Abstract
Women in the Middle East and North Africa (MENA) region are burdened with several risk factors related to gestational diabetes mellitus (GDM) including overweight and high parity. We systematically reviewed the literature and quantified the weighted prevalence of GDM in MENA at the regional, subregional, and national levels. Studies published from 2000 to 2019 reporting the prevalence of GDM in the MENA region were retrieved and were assessed for their eligibility. Overall and subgroup pooled prevalence of GDM was quantified by random-effects meta-analysis. Sources of heterogeneity were investigated by meta-regression. The risk of bias (RoB) was assessed by the National Heart, Lung, and Blood Institute’s tool. One hundred and two research articles with 279,202 tested pregnant women for GDM from 16 MENA countries were included. Most of the research reports sourced from Iran (36.3%) and Saudi Arabia (21.6%), with an overall low RoB. In the 16 countries, the pooled prevalence of GDM was 13.0% (95% confidence interval [CI], 11.5–14.6%, I2, 99.3%). Nationally, GDM was highest in Qatar (20.7%, 95% CI, 15.2–26.7% I2, 99.0%), whereas subregionally, GDM was highest in Gulf Cooperation Council (GCC) countries (14.7%, 95% CI, 13.0–16.5%, I2, 99.0%). The prevalence of GDM was high in pregnant women aged ≥30 years (21.9%, 95% CI, 18.5–25.5%, I2, 97.1%), in their third trimester (20.0%, 95% CI, 13.1–27.9%, I2, 98.8%), and who were obese (17.2%, 95% CI, 12.8–22.0%, I2, 93.8%). The prevalence of GDM was 10.6% (95% CI, 8.1–13.4%, I2, 98.9%) in studies conducted before 2009, whereas it was 14.0% (95% CI, 12.1–16.0%, I2, 99.3%) in studies conducted in or after 2010. Pregnant women in the MENA region are burdened with a substantial prevalence of GDM, particularly in GCC and North African countries. Findings have implications for maternal health in the MENA region and call for advocacy to unify GDM diagnostic criteria.
Systematic Review Registration
PROSPERO CRD42018100629
Keywords: gestational diabetes mellitus, MENA region, prevalence, meta-analysis, systematic review
Introduction
Gestational diabetes mellitus (GDM) (1) is usually diagnosed during the second and third trimesters of pregnancy (2). Risk factors of GDM include excessive body weight, low level of physical activity, consanguineous marriage, previous history of GDM, glycated hemoglobin >5.7%, and history of cardiovascular disease (3). As the toll of overweight and obese reproductive-age females soars, the risk of developing hyperglycemia in pregnancy increases (4).
GDM has a global public health burden (5) with both short- and long-term consequences on health. The short-term ramifications of GDM include adverse perinatal outcomes for the affected women (e.g., preeclampsia, polyhydramnios, and increased cesarean section [“C-section”] risk) and their neonates (e.g., macrosomia and shoulder dystocia) (1, 6), whereas the long-term complications of GDM incorporate the risk of type 2 diabetes mellitus (T2DM) for the mother and the risk of childhood obesity, impaired glucose tolerance, and/or metabolic syndrome for their neonates (6). Since increased blood glucose levels are associated with certain perinatal complications, gestational blood glucose control is vital (7).
Understanding population-specific healthcare needs at specific points of time is essential, and prevalence estimates are ideal for such purposes (8). Unfortunately, the global GDM prevalence estimates (<1%–28%) show a wide variation due to ethnicity, ethnic variation among various populations, and inconsistent use of screening and diagnostic criteria (4, 9). To precisely estimate the burden of GDM of a particular geographic area, it is essential to determine the region-specific prevalence estimate. There is scant literature on the prevalence of GDM in the Middle East and North Africa (MENA) region, although two of the main risk factors [physical inactivity and above-normal body mass index (BMI)] are identified as being highly prevalent in this region (10). Moreover, three of the world’s top ten most prevalent countries for diabetes mellitus belong to this region: Saudi Arabia (24%), Kuwait (23%), and Qatar (23%) (11). For the entire Eastern Mediterranean region, the existing prevalence estimate of GDM is 14.5%, although this includes only cases diagnosed according to the World Health Organization (WHO) 1999 criteria (4). One previous survey showed that physicians and hospitals in this region use different criteria to diagnose GDM (12).
A systematic review and meta-analysis of prevalence studies is considered to be an ideal method to understand the burden of GDM at regional and national levels. In this systematic review, meta-analysis, and meta-regression, we estimated the weighted pooled prevalence of GDM in the MENA region, at the regional, subregional, and national levels, based on literature published between January 2000 and December 2019.
Methods
This review follows the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2009 guidelines (13). The PRISMA checklist is provided elsewhere (Supplementary File 1). Following our published protocol, we report here “systematic review 2” (14). We implemented minor amendments whenever needed, including an updated database search.
Data Source and Searches
To identify eligible studies reporting the prevalence of GDM in the MENA countries, we conducted a comprehensive search of five electronic databases (MEDLINE, EMBASE, Web of Science, SCOPUS, and Cochrane library) from January 1, 2000, to December 31, 2019, using variant Medical Subject Headings and free-text terms. Restricting the literature search to 2000 was to estimate changes in the GDM prevalence over the past two decades (before and after 2010), at national, sub-regional, and regional levels, whenever enough data is available for the meta-analysis. The literature search strategy was developed in consultation with an expert librarian at the National Medical Library at the United Arab Emirates University. The full search strategy available in the published protocol (14). Retrieved references were imported to the Covidence software (Covidence, Melbourne, Australia) (15). Deduplication of similar references was performed automatically by the Covidence software.
Study Selection
To identify and select studies for inclusion, we followed the PECO(T) framework: participants, exposure, comparator, outcome(s), and type of study (16). However, we considered only participants and outcomes because the focus of this review was on studies reporting the prevalence of GDM. Study eligibility criteria are presented in Table 1.
Table 1.
Study eligibility criteria.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Population | Pregnant women regardless of their age, parity, or any maternal or sociodemographic characteristics | Non-pregnant women |
| Outcome | Studies reported quantitative or calculable GDM prevalence estimate(s) regardless of the GDM diagnostic criteria/guidelines or pregnancy trimester | Studies on pregnant women with no information related to GDM prevalence |
| Sample size | Studies with at least ten pregnant women tested for GDM | Studies with less than ten pregnant women tested for GDM |
| Study design | Cross-sectional, cohort studies, case–control studies comparing no-GDM with no-GDM subpopulations, and trials with nonpharmaceutical interventions | Case–control studies comparing GDM with no-GDM populations, qualitative studies, modeling studies, case reports and case series regardless of the number of cases, narrative and systematic reviews, conference abstracts with no full information, editorials, commentaries, letters to the editor, author replies, and other publications that did not include quantitative data on the prevalence of GDM |
| Geographical region | Any of the 18 Arab countries (Algeria, Bahrain, Djibouti, Egypt, Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Qatar, Saudi Arabia, Syria, Tunisia, United Arab Emirates, West Bank and Gaza, and Yemen) in addition to Iran and Malta in the MENA region, according to the definition of the World Bank Country and Lending Groups (17). | All other countries |
| Publication period | January 2000 to December 2019 | Studies conducted before January 2000 or after December 2019 and studies for which the time period of the GDM tests in pregnant women was unclear |
| Language | English language | Non-English studies |
| Setting | No limitations. Hospital based, population based, or clinic based. | No limitations |
| Duplicate studies | – | Studies duplicating or potentially duplicating GDM ascertainment in the same population. In the case of duplicate publications, we included only the study containing the most relevant information in the context of the prevalence of GDM |
Identifying Eligible Studies
Titles and abstracts were screened by RHA, NMA, and MSP to detect eligible research reports on the prevalence of GDM. For studies that appeared eligible, the full text was reviewed (RHA, NMA, and MSP). Screening of all titles and abstracts and full text articles was performed independently by two reviewers. Disagreements among reviewers were resolved by discourse. We also searched the reference lists of eligible studies for studies that might have been missed. Figure 1 shows the PRISMA flowchart of study selection.
Figure 1.
PRISMA flowchart of study selection.
In this review, the term “research report” is used to refer to a full published research document. The term “study” is used to refer to a single study on a specific population group. One big observational study (one research report) provides GDM data stratified into four age groups (four studies). Hence, one research report could contribute several studies on GDM prevalence.
Data Extraction and Quality Assessment
Relevant data from eligible studies were extracted into a predesigned Excel sheet using a predefined list of numerical and string variables. The outcome of interest was the weighted prevalence of GDM in pregnant women in the MENA countries, according to various characteristics including, but not limited to, age, BMI, trimester, and time period. We extracted author names, publication year, country, city, and study setting. In addition, data on the implemented methodology (design, data collection period, sampling strategy, and GDM diagnosis and ascertainment methodology) and characteristics of the studied pregnant women (age, pregnancy trimester, sample size, number of women with GDM and GDM prevalence) were extracted whenever available.
In addition to the overall prevalence of GDM, some research reports also reported the prevalence of GDM stratified according to different characteristics, such as age, parity, comorbidity, pregnancy trimester, and BMI. In such reports, data extraction was performed for the stratified GDM prevalence, following the rule that the study had to have at least ten tested subjects per strata; otherwise, information on the entire tested sample was extracted. A predefined sequential order was established when extracting stratified GDM prevalence estimates as follows: GDM stratified first according to comorbidities followed by parity, age, and BMI. This prioritization was used to identify the strata with more information on the tested pregnant women. When there was no stratification for the prevalence of GDM, we extracted the overall GDM prevalence measured.
For each research report reporting the stratified prevalence of GDM according to more than one category (i.e., age and BMI), one category per research report was considered and included based on the aforementioned prioritization scheme, to avoid double counting. In studies in which GDM was ascertained using different guidelines, the most sensitive and reliable ascertainment assay was considered (i.e., prioritizing fasting blood glucose over self-reported) or was based on the most recent and updated criteria (i.e., prioritizing WHO 2010 over 2006 criteria).
The risk of bias (RoB) assessment was performed at the level of the research report rather than the study. The quality of each research report was evaluated according to criteria of the National Heart, Lung, and Blood Institute (18). Six of 14 items from the quality assessment tool for prevalence studies were used (18). The six quality-related items assessed the research question/objectives, studied population, sample size justification, and outcome measures and assessment. Eight items were not used because they are applicable only to follow-up cohort studies. For additional quality assessment, we also assessed the robustness of the implemented methodology using three additional quality-of-evidence criteria: sampling methodology, GDM ascertainment methodology, and precision of the estimate. Studies were considered to have “high” precision if at least 100 women were tested for GDM. We computed the overall proportion of research reports with potentially low RoB across each of these nine quality criteria and also computed the proportion (out of nine) of quality items with a potentially low RoB for each of the included research reports.
Data abstraction and quality assessment were performed independently by two reviewers (NA and MP) and cross-checked for disagreements. Any discrepancies in the extraction phase or in the quality assessment between the reviewers were discussed and resolved with a consultation of a senior reviewer (RA-R).
Data Synthesis and Analysis
To estimate the weighted pooled prevalence of GDM and the corresponding 95% confidence interval (CI), we performed meta-analyses of the extracted data. The Freeman–Tukey double arcsine transformation method was applied to stabilize the variances of the prevalence measures (19). The inverse variance method was used to weight the estimated pooled prevalence measures (20). Dersimonian–Laird random-effects model was used to estimate the overall pooled GDM prevalence (21). Cochran’s Q statistic and the inconsistency index, I 2, were calculated to measure heterogeneity (22). Along with the pooled estimates, ranges and median were also reported to describe the dispersion of the GDM prevalence measures reported in the literature. The prediction interval, which estimates the 95% interval in which the true effect size in a new prevalence study will lie, was also quantified and reported (22).
For the subgroup meta-analysis, country-level pooled estimates were generated overall and based on time period. In addition, to estimate the change in GDM both at the country level and overall, the data collection period was stratified into two time periods: 2000–2009 and 2010–2019. For studies in which the data collection period overlapped, the collection period was defined as “overlap” so as not to miss any important data when estimating country-level, subregional, and regional prevalence. The median (~2 years) was used in studies with an unclear data collection period. In these studies, the median was subtracted from the year of publication to estimate the year of data collection.
The weighted pooled prevalence, regardless of country, was also estimated according to the age of the pregnant women, trimester, BMI, study period, GDM ascertainment guidelines, and sample size (<100 or ≥100). The provision of pooled estimates regardless of the ascertainment guidelines was justified by the fact that the women were defined and treated as GDM patients following each specific ascertainment guideline.
Accumulated evidence has shown that GDM is associated with an increased risk of C-section (23, 24) and maternal mortality (4). Independent of the research report and the characteristics of the tested pregnant women for GDM, we estimated the pooled GDM prevalence according to the C-section rate and maternal mortality ratio (MMR). Information on the C-section rate (25, 26) and MMR were retrieved from various resources (27). Depending on data availability, information on C-section rate and MMR was extracted in the same or the closest year to the estimated GDM prevalence. For every GDM study, the rate of C-section was then categorized as <15%, 15–29%, >30%, or unclear, whereas the MMR was categorized as either ≤100/100,000 live births, >100/100,000 live births, or unclear.
To provide prevalence estimates at a subregional level, we regrouped MENA countries into four subregions, namely, North Africa, Gulf Cooperation Council (GCC) countries, Levant, and Iran/Iraq region. We estimated the overall pooled prevalence in these subregions and according to patient age, trimester, BMI, study period, GDM ascertainment guidelines, rate of C-section, and MMR.
Random-effects univariate and multivariable meta-regression models were implemented to identify sources of between-study heterogeneity and to quantify their contribution to variability in the prevalence of GDM. In univariate meta-regression models, analysis was performed by country, age, pregnancy trimester, BMI, and sample size. All variables with a p-value <0.1 in the univariate models were included in the multivariable model. In the final multivariable model, a p-value ≤0.05 was considered statistically significant, which contributed to the heterogeneity in prevalence estimates.
Publication Bias
A funnel plot was generated to explore the small-study effect on the pooled GDM prevalence estimates. The funnel plot was created by plotting each GDM prevalence measure against its standard error. The asymmetry of the funnel plot was tested using Egger’s test (28).
All analyses were performed using the metaprop (29) and metareg packages in Stata/SE v15 (30).
The study is registered with PROSPERO, number CRD42018100629.
Results
Database Search and Scope of the Review
Of the 13,139 citations retrieved from the 5 databases, 102 research reports were deemed eligible and included in this review (Figure 1).
The research reports were from 16 countries in the MENA region: Algeria (one), Bahrain (two), Egypt (four), Iraq (three), Iran (37), Jordan (four), Lebanon (two), Libya (one), Morocco (one), Oman (five), Qatar (six), Saudi Arabia (22), Sudan (two), Tunisia (one), United Arab Emirates (UAE) (eight), and Yemen (one). The prevalence data for both decades (time periods) were available from six countries (Bahrain, Iran, Oman, Qatar, Saudi Arabia, and the UAE); for the other countries, data were available for the time period 2010–2019 (Table 2). Self-reported GDM status was documented in five research reports (31, 73, 83, 90, 119). The predominantly used GDM diagnostic criteria in the MENA region were from the American Diabetes Association and the International Association of Diabetes and Pregnancy Study Group (ADA/IADPSG; 48.5% of studies).
Table 2.
