Abstract
Alcohol consumption is mediated by several important neuromodulatory systems, including the endocannabinoid and neuropeptide Y (NPY) systems in the limbic brain circuitry. However, molecular mechanisms through which cannabinoid-1 (CB1) receptors regulate alcohol consumption are still unclear. Here, we investigated the role of the CB1 receptor-mediated downstream regulation of NPY via epigenetic mechanisms in the amygdala. Alcohol drinking behavior was measured in adult male C57BL/6J mice treated with a CB1 receptor neutral antagonist AM4113 using a two-bottle choice paradigm while anxiety-like behavior was assessed in the light-dark box (LDB) test. The CB1 receptor-mediated changes in the protein levels of phosphorylated cAMP-responsive element binding protein (pCREB), CREB binding protein (CBP), H3K9ac, H3K14ac and NPY, and the mRNA levels of Creb1, Cbp, and Npy were measured in amygdaloid brain structures. Npy-specific changes in the levels of acetylated histone (H3K9/14ac) and CBP in the amygdala were also measured. We found that the pharmacological blockade of CB1 receptors with AM4113 reduced alcohol consumption and, in an ethanol-naïve cohort, reduced anxiety-like behavior in the LDB test. Treatment with AM4113 also increased the mRNA levels of Creb1 and Cbp in the amygdala as well as the protein levels of pCREB, CBP, H3K9ac and H3K14ac in the central and medial nucleus of amygdala, but not in the basolateral amygdala. Additionally, AM4113 treatment increased occupancy of CBP and H3K9/14ac at the Npy gene promoter, leading to an increase in both mRNA and protein levels of NPY in the amygdala. These novel findings suggest that CB1 receptor-mediated CREB signaling plays an important role in the modulation of NPY function through an epigenetic mechanism and further support the potential use of CB1 receptor neutral antagonists for the treatment of alcohol use disorder.
Keywords: CB1 receptor, NPY, Histone acetylation, CREB, Anxiety, Alcohol intake, AM4113, Amygdala
1. INTRODUCTION
The cyclic three stages (the binge/intoxication stage, the withdrawal/negative affect stage, and the preoccupation/anticipation or “craving” stage) of alcohol use disorder (AUD) are associated with adaptive changes in various neurocircuits, including the extended amygdala (Koob, 2003; Koob and Mason, 2016). Acute and chronic alcohol exposure has been shown to dysregulate several biological processes including epigenetic mechanisms in the amygdala (Pandey et al., 2017). This occurs particularly in the central nucleus of the amygdala (CeA), which is hypothesized to control the negative affective states experienced during alcohol abstinence, also known as the ‘dark side’ of alcohol addiction (Koob et al., 2014; Koob and Mason, 2016; Kyzar and Pandey, 2015; Pandey et al., 2017).
The endocannabinoid system, a key modulator of synaptic function, serves as a potential biological target for neurological and psychiatric disorders, including AUD (Castellano et al., 2003; Manzanares et al., 2018; Pava and Woodward, 2012; Piomelli, 2003; Vinod and Hungund, 2006). Several studies have shown that recruitment of the endocannabinoid system occurs in the specific limbic brain circuitry that regulates alcohol drinking as well as reinforcement (Manzanares et al., 2018; Pava and Woodward, 2012; Parsons and Hurd, 2015; Varodayan et al., 2016; Vinod and Hungund, 2006). Alcohol exposure has also been shown to dysregulate the central endocannabinoid system (Gonzalez et al., 2002; Mitrirattanakul et al., 2007, Pava and Woodward, 2012; Vinod et al., 2006, 2010, 2012). Moreover, several studies have shown that CB1 receptor antagonists reduce voluntary alcohol consumption whereas activation of CB1 receptor promotes alcohol drinking in rodent models (Blednov et al., 2007; Colombo et al., 2005; Lopez-Moreno et al., 2004; Pava and Woodward, 2012; Vinod et al., 2008a, 2008b). Relapse to alcohol drinking in rats can also be prevented by treatment with the CB1 receptor antagonist SR141716A (rimonabant) (Cippitelli et al., 2005; Maccioni et al., 2010). However, clinical use of rimonabant has been discontinued due to the resulting increased risk of anxiety and depression (Gueye et al., 2016; Kirilly et al., 2012), which may be due to its inverse agonistic property, extended half-life, and activity at other neuronal targets. Recent studies seem to support the potential utility of the CB1 receptor neutral antagonist AM4113 for treating tobacco, heroin, and cannabis dependence as well as binge alcohol drinking without producing anxiety- or depression-like behaviors in animal models (Balla et al., 2018; Gueye et al., 2016; He et al., 2018; Schindler et al., 2016).
The CB1 receptors are one of the most abundant neuromodulatory G-protein coupled receptors in the mammalian brain including in the amygdala and they are negatively coupled to adenylyl cyclase (AC) through Gi protein (Castillo et., 2012; Howlett, 1998). The gene transcription factor cAMP-responsive element-binding protein (CREB) is the common convergence target of signaling cascades. It is regulated via phosphorylation at serine 133 by cAMP-dependent protein kinase A (PKA), Ca2+/calmodulin-dependent protein kinases II and IV, and mitogen-activated protein kinase (Impey et al., 1999; Pandey, 2004; Silva et al., 1998; Soderling et al., 1999). Phosphorylated CREB (pCREB) then regulates the expression of cAMP-inducible genes, such as Npy, by interacting with chromatin (Lonze and Ginty, 2002; Mayr et al., 2001; McClung and Nestler, 2003; Pandey et al., 2017). Previous studies have shown an association between deficiency in CREB function and its related Npy gene in the CeA with anxiety-like and alcohol drinking behaviors in rodent models (Pandey et al., 2004, 2005; Zhang et al., 2010). It has been shown that pCREB recruits CREB binding protein (CBP) and p300 transcriptional regulators to chromatin for the modulation of histone acetylation and gene expression (Bannister and Kouzarides, 1996; Barrett et al., 2011; Berkel and Pandey, 2017; Kouzarides, 2007; Verdone et al., 2005;). We recently showed that changes in chromatin dynamics via the acetylation of histones H3K9/14 (H3K9/14ac) at the Npy promoter modulate its expression in the amygdala, and that deficits in NPY expression in the CeA and the medial nucleus of the amygdala (MeA) promote higher alcohol intake and anxiety-like behaviors in rodent models of AUD (Kokare et al., 2017; Pandey et al., 2015; Sakharkar et al., 2014).
Although all of these studies have demonstrated the importance of the CB1 receptor and CREB related target gene, Npy, in alcohol-related behaviors, the molecular mechanisms through which endocannabinoid and NPY systems interact in the amygdala and regulate alcohol drinking remain unknown. We hypothesized that CB1 receptors have the ability to regulate chromatin remodeling via CREB/CBP signaling, leading to the regulation of NPY expression as well as alcohol drinking and anxiety-like behaviors. This study was designed to test the above hypothesis by examining the effect of the CB1 receptor neutral antagonist AM4113 on alcohol consumption and anxiety-like behavior, and the underlying epigenetic mechanisms of the interaction between CB1 receptors and NPY in the amygdala using an alcohol preferring mouse model.
2. MATERIALS AND METHODS
2.1. Animals
All of the experiments were conducted in adult male C57BL/6J mice (10-14 weeks old; Jackson Laboratory, USA) with body weights ranging from 24 to 30g at the time of the experiment. Mice were given unlimited access to water and standard mouse chow during the entire study. All procedures were conducted in accordance with the National Institutes of Health and Institutional Animal Care and Use Committees’ guidelines.
2.2. CB1 Neutral Antagonist Treatment
All mice in this study were injected intraperitoneally with AM4113 (1 mg/kg body weight) or vehicle (4% DMSO and 1% Tween-80 in saline) once daily for 3 days. This dose is based on our recent study on the pharmacokinetics of drug distribution and elimination rate in brain and blood in mice (Balla et al., 2018). For drinking and immunohistochemistry experiments, AM4113 was kindly provided by Drs. Alexandros Makriyannis and Kiran Vemuri of Northeastern University. For follow-up experiments on immunohistochemistry, biochemistry, and anxiety-like behavior, AM4113 was purchased from Cayman Chemical (No. 20581, Ann Arbor, MI). The drug and vehicle solutions were freshly prepared each day just before administration.
