SUMMARY
Staphylococcus aureus is a common cause of both superficial and invasive infections of humans and animals. Despite a potent host response and apparently appropriate antibiotic therapy, staphylococcal infections frequently become chronic or recurrent, demonstrating a remarkable ability of S. aureus to withstand the hostile host environment. There is growing evidence that staphylococcal DNA repair makes important contributions to the survival of the pathogen in host tissues, as well as promoting the emergence of mutants that resist host defenses and antibiotics. While much of what we know about DNA repair in S. aureus is inferred from studies with model organisms, the roles of specific repair mechanisms in infection are becoming clear and differences with Bacillus subtilis and Escherichia coli have been identified. Furthermore, there is growing interest in staphylococcal DNA repair as a target for novel therapeutics that sensitize the pathogen to host defenses and antibiotics. In this review, we discuss what is known about staphylococcal DNA repair and its role in infection, examine how repair in S. aureus is similar to, or differs from, repair in well-characterized model organisms, and assess the potential of staphylococcal DNA repair as a novel therapeutic target.
KEYWORDS: DNA damage, DNA repair, Staphylococcus aureus, antibiotic resistance, bacteriophage, neutrophil, SOS
INTRODUCTION
In living cells, DNA is continually damaged by numerous factors, ranging from exogenous agents such as ionizing radiation, chemical compounds, and physical stress (1–3) to endogenous threats such as reactive oxygen species (ROS) produced during cellular metabolism (1). This damage can impair replication and transcription within the cell, affecting cell viability.
During normal bacterial growth, DNA damage can result from stalled replication forks, replication past single-strand nicks, or exposure to metabolic by-products such as ROS (1). These molecules are formed during aerobic metabolism and include hydrogen peroxide, superoxide, and the hydroxyl radical. ROS are highly reactive and can damage cellular components, including lipids, proteins, and nucleic acids (4). The most significant target of ROS is believed to be DNA because, unlike proteins or lipids, which can be newly synthesized if damage is too severe for repair, the repair of DNA is essential for replication (5).
The highly reactive and damaging nature of ROS makes them a major part of the host immune defense against Staphylococcus aureus. During infection, staphylococci are rapidly phagocytosed by neutrophils and exposed to ROS as part of the respiratory burst (6). The importance of this host defense mechanism is demonstrated by the high incidence of S. aureus infections in people with chronic granulomatous disease, who lack a functional NADPH oxidase enzyme and cannot generate ROS (7). In addition, deletion of NADPH oxidase in mice is associated with increased susceptibility to S. aureus infections (8), and treatment of isolated human neutrophils with the NADPH-oxidase inhibitor diphenyleneiodonium has been shown to impair the killing of S. aureus (9).
Another cause of DNA damage is antibiotic treatment. For example, antibiotics from the quinolone family, such as ciprofloxacin, cause DNA damage by targeting DNA replication. This is achieved by inhibiting the ligase activity of topoisomerase enzymes, which cut both strands of the DNA helix to introduce negative supercoiling (10). Therefore, the enzyme is unable to religate cut DNA strands, which leads to double-strand breaks (DSBs). It has also been proposed that several classes of antibiotics may cause bacterial killing independent of their drug-target interactions, possibly via DNA damage (11–19). In S. aureus, induction of the SOS response pathway, indicative of DNA damage, was triggered by the β-lactam antibiotics ceftriaxone and cloxacillin, which are used extensively in the treatment of staphylococcal infections (20). Although it is still unclear how this damage occurs, previous reports have suggested that disruption of metabolic processes results in production of ROS, which can in turn damage DNA (11–19).
SOS RESPONSE
For many bacteria, the SOS response is a global transcriptional reaction to address DNA damage and leads to cell cycle arrest and the initiation of DNA repair (1). The SOS response pathway can be triggered by numerous DNA-damaging agents, including UV light, ROS, and certain antibiotics (e.g., fluoroquinolones) (21). In S. aureus, the SOS response has been shown to be induced by H2O2 and various DNA-damaging antibiotics (22–25).
The SOS response is governed by two key protein regulators: the Lee X-ray-sensitive mutant A (LexA) transcriptional repressor and recombinase A (RecA) sensor protein (Fig. 1) (26, 27). In the absence of DNA damage, LexA forms a dimer and negatively regulates expression of genes within the SOS regulon by binding to the promoter regions of genes with the S. aureus-specific consensus sequence CGAACAAATGTTCG, where boldfacing indicates 100% conservation (25). Upon DNA damage, single-stranded DNA (ssDNA) is formed during the replication of damaged DNA templates or after processing by RecBCD/AddAB family enzymes during DNA repair. RecA binds to the ssDNA and polymerizes, forming a nucleoprotein filament that activates LexA for self-cleavage, derepressing SOS genes (26, 27). The importance of autocatalytic LexA cleavage for SOS induction in S. aureus was demonstrated by the construction of a strain with mutation within lexA to change a catalytically important serine residue to alanine [LexA(S130A)] (25, 28). Exposure of this mutant strain to ciprofloxacin failed to trigger the SOS response and was more susceptible to DNA damage caused by UV light or methyl methanesulfonate (25). However, while autocatalysis of LexA to N- and C-terminal domains is required for derepression of genes within the SOS regulon, the N-terminal domain retains some DNA-binding activity. Thus, further degradation of the N-terminal domain of LexA by ClpXp and/or ClpCp proteases is required for full derepression (29). As such, there appear to be some SOS genes, including lexA, which are expressed upon LexA autocatalysis and others, such as sosA, which are only derepressed when the N-terminal domain is further digested by Clp proteases (29, 30). This is similar to Escherichia coli, where ClpXp-mediated degradation of the N-terminal domain of LexA is required for full induction of the SOS response and survival of DNA damaged by UV exposure (31, 32).
FIG 1.
Model for activation of the SOS response in S. aureus. (A) In this model, the SOS response is induced by a double-stranded DNA break (DSB), but SOS induction can occur via other mechanisms that result in a ssDNA being formed during DNA repair or replication of the damaged DNA template. (B) The DSB undergoes end processing to produce ssDNA, carried out by the RecBCD or AddAB (also known as RexAB) complexes. (C) The RecA protein forms filaments on the ssDNA, leading to RecA activation. (D) Activated RecA interacts with the LexA repressor, activating its latent protease activity. (E) This results in autocleavage of LexA, inactivating the LexA repressor and leading to derepression of the SOS genes.
In addition to LexA, RecA activates at least one low-fidelity DNA polymerase in S. aureus, UmuC, also referred to as DNA polymerase V or Pol V. In E. coli, Pol V consists of the trimeric complex UmuD′2C, encoded by the umuDC operon, which enables replication of damaged DNA templates (25, 33, 34). However, neither S. aureus nor Bacillus subtilis appears to have a umuD homologue, and it has been proposed that the function of UmuD is instead performed by YolD (25, 35). S. aureus encodes a LexA-regulated gene that is predicted to encode a YolD homologue (SACOL1986) (25), but it is not clear what, if any, role this plays in UmuC function. Since UmuC lacks proofreading activity, the activity of this DNA polymerase leads to an increase in the mutation rate (36, 37), which has been suggested to promote adaptation at times of environmental stress and contribute to the acquisition of antibiotic resistance (25).
The S. aureus SOS regulon consists of 16 genes, identified by Cirz et al. (25) by comparing the global transcriptional response of wild-type and noncleavable lexA-bearing S. aureus strains after exposure to ciprofloxacin, a fluoroquinolone antibiotic. These genes include recA and lexA; umuC; sosA, which inhibits cell division in response to DNA damage (38); genes involved in nucleotide excision repair (NER) (uvrA and uvrB); topoisomerase IV genes (parC and parE); and two genes encoding the SbcCD endonuclease (sbcC and sbcD) to process stalled replication forks (25). The remaining six genes consist of three encoding hypothetical proteins (SACOL0436, SACOL1986, and SACOL1999) and the polycistronic operon SACOL2162-SACOL2160, which encodes a protein of unknown function, a protein involved in cell wall synthesis (UTP–glucose-1-phosphate uridylyltransferase), and a putative hemolysin (25). The binding of S. aureus LexA to the recA promoter has also been demonstrated, which is consistent with recA regulation in other systems (26, 39).
The number of genes under LexA-regulated control in S. aureus is significantly lower than in the model organisms E. coli and B. subtilis, which contain SOS regulons that consist of at least 43 and 63 genes, respectively (26, 40). Genes that are conserved among all three systems include recA, lexA, genes encoding DNA repair proteins and at least one error-prone polymerase. For example, SOS-induced error-prone DNA repair is performed by UmuC in S. aureus (25); UmuC, DinB (Pol IV), or DnaE (Pol III) in B. subtilis (41–43) and by polymerase B (PolB or Pol II), DinB, or UmuD′2C in E. coli (1). Although no significant sequence similarity is observed between LexA-regulated S. aureus SosA, E. coli SulA, and B. subtilis YneA, they all perform the same function to inhibit cell division to enable DNA damage repair, and this may apply to other SOS-regulated proteins whose function is currently unclear (44). Nevertheless, the consequences of S. aureus having a relatively small SOS regulon are currently unclear.
In addition to DNA repair, the SOS response in S. aureus has been shown to affect virulence by promoting horizontal gene transfer of virulence factors and the expression of chromosomal virulence genes (20, 21, 39, 45–47). Ubeda et al. (45) found that exposure to ciprofloxacin triggered dissemination of S. aureus pathogenicity islands (SaPIs), mobile genetic elements that encode virulence factors, and that this was significantly reduced by inactivation of recA. In addition, Goerke et al. (47) showed that increased transcription of staphylokinase (a phage-encoded virulence factor) in response to ciprofloxacin was strongly linked to upregulation of recA. The SOS response may also affect the ability of S. aureus to bind host surfaces, as Bisognano et al. (39) found that purified LexA can bind to the promoter of the fnbB gene, which encodes a fibronectin-binding adhesin.
DIRECT REVERSAL REPAIR
Bacteria have evolved diverse mechanisms to repair DNA damage caused by endogenous and exogenous agents. These include repair pathways for single-strand damage, as well as for DSBs. In addition, bacteria have several mechanisms by which damage can be resolved by a single repair protein without breakage of the phosphodiester backbone. Although direct reversal mechanisms only repair a small set of lesions, these pathways do not require a DNA template and are error free (48).
Photoreactivation
Exposure to UV light leads to the formation of pyrimidine dimers, where abnormal covalent bonds form between consecutive thymine or cytosine bases. Two types of pyrimidine dimers can be formed: cyclobutane pyrimidine dimers (CPDs) or pyrimidine pyrimidone (6–4) photoproducts (6–4PPs). DNA photolyase enzymes can repair these lesions in a process called photoreactivation, which uses blue and near-UV light (350 to 450 nm) to reverse the damage. Since photolyases are specific to one type of pyrimidine dimer, they are referred to as either CPD photolyases or (6–4) photolyases (48, 49).
Photolyases absorb light via chromophoric cofactors. In particular, the flavin adenine dinucleotide (FAD) cofactor is essential for binding to damaged DNA and catalysis (48, 49). CPD photolyases also contain a second chromophore, either methenyltetrahydrofolate (MTHF) or 8-hydroxy-7,8-didemethyl-5-deazariboflavin (8-HDF). The second chromophore is not required for catalysis, but it may increase the rate of repair under limiting light conditions (49).
DNA photolyases occur in nearly all living organisms exposed to sunlight, with the exception of placental mammals such as humans, where NER systems are used instead (50–53). S. aureus encodes a putative DNA photolyase (SACOL0751), though its function has not yet been confirmed (25, 54, 55).
Alkylation Damage Repair
DNA damage via alkylation or methylation can occur endogenously or in the environment through alkylating agents such as methyl chloride (56). These agents react with the nitrogen and oxygen atoms of DNA bases to generate covalent adducts that can be cytotoxic. Repair of alkylation damage in bacteria is carried out by multiple partially redundant mechanisms, including the base excision repair (BER) system and two direct reversal repair pathways: (i) O6-alkylguanine DNA alkyltransferases to reverse O6-alkylated guanines and (ii) alkylation B (AlkB) family dioxygenases to reverse N-alkylated lesions (48).
In many bacteria, alkylation damage repair is induced by the adaptive response to alkylation damage (Ada response) (1). For example, in E. coli, this is mediated by the N-terminal domain of the Ada protein (N-Ada), which is activated by a DNA methylphosphotriester lesion to induce transcription of Ada response genes: ada, alkA, alkB, and aidB (1, 57, 58). AlkA is a DNA glycosylase involved in BER that removes methyl lesions formed on nitrogen moieties (e.g., N3-methyladenine and N3-methylguanine) (56, 59), whereas Ada and AlkB reverse alkylation damage directly (56, 58). The C terminus of Ada (C-Ada) repairs O6-alkylguanine and O4-alkylthymine base lesions (48, 58, 60), and AlkB repairs N1-methyladenine and N3-methylcytosine lesions (61, 62). Meanwhile, AidB has been proposed to function by preventing alkylation damage (63–65).
The Ada response in B. subtilis occurs though a similar process as described for E. coli, with the key difference that two separate Ada proteins (AdaA and AdaB) mediate the functions performed by E. coli N-Ada and C-Ada, respectively (26). The B. subtilis Ada response also leads to induction of the adaAB operon and the alkA gene only (66–69), and the lack of AlkB may be compensated for by the BER pathway (70). However, while the Ada response is present in many bacteria (1, 26, 71), its presence in S. aureus is still unknown. A report by Ambur et al. (72) identifying homologues of selected genes encoding proteins that mediate DNA repair found that the genome of S. aureus strain EMRSA-16 (MRSA252) did not contain either ada or alkB. However, Zhang et al. (73) found that the clinical VISA isolate XN108 contains a putative O6-methylguanine DNA methyltransferase (AdaB).
SINGLE-STRAND DAMAGE REPAIR
When only one strand of DNA is damaged, excision repair mechanisms can remove and replace damaged bases using the undamaged strand as a template (26). Excision repair mechanisms include base excision repair (BER), nucleotide excision repair (NER), and mismatch repair (MMR). In addition to these mechanisms, the RecF pathway of homologous recombination is used to repair single-strand gaps.
Base Excision Repair
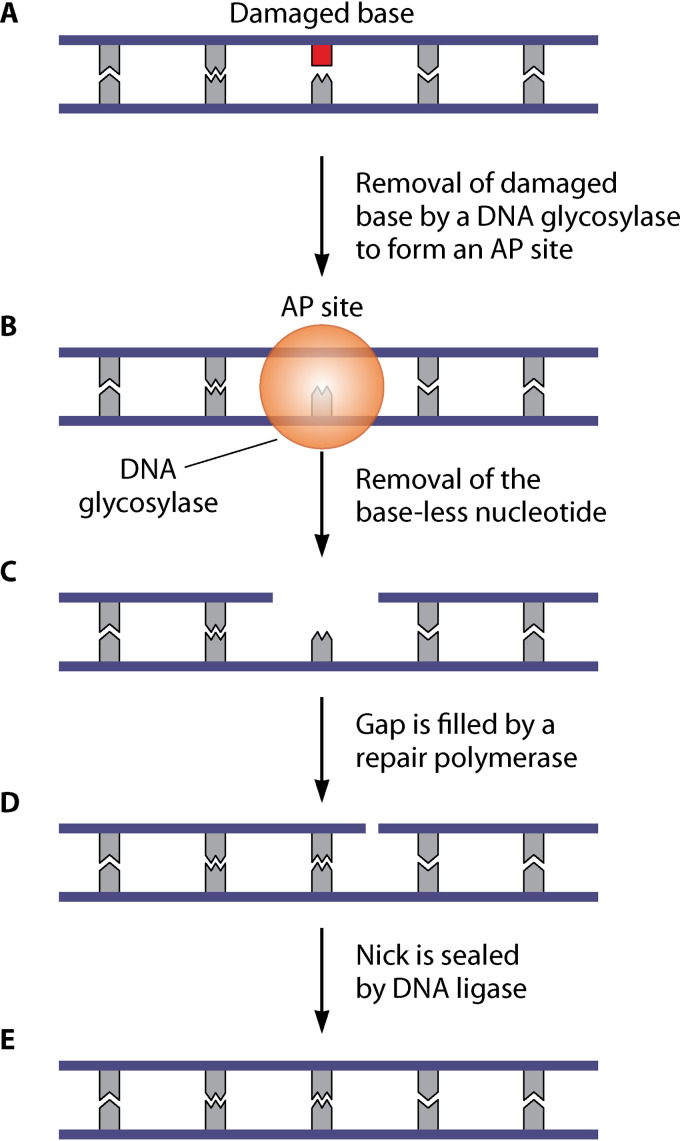
BER is the main pathway for repairing nonbulky single-base lesions in DNA, including alkylated and deaminated bases, oxidized bases, abasic sites, and dUTP incorporation during DNA replication (26, 74). BER is initiated by damage-specific DNA glycosylases that recognize lesions and cleave the N-glycosidic bond (Fig. 2). This removes the damaged base and leads to the formation of abasic or apurinic/apyrimidinic sites (AP sites). The 5′ and 3′ ends of the AP site are nicked by AP endonucleases and AP lyases, respectively, which enables processing by an exonuclease or deoxyribophosphodiesterase (dRpase). The resultant gap is filled by a repair polymerase (such as Pol I, encoded by the polA gene) via short-patch (one nucleotide is replaced) or long-patch (multiple nucleotides are synthesized) pathways, with the remaining nick sealed by DNA ligase (26, 75).
