Abstract
Dysregulated Wnt signaling plays a central role in initiation, progression and metastasis in many types of human cancers. Cancer development and resistance to conventional cancer therapies are highly associated to the tumor microenvironment (TME), which is composed of numerous stable non-cancer cells including immune cells, extracellular matrix (ECM), fibroblasts, endothelial cells (ECs), and stromal cells. Recently increasing evidence suggests that the relationship between Wnt signaling and the TME promotes the proliferation and maintenance of tumor cells including leukemia. Here, we review the Wnt pathway, the role of Wnt signaling in different components of the TME, and therapeutic strategies for targeting Wnt signaling.
Keywords: Wnt, β-catenin, cancer, tumor microenvironment, cancer stem cell, immune cell, immune tolerance, immune evasion, extracellular matrix, fibroblast, endothelial cell, stromal cell, therapy
1. Introduction
The Wnt signaling pathway is a critical regulator of development in embryogenesis, tissue homeostasis, stemness control, wound repair, and malignancy[1]. Recently, it has been found that aberrant Wnt signaling is involved in the pathogenesis of cancer such as immune evasion and immunomodulation[2, 3]. The microenvironment of tumor cells is composed of tumor cells, immune cells, and stromal cells,which contribute to drug resistance and survival of the turnor[4],Here, we review the relationship between aberrant Wnt signaling and the tumor microenvironment (TME), and summarize the potential targeting therapeutic strategies.
2. Wnt Signaling Pathway
The Wnt signaling pathway has been extensively studied and reviewed[1, 5, 6]. In general, there are three pathways: the canonical Wnt pathway, the non-canonical Wnt-planar cell polarity (PCP) pathway, and the non-canonical Wnt-calcium(Ca2+) pathway.
2.1. The Canonical Pathway
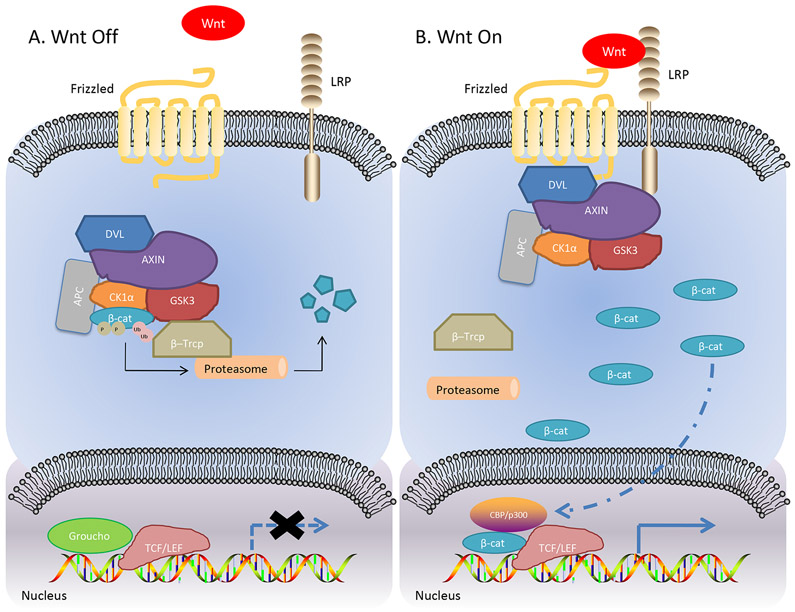
The canonical pathway relies on cytoplasmic β-catenin stabilization [7]. However, β-catenin is a highly unstable protein. In the absence of Wnt ligands, β-catenin is part of the β-catenin destruction complex composed of adenomatous polyposis coli (APC), AXIN, casein kinase 1α (CK1α) and glycogen synthase kinase 3 (GSK-3), which catalyzes the phosphorylation of β-catenin at its N terminus and tags ubiquitin protein ligaseβ–Trcpontoβ-catenin. After poly-ubiquitination, β-catenin is then degraded byproteasomes[8]. Upon the binding of Wnt ligand to Frizzled (FZD) receptors, which is a G-protein-coupled receptor (GPCR) with seven-transmembrane domains[9], and the co-receptor low density lipoprotein receptor-related protein 5/6(LRP5/6)[10]. Next, disheveled segment polarity protein (DVL) is recruited intracellularly as a platform for AXIN to interact with the cytoplasmic domain of LRP5/6.This interaction disassembles the destruction complex, therefore resulting in stabilization of β-catenin. Ultimately β-catenin accumulates and translocates to the nucleus, where it can bind to the transcription factor T-cell factor/lymphoid enhancer factor(TCF/LEF), and recruit the transcriptional Kat3 co-activators p300 and/or CREB-binding protein(CBP) to transcribe Wnt target genes[11]. In contrast, TCF is in an inactive state as the consequence of binding to the repressor Groucho. The genes activated by Wnt have important functions for many processes in oncogenesis and development such as self-renewal, differentiation, proliferation, and metastasis[12, 13]. (Figure 1)
Figure 1. The Canonical Wnt Pathway.
A. Inactivation of Wnt signaling. B. Activation of Wnt signaling. LRP, lipoprotein receptor-related protein; DVL, disheveled segment polarity protein; CK1α, casein kinase 1α; GSK-13, glycogen synthase kinase 3; β-cat, β-catenin; APC, adenomatous polyposis coli; TCF/LEF, T-cell Factor/Lymphoid Enhancer Factor; CBP, CREB-binding protein.
2.2. The Non-canonical Pathways
The non-canonical Wnt pathways coexist with the canonical pathway and are β-catenin-independent[6]. In the non-canonical Wnt-planar cell polarity (PCP) pathway, the Wnt ligand binds to Frizzled receptors and activates the small GTPasesRhoA and Ras-related C3 botulinum toxin substrate 1 (RAC1) via activation of DVL. RhoA upregulates Rho kinase while activated RAC1 enhances c-Jun N-terminal kinase (JNK) expression, triggering the expression of downstream target genes[5]. The non-canonical Wnt-Ca2+ pathway is initiated by G protein–mediated phospholipase C (PLC) activation, which induces the influx of calcium. Calcium acts as a second messenger and further activates of downstream proteins such as calmodulin–dependent protein kinase II (CAMKII) and protein kinase C (PKC), resulting in cell migration[14]. Wnt signaling contributes to the stabilization of proteins other than β-catenin to maintain intracellular functions through these alternative pathways [15].
3. WntSignaling and Cancer Stem Cells
The self-renewal potential of cancer cells is described by the cancer stem cells (CSCs) in solid tumors[16]. There is adequate evidence that Wnt signaling has a vital role for the maintenance and progression of CSCs[17]. Leukemia stem cells (LSCs) share similar properties with CSC, and leukemogenesis is closely related with aberrant Wnt signaling in both leukemic cells and stromal cells in bone marrow microenvironment[18, 19]. One of the hallmarks of CSCs is to sustain long telomeres through high expression of telomerase reverse transcriptase (TERT)[20]. β-catenin directly augments TERT expression through promoter binding[21].Recent studies showed that GSK3β, AXIN binding to tankyrase, and the SOX family transcription factors played key roles in the maintenance of CSC traits in breast cancers through the Wnt signaling pathway[22-24]. One target gene of Wnt is Leucine-rich repeat-containing G-protein-coupled receptor 5 (LGR5), a bona fide marker of adult stem cells in the gastrointestinal tract that acts to enhance Wnt/β catenin signaling [25]. In colorectal cancer, LGR5 has recently been found to also be a marker of colorectal CSCs[26]. Furthermore, intestinal famesoid X receptor (FXR) function is antagonized by bile acids and induces proliferation and DNA damage in LGR5-positive CSCs [27]. Colorectal cancer cell stemness was found to be enhanced by miR-372/373, a cluster of stem cell-specific microRNAs transactivated by the Wnt pathway, by repression of differentiation genes[28]. In addition, LGR5 has a vital oncogenic role in cervical cancer by upregulating Wnt signaling and promoting cervical CSC traits[29]. In non-small cell lung carcinoma, RIF1 and serine-arginine protein kinase 1 (SRPK1) promote tumor growth and CSC-like properties as positive regulators of Wnt/β-catenin signaling [30, 31].The activation of RNA-binding motif on Y chromosome (RBMY), which is only present in male hepatocellular carcinoma, results in the augmentation of CSC traits via stimulation of Wnt-3a[32]. In summary, Wnt signaling affects several downstream targets that are important for the maintenance of CSC function.
