Summary
Background
Ivermectin became a popular choice for COVID-19 treatment among clinicians and the public following encouraging results from pre-print trials and in vitro studies. Early reviews recommended the use of ivermectin based largely on non-peer-reviewed evidence, which may not be robust. This systematic review and meta-analysis assessed the efficacy and safety of ivermectin for treating COVID-19 based on peer-reviewed randomized controlled trials (RCTs) and observational studies (OSs).
Methods
MEDLINE, EMBASE and PubMed were searched from 1 January 2020 to 1 September 2021 for relevant studies. Outcomes included time to viral clearance, duration of hospitalization, mortality, incidence of mechanical ventilation and incidence of adverse events. RoB2 and ROBINS-I were used to assess risk of bias. Random-effects meta-analyses were conducted. GRADE was used to evaluate quality of evidence.
Results
Three OSs and 14 RCTs were included in the review. Most RCTs were rated as having some concerns in regards to risk of bias, while OSs were mainly rated as having a moderate risk of bias. Based on meta-analysis of RCTs, the use of ivermectin was not associated with reduction in time to viral clearance, duration of hospitalization, incidence of mortality and incidence of mechanical ventilation. Ivermectin did not significantly increase incidence of adverse events. Meta-analysis of OSs agrees with findings from RCT studies.
Conclusions
Based on very low to moderate quality of evidence, ivermectin was not efficacious at managing COVID-19. Its safety profile permits its use in trial settings to further clarify its role in COVID-19 treatment.
Protocol registration
The review was prospectively registered in PROSPERO (CRD42021275302).
Introduction
COVID-19 has ravaged the world since its designation as a global pandemic in March 2020. Despite the successful development of SARS-CoV-2 vaccines, such as Comirnaty, vaccine hesitancy and new viral variants, such as delta, threaten to extend the pandemic well into 2022. To manage patients with COVID-19, a disease with no known cure, clinicians and researchers had turned their attention to repurposed drug therapies since the beginning of the pandemic. Repurposed regimens, such as hydroxychloroquine, corticosteroids and lopinavir–ritonavir combination therapies promised to offer great efficacy using established drugs with known pharmacokinetic and pharmacodynamic profiles, thus dramatically reducing the cost and length of drug development amidst the ongoing pandemic. However, these efforts are often marred by misinformation and poorly-conducted research. Apart from corticosteroids,1 tocilizumab2 and remdesivir,3 other repurposed therapies were often found to not offer any benefits to the patients when compared to standard of care.
In late 2020, a new repurposed regimen, ivermectin, began to attract international attention following encouraging results published as a pre-print article by Elgazzar et al.4 Following this publication, an influx of low-quality clinical trials regarding ivermectin began to be disseminated through pre-print servers and independent websites. These publications were subsequently included in systematic reviews and meta-analyses, which generally found that ivermectin had a positive effect on patient outcomes compared to standard of care. However, these early reviews have several methodological limitations. An early meta-analysis by Hill et al.5 was retracted following the withdrawal of an included article, which was determined to contain fraudulent data. In a subsequent meta-analysis by Bryant et al.,6 which assessed the impact of ivermectin on mortality, the Elgazzar pre-print accounted for 15% of the study weight despite being later withdrawn by the pre-print server. In yet another review, the meta-analysis relied almost exclusively on pre-print articles.7 Evidently, positive results yielded from these reviews are not entirely reliable, and further investigations in the efficacy and safety of ivermectin are needed. To clarify the role of ivermectin in the treatment of COVID-19 patients, we conducted this systematic review and meta-analysis to determine the impact of ivermectin on the duration of viral clearance, duration of hospitalization, mortality incidence, incidence of mechanical ventilation, as well as incidence of adverse events, using peer-reviewed randomized controlled trials (RCTs) and observational studies (OSs).
Methods
We performed this systematic review and meta-analysis following recommendations from the Cochrane Handbook8 and in accordance with the latest Preferred Reporting Items for Systematic Reviews of Interventions (PRISMA 2020) statements.9 The completed PRISMA 2020 checklist is included as Supplementary Table S1. This review was prospectively registered on PROSPERO (CRD42021275302).
Study identification
Databases including MEDLINE, EMBASE and PubMed were searched from 1 January 2020 to 1 September 2021 for relevant articles. The search strategy was developed based on database-specific COVID-19 search strings provided by the Rudolph Matas Library of the Health Sciences of Tulane University10 with keywords, such as ‘ivermectin*’, ‘stromectol*’ and ‘ivomec’, etc. The complete search strategy is tabulated in Supplementary Tables S2–S4. We also hand-searched the reference sections of previous meta-analyses for relevant articles. Due to concerns regarding the quality of non-peer-reviewed articles published during the pandemic,11 especially surrounding ivermectin, we did not search pre-print sources and we also excluded all non-peer-reviewed articles.
Eligibility criteria
We included both randomized and non-randomized comparative studies that met the following criteria: (i) compared ivermectin to standard of care or a control group receiving placebo; (ii) included adult COVID-19 inpatients and/or outpatients; and (iii) reported any of our outcomes of interest.
Outcome measures
Our efficacy outcomes included: (i) time to viral clearance; (ii) duration of hospitalization; (iii) mortality incidence; and (iv) incidence of progression to mechanical ventilation. Our safety outcomes included incidence of all-cause adverse events and incidence of investigator-defined serious adverse events.
Study selection and data extraction
Abstract screening and subsequent full-text screening were performed in duplicate by four reviewers (J.D., W.H., C.Y.W. and E.H.) based on the aforementioned eligibility criteria. Disagreements were resolved by recruiting a third author to attain consensus. Data extraction were performed in duplicate by four reviewers (J.D., F.Z., S.A. and K.H.) using extraction sheets developed a priori. Data items extracted include: (i) study meta-data (author name, publication year, country of origin and doi); (ii) study design (registration, number of centers, blinding and allocation methods); (iii) inclusion criteria (hospitalization status, disease severity and severity definition); (iv) baseline information and patient characteristics (sex distribution and age); (v) treatment arm descriptions (ivermectin dose and duration, descriptions of adjuvant therapies and standard of care); and (vi) outcome data.
