Abstract
Antimicrobial resistance (AMR) can be highlighted as one of the most significant health concerns among the last decades, for which antimicrobial drug use in food-producing animals has contributed as one of the major drivers. Food-producing animals are one of the most important and rapidly expanding commercial agricultural sectors worldwide but there is currently limited knowledge on the temporal and geographical distribution of scientific research on antimicrobial resistance in food-producing animals. We provide a global overview of the spatial and temporal trends of scientific knowledge on AMR in food-producing animals. Peer-reviewed papers of AMR on food-producing animals were retrieved from the Web of Science, systemized and dissected. The final validated dataset contained 1341 occurrences observations covering the 1957–2018 period. There has been a shift of research efforts, both geographically and temporally, emphasizing regional differences in food animal production and changing practices in the food production industry. It becomes evident that many regions have been poorly surveyed, wherein intensified sampling and testing efforts should be most valuable. This systematization of knowledge will be crucial in helping to determine how to optimally allocate limited resources available for AMR monitor and control, aiding in the prediction where the threat of new resistant infections will be greatest. AMR research in food-producing animals in developing countries is markedly growing, reflecting changes in food animals production systems but also posing a particularly significant threat, not only due to intensive animal production, but also exacerbated by poor sanitation. We highlight that the use of antibiotics in food producing animals is pervasive, calling for urgent action. These findings raise the possibility to finetuning key priorities on AMR global issues.
Keywords: AMR, Food -producing animals, Global trends, Health, Mapping
Highlights
-
•
This is the first study providing a global overview of the spatial and temporal trends of research related to AMR in food-producing animals.
-
•
There is a clear rising interest on AMR research in food-producing animals worldwide but there is a spatial bias.
-
•
This study highlights poorly surveyed countries, where intensified sampling efforts are crucial.
-
•
Research trends and scientific areas are unearthed that should be invested in and explored in the future.
1. Introduction
“The time may come when penicillin can be bought by anyone in the shops. Then there is the danger that the ignorant man may easily underdose himself and by exposing his microbes to non-lethal quantities of the drug make them resistant. (…) If you use penicillin, use enough.”
This extract from the 1945 Nobel lecture of Sir Alexander Fleming is remarkable, as the scientist who discovered penicillin vaticinated an era wherein antibiotics would become less effective. More than 70 years after Fleming's Nobel speech, antimicrobial resistance (AMR) is at alarming levels, posing serious global health problems [1]. Despite Fleming's warnings, it was not long after the discovery of penicillin, that resistance became a serious clinical problem jeopardizing the conquests of the prior decade [2]. Today, bacterial resistance is reported across nearly all classes of antibiotics commercially available [3,4]. In 2014, the World Health Organization (WHO) provided a report in which, for the first time, the extent of antimicrobial resistance was globally assessed. The results were dramatic, showing that in many areas of the globe (e.g., Eastern Mediterranean, South-East Asia Region, among others) resistance levels displayed by common bacteria had reached worrying levels (WHO, 2014).
Antibiotics are essential for the treatment of bacterial infections in humans; they have changed medical practices and saved millions of lives [5]. Antibiotics were also a stepping stone supporting routine medical procedures varying from surgeries to chemotherapy, organ transplantation, among others [6]. Whilst antibiotic consumption and overuse is considered the primary driver of antibiotic resistance [7], several factors have directly and/or indirectly promoted resistance emergence and consolidation along the years, including demographic changes associated with urbanization and poor sanitation, rising life expectancy, discharge of antibiotic residues through environmental wast and use of animal manure, immunosuppression and increased opportunistic infection [8]. In fact, the intensification of resistance in recent decades is now jeopardizing the control of infectious diseases [5,9]. Vancomycin-resistant enterococci (VRE) and methicillin-resistant Staphylococcus aureus (MRSA) are nowadays global problems [10,11]. For instance, MRSA is responsible for more deaths, yearly, in U.S.A. (∼19,000) than the combination of HIV/AIDS, Parkinson's disease, emphysema and homicide (Spellberg et al., 2016; Ventola, 2015). Resistant bacterial pathogens are credited for more than 700,000 deaths per year, including 214,000 neonatal sepsis deaths [12]. The loss of efficacy of antibiotics against common pathogens led to increasing health costs in high-income countries [12], but also to an increase of morbidity and mortality in developing countries [8,13].
In the European Union (EU), economic losses associated with resistant bacterial infections are on the order of €1.5 billion annually and in the U.S.A. these losses are estimated to be US$20 billion a year (Transatlantic Taskforce on Antimicrobial Resistance). Infections with bacteria acquiring multidrug-resistant phenotypes are responsible, yearly, for the death of 25,000 patients in the EU (ECDC/EMEA Joint Working Group, 2009) and over 63,000 people in the U.S.A.. Recent estimates are alarming, suggesting that nearly 10 million people will die due to antimicrobial resistance by 2050 [14], however such “broad brush estimates” require caution to project future scenarios [15]. Undoubtedly, the burden of antimicrobial resistance on human health is increasing but it is difficult to precisely quantify, as detailed and reliable data are missing [16], but see [17]. Boeckel et al. [18] mapped, for the first-time, antibiotic consumption by humans, showing that antibiotics demand has increased worldwide by 36%, between 2000 and 2010. BRICS countries (Brazil, Russia, India, China and South Africa) are responsible for 76% of that increase. These are impressive figures, particularly when these countries represent 40% of the world's population [18]. The same trends were reported by Klein et al. (2018) in an expanded temporal scale (2000–2015), where antibiotic consumption increased by 65%, mainly driven by low- and middle-income countries. As global human population is expected to increase from approximately 7.7 billion in 2019 to approximately 9.2 billion by 2050 (United Nations Department of Economic and Social Affairs, 2015), antibiotic consumption is expected to increase by 50% in 2030 [19]. Such human population increase will claim more animal-based protein and will demand more industrialized methods of food-animal production, including management tools such as subtherapeutic dose antibiotics for growth promotion and disease prevention [20]. Furthermore, 34% of the projected global consumption of antibiotics in livestock by 2030 is due to a shift in farming practices as intensive animal husbandry will increase [13]. A substantial part of the resistance burden in humans is attributable to antimicrobial use in livestock production, primarily for disease prevention and growth promotion [[21], [22], [23]], even if the mechanisms underlying growth promotion remains uncertain [23]. For example, antimicrobials used in food-producing animals are expected to account for circa 80% of the U.S.A. annual antimicrobials consumption [6,23] and for circa 84% in China [24]. There is also expanding awareness that the use of antimicrobials in food-producing animals may add to the emergence of resistance to antibiotics regularly used by humans, mainly due to the overlap of molecules from the same antibiotic classes that are used both in human and