FIG 2.
Model for base excision repair (BER) in S. aureus. (A and B) BER is initiated by specific DNA glycosylases that recognize and remove damaged bases (shown in red) (A), generating abasic or apurinic/apyrimidinic sites (AP sites) (B). (C) Next, the 5′ and 3′ ends of the AP sites are nicked by AP endonucleases and AP lyases, followed by processing by an exonuclease or deoxyribophosphodiesterase (dRpase) to remove the baseless nucleotide. (D) This leaves a gap, which is filled by a repair polymerase (such as Pol I). (E) The remaining nick is sealed by DNA ligase.
In E. coli, AP endonuclease activity is provided by exonuclease III (ExoIII or Xth) and endonuclease IV (EndoIV or Nfo), whereas dRpase activity is due to RecJ and exonuclease I (ExoI) (27). In B. subtilis, AP endonuclease activity is provided by the products of three genes: exoA, yqfS, and yshC (26, 76, 77). ExoA and YqfS are homologous to E. coli Xth and Nfo, respectively (78, 79). Meanwhile, yshC encodes DNA polymerase X (PolX), a low-processivity DNA polymerase that possesses AP endonuclease activity and acts preferentially on small gaps in DNA (76, 80). As a result, PolX has been suggested as the primary polymerase for repairing small lesions (26). Although Xth/ExoA is not present within the staphylococcal genome, S. aureus contains homologues of Nfo, RecJ, PolX, and Pol I (72, 81, 82). Recently, Nagpal et al. (83) showed that staphylococcal PolX aids high-fidelity DNA synthesis by removing misincorporated nucleotides using its C-terminal polymerase and histidinol phosphatase (PHP) domain.
The most common lesion formed in DNA during oxidative stress is 7,8-dihydro-8-oxoguanine (8-oxoG or GO lesion), which can mispair with adenine and cause G-to-T transversions (84, 85). In E. coli, repair of this lesion is mediated by the GO system via DNA glycosylases (86). Formamidopyrimidine DNA glycosylase (Fpg, also known as MutM) removes 8-oxoG prior to a mismatch, whereas MutY removes misincorporated adenine from an 8-oxoG-A mismatch (87, 88). The GO system also removes oxidized guanines (8-oxo-dGTP) from the nucleotide pool, preventing their misincorporation into DNA. This function is provided by MutT, which selectively hydrolyses 8-oxo-dGTP to 8-oxo-dGMP, with the concomitant release of pyrophosphate (89).
Removal of 8-oxoG lesions and 8-oxoG-A mismatches in S. aureus and B. subtilis is performed by homologues of E. coli MutM and MutY (90, 91), and S. aureus has an active GO system (92). Canfield et al. (91) showed that S. aureus mutM and mutY mutants displayed increased mutation frequencies compared to the wild type, and confirmed MutM and MutY glycosylase activities in the clearance of 8-oxoG-associated DNA lesions. In addition to these lesions, Endutkin et al. (92) reported that S. aureus MutM cleaves duplexes containing 5-hydroxyuracil, 5,6-dihydrouracil, or apurinic/apyrimidinic site paired with C, T, or G—but not with A. Furthermore, S. aureus MutY is capable of efficiently excising adenine from double-stranded substrates containing A:8-oxoG or A:G pairs (92). However, a clear functional homologue for MutT has not been identified in either S. aureus or B. subtilis. Canfield et al. (91) identified five potential mutT homologues in S. aureus, but inactivation of these genes did not increase the mutation frequency, as would be expected from a mutT mutant.
Oxidative stress can also lead to the generation of pyrimidine lesions, such as thymine glycol (93). In E. coli, these lesions are recognized by endonuclease III (EndoIII or Nth) or endonuclease VIII (EndoVIII or Nei), both of which are DNA glycosylases (94–96). However, less is known about these enzymes in B. subtilis and S. aureus. Ambur et al. (72) showed that these two species do not contain a homologue of E. coli Nei but do contain homologues of Nth. S. aureus Nth (SAUSA300_1343) is induced by H2O2 stress (22), and inactivation of this gene has been shown to increase the mutation rate (97). Meanwhile, B. subtilis Nth has been shown to nick substrates with AP sites and protect the bacterium against H2O2 stress (98, 99), consistent with a conserved role of this enzyme in repair of oxidative damage.
Finally, BER is responsible for the removal of misincorporated dUMP during DNA replication (100). Although dUMP incorporation is rare due to the small pool of dUTP compared to that of dTTP, the integration of uracil (instead of thymine) can lead to transition mutations (100). These dUMP lesions can also occur via deamination of cytosine residues (dCMP to dUMP), which arises spontaneously but is promoted by the presence of •OH (1). Many bacteria (including E. coli, B. subtilis, and S. aureus) counteract dUMP incorporation by removal of dUMP from DNA, which is performed by uracil-DNA glycosylase (UDG, encoded by ung) (26, 72, 100–103). The function of S. aureus UDG was confirmed by Wang et al. (103) via direct testing of the purified protein for uracil-removing activity.
Nucleotide Excision Repair
The nucleotide excision repair (NER) pathway is responsible for the high-fidelity repair of bulky helix-distorting lesions in DNA, such as thymine dimers or DNA cross-links (104). This pathway is conserved in prokaryotes and eukaryotes, and lesions are repaired in 10- to 15-nucleotide segments (26). This process requires the highly conserved UvrABC endonuclease complex (1), the expression of which is induced by the SOS response in S. aureus, as well as E. coli and B. subtilis (25, 26, 40).
The function of this system has been elucidated in E. coli but is very likely conserved in S. aureus. First, damaged DNA is recognized by a complex of UvrA and UvrB (Fig. 3). This leads to UvrA dissociation and enables UvrC to complex with UvrB. Then, the UvrBC complex cleaves the phosphodiester backbone, leading to the removal of approximately 10 to 15 nucleotides surrounding the lesion. This removal is facilitated by PcrA in S. aureus (105, 106) or UvrD (DNA helicase II) in E. coli, which enables release of the nucleotide segment. Finally, the gap is filled by Pol I using the intact strand as a template, and the remaining nick is sealed by DNA ligase (26, 107). In S. aureus, UvrABC has been reported to contribute to nitrosative stress resistance, which has been suggested previously as a role for NER in E. coli (108, 109).
FIG 3.
Model for the nucleotide excision repair (NER) pathway in S. aureus. (A) The NER pathway repairs bulky helix-distorting DNA lesions, such as thymine dimers and DNA cross-links. (B) In this pathway, damaged DNA is recognized by the UvrAB complex. (C and D) This leads to UvrA dissociation to enable formation of the UvrBC complex (C), which removes approximately 10 to 15 nucleotides surrounding the lesion (D). This removal is facilitated by PcrA, which enables release of the nucleotide segment. (E and F) The resultant gap is filled by Pol I (E), with the nick sealed by DNA ligase (F).
The NER pathway is also triggered in response to bulky DNA lesions that cause stalling of RNA polymerase (107). This subpathway of NER is called transcription-coupled repair and involves the mutation frequency decline (Mfd) protein, also known as the transcription repair coupling factor. Mfd releases stalled RNA polymerase from the transcribed strand and recruits UvrA, which initiates the rest of the NER pathway as described above (52, 72, 107).
Mismatch Repair
Mismatch repair (MMR) is responsible for correcting replication errors that escape DNA polymerase proofreading activity (110, 111). In Gram-negative bacteria, the replicative DNA polymerase is DnaE (Pol III) (112), but B. subtilis and many Gram-positive bacteria contain two replicative Pol III polymerases: DNA polymerase C (PolC) and DnaE (26, 112, 113). PolC is the main polymerase, responsible for the majority of leading and lagging strand synthesis, but it cannot extend from the 3′-OH ends of RNA primers, which is instead carried out by DnaE before handing over to PolC (114, 115). In Gram-negative bacteria, DnaE is associated with the proofreading ε subunit (DnaQ) to provide 3′–5′ exonuclease activity (116). However, this subunit is not present in Gram-positive bacteria and high-fidelity DNA replication is enabled by PolC instead, which has endogenous proofreading activity (112). This corrects most errors that occur during replication. Any remaining errors are corrected by MMR, which is coupled to DNA synthesis and increases the replication fidelity by up to 1,000-fold (111, 117–119).
In E. coli, mismatched bases generated from DNA synthesis are recognized and bound by MutS, which recruits MutL to stabilize the MutS-mismatch complex (110, 111), and the process is likely conserved in S. aureus, which encodes homologues of MutS and MutL (72, 111, 120–124). However, while MutL activates MutH endonuclease in E. coli to nick the nascent strand carrying the mismatch, identified by its absence of methylation (110, 111), homologues of E. coli MutH have not been identified in most other bacteria (125). In some MutH-less bacteria, homologues of MutL have been shown to exhibit endonuclease activity (126–129), and so the nascent strand is identified by recognizing strand discontinuities near DNA replication forks (26, 130, 131). Although endonuclease activity has not yet been functionally demonstrated in S. aureus MutL, Fukui et al. (132) showed that the sequence motifs essential for MutL endonuclease activity are conserved and present. In addition, d(GATC) sequences in S. aureus and B. subtilis are not methylated, which supports the use of methylation-independent MMR in these species (133).
The error-containing region is excised by UvrD helicase and degraded by one of several exonucleases, depending on the polarity of the strand (RecJ, Exo I, ExoVII, and ExoX) (110, 134). In contrast, only RecJ is present in S. aureus (81), and only one exonuclease is present in B. subtilis, WalJ (134). Next, the remaining gap is filled by Pol III and the nick sealed by DNA ligase (110, 111). The daughter strand is then methylated at adenine in the d(GATC) sequence by Dam methylase (26). The importance of this system is exemplified in B. subtilis, where the loss of mutSL has been shown to increase the overall mutation rate by 60-fold (123, 124). In addition, the inactivation of MMR is associated with mutator phenotypes that have been shown to play a role in the adaptation of bacterial populations to stressful environments (135), including for S. aureus (91, 121, 122).
DOUBLE-STRAND BREAK REPAIR
DNA DSBs in S. aureus can be caused by a variety of factors, including DNA-damaging agents (e.g., antibiotics or ROS from immune cells [136, 137]) and replication fork collapse. These DNA lesions are particularly dangerous, because they can interrupt the coding region of a gene, alter chromosome organization, and lead to mutagenic DNA rearrangements (138). If not repaired, DSBs can also become substrates for exonucleases, leading to loss of vital genetic information and driving genetic instability (5, 138). In bacteria, including S. aureus, DSBs are repaired via homologous recombination, which involves the exchange of genetic material between two homologous DNA sequences (5). In some bacterial species, but not S. aureus, a second pathway of DSB repair is present, called nonhomologous end joining (NHEJ) (26, 139). This pathway is mutagenic since the two broken DNA ends are directly ligated using minimal or no sequence homology, but unlike homologous recombination, only a single copy of the genome is required (139). NHEJ is performed by the Ku protein, which binds the DSB ends, and an ATP-dependent DNA ligase (usually ligase D) to join the broken segments (139).
The NHEJ pathway has been identified in a number of bacteria, including B. subtilis, M. tuberculosis, M. smegmatis, and P. aeruginosa (140–143). Interestingly, many of these bacteria are capable of sporulation (B. subtilis) and/or spend long periods of their life cycle in the stationary phase (M. tuberculosis), and it is speculated that the NHEJ system could be particularly important for repairing DSBs that arise during states of relative inactivity, where homologous recombination is less effective (140). Therefore, it is perhaps not surprising that NHEJ is not present in S. aureus, which is believed to rely on homologous recombination (143), although it may also employ the alternative end-joining (A-EJ) mechanism found in E. coli since it encodes homologues of the necessary proteins (RexAB and LigA, respectively) (144–146). This is a modified version of NHEJ that involves minor processing of the broken ends in E. coli by the RecBCD complex, followed by direct religation by the essential replicative DNA ligase A (LigA).
Another potential pathway that can be used to repair DSBs in some bacteria is single-strand annealing (SSA), which is RecA independent and uses homologous repeat sequences flanking the break site for repair (147). DNA ends undergo extensive resection and exposed complementary sequences are annealed, followed by DNA processing of remaining tails, gap filling, and ligation. As a result, SSA leads to deletions between the repeats and is relatively mutagenic (147). However, although SSA is believed to be present in bacteria and has been reported in both E. coli and B. subtilis, it has not been well characterized in prokaryotes, and its presence in S. aureus is unknown (148–150).
Homologous Recombination
Homologous recombination (also known as recombinational repair) is the major route for DSB repair in bacteria, resulting in high-fidelity DNA repair (5). This pathway requires a second copy of the genome as a template for repair, meaning that it can only occur when the damaged region has already been copied prior to cell division. This is usually not prohibitive for many bacteria, which often contain partially replicated copies of the genome, unless grown under conditions where the doubling time is longer than the time taken to replicate the chromosome and segregate the products (151, 152). Homologous recombination consists of several steps: (i) break recognition and end resection, (ii) loading of RecA and strand invasion, (iii) DNA synthesis and branch migration, and (iv) Holliday junction resolution (Fig. 4). Although the details of each step and the components involved may differ between organisms, these steps are conserved in both prokaryotes and eukaryotes (138, 144). In addition to DSB repair, homologous recombination is used to repair single-strand gaps as part of the RecF pathway, which will also be described below.
FIG 4.
Model for DSB repair by homologous recombination in S. aureus. (A and B) When an active replication fork encounters a single-strand nick (A), this produces a double-strand break (DSB) and the replication fork collapses (B). (C) The DSB is processed by the RexAB complex, generating a 3′-ssDNA overhang. (D) RecOR is recruited to load the RecA recombinase onto the ssDNA region, with RecF increasing the loading efficiency. (E) RecA pairs with the homologous DNA sequence and mediates strand invasion, producing a D-loop structure. This is aided by the accessory proteins RecX and RarA. (F) DNA polymerase extends the 3′ end of the filament to form a stable four-stranded DNA structure called a Holliday junction, which is moved along the DNA by RuvAB or RecG. (G) In the final step, this junction is cleaved by RecU, and replication restarts.
The homologous recombination pathway is initiated by recognition of the break, followed by end resection, in which broken DNA ends are processed to generate ssDNA (26, 153). In B. subtilis, it has been reported that RecN is one of the first responders to a DSB and may participate in the assembly of a DNA repair center (154–157). However, although recN is present in S. aureus, its rule in DSB repair DSBs is unknown, and it does not appear to be LexA regulated or induced in response to DSBs (25). In E. coli, RecN is kept at very low levels prior to SOS induction, suggesting that it cannot act before end resection and induction of SOS (153).
After a DSB is identified, the ends are processed by the RexAB helicase-nuclease complex. During this process, S. aureus RexAB (homologue of AddAB in Bacillus; see section below) binds to the DSB and unwinds the DNA using the helicase activity in its RexA subunit, while simultaneously digesting the strand (via both subunits) to generate a 3′-ssDNA overhang (26, 151, 158). In the RecFOR pathway, end resection is performed by the RecQ helicase and RecJ exonuclease, which unwind and degrade the DNA, respectively (159–161).
