Abstract
Objectives
The purpose of the ongoing follow‐up of ReActiv8‐A clinical trial is to document the longitudinal benefits of episodic stimulation of the dorsal ramus medial branch and consequent contraction of the lumbar multifidus in patients with refractory mechanical chronic low back pain (CLBP). We report the four‐year outcomes of this trial.
Materials and Methods
ReActiv8‐A is a prospective, single‐arm trial performed at nine sites in the United Kingdom, Belgium, and Australia. Eligible patients had disabling CLBP (low back pain Numeric Rating Scale [NRS] ≥6; Oswestry Disability Index [ODI] ≥25), no indications for spine surgery or spinal cord stimulation, and failed conventional management including at least physical therapy and medications for low back pain. Fourteen days postimplantation, stimulation parameters were programmed to elicit strong, smooth contractions of the multifidus, and participants were given instructions to activate the device for 30‐min stimulation‐sessions twice daily. Annual follow‐up through four years included collection of NRS, ODI, and European Quality of Life Score on Five Dimensions (EQ‐5D). Background on mechanisms, trial design, and one‐year outcomes were previously described.
Results
At baseline (N = 53) (mean ± SD) age was 44 ± 10 years; duration of back pain was 14 ± 11 years, NRS was 6.8 ± 0.8, ODI 44.9 ± 10.1, and EQ‐5D 0.434 ± 0.185. Mean improvements from baseline were statistically significant (p < 0.001) and clinically meaningful for all follow‐ups. Patients completing year 4 follow‐up, reported mean (±standard error of the mean) NRS: 3.2 ± 0.4, ODI: 23.0 ± 3.2, and EQ‐5D: 0.721 ± 0.035. Moreover, 73% of participants had a clinically meaningful improvement of ≥2 points on NRS, 76% of ≥10 points on ODI, and 62.5% had a clinically meaningful improvement in both NRS and ODI and 97% were (very) satisfied with treatment.
Conclusions
In participants with disabling intractable CLBP who receive long‐term restorative neurostimulation, treatment satisfaction remains high and improvements in pain, disability, and quality‐of‐life are clinically meaningful and durable through four years.
Keywords: Chronic low back pain, long‐term effect, multifidus muscle impaired neuromuscular control, restorative neurostimulation
INTRODUCTION
Patients presenting with mechanical low back pain that has persisted more than 12 months have a poor prognosis for recovery (1, 2). In most cases, no obvious pathology can be identified, and the source of the pain is ill‐defined (3). The symptoms associated with long standing low back pain are an overlap of physiological pain and psychological and social sequelae resulting in a complex biopsychosocial phenomenon. In the absence of etiological pathology or clear surgical targets, significant effort has been directed at physical rehabilitation, behavioral modification, and coping strategies (4). While these approaches provide relief to some patients, benefits are minimal or transient in many (5, 6).
The multifidus and transversus abdominis muscles are directly responsible for stabilizing the spine at the ends of it range of motion and for dynamic stability through the midrange of motion (7). Multiple studies have shown that these muscles are dysfunctional in patients with low back pain, and in particular the multifidus has been shown to rapidly atrophy after acute episodes of pain (8). The mechanism behind this atrophy is believed to be the result of peripheral and centrally mediated inhibitory mechanisms, resulting in fatty infiltration and persistent inflammatory changes (9, 10, 11, 12, 13, 14, 15, 16). This leads to a deficit in involuntary muscle activation in response to perturbation and a loss of proprioceptive sensitivity (17), allowing the patient to adopt postures and motions that result in deviations from the safe range of motion (18, 19), leading to additional injury and a negative feedback loop of further pain, disability, and muscular inhibition (20, 21).
Current best practice therapies prescribed to target the physiological causes of mechanical low back pain consist predominantly of physiotherapy and exercise therapy, with a particular focus on attaining spinal stability through improvements in strength and motor control of the key stabilizing muscles. A major research focus has been the targeting the functional restoration of both the multifidus and transversus abdominis muscles (7). The multifidus presents itself as an obvious therapeutic target for the treatment of mechanical chronic low back pain (CLBP). Restoring motor control and strength to the multifidus muscles through the prescription of specific functional exercises has been a frontline approach for physiotherapy for more than 25 years (22, 23, 24). These muscles are tonic spinal stabilizers, so voluntary activation can be difficult to achieve; however, in a research setting, under direct clinician supervision this strategy has been moderately effective. The translation to clinical practice has led to more equivocal results, especially at the more severe end of the disease spectrum (23, 25, 26). As such, physiotherapy‐based methods for restoration of multifidus motor control remain at the forefront of treatment for subacute and CLBP, yet there remains a significant cohort of patients that receive nil or only temporary benefit (7).
