Abstract
Study Objectives
To examine associations between positive airway pressure (PAP) therapy, adherence and incident diagnoses of Alzheimer’s disease (AD), mild cognitive impairment (MCI), and dementia not otherwise specified (DNOS) in older adults.
Methods
This retrospective study utilized Medicare 5% fee-for-service claims data of 53,321 beneficiaries, aged 65 and older, with an obstructive sleep apnea (OSA) diagnosis prior to 2011. Study participants were evaluated using ICD-9 codes for neurocognitive syndromes (AD [n = 1,057], DNOS [n = 378], and MCI [n = 443]) that were newly identified between 2011 and 2013. PAP treatment was defined as the presence of at least one durable medical equipment (Healthcare Common Procedure Coding System [HCPCS]) code for PAP supplies. PAP adherence was defined as at least two HCPCS codes for PAP equipment, separated by at least 1 month. Logistic regression models, adjusted for demographic and health characteristics, were used to estimate associations between PAP treatment or adherence and new AD, DNOS, and MCI diagnoses.
Results
In this sample of Medicare beneficiaries with OSA, 59% were men, 90% were non-Hispanic whites and 62% were younger than 75 years. The majority (78%) of beneficiaries with OSA were prescribed PAP (treated), and 74% showed evidence of adherent PAP use. In adjusted models, PAP treatment was associated with lower odds of incident diagnoses of AD and DNOS (odds ratio [OR] = 0.78, 95% confidence interval [95% CI]: 0.69 to 0.89; and OR = 0.69, 95% CI: 0.55 to 0.85). Lower odds of MCI, approaching statistical significance, were also observed among PAP users (OR = 0.82, 95% CI: 0.66 to 1.02). PAP adherence was associated with lower odds of incident diagnoses of AD (OR = 0.65, 95% CI: 0.56 to 0.76).
Conclusions
PAP treatment and adherence are independently associated with lower odds of incident AD diagnoses in older adults. Results suggest that treatment of OSA may reduce the risk of subsequent dementia.
Keywords: obstructive sleep apnea, CPAP, Medicare, Alzheimer’s disease, mild cognitive impairment
Statement of Significance.
Emerging evidence has linked obstructive sleep apnea (OSA) to cognitive impairment and dementia incidence. However, research focused on the impact of OSA treatment on dementia is scarce. This analysis of Medicare Claims Data investigates associations between positive airway pressure (PAP) therapy and incident dementia diagnosis, at the population level. We demonstrate that treatment with PAP therapy was associated during a subsequent period of 3 years with lower odds of incident diagnoses of Alzheimer’s dementia (AD) and dementia not otherwise specified. Adherence to PAP therapy was also associated with lower odds of incident AD diagnosis. These findings suggest a protective role of PAP therapy with respect to dementia among older adults with OSA.
Introduction
Obstructive sleep apnea (OSA) is a common disorder characterized by repeated episodes of upper airway obstruction, sleep fragmentation, and hypoxia during sleep. This disorder is associated with many serious health and socioeconomic consequences, including cognitive impairment [1].
The risk of OSA increases with age [2–4]. Up to 56% of older Americans may suffer from OSA, even though the majority remain undiagnosed [5, 6]. Older individuals are also more likely to experience many of the health consequences that are associated with OSA, including adverse neurocognitive outcomes [7]. Prior work has revealed compelling relationships between OSA and objective measures of cognitive performance (e.g. executive function, attention, and memory) [8–12], both incident dementia [13] and Alzheimer’s disease (AD) [14], as well as mild cognitive impairment (MCI) [15]. Still, data regarding the potential benefits of OSA treatment on cognitive performance are inconclusive [16–20], and any associations between OSA treatment and risk of incident neurodegenerative conditions, in particular, are lacking.
Although some evidence suggests a possible therapeutic benefit of positive airway pressure (PAP) therapy on cognitive performance in older individuals [21], advancement of knowledge on this topic has been limited by the use of small, single-center, or regional samples, heterogeneity in outcome measures, and limited enrollment of older individuals. Furthermore, the role of OSA treatment in the development of specific neurodegenerative conditions such as AD—the most frequent cause of dementia in older adults—has not been sufficiently studied. Specifically, whether the use of PAP therapy in older adults with OSA mitigates the risk of development of MCI or dementia syndromes remains unknown, as longitudinal, population-level data are lacking.
The purpose of this study was to determine, within a cohort of older Americans with OSA, longitudinal associations between OSA treatment and odds of new dementia diagnosis. We hypothesized that older adults who received and adhered to treatment for OSA (PAP therapy) would have a lower risk of incident diagnoses of MCI, AD, or other dementia subtypes compared to untreated or nonadherent individuals.
Methods
This was a retrospective cohort study of Medicare fee-for-service claims files (outpatient, durable medical equipment [DME], and carrier files). Data were extracted from a representative sample of older adults with OSA, aged 65 or older, derived from a 5% random sample (n = 2,758,197) from the Medicare Beneficiary Summary File in 2011, 2012, and 2013.