Summary of the included studies reporting the prevalence of GDM in pregnant women in the MENA region, 2000–2019, stratified by country (102 reports with 198 prevalence measures).
| Author, year [Ref] | Duration of data collection | Country, city | Setting | Design | Sampling | Population | Strata | Ascertainment method | Tested sample | GDM | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | % | ||||||||||
| Tebbani F. et al. (31) | 12/2013–12/2015 | Algeria, Constantine | Maternities, antenatal and private gynecologists | PC | Unclear | Algerian pregnant women aged 19–41 years who entered prenatal care before 16 weeks of amenorrhea | All | Face-to-face interview | 200 | 6 | 3.0 |
| Rajab K. et al. (32) | 2002–2010 | Bahrain | Government central hospital that is responsible for approximately 80% of all births in Bahrain | CS | Whole population | Pregnant women | All 2002–2010 |
NDDG 1979 guidelines | 49,552 | 4,982 | 10.1 |
| Al Mahroos S. et al. (33) | 1/2001–12/2002 | Bahrain | ANC clinics at health centers and at Salmaniya Medical Complex | CS | All women during the study period | Nondiabetic pregnant women | All | Fourth International Workshop-Conference on GDM | 10,495 | 1,394 | 13.7 |
| Bahraini | 7,575 | 1,175 | 15.5 | ||||||||
| Expatriate | 2,920 | 219 | 7.5 | ||||||||
| Rakha S and El Marsafawy H (34) | 01/2011 – 01/2019 | Egypt, Mansoura | Pediatric cardiology unit in Mansoura University Children’s Hospital | CS | Whole population | Pregnant with at least one high risk indication of fetal echocardiography | All | Unclear | 458 | 57 | 12.5 |
| Rezk M and Omar Z (35) | 05/2012–05/2017 | Egypt | Shibin El-Kom | PS | Whole Population | Pregnant women with chronic HCV infection | All | Unclear | 342 | 90 | 26.3 |
| Pregnant women with no HCV infection | 170 | 10 | 5.9 | ||||||||
| Maged AM. et al. (36) | 01/2011–02/2013 | Egypt, Cairo | Kasr El Aini Hospital | PS | Unclear | Pregnant women in their first trimester with a singleton living fetus, excluding women with preexisting type 1 or 2 diabetes mellitus, hypertension, liver disease, renal disease, or the presence of active infection | All | ADA 2002 | 269 | 27 | 10.0 |
| Elkholi DGEY and Nagy HM (37) | 3/2007–3/2013 | Egypt, Tanta | Infertility Clinic, Tanta University Hospitals | CS | Unclear | Obese pregnant women (BMI ≥30 kg/m2) with PCOS before treatment for infertility, attending 100 patients with android obesity and 100 patients with gynoid obesity | All | Fifth International Workshop Conference on Gestational Diabetes criteria | 131 | 10 | 7.6 |
| Outpatient Clinic of Department of Obstetric | Non-PCOS pregnant women with android obesity were controls for group 1 and 100 non-PCOS pregnant women with gynoid obesity who were free of DM before pregnancy | 177 | 14 | 7.9 | |||||||
| Mohammed AK and Alqani VHA (38) | 06/2016–07/2017 | Iraq, Al-Diwaniyah | Child and Maternity Teaching Hospital | CS | Unclear | Pregnant women with a mean age of 30.02 ± 6.37 years | All | Unclear | 49 | 12 | 24.5 |
| Alawad ZM and Al-Omary HL (39) | 09/2018–12/2018 | Iraq, Baghdad | Baghdad teaching hospital | PC | Unclear | Women between 18 and 40 years of age, normal vaginal deliveries to live singletons with no congenital anomalies, women with normal thyroid function test | All | Unclear | 35 | 7 | 20.0 |
| Safari K et al. (40) | 10/2017–01/2018 | Iraq, Erbil | Hawler Maternity Teaching Hospital | CC | Unclear | Singleton Muslim pregnant women aged 18–35 years who fasted in Ramadan during the second trimester | All | Unclear | 155 | 4 | 2.6 |
| 144 | 12 | 8.2 | |||||||||
| Maghbooli Z et al. (41) | 2005 | Iran, Tehran | Five university hospital clinics of the Tehran University of Medical Sciences | CS | Unclear | Pregnant women with no previous history of DM and who sought prenatal care during the first half of their pregnancies | All | Carpenter and Coustan criteria | 741 | 52 | 7.0 |
| Abolfazl M et al. (42) | 2006 | Iran, Shiraz | Shiraz Hospital | Unclear | Random | Pregnant women with a mean age of 31.2 years | All | Unclear | 420 | 70 | 16.6 |
| Keshavarz M et al. (43) | 12/1999–01/2001 | Iran, Shahrood | Fatemiyeh Hospital | PC | Consecutive | All pregnant women within the catchment area of the hospital were referred to this antenatal service; twin pregnancies, miscarriages, terminations, and women with preexisting diabetes were excluded from our study | All | Carpenter and Coustan criteria | 1,310 | 63 | 4.8 |
| Hadaegh F et al. (44) | 3/2002–3/2004 | Iran, Bandar Abbas | Obstetrics clinics in various parts of Bandar Abbas city in southern Iran | CS | All women during the study period | Pregnant women with a mean age of 24.9 years in the 24th to the 28th week of pregnancy excluding women with history of diabetes, using drugs that affect glucose metabolism, with chronic liver disease, endocrine disorders (such as hyperthyroidism), or connective tissue disorders, and with major medical conditions, such as persistent hypertension | All | Carpenter and Coustan criteria | 700 | 62 | 8.9 |
| <20 years | 93 | 2 | 2.2 | ||||||||
| 20–24 years | 279 | 15 | 5.4 | ||||||||
| 25–29 years | 184 | 22 | 12.0 | ||||||||
| 30–34 years | 103 | 13 | 12.6 | ||||||||
| 35–≥45 years | 41 | 10 | 24.3 | ||||||||
| Amooee S et al. (45) | 2006–2008 | Iran, Sheraz | Hafez and Zeinabieh Hospitals of Shiraz University of Medical Sciences | CS | Unclear | All singleton pregnancies with and without minor β-thalassemia | With minor β-thalassemia | Unclear | 510 | 16 | 3.5 |
| Without minor β-thalassemia | 512 | 20 | 20.0 | ||||||||
| Lamyian M et al. (46) | 08/2010– 01/2011 | Iran, Tehran | Prenatal clinics in five hospitals affiliated with universities of medical sciences in different districts | PS | Random | Singleton pregnant women age 18–45 years, excluding preexisting diabetes and smokers | All | ADA 2016 | 1,026 | 71 | 6.9 |
| Soheilykhah S et al. (47) | 2007–2009 | Iran, Yazd | Two prenatal clinics in Yazd | PS | Unclear | Iranian pregnant women with a mean age of 27 years, excluding those with prepregnancy DM | All | ADA 2004 | 734 | 95 | 13.0 |
| <25 years | 247 | 19 | 7.7 | ||||||||
| 25–29 years | 202 | 30 | 14.9 | ||||||||
| ≥30 years | 285 | 46 | 16.1 | ||||||||
| Pirjani R et al. (48) | 2012–2013 | Iran, Tehran | Dr Shariati and Arash Hospitals | PS | Convenience | Pregnant women with a mean age of 28.70 ± 5.57 years (range 17–44 years) excluding women with a history of diabetes (type 1 or 2), tested for GDM at the 24th–28th weeks of pregnancy | All | ADA 2012 | 256 | 78 | 30.5 |
| Soheilykhah S et al. (49) | 01/2010–02/2013 | Iran, Yazd | Two prenatal clinics (Mojibian and Shahid Sadoughi Hospitals |
CS | Unclear | Pregnant women tested for GDM at 24–28 weeks of pregnancy, excluding women with type 1 or 2 diabetes, malignancies, acute or chronic inflammatory or infective diseases, acute or chronic liver disease, and iron deficiency anemia | All | ADA 2013 | 1,279 | 281 | 21.9 |
| Shahbazian H et al. (50) | 08/2014–02/2015 | Iran, Ahvaz | Prenatal clinic of a public medical hospital and four private prenatal clinics | PS | Unclear | Pregnant women tested for GDM between 24 and 32 weeks of gestation | All | IADPSG | 750 | 224 | 29.9 |
| 15–24 years | 190 | 32 | 16.8 | ||||||||
| 25–34 years | 452 | 145 | 32.1 | ||||||||
| 35–44 years | 108 | 47 | 43.5 | ||||||||
| Yassaee F et al. (51) | 10/2008–2/2010 | Iran, Tehran | Teaching hospital in the North of Tehran | PS | Unclear | Pregnant women with idiopathic thrombocytopenic purpura at a mean age of 28.9 years | Unclear | 21 | 6 | 28.6 | |
| Ashrafi M et al. (52) | 2012–2013 | Iran, Tehran | Reproductive biomedicine research center, Royan Institute | CS | Unclear | Non-PCOS pregnant women who conceived spontaneously with a mean age of 26.4 years | All | Fifth International Workshop on GDM | 234 | 17 | 7.3 |
| Non-PCOS pregnant women conceived with RT with a mean age of 30.7 years | All | 234 | 70 | 29.9 | |||||||
| PCOS pregnant women with ART with a mean age of 29.6 years | All | 234 | 104 | 44.4 | |||||||
| Goshtasebi A et al. (53) | 8/2010–1/2011 | Iran, Tehran | Prenatal clinics in five hospitals affiliated with universities of medical sciences | CS | Consecutive | Pregnant women aged 18–45 years, singleton pregnancy, gestational age ≤6 weeks, gestations ≤2, and nonsmokers | All | ADA 2016 | 1,026 | 71 | 6.9 |
| Ashrafi M et al. (54) | 11/2011–10/2012 | Iran, Tehran | Reproductive Biomedicine Research Centre of the Royan Institute, | CS | Unclear | Pregnant women who conceived after fresh IVF/ICSI or intrauterine insemination at a mean age of 31.3 years with no history of DM, family history of DM, GDM | All | ADA 2005 | 145 | 54 | 15.7 |
| Akbarabadi Women’s Hospital, affiliated with Tehran University of Medical Science | CS | Unclear | Pregnant women with singleton spontaneous pregnancies at a mean age of 26.6 years and with no history of DM, family history of DM, or GDM | All | 215 | 22 | 25.1 | ||||
| Jamali S et al. (55) | 4/2012–10/2015 | Iran, Jahrom | Paymaneh Hospital Jahrom, Iran | CS | Unclear | Inclusion criterion was all women aged 15–45 years; incomplete and doubtful data were excluded; the study compared 154 women in the first group (teenage group), 400 women in the second group (control group), and 196 women in the third group (adult women) | All 15–45 years |
Medical Records | |||
| 750 | 16.2 | 2.1 | |||||||||
| 15–19 years | 154 | 1 | 0.6 | ||||||||
| 20–34 years | 400 | 7 | 1.8 | ||||||||
| 35–45 years | 196 | 8 | 4.1 | ||||||||
| Pourali L et al. (56) | 7/2009–7/2014 | Iran, Mashad | Ghaem Hospital | CS | Convenience | Women with dichorionic spontaneous twin pregnancy with a mean age of 27.1 years | All | Medical records | 96 | 8 | 8.3 |
| Women with dichorionic pregnancy following ART with a mean age of 28.9 years | 31 | 8 | 25.8 | ||||||||
| Mehrabian F and Rezae M (57) | 1/2009–3/2013 | Iran, Isfahan | Shahid Beheshti Hospital | CS | Unclear | Pregnant women who were infertile due to PCOS with an age range of 18–42 years | All | ADA 2011 | 180 | 50 | 27.8 |
| Mehrabian F and Hosseini SM (58) | 2011–2012 | Iran, Isfahan | Isfahan University of Medical Sciences | CS | Convenience | Pregnant women without preexisting diabetes, mean age 27.6 years | All | Unclear | 944 | 72 | 7.6 |
| Hosseini E et al. (59) | 10/2015–01/2017 | Iran, Isfahan | 10 community health care centers | CS | Consecutive | Women 18–45 years old with singleton pregnancy | All | IADSPG two-step approach | 929 | 93 | 10.0 |
| Hantoushzadeh S et al. (60) | 2/2012–3/2015 | Iran, Tehran | Maternal, Fetal and Neonatal Research Center, Vali-asr Teaching Hospital | CS | Unclear | Pregnant women aged 20–32 years with singleton pregnancies screened for GDM at 28 weeks. excluding women with a history of type 1 or type 2 diabetes mellitus, missing information about prepregnancy diabetes status or BMI, incomplete data on glucose tolerance testing or weight gain during pregnancy | All | ACOG | 1,279 | 100 | 7.8 |
| Underweight | 27 | 0 | 0.0 | ||||||||
| Normal weight | 751 | 45 | 3.3 | ||||||||
| Overweight | 381 | 35 | 9.2 | ||||||||
| Obese | 120 | 20 | 16.7 | ||||||||
| Niromanesh S et al. (61) | 2008–2010 | Iran, Tehran | Tehran Women General Hospital | CS | Consecutive | Normal pregnant women 20–35 years of age with gestational age 16–20 weeks, gravid >2, BMI of 20–25 kg/m² were included in the study, excluding women with a history of PTB, preeclampsia, diabetes, GDM, primigravida, those with a BMI >25, and high maternal age (>35 years) | High triglyceride level (>195 mg/dL) | Unclear | 45 | 9 | 20.0 |
| Normal triglyceride level (<195 mg/dL) | 135 | 8 | 5.9 | ||||||||
| Vaezi A et al. (62) | 2009–2012 | Iran, Tehran | Akbarabadi Hospital | RC | Convenient | Medical records of pregnant women aged between 18 and 50 years admitted to the hospital to obtain prenatal care | All | Unclear | 580 | 56 | 9.6 |
| With asthma | 274 | 37 | 13.5 | ||||||||
| Without asthma | 306 | 19 | 6.2 | ||||||||
| Hossein–Nezhad A et al. (63) | Unclear | Iran, Tehran | Five teaching hospitals affiliated with Tehran University of Medical Sciences | CS | Consecutive | Pregnant women referred to ANC visits with no known history with known diabetes were excluded from the study | All 15–45 years |
Carpenter and Coustan | |||
| 2,416 | 114 | 4.7 | |||||||||
| 15–24 years | 1,209 | 27 | 2.2 | ||||||||
| 25–34 years | 1,001 | 56 | 5.6 | ||||||||
| 35–45 years | 206 | 31 | 15.0 | ||||||||
| Nastaran SA et al. (64) | 10/2009–8/2010 | Iran, Tehran | Milad Hospital | PS | Convenience | Pregnant woman referred to the pregnancy care clinics with a single fetus, aged 18–35 years with a gestational age of 1–13 weeks, a parity of 3 or less, lack of known systemic diseases, and lack of gestational diabetes during previous pregnancies | All | Carpenter and Coustan | 600 | 49 | 8.2 |
| Talebian A et al. (65) | 2/2007–12/2012 | Iran, Kashan | Shabihkhani, Shahid Beheshti and Milad hospitals | CS | Unclear | Pregnant women with normal pregnancies and with neural tube defects | All | Unclear | 300 | 21 | 7.3 |
| Kouhkan A, et al. 2018 (66) | 11/2014–1/2017 | Iran, Tehran | Royan Institute and maternity teaching hospital located in Tehran | PC | Whole population | Singleton pregnant women aged 20–42 years, who conceived via ART or SC | All | ADA/IAPDSG | 574 | 287 | 50 |
| Abedi P et al. (67) | 08/2013–10/2014 | Iran, Ahfav | Four centers from the east and three centers from the west of Ahvaz | CS | Unclear | Pregnant women | All | Medical records | 700 | 43 | 6.1 |
| Pezeshki B et al. (68) | 04/2015–04/2016 | Iran, Zanjan | Seven health care centers affiliated with Zanjan University of Medical Sciences | PC | Whole population | Pregnant women between the ages of 18 and 35 years, gestational age of equal or less than 12 weeks at first visit, a BMI of between 30 and 18.5 kg/m2, and a blood pressure of less than 140/90 mm Hg during first visit, tested for GDM in the first trimester | All | ADA 2016 | 356 | 25 | 7.0 |
| Heydarpour F et al. (69) | 2015–2017 | Iran, four cities were selected from each province | One rural and one urban health clinic were selected in each city | RC | Multistage | Pregnant women with: a hemoglobin level less than 11 g/dL during the first trimester | All | Medical records | 1,038 | 27 | 2.6 |
| a hemoglobin level more than 11 g/dL during the first trimester | 2,463 | 106 | 4.3 | ||||||||
| a hemoglobin level less than 11 g/dL during the third trimester | 756 | 28 | 3.8 | ||||||||
| a hemoglobin level more than 11 g/dL during the third trimester | 1,986 | 68 | 3.4 | ||||||||
| Fazel N et al. (70) | 08/2014–04/2015 | Iran, Sabzevar | From 18 obstetric clinics associated with Mobini Hospital | PC | Cluster random sampling | Pregnant women in gestational week 24 or less | All | Medical records | 1603 | 30 | 1.87 |
| Nouhjah S. et al. (71) | 03/2015–01/2016 | Iran, Ahvaz | 25 urban and public and private prenatal care clinics | PC | Unclear | Pregnant women | All | IADPSG | 800 | 176 | 22.0 |
| Maghbooli Z et al. (72) | 04/2016–03/2017 | Iran, Tehran | Prenatal care clinics in two regions in Tehran, Iran | CC | Unclear | Pregnant women living in nonpolluted areas | All | Unclear | 44 | 3 | 6.8 |
| Salehi-Pourmehr H et al. (73) | 12/2012–01/2016 | Iran, Tabriz | All health centers in Tabriz (65 centers and subcenters) | PC | Unclear | Obese (BMI ≥ 35 kg/m2) pregnant women in the first trimester of pregnancy, aged 18–35 years | All | Self-reported | 62 | 7 | 11.0 |
| Zargar M et al. (74) | 2011–2016 | Iran, Ahvaz | Pregnant women referring to three infertility centers in Ahvaz city | CC | Randomly | All women undergoing ART | All | Unclear | 318 | 33 | 10.4 |
| Mojtahedi SY et al. (75) | 04/2010–05/2016 | Iran, Tehran | Ziaeean and Imam Khomeini hospitals in Tehran | CS | Random | Mothers of neonates (<15 days) with hyperbilirubinemia (> 15 mg/dL) | All | Medical records | 163 | 41 | 25.2 |
| Eslami E et al. (76) | 07/2016–04/2016/ 12/2017–02/2017 | Iran, Tehran | 12 health centers of Tehran | RCTs | Unclear | Singleton pregnant females with BMI greater than 25 aged 18 and older, gestational age of 16–20 weeks | All | Unclear | 70 | 17 | 24.3 |
| Singleton pregnant females with BMI greater than 25, aged 18 and older, gestational age of 16–20 weeks receiving lifestyle training | All | 70 | 15 | 21.4 | |||||||
| Mardani M et al. (77) | 2015–2016 | Iran | Health care centers | CC | Whole population | Pregnant women with severe acute respiratory illness | All | Medical records | 24 | 3 | 12.5 |
| Randomly | Living pregnant women with severe acute respiratory illness | All | 100 | 4 | 4.0 | ||||||
| Basha S et al. (78) | 01/2015–01/2016 | Jordan | Jordan University Hospital | CS | Consecutive | Women with singleton pregnancies tested for GDM at 24–28 weeks of pregnancy | All 15–49 years |
IADPSG | 644 | 87 | 13.5 |
| 15–29 years | 301 | 24 | 8.0 | ||||||||
| 30–39 years | 302 | 50 | 16.5 | ||||||||
| 40–49 years | 41 | 13 | 31.7 | ||||||||
| Abdel Razeq NM et al. (79) | 2012/2013 | Jordan | Nationwide in 18 maternity hospitals | CS | Unclear | All women who gave birth to dead or live neonates at 20 or more weeks of gestation | All | Medical records | 21,075 | 253 | 1.2 |
| Clouse K et al. (80) | 04/2015–05/2015 | Jordan, Amman | Al-Bashir Hospital | CS | Unclear | Pregnant women | All | Medical records and interviews | 200 | 3 | 1.5 |
| Khader YS et al. (81) | 03/2011–04/2012 | Jordan, nationwide | 18 hospitals with maternity departments in three regions of Jordan (South, Middle, and North) | CS | Whole population | Deliveries with a gestational age ≥20 weeks | All | Medical records and interviews | 21,928 | 261 | 1.2 |
