Abstract
Schizophrenia is a complex condition associated with perceptual disturbances, decreased motivation and affect, and disrupted cognition. Individuals living with schizophrenia may experience myriad poor outcomes, including impairment in independent living and function as well as decreased life expectancy. Though existing treatments may offer benefit, many individuals still experience treatment resistant and disabling symptoms. In light of the negative outcomes associated with schizophrenia and the limitations in currently available treatments, there is a significant need for novel therapeutic interventions. Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive brain stimulation technique that can modulate the activity of discrete cortical regions, allowing direct manipulation of local brain activation and indirect manipulation of the target’s associated neural networks. rTMS has been studied in schizophrenia for the treatment of auditory hallucinations, negative symptoms, and cognitive deficits, with mixed results. The field’s inability to arrive at a consensus on the use rTMS in schizophrenia has stemmed from a variety of issues, perhaps most notably the significant heterogeneity amongst existing trials. In addition, it is likely that factors specific to schizophrenia, rather than the rTMS itself, have presented barriers to the interpretation of existing results. However, advances in approaches to rTMS as a biologic probe and therapeutic, many of which include the integration of neuroimaging with rTMS, offer hope that this technology may still play a role in improving the understanding and treatment of schizophrenia.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-021-01046-1.
Key Words: Repetitive transcranial magnetic stimulation, Schizophrenia, fMRI, MRI
Introduction to Schizophrenia
Schizophrenia is a complex and disabling illness that is estimated to affect over 20 million people across the globe [1]. Since Emil Kraepelin’s coining of the term dementia praecox in the late nineteenth century [2], much has been discovered about the condition. However, in spite of advances in our understanding of schizophrenia, it nonetheless remains one of the top 10 causes of disability worldwide with detrimental effects on individual, family, and societal levels [3].
Schizophrenia is a heterogeneous condition consisting of “positive” and “negative” symptoms as well as cognitive dysfunction. Positive symptoms include delusional thinking, disorganized speech and behavior, and perceptual disturbances such as auditory hallucinations, which have been associated with disrupted function of temporal-parietal cortical circuitry [4]. Negative symptoms include avolition, blunted affect, and asociality. Cognitive deficits are commonly observed in domains including working, episodic and verbal memory, processing speed, and social cognition. Negative symptoms and cognitive deficits are major drivers of poor outcomes associated with the illness [5], and both have been associated with decreased function of the dorsolateral prefrontal cortex (DLPFC) [4, 6–8]. The mainstay of schizophrenia treatment is antipsychotic pharmacotherapy, which is generally effective for positive symptoms but less so for negative symptoms [9] and cognitive deficits [10, 11]. While approximately a third of affected individuals will adequately respond to antipsychotics, functionally impairing symptoms persist in a third, and up to another third will see little benefit [12]. There is a clear need for novel therapeutics for schizophrenia, as evidenced by associated morbidity and poor outcomes more than 60 years after antipsychotic medications were introduced [13]. Non-invasive brain modulation techniques, such as repetitive transcranial magnetic stimulation (rTMS), are potential novel therapeutic options.
Introduction to Repetitive Transcranial Magnetic Stimulation
Background and Mechanism
Transcranial magnetic stimulation (TMS) was first described by Anthony Barker [14] in a letter to Lancet in 1985, following his observation that running an electrical current through a circular magnetic coil applied to the human motor cortex could produce movements of the contralateral limb. Single pulse TMS has since been used to measure cortical excitability as well as to create a “virtual lesion” where investigators stimulate a cortical target, creating a short-term disruption in function in order to simulate the effects of a brain insult. This was first demonstrated by Amassian et al. who applied TMS over the visual cortex, observing a temporary impairment in visual perception [15]. The observation that repetitively pulsed TMS, coined repetitive transcranial magnetic stimulation (rTMS), could produce effects that extend beyond the period of stimulation raised the possibility of TMS as a clinical intervention in addition to an investigational tool [16].
rTMS applies a repetitively pulsed magnetic field over the scalp to induce an electric field within a discrete area of the brain. This electric field modulates ion flow across the neuronal cellular membrane with resultant changes in neuronal polarization. The end result is altered neuronal activity in the area where the rTMS is applied [17, 18] (Fig. 1). It is thought that rTMS causes lasting neural effects through long-term potentiation (LTP) and long-term depression (LTD) like mechanisms [19]. rTMS is a malleable neuromodulation technique that can exert varying physiologic effects depending on how it is administered. Early studies demonstrated that using different stimulation frequencies (Hz), or pulses per second, may result in different effects. Investigators observed that rTMS administered at 10–25 Hz, or high-frequency rTMS, led to an increase in cortical excitability. Stimulation at 1 Hz, or low frequency rTMS, is thought to result in cortical inhibition [20, 21]. Though the precise mechanisms are unclear, it is believed that these disparate effects may be related to differences in calcium influx resulting from application of these stimulation fields [22–24].
Fig. 1.
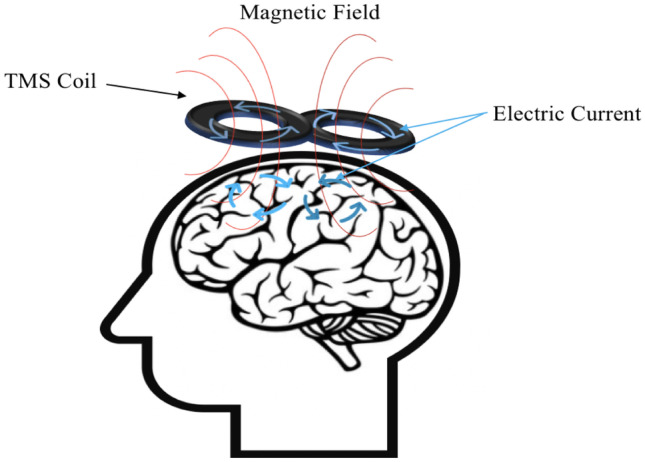
During repetitive transcranial magnetic stimulation (rTMS), an electric current is used to create a repetitively pulsed magnetic field. When applied over the scalp, this induces an electric current within a discrete area of the brain with resultant changes in neuronal polarization. The end result is altered neuronal activity in the area where the rTMS is applied
Other variables, including the intensity of stimulation, pulse pattern, and coil type have been used to modify the effects of rTMS. The stimulation intensity is believed to inform rTMS induced changes in cortical excitability. Intensity is defined as a percentage of an individual’s resting motor threshold (MT), which is the stimulation output required to produce a motor-evoked potential [25]. Stimulating at intensities greater than an individual’s resting MT is believed to result in more sustained effects, while stimulation below MT threshold may result in weaker, less consistent effects [20, 26, 27].
The coil type used in rTMS is important, as this influences both the depth and focality of stimulation. The figure-8 coil initially came into use as it offered a focality advantage over the circular coil [28]. Since then, the H-coil has been shown to penetrate more deeply [29], though focality is lost. There are many different coil types [30], and investigations remain underway to clarify optimized depth-focality tradeoff [31, 32].
Different stimulation patterns are observed to result in varying physiologic effects. Theta burst stimulation (TBS) is an increasingly common variant of rTMS that was developed based on studies in rodent and human brains which indicated that theta rhythms are associated with LTP [33–37]. TBS consists of lower intensity stimuli applied at a high frequency over a shorter period of time, with effects potentially lasting longer than standard rTMS [38]. TBS is often administered in bursts of 3 stimuli at 50 Hz (20 ms between each stimulus) at 80% MT, with repeated bursts at intervals of 200 ms (5 Hz). When applied intermittently (intermittent theta burst stimulation or iTBS), e.g., 2 s at a time with an 8 s break, it results in increased cortical excitability. When applied continuously (continuous theta burst stimulation, cTBS), it results in cortical inhibition, though recent work has suggested that cTBS may be associated with inconsistent effects [39, 40]. Another pattern is quadripulse stimulation (QPS), which consists of stimulation at 90% of MT, arranged in 4 pulses separated by a given interstimulus interval. Shorter interstimulus intervals lead to long-lasting facilitation, whereas longer interstimulus intervals led to long-lasting suppression. Investigators have observed that an interval of 50 ms, corresponding to a frequency of 20 Hz, resulted in suppression lasting over an hour. Without the QPS pattern, rTMS at this frequency would result in a few minutes of facilitation [41]. Relative to TBS, QPS has demonstrated stronger and more predictable (smaller number of opposite responders) excitatory and inhibitory effects [42].
Effects on Neural Circuitry
rTMS has many clinical applications because it impacts not only the region that is directly stimulated but also associated downstream circuitry. In her 2018 review, Hanlon [43] reasons that even the earliest studies of TMS demonstrate a polysynaptic effect, because the impulse travels from the upper motor neuron to the lower motor neuron, resulting in a motor evoked potential. PET studies have provided data elucidating the effects of rTMS at a brain circuit level. Investigations in healthy subjects have demonstrated that rTMS targeting the prefrontal cortex (PFC) induced dopamine release in the anterior cingulate cortex, orbitofrontal cortex, caudate nucleus, and striatum [44–46]. Vidal-Pineiro and colleagues [47] demonstrated via magnetic resonance spectroscopy that iTBS applied to the inferior parietal lobule of healthy volunteers was associated with increased gamma aminobutyric acid (GABA) throughout the posteromedial cortex, with the strength of GABA release at the posterior cingulate cortex correlating to baseline functional connectivity between these regions.
Functional magnetic resonance imaging (fMRI) studies have also demonstrated the activation of neural circuitry following TMS. Denslow et al. [48] demonstrated that single-pulse TMS applied to the motor cortex resulted in motor circuit activation. A recent study by Dowdle and colleagues [49] demonstrated that single-pulse TMS to the left DLPFC, compared to sham TMS, resulted in increased activity in the anterior cingulate, caudate, and thalamus. These results provide support for the belief that multiple networks involved in psychopathology can be modulated by TMS and have opened the door for various therapeutic applications.
Safety and Tolerability of rTMS
A strength of rTMS is its safety profile. The most severe known adverse effect is seizures, and these are remarkably rare, occurring in fewer than 1 per 60,000 sessions [50]. Syncope has been observed and can be distressing, especially when convulsive features may make the episode appear to be a seizure [18]. The most common side effects are local pain and headaches [18]. A 2008 review of sham-controlled studies found that 28% experienced headache and 39% experienced pain with active rTMS, versus 16% headache and 15% pain with sham treatment [51]. Fortunately, these symptoms commonly resolve without clinical intervention.
rTMS in Schizophrenia
The majority of studies examining rTMS in schizophrenia fall into one of two camps. The first are investigations modulating auditory cortical function via stimulation of the temporal-parietal cortex (TPC), an area for which there had traditionally been less data regarding tolerability or efficacy. The second are investigations that retained the same or similar protocols as studies in major depressive disorder, targeting the DLPFC. Though few in number, there have also been investigations studying other rTMS targets in schizophrenia, such as the inferior parietal lobule and cerebellum. The scope of this review will include studies targeting the TPC and DLPFC in schizophrenia, with a focus on trials leveraging neuroimaging in either study design or outcomes.
rTMS of the Temporal-Parietal Cortex in Schizophrenia
Aberrant TPC function has been hypothesized to contribute to a cardinal symptom of schizophrenia, auditory hallucinations. This theory has been supported by neuroimaging investigations demonstrating associations between auditory hallucinations and altered TPC function [52]. Leveraging the ability of rTMS to modulate neural function and connectivity, investigators have questioned whether stimulation of the TPC may reverse these neural abnormalities and thus ameliorate auditory hallucinations associated with schizophrenia (Table 1).