4. Wnt Signaling and Immune Cells
Ample vital advancements in studying the relationship between tumors and immune system have been illuminated over the past two decades. The immune system is known to play a role not only in tumor suppression but also in cancer progression[33]. An extensive immunogenomic analysis of more than 10,000 tumors of 33 cancer types from The Cancer Genome Atlas (TCGA) found six immune subtypes—wound healing, IFN-gamma dominant, inflammatory, lymphocyte-depleted, immunologically quiet, and TGF-beta dominant—characterized by differences in TME signatures[34]. Wnt signaling not only affects the differentiation of T-cells[35-39] but also influences other immune cells such as natural killer (NK) cells[40, 41] and dendritic cells (DC)[42-44].
4.1. Wnt Signaling in Immune Tolerance
Some lymphocytes within the TME that infiltrate solid tumors regulate the immune tolerance of tumors. For example, β-catenin in DCs serves as a key mediator in promoting both CD4+ and CD8+ T-cell tolerance. One possible reason is that DCs can be modulated by denileukin diftitox (DD) , a diphtheria toxin fragment-IL2 fusion protein, leading to upregulation of the immune tolerance-associated β-catenin pathway[45]. Inhibition of interactions between Wnt with its cognate co-receptor LRP5/6 and Frizzled suggested that LRP5 and LRP6 in DCs play a critical role in immune tolerance[46]. Another study demonstrated that β-catenin/mTOR/IL-10 signaling impairs the ability of DCs to cross-prime CD8+ T-cell immunity[43]. A recent study showed that WNT5a released from melanoma cells resulted in paracrine WNT5β-catenin signaling in DC, leading to an increase in the immunoregulatory enzyme, indoleamine 2,3-dioxygenase-1 (IDO), which plays a vital role in tumor-mediated immune tolerization[47]. On the other hand, in colon cancer, activation of β-catenin resulted in production of T helper 17 (TH17) cells-mediated inflammation that promoted cancer function[48].
4.2. WntSignaling in Immune Evasion
Active canonical Wnt signaling in immune cells in the TME is a crucial cause of resistance to cancer immunotherapies called immune checkpoint inhibitors [2]. Effective recognition of tumor-associated antigens and thus eradication of cancer by cytotoxic T lymphocytes (CTLs) can be interfered via cytotoxic T lymphocyte-associated protein 4 (CTLA-4) or the programmed cell death 1 (PD-1) and programmed death-ligand 1 (PD-L1) pathway[49]. In melanoma, T-cell exclusion and resistance to anti-PD-L1/anti-CTLA-4 monoclonal antibody therapy were mediated via tumor-intrinsic active β-catenin signaling[50]. One of the key factors was GSK-3 which could potentially decrease CD8+ T-cell function in cancer therapy via upregulation of PD-1 expression[51]. Moreover, in a prospective genotyping of hepatocellular carcinoma clinical study, active Wnt/β-catenin signaling was associated with lower disease control rates (DCR), shorter median progression-free survival (PFS), and shorter median overall survival (OS), even though the 31 patients were treated with immune checkpoint inhibitors[52].A new evidence was found that not only the T-cell-inflamed phenotype correlated with efficacy of immune-checkpoint blockade but also non-T-cell-inflamed tumors via activation of tumor-intrinsic Wnt/β-catenin signaling[53]. Furthermore, stroma-derived Dickkopf-1 (Dkk1) targeted β-catenin in myeloid-derived suppressor cells (MDSCs), hence exerting immune suppressive effects during tumor progression[54].
5. Wnt Signaling and Extracellular Matrix
The extracellular matrix (ECM) is a strikingly dynamic structure that undergoes mechanical remodeling by most tumor cells which is critical for cancer progression from a primary tumor to metastatic disease[55, 56]. Stiff ECM activates the integrin/focal adhesion kinase (FAK) pathway, which elevates the expression of members of the Wnt/β-catenin pathway and in turn enhances regulation of mesenchymal stem cell differentiation and primary chondrocyte phenotype maintenance[57]. Interestingly, the expression levels of B-cell lymphoma 2 (Bcl-2) associated X protein (Bax), procaspase-3 and −9, matrix metalloproteinase 1 (MMP1), MMP3, MMP13, WNT3a, WNT5a, WNT7a and β-catenin were significantly inhibited with resveratrol (RES) in osteoarthritis chondrocytes[58]. This indicates that sirtuin 1 (Sirt1), which can be upregulated by RES, may regulate ECM degradation in RES-treated osteoarthritis chondrocytes through the Wnt/β-catenin signaling pathway[58]. In cervical cancer, the ECM protein collagen triple helix repeat containing 1 (CTHRC1) is regulated by E6/E7, which are the early genes of the high-risk mucosal human papillomavirus type[59]. Through activation of the noncanonical Wnt/PCP signaling pathway, ultimately the E6/E7-p53-POU2F1-CTHRC1 axis promoted cervical cancer cell invasion and metastasis[59].In a urinary bladder cancer study, increased WNT7a expression was associated with metastasis and poor prognosis. Mechanically, WNT7a-mediated MMP10 activation is mediated by the canonical Wnt/β-catenin pathway[60]. In breast cancer, deposition of type I collagen in the ECM played an important role in metastasis due to Wnt signaling causing an increase in expression of MRTF-A, which was critical for regulation of the type I collagen gene COL1A1 in breast cancer cells [61]. Moreover, MRTF-A integrated signals from the Rho-ROCK-actinandWnt/β-catenin pathways to regulate migration-related genes including MYL9, CYR61 and lncRNA HOTAIR that in turn stimulate breast cancer cell migration[62]. In glioma, MYH10 gene silencing resulted in reduced expression of MTA-1, MPP-2, MMP-9 and vimentin, and increased expression of TIMP-2, E-cadherin and collagen 1 through inhibition of the Wnt/β-catenin pathway[63]. HEmT-DCN/sLRP6, an oncolytic adenovirus (Ad) co-expressing decorin and soluble Wnt decoy receptor, eradicated excessive accumulation of ECM in pancreatic cancer via inhibition of the Wnt/β-catenin signaling pathway[64]. Previously another study demonstrated a similar effect using an ECM-degrading and Wnt signal-disrupting oncolytic adenovirus (oAd/DCN/LRP)[65]. Wnt/β-catenin activated Ewing sarcoma cells upregulated secretion of ECM proteins such as structural collagens, matricellular proteins and tenascin C (TNC)[66]. Hence, ECM is secreted excessively and remodeled by cancer cells via Wnt/β-catenin pathway in the TME.