veterinary medicine [25]. The antibiotics used in food-producing animals can spread to humans through various direct and/or indirect routes. It can occur directly through food consumption and handling, − the food chain is actually considered the main route of transmission of resistance genes between animal and human populations [26], or indirect exposure through the environment, as 90% of the antibiotics given to food-producing animals are excreted in their active form in the urine and faeces of food-producing animals [27]. Remarkably, 90% of the antibiotics administered to food-producing animals are ultimately dispersed through fertilizer, groundwater, and surface runoff in the environment [[28], [29], [30]]. Chemical and physical treatments of water for human consumption do not effectively remove all antibiotic traces. [30]. Exposure of environmental niches to stressors, such as bacterial commensals or pathogens harboring multidrug resistant genes, creates a complex scenario that promotes adaptation mechanisms of commensal or environmental bacteria and horizontal transfer of resistance determinants. Antimicrobial use in food-producing animals is therefore responsible for antimicrobial resistance worldwide [29,31] and, even though controversially, consumption of food that was carrying antimicrobial resistant bacteria has developed in acquisition of antibiotic resistant infections [32]. Antibiotics used in food-producing animals are therefore a serious threat to human health. Using global datasets of antibiotic veterinary use, livestock densities and socio-economic projections of meat demand, Boeckel et al. [18] projected that antimicrobial use in food-producing animals will increase by 67% by 2030. This study was the first to set a global picture of antibiotic use in livestock production on a worldwide scale. Although these are admirable exercises to tackle difficult questions, they rely on limited datasets of available data as most countries are reluctant to provide or do not have antibiotic consumption data therefore hampering the development of efficient strategies. Still, the [18] projections were derived from data concerning 32 countries [33]. As the livestock sector is one of the most swiftly growing commercial agricultural system globally [34], it is fundamental to evaluate the state and the geographical distribution of scientific knowledge on antimicrobial resistance in food-producing animals. Such a global map is essential to swiftly inspect trends, to understand the contribution of food-producing animals to global health problems, to identify regions in which committed research is intense or rapidly increasing and those that urgently need to generate new data. This systematization of knowledge will be crucial in helping to determine how to optimally allocate limited resources available for AMR monitor and control, aiding in the prediction where the threat of new resistant infections will be greatest (CDDEP 2018). Current understanding about the global distribution of AMR burden is surprisingly limited. Moreover, the frequency with which new bacteria with (multi)resistance are emerging underscores the inevitability of shifting from a reactionary to a pro-active preventive approach to mitigating AMR.
To meet these demands, this study aimed at mapping the spatial and temporal trends of scientific knowledge on antibiotic resistance in food-producing animals. Specifically, we wanted to assemble and analyze published peer-reviewed evidence on AMR in the main livestock taxa at different time points and geographical regions. This will allow to identify hotspots of AMR research, from which important research gaps can be identified. As far as we know, this is the first study mapping, at a global scale, the scientific knowledge on antimicrobial resistance in food animals.
2. Material and methods
2.1. Data assembly
2.1.1. Overview
The review question was defined as “What is the global distribution of published scientific literature of antimicrobial resistance in livestock?” We complied an extensive database comprising spatial and temporal occurrence data derived from the peer-reviewed literature. To gather this database, a systematic literature review was performed using online search engines and the resultant articles were manually reviewed. Reports by national or international authorities concerning official data submission by each country were not considered.
2.2. Peer-reviewed literature search
2.2.1. Data collection
A systematic literature review was performed using a rigorous search strategy in the online version of the Science Citation Index Expanded (SCI-EXPANDED) from the Web of Science (WoS) database (http://www.isiknowledge.com). No time and geographical location restrictions were placed on these searches, and only those published in English retrieved with full title and abstract. The searches were last updated on 19th January 2018. Search results were restricted to the following Boolean query, executed within a single search of assembling three different search strings, one for each category (antimicrobial resistance and livestock) and combining these by the Boolean operator “AND” to obtain only the intersection. Specifically, we used the following Boolean search statement: #1 “antimicrobial resistance”: “ANTIMICROBIAL” OR “ANTIBIOTIC” AND “RESISTANT” OR “RESISTANCE” and #2 “livestock”: “PIG” “SUID” “BOVINE” “CATTLE” “POULTRY” “BROILERS” and the interception consisted in #1 AND #2. Our search was only focused food-producing animals that were either terrestrial mammals and birds; henceforth other groups (fish, amphibians, reptiles, and invertebrates) were excluded. All the resulted publication records were imported into a bibliographic referencing tool and manually assessed for relevance, removing articles that did not contain information relating to AMR in livestock (e.g., methodology, environmental AMR, clinical AMR). All results were manually checked before excluding duplicates. To verified duplicates, queries targeting identical titles and authors were performed and hereafter removed. Incomplete publications e.g., without an abstract and publications clearly indexed as review, editorial, or errata were excluded. To create the final dataset, papers were entered into an Excel spreadsheet for further analysis. Key detail variables in the Excel database included the publication date, country where the study was performed, food-producing animal species, bacterial species, class of antibiotic, citation. All the selection process was made by two independent readers using predefined exclusion criteria.
2.2.2. Linking locations to data from the literature
From each peer-reviewed article, all available location information (coordinates) was extracted and included in the database. From the publications, we compiled a geo-positioning occurrence database of confirmed AMR presence, comprising either points, in the case of cities, towns or villages, or polygons in the case of counties or provinces. Geo-positioning was extracted to the smallest possible level (e.g., country, province, district, city/town or region) and the coordinates were extracted using Google Maps (https://www.maps.google.pt/). If the peer-reviewed article clearly stated a specific geo-positioned place (e.g., city, town or village), its central coordinate was recorded. But if the study area could only be identified at an administrative area level (e.g., province or district), the centroid of the polygon was recorded. The assigning of geo-positions to data from the peer-review literature was done in a geographic information system (ArcGIS Desktop: Release 10.6. Redlands, CA: Environmental Systems Research Institute).
3. Results and discussion
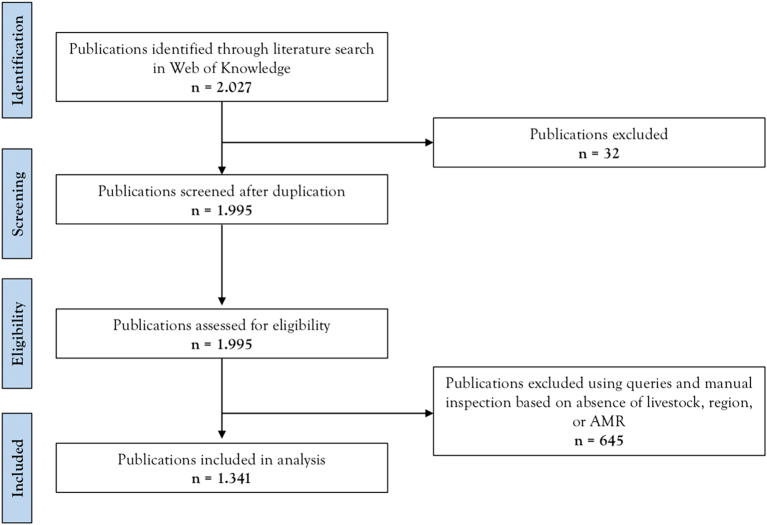
The systematic analysis of peer-reviewed papers reporting antimicrobial resistance in livestock species for the last 60 years shows that research in this topic has been exponentially growing in interest by the scientific community and that the use of antibiotics in food producing animals is pervasive. In total, we retrieved 1341 valid publications covering a period from 1957 to 2018 (Fig. 1).