Table 1.
Temporoparietal cortex rTMS for the treatment of schizophrenia
| First author, year | Design | Control | Sample size | Frequency, %MT, sessions | Pulses per session | Side | Summary |
|---|---|---|---|---|---|---|---|
| Hoffman, 2000 | Double-blind, crossover | Tilted coil | 12 | 1 Hz, 80%, 4 |
Day 1: 240 Day 2: 480 Day 3: 720 Day 4: 960 |
L |
Improvement on individualized AH composite scale |
| d’Alfonso, 2002 | Open label, single arm | No | 9 | 1 Hz, 80%, 10 | 1200 | L | Decreased severity of AH measured by Topography of Voices Rating Scale |
| Hoffman, 2003 | Double-blind, randomized | Tilted coil | 24 | 1 Hz, 90%, 10 |
Day 1: 480 Day 2: 720 Days 3–7: 960 |
L | Improved AH, including decreased frequency and attentional salience, measured by Auditory Hallucinations rating Scale developed by same group |
| McIntosh, 2004 | Double-blind, randomized | Tilted coil | 16 | 1 Hz, 80%, 4 |
Day 1: 240 Day 2: 480 Day 3: 720 Day 4: 960 |
L | Improvement in active and sham groups, no significant between group differences |
| Schonfeldt-Lecuona, 2004 | Double-blind, crossover | Alternate site | 12 | 1 Hz, 90%, 5 | 960 | L | No significant improvement in AH, though trend towards improvement in subset receiving fMRI-guided rTMS |
| Poulet, 2005 | Double-blind, crossover | Sham coil | 10 | 1 Hz, 90%, 10 | 1000 | L | Significant reduction in AHRS score |
| Lee, 2005 | Double-blind, randomized | Tilted coil | 39 | 1 Hz, 100%, 10 | 1200 | L, R | Improved AH severity, improved PANSS positive score and CGI |
| Hoffman, 2005 | Double-blind, randomized | Tilted coil | 50 | 1 Hz, 90%, 10 |
Day 1: 480 Day 2: 720 Days 3–7: 960 |
L | Improved HCS, CGI |
| Chibbaro,2005 | Single-blind, randomized | Tilted coil | 16 | 1 Hz, 90%, 4 | 900 | L | Significant improvements in SAPS and SANS scores, improved SAH score |
| Saba, 2005 | Double-blind, randomized | Sham coil | 18 | 1 Hz, 80%, 10 | 300 | L | No significant difference between groups on PANSS, CGI |
| Fitzgerald 2005 | Double-blind, randomized | Tilted coil | 33 | 1 Hz, 90%, 10 | 900 | L | No significant effect on HCS, PANNS, or PSYRATS, did find decreased volume of voices |
| Jandl, 2006 | Double-blind, randomized | Tilted coil | 16 | 1 Hz, 100%, 5 | 900 | L, R | No effect on PSYRATS |
| Rosa, 2007 | Double-blind, randomized | Sham coil | 1 Hz | L | No significant effect on AHRS, PANSS, CGI | ||
| Vercammen, 2009 | Double-blind, randomized | Sham coil | 36 | 1 Hz, 90%, 12 | 1200 | L, B/L | Reduced AH frequency in L TPC group, decrease PANSS AH in B/L group |
| Montagne-Larmurier, 2009 | No control | No | 11 | 20 Hz, 80%, 4 | 2600 | L | Improved AHRS and CGI |
| Loo, 2010 | Double-blind, crossover | Tilted coil | 18 | 1 Hz, 110%, 3 | 960 | L, R, vertex | None during double-blind, improved AHRS in L, R groups during open-label |
| Vercammen, 2010 | Double-blind, randomized | Sham coil | 18 | 1 Hz, 90%, 12 | 1200 | L | No significant effects between groups, trend level improvement in AH for active group |
| De Jesus, 2011 | Double-blind, randomized | Tilted coil | 17 | 1 Hz, 90%, 20 |
Day 1: 480 Day 2:960 Days 3–18: 1200 |
L | Improved BPRS, no effects on AHRS, CGI |
| Slotema, 2011 | Double-blind, randomized | Tilted coil | 62 | 1 Hz, 90%, 15 | 1200 | L | No effects on AHRS, PANSS |
| Blumberger, 2012 | Double-blind, randomized | Tilted coil | 51 | 6 Hz priming before 1 Hz vs. 1 Hz, 115%, 20 | 1200 | L | No effect on PSYRATS |
| Kindler, 2013 | Single-blind, randomized | No | 24 | cTBS vs. 1 Hz, both 90%, 10 |
cTBS: 3204 pulses on days 1–3, 1602 pulses on days 4–10 LF: 480–960 pulses |
L | No differences in PANSS, AHRS, PSYRATS, HCS between 1 Hz and cTBS groups |
| Kindler, 2013 | Single-blind, randomized | No | 30 | cTBS vs 1 Hz, 90%, 10 |
cTBS: 3204 pulses on days 1–3, 1602 pulses on days 4–10 LF: 480–960 pulses |
L | Compared to control, improved PSYRAT score, no difference between 1 Hz and cTBS groups |
| Bais, 2014 | Double-blind, randomized | Sham coil | 51 | 1 Hz, 90%, 12 | 1200 | L, L/R |
Trend effect on PANSS hallucination Item, no significant difference between groups on AHRS |
| de Weijer, 2014 | Randomized | No | 28 | 1 Hz, 90% vs. 20 Hz 80%, 5, then one weekly follow-up session over 3 weeks |
LF: 1200 pulses HF: 2600 pulses |
L, R | Improvement on AHRS in both groups |
| Koops, 2016 | Double-blind, randomized | Sham coil | 71 | cTBS, 80%, 10 | 900 | L | Improvement in PSYRATS, AHRS and PANSS positive and general sub-scores that did not differ between groups |
| Kimura, 2016 | Double-blind, randomized | Sham coil | 30 | 20 Hz, 80%, 4 | 2600 | L | No effects on AHRS |
| Paillere-Martinot, 2017 | Double-blind, randomized | Sham coil | 27 | 1 Hz, 100%, 10 | 1200 | L | Reduction in SAPS hallucination subscale score in both groups, no difference between two groups |
| Dollfus, 2018 | Double-blind, randomized | Sham coil | 74 | 20 Hz, 80%, 4 sessions over 2 days | 2600 | L | No response to treatment, defined as 30% reduction in AHRS frequency item |
| Chen, 2019 | Non-randomized | Used control group from another study who had received sham TMS with a sham coil | 27 | cTBS, 80% MT, 42 sessions over 14 days | 1800 | L | Improvement in AHRS, PANSS negative and total scores |
AHRS auditory hallucination rating scale, MT motor threshold, L left, R right, B/L bilateral, L/R left then right, TPC temporal-parietal cortex, DLPFC, dorsolateral prefrontal cortex, cTBS continuous theta burst stimulation, post-mid posterior middle, fMRI functional magnetic resonance imaging, AH auditory hallucinations, PANSS positive and negative syndrome scale, CGI clinical global impression scale, HCS hallucination change scale, PSYRATS psychotic symptom rating scales, BPRS brief psychiatric rating scale, SAPS scale for the assessment of positive symptoms, SANS scale for the assessment of negative symptoms, SAH scale for auditory hallucinations
The first to publish in this area was Hoffman et al. [53], who used a 1 Hz rTMS protocol in three subjects, stimulating directly over the midway point between the left temporal (T3) and left parietal (P3) positions defined by the International 10–20 EEG system, an approach that has been replicated in trials that followed. All subjects reported improvements in auditory hallucinations following rTMS, and two of the three experienced a maintained reduction in hallucination severity two weeks later. Hoffman et al. [54–56] later replicated these findings, extending the duration of treatment. Other investigators began to examine the effects of rTMS on auditory hallucinations, with mixed results. Chibbaro and colleagues [57] observed maintained reduction of auditory hallucinations at follow-up 8 weeks post-stimulation, while work by Poulet et al. [58] reported significantly reduced scores on the auditory hallucinations rating scale (AHRS) after rTMS twice per day over 5 days. However, in other trials, the results demonstrated either similar improvement in rTMS and sham groups [59–63] or no effect in either [64–67].
While the majority of studies focusing on the TPC as a rTMS target involve a 1 Hz protocol, there are a few exceptions. Montagne-Larmurier and colleagues [68] administered 20 Hz rTMS targeting the left posterior superior temporal sulcus twice daily over 2 days. Subjects experienced a significant reduction in combined auditory and visual hallucinations at 12-day follow-up, though there was no control group. Kimura et al. [69] and Dollfus et al. [70] also used 20 Hz protocols directed at the left TPC but did not find significant reductions in auditory hallucinations. cTBS protocols targeting the left TPC have likewise produced mixed results, showing either no effect [71] or a reduction in auditory hallucinations relative to sham treatment [72]. Though initial studies targeting the TPC for the treatment of auditory hallucinations were promising, subsequent work revealed mixed results. This prompted investigators to consider alternative approaches, such as imaging guided rTMS.
Role of Neuroimaging in Studies of rTMS Targeting the TPC in Schizophrenia
Neuronavigation
While some studies have used traditional EEG scalp lead placement to guide and standardize target location, others have employed neuroimaging, commonly structural magnetic resonance imaging (MRI), to more precisely target areas of interest. The selected target area can also be specified based on brain function, as revealed by fMRI data collected at rest or during task performance.
Schönfeldt-Lecuona and colleagues [73] were the first to use image-guided, neuronavigated rTMS in schizophrenia. In this small study (n = 11), T1-weighted structural MRI was employed to identify the superior temporal gyrus and Broca’s area. Five subjects also had fMRI data collected during a delayed match-to-sample task, where subjects were asked to memorize and later recall previously shown syllables and geometric figures, which activated regions within the left superior temporal sulcus. In a blinded crossover trial between 4 conditions (left superior temporal gyrus, Broca’s area, midline parieto-occipital sham stimulation, and no stimulation), no significant effects on auditory hallucinations were observed in any of the four groups, though there was a trend toward improvement in the subjects whose left superior temporal sulcus was targeted via fMRI.
In a larger randomized control trial of subjects experiencing auditory-visual hallucinations, fMRI was used to identify the area of maximal activation in subjects experiencing auditory-visual hallucinations within left or right superior, medial, inferior, and transversal temporal gyri, supramarginal gyrus, and angular gyrus. However, administering 1 Hz rTMS to this target had no effect on positive symptoms compared to a structurally defined anatomical target or sham treatment [74]. Blumberger et al. [75] examined MRI guided stimulation of Herschel’s gyrus in three groups, a 6 Hz priming lead-in to 1 Hz stimulation, 1 Hz, and sham, but did not observe an effect in any of the three groups. Paillere-Martinot et al. [76] targeted the region of greatest temporal activation during a language-fragment detection task but did not observe an effect on hallucinations.
