Abstract
Background
The development of sarcopenia is attributed to normal aging and factors like type 2 diabetes, obesity, inactivity, reduced testosterone levels, and malnutrition, which are factors of poor prognosis in patients with coronary artery disease (CAD). This study aimed to perform a meta-analysis to assess whether preoperative sarcopenia can be used to predict the outcomes after cardiac surgery in elderly patients with CAD.
Methods
PubMed, Embase, the Cochrane library, and Web of Science were searched for available papers published up to December 2020. The primary outcome was major adverse cardiovascular outcomes (MACE). The secondary outcomes were mortality and heart failure (HF)-related hospitalization. The random-effects model was used. Hazard ratios (HRs) with 95% confidence intervals (95%CIs) were estimated.
Results
Ten studies were included, with 3707 patients followed for 6 months to 4.5 ± 2.3 years. The sarcopenia population had a higher rate of MACE compared to the non-sarcopenia population (HR = 2.27, 95%CI: 1.58–3.27, P < 0.001; I2 = 60.0%, Pheterogeneity = 0.02). The association between sarcopenia and MACE was significant when using the psoas muscle area index (PMI) to define sarcopenia (HR = 2.86, 95%CI: 1.84–4.46, P < 0.001; I2 = 0%, Pheterogeneity = 0.604). Sarcopenia was not associated with higher late mortality (HR = 2.15, 95%CI: 0.89–5.22, P = 0.090; I2 = 91.0%, Pheterogeneity < 0.001), all-cause mortality (HR = 1.35, 95%CI: 0.14–12.84, P = 0.792; I2 = 90.5%, Pheterogeneity = 0.001), and death, HF-related hospitalization (HR = 1.37, 95%CI: 0.59–3.16, P = 0.459; I2 = 62.0%, Pheterogeneity = 0.105). The sensitivity analysis revealed no outlying study in the analysis of the association between sarcopenia and MACE after coronary intervention.
Conclusion
Sarcopenia is associated with poor MACE outcomes in patients with CAD. The results could help determine subpopulations of patients needing special monitoring after CAD surgery. The present study included several kinds of participants; although non-heterogeneity was found, interpretation should be cautious.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-021-02438-w.
Keywords: Sarcopenia, Coronary artery disease, Outcome, Elderly, meta-analysis
Background
Sarcopenia is a progressive, generalized skeletal muscle disorder characterized by low muscle strength, low muscle quantity or quality, and low physical performance [1, 2]. The prevalence of sarcopenia is estimated at 5–13% in patients of > 60 years of age and 11–50% in patients of > 80 years of age [3, 4]. Multiple definitions and criteria of sarcopenia are available, using different cutoff points and leading to a lack of standardization and poor application of these definitions in clinical practice [1, 2]. Still, the diagnosis of sarcopenia, using any definition of sarcopenia, is relatively straightforward since it requires the measurement of a combination of muscle mass, muscle strength, and physical performance, and since all definitions use at least two of these parameters [1, 2]. The disease burden from sarcopenia arises from the fact that it is a relatively common condition associated with short-term and long-term adverse effects. Sarcopenia is associated with higher risks of falls and fractures [5] and is a major risk factor for loss of independence in the elderly [6]. The muscular degeneration observed in sarcopenia might ultimately impair daily life activities and adversely affect major surgery outcomes in terms of complications, morbidity, and mortality [7–12].
The development of sarcopenia is attributed to normal aging, but it has multiple aspects [13]. These aspects include type 2 diabetes, obesity, inactivity, reduced number and size of type II muscle fibers, reduced testosterone levels, malnutrition, reduced growth factor levels, and decreased muscle proteins [13–19]. In addition, any disease or condition that will decrease physical activity will contribute to sarcopenia [20–26]. Some risk factors for sarcopenia (i.e., type 2 diabetes, obesity, and inactivity) are also risk factors for coronary artery disease (CAD) [27]. Furthermore, type 2 diabetes, obesity, inactivity, reduced testosterone levels, malnutrition, and reduced growth factor levels are also factors for poor outcomes after a coronary event or after surgery [28–32]. Sarcopenia is associated with lower physical activity and respiratory muscle strength in patients with CAD [33, 34].
To date, several studies have examined sarcopenia as a prognostic factor in patients with CAD [35–37]. Still, the available studies about the impact of sarcopenia on CAD outcomes yield conflicting results [35–44], with studies suggesting a poorer prognosis of CAD in patients with sarcopenia, while other studies suggest no association or associations no longer significant after adjustment for traditional risk factors of poor prognosis. Hence, the exact contributions of sarcopenia to CAD-related health and outcomes are unknown.
We hypothesized that sarcopenia negatively affects the outcomes of elderly patients with CAD who undergo cardiac surgery. Therefore, this meta-analysis aimed to assess whether preoperative sarcopenia can be used to predict the outcomes after cardiac surgery in elderly patients with CAD. The results could help determine subpopulations of patients needing special monitoring after surgery.
Methods
Literature search
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [45]. The relevant articles were identified based on the PICO principle [46], followed by screening using the eligibility criteria. PubMed, Embase, the Cochrane library, and Web of Science were searched for available papers published up to December 2020 using the MeSH terms of ‘Coronary artery disease’, ‘Coronary heart disease’, and ‘Sarcopenia’, as well as relevant key words.
Eligibility criteria
The eligibility criteria were 1) population: CAD/CHD patients > 65 years of age, 2) exposure: sarcopenia, 3) non-exposure: non-sarcopenia, 4) primary outcome: major adverse cardiovascular event (MACE), 5) study type: cohort studies, and 6) language: English. Conference abstracts, editorials, comments to the editor, reviews, meta-analyses, and papers with inaccessible full-text were excluded.