For studies with missing outcome data, we made attempts to contact the corresponding author to obtain unpublished data. If a study reported median and interquartile range, we used methods recommended by Luo et al.12 and Wan et al.13 to estimate the mean and standard deviation for data pooling if there were no significant skewness based on the test by Shi et al.14
Risk of bias assessment
We assessed the risk of bias of RCTs using the revised Cochrane risk of bias tool for randomized trials (RoB2).15 The risk of bias of non-randomized comparative studies was assessed using the risk of bias in non-randomized studies of interventions (ROBINS-I) tool.16 All risk of bias assessments were conducted in duplicate by four reviewers (J.D., F.Z., S.A. and K.H.). Disagreements were resolved by recruiting a third author to attain consensus.
Quality of evidence
We assessed the quality of evidence for our primary outcomes using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework.17 A summary of our outcomes and their associated GRADE ratings are presented in a GRADE summary of findings table generated using GRADEpro (https://gradepro.org/).
Statistical analysis
We conducted all statistical analyses using R 3.6.3 and the meta 4.18 library. RCTs and OSs were analyzed separately. We performed a random-effects meta-analysis after expressing the treatment effects of dichotomous outcomes as odds ratios (ORs) and the treatment effects of continuous outcomes as mean differences (MDs). For studies reporting zero events in one or both of its treatment arms, we applied treatment arm continuity correction18 to complete the meta-analysis. Heterogeneity was examined using Cochran’s Q test with a significance level of P < 0.10 and further quantified using I2 statistics. We interpreted 30% < I2< 75% as moderate heterogeneity and I2 ≥ 75% as serious heterogeneity.8 Publication bias was assessed using funnel plots and Egger’s test for outcomes with 10 or more included studies.
If meta-analysis was not possible due to insufficient data, the results of the included studies were narratively described.
Meta-regression and subgroup analysis
We performed meta-regression analysis by cumulative ivermectin dose and subgroup analysis by investigator-defined disease severity (severe vs. non-severe). Given that we included several studies using doxycycline as an adjuvant to ivermectin and/or used hydroxychloroquine and lopinavir–ritonavir as the control arm, we performed post hoc sensitivity analyses excluding these studies to examine their impact on the pooled effect. Additionally, as a wide variety of follow-up durations were reported for dichotomous outcomes, we performed post hoc meta-regression analysis by follow-up duration for the outcome of mortality incidence, incidence of mechanical ventilation and incidence of adverse events. Although we planned to conduct the same set of meta-regression and subgroup analyses in both RCTs and OSs, these analyses were not conducted for OSs due to the low number of analyzed studies.
Results
Included studies
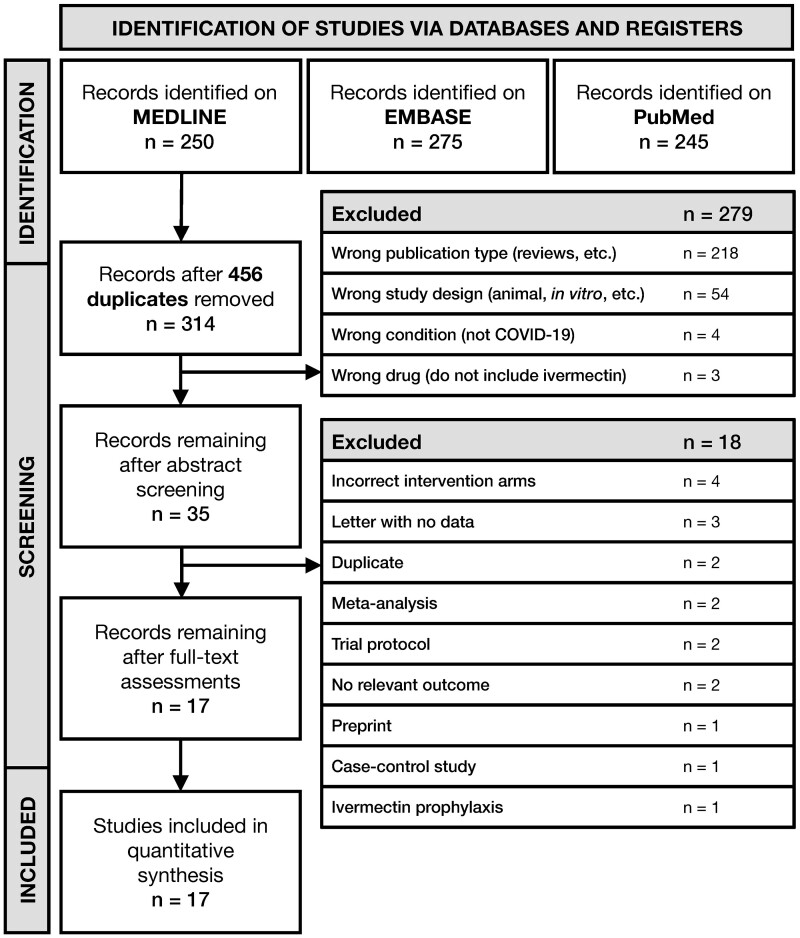
We identified and screened 314 potentially eligible titles and abstracts following deduplication (Figure 1). A total of 35 full-text articles were subsequently retrieved and screened. Finally, 3 OSs19–21 and 14 RCTs22–35 with 2724 adult COVID-19 patients were included in the review. All included studies generally compared standard of care with ivermectin + standard of care, with the exception of Ahmed et al.23 and Mahmud et al.,28 which used doxycycline as an adjuvant to ivermectin; Babalola et al.,24 which used lopinavir–ritonavir as the control arm; and Galan et al.,35 which used hydroxychloroquine and chloroquine as the control arm. These studies were included with the assumption that doxycycline, lopinavir–ritonavir and chloroquine compounds did not have a significant impact on patient outcomes, as shown by previous studies.36–38 Detailed characteristics of each included study are listed in Table 1.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart for the identification and selection of studies.