Nathou and colleagues [77], observing the inconsistency of findings in the literature regarding rTMS efficacy in schizophrenia, sought to determine if individual anatomical variations were a possible contributor. Here, 15 subjects received 20 Hz rTMS targeting the left TPC after undergoing MRI. The authors observed that treatment response, i.e., improved AHRS scores, was predicted by the scalp-to-cortex distance and the gray matter density in the target region. Interestingly, the resting MT was not at all predicted by the same measures in the primary hand cortex, leading the authors to argue that individualized resting MT may not be as useful a tool for gauging stimulation intensity in this population.
In summary, structural MRI that focuses on anatomical landmarks, scalp-to-cortex distance, and gray matter density at the target regions may predict clinical response, though the results are varied. fMRI has been employed as a means of more precise targeting of rTMS, though the impact of the practice on efficacy has yet to be fully explored.
Effects of rTMS on Brain Structure or Function
As the field’s understanding of rTMS expanded, studies began to employ neuroimaging for purposes other than navigation. Functional imaging before and after rTMS may be useful for assessing how brain activity is impacted by the stimulation. Further, functional connectivity analysis can characterize downstream, network-related changes in brain function.
In one study, Fitzgerald and colleagues [78] had three subjects perform a repeated block-design word generation fMRI task before and after 1 Hz rTMS of the left TPC. These subjects experienced significantly reduced auditory hallucination severity as well as increased blood oxygen level dependent (BOLD) signal in the left TPC (angular gyrus), left frontal-precentral cortex, and left inferior frontal gyrus compared to imaging from four healthy controls that did not undergo rTMS. Subjects also showed reduced task-related activation in brain regions including left superior temporal gyrus, left inferior frontal gyrus, right inferior frontal gyrus, anterior cingulate cortex, and parietal regions compared to controls. Kindler et al. [79] measured cerebral blood flow (CBF) in patients versus controls with pseudo-continuous magnetic resonance-arterial spin labeling before and after either 1 Hz rTMS or cTBS to the left TPC. The 1 Hz rTMS and cTBS groups exhibited decreased auditory hallucination scores as well as reductions in CBF in the primary auditory cortex, Broca’s area, and cingulate gyrus, compared to controls.
Vercammen and colleagues [80] studied 1 Hz rTMS or sham targeting the left TPC in 18 subjects, obtaining fMRI at baseline and following study intervention. While no significant reduction in auditory hallucinations were observed, there was a trend-level effect (p = 0.068) in the active group. In the active arm, there was significantly increased functional connectivity (FC) between the left TPC and the right insula, but no FC change between pre-determined regions of interest that were hypothesized to be elements of a network substrate for auditory hallucinations. Gromann et al. [81] demonstrated that rTMS, compared to sham, was associated with increased FC between the right TPC, the DLPFC, and the angular gyrus, supporting claims that rTMS may be a mechanism with which to modulate abnormal FC in schizophrenia. Bais et al. [82] measured changes in FC as a result of 1 Hz rTMS targeting either left or bilateral TPC, compared to sham. Results revealed varying effects on network FC, including frontotemporal, auditory-sensorimotor, and default mode network changes. Chen and colleagues [72] performed fMRI before and after active vs sham cTBS of the left TPC, examining changes in FC in addition to auditory hallucinations. The active cTBS group had a greater improvement in symptoms and a larger decrease in FC within the left cerebellum. The investigators observed that greater FC of the cerebellar cluster at baseline was associated with a lesser response to treatment, a potential biomarker of treatment response. In summary, though existing work have successfully employed rTMS as a means of exploring the relationship between TPC structure–function and hallucinations in schizophrenia, the optimized approaches for doing so have yet to be elucidated. This remains a fertile area for continued investigation.
rTMS of the Dorsolateral Prefrontal Cortex in Schizophrenia
In contrast to studies involving the TPC, rTMS investigations targeting the DLPFC in schizophrenia have generally focused on negative symptoms or cognitive dysfunction. Thus far these results have been mixed (Table 2). A pilot study by Cohen et al. [83] treated 6 patients with 20 Hz rTMS over the left DLPFC for 2 weeks. The investigators observed a significant reduction in positive and negative syndrome scale (PANSS) negative symptom score as well as improved performance during a delayed visual memory task, though the study suffered from lack of a control group. Hajak et al. [84] were the first to clearly demonstrate improved negative symptoms following a sham controlled trial of 10 Hz rTMS targeting the left DLPFC, with subsequent investigations similarly observing benefit [85–89]. Li 2016 et al. [90] failed to show negative symptom improvement after four weeks of rTMS, but did report a delayed effect in the rTMS, but not sham group, at 4-week follow-up. Quan and colleagues [91] not only showed improvement in negative symptoms following 10 Hz rTMS of the DLPFC, compared to sham, but also that this was maintained at 24-week follow-up. Schneider et al. [92] targeted the DLPFC with 10 Hz, 1 Hz, and sham stimulation, observing significant improvements in negative symptoms and a trend-level effect on the Wisconsin Card Sorting Task in the 10 Hz group.
Table 2.
Dorsolateral prefrontal cortex rTMS for the treatment of schizophrenia
| First author, year | Design | Control | Sample size | Frequency, %MT, sessions | Pulses per session | Side | Summary |
|---|---|---|---|---|---|---|---|
| Rollnik, 2000 | Double-blind, crossover | Tilted coil | 12 | 20 Hz, 80%, 10 | 800 | L | Improvements in BPRS |
| Holi, 2004 | Double-blind, randomized | Tilted coil | 22 | 10 Hz, 100%, 10 | 1000 | L | No between-group difference in PANSS; no significant change in MMSE or hormones (cortisol, prolactin, TSH) |
| Hajak, 2004 | Double-blind, randomized | Sham coil | 20 | 10 Hz, 110%, 10 | 1,000 | L | Improvement in PANSS negative scores, trend toward improved general and worse positive scores; no cerebral blood flow differences on ECD-SPECT |
| Novak, 2006 | Double-blind, randomized | Tilted coil | 16 | 20 Hz, 90%, 10 | 2,000 | L | No significant effect on PANSS, CGI, MADRS, neuropsychological testing; sham showed a trend toward improvement on positive and negative subscales of PANSS and MADRS; between-group comparisons showed a positive subscale of PANSS after 8 weeks |
| Mogg, 2007 | Double-blind, randomized | Sham coil | 17 | 10 Hz, 110%, 10 | 2,000 | L | No significant difference on PANSS negative scores; active rTMS group had better delayed recall on HVLT |
| Prikryl, 2007 | Double-blind, randomized | Tilted coil | 22 | 10 Hz, 110%, 15 | 1500 | L | Negative symptom improvement on PANSS and SANS |
| Goyal, 2007 | Double-blind, randomized | Tilted coil | 10 | 10 Hz, 110%, 10 | 980 | L | Inpatients without antipsychotic medication for past 2 months- active rTMS group improved negative symptoms on PANSS even when factoring out depressive symptoms with CDSS |
| Fitzgerald, 2008 | Double-blind, randomized | Tilted coil | 20 | 10 Hz, 110%, 15 | 1,000 per hemisphere | B/L | No difference of negative symptoms on SANS or cognitive outcomes on ST, COWAT, and TMT-A/B; trend toward reduction in autistic preoccupation component of PANSS |
| Schneider, 2008 | Double-blind, randomized | Coil cover for sham group | 17 | 1 Hz vs 10 Hz, 110%, 20 | 100 of 1 Hz, 1,000 of 10 Hz | L | 10 Hz group showed improved negative symptoms on SANS at weeks 4 and 8; trend toward improved WCST in 10 Hz group |
| Cordes, 2010 | Double-blind, randomized | Sham coil | 95 | 10 Hz, 110%, 10 | 1,000 | L | No change in PANSS, CGI, or GAF except subgroup with pronounced negative symptoms showed improvements in GAF |
| Mittrach, 2010 | Double-blind, randomized | Sham coil | 32 | 10 Hz, 110%, 10 | 1,000 | L | No effect on cognition based on TMT-A/B, WCST, D2 attention task, and KAI |
| Barr, 2012 | Double-blind, randomized | Tilted coil | 25 | 20 Hz, 90% MT, 20 | 750 per hemisphere | B/L | No improvement of negative symptoms on PANSS or SANS, or depression on CDSS |
| Prikyrl, 2012 | Double-blind, randomized | Sham coil | 30 | 10 Hz, 110%, 15 | 1500 | L | Improved negative and general psychopathology scores on PANSS; improved cognition based on VFT scores but not statistically significant |
| Prikryl, 2013 | Double-blind, randomized | Sham coil | 40 | 10 Hz, 110%, 15 | 2,000 | L | Improved negative symptoms on SANS |
| Guse, 2013 | Double-blind, randomized | Tilted coil | 25 | 10 Hz, 110%, 15 | 1,000 | L | No change in brain activation patterns seen on fMRI during VWMT or cognitive improvement based on TMT-A/B, TAP, WCST |
| Barr, 2013 | Double-blind, randomized | Tilted coil | 27 | 20 Hz, 90%, 20 | 750 per hemisphere | L/R | Cognition improved on n-back working memory task |
| Wolwer, 2014 | Double-blind, randomized | Sham coil | 35 | 10 Hz, 110%, 10 | 1,000 | L | Improved facial affect recognition assessed with 30 digitally reworked photographs of faces |
| Zhao, 2014 | Double-blind, randomized | Tilted coil | 96 |
iTBS,80%, 10 Hz, 20 Hz, 110%, 20 |
2,400 (iTBS) 1500 (10/20 Hz) |
Decreased PANSS negative and general psychopathology, SANS scores in iTBS, 10 Hz, 20 Hz groups. Decrease greater in iTBS compared to 10 and 20 Hz groups | |
| Quan, 2015 | Double-blind, randomized | Tilted coil | 117 | 10 Hz, 80%, 10 | 800 | L | Improved negative symptoms at end of treatment and 24 week follow up based on PANSS and SANS; no effect on CGI |
| Wobrock, 2015 | Double-blind, randomized | Tilted coil | 197 | 10 Hz, 110%, 15 | 1,000 | L | Small but significant improvement in positive symptoms on PANSS; no effect on negative symptoms on PANSS, depression on MADRS or CDSS, symptoms severity on CGI or GAF, or cognitive function on TMT-A/B |
| Dlabac-de lange, 2015 | Double-blind, randomized | Tilted coil | 32 | 10 Hz, 90%, 30 (2 times daily for 15 days) | 2,000 per hemisphere | L/R | Negative symptoms improved on SANS but not PANSS; no change in depressive symptoms on MADRS; cognitive improvement on VFT but not DSST, TMT- A/B, WCST, RAVL; insight improved based on BIS |
| Li, 2016 | Double-blind, randomized | Sham coil | 47 | 10 Hz, 110%, 20 | 1,500 | L | Decreased negative symptoms on SANS at 8 weeks but not 4 weeks |
| Kamp, 2016 | Randomized | Sham coil | 35 | 10 Hz, 110%, 10 | 1,000 | L | Decreased delta band activity thereby decreasing hypofrontality; trend towards a correlation between this and improvement of facial affect recognition |
| Hasan, 2016 | Double-blind, randomized | Tilted coil | 156 | 10 Hz, 110%, 15 | 1,000 | L | No cognitive improvement on RAVL, TMT-A/B, WCST, DST, RWFT |
| Hasan, 2017 | Double-blind, randomized | Tilted coil | 73 | 10 Hz, 110%, 15 | 1,000 | L | Significant correlations between left hippocampal, parahippocampal, and precuneal volume increases measured by MRI; negative symptom improvement on PANSS |
| Francis, 2019 | Double-blind, randomized | Sham coil | 20 | 20 Hz, 110%, 10 | 600 per hemisphere | B/L | Cognitive improvement based on BACS; no significant changes in cortical thickness; thicker L prefrontal cortex predicted greater improvement in cognitive function |
