Abstract
Cardiovascular disease (CVD) is the leading cause of death and disability worldwide. Influenza infection is associated with an increased risk of cardiovascular events (myocardial infarction, stroke, and heart failure exacerbation) and mortality, and all‐cause mortality in patients with CVD. Infection with influenza leads to a systemic inflammatory and thrombogenic response in the host body, which further causes destabilization of atherosclerotic plaques. Influenza vaccination has been shown to be protective against cardiovascular and cerebrovascular events in several observational and prospective studies of at‐risk populations. Hence, many international guidelines recommend influenza vaccination for adults of all ages, especially for individuals with high‐risk conditions such as CVD. Despite these long‐standing recommendations, influenza vaccine uptake among US adults with CVD remains suboptimal. Specifically, vaccination uptake is strikingly low among patients aged <65 years, non‐Hispanic Black individuals, those without health insurance, and those with diminished access to healthcare services. Behavioral factors such as perceived vaccine efficacy, vaccine safety, and attitudes towards vaccination play an important role in vaccine acceptance at the individual and community levels. With the ongoing COVID‐19 pandemic, there is a potential threat of a concurrent epidemic with influenza. This would be devastating for vulnerable populations such as adults with CVD, further stressing the need for ensuring adequate influenza vaccination coverage. In this review, we describe a variety of strategies to improve the uptake of influenza vaccination in patients with CVD through improved understanding of key sociodemographic determinants and behaviors that are associated with vaccination, or the lack thereof. We further discuss the potential use of relevant strategies for COVID‐19 vaccine uptake among those with CVD.
Keywords: behavioral factors, cardiovascular disease, influenza, interventions, sociodemographic factors, vaccination
Subject Categories: Cardiovascular Disease, Epidemiology
Nonstandard Abbreviations and Acronyms
- ASCVD
atherosclerotic cardiovascular disease
- aPR
adjusted prevalence ratio
- CDC
Centers for Disease Control and Prevention
- COVID‐19
Coronavirus Disease 2019
Cardiovascular disease (CVD) is the leading cause of mortality, with about 800 000 lives lost to CVD annually in the United States.1, 2 While there has been a reduction in age‐specific cardiovascular mortality, the burden of CVD in the United States has been rising steadily, leading to massive economic costs and loss of disability‐adjusted life years. Annual influenza epidemics lead to significant rises in cardiovascular events and deaths every year.3, 4 Since 2010, the Centers for Disease Control and Prevention (CDC) estimated 140 000 to 810 000 hospitalizations and 12 000 to 61 000 deaths annually in the United States attributable to influenza.5 Apart from its well‐recognized respiratory complications, about one in eight patients admitted with influenza has an acute cardiovascular event, with 31% of those requiring intensive care and 7% eventually dying.6 Individuals with preexisting CVD have even greater risk of cardiovascular events and mortality associated with influenza than in the general population.6
Many observational and prospective studies have demonstrated the influenza vaccine to be protective against cardiovascular mortality, especially in this high‐risk population.7, 8 Given the benefits conferred by influenza vaccination and the risks posed by influenza infection among patients with established CVD, the CDC's Advisory Committee on Immunization Practices and many international societies strongly recommend annual influenza vaccination in patients with CVD.9 However, the uptake of influenza vaccination remains below the national target in the general population as well as in high‐risk groups such as patients with CVD. Specifically, in US adults aged 18 to 64 years, the rate of influenza vaccination was 45% and 33% among those with and without high‐risk conditions, respectively, well below the target of 90% set by the Healthy People 2020 initiative.10 Insufficient vaccination uptake is particularly concerning this season amid the current COVID‐19 pandemic, potentially leading to a concurrent epidemic.
In this review, we describe the epidemiology of influenza infection and the pathophysiology of cardiovascular events in patients with CVD. We then discuss current recommendations for annual seasonal influenza vaccination, published data on uptake of influenza vaccination in the United States, and sociodemographic‐ and behavior‐related factors associated with influenza vaccination. Further, we outline strategies to improve vaccination coverage, especially among vulnerable populations including patients with CVD.
CVD and Influenza Infection
CVD remains the leading cause of mortality worldwide accounting for >17 million deaths yearly, which is expected to increase to >24 million by 2030.11 Despite reductions in age‐specific CVD mortality in recent decades, the prevalence, complications, and economic burden of CVD have been expansive because of progressively aging populations and global population growth.12 An estimated USD 351 billion of economic loss between 2014 and 2015 in the United States is attributed to CVD.13, 14 Influenza infection typically presents with upper and/or lower respiratory symptoms which are accompanied by non‐specific systemic symptoms. Influenza is usually a self‐limiting infection, but can cause severe and fatal complications in high‐risk groups, including pregnant women, children, adults aged ≥65 years, and individuals with chronic medical conditions like CVD, diabetes mellitus, chronic kidney disease, and others (Table 1).15 Influenza affects 5% to 10% of the general population annually. Globally, 290 000 to 650 000 deaths are attributed to influenza‐associated illness every year, with disproportionately higher mortality rates among older adults aged ≥65 years and those with chronic diseases.16
Table 1.
High‐Risk Groups for Influenza Complications15
| All children aged 6–59 mo |
| Adult of age ≥50 y |
| Women who are or will be pregnant during influenza season |
|
Adults with chronic medical conditions which include:
|
| Immunocompromised adults (including HIV/AIDS) or those on immunosuppressive treatment |
| Individuals with body mass index ≥40 |
| Individuals aged <19 y on long‐term aspirin‐ or salicylate‐containing medications and who might be at risk for Reye syndrome after influenza infection |
| American Indians/Alaskan Natives |
| Residents of nursing homes and other long‐term care facilities |
The listing of risk groups is not in any order of hierarchy.
Besides respiratory manifestations, influenza infection is associated with significant increases in both fatal and non‐fatal cardiovascular events such as acute myocardial infarction and stroke.17 A few self‐controlled case‐series studies have demonstrated a 3‐ to 6‐fold increased risk of acute myocardial infarction, stroke, and hospitalization following influenza infection compared with the rest of the year.18, 19 The pathophysiology of influenza leading to acute coronary syndromes is mainly because of systemic inflammatory and thrombogenic states from the surge in several acute phase reactants in response to viral infection.20, 21 The acute inflammatory reaction that evolves in the coronary bed and atherosclerotic plaque leads to endothelial dysfunction, vasoconstriction, platelet activation, and dysregulation of the coagulation system. Further, the increased sympathetic activity and metabolic demand, changes in circulatory volume, and variations in systemic and coronary vascular tone cause plaque destabilization and disruption leading to acute myocardial infarction.22
Influenza Vaccine Effectiveness
There is now substantial evidence that influenza vaccination is associated with a reduction in CVD events and mortality.23 In a population‐based case‐control study of individuals without prior heart disease, adults who received influenza vaccine in the past 12 months were 50% less likely to have an out‐of‐hospital primary cardiac arrest compared with those who had not received influenza vaccination (odds ratio [OR], 0.51; 95% CI, 0.33–0.79).24 Additionally, influenza vaccination is associated with lower rates of hospitalization for cardiovascular and cerebrovascular diseases.25 In a meta‐analysis of case‐control studies, the effectiveness of influenza vaccine in preventing acute myocardial infarction among those with CVD was found to be 29% (95% CI, 9%–44%), similar to that of standard interventions such as statin therapy, anti‐hypertensive medications, and smoking cessation.26, 27, 28 Similarly, individuals who received influenza vaccination had a decreased risk of stroke compared with individuals who did not receive influenza vaccination (OR, 0.82; 95% CI, 0.75–0.91; P<0.001).29
Current Recommendations for Influenza Vaccination
The US CDC's Advisory Committee on Immunization Practices recommends annual influenza vaccination for all adults, especially among those who are at increased risk of severe illness including adults aged ≥50 years, individuals with chronic conditions, and pregnant women. Additionally, emphasis has been placed on vaccination of household contacts and caregivers of high‐risk individuals and healthcare workers to promote herd immunity.15 Various cardiology societies have also provided recommendations for influenza vaccination among individuals with CVD. The American Heart Association and American College of Cardiology recommend annual influenza vaccination with inactivated vaccine among people with atherosclerotic CVD (ASCVD) as part of a comprehensive secondary prevention strategy (Class I, Level B).9 Similarly, the Sixth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention provided a class IIb recommendation for annual influenza vaccination among patients with established CVD (Table 2).15, 30, 31, 32, 33, 34
Table 2.
