Abstract
Alcohol use disorder (AUD) is widely associated with cerebellar dysfunction and altered cerebro-cerebellar functional connectivity (FC) that lead to cognitive impairments. Evidence for this association comes from resting-state functional magnetic resonance imaging (rsfMRI) studies that assess time-averaged measures of FC across the duration of a typical scan. This approach, however, precludes the assessment of potentially FC dynamics happening at faster timescales. In this study, using rsfMRI data, we aim at exploring cerebro-cerebellar FC dynamics in AUD patients (N = 18) and age- and sex-matched controls (N = 18). In particular, we quantified group-level differences in the temporal variability of FC between the posterior cerebellum and large-scale cognitive systems, and we investigated the role of the cerebellum in large-scale brain dynamics in terms of the temporal flexibility and integration of its regions. We found that, relative to controls, the AUD group exhibited significantly greater FC variability between the cerebellum and both the frontoparietal executive control (F1,31 = 7.01, p(FDR) = 0.028) and ventral attention (F1,31 = 7.35, p(FDR) = 0.028) networks. Moreover, the AUD group exhibited significantly less flexibility (F1,31 = 8.61, p(FDR) = 0.028) and greater integration (F1,31 = 9.11, p(FDR) = 0.028) in the cerebellum. Finally, in an exploratory analysis, we found distributed changes in the dynamics of canonical large-scale networks in AUD. Overall, this study brings evidence of AUD-related alterations in dynamic FC within major cerebro-cerebellar networks. This pattern has implications for explaining the development and maintenance of this disorder and improving our understating of the cerebellum’s involvement in addiction.
Keywords: Cerebellum, Alcohol use disorder, Resting-state fMRI, Cerebro-cerebellar networks, Dynamic functional connectivity
Introduction
Alcohol use disorder (AUD) is a chronic and relapsing medical condition characterized by uncontrolled consumption of alcohol that induces a spectrum of negative effects on the brain [1]. Three decades of magnetic resonance imaging (MRI) have extensively highlighted the negative consequences of AUD on selective brain regions and large-scale brain networks, providing a powerful non-invasive, in vivo view of the neurobiological underpinnings of cognitive impairments, impulsive decision making, compulsive alcohol seeking, and the emotional distress associated with alcoholism [2–4]. In this context, evidence suggests that the cerebellum and its functional and structural circuits, notably with prefrontal cortex, are highly vulnerable to alcohol-related damage [5–7]. In short, structural MRI studies have reported significant reduction in grey matter volumes in the cerebellum [6], and have found substantial damage to frontal and cerebellar white matter pathways associated with the severity of alcohol dependence [8–10]. Moreover, task activation and resting-state functional MRI (rsfMRI) studies have consistently reported abnormal activity in the cerebellum of AUD patients, coupled with altered frontocerebellar functional connectivity (FC) and associated with cognitive impairments mainly affecting executive functions [5, 11–15].
The cerebellum has been traditionally associated with motor control, but recent functional neuroimaging studies point to its involvement in cognitive and affective processing [16–19]. In addition, damage to the posterior cerebellum, which can induce disruptions in connectivity between the cerebellum and association and limbic cortices, has been shown to cause the cerebellar cognitive and affective syndrome (CCAS), a constellation of cognitive deficits in executive functioning, language, behavioural inhibition, and emotion regulation [20]. Thus, the cerebellum is believed to monitor and control cognitive processes, thereby maintaining healthy cognitive processing and harmonious behavior [19, 21, 22]. In AUD, selective cognitive, and affective processes are impaired and are deemed inefficient. This inefficiency possibly arises from functional disorganization caused, at least in part, by dysfunction in the connections of the posterior cerebellum to prefrontal association regions and the limbic system, as described by the CCAS paradigm [11, 20, 23, 24]. Furthermore, studies have shown that AUD patients performing well on cognitively demanding tasks tend to recruit unaffected intact loops to compensate for functionally degraded ones normally used by healthy controls for task performance, suggesting compensatory functional reorganization within the cerebellum of those patients [12]. These findings are predicated on observations of reduced FC within certain frontocerebellar executive control loops paralleled by an increase in FC strength within adjacent unaffected loops [11, 12]. Thus, the disruption of cerebro-cerebellar functional coupling contributes, on one hand, to the characteristic behaviors of AUD, and on the other hand, lead to compensation for the so-called frontally based dysfunctions in alcoholism [25].
So far, most of what we know about the role of the cerebellum and cerebro-cerebellar circuits in AUD comes from studies treating FC as a “static” quantity that does not change across an fMRI scanning session. However, the brain is dynamic, continuously integrating information and reconfiguring to adapt to various changes in the internal and external milieu [26]. Therefore, the static FC approach is believed to average out meaningful variations in FC happening at timescales much shorter than a typical fMRI scan [27, 28]. In response to the limitations of traditional approaches, researchers have begun exploring “dynamic” FC, with findings indicating that the brain navigates through a set of transiently recurring FC configurations that encode ongoing fluctuations in cognitive state, even during rest [27, 29–31]. Measures of dynamic FC have been shown to outperform those of static FC in predicting individual differences in learning capabilities [32], executive functioning [33], attention [34], and cognitive flexibility [35], to name a few. In addition, patients with brain disorders including, among others, autism [36], schizophrenia [37, 38], and substance use disorders [39] have been shown to exhibit alterations in dynamic FC, associated with disease symptoms.
A handful of studies have investigated the impact of excessive and prolonged use of alcohol on dynamic FC in the brain, with findings showing alterations in sensorimotor control regions [39], the orbitofrontal cortex and insula [40], and, recently, fronto-striatal circuits [41], associated with the severity of alcohol dependence and cognitive decline in alcoholics. Furthermore, a recent study [42] of cerebro-cerebellar dynamic FC in healthy individuals shows significant associations with sub-facets of trait impulsivity, especially sensation seeking, which is a well-known predisposing factor for addiction [43] and a key component in the disease pattern of both AUD and the CCAS [4, 20, 44, 45]. Together, these findings suggest that dynamic FC may have implications for explaining features of the development and maintenance of AUD that traditional methods are blind to. So far, however, evidence for a role of cerebro-cerebellar FC dynamics in AUD remains limited.
In this study, we explored between-group differences in cerebro-cerebellar dynamic FC between a group of AUD patients and a group of unaffected controls, matched to the AUD group on age and sex. Toward this goal, we adopted the sliding window approach [46] to estimate FC within overlapping segments (≈ 1 min) of the BOLD timeseries extracted from a fine-grained map of cerebral and cerebellar regions [47]. With a focus on temporal variability of FC, we examined the extent of variation in FC between the posterior cerebellum and the executive control, attention, salience, and default mode networks. Then, we applied a graph theory-based dynamic network analysis, known as multilayer community detection [33, 48, 49], to characterize AUD-related changes in the dynamics of the brain’s network structure. In particular, we estimated measures, such as flexibility and integration that characterize the network role of the cerebellum in those dynamics [50, 51]. In an exploratory analysis, we computed those measures at the level of large-scale canonical networks to explore AUD-related changes in their connectivity dynamics. Our specific hypothesis was that dynamic FC within cerebro-cerebellar networks, especially those connecting the cerebellum to the prefrontal cortex, would differ between AUD patients and healthy controls, such that AUD would confer an overall reduction in frontocerebellar FC.
Methods and Materials
Participants and Neuropsychological Tests
This study was approved by the Institutional Review Boards of Stanford University and SRI International and included 18 AUD patients and 18 controls who had participated in previous studies [52–56]. AUD patients were recruited from local outpatient and treatment centers, or during presentations in clinics by project staff and by distribution of flyers at community events. Healthy, control participants were recruited from the local community by flyers. All participants were free of any serious medical, psychiatric, or neurological diseases (e.g., neurodegenerative diseases) at the time of scanning, and provided written informed consent to participate in this study, in accordance with the Declaration of Helsinki, by the signing of consent documents in the presence of staff. All AUD patients were screened using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders to assess the DSM-5 criteria for AUD [57], and were given the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) to assess the severity of alcohol withdrawal. Demographic data of study groups are summarized in Table 1.
Table 1.
