Abstract
Skeletal muscle is the most abundant tissue in healthy individuals and it has important roles in health beyond voluntary movement. The overall mass and energy requirements of skeletal muscle require it to be metabolically active and flexible to multiple energy substrates. The tissue has evolved to be largely load dependent and it readily adapts in a number of positive ways to repetitive overload, such as various forms of exercise training. However, unloading from extended bed rest and/or metabolic derangements in response to trauma, acute illness, or severe pathology, commonly results in rapid muscle wasting. Decline in muscle mass contributes to multimorbidity, reduces function, and exerts a substantial, negative impact on the quality of life. The principal mechanisms controlling muscle mass have been well described and these cellular processes are intricately regulated by exercise. Accordingly, exercise has shown great promise and efficacy in preventing or slowing muscle wasting through changes in molecular physiology, organelle function, cell signaling pathways, and epigenetic regulation. In this review, we focus on the role of exercise in altering the molecular landscape of skeletal muscle in a manner that improves or maintains its health and function in the presence of unloading or disease.
epigenetics; exercise; muscle wasting; resistance training; skeletal muscle
Keywords: epigenetics, exercise, muscle wasting, resistance training, skeletal muscle
INTRODUCTION
Exercise training has been established as an efficacious and multipotent health intervention (1, 2) that can limit or treat the development of cognitive, psychological, cardiovascular, musculoskeletal, metabolic, and age-related diseases, as well as many cancers (3, 4). These systemic improvements are driven by acute and chronic molecular responses to exercise in skeletal muscle and a host of other organ systems and tissues. The changes in skeletal muscles are important as it is the most abundant tissue in healthy individuals and, outside of its role in movement, is an essential metabolic regulator and endocrine organ. Exercising skeletal muscle acts to clear glucose and other metabolic substrates from the blood (5). In addition, its robust mitochondrial density and energy capacity provide metabolic flexibility (6). Actively working large muscle groups release factors into the system, often called myokines or exerkines, which act in autocrine, paracrine, and endocrine manners (7). The diversity of secreted factors during exercise is likely related to the heterogeneity of skeletal muscle. Single fiber proteomics of trained skeletal muscle shows ∼200 proteins differentially expressed between slow and fast twitch fibers, with fiber-type dependent enrichment profiles of proteins associated with the secretome (8). Taken together, it is clear that healthy, active skeletal muscle has the capacity to alter local and systemic environments both after acute exercise and chronic training. This dynamic regulation by muscle is a crucial factor in how exercise improves overall health and well-being.
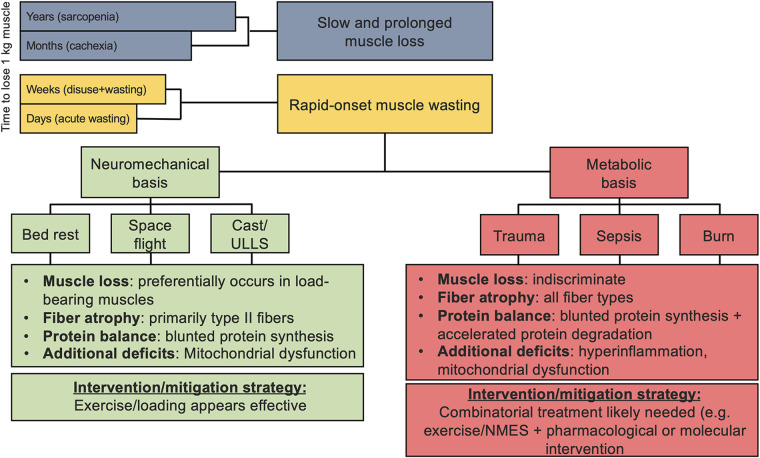
The ability of exercise to prevent or slow disease states is intimately related to its ability to prevent or slow muscle wasting. Muscle atrophy is a natural consequence of the aging process with consistent and appreciable muscle loss beginning around the age of 40 (9). More dramatic and accelerated muscle loss (i.e., muscle wasting) occurs as a by-product of disuse/unloading (e.g., cast immobilization, in-patient hospitalization/bed rest, spaceflight) or as a result of pathology or trauma (e.g., burn injury, spinal cord injury (SCI), neurodegeneration, and amyotrophic lateral sclerosis (ALS), trauma/sepsis) and is summarized in Fig. 1. The balance of limiting muscle atrophy and promoting muscle hypertrophy is controlled by intracellular signaling cascades that control protein synthesis and translation efficiency/capacity while also coordinating the regulation of the proteolytic and autophagic degradation systems. This review aims to describe how exercise alters the molecular state of skeletal muscle to combat muscle atrophy and muscle wasting, with an emphasis on the state of knowledge in human participants.
Figure 1.
Conceptual model of muscle wasting as a function of rate of muscle loss. In contrast to pathologies that lead to slow but prolonged muscle loss, rapid onset muscle wasting is aggressive and can be categorized into two distinct types of muscle wasting: one that is primarily neuromechanical and the other based in metabolic dysfunction. Relevant examples, characteristics, and strategies for intervention and/or mitigation are shown. NMES, neuromuscular electrical stimulation; ULLS, unilateral lower limb suspension.
MECHANISMS OF MUSCLE WASTING
Muscle mass is maintained by balancing the rates of protein synthesis and protein degradation. When synthesis exceeds degradation over extended periods, the result is often an increase in the size and performance of muscle fibers. Conversely, when long-term net balance favors protein degradation, muscle size and performance are reduced. Excess protein breakdown during muscle wasting can occur rapidly even in healthy individuals and there is a spirited debate regarding the principal mechanisms of atrophy in the early timespan of disuse (10–12). Short-term conditions such as bedrest or immobilization result in rapid losses in muscle mass [∼3%–5% in the first week; (13, 14)] and these losses can be compounded when sustained following severe injuries due to burn (15) or following SCI (16). Several excellent reviews have been dedicated to the detailed mechanisms of muscle hypertrophy and muscle wasting (17–23). In this review, we summarize the state of the field on mechanisms of muscle wasting and exercise-induced countermeasures, identify knowledge gaps, and suggest innovative future directions to advance the science and ultimately the clinical application.
Protein Synthesis
Regulation of protein synthesis is a highly coordinated and conserved process that has evolved to be sensitive to nutrient availability, mechanotransduction, intracellular stress, and numerous other factors. Skeletal muscle fibers are unique as they are postmitotic cells created from the fusion of 100–1,000s of differentiating myogenic progenitor cells. The relatively limited myofiber turnover in the absence of damage means any consistent net gain in protein synthesis results in a larger muscle cell, in other words, hypertrophy, of the myofiber.
The nexus of cellular protein synthesis is the mechanistic (or mammalian) target of rapamycin (mTOR) (24). mTOR is a serine/threonine kinase that is a key mediator of many anabolic signals in skeletal muscle. There are two main mTOR complexes, each defined by their principal regulatory protein: mTORC1 contains raptor (rapamycin-sensitive) whereas mTORC2 contains rictor (rapamycin-insensitive). mTORC1 has generally been associated with overload and resistance exercise-induced protein synthesis and resultant hypertrophy. However, recent work has highlighted the role of mTOR-dependent but rapamycin-independent roles for protein synthesis (25, 26) and regulation of exercise-induced elevations in protein synthesis controlled by mTORC2 (27). The differential effects of mTORC1/2 in relation to resistance exercise in skeletal muscle have been recently reviewed (28). The most well-described roles of mTORC1 in regulating protein synthesis in skeletal muscle are also the general downstream markers of its activity, phosphorylation of p70S6KT389 and 4eBP1T36/45 to improve the capacity (i.e., ribosome biogenesis) and efficiency (i.e., initiation, elongation, and termination) of mRNA translation, though phosphorylation of 4eBP1 has been shown to be rapamycin-insensitive but raptor-dependent in mice (26). Growth factor signaling, downstream of the potent insulin and insulin-like growth factor-1 (IGF-1) receptor, activates mTORC1 through a PI3K/PDK1/Akt-mediated process that prevents TSC2 from inhibiting mTORC1/2, phosphorylates PRAS40, and also allows mTORC2 to further activate Akt (29). Activated Akt phosphorylates and sequesters the atrophic transcription factors FOXO1 and 3a in the cytosol and inactivates GSK3β, another atrophy-inducing protein. Furthermore, amino acids, especially the branched-chain amino acid leucine, also activate mTORC1, stimulating protein synthesis. Activation of PI3K through insulin receptor binding also initiates signaling through Akt that leads to GLUT4 translocation and the principal mode of skeletal muscle glucose uptake.
It is evident that having proper caloric and nutrient intake, and the hormonal profile that responds to them, is crucial for mTORC1/2 activation and protein synthesis. However, myofibrillar protein synthesis is greatly impaired in the absence of skeletal muscle load (13, 30), implying a crucial link between mechanical loading and mTORC1 and potentially mTORC2 activity. However, recent evidence probing the proteome and phosphoproteome of immobilized mouse muscle suggests reduced translation efficiency, not changes in protein abundance or phosphorylation state, is the primary driver of reduced protein synthesis in the immediate days after disuse (31). For overload-induced increases in protein synthesis, activation of both mTORC complexes is needed (26, 27) but mTORC1/raptor seems to be necessary for hypertrophy (26). The mechanisms behind this have been linked to phosphorylation of key residues on the potent mTOR inhibitor TSC2 (32) as well as mechanically induced increases in phosphatidic acid (33, 34).
The direct mechanical signaling pathways that lead to mTORC1/2 activation have not been precisely mapped but are probably related to activation of the mechanosensory apparatus of skeletal muscle. The best described mechanosensors in mature skeletal muscle are located in the costamere. The costamere is composed of the dystroglycan complex (DGC) and the α7β1 integrin/focal adhesion complex. Their importance has been extensively characterized as genetic defects in major components of these complexes (dystrophin in the DGC and α7 integrin, for example) lead to severe myopathies (35). The role of the costamere is to physically anchor the sarcolemma to both the extracellular matrix (ECM) and the intracellular cytoskeleton to coordinate signals between these regions. Transgenic overexpression of the α7 integrin results in hypertrophic responses in mouse muscles after acute and chronic eccentric exercise training (36, 37) and it may work through integrin-linked kinase, a protein which binds to the α7 subunit, that has been related to activation of Akt and p70S6K following α7 integrin overexpression (38). Focal adhesion kinase (FAK) is another well-described effector of integrin activation in skeletal muscle (39). It is a key signaling factor within the focal adhesion complex in skeletal muscle and it is rapidly activated after both aerobic and anaerobic exercise (40). FAK activation is reduced after disuse (30, 41) and its activation is linked to improved IGF-1 signaling in C2C12 cell culture models (42). Other potential mechanosensors, such as the previously mentioned phosphatidic acid, the sarcomeric protein titin, and components of the z-disk, have been implicated and reviewed in detail (43–45).
Protein Degradation
Proteolysis through the proteasome appears to be the predominant way muscle catabolizes protein. Individual proteins that are targeted for proteasomal degradation are ubiquitinated through a multistep process. E1 enzymes first activate ubiquitin, then E2 conjugating enzymes transfer and chaperone activated ubiquitin. Lastly, E3 ligases transfer activated ubiquitin to the targeted substrate. Polyubiquitinated chains of lysine residues are a major cue for a protein to be degraded by the proteasome. The most well-described E3 ligases associated with muscle wasting are muscle ring finger1 (MuRF1) and muscle atrophy F-box (MAFbx) or atrogin-1. These E3 ligases were discovered in the early 2000s (46) and numerous publications under both transgenic, preclinical and clinical conditions highlight their crucial role in muscle atrophy (19). Whole body mouse knockout models suggest that these proteins may have additional roles outside of muscle atrophy. Six-month-old MuRF1 knockout mice have reduced markers of oxidized proteins, whereas 24-month-old MuRF1 knockouts have increased capillary density, elevations in caspase-3 activity, and reduced markers of sarco/endoplasmic reticulum stress (47). MAFbx knockout seems to be more detrimental as mice have a shortened life span, histological and electron microscopy evidence of sarcomeric and cross-sectional ventricular abnormalities, as well as consistent arrhythmias and hypertrophic cardiomyopathy (48). Despite these potential alternative roles of MuRF1 and MAFbx, they have largely been discussed in the context of muscle wasting. The transcription factors best described in regulating MuRF1 and MAFbx expression are FOXOs (1, 3a, and 4), KLF-15, NF-κB, C/EBPβ, and Smad3 (19). A number of atrophic stimuli can activate distinct pathways that result in upregulation of MuRF1 and MAFbx (19). Disuse, cytokines, glucocorticoids, TGF-β family members (myostatin and GDF11), oxidative stress, and caloric deficits can all work independently as well as together through their own distinct cellular receptors and signaling pathways to regulate the activity and expression of these canonical E3 ligases. MuRF1 and MAFbx target different components of the myofiber with MuRF1 largely assumed to target contractile proteins and MAFbx key muscle regulatory proteins like MyoD and eIF3-f (as reviewed in Refs. 19, 20). In hypermetabolic muscle atrophy, such as the flow phase following human burn injury, the systemic effects lead to markedly upregulated ubiquitin-proteasome activity even in the skeletal muscle of a nonburned limb, suggesting muscle could be releasing circulating factors (49). Among atrophied older adults, acute resistance exercise appears to reduce the gene expression of FBOX32 and TRIM63, the genes that encode MAFbx and MuRF1, respectively (50). It should be noted, however, that although MuRF1 and MAFbx are often seen as the default markers of muscle atrophy, there are hundreds of other E3 ligases, as well as other muscle and striated tissue-specific E3 ligases. These are detailed elsewhere (19, 21, 23).
The other major degradation system that exists in muscle fibers is the autophagic system. Autophagy was first associated with activation during nutrient stress, thus, it is not surprising it works through a combined regulatory system involving AMPK and mTOR, principally mTORC1 (51). It acts like a large-scale recycling system that is able to use autophagasome formation to encapsulate dysfunctional organelles and proteins to promote their degradation by delivering their cargo to the lysosome (24, 52–54). Autophagy is often renamed based on specific targets like the mitochondria (mitophagy) or intracellular fat stores (lipophagy). Regardless of the ultimate substrate, the upstream signaling molecules share common characteristics. When active, AMPK directly phosphorylates key residues of unc-51-like kinase 1 (ULK1) to activate the ULK1 complex, which contains ULK1, autophagy-related gene 13 (ATG13), and FAK family-interacting protein of 200 kDa (FIP200). mTORC1 phosphorylates ULK1 at its inhibitory site to prevent AMPK-ULK1 interactions. The development and progression of the phagophore to a mature autophagosome starts with the activation of the ULK1 complex. The phagophore progresses and expands following activation of the beclin-1 complex and LC3 conjugation and lipidation. Protein clusters or membrane proteins with ubiquitin chains are targeted by the autophagasome through p62-LC3 scaffolding. Mature autophagosomes, with cargo inside, are then transported to the lysosome where they fuse into the autolysosome and the protein contents are then catabolized by cathepsins, peptidases, and other enzymes that function in low pH environments (54).
