manufacture (255, 283, 284). An AOM, functioning as a filter, allows nucleic acid extraction, with the nucleic acids being localized to the surface (255, 283, 284).
Since their development, AOMs have been widely studied for detecting viral and bacterial infections from extracted genomic DNA (282, 285–290). Single-tube nucleic acid extraction methods using AOMs have been designed wherein the membrane is built into a 0.2-ml PCR tube (Fig. 6) (285, 286). This has successfully been used for hepatitis C virus detection with accuracy comparable to that of conventional extraction methods to which it was compared (285, 286). PCR amplification of DNA extracted using AOM filtration provided results that were close to 100% efficient (282). AOMs can be incorporated into microfluidic chips for DNA extraction from blood and saliva, a procedure that has been used to detect both methicillin-resistant and -susceptible strains of Staphylococcus aureus and HIV (282, 285–290). AOMs have yet to be utilized for parasite DNA extraction and, given the requirement for blood collected by venipuncture, these methods may not be ideal for use in parasite diagnosis due to cost (282). As such, for AOMs to be considered for wide-scale POC diagnostic application, further optimization would be required to allow DNA extraction from small sample volumes or from dried blood spots.
FIG 6.
PCR tube (0.2 ml) with AOM insert. (Reprinted from reference 286 with permission of Elsevier.)
ANIMAL HOSTS AND RDTs
Many of the parasitic NTDs are zoonotic, with animal reservoir hosts important in transmission and maintaining the parasite in the environment (18). Schistosoma japonicum, as an example, has 46 potential mammalian definitive hosts (291), as well as a molluscan intermediate host, that complicate control efforts. Dracunculus medinensis, otherwise known as guinea worm, has almost been eliminated. However, complications in Chad, one of the last remaining countries where the guinea worm is still endemic, including potential paratenic hosts and zoonotic transmission in dogs, may set back elimination of this disease (292–294).
It is therefore highly important that animals are also included in control programs by testing and treating infected animals, as well as using other control interventions to prevent transmission of parasites. For example, in China, bovines have been identified as major reservoir hosts, and these animals have been removed from areas of endemicity, while in the Philippines interventions to prevent fascioliasis transmission from bovines, such as drying and storing animal feces for a set amount of time before using on fields and keeping bovines away from flooded fields, have been implemented (295–297). For some parasite-related diseases, such as schistosomiasis, fascioliasis, taeniasis, dracunculiasis, and STH, environmental monitoring of water and soil is appropriate and can indicate active transmission sites for formation of risk maps (298–305).
In control programs and field research studies of zoonotic parasites, a number of diagnostic methods, including microscopy, immunodiagnostics, and molecular assays, have been used to diagnose animal infections (71, 306–321). RDTs used on humans would ideally also be able to identify animal infections for rapid treatment and management of animal cases, as well as prevent transmission to humans from animal reservoirs of infection in cases of zoonotic transmission.
Several RDTs have been developed for canine visceral leishmaniasis. The Kalazar Detect Canine Rapid Test (Inbios; rK39) and the Canine and DPP CVL (BioManguinhos) RDTs both detect leishmaniasis from serum samples (146, 147). The sensitivity of the Inbios rK39 increases with increasing parasitemia, and it is therefore more sensitive in animals exhibiting clinical disease symptoms than in those who are asymptomatic—86.7 and 59.3%, respectively—from a meta-analysis on rK39 (146).
Animal African trypanosomiasis (AAT) is caused by species that generally do not cause infections in humans (T. congolense, T. vivax, and T. brucei brucei), although there are some cases of AAT causing human infections (322). AAT can cause significant economic losses in Africa (322). Two RDTs developed for detecting human African trypanosomiasis (HAT) have been tested in cattle for detecting AAT using fresh whole blood from cattle in Kenya and thawed serum samples from cattle in Uganda (323). The first RDT, the 1G RDT (SD BIOLINE HAT; FIND and Alere/Standard Diagnostics), is available commercially, while the second, p2G RDT, is a prototype. The specificity in animals from regions of endemicity was low for both tests (14.6 to 22.6%), while the specificity in areas of nonendemicity was high (97.5%) for the p2G RDT but low for the 1G RDT (57.9%). In both cases neither RDT is suggested for use in detecting AAT (323).
There are a number of immunochromatography (ICT) RDTs that detect Toxoplasma, a zoonotic protozoan parasite, in animals and humans (324). The majority detect antibodies in sera, with three of the available ICT tests commercially available for use in humans (324).
REGULATORY APPROVAL
In vitro diagnostics (IVDs) utilize human clinical samples to diagnose diseases and can be in the form of commercial test kits or laboratory-developed tests (LDTs). Although the cost of diagnostic testing comprises a fraction, <5%, of overall of medical and hospital costs, IVDs contribute to up to 70% of treatment decisions (325, 326). This highlights the critical requirements for highly accurate IVDs that are reliable, effective, and accessible. IVDs are constantly evolving and advancing due to increasingly sophisticated scientific techniques, biomarkers, and available assays. Further, there is an increased demand for point-of-care and home-based testing kits that provide rapid results, aiming to reduce the diagnosis times and ensure that patients receive appropriate medical treatment as quickly as possible (325, 326). However, many barriers affect the adoption of new innovative diagnostic tests in routine medical practice.
The U.S. Food and Drug Administration (FDA), the Therapeutic Goods Administration (TGA; Australia), and the European Medicines Agency (EMA) regulate the development of IVDs. IVDs are divided into three classes: class I (low to moderate risk), class II (moderate to high risk), and class III (high risk). There are three main approval routes available for IVDs, 510(k), premarket approval (PMA), and de novo reclassification, each of which requires the availability of samples and associated data, the use of relevant samples for the market, compliance with informed consent, and lack of any ethical issues (327, 328). The 510(k) is a regulatory pathway for tests that are shown to be equivalent to an existing test, while PMA is required when a test is not similar to an existing test. De novo reclassification allows for a low to moderate risk test that does not have a comparable device, which is usually required when 510(k) applications are rejected due to not being similar enough. These regulatory hurdles are complex, and the process of approval can be lengthy, taking up to 5 years from time of submission. Laboratories can avoid these hurdles by developing in-house diagnostic tests (LDTs); however, these tests have recently begun being more closely scrutinized by the FDA, which threatens to significantly slow down the development and use of innovative LDTs.
Of all the NTDs, only the following three have FDA-approved molecular diagnostics: Dengue (CDC DENV-1-4 Real-Time PCR Assay); Leishmania spp. (SMART Leish), which has limited availability (military use only); and Trachoma (multiple tests) (329). The EMA and TGA currently do not have lists of approved IVDs; however, the TGA indicates that one will be available by July 2022.
As part of the new roadmap for NTDs 2021 to 2030, the WHO has highlighted diagnostics as one of four key priority areas (330, 331). As part of this the WHO has established a diagnostics technical advisory group (DTAG) for collaborative development of new diagnostic tools. Key areas of responsibility for the DTAG include reviewing diagnostics needs in NTD programs, defining use cases and target product profiles, and linking with key partners to support test development and validation. A “silver lining” of the COVID-19 pandemic has been an increase in molecular testing capacity in countries that previously had low capacity, increasing the likelihood of adoption of molecular methods, at least in the short term. However, reagent costs may still place traditional molecular diagnostics for NTDs out of reach.
CONCLUSION
The ideal diagnostic test should be simple, rapid, and low in cost; require few steps; and exhibit high sensitivity and specificity—features particularly relevant in areas of endemicity where infection intensity is reduced as the result of MDA programs operating. Although serological tests, especially dipstick assays, provide diagnostic simplicity, they can lack sensitivity and specificity and may not distinguish between past and current infections, since antibodies generally remain in circulation after drug treatment. Furthermore, antibody levels do not accurately correlate with parasite burden (61–64, 187). Moreover, the current costs associated with LFIAs prohibit their extensive use in resource-poor areas of endemicity.
Cellulose-based Whatman FTA cards have been utilized extensively for parasite diagnosis and in epidemiological studies. These cards can maintain nucleic acid stability under less-than-optimal conditions, providing low-cost storage and transport of clinical samples for subsequent nucleic acid purification. Fusion 5 technology, AOMs, and the DjinniChip have yet to be tested or utilized for parasite nucleic acid extraction, but with such promising results obtained for other pathogens using whole blood, saliva, and buccal swabs, they represent a promising advance (274, 276, 278). These methods allow for rapid and simple nucleic acid extraction and require only small sample volumes which can be stored at temperatures up to 37°C, while maintaining DNA stability (276, 278). Given their efficiency for DNA extraction from blood, these methods may be particularly useful for diagnosing blood-borne parasites.
A major consideration in molecular diagnosis remains the high cost of DNA extraction. Many NAATs, except for LAMP, remain expensive and complicated, making them generally inaccessible for POC diagnosis. Therefore, as countries look toward NTD elimination, new nucleic acid purification techniques that are simple, rapid, and more cost-effective must be developed. The use of membrane technology provides a novel avenue for simple and cost-effective parasite DNA purification. Further optimization of this technology could pave the way for significant improvements in the accessibility of highly sensitive molecular diagnostic techniques applicable to the most affected regions of NTD endemicity globally.
ACKNOWLEDGMENTS
Our studies on schistosomiasis and other NTDs receive financial support from The National Health and Medical Research Council of Australia.
We declare that we have no conflicts of interest.
Biographies

Madeleine J. Rogers, B.Sc. (Hons), is a Ph.D. candidate at QIMR Berghofer Medical Research institute and the University of Queensland. Madeleine researches the molecular biology and immunology of parasitic worms and has published one article. Madeleine graduated from a Bachelor of Science with first-class honors in 2020, during which they were awarded the QIMR Berghofer Honours Scholarship and the University of Queensland Dean’s Commendation for Academic Excellence. In 2020, Madeleine began their Ph.D. project by studying the immunomodulatory properties of helminth egg-derived products in food allergy, with the aim of developing novel therapeutics derived from parasitic worms for the treatment of allergic disease.

Donald P. McManus, B.Sc., Ph.D., D.Sc., is a National Health and Medical Research Senior Leadership Fellow and Senior Principal Research Fellow and Senior Scientist at QIMR Berghofer Medical Research Institute, Professor of Tropical Health, University of Queensland, and Professor, Australian National University. He researches the molecular biology, immunology, diagnosis, and epidemiology of parasitic worms. Professor McManus has published over 650 articles, cited more than 38,000 times. He is Honorary International Fellow of the American Society of Tropical Medicine and Hygiene, honorary member of the American and British Societies of Parasitologists, and Fellow of the Royal Society of Biology (United Kingdom) and the Australian Academy of Health and Medical Sciences. He was recipient of the Ralph Doherty QIMR Berghofer Prize for Outstanding Achievement and Leadership in Medical Research, the 2018 Sornchai Looareesuwan Medal “for outstanding achievements in experimental and clinical topical medicine research” and the 2020 NHMRC Peter Doherty Investigator Grant Award for the top ranked application in the Leadership category.

Stephen Muhi, M.D., is an Infectious Diseases Physician at the Royal Melbourne Hospital and Ph.D. Candidate at the Peter Doherty Institute for Infection and Immunity. His Ph.D. research includes novel strategies for diagnosing and controlling emerging infectious diseases, including the evaluation of rapid, point-of-care diagnostics for COVID-19 and vaccine testing strategies for Buruli ulcer. He is a Fellow of the Australasian College of Physicians and the Australasian College of Tropical Medicine. He also has several roles in medical education, including an appointment at the University of Melbourne Medical School. He also works as a Senior Medical Advisor to the Victorian Department of Health in COVID-19 response. He sits on a number of councils and committees related to tropical medicine and medical education and has supervised a number of medical students and junior doctors in infectious-diseases-related research.

Catherine A. Gordon, B.Sc., Ph.D., is a postdoctoral researcher at the QIMR Berghofer Medical Research Institute and lecturer in Parasitology at the University of Queensland and the Queensland University of Technology. Her research is focused on using molecular tools to understand the biology and epidemiology of a range of parasitic diseases including schistosomiasis, soil-transmitted helminths, and intestinal protozoa. She is particularly focused on developing rapid point-of-care diagnostics for use “in the field,” where these parasitic diseases occur to allow for rapid treatment and accurate prevalence data to better understand epidemiology of disease.
Contributor Information
Donald P. McManus, Email: Don.McManus@qimrberghofer.edu.au.
Catherine A. Gordon, Email: Catherine.Gordon@qimrberghofer.edu.au.
REFERENCES
- 1.WHO. 2019. Neglected tropical disease. World Health Organization, Geneva, Switzerland. https://www.who.int/neglected_diseases/diseases/en/. Accessed 19 August 2019. [Google Scholar]
- 2.CDC. 2016. About parasites. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/parasites/about.html. Accessed 17 November 2019. [Google Scholar]
- 3.Gordon CA, Kurscheid J, Williams GM, Clements ACA, Li Y, Zhou XN, Utzinger J, McManus DP, Gray DJ. 2019. Asian schistosomiasis: current status and prospects for control leading to elimination. Trop Med Infect Dis 4:40. 10.3390/tropicalmed4010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mationg MLS, Gordon CA, Tallo VL, Olveda RM, Alday PP, Reñosa MDC, Bieri FA, Williams GM, Clements ACA, Steinmann P, Halton K, Li Y, McManus DP, Gray DJ. 2017. Status of soil-transmitted helminth infections in schoolchildren in Laguna Province, the Philippines: determined by parasitological and molecular diagnostic techniques. PLoS Negl Trop Dis 11:e0006022. 10.1371/journal.pntd.0006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray CJ, Barber RM, Foreman KJ, Abbasoglu Ozgoren A, Abd-Allah F, Abera SF, Aboyans V, Abraham JP, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NM, Achoki T, Ackerman IN, Ademi Z, Adou AK, Adsuar JC, Afshin A, Agardh EE, Alam SS, Alasfoor D, Albittar MI, Alegretti MA, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Alla F, Allebeck P, Almazroa MA, Alsharif U, Alvarez E, Alvis-Guzman N, Amare AT, Ameh EA, Amini H, Ammar W, Anderson HR, Anderson BO, Antonio CA, Anwari P, Arnlöv J, Arsic Arsenijevic VS, Artaman A, Asghar RJ, Assadi R, Atkins LS, Avila MA, Awuah B, Bachman VF, Badawi A, GBD 2013 DALYs and HALE Collaborators, et al. 2015. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet 386:2145–2191. 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitra AK, Mawson AR. 2017. Neglected tropical diseases: epidemiology and global burden. Trop Med Infect Dis 2:36. 10.3390/tropicalmed2030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McManus DP, Bieri FA, Li Y-S, Williams GM, Yuan L-P, Henglin Y, Du Z-W, Clements AC, Steinmann P, Raso G, Yap P, Magalhães RJS, Stewart D, Ross AG, Halton K, Zhou X-N, Olveda RM, Tallo V, Gray DJ. 2014. Health education and the control of intestinal worm infections in China: a new vision. Parasit Vectors 7:344–344. 10.1186/1756-3305-7-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bieri FA, Gray DJ, Williams GM, Raso G, Li YS, Yuan L, He Y, Li RS, Guo FY, Li SM, McManus DP. 2013. Health-education package to prevent worm infections in Chinese schoolchildren. N Engl J Med 368:1603–1612. 10.1056/NEJMoa1204885. [DOI] [PubMed] [Google Scholar]
- 9.Piper JD, Chandna J, Allen E, Linkman K, Cumming O, Prendergast AJ, Gladstone MJ. 2017. Water, sanitation and hygiene (WASH) interventions: effects on child development in low- and middle-income countries. Cochrane Database Systematic Rev 2017:CD012613. [Google Scholar]