This study used only de-identified claims data. All study procedures were approved by the University of Michigan Institutional Review Board (IRBMED).
Study population
Medicare beneficiaries aged 65 and older who carried an OSA ICD-9 diagnosis code prior to 2011 were selected for analyses. To examine the temporal associations between preexisting OSA, OSA treatment patterns, and new dementia diagnoses, we excluded Medicare beneficiaries who were given an incident diagnosis of OSA (as reflected by the presence of polysomnography or home sleep apnea testing claims) during the 2011–2013 study period.
Beneficiaries with OSA who were offered and issued treatment with PAP were labeled as “treated” based on the presence of one or more PAP Healthcare Common Procedure Coding System (HCPCS) claims codes, indicating the presence of at least one prescription fill for PAP equipment, in the DME file for the 2011–2013 period under study. The HCPCS is a standardized code system used by medical providers to submit healthcare claims to Medicare and other health insurances. Beneficiaries were considered “adherent” to PAP treatment, if two more HCPCS claims for PAP supplies (e.g. filters, cushions, masks, or tubing), separated by at least 1 month from each other, were present during the study period (Supplementary Table 1). At least two equipment claims over the 3-year follow-up interval were chosen to be consistent with the work by Patel et al. [22], which validated a threshold value of 0.7 PAP equipment refills (claims) per year to best discriminate between PAP-adherent and nonadherent patients (using the Medicare definition of adherence of at least 4 h/night, at least 70% of nights).
New neurocognitive diagnoses of interest—AD, dementia not otherwise specified (DNOS—a category that included vascular dementia, dementia with Parkinsonism/Lewy bodies, and frontotemporal dementia), and MCI—were also identified with ICD-9 codes (Supplementary Table 2). These neurocognitive diagnoses were mutually exclusive. Beneficiaries with a prevalent neurocognitive diagnosis who were identified in the 2011 files were excluded from the analysis. In a recent sensitivity analysis of Medicare claims, this methodology correctly classifies dementia diagnoses in 85% of patients with dementia who were diagnosed clinically [23].
Additional comorbidity data extracted from Medicare files included diagnoses of hypertension, stroke, diabetes mellitus, and depression. Cardiovascular disease included a diagnosis of arrhythmia, congestive heart failure, or acute myocardial infarction (Table 1). Demographic information, i.e. age, sex, race/ethnicity, and state of residence, was also obtained from Medicare files. Age was classified into five categories: 65–69, 70–74, 75–79, 80–84, and 85 years or older. Self-reported race and ethnicity, as recorded by Medicare beneficiaries at the time of social security enrollment, included three categories: non-Hispanic white, non-Hispanic black, and Hispanic/Asian/other.
Table 1.
Demographic characteristics and comorbidities among Medicare beneficiaries with obstructive sleep apnea
| Demographic and health characteristics | All participants | Alzheimer’s disease | DNOS | MCI |
|---|---|---|---|---|
| N (column %) | N (row %) | N (row %) | N (row %) | |
| Sample size | 53,321 (100) | 1,057 (2.0) | 378 (0.7) | 443 (0.8) |
| Sex | ||||
| Men | 31,627 (59) | 567 (1.8) | 241 (0.8) | 267 (0.8) |
| Women | 21,694 (41) | 490 (2.3) | 137 (0.6) | 176 (0.8) |
| Age (years) | ||||
| 65–69 | 18,099 (34) | 82 (0.5) | 46 (0.3) | 85 (0.5) |
| 70–74 | 15,100 (28) | 162 (1.1) | 86 (0.6) | 100 (0.7) |
| 75–79 | 10,266 (19) | 241 (2.4) | 85 (0.8) | 119 (1.2) |
| 80–84 | 6,232 (12) | 288 (4.6) | 87 (1.4) | 78 (1.3) |
| 85+ | 3,624 (7) | 284 (7.8) | 74 (2.0) | 61 (1.7) |
| Race/ethnicity | ||||
| Non-Hispanic white | 48,097 (90) | 936 (2.0) | 340 (0.7) | 404 (0.8) |
| Non-Hispanic black | 3,353 (6) | 67 (2.0) | 24 (0.7) | 27 (0.8) |
| Hispanic/Asian/Other* | 1,871 (4) | 54 (2.9) | 14 (0.8) | 12 (0.6) |
| Stroke | 3,530 (7) | 208 (5.9) | 110 (3.1) | 73 (2) |
| Hypertension | 44,370 (83) | 951 (2.0) | 343 (0.8) | 385 (0.9) |
| Diabetes mellitus | 24,670 (46) | 497 (2.0) | 185 (0.8) | 214 (0.9) |
| Cardiovascular diseases | 23,147 (43) | 653 (2.8) | 237 (1.0) | 233 (1) |
| Depression | 9,353 (18) | 442 (4.7) | 164 (1.8) | 176 (1.9) |
*Other ethnicity = Pacific Islander and Native American.