Epigenetics
Epigenetics is the regulation of gene expression without changes in the genetic code (55). The complex regulatory mechanisms of epigenetic modulation in prolonging or protecting skeletal muscle from active wasting, and how exercise alters these effects, include DNA methylation, histone modifications, changes in the expression of a range of RNA species including micro- (miRNA), circular (circRNA), and long noncoding (lncRNA), and (potentially) nuclear mRNA methylation cycling. These regulators of gene expression have not been extensively described in exercised skeletal muscle during conditions of wasting, but existing evidence supports that they can influence muscle development, hypertrophy, atrophy, and wasting (56–59). We highlight and summarize well-known epigenetic regulators below and direct the reader to resources that cover these factors in depth (60–65). miRNAs are short ∼22 nucleotide, noncoding RNAs which bind to the 3′ UTR of target mRNA to inhibit or impair translation. They have been implicated in many aspects of skeletal muscle formation, development, health, and responses to exercise (66). Skeletal muscle produces tissue-specific miRNAs known as myomiRs (61, 66). The first myomiRs identified include miR-1, miR-133a/b, and miR-206. They appear to be under the regulation of key transcription factors (MyoD, myogenin, Myf5) in muscle development (67, 68) and their downstream effect in vitro is generally enhancement of skeletal muscle development (69). For example, upon exposure to an acute inflammatory stimulus, C2C12 myotubes exhibit decreased expression of miR-1, -133a/b, and -206, among others (70). However, in preclinical models of dexamethasone-induced atrophy, high activity of miR-1 was associated with FOXO3-mediated proteolysis (71). Although myomiRs exert effects within muscle tissue, they may also be released into circulation. miR-206 has been found elevated in serum of individuals undergoing unloading-induced atrophy, as well as other potential serum miRNA biomarkers (72). The same effect has been reported in Duchenne muscular dystrophy (73). These findings suggest that miR-206 might be released by muscle under conditions of atrophy or damage. Other circulating indicators of muscle health during wasting and other pathophysiological states are obviously of great interest for their potential prognostic use as biomarkers.
Recent investigations focusing on circulating factors contained in extracellular vesicles (EV) and their miRNA cargo may help facilitate understanding how tissues distant from each other can communicate and potentially alter muscle mass (74). EVs are membrane-enclosed bodies that can be secreted by tissues with varying cargo (miRNA, mRNA, and proteins) depending on the stimulus. Circulating levels of miR-203a-3p have been associated with muscle disuse following acute limb suspension, with potential links to the liver (75). Relatedly, circulating miRNA are different at baseline between sedentary and trained individuals, and a 40 min cycling session was able to further alter the circulating miRNA cargo of EVs, with pathway enrichment showing potential alterations in miRNA regulation of IGF-1 between trained and untrained individuals (76).
Another principal epigenetic regulator is DNA methylation. Changes in DNA methylation alter the accessibility of transcription factors and coactivators to key sites on the genome to promote or repress the expression of target genes. DNA is most often methylated on cytosine residues directly followed by a guanine in the 5′-3′ direction. These sites are termed CpGs and they often cluster together near promoter regions (77). Heavily methylated CpG elements generally implicate poor downstream gene transcription by limiting chromatin accessibility. Acute cycling exercise can reduce global methylation status of vastus lateralis muscle and resultant hypomethylation of promoter regions of important metabolic regulators (78). Chronic resistance training appears to have an “epi-memory,” in which the methylation status of skeletal muscle is preserved even with a long period of unloading and subsequent retraining (79). Although exercise training and the metabolic stresses associated with it would be expected to change the methylome, individuals that report taking part in life-long physical activity also demonstrate distinct muscle methylation profiles and reduced methylation in gene promoter regions associated with energy metabolism (80). Future studies examining global patterns in DNA methylation and other epigenetic mechanisms are likely to reveal important processes influencing known muscle wasting mechanisms as well as connections to novel pathways that remain incompletely understood in this context.
Changes in DNA methylation and noncoding RNA expression have been the most investigated epigenetic regulators of skeletal muscle in regard to exercise and disuse or pathology. Another intriguing direction is modification of the epitranscriptome through post-translational modifications of nuclear mRNA. Methylation of mRNA to destabilize and limit its translation has been a known functional outcome for decades, but improvements in detection assays and techniques, as well as sequencing, mapping, and identification of key methylation sites, have greatly improved resolution and throughput. N6-methyladenosine (m6A) is the most common of the mRNA alterations. Methyltransferase-like protein 3 (METTL3) and adaptor proteins of the writer complex are responsible for this methylation process, with additional nuclear reading and erasing processing occurring before export to the cytoplasm (81). Most m6A sites have been identified in the 3′ UTR, similar to miRNA target sites, but abundant sites have also been described in the coding region, 5′ UTR as well as the cap (81). To our knowledge, the epitranscriptome has not been investigated in mature muscle from preclinical models or from human skeletal muscle biopsies. Cell culture studies do suggest changes in m6A profiles in proliferating and differentiating C2C12 cells, and knockdown of METTL3 can induce premature myoblast differentiation (82), insinuating an important role for mRNA methylation in muscle development and likely key roles during wasting and exercise.
Exercise to Combat Muscle Wasting
The regulation of muscle mass involves a complex biochemical interplay of factors that are highly sensitive to external and intracellular cues; thus, any imbalance greatly challenges protein homeostasis. Exercise training of various modes and prescriptions is a powerful stimulus that mitigates muscle loss in a variety of pathologies. The following sections describe how exercise alters the key molecular profile and ultimately the phenotype.
Unloading and Disuse Atrophy
Targeting muscle health and function during disuse has largely focused on astro/cosmonauts experiencing microgravity during spaceflight and individuals undergoing extended bedrest due to hospitalization, illness, injury, or as a ground-based spaceflight analog. Due to the extent of its effects on physiological systems such as skeletal muscle (83), this type of disuse has been proposed as a model to quantify aspects of accelerated aging (84). There are multiple methodologies that have aimed to simulate these environmental/situational effects in research settings. A 6° head-tilt model is often used to understand how microgravity affects hemodynamics, the cardiovascular system, and skeletal muscle (85–87). Unilateral lower limb suspension (ULLS) is another model which allows comparison of one leg with another within an individual without overly constricting daily living. Skeletal muscle mass is reduced by up to 20%–30% with long-term bedrest (4), and strength may be reduced up to 40% (88). Muscle atrophy under these conditions appears to be impacted by the role of the muscle groups affected (89, 90). For example, upper body muscles tend to be less affected by unloading (91, 92) whereas postural muscles such as the extensors of the trunk, knee, and ankle (i.e., plantar flexors) are highly impacted (89, 93–97). Some individuals experience additional metabolic disturbances, including upregulation of genes related to lipogenesis and reduced lipid export, and insulin resistance (98). Histologically, unloading induces a slow-to-fast myofiber type shift, often contributing to a larger hybrid pool (99, 100). At the myofiber level, decreases in power and force are apparent (101, 102) and unloaded shortening velocity often increases (103). Furthermore, a 40% reduction in the muscle satellite cell pool (104) may have implications for regenerative capacity upon reloading.
Mechanistic investigations into large-scale molecular profiling of unloaded muscle are beginning to emerge (105). A recent multiomics meta-analysis of spaceflight datasets in humans and animals highlighted the importance of mitochondrial function in response to unloading and revealed mechanisms (e.g., DNA methylation) regulating the behavior of innate immune components (106), which may be critical for the maintenance of muscle mass during stress. Interestingly, knockout of MuRF1 in mice showed protection against declines in soleus and myofiber cross-sectional area on earth (107) but not in space (108), suggesting unique challenges in microgravity that may be difficult to fully mirror with Earth-based analogs (78). Another recent transcriptome-wide study found that 5 days of bed rest elicited expression of inflammatory genes in the muscle of older adults but not in younger counterparts (109), including inflammatory factors (NFKB1, IL6R, CXCL2) and growth arrest and DNA-damage-inducible protein GADD45α (GADD45A), often implicated in muscle denervation (110). Likewise, older adults undergoing bedrest had marked reductions in muscle protein synthesis that were not seen in young adults after 5 days, despite lower mTOR activity in both groups (111). The concept that age introduces variation into the response to unloading is intuitive, and it is likely that other intrinsic and extrinsic factors play a role, as has been extensively investigated in the context of exercise-induced muscle hypertrophic response (112, 113). It may be of value to establish a better understanding of heterogeneity in resilience and susceptibility to unloading atrophy and its array of molecular, metabolic, and mechanical consequences in order to design more personalized countermeasures for preservation of muscle health during immobilization.
Exercise training upon reloading promotes eventual restoration of muscle mass and function, although the duration is considerably longer than the time in which they are lost (114–116). The most effective approach to combat unloading-induced atrophy is exercise during unloading. Exercise is surprisingly protective in very small doses as even the minor degree of muscle loading in exercise testing is potent enough to serve as a countermeasure (117). Structured exercise interventions are partially or completely protective against whole muscle atrophy and strength losses (90, 118, 119) and may curtail the increase in hybrid myofibers (88) during unloading. Resistance exercise to volitional fatigue during 14 days of continual head-tilt bedrest was able to prevent reductions in circulating creatine kinase and FGF levels (120) and muscle protein synthesis (121). In addition, myofiber size and leg press 1-repetition max strength (122), as well as other contractile and functional parameters (123), were preserved. In a 70-day head-tilt bed rest study that coupled aerobic and resistance training with placebo or low dose testosterone enanthate (TE) supplementation, exercise, in both placebo and TE groups, was able to preserve leg press power, vertical jump power, and whole muscle CSA compared with nonexercised controls, though there were not any clear benefits of TE (124). Although complete preservation of postural muscles remains a challenge, studies in microgravity have shown that exercise exerts an incrementally protective effect on postural muscles and counteracts unloading-induced decrements in myofiber lipid availability and cytochrome C oxidase activity (125). Efforts are being directed to design smaller, lighter, more efficient microgravity exercise devices and time-saving prescriptions, such as the NASA Sprint interval training protocol (124, 126), to maintain astronaut health in the face of more intense stressors (e.g., hypoxic conditions, longer duration flights, extravehicular activities) (125, 127–129).
Factors that mechanistically drive the protective effects of exercise are less well-established. Recent work has demonstrated that concurrent flywheel exercise during 85 days of bedrest normalized the expression of transcripts related to mitochondrial function, oxidative metabolism, and circadian rhythm in the vastus lateralis muscle of young men (130), which was associated an exercise-induced protection against muscle loss previously reported from these individuals (119). Despite this effect, 209 of 650 genes dysregulated by bedrest were unaffected by exercise, primarily related to protein synthesis and degradation, cell cycle dynamics, and nucleotide metabolism (130). In the soleus, resistance exercise with and without vibration stimulation induces a differential transcriptomic profile versus bedrest alone (117). Taken together, these two investigations point towards a potential regulatory role of the transcription factor prospero homeobox 1 (PROX1) in slow-to-fast fiber type shifting. Specifically, bed rest without exercise induces a reduction in PROX1 expression in the vastus lateralis (130), whereas expression is reduced in the soleus even with concurrent exercise (117). This may be due to the likely higher baseline expression of PROX1, a type-I myofiber specific marker (131) in the soleus tissue. Future investigations at other ‘omics levels are likely to provide valuable insight into regulatory mechanisms and molecular profile of the muscle-specific effects of unloading.
Other approaches to combat muscle wasting during unloading have included blood flow-restricted exercise, nutritional supplementation, and neuromuscular electrical stimulation (NMES) (132). In a recent clinical trial, NMES combined with protein supplementation preserved muscle mass but not function during 5 days of bedrest (133). However, during longer periods of unloading, protein supplementation without muscle contraction appears counterproductive (90, 134). Thus, the appropriate implementation of these countermeasures is likely dependent on a range of factors, including the duration of mechanical unloading, participant demographics, and intrinsic molecular profile of the individual. Continued efforts to characterize the molecular mechanisms underlying disuse atrophy are likely to aid in the development of more effective strategies to mitigate muscle mass and strength losses in space and on earth.
Spinal Cord Injury
SCI is a devastating systemic injury that results in rapid muscle atrophy and deterioration in muscle contractile and metabolic function below the spinal lesion. Skeletal muscle health is greatly affected post SCI due to being at the crossroads of major atrophy inducers such as immediate disuse, systemic stress from the trauma, and potential loss of innervating motor neuron regulation below the area of the lesion. Individuals with long-standing SCI also have higher rates of general physical inactivity (135, 136). Acute SCI is associated with elevations in the gene expression of TRIM63, FBOX32, and pathway analyses of differentially regulated genes as measured by microarray show SCI-induced changes in factors associated with the proteasome (137). A physiologically or functionally complete SCI means any modest contractile activity in paralyzed muscle is likely beneficial. For example, excess involuntary spasticity in those with more severe SCI is often considered problematic and impacts quality of life, but these consistent contractions are able to preserve sarcomere integrity, mitochondrial localization, and t-tubule/ryanodine receptor organization (138). Thus, any consistent loading for those with paresis/paralysis is likely to lead to drastic improvements in muscle health. Exercise training through NMES and/or functional electrical stimulation (FES) (139–143) and body weight-supported training (BWST; refs. 149, 150, 151, 249, 250) have been the most effective strategies to date for those with SCI.
Multiple rehabilitative strategies have been developed to improve muscle health and performance in individuals with SCI. FES, a specialized methodology of NMES in which the electrical stimulation paradigm is meant to mimic the firing patterns of some functional task like cycling or walking, is one that has been used extensively. Early FES studies in individuals with motor complete SCI show 2 mo of triweekly FES cycling can increase protein expression of both GLUT1 and GLUT4 and improve function of citrate synthase, a key metabolic enzyme that is commonly used as a marker of mitochondrial biogenesis and function in skeletal muscle (144). Similarly, 1 yr of a similar training design elevated GLUT4 protein expression, which decreased 6 mo after exercise volume reduction (145). In a 16-wk study where individuals with SCI did 40 min of FES for 5 days/wk at 75% of their predicted max heart rate, there were beneficial responses in protein expression of GLUT4, PGC1α, and AMPK although no improvements in lean body mass were detected (146). Passive cycling, where individuals with SCI have their lower limbs mechanically moved through the cycling range of motion by the apparatus, has been associated with reductions in mRNA and protein markers of proteasome activity (147, 148).
BWST, using treadmills with support devices, is also an established way to improve muscle health in those with SCI. In a case study, 4 mo of BWST training was able to increase muscle fiber size by 25% and increase the percentage of slow-twitch myofiber populations (149). These data are supported by a 6-mo study with a larger cohort showing similar outcomes (150). This same 6-mo cohort used males with incomplete SCI (ASIA C) and trained 3 days/wk with 20–30 min sessions. They reduced their need for weight-bearing assistance over the course of the study (65% pre- to 23% post-), and muscle GLUT4 protein expression and hexokinase enzymatic activity were greater posttraining, suggesting improved glucose utilization (151).