Demographic data of study groups: mean ± SD/frequency count
| Demographic variable | Controls (n = 18) | AUD (n = 18) | p value |
|---|---|---|---|
| Sex (male/female) | 10/8 | 13/5 | 0.49 |
| Age (years) | 48.8 ± 8.81 | 53.2 ± 8.76 | 0.13 |
| Handedness (right/left) | 18/0 | 15/3 | 0.23 |
| Ethnicity (Caucasian/African/American/Asian) | 10/2/6 | 13/4/1 | 0.1 |
| Education (years) | 17.2 ± 2.57 | 12.7 ± 1.82 | < .0001 |
| Socioeconomic status | 19.72 ± 11.46 | 41.28 ± 12.8 | < .0001 |
| ‡WTAR predicted IQ | 104.23 ± 6.2 | 96.7 ± 8.34 | 0.01 |
| ‡Beck Depression Index | 4 ± 4.08 | 13.6 ± 11.12 | < .0001 |
| AUD onset age | — | 21.83 ± 6.91 | — |
| Lifetime alcohol consumption (kg) | 61.4 ± 93.2 | 1567.67 ± 1165.86 | < .0001 |
| Days since last drink | — | 105.26 ± 102.63 | — |
| CIWA total score | — | 27.68 ± 15.5 | — |
| DSM-5 AUD total score | — | 9.37 ± 2.26 | — |
| Smoker (never/past/current) | 18/0/0 | 4/5/9 | < .0001 |
| In-scanner head motion (mm) | 0.1 ± 0.04 | 0.18 ± 0.09 | .0003 |
Some subjects in both groups had missing data on WTAR-IQ and Beck Depression Index
t tests were used on continuous variables (e.g., age); χ2 tests were used on nominal variables to assess group differences
Bold indicates significant differences between groups
Participants completed a comprehensive neuropsychological battery to assess attention, memory and learning, visuospatial abilities, and executive functions. Raw test scores were statistically corrected for age on the control group [mean and standard deviation for control group = 0 ± 1], allowing averaging across tests. Composite scores were then calculated as the mean of all Z- scores of tests comprising each of the functional domains. Neuropsychological data were available for all 18 subjects with AUD and 12 of the 18 controls. The comprehensive list of the included tests is as follows:
Attention:
Wechsler Memory Scale-Revised (WMS-R) block forward total
WMS-R block forward span
Memory and Learning:
Wechsler Adult Intelligence Scale (WAIS) digit symbol incidental recall of symbols
WAIS digit symbol incidental recall of numbers
California Verbal Learning Test (CVLT) short delay free recall
CVLT long delay free recall
Montreal Cognitive Assessment (MOCA) delayed recall
WMS-R logical memory story A raw score
WMS-R logical memory story B raw score
Visuospatial Abilities:
Rey-Osterrieth copy raw score
WMS-R visual reproduction item 1 raw score
WMS-R visual reproduction item 2 raw score
Executive Functions:
Controlled Oral Word Association Test (F + A + S total)
Semantic fluency (inanimate objects, animals)
WAIS digit symbol total time to complete set
WAIS digit symbol standard score at 90 seconds
MoCA abstraction score
MRI Acquisition
For each participant, a single resting-state fMRI scan and a T1-weighted MRI scan were performed. The resting-state scans were collected with a 1.71 × 1.71 × 3-mm spatial resolution and 200 time-points (i.e., repetition times; TRs) for each participant. Three protocols associated with slightly different repetition times (TRs) ranging from 2.65 to 2.86 s were used during the study. In particular, 28 participants (16 controls, 12 AUD) were scanned with a TR = 2.65 s, 4 participants (2 controls, 2 AUD) were scanned with a TR = 2.75 s, and 4 participants (4 AUD) were scanned with a TR = 2.86 s. Statistical analysis revealed no effect of TR on group differences (F-tests < 0.1, p values > 0.05). Therefore, scans from all protocols were included, and TR was not considered a confounding factor in group comparisons.
MRI Data Preprocessing
Functional and structural MRI data were preprocessed using fmriprep 20.0.1 [58]. For the structural T1-weighted scans, we performed skull-stripping, segmentation, and normalization to the Montreal Neurological Institute (MNI152) standard space, whereas for the resting-state fMRI scans, we performed slice timing correction, distortion correction, motion correction, co-registration to each subject’s T1-weighted scan, normalization to MNI152 space, resampling to 2 × 2 × 2 mm grid, spatial smoothing (FWHM = 4 mm), and bandpass filtering [0.02–0.1 Hz]. Finally, we regressed out the confounding effects of head motion using Friston 24-parameter model, white matter and cerebrospinal fluid signal using the aCompCor method [59], and linear and quadratic trends.
Further details for “MRI Data Preprocessing” to “Dynamic Network Analysis: Flexibility and Integration of the Cerebellum” sections are found in Supplementary Material.
Definition of Functional Brain Regions and Networks
We used 300 spherical regions positioned around suggested coordinates in MNI space (see Fig. 1a) to define functional brain regions. This functional map offers a comprehensive view for the study of brain functional modules and their interactions with improved representations of the cerebellum and the subcortex [47]. In this map, the whole brain is divided into 13 functional systems. Here, however, we focused on the dynamics of FC within the cerebellum and between regions of the cognitive posterior cerebellum and seven large-scale cerebral cognitive systems, namely, the frontoparietal network (FPN), default mode network (DMN), reward network (RN), dorsal and ventral attention networks (DAN and VAN), salience network (SN), and the cingulo-opercular network (CON).
Fig. 1.
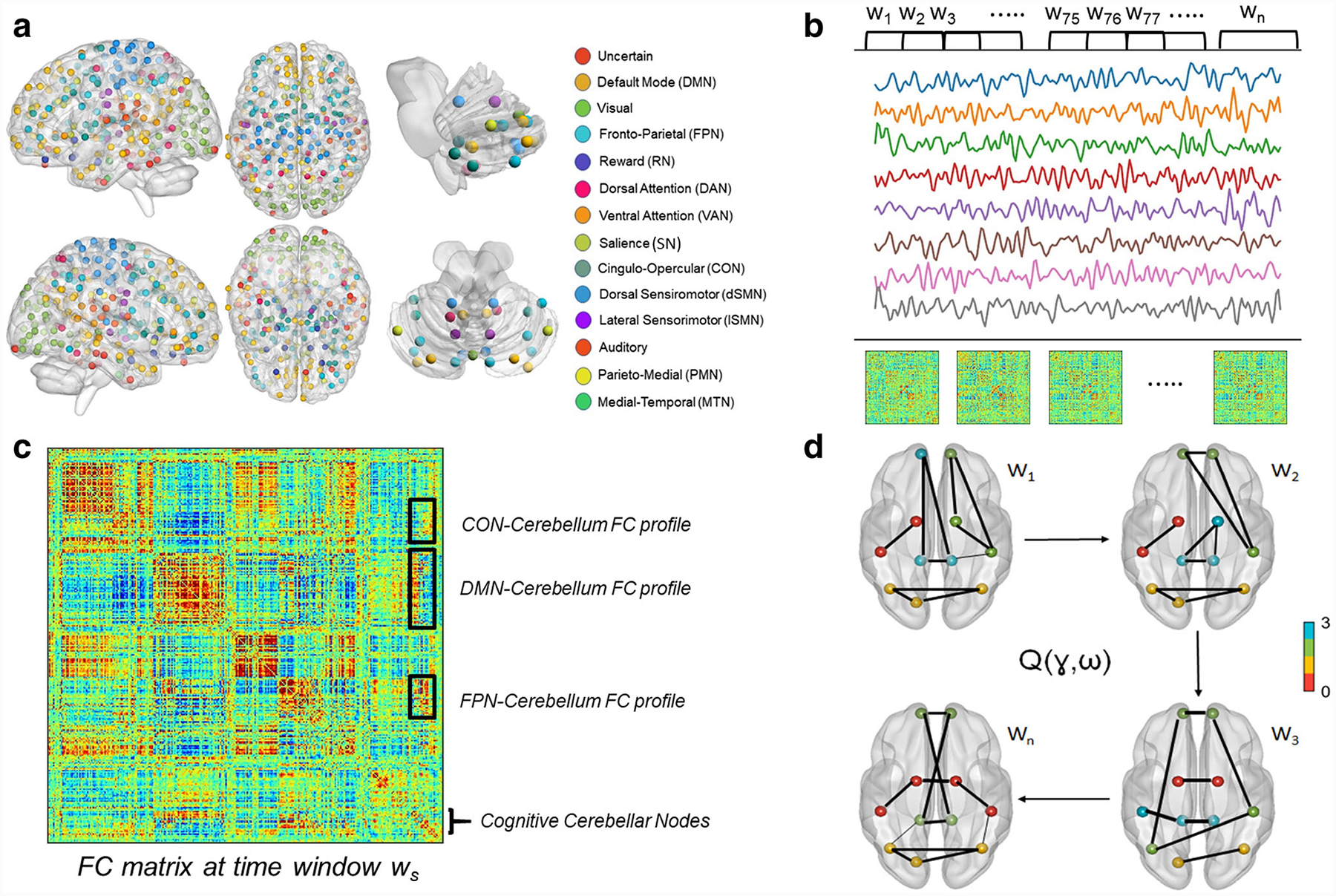
Schematic overview of the main methods used in this study. a Whole-brain parcellation using functionally defined regions of interest with an improved representation of the cerebellum and the subcortex. b BOLD signals from all ROIs were segmented into overlapping windows using the tapered sliding window analysis, and a whole-brain FC matrix was constructed by computing pairwise Pearson’s correlation in each window. c An example FC matrix in an arbitrary time window ws. The FC profile between distinct cerebral cognitive networks and the cerebellum is a sub-matrix (shown as boxes marked by black lines) containing the FC weights between its constituent nodes and every node in the cognitive cerebellum. Then, temporal variability of FC was calculated as the extent of variation of cerebro-cerebellar FC profiles across time windows. d Maximizing the multilayer modularity quality function Q(γ,ω) helps detecting the optimal community assignment for each node in each window. Using the optimal community structure, we calculated flexibility as the proportion of time a node changes its community assignment in time. Also, we calculated integration as the average probability for a node to be assigned to the same community with nodes outside its native system. Brain networks were visualized with the BrainNet Viewer toolbox http://www.nitrc.org/projects/bnv/ [60]
Dynamic Functional Connectivity: Sliding Window Approach
For each participant, whole-brain dynamic FC was modelled using the sliding window approach [46]. In this setup, the BOLD time series from 300 brain regions were divided into overlapping segments (i.e., windows), each spanning 20 timepoints (i.e., TRs). Then within each window, pairwise connectivity was estimated using Pearson’s correlation between the time series followed by Fisher r-to-z transform of the coefficients to stabilize their variance. We also performed a supplementary validation analysis using windows of different lengths (15 TR and 25 TR) to assess the robustness of our findings to different parameters.