Definition of MACE
The definition of MACE could vary among studies, but MACE is generally defined as a composite endpoint including nonfatal stroke, nonfatal myocardial infarction, cardiovascular and cerebrovascular death, revascularization, and heart failure in this study [47, 48].
Data extraction
The data were extracted by two investigators according to a pre-specified protocol. The study characteristics (authors, year of publication, country, study design, sex, sample size, sarcopenia index, cutoff value to define sarcopenia, and follow-up duration) and outcomes (MACE, mortality, and heart failure (HF)-related hospitalization) were extracted. If a study reported hazard ratios (HRs), the adjusted HRs with 95% confidence intervals (CIs) were extracted; otherwise, the crude HRs with 95%CIs were obtained.
Quality of the evidence
Ten studies were included. The level of evidence of all articles was assessed independently by two authors according to the Newcastle-Ottawa Scale (NOS) criteria for quality assessment of cohort studies [49]. Discrepancies in the assessment were resolved through discussion until a consensus was reached. The details were summarized in Supplementary Table S1.
Statistical analysis
HRs and corresponding 95%CIs were used to summarize the results. Statistical heterogeneity among studies was calculated using Cochran’s Q-test and the I2 index. An I2 > 50% and Q-test P < 0.10 indicated high heterogeneity. The random-effects model was used to avoid possible heterogeneity among studies. The possible publication bias was not assessed by funnel plots and Egger’s test because the number of studies with MACE as the primary outcome was less than 10, in which case the funnel plots and Egger’s test could yield misleading results [50, 51]. A sensitivity analysis was performed by sequentially excluding each study in turn. If the 95%CI of each analysis still included the HR of the initial whole analysis, the results revealed that no single study was outlying and driving the results by itself. All analyses were performed using STATA SE 14.0 (StataCorp, College Station, Texas, USA).
Results
Selection of the studies
Figure 1 and Supplementary Table S1 present the study selection process. The initial search yielded 483 records, and 427 records were screened after removing the duplicates. After excluding 250 records, 177 full text articles or abstracts were assessed for eligibility, and 167 were excluded (study aim/design, n = 94; population, n = 32; outcomes, n = 7; exposure, n = 31; and animal study, n = 3).
Fig. 1.
Flow diagram of the study selection process
Ten studies were included [35, 38–44, 52, 53], with a total of 3707 patients who were followed for 6 months to 4.5 ± 2.3 years (Supplementary Table S2). Nine studies were from Asia [35, 39–44, 52, 53] and one from the United States of America [38]. Supplementary Table S2 shows that the definition of sarcopenia varied among the studies.
Supplementary Table S3 shows that four studies [38–40, 42] scored 8 points on the NOS, and six studies [35, 41, 43, 44, 52, 53] scored 9 points.
Sarcopenia and MACE after coronary intervention
Seven studies [39–44, 52] analyzed the occurrence of MACE after coronary intervention. The sarcopenia population had a higher rate of MACE compared to the non-sarcopenia population (HR = 2.27, 95%CI: 1.58–3.27, P < 0.001; I2 = 60.0%, Pheterogeneity = 0.02) (Fig. 2A and Table 1). Figure 2B and Table 1 show that this association was observed in prospective cohort studies [41, 43, 44, 52] (HR = 2.23, 95%CI: 1.28–3.90, P = 0.005; I2 = 78.8%, Pheterogeneity = 0.003) and retrospective cohort studies [39, 40, 42] (HR = 2.32, 95%CI: 1.46–3.67, P < 0.001; I2 = 0%, Pheterogeneity = 0.665). When considering the definitions of sarcopenia, the results showed that the association between sarcopenia and MACE was significant when using the psoas muscle area index (PMI) to define sarcopenia [39, 40, 52] (HR = 2.86, 95%CI: 1.84–4.46, P < 0.001; I2 = 0%, Pheterogeneity = 0.604) (Fig. 2C and Table 1), but not when using the skeletal muscle area index (SMI)/height squared [41, 42] (HR = 1.32, 95%CI: 0.57–3.05, P = 0.518; I2 = 68.2%, Pheterogeneity = 0.076) (Fig. 2C and Table 1); the association was also observed when using definitions other than the PMI or SMI/height squared [43, 44] (HR = 2.77, 95%CI: 1.63–4.71, P < 0.001; I2 = 67.5%, Pheterogeneity = 0.079) (Fig. 2C and Table 1).
Fig. 2.
A. Forest plot of MACE. B. Forest plot of Subgroup analysis of MACE by study type. C. Forest plot of subgroup analysis of MACE by sarcopenia index D. Forest plot of subgroup analysis of MACE by diagnosis
Table 1.