NMES has been the predominant rehabilitative modality to improve muscle function in those with SCI (152). In two individuals with over 6 yr of chronic unilateral soleus NMES (right soleus: 4,000 contractions/month; left soleus: untrained control) the trained right leg showed expected hallmarks of training effects compared with the left: greater slow twitch muscle mRNA profiles and greater mRNA expression of electron transport chain subunits and glycolytic markers (153). When NMES was removed from the right side, mRNA expression of MSTN, the gene encoding myostatin, was still reduced after 6 mo compared with the continually untrained left soleus, and the oxidative type I muscle genes MYH7 and MYL3 were still elevated (153). This same group did a more comprehensive exon array and methylation analysis of both short (avg: 4 mo) and long-term (avg. 4 yr) unilateral NMES training. Gene set enrichment scores showed training-induced clustering of genes that are well-associated with exercise and oxidative metabolism (141). They also probed targeted CpG sites within the PPARGC1A gene, which encodes PGC1α, and demonstrate long-term NMES resulted in demethylation when compared with the untrained muscle (141). Acute NMES has been shown to result in major changes in the transcriptome, with a single bout of 4 × 125 contractions resulting in ontological profiles associated with cell signaling and metabolism, and follow-up mRNA analyses showing upregulation in PPARGC1A and NR4A3 (142).
Outcomes at the protein level also demonstrate exercise-induced benefits. Acute NMES at 30% electrically evoked maximum torque in those with ASIA A or B SCI was able to increase phosphorylation of key activation sites for Akt, Ca2+/calmodulin-dependent protein kinase, and AMPK at 10 or 60 min post-NMES (154). This same cohort showed greater activation of FAK and RPS6, and total α7 integrin expression compared with able-bodied controls at the same relative contraction force, implying a lower threshold for the mechanosensory program in chronically paralyzed muscle (155). Chronic NMES has shown more varied responses to the molecular signals associated with hypertrophy. Eight weeks of NMES of the vastus lateralis in individuals with SCI given testosterone replacement (TRT) showed improved GLUT4 expression and modest trends for elevated total and activated Akt compared with TRT-only controls, but no NMES-induced changes were seen in PGC1α, FAK, mTOR or AMPK (156). Similarly, there were no changes in expression of total or phosphorylated hypertrophic protein markers (Akt, p70S6K, and RPS6) after 8 wk of NMES of the vastus lateralis despite gains in myofiber size (157).
Denervation Atrophy
The neuromuscular junction (NMJ) is the primary communication site between the innervating motor neuron and each myofiber. Loss of neural input by pathology or trauma results in fiber atrophy and eventual death (158, 159). Denervated fibers can be distinguished histologically from nearby healthy fibers through their severe atrophy and triangular shape but direct observations of the NMJ are necessary to confirm denervation. In animal models, longitudinally sectioned muscle can reveal disruption of the NMJ (160) but this is notoriously challenging in human biopsy specimens. Denervated muscle displays a reprogramming signature that recapitulates the muscle environment during development (161). This commonly involves the neural cell adhesion molecule (162), sodium channel 1.5 (161), and the neonatal myosin heavy chain isoform (163, 164).
At the cellular level, denervation is accompanied by disassembly of the acetylcholine receptor (AChR) complex on the motor end plate of the NMJ (165), heightened activity of histone deacetylases (HDACs) (110, 166), and increased proteolysis via traditional catabolic mechanisms (24, 79, 167). Preclinical studies using proteomics have suggested that calcium handling and contractility are also negatively impacted by denervation (168). Denervation is also associated with impaired mitochondrial function (162, 169–171) and mTOR activity, specifically mTORC1, has been linked to muscle denervation in animal models (172, 173).
Denervation in primary aging.
There is a significant body of literature to suggest that neuromuscular communication is negatively impacted by aging (167, 174, 175) but whether the process is initiated by the muscle or nerve is unclear (160). In primary aging, denervation largely occurs with type II myofibers (176) with reinnervation typically involving rescue by a neighboring type I motor neuron. This gives rise to a clustered configuration (177, 178) in which grouped type I myofibers exhibit some characteristic features of their previous type II identity (174). At least during development, formation of a new NMJ is thought to involve muscle-nerve exchange of low-density lipoprotein receptor-related protein 4 (Lrp4) and muscle-specific kinase (MuSK), although the direction of the communication is still being debated (179). Evidence in humans supports that muscle of individuals with a higher degree of type I myofiber grouping exhibits a higher abundance of MuSK (165), supporting this mechanistic link.
At the whole muscle level, myofiber grouping translates into larger motor unit size and lower estimates of motor unit number in old versus young adults (180). Consequentially, a greater proportion of myofibers is activated at a given intensity (relative to maximum voluntary contraction) (165) and motor unit activation exhibits greater synchronization (181). However, reinnervation is protective against age-related muscle loss and associated functional deficits (182, 183). Some evidence suggests that males are less successful at reinnervation than females (184, 185) but may be more likely to undergo denervation atrophy and compensatory type I myofiber hypertrophy (186). Long-term exercise is thought to heighten reinnervation success in both sexes throughout aging (182, 183, 187, 188); however, recent evidence to the contrary has emerged (189). A more complete understanding of the dynamics of denervation/reinnervation and an optimized approach to quantifying fiber grouping may aid in establishing whether regular exercise offsets any deleterious effects of NMJ remodeling in aging.
Denervation in disease states.
Amyotrophic lateral sclerosis (ALS) is a motor neuron disorder characterized by extensive dismantling of NMJs (179) and sustained progressive loss in muscle mass. Although the precise mechanisms are still unclear, recently proposed biomarkers are low muscle expression of HDAC4 (190), the vitamin D activator CYP27B1 (191), TGF-β (192), and expression of Smad8 (192). Furthermore, glial cell dysfunction is thought to contribute to the pathology (179, 193) and recent research has focused on the potentially protective role of synaptotagmin for motor neuron survival under ALS and related conditions (194, 195). Descriptive, well-designed exercise training studies focused on individuals with ALS are difficult due to the rarity of disease and aggressive disease progression; thus molecular outcomes from these types of studies are lacking. Resistance exercise training in this population has been associated with improved functional outcomes and a reduced loss in muscular strength (196) whereas changes in lifestyle behaviors may also play a role in modifying disease progression (197). Six weeks of progressive rehabilitative exercise training in combination with stretching in individuals with ALS was able to reduce circulating levels of myomiRs, suggesting limitations in muscle damage or breakdown (198).
In Huntington’s disease, a hereditary neurodegenerative disorder, NMJ defects appear to arise from the muscle itself, wherein ion channel disturbances result in inappropriate polarization of the sarcolemma (199–201). Interestingly, the motor neuron compensates for this hyperexcitable muscle state (202), potentially reducing the severity of myopathy. In a human exercise trial, endurance training improved muscle metabolic capacity and function of enzymes of the electron transport chain (203), but not muscle satellite cell abundance (204), in individuals with Huntington’s. Thus, the propensity of exercise interventions to correct muscle-level deficits is not well-established, and some evidence from animal models suggests that it may actually be contraindicated (205).
In individuals with Parkinson’s disease, primary neurodegeneration in the midbrain manifests in motor control abnormalities (e.g., tremor). Within skeletal muscle, type II myofibers appear to undergo extensive denervation, leading to type I myofiber grouping in an apparently exaggerated version of what occurs with normal aging (165, 206). This phenotype, however, is partially plastic when stimulated with combination high-intensity resistance exercise rehabilitation (165, 207). Due to the reversal of such a strong phenotype, it is postulated that grouped type I myofibers are dually innervated at some point in their transition, as demonstrated in animals (208). Mechanistically, this effect appears to be accomplished by exercise-induced expression of skeletal muscle genes linked to axon guidance, remodeling, and neuromuscular communication (209). Importantly, a high-intensity exercise intervention reduced the apparent histopathology in a manner concomitant with improvements in motor, cognitive, and emotional health domains (207).
Inflammatory States
Acute inflammation plays a vital role for muscle regeneration and repair, but sustained elevation in systemic or local tissue levels can lead to muscle wasting or blunted anabolic responses. For example, as an extreme case, primary human myotubes treated with proinflammatory serum from burn victims with hypermetabolic muscle wasting show heightened IL-6 signaling via STAT3 phosphorylation, blunted myogenesis, and impaired anabolic signaling and protein synthesis rates (210). Aged muscle shows inflammatory susceptibility and sensitivity to proinflammatory cytokines (50, 211) and may provide at least some explanation for the diminished response to exercise training seen in aged individuals compared with young individuals. Lifestyle-induced increases in visceral adiposity (212) and age-related decreases in cellular function can slowly increase basal inflammation and disrupt skeletal muscle regeneration capacity over time, resulting in age-related muscle wasting in otherwise healthy individuals (213). In addition, the onset of sarcopenia was inhibited in old mice transplanted with bone marrow from young mice, suggesting that aging of the immune system directly contributes to muscle loss (214). Most analyses of skeletal muscle are from whole muscle lysates that include numerous other cell types that are capable of releasing proatrophy cytokines (215), so elucidating which exact cell types are releasing inflammatory factors can be a challenge. What has been established is that inflamed muscle exists within a multicellular inflammatory environment which can lead to muscle wasting, and that long-term resistance training or lifelong exercise training can create an anti-inflammatory profile and beneficial exercise response (216, 217).
TNFα.
TNFα is one of the major proinflammatory cytokines involved in muscle wasting, exerting effects via multiple mechanisms. It contributes to intramuscular insulin resistance (218) and is a likely component of muscle atrophy in those with type II diabetes (219). Elevated levels of intramuscular TNFα activate the apoptosis pathway (220) and suppress anabolic signaling (221). TNFα may also interfere with the regenerative capacity of muscle by inhibiting myoblast fusion via FAK signaling (222) and contributing to impairment of endothelial responsiveness near muscle by disrupting dilation and intracellular communication. Among atrophied older adults, muscle tissue TNFα and its receptor, TNFα-R1B, have elevated mRNA expression and elevated downstream protein signaling components IκBα and p50-NFκB. In addition, cell culture studies using primary satellite cells show older adults have heightened basal protein levels of TNFα-R1 and an exaggerated signaling response to TNFα treatment compared with young adults (50). The evidence of exercise altering TNFα in muscle is varied. Resistance exercise training reduces whole muscle TNFα protein and mRNA in frail individuals (223, 224) but endurance exercise training elevated TNF mRNA in 50-yr-old individuals (225).
TWEAK.
TNF-like weak inducer of apoptosis (TWEAK) is a proatrophy cytokine that has similar characteristics to TNFα, such as activating p65-NFκB, but binds to its own TNF super family cognate receptor, Fn14 (226). It can be upregulated in diseased muscle without any systemic marker of inflammation (227). It is a potent muscle wasting stimulus as evidenced in cell culture and preclinical mouse models (226, 228, 229) and is associated with pathological muscle wasting conditions (227, 230, 231). In human burn injury, markedly elevated muscle mRNA expression of TNFSF12, the gene that encodes TWEAK, and TNFRSF12A, the gene encoding Fn14, has been found in conjunction with elevated MAFbx and MuRF1 in a nonburned limb (230). Similar elevated expression profiles of these proinflammatory genes have been noted in the skeletal muscle of patients with hip fracture (227) and individuals with SCI (231). Despite the clear links to inflammation and TNFα, there is varied evidence about the role of TWEAK-Fn14 and signaling capacity through noncanonical molecular pathways (232). The role of exercise in altering TWEAK-Fn14 is not fully understood but in general there appears to be transient changes in Fn14 in nondiseased individuals that may be beneficial and distinct from the deleterious consequences of chronic elevation. (225, 233).
IL-6.
The acute release of IL-6 from exercising skeletal muscle established muscle as a key secretory organ and IL-6 as a key molecule in regulating the local and systemic response to exercise (7). However, chronic elevations of IL-6 due to obesity, physical inactivity, or pathology are detrimental (234). In support of IL-6 contributing to muscle loss, studies found that IL-6 levels are heightened in various disease states, including chronic kidney disease (CKD) (235) and cancer cachexia (236), as well as in aged individuals (237). Beyond circulating IL-6, muscle-specific expression of IL-6 related genes, along with some IL-6 signaling components, are markedly elevated in acute muscle wasting conditions such as trauma (227) and burn injury (230). Although to a lesser magnitude, likely biologically significant elevations are also found in muscle from aging humans (50). In contrast, anti-inflammatory effects of IL-6 can inhibit muscle wasting particularly through exercise-induced means (i.e., calcineurin pathway, IL-10 production) rather than low-grade inflammation induction (220, 238, 239). It is possible the location and mode of induction of IL-6 (i.e., serum, muscle; exercise, immune system) may influence its involvement in muscle wasting. Baseline total and activated STAT3 levels, the canonical intracellular signaling node for IL-6, are elevated in aged individuals compared with young, although an acute bout of moderate resistance exercise was not able to alter these levels (240). IL-6 regulation of myostatin, a potent negative regulator of muscle mass, has been shown to work through the C/EBP transcription factor, which was correlated to patients with CKD-induced muscle wasting (235). This was further supported using a preclinical approach showing STAT3 inhibition prevented muscle wasting via myostatin signaling (235).
Sepsis.
Hyperinflammation during sepsis is a critical medical event that often results in organ damage, hypermetabolism and, often Intensive Care Unit-mediated mechanical ventilation. Those with sepsis that suffer severe muscle loss have elevated risks for 28-day mortality, demonstrating a great need for interventions to protect muscle in this condition (241). NMES/FES interventions are strategies that have been used to mitigate the rapid muscle wasting under these conditions. Upper and lower body NMES, with or without additional whole body vibration therapy, was not able to protect muscle strength but was able to protect vastus lateralis fCSA and increase slow MYH1 and MYH4 gene expression, as well as fast and slow myosin protein expression compared with patients that received standard of care only (242). In addition, serum levels of TNFα were reduced in the intervention group while serum amyloid A1/2 was elevated (242). Unilateral FEC cycling was not effective in preserving quadriceps strength and resulted in minimal differences in changes in rectus femoris CSA compared with usual care, despite long-duration (up to 60 min) and frequency (>5 days/wk) (243); however, no molecular work was reported for this study. NMES training (1–2 bouts/day) of the quadriceps and tibialis anterior of ICU-bound individuals resulted in reduced blood concentrations of IL-6 and elevated IL-10, improved self-reported physical function, but no changes in fat-free mass or muscle strength (244).
CURRENT KNOWLEDGE GAPS AND FUTURE DIRECTIONS
The discovery of the secretory capacity of exercising muscle has led to focused investigations aimed at identifying novel factors that promote muscle and systemic health. A number of peptides have been described to be released from exercising skeletal muscle that improve metabolic function, coordinate inflammatory responses, and protect muscle mass (245, 246). Some of these factors, such as irisin/FNDC5, apelin, SPARC, myonectin and FGF21, among others, likely have crucial roles in coordinating integrative responses to exercise. However, their roles in muscle loss have largely been in context of transgenic mouse models or preclinical models of aging, cachexia, and heart failure (245). Future research will likely uncover important roles for these factors during acute muscle wasting. Another consideration is the role of nonmuscle exerkines that may exert an effect on skeletal muscle. Adipose tissue and the liver have secretory roles important for the exercise response and there are likely important factors released from the cardiovascular and peripheral nervous systems (247).
There are some major limitations to investigating how exercise can treat muscle wasting, especially in acute conditions of a metabolic origin where they may not be ready access to patients and determining thresholds for necessary exercise volumes are not established. Well-controlled, multisite randomized clinical trials that can leverage diverse investigator expertise and provide sufficient access to specific populations would greatly improve our understanding of the role of exercise in clinical conditions of muscle wasting across various demographics, especially in those that are underrepresented (e.g., racial/ethnic minorities). Emphasis on discovering biomarkers correlated with important clinical outcomes that are also directly regulated by exercise may accelerate the implementation of exercise prescription as bedside standard of care. Synergistic approaches to improve muscle health using exercise and pharmaceuticals simultaneously may be useful in treating pathologies that do not have promising drug candidates. Lastly, identifying mechanisms behind heterogeneity in resiliency to muscle wasting and/or muscle mass adaptation to exercise, could direct personalized exercise prescriptions to optimize recovery.