- 10.Cairncross S, Muller R, Zagaria N. 2002. Dracunculiasis (Guinea worm disease) and the eradication initiative. Clin Microbiol Rev 15:223–246. 10.1128/CMR.15.2.223-246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun L-P, Wang W, Hong Q-B, Li S-Z, Liang Y-S, Yang H-T, Zhou X-N. 2017. Approaches being used in the national schistosomiasis elimination programme in China: a review. Infect Dis Poverty 6:55. 10.1186/s40249-017-0271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weerakoon KG, Gobert GN, Cai P, McManus DP. 2015. Advances in the diagnosis of human schistosomiasis. Clin Microbiol Rev 28:939–967. 10.1128/CMR.00137-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krentel A, Fischer PU, Weil GJ. 2013. A review of factors that influence individual compliance with mass drug administration for elimination of lymphatic filariasis. PLoS Negl Trop Dis 7:e2447-e2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krentel A, Damayanti R, Titaley CR, Suharno N, Bradley M, Lynam T. 2016. Improving coverage and compliance in mass drug administration for the elimination of LF in two “endgame” districts in Indonesia using micronarrative surveys. PLoS Negl Trop Dis 10:e0005027. 10.1371/journal.pntd.0005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inobaya MT, Chau TN, Ng SK, MacDougall C, Olveda RM, Tallo VL, Landicho JM, Malacad CM, Aligato MF, Guevarra JB, Ross AG. 2018. Mass drug administration and the sustainable control of schistosomiasis: an evaluation of treatment compliance in the rural Philippines. Parasit Vectors 11:441. 10.1186/s13071-018-3022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inobaya MT, Olveda RM, Tallo V, McManus DP, Williams GM, Harn DA, Li Y, Chau TN, Olveda DU, Ross AG. 2015. Schistosomiasis mass drug administration in the Philippines: lessons learnt and the global implications. Microbes Infect 17:6–15. 10.1016/j.micinf.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Ostan I, Kilimcioğlu AA, Girginkardeşler N, Ozyurt BC, Limoncu ME, Ok UZ. 2007. Health inequities: lower socio-economic conditions and higher incidences of intestinal parasites. BMC Public Health 7:342–342. 10.1186/1471-2458-7-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon CA, McManus DP, Jones MK, Gray DJ, Gobert GN. 2016. The increase of exotic zoonotic helminth infections: the impact of urbanization, climate change, and globalization. Adv Parasitol 91:311–397. 10.1016/bs.apar.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Genchi C, Mortarino M, Rinaldi L, Cringoli G, Traldi G, Genchi M. 2011. Changing climate and changing vector-borne disease distribution: the example of Dirofilaria in Europe. Vet Parasitol 176:295–299. 10.1016/j.vetpar.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 20.York EM, Butler CJ, Lord WD. 2014. Global decline in suitable habitat for Angiostrongylus ( = Parastrongylus) cantonensis: the role of climate change. PLoS One 9:e103831. 10.1371/journal.pone.0103831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montarsi F, Ciocchetta S, Devine G, Ravagnan S, Mutinelli F, di Regalbono A, Otranto D, Capelli G. 2015. Development of Dirofilaria immitis within the mosquito Aedes (Finlaya) koreicus, a new invasive species for Europe. Parasit Vectors 8:177. 10.1186/s13071-015-0800-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plumer L, Davison J, Saarma U. 2014. Rapid urbanization of red foxes in Estonia: distribution, behaviour, attacks on domestic animals, and health-risks related to zoonotic diseases. PLoS One 9:e115124. 10.1371/journal.pone.0115124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellner KF, Page LK, Downey M, McCord SE. 2012. Effects of urbanization on prevalence of Baylisascaris procyonis in intermediate host populations. J Wildl Dis 48:1083–1087. 10.7589/2011-09-267. [DOI] [PubMed] [Google Scholar]
- 24.Page K, Smyser TJ, Dunkerton E, Gavard E, Larkin B, Gehrt S. 2014. Reduction of Baylisascaris procyonis eggs in raccoon latrines, Suburban Chicago, Illinois, USA. Emerg Infect Dis 20:2137–2140. 10.3201/eid2012.140977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muhumuza S, Olsen A, Katahoire A, Nuwaha F. 2013. Uptake of preventive treatment for intestinal schistosomiasis among school children in Jinja district, Uganda: a cross sectional study. PLoS One 8:e63438. 10.1371/journal.pone.0063438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross AG, Olveda RM, Li Y. 2015. An audacious goal: the elimination of schistosomiasis in our lifetime through mass drug administration. Lancet 385:2220–2221. 10.1016/S0140-6736(14)61417-3. [DOI] [PubMed] [Google Scholar]
- 27.Colley DG, Andros TS, Campbell CH, Jr.. 2017. Schistosomiasis is more prevalent than previously thought: what does it mean for public health goals, policies, strategies, guidelines and intervention programs? Infect Dis Poverty 6:63. 10.1186/s40249-017-0275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldmeier H, Poggensee G. 1993. Diagnostic techniques in schistosomiasis control: a review. Acta Trop 52:205–220. 10.1016/0001-706X(93)90009-Z. [DOI] [PubMed] [Google Scholar]
- 29.Bieri FA, Yuan L-P, Li Y-S, He Y-K, Bedford A, Li RS, Guo F-Y, Li S-M, Williams GM, McManus DP, Raso G, Gray DJ. 2013. Development of an educational cartoon to prevent worm infections in Chinese schoolchildren. Infect Dis Poverty 2:29. 10.1186/2049-9957-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bieri FA, Li Y-S, Yuan L-P, He Y-K, Gray DJ, Williams GM, McManus DP. 2014. School-based health education targeting intestinal worms-further support for integrated control. PLoS Negl Trop Dis 8:e2621. 10.1371/journal.pntd.0002621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross AG, Chau TN, Inobaya MT, Olveda RM, Li Y, Harn DA. 2017. A new global strategy for the elimination of schistosomiasis. Int J Infect Dis 54:130–137. 10.1016/j.ijid.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 32.Krentel A, Gyapong M, Mallya S, Boadu NY, Amuyunzu-Nyamongo M, Stephens M, McFarland DA. 2017. Review of the factors influencing the motivation of community drug distributors towards the control and elimination of neglected tropical diseases (NTDs). PLoS Negl Trop Dis 11:e0006065. 10.1371/journal.pntd.0006065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori M, Ravinetto R, Jacobs J. 2011. Quality of medical devices and in vitro diagnostics in resource-limited settings. Trop Med Int Health 16:1439–1449. 10.1111/j.1365-3156.2011.02852.x. [DOI] [PubMed] [Google Scholar]
- 34.Peeling RW, Holmes KK, Mabey D, Ronald A. 2006. Rapid tests for sexually transmitted infections (STIs): the way forward. Sex Transm Infect 82(Suppl 5):v1–v6. 10.1136/sti.2006.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunningham J, Jones S, Gatton ML, Barnwell JW, Cheng Q, Chiodini PL, Glenn J, Incardona S, Kosack C, Luchavez J, Menard D, Nhem S, Oyibo W, Rees-Channer RR, Gonzalez I, Bell D. 2019. A review of the WHO malaria rapid diagnostic test product testing programme (2008–2018). Malar J 18:387–387. 10.1186/s12936-019-3028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO. 2020. WHO list of prequalified in vitro diagnostic products. World Health Organization, Geneva, Switzerland. https://www.who.int/diagnostics_laboratory/evaluations/200424_prequalified_product_list.pdf?ua=1. Accessed 25 July 2021. [Google Scholar]
- 37.WHO. 2011. Visceral leishmaniasis rapid diagnostic test performance: diagnostics evaluation series no. 4. World Health Organization, Geneva, Switzerland. https://www.who.int/tdr/publications/documents/vl-rdt-evaluation.pdf?ua=1. Accessed 3 June 2020. [Google Scholar]
- 38.García-Bernalt Diego J, Fernández-Soto P, Febrer-Sendra B, Crego-Vicente B, Muro A. 2021. Loop-mediated isothermal amplification in schistosomiasis. J Clin Microbiol 10:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Land KJ, Boeras DI, Chen X-S, Ramsay AR, Peeling RW. 2019. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat Microbiol 4:46–54. 10.1038/s41564-018-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO. 2021. TPP scope schistosomiasis monitoring and evaluation narrative. World Health Organization, Geneva, Switzerland. https://www.who.int/news-room/articles-detail/public-consultation-target-product-profiles-for-diagnostic-tests-to-meet-schistosomiasis-and-soil-transmitted-helminth-programme-needs. Accessed 11.02.2021. [Google Scholar]
- 41.Ndao M. 2009. Diagnosis of parasitic diseases: old and new approaches. Interdiscip Perspect Infect Dis 2009:278246. 10.1155/2009/278246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia LS, Arrowood M, Kokoskin E, Paltridge GP, Pillai DR, Procop GW, Ryan N, Shimizu RY, Visvesvara G. 2018. Practical guidance for clinical microbiology laboratories: laboratory diagnosis of parasites from the gastrointestinal tract. Clin Microbiol Rev 31:e00025-17. 10.1128/CMR.00025-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou X-N. 2018. Schistosomiasis. Nat Rev Dis Primers 4:13. 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 44.Hancock K, Tsang VC. 1986. Development and optimization of the FAST-ELISA for detecting antibodies to Schistosoma mansoni. J Immunol Methods 92:167–176. [DOI] [PubMed] [Google Scholar]
- 45.Katz N, Chaves A, Pellegrino J. 1972. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo 14:397–400. [PubMed] [Google Scholar]
- 46.Pardo J, Arellano JL, Lopez-Velez R, Carranza C, Cordero M, Muro A. 2007. Application of an ELISA test using Schistosoma bovis adult worm antigens in travellers and immigrants from a schistosomiasis endemic area and its correlation with clinical findings. Scand J Infect Dis 39:435–440. 10.1080/00365540601105806. [DOI] [PubMed] [Google Scholar]
- 47.Rabello A. 1997. Diagnosing schistosomiasis. Mem Inst Oswaldo Cruz 92:669–676. 10.1590/S0074-02761997000500021. [DOI] [PubMed] [Google Scholar]
- 48.Drain PK, Hyle EP, Noubary F, Freedberg KA, Wilson D, Bishai WR, Rodriguez W, Bassett IV. 2014. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis 14:239–249. 10.1016/S1473-3099(13)70250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajchgot J, Coulibaly JT, Keiser J, Utzinger J, Lo NC, Mondry MK, Andrews JR, Bogoch II. 2017. Mobile-phone and handheld microscopy for neglected tropical diseases. PLoS Negl Trop Dis 11:e0005550. 10.1371/journal.pntd.0005550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saeed MA, Jabbar A. 2018. Smart diagnosis of parasitic diseases by use of smartphones. J Clin Microbiol 56:e01469-17. 10.1128/JCM.01469-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. 2018. Schistosomiasis. Nat Rev Dis Primers 4:13. 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz C, Fallon PG. 2018. Schistosoma “eggs-Iting” the host: granuloma formation and egg excretion. Front Immunol 9:2492. 10.3389/fimmu.2018.02492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joseph H, Speare R, Melrose W. 2012. Laboratory diagnosis of lymphatic filariasis in Australia: available tools and interpretation. Aust J Med Sci 33:2–9. [Google Scholar]
- 54.Gordon CA, Jones M, McManus DP. 2018. The history of Bancroftian lymphatic gilariasis in Australasia and Oceania: is there a threat of re-occurrence in mainland Australia? Tropical Med 3:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deelder AM, Kornelis D, Van Marck EA, Eveleigh PC, Van Egmond JG. 1980. Schistosoma mansoni: characterization of two circulating polysaccharide antigens and the immunological response to these antigens in mouse, hamster, and human infections. Exp Parasitol 50:16–32. 10.1016/0014-4894(80)90004-1. [DOI] [PubMed] [Google Scholar]
- 56.van Dam GJ, de Dood CJ, Lewis M, Deelder AM, van Lieshout L, Tanke HJ, van Rooyen LH, Corstjens PL. 2013. A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen. Exp Parasitol 135:274–282. 10.1016/j.exppara.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weerakoon KG, McManus DP. 2016. Cell-Free DNA as a diagnostic tool for human parasitic infections. Trends Parasitol 32:378–391. 10.1016/j.pt.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Weerakoon KG, Gordon CA, Williams GM, Cai P, Gobert GN, Olveda RM, Ross AG, Olveda DU, McManus DP. 2017. Droplet digital PCR diagnosis of human schistosomiasis: parasite cell-free DNA detection in diverse clinical samples. J Infect Dis 216:1611–1622. 10.1093/infdis/jix521. [DOI] [PubMed] [Google Scholar]
- 59.Cai P, Weerakoon KG, Mu Y, Olveda DU, Piao X, Liu S, Olveda RM, Chen Q, Ross AG, McManus DP. 2017. A parallel comparison of antigen candidates for development of an optimized serological diagnosis of schistosomiasis Japonica in the Philippines. EBioMedicine 24:237–246. 10.1016/j.ebiom.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caballero ZC, Sousa OE, Marques WP, Saez-Alquezar A, Umezawa ES. 2007. Evaluation of serological tests to identify Trypanosoma cruzi infection in humans and determine cross-reactivity with Trypanosoma rangeli and Leishmania spp. Clin Vaccine Immunol 14:1045–1049. 10.1128/CVI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Lieshout L, Polderman AM, Visser LG, Verwey JJ, Deelder AM. 1997. Detection of the circulating antigens CAA and CCA in a group of Dutch travelers with acute schistosomiasis. Trop Med Int Health 2:551–557. 10.1046/j.1365-3156.1997.d01-324.x. [DOI] [PubMed] [Google Scholar]
- 62.Doenhoff MJ, Chiodini PL, Hamilton JV. 2004. Specific and sensitive diagnosis of schistosome infection: can it be done with antibodies? Trends Parasitol 20:35–39. 10.1016/j.pt.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 63.Tchuenté LA. 2011. Control of soil-transmitted helminths in sub-Saharan Africa: diagnosis, drug efficacy concerns, and challenges. Acta Trop 120:S4–S11. 10.1016/j.actatropica.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 64.Stothard JR, Stanton MC, Bustinduy AL, Sousa-Figueiredo JC, Van Dam GJ, Betson M, Waterhouse D, Ward S, Allan F, Hassan AA, Al-Helal MA, Memish ZA, Rollinson D. 2014. Diagnostics for schistosomiasis in Africa and Arabia: a review of present options in control and future needs for elimination. Parasitology 141:1947–1961. 10.1017/S0031182014001152. [DOI] [PubMed] [Google Scholar]
- 65.Afrin T, Murase K, Kounosu A, Hunt VL, Bligh M, Maeda Y, Hino A, Maruyama H, Tsai IJ, Kikuchi T. 2019. Sequential changes in the host gut microbiota during infection with the intestinal parasitic nematode Strongyloides venezuelensis. Front Cell Infect Microbiol 9:217–217. 10.3389/fcimb.2019.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alkajj M, Farhana A. 2020. Enzyme-linked immunoabsorbent assay (ELISA). StatPearls. StatPearls Publishing, Treasure Island, FL. [Google Scholar]
- 67.Sakamoto S, Putalun W, Vimolmangkang S, Phoolcharoen W, Shoyama Y, Tanaka H, Morimoto S. 2018. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J Nat Med 72:32–42. 10.1007/s11418-017-1144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hawkes R, Niday E, Gordon J. 1982. A Dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem 119:142–147. 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- 69.Piña R, Gutiérrez AH, Gilman RH, Rueda D, Sifuentes C, Flores M, Sheen P, Rodriguez S, García HH, Zimic M. 2011. A Dot-ELISA using a partially purified cathepsin-L-like protein fraction from Taenia solium cysticerci, for the diagnosis of human neurocysticercosis. Ann Trop Med Parasitol 105:311–318. 10.1179/136485911X12987676649782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weerakoon KG, Gordon CA, Cai P, Gobert GN, Duke M, Williams GM, McManus DP. 2017. A novel duplex ddPCR assay for the diagnosis of schistosomiasis japonica: proof of concept in an experimental mouse model. Parasitology 144:1005–1015. 10.1017/S003118201700021X. [DOI] [PubMed] [Google Scholar]
- 71.Gordon CA, Acosta LP, Gobert GN, Olveda RM, Ross AG, Williams GM, Gray DJ, Harn D, Li Y, McManus DP. 2015. Real-time PCR demonstrates high prevalence of Schistosoma japonicum in the Philippines: implications for surveillance and control. PLoS Negl Trop Dis 9:e0003483. 10.1371/journal.pntd.0003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parida M, Sannarangaiah S, Dash PK, Rao PV, Morita K. 2008. Loop mediated isothermal amplification (LAMP): a new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev Med Virol 18:407–421. 10.1002/rmv.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meurs L, Brienen E, Mbow M, Ochola EA, Mboup S, Karanja DM, Secor WE, Polman K, van Lieshout L. 2015. Is PCR the next reference standard for the diagnosis of Schistosoma in stool? A comparison with microscopy in Senegal and Kenya. PLoS Negl Trop Dis 9:e0003959. 10.1371/journal.pntd.0003959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Momčilović S, Cantacessi C, Arsić-Arsenijević V, Otranto D, Tasić-Otašević S. 2019. Rapid diagnosis of parasitic diseases: current scenario and future needs. Clin Microbiol Infect 25:290–309. 10.1016/j.cmi.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 75.Murray TS, Cappello M. 2008. The molecular diagnosis of parasitic diseases. Pediatr Infect Dis J 27:163–164. 10.1097/INF.0b013e3181658af0. [DOI] [PubMed] [Google Scholar]
- 76.Derrick TR, Holland M. 2020. Evaluation of DjinniChip, a simple, fast, and low-cost diagnostic assay for the detection of ocular Chlamydia trachomatis. http://blogs.biomedcentral.com/bugbitten/2020/12/01/evaluation-of-djinnichip-a-simple-fast-and-low-cost-diagnostic-assay-for-the-detection-of-ocular-chlamydia-trachomatis/. Accessed 1 December 2020.