Fig. 1.
Scoping review flowchart of the peer-reviewed dataset selection process. All search categories (livestock and antimicrobial resistance) were joined by the Boolean expression “AND,” resulting in the intersection used for analysis. The PRISMA flow diagram of the search strategy, study selection and data management procedure.
3.1. Temporal and geographical evolution of publications
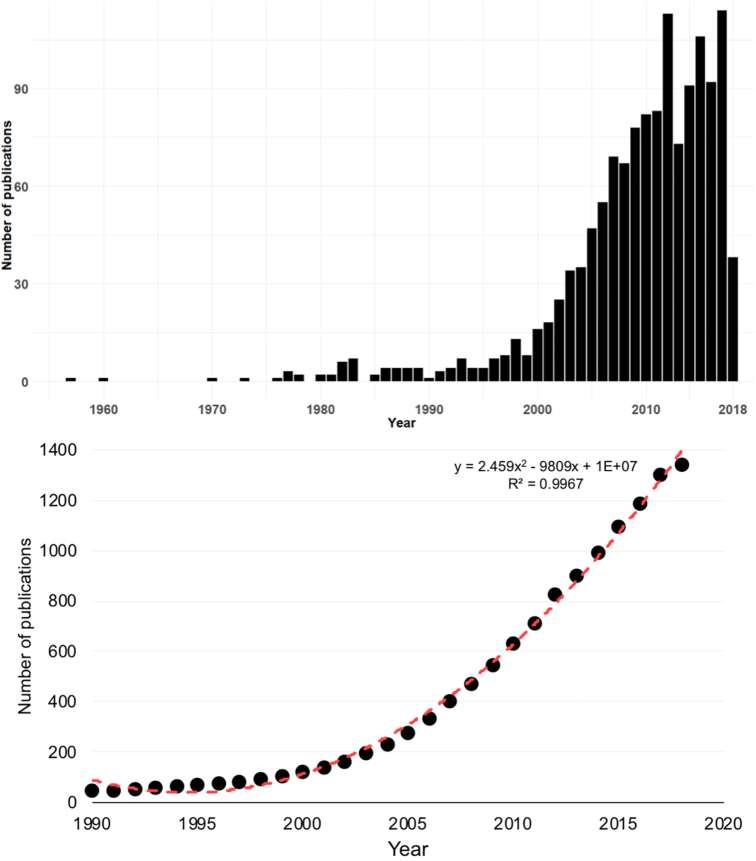
The number of publications on antimicrobial resistance in food-producing animals has steadily increased through time, reflecting the amount of research efforts worldwide (Fig. 2). The first article dealing with this topic appeared in 1957 and the number of articles produced afterwards grew overall. Perhaps the initial interests in AMR research in food animals are linked to the “alarm call” made during the 1950's by a British microbiologist who drew attention into the human health implications of the careless use of antibiotics in animal farming. E.S. “Andy” Anderson described the increase of antibiotic resistance genes horizontally from animals to humans [35]. However, it was not until after 1985 that publications began to appear annually. Since the 90s', the annual average growth rate of scientific publications dealing with antimicrobial resistance in food-producing animals was 26.61% (Fig. 2).
Fig. 2.
Global publication trends of antimicrobial resistance in food animals, n = 1341 (a) and the cumulative number of publications for the 1957–2018 period (b) (n = 1341).
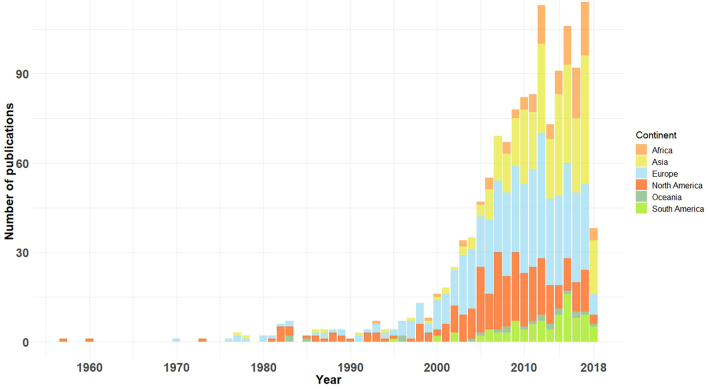
After the first published study on AMR and food-producing animals in the U.S.A., subsequent reports emerged from Europe (United Kingdom) in 1970 and Asia (India) in 1977. It was first reported in Oceania (Australia) in 1983 and, finally, in Africa (Zambia), in 1993 (Fig. 3). After the 2000's, AMR research in food-producing animals showed a remarkable increasing trend (Fig. 2). Interestingly, after 2000, meat production has stabilized in most developed countries and has, contrastingly, increased in Africa (68%), Asia (64%) and South America (40%) [36]. As developing countries have become richer, there was an increase in the demand for animal protein diets. Such shift was responsible for a major transformation of global food animals production, where antimicrobials are routinely used to maintain health and productivity [35; 36], thereafter the increase in publications can be a consequence of this. For example, global meat production has tripled in developing countries between 1980 and 2002, from 45 to 134 million tons [34]. This expansion was mostly supported by countries experiencing rapid economic growth, particularly in East Asia, accompanied by a remarkable increase of the published studies in this region (Fig. 3, Fig. 1 Supplementary material).
Fig. 3.
Temporal and geographical (per continent) distribution of publications for the 1957–2018 period (n = 1341).
Publications were not distributed evenly on all continents: the majority of publications were spatialized into Europe (38%), followed by Asia (24%), north America (23%), Africa (8%), south America (6%), and Oceania (2%) (Fig. 3). It is clear that in Asia, Africa and South America the increase of publications only occurred in the last decade, which can be a consequence of the increasing importance of the AMR topic at a global level, but also of governmental investment in science, particularly into the One Health prism and the advent of high throughput technologies. Several critical points are crucial for understanding our results that are deeply linked to the national politics of approvals, restrictions and bans of antibiotics use in food production. Antibiotics use in food production was already an established practice in the 1960's. For example, in 1938, Prontosil (the first effective drug against Gram-positive infections) was marketed in Britain for use in animals [38], followed by U.S.A.. Soon after, nontherapeutic use of antibiotic as growth promoters (AGPs) also proved to be effective to protect against bacterial infections and its use grew after 1949 where it became licensed to be used without veterinary prescription in 1951 in West Germany. The expansion of antibiotic use was even more dramatic in China but routine use of antibiotics in farms was not before the 1980s. During the late 1990s and early 2000s, the European Union (EU) phased in more stringent regulations on the use of antimicrobial substances in animal production, particularly their use for growth promotion. Also in the early 2000s, the EU enacted policies for on-farm surveillance. It was in 1996, that U.S.A. began to systematically monitor resistance in foodborne pathogens, with the implementation of the National Antimicrobial Resistance Monitoring System (NARMS).