2.3. Alcohol Consumption Study
Mice were housed individually and habituated to a reverse light-dark cycle for a week prior to the test. Alcohol drinking behavior was measured using a two-bottle choice daily drinking paradigm following the acclimatization of mice to water and escalating concentrations of alcohol [3% (3 days), 6% (3 days), and 10% (6 days)] as previously described (Vinod et al., 2008a, 2008b). Animals were assigned to vehicle and AM4113 groups after alcohol habituation. They were then injected intraperitoneally with AM4113 (1 mg/kg) or vehicle (Balla et al., 2018) once daily for an additional 3 days. After 40 minutes of drug or vehicle treatment, mice were offered fresh bottles, one containing water and other containing 10% alcohol daily, and during this time consumption of alcohol and water of the previous day was also measured. The positions of the bottles were alternated each day to avoid side preference. Data were corrected for the loss of liquid due to spillage and evaporation by comparing weights of water and alcohol bottles in a control cage without a mouse. The mean alcohol intake is expressed as grams of alcohol/kg of body weight/day.
2.4. Anxiety-like Behavior Test
A separate cohort of ethanol-naïve mice was group-housed and habituated to regular handling and weighing for a week prior to test. Mice were injected with 1 mg/kg AM4113 or vehicle once daily for 3 days as described and tested for anxiety-like behavior twenty-four hours following the final injection. Anxiety-like behavior was assessed in the light-dark box (LDB) apparatus, as previously described by us (Dong et al., 2018; Sakharkar et al., 2014). Briefly, mice were acclimated to the anxiety testing room for 1 hr prior to being tested. Mice were tested individually and allowed to explore the LDB for 5 min. The LDB test sessions were video recorded and analyzed using ANY-maze behavioral video scoring software (Stoelting, Wood Dale, IL) which was programmed to track the center of the mouse to derive the following measurements: time spent in each chamber and entries into each chamber. The percentage of time spent in either the light or the dark compartment was then calculated for each mouse; more time exploring the light compartment is interpreted as decreased anxiety-like behavior (Sakharkar et al., 2014). We used the number of entries into the dark compartment as an index of general activity of the mouse.
2.5. Biochemical Studies
For biochemical studies, alcohol-naïve adult mice were injected with either AM4113 (1 mg/kg i.p.) or vehicle (4% DMSO and 1% Tween 80 in saline, i.p.) once daily for 3 days. Twenty-four hours after the last injections, mice were either perfused with saline followed by 4% paraformaldehyde or directly decapitated without perfusion under anesthesia (ketamine [100 mg/kg] and xylazine [10 mg/kg], i.p.) or isoflurane. Brains were collected and amygdaloid brain regions containing all major nuclei (central, medial, and basolateral amygdala) were immediately dissected out. For immunohistochemistry, brains were placed in fixative overnight at 4°C and were then cryoprotected in 10%, 20%, and 30% sucrose (in 0.1 M PBS buffer, pH 7.4). All tissues were frozen at −80°C until use.
2.6. Measurement of Protein levels using Immunohistochemistry
Gold immunolabeling procedure was employed to measure pCREB, CBP, NPY, H3K9ac, and H3K14ac protein levels in the amygdaloid brain structures as previously described by us (Pandey et al., 2004, 2008; Sakharkar et al., 2014). The bregma-matched coronal sections (20 μm-thick) were used and incubated in RPMI 1640 medium containing l-glutamine (Life Technologies, Grand Island, NY) for 30 minutes. Sections were incubated with 10% normal goat serum in PBS containing 0.25% Triton X-100 (PBST) for 30 minutes at room temperature. The sections were then incubated with antibodies against pCREB (1:500 dilution; 06-519, Millipore, Billerica, MA), CBP (1:200 dilution; sc-7300, Santa Cruz Biotechnology, Santa Cruz, CA), NPY (1:500 dilution; 22940, Immunostar, Hudson, WI), H3K9ac (06-942) and H3K14ac (06-911) (1:500 dilution; Millipore, Billerica, MA) in PBST containing 1% BSA for 18 hours at room temperature. Sections were then washed with PBS containing 1% BSA and incubated with gold particle (1.4 nm)-conjugated anti-rabbit secondary antibody (1:200 dilution in PBS containing 1% BSA; Nanoprobes, Inc., Yaphank, NY) for 1 hour at room temperature. The sections were washed with PBS containing 1% BSA and with distilled water. Gold particles were then silver enhanced (Ted Pella Inc., Redding, CA) followed by washing with water. Immunogold particles were visualized and counted using the Image Analyzer program (Loats Associates, Westminster, MD). The threshold for non-immunostained areas in the amygdalar region was set to zero. After threshold determination, the software counted the numbers of immuno-gold particles per 100 μm2 brain area at higher magnification (100X). Three object areas of defined amygdaloid nuclei (CeA, MeA and the basolateral nucleus of the amygdala [BLA]) from each of the three brain sections for each animal were counted, and the values were averaged. Results are represented as number of immunogold particles per 100 μm2 brain area.
2.7. Chromatin Immunoprecipitation Assay
The occupancy of CBP and acetylated histone (H3K9/14ac) at the Npy gene promoter was measured in the amgydala using the chromatin immunoprecipitation (ChIP) assay as previously described by us (Kyzar et al., 2017; Zhang et al., 2018). Briefly, amygdaloid tissues containing all major nuclei (CeA, MeA and BLA) were fixed in methanol free formaldehyde and subjected to DNA sonication to achieve DNA fragment size of 200-500 base pairs. The resulting DNA-chromatin complex was immunoprecipitated with 5 μg of either H3K9/14ac antibody (Millipore 06-599, Billerica, MA) or CBP antibody (Abcam 2832, Cambridge, MA) and then chromatin was eluted. DNA fragments were isolated and quantified by real-time quantitative PCR using primers designed within the promoter region of Npy gene (Set 1 F: ACTCGGGACTTCACAGGGTTTACA; R: GCGGTCCATGAGCTTCTGTATCTA; Set 2 F: TGCCCTTGCAGAGTTAGTCACAGT; R: AGACTCACCCAAATGTCCACAGCA; Set 3 F: GCGGGCCGCTTAGAATTGG; R: TTTATGGAGCGCCCTTGTCGC). Input DNA was used for the normalization as an internal control. After subtraction of the input DNA Ct value from the Ct value of each respective sample, the ΔΔCt method (Schmittgen and Livak, 2008) was used to determine the fold change in the levels of H3K9/14ac and CBP at the Npy gene promoter.
2.8. RNA Isolation and Real-Time Quantitative PCR
Total RNA was extracted from amygdala and was isolated using miRNeasy kit (Qiagen, Valencia, CA, USA). RNA was reverse transcribed using random hexamer primers and reverse transcriptase (Thermo Fisher Scientific, Waltham, MA, USA). Real-Time qPCR was performed on a CFX connect qPCR system (Bio-Rad, Hercules, CA) with SYBR Green Master Mix (Thermo Fisher Scientific) using specific primers for Npy (F: CCAGACAGAGATATGGCAAGAG; R: GGGTCTTCAAGCCTTGTTCT), Creb1 (F: TGTACCACCGGTATCCATGC; R: CCATGGACCTGGACTGTCTG), and Cbp (F: GACCTGGGATCTGCATGAAT; R: TGACTTGAGCTTGCCCTTGTT). Ct values for Npy, Creb1, and Cbp genes were normalized to the Ct value of Gapdh (F: CAATGTGTCCGTCGTGGATCT; R: GTCCTCAGTGTAGCCCAAGATG) as an internal control. Relative expression levels were calculated using the ΔΔCt method (Schmittgen and Livak 2008).
2.9. Data Analysis
The group differences in alcohol consumption were analyzed using a two-way repeated measures ANOVA followed by a Bonferroni post-hoc multiple comparison test. The effect of AM4113 on mean alcohol consumption, anxiety-like behavior and neurochemical data were evaluated by a Student t-test (two-tailed). Differences were considered statistically significant at p<0.05.