Temporal Variability of Cerebro-Cerebellar FC
The connectivity patterns between brain regions/networks fluctuate dynamically over short periods of time. Temporal variability of FC is a straightforward measure that can be used to quantify these fluctuations [61]. Here, we estimated the temporal variability of the FC between cerebral regions, belonging to seven large-scale cognitive networks of interest, and the cognitive cerebellum.
For a given cerebral region k, a FC profile at time window ws is defined as a vector with M values that quantify the connectivity map between region k and all regions of the cognitive cerebellum. Then, temporal variability was computed as an average measure of “dissimilarity” among the FC profiles between region k and the cerebellum extracted from all time windows. Finally, the temporal variability between each large-scale cognitive network and the cerebellum is calculated by averaging the temporal variability scores over all regions belonging to the same network (see Fig. 1c).
Dynamic Network Analysis: Multilayer Community Detection
For each participant, the sliding window approach yielded a time series of three-dimensional 300 × 300 FC matrices that can be thought of as a large multilayer connectivity matrix. In each layer, there exists a different network organization, reflecting the transient emergence and dissolution of functional communities as patterns of FC change from one window to another. A functional community, in this case, is defined as an ensemble of brain regions that have a strong FC among them and a weak FC with other regions during a short time period (≈ 50 − 60 s).
By using a dynamic graph theory method known as multilayer community detection, we delineated the time-varying functional communities across time windows for each participant. To achieve this, the algorithm maximizes a Louvain-like modularity function Q, resulting in the optimal community assignment for each region in each window (see Fig. 1d; [49, 51, 62–64]).
Dynamic Network Analysis: Flexibility and Integration of the Cerebellum
Multilayer community detection also permitted the quantification of the dynamic role of each of the 300 brain regions, as communities emerge, dissolve, and reemerge, using two measures:
The first measure is flexibility, defined as the proportion of time a brain region switches its community affiliation across time windows [32, 33]. For each participant, we computed the flexibility of the cerebellum by averaging flexibility scores over all cerebellar regions.
The second measure is integration, defined as the average probability for a brain region to be assigned to the same community with regions outside its “native” functional system [51, 65]. We computed the integration of the cerebellum for each participant by averaging integration scores over all cerebellar regions.
Finally, in an exploratory analysis, we estimated those measures for each of the seven large-scale cognitive networks by averaging the scores over their constituent regions.
Statistical Analysis
Two-sample t tests assessed between-group differences on continuous variables and chi-squared tests for categorical variables. Univariate analyses of covariance (ANCOVAs) tested between-group differences on temporal variability, flexibility, and integration, while controlling for the confounding effects of age, sex, and overall head motion in the scanner. Finally, the false discovery rate (FDR) procedure controlled for the rate of type-Ⅰ errors at α = 0.05 [66]. We did not, however, correct for multiple comparisons in the exploratory analysis. Because the different hypotheses tested were not pre-registered on any online platform, study findings should be considered exploratory.
Results
Group Comparisons: Demographics and Neuropsychological Tests
Groups significantly differed in terms of yeas of education, socioeconomic status, IQ, Beck depression index, lifetime consumption of alcohol, smoking, and in-scanner head motion. Moreover, the AUD group performed worse than controls on all 4 neuropsychological composite scores: attention (t = − 3.2, p = 0.003), learning and memory (t = − 4.9, p < 0.0001), visuospatial abilities (t = − 3.9, p = 0.0007), and executive functions (t = − 6.9, p < 0.0001). The directionality of these group differences is expected and consistent with previous findings on the typical epidemiology of the AUD group. Demographic data of study groups are summarized in Table 1.
Group Comparisons: Temporal Variability of Cerebro- Cerebellar FC
Results of group comparisons showed that, relative to the controls, the AUD group exhibited significantly greater temporal variability of FC between the cerebellum and two large-scale cognitive networks: FPN (F1,31 = 7.01, p(FDR) = 0.028) and VAN (F1,31 = 7.35, p(FDR) = 0.028). Group differences were not forthcoming in other networks: DMN (F1,31 = 0.144, p(FDR) = 0.71), RN (F1,31 = 0.7, p(FDR) = 0.45), DAN (F1,31 = 0.72, p(FDR) = 0.45), SN (F1,31 = 1.28, p(FDR) = 0.45), and CON (F1,31 = 0.93, p(FDR) = 0.45). Statistical details of group comparisons in temporal variability of cerebro-cerebellar FC are summarized in Table 2 and illustrated in Fig. 2.
Table 2.
Results of group comparisons in temporal variability of FC between seven large-scale networks and the cerebellum
| Network | Controls mean (SD) | AUD mean (SD) | F 1,31 | p value (FDR) | η p 2 |
|---|---|---|---|---|---|
| DMN | 0.76(0.03) | 0.77(0.046) | 0.14 | 0.71 | 0.005 |
| FPN | 0.71(0.055) | 0.76(0.046) | 7.01 | 0.028 | 0.18 |
| RN | 0.82(0.036) | 0.82(0.048) | 0.7 | 0.45 | 0.02 |
| DAN | 0.77(0.04) | 0.76(0.07) | 0.72 | 0.45 | 0.02 |
| VAN | 0.79(0.04) | 0.82(0.052) | 7.34 | 0.028 | 0.19 |
| SN | 0.8(0.035) | 0.82(0.045) | 1.28 | 0.45 | 0.04 |
| CON | 0.8(0.033) | 0.81(0.027) | 0.93 | 0.45 | 0.03 |
AUD alcohol use disorder, SD standard deviation, FDR false discovery rate, F F-statistic, ηp2 partial eta-squared effect size, DMN default mode network, FPN fronto-parietal network, RN reward network, DAN dorsal attention network, VAN ventral attention network, SN salience network, CON cingulo-opercular network
Bold indicates significant between-group difference
Fig. 2.