International Scientific Society Recommendations for Influenza Vaccination in Patients With Cardiovascular Diseases
| Society | Recommendation for Influenza Vaccination |
|---|---|
| ACIP/CDC | Routine annual influenza vaccination is recommended for all people aged ≥6 mo who do not have contraindications, with increased priority for high‐risk groups including patients with CVD.15 |
| AHA/ACC | Influenza immunization with inactivated vaccine is recommended as part of comprehensive secondary prevention in people with coronary and other atherosclerotic vascular disease (Class 1, Level B).31 |
| ECDC | All adults who are recommended to have the influenza vaccine should get vaccinated. Vaccination is especially important for people at higher risk of serious influenza complications: adults with specific chronic medical conditions, pregnant women, and children aged 6–59 mo, the elderly and healthcare workers.32 |
| ESC | Annual influenza vaccination may be considered in patients with established CVD (Class IIB, Level C).30 |
| NACI‐Canada | Influenza vaccination should be offered annually to anyone 6 mo of age and older who does not have contraindications to the vaccine, with priority to those who are at increased of complications or hospitalization, and those capable of transmitting to high‐risk population. Patients with CVD are included in high‐risk groups.33 |
| WHO | Annual influenza vaccination is recommended with highest priority for pregnant women. Additional risk groups to be considered include children aged 6–59 mo, adults of age ≥65 y, those with specific chronic medical conditions and healthcare workers.34 |
ACC indicates American College of Cardiology; ACIP, Advisory Committee on Immunization Practices; AHA, American Heart Association; CDC, Centers for Disease Control and Prevention; CVD, cardiovascular disease; ECDC, European Center for Disease Prevention and Control; ESC, European Society of Cardiology; NACI, National Advisory Committee on Immunization; and WHO, World Health Organization.
Prevalence and Determinants of Influenza Vaccination
Despite well‐established recommendations of annual influenza vaccination for adults with CVD, the overall prevalence of influenza vaccination in the United States was 67% among those with ASCVD, which is much lower than the national target of 90% set by the Healthy People 2020 initiative.35 In this observational study, Grandhi et al found that individuals with a greater cumulative number of the following sociodemographic characteristics (aged 40–64 years, non‐Hispanic Black race/ethnicity, uninsured status, low income, and lower education level) and healthcare contextual and accessibility barriers (lack of usual source of care) were at a greater risk of lacking influenza vaccination.35 Among individuals with ≥4 of these risk factors, 60% (95% CI, 55.8%–63.5%) did not receive annual influenza vaccination.35 Usual source of care is defined as a particular healthcare professional or facility that a person usually goes to if sick or in need of healthcare advice.36 In addition to sociodemographic and physical determinants (eg, age, race/ethnicity, and income), other factors like access to healthcare (eg, insurance coverage and usual source of care) and patients' attitudes and behaviors towards vaccination (eg, perceptions of vaccine efficacy and safety) play important roles in determining vaccine acceptance. We have discussed these determinants of vaccine uptake in detail and categorized them into 4 groups: sociodemographic, health behavioral, contextual, and psychological determinants (Figure 1).
Figure 1. Determinants of influenza vaccine uptake in adults with cardiovascular diseases.
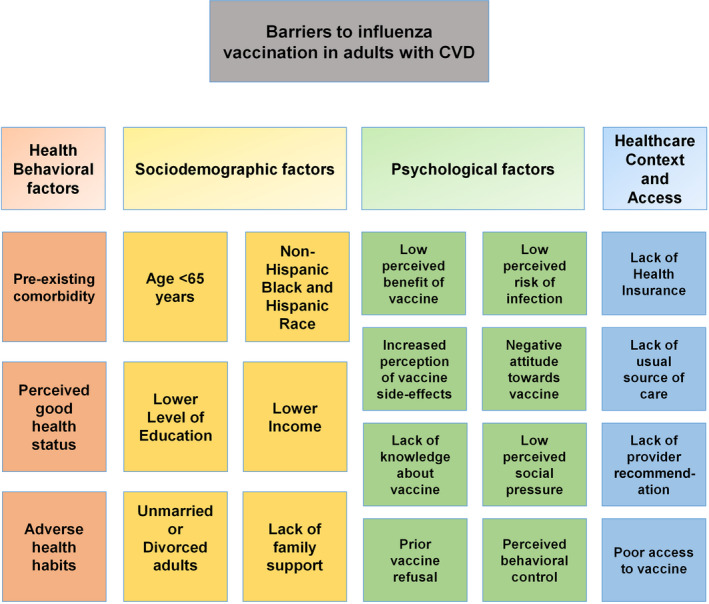
CVD indicates cardiovascular disease.
Sociodemographic Determinants
Age
Age is an important determinant of influenza vaccination in US adults, with younger adults being less likely to receive vaccination compared with older adults aged ≥65 years. In a cross‐sectional study using data from National Health Interview Survey, Singleton et al reported that influenza vaccination rates among adults with heart disease were significantly lower in younger age groups (18–49 years) compared with older populations (≥50 years), during 1996 to 1997 through 2000 to 2001 influenza seasons.37 More recently, using pooled National Health Interview Survey data from 2008 to 2016, Grandhi et al demonstrated that, among individuals with ASCVD, younger adults aged 40 to 64 years were more likely to lack vaccination than older adults aged ≥65 years, with vaccination rates at 54% among adults aged 40 to 64 years versus 76% among adults aged ≥65 years.35 International studies using observational data have shown similar age‐based differences in influenza vaccination, notably that adults with CVD who were aged <65 years had lower vaccination rates when compared with older populations.38, 39
Race/Ethnicity
In addition to age‐based differences, racial disparities in influenza vaccination have been also recognized, with non‐Hispanic Black and Hispanic populations noted to be at an increased risk of lacking vaccination when compared with non‐Hispanic White and Asian populations.40 In a recent study of adults aged ≥40 years with ASCVD, non‐Hispanic Black individuals were 1.2 times more likely to miss vaccination when compared with non‐Hispanic White individuals.35 In another cross‐sectional study of adults with CVD aged 18 to 64 years, racial differences in vaccine coverage were only significant in older adults aged 50 to 64 years, with lower uptake in non‐Hispanic Black and Hispanic individuals.37 In a retrospective study by Takayama et al, racial disparities in vaccine uptake were greater in older adults aged ≥65 years when compared with adults aged 18 to 64 years.41
Sex
Overall, various studies have demonstrated influenza vaccination rates by sex to be variable in the general population and among high‐risk subgroups. Female sex was associated with lower vaccination rates among some studies and higher vaccination rate in others.42 In our recent cross‐sectional study, women had a similar likelihood of influenza vaccination as that of men with ASCVD.35
Family Support
Marital status was noted to influence vaccine uptake, with divorced and widowed adults having reduced vaccination rates when compared with married adults.40 Additionally, individuals who live with family members, particularly older adults aged ≥65 years, were more likely to receive vaccination.43
Income
Adults with lower family income have been shown to be less likely to receive influenza vaccination compared with those with middle to high family income.35 In an observational study of US adults using data from 2009 Behavioral Risk Factor Surveillance System, adults with annual household income of <$35 000 were less likely to be vaccinated than those with higher income within each age group studied. Moreover, the impact of lower income on influenza vaccination was more pronounced in younger adults aged 18 to 64 years (adjusted prevalence ratio [aPR], 0.84; 95% CI, 0.81–0.89) when compared with those aged ≥65 years (aPR, 0.94; 95% CI, 0.92–0.96).41
Education
The positive association between maternal education and childhood vaccination is well‐known.44 However, an observational study in Europe over 7 influenza seasons showed an inconsistent relationship between level of education and influenza vaccine uptake in individuals of age >14 years. There was a positive correlation with higher educational status and vaccination in some countries like Austria and Poland while in other countries including Ireland, Italy, and Spain, there was a negative correlation.45 In our recent study of US adults with ASCVD, a lower level of completed education was identified as a significant barrier to influenza vaccination, with those having less than a high‐school level education being 1.25 times more likely to miss vaccination than those with higher relative level of completed education.35
