Keywords: complement system, gut microbiome, immunotherapy, mycobiome, pancreatic cancer
Abstract
Advances in -omics analyses have tremendously enhanced our understanding of the role of the microbiome in human health and disease. Most research is focused on the bacteriome, but scientists have now realized the significance of the virome and microbial dysbiosis as well, particularly in noninfectious diseases such as cancer. In this review, we summarize the role of mycobiome in tumorigenesis, with a dismal prognosis, and attention to pancreatic ductal adenocarcinoma (PDAC). We also discuss bacterial and mycobial interactions to the host’s immune response that is prevalently responsible for resistance to cancer therapy, including immunotherapy. We reported that the Malassezia species associated with scalp and skin infections, colonize in human PDAC tumors and accelerate tumorigenesis via activating the C3 complement-mannose-binding lectin (MBL) pathway. PDAC tumors thrive in an immunosuppressive microenvironment with desmoplastic stroma and a dysbiotic microbiome. Host-microbiome interactions in the tumor milieu pose a significant threat in driving the indolent immune behavior of the tumor. Microbial intervention in multimodal cancer therapy is a promising novel approach to modify an immunotolerant (“cold”) tumor microenvironment to an immunocompetent (“hot”) milieu that is effective in eliminating tumorigenesis.
HUMAN MICROBIOME: FRIENDS OR FOES
The human microbiome consists of trillions of microorganisms, including bacteria, archaea, fungi, protists, helminths, and viruses that inhabit and establish a relationship with the human body. Advances in the -omics analyses have revealed the diverse phylogenetic community within the human body, informing us with “friendly commensal” distribution to disease-causing dysbiosis in species’ diversity and abundance. The prominent players in the microbial ecosystem in the human body, for example, the bacteriome (1, 2), virome (3, 4), and mycobiome (5) in fact play a vital role not only in developing the immune system and maintaining cellular immune homeostasis but also in disease progression. The immune system, however, restricts resident microbial populations to their natural niches, such as skin and gut mucosa; a breach at this checkpoint is the root cause of many inflammatory diseases of noninfectious origin, including many cancer types.
Humans are exposed to a myriad of microorganisms in the birth canal, which continues through life, via the food we eat, the physical space we live in, and the microenvironment of the body (6). Other individual factors, for example, gender, ethnicity, lifestyle, hormonal, and immune system status further determine some degree of microbial dominance in individuals. It is conceivable that areas such as the skin, the respiratory system, and the gastrointestinal tract are frequent sites of microbial colonization. Extensive bacteriome analyses have exhibited bacterial signatures unique to specific anatomical sites. Actinobacteria and Proteobacteria found on the skin, with lipophilic Propionibacterium spp. are prominent in areas of higher density sebaceous glands (face or back). The oral cavity is home to anaerobic or facultative bacteria, such as Actinomyces, Fusobacterium, Porphyromonas, Treponema, and Veillonella spp. The human gut bacteriome consists of Firmicutes, Bacteriodetes, Actinobacteria, and Proteobacteria in which the former two prevail in the anaerobic distal gut region. Bacteria that colonize the colon have mutualistic functions in digestion, including the production of beneficial metabolites (7–9). Yet, dysbiotic changes in the gut microbiome involve distinct and universal predominance of pathogenic species, in creating conditions such as inflammatory bowel disease (10), metabolic syndrome, cardiovascular disease, and many cancer types, including pancreatic cancer (PC) (1). As such, supporting the “germ theory of cancer” phenomenon, Helicobacter pylori and Fusobacterium nucleatum have been implicated as “sole” infections driving gastric and colorectal tumorigenesis, respectively (11, 12). Thus, like pathogenic infections and dynamic changes in the bacteriome, their role in diseases, including cancer, are constantly evolving.
The virome in health has been understudied, although viral infections and their role in disease provide global insight about their possible significance, especially bacteriophages that harbor gut bacterial populations, with ample opportunity to guide immune responses and maintain immune health of the gut (13). The human virome dysbiosis may cause the opportunistic oncogenic bacteriome to prevail, whereas cytomegalovirus (CMV), human herpesvirus 8 (HHV-8), human papilloma virus (HPV), hepatitis B virus (HBV), hepatitis C virus (HCV), and Epstein–Barr virus (EBV) have direct implications in carcinogenesis (4, 14). Coinfections provide pathogenic synergy in which bacterial products remodel chromatin to trigger a latent infection into an active one, leading to a hypermethylation state of tumor suppressor genes, in turn inducing tumor progression (15). Thus, engagement between the virome and bacteriome may yield complex results in immune health to disease conditions.
The human fungal landscape, on the other hand, is less populated in the body, but can greatly affect human health (16, 17). Diverse groups of fungi have been found associated with the human digestive tract. Ghannoum et al. (18) showed that there are over 101 species belonging to 85 fungal genera in the oral cavity of healthy people, whereas there are 66–247 genera present in the gut (19–21). Commensal human mycobiota comprises fungi to assist in immune system recognition and memory, combating pathogenic microorganisms, while being involved in important physiological processes, including metabolic functions (22). Like an individual bacteriome profile, the differential fungal composition within a population is derived from a complex interplay of singular factors that include geographic location, gender, ethnicity, and lifestyle (23). Although fungal types are not specific to healthy people, phyla Ascomycota, Basidiomycota, and Zygomycota are quite common (24). The lower gastrointestinal tract has diverse fungal genera, for example, Aspergillus, Candida, Cladosporium, Cryptococcus, Galactomyces, Malassezia, Saccharomyces, and Trichosporon (16). Although Malassezia spp. are prevalent on the skin (25), the gut harbors Candida, Aspergillus, and Cladosporium spp. (19). The presence of certain fungi further play a role in establishing a healthy microbiome at specific sites.
As with the bacteriome, fungal dysbiosis has been identified as a key comorbidity factor in many human diseases, including cancer. The use of antibiotics, known to decrease commensal bacteria, further exacerbate fungal colonization and infection (26). Fungal dysbiosis of the gut is often characterized by overgrowth of Candida albicans, followed by its invasion into the bloodstream, which is strongly connected to irritable bowel syndrome (IBS) (27–30), Crohn’s disease (31), allergic diseases, colitis (17), ulcerative colitis (16, 32), and gastric carcinogenesis (33). In contrast, fungal infections are considered as secondary infections to cancer development and/or a result of chemotherapy. The mycobiome dysbiosis, as a driving force in oncogenesis is an emerging idea as we demonstrated recently that the prevalence of Malassezia spp. promotes pancreatic tumorigenesis via activation of components in the innate immune response (5). Zhu et al. (34) recently showed that Candida albicans could promote tumorigenesis in colon cancer, suggesting a cross talk between C. albicans, macrophages, and innate lymphoid cells in the intestine.
Thus, the -virome, -bacteriome, and -mycobiome predominantly constitute the human microbiome, and while these are shaping our healthy immunity, also strongly implicated in diseases. These alien entities are entangled with human cellular processes so deeply that one can only distinguish their behavior as a “friend or foe” during dysbiosis (2, 35). In the oral-gut axis, local inflammatory response to the commensal microbiome facilitates innate and adaptive immune responses—though prolonged inflammation can ultimately lead to dysbiosis (36) (Fig. 1). The gut is vulnerable to opportunistic pathogens during dysbiosis, which can cause breakdown of mucosal barriers, resulting in leaky-gut syndrome, translocation to peripheral circulation, and inciting a local as well as systemic inflammatory state (37) that include various tissues such as the brain (38, 39), liver (40), and pancreas (41). Widespread inflammation is an underlining trigger in the colonization of microorganisms and aggressive behavior of several cancer types (12).