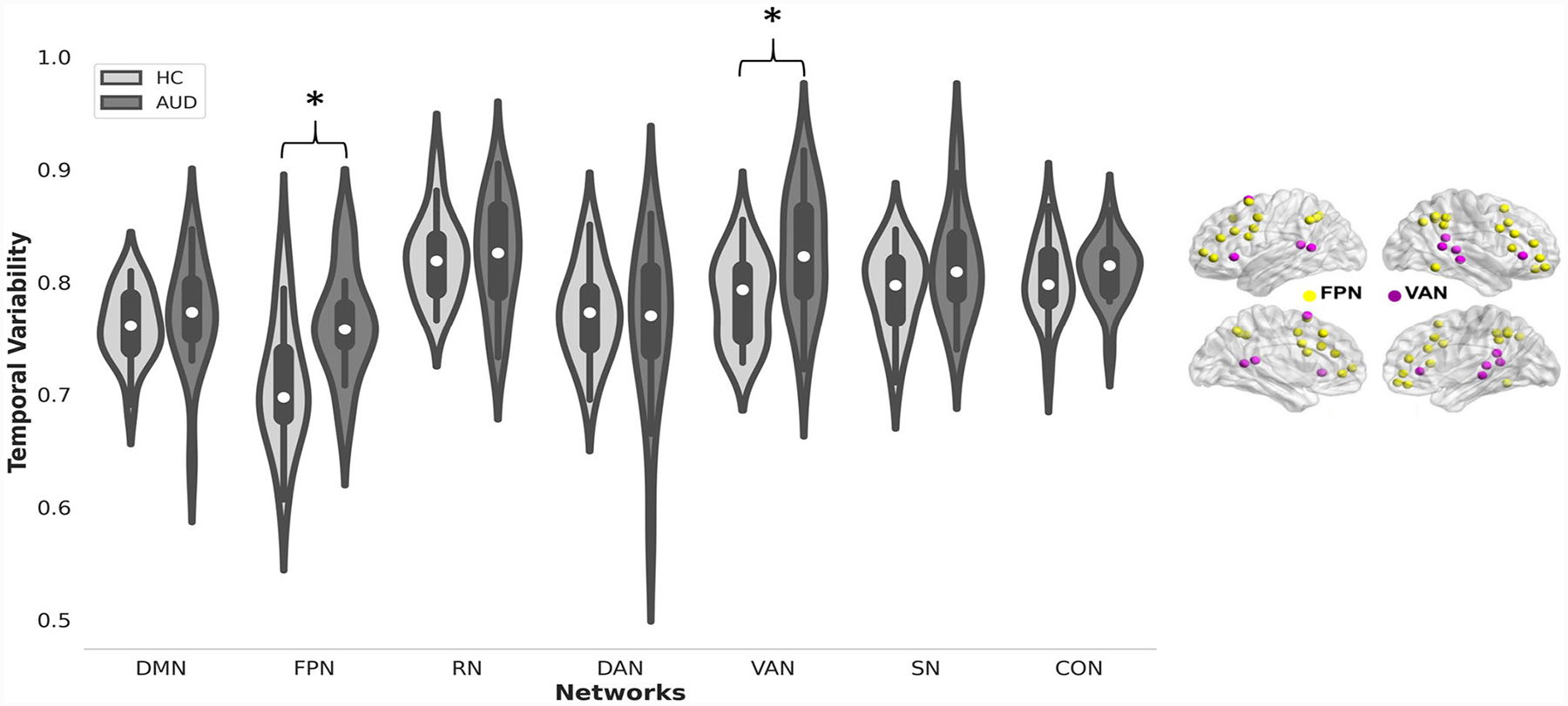
(Left) Violin plots of temporal variability of FC between the cerebellum and seven large-scale cognitive networks for the AUD group and controls. Asterisk indicates p < 0.05 (FDR corrected) for group differences. (Right) Brain plot of the frontoparietal and ventral attention networks
Group Comparisons: Cerebellar Flexibility and Integration
On average, the AUD group exhibited significantly less cerebellar flexibility than controls across the fMRI scanning interval (F1,31 = 8.61, p(FDR) = 0.028), indicating that over time, cerebellar nodes switched their community affiliation less frequently in the AUD group than the controls. By contrast, the AUD group demonstrated significantly greater cerebellar integration than controls across the scanning interval (F1,31 = 9.11, p(FDR) = 0.028), indicating that over time, cerebellar nodes that belong to different functional systems tend to communicate with nodes outside their native functional systems more often in the AUD group than in controls. Statistical details of group comparisons in cerebellar flexibility and integration are summarized in Table 3 and illustrated in Fig. 3.
Table 3.
Results of group comparisons in flexibility and integration in the cerebellum
| Score | Controls mean (SD) | AUD mean (SD) | F 1,31 | p value (FDR) | η p 2 |
|---|---|---|---|---|---|
| Flexibility | 0.45(0.033) | 0.4(0.06) | 8.61 | 0.028 | 0.22 |
| Integration | 0.28(0.012) | 0.3(0.019) | 9.11 | 0.028 | 0.23 |
Fig. 3.
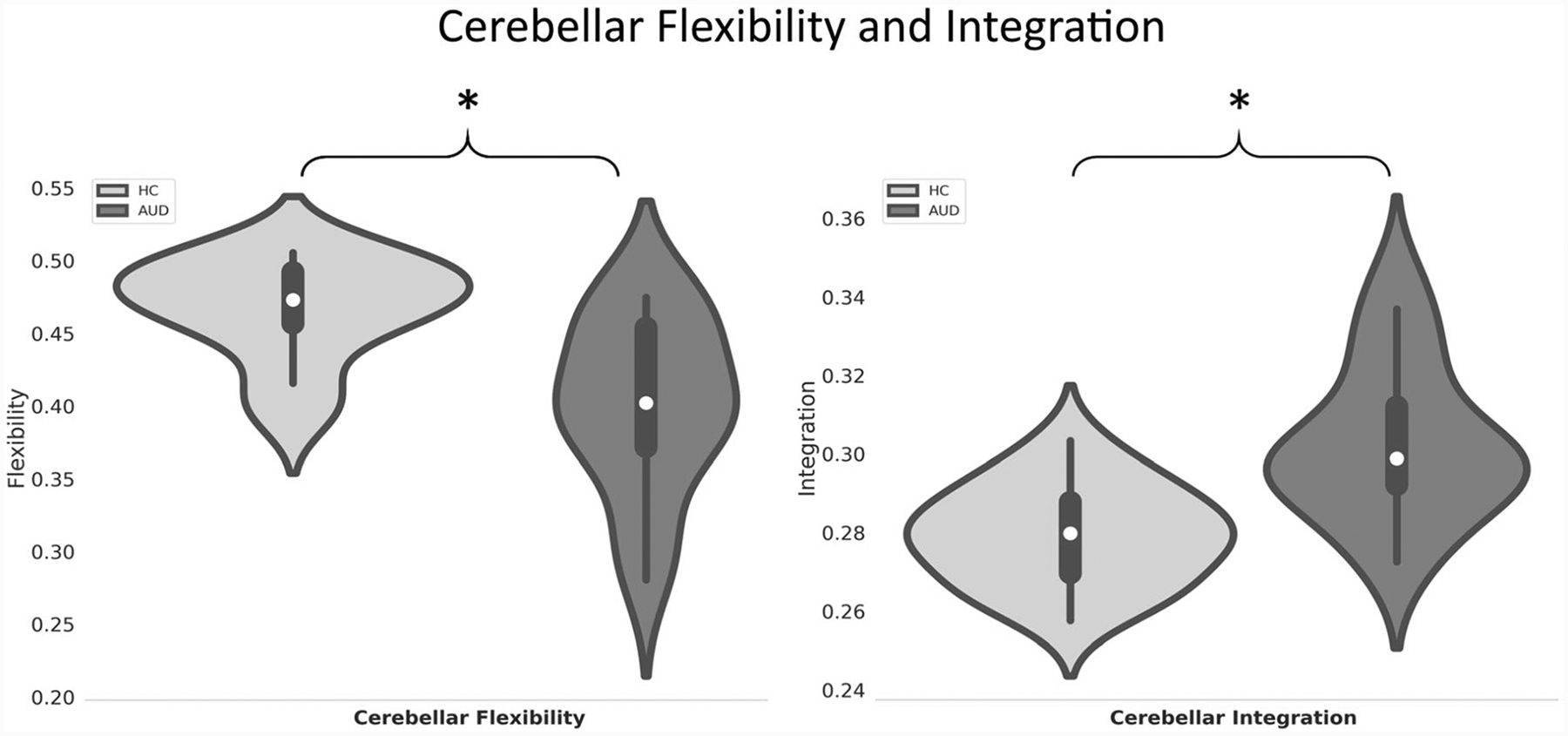
Violin plots of cerebellar flexibility and integration (right) for the AUD group and controls. Asterisk indicates p < 0.05 (FDR corrected) for group differences
Group Comparisons: Network Flexibility and Integration
Results of group comparisons in the exploratory analysis revealed statistically significant group differences in network flexibility and integration (see Figs. 4 and 5). Specifically, relative to controls, the AUD group exhibited significantly less flexibility in the SN (F1,31 = 21.6, p < 0.0001 uncorrected) and CON (F1,31 = 6.01, p = 0.02 uncorrected).Furthermore, relative to controls, the AUD group exhibited significantly greater integration in the FPN (F1,31 = 5.4, p = 0.026 uncorrected) (Table 4).
