Abstract
The relationship between diabetes mellitus and Parkinson’s disease has been described in several epidemiological studies over the 1960s to date. Molecular studies have shown the possible functional link between insulin and dopamine, as there is strong evidence demonstrating the action of dopamine in pancreatic islets, as well as the insulin effects on feeding and cognition through central nervous system mechanism, largely independent of glucose utilization. Therapies used for the treatment of type 2 diabetes mellitus appear to be promising candidates for symptomatic and/or disease-modifying action in neurodegenerative diseases including Parkinson’s disease, while an old dopamine agonist, bromocriptine, has been repositioned for the type 2 diabetes mellitus treatment. This review will aim at reappraising the different studies that have highlighted the dangerous liaisons between diabetes mellitus and Parkinson’s disease.
Key Words: dopamine, GLP-1, insulin, Parkinson's disease, type 2 diabetes mellitus
Introduction
The relationship between type 2 diabetes mellitus (T2DM) and Parkinson’s disease (PD) has been already demonstrated over the ‘60s, disclosing a significantly high prevalence of T2DM in parkinsonian patients (Sandyk, 1993). These initial epidemiological studies opened the field to further experimental works, investigating the possible functional link between insulin and dopamine (DA). Evidence for the presence of biogenic amines in the mammalian endocrine pancreas was originally reported by Falck and Hellman (1963), while functional studies supported a possible role of intracellular β cell monoamines in the regulation of insulin secretion (Wilson et al., 1974; Ericson et al., 1977). Subsequently, the development of molecular biology allows us to identify entire functional DA signaling machinery in pancreatic β-cells (Rubì et al., 2005; Saisho et al., 2008). More recently, a role in insulin signaling has also been shown for α-synuclein (α-syn) and leucine-rich repeat kinase 2, proteins whose mutations are involved in the pathogenesis of PD (Gao et al., 2015; Funk et al., 2019). On the other hand, insulin receptors were localized throughout the brain (Unger et al., 1991; Schulingkamp et al., 2000), allowing the identification of the insulin signaling pathways. At present, there is no doubt about the presence of cerebral insulin and its action as a neuromodulator, even if it is still debated if insulin is brain-derived or produced outside the central nervous system (CNS) (Havrankova et al., 1979; Kleinridders et al., 2014; Zeng et al., 2016).
Key treatments for T2DM have been investigated in clinical trials for their possible neuroprotective effects in PD, opening a new field in the pharmacological research of this disease. At the same time, bromocriptine, a DA agonist treating PD, has been approved by the United States Food and Drug Administration (FDA) for T2DM therapy. Here, we will review the studies that have traced a red thread through glucose metabolism and neurodegenerative diseases, specifically addressing the link between T2DM and PD.
Literature Search Strategy and Selection Criteria
The articles included in this review were retrieved by an electronic search of the PubMed database from inception to September 30, 2020 for literature describing epidemiological, experimental, and therapeutic studies on this topic. Searches were conducted using the following key words: insulin and dopamine, glucose and Parkinson’s disease, diabetes and neurodegeneration. Articles were included if they were deemed to contribute to the understanding of the possible functional link between insulin and dopamine.
Epidemiological Studies
Is T2DM a predisposing factor for PD?
The analysis of the initial epidemiological studies, concerning the association between T2DM and PD, led Sandyk to assert there is a wide agreement that PD is associated with a significant prevalence of diabetes mellitus (Sandyk, 1993).
The main question emerging from this analysis was related to the impact of hyperglycemia on the motor symptoms of PD, a matter still debated today. Subsequent epidemiological investigations, based on the strong biological evidence emerging from functional and molecular studies, have analyzed larger cohorts of patients aiming to evaluate a real connection between PD and T2DM.
T2DM as a risk factor of developing PD has been confirmed in different ethnic groups (Hu et al., 2007; Schernhammer et al., 2011; Bosco et al., 2012; Sun et al., 2012; Yue et al., 2016; Yang et al., 2017; De Pablo-Fernandez et al., 2018), and PD patients resulted to be insulin-resistant (Bosco et al., 2012). Other investigations emphasized the role of comorbid diabetes in impairing motor and/or cognitive domains (Cereda et al., 2012; Kotagal et al., 2013), and the data were confirmed by imaging studies (Ong et al., 2017; Chung et al., 2018; Table 1).
Table 1.
Epidemiological studies supporting the association between T2DM and PD
| Study | Study design | Main results |
|---|---|---|
| Hu et al., 2007 | Prospective, cohort study, Finnish population | T2DM associated with an elevated risk of PD in both sex |
| Schernhamer et al., 2011 | Case-control, Danish population | T2DM is associated with an increased risk of PD, especially younger onset PD |
| Bosco et al., 2012 | Case-control, Italian population | Insulin resistance is significantly higher in PD patients with dementia than PD patients without dementia |
| Cereda et al., 2012 | Retrospective, case-control | T2DM onset before PD onset is a risk factor for more severe PD symptoms |
| Sun et al., 2012 | Retrospective, cohort study, Chinese population | T2DM is associated with an increased risk of PD onset, stronger in women and younger patients |
| Kotagal et al., 2013 | Case-control | PD patients with T2DM displayed greater postural instability and gait difficulty |
| Bohnen et al., 2014 | Case-control | T2DM is associated with more severe cognitive impairment in PD |
| Yue et al., 2016 | Cohort study meta-analysis | T2DM is associated with an increased 38% PD risk |
| De Pablo-Fernandez et al., 2017 | Cross-sectional study, Spanish population | The risk of PD in T2DM might be limited to longer disease duration |
| Ong et al., 2017 | Prospective | Longitudinally T2DM in PD results in a greater rate of cognitive decline with higher white matter atrophy |
| Yang et al., 2017 | Retrospective, case-control, Chinese population | T2DM increases the risk of PD. The magnitude of association is higher in women |
| Chung et al., 2018 | Inter-group comparative analysis | Coexistent T2DM in PD patients may have a detrimental effect on the baseline striatal dopamine loss, and cognitive performance |
| De Pablo-Fernandez et al., 2018 | Retrospective, cohort study | Increased rate of subsequent PD in T2DM patients |
| Pagano et al., 2018 | Cross-sectional | T2DM may predispose toward a PD-like pathology. T2DM present in PD patients can induce a more aggressive phenotype |
PD: Parkinson’s disease; T2DM: type 2 diabetes mellitus.