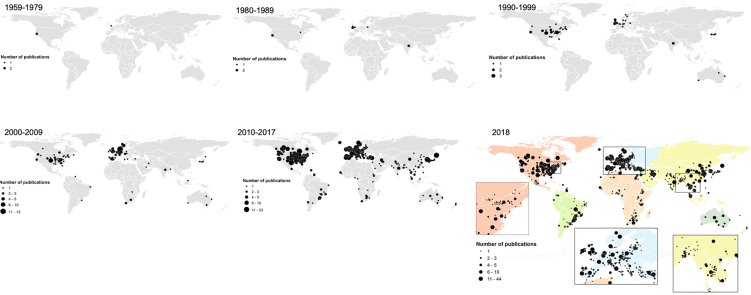
When the publication trends are analyzed by decade, there is a visible consistent increase in publications globally but also significant shift points in the geographical area of research production (Fig. 4), which are naturally coincident with important key points in the antibiotic resistance history [39]. Even though the WHO organized some working groups and meetings focused on antibiotic resistance during the late 70's (WHO 1976; WHO 1978), it was only when a clinician-scientist from Tufts University (U.S.A.), Stuart Levy, in 1981, gathered 147 scientists from 27 countries (but most of them from U.S.A.) on a conference in Santo Domingo (Dominican Republic) to sign the “Statement Regarding Worldwide Antibiotic Misuse”, emphasizing that AMR was a “worldwide public health problem” – this conference was a on the basis for the Alliance for the Prudent Use of Antibiotics (APUA) [39]. Throughout the decade, there was an increase of the U.S.A. government agency funding for AMR but the headship on AMR research shifted to Europe by the mid-1990s. Alarmed by the increase in multidrug-resistant Salmonella in the 1960s, the “Swann report” (1969) banned the therapeutic use of important antibiotics (e.g., penicillin and tetracyclines) for agricultural growth promotion, in Great Britain [38]. Despite the warnings, responses to antibiotic resistance as a shared, global problem were muffled. But Great Britain, quaked by the bovine spongiform encephalitis (“mad cow disease”) gathered action, which peaked in a 1998 report by the House of Lords, that finally led in 2001 to the launching of the European Antimicrobial Resistance Surveillance System (EARS-Net) [39].
Fig. 4.
Global spatial distribution of AMR peer-reviewed reports per selected time intervals (a) and since 1957 (b) (n = 1341).
Benefiting by the government interest and awareness of the antibiotic resistance problem, up until the 90's most of the research was mostly focused on the U.S.A. and some European countries, perfectly reflected by Fig. 4. Nevertheless, the low development aid for health, and particularly of AMR, in developing countries surely led to a slow implementation of national surveillance frameworks [40], which then translated into a small number of publications during this decade. By the 1990's, the WHO was fully promised with the global AMR problem and organized several working groups and meetings on the “major public health problem in both developed and developing countries” (WHO, 1994; WHO, 1997; WHO, 1998), probably motivating further research interest on this problematic in other countries. The WHO reports stressing the importance of including AMR on a global research agenda, and the relevance of tackling this topic on developing countries, was an important turning point. This, in combination with the increased infrastructures and qualified researches in these countries (e.g., India, China), created new perspectives and opportunities, which likely fed into the shifts of national agendas, translated into the consistent increase in publications in these countries. For example, China, one of the world's largest producers and consumers of antibiotics, began monitoring bacterial resistance nationwide since 2005 through the China Antimicrobial Surveillance Network [41] (Fig. 5). Finally, in 2013, AMR was indicated as one of the major global risks at the World Economic Forum: “While viruses may capture more headlines, arguably the greatest risk of hubris to human health comes in the form of antibiotic-resistant bacteria” (WEF 2013).
Fig. 5.
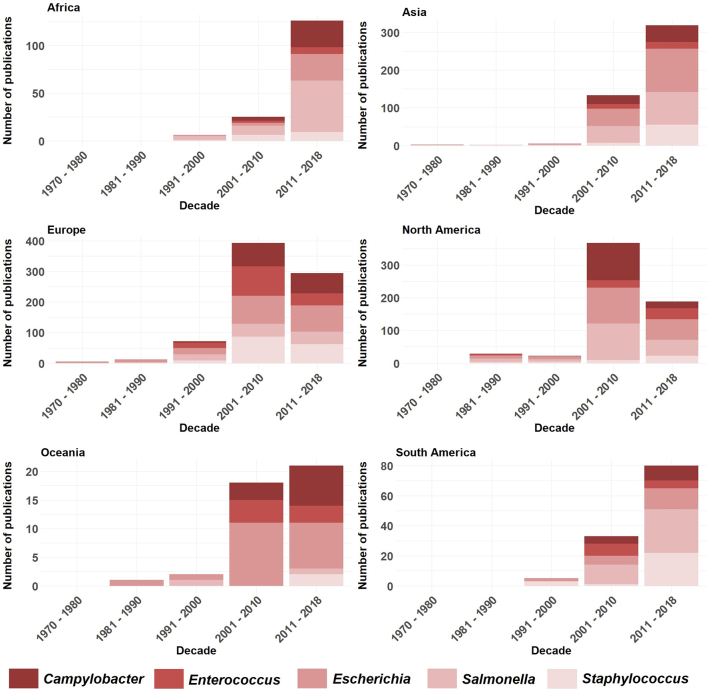
Temporal (per decade) and geographical (per continent) evolution of AMR publications focusing on the top-five bacterial genera (studies before 1970 are included in the 1970–1980 decade) (n = 1341).
The increased number of publications focused of AMR on food-producing animals in developing countries is likely a reflection of overall shifting research priorities and funding availability [41] but also a reflection of the increase in relevance of the use and misuse of antimicrobials in food-producing animals and their potential impact on human health [32]. A recent review just highlighted this observation in that reducing the level of antibiotic use in food-producing animals is likely to be beneficial to both animals and human beings [32]. It also underlines the One Health paradigm and possibly the sensitization of researchers in the veterinary field for conducting studies on AMR-related topics. In fact, AMR perfectly fits the One Health concept, defined as “…the collaborative effort of multiple disciplines – working locally, nationally, and globally – to attain optimal health for people, animals and our environment…” [42,43]. The recognition of the interdisciplinary nature of AMR led the Food and Agriculture Organization of the United Nations (FAO) and World Organization for Animal Health (OIE) to support the Global Action Plan on Antimicrobial Resistance (GAP) defined on the 68th World Health Assembly in May 2015 (44). But while the number of publications has steadily increased through time, reflecting the amount of research efforts worldwide, it also highlights poor investigation, and therefore surveillance, in developed countries. The management strategies of antimicrobial resistance in these resource-limited areas are specially challenging because strategies to fight and manage AMR cannot mirror those used in developing countries for the sake of depleting resources.