Next, RecA binds to the 3′-ssDNA overhang and searches for the intact homologous DNA sequence to initiate strand invasion (26, 153). Although mechanisms for RecA loading are well established in E. coli, they are poorly understood in many other bacteria. In E. coli, RecA loading can be carried out by RecBCD or RecFOR, which are part of the DSB and single-strand gap repair mechanisms, respectively (159, 162). Meanwhile in B. subtilis, AddAB (RexAB homologue) is not involved in RecA loading, which is instead carried out by RecOR, with RecF not essential for loading but increasing its efficiency (163). In a report by Alonso et al. (164), the S. aureus recF gene was shown to partially restore the activity of a B. subtilis recF mutant, indicating that RecF protein function is conserved in S. aureus. The steps following RecA loading are the same in both RecBCD and RecFOR pathways and, as such, are assumed to also apply to S. aureus even though they have not yet been experimentally confirmed in this species.
After binding of RecA to the ssDNA overhang, the recombinase pairs with the homologous DNA sequence and mediates strand invasion, where the broken strand exchanges places with the intact strand. This involves local denaturation of the dsDNA in the region of homology to produce a D-loop structure (26, 159). In B. subtilis, this is aided by the accessory proteins RecX and RarA, both of which are present in S. aureus (165, 166).
After strand invasion, regular DNA synthesis occurs via Pol III using the intact strand as a template to form stable four-stranded DNA structures called Holliday junctions (26, 52, 159). These are moved along the DNA in a branch migration process, where base pairs between the two homologous DNA strands are exchanged. In E. coli, this process is mediated by the RuvAB protein complex (159, 167). RuvA binds to the Holliday junction and recruits RuvB, which then translocates the junction along the DNA. Finally, the endonuclease RuvC cleaves the Holliday junction to separate the two repaired DNA duplexes, and the nicks at the cleavage site are sealed by DNA ligase (159, 167). Branch migration and resolution can also be catalyzed by RecG (159, 167). Although homologues of RuvA, RuvB, and RecG proteins are present in many Gram-positive bacteria, including B. subtilis and S. aureus, the RuvC protein is not (26, 72, 168). Instead, Holliday junction resolution in Gram-positive bacteria is carried out by the RecU protein, which is absent in most Gram-negative species (169). S. aureus also encodes a putative endonuclease, MutS2, the role of which is unknown. However, in B. subtilis MutS2 serves as an alternative Holliday junction endonuclease (170). However, deletion of RecU in S. aureus leads to defects in chromosome segregation and DNA damage repair, which is in agreement with the role of a Holliday junction resolvase (171).
RecBCD/AddAB Enzyme Family
In bacteria, processing of DSBs is mediated by the RecBCD/AddAB family of enzymes. This consists of three members: RecBCD, AddAB (called RexAB in S. aureus), and AdnAB. These multisubunit complexes use different combinations of helicase and nuclease activities to resect DNA ends, resulting in a 3′ ssDNA extension onto which RecA is loaded (151). Therefore, these enzymes enable (and are required for) the induction of the SOS response (via RecA) in response to DSBs (136). RecBCD and AddAB enzymes have been well studied, mostly using E. coli RecBCD and B. subtilis AddAB, respectively. The third member of the family, AdnAB, was identified more recently in mycobacterial species (172). RecBCD is generally found in Gram-negative species, although there are exceptions, while Gram-positive bacteria use AddAB. In S. aureus, AddAB is called RexAB, and has been confirmed as a member of the AddAB family and displays the characteristic helicase and nuclease activities (136).
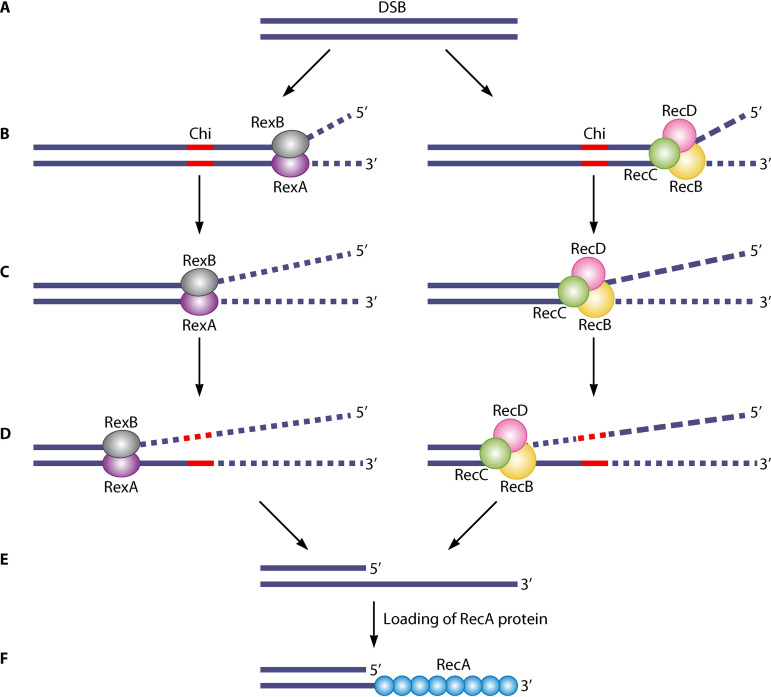
Enzymes from the RecBCD/AddAB family (including RexAB) initiate recombinational repair by binding to free DNA ends and unwinding the DNA using their helicase activity (Fig. 5A and B), located in the RecB and RecD, or RexA subunits (173, 174). Then, the enzyme translocates along the DNA, using its nuclease activity to degrade both strands until a crossover hot spot instigator (Chi or χ) site is reached (Fig. 5C). Nuclease activity is located in the RecB subunit in RecBCD, or both subunits in RexAB. When a Chi site is encountered, structural changes are induced in the complex that lead to attenuated cleavage of DNA in the 3′–5′ direction (Fig. 5D) (158). Recognition of the Chi site is polar, only occurring when the enzyme is travelling though the DNA from the 3′ end (151). The Chi sequence recognized by E. coli RecBCD is 8 bases long (5′‐GCTGGTGG‐3′) (175, 176), whereas the Chi site for B. subtilis AddAB is shorter at 5 bases (5′-AGCGG-3′) (177), and S. aureus RexAB is predicted to recognize a 7-nucleotide Chi site (5′-GAAGCGG-3′), but this has not yet been functionally confirmed (178). Chi sequences are specific to a bacterial species, although those recognized by RecBCD homologues do share some similarity (151, 178).
FIG 5.
Model for processing of DNA ends by S. aureus RexAB or E. coli RecBCD. (A and B) DNA damage can lead to a double-strand break (DSB) (A), which can be lethal if not repaired. RexAB or RecBCD binds to the broken end and unwinds the DNA (B). A Chi (crossover hot spot instigator) site is denoted in red. (C) The enzyme translocates along the DNA, degrading both DNA strands. Degradation is performed by the RecB and RecD subunits in RecBCD, or both subunits in RexAB. (D and E) When a Chi site is encountered in the 3′ strand, this induces changes that attenuate degradation of the 3′–5′ nuclease (D), resulting in a 3′ overhang (E). (F) The RecA protein is loaded onto the 3′ overhang to form a filament (green circles) for the next step of the recombinational repair pathway.
Although recognition of Chi leads to attenuated 3′–5′ nuclease activity, degradation continues in the 5′–3′ direction, resulting in the production of a 3′ single-strand overhang (Fig. 5E) (158). Chi recognition also enhances 5′–3′ nuclease activity in RecBCD, leading to faster degradation of the 5′ strand (179), but this does not occur in B. subtilis AddAB (180). Finally, RecA protein is loaded onto the 3′ overhang for the next step of recombination (Fig. 5F). In the case of RecBCD, this is performed by the RecB subunit (181, 182). In B. subtilis, AddAB is not involved in RecA loading, which is carried out by RecOR and presumed to be the same in S. aureus (163).
RecBCD and AddAB/RexAB enzymes have similar biochemical properties, and both lead to the formation of a RecA filament, but there are significant differences in the structure of these two protein complexes. The most obvious difference is in their subunit composition. RecBCD consists of three subunits, whereas AddAB/RexAB only has two (173, 183). In addition, these subunits have different combinations of helicase and nuclease activities. Although the activities of individual RexA and RexB subunits have not yet been confirmed experimentally in S. aureus, in silico structural analysis of RexAB reported high similarity with B. subtilis AddAB (Fig. 6) (136). As such, details of B. subtilis AddAB subunit function are also expected to apply to S. aureus RexAB and are presumed to be in this review.
FIG 6.
Structural homology of S. aureus RexAB to B. subtilis AddAB. Predicted structural models of S. aureus RexA and RexB superimposed onto AddA and AddB subunits of the B. subtilis AddAB crystal structure. Ribbon representation of S. aureus and B. subtilis proteins individually and superimposed. Individual protein structures are colored from blue to red from the N terminus to the C terminus. When superimposed, S. aureus proteins are shown in blue, and B. subtilis proteins are shown in orange. The predicted three-dimensional models were generated by Phyre2 and protein structures visualized using PyMOL. PDB 3U4Q was used for the B. subtilis AddAB structure.
RexAB contains a single helicase domain located in the RexA subunit and two homologous nuclease domains, one in each subunit. The RexB subunit is also responsible for Chi recognition. Each nuclease cleaves a different DNA strand, with the RexA subunit displaying 3′–5′ nuclease activity, while RexB has 5′–3′ activity (180). In RecBCD, the RecB subunit contains a helicase domain with 3′–5′ directionality and a nuclease domain that also loads RecA onto the 3′ ssDNA overhang (181, 183). The RecD subunit contains a helicase domain but has 5′–3′ specificity. Meanwhile, RecC is responsible for recognizing Chi sites (184, 185). DNA degradation occurs symmetrically in RexAB but not in RecBCD, which is consistent with the fact that RexAB contains two nuclease domains, while RecBCD contains only one (186).
Meanwhile, the third member of the RecBCD/AddAB enzyme family, AdnAB, consists of two subunits, each displaying both helicase and nuclease activities (172). The arrangement and cleavage activity of AdnAB corresponds to those of AddAB/RexAB (with AdnA and AdnB being equivalent to AddB/RexB and AddA/RexA, respectively), in that both helicase subunits move along the same DNA strand, but each nuclease subunit cleaves a different strand (151, 187).
CONSEQUENCES OF DNA REPAIR AND INDUCTION OF THE SOS RESPONSE
Survival during Infection
The ability of S. aureus to efficiently repair DNA damage is of critical importance in the context of infection, where the pathogen faces the twin threats of host immune defenses and antibiotics. The key host defense against S. aureus infection is the respiratory (oxidative) burst of neutrophils, which causes various types of DNA damage, including DSBs (9, 136, 188–192). Processing of DNA DSBs by RexAB results in induction of the SOS response within 30 min of incubation of bacteria with neutrophils, which contributes significantly to bacterial survival (136).
In addition to the oxidative burst of neutrophils, phagocytosis of S. aureus by macrophages led to induction of the SOS response via a process that was largely was dependent upon the stringent response that is triggered by amino acid starvation (193). The basis for this observation is unknown but induction of the stringent response leads to expression of genes associated with oxidative stress resistance suggesting that nutrient limitation may cause DNA damage via the endogenous generation of ROS (194). In support of this, the LexA-regulated gene umuC was found to contribute to survival of S. aureus during amino acid starvation (195).
Besides immune cells, there is also evidence that some antimicrobial peptides (AMPs) trigger the SOS response in S. aureus by inhibiting DNA replication (196, 197). While it is not clear whether this observation extends to all AMPs or just specific classes, the lipopeptide antibiotic daptomycin also triggers the SOS response, suggesting that DNA damage may be a consistent consequence of AMP-mediated membrane damage (137).
Bacterial DNA damage during infection includes DNA DSBs, the repair of which by RexAB was found to be required for the survival of S. aureus in an ex vivo whole human blood model and in both systemic and superficial murine infections (136). Along with rexAB, several other genes associated with DNA repair were found to enhance staphylococcal survival in whole human blood, including recA and recF (136).
Additional support for the critical role of DNA repair in staphylococcal virulence comes from a Tn-seq experiment that showed mutants lacking rexA, rexB, recF, recN, recO, or recF were unable to survive in a murine skin and soft tissue infection model (108). Another Tn-seq study demonstrated that RexAB contributed to staphylococcal survival in a murine lung infection model, confirming the importance of DNA DSB repair for staphylococcal survival at distinct anatomical sites (198).
Staphylococcal DNA can also be damaged during infection by exposure to therapeutic antibiotics, of which several trigger the SOS response, including ciprofloxacin, co-trimoxazole, daptomycin, nitrofurantoin, chloramphenicol, linezolid, and some beta-lactams (136, 137, 199, 200). The combination antibiotic co-trimoxazole and the quinolone ciprofloxacin are both known to cause DNA DSBs, so induction of the SOS response is unsurprising (10, 136, 199). However, several of the other antibiotics that trigger the SOS response do not directly target DNA replication but instead cause metabolic perturbations that lead to endogenous ROS production and thus DNA damage (11). For example, beta-lactam antibiotics cause DNA damage in S. aureus via enhanced TCA cycle activity and endogenous ROS production, which leads to SOS induction (200). DNA DSBs appear to be a common manifestation of antibiotic-mediated DNA damage in S. aureus, since RexAB has been shown to promote the survival of S. aureus exposed to ciprofloxacin, co-trimoxazole, nitrofurantoin, daptomycin, and oxacillin (137). Processing of DNA DSBs by RexAB also appeared to make an important contribution to induction of the SOS response by these antibiotics (137).
Virulence
In addition to promoting S. aureus survival in vivo, there is also evidence that the SOS response contributes to the regulation of some of the many virulence factors produced by the pathogen. These include a raft of adhesins, such as the fibronectin-binding proteins that promote invasion of host cells, biofilm formation, and disseminated infection (201). SOS activation leads to increased production of fibronectin-binding protein B, resulting in enhanced biofilm formation (202). This is because LexA appears to be a transcriptional regulator of the fnbB gene, suggesting that biofilm formation may be an important response by which S. aureus can withstand genotoxic stress (39).
There is also evidence that staphylococcal capsule is negatively regulated via the SOS response, though it is not clear what advantage this provides the bacterium (203). SOS-mediated activation of prophage ϕ13 by the antibiotics ciprofloxacin and trimethoprim led to increased expression of the phage-encoded virulence factor staphylokinase, which inhibits the antibacterial activity of AMPs and also converts plasminogen to plasmin (47, 204).
Induction of the SOS response results in an elevated mutation rate due to the expression of the UmuC error-prone DNA polymerase (205, 206). This increases the rate of emergence of small-colony variants (SCVs), which are associated with chronic and relapsing infection (205–207). SCVs originate by mutations in the genes encoding components of the electron-transport chain and have lowered metabolic activity as a result, forming small colonies as a result of slow growth (208). In addition, SCVs have other phenotypic properties that enable their long-term survival in the host, including a high degree of antibiotic tolerance, strong expression of surface proteins and enhanced biofilm formation, and an ability to persist within host cells, and they are highly resistant to killing by neutrophils (209–213). Therefore, the exposure of S. aureus to genotoxic stresses such as the respiratory burst and antibiotics in the host may drive the development of chronic infection by promoting the rate of emergence of, and selecting for, SCVs.
Antimicrobial Resistance and Tolerance
As might be expected from its effect on the mutation rate, SOS induction leads to higher rates of spontaneous resistance to rifampin, co-trimoxazole, and ciprofloxacin (199, 205, 214). SOS induction can also affect methicillin resistance, which is mediated by acquisition of the SCCmec element that contains the mecA gene encoding Pbp2A (215). Many MRSA strains are heteroresistant to beta-lactams, with only a small subpopulation of cells being phenotypically resistant to this class of antibiotic. However, beta-lactams trigger the induction of the SOS response via endogenous ROS production, and this results in a shift from heteroresistance to homogeneous resistance (200, 216, 217). The role of SOS induced mutation in this process has been confirmed using ciprofloxacin to trigger SOS, which also leads to emergence of homogeneous beta-lactam resistance in S. aureus, confirming that this phenomenon is not simply due to antibiotic selection (218).