Figure 1.
Mouth-gut microbiome as commensals and/or pathobionts. The flowchart represents diverse, anatomical site-specific predominance of a healthy microbiota profile in the oral-gut axis. Acute inflammatory response directs the development of a normal immune system, whereas chronic low-grade inflammation causes leaky-gut resulting specific microbial colonization in various tissues, leading to disease conditions. HCC, hepatocellular carcinoma; PDAC, pancreatic ductal adenocarcinoma.
MYCOBIOME: APPROACHING THE FOREFRONT IN HUMAN HEALTH
Of millions of fungal species identified worldwide, only a few hundred fungi are known to cause disease phenotypes, with a handful frequently affecting humans. In the same light, fungal infectious diseases are limited, whereas invasive opportunistic fungal infections are constantly expressed due to several contributing factors, for example, nosocomial exposure, emergence of antifungal resistance, advances in fungal identification, and zoonotic fungal epidemics that have human impact and immunotherapies (42). In our own ecosystem, many physiological and socioeconomic factors influence fungal diversity contributing to the human fungal landscape. Two groups of fungi, Dermatophytes and Malassezia, are a common cause of skin and mucous membrane infections such as dandruff, atopic dermatitis/eczema, ringworm, athlete’s foot, jock itch, and nail infections (43). The class of Malasseziomycetes are mostly comprised of plant pathogens, whereas Malassezia is uniquely associated with common skin disorders.
Opportunistic mycobial pathogens often cause systemic infections in at-risk individuals; for example, pneumocystis pneumonia and cryptococcosis are frequently associated with HIV/AIDS outbreaks (44). Paracoccidioidomycosis is one of the most prevalent systemic mycoses in Latin America, whereas invasive candidiasis and allergic bronchopulmonary disease are commonly associated with postsurgical infections. Other opportunistic fungal pathogens include Acremonium, which is present in the blood, sputum, and tracheal aspirates, whereas Rhodotorula is capable of colonizing, infecting, and disrupting the essential microflora of the human digestive system. Rhodotorula is also reported in the meninges, skin, ocular, peritoneal, and prosthetic joint infections (45). Overgrowth of Saccharomyces occurs in Crohn’s disease, whereas Candida spp. colonize in IBS and colitis during the disease progression (17).
Evolution of metagenomic studies further reveal mycobial signature associated with several different types of cancers, including colorectal cancer (CRC) (46), colitis-associated colorectal cancer (47), skin cancer (25, 48, 49), and more recently, pancreatic ductal adenocarcinoma (PDAC) (5). Malassezia, a common skin commensal is implicated in many skin disorders, as well as asthma, Alzheimer’s disease, and many cancers (e.g., skin, CRC, and PDAC); it is also strongly linked to the immunosuppressive pancreatic tumor milieu (5, 50). Malassezia, Rhodotorula, and Acremonium are predominant species in colorectal cancer patients. Candida, another skin-inhabiting fungus mentioned earlier, also colonizes, causes candidiasis, and then invades other tissues. Candida is thus implicated in many disorders, including multiple sclerosis, Alzheimer’s, cystic fibrosis, IBS, and cancer (51).
Fungal pathogens, therefore, are no longer viewed as causative agents for primary and secondary infectious disorders but are key factors in driving different types of cancers.
SENSING FUNGAL “DANGER”
Components of both innate and adaptive immunity provide tolerance to commensals while protecting against pathogenic fungi (52). Pattern recognition receptors (PRRs) are strategically and predominantly localized on the surface of myeloid cells, including neutrophils, monocytes, antigen-presenting cells like dendritic cells (DC), and macrophages, as well as some B- and T-cell lymphocytes. C-type lectin receptors (CLRs) are specialized PRRs, containing one or more carbohydrate recognition domain (CRD), recognize pathogen-associated molecular patterns (PAMPs) such as mannans and β-glucans on the fungal cell wall and requires calcium for binding (23) (Fig. 2). CLR and fungal carbohydrate associations thus trigger an intracellular “proinflammatory storm” to curtail fungal invasion (53). CLR signaling involves distinct types of surface receptors, such as Dectin-1, Dectin-2, Mincle, and DC-SIGN, and soluble mannose-binding lectin (MBL)-mediated sensing (23). These innate immune system molecular players activate myriads of downstream signaling pathways (16), which further activate/recruit other defenders, for example, monocyte, neutrophils, and natural killer (NK) cells to produce reactive oxygen species (ROS), proinflammatory cytokines and chemokines to kill fungi, and secrete perforin and granulocyte-macrophage colony-stimulating factors (GM-CSF), which exhibit tumoricidal activity (23). Myeloid cells stimulate naïve T-cell maturation into several types of effector T-cells that join cytokine production that induce infections, allowing epigenetic reprogramming of myeloid cells with adaptive characteristics of “trained immunity.” In contrast, reprograming of innate immune cells can lead to misguided trained immunity that results in a persistent immunotolerant state that on one hand is essential for homeostasis, but is also responsible for the disease progression. We reported that free-living fungal species of Malassezia secrete hydrolases to release host lipids and activate the C3 complement-MBL pathway, promoting an immunosuppressive tumor milieu in PC (5).
Figure 2.
Fungal pathogens induce inflammatory microenvironments. C-type lectin receptors recognize fungal invasion through surface receptors on molecules of the innate immune system or via soluble mannose-binding lectin (MBL)-mediated sensing. The proinflammatory storm further “trains” an adaptive immune system. Malassezia spp. secrete hydrolase to release host lipids and indoles, which act as potent agonists for AhRs that can trigger immune cell activation. Malassezia spp. can also activate the C3 complement-MBL pathway to promote an immunosuppressive tumor milieu, as in PC. AhR, aryl hydrocarbon receptor; CD4+, cluster of differentiation 4 positive; CLR, C-type lectin receptor; GM-CSF, granulocyte-macrophage colony-stimulating factor; PC, pancreatic cancer; ROS, reactive oxygen species; Treg, regulatory T-cells.