6. Wnt Signaling and Fibroblasts
Cancer associated fibroblasts (CAFs) are important stromal cells components in the TME that play critical roles in tumor initiation, progression and metastasis[55, 67, 68]. There have been a tremendous number of studies that identify how Wnt signaling is involved in the formation and regulation of CAFs in different tumor types. In contrast to the expression in cancer cells as a Wnt antagonist to suppress various cancers progression[69-71], the stromal expression of Dickkopf-3 (DKK3), a HSF1 effector, regulated the pro-turnorigenic behavior of CAFs via Wnt signaling and the activation of Hippo pathway transducers YAP/TAZ[72]. CAFs also promoted the stemness, metastasis, and chemoresistance of colorectal cancer cells by secreting exosomes to increase miR-92a-3p, a microRNA, activating the Wnt/β-catenin pathway and inhibiting mitochondrial apoptosis by directly inhibiting FBXW7 and MOAP1[73]. Furthermore, CAFs upregulated T-lymphoma invasion and metastasis-inducing protein-1 (TIAM1), one of the Wnt-signaling associated genes, resulting in chemoresistance in colorectal cancer[74]. Moreover, desmosomal protein Plakophilin-2 encoded by PKP2 gene is a target gene of Wnt/β-catenin and acts to inhibit the pathway, suggesting that PKP2 plays a role in the negative feedback control of Wnt/β-catenin in normal and colon CAFs[75]. It was found that high levels of WNT2, which are associated with a poor prognosis in colorectal cancer, binds to its putative receptor FZD8 and activates autocrine canonical Wnt signaling in CAFs, which resulted in promoting colorectal cancer progression, invasion, and metastasis[76]. In head and neck squamous cell carcinoma, CAF-derived POSTN was an upstream ligand of protein tyrosine kinase 7 (PTK7) and promoted the CSC-like phenotype via PTK7-Wnt/β-Catenin signaling, inducing proliferation and invasion [77].Deletion of hypoxia-inducible transcription factor (HIF-1) was associated with inactivation of Wnt/β-catenin, significantly inhibiting tumor progression and decreasing production of CAFs in the TME[78, 79]. It has been reported that overexpression of phospholipase D2 (PLD2) in colon tumors induces senescence in neighboring fibroblasts and leads to senescence-associated secretory phenotype (SASP), contributing to tumor development by Wnt pathway activation in colon cancer[80]. A 3-dimensional multi-culture tumor-CAF spheroid phenotypic screening platform was developed to profile 1,024 candidate genes for CAF-intrinsic anti-spheroid activity, in which it was found that CAF-derived products such as Wnt and the G-protein coupled-receptor OGR1 are potential therapeutic targets for colorectal cancer[81].The activation of Wnt/β-catenin and Hgf/Met signaling significantly mediates interactions between CSCs and CAFs in mammary gland tumors[82].In epithelial ovarian cancer, STAT4 overexpression induced normal omental fibroblasts to obtain CAF-like features via tumor-derived WNT7a [83].On the other hand, WNT7a-mediated fibroblast activation was not only dependent on canonical Wnt signaling but also TGF-β receptor signaling in breast cancer[84].Interestingly, aged fibroblasts can secrete a Wnt antagonist, sFRP2, which activates a multi-step signaling cascade that results in melanoma metastasis and therapy resistance [85]. Paracrine WNT10b from p85α-deficient fibroblasts regulated breast cancer tumorigenesis and progression via TME remodeling and epithelial-to-mesenchymal transition induced by the canonical Wnt pathway[86].Also in breast cancer, the effect of oxidative stress by oxidized ataxia-telangiectasia mutated protein kinase (ATM) on aberrant CAF proliferation is regulated through ERK, PI3K-AKT, and Wnt signaling pathways[87].
7. Wnt Signaling and Endothelial Cells
A vascular network is imperative in tumor development and metastasis, and the endothelial cells (ECs) in the TME are highly responsive to cues to promote angiogenesis of tumor-infiltrating blood vessels. Firstly, differentiated ECs undergo phenotypic transition to mesenchymal cells through a complex process named endothelial-mesenchymal transition (EndMT)[88]. TGF-β and Snail transcription factor are two important stimulators of EndMT via Notch and Wnt signaling pathways utilizing Frizzled-2 (FZD2), FZD9, and Wnt5B in the induction process[89]. A similar finding from oral squamous cell carcinoma reports that Wnt5B functions in EndMT and regulates the expression of Snail and Slug proteins through activation of canonical and non-canonical Wnt signaling pathways[90]. Secondly, many advances intumor-angiogenesis via Wnt signaling have been reported. Regulators of Wnt signaling including FZD7[71] and R-spondin3 play important roles in vascular endothelial cells[91].Increased non-canonical WNT5a in squamous cell lung carcinoma inhibits endothelial cell growth and motility[92], implicating itspotential asa therapeutic target of angiogenesis-related diseases[93]. In malignant glioma, increased WNT7 expression in Olig2+ oligodendrocyte precursor-like cells (OPLCs) allowed for the invasion of glioma cells in the vasculature, and inhibition of Wnt blocked invasion and enhanced the response to temozolomide therapy[94]. Microparticles (MPs) released from ovarian cancer cells mediated activation of Wnt/β-catenin in which RAC1 and AKT were responsible for phosphorylation and nuclear translocation in ECs for neo-angiogenesis[95]. Ribosomal protein s15a (RPS15A) promoted angiogenesis in primary hepatocellular carcinoma (HCC) by enhancing Wnt/β-catenin-induced FGF18 expression, suggesting that the RPS15A/FGF18 pathway may be a target for anti-angiogenic therapy of HCC[96]. Importantly, it has been demonstrated that FZD5 – aWnt/FZD family member - was a receptor for secreted frizzled-related protein 2 (SFRP2) and mediates SFRP2-induced angiogenesis through calcineurin / nuclear factor of activated T-cells cytoplasmic 3 (NFATc3) pathway in ECs[97]. Furthermore, hypoxic colorectal cancer cells secreted exosomes enriched with WNT4 and were dependent on hypoxia-inducible factor α (HIF1α), and promoted the proliferation and migration of ECs through increased β-catenin nuclear translocation[98]. Interestingly, HIF-1α activated via Wnt/β-catenin pathway promoted vasculogenesis and angiogenesis even in normoxic conditions due to lactate released by glioma cells[99]. Other important mediators such as vascular endothelial growth factor-A (VEGF-A) [100], and pro-inflammatory factor TNF-α[101] were associated with activation of Wnt/β-catenin signal pathway in ECs. On the other hand, loss of Norrin (an atypical Wnt)/FZD4 -mediated signaling in ECs created a tumor-permissive microenvironment at the earliest, preneoplastic stages of medulloblastoma[102].In addition, in colorectal cancer Norrin/FZD4 also played a vital role in the regulation of angiogenesis as well[103].
8. Wnt and Mesenchymal Stem Cells
The stroma of the TME is comprised of a heterogeneous population of connective tissue cells such as fibroblasts, epithelial cells, and mesenchymal stem cells (MSCs), also referred to as mesenchymal stromal cells. The bone marrow microenvironment also contains MSCs which make up the bone marrow niche. Within the niche, the MSCs function to regulate and maintain hematopoietic stem cells (HSCs) and give rise to the majority of bone marrow stromal cell lineages, including chondrocytes, osteoblasts, fibroblasts, adipocytes, endothelial cells, and myocytes[104]. Wnt signaling is important for the proliferation and differentiation of MSCs but its exact role has not yet been elucidated. Huang et al. found that in the chondrogenic differentiation of MSCs, Wnt-signaling modulator BIO activated canonical Wntsignaling and activated genes important for stemness and proliferation, while the PFK compound inhibited signaling-induced chondrogenic differentiation and inhibitedβ-catenin translocation into the nucleus [105]. Another study showed that by inhibiting GSK-3–the kinase which mediates phosphorylation and degradation ofβ-catenin–upregulation of the Wnt/β-catenin pathway was observed, as well as increased chondrogenic differentiation[106, 107]. These results indicate that the Wnt pathway is critical for MSC developmental function with regards to proliferation and differentiation. In terms of the role of Wnt signaling in MSCs and cancer, it has been found that in many cancers, Ror2 receptor tyrosine kinase can act as a receptor for WNT5a to mediate β-catenin-independent non-canonical Wnt signaling. The constitutive WNT5a-Ror2 signaling in MSCs promotes proliferation and aggressiveness of gastric cancer cells by enhancing expression of CXCL16, thereby activating the CXCL16-CXCR6 axis in a cell-autonomous manner [108]. One study demonstrated by qPCR that MSCs had detectable mRNA expression of all 16 Wnt ligands, with the highest expressed being WNT5a, WNT3, WNT10a, and WNT7b[109]. The authors found that co-culture of acute lymphoblastic leukemia (ALL) with MSCs provided a protective effect to the ALL cells by changes in MYC, LEF1, CCNDBP1, and GSK3b levels, which led to activation of the Wnt pathway and promoted leukemic cell proliferation in ALL[109]. In acute myeloid leukemia (AML), it has been suggested that AML-MSCs have dysregulation of the canonical Wnt signaling pathway, in which there is a decrease in β-catenin/TCF-LEF complex formation and decreased bone morphogenetic protein 4 (BMP4) expression[110]. MSC-derived WNT5a inhibits proliferation and promotes differentiation of HL60, an AML cell line, via activation of the non-canonical Wnt signaling pathway due to significantly increased expression of Ror2 and Calcium/calmodulin-dependent protein kinase II (CaMKII) and decreased expression of β-catenin and cyclin D1[111]. Healthy donor hematopoietic stem/progenitor cells (HSPCs) co-cultured on MSCs derived from Fanconi anemia patients with AML showed reduced secretion of prostaglandins (PGs) by mesenchymal inhibition of COX2 and led to decreased expression of NR4A transcription factors and β-catenin, representing a novel COX2/PG/NR4A/Wnt signaling axis[112]. In summary, Wnt signaling is implicated in MSC function in development and cancer progression and can significantly remodel the TME to promote oncogenesis.