3.2. Temporal and geographical evolution of publications per bacterial genus
Publications tend to center more on bacteria related to zoonotic, foodborne casualties, reproducing policy and priorities worldwide and a focus on public health. Campylobacter spp., E. coli, Enterococcus spp., Salmonella spp. and Staphylococcus spp. are the top five bacteria published globally, with E. coli and Salmonella spp. representing the majority of bacteria under focus (Fig. 5). The top five bacteria in terms of publication counts were all zoonotic, which likely illustrates the engagedment of the research community in human health research and/or the way funding for infectious disease research is driven by human health.
The differences on the proportion of publication numbers in the top-five bacteria mirrors the reality of each country concerning food-producing animals and development status of the production systems (Fig. 2 Supplementary material). For example, in Europe and USA the difference between the number of studies focused on Salmonella sp. (>USA) and Campylobacter sp. (>Europe) is a consequence of the foodborne disease reality in each country. In Europe, the most common bacterial foodborne disease is campylobacteriosis, followed by salmonellosis, and non-typhoidal Salmonella spp. followed by Campylobacter spp. cause the highest burden (WHO, 2020). Conversely, the main foodborne disease (e.g., diarrhea, nausea, abdominal pain, vomiting) in USA is caused by Salmonella spp. [45]. Representation of the numbers for Africa, Asia and South America show Salmonella as the bacteria second most reported, highlighting the developing status of these continents, where the surveillance and effective measures for food safety may not be as efficient when comparing with developed countries [46]. E. coli was the most reported in Asia, Europe, North America and Oceania, which was expected since E. coli is the most abundant commensal and indicator bacteria of the intestinal tract of mammals, with some extra-intestinal pathogenic strains being a public health problem and its ubiquity and adaptive behavior playing a key role in the spread of AMR through the human-animal-environment interface [47]. Since E. coli are potential reservoirs of resistance determinants, it has been regularly monitored in food animals to assess the prevalence of antimicrobial resistance (EFSA & ECDC, 2014).
3.3. Class of antibiotics
Antibiotics have been routinely used in food-producing animals to control diseases (therapeutic use), to prevent diseases in healthy animals with somehow risk of infection (prophylactic use) and to improve feed efficiency (growth promotion use) [44]. With the risk of losing their efficiency, which would lead to a global public health problem, and to preserve the success of medically important antimicrobials, particularly those critically important to human medicine, the WHO developed guidelines on use of antimicrobials in food producing animals.
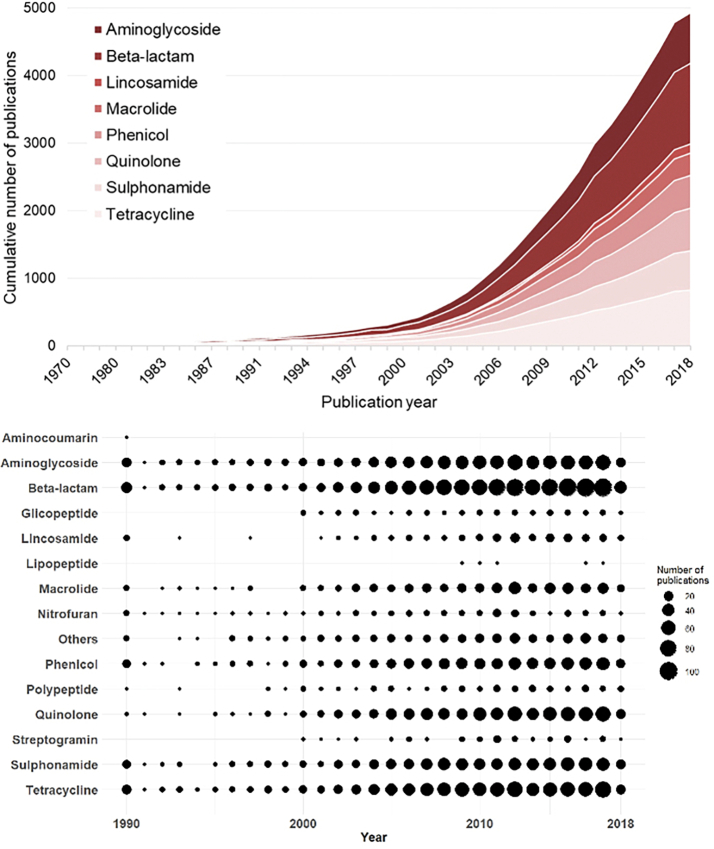
Our results show that several classes of antibiotics are widely found in food-producing animals and that their use has been increasing through time, particularly in the last 15 years, with highlight for tetracycline, quinolones and beta-lactams (Fig. 6). Even though tetracyclines are not, currently, a first option antimicrobial in the human clinic, they remain as one of those most used antibiotic classes in food-producing animals, representing a classical example of AMR emergence due to the selective pressure exerted by extended use. Quinolones are among the “Highest Priority Critically Important Antimicrobials“ (WHO, 2019). Quinolones and beta-lactams are first-line choice antimicrobials to treat infections in humans, due to their broad-spectrum action and low toxicity, also commonly administered to food-producing animals. Resistance to extended-spectrum beta-lactams (ESBLs), especially third- and fourth-generation cephalosporins and carbapenems, have worried medical practice and scientists in the last decade. WHO recently defined third- and fourth-generation cephalosporins as being “critically important” for human medicine since increasing resistance to this antimicrobial could compromise the treatment of bacteremia and bacterial meningitis [49]. But third-generation (ceftiofur) and fourth-generation cephalosporin (cefquinome) have been developed for veterinary use [49].
Fig. 6.
Temporal distribution of publications per class of antibiotics.
From a perspective of the number of publications by class of antibiotics in the last three decades (1990–2018), the Fig. 6 shows an increase in the number of publications globally. However, some classes of antibiotics call attention for their particularities, such as polypeptides and nitrofurans. Colistin is currently one of the last therapeutic options for severe infections by MDR bacteria in hospitals, it is an antibiotic that has been clinically used since the 60's and whose use has also been reported food-producing animals. Strikingly, an increase in the number of publications concerning colistin is registered only since the 2000's, probably due to its notoriety as a therapeutic option [50]. Nitrofuran antibiotics, used for treatment of urinary infections, have not shown an increase in the number of publications in the last few years when compared with the other classes, which is probably related with the progressive abolition of their use in food-producing animals but also as these are preferred antibiotics used in poultry. Works related with nitrofuran resistance are mainly centred in the description of cross-resistance with other antibiotic classes [51]. The continued use of the abovementioned antimicrobials that could potentially select for resistant organisms is worrying because of the importance of food animals in spreading and maintenance of resistance genes into the environment. In this line, WHO has been recommended the reduction or restriction of use, either for growth promotion or prophylactical purposes, of all classes of medically important antimicrobials in food-producing animals (WHO, 2017). The available data suggests that abolition of these antimicrobials in food-producing animals would represent minimal undesired consequences (e.g., less efficient animal health management or increased production cost) compared to the advantages thereof [52].