Statistical analyses
Descriptive statistics were used to estimate the frequencies and proportions of demographic and health characteristics of Medicare beneficiaries with OSA within three neurocognitive subgroups of interest: AD, DNOS, or MCI, as well as within subgroups defined by PAP treatment or adherence.
Bivariate analyses were used to estimate the frequencies of beneficiaries who were prescribed PAP treatment or were PAP-adherent within each of the neurocognitive groups.
Logistic regression was applied to estimate associations between PAP treatment and adherence to treatment in Medicare beneficiaries with an OSA diagnosis that preceded the diagnosis of AD, DNOS, or MCI. To account for the clustering of Medicare beneficiaries by state and county, we estimated the odds ratio (OR) and 95% confidence intervals (95% CIs) with regression procedures (generalized estimating equation) for correlated data. Adjustment for state and county was informed by data from our group that suggested variation in OSA treatment and treatment adherence among Medicare beneficiaries in different states and within states [24]. In adjusted analyses, we controlled for potential confounders, including age, sex, race, stroke, hypertension, cardiovascular disease, and depression. SAS 9.4 (Cary, NC) was used for descriptive and regression analyses.
Results
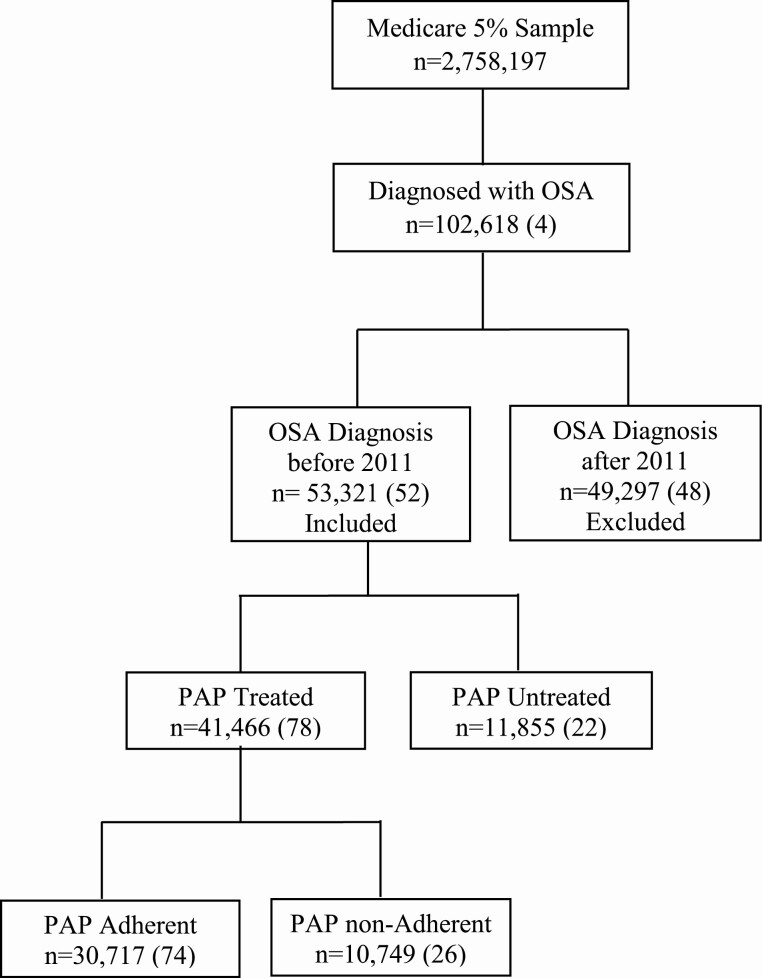
Of the entire 5% sample of Medicare beneficiaries (n = 2,758,197), approximately 4% (n = 102,618) had an OSA diagnosis. Among those beneficiaries with OSA, 53,321 received an OSA diagnosis prior to 2011 and therefore were included in this analysis (Figure 1). In this sample, 59% were men, 90% were non-Hispanic whites, and 62% were younger than 75 years. From 2011 to 2013 (inclusive), incident neurocognitive diagnoses were rare, with AD, DNOS, and MCI identified in 1,057 (2%), 378 (<1%), and 443 (<1%) of the Medicare beneficiaries with OSA diagnoses, respectively. AD was more frequent in women than men and in Hispanics and Asians than other races/ethnicities. AD frequency increased with age. Distributions of race/ethnicity and sex were similar among beneficiaries with DNOS or MCI. As with AD, the proportions of Medicare beneficiaries with DNOS and MCI increased with age (Table 1).
Figure 1.
Distribution of PAP treatment and treatment adherence among Medicare beneficiaries with OSA; frequencies and proportions (in parenthesis).
