Abstract
Diabetes mellitus (DM) is a chronic, progressive, and multifaceted illness resulting in significant physical and psychological detriment to patients. As of 2019, 463 million people are estimated to be living with DM worldwide, out of which 90% have type-2 diabetes mellitus (T2DM). Over the years, significant progress has been made in identifying the risk factors for developing T2DM, understanding its pathophysiology and uncovering various metabolic pathways implicated in the disease process. This has culminated in the implementation of robust prevention programmes and the development of effective pharmacological agents, which have had a favourable impact on the management of T2DM in recent times. Despite these advances, the incidence and prevalence of T2DM continue to rise. Continuing research in improving efficacy, potency, delivery and reducing the adverse effect profile of currently available formulations is required to keep pace with this growing health challenge. Moreover, new metabolic pathways need to be targeted to produce novel pharmacotherapy to restore glucose homeostasis and address metabolic sequelae in patients with T2DM. We searched PubMed, MEDLINE, and Google Scholar databases for recently included agents and novel medication under development for treatment of T2DM. We discuss the pathophysiology of T2DM and review how the emerging anti-diabetic agents target the metabolic pathways involved. We also look at some of the limiting factors to developing new medication and the introduction of unique methods, including facilitating drug delivery to bypass some of these obstacles. However, despite the advances in the therapeutic options for the treatment of T2DM in recent years, the industry still lacks a curative agent.
Keywords: Type 2 diabetes mellitus, novel agents, treatment for type 2 diabetes mellitus
Introduction
Diabetes mellitus (DM) is a global epidemic posing a significant health threat to people around the world. According to the International Diabetes Federation (IDF) estimates in 2019, 463 million people aged 20–79 years suffered from DM worldwide, which is projected to rise to a staggering 700 million in the next 25 years1 (Figure 1). DM is the fastest growing health challenge of the 21st century, and its scale is such that 1 in 11 adults (20–79 years) have DM, bleeding $760 billion in funds, which is 10% of the worldwide health expenditure.2 DM confers a risk of early mortality, and in 2019 the IDF projected that 4.2 million adults would die as a result of DM and related complications, which, when put into perspective, is equivalent to one death every 8 s.2 T2DM accounts for approximately 90% of all DM cases, and the global exponential growth of this epidemic is mainly attributable to a sedentary lifestyle, obesity, an ageing populace, urbanization, and economic development.3,4
Figure 1.
A graphical description of the IDF-estimated number of adults living with diabetes globally since the year 2000 which is projected to rise to 700 million by 2045.1,2
Management of T2DM requires a patient-centred approach in bringing about lifestyle changes and optimizing glycaemic control using the available pharmacological options to preserve the quality of life and minimize complications.5,6 The Kumamoto7 and UK Prospective Diabetes Study (UKPDS)8,9 confirmed that intensive glycaemic control in patients with newly diagnosed T2DM significantly decreased rates of microvascular complications and, therefore, early and adequate treatment is of paramount importance. T2DM is a progressive condition, and to prevent long-term complications, improving and sustaining glycaemic control is vital. Timely treatment escalation can help achieve this; however, this is often delayed, and people remain with suboptimal glycaemic control for several years.10 Comorbid state, insulin resistance (IR), and beta-cell dysfunction render many available treatments, either inadequate or contra-indicated. An intricate scenario is further complicated when treatment choices have to be informed to avoid hypoglycaemia and weight gain.11
Due to the introduction of new oral and injectable pharmacological agents, which are potent and well tolerated, clinicians nowadays have more options for treating T2DM. However, based on the commonly encountered constraints mentioned above, there is a requirement for developing new agents which are effective individually and can be safely co-prescribed with the currently available array of treatment options. In addition to this, the medical community is longing for preparations that positively impact complications arising from T2DM and have little or no limitations to their use in patients with significant multiorgan comorbid states.
In this article, we discuss the pathophysiology of hyperglycaemia in T2DM, followed by recently included therapeutic agents and novel medication under development for the treatment of T2DM.
Methodology
We systematically searched PubMed, MEDLINE, and Google Scholar for original articles, review articles, systematic reviews, randomized control trials (RCTs), and meta-analysis published in English from 01 January 2011 to 05 March 2021. Relevant and important articles published before 2011 were also included. We developed a search string of medical subject headings (MeSH) including the terms ‘diabetes’ OR ‘non-insulin-dependent diabetes mellitus’ OR ‘hyperglycaemia’ OR ‘T2DM’, ‘insulin resistance’, ‘novel agents’ OR ‘future medications’ AND ‘updates’, ‘medical treatment’ OR ‘pharmacological treatment’ OR ‘therapy’, ‘novel delivery methods’ OR ‘administration’, ‘genome-wide association and type 2 diabetes’, ‘insulin pump systems’ AND ‘closed-loop pump systems’.
Pathophysiology of hyperglycaemia in T2DM
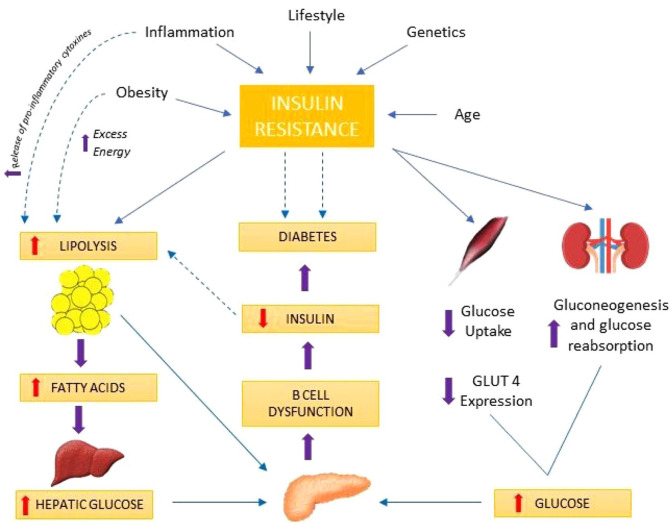
In contrast to type-1 diabetes mellitus (T1DM), where the driving force behind hyperglycaemia is autoimmune destruction of β-cells, T2DM results from β-cell dysfunction and IR. There is high insulin demand from the peripheral tissues due to IR resulting in β-cell expansion and hyperinsulinemia.12 As a result of this compensatory response from the β-cells, in the presence of a hyperglycaemic milieu, there is a steady loss of β-cell mass thought to occur from accentuated apoptosis.13–15 The development of T2DM is a continuum from a state of impaired glucose tolerance (IGT), where patients exhibit a very high level of IR and have lost an estimated 80% of their β-cell function to T2DM. To explain the pathophysiologic defects in T2DM, eight key collaborators have been described which include pancreatic β-cells (decreased insulin secretion), pancreatic α-cells (increased glucagon secretion), liver (increased hepatic glucose output), muscle (decreased glucose uptake), adipose tissue (increased lipolysis), kidney (increased glucose re-absorption), gut (decreased incretin effect), and the brain (impaired appetite regulation) which were collectively called the ominous octet.16 In DM, the complex system required to maintain euglycemia independent of glucose load and clearance fails17 (Figure 2) and the fascinating interplay between IR, impaired insulin secretion and loss of β-cell mass, have long been targeted with therapeutic agents to treat this condition.
Figure 2.
A brief illustrative explanation of molecular mechanisms responsible for insulin resistance in T2DM followed by a discussion on organ-specific contributions. Insulin resistance in the muscles; defective insulin signalling, glucose transport, glucose phosphorylation, glycogen synthesis, pyruvate dehydrogenase complex activity, and mitochondrial oxidative activity.16,18,19 Events in the liver; insulin resistance/deficiency, hyperglucagonaemia, enhanced glucagon sensitivity, and increased substrate (fatty acids, lactate, glycerol, and amino acids) delivery, leads to increased gluconeogenesis, which is responsible for the increased basal rate of glucose production and fasting hyperglycaemia.20–22 Renal contribution; renal insulin resistance and augmented renal gluconeogenesis contribute to fasting hyperglycaemia.23 Vascular endothelium; impaired vasodilation due to insulin resistance resulting in reduced insulin and glucose delivery.24 Finally, post-prandial hyperglycaemia ensues due to increased hepatic glucose output, muscle insulin resistance, reduced non-insulin-mediated glucose uptake, and excessive renal glucose re-absorption.25,26
Combined peptide injectables
By increasing β-cell insulin secretion, delaying gastric emptying, and reducing glucagon secretion, glucagon-like peptide-1 (GLP-1) analogues successfully target and suppress post-prandial hyperglycaemia in T2DM.27,28 On the contrary, basal insulin therapy targets fasting hyperglycaemia and theoretically, when combined, their complimentary action should result in significant improvement in glycaemic control. This materialized with the introduction of IDegLira (50 U insulin degludec and 1.8 mg liraglutide) and iGlarLixi (100 U insulin glargine and 50 μg lixisenatide) in a titratable fixed-ratio combination. The FDA approved both combination injections for clinical use in 2016. Results from the DUAL (Dual Action of liraglutide and Insulin Degludec in T2DM) clinical development programme demonstrated the efficacy and safety of iDegLira in 1393 adults with T2DM, who had inadequate glycaemic control on liraglutide or basal insulin alone. Switching to iDegLira resulted in a mean reduction in HbA1c up to 1.94%. Lixilan-O (ClinicalTrials.gov NCT02058147) compared iGlarLixi with insulin glargine or lixisenatide alone for treatment of insulin-naive patients on metformin29,30 while lixiLan-L (ClinicalTrials.gov NCT02058160) compared iGlarLixi with insulin glargine alone in patients already using basal insulin with or without oral hypoglycaemic agents.30,31 As evidenced by several studies,29,31–33 both IDegLira and iGlarLixi achieved more significant HbA1c reductions without increasing the risk of hypoglycaemia in patients with T2DM, compared to individual insulin or GLP-1 analogues. In addition to the described benefits, there is a lower risk of hypoglycaemia, weight gain, and gastrointestinal side effects.34,35 All these attributes make these injections suited for the management of T2DM and can be utilized at the treatment intensification stage of the management algorithm.
GLP-1 and GIP dual-receptor agonists
GIP is an incretin hormone like the much talked about GLP-1. Together, they are responsible for enhancing glucose-dependant insulin secretion from the pancreatic β-cells termed the incretin effect.36,37 Under normal physiological circumstances, the incretin effect is mainly driven by GIP.38,39 Developed by Eli Lilly, Tirzepatide (TZP), LY3298176, is a GLP-1 receptor and GIP receptor (GLP-1R/GIPR) dual agonist, which has achieved superior HbA1c and weight reductions compared to injectable semaglutide as an add-on to metformin in adults with T2DM in the phase-3 SURPASS-2 clinical trial (ClinicalTrials.gov NCT03987919). In this 40-week, multi-centre, randomized, parallel, open-label trial, participants taking weekly TZP 15 mg (highest dose) achieved an HbA1c reduction of 2.46% and weight loss of 13.1% compared to weekly semaglutide 1 mg. An estimated 51% of patients who received the higher TZP dose achieved an HbA1c of <5.7% (non-diabetic range) compared to 20% in the semaglutide arm.40 Previously in patients with T2DM, once weekly TZP at doses of 1, 5, 10, or 15 mg has been compared to once weekly dulaglutide 1.5 mg; in a double-blind phase-2 study. Patients were randomly assigned (1:1:1:1:1:1) to receive either once weekly subcutaneous (SC) TZP (1, 5, 10, or 15 mg), dulaglutide (1.5 mg), or a placebo for 26 weeks. The change in HbA1c with TZP at 26 weeks was −1.06% (1 mg), −1.73% (5 mg), −1.89% (10 mg), and −1.94% (15 mg) compared with −0.06% for placebo and −1.21%. Reassuringly, the change in HbA1c with TZP did not plateau. At 26 weeks, 33%–90% of patients treated with TZP achieved the HbA1c target <7% versus (vs) 52% with dulaglutide and 12% with placebo. At 26 weeks, changes in mean body weight ranged from −0.9 to −11.3 kg for TZP versus −0.4 kg for placebo versus −2.7 kg for dulaglutide. Gastrointestinal events including nausea, diarrhea, and vomiting were the most reported side effects, were dose dependent, mostly mild-moderate and self-limiting. There were no reports of severe hypoglycaemia.41 Although the mechanism of its action is not fully understood, studies suggest that TZP binds to the GIPR with the same affinity as native GIP while its affinity with the GLP-1R is five times lower than the endogenous molecule.42 As a result, TZP engages GIPR more than the GLP-1R, and the unique signalling properties at the GLP-1R may form the basis of its profound efficacy.43,44 GLP-1R/GIPR dual agonists (Table 1) have shown significantly better efficacy in terms of glycaemic control and weight loss compared to GLP-1R agonists with satisfactory tolerability and so will be a welcome addition to the therapeutic options in T2DM.
Table 1.
A list of recently developed GLP-1R/GIPR dual agonists.45.
| Drug | Developer | Route of administration | Status |
|---|---|---|---|
| LYS3298176 (TZP) | Eli Lilly | Once weekly SC injection | Phase 3 |
| NNCOO90-2746 | Novo Nordisk | Daily SC injection | Phase 2 |
| Eli Lilly | – | Preclinical | |
| ZP-I-98 | Zealand Pharma | – | Preclinical |
| ZP-DI-70 | Zealand Pharma | Preclinical | |
| SAR438335 | Sanofi | – | Discontinued in phase-2 clinical trials. |
GLP-1R, GLP-1 receptor; GIPR, GIP receptor.
Sodium-glucose co-transporter and dual SGLT1 and SGLT2 inhibitors
Sodium-glucose co-transporters (SGLTs) regulate sodium and glucose transport across cell membranes. Six SGLT isoforms have been identified in human beings;46 however, only SGLT1 and SGLT2 inhibition has translated into pharmacotherapy in T2DM so far. SGLT1 is a high-affinity but low-capacity transporter,47 which mediates the absorption of glucose in the small intestine and accounts for the re-absorption of approximately 3% of the filtered glucose from the S3 segment of the proximal convulated tubule (PCT) in the nephrons.48,49 In contrast, SGLT2 is a low-affinity but high-capacity transporter,47 which is responsible for the bulk (>90%)48,49 of glucose re-absorption from the S1 and S2 segments of the PCT.50,51 Targeting the inhibition of SGLT2 could lead to glucosuria and improved glycemia.