Although several key mechanisms have been identified, the complex molecular interactions that promote muscle wasting and/or adaptation to exercise have not been entirely elucidated. Thus, a promising direction for future work would be to leverage the reductions in costs of sequencing and mass spectroscopy technology to direct non-hypothesis driven discovery and systems biology. Initiatives such as the Molecular Transducers of Physical Activity Consortium [MoTrPAC; (248)] aim to do this in the context of exercise on the largest scale to date. The data generated by MoTrPAC will be a valuable public resource, providing researchers specializing across multiple disciplines in muscle wasting research access to robust multiomic data from a healthy reference population. MoTrPAC and other future multiomic work is likely to be critical in the identification of biomarkers and candidate targets for exercise + pharmacotherapy interventions under conditions characterized by muscle wasting.
CONCLUSIONS
Many of the adaptations in skeletal muscle in response to exercise directly oppose factors that lead to muscle wasting (Fig. 2). The power of exercise and relative ease of accessibility clearly implicate exercise as a crucial frontline intervention to treat muscle loss in many disease states. In individuals without easy access to exercise, such as those with advance neurological diseases or injuries, improvements in exoskeleton design and battery technology may allow for rehabilitative or home use to improve health and quality of life. It would be anticipated that in the near future, exercise will be treated as a usual care ‘drug’ with a proper dose and proper prescription. In cases where unloading and disuse atrophy are predicted, such as a planned surgery or spaceflight, prescribed exercise to increase muscle mass and combat muscle loss should be considered. Under metabolically based wasting conditions, exercise likely has to be used synergistically with a combinatorial intervention strategy, e.g., complemented by pharmacological treatments. The plentiful benefits of exercise, paired with its minimal side effects and rare contra-indications, clearly implicate it both as a preventative and therapeutic measure that can safely be deployed to combat multiple mechanisms of muscle wasting.
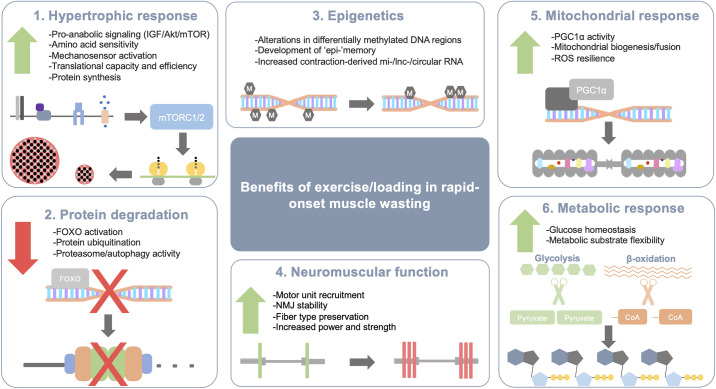
Figure 2.
Range of benefits of exercise for pathophysiology during rapid onset muscle wasting. Acute and chronic exercise training involves the interaction of multiple molecular systems that act together to improve contractile and metabolic function. 1) Activation of pro-anabolic signaling pathways as a result of exercise converges upon mTORC1/2 to increase ribosomal initiation and efficiency to increase protein synthesis. 2) The activation of mTORC1/2 and related anabolic pathways inhibit activation of transcription factors that begin the muscle atrophy program (e.g., FOXO family) to prevent excess protein degradation. When protein synthesis exceeds protein degradation over time, appreciable muscle mass can be gained. 3) Exercise can create long-standing changes in the epigenetic profile of muscle by hypermethylating DNA in promoter regions of deleterious genes to limit their expression while also demethylating DNA regions to increase transcription of beneficial genes. Contraction-mediated increases in noncoding RNA species may regulate gene expression to limit factors that induce muscle wasting. 4) Improved neuromuscular function can result in protected or increased muscle strength and power. Strength and power are two critical clinical outcomes that may be a result of exercise training even if there is no gain in muscle mass. 5) A primary result of exercise is improved mitochondrial density and function. This is mostly mediated by PGC1α nuclear translocation and transcription of key proteins needed for oxidative phosphorylation and mitochondrial fusion. 6) Muscle contractions and sustained exercise require ample amounts of ATP. Events that result in muscle wasting can create metabolic hurdles to proper energy regulation such as insulin resistance. Exercise can improve glucose uptake regardless of insulin sensitivity to help control systemic glucose homeostasis while also using additional fuels such as fatty acids to generate substrate for oxidative phosphorylation and ATP production. lnc, long noncoding; miRNA, microRNA; mTORC1/2, mechanistic target of rapamycin complex 1/2; NMJ, neuromuscular junction; PGC1α, PPARG coactivator 1 alpha; ROS, reactive oxygen species.
GRANTS
This work was supported in part by the Department of Veterans Rehabilitation Research and Development Service Career Development Award-2 (IK2RX002781); NIH Grants T32HD071866, P2CHD086851, R01HD084124, U01AR071133; and DoD awards FA8650-19-C-7944, N000141613159.
DISCLAIMERS
This work does not represent the views of the Department of Veterans Affairs or the US Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.A.G., K.M.L., S.M.O., and M.M.B. prepared figures; Z.A.G., K.M.L., and S.M.O. drafted manuscript; Z.A.G., K.M.L., S.M.O., A.E.T.-M., T.W.B., K.M.F., T.J.B., and M.M.B. edited and revised manuscript; Z.A.G., K.M.L., S.M.O., A.E.T.-M., T.W.B., K.M.F., T.J.B., and M.M.B. approved final version of manuscript.
REFERENCES
- 1.Franklin BA, Thompson PD, Al-Zaiti SS, Albert CM, Hivert MF, Levine BD, Lobelo F, Madan K, Sharrief AZ, Eijsvogels TMH. Exercise-related acute cardiovascular events and potential deleterious adaptations following long-term exercise training: placing the risks into perspective-an update: a scientific statement from the American Heart Association. Circulation 141: e705–e736, 2020. doi: 10.1161/cir.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 2.Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NA 3rd, Fulton JE, Gordon NF, Haskell WL, Link MS, Maron BJ, Mittleman MA, Pelliccia A, Wenger NK, Willich SN, Costa F. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 115: 2358–2368, 2007. doi: 10.1161/circulationaha.107.181485. [DOI] [PubMed] [Google Scholar]
- 3.McLeod JC, Stokes T, Phillips SM. Resistance exercise training as a primary countermeasure to age-related chronic disease. Front Physiol 10: 645, 2019. doi: 10.3389/fphys.2019.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 25: 1–72, 2015. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 5.Sylow L, Kleinert M, Richter EA, Jensen TE. Exercise-stimulated glucose uptake - regulation and implications for glycaemic control. Nat Rev Endocrinol 13: 133–148, 2017. doi: 10.1038/nrendo.2016.162. [DOI] [PubMed] [Google Scholar]
- 6.Hood DA, Tryon LD, Carter HN, Kim Y, Chen CC. Unravelling the mechanisms regulating muscle mitochondrial biogenesis. Biochem J 473: 2295–2314, 2016. doi: 10.1042/BCJ20160009. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8: 457–465, 2012. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 8.Deshmukh AS, Steenberg DE, Hostrup M, Birk JB, Larsen JK, Santos A, Kjøbsted R, Hingst JR, Schéele CC, Murgia M, Kiens B, Richter EA, Mann M, Wojtaszewski JFP. Deep muscle-proteomic analysis of freeze-dried human muscle biopsies reveals fiber type-specific adaptations to exercise training. Nat Commun 12: 304, 2021. doi: 10.1038/s41467-021-22015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 13: 34–39, 2010. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atherton PJ, Greenhaff PL, Phillips SM, Bodine SC, Adams CM, Lang CH. Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence. Am J Physiol Endocrinol Metab 311: E594–E604, 2016. doi: 10.1152/ajpendo.00257.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips SM, McGlory C. CrossTalk proposal: The dominant mechanism causing disuse muscle atrophy is decreased protein synthesis. J Physiol 592: 5341–5343, 2014. doi: 10.1113/jphysiol.2014.273615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid MB, Judge AR, Bodine SC. Rebuttal from Michael B. Reid, Andrew R. Judge and Sue C. Bodine. J Physiol 592: 5351–5351, 2014. doi: 10.1113/jphysiol.2014.284398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol 585: 241–251, 2007. doi: 10.1113/jphysiol.2007.142828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dirks ML, Wall BT, van de Valk B, Holloway TM, Holloway GP, Chabowski A, Goossens GH, van Loon LJ. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes 65: 2862–2875, 2016. doi: 10.2337/db15-1661. [DOI] [PubMed] [Google Scholar]
- 15.Ogunbileje JO, Porter C, Herndon DN, Chao T, Abdelrahman DR, Papadimitriou A, Chondronikola M, Zimmers TA, Reidy PT, Rasmussen BB, Sidossis LS. Hypermetabolism and hypercatabolism of skeletal muscle accompany mitochondrial stress following severe burn trauma. Am J Physiol Endocrinol Metab 311: E436–E448, 2016. doi: 10.1152/ajpendo.00535.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castro MJ, Apple DF Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol 80: 373–378, 1999. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- 17.Bamman MM, Roberts BM, Adams GR. Molecular regulation of exercise-induced muscle fiber hypertrophy. Cold Spring Harb Perspect Med 8: a029751, 2018. doi: 10.1101/cshperspect.a029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodine SC. Disuse-induced muscle wasting. Int J Biochem Cell Biol 45: 2200–2208, 2013. doi: 10.1016/j.biocel.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab 307: E469–E484, 2014. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Critical Rev Biochem Mol Biol 49: 59–68, 2014. doi: 10.3109/10409238.2013.857291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sartori R, Romanello V, Sandri M. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat Commun 12: 330, 2021. doi: 10.1038/s41467-020-20123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 280: 4294–4314, 2013. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 23.Vainshtein A, Sandri M. Signaling pathways that control muscle mass. Int J Mol Sci 21: 4759, 2020. doi: 10.3390/ijms21134759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 168: 960–976, 2017. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang SA, Pacold ME, Cervantes CL, Lim D, Lou HJ, Ottina K, Gray NS, Turk BE, Yaffe MB, Sabatini DM. mTORC1 phosphorylation sites encode their sensitivity to starvation and rapamycin. Science 341: 1236566–1236566, 2013. doi: 10.1126/science.1236566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.You J-S, McNally RM, Jacobs BL, Privett RE, Gundermann DM, Lin K-H, Steinert ND, Goodman CA, Hornberger TA. The role of raptor in the mechanical load-induced regulation of mTOR signaling, protein synthesis, and skeletal muscle hypertrophy. FASEB J 33: 4021–4034, 2019. doi: 10.1096/fj.201801653RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogasawara R, Suginohara T. Rapamycin-insensitive mechanistic target of rapamycin regulates basal and resistance exercise-induced muscle protein synthesis. FASEB J 32: 5824–5834, 2018. doi: 10.1096/fj.201701422R. [DOI] [PubMed] [Google Scholar]
- 28.Ogasawara R, Jensen TE, Goodman CA, Hornberger TA. Resistance exercise-induced hypertrophy: a potential role for rapamycin-insensitive mTOR. Exerc Sport Sci Rev 47: 188–194, 2019. doi: 10.1249/jes.0000000000000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiza C, Nascimento EBM, Ouwens DM. Role of PRAS40 in Akt and mTOR signaling in health and disease. Am J Physiol Endocrinol Metab 302: E1453–E1460, 2012. doi: 10.1152/ajpendo.00660.2011. [DOI] [PubMed] [Google Scholar]
- 30.Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol 586: 6049–6061, 2008. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin K-H, Wilson GM, Blanco R, Steinert ND, Zhu WG, Coon JJ, Hornberger TA. A deep analysis of the proteomic and phosphoproteomic alterations that occur in skeletal muscle after the onset of immobilization. J Physiol 599: 2887–2906. doi: 10.1113/JP281071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobs BL, McNally RM, Kim KJ, Blanco R, Privett RE, You JS, Hornberger TA. Identification of mechanically regulated phosphorylation sites on tuberin (TSC2) that control mechanistic target of rapamycin (mTOR) signaling. J Biol Chem 292: 6987–6997, 2017. doi: 10.1074/jbc.M117.777805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You JS, Frey JW, Hornberger TA. Mechanical stimulation induces mTOR signaling via an ERK-independent mechanism: implications for a direct activation of mTOR by phosphatidic acid. PLoS One 7: e47258, 2012. doi: 10.1371/journal.pone.0047258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.You JS, Lincoln HC, Kim CR, Frey JW, Goodman CA, Zhong XP, Hornberger TA. The role of diacylglycerol kinase ζ and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. J Biol Chem 289: 1551–1563, 2014. doi: 10.1074/jbc.M113.531392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashi YK, Chou FL, Engvall E, Ogawa M, Matsuda C, Hirabayashi S, Yokochi K, Ziober BL, Kramer RH, Kaufman SJ, Ozawa E, Goto Y, Nonaka I, Tsukahara T, Wang JZ, Hoffman EP, Arahata K. Mutations in the integrin alpha7 gene cause congenital myopathy. Nat Genet 19: 94–97, 1998. doi: 10.1038/ng0598-94. [DOI] [PubMed] [Google Scholar]
- 36.Lueders TN, Zou K, Huntsman HD, Meador B, Mahmassani Z, Abel M, Valero MC, Huey KA, Boppart MD. The α7β1-integrin accelerates fiber hypertrophy and myogenesis following a single bout of eccentric exercise. Am J Physiol Cell Physiol 301: C938–C946, 2011. doi: 10.1152/ajpcell.00515.2010. [DOI] [PubMed] [Google Scholar]
- 37.Zou K, Meador BM, Johnson B, Huntsman HD, Mahmassani Z, Valero MC, Huey KA, Boppart MD. The α7β1-integrin increases muscle hypertrophy following multiple bouts of eccentric exercise. J Appl Physiol 111: 1134–1141, 2011. doi: 10.1152/japplphysiol.00081.2011. [DOI] [PubMed] [Google Scholar]