Among OSA-diagnosed beneficiaries, 78% were prescribed PAP treatment and 74% of those adhered to their treatment. The percentages of beneficiaries who received PAP treatment and were adherent—by gender, age, race, and comorbidities—are given in Table 2.
Table 2.
Demographic characteristics and comorbidities among Medicare beneficiaries with a diagnosis of obstructive sleep apnea by treatment and treatment adherence (n = 53,321)
| Demographic and health characteristics | Treated among diagnosed | Adhered among treated |
|---|---|---|
| Sample size N (%) | 41,466 (78) | 30,717 (74) |
| Gender | ||
| Men | 25,462 (81) | 19,101 (75) |
| Women | 16,004 (74) | 11,616 (73) |
| Age (years) | ||
| 65–69 | 14,134 (78) | 10,191 (72) |
| 70–74 | 12,137 (80) | 9,271 (76) |
| 75–79 | 8,065 (79) | 6,103 (76) |
| 80–84 | 4,690 (75) | 3,451 (74) |
| 85+ | 2,440 (67) | 1,701 (69) |
| Race/ethnicity | ||
| Non-Hispanic white | 37,676 (78) | 28,268 (75) |
| Non-Hispanic black | 2,504 (75) | 1,602 (64) |
| Hispanic | 314 (64) | 170 (54) |
| Asian | 204 (56) | 131 (64) |
| Other | 768 (76) | 546 (71) |
| Stroke | 2,546 (72) | 1,815 (71) |
| Hypertension | 34,622 (78) | 26,167 (76) |
| Diabetes mellitus | 19,547 (79) | 14,653 (75) |
| Cardiovascular diseases | 17,573 (76) | 13,259 (75) |
| Depression | 6,831 (73) | 5,010 (73) |
Other ethnicity = Pacific Islander and Native American.
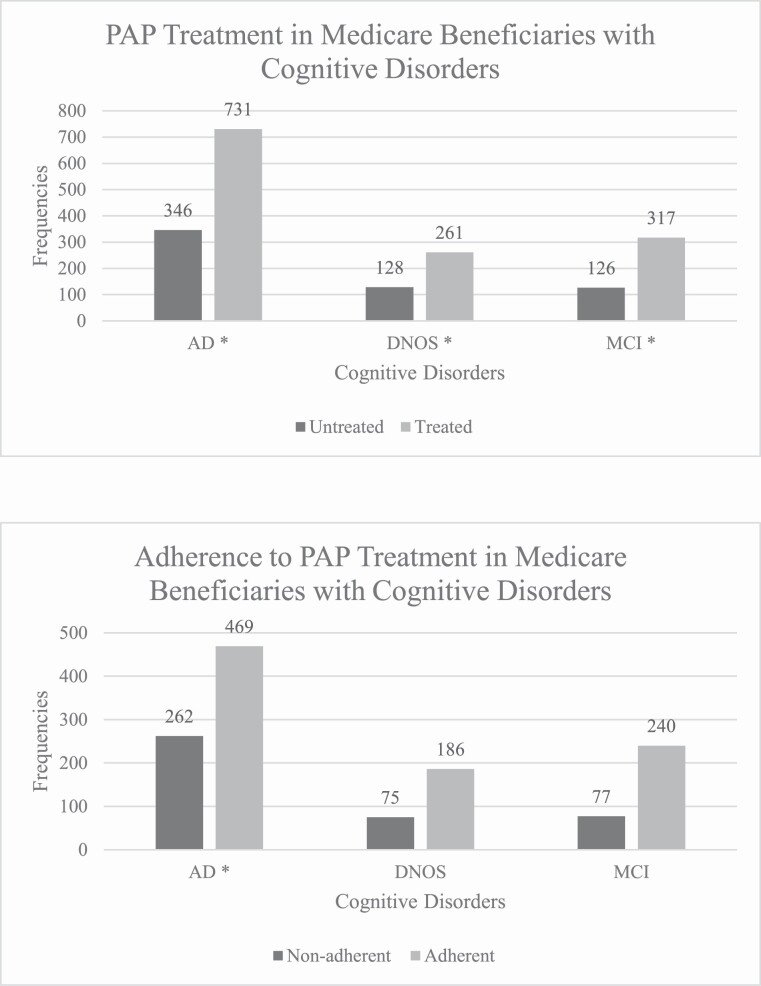
Bivariate estimates of PAP treatment and adherence suggest that among the 1,909 Medicare beneficiaries with dementia and OSA diagnoses, 1,309 (69%) were PAP-treated and of those, 895 (68%) were PAP-adherent (Figure 2). In analyses adjusted for demographic characteristics and comorbidities, beneficiaries who were prescribed PAP (in comparison to beneficiaries who were not prescribed PAP) had a significantly lower odds of developing AD and DNOS (OR = 0.78, 95% CI: 0.69 to 0.89; and OR = 0.69, 95% CI: 0.55 to 0.85), respectively. Lower odds of MCI, approaching statistical significance, were also observed among beneficiaries who were prescribed PAP, median OR = 0.82 (95% CI: 0.66 to 1.02). Attenuation of this association in the adjusted model was attributable mainly to the inclusion of age, stroke, and depression as covariates. Adherence with PAP treatment was associated with lower odds of developing AD, OR = 0.65 (95% CI: 0.56 to 0.76), though not DNOS or MCI (Table 3). Exclusion of stroke-diagnosed or depression-diagnosed beneficiaries did not change the relationships between PAP therapy and dementia (data not shown).