In 2012, dapagliflozin was approved by the European Medicines Agency (EMA) for clinical use, marking the first approval of a drug from this class anywhere around the world.52 The following year, canagliflozin was approved by the Food and Drug Administration (FDA) for clinical use in the United States,53 and thereafter several agents have been approved and marketed around the world. These approvals came on the back of large-scale clinical trials, where, in addition to improving glycaemic control, SGLT2 inhibitors led to weight loss and demonstrated cardio-renal benefits. For instance, not only did empagliflozin reduce the 3-point major adverse cardiac events (cardiovascular death, non-fatal myocardial infarction, and stroke), it was shown to reduce cardiovascular death (by 38%), hospitalization with heart failure (by 35%), and all-cause mortality (by 32%) aswell.54–57 These remarkable results have led to a rapid uptake of this class in the management of T2DM.
Recent pharmacological research suggested that combined SGLT1 and SGLT2 inhibition resulted in significant post-prandial glucose reduction, the elevation of endogenous GLP-1, and urinary glucose excretion. First-in-class of dual SGLT1 and SGLT2 inhibitors, sotagliflozin has been developed.58 It has 20-fold higher selectivity for SGLT2 than SGLT1, is as effective as dapagliflozin and canagliflozin in inhibiting SGLT2 but is greater than 10-fold more potent than them in inhibiting SGLT1.59–61 From phase-2 and -3 clinical trials, sotagliflozin has demonstrated improved glycaemic control, reduced post-prandial glucose, reduced insulin requirements, appetite suppression, and weight loss in patients with type-162 and type-2 diabetes.63,64
Encouraging results from phase 3, inTandem 1–3 trials65 among others,62 have led to its approval in the EU as an adjunct to insulin in patients with T1DM with a body mass index (BMI) ⩾ 27 kg/m2 who have failed to achieve adequate glycaemic control despite optimal insulin therapy.66 A higher incidence of ketoacidosis with sotagliflozin (3%) compared to placebo (0.6%) was seen in the inTandem study which is of some concern,61,65 an effect also seen with dapagliflozin in the past.67 Goldenberg and colleagues have developed the ‘STOP DKA Protocol’, a practical tool that may potentially help in reducing this risk in clinical pratice68 in addition to appropriate patient selection and down titration of basal insulin. In the SOLOIST-WHF clinical trial (ClinicalTrials.gov NCT03521934), sotagliflozin therapy resulted in a significantly lower total number of deaths from cardiovascular causes, hospitalizations, and urgent visits for heart failure compared to placebo in patients with T2DM.69 Moreover, in the SCORED clinical trial (ClinicalTrials.gov NCT03315143), treatment with sotagliflozin resulted in a similar effect in patients with T2DM and chronic kidney disease with or without albuminuria.70 Another dual SGLT1 and SGLT2 inhibitor, licogliflozin has been shown to exert a favourable metabolic effect leading to weight loss, reduced PPG excursion, and elevation of total GLP-1 levels in patients with obesity and T2DM.71,72 Based on the overwhelmingly encouraging results from sotagliflozin, it seems that approval for use in T2DM is not far away. At the same time, other agents from this novel class can emerge as clinically beneficial entities for the treatment of T2DM in the years to come.
Peroxisome proliferator–activated receptor α (alpha)/γ (gamma)/δ (delta) pan-agonists
Peroxisome proliferator–activated receptors (PPARs) are ligand-activated transcription factors of nuclear hormone receptors. Their subtypes include PPAR-α (liver, muscle, and heart); PPAR-γ (adipose tissue and vascular endothelial cells), and PPAR-δ (widespread whole-body distribution) which play an integral part in energy metabolism, where PPAR- δ regulates energy expenditure while PPAR-γ mediates its storage.73–75 Thiazolidinediones (TZDs) are anti-diabetic agents in clinical use since 1997.76 They are PPAR-γ agonists, which enhance insulin sensitivity by increasing adiponectin, GLUT4 expression and oppose the effect of tumour necrosis factor (TNF)-alpha in adipocytes. These actions result in reduced hepatic gluconeogenesis and increase insulin-dependent glucose uptake in muscle and fat.77 Chiglitazar, a novel PPARα/γ/δ pan-agonist, has been investigated in phase-3 multi-center, randomized, double-blind, and placebo-controlled trials (ClinicalTrials.gov NCT02121717),78 as well as sitagliptin, controlled (ClinicalTrials.gov NCT02173457)79 trials in patients with T2DM with insufficient glycaemic control despite diet and exercise. Compared to placebo, at 24 weeks, both doses of chiglitazar were superior to placebo in HbA1c reduction and the effects were sustained for 52 weeks. The mean change in HbA1c from baseline was −0.45% ± 1.22% with placebo, −1.30% ± 1.07% with chiglitazar 32 mg, and −1.52% ± 1.19% with chiglitazar 48 mg. Although hypoglycaemia, weight gain, and oedema were relatively more common with chiglitazar, the overall adverse events were comparable across all groups with no major safety concerns.80 MHY2013 and IVA337 are other examples of PPAR pan-agonists that have been shown to suppress inflammation and hepatic lipid accumulation and may emerge as useful candidates for treating non-alcoholic fatty liver disease (NAFLD) in the future.81,82
GLIMINS
A novel oral anti-diabetic drug, imeglimin is a tetrahydrotriazine compound that is the first member of the ‘glimins’ class of agents and has been investigated in many landmark clinical trials.83 In patients with T2DM, imeglimin has shown sustained improvement in hyperglycaemia without disabling hypoglycaemia and was not associated with a concerning side-effect profile.84,85 Its unique mechanism of action (MOA) (Figure 3) not only increases glucose-dependant insulin secretion but also reduces IR.86,87 It acts by inhibiting the process of oxidative phosphorylation in the mitochondria of aerobic cells which in turn leads to favourable metabolic effects.86 Imeglimin counteracts a variety of metabolic disruptions at play in T2DM. It accelerates the phosphorylation of Akt (protein kinase B) leading to enhanced insulin signalling88 and has also been linked to the induction of insulin sensitivity in the β-cells.89 In addition to this, imeglimin reduces hepatic gluconeogenesis,86,90 is protective against β-cell death, increases the β-cell mass and improves glucose-induced insulin secretion (GSIS) from islets of Langerhan cells in the pancreas.87,89,91 Interestingly, Lachaux et al.92 reported a reduction in left ventricle (LV) end-diastolic pressure and increased LV tissue perfusion with imeglimin treatment in Zucker fa/fa rats. These findings suggest that imeglimin is potentially cardioprotective in addition to its anti-diabetic properties and thus once approved for commercial use, will be a useful inclusion to the arsenal against T2DM.
Figure 3.
Potential mechanisms via which glucose homeostasis is improved by imeglimin therapy.86
A systematic review and meta-analysis of the available evidence from Crabtree et al.93 showed that imeglimin therapy, at a dose of 1500 mg twice a day, lead to a reduction in HbA1c (0.63%) and fasting plasma glucose (FPG) (0.52 mmol/L) in patients with T2DM when used alone or in combination with metformin or sitagliptin. These results are similar to those reported for other classes of glucose-lowering therapies inducted in clinical practice in recent times. Based on the available evidence, imeglimin use is not related to any significant adverse effects and is overall well tolerated.94 Based on its potent anti-diabetic effect as monotherapy and as an adjunctive treatment,93 the lack of major adverse effects and potential cardioprotection, imeglimin is poised to occupy a crucial role in the future of T2DM pharmacotherapy.
Therapies targeting the glucagon receptor
GLP-1R and glucagon receptor dual agonists
Around 90% of patients with T2DM are overweight, making it an important risk factor for its development.95 GLP-1R agonists improve glycaemic control and reduce weight in patients with T2DM, making them an effective pharmacological agent.96 Glucagon is a peptide hormone, produced by the α-cells of the islets of Langerhans. Acting via the glucagon receptor (GCGR), it increases glucose concentration in the bloodstream via gluconeogenesis and glycogenolysis, increases lipolysis, improves energy expenditure and can activate the GLP-1R leading to a glucose-lowering effect.97,98 In diet-induced obese (DIO) mice, Pocai et al. demonstrated that GLP-1 and GCGR dual agonism lead to superior weight loss compared to GLP-1R agonists alone while the lipid-lowering and antihyperglycemic activity of both classes were comparable. Chronic administration of GLP-1/GCGR dual agonist leads to a more pronounced improvement in plasma metabolic parameters (insulin, leptin, and adiponectin) compared to GLP-1R agonist alone.99 Oxyntomodulin, JNJ-64565111, and MEDI-0382100,101 are examples of some agents being developed in this emerging class with some encouraging results being reported with MEDI-0382 recently.102
GCGR antagonist
Glucagon and insulin exhibit opposite metabolic effects. Glucagon regulates glucose homeostasis through hepatic glucose production via gluconeogenesis and glycogenolysis. Glucagon receptor antagonists (GRAs) are a novel class of anti-hyperglycaemic agents designed to target hyperglycaemia in diabetes by blocking glucagon action through its receptor.103,104 GRAs differ chemically, and on this basis, can be classified into small molecule, monocloncal antibody, and anti-sense oligonucleotide agents. A variety of GRAs studied in clinical trials have shown to improve glycaemic control in terms of HbA1c, but clinically significant adverse effects have precluded their use as therapeutic options in clinical practice. Weight gain, elevated hepatic enzymes, increase in low-density-lipoprotein-C (LDL-C) levels and liver fat content, rise in systolic blood pressure, and α-cell hyperplasia are some of the concerns reported with GRA use.105,106 Efforts are underway to minimize side effects of GRAs,107 and many agents are currently being developed from this class101(Table 2).
Table 2.
Examples of GRAs in various stages of development.
| Chemical nature | Name | Developer | Status |
|---|---|---|---|
| Small molecules | LGD 6972108,109 | Metavant Sciences and Ligand Pharmaceuticals | Phase 2 |
| Monoclonal antibodies | PF 06293620110 | Pfizer | Phase 1 |
| Anti-sense oligonucleotide agents | IONIS-GCGRRx111 | Isis Pharmaceuticals and Ionis Pharmaceuticals | Phase 2 |
GRA, glucagon receptor antagonists.
GLP-1R/GCGR/GIPR triple agonists
Following Roux-en-Y gastric bypass (RYGBP) surgery, it was noted that the endogenous GLP-1, GIP, and glucagon (Incretins) secretion was increased. It was theorized that these incretins’ synergistic metabolic action could lead to weight reduction.112,113 HM15211, currently in phase-1 clinical trials, is a weekly SC GLP-1R/GCGR/GIPR tri-agonist peptide being developed by Hanmi Pharma to treat obesity, NAFLD, and T2DM. It is a modified human glucagon molecule formed by conjugating to human immunoglobulin G Fc fragment through PEG linkage.
To assess the efficacy in treatment of obesity, HM15211 was compared with liraglutide in DIO mice, where early results have shown a ~three-fold reduction in weight and increased energy expenditure with HM15211 compared to liraglutide. Moreover, in methionine choline–deficient (MCD) mice, HM15211 administration resulted in reduction in hepatic TGs (−82.6% vs vehicle) and thiobarbituric acid reactive substances (TBARS) (−60.7% vs vehicle). A concurrent fall in serum alanine-aminotransferase (ALT) and bilirubin was also observed. HM15211 resulted in increased intracellular cAMP through GLP-1R, GCGR and GIPR. Its robust glucagon activity caused weight loss and improved lipid profiles by accelerated lipid oxidation. GLP-1R and GIP served to neutralize the glucagon-induced hyperglycaemia.101,114,115 By affecting weight loss and improving lipid profiles, this class could provide an attractive therapeutic option for treating obesity and related diseases, for example, T2DM and NAFLD. NN9423 (NNC9204-1706) by Novo Nordisk and GGG tri-agonist by Eli Lilly are examples of some other GLP-1R/GCGR/GIPR triple agonists which are currently under development.101
G-protein-coupled receptor ligands
A variety of mediators acting through G-protein-coupled receptors (GPCRs) can enhance and inhibit GSIS.116 Gs, Gi, Gq, and G12 are involved in signalling pathways modulating insulin secretion. Gs and Gq stimulate insulin release while Gi inhibits it.117
GPR119 agonists
GPR119 is a Class-A orphan GPCR found in the pancreas and the gastrointestinal tract and can regulate insulin and incretin release. Its activation leads to the intracellular accumulation of cAMP, resulting in GSIS from the pancreatic β-cells and incretin release from the gut.118,119 Due to the dual effect of GSIS and incretin release coupled with a low risk of hypoglycaemia, these agents have drawn considerable interest as a therapeutic entity in the management of T2DM. Unfortunately, no synthetic GPR119 ligand has been approved for treatment, nor has any passed beyond phase-2 clinical studies.120,121 DS-8500 is a GPR119 agonist being developed by Daiichi Sankyo Company and is currently in phase-2 clinical trials.122
Free fatty acid receptor
Several agents have been identified to have anti-hyperglycaemic action by stimulating or blocking the free fatty acid receptor (FFAR). Among the FFARs, FFAR1 (GPR40), FFAR2 (GPR43), FFAR3 (GPR41), and FFAR4 (GPR120) have emerged as anti-diabetic targets.123 Long-chain free fatty acids (FFAs) activate FFAR1 and FFAR4, and agonists of these two receptors have been proven to enhance insulin and incretin release.124 Short-chain FFA activates FFAR2 and FFAR3 and as opposed to FFAR1 and 4, antagonism of these receptors has shown anti-diabetic potential.125 Mice studies have demonstrated that FFAR2 and FFAR3 antagonists can enhance insulin secretion and glucose tolerance.126 Agents targeting the FFAR are still in the early stages of development, and it remains to be seen whether an effective and safe drug can be delivered and inducted into clinical practice from this class in the future. P1736 and P11187 by Piramal Enterprises and LY2922470 by Eli Lilly are examples of recently studied FFAR1 agonists.123,127
Takeda G-protein-coupled receptor 5 (TGR5 agonists)
Discovered in 2002, TGR5 (also known as GPBAR1 and GPR131) is a bile acid–specific receptor found in the gastrointestinal tract, pancreas, liver, gallbladder, and adipose tissue.128 A lot of research has gone into establishing a link between bile acids and glucose metabolism, with numerous studies showing bile acid–mediated TGR5 activation resulting in impactful gluco-metabolic sequelae including GLP-1, insulin, and glucagon release.129–132 The ability to stimulate GLP-1 secretion133 has attracted considerable interest; however, this effect may be limited as TGR5 receptors are predominantly present in L-cells’ basolateral membrane, leading to insufficient luminal activation required to produce the desired metabolic impact.134,135 Studies in rodents suggest an important role for TGR5 in GLP-1 secretion,129 and therefore, it is an important target for the development of pharmacotherapy directed against metabolic disease, including T2DM.136 However, data from animal studies showed significant safety concerns with TGR5 agonists treatment, foremost of which were gall bladder dilation, pancreatitis, and hepatic necrosis. Human studies have reported no beneficial effects on GLP-1 secretion or plasma glucose levels, and as such, currently, a viable pharmacological option using this concept is not on the horizon.137,138 SB 756050, originated by GlasgowSmithKline, was TGR5 agonist studied for the treatment of T2DM. Fifty-one participants were randomized to receive either placebo or one of four doses of SB-756050 for 6 days. A single 100 mg dose of sitagliptin was co-administered on Day 6 to all participants. It was well tolerated, however, exhibited variable pharmacodynamic effects with rise in glucose at the two lowest doses and no fall in glucose at the two highest doses. The glucose effects of SB-756050 and sitagliptin were comparable to those of sitagliptin alone (ClinicalTrials.gov NCT00733577).139 Studies on SB-756050 were eventually discontinued in Phase-2 clinical trials.140
Melatonin receptor
Peripheral and central IR, glucose intolerance and reduced expression of the GLUT4 gene have been observed in pinealectomized animals, which improved with melatonin administration.141,142 Furthermore, a reduction in melatonin formation has been reported in animal models with T2DM.143,144 MT1 and MT2 are isoforms of melatonin receptors expressed on hepatocytes and pancreatic cells, forming part of the glucose regulation pathway. Various studies demonstrate that melatonin-mediated activation of these receptors improves insulin sensitivity and suppress gluconeogenesis.145,146 In the past, MT1 and MT2 knockout mice have been shown to have systemic IR and reduced insulin sensitivity, with the latter exhibiting increased insulin release.147–149 Ramelteon is a selective melatonin receptor (MT1 and MT2) agonist, approved by the FDA for the treatment of insomnia;150 however, as noted by Tsunoda et al.,151 its use did not improve glycaemic control in patients with T2DM suffering from insomnia.