Healthcare Context and Access
Health Insurance
A lack of health insurance coverage has been identified as one of the strongest barriers to influenza vaccine uptake, with uninsured individuals being nearly twice as likely to miss vaccination than those with insurance.35, 37, 39 Additionally, among those with ASCVD, unvaccinated rates have been found to be as high as 65% among individuals without insurance versus 31% among those with insurance.35 Lack of health insurance may be of greater consequence in younger adults of age 19 to 64 years with an uninsured rate of nearly 15% compared with <2% for those of age ≥65 years.46
Contact With Healthcare System
Having a usual source of care either alone or in combination with a usual provider has been consistently linked with improved influenza vaccine uptake.47 Among individuals with ASCVD, those without a usual source of care had vaccination rates at 43% (versus 69% among those with a usual source of care) and they were twice as likely to miss influenza vaccination than their counterparts with a usual source of care.35 Similarly, fewer recent visits to a doctor and hospitalization follow‐ups were associated with lower vaccine uptake.48 Use of preventive medical services like colon cancer screening was associated with increased influenza vaccination as well.49
Provider Cues to Action
Direct recommendation and education from healthcare professionals was one of the strongest motivators for vaccine uptake.50 In a self‐administered, anonymous questionnaire of randomly selected US physicians from the American Medical Association's physician master file, 14% of generalists and 25% of subspecialists failed to provide strong recommendation for influenza vaccination to their older and higher‐risk patients despite recognizing the importance of vaccination. In addition, providers' vaccination status and beliefs about vaccination had a strong positive correlation with their likelihood of recommending vaccine to their patients.51
Vaccine Access
General access to vaccines and reliability of vaccine supply have not been identified as barriers to influenza vaccination. However, factors such as physical disability, lack of transportation, and financial burden are impediments to vaccine access and uptake, especially in older adults.52 Individuals are more likely to consider vaccination if it is provided free of cost.53 Lack of time to obtain influenza vaccination has also been reported as a barrier, especially in healthcare workers.54 Vaccine access has now become a critical issue with decline in routine primary care visits amid the ongoing COVID‐19 pandemic.55, 56
Health Behavioral Barriers
Preexisting Comorbidities
There is an increased likelihood of influenza vaccination in adults with preexisting medical conditions, which could be explained by their increased contact with the healthcare system. In a cross‐sectional study of patients with CVD in Spain, higher vaccination rates were observed in those with accompanying pulmonary disease.38 Similarly, in a US population with CVD, adults having a concomitant medical indication for influenza vaccine were more likely to get vaccinated.37 In addition, prior history of myocardial infarction is positively associated with vaccination in younger adults, aged 18 to 64 years group (aPR, 1.08; 95% CI, 1.01–1.16) and negatively associated in older age groups (≥65 years) (aPR, 0.97; 95% CI, 0.94–0.99). Although older adults aged ≥65 years with a previous history of stroke were less likely to be vaccinated compared with those without history of stroke (aPR, 0.97; 95% CI, 0.94–1.00), there is no evidence for a clear relationship between prior stroke and vaccination in younger adults aged 18 to 64 years.41
Perceived Health Status
Individuals' judgement of their health status is a strong predictor of influenza vaccination, with perceived good health status being associated with lower vaccine uptake.57, 58 In an observational study using data from the Canadian Community Health Survey, there was a progressive increase in influenza vaccination rates with worsening perceived health status of the study individuals. In this study, individuals with poor perceived health status were 2.5 times more likely to receive vaccination compared with those with excellent perceived health status.59 Similarly, in a study of adults aged ≥65 years in the United Kingdom, the likelihood of receiving influenza vaccination increased as the self‐perceived health status worsened from excellent to poor.58
Health Habits
Several studies have consistently found that detrimental health habits such as physical inactivity and diet consisting of <5 servings of fruits or vegetables per day had a negative impact on vaccine uptake.41, 42, 60 However, the association between current smoking and influenza vaccine uptake was inconsistent.42 Nevertheless, smoking cessation was unvaryingly associated with increased uptake of vaccine, which could be explained by individual's motivation for and adoption of positive health habits in general.42
Psychological Barriers
To date, there is limited information about potential psychological barriers for influenza vaccination uptake among patients with CVD. In the general population, perceived lack of benefit of vaccination and increased perception of vaccine‐associated adverse events are negatively associated with vaccine uptake, with concern about vaccine safety being one of the most common reasons for vaccine refusal.42 Further, prior refusal to vaccination, negative attitudes towards vaccination, and lack of trust in the healthcare system have been identified as significant barriers to influenza vaccination.42, 48 In general, the misconception that the influenza vaccine itself can cause infection, as well as low perceived susceptibility and severity of infection, are associated with reduced vaccine acceptance.42 Encouragement to receive vaccination from family members and friends has been demonstrated to favorably impact influenza vaccination uptake especially in the elderly population.48 Moreover, healthcare professionals who do not believe that vaccination is their ethical and professional obligation, had a lower likelihood of receiving influenza vaccine themselves compared with their counterparts.42 Lack of perceived behavioral control, which is the perception of the difficulty in enacting a behavior, has been recognized as a barrier to vaccine uptake.42 Self‐efficacy, or the confidence in one's capability to get vaccinated, was also determined as an important predictor of vaccine uptake in a longitudinal observational study of university students in France.61 In another observational study in university students, “too lazy to get the influenza vaccine” or lack of motivation was cited as one of the major reasons for non‐vaccination.62
Strategies to Improve Influenza Vaccination Coverage
Understanding each of the above‐mentioned determinants of influenza vaccination is crucial to improve vaccination uptake among adults, and in particular those with or at‐risk of CVD. With the COVID‐19 pandemic, there is an increased probability of a concurrent epidemic of COVID‐19 and influenza infections later this influenza season. There is a pressing need for intensifying influenza vaccine uptake and simultaneously laying the groundwork for wide‐reaching strategies as more COVID‐19 vaccines become available. A combination of strategies directed at patients, providers, practices, and policymakers, which can help optimize vaccination coverage and uptake, are depicted in Figure 2.
Figure 2. Interventions to improve influenza vaccine uptake.