| Wagner, 2019 | Double-blind, randomized | Tilted coil | 26 | 10 Hz, 110%, 15 | 1,000 | L | Improved positive symptoms and general psychopathology PANSS scores in patients on clozapine |
MT motor threshold, L left, R right, B/L bilateral, L/R left then right, DLPFC dorsolateral prefrontal cortex, BACS Brief Assessment of Cognition in Schizophrenia, BIS Birchwood Insight Scale, BPRS Brief Psychiatric Rating Scale, CDSS Calgary Depression Scale for Schizophrenia, CGI Clinical Global Impression scale, COWAT Controlled Oral Word Association Test, DSST Digit Symbol Substitution Test, DST Digit Span Test, ECD-SPECT technetium-99 bicisate Single Photon Emission Computed Tomography, fMRI functional Magnetic Resonance Imaging, GAF Globalized Assessment of Functioning, HVLT Hopkins Verbal Learning Task, KAI Short test of general intelligence, MADRS Montgomery–Åsberg Depression Rating Scale, MMSE Mini-Mental Status Exam, PANSS Positive and Negative Syndrome Scale, RAVL Rey Auditory Verbal Learning Test, RWFT Regensburg Word Fluency Test, SANS Scale for the Assessment of Negative Symptoms, SAPS Scale for the Assessment of Positive Symptoms, ST Stroop Test, TAP Tübinger Aufmerksamkeitsprüfung (computer-based test battery) , iTBS Intermittent Theta Burst Stimulation, TMT- A/B Trail Making Test parts A and B, VFT Verbal Fluency Test, VWMT Verbal Working Memory Task, WCST Wisconsin Card Sorting Test
In a large clinical trial examining three different rTMS paradigm, Zhao et al. [93] divided 96 subjects into 4 groups for stimulation of the left DLPFC: 20 Hz rTMS, 10 Hz rTMS, iTBS, and sham. After 4 weeks of treatment, the three rTMS groups had decreased scores on the PANSS negative and general psychopathology subscales as well as the scale for the assessment of negative symptoms (SANS). The iTBS group experienced a greater decrease in these scores than the 10 and 20 Hz arms. There was no difference between the 10 and 20 Hz groups. Wolwer et al. [94] and Kamp et al. [95] both showed improvement in facial affect recognition after 10 Hz rTMS protocols targeting the left DLPFC.
Though some trials have reported promising results of DLPFC stimulation, others have failed to demonstrate clinical benefit. Holi and colleagues [96] observed improvement in negative symptoms and cognitive deficits in both 10 Hz rTMS and sham conditions, without any significant difference between the two groups. Cordes et al. [97] found no significant improvement in negative symptoms, although a subset of patients with worse negative symptoms at baseline had significantly improved global assessment of functioning (GAF) scores. Mittrach and colleagues [98] performed a double-blind, sham-controlled study with a larger sample (n = 32) to assess for changes in cognition. Subjects received 10 Hz rTMS of the left DLPFC daily over 2 weeks. There were no significant effects of stimulation, though a subgroup with poor baseline performance on the Wisconsin Card Sorting Task demonstrating trend-level improvement (p = 0.059). The authors also argued that the correlational data, while not significant, suggested that “inferior performance in certain neuropsychological aspects before treatment predicts a better response to active rTMS.” Barr et al. [99] administered 20 Hz sequential, bilateral DLPFC rTMS vs sham in a study of 25 subjects over four weeks and detected no significant effect on negative symptoms. In a large, multi-center, double-blinded randomized controlled trial (RESIS, Repetitive Transcranial Magnetic Stimulation for the Treatment of Negative Symptoms in Schizophrenia), 76 patients with high illness severity were treated with 10 Hz rTMS applied 5 days per week for three weeks to the left DLPFC, compared to 81 well-matched patients who received sham rTMS. There were no significant differences between groups in negative symptoms, depressive symptoms, or cognition at 21-day and 105-day follow-up, although there was a statistically significant, but small improvement in positive symptoms for the active rTMS group at day 21 [100]. This was shortly followed in publication by Hasan and colleagues [101], who reported a lack of rTMS effects on cognition in the RESIS trial at 21, 45, or 105-day follow-up. In summary, while early studies targeting the DLPFC with rTMS demonstrated efficacy for negative symptoms or cognitive deficits, later trials did not consistently replicate these results [102].
Though most studies targeting the DLPFC have focused on negative symptoms or cognitive deficits, some have identified effects of rTMS on auditory hallucinations. For instance, Rollnik et al. [103] used a crossover design with 20 Hz rTMS targeting the left DLPFC compared to sham, finding an improvement in brief psychiatric rating scale (BPRS) score. Similarly, Novak and colleagues [104] examined 20 Hz rTMS of the left DLPFC, compared to sham, finding a small but significant improvement in the PANSS positive symptom subscale score. Wagner et al. [105] performed a secondary analysis on the subset of RESIS trial patients treated with clozapine during active or sham rTMS, and reported significant improvements on PANSS total and positive symptom subscale, but not negative subscale, scores. This suggested a potential add-on role for DLPFC rTMS in patients on clozapine.
Role of Neuroimaging in Studies of rTMS Targeting the DLPFC in Schizophrenia
Neuronavigation
There are relatively few studies utilizing neuronavigated approaches to targeting the DLPFC, compared to the TPC, which can presumably be attributed to the relative standardization of DLPFC stimulation in the field. Barr et al. [106] used MRI-guided neuronavigation to target sequential bilateral DLPFC with higher precision in 27 subjects who performed a working memory task before and after four weeks 20 Hz rTMS vs sham, with results demonstrating significantly improved 3-back accuracy for rTMS, compared to sham.
Effects of rTMS on Brain Structure or Function
Although there has been less use of imaging to guide rTMS targeting the DLPFC in schizophrenia, there has been interest in using imaging to identify potential treatment biomarkers and to clarify functional network-related responses to stimulation of the DLPFC or other regions (Table 3). Hasan and colleagues [107] analyzed structural MRI data collected before and after 10 Hz rTMS of the left DLPFC, compared to sham, and correlated it with negative symptom response. Volumetric gains in the left hippocampal, parahippocampal, and precuneal cortices following active rTMS predicted negative symptom improvement. The authors suggested that the observed heterogeneity in negative symptom response to rTMS may be in part mediated by the variable capacity for structural plasticity, especially in the left hippocampus and precuneus. Koutsouleris et al. [108] also suggested that individual variation in responsiveness to 10 Hz rTMS in patients with negative symptom predominant schizophrenia may be predicted through the use of structural biomarkers. Using data from a trial examining DLPFC directed 10 Hz vs sham rTMS, they fed pre- and post-experiment structural MRI data into a machine learning tool to identify patterns and make predictions. Their prediction tool was able to use pre-treatment MRI information related to gray matter density in a variety of cortical and subcortical areas to predict with some degree of specificity, which patients were most likely to respond to rTMS selectively in regard to negative symptoms.
Table 3.
Studies examining the effects of rTMS on structure, functional activation, and functional connectivity in schizophrenia
| First author, year | rTMS | Target | Summary |
|---|---|---|---|
| Fitzgerald, 2007 | 1 Hz | L TPC | Increased post-treatment activation in left medial frontal gyrus, right cingulate gyrus, left middle frontal gyrus, left fronto-temporal regions, left dorsal inferior frontal gyrus, and left inferior parietal gyrus in patient group |
| Vercammen, 2010 | 1 Hz | L TPC | Increased FC between L TPC and R insula post-rTMS; but no FC change in a proposed “auditory hallucination network” between L TPC and B/L cingulate and amygdala |
| Prikryl, 2012 | 10 Hz | L DLPFC | No statistically significant changes in activation following rTMS or sham |
| Guse, 2013 | 10 Hz | L post-mid-frontal gyrus | No change in activation patterns of common frontoparietal and subcortical working memory networks following treatment |
| Kindler, 2013 | 1 Hz, iTBS | L TPC | Reduced CBF in primary auditory cortex, as well as Broca’s area and cingulate gyrus |
| Dlabac-de Lange, 2015 | 10 Hz | B/L DLPFC | Increased activation of R DLPFC and R medial frontal gyrus in the active group. Also, the active group had decreased activation in the left posterior cingulate, while sham had increased activation in this region |
| Bais, 2017 | 1 Hz | L TPC, B/L TPC | Varying effects on network FC, including frontotemporal, auditory-sensorimotor and default mode network changes |
| Hasan, 2017 | 10 Hz | L DLPFC | Volumetric gains observed in the left hippocampal, parahippocampal, and precuneal cortices, that were predictive of negative symptom improvement in the active group following rTMS |
| Leimburg, 2018 | 10 Hz | L DLPFC | Decreased striato-fronto-parietal activity seen on fMRI during WoF task |
| Chen, 2019 | cTBS | L TPC | Larger reduction of FC within the L cerebellum following cTBS |
iTBS intermittent theta burst stimulation, cTBS continuous theta burst stimulation, TPC temporal-parietal cortex, DLPFC dorsolateral prefrontal cortex, L left, R right, B/L bilateral, post-mid- posterior-middle, rTMS repetitive transcranial magnetic stimulation, FC functional connectivity, CBF cerebral blood flow, WoF wall of faces
Guse et al. [109] studied the effects of 10 Hz rTMS of the left posterior middle frontal gyrus on working memory performance and task related activation during fMRI. The active rTMS group did not perform significantly better than sham following treatment, and there was no observed difference in working memory network activation during task performance between the two groups. Dlabac-de Lange and colleagues [89] performed a multicenter, randomized control trial with 32 patients targeting bilateral DLPFC vs sham. Although there were no changes in the PANSS negative symptom subscale score, the active rTMS group did show significant improvement on the SANS. Overall, there was no significant effect on cognition. A subset of these patients also underwent fMRI while performing the Tower of London task. The active group demonstrated increased activation of the right DLPFC and right medial frontal gyrus, as well as decreased activation of the left posterior cingulate, while the sham group had increased activation of the left posterior cingulate [110]. Liemburg et al. [111] had subjects perform the Wall of Faces (WOF) task [112], where both gender and emotional valence (happy or angry) were identified on an array of displayed faces, before and after active and sham rTMS. There was no significant difference in performance between active and sham arms, though the active group was found to have decreased activation in frontal, parietal, and striatal areas compared to before treatment, whereas sham was associated with increased activation of these areas. The authors were hesitant to draw conclusions due to the small sample size but suggested that 10 Hz rTMS of the DLPFC may lead to a normalization of a heightened neural response to ambiguous emotional stimuli. In their early phase psychosis pilot study, while they did observe a beneficial effect of rTMS on cognition, Francis et al. [113] did not observe changes in BOLD signal or functional connectivity during in-scanner working or episodic memory tasks. However, investigators did observe that thicker left frontal cortex predicted a greater cognitive response to rTMS.