| Zein S et al. (82) | 12/2012–11/2013 | Lebanon, Beirut | Bahman hospital | CS | Unclear | Singleton pregnancies, nonanemic, having first prenatal visit before 12 weeks | All | IADPSG | 104 | 16 | 15.4 |
| Ghaddar N et al. (83) | 09/2016–08/2017 | Lebanon, Beirut and South Lebanon | Outpatient clinic of obstetrics and gynecology department of different hospitals and peripheral clinics in Lebanon | CS | Consecutive | Pregnant women, at 35–37 weeks of gestation | All | Self-reported or reported by physician | 107 | 7 | 6.5 |
| Khalil MM and Alzahra E (84) | 1/2009–12/2010 | Libya, Tripoli | Al-Jalaa Maternity Hospital | CS | Consecutive | Pregnant women with singleton pregnancies who completed 28 weeks of gestation excluding stillbirths, neonatal deaths, and infants with congenital anomalies | All | Medical records | 28,140 | 405 | 1.4 |
| Utz B et al. (85) | 12/2016–03/2017 | Morocco, Marrakech-Safi | 10 health centers per district; two districts, Marrakech and Al Haouz | CS | Whole population | Pregnant women attending ANC with GDM screening and management intervention | All | WHO 2013 | 846 | 155 | 18.3 |
| Pregnant women attending ANC with GDM screening and initial management | 1034 | 138 | 13.4 | ||||||||
| Abdwani R et al. (86) | 01/2007–12/2013 | Oman, Seeb | Sultan Qaboos University Hospital | RS | Consecutive | Mothers with systemic lupus erythematosus | All | Medical Records | 56 | 15 | 26.8 |
| Healthy mothers | 91 | 9 | 9.9 | ||||||||
| Al-Hakmani FM et al. (87) | 3/2011–4/2012 | Oman, Seeb | All primary health care centers | PS | Consecutive | Pregnant women without preexisting diabetes or chronic disease tested in their second trimester | All | WHO 1999 | 638 | 100 | 15.7 |
| BMI: 18.5–24.9 kg/m2 | 229 | 27 | 11.8 | ||||||||
| BMI: 25–29.9 kg/m2 | 197 | 35 | 17.8 | ||||||||
| BMI: ≥30 kg/m2 | 212 | 38 | 17.9 | ||||||||
| Abu-Heija AT et al. (88) | 09/15/2013–09/14/2014 | Oman, Muscat | Sultan Qaboos University Hospital | CS | Whole population | Healthy singleton Omani nondiabetic pregnant women attending the antenatal clinic at SQUH were studied | All | Unclear | 306 | 23 | 7.5 |
| BMI: 18–20 kg/m2 | 32 | 1 | 3.1 | ||||||||
| BMI: 21–25 Kg/m2 | 74 | 3 | 4.1 | ||||||||
| BMI: 26–30 kg/m2 | 102 | 8 | 7.8 | ||||||||
| BMI: 31–35 kg/m2 | 47 | 5 | 10.6 | ||||||||
| BMI: >35 kg/m2 | 51 | 6 | 11.8 | ||||||||
| Zutshi A et al. (89) | 11/2011–04/2012 | Oman, Muscat | Royal Hospital in Muscat | RC | Whole population | All pregnant Omani women with available weight/height or BMI data at <12 gestational weeks (obese and normal weight) | All | Medical records | 1813 | 221 | 12.2 |
| Normal weight | 912 | 69 | 7.6 | ||||||||
| Obese | 901 | 152 | 16.9 | ||||||||
| Islam M et al. (90) | 2000–2000 | Oman | National Health household survey | CS | Multistage sampling | 15–49-year-old pregnant women | All | Self–reported | 1,345 | 44 | 3.3 |
| 20–34 years | 1,030 | 30 | 2.9 | ||||||||
| ≥35 years | 315 | 14 | 4.4 | ||||||||
| Al–Kuwari MG et al. (91) | 1/3–30/6/2010 | Qatar | Sixteen primary health care centers that offer ANC care services | CS | Unclear | All pregnant women attending ANC clinics with a mean age of 28.3 years | All | ADA 2003 | 4,295 | 275 | 6.4 |
| <24 years | 1,140 | 27 | 2.4 | ||||||||
| 25–29 years | 1,537 | 89 | 5.8 | ||||||||
| 30–34 years | 1,007 | 70 | 7.0 | ||||||||
| ≥35 years | 611 | 89 | 14.6 | ||||||||
| Bener A et al. (92) | 1/2010–4/2011 | Qatar | Women’s Hospital in Doha | CS | Whole population | All pregnant women who attended the ANC clinics, excluding women with diabetes before pregnancy | All | Unclear | 1,608 | 262 | 16.3 |
| BMI: <25 kg/m2 | 513 | 35 | 6.8 | ||||||||
| BMI: 25–30 kg/m2 | 601 | 72 | 12.0 | ||||||||
| BMI: >30 kg/m2 | 494 | 155 | 31.4 | ||||||||
| Abu Yaacob S et al. (93) | 01/2001–06/2001 | Doha, Qatar | Women’s Hospital | CS | Random | Postnatal women at the Women’s Hospital; multiple pregnancies were not included | All | Medical records | 150 | 35 | 23.3 |
| BMI: >30 kg/m2 | 75 | 26 | 34.7 | ||||||||
| BMI: 20–28 kg/m2 | 75 | 9 | 12.0 | ||||||||
| Bashir M et al. (94) | 03/2015–12/2016 | Qatar, Doha | Women’s Hospital of Hamad Medical Corporation | CS | Whole population | Pregnant women | All | Medical records, FBG at first trimester and OGTT at second trimester according to WHO | 2,221 | 801 | 36.1 |
| Shaukat S and Nur U (95) | 06/01/2016–11/10/2017 | Qatar | Primary Healthcare Corporation Database | RC | Whole population | Nulliparous women with singleton pregnancies who had their first antenatal visit at the Primary Healthcare Corporation | All | Medical records | 1,134 | 407 | 35.9 |
| BMI: <25 Kg/m2 | 404 | 118 | 29.2 | ||||||||
| BMI: 25–29.99 Kg/m2 | 399 | 140 | 35.1 | ||||||||
| BMI: ≥30 kg/m2 | 230 | 108 | 47.00 | ||||||||
| Missing | 101 | 41 | 40.6 | ||||||||
| Soliman A et al. (96) | 01/2017–08/2017 | Qatar, All Qatar | Perinatal registry | CS | Whole population | Women with singleton births and completed record abstraction | All | IADPSG | 12,255 | 3027 | 24.7 |
| ≤19 years | 256 | 35 | 13.7 | ||||||||
| 20–24 years | 2,075 | 332 | 16.0 | ||||||||
| 25–29 years | 4,035 | 909 | 22.5 | ||||||||
| 30–34 years | 3,641 | 964 | 26.7 | ||||||||
| ≥35 years | 2,275 | 787 | 34.6 | ||||||||
| Kurdi AM et al. (97) | 07/01/2010–06/30/2013 | Saudi Arabia, Riyadh | The Prince Sultan Military Medical City (PSMMC) is a tertiary teaching institution | PC | Random | Healthy pregnant women | All | IADPSG | 1262 | 188 | 14.9 |
| Whole population | Pregnant women with congenital anomalies | All | 1179 | 187 | 15.9 | ||||||
| El–Gilany AH and Hammad S (98) | 2007 | Saudi Arabia, Al–Hassa | Primary health care centers | PS | Unclear | Pregnant women initiated into ANC in the first month of pregnancy, excluding any prepregnancy chronic medical disease (e.g., hypertension, diabetes, renal or cardiac disease, and sickle cell disease) and multiple pregnancies | All | Unclear | 787 | 30 | 3.8 |
| BMI: 18.5–24.99 kg/m2 | 307 | 3 | 1.0 | ||||||||
| BMI: <18 kg/m2 | 67 | 0 | 0.0 | ||||||||
| BMI: ≥25–29.99 kg/m2 | 187 | 8 | 4.3 | ||||||||
| BMI: ≥30 kg/m2 | 226 | 19 | 8.4 | ||||||||
| Lasheen AE et al. (99) | 1/2011–11/2011 | Saudi Arabia, Riyadh | Security Forces Hospital | CS | Unclear | Pregnant women | All | Unclear | 601 | 153 | 25.5 |
| Wahabi HA et al. (100) | 2013–2015 | Saudi Arabia, Riyadh | Three hospitals, part of RAHMA study | CS | Random | Saudi mothers | All <20–≥45 years |
WHO 2013 | |||
| 9,723 | 345 | 3.5 | |||||||||
| <20 years | 216 | 38 | 17.6 | ||||||||
| 20–24 years | 1,625 | 271 | 16.7 | ||||||||
| 25–29 years | 2,850 | 596 | 20.9 | ||||||||
| 30–34 years | 2,603 | 688 | 26.4 | ||||||||
| 35–39 years | 1,769 | 537 | 30.4 | ||||||||
| 40–44 years | 601 | 208 | 34.6 | ||||||||
| ≥45 years | 59 | 16 | 27.1 | ||||||||
| Wahabi HA et al. (101) | 1/1/–31/12/2008 | Saudi Arabia, Riyadh | King Khalid University Hospital | RS | Unclear | Women who were admitted to the labor ward in King Khalid University Hospital | All | IADPSG | 3,157 | 569 | 18.0 |
| Wahabi HA et al. (102) | 1/1–31/12/2010 | Saudi Arabia, Riyadh | King Khalid University Hospital | RS | Unclear | Pregnant women with singleton pregnancies at gestational age of at least 24 months excluding women with preexisting diabetes | All | IADPSG | 3,041 | 569 | 18.7 |
| Wahabi HA et al. (103) | 1/7/2011–30/6/2012 | Saudi Arabia, Riyadh | King Khalid University hospital | RS | All subjects during the study period | Women booked for ANC care services who were with singleton pregnancies and with no history of T1DM or T2DM | All | Carpenter and Coustan | 2,701 | 415 | 15.4 |
| Obese | 1,185 | 260 | 21.9 | ||||||||
| Not obese | 1,516 | 155 | 10.2 | ||||||||
| Al-Rowaily MA and Abolfotouh MA (104) | 7/2005–7/2006 | Saudi Arabia, Riyadh | ANC clinic of King Fahd hospital, part of the National Guard Health Affairs services | CS | Consecutive | All pregnant women who had no previous history of diabetes without pregnancy excluding women who suffered an abortion before reaching 24–28 weeks gestation; 50.1% of pregnant women were grand multiparas | All | WHO 1985 | 633 | 79 | 12.5 |
| <20 years | 21 | 0 | 0.0 | ||||||||
| 20–29 years | 180 | 10 | 5.6 | ||||||||
| 30–39 years | 379 | 54 | 14.2 | ||||||||
| ≥40 years | 53 | 15 | 28.3 | ||||||||
| Almarzouki AA (105) | 1/11/2007–30/4/2008 | Saudi Arabia, Makkah | Department of endocrinology, Al-Noor Specialist Hospital | RS | All pregnant women during the study period | All singleton pregnant women excluding pregnant women known to have DM before pregnancy or who have OGTT positive in first trimester of pregnancy with unknown prepregnancy DM status were also excluded | All | O’Sullivan and NDDG | 1,550 | 94 | 6.1 |
| Al–Shaikh G et al. (106) | 2014–2014 | Saudi Arabia, Riyadh | Labour ward of King Khaled University Hospital | CS | Consecutive | 17–47-year-old pregnant women who were admitted for delivery | All | Unclear | 1,000 | 111 | 11.1 |
| Al-Daghri N et al. (107) | Unclear | Saudi Arabia, Riyadh | Patients recruited from homes and invited to visit primary healthcare centers. | CS | Random | 18–45-year-old pregnant women attending clinics | All | WHO 1999 | 2,373 | 33 | 1.4 |
| Wahabi H et al. (108) | 2013–2015 | Saudi Arabia, Riyadh | Large tertiary care public hospitals | CS | Whole population | Women delivered at participating hospitals with a mean age of 29.1 years | <20–≥40 years | WHO 2013 | 9,723 | 2,354 | 24.2 |
| Alfadhli E et al. (109) | 2011–2014 | Saudi Arabia, Medina | Maternity and Children hospital | PC | Consecutive | Singleton Saudi pregnant women without DM and with mean age 30.5 years | All | ADA 2010 | 573 | 93 | 16.2 |
| Al Serehi A et al. (110) | 2011–2013 | Saudi Arabia, Riyadh | Single-center study conducted at King Fahad Medical City | CS | Whole population | Pregnant women with a mean age of 29.9 years; trimester not mentioned | All | Medical records | 1,718 | 238 | 13.8 |
| Al–Rubeaan K et al. (111) | 2007–2009 | Saudi Arabia, Nationwide | SAUDI–DM national level household survey. | CS | Random | Pregnant women in different trimesters, recruited from general population with an age range of 18–49 years | All 18–49 years |
IADPSG criteria | |||
| 549 | 201 | 36.6 | |||||||||
| 18–29 years | 264 | 79 | 29.9 | ||||||||
| 30–39 years | 212 | 85 | 40.1 | ||||||||
| 40–49 years | 73 | 37 | 50.7 | ||||||||
| Gasim T et al. (112) | 2001–2008 | Saudi Arabia | King Fahad Hospital | CC | Matched random sampling | Pregnant women in their second trimester with a mean age of 32.4 years | All | IADPSG | 8,075 | 220 | 2.7 |
| Kurdi MA et al. (113) | 01/2000–12/2001 | Saudi Arabia, Riyadh | Armed Forces Hospital and King Khalid University Hospital | CS | Consecutive | Pregnant women with multiple pregnancies | All | Unclear | 375 | 60 | 16.0 |
| Abdelmola AO et al. (114) | 11/2014 | Saudi Arabia, Jazan | Sabya, Jazan, and Abuarish hospitals | CS | Random | Pregnant women aged 15–49 years in the second and third trimester tested for GDM at 24–28 weeks | 15–20 years | Medical records | 48 | 6 | 12.5 |
| 21–25 years | 145 | 3 | 2.1 | ||||||||
| 26–30 years | 136 | 13 | 9.6 | ||||||||
| 31–35 years | 76 | 10 | 13.2 | ||||||||
| 36–50 years | 35 | 4 | 11.4 | ||||||||
| Al-Shaikh GK et al. (115) | 11/2013- 11/2014 | Saudi Arabia, Riyadh | King Khaled University Hospital | CS | Whole population | Women who had singleton births | All | Medical records | 3,327 | 415 | 12.5 |
| Primipara | 1,889 | 174 | 9.3 | ||||||||
| Multipara | 1,097 | 156 | 14.4 | ||||||||
| Grand multipara | 341 | 85 | 25.2 | ||||||||
| Fayed AA et al. (116) | 11/2013–03/2015 | Saudi Arabia, Riyadh | Multicenter Mother and Child Cohort Study RAHMA, three hospitals in Riyadh | CS | Systematic | RAHMA study recruited more than 14,000 pregnant women and their newborns from three hospitals representing the ministry of health, military and university hospitals; all Saudi women were eligible to participate, and 14,568 consented | All 15–39 years |
WHO 2013 | |||
| 9,022 | 2,124 | 23.5 | |||||||||
| 15–20 years | 181 | 32 | 17.7 | ||||||||
| 20–29 years | 4,469 | 867 | 19.4 | ||||||||
| 30–34 years | 2,606 | 688 | 26.4 | ||||||||
| 35–39 years | 1,766 | 537 | 30.4 | ||||||||
| Subki AH et al. (117) | 01/2015–06/2017 | Saudi Arabia, Jeddah | King Abdulaziz University Hospital, a teaching hospital and tertiary health center located in the city of Jeddah in the western province of Saudi Arabia | CS | Whole population | All patients diagnosed with HDP | All | Medical records | 244 | 59 | 26.3 |
| Primigravida | 97 | 18 | 18.6 | ||||||||
| Multigravida | 127 | 41 | 32.3 | ||||||||
| Al Shanqeeti SA et al. (118) | 01/2016–08/2016 | Saudi Arabia, Riyadh | King Abdulaziz Medical City | CS | Whole population | Pregnant women attending the antenatal clinic at the tertiary hospital as well as those admitted for OB/GYN care and women attending the antenatal clinic at the primary care center were invited to participate in this study | All | Unclear | 384 | 35 | 9.1 |
| Dafa Elseed EB and Khougali HS (119) | 01/01/2016–06/01/2017 | Sudan Omdurman | Outpatient clinical at Omdurman Maternity Hospital, Omdurman, Sudan | CS | Unclear | Women with diabetes aged 18–45 years | All | Self-reported | 119 | 55 | 46.2 |
| Naser W et al. (120) | 01/2015–11/2015 | Sudan, Khartoum | ANC clinic of Saad Abualila Hospital | PC | Whole population | Singleton pregnant, started ANC follow-up in the first trimester (≤14 weeks of gestation) | All | IADPSG and ADA | 126 | 19 | 15.0 |
| Alshareef SA et al. (121) | 07/01/2017–01/31/2018 | Sudan, Khartoum | Saad Abuelela hospital | CS | Unclear | Pregnant women | All | IADPSG | 166 | 20 | 12.0 |
| Mallouli M et al. (122) | 01/01–31/12, 2013 | Tunisia, Sfax | University Hospital, HediChaker | CS | Whole population | Mothers of macrosomic newborn | All | ADA 2015 | 821 | 76 | 9.3 |
| Radwan H et al. (123) | 6/2016 | UAE, Sharjah, Dubai and Ajman | Three main public governmental hospitals and seven rimary health care (PHC) clinics and mother and child centers (MCH) | PC | Convenient | Singleton Arab aged 19–40 years within the third trimester of pregnancy (27–42 weeks of gestation) | All | NICE | 256 | 49 | 19.2 |
| Agarwal MM et al. (124) | 1/1998–12/2002 | UAE, Al Ain | Obstetric clinics at the Al Ain Hospital | RS | Unclear | Pregnant women attending routine obstetric clinics at the Al Ain Hospital with a mean maternal age of 32 years | All | ADA 1997 | 5,347 | 1,641 | 30.7 |
| Agarwal MM et al. (125) | 1/1/2012–31/12/2012 | UAE, Al Ain | Tawam Hospital | CS | Unclear | Pregnant women attending the routine ANC clinics | All | ADA 2003 | 2,337 | 310 | 13.2 |
| Agarwal MM et al. (126) | 2003–2008 | UAE, Al Ain | Antenatal clinics of two tertiary care hospitals | PC | Whole population | Pregnant women attending antenatal clinics | All | ADA 2010 | 10,283 | 1328 | 12.9 |
| Agarwal MM et al. (127) | 1/07/2007–30/06/2008 | UAE, Al Ain | Al Ain Hospital | CS | Unclear | Pregnant women attending routine antenatal clinics tested for GDM at 24–28 weeks’ gestation | All | ADA 2007 | 1,465 | 196 | 13.4 |
| Mirghani MH et al. (128) | 01/2002–05/2004 | UAE, Al Ain | Al-Ain Hospital, Al Ain District | CS | Consecutive | Healthy pregnant women fasting in the month of Ramadan | All | WHO 1999 | 168 | 34 | 20.2 |
| Healthy pregnant women not fasting in the month of Ramadan | 156 | 11 | 7.1 | ||||||||
| Agarwal MM et al. (129) | 1/5/2003–31/7/2003 | UAE, Al Ain | Tawam Hospital, Al Ain | CS | Consecutive | All pregnant women undergoing one-step universal screening protocol for GDM between 24–28 weeks gestation | All | ADA 2004 | 442 | 49 | 11.1 |
| Vaswani PR et al. (130) | 12/2010–10/2011 | UAE, Abu Dhabi | Mafraq hospital | CS | Consecutive | Pregnant women except the ones with multiple pregnancies or BMI less than 18.5 kg/m2 or preexisting hypertension or diabetes | All | Medical records | 1,985 | 171 | 8.6 |
| Overweight | 635 | 36 | 5.6 | ||||||||
| Obese class I | 520 | 53 | 10.1 | ||||||||
| Obese class II | 280 | 42 | 1.0 | ||||||||
| Obese class III | 130 | 23 | 17.6 | ||||||||
| Normal weight | 420 | 17 | 4.0 | ||||||||
| Abdel–Wareth OL et al. (131) | 11/1999–04/2001 | UAE, Abu Dhabi | Mafraq Hospital | CS | Consecutive | Women delivering at Mafraq Hospital during the time period were included; women who could not perform the test due to vomiting were excluded from the study | <25–≥35 years | ADA criteria | 877 | 143 | 16.3 |
| Ali AD. et al. (132) | 08/2013–03/2014 | Yemen, Dhamar | Antenatal care clinics associated with several hospitals | CS | Systematic | Pregnant women visiting antenatal clinics with a mean age of 25.1 years | Obese | ADA criteria | 18 | 3 | 16.7 |
| Others | 293 | 13 | 4.4 | ||||||||
ACOG, American College of Obstetricians and Gynecologists; ADA, American Diabetes Association; ANC, antenatal care; ART, assisted reproductive technology; BMI, body mass index; CC, case control; CS, cross-sectional; DM, diabetes mellitus; FIGO, Federation of Gynecology and Obstetrics; GDM, gestational diabetes mellitus; HCV, hepatitis C virus; HDP, hypertension disorder in pregnancy; IADPSG, International Association of Diabetes and Pregnancy Study Groups; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; NDDG, National Diabetes Data Group; OGTT, oral glucose tolerance test; PC, prospective cohort; PCOS, polycystic ovary syndrome; PS, prospective; PTB, preterm birth; RC, retrospective cohort; RS, retrospective; SC, spontaneous conception; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; WHO, World Health Organization.