9. Targeting Wnt Signaling
New agents that target Wnt signaling are emerging in research. For example, there are porcupine (PORCN) inhibitors (e.g., LGK974 or WNT974, IWP-L6, CGX1321, ETC1922159, RXC004), AXIN1 activators (e.g., XAV939, niclosamide), Dickkopf1(DKK1) antibodies (e.g., DKN-01,CKAP4), FZD receptors inhibitors (e.g., OMP-18R5, OMP54F28), CBP/β-catenin inhibitors (e.g., PRI-724,ICG-001) and a WNT5A mimic (Foxy-5)[2, 113]. Table 1 lists current Wnt modulators for cancer therapy in clinical trials from the U.S. National Library of Medicine database (https://www.clinicaltrials.gov/ access on June 23, 2019).
Table1.
Clinical Trials of Wnt Modulators in cancer.
| Mechanism | Agent | Disease | Status | Identifier |
|---|---|---|---|---|
| PORCN inhibitor | WNT974/LGK974 | Squamous Cell Carcinoma, Head And Neck | Withdrawn | NCT02649530 |
| Metastatic Colorectal Cancer | Completed | NCT02278133 | ||
| Pancreatic CancerBRAF Mutant Colorectal CancerMelanomaTriple Negative Breast CancerHead and Neck Squamous Cell CancerCervical Squamous Cell CancerEsophageal Squamous Cell CancerLung Squamous Cell Cancer |
Recruiting | NCT01351103 | ||
| CGX1321 | Solid Tumors GI Cancer |
Recruiting | NCT02675946 | |
| RXC004 | Cancer Solid Tumor |
Not yet recruiting | NCT03447470 | |
| ETC-1922159 | Solid Tumors | Active, not recruiting | NCT02521844 | |
| Wnt-5a protein | Foxy-5 | Colon Cancer | Recruiting | NCT03883802 |
| Metastatic Breast Cancer Colorectal Cancer Prostate Cancer |
Completed | NCT02020291 | ||
| DKK1 antibody | DKN-01 | Hepatocellular Carcinoma | Recruiting | NCT03645980 |
| Endometrial Cancer Uterine Cancer Ovarian Cancer |
Recruiting | NCT03395080 | ||
| Esophageal Neoplasms Adenocarcinoma of the Gastroesophageal Junction Gastroesophageal Cancer Squamous Cell Carcinoma Gastric Adenocarcinoma |
Recruiting | NCT02013154 | ||
| Wnt signaling pathway inhibitor | SM08502 | Solid Tumor | Recruiting | NCT03355066 |
| XNW7201 | Advanced Solid Tumors | Not yet recruiting | NCT03901950 | |
| CBP/beta-catenin Antagonist | PRI-724 | Colorectal Adenocarcinoma Stage IVA Colorectal Cancer Stage IVB Colorectal Cancer |
Withdrawn | NCT02413853 |
| Acute Myeloid Leukemia Chronic Myeloid Leukemia |
Completed | NCT01606579 | ||
| CWP232291 | Acute Myeloid Leukemia Chronic Myelomonocytic Leukemia Myelodysplastic Syndrome Myelofibrosis Colon Cancer |
Completed | NCT01398462 | |
| AXIN1 activator | Niclosamide | Colon Cancer | Recruiting | NCT02687009 |
| Wnt signaling modulator | Resveratrol | Colon Cancer Cancer |
Completed | NCT00256334 |
9.1. PORCN Inhibitors
PORCN inhibitors block secretion of Wnt by inhibiting palmitoylation in the TME. The efficiency of LGK974 was determined in different tumors such as squamous cell carcinoma[114],colorectal cancer[115], prostate cancer[116] and chronic myeloid leukemia (CML)[117]. However, LGK974 has low solubility and high toxicity in different tissues, a potent cyclodextrins: LGK974 complex was investigated in lung cancer[118]. WNT974in combination with a tyrosine kinase inhibitor(TKI) enhanced the targeting effect of CML stem and progenitor cells[117].IWP-L6 blocked the Wnt-LRP5/6 pathway and delayed tumor growth via the promotion of a strong tumor-specific T cell responseby regulating DC function[46].
9.2. AXIN1 Activators
AXIN1 activators play a role in the induction of β-catenin degradation. Niclosamide, an FDA-approved anthelmintic drug, eradicated cancer stemness and elicited therapeutic effects on colorectal cancer via disruption of the LEF1/DCLK1-B axis[119]. However, due to the low solubility, bioavailability and systemic exposure of niclosamide, niclosamide conjugated polypeptide nanoparticles were introduced for study in colon cancer[120]. Repression of the microRNA miR-31-5p suppressed proliferation, invasion and tumorigenesis of osteosarcoma cells via promoting AXIN1[121].
9.3. DKK1 antibodies
Although DKK1 is a secreted inhibitor of β-catenin-dependent Wnt signaling, DKK1 appears to increase tumor growth and metastasis in preclinical models due to activation of β-catenin-independent Wnt signaling and mediation of immunosuppressive TME and immune evasion[122].A monoclonal antibody (mAb) against cytoskeleton-associated protein 4 (CKAP4), a novel DKK1 receptor mAbs suppressed xenograft tumor formation and extended the survival of pancreatic ductal adenocarcinoma mice [123]. Moreover, Dickkopf-related protein 2 (DKK2), anantagonist of Wnt/β-catenin signaling, suppressed tumor cell migration by reversing EndMT and downregulating stem cell markers in breast cancer[124].
9.4. FZD Receptors Inhibitors
Wnt receptor decoys function to prevent Wnt binding to FZD receptors. A phase 1b dose escalation study of ipafricept (OMP54F28) , a recombinant protein that inhibits Wnt signaling, in platinum-sensitive ovarian cancer was recently released, and the results indicated that bone toxicity at efficacy doses limited its use in ovarian cancer[125].
9.5. CBP/β-catenin Inhibitors
CBP/β-catenin inhibitors disrupt the interaction between CBP and β-catenin. One inhibitor, ICG-001, partially reversed EndMT and attenuated cancer stemness[126]. In addition, ICG-001 suppressed pancreatic cancer growth and significantly prolonged survival in an in vivo pancreatic ductal adenocarcinoma xenograft mouse model[127]. ICG-001 significantly prolonged the survival of NOD/SCID mice engrafted with drug-resistant primary ALL[128], and inhibited colony formation in sorted CD34+ CML progenitors in combination with imatinibmesylate[129].Moreover, inhibition by the CBP/β-catenin antagonist C-82/PRI-724and the use of FLT3 TKI decreased Wnt/β-catenin signaling in FLT3-mutant AML[130].