A Place for rTMS in Schizophrenia?
Since Hoffman’s first published work describing the effects on auditory hallucinations [53], there has been great interest in rTMS as an investigational and therapeutic tool for schizophrenia. However, though many investigators have studied rTMS as a treatment of hallucinations, negative symptoms, or cognitive deficits, with some reporting promising results, questions about the utility of rTMS in schizophrenia remain.
Reaching a consensus on the efficacy of rTMS in schizophrenia has been challenging for a variety of reasons. Early studies relied on potentially imprecise targeting strategies, such as locating the DLPFC by positioning the coil 5–6 cm anterolaterally from the point of maximal stimulation of the abductor pollicus brevis muscle (thumb) [92, 94, 103, 113, 114]. Unfortunately, this technique does not accommodate subject-to-subject anatomic variance and may result in imprecise stimulation delivery [115]. As the field has evolved, many studies now use neuronavigation techniques, including infrared cameras and trackers that display the location and orientation of the coil in real time over a subject’s magnetic resonance image. Investigators can use image guidance to map specific stimulation targets based off standardized coordinates, individually specified anatomic regions, or regions of functional activation during fMRI.
The use of discrepant stimulation paradigms across trials has been a notable source of heterogeneity in the field. Studies targeting auditory hallucinations have, relative to work in other symptom domains, used more uniform stimulation settings. However, trials examining rTMS for negative symptoms and cognitive dysfunction have varied more widely in approach. Positive and negative results with 10 Hz [84, 86, 114, 116] and 20 Hz [99, 104, 106, 113] paradigms have been reported, though many of these studies have suffered from small sample sizes. Larger multi-site clinical trials have generally been yielded negative results [100, 101], but they are limited in number. Additional multi-site trials with large sample sizes will be necessary to move toward a consensus on rTMS as a treatment for schizophrenia.
There remain unanswered questions regarding to the optimal “dose” of rTMS needed to elicit a clinical response. Though it may be intuitive to think of rTMS trials in a drug development sense, where increased “dose” may have a better chance of engaging the target and exerting an effect, which is not clearly the case. A recent meta-analysis indicated that beyond a certain point, increasing the number of pulses delivered does not translate to a greater biologic effect [117]. Many studies have thus far focused on 1 to 2 weeks of stimulation, perhaps balancing the practical need for subjects to successfully complete a trial with the understanding that multiple sessions are required to affect some type of behavioral or symptom response, presumably related to LTP/LTD-like effects of rTMS [19, 22–24]. This issue could be clarified by further investigating the mechanisms underlying the proposed LTP/LTD-like effects or rTMS. Though it has been suggested that rTMS induced changes in presynaptic calcium concentration, where by increased or decreased calcium leads to the respective faciliatory or inhibitory changes in plasticity [38, 118], the precise means by which plasticity may be changed are unclear. Elucidating this point may be important, as a more precise understanding of the biologic mechanism of lasting rTMS effects could inform standardized study design. Another question involves durability of rTMS effects. More is known about the duration of anti-depressant effects of rTMS, highlighted by a recent meta-analysis by Senova et al. showing response rates of up to 50% 1 year after initial response [119]. However, less is known about the durability of effects in schizophrenia populations where differences in study design and underlying illness biology complicate comparisons. Considering the implications for trial design and subject safety, clarifying these questions is an important task for future research.
Illness-Related Challenges
Beyond the issues associated with rTMS study design, it is worth considering disease-specific factors which may complicate the interpretation of findings. It is unknown whether changes in brain structure and function that occur during the course of schizophrenia could influence response to rTMS. If so, perhaps brain stimulation could be more impactful earlier in the course of illness. Relatively little work has examined response early in the course of schizophrenia. Francis et al. [113] demonstrated a positive effect of bilateral 20 Hz rTMS on cognitive function in an early-phase psychosis cohort, though the sample size was small. Related to the duration of illness, it is unclear what impact antipsychotic medication exposure may have on the response to rTMS. It has been observed that antipsychotic medications may be associated with changes in brain structure [120–122] and function [120, 121, 123, 124]. If this is the case, it is possible that factors such as the duration or amount of antipsychotic medication exposure may mitigate or predict potential therapeutic effects of rTMS. Clarifying these points will inform our interpretation of current results and the design of future trials.
Future Directions
Interleaved TMS and fMRI
Schizophrenia is an illness associated with neural dysconnectivity [125–127], which pairs nicely with the ability of rTMS to modulate activity in target structures and connectivity with associated circuitry. Interleaved TMS and fMRI is an important direction for research in schizophrenia, as it expands the ability of investigators to determine how rTMS modulates brain circuitry. Interleaved TMS/fMRI enables investigators to sequentially perform TMS and fMRI to look at the immediate effects of the stimulation. Hanlon and colleagues [128] have developed innovative paradigms to explore the associations between neural circuitry and response to alcohol cue exposure in individuals with alcohol use disorder. Chen and colleagues [129] have employed interleaved rTMS and fMRI to examine the interplay between disparate large-scale networks, displaying the potential utility of the technology. Intentional exploration of neural circuit function is particularly relevant in schizophrenia and future investigations should replicate approaches that have been successfully used in other diseases to clarify the role of dysconnectivity in psychosis.
Precision Medicine Approach
In addition to more robust exploration of the pathophysiology of schizophrenia, movement toward a precision medicine approach will enable investigators to draw more definitive conclusions about the effectiveness of rTMS for the condition. By identifying patient-specific factors that predict response to neuromodulation, we can ultimately use the technology to greater clinical effect. This is an area where the combination of neuroimaging and non-invasive brain stimulation holds the most promise. Investigators have begun incorporating fMRI either at rest or during task performance to clarify the optimal location of intended stimulation targets on a subject-by-subject basis. This is important considering the individual variability in neuroanatomy and should move the field in a personalized direction. Though findings using this approach are mixed [73, 130], it represents an important area for continued innovation. For instance, work by our group employs this technique to explore the effects of precuneus rTMS on episodic memory neurocircuitry in early-phase psychosis by having subjects perform a scene recognition task during fMRI, which reliably activates the precuneus [131] and enables precise targeting (Fig. 2) [132].
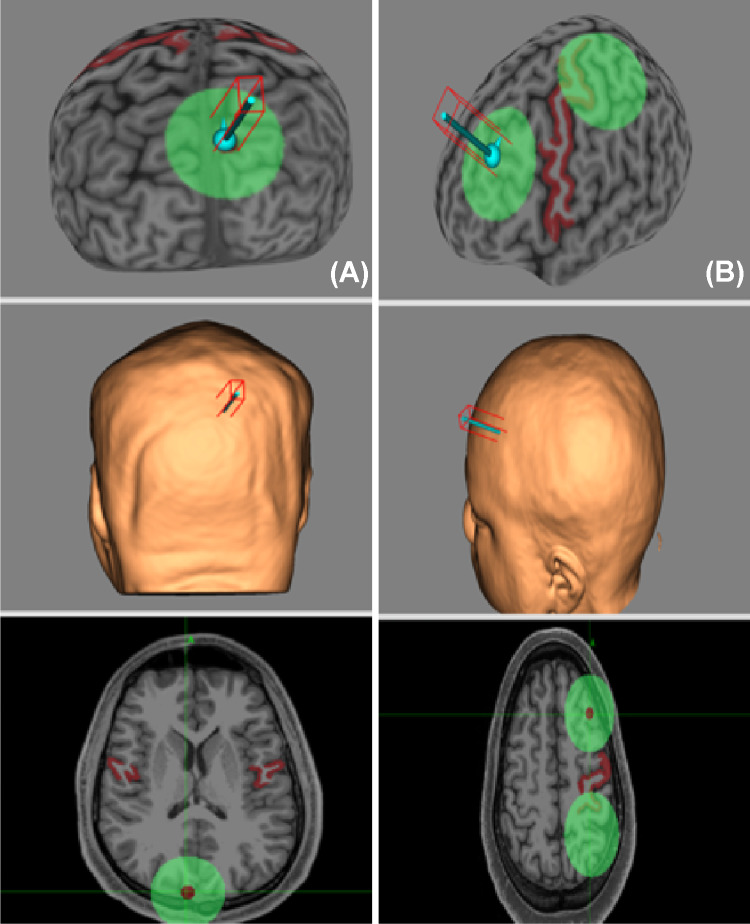
Fig. 2.