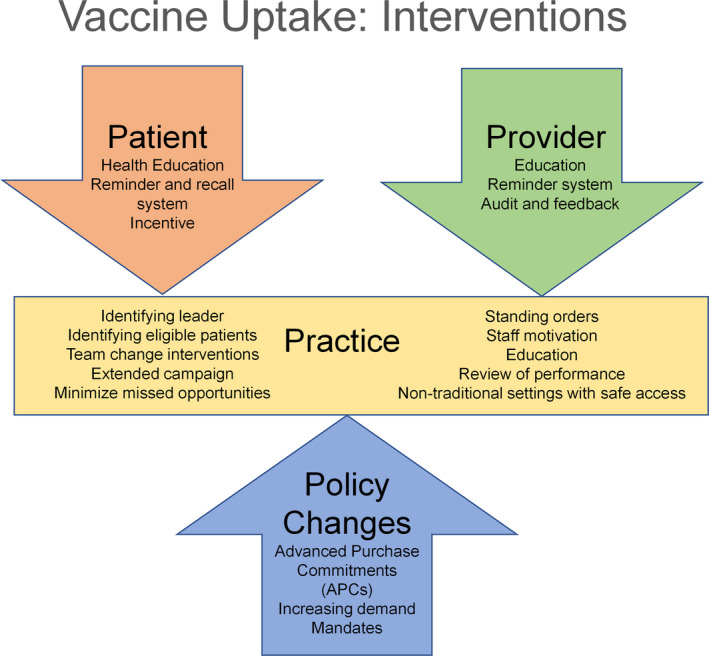
Patient‐Centered Interventions
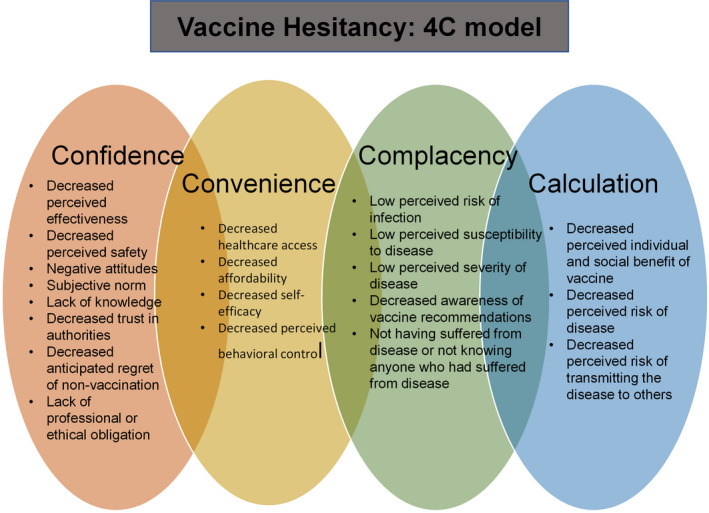
From a patient perspective, it is critical to understand patient‐related barriers to appropriate vaccination uptake. Vaccine hesitancy, described by the World Health Organization Strategic Advisory Group of Experts Working Group, as a delay in acceptance or refusal of vaccination despite availability of vaccination services, is an important factor in studying vaccine behaviors and perceptions.63 One model commonly used to describe the determinants of individual vaccination decisions is the 4C model, which includes confidence, complacency, calculation, and convenience (Figure 3).64 Complacency and lack of confidence have been identified as important barriers to influenza vaccine uptake during seasonal as well as pandemic influenza outbreaks. Moreover, convenience and calculation have been found to play more minor roles.42 Vaccine hesitancy is extremely concerning and relevant given the current COVID‐19 pandemic and its anticipated concurrent epidemic with influenza. Specifically, recent surveys have suggested that 1 out of 4 US adults were unwilling to receive the COVID‐19 vaccine, and only 30% of US adults would want to receive it as soon as it is available.65, 66, 67
Figure 3. 4C model of vaccine hesitancy.
Patient Education
Direct recommendations for vaccination by healthcare providers have been shown to improve influenza vaccine uptake in adults of all age groups, especially among elderly and high‐risk groups.48 In addition, healthcare providers can help educate their patient population on efficacy and safety of the influenza vaccine and increased susceptibility to infection and complications among unvaccinated individuals. Besides provider recommendations, other channels of communication such as posters in waiting areas, brochures, emails, and website resources, can be used to educate patients and caregivers about influenza infection and vaccination.68, 69 Outreach through online web portals and social media campaigns focusing on the safety and efficacy of vaccines and their relatively limited side effects have been shown to be effective in improving vaccination rates by alleviating concerns and dispelling myths about vaccination.70 These campaigns should further emphasize the social benefit, personal obligation, and collective responsibility of communities for protecting the most vulnerable from epidemics of influenza and COVID‐19.
Patient Reminder and Recall
A systematic review showed that influenza vaccination reminders via telephone, in person (eg, through home visits), and by sending emails, letters, and postcards have been found to be effective interventions in increasing influenza vaccine uptake among the general population.71 Multiple reminders were found be more effective than single reminders for influenza vaccination.71, 72 Additionally, using a patient reminder and recall system, which involves sending notifications to patients who are past due for vaccination, can help capture missed appointments and vaccinations.71
Financial Incentive
Incentives that reduce or eliminate out‐of‐pocket costs to the patients are associated with increased vaccination rates.73, 74 Additionally, providing vouchers for free vaccination has been associated with increased uptake in older adults aged ≥65 years in a study conducted in a European population.75
Sharing Experience
Knowing someone with influenza infection is an important positive predictor of vaccination, especially among older adults aged ≥65 years.48 Visit to a social center was also reported as a significant positive predictor for first time vaccination in older adults aged ≥65 years48, 76 Sharing positive experiences of vaccination at elderly centers can further help change negative perceptions about influenza vaccines in this at‐risk population.
Provider‐Centered Interventions
Provider Education
Educating healthcare providers and their staff about influenza infection, current vaccination guidelines, vaccine efficacy, types of vaccines available, and extended vaccine schedule can help increase vaccine recommendations and patient education in clinical practice.70, 77, 78, 79
Provider Reminder and Audit
Reminders through a range of different channels for healthcare providers represent effective tools for improving compliance with vaccination.69 Reminders include updated vaccination guidelines, recommended strategies for patient discussions, and procedures for documentation and billing. The best‐practice alerts generated in an electronic health record can alert the physician about vaccination status of the patient and thus help reduce the missed opportunities for vaccination. In the absence of electronic health records, physical documents such as tagged notes and stickers in front of the charts can be effective point‐of‐care reminders.80 Regularly evaluating provider‐level vaccination rates, and providing feedback and guidance to healthcare providers with lower rates, can improve vaccine uptake as well.69
Practice‐Centered Interventions
Identifying Practice Champion
Identifying a lead member of the staff to arrange the annual influenza vaccination campaign for the clinical practice has been associated with improved vaccine uptake.81 This leader could be a physician or another healthcare professional who is familiar with the vaccination guidelines and is strongly motivated to adopt strategies to improve vaccine uptake at the practice.
Identifying Eligible Patients
Potential chart reviews and digital care processes can help identify patients eligible for vaccination, especially older and at‐risk patients from the practice's patient database. These data can be used to monitor the level of appointments and vaccine uptake in real time, generate patient reminders, and create alert flags on patient charts.69
Team Change
Team change interventions such as assuming responsibilities of vaccine administration by other members of the healthcare team including nurses, has been shown to be an effective strategy in improving influenza vaccination in the community.82
Extended Vaccination Campaign
As per CDC guidelines, annual influenza vaccination must be administered preferably early in the fall before the onset of influenza activity in the community. However, vaccination should be offered throughout the influenza season for those who may have missed early vaccination because of continuing benefit of vaccination later in the season, even after the onset of influenza activity in the community.15 Thus, practices should offer influenza vaccines throughout the influenza season and not just the early months of October and November.