Neuromuscular stimulation of the medial branch of the dorsal ramus to elicit contractions of the multifidus has been suggested as a therapeutic approach for CLBP patients who fail traditional conservative treatment (12). It was proposed that electrical stimulation of the nerve could override its inhibition, restore segmental spine control, and ultimately reduce the severity of symptoms (12, 27, 28, 29). This restorative neurostimulation mechanism was predicted to provide progressive improvement in CLBP with durable outcomes, especially when compared to analgesic approaches such as spinal cord stimulation with outcomes that diminish over time. Recently, this approach has been validated in a pivotal, double‐blind, active sham, randomized controlled trial (ReActiv8‐B; ClinicalTrials.gov NCT02577354). In patients with an average of more than 14 years of nociceptive low back pain, this therapy demonstrated a significant reduction in pain, disability, and improvement in the quality of life at 120 days, with accrual over time (30). The pivotal study was preceded by the original international, multicenter, prospective, single‐arm trial (ReActiv8‐A) that similarly showed significant and clinically meaningful improvements reduction in pain, disability, and improvement in the quality of life (1). The data reported here represent the long‐term follow‐up (12–48 months) from the original one‐year cohort data of the trial presented by Deckers et al. (1).
MATERIALS AND METHODS
Trial Design
ReActiv8‐A is an international, multicenter, prospective, single‐arm trial to characterize the technical feasibility, performance, and safety of a restorative neurostimulation device (ReActiv8® Mainstay Medical, Dublin, Ireland) in the treatment of patients with refractory CLBP (ClinicalTrials.gov NCT01985230). The long‐term follow‐up was conducted at nine clinical sites (Australia—4, Belgium—2, and United Kingdom—3) in accordance with ISO 14155:2011, and enrolled patients from July 2014 to October 2015. The protocol and associated data collection forms were reviewed and approved by the ethics committee for each site, and informed consent was obtained from each subject. A detailed discussion of the trial design and implementation is included with the publication of the 12‐month data (1). The inclusion and exclusion criteria are presented in Table 1.
Table 1.
Key Inclusion and Exclusion Criteria.
| Inclusion criteria |
|
| Exclusion criteria |
|
|
|
| Back pain exclusions |
|
|
|
| Drug use exclusions |
|
|
| Surgical exclusions |
|
|
|
|
| Psychosocial exclusions |
|
|
|
Device and Surgical Technique
A detailed description of the device and surgical technique have been reported elsewhere (1). Briefly, the device consists of an implanted pulse generator (IPG) and two leads, each with a four‐electrode array. In the first 47 subjects, the leads were placed via two incisions under fluoroscopic guidance with the lead body positioned parallel to the spinal column (the “lateral approach”). For the last six subjects and all subsequent lead implants (including lead replacements), the surgical approach was modified, so leads were placed from a single midline incision directed laterally to the target (the “midline approach”). For both the midline and the lateral approaches, the distal ends of the leads were placed over the left and right medial branches of the L2 dorsal ramus and fixed in position using a self‐deploying anchor (tines) placed into the intertransversarius muscles. The IPG was positioned in a subcutaneous pocket and connected to the leads through a subcutaneous tunnel.
Two weeks following implantation, devices were activated and programmed via radio frequency telemetry. Each lead has four individually programmable electrodes, enabling selective stimulation of the medial branch of the dorsal ramus to elicit isolated multifidus contractions. The devices were programmed to deliver patient specific values resulting in strong smooth bilateral contractions of the multifidus muscles. The onset of the stimulation occurred with a pre‐ and postramp up to a fused tetanic contraction intensity that was determined by the patient to be strong yet comfortable. Using this smooth ramped waveform eliminated the sudden uncomfortable onset of stimulation and allowed patients to comfortably sustain a higher peak contraction intensity. It was recommended that participants perform two 30‐min stimulation sessions per day with the program cycling through 10 sec of stimulated contractions followed by 20 sec of relaxation.
Follow‐Up and Outcome Measures
Participants enrolled in the trial were followed at 45, 90, 180, and 270 days, then annually for 48 months.
At each follow‐up, participants were asked to report the following outcomes:
Low back pain using a single day recall Numerical Rating Scale (NRS)
Back pain‐related disability using the Oswestry Disability Index (ODI; version 2.1a) (31)
General health‐related quality of life using the European Quality of Life Score on Five Dimensions (EQ‐5D; EuroQol Group; https://euroqol.org/)
Treatment satisfaction on a 5‐point Likert scale
Statistical Analysis
Continuous demographic variables were reported as mean and standard deviation, patient‐reported outcome measures (PROMs) were reported as mean and standard error of the mean (SEM), or median and range, and categorical variables were reported as counts and percentages where possible.
In order to accurately represent the long‐term performance of the restorative neurostimulation and the attrition in the population, we present these data as completed case (CC) cohorts. For each of the CC cohorts (one, two, three, and four years), the analysis matches all complete data with that of the same cohort at prior follow‐ups (e.g., the four‐year completed case analysis looks at the data at each time point only from participants with complete data at the four‐year follow‐up) (32, 33).
Parametric data were compared with the two‐tailed Student's t‐test, nonparametric data with the Mann‐Whitney U test, and proportional differences with the Chi‐squared test. Statistical significance was defined as α <0. All statistical analyses were conducted using R version 3.6.1 (https://www.R-project.org).
