Abstract
Skeletal muscle (muscle) is essential for physical health and for metabolic integrity, with sarcopenia (progressive muscle mass loss and weakness), a pre-curser of aging and chronic disease. Loss of lean mass and muscle quality (force generation per unit of muscle) in the general population are associated with fatigue, weakness, and slowed walking speed, eventually interfering with the ability to maintain physical independence, and impacting participation in social roles and quality of life. Muscle mass and strength impairments are also documented during childhood cancer treatment, which often persist into adult survivorship, and contribute to an aging phenotype in this vulnerable population. Although several treatment exposures appear to confer increased risk for loss of mass and strength that persists after therapy, the pathophysiology responsible for poor muscle quantity and quality is not well understood in the childhood cancer survivor population. This is partly due to limited access to both pediatric and adult survivor muscle tissue samples, and to difficulties surrounding non-invasive investigative approaches for muscle assessment. Because muscle accounts for just under half of the body’s mass, and is essential for movement, metabolism and metabolic health, understanding mechanisms of injury responsible for both initial and persistent dysfunction is important, and will provide a foundation for intervention. The purpose of this review is to provide an overview of the available evidence describing associations between childhood cancer, its treatment, and muscle outcomes, identifying gaps in current knowledge.
Keywords: childhood cancer, skeletal muscle, muscle outcomes, muscle health, muscle quality, muscle fitness, muscle mass
INTRODUCTION
Increased survival rates for childhood cancer are a direct reflection of major therapeutic and diagnostic advances made in recent decades. Today, the expected five year survival rate for childhood cancer exceeds 85%.1,2 As the number of childhood cancer survivors continues to grow, so does the number of investigations designed to identify risk factors for limitations in physical function,3–5 adverse health outcomes,6,7 and reduced quality of life in long-term survivors.5,8 In the general population, skeletal muscle (muscle) health is a clinical indicator of metabolic disease and fall risk. Poor muscle health is associated with impaired mobility and early mortality.9–11 In children, low muscle mass and fitness are associated with metabolic risk.12 Muscle strength in both children and adolescents is also a powerful predictor of insulin sensitivity and metabolic risk in adulthood.13 Childhood cancer patients receive aggressive chemotherapy and radiation treatment during crucial physiological development. Survivors are at risk for treatment-related musculoskeletal late effects.14 While much attention has been directed at bone health, the acute and long-term effects of childhood cancer and treatment on muscle health has not been fully elucidated.
There are over 600 muscles in the human body, accounting for 40% of total body mass. Muscle serves a variety of health and functional purposes across the lifespan. It is the mechanistic machinery of daily movement and protects other organs against trauma. Muscle is the largest source of body protein and the main reservoir of amino acids used by other tissues for regeneration and repair. Further, it is a major site of glucose homeostasis, energy mobilization, and even has the capacity to act as a secretory organ, releasing factors into circulation with autocrine and paracrine effects. Healthy muscle is resilient; it is strong, flexible, abundant, and adaptable. Muscle quality and function are the primary measures of muscle health (Figure 1), integrating the lifetime impact of a variety of influences such as diet, exercise, and disease. Muscle fitness integrates the quantity and quality of muscle and neurological coordination to provide a measure of functional capacity, measured as strength, endurance, and power. Given that key muscle development occurs early in life, a time when childhood cancer patients are exposed to aggressive chemotherapy and radiotherapy treatments, they are at great risk for early declines in muscle health that may contribute to adverse outcomes later in life.
Figure 1.
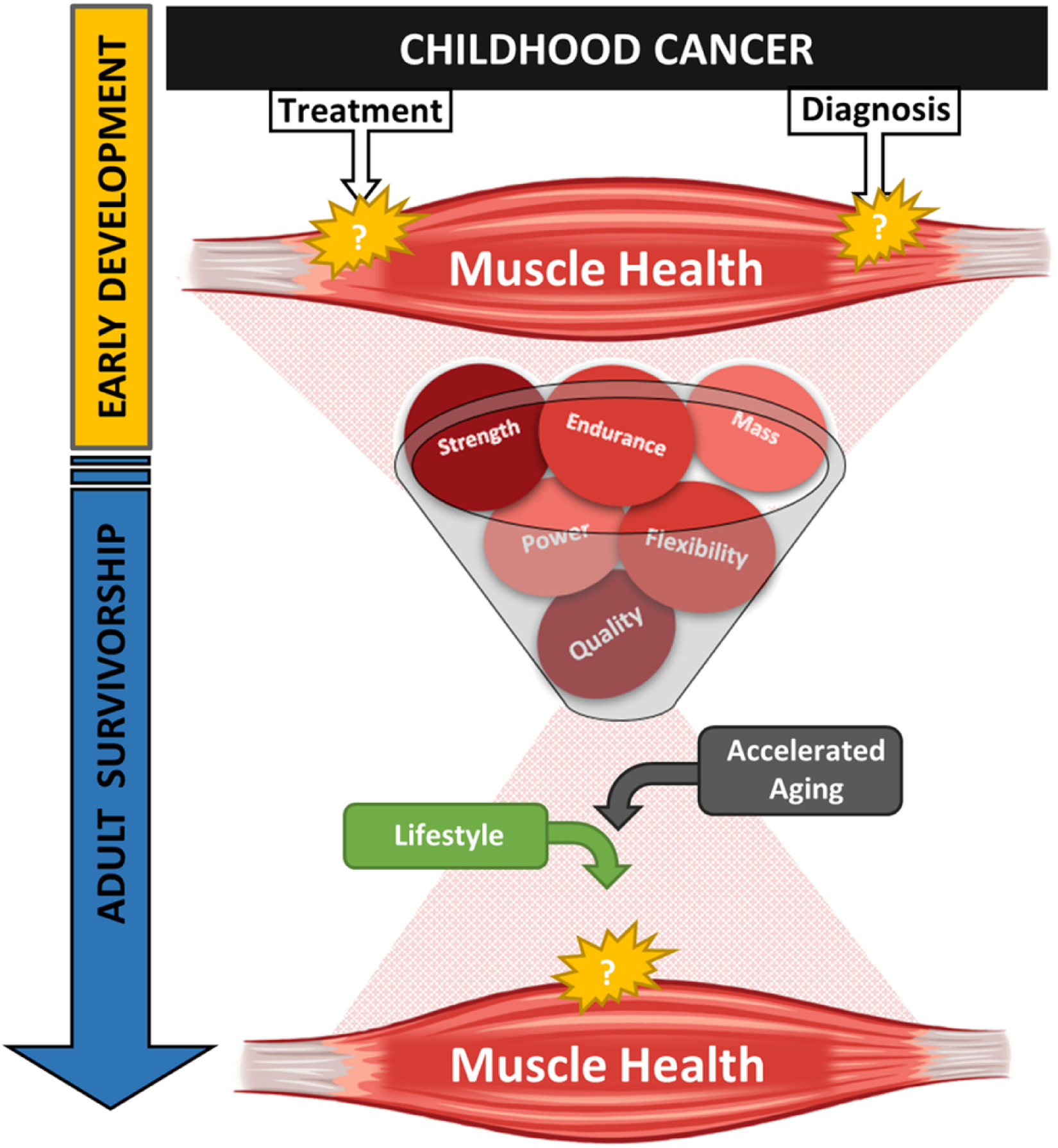
Complexity of muscle health and childhood cancer
Impaired muscle quality and function are noted in both childhood cancer patients15–19 and survivors.3,5,17,20–28 However, the pathophysiologic mechanisms responsible for these impairments are not well documented. This is partly due to limited research access to muscle tissue samples, and to difficulties surrounding non-invasive investigative approaches for muscle assessment. Given muscle’s contribution to mobility, cardiometabolic health and quality of life, identifying treatment and diagnostic specific risk for muscle impairments will undoubtedly improve functional outcomes for both patients and long-term survivors. The purpose of this review is to provide an overview of the available evidence describing associations between childhood cancer, its treatment, and muscle outcomes, identifying gaps in current knowledge.
MUSCLE ASSESSMENT METHODS
I. Mass
Both imaging and non-imaging techniques are used to measure muscle mass. Bioelectrical impedance analysis (BIA) is a non-imaging technique routinely used in clinical settings. Despite BIA being inexpensive, quick, and easy to perform, BIA may not provide the most accurate estimation of muscle mass in children with cancer; BIA measurements are affected by hydration status, often compromised in hospitalized children. Also, no population specific equation for children with cancer to predict fat-free mass exists. Air displacement plethysmography (ADP) is another non-imaging method previously used to evaluate body composition in children with cancer.29,30 While ADP is non-invasive and time efficient, estimates of fat and fat free mass can be affected by a patient’s clothing, movement or body temperature.31 ADP is also unable to identify regional differences in body composition which is important when evaluating relationships between muscle mass and function to understand the whole sarcopenic phenotype in this population.
Fortunately, multiple imaging techniques are available, offering advantages over BIA and ADP. Dual-energy x-ray absorptiometry (DXA) is commonly used to assess body composition and fat free mass in clinical settings. A DXA scan has a modest cost, short scan time and is relatively accessible in a hospital setting. However, despite these advantages, DXA can only distinguish regional differences in body composition in 2-dimensions. Given that development of sarcopenia is undoubtedly complex, more detailed, 3-dimensional images are better suited to divulging the pathophysiological processes underlying muscle wasting in children with cancer. Magnetic resonance (MR) and computed tomography (CT) are considered gold standard imaging techniques to assess regional changes in lean mass, specifically muscle. Both these methods can quantify change in intramuscular structure such as cross-sectional area, volume or intramuscular adiposity.32,33 Regional 3-dimensional images at predefined anatomical landmarks can also be used to estimate whole body muscle mass.34,35 Unfortunately, both MR and CT imaging are costly, require highly sophisticated machinery, and are resource intensive. Alternatively, ultrasound has emerged as a potential alternative for skeletal muscle imaging. Muscle ultrasound has been performed previously in hospitalized children to assess diaphragm and lower leg muscle thickness.36–40 Panoramic ultrasound is a reliable and valid tool, comparable to MRI, to quantify change in muscle structure and mass in adults.41–43 Although not yet validated in children, panoramic ultrasound may be a cost efficient, bedside capable, less invasive alternative to MR and CT techniques in children with cancer. Other ultrasound methodologies such as elastography and contrast enhanced ultrasound can be used to assess mechanistical properties and microvascular blood flow of muscle,44–47 and are promising methods to provide insight into the pathophysiological processes underlying muscle wasting in children with cancer.
One of the major processes that underlies muscle mass homeostasis is muscle protein synthesis. Traditionally, muscle biopsies are used in combination with radioisotopes to evaluate muscle protein synthesis in adults.48,49 However, in children access to muscle tissue is limited. Thus, there is very limited knowledge of muscle protein synthesis and whether it is altered in children with cancer. Positron-emission tomography (PET) is a functional imaging technique that is routinely used in the diagnosis and treatment of children with cancer. Sometimes, a PET and CT scan are performed simultaneously to provide information about function, size and shape of tumors and the surrounding structures. Furthermore, PET imaging can also provide dynamic and quantifiable information of other tissues beside tumors. PET with L-[methyl-11C] methionine can be used to assess both basal and responsive muscle protein synthesis rates,50–52 and more recently has been validated as a less-invasive alternative to the traditional L-[ring 13C6] phenylalanine infusion with serial muscle biopsy in adults.53 However, the use of PET to evaluate muscle protein synthesis has not been validated in children.
II. Strength
A wide variety of methods are available to evaluate muscle function. Muscle strength is a component of both sarcopenia32,54 and frailty,55 two phenotypes indicating comprised muscle health. Manual muscle testing (MMT) is readily used in clinic to determine the grade of strength in patients with neuromuscular dysfunction.56 Unfortunately, this method provides more qualitative information and relies on subjective interpretation of muscle performance. Thus, it is a less than ideal method to quantify muscle strength changes in children with cancer, especially when performed by different testers. Hand-held dynamometry (HHD) is a cost and time-efficient method that can provide a basic quantifiable measure of strength. Strength is assessed by a “make” or “break” test by which a patient has to meet the resistance or over-power the force imparted on them by the test administrator using a hand-held dynamometer. While this method can be used in patients as young as 2 years old,57 it can be difficult to perform when a patient has a body-size advantage over the test administer or the patient is not positioned correctly, potentially affecting the validity of the test58,59 Fixed and portable dynamometers are popular alternative methods in both research and clinical settings. They are easy to use, cost-effective, and provide reliable and valid measures of strength.60–65 They limit the margin of user error compared to HHD. While these methods are well suited to both clinical and research environments, using minimal space and requiring minimal set-up time, they are unable to assess dynamic movement. As such, isokinetic dynamometry is the gold standard method to assess muscle strength. This computerized method can capture concentric, eccentric, and isotonic/isometric maximal and endurance strength of multiple muscle groups . Unfortunately, this equipment is costly, requires specialized training and substantial physical space. Alternatively, hand-grip strength via HHD is used in the diagnostic criteria for aging muscle phenotypes.32,54,55 Not only is it easily administered and does not require advanced training, it is also portable making it well suited for use in the hospital setting. While hand-grip strength is associated with long-term health in adolescents,66 it is not a direct measure of the major locomotive muscle groups in the legs. Thus, hand-grip strength may inaccurately estimate the prevalence and impact of muscle weakness in children with cancer. Overall, the interpretation of muscle strength and changes there-of should be considered in the context of the advantages and disadvantages of the assessment methods used.