Figure 2.
Frequencies of OSA-diagnosed Medicare beneficiaries treated with and adherent to PAP, by dementia diagnoses.
Table 3.
Associations of OSA treatment and treatment adherence with dementia among 53,321 Medicare beneficiaries with OSA diagnosis
| Alzheimer’s disease | DNOS | Mild cognitive disorder | ||||
|---|---|---|---|---|---|---|
| Unadjusted, odds ratio (95% CI) | Adjusted*, odds ratio (95% CI) | Unadjusted, odds ratio (95% CI) | Adjusted*, odds ratio (95% CI) | Unadjusted, odds ratio (95% CI) | Adjusted*, odds ratio (95% CI) | |
| OSA treatment | 0.60 (0.52 to 0.69) | 0.78 (0.69 to 0.89) | 0.58 (0.47 to 0.71) | 0.69 (0.55 to 0.85) | 0.72 (0.58 to 0.90) | 0.82 (0.66 to 1.02) |
| OSA adherence | 0.62 (0.53 to 0.72) | 0.65 (0.56 to 0.76) | 0.87 (0.66 to 1.14) | 0.92 (0.70 to 1.21) | 1.09 (0.84 to 1.40) | 1.13 (0.87 to 1.45) |
*Adjusted for sex, age, race/ethnicity, stroke, hypertension, cardiovascular disease, and depression.
Discussion
In a large, retrospective cohort of Medicare beneficiaries with OSA, 2%, 0.7%, and 0.8% received a new diagnosis of AD, DNOS, or MCI, respectively. Medicare beneficiaries with OSA who were given OSA treatment, in comparison to others who were not, had significantly lower odds of developing AD, DNOS, and MCI. Adherence to treatment was significantly associated with lower odds of AD, but not DNOS or MCI. These population-based data suggest temporal associations between PAP use and dementia, highlighting a potentially protective role for PAP therapy and continuous treatment with PAP on dementia risk in older adults with OSA.
The growing prevalence and impact of dementia syndromes such as AD, coupled with a lack of effective treatments to prevent, cure, or slow dementia progression, have galvanized the need to identify modifiable risk factors for these conditions. To this end, mounting evidence has implicated OSA as a potential risk factor for poor cognitive outcomes or incident dementia. In the Study of Osteoporotic Fractures, Yaffe et al. [25] found that women with evidence of sleep-disordered breathing (defined in this study as an apnea–hypopnea index [AHI] of 15 or more) were 85% more likely to develop MCI or dementia, as compared to women with an AHI less than 15. In a separate study by Osorio et al. [26], the presence of self-reported sleep-disordered breathing in late life was also associated with a younger age of onset for MCI and AD. Numerous studies have also identified associations between impaired performance on cognitive testing and OSA, as well as relationships between OSA and AD biomarkers [27, 28].
The aforementioned work allows speculation that OSA may serve as a potential therapeutic target to slow or prevent the development of AD and related dementias or other poor neurocognitive outcomes. To date, however, studies that have examined this possibility have yielded conflicting results. In a multicenter, landmark randomized controlled trial that studied the effects of active or sham continuous positive airway pressure (CPAP) on cognitive performance in adults, treatment with active CPAP for 6 months had no significant effect on primary neurocognitive outcomes of interest [29]. This study, however, did not focus on the incidence of specific neurodegenerative conditions, and the study sample was not restricted to older individuals (mean age of active and sham CPAP groups was 52.2 and 50.8 years, respectively). In another study that examined the effects of PAP on several cognitive function tests in older adults (as secondary outcomes), PAP (vs a supportive care comparator) was not shown to have an effect on cognitive performance [30]. Conversely, other studies suggest that PAP use may improve performance in specific cognitive domains or delay cognitive decline [16, 31] in adults with OSA. A pilot study that evaluated the effects of PAP versus conservative care on cognitive function in older adults with severe OSA showed an improvement in multiple cognitive domains in those randomized to PAP [32]. Furthermore, in an exploratory analysis, the aforementioned study by Osorio et al. also identified an association between older age of onset for MCI (though not AD) in adults with OSA who endorsed the use of CPAP as compared with those who did not endorse treatment [11]. Although these prior observations were limited by self-reported data and small sample size, they were among the first to report a potential benefit of CPAP to delay cognitive decline. Our study supports and advances this line of inquiry by providing new, population-based evidence through the use of objective ICD-9 diagnoses and HCPCS codes to demonstrate an association between OSA treatment and dementia, in adults older than age 65 who have OSA.
