Abstract
Purpose of Review
The purpose of this review is to discuss current knowledge of brain-gut therapies (BGT) in pediatric functional gastrointestinal disorders (FGID) and inflammatory bowel disease (IBD), including their evidence base, the common psychopathology that they address, and the integration of this knowledge into medical settings.
Recent Findings
Cognitive behavioral therapy (CBT), hypnotherapy (HT), mindfulness-based therapy (MBT), and exposure-based therapy (EBT) have the most data supporting their use in children, particularly in FGID, more so than in IBD. This difference is most likely because of the increased role of psychological factors in FGID, though these same factors can be seen comorbidly in IBD. Integrative BGT treatment strategies with the collaboration of clinicians across disciplines may provide the most benefit to patients.
Summary
This review details our current understanding of the evidence for BGT in pediatric FGID and IBD and how they may best be used in treatment strategies.
Keywords: Functional gastrointestinal disorders, Pediatrics, Brain-gut therapies, Irritable bowel syndrome, Inflammatory bowel disease, Psychological treatments, Cognitive behavioral therapy
Introduction
Brain-gut therapies (BGTs) are psychotherapeutic modalities developed to address the psychological and stress-related factors that are intrinsic to pediatric functional gastrointestinal disorders (FGIDs) and sometimes part of the symptomatology of inflammatory bowel disease (IBD). Common pediatric FGIDs include irritable bowel syndrome (IBS), functional abdominal pain (FAP), functional constipation, and functional dyspepsia (FD). The etiology of FGID is seen as multifactorial, relating to a disturbance in the brain-gut axis (BGA) due to genetic, environmental, and psychosocial factors that may lead to changes in endocrine pathways, immune response, motility, and sensation (Fig. 1) [1•, 2, 3]. Conversely, IBD (e.g., Crohn’s disease and ulcerative colitis) is seen as an organic and immune-mediated inflammatory disorder, though a diagnosis of IBD does not preclude a diagnosis of FGID [4, 5•]. Treatment for both FGID and IBD should include an emphasis on both biologic and psychological aspects of these diseases, as both aspects are associated with low quality of life and may benefit from BGT [6, 7]. While BGTs may sometimes serve as the sole therapy for FGID, they are an adjunctive treatment in IBD to improve quality of life and enhance medical therapies.
Fig. 1.
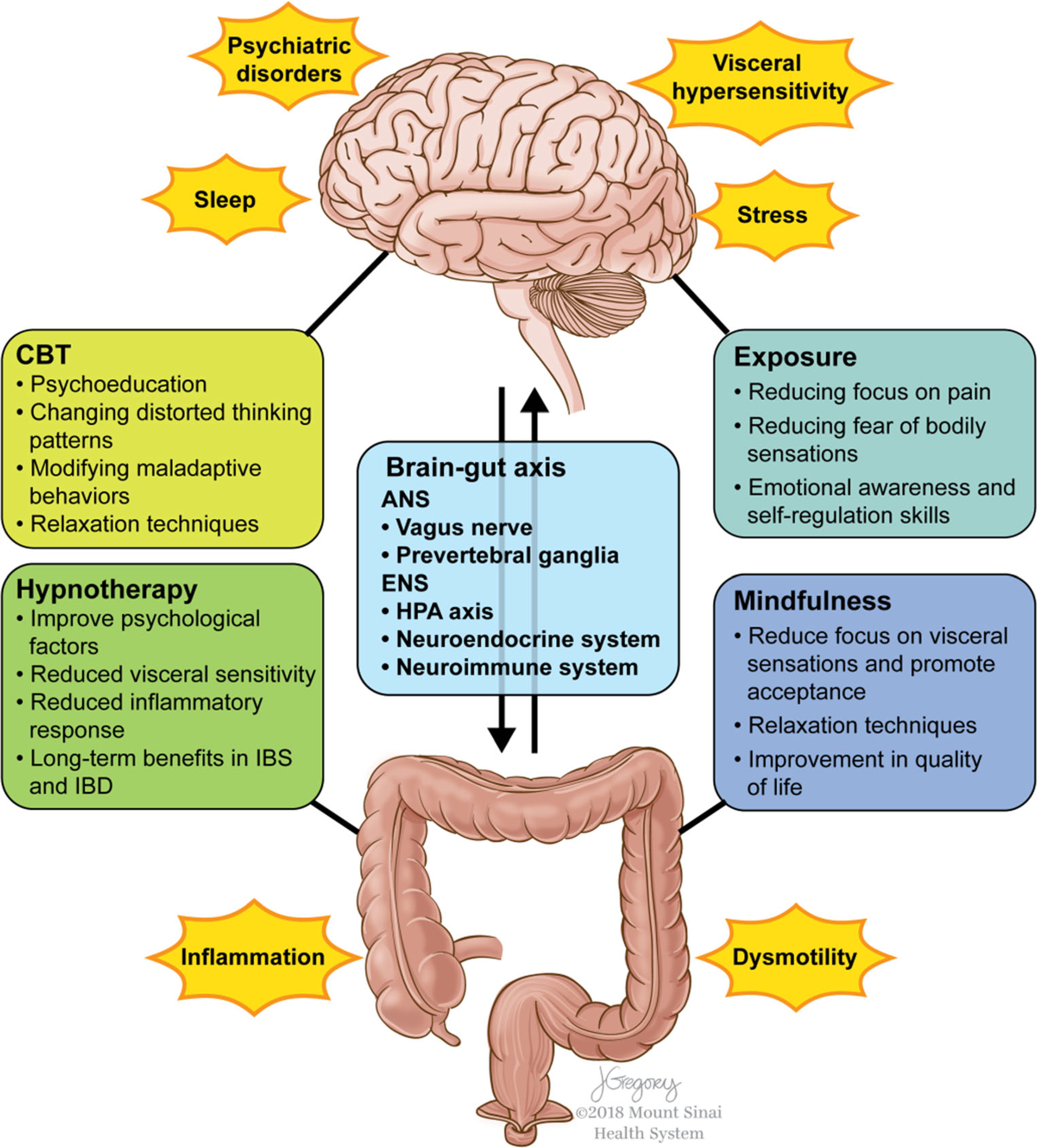
The BGA consists of bidirectional communication from the central nervous system to the enteric nervous system of the gut via vagal and spinal pathways. The hypothalamic-pituitary-adrenal axis, neuroendocrine, and neuroimmune systems are also important pathways of communication. These processes can be influenced by the gut microbiome, and, in turn, alter gut microbial composition. Dysregulation of the BGA is believed to be part of both the pathology of FGID and the symptomatology of IBD. BGT, including CBT, HT, MBT, and EBT, is believed to address the BGA dysregulation seen in FGID and IBD. While the potential of these therapies to alter the BGA at the level of the CNS is clearer, emerging data suggests they may also have a role in modification at the level of the gut
While numerous psychotherapeutic interventions have been proposed to be useful in these conditions, cognitive behavioral therapy (CBT), hypnotherapy (HT), mindfulness-based therapies (MBT), and exposure-based therapies (EBT) have the most evidence for their effectiveness in pediatric FGID and IBD. Emerging research is also exploring using internet-based and other platforms for the delivery of these modalities. Other psychotherapies, including psychodynamic psychotherapy, are not only much less researched but are lacking in robust supportive findings in pediatric FGID and IBD. Overall, there is less research about BGTs in pediatric IBD than in FGID, likely due to the differing etiologies of these illnesses, including increased psychiatric comorbidity in FGID. It is important to note that there is increasing literature suggesting the overlap of IBD and IBS, and to our knowledge, no research has been done to understand the differing effectiveness of BGTs in IBS-IBD versus IBD alone. Also discussed in this review, current clinical experience and literature support increased integration of BGT into medical settings. It is likely that this integration improves patient experience, patient care, and outcome.