MUSCLE HEALTH AND CHILDHOOD CANCER
I. Acute Outcomes
Muscle weakness and low lean mass in childhood cancer patients and survivors are consistently reported (Table 1).5,20,22–24,67–73 These acute outcomes likely result from a combination of factors, including the cancer itself, aggressive multimodal-therapies, as well as systemic changes including inflammation, hormone concentrations and nutritional status. Unfortunately, few studies have focused on how these factors directly affect skeletal muscle. However, in children with cancer, muscle mass changes occur independently of changes in bone mineral content, fat mass and total body mass, suggesting that muscle can and should be considered explicitly and independently from BMI or adiposity. Yang and Choi74 illustrated this in a propsective study for longtitudinal assessment of nutritonal status and body composition in newly diagnosed heamatologic malignancy (n=19) and solid tumor (n=11) patients. Among 30 children (mean age: 10.9±3.8 years, 70% male), DXA showed that median fat free mass significantly decreased from 27.4 kg (range, 11.5–53.5 kg) to 26.9 kg (14.4–50.6 kg, p=0.008) during the first month of cancer treatment, occurring in the absence of total body mass change.
Table 1:
Summary of key clinical evaluations of muscle health and childhood cancer
| Author Country |
Year published | Population | Study design | N | Age | Muscle mass assessment method | Muscle function assessment method | Results |
|---|---|---|---|---|---|---|---|---|
| Children with cancer | ||||||||
| Yang Korea |
2019 | Children newly diagnosed with cancer: 70% male Controls: 73 % male |
Prospective | 30 patients: 19 hematological malignancy,11 solid tumor 30 controls |
Patient mean (SD): 10.9 (3.8) y Control mean (range):12.3 (6.3–17.2) y |
FFM mass by DXA | Leg FFM differed between groups Patients’ median fat free mass ↓ during treatment |
|
| Brinksma The Netherlands |
2015 | Children newly diagnosed with cancer: 48.4% male |
Prospective | 133 patients: 39.8% hematological malignancy, 33.1% solid tumor, 27.1% brain tumor |
Median (range) 8.1 (0.1–17.7) y | FFM via BIA | FFM was low at diagnosis, and remained low during treatment FFM was lowest in children with brain malignancies BMI ↑ during treatment |
|
| Rayar Canada |
2013 | Children with ALL: 63% male |
Prospective | 91 patients | Mean 6.1 y, median (range) 5.0 (1.2–17.6) y | SMM by DXA | SMM was low at diagnosis, ↓with treatment, and remained low 12 months after diagnosis | |
| Ness United States Canada |
2015 | Children newly diagnosed with ALL: 65.1% male 63.3% white |
Cross-sectional | 109 patients | Median (range) 10 (4–18) y | Lower extremity isometric strength by HHD “break” test Hand grip strength by HHD Motor development by BOT2-SF |
Patients had weaker knee extensors and hands at start of treatment Patients had worse motor abilities at diagnosis than expected |
|
| Gocha United States |
2003 | Children with ALL Controls |
Prospective | 16 patients 8 controls | Patient median (range): 9.6 (4–15) y Controls: age-and gender matched |
Knee extension and ankle dorsiflexion strength by HHD Functional mobility by TUG |
Patients were weaker in knee extensor and ankle dorsiflexor muscles, and had lower functional mobility than controls before start of treatment Patients’ ankle weakness worsened over 28 days of treatment Knee extensor strength was negatively correlated with functional mobility in patients |
|
| Akyay Turkey |
2014 | Children with ALL, on-therapy: 60% male Children with ALL, off therapy: 55.6% male On-therapy controls: 60% male Off-therapy controls: 55.6% male |
Prospective | 15 on-therapy patients 18 off-therapy patients 15 on-therapy controls 18 off-therapy controls |
Children on-therapy mean (SD): 9.7(4.5) y Children off-therapy mean (SD): 12.5 (5.2) y On-therapy control mean (SD): 9.7 (4.5) y Off-therapy control mean (SD): 12.5 (5.2) y |
Hand grip strength by HHD Functional mobility by TUG |
On-therapy patients: Strength and functional mobility ↓ during treatment and was lower than off-therapy patients and controls Off-therapy patients had lower functional mobility than controls |
|
| Schoenmaker The Netherlands |
2006 | Children with cancer: 50% male |
Prospective | 18 patients: 17 ALL, 1 T-NHL | Range 0–18 y | Upper and lower extremity strength by MMT and HHD | Muscle weakness and mobility issues were most severe in the first two months of treatment All patients had muscle weakness in at least one muscle measured by MMT Lower extremity muscle weakness measured by HHD persisted 6 months after therapy in patients older than 5.5 y |
|
| Survivors of childhood cancer | ||||||||
| Tonorezos United States |
2013 | Survivors of childhood ALL: 44.4% male 72% white |
Cross-sectional | 117 survivors | Median (range) 23 (18–37) y | Lean mass by DXA | Survivors who received cranial radiation had less lean body mass than survivors without cranial radiation | |
| Boland United States |
2016 | Survivors of childhood ALL: 47.7% male 87% white Community controls: 47.7% male 87% white |
Cross-sectional | 365 survivors 365 controls |
Survivor median (range): 28.5 (18.4–44.6) y Controls: 5-year age matched |
Relative lean muscle mass by DXA | Survivors had lower absolute and relative lean mass than controls | |
| Ness United States |
2012 | Survivors of childhood ALL: 48.9% male 93.7% white |
Cross-sectional | 415 survivors | Median (range): 35.6 (21.9–52.3) y | Lower extremity muscle strength by isokinetic dynamometry Functional mobility by TUG |
Survivors had dorsiflexion weakness (16.9%), plantarflexion weakness (24.6%), knee extensor weakness (30.1%) and limited functional mobility (3.6%) Knee extensor weakness increased risk for limited physical performance |
|
| Hovi Finland |
1993 | Female survivors of childhood ALL Controls |
Cross-sectional | 43 survivors 37 controls |
Survivor mean (range): 19 (14–30) y Control mean (range): 19.4 (14 – 30 ) y |
Elbow, knee and hand grip isometric strength by custom-made dynamometer chair Abdominal muscular endurance by maximal sit-up test Upper body muscular endurance by maximal push-up test |
Survivors had less abdominal and upper body endurance and weaker elbow and knee extensors than controls | |
| Ness United States |
2015 | Survivors of childhood ALL: 47.7% male 12.1% black Community controls: 47.7% male 12.1% black |
Cross-sectional | 365 survivors 365 controls |
Survivor mean (SD): 28.6 (5.9) y Control mean (SD): 28.9 (7.5) y |
% FFM and relative lean mass by DXA | Hand grip strength by HHD Knee extensor and ankle dorsiflexion strength by isokinetic dynamometry |
Compared to controls: Female survivors with a history of cranial radiation had lower % FFM and relative lean mass (g/m2) Male survivors had lower % FFM Survivors were weaker and had less endurance in their quadriceps muscles Survivors with a history of cranial radiation had hand-grip weakness |
| Van Brussel The Netherlands |
2006 | Survivors of childhood ALL: 46.2% male |
Cross-sectional | 13 survivors | Mean (SD): 15.5_(5.8) y | Grip strength by HHD “make” test Upper and lower extremity strength by HHD “break” test |
Survivors had knee extensor weakness |
|
| Ness United States |
2019 | Survivors of childhood cancer: 51.1% male 84.1% non-Hispanic white Community controls: 48.8% male 90.2% non-Hispanic white |
Cross-sectional | 1260 survivors 285 controls |
Survivor mean (SD): 36.4.6 (9.2) y | % lean mass and relative lean mass by DXA | Isokinetic quadriceps strength by isokinetic dynamometry | Survivors had lower % lean mass and lower relative lean mass z-scores that controls Cranial radiation exposure >20 Gy was associated with quadriceps weakness Quadriceps weakness (−1 SD) increased risk for exercise intolerance (OR, 1.49; 95% CI, 1.23 to 1.82 |
Abbreviations: ALL, Acute lymphoblastic leukemia; T-NHL, T-cell Non-Hodgkin lymphoma ;y, years; %, percent; TUG, Time Up and Go; DXA, dual-energy x-ray absorptiometry; BIA, bioelectrical impedance analysis; HHD, Hand-held dynamometer; MMT, manual muscle testing; PEDI, pediatric evaluation of disability inventory; FFM, fat free mass; SMM, skeletal muscle mass; BOT2-SF, Bruininks-Oseretsky Test of Motor Proficiency Version 2 Short Form; ROM, range of motion; ↑, increase; ↓, decrease; ≥, equal to or greater than; OR, odds ratio; CI, confidence interval;
In children with cancer, both the disease and associated treatments place increased energy-substrate demands on the body to fight infection and fatigue, beyond what is normally required for normal growth and development. Further, children often experience treatment induced nausea and vomiting. While both over-and under-nutrition in the childhood cancer population is reported,75–77 there is limited information on associations between nutritional status and body composition. There is little to no evidence that suggests nutritional status or dietary protein intake is a mediator of muscle wasting in children with cancer. Brinksma et al78 prospectively observed significant increases in fat mass despite low energy intake during the first year following diagnosis. In this study, a total of 115 Dutch children (median age: 8.1 (range 0.1–17.7) years, 47.0% male) recently diagnosed with cancer (41% hematological, 33% solid tumor, 26% brain) participated in assessments of dietary energy and protein intake during various treatment protocols. Dietary intake was collected using 3-day (consecutive) food records (included 1 weekend day) within the first 1–3 weeks following diagnosis, and was repeated at 3, 6, and 12 months post diagnosis, in between chemotherapy treatments. Food records were then transferred to a computer calculation software to quantify intakes by standard measurement units (i.e. mg, IU). To standardize for each child’s specific nutritional needs, individual energy requirement (kcal/day) and individual protein requirement (g/day) were calculated and adjusted for body weight.79 Daily intakes were compared to the recommended daily allowance,80 intakes of healthy children in the Netherlands,81,82 and calculated individual requirements. Mean energy intake (kcal) in patients was significantly lower than both recommended daily allowance and intakes of healthy children regardless of timepoint; mean percent of individual energy requirement throughout the study period was 105%. Even though overall intake was lower than required among patients, at all assessments, percent individual protein requirement (145%, p<0.05) and recommended daily allowance of protein (181%, p<0.01) intake were more than adequate. However, percent individual protein intake was not associated with preservation of lean mass, indicating an underlying desensitization or resistance to the nutrition-stimulated anabolism necessary for muscle mass homeostasis.83 Some patients also have lowered resting energy expenditure suggesting decreased metabolic activity.84,85 Given that muscle has high metabolic activity,86 decreased resting energy expenditure supports the observed losses in lean mass during treatment and further underlines an insensitivity to dietary protein known to stimulate the anabolic growth process in muscle.87
Muscle wasting may vary by diagnosis, suggesting a direct effect of different malignancies. Brinksma et al88 noted lower fat free mass in patients with brain malignancies than patients with hematological or solid malignancies. This may be due to differences in tumor derived cytokines89 or inflammation90–93 altering the muscle’s microenvironment.94,95 Further, the decline in muscle mass that occurs acutely has also been associated with the duration of hospitalization during induction therapy. Rayar et al96 recently examined the patterns of change in appendicular muscle mass throughout therapy in 91 children who were less than 17 years of age at diagnosis with acute lymphoblastic leukemia (ALL). Patients received DXA scans at 5 separate time points during treatment with change in mass converted to Z-scores. In this study, mean muscle mass loss at diagnosis was small (−0.18 SD), and substantially increased within 6 months (−1.08 SD). This increase corresponds with the completion of remission induction therapy which includes high dose glucocorticoid administration. Further, the children did not fully regain their muscle mass within 12 months of diagnoses (−0.5 SD). In an evaluation of the relationship between change in muscle mass and the burden of illness (days of hospitalization), there was a significant association between mass loss at 6 months post diagnosis and the number of inpatient days during induction therapy (r=0.31; p<0.05).
Muscle mass contributes to functional capacity and is implicated in body compositional changes that occur with treatment.23,30,97–99 Lower extremity strength is required for safe ambulation, balance and many daily functional activities such as sitting and standing from a chair, climbing stairs, as well as maintenance of independence in daily living. There are a limited number of studies to date that have focused on muscle quality and function in patients, most of which are in children diagnosed with ALL. The vast majority of the literature has evaluated muscle strength.15–17,22,67
Children newly diagnosed with ALL present with muscle weakness that can worsen as treatment progresses, and may never fully recover.15–17,67 We found strength loss in the lower extremities in 109 children (median age: 10 (range 4–18) years, 65.1% male, 63.3% white) newly diagnosed with ALL enrolled on a physical activity trial.17 Participants were assessed for skeletal, neuromuscular, and fitness impairments within 7–10 days of initiation of chemotherapy. HHD was used to administer a “break test” for knee extension (proximal lower extremity) strength. On average, knee extensor strength was 34.2 Newtons less than expected (p<0.01). Gocha et al16 reported similar findings in 8 children with ALL (median age: 9.6 (range 3–15) years), assessed for strength and functional mobility before (day 0, baseline) and following delayed intensification therapy (day 28). HHD was used to measure knee extension and ankle dorsiflexion strength, and the Timed Up and Go (TUG) test to assess functional mobility. The TUG captures the time needed to stand from a seated position, walk three meters, turn around, return, and sit back down in the chair.100 In this study, ALL patients on average had significantly lower knee and ankle strength, and higher TUG times (indicating impaired function) than age-matched controls. Patients with greater strength performed the TUG task faster. In this study, weakness in ALL patients progressed with delayed intensification therapy, with significant dorsiflexion strength loss (baseline 0.23±0.09 kg vs. DI 0.15±0.06 kg) noted within 4 weeks. Akyay et al15 studied 15 newly diagnosed ALL patients and 18 off-therapy patients, each with respective control-group counterparts. Hand grip strength was measured with dynamometry and functional mobility with the TUG test. Children with cancer had grip weakness at diagnosis in the right (median (min–max), 9.5 (1.0–20.0) vs. 16.0 (7.0–34.6) kg, p=0.039) and left (median (min– max), 9.2 (0.5–22.5) vs. 15.3 (6.8–33.6) kg, p=0.042) hands prior to starting chemotherapy, compared to controls. Newly diagnosed patients also experienced progressive strength loss during induction therapy in both right (median (min-max), 11.4 (6.3–28.6) kg vs. 9.7 (1.0–20.0) kg, p<0.001) and left hands (12.1 (4.3–33.1) kg vs. 9.7 (0.5–22.5) kg), p=0.009) compared to their baseline measures, and took longer to complete the TUG test following the completion of induction therapy than they did prior to starting chemotherapy (median (min–max), 8.4 (6.9–10.2) seconds vs. 11.0 (8.3–22.8) seconds, p=0.008). These deficits improved following cessation of treatment. Off-therapy patients, compared to newly diagnosed patients, had significantly stronger grip strength in both right (median (min-max), 18.9 (8.0–37.6) kg vs. 9.5 (1.0–20.0) kg, p<0.001) and left hands (19.4 (7.2–37.6) kg vs. 9.2 (0.5–22.5) kg, p<0.001), as well as faster TUG times (median (min-max), 6.8 (6.0–11.5) seconds vs. 11.0 (8.3–22.8) seconds, p<0.001). Unfortunately, off-therapy patients were still weaker and had impaired mobility compared to controls (children without cancer). These data suggest that impaired motor performance is related to diagnosis and acute treatment, and has the potential to improve following therapy cessation, but does not completely recover.