Minimizing Missed Opportunities
Influenza vaccination should be offered to patients on every contact with the healthcare system, including routine healthcare visits, during hospitalizations, and post‐hospitalization follow‐up visits. Selected office hours can be dedicated to only influenza vaccination either in a parallel‐track during daytime or during after‐hours or weekends.69 These vaccine‐only clinics should be scheduled during influenza season to broaden vaccine coverage.69, 74, 83 Eliminating the need for appointments at these clinics can further encourage patients to obtain their vaccination. Further, emergency departments have also been identified as potential settings for increasing influenza vaccination for at‐risk patients.84
Standing Orders
Standing orders authorize nurses, pharmacists, and other healthcare personnel to assess vaccine status and administer vaccines to patients without examination or direct order from the attending physician based on the state laws of the practice location. Standing order is a powerful tool for reducing missed opportunities for vaccination, both in outpatient and hospital settings85, 86 and are endorsed by Community Preventive Services Task and Advisory Committee on Immunization Practices.87, 88 Standing order consists of information about eligibility for the vaccine, contraindications of the vaccine, method of administration, vaccine information, vaccine documentation, adverse effects from the vaccine and their management, and information about reporting adverse events.86
Review of Performance
The written review of influenza vaccination rate of a clinical practice helps increase the vaccination rate by identifying areas of improvement, increasing motivation of the staff, and organizing a more effective vaccination campaign.81
Non‐Traditional Settings for Vaccination
In an economic analysis by Prosser et al, the mean cost of vaccination in non‐traditional settings including pharmacies, workplace, retail stores, and other locations was significantly lower than in the physician's office.89 Further, vaccination in non‐traditional settings was projected to be cost saving for high‐risk adults of all ages and healthy adults aged ≥50 years.89 Non‐traditional vaccine settings can potentially play an instrumental role in improving vaccination and awareness rates in marginalized communities and medically underserved populations.90, 91 Other innovative efforts by healthcare institutions such as express vaccination clinics, home visits for high‐risk patients, and refitted ambulances known as “mobile flu stops” can also help overcome accessibility barriers and increase coverage of influenza vaccination in these communities.48 More importantly, these non‐traditional sites can play an important role in expanding outreach and capacity for administering vaccines as routine visits to doctors' offices are drastically reduced in the current COVID‐19 pandemic.92 While there is a plausible risk of infectious transmission at these sites during the ongoing pandemic, adherence to CDC's guidance for administration of vaccines at these alternative sites such as scheduling appointments, limiting attendees, arranging separate vaccination areas, and prohibiting adults who have tested positive for COVID‐19 or their contacts from visiting the site can help ensure safe administration of the vaccine.93
Policy‐Centered Interventions
Incentivize Production
The production, supply, and distribution of vaccines is a highly complex and coordinated process involving multiple steps, including detection of annual changes in influenza virus strains, production of updated vaccine formulation, vaccine distribution to both public and private providers, and meeting the differential demand of the vaccine every year. Apart from its complexity, there are multiple stakeholders in the process such as government agencies; federal, state and local policymakers; vaccine manufacturers, vaccine distributors, and vaccine providers.94 In view of uncertainty of demand for the vaccine every year and need for annual reformulation, the federal government should absorb the financial risk to the vaccine manufacturers through various measures such as advanced purchase commitments, tax credits, buy‐back mechanisms, or surplus purchase programs, while also ensuring that cost is not a barrier to vaccine access.92, 95
Increasing Demand
Increasing the demand for vaccines will enhance coverage among members of the general population as well as help mitigate the financial risk, thus encouraging participation of vaccine manufacturers and distributors.94 Although vaccination is recommended throughout the influenza season, demand for the vaccine is typically higher in the fall. Offering the vaccine throughout the season including January and beyond can help increase the demand and decrease the proportion of unused vaccines.94 As previously mentioned, mass education campaigns about the benefit and safety of vaccination can help enhance the demand for vaccines. In light of the current COVID‐19 pandemic, there is a need for a comprehensive plan for universal coverage of the COVID‐19 vaccine, including increasing awareness, routine offering, and ongoing evaluation.92
Mandates
Currently, all states, except Massachusetts, do not require influenza vaccination for grades K‒12 despite evidence of lower infection rates among vaccinated children.96, 97 Preliminary data released by the American College Health Association found that only 14% of colleges and universities require influenza vaccination in the United States while 67% encourage vaccination without mandating it.98 Recently, the state of Massachusetts mandated influenza vaccination for college and university students as well.97 As educational facilities make plans for safe reopening, mandates for influenza vaccination will become necessary along with other protective and surveillance measures.92 This mandate will further require expansion as more COVID‐19 vaccines become available. In preparation for a potential concurrent epidemic of influenza and COVID‐19, employers can make vaccination a requisite for its employees, with appropriate exemptions and considerations. Further, prioritizing influenza and COVID‐19 vaccination among healthcare personnel is crucial owing to their indispensable role in overcoming the pandemic and ensuring patient safety. Newer state mandates and strong incentives to healthcare facilities could be used to provide routine vaccination to consenting healthcare workers.92
Implications for COVID‐19 Vaccination
As per World Health Organization estimates, around 300 COVID‐19 vaccines are currently under development, with 102 in phase I‐III trials and 185 in preclinical stage. While SAGE/WHO has provided interim recommendations for 6 vaccines so far, only 3 vaccines have been granted emergency use authorization by FDA.15, 99 Adopting approaches to improve influenza vaccination rates discussed in this review may also help optimize the coverage of COVID‐19 vaccination among patients with established CVD as more vaccines are approved and available in the United States. Similar barriers to those affecting influenza vaccination uptake in the United States might also preclude optimal COVID‐19 vaccine coverage and uptake, particularly among marginalized subgroups with unmet health needs, and will have to be addressed and reconciled. We hope this review will help enhance vaccination coverage in patients with CVD, and increase access to and use of vaccines given key patient‐ and population‐level implications.
Conclusions
The rate of influenza vaccination in US adults with CVD remains much lower than the national target despite known incidence of increased influenza‐related complications, cardiovascular events, and deaths in this high‐risk population. Vaccine uptake is a complex interplay of sociodemographic factors including age, insurance status, and healthcare access, as well as behavioral factors such as perception of vaccine efficacy and safety. Multi‐level strategies to increase awareness of vaccine effectiveness and its limited side effect profile, and efforts to improve accessibility are urgently warranted in this vulnerable and expansive patient population. In the context of the ongoing health crisis attributable to the COVID‐19 pandemic, increasing uptake of the influenza vaccine is even more crucial in preventing a concurrent epidemic. Lessons learned from enhanced influenza vaccine uptake in patients with CVD will most likely inform vaccination efforts against COVID‐19 once vaccines become more widely available and accessible.
Sources of Funding
None.
Disclosures
Dr. Virani has received research grants from the Department of Veterans Affairs, American Heart Association, American Diabetes Association, and World Heart Federation; and has received honoraria from the American College of Cardiology (Associate Editor for Innovations, acc.org). Dr. Nasir is supported by the Katz Academy of Translational Research. The remaining authors have no disclosures to report.
Acknowledgments
Author contributions: Drs. Bhugra, Cainzos‐Achirica, Nasir were responsible for study conception and design. Drs. Cainzos‐Achirica, Nasir provided administrative support. Drs. Bhugra, Grandhi, Mszar, Satish, Singh, Blaha, Blankstein, Virani, Cainzos‐Achirica, Nasir were responsible for manuscript writing. All authors were involved in critical revision for important intellectual content and all authors reviewed and approved the final version of the review.
(J Am Heart Assoc. 2021;10:e019671. DOI: 10.1161/JAHA.120.019671.)