3.4. Geographical distribution of publications by livestock species
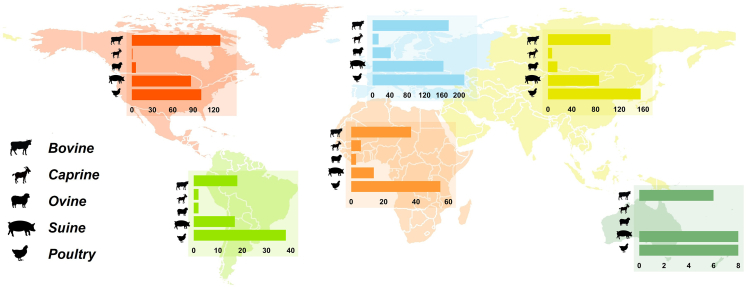
The most common livestock species being reported in the selected publications includes poultry, cattle (beef and buffalo meat), pig, sheep and goat to a lesser extent (Fig. 7). Interestingly, these figures are coincident with worldwide food animal production numbers (FAO, 2018). With the exception of north America, poultry was the group of animals with more AMR studies (Fig. 7); this greater prominence of publications in the poultry sector is probably related to the fact that this is the most produced meat worldwide (FAO, 2019) but also the one whose growing intensification was more profound in the last decades [53].
Fig. 7.
Geographic distribution of the number of AMR publications per specific livestock species (n = 1341).
Between 1961 and 2016, poultry meat production has increased globally from 9 to 120 million tones, and in 2016 this represented 36% of global meat production (FAO, 2020). Likewise, in the last three decades, egg production has increased by more than 150% worldwide, from 15 to 81 million tones (FAO, 2020). Most of this increase has occurred in Asia, where production rose almost fourfold [54]. In United States of America, cattle was under focus of most publications, probably because this country is the world's largest bovine (e.g., beef and buffalo) producer, generating 11–12 million tones in 2014 (USDA-FAS, 2019). Between 1961 and 2014, cattle meat production has more than doubled globally, from 28 million tones yearly to 68 million tones. Other major producers are Brazil and China.
Globally, pig and poultry production are the fastest growing food-producing animals segments. Increasingly, pigs and cattle are also raised under similar conditions of confinement and high density like poultry (FAO, 2018) which poses a particularly significant threat, not only due to intensive animal production, but also exacerbated by poor sanitation infrastructure [55]. Several developing countries are also showing a shift towards industrialization of food animals production by replacing traditional systems for intensive units, with much of that rise occurring in Asia, South America and North Africa [34]. Our analyses suggests that there has been an increased interest in food-producing animals research issues in developed countries, which are those precisely where there has been an increment of meat production and where human population growth is expected to increase. In one sense, this means that antimicrobial resistance in food-producing animals and their threat to pass AMR determinants to human via food chain is now acknowledged and that effective control and biosecurity measures associated may have been applied in the meanwhile.
3.5. Study limitations
We recognize that our study has some limitations. Firstly, we only focused on articles that have been published in academic journals indexed in Web of Science, excluding substantial proportion of knowledge that may have been published in other formats (e.g., books, reports, and national journals) and in government and industry reports, summarizing significant surveillance efforts around the globe. Publications that did not include the used search terms in the title might have been left out of our analysis, although we suspect that our results reflect the general trends within the thematic research landscape. Whereas our results are based only on peer-review literature, they likely reflect the observed interest by the scientific community, measured here by the number of publications, and the global dynamics within this research field itself, and not the actual levels of antimicrobial resistance and/or use. However it is also important to note that research interest should not be confused with incidence of antimicrobial resistance. In addition, this analysis was restricted to international journals in English, therefore a linguistic bias may exist. But the caveats discussed are outweighed by the findings assembled. We have reviewed a large body of evidence suggesting a clear temporal and geographical bias towards the pervasive use of antibiotics in food-producing animals, offering a valid representation within this research field at a global level.
3.6. Conclusions
There was an increase in the number of publications on the AMR in the last decade which is probably a reflection of the profound changes that food-animal production systems have undergone over the past decades: under the umbrella of rapid population turnover, large number of animals are raised in confinement (56). In modern food-animal production, antimicrobial agents are regularly used as health management tools. Today, food animals and animal-derived products are traded worldwide. Thus, the selection of antimicrobial resistance in one country becomes a global problem. This has alerted governments and stakeholders emphasizing the need for broad initiatives, control and monitoring systems for containment of antimicrobial resistance, which is laid out in the trend of publications. Our results show that throughout the time there was a shifting in research priorities and this was most likely related to regional differences in food-producing animals production and changing practices in the food production industry. Antimicrobial resistance research in food-producing animals in developed countries is still growing, reflecting either changes in livestock production systems [57] but also because they realized the negative effect of the resistance emergency globally.
Our assessment outlines three global priorities for action. First, our maps show regions that are poorly surveyed and where sampling intensification efforts could be most valuable, namely north Asia and Africa. Second, our findings clearly indicate that the highest publication rate is currently allocated to India and China. The increase in meat production and the shift in food animals production systems stress the importance of these countries to implement actions to prevent further aggravation of the AMR problem. Third, countries such as the U.S.A. and some European countries, where national efforts for harmonized surveillance, uniform reporting and proactive policy actions occurred earlier, and where antibiotic ban and restrictions for growth promotion is at least voluntary, should serve as encouraging examples for the change to new strategies in food animal production [17]. Since current trends towards intensive livestock production are unlikely to reverse, the research in food-producing animals will be an ongoing area of interest. Our review shows where research in this area has been focused over the last decades and where it should move forward. We conclude that the global effort for AMR monitoring and research is poorly allocated, with most of the scientific tools and resources focused on regions where meat production has plateaued and strong national plans are ongoing, therefore where the next important problems are least likely to prevail [17,37]. Systematic efforts should include antibiotic surveillance in regions or countries where no, or little data, is available. However, the lack of these structures in developing countries, where there is often inadequate surveillance and minimal laboratory capacity and where livestock producing is swiftly increasing, can be a serious setback [16]. Alternative models on “smart surveillance” on middle and low-income countries must focus on inexpensive testing, developed by trained workers which could feed a web-based system, national and internationally connected. This will obviously not replace the necessity for laboratories and standardized practical procedures but instead would allow a sense of monitoring and surveillance in areas where no laboratories are available. Additionally, international collaboration is essential as AMR is a global problem, with no borders, and we encouraged this collaboration between developing and developed countries. As the threat of AMR continues to grow, more work is needed to overcome financial and regional gaps of surveillance, while improving and harmonizing methodologies towards an integrated research and action framework on AMR. This need goes beyond the food producing animals compartment and should expand into the wildlife compartment, where i) rising interest in scientific community is notorious, ii) poorly surveyed countries should intensified sampling efforts and iii) taxonomic and geographical research gaps have been identified [58].
Ethical statement
Not applicable.