- 77.Derrick TR, Sandetskaya N, Pickering H, Kölsch A, Ramadhani A, Mafuru E, Massae P, Malisa A, Mtuy T, Burton MJ, Holland MJ, Kuhlmeier D. 2020. DjinniChip: evaluation of a novel molecular rapid diagnostic device for the detection of Chlamydia trachomatis in trachoma-endemic areas. Parasit Vectors 13:533. 10.1186/s13071-020-04414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hermans S, Caldwell J, Kaplan R, Cobelens F, Wood R. 2017. The impact of the roll-out of rapid molecular diagnostic testing for tuberculosis on empirical treatment in Cape Town, South Africa. Bull World Health Organ 95:554–563. 10.2471/BLT.16.185314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cepheid. 2020. Introducing a single test for SARS-CoV-2, Flu A, Flu B, and RSV. Cepheid, Sunnyvale, CA. https://www.cepheid.com/en/about/SARS-CoV-2-Test-Development-Information. Accessed 5 May 2021. [Google Scholar]
- 80.Brasil PE, De Castro L, Hasslocher-Moreno AM, Sangenis LH, Braga JU. 2010. ELISA versus PCR for diagnosis of chronic Chagas disease: systematic review and meta-analysis. BMC Infect Dis 10:337. 10.1186/1471-2334-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. 1990. Rapid and simple method for purification of nucleic acids. J Clin Microbiol 28:495–503. 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Esser K-H, Marx WH, Lisowsky T. 2006. maxXbond: first regeneration system for DNA binding silica matrices. Nat Methods 3:i–ii. 10.1038/nmeth845. [DOI] [Google Scholar]
- 83.Hoff-Olsen P, Mevag B, Staalstrom E, Hovde B, Egeland T, Olaisen B. 1999. Extraction of DNA from decomposed human tissue: an evaluation of five extraction methods for short tandem repeat typing. Forensic Sci Int 105:171–183. 10.1016/s0379-0738(99)00128-0. [DOI] [PubMed] [Google Scholar]
- 84.Elkins KM. 2013. DNA extraction, p 39–52. In Elkins KM (ed), Forensic DNA biology. Academic Press, San Diego, CA. 10.1016/B978-0-12-394585-3.00004-3. [DOI] [Google Scholar]
- 85.Berensmeier S. 2006. Magnetic particles for the separation and purification of nucleic acids. Appl Microbiol Biotechnol 73:495–504. 10.1007/s00253-006-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nargessi D, Ou CY. 2010. MagaZorb: a simple tool for rapid isolation of viral nucleic acids. J Infect Dis 201:S37–S41. 10.1086/650391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Allen GC, Flores-Vergara MA, Krasynanski S, Kumar S, Thompson WF. 2006. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat Protoc 1:2320–2325. 10.1038/nprot.2006.384. [DOI] [PubMed] [Google Scholar]
- 88.Tan SC, Yiap BC. 2009. DNA, RNA, and protein extraction: the past and the present. J Biomed Biotechnol 2009:574398. 10.1155/2009/574398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.CDC. 20. November 2019. DPDx: laboratory identification of parasite of public health concern. Centers for Disease Control and Prevention, Atlanta, GA. cdc.gov/dpdx/az.html. Accessed 3 June 2020. [Google Scholar]
- 90.CDC. 2016. About parasites. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/parasites/about.html. Accessed 17 November 2019. [Google Scholar]
- 91.Khurana S, Chaudhary P. 2018. Laboratory diagnosis of cryptosporidiosis. Trop Parasitol 8:2–7. 10.4103/tp.TP_34_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hooshyar H, Rostamkhani P, Arbabi M, Delavari M. 2019. Giardia lamblia infection: review of current diagnostic strategies. Gastroenterol Hepatol Bed Bench 12:3–12. [PMC free article] [PubMed] [Google Scholar]
- 93.Werneck-Silva AL, Sipahi AM, Damião AO, Buchpigue CA, Iriya K, Laudanna AA. 2001. Intestinal permeability in strongyloidiasis. Braz J Med Biol Res 34:353–357. 10.1590/S0100-879X2001000300009. [DOI] [PubMed] [Google Scholar]
- 94.Page W, Judd JA, Bradbury RS. 2018. The unique life cycle of Strongyloides stercoralis and implications for public health action. Tropical Med 3:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dougall AM, Pearson MS, Loukas A. 2013. Na-APR-1 (aka Necepsin-2), p 113–118. In Rawlings ND, Salvesen G (ed), Handbook of proteolytic enzymes, 3rd ed. Academic Press, New York, NY. 10.1016/B978-0-12-382219-2.00022-3. [DOI] [Google Scholar]
- 96.Brooker SJ, Bundy DAP. 2014. Soil-transmitted helminths (geohelminths), p 766–794. In Farrar J, Hotez PJ, Junghanss T, Kang G, Lalloo D, White NJ (ed), Manson’s tropical infectious diseases, 23rd ed. W.B. Saunders, London, United Kingdom. 10.1016/B978-0-7020-5101-2.00056-X. [DOI] [Google Scholar]
- 97.Holland CV, Behnke JM, Dold C. 2013.Larval ascariasis: impact, significance, and model organisms, p 107–125. In Holland C (ed), Ascaris: the neglected parasite. Elsevier, Amsterdam, The Netherlands. 10.1016/B978-0-12-396978-1.00005-7. [DOI] [Google Scholar]
- 98.WHO. 2015. Investigating the global impact of neglected tropical diseases: third WHO report on neglected diseases. WHO Press, Geneva, Switzerland. [Google Scholar]
- 99.Gordon CA, Kurscheid J, Jones MK, Gray DJ, McManus DP. 2017. Soil-transmitted helminths in tropical Australia and Asia. Trop Med Infect Dis 2:56. 10.3390/tropicalmed2040056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khurana S, Sethi S. 2017. Laboratory diagnosis of soil transmitted helminthiasis. Trop Parasitol 7:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Papaiakovou M, Pilotte N, Baumer B, Grant J, Asbjornsdottir K, Schaer F, Hu Y, Aroian R, Walson J, Williams SA. 2018. A comparative analysis of preservation techniques for the optimal molecular detection of hookworm DNA in a human fecal specimen. PLoS Negl Trop Dis 12:e0006130. 10.1371/journal.pntd.0006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Picozzi K, Tilley A, Fèvre EM, Coleman PG, Welburn JW, Magona M, Odiit MC, Eisler SC. 2002. The diagnosis of trypanosome infections: applications of novel technology for reducing disease risk. Afr J Biotechnol 1:20–28. [Google Scholar]
- 103.Reck M, Tomasch J, Deng Z, Jarek M, Husemann P, Wagner-Dobler I, COMBACTE Consortium. 2015. Stool metatranscriptomics: a technical guideline for mRNA stabilization and isolation. BMC Genomics 16:494. 10.1186/s12864-015-1694-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liang S, Yang C, Zhong B, Qiu D. 2006. Re-emerging schistosomiasis in hilly and mountainous areas of Sichuan, China. Bull World Health Organ 84:139–144. 10.2471/BLT.05.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kato H, Caceres AG, Mimori T, Ishimaru Y, Sayed ASM, Fujita M, Iwata H, Uezato H, Velez LN, Gomez EAL, Hashiguchi Y. 2010. Use of FTA cards for direct sampling of patients’ lesions in the ecological study of cutaneous leishmaniasis. J Clin Microbiol 48:3661–3665. 10.1128/JCM.00498-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Engels D, Sinzinkayo E, Gryseels B. 1996. Day-to-day egg count fluctuation in Schistosoma mansoni infection and its operational implications. Am J Trop Med Hyg 54:319–324. 10.4269/ajtmh.1996.54.319. [DOI] [PubMed] [Google Scholar]
- 107.CDC. 2016. Blood specimen collection. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/dpdx/diagnosticprocedures/blood/specimencoll.html. Accessed 28 November 2019. [Google Scholar]
- 108.Pietrzak-Johnston SM, Bishop H, Wahlquist S, Moura H, Da Silva ND, Da Silva SP, Nguyen-Dinh P. 2000. Evaluation of commercially available preservatives for laboratory detection of helminths and protozoa in human fecal specimens. J Clin Microbiol 38:1959–1964. 10.1128/JCM.38.5.1959-1964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.CDC. 2016. Stool Specimen Collection. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/dpdx/diagnosticprocedures/stool/specimencoll.html. Accessed 28 November 2019. [Google Scholar]
- 110.VWR. 2021. Para-Pak stool transport systems. Meridian Bioscience, Cincinnati, OH. https://us.vwr.com/store/product/4636590/para-pak-stool-transport-systems-meridian-bioscience. Accessed 5 May 2021. [Google Scholar]
- 111.Ayana M, Cools P, Mekonnen Z, Biruksew A, Dana D, Rashwan N, Prichard R, Vlaminck J, Verweij JJ, Levecke B. 2019. Comparison of four DNA extraction and three preservation protocols for the molecular detection and quantification of soil-transmitted helminths in stool. PLoS Negl Trop Dis 13:e0007778. 10.1371/journal.pntd.0007778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Francis J, Barrett SP, Chiodini PL. 2003. Best Practice No 174: best practice guidelines for the examination of specimens for the diagnosis of parasitic infections in routine diagnostic laboratories. J Clin Pathol 56:888–891. 10.1136/jcp.56.12.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.CDC. 2016. Other specimens: urine specimens. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/dpdx/diagnosticprocedures/other/urine.html. Accessed 6 April 2020. [Google Scholar]
- 114.CDC. 2016. Other specimens: sputum specimens. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/dpdx/diagnosticprocedures/other/sputum.html. Accessed 6 April 2019. [Google Scholar]
- 115.Rajendram D, Ayenza R, Holder FM, Moran B, Long T, Shah HN. 2006. Long-term storage and safe retrieval of DNA from microorganisms for molecular analysis using FTA matrix cards. J Microbiol Methods 67:582–592. 10.1016/j.mimet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 116.Gan W, Zhuang B, Zhang P, Han J, Li C-X, Liu P. 2014. A filter paper-based microdevice for low-cost, rapid, and automated DNA extraction and amplification from diverse sample types. Lab Chip 14:3719–3728. 10.1039/C4LC00686K. [DOI] [PubMed] [Google Scholar]
- 117.Harty LC, Garcia-Closas M, Rothman N, Reid YA, Tucker MA, Hartge P. 2000. Collection of buccal cell DNA using treated cards. Cancer Epidemiol Biomarkers Prev 9:501–506. [PubMed] [Google Scholar]
- 118.Kline MC, Duewer DL, Redman JW, Butler JM, Boyer DA. 2002. Polymerase chain reaction amplification of DNA from aged blood stains: quantitative evaluation of the “suitability for purpose” of four filter papers as archival media. Anal Chem 74:1863–1869. 10.1021/ac015715e. [DOI] [PubMed] [Google Scholar]
- 119.Smith LM, Burgoyne LA. 2004. Collecting, archiving and processing DNA from wildlife samples using FTA databasing paper. BMC Ecol 4:4. 10.1186/1472-6785-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mas S, Crescenti A, Gassó P, Vidal‐Taboada JM, Lafuente A. 2007. DNA cards: determinants of DNA yield and quality in collecting genetic samples for pharmacogenetic studies. Basic Clin Pharmacol Toxicol 101:132–137. 10.1111/j.1742-7843.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- 121.Rogers C, Burgoyne L. 1997. Bacterial typing: storing and processing of stabilized reference bacteria for polymerase chain reaction without preparing DNA: an example of an automatable procedure. Anal Biochem 247:223–227. 10.1006/abio.1997.2031. [DOI] [PubMed] [Google Scholar]
- 122.Mulot C, Stucker I, Clavel J, Beaune P, Loriot MA. 2005. Collection of human genomic DNA from buccal cells for genetics studies: comparison between cytobrush, mouthwash, and treated card. J Biomed Biotechnol 2005:291–296. 10.1155/JBB.2005.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Johanson HC, Hyland V, Wicking C, Sturm RA. 2009. DNA elution from buccal cells stored on Whatman FTA Classic Cards using a modified methanol fixation method. Biotechniques 46:309–311. 10.2144/000113077. [DOI] [PubMed] [Google Scholar]
- 124.Lalani T, Tisdale MD, Maguire JD, Wongsrichanalai C, Riddle MS, Tribble DR. 2015. Detection of enteropathogens associated with travelers’ diarrhea using a multiplex Luminex-based assay performed on stool samples smeared on Whatman FTA Elute cards. Diagn Microbiol Infect Dis 83:18–20. 10.1016/j.diagmicrobio.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nogueira TLS, Oliveira TP, Braz EBV, Santos OCL, Silva DA, Amaral CRL, Carvalho EF. 2015. Mitochondrial DNA direct PCR sequencing of blood FTA paper. Forensic Sci Int Genetics Suppl Ser 5:e611–e613. 10.1016/j.fsigss.2015.09.241. [DOI] [Google Scholar]
- 126.Kirgiz IA, Calloway C. 2017. Increased recovery of touch DNA evidence using FTA paper compared to conventional collection methods. J Forensic Leg Med 47:9–15. 10.1016/j.jflm.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 127.Da Cunha Santos G, Liu N, Tsao MS, Kamel‐Reid S, Chin K, Geddie WR. 2010. Detection of EGFR and KRAS mutations in fine‐needle aspirates stored on Whatman FTA cards. Cancer Cytopathol 118:450–456. 10.1002/cncy.20102. [DOI] [PubMed] [Google Scholar]
- 128.Barbé B, Verdonck K, El-Safi S, Khanal B, Teav S, Lilo Kalo JR, Ravinetto R, Chappuis F, Boelaert M, Jacobs J. 2016. Rapid diagnostic tests for neglected infectious diseases: case study highlights need for customer awareness and postmarket surveillance. PLoS Negl Trop Dis 10:e0004655. 10.1371/journal.pntd.0004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Büscher P, Mertens P, Leclipteux T, Gilleman Q, Jacquet D, Mumba-Ngoyi D, Pyana PP, Boelaert M, Lejon V. 2014. Sensitivity and specificity of HAT Sero-K-SeT, a rapid diagnostic test for serodiagnosis of sleeping sickness caused by Trypanosoma brucei gambiense: a case-control study. Lancet Glob Health 2:e359–e363. 10.1016/S2214-109X(14)70203-7. [DOI] [PubMed] [Google Scholar]
- 130.Lumbala C, Bessell PR, Lutumba P, Baloji S, Biéler S, Ndung’u JM. 2017. Performance of the SD BIOLINE HAT rapid test in various diagnostic algorithms for gambiense human African trypanosomiasis in the Democratic Republic of the Congo. PLoS One 12:e0180555. 10.1371/journal.pone.0180555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barfield CA, Barney RS, Crudder CH, Wilmoth JL, Stevens DS, Mora-Garcia S, Yanovsky MJ, Weigl BH, Yanovsky J. 2011. A highly sensitive rapid diagnostic test for Chagas disease that utilizes a recombinant Trypanosoma cruzi antigen. IEEE Trans Biomed Eng 58:814–817. 10.1109/TBME.2010.2087334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lozano D, Rojas L, Méndez S, Casellas A, Sanz S, Ortiz L, Pinazo MJ, Abril M, Gascón J, Torrico F, Alonso-Padilla J. 2019. Use of rapid diagnostic tests (RDTs) for conclusive diagnosis of chronic Chagas disease: field implementation in the Bolivian Chaco region. PLoS Negl Trop Dis 13:e0007877. 10.1371/journal.pntd.0007877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.WHO. 2011. Visceral leishmaniasis rapid diagnostic test performance. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 134.WHO. 2016. Improved availability of new test to enhance global lymphatic filariasis elimination. World Health Organization, Geneva, Switzerland. https://www.who.int/news/item/05-02-2016-improved-availability-of-new-test-to-enhance-global-lymphatic-filariasis-elimination. Accessed 26 July 2021. [Google Scholar]
- 135.Hertz MI, Nana-Djeunga H, Kamgno J, Jelil Njouendou A, Chawa Chunda V, Wanji S, Rush A, Fischer PU, Weil GJ, Budge PJ. 2018. Identification and characterization of Loa loa antigens responsible for cross-reactivity with rapid diagnostic tests for lymphatic filariasis. PLoS Negl Trop Dis 12:e0006963. 10.1371/journal.pntd.0006963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yunus MH, Arifin N, Balachandra D, Anuar NS, Noordin R. 2019. Lateral flow dipstick test for serodiagnosis of strongyloidiasis. Am J Trop Med Hyg 101:432–435. 10.4269/ajtmh.19-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sadaow L, Sanpool O, Rodpai R, Boonroumkaew P, Maleewong W, Intapan PM. 2020. Development of immunochromatographic device as a point-of-care tool for serodiagnosis of human strongyloidiasis cases. Eur J Clin Microbiol Infect Dis 39:465–470. 10.1007/s10096-019-03745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.NTD Support Center. 2016. Bench aid for the Onchocerciasis OV16 rapid diagnostic test. Neglected Tropical Diseases Support Center, Decatur, GA. https://www.ntdsupport.org/resources/bench-aid-onchocerciasis-ov16-rapid-diagnostic-test. Accessed 12 February 2020. [Google Scholar]