Adverse outcomes for sarcopenia versus non-sarcopenia
| N | HR (95%CI) | P | I2, % | P(Heterogeneity) | |
|---|---|---|---|---|---|
| MACE overall | 7 | 2.273 (1.581–3.268) | < 0.001 | 60 | 0.02 |
| Other | 2 | 2.769 (1.630–4.706) | < 0.001 | 67.5 | 0.079 |
| PMI | 3 | 2.861 (1.835–4.460) | < 0.001 | 0 | 0.604 |
| SMI/height squared | 2 | 1.319 (0.570–3.050) | 0.518 | 68.2 | 0.076 |
| Prospective cohort | 4 | 2.232 (1.278–3.898) | 0.005 | 78.8 | 0.003 |
| Retrospective cohort | 3 | 2.315 (1.460–3.670) | < 0.001 | 0 | 0.665 |
| CAD | 3 | 1.979 (0.984–3.983) | 0.056 | 84.2 | 0.002 |
| Non-STEMI | 1 | 3.320 (1.727–6.381) | < 0.001 | ||
| CABG | 1 | 1.960 (0.827–4.644) | 0.126 | ||
| Heart valve surgery | 1 | 3.210 (1.374–7.501) | 0.007 | ||
| STEMI | 1 | 2.060 (1.011–4.196) | 0.046 | ||
| Late mortality | 3 | 2.152 (0.887–5.223) | 0.09 | 91 | < 0.001 |
| Heart valve surgery | 2 | 1.548 (0.7–3.422) | 0.281 | 85.2 | 0.009 |
| CABG | 1 | 4.250 (2.181–8.283) | < 0.001 | ||
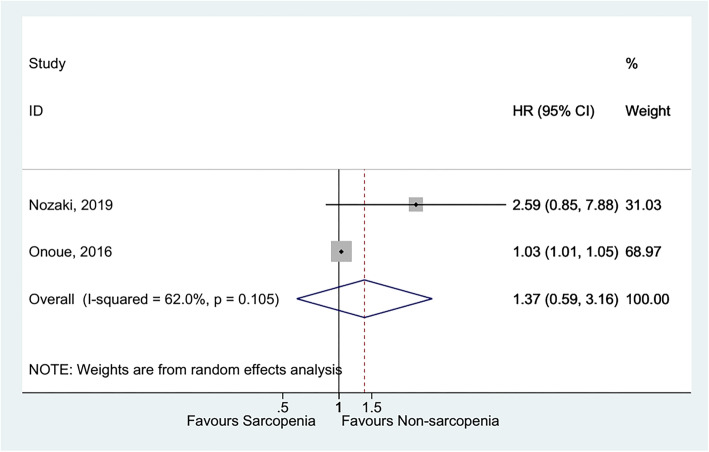
| Death, HF-related hospitalization | 2 | 1.370 (0.594–3.164) | 0.459 | 62 | 0.105 |
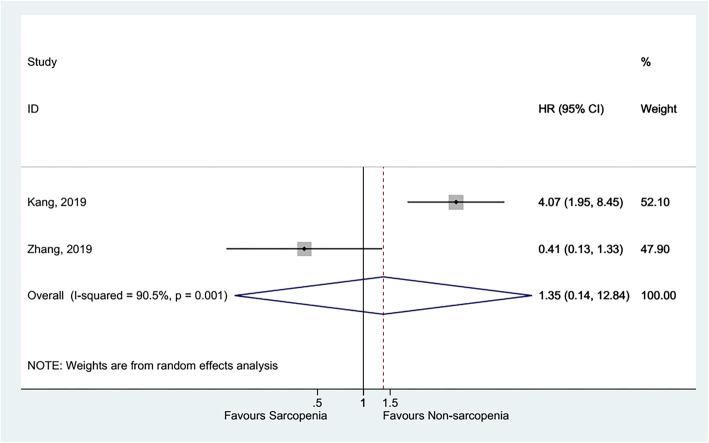
| All-cause mortality | 2 | 1.354 (0.143–12.842) | 0.792 | 90.5 | 0.001 |
HR hazard ratio, CI confidence interval, MACE major adverse cardiovascular event, PMI psoas muscle area index, SMI skeletal muscle area index, CAD coronary artery disease, STEMI ST-elevated myocardial infarction, CABG coronary artery bypass graft, HF heart failure
Sarcopenia, mortality, and HF-related hospitalization after coronary intervention
Sarcopenia was not associated with higher late mortality [38–40] (HR = 2.15, 95%CI: 0.89–5.22, P = 0.090; I2 = 91.0%, Pheterogeneity < 0.001) (Fig. 3 and Table 1), all-cause mortality [41, 43] (HR = 1.35, 95%CI: 0.14–12.84, P = 0.792; I2 = 90.5%, Pheterogeneity = 0.001) (Fig. 4 and Table 1), and death, HF-related hospitalization [35, 53] (HR = 1.37, 95%CI: 0.59–3.16, P = 0.459; I2 = 62.0%, Pheterogeneity = 0.105) (Fig. 5 and Table 1).
Fig. 3.
A. Forest plot of late mortality. B. Forest plot of subgroup analysis of late mortality by diagnosis
Fig. 4.
Forest plot of All-cause mortality
Fig. 5.
Forest plot of Death, HF-related hospitalization
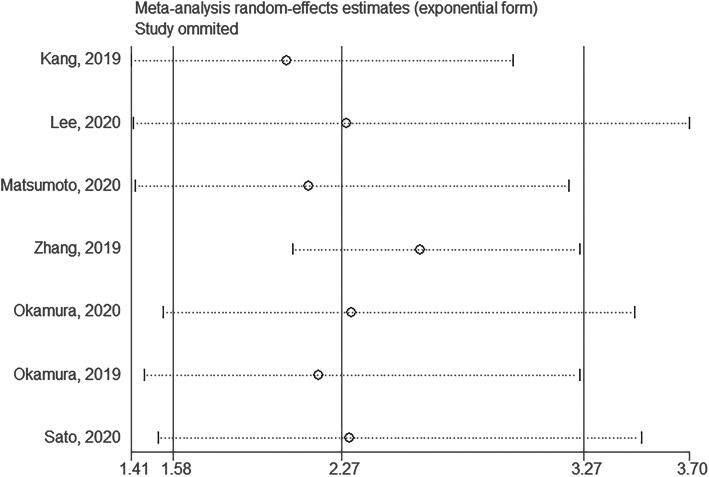
Sensitivity analysis
The sensitivity analysis suggested that there was no outlying study in the analysis of the association between sarcopenia and MACE after coronary intervention (Fig. 6).