Fig. 4.
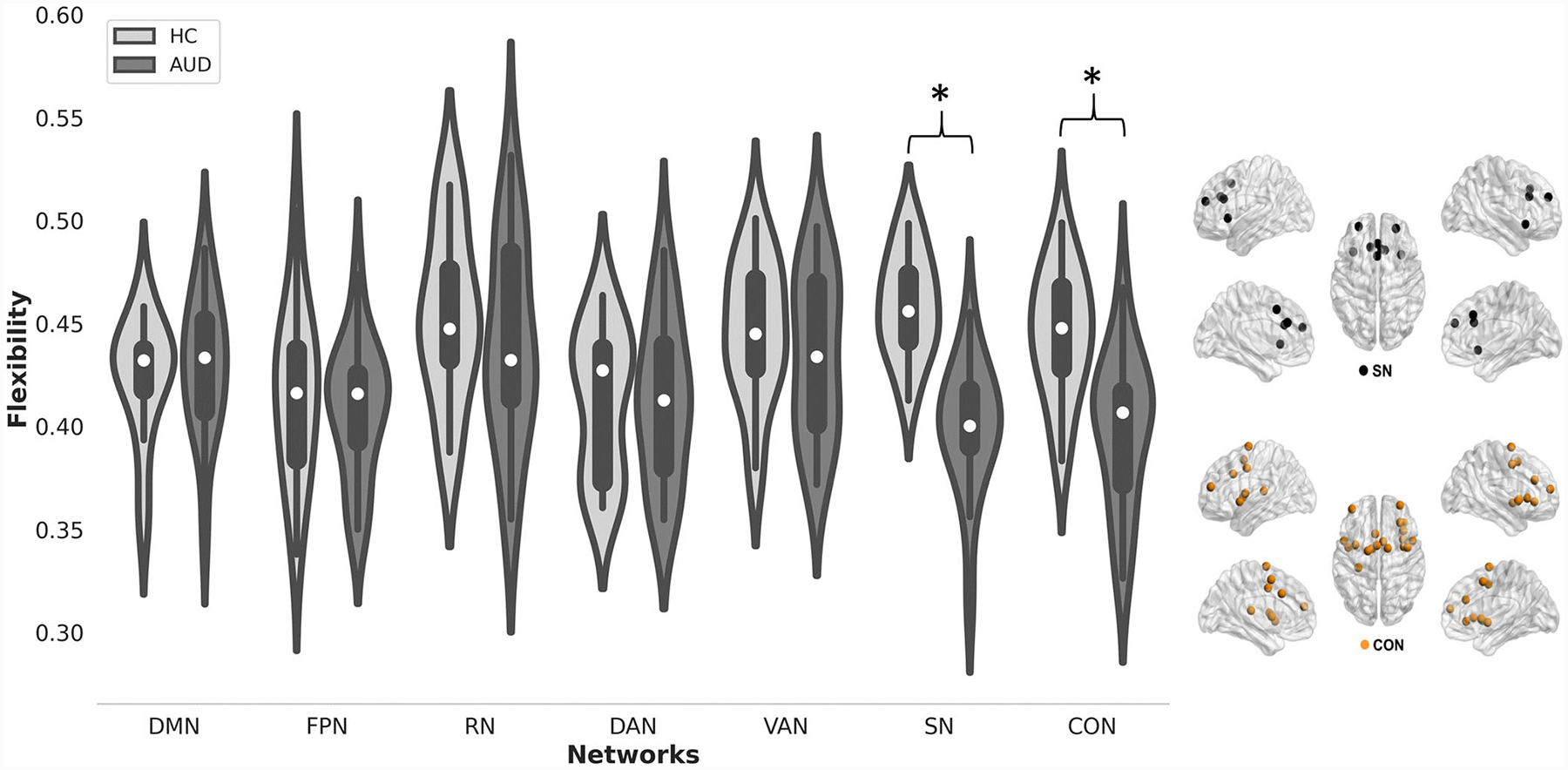
(Left) Violin plots of network flexibility for the AUD group and controls. Asterisk indicates p < 0.05 (uncorrected) for group differences. (Right) Brain plots of the salience and cingulo-opercular networks
Fig. 5.
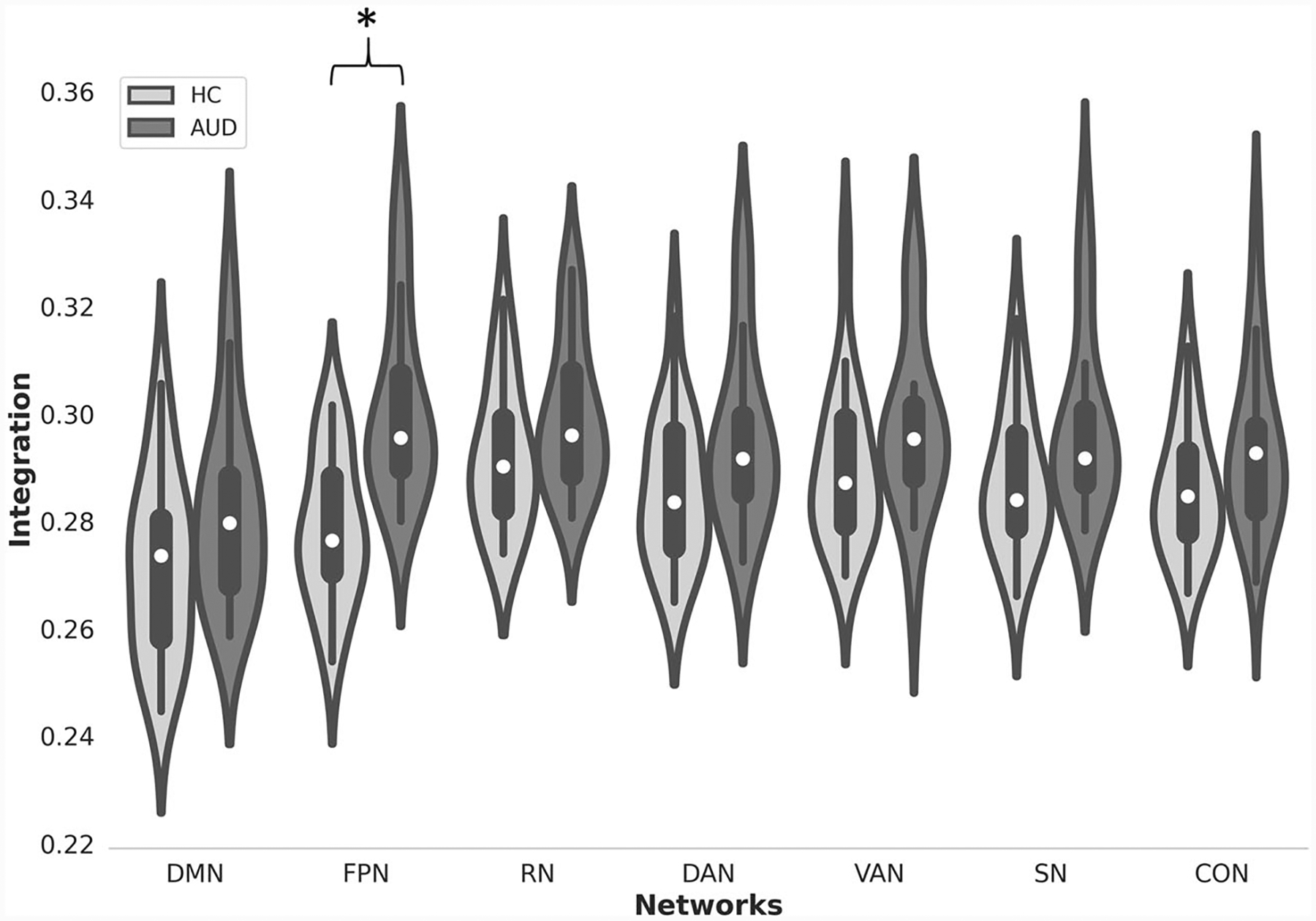
Violin plots of network integration for the AUD group and controls. Asterisk indicates p < 0.05 (uncorrected) for group differences
Table 4.