3. RESULTS
3.1. Effect of AM4113 on Alcohol Consumption and Anxiety-Like Behavior
We recently reported that AM4113 treatment decreases binge-like alcohol consumption in the limited-access drinking in the dark mouse model (Balla et al., 2018). Here, we used unlimited (24-hour) two-bottle choice paradigm to examine the effect of daily treatment of AM4113 (1 mg/kg, i.p.) on alcohol consumption for three days. A two-way repeated measures ANOVA revealed a main effect of day (F8,128=2.470; p=0.0160), a trend toward a main effect of AM4113 treatment on alcohol consumption (F1,16=3.558; p=0.0775), and a significant interaction between AM4113 treatment and day (F8,128=3.316; p=0.0018) (Fig. 1A). A Bonferroni post-hoc multiple comparison analysis showed a significant reduction in alcohol intake on day 7 (t =3.618; p=0.0037), day 8 (t=2.868; p=0.0428) and day 9 (t=2.877; p=0.0417) in AM4113 treated group compared to the vehicle treated control group. Treatment with AM4113 reduced mean daily alcohol consumption during the three treatment days compared to the vehicle treated group (t16=2.966; p=0.009) (Fig. 1B). AM4113-treated mice spent more time exploring the light compartment of the LDB compared to vehicle-treated mice (t21=2.104; p=0.0476) suggesting a small but statistically significant anxiolytic effect of AM4113 (Fig. 1C). Mice tested in the LDB do not differ in their general activity as entries to the dark compartment are not significantly different between treatment groups (Fig. 1D). These results suggest that AM4113 treatment is effective in attenuating continuous access alcohol consumption in male C57 mice and is anxiolytic in ethanol-naive mice. We then investigated the possible molecular mechanisms underlying these behavioral actions of the CB1 receptor neutral antagonist AM4113 as described below.
Figure 1.
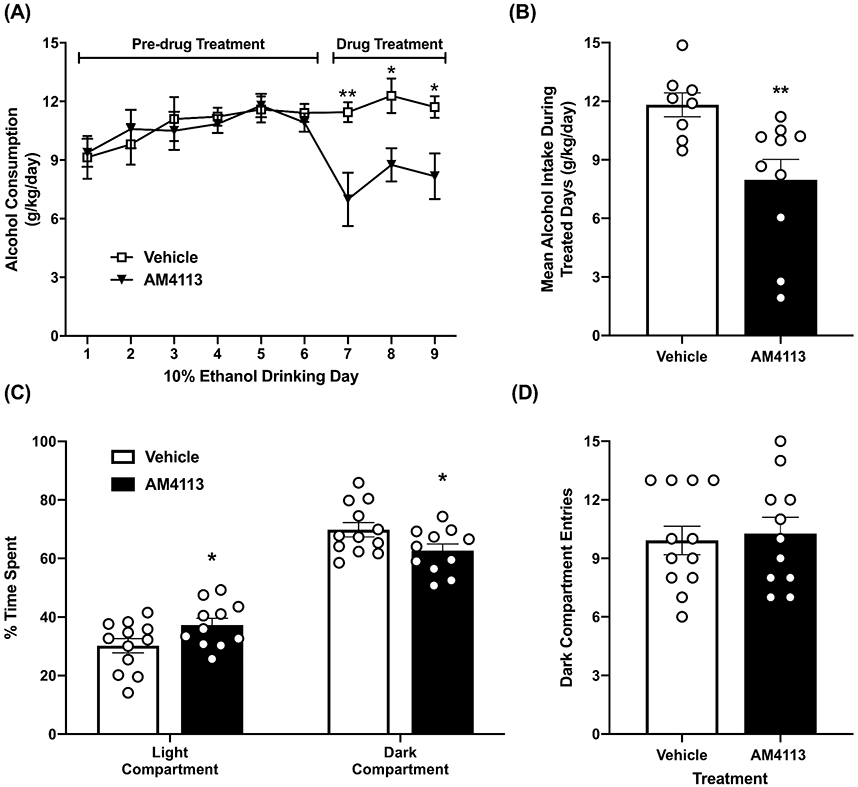
Effect of AM4113 (1 mg/kg, i.p.) on alcohol drinking and anxiety-like behaviors in mice. (A) In a two-bottle free choice paradigm, AM4113 reduced consumption of 10% ethanol compared to vehicle treated group (*p<0.05; **p<0.01). (B) Bar graph shows the effect of AM4113 on mean alcohol intake (n=8-10 in each group). (C) In the light-dark box (LDB) exploration test, AM4113 treatment increased time spent exploring the light compartment compared to vehicle treatment. (*p<0.05). (D) AM4113 had no effect on general activity as entries to the dark compartment do not differ between AM4113-treated and vehicle-treated mice (n=11-12 in each group). Overall values are represented as mean ± SEM and individual values are shown with open circles.
3.2. Effect of AM4113 on CREB Signaling in the Amygdala
Since CB1 receptors are negatively coupled to the cAMP second messenger pathway (Howlett, 1998; Pacher et al., 2006), we first examined the effect of AM4113 on protein levels of pCREB and CBP in amygdaloid brain structures. It was found that AM4113 treatment significantly increased the protein levels of pCREB in the CeA (t8=6.392, p<0.001) and MeA (t8=4.337, p=0.002) but not in the BLA as compared with the vehicle treated control group (Fig. 2A and 2B). In addition, we found a significant elevation in CBP levels in the CeA (t10=13.294, p<0.001) and MeA (t10=13.350, p<0.001) of AM4113 treated mice relative to the vehicle treated control mice (Fig. 2C and 2D). We also observed significant increases in mRNA levels of Creb1 (t11 =2.317, p=0.041) and Cbp (t11 =2.921, p=0.014) in the amygdala after AM4113 treatment (Fig. 2E). These findings indicate that neutral antagonism of the CB1 receptor activates CREB and increases CBP levels in the amygdaloid structures of mice.
Figure 2.
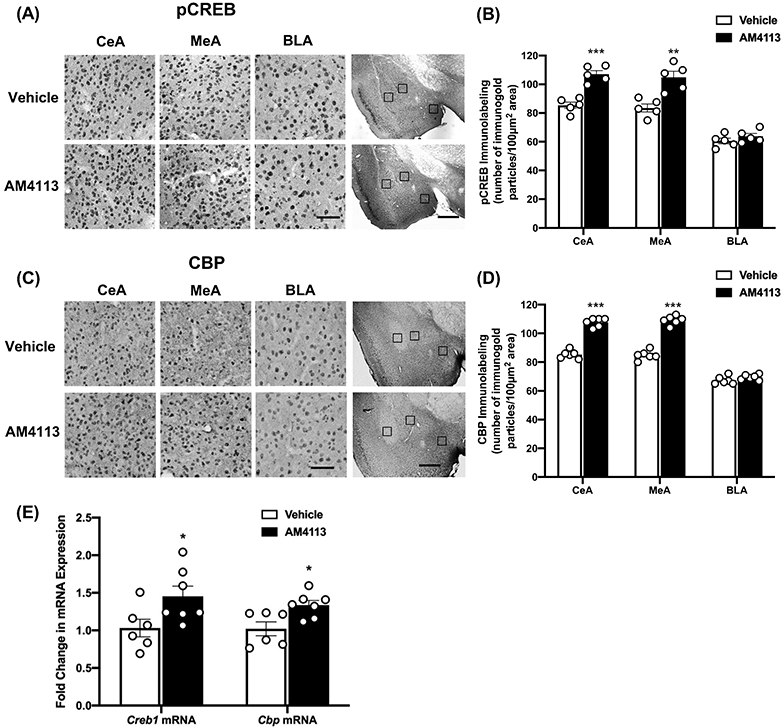
Alteration in the protein levels of phosphorylated CREB (pCREB) and CREB binding protein (CBP) and the mRNA levels of Creb1 and Cbp in amygdala of mice treated with AM4113 (1 mg/kg, i.p.) or vehicle. (A) Representative photomicrographs of high magnification (scale bar = 50 μm) show the gold immunolabeling of pCREB in the central (CeA), medial (MeA), and basolateral (BLA) nuclei of amygdala of AM4113 or vehicle treated mice. The locations of these amygdaloid areas are shown by box at low magnification (scale bar = 1mm) images of amygdala of AM4113 or vehicle treated mice. (B) Bar graph depicts pCREB protein levels in amygdaloid brain regions after AM4113 treatment compared to vehicle group (**p<0.01; ***p<0.001; n=5 in each group). (C) Representative photomicrographs of high magnification (scale bar = 50 μm) show the gold immunolabeling of CBP in CeA, MeA and BLA in mice treated with AM4113 or vehicle. The locations of these amygdaloid areas are shown by box at low magnification (scale bar = 1mm) images of amygdala of AM4113 or vehicle treated mice. (D) Bar graph depicts levels of CBP protein in CeA, MeA and BLA following AM4113 treatment compared to vehicle group (***p<0.001; n=6 in each group). (E) Bar graph depicts Creb1 and Cbp mRNA expression levels in the amygdala of mice treated with AM4113 or vehicle (*p<0.05, n=6-7 in each group). Individual values are shown with open circles and overall values are represented as mean ± SEM.