Table 1.
Characteristics of included studies and patients
| Study | Design | Registration | Country | Population | Sample size | Sex (females/ males) | Agea | Treatment arm | Treatment description |
|---|---|---|---|---|---|---|---|---|---|
| Abd-Elsalam et al. (2021)22 | Open-label, Parallel RCT | NCT04403555 | Egypt | Mild/moderate COVID-19 inpatients | 82 | 45/37 | 42.4 ± 16 | IVM | IVM 12 mg/d po for 3 days + SOC |
| 82 | 37/45 | 39.4 ± 16.9 | SOC | Egypt Ministry of Health guidelines [paracetamol, O2, fluids if needed, empiric antibiotic, oseltamivir if needed (75 mg q12h for 5 days), invasive mechanical ventilation if PaO2<60 mmHg, O2 saturation <90% despite oxygen or non-invasive ventilation, progressive hypercapnia, respiratory acidosis (pH < 7.3) and progressive or refractory septic shock] for 14 days | |||||
| Ahmed et al. (2021)23 | Double-blind, Parallel RCT | - | Bangladesh | Mild COVID-19 inpatients | 22 | 37/31 | 42b | IVM | IVM 12 mg/d po for 5 days |
| 23 | IVM + DOXY | IVM 12 mg po single dose + DOXY 200 mg on Day 1 and 100 mg q12h for 4 days | |||||||
| 23 | PBO | – | |||||||
| Babalola et al. (2021)24 | Double-blind, Parallel RCT | - | Nigeria | Asymptomatic/mild/moderate COVID-19 inpatients | 21 | 6/15 | 48.3b | IVM 6 mg | IVM 6 mg IV q84h for 14 days |
| 21 | 7/14 | 39.7b | IVM 12 mg | IVM 12 mg IV q84h for 14 days | |||||
| 20 | 6/14 | 44.8b | PBO + LPV/r | PBO + LPV/r for 14 days | |||||
| Camprubi et al. (2020)19 | Retrospective cohort | - | Spain | Severe COVID-19 inpatients | 13 | 4/9 | 43 (41–49) | IVM | IVM 200 μg/kg single dose + SOC |
| 13 | 5/8 | 54 (48–58) | SOC | HCQ + AZM + supportive treatment including HFNC + LPV/r | |||||
| Chaccour et al. (2021)25 | Double-blind, Parallel RCT | NCT04390022 | Spain | Non-severe COVID-19 outpatients | 12 | 5/7 | 26 (19–36) | IVM | IVM 400 μg/kg single dose po |
| 12 | 7/5 | 26 (21–44) | PBO | – | |||||
| Khan et al. (2020)20 | Retrospective cohort | - | Bangladesh | Mild/moderate COVID-19 inpatients | 115 | 35/80 | 34 (30–42) | IVM | IVM 12 mg single dose within 24 h of admission + SOC |
| 133 | 64/69 | 35 (30–45) | SOC | Antipyretics, antihistamines, antibiotics | |||||
| Krolewiecki et al. (2021)26 | Open-label, Assessor blinded, Parallel RCT | NCT04381884 | Argentina | Mild/moderate COVID-19 inpatients | 30 | 15/15 | 42.3 ± 12.8 | IVM | IVM 600 μg/kg/d po for 5 days |
| 15 | 5/10 | 38.1 ± 11.7 | SOC | – | |||||
| López-Medina et al. (2021)27 | Double-blind, Parallel RCT | NCT04405843 | Colombia | Mild COVID-19 patients | 200 | 122/78 | 37 (29–48) | IVM | IVM 300 μg/kg/d po for 5 days |
| 198 | 109/89 | 37 (29–49) | PBO | – | |||||
| Mahmud et al. (2021)28 | Double-blind, Parallel RCT | NCT04523831 | Bangladesh | Mild/moderate COVID-19 patients | 200 | 77/123 | 41 ± 14 | IVM + DOXY | IVM 12 mg single dose and DOXY 100 mg bid for 5 days + SOC |
| 200 | 88/112 | 38 ± 12 | SOC | Paracetamol, antihistamines, cough suppressants, vitamins, oxygen therapy according to indication and need, LMWH according to indication, appropriate other broad-spectrum antibiotics, remdesivir injection, other antiviral drugs and other drugs for associated comorbid conditions | |||||
| Mohan et al. (2021)29 | Double-blind, Parallel RCT | CTRI/2020/ 06/026001 | India | Mild/moderate COVID-19 inpatients | 40 | 3/37 | 34.3 ± 10.5 | IVM 24 mg | IVM 24 mg single dose po |
| 40 | 5/35 | 36.3 ± 10.5 | IVM 12 mg | IVM 12 mg single dose po | |||||
| 45 | 6/39 | 35.3 ± 10.5 | PBO | – | |||||