Crude GDM Prevalence
The 102 research reports (31–67, 69–132) yielded 198 GDM prevalence studies. Iran (32.3%) (41, 43–67, 69–77) and Saudi Arabia (24.2%) (97–118) contributed to most of the prevalence studies, followed by Qatar (9.7%). In these prevalence studies, a total of 279,202 pregnant women were tested for GDM between 2000 and 2019, and the crude GDM prevalence was estimated to be about 11.0%. The prevalence of GDM ranged from 0.0% in three studies (60, 98, 104) to 50.7% in pregnant women aged 40–49 years in Saudi Arabia tested between 2007 and 2009 (111). The GDM prevalence range was identical in studies reported in the two decades (Tables 2 and 3).
Table 3.
Weighted national prevalence of GDM in pregnant women in 16 MENA countries by study period and overall.
| Country/study period | No. of studies | Tested sample | GDM | GDM prevalence | Heterogeneity measures | p–value4 (fixed model) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Range (%) | Median (%) | Weighted prevalence % | 95% CI | Q (p–value)1 | I2 (%)2 | 95% prediction interval (%)3 | |||||
| Algeria | — | ||||||||||
| 2010–2019 | 1 | 200 | 6 | — | — | 3.0 | 1.4–6.4 | — | — | — | |
| Bahrain | <0.001 (<0.001) | ||||||||||
| 2000–2009 | 2 | 10,495 | 1,394 | 7.5–15.5 | 11.5 | 13.0 | 12.4–13.7 | — | — | — | |
| 2010–2019 | 9 | 49,552 | 4,982 | 6.9–13.3 | 9.5 | 9.7 | 8.1–11.6 | 352.4 (p<0.001) | 97.7 | 4.2 – 17.2 | |
| Overall | 11 | 60,047 | 6,376 | 6.9–15.5 | 9.5 | 10.0 | 8.3–11.9 | 572.3 (p<0.001) | 98.3 | 4.0–18.3 | |
| Egypt | 0.21 (0.002) | ||||||||||
| 2010–2019 | 4 | 1,239 | 184 | 5.9–26.3 | 11.2 | 13.5 | 6.2–21.8 | 49.9 (p<0.001) | 94.0 | 0.0–63.8 | |
| Overlapping | 2 | 308 | 24 | 7.6–7.9 | 7.8 | 7.8 | 5.0–11.1 | — | — | — | |
| Overall | 6 | 1,547 | 208 | 5.9–26.3 | 9.0 | 11.2 | 6.2–17.4 | 59.7 (p<0.001) | 91.6 | 0.0–37.7 | |
| Iran | 0.07 (<0.001) | ||||||||||
| 2000–2009 | 16 | 7,343 | 492 | 2.2–24.4 | 7.4 | 8.2 | 5.9–11.0 | 215.3 (p<0.001) | 93.0 | 0.8–21.9 | |
| 2010–2019 | 39 | 21,028 | 2,235 | 0.0–50.0 | 9.2 | 12.3 | 9.0–16.0 | 2,135 (p<0.001) | 98.2 | 0.0–41.0 | |
| Overlapping | 9 | 1,388 | 166 | 5.9–28.6 | 13.5 | 13.5 | 8.2–19.7 | 67.8 (p<0.001) | 88.2 | 0.3–38.4 | |
| Overall | 64 | 29,759 | 2,893 | 0.0–50.0 | 8.8 | 11.4 | 9.2–13.9 | 2,491 (p<0.001) | 97.5 | 0.1–35.8 | |
| Iraq | — | ||||||||||
| 2010–2019 | 4 | 383 | 35 | 2.6–24.5 | 14.2 | 11.5 | 3.3–23.3 | 24.5 (p<0.001) | 87.8 | 0.0–76.6 | |
| Jordan | — | ||||||||||
| 2010–2019 | 6 | 43,847 | 604 | 1.2–31.7 | 4.7 | 4.7 | 3.0–6.7 | 193.7 (p<0.001) | 97.4 | 0.4–12.5 | |
| Lebanon | — | ||||||||||
| 2010–2019 | 2 | 211 | 23 | 6.5–15.4 | 11.0 | 10.5 | 6.7–15.1 | — | — | — | |
| Libya | — | ||||||||||
| Overlapping | 1 | 28,140 | 405 | – | – | 1.4 | 1.3–1.6 | — | — | — | |
| Morocco | — | ||||||||||
| 2010–2019 | 2 | 1,880 | 393 | 13.3–18.3 | 15.8 | 15.5 | 13.9–17.2 | — | — | — | |
| Oman | <0.001 (<0.001) | ||||||||||
| 2000–2009 | 2 | 1,345 | 44 | 2.9–4.4 | 3.7 | 3.2 | 2.3–4.2 | — | — | — | |
| 2010–2019 | 10 | 2,757 | 344 | 3.1–17.9 | 11.2 | 11 | 8.0–15.0 | 59.2 (p<0.001) | 84.8 | 1.9–25.8 | |
| Overlapping | 2 | 147 | 24 | 9.9–26.8 | 18.3 | 15.5 | 10–21.9 | – | – | – | |
| Overall | 14 | 4,249 | 412 | 2.9–26.8 | 10.3 | 10.1 | 6.5–14.3 | 184.5 (p<0.001) | 93.0 | 0.2–29.7 | |
| Qatar | 0.65 (0.59) | ||||||||||
| 2000–2009 | 2 | 150 | 35 | 12.0–34.7 | 23.3 | 22.3 | 15.9–29.4 | – | – | – | |
| 2010–2019 | 17 | 21,513 | 4,772 | 2.4–47.0 | 22.5 | 20.5 | 14.8–26.9 | 1,869.0 (p<0.001) | 99.1 | 1.6–52.6 | |
| Overall | 19 | 21,663 | 4,807 | 2.4–47.0 | 22.5 | 20.7 | 15.2–26.7 | 1,880.3 (p<0.001) | 99.0 | 1.7–52.4 | |
| Saudi Arabia | 0.02 (<0.001) | ||||||||||
| 2000–2009 | 16 | 17,499 | 1,286 | 0.0–50.7 | 7.2 | 10.8 | 6.2–16.5 | 1,330.5 (p<0.001) | 98.9 | 0.0–41.1 | |
| 2010–2019 | 32 | 44,918 | 9,331 | 2.1–34.6 | 17.6 | 18.2 | 15.9–20.6 | 1,116.5 (p<0.001) | 97.2 | 7.1–32.9 | |
| Overall | 48 | 62,417 | 10,617 | 0.0–50.7 | 16.1 | 15.5 | 12.6–18.8 | 4,989.3 (p<0.001) | 99.1 | 1.0–41.9 | |
| Sudan | — | ||||||||||
| 2010–2019 | 3 | 411 | 94 | 12.0–46.2 | 15.1 | 23.0 | 3.3–45.2 | 47.2 (p<0.001) | 95.8 | — | |
| Tunisia | — | ||||||||||
| 2010–2019 | 1 | 821 | 76 | — | — | 9.3 | 7.5–11.4 | — | — | — | |
| United Arab Emirates | 0.3 (<0.001) | ||||||||||
| 2000–2009 | 7 | 18,738 | 3,402 | 7.1–30.7 | 13.4 | 15.5 | 9.2–23.0 | 736.7 (p<0.001) | 99.2 | 0.2–46.9 | |
| 2010–2019 | 7 | 4,578 | 530 | 4.0–19.1 | 13.3 | 11.3 | 7.6–15.69 | 87.8 (p<0.001) | 93.2 | 1.3–28.8 | |
| Overall | 14 | 23,316 | 3,932 | 4.0–30.7 | 13.3 | 13.4 | 9.4–18.0 | 945.1 (p<0.001) | 98.6 | 1.1–35.6 | |
| Yemen | |||||||||||
| 2010–2019 | 2 | 311 | 16 | — | — | — | — | — | — | — | — |
| Overall5 | 198 | 279,202 | 30,797 | 0.0–50.7 | 12.3 | 13.0 | 11.5–14.6 | 28,154 (p<0.001) | 99.3 | 0.1–40.6 | — |
CI, confidence interval calculated using the exact binomial method; GDM, gestational diabetes mellitus; MENA, Middle East and North Africa.
1Q: Cochran’s Q statistic is a measure assessing the existence of heterogeneity in estimates of GDM prevalence.
2I2 is a measure assessing the percentage of between-study variation due to differences in GDM prevalence estimates across studies rather than chance.
3Prediction intervals estimate the 95% confidence interval in which the true GDM prevalence estimate in a new study is expected to fall.
4Heterogeneity between subgroups using random-effects model (fixed-effect model).
5Overall pooled estimates in the 16 countries regardless of the tested population, sample size, and data collection period, using the most updated criteria when GDM is ascertained using different criteria in the same population.
Regional and National Pooled GDM Prevalence
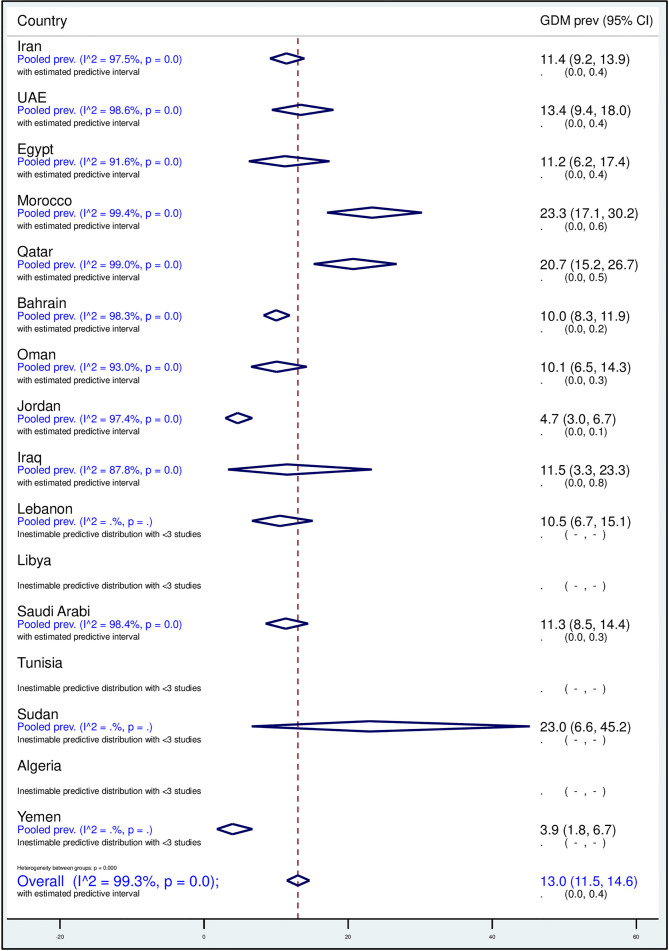
The overall pooled weighted GDM prevalence in the MENA region was 13.0% (95% CI, 11.5–14.6%, I2, 99.3%; Table 3; Figure 2). The highest GDM prevalence was observed in Qatar (20.7%, 95% CI, 15.2–26.7%; 19 studies), followed by 15.5% in Saudi Arabia (95% CI, 12.6–18.8%; 48 studies) and 13.4% in the UAE (95% CI, 9.4–18.0%; 14 studies; Table 3). The lowest pooled GDM prevalence was 4.7% in Jordan (95% CI, 3.0–6.7%; six studies) reported between 2010 and 2019. In the studies conducted between 2000 and 2009, the prevalence estimates ranged from 3.2% in Oman (95% CI, 2.3–4.2%) to 22.3% in Qatar (95% CI, 15.9–29.4%), and in the studies conducted between 2010 and 2019, it ranged from 3.0% in Algeria (95% CI, 1.4–6.4%) to 23.0% in Sudan (95% CI, 3.3–45.2%; Table 3).
Figure 2.
Forest plot of the meta-analyses of the studies on GDM from 16 MENA countries.
For the six countries reporting data on both decades, the overall GDM prevalence was estimated separately for each decade. There was a rise in the prevalence of GDM by 4% to 8% in Iran, Oman, and Saudi Arabia and a decrease of 2% to 4% in Bahrain, Qatar, and the UAE from 2000–2009 to 2010–2019 periods. The largest increase in prevalence occurred in Oman: from 3.2% in 2000 (95% CI, 2.3–4.2%) to 11.0% in 2019 (95% CI, 8.0–15.0%, I2, 84.2%). An appreciable reduction in the prevalence of GDM was observed in the UAE: from 15.5% in 2000 (95% CI, 9.2–23.0%, I2, 99.2%) to 11.3% in 2019 (95% CI, 7.6–15.69, I2, 93.2%; Tables 2 and 3).