In addition to antibiotic resistance, SOS induction may play a role in antibiotic tolerance (193). SOS induction results in inhibition of cell growth via SosA which may contribute to the formation of persisters, nongrowing antibiotic tolerant bacteria, although this remains to be tested (38, 193). Activation of the SOS response has also been linked to the acquisition of tolerance to photodynamic therapy (219).
SOS and Phage Induction
The genomes of many staphylococcal strains contain prophages, which give rise to functional bacteriophages when activated (220). Activation can be spontaneous but often occurs as a response to RecA activation (such as during SOS induction) and results in the phage entering the lytic cycle (20, 221).
The induction of phage by the SOS response has been exploited by the human pathogen Streptococcus pneumoniae during interspecies competition against S. aureus (221). In this case, phage induction triggered SOS induction in S. aureus via the production of H2O2, leading to staphylococcal destruction by phage-mediated lysis (221). There is also evidence that the bactericidal activity of some antibiotics is enhanced by phage activation (222). However, while SOS-mediated phage induction appears to be an Achilles heel for S. aureus, phages make an enormous contribution to the success of the pathogen (220). For example, antibiotic-induced prophage activation leads to elevated horizontal gene transfer of antibiotic resistance determinants, suggesting that antibiotics not only select for resistance but also are very likely to trigger its dissemination (20, 45).
In addition to transfer of resistance determinants, phages can aid the dissemination of S. aureus pathogenicity islands (SaPIs) that contain toxins such as toxic-shock syndrome toxin 1 (223). SaPIs have evolved to disseminate via phages and promote this by encoding proteins that promote SaPI packaging into phage (encapsidation) (224). To ensure that production of these encapsidation proteins is coordinated with phage induction, their expression on the SaPIbov1 pathogenicity island is regulated by LexA and thus activated during the SOS response, together with prophages (46, 220).
To summarize, DNA repair and the SOS response are not only important processes that enable staphylococcal survival in the host. They also enhance genotypic variation via enhanced mutation rates and horizontal gene transfer, thereby contributing to the emergence of strains that can resist host defenses and antibiotics.
INHIBITORS OF DNA REPAIR
Several studies have examined various aspects of bacterial DNA repair as targets for the discovery and development of new antibiotics (225–231). Since these have recently been reviewed in detail (232, 233), we provide only a brief overview here.
The redundancy of diverse bacterial DNA repair pathways has made it difficult to identify suitable targets. Special attention has been given to inhibition of the SOS response, which is responsible for induction of excision repair, recombinational repair, and DNA mutagenesis. Most of these studies use E. coli as a model, targeting LexA or RecA (228, 230, 231). Phthalocyanine tetrasulfonic acid-based RecA inhibitors block SOS induction in E. coli and inhibited acquisition of ciprofloxacin resistance in a neutropenic murine thigh infection model, demonstrating the potential effectiveness of targeting the SOS response (228). However, RecA homologues are present in humans (Rad51 and Dmc1) (234), meaning that inhibitors of RecA may exhibit host toxicity.
Regarding SOS suppression in S. aureus, a report by Peng et al. (235) found that baicalein, the main component of the Chinese herb Scutellaria baicalensis, inhibited expression of several SOS genes (recA, lexA, and umuC). This effect correlated with a reduction in intracellular ATP production, suggesting that baicalein may act on ATP synthase. Another study identified an additional effect of novobiocin in suppressing the ciprofloxacin-induced SOS response in S. aureus by inhibiting recA expression (214). Since novobiocin is an aminocoumarin, a class of antibiotics that inhibit DNA gyrase without inducing DSBs, this finding suggests that clinical reevaluation of existing antibiotics may help to combat the development of antibiotic resistance (214). Another SOS inhibitor is betulinic acid, a plant-derived triterpenoid, which reduces ciprofloxacin-induced activation of the SOS response and potentiates ciprofloxacin activity in S. aureus (236).
Inhibitors of RecBCD and/or AddAB have also been reported. RecBCD inhibitors include dozelesin, ecteinascidin 743, hedamycin, cisplatin, psoralen, and the Gam protein of bacteriophage lambda (237–240). However, issues with these inhibitors include limited in vivo stability, poor oral bioavailability, and a suboptimal mechanism of action such as DNA alkylation, making them unselective and highly cytotoxic (237–240). More promisingly, Amundsen et al. (241) identified a number of potent inhibitors of E. coli RecBCD and H. pylori AddAB in a screen of 326,100 small molecules from the National Institutes of Health (NIH) molecular library sample collection. Their screen employed a cell-based assay using phage infection as a marker for inhibition of RecBCD/AddAB activity, based on the observation that the gene 2 protein of T4 phage protects viral DNA from RecBCD/AddAB-mediated degradation (242). Inhibition of RecBCD or AddAB by an active compound promoted growth of the T4 gene 2 mutant, leading to killing of bacteria. After 12 hits were tested for inhibition of purified E. coli RecBCD and H. pylori AddAB enzyme activities, similar tests were performed on a further 40 compounds structurally related to the two most active molecules, which identified six more potent inhibitors (241). Sixty compounds from the “iminobenzothiazoles” class, previously reported in a large-scale screen of DNA helicase inhibitors (243), were also tested for inhibition of RecBCD nuclease activity, which identified three more active compounds. In total, 21 potent inhibitors were identified, from which CID 1517823 was most active against E. coli RecBCD, and CID 697851 was most active against H. pylori AddAB (241).
To treat S. aureus infections, RexAB inhibitors could be used as monotherapy for superficial infections in otherwise healthy patients or in combination with ciprofloxacin or other antibiotics for invasive infections. RexAB inhibition may also resensitize bacteria to ciprofloxacin, providing an additional therapeutic against MRSA strains, many of which are resistant to fluoroquinolone antibiotics (244).
In addition, a key feature of the immune response is the production of ROS, which kill bacteria by damaging biological molecules such as DNA (136). Although RexAB inhibitors would not be able to discriminate between pathogenic and beneficial bacteria due to RexAB’s conserved nature, RexAB is not essential for bacterial growth and is only required upon DNA damage (136). This means that during infection, RexAB inhibitors would be expected to have the greatest effect on the pathogenic bacteria that are targeted by immune response. Therefore, undesired damage to the commensal gut microbiota would be reduced, unlike with conventional antibiotic therapy (245). This would reduce susceptibility to opportunistic secondary infections caused by pathogens such as C. difficile (246).
Another advantage of targeting RexAB is that the mutagenic SOS response would be inhibited because of the failure to process DNA DSBs to ssDNA and thus the RecA nucleoprotein filament. Induction of the SOS regulon leads to expression of the low-fidelity DNA polymerase UmuC (Pol V), which enables bypass of DNA lesions during DNA replication and results in an increased rate of mutagenesis (33, 247). By blocking this mutagenic pathway, host adaptation and acquisition of resistance could be reduced (248). In addition, homologues of S. aureus RexAB are present in more than 90% of sequenced bacteria (249). This means that inhibition of RexAB has potential as a broad-spectrum therapeutic approach. RexAB homologues are also not present in eukaryotes, which reduces the likelihood of host toxicity.
In summary, there is growing evidence of the importance of DNA repair for the success of S. aureus as a pathogen of humans and animals, and ever-increasing understanding of how DNA repair in this bacterium differs from model organisms. This information is already being used to inform the development of next-generation antistaphylococcal therapeutics that sensitize the pathogen to host defenses, antibiotics, to reduce the emergence of drug-resistant and host-adapted strains.
OUTSTANDING QUESTIONS
Recent years have seen significant advances in our understanding of mechanisms of staphylococcal DNA repair and the roles these processes play in the success of this pathogen. However, progress lags behind that seen for the model organisms E. coli and B. subtilis, and there are still substantial gaps in our knowledge. We consider the following to be the major research questions that warrant attention in the near future.
(i) What determines the size and composition of the SOS regulon in different bacteria? SOS regulons vary in both size and composition between bacteria, with only a few genes in common between them (25, 250). Why is this? Is it due to exposure to different genotypic stresses and/or pathogenesis?
(ii) What do all the genes in the S. aureus SOS regulon do? Although S. aureus has a relatively small SOS regulon, it is still not clear what all these gene products do or why it is beneficial to S. aureus that they are regulated by LexA (25). For example, SACOL1986 encodes a putative YolD protein, which may contribute to the function of the error-prone polymerase UmuC, but this has not been tested experimentally.
(iii) What regulates genes encoding DNA repair proteins that are not within the SOS regulon? Several of the DNA repair-associated genes in the B. subtilis and E. coli SOS regulons are present in the S. aureus genome but are not regulated by LexA (25, 26, 251). What regulates the expression of these genes, and why is it advantageous that they are not controlled by LexA?
(iv) What are the mechanistic links between SOS and other stress responses, and what are the consequences for S. aureus? S. aureus employs numerous stress-response systems that enable survival in hostile environments (24, 193, 252). At least one of these, the stringent response, appears to enhance the magnitude of the SOS response within macrophages, but it is not clear how or why this benefits S. aureus (193). Furthermore, SOS induction is often coincident with other stress responses such as the PerR-mediated oxidative stress response, but it is not known whether there is any cross talk between these systems (193, 194).
(v) Does the SOS response confer antibiotic tolerance? The SOS response has been linked to antibiotic tolerance and persistence in several different bacterial species, in some cases via activation of toxin-antitoxin (TA) systems that arrest growth (253, 254). While TA systems do not appear to contribute to antibiotic tolerance in S. aureus, SOS-mediated growth arrest via SosA could feasibly confer antibiotic tolerance (38, 44, 255).
(vi) Is staphylococcal DNA repair a viable target for novel therapeutic approaches? DNA repair enables S. aureus to cause infection and tolerate many different classes of antibiotic. Induction of the mutagenic SOS response is also associated with increased emergence of bacteria with host-adapted phenotypes and/or antibiotic resistance (205, 213). Can these repair processes be targeted via novel inhibitors to deliver clinical benefit (232)? Importantly, can we target bacterial DNA repair without compromising mammalian DNA repair systems?
(vii) How heterogeneous is the SOS response in S. aureus, and what are the consequences of this? There is good evidence that the magnitude of the SOS response is heterogenous within a clonal bacterial population during exposure to genotoxic stress (136, 256, 257). However, it is not clear why this is, whether it applies to all genotoxic stresses, and whether this heterogeneity confers a selective advantage at the single cell and/or population level.
ACKNOWLEDGMENTS
K.P.H. was supported by a Ph.D. scholarship funded by a Medical Research Council award to the Centre for Molecular Bacteriology and Infection (MR/J006874/1). A.M.E. acknowledges funding from Shionogi & Co., Ltd., and support from the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC). The funders had no role in the study design, interpretation of the findings or the writing of the manuscript.
We declare there are no conflicts of interest.
Biographies

Kam Pou Ha graduated from Imperial College London in 2014 with a B.Sc. in Biochemistry with a placement year at AstraZeneca. She continued her studies at Imperial College London, obtaining an M.Res. in Biomedical Research, specializing in bacterial pathogenesis and infection, and a Ph.D. working on staphylococcal DNA repair and host immune defenses. She is currently working as a postdoc at the Institute for Integrative Cell Biology (France), working on small regulatory RNA in Staphylococcus aureus and mechanisms of antibiotic resistance.

Andrew M. Edwards graduated from University College London in 1998 with a B.Sc. in Medical Microbiology before studying Treponema spirochetes during a Ph.D. at the University of Bristol. He then spent time at the University of Minnesota, working on bacterial cooperation and host cell invasion; Novartis Vaccines, where he studied the pili of group A streptococci; and the University of Bath, where he began working with Staphylococcus aureus. He established his own group in the MRC Centre for Molecular Bacteriology and Infection at Imperial College London in 2011. He studies fundamental mechanisms of resistance to antibiotics and host defenses, with a long-term aim of developing novel therapeutics to combat bacterial pathogens.