RESULTS
Demographics and Participant Disposition
Participant follow‐up and disposition is documented in Figure 1 and the demographics at baseline for the entire cohort, and the four‐year completed case cohort (four‐year NRS CC) are reported in Table 2. There were no statistically significant differences between the four‐year NRS CC and the noncompleters at four years in gender, age, body mass index, pain duration, or baseline PROMs, suggesting that the various CC cohorts are demographically representative of the full cohort.
Figure 1.
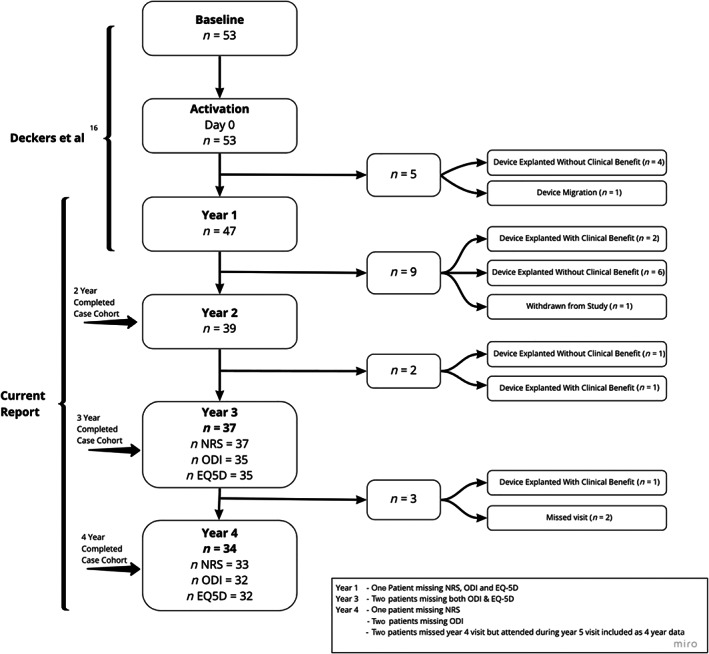
Patient disposition over the four‐year study duration.
Table 2.
Baseline Demographics for Complete Cohort and Four‐Year NRS Completed Case Cohort.
| Complete cohort | Four‐year NRS CC cohort | |
|---|---|---|
| N | 53 | 33 |
| Sex (% female) | 57% | 55% |
| Age (years) (SD) | 44.1 (10.2) | 44.8 (9.0) |
| Mean BMI (kg/m2) SD) | 27.3 (4.5) | 26.8 (4.6) |
| Mean pain duration (years) (SD) | 14.3 (10.5) | 14.1 (9.6) |
| Baseline NRS (mean) (SD) | 6.8 (0.8) | 6.7 (1.2) |
| Baseline ODI (mean) (SD) | 44.9 (10.1) | 43.8 (9.9) |
| Baseline EQ‐5D (mean) (SD) | 0.434 (0.185) | 0.444 (0.186) |
Over the four years of follow‐up, one patient was lost to follow‐up and could no longer be contacted. The remaining patients exiting the study did so following explant, either without clinical benefit (n = 11) or with clinical benefit (n = 4) or because of a device migration that could not be repositioned (n = 1). The patients that elected to receive explant with clinical benefit purportedly did so as they felt their symptoms had resolved to a point where they no longer required the device. After patients exited the study, they were not contacted again as per the protocol, so we are unable to speculate on the durability of effect on explanted responders.
Early dropouts were generally associated with need for lead revisions which prompted a change in surgical techniques and lead design (as discussed in the original paper (1)), while later stage dropouts were for more varied reasons.
Durability: Completed Cases
The completed case analysis presents four cohorts, defined by completers of each annual follow‐up, for which matched data from previous follow‐ups are available. The advantage of reporting outcomes for each of these four cohorts is that the impact of participant attrition is quantified by the difference in the cohort means at each follow‐up period (Table 3). Figure 2a–c shows thce means at one, two, three, and four years (n = 47, 39, 37, and 33 (31), respectively) for NRS, ODI, and EQ‐5D. Similarly, matched completed cases as a proportion of the overall response to therapy at each time point aggregated as participants responding with greater than minimally clinically important change (MCIC) in both NRS, ODI, or EQ‐5D is shown in Figure 2d. Sixty‐two percent of participants experienced a clinical meaningful benefit of at least the MCIC in both pain and disability, 73% of patients had a clinically meaningful improvement of ≥2 points on NRS, and 76% of ≥10 points on ODI.
Table 3.