In contrast, piromelatine, a novel melatonin receptor agonist, has been shown to exert a protective effect on insulin sensitivity and lipid metabolism in sleep-deprived rats.152 Melatonin receptor is a potential target for developing therapies to address hyperglycaemia. However, substantial research is required in this class to produce a clinically relevant agent in the future. Shiraz University of Medical Sciences is conducting a clinical study on Melatonin’s Effects on the Treatment of Diabetes Mellitus (METOD), which is currently in the early phase-1 stage.153
Enzymes, hormones, and receptors as therapeutic targets
Protein tyrosine phosphatase-1B inhibitor
Protein tyrosine phosphatases (PTPs) lead to the inactivation of the insulin receptor via dephosphorylation. PTP-1B down-regulates insulin and leptin pathway and therefore has been seen as a therapeutic target for the treatment of diabetes and obesity.154,155 PTP-1B-deficient mice were observed to be sensitive to insulin and had a degree of resistance to weight gain.156,157 Selective, reversible, and high-affinity PTP-1B inhibitors have been tested in animal models, but being highly charged molecules, they could not be developed into medicinal products.158–160 KQ-791, developed by Kaneq Bioscience, has been studied in a phase-1 interventional randomized placebo control trial for treatment of T2DM (ClinicalTrials.gov NCT02445911). The study consisted of 81 participants which were divided into four groups based on the regimen of KQ-791 they receive over a period of 4 weeks. Group 1 (n = 20) received a loading dose of 100 mg on day 1, followed by single 50 mg doses on days 8, 15, 22, and 29. Group 2 (n = 20) received a loading dose of 250 mg on day 1, followed by a daily dose of 25 mg for 28 days and group 3 (n = 21) received a loading dose of 1500 mg on day 1, followed by a daily dose of 150 mg for 28 days. Group 4 (n = 20) received multiple ascending doses matching KQ-791 dose (placebo). The primary outcome measure was the difference in change in fasting blood glucose between KQ-791 and placebo from baseline to day 29. The results suggested superior improvement in fasting blood glucose in group 1 −3.28 ± 22.99 mg/dL and group 2 −3.93 ± 30.63 mg/dL compared to group 3 0.67 ± 30.82 and placebo 3.17 ± 23.17. No serious adverse event (SAE) was recorded with any intervention group.161
Human pro-islet peptide
DM is a chronic illness and is characterized by a lack of functioning pancreatic β-cells at advanced stages. Increasing β-cell numbers to alleviate DM is the logical answer; however, this is cumbersome to deliver in clinical practice. Transplantation of donor islets has been explored but has been limited by insufficient donors. Where successful islet transplantation has been achieved, patients have been plagued with post-transplantation sequelae, including rejection and adverse effects of immunosuppressive therapies.162–166 The human pro-islet peptide (HIP) is a 14-amino acid, biologically active fragment encoded by the human REG3a gene with the ability to increase β-cell mass whereby improving glycaemic control.167 In streptozotocin-induced diabetic mice, Jiang et al.168 have demonstrated that HIP promotes differentiation of human foetus–derived pancreatic progenitor cells (HFPPCs) into insulin-secreting cells leading to a significant reduction in hyperglycaemia. Evidence from this landmark effort from Jiang et al.168 suggests that ex vivo HIP-treated HFPPCs can differentiate into functional β-cells and can potentially cure diabetes in their recipients. Phase-1 clinical trials with BTI-410 (first agent in the class of HIPs) established good safety and tolerability in patients with T2DM with promising results. Boston therapeutics have now planned two 90-day phase-2 clinical trials for the study of BTI-410; one 36 patient study in immunocompromised T1DM patients post–renal transplant and another 120 patient study in otherwise healthy T2DM patients, marking the beginning of an exciting prospect.169
Fibroblast growth factor 21 analogues
Fibroblast growth factors (FGFs) are signalling molecules responsible for wide-ranging functions from development and regeneration to maintaining metabolic homeostasis.170 FGF-21 is an atypical member of this family, produced from the liver; it is involved in regulating glucose and lipid metabolism.171 Elevated FGF-21 levels in obese animals have been observed to reduce hyperglycaemia, body weight, and lipid concentration.172 Recombinant FGF-21 also demonstrates a similar effect in various diabetic animal models.173–175 Pegbelfermin (BMS-986036) is a recombinant PEGylated FGF-21 that is well tolerated and significantly reduces fat fraction in patients with non-alcoholic steatohepatitis.176 A randomized, double-blind, placebo-controlled trial was carried out in which patients with T2DM and obesity (BMI 30–50 kg/m2) received either SC pegbelfermin (n = 96) at doses of 1, 5, or 20 mg daily or 20 mg weekly or placebo (n = 24) for a duration of 12 weeks (ClinicalTrials.gov NCT02097277). At 12 weeks, pegbelfermin therapy did not impact HbA1c concentrations compared to placebo; however, significant differences (p < 0.05) versus placebo were noted for a reduction in FPG (20 mg weekly) and an increase in whole-body insulin sensitivity (Matsuda index; 20 mg daily). In addition, significant improvements were observed in high-density lipoproteins (HDLs) and TG for the 20 mg daily regime versus placebo. Injection site bruising (5%) and reaction (4%) together with diarrhoea were among the commonly reported adverse events.177 Based on these results, pegbelfermin warrants further investigation in evaluation of its efficacy in obesity-induced metabolic conditions.
Adenosine monophosphate analogues
Adenosine monophosphate (AMP) predominantly activates adenosine monophosphate–activated protein kinase (AMPK). Once activated, AMPK exhibits an insulin-sensitizing effect, reducing hepatic gluconeogenesis and lipogenesis via negative regulation of specific genes178 while promoting peripheral glucose uptake and lipid oxidation in skeletal muscles. Low levels of AMPK have been noted in patients with diabetes,179 and its activation is one of the cellular mechanisms through which metformin exerts its anti-diabetic effect.180,181 Novel agents which can activate AMPK or serve as AMP analogues have been explored as potential therapeutic modalities in T2DM. First in class, AMPK activator, O304, is a PAN-AMPK activator studied in a proof-of-concept phase-2(a), single-centre, randomized, parallel-group, double-blinded, placebo-controlled trial in patients with T2DM on metformin (TELLUS). Sixty-five patients with T2DM were studied for 28 days and O304 administration resulted in a mean absolute reduction in FPG at day 28 compared with day 1 of −0.60 mM (p = 0.010) compared to −0.10 mM in the placebo group. At day 28, O304 use resulted in a mean absolute reduction in systolic blood pressure (BP) by 5.8 mmHg (p = 0.030) and diastolic BP by −3.8 mmHg (p = 0.009). In addition to these findings, O304 use resulted in an increase in microvascular perfusion as well. These beneficial effects may allow it to play an important role in treating patient with T2DM complicated with cardiovascular disease.182,183
Glucokinase activators
Glucokinase (GK), also known as hexokinase type-IV, is part of the hexokinase enzyme family. Mainly expressed in the pancreas, liver, brain, and gastrointestinal tract,184 it facilitates phosphorylation of glucose to glucose-6-phosphate.185,186 It regulates GSIS, hepatic glucose uptake and glycogenesis, thus regulating whole-body glucose homeostasis.187,188 Based on the characteristics mentioned above, agents activating GK have a therapeutic potential in T2DM.189 Numerous GK activators developed thus far have shown significant glucose-lowering capabilities. However, a concomitant increase in plasma (TGs) and loss of efficacy with continued administration meant that they could not be used as therapeutic entities.190–192 TMG-123, a novel GK activator, has been shown to overcome these limitations and, in animal studies, has demonstrated glucose-lowering effects without affecting hepatic and plasma TG. As the plasma insulin levels were not increased, it is likely that TMG-123 mainly acts in the liver.193 Although it is a viable candidate for development as a new treatment for T2DM, its safety needs to be evaluated further as Kobayashi and colleagues have shown that TMG-123 causes long-lasting hypoglycaemia and irreversibly impairs spermatogenesis in rats.194 TTP-399 is another GK activator being investigated as a potential therapy in both T1DM and T2DM.195
Sirtuins
Sirtuins (SIRT1-SIRT7) are a family of NAD+-dependent deacetylases which modulate a range of metabolic processes together with age-related conditions, including obesity and T2DM.196,197 SIRT1 and SIRT6 have been extensively studied and have been identified to have a role in maintaining insulin sensitivity.198,199 In the pancreatic β-cells, overexpression of SIRT1 was noted to augment insulin secretion, leading to improved glucose tolerability.200 And by inhibiting PTP-1B (which blocks the insulin receptor), SIRT1 enhanced insulin sensitivity.156,201 Metformin is recommended as the first-line treatment for T2DM202 and has been shown to directly activate SIRT1,203 among various other recognized metabolic effects. This lends credence to the potential usefulness of SIRT1 activating agents in the treatment of T2DM. SIRT2, SIRT3, and SIRT6 also sustain insulin sensitivity, whereas SIRT4 and SIRT7 downregulate insulin secretion.204 Resveratrol, a polyphenol found in grapes, has been shown to activate SIRT1.205 It has been shown to have a positive impact on glucose homeostasis in rodents,206–208 and several studies have reported improvements in insulin sensitivity, glycaemic control, and other metabolic parameters in patients with T2DM.209–211 Jeyaraman et al.212 reviewed RCTs comparing the effects of oral resveratrol with placebo, no treatment, other anti-diabetic medications, or diet or exercise, in adults with a diagnosis of T2DM and found that the available research was insufficient for evaluation of safety and potency of resveratrol therapy for treating T2DM. Recently, De Ligt et al. studied the effects of resveratrol 150 mg daily (n = 20) compared to placebo (n = 21), in a randomized, double-blind, placebo-controlled trial over 6 months in overweight participants with pre-diabetes (BMI 27–35 kg/m2) (ClinicalTrials.gov NCT02565979). At 6 months, although treatment with resveratrol did not affect insulin sensitivity, there was a significant (p = 0.007) difference in the post-intervention HbA1c between the two groups with the adjusted means showing a lower post-intervention HbA1c in the resveratrol group ((35.8 ± 0.43 mmol/mol) compared to placebo (37.6 ± 0.44 mmol/mol).213 These results point towards a positive impact of resveratrol on glycaemic control; however, despite rigorous research, a clinically safe and effective SIRT activator for treating T2DM is yet to be found.214–216
11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) inhibitors
11β-HSD1 is a reductive nicotinamide adenine dinucleotide phosphate (NADPH)-dependent enzyme, predominantly expressed in the liver, adipose tissue, gonads, and brain and is responsible for the formation of cortisol from its inactive metabolite, cortisone.217,218 Cortisol regulates energy homeostasis (liver and adipose tissue) and has been well characterized to impair peripheral glucose uptake and increase hepatic IR.219 Stimson and colleagues have shown that the whole body 11β-HSD1 activity is increased in obese men with T2DM,220 making the inhibition of 11β-HSD1 a target for treating T2DM, especially in those with high fasting blood glucose as shown by Shukla and colleagues.221 Several compounds have entered clinical trials and have demonstrated modest improvement in glycaemic control, together with favourable changes in some metabolic syndrome parameters.222,223 11β-HSD1 inhibition will reduce the levels of circulating cortisol and, due to a lack of negative feedback, will increase levels of adrenocorticotropic hormone (ACTH). This can result in adrenal hypertrophy, accumulation of adrenal androgen precursors and depression.224,225 These unfavourable sequelae must be addressed while ensuring significant clinical benefit before a medicinal product from this class can be introduced into clinical practice. BI 135585, a selective 11β-HSD1 inhibitor, was evaluated in an open-label administration of BI 135585 200 mg as a single dose in nine healthy volunteers (ClinicalTrials.gov NCT01652742) and in a multiple-dose study with randomized, double-blind administration of BI 135585 5, 12.5, 25, 50, 100, and 200 mg or placebo once daily in 72 participants with T2DM over a period of 2 weeks (ClinicalTrials.gov NCT01282970). It was found to be safe and was well tolerated; however, adrenocorticotropic hormone was noted to be slightly raised but within the normal range. Serum cortisol levels remained unchanged and increased total urinary corticoid excretion was noted.226 While these are positive results, its safety profile and therapeutic potency need to be investigated in further trials over a longer period of time.