9.6. WNT5A Mimic
In addition, WNT5A upregulated the durable expression and activity of the indoleamine 2,3-dioxygenase-1 (IDO) enzyme by local DCs and induced immunotolerance[131]. WNT5A is an β-catenin-independent ligand that has been shown to induce tumor suppression [132]. Foxy-5, a WNT5A-mimicking peptide, which has been recently used in clinical trials, significantly inhibited the initial metastatic dissemination of prostate cancer cells with absent or low WNT5A expression[133]. In human colonic cancer, the number of colonic CSCs was decreased by Foxy-5[134]. .
10. Concluding Remarks
Herein, we have systemically reviewed the complex relationship between Wnt signaling and many factors in the TME (Figure 2). As discussed, most research has focused on canonical Wnt signaling which is based on Wnt/β-catenin interaction. Meanwhile, Wnt signaling plays an important role in sustaining the self-renewal potential of CSCs as well as LSCs, and subsequently enhances CSCs promotion. The immune cells in the TME mediate theimmune tolerance and immune evasion of cancer cells via Wnt signaling. In addition, Wnt signaling regulates ECM remodeling and causes overproduction of extracellular proteins in the TME to protect cancer cells from eradication. The transformation of stromal normal fibroblasts into the CAF phenotype has been implicated in promoting primary tumor growth and progression to metastatic disease. ECs in the TME induce tumor angiogenesis through Wnt signaling activation. There are several potential Wnt signaling modulator targets including but not limited to PORCN inhibitors, AXIN1 activators, Dickkopf1 antibodies, Wnt receptor decoys, CBP/β-catenin inhibitors and a WNT5A mimic. However, Wnt signaling also plays a crucial homeostatic role in normal cells and restricts the administration of potent Wnt signaling inhibitors due to off-target toxicities. In the future, the dual targeting of Wnt signaling and the TME may efficiently eradicate cancer cells. Taken together, Wnt signaling tightly correlates to the TME, and targeting the Wnt signaling pathway in the TME is a promising tumor therapeutic strategy.
Figure 2. Wnt signaling in the tumor microenvironment (TME).
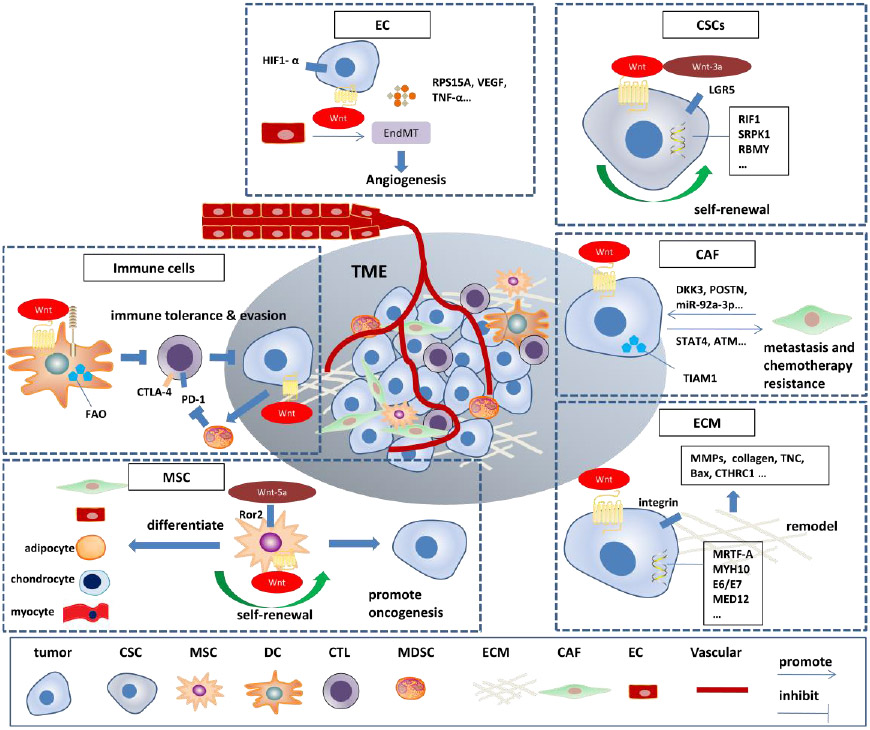
ECs, endothelial cells; EndMT, endothelial-mesenchymal transition; CSCs, cancer stem cells; LGR5, Leucine-rich repeat-containing G-protein-coupled receptor 5; ECM, extracellular matrix; CAF, Cancer associated fibroblast; TIAM1, T-lymphoma invasion and metastasis-inducing protein-1; FAO, fatty acid oxidation; CTLA-4, cytotoxic T lymphocyte-associated protein 4; PD-1, programmed cell death-1; CTL, cytotoxic T lymphocyte; MSC, mesenchymal stem cell; DC, dendritic cell; MDSC, myeloid-derived suppressor cell.
References
- 1.Nusse R and Clevers H, Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell, 2017. 169(6): p. 985–999. [DOI] [PubMed] [Google Scholar]
- 2.Galluzzi L, et al. , WNT Signaling in Cancer Immunosurveillance. Trends Cell Biol, 2019. 29(1): p. 44–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldsberry WN, et al. , A Review of the Role of Wnt in Cancer Immunomodulation. Cancers (Basel), 2019. 11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu T and Dai Y, Tumor microenvironment and therapeutic response. Cancer Lett, 2017. 387: p. 61–68. [DOI] [PubMed] [Google Scholar]
- 5.Kahn M, Can we safely target the WNT pathway? Nat Rev Drug Discov, 2014. 13(7): p. 513–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duchartre Y, Kim YM, and Kahn M, The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol, 2016. 99: p. 141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alok A, et al. , Wnt proteins synergize to activate beta-catenin signaling. J Cell Sci, 2017. 130(9): p. 1532–1544. [DOI] [PubMed] [Google Scholar]
- 8.Stamos JL and Weis WI, The beta-catenin destruction complex. Cold Spring Harb Perspect Biol, 2013. 5(1): p. a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulte G, Frizzleds and WNT/beta-catenin signaling--The black box of ligand-receptor selectivity, complex stoichiometry and activation kinetics. Eur J Pharmacol, 2015. 763(Pt B): p. 191–5. [DOI] [PubMed] [Google Scholar]
- 10.Janda CY, et al. , Surrogate Wnt agonists that phenocopy canonical Wnt and beta-catenin signalling. Nature, 2017. 545(7653): p. 234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniels DL and Weis WI, Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol, 2005. 12(4): p. 364–71. [DOI] [PubMed] [Google Scholar]
- 12.Prasetyanti PR, et al. , Regulation of stem cell self-renewal and differentiation by Wnt and Notch are conserved throughout the adenoma-carcinoma sequence in the colon. Mol Cancer, 2013. 12(1): p. 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, et al. , Roles of Wnt Target Genes in the Journey of Cancer Stem Cells. Int J Mol Sci, 2017. 18(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De A, Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin (Shanghai), 2011. 43(10): p. 745–56. [DOI] [PubMed] [Google Scholar]
- 15.Stolz A, et al. , Wnt-mediated protein stabilization ensures proper mitotic microtubule assembly and chromosome segregation. EMBO Rep, 2015. 16(4): p. 490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck B and Blanpain C, Unravelling cancer stem cell potential. Nat Rev Cancer, 2013. 13(10): p. 727–38. [DOI] [PubMed] [Google Scholar]
- 17.Reya T and Clevers H, Wnt signalling in stem cells and cancer. Nature, 2005. 434(7035): p. 843–50. [DOI] [PubMed] [Google Scholar]
- 18.Chattopadhyay S, Chaklader M, and Law S, Aberrant Wnt Signaling Pathway in the Hematopoietic Stem/Progenitor Compartment in Experimental Leukemic Animal. J Cell Commun Signal, 2019. 13(1): p. 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staal FJ, et al. , Aberrant Wnt Signaling in Leukemia. Cancers (Basel), 2016. 8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhan T, Rindtorff N, and Boutros M, Wnt signaling in cancer. Oncogene, 2017. 36(11): p. 1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JI, et al. , Telomerase modulates Wnt signalling by association with target gene chromatin. Nature, 2009. 460(7251): p. 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vijay GV, et al. , GSK3beta regulates epithelial-mesenchymal transition and cancer stem cell properties in triple-negative breast cancer. Breast Cancer Res, 2019. 21(1): p. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, et al. , Angiomotin-p130 inhibits beta-catenin stability by competing with Axin for binding to tankyrase in breast cancer. Cell Death Dis, 2019. 10(3): p. 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domenici G, et al. , A Sox2-Sox9 signalling axis maintains human breast luminal progenitor and breast cancer stem cells. Oncogene, 2019. 38(17): p. 3151–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmon KS, et al. , LGR5 interacts and cointernalizes with Wnt receptors to modulate Wnt/beta-catenin signaling. Mol Cell Biol, 2012. 32(11): p. 2054–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schepers AG, et al. , Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science, 2012. 337(6095): p. 730–5. [DOI] [PubMed] [Google Scholar]