Improvement* From Baseline for Each Completed Case Cohort One to Four Years Postactivation.
| Follow‐up period | One‐year completed case cohort | Two‐year completed case cohort | Three‐year completed case cohort | Four‐year completed case cohort | Standard deviation | |
|---|---|---|---|---|---|---|
| ∆NRS | One year | 2.4 | 2.6 | 2.7 | 2.6 | 0.14 |
| Two years | 2.7 | 2.8 | 2.8 | 0.08 | ||
| Three years | 3.3 | 3.2 | 0.10 | |||
| Four years | 3.5 | ‐ | ||||
| ∆ODI | One year | 14.3 | 16.4 | 17.1 | 17.4 | 1.39 |
| Two years | 17.0 | 17.9 | 18.9 | 0.95 | ||
| Three years | 19.7 | 20.3 | 0.37 | |||
| Four years | 22.2 | ‐ | ||||
| ∆EQ‐5D | One year | 0.219 | 0.247 | 0.245 | 0.235 | 0.01 |
| Two years | 0.244 | 0.256 | 0.263 | 0.01 | ||
| Three years | 0.288 | 0.286 | 0.00 | |||
| Four years | 0.285 | ‐ |
This shows, for example, from left to right, the mean change in NRS at one year from baseline for the one‐year completed cohort (n = 47), the two‐year completed cohort (n = 39), the three‐year completed cohort (n = 37), and the four‐year completed cohort (n = 33) followed by the standard deviation of those four means.
Positive values represent the magnitude of the improvement in outcome over baseline.
Figure 2.
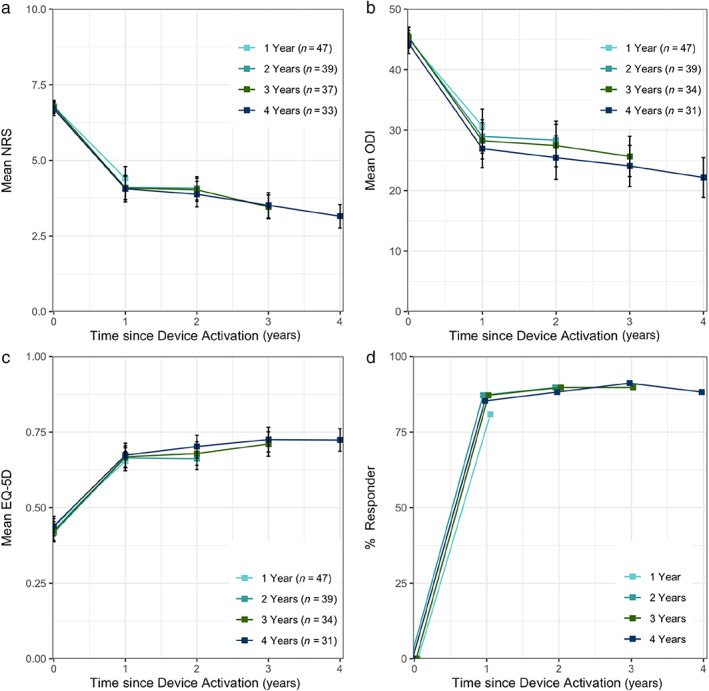
Mean ± standard error of the mean (SEM) (a) NRS, (b) ODI, and (c) EQ‐5D, and (d) proportion of participants benefiting by more than one MCIC in either NRS, ODI, or EQ‐5D, in completed cases at one to four years. [Color figure can be viewed at wileyonlinelibrary.com]
In this study, we observed a pattern where early dropouts were generally associated with need for lead revisions which prompted a change in surgical technique for implantation and an update to the lead design. While the loss of some patients to long‐term follow‐up is a confounding factor in the analysis, we have attempted to provide these unique long‐term data in a manner that is reasonable. Table 3 presents an analysis of each of the completed cohorts with the change from baseline for each measure at each available time. By calculating for example, the changes from baseline mean NRS for the one, two, three, and four year completed case cohorts (i.e., 2.4, 2.6, 2.7, and 2.6, respectively), it can be demonstrated that the pain reduction across groups is consistent, with only small variance (standard deviation = 0.1). The small difference in mean outcome scores between completed case cohorts is observed for each outcome measure at each time point and suggests that the overall impact of patient dropouts on the mean reported scores was also small. This in turn indicates that this analytical approach is appropriate to describe a therapy such as restorative neurostimulation where the effects accumulate over time.
In the completed case cohort treatment, satisfaction at four years was reported as “Very Satisfied” in 32/33 (97%) of participants. To date, no participants have required a procedure to replace a discharged battery.
DISCUSSION
Restorative neurostimulation for the treatment of mechanical CLBP is characterized by improvements in pain and function that accrue over time. Therefore, the long‐term effect is an important point of differentiation from palliative analgesic treatment approaches. In this paper, we report the four‐year data as a follow‐up to the publication of the one‐year results.
In the time between implantation and the primary endpoint, patient compliance was particularly high with 84.5 ± 22.6% (n = 50) of the maximum number of therapy sessions being completed. Four years after implantation, 48.8 ± 34.0% (n = 27) or approximately half of maximum number of stimulation sessions are being completed across the patient cohort. Early compliance was in line with strict instructions to complete the therapy and then a decline over time as would be broadly anticipated in a restorative therapy with accruing effect.