PANCREATIC TUMORIGENESIS AND MICROBIAL INFLUENCE (BACTERIAL AND FUNGAL INTERACTOME)
It is perceived that obesity induces visceral fat deposition and results in metabolic disorders (54), leading to a pathophysiology known as fatty pancreas which is implicated in severe acute pancreatitis (55) and PDAC progression (56). One can hypothesize that pancreatic colonization of Malassezia is possibly favored by the lipophilic microenvironment of the pancreas, which then seizes the host-immune response directly via MBL-signaling. Malassezia, a basidiomycetous yeast that feeds on an exogenous fat/lipid source, lacks the genes for fatty acid synthesis or carbohydrate metabolism (57), and has a predilection for seborrheic skin sites such as the scalp and trunk. We recently observed that Ascomycota and Basidiomycota were the most common phyla in the gut and pancreatic tumors of mice and humans. Parallel to our mice data, Malassezia is the most prevalent genus in human PDAC tissues than in the gut. Malassezia’s secretome can participate in potential indirect effects. Potent indolic ligands produced by these yeasts are known to target the aryl hydrocarbon receptor (AhR), functioning as a transcription factor in UV damage and host immune response pathways (58) (Fig. 2). Increased AhR expression is a strong predictor of gastric cancer progression and metastasis (59), whereas higher plasma AhR levels are correlated with PC (60). We demonstrated that M. globosa oral gavages in mice enable fungal translocation from the gut to the pancreas, while accelerating the progression of PDAC via the C3 complement-MBL pathway (5). Transcriptomic data from The Cancer Genome Atlas (TCGA) were also correlated to higher MBL2 expression in patients with PDAC, and thus reduced survival. Thus, a common skin commensal that causes dermal disorders can create havoc in the host immune response, enabling tumorigenesis. Besides fungal interference, evidence suggests that the altered tumor bacteriome aggravates innate and adaptive responses that in turn promote PDAC (1). Pseudomonas genus in human PDAC tissues (1) produces phenazine pigments that target AhRs, activating downstream pathways (61, 62). A recent study suggests that periodontal infections can be a risk factor for PDAC, in which an oral pathogen, Fusobacterium species, leads to a heightened prognosis for pancreatic tumors (63). Fungal and bacterial dysbiosis and immunomodulating events thus congregate to facilitate PDAC tumorigenesis.
The immunosuppressive phenomenon is observed in several other cancers, especially with acute or chronic inflammation. Helicobacter pylori is recognized to cause chronic gastritis, peptic ulcers, and gastric adenocarcinoma (64, 65). An oral commensal, Fusobacterium nucleatum is similarly associated with colon cancer, whereas the presence of Bacteroides fragilis can promote an immunosuppressive environment that enhances CRC development. In addition, the microbiome can influence tumor progression and modulate inflammatory receptor pathways. A recent study suggests that the intracellular survival of bacterium P. gingivalis increases pancreatic tumorigenesis through direct activation of cancer signaling pathways (66).
A comprehensive list of bacterial and fungal dysbiosis implicated in several diseases including cancer are categorized based on the anatomical sites, as indicated in Table 1.
Table 1.
Bacterial and fungal species associated with human diseases
| Microbial Species | Fungi or Bacteria |
Host Tested | Body Site | Related Diseases | References |
|---|---|---|---|---|---|
| Oral microbiota | |||||
| Fusobacterium spp. | B | Human | Oral | Pancreatic cancer prognosis | (63) |
| Candida albicans | F | Human | Esophagus | Esophageal cancer | (67) |
| Epithelial surfaces | |||||
| Malassezia spp. | F | Human | Skin | Dandruff, atopic dermatitis/eczema | (43) |
| Cladosporium | F | Human | Reproductive tissue | Ovarian cancer | (68) |
| Lung | |||||
| Malassezia | F | Human | Lung | Asthma | (5, 50) |
| Breast | |||||
| Methylobacterium radiotolerans | B | Human | Breast | Breast cancer | (69) |
| Brain | |||||
| Malassezia | F | Human | Brain | Alzheimer’s disease | (5, 50) |
| Stomach | |||||
| Helicobacter pylori | B | Human | Stomach | Gastric cancer | (47) |
| Pancreas | |||||
| Porphyromonas gingivalis | B | Pancreatic Carcinoma cells | Intracellular | Pancreatic tumorigenesis | (66) |
| Rhodotorula | F | Human | Digestive system | Colorectal cancer | (45) |
| Malassezia spp. | F | Mouse and human | Pancreas | Pancreatic cancer | (5) |
| Gut | |||||
| Fusobacterium nucleatum | B | Mouse and human | Colon | Colorectal cancer | (12) |
| Enterobacteriaceae | B | Human | Colon | Colitis-associated colorectal cancer | (70) |
| Fusobacterium | B | Human | Colon | Sporadic colorectal cancer | (70) |
| Fusobacterium nucleatum | B | Colon Carcinoma cells | Gut | Colorectal cancer stimulation | (67) |
| Bacteroides fragilis | B | Human | Fecal | CRC development | (71) |
| Candida spp. | F | Human | Gut | IBS | (17) |
| Candida albicans | F | Human | Gut | Crohn’s, UC, colitis | (16,17) |
| Saccharomyces | F | Human | Gut | Crohn’s disease | (17) |
B, bacteria; CRC, colorectal cancer; F, fungi; IBS, irritable bowel syndrome; UC, ulcerative colitis.
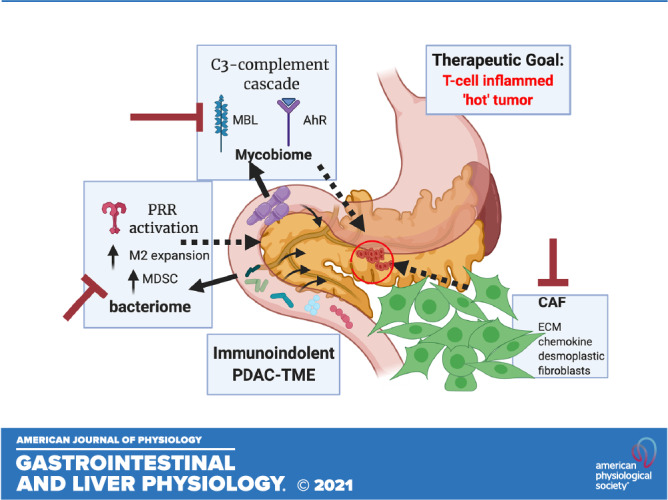
IMMUNE-INDOLENT PDAC TUMOR MICROENVIRONMENT
Multifaceted components in a pancreatic tumor support the Paget’s classical “seed” and “soil” hypothesis: fungal-bacterial (1, 5) interactions, host immunosuppressive factors, desmoplastic stroma with enriched deposition of extracellular matrix components lead to reduced elasticity of tumor tissue. Highly fibrotic stroma and extensive infiltration by immunosuppressive cell populations, such as tumor-associated macrophages (TAMs), regulatory T-cells (Tregs), and myeloid-derived suppressor cells (MDSCs), makes PDAC tumor milieu profoundly immunosuppressive and protumorigenic (72) that is resistant to single-agent immunotherapies (73). Tregs are often recruited early in premalignant pancreatic lesions and progressively dominate in PDAC; as such, Tregs are poor prognostic markers for PDAC. Thus, Tregs that under normal physiology maintain immune homeostasis and establish peripheral tolerance in the context of autoimmunity, allergies, and microbial infections, when employed at the precancerous lesions, establish a “cold tumor” that is refractory to cancer therapeutics, especially immunotherapy.