- 139.Tamarozzi F, Covini I, Mariconti M, Narra R, Tinelli C, De Silvestri A, Manzoni F, Casulli A, Ito A, Neumayr A, Brunetti E. 2016. Comparison of the diagnostic accuracy of three rapid tests for the serodiagnosis of hepatic cystic echinococcosis in humans. PLoS Negl Trop Dis 10:e0004444. 10.1371/journal.pntd.0004444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gao CH, Wang JY, Shi F, Steverding D, Wang X, Yang YT, Zhou XN. 2018. Field evaluation of an immunochromatographic test for diagnosis of cystic and alveolar echinococcosis. Parasit Vectors 11:311. 10.1186/s13071-018-2896-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mubanga C, Mwape KE, Phiri IK, Trevisan C, Zulu G, Chabala C, van Damme I, Schmidt V, Dorny P, Gabriël S. 2019. Progress on the development of rapid diagnostic tests for foodborne neglected zoonotic helminthiases: a systematic review. Acta Trop 194:135–147. 10.1016/j.actatropica.2019.03.030. [DOI] [PubMed] [Google Scholar]
- 142.Coulibaly JT, N’Goran EK, Utzinger J, Doenhoff MJ, Dawson EM. 2013. A new rapid diagnostic test for detection of anti-Schistosoma mansoni and anti-Schistosoma haematobium antibodies. Parasit Vectors 6:29. 10.1186/1756-3305-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Elbasheir MM, Karti IA, Elamin EM. 2020. Evaluation of a rapid diagnostic test for Schistosoma mansoni infection based on the detection of circulating cathodic antigen in urine in Central Sudan. PLoS Negl Trop Dis 14:e0008313. 10.1371/journal.pntd.0008313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Anonymous. 2018. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1789–1858. 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Karimkhani C, Colombara DV, Drucker AM, Norton SA, Hay R, Engelman D, Steer A, Whitfeld M, Naghavi M, Dellavalle RP. 2017. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis 17:1247–1254. 10.1016/S1473-3099(17)30483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Quinnell RJ, Carson C, Reithinger R, Garcez LM, Courtenay O. 2013. Evaluation of rK39 rapid diagnostic tests for canine visceral leishmaniasis: longitudinal study and meta-analysis. PLoS Negl Trop Dis 7:e1992. 10.1371/journal.pntd.0001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Herrera G, Castillo A, Ayala MS, Flórez C, Cantillo-Barraza O, Ramirez JD. 2019. Evaluation of four rapid diagnostic tests for canine and human visceral Leishmaniasis in Colombia. BMC Infect Dis 19:747. 10.1186/s12879-019-4353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Michaels MG, Sanchez P, Lin PL. 2018. Congenital toxoplasmosis, syphilis, malaria, and tuberculosis, p 527–552. In Gleason CA, Juul SE (ed), Avery’s diseases of the newborn, 10th ed. Elsevier, Philadelphia, PA. 10.1016/B978-0-323-40139-5.00038-3. [DOI] [Google Scholar]
- 149.Osei-Bimpong A, Burthem J. 2017. Supplementary techniques including blood parasite diagnosis, p 93–111. In Bain BJ, Bates I, Laffan MA (ed), Dacie and Lewis practical hematology , 12th ed. Elsevier, New York, NY. 10.1016/B978-0-7020-6696-2.00006-0. [DOI] [Google Scholar]
- 150.Pappas MG, Hajkowski R, Hockmeyer WT. 1983. Dot enzyme-linked immunosorbent assay (Dot-ELISA): a micro technique for the rapid diagnosis of visceral leishmaniasis. J Immunol Methods 64:205–214. 10.1016/0022-1759(83)90399-X. [DOI] [PubMed] [Google Scholar]
- 151.Pappas MG. 1988. Recent applications of the Dot-ELISA in immunoparasitology. Vet Parasitol 29:105–129. 10.1016/0304-4017(88)90120-3. [DOI] [PubMed] [Google Scholar]
- 152.de Puig H, Bosch I, Gehrke L, Hamad-Schifferli K. 2017. Challenges of the nano–bio interface in lateral flow and dipstick immunoassays. Trends Biotechnol 35:1169–1180. 10.1016/j.tibtech.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pappas MG. 1986. Rapid serodiagnosis of parasitic infections by Dot-ELISA using dipsticks. Trans R Soc Trop Med Hyg 80:1006. 10.1016/0035-9203(86)90302-0. [DOI] [PubMed] [Google Scholar]
- 154.Al-Sherbiny MM, Farrag AA, Fayad MH, Makled MK, Tawfeek GM, Ali NM. 2004. Application and assessment of a dipstick assay in the diagnosis of hydatidosis and trichinosis. Parasitol Res 93:87–95. 10.1007/s00436-004-1076-x. [DOI] [PubMed] [Google Scholar]
- 155.Chun P. 2009. Colloidal gold and other labels for lateral flow immunoassays, p 1–19. In Wong R, Tse H (ed), Lateral flow immunoassay. Humana Press, Totowa, NJ. 10.1007/978-1-59745-240-3_5. [DOI] [Google Scholar]
- 156.Jamail M, Andrew K, Junaidi D, Krishnan AK, Faizal M, Rahmah N. 2005. Field validation of sensitivity and specificity of rapid test for detection of Brugia malayi infection. Trop Med Int Health 10:99–104. 10.1111/j.1365-3156.2004.01334.x. [DOI] [PubMed] [Google Scholar]
- 157.Lammie PJ, Weil G, Noordin R, Kaliraj P, Steel C, Goodman D, Lakshmikanthan VB, Ottesen E. 2004. Recombinant antigen-based antibody assays for the diagnosis and surveillance of lymphatic filariasis: a multicenter trial. Filaria J 3:9. 10.1186/1475-2883-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rahmah N, Taniawati S, Shenoy RK, Lim BH, Kumaraswami V, Anuar AK, Hakim SL, Hayati MI, Chan BT, Suharni M, Ramachandran CP. 2001. Specificity and sensitivity of a rapid dipstick test (Brugia Rapid) in the detection of Brugia malayi infection. Trans R Soc Trop Med Hyg 95:601–604. 10.1016/S0035-9203(01)90091-4. [DOI] [PubMed] [Google Scholar]
- 159.Mason M, Botella J. 2019. A simple, robust, and equipment-free DNA amplification readout in less than 30 seconds. RSC Adv 9:24440–24450. 10.1039/C9RA04725E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mason MG, Botella JR. 2020. Rapid (30-second), equipment-free purification of nucleic acids using easy-to-make dipsticks. Nat Protoc 15:3663–3677. 10.1038/s41596-020-0392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zou Y, Mason MG, Wang Y, Wee E, Turni C, Blackall PJ, Trau M, Botella JR. 2017. Nucleic acid purification from plants, animals and microbes in under 30 seconds. PLoS Biol 15:e2003916. 10.1371/journal.pbio.2003916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu C, Liao SC, Song J, Mauk MG, Li X, Wu G, Ge D, Greenberg RM, Yang S, Bau HH. 2016. A high-efficiency superhydrophobic plasma separator. Lab Chip 16:553–560. 10.1039/C5LC01235J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Aydin S. 2015. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 72:4–15. 10.1016/j.peptides.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 164.Zheng HJ, Tao ZH, Cheng WF, Piessens WF. 1990. Comparison of Dot-ELISA with sandwich-ELISA for the detection of circulating antigens in patients with Bancroftian filariasis. Am J Trop Med Hyg 42:546–549. [PubMed] [Google Scholar]
- 165.Koh B, Kim KR. 2019. Long-term stability monitoring of printed proteins on paper-based membranes. ACS Omega 4:15134–15138. 10.1021/acsomega.9b02021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Intapan PM, Maleewong W, Nateeworanart S, Wongkham C, Pipitgool V, Sukolapong V, Sangmaneedet S. 2003. Immunodiagnosis of human fascioliasis using an antigen of Fasciola gigantica adult worm with the molecular mass of 27 kDa by a Dot-ELISA. Southeast Asian J Trop Med Public Health 34:713–717. [PubMed] [Google Scholar]
- 167.Pontes LA, Dias-Neto E, Rabello A. 2002. Detection by polymerase chain reaction of Schistosoma mansoni DNA in human serum and feces. Am J Trop Med Hyg 66:157–162. 10.4269/ajtmh.2002.66.157. [DOI] [PubMed] [Google Scholar]
- 168.Rabello AL, Garcia MM, Dias Neto E, Rocha RS, Katz N. 1993. Dot-dye-immunoassay and Dot-ELISA for the serological differentiation of acute and chronic schistosomiasis mansoni using keyhole limpet haemocyanin as antigen. Trans R Soc Trop Med Hyg 87:279–281. 10.1016/0035-9203(93)90127-C. [DOI] [PubMed] [Google Scholar]
- 169.Courtioux B, Bisser S, M’Belesso P, Ngoungou E, Girard M, Nangouma A, Josenando T, Jauberteau-Marchan MO, Bouteille B. 2005. Dot enzyme-linked immunosorbent assay for more reliable staging of patients with human African trypanosomiasis. J Clin Microbiol 43:4789–4795. 10.1128/JCM.43.9.4789-4795.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kumar N, Ghosh S, Gupta SC. 2008. Early detection of Fasciola gigantica infection in buffaloes by enzyme-linked immunosorbent assay and dot enzyme-linked immunosorbent assay. Parasitol Res 103:141–150. 10.1007/s00436-008-0941-4. [DOI] [PubMed] [Google Scholar]
- 171.Jafar Pour Azami S, Keshavarz H, Rezaian M, Mohebali M, Shojaee S. 2011. Rapid detection of Toxoplasma gondii antigen in experimentally infected mice by Dot-ELISA. Iran J Parasitol 6:28–33. [PMC free article] [PubMed] [Google Scholar]
- 172.Taher EE, Meabed EMH, El Akkad DMH, Kamel NO, Sabry MA. 2017. Modified Dot-ELISA for diagnosis of human trichinellosis. Exp Parasitol 177:40–46. 10.1016/j.exppara.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 173.Lakshmanan B, Devada K, Joseph S, Binu MB, Kuttan K. 2016. Efficacy of Dot-ELISA using different antigens in detecting anti-schistosome antibodies among bovines in field conditions. J Parasit Dis 40:189–193. 10.1007/s12639-014-0476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Boctor FN, Stek MJ, Jr, Peter JB, Kamal R. 1987. Simplification and standardization of Dot-ELISA for human schistosomiasis mansoni. J Parasitol 73:589–592. 10.2307/3282141. [DOI] [PubMed] [Google Scholar]
- 175.Bojanich MV, Marino GL, Lopez MA, Alonso JM. 2012. An evaluation of the Dot-ELISA procedure as a diagnostic test in an area with a high prevalence of human Toxocara canis infection. Mem Inst Oswaldo Cruz 107:194–197. 10.1590/S0074-02762012000200007. [DOI] [PubMed] [Google Scholar]
- 176.Zimmerman GL, Nelson MJ, Clark CR. 1985. Diagnosis of ovine fascioliasis by a dot enzyme-linked immunosorbent assay: a rapid microdiagnostic technique. Am J Vet Res 46:1513–1515. [PubMed] [Google Scholar]
- 177.Pappas MG, Schantz PM, Cannon LT, Sr, Wahlquist SP. 1986. Dot-ELISA for the rapid serodiagnosis of human hydatid disease. Diagn Immunol 4:271–276. [PubMed] [Google Scholar]
- 178.Murray CK, Gasser RA, Jr, Magill AJ, Miller RS. 2008. Update on rapid diagnostic testing for malaria. Clin Microbiol Rev 21:97–110. 10.1128/CMR.00035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.van Hellemond JJ, Rutten M, Koelewijn R, Zeeman AM, Verweij JJ, Wismans PJ, Kocken CH, van Genderen PJ. 2009. Human Plasmodium knowlesi infection detected by rapid diagnostic tests for malaria. Emerg Infect Dis 15:1478–1480. 10.3201/eid1509.090358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yerlikaya S, Campillo A, Gonzalez IJ. 2018. A systematic review: performance of rapid diagnostic tests for the detection of Plasmodium knowlesi, Plasmodium malariae, and Plasmodium ovale monoinfections in human blood. J Infect Dis 218:265–276. 10.1093/infdis/jiy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK. 2009. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J Clin Microbiol 47:975–980. 10.1128/JCM.01858-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Martinez AW, Phillips ST, Whitesides GM, Carrilho E. 2010. Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal Chem 82:3–10. 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- 183.Sebastian C, William H. 2016. Immunochromatography: formats and applications. Indo American J Pharmaceutical Res 6:6400–6417. [Google Scholar]
- 184.Peruski AH, Peruski LF, Jr.. 2003. Immunological methods for detection and identification of infectious disease and biological warfare agents. Clin Diagn Lab Immunol 10:506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Savage HM, Duncan JF, Roberts DR, Sholdt LL. 1991. A dipstick ELISA for rapid detection of human blood meals in mosquitoes. J Am Mosq Control Assoc 7:16–23. [PubMed] [Google Scholar]
- 186.Allan JC, Mencos F, Garcia-Noval J, Sarti E, Flisser A, Wang Y, Liu D, Craig PS. 1993. Dipstick dot ELISA for the detection of Taenia coproantigens in humans. Parasitology 107:79–85. 10.1017/S0031182000079439. [DOI] [PubMed] [Google Scholar]
- 187.van Etten L, Folman CC, Eggelte TA, Kremsner PG, Deelder AM. 1994. Rapid diagnosis of schistosomiasis by antigen detection in urine with a reagent strip. J Clin Microbiol 32:2404–2406. 10.1128/jcm.32.10.2404-2406.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ali NM. 2012. Development and evaluation of a dipstick assay in diagnosis of human fasciolosis. Parasitol Res 110:1649–1654. 10.1007/s00436-011-2678-8. [DOI] [PubMed] [Google Scholar]
- 189.Chappuis F, Rijal S, Soto A, Menten J, Boelaert M. 2006. A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ 333:723. 10.1136/bmj.38917.503056.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Demerdash Z, Mohamed S, Hendawy M, Rabia I, Attia M, Shaker Z, Diab TM. 2013. Monoclonal antibody-based dipstick assay: a reliable field applicable technique for diagnosis of Schistosoma mansoni infection using human serum and urine samples. Korean J Parasitol 51:93–98. 10.3347/kjp.2013.51.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.van Doorn HR, Hofwegen H, Koelewijn R, Gilis H, Peek R, Wetsteyn JC, van Genderen PJ, Vervoort T, van Gool T. 2005. Use of rapid dipstick and latex agglutination tests and enzyme-linked immunosorbent assay for serodiagnosis of amebic liver abscess, amebic colitis, and Entamoeba histolytica cyst passage. J Clin Microbiol 43:4801–4806. 10.1128/JCM.43.9.4801-4806.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rahmah N, Shenoy RK, Nutman TB, Weiss N, Gilmour K, Maizels RM, Yazdanbakhsh M, Sartono E. 2003. Multicentre laboratory evaluation of Brugia Rapid dipstick test for detection of Brugian filariasis. Trop Med Int Health 8:895–900. 10.1046/j.1365-3156.2003.01102.x. [DOI] [PubMed] [Google Scholar]
- 193.El-Moamly A, El-Sweify M, Hafeez M. 2012. Performance of rK39 immunochromatography and freeze-dried direct agglutination tests in the diagnosis of imported visceral leishmaniasis. Parasitol Res 110:349–354. 10.1007/s00436-011-2499-9. [DOI] [PubMed] [Google Scholar]
- 194.El-Moamly AA, El-Sweify MA. 2012. ImmunoCard STAT! cartridge antigen detection assay compared to microplate enzyme immunoassay and modified Kinyoun’s acid-fast staining technique for detection of Cryptosporidium in fecal specimens. Parasitol Res 110:1037–1041. 10.1007/s00436-011-2585-z. [DOI] [PubMed] [Google Scholar]
- 195.El-Moamly AM, Rashad SM. 2008. Trichomonas vaginalis antigens in vaginal and urine specimens by immunochromatography, compared to culture and microscopy. J Egypt Soc Parasitol 38:573–584. [PubMed] [Google Scholar]
- 196.Gao CH, Yang YT, Shi F, Wang JY, Steverding D, Wang X. 2015. Development of an immunochromatographic test for diagnosis of visceral leishmaniasis based on detection of a circulating antigen. PLoS Negl Trop Dis 9:e0003902. 10.1371/journal.pntd.0003902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Garcia LS, Shimizu RY, Novak S, Carroll M, Chan F. 2003. Commercial assay for detection of Giardia lamblia and Cryptosporidium parvum antigens in human fecal specimens by rapid solid-phase qualitative immunochromatography. J Clin Microbiol 41:209–212. 10.1128/JCM.41.1.209-212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Goni P, Martin B, Villacampa M, Garcia A, Seral C, Castillo FJ, Clavel A. 2012. Evaluation of an immunochromatographic dip strip test for simultaneous detection of Cryptosporidium spp, Giardia duodenalis, and Entamoeba histolytica antigens in human faecal samples. Eur J Clin Microbiol Infect Dis 31:2077–2082. 10.1007/s10096-012-1544-7. [DOI] [PubMed] [Google Scholar]