Insulin
For the management of T2DM, insulin therapy is initiated generally as a last resort when patients fail to achieve their glycaemic targets with two or more oral hypoglycaemic agents (OHAs) or with non-insulin injectable medication. Various insulin preparations are now available in clinical practice, ranging from rapid to long-acting preparations.227 As with other areas in the management of T2DM, insulin therapy has also been a focus of research, and work has been done to devise insulin preparations with alternate delivery methods. Compounds have been developed with a longer duration of action to reduce the frequency of injections.
Oral insulin
Following its endogenous release from the β-cells in the pancreas, insulin is transferred to the liver, where it undergoes degradation from the hepatic first-pass metabolism resulting in a concentration gradient between the hepatic portal and peripheral systemic circulation.228 As parenteral insulin preparations are directly delivered to the peripheral circulation, they fail to achieve the described concentration gradient resulting in the reversal of normal physiology. Therefore, when injected, insulin reaches the liver at a lower concentration, and insulin-treated diabetic patients tend to develop weight gain and hypoglycaemia as a manifestation of peripheral hyperinsulinaemia.229 Oral insulin, on the contrary, mimics normal physiology leading to better glycaemic regulation. Once absorbed from the gut, insulin is delivered to the liver via the portal circulation building a high portosystemic gradient. Reduced levels of systemic insulin alleviate hypoglycaemia and issues with weight gain.230–233 As a peptide hormone, insulin is susceptible to degradation from acid hydrolysis in the stomach and proteolytic cleavage in the intestine. These factors have made the production of a viable oral insulin preparation challenging.232,234 There is an emphasis on protecting and enhancing peptide drug absorption. To achieve this, researchers have employed innovative measures such as mucoadhesive polymers, micronization, absorption enhancers, protease inhibitors, and particulate carrier systems.233,235,236 A recent randomized, placebo-controlled, phase-2b trial with the novel oral human insulin, ORMD-0801, has shown promising results in subjects with poorly controlled T2DM. ORMD-0801 achieved a significant reduction in HbA1c without an increase in the frequency of hypoglycaemia or weight.237 It can be the first commercial oral insulin preparation and has currently entered the FDA phase-3 clinical trials.
Oral devices comprising mucoadhesive patches and enterically coated capsules are another insulin delivery technique on the horizon.238 Gupta et al. developed a mucoadhesive patch of Carbopol934, pectin, sodium carboxymethyl cellulose in 1:1:2 ratio. When loaded with bovine insulin, human insulin, and exenatide to compare the efficacies when administered through the oral route, the results indicated that mucoadhesive patches increased oral insulin absorption. They concluded that the release of the insulin from the patches was time-dependent, and the insulin loaded into the delivery system was wholly released from the patch within 5 h of administration. The results are an encouraging step towards the development of another non-invasive anti-diabetic therapeutic option in the form of sustained-release mucoadhesive patches promoting oral peptide delivery to improve patient compliance and drug adherence.239,240
Transdermal insulin
The use of the transdermal route for drug delivery has been around for a long time. In terms of insulin delivery, the transdermal route alleviates the discomfort and inconvenience of regular SC injections. This is especially true for patients on multiple daily injections (MDIs) of insulin. It bolsters compliance among patients while ensuring controlled insulin release over a while.241 Lack of adequate permeability of the stratum corneum is the main limiting factor with this route.242,243 Over the years, researchers have been working on methods to bypass this obstacle, leading to discovering various novel techniques to achieve this.244–246 Abdul Ahad et al.247 have summarized various transdermal insulin delivery techniques, including microneedle (MN), a chemical permeation enhancer, patches, sonophoresis, iontophoresis, nanoparticles, and microemulsions, among others. In this article, we will focus on insulin patches as a novel insulin delivery method.
Subcutaneous insulin patch-pump system
Using novel technologies, the insulin patch-pump devices provide accurate and flexible insulin delivery. Omnipod, a full-featured device available worldwide and V-GoTM, a simple specific patch pump available in the US/Europe,248 can be seen as a sophisticated modification of the already widely used continuous SC Insulin infusion (CSII) systems (Table 3 shows examples of fully featured and simple mechanical patch pumps). Patch pumps are smaller, flexible, discreet, without tubing and cheaper, making them more attractive than CSII systems.249 These devices do not use tubing, thus improving the user experience. The Omnipod system comprises an insulin reservoir and a handheld Personal Diabetes Manager (PDM). Using wireless technology, PDM directs the delivery of continuous basal insulin from the reservoir via a discreet needle. The V-GoTM device, on the other hand, can deliver basal and bolus insulin over 24 h and is suitable for patients on MDI insulin regimes. It replaces the insulin pens, low in price without an upfront cost and is disposable.248 Using an adapter, the device is loaded with rapid-acting insulin analogues and then attached to the skin with a hypoallergenic adhesive strip. The basal rates are pre-set and are available as 20, 30, or 40 units of insulin per 24 h and each one also allows for 36 units of bolus insulin daily. Mader et al. evaluated PAQ®, which is a simple specific patch pump providing set basal and on demand insulin delivery. The study included 28 adults with T2DM with HbA1c ⩾53 and ⩽97 mmol/mol while treated with ⩾2 insulin injections/day. Participants were investigated in three phases: baseline (on usual insulin injections), transition, and a PAQ treatment phase. After 12 weeks of PAQ wear, HbA1c improved significantly (HbA1c –16 ± 9 mmol/mol (p < 0.0001)) while the body weight remained stable with no episodes of severe hypoglycaemia (ClinicalTrials.gov NCT02158078 & NCT02419859).250 The patch-pump targets a large cohort within the DM population however, for patients with T2DM who are on simple insulin regimes, the simple specific patch pump is an exciting prospect as in clinical studies they have demonstrated improved glycaemic control, user satisfaction, and were cost-effective compared to MDI insulin regimes.251,252
Table 3.
Examples of full-featured and simple mechanical patch pumps.248
| Full-featured mechanical patch pumps | Simplified mechanical patch pump systems |
|---|---|
| Omnipod | V- GoTM |
| Cellnovo | PAQ |
| JewelPump | OneTouch Via |
Closed-loop pump systems
Over the last few years, particularly in the domain of blood glucose monitoring and insulin delivery, advances in technology have revolutionized diabetes care. Introduction of closed-loop (CL) pump systems are one such technological marvel, where continuous glucose monitoring (CGM) and an insulin pump function in a unified manner, achieving automation in glucose-responsive insulin delivery by the help of a computerized algorithm which adjusts basal insulin in response to data obtained from the CGM helping to maintain glucose levels in the target range. For mealtime insulin delivery, users input their carbohydrate counts into the algorithm which calculates the required insulin dose and signals the pump to deliver the dose (single hormone system). Dual hormone CL pump systems can also deliver glucagon in a glucose-responsive manner, and therefore, the CL pump systems reduce the user burden by automatically adjusting insulin delivery based on sensed glucose levels (CGM).
With the hybrid closed-loop (HCL) systems, algorithm-mediated and user-initiated insulin delivery co-exists as basal insulin delivery is adjusted automatically; however, meal time boluses are required to be programmed manually.253 The HCL system can make regular self-directed adjustments to the basal insulin delivery rate which prevents large fluctuations in blood glucose, and overall, these novel insulin delivery systems have resulted in improved glycaemic control and reduction in hypoglycaemic events in people with T1DM.254,255 In the last 2 years numerous commercial HCL pump systems have been marketed (Table 4 describes examples of commercially available HCL pump systems and their properties while Figure 4 depicts the Medtronic 670G with the guardian 3 sensor).
Table 4.
Salient features of various commercially available HCL pump systems.256
| Hybrid closed-loop system | Medtronic 670G- Guardian 3 sensor | CamAPS FX DanaRS-Dexcom G6 | Tandem t: slimX2- Dexcom G6 -Control IQ |
|---|---|---|---|
| Insulin pump | 670 G | Dana RS pump | Tandem t: slimX2 |
| Sensor | Guardian 3 | Dexcom G6 | Dexcom G6 |
| Sensor duration | 7 days | 10 days | 10 days |
| Algorithm | Treat to target, proportional integral derivative (PID) with insulin feedback | Treat to target, Adaptive Model, Predictive Control | Treat to range, Predictive algorithm |
| Advantages | Well-established pump system with significant clinical experience | Robust evidence base, no finger-prick tests required, licensed for use in pregnancy | Strong evidence base, no need for fingerstick |
| Disadvantages | Multiple daily finger-prick tests required, alarm fatigue, sparse RCT evidence | Limited real-world clinical experience | Limited real-world clinical experience |
HCL, hybrid closed-loop; RCT, randomized control trials.
Figure 4.

The Medtronic 670G with a guardian 3 sensor; an example of a hybrid closed-loop pump system.
Interestingly, people with T1DM around the world have developed ‘Do-It-Yourself’ (DIY) artificial pancreas systems by creating their own algorithm which runs on a smartphone app and automates insulin delivery while integrating with existing CGM and insulin pumps.256 Several studies have shown that DIY artificial pancreas systems increase the time in range (TIR) leading to a fall in HbA1c without increasing the incidence of hypoglycaemia.259 Examples of these DIY artificial pancreas systems include OpenAPS (runs on a Linux computer); AndroidAPS (runs on an Android phone); and Loop (runs on an iPhone and communicates to the pump with the RileyLink).259 However, these are unlicenced devices and users are responsible for any untoward events related to their use.
The HCL pump system technology is undergoing rapid development and with active trials being conducted on Insulin Only Bionic Pancreas (iLET) (ClinicalTrials.gov NCT04200313) and Omnipod Horizon™ Automated Glucose Control System (ClinicalTrials.gov NCT04196140),256 and the future of closed-loop pump system technology appears to be bright. Although these systems are relevant to the management of T1DM which is beyond the scope of this article, we have described these systems due their novel insulin delivery method and in future we may see them being employed for treatment of people with T2DM on MDI insulin regimes.
MN patch
The MN technology provides an innovative method for transdermal delivery of proteins.260,261 The MN patch forms temporary cutaneous channels, which provide access to the epidermis and dermis for biotherapeutics delivery. Once the MN patch is removed, the micro-channels close up quickly, preventing long-term skin damage.262,263 As opposed to the 26-G hypodermic needles, MN use is tolerated much better as it causes significantly less pain, anxiety, and local tissue irritation/damage.264 Based on the material and mechanism of drug delivery, MN’s can be classified into solid, degradable, hollow, dissolvable, and recently developed bioresponsive MNs that can respond to physiological glucose levels on-demand delivery of insulin.265 The MN developed by Zhang et al. using cross-linked alginate coupled with maltose, were functionally robust with high cytocompatibility. These MN’s demonstrated a sustained hypoglycaemic effect in diabetic SD rats with the relative pharmacological availability (RPA) and relative bioavailability (RBA) at 94.1 ± 5.6% and 93.7 ± 4.7%, respectively, compared with that of SC injection route at similar insulin doses demonstrating their efficacy in the treatment of DM.266 Being convenient and painless, MN is ideal for domiciliary use. However, before induction into clinical practice, a thorough investigation is required to look at their safety with regards to the potential for skin infection and irritation.
Inhaled insulin
Dance 501, from Dance Biopharm, is a front runner in the domain of pulmonary insulin delivery. It is a mist formulation of human insulin administered with a pocket-sized smart inhaler using vibrating mesh micropump technology developed by Aerogen.267 It is small, discreet, portable, and battery-operated hence overcoming some of the major drawbacks encountered with Exubera which lead to its withdrawal from the market in 2007.268 Data from phase-2 randomized, controlled study comparing Dance 501 to SC insulin lispro in T2DM are encouraging. Dance 501 inhaled human insulin demonstrated faster onset and greater action in the first hour of administration than SC insulin lispro, with good tolerability and without any pulmonary safety concerns.269 The insulin release with Dance 501 is breath actuated and so requires training before use. In addition to this, before use, the inhaler reservoir needs to be loaded with insulin and may present some difficulty to patients with compromised manual dexterity, for example, in cases of tremors or severe deforming arthritis.270 Nevertheless, if it continues to provide positive results in clinical trials, Dance 501 will be a potent option in managing T2DM.
Once weekly insulin
T2DM is a chronic illness, and to reduce the long-term morbidity burden, patients must achieve adequate glycaemic control. Due to the lack of compliance and acceptance in some patients, multiple daily basal insulin injections prove to hinder this venture. Novo Nordisk has developed a long-acting insulin analogue, insulin Icodec, which has now entered phase-3 clinical trials in the United States, United Kingdom, and Europe. By three amino acid substitutions (A14E, B16H, and B25 H) and an addition of a C20 fatty diacid containing side chain at B29 K, insulin icodec undergoes reduced enzymatic degradation and robust reversible albumin binding resulting in reduced clearance which stretches its terminal elimination half-life to 196 h.271 These properties allow it to be used as a single weekly injection covering the basal insulin requirements for an entire week. In a randomized, double-blind, double-dummy, phase-2 clinical trial, Rosenstock et al. compared the safety and efficacy of insulin icodec with once-daily insulin glargine U100 in insulin-naïve patients with T2DM. At 26 weeks, the estimated mean change from baseline in the HbA1c was −1.33% in the insulin icodec group and −1.15% in the insulin glargine group; thus insulin icodec demonstrated similar glucose-lowering efficacy to once-daily insulin glargine U100. There were no significant safety concerns with insulin icodec either, with observed hypoglycaemia rates similar to those in the insulin glargine group.272 From the available evidence so far, without compromising glycaemic control, insulin icodec can reduce the frequency of basal insulin injections and improve compliance, making it an exciting prospect for the future.
Genome-Wide Association Study and T2DM
Genome-Wide Association Study (GWAS) is a method used in genetics research, which can identify specific genetic variations in the form of single nucleotide polymorphisms (SNPs) linked to a variety of disease processes.273 A recent meta-analysis of GWAS with ~16 million genetic variants in 62,892 T2DM cases and 596,424 controls of European ancestry has led to the identification of 143 variants associated with T2DM, over three dozen of which were previously unknown.274 Impairment of pancreatic β-cell glucose sensitivity in the non-diabetic population is an independent and robust predictor of abnormal glucose tolerance and progression to T2DM.275,276 Following a genome-wide association meta-analysis, Deshmukh and Madsen et al. identified CDKAL1 and GIPR-QPCTL loci as important determinants of beta-cell glucose sensitivity.277 Similar studies have uncovered genes that are targets for current therapeutics. KCNJ11, GLP1R, and PPARG are gene variants identified by this method for T2DM targeted by sulfonylureas (SU), GLP-1 analogues, and TZD’s, respectively.278–280 Using the knowledge obtained from GWAS, new modalities in the form of ivacaftor and lumacaftor have been developed to treat cystic fibrosis (CF).281,282 Identification of genetic variants implicated in complex pathologies, the presence of successful drug therapies against these targets and the development of new treatments using knowledge from GWAS has lent substantial credence to this approach. In the future, advances in this strategy can inform the development of effective and may be curative pharmacotherapy for T2DM.