- 38.Boppart MD, Burkin D, Kaufman SJ. Activation of AKT signaling promotes cell growth and survival in α7β1 integrin-mediated alleviation of muscular dystrophy. Biochimica et Biophysica Acta 1812: 439–436, 2011. doi: 10.1016/j.bbadis.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graham ZA, Gallagher PM, Cardozo CP. Focal adhesion kinase and its role in skeletal muscle. J Muscle Res Cell Motil 36: 305–315, 2015. doi: 10.1007/s10974-015-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586: 3701–3717, 2008. doi: 10.1113/jphysiol.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durieux AC, D’Antona G, Desplanches D, Freyssenet D, Klossner S, Bottinelli R, Fluck M. Focal adhesion kinase is a load-dependent governor of the slow contractile and oxidative muscle phenotype. J Physiol 587: 3703–3717, 2009. doi: 10.1113/jphysiol.2009.171355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crossland H, Kazi AA, Lang CH, Timmons JA, Pierre P, Wilkinson DJ, Smith K, Szewczyk NJ, Atherton PJ. Focal adhesion kinase is required for IGF-I-mediated growth of skeletal muscle cells via a TSC2/mTOR/S6K1-associated pathway. Am J Physiol Endocrinol Metab 305: E183–E193, 2013. doi: 10.1152/ajpendo.00541.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braun T, Gautel M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat Rev Mol Cell Biol 12: 349–361, 2011. doi: 10.1038/nrm3118. [DOI] [PubMed] [Google Scholar]
- 44.Gautel M. Cytoskeletal protein kinases: titin and its relations in mechanosensing. Pflugers Arch 462: 119–134, 2011. doi: 10.1007/s00424-011-0946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirby TJ. Mechanosensitive pathways controlling translation regulatory processes in skeletal muscle and implications for adaptation. J Appl Physiol (1985) 127: 608–618, 2019. doi: 10.1152/japplphysiol.01031.2018. [DOI] [PubMed] [Google Scholar]
- 46.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 47.Hwee DT, Baehr LM, Philp A, Baar K, Bodine SC. Maintenance of muscle mass and load-induced growth in muscle RING Finger 1 null mice with age. Aging Cell 13: 92–101, 2014. doi: 10.1111/acel.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaglia T, Milan G, Ruhs A, Franzoso M, Bertaggia E, Pianca N, Carpi A, Carullo P, Pesce P, Sacerdoti D, Sarais C, Catalucci D, Krüger M, Mongillo M, Sandri M. Atrogin-1 deficiency promotes cardiomyopathy and premature death via impaired autophagy. J Clin Invest 124: 2410–2424, 2014. doi: 10.1172/JCI66339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merritt EK, Cross JM, Bamman MM. Inflammatory and protein metabolism signaling responses in human skeletal muscle after burn injury. J Burn Care Res 33: 291–297, 2012. doi: 10.1097/BCR.0b013e3182331e4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merritt EK, Stec MJ, Thalacker-Mercer A, Windham ST, Cross JM, Shelley DP, Craig Tuggle S, Kosek DJ, Kim JS, Bamman MM. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Appl Physiol (1985) 115: 937–948, 2013. doi: 10.1152/japplphysiol.00019.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13: 132–141, 2011. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19: 121–135, 2018. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol 16: 461–472, 2015. doi: 10.1038/nrm4024. [DOI] [PubMed] [Google Scholar]
- 54.Vainshtein A, Hood DA. The regulation of autophagy during exercise in skeletal muscle. J Appl Physiol (1985) 120: 664–673, 2016. doi: 10.1152/japplphysiol.00550.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharples AP, Stewart CE, Seaborne RA. Does skeletal muscle have an ’epi’-memory? The role of epigenetics in nutritional programming, metabolic disease, aging and exercise. Aging cell 15: 603–616, 2016. doi: 10.1111/acel.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Breuls N, Giacomazzi G, Sampaolesi M. (Epi)genetic modifications in myogenic stem cells: from novel insights to therapeutic perspectives. Cells 8: 429, 2019. doi: 10.3390/cells8050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hitachi K, Nakatani M, Funasaki S, Hijikata I, Maekawa M, Honda M, Tsuchida K. Expression levels of long non-coding RNAs change in models of altered muscle activity and muscle mass. Int J Mol Sci 21: 1628, 2020. doi: 10.3390/ijms21051628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santos JMO, Peixoto da Silva S, Gil da Costa RM, Medeiros R. The emerging role of microRNAs and other non-coding RNAs in cancer cachexia. Cancers (Basel) 12: 1004, 2020. doi: 10.3390/cancers12041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vangoor VR, Gomes-Duarte A, Pasterkamp RJ. Long non-coding RNAs in motor neuron development and disease. J Neurochem 156: 777–801, 2021. doi: 10.1111/jnc.15198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greco S, Cardinali B, Falcone G, Martelli F. Circular RNAs in muscle function and disease. Int J Mol Sci 19: 3454, 2018. doi: 10.3390/ijms19113454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suzuki T, Springer J. MicroRNAs in muscle wasting. J Cachexia Sarcopenia Muscle 9: 1209–1212, 2018. doi: 10.1002/jcsm.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Y, Chen M, Lian D, Li Y, Li Y, Wang J, Deng S, Yu K, Lian Z. Non-coding RNA regulates the myogenesis of skeletal muscle satellite cells injury repair and diseases. Cells 8: 988, 2019. doi: 10.3390/cells8090988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martone J, Mariani D, Desideri F, Ballarino M. Non-coding RNAs shaping muscle. Front Cell Dev Biol 7: 394, 2019. doi: 10.3389/fcell.2019.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marceca GP, Nigita G, Calore F, Croce CM. MicroRNAs in skeletal muscle and hints on their potential role in muscle wasting during cancer cachexia. Front Oncol 10: 607196, 2020. doi: 10.3389/fonc.2020.607196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo H, Lv W, Tong Q, Xu JJ, Zuo Z. Functional non-coding RNA during embryonic myogenesis and postnatal muscle development and disease. Front Cell Dev Biol 9: 628339, 2021. doi: 10.3389/fcell.2021.628339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirby TJ, Chaillou T, McCarthy JJ. The role of microRNAs in skeletal muscle health and disease. Front Biosci 20: 37–77, 2015. doi: 10.2741/4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piasecka A, Sekrecki M, Szcześniak MW, Sobczak K. MEF2C shapes the microtranscriptome during differentiation of skeletal muscles. Sci Rep 11: 3476, 2021. doi: 10.1038/s41598-021-82706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCarthy JJ. MicroRNA-206: the skeletal muscle-specific myomiR. Biochim Biophys Acta 1779: 682–691, 2008. doi: 10.1016/j.bbagrm.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 38: 228–233, 2006. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Panguluri SK, Bhatnagar S, Kumar A, McCarthy JJ, Srivastava AK, Cooper NG, Lundy RF, Kumar A. Genomic profiling of messenger RNAs and microRNAs reveals potential mechanisms of TWEAK-induced skeletal muscle wasting in mice. PLoS One 5: e8760, 2010. doi: 10.1371/journal.pone.0008760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kukreti H, Amuthavalli K, Harikumar A, Sathiyamoorthy S, Feng PZ, Anantharaj R, Tan SLK, Lokireddy S, Bonala S, Sriram S, McFarlane C, Kambadur R, Sharma M. Muscle-specific microRNA1 (miR1) targets heat shock protein 70 (HSP70) during dexamethasone-mediated atrophy. J Biol Chem 288: 6663–6678, 2013. doi: 10.1074/jbc.M112.390369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang F, Wang J, He J, Li W, Li J, Chen S, Zhang P, Liu H, Chen X. Serum miRNAs miR-23a, 206, and 499 as potential biomarkers for skeletal muscle atrophy. Biomed Res Int 2017: 1–9, 2017. doi: 10.1155/2017/8361237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu J, Kong M, Ye Y, Hong S, Cheng L, Jiang L. Serum miR-206 and other muscle-specific microRNAs as non-invasive biomarkers for Duchenne muscular dystrophy. J Neurochem 129: 877–883, 2014. doi: 10.1111/jnc.12662. [DOI] [PubMed] [Google Scholar]
- 74.Nederveen JP, Warnier G, Di Carlo A, Nilsson MI, Tarnopolsky MA. Extracellular vesicles and exosomes: insights from exercise science. Front Physiol 11: 604274, 2021. doi: 10.3389/fphys.2020.604274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Pelt DW, Vechetti IJJ, Lawrence MM, Van Pelt KL, Patel P, Miller BF, Butterfield TA, Dupont-Versteegden EE. Serum extracellular vesicle miR-203a-3p content is associated with skeletal muscle mass and protein turnover during disuse atrophy and regrowth. Am J Physiol Cell Physiol 319: C419–C431, 2020. doi: 10.1152/ajpcell.00223.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nair VD, Ge Y, Li S, Pincas H, Jain N, Seenarine N, Amper MAS, Goodpaster BH, Walsh MJ, Coen PM, Sealfon SC. Sedentary and trained older men have distinct circulating exosomal microRNA profiles at baseline and in response to acute exercise. Front Physiol 11: 605, 2020. doi: 10.3389/fphys.2020.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seaborne RA, Sharples AP. The interplay between exercise metabolism, epigenetics, and skeletal muscle remodeling. Exerc Sport Sci Rev 48: 188–200, 2020. doi: 10.1249/JES.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 78.Barres R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O’Gorman DJ, Zierath JR. Acute exercise remodels promoter methylation in human skeletal muscle. Cell metabolism 15: 405–411, 2012. doi: 10.1016/j.cmet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 79.Seaborne RA, Strauss J, Cocks M, Shepherd S, O’Brien TD, van Someren KA, Bell PG, Murgatroyd C, Morton JP, Stewart CE, Sharples AP. Human skeletal muscle possesses an epigenetic memory of hypertrophy. Sci Rep 8: 1898, 2018. doi: 10.1038/s41598-018-20287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sailani MR, Halling JF, Møller HD, Lee H, Plomgaard P, Pilegaard H, Snyder MP, Regenberg B. Lifelong physical activity is associated with promoter hypomethylation of genes involved in metabolism, myogenesis, contractile properties and oxidative stress resistance in aged human skeletal muscle. Sci Rep 9: 3272, 2019. doi: 10.1038/s41598-018-37895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zaccara S, Ries RJ, Jaffrey SR. Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell Biol 20: 608–624, 2019. doi: 10.1038/s41580-019-0168-5. [DOI] [PubMed] [Google Scholar]
- 82.Gheller BJ, Blum JE, Fong EHH, Malysheva OV, Cosgrove BD, Thalacker-Mercer AE. A defined N6-methyladenosine (m6A) profile conferred by METTL3 regulates muscle stem cell/myoblast state transitions. Cell Death Discov 6: 95, 2020. doi: 10.1038/s41420-020-00328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coker RH, Wolfe RR. Bedrest and sarcopenia. Curr Opin Clin Nutr Metab Care 15: 7–11, 2012. doi: 10.1097/MCO.0b013e32834da629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kehler DS, Theou O, Rockwood K. Bed rest and accelerated aging in relation to the musculoskeletal and cardiovascular systems and frailty biomarkers: a review. Exp Gerontol 124: 110643, 2019. doi: 10.1016/j.exger.2019.110643. [DOI] [PubMed] [Google Scholar]
- 85.Kakurin LI, Lobachik VI, Mikhailov VM, Senkevich YA. Antiorthostatic hypokinesia as a method of weightlessness simulation. Aviat Space Environ Med 47: 1083–1086, 1976. [PubMed] [Google Scholar]
- 86.Trappe SW, Trappe TA, Lee GA, Widrick JJ, Costill DL, Fitts RH. Comparison of a space shuttle flight (STS-78) and bed rest on human muscle function. J Appl Physiol 91: 57–64, 2001. doi: 10.1152/jappl.2001.91.1.57. [DOI] [PubMed] [Google Scholar]
- 87.Widrick JJ, Knuth ST, Norenberg KM, Romatowski JG, Bain JL, Riley DA, Karhanek M, Trappe SW, Trappe TA, Costill DL, Fitts RH. Effect of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J Physiol 516: 915–930, 1999. doi: 10.1111/j.1469-7793.1999.0915u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol 557: 501–513, 2004. doi: 10.1113/jphysiol.2004.062166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miokovic T, Armbrecht G, Felsenberg D, Belavy DL. Heterogeneous atrophy occurs within individual lower limb muscles during 60 days of bed rest. J Appl Physiol (1985) 113: 1545–1559, 2012. doi: 10.1152/japplphysiol.00611.2012. [DOI] [PubMed] [Google Scholar]
- 90.Trappe TA, Burd NA, Louis ES, Lee GA, Trappe SW. Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol (Oxf) 191: 147–159, 2007. doi: 10.1111/j.1748-1716.2007.01728.x. [DOI] [PubMed] [Google Scholar]
- 91.Krainski F, Hastings JL, Heinicke K, Romain N, Pacini EL, Snell PG, Wyrick P, Palmer MD, Haller RG, Levine BD. The effect of rowing ergometry and resistive exercise on skeletal muscle structure and function during bed rest. J Appl Physiol (1985) 116: 1569–1581, 2014. doi: 10.1152/japplphysiol.00803.2013. [DOI] [PubMed] [Google Scholar]
- 92.McNamara KP, Greene KA, Tooze JA, Dang J, Khattab K, Lenchik L, Weaver AA. Neck muscle changes following long-duration spaceflight. Front Physiol 10: 1115, 2019. doi: 10.3389/fphys.2019.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Belavy DL, Miokovic T, Armbrecht G, Richardson CA, Rittweger J, Felsenberg D. Differential atrophy of the lower-limb musculature during prolonged bed-rest. Eur J Appl Physiol 107: 489–499, 2009. doi: 10.1007/s00421-009-1136-0. [DOI] [PubMed] [Google Scholar]
- 94.Chang DG, Healey RM, Snyder AJ, Sayson JV, Macias BR, Coughlin DG, Bailey JF, Parazynski SE, Lotz JC, Hargens AR. Lumbar spine paraspinal muscle and intervertebral disc height changes in astronauts after long-duration spaceflight on the International Space Station. Spine 41: 1917–1924, 2016. doi: 10.1097/BRS.0000000000001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.LeBlanc A, Lin C, Shackelford L, Sinitsyn V, Evans H, Belichenko O, Schenkman B, Kozlovskaya I, Oganov V, Bakulin A, Hedrick T, Feeback D. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J Appl Physiol 89: 2158–2164, 2000. doi: 10.1152/jappl.2000.89.6.2158. [DOI] [PubMed] [Google Scholar]
- 96.Miokovic T, Armbrecht G, Felsenberg D, Belavy DL. Differential atrophy of the postero-lateral hip musculature during prolonged bedrest and the influence of exercise countermeasures. J Appl Physiol (1985) 110: 926–934, 2011. doi: 10.1152/japplphysiol.01105.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tesch PA, Berg HE, Bring D, Evans HJ, LeBlanc AD. Effects of 17-day spaceflight on knee extensor muscle function and size. Eur J Appl Physiol 93: 463–468, 2005. doi: 10.1007/s00421-004-1236-9. [DOI] [PubMed] [Google Scholar]