3.3. Effect of AM4113 on Global Histone Acetylation in the Amygdala
It has been shown that CBP has acetyltransferase activity that regulates gene expression by acetylation of histones (Bannister and Kouzarides,1996; Berkel and Pandey, 2017). Thus, we examined the effects of AM4113 on the status of two epigenetic marks of transcriptional activation in the amygdaloid structures: H3K9ac and H3K14c. It was found that AM4113 treatment significantly increased the protein levels of acetylated histones in the CeA (t10 =8.687, p<0.001 for H3K9ac; t10 =3.815, p=0.003 for H3K14ac) and MeA (t10 =9.743, p<0.001 for H3K9ac; t10=4.276, p=0.002 for H3K14ac) compared to the vehicle group (Fig. 3). There were no significant changes in the levels of these proteins in the BLA (Fig. 3). These results reveal that treatment with a neutral antagonist of CB1 receptors increased acetylation at two active epigenetic histone lysine marks, and further suggest that CB1 receptor modulation may impact downstream gene expression in the amygdala via global effects on the epigenome.
Figure 3.
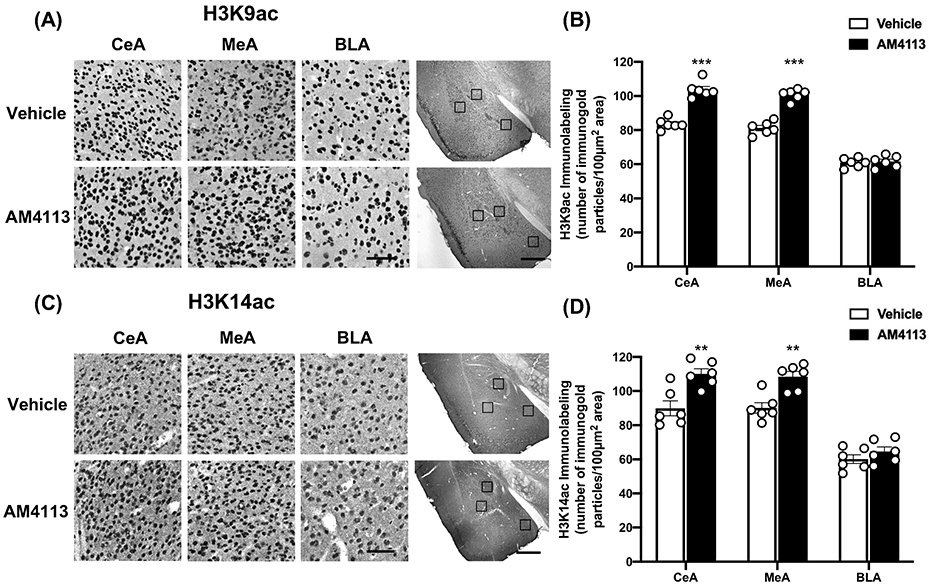
Effect of AM4113 treatment on the global protein levels of acetylated histone H3K9 (H3K9ac) and acetylated histone H3K14 (H3K14ac). (A) Representative photomicrographs of high magnification (scale bar = 50 μm) of H3K9ac gold-immunolabeling in central (CeA), medial (MeA) and basolateral (BLA) nuclei of amygdala of mice treated with either vehicle or AM4113 (1 mg/kg, i.p.). The location of these amygdaloid areas are shown by box at low magnification (scale bar = 1mm) images of amygdala of AM4113 or vehicle treated mice. (B) Bar graph shows levels of H3K9ac in CeA, MeA and BLA of AM4113 and vehicle treated mice (***p<0.001; n=6 in each group). (C) Representative photomicrographs of high magnification (scale bar = 50 μm) of H3K14ac gold-immunolabeling in CeA, MeA and BLA in mice treated with either vehicle or AM4113 (1 mg/kg). The locations of these amygdaloid areas are shown by box at low magnification (scale bar = 1mm) images of amygdala of AM4113 or vehicle treated mice. (D) Bar graph shows levels of H3K14ac in CeA, MeA and BLA following administration of AM4113 compared to vehicle group (**p<0.01; n=6 in each group). Individual values are shown with open circles and overall values are represented as mean ± SEM.
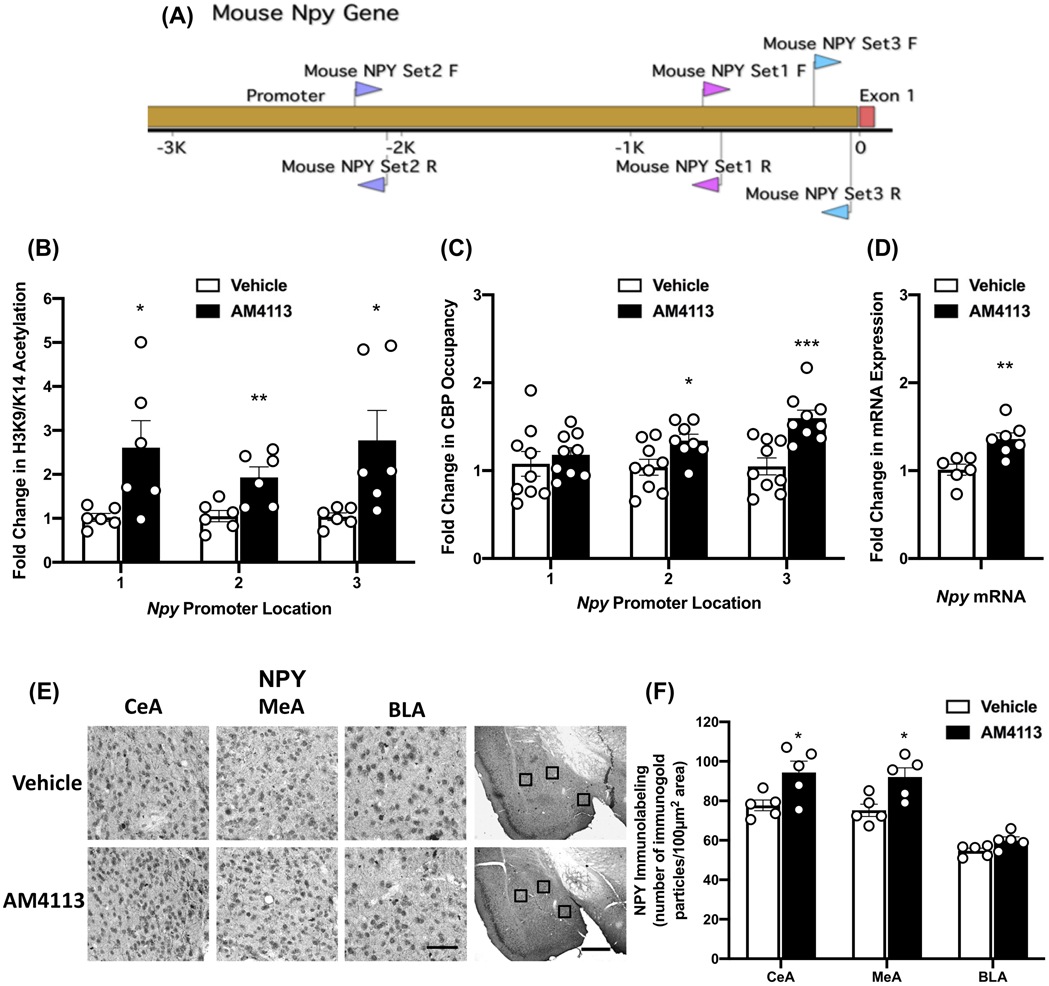
3.4. Effect of AM4113 on Occupancy of Acetylated Histone and CBP at Npy Gene Promoter and NPY Expression
NPY has been shown to regulate alcohol drinking behaviors (Pandey et al., 2003; Thiele et al., 1998; Robinson and Thiele, 2017), and higher levels of NPY in the CeA is associated with reduction in alcohol drinking and anxiety-like behaviors (Gilpin et al., 2008; Zhang et al., 2010). To determine the modulatory effects of CB1 receptor neutral antagonism on NPY expression through epigenetic mechanisms, ChIP analysis was performed using an H3K9/14ac and CBP antibodies at three locations of the Npy gene promoter in the amygdala (Fig. 4A) after AM4113 treatment. We found an elevation in H3K9/14ac levels at the Npy gene promoter location 1 (t10=2.567, p=0.0280); location 2 (t10=3.248, p=0.0088); location 3 (t10=2.528, p=0.03) compared to vehicle control group (Fig. 4B). In addition, AM4113 treatment increased occupancy of CBP at the Npy gene promoter location 2 (t15=2.589, p=0.0205) and location 3 (t16=4.193, p=0.0007) but produced no change at location 1 (t16=0.6325, p=0.5360) compared to the vehicle control group (Fig. 4C). Treatment with AM4113 also led to a significant increase in Npy mRNA levels in the amygdala as compared to the vehicle treated group (t11=3.641, p=0.0039; Fig. 4D). This increase in Npy mRNA levels is associated with an enhancement of NPY protein expression in the CeA (t8 =2.622, p=0.031) and MeA (t8=3.031, p=0.016), but not in the BLA of mice (Fig. 4E and 4F). These findings demonstrate that AM4113 treatment increases histone acetylation (H3K9/14ac) via increases in CBP at the Npy gene promoter, thereby increasing expression of Npy mRNA levels and ultimately NPY protein levels in the CeA and MeA. These molecular effects of AM4113 treatment in the amygdala may be responsible for reducing alcohol consumption and decreasing anxiety-like behavior.
Figure 4.