Conversely, these data were not confirmed by other authors who found no association at all (Palacios et al., 2011; Savica et al., 2012) or even inverse association (Driver et al., 2008; D’Amelio et al., 2009; Miyake et al., 2010; Cereda et al., 2011; Lu et al., 2014; Table 2). Those inconsistent results may be due to study heterogeneity (case-control vs. cohort), the definition of PD (direct examination vs. self-report), the definition of T2DM (formal glucose/insulin measurement, medical records, or self-report), and disease duration since the diagnosis of T2DM.
Table 2.
Epidemiological studies against the association between T2DM and PD
| Study | Study design | Main results |
|---|---|---|
| Driver et al., 2008 | Prospective, cohort study, US population | T2DM is associated with an increased risk of PD, but it is not a preceding risk factor of PD |
| D’Amelio et al., 2009 | Case-control | T2DM consistently lower among PD cases compared to controls |
| Miyake et al., 2010 | Case-control | Inverse associations of hypertension, hypercholesterolemia, and diabetes mellitus with the risk of PD in Japan |
| Cereda et al., 2011 | Meta-analysis | No conclusive evidence of the association between T2DM and PD |
| Palacios et al., 2011 | Prospective | No evidence between baseline T2DM and PD |
| Xu et al., 2011 | Prospective, cohort study, US population | T2DM in older patients is associated with a modest increase in PD |
| Savica et al., 2012 | Case-control | No association between T2DM and later development of PD |
| Lu et al., 2014 | Case-control meta-analysis | T2DM patients may have a decreased incidence of PD |
PD: Parkinson’s disease; T2DM: type 2 diabetes mellitus.
Interestingly, multi-center analysis of T2DM patients with and without PD compared many diabetes endpoints. T2DM patients with PD showed a better metabolic control, probably due to more rigorous follow-ups (Scheuing et al., 2013).
Despite these conflicting results, in the last decade, there has been a rapidly increasing interest in investigating the connection between T2DM and PD, leading to the hypothesis that new drugs for diabetes mellitus might alter the natural history of PD itself. Furthermore, a new formulation of bromocriptine, an old antiparkinsonian drug, has been specifically approved by the FDA for the treatment of T2DM.
Functional Studies
DA in the endocrine pancreas
Several in vitro studies and preclinical investigations on animal models have confirmed the seminal histochemical and histological reports of Falck and Hellman (1963). These scientists demonstrated, by using the formaldehyde-induced fluorescence method, the intracellular presence of catecholamines in pancreatic islets of different mammalian species, in which adrenergic and cholinergic fibers were identified (Lakomy and Chodkowska, 1984). The pancreatic beta cells appear to work such as the neurons, as they was able to decarboxylate l-DOPA to DA, as observed in experimental animals (Cegrell, 1968).
The main question derived from these experimental observations concerned the role and the mechanism underlying dopaminergic transmission in the endocrine pancreas. In the first instance, the in vitro effects of adrenergic and cholinergic drugs on pancreatic tissues, observed by Malaisse et al. (1967) showed the insulin secretion to be stimulated by acetylcholine and β-adrenergic receptors, and inhibited by α-adrenergic receptors. Possible roles of intracellular β cells monoamines in regulating insulin secretion have been subsequently provided from in vitro and in vivo experiments (Feldman et al, 1970; Wilson et al., 1974). The presence of DA in secretory granules of the pancreatic β-cells was later confirmed by electron microscopy (Ericson et al., 1977). This histological localization, according to the authors, demonstrated that hyperglycemic effects might have been driven by DA through insulin storage or releasing mechanism.
Currently, this hypothesis has been validated by molecular studies that highlighted the mechanisms of DA stimulation on the endocrine pancreas. The whole DA secretion mechanisms and signaling machinery of pancreatic β cells have been better characterized. Tyrosine hydroxylase, the rate-limiting enzyme in DA biosynthesis, converting I-DOPA in DA and specific marker for catecholamine-synthesizing cells, was identified in β-cells. Moreover, electron microscopy studies showed how the enzyme monoamine oxidase (MAO), involved in the deamination of catecholamines, is co-localized along with insulin in secretory granules of β-cells. Vesicular monoamine type 1 (VMAT-1) and type 2 (VMAT-2) transporters, essential for the vesicular storage of DA, were respectively identified in rat and human pancreas (Mezey et al., 1996; Anlauf et al., 2003). DA transporter (DAT), a plasma membrane protein, which transports released DA back into the cell or may also function in reverse mode releasing DA, was found in exocrine and endocrine pancreatic cells (Mezey et al., 1996). DA receptors, classified as D1-like (D1 and D5) and D2-like receptors (D2, D3, and D4), possible mediators of the DA action on the insulin secretion, are expressed in β-cells (Rubì et al., 2005; Bini et al., 2020).
The demonstrated presence of the DA machinery leaves unsolved the following problems: a) From where the DA comes in the endocrine pancreas? b) How that machinery works on insulin secretion?
Here are two possible explanations: a) The presence of DA in the pancreas seems to exclude a neuronal production. The existence of a peripheral DA system independent of the sympathoadrenal system was first suggested by the detection of a considerable DA concentration in the body not metabolized to other catecholamines (Mezey et al., 1996).
Eisenhofer et al. (1997) inferred a production of DA by mesenteric organs because the higher concentrations of DA and metabolites were found in portal venous than arterial plasm. They introduced the concept of a “third catecholamine system” in which the DA, synthesized from decarboxylation of I-DOPA in nonneuronal cells, would act locally as an autocrine/paracrine effector and undergo inactivation by sulfoconjugation before entering the bloodstream (Goldstein et al., 1999). Clinical studies evaluating the plasma concentrations of DA sulfate after fasting and food intake showed increased DA sulfate and I-DOPA, after meal ingestion, but normal concentrations after fasting and I-DOPA infusion, in patients with peripheral autonomic failure and patients undergoing gastrointestinal surgery, compared with healthy subjects. In patients deficient of L-aromatic amino acid decarboxylase (L-AADC), the I-DOPA converting enzyme in DA, they registered very low concentrations of DA sulfate in the same conditions (Goldestein et al., 1999, 2003).
Despite the robust evidence of the presence of DA, strengthened by in vitro and in vivo studies (Mezey et al., 1996; Simpson et al., 2012; Ustione and Piston, 2012), the identification of an endogenous synthesis of DA in β-cells, is still a complex work. Farino et al. (2020) investigated the possible mechanism underlying metabolic disturbances induced by the antipsychotic drug and argued a DA production and secretion by β-cells when provided DA precursors, such as I-DOPA deriving from protein and carbohydrate meals. This release would be regulated by glucose stimulation (Farino et al., 2020). Lastly, β-cells appear to be able to de novo synthesize DA in two kinds of mouse strains (Mitok et al., 2018).
b) The role of DA in insulin secretion seems to be inhibitory, modulated by DA signaling machinery. The insulin granules express D2 receptors (D2R) and, during insulin secretion glucose-stimulated (GSIS), DA and insulin appear delivered to the cell surface, where D2R binds to DA, inhibiting the GSIS insulin secretion (Rubì et al., 2005; Simpson et al., 2012). Other scholars localized D2R on the β-cell plasma membrane but showed D3Rs to be involved in β-cell DA signaling (Ustione and Piston 2012; Farino et al., 2020; Figure 1).