These studies are supported by data from Schoenmaker et al67 who documented severe muscle weakness and mobility problems (such as transfers, walking, navigating stairs) in children treated for standard risk ALL or T-cell Non-Hodgkin lymphoma. In 18 Dutch children, 9 children (mean age: 8.7 years, range 1–16 years, 55.5% male), were treated according to a German Berlin, Frankfurt, Muenster (BFM) based protocol (ALL-8), with the other 9 children (mean age: 7.5 years, range 2–15 years, 44.4% male) treated on protocol ALL-9, an antimetabolite treatment high in dexamethasone and vincristine. MMT of the upper and lower extremities, scored by the criteria of the Medical Research Council, using a 6-point scale (grade range 0–5),101 was conducted prospectively at time of diagnosis (T1=week 0), twice during treatment (T2=week 7 and T3=week 28), at the end of treatment (T4=week 105), and 6 months after completion of treatment (T5=week 131). HHD was also used to evaluate isometric force of upper and lower extremity muscles on the non-dominant side at diagnosis and 6 months after treatment. Investigators also measured functional skills, including mobility, with the adapted Dutch version of the Pediatric Evaluation of Disability Inventory (PEDI).102 In pooled analysis, muscle weakness and mobility issues were most severe in the first two months of treatment. Specifically, MMT revealed weakness in all patients (grade ≤4, “active movement”, against gravity with some resistance) in at least one muscle group, both proximally and distally, and was most apparent at 7 weeks following chemotherapy induction. At this same assessment, patients also presented with functional mobility problems scoring below the normal range (30–70 points) on the PEDI mobility scale. At 6 months after completion of treatment, MMT strength improved to a grade 5 (“normal power”) in 15 of 18 participants although HHD revealed that muscle strength of the knee- and foot extensors remained significantly decreased compared to reference values,103 again suggesting that impaired motor performance (weakness) persists after cessation of therapy.
Overall, the sequence of events that contribute to low muscle mass and weakness in children with cancer is difficult to discern. This is in part due to limited investigations that assess muscle-centric outcomes, small samples sizes in existing investigations, differences in evaluation techniques, and different diagnostic populations studied. However, it is likely that muscle wasting and weakness begin early at diagnosis15–17,30,104,105 and worsens with treatment (Figure 2).15,16,67,96,104 While some children may partially recover their muscle mass,96,104 muscle quality is likely compromised. Such, these children enter survivorship with poor muscle health, likely contributing to the early onset of chronic conditions.6,7 Furthermore, survivors have muscle mass decreases independent of- or preceding strength loss71 which is unlike older adults without a history of childhood cancer who experience decreases in muscle strength preceding mass loss. Whether this pattern in present during childhood cancer treatment remains unclear. Thus, the interrelationship between mass and function in children with cancer deserves attention in future research to better understand the phenotype and develop strategies to limit its consequences.
Figure 2.
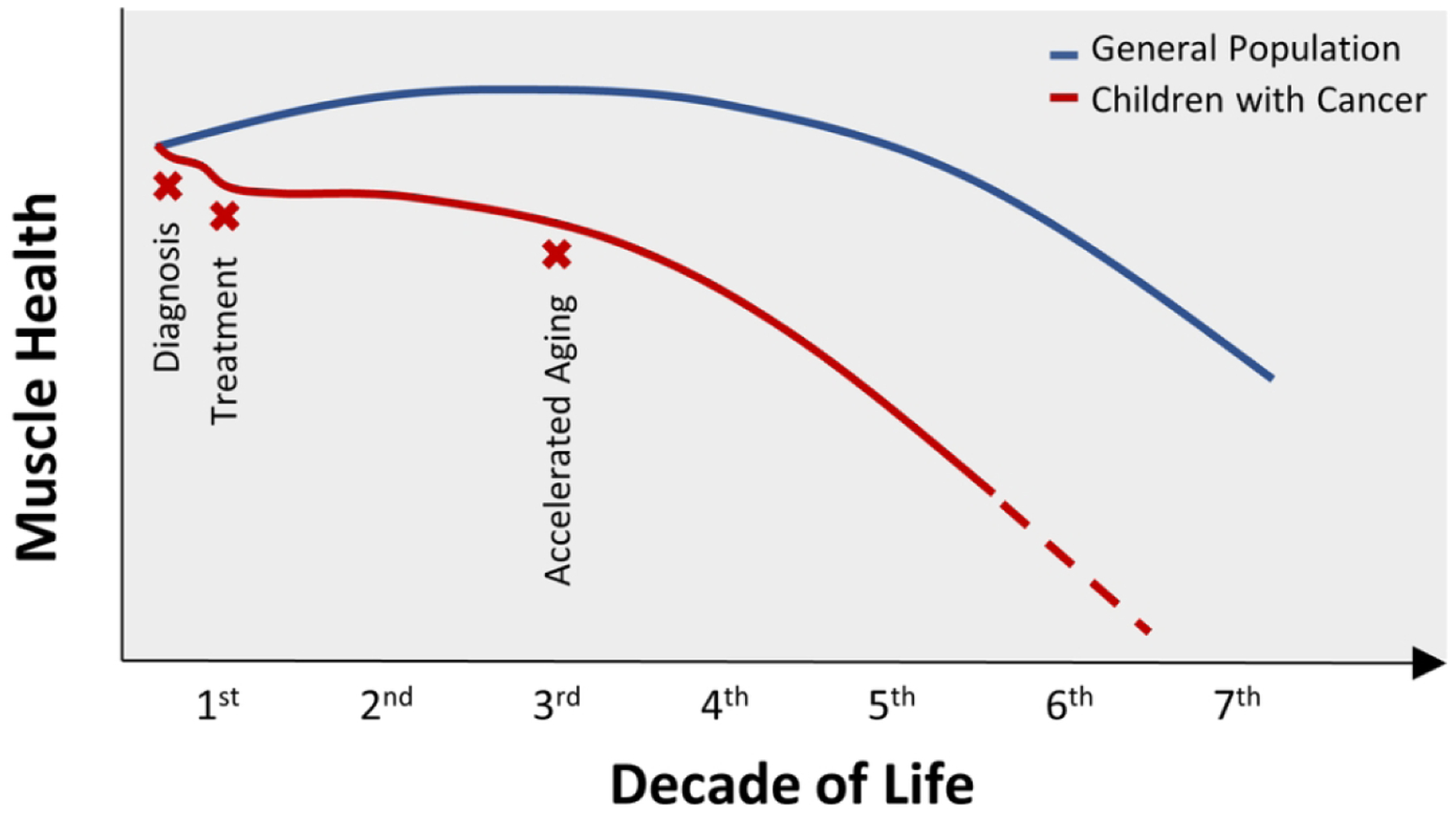
Decline in muscle health with childhood cancer
II. Long-Term Outcomes
Impaired muscle quality or function following cancer treatment may contribute to poor function and reduced fitness in survivorship. Most investigations observing muscle outcomes in childhood cancer survivors have been conducted in childhood ALL survivors. Low lean mass is one of the most commonly reported outcomes in long-term ALL survivors.5,24,68–73,106 Boland et al69 observed lower relative (68.6% vs. 71.4%) lean mass and lower absolute (55.0 vs. 57.2 kg) lean muscle in childhood ALL survivors compared to age-, sex-, and race-matched controls. This study also noted that survivors (N=365, median age:28.5 (range 23.6–31.7) years, 52% male, 87% white) are less likely to report participating in resistance training than controls (45.4% vs. 53.8%), suggesting that lifestyle choices may contribute to low muscle mass in this population.
The degree of muscle mass and strength loss in survivors can differ with previous treatment exposures. Tonorezos et al68 evaluated the contribution of diet and physical activity to metabolic parameters among 117 long-term survivors of childhood cancer (median age: 23 (range 18–37) years, 44% male,) with and without a history of cranial radiation therapy (CRT). This study found that survivors with a history of CRT had lower lean body mass compared to survivors without CRT exposure (47.8±12.4 vs. 52.7±11.0 kg, p=0.04) despite no difference in physical activity or total daily protein intake. We captured an association between CRT exposure and muscle strength in 75 young adults (mean age: 30.2±7.1 years, 41.3% male, 98.7% white) previously treated for childhood ALL (mean diagnosis age: 5.6±4.3 years).24 Using HHD to measure lower extremity strength, we found that males were 76.7 Newtons and females were 58.6 Newtons weaker on average in their knee extensor muscles than expected (expected males 569.87 N, females 464.67 N). Survivors exposed to CRT were weaker than survivors who were not exposed, although a significant association was only found among females. Further, knee extensor strength was positively correlated with percent skeletal muscle mass (r=0.27, p=0.02) assessed by DXA. Male survivors had 2.6% and females had 2.2% less muscle mass than expected (expected males 40.7%, females 32.3%).107–110 Treatment exposures such as CRT may contribute to tissue compositional changes within a muscle (i.e. contractile tissue vs. non-contractile tissue), influencing strength capabilities and muscle mass maintenance. However, it is unclear whether strength loss in survivors is due to an infiltration of fat or fibrotic tissue into skeletal muscle, and whether muscle mass is compromised as a result.
Muscle strength and flexibility are also known to be impaired following childhood cancer treatment and contribute to reduced mobility and walking efficiency. We clinically assessed neuromuscular impairments and physical performance limitations in 415 childhood ALL survivors (median age: 35 (range 21–52) years, 48.9%, 97.3%) 10+ years from diagnosis (median time since diagnosis: 29.9 (range 13.7–46.5) years).26 Knee extension strength and active dorsiflexion range of motion (ROM) was impaired in survivors (30.1% and 33.5%, respectively). In this study, survivors with impaired knee extension strength were 8.2 times (95% Confidence Interval (CI) 2.3–29.0) more likely to have limited mobility, and 2.3 times (95% CI 1.4–4.1) more likely to have limited walking efficiency compared to survivors without impaired knee extensor strength. Survivors with ankle ROM deficits were more likely to have impaired walking efficiency (OR 3.9, 95% CI 2.4–6.3) compared to those without ROM deficits. These findings are consistent with a study by Gerber et al111 who assessed ROM, strength, and walking velocity in 32 survivors (mean age: 35.4±10.6 years, 53.1%) of pediatric sarcoma. Walking velocity was positively correlated with ROM deficits (r=0.50, p=0.06) and strength loss (r=0.74, p=0.002) in female survivors, suggesting likelihood for reduced exercise tolerance and elevated risk for functional loss. Unlike survivors of childhood ALL, pediatric sarcoma survivors often receive surgical intervention as part of the treatment plan. Thus, they have added risk for muscular complications in survivorship that can influence their daily mobility and physical activity levels. A recent study by Marina et al8 found that survivors who were surgically treated for lower extremity sarcomas during childhood cancer treatment had a 50% increased risk for activity limitations during survivorship compared to upper extremity sarcoma survivors.
Muscle endurance, the ability to offset fatigue, is an important aspect of muscle fitness. Poor muscle endurance manifests as a decreased capacity to do work and thus reduced efficiency of performance that normally follows a period of activity. As an indicator of muscle fitness, good endurance is evident in a quicker recovery from physical exertion and longer time to fatigue. Hovi et al22 included an evaluation of muscle endurance in their strength assessments of 43 young female survivors of childhood ALL (mean age: 19 (range 14–30) years) that were off therapy (mean time: 8 (range 1–19) years). Arm extension endurance was determined by maximum number of push-ups participants could complete within 1 minute. Trunk flexion endurance was determined by the maximum number of sit-ups performed in 1 minute. Performance numbers were compared to 69 healthy women (mean age: 19.4 (range 14–30) years). The average number of completed repetitions for push-ups (7.2 (range 2.0–12.4), p=0.008) and sit-ups (5.8 (range −0.2–11.8), p=0.06) were significantly less in survivors compared to controls. This study also found muscle endurance to be associated with treatment exposures. Survivors exposed to radiation did, on average, 7.1 (95% CI −1.5 to 15.8 reps, p=0.10) fewer push-ups, and those exposed to asparaginase 7.6 (95% CI 1.3–13.9, p=0.019) fewer push-ups and 10.6 fewer sit-ups (95% CI −4.3 to 16.9 reps, p=0.002) than those not exposed. We also compared muscle endurance of the lower extremities in a large cohort of childhood ALL survivors (n=365, median age: 28.5 (range 23.6–31.7) years, 52% male, 87% white) with and without CRT exposure during cancer treatment, to age-, sex- and race-matched controls.25 In this study, lower extremity muscle endurance was evaluated using an isokinetic dynamometer. In a seated position, participants performed repeated isokinetic knee extensions (Newton-meters [Nm]/kg at and 300°/s). Consistent with Hovi et al,22 this study reported lower endurance in quadricep muscles in both CRT exposed (69.4±24.2 vs. 93.1±35.1 Nm/kg, p<0.001) and non-CRT exposed (81.7±30.5 vs. 93.1±35.1 Nm/kg, p<0.001) survivors compared to normative values. Muscle fatigue results from adenosine triphosphate depletion and is related to energy availability during repeated movement. Reduced muscle endurance suggests that mitochondrial function and/or substrate oxidation may be compromised in this population, translating to imitations in repeated or prolonged functional movements (i.e. walking, running), interfering with ability to fully participate in sport and/or recreational activities.