Psychopathology in Gastrointestinal Disease
Psychosocial factors are seen as core to the development and maintenance of FGID, and recent investigations in IBD not only suggest increased comorbidity of psychiatric illness but also emphasize the role of stress in exacerbating symptoms and reducing quality of life [7–9]. For this reason, it is important to examine both FGID and IBD through a biopsychosocial lens. The biopsychosocial model of illness takes into account the complex interactions of the biologic underpinnings of disease as they interact with the patient’s psychological state and social context. For example, children whose illness behaviors are reinforced by their mothers are more likely to miss school and experience more severe abdominal pain symptoms [10]. Management of these children will remain incomplete if this psychosocial dimension of these patients’ symptoms is not addressed, including education of their parents about the role of their reinforcement in contributing to symptom severity. It is also useful to introduce families to the BGA, explaining how disruption of bidirectional communication between the brain and the gut via the autonomic nervous system, endocrine, and immunologic pathways, in conjunction with changes to gut microbiota, can lead to disruption at the level of both the brain and gut. This disruption can include perception of visceral pain, changes in behavior, and changes in motility and secretion [3, 11, 12].
Explaining the biopsychosocial model to patients and their families is important to the management of pediatric FGID and IBD for multiple reasons. First, it introduces the rationale behind the introduction of BGT into the treatment of these disorders, hopefully reducing stigma and encouraging the patient and family’s acceptance of behavioral interventions. Further, it also may work to counter feelings of helplessness surrounding symptoms. This model allows the patient and family to identify and understand modifiable factors contributing to symptoms and disability, potentially empowering them to take steps to address these factors. One common process seen in IBS, FAP, and IBD is symptom-specific anxiety. When a patient becomes hypervigilant of any gastrointestinal sensation, ranging from normal bodily functions to symptoms related to underlying disease, this hypervigilance can evolve into feelings of fear and anxiety, as this sensation is perceived as threatening. Reinforcing this process, patients may then modify their behaviors to reflect this feeling of fear. Examples include not going to school for fear of vomiting, feeling worsening pain, or having urgency to defecate. Such behavioral changes not only work to reinforce the misinterpreted sensation as threatening but also impair overall functioning.
Psychiatric conditions, stress, and impairments in quality of life are increasingly common in both FGID and IBD. Children with FAP and IBS are more likely to be diagnosed with a depressive or anxiety disorder [13]. Anxiety in itself can mimic gastrointestinal symptoms, but also may heighten hypervigilance to body sensations and reduce the ability to tolerate discomfort. Depression in children can also manifest with somatic complaints, especially in children who internalize, and may also promote feelings of hopeless, helplessness, and worthlessness that promote disengagement from treatment and maladaptive coping. Pediatric IBD is also associated with increased risk for anxiety and depression, and debate exists about the possibility of inflammation causing or worsening these disorders [14–16]. Sleep disruption with worsening IBD symptom severity has been shown [17]. As in FGID, anxiety and depression may negatively impact functioning and engagement in medical treatment.