Restorative neurostimulation is intended to be a rehabilitative procedure where over time the patient regains their innate motor control. The amount of stimulation sessions and recovery trajectory varies by patient.
Patients who were eligible for the trial reported an average of 14 years of low back pain. Kongsted et al. (32) identified several common clinical trajectories that describe the pattern and persistence of CLBP symptoms. This phenotypic description suggests that the clinical course for patients suffering from long lasting CLBP is invariably poor and that spontaneous remission of pain does not occur.
This study showed improved outcomes in comparison to baseline across the reported four‐year follow‐up period. The ReActiv8‐B study also demonstrated that long‐term therapeutic stimulation of the medial branch of the dorsal ramus could significantly reduce pain in a large cohort of CLBP patients, with significant and clinically meaningful differences in visual analog scale, ODI, and EQ‐5D between treatment and active‐sham at 120 days (30). The durability data presented here should give insight into the long‐term durability of the improvements demonstrated in the larger and randomized controlled cohort.
Impact of Surgical Approach
As noted in the publication of the one‐year data, there was a significant impact of the surgical approach on early therapy performance. Leads which were placed via the “lateral trajectory” (n = 47/53) crossed the two main layers of the posterior thoracolumbar fascia, plus investing fascia on the multifidus and erector spinae muscles. The shear stresses exerted on the lead by the relative movements of these fascial planes in some cases resulted in conductor fracture and consequent loss of therapeutic stimulation. Participants with lead fractures were given the opportunity to have the leads repositioned to a medial trajectory. Figure 1 shows timing and impact of explanation on the number of participants remaining in the study. In year 1, before the introduction of the medial implant approach, six patients opted to have the system explanted. During years 1 through 4, seven patients opted to have the system explanted without clinical benefit and three opted to have the system explanted even though they had reported clinically meaningful benefits at their last follow‐up visit. Additionally, one patient was explanted after lead migration, one patient elected to withdraw from the study, and two participants missed their four‐year visit. The recent publication of the ReActiv8‐B study reports that medial trajectory which avoids the facial planes demonstrably mitigates the risk of lead fracture with a rate of less than 3% at one year (30).
Additional Analyses of Noncompleters
The CC analysis is presented as the mean outcome score and the proportion of participants who reported improvements greater than a MCIC from baseline. Threshold MCICs have been defined as a change in NRS and ODI of 2 and 10, respectively. The MCIC for EQ‐5D was 0.1 acknowledging that there is a lack of consensus on the MCIC threshold for CLBP with this instrument (33, 34). The standard deviation was used to estimate the residual change in mean scores between CC cohorts and indicate the impact of different completer rates at each follow‐up time point (Table 3).
A main limitation of these data is the proportion of patients exiting the study in the early portion of the study period. The patient disposition detailed in Figure 1 shows that most explants were conducted in year 1 and 2 with 71% (12/17) occurring within 18 months of activation. The reasons for these have been detailed above but this early loss presents a unique complexity in the analysis of the long‐term effect, especially given that the mechanism of restorative neurostimulation is one that accumulates efficacy over time. The simplest method for imputing missing data is to carry the last observation forward as far as possible and include a confidence range of ±1 MCIC for each missing datapoint. For completeness, this is included in Figure 3; however, the authors would contend that this methodology, although appropriate for a palliative pain device, may not represent long‐term outcomes in the context of a restorative therapy where gains accrue over time. Thus, the intervention given to a patient exiting the study at one year is quantifiably different to one still using stimulation after four years. As the effect of the intervention improves over time, this difference in the quantity of therapy (i.e., number of stimulation sessions) and timing of follow‐up does not inform the long‐term impact of stimulation. We considered alternative methods for analyzing missing data, such as only carrying forward the last observation for only 12 months and multiple imputation (MI). These are also included in Figure 3, for illustrative purposes. Limiting the length of time, an observation can be extrapolated has some merit as it partly attempts to minimize the differences in the amount of therapy delivered, but this method makes the number of patients being analyzed at a particular time point variable and thus difficult for readers to account for all the patients in this trial. In many cases, MI is arguably the most statistically sound approach to missing data, though in this case MI was not appropriate as the underlying assumption is the missing values can be modeled based on the available data. This is not necessarily a correct assumption with these data, as at the later time points the missing data are imputed with the outcomes of those patients remaining in the study who had received a larger amount of therapy and therefore the imputed mean trends closely to the observed mean, potentially overestimating the response in noncompleters. With larger data sets, more sophisticated analyses such as these are more reliable.
Figure 3.