REFERENCES
- 1.Friedberg EC, Wood RD, Walker GC, Schultz RA, Siede W, Ellenberger T. 2006. DNA repair and mutagenesis. American Society for Microbiology Press, Washington, DC. [Google Scholar]
- 2.Rosen R, Buchinger S, Pfänder R, Pedhazur R, Reifferscheid G, Belkin S. 2016. SOS gene induction and possible mutagenic effects of freeze-drying in Escherichia coli and Salmonella Typhimurium. Appl Microbiol Biotechnol 100:9255–9264. doi: 10.1007/s00253-016-7751-x. [DOI] [PubMed] [Google Scholar]
- 3.Maeda T, Horinouchi T, Sakata N, Sakai A, Furusawa C. 2019. High-throughput identification of the sensitivities of an Escherichia coli ΔrecA mutant strain to various chemical compounds. J Antibiot (Tokyo) 72:566–573. doi: 10.1038/s41429-019-0160-5. [DOI] [PubMed] [Google Scholar]
- 4.Imlay JA. 2003. Pathways of oxidative damage. Annu Rev Microbiol 57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 5.Chapman JR, Taylor MRG, Boulton SJ. 2012. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell 47:497–510. doi: 10.1016/j.molcel.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 6.Sbarra A, Karnovsky M. 1959. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem 234:1355–1362. doi: 10.1016/S0021-9258(18)70011-2. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Ari J, Wolach O, Gavrieli R, Wolach B. 2012. Infections associated with chronic granulomatous disease: linking genetics to phenotypic expression. Expert Rev Anti Infect Ther 10:881–894. doi: 10.1586/eri.12.77. [DOI] [PubMed] [Google Scholar]
- 8.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. 1995. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet 9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 9.Hampton MB, Kettle AJ, Winterbourn CC. 1996. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect Immun 64:3512–3517. doi: 10.1128/iai.64.9.3512-3517.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drlica K. 1999. Mechanism of fluoroquinolone action. Curr Opin Microbiol 2:504–508. doi: 10.1016/S1369-5274(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 11.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 12.Dwyer DJ, Belenky PA, Yang JH, et al. 2014. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc Natl Acad Sci USA 111. doi: 10.1073/pnas.1401876111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Zhao X. 2009. Contribution of oxidative damage to antimicrobial lethality. Antimicrob Agents Chemother 53:1395–1402. doi: 10.1128/AAC.01087-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeom J, Imlay JA, Park W. 2010. Iron homeostasis affects antibiotic-mediated cell death in Pseudomonas species. J Biol Chem 285:22689–22695. doi: 10.1074/jbc.M110.127456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shatalin K, Shatalina E, Mironov A, Nudler E. 2011. H2S: a universal defense against antibiotics in bacteria. Science 334:986–990. doi: 10.1126/science.1209855. [DOI] [PubMed] [Google Scholar]
- 16.Calhoun LN, Kwon YM. 2011. The ferritin-like protein Dps protects Salmonella enterica serotype Enteritidis from the Fenton-mediated killing mechanism of bactericidal antibiotics. Int J Antimicrob Agents 37:261–265. doi: 10.1016/j.ijantimicag.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 17.Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. 2012. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science 336:315–319. doi: 10.1126/science.1219192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Liu X, Qu Y, Wang X, Li L, Zhao X. 2012. Inhibitors of reactive oxygen species accumulation delay and/or reduce the lethality of several antistaphylococcal agents. Antimicrob Agents Chemother 56:6048–6050. doi: 10.1128/AAC.00754-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sampson TR, Liu X, Schroeder MR, Kraft CS, Burd EM, Weiss DS. 2012. Rapid killing of Acinetobacter baumannii by polymyxins is mediated by a hydroxyl radical death pathway. Antimicrob Agents Chemother 56:5642–5649. doi: 10.1128/AAC.00756-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiques E, Ubeda C, Campoy S, Salvador N, Lasa I, Novick RP, Barbé J, Penadés JR. 2006. Beta-lactam antibiotics induce the SOS response and horizontal transfer of virulence factors in Staphylococcus aureus. J Bacteriol 188:2726–2729. doi: 10.1128/JB.188.7.2726-2729.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelley WL. 2006. Lex marks the spot: the virulent side of SOS and a closer look at the LexA regulon. Mol Microbiol 62:1228–1238. doi: 10.1111/j.1365-2958.2006.05444.x. [DOI] [PubMed] [Google Scholar]
- 22.Chang W, Small DA, Toghrol F, Bentley WE. 2006. Global transcriptome analysis of Staphylococcus aureus response to hydrogen peroxide. J Bacteriol 188:1648–1659. doi: 10.1128/JB.188.4.1648-1659.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf C, Hochgräfe F, Kusch H, Albrecht D, Hecker M, Engelmann S. 2008. Proteomic analysis of antioxidant strategies of Staphylococcus aureus: diverse responses to different oxidants. Proteomics 8:3139–3153. doi: 10.1002/pmic.200701062. [DOI] [PubMed] [Google Scholar]
- 24.Anderson KL, Roberts C, Disz T, Vonstein V, Hwang K, Overbeek R, Olson PD, Projan SJ, Dunman PM. 2006. Characterization of the Staphylococcus aureus heat shock, cold shock, stringent, and SOS responses and their effects on log-phase mRNA turnover. J Bacteriol 188:6739–6756. doi: 10.1128/JB.00609-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cirz RT, Jones MB, Gingles NA, Minogue TD, Jarrahi B, Peterson SN, Romesberg FE. 2007. Complete and SOS-mediated response of Staphylococcus aureus to the antibiotic ciprofloxacin. J Bacteriol 189:531–539. doi: 10.1128/JB.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenhart JS, Schroeder JW, Walsh BW, Simmons LA. 2012. DNA repair and genome maintenance in Bacillus subtilis. Microbiol Mol Biol Rev 76:530–564. doi: 10.1128/MMBR.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaupp R, Ledala N, Somerville GA. 2012. Staphylococcal response to oxidative stress. Front Cell Infect Microbiol 2:33. doi: 10.3389/fcimb.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Little JW, Kim B, Roland KL, Smith MH, Lin LL, Slilaty SN. 1994. Cleavage of LexA repressor. Methods Enzymol 244:266–284. doi: 10.1016/0076-6879(94)44022-0. [DOI] [PubMed] [Google Scholar]
- 29.Cohn MT, Kjelgaard P, Frees D, Penadés JR, Ingmer H. 2011. Clp-dependent proteolysis of the LexA N-terminal domain in Staphylococcus aureus. Microbiology (Reading) 157:677–684. doi: 10.1099/mic.0.043794-0. [DOI] [PubMed] [Google Scholar]
- 30.Frees D, Gerth U, Ingmer H. 2014. Clp chaperones and proteases are central in stress survival, virulence and antibiotic resistance of Staphylococcus aureus. Int J Med Microbiol 304:142–149. doi: 10.1016/j.ijmm.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Neher SB, Flynn JM, Sauer RT, Baker TA. 2003. Latent ClpX-recognition signals ensure LexA destruction after DNA damage. Genes Dev 17:1084–1089. doi: 10.1101/gad.1078003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butala M, Žgur-Bertok D, Busby SJW. 2009. The bacterial LexA transcriptional repressor. Cell Mol Life Sci 66:82–93. doi: 10.1007/s00018-008-8378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reuven NB, Arad G, Maor-Shoshani A, Livneh Z. 1999. The mutagenesis protein UmuC is a DNA polymerase activated by UmuD′, RecA, and SSB and is specialized for translesion replication. J Biol Chem 274:31763–31766. doi: 10.1074/jbc.274.45.31763. [DOI] [PubMed] [Google Scholar]
- 34.Walsh JM, Hawver LA, Beuning PJ. 2011. Escherichia coli Y family DNA polymerases. Front Biosci (Landmark Ed) 16:3164–3182. doi: 10.2741/3904. [DOI] [PubMed] [Google Scholar]
- 35.Permina EA, Mironov AA, Gelfand MS. 2002. Damage-repair error-prone polymerases of eubacteria: association with mobile genome elements. Gene 293:133–140. doi: 10.1016/S0378-1119(02)00701-1. [DOI] [PubMed] [Google Scholar]
- 36.McKenzie GJ, Harris RS, Lee PL, Rosenberg SM. 2000. The SOS response regulates adaptive mutation. Proc Natl Acad Sci USA 97:6646–6651. doi: 10.1073/pnas.120161797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sale JE, Lehmann AR, Woodgate R. 2012. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat Rev Mol Cell Biol 13:141–152. doi: 10.1038/nrm3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bojer MS, Wacnik K, Kjelgaard P, Gallay C, Bottomley AL, Cohn MT, Lindahl G, Frees D, Veening J-W, Foster SJ, Ingmer H. 2019. SosA inhibits cell division in Staphylococcus aureus in response to DNA damage. Mol Microbiol 112:1116–1130. doi: 10.1111/mmi.14350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bisognano C, Kelley WL, Estoppey T, Francois P, Schrenzel J, Li D, Lew DP, Hooper DC, Cheung AL, Vaudaux P. 2004. A RecA-LexA-dependent pathway mediates ciprofloxacin-induced fibronectin binding in Staphylococcus aureus. J Biol Chem 279:9064–9071. doi: 10.1074/jbc.M309836200. [DOI] [PubMed] [Google Scholar]
- 40.Janion C. 2008. Inducible SOS response system of DNA repair and mutagenesis in Escherichia coli. Int J Biol Sci 4:338–344. doi: 10.7150/ijbs.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sung H-M, Yeamans G, Ross CA, Yasbin RE. 2003. Roles of YqjH and YqjW, homologs of the Escherichia coli UmuC/DinB or Y superfamily of DNA polymerases, in stationary-phase mutagenesis and UV-induced mutagenesis of Bacillus subtilis. J Bacteriol 185:2153–2160. doi: 10.1128/JB.185.7.2153-2160.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Au N, Kuester-Schoeck E, Mandava V, Bothwell LE, Canny SP, Chachu K, Colavito SA, Fuller SN, Groban ES, Hensley LA, O’Brien TC, Shah A, Tierney JT, Tomm LL, O’Gara TM, Goranov AI, Grossman AD, Lovett CM. 2005. Genetic composition of the Bacillus subtilis SOS system. J Bacteriol 187:7655–7666. doi: 10.1128/JB.187.22.7655-7666.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Chatelier E, Bécherel OJ, d’Alençon E, Canceill D, Ehrlich SD, Fuchs RPP, Jannière L. 2004. Involvement of DnaE, the second replicative DNA polymerase from Bacillus subtilis, in DNA mutagenesis. J Biol Chem 279:1757–1767. doi: 10.1074/jbc.M310719200. [DOI] [PubMed] [Google Scholar]
- 44.Bojer MS, Frees D, Ingmer H. 2020. SosA in staphylococci: an addition to the paradigm of membrane-localized, SOS-induced cell division inhibition in bacteria. Curr Genet 66:495–499. doi: 10.1007/s00294-019-01052-z. [DOI] [PubMed] [Google Scholar]
- 45.Úbeda C, Maiques E, Knecht E, Lasa Í, Novick RP, Penadés JR. 2005. Antibiotic-induced SOS response promotes horizontal dissemination of pathogenicity island-encoded virulence factors in staphylococci. Mol Microbiol 56:836–844. doi: 10.1111/j.1365-2958.2005.04584.x. [DOI] [PubMed] [Google Scholar]
- 46.Ubeda C, Maiques E, Tormo MA, Campoy S, Lasa I, Barbé J, Novick RP, Penadés JR. 2007. SaPI operon I is required for SaPI packaging and is controlled by LexA. Mol Microbiol 65:41–50. doi: 10.1111/j.1365-2958.2007.05758.x. [DOI] [PubMed] [Google Scholar]
- 47.Goerke C, Köller J, Wolz C. 2006. Ciprofloxacin and trimethoprim cause phage induction and virulence modulation in Staphylococcus aureus. Antimicrob Agents Chemother 50:171–177. doi: 10.1128/AAC.50.1.171-177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yi C, He C. 2013. DNA repair by reversal of DNA damage. Cold Spring Harb Perspect Biol 5:a012575. doi: 10.1101/cshperspect.a012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sancar A. 2003. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem Rev 103:2203–2238. doi: 10.1021/cr0204348. [DOI] [PubMed] [Google Scholar]
- 50.Li YF, Kim ST, Sancar A. 1993. Evidence for lack of DNA photoreactivating enzyme in humans. Proc Natl Acad Sci USA 90:4389–4393. doi: 10.1073/pnas.90.10.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu DS, Zhao X, Zhao S, et al. 1996. Putative human blue-light photoreceptors hCRY1 and hCRY2 are flavoproteins. Biochemistry 35:13871–13877. doi: 10.1021/BI962209O. [DOI] [PubMed] [Google Scholar]
- 52.Morita R, Nakane S, Shimada A, Inoue M, Iino H, Wakamatsu T, Fukui K, Nakagawa N, Masui R, Kuramitsu S. 2010. Molecular mechanisms of the whole DNA repair system: a comparison of bacterial and eukaryotic systems. J Nucleic Acids 2010:179594. doi: 10.4061/2010/179594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Essen LO, Klar T. 2006. Light-driven DNA repair by photolyases. Cell Mol Life Sci 63:1266–1277. doi: 10.1007/s00018-005-5447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riordan JT, Dupre JM, Cantore-Matyi SA, Kumar-Singh A, Song Y, Zaman S, Horan S, Helal NS, Nagarajan V, Elasri MO, Wilkinson BJ, Gustafson JE. 2011. Alterations in the transcriptome and antibiotic susceptibility of Staphylococcus aureus grown in the presence of diclofenac. Ann Clin Microbiol Antimicrob 10:30. doi: 10.1186/1476-0711-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sung JM-L, Lloyd DH, Lindsay JA. 2008. Staphylococcus aureus host specificity: comparative genomics of human versus animal isolates by multi-strain microarray. Microbiology (Reading) 154:1949–1959. doi: 10.1099/mic.0.2007/015289-0. [DOI] [PubMed] [Google Scholar]
- 56.Sedgwick B, Lindahl T. 2002. Recent progress on the Ada response for inducible repair of DNA alkylation damage. Oncogene 21:8886–8894. doi: 10.1038/sj.onc.1205998. [DOI] [PubMed] [Google Scholar]
- 57.Kreuzer KN. 2013. DNA damage responses in prokaryotes: regulating gene expression, modulating growth patterns, and manipulating replication forks. Cold Spring Harb Perspect Biol 5:a012674. doi: 10.1101/cshperspect.a012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mishina Y, Duguid EM, He C. 2006. Direct reversal of DNA alkylation damage. Chem Rev 106:215–232. doi: 10.1021/cr0404702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krokan HE, Bjørås M. 2013. Base excision repair. Cold Spring Harb Perspect Biol 5:a012583. doi: 10.1101/cshperspect.a012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kleibl K. 2002. Molecular mechanisms of adaptive response to alkylating agents in Escherichia coli and some remarks on O6-methylguanine DNA-methyltransferase in other organisms. Mutat Res 512:67–84. doi: 10.1016/s1383-5742(02)00025-x. [DOI] [PubMed] [Google Scholar]
- 61.Trewick SC, Henshaw TF, Hausinger RP, Lindahl T, Sedgwick B. 2002. Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature 419:174–178. doi: 10.1038/nature00908. [DOI] [PubMed] [Google Scholar]
- 62.Falnes PØ, Johansen RF, Seeberg E. 2002. AlkB-mediated oxidative demethylation reverses DNA damage in Escherichia coli. Nature 419:178–182. doi: 10.1038/nature01048. [DOI] [PubMed] [Google Scholar]
- 63.Rippa V, Duilio A, di Pasquale P, Amoresano A, Landini P, Volkert MR. 2011. Preferential DNA damage prevention by the Escherichia coli AidB gene: a new mechanism for the protection of specific genes. DNA Repair (Amst) 10:934–941. doi: 10.1016/j.dnarep.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rohankhedkar MS, Mulrooney SB, Wedemeyer WJ, Hausinger RP. 2006. The AidB component of the Escherichia coli adaptive response to alkylating agents is a flavin-containing, DNA-binding protein. J Bacteriol 188:223–230. doi: 10.1128/JB.188.1.223-230.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bowles T, Metz AH, O’Quin J, Wawrzak Z, Eichman BF. 2008. Structure and DNA binding of alkylation response protein AidB. Proc Natl Acad Sci USA 105:15299–15304. doi: 10.1073/pnas.0806521105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morohoshi F, Hayashi K, Munakata N. 1991. Molecular analysis of Bacillus subtilis ada mutants deficient in the adaptive response to simple alkylating agents. J Bacteriol 173:7834–7840. doi: 10.1128/jb.173.24.7834-7840.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aamodt RM, Falnes PØ, Johansen RF, Seeberg E, Bjørås M. 2004. The Bacillus subtilis counterpart of the mammalian 3-methyladenine DNA glycosylase has hypoxanthine and 1,N6-ethenoadenine as preferred substrates. J Biol Chem 279:13601–13606. doi: 10.1074/jbc.M314277200. [DOI] [PubMed] [Google Scholar]