Author's contribution
R.T.T. and M.V.C. designed the work. R.T.T. wrote the manuscript. J.F and J.D.P. prepared the database. J.C. analyzed the data. All authors gave final approval of the version to be published.
Funding statement
This work was supported by the project EcoARUn: POCI-01-0145-FEDER-030310- funded by FEDER, through COMPETE2020 - Programa Operacional Competitividade e Internacionalização (POCI), and by national funds (OE), through FCT/MCTES.
Funding
This research was supported by the project EcoARUn: POCI-01-0145-FEDER-030310- funded by FEDER, through COMPETE2020 - Programa Operacional Competitividade e Internacionalização (POCI), and by national funds (OE), through FCT/MCTES.
Author's contribution
R.T.T. and M.V.C. designed the work. R.T.T. wrote the manuscript. J.F and J.D.P. prepared the database. J.C. analyzed the data. All authors gave final approval of the version to be published.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
R. T. Torres is funded by national funds (OE), through FCT – Fundação para a Ciência e a Tecnologia, I.P., in the scope of the framework contract foreseen in the numbers 4, 5 and 6 of the article 23, of the Decree-Law 57/2016, of August 29, changed by Law 57/2017, of July 19. J. Fernandes was supported by a grant (BI/CESAM/00056/POCI-01-0145-FEDER-030310) within the research project EcoARUn (POCI-01-0145-FEDER-030310).Thanks are due to FCT/MCTES for the financial support to CESAM (UID/AMB/50017/2019), through national funds. Strategic funding to cE3c (UID/BIA/00329/2020) and BioISI (UID/Multi/04046/2020) from FCT is also acknowledged. We apologize to the authors of many significant papers in the field for not citing their work because of linguistic restrictions. JC was supported by a research contract (CEECIND/01428/2018) from the Fundação para a Ciência e a Tecnologia (FCT).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2021.100324.
Appendix A. Supplementary data
Supplementary material
References
- 1.Woolhouse M., Farrar J. An intergovernmental panel on antimicrobial resistance. Nature. 2014;509:555–557. doi: 10.1038/509555a. [DOI] [PubMed] [Google Scholar]
- 2.Ventola C.L. The antibiotic resistance crisis: part 1: causes and threats. Pharm Ther. 2015;40(4):277–283. [PMC free article] [PubMed] [Google Scholar]
- 3.Monroe S., Polk R. Antimicrobial use and bacterial resistance. Curr. Opin. Microbiol. 2000;3(5):496–501. doi: 10.1016/s1369-5274(00)00129-6. [DOI] [PubMed] [Google Scholar]
- 4.Gould I.M., Bal A.M. New antibiotic agents in the pipeline and how they can help overcome microbial resistance. Virulence. 2013;4(2):185–191. doi: 10.4161/viru.22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laxminarayan R., Matsoso P., Pant S., Brower C., Røttingen J., Klugman K. Antimicrobials : access and sustainable effectiveness 1 access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387:168–175. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- 6.Spellberg B., Hansen G.R., Kar A., Cordova C.D., Price L.B., Johnson J.R. National Academy of Medicine; Washington, DC: 2016. Antibiotic resistance in humans and animals. Discussion paper.http://www.nam.edu/antibiotic-resistance-in-humans-and-animals [Google Scholar]
- 7.Klein E.Y., Van Boeckel T.P., Martinez E.M., Pant S., Gandra S., Levin S.A. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. PNAS. 2018;115(15):3463–3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laxminarayan R., Duse A., Wattal C., AK M. Zaidi, HF L. Wertheim, Sumpradit N. The Lancet Infectious Diseases Commission Antibiotic resistance—the need for global solutions Part 1: Global epidemiology of antibiotic resistance and use. Lancet Infect Dis [Internet] 2013;13(13):1057–1098. doi: 10.1016/S1473-3099(13)70318-9. www.thelancet.com/infection Available from: [DOI] [PubMed] [Google Scholar]
- 9.Okeke I.N., Laxminarayan R., Bhutta Z.A., Duse A.G., Jenkins P., Brien T.F.O. Review antimicrobial resistance in developing countries. Part I : recent trends and current status. 2005;5(August):481–493. doi: 10.1016/S1473-3099(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 10.Chambers H.F., Deleo F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009;7(September):629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nordmann P., Poirel L., Dortet L. Global spread of Carbapenemase- producing Enterobacteriaceae. Emerg. Infect. Dis. 2011;17(10):1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandra S., Barter D.M., Laxminarayan R. Economic burden of antibiotic resistance : how much do we really know ? Clin. Microbiol. Infect. 2014;20:973–979. doi: 10.1111/1469-0691.12798. [DOI] [PubMed] [Google Scholar]
- 13.Van Boeckel T.P., Gandra S., Ashok A., Caudron Q., Grenfell B.T., Levin S.A. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet. 2014;14:742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 14.O’Neill J. 2014. Antimicrobial Resistance : Tackling a Crisis for the Health and Wealth of Nations. [Google Scholar]
- 15.De Kraker M.E.A., Stewardson A.J., Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050 ? PLoS Med. 2016;13(11):1–6. doi: 10.1371/journal.pmed.1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hay S.I., Rao P.C., Dolecek C., Day N.P.J., Stergachis A., Lopez A.D. Measuring and mapping the global burden of antimicrobial resistance. BMC Med. 2018;16(78):1–3. doi: 10.1186/s12916-018-1073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Boeckel T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P. Global trends in antimicrobial use in food animals. PNAS. 2015;112(18):5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein E.Y., Van Boeckel T.P., Martinez E.M., Pant S., Gandra S., Levin S.A. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. U. S. A. 2018;115(15):E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomley F.M., Shirley M.W. Livestock infectious diseases and zoonoses. Philos. Trans. R. Soc. B. 2009;364:2637–2642. doi: 10.1098/rstb.2009.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall B.M., Levy S.B. Food animals and antimicrobials : impacts on human health. Clin. Microbiol. Rev. 2011;24(4):718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woolhouse M., Ward M., Van Bunnik B., Farrar J. Antimicrobial resistance in humans, livestock and the wider environment. Philos Trans R Soc B Biol Sci. 2015;370(1670) doi: 10.1098/rstb.2014.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy S.B., Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 2004;10(12):S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 23.Bartlett J.G., Gilbert D.N., Spellberg B. Seven ways to preserve the miracle of antibiotics. Clin. Infect. Dis. 2013;56(10):1445–1450. doi: 10.1093/cid/cit070. [DOI] [PubMed] [Google Scholar]