Subgroup Pooled GDM Prevalence
The prevalence of GDM in pregnant women aged ≥30 years was 2.26 times higher (21.9%, 95% CI, 18.5–25.5%, I2, 97.1%) than that estimated in younger (15–29 years) pregnant women (9.7%, 95% CI, 6.7–13.2%, I2, 98.0%). A trend was observed between GDM and pregnancy trimester. The weighted GDM prevalence increased by 45.0%, from 8.9% in the first trimester to 12.9% in the second trimester, and by 55.0% in the third trimester (20.0%, 95% CI, 13.1–27.9%, I2, 98.8%) compared with the second trimester. It was also noticeable that, as the BMI increased, the prevalence of GDM increased by 54% in overweight (12.0%, 95% CI, 5.7–20.1%, I2, 96.7) and by 120% in obese (17.2%, 95% CI, 12.8–22.0%, I2, 93.8%) compared with normal-weight pregnant women (7.8%, 95% CI, 4.1–12.4%, I2, 95.0%). No GDM cases were reported in two studies that included underweight women (Table 4).
Table 4.
Subgroup weighted prevalence of GDM in pregnant women in 16 MENA countries by age, pregnancy trimester, body mass index, study period, ascertainment methodology, tested sample, C-section, and maternal mortality ratio.
| No. of studies | Tested sample | GDM | GDM prevalence | Heterogeneity measures | p–value4(fixed model) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Range (%) | Median (%) | Weighted prevalence % | 95% CI | Q (p–value)1 | I2(%)2 | 95% prediction interval (%)3 | |||||
| Age | <0.001 (<0.001) | ||||||||||
| 15–29 years | 24 | 19,187 | 2,883 | 0.0–29.9 | 10.8 | 9.7 | 6.7–13.2 | 1,140.7 (p<0.001) | 98.0 | 0.0–31.4 | |
| ≥30 years | 26 | 22,186 | 5,617 | 4.1–50.7 | 25.4 | 21.9 | 18.5–25.5 | 868.6 (p<0.001) | 97.1 | 7.0–42.0 | |
| Unclear age | 148 | 237,518 | 22,281 | 0.0–50.0 | 11.2 | 12.3 | 10.6–14.0 | 20,967.2 (p<0.001) | 99.3 | 0.1–37.6 | |
| Trimester | 0.06 (<0.001) | ||||||||||
| First | 11 | 5,807 | 387 | 2.2–37.2 | 7.6 | 8.9 | 5.3–13.3 | 272.5 (p<0.001) | 96.3 | 0.0–29.7 | |
| Second | 85 | 134,792 | 14,378 | 0.0–50.0 | 12.0 | 12.9 | 10.9–15.0 | 9,687.2 (p<0.001) | 99.1 | 0.6–36.3 | |
| Third | 18 | 14,146 | 1,354 | 2.7–50.7 | 18.5 | 20.0 | 13.1–27.9 | 1,428.2 (p<0.001) | 98.8 | 0.0–60.6 | |
| Not reported | 84 | 124,457 | 14,678 | 0.0–47.0 | 12.5 | 12.5 | 9.8–15.5 | 16,618.8 (p<0.001) | 99.5 | 0.0–46.1 | |
| BMI | <0.001 (<0.001) | ||||||||||
| Underweight | 2 | 94 | 0 | 0 | 0 | 0 | — | — | — | — | |
| Normal weight | 11 | 3,822 | 335 | 1.0–29.2 | 6.0 | 7.8 | 4.1–12.4 | 200.8 (p<0.001) | 95.0 | 0.0–29.5 | |
| Overweight | 7 | 2,502 | 334 | 4.3–35.1 | 9.2 | 12.0 | 5.7–20.1 | 182.2 (p<0.001) | 96.7 | 0.0–47.5 | |
| Obese | 17 | 4,8459 | 941 | 7.6–47.0 | 15.8 | 17.2 | 12.8–22.0 | 241.5 (p<0.001) | 93.8 | 2.6–40.2 | |
| Unclear | 161 | 267,6925 | 29,187 | 0.0–50.7 | 12.8 | 13.4 | 11.7–15.2 | 27,066.0 (p<0.001) | 99.4 | 0.1–41.2 | |
| Study period | 0.14 (<0.001) | ||||||||||
| 2000–2009 | 45 | 55,570 | 6,653 | 0.0–50.7 | 11.1 | 10.6 | 8.1–13.4 | 4,118.0 (p<0.001) | 98.9 | 0.0–34.2 | |
| 2010–2019 | 139 | 193,3649 | 23,527 | 0.0–50.0 | 12.7 | 14.0 | 12.1–16.0 | 19,613.9 (p<0.001) | 99.3 | 0.2–42.2 | |
| Overlapping | 14 | 29,983 | 619 | 1.4–28.6 | 9.1 | 12.0 | 6.5–18.7 | 414.1 (p<0.001) | 96.9 | 0.0–45.3 | |
| GDM ascertainment 5 | <0.001 (<0.001) | ||||||||||
| WHO guidelines | |||||||||||
| WHO 1985 | 4 | 633 | 79 | 0.0–28.3 | 9.9 | 10.4 | 3.2–20.5 | 25.4 (p<0.001) | 88.2 | 0.0–67.5 | |
| WHO 1999 | 6 | 3,335 | 178 | 1.4–20.2 | 14.8 | 11.4 | 3.6–22.8 | 228.9 (p<0.001) | 97.8 | 0.0–62.4 | |
| WHO 2013 | 14 | 30,348 | 7,125 | 13.3–34.6 | 22.6 | 22.8 | 20.2–25.5 | 344.5 (p<0.001) | 96.2 | 13.0–34.5 | |
| WHO year not mentioned | 1 | 2,221 | 801 | — | — | 36.1 | 34.1–38.1 | — | — | — | |
| ADA guidelines | — | — | — | — | — | ||||||
| ADA 1997 | 1 | 5,347 | 1,641 | — | — | 30.7 | 29.5–31.9 | — | — | — | |
| ADA 2002–2010 | 16 | 19,604 | 2,269 | 2.4–37.2 | 12.0 | 11.7 | 9.0–14.7 | 364.6 (p<0.001) | 96.4 | 2.6–25.9 | |
| ADA 2011–2013 | 4 | 3,180 | 605 | 13.4–30.5 | 24.9 | 22.7 | 15.4–30.9 | 67.5 (p<0.001) | 95.6 | 0.2–65.0 | |
| ADA 2015–2016 | 4 | 3,229 | 243 | 6.9–9.3 | 7.0 | 7.5 | 6.4–8.7 | 4.435 (p=0.218) | 32.4 | 4.2–11.7 | |
| ADA year not mentioned | 1 | 877 | 143 | — | — | 16.3 | 14.0–18.9 | — | — | — | |
| ADA/IADPSG | 2 | 700 | 306 | 15.1–50.0 | 32.5 | 43.1 | 39.4–46.8 | — | — | — | |
| Self-reported | 6 | 1,833 | 119 | 2.9–46.2 | 5.5 | 9.6 | 2.7–19.8 | 148.2 (p<0.001) | 96.6 | 0.0–56.2 | |
| Medical records | 45 | 70,833 | 2,803 | 0.6–47.0 | 11.4 | 11.5 | 9.1–14.2 | 3,588.1 (p<0.001) | 98.8 | 0.4–33.1 | |
| Unclear | 36 | 31,541 | 1,319 | 0.0–31.4 | 8.4 | 9.3 | 6.2–12.9 | 1,770.5 (p<0.001) | 98.0 | 0.0–36.9 | |
| IADPSG | 23 | 32,911 | 5,577 | 2.7–50.7 | 18.0 | 20.9 | 15.6–26.6 | 3,071.8 (p<0.001) | 99.3 | 1.5–53.5 | |
| Carpenter and Coustan | 13 | 8,468 | 755 | 2.2–24.4 | 8.2 | 8.8 | 5.6–12.7 | 356.1 (p<0.001) | 96.6 | 0.1–27.4 | |
| NDDG | 10 | 51,102 | 5,076 | 6.1–13.3 | 8.7 | 9.4 | 7.8–11.1 | 382.7 (p<0.001) | 97.6 | 4.0–16.7 | |
| Fourth International Workshop–Conference | 2 | 10,495 | 1,394 | 7.5–15.5 | 11.5 | 13.0 | 12.4–13.7 | — | — | — | |
| Fifth International Workshop–Conference | 5 | 1,010 | 215 | 7.3–44.4 | 7.9 | 17.4 | 5.6–33.9 | 149.9 (p<0.001) | 97.3 | 0.0–85.6 | |
| ACOG | 4 | 1,279 | 100 | 0.0–16.7 | 7.6 | 7.7 | 3.7–12.9 | 18.11 (p<0.001) | 83.4 | 0.0–36.8 | |
| NICE | 1 | 256 | 49 | — | — | 19.1 | 14.8–24.4 | — | — | — | |
| Sample size | 0.25 (<0.001) | ||||||||||
| <100 | 32 | 1,779 | 300 | 0.0–50.7 | 12.8 | 14.8 | 10.7–19.5 | 198.8 (p<0.001) | 84.4 | 0.0–44.3 | |
| ≥100 | 166 | 277,423 | 30,497 | 0.6–50.0 | 12.0 | 12.8 | 11.2–14.5 | 27,873.7 (p<0.001) | 99.4 | 0.1–40.1 | |
| C-section rate | <0.001 (<0.001) | ||||||||||
| <15% | 7 | 10,206 | 481 | 2.7–46.2 | 12.0 | 11.5 | 5.6–19.0 | 285.6 (p<0.001) | 97.9 | 0.0–44.2 | |
| 15–29% | 118 | 235,106 | 27,222 | 0.0–50.7 | 13.5 | 14.4 | 12.3–16.6 | 24,307.1 (p<0.001) | 99.5 | 0.2–43.3 | |
| >30% | 69 | 29,101 | 3,010 | 0.0–50.0 | 9.2 | 11.6 | 9.4–14.1 | 2,461.8 (p<0.001) | 97.2 | 0.1–36.1 | |
| Unclear | 4 | 4,789 | 147 | 1.4–15.0 | 3.9 | 4.8 | 1.8–9.0 | 89.2 (p<0.001) | 96.6 | 0.0–34.3 | |
| Maternal mortality ratio | <0.001 (<0.001) | ||||||||||
| ≤100/100,000 | 188 | 273,491 | 30,534 | 0.0–50.7 | 12.5 | 13.2 | 11.6–14.9 | 27,551.7 (p<0.001) | 99.3 | 0.1–40.8 | |
| >100/100,000 | 6 | 922 | 1116 | 3.0–46.2 | 13.6 | 16.5 | 3.4–36.3 | 97.1 (p<0.001) | 96.9 | 0.0–100.0 | |
| Unclear | 4 | 4,789 | 147 | 1.4–15.0 | 3.9 | 4.8 | 1.8–9.0 | 89.2 (p<0.001) | 96.6 | 0.0–34.3 | |
| Overall6 | 198 | 279,202 | 30,797 | 0.0–50.7 | 12.3 | 13.0 | 11.5–14.6 | 28154 (p<0.001) | 99.3 | 0.1–40.6 | — |
CI, confidence interval calculated using the exact binomial method; ACOG, American College of Obstetricians and Gynecologists; ADA, American Diabetes Association; GDM, gestational diabetes mellitus; IADPSG, International Association of Diabetes and Pregnancy Study Groups; NDDG, National Diabetes Data Group; NICE, National Institute for Health and Care Excellence; WHO: World Health Organization.
1Q: Cochran’s Q statistic is a measure assessing the existence of heterogeneity in estimates of GDM prevalence.
2I2 is a measure assessing the percentage of between-study variation due to differences in GDM prevalence estimates across studies rather than chance.
3Prediction intervals estimate the 95% confidence interval in which the true GDM prevalence estimate in a new study is expected to fall.
4Heterogeneity between subgroups using random-effects model (fixed-effect model).
5Regardless of the year of the guidelines for the most updated criteria when GDM was ascertained, based on different criteria in the same population.
6Overall pooled estimates in the 16 countries regardless of the tested population, sample size, and data collection period, using the most updated criteria when GDM was ascertained using different criteria in the same population.
From the 137 studies conducted between 2010 and 2019, the pooled GDM prevalence (14.0%, 95% CI, 12.1–16.0%) was 32.0% higher than that reported in the 45 studies conducted in the previous decade (2000–2009; 10.6%, 95% CI, 8.1–13.4%). The pooled GDM prevalence was relatively higher in 32 studies with a sample size of <100 pregnant women (14.8%, 95% CI, 10.7–19.5%) compared with that in 164 studies with a sample size of ≥100 pregnant women (12.8%, 95% CI, 11.2–14.8%; Table 4).
The prevalence of GDM was 25.2% higher in countries with a C-section rate of 15–29% (weighted estimate of 14.4%, 95% CI, 12.3–16.6%, I2, 99.5%) than countries with a C-section rate of <15% (weighted estimate of 11.5%, 95% CI, 5.36–19.0%, I2, 97.9%; Table 4). In addition, in four studies in countries with high MMR (i.e., >100 per 100,000 live births), the prevalence of GDM was 25.0% higher than in countries with MMR ≤100 per 100,000 live births (weighted estimates of 16.5%, 95% CI, 3.4–36.3%, and 14.4%, 95% CI, 12.3–16.6%, respectively; Table 4).
Subregional Specific Pooled GDM Prevalence
In Sudan, one of the North African countries with a C-section rate of 15–29%, a lower GDM prevalence (weighted prevalence of 7.9%) was observed compared with countries with a C-section rate of <15% (weighted prevalence of 23.0%). In North African countries with an MMR of >100/100,000 live births, the prevalence of GDM was 32.0% higher than in countries with an MMR of ≤100/100,000 live births (Supplementary File 2).
The highest weighted GDM prevalence was in the GCC countries (14.7%, 95% CI, 13.0–16.5%, I2, 99.0%), followed by North African countries (13.5%, 95% CI, 7.4–20.9%, I2, 98.9%) and Iran/Iraq 11.2% (95% CI, 9.0–13.5%, I2, 97.4%), whereas the lowest prevalence was estimated in the Levant region countries (5.8%, 95% CI, 3.9–7.9%, I2, 97.1%; Supplementary File 3).
In GCC countries, the prevalence of GDM rose from 11.9% to 15.9% over the two successive decades. Overweight (12.5%) and obese (18.5%) pregnant women and pregnant women with a C-section rate of 15–29% (15.5%) were burdened with high GDM prevalence (Supplementary File 3). In these countries, pregnant women aged ≥30 years were burdened with higher GDM prevalence than the other subregions. As compared with the first decade, the weighted GDM prevalence in the subsequent decade increased by almost 4% in Iraq.
Tables 2–4 in the appendix provide additional weighted GDM prevalence estimates in each subregion according to different measured characteristics (Supplementary Files 2 –5).
Predictors of Heterogeneity in GDM
In the univariate meta-regression models, country, age, pregnancy trimester, BMI, and sample size were associated with variability in the prevalence of GDM at p<0.1. In the multivariate meta-regression model, only pregnancy trimester was retained, with no significant association with the prevalence of GDM at p<0.05. Compared with Saudi Arabia, the adjusted GDM prevalence was 135% (adjusted odds ratio [aOR], 2.35, 95% CI, 1.39–3.95) and 122% (aOR, 2.22, 95% CI, 1.30–3.76) higher in Qatar and Morocco, respectively, but lower in Libya (aOR, 0.09, 95% CI, 0.02–0.52) and Jordan (aOR, 0.38, 95% CI, 0.18–0.80). Pregnant women aged ≥30 years had a 152% higher prevalence of GDM (aOR, 2.52, 95% CI, 1.51–4.21) relative to younger pregnant women. Obese pregnant women were burdened with a 192% higher prevalence of GDM relative to normal-weight pregnant women (aOR, 2.92, 95% CI, 1.50–5.69; Supplementary File 6).
Publication Bias in GDM Prevalence
Both the visual (funnel plot asymmetry) and statistical assessment (Egger’s test, p<0.001) of publication bias suggested the role of a small-study effect (Supplementary File 7).
Quality Assessment of the GDM Research Reports
Supplementary Figure 2 presents the findings of the research report-specific quality assessment for relevant GDM prevalence studies. In all 102 research reports, the research question(s) and/or objective(s) were clearly stated, and the study population group was clearly specified and defined. Half of the research reports (49.5%) did not provide information on the sample size calculation or justification. Most (79.2%) of the research reports used biological assays or extracted data from medical records to ascertain GDM, whereas the GDM status was self-reported in only five reports. In more than half (58.4%) of the 102 research reports, the tested sample size was at least 100 pregnant women. Overall, the research reports were judged to be of potentially low RoB, with an average of seven of the nine measured assessment items. Four (4.0%) of the reports (70, 85, 105, 120) were of low RoB in all of the assessed RoB items (Supplementary File 8).
Discussion
Main Findings
A total of 102 eligible research reports comprising 198 GDM prevalence studies were reported in 16 countries in the MENA region between 2000 and 2019. Most of these reports (58.41%) were from Iran and Saudi Arabia. The pooled prevalence of GDM in the 16 MENA countries was appreciably high (13.0%, 95% CI, 11.5–14.6%, I2, 99.3%), particularly in the GCC and North African countries. The prevalence of GDM increased with maternal age, gestational age, and BMI. It was also high in countries with a C-section rate of 15–29% and an MMR of >100/100,000 live births.
The pooled GDM prevalence (13.0%) was alarmingly higher than that of European countries (2–6%) (133) but was similar to the sub-Saharan Africa region (14.0%). In contrast to the pooled prevalence estimates of Asia (11.5%) (134), the prevalence estimated in the present meta-analysis was slightly higher. The Asian meta-analysis included prevalence estimates from Saudi Arabia, Iran, and Qatar, and when compared with our estimates, they were 3.5% and 7.4% lower for Iran and Saudi Arabia, respectively, and 7.4% higher for Qatar (134). Such variations might be due to the differences in the literature search dates and languages, eligible sample size, GDM ascertainment criteria, and differences in the type of observational studies used for the prevalence estimation.