Muscle strength is the most commonly measured functional outcome in survivors of childhood cancer. Strength deficits often present concurrently with impaired exercise capacity as the skeletal muscle system is one of several organ systems that integrate functions during exercise. Van Brussel et al28 evaluated physical function and fitness in 13 young survivors of childhood ALL (mean age: 15.5±5.8 years, 46.2% male) who were within 5 to 6 years of chemotherapy cessation. On average, muscle strength determined by HHD was significantly lower in the knee extensor muscles of survivors compared to controls (252.1±81.13 vs. 299.7±98.9 kg, p=0.001). Anerobic fitness was evaluated using a wingate test,112 and aerobic fitness with a cardio-pulmonary exercise test. Compared to controls, survivors, on average, generated lower peak power (538.6±289.6 vs. 867.32±508.2 W, p<0.001) and mean power (376.1±171.9 W vs. 492.9±276.9 W, p<0.001) outputs. Survivors also had a lower exercise capacity on average than controls (VO2 peak, 36.64±18.3 vs. 49.58±21.22 ml·kg−1·min−1). Recently, we evaluated exercise intolerance, mortality, and organ system impairment in 1,041 10+ years survivors of childhood cancer (mean age: 35.6±8.8 years, 49.3% male, 85.15 non–Hispanic white), and 285 community controls (mean age: 34.5±10.0 years, 48.8% male, 90.2% non-Hispanic white),113 and found that a one standard deviation decrease in quadricep strength increased the odds of having an ejection fraction <53% (OR 1.50, 95% CI 1.30 to 1.72), and of having an exercise tolerance <85% of predicted (OR 1.49, 95% CI 1.23 to 1.82). These data indicate that continued monitoring of muscle fitness in childhood cancer survivors is important as reduced strength and aerobic fitness are associated with mortality in healthy aging individuals, and among adults undergoing cancer therapy.114,115–117
III. Accelerated Aging in Childhood Cancer Survivors
In the general population, muscle mass declines at a rate of 5% per decade beginning in the 4th decade, accelerating in the 5th and 6th decades of life, and is prevalent in both healthy and frail populations.118–120 Muscle strength also decreases by 10% to 15% per decade of life until 70 years when losses are further accelerated.121,122 Loss of muscle mass accounts for 5% of the change in strength. Together, the loss of muscle mass and strength are used to describe sarcopenia. Sarcopenia is a progressive and generalized skeletal muscle disorder with known adverse outcomes that includes both impaired function (low muscle strength) and structural damage (low mass/quality) as its primary diagnostic components. The presence of sarcopenia has been linked to increased risk for fall and fractures,123–125 impaired mobility126 and activities of daily living,127,128 and loss of independence.129 It is further associated with cardiac disease,130 respiratory disease,131 and cognitive impairment,132 contributing to lowered quality of life133 and even death.134–136 This current operational definition of sarcopenia is an evolution from very early works which referenced decreased muscle mass with age as the sole indicator of sarcopenia.137,138 Since it is now known that mass loss can occur independent of age, resultant from conditions such as cancer and malnutrition, the scientific community no longer utilizes muscle mass as the sole-defining parameter of sarcopenia. Although loss of muscle mass is still important, muscle strength is the best predictor of health outcomes and is the central diagnostic criteria of sarcopenia.139–141 Muscle quality and physical performance are indicators of sarcopenia severity.
Recently, childhood cancer survivors have been identified at risk for early onset of aging phenotypes that are heavily dependent on muscle mass and strength.28,71,142 Frailty, a phenotype that uses sarcopenia in its diagnostic criteria, is prevalent in 13.1% of young adult childhood cancer survivors (mean age: 33.6±8.1 years, 50.3% male, 43.8% leukemia diagnosis).71 However, in this study, declines in muscle mass were independent of muscle strength, with some survivors demonstrating similar strength relative to sex- and age- matched controls, perhaps indicating that frailty differs among young survivors when compared to aging adults, who typically lose strength before they lose mass.143 In addition, it is not yet clear the exact nature and timing of muscle decline among children with cancer. Current evidence suggests that it begins early, emerges during cancer therapy, and can extend into survivorship immediately or reappear early in young adulthood.17,24,144,145 Nevertheless, as in older populations, frailty in young adult survivors of childhood cancer is associated with new onset chronic disease and increased hazard of death over three years. It is a significant problem.
In adults, muscle protein is maintained by synchronized periods of net catabolism in post absorptive states and net anabolism in postprandial states,146 with balance in protein turnover closely correlated with fat-free mass. However, protein turnover declines with advancing age, even when adjusted for fat-free mass,147 suggesting that with age, synchrony between protein synthesis and proteolysis is altered or resistant. Results from a recent exercise and nutrition intervention suggests there is an underlying resistance to nutrition-stimulated anabolism in the muscle of childhood cancer survivors, similar to that seen in older adults. Krull et al148 conducted a randomized placebo-control trial in 70 adult survivors of childhood cancer with low muscle mass. Participants were randomized to resistance training with protein supplement (21 g whey protein per day) (median age: 33.0 (range 20.6–44.2) years, 55.2% male) or resistance training with placebo (sucrose) (median age: 33.7 (range 21.1–44.9) years, 50.0% male) group. The training component consisted of whole-body resistance training, 3 times week for 24 weeks. At the end of the intervention, lean body mass improved in both groups (supplement 1.05±2.34 kg, p=0.04; placebo 0.13±2.19 kg, p=0.74; p=0.11 for comparison of change between groups) compared to DXA baseline lean mass. This study demonstrated that resistance training supplemented by protein supplementation is not better than resistance training alone, and that response to resistance training is only moderate in adult survivors of childhood cancer with low lean muscle mass.
In addition to age related decreased anabolic response and increased catabolic state, senescent skeletal muscle has impaired regenerative capacity.149 Among childhood cancer survivors, it is possible that treatment may damage or alter proteins, and contribute to slower tissue repair/recovery throughout life.150 Other potential mechanisms of age-associated declines in muscle quality and function include, but are not limited to, fiber atrophy and distribution,151–153 fat and fibrotic infiltration,154,155 anabolic resistance and altered protein synthesis,156,157 impaired metabolism and mitochondrial function,158,159 neuromuscular remodeling,160,161 oxidative damage,162,163 regenerative impairment,164 and dysregulated cell maintenance.165–167 Aging associated physiological impairments such as chronic inflammation, atherosclerosis, insulin resistance, and neurological deficiencies may also contribute to systemic decline.168–171 Specifically, chronic inflammation, the result of cellular damage incurred from aggressive multimodal treatments received early in life, and characterized by prolonged exposure to pro-inflammatory cytokines in systemic circulation, coincides with shortened leukocyte telomere length in childhood cancer survivors, and increases risk for metabolic syndrome.172 We hypothesize that these conditions, experienced by survivors of childhood cancer much sooner than expected when compared to their peers,173 also contribute to early loss of muscle health.
CANCER AND TREATMENT RELATED BIOMECHANISMS
I. Drug Therapy
Chemotherapy delivery in children with cancer is multi-modal and includes agents that acutely impact neuromuscular control, protein synthesis, and mitochondrial function, and that accelerate protein degradation. While research activity is underway to incorporate host, treatment, and genetic predictors of treatment related toxicities, chemotherapy administration is still required to achieve cure. Acute toxicities do not always resolve, leaving survivors at risk for poor muscle health later in life. Because children with cancer are exposed to multiple agents, toxicities that impact muscle health are likely at least additive.
Neurotoxic agents such as methotrexate and vincristine174 are exposures among children treated for cancer that contribute to long-term muscle impairments.14 Vincristine, a vinca alkaloid, induces mitotic arrest and cell death in lymphoid malignant cells by interfering with microtubule formation and mitotic spindle dynamics.175–177 Vincristine-induced peripheral neuropathy is a common side effect in children with cancer, typically manifesting as distal muscle weakness, absent reflexes and impaired flexibility.26,27 It is both dose dependent and associated with a homozygous T-allele in the CEP72 gene.178–182 Methotrexate is an antimetabolite that inhibits DNA synthesis and arrests cellular proliferation by competitively inhibiting dihydrofolate reductase activity.183–185 When it is administered directly to the central nervous system, or given in high enough doses to cross the blood brain barrier, it may interfere with folate homeostasis in normal tissue.186,187 Cumulative doses of intrathecal methotrexate in the range of 215–225 mg/m2 are associated with increased risk of impaired strength and reduced ankle ROM in children treated for ALL.25,26 Inadequate nervous system signals acutely reduce motor recruitment and limit muscle force production, which, over time, likely blunts muscle sensitivity to contractile anabolic stimuli that facilitates myofibril synthesis and muscle growth
L-asparaginase is also implicated as a potential risk factor for reduced muscle health in survivors of childhood ALL, acute myeloid leukemia and non-Hodgkin lymphoma. It is an enzyme that hydrolyzes asparagine, the alpha-amino acid that promotes cell proliferation in both healthy and cancerous cells. Leukemia cells have little to no expression of the enzyme responsible for synthesizing asparagine. Thus, L-asparaginase elicits its cytotoxic effect by decreasing asparagine levels in serum and cerebrospinal fluid, blocking biosynthesis of DNA and RNA, ultimately inhibiting cell profleration.188 Childhood cancer survivors with a cumulative dose of ≥120,000 IU/m experience muscle weakness22 and impaired flexibility during long-term survivorship.25 The exact mechanism underlying impaired muscle function with asparaginase exposure during childhood cancer treatment is currently unknown. Given that L-asparaginase has an inhibitory effect on protein synthesis in cancer cells,189,190 muscle protein synthesis rates in muscle cells may also be compromised. Asparaginase also metabolizes glutamine, a key intramuscular amino acid involved in the regulation of muscle protein synthesis and breakdown.191 Prolonged asparaginase treatment may result in decreased muscle cell anabolic signaling,192 mRNA transcription and translation, resulting in reduced protein accumulation and cellular mass. Indirect actions of asparaginase may include increased muscle damage during combined treatment with glucocorticoids,193,194 or altered muscle mass homeostasis and quality (i.e. intramural adiposity) through impaired β-cell function and increased fat infiltration.195–197
Mitochondrial dysfunction is implicated in the pathophysiological progression of many diseases including sarcopenia. Childhood cancer survivors are exposed to therapies capable of inducing mitochondrial damage.198 Prolonged high dose exposures to anthracycline antibiotics impair mitochondrial respiration and increase reactive oxygen species release in muscle,199 compromising metabolic function of muscle cells, contributing to loss of muscle cells200–204 and to impaired glucose metabolism.205,206 Doxorubicin, a specific anthracycline, is associated with long-term loss, disruption, and disassembly of myofibrils, and with mitochondrial swelling. Because mitochondrial dysfunction is implicated as a driver of aging,207–209 treatment-induced mitochondrial dysfunction and myofibril disassembly may be responsible for premature physiological aging in childhood cancer survivors.71,210–212
Corticosteroids are the most common cause of drug-induced myopathy, characterized by proximal weakness and associated with duration of therapy, cumulative drug dose and drug type.213 Corticosteroids induce muscle atrophy by increasing protein degradation rates via ubiquitin-proteasome autophagy lysosome systems,214,215 and by directly inhibiting the activation of the mechanistic target of rapamycin, the main cellular pathway regulating muscle protein synthesis.216 Muscle mass and strength loss during cancer treatment involves preferential atrophy of type II force generating, glycolytic muscle fibers.217,218 Among children or adults with cancer and among survivors, higher cumulative doses of corticosteroids and prolonged exposure increase risk for muscle wasting and weakness.219,220 DeAnglelis et al221 assessed the effect of corticosteroid dose on muscle health in adults (age range: 24–71 years) with non-Hodgkin lymphoma, and demonstrated that weakness was more prevalent in proximal extremity muscles among those treated with a high-dose schedule of dexamethasone (50 mg/day, 3 days/week), and that weakness progressed with prolonged treatment. Schoenmaker et al67 also reported persistent proximal muscle weakness in pediatric patients of ALL and T-NHL treated with high doses of dexamethasone. In a randomized study, the United Kingdom Medical Research Council ALL97 evaluated survival benefit of dexamethasone compared with prednisolone for childhood ALL,222 reporting lower limb weakness in the quadriceps and/or glutei muscles among all children, and a five-fold higher incidence of upper arm weakness in those treated with dexamethasone compared to prednisolone (2.8% vs. 0.5%).
In adults, drug-induced myopathies primarily cause weakness in proximal muscles of the legs, and arms.223 Whether this is consistent for children with cancer is unknown. Yang and Choi74 followed 30 children (mean age: 10.9±3.8) newly diagnosed with cancer (hematologic malignancies n=19, solid tumors n=11) during their first year of cancer treatment. DXA scans showed only a significant difference in lower leg lean mass in patients compared to healthy controls. However, whether certain muscle fiber types (i.e. “slow” vs “fast” twitch) or whole muscle groups consistently experience preferential wasting as a result of diagnosis or treatment is unknown. Given that low muscle mass is related to treatment intolerance, infection risk, and overall worse outcomes,30 there is a need for investigation into wasting specificity for children diagnosed with cancer.