Effects of AM4113 treatment (1 mg/kg, i.p.) on NPY-specific changes in CBP and H3K9/14ac as well as NPY protein and mRNA expression in the amygdala. (A) Gene diagram showing three locations of primers (location 1-primer set 1; location 2-primer set 2; and location 3-primer set 3) used to measure occupancy of CBP and H3K9/14ac at NPY gene promoter in amygdala using chromatin immunoprecipitation (ChIP) assay. (B) A significant increase in the occupancy of H3K9/14ac at all three locations was observed following treatment with AM4113 compared to vehicle treated group (*p<0.05; **p<0.01; n=6 in each group). (C) A significant increase in the occupancy of CBP at locations 2 and 3 but not at location 1 of Npy promoter was observed following treatment with AM4113 compared to vehicle treated group (*p<0.05; ***p<0.001; n=8-9 in each group). (D) The measurement of Npy mRNA level revealed an increase in Npy mRNA levels in amygdala following treatment with AM4113 (**p<0.01; n=6-7 in each group). (E) Representative photomicrographs of high magnification (scale bar = 50 μm) of gold immunolabeling of NPY in central (CeA), medial (MeA), and basolateral (BLA) nuclei of amygdala of mice. The locations of these amygdaloid areas are shown by box at low magnification (scale bar = 1mm) images of amygdala of AM4113 or vehicle treated mice. (F) Bar graph shows NPY protein levels in CeA, MeA, and BLA of mice treated with AM4113 compared to vehicle treated group (*p<0.05; n=5 in each group). Individual values are shown with open circles and overall values are represented as mean ± SEM.
4. DISCUSSION
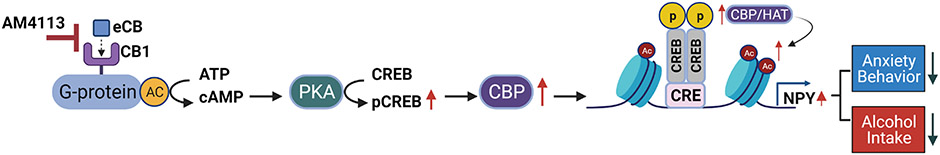
The goal of this study was to investigate the underlying molecular mechanisms of the attenuation of alcohol drinking behavior by CB1 receptor neutral antagonist AM4113. Our novel findings demonstrate that CB1 receptor blockade increases NPY expression via epigenetic mechanisms in the amygdala, which may be an important neuronal mechanism in the reduction of alcohol intake. Our data also underscore the ability of CB1 receptor signaling to modulate chromatin remodeling, and thereby regulate the expression of downstream genes. Particularly, we found that CB1 receptor blockade decreases alcohol consummatory and anxiety-like behaviors while producing increases in pCREB, CBP, global and Npy-specific histone acetylation, and NPY expression (Fig. 5).
Figure 5.
Molecular mechanism of CB1 receptor-mediated attenuation of alcohol drinking and anxiety-like behavior. The blockade of CB1 receptors with neutral CB1 antagonist AM4113 leads to an increase in cAMP mediated CREB phosphorylation as these receptors are negatively coupled to adenylate cyclase (AC) and increase the levels of CREB binding protein (CBP). Direct or indirect activation of CBP/CREB signaling increases CBP and histone acetylation globally and at the Npy gene specifically leading to increased NPY expression in the amygdala. This CB1 receptor-mediated modulation of NPY expression through epigenetic modification via histone acetylation may be one of the important molecular mechanisms in the amygdala underlying regulation of alcohol consumption and anxiety-like behavior. eCB, endocannabinoid; CB1, Cannabinoid Receptor 1; cAMP, Cyclic adenosine monophosphate; PKA, Protein kinase A; CREB, cAMP-response element binding protein; pCREB, phosphorylated CREB; CBP, CREB binding protein; CRE, cAMP response element site; NPY, Neuropeptide Y.
Amygdaloid structures serve as important neuroanatomical substrates for anxiety and alcohol drinking behaviors (McBride, 2002; Koob et al., 2014; Koob and Mason, 2016; Pandey et al., 2006). The CeA represents a critical hub for the interaction of several neuromodulators such as NPY, corticotropin-releasing factor (CRF), and CB1 receptor signaling to regulate anxiety and alcohol drinking behaviors (Roberto et al., 2010; 2012; Gilpin, 2012). Optogenetic activation of projections from BLA to CeA produces anxiolytic effects, and inhibition of these projections causes anxiety-like behaviors (Tye et al., 2011). Furthermore, CeA neurons consist of mainly GABAergic projection and interneurons, and the lateral CeA sends projections to medial CeA internally; dynamic interactions between these synaptic arrangements also regulate anxiety and fear (Roberto et al., 2012; Gilpin, 2012, Janak and Tye, 2015). NPY and GABA are colocalized together and NPY modulates GABAergic neurotransmission in the CeA during alcohol exposure (Gilpin, 2012; Gilpin et al., 2011). Interestingly, GABAergic function in the CeA is also modulated by alcohol-induced disruptions of CB1 receptors (Varodayan et al., 2016). Our study extends these important functional studies in the CeA to the molecular level to suggest that CB1 receptor antagonist (AM4113) treatment upregulates NPY expression in the CeA and MeA to regulate anxiety and alcohol drinking behaviors, most likely via an epigenetic mechanism.