New agents and delivery methods in pre-existing classes of medication for the treatment of T2DM
SGLT2 inhibitors, GLP-1 analogues, and dipeptidyl peptidase-4 (DPP-4) inhibitors are among the new classes of medication for the treatment of T2DM, developed over the last 15 years. Their novel MOA, ease of administration, effective glycaemic control, weight reduction capability, cardiovascular, and renal protection have had an overwhelmingly positive impact on the management of T2DM.283 A description of these agents is beyond the scope of this review; however, we detail some of the new developments to these classes in Tables 5 and 6.
Table 5.
Examples of new developments in pre-existing therapeutic classes in T2DM.
| Class | Novelty |
|---|---|
| GLP-1 analogues | Efpeglenatide, once monthly SC.257 |
| DPP-4 inhibitors | Trelagliptin, once weekly oral administration.258 |
DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; SC, subcutaneous; T2DM, type-2 diabetes mellitus.
Table 6.
The table summarizes some examples of new therapeutic classes, recently approved, and novel agents in pre-existing groups for the treatment of T2DM.
| CLASS | DRUG | NOVELTY | Clinical status |
|---|---|---|---|
| ORMD-0801 | Oral | Phase 3 | |
| Insulin | Omnipod V-GoTM | Transdermal | Approved for clinical use |
| Dance 501 | Inhaled | Phase 2 | |
| Icodec | Once weekly administration | Phase 3 | |
| Combined peptide injections | IDegLira iGLarLixi | Basal insulin and GLP-1 agonists combination | Approved for clinical use |
| Glimins | Imeglimin | Inhibits oxidative phosphorylation and improves insulin resistance | Phase 3 |
| Dual GLP-1/GIP agonists | Tirzepatide | Glucagon pathway | Phase 3 |
| GLP-1/GCGR dual agonist | MEDI-0382 | Glucagon pathway | Phase 2 |
| GCGR antagonist | LGD 6972 IONIS-GCGRRx | Glucagon receptor blockade | Phase 2 |
| GLP-1R/GCGR/GIPR triple agonist | HM15211 NN9423 GGG tri-agonist |
Synergistic incretin effect | Phase 1 |
| GPR119 agonists | DS-8500 | Stimulates insulin release | Phase 2 |
| FFAR1 agonists | P1736 P11187 LY2922470 |
Enhance insulin and incretin release | Phase 2 Phase 1 Phase 1 |
| TGR5 agonists | SB-756050 | Bile acid-specific receptor-mediated gluco-metabolic effects | Phase 2 (discontinued) |
| Melatonin receptor agonists | Melatonin | Improve insulin sensitivity and suppress gluconeogenesis | Phase 1 |
| PTP-1B inhibitors | KQ-791 | Protection of insulin receptor from inactivation | Phase 1 |
| HIP | BTI-410 | Increases β-cell mass | Phase 2 |
| FGF-21 analogues | Pegbelfermin | Promotes insulin-dependent glucose uptake | Phase 2 |
| AMP analogues | O304 | AMPK activation, insulin-sensitizing effect | Phase 2(a) |
| Glucokinase activators | TTP-399 | Promotes conversion of glucose to glucose-6-phosphate | Phase 2 |
| Sirtuins | – | SIRT1 activation increased insulin secretion and sensitivity. | – |
| 11β-HSD1 inhibitors | BI 135585 | Prevents conversion of cortisone to cortisol | Phase 1 |
| PPAR pan-agonists | Chiglitazar | Pan α/γ/δ agonists leading to improvement in glycaemic control, inflammation and hepatic lipid accumulation | Phase 3 |
| Dual SGLT1/SGLT2 inhibitors | Sotagliflozin | Blocking both SGLT1 and SGLT2 receptors | Approved for use in T1DM as an adjunct to insulin in the European Union (EU); in Phase 3 clinical trials for use in T2DM |
AMP, Adenosine monophosphate; AMPK, adenosine monophosphate-activated protein kinase; EU, European Union; FFAR, free fatty acid receptor; FGF-21, fibroblast growth factor 21; GCGR, glucagon receptor; GIP, gastric inhibitory polypeptide; GIPR, GIP receptor; GLP-1, glucagon-like peptide-1; GLP-1R, GLP-1 receptor; HIP, human pro-islet peptide; 11β-HSD1, 11β-hydroxysteroid dehydrogenase 1; PPAR, peroxisome proliferator-activated receptor; PTP-1B, protein tyrosine phosphatase -1B; SGLT, sodium-glucose co-transporter; SIRT, sirtuins; T2DM, type-2 diabetes mellitus; TGR5, Takeda G protein-coupled receptor 5.
Conclusion
Since the ground-breaking work from the French physician Jean Sterne, who studied metformin for the first time in humans in the 1950s to treat DM,284 the industry has come a long way in investigating, developing, and delivering beneficial treatment modalities in T2DM. Most novel compounds produced for treatment have shown a potent anti-hyperglycaemic effect; however, they need to be examined further to ensure that their use is safe and well-tolerated in clinical practice. Various new therapeutic targets have been explored; however, an extension of previously known mechanisms has yielded greater success as in the case of sotagliflozin, for example. Innovations in delivery methods and reduction in dosing frequency will undoubtedly improve compliance with these formulations in the real world. Oral and once-weekly insulin, insulin patch pumps and once monthly GLP-1 analogues are an exciting prospect with the potential of conferring significant clinical benefits to patients.
The future appears to be bright; there are 129 active interventional studies in T2DM registered over the last 10 years, currently in phase 1 to phase 4 globally, based on data from ClinicalTrials.gov from March 2021. Can the academic and pharmaceutical industry come together and uncover an agent which reverses the underlying mechanisms fuelling dysglycaemia and normalizes glucose homoeostasis resulting in the cure of T2DM? Perhaps the advances in monoclonal antibody and gene therapy will provide this answer in the future.
Footnotes
Author contributions: NS: conceptualization; writing—original draft; writing—review and editing. MAA: writing—review and editing. HD: writing—review and editing. TS: supervision and writing—review and editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Thozhukat Sathyapalan  https://orcid.org/0000-0003-3544-2231
https://orcid.org/0000-0003-3544-2231
Contributor Information
Najeeb Shah, Hull University Teaching Hospitals NHS Trust, Hull, UK; Department of Academic Diabetes, Endocrinology & Metabolism, Hull York Medical School, University of Hull, Brocklehurst Building, 220-236 Anlaby Road, Hull, HU3 2RW, UK.
Mohammed Altigani Abdalla, Department of Academic Diabetes, Endocrinology & Metabolism, Hull York Medical School, University of Hull, Hull, UK.
Harshal Deshmukh, University Teaching Hospitals NHS Trust and Department of Academic Diabetes, Endocrinology & Metabolism, Hull York Medical School, University of Hull, Hull, UK.
Thozhukat Sathyapalan, University Teaching Hospitals NHS Trust and Department of Academic Diabetes, Endocrinology & Metabolism, Hull York Medical School, University of Hull, Hull, UK.
References
- 1.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 2019; 157: 107843. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF diabetes atlas, https://www.diabetesatlas.org/en/ (2019, accessed 22 February 2021).
- 3.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet 2017; 389: 2239–2251. [DOI] [PubMed] [Google Scholar]
- 4.Zimmet PZ. Diabetes and its drivers: the largest epidemic in human history. Clin Diabetes Endocrinol 2017; 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 6.Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology – clinical practice guidelines for developing a diabetes mellitus comprehensive care plan – 2015. Endocr Pract 2015; 21(Suppl. 1): 1–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995; 28: 103–117. [DOI] [PubMed] [Google Scholar]
- 8.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33).UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 9.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 854–865. [PubMed] [Google Scholar]
- 10.Khunti K, Wolden ML, Thorsted BL, et al. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care 2013; 36: 3411–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey CJ, Tahrani AA, Barnett AH. Future glucose-lowering drugs for type 2 diabetes. Lancet Diabetes Endocrinol 2016; 4: 350–359. [DOI] [PubMed] [Google Scholar]
- 12.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 2005; 365: 1333–1346. [DOI] [PubMed] [Google Scholar]
- 13.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 2008; 29: 351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler AE, Janson J, Bonner-Weir S, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52: 102–110. [DOI] [PubMed] [Google Scholar]
- 15.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest 2006; 116: 1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58: 773–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988; 37: 667–687. [DOI] [PubMed] [Google Scholar]
- 18.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 2010; 53: 1270–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell 2012; 148: 852–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magnusson I, Rothman DL, Katz LD, et al. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest 1992; 90: 1323–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuda M, Defronzo RA, Glass L, et al. Glucagon dose-response curve for hepatic glucose production and glucose disposal in type 2 diabetic patients and normal individuals. Metabolism 2002; 51: 1111–1119. [DOI] [PubMed] [Google Scholar]
- 22.Baron AD, Schaeffer L, Shragg P, et al. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 1987; 36: 274–283. [DOI] [PubMed] [Google Scholar]
- 23.Gerich JE, Meyer C, Woerle HJ, et al. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 2001; 24: 382–391. [DOI] [PubMed] [Google Scholar]
- 24.Baron AD. Hemodynamic actions of insulin. Am J Physiol 1994; 267: E187–E202. [DOI] [PubMed] [Google Scholar]
- 25.DeFronzo RA, Ferrannini E, Hendler R, et al. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci USA 1978; 75: 5173–5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrannini E, Simonson DC, Katz LD, et al. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism 1988; 37: 79–85. [DOI] [PubMed] [Google Scholar]
- 27.Sharma D, Verma S, Vaidya S, et al. Recent updates on GLP-1 agonists: current advancements & challenges. Biomed Pharmacother 2018; 108: 952–962. [DOI] [PubMed] [Google Scholar]
- 28.Meloni A, DeYoung M, Lowe C, et al. GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes Obes Metab 2013; 15: 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O randomized trial. Diabetes Care 2016; 39: 2026–2035. [DOI] [PubMed] [Google Scholar]
- 30.Hinnen D, Strong J. iGlarLixi : a new once-daily fixed-ratio combination of basal insulin glargine and lixisenatide for the management of type 2 diabetes. Diabetes Spectr 2018; 31: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care 2016; 39: 1972–1980. [DOI] [PubMed] [Google Scholar]
- 32.Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol 2014; 2: 885–893. [DOI] [PubMed] [Google Scholar]
- 33.Buse JB, Vilsboll T, Thurman J, et al. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care 2014; 37: 2926–2933. [DOI] [PubMed] [Google Scholar]
- 34.Perreault L, Rodbard H, Valentine V, et al. Optimizing fixed-ratio combination therapy in type 2 diabetes. Adv Ther 2019; 36: 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gough SC, Bode BW, Woo VC, et al. One-year efficacy and safety of a fixed combination of insulin degludec and liraglutide in patients with type 2 diabetes: results of a 26-week extension to a 26-week main trial. Diabetes Obes Metab 2015; 17: 965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 2013; 17: 819–837. [DOI] [PubMed] [Google Scholar]
- 37.Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab 2018; 20(Suppl. 1): 5–21. [DOI] [PubMed] [Google Scholar]
- 38.Nauck MA, Meier JJ. GIP and GLP-1: stepsiblings rather than monozygotic twins within the incretin family. Diabetes 2019; 68: 897–900. [DOI] [PubMed] [Google Scholar]
- 39.Gasbjerg LS, Bergmann NC, Stensen S, et al. Evaluation of the incretin effect in humans using GIP and GLP-1 receptor antagonists. Peptides 2020; 125: 170183. [DOI] [PubMed] [Google Scholar]
- 40.Eli Lilly and Company. Tirzepatide achieved superior A1C and body weight reductions across all three doses compared to injectable semaglutide in adults with type 2 diabetes, https://investor.lilly.com/news-releases/news-release-details/tirzepatide-achieved-superior-a1c-and-body-weight-reductions (2021, accessed 10 March 2021).
- 41.Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018; 392: 2180–2193. [DOI] [PubMed] [Google Scholar]
- 42.Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab 2018; 18: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willard FS, Douros JD, Gabe MB, et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight 2020; 5: e140532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frías JP. Tirzepatide: a glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) dual agonist in development for the treatment of type 2 diabetes. Expert Rev Endocrinol Metab 2020; 15: 379–394. [DOI] [PubMed] [Google Scholar]
- 45.Bastin M, Andreelli F. Dual GIP-GLP1-receptor agonists in the treatment of type 2 diabetes: a short review on emerging data and therapeutic potential. Diabetes Metab Syndr Obes 2019; 12: 1973–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res 2015; 12: 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanai Y, Lee WS, You G, et al. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest 1994; 93: 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gorboulev V, Schürmann A, Vallon V, et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012; 61: 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rieg T, Masuda T, Gerasimova M, et al. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 2014; 306: F188–F193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011; 91: 733–794. [DOI] [PubMed] [Google Scholar]
- 51.Vrhovac I, Balen Eror D, Klessen D, et al. Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch 2015; 467: 1881–1898. [DOI] [PubMed] [Google Scholar]
- 52.European Medicines Agency. Forxiga, https://www.ema.europa.eu/en/medicines/human/EPAR/forxiga#overview-section (2012, accessed 8 June 2021).