Fig. 6.
Sensitivity analysis of MACE
Discussion
The available studies about the impact of sarcopenia on the outcomes of CAD yield conflicting results. Hence, this meta-analysis aimed to assess whether preoperative sarcopenia can be used to predict the outcomes after cardiac surgery in elderly patients with CAD. The results showed that sarcopenia is associated with poor MACE outcomes in patients with CAD.
These findings highlight the importance of performing a routine physical assessment for risk stratification and sarcopenia in CAD patients. In this context, sarcopenia is a functional status to be detected early in clinical practice, and the importance of PMI is indispensable for identifying simple methods. This is important since a recent meta-analysis showed that sarcopenia could be observed in 31.4% of patients with CAD [54]. Sarcopenia has also been associated with hypertension in older adults [55], with type 2 diabetes [26, 56, 57], and obesity [58, 59]. Hypertension, diabetes, and obesity are also risk factors for the occurrence of CAD [27, 60–62] but are also well-known factors for adverse outcomes in patients with CAD [62–65]. In addition, other factors associated with the development of sarcopenia are also risk factors for poor outcomes after surgery. Indeed, low testosterone levels are associated with increased mortality after CAD and after surgery for CAD [66, 67]. Malnutrition is a factor of poor prognosis in hospitalized patients [68, 69]. Low levels of growth factors are also related to poor outcomes after CAD and surgery [70, 71].
Still, this study showed that only the MACEs after coronary intervention for CAD were affected by sarcopenia, while mortality was not associated. Previous meta-analyses showed that sarcopenia is a risk factor for mortality in the general elderly population [72–74] and a factor of poor prognosis in cancer patients [75, 76]. No previous meta-analysis examined specifically the impact of sarcopenia on MACEs and mortality after coronary intervention. Still, a previous meta-analysis showed that handgrip strength was associated with CAD in the community [77], but a prospective study showed no such association [78]. Still, an association with an increased risk of MACE, even in the absence of increased mortality, signifies a higher disease burden and lower quality of life for the patients [79, 80] and higher healthcare costs for the patients, their family, and society [81].
This meta-analysis revealed wide differences among studies (and even within a single country) regarding the definition of sarcopenia, and these definitions affected the association of sarcopenia with the primary outcome (MACEs). Liu et al. [72] also showed that the method for determining sarcopenia, dual X-ray absorptiometry vs. bioelectrical impedance analysis in their case, affected their results of the association between sarcopenia and all-cause mortality. A recent meta-analysis highlighted the need for the proper diagnosis of sarcopenia and that properly diagnosed sarcopenia was associated with the outcomes after major gastrointestinal surgery [82].
The conclusions of this meta-analysis must be considered along with its limitations. Indeed, meta-analyses inherit all the included studies’ limitations, and some caveats should be considered while interpreting the findings. Because of non-randomized registry data’s intrinsic limitations, the differences in baseline characteristics between groups can affect the outcome. To minimize such biases, two studies [40, 43] performed propensity score-matched analyses, and others used a multivariable logistic regression model. Second, the included sample of 3707 patients was relatively large. Finally, studies that used other and PMI to define sarcopenia demonstrated that the sarcopenia population has a higher MACE rate compared with the non-sarcopenia population. Still, studies performed using SMI/height squared index did not observe the statistical difference between the two groups. Nevertheless, the sensitivity analysis demonstrated no outlying study. Future studies should look to reconcile these definitions of sarcopenia.
A major limitation is that suitable cutoff values for sarcopenia potentially differ among races, sexes, and age groups. In addition, there are many different cutoff values used for the definition of sarcopenia in the literature. Thus, it is difficult to obtain a universal definition of sarcopenia. The cutoff value for sarcopenia was defined as the lowest quartile in most studies [38, 40, 42, 44]. In contrast, some of the other studies performed a receiver operating characteristic (ROC) curve analysis to obtain the optimal cutoff value to define sarcopenia [43, 52]. Because of heterogeneity, a sensitivity analysis was performed and demonstrated no outlying study. In addition, the present study included several kinds of participants such as CAD, NSTEMI/STMI, HF, off-pump CABG, and heart valve surgery. Although non-heterogeneity was found, interpretation should be cautious.
Conclusion
In conclusion, sarcopenia is associated with poor MACE outcomes in patients with CAD. Still, the definitions of sarcopenia were different among the included studies. Future studies should look to standardize the definition of sarcopenia to achieve better estimations of the associations of sarcopenia with adverse outcomes. Although non-heterogeneity was found, interpretation should be cautious because various types of patients were included.
Supplementary Information
Additional file 1: Supplementary Table S1. Search terms and strategy.
Additional file 2: Supplementary Table S2. Literature search and study characteristic.
Additional file 3: Supplementary Appendix S3. NOS criteria for quality of cohort studies.
Acknowledgments
Not applicable.