- 199.Huang X, Xuan X, Verdida RA, Zhang S, Yokoyama N, Xu L, Igarashi I. 2006. Immunochromatographic test for simultaneous serodiagnosis of Babesia caballi and B. equi infections in horses. Clin Vaccine Immunol 13:553–555. 10.1128/CVI.13.5.553-555.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huang X, Xuan X, Xu L, Zhang S, Yokoyama N, Suzuki N, Igarashi I. 2004. Development of an immunochromatographic test with recombinant EMA-2 for the rapid detection of antibodies against Babesia equi in horses. J Clin Microbiol 42:359–361. 10.1128/JCM.42.1.359-361.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Iqbal J, Sher A. 2006. Determination of the prevalence of lymphatic filariasis among migrant workers in Kuwait by detecting circulating filarial antigen. J Med Microbiol 55:401–405. 10.1099/jmm.0.46376-0. [DOI] [PubMed] [Google Scholar]
- 202.Kim C, Alhassan A, Verdida RA, Yokoyama N, Xuan X, Fujisaki K, Kawazu S, Igarashi I. 2007. Development of two immunochromatographic tests for the serodiagnosis of bovine babesiosis. Vet Parasitol 148:137–143. 10.1016/j.vetpar.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 203.Mason DP, Kawamoto F, Lin K, Laoboonchai A, Wongsrichanalai C. 2002. A comparison of two rapid field immunochromatographic tests to expert microscopy in the diagnosis of malaria. Acta Trop 82:51–59. 10.1016/S0001-706X(02)00031-1. [DOI] [PubMed] [Google Scholar]
- 204.Mishra MN, Misra RN. 2007. Immunochromatographic methods in malaria diagnosis. Med J Armed Forces India 63:127–129. 10.1016/S0377-1237(07)80054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Phantana S, Sensathein S, Songtrus J, Klagrathoke S, Phongnin K. 1999. ICT filariasis test: a new screening test for Bancroftian filariasis. Southeast Asian J Trop Med Public Health 30:47–51. [PubMed] [Google Scholar]
- 206.Playford EG, Walker J. 2002. Evaluation of the ICT malaria P.f/P.v and the OptiMal rapid diagnostic tests for malaria in febrile returned travelers. J Clin Microbiol 40:4166–4171. 10.1128/JCM.40.11.4166-4171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ponce C, Ponce E, Vinelli E, Montoya A, de Aguilar V, Gonzalez A, Zingales B, Rangel-Aldao R, Levin MJ, Esfandiari J, Umezawa ES, Luquetti AO, da Silveira JF. 2005. Validation of a rapid and reliable test for diagnosis of Chagas’ disease by detection of Trypanosoma cruzi-specific antibodies in blood of donors and patients in Central America. J Clin Microbiol 43:5065–5068. 10.1128/JCM.43.10.5065-5068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ritmeijer K, Melaku Y, Mueller M, Kipngetich S, O’Keeffe C, Davidson RN. 2006. Evaluation of a new recombinant K39 rapid diagnostic test for Sudanese visceral leishmaniasis. Am J Trop Med Hyg 74:76–80. 10.4269/ajtmh.2006.74.76. [DOI] [PubMed] [Google Scholar]
- 209.Rocha A, Braga C, Belem M, Carrera A, Aguiar-Santos A, Oliveira P, Texeira MJ, Furtado A. 2009. Comparison of tests for the detection of circulating filarial antigen (Og4C3-ELISA and AD12-ICT) and ultrasound in diagnosis of lymphatic filariasis in individuals with microfilariae. Mem Inst Oswaldo Cruz 104:621–625. 10.1590/S0074-02762009000400015. [DOI] [PubMed] [Google Scholar]
- 210.Simonsen PE, Dunyo SK. 1999. Comparative evaluation of three new tools for diagnosis of Bancroftian filariasis based on detection of specific circulating antigens. Trans R Soc Trop Med Hyg 93:278–282. 10.1016/S0035-9203(99)90022-6. [DOI] [PubMed] [Google Scholar]
- 211.Verdida RA, Xuan X, Fukumoto S, Huang X, Zhou J, Igarashi I, Claveria FG, Nagasawa H. 2005. Development of a practical immunochromatographic test with recombinant P50 for the diagnosis of Babesia gibsoni infection in dogs. Parasitology 131:769–774. 10.1017/S0031182005008401. [DOI] [PubMed] [Google Scholar]
- 212.Weil GJ, Lammie PJ, Weiss N. 1997. The ICT filariasis test: a rapid-format antigen test for diagnosis of Bancroftian filariasis. Parasitol Today 13:401–404. 10.1016/S0169-4758(97)01130-7. [DOI] [PubMed] [Google Scholar]
- 213.Wongsrichanalai C. 2001. Rapid diagnostic techniques for malaria control. Trends Parasitol 17:307–309. 10.1016/S1471-4922(01)01925-0. [DOI] [PubMed] [Google Scholar]
- 214.Cooke AH, Chiodini PL, Doherty T, Moody AH, Ries J, Pinder M. 1999. Comparison of a parasite lactate dehydrogenase-based immunochromatographic antigen detection assay (OptiMAL) with microscopy for the detection of malaria parasites in human blood samples. Am J Trop Med Hyg 60:173–176. 10.4269/ajtmh.1999.60.173. [DOI] [PubMed] [Google Scholar]
- 215.Orish VN, De-Gaulle VF, Sanyaolu AO. 2018. Interpreting rapid diagnostic test (RDT) for Plasmodium falciparum. BMC Res Notes 11:850. 10.1186/s13104-018-3967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Weil GJ, Curtis KC, Fakoli L, Fischer K, Gankpala L, Lammie PJ, Majewski AC, Pelletreau S, Won KY, Bolay FK, Fischer PU. 2013. Laboratory and field evaluation of a new rapid test for detecting Wuchereria bancrofti antigen in human blood. Am J Trop Med Hyg 89:11–15. 10.4269/ajtmh.13-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schlabe S, Reiter-Owona I, Nordmann T, Dolscheid-Pommerich R, Tannich E, Hoerauf A, Rockstroh J. 2021. Rapid diagnostic test negative Plasmodium falciparum malaria in a traveller returning from Ethiopia. Malar J 20:145. 10.1186/s12936-021-03678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tamer GS, Dündar D, Uzuner H, Baydemir C. 2015. Evaluation of immunochromatographic test for the detection of antibodies against Echinococcosis granulosus. Med Sci Monit 21:1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yu L-L, Ding J-Z, Wen L-Y, Lou D, Yan X-L, Lin L-J, Lu S-H, Lin D-D, Zhou X-N. 2011. Development of a rapid dipstick with latex immunochromatographic assay (DLIA) for diagnosis of schistosomiasis japonica. Parasit Vectors 4:157. 10.1186/1756-3305-4-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Jiang SF, Zhang XP, Li BL, He YY, Liu J, Tang YH, Cai L. 2014. Evaluation of partially purified soluble egg antigens in colloidal gold immunochromatography assay card for rapid detection of anti-Schistosoma japonicum antibodies. Southeast Asian J Trop Med Public Health 45:568–575. [PubMed] [Google Scholar]
- 221.Steel C, Golden A, Kubofcik J, LaRue N, de Los Santos T, Domingo GJ, Nutman TB. 2013. Rapid Wuchereria bancrofti-specific antigen Wb123-based IgG4 immunoassays as tools for surveillance following mass drug administration programs on lymphatic filariasis. Clin Vaccine Immunol 20:1155–1161. 10.1128/CVI.00252-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Golden A, Steel C, Yokobe L, Jackson E, Barney R, Kubofcik J, Peck R, Unnasch TR, Nutman TB, de los Santos T, Domingo GJ. 2013. Extended result reading window in lateral flow tests detecting exposure to Onchocerca volvulus: a new technology to improve epidemiological surveillance tools. PLoS One 8:e69231. 10.1371/journal.pone.0069231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.RMD. 2019. Schisto POC-CCA cassette based test. Rapid Diagnostics, Charlotte, NC. https://www.rapid-diagnostics.com/products.html. Accessed 2 November 2021. [Google Scholar]
- 224.Thomas JE, Bassett MT, Sigola LB, Taylor P. 1990. Relationship between bladder cancer incidence, Schistosoma haematobium infection, and geographical region in Zimbabwe. Trans R Soc Trop Med Hyg 84:551–553. 10.1016/0035-9203(90)90036-E. [DOI] [PubMed] [Google Scholar]
- 225.Peralta JM, Cavalcanti MG. 2018. Is POC-CCA a truly reliable test for schistosomiasis diagnosis in low endemic areas? The trace results controversy. PLoS Negl Trop Dis 12:e0006813. 10.1371/journal.pntd.0006813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Huyse T, Van den Broeck F, Hellemans B, Volckaert FAM, Polman K. 2013. Hybridization between the two major African schistosome species of humans. Int J Parasitol 43:687–689. 10.1016/j.ijpara.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 227.Leger E, Garba A, Hamidou AA, Webster BL, Pennance T, Rollinson D, Webster JP. 2016. Introgressed animal schistosomes Schistosoma curassoni and S. bovis naturally infecting humans. Emerg Infect Dis 22:2212–2214. 10.3201/eid2212.160644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Leger E, Webster JP. 2017. Hybridizations within the genus Schistosoma: implications for evolution, epidemiology and control. Parasitology 144:65–80. 10.1017/S0031182016001190. [DOI] [PubMed] [Google Scholar]
- 229.Stothard JR, Kayuni SA, Al-Harbi MH, Musaya J, Webster BL. 2020. Future schistosome hybridizations: will all Schistosoma haematobium hybrids please stand-up!. PLoS Negl Trop Dis 14:e0008201. 10.1371/journal.pntd.0008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Homsana A, Odermatt P, Southisavath P, Yajima A, Sayasone S. 2020. Cross-reaction of POC-CCA urine test for detection of Schistosoma mekongi in Lao PDR: a cross-sectional study. Infect Dis Poverty 9:114. 10.1186/s40249-020-00733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Siqueira LM, Couto FF, Taboada D, Oliveira ÁA, Carneiro NF, Oliveira E, Coelho PM, Katz N. 2016. Performance of POC-CCA in diagnosis of schistosomiasis mansoni in individuals with low parasite burden. Rev Soc Bras Med Trop 49:341–347. 10.1590/0037-8682-0070-2016. [DOI] [PubMed] [Google Scholar]
- 232.Bezerra FSM, Leal JKF, Sousa MS, Pinheiro MCC, Ramos AN, Jr, Silva-Moraes V, Katz N. 2018. Evaluating a point-of-care circulating cathodic antigen test (POC-CCA) to detect Schistosoma mansoni infections in a low endemic area in northeastern Brazil. Acta Trop 182:264–270. 10.1016/j.actatropica.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 233.Graeff-Teixeira C, Favero V, Pascoal VF, de Souza RP, Rigo FV, Agnese LHD, Bezerra FSM, Coelho PMZ, Enk MJ, Favre TC, Katz N, Oliveira RR, Dos Reis MG, Pieri OS. 2021. Low specificity of point-of-care circulating cathodic antigen (POCCCA) diagnostic test in a non-endemic area for schistosomiasis mansoni in Brazil. Acta Trop 217:105863. 10.1016/j.actatropica.2021.105863. [DOI] [PubMed] [Google Scholar]
- 234.ClinicalTrials.gov. 2019. Validation of POC-CCA rapid urine test for qualitative detection of Schistosoma japonicum (SchisCCA). https://clinicaltrials.gov/ct2/show/NCT03870204. Accessed 2 November 2021.
- 235.Lorey F, Cunningham G, Vichinsky EP, Lubin BH, Witkowska HE, Matsunaga A, Azimi M, Sherwin J, Eastman J, Farina F, Waye JS, Chui DH. 2001. Universal newborn screening for Hb H disease in California. Genet Test 5:93–100. 10.1089/109065701753145538. [DOI] [PubMed] [Google Scholar]
- 236.Chaisomchit S, Wichajarn R, Janejai N, Chareonsiriwatana W. 2005. Stability of genomic DNA in dried blood spots stored on filter paper. Southeast Asian J Trop Med Public Health 36:270–273. [PubMed] [Google Scholar]
- 237.Zhong XB, Leng L, Beitin A, Chen R, McDonald C, Hsiao B, Jenison RD, Kang I, Park SH, Lee A, Gregersen P, Thuma P, Bray-Ward P, Ward DC, Bucala R. 2005. Simultaneous detection of microsatellite repeats and SNPs in the macrophage migration inhibitory factor (MIF) gene by thin-film biosensor chips and application to rural field studies. Nucleic Acids Res 33:e121. 10.1093/nar/gni123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hannelius U, Lindgren CM, Melén E, Malmberg A, von Dobeln U, Kere J. 2005. Phenylketonuria screening registry as a resource for population genetic studies. J Med Genet 42:e60–e60. 10.1136/jmg.2005.032987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mitterer G, Bodamer O, Harwanegg C, Maurer W, Mueller MW, Schmidt WM. 2005. Microarray-based detection of mannose-binding lectin 2 (MBL2) polymorphisms in a routine clinical setting. Genet Test 9:6–13. 10.1089/gte.2005.9.6. [DOI] [PubMed] [Google Scholar]
- 240.Lin Z, Suzow JG, Fontaine JM, Naylor EW. 2004. A high throughput beta-globin genotyping method by multiplexed melting temperature analysis. Mol Genet Metab 81:237–243. 10.1016/j.ymgme.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 241.Fitness J, Dixit N, Webster D, Torresani T, Pergolizzi R, Speiser PW, Day DJ. 1999. Genotyping of CYP21, linked chromosome 6p markers, and a sex-specific gene in neonatal screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab 84:960–966. [DOI] [PubMed] [Google Scholar]
- 242.He L, Deng T, Luo H-S. 2015. Genetic polymorphism in alcohol dehydrogenase 2 (ADH2) gene and alcoholic liver cirrhosis risk. Int J Clin Exp Med 8:7786–7793. [PMC free article] [PubMed] [Google Scholar]
- 243.Sjöholm MIL, Dillner J, Carlson J. 2007. Assessing quality and functionality of DNA from fresh and archival dried blood spots and recommendations for quality control guidelines. Clin Chem 53:1401–1407. 10.1373/clinchem.2007.087510. [DOI] [PubMed] [Google Scholar]
- 244.Hoffman BR, Yu H, Diamandis EP. 1996. Assay of prostate-specific antigen from whole blood spotted on filter paper and application to prostate cancer screening. Clin Chem 42:536–544. 10.1093/clinchem/42.4.536. [DOI] [PubMed] [Google Scholar]
- 245.Formenti F, Buonfrate D, Prandi R, Marquez M, Caicedo C, Rizzi E, Guevara AG, Vicuña Y, Huerlo FR, Perandin F, Bisoffi Z, Anselmi M. 2016. Comparison of Strongyloides stercoralis serology performed on dried blood spots and on conventional serum samples. Front Microbiol 7:1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Masson J, Douglass J, Roineau M, Aye KS, Htwe KM, Warner J, Graves PM. 2017. Relative performance and predictive values of plasma and dried blood spots with filter paper sampling techniques and dilutions of the lymphatic filariasis Og4C3 antigen ELISA for samples from Myanmar. Tropical Med 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mounsey K, Kearns T, Rampton M, Llewellyn S, King M, Holt D, Currie BJ, Andrews R, Nutman T, McCarthy J. 2014. Use of dried blood spots to define antibody response to the Strongyloides stercoralis recombinant antigen NIE. Acta Trop 138:78–82. 10.1016/j.actatropica.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.de Almeida PP, Ndao M, Van Meirvenne N, Geerts S. 1998. Diagnostic evaluation of PCR on dried blood samples from goats experimentally infected with Trypanosoma brucei brucei. Acta Trop 70:269–276. 10.1016/S0001-706X(98)00031-X. [DOI] [PubMed] [Google Scholar]
- 249.Sonja H-M, Scott AR, Cheryl AJ, Paul Z, Giles C, Scott D, Roy AH, Andrew FDH. 2010. Exploiting mosquito sugar feeding to detect mosquito-borne pathogens. Proc Natl Acad Sci U S A 107:11255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lusby PE, Coombes AL, Wilkinson JM. 2005. Bactericidal activity of different honeys against pathogenic bacteria. Arch Med Res 36:464–467. 10.1016/j.arcmed.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 251.Zhong KJ, Salas CJ, Shafer R, Gubanov A, Gasser RA, Jr, Magill AJ, Forney JR, Kain KC. 2001. Comparison of IsoCode STIX and FTA Gene Guard collection matrices as whole-blood storage and processing devices for diagnosis of malaria by PCR. J Clin Microbiol 39:1195–1196. 10.1128/JCM.39.3.1195-1196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Okabayashi T, Hagiya J, Tsuji M, Ishihara C, Satoh H, Morita C. 2002. Detection of Babesia microti-like parasite in filter paper-absorbed blood of wild rodents. J Vet Med Sci 64:145–147. 10.1292/jvms.64.145. [DOI] [PubMed] [Google Scholar]
- 253.Becker S, Franco JR, Simarro PP, Stich A, Abel PM, Steverding D. 2004. Real-time PCR for detection of Trypanosoma brucei in human blood samples. Diagn Microbiol Infect Dis 50:193–199. 10.1016/j.diagmicrobio.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 254.Mangold KA, Manson RU, Koay ES, Stephens L, Regner M, Thomson RB, Jr, Peterson LR, Kaul KL. 2005. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol 43:2435–2440. 10.1128/JCM.43.5.2435-2440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Swan H, Sloan L, Muyombwe A, Chavalitshewinkoon-Petmitr P, Krudsood S, Leowattana W, Wilairatana P, Looareesuwan S, Rosenblatt J. 2005. Evaluation of a real-time polymerase chain reaction assay for the diagnosis of malaria in patients from Thailand. Am J Trop Med Hyg 73:850–854. 10.4269/ajtmh.2005.73.850. [DOI] [PubMed] [Google Scholar]