| Okumuş et al. (2021)30 | Single-blind, Parallel RCT | NCT04646109 | Turkey | Severe COVID-19 inpatients | 30 | 9/21 | 58.2 ± 11.5 | IVM | IVM 200 µg/kg/d for 5 days + SOC |
| 30 | 11/19 | 66.2 ± 13.3 | SOC | HCQ 400 mg bid loading dose then HCQ 200 mg bid for 5 days, favipiravir 1600 mg bid loading dose then favipiravir 600 mg bid for 5 days, AZM 500 mg/d loading dose then 250 mg/d for 5 days | |||||
| Pott-Junior et al. (2021)31 | Open-label, Parallel RCT | NCT04431466 | Brazil | Mild COVID-19 inpatients | 6 | 4/2 | 50 ± 9 | IVM 100 µg/kg | IVM 100 µg/kg for 7 days |
| 14 | 5/9 | 49 ± 13.5 | IVM 200 µg/kg | IVM 200 µg/kg for 7 days | |||||
| 7 | 4/3 | 47 ± 22.9 | IVM 400 µg/kg | IVM 400 µg/kg for 7 days | |||||
| 4 | 4/0 | 54.2 ± 9.6 | SOC | – | |||||
| Rajter et al. (2021)21 | Retrospective cohort | - | USA | COVID-19 inpatients | 98 | 39/59 | 60.1 ± 17.4 | IVM | IVM 200 µg/kg single dose po, with or without a second dose at physician discretion + SOC |
| 98 | 39/59 | 59 ± 17.7 | SOC | GC, HCQ, AZM | |||||
| Ravikirti et al. (2021)32 | Double-blind, Parallel RCT | - | India | Mild/moderate COVID-19 inpatients | 55 | 15/40 | 50.7 ± 12.7 | IVM | IVM 12 mg/d for 2 days + SOC |
| 57 | 16/41 | 54.2 ± 16.3 | SOC | HCQ, GC, enoxaparin, antibiotics, remdesivir, convalescent plasma, tocilizumab, etc. | |||||
| Shahbaznejad et al. (2021)33 | Double-blind, Parallel RCT | IRCT2011122 4008507N3 | Iran | Severe COVID-19 inpatients | 35 | 18/17 | 47.6 ± 22.2 | IVM | IVM single dose, weight-adjusted dose, 3 mg for 15–24 kg, 6 mg for 25–30 kg, 9 mg for 36–50 kg, 12 mg for 51–80 kg, 0.2 mg/kg for >80 kg + SOC |
| 34 | 18/16 | 45.2 ± 23.2 | SOC | HCQ, CQ, LPV/r, oseltamivir, ribavirin, antibiotics (ceftriaxone, AZM, meropenem, vancomycin) and supplemental oxygen | |||||
| Vallejos et al. (2021)34 | Double-blind, Parallel RCT | NCT04529525 | Argentina | Mild/moderate COVID-19 outpatients | 250 | 111/139 | 42.6 ± 15.3 | IVM | IVM weight-adjusted dose, 12 mg for 2 days for ≤80 kg, 18 mg for 2 days for 81–110 kg, 24 mg for 2 days for >110 kg + SOC |
| 251 | 126/125 | 42.4 ± 15.8 | SOC | In accordance with the recommendations of the Argentine Ministry of Health | |||||
| Galan et al. (2021)35 | Double-blind, Parallel RCT | RBR-8h7q82 | Brazil | Severe COVID-19 inpatients | 53 | 22/31 | 53.2 ± 17.3 | IVM | IVM 42 mg over 4 days |
| 54 | 25/29 | 54.8 ± 15.5 | HCQ | HCQ 400 mg bid loading dose, then HCQ 400 mg/d for 4 days | |||||
| 61 | 26/35 | 51.9 ± 14 | CQ | CQ 450 mg bid loading dose, then 450 mg/d for 4 days |
Cells containing ‘-’ indicate that no relevant data were reported.
RCT, randomized controlled trial; SOC, standard of care; LPV/r: lopinavir–ritonavir combination therapy; IVM, ivermectin; DOXY, doxycycline; PBO, placebo; HCQ, hydroxychloroquine; AZM, azithromycin; HFNC, high flow nasal cannula; LMWH, low molecular weight heparin; GC, glucocorticoid; CQ, chloroquine; SD, standard deviation; IQR, interquartile range.
Age is presented as mean (SD) or median (IQR) unless otherwise specified.
Only the mean age was reported with no variance.
Risk of bias
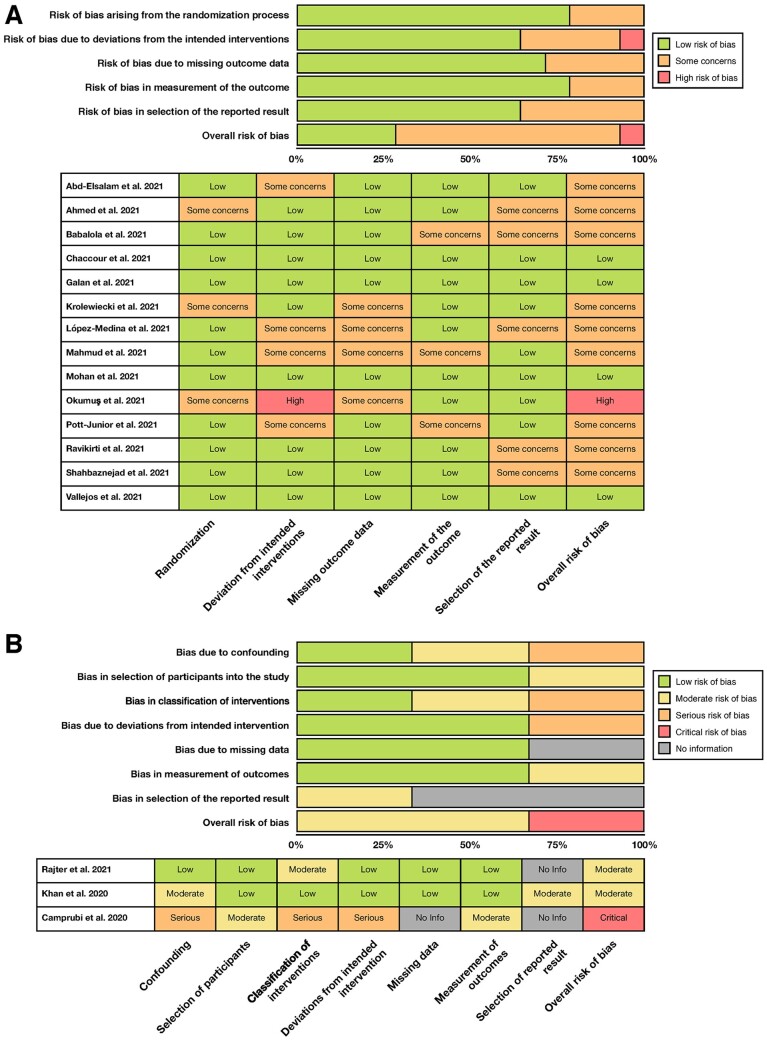
According to RoB2, nine RCTs were rated as having some concerns regarding the risk of bias,22–24,26–28,31–33 and the RCT by Okumuş et al.30 was rated as having a high risk of bias. Major sources of concerns include open-label designs leading to treatment deviations, and a lack of prospectively developed analysis plans, which could have contributed to possible selection of reported results. The remaining four RCTs25,29,34,35 were rated as having a low risk of bias.