Our overall weighted GDM prevalence estimate depicted substantial heterogeneity (I2, 99.3%). This could be attributable to the less restrictive inclusion criteria in this review. In addition, the prevalence estimates of GDM can significantly differ with the variation in the GDM diagnostic criteria (135, 136). We noted clinical inconsistency in GDM diagnostic criteria used in the prevalence studies we reviewed (Table 4). This corresponds to the common use of existing nonuniform GDM diagnostic criteria in different countries (12, 134). Given the importance of the prevalence of GDM in meaningful intervention development, its estimation can be affected by the inclusion of studies that use different GDM diagnosing criteria (137, 138). The prevalence of GDM estimated based on the IADPSG criteria is usually high due to the low threshold for fasting blood glucose level relevant to other criteria. In our study, more than 25% of the studies used IADPSG criteria. To obtain homogenous and comparable prevalence estimates and to avoid confusion in practices of screening, diagnosis, and follow-up of GDM, health authorities should consider implementing uniform GDM diagnostic criteria nationally and across the MENA region.
The GDM prevalence estimates in our analysis suggested an increasing trend, parallel to the increase in BMI, correlating with the known fact that overweight and obesity are risk factors of GDM (139, 140). Although this does not prove a causal link between these parameters, it inevitably might significantly reflect the impact of the high burden of overweight and obesity in several countries in the MENA region, such as Egypt and the six GCC countries (141). This highlights the importance of investigating dietitians’ role in ensuring the appropriate caloric intake of GDM patients based on their BMI as per the recommendations of the ADA (142) and promoting exercise, especially among those with increased BMI (143).
GDM can have devastating maternal and birth consequences. Mothers with GDM are at higher risk of developing T2DM, dying, and undergoing C-section (23, 24, 144). Children born to mothers with untreated GDM face an increased risk of neonatal death and long-term disability (145, 146). Notably, diabetes in pregnancy is a neglected cause of maternal mortality globally, affecting one of every sixth pregnancy in the world, and some of the known GDM morbidities that may cause maternal death are postpartum hemorrhage, obstructed labor, and preeclampsia (147). In our analysis, although the prevalence of GDM was higher (16.35%) in countries with high MMR (>100/100,000 live births), it was also substantial in countries with lower MMR (≤100/100,000 live births). Although this does not prove temporality, it highlights the importance of researching complications of GDM (if any) leading to maternal deaths, to help healthcare providers in the MENA region establish protocols to prevent these anticipated adversities. GCC countries with the highest GDM prevalence, as presented in this study, are also burdened with high T2DM (148). There is no doubt that controlling GDM would have multiple benefits in avoiding unfavorable health consequences for both mothers and their babies.
Strengths, Implications, and Limitations
The strengths of our review included its comprehensive characterization of the burden of GDM among pregnant women in several MENA countries. The review provides several weighted estimates in different population groups of the pregnant women at national, subregional, and regional levels that could be used, in addition to future work, to guide the planning, implementation, and evaluation of programs to prevent and control GDM. The overall and national-based pooled prevalence estimates might help policy makers of the respective MENA countries to contrast and quantify the local burden of GDM and introduce better policy initiatives regarding the flow of resources and funds for GDM care and management. Moreover, the finding of higher GDM prevalence corresponding to higher BMI categories might help in developing BMI-specific dietary and exercise guidelines. Furthermore, health authorities and organizations in the region are encouraged to review and consider standardizing the GDM diagnostic criteria at least at the national levels to improve the measurability and comparability of GDM rates and burden across the country and over time. Since we found a wide range of GDM diagnostic criteria used in the MENA region, health organizations across this region might consider moving toward the use of uniform GDM diagnostic criteria to produce better comparable statistical estimates in the future. For instance, in the UAE, different hospitals within the country use different GDM screening and ascertainment criteria (12). Having different GDM diagnostic criteria will preclude understanding the exact burden of the GDM.
Limitations included that our review did not provide any prevalence estimate for about 29% of the MENA region countries, as no prevalence data were available. This might have compromised the comprehensiveness of our prevalence estimates at the regional level. Since we believe that this study is the first to determine the prevalence of GDM in the MENA region, a comparison with previous similar estimates was not possible. This study offers scarce help regarding the prevalence of GDM with its associated comorbidities, such as gestational hypertension, preterm birth, and traumatic vaginal delivery (149), and separate review articles are warranted. The prevalence of GDM can also vary depending on several sociodemographic and maternal characteristics as well as within [urban or rural setting (150, 151)] and between countries and regions; however, our study does not provide such distinction on the prevalence data. In some of the reviewed studies, detailed information on the methodology and GDM measurement procedures was missing, and this limits the category-based generalizability of the measured pooled GDM prevalence. For instance, the 3.35-times increase in the prevalence of GDM in studies reported before 2009 compared with studies reported after 2009 should be cautiously interpreted, as there was an overlap in the time period in 14 studies that tested 29,983 women. The various thresholds for fasting blood glucose level to diagnose GDM, applied on the several criteria considered from the studies, might suggest a bias in the estimated GDM prevalence. Unless estimated by rigorous comparable survey and testing methodology in individual population-based studies, the burden of GDM at the country, subregional, or regional level should not be interpreted as the burden of the measured outcomes at the population level. Moroever, this review did not explore the associations between various maternal and neaonatal characterstics and GDM. Therefore, future systematic reviews and meta-analyses studies focusing on the burden of GDM according to different maternal and neonatal characteristics as well as on the strength of association between various maternal characteristics and GDM are warranted.
Conclusions
Pregnant women in the MENA region are burdened with a relatively high GDM prevalence. Particularly, in the GCC and North African countries, the observed high burden of GDM may be mainly driven by the high prevalence of several risk factors for DM including overweight and obesity, parity, and late maternal age. To avoid maternal and newborn consequences, vigilant risk factor prevention programs and screening and management programs are necessary in the context of GDM. Moreover, unifying the GDM screening and diagnostic criteria, at least at the country level, is warranted to understand the precise burden of GDM. In countries that lack GDM burden data, high-quality research and surveillance programs are also warranted.
Data Availability Statement
The data sets used and/or analyzed in the current study and the supplementary information files are available from the corresponding author on reasonable request.
Author Contributions
Conceptualization, RHA. Methodology, RHA, NMA and MSP. Software, RHA. Validation, RHA. Formal analysis, RHA, NMA, and LAA. Resources, RHA. Writing—original draft preparation, SS and MSP. Writing—review and editing, NMA, MSP, SS, and LAA. Supervision, RHA. Project administration, RHA. Funding acquisition, RHA. All authors contributed to the article and approved the submitted version.
Funding
This systematic review was funded by the Summer Undergraduate Research Experience (SURE) PLUS-Grant of the United Arab Emirates University, 2017 (research grant 31M348). The funding source had no role in the study design, collection, analysis, or interpretation of the data, nor in writing or the decision to submit this article for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.668447/full#supplementary-material
PRISMA checklist.
Overall weighted prevalence of GDM in pregnant women in North African countries (Morocco, Algeria, Tunisia, Libya, Egypt) by pregnancy trimester, body mass index, study period, ascertainment methodology, rate of cesarean section deliveries, and maternal mortality.
Overall weighted prevalence of GDM in pregnant women in GCC countries and Yemen (the UAE, Qatar, Saudi Arabia, Kuwait, Yemen, Oman, Bahrain) by pregnancy trimester, body mass index, study period, ascertainment methodology, rate of cesarean section deliveries, and maternal mortality.
Overall weighted prevalence of GDM in pregnant women in Iran/Iraq by pregnancy trimester, body mass index, study period, ascertainment methodology, rate of cesarean section deliveries, and maternal mortality.
Overall weighted prevalence of GDM in pregnant women in the Levant region (Jordan, Syria, Palestine, Lebanon) by pregnancy trimester, body mass index, study period, ascertainment methodology, rate of cesarean section deliveries, and maternal mortality.
Univariate and multivariable meta-regression analyses to identify sources of heterogeneity in studies reporting on the prevalence of GDM in pregnant women by different measured characteristics.
Funnel plots (A) and Egger’s publication bias plot (B) examining small-study effects on the pooled GDM prevalence among pregnant women in the MENA region, 2000–2019.
Risk of bias assessment of the 102 reviewed research reports on GDM.
References
- 1.Quintanilla Rodriguez BS, Mahdy H. Gestational Diabetes. In: StatPearls. Treasure Island (FL) (2020). [PubMed] [Google Scholar]
- 2.Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on Gestational Diabetes Mellitus: A Pragmatic Guide for Diagnosis, Management, and Care. Int J Gynaecol Obstet (2015) 131(Suppl 3):S173–211. 10.1016/S0020-7292(15)30033-3 [DOI] [PubMed] [Google Scholar]
- 3.Spaight C, Gross J, Horsch A, Puder JJ. Gestational Diabetes Mellitus. Endocr Dev (2016) 31:163–78. 10.1159/000439413 [DOI] [PubMed] [Google Scholar]
- 4.Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on Gestational Diabetes Mellitus: A Pragmatic Guide for Diagnosis, Management, and Care(). Int J Gynaecol Obstet (2015) 131(Suppl 3):S173–211. 10.1016/S0020-7292(15)30033-3 [DOI] [PubMed] [Google Scholar]
- 5.Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global Estimates of the Prevalence of Hyperglycaemia in Pregnancy. Diabetes Res Clin Pract (2014) 103(2):176–85. 10.1016/j.diabres.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 6.Mack LR, Tomich PG. Gestational Diabetes: Diagnosis, Classification, and Clinical Care. Obstet Gynecol Clin North Am (2017) 44(2):207–17. 10.1016/j.ogc.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 7.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of Treatment of Gestational Diabetes Mellitus on Pregnancy Outcomes. N Engl J Med (2005) 352(24):2477–86. 10.1056/NEJMoa042973 [DOI] [PubMed] [Google Scholar]
- 8.Ward MM. Estimating Disease Prevalence and Incidence Using Administrative Data: Some Assembly Required. J Rheumatol (2013) 40(8):1241–3. 10.3899/jrheum.130675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiwani A, Marseille E, Lohse N, Damm P, Hod M, Kahn JG. Gestational Diabetes Mellitus: Results From a Survey of Country Prevalence and Practices. J Matern Fetal Neonatal Med (2012) 25(6):600–10. 10.3109/14767058.2011.587921 [DOI] [PubMed] [Google Scholar]
- 10.World health Organization . Global Health Observatory (GHO) Data. Overweight and Obesity. Noncommunicable Diseases. Obesity Among Adults (2016). Available at: https://www.who.int/gho/ncd/risk_factors/overweight/en/ (Accessed 10.06.2020).
- 11.International Diabetes Federation . IDF Diabetes Atlas 6th Edition (2013). Available at: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/19-atlas-6th-edition.html (Accessed June 3, 2020).
- 12.Agarwal MM, Shah SM, Al Kaabi J, Saquib S, Othman Y. Gestational Diabetes Mellitus: Confusion Among Medical Doctors Caused by Multiple International Criteria. J Obstet Gynaecol Res (2015) 41(6):861–9. 10.1111/jog.12661 [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Rifai RH, Aziz F. Prevalence of Type 2 Diabetes, Prediabetes, and Gestational Diabetes Mellitus in Women of Childbearing Age in Middle East and North Africa, 2000-2017: Protocol for Two Systematic Reviews and Meta-Analyses. Syst Rev (2018) 7(1):96. 10.1186/s13643-018-0763-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Covidence . Covidence – Better Systematic Review Management. Melbourne, Victoria, Australia: Covidence; (2018). Available at: https://www.covidence.org/home. [Google Scholar]
- 16.Woodruff TJ, Sutton P. The Navigation Guide Systematic Review Methodology: A Rigorous and Transparent Method for Translating Environmental Health Science Into Better Health Outcomes. Environ Health Perspect (2014) 122(10):1007–14. 10.1289/ehp.1307175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Bank Definition of Middle East and North Africa Countries. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519.
- 18.National Heart, Lung, and Blood Institute . Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 19.Tukey JW, Murray FF. Transformations Related to the Angular and the Square Root. Ann Math Stat (1950) 21(4):607–11. 10.1214/aoms/1177729756 [DOI] [Google Scholar]
- 20.Miller JJ. The Inverse of the Freeman – Tukey Double Arcsine Transformation. Am Stat (1978) 32(4):138. 10.2307/2682942 [DOI] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-Analysis in Clinical Trials Revisited. Contemp Clin Trials (2015) 45(Pt A):139–45. 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borenstein M, Hedges LV, Higgins J, Rothstein HR. Introduction to Meta-Analysis. Chichester, U.K: John Wiley & Sons, Ltd. (2009). 10.1002/9780470743386 [DOI] [Google Scholar]
- 23.Billionnet C, et al. Gestational Diabetes and Adverse Perinatal Outcomes From 716,152 Births in France in 2012. Diabetologia (2017) 60(4):636–44. 10.1007/s00125-017-4206-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bawah AT, Ngala RA, Alidu H, Seini MM, Wumbee JDK, Yeboah FA. Gestational Diabetes Mellitus and Obstetric Outcomes in a Ghanaian Community. Pan Afr Med J (2019) 32:94. 10.11604/pamj.2019.32.94.17334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betran AP, Ye J, Moller A, Zhang J, Gülmezoglu AM, Torloni MR. The Increasing Trend in Caesarean Section Rates: Global, Regional and National Estimates: 1990-2014. PloS One (2016) 11(2):e0148343. 10.1371/journal.pone.0148343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization . World Health Statistics 2015. Available at: https://apps.who.int/iris/bitstream/handle/10665/170250/9789240694439_eng.pdf;jsessionid=EF2A3F289024FCB434BE9A04B2C617DA?sequence=1 (Accessed February 2020).