- 256.Kuboki N, Inoue N, Sakurai T, Di Cello F, Grab DJ, Suzuki H, Sugimoto C, Igarashi I. 2003. Loop-mediated isothermal amplification for detection of African trypanosomes. J Clin Microbiol 41:5517–5524. 10.1128/JCM.41.12.5517-5524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Jefferies R, Ryan UM, Irwin PJ. 2007. PCR–RFLP for the detection and differentiation of the canine piroplasm species and its use with filter paper-based technologies. Vet Parasitol 144:20–27. 10.1016/j.vetpar.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 258.Sant’anna MRV, Jones NG, Hindley JA, Mendes-Sousa AF, Dillon RJ, Cavalcante RR, Alexander B, Bates PA. 2008. Blood meal identification and parasite detection in laboratory-fed and field-captured Lutzomyia longipalpis by PCR using FTA databasing paper. Acta Trop 107:230–237. 10.1016/j.actatropica.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Jones S, Sutherland CJ, Hermsen C, Arens T, Teelen K, Hallett R, Corran P, van der Vegte-Bolmer M, Sauerwein R, Drakeley CJ, Bousema T. 2012. Filter paper collection of Plasmodium falciparum mRNA for detecting low-density gametocytes. Malar J 11:266. 10.1186/1475-2875-11-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Brugman VA, Kristan M, Gibbins MP, Angrisano F, Sala KA, Dessens JT, Blagborough AM, Walker T. 2018. Detection of malaria sporozoites expelled during mosquito sugar feeding. Sci Rep 8:7545. 10.1038/s41598-018-26010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.MacLean L, Chisi JE, Odiit M, Gibson WC, Ferris V, Picozzi K, Sternberg JM. 2004. Severity of human African trypanosomiasis in East Africa is associated with geographic location, parasite genotype, and host inflammatory cytokine response profile. Infect Immun 72:7040–7044. 10.1128/IAI.72.12.7040-7044.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Duscher G, Peschke R, Wille-Piazzai W, Joachim A. 2009. Parasites on paper: the use of FTA Elute® for the detection of Dirofilaria repens microfilariae in canine blood. Vet Parasitol 161:349–351. 10.1016/j.vetpar.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 263.Farnert A, Arez AP, Correia AT, Bjorkman A, Snounou G, do Rosario V. 1999. Sampling and storage of blood and the detection of malaria parasites by polymerase chain reaction. Trans R Soc Trop Med Hyg 93:50–53. 10.1016/S0035-9203(99)90177-3. [DOI] [PubMed] [Google Scholar]
- 264.Cox AP, Tosas O, Tilley A, Picozzi K, Coleman P, Hide G, Welburn SC. 2010. Constraints to estimating the prevalence of trypanosome infections in East African zebu cattle. Parasit Vectors 3:82. 10.1186/1756-3305-3-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Silbermayr K, Eigner B, Duscher GG, Joachim A, Fuehrer HP. 2014. The detection of different Dirofilaria species using direct PCR technique. Parasitol Res 113:513–516. 10.1007/s00436-013-3682-y. [DOI] [PubMed] [Google Scholar]
- 266.Lalani T, Tisdale MD, Liu J, Mitra I, Philip C, Odundo E, Reyes F, Simons MP, Fraser JA, Hutley E, Connor P, Swierczewski BE, Houpt E, Tribble DR, Riddle MS. 2018. Comparison of stool collection and storage on Whatman FTA Elute cards versus frozen stool for enteropathogen detection using the TaqMan Array Card PCR assay. PLoS One 13:e0202178. 10.1371/journal.pone.0202178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Charles E, Mingbo Y, Hongyan L, Ting Z, Bin X, Moussa S, Zheng F, Wei H. 2015. High genetic variability of Schistosoma haematobium in Mali and Nigeria. Korean J Parasitol 53:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Van den Broeck F, Geldof S, Polman K, Volckaert FA, Huyse T. 2011. Optimal sample storage and extraction protocols for reliable multilocus genotyping of the human parasite Schistosoma mansoni. Infect Genet Evol 11:1413–1418. 10.1016/j.meegid.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 269.Xiao N, Remais J, Brindley P, Qiu D-C, Carlton E, Li R-Z, Lei Y, Blair D. 2013. Approaches to genotyping individual miracidia of Schistosoma japonicum. Parasitol Res 112:3991–3999. 10.1007/s00436-013-3587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Webster BL, Rabone M, Pennance T, Emery AM, Allan F, Gouvras A, Knopp S, Garba A, Hamidou AA, Mohammed KA, Ame SM, Rollinson D, Webster JP. 2015. Development of novel multiplex microsatellite polymerase chain reactions to enable high-throughput population genetic studies of Schistosoma haematobium. Parasit Vectors 8:432. 10.1186/s13071-015-1044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gao Y-M, Lu D-B, Ding H, Lamberton PHL. 2015. Detecting genotyping errors at Schistosoma japonicum microsatellites with pedigree information. Parasit Vectors 8:452. 10.1186/s13071-015-1074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Shortt J, Card D, Schield D, Liu Y, Zhong B, Castoe T, Carlton E, Pollock D. 2017. Whole genome amplification and reduced-representation genome sequencing of Schistosoma japonicum miracidia. PLoS Negl Trop Dis 11:e0005292. 10.1371/journal.pntd.0005292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Aula OP, McManus DP, Mason MG, Botella JR, Gordon CA. 2021. Rapid parasite detection utilizing a DNA dipstick. Exp Parasitol 224:108098. 10.1016/j.exppara.2021.108098. [DOI] [PubMed] [Google Scholar]
- 274.Derrick TR, Sandetskaya N, Pickering H, Kölsch A, Ramadhani A, Mafuru E, Massae P, Malisa A, Mtuy T, Burton MJ, Holland MJ, Kuhlmeier D. 2020. DjinniChip: evaluation of a novel molecular rapid diagnostic device for the detection of Chlamydia trachomatis in trachoma-endemic areas. Parasit Vectors 13:533. 10.1186/s13071-020-04414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Liu C, Mauk M, Gross R, Bushman FD, Edelstein PH, Collman RG, Bau HH. 2013. Membrane-based, sedimentation-assisted plasma separator for point-of-care applications. Anal Chem 85:10463–10470. 10.1021/ac402459h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Jangam SR, Yamada DH, McFall SM, Kelso DM. 2009. Rapid, point-of-care extraction of human immunodeficiency virus type 1 proviral DNA from whole blood for detection by real-time PCR. J Clin Microbiol 47:2363–2368. 10.1128/JCM.r00092-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Shearer WT, Quinn TC, LaRussa P, Lew JF, Mofenson L, Almy S, Rich K, Handelsman E, Diaz C, Pagano M, Smeriglio V, Kalish LA. 1997. Viral load and disease progression in infants infected with human immunodeficiency virus type 1: women and infants transmission study group. N Engl J Med 336:1337–1342. 10.1056/NEJM199705083361901. [DOI] [PubMed] [Google Scholar]
- 278.McFall SM, Wagner RL, Jangam SR, Yamada DH, Hardie D, Kelso DM. 2015. A simple and rapid DNA extraction method from whole blood for highly sensitive detection and quantitation of HIV-1 proviral DNA by real-time PCR. J Virol Methods 214:37–42. 10.1016/j.jviromet.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 279.Govindarajan AV, Ramachandran S, Vigil GD, Yager P, Böhringer KF. 2012. A low cost point-of-care viscous sample preparation device for molecular diagnosis in the developing world; an example of microfluidic origami. Lab Chip 12:174–181. 10.1039/C1LC20622B. [DOI] [PubMed] [Google Scholar]
- 280.Keller F, Hunter MS, Robinson DL. 1953. Structural features of oxide coatings on aluminum. J Electrochem Soc 100:411–419. 10.1149/1.2781142. [DOI] [Google Scholar]
- 281.Furneaux RC, Rigby WR, Davidson AP. 1989. The formation of controlled-porosity membranes from anodically oxidized aluminum. Nature 337:147–149. 10.1038/337147a0. [DOI] [Google Scholar]
- 282.Elgort MG, Herrmann MG, Erali M, Durtschi JD, Voelkerding KV, Smith RE. 2004. Extraction and amplification of genomic DNA from human blood on nanoporous aluminum oxide membranes. Clin Chem 50:1817–1819. 10.1373/clinchem.2004.035162. [DOI] [PubMed] [Google Scholar]
- 283.Sander MS, Tan L-S. 2003. Nanoparticle arrays on surfaces fabricated using anodic alumina films as templates. Adv Funct Mater 13:393–397. 10.1002/adfm.200304290. [DOI] [Google Scholar]
- 284.Karlsson M, Palsgard E, Wilshaw PR, Di Silvio L. 2003. Initial in vitro interaction of osteoblasts with nano-porous alumina. Biomaterials 24:3039–3046. 10.1016/S0142-9612(03)00146-7. [DOI] [PubMed] [Google Scholar]
- 285.Margraf RL, Page S, Erali M, Wittwer CT. 2004. Single-tube method for nucleic acid extraction, amplification, purification, and sequencing. Clin Chem 50:1755–1761. 10.1373/clinchem.2004.035808. [DOI] [PubMed] [Google Scholar]
- 286.Dames S, Bromley LK, Herrmann M, Elgort M, Erali M, Smith R, Voelkerding KV. 2006. A single-tube nucleic acid extraction, amplification, and detection method using aluminum oxide. J Mol Diagn 8:16–21. 10.2353/jmoldx.2006.040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kim J, Gale BK. 2008. Quantitative and qualitative analysis of a microfluidic DNA extraction system using a nanoporous AlOx membrane. Lab Chip 8:1516–1523. 10.1039/b804624g. [DOI] [PubMed] [Google Scholar]
- 288.Kim J, Mauk M, Chen D, Qiu X, Kim J, Gale B, Bau HH. 2010. A PCR reactor with an integrated alumina membrane for nucleic acid isolation. Analyst 135:2408–2414. 10.1039/c0an00288g. [DOI] [PubMed] [Google Scholar]
- 289.Liu C, Geva E, Mauk M, Qiu X, Abrams WR, Malamud D, Curtis K, Owen SM, Bau HH. 2011. An isothermal amplification reactor with an integrated isolation membrane for point-of-care detection of infectious diseases. Analyst 136:2069–2076. 10.1039/c1an00007a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Oblath EA, Henley WH, Alarie JP, Ramsey JM. 2013. A microfluidic chip integrating DNA extraction and real-time PCR for the detection of bacteria in saliva. Lab Chip 13:1325–1332. 10.1039/c3lc40961a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.He Y, Salafsky B, Ramaswamy K. 2001. Host-parasite relationships of Schistosoma japonicum in mammalian hosts. Trends Parasitol 17:320–324. 10.1016/S1471-4922(01)01904-3. [DOI] [PubMed] [Google Scholar]
- 292.Eberhard ML, Brandt FH. 1995. The role of tadpoles and frogs as paratenic hosts in the life cycle of Dracunculus insignis (Nematoda: Dracunculoidea). J Parasitol 81:792–793. 10.2307/3283979. [DOI] [PubMed] [Google Scholar]
- 293.Eberhard ML, Ruiz-Tiben E, Hopkins DR, Farrell C, Toe F, Weiss A, Withers PC, Jr, Jenks MH, Thiele EA, Cotton JA, Hance Z, Holroyd N, Cama VA, Tahir MA, Mounda T. 2014. The peculiar epidemiology of dracunculiasis in Chad. Am J Trop Med Hyg 90:61–70. 10.4269/ajtmh.13-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Cleveland CA, Eberhard ML, Thompson AT, Garrett KB, Swanepoel L, Zirimwabagabo H, Moundai T, Ouakou PT, Ruiz-Tiben E, Yabsley MJ. 2019. A search for tiny dragons (Dracunculus medinensis third-stage larvae) in aquatic animals in Chad, Africa. Sci Rep 9:375. 10.1038/s41598-018-37567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Gray DJ, Williams GM, Li Y, Chen H, Forsyth SJ, Li RS, Barnett AG, Guo J, Ross AG, Feng Z, McManus DP. 2009. A cluster-randomised intervention trial against Schistosoma japonicum in the Peoples’ Republic of China: bovine and human transmission. PLoS One 4:e5900. 10.1371/journal.pone.0005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Gray DJ, Williams GM, Li Y, McManus DP. 2008. Transmission dynamics of Schistosoma japonicum in the lakes and marshlands of China. PLoS One 3:e4058. 10.1371/journal.pone.0004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Gray GD, Copland RS, Copemand DB. 2008. Overcoming liver fluke as a constraint to ruminant production in South-East Asia. Australian Centre for International Agricultural Research, Canberra, Australia. [Google Scholar]
- 298.Sato MO, Rafalimanantsoa A, Ramarokoto C, Rahetilahy AM, Ravoniarimbinina P, Kawai S, Minamoto T, Sato M, Kirinoki M, Rasolofo V, De Calan M, Chigusa Y. 2018. Usefulness of environmental DNA for detecting Schistosoma mansoni occurrence sites in Madagascar. Int J Infect Dis 76:130–136. 10.1016/j.ijid.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 299.Fornillos RJC, Sato MO, Tabios IKB, Sato M, Leonardo LR, Chigusa Y, Minamoto T, Kikuchi M, Legaspi ER, Fontanilla IKC. 2019. Detection of Schistosoma japonicum and Oncomelania hupensis quadrasi environmental DNA and its potential utility to schistosomiasis japonica surveillance in the Philippines. PLoS One 14:e0224617. 10.1371/journal.pone.0224617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Knapp J, Millon L, Mouzon L, Umhang G, Raoul F, Ali ZS, Combes B, Comte S, Gbaguidi-Haore H, Grenouillet F, Giraudoux P. 2014. Real time PCR to detect the environmental faecal contamination by Echinococcus multilocularis from red fox stools. Vet Parasitol 201:40–47. 10.1016/j.vetpar.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 301.Sánchez Thevenet P, Jensen O, Mellado I, Torrecillas C, Raso S, Flores ME, Minvielle MC, Basualdo JA. 2003. Presence and persistence of intestinal parasites in canine fecal material collected from the environment in the province of Chubut, Argentine Patagonia. Vet Parasitol 117:263–269. 10.1016/j.vetpar.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 302.Sun LP, Wang W, Zuo YP, Zhang ZQ, Hong QB, Yang GJ, Zhu HR, Liang YS, Yang HT. 2017. An integrated environmental improvement of marshlands: impact on control and elimination of schistosomiasis in marshland regions along the Yangtze River, China. Infect Dis Poverty 6:72. 10.1186/s40249-017-0287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Tong QB, Chen R, Zhang Y, Yang GJ, Kumagai T, Furushima-Shimogawara R, Lou D, Yang K, Wen LY, Lu SH, Ohta N, Zhou XN. 2015. A new surveillance and response tool: risk map of infected Oncomelania hupensis detected by loop-mediated isothermal amplification (LAMP) from pooled samples. Acta Trop 141:170–177. 10.1016/j.actatropica.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 304.Verbyla ME, Oakley SM, Lizima LA, Zhang J, Iriarte M, Tejada-Martinez AE, Mihelcic JR. 2013. Taenia eggs in a stabilization pond system with poor hydraulics: concern for human cysticercosis? Water Sci Technol 68:2698–2703. 10.2166/wst.2013.556. [DOI] [PubMed] [Google Scholar]
- 305.Worrell CM, Wiegand RE, Davis SM, Odero KO, Blackstock A, Cuéllar VM, Njenga SM, Montgomery JM, Roy SL, Fox LM. 2016. A cross-sectional study of water, sanitation, and hygiene-related risk factors for soil-transmitted helminth infection in urban school- and preschool-aged children in Kibera, Nairobi. PLoS One 11:e0150744. 10.1371/journal.pone.0150744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Aula OP, McManus DP, Weerakoon KG, Olveda R, Ross AG, Rogers MJ, Gordon CA. 2020. Molecular identification of Ancylostoma ceylanicum in the Philippines. Parasitology 147:1718–1722. 10.1017/S0031182020001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Gordon CA, Acosta LP, Gray DJ, Olveda R, Jarilla B, Gobert GN, Ross AG, McManus DP. 2012. High prevalence of Schistosoma japonicum infection in Carabao from Samar province, the Philippines: implications for transmission and control. PLoS Negl Trop Dis 6:e1778. 10.1371/journal.pntd.0001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Xu B, Gordon CA, Hu W, McManus DP, Chen H, Gray DJ, Ju C, Zeng X, Gobert GN, Ge J, Lan W, Xie S, Jiang S, Ross AG, Acosta LP, Olveda R, Feng Z. 2012. A novel procedure for precise quantification of Schistosoma japonicum eggs in bovine feces. PLoS Negl Trop Dis 6:e1885. 10.1371/journal.pntd.0001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Adinezadeh A, Kia EB, Mohebali M, Shojaee S, Rokni MB, Zarei Z, Mowlavi G. 2013. Endoparasites of stray dogs in Mashhad, Khorasan Razavi province, northeast Iran, with special reference to zoonotic parasites. Iran J Parasitol 8:459–466. [PMC free article] [PubMed] [Google Scholar]