Strengths of our study included the use of a large, representative sample of older Americans, longitudinal design that allowed for new dementia diagnosis claims in an OSA cohort, adjustment for other comorbid conditions know to affect dementia risk, and use of fee-for-service data that maximized the likelihood of capturing all relevant claims.
Some limitations should also be acknowledged. This study has a relatively short follow-up interval (3 years) and—necessarily in the absence of a large prospective study over many years—could not take into account duration of OSA, length of treatment, or degree of adherence to therapy. However, as the typical onset of OSA is in middle age, whereas dementia typically appears later in life, the present findings may well reflect an influence of OSA on dementia decades after its onset [33, 34]. Demographic data, health characteristics, and behavior, i.e. education, diet, socioeconomic status, body mass index, and smoking, which may be independent risk factors for dementia, are not available in claims datasets. These risk factors may also reflect motivation and a health-conscious lifestyle in a manner that could influence both PAP therapy initiation and dementia risk. That said, several sociodemographic factors—race, sex, and age—are included in Medicare files and adjusted for in the present analysis. The role of education and socioeconomic status as potential confounders in the association between PAP treatment and cognitive outcomes has been examined previously with mixed results. A prospective study reported no relationship between PAP adherence and socioeconomic status, education, or type of personality in adults with a median age of 53 years [35]. Furthermore, a recent population-level analysis that examined associations between household income and PAP adherence indicated above 4-h average PAP use for those in both lowest and highest quartile of median household income, with respective adherence rates of 40% and 47%, with a 24-min difference of average nightly use [36]. In contrast, other studies suggested that lower socioeconomic status and smoking influence nonadherence of PAP treatment among older men with OSA [37–39]. Taken together, these data suggest that socioeconomic factors could play a role in PAP treatment and treatment adherence.
Data regarding the number of PAP equipment refills—beyond at least two fills separated by at least 1 month—were not available from the dataset, precluding assessment of dose–response associations between equipment refills and dementia.
The coexistence of several dementia diagnoses is plausible, although its prevalence in older adults may be lower than expected based on a recent study [40]. A possibility also exists that those who seek and are adherent to medical care for their OSA are also more likely to be evaluated and diagnosed with a neurocognitive disorder than those who do not seek treatment and therefore likely to receive better care and have better outcomes. In this case, however, we believe that our current findings may in fact represent an underestimation of the risk of neurocognitive disorders in untreated or nonadherent patients.
Information on symptoms of sleepiness and OSA severity, which may have differential roles in cognitive function [41–43], and could affect beneficiaries’ eligibility for PAP coverage by Medicare, are not available from claims data. However, while sleep laboratories consider an AHI of 5 or more to qualify a patient for PAP treatment, Medicare rules require a minimum AHI of 15 for CPAP coverage, unless a secondary diagnosis is also present (including excessive daytime sleepiness, mood disorders, and hypertension).
We cannot rule out the possibility that beneficiaries whom we labeled as “untreated” or “nonadherent” could have obtained PAP equipment through other payers, or elected not to refill equipment prescribed from 2011 through 2013, in which case PAP treatment would not be captured in Medicare claims files. However, we believe that chances of such misclassification were reduced by restricting our analysis to fee-for-service beneficiaries.
Misclassification of neurocognitive diagnoses is also possible with claims data. Although a recent report estimated the sensitivity and specificity of Medicare claims data to detect AD diagnoses to be relatively high (85% and 77%, respectively), the sensitivity and specificity of Medicare claims algorithms to correctly classify MCI diagnoses are less robust [23]. Similarly, given the lack of confirmation of amyloid pathology from these Medicare claims data (e.g. lack of cerebrospinal fluid assays or amyloid neuroimaging), AD cannot be fully distinguished from other dementia subtypes with Medicare claims data.
Although we adjusted for several comorbidities (i.e. hypertension, CVD, stroke, and depression diagnoses), we should acknowledge that treatment for these comorbidities could potentially influence associations between PAP therapy and cognitive outcomes. That said, although information regarding the treatment of these comorbidities was not available in these claims, post-hoc findings showed that the association between PAP therapy and dementia remained essentially unchanged following exclusion of stroke-diagnosed beneficiaries, and those with a diagnosis of depression, suggesting that confounding by stroke or depression treatment would be comparatively low (data not shown). Adjustment for hypertensive therapy through the exclusion of beneficiaries with hypertension diagnosis was not feasible in these post-hoc analyses, as more than 84% of beneficiaries carried a diagnosis of hypertension. Nonetheless, prior reports suggest that the proportion of beneficiaries with hypertension who are prescribed hypertensive therapy exceeds 90% [44]. In this case, it is plausible that hypertension diagnosis could serve as a proxy for hypertensive therapy in adjusted models.