- 98.Mahmassani ZS, Reidy PT, McKenzie AI, Stubben C, Howard MT, Drummond MJ. Disuse-induced insulin resistance susceptibility coincides with a dysregulated skeletal muscle metabolic transcriptome. J Appl Physiol (1985) 126: 1419–1429, 2019. doi: 10.1152/japplphysiol.01093.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Edgerton VR, Zhou MY, Ohira Y, Klitgaard H, Jiang B, Bell G, Harris B, Saltin B, Gollnick PD, Roy RR, et al. Human fiber size and enzymatic properties after 5 and 11 days of spaceflight. J Appl Physiol 78: 1733–1739, 1995. doi: 10.1152/jappl.1995.78.5.1733. [DOI] [PubMed] [Google Scholar]
- 100.Gallagher P, Trappe S, Harber M, Creer A, Mazzetti S, Trappe T, Alkner B, Tesch P. Effects of 84-days of bedrest and resistance training on single muscle fibre myosin heavy chain distribution in human vastus lateralis and soleus muscles. Acta Physiol Scand 185: 61–69, 2005. doi: 10.1111/j.1365-201X.2005.01457.x. [DOI] [PubMed] [Google Scholar]
- 101.Brocca L, Longa E, Cannavino J, Seynnes O, de Vito G, McPhee J, Narici M, Pellegrino MA, Bottinelli R. Human skeletal muscle fibre contractile properties and proteomic profile: adaptations to 3 weeks of unilateral lower limb suspension and active recovery. J Physiol 593: 5361–5385, 2015. doi: 10.1113/JP271188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Widrick JJ, Norenberg KM, Romatowski JG, Blaser CA, Karhanek M, Sherwood J, Trappe SW, Trappe TA, Costill DL, Fitts RH. Force-velocity-power and force-pCa relationships of human soleus fibers after 17 days of bed rest. J Appl Physiol 85: 1949–1956, 1998. doi: 10.1152/jappl.1998.85.5.1949. [DOI] [PubMed] [Google Scholar]
- 103.Fitts RH, Riley DR, Widrick JJ. Functional and structural adaptations of skeletal muscle to microgravity. J Exp Biol 204: 3201–3208, 2001. doi: 10.1242/jeb.204.18.3201. [DOI] [PubMed] [Google Scholar]
- 104.Arentson-Lantz EJ, English KL, Paddon-Jones D, Fry CS. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J Appl Physiol (1985) 120: 965–975, 2016. doi: 10.1152/japplphysiol.00799.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ray S, Gebre S, Fogle H, Berrios DC, Tran PB, Galazka JM, Costes SV. GeneLab: Omics database for spaceflight experiments. Bioinformatics 35: 1753–1759, 2019. doi: 10.1093/bioinformatics/bty884. [DOI] [PubMed] [Google Scholar]
- 106.da Silveira WA, Fazelinia H, Rosenthal SB, Laiakis EC, Kim MS, Meydan C, Kidane Y, et al. Comprehensive multi-omics analysis reveals mitochondrial stress as a central biological hub for spaceflight impact. Cell 183: 1185–1201, 2020. doi: 10.1016/j.cell.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Labeit S, Kohl CH, Witt CC, Labeit D, Jung J, Granzier H. Modulation of muscle atrophy, fatigue and MLC phosphorylation by MuRF1 as indicated by hindlimb suspension studies on MuRF1-KO mice. J Biomed Biotechnol 2010: 1–9, 2010. doi: 10.1155/2010/693741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cadena SM, Zhang Y, Fang J, Brachat S, Kuss P, Giorgetti E, Stodieck LS, Kneissel M, Glass DJ. Skeletal muscle in MuRF1 null mice is not spared in low-gravity conditions, indicating atrophy proceeds by unique mechanisms in space. Sci Rep 9: 9397, 2019. doi: 10.1038/s41598-019-45821-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mahmassani ZS, Reidy PT, McKenzie AI, Stubben C, Howard MT, Drummond MJ. Age-dependent skeletal muscle transcriptome response to bed rest-induced atrophy. J Appl Physiol (1985) 126: 894–902, 2019. doi: 10.1152/japplphysiol.00811.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bongers KS, Fox DK, Ebert SM, Kunkel SD, Dyle MC, Bullard SA, Dierdorff JM, Adams CM. Skeletal muscle denervation causes skeletal muscle atrophy through a pathway that involves both Gadd45a and HDAC4. Am J Physiol Endocrinol Metab 305: E907–E915, 2013. doi: 10.1152/ajpendo.00380.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tanner RE, Brunker LB, Agergaard J, Barrows KM, Briggs RA, Kwon OS, Young LM, Hopkins PN, Volpi E, Marcus RL, LaStayo PC, Drummond MJ. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol 593: 4259–4273, 2015. doi: 10.1113/JP270699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sparks LM. Exercise training response heterogeneity: physiological and molecular insights. Diabetologia 60: 2329–2336, 2017. doi: 10.1007/s00125-017-4461-6. [DOI] [PubMed] [Google Scholar]
- 113.Thalacker-Mercer A, Stec M, Cui X, Cross J, Windham S, Bamman M. Cluster analysis reveals differential transcript profiles associated with resistance training-induced human skeletal muscle hypertrophy. Physiol Genomics 45: 499–507, 2013. doi: 10.1152/physiolgenomics.00167.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Galvan E, Arentson-Lantz E, Lamon S, Paddon-Jones D. Protecting skeletal muscle with protein and amino acid during periods of disuse. Nutrients 8: 404, 2016. doi: 10.3390/nu8070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reidy PT, Lindsay CC, McKenzie AI, Fry CS, Supiano MA, Marcus RL, LaStayo PC, Drummond MJ. Aging-related effects of bed rest followed by eccentric exercise rehabilitation on skeletal muscle macrophages and insulin sensitivity. Exp Gerontol 107: 37–49, 2018. doi: 10.1016/j.exger.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scott JM, Downs M, Buxton R, Goetchius E, Crowell B, Ploutz-Snyder R, Hackney KJ, Ryder J, English K, Ploutz-Snyder LL. Disuse-induced muscle loss and rehabilitation: the National Aeronautics and Space Administration Bed Rest Study. Crit Care Explor 2: e0269, 2020. doi: 10.1097/CCE.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Salanova M, Gambara G, Moriggi M, Vasso M, Ungethuem U, Belavy DL, Felsenberg D, Cerretelli P, Gelfi C, Blottner D. Vibration mechanosignals superimposed to resistive exercise result in baseline skeletal muscle transcriptome profiles following chronic disuse in bed rest. Sci Rep 5: 17027, 2015. doi: 10.1038/srep17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alkner BA, Tesch PA. Efficacy of a gravity-independent resistance exercise device as a countermeasure to muscle atrophy during 29-day bed rest. Acta Physiol Scand 181: 345–357, 2004. doi: 10.1111/j.1365-201X.2004.01293.x. [DOI] [PubMed] [Google Scholar]
- 119.Alkner BA, Tesch PA. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur J Appl Physiol 93: 294–305, 2004. doi: 10.1007/s00421-004-1172-8. [DOI] [PubMed] [Google Scholar]
- 120.Clarke MS, Bamman MM, Feeback DL. Bed rest decreases mechanically induced myofiber wounding and consequent wound-mediated FGF release. J Appl Physiol 85: 593–600, 1998. doi: 10.1152/jappl.1998.85.2.593. [DOI] [PubMed] [Google Scholar]
- 121.Ferrando AA, Tipton KD, Bamman MM, Wolfe RR. exercise maintains skeletal muscle protein synthesis during bed rest. J Appl Physiol 82: 807–810, 1997. doi: 10.1152/jappl.1997.82.3.807. [DOI] [PubMed] [Google Scholar]
- 122.Bamman MM, Clarke MS, Feeback DL, Talmadge RJ, Stevens BR, Lieberman SA, Greenisen MC. Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol 84: 157–163, 1998. doi: 10.1152/jappl.1998.84.1.157. [DOI] [PubMed] [Google Scholar]
- 123.Bamman MM, Hunter GR, Stevens BR, Guilliams ME, Greenisen MC. Resistance exercise prevents plantar flexor deconditioning during bed rest. Med Sci Sports Exerc 29: 1462–1468, 1997. doi: 10.1097/00005768-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 124.Ploutz-Snyder LL, Downs M, Goetchius E, Crowell B, English KL, Ploutz-Snyder R, Ryder JW, Dillon EL, Sheffield-Moore M, Scott JM. Exercise training mitigates multisystem deconditioning during bed rest. Med Sci Sports Exerc 50: 1920–1928, 2018. doi: 10.1249/MSS.0000000000001618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fitts RH, Colloton PA, Trappe SW, Costill DL, Bain JL, Riley DA. Effects of prolonged space flight on human skeletal muscle enzyme and substrate profiles. J Appl Physiol 115: 667–679, 2013. doi: 10.1152/japplphysiol.00489.2013. [DOI] [PubMed] [Google Scholar]
- 126.English KL, Downs M, Goetchius E, Buxton R, Ryder JW, Ploutz-Snyder R, Guilliams M, Scott JM, Ploutz-Snyder LL. High intensity training during spaceflight: results from the NASA Sprint Study. NPJ Microgravity 6: 21, 2020. doi: 10.1038/s41526-020-00111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.English KL, Bloomberg JJ, Mulavara AP, Ploutz-Snyder LL. Exercise countermeasures to neuromuscular deconditioning in spaceflight. Compr Physiol 10: 171–196, 2019. doi: 10.1002/cphy.c190005. [DOI] [PubMed] [Google Scholar]
- 128.Hackney KJ, Scott JM, Hanson AM, English KL, Downs ME, Ploutz-Snyder LL. The astronaut-athlete: optimizing human performance in space. J Strength Cond Res 29: 3531–3545, 2015. doi: 10.1519/JSC.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 129.Murach KA, Minchev K, Grosicki GJ, Lavin KM, Perkins RK, Ryder JW, Scott J, Ploutz-Snyder L, Trappe TA, Trappe S. Myocellular responses to concurrent flywheel training during 70 days of bed rest. Med Sci Sports Exerc 50: 1950–1960, 2018. doi: 10.1249/MSS.0000000000001620. [DOI] [PubMed] [Google Scholar]
- 130.Fernandez-Gonzalo R, Tesch PA, Lundberg TR, Alkner BA, Rullman E, Gustafsson T. Three months of bed rest induce a residual transcriptomic signature resilient to resistance exercise countermeasures. FASEB J 34: 7958–7969, 2020. doi: 10.1096/fj.201902976R. [DOI] [PubMed] [Google Scholar]
- 131.Kivela R, Salmela I, Nguyen YH, Petrova TV, Koistinen HA, Wiener Z, Alitalo K. The transcription factor Prox1 is essential for satellite cell differentiation and muscle fibre-type regulation. Nat Commun 7: 13124, 2016. doi: 10.1038/ncomms13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Valenzuela PL, Morales JS, Pareja-Galeano H, Izquierdo M, Emanuele E, de la Villa P, Lucia A. Physical strategies to prevent disuse-induced functional decline in the elderly. Ageing Res Rev 47: 80–88, 2018. doi: 10.1016/j.arr.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 133.Reidy PT, McKenzie AI, Brunker P, Nelson DS, Barrows KM, Supiano M, LaStayo PC, Drummond MJ. Neuromuscular electrical stimulation combined with protein ingestion preserves thigh muscle mass but not muscle function in healthy older adults during 5 days of bed rest. Rejuvenation Res 20: 449–461, 2017. doi: 10.1089/rej.2017.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lemoine JK, Haus JM, Trappe SW, Trappe TA. Muscle proteins during 60-day bedrest in women: impact of exercise or nutrition. Muscle Nerve 39: 463–471, 2009. doi: 10.1002/mus.21189. [DOI] [PubMed] [Google Scholar]
- 135.Martin Ginis KA, van der Scheer JW, Latimer-Cheung AE, Barrow A, Bourne C, Carruthers P, Bernardi M, Ditor DS, Gaudet S, de Groot S, Hayes KC, Hicks AL, Leicht CA, Lexell J, Macaluso S, Manns PJ, McBride CB, Noonan VK, Pomerleau P, Rimmer JH, Shaw RB, Smith B, Smith KM, Steeves JD, Tussler D, West CR, Wolfe DL, Goosey-Tolfrey VL. Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord 56: 308–321, 2018. doi: 10.1038/s41393-017-0017-3. [DOI] [PubMed] [Google Scholar]
- 136.Rocchi M, Routhier F, Latimer-Cheung AE, Ginis KAM, Noreau L, Sweet SN. Are adults with spinal cord injury meeting the spinal cord injury-specific physical activity guidelines? A look at a sample from a Canadian province. Spinal cord 55: 454–459, 2017. doi: 10.1038/sc.2016.181. [DOI] [PubMed] [Google Scholar]
- 137.Urso ML, Chen YW, Scrimgeour AG, Lee PC, Lee KF, Clarkson PM. Alterations in mRNA expression and protein products following spinal cord injury in humans. J Physiol 579: 877–892, 2007. doi: 10.1113/jphysiol.2006.118042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kern H, Hofer C, Modlin M, Mayr W, Vindigni V, Zampieri S, Boncompagni S, Protasi F, Carraro U. Stable muscle atrophy in long-term paraplegics with complete upper motor neuron lesion from 3- to 20-year SCI. Spinal cord 46: 293–304, 2008. doi: 10.1038/sj.sc.3102131. [DOI] [PubMed] [Google Scholar]
- 139.Bochkezanian V, Newton RU, Trajano GS, Blazevich AJ. Effects of neuromuscular electrical stimulation in people with spinal cord injury. Med Sci Sports Exerc 50: 1733–1739, 2018. doi: 10.1249/MSS.0000000000001637. [DOI] [PubMed] [Google Scholar]
- 140.Erickson ML, Ryan TE, Backus D, McCully KK. Endurance neuromuscular electrical stimulation training improves skeletal muscle oxidative capacity in individuals with motor-complete spinal cord injury. Muscle Nerve 55: 669–675, 2017. doi: 10.1002/mus.25393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Petrie MA, Sharma A, Taylor EB, Suneja M, Shields RK. Impact of short- and long-term electrically induced muscle exercise on gene signaling pathways, gene expression, and PGC1a methylation in men with spinal cord injury. Physiol Genomics 52: 71–80, 2020. doi: 10.1152/physiolgenomics.00064.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Petrie MA, Suneja M, Faidley E, Shields RK. A minimal dose of electrically induced muscle activity regulates distinct gene signaling pathways in humans with spinal cord injury. PLoS One 9: e115791, 2014. doi: 10.1371/journal.pone.0115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ryan TE, Brizendine JT, Backus D, McCully KK. Electrically induced resistance training in individuals with motor complete spinal cord injury. Arch Phys Med Rehabil 94: 2166–2173, 2013. doi: 10.1016/j.apmr.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 144.Chilibeck PD, Bell G, Jeon J, Weiss CB, Murdoch G, MacLean I, Ryan E, Burnham R. Functional electrical stimulation exercise increases GLUT-1 and GLUT-4 in paralyzed skeletal muscle. Metabolism 48: 1409–1413, 1999. doi: 10.1016/S0026-0495(99)90151-8. [DOI] [PubMed] [Google Scholar]
- 145.Mohr T, Dela F, Handberg A, Biering-Sørensen F, Galbo H, Kjaer M. Insulin action and long-term electrically induced training in individuals with spinal cord injuries. Med Sci Sports Exerc 33: 1247–1252, 2001. doi: 10.1097/00005768-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 146.Gorgey AS, Graham ZA, Bauman WA, Cardozo C, Gater DR. Abundance in proteins expressed after functional electrical stimulation cycling or arm cycling ergometry training in persons with chronic spinal cord injury. J Spinal Cord Med 40: 439–448, 2017. doi: 10.1080/10790268.2016.1229397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Willoughby DS, Priest JW, Jennings RA. Myosin heavy chain isoform and ubiquitin protease mRNA expression after passive leg cycling in persons with spinal cord injury. Arch Phys Med Rehabil 81: 157–163, 2000. doi: 10.1053/apmr.2000.0810157. [DOI] [PubMed] [Google Scholar]