Figure 1.
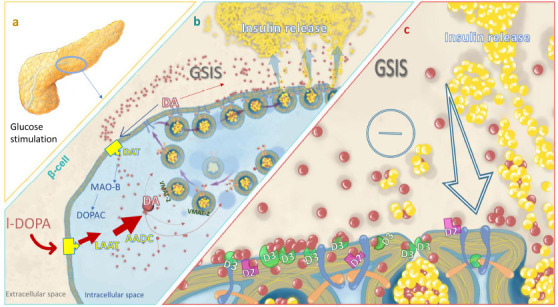
A schematic overview of the possible inhibitory role of DA in the pancreas.
(a) Glucose stimulation increases I-DOPA uptake, forming intracellular DA in β-cells. (b) VMAT-2 transports cytoplasmic DA into insulin-containing vesicles. On GSIS, insulin and DA are delivered to the extracellular space. (c) DA diffuses to neighboring β-cells and binds to D2R and/or D3R, inhibiting further insulin secretion. By DAT, DA is recycled and degraded by MAO-B. AADC: Amino acid decarboxylase; D2: dopamine receptor 2; D3: dopamine receptor 3; DA: dopamine; DAT: dopamine transporter; DOPAC: 3,4-dihydroxyphenylacetic acid; GSIS: glucose-stimulated insulin secretion; I-DOPA: l-3-4-dihydroxyphenylalanine; LAAT: l-type amino acid transporter; MAO-B: monoamine oxidase-B; VMT-2: vesicular monoamine transporter 2.
A functional role of DAT and VMAT-2 has been hypothesized by the enhancement of insulin secretion after VMAT-2 and DAT inhibition (Ustione and Piston, 2012). The role of DA in modulating islet survival has also been demonstrated (Garcia-Barrado et al., 2015). In an imaging study, D2/D3 receptor agonist radioligand 11C-(1)-4-propyl-9-hydroxynaphthoxazine (PHNO) was identified as a tracer to measure β-cell mass (Bini et al., 2018). The same radioligand has been subsequently used to evaluate residual β-cell mass in type 1 diabetes mellitus (T1DM) and, in the same study, by using immunohistochemistry, it was demonstrated a lack of staining for the D2R, DA D3 receptor (D3R), and insulin in a T1DM pancreatic tissue, whereas D2R, D3R, and insulin were colocalized in the pancreas of healthy controls (Bini et al., 2020).
Insulin in the brain
If the DA in the gut appears to act as a metabolic regulator, the insulin in the brain shows a neuromodulatory action through the insulin signaling pathways. Different studies have shown the reciprocal regulation that insulin and DA can exert each other.
Intracerebral insulin comes from pancreatic β-cells, crossing the blood-brain barrier via a saturable transport process (Havrankova et al., 1978). Neuronal de novo synthesis of insulin was also demonstrated in primary cultures from rat brains (Clarke et al., 1986). Molecular studies, performed in experimental animals, identified insulin transcripts in specific brain regions, as pyramidal cells of the hippocampus, dentate gyrus, prefrontal cortex, entorhinal cortex, thalamus, and granular cell layer of the olfactory bulbs (Schechter et al., 1988; Devaskar et al., 1994; Kuwabara et al., 2011). Insulin mRNA has been shown to be strongly expressed in GABAergic neurogliaform cells in the cerebral cortex, which showed insulin-like effects according to the detection of excitatory postsynaptic currents (Molnár et al., 2014). Insulin protein expression was detected in rat astrocytes, as well, where appeared to recover neuronal activity after hypoglycemia, in synergistic action with insulin growth factor-1 (Fernandez, 2017), and to exert neuroprotective effects (Pitt et al., 2017; Takano et al., 2018). The uneven distribution through the brain of insulin signaling machinery should give the reason for the different actions that insulin plays in the CNS, beyond the glucose metabolism regulation. The brain insulin receptors (IRs) have been identified in neurons and astrocytes from human cerebral cortex, rat olfactory bulb, hypothalamus, hippocampus, and cerebellum (Havrankova et al., 1978; Unger et al., 1991; Shulingkamp et al., 2000), DA neurons of the ventral tegmental area (VTA) and substantia nigra (SN) (Figlewicz et al., 2003). Among the glucose transporters, distributed through the brain (Mc Ewen and Reagan, 2004), insulin regulates glucose transporter 4 (GLUT4) and glucose transporter 8 (GLUT8), localized in neuronal cell bodies in cortex, cerebellum, hypothalamus, hippocampus, and amygdala (Jurcovicova et al., 2014)
Binding to its specific receptors and through the consequent tyrosine phosphorylation of insulin receptor substrate (IRS) docking proteins, insulin can trigger two main downstream pathways: the phosphoinositide-3-kinase (PI3K/Akt) cascade, involved in neuronal survival, and the Ras-Raf-mitogen-activated protein kinase (MAPK/ERK) signaling, involved in cell death (White and Kahn, 1994). Alternatively, through the SHC/ERK1/2 pathway, insulin promotes neuronal survival and plasticity (van der Heide et al., 2006), improving neuronal antioxidant defense and protecting against neuronal apoptosis (Duarte et al., 2008).
Insulin in brain reward circuitry
The important relationship between insulin and DA emerges even more in the brain reward system, involving the mesolimbic DA pathway (Ikemoto, 2007). DA plays a central role in food reward, energizing feeding, and reinforcing food-seeking behavior (Morton, 2006; Wise, 2006) and substantial body of evidence showed that insulin modulates the reward circuitry. In dopaminergic neurons located in VTA and substantia nigra pars compacta (SNpc), the IRs are coexpressed with TH (Figlewicz et al., 2006). The inactivation of the IRs signaling in mouse TH-expressing cells resulted in a hyperphagic phenotype, increased body weight, and fat mass (Konner et al., 2011). In previous studies, it was observed by using intracerebroventricular administration of insulin (icv), the synergistic action of this hormone when paired with D2R antagonism in reducing sucrose consumption (Sipols et al., 2000). Increasing mRNA levels for the DAT, responsible for the DA reuptake have been detected in VTA and SNpc of rats chronically treated with icv insulin infusion and in hyperinsulinemic obese rats. Thus, insulin appeared to decrease DA concentration in VTA via increased reuptake of DA through DAT, reducing conditioned place preference to high-fat food and modulating the reward-related feeding behavior (Figlewicz et al., 2006). Moreover, an insulin-mediated decrease of DA in VTA suppressed the salience of food once satiety had reached (Mebel et al., 2012). Insulin as a reward signal has been well demonstrated by Stouffer et al. (2015). They observed an increased release of DA in nucleus accumbens (Nac) and caudate-putamen (Cpu) stimulated by insulin through striatal cholinergic interneurons expressing IRs, in food-restricted rats but not in obesogenic rats. According to the authors (Stouffer et al., 2015), such striatal insulin signaling enhances DA release to influence food choice.