Brain-Gut Therapies
Cognitive Behavioral Therapy
CBT began as a psychotherapy for depression and since has been adapted for various psychiatric disorders and for use in different disease states and clinical settings [18, 19]. This therapy encourages the patient to recognize associations of situations, thoughts, emotions, behaviors, and sensations, recognizing maladaptive patterns that contribute to mood symptoms and other dysfunctions. Patients are encouraged to identify and challenge distorted thoughts, engage in healthy coping strategies, and change behaviors to promote psychological well-being.
FGID
CBT is the most widely studied BGT in pediatric FGID, with numerous studies supporting its use as the primary intervention for IBS and FAP [20, 21]. There is less literature on the role of CBT in pediatric FD, though early work is promising [22••]. Functional constipation is often treated through both medical and medical-behavioral therapy, and there is less evidence that CBT provides a clear benefit over medical-behavioral interventions or enhanced toilet training [23]. This is likely because children suffering from functional constipation are often too young for cognitive restructuring, though this modality can be applied to their parents. CBT for cyclic vomiting syndrome in conjunction with biofeedback has been explored, but it is understudied and not considered part of standard management [24].
No single protocol exists for the delivery of CBT in IBS and FAP, though most courses of treatment involve 6–12 therapy sessions. These sessions consist of psychoeducation about the BGA and its response to stress, building understanding about the patient’s cognitive and behavioral responses to symptoms, and modifying these responses to decrease disease-related distress and stress-driven symptoms. Several studies have demonstrated CBT to be superior to standard medical interventions for pediatric IBS and FAP, reducing bowel symptoms and improving quality of life [25]. These studies also suggest that the benefits of CBT are lasting in adult studies [26]. More recent research has also demonstrated that CBT can successfully be delivered through the internet [27]. Ongoing research also seeks to explore the role of patients self-administering CBT through phone applications or web-based platforms as part of treatment [28].
IBD
Several studies have explored the role of CBT in pediatric IBD, with mixed results. Early research using disease-specific CBT supports its role in reducing depressive and anxiety symptoms and improving health-related quality of life (HRQOL) [29–31]. However, it is important to note that nondirective therapy had similar outcomes as disease-specific therapy in one of these studies [30]. Overall, this work suggests that CBT is helpful in pediatric IBD, though studies are limited in terms of their heterogeneity, including whether the focus of CBT was depression or anxiety vs. GI symptoms, and whether patients experienced subclinical vs. clinical mood symptoms.
More recently, Levy et al. conducted an RCT in which patients, not selected based on having psychological or somatic symptoms, were randomized to either three sessions of social learning and cognitive behavioral therapy (SLCBT) or educational support [31]. SLCBT was demonstrated to outperform educational support in improving IBD-related quality of life and school attendance, though no differences in anxiety or depression were found. Also, Stapersma et al. randomized patients with subclinical symptoms of depression and anxiety to either Primary and Secondary Control Enhancement Training for Physical Illness (PASCET-PI), a disease-specific CBT, or care as usual (CAU) [32••]. They found that participants in both groups experienced significant improvement in depression, anxiety, and HRQOL. While they drew the conclusion that PASCET-PI did not outperform CAU, they do discuss that CAU patients may have benefitted from psychological assessments, psychoeducation, and increased symptom awareness through study participation, and the fact that their population had a low psychiatric and medical disease burden. Overall, more research will be necessary to better understand the role of IBD-specific CBT and it may be more efficacious in patients with higher psychiatric comorbidity or more severe symptoms of IBD.
Exposure-Based Therapies
EBTs are part of CBT and are built out of the concept that avoidance behaviors can exacerbate gastrointestinal symptoms and overall distress. For example, a child afraid of experiencing abdominal pain may reinforce this symptom by avoiding symptom-provoking stimuli and thus perpetuate and exacerbate the underlying process. Through exposure, patients are encouraged to face sensation and situations that make them fearful, thus alleviating their distress through habituation and discouraging further avoidance.
FGID
EBTs have been applied to pediatric IBS and FAP, with several recent studies suggesting they are effective at improving gastrointestinal symptoms and quality of life [22••, 27, 33, 34]. School absenteeism is also frequently seen in FGID, and in one study of 20 children with FAP, Lalouni et al. demonstrated that ten weekly sessions of exposure-based CBT not only improved pain intensity and mood symptoms but also reduced school absenteeism [34]. In an uncontrolled trial of 31 adolescents with FAP or FD, Bonnert et al. recently demonstrated that participation in ten weekly online modules reduced pain symptoms for 6 months of post-treatment follow-up [27]. They also showed a reduction in gastrointestinal symptoms and improvement in the quality of life. Similarly, in an RCT of 101 adolescents with IBS randomized to either ten sessions of internet-delivered exposure-based CBT or wait-list control, Bonnert et al. demonstrated significant improvement in gastrointestinal symptoms and quality of life in the treatment group, which remained until at least 6 months post-treatment [22••]. Taken together, there is a growing body of research supporting the use of exposure-based therapies in pediatric FGID, including through internet delivery.
IBD
EBT can be part of CBT for IBD; however, such therapy has not been specifically studied in pediatric IBD. Theoretically, through similar mechanisms as in treating IBS, exposure-based therapy could potentially aid in addressing symptom avoidance. More research will be necessary to explore this possibility.