Abbreviations
- CAD
Coronary artery disease
- CIs
Confidence intervals
- HF
Heart failure
- HRs
Hazard ratios
- MACE
Major adverse cardiovascular event
- NOS
Newcastle-Ottawa Scale
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- ROC
Receiver operating characteristic
- SMI
Skeletal muscle area index
Authors’ contributions
Study concept: JMC and KY. Study design: JMC, KY, and QQX. Data acquisition: QQX, JW, and YR. Quality control of data and algorithms: JMC and KY. Data analysis and interpretation: QQX, YR, and JAH. Manuscript preparation: QQX and JW. Manuscript editing: YR and JAH. Manuscript review: JMC and KY. All authors (QQX, JW, YR, JAH, KY, and JMC) have read and approved the final version of the manuscript.
Funding
Shanghai Jiaotong University School of Medicine [TM201805], The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qiqi Xue, Jie Wu and Yan Ren contributed equally to this work.
Contributor Information
Ke Yang, Email: ykk_ykkk@126.com.
Jiumei Cao, Email: cjm11261@rjh.com.cn.
References
- 1.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardeljan AD, Hurezeanu R. Sarcopenia. In: StatPearls: Treasure Island (FL); 2020.
- 3.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1(2):129–133. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. 2017;16(1):21. doi: 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, Maier AB. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10(3):485–500. doi: 10.1002/jcsm.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dos Santos L, Cyrino ES, Antunes M, Santos DA, Sardinha LB. Sarcopenia and physical independence in older adults: the independent and synergic role of muscle mass and muscle function. J Cachexia Sarcopenia Muscle. 2017;8(2):245–250. doi: 10.1002/jcsm.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11(3):177–180. doi: 10.11138/ccmbm/2014.11.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim EY, Lee HY, Kim KW, Lee JI, Kim YS, Choi WJ, Kim JH. Preoperative computed tomography-determined sarcopenia and postoperative outcome after surgery for non-small cell lung Cancer. Scand J Surg. 2018;107(3):244–251. doi: 10.1177/1457496917748221. [DOI] [PubMed] [Google Scholar]
- 9.Rangel EL, Rios-Diaz AJ, Uyeda JW, Castillo-Angeles M, Cooper Z, Olufajo OA, Salim A, Sodickson AD. Sarcopenia increases risk of long-term mortality in elderly patients undergoing emergency abdominal surgery. J Trauma Acute Care Surg. 2017;83(6):1179–1186. doi: 10.1097/TA.0000000000001657. [DOI] [PubMed] [Google Scholar]
- 10.El Amrani M, Vermersch M, Fulbert M, Prodeau M, Lecolle K, Hebbar M, Ernst O, Pruvot FR, Truant S. Impact of sarcopenia on outcomes of patients undergoing pancreatectomy: a retrospective analysis of 107 patients. Medicine (Baltimore) 2018;97(39):e12076. doi: 10.1097/MD.0000000000012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaido T, Hamaguchi Y, Uemoto S. Significance of preoperative sarcopenia to liver surgery. Hepatobiliary Surg Nutr. 2019;8(1):59–62. doi: 10.21037/hbsn.2018.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang DD, Wang SL, Zhuang CL, Zheng BS, Lu JX, Chen FF, Zhou CJ, Shen X, Yu Z. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Color Dis. 2015;17(11):O256–O264. doi: 10.1111/codi.13067. [DOI] [PubMed] [Google Scholar]
- 13.Lexell J, Henriksson-Larsen K, Winblad B, Sjostrom M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve. 1983;6(8):588–595. doi: 10.1002/mus.880060809. [DOI] [PubMed] [Google Scholar]
- 14.Visser M, Harris TB, Langlois J, Hannan MT, Roubenoff R, Felson DT, Wilson PW, Kiel DP. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham heart study. J Gerontol A Biol Sci Med Sci. 1998;53(3):M214–M221. doi: 10.1093/gerona/53A.3.M214. [DOI] [PubMed] [Google Scholar]
- 15.Morley JE. Hormones and the aging process. J Am Geriatr Soc. 2003;51(7 Suppl):S333–S337. doi: 10.1046/j.1365-2389.2003.51344.x. [DOI] [PubMed] [Google Scholar]
- 16.Vermeulen A, Goemaere S, Kaufman JM. Testosterone, body composition and aging. J Endocrinol Investig. 1999;22(5 Suppl):110–116. [PubMed] [Google Scholar]
- 17.Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, Ferrucci L. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol (1985) 2007;102(3):919–925. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickinson JM, Volpi E, Rasmussen BB. Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exerc Sport Sci Rev. 2013;41(4):216–223. doi: 10.1097/JES.0b013e3182a4e699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granic A, Mendonca N, Sayer AA, Hill TR, Davies K, Siervo M, Mathers JC, Jagger C. Effects of dietary patterns and low protein intake on sarcopenia risk in the very old: the Newcastle 85+ study. Clin Nutr. 2020;39(1):166–173. doi: 10.1016/j.clnu.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SH, Shin MJ, Shin YB, Kim KU. Sarcopenia associated with chronic obstructive pulmonary disease. J Bone Metab. 2019;26(2):65–74. doi: 10.11005/jbm.2019.26.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdul Aziz SA, McStea M, Ahmad Bashah NS, Chong ML, Ponnampalavanar S, Syed Omar SF, Sulaiman H, Azwa I, Tan MP, Kamarulzaman A, et al. Assessment of sarcopenia in virally suppressed HIV-infected Asians receiving treatment. AIDS. 2018;32(8):1025–1034. doi: 10.1097/QAD.0000000000001798. [DOI] [PubMed] [Google Scholar]