For OSs, ROBINS-I indicated that Camprubi et al.19 had a critical risk of bias due to serious concerns regarding confounding factors, intervention classifications and potential deviations from assigned intervention. The remaining studies20,21 were rated as having moderate risk of bias.
The detailed results of the risk of bias analyses are available in Figure 2.
Figure 2.
Results of the risk of bias assessment using RoB2 and ROBINS-I.
(A) Bar chart overview and per-study risk of bias rating for RCT studies. (B) Bar chart overview and per-study risk of bias rating for observational studies.
Efficacy outcomes
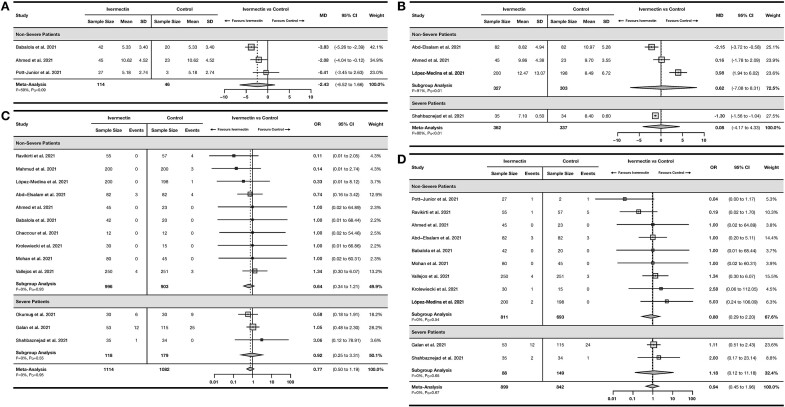
Total of 3 RCTs23,24,31 including 160 non-severe COVID-19 patients reported time to viral clearance (Figure 3A). The pooled MD was −2.43 days, although this finding was not significant [95% confidence interval (95% CI) −6.52 to 1.66] with moderate heterogeneity (I2= 59%, PQ < 0.10). Only one OS by Khan et al.20 reported time to viral clearance, which reported that ivermectin significantly reduced time to viral clearance by 9.78 days (95% CI −10.59 to −8.97).
Figure 3.
Forest plot showing the results of meta-analyses for efficacy outcomes using RCT studies.
(A) Forest plot showing mean difference of time to viral clearance in the ivermectin arm vs. control/standard of care arm. (B) Forest plot showing mean difference of duration of hospitalization in the ivermectin arm vs. control/standard of care arm. (C) Forest plot showing the odds of death among patients receiving ivermectin compared to control/standard of care. (D) Forest plot showing the odds of progression to mechanical ventilation among patients receiving ivermectin compared to control/standard of care.
OR, odds ratio; MD, mean difference; 95% CI, 95% confidence interval.
For duration of hospitalization, 4 RCTs22,23,27,33 including 699 patients reported a pooled MD of 0.08 days (95% CI −4.17 to 4.33) with serious heterogeneity (I2 = 90%, PQ < 0.01) (Figure 3B). Two OSs20,21 also reported duration of hospitalization (Supplementary Figure S1), with a pooled MD of 3.54 days (95% CI −32.01 to 39.09) with serious heterogeneity (I2 = 96%, PQ<0.01).
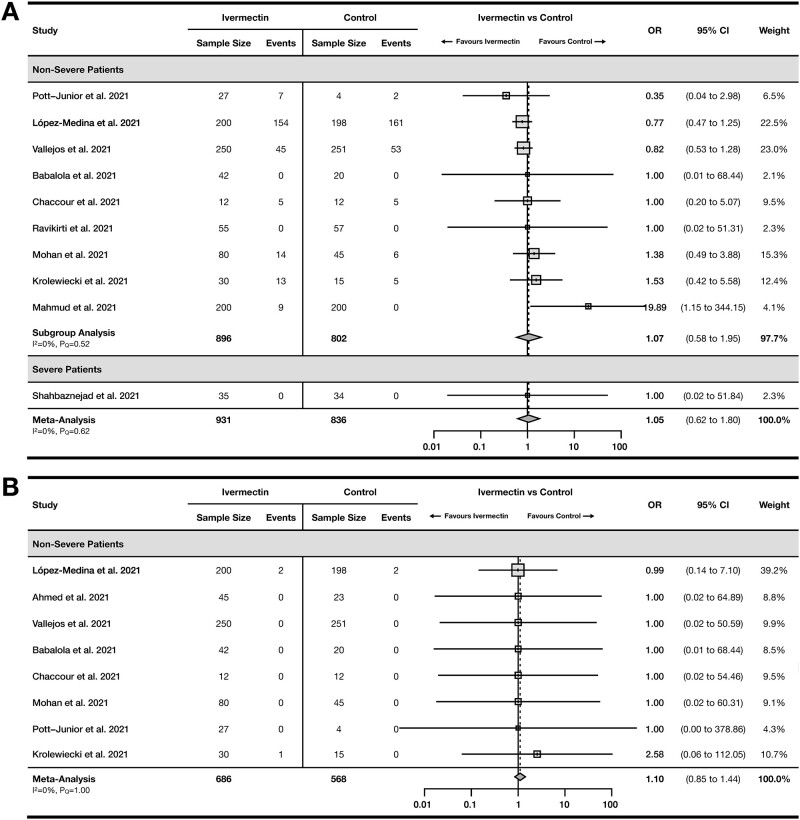
A total of 13 RCTs22–30,32–35 with 2196 COVID-19 patients reported incidence of mortality in their studies (Figure 3C). The pooled OR was 0.77 (95% CI 0.50–1.19) with no heterogeneity (I2= 0%, PQ = 0.95). Two OSs20,21 reported a pooled OR of 0.29 (95% CI 0.01–13.08) with serious heterogeneity (I2= 77%, PQ<0.05; Supplementary Figure S2).