Hypnotherapy
Gut-directed HT focuses post-hypnotic suggestions on the gastrointestinal tract. HT has been applied in a variety of settings for different medical conditions, including management of chronic pain [35]. Patients first learn to achieve and deepen a hypnotic state. They are introduced to gut-focused imagery and hypnotic suggestions, practicing the exercises at home and completing self-monitoring forms. While the mechanism of action of HT is not fully understood, it has been associated with the activation of the left frontal, right cingulate, and the right insular cortices and the deactivation of the thalamus, pathways important in pain processing [36]. It also is hypothesized to have direct effects on gut function and psychological symptoms [37, 38].
FGID
To date, studies on HT in pediatric FGID have focused on its role in FAP and IBS. There is literature in adults indicating that it may also be beneficial in both functional dyspepsia and esophageal disorders [39, 40]. Early RCTs in therapist administered HT in children with FAP all demonstrated significantly lower levels of abdominal pain and symptom scores in children receiving HT versus controls [41–44]. Two trials have examined the benefit of patients performing self-exercises at home using an audio CD, one examining self-exercises vs. standard medical care [41, 43], and the other comparing in-person HT vs. self-exercises in 260 children with FAP. Both these studies suggest the efficacy of CD-recorded self-exercises in reducing abdominal pain frequency and intensity, supporting the use of this low-cost option. There are data to suggest that the effects of HT are lasting, and one study demonstrated that 68% of children in the treatment arm vs. only 20% of those in the control group remained in remission from FAP at 5 years [45•]. Overall, while more studies, particularly evaluating mechanisms of self-delivery of HT, are needed in pediatric populations, those to date suggest that HT is highly effective for pediatric IBS and FAP.
IBD
There is less literature examining the role of hypnotherapy in IBD, particularly in the pediatric setting, though the research that exists is promising [46]. Shaoul et al. enrolled seven patients (ages 10–17) with IBD and emotional distress [47]. Patients who continued to have gastrointestinal symptoms after 6 months of standard medical treatment underwent HT tailored to their age and symptoms. This group continued on the same medical treatment and experienced significant improvement in pain, diarrhea, and inflammatory markers. This concept of HT having anti-inflammatory effects is supported elsewhere in the adult literature, including rectal mucosal reduction in inflammatory markers in ulcerative colitis after one HT session [48, 49]. A study from our own group suggested that HT in adults is useful in maintaining remission in IBD. While further investigation of HT in IBD is needed, the potential for this therapy to directly address inflammation while lessening psychological distress is compelling.
Mindfulness-Based Therapies
MBT center around complete and non-judgmental awareness of the current moment through meditative action and relaxation. Mindfulness is seen as a skill that must be taught and practiced, eventually being able to be applied to any everyday action. For example, a patient might learn to “mindfully” notice and accept abdominal discomfort, moving away from prior reactions of distress and negative mood. These MBTs were not developed to be gut-specific; thus, this therapeutic skill set has broad applications.
FGID
Few studies have been performed on the efficacy of MBT in pediatric FGID, though, generally, these therapies have shown promise in both adult FGID and many other medical and psychiatric conditions [50, 51]. Korterink et al. examined yoga therapy in a RCT of 69 pediatric patients with FAP, randomized to either 10 weeks of 1.5 h, developmentally tailored yoga sessions, or standard medical care [52]. They were able to demonstrate that children receiving yoga therapy had significant treatment response of ≥ 50% reduction in weekly pain scores at 1 year of follow-up; however, this difference was not significant prior to a year and there was no difference in pain frequency scores or quality of life. Another study by Ali et al. utilized a mindfulness-based stress reduction (MBSR) protocol of eight weekly sessions for 15 pediatric patients with functional somatic symptoms and their parents [53•]. Functional somatic symptoms included abdominal pain-related bowel dysfunction. They were able to demonstrate, with consistency between child and parent measures, significant reduction in functional disability, symptom impact, and anxiety. Given stronger adult literature suggesting MBT may be useful in decreasing hypervigilance to visceral sensations, reduce symptom-related psychological distress, and improve IBS symptoms, more research will be necessary in pediatric FGID to see if these results can be consistently replicated [54, 55].
IBD
There is a lack of pediatric studies surrounding MBT in IBD. One study examining the mindfulness practices and attitudes of 61 adolescent IBD patients demonstrated females were more likely to already use relaxation techniques and younger adolescents were more open to or already practicing meditation [56]. Adolescents with worse HRQOL were more willing to consider using some forms of mindfulness, and significant portions of the population sampled regularly used mindfulness-based practices, including prayer (62%). Studies in adults have shown mixed results, though a recent controlled trial by Neilson et al. using an 8-week MBSR protocol for 60 patients with IBD demonstrated improved anxiety and quality of life after treatment versus control, and this improvement in quality of life was maintained at 6 months [57]. More investigation of these therapies is needed in pediatric IBD, and it is possible that their further development to be more gut-directed may yield even greater therapeutic responses.
Integrative Care Models
There are numerous benefits to the integration of psychological evaluation and management into the medical care of pediatric FGID and IBD, most notably reducing the stigma of engagement in behavioral healthcare. Further, this integration may increase patient satisfaction, reduce healthcare costs, and foster better interdisciplinary communication and collaboration [58••, 59]. Not all patients require psychological evaluation, and typically, this should be reserved for patients with more severe symptoms or a known or suspected comorbid psychiatric disorder. Medical providers should provide early education to the patient and their family about the biopsychosocial model of illness as it relates to FGID or IBD. Patients and their families may interpret referral for psychological evaluation as an invalidating message that the patient’s symptoms are psychogenic or even feigned. Waiting until after medical evaluation is complete to introduce the biopsychosocial model may further foster this sense of invalidation, as it implies that all medical causes have been excluded, leaving only a psychiatric process. Instead, discussion of the role of BGT in management of both of these conditions early in the process of evaluation can not only encourage family “buy-in” to psychotherapeutic treatment but may also expedite engagement in BGT, which can occur in conjunction with the remaining medical evaluation. Ultimately, this referral should be explained as the standard of care for the patient’s condition.