Deficits in NPY expression in the CeA are associated with alcohol-related behaviors, particularly an increase in alcohol intake (Gilpin et al., 2008; Thorsell et al., 2005; 2007; Zhang et al., 2010). For example, expression of NPY in the CeA of P rats is lower than that of alcohol non-preferring (NP) rats, and infusion of NPY into the CeA has been shown to attenuate alcohol consumption in P rats (Pandey et al., 2005; Suzuki et al., 2004; Zhang et al., 2010) and notably also reduces anxiety-like behaviors (Pandey et al., 2005; Zhang et al., 2010). Viral-mediated overexpression of the Npy gene in the CeA has also been shown to suppress alcohol preference in alcohol dependent rats, as well as in anxious rats (Gilpin et al., 2008: Primeaux et al., 2006; Thorsell et al., 2005; 2007) while additionally reducing anxiety-like behavior in the openfield test (Thorsell et al., 2007). We previously showed that deficits in NPY expression in the amygdala of P rats correspond with lower histone acetylation of the Npy gene as compared with NP rats (Sakharkar et al., 2014). Interestingly, deficits in Npy mRNA and protein levels in the CeA and MeA were normalized by increasing histone acetylation after treatment with a histone deacetylase inhibitor, trichostatin A (TSA). Behaviorally, anxiety and alcohol consumption in P rats were both attenuated by TSA treatment (Sakharkar et al., 2014). Ethanol withdrawal-induced decrease of NPY levels in the CeA and MeA can also be reversed by TSA treatment, which is associated with a reduction of anxiety-like behavior during ethanol withdrawal in rats (Pandey et al., 2008; Roy and Pandey, 2002). These data suggest that histone acetylation mediated changes in NPY expression in the amygdala are crucially involved in alcohol drinking and anxiety-like behaviors and our present data further highlights the importance of these histone acetylation mechanisms driving changes in amygdaloid NPY expression and subsequent behavior.
CREB also plays an important role in the neurobiological effects of alcohol and drugs of abuse (McClung and Nestler, 2003; Pandey, 2003). For instance, a decrease in amygdalar CREB function is causally linked to reduced NPY expression and increased alcohol drinking and anxiety-like behaviors (Pandey et al., 2004, 2005). These studies suggest that NPY is a CREB target and that decreased CREB function leads to a decrease in NPY, in turn promoting alcohol intake and anxiety-like behaviors (Pandey, 2003). We report here that CB1 receptor antagonism led to a reduction in alcohol consumption and anxiety-like behavior. Furthermore, inhibition of CB1 receptor function by AM4113 resulted in increased NPY expression via increases in both global and Npy-specific CBP and histone acetylation (H3K9/14ac) in the amygdala. The CB1 receptors are expressed in amygdala and are negatively coupled to the cAMP signaling pathway (Howlett, 1998; McDonald and Mascagni, 2001; Pacher et al., 2006; Stincic and Hyson, 2008). CREB appears to be a common denominator for the interaction of both CB1 and NPY signaling. This notion is supported by the findings that CB1 antagonism leads to increases in the mRNA levels of Creb1 and Cbp and their protein levels in the amygdala. Thus, the decrease in ethanol consumption and anxiolytic effects produced by AM4113 are most likely due to the observed increase in CREB phosphorylation, as well as increased NPY expression in the CeA and MeA of mice.
The epigenetic modifications mediated by histone acetylation in the amygdaloid circuitry and other brain regions have been shown to regulate alcohol drinking behaviors (Moonat et al., 2013; Sakharkar et al., 2014; Starkman et al., 2012; Renthal et al., 2008). In the present study, we observed increased acetylation of H3K9 and H3K14 globally in the CeA and MeA by AM4113 treatment. This somewhat concurs with the results of a previous study that associated lower H3K9ac in the CeA and MeA with a phenotype of higher alcohol intake in rodent models of AUD (Kokare et al., 2017; Moonat et al., 2013; Pandey et al., 2015). To further explore the relationship between histone acetylation and NPY expression after CB1 receptor blockade, ChIP assays revealed an increase in occupancy of CBP and H3K9/14ac at multiple sites in the Npy gene promoter in the amygdala. These changes were associated with increased mRNA levels in the amygdala and protein levels of NPY in the CeA and MeA, but not in the BLA of mice. The data indicate that blocking CB1 receptors leads to enhanced phosphorylation of CREB which in turn activates CBP and leads to higher histone acetylation at the Npy gene promoter and enhancement of the transcriptional machinery of the Npy gene. Collectively, the present study clearly demonstrates enhancement of NPY expression at the transcriptional level is driven by epigenetic mechanisms after CB1 receptor blockade involving CREB-CBP mediated histone acetylation specifically in the CeA and MeA (Fig. 5).
Although CB1 receptors are highly expressed in the BLA (McDonald and Mascagni, 2001; Stincic and Hyson, 2008), their blockade did not produce any significant effect on the signaling cascade studied in this brain region. This finding seems to coincide with the results of an earlier study that showed that lesions to the CeA, but not to the BLA, caused decreased anxiety levels and alcohol intake in rats (Möller et al., 1997). The reason for this region-specific change is unclear at present, but it may be related to the existence of different cell types (Polepalli et al., 2020) and the output of presynaptic CB1-expressing cells onto postsynaptic neurons. While endocannabinoid signaling classically occurs in a retrograde manner (Castillo et al., 2012), emerging evidence suggests that interneurons can signal via an autocrine endocannabinoid mechanism (Bacci et al., 2004; Marinelli et al., 2009), and that astrocytes expressing CB1 receptors can modulate responses at more distant synapses (Navarrete and Araque, 2010). Since CB1 receptors are largely expressed presynaptically (Piomelli, 2003) and epigenetic alterations require access to DNA in the nucleus (Kouzarides, 2007), the epigenetic changes induced by CB1 receptor blockade observed in the current study may be due to autocrine CB1 receptor blockade or the cumulative effect of multiple actions of presynaptic CB1 receptors on postsynaptic signaling. Here, we report that treatment with AM4113 (1 mg/kg) produces subtle anxiolytic effects in LDB test in mice. This behavioral finding is in concordance with increased levels of NPY in the amygdala. One earlier study reported that lower (1 mg/kg) and higher doses of AM4113 (3 mg/kg or 10 mg/kg) produce no effects on anxiety-like behavior in the elevated plus maze in rats, but there is an antidepressant effect in the forced swim test at all three doses (Gueye et al., 2016). The differences in anxiety measures between the present findings and those of Gueye et al. (2016) could be related to differences in dose and duration of drug treatment as well as animal species and behavioral model. Nonetheless, these behavioral findings suggest that AM4113 treatment, unlike rimonabant (Kirilly et al., 2012), is devoid of liability to provoke anxiety or depression-like behaviors but still retains efficacy in decreasing alcohol intake in mice. The reduction of alcohol intake by CB1 neutral antagonist AM4113 treatment as observed here is consistent with the results of previous studies performed using CB1 inverse agonists (Femenia et al., 2010; Pava and Woodward, 2012; Vinod et al., 2008a; Wang et al., 2003; Zhou et al., 2016). Our recent study showed that AM4113 decreases binge-like alcohol consumption and alcohol-induced dopamine (DA) release in the nucleus accumbens (NAc) (Balla et al., 2018), which suggests that AM4113 may also regulate reward properties of alcohol drinking by blocking the release of accumbal DA. It remains to be examined whether AM4113 treatment is effective in reducing alcohol intake in female mice as well as treating alcohol dependence. Also, further study is needed to elucidate how epigenetic consequences of AM4113 treatment alter other amygdaloid gene network pathways or NPY signaling and non-neuronal immune responses in other brain regions of interest to addiction.
In summary, the present study identifies an important molecular mechanism by which CB1 receptors epigenetically regulate NPY function via histone acetylation in the amygdala. Furthermore, our findings suggest that AM4113 treatment increases CREB-CBP signaling and increases two active marks of histone acetylation both globally and specifically at the NPY promoter. Taken together, the data supports the potential use of CB1 receptor neutral antagonists for the treatment of excessive alcohol drinking.
Highlights:
CB1 receptor neutral antagonist decreases alcohol drinking and anxiety-like behavior.
CB1 receptor blockade increases CREB and CBP mRNA and protein levels in the amygdala.
CB1 receptor blockade causes chromatin remodeling by increasing histone H3K9 and H3K14 acetylation in the amygdala.