Evidence of the insulin action in the human brain comes from neuroimaging studies mirroring molecular studies. Former studies applying auditory evoked brain potentials (AEP) and behavioral tests, after insulin infusion, suggested a direct influence of the hormone in improving cognitive functions in human subjects (Kern et al., 2001). Such increased neuronal activity was specifically localized in the medial temporal lobe, including the hippocampus, by functional magnetic resonance imaging (Rotte et al., 2005).
Studies of insulin in the human brain rely on intranasal administration. Via this delivery, insulin rapidly reaches the CNS through the olfactory nerve and it is possible to evaluate its direct effects within the brain, overcoming systemic effects on peripherally blood glucose or insulin levels (Kern et al. 1999; Born et al., 2002). Insulin appears as a metabolic key signal in the homeostatic mediation of satiety as resulted from the comparison between the effect of intranasal insulin administration and placebo on the brain of subjects processing food pictures analyzed by fMRI (Guthoff et al., 2010).
As also observed in experimental animals (Szczypka et al., 2001), intranasal insulin action in the human brain regulates the peripheral insulin sensitivity in a time-dependent manner, fitting with insulin kinetics after a meal (Heni et al., 2012). Moreover, Heni et al. (2014) investigated the eating behavior in response to food cues and demonstrated an enhanced activity of the putamen after glucose ingestion, without difference from water ingestion in overweight subjects, contrary to lean people that showed different activity patterns to low caloric and high caloric stimuli.
Insulin in the Pathophysiology of Parkinson’s Disease
The emerging role of insulin as a neuromodulator appears a possible puzzle piece contributing to the neurodegeneration pathway of PD. The etiology of PD remains unknown despite genetic and environmental factors, affecting fundamental cellular processes, have been identified and correlated to the specific neurodegenerative process of PD. One of these is oxidative stress, intimately linked to other components of the degenerative process, such as mitochondrial dysfunction, excitotoxicity, nitric oxide toxicity, and inflammation, even if it is still difficult to determine whether oxidative stress leads to, or is a consequence of these events (Kalia and Lang, 2015).
Ramalingam and Kim demonstrated that insulin reduces the hydrogen peroxide (H2O2)-induced oxidative damage in neuronal and glial cells (Ramalingam and Kim 2014, 2016a). The same authors observed the neuroprotective action of insulin in cell cultures treated with 1-methyl-4-phenyl pyridinium (MPP+), a neurotoxin derived from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which causes oxidative stress, mitochondrial dysfunction, and ultimately apoptotic cell death in vitro. In this case, pretreatment of insulin prevented cell death and lowered nitric oxide (NO) release, reactive oxygen species (ROS), and calcium (Ca2+) influx induced by MPP+ (Ramalingam and Kim, 2016b). The neuroprotective effects of insulin were confirmed in the 6-hydroxydopamine (6-OHDA)-based rat PD model. Treatment with intranasal insulin significantly ameliorated 6-OHDA-induced motor impairments, providing potent protection of DA neurons in the SNpc against 6-OHDA neurotoxicity (Pang et al., 2016).
Such actions of insulin are ascribed to the suppression of the glycogen-synthase kinase3 (GSK-3) activity, a crucial multi-target kinase, whose dysregulation is implicated in PD pathogenesis and promotion of Lewy bodies formation, the neuronal inclusions representing the pathological hallmark of PD (van der Heide et al., 2006; Albeley et al., 2018)
The insulin signaling pathway embraces two proteins, whose mutations are closely involved in the pathogenesis of PD: alpha-synuclein (α-syn) (Polymeropoulos et al., 1997) and Leucine-Rich Repeat Kinase 2 (LRRK2) (Paisàn-Ruiz et al., 2004). The physiological roles of α-syn, which is the main component of Lewy bodies and Lewy neurites in the PD brain, are not completely clear, ranging from chaperone activity to management of synaptic vesicle trafficking (Bendor et al., 2013). Expressed in presynaptic sites in the CNS, α-syn was found to be present also in several non-neuronal tissues (Askanas et al., 2000; Baltic et al., 2004; Barbour et al., 2008), and its possible spreading through different organs in PD has determined an unsolved discussion among the researchers (Braak et al., 2003; Olanow and Prusiner 2009; Bendor et al., 2013).
Alpha-syn has also been identified in the pancreas (Ueda et al., 1994) and successively localized on the insulin-secretory granule interacting with the K+ATP channel. This protein has been shown to functionally act as a brake on insulin secretion; thus in diabetes, when α-syn interaction with secretory granules becomes excessive, it should be able to compromise insulin secretion (Geng et al., 2011). Alpha-syn overexpression appears to negatively regulate the insulin receptor substrate-1 (IRS-1), impairing the PI3/Akt insulin signaling via mTORC1/S6K1 activation and promoting insulin resistance (Gao et al., 2015). On the other hand, it has been observed that the acute administration of α-syn in experimental animals promotes glucose metabolism in adipose tissue and skeletal muscle, through GAb1/PI3K/AkT transduction pathway (Rodriguez-Arujo et al., 2013). Metabolic changes were detected in α-syn A53T mutant mouse model of PD, as they are resistant to both high fat-induced obesity and high fat diet-induced insulin resistance (Rothmann et al., 2013). In a study analyzing the serum of 1152 subjects from medical check-ups, low plasma levels of α-syn were associated with insulin resistance, as serum α-syn levels were inversely correlated with insulin resistance indicator such as homeostatic model assessment for insulin resistance (HOMA-IR). In the same study, α-syn deficient mice displayed alterations in glucose and insulin response during diet-induced insulin resistance. Moreover, the measurement of insulin sensitivity by hyperinsulinemic-euglycemic clamp assessment in α-syn knockout mice fed with a high-fat diet has shown severe insulin resistance in adipose tissue and skeletal muscle (Rodriguez-Araujo et al., 2015).