- 310.Afonso SM, Vaz Y, Neves L, Pondja A, Dias G, Willingham AL, Vilhena M, Duarte PC, Jost CC, Noormahomed EV. 2011. Human and porcine Taenia solium infections in Mozambique: identifying research priorities. Anim Health Res Rev 12:123–129. 10.1017/S1466252311000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Allepuz A, Gabriel S, Dorny P, Napp S, Jansen F, Vilar MJ, Vives L, Picart L, Ortuno A, Gutierrez J, Casal J. 2012. Comparison of bovine cysticercosis prevalence detected by antigen ELISA and visual inspection in the North East of Spain. Res Vet Sci 92:393–395. 10.1016/j.rvsc.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 312.Beiromvand M, Akhlaghi L, Fattahi Massom SH, Meamar AR, Motevalian A, Oormazdi H, Razmjou E. 2013. Prevalence of zoonotic intestinal parasites in domestic and stray dogs in a rural area of Iran. Prev Vet Med 109:162–167. 10.1016/j.prevetmed.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 313.Angeles JM, Goto Y, Kirinoki M, Asada M, Leonardo LR, Rivera PT, Villacorte EA, Inoue N, Chigusa Y, Kawazu S. 2012. Utilization of ELISA using thioredoxin peroxidase-1 and tandem repeat proteins for diagnosis of Schistosoma japonicum infection among water buffaloes. PLoS Negl Trop Dis 6:e1800. 10.1371/journal.pntd.0001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Ayaz S, Ullah R, AbdEl-Salam NM, Shams S, Niaz S. 2014. Fasciola hepatica in some Buffaloes and cattle by PCR and microscopy. ScientificWorldJournal 2014:462084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Chaudhry U, van Paridon B, Shabbir MZ, Shafee M, Ashraf K, Yaqub T, Gilleard J. 2015. Molecular evidence shows that the liver fluke Fasciola gigantica is the predominant Fasciola species in ruminants from Pakistan. J Helminthol 90:206–213. 10.1017/S0022149X15000176. [DOI] [PubMed] [Google Scholar]
- 316.Gordon DK, Zadoks RN, Stevenson H, Sargison ND, Skuce PJ. 2012. On farm evaluation of the coproantigen ELISA and coproantigen reduction test in Scottish sheep naturally infected with Fasciola hepatica. Vet Parasitol 187:436–444. 10.1016/j.vetpar.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 317.Abbasi I, Branzburg A, Campos-Ponce M, Abdel Hafez SK, Raoul F, Craig PS, Hamburger J. 2003. Copro-diagnosis of Echinococcus granulosus infection in dogs by amplification of a newly identified repeated DNA sequence. Am J Trop Med Hyg 69:324–330. 10.4269/ajtmh.2003.69.324. [DOI] [PubMed] [Google Scholar]
- 318.Adediran OA, Kolapo TU, Uwalaka EC. 2014. Echinococcus granulosus Prevalence in Dogs in southwest Nigeria. J Parasitol Res 2014:1–5. 10.1155/2014/124358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Balkaya I, Simsek S. 2011. A serological survey of cystic echinococcosis in equids in East of Turkey. Iran J Parasitol 6:46–50. [PMC free article] [PubMed] [Google Scholar]
- 320.Beiromvand M, Akhlaghi L, Fattahi Massom SH, Meamar AR, Darvish J, Razmjou E. 2013. Molecular identification of Echinococcus multilocularis infection in small mammals from northeastern Iran. PLoS Negl Trop Dis 7:e2313. 10.1371/journal.pntd.0002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Bottcher D, Bangoura B, Schmaschke R, Muller K, Fischer S, Vobis V, Meiler H, Wolf G, Koller A, Kramer S, Overhoff M, Gawlowska S, Schoon HA. 2013. Diagnostics and epidemiology of alveolar echinococcosis in slaughtered pigs from large-scale husbandries in Germany. Parasitol Res 112:629–636. 10.1007/s00436-012-3177-2. [DOI] [PubMed] [Google Scholar]
- 322.CFSPH. 2018. African animal trypanosomiasis. Center for Food Security and Public Health, Iowa State University, Ames, IA. [Google Scholar]
- 323.Matovu E, Kitibwa A, Picado A, Biéler S, Bessell PR, Ndung’u JM. 2017. Serological tests for gambiense human African trypanosomiasis detect antibodies in cattle. Parasit Vectors 10:546. 10.1186/s13071-017-2487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Khan AH, Noordin R. 2020. Serological and molecular rapid diagnostic tests for toxoplasma infection in humans and animals. Eur J Clin Microbiol Infect Dis 39:19–30. 10.1007/s10096-019-03680-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Ivanov A. 2013. Barriers to the introduction of new medical diagnostic tests. Lab Med 44:e132–e136. 10.1309/LMMHGYKY7LIUEEQ6. [DOI] [Google Scholar]
- 326.The Lewin Group. 2005. The value of diagnostics innovation. In Adoption and diffusion into health care. The Lewin Group, Washington, DC. [Google Scholar]
- 327.Gibbs J. 2008. Regulatory pathways for molecular Dx: detailing the various options available and what each requires. Genet Eng Biotechnol News 28. [Google Scholar]
- 328.Liotta LA, Petricoin EF. 2012. Regulatory approval pathways for molecular diagnostic technology, p 409–420. In Espina V, Liotta LA (ed), Molecular profiling: methods and protocols. Humana Press, Totowa, NJ. 10.1007/978-1-60327-216-2_27. [DOI] [PubMed] [Google Scholar]
- 329.FDA. 2021. Nucleic acid based tests. Food and Drug Administration, Bethesda, MD. https://www.fda.gov/medical-devices/vitro-diagnostics/nucleic-acid-based-tests. Accessed 8 February 2021. [Google Scholar]
- 330.WHO. 2020. Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases, 2021–2030. World Health Organization, Geneva, Switzerland. https://www.who.int/neglected_diseases/Revised-Draft-NTD-Roadmap-23Apr2020.pdf?ua=1. [Google Scholar]
- 331.Souza AA, Ducker C, Argaw D, King JD, Solomon AW, Biamonte MA, Coler RN, Cruz I, Lejon V, Levecke B, Marchini FK, Marks M, Millet P, Njenga SM, Noordin R, Paulussen R, Sreekumar E, Lammie PJ. 2021. Diagnostics and the neglected tropical diseases roadmap: setting the agenda for 2030. Trans R Soc Trop Med Hyg 115:129–135. 10.1093/trstmh/traa118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.WHO. 2020. Health topics. World Health Organization, Geneva, Switzerland. https://www.who.int/health-topics/#I. Accessed 12 April 2020. [Google Scholar]
- 333.WHO. 2015. WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007–2015. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 334.WHO. 2020. Strongyloides. World Health Organization, Geneva, Switzerland. https://www.who.int/teams/control-of-neglected-tropical-diseases/soil-transmitted-helminthiases/strongyloidiasis). Accessed 26 July 2021. [Google Scholar]
- 335.GBD. 2018. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 18:1211–1228. 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Khalil IA, Troeger C, Rao PC, Blacker BF, Brown A, Brewer TG, Colombara DV, De Hostos EL, Engmann C, Guerrant RL, Haque R, Houpt ER, Kang G, Korpe PS, Kotloff KL, Lima AAM, Petri WA, Jr, Platts-Mills JA, Shoultz DA, Forouzanfar MH, Hay SI, Reiner RC, Jr, Mokdad AH. 2018. Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: a meta-analyses study. Lancet Glob Health 6:e758–e768. 10.1016/S2214-109X(18)30283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Lane S, Lloyd D. 2002. Current trends in research into the waterborne parasite Giardia. Crit Rev Microbiol 28:123–147. 10.1080/1040-840291046713. [DOI] [PubMed] [Google Scholar]
- 338.Mukadi P, Lejon V, Barbé B, Gillet P, Nyembo C, Lukuka A, Likwela J, Lumbala C, Mbaruku J, Vander Veken W, Mumba D, Lutumba P, Muyembe J-J, Jacobs J. 2016. Performance of microscopy for the diagnosis of malaria and human African trypanosomiasis by diagnostic laboratories in the Democratic Republic of the Congo: results of a nation-wide external quality assessment. PLoS ne 11:e0146450. 10.1371/journal.pone.0146450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Bonnet J, Boudot C, Courtioux B. 2015. Overview of the diagnostic methods used in the field for human African trypanosomiasis: what could change in the next years? Biomed Res Int 2015:583262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Njamnshi AK, Seke Etet PF, Ngarka L, Perrig S, Olivera GC, Nfor LN, Njamnshi WY, Acho A, Muyembe JJ, Bentivoglio M, Rottenberg M, Kennedy PGE. 2020. The actigraphy sleep score: a new biomarker for diagnosis, disease staging, and monitoring in human African trypanosomiasis. Am J Trop Med Hyg 103:2244–2252. 10.4269/ajtmh.20-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.WHO. 2002. Control of Chagas Disease: second report of the WHO expert committee. World Health Organization, Geneva, Switzerland. http://apps.who.int/iris/bitstream/10665/42443/1/WHO_TRS_905.pdf Accessed April 15 2021. [Google Scholar]
- 342.PAHO. 2019. Guidelines for the diagnosis and treatment of Chagas disease. Pan American Health Organization/World Health Organization, Geneva, Switzerland. http://iris.paho.org/xmlui/bitstream/handle/123456789/49653/9789275120439_eng.pdf?sequence=6&isAllowed=y. [Google Scholar]
- 343.Sullivan L, Fleming J, Sastry L, Mehlert A, Wall SJ, Ferguson MA. 2014. Identification of sVSG117 as an immunodiagnostic antigen and evaluation of a dual-antigen lateral flow test for the diagnosis of human African trypanosomiasis. PLoS Negl Trop Dis 8:e2976. 10.1371/journal.pntd.0002976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Magnus E, Vervoort T, Van Meirvenne N. 1978. A card-agglutination test with stained trypanosomes (C.A.T.T.) for the serological diagnosis of T. b. gambiense trypanosomiasis. Ann Soc Belg Med Trop 58:169–176. [PubMed] [Google Scholar]
- 345.Bisser S, Lumbala C, Nguertoum E, Kande V, Flevaud L, Vatunga G, Boelaert M, Büscher P, Josenando T, Bessell PR, Biéler S, Ndung’u JM. 2016. Sensitivity and specificity of a prototype rapid diagnostic test for the detection of Trypanosoma brucei gambiense infection: a multi-centric prospective study. PLoS Negl Trop Dis 10:e0004608. 10.1371/journal.pntd.0004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Boodman C, Libman M, Ndao M, Yansouni CP. 2019. Case report: Trypanosoma brucei gambiense human African trypanosomiasis as the cause of fever in an inpatient with multiple myeloma and HIV-1 coinfection. Am J Trop Med Hyg 101:123–125. 10.4269/ajtmh.18-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Büscher P, Deborggraeve S. 2015. How can molecular diagnostics contribute to the elimination of human African trypanosomiasis? Expert Rev Mol Diagn 15:607–615. 10.1586/14737159.2015.1027195. [DOI] [PubMed] [Google Scholar]
- 348.Schijman AG, Bisio M, Orellana L, Sued M, Duffy T, Mejia Jaramillo AM, Cura C, Auter F, Veron V, Qvarnstrom Y, Deborggraeve S, Hijar G, Zulantay I, Lucero RH, Velazquez E, Tellez T, Sanchez Leon Z, Galvão L, Nolder D, Monje Rumi M, Levi JE, Ramirez JD, Zorrilla P, Flores M, Jercic MI, Crisante G, Añez N, De Castro AM, Gonzalez CI, Acosta Viana K, Yachelini P, Torrico F, Robello C, Diosque P, Triana Chavez O, Aznar C, Russomando G, Büscher P, Assal A, Guhl F, Sosa Estani S, DaSilva A, Britto C, Luquetti A, Ladzins J. 2011. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis 5:e931. 10.1371/journal.pntd.0000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Guggenbuhl Noller JM, Froeschl G, Eisermann P, Jochum J, Theuring S, Reiter-Owona I, Bissinger AL, Hoelscher M, Bakuli A, von Sonnenburg FF, Rothe C, Bretzel G, Albajar-Vinas P, Grout L, Pritsch M. 2020. Describing nearly two decades of Chagas disease in Germany and the lessons learned: a retrospective study on screening, detection, diagnosis, and treatment of Trypanosoma cruzi infection from 2000–2018. BMC Infect Dis 20:919. 10.1186/s12879-020-05600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Bern C. 2015. Chagas’ disease. N Engl J Med 373:456–466. 10.1056/NEJMra1410150. [DOI] [PubMed] [Google Scholar]
- 351.Turabelidze G, Vasudevan A, Rojas-Moreno C, Montgomery SP, Baker M, Pratt D, Enyeart S. 2020. Autochthonous Chagas disease—Missouri, 2018. MMWR Morb Mortal Wkly Rep 69:193–195. 10.15585/mmwr.mm6907a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Brashear RJ, Winkler MA, Schur JD, Lee H, Burczak JD, Hall HJ, Pan AA. 1995. Detection of antibodies to Trypanosoma cruzi among blood donors in the southwestern and western United States. I. Evaluation of the sensitivity and specificity of an enzyme immunoassay for detecting antibodies to T. cruzi. Transfusion 35:213–218. 10.1046/j.1537-2995.1995.35395184277.x. [DOI] [PubMed] [Google Scholar]
- 353.Kun H, Moore A, Mascola L, Steurer F, Lawrence G, Kubak B, Radhakrishna S, Leiby D, Herron R, Mone T, Hunter R, Kuehnert M, Chagas Disease in Transplant Recipients Investigation Team. 2009. Transmission of Trypanosoma cruzi by heart transplantation. Clin Infect Dis 48:1534–1540. 10.1086/598931. [DOI] [PubMed] [Google Scholar]
- 354.Bosch-Nicolau P, Salvador F, Sánchez-Montalvá A, Sulleiro E, Burgos J, Molina I. 2019. A case report of long treatment with Itraconazole in a patient with chronic Chagas disease. BMC Infect Dis 19:956. 10.1186/s12879-019-4608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Rangel-Gamboa L, López-García L, Moreno-Sánchez F, Hoyo-Ulloa I, Vega-Mémije ME, Mendoza-Bazán N, Romero-Valdovinos M, Olivo-Díaz A, Villalobos G, Martínez-Hernández F. 2019. Trypanosoma cruzi infection associated with atypical clinical manifestation during the acute phase of the Chagas disease. Parasit Vectors 12:506. 10.1186/s13071-019-3766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Gray EB, La Hoz RM, Green JS, Vikram HR, Benedict T, Rivera H, Montgomery SP. 2018. Reactivation of Chagas disease among heart transplant recipients in the United States, 2012–2016. Transpl Infect Dis 20:e12996. 10.1111/tid.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Duffy T, Bisio M, Altcheh J, Burgos JM, Diez M, Levin MJ, Favaloro RR, Freilij H, Schijman AG. 2009. Accurate real-time PCR strategy for monitoring bloodstream parasitic loads in Chagas disease patients. PLoS Negl Trop Dis 3:e419. 10.1371/journal.pntd.0000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Diez M, Favaloro L, Bertolotti A, Burgos JM, Vigliano C, Lastra MP, Levin MJ, Arnedo A, Nagel C, Schijman AG, Favaloro RR. 2007. Usefulness of PCR strategies for early diagnosis of Chagas’ disease reactivation and treatment follow-up in heart transplantation. Am J Transplant 7:1633–1640. 10.1111/j.1600-6143.2007.01820.x. [DOI] [PubMed] [Google Scholar]
- 359.Thakur S, Joshi J, Kaur S. 2020. Leishmaniasis diagnosis: an update on the use of parasitological, immunological, and molecular methods. J Parasit Dis 44:253–220. 10.1007/s12639-020-01212-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Shirian S, Oryan A, Hatam GR, Panahi S, Daneshbod Y. 2014. Comparison of conventional, molecular, and immunohistochemical methods in diagnosis of typical and atypical cutaneous leishmaniasis. Arch Pathol Lab Med 138:235–240. 10.5858/arpa.2013-0098-OA. [DOI] [PubMed] [Google Scholar]
- 361.Aronson N, Herwaldt BL, Libman M, Pearson R, Lopez-Velez R, Weina P, Carvalho E, Ephros M, Jeronimo S, Magill A. 2017. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am J Trop Med Hyg 96:24–45. 10.4269/ajtmh.16-84256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Boggild AK, Ramos AP, Espinosa D, Valencia BM, Veland N, Miranda-Verastegui C, Arevalo J, Low DE, Llanos-Cuentas A. 2010. Clinical and demographic stratification of test performance: a pooled analysis of five laboratory diagnostic methods for American cutaneous leishmaniasis. Am J Trop Med Hyg 83:345–350. 10.4269/ajtmh.2010.09-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Antinori S, Calattini S, Piolini R, Longhi E, Bestetti G, Cascio A, Parravicini C, Corbellino M. 2009. Is real-time polymerase chain reaction (PCR) more useful than a conventional PCR for the clinical management of leishmaniasis? Am J Trop Med Hyg 81:46–51. 10.4269/ajtmh.2009.81.46. [DOI] [PubMed] [Google Scholar]
- 364.Marfurt J, Niederwieser I, Makia ND, Beck HP, Felger I. 2003. Diagnostic genotyping of Old and New World Leishmania species by PCR-RFLP. Diagn Microbiol Infect Dis 46:115–124. 10.1016/S0732-8893(03)00040-3. [DOI] [PubMed] [Google Scholar]
- 365.Anonymous. 2020. The SMART Leish. https://www.accessdata.fda.gov/cdrh_docs/pdf8/K081868.pdf.