It is essential to collaborate with patients and their families regarding the goals of care within this interdisciplinary model. Goals of care may vary dramatically among families, and some may have unrealistic expectations of finding a “cure” to achieve complete resolution of all symptoms. While symptom remission is sometimes a possibility in both FGID and IBD, many patients with both conditions may continue to have periods of worsening symptoms and neither process is curable. Also, the constellation of symptoms of various FGIDs and IBDs may overlap, making identifying and addressing debilitating symptoms through both medical management and BGT daunting. Parents of children with FGID may often seek multiple opinions, and these children may undergo unnecessary testing. While this testing may be ordered in an attempt to convince the parent that there is no serious pathology, it can actually work to increase parental anxiety and reinforce the idea that there is an undiagnosed underlying illness. Interdisciplinary care requires the involvement of all providers throughout treatment, including medical providers remaining involved and supportive of behavioral healthcare treatment. Additionally, behavioral healthcare providers must remain knowledgeable and vigilant regarding pediatric gastrointestinal disease, referring a patient back for medical reevaluation if there is a concern that further evaluation is necessary.
Different models of integration are possible, including co-located medical and behavioral healthcare providers, providers in separate clinics within the same healthcare institution, or medical providers referring to a community-based mental health practitioner. Emphasizing the importance of collaboration, some patients may benefit from same-day appointments with these providers or standing appointments to facilitate close follow-up. It is also important that the care plan reflects the intensity of care dictated by the clinical picture, and some patients may benefit from engagement in frequent visits and even daily intensive psychotherapy programs. Potential barriers to this integrated treatment include logistical barriers and the lack of access to behavioral healthcare practitioners with BGT expertise. There is a growing body of literature supporting the use of Internet-based or tele-psychiatry platforms for the delivery of BGT, and this may be an attractive and low-cost option for many medical providers and patients without readily available access to in-person BGT [60].
Conclusion
In summary, a growing body of literature supports the use of BGT in pediatric FGID and, to a lesser extent, in pediatric IBD. This review details the current evidence for CBT, HT, MBT, and ET, though other therapies have been studied with less successful results. Reasons why BGT have shown greater impact in FGID vs. IBD, are likely related to the increased importance of psychopathology in the genesis and maintenance of FGID. However, BGT may help address psychopathology commonly seen in IBD, including mood symptoms, distorted cognition, and interoceptive preoccupation. Further, new data suggest significant comorbidity of IBD and IBS, and these patients may particularly benefit from engagement in FGID over IBD patients without IBS. Most importantly, these psychotherapies not only improve the quality of life and mental health, but also address disease by modifying the BGA through reducing stress response and inflammation.
Integration of BGT into medical settings is important to comprehensive care, and this integration may increase patient understanding and acceptance of the role of psychotherapies in their treatment. Collaborative care can be delivered through different mechanisms, including co-located medical and behavioral healthcare providers or fostering relationships between the medical team and a community-based mental health practitioner. Regardless of how this care is organized, the patient’s early introduction to the concept of the BGA and the biopsychosocial model of illness and engagement in behavioral evaluation and treatment is essential. Barriers to integrated care include shortages of mental health practitioners with expertise in BGT, though increasing research has demonstrated efficacy of delivery of BGT through tele-psychiatry and other online platforms.
Further research will be necessary to understand better the most effective implementation of BGT in the treatment of pediatric FGID and IBD. Specific goals are to better understand the process of dysregulation of the BGA in these diseases and to identify psychotherapeutic targets in order to better refine and optimize these psychotherapies.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Laurie Keefer reports personal fees from MetaMe Connect and non-financial support from Rome Foundation Board of Directors outside the submitted work.
Hannibel Person declares no conflict of interest.
Resources The following organizations provide further resources to providers and patients:
Association for Behavioral and Cognitive Therapies (www.abct.org)
Rome Foundation Psychogastroenterology Section (www.romegipsych.org)
International Foundation for Functional Disorders (www.iffgd.org)
University of North Carolina Functional GI and Motility Disorders (www.med.unc.edu/ibs/patient-education/educational-gi-handouts)
“IBShypnosis.com” (www.ibshypnosis.com)
Society of Behavioral Medicine (www.sbm.org)
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.•. Van Oudenhove L, Crowell MD, Drossman DA, Halpert AD, Keefer L, Lackner JM, et al. Biopsychosocial aspects of functional gastrointestinal disorders. Gastroenterology. 2016. 10.1053/j.gastro.2016.02.027. This is a review of our current understanding of the biopsychosocial mechanisms that create and maintain FGID.
- 2.Bernstein CN. The brain-gut axis and stress in inflammatory bowel disease. Gastroenterol Clin N Am 2017;46(4):839–46. 10.1016/j.gtc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Khlevner J, Park Y, Margolis KG. Brain-gut axis: clinical implications. Gastroenterol Clin N Am 2018;47(4):727–39. 10.1016/j.gtc.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombel JF, Shin A, Gibson PR. Functional gastrointestinal symptoms in patients with inflammatory bowel disease; Clin Gastroenterol Hepatol 2018. 10.1016/j.cgh.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.•. Watson KL Jr, Kim SC, Boyle BM, Saps M. Prevalence and impact of functional abdominal pain disorders in children with inflammatory bowel diseases (IBD-FAPD). J Pediatr Gastroenterol Nutr 2017;65(2):212–7. 10.1097/MPG.0000000000001479. This is the first study to use Rome III criteria to assess the prevalence of FAP in pediatric IBD, also suggesting increased prevalence of mood symptoms in children with IBD-FAP.