Interplay between immunosuppressive cells and stromal tissue further aid PDAC tumorigenesis. Although depletion of Tregs reduces tumor size, depletion of MDSC synergistically reduces the Tregs load on pancreatic tumors. PDAC-derived granulocyte macrophage-colony-stimulating factor (GM-CSF) and the chemokine (C-C motif) ligand 2 (CCL2) recruit MDSC and TAMs. Both MDSC and tumor-promoting M2 macrophages further suppress cluster of differentiation 8 positive (CD8+) T-cell functions through secretion of cytokines that lead to cytotoxic T-cell lymphocyte (CTL) exhaustion. Myeloid-inflamed stroma recruit regulatory B cells (Bregs) to produce cytokine interleukin 35 (IL35), which further drives PDAC progression (73, 74). Moreover, highly desmoplastic stroma in PDAC is enriched in growth factors and myofibroblast-like pancreatic stellate cells that secrete collagen for stromal cell proliferation. Three subtypes of cancer-associated fibroblasts (CAFs): inflammatory CAFs (iCAFs), myofibroblastic CAFs (myCAFs), and antigen-presenting CAFs (apCAFs) arise with environmental cues (25, 75) and play an important role in the tumor microenvironment (TME). These CAFs provide stiffness in the tumor environment and produce anti-inflammatory cytokines that elevate immunosuppressive “soil” surrounding the tumor mass. The infiltrating lymphocytes influence the extracellular matrix and vasculature, directly contributing to tumor progression and metastasis (76).
In this already complex cross talk, selective colonization of both the bacteriome (1) and mycobiome (5) in PDAC aid in inhibiting the antitumor immune response. Stromal stiffness concomitantly increases tumor interstitial fluid pressure. We recently identified role of mechanosensitive ion channels in innate immunity (77); the myeloid cells sense the mechanical force around the tumor mass, leading to its expansion via the retinoblastoma tumor suppressor pathway. Thus, a concerted attack from many factors establishes an immunosuppressive TME that fail therapeutics in PDAC and promotes tumorigenesis.
STRATEGIES TO “TURN ON” A COMPETENT IMMUNE SYSTEM IN PDAC
Given the retroperitoneal position of the pancreas and the lack of highly sensitive diagnostic imaging technology, we fail to clearly capture early-stage pancreatic cancer (PC), clinically. PDAC is an aggressive form that involves exocrine glands and constitutes 90% of all PCs. There are no specific tumor markers or symptoms to detect early or distant metastases in PDAC; surgical resection is, therefore, not possible due to advanced stage detection. Diagnosis of a pancreatic tumor generally leads to a very poor prognosis, as even with identification of resectable tumors often relapse with lung and liver metastases, resulting in a highly lethal cancer.
Like the pancreatic tumor milieu, cancer progression is complex and multifaceted that include genetic, epigenetic, and metabolic alterations along with cross talk of multiple signaling pathways. Intriguingly, PC exhibits very few genetic mutations; Kirsten rat sarcoma (KRAS) oncogene activation is a dominant feature in more than 90% of PCs, in which inactivation of tumor suppressor genes, for example, cyclin-dependent kinase inhibitor 2A (CDKN2A) (encoding p16), tumor protein 53 (TP53), and SMAD family member 4 (SMAD4) occur in 50%–80% of all cases. Angiogenesis as a hallmark of all types of tumors, paradoxically PDAC tumors are atypically hypovascular. It has been proposed that pancreatic tumor cells in fact metastasize rapidly by endothelial ablation via the activin-ALK7 pathway in the three-dimensional (3-D) organotypic model (78). The more complex TME, as mentioned earlier with CAFs, involves microorganisms that modulate the immune cell infiltration in PC (74, 79, 80) pose further challenges, at the same time provide opportunities in targeting this cancer with precision. Experimental platforms, for example, 3-D organoids, syngeneic xenografts, with genetically engineered mice models (GEMMs), and the most translatable patient-derived xenografts (PDXs) provide useful tools in identifying effective therapies (80, 81).
Multimodal strategies are under investigation, with a focus on reprogramming the stromal and immune cell milieu into an antitumor environment in PDAC (79). Targeting stromal desmoplasia, a histopathological hallmark of PDAC, is an attractive approach, but has mainly failed due to the dual personality of tumor stroma that promote or resist tumor progression. Approaches to deconstruct the stroma include matrix metalloproteinase (MMP) inhibitors, hyaluronidase, sonic hedgehog (SHH) inhibitors, fibroblast activation protein (FAP) targeting agents, and C-X-C motif chemokine receptor 4 (CXCR4) inhibitors.
Awakening the competent immune response in PDAC is an attractive approach. Considering the immunosuppressive TME in PDAC, obvious attempts are targeted toward remodeling the immune cells to become proinflammatory to trigger innate and adaptive responses that could clear MDSC, anti-inflammatory tumor-associated macrophages (TAM M2), along with Tregs to relieve CTL-exhaustion (Fig. 3). These treatments include allogenic, granulocyte-macrophage colony-stimulating factor (GM-CSF) secreting cell vaccines (GVAX) (82), inhibit receptor CTL-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), or programmed death-ligand 1 (PD-L1) using specific antibodies to activate cytotoxic T-cell response and reverse Treg-mediated immunosuppression (83). Similarly, agonists that stimulate proinflammatory antitumor-associated macrophages M1 (TAM M1) will enhance cytotoxic CD8+ T-cell response. Since differentiation of CD4+ T-cells into a variety of subsets of T-cells result in pro- and anti-inflammatory cytokines, the success of an immune treatment is largely dependent on the type of cell it targets (84, 85). CD23, structurally belonging to the C-type lectin family, plays an important role in B- and T-cell differentiation to induce proinflammation; therapeutic monoclonal antibodies against CD23 have been effective in combating chronic lymphocytic leukemia (86). Recently, it was demonstrated that targeting DC paucity early on, shows promise in controlling PDAC progression by activating the CTL and Th1 (T helper type 1) response to neoantigens of PDAC (87). Currently, combinatorial therapy for PDAC is underway in multiple trials, for which vaccine therapy is used to induce accumulation of lymphoid aggregates. Lymphocytes with interferon-γ also activate PD-1-PD-L1 signaling pathways, which can be targeted by a checkpoint inhibitor to yield cytotoxic T-cell proliferation to remove tumors. This synergistic effect can unleash the full therapeutic potential soon to be revealed in clinical trials (88).
Figure 3.
Treating fungal-induced inflammatory microenvironments. Immune-indolent tumors can be targeted in anti-CTL-4 and anti-PD-1 therapy that downregulates immunosuppressive Tregs and upregulates T-cell activity. In contrast, CD23 inhibitors target a wide range of immune responses, including activation of macrophages, subsequent secretion of proinflammatory mediators, which include TNF-α, IL-6, and NO, as well as B- and T-cell diffentiation. CD23, a low-affinity receptor for IgE; CTL, cytotoxic T lymphocytes; IL, interleukin; NO, nitric oxide; PD-1, programmed cell death protein 1.
Emerging new therapies include focusing on the microbiome in TME, often associated with immune cells that modulate their response in PDAC progression. Recognizing commensal microbiota and their role in pathogenicity provide the opportunity to target or replenish them during disease progression to relieve symptoms and/or increase the efficacy of resistant treatment plans (89). Microbial interventions, such as cancer therapeutics, are gaining increased attention (90), whereas recent studies suggest this might be effective in combating PC. We have demonstrated that intrapancreatic microbiota promotes the crippling immunosuppression characteristics of PDAC (1). We and others show distinct bacterial communities in early and advanced PDAC linked to survival outcomes in patients (1, 91). Geller et al. (92) also evidenced the presence of the bacterial Class Gammaproteobacteria, in 76% of human PDACs. They further demonstrated in colon cancer mouse model that intratumor Gammaproteobacteria provided gemcitabine resistance. Such chemo resistance was abrogated by cotreatment with the antibiotic ciprofloxacin. Ablation of the bacteriome also results in reprogramming of immune cells and the promotion of antitumor activities of CD8+ T-cells, NK cells, M1-type macrophages, Th1 cells, and dendritic cells (DCs)—whereas the fecal microbial transplant (FMT) from PDAC-bearing mice enhance tumor-promoting activities with M2-type tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), Tregs, and Th2 cells (1). Using a similar investigation, modulation of the gut microbiome allows the intratumor microbiome to connect to short- or long-term survival signature in PDAC (91). Furthermore, a combination of antibiotic and anti-PD-1 therapy result in a synergistic effect in enhanced intratumoral CD4+ and CD8+ T-cell activation. These studies highlight microbial intervention as an effective approach in turning on the immune checkpoint to halt PDAC progression.