- 23.Curcio F, Testa G, Liguori I, Papillo M, Flocco V, Panicara V, et al. Sarcopenia and Heart Failure. Nutrients. 2020;12(1). 10.3390/nu12010211. [DOI] [PMC free article] [PubMed]
- 24.Souza VA, Oliveira D, Mansur HN, Fernandes NM, Bastos MG. Sarcopenia in chronic kidney disease. J Bras Nefrol. 2015;37(1):98–105. doi: 10.5935/0101-2800.20150014. [DOI] [PubMed] [Google Scholar]
- 25.Dunne RF, Loh KP, Williams GR, Jatoi A, Mustian KM, Mohile SG. Cachexia and Sarcopenia in Older Adults with Cancer: A Comprehensive Review. Cancers (Basel). 2019;11(12). 10.3390/cancers11121861. [DOI] [PMC free article] [PubMed]
- 26.Mesinovic J, Zengin A, De Courten B, Ebeling PR, Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab Syndr Obes. 2019;12:1057–1072. doi: 10.2147/DMSO.S186600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Authors/Task Force M. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur J Prev Cardiol. 2016;23(11):NP1–NP96. doi: 10.1177/2047487316653709. [DOI] [PubMed] [Google Scholar]
- 28.Karaye KM, Sani MU. Factors associated with poor prognosis among patients admitted with heart failure in a Nigerian tertiary medical Centre: a cross-sectional study. BMC Cardiovasc Disord. 2008;8(1):16. doi: 10.1186/1471-2261-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iscen S. Prognostic factors in patients with acute coronary syndrome without ST-segment elevation. Arq Bras Cardiol. 2013;101(4):373–374. doi: 10.5935/abc.20130198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang R, Mei B, Liao X, Lu X, Yan L, Lin M, Zhong Y, Chen Y, You T. Determination of risk factors affecting the in-hospital prognosis of patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention. BMC Cardiovasc Disord. 2017;17(1):243. doi: 10.1186/s12872-017-0660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakravarthy M. Modifying risks to improve outcome in cardiac surgery: an anesthesiologist’s perspective. Ann Card Anaesth. 2017;20(2):226–233. doi: 10.4103/aca.ACA_20_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeitouni M, Clare RM, Chiswell K, Abdulrahim J, Shah N, Pagidipati NP, Shah SH, Roe MT, Patel MR, Jones WS. Risk factor burden and long-term prognosis of patients with premature coronary artery disease. J Am Heart Assoc. 2020;9(24):e017712. doi: 10.1161/JAHA.120.017712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izawa KP, Watanabe S, Oka K, Kasahara Y, Morio Y, Hiraki K, Hirano Y, Omori Y, Suzuki N, Kida K, Suzuki K, Akashi YJ. Sarcopenia and physical activity in older male cardiac patients. Int J Cardiol. 2016;222:457–461. doi: 10.1016/j.ijcard.2016.07.167. [DOI] [PubMed] [Google Scholar]
- 34.Izawa KP, Watanabe S, Oka K, Kasahara Y, Morio Y, Hiraki K, Hirano Y, Omori Y, Suzuki N, Kida K, Suzuki K, Akashi YJ. Respiratory muscle strength in relation to sarcopenia in elderly cardiac patients. Aging Clin Exp Res. 2016;28(6):1143–1148. doi: 10.1007/s40520-016-0534-5. [DOI] [PubMed] [Google Scholar]
- 35.Onoue Y, Izumiya Y, Hanatani S, Tanaka T, Yamamura S, Kimura Y, Araki S, Sakamoto K, Tsujita K, Yamamoto E, Yamamuro M, Kojima S, Kaikita K, Hokimoto S. A simple sarcopenia screening test predicts future adverse events in patients with heart failure. Int J Cardiol. 2016;215:301–306. doi: 10.1016/j.ijcard.2016.04.128. [DOI] [PubMed] [Google Scholar]
- 36.Narumi T, Watanabe T, Kadowaki S, Takahashi T, Yokoyama M, Kinoshita D, Honda Y, Funayama A, Nishiyama S, Takahashi H, Arimoto T, Shishido T, Miyamoto T, Kubota I. Sarcopenia evaluated by fat-free mass index is an important prognostic factor in patients with chronic heart failure. Eur J Intern Med. 2015;26(2):118–122. doi: 10.1016/j.ejim.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349(9058):1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 38.Hawkins RB, Mehaffey JH, Charles EJ, Kern JA, Lim DS, Teman NR, Ailawadi G. Psoas muscle size predicts risk-adjusted outcomes after surgical aortic valve replacement. Ann Thorac Surg. 2018;106(1):39–45. doi: 10.1016/j.athoracsur.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okamura H, Kimura N, Mieno M, Yuri K, Yamaguchi A. Preoperative sarcopenia is associated with late mortality after off-pump coronary artery bypass grafting. Eur J Cardiothorac Surg. 2020;58(1):121–129. doi: 10.1093/ejcts/ezz378. [DOI] [PubMed] [Google Scholar]
- 40.Okamura H, Kimura N, Tanno K, Mieno M, Matsumoto H, Yamaguchi A, Adachi H. The impact of preoperative sarcopenia, defined based on psoas muscle area, on long-term outcomes of heart valve surgery. J Thorac Cardiovasc Surg. 2019;157(3):1071–1079. doi: 10.1016/j.jtcvs.2018.06.098. [DOI] [PubMed] [Google Scholar]