- 6.Chiou E, Nurko S. Functional abdominal pain and irritable bowel syndrome in children and adolescents. Therapy. 2011;8(3):315–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackner LM, Greenley RN, Szigethy E, Herzer M, Deer K, Hommel KA. Psychosocial issues in pediatric inflammatory bowel disease: report of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2013;56(4):449–58. 10.1097/MPG.0b013e3182841263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54(10):1481–91. 10.1136/gut.2005.064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonney Reed-Knight LMM, Wallace V. Crandall Psychological aspects of inflammatory bowel disease in children and adolescents. Pediatric inflammatory bowel disease. New York: Springer International Publishing; 2017. p. 615–23. [Google Scholar]
- 10.Levy RL, Whitehead WE, Walker LS, Von Korff M, Feld AD, Garner M, et al. Increased somatic complaints and health-care utilization in children: effects of parent IBS status and parent response to gastrointestinal symptoms. Am J Gastroenterol 2004;99(12):2442–51. 10.1111/j.1572-0241.2004.40478.x. [DOI] [PubMed] [Google Scholar]
- 11.Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome Axis. Cell Mol Gastroenterol Hepatol 2018;6(2): 133–48. 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med 2011;62:381–96. 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campo JV, Bridge J, Ehmann M, Altman S, Lucas A, Birmaher B, et al. Recurrent abdominal pain, anxiety, and depression in primary care. Pediatrics. 2004;113(4):817–24. [DOI] [PubMed] [Google Scholar]
- 14.Kilroy S, Nolan E, Sarma KM. Quality of life and level of anxiety in youths with inflammatory bowel disease in Ireland. J Pediatr Gastroenterol Nutr 2011;53(3):275–9. 10.1097/MPG.0b013e318214c131. [DOI] [PubMed] [Google Scholar]
- 15.Reigada LC, Hoogendoorn CJ, Walsh LC, Lai J, Szigethy E, Cohen BH, et al. Anxiety symptoms and disease severity in children and adolescents with Crohn disease. J Pediatr Gastroenterol Nutr 2015;60(1):30–5. 10.1097/MPG.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 16.Clark JG, Srinath AI, Youk AO, Kirshner MA, McCarthy FN, Keljo DJ, et al. Predictors of depression in youth with Crohn disease. J Pediatr Gastroenterol Nutr 2014;58(5):569–73. 10.1097/MPG.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benhayon D, Youk A, McCarthy FN, Davis S, Keljo DJ, Bousvaros A, et al. Characterization of relations among sleep, inflammation, and psychiatric dysfunction in depressed youth with Crohn disease. J Pediatr Gastroenterol Nutr 2013;57(3):335–42. 10.1097/MPG.0b013e31829641df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck AT. The past and future of cognitive therapy. J Psychother Pract Res 1997;6(4):276–84. [PMC free article] [PubMed] [Google Scholar]
- 19.White CA. Cognitive behavioral principles in managing chronic disease. West J Med 2001;175(5):338–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinsinger SW. Cognitive-behavioral therapy for patients with irritable bowel syndrome: current insights. Psychol Res Behav Manag 2017;10:231–7. 10.2147/PRBM.S120817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galdston MR, John RM. Mind over gut: psychosocial management of pediatric functional abdominal pain. J Pediatr Health Care. 2016;30(6):535–45. 10.1016/j.pedhc.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 22.••. Bonnert M, Olen O, Lalouni M, Hedman-Lagerlof E, Sarnholm J, Serlachius E et al. Internet-delivered exposure-based cognitive behavioral therapy for adolescents with functional abdominal pain or functional dyspepsia: a feasibility study. Behav Ther 2019;50(1): 177–88. 10.1016/j.beth.2018.05.002. This study demonstrates that internet-delivered EBT is beneficial in adolescents with FAP and FD.