Conversely, it has been demonstrated action on α-syn from molecules structurally very close to insulin, the insulin-like growth factor 1 (IGF-1), which, together with insulin and IGF-2, is referred to insulin-like peptides (ILPs), belonging to the insulin superfamily of peptides (Fernandez and Torres-Alemàn, 2012) and the insulin-degrading enzyme (IDE) (Duckworth et al., 1998). IGF-1 is a potent growth factor in the CNS (Dyer et al., 2016), and its protective effects against loss of dopaminergic neurons have been proved in cell culture and animal model of PD studies (Offen et al., 2001). The IGF-1 receptor (IGF-1R) and the IR can form functional hybrids with similar affinities for IGF1 and insulin, indicative of cooperation between these two ILPs. IGF-1 signaling, like insulin, can proceed through the RAS–ERK and PI3K/Akt cascades. Through the activation of PI3K/Akt pathways, IGF-1 rescued α-synuclein toxicity and suppressed α-syn aggregation (Chung et al., 2011). Higher IGF-1 serum levels were found in newly diagnosed untreated PD patients comparing to healthy controls, providing evidence for IGF-1 as a possible biomarker for early PD. Given the neuroprotective action of IGF-1, it has also been hypothesized that increased serum IGF-1 levels in early PD may reflect a compensatory effort to protect dopaminergic neurons from neurodegeneration (Godau et al., 2010, 2011). Moreover, Picillo and co-workers demonstrated a significant correlation between IGF-1 serum levels and progression of motor symptoms in a cohort of early, drug-naive PD patients, in whom higher IGF-1 levels matched with a higher dopaminergic score (Picillo et al., 2013).
The IDE, a zinc metalloprotease, so named by its degradation action on receptor bound-insulin, has multiple cellular functions including binding and regulatory functions. Insulin degradation is tightly linked to insulin action and it has been suggested that intracellular interactions of insulin-degrading enzyme (IDE) may be involved in insulin control of cellular protein degradation and fat oxidation (Duckworth et al., 1998). Intriguingly, IDE in vitro prevented the formation of α-syn pathogenic amyloid fibrils in a mutual interaction considering that the binding of α-syn to IDE enhanced the proteolytical activity of IDE (Sharma et al., 2015).
A role in insulin signaling on LRRK2, a crucial protein in the synaptic vesicle recycling regulation (Maas et al., 2017) and DA receptor trafficking (Rassu et al., 2017), has been shown in LRRK2 deficient cells from experimental animals and fibroblasts of PD patients with G2019S mutated-LRRK2. The insulin-induced translocation of GLUT 4 containing vesicles to the cell surface appeared to fail in LRRK2 deficiency, highlighting an essential role of LRRK2 in the phosphorylation of Rab10, a RabGTPase required in the PI3/Akt signaling pathway for the insulin-induced translocation of GLUT 4 (Funk et al., 2019).
Dysregulated Pathways in Type 2 Diabetes Mellitus and Parkinson’s Disease
Experimental pieces of evidence show pathways intersecting between T2DM and PD (Santiago and Potashkin, 2013). Hyperglycemia in diabetes appears to be the unifying mechanism inducing dysregulation of pathways essential for cell life (Bronwlee, 2001). Excess of the intracellular glucose saturates the polyol pathway, lowering the reduced form of glutathione (GSH) and consequently increasing the cell susceptibility to oxidative stress. Increased oxidative stress, due to overproduction of reactive species of oxygen (ROS) in dopaminergic neurons, with a low level of GSH, is also described as a possible mechanism of neurodegeneration in the pathogenesis of PD (Lang and Lozano, 1999). Hyperglycemia causes mitochondrial superoxide overproduction with an increased formation of advanced glycation end products (AGEs), modifying circulating proteins that, bound to AGE receptors, trigger the production of inflammatory cytokines (Bronwlee, 2005; Giacco and Bronwlee, 2010). Neuroinflammation is involved in the neurodegeneration pathways (Kalia and Lang, 2015) and glycation is considered an unavoidable age-associated process that might play a role in PD. The levels of AGEs were found to be increased in the brains of patients suffering from synucleinopathy (Dalfo et al., 2005) and glycation of a-syn enhanced its toxicity, in vitro and in vivo, in Drosophila and mice (Miranda et al., 2017). Moreover, methylglyoxal, a highly reactive glycating agent was described to show chemical similarity with DA oxidation products, highly toxic for dopaminergic neurons when they are not physiologically removed (Biosa et al., 2018). The formation of toxic aggregates of the islet amyloid polypeptide (IAPP) in diabetes has been observed to contribute to the β-cell dysfunction, thus T2DM can be included in the group of protein misfolding disorders, together with PD and other neurodegenerative diseases. Such as in PD, also in T2DM the alterations of the autophagy, the major clearance mechanism for lysosomal degradation of damaged proteins, lead to the accumulation of protein aggregates which results in cytotoxicity (Mukherjee et al., 2016).
Antidiabetic Drugs in Parkinson’s Disease
Tolbutamide
Already back in 1960, Gates and Hyman (1960) reported an open-label treatment of 15 parkinsonian patients with tolbutamide, a drug belonging to the sulfonylurea class of insulin secretagogues, which act by stimulating pancreatic cells to release insulin. This compound was able to markedly improve tremor and rigidity in 12 of the patients. A similar study, with analogous conclusions, was reported by Barbeau et al. (1961).