Like the bacteriome, we observed a distinct fungal community, predominantly the Malassezia genus that weakens the innate immune system; the MBL2-C3 cascade provides a novel druggable pathway in PDAC tumorigenesis (5). It has been perceived that antibacterial regime (Fig. 3) could be the root cause of fungal dysbiosis, or the initial inflammatory milieu that triggers fungal imbalance (or more accurately, gut-dysbiosis can modulate the pancreatic microbiome via the sphincter of oddi). Cross talk between tissues (e.g., gut-pancreas axis), immunomodulation (CAF/microorganisms-immune cells), and underlying metabolic pathways converge in PDAC progression. We must forge ahead to design syngeneic therapeutics to combat PDAC.
CONCLUSIONS
We believe that microbiome-targeted therapies are the next frontier in clinical cancer treatment. Since the microbiome is modifiable, new findings may lead to prophylactic, preventative therapy for at-risk individuals that are genetically predisposed. An indolent immune system is a hallmark in PDAC progression; the immunomodulation effect of the tumor microbiome thus becomes significant driving force for tumorigenesis. Targeting the mycobiome may have potential clinical implications. Aykut et al. (5) reported that the ablation of mycobiome enhanced the activity of gemcitabine, which is still among the most prescribed drugs in patients with PDAC; however, the response to this chemotherapeutic as monotherapy is rather poor. The mechanism underpinning these observations are incompletely understood. Nevertheless, lessons learned from the bacterial microbiome may be relevant here. Geller et al. (92) have shown that several bacterial taxa that express the enzyme cytidine deaminase can metabolize gemcitabine into its inactive metabolite. Consequently, ablation of these microbes bolsters the activity of gemcitabine. Similarly, targeting the bacterial microbiome has been reported to enhance the efficacy of PD-1-based immunotherapy by reprograming the tumor-associated macrophage toward a more immunogenic M1-like phenotype (1). The potential of targeting fungi to enhance responsiveness to traditional cancer therapeutics or immunotherapy requires more exact study. While tumor-associated bacteriome knowledge has advanced drastically in recent years (93), mycobiome in oncogenesis is only evolving (5). The microbial ecosystem (bacterial, fungal, and viral among others) further cross talk either synergistically or control the outgrowth of one species and maintain diversity. Therefore, not only probiotics but also prebiotics hold great promises in maintaining healthy microbial homeostasis while targeting tumor-associated dominant species. Optimizing microbial-based therapies in patients will require exploratory clinical trials aimed at basic questions, which can culminate in viable therapeutic strategies.
GRANTS
This work was supported by City University of New York Enhanced Research Grant No. 63814-00 51 (to A.S.), NCI CA206105 and DOD W81XWH-19-1-0605 (to D.S.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.S. and A.S. conceived and designed research; D.S. and A.S. performed experiments; M.P., D.S., and A.S. analyzed data; M.E., M.P., D.S., and A.S. interpreted results of experiments; R.P., M.P., D.S., and A.S. prepared figures; M.E., R.P., M.P., D.S., and A.S. drafted manuscript; M.E., R.P., M.P., G.M., D.S. and A.S. edited and revised manuscript; M.E., R.P., M.P., G.M., D.S., and A.S. approved final version of manuscript.
REFERENCES
- 1.Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres LE, Werba G, Zhang K, Guo Y, Li Q, Akkad N, Lall S, Wadowski B, Gutierrez J, Kochen Rossi JA, Herzog JW, Diskin B, Torres-Hernandez A, Leinwand J, Wang W, Taunk PS, Savadkar S, Janal M, Saxena A, Li X, Cohen D, Sartor RB, Saxena D, Miller G. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov 8: 403–416, 2018[Erratum inCancer Discov10: 1988, 2020] doi: 10.1158/2159-8290.CD-17-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sethi V, Vitiello GA, Saxena D, Miller G, Dudeja V. The role of the microbiome in immunologic development and its implication for pancreatic cancer immunotherapy. Gastroenterology 156: 2097–2115.e2,2019. doi: 10.1053/j.gastro.2018.12.045. [DOI] [PubMed] [Google Scholar]
- 3.Saxena D, Li Y, Devota A, Pushalkar S, Abrams W, Barber C, Corby P, Poles M, Phelan J, Malamud D. Modulation of the orodigestive tract microbiome in HIV-infected patients. Oral Dis 22, Suppl1: 73–78, 2016. doi: 10.1111/odi.12392. [DOI] [PubMed] [Google Scholar]
- 4.Stern J, Miller G, Li X, Saxena D. Virome and bacteriome: two sides of the same coin. Curr Opin Virol 37: 37–43, 2019. doi: 10.1016/j.coviro.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aykut B, Pushalkar S, Chen R, Li Q, Abengozar R, Kim JI, Shadaloey SA, Wu D, Preiss P, Verma N, Guo Y, Saxena A, Vardhan M, Diskin B, Wang W, Leinwand J, Kurz E, Kochen Rossi JA, Hundeyin M, Zambrinis C, Li X, Saxena D, Miller G. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 574: 264–267, 2019. doi: 10.1038/s41586-019-1608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albhaisi SAM, Bajaj JS, Sanyal AJ. Role of gut microbiota in liver disease. Am J Physiol Gastrointest Liver Physiol 318: G84–G98, 2020. doi: 10.1152/ajpgi.00118.2019. [DOI] [PubMed] [Google Scholar]
- 7.Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. Nature 569: 641–648, 2019. doi: 10.1038/s41586-019-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Rosa GRM, Gattuso G, Pedullà E, Rapisarda E, Nicolosi D, Salmeri M. Association of oral dysbiosis with oral cancer development. Oncol Lett 19: 3045–3058, 2020. doi: 10.3892/ol.2020.11441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 449: 804–810, 2007. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol 14: 573–584, 2017. doi: 10.1038/nrgastro.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elinav E, Garrett WS, Trinchieri G, Wargo J. The cancer microbiome. Nat Rev Cancer 19: 371–376, 2019. doi: 10.1038/s41568-019-0155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xavier JB, Young VB, Skufca J, Ginty F, Testerman T, Pearson AT, Macklin P, Mitchell A, Shmulevich I, Xie L, Caporaso JG, Crandall KA, Simone NL, Godoy-Vitorino F, Griffin TJ, Whiteson KL, Gustafson HH, Slade DJ, Schmidt TM, Walther-Antonio MRS, Korem T, Webb-Robertson BM, Styczynski MP, Johnson WE, Jobin C, Ridlon JM, Koh AY, Yu M, Kelly L, Wargo JA. The cancer microbiome: distinguishing direct and indirect effects requires a systemic view. Trends Cancer 6: 192–204, 2020. doi: 10.1016/j.trecan.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo SU, Kweon MN. Virome-host interactions in intestinal health and disease. Curr Opin Virol 37: 63–71, 2019. doi: 10.1016/j.coviro.