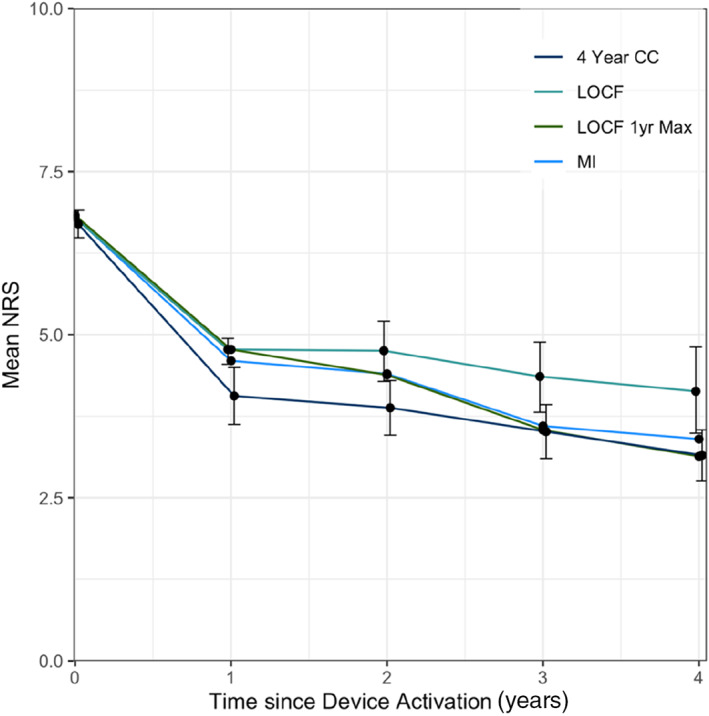
Multiple methods used to describe the impact of patients exiting the study on the long‐term outcomes; four‐year completed case cohort ± SEM (n = 33) (four year CC), last observation carried forward ± MCIC for imputed values (n = 53) (LOCF), last observation carried forward for one year (n = 53, 53, 47, 39, 37) (LOCF 1yr Max), and multiple imputation (n = 53) (MI). [Color figure can be viewed at wileyonlinelibrary.com]
The completed case analysis used here best represents the available data in describing the impact of long‐term stimulation of the medial branch of the dorsal ramus in CLBP patients. This approach also allows visualization of the trajectory of response in patients who are still being actively treated, while also demonstrating the impact of patients who have exited from the study.
Strengths
There are very few studies, which have reported four‐year durability data for treatments for mechanical CLBP.
Limitations
The longitudinal analysis presented is not without limitations. After four years, 19/53 patients were missing data for various reasons. Also, the relatively high lead revision rate which contributed to early attrition may also have impacted reported outcomes. While attrition is consistent with that seen in other studies (35), we have nevertheless presented the outcomes and conclusions conservatively. Despite these limitations, there are some important findings pertaining to long‐term effectiveness.
CONCLUSION
The totality of data demonstrates that in this difficult‐to‐treat population of patients with refractory CLBP, durable benefits are accrued over time and sustained through four years post‐therapy activation. Pain and disability in the four‐year completed case cohort were on average 53% and 50% lower than baseline, respectively, suggesting that the effects are durable over the long term.
Knowledge of restorative neurostimulation has evolved considerably over the past six years including patient selection, safety, and complication profiles and associated surgical techniques, and this is the subject of further research.
Authorship Statement
Drs. Mitchell, Deckers, De Smedt, Russo, Georgius, Green, Gulve, van Buyten, Smet, Mehta, Baranidharan, and Eldabe conducted the study, including patient recruitment, data collection, and data analysis. Dr. Rathmell chaired the CEC and Dr. Gilligan chaired the Data Safety Monitoring Committee. Both Drs. Rathmell and Gilligan reviewed the incoming data. Drs. Mitchell, Goss, and Eldabe conducted the data analysis and prepared the manuscript draft. All authors reviewed the manuscript drafts and approved the final manuscript.
Comments
The authors present the results of 4‐year follow‐up of patients in a multicenter clinical trial investigating the effects of stimulation of the multifidus muscle via electrode implantation at the medial branch of the dorsal ramus of L2 bilaterally for chronic back pain. In the original study, leads were placed via two surgical approaches, with a later revised approach intending to reduce the incidence of lead fracture requiring revision. The authors followed the cohort of patients who were previously reported at 1‐year follow‐up and obtained their pain, disability, and satisfaction scores with the device after 4 years. Overall, the majority of patients who were followed reported satisfaction with the device and continued improvement at 4 years. In addition, there were significantly fewer lead revisions due to lead fracture with the updated surgical approach. Although the authors only followed subjects who were in the completed case cohort at 1‐year follow‐up in the prior study, of which many more subjects were lost to follow‐up since that time, this study nonetheless demonstrates that multifidus stimulation may offer long‐term benefit for patients suffering from chronic low back pain.
Jennifer Sweet, MD
Cleveland, OH USA
***
The authors' thorough discussion of the problems of analyzing cohort studies in the face of significant dropout is very interesting. This will be useful to those looking at similar problems in a wide range of other applications where outcomes are liable to change over time, rather than remaining stable after the initial intervention.
James FitzGerald, MA, BM, BCh, PhD
Oxford, UK
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: The ReActiv8‐A study was sponsored by Mainstay Medical Limited.