fMRI-guided rTMS. Neuronavigated rTMS targeting (A) episodic memory (EM) circuitry and (B) cognitive control in early-phase psychosis. Here, rTMS is applied to the precuneus and left dorsolateral prefrontal cortex, respectively. Baseline fMRI during task performance allows for identification of the point of maximal BOLD signal change during EM retrieval and cognitive control tasks, enabling individualized stimulation for each subject. In both sets of images, the motor strip has been highlighted for use during motor threshold determination
In conclusion, the evidence supporting rTMS as a therapeutic for schizophrenia is mixed. Though it is reasonable to believe that rTMS holds promise as a treatment option for individuals living with psychosis, larger replicative studies are needed to define optimized stimulation paradigms and appropriate follow-up intervals to assess the duration of rTMS induced effects. Beyond its therapeutic potential, rTMS also holds significant promise as a probe of the role neural circuit dysconnectivity in psychosis. Greater use of interleaved TMS and fMRI, a practice employed successfully in other populations, will move the field forward. This may be important not only for future rTMS-schizophrenia trial development but also for areas such as identifying biomarkers of illness progression or even onset, in populations such as those at clinical high risk for psychosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by funding from the National Institute of Mental Health R21MH119564 and a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ban TA. Neuropsychopharmacology and the genetics of schizophrenia: a history of the diagnosis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(5):753–762. doi: 10.1016/j.pnpbp.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Fleischhacker WW, Arango C, Arteel P, et al. Schizophrenia–time to commit to policy change. Schizophr Bull. 2014;40(Suppl 3):S165–S194. doi: 10.1093/schbul/sbu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennox BR, Park SB, Medley I, Morris PG, Jones PB. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res. 2000;100(1):13–20. doi: 10.1016/S0925-4927(00)00068-8. [DOI] [PubMed] [Google Scholar]
- 5.Breier A, Schreiber JL, Dyer J, et al. National Institute of Mental Health longitudinal study of chronic schizophrenia. Prognosis and predictors of outcome. Arch Gen Psychiatry. 1991;48(3):239–46. [DOI] [PubMed]
- 6.Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155(9):1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- 7.Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158(7):1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- 8.Yoon JH, Minzenberg MJ, Ursu S, Ryan Walter BS, Wendelken C, Ragland JD, et al. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry. 2008;165(8):1006–1014. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, et al. Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophr Bull. 2015;41(4):892–899. doi: 10.1093/schbul/sbu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowie CR, Harvey PD. Treatment of cognitive deficits in schizophrenia. Curr Opin Investig Drugs. 2006;7(7):608–613. [PubMed] [Google Scholar]
- 11.Bowie CR, Harvey PD. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr Dis Treat. 2006;2(4):531–536. doi: 10.2147/nedt.2006.2.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kane JM, Agid O, Baldwin ML, et al. Clinical Guidance on the Identification and Management of Treatment-Resistant Schizophrenia. J Clin Psychiatry. 2019;80(2). [DOI] [PubMed]
- 13.Ramachandraiah CT, Subramaniam N, Tancer M. The story of antipsychotics: Past and present. Indian J Psychiatry. 2009;51(4):324–326. doi: 10.4103/0019-5545.58304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–1107. doi: 10.1016/S0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 15.Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell A, Eberle L. Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalogr Clin Neurophysiol. 1989;74(6):458–462. doi: 10.1016/0168-5597(89)90036-1. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick SM, Rothman DL. Meeting report: transcranial magnetic stimulation and studies of human cognition. J Cogn Neurosci. 2000;12(4):704–709. doi: 10.1162/089892900562327. [DOI] [PubMed] [Google Scholar]
- 17.Chen R. Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve Suppl. 2000;9:S26–32. doi: 10.1002/1097-4598(2000)999:9<::AID-MUS6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 18.Rossi S, Hallett M, Rossini PM, et al. Safety of TMSCG. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–39. [DOI] [PMC free article] [PubMed]
- 19.Wang H, Wang X, Scheich H. LTD and LTP induced by transcranial magnetic stimulation in auditory cortex. Neuroreport. 1996;7(2):521–525. doi: 10.1097/00001756-199601310-00035. [DOI] [PubMed] [Google Scholar]
- 20.Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48(5):1398–1403. doi: 10.1212/WNL.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 21.Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(Pt 4):847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- 22.Chervyakov AV, Chernyavsky AY, Sinitsyn DO, Piradov MA. Possible Mechanisms Underlying the Therapeutic Effects of Transcranial Magnetic Stimulation. Front Hum Neurosci. 2015;9:303. doi: 10.3389/fnhum.2015.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain stimulation. 2010;3(2):95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res. 2007;180(4):583–593. doi: 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- 25.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108(1):1–16. doi: 10.1016/S0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 26.Lang N, Harms J, Weyh T, Lemon RN, Paulus W, Rothwell JC, et al. Stimulus intensity and coil characteristics influence the efficacy of rTMS to suppress cortical excitability. Clin Neurophysiol. 2006;117(10):2292–2301. doi: 10.1016/j.clinph.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 27.Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol. 2000;111(6):1002–1007. doi: 10.1016/S1388-2457(00)00284-4. [DOI] [PubMed] [Google Scholar]
- 28.Cohen LG, Roth BJ, Nilsson J, Dang N, Panizza M, Bandinelli S, et al. Effects of coil design on delivery of focal magnetic stimulation. Technical considerations. Electroencephalogr Clin Neurophysiol. 1990;75(4):350–357. doi: 10.1016/0013-4694(90)90113-X. [DOI] [PubMed] [Google Scholar]
- 29.Roth Y, Pell GS, Chistyakov AV, et al. Motor cortex activation by H-coil and figure-8 coil at different depths. Combined motor threshold and electric field distribution study. Clin Neurophysiol. 2014;125(2):336–43. [DOI] [PubMed]
- 30.Deng ZD, Lisanby SH, Peterchev AV. Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul. 2013;6(1):1–13. doi: 10.1016/j.brs.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez LJ, Goetz SM, Peterchev AV. Design of transcranial magnetic stimulation coils with optimal trade-off between depth, focality, and energy. J Neural Eng. 2018;15(4):046033. doi: 10.1088/1741-2552/aac967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei X, Li Y, Lu M, et al. Comprehensive Survey on Improved Focality and Penetration Depth of Transcranial Magnetic Stimulation Employing Multi-Coil Arrays. Int J Environ Res Public Health. 2017;14(11). [DOI] [PMC free article] [PubMed]
- 33.Hill AJ. First occurrence of hippocampal spatial firing in a new environment. Exp Neurol. 1978;62(2):282–297. doi: 10.1016/0014-4886(78)90058-4. [DOI] [PubMed] [Google Scholar]
- 34.Klimesch W, Doppelmayr M, Russegger H, Pachinger T. Theta band power in the human scalp EEG and the encoding of new information. Neuroreport. 1996;7(7):1235–1240. doi: 10.1097/00001756-199605170-00002. [DOI] [PubMed] [Google Scholar]
- 35.Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368(2):347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- 36.Oberman L, Edwards D, Eldaief M, Pascual-Leone A. Safety of theta burst transcranial magnetic stimulation: a systematic review of the literature. J Clin Neurophysiol. 2011;28(1):67–74. doi: 10.1097/WNP.0b013e318205135f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staubli U, Lynch G. Stable hippocampal long-term potentiation elicited by 'theta' pattern stimulation. Brain Res. 1987;435(1–2):227–234. doi: 10.1016/0006-8993(87)91605-2. [DOI] [PubMed] [Google Scholar]
- 38.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 39.Jannati A, Fried PJ, Block G, Oberman LM, Rotenberg A, Pascual-Leone A. Test-Retest Reliability of the Effects of Continuous Theta-Burst Stimulation. Front Neurosci. 2019;13:447. doi: 10.3389/fnins.2019.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suppa A, Huang YZ, Funke K, Ridding MC, Cheeran B, Di Lazzaro V, et al. Ten Years of Theta Burst Stimulation in Humans: Established Knowledge. Unknowns and Prospects. Brain stimulation. 2016;9(3):323–335. doi: 10.1016/j.brs.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Hamada M, Terao Y, Hanajima R, Shirota Y, Nakatani-Enomoto S, Furubayashi T, et al. Bidirectional long-term motor cortical plasticity and metaplasticity induced by quadripulse transcranial magnetic stimulation. J Physiol. 2008;586(16):3927–3947. doi: 10.1113/jphysiol.2008.152793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiksnadi A, Murakami T, Wiratman W, Matsumoto H, Ugawa Y. Direct comparison of efficacy of the motor cortical plasticity induction and the interindividual variability between TBS and QPS. Brain Stimul. 2020;13(6):1824–1833. doi: 10.1016/j.brs.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 43.Hanlon CA, Dowdle LT, Henderson JS. Modulating Neural Circuits with Transcranial Magnetic Stimulation: Implications for Addiction Treatment Development. Pharmacol Rev. 2018;70(3):661–683. doi: 10.1124/pr.116.013649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. 2009;4(8):e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21(15):RC157. [DOI] [PMC free article] [PubMed]
- 46.Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126(Pt 12):2609–2615. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- 47.Vidal-Pineiro D, Martin-Trias P, Falcon C, Bargallo N, Clemente IC, Valls-Sole J, et al. Neurochemical Modulation in Posteromedial Default-mode Network Cortex Induced by Transcranial Magnetic Stimulation. Brain Stimul. 2015;8(5):937–944. doi: 10.1016/j.brs.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Denslow S, Lomarev M, George MS, Bohning DE. Cortical and subcortical brain effects of transcranial magnetic stimulation (TMS)-induced movement: an interleaved TMS/functional magnetic resonance imaging study. Biol Psychiatry. 2005;57(7):752–760. doi: 10.1016/j.biopsych.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 49.Dowdle LT, Brown TR, George MS, Hanlon CA. Single pulse TMS to the DLPFC, compared to a matched sham control, induces a direct, causal increase in caudate, cingulate, and thalamic BOLD signal. Brain Stimul. 2018;11(4):789–796. doi: 10.1016/j.brs.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lerner AJ, Wassermann EM, Tamir DI. Seizures from transcranial magnetic stimulation 2012–2016: Results of a survey of active laboratories and clinics. Clin Neurophysiol. 2019;130(8):1409–1416. doi: 10.1016/j.clinph.2019.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loo CK, McFarquhar TF, Mitchell PB. A review of the safety of repetitive transcranial magnetic stimulation as a clinical treatment for depression. Int J Neuropsychopharmacol. 2008;11(1):131–147. doi: 10.1017/S1461145707007717. [DOI] [PubMed] [Google Scholar]