Increased CBP levels and histone acetylation at Npy gene promoter following CB1 receptor blockade is associated with increases in NPY mRNA and protein levels.
ACKNOWLEDGMENTS
We greatly appreciate Drs. Alexandros Makriyannis and Kiran Vemuri of Northeastern University, Boston for kindly providing AM4113 for this study. Some of the data is part of PhD thesis of Russell S. Dulman, MD/PhD student in the College of Medicine and Graduate College of University of Illinois at Chicago
FUNDING AND DISCLOSURE
This work was supported by the National Institute on Alcohol Abuse and Alcoholism [R01AA021662 (SCP and KYV), P50AA-022538 (SCP), R01AA-010005 (SCP), U01AA-019971 (SCP), U24AA-024605 (SCP), R21AA018709 (KYV), F30AA027936 (RSD)] and the department of Veterans Affairs [I01BX004517 (SCP) and Senior Research Career Scientist Award (SCP)]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or US department of Veterans Affairs. All authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bacci A, Huguenard JR and Prince DA (2004). Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature 431: 312–316. [DOI] [PubMed] [Google Scholar]
- Balla A, Dong B, Shilpa BM, Vemuri K, Makriyannis A, Pandey SC, Sershen H, Suckow RF and Vinod KY (2018). Cannabinoid-1 receptor neutral antagonist reduces binge-like alcohol consumption and alcohol-induced accumbal dopaminergic signaling. Neuropharmacol 131: 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister AJ and Kouzarides T (1996). The CBP co-activator is a histone acetyltransferase. Nature 384: 641–643. [DOI] [PubMed] [Google Scholar]
- Barrett RM, Malvaez M, Kramar E Matheos DP, Arrizon A, Cabrera SM, Lynch G Greene RW and Wood MA (2011). Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. Neuropsychopharmacol 36: 1545–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkel TD and Pandey SC (2017). Emerging role of epigenetic mechanisms in alcohol addiction. Alcohol Clin Exp Res 41:666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Cravatt BF, Boehm SL 2nd, Walker D and Harris RA (2007). Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacol 32: 1570–1582. [DOI] [PubMed] [Google Scholar]
- Castellano C, Rossi-Arnaud C, Cestari V and Costanzi M (2003). Cannabinoids and memory: animal studies. Curr Drug Targets CNS Neurol Disord 2: 389–402. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chávez AE and Hashimotodani Y (2012). Endocannabinoid signaling and synaptic function. Neuron 76: 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Hansson AC, del Arco I, Sommer W, Heilig M, Massi M, Bermúdez-Silva FJ, Navarro M, Ciccocioppo R and de Fonseca FR (2005). Cannabinoid CB1 receptor antagonism reduces conditioned reinstatement of ethanol-seeking behavior in rats. Eur J Neurosci 21: 2243–2251. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra B, Vacca G, Carai MA and Gessa GL (2005). Endocannabinoid system and alcohol addiction: Pharmacological studies. Pharmacol Biochem Behav 81: 369–380. [DOI] [PubMed] [Google Scholar]
- Dong E, Guidotti A, Zhang H and Pandey SC (2018). Prenatal stress leads to chromatin and synaptic remodeling and excessive alcohol intake comorbid with anxiety-like behaviors in adult offspring. Neuropharmacology 140: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femenía T, García-Gutiérrez MS and Manzanares J (2010). CB1 receptor blockade decreases ethanol intake and associated neurochemical changes in fawn-hooded rats. Alcohol Clin Exp Res 34: 131–141. [DOI] [PubMed] [Google Scholar]
- Gilpin NW (2012). Neuropeptide Y (NPY) in the extended amygdala is recruited during the transition to alcohol dependence. Neuropeptides 46:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K and Koob GF (2008). Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol Biochem Behav 90:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF and Roberto M (2011). Neuropeptide Y opposes alcohol effects on GABA release in amygdala and blocks the transition to alcohol dependence. Biol Psychiatry 69: 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González S, Cascio MG, Fernández-Ruiz J, Fezza F, Di Marzo V and Ramos JA (2002). Changes in endocannabinoid content in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res 954: 73–81. [DOI] [PubMed] [Google Scholar]
- Gueye AB, Pryslawsky Y, Trigo JM, Poulia N, Delis F, Antoniou K. Loureiro M, Laviolette SR, Vemuri K, Makriyannis A and Le Foll B (2016). The CB1 neutral antagonist AM4113 retains the therapeutic efficacy of the inverse agonist rimonabant for nicotine dependence and weight loss, with better psychiatric tolerability. Int J Neuropsychopharmacol 19:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XH, Jordan CJ, Vemuri K, Bi GH, Zhan J, Gardner EL, Makriyannis A, Wang YL and Xi ZX (2019). Cannabinoid CB1 receptor neutral antagonist AM4113 inhibits heroin self-administration without depressive side effects in rats. Acta Pharmacol Sin 40:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC (1998). The CB1 cannabinoid receptor in the brain. Neurobiol Dis 5: 405–416. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K and Storm DR (1999). Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron 23: 11–14. [DOI] [PubMed] [Google Scholar]
- Janak PH and Tye KM (2015). From circuits to behaviour in the amygdala. Nature 517: 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilly E, Gonda X and Bagdy G (2012). CB1 receptor antagonists: new discoveries leading to new perspectives. Acta Physiol (Oxf) 205: 41–60. [DOI] [PubMed] [Google Scholar]
- Kokare DM, Kyzar EJ, Sakharkar AJ, Pandey SC (2017) Adolescent alcohol exposure-induced changes in alpha-melanocyte stimulating hormone and neuropeptide Y pathways via histone acetylation in the brain during adulthood. Int. J Neuropyschopharmacology, 20(9):758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2003). Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol 13: 442–452. [DOI] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW Jr and George O (2014). Addiction as a stress surfeit disorder. Neuropharmacol 76 Pt B: 370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF and Mason BJ (2016). Existing and Future Drugs for the Treatment of the Dark Side of Addiction. Annu Rev Pharmacol Toxicol 56: 299–322. [DOI] [PubMed] [Google Scholar]
- Kouzarides T (2007). Chromatin modifications and their function. Cell 128: 693–705. [DOI] [PubMed] [Google Scholar]
- Kyzar EJ and Pandey SC (2015). Molecular mechanisms of synaptic remodeling in alcoholism. Neurosci Lett 601: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Zhang H, Sakharkar AJ and Pandey SC (2017). Adolescent alcohol exposure alters lysine demethylase 1 (LSD1) expression and histone methylation in the amygdala during adulthood. Addict Biol 22: 1191–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE and Ginty DD (2002). Function and regulation of CREB family transcription factors in the nervous system. Neuron 35: 605–623 [DOI] [PubMed] [Google Scholar]
- López-Moreno JA, González-Cuevas G, Rodríguez de Fonseca F and Navarro M (2004). Long-lasting increase of alcohol relapse by the cannabinoid receptor agonist WIN 55,212-2 during alcohol deprivation. J Neurosci 24: 8245–8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni P, Colombo G and Carai MA (2010). Blockade of the cannabinoid CB1 receptor and alcohol dependence: preclinical evidence and preliminary clinical data. CNS Neurol Disord Drug Targets 9: 55–59. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Cabañero D, Puente N, García-Gutiérrez MS, Grandes P, Maldonado R (2018). Role of the endocannabinoid system in drug addiction. Biochem Pharmacol. 157: 108–121. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Pacioni S, Cannich A, Marsicano G and Bacci A (2009). Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat. Neurosci 12:1488–1490. [DOI] [PubMed] [Google Scholar]
- Mayr BM, Canettieri G and Montminy MR (2001). Distinct effects of cAMP and mitogenic signals on CREB-binding protein recruitment impart specificity to target gene activation via CREB. Proc Natl Acad Sci 98: 10936–10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ (2002). Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav 71:509–515. [DOI] [PubMed] [Google Scholar]
- McClung CA and Nestler EJ (2003). Regulation of gene expression and cocaine reward by CREB and Delta FosB. Nat Neurosci 6: 1208–1215. [DOI] [PubMed] [Google Scholar]
- McDonald AJ and Mascagni F (2001). Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin containing interneurons. Neuroscience 107: 641–652. [DOI] [PubMed] [Google Scholar]
- Mitrirattanakul S, López-Valdés HE, Liang J, Matsuka Y, Mackie K, Faull KF and Spigelman I (2007). Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol Clin Exp Res 31: 855–67. [DOI] [PubMed] [Google Scholar]
- Möller C, Wiklund L, Sommer W, Thorsell A and Heilig M (1997). Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res 760: 94–101. [DOI] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Tang L and Pandey SC (2013). Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biol Psychiatry 73: 763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M and Araque A (2010). Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68:113–126. [DOI] [PubMed] [Google Scholar]