A total of 11 RCTs22–24,26,27,29,31–35 with 1741 COVID-19 patients reported incidence of mechanical ventilation (Figure 3D). The pooled OR was 0.94 (95% CI 0.45–1.96) with no heterogeneity (I2= 0%, PQ = 0.67). One OS by Camprubi et al.19 reported incidence of mechanical ventilation, with an OR of 0.48 (95% CI 0.09–2.65).
Safety outcomes
A total of 10 RCTs24–29,31–34 with 1767 COVID-19 patients reported incidence of adverse events with a pooled OR of 1.05 (95% CI 0.62–1.80) with no heterogeneity (I2= 0%, PQ = 0.62) (Figure 4A) and 8 RCTs23–27,29,31,34 with 1254 non-severe COVID-19 patients reported incidence of serious adverse events with a pooled OR of 1.10 (95% CI 0.85–1.44) with no heterogeneity (I2= 0%, PQ = 1.00) (Figure 4B). Only one OS by Camprubi et al.19 reported safety outcomes, yielding an OR of 0.68 (95% CI 0.12–3.87) for serious adverse events.
Figure 4.
Forest plot showing the results of meta-analyses for safety outcomes using RCT studies.
(A) Forest plot showing the odds of developing at least one adverse event among patients receiving ivermectin compared to control/standard of care. (B) Forest plot showing the odds of developing at least one serious adverse event among patients receiving ivermectin compared to control/standard of care.
OR, odds ratio; 95% CI, 95% confidence interval.
Additional analyses
None of the meta-regression analyses by follow-up duration and cumulative ivermectin dose yielded a significant correlation (Supplementary Figure S3). There were also no significant between-group differences in the subgroup analysis by disease severity (Figures 3 and 4) for duration of hospitalization (P = 0.29), mortality (P = 0.25), incidence of mechanical ventilation (P = 0.25) and incidence of adverse events (P = 0.97). Subgroup analysis by severity was not performed for time to viral clearance and incidence of serious adverse events as only non-severe patients were included in these analyses. Sensitivity analyses excluding studies using doxycycline adjuvants and lopinavir–ritonavir/chloroquine control arms did not yield substantially different pooled effects compared to the original analyses (Supplementary Figure S4). We did not perform sensitivity analysis for time to viral clearance, as two out of the three included studies would have been excluded in the sensitivity analysis.
Publication bias assessment
Publication bias was assessed for the RCT meta-analysis of mortality incidence, incidence of mechanical ventilation and incidence of adverse events. This was not conducted for other analyses as fewer than 10 studies were included. Visual inspection of the funnel plots and results of the Egger’s test showed no significant small study effects as an indication for publication bias in these outcomes (Supplementary Figure S5).
Quality of evidence
The summary of findings and quality of evidence for study outcomes is tabulated in Table 2.
Table 2.
Summary of findings, ivermectin compared to standard of care for the management of COVID-19 patients
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects (95% CI)g |
No. of patients (No. of studies) | Quality of evidence (GRADE) | |||
|---|---|---|---|---|---|---|---|
| Risk without ivermectin | Risk with ivermectin | Risk difference (95% CI) | |||||
| Time to viral clearance | RCT | The mean time in the control group was 10.69 days | MD 2.43 fewer days | 160 | ⨁◯◯◯ | ||
| (6.52 fewer to 1.66 more) | (3 RCTs) | Very lowb,c,d | |||||
| Duration of hospitalization | RCT | The mean time in the control group was 9.17 days | MD 0.08 more days | 699 | ⨁◯◯◯ | ||
| (4.17 fewer to 4.33 more) | (4 RCTs) | Very lowb,c,e | |||||
| OS | The mean time in the control group was 7.00 daysa | MD 3.54 more days | 444 | ⨁◯◯◯ | |||
| (32.01 fewer to 39.09 more) | (2 OSs) | Very lowb,e,f | |||||
| Mortality incidence | RCT | OR 0.77 | 45 per 1000 | 35 per 1000 | 10 fewer per 1000 | 2196 | ⨁⨁⨁◯ |
| (0.50–1.19) | (23–53) | (22 fewer to 8 more) | (13 RCTs) | Moderateb | |||
| OS | OR 0.29 | 135 per 1000 | 43 per 1000 | 92 fewer per 1000 | 445 | ⨁◯◯◯ | |
| (0.01–13.08) | (1–672) | (134 fewer to 537 more) | (2 OSs) | Very lowb,e,f | |||
| Incidence of mechanical ventilation | RCT | OR 0.94 | 44 per 1000 | 41 per 1000 | 3 fewer per 1000 | 1741 | ⨁⨁⨁◯ |
| (0.45–1.96) | (20–83) | (24 fewer to 39 more) | (11 RCTs) | Moderateb | |||
| Incidence of adverse events | RCT | OR 1.05 | 278 per 1000 | 288 per 1000 | 10 more per 1000 | 1767 | ⨁⨁⨁◯ |
| (0.62–1.80) | (193–409) | (85 fewer to 131 more) | (10 RCTs) | Moderateb | |||
| Incidence of serious adverse events | RCT | OR 1.10 | 4 per 1000 | 4 per 1000 | 0 fewer per 1000 | 1254 | ⨁⨁⨁◯ |
| (0.85–1.44) | (3–6) | (1 fewer to 2 more) | (8 RCTs) | Moderateb | |||
GRADE Working Group quality of evidence rating17.
High quality: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
95% CI, 95% confidence interval; GRADE, Grading of Recommendations, Assessment, Development and Evaluations; OR, odds ratio; MD, mean difference; RCT, randomized controlled trial; OS, observational study.