- 148.Willoughby DS, Priest JW, Nelson M. Expression of the stress proteins, ubiquitin, heat shock protein 72, and myofibrillar protein content after 12 weeks of leg cycling in persons with spinal cord injury. Arch Phys Med Rehabil 83: 649–654, 2002. doi: 10.1053/apmr.2002.31184. [DOI] [PubMed] [Google Scholar]
- 149.Adams MM, Ditor DS, Tarnopolsky MA, Phillips SM, McCartney N, Hicks AL. The effect of body weight-supported treadmill training on muscle morphology in an individual with chronic, motor-complete spinal cord injury: a case study. J Spinal Cord Med 29: 167–171, 2006. doi: 10.1080/10790268.2006.11753860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stewart BG, Tarnopolsky MA, Hicks AL, McCartney N, Mahoney DJ, Staron RS, Phillips SM. Treadmill training-induced adaptations in muscle phenotype in persons with incomplete spinal cord injury. Muscle Nerve 30: 61–68, 2004. doi: 10.1002/mus.20048. [DOI] [PubMed] [Google Scholar]
- 151.Phillips SM, Stewart BG, Mahoney DJ, Hicks AL, McCartney N, Tang JE, Wilkinson SB, Armstrong D, Tarnopolsky MA. Body-weight-support treadmill training improves blood glucose regulation in persons with incomplete spinal cord injury. J Appl Physiol (1985) 97: 716–724, 2004. doi: 10.1152/japplphysiol.00167.2004. [DOI] [PubMed] [Google Scholar]
- 152.Dudley-Javoroski S, Shields RK. Muscle and bone plasticity after spinal cord injury: review of adaptations to disuse and to electrical muscle stimulation. J Rehabil Res Dev 45: 283–296, 2008. doi: 10.1682/jrrd.2007.02.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Adams CM, Suneja M, Dudley-Javoroski S, Shields RK. Altered mRNA expression after long-term soleus electrical stimulation training in humans with paralysis. Muscle Nerve 43: 65–75, 2011. doi: 10.1002/mus.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yarar-Fisher C, Bickel CS, Windham ST, McLain AB, Bamman MM. Skeletal muscle signaling associated with impaired glucose tolerance in spinal cord-injured men and the effects of contractile activity. J Appl Physiol 115: 756–764, 2013. doi: 10.1152/japplphysiol.00122.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yarar-Fisher C, Bickel CS, Kelly NA, Windham ST, McLain AB, Bamman MM. Mechanosensitivity may be enhanced in skeletal muscles of spinal cord-injured versus able-bodied men. Muscle Nerve 50: 599–601, 2014. doi: 10.1002/mus.24248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gorgey AS, Graham ZA, Chen Q, Rivers J, Adler RA, Lesnefsky EJ, Cardozo CP. Sixteen weeks of testosterone with or without evoked resistance training on protein expression, fiber hypertrophy and mitochondrial health after spinal cord injury. J Appl Physiol (1985) 128: 1487–1496, 2020. doi: 10.1152/japplphysiol.00865.2019. [DOI] [PubMed] [Google Scholar]
- 157.Yarar-Fisher C, Polston KF, Eraslan M, Henley KY, Kinikli GI, Bickel CS, Windham ST, McLain AB, Oster RB, Amman MM. Paralytic and non-paralytic muscle adaptations to exercise training vs. high protein diet in individuals with long-standing spinal cord injury. J Appl Physiol (1985) 125: 64–72, 2018. doi: 10.1152/japplphysiol.01029.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Carlson BM. Skeletal muscle regeneration during aging and after long-term denervation. Tsitologiia 39: 965–968, 1997. [PubMed] [Google Scholar]
- 159.Moresi V, Williams AH, Meadows E, Flynn JM, Potthoff MJ, McAnally J, Shelton JM, Backs J, Klein WH, Richardson JA, Bassel-Duby R, Olson EN. Myogenin and class II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell 143: 35–45, 2010. doi: 10.1016/j.cell.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jones RA, Harrison C, Eaton SL, Llavero Hurtado M, Graham LC, Alkhammash L, Oladiran OA, Gale A, Lamont DJ, Simpson H, Simmen MW, Soeller C, Wishart TM, Gillingwater TH. Cellular and molecular anatomy of the human neuromuscular junction. Cell Rep 21: 2348–2356, 2017. doi: 10.1016/j.celrep.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT. Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS One 7: e29082, 2012. doi: 10.1371/journal.pone.0029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sonjak V, Jacob KJ, Spendiff S, Vuda M, Perez A, Miguez K, Minozzo FC, Spake C, Morais JA, Hepple RT. Reduced mitochondrial content, elevated reactive oxygen species, and modulation by denervation in skeletal muscle of prefrail or frail elderly women. J Gerontol A Biol Sci Med Sci 74: 1887–1895, 2019. doi: 10.1093/gerona/glz066. [DOI] [PubMed] [Google Scholar]
- 163.Davis CE, Harris JB, Nicholson LV. Myosin isoform transitions and physiological properties of regenerated and re-innervated soleus muscles of the rat. Neuromuscul Disord 1: 411–421, 1991. doi: 10.1016/0960-8966(91)90004-C. [DOI] [PubMed] [Google Scholar]
- 164.Schiaffino S, Gorza L, Pitton G, Saggin L, Ausoni S, Sartore S, Lomo T. Embryonic and neonatal myosin heavy chain in denervated and paralyzed rat skeletal muscle. Dev Biol 127: 1–11, 1988. doi: 10.1016/0012-1606(88)90183-2. [DOI] [PubMed] [Google Scholar]
- 165.Kelly NA, Hammond KG, Bickel CS, Windham ST, Tuggle SC, Bamman MM. Effects of aging and Parkinson’s disease on motor unit remodeling: influence of resistance exercise training. J Appl Physiol (1985) 124: 888–898, 2018. doi: 10.1152/japplphysiol.00563.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bruneteau G, Simonet T, Bauche S, Mandjee N, Malfatti E, Girard E, Tanguy ML, Behin A, Khiami F, Sariali E, Hell-Remy C, Salachas F, Pradat PF, Fournier E, Lacomblez L, Koenig J, Romero NB, Fontaine B, Meininger V, Schaeffer L, Hantai D. Muscle histone deacetylase 4 upregulation in amyotrophic lateral sclerosis: potential role in reinnervation ability and disease progression. Brain 136: 2359–2368, 2013. doi: 10.1093/brain/awt164. [DOI] [PubMed] [Google Scholar]
- 167.Oda K. Age changes of motor innervation and acetylcholine receptor distribution on human skeletal muscle fibres. J Neurol Sci 66: 327–338, 1984. doi: 10.1016/0022-510X(84)90021-2. [DOI] [PubMed] [Google Scholar]
- 168.Lang F, Khaghani S, Turk C, Wiederstein JL, Holper S, Piller T, Nogara L, Blaauw B, Gunther S, Muller S, Braun T, Kruger M. Single muscle fiber proteomics reveals distinct protein changes in slow and fast fibers during muscle atrophy. J Proteome Res 17: 3333–3347, 2018. doi: 10.1021/acs.jproteome.8b00093. [DOI] [PubMed] [Google Scholar]
- 169.Anagnostou M-E, Hepple RT. Mitochondrial mechanisms of neuromuscular junction degeneration with aging. Cells 9: 197, 2020. doi: 10.3390/cells9010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Picard M, Ritchie D, Thomas MM, Wright KJ, Hepple RT. Alterations in intrinsic mitochondrial function with aging are fiber type-specific and do not explain differential atrophy between muscles. Aging Cell 10: 1047–1055, 2011. doi: 10.1111/j.1474-9726.2011.00745.x. [DOI] [PubMed] [Google Scholar]
- 171.Spendiff S, Vuda M, Gouspillou G, Aare S, Perez A, Morais JA, Jagoe RT, Filion ME, Glicksman R, Kapchinsky S, MacMillan NJ, Pion CH, Aubertin-Leheudre M, Hettwer S, Correa JA, Taivassalo T, Hepple RT. Denervation drives mitochondrial dysfunction in skeletal muscle of octogenarians. J Physiol 594: 7361–7379, 2016. doi: 10.1113/JP272487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Baraldo M, Geremia A, Pirazzini M, Nogara L, Solagna F, Turk C, Nolte H, Romanello V, Megighian A, Boncompagni S, Kruger M, Sandri M, Blaauw B. Skeletal muscle mTORC1 regulates neuromuscular junction stability. J Cachexia Sarcopenia Muscle 11: 208–225, 2020. doi: 10.1002/jcsm.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tang H, Inoki K, Lee M, Wright E, Khuong A, Khuong A, Sugiarto S, Garner M, Paik J, DePinho RA, Goldman D, Guan KL, Shrager JB. mTORC1 promotes denervation-induced muscle atrophy through a mechanism involving the activation of FoxO and E3 ubiquitin ligases. Sci Signal 7: ra18–ra18, 2014. doi: 10.1126/scisignal.2004809. [DOI] [PubMed] [Google Scholar]
- 174.Kelly NA, Hammond KG, Stec MJ, Bickel CS, Windham ST, Tuggle SC, Bamman MM. Quantification and characterization of grouped type I myofibers in human aging. Muscle Nerve 57: E52–E59, 2018. doi: 10.1002/mus.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Soendenbroe C, Heisterberg MF, Schjerling P, Karlsen A, Kjaer M, Andersen JL, Mackey AL. Molecular indicators of denervation in aging human skeletal muscle. Muscle Nerve 60: 453–463, 2019. doi: 10.1002/mus.26638. [DOI] [PubMed] [Google Scholar]
- 176.Nilwik R, Snijders T, Leenders M, Groen BB, van Kranenburg J, Verdijk LB, van Loon LJ. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 48: 492–498, 2013. doi: 10.1016/j.exger.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 177.Jennekens FG, Tomlinson BE, Walton JN. Histochemical aspects of five limb muscles in old age. An autopsy study. J Neurol Sci 14: 259–276, 1971. doi: 10.1016/0022-510X(71)90216-4. [DOI] [PubMed] [Google Scholar]
- 178.Lexell J, Downham DY. The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol 81: 377–381, 1991. doi: 10.1007/BF00293457. [DOI] [PubMed] [Google Scholar]
- 179.Cappello V, Francolini M. Neuromuscular junction dismantling in amyotrophic lateral sclerosis. Int J Mol Sci 18: 2092, 2017. doi: 10.3390/ijms18102092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Piasecki M, Ireland A, Jones DA, McPhee JS. Age-dependent motor unit remodelling in human limb muscles. Biogerontology 17: 485–496, 2016. doi: 10.1007/s10522-015-9627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Boccia G, Dardanello D, Beretta-Piccoli M, Cescon C, Coratella G, Rinaldo N, Barbero M, Lanza M, Schena F, Rainoldi A. Muscle fiber conduction velocity and fractal dimension of EMG during fatiguing contraction of young and elderly active men. Physiol Meas 37: 162–174, 2016. doi: 10.1088/0967-3334/37/1/162. [DOI] [PubMed] [Google Scholar]
- 182.Piasecki M, Ireland A, Piasecki J, Stashuk DW, Swiecicka A, Rutter MK, Jones DA, McPhee JS. Failure to expand the motor unit size to compensate for declining motor unit numbers distinguishes sarcopenic from non-sarcopenic older men. J Physiol 596: 1627–1637, 2018. doi: 10.1113/JP275520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sonjak V, Jacob K, Morais JA, Rivera-Zengotita M, Spendiff S, Spake C, Taivassalo T, Chevalier S, Hepple RT. Fidelity of muscle fibre reinnervation modulates ageing muscle impact in elderly women. J Physiol 597: 5009–5023, 2019. doi: 10.1113/JP278261. [DOI] [PubMed] [Google Scholar]
- 184.Gawel M, Kostera-Pruszczyk A. Effect of age and gender on the number of motor units in healthy subjects estimated by the multipoint incremental MUNE method. J Clin Neurophysiol 31: 272–278, 2014. doi: 10.1097/WNP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 185.Roberts BM, Lavin KM, Many GM, Thalacker-Mercer A, Merritt EK, Bickel CS, Mayhew DL, Tuggle SC, Cross JM, Kosek DJ, Petrella JK, Brown CJ, Hunter GR, Windham ST, Allman RM, Bamman MM. Human neuromuscular aging: Sex differences revealed at the myocellular level. Exp Gerontol 106: 116–124, 2018. doi: 10.1016/j.exger.2018.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Frontera WR, Reid KF, Phillips EM, Krivickas LS, Hughes VA, Roubenoff R, Fielding RA. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol 105: 637–642, 2008. doi: 10.1152/japplphysiol.90332.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mosole S, Carraro U, Kern H, Loefler S, Fruhmann H, Vogelauer M, Burggraf S, Mayr W, Krenn M, Paternostro-Sluga T, Hamar D, Cvecka J, Sedliak M, Tirpakova V, Sarabon N, Musaro A, Sandri M, Protasi F, Nori A, Pond A, Zampieri S. Long-term high-level exercise promotes muscle reinnervation with age. J Neuropathol Exp Neurol 73: 284–294, 2014. doi: 10.1097/NEN.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 188.Soendenbroe C, Bechshoft CJL, Heisterberg MF, Jensen SM, Bomme E, Schjerling P, Karlsen A, Kjaer M, Andersen JL, Mackey AL. Key components of human myofibre denervation and neuromuscular junction stability are modulated by age and exercise. Cells 9: 893, 2020. doi: 10.3390/cells9040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Messa GAM, Piasecki M, Rittweger J, McPhee JS, Koltai E, Radak Z, Simunic B, Heinonen A, Suominen H, Korhonen MT, Degens H. Absence of an aging-related increase in fiber type grouping in athletes and non-athletes. Scand J Med Sci Sports 30: 2057–2069, 2020. doi: 10.1111/sms.13778. [DOI] [PubMed] [Google Scholar]
- 190.Pegoraro V, Marozzo R, Angelini C. MicroRNAs and HDAC4 protein expression in the skeletal muscle of ALS patients. Clin Neuropathol 39: 105–114, 2020. doi: 10.5414/NP301233. [DOI] [PubMed] [Google Scholar]
- 191.Si Y, Kazamel M, Kwon Y, Lee I, Anderson T, Zhou S, Bamman M, Wiggins D, Kwan T, King PH. The vitamin D activator CYP27B1 is upregulated in muscle fibers in denervating disease and can track progression in amyotrophic lateral sclerosis. J Steroid Biochem Mol Biol 200: 105650, 2020. doi: 10.1016/j.jsbmb.2020.105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Si Y, Kim S, Cui X, Zheng L, Oh SJ, Anderson T, AlSharabati M, Kazamel M, Volpicelli-Daley L, Bamman MM, Yu S, King PH. Transforming growth factor beta (TGF-β) is a muscle biomarker of disease progression in ALS and correlates with Smad expression. PLoS One 10: e0138425, 2015. doi: 10.1371/journal.pone.0138425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Martineau E, Arbour D, Vallee J, Robitaille R. Properties of glial cell at the neuromuscular junction are incompatible with synaptic repair in the SOD1(G37R) ALS mouse model. J Neurosci 40: 7759–7777, 2020. doi: 10.1523/JNEUROSCI.1748-18.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fyfe I. Synaptotagmin 13 - the key to cell resilience in motor neuron disease? Nat Rev Neurol 16: 186, 2020. doi: 10.1038/s41582-020-0336-4. [DOI] [PubMed] [Google Scholar]
- 195.Nizzardo M, Taiana M, Rizzo F, Aguila Benitez J, Nijssen J, Allodi I, Melzi V, Bresolin N, Comi GP, Hedlund E, Corti S. Synaptotagmin 13 is neuroprotective across motor neuron diseases. Acta Neuropathol 139: 837–853, 2020. doi: 10.1007/s00401-020-02133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bello-Haas VD, Florence JM, Kloos AD, Scheirbecker J, Lopate G, Hayes SM, Pioro EP, Mitsumoto H. A randomized controlled trial of resistance exercise in individuals with ALS. Neurology 68: 2003–2007, 2007. doi: 10.1212/01.wnl.0000264418.92308.a4. [DOI] [PubMed] [Google Scholar]