- 27.Global Health Observatory (GHO) . Maternal Mortality Country Profiles. World Health Organization. Available at: https://www.who.int/gho/mdg/maternal_health/countries/en/ (Accessed February 2020). [Google Scholar]
- 28.Sterne JA, Egger M. Funnel Plots for Detecting Bias in Meta-Analysis: Guidelines on Choice of Axis. J Clin Epidemiol (2001) 54(10):1046–55. 10.1016/S0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- 29.Nyaga VN, Arbyn M, Aerts M. Metaprop: A Stata Command to Perform Meta-Analysis of Binomial Data. Arch Public Health (2014) 72(1):39. 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.StataCorp . Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; (2017). [Google Scholar]
- 31.Tebbani F, Oulamara H, Agli A. Effects of Gestational Weight Gain on Pregnancy Complications. Nutr Clinique Metabolisme (2017) 32:27 – 32. 10.1016/j.nupar.2017.09.011 [DOI] [Google Scholar]
- 32.Rajab KE, Issa AA, Hasan ZA, Rajab E, Jaradat AA. Incidence of Gestational Diabetes Mellitus in Bahrain From 2002 to 2010. Int J Gynaecol Obstet (2012) 117(1):74–7. 10.1016/j.ijgo.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 33.Al Mahroos S, Nagalla DS, Yousif W, Sanad H. A Population-Based Screening for Gestational Diabetes Mellitus in non-Diabetic Women in Bahrain. Ann Saudi Med (2005) 25(2):129–33. 10.5144/0256-4947.2005.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rakha S, El Marsafawy H. Sensitivity, Specificity, and Accuracy of Fetal Echocardiography for High-Risk Pregnancies in a Tertiary Center in Egypt. Arch Pediatr (2019) 26(6):337–41. 10.1016/j.arcped.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 35.Rezk M, Omar Z. Deleterious Impact of Maternal Hepatitis-C Viral Infection on Maternal and Fetal Outcome: A 5-Year Prospective Study. Arch Gynecol Obstet (2017) 296(6):1097–102. 10.1007/s00404-017-4550-2 [DOI] [PubMed] [Google Scholar]
- 36.Maged AM, Moety GAF, Mostafa WA, Hamed DA. Comparative Study Between Different Biomarkers for Early Prediction of Gestational Diabetes Mellitus. J Matern Fetal Neonatal Med (2014) 27(11):1108–12. 10.3109/14767058.2013.850489 [DOI] [PubMed] [Google Scholar]
- 37.Elkholi DGEY, Nagy HM. The Effects of Adipocytokines on the Endocrino-Metabolic Features and Obstetric Outcome in Pregnant Obese Women With Polycystic Ovary Syndrome. Middle East Fertil Soc J (2014) 19:292–302. 10.1016/j.mefs.2014.02.001 [DOI] [Google Scholar]
- 38.Mohammed AK, Algani VHA. The Correlation Between Serum Ferritin and Fasting Blood Sugar in Iraqi Women With Gestational Diabetes. J Pharm Sci (2017) 9(9):1654–8. [Google Scholar]
- 39.Alawad ZM, Al-Omary HL. Maternal and Cord Blood Prolactin Level and Pregnancy Complications. Pak J Med Sci (2019) 35(4):1122–7. 10.12669/pjms.35.4.558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Safari K, Piro TJ, Ahmad HM. Perspectives and Pregnancy Outcomes of Maternal Ramadan Fasting in the Second Trimester of Pregnancy. BMC Pregnancy Childbirth (2019) 19(1):128. 10.1186/s12884-019-2275-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maghbooli Z, Hossein-Nezhad A, Karimi F, Shafaei A, Larijani B. Correlation Between Vitamin D3 Deficiency and Insulin Resistance in Pregnancy. Diabetes Metab Res Rev (2008) 24(1):27–32. 10.1002/dmrr.737 [DOI] [PubMed] [Google Scholar]
- 42.Abolfazl M, Hamidreza TS, Narges M, Maryam Y. Gestational Diabetes and its Association With Unpleasant Outcomes of Pregnancy. Pak J Med Sci (2008) 24(4):566–70. [Google Scholar]
- 43.Keshavarz M, Cheung NW, Babaee GR, Moghadam HK, Ajami ME, Shariati M. Gestational Diabetes in Iran: Incidence, Risk Factors and Pregnancy Outcomes. Diabetes Res Clin Pract (2005) 69(3):279–86. 10.1016/j.diabres.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 44.Hadaegh F, Tohidi M, Harati H, Kheirandish M, Rahimi S. Prevalence of Gestational Diabetes Mellitus in Southern Iran (Bandar Abbas City). Endocr Pract (2005) 11(5):313–8. 10.4158/EP.11.5.313 [DOI] [PubMed] [Google Scholar]
- 45.Amooee S, Samsami A, Jahanbakhsh J, Karimi M. The Pregnancy Outcome in Patients With Minor Beta-Thalassemia. Iran J Reprod Med (2011) 9(1):9–14. [PMC free article] [PubMed] [Google Scholar]
- 46.Lamyian M, Hosseinpour-Niazi S, Mirmiran P, Banaem LM, Goshtasebi A, Azizi F, et al. Pre-Pregnancy Fast Food Consumption Is Associated With Gestational Diabetes Mellitus Among Tehranian Women. Nutrients (2017) 9(3):216. 10.3390/nu9030216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soheilykhah S, Mogibian M, Rahimi-Saghand S, Rashidi M, Soheilykhah S, Piroz M. Incidence of Gestational Diabetes Mellitus in Pregnant Women. Iran J Reprod Med (2010) 8(1):24–8. [Google Scholar]
- 48.Pirjani R, Shirzad N, Qorbani M, Phelpheli M, Nasli-Esfahani E, Bandarian F, et al. Gestational Diabetes Mellitus Its Association With Obesity: A Prospective Cohort Study. Eat Weight Disord (2017) 22(3):445–50. 10.1007/s40519-016-0332-2 [DOI] [PubMed] [Google Scholar]
- 49.Soheilykhah S, Mojibian M, Jannati Moghadam M. Serum Ferritin Concentration in Early Pregnancy and Risk of Subsequent Development of Gestational Diabetes: A Prospective Study. Int J Reprod BioMed (Yazd) (2017) 15(3):155–60. 10.29252/ijrm.15.3.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shahbazian H, Nouhjah S, Shahbazian N, Jahanfar S, Latifi SM, Aleali A, et al. Gestational Diabetes Mellitus in an Iranian Pregnant Population Using IADPSG Criteria: Incidence, Contributing Factors and Outcomes. Diabetes Metab Syndr (2016) 10(4):242–6. 10.1016/j.dsx.2016.06.019 [DOI] [PubMed] [Google Scholar]
- 51.Yassaee F, Eskandari R, Amiri Z. Pregnancy Outcomes in Women With Idiopathic Thrombocytopenic Purpura. Iran J Reprod Med (2012) 10(5):489–92. [PMC free article] [PubMed] [Google Scholar]
- 52.Ashrafi M, Sheikhan F, Arabipoor A, Hosseini R, Nourbakhsh F, Zolfaghari Z. Gestational Diabetes Mellitus Risk Factors in Women With Polycystic Ovary Syndrome (PCOS). Eur J Obstet Gynecol Reprod Biol (2014) 181:195–9. 10.1016/j.ejogrb.2014.07.043 [DOI] [PubMed] [Google Scholar]
- 53.Goshtasebi A, Hosseinpour-Niazi S, Mirmiran P, Lamyian M, Banaem LM, Azizi F. Pre-Pregnancy Consumption of Starchy Vegetables and Legumes and Risk of Gestational Diabetes Mellitus Among Tehranian Women. Diabetes Res Clin Pract (2018) 139:131–8. 10.1016/j.diabres.2018.02.033 [DOI] [PubMed] [Google Scholar]
- 54.Ashrafi M, Gosili R, Hosseini R, Arabipoor A, Ahmadi J, Chehrazi M. Risk of Gestational Diabetes Mellitus in Patients Undergoing Assisted Reproductive Techniques. Eur J Obstet Gynecol Reprod Biol (2014) 176:149–52. 10.1016/j.ejogrb.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 55.Jamali S, Sh J, RakeshJahromi A, Haghbeen M. Teenage Versus Adult Pregnancy: Maternal and Neonatal Outcomes. J Fundam Appl Sci (2017) 9(2):1170–82. 10.4314/jfas.v9i2.36 [DOI] [Google Scholar]
- 56.Pourali L, Ayati S, Jelodar S, Zarifian A, Andalibi MSS. Obstetrics and Perinatal Outcomes of Dichorionic Twin Pregnancy Following ART Compared With Spontaneous Pregnancy. Int J Reprod BioMed (Yazd) (2016) 14(5):317–22. [PMC free article] [PubMed] [Google Scholar]
- 57.Mehrabian F, Rezae M. Sex Hormone Binding Globulin Measurement Before Conception as a Predictor of Gestational Diabetes in Women With Polycystic Ovarian Syndrome. J Res Med Sci (2013) 18(8):637–40. [PMC free article] [PubMed] [Google Scholar]
- 58.Mehrabian F, Hosseini SM. Comparison of Gestational Diabetes Mellitus and Pre-Eclampsia in Women With High Hemoglobin in the First Trimester of Pregnancy: A Longitudinal Study. Pak J Med Sci (2013) 29(4):986–90. 10.12669/pjms.294.4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hosseini E, Janghorbani M, Aminorroaya A. Incidence, Risk Factors, and Pregnancy Outcomes of Gestational Diabetes Mellitus Using One-Step Versus Two-Step Diagnostic Approaches: A Population-Based Cohort Study in Isfahan, Iran. Diabetes Res Clin Pract (2018) 140:288–94. 10.1016/j.diabres.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 60.Hantoushzadeh S, Sheikh M, Bosaghzadeh Z, Ghotbizadeh F, Tarafdari A, Panahi Z, et al. The Impact of Gestational Weight Gain in Different Trimesters of Pregnancy on Glucose Challenge Test and Gestational Diabetes. Postgrad Med J (2016) 92(1091):520–4. 10.1136/postgradmedj-2015-133816 [DOI] [PubMed] [Google Scholar]
- 61.Niromanesh S, Shirazi M, Dastgerdy E, Sharbaf FR, Shirazi M, Khazaeipour Z. Association of Hypertriglyceridaemia With Pre-Eclampsia, Preterm Birth, Gestational Diabetes and Uterine Artery Pulsatility Index. Natl Med J India (2012) 25(5):265–7. [PubMed] [Google Scholar]
- 62.Vaezi A, Haghighi L, Beigmohammadi F, Nojomi M. Maternal Asthma, Pregnancy, Delivery and Birth Outcomes: A Retrospective Cohort Study. Iran J Allergy Asthma Immunol (2017) 16(2):92–8. [PubMed] [Google Scholar]
- 63.Hossein-Nezhad A, Maghbooli Z, Vassigh A, Larijani B. Prevalence of Gestational Diabetes Mellitus and Pregnancy Outcomes in Iranian Women. Taiwan J Obstet Gynecol (2007) 46(3):236–41. 10.1016/S1028-4559(08)60026-1 [DOI] [PubMed] [Google Scholar]
- 64.Nastaran SA, Nourossadat K, Abbas HF, Hamid AM. Hemoglobin Level During the First Trimester of Pregnancy in Gestational Diabetes. Ginekol Pol (2012) 83(12):929–33. [PubMed] [Google Scholar]
- 65.Talebian A, Soltani B, Sehat M, Ai Z, Noorian A, Talebian M. Incidence and Risk Factors of Neural Tube Defects in Kashan, Central Iran. Iran J Child Neurol (2015) 9(3):50–6. [PMC free article] [PubMed] [Google Scholar]
- 66.Kouhkan A, Khamseh ME, Pirjani R, Moini A, Arabipoor A, Maroufizadeh S, et al. Obstetric and Perinatal Outcomes of Singleton Pregnancies Conceived via Assisted Reproductive Technology Complicated by Gestational Diabetes Mellitus: A Prospective Cohort Study. BMC Pregnancy Childbirth (2018) 18(1):495. 10.1186/s12884-018-2115-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abedi P, Bagheri R, Qorbani M, Ansari S. Is There a Relationship Between Restless Legs Syndrome and Medical Problems in Pregnant Women? A Cross-Sectional Study in Iran. Acta Neurol Belg (2018) 120(5):1091–6. 10.1007/s13760-018-01062-7 [DOI] [PubMed] [Google Scholar]
- 68.Pezeshki B, Chiti H, Arasteh P, Mazloomzadeh S. Early Screening of Gestational Diabetes Mellitus Using Hemoglobin A1C: Revising Current Screening Guidelines. Caspian J Intern Med (2019) 10(1):16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heydarpour F, Soltani M, Najafi F, Tabatabaee HR, Etemad K, Hajipour M, et al. Maternal Anemia in Various Trimesters and Related Pregnancy Outcomes: Results From a Large Cohort Study in Iran. Iran J Pediatr (2019) 29(1):e69741. 10.5812/ijp.69741 [DOI] [Google Scholar]
- 70.Fazel N, Kundi M, Jensen-Jarolim E, Pali-Schöll I, Kazemzadeh, et al. Prospective Cohort Study of Pregnancy Complications and Birth Outcomes in Women With Asthma. Arch Gynecol Obstet (2018) 298(2):279–87. 10.1007/s00404-018-4800-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nouhjah S, Shahbazian H, Shahbazian N, Jahanfar S, Jahanshahi A, Cheraghian B, et al. Early Postpartum Metabolic Syndrome in Women With or Without Gestational Diabetes: Results From Life After Gestational Diabetes Ahvaz Cohort Study. Diabetes Metab Syndr (2018) 12(3):317–23. 10.1016/j.dsx.2017.12.027 [DOI] [PubMed] [Google Scholar]
- 72.Maghbooli Z, Hossein-Nezhad A, Adabi E, Asadollah-Pour E, Sadeghi M, Mohammad-Nabi S, et al. Air Pollution During Pregnancy and Placental Adaptation in the Levels of Global DNA Methylation. PloS One (2018) 13(7):e0199772. 10.1371/journal.pone.0199772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salehi-Pourmehr H, Mohammad-Alizadeh S, Jafarilar-Agdam N, Rafiee S, Farshbaf-Khalili A, et al. The Association Between Pre-Pregnancy Obesity and Screening Results of Depression for All Trimesters of Pregnancy, Postpartum and 1 Year After Birth: A Cohort Study. J Perinat Med (2018) 46(1):87–95. 10.1515/jpm-2016-0277 [DOI] [PubMed] [Google Scholar]
- 74.Zargar M, Razmkhah N, Nikbakht R. Evaluating the Factors Associated With Pregnancy Loss in Pregnant Women Undergoing Assisted Reproductive Techniques. Middle East Fertil Soc J (2018) 23:342 – 345. 10.1016/j.mefs.2018.04.009 [DOI] [Google Scholar]
- 75.Mojtahedi SY, Izadi A, Seirafi G, Khedmat L, Tavakolizadeh R, et al. Risk Factors Associated With Neonatal Jaundice: A Cross-Sectional Study From Iran. Open Access Maced J Med Sci (2018) 6(8):1387–93. 10.3889/oamjms.2018.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eslami E, Charandabi SMA, Khalili AF, Jafarabadi MA, Mirghafourvand M. The Effect of a Lifestyle-Based Training Package on Weight Gain and Frequency of Gestational Diabetes in Obese and Over Weight Pregnant Females. Iran Red Crescent Med J (2018) 20(S1):e62576. 10.5812/ircmj.62576 [DOI] [Google Scholar]
- 77.Mardani M, Yazdani A, Rezaei F, Gachkar L, Shokouhi S, Pourkaveh B, et al. Risk Factors Associated With Outcomes of Seasonal Influenza in Pregnant Women Referring to Health Care Centers in Iran in 2015-2016. Arch Clin Infect Dis (2019) 14(3):e96403. 10.5812/archcid.96403 [DOI] [Google Scholar]
- 78.Basha AS, Fram KM, Thekrallah FM, Irshaid ZA, Maswady AM, Obeidat ZN. Prevalence of Gestational Diabetes and Contributing Factors Among Pregnant Jordanian Women Attending Jordan University Hospital. Int J Diabetes Developing Countries (2018) 9(9):132–8. 10.1007/s13410-018-0635-0 [DOI] [Google Scholar]
- 79.Abdel Razeq NM, Khader YS, Batieha AM. The Incidence, Risk Factors, and Mortality of Preterm Neonates: A Prospective Study From Jordan (2012-2013). Turk J Obstet Gynecol (2017) 14(1):28–36. 10.4274/tjod.62582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clouse K, Shehabi A, Suleimat AM, Faouri S, Khuri-Bulos N, Al Jammal A, et al. High Prevalence of Group B Streptococcus Colonization Among Pregnant Women in Amman, Jordan. BMC Pregnancy Childbirth (2019) 19(1):177. 10.1186/s12884-019-2317-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khader YS, Batieha A, Al-Njadat RA, Hijazi SS. Preeclampsia in Jordan: Incidence, Risk Factors, and its Associated Maternal and Neonatal Outcomes. J Matern Fetal Neonatal Med (2018) 31(6):770–6. 10.1080/14767058.2017.1297411 [DOI] [PubMed] [Google Scholar]
- 82.Zein S, Rachidi S, Awada S, Osman M, Al-Hajje A, Shami N, et al. High Iron Level in Early Pregnancy Increased Glucose Intolerance. J Trace Elem Med Biol (2015) 30:220–5. 10.1016/j.jtemb.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 83.Ghaddar N, Roz AE, Ghssein G, Ibrahim J. Emergence of Vulvovaginal Candidiasis Among Lebanese Pregnant Women: Prevalence, Risk Factors, and Species Distribution. Infect Dis Obstet Gynecol (2019) 2019:5016810. 10.1155/2019/5016810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khalil MM, Alzahra E. Fetal Gender and Pregnancy Outcomes in Libya: A Retrospective Study. Libyan J Med (2013) 8. 10.3402/ljm.v8i0.20008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Utz B, Assarag B, Smekens T, Ennassiri H, Lekhal T, El Ansari N, et al. Detection and Initial Management of Gestational Diabetes Through Primary Health Care Services in Morocco: An Effectiveness-Implementation Trial. PloS One (2018) 13(12):e0209322. 10.1371/journal.pone.0209322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abdwani R, Al Shaqsi L, Al-Zakwani I. Neonatal and Obstetrical Outcomes of Pregnancies in Systemic Lupus Erythematosus. Oman Med J (2018) 33(1):15–21. 10.5001/omj.2018.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Al-Hakmani FM, Al-Fadhil FA, Al-Balushi LH, Al-Harthy NA, Al-Bahri ZA, Al-Rawahi NA, et al. The Effect of Obesity on Pregnancy and Its Outcome in the Population of Oman, Seeb Province. Oman Med J (2016) 31(1):12–7. 10.5001/omj.2016.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abu-Heija AT, Al-Bash MR, Al-Kalbani MA. Effects of Maternal Age, Parity and Pre-Pregnancy Body Mass Index on the Glucose Challenge Test and Gestational Diabetes Mellitus. J Taibah Univ Med Sci (2017) 12(4):338342. 10.1016/j.jtumed.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zutshi A, Santhosh J, Sheikh J, Naeem F, Al-Hamedi A, Khan S, et al. Implications of Early Pregnancy Obesity on Maternal, Fetal and Neonatal Health: Retrospective Cohort Study From Oman. Sultan Qaboos Univ Med J (2018) 18(1):e47–53. 10.18295/squmj.2018.18.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Islam MM, Bakheit CS. Advanced Maternal Age and Risks for Adverse Pregnancy Outcomes: A Population-Based Study in Oman. Health Care Women Int (2015) 36(10):1081–103. 10.1080/07399332.2014.990560 [DOI] [PubMed] [Google Scholar]
- 91.Al-Kuwari MG, Al-Kubaisi BS. Prevalence and Predictors of Gestational Diabetes in Qatar. Diabetol Croatica (2011) 40(3):65–70. [Google Scholar]