One study was excluded from the calculation as it only reported mean difference and did not report mean duration in the control group.
Downgraded by 1 level due to imprecision; confidence intervals could not rule out the possibility of no effect (crosses null).
Downgraded by 1 level due to risk of bias; all included studies were rated as having ‘some concerns’ on RoB2 regarding risk of bias.
Downgraded by 1 level due to inconsistency; moderate heterogeneity was observed in the analysis.
Downgraded by 2 level due to inconsistency; serious heterogeneity was observed in the analysis.
Quality of study was rated as low prior to downgrading or upgrading as the included studies were observational studies.
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Discussions
Our systematic review and meta-analysis included 14 RCTs and 3 OSs to assess the efficacy and safety of ivermectin for the treatment of patients with COVID-19. Ivermectin did not significantly reduce time to viral clearance and duration of hospitalization based on very low-quality RCT evidence, nor did it reduce incidence of mortality and incidence of mechanical ventilation based on moderate quality RCT evidence. These non-significant findings were maintained among meta-analyses of OSs. Additionally, ivermectin use was not associated with increased odds of adverse events or serious adverse events based on moderate quality of evidence from RCTs. Nevertheless, given that our findings demonstrate a lack of efficacy, we cannot recommend the use of ivermectin for treatment of COVID-19 beyond the context of clinical trials.
The current review was conducted during an influx of misinformation regarding the efficacy of ivermectin. Optimistic results from early non-peer-reviewed clinical trials and in vitro studies had led to extensive off-label use of ivermectin for COVID-19 treatment by both clinicians and the general public. As an antiparasitic agent, ivermectin had been found to exert diverse effects on the human immune system; thus, it was proposed that ivermectin may be efficacious in the treatment of many diseases, including cancer,39 bacterial infections40 and viral infections. Investigations into the antiviral effects of ivermectin began long before the COVID-19 pandemic, with several projects assessing the in vitro efficacy of ivermectin against other RNA viruses, such as the Zika virus, dengue virus and the West Nile virus, among others.41 These investigations generally yielded optimistic results, associating ivermectin with reductions in viral replication by inhibiting multiple replication mechanisms.
In early 2020, a landmark in vitro study conducted by Caly et al.42 showed that Vero cells infected with SARS-CoV-2 demonstrated a 5000-fold reduction in viral RNA after exposure to 5 µM of ivermectin. The results suggested that ivermectin effectively disables all viral particles within 48 h by inhibiting the importin α/β receptor, thereby preventing transmission of viral proteins into the host cell nucleus. However, the clinical applicability of this research is limited; previous research had shown that even with the highest reported ivermectin dose of 1700 μg/kg, the maximum plasma concentration was only 0.28 μM. This figure is further diminished by the blinding of ivermectin to plasma proteins, which limits its uptake by endothelial cells, as well as its low accumulation in human lungs.43 The ivermectin doses reported in this review is substantially lower than the highest reported dose of ivermectin, ranging from a weight-adjusted dose of 400 µg/kg to as low as 100 µg/kg. Additionally, we did not observe a significant correlation between cumulative ivermectin dose and patient outcome. To replicate the efficacy observed in in vitro studies, an unsafely high dosage of ivermectin may be needed that is not appropriate for clinical use.43
As the pandemic continues to persist into 2022, management strategies for patients with COVID-19 need to be based upon valid, high-quality evidence in order to both improve patient outcomes and conserve hospital resources. While previous meta-analyses reported beneficial outcomes associated with ivermectin based on pre-print studies,5–7 our review found that the current peer-reviewed evidence does not support the use of ivermectin for the treatment of COVID-19. However, ivermectin may be safely used in clinical trials to further establish its potential role in the management of the disease as it did not significantly increase the incidence of adverse events compared to standard of care.
Limitations
We observed significant heterogeneity for the meta-analyses of RCTs for time to viral clearance and duration of hospitalization, as well as for all meta-analyses of OSs. Additionally, there were a low number of studies reporting time to viral clearance and duration of hospitalization, thus these outcomes should be interpreted with caution. Lastly, we could not assess publication bias for the meta-analysis of RCTs for time to viral clearance, duration of hospitalization and incidence of serious adverse events, as well as all meta-analyses of OSs, as <10 studies were included in these analyses.
Conclusion
The use of ivermectin in COVID-19 patients was not significantly associated with reductions in time to viral clearance, duration of hospitalization, incidence of mortality and incidence of mechanical ventilation. Based on a lack of efficacy, ivermectin is not recommended for use in the treatment of COVID-19 based on the currently available evidence. However, because ivermectin was not associated with increased incidence of adverse events or serious adverse events, it can be safely used in larger clinical trials to further clarify its role in the management of COVID-19.
Supplementary material
Supplementary material is available at QJMED online.
Conflict of interest. None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data sharing statement
All relevant data are disclosed in the manuscript and its associated figures and Supplementary Materials. Further inquiries related to the study should be directed to the corresponding author.
Supplementary Material
References
- 1. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsel L, et al. ; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021; 397:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Elgazzar A, Eltaweel A, Youssef SA, Hany B, Hafez M, Moussa H. Efficacy and safety of ivermectin for treatment and prophylaxis of COVID-19 pandemic. Res Sq DOI: 10.21203/rs.3.rs-100956/v4. [DOI] [Google Scholar]
- 5. Hill A, Garratt A, Levi J, Falconer J, Ellis L, McCann K, et al. Erratum: expression of concern: ‘Meta-analysis of Randomized Trials of Ivermectin to Treat SARS-CoV-2 Infection’. Open Forum Infect Dis 2021; 8:ofab394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. Kory P, Meduri GU, Varon J, Iglesias J, Marik PE. Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19. Am J Ther 2021; 28:e299–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hariyanto TI, Halim DA, Rosalind J, Gunawan C, Kurniawan A. Ivermectin and outcomes from COVID-19 pneumonia: a systematic review and meta-analysis of randomized clinical trial studies. Rev Med Virol 2021; e2265. [Google Scholar]
- 8. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. New Jersey, Wiley, 2008. [Google Scholar]
- 9. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hicks E. Library Guides: TU GOARN: COVID-19 Search Strategies. 2020. https://libguides.tulane.edu/TESTCovid-19/COVID-19SearchStrings (15 March 2021, date last accessed).