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti Després J‐P, Fullerton HJ, Howard VJ, Huffman MD, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. DOI: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.CDC . Million Hearts® Costs & Consequences. Centers for Disease Control and Prevention; 2019. [Google Scholar]
- 3.Nguyen JL, Yang W, Ito K, Matte TD, Shaman J, Kinney PL. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol. 2016;1:274–281. DOI: 10.1001/jamacardio.2016.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arora A, Rout A, Satya K. Cardiovascular disease burden of influenza syndrome: national trends and outcomes from a United States population study from 2011 to 2014. J Am Coll Cardiol. 2019;73:1794.– 10.1016/S0735-1097(19)32400-3. [DOI] [Google Scholar]
- 5.CDC . Burden of influenza. Centers for Disease Control and Prevention; 2020. [Google Scholar]
- 6.Chow EJ, Rolfes MA, O'Halloran A, Anderson EJ, Bennett NM, Billing L, Chai S, Dufort E, Herlihy R, Kim S, et al. Acute cardiovascular events associated with influenza in hospitalized adults. Ann Intern Med. 2020;173:605–613. DOI: 10.7326/M20-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Udell JA, Zawi R, Bhatt DL, Keshtkar‐Jahromi M, Gaughran F, Phrommintikul A, Ciszewski A, Vakili H, Hoffman EB, Farkouh ME, et al. Association between influenza vaccination and cardiovascular outcomes in high‐risk patients: a meta‐analysis. JAMA. 2013;310:1711–1720. DOI: 10.1001/jama.2013.279206. [DOI] [PubMed] [Google Scholar]
- 8.Phrommintikul A, Kuanprasert S, Wongcharoen W, Kanjanavanit R, Chaiwarith R, Sukonthasarn A. Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Eur Heart J. 2011;32:1730–1735. DOI: 10.1093/eurheartj/ehr004. [DOI] [PubMed] [Google Scholar]
- 9.Davis MM, Taubert K, Benin AL, Brown DW, Mensah GA, Baddour LM, Dunbar S, Krumholz HM. Influenza vaccination as secondary prevention for cardiovascular disease. Circulation. 2006;114:1549–1553. DOI: 10.1161/CIRCULATIONAHA.106.178242. [DOI] [PubMed] [Google Scholar]
- 10.O’Halloran AC, Lu P‐J, Williams WW, Bridges CB, Singleton JA. Influenza vaccination coverage among people with high‐risk conditions in the U.S. Am J Prev Med. 2016;50:e15–e26. DOI: 10.1016/j.amepre.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SC, Collins A, Ferrari R, Holmes DR, Logstrup S, McGhie DV, Ralston J, Sacco RL, Stam H, Taubert K, et al. Our time: a call to save preventable death from cardiovascular disease (heart disease and stroke). J Am Coll Cardiol. 2012;60:2343–2348. DOI: 10.1016/j.jacc.2012.08.962. [DOI] [PubMed] [Google Scholar]
- 12.Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Flaxman A, Murray CJL, Naghavi M. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1493–1501. DOI: 10.1161/CIRCULATIONAHA.113.004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. DOI: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 14.Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, Mussolino ME, Hsu LL, Addou E, Engelgau MM, et al. Decline in cardiovascular mortality: possible causes and implications. Circ Res. 2017;120:366–380. DOI: 10.1161/CIRCRESAHA.116.309115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grohskopf LA, Alyanak E, Broder KR, Blanton LH, Fry AM, Jernigan DB, Atmar RL. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2020–21 influenza season. MMWR Recomm Rep. 2020;69:1–24. DOI: 10.15585/mmwr.rr6908a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, et al. Estimates of global seasonal influenza‐associated respiratory mortality: a modelling study. Lancet. 2018;391:1285–1300. DOI: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren‐Gash C, Smeeth L, Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis. 2009;9:601–610. DOI: 10.1016/S1473-3099(09)70233-6. [DOI] [PubMed] [Google Scholar]
- 18.Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow T, Katz K, Ko DT, McGeer AJ, McNally D, et al. Acute myocardial infarction after laboratory‐confirmed influenza infection. N Engl J Med. 2018;378:345–353. DOI: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 19.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. DOI: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 20.Falsey AR, Walsh EE, Francis CW, Looney RJ, Kolassa JE, Hall WJ, Abraham GN. Response of C‐reactive protein and serum amyloid A to influenza A infection in older adults. J Infect Dis. 2001;183:995–999. DOI: 10.1086/319275. [DOI] [PubMed] [Google Scholar]
- 21.Julkunen I, Sareneva T, Pirhonen J, Ronni T, Melén K, Matikainen S. Molecular pathogenesis of influenza A virus infection and virus‐induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 2001;12:171–180. DOI: 10.1016/S1359-6101(00)00026-5. [DOI] [PubMed] [Google Scholar]
- 22.Corrales‐Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis. 2010;10:83–92. DOI: 10.1016/S1473-3099(09)70331-7. [DOI] [PubMed] [Google Scholar]
- 23.Clar C, Oseni Z, Flowers N, Keshtkar‐Jahromi M, Rees K. Influenza vaccines for preventing cardiovascular disease. Cochrane Database Syst Rev. 2015:CD005050. DOI: 10.1002/14651858.CD005050.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siscovick DS, Raghunathan TE, Lin D, Weinmann S, Arbogast P, Lemaitre RN, Psaty BM, Alexander R, Cobb LA. Influenza vaccination and the risk of primary cardiac arrest. Am J Epidemiol. 2000;152:674–677. DOI: 10.1093/aje/152.7.674. [DOI] [PubMed] [Google Scholar]
- 25.Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348:1322–1332. DOI: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 26.Barnes M, Heywood AE, Mahimbo A, Rahman B, Newall AT, Macintyre CR. Acute myocardial infarction and influenza: a meta‐analysis of case–control studies. Heart. 2015;101:1738–1747. DOI: 10.1136/heartjnl-2015-307691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonelli M, Lloyd A, Clement F, Conly J, Husereau D, Hemmelgarn B, Klarenbach S, McAlister FA, Wiebe N, Manns B, et al. Efficacy of statins for primary prevention in people at low cardiovascular risk: a meta‐analysis. CMAJ. 2011;183:E1189–E1202. DOI: 10.1503/cmaj.101280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neal B, MacMahon S, Chapman N; Blood Pressure Lowering Treatment Trialists’ Collaboration . Effects of ACE inhibitors, calcium antagonists, and other blood‐pressure‐lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. Lancet. 2000;356:1955–1964. DOI: 10.1016/s0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 29.Lee KR, Bae JH, Hwang IC, Kim KK, Suh HS, Ko KD. Effect of influenza vaccination on risk of stroke: a systematic review and meta‐analysis. Neuroepidemiology. 2017;48:103–110. DOI: 10.1159/000478017. [DOI] [PubMed] [Google Scholar]
- 30.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M‐T, Corrà U, Cosyns B, Deaton C, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381. DOI: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. DOI: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.European Centre for Disease Prevention and Control . Seasonal influenza vaccines. 2018. Available at: https://www.ecdc.europa.eu/en/seasonal‐influenza/prevention‐and‐control/seasonal‐influenza‐vaccines. Accessed October 01, 2020.
- 33.Young K, Gemmill I, Harrison R, on behalf of the National Advisory Committee on Immunization (NACI) . Summary of the NACI Seasonal Influenza Vaccine Statement for 2020–2021. Can Commun Dis Rep. 2020;46:132–137. DOI: 10.14745/ccdr.v46i05a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO | WHO recommendations for routine immunization—summary tables. WHO. [Google Scholar]
- 35.Grandhi GR, Mszar R, Vahidy F, Valero‐Elizondo J, Blankstein R, Blaha MJ, Virani SS, Andrieni JD, Omer SB, Nasir K. Sociodemographic disparities in influenza vaccination among adults with atherosclerotic cardiovascular disease in the United States. JAMA Cardiol. 2021;6:87–89. DOI: 10.1001/jamacardio.2020.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medical Expenditure Panel Survey. Available at: http://meps.ahrq.gov/mepsweb/about_meps/survey_back.jsp. Accessed October 1, 2020.