- 366.Sakkas H, Gartzonika C, Levidiotou S. 2016. Laboratory diagnosis of human visceral leishmaniasis. J Vector Borne Dis 53:8–16. [PubMed] [Google Scholar]
- 367.Harith AE, Awad Y, Mahamoud A, Abass E, Mansour D, Moura de Melo C, Madi RR, Semiao-Santos SJ, Osman HA. 2020. Modifications in a reference freeze-dried direct agglutination test to improve visceral leishmaniasis detection. Am J Trop Med Hyg 102:782–787. 10.4269/ajtmh.19-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Souza Filho JA, Barbosa JR, Figueiredo FB, Mendes AAJ, Silva SR, Coelho GL, Marcelino AP. 2016. Performance of Alere™ immunochromathographic test for the diagnosis of canine visceral leishmaniasis. Vet Parasitol 225:114–116. 10.1016/j.vetpar.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 369.Sakru N, Ozensoy Toz S, Korkmaz M, Ozbel Y. 2011. Serological monitoring of paediatric visceral leishmaniasis by IFA and ELISA methods. TurkiyeParazitolDerg 35:125–128. 10.5152/tpd.2011.31. [DOI] [PubMed] [Google Scholar]
- 370.Sundar S, Singh OP. 2018. Molecular diagnosis of visceral leishmaniasis. Mol Diagn Ther 22:443–457. 10.1007/s40291-018-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Mallawarachchi CH, Nilmini Chandrasena TGA, Premaratna R, Mallawarachchi S, de Silva NR. 2018. Human infection with sub-periodic Brugia spp. in Gampaha District, Sri Lanka: a threat to filariasis elimination status? Parasit Vectors 11:68. 10.1186/s13071-018-2649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Sasa M. 1976. Human filariasis. University of Tokyo Press, Tokyo, Japan. [Google Scholar]
- 373.Chandrashekar R, Curtis KC, Ramzy RM, Liftis F, Li BW, Weil GJ. 1994. Molecular cloning of Brugia malayi antigens for diagnosis of lymphatic filariasis. Mol Biochem Parasitol 64:261–271. 10.1016/0166-6851(94)00035-2. [DOI] [PubMed] [Google Scholar]
- 374.Ramzy RM, Gad AM, Faris R, Weil GJ. 1991. Evaluation of a monoclonal-antibody based antigen assay for diagnosis of Wuchereria bancrofti infection in Egypt. Am J Trop Med Hyg 44:691–695. 10.4269/ajtmh.1991.44.691. [DOI] [PubMed] [Google Scholar]
- 375.Weil GJ, Jain DC, Santhanam S, Malhotra A, Kumar H, Sethumadhavan KV, Liftis F, Ghosh TK. 1987. A monoclonal antibody-based enzyme immunoassay for detecting parasite antigenemia in Bancroftian filariasis. J Infect Dis 156:350–355. 10.1093/infdis/156.2.350. [DOI] [PubMed] [Google Scholar]
- 376.Wanji S, Amvongo-Adjia N, Njouendou AJ, Kengne-Ouafo JA, Ndongmo WP, Fombad FF, Koudou B, Enyong PA, Bockarie M. 2016. Further evidence of the cross-reactivity of the Binax NOW® Filariasis ICT cards to non-Wuchereria bancrofti filariae: experimental studies with Loa loa and Onchocerca ochengi. Parasit Vectors 9:267. 10.1186/s13071-016-1556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Thanchomnang T, Intapan PM, Tantrawatpan C, Lulitanond V, Chungpivat S, Taweethavonsawat P, Kaewkong W, Sanpool O, Janwan P, Choochote W, Maleewong W. 2013. Rapid detection and identification of Wuchereria bancrofti, Brugia malayi, B. pahangi, and Dirofilaria immitis in mosquito vectors and blood samples by high resolution melting real-time PCR. Korean J Parasitol 51:645–650. 10.3347/kjp.2013.51.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Yahathugoda TC, Supali T, Rao RU, Djuardi Y, Stefani D, Pical F, Fischer PU, Lloyd MM, Premaratne PH, Weerasooriya MV, Weil GJ. 2015. A comparison of two tests for filarial antigenemia in areas in Sri Lanka and Indonesia with low-level persistence of lymphatic filariasis following mass drug administration. Parasit Vectors 8:369. 10.1186/s13071-015-0979-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Chanteau S, Moulia-Pelat JP, Glaziou P, Nguyen NL, Luquiaud P, Plichart C, Martin PM, Cartel JL. 1994. Og4C3 circulating antigen: a marker of infection and adult worm burden in Wuchereria bancrofti filariasis. J Infect Dis 170:247–250. 10.1093/infdis/170.1.247. [DOI] [PubMed] [Google Scholar]
- 380.Goka AKJ, Rolston DDK, Mathan VI, Farthing MJG. 1990. Diagnosis of Strongyloides and hookworm infections: comparison of faecal and duodenal fluid microscopy. Trans R Soc Trop Med Hyg 84:829–831. 10.1016/0035-9203(90)90098-Y. [DOI] [PubMed] [Google Scholar]
- 381.Paula FMd, Malta FM, Corral MA, Marques PD, Gottardi M, Meisel DMCL, Yamashiro J, Pinho JRR, Castilho VLP, Gonçalves EMdN, Gryschek RCB, Chieffi PP. 2016. Diagnosis of Strongyloides stercoralis infection in immunocompromised patients by serological and molecular methods. Rev Inst Med Trop Sao Paulo 58:63–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Salazar SA, Gutierrez C, Berk SL. 1995. Value of the agar plate method for the diagnosis of intestinal strongyloidiasis. Diagn Microbiol Infect Dis 23:141–145. 10.1016/0732-8893(95)00247-2. [DOI] [PubMed] [Google Scholar]
- 383.Siddiqui AA, Berk SL. 2001. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis 33:1040–1047. 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 384.ten Hove RJ, van Esbroeck M, Vervoort T, van den Ende J, van Lieshout L, Verweij JJ. 2009. Molecular diagnostics of intestinal parasites in returning travellers. Eur J Clin Microbiol Infect Dis 28:1045–1053. 10.1007/s10096-009-0745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Buonfrate D, Perandin F, Formenti F, Bisoffi Z. 2017. A retrospective study comparing agar plate culture, indirect immunofluorescence and real-time PCR for the diagnosis of Strongyloides stercoralis infection. Parasitology 144:812–816. 10.1017/S0031182016002559. [DOI] [PubMed] [Google Scholar]
- 386.de Kaminsky RG. 1993. Evaluation of three methods for laboratory diagnosis of Strongyloides stercoralis infection. J Parasitol 79:277–280. 10.2307/3283519. [DOI] [PubMed] [Google Scholar]
- 387.Glinz D, Silué KD, Knopp S, Lohourignon LK, Yao KP, Steinmann P, Rinaldi L, Cringoli G, N’Goran EK, Utzinger J. 2010. Comparing diagnostic accuracy of Kato-Katz, Koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soli-transmitted helminths. PLoS Negl Trop Dis 4:e754. 10.1371/journal.pntd.0000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Gottardi M, Paula FM, Corral MA, Meisel DM, Costa SF, Abdala E, Pierrotti LC, Yamashiro J, Chieffi PP, Gryschek RC. 2015. Immunofluorescence assay for diagnosis of strongyloidiasis in immunocompromised patients. Infect Dis (Lond) 47:550–554. 10.3109/23744235.2015.1028096. [DOI] [PubMed] [Google Scholar]
- 389.Machado ER, Ueta MT, Goncalves-Pires MR, de Oliveira JB, Faccioli LH, Costa-Cruz JM. 2001. Diagnosis of human strongyloidiasis using particulate antigen of two strains of Strongyloides venezuelensis in indirect immunofluorescence antibody test. Exp Parasitol 99:52–55. 10.1006/expr.2001.4632. [DOI] [PubMed] [Google Scholar]
- 390.Watts MR, James G, Sultana Y, Ginn AN, Outhred AC, Kong F, Verweij JJ, Iredell JR, Chen SCA, Lee R. 2014. A loop-mediated isothermal amplification (LAMP) assay for Strongyloides stercoralis in a tool that uses a visual detection method with SYTO-82 fluorescent dye. Am J Trop Med Hyg 90:306–311. 10.4269/ajtmh.13-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Watts MR, Kim R, Ahuja V, Robertson GJ, Sultana Y, Wehrhahn MC, Bradbury RS, Gilbert GL, Lee R. 2019. Comparison of loop-mediated isothermal amplification and real-time PCR assays for detection of Strongyloides larvae in different specimen matrices. J Clin Microbiol 57:e01173-18. 10.1128/JCM.01173-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Verweij JJ, Canales M, Polman K, Ziem J, Brienen EAT, Polderman AM, van Lieshout L. 2009. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans R Soc Trop Med Hyg 103:342–346. 10.1016/j.trstmh.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 393.Knopp S, Glinz D, Rinaldi L, Mohammed KA, N’Goran EK, Stothard JR, Marti H, Cringoli G, Rollinson D, Utzinger J. 2009. FLOTAC: a promising technique for detecting helminth eggs in human faeces. Trans R Soc Trop Med Hyg 103:1190–1194. 10.1016/j.trstmh.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 394.Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, Rollinson D, Marti H, Utzinger J. 2008. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis 2:e331. 10.1371/journal.pntd.0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Vadlejch J, Petrtyl M, Zaichenko I, Cadkova Z, Jankovska I, Langrova I, Moravec M. 2011. Which McMaster egg counting technique is the most reliable? Parasitol Res 109:1387–1394. 10.1007/s00436-011-2385-5. [DOI] [PubMed] [Google Scholar]
- 396.Stracke K, Clarke N, Awburn CV, Vaz Nery S, Khieu V, Traub RJ, Jex AR. 2019. Development and validation of a multiplexed-tandem qPCR tool for diagnostics of human soil-transmitted helminth infections. PLoS Negl Trop Dis 13:e0007363. 10.1371/journal.pntd.0007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.O’Connell EM, Nutman TB. 2016. Molecular diagnostics for soil-transmitted helminths. Am J Trop Med Hyg 95:508–513. 10.4269/ajtmh.16-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Mand S, Marfo-Debrekyei Y, Debrah A, Buettner M, Batsa L, Pfarr K, Adjei O, Hoerauf A. 2005. Frequent detection of worm movements in onchocercal nodules by ultrasonography. Filaria J 4:1. 10.1186/1475-2883-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Kale OO, Bammeke AO, Ayeni O. 1974. An evaluation of skin snip techniques used in the quantitative assessment of microfilarial densities of Onchocerca volvulus. Bull World Health Organ 51:547–549. [PMC free article] [PubMed] [Google Scholar]
- 400.Unnasch TR, Golden A, Cama V, Cantey PT. 2018. Diagnostics for onchocerciasis in the era of elimination. Int Health 10:i20–i26. 10.1093/inthealth/ihx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Henry NL, Law M, Nutman TB, Klion AD. 2001. Onchocerciasis in a nonendemic population: clinical and immunologic assessment before treatment and at the time of presumed cure. J Infect Dis 183:512–516. 10.1086/318088. [DOI] [PubMed] [Google Scholar]
- 402.Lagatie O, Granjon E, Odiere MR, Zrein M, Stuyver LJ. 2019. Assessment of multiplex Onchocerca volvulus peptide ELISA in non-endemic tropical regions. Parasit Vectors 12:570. 10.1186/s13071-019-3824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Cama VA, McDonald C, Arcury-Quandt A, Eberhard M, Jenks MH, Smith J, Feleke SM, Abanyie F, Thomson L, Wiegand RE, Cantey PT. 2018. Evaluation of an OV-16 IgG4 enzyme-linked immunosorbent assay in humans and its application to determine the dynamics of antibody responses in a non-human primate model of Onchocerca volvulus infection. Am J Trop Med Hyg 99:1041–1048. 10.4269/ajtmh.18-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Lipner EM, Dembele N, Souleymane S, Alley WS, Prevots DR, Toe L, Boatin B, Weil GJ, Nutman TB. 2006. Field applicability of a rapid-format anti-Ov-16 antibody test for the assessment of onchocerciasis control measures in regions of endemicity. 194:216–221. 10.1086/505081. [DOI] [PubMed] [Google Scholar]
- 405.CDC. 2018. Dracunculiasis. Centers for Disease Control and Prevention, Atlanta, GA. https://www.cdc.gov/dpdx/dracunculiasis/index.html. Accessed 11 December 2020. [Google Scholar]
- 406.McManus DP, Zhang W, Li J, Bartley PB. 2003. Echinococcosis. Lancet 362:1295–1304. 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- 407.Giorgio A, Tarantino L, Francica G, Mariniello N, Aloisio T, Soscia E, Pierri G. 1992. Unilocular hydatid liver cysts: treatment with US-guided, double percutaneous aspiration and alcohol injection. Radiology 184:705–710. 10.1148/radiology.184.3.1509053. [DOI] [PubMed] [Google Scholar]
- 408.Filice C, Di Perri G, Strosselli M, Pirola F, Brunetti E, Dughetti S, Concia E. 1990. Parasitologic findings in percutaneous drainage of human hydatid liver cysts. J Infect Dis 161:1290–1295. 10.1093/infdis/161.6.1290. [DOI] [PubMed] [Google Scholar]
- 409.Riganò R, Profumo E, Ioppolo S, Notargiacomo S, Ortona E, Teggi A, Siracusano A. 1995. Immunological markers indicating the effectiveness of pharmacological treatment in human hydatid disease. Clin Exp Immunol 102:281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Riganò R, Ioppolo S, Ortona E, Margutti P, Profumo E, Ali MD, Di Vico B, Teggi A, Siracusano A. 2002. Long-term serological evaluation of patients with cystic echinococcosis treated with benzimidazole carbamates. Clin Exp Immunol 129:485–492. 10.1046/j.1365-2249.2002.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Sulima M, Szostakowska B, Nahorski W, Sikorska K, Wołyniec W, Wąż P. 2019. The usefulness of commercially available serological tests in the diagnosis and monitoring of treatment in patients with alveolar echinococcosis. Clin Exp Hepatol 5:327–333. 10.5114/ceh.2019.89480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Zarzosa MP, Orduña Domingo A, Gutiérrez P, Alonso P, Cuervo M, Prado A, Bratos MA, García-Yuste M, Ramos G, Rodríguez Torres A. 1999. Evaluation of six serological tests in diagnosis and postoperative control of pulmonary hydatid disease patients. Diagn Microbiol Infect Dis 35:255–262. 10.1016/S0732-8893(99)00079-6. [DOI] [PubMed] [Google Scholar]
- 413.Biava MF, Dao A, Fortier B. 2001. Laboratory diagnosis of cystic hydatic disease. World J Surg 25:10–14. 10.1007/s002680020002. [DOI] [PubMed] [Google Scholar]
- 414.White AC, Jr, Coyle CM, Rajshekhar V, Singh G, Hauser WA, Mohanty A, Garcia HH, Nash TE. 2018. Diagnosis and treatment of neurocysticercosis: 2017 Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis 66:1159–1163. 10.1093/cid/ciy157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Bustos JA, Rodriguez S, Jimenez JA, Moyano LM, Castillo Y, Ayvar V, Allan JC, Craig PS, Gonzalez AE, Gilman RH, Tsang VCW, Garcia HH, Cysticercosis Working Group in Peru. 2012. Detection of Taenia solium taeniasis coproantigen is an early indicator of treatment failure for taeniasis. Clin Vaccine Immunol 19:570–573. 10.1128/CVI.05428-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Del Brutto OH, Nash TE, White AC, Jr, Rajshekhar V, Wilkins PP, Singh G, Vasquez CM, Salgado P, Gilman RH, Garcia HH. 2017. Revised diagnostic criteria for neurocysticercosis. J Neurol Sci 372:202–210. 10.1016/j.jns.2016.11.045. [DOI] [PubMed] [Google Scholar]
- 417.Tsang VC, Brand JA, Boyer AE. 1989. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis 159:50–59. 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 418.Gabriël S, Blocher J, Dorny P, Abatih EN, Schmutzhard E, Ombay M, Mathias B, Winkler AS. 2012. Added value of antigen ELISA in the diagnosis of neurocysticercosis in resource poor settings. PLoS Negl Trop Dis 6:e1851. 10.1371/journal.pntd.0001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.O’Connell EM, Harrison S, Dahlstrom E, Nash T, Nutman TB. 2020. A novel, highly sensitive quantitative polymerase chain reaction assay for the diagnosis of subarachnoid and ventricular neurocysticercosis and for assessing responses to treatment. Clin Infect Dis 70:1875–1881. 10.1093/cid/ciz541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Mas-Coma S, Bargues MD, Valero MA. 2014. Diagnosis of human fascioliasis by stool and blood techniques: update for the present global scenario. Parasitology 141:1918–1946. 10.1017/S0031182014000869. [DOI] [PubMed] [Google Scholar]
- 421.Zárate-Rendón DA, Vlaminck J, Levecke B, Briones-Montero A, Geldhof P. 2019. Comparison of Kato-Katz Thick Smear, Mini-FLOTAC, and Flukefinder for the detection and quantification of Fasciola hepatica eggs in artificially spiked human stool. Am J Trop Med Hyg 101:59–61. 10.4269/ajtmh.18-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Cordova M, Reategui L, Espinoza JR. 1999. Immunodiagnosis of human fascioliasis with Fasciola hepatica cysteine proteinases. Trans R Soc Trop Med Hyg 93:54–57. 10.1016/S0035-9203(99)90178-5. [DOI] [PubMed] [Google Scholar]
- 423.Espinoza JR, Maco V, Marcos L, Saez S, Neyra V, Terashima A, Samalvides F, Gotuzzo E, Chavarry E, Huaman MC, Bargues MD, Valero MA, Mas-Coma S. 2007. Evaluation of Fas2-ELISA for the serological detection of Fasciola hepatica infection in humans. Am J Trop Med Hyg 76:977–982. 10.4269/ajtmh.2007.76.977. [DOI] [PubMed] [Google Scholar]
- 424.Shin SH, Hsu A, Chastain HM, Cruz LA, Elder ES, Sapp SG, McAuliffe I, Espino AM, Handali S. 2016. Development of two FhSAP2 recombinant-based assays for immunodiagnosis of human chronic fascioliasis. Am J Trop Med Hyg 95:852–855. 10.4269/ajtmh.16-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Sarkari B, Khabisi SA. 2017. Immunodiagnosis of human fascioliasis: an update of concepts and performances of the serological assays. J Clin Diagn Res 11:Oe05–Oe10. 10.7860/JCDR/2017/26066.10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Kayuni SA, LaCourse EJ, Makaula P, Lampiao F, Juziwelo L, Fawcett J, Shaw A, Alharbi M, Verweij JJ, Stothard JR. 2019. Case report: highlighting male genital schistosomiasis (MGS) in fishermen from the southwestern shoreline of Lake Malawi, Mangochi district. Am J Trop Med Hyg 101:1331–1335. 10.4269/ajtmh.19-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Kjetland EF, Hove RJT, Gomo E, Midzi N, Gwanzura L, Mason P, Friis H, Verweij JJ, Gundersen SG, Ndhlovu PD, Mduluza T, Van Lieshout L. 2009. Schistosomiasis PCR in vaginal lavage as an indicator of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg 81:1050–1055. 10.4269/ajtmh.2009.09-0081. [DOI] [PubMed] [Google Scholar]
- 428.WHO. 2014. Female genital schistosomiasis: a pocket atlas for clinical health-care professionals. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 429.Al-Sherbiny MM, Osman AM, Hancock K, Deelder AM, Tsang VC. 1999. Application of immunodiagnostic assays: detection of antibodies and circulating antigens in human schistosomiasis and correlation with clinical findings. Am J Trop Med Hyg 60:960–966. 10.4269/ajtmh.1999.60.960. [DOI] [PubMed] [Google Scholar]
- 430.Ashton RA, Stewart BT, Petty N, Lado M, Finn T, Brooker S, Kolaczinski JH. 2011. Accuracy of circulating cathodic antigen tests for rapid mapping of Schistosoma mansoni and S. haematobium infections in Southern Sudan. Trop Med Int Health 16:1099–1103. 10.1111/j.1365-3156.2011.02815.x. [DOI] [PubMed] [Google Scholar]
- 431.van Gool T, Vetter H, Vervoort T, Doenhoff MJ, Wetsteyn J, Overbosch D. 2002. Serodiagnosis of imported schistosomiasis by a combination of a commercial indirect hemagglutination test with Schistosoma mansoni adult worm antigens and an enzyme-linked immunosorbent assay with S. mansoni egg antigens. J Clin Microbiol 40:3432–3437. 10.1128/JCM.40.9.3432-3437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.ten Hove RJ, van Esbroeck M, Vervoort T, van den Ende J, Van Lieshout L, Verweij JJ. 2009. Molecular diagnostics of intestinal parasites in returning travellers. Eur J Clin Microbiol Infect Dis 28:1045–1053. 10.1007/s10096-009-0745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.ten Hove RJ, Verweij JJ, Vereecken K, Polman K, Dieye L, van Lieshout L. 2008. Multiplex real-time PCR for the detection and quantification of Schistosoma mansoni and S. haematobium infection in stool samples collected in northern Senegal. Trans R Soc Trop Med Hyg 102:179–185. 10.1016/j.trstmh.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 434.Arlian LG, Morgan MS. 2017. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors 10:297. 10.1186/s13071-017-2234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Alasaad S, Sarasa M, Heukelbach J, Mijele D, Soriguer RC, Zhu XQ, Rossi L. 2014. Advances in studies of disease-navigating webs: Sarcoptes scabiei as a case study. Parasit Vectors 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]