As referrals for evaluation and treatment are intrinsic components of any medical claims data, their absence may introduce systematic bias in these datasets. Indeed, OSA is often underdiagnosed in older adults [5] and those with OSA may not obtain PAP treatment. To alleviate concerns of OSA underdiagnosis, we designed this study to only include Medicare beneficiaries with ICD-9-diagnosed OSA.
Finally, as with any observational study, causality and its direction cannot be established beyond doubt. While OSA diagnosis preceded the diagnosis of dementia, reverse causality cannot be fully ruled out. As noted above, the typical age of OSA onset greatly precedes the typical age of dementia onset, but future studies over longer time intervals may be needed to confirm that the association in the present data between OSA treatment or adherence and lower incidence of dementia does not arise because dementia impedes subsequent OSA treatment and adherence.
Conclusions
This population-based data highlight a potentially protective role for PAP therapy on short-term dementia risk in older adults with OSA. Additional research is necessary to explore mechanisms that may underlie this association; however, if a causal pathway exists, treatment of OSA may offer new opportunities to improve cognitive outcomes in older adults with OSA.
Funding
This study was supported by the American Sleep Medicine Foundation Strategic Research Award 115-SR-15 (PI: Braley).
Conflict of interest statement. None declared.
Disclosure Statement
Financial disclosure: T.J.B. and G.L.D. received funding from the American Academy of Sleep Medicine Foundation to complete the current study (award #115-SR-15, PI Braley). T.J.B. is the principal investigator for investigator-initiated studies funded by the National Multiple Sclerosis Society and the Patient-Centered Outcomes Research Institute. She is named in a patent, held by the University of Michigan, concerning a treatment for sleep apnea. She completed a sleep apnea clinical trial that received material support, but no financial support, from Biogen-Idec. She is also site principal investigator for several industry-funded studies of MS immunotherapeutics at the University of Michigan (sponsors include Genzyme-Sanofi and Genentech-Roche). She has previously done consulting work for Jazz Pharmaceuticals and CVS Caremark. R.D.C. has received research funding from the National Institutes of Health (NIH; HL105999 and NS099043). He is named in or has developed materials, patented and copyrighted by the University of Michigan, and designed to assist with assessment or treatment of sleep disorders. He has served on the boards of the American Academy of Sleep Medicine, Associated Professional Sleep Societies, American Board of Sleep Medicine, American Academy of Sleep Medicine Foundation (which funded the current research), International Pediatric Sleep Society, and the nonprofit Sweet Dreamzzz. He is an author and editor for UpToDate, has edited a book for Cambridge University Press, and has consulted for Zansors. J.F.B. is funded by the NIH (R01s AG059733 and MD008879). A.S.C. is the Deputy Director of the nonprofit organization Science Debate, Inc.
Supplementary Material
The work was performed at the University of Michigan.
References
- 1. Goel N, et al. . Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29(4):320–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ancoli-Israel S, et al. . Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14(6):486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bixler EO, et al. . Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157(1):144–148. [DOI] [PubMed] [Google Scholar]
- 4. Lee SD, et al. . The prevalence of and risk factors for sleep-disordered breathing in an elderly Korean population. Respiration. 2014;87(5):372–378. [DOI] [PubMed] [Google Scholar]
- 5. Braley TJ, et al. . Recognition and diagnosis of obstructive sleep apnea in older Americans. J Am Geriatr Soc. 2018;66(7):1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Young T, et al.. Epidemiology of obstructive sleep apnea—a population health perspective. Am J Resp Crit Care. 2002;165(9):1217–1239. [DOI] [PubMed] [Google Scholar]
- 7. Antonelli Incalzi R, et al. . Does cognitive dysfunction conform to a distinctive pattern in obstructive sleep apnea syndrome? J Sleep Res. 2004;13(1):79–86. [DOI] [PubMed] [Google Scholar]
- 8. Beebe DW, et al. . The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26(3):298–307. [DOI] [PubMed] [Google Scholar]
- 9. Décary A, et al. . Cognitive deficits associated with sleep apnea syndrome: a proposed neuropsychological test battery. Sleep. 2000;23(3):369–381. [PubMed] [Google Scholar]
- 10. Engleman H, et al. . Neuropsychological function in obstructive sleep apnoea. Sleep Med Rev. 1999;3(1):59–78. [DOI] [PubMed] [Google Scholar]
- 11. Richards KC, et al. . CPAP adherence may slow 1-year cognitive decline in older adults with mild cognitive impairment and apnea. J Am Geriatr Soc. 2019;67(3):558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saunamäki T, et al. . A review of executive functions in obstructive sleep apnea syndrome. Acta Neurol Scand. 2007;115(1):1–11. [DOI] [PubMed] [Google Scholar]