- 11. King A. Fast news or fake news? The advantages and the pitfalls of rapid publication through pre-print servers during a pandemic. EMBO Rep 2020; 21:e50817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018; 27:1785–805. [DOI] [PubMed] [Google Scholar]
- 13. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi J, Luo D, Wan X, Liu Y, Liu J, Bian Z, et al. Detecting the skewness of data from the sample size and the five-number summary. arXiv 2020. http://arxiv.org/abs/2010.05749. [DOI] [PubMed] [Google Scholar]
- 15. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366:l4898. [DOI] [PubMed] [Google Scholar]
- 16. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 2004; 23:1351–75. [DOI] [PubMed] [Google Scholar]
- 19. Camprubí D, Almuedo-Riera A, Martí-Soler H, Soriano A, Hurtado JC, Subirà C, et al. Lack of efficacy of standard doses of ivermectin in severe COVID-19 patients. PLoS One 2020; 15:e0242184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khan MSI, Khan MSI, Debnath CR, Nath PN, Mahtab MA, Nabeka H, et al. Ivermectin treatment may improve the prognosis of patients with COVID-19. Arch Bronconeumol 2020; 56:828–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rajter JC, Sherman MS, Fatteh N, Vogel F, Sacks J, Rajter J-J. Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ivermectin in COVID nineteen study. Chest 2021; 159:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abd-Elsalam S, Noor RA, Badawi R, Khalaf M, Esmail ES, Soliman S, et al. Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: a randomized controlled study. J Med Virol 2021; 93:5833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahmed S, Karim MM, Ross AG, Hossain MS, Clemens JD, Sumiya MK, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis 2021; 103:214–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Babalola OE, Bode CO, Ajayi AA, Alakaloko FM, Akase IE, Otrofanowei E, et al. Ivermectin shows clinical benefits in mild to moderate COVID19: a randomised controlled double-blind, dose-response study in Lagos. QJM 2021; DOI: 10.1093/qjmed/hcab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaccour C, Casellas A, Blanco-Di Matteo A, Pineda I, Fernandez-Montero A, Ruiz-Castillo P, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine 2021; 32:100720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krolewiecki A, Lifschitz A, Moragas M, Travacio M, Valentini R, Alonso DF, et al. Antiviral effect of high-dose ivermectin in adults with COVID-19: a proof-of-concept randomized trial. EClinicalMedicine 2021; 37:100959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA 2021; 325:1426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mahmud R, Rahman MM, Alam I, Ahmed KGU, Kabir AKMH, Sayeed SKJB, et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. J Int Med Res 2021; 49:3000605211013550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mohan A, Tiwari P, Suri TM, Mittal S, Patel A, Jain A, et al. Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): a single-centre randomized, placebo-controlled trial. J Infect Chemother 2021; DOI: 10.1016/j.jiac.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Okumuş N, Demirtürk N, Çetinkaya RA, Güner R, Avcı İY, Orhan S, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect Dis 2021; 21:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pott-Junior H, Paoliello MMB, Miguel AdQ, da Cunha AF, de Melo Freire CC, Neves FF, et al. Use of ivermectin in the treatment of Covid-19: a pilot trial. Toxicol Rep 2021; 8:505–10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32. Ravikirti RR, Pattadar C, Raj R, Agarwal N, Biswas B, et al. Evaluation of ivermectin as a potential treatment for mild to moderate COVID-19: a double-blind randomized placebo controlled trial in Eastern India. J Pharm Pharm Sci 2021; 24:343–50. [DOI] [PubMed] [Google Scholar]
- 33. Shahbaznejad L, Davoudi A, Eslami G, Markowitz JS, Navaeifar MR, Hosseinzadeh F, et al. Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized, controlled clinical trial. Clin Ther 2021; 43:1007–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vallejos J, Zoni R, Bangher M, Villamandos S, Bobadilla A, Plano F, et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect Dis 2021; 21:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Galan LEB, Santos NMD, Asato MS, Araújo JV, de Lima Moreira A, Araújo AMM, et al. Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection. Pathog Glob Health 2021; 115:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Butler CC, Yu L-M, Dorward J, Gbinigie O, Hayward G, Saville BR, et al. ; PRINCIPLE Trial Collaborative Group. Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet Respir Med 2021; 9:1010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Amani B, Khanijahani A, Amani B, Hashemi P. Lopinavir/ritonavir for COVID-19: a systematic review and meta-analysis. J Pharm Pharm Sci 2021; 24:246–57. [DOI] [PubMed] [Google Scholar]
- 38. Kashour Z, Riaz M, Garbati MA, AlDosary O, Tlayjeh H, Gerberi D, et al. Efficacy of chloroquine or hydroxychloroquine in COVID-19 patients: a systematic review and meta-analysis. J Antimicrob Chemother 2021; 76:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang M, Hu X, Wang Y, Yao X, Zhang W, Yu C, et al. Ivermectin, a potential anticancer drug derived from an antiparasitic drug. Pharmacol Res 2021; 163:105207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ashraf S, Chaudhry U, Raza A, Ghosh D, Zhao X. In vitro activity of ivermectin against Staphylococcus aureus clinical isolates. Antimicrob Resist Infect Control 2018; 7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heidary F, Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot 2020; 73:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 2020; 178:104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peña-Silva R, Duffull SB, Steer AC, Jaramillo-Rincon SX, Gwee A, Zhu X. Pharmacokinetic considerations on the repurposing of ivermectin for treatment of COVID-19. Br J Clin Pharmacol 2021; 87:1589–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.