- 37.Singleton JA, Wortley P, Lu P‐J. Influenza vaccination of persons with cardiovascular disease in the United States. Tex Heart Inst J. 2004;31:22–27. [PMC free article] [PubMed] [Google Scholar]
- 38.Jiménez‐García R, Hernández‐Barrera V, Carrasco Garrido P, del Pozo SV‐F, de Miguel ÁG. Influenza vaccination among cardiovascular disease sufferers in Spain: related factors and trend, 1993–2003. Vaccine. 2006;24:5073–5082. DOI: 10.1016/j.vaccine.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 39.Kim EY, Ko JH, Kim YS, Oh PC. Prevalence and associated factors of influenza vaccination coverage in Korean adults with cardiovascular disease. Medicine (Baltimore). 2020;99:e18540. DOI: 10.1097/MD.0000000000018540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ajani UA, Ford ES, Mokdad AH. Examining the coverage of influenza vaccination among people with cardiovascular disease in the United States. Am Heart J. 2005;149:254–259. DOI: 10.1016/j.ahj.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 41.Takayama M, Wetmore CM, Mokdad AH. Characteristics associated with the uptake of influenza vaccination among adults in the United States. Prev Med. 2012;54:358–362. DOI: 10.1016/j.ypmed.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Schmid P, Rauber D, Betsch C, Lidolt G, Denker M‐L. Barriers of influenza vaccination intention and behavior—a systematic review of influenza vaccine hesitancy, 2005–2016. PLoS One. 2017;12:e0170550. DOI: 10.1371/journal.pone.0170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau JTF, Kim JH, Choi KC, Tsui HY, Yang X. Changes in prevalence of influenza vaccination and strength of association of factors predicting influenza vaccination over time—results of two population‐based surveys. Vaccine. 2007;25:8279–8289. DOI: 10.1016/j.vaccine.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 44.Forshaw J, Gerver SM, Gill M, Cooper E, Manikam L, Ward H. The global effect of maternal education on complete childhood vaccination: a systematic review and meta‐analysis. BMC Infect Dis. 2017;17:801. DOI: 10.1186/s12879-017-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Endrich MM, Blank PR, Szucs TD. Influenza vaccination uptake and socioeconomic determinants in 11 European countries. Vaccine. 2009;27:4018–4024. DOI: 10.1016/j.vaccine.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 46.Keisler‐Starkey K, Bunch LN. U.S. Census Bureau Current Population Reports, P60‐271, Health Insurance Coverage in the United States: 2019. U.S. Government Publishing Office, Washington, DC, 2020. [Google Scholar]
- 47.Blewett LA, Johnson PJ, Lee B, Scal PB. When a usual source of care and usual provider matter: adult prevention and screening services. J Gen Intern Med. 2008;23:1354–1360. DOI: 10.1007/s11606-008-0659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kan T, Zhang J. Factors influencing seasonal influenza vaccination behaviour among elderly people: a systematic review. Public Health. 2018;156:67–78. DOI: 10.1016/j.puhe.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmerman RK, Nowalk MP, Bardella IJ, Fine MJ, Janosky JE, Santibanez TA, Wilson SA, Raymund M. Physician and practice factors related to influenza vaccination among the elderly. Am J Prev Med. 2004;26:1–10. DOI: 10.1016/j.amepre.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 50.Evans MR, Prout H, Prior L, Tapper‐Jones LM, Butler CC. A qualitative study of lay beliefs about influenza immunisation in older people. Br J Gen Pract. 2007;57:352–358. [PMC free article] [PubMed] [Google Scholar]
- 51.Nichol KL, Zimmerman R. Generalist and subspecialist physicians’ knowledge, attitudes, and practices regarding influenza and pneumococcal vaccinations for elderly and other high‐risk patients: a nationwide survey. Arch Intern Med. 2001;161:2702–2708. DOI: 10.1001/archinte.161.22.2702. [DOI] [PubMed] [Google Scholar]
- 52.Burns VE, Ring C, Carroll D. Factors influencing influenza vaccination uptake in an elderly, community‐based sample. Vaccine. 2005;23:3604–3608. DOI: 10.1016/j.vaccine.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 53.Payaprom Y, Bennett P, Burnard P, Alabaster E, Tantipong H. Understandings of influenza and influenza vaccination among high‐risk urban dwelling Thai adults: a qualitative study. J Public Health. 2010;32:26–31. DOI: 10.1093/pubmed/fdp086. [DOI] [PubMed] [Google Scholar]
- 54.Smedley J, Poole J, Waclawski E, Stevens A, Harrison J, Watson J, Hayward A, Coggon D. Influenza immunisation: attitudes and beliefs of UK healthcare workers. Occup Environ Med. 2007;64:223–227. DOI: 10.1136/oem.2005.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.What impact has COVID‐19 had on outpatient visits? Available at: https://www.commonwealthfund.org/publications/2020/apr/impact‐covid‐19‐outpatient‐visits. Accessed October 1, 2020.
- 56.Basu S, Phillips RS, Phillips R, Peterson LE, Landon BE. Primary care practice finances in the United States Amid the COVID‐19 pandemic. Health Aff (Millwood). 2020;39:1605–1614. DOI: 10.1377/hlthaff.2020.00794. [DOI] [PubMed] [Google Scholar]
- 57.van Essen GA, Kuyvenhoven MM, de Melker RA. Why do healthy elderly people fail to comply with influenza vaccination? Age Ageing. 1997;26:275–279. [DOI] [PubMed] [Google Scholar]
- 58.Evans MR, Watson PA. Why do older people not get immunised against influenza? A community survey. Vaccine. 2003;21:2421–2427. DOI: 10.1016/S0264-410X(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y, Yi Q‐L, Wu J, Li F. Chronic disease status, self‐perceived health and hospital admissions are important predictors for having a flu shot in Canada. Vaccine. 2007;25:7436–7440. DOI: 10.1016/j.vaccine.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Kee SY, Lee JS, Cheong HJ, Chun BC, Song JY, Choi WS, Jo YM, Seo YB, Kim WJ. Influenza vaccine coverage rates and perceptions on vaccination in South Korea. J Infect. 2007;55:273–281. DOI: 10.1016/j.jinf.2007.04.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fall E, Izaute M, Chakroun‐Baggioni N. How can the health belief model and self‐determination theory predict both influenza vaccination and vaccination intention ? A longitudinal study among university students. Psychol Health. 2018;33:746–764. DOI: 10.1080/08870446.2017.1401623. [DOI] [PubMed] [Google Scholar]
- 62.Bednarczyk RA, Chu SL, Sickler H, Shaw J, Nadeau JA, McNutt L‐A. Low uptake of influenza vaccine among university students: evaluating predictors beyond cost and safety concerns. Vaccine. 2015;33:1659–1663. DOI: 10.1016/j.vaccine.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 63.MacDonald NE; SAGE Working Group on Vaccine Hesitancy . Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33:4161–4164. DOI: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 64.Betsch C, Böhm R, Chapman GB. Using behavioral insights to increase vaccination policy effectiveness. Policy Insights Behav Brain Sci. 2015;2:61–73. DOI: 10.1177/2372732215600716. [DOI] [Google Scholar]
- 65.DeRoo SS, Pudalov NJ, Fu LY. Planning for a COVID‐19 vaccination program. JAMA. 2020;323:2458–2459. DOI: 10.1001/jama.2020.8711. [DOI] [PubMed] [Google Scholar]
- 66.National Tracking Poll 200395. 2020;38.
- 67.Vaccine hesitancy. KFF COVID‐19 Vaccine Monitor. January 27, 2021. Available at: https://www.kff.org/report‐section/kff‐covid‐19‐vaccine‐monitor‐january‐2021‐vaccine‐hesitancy/. Accessed June 3, 2021.