- 52.Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168(1):73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- 53.Hoffman RE, Boutros NN, Berman RM, Roessler E, Belger A, Krystal JH, et al. Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated "voices". Biol Psychiatry. 1999;46(1):130–132. doi: 10.1016/S0006-3223(98)00358-8. [DOI] [PubMed] [Google Scholar]
- 54.Hoffman RE, Boutros NN, Hu S, Berman RM, Krystal JH, Charney DS. Transcranial magnetic stimulation and auditory hallucinations in schizophrenia. Lancet. 2000;355(9209):1073–1075. doi: 10.1016/S0140-6736(00)02043-2. [DOI] [PubMed] [Google Scholar]
- 55.Hoffman RE, Gueorguieva R, Hawkins KA, Varanko M, Boutros NN, Wu YT, et al. Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a fifty patient sample. Biol Psychiatry. 2005;58(2):97–104. doi: 10.1016/j.biopsych.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 56.Hoffman RE, Hawkins KA, Gueorguieva R, Boutros NN, Rachid F, Carroll K, et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry. 2003;60(1):49–56. doi: 10.1001/archpsyc.60.1.49. [DOI] [PubMed] [Google Scholar]
- 57.Chibbaro G, Daniele M, Alagona G, Di Pasquale C, Cannavo M, Rapisarda V, et al. Repetitive transcranial magnetic stimulation in schizophrenic patients reporting auditory hallucinations. Neurosci Lett. 2005;383(1–2):54–57. doi: 10.1016/j.neulet.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 58.Poulet E, Brunelin J, Bediou B, Bation R, Forgeard L, Dalery J, et al. Slow transcranial magnetic stimulation can rapidly reduce resistant auditory hallucinations in schizophrenia. Biol Psychiatry. 2005;57(2):188–191. doi: 10.1016/j.biopsych.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 59.de Jesus DR, Gil A, Barbosa L, Lobato MI, Magalhaes PV, Favalli GP, et al. A pilot double-blind sham-controlled trial of repetitive transcranial magnetic stimulation for patients with refractory schizophrenia treated with clozapine. Psychiatry Res. 2011;188(2):203–207. doi: 10.1016/j.psychres.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 60.Fitzgerald PB, Benitez J, Daskalakis JZ, Brown TL, Marston NA, de Castella A, et al. A double-blind sham-controlled trial of repetitive transcranial magnetic stimulation in the treatment of refractory auditory hallucinations. J Clin Psychopharmacol. 2005;25(4):358–362. doi: 10.1097/01.jcp.0000168487.22140.7f. [DOI] [PubMed] [Google Scholar]
- 61.McIntosh AM, Semple D, Tasker K, Harrison LK, Owens DG, Johnstone EC, et al. Transcranial magnetic stimulation for auditory hallucinations in schizophrenia. Psychiatry Res. 2004;127(1–2):9–17. doi: 10.1016/j.psychres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Rosa MO, Gattaz WF, Rosa MA, Rumi DO, Tavares H, Myczkowski M, et al. Effects of repetitive transcranial magnetic stimulation on auditory hallucinations refractory to clozapine. J Clin Psychiatry. 2007;68(10):1528–1532. doi: 10.4088/JCP.v68n1009. [DOI] [PubMed] [Google Scholar]
- 63.Saba G, Verdon CM, Kalalou K, Rocamora JF, Dumortier G, Benadhira R, et al. Transcranial magnetic stimulation in the treatment of schizophrenic symptoms: a double blind sham controlled study. J Psychiatr Res. 2006;40(2):147–152. doi: 10.1016/j.jpsychires.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 64.Bais L, Vercammen A, Stewart R, van Es F, Visser B, Aleman A, et al. Short and long term effects of left and bilateral repetitive transcranial magnetic stimulation in schizophrenia patients with auditory verbal hallucinations: a randomized controlled trial. PLoS One. 2014;9(10):e108828. doi: 10.1371/journal.pone.0108828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jandl M, Steyer J, Weber M, Linden DE, Rothmeier J, Maurer K, et al. Treating auditory hallucinations by transcranial magnetic stimulation: a randomized controlled cross-over trial. Neuropsychobiology. 2006;53(2):63–69. doi: 10.1159/000091721. [DOI] [PubMed] [Google Scholar]
- 66.Lee SH, Kim W, Chung YC, Jung KH, Bahk WM, Jun TY, et al. A double blind study showing that two weeks of daily repetitive TMS over the left or right temporoparietal cortex reduces symptoms in patients with schizophrenia who are having treatment-refractory auditory hallucinations. Neurosci Lett. 2005;376(3):177–181. doi: 10.1016/j.neulet.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 67.Loo CK, Sainsbury K, Mitchell P, Hadzi-Pavlovic D, Sachdev PS. A sham-controlled trial of left and right temporal rTMS for the treatment of auditory hallucinations. Psychol Med. 2010;40(4):541–546. doi: 10.1017/S0033291709990900. [DOI] [PubMed] [Google Scholar]
- 68.Montagne-Larmurier A, Etard O, Razafimandimby A, Morello R, Dollfus S. Two-day treatment of auditory hallucinations by high frequency rTMS guided by cerebral imaging: a 6 month follow-up pilot study. Schizophr Res. 2009;113(1):77–83. doi: 10.1016/j.schres.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 69.Kimura H, Kanahara N, Takase M, Yoshida T, Watanabe H, Iyo M. A randomized, sham-controlled study of high frequency rTMS for auditory hallucination in schizophrenia. Psychiatry Res. 2016;241:190–194. doi: 10.1016/j.psychres.2016.04.119. [DOI] [PubMed] [Google Scholar]
- 70.Dollfus S, Jaafari N, Guillin O, Trojak B, Plaze M, Saba G, et al. High-Frequency Neuronavigated rTMS in Auditory Verbal Hallucinations: A Pilot Double-Blind Controlled Study in Patients With Schizophrenia. Schizophr Bull. 2018;44(3):505–514. doi: 10.1093/schbul/sbx127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koops S, van Dellen E, Schutte MJ, Nieuwdorp W, Neggers SF, Sommer IE. Theta Burst Transcranial Magnetic Stimulation for Auditory Verbal Hallucinations: Negative Findings From a Double-Blind-Randomized Trial. Schizophr Bull. 2016;42(1):250–257. doi: 10.1093/schbul/sbv100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen X, Ji GJ, Zhu C, Bai X, Wang L, He K, et al. Neural Correlates of Auditory Verbal Hallucinations in Schizophrenia and the Therapeutic Response to Theta-Burst Transcranial Magnetic Stimulation. Schizophr Bull. 2019;45(2):474–483. doi: 10.1093/schbul/sby054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schonfeldt-Lecuona C, Gron G, Walter H, Buchler N, Wunderlich A, Spitzer M, et al. Stereotaxic rTMS for the treatment of auditory hallucinations in schizophrenia. Neuroreport. 2004;15(10):1669–1673. doi: 10.1097/01.wnr.0000126504.89983.ec. [DOI] [PubMed] [Google Scholar]
- 74.Slotema CW, Blom JD, de Weijer AD, Diederen KM, Goekoop R, Looijestijn J, et al. Can low-frequency repetitive transcranial magnetic stimulation really relieve medication-resistant auditory verbal hallucinations? Negative results from a large randomized controlled trial. Biol Psychiatry. 2011;69(5):450–456. doi: 10.1016/j.biopsych.2010.09.051. [DOI] [PubMed] [Google Scholar]
- 75.Blumberger DM, Christensen BK, Zipursky RB, Moller B, Chen R, Fitzgerald PB, et al. MRI-targeted repetitive transcranial magnetic stimulation of Heschl's gyrus for refractory auditory hallucinations. Brain Stimul. 2012;5(4):577–585. doi: 10.1016/j.brs.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 76.Paillere-Martinot ML, Galinowski A, Plaze M, Andoh J, Bartres-Faz D, Bellivier F, et al. Active and placebo transcranial magnetic stimulation effects on external and internal auditory hallucinations of schizophrenia. Acta Psychiatr Scand. 2017;135(3):228–238. doi: 10.1111/acps.12680. [DOI] [PubMed] [Google Scholar]
- 77.Nathou C, Simon G, Dollfus S, Etard O. Cortical Anatomical Variations and Efficacy of rTMS in the Treatment of Auditory Hallucinations. Brain Stimul. 2015;8(6):1162–1167. doi: 10.1016/j.brs.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 78.Fitzgerald PB, Sritharan A, Benitez J, Daskalakis ZJ, Jackson G, Kulkarni J, et al. A preliminary fMRI study of the effects on cortical activation of the treatment of refractory auditory hallucinations with rTMS. Psychiatry Res. 2007;155(1):83–88. doi: 10.1016/j.pscychresns.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 79.Kindler J, Homan P, Jann K, Federspiel A, Flury R, Hauf M, et al. Reduced neuronal activity in language-related regions after transcranial magnetic stimulation therapy for auditory verbal hallucinations. Biol Psychiatry. 2013;73(6):518–524. doi: 10.1016/j.biopsych.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 80.Vercammen A, Knegtering H, Liemburg EJ, den Boer JA, Aleman A. Functional connectivity of the temporo-parietal region in schizophrenia: effects of rTMS treatment of auditory hallucinations. J Psychiatr Res. 2010;44(11):725–731. doi: 10.1016/j.jpsychires.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 81.Gromann PM, Tracy DK, Giampietro V, Brammer MJ, Krabbendam L, Shergill SS. Examining frontotemporal connectivity and rTMS in healthy controls: implications for auditory hallucinations in schizophrenia. Neuropsychology. 2012;26(1):127–132. doi: 10.1037/a0026603. [DOI] [PubMed] [Google Scholar]
- 82.Bais L, Liemburg E, Vercammen A, Bruggeman R, Knegtering H, Aleman A. Effects of low frequency rTMS treatment on brain networks for inner speech in patients with schizophrenia and auditory verbal hallucinations. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:105–113. doi: 10.1016/j.pnpbp.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 83.Cohen E, Bernardo M, Masana J, Arrufat FJ, Navarro V, Valls S, et al. Repetitive transcranial magnetic stimulation in the treatment of chronic negative schizophrenia: a pilot study. J Neurol Neurosurg Psychiatry. 1999;67(1):129–130. doi: 10.1136/jnnp.67.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hajak G, Marienhagen J, Langguth B, Werner S, Binder H, Eichhammer P. High-frequency repetitive transcranial magnetic stimulation in schizophrenia: a combined treatment and neuroimaging study. Psychol Med. 2004;34(7):1157–1163. doi: 10.1017/S0033291704002338. [DOI] [PubMed] [Google Scholar]
- 85.Goyal N, Nizamie SH, Desarkar P. Efficacy of adjuvant high frequency repetitive transcranial magnetic stimulation on negative and positive symptoms of schizophrenia: preliminary results of a double-blind sham-controlled study. J Neuropsychiatry Clin Neurosci. 2007;19(4):464–467. doi: 10.1176/jnp.2007.19.4.464. [DOI] [PubMed] [Google Scholar]
- 86.Prikryl R, Kasparek T, Skotakova S, Ustohal L, Kucerova H, Ceskova E. Treatment of negative symptoms of schizophrenia using repetitive transcranial magnetic stimulation in a double-blind, randomized controlled study. Schizophr Res. 2007;95(1–3):151–157. doi: 10.1016/j.schres.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 87.Prikryl R, Mikl M, Prikrylova Kucerova H, Ustohal L, Kasparek T, Marecek R, et al. Does repetitive transcranial magnetic stimulation have a positive effect on working memory and neuronal activation in treatment of negative symptoms of schizophrenia? Neuro Endocrinol Lett. 2012;33(1):90–97. [PubMed] [Google Scholar]
- 88.Prikryl R, Ustohal L, Prikrylova Kucerova H, Kasparek T, Venclikova S, Vrzalova M, et al. A detailed analysis of the effect of repetitive transcranial magnetic stimulation on negative symptoms of schizophrenia: a double-blind trial. Schizophr Res. 2013;149(1–3):167–173. doi: 10.1016/j.schres.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 89.Dlabac-de Lange JJ, Bais L, van Es FD, Visser BG, Reinink E, Bakker B, et al. Efficacy of bilateral repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: results of a multicenter double-blind randomized controlled trial. Psychol Med. 2015;45(6):1263–1275. doi: 10.1017/S0033291714002360. [DOI] [PubMed] [Google Scholar]