Results of group comparisons in network flexibility and integration
| Network | Score | Controls mean (SD) | AUD mean (SD) | F 1,31 | p value | η p 2 |
|---|---|---|---|---|---|---|
| SN | Flexibility | 0.46(0.025) | 0.4(0.03) | 21.6 | < 0.001 | 0.42 |
| CON | Flexibility | 0.45(0.03) | 0.42(0.035) | 6.01 | 0.02 | 0.15 |
| FPN | Integration | 0.28(0.013) | 0.3(0.017) | 5.4 | 0.026 | 0.12 |
Discussion
Previous studies that reported altered cerebro-cerebellar FC in AUD based their findings on measures of static FC that is blind to fast variations in the brain’s network structure. Herein, we explored cerebro-cerebellar dynamic FC in AUD using sliding window approach and multilayer community detection. Relative to controls, the AUD group exhibited significantly greater temporal variability of FC between the cerebellum and two cognitive networks: FPN and VAN. Moreover, the AUD group showed significantly less flexibility and greater integration in the cerebellum compared with controls. Finally, exploratory analysis revealed that the AUD group exhibited less flexibility in the SN and CON and greater integration in the FPN. Overall, these results provide evidence of AUD-related alterations in dynamic FC, complementing existing literature on the adverse effects of prolonged, excessive use of alcohol on the brain in general and cerebro-cerebellar networks in particular.
Hypervariability of Cerebro-Cerebellar FC in AUD
Temporal variability of FC is thought to reflect a general readiness of the brain to reorganize in response to changing attentional demands [33, 35, 61, 67, 68]. However, previous studies have shown that hypervariability of FC during rest is a hallmark of brain disorders including schizophrenia, bipolar disorder, autism spectrum disorder, Alzheimer’s disease, and Parkinson’s disease, potentially reflecting a frequent emergence of a state of dysconnectivity and disrupted exchange of information among brain regions [36, 69–73].
Likewise, our results revealed that, relative to controls, the AUD group exhibited hypervariability of FC between the cerebellum and both the FPN and VAN, suggesting a disorganized FC dynamic and reduced overall connectivity strength within cerebro-cerebellar executive control and attention networks. These networks are involved in top-down cognitive control, working memory, and attention shifting [74–76], which have been shown to be impaired in AUD [77–80]. Although these networks are anchored in the frontal lobe, the coordinated interaction among distributed brain regions, including the cerebellum, is believed to be a critical component for maintaining efficient cognitive functioning [81]. Thus, we hypothesize that the observed hypervariability of cerebro-cerebellar FC within these networks would be associated with deficits in executive function and attention, often attributed to the neurotoxic effects of excessive use of alcohol [1].
Abnormal Cerebellar Flexibility and Integration in AUD
The AUD group showed significantly less flexibility in the cerebellum than controls. Flexibility has been previously associated with diverse cognitive processes including working memory [33], learning [32], attention [82], cognitive flexibility, planning, and processing speed [48, 83], supporting its relevance to cognition and behavior. Moreover, studies of brain disorders have reported abnormally high levels of whole-brain flexibility in patients with schizophrenia and autism, which is thought to reflect temporally less stable, disintegrated, and disordered network dynamics associated with the unique pathophysiology of these disorders [36, 37, 48].
Given that the cerebellum is implicated in AUD, the relative cerebellar inflexibility, during rest, in the AUD group is unlikely to indicate more organized cerebro-cerebellar network dynamics, but rather increased functional rigidity of cerebellar regions across time-varying functional communities. This is likely to reflect a compromised capacity of the cerebellum in AUD to flexibly adapt to environmental changes that require fast reconfiguration of FC patterns and re-organization of brain networks. Nonetheless, this interpretation remains speculative and should be further explored in future studies using externally cued cognitive tasks interspersed by epochs of resting-state, which enable a direct assessment of cerebellar flexibility as environmental conditions change.
We also found that the AUD group exhibited significantly greater integration in the cerebellum than controls. This might reflect compensatory functional remapping in the cerebellum [12], whereby cerebellar regions tend to connect for longer periods with other regions outside their “native” functional systems to promote normal performance on tasks. Recent findings, however, suggest that abnormal cerebellar activity in AUD patients, who are early in abstinence, is unlikely to reflect compensatory re-organization but rather pathological or maladaptive plasticity, especially in the frontal lobe and the cerebellum [84]. In this sense, results from our exploratory analysis also revealed significantly greater integration in the FPN in the AUD group compared to controls. As such, we could also interpret greater integration in the cerebellum and the FPN as a signature of pathological, rather than compensatory, functional re-organization. However, this remains an open question for future studies that explore task-evoked dynamics within the frontocerebellar circuitry.
Exploratory Analysis: Abnormal Large-Scale Network Flexibility in AUD
Results of the exploratory analysis revealed that the AUD group exhibited relatively less flexibility than controls in the salience network (SN) and the cingulo-opercular network (CON). These networks subtend portions of the anterior cingulate cortex, anterior insula/operculum, supplementary motor area, thalamus, and basal ganglia (see Fig. 4c, d), which are highly vulnerable to alcohol-related damage and are purported to take part in the formation of the addiction cycle [81]. Of particular importance, the SN is known to be a highly flexible and versatile network that facilitates the antagonistic activity of the DMN and the executive control and attention networks, promoting cognitive flexibility [85–87]. Altered dynamics between those networks is associated with delayed shifting from internally to externally focused attention, leading to inefficient cognitive processing and delayed responses to changing environmental changes [88]. Considering this, we hypothesize that altered SN dynamics in AUD contributes to commonly reported deficits in cognitive control, memory, and reward/motivation processes [85, 89].
Taken together, the findings from this study corroborate those from the literature suggesting selective and far-reaching alterations in major brain systems encompassing the cerebellum, frontal lobe, and the basal forebrain in AUD [1, 6, 90]. In particular, this study comes in line with previous findings showing that the disruption of cognitive cerebro-cerebellar networks, especially the executive frontocerebellar circuitry, is associated with characteristic behaviors of alcoholism that, on the one hand, enable alcohol use disorders, and on the other hand, may lead to compensation for so-called frontally based dysfunctions in alcoholism. Specifically considered, is the possibility that in AUDcerebellar degeneration and the compromise of the functional dynamics in executive loops may contribute to dysfunction in standard neuropsychological testing as well as inefficiency of information processing, interpersonal problem solving, impulse control [44, 91], conflict processing [92], and behavioral disinhibition [93] that enable maintenance of the addictive behavior. In this context, present findings report AUD-related deficits in all four neuropsychological domains assessed, which is in accordance with the literature that AUD individuals have detectable cognitive impairments, often involving executive dysfunction, attention deficits, and reduced cognitive control (see reviews [1, 79]).
Limitations
Important limitations that could affect the reliability of current findings are the small sample sizes and number of timepoints, which may be sub-optimal for dynamic FC analysis. Future studies should validate current findings using larger sample sizes, longer scanning periods, and multiple fMRI sessions. Another limitation is that the groups were not matched with respect to years of education, socioeconomic status, IQ, depressive symptoms, and smoking status. However, the directionality of these group differences is expected based on the typical epidemiology of the AUD group. A third limitation is the lack of detail at the level of the cerebellum and large-scale brain networks. Therefore, future studies, guided by specific hypotheses, should explore features of dynamic FC at the level of cerebellar sub-modules for a more comprehensive view of AUD-related alterations in the cerebellum.
Conclusion
To the best of our knowledge, this is the first study to bring evidence of altered cerebro-cerebellar dynamic FC in AUD. Our analysis shows that AUD patients exhibit hypervariability in cerebro-cerebellar networks subserving executive control and attention as well as abnormalities in the network role of the cerebellum at fast timescales. Adding to previous findings, our results suggest that AUD confers alterations to cognitive cerebro-cerebellar networks at different temporal and topological scales. Longitudinal studies will be essential for determining whether the features of cerebro-cerebellar dynamic FC are trait characteristics for the development and maintenance of AUD or are state characteristics that may change with sustained sobriety.
Finally, the present findings may provide an impetus for further studies on the dynamic interactions among functional brain networks that subserve executive functioning, cognitive flexibility, and attention and their role in the etiology and pathophysiology of substance use disorders.
Supplementary Material
Funding
The study was supported by the following grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA): AA010723, AA017923, and R21 AA023582. Majd Abdallah was supported by a research grant from the Translational Research and Advanced Imaging Laboratory (TRAIL), University of Bordeaux, France, within the context of the Laboratory of Excellence (Labex) TRAIL-ANR-10-LABX-57.