- 27.Fu T, et al. , FXR Regulates Intestinal Cancer Stem Cell Proliferation. Cell, 2019. 176(5): p. 1098–1112 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang LQ, et al. , miR-372 and miR-373 enhance the stemness of colorectal cancer cells by repressing differentiation signaling pathways. Mol Oncol, 2018. 12(11): p. 1949–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao HZ, et al. , LGR5 promotes cancer stem cell traits and chemoresistance in cervical cancer. Cell Death Dis, 2017. 8(9): p. e3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mei Y, et al. , RIF1 promotes tumor growth and cancer stem cell-like traits in NSCLC by protein phosphatase 1-mediated activation of Wnt/beta-catenin signaling. Cell Death Dis, 2018. 9(10): p. 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong L, et al. , Serine-arginine protein kinase 1 promotes a cancer stem cell-like phenotype through activation of Wnt/beta-catenin signalling in NSCLC. J Pathol, 2016. 240(2): p. 184–96. [DOI] [PubMed] [Google Scholar]
- 32.Chua HH, et al. , RBMY, a novel inhibitor of glycogen synthase kinase 3beta, increases tumor stemness and predicts poor prognosis of hepatocellular carcinoma. Hepatology, 2015. 62(5): p. 1480–96. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber RD, Old LJ, and Smyth MJ, Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science, 2011. 331(6024): p. 1565–70. [DOI] [PubMed] [Google Scholar]
- 34.Thorsson V, et al. , The Immune Landscape of Cancer. Immunity, 2018. 48(4): p. 812–830 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staal FJ, Luis TC, and Tiemessen MM, WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol, 2008. 8(8): p. 581–93. [DOI] [PubMed] [Google Scholar]
- 36.Zhao DM, et al. , Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J Immunol, 2010. 184(3): p. 1191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forget MA, et al. , Stimulation of Wnt/ss-catenin pathway in human CD8+ T lymphocytes from blood and lung tumors leads to a shared young/memory phenotype. PLoS One, 2012. 7(7): p. e41074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Driessens G, et al. , Beta-catenin inhibits T cell activation by selective interference with linker for activation of T cells-phospholipase C-gamma1 phosphorylation. J Immunol, 2011. 186(2): p. 784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma J, et al. , beta-catenin/TCF-1 pathway in T cell development and differentiation. J Neuroimmune Pharmacol, 2012. 7(4): p. 750–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeevan-Raj B, et al. , The Transcription Factor Tcf1 Contributes to Normal NK Cell Development and Function by Limiting the Expression of Granzymes. Cell Rep, 2017. 20(3): p. 613–626. [DOI] [PubMed] [Google Scholar]
- 41.Kling JC, et al. , Temporal Regulation of Natural Killer T Cell Interferon Gamma Responses by beta-Catenin-Dependent and -Independent Wnt Signaling. Front Immunol, 2018. 9: p. 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou J, et al. , Notch and wingless signaling cooperate in regulation of dendritic cell differentiation. Immunity, 2009. 30(6): p. 845–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu C, et al. , beta-Catenin in dendritic cells exerts opposite functions in cross-priming and maintenance of CD8+ T cells through regulation of IL-10. Proc Natl Acad Sci U S A, 2015. 112(9): p. 2823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swafford D and Manicassamy S, Wnt signaling in dendritic cells: its role in regulation of immunity and tolerance. Discov Med, 2015. 19(105): p. 303–10. [PMC free article] [PubMed] [Google Scholar]
- 45.Baur AS, et al. , Denileukin diftitox (ONTAK) induces a tolerogenic phenotype in dendritic cells and stimulates survival of resting Treg. Blood, 2013. 122(13): p. 2185–94. [DOI] [PubMed] [Google Scholar]
- 46.Hong Y, et al. , Deletion of LRP5 and LRP6 in dendritic cells enhances antitumor immunity. Oncoimmunology, 2016. 5(4): p. e1115941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao F, et al. , Paracrine Wnt5a-beta-Catenin Signaling Triggers a Metabolic Program that Drives Dendritic Cell Tolerization. Immunity, 2018. 48(1): p. 147–160 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keerthivasan S, et al. , beta-Catenin promotes colitis and colon cancer through imprinting of proinflammatory properties in T cells. Sci Transl Med, 2014. 6(225): p. 225ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ribas A and Wolchok JD, Cancer immunotherapy using checkpoint blockade. Science, 2018. 359(6382): p. 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spranger S, Bao R, and Gajewski TF, Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature, 2015. 523(7559): p. 231–5. [DOI] [PubMed] [Google Scholar]
- 51.Taylor A, Rothstein D, and Rudd CE, Small-Molecule Inhibition of PD-1 Transcription Is an Effective Alternative to Antibody Blockade in Cancer Therapy. Cancer Res, 2018. 78(3): p. 706–717. [DOI] [PubMed] [Google Scholar]
- 52.Harding JJ, et al. , Prospective Genotyping of Hepatocellular Carcinoma: Clinical Implications of Next-Generation Sequencing for Matching Patients to Targeted and Immune Therapies. Clin Cancer Res, 2019. 25(7): p. 2116–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luke JJ, et al. , WNT/beta-catenin Pathway Activation Correlates with Immune Exclusion across Human Cancers. Clin Cancer Res, 2019. 25(10): p. 3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D'Amico L, et al. , Dickkopf-related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer. J Exp Med, 2016. 213(5): p. 827–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erdogan B and Webb DJ, Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans, 2017. 45(1): p. 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J, et al. , Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-beta pathways. Cancer Lett, 2016. 379(1): p. 49–59. [DOI] [PubMed] [Google Scholar]
- 57.Du J, et al. , Extracellular matrix stiffness dictates Wnt expression through integrin pathway. Sci Rep, 2016. 6: p. 20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu S, et al. , Sirt1 regulates apoptosis and extracellular matrix degradation in resveratrol-treated osteoarthritis chondrocytes via the Wnt/beta-catenin signaling pathways. Exp Ther Med, 2017. 14(5): p. 5057–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang R, et al. , E6/E7-P53-POU2F1-CTHRC1 axis promotes cervical cancer metastasis and activates Wnt/PCP pathway. Sci Rep, 2017. 7: p. 44744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang X, et al. , Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by miR-370-3p. J Biol Chem, 2018. 293(18): p. 6693–6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meng C, et al. , MRTF-A mediates the activation of COL1A1 expression stimulated by multiple signaling pathways in human breast cancer cells. Biomed Pharmacother, 2018. 104: p. 718–728. [DOI] [PubMed] [Google Scholar]
- 62.He H, et al. , The Wnt-beta-catenin signaling regulated MRTF-A transcription to activate migration-related genes in human breast cancer cells. Oncotarget, 2018. 9(20): p. 15239–15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, et al. , Myosin Heavy Chain 10 (MYH10) Gene Silencing Reduces Cell Migration and Invasion in the Glioma Cell Lines U251, T98G, and SHG44 by Inhibiting the Wnt/beta-Catenin Pathway. Med Sci Monit, 2018. 24: p. 9110–9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Y, et al. , Oncolytic Ad co-expressing decorin and Wnt decoy receptor overcomes chemoresistance of desmoplastic tumor through degradation of ECM and inhibition of EMT. Cancer Lett, 2019. 459: p. 15–29. [DOI] [PubMed] [Google Scholar]