- 68.Jurelevicius D, Alvarez VM, Peixoto R, Rosado AS, Seldin L. 2013. The use of a combination of alkB primers to better characterize the distribution of alkane-degrading bacteria. PLoS One 8:e66565. doi: 10.1371/journal.pone.0066565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li S-W, Liu M-Y, Yang R-Q. 2019. Comparative genome characterization of a petroleum-degrading Bacillus subtilis strain DM2. Int J Genomics 2019:7410823. doi: 10.1155/2019/7410823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi R, Mullins EA, Shen X-X, Lay KT, Yuen PK, David SS, Rokas A, Eichman BF. 2018. Selective base excision repair of DNA damage by the non‐base‐flipping DNA glycosylase AlkC. EMBO J 37:63–74. doi: 10.15252/embj.201797833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang M, Aamodt RM, Dalhus B, Balasingham S, Helle I, Andersen P, Tønjum T, Alseth I, Rognes T, Bjørås M. 2011. The ada operon of Mycobacterium tuberculosis encodes two DNA methyltransferases for inducible repair of DNA alkylation damage. DNA Repair (Amst) 10:595–602. doi: 10.1016/j.dnarep.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 72.Ambur OH, Davidsen T, Frye SA, Balasingham SV, Lagesen K, Rognes T, Tønjum T. 2009. Genome dynamics in major bacterial pathogens. FEMS Microbiol Rev 33:453–470. doi: 10.1111/j.1574-6976.2009.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X, Xu X, Yuan W. 2014. Complete genome sequence of Staphylococcus aureus XN108, an ST239-MRSA-SCCmec III strain with intermediate vancomycin resistance isolated in mainland China. Genome Announc 2:e00449-14. doi: 10.1128/genomeA.00449-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krwawicz J, Arczewska KD, Speina E, Maciejewska A, Grzesiuk E. 2007. Bacterial DNA repair genes and their eukaryotic homologues. 1. Mutations in genes involved in base excision repair (BER) and DNA-end processors and their implication in mutagenesis and human disease. Acta Biochim Pol 54:413–434. doi: 10.18388/abp.2007_3219. [DOI] [PubMed] [Google Scholar]
- 75.Dalhus B, Laerdahl JK, Backe PH, Bjørås M. 2009. DNA base repair: recognition and initiation of catalysis. FEMS Microbiol Rev 33:1044–1078. doi: 10.1111/j.1574-6976.2009.00188.x. [DOI] [PubMed] [Google Scholar]
- 76.Baños B, Lázaro JM, Villar L, Salas M, de Vega M. 2008. Characterization of a Bacillus subtilis 64-kDa DNA polymerase X potentially involved in DNA repair. J Mol Biol 384:1019–1028. doi: 10.1016/j.jmb.2008.09.081. [DOI] [PubMed] [Google Scholar]
- 77.Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessières P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell SC, Bron S, Brouillet S, Bruschi CV, Caldwell B, Capuano V, Carter NM, Choi SK, Cordani JJ, Connerton IF, Cummings NJ, Daniel RA, Denziot F, Devine KM, Düsterhöft A, Ehrlich SD, Emmerson PT, Entian KD, Errington J, Fabret C, Ferrari E, Foulger D, Fritz C, Fujita M, Fujita Y, Fuma S, Galizzi A, Galleron N, Ghim SY, Glaser P, Goffeau A, Golightly EJ, Grandi G, Guiseppi G, Guy BJ, Haga K, et al. 1997. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 78.Salas-Pacheco JM, Urtiz-Estrada N, Martínez-Cadena G, Yasbin RE, Pedraza-Reyes M. 2003. YqfS from Bacillus subtilis is a spore protein and a new functional member of the type IV apurinic/apyrimidinic-endonuclease family. J Bacteriol 185:5380–5390. doi: 10.1128/JB.185.18.5380-5390.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shida T, Kaneda K, Ogawa T, Sekiguchi J. 1999. Abasic site recognition mechanism by the Escherichia coli exonuclease III. Nucleic Acids Symp Ser (Oxf) 42:195–196. doi: 10.1093/nass/42.1.195. [DOI] [PubMed] [Google Scholar]
- 80.Baños B, Villar L, Salas M, de Vega M. 2010. Intrinsic apurinic/apyrimidinic (AP) endonuclease activity enables Bacillus subtilis DNA polymerase X to recognize, incise, and further repair abasic sites. Proc Natl Acad Sci USA 107:19219–19224. doi: 10.1073/pnas.1013603107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e00537-12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Leary NA, Wright MW, Brister JR, Ciufo S, Haddad D, McVeigh R, Rajput B, Robbertse B, Smith-White B, Ako-Adjei D, Astashyn A, Badretdin A, Bao Y, Blinkova O, Brover V, Chetvernin V, Choi J, Cox E, Ermolaeva O, Farrell CM, Goldfarb T, Gupta T, Haft D, Hatcher E, Hlavina W, Joardar VS, Kodali VK, Li W, Maglott D, Masterson P, McGarvey KM, Murphy MR, O’Neill K, Pujar S, Rangwala SH, Rausch D, Riddick LD, Schoch C, Shkeda A, Storz SS, Sun H, Thibaud-Nissen F, Tolstoy I, Tully RE, Vatsan AR, Wallin C, Webb D, Wu W, Landrum MJ, Kimchi A, et al. 2016. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res 44:D733–D745. doi: 10.1093/nar/gkv1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nagpal S, Nair DT. 2021. The PHP domain of PolX from Staphylococcus aureus aids high fidelity DNA synthesis through the removal of misincorporated deoxyribo-, ribo-, and oxidized nucleotides. Sci Rep 11:4178. doi: 10.1038/s41598-021-83498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shibutani S, Takeshita M, Grollman AP. 1991. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 349:431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- 85.Hsu GW, Ober M, Carell T, Beese LS. 2004. Error-prone replication of oxidatively damaged DNA by a high-fidelity DNA polymerase. Nature 431:217–221. doi: 10.1038/nature02908. [DOI] [PubMed] [Google Scholar]
- 86.Michaels ML, Miller JH. 1992. The GO system protects organisms from the mutagenic effect of the spontaneous lesion 8-hydroxyguanine (7,8-dihydro-8-oxoguanine). J Bacteriol 174:6321–6325. doi: 10.1128/jb.174.20.6321-6325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Michaels ML, Pham L, Cruz C, Miller JH. 1991. MutM, a protein that prevents GC→TA transversions, is formamidopyrimidine-DNA glycosylase. Nucleic Acids Res 19:3629–3632. doi: 10.1093/nar/19.13.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Michaels ML, Cruz C, Grollman AP, Miller JH. 1992. Evidence that MutY and MutM combine to prevent mutations by an oxidatively damaged form of guanine in DNA. Proc Natl Acad Sci USA 89:7022–7025. doi: 10.1073/pnas.89.15.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maki H, Sekiguchi M. 1992. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature 355:273–275. doi: 10.1038/355273a0. [DOI] [PubMed] [Google Scholar]
- 90.Sasaki M, Kurusu Y. 2004. Analysis of spontaneous base substitutions generated in mutator strains of Bacillus subtilis. FEMS Microbiol Lett 234:37–42. doi: 10.1016/j.femsle.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 91.Canfield GS, Schwingel JM, Foley MH, Vore KL, Boonanantanasarn K, Gill AL, Sutton MD, Gill SR. 2013. Evolution in fast forward: a potential role for mutators in accelerating Staphylococcus aureus pathoadaptation. J Bacteriol 195:615–628. doi: 10.1128/JB.00733-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Endutkin AV, Panferova EP, Barmatov AE, Zharkov DO. 2021. DNA glycosylases for 8-oxoguanine repair in Staphylococcus aureus. DNA Repair (Amst) 105:103160. doi: 10.1016/j.dnarep.2021.103160. [DOI] [PubMed] [Google Scholar]
- 93.Wallace SS. 2013. DNA glycosylases search for and remove oxidized DNA bases. Environ Mol Mutagen 54:691–704. doi: 10.1002/em.21820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Strniste GF, Wallace SS. 1975. Endonucleolytic incision of X-irradiated deoxyribonucleic acid by extracts of Escherichia coli. Proc Natl Acad Sci USA 72:1997–2001. doi: 10.1073/pnas.72.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Melamede RJ, Hatahet Z, Kow YW, Ide H, Wallace SS. 1994. Isolation and characterization of endonuclease VIII from Escherichia coli. Biochemistry 33:1255–1264. doi: 10.1021/bi00171a028. [DOI] [PubMed] [Google Scholar]
- 96.Jiang D, Hatahet Z, Melamede RJ, Kow YW, Wallace SS. 1997. Characterization of Escherichia coli endonuclease VIII. J Biol Chem 272:32230–32239. doi: 10.1074/jbc.272.51.32230. [DOI] [PubMed] [Google Scholar]
- 97.Roberts CA, Al-Tameemi HM, Mashruwala AA, et al. 2017. The Suf iron-sulfur cluster biosynthetic system is essential in Staphylococcus aureus, and decreased Suf function results in global metabolic defects and reduced survival in human neutrophils. Infect Immun 85:e00100-17. doi: 10.1128/IAI.00100-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Collier C, Machón C, Briggs GS, Smits WK, Soultanas P. 2012. Untwisting of the DNA helix stimulates the endonuclease activity of Bacillus subtilis Nth at AP sites. Nucleic Acids Res 40:739–750. doi: 10.1093/nar/gkr785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barajas-Ornelas RDC, Ramírez-Guadiana FH, Juárez-Godínez R, Ayala-García VM, Robleto EA, Yasbin RE, Pedraza-Reyes M. 2014. Error-prone processing of apurinic/apyrimidinic (AP) sites by PolX underlies a novel mechanism that promotes adaptive mutagenesis in Bacillus subtilis. J Bacteriol 196:3012–3022. doi: 10.1128/JB.01681-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pearl LH. 2000. Structure and function in the uracil-DNA glycosylase superfamily. Mutat Res 460:165–181. doi: 10.1016/S0921-8777(00)00025-2. [DOI] [PubMed] [Google Scholar]
- 101.Lindahl T. 1974. An N-glycosidase from Escherichia coli that releases free uracil from DNA containing deaminated cytosine residues. Proc Natl Acad Sci USA 71:3649–3653. doi: 10.1073/pnas.71.9.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cone R, Duncan J, Hamilton L, Friedberg EC. 1977. Partial purification and characterization of a uracil DNA N-glycosidase from Bacillus subtilis. Biochemistry 16:3194–3201. doi: 10.1021/bi00633a024. [DOI] [PubMed] [Google Scholar]
- 103.Wang H-C, Hsu K-C, Yang J-M, Wu M-L, Ko T-P, Lin S-R, Wang AH-J. 2014. Staphylococcus aureus protein SAUGI acts as a uracil-DNA glycosylase inhibitor. Nucleic Acids Res 42:1354–1364. doi: 10.1093/nar/gkt964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Van Houten B. 1990. Nucleotide excision repair in Escherichia coli. Microbiol Rev 54:18–51. doi: 10.1128/mr.54.1.18-51.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Petit MA, Dervyn E, Rose M, Entian KD, McGovern S, Ehrlich SD, Bruand C. 1998. PcrA is an essential DNA helicase of Bacillus subtilis fulfilling functions both in repair and rolling-circle replication. Mol Microbiol 29:261–273. doi: 10.1046/j.1365-2958.1998.00927.x. [DOI] [PubMed] [Google Scholar]
- 106.Chang TL, Naqvi A, Anand SP, Kramer MG, Munshi R, Khan SA. 2002. Biochemical characterization of the Staphylococcus aureus PcrA helicase and its role in plasmid rolling circle replication. J Biol Chem 277:45880–45886. doi: 10.1074/jbc.M207383200. [DOI] [PubMed] [Google Scholar]
- 107.Sancar A. 1996. DNA excision repair. Annu Rev Biochem 65:43–81. doi: 10.1146/annurev.bi.65.070196.000355. [DOI] [PubMed] [Google Scholar]
- 108.Grosser MR, Paluscio E, Thurlow LR, Dillon MM, Cooper VS, Kawula TH, Richardson AR. 2018. Genetic requirements for Staphylococcus aureus nitric oxide resistance and virulence. PLoS Pathog 14:e1006907. doi: 10.1371/journal.ppat.1006907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nakano T, Katafuchi A, Shimizu R, Terato H, Suzuki T, Tauchi H, Makino K, Skorvaga M, Van Houten B, Ide H. 2005. Repair activity of base and nucleotide excision repair enzymes for guanine lesions induced by nitrosative stress. Nucleic Acids Res 33:2181–2191. doi: 10.1093/nar/gki513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kunkel TA, Erie DA. 2005. DNA mismatch repair. Annu Rev Biochem 74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- 111.Schofield MJ, Hsieh P. 2003. DNA mismatch repair: molecular mechanisms and biological function. Annu Rev Microbiol 57:579–608. doi: 10.1146/annurev.micro.57.030502.090847. [DOI] [PubMed] [Google Scholar]
- 112.McHenry CS. 2011. Breaking the rules: bacteria that use several DNA polymerase IIIs. EMBO Rep 12:408–414. doi: 10.1038/embor.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bruck I, Georgescu RE, O’Donnell M. 2005. Conserved interactions in the Staphylococcus aureus DNA PolC chromosome replication machine. J Biol Chem 280:18152–18162. doi: 10.1074/jbc.M413595200. [DOI] [PubMed] [Google Scholar]
- 114.Sanders GM, Dallmann HG, McHenry CS. 2010. Reconstitution of the Bacillus subtilis replisome with 13 proteins, including two distinct replicases. Mol Cell 37:273–281. doi: 10.1016/j.molcel.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 115.Paschalis V, Le Chatelier E, Green M, Képès F, Soultanas P, Janniere L. 2017. Interactions of the Bacillus subtilis DnaE polymerase with replisomal proteins modulate its activity and fidelity. Open Biol 7:170146. doi: 10.1098/rsob.170146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scheuermann RH, Echols H. 1984. A separate editing exonuclease for DNA replication: the epsilon subunit of Escherichia coli DNA polymerase III holoenzyme. Proc Natl Acad Sci USA 81:7747–7751. doi: 10.1073/pnas.81.24.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Klocko AD, Schroeder JW, Walsh BW, Lenhart JS, Evans ML, Simmons LA. 2011. Mismatch repair causes the dynamic release of an essential DNA polymerase from the replication fork. Mol Microbiol 82:648–663. doi: 10.1111/j.1365-2958.2011.07841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smith BT, Grossman AD, Walker GC. 2001. Visualization of mismatch repair in bacterial cells. Mol Cell 8:1197–1206. doi: 10.1016/S1097-2765(01)00402-6. [DOI] [PubMed] [Google Scholar]
- 119.Schaaper RM. 1993. Base selection, proofreading, and mismatch repair during DNA replication in Escherichia coli. J Biol Chem 268:23762–23765. doi: 10.1016/S0021-9258(20)80446-3. [DOI] [PubMed] [Google Scholar]
- 120.Ginetti F, Perego M, Albertini AM, Galizzi A. 1996. Bacillus subtilis mutS mutL operon: identification, nucleotide sequence and mutagenesis. Microbiology 142:2021–2029. doi: 10.1099/13500872-142-8-2021. [DOI] [PubMed] [Google Scholar]
- 121.Prunier A-L, Leclercq R. 2005. Role of mutS and mutL genes in hypermutability and recombination in Staphylococcus aureus. J Bacteriol 187:3455–3464. doi: 10.1128/JB.187.10.3455-3464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O’Neill AJ, Chopra I. 2002. Insertional inactivation of mutS in Staphylococcus aureus reveals potential for elevated mutation frequencies, although the prevalence of mutators in clinical isolates is low. J Antimicrob Chemother 50:161–169. doi: 10.1093/jac/dkf118. [DOI] [PubMed] [Google Scholar]
- 123.Schroeder JW, Hirst WG, Szewczyk GA, Simmons LA. 2016. The effect of local sequence context on mutational bias of genes encoded on the leading and lagging strands. Curr Biol 26:692–697. doi: 10.1016/j.cub.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sung W, Ackerman MS, Gout J-F, Miller SF, Williams E, Foster PL, Lynch M. 2015. Asymmetric context-dependent mutation patterns revealed through mutation-accumulation experiments. Mol Biol Evol 32:1672–1683. doi: 10.1093/molbev/msv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Putnam CD. 2016. Evolution of the methyl directed mismatch repair system in Escherichia coli. DNA Repair (Amst) 38:32–41. doi: 10.1016/j.dnarep.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fukui K, Nishida M, Nakagawa N, Masui R, Kuramitsu S. 2008. Bound nucleotide controls the endonuclease activity of mismatch repair enzyme MutL. J Biol Chem 283:12136–12145. doi: 10.1074/jbc.M800110200. [DOI] [PubMed] [Google Scholar]
- 127.Mauris J, Evans TC. 2009. Adenosine triphosphate stimulates Aquifex aeolicus MutL endonuclease activity. PLoS One 4:e7175. doi: 10.1371/journal.pone.0007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu L, Ortiz Castro MC, Rodríguez González J, Pillon MC, Guarné A. 2019. The endonuclease domain of Bacillus subtilis MutL is functionally asymmetric. DNA Repair (Amst) 73:1–6. doi: 10.1016/j.dnarep.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 129.Duppatla V, Bodda C, Urbanke C, Friedhoff P, Rao DN. 2009. The C-terminal domain is sufficient for endonuclease activity of Neisseria gonorrhoeae MutL. Biochem J 423:265–277. doi: 10.1042/BJ20090626. [DOI] [PubMed] [Google Scholar]