List of Acronyms
- AUD
alcohol use disorder
- CON
cingulo-opercular network
- DAN
dorsal attention network
- DMN
default mode network
- FC
functional connectivity
- FPN
fronto-parietal network
- RN
reward network
- ROI
region of interest
- RsfMRI
resting-state functional magnetic resonance imaging
- SN
salience network
- VAN
ventral attention network
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s12311-021-01241-y.
Conflict of Interest The authors declare no competing interests.
References
- 1.Sullivan EV, Harris RA, Pfefferbaum A. Alcohol’s effects on brain and behavior. Alcohol Res Health. 2010;33(1–2):127–43. [PMC free article] [PubMed] [Google Scholar]
- 2.Chanraud S, Bernard C. Neuroimagerie de l’alcoolisme chronique. In: Annales Médico-psychologiques, revue psychiatrique, vol. 173: Elsevier; 2015. p. 249–54. [Google Scholar]
- 3.Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28(18):4583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung Y-C, Schulte T, Müller-Oehring EM, Namkoong K, Pfefferbaum A, Sullivan EV. Compromised frontocerebellar circuitry contributes to nonplanning impulsivity in recovering alcoholics. Psychopharmacology. 2014;231(23):4443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers BP, Parks MH, Nickel MK, Katwal SB, Martin PR. Reduced fronto-cerebellar functional connectivity in chronic alcoholic patients. Alcohol Clin Exp Res. 2012;36(2):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology. 2005;180(4): 583–94. [DOI] [PubMed] [Google Scholar]
- 7.Zahr NM, Pfefferbaum A, Sullivan EV. Perspectives on frontofugal circuitry from human imaging of alcohol use disorders. Neuropharmacology. 2017;122:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29(9):1590–600. [DOI] [PubMed] [Google Scholar]
- 9.Pfefferbaum A, Rosenbloom MJ, Chu W, Sassoon SA, Rohlfing T, Pohl KM, et al. White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal dti study. Lancet Psychiatry. 2014;1(3):202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeh P-H, Simpson K, Durazzo TC, Gazdzinski S, Meyerhoff DJ. Tractbased spatial statistics (tbss) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res Neuroimaging. 2009;173(1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chanraud S, Pitel A, Pfefferbaum A, Sullivan E. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex. 2011;21(10):2272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chanraud S, Pitel A-L, Müller-Oehring EM, Pfefferbaum A, Sullivan EV. Remapping the brain to compensate for impairment in recovering alcoholics. Cereb Cortex. 2013;23(1):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desmond JE, Chen SA, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: an fmri study. Neuroimage. 2003;19(4):1510–20. [DOI] [PubMed] [Google Scholar]
- 14.Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fmri measurement of brain dysfunction in alcohol dependent young women. Alcohol Clin Exp Res. 2001;25(2): 236–45. [PubMed] [Google Scholar]
- 15.Wilcox CE, Dekonenko CJ, Mayer AR, Bogenschutz MP, Turner JA. Cognitive control in alcohol use disorder: deficits and clinical relevance. Rev Neurosci. 2014;25(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80:807–15. [DOI] [PubMed] [Google Scholar]
- 18.Manto M, Bower JM, Conforto AB, Delgado-García JM, Da Guarda SNF, Gerwig M, et al. Consensus paper: roles of the cerebellum in motor control—the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2012;11(2):457–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmahmann JD. The cerebellum and cognition. Neurosci Lett. 2019;688:62–75. [DOI] [PubMed] [Google Scholar]
- 20.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998; 121:561–79. [DOI] [PubMed] [Google Scholar]
- 21.Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80(3):807–15. [DOI] [PubMed] [Google Scholar]
- 22.Sokolov AA, Miall RC, Ivry RB. The cerebellum: adaptive prediction for movement and cognition. Trends Cogn Sci. 2017;21(5): 313–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernardin F, Maheut-Bosser A, Paille F. Cognitive impairments in alcohol dependent subjects. Front Psychiatry. 2014;5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oscar-Berman M, Marinković K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17(3):239–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zahr NM, Pitel A-L, Chanraud S, Sullivan EV. Contributions of Studies on Alcohol Use Disorders to Understanding Cerebellar Function. Neuropsychol Rev. 2010;20:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breakspear M Dynamic models of large-scale brain activity. Nat Neurosci. 2017;20(3):340–52. [DOI] [PubMed] [Google Scholar]
- 27.Calhoun VD, Miller R, Pearlson G, Adalı T. The chronnectome: time-varying connectivity networks as the next frontier in fmri data discovery. Neuron. 2014;84(2):262–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zalesky A, Fornito A, Cocchi L, Gollo LL, Breakspear M. Timeresolved resting-state brain networks. Proc Natl Acad Sci. 2014;111(28):10341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex. 2014;24(3):663–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rashid B, Damaraju E, Pearlson GD, Calhoun VD. Dynamic connectivity states estimated from resting fmri identify differences among schizophrenia, bipolar disorder, and healthy control subjects. Front Hum Neurosci. 2014;8:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vidaurre D, Abeysuriya R, Becker R, Quinn AJ, Alfaro-Almagro F, Smith SM, et al. Discovering dynamic brain networks from big data in rest and task. NeuroImage. 2018;180:646–56 Brain Connectivity Dynamics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci. 2011;108(18):7641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braun U, Schäfer A, Walter H, Erk S, Romanczuk-Seiferth N, Haddad L, et al. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci. 2015;112(37):11678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fong AHC, Yoo K, Rosenberg MD, Zhang S, Li C-SR, Scheinost D, et al. Dynamic functional connectivity during task performance and rest predicts individual differences in attention across studies. NeuroImage. 2019;188:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Douw L, Wakeman DG, Tanaka N, Liu H, Stufflebeam SM. State dependent variability of dynamic functional connectivity between frontoparietal and default networks relates to cognitive flexibility. Neuroscience. 2016;339:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harlalka V, Bapi RS, Vinod P, Roy D. Atypical flexibility in dynamic functional connectivity quantifies the severity in autism spectrum disorder. Front Hum Neurosci. 2019;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braun U, Schäfer A, Bassett DS, Rausch F, Schweiger JI, Bilek E, et al. Dynamic brain network reconfiguration as a potential schizophrenia genetic risk mechanism modulated by nmda receptor function. Proc Natl Acad Sci. 2016;113(44):12568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakoğlu Ü, Pearlson GD, Kiehl KA, Wang YM, Michael AM, Calhoun VD. A method for evaluating dynamic functional network connectivity and taskmodulation: application to schizophrenia. MAGMA. 2010;23(5–6):351–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vergara VM, Weiland BJ, Hutchison KE, Calhoun VD. The impact of combinations of alcohol, nicotine, and cannabis on dynamic brain connectivity. Neuropsychopharmacology. 2018;43(4):877–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong J-Y, Müller-Oehring EM, Pfefferbaum A, Sullivan EV, Kwon D, Schulte T. Aberrant blood-oxygen-level-dependent signal oscillations across frequency bands characterize the alcoholic brain. Addict Biol. 2018;23(2):824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerchen MF, Weiss F, Kirsch M, Rentsch A, Halli P, Kiefer F, et al. Dynamic frontostriatal functional peak connectivity (in alcohol use disorder). Hum Brain Mapp. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdallah M, Farrugia N, Chirokoff V, Chanraud S. Static and dynamic aspects of cerebro-cerebellar functional connectivity are associated with self-reported measures of impulsivity: a resting-state fmri study. Netw Neurosci. 2020;4(3):891–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, et al. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol. 2010;15(2):217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fein G, Di Sclafani V, Finn P. Sensation seeking in long-term abstinent alcoholics, treatment-naive active alcoholics, and nonalcoholic controls. Alcohol Clin Exp Res. 2010;34:1045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miquel M, Nicola SM, Gil-Miravet I, Guarque-Chabrera J, Sanchez-Hernandez A. A working hypothesis for the role of the cerebellum in impulsivity and compulsivity. Front Behav Neurosci. 2019;13:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hindriks R, Adhikari MH, Murayama Y, Ganzetti M, Mantini D, Logothetis NK, et al. Can sliding-window correlations reveal dynamic functional connectivity in resting-state fmri. Neuroimage. 2016;127:242–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seitzman BA, Gratton C, Marek S, Raut RV, Dosenbach NU, Schlaggar BL, et al. A set of functionally-defined brain regions with improved representation of the subcortex and cerebellum. Neuroimage. 2020;206:116290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gifford G, Crossley N, Kempton MJ, Morgan S, Dazzan P, Young J, et al. Resting state fMRI based multilayer network configuration in patients with schizophrenia. NeuroImage: Clinical. 2020;25: 102169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mucha PJ, Richardson T, Macon K, Porter MA, Onnela J-P. Community structure in time-dependent, multiscale, and multiplex networks. Science. 2010;328(5980):876–8. [DOI] [PubMed] [Google Scholar]