- 65.Na Y, et al. , Potent antitumor effect of neurotensin receptor-targeted oncolytic adenovirus co-expressing decorin and Wnt antagonist in an orthotopic pancreatic tumor model. J Control Release, 2015. 220(Pt B): p. 766–82. [DOI] [PubMed] [Google Scholar]
- 66.Hawkins AG, et al. , The Ewing Sarcoma Secretome and Its Response to Activation of Wnt/beta-catenin Signaling. Mol Cell Proteomics, 2018. 17(5): p. 901–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCarthy JB, El-Ashry D, and Turley EA, Hyaluronan, Cancer-Associated Fibroblasts and the Tumor Microenvironment in Malignant Progression. Front Cell Dev Biol, 2018. 6: p. 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sasaki S, et al. , Crucial involvement of the CCL3-CCR5 axis-mediated fibroblast accumulation in colitis-associated carcinogenesis in mice. Int J Cancer, 2014. 135(6): p. 1297–306. [DOI] [PubMed] [Google Scholar]
- 69.Lorsy E, et al. , Loss of Dickkopf 3 Promotes the Tumorigenesis of Basal Breast Cancer. PLoS One, 2016. 11 (7): p. e0160077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caffo M, et al. , Modulation of Dkk-3 and claudin-5 as new therapeutic strategy in the treatment of meningiomas. Oncotarget, 2017. 8(40): p. 68280–68290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferreira Tojais N, et al. , Frizzled7 controls vascular permeability through the Wnt-canonical pathway and cross-talk with endothelial cell junction complexes. Cardiovasc Res, 2014. 103(2): p. 291–303. [DOI] [PubMed] [Google Scholar]
- 72.Ferrari N, et al. , Dickkopf-3 links HSF1 and YAP/TAZ signalling to control aggressive behaviours in cancer-associated fibroblasts. Nat Commun, 2019. 10(1): p. 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu JL, et al. , CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol Cancer, 2019. 18(1): p. 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Izumi D, et al. , TIAM1 promotes chemoresistance and tumor invasiveness in colorectal cancer. Cell Death Dis, 2019. 10(4): p. 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niell N, et al. , The human PKP2/plakophilin-2 gene is induced by Wnt/beta-catenin in normal and colon cancer-associated fibroblasts. Int J Cancer, 2018. 142(4): p. 792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kramer N, et al. , Autocrine WNT2 signaling in fibroblasts promotes colorectal cancer progression. Oncogene, 2017. 36(39): p. 5460–5472. [DOI] [PubMed] [Google Scholar]
- 77.Yu B, et al. , Periostin secreted by cancer-associated fibroblasts promotes cancer stemness in head and neck cancer by activating protein tyrosine kinase 7. Cell Death Dis, 2018. 9(11): p. 1082. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Rohwer N, et al. , Non-canonical HIF-1 stabilization contributes to intestinal tumorigenesis. Oncogene, 2019. [DOI] [PubMed] [Google Scholar]
- 79.Rupp C, et al. , IGFBP7, a novel tumor stroma marker, with growth-promoting effects in colon cancer through a paracrine tumor-stroma interaction. Oncogene, 2015. 34(7): p. 815–25. [DOI] [PubMed] [Google Scholar]
- 80.Munoz-Galvan S, et al. , Tumor cell-secreted PLD increases tumor stemness by senescence-mediated communication with microenvironment. Oncogene, 2019. 38(8): p. 1309–1323. [DOI] [PubMed] [Google Scholar]
- 81.Horman SR, et al. , Functional profiling of microtumors to identify cancer associated fibroblast-derived drug targets. Oncotarget, 2017. 8(59): p. 99913–99930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Valenti G, et al. , Cancer Stem Cells Regulate Cancer-Associated Fibroblasts via Activation of Hedgehog Signaling in Mammary Gland Tumors. Cancer Res, 2017. 77(8): p. 2134–2147. [DOI] [PubMed] [Google Scholar]
- 83.Zhao L, et al. , An integrated analysis identifies STAT4 as a key regulator of ovarian cancer metastasis. Oncogene, 2017. 36(24): p. 3384–3396. [DOI] [PubMed] [Google Scholar]
- 84.Avgustinova A, et al. , Tumour cell-derived Wnt7a recruits and activates fibroblasts to promote tumour aggressiveness. Nat Commun, 2016. 7: p. 10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaur A, et al. , sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature, 2016. 532(7598): p. 250–4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Chen Y, et al. , Aberrant low expression of p85alpha in stromal fibroblasts promotes breast cancer cell metastasis through exosome-mediated paracrine Wnt10b. Oncogene, 2017. 36(33): p. 4692–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang S, et al. , Oxidized ATM promotes abnormal proliferation of breast CAFs through maintaining intracellular redox homeostasis and activating the PI3K-AKT, MEK-ERK, and Wnt-beta-catenin signaling pathways. Cell Cycle, 2015. 14(12): p. 1908–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Piera-Velazquez S and Jimenez SA, Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol Rev, 2019. 99(2): p. 1281–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pinto MT, et al. , Endothelial cells from different anatomical origin have distinct responses during SNAIL/TGF-beta2-mediated endothelial-mesenchymal transition. Am J Transl Res, 2018. 10(12): p. 4065–4081. [PMC free article] [PubMed] [Google Scholar]
- 90.Wang SH, et al. , Tumour cell-derived WNT5B modulates in vitro lymphangiogenesis via induction of partial endothelial-mesenchymal transition of lymphatic endothelial cells. Oncogene, 2017. 36(11): p. 1503–1515. [DOI] [PubMed] [Google Scholar]
- 91.Scholz B, et al. , Endothelial RSPO3 Controls Vascular Stability and Pruning through Non-canonical WNT/Ca(2+)/NFAT Signaling. Dev Cell, 2016. 36(1): p. 79–93. [DOI] [PubMed] [Google Scholar]
- 92.Rapp J, et al. , Increased Wnt5a in squamous cell lung carcinoma inhibits endothelial cell motility. BMC Cancer, 2016. 16(1): p. 915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shi YN, et al. , Wnt5a and its signaling pathway in angiogenesis. Clin Chim Acta, 2017. 471: p. 263–269. [DOI] [PubMed] [Google Scholar]
- 94.Griveau A, et al. , A Glial Signature and Wnt7 Signaling Regulate Glioma-Vascular Interactions and Tumor Microenvironment. Cancer Cell, 2018. 33(5): p. 874–889 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al Thawadi H, et al. , VE-cadherin cleavage by ovarian cancer microparticles induces beta-catenin phosphorylation in endothelial cells. Oncotarget, 2016. 7(5): p. 5289–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guo P, et al. , Ribosomal protein S15a promotes tumor angiogenesis via enhancing Wnt/beta-catenin-induced FGF18 expression in hepatocellular carcinoma. Oncogene, 2018. 37(9): p. 1220–1236. [DOI] [PubMed] [Google Scholar]
- 97.Peterson YK, et al. , Frizzled-5: a high affinity receptor for secreted frizzled-related protein-2 activation of nuclear factor of activated T-cells c3 signaling to promote angiogenesis. Angiogenesis, 2017. 20(4): p. 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang Z and Feng Y, Exosomes Derived From Hypoxic Colorectal Cancer Cells Promote Angiogenesis Through Wnt4-Induced beta-Catenin Signaling in Endothelial Cells. Oncol Res, 2017. 25(5): p. 651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vallee A, Guillevin R, and Vallee JN, Vasculogenesis and angiogenesis initiation under normoxic conditions through Wnt/beta-catenin pathway in gliomas. Rev Neurosci, 2018. 29(1): p. 71–91. [DOI] [PubMed] [Google Scholar]