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Rickinson AB. Co-infections, inflammation and oncogenesis: future directions for EBV research. Semin Cancer Biol 26: 99–115, 2014. doi: 10.1016/j.semcancer.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Iizasa H, Ishihara S, Richardo T, Kanehiro Y, Yoshiyama H. Dysbiotic infection in the stomach. World J Gastroenterol 21: 11450–11457, 2015. doi: 10.3748/wjg.v21.i40.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iliev ID, Leonardi I. Fungal dysbiosis: immunity and interactions at mucosal barriers. Nat Rev Immunol 17: 635–646, 2017. doi: 10.1038/nri.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai GC, Tan TG, Pavelka N. The mammalian mycobiome: A complex system in a dynamic relationship with the host. Wiley Interdiscip Rev Syst Biol Med 11: e1438, 2019. doi: 10.1002/wsbm.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, Gillevet PM. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog 6: e1000713, 2010. doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallen-Adams HE, Suhr MJ. Fungi in the healthy human gastrointestinal tract. Virulence 8: 352–358, 2017. doi: 10.1080/21505594.2016.1247140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann C, Dollive S, Grunberg S, Chen J, Li H, Wu GD, Lewis JD, Bushman FD. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS One 8: e66019, 2013. doi: 10.1371/journal.pone.0066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nash AK, Auchtung TA, Wong MC, Smith DP, Gesell JR, Ross MC, Stewart CJ, Metcalf GA, Muzny DM, Gibbs RA, Ajami NJ, Petrosino JF. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 5: 153, 2017. doi: 10.1186/s40168-017-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leal-Lopes C, Velloso FJ, Campopiano JC, Sogayar MC, Correa RG. Roles of commensal microbiota in pancreas homeostasis and pancreatic pathologies. J Diabetes Res 2015: 284680, 2015. doi: 10.1155/2015/284680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salazar F, Brown GD. Antifungal innate immunity: a perspective from the last 10 years. J Innate Immun 10: 373–397, 2018. doi: 10.1159/000488539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gouba N, Hien Y, Guissou M-L, Mbogning FM, Traoré Y, Tarnagda Z. Digestive tract mycobiota and microbiota and the effects on the immune system. Hum Microbiome J 12: 100056.2019. doi: 10.1016/S1473-3099(21)00397-2. [DOI] [Google Scholar]
- 25.Saunte DML, Gaitanis G, Hay RJ. Malassezia-associated skin diseases, the use of diagnostics and treatment. Front Cell Infect Microbiol 10: 112, 2020. doi: 10.3389/fcimb.2020.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galloway-Peña JR, Kontoyiannis DP. The gut mycobiome: The overlooked constituent of clinical outcomes and treatment complications in patients with cancer and other immunosuppressive conditions. PLoS Pathog 16: e1008353, 2020. doi: 10.1371/journal.ppat.1008353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berstad A, Hauso O, Berstad K, Berstad JER. From IBS to ME - the dysbiotic march hypothesis. Med Hypotheses 140: 109648, 2020. doi: 10.1016/j.mehy.2020.109648. [DOI] [PubMed] [Google Scholar]
- 28.Botschuijver S, Roeselers G, Levin E, Jonkers DM, Welting O, Heinsbroek SEM, de Weerd HH, Boekhout T, Fornai M, Masclee AA, Schuren FHJ, de Jonge WJ, Seppen J, van den Wijngaard RM. Intestinal fungal dysbiosis is associated with visceral hypersensitivity in patients with irritable bowel syndrome and rats. Gastroenterology 153: 1026–1039, 2017. doi: 10.1053/j.gastro.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Hong G, Li Y, Yang M, Li G, Qian W, Xiong H, Bai T, Song J, Zhang L, Hou X. Gut fungal dysbiosis and altered bacterial-fungal interaction in patients with diarrhea-predominant irritable bowel syndrome: an explorative study. Neurogastroenterol Motil 32: e13891, 2020. doi: 10.1111/nmo.13891. [DOI] [PubMed] [Google Scholar]
- 30.Sciavilla P, Strati F, Di Paola M, Modesto M, Vitali F, Cavalieri D, Prati GM, Di Vito M, Aragona G, De Filippo C, Mattarelli P. Gut microbiota profiles and characterization of cultivable fungal isolates in IBS patients. Appl Microbiol Biotechnol 105: 3277–3288, 2021. doi: 10.1007/s00253-021-11264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sendid B, Salvetat N, Sarter H, Loridant S, Cunisse C, Francois N, Aijjou R, Gelé P, Leroy J, Deplanque D, Jawhara S, Weissmann D, Desreumaux P, Gower-Rousseau C, Colombel JF, Poulain D. A pilot clinical study on post-operative recurrence provides biological clues for a role of candida yeasts and fluconazole in Crohn's disease. J Fungi (Basel) 7: 324, 2021. doi: 10.3390/jof7050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fritsch J, Abreu MT. Candida in IBD: friend or foe? Cell Host Microbe 27: 689–691, 2020. doi: 10.1016/j.chom.2020.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Zhong M, Xiong Y, Zhao J, Gao Z, Ma J, Wu Z, Song Y, Hong X. Candida albicans disorder is associated with gastric carcinogenesis. Theranostics 11: 4945–4956, 2021. doi: 10.7150/thno.55209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Y, Shi T, Lu X, Xu Z, Qu J, Zhang Z, Shi G, Shen S, Hou Y, Chen Y, Wang T. Fungal-induced glycolysis in macrophages promotes colon cancer by enhancing innate lymphoid cell secretion of IL-22. EMBO J 40: e105320, 2021. doi: 10.15252/embj.2020105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell 33: 570–580, 2018. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitamoto S, Nagao-Kitamoto H, Jiao Y, Gillilland MG 3rd, Hayashi A, Imai J, Sugihara K, Miyoshi M, Brazil JC, Kuffa P, Hill BD, Rizvi SM, Wen F, Bishu S, Inohara N, Eaton KA, Nusrat A, Lei YL, Giannobile WV, Kamada N. The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell 182: 447–462.e14, 2020. doi: 10.1016/j.cell.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rumba R, Cipkina S, Cukure F, Vanags A. Systemic and local inflammation in colorectal cancer. Acta Med Litu 25: 185–196, 2018. doi: 10.6001/actamedica.v25i4.3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, Longman RS, Honda K, Littman DR, Choi GB, Huh JR. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549: 528–532, 2017. doi: 10.1038/nature23910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell 167: 1469–1480.e12, 2016. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol 15: 397–411, 2018[Erratum inNat Rev Gastroenterol Hepatol15: 785, 2018]. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pagliari D, Saviano A, Newton EE, Serricchio ML, Dal Lago AA, Gasbarrini A, Cianci R. Gut microbiota-immune system crosstalk and pancreatic disorders. Mediators Inflamm 2018: 7946431, 2018. doi: 10.1155/2018/7946431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CDC. Antibiotic resistance threats in the United States. Atlanta, GA: US Dept of Health and Human Services CDC, 2019. [Google Scholar]