- 130.Pillon MC, Lorenowicz JJ, Uckelmann M, Klocko AD, Mitchell RR, Chung YS, Modrich P, Walker GC, Simmons LA, Friedhoff P, Guarné A. 2010. Structure of the endonuclease domain of MutL: unlicensed to cut. Mol Cell 39:145–151. doi: 10.1016/j.molcel.2010.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kadyrov FA, Dzantiev L, Constantin N, Modrich P. 2006. Endonucleolytic function of MutLα in human mismatch repair. Cell 126:297–308. doi: 10.1016/j.cell.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 132.Fukui K, Shimada A, Iino H, Masui R, Kuramitsu S. 2011. Biochemical properties of MutL, a DNA mismatch repair endonuclease. In DNA repair: on the pathways to fixing DNA damage and errors. IntechOpen; doi: 10.5772/23758. [DOI] [Google Scholar]
- 133.Dreiseikelmann B, Wackernagel W. 1981. Absence in Bacillus subtilis and Staphylococcus aureus of the sequence-specific deoxyribonucleic acid methylation that is conferred in Escherichia coli K-12 by the Dam and Dcm enzymes. J Bacteriol 147:259–261. doi: 10.1128/jb.147.1.259-261.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lenhart JS, Pillon MC, Guarné A, Biteen JS, Simmons LA. 2016. Mismatch repair in Gram-positive bacteria. Res Microbiol 167:4–12. doi: 10.1016/j.resmic.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 135.Chopra I, O’Neill AJ, Miller K. 2003. The role of mutators in the emergence of antibiotic-resistant bacteria. Drug Resist Updat 6:137–145. doi: 10.1016/S1368-7646(03)00041-4. [DOI] [PubMed] [Google Scholar]
- 136.Ha KP, Clarke RS, Kim GL. 2020. Staphylococcal DNA repair is required for infection. mBio 11:e02288‐20. doi: 10.1128/mBio.02288-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Clarke RS, Ha KP, Edwards AM. 2021. RexAB promotes the survival of Staphylococcus aureus exposed to multiple classes of antibiotics. Antimicrob Agents Chemother doi: 10.1128/AAC.00594-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cromie GA, Connelly JC, Leach DRF. 2001. Recombination at double-strand breaks and DNA ends: conserved mechanisms from phage to humans. Mol Cell 8:1163–1174. doi: 10.1016/S1097-2765(01)00419-1. [DOI] [PubMed] [Google Scholar]
- 139.Shuman S, Glickman MS. 2007. Bacterial DNA repair by non-homologous end joining. Nat Rev Microbiol 5:852–861. doi: 10.1038/nrmicro1768. [DOI] [PubMed] [Google Scholar]
- 140.Weller GR, Kysela B, Roy R, Tonkin LM, Scanlan E, Della M, Devine SK, Day JP, Wilkinson A, d’Adda di Fagagna F, Devine KM, Bowater RP, Jeggo PA, Jackson SP, Doherty AJ. 2002. Identification of a DNA nonhomologous end-joining complex in bacteria. Science 297:1686–1689. doi: 10.1126/science.1074584. [DOI] [PubMed] [Google Scholar]
- 141.Doherty AJ, Jackson SP, Weller GR. 2001. Identification of bacterial homologues of the Ku DNA repair proteins. FEBS Lett 500:186–188. doi: 10.1016/s0014-5793(01)02589-3. [DOI] [PubMed] [Google Scholar]
- 142.Aravind L, Koonin EV. 2001. Prokaryotic homologs of the eukaryotic DNA-end-binding protein Ku, novel domains in the Ku protein and prediction of a prokaryotic double-strand break repair system. Genome Res 11:1365–1374. doi: 10.1101/gr.181001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wilson TE, Topper LM, Palmbos PL. 2003. Non-homologous end-joining: bacteria join the chromosome breakdance. Trends Biochem Sci 28:62–66. doi: 10.1016/S0968-0004(03)00005-7. [DOI] [PubMed] [Google Scholar]
- 144.Rocha EPC, Cornet E, Michel B. 2005. Comparative and evolutionary analysis of the bacterial homologous recombination systems. PLoS Genet 1:e15. doi: 10.1371/journal.pgen.0010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zuñiga-Castillo J, Romero D, Martínez-Salazar JM. 2004. The recombination genes addAB are not restricted to Gram-positive bacteria: genetic analysis of the recombination initiation enzymes RecF and AddAB in Rhizobium etli. J Bacteriol 186:7905–7913. doi: 10.1128/JB.186.23.7905-7913.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Podos SD, Thanassi JA, Pucci MJ. 2012. Mechanistic assessment of DNA ligase as an antibacterial target in Staphylococcus aureus. Antimicrob Agents Chemother 56:4095–4102. doi: 10.1128/AAC.00215-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bhargava R, Onyango DO, Stark JM. 2016. Regulation of single-strand annealing and its role in genome maintenance. Trends Genet 32:566–575. doi: 10.1016/j.tig.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tomso DJ, Kreuzer KN. 2000. Double-strand break repair in tandem repeats during bacteriophage T4 infection. Genetics 155:1493–1504. doi: 10.1093/genetics/155.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yadav T, Carrasco B, Hejna J, Suzuki Y, Takeyasu K, Alonso JC. 2013. Bacillus subtilis DprA recruits RecA onto single-stranded DNA and mediates annealing of complementary strands coated by SsbB and SsbA. J Biol Chem 288:22437–22450. doi: 10.1074/jbc.M113.478347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ithurbide S, Bentchikou E, Coste G, Bost B, Servant P, Sommer S. 2015. Single strand annealing plays a major role in RecA-independent recombination between repeated sequences in the radioresistant Deinococcus radiodurans bacterium. PLoS Genet 11:e1005636. doi: 10.1371/journal.pgen.1005636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wigley DB. 2013. Bacterial DNA repair: recent insights into the mechanism of RecBCD, AddAB and AdnAB. Nat Rev Microbiol 11:9–13. doi: 10.1038/nrmicro2917. [DOI] [PubMed] [Google Scholar]
- 152.Hildenbrand C, Stock T, Lange C, Rother M, Soppa J. 2011. Genome copy numbers and gene conversion in methanogenic archaea. J Bacteriol 193:734–743. doi: 10.1128/JB.01016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ayora S, Carrasco B, Cárdenas PP, César CE, Cañas C, Yadav T, Marchisone C, Alonso JC. 2011. Double-strand break repair in bacteria: a view from Bacillus subtilis. FEMS Microbiol Rev 35:1055–1081. doi: 10.1111/j.1574-6976.2011.00272.x. [DOI] [PubMed] [Google Scholar]
- 154.Kidane D, Sanchez H, Alonso JC, Graumann PL. 2004. Visualization of DNA double-strand break repair in live bacteria reveals dynamic recruitment of Bacillus subtilis RecF, RecO, and RecN proteins to distinct sites on the nucleoids. Mol Microbiol 52:1627–1639. doi: 10.1111/j.1365-2958.2004.04102.x. [DOI] [PubMed] [Google Scholar]
- 155.Sanchez H, Alonso JC. 2005. Bacillus subtilis RecN binds and protects 3′-single-stranded DNA extensions in the presence of ATP. Nucleic Acids Res 33:2343–2350. doi: 10.1093/nar/gki533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sanchez H, Cardenas PP, Yoshimura SH, Takeyasu K, Alonso JC. 2008. Dynamic structures of Bacillus subtilis RecN-DNA complexes. Nucleic Acids Res 36:110–120. doi: 10.1093/nar/gkm759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cardenas PP, Gándara C, Alonso JC. 2014. DNA double strand break end-processing and RecA induce RecN expression levels in Bacillus subtilis. DNA Repair (Amst) 14:1–8. doi: 10.1016/j.dnarep.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 158.Yeeles JTP, Dillingham MS. 2010. The processing of double-stranded DNA breaks for recombinational repair by helicase–nuclease complexes. DNA Repair (Amst) 9:276–285. doi: 10.1016/j.dnarep.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 159.Kuzminov A. 1999. Recombinational repair of DNA damage in Escherichia coli and bacteriophage lambda. Microbiol Mol Biol Rev 63:751–813. doi: 10.1128/MMBR.63.4.751-813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Umezu K, Nakayama K, Nakayama H. 1990. Escherichia coli RecQ protein is a DNA helicase. Proc Natl Acad Sci USA 87:5363–5367. doi: 10.1073/pnas.87.14.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lovett ST, Kolodner RD. 1989. Identification and purification of a single-stranded-DNA-specific exonuclease encoded by the recJ gene of Escherichia coli. Proc Natl Acad Sci USA 86:2627–2631. doi: 10.1073/pnas.86.8.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Morimatsu K, Kowalczykowski SC. 2003. RecFOR proteins load RecA protein onto gapped DNA to accelerate DNA strand exchange. Mol Cell 11:1337–1347. doi: 10.1016/S1097-2765(03)00188-6. [DOI] [PubMed] [Google Scholar]
- 163.Lenhart JS, Brandes ER, Schroeder JW, Sorenson RJ, Showalter HD, Simmons LA. 2014. RecO and RecR are necessary for RecA loading in response to DNA damage and replication fork stress. J Bacteriol 196:2851–2860. doi: 10.1128/JB.01494-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Alonso JC, Fisher LM. 1995. Nucleotide sequence of the recF gene cluster from Staphylococcus aureus and complementation analysis in Bacillus subtilis recF mutants. Mol Gen Genet 246:680–686. doi: 10.1007/BF00290713. [DOI] [PubMed] [Google Scholar]
- 165.Cárdenas PP, Carrasco B, Defeu Soufo C, César CE, Herr K, Kaufenstein M, Graumann PL, Alonso JC. 2012. RecX facilitates homologous recombination by modulating RecA activities. PLoS Genet 8:e1003126. doi: 10.1371/journal.pgen.1003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Romero H, Serrano E, Hernández-Tamayo R, Carrasco B, Cárdenas PP, Ayora S, Graumann PL, Alonso JC. 2020. Bacillus subtilis RarA acts as a positive RecA accessory protein. Front Microbiol 11:92. doi: 10.3389/fmicb.2020.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.West SC. 1997. Processing of recombination intermediates by the RuvABC proteins. Annu Rev Genet 31:213–244. doi: 10.1146/annurev.genet.31.1.213. [DOI] [PubMed] [Google Scholar]
- 168.Niga T, Yoshida H, Hattori H, Nakamura S, Ito H. 1997. Cloning and sequencing of a novel gene (recG) that affects the quinolone susceptibility of Staphylococcus aureus. Antimicrob Agents Chemother 41:1770–1774. doi: 10.1128/AAC.41.8.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McGregor N, Ayora S, Sedelnikova S, Carrasco B, Alonso JC, Thaw P, Rafferty J. 2005. The structure of Bacillus subtilis RecU Holliday junction resolvase and its role in substrate selection and sequence-specific cleavage. Structure 13:1341–1351. doi: 10.1016/j.str.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 170.Burby PE, Simmons LA. 2017. MutS2 promotes homologous recombination in Bacillus subtilis. J Bacteriol 199:e00682-16. doi: 10.1128/JB.00682-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pereira AR, Reed P, Veiga H, Pinho MG. 2013. The Holliday junction resolvase RecU is required for chromosome segregation and DNA damage repair in Staphylococcus aureus. BMC Microbiol 13:18. doi: 10.1186/1471-2180-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sinha KM, Unciuleac M-C, Glickman MS, Shuman S. 2009. AdnAB: a new DSB-resecting motor-nuclease from mycobacteria. Genes Dev 23:1423–1437. doi: 10.1101/gad.1805709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Saikrishnan K, Yeeles JT, Gilhooly NS, Krajewski WW, Dillingham MS, Wigley DB. 2012. Insights into Chi recognition from the structure of an AddAB-type helicase-nuclease complex. EMBO J 31:1568–1578. doi: 10.1038/emboj.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Singleton MR, Dillingham MS, Wigley DB. 2007. Structure and mechanism of helicases and nucleic acid translocases. Annu Rev Biochem 76:23–50. doi: 10.1146/annurev.biochem.76.052305.115300. [DOI] [PubMed] [Google Scholar]
- 175.Smith GR, Kunes SM, Schultz DW, Taylor A, Triman KL. 1981. Structure of chi hotspots of generalized recombination. Cell 24:429–436. doi: 10.1016/0092-8674(81)90333-0. [DOI] [PubMed] [Google Scholar]
- 176.Bianco PR, Kowalczykowski SC. 1997. The recombination hotspot Chi is recognized by the translocating RecBCD enzyme as the single strand of DNA containing the sequence 5′-GCTGGTGG-3′. Proc Natl Acad Sci USA 94:6706–6711. doi: 10.1073/pnas.94.13.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chédin F, Noirot P, Biaudet V, Ehrlich SD. 1998. A five‐nucleotide sequence protects DNA from exonucleolytic degradation by AddAB, the RecBCD analogue of Bacillus subtilis. Mol Microbiol 29:1369–1377. doi: 10.1046/j.1365-2958.1998.01018.x. [DOI] [PubMed] [Google Scholar]
- 178.Halpern D, Chiapello H, Schbath S, Robin S, Hennequet-Antier C, Gruss A, El Karoui M. 2007. Identification of DNA motifs implicated in maintenance of bacterial core genomes by predictive modeling. PLoS Genet 3:e153–1621. doi: 10.1371/journal.pgen.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dixon DA, Kowalczykowski SC. 1993. The recombination hotspot χ is a regulatory sequence that acts by attenuating the nuclease activity of the Escherichia coli RecBCD enzyme. Cell 73:87–96. doi: 10.1016/0092-8674(93)90162-J. [DOI] [PubMed] [Google Scholar]
- 180.Yeeles JTP, Dillingham MS. 2007. A dual-nuclease mechanism for DNA break processing by AddAB-type helicase-nucleases. J Mol Biol 371:66–78. doi: 10.1016/j.jmb.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 181.Spies M, Kowalczykowski SC. 2006. The RecA binding locus of RecBCD is a general domain for recruitment of DNA strand exchange proteins. Mol Cell 21:573–580. doi: 10.1016/j.molcel.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 182.Anderson DG, Kowalczykowski SC. 1997. The translocating RecBCD enzyme stimulates recombination by directing RecA protein onto ssDNA in a Chi-regulated manner. Cell 90:77–86. doi: 10.1016/S0092-8674(00)80315-3. [DOI] [PubMed] [Google Scholar]
- 183.Singleton MR, Dillingham MS, Gaudier M, Kowalczykowski SC, Wigley DB. 2004. Crystal structure of RecBCD enzyme reveals a machine for processing DNA breaks. Nature 432:187–193. doi: 10.1038/nature02988. [DOI] [PubMed] [Google Scholar]
- 184.Handa N, Yang L, Dillingham MS, Kobayashi I, Wigley DB, Kowalczykowski SC. 2012. Molecular determinants responsible for recognition of the single-stranded DNA regulatory sequence, χ, by RecBCD enzyme. Proc Natl Acad Sci USA 109:8901–8906. doi: 10.1073/pnas.1206076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang L, Handa N, Liu B, Dillingham MS, Wigley DB, Kowalczykowski SC. 2012. Alteration of χ recognition by RecBCD reveals a regulated molecular latch and suggests a channel-bypass mechanism for biological control. Proc Natl Acad Sci USA 109:8907–8912. doi: 10.1073/pnas.1206081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chédin F, Ehrlich SD, Kowalczykowski SC. 2000. The Bacillus subtilis AddAB helicase/nuclease is regulated by its cognate Chi sequence in vitro. J Mol Biol 298:7–20. doi: 10.1006/jmbi.2000.3556. [DOI] [PubMed] [Google Scholar]
- 187.Unciuleac M-C, Shuman S. 2010. Double strand break unwinding and resection by the mycobacterial helicase-nuclease AdnAB in the presence of single strand DNA-binding protein (SSB). J Biol Chem 285:34319–34329. doi: 10.1074/jbc.M110.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Buvelot H, Posfay-Barbe KM, Linder P, Schrenzel J, Krause K-H. 2017. Staphylococcus aureus, phagocyte NADPH oxidase and chronic granulomatous disease. FEMS Microbiol Rev 41:139–157. doi: 10.1093/femsre/fuw042. [DOI] [PubMed] [Google Scholar]
- 189.Ellson CD, Davidson K, Ferguson GJ, O’Connor R, Stephens LR, Hawkins PT. 2006. Neutrophils from p40phox–/− mice exhibit severe defects in NADPH oxidase regulation and oxidant-dependent bacterial killing. J Exp Med 203:1927–1937. doi: 10.1084/jem.20052069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hirsch J, Cohn Z. 1960. Degranulation of polymorphonuclear leucocytes following phagocytosis of microorganisms. J Exp Med 112:1005–1014. doi: 10.1084/jem.112.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Babior BM. 1987. The respiratory burst oxidase. Trends Biochem Sci 12:241–243. doi: 10.1016/0968-0004(87)90118-6. [DOI] [Google Scholar]
- 192.Fridovich I. 1995. Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 193.Peyrusson F, Varet H, Nguyen TK, Legendre R, Sismeiro O, Coppée J-Y, Wolz C, Tenson T, Van Bambeke F. 2020. Intracellular Staphylococcus aureus persisters upon antibiotic exposure. Nat Commun 11:2200–2214. doi: 10.1038/s41467-020-15966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Horvatek P, Salzer A, Hanna AMF, Gratani FL, Keinhörster D, Korn N, Borisova M, Mayer C, Rejman D, Mäder U, Wolz C. 2020. Inducible expression of (pp)pGpp synthetases in Staphylococcus aureus is associated with activation of stress response genes. PLoS Genet 16:e1009282. doi: 10.1371/journal.pgen.1009282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Watson SP, Antonio M, Foster SJ. 1998. Isolation and characterization of Staphylococcus aureus starvation-induced, stationary-phase mutants defective in survival or recovery. Microbiology (Reading) 144(Pt 11):3159–3169. doi: 10.1099/00221287-144-11-3159. [DOI] [PubMed] [Google Scholar]