- Pacher P, Bátkai S and Kunos G (2006). The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58: 389–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC (2003). Anxiety and alcohol abuse disorders: a common role for CREB and its target, the neuropeptide Y gene. Trends Pharmacol Sci 24: 456–460. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Carr LG, Heilig M, Ilveskoski E and Thiele TE (2003). Neuropeptide Y and alcoholism: genetic, molecular, and pharmacological evidence. Alcohol Clin Exp Res 27: 149–154. [DOI] [PubMed] [Google Scholar]
- Pandey SC (2004). The gene transcription factor cyclic AMP-responsive element binding protein: role in positive and negative affective states of alcohol addiction. Pharmacol Ther 104: 47–58. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H and Xu T (2004). Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinking behaviors. J Neurosci 24: 5022–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A and Xu T (2005). Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest 115: 2762–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A and Misra K (2006). Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol–drinking and anxiety-like behaviors. J Neurosci 26: 8320–8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L and Prakash A (2008). Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci 28: 3729–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Sakharkar AJ, Tang L and Zhang H (2015). Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol Dis 82: 607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Kyzar EJ, Zhang H (2017) Epigenetic basis of the dark side of alcohol addiction. Neuropharmacology 122:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH and Hurd YL (2015). Endocannabinoid signaling in reward and addiction. Nat Rev Neurosci 16: 579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pava MJ and Woodward JJ (2012). A review of the interactions between alcohol and the endocannabinoid system: implications for alcohol dependence and future directions for research. Alcohol 46: 185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D (2003). The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4: 873–884. [DOI] [PubMed] [Google Scholar]
- Polepalli JS, Gooch H and Sah P (2020). Diversity of interneurons in the lateral and basolateral amygdala. NPJ Sci Learn 5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, Bray GA, York DA and Wilson MA (2006). Over expression of neuropeptide Y in the central nucleus of the amygdala decreases ethanol self-administration in “anxious” rats. Alcohol Clin Exp Res 30: 791–801. [DOI] [PubMed] [Google Scholar]
- Renthal W and Nestler EJ (2008). Epigenetic mechanisms in drug Addiction. Trends Mol Med 14: 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SL and Thiele TE (2017). The role of neuropeptide Y (NPY) in alcohol and drug abuse disorders. Int Rev Neurobiol 136: 177–197. [DOI] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW and Siggins GR (2012). The central amygdala and alcohol: Role of γ-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb Perspect Med 2: a012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH and Schweitzer P (2010). The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacology 35:1962–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A and Pandey SC (2002). The decreased cellular expression of neuropeptide Y protein in rat brain structures during ethanol withdrawal after chronic ethanol exposure. Alcohol Clin Exp Res 26: 796–803. [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Baxstrom K, Shi G, Moonat S and Pandey SC (2014). Effects of histone deacetylase inhibitors on amygdaloid histone acetylation and neuropeptide Y expression: a role in anxiety-like and alcohol-drinking behaviours. Int J Neuropsychopharmacology 17: 1207–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Redhi GH, Vemuri K, Makriyannis A, Le Foll B, Bergman J, Goldberg SR and Justinova Z (2016). Blockade of nicotine and cannabinoid reinforcement and relapse by a cannabinoid CB1-receptor neutral antagonist AM4113 and inverse agonist rimonabant in squirrel monkeys. Neuropsychopharmacol 41: 2283–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD and Livak KJ (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW and Kida S (1998). CREB and memory. Annu Rev Neurosci 21: 127–148. [DOI] [PubMed] [Google Scholar]
- Soderling TR (1999). The Ca2+-calmodulin-dependent protein kinase cascade. Trends Biochem Sci 24: 232–236. [DOI] [PubMed] [Google Scholar]
- Starkman BG, Sakharkar AJ and Pandey SC (2012). Epigenetics-beyond the genome in alcoholism. Alcohol Res 34: 293–305. [PMC free article] [PubMed] [Google Scholar]
- Stincic TL and Hyson RL (2008). Localization of CB1 cannabinoid receptor mRNA in the brain of the chick (Gallus domesticus). Brain Res 1245: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Lumeng L, McBride WJ, Li TK and Hwang BH (2004). Reduced neuropeptide Y mRNA expression in the central nucleus of amygdala of alcohol preference and anxiety. Brain Res 1014: 251–254. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL and Palmiter RD (1998). Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 396: 366–369. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ and Ehlers CL (2005). Effects of neuropeptide Y on appetitive and consummatory behaviors associated with alcohol drinking in Wistar rats with a history of ethanol exposure. Alcohol Clin Exp Res 29: 584–590. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Repunte-Canonigo V, O'Dell LE, Chen SA, King AR, Lekic D, Koob GF and Sanna PP (2007). Viral vector-induced amygdala NPY over expression reverses increased alcohol intake caused by repeated deprivations in Wistar rats. Brain 130:1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Viviana Gradinaru V, Ramakrishnan C and Deisseroth K (2011) Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471: 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Soni N, Bajo M, Luu G, Madamba SG, Schweitzer P, Parsons LH and Roberto M (2016). Chronic ethanol exposure decreases CB1 receptor function at GABAergic synapses in the rat central amygdala. Addict Biol 21: 788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdone L, Caserta M and Di Mauro E (2005). Role of histone acetylation in the control of gene expression. Biochem Cell Biol 83: 344–353. [DOI] [PubMed] [Google Scholar]
- Vinod KY and Hungund BL (2006). Cannabinoid-1 receptor: a novel target for the treatment of neuropsychiatric disorders. Expert Opin Ther Targets 10: 203–210. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Thanos PK, Vadasz C, Cooper TB, Volkow ND and Hungund BL (2008a). Genetic and pharmacological manipulations of the CB (1) receptor alter ethanol preference and dependence in ethanol preferring and nonpreferring mice. Synapse 62: 574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Sanguino E, Yalamanchili R, Manzanares J and Hungund BL (2008b). Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J Neurochem 104: 233–243. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Kassir SA, Hungund BL, Cooper TB, Mann JJ and Arango V (2010). Selective alterations of the CB1 receptors and the fatty acid amide hydrolase in the ventral striatum of alcoholics and suicides. J Psychiatr Res 44: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Maccioni P, Garcia-Gutierrez MS, Femenia T, Xie S, Carai MA, Manzanares J, Cooper TB, Hungund BL and Colombo G (2012). Innate difference in the endocannabinoid signaling and its modulation by alcohol consumption in alcohol-preferring sP rats. Addict Biol 17: 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Xie S, Cooper TB and Hungund BL (2006). Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int 49: 619–625. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-white J, Zimmer A and Kunos G (2003). Endocannabinoid signaling via CB1 receptors is involved in alcohol preference and its age-dependent decline in mice. Proc Natl Acad Sci U S A 100: 1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sakharkar AJ, Shi G, Ugale R, Prakash A and Pandey SC (2010). Neuropeptide Y signaling in the central nucleus of amygdala regulates alcohol-drinking and anxiety-like behaviors of alcohol-preferring rats. Alcohol Clin Exp Res 34: 451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kyzar EJ, Bohnsack JP, Kokare DM, Teppen T, Pandey SC (2018) Adolescent alcohol exposure epigenetically regulates CREB signaling in adult amygdala. Sci Rep July 10; 8:10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Huang T, Lee F and Kreek MJ (2016). Involvement of endocannabinoids in alcohol “binge” drinking: studies of mice with human fatty acid amide hydrolase genetic variation and after CB1 receptor antagonists. Alcohol Clin Exp Res 40: 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]