- 23.Freeman KA, Riley A, Duke DC, Fu R. Systematic review and meta-analysis of behavioral interventions for fecal incontinence with constipation. J Pediatr Psychol 2014;39(8):887–902. 10.1093/jpepsy/jsu039. [DOI] [PubMed] [Google Scholar]
- 24.Slutsker B, Konichezky A, Gothelf D. Breaking the cycle: cognitive behavioral therapy and biofeedback training in a case of cyclic vomiting syndrome. Psychol Health Med 2010;15(6):625–31. 10.1080/13548506.2010.498893. [DOI] [PubMed] [Google Scholar]
- 25.van Tilburg MAL. Cognitive behavioral therapy for functional gastrointestinal disorders. In: Faure C, Thapar N, De Lorenzo C, editors. Pediatric neurogastroenterology. Cham, Springer; 2017. p. 507–13. [Google Scholar]
- 26.Lackner JM, Jaccard J, Radziwon CD, Firth RS, Gudleski GD, Hamilton F, et al. Durability and decay of treatment benefit of cognitive behavioral therapy for irritable bowel syndrome: 12month follow-up. Am J Gastroenterol 2018. 10.1038/s41395-018-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonnert M, Olen O, Lalouni M, Benninga MA, Bottai M, Engelbrektsson J, et al. Internet-delivered cognitive behavior therapy for adolescents with irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol 2017;112(1):152–62. 10.1038/ajg.2016.503. [DOI] [PubMed] [Google Scholar]
- 28.Rathbone AL, Clarry L, Prescott J. Assessing the efficacy of mobile health apps using the basic principles of cognitive behavioral therapy: systematic review. J Med Internet Res 2017;19(11):e399. 10.2196/jmir.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szigethy E, Kenney E, Carpenter J, Hardy DM, Fairclough D, Bousvaros A, et al. Cognitive-behavioral therapy for adolescents with inflammatory bowel disease and subsyndromal depression. J Am Acad Child Adolesc Psychiatry. 2007;46(10):1290–8. 10.1097/chi.0b013e3180f6341f. [DOI] [PubMed] [Google Scholar]
- 30.Szigethy E, Bujoreanu SI, Youk AO, Weisz J, Benhayon D, Fairclough D, et al. Randomized efficacy trial of two psychotherapies for depression in youth with inflammatory bowel disease. J Am Acad Child Adolesc Psychiatry. 2014;53(7):726–35. 10.1016/j.jaac.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy RL, van Tilburg MA, Langer SL, Romano JM, Walker LS, Mancl LA, et al. Effects of a cognitive behavioral therapy intervention trial to improve disease outcomes in children with inflammatory bowel disease. Inflamm Bowel Dis 2016;22(9):2134–48. 10.1097/MIB.0000000000000881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.••. Stapersma L, van den Brink G, van der Ende J, Szigethy EM, Beukers R, Korpershoek TA, et al. Effectiveness of disease-specific cognitive behavioral therapy on anxiety, depression, and quality of life in youth with inflammatory bowel disease: a randomized controlled trial. J Pediatr Psychol 2018;43(9):967–80. 10.1093/jpepsy/jsy029. The most recent randomized control trial comparing disease-specific CBT to care as usual in pediatric IBD, suggesting no difference in improving psychological symptoms or HRQOL.
- 33.Ljotsson B, Hedman E, Andersson E, Hesser H, Lindfors P, Hursti T, et al. Internet-delivered exposure-based treatment vs. stress management for irritable bowel syndrome: a randomized trial. Am J Gastroenterol 2011;106(8):1481–91. 10.1038/ajg.2011.139. [DOI] [PubMed] [Google Scholar]
- 34.Lalouni M, Olen O, Bonnert M, Hedman E, Serlachius E, Ljotsson B. Exposure-based cognitive behavior therapy for children with abdominal pain: a pilot trial. PLoS One. 2016;11(10):e0164647. 10.1371/journal.pone.0164647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brugnoli MP, Pesce G, Pasin E, Basile MF, Tamburin S, Polati E. The role of clinical hypnosis and self-hypnosis to relief pain and anxiety in severe chronic diseases in palliative care: a 2-year longterm follow-up of treatment in a nonrandomized clinical trial. Ann Palliat Med 2018;7(1):17–31. 10.21037/apm.2017.10.03. [DOI] [PubMed] [Google Scholar]
- 36.Del Casale A, Ferracuti S, Rapinesi C, De Rossi P, Angeletti G, Sani G, et al. Hypnosis and pain perception: an activation likelihood estimation (ALE) meta-analysis of functional neuroimaging studies. J Physiol Paris. 2015;109(4–6):165–72. 10.1016/j.jphysparis.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Chiarioni G, Vantini I, De Iorio F, Benini L. Prokinetic effect of gut-oriented hypnosis on gastric emptying. Aliment Pharmacol Ther 2006;23(8):1241–9. 10.1111/j.1365-2036.2006.02881.x. [DOI] [PubMed] [Google Scholar]
- 38.Gonsalkorale WM, Toner BB, Whorwell PJ. Cognitive change in patients undergoing hypnotherapy for irritable bowel syndrome. J Psychosom Res 2004;56(3):271–8. 10.1016/S00223999(03)00076-X. [DOI] [PubMed] [Google Scholar]
- 39.Riehl ME, Keefer L. Hypnotherapy for esophageal disorders. Am J Clin Hypn 2015;58(1):22–33. 10.1080/00029157.2015.1025355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calvert EL, Houghton LA, Cooper P, Morris J, Whorwell PJ. Long-term improvement in functional dyspepsia using hypnotherapy. Gastroenterology. 2002;123(6):1778–85. 10.1053/gast.2002.37071. [DOI] [PubMed] [Google Scholar]
- 41.Rutten JM, Vlieger AM, Frankenhuis C, George EK, Groeneweg M, Norbruis OF, et al. Gut-directed hypnotherapy in children with irritable bowel syndrome or functional abdominal pain (syndrome): a randomized controlled trial on self exercises at home using CD versus individual therapy by qualified therapists. BMC Pediatr. 2014;14:140. 10.1186/1471-2431-14-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vlieger AM, Menko-Frankenhuis C, Wolfkamp SC, Tromp E, Benninga MA. Hypnotherapy for children with functional abdominal pain or irritable bowel syndrome: a randomized controlled trial. Gastroenterology. 2007;133(5):1430–6. 10.1053/j.gastro.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 43.van Tilburg MA, Chitkara DK, Palsson OS, Turner M, Blois-Martin N, Ulshen M, et al. Audio-recorded guided imagery treatment reduces functional abdominal pain in children: a pilot study. Pediatrics. 2009;124(5):e890–7. 10.1542/peds.2009-0028. [DOI] [PubMed] [Google Scholar]
- 44.Weydert JA, Shapiro DE, Acra SA, Monheim CJ, Chambers AS, Ball TM. Evaluation of guided imagery as treatment for recurrent abdominal pain in children: a randomized controlled trial. BMC Pediatr 2006;6:29. 10.1186/1471-2431-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.•. Vlieger AM, Rutten JM, Govers AM, Frankenhuis C, Benninga MA. Long-term follow-up of gut-directed hypnotherapy vs. standard care in children with functional abdominal pain or irritable bowel syndrome. Am J Gastroenterol 2012;107(4):627–31. 10.1038/ajg.2011.487. Randomized control trial suggesting that HT is more effective than supportive therapy in reducing pain and somatisization scores in pediatric FAP and IBS after 5 years of follow-up.