- 100.Linke F, et al. , Microenvironmental interactions between endothelial and lymphoma cells: a role for the canonical WNT pathway in Hodgkin lymphoma. Leukemia, 2017. 31(2): p. 361–372. [DOI] [PubMed] [Google Scholar]
- 101.Yan TL, et al. , Up-regulation of syncytin-1 contributes to TNF-alpha-enhanced fusion between OSCC and HUVECs partly via Wnt/beta-catenin-dependent pathway. Sci Rep, 2017. 7: p. 40983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bassett EA, et al. , Norrin/Frizzled4 signalling in the preneoplastic niche blocks medulloblastoma initiation. Elife, 2016. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Planutis K, Planutiene M, and Holcombe RF, A novel signaling pathway regulates colon cancer angiogenesis through Norrin. Sci Rep, 2014. 4: p. 5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yin T and Li L, The stem cell niches in bone. J Clin Invest, 2006. 116(5): p. 1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang X, et al. , The Effects of the WNT-Signaling Modulators BIO and PKF118-310 on the Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Int J Mol Sci, 2018. 19(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eslaminejad MB, Karimi N, and Shahhoseini M, Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells treated by GSK-3 inhibitors. Histochem Cell Biol, 2013. 140(6): p. 623–33. [DOI] [PubMed] [Google Scholar]
- 107.Wu D and Pan W, GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci, 2010. 35(3): p. 161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takiguchi G, et al. , Wnt5a-Ror2 signaling in mesenchymal stem cells promotes proliferation of gastric cancer cells by activating CXCL16-CXCR6 axis. Cancer Sci, 2016. 107(3): p. 290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Y, et al. , Wnt pathway contributes to the protection by bone marrow stromal cells of acute lymphoblastic leukemia cells and is a potential therapeutic target. Cancer Lett, 2013. 333(1): p. 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Azevedo PL, et al. , Canonical WNT Signaling Pathway is Altered in Mesenchymal Stromal Cells From Acute Myeloid Leukemia Patients And Is Implicated in BMP4 Down-Regulation. Transl Oncol, 2019. 12(4): p. 614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shen YL, et al. , Bone marrow mesenchymal stem cell-derived Wnt5a inhibits leukemia cell progression in vitro via activation of the non-canonical Wnt signaling pathway. Oncol Lett, 2014. 8(1): p. 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu L, et al. , Mesenchymal COX2-PG secretome engages NR4A-WNT signalling axis in haematopoietic progenitors to suppress anti-leukaemia immunity. Br J Haematol, 2018. 183(3): p. 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim YM, Gang EJ, and Kahn M, CBP/Catenin antagonists: Targeting LSCs'Achilles heel. Exp Hematol, 2017. 52: p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zimmerli D, et al. , WNT ligands control initiation and progression of human papillomavirus-driven squamous cell carcinoma. Oncogene, 2018. 37(27): p. 3753–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Picco G, et al. , Loss of AXIN1 drives acquired resistance to WNT pathway blockade in colorectal cancer cells carrying RSPO3 fusions. EMBO Mol Med, 2017. 9(3): p. 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ma F, et al. , SOX9 drives WNT pathway activation in prostate cancer. J Clin Invest, 2016. 126(5): p. 1745–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Agarwal P, et al. , Enhanced targeting of CML stem and progenitor cells by inhibition of porcupine acyltransferase in combination with TKI. Blood, 2017. 129(8): p. 1008–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guimaraes PPG, et al. , Potent in vivo lung cancer Wnt signaling inhibition via cyclodextrin-LGK974 inclusion complexes. J Control Release, 2018. 290: p. 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Park SY, et al. , Inhibition of LEF1-Mediated DCLK1 by Niclosamide Attenuates Colorectal Cancer Stemness. Clin Cancer Res, 2019. 25(4): p. 1415–1429. [DOI] [PubMed] [Google Scholar]
- 120.Bhattacharyya J, et al. , Niclosamide-conjugated polypeptide nanoparticles inhibit Wnt signaling and colon cancer growth. Nanoscale, 2017. 9(34): p. 12709–12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen X, et al. , Down-regulation of microRNA-31-5p inhibits proliferation and invasion of osteosarcoma cells through Wnt/beta-catenin signaling pathway by enhancing AXIN1. Exp Mol Pathol, 2019. 108: p. 32–41. [DOI] [PubMed] [Google Scholar]
- 122.Kagey MH and He X, Rationale for targeting the Wnt signalling modulator Dickkopf-1 for oncology. Br J Pharmacol, 2017. 174(24): p. 4637–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kimura H, et al. , CKAP4, a DKK1 Receptor, Is a Biomarker in Exosomes Derived from Pancreatic Cancer and a Molecular Target for Therapy. Clin Cancer Res, 2019. 25(6): p. 1936–1947. [DOI] [PubMed] [Google Scholar]
- 124.Mu J, et al. , Dickkopf-related protein 2 induces G0/G1 arrest and apoptosis through suppressing Wnt/beta-catenin signaling and is frequently methylated in breast cancer. Oncotarget, 2017. 8(24): p. 39443–39459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moore KN, et al. , A phase 1b dose escalation study of ipafricept (OMP54F28) in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Gynecol Oncol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen Y, et al. , TNF-alpha derived from M2 tumor-associated macrophages promotes epithelial-mesenchymal transition and cancer stemness through the Wnt/beta-catenin pathway in SMMC-7721 hepatocellular carcinoma cells. Exp Cell Res, 2019. 378(1): p. 41–50. [DOI] [PubMed] [Google Scholar]
- 127.Arensman MD, et al. , The CREB-binding protein inhibitor ICG-001 suppresses pancreatic cancer growth. Mol Cancer Ther, 2014. 13(10): p. 2303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gang EJ, et al. , Small-molecule inhibition of CBP/catenin interactions eliminates drug-resistant clones in acute lymphoblastic leukemia. Oncogene, 2014. 33(17): p. 2169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim YM, et al. , The gamma catenin/CBP complex maintains survivin transcription in beta-catenin deficient/depleted cancer cells. Curr Cancer Drug Targets, 2011. 11 (2): p. 213–25. [DOI] [PubMed] [Google Scholar]
- 130.Jiang X, et al. , Disruption of Wnt/beta-Catenin Exerts Antileukemia Activity and Synergizes with FLT3 Inhibition in FLT3-Mutant Acute Myeloid Leukemia. Clin Cancer Res, 2018. 24(10): p. 2417–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Holtzhausen A, et al. , Melanoma-Derived Wnt5a Promotes Local Dendritic-Cell Expression of IDO and Immunotolerance: Opportunities for Pharmacologic Enhancement of Immunotherapy. Cancer Immunol Res, 2015. 3(9): p. 1082–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Prasad CP, et al. , WNT5A as a therapeutic target in breast cancer. Cancer Metastasis Rev, 2018. 37(4): p. 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Canesin G, et al. , Treatment with the WNT5A-mimicking peptide Foxy-5 effectively reduces the metastatic spread of WNT5A-low prostate cancer cells in an orthotopic mouse model. PLoS One, 2017. 12(9): p. e0184418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Osman J, et al. , The WNT5A Agonist Foxy5 Reduces the Number of Colonic Cancer Stem Cells in a Xenograft Mouse Model of Human Colonic Cancer. Anticancer Res, 2019. 39(4): p. 1719–1728. [DOI] [PubMed] [Google Scholar]