Conflict of Interest: Dr. Mitchell reports grants from Mainstay Medical, during the conduct of the study; personal fees from Mainstay Medical, outside the submitted work; Dr. Deckers reports grants and personal fees from Mainstay Medical, during the conduct of the study; Dr. De Smedt has nothing to disclose; Dr. Russo reports grants from Mainstay Medical, during the conduct of the study; personal fees from Mainstay Medical, outside the submitted work; Dr. Georgius reports personal fees from Boston Scientific, personal fees from Abbott Medical, personal fees from Seqirus CSL Australia, personal fees from Spectrum Therapeutics, personal fees from AusCann, personal fees from Tilray, outside the submitted work; Dr. Green has nothing to disclose; Dr. Gulve reports grants and nonfinancial support from Mainstay Medical, during the conduct of the study; grants and personal fees from Boston Scientific, grants and personal fees from Medtronic, grants and personal fees from Mainstay Medical, grants and personal fees from Saluda, grants and personal fees from Abbott, grants and personal fees from Nevro, outside the submitted work; Prof. Dr. van Buyten received consultation fees from research grants from Medtronic, Nevro, Boston Scientific, Abbott and Mainstay Medical; Dr. Smet reports personal fees from Medtronic, personal fees from Nevro, personal fees from Wise, outside the submitted work; Dr. Mehta reports grants and personal fees from Mainstay Medical, during the conduct of the study; grants and personal fees from Boston Scientific, grants and personal fees from Medtronic, outside the submitted work; Dr. Baranidharan reports grants and personal fees from Mainstay Medical, during the conduct of the study; grants and personal fees from Abbott, personal fees from Nalu, personal fees from Pain Consultancy, grants and personal fees from Nevro Corp, personal fees from Saluda, grants and personal fees from Boston Scientific, outside the submitted work; Dr. Rathmell reports personal fees from Mainstay Medical, LLP, during the conduct of the study; Dr. Gilligan reports grants and personal fees from Mainstay Medical, during the conduct of the study; personal fees from Medtronic Ltd, personal fees from Abbott, personal fees from Saluda, personal fees from Nuvectra, personal fees from Medasense, personal fees from Eli Lilly, grants from Sollis Therapeutics, outside the submitted work; Dr. Goss reports personal fees from Mainstay Medical, during the conduct of the study; Dr. Eldabe reports grants, personal fees and nonfinancial support from Mainstay Medical, during the conduct of the study; grants and personal fees from Medtronic Ltd, personal fees from Boston Scientific, from Abbott, grants from Nevro Corp, outside the submitted work.
REFERENCES
- 1.Deckers K, De Smedt K, Mitchell B et al. New therapy for refractory chronic mechanical low back pain—restorative neurostimulation to activate the lumbar multifidus: one year results of a prospective multicenter clinical trial. Neuromodulation 2018;21:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maher C, Underwood M, Buchbinder R. Non‐specific low back pain. Lancet 2017;389:736–747. [DOI] [PubMed] [Google Scholar]
- 3.Hartvigsen J, Hancock MJ, Kongsted A et al. What low back pain is and why we need to pay attention. Lancet 2018;391:2356–2367. [DOI] [PubMed] [Google Scholar]
- 4.Foster NE, Anema JR, Cherkin D et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet 2018;391:2368–2383. [DOI] [PubMed] [Google Scholar]
- 5.Hayden JA, Van Tulder MW, Malmivaara AV et al. Meta‐analysis: exercise therapy for nonspecific low back pain. Ann Intern Med 2005;142:765–775. [DOI] [PubMed] [Google Scholar]
- 6.Steffens D, Maher CG, Pereira LSM et al. Prevention of low back pain: a systematic review and meta‐analysis. JAMA Intern Med 2016;176:199–208. [DOI] [PubMed] [Google Scholar]
- 7.Macedo LG, Latimer J, Maher CG et al. Effect of motor control exercises versus graded activity in patients with chronic nonspecific low back pain: a randomized controlled trial. Phys Ther 2012;92:363–377. [DOI] [PubMed] [Google Scholar]
- 8.Hodges P, Holm AK, Hansson T, Holm S. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine 2006;31:2926–2933. [DOI] [PubMed] [Google Scholar]
- 9.Indahl A, Kaigle AM, Reikerås O, Holm SH. Interaction between the porcine lumbar intervertebral disc, zygapophysial joints, and paraspinal muscles. Spine 1997;22:2834–2840. [DOI] [PubMed] [Google Scholar]
- 10.James G, Sluka KA, Blomster L et al. Macrophage polarization contributes to local inflammation and structural change in the multifidus muscle after intervertebral disc injury. Eur Spine J 2018;27:1744–1756. [DOI] [PubMed] [Google Scholar]
- 11.Brumagne S, Diers M, Danneels L, Moseley GL, Hodges PW. Neuroplasticity of sensorimotor control in low back pain. J Orthop Sports Phys Ther 2019;49:402–414. [DOI] [PubMed] [Google Scholar]