- 53.U.S. Food & Drug Administration (FDA). Drug approval package: Invokana (canagliflozin) tablets, https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204042Orig1s000TOC.cfm (2013, accessed 8 June 2021). [Google Scholar]
- 54.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 55.Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J 2016; 37: 1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 57.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 2018; 61: 2108–2117. [DOI] [PubMed] [Google Scholar]
- 58.Lapuerta P, Zambrowicz B, Strumph P, et al. Development of sotagliflozin, a dual sodium-dependent glucose transporter 1/2 inhibitor. Diab Vasc Dis Res 2015; 12: 101–110. [DOI] [PubMed] [Google Scholar]
- 59.Zambrowicz B, Freiman J, Brown PM, et al. LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial. Clin Pharmacol Ther 2012; 92: 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Washburn WN, Poucher SM. Differentiating sodium-glucose co-transporter-2 inhibitors in development for the treatment of type 2 diabetes mellitus. Expert Opin Investig Drugs 2013; 22: 463–486. [DOI] [PubMed] [Google Scholar]
- 61.Cefalo CMA, Cinti F, Moffa S, et al. Sotagliflozin, the first dual SGLT inhibitor: current outlook and perspectives. Cardiovasc Diabetol 2019; 18: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Musso G, Gambino R, Cassader M, et al. Efficacy and safety of dual SGLT 1/2 inhibitor sotagliflozin in type 1 diabetes: meta-analysis of randomised controlled trials. BMJ 2019; 365: l1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sims H, Smith KH, Bramlage P, et al. Sotagliflozin: a dual sodium-glucose co-transporter-1 and -2 inhibitor for the management of type 1 and type 2 diabetes mellitus. Diabet Med 2018; 35: 1037–1048. [DOI] [PubMed] [Google Scholar]
- 64.Powell DR, Zambrowicz B, Morrow L, et al. Sotagliflozin decreases postprandial glucose and insulin concentrations by delaying intestinal glucose absorption. J Clin Endocrinol Metab 2020; 105: e1235–e1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deeks ED. Sotagliflozin: a review in type 1 diabetes. Drugs 2019; 79: 1977–1987. [DOI] [PubMed] [Google Scholar]
- 66.Markham A, Keam SJ. Sotagliflozin: first global approval. Drugs 2019; 79: 1023–1029. [DOI] [PubMed] [Google Scholar]
- 67.Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5: 864–876. [DOI] [PubMed] [Google Scholar]
- 68.Goldenberg RM, Gilbert JD, Hramiak IM, et al. Sodium-glucose co-transporter inhibitors, their role in type 1 diabetes treatment and a risk mitigation strategy for preventing diabetic ketoacidosis: the STOP DKA protocol. Diabetes Obes Metab 2019; 21: 2192–2202. [DOI] [PubMed] [Google Scholar]
- 69.Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2020; 384: 117–128. [DOI] [PubMed] [Google Scholar]
- 70.Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med 2020; 384: 129–139. [DOI] [PubMed] [Google Scholar]
- 71.He YL, Haynes W, Meyers CD, et al. The effects of licogliflozin, a dual SGLT1/2 inhibitor, on body weight in obese patients with or without diabetes. Diabetes Obes Metab 2019; 21: 1311–1321. [DOI] [PubMed] [Google Scholar]
- 72.Bays HE, Kozlovski P, Shao Q, et al. Licogliflozin, a novel SGLT1 and 2 inhibitor: body weight effects in a randomized trial in adults with overweight or obesity. Obesity 2020; 28: 870–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tyagi S, Gupta P, Saini AS, et al. The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. J Adv Pharm Technol Res 2011; 2: 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michalik L, Wahli W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. J Clin Invest 2006; 116: 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med 2004; 10: 355–361. [DOI] [PubMed] [Google Scholar]
- 76.Quinn CE, Hamilton PK, Lockhart CJ, et al. Thiazolidinediones: effects on insulin resistance and the cardiovascular system. Br J Pharmacol 2008; 153: 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hauner H. The mode of action of thiazolidinediones. Diabetes Metab Res Rev 2002; 18(Suppl. 2): S10–S15. [DOI] [PubMed] [Google Scholar]
- 78.ClinicalTrials.gov. Study of chiglitazar compare with placebo in type 2 diabetes patients (CMAP), https://clinicaltrials.gov/ct2/show/NCT02121717 (2019, accessed 11 March 2021).
- 79.ClinicalTrials.gov. Study of chiglitazar compare with sitagliptin in type 2 diabetes patients (CMAS), https://clinicaltrials.gov/ct2/show/NCT02173457 (2019, accessed 11 March 2021).
- 80.Ji L, Song W, Fang H, et al. 17-OR: efficacy and safety of chiglitazar, a novel PPARα/γ/d pan-agonist, in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled phase 3 superiority trial (CMAP). Diabetes 2019; 68: 17-OR. [DOI] [PubMed] [Google Scholar]
- 81.Wettstein G, Luccarini J-M, Poekes L, et al. The new-generation pan-peroxisome proliferator-activated receptor agonist IVA337 protects the liver from metabolic disorders and fibrosis. Hepatol Commun 2017; 1: 524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.An HJ, Lee B, Kim SM, et al. A PPAR pan agonist, MHY2013 alleviates age-related hepatic lipid accumulation by promoting fatty acid oxidation and suppressing inflammation. Biol Pharm Bull 2018; 41: 29–35. [DOI] [PubMed] [Google Scholar]
- 83.Pirags V, Lebovitz H, Fouqueray P. Imeglimin, a novel glimin oral antidiabetic, exhibits a good efficacy and safety profile in type 2 diabetic patients. Diabetes Obes Metab 2012; 14: 852–858. [DOI] [PubMed] [Google Scholar]
- 84.Fouqueray P, Pirags V, Inzucchi SE, et al. The efficacy and safety of imeglimin as add-on therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care 2013; 36: 565–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fouqueray P, Pirags V, Diamant M, et al. The efficacy and safety of imeglimin as add-on therapy in patients with type 2 diabetes inadequately controlled with sitagliptin monotherapy. Diabetes Care 2014; 37: 1924–1930. [DOI] [PubMed] [Google Scholar]
- 86.Yaribeygi H, Maleki M, Sathyapalan T, et al. Molecular mechanisms by which imeglimin improves glucose homeostasis. J Diabetes Res 2020; 2020: 8768954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hallakou-Bozec S, Bolze S, Kergoat M, et al. Imeglimin increases insulin secretion in response to glucose as a unique mechanism of action depending on NAD synthesis. Insulin 2016; 40: 20. [Google Scholar]
- 88.Vial G, Chauvin MA, Bendridi N, et al. Imeglimin normalizes glucose tolerance and insulin sensitivity and improves mitochondrial function in liver of a high-fat, high-sucrose diet mice model. Diabetes 2015; 64: 2254–2264. [DOI] [PubMed] [Google Scholar]
- 89.Pacini G, Mari A, Fouqueray P, et al. Imeglimin increases glucose-dependent insulin secretion and improves beta-cell function in patients with type 2 diabetes. Diabetes Obes Metab 2015; 17: 541–545. [DOI] [PubMed] [Google Scholar]
- 90.Fouqueray P. Imeglimin – a new oral anti diabetic that targets the three key defects of type 2 diabetes. J Diabetes Metab 2011; 2: 126. [Google Scholar]
- 91.Konkwo C, Perry RJ. Imeglimin: current development and future potential in type 2 diabetes. Drugs 2021; 81: 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lachaux M, Soulié M, Hamzaoui M, et al. Short-and long-term administration of imeglimin counters cardiorenal dysfunction in a rat model of metabolic syndrome. Endocrinol Diabetes Metab 2020; 3: e00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crabtree TSJDR, Ryder REJ, Bailey CJ. Imeglimin, a novel, first in-class, blood glucose-lowering agent: a systematic review and meta-analysis of clinical evidence. Br J Diabetes 2020; 20: 28–31. [Google Scholar]
- 94.Bailey CJ. The current drug treatment landscape for diabetes and perspectives for the future. Clin Pharmacol Ther 2015; 98: 170–184. [DOI] [PubMed] [Google Scholar]
- 95.Karnieli E. The growing prevalence of obesity worldwide is an increasing concern. Preface. Endocrinol Metab Clin North Am 2008; 37: xvii–xviii. [DOI] [PubMed] [Google Scholar]
- 96.Cvetkovic RS, Plosker GL. Exenatide: a review of its use in patients with type 2 diabetes mellitus (as an adjunct to metformin and/or a sulfonylurea). Drugs 2007; 67: 935–954. [DOI] [PubMed] [Google Scholar]
- 97.Cho YM, Merchant CE, Kieffer TJ. Targeting the glucagon receptor family for diabetes and obesity therapy. Pharmacol Ther 2012; 135: 247–278. [DOI] [PubMed] [Google Scholar]
- 98.Habegger KM, Heppner KM, Geary N, et al. The metabolic actions of glucagon revisited. Nat Rev Endocrinol 2010; 6: 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pocai A, Carrington PE, Adams JR, et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes 2009; 58: 2258–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rajput R, Prakash A, Aggarwal R. Newer antidiabetic drugs in the pipeline. Int J Diab 2019; 29: 28–33. [Google Scholar]
- 101.Patil M, Deshmukh NJ, Patel M, et al. Glucagon-based therapy: past, present and future. Peptides 2020; 127: 170296. [DOI] [PubMed] [Google Scholar]
- 102.Ambery P, Parker VE, Stumvoll M, et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet 2018; 391: 2607–2618. [DOI] [PubMed] [Google Scholar]
- 103.Quesada I, Tuduri E, Ripoll C, et al. Physiology of the pancreatic alpha-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol 2008; 199: 5–19. [DOI] [PubMed] [Google Scholar]
- 104.Bagger JI, Knop FK, Holst JJ, et al. Glucagon antagonism as a potential therapeutic target in type 2 diabetes. Diabetes Obes Metab 2011; 13: 965–971. [DOI] [PubMed] [Google Scholar]
- 105.Kazierad DJ, Chidsey K, Somayaji VR, et al. Efficacy and safety of the glucagon receptor antagonist PF-06291874: a 12-week, randomized, dose-response study in patients with type 2 diabetes mellitus on background metformin therapy. Diabetes Obes Metab 2018; 20: 2608–2616. [DOI] [PubMed] [Google Scholar]
- 106.Guzman CB, Zhang XM, Liu R, et al. Treatment with LY2409021, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes Obes Metab 2017; 19: 1521–1528. [DOI] [PubMed] [Google Scholar]
- 107.Morgan ES, Tai L-J, Pham NC, et al. Antisense inhibition of glucagon receptor by IONIS-GCGRRx improves type 2 diabetes without increase in hepatic glycogen content in patients with type 2 diabetes on stable metformin therapy. Diabetes Care 2019; 42: 585–593. [DOI] [PubMed] [Google Scholar]
- 108.ClinicalTrials.gov. A study of LGD-6972 in patients with type 2 diabetes mellitus, https://clinicaltrials.gov/ct2/show/NCT02851849 (2018, accessed 16 March 2021).
- 109.Vajda EG, Logan D, Lasseter K, et al. Pharmacokinetics and pharmacodynamics of single and multiple doses of the glucagon receptor antagonist LGD-6972 in healthy subjects and subjects with type 2 diabetes mellitus. Diabetes Obes Metab 2017; 19: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gumbiner B, Esteves B, Dell V, et al. Single and multiple ascending-dose study of glucagon-receptor antagonist RN909 in type 2 diabetes: a phase 1, randomized, double-blind, placebo-controlled trial. Endocrine 2018; 62: 371–380. [DOI] [PubMed] [Google Scholar]
- 111.Luu KT, Morgan ES, Bhanot S, et al. Population pharmacokinetics and pharmacodynamics of IONIS-GCGR(Rx), an antisense oligonucleotide for type 2 diabetes mellitus: a red blood cell lifespan model. J Pharmacokinet Pharmacodyn 2017; 44: 179–191. [DOI] [PubMed] [Google Scholar]
- 112.Jiménez A, Casamitjana R, Viaplana-Masclans J, et al. GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes after gastric bypass surgery. Diabetes Care 2013; 36: 2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brandt SJ, Kleinert M, Tschöp MH, et al. Are peptide conjugates the golden therapy against obesity? J Endocrinol 2018; 238: R109–R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Choi IY, Lee JS, Kim JK, et al. Potent body weight loss and efficacy in a NASH animal model by a novel long-acting GLP-1/Glucagon/GIP triple-agonist (HM15211). In: American Diabetes Association’s 77th scientific session, San Diego, CA, 9–13June2017. [Google Scholar]
- 115.Kim JK, Lee JS, Park E, et al. Therapeutic effect of a novel long-acting GLP-1/GIP/Glucagon triple agonist (HM15211) in NASH and fibrosis animal models. In: EASD annual meeting, Berlin, 1–5October2018. [Google Scholar]
- 116.Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov 2009; 8: 369–385. [DOI] [PubMed] [Google Scholar]
- 117.Kimple ME, Neuman JC, Linnemann AK, et al. Inhibitory G proteins and their receptors: emerging therapeutic targets for obesity and diabetes. Exp Mol Med 2014; 46: e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Overton HA, Babbs AJ, Doel SM, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab 2006; 3: 167–175. [DOI] [PubMed] [Google Scholar]
- 119.Shah U, Kowalski TJ. GPR119 agonists for the potential treatment of type 2 diabetes and related metabolic disorders. Vitam Horm 2010; 84: 415–448. [DOI] [PubMed] [Google Scholar]
- 120.Yang JW, Kim HS, Choi YW, et al. Therapeutic application of GPR119 ligands in metabolic disorders. Diabetes Obes Metab 2018; 20: 257–269. [DOI] [PubMed] [Google Scholar]
- 121.Yoshida S, Ohishi T, Matsui T, et al. Novel GPR119 agonist AS1535907 contributes to first-phase insulin secretion in rat perfused pancreas and diabetic db/db mice. Biochem Biophys Res Commun 2010; 402: 280–285. [DOI] [PubMed] [Google Scholar]
- 122.ClinicalTrials.gov. Phase 2 study of DS-8500a in patients with type 2 diabetes, https://clinicaltrials.gov/ct2/show/NCT02222350 (2019, accessed 12 March 2021).