II. Radiotherapy
Radiation exposure also increases risk for muscle impairment in childhood cancer survivors. Soft tissue fibrosis occurs in 80% of pediatric sarcoma patients treated with radiation therapy,224 and can also manifest as asymmetrical muscle growth and reduced ROM. Hypoplasia is associated with direct radiation exposure >20 Gy at a young age.226 Survivors treated with total body irradiation have lower muscle strength on average than survivors with no irradiation exposure in the upper arm (elbow flexion strength: 30 N, 95% CI 0.3 to 59 N, p=0.048), hand (grip strength: 181.7 N, 95% CI 114.3 to 249.1 N, p<0.001), and knee extensors (quadricep strength: 114 N m, 95% CI 50 to 179 N, p<0.001).22 Childhood ALL survivors treated with CRT have weaker hand grip strength (38.4±13.9 kg vs 38.2±12.3 kg, p=0.02), quadricep strength (158.3±54.7 Nm/kg vs 182.5±57.1 Nm/kg , p<0.001) and muscular endurance (69.4±24.2 Nm/kg vs 81.7±30.5 Nm/kg, p=0.002) than survivors without CRT exposure.25 In addition, female survivors of childhood ALL treated with CRT have lower fat-free (%) and relative lean mass (g/m2) (58.7±6.2%, 18.4±3.0 g/m2) when compared to survivors without CRT exposure (66.0±7.5%, 17.2±2.9 g/m2) and age and sex-matched controls (65.1±8.5%, 17.5±3.7 g/m2).
While hormonal dysregulation is largely responsible for reduced lean mass in childhood cancer survivors exposed to cranial radiation,20,24,70,225,227–230 the exact mechanism responsible for muscle impairment has not been studied extensively in children whose muscle tissue is directly exposed to radiation during therapy. However, damage to muscle satellite cells (SC) is likely. SCs are responsible for early muscle development, muscle growth in response to external stimuli (for example resistance training),231 and muscle repair following myocyte injury. Information from animal studies indicate that acute radiation exposure at doses of radiation ≥5 Gy reduces muscle SC number by 70% in young adult rats,232 muscle exposed to radiation fails to regain pre-radiation myonuclei count and DNA content in response to overloading,233 and fibro/adipogenic progenitor cells necessary for initial phases of SC mediated muscle repair fail to adequately clear from the regenerative niche.234,235 As such, further research is needed to elucidate the acute effects of radiation on muscle SCs in children with cancer, and the long-term implications on muscle health during survivorship.
Currently it is unknown whether drug or radiotherapy-induced muscle wasting in children with cancer is reversible. This is primarily due to the limited knowledge of whether the biological mechanisms discussed above are similar among children with cancer. However, epidemiological evidence supports that children with low lean mass at or before diagnosis have continually low lean mass during treatment.88 Children with cancer do not seem to return to pre-treatment muscle mass amounts, nor recover to the amount of muscle mass of their healthy peers.74,96 Given limited access to skeletal muscle tissue in children, there is a need for future research to incorporate molecular imaging techniques and animal modeling methods to begin to answer these important mechanistic questions.
INTERVENTIONS TO IMPROVE MUSCLE HEALTH
Interventions during primary cancer treatment have had mixed success in improving muscle function. One of the largest investigations was by Morales et al236 who evaluated the efficacy of exercise training in 24 children (mean age: 10±4 years) with solid tumors (NCT01645436). Children completed aerobic and strength exercise sessions 3 times a week over the 19±8 week intervention period, spanning chemotherapy treatment. Maximum muscle strength was evaluated by a 5 rep-maximum test on pediatric-specific weight training machines.237 Compared to baseline, muscle strength across all muscle groups (chest, back, legs) improved (p<0.01). However, no significant improvements in functional performance on the TUG and timed up and down stairs (TUDS) tests was observed. In a smaller sample of patients, Keats and Culos-Reed238 conducted a physical activity intervention in 10 adolescents (mean age: 16.2±1.6 years) with mixed diagnoses (lymphoma n=4, leukemia n=4, CNS tumor n=1, germ cell tumor n=1). Over the 16 week intervention, participants performed 45 minutes of aerobic and 15 minutes of strength and flexibility training. Significant improvements in upper body strength, indicated by more push-ups completed on the 90° push-up test,239 was observed as early as 8 weeks into the intervention.
Physical therapy is also capable of improving muscle function in this population. Marchese et al240 evaluated the effects of 4 months of physical therapy compared to no physical therapy in 28 children (age range: 4–18 years) with ALL. The intervention consisted of 3 sessions of supervised physical therapy and an individualized home exercise program that combined functional, strength, flexibility, and aerobic exercise 5 days a week for 12 weeks during maintenance therapy. After 4 months, both ankle dorsiflexion and knee extension strength measured by HHD improved significantly, compared to controls. Pre-operative physical therapy sessions have also proven to be beneficial in maintaining or restoring physical function in children with lower extremity malignancies. In a prospective clinical trial (NCT01674101),19 14 children and adolescents (mean age: 13.5±3.5 years) with lower extremity malignancies completed 60-minute physical therapy sessions focused on endurance, strength, and flexibility 3 times a week for 10-weeks prior to local control. Physical function was evaluated after 10–12 weeks of preoperative physical therapy, and at 20–22 weeks post local control using the Functional Mobility Assessment,241 a break test via HHD to assess lower extremity strength and a goniometer to quantify joint ROM. The patients who received the pre-operative physical therapy had better mobility reflected in significantly higher scores on the Functional Mobility Assessment than controls (35.6±10.3 vs 25.7±13.7 points, p=0.0267). However, the intervention did not result in strength improvements in the lower limbs. This suggests that either the training intensity was insufficient in activating the physiologic pathways contributing to strength gains, or preoperative chemotherapy limits contraction capability and/ or results in a resistance to neuromuscular adaptations.
Collectively, these studies re-enforce that the effect of childhood cancer on muscle function is complex, and that physical function can improve despite continued weakness. These studies also highlight that changes in muscle mass are not evaluated alongside changes in muscle function. In fact, we are unaware of any clinical trial directed at mitigating muscle wasting in children with cancer. Given that muscle mass is a key criterion of sarcopenia, and is protective again treatment associated toxicity, there is a need to evaluate whether interventions administered during treatment can rescue muscle mass losses in this population. However, without an understanding of the pathobiological mechanisms driving these losses, it is difficult to develop such interventions.
SUMMARY
In the year 2020, it is estimated that 15,590 children and adolescents under the age of 19 will be diagnosed with cancer.242 In survivorship, these children will be burdened by long-term morbidity and mortality associated with late term treatment effects; nearly two thirds of childhood cancer survivors have at least one chronic health condition 30 years after diagnosis.6 Muscle is implicated in many of these conditions and loss of mass and strength continue to be reported.5,20,22–24,67 Children with cancer often have poor muscle health and function that can progressively worsen with treatment, persisting into survivorship, increasing risk for reduced physiologic reserve, insulin resistance, and exercise intolerance. Epidemiological evidence to support poor muscle health as an adverse outcome of childhood cancer is strong; weakness, muscle wasting, peripheral neuropathy, poor muscle endurance, and impaired flexibility and functional mobility are commonly reported across diagnoses. Further, key chemotherapy (methotrexate, vincristine, L-asparaginase, doxorubicin, corticosteroids) and radiation (total body, cranial) exposures have both been associated with long-term impairments in muscle mass and function. Biomechanistic investigations have identified key biological processes altered by cancer therapy; mitochondrial dysfunction, satellite cell damage, hormone dysregulation, impaired cellular anabolism, altered cell cycle, and neurophysiologic dysfunction can result in compromised muscle. We hypothesize that these biological mechanisms are altered in children with cancer, contribute to poor muscle following treatment, accelerating the onset of premature physiological aging and chronic health conditions during survivorship. Future research is needed to evaluate these mechanisms in the children with cancer in order to design and implement targeted lifestyle and therapeutic interventions directed to improving muscle health and quality of life across the childhood cancer continuum.
ACKNOWLEDGEMENTS
Financial support for this work provided by a Cancer Center Core Grant, P30CA021765 Charles Roberts and by the American Lebanese Syrian Associated Charities (ALSAC) from the National Cancer Institute. The authors would like to acknowledge Tracie Gatewood for her assistance with article preparation.
Footnotes
Conflicts of Interest (CoI)
CGG has no conflicts of interest to declare.
REP has no conflicts of interest to declare.
KKN has no conflicts of interest to declare
Data Availability Statement
Data sharing not applicable – no new data generated
REFERENCE
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.SEER Cancer Statistics Review, 1975–2017. National Cancer Institute. https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site, April2020. AccessedAugust 2017. [Google Scholar]
- 3.Ness KK, Hudson MM, Ginsberg JP, et al. Physical performance limitations in the Childhood Cancer Survivor Study Cohort. J Clin Oncol. 2009;27(14):2382–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wampler MA, Galantino ML, Huang S, et al. Physical activity among adult survivors of childhood lower-extremity sarcoma. J Cancer Surviv. 2012;6(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Pineda I, Hudson MM, Pappo AS, et al. Long-term functional outcomes and quality of life in adult survivors of childhood extremity sarcomas: A report from the St. Jude Lifetime Cohort Study. J Cancer Surviv. 2017;11(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. [DOI] [PubMed] [Google Scholar]
- 7.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: Life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marina N, Hudson MM, Jones KE, et al. Changes in health status among aging survivors of pediatric upper and lower extremity sarcoma: A report from the childhood cancer survivor study. Arch Phys Med Rehabil. 2013;94(6):1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker DJ, Kilgore ML, Morrisey MA. The societal burden of osteoporosis. Curr Rheumatol Rep. 2010;12(3):186–191. [DOI] [PubMed] [Google Scholar]
- 10.Weaver CM, Gordon CM, Janz KF, et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporosis Int. 2016;27(4):1281–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasilewski-Masker K, Kaste SC, Hudson MM, Esiashvili N, Mattano LA, Meacham LR. Bone mineral density deficits in survivors of childhood cancer: Long-term follow-up guidelines and review of the literature. Pediatrics. 2008;121(3):e705–713. [DOI] [PubMed] [Google Scholar]
- 12.Steene-Johannessen J, Anderssen SA, Kolle E, Andersen LB. Low muscle fitness is associated with metabolic risk in youth. Med Sci Sports Exerc. 2009;41(7):1361–1367. [DOI] [PubMed] [Google Scholar]
- 13.Benson AC, Torode ME, Singh MA. Muscular strength and cardiorespiratory fitness is associated with higher insulin sensitivity in children and adolescents. Int J Pediatr Obes. 2006;1(4):222–231. [DOI] [PubMed] [Google Scholar]
- 14.Gawade PL, Hudson MM, Kaste SC, et al. A systematic review of selected musculoskeletal late effects in survivors of childhood cancer. Curr Pediatr Rev. 2014;10(4):249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akyay A, Olcay L, Sezer N, Sönmez Ç. Muscle Strength, Motor Performance, Cardiac and Muscle Biomarkers in Detection of Muscle Side Effects During and After Acute Lymphoblastic Leukemia Treatment in Children. J Pediatr Hematol Oncol. 2014;36:594–598. [DOI] [PubMed] [Google Scholar]
- 16.Gocha Marchese V, Chiarello LA, Lange BJ. Strength and functional mobility in children with acute lymphoblastic leukemia. Med Pediatr Oncol. 2003;40(4):230–232. [DOI] [PubMed] [Google Scholar]
- 17.Ness KK, Kaste SC, Zhu L, et al. Skeletal, neuromuscular and fitness impairments among children with newly diagnosed acute lymphoblastic leukemia. Leuk Lymphoma. 2015;56(4):1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taskinen M, Saarinen-Pihkala UM. Evaluation of muscle protein mass in children with solid tumors by muscle thickness measurement with ultrasonography, as compared with anthropometric methods and visceral protein concentrations. Eur J Clin Nutr. 1998;52(6):402–406. [DOI] [PubMed] [Google Scholar]