- 196.Gottschalk S, Ifrah D, Lerche S, Gottlieb CT, Cohn MT, Hiasa H, Hansen PR, Gram L, Ingmer H, Thomsen LE. 2013. The antimicrobial lysine-peptoid hybrid LP5 inhibits DNA replication and induces the SOS response in Staphylococcus aureus. BMC Microbiol 13:192–198. doi: 10.1186/1471-2180-13-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gottschalk S, Gottlieb CT, Vestergaard M, Hansen PR, Gram L, Ingmer H, Thomsen LE. 2015. Amphibian antimicrobial peptide Fallaxin analogue FL9 affects virulence gene expression and DNA replication in Staphylococcus aureus. J Med Microbiol 64:1504–1513. doi: 10.1099/jmm.0.000177. [DOI] [PubMed] [Google Scholar]
- 198.Kim G-L, Hooven TA, Norambuena J, Li B, Boyd JM, Yang JH, Parker D. 2021. Growth and stress tolerance comprise independent metabolic strategies critical for Staphylococcus aureus infection. mBio 12:e00814-21. doi: 10.1128/mBio.00814-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Clarke RS, Bruderer MS, Ha KP, Edwards AM. 2019. RexAB is essential for the mutagenic repair of DNA damage caused by co-trimoxazole. Antimicrob Agents Chemother 63:e00944-19. doi: 10.1128/AAC.00944-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rosato RR, Fernandez R, Paz LI, Singh CR, Rosato AE. 2014. TCA cycle-mediated generation of ROS is a key mediator for HeR-MRSA survival under β-lactam antibiotic exposure. PLoS One 9:e99605. doi: 10.1371/journal.pone.0099605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Foster TJ. 2019. Surface proteins of Staphylococcus aureus. Microbiol Spectr 7:GPP3_0046_2018. doi: 10.1128/microbiolspec.GPP3-0046-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vergara-Irigaray M, Valle J, Merino N, Latasa C, García B, Ruiz de Los Mozos I, Solano C, Toledo-Arana A, Penadés JR, Lasa I. 2009. Relevant role of fibronectin-binding proteins in Staphylococcus aureus biofilm-associated foreign-body infections. Infect Immun 77:3978–3991. doi: 10.1128/IAI.00616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen Z, Luong TT, Lee CY. 2007. The sbcDC locus mediates repression of type 5 capsule production as part of the SOS response in Staphylococcus aureus. J Bacteriol 189:7343–7350. doi: 10.1128/JB.01079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bokarewa MI, Jin T, Tarkowski A. 2006. Staphylococcus aureus: staphylokinase. Int J Biochem Cell Biol 38:504–509. doi: 10.1016/j.biocel.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 205.Painter KL, Strange E, Parkhill J, Bamford KB, Armstrong-James D, Edwards AM. 2015. Staphylococcus aureus adapts to oxidative stress by producing H2O2-resistant small-colony variants via the SOS response. Infect Immun 83:1830–1844. doi: 10.1128/IAI.03016-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vestergaard M, Paulander W, Ingmer H. 2015. Activation of the SOS response increases the frequency of small colony variants. BMC Res Notes 8:749. doi: 10.1186/s13104-015-1735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lacoma A, Edwards AM, Young BC, Domínguez J, Prat C, Laabei M. 2019. Cigarette smoke exposure redirects Staphylococcus aureus to a virulence profile associated with persistent infection. Sci Rep 9:10798–10715. doi: 10.1038/s41598-019-47258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Garzoni C, Kelley WL. 2011. Return of the Trojan horse: intracellular phenotype switching and immune evasion by Staphylococcus aureus. EMBO Mol Med 3:115–117. doi: 10.1002/emmm.201100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Proctor RA, van Langevelde P, Kristjansson M, Maslow JN, Arbeit RD. 1995. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin Infect Dis 20:95–102. doi: 10.1093/clinids/20.1.95. [DOI] [PubMed] [Google Scholar]
- 210.Massey RC, Buckling A, Peacock SJ. 2001. Phenotypic switching of antibiotic resistance circumvents permanent costs in Staphylococcus aureus. Curr Biol 11:1810–1814. doi: 10.1016/S0960-9822(01)00507-3. [DOI] [PubMed] [Google Scholar]
- 211.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 212.Tuchscherr L, Heitmann V, Hussain M, Viemann D, Roth J, von Eiff C, Peters G, Becker K, Löffler B. 2010. Staphylococcus aureus small‐colony variants are adapted phenotypes for intracellular persistence. J Infect Dis 202:1031–1040. doi: 10.1086/656047. [DOI] [PubMed] [Google Scholar]
- 213.Painter KL, Hall A, Ha KP, Edwards AM. 2017. The electron transport chain sensitizes Staphylococcus aureus and Enterococcus faecalis to the oxidative burst. Infect Immun 85:e00659-17. doi: 10.1128/IAI.00659-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Schröder W, Goerke C, Wolz C. 2013. Opposing effects of aminocoumarins and fluoroquinolones on the SOS response and adaptability in Staphylococcus aureus. J Antimicrob Chemother 68:529–538. doi: 10.1093/jac/dks456. [DOI] [PubMed] [Google Scholar]
- 215.Chambers HF, DeLeo FR. 2009. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Keaton MA, Rosato RR, Plata KB, Singh CR, Rosato AE. 2013. Exposure of clinical MRSA heterogeneous strains to β-lactams redirects metabolism to optimize energy production through the TCA cycle. PLoS One 8:e71025. doi: 10.1371/journal.pone.0071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cuirolo A, Plata K, Rosato AE. 2009. Development of homogeneous expression of resistance in methicillin-resistant Staphylococcus aureus clinical strains is functionally associated with a β-lactam-mediated SOS response. J Antimicrob Chemother 64:37–45. doi: 10.1093/jac/dkp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tattevin P, Basuino L, Chambers HF. 2009. Subinhibitory fluoroquinolone exposure selects for reduced beta-lactam susceptibility in methicillin-resistant Staphylococcus aureus and alterations in the SOS-mediated response. Res Microbiol 160:187–192. doi: 10.1016/j.resmic.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 219.Rapacka-Zdonczyk A, Wozniak A, Pieranski M, Woziwodzka A, Bielawski KP, Grinholc M. 2019. Development of Staphylococcus aureus tolerance to antimicrobial photodynamic inactivation and antimicrobial blue light upon sublethal treatment. Sci Rep 9:9423–9418. doi: 10.1038/s41598-019-45962-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Haaber J, Penadés JR, Ingmer H. 2017. Transfer of antibiotic resistance in Staphylococcus aureus. Trends Microbiol 25:893–905. doi: 10.1016/j.tim.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 221.Selva L, Viana D, Regev-Yochay G, Trzcinski K, Corpa JM, Lasa I, Novick RP, Penadés JR. 2009. Killing niche competitors by remote-control bacteriophage induction. Proc Natl Acad Sci USA 106:1234–1238. doi: 10.1073/pnas.0809600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ronayne EA, Wan YCS, Boudreau BA, Landick R, Cox MM. 2016. P1 Ref endonuclease: a molecular mechanism for phage-enhanced antibiotic lethality. PLoS Genet 12:e1005797. doi: 10.1371/journal.pgen.1005797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lindsay JA, Ruzin A, Ross HF, Kurepina N, Novick RP. 1998. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol Microbiol 29:527–543. doi: 10.1046/j.1365-2958.1998.00947.x. [DOI] [PubMed] [Google Scholar]
- 224.Ruzin A, Lindsay J, Novick RP. 2001. Molecular genetics of SaPI1: a mobile pathogenicity island in Staphylococcus aureus. Mol Microbiol 41:365–377. doi: 10.1046/j.1365-2958.2001.02488.x. [DOI] [PubMed] [Google Scholar]
- 225.Amarh V, Arthur PK. 2019. DNA double-strand break formation and repair as targets for novel antibiotic combination chemotherapy. Future Sci OA 5:FSO411. doi: 10.2144/fsoa-2019-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Reiche MA, Warner DF, Mizrahi V. 2017. Targeting DNA replication and repair for the development of novel therapeutics against tuberculosis. Front Mol Biosci 4:75. doi: 10.3389/fmolb.2017.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Su LY, Willner DL, Segall AM. 2010. An antimicrobial peptide that targets DNA repair intermediates in vitro inhibits Salmonella growth within murine macrophages. Antimicrob Agents Chemother 54:1888–1899. doi: 10.1128/AAC.01610-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Alam MK, Alhhazmi A, Decoteau JF, Luo Y, Geyer CR. 2016. RecA inhibitors potentiate antibiotic activity and block evolution of antibiotic resistance. Cell Chem Biol 23:381–391. doi: 10.1016/j.chembiol.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 229.da Silva LCN, Diniz RC, Lima IMDSF. 2017. SOS response and Staphylococcus aureus: implications for drug development. In The rise of virulence and antibiotic resistance in Staphylococcus aureus. IntechOpen; doi: 10.5772/65960. [DOI] [Google Scholar]
- 230.Sexton JZ, Wigle TJ, He Q, Hughes MA, Smith GR, Singleton SF, Williams AL, Yeh L-A. 2010. Novel inhibitors of Escherichia coli RecA ATPase activity. Curr Chem Genomics 4:34–42. doi: 10.2174/1875397301004010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Peterson EJR, Janzen WP, Kireev D, Singleton SF. 2012. High-throughput screening for RecA inhibitors using a transcreener adenosine 5′-O-diphosphate assay. Assay Drug Dev Technol 10:260–268. doi: 10.1089/adt.2011.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lanyon-Hogg T. 2021. Targeting the bacterial SOS response for new antimicrobial agents: drug targets, molecular mechanisms and inhibitors. Future Med Chem 13:143–155. doi: 10.4155/fmc-2020-0310. [DOI] [PubMed] [Google Scholar]
- 233.Memar MY, Yekani M, Celenza G, Poortahmasebi V, Naghili B, Bellio P, Baghi HB. 2020. The central role of the SOS DNA repair system in antibiotics resistance: a new target for a new infectious treatment strategy. Life Sci 262:118562. doi: 10.1016/j.lfs.2020.118562. [DOI] [PubMed] [Google Scholar]
- 234.Noirot P, Gupta RC, Radding CM, Kolodner RD. 2003. Hallmarks of homology recognition by RecA-like recombinases are exhibited by the unrelated Escherichia coli RecT protein. EMBO J 22:324–334. doi: 10.1093/emboj/cdg027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Peng Q, Zhou S, Yao F, Hou B, Huang Y, Hua D, Zheng Y, Qian Y. 2011. Baicalein suppresses the SOS response system of Staphylococcus aureus induced by ciprofloxacin. Cell Physiol Biochem 28:1045–1050. doi: 10.1159/000335791. [DOI] [PubMed] [Google Scholar]
- 236.Carvalho AR, Martins ALDB, Cutrim BDS, et al. 2019. Betulinic acid prevents the acquisition of ciprofloxacin-mediated mutagenesis in Staphylococcus aureus. Molecules 24. doi: 10.3390/molecules24091757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dziegielewska B, Beerman TA, Bianco PR. 2006. Inhibition of RecBCD enzyme by antineoplastic DNA alkylating agents. J Mol Biol 361:898–919. doi: 10.1016/j.jmb.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 238.Karu AE, Linn S. 1972. Uncoupling of the recBC ATPase from DNase by DNA cross-linked with psoralen. Proc Natl Acad Sci USA 69:2855–2859. doi: 10.1073/pnas.69.10.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wilkinson M, Troman L, Wan Nur Ismah WA, Chaban Y, Avison MB, Dillingham MS, Wigley DB. 2016. Structural basis for the inhibition of RecBCD by Gam and its synergistic antibacterial effect with quinolones. Elife 5. doi: 10.7554/eLife.22963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sun D, Hurley LH. 1992. Structure-activity relationships of (+)-CC-1065 analogs in the inhibition of helicase-catalyzed unwinding of duplex DNA. J Med Chem 35:1773–1782. doi: 10.1021/jm00088a012. [DOI] [PubMed] [Google Scholar]
- 241.Amundsen SK, Spicer T, Karabulut AC, Londoño LM, Eberhart C, Fernandez Vega V, Bannister TD, Hodder P, Smith GR. 2012. Small-molecule inhibitors of bacterial AddAB and RecBCD helicase-nuclease DNA repair enzymes. ACS Chem Biol 7:879–891. doi: 10.1021/cb300018x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Oliver DB, Goldberg EB. 1977. Protection of parental T4 DNA from a restriction exonuclease by the product of gene 2. J Mol Biol 116:877–881. doi: 10.1016/0022-2836(77)90276-5. [DOI] [PubMed] [Google Scholar]
- 243.Aiello D, Barnes MH, Biswas EE, Biswas SB, Gu S, Williams JD, Bowlin TL, Moir DT. 2009. Discovery, characterization, and comparison of inhibitors of Bacillus anthracis and Staphylococcus aureus replicative DNA helicases. Bioorg Med Chem 17:4466–4476. doi: 10.1016/j.bmc.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Uhlemann A-C, Otto M, Lowy FD, DeLeo FR. 2014. Evolution of community- and healthcare-associated methicillin-resistant Staphylococcus aureus. Infect Genet Evol 21:563–574. doi: 10.1016/j.meegid.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Jernberg C, Lofmark S, Edlund C, Jansson JK. 2010. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology (Reading) 156:3216–3223. doi: 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- 246.Falcone M, Russo A, Iraci F, Carfagna P, Goldoni P, Vullo V, Venditti M. 2016. Risk factors and outcomes for bloodstream infections secondary to Clostridium difficile infection. Antimicrob Agents Chemother 60:252–257. doi: 10.1128/AAC.01927-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Cirz RT, Chin JK, Andes DR, de Crécy-Lagard V, Craig WA, Romesberg FE. 2005. Inhibition of mutation and combating the evolution of antibiotic resistance. PLoS Biol 3:e176. doi: 10.1371/journal.pbio.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Recacha E, Machuca J, Díaz-Díaz S, García-Duque A, Ramos-Guelfo M, Docobo-Pérez F, Blázquez J, Pascual A, Rodríguez-Martínez JM. 2019. Suppression of the SOS response modifies spatiotemporal evolution, post-antibiotic effect, bacterial fitness and biofilm formation in quinolone-resistant Escherichia coli. J Antimicrob Chemother 74:66–73. doi: 10.1093/jac/dky407. [DOI] [PubMed] [Google Scholar]
- 249.Cromie GA. 2009. Phylogenetic ubiquity and shuffling of the bacterial RecBCD and AddAB recombination complexes. J Bacteriol 191:5076–5084. doi: 10.1128/JB.00254-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Cirz RT, O’Neill BM, Hammond JA, Head SR, Romesberg FE. 2006. Defining the Pseudomonas aeruginosa SOS response and its role in the global response to the antibiotic ciprofloxacin. J Bacteriol 188:7101–7110. doi: 10.1128/JB.00807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. Lancet 367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 252.Rapun-Araiz B, Haag AF, De Cesare V, Gil C, Dorado-Morales P, Penades JR, Lasa I. 2020. Systematic reconstruction of the complete two-component sensorial network in Staphylococcus aureus. mSystems 5:e00511-20. doi: 10.1128/mSystems.00511-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gollan B, Grabe G, Michaux C, Helaine S. 2019. Bacterial persisters and infection: past, present, and progressing. Annu Rev Microbiol 73:359–385. doi: 10.1146/annurev-micro-020518-115650. [DOI] [PubMed] [Google Scholar]
- 254.Podlesek Z, Žgur Bertok D. 2020. The DNA damage-inducible SOS response is a key player in the generation of bacterial persister cells and population wide tolerance. Front Microbiol 11:1785. doi: 10.3389/fmicb.2020.01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Conlon BP, Rowe SE, Gandt AB, Nuxoll AS, Donegan NP, Zalis EA, Clair G, Adkins JN, Cheung AL, Lewis K. 2016. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat Microbiol 1:16051–16057. doi: 10.1038/nmicrobiol.2016.51. [DOI] [PubMed] [Google Scholar]
- 256.Kamenšek S, Podlesek Z, Gillor O, Žgur-Bertok D. 2010. Genes regulated by the Escherichia coli SOS repressor LexA exhibit heterogenous expression. BMC Microbiol 10:283–289. doi: 10.1186/1471-2180-10-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Jones EC, Uphoff S. 2021. Single-molecule imaging of LexA degradation in Escherichia coli elucidates regulatory mechanisms and heterogeneity of the SOS response. Nat Microbiol 6:981–910. doi: 10.1038/s41564-021-00930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]