- 197.Park D, Kwak SG, Park JS, Choo YJ, Chang MC. Can therapeutic exercise slow down progressive functional decline in patients with amyotrophic lateral sclerosis? Front Neurol 11: 853, 2020. doi: 10.3389/fneur.2020.00853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pegoraro V, Merico A, Angelini C. MyomiRNAs dysregulation in ALS rehabilitation. Brain Sci 9: 8, 2019. doi: 10.3390/brainsci9010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Miranda DR, Wong M, Romer SH, McKee C, Garza-Vasquez G, Medina AC, Bahn V, Steele AD, Talmadge RJ, Voss AA. Progressive Cl- channel defects reveal disrupted skeletal muscle maturation in R6/2 Huntington’s mice. J Gen Physiol 149: 55–74, 2017. doi: 10.1085/jgp.201611603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ribchester RR, Thomson D, Wood NI, Hinks T, Gillingwater TH, Wishart TM, Court FA, Morton AJ. Progressive abnormalities in skeletal muscle and neuromuscular junctions of transgenic mice expressing the Huntington’s disease mutation. Eur J Neurosci 20: 3092–3114, 2004. doi: 10.1111/j.1460-9568.2004.03783.x. [DOI] [PubMed] [Google Scholar]
- 201.Waters CW, Varuzhanyan G, Talmadge RJ, Voss AA. Huntington disease skeletal muscle is hyperexcitable owing to chloride and potassium channel dysfunction. Proc Natl Acad Sci USA 110: 9160–9165, 2013. doi: 10.1073/pnas.1220068110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Khedraki A, Reed EJ, Romer SH, Wang Q, Romine W, Rich MM, Talmadge RJ, Voss AA. Depressed synaptic transmission and reduced vesicle release sites in Huntington’s disease neuromuscular junctions. J Neurosci 37: 8077–8091, 2017. doi: 10.1523/JNEUROSCI.0313-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mueller SM, Gehrig SM, Petersen JA, Frese S, Mihaylova V, Ligon-Auer M, Khmara N, Nuoffer JM, Schaller A, Lundby C, Toigo M, Jung HH. Effects of endurance training on skeletal muscle mitochondrial function in Huntington disease patients. Orphanet J Rare Dis 12: 184, 2017. doi: 10.1186/s13023-017-0740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mueller SM, Mihaylova V, Frese S, Petersen JA, Ligon-Auer M, Aguayo D, Fluck M, Jung HH, Toigo M. Satellite cell content in Huntington’s disease patients in response to endurance training. Orphanet J Rare Dis 14: 135, 2019. doi: 10.1186/s13023-019-1115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Corrochano S, Blanco G, Acevedo-Arozena A. Skeletal muscle modulates Huntington’s disease pathogenesis in mice: role of physical exercise. J Exp Neurosci 12: 117906951880905, 2018. doi: 10.1177/1179069518809059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lavin KM, Sealfon SC, McDonald MN, Roberts BM, Wilk K, Nair VD, Ge Y, Lakshman Kumar P, Windham ST, Bamman MM. Skeletal muscle transcriptional networks linked to type I myofiber grouping in Parkinson’s disease. J Appl Physiol (1985) 128: 229–240, 2020. doi: 10.1152/japplphysiol.00702.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kelly NA, Ford MP, Standaert DG, Watts RL, Bickel CS, Moellering DR, Tuggle SC, Williams JY, Lieb L, Windham ST, Bamman MM. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson’s disease. J Appl Physiol (1985) 116: 582–592, 2014. doi: 10.1152/japplphysiol.01277.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Obongo R, Bon-Mardion N, Duclos C, Strunski V, Guerout N, Marie JP. Dual innervation may occur in a partially denervated muscle. Muscle Nerve 59: 108–115, 2019. doi: 10.1002/mus.26323. [DOI] [PubMed] [Google Scholar]
- 209.Lavin KM, Ge Y, Sealfon SC, Nair VD, Wilk K, McAdam JS, Windham ST, Kumar PL, McDonald MN, Bamman MM. Rehabilitative impact of exercise training on human skeletal muscle transcriptional programs in Parkinson’s. Disease. Front Physiol 11: 653, 2020. doi: 10.3389/fphys.2020.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Corrick KL, Stec MJ, Merritt EK, Windham ST, Thomas SJ, Cross JM, Bamman MM. Serum from human burn victims impairs myogenesis and protein synthesis in primary myoblasts. Front Physiol 6: 184, 2015. doi: 10.3389/fphys.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, Gundermann DM, Volpi E, Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol (1985) 111: 135–142, 2011. doi: 10.1152/japplphysiol.01408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 156: 20–44, 2014. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani G, Salvioli S. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128: 92–105, 2007. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 214.Wang Y, Wehling-Henricks M, Welc S, Fisher A, Zuo Q, Tidball J. Aging of the immune system causes reductions in muscle stem cell populations, promotes their shift to a fibrogenic phenotype, and modulates sarcopenia. FASEB J 33: 1415–1427, 2019. doi: 10.1096/fj.201800973R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rubenstein AB, Smith GR, Raue U, Begue G, Minchev K, Ruf-Zamojski F, Nair VD, Wang X, Zhou L, Zaslavsky E, Trappe TA, Trappe S, Sealfon SC. Single-cell transcriptional profiles in human skeletal muscle. Sci Rep 10: 229, 2020. doi: 10.1038/s41598-019-57110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J Appl Physiol (1985) 128: 87–99, 2020. doi: 10.1152/japplphysiol.00495.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ziegler AK, Jensen SM, Schjerling P, Mackey AL, Andersen JL, Kjaer M. The effect of resistance exercise upon age-related systemic and local skeletal muscle inflammation. Exp Gerontol 121: 19–32, 2019. doi: 10.1016/j.exger.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 218.Bouzakri K, Zierath J. MAP4K4 gene silencing in human skeletal muscle prevents tumor necrosis factor-alpha-induced insulin resistance. J Biol Chem 282: 7783–7789, 2007. doi: 10.1074/jbc.M608602200. [DOI] [PubMed] [Google Scholar]
- 219.Meex RCR, Blaak EE, Loon LJC. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes Rev 20: 1205–1217, 2019. doi: 10.1111/obr.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yarasheski K, Zachwieja J, Campbell J, Bier D. Effect of growth hormone and resistance exercise on muscle growth and strength in older men. Am J Physiol Endocrinol Metab 268: E268–E276, 1995. doi: 10.1152/ajpendo.1995.268.2.E268. [DOI] [PubMed] [Google Scholar]
- 221.Budui SL, Rossi AP, Zamboni M. The pathogenetic bases of sarcopenia. Clin Cases Miner Bone Metab 12: 22–26, 2015. doi: 10.11138/ccmbm/2015.12.1.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ebhardt A, Degen S, Tadini V, Schilb A, Johns N, Greig C, Fearon K, Aebersold R, Jacobi C. Comprehensive proteome analysis of human skeletal muscle in cachexia and sarcopenia: a pilot study. J Cachexia Sarcopenia Muscle 8: 567–582, 2017. doi: 10.1002/jcsm.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. Faseb j 15: 475–482, 2001. doi: 10.1096/fj.00-0274com. [DOI] [PubMed] [Google Scholar]
- 224.Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol 105: 473–478, 2008. doi: 10.1152/japplphysiol.00006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Walton RG, Kosmac K, Mula J, Fry CS, Peck BD, Groshong JS, Finlin BS, Zhu B, Kern PA, Peterson CA. Human skeletal muscle macrophages increase following cycle training and are associated with adaptations that may facilitate growth. Sci Rep 9: 969, 2019. doi: 10.1038/s41598-018-37187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sato S, Ogura Y, Kumar A. TWEAK/Fn14 signaling axis mediates skeletal muscle atrophy and metabolic dysfunction. Front Immunol 5: 18, 2014. doi: 10.3389/fimmu.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bamman MM, Ferrando AA, Evans RP, Stec MJ, Kelly NA, Gruenwald JM, Corrick KL, Trump JR, Singh JA. Muscle inflammation susceptibility: a prognostic index of recovery potential after hip arthroplasty? Am J Physiol Endocrinol Metab 308: E670–E679, 2015. doi: 10.1152/ajpendo.00576.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dogra C, Changoua H, Wedhas N, Qin X, Wergedal JE, Kumar A. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB j 21: 1857–1869, 2007. doi: 10.1096/fj.06-7537com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mittal A, Bhatnagar S, Kumar A, Lach-Trifilieff E, Wauters S, Li H, Makonchuk DY, Glass DJ, Kumar A. The TWEAK-Fn14 system is a critical regulator of denervation-induced skeletal muscle atrophy in mice. J Cell Biol 188: 833–849, 2010. doi: 10.1083/jcb.200909117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Merritt EK, Thalacker-Mercer A, Cross JM, Windham ST, Thomas SJ, Bamman MM. Increased expression of atrogenes and TWEAK family members after severe burn injury in nonburned human skeletal muscle. J Burn Care Res 34: e297-304–e304, 2013. doi: 10.1097/BCR.0b013e31827a2a9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yarar-Fisher C, Bickel CS, Kelly NA, Stec MJ, Windham ST, McLain AB, Oster RA, Bamman MM. Heightened TWEAK-NF-kappaB signaling and inflammation-associated fibrosis in paralyzed muscles of men with chronic spinal cord injury. Am J Physiol Endocrinol Metab 310: E754–E761, 2016. doi: 10.1152/ajpendo.00240.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Pascoe AL, Johnston AJ, Murphy RM. Controversies in TWEAK-Fn14 signaling in skeletal muscle atrophy and regeneration. Cell Mol Life Sci 77: 3369–3381, 2020. doi: 10.1007/s00018-020-03495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Raue U, Jemiolo B, Yang Y, Trappe S. TWEAK-Fn14 pathway activation after exercise in human skeletal muscle: insights from two exercise modes and a time course investigation. J Appl Physiol 118: 569–578, 2015. doi: 10.1152/japplphysiol.00759.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Belizário JE, Fontes-Oliveira CC, Borges JP, Kashiabara JA, Vannier E. Skeletal muscle wasting and renewal: a pivotal role of myokine IL-6. Springerplus 5: 619, 2016. doi: 10.1186/s40064-016-2197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhang L, Pan J, Dong Y, Tweardy D, Dong Y, Garibotto G, Mitch W. Stat3 activation links a C/EBPδ to myostatin pathway to stimulate loss of muscle mass. Cell Metab 18: 368–379, 2013. doi: 10.1016/j.cmet.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tisdale MJ. Cancer cachexia. Curr Opin Gastroenterol 26: 146–151, 2010. doi: 10.1097/MOG.0b013e3283347e77. [DOI] [PubMed] [Google Scholar]
- 237.Rong Y-D, Bian A-L, Hu H-Y, Ma Y, Zhou X-Z. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatrics 18: 308, 2018. doi: 10.1186/s12877-018-1007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Nelke C, Dziewas R, Minnerup J, Meuth S, Ruck T. Skeletal muscle a potenital central link between sarcopenia and immune senescence. EBioMedicine 49: 381–388, 2019. doi: 10.1016/j.ebiom.2019.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Saxena A, Khosraviani S, Noel S, Mohan D, Donner T, Hamad A. Interleukin-10 paradox: A potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine 74: 27–34, 2015. doi: 10.1016/j.cyto.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Thalacker-Mercer AE, Dell'Italia LJ, Cui X, Cross JM, Bamman MM. Differential genomic responses in old vs. young humans despite similar levels of modest muscle damage after resistance loading. Physiol Genomics 40: 141–149, 2010. doi: 10.1152/physiolgenomics.00151.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lee Y, Park HK, Kim WY, Kim MC, Jung W, Ko BS. Muscle mass depletion associated with poor outcome of sepsis in the emergency department. Ann Nutr Metab 72: 336–344, 2018. doi: 10.1159/000488994. [DOI] [PubMed] [Google Scholar]
- 242.Wollersheim T, Grunow JJ, Carbon NM, Haas K, Malleike J, Ramme SF, Schneider J, Spies CD, Märdian S, Mai K, Spuler S, Fielitz J, Weber-Carstens S. Muscle wasting and function after muscle activation and early protocol-based physiotherapy: an explorative trial. J Cachexia Sarcopenia Muscle 10: 734–747, 2019. doi: 10.1002/jcsm.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Berney S, Hopkins RO, Rose JW, Koopman R, Puthucheary Z, Pastva A, Gordon I, Colantuoni E, Parry SM, Needham DM, Denehy L. Functional electrical stimulation in-bed cycle ergometry in mechanically ventilated patients: a multicentre randomised controlled trial. Thorax 76: 656–663, 2021. doi: 10.1136/thoraxjnl-2020-215093. [DOI] [PubMed] [Google Scholar]
- 244.Kayambu G, Boots R, Paratz J. Early physical rehabilitation in intensive care patients with sepsis syndromes: a pilot randomised controlled trial. Intensive Care Med 41: 865–874, 2015. doi: 10.1007/s00134-015-3763-8. [DOI] [PubMed] [Google Scholar]
- 245.Piccirillo R. Exercise-induced myokines with therapeutic potential for muscle wasting. Front Physiol 10: 287, 2019. doi: 10.3389/fphys.2019.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lee JH, Jun H-S. Role of myokines in regulating skeletal muscle mass and function. Front Physiol 10: 42, 2019. doi: 10.3389/fphys.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Thyfault JP, Bergouignan A. Exercise and metabolic health: beyond skeletal muscle. Diabetologia 63: 1464–1474, 2020. doi: 10.1007/s00125-020-05177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sanford JA, Nogiec CD, Lindholm ME, Adkins JN, Amar D, Dasari S, Drugan JK, Fernández FM, Radom-Aizik S, Schenk S, Snyder MP, Tracy RP, Vanderboom P, Trappe S, Walsh MJ. Molecular transducers of physical activity consortium. Molecular Transducers of Physical Activity Consortium (MoTrPAC): Mapping the dynamic responses to exercise. Cell 181: 1464–1474, 2020. doi: 10.1016/j.cell.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, Ferreira CK, Harkema SJ. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med 379: 1244–1250, 2018. doi: 10.1056/NEJMoa1803588. [DOI] [PubMed] [Google Scholar]
- 250.Giangregorio LM, Webber CE, Phillips SM, Hicks AL, Craven BC, Bugaresti JM, McCartney N. Can body weight supported treadmill training increase bone mass and reverse muscle atrophy in individuals with chronic incomplete spinal cord injury? Appl Physiol Nutr Metab 31: 283–291, 2006. doi: 10.1139/h05-036. [DOI] [PubMed] [Google Scholar]