- 19.Corr AM, Liu W, Bishop M, et al. Feasibility and functional outcomes of children and adolescents undergoing preoperative chemotherapy prior to a limb-sparing procedure or amputation. Rehabil Oncol. 2017;35(1):38–45. [PMC free article] [PubMed] [Google Scholar]
- 20.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107(6):1303–1312. [DOI] [PubMed] [Google Scholar]
- 21.Hartman A, van den Bos C, Stijnen T, Pieters R. Decrease in peripheral muscle strength and ankle dorsiflexion as long-term side effects of treatment for childhood cancer. Pediatr Blood Cancer. 2008;50(4):833–837. [DOI] [PubMed] [Google Scholar]
- 22.Hovi L, Era P, Rautonen J, Siimes MA. Impaired muscle strength in female adolescents and young adults surviving leukemia in childhood. Cancer. 1993;72(1):276–281. [DOI] [PubMed] [Google Scholar]
- 23.Meacham LR, Gurney JG, Mertens AC, et al. Body mass index in long-term adult survivors of childhood cancer: A report of the Childhood Cancer Survivor Study. Cancer. 2005;103(8):1730–1739. [DOI] [PubMed] [Google Scholar]
- 24.Ness KK, Baker KS, Dengel DR, et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49(7):975–981. [DOI] [PubMed] [Google Scholar]
- 25.Ness KK, Delany JP, Kaste SC, et al. Energy balance and fitness in adult survivors of childhood acute lymphoblastic leukemia. Blood. 2015;125(22):3411–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ness KK, Hudson MM, Pui CH, et al. Neuromuscular impairments in adult survivors of childhood acute lymphoblastic leukemia: Associations with physical performance and chemotherapy doses. Cancer. 2012;118(3):828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ness KK, Jones KE, Smith WA, et al. Chemotherapy-related neuropathic symptoms and functional impairment in adult survivors of extracranial solid tumors of childhood: Results from the St. Jude Lifetime Cohort Study. Arch Phys Med Rehabil. 2013;94(8):1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Brussel M, Takken T, van der Net J, et al. Physical function and fitness in long-term survivors of childhood leukaemia. Pediatr Rehabil. 2006;9(3):267–274. [DOI] [PubMed] [Google Scholar]
- 29.Murphy-Alford AJ, White M, Lockwood L, Hallahan A, Davies PSW. Body composition, dietary intake and physical activity of young survivors of childhood cancer. Clin Nutr. 2019;38(2):842–847. [DOI] [PubMed] [Google Scholar]
- 30.Murphy AJ, White M, Davies PS. Body composition of children with cancer. Am J Clin Nutr. 2010;92(1):55–60. [DOI] [PubMed] [Google Scholar]
- 31.Fields DA, Higgins PB, Hunter GR. Assessment of body composition by air-displacement plethysmography: influence of body temperature and moisture. Dyn Med. 2004;3(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faron A, Sprinkart AM, Kuetting DLR, et al. Body composition analysis using CT and MRI: intra-individual intermodal comparison of muscle mass and myosteatosis. Sci Rep. 2020;10(1):11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985). 2004;97(6):2333–2338. [DOI] [PubMed] [Google Scholar]
- 35.Faron A, Luetkens JA, Schmeel FC, Kuetting DLR, Thomas D, Sprinkart AM. Quantification of fat and skeletal muscle tissue at abdominal computed tomography: associations between single-slice measurements and total compartment volumes. Abdom Radiol (NY). 2019;44(5):1907–1916. [DOI] [PubMed] [Google Scholar]
- 36.Tillquist M, Kutsogiannis DJ, Wischmeyer PE, et al. Bedside ultrasound is a practical and reliable measurement tool for assessing quadriceps muscle layer thickness. JPEN J Parenter Enteral Nutr. 2014;38(7):886–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng KWP, DIETZ AR, Johnson R, Shoykhet M, Zaidman CM. Reliability of bedside ultrasound of limb and diaphragm muscle thickness in critically ill children. Muscle Nerve. 2019;59(1):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pillen S, Verrips A, van Alfen N, Arts IMP, Sie LTL, Zwarts MJ. Quantitative skeletal muscle ultrasound: Diagnostic value in childhood neuromuscular disease. Neuromuscul Disord. 2007;17(7):509–516. [DOI] [PubMed] [Google Scholar]
- 39.Lori S, Lolli F, Molesti E, et al. Muscle-ultrasound evaluation in healthy pediatric subjects: Age-related normative data. Muscle Nerve. 2018;58(2):245–250. [DOI] [PubMed] [Google Scholar]
- 40.Valla FV, Young DK, Rabilloud M, et al. Thigh Ultrasound Monitoring Identifies Decreases in Quadriceps Femoris Thickness as a Frequent Observation in Critically Ill Children. Pediatr Crit Care Med. 2017;18(8):e339–e347. [DOI] [PubMed] [Google Scholar]
- 41.Scott JM, Martin DS, Ploutz-Snyder R, et al. Reliability and validity of panoramic ultrasound for muscle quantification. Ultrasound Med Biol. 2012;38(9):1656–1661. [DOI] [PubMed] [Google Scholar]
- 42.Scott JM, Martin DS, Ploutz-Snyder R, et al. Panoramic ultrasound: a novel and valid tool for monitoring change in muscle mass. J Cachexia Sarcopenia Muscle. 2017;8(3):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothwell DT, Fong DTP, Stapley SA, Williams DJ. A clinically applicable tool for rapidly estimating muscle volume using ultrasound images. Eur J Appl Physiol. 2019;119(11):2685–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunford EC, Au JS, Devries MC, Phillips SM, MacDonald MJ. Cardiovascular aging and the microcirculation of skeletal muscle: using contrast-enhanced ultrasound. Am J Physiol Heart Circ Physiol. 2018;315(5):H1194–h1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garra BS. Imaging and estimation of tissue elasticity by ultrasound. Ultrasound Q. 2007;23(4):255–268. [DOI] [PubMed] [Google Scholar]
- 46.Hildebrandt W, Schwarzbach H, Pardun A, et al. Age-related differences in skeletal muscle microvascular response to exercise as detected by contrast-enhanced ultrasound (CEUS). PLoS One. 2017;12(3):e0172771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Snedeker JG. Elastography: modality-specific approaches, clinical applications, and research horizons. Skeletal Radiol. 2011;40(4):389–397. [DOI] [PubMed] [Google Scholar]
- 48.Bergstrom J Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35(7):609–616. [PubMed] [Google Scholar]
- 49.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286(3):E321–328. [DOI] [PubMed] [Google Scholar]
- 50.Hsu H, Yu YM, Babich JW, et al. Measurement of muscle protein synthesis by positron emission tomography with L-[methyl-11C]methionine. Proc Natl Acad Sci U S A. 1996;93(5):1841–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fischman AJ, Yu YM, Livni E, et al. Muscle protein synthesis by positron-emission tomography with L-[methyl-11C]methionine in adult humans. Proc Natl Acad Sci U S A. 1998;95(22):12793–12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harnish R, Streeper T, Saeed I. Quantification of Changes in Skeletal Muscle Amino Acid Kinetics in Adult Humans in Response to Exercise via Positron-emission Tomography with L-[methyl-11C] methionine. J Mol Imaging Dyn. 2012;02. [Google Scholar]
- 53.Arentson-Lantz EJ, Saeed IH, Frassetto LA, et al. (11)C-L-methyl methionine dynamic PET/CT of skeletal muscle: response to protein supplementation compared to L-[ring (13)C6] phenylalanine infusion with serial muscle biopsy. Ann Nucl Med. 2017;31(4):295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 56.Robertson JA. Kendall FP and McCreary EK “Muscles, Testing and Function” (Third Edition). Br J Sports Med. 1984;18(1):25–25. [Google Scholar]
- 57.Rose KJ, Burns J, Ryan MM, Ouvrier RA, North KN. Reliability of quantifying foot and ankle muscle strength in very young children. Muscle Nerve. 2008;37(5):626–631. [DOI] [PubMed] [Google Scholar]
- 58.Bohannon RW. Manual muscle test scores and dynamometer test scores of knee extension strength. Arch Phys Med Rehabil. 1986;67(6):390–392. [PubMed] [Google Scholar]
- 59.Mahony K, Hunt A, Daley D, Sims S, Adams R. Inter-tester reliability and precision of manual muscle testing and hand-held dynamometry in lower limb muscles of children with spina bifida. Phys Occup Ther Pediatr. 2009;29(1):44–59. [DOI] [PubMed] [Google Scholar]
- 60.Toonstra J, Mattacola CG. Test-retest reliability and validity of isometric knee-flexion and -extension measurement using 3 methods of assessing muscle strength. J Sport Rehabil. 2013;22(1). [DOI] [PubMed] [Google Scholar]
- 61.Hirano M, Katoh M, Gomi M, Arai S. Validity and reliability of isometric knee extension muscle strength measurements using a belt-stabilized hand-held dynamometer: a comparison with the measurement using an isokinetic dynamometer in a sitting posture. J Phys Ther Sci. 2020;32(2):120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nordin F, Nyberg A, Sandberg C. Concurrent validity of a fixated hand-held dynamometer for measuring isometric knee extension strength in adults with congenital heart disease. Eur J Physiother. 2020;22(4):206–211. [Google Scholar]
- 63.Kollock RO Jr., Onate, Van Lunen B. The reliability of portable fixed dynamometry during hip and knee strength assessments. J Athl Train. 2010;45(4):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scott DA, Bond EQ, Sisto SA, Nadler SF. The intra- and interrater reliability of hip muscle strength assessments using a handheld versus a portable dynamometer anchoring station 11No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated. Arch Phys Med Rehabil. 2004;85(4):598–603. [DOI] [PubMed] [Google Scholar]
- 65.Nadler SF, DePrince ML, Hauesien N, Malanga GA, Stitik TP, Price E. Portable dynamometer anchoring station for measuring strength of the hip extensors and abductors. Arch Phys Med Rehabil. 2000;81(8):1072–1076. [DOI] [PubMed] [Google Scholar]
- 66.Peterson MD, Gordon PM, Smeding S, Visich P. Grip Strength Is Associated with Longitudinal Health Maintenance and Improvement in Adolescents. J Pediatr. 2018;202:226–230. [DOI] [PubMed] [Google Scholar]
- 67.Schoenmakers M, Takken T, Gulmans V, et al. Muscle strength and functional ability in children during and after treatment for acute lymphoblastic leukemia or T-cell Non-Hodgkin lymphoma: A pilot study. Cancer Therapy. 2006;4:241–248. [Google Scholar]
- 68.Tonorezos ES, Robien K, Eshelman-Kent D, et al. Contribution of diet and physical activity to metabolic parameters among survivors of childhood leukemia. Cancer Cause Control. 2013;24(2):313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boland AM, Gibson TM, Lu L, et al. Dietary Protein Intake and Lean Muscle Mass in Survivors of Childhood Acute Lymphoblastic Leukemia: Report From the St. Jude Lifetime Cohort Study. Phys Ther. 2016;96(7):1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chemaitilly W, Li Z, Huang S, et al. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: A report from the St Jude Lifetime Cohort Study. J Clin Oncol. 2015;33(5):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: A report from the St Jude Lifetime Cohort Study. J Clin Oncol. 2013;31(36):4496–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scheede-Bergdahl C, Jagoe RT. After the chemotherapy: Potential mechanisms for chemotherapy-induced delayed skeletal muscle dysfunction in survivors of acute lymphoblastic leukaemia in childhood. Front Pharmacol. 2013;4:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Talvensaari KK, Jämsen A, Vanharanta H, Lanning M. Decreased isokinetic trunk muscle strength and performance in long-term survivors of childhood malignancies: Correlation with hormonal defects. Arch Phys Med Rehabil. 1995;76(11):983–988. [DOI] [PubMed] [Google Scholar]
- 74.Yang HR, Choi HS. A prospective study on changes in body composition and fat percentage during the first year of cancer treatment in children. Nutr Res Pract. 2019;13(3):214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reilly JJ, Weir J, McColl JH, Gibson BE. Prevalence of protein-energy malnutrition at diagnosis in children with acute lymphoblastic leukemia. J Pediatr Gastroenterol Nutr. 1999;29(2):194–197. [DOI] [PubMed] [Google Scholar]
- 76.Sala A, Pencharz P, Barr RD. Children, cancer, and nutrition—A dynamic triangle in review. Cancer. 2004;100(4):677–687. [DOI] [PubMed] [Google Scholar]
- 77.Brinksma A, Huizinga G, Sulkers E, Kamps W, Roodbol P, Tissing W. Malnutrition in childhood cancer patients: A review on its prevalence and possible causes. Crit Rev Oncol Hematol. 2012;83(2):249–275. [DOI] [PubMed] [Google Scholar]
- 78.Brinksma A, Roodbol PF, Sulkers E, et al. Finding the right balance: An evaluation of the adequacy of energy and protein intake in childhood cancer patients. Clin Nutr. 2015;34(2):284–290. [DOI] [PubMed] [Google Scholar]
- 79.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39.Suppl 1: 5–41. [PubMed] [Google Scholar]
- 80.Health Council of the Netherlands. Dietary Reference Intakes: Energy, Proteins, Fats and Digestible Carbohydrates. 2001; https://www.healthcouncil.nl/documents/advisory-reports/2001/07/18/dietary-reference-intakes-energy-proteins-fats-and-digestible-carbohydrates.AccessedJanuary 1, 2020. [DOI] [PubMed]
- 81.Ocke MC, van Rossum CTM, Fransen HP, et al. Dutch National Food Consumption Survey Young Children 2005/2006. 2008; https://www.rivm.nl/bibliotheek/rapporten/350070001.pdf.AccessedJanuary 1, 2020.
- 82.van Rossum CTM, Fransen HP, Verkaik-Kloosterman J, Buurma-Rethans EJM, Ocke MC. Dutch National Food Consumption Survey 2007–2010: Diet of children and adults aged 7 to 69 years. 2011; https://www.rivm.nl/bibliotheek/rapporten/350050006.pdf.AccessedJanuary 1, 2020.