- 123.Chellappan DK, Yap WS, Bt Ahmad Suhaimi NA, et al. Current therapies and targets for type 2 diabetes mellitus. Panminerva Med 2018; 60: 117–131. [DOI] [PubMed] [Google Scholar]
- 124.Watterson KR, Hudson BD, Ulven T, et al. Treatment of type 2 diabetes by free fatty acid receptor agonists. Front Endocrinol 2014; 5: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brown AJ, Goldsworthy SM, Barnes AA, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 2003; 278: 11312–11319. [DOI] [PubMed] [Google Scholar]
- 126.Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012; 61: 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Z, Qiu Q, Geng X, et al. Free fatty acid receptor agonists for the treatment of type 2 diabetes: drugs in preclinical to phase II clinical development. Expert Opin Investig Drugs 2016; 25: 871–890. [DOI] [PubMed] [Google Scholar]
- 128.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem 2003; 278: 9435–9440. [DOI] [PubMed] [Google Scholar]
- 129.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 2009; 10: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Trabelsi M-S, Daoudi M, Prawitt J, et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat Commun 2015; 6: 7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kumar DP, Rajagopal S, Mahavadi S, et al. Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic β cells. Biochem Biophys Res Commun 2012; 427: 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kumar DP, Asgharpour A, Mirshahi F, et al. Activation of transmembrane bile acid receptor TGR5 modulates pancreatic islet α cells to promote glucose homeostasis. J Biol Chem 2016; 291: 6626–6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Duboc H, Taché Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis 2014; 46: 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Duan H, Ning M, Zou Q, et al. Discovery of intestinal targeted TGR5 agonists for the treatment of type 2 diabetes. J Med Chem 2015; 58: 3315–3328. [DOI] [PubMed] [Google Scholar]
- 135.Bailey CJ, Day C. Treatment of type 2 diabetes: future approaches. Br Med Bull 2018; 126: 123–137. [DOI] [PubMed] [Google Scholar]
- 136.van Nierop FS, Scheltema MJ, Eggink HM, et al. Clinical relevance of the bile acid receptor TGR5 in metabolism. Lancet Diabetes Endocrinol 2017; 5: 224–233. [DOI] [PubMed] [Google Scholar]
- 137.Briere DA, Ruan X, Cheng CC, et al. Novel small molecule agonist of TGR5 possesses anti-diabetic effects but causes gallbladder filling in mice. PLoS ONE 2015; 10: e0136873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hodge RJ, Nunez DJ. Therapeutic potential of Takeda-G-protein-receptor-5 (TGR5) agonists. Hope or hype? Diabetes Obes Metab 2016; 18: 439–443. [DOI] [PubMed] [Google Scholar]
- 139.Hodge RJ, Lin J, Vasist Johnson LS, et al. Safety, pharmacokinetics, and pharmacodynamic effects of a selective TGR5 agonist, SB-756050, in type 2 diabetes. Clin Pharmacol Drug Dev 2013; 2: 213–222. [DOI] [PubMed] [Google Scholar]
- 140.Adis Insight. SB 756050, https://adisinsight.springer.com/drugs/800028284 (2021, accessed 6 June 2021).
- 141.Lima FB, Machado UF, Bartol I, et al. Pinealectomy causes glucose intolerance and decreases adipose cell responsiveness to insulin in rats. Am J Physiol 1998; 275: E934–E941. [DOI] [PubMed] [Google Scholar]
- 142.Nogueira TC, Lellis-Santos C, Jesus DS, et al. Absence of melatonin induces night-time hepatic insulin resistance and increased gluconeogenesis due to stimulation of nocturnal unfolded protein response. Endocrinology 2011; 152: 1253–1263. [DOI] [PubMed] [Google Scholar]
- 143.Champney TH, Brainard GC, Richardson BA, et al. Experimentally-induced diabetes reduces nocturnal pineal melatonin content in the Syrian hamster. Comp Biochem Physiol A Comp Physiol 1983; 76: 199–201. [DOI] [PubMed] [Google Scholar]
- 144.Peschke E, Frese T, Chankiewitz E, et al. Diabetic Goto Kakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin-receptor status. J Pineal Res 2006; 40: 135–143. [DOI] [PubMed] [Google Scholar]
- 145.Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 1994; 13: 1177–1185. [DOI] [PubMed] [Google Scholar]
- 146.She M, Laudon M, Yin W. Melatonin receptors in diabetes: a potential new therapeutical target? Eur J Pharmacol 2014; 744: 220–223. [DOI] [PubMed] [Google Scholar]
- 147.Karamitri A, Jockers R. Melatonin in type 2 diabetes mellitus and obesity. Nat Rev Endocrinol 2019; 15: 105–125. [DOI] [PubMed] [Google Scholar]
- 148.Tuomi T, Nagorny CLF, Singh P, et al. Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab 2016; 23: 1067–1077. [DOI] [PubMed] [Google Scholar]
- 149.Contreras-Alcantara S, Baba K, Tosini G. Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity 2010; 18: 1861–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Neubauer DN. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr Dis Treat 2008; 4: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tsunoda T, Yamada M, Akiyama T, et al. The effects of ramelteon on glucose metabolism and sleep quality in type 2 diabetic patients with insomnia: a pilot prospective randomized controlled trial. J Clin Med Res 2016; 8: 878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.She M, Hu X, Su Z, et al. Piromelatine, a novel melatonin receptor agonist, stabilizes metabolic profiles and ameliorates insulin resistance in chronic sleep restricted rats. Eur J Pharmacol 2014; 727: 60–65. [DOI] [PubMed] [Google Scholar]
- 153.Clincosm. METOD: melatonin’s effects on treatment of diabetes mellitus, https://www.clincosm.com/trial/diabetes-shiraz-melatonin (2019, accessed 9 March 2021).
- 154.Crunkhorn S. Protein tyrosine phosphatase inhibitor reverses diabetes. Nat Rev Drug Discov 2017; 16: 312–313. [DOI] [PubMed] [Google Scholar]
- 155.Krishnan N, Konidaris KF, Gasser G, et al. A potent, selective, and orally bioavailable inhibitor of the protein-tyrosine phosphatase PTP1B improves insulin and leptin signaling in animal models. J Biol Chem 2018; 293: 1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Elchebly M, Payette P, Michaliszyn E, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 1999; 283: 1544–1548. [DOI] [PubMed] [Google Scholar]
- 157.Klaman LD, Boss O, Peroni OD, et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol 2000; 20: 5479–5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stanford SM, Bottini N. Targeting tyrosine phosphatases: time to end the stigma. Trends Pharmacol Sci 2017; 38: 524–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.He RJ, Yu ZH, Zhang RY, et al. Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol Sin 2014; 35: 1227–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Maheshwari N, Karthikeyan C, Trivedi P, et al. Recent advances in protein tyrosine phosphatase 1B targeted drug discovery for type II diabetes and obesity. Curr Drug Targets 2018; 19: 551–575. [DOI] [PubMed] [Google Scholar]
- 161.Adis Insight. KQ 791, https://adisinsight.springer.com/drugs/800042103 (2018, accessed 12 March 2021).
- 162.Butler AE, Janson J, Bonner-Weir S, et al. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52: 102–110. [DOI] [PubMed] [Google Scholar]
- 163.Trucco M. Regeneration of the pancreatic beta cell. J Clin Invest 2005; 115: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343: 230–238. [DOI] [PubMed] [Google Scholar]
- 165.Fridell JA, Rogers J, Stratta RJ. The pancreas allograft donor: current status, controversies, and challenges for the future. Clin Transplant 2010; 24: 433–449. [DOI] [PubMed] [Google Scholar]
- 166.Kaestner KH. Beta cell transplantation and immunosuppression: can’t live with it, can’t live without it. J Clin Invest 2007; 117: 2380–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Levetan CS, Upham LV, Deng S, et al. Discovery of a human peptide sequence signaling islet neogenesis. Endocr Pract 2008; 14: 1075–1083. [DOI] [PubMed] [Google Scholar]
- 168.Jiang Z, Shi D, Tu Y, et al. Human proislet peptide promotes pancreatic progenitor cells to ameliorate diabetes through FOXO1/menin-mediated epigenetic regulation. Diabetes 2018; 67: 1345–1355. [DOI] [PubMed] [Google Scholar]
- 169.Boston Therapeutics. Human proislet peptide, https://www.bostonti.com/the-science/human-proislet-peptide (2021, accessed 17 March 2021).
- 170.Zhang J, Li Y. Fibroblast growth factor 21 analogs for treating metabolic disorders. Front Endocrinol 2015; 6: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fukumoto S. Actions and mode of actions of FGF19 subfamily members. Endocr J 2008; 55: 23–31. [DOI] [PubMed] [Google Scholar]
- 172.Ge X, Wang Y, Lam KSL, et al. Metabolic actions of FGF21: molecular mechanisms and therapeutic implications. Acta Pharm Sin B 2012; 2: 350–357. [Google Scholar]
- 173.Coskun T, Bina HA, Schneider MA, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008; 149: 6018–6027. [DOI] [PubMed] [Google Scholar]
- 174.Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest 2005; 115: 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xu J, Lloyd DJ, Hale C, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009; 58: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sanyal A, Charles ED, Neuschwander-Tetri BA, et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 2019; 392: 2705–2717. [DOI] [PubMed] [Google Scholar]
- 177.Charles ED, Neuschwander-Tetri BA, Pablo Frias J, et al. Pegbelfermin (BMS-986036), PEGylated FGF21, in patients with obesity and type 2 diabetes: results from a randomized phase 2 study. Obesity 2019; 27: 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Horike N, Sakoda H, Kushiyama A, et al. AMP-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3beta and thereby reduces cAMP-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver. J Biol Chem 2008; 283: 33902–33910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov 2004; 3: 340–351. [DOI] [PubMed] [Google Scholar]
- 180.Jäger S, Handschin C, St-Pierre J, et al. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 2007; 104: 12017–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Coughlan KA, Valentine RJ, Ruderman NB, et al. AMPK activation: a therapeutic target for type 2 diabetes. Diabetes Metab Syndr Obes 2014; 7: 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Steneberg P, Lindahl E, Dahl U, et al. PAN-AMPK activator O304 improves glucose homeostasis and microvascular perfusion in mice and type 2 diabetes patients. JCI Insight 2018; 3: e99114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.BioSpace. Betagenon/Baltic Bio announces positive results from a 28-day phase IIa trial of the first-in-class AMPK activator O304 in type 2 diabetics, https://www.biospace.com/article/releases/betagenon-baltic-bio-announces-positive-results-from-a-28-day-phase-iia-trial-of-the-first-in-class-ampk-activator-o304-in-type-2-diabetics/?s=95 (2017, accessed 13 March 2021).
- 184.Matschinsky FM, Magnuson MA, Zelent D, et al. The network of glucokinase-expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes 2006; 55: 1–12. [PubMed] [Google Scholar]
- 185.Matschinsky FM. Banting lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes 1996; 45: 223–241. [DOI] [PubMed] [Google Scholar]
- 186.Massa ML, Gagliardino JJ, Francini F. Liver glucokinase: an overview on the regulatory mechanisms of its activity. IUBMB Life 2011; 63: 1–6. [DOI] [PubMed] [Google Scholar]
- 187.Matschinsky FM. Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes 1990; 39: 647–652. [DOI] [PubMed] [Google Scholar]
- 188.Ferre T, Riu E, Bosch F, et al. Evidence from transgenic mice that glucokinase is rate limiting for glucose utilization in the liver. FASEB J 1996; 10: 1213–1218. [DOI] [PubMed] [Google Scholar]
- 189.Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov 2009; 8: 399–416. [DOI] [PubMed] [Google Scholar]
- 190.Meininger GE, Scott R, Alba M, et al. Effects of MK-0941, a novel glucokinase activator, on glycemic control in insulin-treated patients with type 2 diabetes. Diabetes Care 2011; 34: 2560–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Amin N, Aggarwal N, Pall D, et al. Two dose-ranging studies with PF-04937319, a systemic partial activator of glucokinase, as add-on therapy to metformin in adults with type 2 diabetes. Diabetes Obes Metab 2015; 17: 751–759. [DOI] [PubMed] [Google Scholar]
- 192.Kiyosue A, Hayashi N, Komori H, et al. Dose-ranging study with the glucokinase activator AZD1656 as monotherapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab 2013; 15: 923–930. [DOI] [PubMed] [Google Scholar]
- 193.Tsumura Y, Tsushima Y, Tamura A, et al. TMG-123, a novel glucokinase activator, exerts durable effects on hyperglycemia without increasing triglyceride in diabetic animal models. PLoS ONE 2017; 12: e0172252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kobayashi T, Shimomoto T, Tamura A, et al. A novel glucokinase activator TMG-123 causes long-lasting hypoglycemia and impairs spermatogenesis irreversibly in rats. J Toxicol Sci 2021; 46: 115–123. [DOI] [PubMed] [Google Scholar]
- 195.Egan A, Vella A. TTP399: an investigational liver-selective glucokinase (GK) activator as a potential treatment for type 2 diabetes. Expert Opin Investig Drugs 2019; 28: 741–747. [DOI] [PubMed] [Google Scholar]
- 196.Guarente L. Sirtuins, aging, and medicine. N Engl J Med 2011; 364: 2235–2244. [DOI] [PubMed] [Google Scholar]
- 197.Morris BJ. Seven sirtuins for seven deadly diseases of aging. Free Radic Biol Med 2013; 56: 133–171. [DOI] [PubMed] [Google Scholar]
- 198.Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nat Rev Endocrinol 2009; 5: 367–373. [DOI] [PubMed] [Google Scholar]
- 199.Cao Y, Jiang X, Ma H, et al. SIRT1 and insulin resistance. J Diabetes Complications 2016; 30: 178–183. [DOI] [PubMed] [Google Scholar]
- 200.Moynihan KA, Grimm AA, Plueger MM, et al. Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab 2005; 2: 105–117. [DOI] [PubMed] [Google Scholar]
- 201.Sun C, Zhang F, Ge X, et al. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 2007; 6: 307–319. [DOI] [PubMed] [Google Scholar]
- 202.American Diabetes A. (7) Approaches to glycemic treatment. Diabetes Care 2015; 38(Suppl.): S41–S48. [DOI] [PubMed] [Google Scholar]
- 203.Cuyàs E, Verdura S, Llorach-Parés L, et al. Metformin is a direct SIRT1-activating compound: computational modeling and experimental validation. Front Endocrinol 2018; 9: 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Song J, Yang B, Jia X, et al. Distinctive roles of sirtuins on diabetes, protective or detrimental? Front Endocrinol 2018; 9: 724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003; 425: 191–196. [DOI] [PubMed] [Google Scholar]
- 206.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 2006; 5: 493–506. [DOI] [PubMed] [Google Scholar]
- 207.González-Rodríguez Á, Santamaría B, Mas-Gutierrez JA, et al. Resveratrol treatment restores peripheral insulin sensitivity in diabetic mice in a sirt1-independent manner. Mol Nutr Food Res 2015; 59: 1431–1442. [DOI] [PubMed] [Google Scholar]
- 208.Lee Y-E, Kim J-W, Lee E-M, et al. Chronic resveratrol treatment protects pancreatic islets against oxidative stress in db/db mice. PLoS ONE 2012; 7: e50412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab Syndr Relat Disord 2014; 12: 497–501. [DOI] [PubMed] [Google Scholar]
- 210.Brasnyó P, Molnár GA, Mohás M, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr 2011; 106: 383–389. [DOI] [PubMed] [Google Scholar]
- 211.Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res 2012; 32: 537–541. [DOI] [PubMed] [Google Scholar]
- 212.Jeyaraman MM, Al-Yousif NSH, Singh Mann A, et al. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev 2020; 1: CD011919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.de Ligt M, Bergman M, Fuentes RM, et al. No effect of resveratrol supplementation after 6 months on insulin sensitivity in overweight adults: a randomized trial. Am J Clin Nutr 2020; 112: 1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.ClinicalTrials.gov. Resveratrol in type 2 diabetes and obesity, https://clinicaltrials.gov/ct2/show/NCT01158417?term=NCT01158417 (2012, accessed 14 March 2021).