- 92.Bener A, Saleh NM, Al-Hamaq A. Prevalence of Gestational Diabetes and Associated Maternal and Neonatal Complications in a Fast-Developing Community: Global Comparisons. Int J Womens Health (2011) 3:367–73. 10.2147/IJWH.S26094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abu Yaacob S, Saad FA, Sharara HA, Khalifa L, Manther AA, Rashed YA. The Effect of Obesity in Pregnancy on Perinatal Outcome in Qatar. Qatar Med J (2002) 11(2):32–5. 10.5339/qmj.2002.2.16 [DOI] [Google Scholar]
- 94.Bashir M, Aboulfotouh M, Dabbous Z, Mokhtar M, Siddique M, Wahba R, et al. Metformin-Treated-GDM has Lower Risk of Macrosomia Compared to Diet-Treated GDM- A Retrospective Cohort Study. J Matern Fetal Neonatal Med (2018) 1–141. 10.1080/14767058.2018.1550480 [DOI] [PubMed] [Google Scholar]
- 95.Shaukat S, Nur U. Effect of Prepregnancy Maternal BMI on Adverse Pregnancy and Neonatal Outcomes: Results From a Retrospective Cohort Study of a Multiethnic Population in Qatar. BMJ Open (2019) 9(9):e029757. 10.1136/bmjopen-2019-029757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Soliman A, Salama H, Rifai HA, Sanctis VD, Al-Obaidly S, Qubasi MA, et al. The Effect of Different Forms of Dysglycemia During Pregnancy on Maternal and Fetal Outcomes in Treated Women and Comparison With Large Cohort Studies. Acta BioMed (2018) 89(S5):11–21. 10.23750/abm.v89iS4.7356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kurdi AM, Majeed-Saidan MA, Rakaf MSA, AlHashem AM, Botto LD, Baaqeel HS, et al. Congenital Anomalies and Associated Risk Factors in a Saudi Population: A Cohort Study From Pregnancy to Age 2 Years. BMJ Open (2019) 9(9):e026351. 10.1136/bmjopen-2018-026351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.El-Gilany AH, Hammad S. Body Mass Index and Obstetric Outcomes in Pregnant in Saudi Arabia: A Prospective Cohort Study. Ann Saudi Med (2010) 30(5):376–80. 10.4103/0256-4947.67075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lasheen AE, Abdelbasit OB, Seidahmed MZ, Hussein KA, Miqdad AM, Zahrani MHA, et al. Infants of Diabetic Mothers. A Cohort Study. Saudi Med J (2014) 35(6):572–7. [PubMed] [Google Scholar]
- 100.Wahabi H, Fayed A, Esmaeil S, Mamdouh H, Kotb R. Prevalence and Complications of Pregestational and Gestational Diabetes in Saudi Women: Analysis From Riyadh Mother and Baby Cohort Study (RAHMA). BioMed Res Int (2017) 2017:6878263. 10.1155/2017/6878263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wahabi HA, Esmaeil SA, Fayed A, Al-Shaikh G, Alzeidan RA. Pre-Existing Diabetes Mellitus and Adverse Pregnancy Outcomes. BMC Res Notes (2012) 5:496. 10.1186/1756-0500-5-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wahabi HA, Esmaeil SA, Fayed A, Alzeidan RA. Gestational Diabetes Mellitus: Maternal and Perinatal Outcomes in King Khalid University Hospital, Saudi Arabia. J Egypt Public Health Assoc (2013) 88(2):104–8. 10.1097/01.EPX.0000430392.57811.20 [DOI] [PubMed] [Google Scholar]
- 103.Wahabi HA, Esmaeil SA, Fayed A, Mandil AA. The Independent Effects of Maternal Obesity and Gestational Diabetes on the Pregnancy Outcomes. BMC Endocr Disord (2014) 14:47. 10.1186/1472-6823-14-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Al-Rowaily MA, Abolfotouh MA. Predictors of Gestational Diabetes Mellitus in a High-Parity Community in Saudi Arabia. East Mediterr Health J (2010) 16(6):636–41. 10.26719/2010.16.6.636 [DOI] [PubMed] [Google Scholar]
- 105.Almarzouki AA. Pregnancy Outcome With Controlled Gestational Diabetes: A Single Centre Experience. Pak J Med Sci (2012) 28(5):887–90. [Google Scholar]
- 106.Al-Shaikh GK, Ibrahim GH, Fayed AA, Al-Mandeel H. Impact of Vitamin D Deficiency on Maternal and Birth Outcomes in the Saudi Population: A Cross-Sectional Study. BMC Pregnancy Childbirth (2016) 16:119. 10.1186/s12884-016-0901-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Al-Daghri NM, Al-Attas OS, Alokail MS, Alkharfy KM, Yousef M, Sabico SL, et al. Diabetes Mellitus Type 2 and Other Chronic non-Communicable Diseases in the Central Region, Saudi Arabia (Riyadh Cohort 2): A Decade of an Epidemic. BMC Med (2011) 9:76. 10.1186/1741-7015-9-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wahabi H, Fayed A, Esmaeil S, Alzeidan R, Elawad M, Tabassum R, et al. Riyadh Mother and Baby Multicenter Cohort Study: The Cohort Profile. PloS One (2016) 11(3):e0150297. 10.1371/journal.pone.0150297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alfadhli EM, Osman EN, Basri TH, Mansuri NS, Youssef MH, Assaaedi SA, et al. Gestational Diabetes Among Saudi Women: Prevalence, Risk Factors and Pregnancy Outcomes. Ann Saudi Med (2015) 35(3):222–30. 10.5144/0256-4947.2015.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Serehi AA, Ahmed AM, Shakeel F, Alkhatani K, El-Bakri NK, Buhari BAM, et al. A Comparison on the Prevalence and Outcomes of Gestational Versus Type 2 Diabetes Mellitus in 1718 Saudi Pregnancies. Int J Clin Exp Med (2015) 8(7):11502–7. [PMC free article] [PubMed] [Google Scholar]
- 111.Al-Rubeaan K, Al-Manaa HA, Khoja TA, Youssef AM, Al-Sharqawi AH, Siddiqui K, et al. A Community-Based Survey for Different Abnormal Glucose Metabolism Among Pregnant Women in a Random Household Study (SAUDI-Dm). BMJ Open (2014) 4(8):e005906. 10.1136/bmjopen-2014-005906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gasim T. Gestational Diabetes Mellitus: Maternal and Perinatal Outcomes in 220 Saudi Women. Oman Med J (2012) 27(2):140–4. 10.5001/omj.2012.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kurdi AM, Mesleh RA, Al-Hakeem MM, Khashoggi TY, Khalifa HM. Multiple Pregnancy and Preterm Labor. Saudi Med J (2004) 25(5):632–7. [PubMed] [Google Scholar]
- 114.Abdelmola AO, Mahfouz MS, Gahtani MAM, Mouharrq YJ, Hakami BHO, Daak OI, et al. Gestational Diabetes Prevalence and Risk Factors Among Pregnant Women — Jazan Region, Saudi Arabia. Clin Diabetol (2017) 6(5):172–7. 10.5603/DK.2017.0028 [DOI] [Google Scholar]
- 115.Al-Shaikh GK, Ibrahim GH, Fayed AA, Al-Mandeel H. Grand Multiparity and the Possible Risk of Adverse Maternal and Neonatal Outcomes: A Dilemma to be Deciphered. BMC Pregnancy Childbirth (2017) 17(1):310. 10.1186/s12884-017-1508-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fayed AA, Wahabi H, Mamdouh H, Kotb R, Esmaeil S. Demographic Profile and Pregnancy Outcomes of Adolescents and Older Mothers in Saudi Arabia: Analysis From Riyadh Mother (RAHMA) and Baby Cohort Study. BMJ Open (2017) 7(9):e016501. 10.1136/bmjopen-2017-016501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Subki AH, Algethami HR, Baabdullah WM, Alnefaie MN, Alzanbagi MA, Alsolami RM, et al. Prevalence, Risk Factors, and Fetal and Maternal Outcomes of Hypertensive Disorders of Pregnancy: A Retrospective Study in Western Saudi Arabia. Oman Med J (2018) 33(5):409–15. 10.5001/omj.2018.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Al Shanqeeti SA, Alkhudairy YN, Alabdulwahed AA, Ahmed AE, Al-Adham MS, Mahmood NM. Prevalence of Subclinical Hypothyroidism in Pregnancy in Saudi Arabia. Saudi Med J (2018) 39(3):254–60. 10.15537/smj.2018.3.21621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dafa Elseed EB, Khougali HS. Preconceptions and Awareness of Good Glycemic Control, Pregnancy Contraindications, and Maternal and Fetal Adverse Events in Sudan. Int J Gynaecol Obstet (2019) 144(2):233–5. 10.1002/ijgo.12720 [DOI] [PubMed] [Google Scholar]
- 120.Naser W, Adam I, Rayis DA, Ahmed MA, Hamdan HZ. Serum Magnesium and High-Sensitivity C-Reactive Protein as a Predictor for Gestational Diabetes Mellitus in Sudanese Pregnant Women. BMC Pregnancy Childbirth (2019) 19(1):301. 10.1186/s12884-019-2429-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alshareef SA, Rayis DA, Adam I, Gasim GI. Helicobacter Pylori Infection, Gestational Diabetes Mellitus and Insulin Resistance Among Pregnant Sudanese Women. BMC Res Notes (2018) 11(1):517. 10.1186/s13104-018-3642-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mallouli M, Derbel M, Ingrid A, Sahli J, Zedini C, Ajmi T, et al. Associated Outcomes to Fetal Macrosomia: Effect of Maternal Diabetes. Tunis Med (2017) 95(2):120–5. [PubMed] [Google Scholar]
- 123.Radwan H, Hashim M, Obaid RS, Hasan H, Naja F, Ghazal HA, et al. The Mother-Infant Study Cohort (MISC): Methodology, Challenges, and Baseline Characteristics. PloS One (2018) 13(5):e0198278. 10.1371/journal.pone.0198278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Agarwal MM, Punnose J, Dhatt GS. Gestational Diabetes: Implications of Variation in Post-Partum Follow-Up Criteria. Eur J Obstet Gynecol Reprod Biol (2004) 113(2):149–53. 10.1016/j.ejogrb.2003.09.021 [DOI] [PubMed] [Google Scholar]
- 125.Agarwal MM, Dhatt GS, Othman Y. Gestational Diabetes Mellitus Prevalence: Effect of the Laboratory Analytical Variation. Diabetes Res Clin Pract (2015) 109(3):493–9. 10.1016/j.diabres.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 126.Agarwal MM, Dhatt GS, Shah SM. Gestational Diabetes Mellitus: Simplifying the International Association of Diabetes and Pregnancy Diagnostic Algorithm Using Fasting Plasma Glucose. Diabetes Care (2010) 33(9):2018–20. 10.2337/dc10-0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Agarwal MM, Dhatt GS, Othman Y, Gupta R. Gestational Diabetes: Fasting Capillary Glucose as a Screening Test in a Multi-Ethnic, High-Risk Population. Diabetes Med (2009) 26(8):760–5. 10.1111/j.1464-5491.2009.02765.x [DOI] [PubMed] [Google Scholar]
- 128.Mirghani HM, Hamud OA. The Effect of Maternal Diet Restriction on Pregnancy Outcome. Am J Perinatol (2006) 23(1):21–4. 10.1055/s-2005-923435 [DOI] [PubMed] [Google Scholar]
- 129.Agarwal MM, Dhatt GS, Punnose J, Koster G. Gestational Diabetes: A Reappraisal of HBA1c as a Screening Test. Acta Obstet Gynecol Scand (2005) 84(12):1159–63. 10.1111/j.0001-6349.2005.00650.x [DOI] [PubMed] [Google Scholar]
- 130.Vaswani PR, Balachandran L. Pregnancy Outcomes in a Population With High Prevalence of Obesity: How Bad Is It? Clin Epidemiol Global Health I (2013), 5–11. 10.1016/j.cegh.2012.11.006 [DOI] [Google Scholar]
- 131.Abdel-Wareth LO, Kumari AS, Haq A, Bakir A, Sainudeen A, Sedaghatian MR, et al. An Evaluation of the Latest ADA Criteria for Screening and Diagnosing Gestational Diabetes at a Tertiary Care Hospital in the United Arab Emirates. Int J Diabetes Metab (2006) 14:55–60. 10.1159/000497593 [DOI] [Google Scholar]
- 132.Ali AD, Mehrass AAO, Al-Adhroey AH, Al-Shammakh AA, Amran AA. Prevalence and Risk Factors of Gestational Diabetes Mellitus in Yemen. Int J Womens Health (2016) 8:35–41. 10.2147/IJWH.S97502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Buckley BS, Harreiter J, Damm P, Corcoy R, Chico A, Simmons D, et al. Gestational Diabetes Mellitus in Europe: Prevalence, Current Screening Practice and Barriers to Screening. A Review. Diabetes Med (2012) 29(7):844–54. 10.1111/j.1464-5491.2011.03541.x [DOI] [PubMed] [Google Scholar]
- 134.Lee KW, Ching SM, Ramachandran V, Hoo AYFK, Chia YC, et al. Prevalence and Risk Factors of Gestational Diabetes Mellitus in Asia: A Systematic Review and Meta-Analysis. BMC Pregnancy Childbirth (2018) 18(1):494. 10.1186/s12884-018-2131-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Harper LM, Mele L, Landon MB, Carpenter MW, Ramin SM, Reddy UM, et al. Carpenter-Coustan Compared With National Diabetes Data Group Criteria for Diagnosing Gestational Diabetes. Obstet Gynecol (2016) 127(5):893–8. 10.1097/AOG.0000000000001383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.American Diabetes, A . Standards of Medical Care in Diabetes–2012. Diabetes Care (2012) 35(Suppl 1):S11–63. 10.2337/dc12-s011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Olarinoye JK, Ohwovoriole AE, Ajayi GO. Diagnosis of Gestational Diabetes Mellitus in Nigerian Pregnant Women–Comparison Between 75G and 100G Oral Glucose Tolerance Tests. West Afr J Med (2004) 23(3):198–201. 10.4314/wajm.v23i3.28120 [DOI] [PubMed] [Google Scholar]
- 138.Ranchod HA, Vaughan JE, Jarvis P. Incidence of Gestational Diabetes at Northdale Hospital, Pietermaritzburg. S Afr Med J (1991) 80(1):14–6. [PubMed] [Google Scholar]
- 139.Kim SY, Saraiva C, Curtis M, Wilson HG, Troyan J, Sharma AJ. Fraction of Gestational Diabetes Mellitus Attributable to Overweight and Obesity by Race/Ethnicity, California, 2007-2009. Am J Public Health (2013) 103(10):e65–72. 10.2105/AJPH.2013.301469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim SY, England L, Wilson HG, Bish C, Satten GA, Dietz P. Percentage of Gestational Diabetes Mellitus Attributable to Overweight and Obesity. Am J Public Health (2010) 100(6):1047–52. 10.2105/AJPH.2009.172890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, Regional, and National Prevalence of Overweight and Obesity in Children and Adults During 1980-2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet (2014) 384(9945):766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.American Diabetes, A . 13. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2018. Diabetes Care (2018) 41(Suppl 1):S137–43. 10.2337/dc18-S013 [DOI] [PubMed] [Google Scholar]
- 143.Committee on Practice, B.-O . ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol (2018) 131(2):e49–64. 10.1097/AOG.0000000000002501 [DOI] [PubMed] [Google Scholar]
- 144.Zhu Y, Zhang C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr Diabetes Rep (2016) 16(1):7. 10.1007/s11892-015-0699-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alfadhli EM. Gestational Diabetes Mellitus. Saudi Med J (2015) 36(4):399–406. 10.15537/smj.2015.4.10307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guillen-Sacoto MA, Barquiel B, Hillman N, Burgos MA, Herranz L. Gestational Diabetes Mellitus: Glycemic Control During Pregnancy and Neonatal Outcomes of Twin and Singleton Pregnancies. Endocrinol Diabetes Nutr (2018) 65(6):319–27. 10.1016/j.endien.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 147.Iversen K. Diabetes in Pregnancy—A Neglected Cause of Maternal Mortality In: BMJ Open (2017). Available at: https://blogs.bmj.com/bmj/2017/05/02/katja-iversen-diabetes-in-pregnancy-a-neglected-cause-of-maternal-mortality/ (Accessed June 1, 2020).
- 148.Al-Rifai RH, Majeed M, Qambar MA, Ibrahim A, AlYammahi KM, Aziz F. Type 2 Diabetes and Pre-Diabetes Mellitus: A Systematic Review and Meta-Analysis of Prevalence Studies in Women of Childbearing Age in the Middle East and North Africa, 2000-2018. Syst Rev (2019) 8(1):268. 10.1186/s13643-019-1187-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mirghani Dirar A, Doupis J. Gestational Diabetes From A to Z. World J Diabetes (2017) 8(12):489–511. 10.4239/wjd.v8.i12.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Seshiah V, Balaji V, Balaji MS, Paneerselvam A, Arthi T, Thamizharasi M, et al. Prevalence of Gestational Diabetes Mellitus in South India (Tamil Nadu)–a Community Based Study. J Assoc Phys India (2008) 56:329–33. [PubMed] [Google Scholar]
- 151.Zargar AH, Sheikh MI, Bashir MI, Masoodi SR, Laway BA, Wani AI, et al. Prevalence of Gestational Diabetes Mellitus in Kashmiri Women From the Indian Subcontinent. Diabetes Res Clin Pract (2004) 66(2):139–45. 10.1016/j.diabres.2004.02.023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
Overall weighted prevalence of GDM in pregnant women in North African countries (Morocco, Algeria, Tunisia, Libya, Egypt) by pregnancy trimester, body mass index, study period, ascertainment methodology, rate of cesarean section deliveries, and maternal mortality.
Overall weighted prevalence of GDM in pregnant women in GCC countries and Yemen (the UAE, Qatar, Saudi Arabia, Kuwait, Yemen, Oman, Bahrain) by pregnancy trimester, body mass index, study period, ascertainment methodology, rate of cesarean section deliveries, and maternal mortality.
Overall weighted prevalence of GDM in pregnant women in Iran/Iraq by pregnancy trimester, body mass index, study period, ascertainment methodology, rate of cesarean section deliveries, and maternal mortality.
Overall weighted prevalence of GDM in pregnant women in the Levant region (Jordan, Syria, Palestine, Lebanon) by pregnancy trimester, body mass index, study period, ascertainment methodology, rate of cesarean section deliveries, and maternal mortality.
Univariate and multivariable meta-regression analyses to identify sources of heterogeneity in studies reporting on the prevalence of GDM in pregnant women by different measured characteristics.
Funnel plots (A) and Egger’s publication bias plot (B) examining small-study effects on the pooled GDM prevalence among pregnant women in the MENA region, 2000–2019.
Risk of bias assessment of the 102 reviewed research reports on GDM.
Data Availability Statement
The data sets used and/or analyzed in the current study and the supplementary information files are available from the corresponding author on reasonable request.