- 12.Russo M, Deckers K, Eldabe S et al. Muscle control and non‐specific chronic low back pain. Neuromodulation 2018;21:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James G, Chen X, Diwan A et al. Fat infiltration in the multifidus muscle is related to inflammatory cytokine expression in the muscle and epidural adipose tissue in individuals undergoing surgery for intervertebral disc herniation. Eur Spine J 2020;30:837–845. [DOI] [PubMed] [Google Scholar]
- 14.Hodges PW, James G, Blomster L et al. Can proinflammatory cytokine gene expression explain multifidus muscle fiber changes after an intervertebral disc lesion? Spine 2014;39:1010–1017. [DOI] [PubMed] [Google Scholar]
- 15.Hodges PW, James G, Blomster L et al. Multifidus muscle changes after back injury are characterized by structural remodeling of muscle, adipose and connective tissue, but not muscle atrophy: molecular and morphological evidence. Spine 2015;40:1057–1071. [DOI] [PubMed] [Google Scholar]
- 16.James G, Millecamps M, Stone LS, Hodges PW. Dysregulation of the inflammatory mediators in the multifidus muscle after spontaneous intervertebral disc degeneration sparc‐null mice is ameliorated by physical activity. Spine 2018;43:E1184–E1194. [DOI] [PubMed] [Google Scholar]
- 17.Brumagne S, Cordo P, Lysens R, Verschueren S, Swinnen S. The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without low back pain. Spine 2000;25:989–994. [DOI] [PubMed] [Google Scholar]
- 18.van Dieën JH, Peter Reeves N, Kawchuk G et al. Motor control changes in low back pain: divergence in presentations and mechanisms. J Orthop Sports Phys Ther 2019;49:370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cholewicki J, Silfies SP, Shah RA et al. Delayed trunk muscle reflex responses increase the risk of low back injuries. Spine 2005;30:2614–2620. [DOI] [PubMed] [Google Scholar]
- 20.Van Dieën JH, Selen LPJ, Cholewicki J. Trunk muscle activation in low‐back pain patients, an analysis of the literature. J Electromyogr Kinesiol 2003;13:333–351. [DOI] [PubMed] [Google Scholar]
- 21.Kiesel KB, Uhl T, Underwood FB, Nitz AJ. Rehabilitative ultrasound measurement of select trunk muscle activation during induced pain. Man Ther 2008;13:132–138. [DOI] [PubMed] [Google Scholar]
- 22.Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first‐episode low back pain. Spine 1996;21:2763–2769. [DOI] [PubMed] [Google Scholar]
- 23.Hides JA, Jull GA, Richardson CA. Long‐term effects of specific stabilizing exercises for first‐episode low back pain. Spine 2001;26:e243–e248. [DOI] [PubMed] [Google Scholar]
- 24.Hodges PW. Core stability exercise in chronic low back pain. Orthop Clin North Am 2003;34:245–254. [DOI] [PubMed] [Google Scholar]
- 25.Hides J, Stanton W, Mcmahon S, Sims K, Richardson C. Effect of stabilization training on multifidus muscle cross‐sectional area among young elite cricketers with low back pain. J Orthop Sports Phys Ther 2008;38:101–108. [DOI] [PubMed] [Google Scholar]
- 26.Danneels LA, Vanderstraeten GG, Cambier DC et al. Effects of three different training modalities on the cross‐sectional area of the lumbar multifidus muscle in patients with chronic low back pain. Br J Sports Med 2001;35:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stokes M, Young A. The contribution of reflex inhibition to arthrogenous muscle weakness. Clin Sci 1984;67:7–14. [DOI] [PubMed] [Google Scholar]
- 28.Freeman MD, Woodham MA, Woodham AW. The role of the lumbar multifidus in chronic low back pain: a review. PM R 2010;2:142–146. [DOI] [PubMed] [Google Scholar]
- 29.Rice DA, McNair PJ. Quadriceps arthrogenic muscle inhibition: neural mechanisms and treatment perspectives. Semin Arthritis Rheum 2010;40:250–266. [DOI] [PubMed] [Google Scholar]
- 30.Gilligan C, Volschenk W, Russo M et al. An implantable restorative‐neurostimulator for refractory mechanical chronic low back pain: a randomized sham‐controlled clinical trial. Pain 2021; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fairbank JCT, Pynsent PB. The Oswestry Disability Index. Spine 2000;25:2940–2953. [DOI] [PubMed] [Google Scholar]
- 32.Kongsted A, Hestbæk L, Kent P. How can latent trajectories of back pain be translated into defined subgroups? BMC Musculoskelet Disord 2017;18:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ostelo RWJG, Deyo RA, Stratford P et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine 2008;33:90–94. [DOI] [PubMed] [Google Scholar]
- 34.Mcdonough CM, Tosteson TD, Tosteson ANA et al. A longitudinal comparison of 5 preference‐weighted health state classification systems in persons with intervertebral disk herniation. Med Decis Making 2011;31:270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedrich M, Gittler G, Arendasy M, Friedrich KM. Long‐term effect of a combined exercise and motivational program on the level of disability of patients with chronic low back pain. Spine 2005;30:995–1000. [DOI] [PubMed] [Google Scholar]