- 43.White TC, Findley K, Dawson TL Jr., Scheynius A, Boekhout T, Cuomo CA, Xu J, Saunders CW. Fungi on the skin: dermatophytes and Malassezia. Cold Spring Harb Perspect Med 4: a019802, 2014. doi: 10.1101/cshperspect.a019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garber G. An overview of fungal infections. Drugs 61Suppl1: 1–12, 2001. doi: 10.2165/00003495-200161001-00001. [DOI] [PubMed] [Google Scholar]
- 45.Wirth F, Goldani LZ. Epidemiology of Rhodotorula: an emerging pathogen. Interdiscip Perspect Infect Dis 2012: 465717, 2012. doi: 10.1155/2012/465717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coker OO, Nakatsu G, Dai RZ, Wu WKK, Wong SH, Ng SC, Chan FKL, Sung JJY, Yu J. Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut 68: 654–662, 2019. doi: 10.1136/gutjnl-2018-317178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang T, Fan C, Yao A, Xu X, Zheng G, You Y, Jiang C, Zhao X, Hou Y, Hung MC, Lin X. The adaptor protein CARD9 protects against colon cancer by restricting mycobiota-mediated expansion of myeloid-derived suppressor cells. Immunity 49: 504–514.e4, 2018. doi: 10.1016/j.immuni.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev 25: 106–141, 2012. doi: 10.1128/CMR.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Theelen B, Cafarchia C, Gaitanis G, Bassukas ID, Boekhout T, Dawson TL Jr.. Malassezia ecology, pathophysiology, and treatment. Med Mycol 56: S10–S25, 2018[Erratum inMed Mycol57: e2, 2019]. doi: 10.1093/mmy/myx134. [DOI] [PubMed] [Google Scholar]
- 50.Tiew PY, Mac Aogain M, Ali NABM, Thng KX, Goh K, Lau KJX, Chotirmall SH. The mycobiome in health and disease: emerging concepts, methodologies and challenges. Mycopathologia 185: 207–231, 2020. doi: 10.1007/s11046-019-00413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janbon G, Quintin J, Lanternier F, d'Enfert C. Studying fungal pathogens of humans and fungal infections: fungal diversity and diversity of approaches. Genes Immun 20: 403–414, 2019. doi: 10.1038/s41435-019-0071-2. [DOI] [PubMed] [Google Scholar]
- 52.Underhill DM, Pearlman E. Immune interactions with pathogenic and commensal fungi: a two-way street. Immunity 43: 845–858, 2015. doi: 10.1016/j.immuni.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vautier S, MacCallum DM, Brown GD. C-type lectin receptors and cytokines in fungal immunity. Cytokine 58: 89–99, 2012. doi: 10.1016/j.cyto.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 54.Szentesi A, Párniczky Á, Vincze A, Bajor J, Gódi S, Sarlós P, Gede N, Izbéki F, Halász A, Marta K, Dobszai D, Török I, Farkas H, Papp M, Varga M, Hamvas J, Novák J, Mickevicius A, Maldonado ER, Sallinen V, Illés D, Kui B, Erőss B, Czakó L, Takács T, Hegyi P. Multiple hits in acute pancreatitis: components of metabolic syndrome synergize each other's deteriorating effects. Front Physiol 10: 1202, 2019. doi: 10.3389/fphys.2019.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Majumder S, Philip NA, Takahashi N, Levy MJ, Singh VP, Chari ST. Fatty pancreas: should we be concerned? Pancreas 46: 1251–1258, 2017. doi: 10.1097/MPA.0000000000000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi M, Hori M, Ishigamori R, Mutoh M, Imai T, Nakagama H. Fatty pancreas: A possible risk factor for pancreatic cancer in animals and humans. Cancer Sci 109: 3013–3023, 2018. doi: 10.1111/cas.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu G, Zhao H, Li C, Rajapakse MP, Wong WC, Xu J, Saunders CW, Reeder NL, Reilman RA, Scheynius A, Sun S, Billmyre BR, Li W, Averette AF, Mieczkowski P, Heitman J, Theelen B, Schröder MS, De Sessions PF, Butler G, Maurer-Stroh S, Boekhout T, Nagarajan N, Dawson TL Jr.. Genus-wide comparative genomics of Malassezia delineates its phylogeny, physiology, and niche adaptation on human skin. PLoS Genet 11: e1005614, 2015. doi: 10.1371/journal.pgen.1005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quintana FJ. The aryl hydrocarbon receptor: a molecular pathway for the environmental control of the immune response. Immunology 138: 183–189, 2013. doi: 10.1111/imm.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yin XF, Chen J, Mao W, Wang YH, Chen MH. Downregulation of aryl hydrocarbon receptor expression decreases gastric cancer cell growth and invasion. Oncol Rep 30: 364–370, 2013. doi: 10.3892/or.2013.2410. [DOI] [PubMed] [Google Scholar]
- 60.Masoudi S, Hassanzadeh Nemati A, Fazli HR, Beygi S, Moradzadeh M, Pourshams A, Mohamadkhani A. An increased level of aryl hydrocarbon receptor in patients with pancreatic cancer. Middle East J Dig Dis 11: 38–44, 2018. doi: 10.15171/mejdd.2018.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li S, Bostick JW, Zhou L. Regulation of innate lymphoid cells by aryl hydrocarbon receptor. Front Immunol 8: 1909, 2018. doi: 10.3389/fimmu.2017.01909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moura-Alves P, Faé K, Houthuys E, Dorhoi A, Kreuchwig A, Furkert J, Barison N, Diehl A, Munder A, Constant P, Skrahina T, Guhlich-Bornhof U, Klemm M, Koehler A-B, Bandermann S, Goosmann C, Mollenkopf H-J, Hurwitz R, Brinkmann V, Fillatreau S, Daffe M, Tümmler B, Kolbe M, Oschkinat H, Krause G, Kaufmann SHE. AhR sensing of bacterial pigments regulates antibacterial defence. Nature 512: 387–392, 2014. doi: 10.1038/nature13684. [DOI] [PubMed] [Google Scholar]
- 63.Mitsuhashi K, Nosho K, Sukawa Y, Matsunaga Y, Ito M, Kurihara H, Kanno S, Igarashi H, Naito T, Adachi Y, Tachibana M, Tanuma T, Maguchi H, Shinohara T, Hasegawa T, Imamura M, Kimura Y, Hirata K, Maruyama R, Suzuki H, Imai K, Yamamoto H, Shinomura Y. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 6: 7209–7220, 2015. doi: 10.18632/oncotarget.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amieva M, Peek RM. Jr.. Pathobiology of helicobacter pylori-induced gastric cancer. Gastroenterology 150: 64–78, 2016.doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett 345: 196–202, 2014. doi: 10.1016/j.canlet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 66.Gnanasekaran J, Binder Gallimidi A, Saba E, Pandi K, Eli Berchoer L, Hermano E, Angabo S, Makkawi HA, Khashan A, Daoud A, Elkin M, Nussbaum G. Intracellular porphyromonas gingivalis promotes the tumorigenic behavior of pancreatic carcinoma cells. Cancers (Basel) 12: 2331, 2020. doi: 10.3390/cancers12082331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gholizadeh P, Eslami H, Yousefi M, Asgharzadeh M, Aghazadeh M, Kafil HS. Role of oral microbiome on oral cancers, a review. Biomed Pharmacother 84: 552–558, 2016. doi: 10.1016/j.biopha.2016.09.082. [DOI] [PubMed] [Google Scholar]