- 83.Guadagni M, Biolo G. Effects of inflammation and/or inactivity on the need for dietary protein. Curr Opin Clin Nutr Metab Care. 2009;12(6):617–622. [DOI] [PubMed] [Google Scholar]
- 84.Duggan C, Bechard L, Donovan K, et al. Changes in resting energy expenditure among children undergoing allogeneic stem cell transplantation. Am J Clin Nutr. 2003;78(1):104–109. [DOI] [PubMed] [Google Scholar]
- 85.Roberts SB, Rosenberg I. Nutrition and Aging: Changes in the Regulation of Energy Metabolism With Aging. Physiol Rev. 2006;86(2):651–667. [DOI] [PubMed] [Google Scholar]
- 86.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. 1990;86(5):1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr Metab (Lond). 2011;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brinksma A, Roodbol PF, Sulkers E, et al. Changes in nutritional status in childhood cancer patients: a prospective cohort study. Clin Nutr. 2015;34(1):66–73. [DOI] [PubMed] [Google Scholar]
- 89.Hogan KA, Cho DS, Arneson PC, et al. Tumor-derived cytokines impair myogenesis and alter the skeletal muscle immune microenvironment. Cytokine. 2018;107:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. [DOI] [PubMed] [Google Scholar]
- 91.Argilés JM, López-Soriano FJ, Busquets S. Mediators of cachexia in cancer patients. Nutrition. 2019;66:11–15. [DOI] [PubMed] [Google Scholar]
- 92.Deans C, Wigmore SJ. Systemic inflammation, cachexia and prognosis in patients with cancer. Curr Opin Clin Nutr Metab Care. 2005;8(3):265–269. [DOI] [PubMed] [Google Scholar]
- 93.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 94.VanderVeen BN, Murphy EA, Carson JA. The Impact of Immune Cells on the Skeletal Muscle Microenvironment During Cancer Cachexia. Front Physiol. 2020;11:1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nguyen JH, Chung JD, Lynch GS, Ryall JG. The Microenvironment Is a Critical Regulator of Muscle Stem Cell Activation and Proliferation. Front Cell Dev Biol. 2019;7:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rayar M, Webber CE, Nayiager T, Sala A, Barr RD. Sarcopenia in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2013;35(2):98–102. [DOI] [PubMed] [Google Scholar]
- 97.Murphy AJ, White M, Elliott SA, Lockwood L, Hallahan A, Davies PS. Body composition of children with cancer during treatment and in survivorship. Am J Clin Nutr. 2015;102(4):891–896. [DOI] [PubMed] [Google Scholar]
- 98.Halton JM, Atkinson SA, Barr RD. Growth and body composition in response to chemotherapy in children with acute lymphoblastic leukemia. Int J Cancer Suppl. 1998;11:81–84. [PubMed] [Google Scholar]
- 99.Browne EK, Zhou Y, Chemaitilly W, et al. Changes in body mass index, height, and weight in children during and after therapy for acute lymphoblastic leukemia. Cancer. 2018;124(21):4248–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.American Alliance for Health, Physical Education Recreation and Dance. Health Related Physical Fitness Test Manual. Reston, VA: AAHPERD; 1980. [Google Scholar]
- 101.Riddoch BG. Aids to the Investigation of Peripheral Nerve Injuries. In: Council MR, ed. Nerve Injuries Research Committee. 7 ed.London, UK,1950. [Google Scholar]
- 102.Custers JW, Wassenberg-Severijnen JE, Van der Net J, Vermeer A, Hart HT, Helders PJ. Dutch adaptation and content validity of the ‘Pediatric Evaluation Of Disability Inventory (PEDI)’. Disabil Rehabil. 2002;24(5):250–258. [DOI] [PubMed] [Google Scholar]
- 103.Bäckman E, Odenrick P, Henriksson KG, Ledin T. Isometric muscle force and anthropometric values in normal children aged between 3.5 and 15 years. Scand J Rehabil Med. 1989;21(2):105–114. [PubMed] [Google Scholar]
- 104.Koskelo EK, Saarinen UM, Siimes MA. Skeletal muscle wasting and protein-energy malnutrition in children with a newly diagnosed acute leukemia. Cancer. 1990;66(2):373–376. [DOI] [PubMed] [Google Scholar]
- 105.van der Sluis IM, van den Heuvel-Eibrink MM, Hählen K, Krenning EP, de Muinck Keizer-Schrama SM. Altered bone mineral density and body composition, and increased fracture risk in childhood acute lymphoblastic leukemia. J Pediatr. 2002;141(2):204–210. [DOI] [PubMed] [Google Scholar]
- 106.Janiszewski PM, Oeffinger KC, Church TS, et al. Abdominal obesity, liver fat, and muscle composition in survivors of childhood acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2007;92(10):3816–3821. [DOI] [PubMed] [Google Scholar]
- 107.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72(3):694–701. [DOI] [PubMed] [Google Scholar]
- 108.Gallagher D, Visser M, De Meersman RE, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol (1985). 1997;83(1):229–239. [DOI] [PubMed] [Google Scholar]
- 109.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol (1985). 2000;89(1):81–88. [DOI] [PubMed] [Google Scholar]
- 110.McDowell MA, Fryar CD, Hirsch R, Ogden CL. Anthropometric reference data for children and adults: U.S. population, 1999–2002. Adv Data. 2005(361):1–5. [PubMed] [Google Scholar]
- 111.Gerber LH, Hoffman K, Chaudhry U, et al. Functional outcomes and life satisfaction in long-term survivors of pediatric sarcomas. Arch Phys Med Rehabil. 2006;87(12):1611–1617. [DOI] [PubMed] [Google Scholar]
- 112.Bar-Or O The Wingate anaerobic test. An update on methodology, reliability and validity. Sports Med. 1987;4(6):381–394. [DOI] [PubMed] [Google Scholar]
- 113.Ness KK, Plana JC, Joshi VM, et al. Exercise Intolerance, Mortality, and Organ System Impairment in Adult Survivors of Childhood Cancer. J Clin Oncol. 2020;38(1):29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Newman AB, Kupelian V, Visser M, et al. Strength, But Not Muscle Mass, Is Associated With Mortality in the Health, Aging and Body Composition Study Cohort. J Gerontol A-Biol. 2006;61(1):72–77. [DOI] [PubMed] [Google Scholar]
- 115.Caan BJ, Cespedes Feliciano EM, Prado CM, et al. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncology. 2018;4(6):798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cortellini A, Bozzetti F, Palumbo P, et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: A multicenter real-life study. Sci Rep. 2020;10(1):1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rier HN, Jager A, Sleijfer S, van Rosmalen J, Kock M, Levin MD. Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. Breast. 2017;31:9–15. [DOI] [PubMed] [Google Scholar]
- 118.Forbes F, Renia J. Adult lean body mass decrease with age: Some longtitudinal. Metabolism. 1970;19:653–663. [DOI] [PubMed] [Google Scholar]
- 119.Lexell J, Taylor C, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84. [DOI] [PubMed] [Google Scholar]
- 120.Rosenberg I Summary comments. Am J Clin Nutr. 1989;50:1231–1233. [Google Scholar]
- 121.Hughes VA, Frontera WR, Wood M, et al. Longitudinal Muscle Strength Changes in Older Adults: Influence of Muscle Mass, Physical Activity, and Health. J Gerontol A-Biol. 2001;56(5):B209–B217. [DOI] [PubMed] [Google Scholar]
- 122.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. [DOI] [PubMed] [Google Scholar]
- 123.Bischoff-Ferrari HA, Orav JE, Kanis JA, et al. Comparative performance of current definitions of sarcopenia against the prospective incidence of falls among community-dwelling seniors age 65 and older. Osteoporos Int. 2015;26(12):2793–2802. [DOI] [PubMed] [Google Scholar]
- 124.Clynes MA, Edwards MH, Buehring B, Dennison EM, Binkley N, Cooper C. Definitions of Sarcopenia: Associations with Previous Falls and Fracture in a Population Sample. Calcif Tissue Int. 2015;97(5):445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schaap LA, van Schoor NM, Lips P, Visser M. Associations of Sarcopenia Definitions, and Their Components, With the Incidence of Recurrent Falling and Fractures: The Longitudinal Aging Study Amsterdam. J Gerontol A Biol Sci Med Sci. 2018;73(9):1199–1204. [DOI] [PubMed] [Google Scholar]
- 126.Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: An international consensus. J Am Med Dir Assoc. 2011;12(6):403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bahat G, Tufan A, Kilic C, Karan MA, Cruz-Jentoft AJ. Prevalence of sarcopenia and its components in community-dwelling outpatient older adults and their relation with functionality. Aging Male. 2018:1–7. [DOI] [PubMed] [Google Scholar]
- 129.Dos Santos L, Cyrino ES, Antunes M, Santos DA, Sardinha LB. Sarcopenia and physical independence in older adults: The independent and synergic role of muscle mass and muscle function. J Cachexia Sarcopenia Muscle. 2017;8(2):245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bahat G, İlhan B. Sarcopenia and the cardiometabolic syndrome: A narrative review. Eur Geriatr Med. 2016;7. [Google Scholar]
- 131.Bone AE, Hepgul N, Kon S, Maddocks M. Sarcopenia and frailty in chronic respiratory disease. Chron Respir Dis. 2017;14(1):85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Association Between Sarcopenia and Cognitive Impairment: A Systematic Review and Meta-Analysis. J Am Med Dir Assoc. 2016;17(12):1164.e1167–1164.e1115. [DOI] [PubMed] [Google Scholar]
- 133.Beaudart C, Biver E, Reginster JY, et al. Validation of the SarQoL®, a specific health-related quality of life questionnaire for Sarcopenia. J Cachexia Sarcopenia Muscle. 2017;8(2):238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.De Buyser SL, Petrovic M, Taes YE, et al. Validation of the FNIH sarcopenia criteria and SOF frailty index as predictors of long-term mortality in ambulatory older men. Age Ageing. 2016;45(5):602–608. [DOI] [PubMed] [Google Scholar]
- 135.Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J Cachexia Sarcopenia Muscle. 2016;7(3):290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Otten L, Stobäus N, Franz K, et al. Impact of sarcopenia on 1-year mortality in older patients with cancer. Age Ageing. 2019;48(3):413–418. [DOI] [PubMed] [Google Scholar]
- 137.Abellan van Kan G Epidemiology and consequences of sarcopenia. J Nutr Health Aging. 2009;13(8):708–712. [DOI] [PubMed] [Google Scholar]
- 138.Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatr Med. 2011;27(3):387–399. [DOI] [PubMed] [Google Scholar]
- 139.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2018;48(1):16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7(5):512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cawthon PM, Travison TG, Manini TM, et al. Establishing the Link Between Lean Mass and Grip Strength Cut-points With Mobility Disability and Other Health Outcomes: Proceedings of the Sarcopenia Definition and Outcomes Consortium Conference. J Gerontol A Biol Sci Med Sci. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ness KK, Baker KS, Dengel DR, et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49(7):975–981. [DOI] [PubMed] [Google Scholar]
- 143.Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ness KK, Armstrong GT, Kundu M, Wilson CL, Tchkonia T, Kirkland JL. Frailty in childhood cancer survivors. Cancer. 2015;121(10):1540–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sayer AA, Syddall HE, Gilbody HJ, Dennison EM, Cooper C. Does sarcopenia originate in early life? Findings from the Hertfordshire Cohort Study. J Gerontol A Biol Sci Med Sci. 2004;59(9):M930–M934. [DOI] [PubMed] [Google Scholar]
- 146.Rennie M, Edwards R, Halliday D, Matthews D, Wolman S, Millward D. Muscle protein synthesis measured by stable isotope techniques in man: The effects of feeding and fasting. Clin Sci. 1982;63(6):519–523. [DOI] [PubMed] [Google Scholar]
- 147.Short K, Vittone J, Bigelow M, Proctor D, Nair K. Age and aerobic exercise training effects on whole body and muscle protein metabolsim. Am J Physiol Endocrinol Metab. 2004;286(1):E92–101. [DOI] [PubMed] [Google Scholar]
- 148.Krull MR, Howell CR, Partin RE, et al. Protein Supplementation and Resistance Training in Childhood Cancer Survivors. Med Sci Sports Exerc. 2020;52(10):2069–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pereira M, Baptista I, Carlassara E, Moriscot A, Aoki M, Miyabara E. Leucine Supplementation Improves Skeletal Muscle Regeneration after Cryolesion in Rats. PLoS One. 2014;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Minet-Quinard R, Moinard C, Villie F, et al. Kinetic impairment of nitrogen and muscle glutamine metabolisms in old glucocorticoid-treated rats. Am J Physiol. 1999;276(3):E558–564. [DOI] [PubMed] [Google Scholar]
- 151.Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84(2–3):275–294. [DOI] [PubMed] [Google Scholar]
- 152.Lexell J, Henriksson-Larsén K, Winblad B, Sjöström M. Distribution of different fiber types in human skeletal muscles: Effects of aging studied in whole muscle cross sections. Muscle Nerve. 1983;6(8):588–595. [DOI] [PubMed] [Google Scholar]
- 153.Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev. 2018;47:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Brack AS, Conboy MJ, Roy S, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317(5839):807–810. [DOI] [PubMed] [Google Scholar]
- 155.Vettor R, Milan G, Franzin C, et al. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab. 2009;297(5):E987–998. [DOI] [PubMed] [Google Scholar]
- 156.Balagopal P, Schimke JC, Ades P, Adey D, Nair KS. Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Am J Physiol Endocrinol Metab. 2001;280(2):E203–208. [DOI] [PubMed] [Google Scholar]
- 157.Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol. 1997;273(4):E790–800. [DOI] [PubMed] [Google Scholar]
- 158.Evans WJ. Skeletal muscle loss: Cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91(4):1123s–1127s. [DOI] [PubMed] [Google Scholar]
- 159.Short M, Nemenoff RA, Zawada WM, Stenmark KR, Das M. Hypoxia induces differentiation of pulmonary artery adventitial fibroblasts into myofibroblasts. Am J Physiol Cell Physiol. 2004;286(2):C416–C425. [DOI] [PubMed] [Google Scholar]
- 160.Sheth KA, Iyer CC, Wier CG, et al. Muscle strength and size are associated with motor unit connectivity in aged mice. Neurobiol Aging. 2018;67:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45(5):389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Minet AD, Gaster M. Cultured senescent myoblasts derived from human vastus lateralis exhibit normal mitochondrial ATP synthesis capacities with correlating concomitant ROS production while whole cell ATP production is decreased. Biogerontology. 2012;13(3):277–285. [DOI] [PubMed] [Google Scholar]