- 50.Gerraty RT, Davidow JY, Foerde K, Galvan A, Bassett DS, Shohamy D. Dynamic flexibility in striatal-cortical circuits supports reinforcement learning. J Neurosci. 2018;38(10):2442–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattar MG, Cole MW, Thompson-Schill SL, Bassett DS. A functional cartography of cognitive systems. PLoS Comput Biol. 2015;11(12):e1004533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zahr NM, Pohl KM, Saranathan M, Sullivan EV, Pfefferbaum A. Hippocampal subfield CA2+3 exhibits accelerated aging in Alcohol Use Disorder: A preliminary study. NeuroImage: Clinical. 2019;22:101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sullivan EV, Zahr NM, Saranathan M, Pohl KM, Pfefferbaum A. Convergence of three parcellation approaches demonstrating cerebellar lobule volume deficits in Alcohol Use Disorder. NeuroImage: Clinical. 2019;24:101974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sullivan EV, Zhao Q, Pohl KM, Zahr NM, Pfefferbaum A. Attenuated cerebral blood flow in frontolimbic and insular cortices in Alcohol Use Disorder: Relation to working memory. J Psychiatr Res. 2021;136:140–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Honnorat N, Saranathan M, Sullivan EV, Pfefferbaum A, Pohl KM, Zahr NM. Performance ramifications of abnormal functional connectivity of ventral posterior lateral thalamus with cerebellum in abstinent individuals with Alcohol Use Disorder. Drug Alcohol Depend. 2021;220:108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zahr NM, Sullivan EV, Pohl KM, Pfefferbaum A, Saranathan M. Sensitivity of ventrolateral posterior thalamic nucleus to back pain in alcoholism and CD4 nadir in HIV. Hum Brain Mapp. 2020;41(5):1351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Association, A. P. Diagnostic and statistical manual of mental disorders. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- 58.Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fmriprep: a robust preprocessing pipeline for functional mri. Nat Methods. 2019;16(1):111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Behzadi Y, Restom K, Liau J, Liu TT. A component-based noise correction method (compcor) for bold and perfusion based fmri. NeuroImage. 2007;37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xia M, Wang J, He Y. Brainnet viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8(7):e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J, Cheng W, Liu Z, Zhang K, Lei X, Yao Y, et al. Neural, electrophysiological and anatomical basis of brain-network variability and its characteristic changes in mental disorders. Brain. 2016;139(8):2307–21. [DOI] [PubMed] [Google Scholar]
- 62.Blondel VD, Guillaume J-L, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech Theory Exp. 2008;2008(10):P10008. [Google Scholar]
- 63.Newman ME, Girvan M. Finding and evaluating community structure in networks. Phys Rev E. 2004;69(2):026113. [DOI] [PubMed] [Google Scholar]
- 64.Jeub LGS, Bazzi M, Jutla IS, Mucha PJ. A generalized Louvain method for community detection implemented in MATLAB. 2011–2019. Available from: https://github.com/GenLouvain/GenLouvain.
- 65.Bassett DS, Yang M, Wymbs NF, Grafton ST. Learning-induced autonomy of sensorimotor systems. Nat Neurosci. 2015;18(5):744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 67.Davison EN, Schlesinger KJ, Bassett DS, Lynall M-E, Miller MB, Grafton ST, et al. Brain network adaptability across task states. PLoS Comput Biol. 2015;11(1):e1004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fransson P Spontaneous low-frequency bold signal fluctuations: an fmri investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26(1):15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Engels G, Vlaar A, McCoy B, Scherder E, Douw L. Dynamic functional connectivity and symptoms of Parkinson’s disease: a resting-state fmri study. Front Aging Neurosci. 2018;10:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Long Y, Liu Z, Chan CKY, Wu G, Xue Z, Pan Y, et al. Altered temporal variability of local and large-scale resting-state brain functional connectivity patterns in schizophrenia and bipolar disorder. Front Psychiatry. 2020;11:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mash LE, Linke AC, Olson LA, Fishman I, Liu TT, Müller R-A. Transient states of network connectivity are atypical in autism: a dynamic functional connectivity study. Hum Brain Mapp. 2019;40(8):2377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Guo G, Tian Y. Increased temporal dynamics of intrinsic brain activity in sensory and perceptual network of schizophrenia. Front Psychiatry. 2019;10:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu H, Huang J, Deng L, He N, Cheng L, Shu P, et al. Abnormal dynamic functional connectivity associated with subcortical networks in Parkinson’s disease: a temporal variability perspective. Front Neurosci. 2019;13:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marek S, Dosenbach NU. The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues Clin Neurosci. 2018;20(2):133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheffield JM, Repovs G, Harms MP, Carter CS, Gold JM, MacDonald AW III, et al. Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia. 2015;73:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20(2):150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Le Berre A-P, Fama R, Sullivan EV. Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcohol Clin Exp Res. 2017;41(8):1432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Le Berre A-P, Laniepce A, Segobin S, Pitel A-L, Sullivan E. Alcohol use disorder: permanent and transient effects on the brain and neuropsychological functions, chapter 15. 2019; pages 302–332. [Google Scholar]
- 79.Oscar-Berman M, Valmas MM, Sawyer KS, Ruiz SM, Luhar RB, Gravitz ZR. Profiles of impaired, spared, and recovered neuropsychologic processes in alcoholism. In: Handbook of clinical neurology, vol. 125: Elsevier; 2014. p. 183–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sullivan EV, Pfefferbaum A. Brain-behavior relations and effects of aging and common comorbidities in alcohol use disorder: a review. Neuropsychology. 2019;33(6):760–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weiland BJ, Sabbineni A, Calhoun VD, Welsh RC, Bryan AD, Jung RE, et al. Reduced left executive control network functional connectivity is associated with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(9):2445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shine JM, Bissett PG, Bell PT, Koyejo O, Balsters JH, Gorgolewski KJ, et al. The dynamics of functional brain networks: Integrated network states during cognitive task performance. Neuron. 2016;92(2):544–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pedersen M, Zalesky A, Omidvarnia A, Jackson GD. Multilayer network switching rate predicts brain performance. Proc Natl Acad Sci. 2018;115(52):13376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ritz L, Segobin S, Lannuzel C, Laniepce A, Boudehent C, Cabé N, et al. Cerebellar hypermetabolism in alcohol use disorder: compensatory mechanism or maladaptive plasticity. Alcohol Clin Exp Res. 2019;43(10):2212–21. [DOI] [PubMed] [Google Scholar]
- 85.Chen T, Cai W, Ryali S, Supekar K, Menon V. Distinct global brain dynamics and spatiotemporal organization of the salience network. PLoS Biol. 2016;14(6):e1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nomi JS, Farrant K, Damaraju E, Rachakonda S, Calhoun VD, Uddin LQ. Dynamic functional network connectivity reveals unique and overlapping profiles of insula subdivisions. Hum Brain Mapp. 2016;37(5):1770–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steimke R, Nomi JS, Calhoun VD, Stelzel C, Paschke LM, Gaschler R, et al. Salience network dynamics underlying successful resistance of temptation. Soc Cogn Affect Neurosci. 2017;12(12): 1928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bolton TA, Wotruba D, Buechler R, Theodoridou A, Michels L, Kollias S, et al. Triple network model dynamically revisited: lower salience network state switching in pre-psychosis. Front Physiol. 2020;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci. 2008;105(34): 12569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nixon SJ, Tivis R, Ceballos N, Varner JL, Rohrbaugh J. Neurophysiological efficiency in male and female alcoholics. Prog Neuro-Psychopharmacol Biol Psychiatry. 2002;26:919–27. [DOI] [PubMed] [Google Scholar]
- 92.De Rosa E, Desmond JE, Anderson AK, Pfefferbaum A, Sullivan EV. The human basal forebrain integrates the old and the new. Neuron. 2004;41:825–37. [DOI] [PubMed] [Google Scholar]
- 93.Fein G, Di Sclafani V. Cerebral reserve capacity: implications for alcohol and drug abuse. Alcohol. 2004;32:63–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