- 68.Banerjee S, Tian T, Wei Z, Shih N, Feldman MD, Alwine JC, Coukos G, Robertson ES. The ovarian cancer oncobiome. Oncotarget 8: 36225–36245, 2017. doi: 10.18632/oncotarget.16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xuan C, Shamonki JM, Chung A, Dinome ML, Chung M, Sieling PA, Lee DJ. Microbial dysbiosis is associated with human breast cancer. PLoS One 9: e83744, 2014. doi: 10.1371/journal.pone.0083744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richard ML, Liguori G, Lamas B, Brandi G, da Costa G, Hoffmann TW, Pierluigi Di Simone M, Calabrese C, Poggioli G, Langella P, Campieri M, Sokol H. Mucosa-associated microbiota dysbiosis in colitis associated cancer. Gut Microbes 9: 131–142, 2018. doi: 10.1080/19490976.2017.1379637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haghi F, Goli E, Mirzaei B, Zeighami H. The association between fecal enterotoxigenic B. fragilis with colorectal cancer. BMC Cancer 19: 879, 2019. doi: 10.1186/s12885-019-6115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siret C, Collignon A, Silvy F, Robert S, Cheyrol T, Andre P, Rigot V, Iovanna J, van de Pavert S, Lombardo D, Mas E, Martirosyan A. Deciphering the crosstalk between myeloid-derived suppressor cells and regulatory t cells in pancreatic ductal adenocarcinoma. Front Immunol 10: 3070, 2019. doi: 10.3389/fimmu.2019.03070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson BA 3rd, Yarchoan M, Lee V, Laheru DA, Jaffee EM. Strategies for increasing pancreatic tumor immunogenicity. Clin Cancer Res 23: 1656–1669, 2017. doi: 10.1158/1078-0432.CCR-16-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu X, Xu J, Zhang B, Liu J, Liang C, Meng Q, Hua J, Yu X, Shi S. The reciprocal regulation between host tissue and immune cells in pancreatic ductal adenocarcinoma: new insights and therapeutic implications. Mol Cancer 18: 184, 2019. doi: 10.1186/s12943-019-1117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS, Sivajothi S, Armstrong TD, Engle DD, Yu KH, Hao Y, Wolfgang CL, Park Y, Preall J, Jaffee EM, Califano A, Robson P, Tuveson DA. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov 9: 1102–1123, 2019. doi: 10.1158/2159-8290.CD-19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther 6: 1186–1197, 2007. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 77.Aykut B, Chen R, Kim JI, Wu D, Shadaloey SAA, Abengozar R, Preiss P, Saxena A, Pushalkar S, Leinwand J, Diskin B, Wang W, Werba G, Berman M, Lee SKB, Khodadadi-Jamayran A, Saxena D, Coetzee WA, Miller G. Targeting Piezo1 unleashes innate immunity against cancer and infectious disease. Sci Immunol 5: eabb5168, 2020. doi: 10.1126/sciimmunol.abb5168. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen D-HT, Lee E, Alimperti S, Norgard RJ, Wong A, Lee JJ, Eyckmans J, Stanger BZ, Chen CS. A biomimetic pancreatic cancer on-chip reveals endothelial ablation via ALK7 signaling. Sci Adv 5: eaav6789, 2019. doi: 10.1126/sciadv.aav6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol 17: 527–540, 2020. doi: 10.1038/s41571-020-0363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers 2: 16022, 2016. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 81.Ariston Gabriel AN, Jiao Q, Yvette U, Yang X, Al-Ameri SA, Du L, Wang YS, Wang C. Differences between KC and KPC pancreatic ductal adenocarcinoma mice models, in terms of their modeling biology and their clinical relevance. Pancreatology 20: 79–88, 2020. doi: 10.1016/j.pan.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 82.Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology 144: 1230–1240, 2013. doi: 10.1053/j.gastro.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kabacaoglu D, Ciecielski KJ, Ruess DA, Algül H. Immune checkpoint inhibition for pancreatic ductal adenocarcinoma: current limitations and future options. Front Immunol 9: 1878, 2018. doi: 10.3389/fimmu.2018.01878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Foley K, Kim V, Jaffee E, Zheng L. Current progress in immunotherapy for pancreatic cancer. Cancer Lett 381: 244–251, 2016. doi: 10.1016/j.canlet.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koido S, Homma S, Takahara A, Namiki Y, Tsukinaga S, Mitobe J, Odahara S, Yukawa T, Matsudaira H, Nagatsuma K, Uchiyama K, Satoh K, Ito M, Komita H, Arakawa H, Ohkusa T, Gong J, Tajiri H. Current immunotherapeutic approaches in pancreatic cancer. Clin Dev Immunol 2011: 267539, 2011. doi: 10.1155/2011/267539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rambert J, Mamani-Matsuda M, Moynet D, Dubus P, Desplat V, Kauss T, Dehais J, Schaeverbeke T, Ezzedine K, Malvy D, Vincendeau P, Mossalayi MD. Molecular blocking of CD23 supports its role in the pathogenesis of arthritis. PLoS One 4: e4834, 2009. doi: 10.1371/journal.pone.0004834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hegde S, Krisnawan VE, Herzog BH, Zuo C, Breden MA, Knolhoff BL, Hogg GD, Tang JP, Baer JM, Mpoy C, Lee KB, Alexander KA, Rogers BE, Murphy KM, Hawkins WG, Fields RC, DeSelm CJ, Schwarz JK, DeNardo DG. Dendritic cell paucity leads to dysfunctional immune surveillance in pancreatic cancer. Cancer Cell 37: 289–307.e9, 2020. doi: 10.1016/j.ccell.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Behrens D, Walther W, Fichtner I. Pancreatic cancer models for translational research. Pharmacol Ther 173: 146–158, 2017. doi: 10.1016/j.pharmthera.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 89.Fitzgibbon G, Mills KHG. The microbiota and immune-mediated diseases: Opportunities for therapeutic intervention. Eur J Immunol 50: 326–337, 2020. doi: 10.1002/eji.201948322. [DOI] [PubMed] [Google Scholar]
- 90.Inamura K. Gut microbiota contributes towards immunomodulation against cancer: new frontiers in precision cancer therapeutics. Semin Cancer Biol 70: 11–23, 2021. doi: 10.1016/j.semcancer.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 91.Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, Scheet P, Xu H, Hanash SM, Feng L, Burks JK, Do KA, Peterson CB, Nejman D, Tzeng CD, Kim MP, Sears CL, Ajami N, Petrosino J, Wood LD, Maitra A, Straussman R, Katz M, White JR, Jenq R, Wargo J, McAllister F. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178: 795–806.e 12, 2019. doi: 10.1016/j.cell.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357: 1156–1160, 2017. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368: 973–980, 2020. doi: 10.1126/science.aay9189. [DOI] [PMC free article] [PubMed] [Google Scholar]