- 46.Szigethy E. Hypnotherapy for inflammatory bowel disease across the lifespan. Am J Clin Hypn 2015;58(1):81–99. 10.1080/00029157.2015.1040112. [DOI] [PubMed] [Google Scholar]
- 47.Shaoul R, Sukhotnik I, Mogilner J. Hypnosis as an adjuvant treatment for children with inflammatory bowel disease. J Dev Behav Pediatr 2009;30(3):268. 10.1097/DBP.0b013e3181a7eeb0. [DOI] [PubMed] [Google Scholar]
- 48.Keefer L, Taft TH, Kiebles JL, Martinovich Z, Barrett TA, Palsson OS. Gut-directed hypnotherapy significantly augments clinical remission in quiescent ulcerative colitis. Aliment Pharmacol Ther 2013;38(7):761–71. 10.1111/apt.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mawdsley JE, Jenkins DG, Macey MG, Langmead L, Rampton DS. The effect of hypnosis on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Am J Gastroenterol 2008;103(6):1460–9. 10.1111/j.1572-0241.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 50.Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin Psychol Sci Pract 2006. [Google Scholar]
- 51.Ahola Kohut S, Stinson J, Davies-Chalmers C, Ruskin D, van Wyk M. Mindfulness-based interventions in clinical samples of adolescents with chronic illness: a systematic review. J Altern Complement Med 2017;23(8):581–9. 10.1089/acm.2016.0316. [DOI] [PubMed] [Google Scholar]
- 52.Korterink JJ, Ockeloen LE, Hilbink M, Benninga MA, Deckers-Kocken JM. Yoga therapy for abdominal pain-related functional gastrointestinal disorders in children: a randomized controlled trial. J Pediatr Gastroenterol Nutr 2016;63(5):481–7. 10.1097/MPG.0000000000001230. [DOI] [PubMed] [Google Scholar]
- 53.•. Ali A, Weiss TR, Dutton A, McKee D, Jones KD, Kashikar-Zuck S, et al. Mindfulness-based stress reduction for adolescents with functional somatic syndromes: a pilot cohort study. J Pediatr. 2017;183:184–90. 10.1016/j.jpeds.2016.12.053. A pilot study introducing a MBSR program to a cohort of pediatric patients with functional somatic syndromes demonstrating improvements in functional disability, symptom impact, and anxiety.
- 54.Garland EL, Gaylord SA, Palsson O, Faurot K, Douglas Mann J, Whitehead WE. Therapeutic mechanisms of a mindfulness-based treatment for IBS: effects on visceral sensitivity, catastrophizing, and affective processing of pain sensations. J Behav Med 2012;35(6):591–602. 10.1007/s10865-011-9391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaylord SA, Whitehead WE, Coble RS, Faurot KR, Palsson OS, Garland EL, et al. Mindfulness for irritable bowel syndrome: protocol development for a controlled clinical trial. BMC Complement Altern Med 2009;9:24. 10.1186/1472-6882-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cotton S, Humenay Roberts Y, Tsevat J, Britto MT, Succop P, McGrady ME, et al. Mind-body complementary alternative medicine use and quality of life in adolescents with inflammatory bowel disease. Inflamm Bowel Dis 2010;16(3):501–6. 10.1002/ibd.21045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neilson K, Ftanou M, Monshat K, Salzberg M, Bell S, Kamm MA, et al. A controlled study of a group mindfulness intervention for individuals living with inflammatory bowel disease. Inflamm Bowel Dis 2016;22(3):694–701. 10.1097/MIB.0000000000000629. [DOI] [PubMed] [Google Scholar]
- 58.••. Beinvogl B, Burch E, Snyder J, Schechter N, Hale A, Okazaki Y, et al. Multidisciplinary treatment of pediatric functional gastrointestinal disorders results in improved pain and functioning. Clin Gastroenterol Hepatol 2018. 10.1016/j.cgh.2018.07.025. A single-site, retrospective observational cohort study of pediatric patients with FGID demonstrating the importance of comprehensive and multidisciplinary care.
- 59.Hechler T, Kanstrup M, Holley AL, Simons LE, Wicksell R, Hirschfeld G, et al. Systematic review on intensive interdisciplinary pain treatment of children with chronic pain. Pediatrics. 2015;136(1):115–27. 10.1542/peds.2014-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lalouni M, Ljotsson B, Bonnert M, Ssegonja R, Benninga M, Bjureberg J, et al. Clinical and cost effectiveness of online cognitive behavioral therapy in children with functional abdominal pain disorders. Clin Gastroenterol Hepatol 2018. 10.1016/j.cgh.2018.11.043. [DOI] [PubMed] [Google Scholar]