- 68.Berkhout C, Zgorska‐Meynard‐Moussa S, Willefert‐Bouche A, Favre J, Peremans L, Royen PV. Audiovisual aids in primary healthcare settings’ waiting rooms. A systematic review. Eur J Gen Pract. 2018;24:202–210. DOI: 10.1080/13814788.2018.1491964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stinchfield PK. Practice‐proven interventions to increase vaccination rates and broaden the immunization season. Am J Med. 2008;121:S11–S21. DOI: 10.1016/j.amjmed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 70.Monn JL. An evidence‐based project to improve influenza immunization uptake. J Nurse Pract. 2016;12:e159–e162. DOI: 10.1016/j.nurpra.2015.11.030. [DOI] [Google Scholar]
- 71.Vann JCJ, Szilagyi P. Patient reminder and recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;2005:CD003941. DOI: 10.1002/14651858.CD003941.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thomas RE, Lorenzetti DL. Interventions to increase influenza vaccination rates of those 60 years and older in the community. Cochrane Database Syst Rev. 2018;5:CD005188. DOI: 10.1002/14651858.CD005188.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Satterthwaite P. A randomised intervention study to examine the effect on immunisation coverage of making influenza vaccine available at no cost. N Z Med J. 1997;110:58–60. [PubMed] [Google Scholar]
- 74.Stone EG, Morton SC, Hulscher ME, Maglione MA, Roth EA, Grimshaw JM, Mittman BS, Rubenstein LV, Rubenstein LZ, Shekelle PG. Interventions that increase use of adult immunization and cancer screening services: a meta‐analysis. Ann Intern Med. 2002;136:641–651. DOI: 10.7326/0003-4819-136-9-200205070-00006. [DOI] [PubMed] [Google Scholar]
- 75.Blank P, Schwenkglenks M, Szucs TD. The impact of European vaccination policies on seasonal influenza vaccination coverage rates in the elderly. Hum Vaccin Immunother. 2012;8:328–335. DOI: 10.4161/hv.18629. [DOI] [PubMed] [Google Scholar]
- 76.Madhavan SS, Borker RD, Fernandes AW, Amonkar MM, Rosenbluth SA. Assessing predictors of influenza and pneumonia vaccination in rural senior adults. J Health Soc Policy. 2003;18:71–93. DOI: 10.1300/J045v18n02_05. [DOI] [PubMed] [Google Scholar]
- 77.Vlahov D, Coady MH, Ompad DC, Galea S. Strategies for improving influenza immunization rates among hard‐to‐reach populations. J Urban Health. 2007;84:615–631. DOI: 10.1007/s11524-007-9197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winston CA, Wortley PM, Lees KA. Factors associated with vaccination of Medicare beneficiaries in five U.S. communities: results from the racial and ethnic adult disparities in immunization initiative survey, 2003. J Am Geriatr Soc. 2006;54:303–310. DOI: 10.1111/j.1532-5415.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- 79.Briss PA, Rodewald LE, Hinman AR, Shefer AM, Strikas RA, Bernier RR, Carande‐Kulis VG, Yusuf HR, Ndiaye SM, Williams SM. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. the Task Force on Community Preventive Services. Am J Prev Med. 2000;18:97–140. DOI: 10.1016/s0749-3797(99)00118-x. [DOI] [PubMed] [Google Scholar]
- 80.Reminder Systems for Immunizations and Preventive Services. Available at: https://www.ahrq.gov/cahps/quality‐improvement/improvement‐guide/6‐strategies‐for‐improving/health‐promotion‐education/strategy6r‐reminder‐systems.html. Accessed October 1, 2020.
- 81.Dexter LJ, Teare MD, Dexter M, Siriwardena AN, Read RC. Strategies to increase influenza vaccination rates: outcomes of a nationwide cross‐sectional survey of UK general practice. BMJ Open. 2012;2:e000851. Available at: https://bmjopen.bmj.com/content/2/3/e000851.info. Accessed October 1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lau D, Hu J, Majumdar SR, Storie DA, Rees SE, Johnson JA. Interventions to improve influenza and pneumococcal vaccination rates among community‐dwelling adults: a systematic review and meta‐analysis. Ann Fam Med. 2012;10:538–546. DOI: 10.1370/afm.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vaccine‐preventable diseases: improving vaccination coverage in children, adolescents, and adults. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr4808a1.htm. Accessed October 1, 2020. [PubMed]
- 84.Rimple D, Weiss SJ, Brett M, Ernst AA. An emergency department‐based vaccination program: overcoming the barriers for adults at high risk for vaccine‐preventable diseases. Acad Emerg Med. 2006;13:922–930. DOI: 10.1197/j.aem.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 85.Lawson F, Baker V, Au D, McElhaney JE. Standing orders for influenza vaccination increased vaccination rates in inpatient settings compared with community rates. J Gerontol A Biol Sci Med Sci. 2000;55:M522–M526. DOI: 10.1093/gerona/55.9.M522. [DOI] [PubMed] [Google Scholar]
- 86.Cunningham A, Stoeckle J, Diaz V, Valko G, Arenson C. Back to basics: five steps to better influenza vaccination rates. Fam Pract Manag. 2017;24:30–33. [PubMed] [Google Scholar]
- 87.Vaccination programs: standing orders. Available at: https://www.thecommunityguide.org/findings/vaccination‐programs‐standing‐orders. Accessed October 1, 2020.
- 88.Vaccine standing orders for healthcare providers. Available at: https://www.immunize.org/standing‐orders/. Accessed October 1, 2020.
- 89.Prosser LA, O’Brien MA, Molinari N‐AM, Hohman KH, Nichol KL, Messonnier ML, Lieu TA. Non‐traditional settings for influenza vaccination of adults. Pharmacoeconomics. 2008;26:163–178. DOI: 10.2165/00019053-200826020-00006. [DOI] [PubMed] [Google Scholar]
- 90.Vlahov D, Bond KT, Jones KC, Ompad DC. Factors associated with differential uptake of seasonal influenza immunizations among underserved communities during the 2009–2010 influenza season. J Community Health. 2012;37:282–287. DOI: 10.1007/s10900-011-9443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Coady MH, Weiss L, Galea S, Ompad DC, Glidden K, Vlahov D. Rapid vaccine distribution in nontraditional settings: lessons learned from project VIVA. J Community Health Nurs. 2007;24:79–85. DOI: 10.1080/07370010701316163. [DOI] [PubMed] [Google Scholar]
- 92.Gostin LO, Salmon DA. The dual epidemics of COVID‐19 and influenza: vaccine acceptance, coverage, and mandates. JAMA. 2020;324:335–336. DOI: 10.1001/jama.2020.10802. [DOI] [PubMed] [Google Scholar]
- 93.Interim guidance for routine and influenza immunization services during the COVID‐19 pandemic. Available at: https://www.cdc.gov/vaccines/pandemic‐guidance/index.html. Accessed October 1, 2020.
- 94.Orenstein WA, Schaffner W. Lessons learned: role of influenza vaccine production, distribution, supply, and demand—what it means for the provider. Am J Med. 2008;121:S22–S27. DOI: 10.1016/j.amjmed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 95.Lenfestey N, Layton C. Influenza vaccine manufacturing. Issue brief. 2005. Available at: https://www.rti.org/publication/influenza‐vaccine‐manufacturing‐issue‐brief. Accessed October 1, 2020.
- 96.State‐by‐state: vaccinations required for public school kindergarten—Vaccines—ProCon.org. Available at: https://vaccines.procon.org/state‐by‐state‐vaccinations‐required‐for‐public‐school‐kindergarten/. Accessed October 1, 2020.
- 97.Flu vaccine now required for all Massachusetts school students enrolled in child care, pre‐school, K‐12, and post‐secondary institutions | Mass.gov. Available at: https://www.mass.gov/news/flu‐vaccine‐now‐required‐for‐all‐massachusetts‐school‐students‐enrolled‐in‐child‐care‐pre. Accessed October 1, 2020.
- 98.Goldman M. More colleges require flu shots of students and employees. Wall Str J. 2020. Available at: https://www.wsj.com/articles/more‐colleges‐require‐flu‐shots‐of‐students‐and‐employees‐11603116161. Accessed October 1, 2020. [Google Scholar]
- 99.Draft landscape of COVID‐19 candidate vaccines. Available at: https://www.who.int/publications/m/item/draft‐landscape‐of‐covid‐19‐candidate‐vaccines. Accessed October 1, 2020.