Glitazones
More recently, different studies analyzed the effects of glitazones (GTZ) on PD pathology, targeting the inflammatory process presumably involved in neurodegeneration. GTZ are synthetic ligands of the thiazolidinedione class, used as antidiabetic agents by their insulin-sensitizing effects, decreasing insulin resistance and hyperglycemia (Nolan et al., 1994). Such drugs are agonists of peroxisome proliferator-activated receptor-γ (PPAR-γ), a member of the nuclear receptor superfamily that regulates carbohydrate and lipid metabolism (Delerive et al., 2001). In the brain, PPAR-γ displays a regional distribution, high levels being expressed in the piriform cortex, olfactory tubercle, cerebellar cortex, microglia, astrocytes, oligodendrocytes, and neurons (Moreno et al., 2004; Cimini et al., 2005; Bernardo and Minghetti, 2008). In dopaminergic areas, co-localization with TH-positive neurons has been reported in the SNpc and the VTA (Sarruf et al., 2009; Carta et al., 2011). Although the issue of neuroinflammation as cause or consequence in PD pathophysiology remains controversial, an increasing amount of evidence strongly supports the active involvement of reactive microglia in the progression of neurodegeneration and suggests the possibility of targeting microglial response in the treatment of PD (Hirsch and Hunot, 2009; Long-Smith et al., 2009). In vivo, PPAR-γ agonists have been shown to modulate inflammatory responses in the brain (Heneka et al., 2000). Neuroprotective effects were demonstrated in different animal models of PD, with different mechanisms in each of them. Barbiero et al. (2014) showed that pioglitazone prevented the degeneration of dopaminergic neurons in SNpc in the rat MPTP-model of PD and the decrease of DA levels in the striatum, protecting against hypolocomotion, depressive-like behavior, and memory impairment. The specific effect of pioglitazone on neuroinflammation was demonstrated in an animal model of PD (Pinto et al., 2016), in which dopaminergic neuron loss has been induced by a mitochondrial respiratory defect. In this case, I-DOPA treatment triggered atypical motor behavior, without preventing any neurodegeneration, but after the pioglitazone treatment, it was observed an improvement of motor symptoms, with a specific reduction of both microglial cell number in the midbrain and microglial activation in the midbrain and striatum. The action of pioglitazone on neuroinflammation was recently confirmed in a 6-OHDA animal model of PD, in which it was able to attenuate microglial activation, with increased survival of neurons in the hippocampus in rats with nigral lesions, along with a supposed antidepressant-like effect (Bonato et al., 2018).
Despite the numerous experimental pieces of evidence, similar pioglitazone effects were not replicated in PD patients. A clinical multi-center, double-blind, randomized trial assessed the pioglitazone effects in 210 participants with early PD, during 44 weeks of pioglitazone treatment associated with monoamine oxidase type B inhibitors. Pioglitazone did not demonstrate to slow the progression of motor impairment in early PD (NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators, 2015). Cohort studies also showed contradictory results concerning a supposed positive effect of pioglitazone on PD progression (Brauer et al., 2015; Connolly et al., 2015). Finally, a long-term retrospective study, performed on the Norwegian Prescription Database, analyzed the GTZ and metformin prescriptions during a 10-year period, to investigate the putative inverse association between GTZ use and PD occurrence. GTZ use was indeed associated with a decreased risk of developing PD, compared with metformin use in this nationwide study analyzing a T2DM population (Brakedal et al., 2017).
Metformin
Metformin represents the first-choice oral drug for T2DM. It is a biguanide that improves insulin sensitivity with an increased skeletal myocyte glucose uptake and decreased hepatic glucose production (Bailey, 2017). In a large cohort study, the incident PD risk was analyzed in Taiwanese patients with T2DM taking metformin and/or the secretagogue sulfonylurea. Such an analysis confirmed T2DM as a risk factor for PD, which appeared doubled in the enrolled diabetic patients not taking oral antidiabetic agents, but that risk was significantly reduced in patients receiving metformin treatment. Sulfonylurea therapy increased PD risk, but this was reduced by the sulfonylureas association with metformin (Wahlqvist et al., 2012). A recent study on older adult United States veterans with T2DM evaluated the association between the length of metformin exposure and PD, showing that the risk reduction of this neurodegenerative disease seems to be associated with long-term metformin therapy (Shi et al., 2019). Speculating on the other possible mechanisms of action by metformin, this compound appears to activate proteins involved in mitochondrial function, restoring mitochondrial biogenesis and mitochondrial membrane potential, whose alterations are crucial elements of neurodegeneration in PD (Fitzgerald et al., 2017).
The more appealing function of metformin as a possible explanation of its neuroprotective role is its action on the gut hormone glucagon-like peptide 1 (GLP-1). Metformin sensitizes the cells to GLP-1 through PPAR-γ, which increases the circulating levels of GLP-1 and, consequently, helps to control blood glucose (Mulherin et al., 2011).
GLP-1 receptor agonists
GLP-1 is one of the two gut hormones, produced in the enteroendocrine cell of the small intestine that stimulates insulin secretion from the pancreas in response to food (Drucker, 2006; Muller et al., 2019). Named also incretin, by its capacity to potentiate insulin plasma levels after oral administration of glucose compared to intravenous glucose, its action is not restricted to the glucose homeostasis. GLP-1 was observed in vitro to enhance cell survival and inhibit apoptosis (Lovshin and Drucker, 2009). Such effects are mediated by binding to the GLP-1 receptor (GLP-1R), with the subsequent activation of PI3K, phosphorylating and activating different downstream signaling pathways. GLP-1Rs are expressed not only in pancreatic islet cells and peripheral tissues such as the heart, kidney, lungs but also in the frontal cortex, thalamus, hypothalamus, hippocampus, and substantia nigra (Alvarez et al., 2005), in both astrocytes and microglia, in which antioxidative and anti-apoptotic effects by treatment with GLP-1 were observed (Spielman et al., 2017). Turton et al. (1996) demonstrated the presence of an endogenous GLP-1 in the brain able to reduce food intake. The GLP-1R expression was investigated in the hippocampus, analyzing the possible action in learning and memory. It was found that mice lacking GLP-1R had learning deficits (During et al., 2003) and GLP-1 administration restored the spatial learning and memory performance, which were blocked by GLP-1 antagonist (Gault and Hölscher, 2008).
GLP-1R agonists are currently a common treatment for T2DM. Exenatide is the first GLP-1 analog approved by FDA, in 2005. It is a synthetic copy of the exendin-4 (Ex-4), the hormone found in the saliva of the venomous lizard Gila Monster (Heloderma suspectum), resistant to degradation by the circulating dipeptidyl peptidase IV (DPP-IV), which rapidly inactivates GLP-1 in humans. Ex-4 showed the feature of a neurotrophic factor inducing neurite outgrowth in in vitro model of neuronal differentiation and was able to rescue degenerating cells after NGF-mediated withdrawal (Perry et al., 2002).
A previous cell culture study demonstrated the protective effect of GLP-1 onto dopaminergic neurons against hypoxia and 6-OHDA-induced toxicity, while Ex-4 treatment elevated TH levels in those neurons. In the same study, Ex-4 prevented MPTP animal models of PD from neurodegeneration, showing improved motor function in mice (Li et al., 2009). The same protective action by GLP-1 was observed in the 6-OHDA rat animal model of PD (Jalewa et al., 2017). The compelling pieces of evidence of neuroprotective and neurotrophic actions of GLP-1 agonists led to initiate clinical trials on parkinsonian patients. An initial proof of concept with a single-blind trial design evaluated 45 patients with moderate PD who received subcutaneous exenatide injection for 12 months. The treatment resulted in significant and clinically relevant improvements in the motor and cognitive measures (Aviles-Olmos et al., 2013). A subsequent double-blind, placebo-controlled study from the same group confirmed the efficacy of this antidiabetic therapy in ameliorating motor symptom severity in PD patients (Athauda et al., 2014), opening an intense discussion about the development of this novel therapeutic strategy, also as a potential disease-modifying treatment (Simuni and Brundin, 2014; Athauda et al., 2017; Jankovic, 2017).