- 41.Zhang N, Zhu WL, Liu XH, Chen W, Zhu ML, Kang L, Tian R. Prevalence and prognostic implications of sarcopenia in older patients with coronary heart disease. J Geriatr Cardiol. 2019;16(10):756–763. doi: 10.11909/j.issn.1671-5411.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato R, Akiyama E, Konishi M, Matsuzawa Y, Suzuki H, Kawashima C, Kimura Y, Okada K, Maejima N, Iwahashi N, Hibi K, Kosuge M, Ebina T, von Haehling S, Anker SD, Tamura K, Kimura K. Decreased appendicular skeletal muscle mass is associated with poor outcomes after ST-segment elevation myocardial infarction. J Atheroscler Thromb. 2020;27(12):1278–1287. doi: 10.5551/jat.52282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang DO, Park SY, Choi BG, Na JO, Choi CU, Kim EJ, et al. Prognostic Impact of Low Skeletal Muscle Mass on Major Adverse Cardiovascular Events in Coronary Artery Disease: A Propensity Score-Matched Analysis of a Single Center All-Comer Cohort. J Clin Med. 2019;8(5). 10.3390/jcm8050712. [DOI] [PMC free article] [PubMed]
- 44.Lee HS, Park KW, Kang J, Ki YJ, Chang M, Han JK, et al. Sarcopenia Index as a Predictor of Clinical Outcomes in Older Patients with Coronary Artery Disease. J Clin Med. 2020;9(10). 10.3390/jcm9103121. [DOI] [PMC free article] [PubMed]
- 45.Selcuk AA. A guide for systematic reviews: PRISMA. Turkish Arch Otorhinolaryngol. 2019;57(1):57–58. doi: 10.5152/tao.2019.4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aslam S, Emmanuel P. Formulating a researchable question: a critical step for facilitating good clinical research. Indian J Sexually Transmitted Dis AIDS. 2010;31(1):47–50. doi: 10.4103/0253-7184.69003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Jong M, van der Worp HB, van der Graaf Y, Visseren FLJ, Westerink J. Pioglitazone and the secondary prevention of cardiovascular disease. A meta-analysis of randomized-controlled trials. Cardiovasc Diabetol. 2017;16(1):134. doi: 10.1186/s12933-017-0617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arnott C, Li Q, Kang A, Neuen BL, Bompoint S, Lam CSP, Rodgers A, Mahaffey KW, Cannon CP, Perkovic V, Jardine MJ, Neal B. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and Meta-analysis. J Am Heart Assoc. 2020;9(3):e014908. doi: 10.1161/JAHA.119.014908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14(1):45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343(oct18 2):d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions version 6.1. London: Cochrane Collaboration; 2020. [Google Scholar]
- 52.Matsumoto H, Matsumura K, Yamamoto Y, Fujii K, Tsujimoto S, Otagaki M, Morishita S, Hashimoto K, Shibutani H, Sugiura T, Shiojima I. Prognostic value of psoas muscle mass index in patients with NonST-SegmentElevation myocardial infarction: a prospective observational study. J Am Heart Assoc. 2020;9(19):e017315. doi: 10.1161/JAHA.120.017315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nozaki Y, Yamaji M, Nischiguchi S, Fukutani N, Tashiro Y, Shirooka H, Hirata H, Yamaguchi M, Tasaka S, Matsubara K, et al. Sarcopenia predicts adverse outcomes in an elderly outpatient population with New York heart association class II–IV heart failure: a prospective cohort study. Aging Med Healthcare. 2019;10(2):53–61. doi: 10.33879/AMH.2019.1809. [DOI] [Google Scholar]
- 54.Pacifico J, Geerlings MAJ, Reijnierse EM, Phassouliotis C, Lim WK, Maier AB. Prevalence of sarcopenia as a comorbid disease: a systematic review and meta-analysis. Exp Gerontol. 2020;131:110801. doi: 10.1016/j.exger.2019.110801. [DOI] [PubMed] [Google Scholar]
- 55.Bai T, Fang F, Li F, Ren Y, Hu J, Cao J. Sarcopenia is associated with hypertension in older adults: a systematic review and meta-analysis. BMC Geriatr. 2020;20(1):279. doi: 10.1186/s12877-020-01672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trierweiler H, Kisielewicz G, Hoffmann Jonasson T, Rasmussen Petterle R, Aguiar Moreira C, Zeghbi Cochenski Borba V. sarcopenia: a chronic complication of type 2 diabetes mellitus. Diabetol Metab Syndr. 2018;10(1):25. doi: 10.1186/s13098-018-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fukuda T, Bouchi R, Takeuchi T, Nakano Y, Murakami M, Minami I, Izumiyama H, Hashimoto K, Yoshimoto T, Ogawa Y. Association of diabetic retinopathy with both sarcopenia and muscle quality in patients with type 2 diabetes: a cross-sectional study. BMJ Open Diabetes Res Care. 2017;5(1):e000404. doi: 10.1136/bmjdrc-2017-000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouchonville MF, Villareal DT. Sarcopenic obesity: how do we treat it? Curr Opin Endocrinol Diabetes Obes. 2013;20(5):412–419. doi: 10.1097/01.med.0000433071.11466.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin J. JAMA patient page. Obesity and the heart. JAMA. 2013;310(19):2113. doi: 10.1001/jama.2013.281901. [DOI] [PubMed] [Google Scholar]
- 61.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–584. doi: 10.1016/j.cjca.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piche ME, Tchernof A, Despres JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020;126(11):1477–1500. doi: 10.1161/CIRCRESAHA.120.316101. [DOI] [PubMed] [Google Scholar]