- 163.Qaisar R, Bhaskaran S, Premkumar P, et al. Oxidative stress-induced dysregulation of excitation-contraction coupling contributes to muscle weakness. J Cachexia Sarcopenia Muscle. 2018;9(5):1003–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr). 2014;36(2):545–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Drummond MJ, Addison O, Brunker L, et al. Downregulation of E3 ubiquitin ligases and mitophagy-related genes in skeletal muscle of physically inactive, frail older women: A cross-sectional comparison. J Gerontol A Biol Sci Med Sci. 2014;69(8):1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gouspillou G, Sgarioto N, Kapchinsky S, et al. Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J. 2014;28(4):1621–1633. [DOI] [PubMed] [Google Scholar]
- 167.Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41(12):1234–1238. [DOI] [PubMed] [Google Scholar]
- 168.Balage M, Averous J, Remond D, et al. Presence of low-grade inflammation impaired postprandial stimulation of muscle protein synthesis in old rats. J Nutr Biochem. 2010;21(4):325–331. [DOI] [PubMed] [Google Scholar]
- 169.Doherty T, Vandervoot A, Brown W. Effects of ageing on the motor unit: A brief review. Can J Appl Physiol. 1993;18(4):331–358. [DOI] [PubMed] [Google Scholar]
- 170.Rosenberg I Sarcopenia: Origins and Clinical Relevance. J Nutri. 1997;127(5):990S–991S. [DOI] [PubMed] [Google Scholar]
- 171.Short K, Vittone J, Bigelow M, et al. Impact of Aerobic Exercise Training on Age-Related Changes in Insulin Sensitivity and Muscle Oxidative Capacity. Diabetes. 2003;52(8):1888–1896. [DOI] [PubMed] [Google Scholar]
- 172.Ariffin H, Azanan MS, Abd Ghafar SS, et al. Young adult survivors of childhood acute lymphoblastic leukemia show evidence of chronic inflammation and cellular aging. Cancer. 2017;123(21):4207–4214. [DOI] [PubMed] [Google Scholar]
- 173.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Harila-Saari AH, Vainionpää LK, Kovala TT, Tolonen EU, Lanning BM. Nerve lesions after therapy for childhood acute lymphoblastic leukemia. Cancer. 1998;82(1):200–207. [DOI] [PubMed] [Google Scholar]
- 175.Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci U S A. 1993;90(20):9552–9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–265. [DOI] [PubMed] [Google Scholar]
- 177.Jordan MA, Thrower D, Wilson L. Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer Res. 1991;51(8):2212–2222. [PubMed] [Google Scholar]
- 178.Purser MJ, Johnston DL, McMillan HJ. Chemotherapy-induced Peripheral Neuropathy Among Paediatric Oncology Patients. Can J Neurol Sci. 2014;41(4):442–447. [DOI] [PubMed] [Google Scholar]
- 179.Jain P, Gulati S, Seth R, Bakhshi S, Toteja GS, Pandey RM. Vincristine-induced neuropathy in childhood ALL (acute lymphoblastic leukemia) survivors: Prevalence and electrophysiological characteristics. J Child Neurol. 2014;29(7):932–937. [DOI] [PubMed] [Google Scholar]
- 180.van de Velde ME, Kaspers GL, Abbink FCH, Wilhelm AJ, Ket JCF, van den Berg MH. Vincristine-induced peripheral neuropathy in children with cancer: A systematic review. Crit Rev Oncol Hematol. 2017;114:114–130. [DOI] [PubMed] [Google Scholar]
- 181.Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: Opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58(3):334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Green JL, Knight SJ, McCarthy M, De Luca CR. Motor functioning during and following treatment with chemotherapy for pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60(8):1261–1266. [DOI] [PubMed] [Google Scholar]
- 183.Rajagopalan PT, Zhang Z, McCourt L, Dwyer M, Benkovic SJ, Hammes GG. Interaction of dihydrofolate reductase with methotrexate: Ensemble and single-molecule kinetics. Proc Natl Acad Sci U S A. 2002;99(21):13481–13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vezmar S, Becker A, Bode U, Jaehde U. Biochemical and clinical aspects of methotrexate neurotoxicity. Chemotherapy. 2003;49(1–2):92–104. [DOI] [PubMed] [Google Scholar]
- 185.Goodsell DS. The Molecular Perspective: Methotrexate. Oncologist. 1999;4(4):340–341. [PubMed] [Google Scholar]
- 186.Refsum H, Wesenberg F, Ueland PM. Plasma homocysteine in children with acute lymphoblastic leukemia: Changes during a chemotherapeutic regimen including methotrexate. Cancer Res. 1991;51(3):828–835. [PubMed] [Google Scholar]
- 187.Vezmar S, Schüsseler P, Becker A, Bode U, Jaehde U. Methotrexate-associated alterations of the folate and methyl-transfer pathway in the CSF of ALL patients with and without symptoms of neurotoxicity. Pediatr Blood Cancer. 2009;52(1):26–32. [DOI] [PubMed] [Google Scholar]
- 188.Panosyan EH, Wang Y, Xia P, et al. Asparagine Depletion Potentiates the Cytotoxic Effect of Chemotherapy against Brain Tumors. Mol Cancer Res. 2014;12(5):694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Villa P, Corada M, Bartosek I. L-asparaginase effects on inhibition of protein synthesis and lowering of the glutamine content in cultured rat hepatocytes. Toxicol Lett. 1986;32(3):235–241. [DOI] [PubMed] [Google Scholar]
- 190.Richards NGJ, Kilberg MS. Asparagine Synthetase Chemotherapy. Annu Rev Biochem. 2006;75(1):629–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mittendorfer B, Volpi E, Wolfe RR. Whole body and skeletal muscle glutamine metabolism in healthy subjects. Am J Physiol Endocrinol Metab. 2001;280(2):E323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Reinert RB, Oberle LM, Wek SA, et al. Role of Glutamine Depletion in Directing Tissue-specific Nutrient Stress Responses to L-Asparaginase. J Biol Chem. 2006;281(42):31222–31233. [DOI] [PubMed] [Google Scholar]
- 193.Kawedia JD, Liu C, Pei D, et al. Dexamethasone exposure and asparaginase antibodies affect relapse risk in acute lymphoblastic leukemia. Blood. 2012;119(7):1658–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang L, Panetta JC, Cai X, et al. Asparaginase may influence dexamethasone pharmacokinetics in acute lymphoblastic leukemia. J Clin Oncol. 2008;26(12):1932–1939. [DOI] [PubMed] [Google Scholar]
- 195.Zhang FF, Kelly MJ, Must A. Early Nutrition and Physical Activity Interventions in Childhood Cancer Survivors. Curr Obes Rep. 2017;6(2):168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cohen H, Bielorai B, Harats D, Toren A, Pinhas-Hamiel O. Conservative treatment of L-asparaginase-associated lipid abnormalities in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010;54(5):703–706. [DOI] [PubMed] [Google Scholar]
- 197.Mohn A, Di Marzio A, Capanna R, Fioritoni G, Chiarelli F. Persistence of impaired pancreatic beta-cell function in children treated for acute lymphoblastic leukaemia. Lancet. 2004;363(9403):127–128. [DOI] [PubMed] [Google Scholar]
- 198.Sorensen JC, Cheregi BD, Timpani CA, Nurgali K, Hayes A, Rybalka E. Mitochondria: Inadvertent targets in chemotherapy-induced skeletal muscle toxicity and wasting? Cancer Chemother Pharmacol. 2016;78(4):673–683. [DOI] [PubMed] [Google Scholar]
- 199.Gouspillou G, Scheede-Bergdahl C, Spendiff S, et al. Anthracycline-containing chemotherapy causes long-term impairment of mitochondrial respiration and increased reactive oxygen species release in skeletal muscle. Sci Rep. 2015;5:8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sousa-Victor P, Munoz-Canoves P. Regenerative decline of stem cells in sarcopenia. Mol Aspects Med. 2016;50:109–117. [DOI] [PubMed] [Google Scholar]
- 201.Ibebunjo C, Chick JM, Kendall T, et al. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol Cell Biol. 2013;33(2):194–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rygiel KA, Picard M, Turnbull DM. The ageing neuromuscular system and sarcopenia: A mitochondrial perspective. J Physiol. 2016;594(16):4499–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jang YC, Liu Y, Hayworth CR, et al. Dietary restriction attenuates age-associated muscle atrophy by lowering oxidative stress in mice even in complete absence of CuZnSOD. Aging Cell. 2012;11(5):770–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Alway SE, Mohamed JS, Myers MJ. Mitochondria Initiate and Regulate Sarcopenia. Exerc Sport Sci Rev. 2017;45(2):58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bonnard C, Durand A, Peyrol S, et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008;118(2):789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. [DOI] [PubMed] [Google Scholar]
- 207.Zhang H, Menzies KJ, Auwerx J. The role of mitochondria in stem cell fate and aging. Development. 2018;145(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sun N, Youle RJ, Finkel T. The Mitochondrial Basis of Aging. Mol Cell. 2016;61(5):654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Guarente L Mitochondria--a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132(2):171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Armenian SH, Gibson CJ, Rockne RC, Ness KK. Premature Aging in Young Cancer Survivors. J Natl Cancer Inst. 2019;111(3):226–232. [DOI] [PubMed] [Google Scholar]
- 211.Guida JL, Ahles TA, Belsky D, et al. Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors. J Natl Cancer Inst. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ness KK, Kirkland JL, Gramatges MM, et al. Premature Physiologic Aging as a Paradigm for Understanding Increased Risk of Adverse Health Across the Lifespan of Survivors of Childhood Cancer. J Clin Oncol. 2018;36(21):2206–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chawla J Stepwise approach to myopathy in systemic disease. Front Neurol. 2011;2:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Braun TP, Marks DL. The regulation of muscle mass by endogenous glucocorticoids. Front Physiol. 2015;6(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hasselgren PO. Glucocorticoids and muscle catabolism. Curr Opin Clin Nutr Metab Care. 1999;2(3):201–205. [DOI] [PubMed] [Google Scholar]
- 216.Wang X, Jia Q, Xiao J, Jiao H, Lin H. Glucocorticoids retard skeletal muscle development and myoblast protein synthesis through a mechanistic target of rapamycin (mTOR)-signaling pathway in broilers (Gallus gallus domesticus). Stress. 2015;18(6):686–698. [DOI] [PubMed] [Google Scholar]
- 217.Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol. 2008;197(1):1–10. [DOI] [PubMed] [Google Scholar]
- 218.Gupta A, Gupta Y. Glucocorticoid-induced myopathy: Pathophysiology, diagnosis, and treatment. Indian J Endocrinol Metab. 2013;17(5):913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15(6):993–1000. [DOI] [PubMed] [Google Scholar]
- 220.Zikán V, Týblová M, Raška I Jr., et al. Bone mineral density and body composition in men with multiple sclerosis chronically treated with low-dose glucocorticoids. Physiol Res. 2012;61(4):405–417. [DOI] [PubMed] [Google Scholar]
- 221.DeAngelis LM, Gnecco C, Taylor L, Warrell RP Jr., Evolution of neuropathy and myopathy during intensive vincristine/corticosteroid chemotherapy for non-Hodgkin’s lymphoma. Cancer. 1991;67(9):2241–2246. [DOI] [PubMed] [Google Scholar]
- 222.Mitchell CD, Richards SM, Kinsey SE, Lilleyman J, Vora A, Eden TO. Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: Results of the UK Medical Research Council ALL97 randomized trial. Br J Haematol. 2005;129(6):734–745. [DOI] [PubMed] [Google Scholar]
- 223.Janssen L, Allard NAE, Saris CGJ, Keijer J, Hopman MTE, Timmers S. Muscle Toxicity of Drugs: When Drugs Turn Physiology into Pathophysiology. Physiol Rev. 2020;100(2):633–672. [DOI] [PubMed] [Google Scholar]
- 224.Paulino AC. Late effects of radiotherapy for pediatric extremity sarcomas. Int J Radiat Oncol Biol Phys. 2004;60(1):265–274. [DOI] [PubMed] [Google Scholar]
- 225.Schwartz CL, Hobbie WL, Constine LS, Ruccione KS. Survivors of Childhood and Adolescent Cancer: A Multidisciplinary Approach. 3rd ed.New York City, NY: Springer International Publishing; 2015. [Google Scholar]
- 226.Donaldson SS, Kaplan HS. Complications of treatment of Hodgkin’s disease in children. Cancer Treat Rep. 1982;66(4):977–989. [PubMed] [Google Scholar]
- 227.Brignardello E, Felicetti F, Castiglione A, et al. Endocrine health conditions in adult survivors of childhood cancer: The need for specialized adult-focused follow-up clinics. Eur J Endocrinol. 2013;168(3):465–472. [DOI] [PubMed] [Google Scholar]
- 228.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2011;56(5):825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rose SR, Horne VE, Howell J, et al. Late endocrine effects of childhood cancer. Nat Rev Endocrinol. 2016;12(6):319–336. [DOI] [PubMed] [Google Scholar]
- 230.Savage LJ MR. Late effects of cancer therapy on growth and GH. Int Growth Monitor. 2006(16):2–7. [Google Scholar]
- 231.Rosenblatt JD, Yong D, Parry DJ. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve. 1994;17(6):608–613. [DOI] [PubMed] [Google Scholar]
- 232.Caiozzo VJ, Giedzinski E, Baker M, et al. The radiosensitivity of satellite cells: cell cycle regulation, apoptosis and oxidative stress. Radiat Res. 2010;174(5):582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Adams GR, Caiozzo VJ, Haddad F, Baldwin KM. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol. 2002;283(4):C1182–C1195. [DOI] [PubMed] [Google Scholar]
- 234.Judson RN, Low M, Eisner C, Rossi FM. Isolation, Culture, and Differentiation of Fibro/Adipogenic Progenitors (FAPs) from Skeletal Muscle. Methods Mol Biol. 2017;1668:93–103. [DOI] [PubMed] [Google Scholar]
- 235.Biferali B, Proietti D, Mozzetta C, Madaro L. Fibro-Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Front Physiol. 2019;10:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Morales JS, Padilla JR, Valenzuela PL, et al. Inhospital Exercise Training in Children With Cancer: Does It Work for All? Front Pediatr. 2018;6:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kraemer W, Fry A. Strength testing development and evaluation of methodology. In: Mauch PJ, Foster C, eds. Physiological Assessment of Human Fitness. Champaign, IL: Human Kinetics; 1995:115–138. [Google Scholar]
- 238.Keats MR, Culos-Reed SN. A community-based physical activity program for adolescents with cancer (project TREK): program feasibility and preliminary findings. J Pediatr Hematol Oncol. 2008;30(4):272–280. [DOI] [PubMed] [Google Scholar]
- 239.Plowman SA. Muscular Strength, Endurance, and Flexibility Assessments. In: Plowman SA, Meredith MD, eds. Fitnessgram/Activitygram Reference Guide 4th ed.Dallas, TX: The Cooper Institute; 2013:8–1 - 8–55. [Google Scholar]
- 240.Marchese VG, Chiarello LA, Lange BJ. Effects of physical therapy intervention for children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2004;42(2):127–133. [DOI] [PubMed] [Google Scholar]
- 241.Marchese VG, Rai SN, Carlson CA, et al. Assessing functional mobility in survivors of lower-extremity sarcoma: reliability and validity of a new assessment tool. Pediatr Blood Cancer. 2007;49(2):183–189. [DOI] [PubMed] [Google Scholar]
- 242.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable – no new data generated