A post hoc analysis of the double-blind trial evaluating the exenatide effect was then performed to determine possible predictors of the motor response to exenatide. The possible changes in motor severity, non-motor symptoms, cognition, and quality of life after 48-week treatment with exenatide were evaluated among post-hoc subgroups defined by age, motor phenotype, disease duration, disease severity, body mass index, and insulin resistance. Results showed that patients with older age of onset and disease duration over 10 years responded less well to exenatide treatment, supporting the concept of recruiting patients at earlier disease stages in future clinical trials of GLP-1R agonists in PD (Athauda et al., 2019). Whether exenatide affects the underlying disease pathophysiology or simply induces long-lasting symptomatic effects remains uncertain. Clinical trials on the efficacy of liraglutide, a newer GLP-1 analogue, in PD are also ongoing. (ClinicalTrials.gov Identifier: NCT02953665) (Table 3).
Table 3.
Trials with antidiabetic drugs in PD
| Compound | Authors | Study design | Results |
|---|---|---|---|
| Glitazone | Brauer et al., 2015 | Retrospective cohort study | Prescription of glitazone was associated with a lower incidence of PD |
| NINDS Exploratory Trials in Parkinson’s Disease (Net-PD) FS-ZONE Investigators, 2015 | Phase 2, Multicentre, Double-Blind, Randomised Trial | Pioglitazone did not result to slow the motor impair progression in early PD | |
| Connolly et al., 2015 | Cohort study | The use of glitazone might have a therapeutic benefit in slowing the progression of the disease | |
| Brakedal et al., 2017 | Retrospective study | The use of glitazones is associated with a decreased risk of incident PD in populations with diabetes | |
| Metformin | Wahlqvist et al., 2012 | Cohort study | Incident PD risk in T2DM increases 2.2-fold. Sulfonylureas further increase risk by 57%, which is avoided by combination with metformin |
| Shi et al., 2019 | Retrospective longitudinal cohort study | Long-term metformin therapy (> 2 years) was associated with a lower incidence of PD among elderly veterans with T2DM | |
| Exendin-4 | Aviles-Olmos et al., 2013 | Single-blind clinical trial | Improvements of the motor and cognitive measures in PD patients |
| Athauda et al., 2014 | Double-blind, placebo-controlled clinical trial | Confirmation of the efficacy of the Exendin-4 | |
| Athauda et al., 2019 | Post-hoc analysis of the double-blind trial | Efficacy of Exendin-4 treatment is higher in patients with earlier disease | |
| Liraglutide | Clinical Trial not yet completed | ClinicalTrials.gov Identifier: NCT02953665 |
PD: Parkinson’s disease; T2DM: type 2 diabetes mellitus.
Antiparkinsonian Drugs in Type 2 Diabetes Mellitus
A controlled-release bromocriptine formulation (Cycloset), a DA D2 receptor agonist, has been approved by the FDA for the treatment of T2DM in 2009. The action of bromocriptine in T2DM involves the enhancement of morning hypothalamic dopaminergic activity, resulting in improved insulin sensitivity and reduced plasma glucose, triglyceride, and free fatty acid levels.
The mechanism of bromocriptine action has been inferred from animal studies. Mammalian wild species change their metabolism according to the season, developing insulin resistance when food is scarce and surviving by increasing lipolytic activity, fat oxidation, hepatic glucose production, and gluconeogenesis to supply glucose in the CNS. Such insulin resistance reverts when the food becomes available again. The seasonal metabolic changes are regulated by endogenous dopaminergic and serotoninergic action in SCN and ventromedial nucleus of hypothalamus (VMH), which plays a pivotal role in modulating autonomic nervous system function, hormonal secretion, peripheral glucose/lipid metabolism, and feeding behavior. DA levels are low during the insulin-resistant state and increase in the insulin-sensitive state (De Fronzo, 2011). Type 2 diabetic state appears to mimic the seasonal insulin-resistant state observed in mammalians. Death of dopaminergic neurons in the CNS produces insulin resistance (Luo et al., 1997), and animal models of obesity show reduced DA levels in VMH and lateral hypothalamic nuclei (Oltmans, 1983). Systemic and intracerebral bromocriptine administration in insulin-resistant animals improved insulin sensitivity, reducing lipolysis and hepatic glucose production and gluconeogenesis (Luo et al., 1999). Type 2 diabetic individuals seem to have an early morning lowering of dopaminergic tone. Several human trials showed the efficacy of dopaminergic therapy, resulting in improved glycemic control above all in those subjects whose glycemia was poorly controlled by classic antidiabetic drugs such as metformin (Cincotta et al., 1996; Schwartz et al., 1996; Kamath et al., 1997). A just-released meta-analysis and sequential analysis of randomized clinical trials on the bromocriptine and cabergoline effects in T2DM patients showed reduced glycated hemoglobin (HbA1c), fasting plasma glucose, and triglycerides in diabetic patients without causing serious adverse effects (Andersen et al., 2021).
Conclusion
A considerable amount of studies has analyzed the intersecting signaling pathways between DA and insulin, showing the role of the former as a metabolic regulator and the latter as a neuromodulator. Conflicting data come from experimental studies and clinical trials in humans, leaving still undisclosed whether and when this functional link becomes a dangerous liaison. However, increasing pieces of evidence support the relationship between T2DM and PD and the crucial role that insulin and DA exert to each other, possibly opening a new possibility to treat the two diseases.
Acknowledgments:
We are grateful to Dr. Alberto Anderle for drawing the figure.