- 90.Li Z, Yin M, Lyu XL, Zhang LL, Du XD, Hung GC. Delayed effect of repetitive transcranial magnetic stimulation (rTMS) on negative symptoms of schizophrenia: Findings from a randomized controlled trial. Psychiatry Res. 2016;240:333–335. doi: 10.1016/j.psychres.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 91.Quan WX, Zhu XL, Qiao H, Zhang WF, Tan SP, Zhou DF, et al. The effects of high-frequency repetitive transcranial magnetic stimulation (rTMS) on negative symptoms of schizophrenia and the follow-up study. Neurosci Lett. 2015;584:197–201. doi: 10.1016/j.neulet.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 92.Schneider AL, Schneider TL, Stark H. Repetitive transcranial magnetic stimulation (rTMS) as an augmentation treatment for the negative symptoms of schizophrenia: a 4-week randomized placebo controlled study. Brain Stimul. 2008;1(2):106–111. doi: 10.1016/j.brs.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 93.Zhao S, Kong J, Li S, Tong Z, Yang C, Zhong H. Randomized controlled trial of four protocols of repetitive transcranial magnetic stimulation for treating the negative symptoms of schizophrenia. Shanghai Arch Psychiatry. 2014;26(1):15–21. doi: 10.3969/j.issn.1002-0829.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wolwer W, Lowe A, Brinkmeyer J, Streit M, Habakuck M, Agelink MW, et al. Repetitive transcranial magnetic stimulation (rTMS) improves facial affect recognition in schizophrenia. Brain Stimul. 2014;7(4):559–563. doi: 10.1016/j.brs.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 95.Kamp D, Brinkmeyer J, Agelink MW, Habakuck M, Mobascher A, Wolwer W, et al. High frequency repetitive transcranial magnetic stimulation (rTMS) reduces EEG-hypofrontality in patients with schizophrenia. Psychiatry Res. 2016;236:199–201. doi: 10.1016/j.psychres.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 96.Holi MM, Eronen M, Toivonen K, Toivonen P, Marttunen M, Naukkarinen H. Left prefrontal repetitive transcranial magnetic stimulation in schizophrenia. Schizophr Bull. 2004;30(2):429–434. doi: 10.1093/oxfordjournals.schbul.a007089. [DOI] [PubMed] [Google Scholar]
- 97.Cordes J, Thunker J, Agelink MW, Arends M, Mobascher A, Wobrock T, et al. Effects of 10 Hz repetitive transcranial magnetic stimulation (rTMS) on clinical global impression in chronic schizophrenia. Psychiatry Res. 2010;177(1–2):32–36. doi: 10.1016/j.psychres.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 98.Mittrach M, Thunker J, Winterer G, Agelink MW, Regenbrecht G, Arends M, et al. The tolerability of rTMS treatment in schizophrenia with respect to cognitive function. Pharmacopsychiatry. 2010;43(3):110–117. doi: 10.1055/s-0029-1242824. [DOI] [PubMed] [Google Scholar]
- 99.Barr MS, Farzan F, Tran LC, Fitzgerald PB, Daskalakis ZJ. A randomized controlled trial of sequentially bilateral prefrontal cortex repetitive transcranial magnetic stimulation in the treatment of negative symptoms in schizophrenia. Brain Stimul. 2012;5(3):337–346. doi: 10.1016/j.brs.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 100.Wobrock T, Guse B, Cordes J, Wolwer W, Winterer G, Gaebel W, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. 2015;77(11):979–988. doi: 10.1016/j.biopsych.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 101.Hasan A, Guse B, Cordes J, Wolwer W, Winterer G, Gaebel W, et al. Cognitive Effects of High-Frequency rTMS in Schizophrenia Patients With Predominant Negative Symptoms: Results From a Multicenter Randomized Sham-Controlled Trial. Schizophr Bull. 2016;42(3):608–618. doi: 10.1093/schbul/sbv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aleman A, Enriquez-Geppert S, Knegtering H, Dlabac-de Lange JJ. Moderate effects of noninvasive brain stimulation of the frontal cortex for improving negative symptoms in schizophrenia: Meta-analysis of controlled trials. Neuroscience and biobehavioral reviews. 2018;89:111–118. doi: 10.1016/j.neubiorev.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 103.Rollnik JD, Huber TJ, Mogk H, Siggelkow S, Kropp S, Dengler R, et al. High frequency repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex in schizophrenic patients. Neuroreport. 2000;11(18):4013–4015. doi: 10.1097/00001756-200012180-00022. [DOI] [PubMed] [Google Scholar]
- 104.Novak T, Horacek J, Mohr P, Kopecek M, Skrdlantova L, Klirova M, et al. The double-blind sham-controlled study of high-frequency rTMS (20 Hz) for negative symptoms in schizophrenia: negative results. Neuro Endocrinol Lett. 2006;27(1–2):209–213. [PubMed] [Google Scholar]
- 105.Wagner E, Wobrock T, Kunze B, Langguth B, Landgrebe M, Eichhammer P, et al. Efficacy of high-frequency repetitive transcranial magnetic stimulation in schizophrenia patients with treatment-resistant negative symptoms treated with clozapine. Schizophr Res. 2019;208:370–376. doi: 10.1016/j.schres.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 106.Barr MS, Farzan F, Rajji TK, Voineskos AN, Blumberger DM, Arenovich T, et al. Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biol Psychiatry. 2013;73(6):510–517. doi: 10.1016/j.biopsych.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 107.Hasan A, Wobrock T, Guse B, Langguth B, Landgrebe M, Eichhammer P, et al. Structural brain changes are associated with response of negative symptoms to prefrontal repetitive transcranial magnetic stimulation in patients with schizophrenia. Mol Psychiatry. 2017;22(6):857–864. doi: 10.1038/mp.2016.161. [DOI] [PubMed] [Google Scholar]
- 108.Koutsouleris N, Wobrock T, Guse B, Langguth B, Landgrebe M, Eichhammer P, et al. Predicting Response to Repetitive Transcranial Magnetic Stimulation in Patients With Schizophrenia Using Structural Magnetic Resonance Imaging: A Multisite Machine Learning Analysis. Schizophr Bull. 2018;44(5):1021–1034. doi: 10.1093/schbul/sbx114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guse B, Falkai P, Gruber O, Whalley H, Gibson L, Hasan A, et al. The effect of long-term high frequency repetitive transcranial magnetic stimulation on working memory in schizophrenia and healthy controls–a randomized placebo-controlled, double-blind fMRI study. Behav Brain Res. 2013;237:300–307. doi: 10.1016/j.bbr.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 110.Dlabac-de Lange JJ, Liemburg EJ, Bais L, Renken RJ, Knegtering H, Aleman A. Effect of rTMS on brain activation in schizophrenia with negative symptoms: A proof-of-principle study. Schizophr Res. 2015;168(1–2):475–482. doi: 10.1016/j.schres.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 111.Liemburg EJ, Dlabac-De Lange JJ, Bais L, Knegtering H, Aleman A. Effects of bilateral prefrontal rTMS on brain activation during social-emotional evaluation in schizophrenia: A double-blind, randomized, exploratory study. Schizophr Res. 2018;202:210–211. doi: 10.1016/j.schres.2018.06.051. [DOI] [PubMed] [Google Scholar]
- 112.Simmons A, Stein MB, Matthews SC, Feinstein JS, Paulus MP. Affective ambiguity for a group recruits ventromedial prefrontal cortex. NeuroImage. 2006;29(2):655–661. doi: 10.1016/j.neuroimage.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 113.Francis MM, Hummer TA, Vohs JL, Yung MG, Visco AC, Mehdiyoun NF, et al. Cognitive effects of bilateral high frequency repetitive transcranial magnetic stimulation in early phase psychosis: a pilot study. Brain Imaging Behav. 2019;13(3):852–861. doi: 10.1007/s11682-018-9902-4. [DOI] [PubMed] [Google Scholar]
- 114.Mogg A, Purvis R, Eranti S, Contell F, Taylor JP, Nicholson T, et al. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: a randomized controlled pilot study. Schizophr Res. 2007;93(1–3):221–228. doi: 10.1016/j.schres.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 115.Fitzgerald PB, Maller JJ, Hoy KE, Thomson R, Daskalakis ZJ. Exploring the optimal site for the localization of dorsolateral prefrontal cortex in brain stimulation experiments. Brain Stimul. 2009;2(4):234–237. doi: 10.1016/j.brs.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 116.Fitzgerald PB, Herring S, Hoy K, McQueen S, Segrave R, Kulkarni J, et al. A study of the effectiveness of bilateral transcranial magnetic stimulation in the treatment of the negative symptoms of schizophrenia. Brain Stimul. 2008;1(1):27–32. doi: 10.1016/j.brs.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 117.Jiang Y, Guo Z, Xing G, He L, Peng H, Du F, et al. Effects of High-Frequency Transcranial Magnetic Stimulation for Cognitive Deficit in Schizophrenia: A Meta-Analysis. Front Psychiatry. 2019;10:135. doi: 10.3389/fpsyt.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li CT, Chen MH, Juan CH, Huang HH, Chen LF, Hsieh JC, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. 2014;137(Pt 7):2088–2098. doi: 10.1093/brain/awu109. [DOI] [PubMed] [Google Scholar]
- 119.Senova S, Cotovio G, Pascual-Leone A, Oliveira-Maia AJ. Durability of antidepressant response to repetitive transcranial magnetic stimulation: Systematic review and meta-analysis. Brain stimulation. 2019;12(1):119–128. doi: 10.1016/j.brs.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 120.Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68(2):128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Radua J, Borgwardt S, Crescini A, Mataix-Cols D, Meyer-Lindenberg A, McGuire PK, et al. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev. 2012;36(10):2325–2333. doi: 10.1016/j.neubiorev.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 122.Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, et al. The effects of antipsychotics on the brain: what have we learnt from structural imaging of schizophrenia?–a systematic review. Curr Pharm Des. 2009;15(22):2535–2549. doi: 10.2174/138161209788957456. [DOI] [PubMed] [Google Scholar]
- 123.Fusar-Poli P, Broome MR, Matthiasson P, Williams SC, Brammer M, McGuire PK. Effects of acute antipsychotic treatment on brain activation in first episode psychosis: an fMRI study. Eur Neuropsychopharmacol. 2007;17(6–7):492–500. doi: 10.1016/j.euroneuro.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 124.Lui S, Li T, Deng W, Jiang L, Wu Q, Tang H, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by "resting state" functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67(8):783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- 125.Andreasen NC, Paradiso S, O'Leary DS. "Cognitive dysmetria" as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 126.Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3(2):89–97. [PubMed] [Google Scholar]
- 127.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57(7):637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 128.Hanlon CA, Dowdle LT, Correia B, Mithoefer O, Kearney-Ramos T, Lench D, et al. Left frontal pole theta burst stimulation decreases orbitofrontal and insula activity in cocaine users and alcohol users. Drug Alcohol Depend. 2017;178:310–317. doi: 10.1016/j.drugalcdep.2017.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williams LM, et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(49):19944–19949. doi: 10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hoffman RE, Hampson M, Wu K, Anderson AW, Gore JC, Buchanan RJ, et al. Probing the pathophysiology of auditory/verbal hallucinations by combining functional magnetic resonance imaging and transcranial magnetic stimulation. Cereb Cortex. 2007;17(11):2733–2743. doi: 10.1093/cercor/bhl183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Francis MM, Hummer TA, Vohs JL, Yung MG, Liffick E, Mehdiyoun NF, et al. Functional neuroanatomical correlates of episodic memory impairment in early phase psychosis. Brain imaging and behavior. 2016;10(1):1–11. doi: 10.1007/s11682-015-9357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Francis MM. Repetititve Transcranial Magnetic Stimulation as a Probe of Episodic Memory Neurocircuitry in Schizophrenia. Indiana University School of Medicine, Dept. of Psychiatry: National Institute of Mental Health; 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.