- 63.Rosendorff C, Lackland DT, Allison M, Aronow WS, Black HR, Blumenthal RS, Cannon CP, de Lemos JA, Elliott WJ, Findeiss L, Gersh BJ, Gore JM, Levy D, Long JB, O'Connor CM, O'Gara PT, Ogedegbe G, Oparil S, White WB, American Heart Association, American College of Cardiology, and American Society of Hypertension Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Circulation. 2015;131(19):e435–e470. doi: 10.1161/CIR.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elgendy IY, Bavry AA, Gong Y, Handberg EM, Cooper-DeHoff RM, Pepine CJ. Long-term mortality in hypertensive patients with coronary artery disease: results from the US cohort of the international verapamil (SR)/Trandolapril study. Hypertension. 2016;68(5):1110–1114. doi: 10.1161/HYPERTENSIONAHA.116.07854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matheus AS, Tannus LR, Cobas RA, Palma CC, Negrato CA, Gomes MB. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens. 2013;2013:653789–653715. doi: 10.1155/2013/653789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goodale T, Sadhu A, Petak S, Robbins R. Testosterone and the heart. Methodist Debakey Cardiovasc J. 2017;13(2):68–72. doi: 10.14797/mdcj-13-2-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oskui PM, French WJ, Herring MJ, Mayeda GS, Burstein S, Kloner RA. Testosterone and the cardiovascular system: a comprehensive review of the clinical literature. J Am Heart Assoc. 2013;2(6):e000272. doi: 10.1161/JAHA.113.000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leandro-Merhi VA, de Aquino JL. Determinants of malnutrition and post-operative complications in hospitalized surgical patients. J Health Popul Nutr. 2014;32(3):400–410. doi: 10.1016/S0261-5614(13)60457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ben-Ishay O, Gertsenzon H, Mashiach T, Kluger Y, Chermesh I. Malnutrition in surgical wards: a plea for concern. Gastroenterol Res Pract. 2011;2011:1–4. doi: 10.1155/2011/840512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karu I, Starkopf J, Zilmer K, Zilmer M. Growth factors serum levels in coronary artery disease patients scheduled for bypass surgery: perioperative dynamics and comparisons with healthy volunteers. Biomed Res Int. 2013;2013:985404–985405. doi: 10.1155/2013/985404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gola M, Bonadonna S, Doga M, Giustina A. Clinical review: growth hormone and cardiovascular risk factors. J Clin Endocrinol Metab. 2005;90(3):1864–1870. doi: 10.1210/jc.2004-0545. [DOI] [PubMed] [Google Scholar]
- 72.Liu P, Hao Q, Hai S, Wang H, Cao L, Dong B. Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: a systematic review and meta-analysis. Maturitas. 2017;103:16–22. doi: 10.1016/j.maturitas.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 73.Sobestiansky S, Michaelsson K, Cederholm T. Sarcopenia prevalence and associations with mortality and hospitalisation by various sarcopenia definitions in 85-89 year old community-dwelling men: a report from the ULSAM study. BMC Geriatr. 2019;19(1):318. doi: 10.1186/s12877-019-1338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyere O. Health outcomes of sarcopenia: a systematic review and Meta-analysis. PLoS One. 2017;12(1):e0169548. doi: 10.1371/journal.pone.0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. doi: 10.1016/j.ejca.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 76.Zhang XM, Dou QL, Zeng Y, Yang Y, Cheng ASK, Zhang WW. Sarcopenia as a predictor of mortality in women with breast cancer: a meta-analysis and systematic review. BMC Cancer. 2020;20(1):172. doi: 10.1186/s12885-020-6645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu Y, Wang W, Liu T, Zhang D. Association of Grip Strength With Risk of All-Cause Mortality, Cardiovascular Diseases, and Cancer in Community-Dwelling Populations: A Meta-analysis of Prospective Cohort Studies. J Am Med Dir Assoc. 2017;18(6):551 e517–551 e535. doi: 10.1016/j.jamda.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 78.Gubelmann C, Vollenweider P, Marques-Vidal P. No association between grip strength and cardiovascular risk: the CoLaus population-based study. Int J Cardiol. 2017;236:478–482. doi: 10.1016/j.ijcard.2017.01.110. [DOI] [PubMed] [Google Scholar]
- 79.Thompson DR, Yu CM. Quality of life in patients with coronary heart disease-I: assessment tools. Health Qual Life Outcomes. 2003;1(1):42. doi: 10.1186/1477-7525-1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bahall M, Legall G, Khan K. Quality of life among patients with cardiac disease: the impact of comorbid depression. Health Qual Life Outcomes. 2020;18(1):189. doi: 10.1186/s12955-020-01433-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Korsnes JS, Davis KL, Ariely R, Bell CF, Mitra D. Health care resource utilization and costs associated with nonfatal major adverse cardiovascular events. J Manag Care Spec Pharm. 2015;21(6):443–450. doi: 10.18553/jmcp.2015.21.6.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pipek LZ, Baptista CG, Nascimento RFV, Taba JV, Suzuki MO, Do Nascimento FS, Martines DR, Nii F, Iuamoto LR, Carneiro-D’Albuquerque LA et al. The impact of properly diagnosed sarcopenia on postoperative outcomes after gastrointestinal surgery: a systematic review and meta-analysis. PLoS One. 2020;15(8):e0237740. doi: 10.1371/journal.pone.0237740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table S1. Search terms and strategy.
Additional file 2: Supplementary Table S2. Literature search and study characteristic.
Additional file 3: Supplementary Appendix S3. NOS criteria for quality of cohort studies.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.