- 13. Lutsey PL, et al. . Sleep characteristics and risk of dementia and Alzheimer’s disease: the Atherosclerosis Risk in Communities Study. Alzheimers Dement. 2018;14(2):157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Emamian F, et al. . The association between obstructive sleep apnea and Alzheimer’s disease: a meta-analysis perspective. Front Aging Neurosci. 2016;8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mubashir T, et al. . The prevalence of obstructive sleep apnea in mild cognitive impairment: a systematic review. BMC Neurol. 2019;19(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ancoli-Israel S, et al. . Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc. 2008;56(11):2076–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Canessa N, et al. . Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183(10):1419–1426. [DOI] [PubMed] [Google Scholar]
- 18. Castronovo V, et al. . White matter integrity in obstructive sleep apnea before and after treatment. Sleep. 2014;37(9):1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Devita M, et al. . Global cognitive profile and different components of reaction times in obstructive sleep apnea syndrome: effects of continuous positive airway pressure over time. Int J Psychophysiol. 2018;123:121–126. [DOI] [PubMed] [Google Scholar]
- 20. Wang MJ, et al.. Effect of nasal and sinus surgery in patients with and without obstructive sleep apnea. Acta Otolaryngol. 2019;139(5):467–472. [DOI] [PubMed] [Google Scholar]
- 21. Kim H, et al.. Improvement of cognitive function after continuous positive airway pressure treatment for subacute stroke patients with obstructive sleep apnea: a randomized controlled trial. Brain Sci. 2019;9(10):252. doi: 10.3390/brainsci9100252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patel N, et al. . Refill rates of accessories for positive airway pressure therapy as a surrogate measure of long-term adherence. J Clin Sleep Med. 2012;8(2):169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee E, et al. . Evaluation of Medicare claims data as a tool to identify dementia. J Alzheimers Dis. 2019;67(2):769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dunietz GL, et al.. Obstructive sleep apnea in older adults: geographic disparities in PAP treatment and adherence. J Clin Sleep Med. 2020;6(4):534–540. doi: 10.5664/jcsm.8914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yaffe K, et al. . Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Osorio RS, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Przybylska-Kuć S, et al. . Obstructive sleep apnea may increase the risk of Alzheimer’s disease. PLoS One. 2019;14(9):e0221255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharma RA, et al. . Obstructive sleep apnea severity affects amyloid burden in cognitively normal elderly. a longitudinal study. Am J Respir Crit Care Med. 2018;197(7):933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kushida CA, et al. . Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep. 2012;35(12):1593–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McMillan A, et al. ; PREDICT Investigators. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2(10):804–812. [DOI] [PubMed] [Google Scholar]
- 31. Yaffe K, et al. . Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–1028. [DOI] [PubMed] [Google Scholar]
- 32. Dalmases M, et al. . Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. 2015;148(5): 1214–1223. [DOI] [PubMed] [Google Scholar]
- 33. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Plassman BL, et al. . Prevalence of dementia in the United States: the Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29(1–2):125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gulati A, et al. . A prospective observational study to evaluate the effect of social and personality factors on continuous positive airway pressure (CPAP) compliance in obstructive sleep apnoea syndrome. BMC Pulm Med. 2017;17(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pandey A, et al.. Socioeconomic inequities in adherence to positive airway pressure therapy in population-level analysis. J Clin Med. 2020;9(2):442. doi: 10.3390/jcm9020442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Russo-Magno P, et al. . Compliance with CPAP therapy in older men with obstructive sleep apnea. J Am Geriatr Soc. 2001;49(9):1205–1211. [DOI] [PubMed] [Google Scholar]
- 38. Wickwire EM, et al.. Lower socioeconomic status and co-morbid conditions are associated with reduced continuous positive airway pressure adherence among older adult Medicare beneficiaries with obstructive sleep apnea. Sleep. 2020;43(12): zsaa122. doi: 10.1093/sleep/zsaa122 [DOI] [PubMed] [Google Scholar]
- 39. Jacobsen AR, et al. . Determinants for adherence to continuous positive airway pressure therapy in obstructive sleep apnea. PLoS One. 2017;12(12):e0189614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Claus JJ, et al. . Low prevalence of mixed dementia in a cohort of 2,000 elderly patients in a memory clinic setting. J Alzheimers Dis. 2016;50(3):797–806. [DOI] [PubMed] [Google Scholar]
- 41. Devita M, et al. . Associations between the apnea-hypopnea index during REM and NREM sleep and cognitive functioning in a cohort of middle-aged adults. J Clin Sleep Med. 2019;15(7):965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haba-Rubio J, et al. . Sleep characteristics and cognitive impairment in the general population: the HypnoLaus study. Neurology. 2017;88(5):463–469. [DOI] [PubMed] [Google Scholar]
- 43. Olaithe M, et al. . Cognition and nocturnal disturbance in OSA: the importance of accounting for age and premorbid intelligence. Sleep Breath. 2015;19(1):221–230. [DOI] [PubMed] [Google Scholar]
- 44. Fung V, et al. . Hypertension treatment in a Medicare population: adherence and systolic blood pressure control. Clin Ther. 2007;29(5):972–984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.