- 24.Qiao M., Ying G., Singer A.C., Zhu Y. Review of antibiotic resistance in China and its environment. Environ. Int. 2018;110:160–172. doi: 10.1016/j.envint.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Tang K., Ca N., Nóbrega D., Cork S., Ronksley P., Barkema H. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings : a systematic review and meta-analysis. Lancet Planet Heal. 2017;1(8):9–11. doi: 10.1016/S2542-5196(17)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price L.B., Johnson E., Vailes R., Silbergeld E. Fluoroquinolone-resistant Campylobacter isolates from conventional and antibiotic-free chicken products. Environ. Health Perspect. 2005;113(5):557–560. doi: 10.1289/ehp.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ventola C.L. The antibiotic resistance crisis: part 1: causes and threats. Pharm Ther. 2015;40(4):277. [PMC free article] [PubMed] [Google Scholar]
- 28.Landers T.F. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep. 2012;127:4–22. doi: 10.1177/003335491212700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woolhouse M.E.J., Ward M.J. Sources of Antimicrobial Resistance. Science. 2013;341:1460–1461. doi: 10.1126/science.1243444. [DOI] [PubMed] [Google Scholar]
- 30.Wright G.D. Antibiotic resistance in the environment: a link to the clinic ? Curr. Opin. Microbiol. 2010;13:589–594. doi: 10.1016/j.mib.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Silbergeld E.K., Davis M., Leibler J.H., Peterson A.E. One reservoir: redefining the community origins of antimicrobial-resistant infections. Med Clin North Am. 2008 Nov;92(6):1391–1407. doi: 10.1016/j.mcna.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Tang K.L., Caffrey N.P., Nóbrega D.B., Cork S.C., Ronksley P.E., Barkema H.W. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet Heal. 2017;1(8):e316–e327. doi: 10.1016/S2542-5196(17)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price L.B., Stegger M., Hasman H., Aziz M., Larsen J., Andersen P.S. Staphylococcus aureus CC398: Host Adaptation and Emergence of Methicillin Resistance in Livestock. MBio. 2012;3(1) doi: 10.1128/mBio.00305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thornton P.K. Livestock production: recent trends, future prospects. Philos. Trans. R. Soc. B. 2010;365:2853–2867. doi: 10.1098/rstb.2010.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bud R. Oxford University Press on Demand; 2007. Penicillin: Triumph and Tragedy. [Google Scholar]
- 36.Ng H., Vaughn R.H., Stewart G.F., Nagek C.W., Simpson K. Antibiotics in poultry meat preservation: development of resistance among spoilage organisms. Appl. Microbiol. 1957;5(5):331–333. doi: 10.1128/am.5.5.331-333.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boeckel T.P., Van Pires J., Silvester R., Zhao C., Song J., Criscuolo N.G. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science. 2019;365(1266):1. doi: 10.1126/science.aaw1944. [DOI] [PubMed] [Google Scholar]
- 38.Kirchhelle C. Pharming animals: a global history of antibiotics in food production (1935–2017) Palgrave Commun [Internet] 2018;4(1) doi: 10.1057/s41599-018-0152-2. Available from. [DOI] [Google Scholar]
- 39.Podolsky S.H. The evolving response to antibiotic resistance (1945 – 2018) Palgrave Commun. 2018;4(124):1–8. [Google Scholar]
- 40.Farag M., Nandakumar A.K., Wallack S.S., Gaumer G., Hodgkin D. Does funding from donors displace government spending for health in developing countries? Health Aff. 2009;28(4):1045–1055. doi: 10.1377/hlthaff.28.4.1045. [DOI] [PubMed] [Google Scholar]
- 41.Qu J., Huang Y., Lv X. Crisis of antimicrobial resistance in China: Now and the future. Front. Microbiol. 2019;10(SEP):1–6. doi: 10.3389/fmicb.2019.02240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson T.P., Bu D.P., Carrique-Mas J., Fèvre E.M., Gilbert M., Grace D. Antibiotic resistance is the quintessential one health issue. Trans. R. Soc. Trop. Med. Hyg. 2016;110(7):377–380. doi: 10.1093/trstmh/trw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hernando-amado S., Coque T.M., Baquero F., Martínez J.L. One health and Global Health perspectives. Nat Microbiol [Internet] 2019;4(September) doi: 10.1038/s41564-019-0503-9. Available from. [DOI] [PubMed] [Google Scholar]
- 44.Aidara-Kane A., Angulo F.J., Conly J., Minato Y., Silbergeld E.K., McEwen S.A. World Health Organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. Antimicrob. Resist. Infect. Control. 2018;7(1):1–8. doi: 10.1186/s13756-017-0294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mattia D.D., Manikonda K. Vol. 6262. Surveillance Summaries MMWR; 2018. Morbidity and Mortality Weekly Report Surveillance for Foodborne Disease Outbreaks — United States Centers for Disease Control and Prevention MMWR Editorial and Production Staff MMWR Editorial Board [Internet]https://www.cdc.gov/mmwr/pdf/ss/ss6202.pdf Available from: [Google Scholar]
- 46.Havelaar A.H., Kirk M.D., Torgerson P.R., Gibb H.J., Hald T., Lake R.J. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12(12):1–23. doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szmolka A., Nagy B. Multidrug resistant commensal Escherichia coli in animals and its impact for public health. Front. Microbiol. 2013;4(September):1–13. doi: 10.3389/fmicb.2013.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cameron-Veas K., Moreno M.A., Fraile L., Migura-Garcia L. Shedding of cephalosporin resistant Escherichia coli in pigs from conventional farms after early treatment with antimicrobials. Vet. J. 2016 May;211:21–25. doi: 10.1016/j.tvjl.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 50.Rhouma M., Beaudry F., Thériault W., Letellier A. Colistin in pig production: Chemistry, mechanism of antibacterial action, microbial resistance emergence, and one health perspectives. Front. Microbiol. 2016;7(NOV):1–22. doi: 10.3389/fmicb.2016.01789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papich M.G. Elsevier Health Sciences; 2015. Saunders Handbook of Veterinary Drugs-e-Book: Small and Large Animal. [Google Scholar]
- 52.McEwen S.A., Angulo F.J., Collignon P.J., Conly J.M. Unintended consequences associated with national-level restrictions on antimicrobial use in food-producing animals. Lancet Planet Heal. 2018;2(7):e279–e282. doi: 10.1016/S2542-5196(18)30138-4. [DOI] [PubMed] [Google Scholar]
- 53.Windhorst H.W. Changes in poultry production and trade worldwide. Worlds Poult Sci J. 2006;62(4) [Google Scholar]
- 54.Yang Z., Rose S.P., Yang H.M., Pirgozliev V., Wang Z.Y. Egg production in China. Worlds Poult Sci J. 2018;74(3):417–426. [Google Scholar]
- 55.Thakur S., Gray G.C. The mandate for a global “one health” approach to antimicrobial resistance surveillance. Am J Trop Med Hyg. 2019;100(2):227–228. doi: 10.4269/ajtmh.18-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thakur S., Gray G.C. The mandate for a global “ one health ” approach to antimicrobial resistance surveillance. Am J Trop Med Hyg. 2019;100(2):227–228. doi: 10.4269/ajtmh.18-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torres R.T., Carvalho J., Cunha M.V., Serrano E., Palmeira J.D., Fonseca C. Temporal and geographical research trends of antimicrobial resistance in wildlife–a bibliometric analysis. One Health. 2020 Nov;21:100198. doi: 10.1016/j.onehlt.2020.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material