- 215.ClinicalTrials.gov. A clinical study to assess the safety, tolerability, and activity of oral SRT2104 capsules administered for 28 days to subjects with type 2 diabetes mellitus, https://clinicaltrials.gov/ct2/show/study/NCT01018017?term=NCT01018017.&rank=1 (2017, accessed 15 March 2021).
- 216.ClinicalTrials.gov. A clinical study to assess the safety and pharmacokinetics of SRT2379 in normal healthy male volunteers, https://clinicaltrials.gov/ct2/show/NCT01018628?term=NCT01018628&rank=1 (2017, accessed 15 March 2021).
- 217.Gathercole LL, Lavery GG, Morgan SA, et al. 11β-Hydroxysteroid dehydrogenase 1: translational and therapeutic aspects. Endocr Rev 2013; 34: 525–555. [DOI] [PubMed] [Google Scholar]
- 218.Li X, Wang J, Yang Q, et al. 11β-hydroxysteroid dehydrogenase type 1 in obese subjects with type 2 diabetes mellitus. Am J Med Sci 2017; 354: 408–414. [DOI] [PubMed] [Google Scholar]
- 219.Macfarlane DP, Forbes S, Walker BR. Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J Endocrinol 2008; 197: 189–204. [DOI] [PubMed] [Google Scholar]
- 220.Stimson RH, Andrew R, McAvoy NC, et al. Increased whole-body and sustained liver cortisol regeneration by 11beta-hydroxysteroid dehydrogenase type 1 in obese men with type 2 diabetes provides a target for enzyme inhibition. Diabetes 2011; 60: 720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shukla R, Basu AK, Mandal B, et al. 11beta hydroxysteroid dehydrogenase-1 activity in type 2 diabetes mellitus: a comparative study. BMC Endocr Disord 2019; 19: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rosenstock J, Banarer S, Fonseca VA, et al. The 11-β-hydroxysteroid dehydrogenase type 1 inhibitor INCB13739 improves hyperglycemia in patients with type 2 diabetes inadequately controlled by metformin monotherapy. Diabetes Care 2010; 33: 1516–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Feig P, Shah S, Hermanowski-Vosatka A, et al. Effects of an 11β-hydroxysteroid dehydrogenase type 1 inhibitor, MK-0916, in patients with type 2 diabetes mellitus and metabolic syndrome. Diabetes Obes Metab 2011; 13: 498–504. [DOI] [PubMed] [Google Scholar]
- 224.Heise T, Morrow L, Hompesch M, et al. Safety, efficacy and weight effect of two 11β-HSD1 inhibitors in metformin-treated patients with type 2 diabetes. Diabetes Obes Metab 2014; 16: 1070–1077. [DOI] [PubMed] [Google Scholar]
- 225.Wyrwoll CS, Holmes MC, Seckl JR. 11β-hydroxysteroid dehydrogenases and the brain: from zero to hero, a decade of progress. Front Neuroendocrinol 2011; 32: 265–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Freude S, Heise T, Woerle HJ, et al. Safety, pharmacokinetics and pharmacodynamics of BI 135585, a selective 11β-hydroxysteroid dehydrogenase-1 (HSD1) inhibitor in humans: liver and adipose tissue 11β-HSD1 inhibition after acute and multiple administrations over 2 weeks. Diabetes Obes Metab 2016; 18: 483–490. [DOI] [PubMed] [Google Scholar]
- 227.Wallia A, Molitch ME. Insulin therapy for type 2 diabetes mellitus. JAMA 2014; 311: 2315–2325. [DOI] [PubMed] [Google Scholar]
- 228.Satake S, Moore MC, Igawa K, et al. Direct and indirect effects of insulin on glucose uptake and storage by the liver. Diabetes 2002; 51: 1663–1671. [DOI] [PubMed] [Google Scholar]
- 229.Matteucci E, Giampietro O, Covolan V, et al. Insulin administration: present strategies and future directions for a noninvasive (possibly more physiological) delivery. Drug Des Devel Ther 2015; 9: 3109–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hoffman A, Ziv E. Pharmacokinetic considerations of new insulin formulations and routes of administration. Clin Pharmacokinet 1997; 33: 285–301. [DOI] [PubMed] [Google Scholar]
- 231.Owens DR. New horizons – alternative routes for insulin therapy. Nat Rev Drug Discov 2002; 1: 529–540. [DOI] [PubMed] [Google Scholar]
- 232.Arbit E, Kidron M. Oral insulin: the rationale for this approach and current developments. J Diabetes Sci Technol 2009; 3: 562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gedawy A, Martinez J, Al-Salami H, et al. Oral insulin delivery: existing barriers and current counter-strategies. J Pharm Pharmacol 2018; 70: 197–213. [DOI] [PubMed] [Google Scholar]
- 234.Amidon GL, Lee HJ. Absorption of peptide and peptidomimetic drugs. Annu Rev Pharmacol Toxicol 1994; 34: 321–341. [DOI] [PubMed] [Google Scholar]
- 235.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov 2014; 13: 655–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chin J, Mahmud K, Kim SE, et al. Insight of current technologies for oral delivery of proteins and peptides. Drug Discov Today Technol 2012; 9: e71–e174. [DOI] [PubMed] [Google Scholar]
- 237.Eldor R, Fleming GA, Neutel J, et al. 105-LB: evening oral insulin (ORMD-0801) glycemic effects in uncontrolled T2DM patients. Diabetes 2020; 69: 105-LB. [Google Scholar]
- 238.Banerjee A, Lee J, Mitragotri S. Intestinal mucoadhesive devices for oral delivery of insulin. Bioeng Transl Med 2016; 1: 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hashemi M, Ramezani V, Seyedabadi M, et al. Formulation and optimization of oral mucoadhesive patches of myrtus communis by Box Behnken design. Adv Pharm Bull 2017; 7: 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gupta V, Hwang BH, Doshi N, et al. Delivery of exenatide and insulin using mucoadhesive intestinal devices. Ann Biomed Eng 2016; 44: 1993–2007. [DOI] [PubMed] [Google Scholar]
- 241.Ng LC, Gupta M. Transdermal drug delivery systems in diabetes management: a review. Asian J Pharm Sci 2020; 15: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol 2008; 26: 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Scheuplein RJ. Permeability of the skin: a review of major concepts and some new developments. J Invest Dermatol 1976; 67: 672–676. [Google Scholar]
- 244.Cevc G, Gebauer D, Stieber J, et al. Ultraflexible vesicles, transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim Biophys Acta 1998; 1368: 201–215. [DOI] [PubMed] [Google Scholar]
- 245.Sintov AC, Wormser U. Topical iodine facilitates transdermal delivery of insulin. J Control Release 2007; 118: 185–188. [DOI] [PubMed] [Google Scholar]
- 246.Pillai O, Borkute SD, Sivaprasad N, et al. Transdermal iontophoresis of insulin. II. Physicochemical considerations. Int J Pharm 2003; 254: 271–280. [DOI] [PubMed] [Google Scholar]
- 247.Ahad A, Raish M, Bin Jardan YA, et al. Delivery of insulin via skin route for the management of diabetes mellitus: approaches for breaching the obstacles. Pharmaceutics 2021; 13: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ginsberg BH. Patch pumps for insulin. J Diabetes Sci Technol 2019; 13: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Heinemann L, Waldenmaier D, Kulzer B, et al. Patch pumps: are they all the same. J Diabetes Sci Technol 2019; 13: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mader JK, Lilly LC, Aberer F, et al. Improved glycaemic control and treatment satisfaction with a simple wearable 3-day insulin delivery device among people with type 2 diabetes. Diabet Med 2018; 35: 1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Berget C, Messer LH, Forlenza GP. A clinical overview of insulin pump therapy for the management of diabetes: past, present, and future of intensive therapy. Diabetes Spectr 2019; 32: 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lilly LC, Mader JK, Warner J. Developing a simple 3-day insulin delivery device to meet the needs of people with type 2 diabetes. J Diabetes Sci Technol 2019; 13: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Integrated Diabetes Service. Hybrid closed loop systems: what they are, and how they work, https://integrateddiabetes.com/what-is-a-hybrid-closed-loop-system/hybrid-closed-loop-comparisons-options/ (accessed 8 June 2021).
- 254.Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ 2018; 361: k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Weisman A, Bai JW, Cardinez M, et al. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017; 5: 501–512. [DOI] [PubMed] [Google Scholar]
- 256.Leelarathna L, Choudhary P, Wilmot EG, et al. Hybrid closed-loop therapy: where are we in 2021. Diabetes Obes Metab 2021; 23: 655–660. [DOI] [PubMed] [Google Scholar]
- 257.Del Prato S, Kang J, Trautmann ME, et al. Efficacy and safety of once-monthly efpeglenatide in patients with type 2 diabetes: results of a phase 2 placebo-controlled, 16-week randomized dose-finding study. Diabetes Obes Metab 2020; 22: 1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Nishimura R, Osonoi T, Koike Y, et al. A randomized pilot study of the effect of trelagliptin and alogliptin on glycemic variability in patients with type 2 diabetes. Adv Ther 2019; 36: 3096–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kesavadev J, Srinivasan S, Saboo B, et al. The do-it-yourself artificial pancreas: a comprehensive review. Diabetes Ther 2020; 11: 1217–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev 2004; 56: 581–587. [DOI] [PubMed] [Google Scholar]
- 261.Jin X, Zhu DD, Chen BZ, et al. Insulin delivery systems combined with microneedle technology. Adv Drug Deliv Rev 2018; 127: 119–137. [DOI] [PubMed] [Google Scholar]
- 262.Narayan RJ. Transdermal delivery of insulin via microneedles. J Biomed Nanotechnol 2014; 10: 2244–2260. [DOI] [PubMed] [Google Scholar]
- 263.Larraneta E, McCrudden MT, Courtenay AJ, et al. Microneedles: a new frontier in nanomedicine delivery. Pharm Res 2016; 33: 1055–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Gill HS, Denson DD, Burris BA, et al. Effect of microneedle design on pain in human volunteers. Clin J Pain 2008; 24: 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zhang Y, Yu J, Kahkoska AR, et al. Advances in transdermal insulin delivery. Adv Drug Deliv Rev 2019; 139: 51–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhang Y, Jiang G, Yu W, et al. Microneedles fabricated from alginate and maltose for transdermal delivery of insulin on diabetic rats. Mater Sci Eng C Mater Biol Appl 2018; 85: 18–26. [DOI] [PubMed] [Google Scholar]
- 267.Fink JB, Molloy L, Patton JS, et al. Good things in small packages: an innovative delivery approach for inhaled insulin. Pharm Res 2017; 34: 2568–2578. [DOI] [PubMed] [Google Scholar]
- 268.Heinemann L. The failure of exubera: are we beating a dead horse? J Diabetes Sci Technol 2008; 2: 518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Dance Biopharm. Dance Biopharm presents phase 2 clinical data highlighting rapid effects of inhaled human insulin at European association for the study of diabetes meeting, https://www.globenewswire.com/news-release/2019/09/19/1917968/0/en/Dance-Biopharm-Presents-Phase-2-Clinical-Data-Highlighting-Rapid-Effects-of-Inhaled-Human-Insulin-at-European-Association-for-the-Study-of-Diabetes-Meeting.html (2019, accessed 16 March 2021).
- 270.Easa N, Alany RG, Carew M, et al. A review of non-invasive insulin delivery systems for diabetes therapy in clinical trials over the past decade. Drug Discov Today 2019; 24: 440–451. [DOI] [PubMed] [Google Scholar]
- 271.Nishimura E, Kjeldsen T, Hubalek F, et al. 236-OR: molecular and biological properties of insulin icodec, a new insulin analog designed to give a long half-life suitable for once-weekly dosing. Diabetes 2020; 69: 236-OR. [Google Scholar]
- 272.Rosenstock J, Bajaj HS, Janez A, et al. Once-weekly insulin for type 2 diabetes without previous insulin treatment. N Engl J Med 2020; 383: 2107–2116. [DOI] [PubMed] [Google Scholar]
- 273.Manolio TA. Genomewide association studies and assessment of the risk of disease. N Engl J Med 2010; 363: 166–176. [DOI] [PubMed] [Google Scholar]
- 274.Xue A, Wu Y, Zhu Z, et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun 2018; 9: 2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Walker M, Mari A, Jayapaul MK, et al. Impaired beta cell glucose sensitivity and whole-body insulin sensitivity as predictors of hyperglycaemia in non-diabetic subjects. Diabetologia 2005; 48: 2470–2476. [DOI] [PubMed] [Google Scholar]
- 276.Tura A, Grassi A, Winhofer Y, et al. Progression to type 2 diabetes in women with former gestational diabetes: time trajectories of metabolic parameters. PLoS ONE 2012; 7: e50419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Deshmukh HA, Madsen AL, Viñuela A, et al. Genome-wide association analysis of pancreatic beta-cell glucose sensitivity. J Clin Endocrinol Metab 2021; 106: 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gloyn AL, Weedon MN, Owen KR, et al. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes 2003; 52: 568–572. [DOI] [PubMed] [Google Scholar]
- 279.Wessel J, Chu AY, Willems SM, et al. Low-frequency and rare exome chip variants associate with fasting glucose and type 2 diabetes susceptibility. Nat Commun 2015; 6: 5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Altshuler D, Hirschhorn JN, Klannemark M, et al. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet 2000; 26: 76–80. [DOI] [PubMed] [Google Scholar]
- 281.Wainwright CE, Elborn JS, Ramsey BW, et al. Lumacaftor–ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 2015; 373: 220–231. [DOI] [PubMed] [Google Scholar]
- 282.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011; 365: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Libianto R, Ekinci EI. New agents for the treatment of type 2 diabetes. Crit Care Clin 2019; 35: 315–328. [DOI] [PubMed] [Google Scholar]
- 284.Bailey CJ. Metformin: historical overview. Diabetologia 2017; 60: 1566–1576. [DOI] [PubMed] [Google Scholar]