Footnotes
Conflicts of interest: None of the authors have financial conflicts of interests to report in association with the contents of this paper.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Song LP; T-Editor: Jia Y
References
- 1.Abdesalam RM, Safar MM. Neuroprotective effects of vildagliptin in rat rotenone Parkinson’s disease model: role of RAGE-NFκB and Nrf2-antioxidant signaling pathways. J Neurochem. 2015;133:700–707. doi: 10.1111/jnc.13087. [DOI] [PubMed] [Google Scholar]
- 2.Albeely AM, Ryan SD, Perreault ML. Pathogenic feed-forward mechanism in Alzheimer’s and Parkinson’s disease converge on GSK-3. Brain Plast. 2018;26:151–167. doi: 10.3233/BPL-180078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alquier T, Leloup CA, Lorsignol-Desmet A, Penicaud L. Translocable glucose transporters in the brain: where are in 2006. Diabetes. 2006;55:S131-138. [Google Scholar]
- 4.Alvarez E Martinez MD, Roncero I, Chowen JA, Garcia-Cuartero B, Gispert JD, Sanz C, Vàsquez P, Maldonado A, de Càceres J, Desco M, Pozo MA, Blàsquez E. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem. 2005;92:798–806. doi: 10.1111/j.1471-4159.2004.02914.x. [DOI] [PubMed] [Google Scholar]
- 5.Andersen IB, Andreassen M, Jesper Krogh The effect of dopamine agonists on metabolic variables in adults with type 2 diabetes: a systematic review with meta-analysis and trial sequential analysis of randomized clinical trials. Diabetes Obes Metab. 2021;23:58–67. doi: 10.1111/dom.14183. [DOI] [PubMed] [Google Scholar]
- 6.Anlauf M, Eissele R, Schäfer MK, Eiden LE, Arnold R, Pauser U, Klöppel G, Weihe E. Expression of the two isoforms of the vesicular monoamine transporter (VMAT1 and VMAT2) in the endocrine pancreas and pancreatic endocrine tumors. J Histochem Cytochem. 2003;51:1027–1040. doi: 10.1177/002215540305100806. [DOI] [PubMed] [Google Scholar]
- 7.Askanas V, Engel WK, Alvarez RB, McFerrin J, Broccolini A. Novel immunolocalization of alpha-synuclein in human muscle of inclusion-body myositis, regenerating and necrotic muscle fibers, and at neuromuscular junctions. J Neuropathol Exp Neurol. 2000;59:592 598. doi: 10.1093/jnen/59.7.592. [DOI] [PubMed] [Google Scholar]
- 8.Aslan IR, Ranadive SA, Valle I, Kollipara S, Noble JA, Vaisse C. The melanocortin system and insulin resistance in humans: insights from a patient with complete POMC deficiency and type 1 diabetes mellitus. Int J Obes. 2014;38:148–151. doi: 10.1038/ijo.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Athauda D, Maclagan K, Budnik N, Zampedri L, Hibberts S, Aviles-Olmos I, Chowdhury K, Skene SS, Limousin P, Foltynie T. Post hoc analysis of the exenatide-PD trial-factors that predict response. Eur J Neurosci. 2019;49:410–421. doi: 10.1111/ejn.14096. [DOI] [PubMed] [Google Scholar]
- 10.Athauda D, Maclagan K, Skene SS, Bajwa JM, Letchford DD, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson J, Li Y, Aviles-Olmos I, Warner TT, Limousin P, Lees AJ, Greig NH, Tebbs S, Foltynie T. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind , placebo-controlled trial. Lancet. 2017;390:1664–1675. doi: 10.1016/S0140-6736(17)31585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whiton P, Wyse R, Isaacs T, Lees A, Limousin P, Foltynie T. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123:2730–2736. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badawi GA, Abd El Fattah MA, Zaki HF, El Sayed MI. Sitagliptin and Liraglutide modulate L-dopa effect and attenuate dyskinetic movements in rotenone-lesioned rats. Neurotox Res. 2019;35:635–653. doi: 10.1007/s12640-019-9998-3. [DOI] [PubMed] [Google Scholar]
- 13.Bailey C. Metformin: historical overview. Diabetologia. 2017;60:1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 14.Baltic S, Perovic M, Mladenovic A, Raicevic N, Ruzdijic S, Rakic L, Kanazir S. Alpha-synuclein is expressed in different tissues during human fetal development. J Mol Neurosci. 2004;22:199–204. doi: 10.1385/jmn:22:3:199. [DOI] [PubMed] [Google Scholar]
- 15.Barbeau A. Experience clinique avec tolbutamide dans le maladie de Parkinson. Union Medicale Canada. 1961;90:147–151. [PubMed] [Google Scholar]
- 16.Barbiero JK, Santiago RM, Persike DS, Silva Fernandes MJ, Tonin FS, da Cunha C, Boschen SL, Lima MM, Vital MA. Neuroprotective effects of peroxisome proliferator-activated receptor alpha and gamma agonists in model of parkinsonism induced by intranigral 1-methyl-4-phenyl-1, 2 , 3, 6-tetrahydropyridine. Behav Brain Res. 2014;274:390–399. doi: 10.1016/j.bbr.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, Fox M, Goldestien JM, Soriano F, Seubert D, Chilcote TJ. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis. 2008;5:55–59. doi: 10.1159/000112832. [DOI] [PubMed] [Google Scholar]
- 18.Bayliss JA, Lemus MB, Santos VV, Deo M, Davies JS, Kemp BE, Elsworth JD, Andrews ZB. Metformin prevents nigrostriatal dopamine degeneration independent of ampk activation in dopamine neurons. PLoS One. 2016;11:e0159381. doi: 10.1371/journal.pone.0159381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bendor JT, Logan TP, Edwards RH. The function of α-synuclein. Neuron. 2013;79:1044–1066. doi: 10.1016/j.neuron.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernardo A, Minghetti L. Regulation of glial cell functions by PPAR-γ natural and synthetic agonists. PPAR Res. 2008;2008:864140. doi: 10.1155/2008/864140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bingham EM, Hopkins D, Smith D, Pernet A, Hallett W, Reed L, Marsden PK, Amiel SA. The role of insulin in human brain glucose metabolism: an 18-fluoro-deoxyglucose positron emission tomography study. Diabetes. 2002;51:3384–3390. doi: 10.2337/diabetes.51.12.3384. [DOI] [PubMed] [Google Scholar]
- 22.Bini J, Naganawa M, Nabulsi N, Huang Y, Ropchan J, Lim K, Najafzadeh S, Herold KC, Cline GW, Carson RE. Evaluation of PET brain radioligands for imaging pancreatic β-cell mass: potential utility of 11C-(+)-PHNO. J Nucl Med. 2018;59:1249–1254. doi: 10.2967/jnumed.117.197285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bini J, Sanchez-Rangel E, Gallezot JD, Naganawa M, Nabulsi N, Lim K, Najafzadeh S, Shirali A, Ropchan J, Matuskey D, Huang Y, Herold KC, Harris PE, Sherwin RS, Carson RE, Cline GW. PET imaging of pancreatic dopamine D2 and D3 receptor density with 11C-(+)-PHNO in type 1 diabetes. J Nucl Med. 2020;61:570–576. doi: 10.2967/jnumed.119.234013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biosa A, Outeiro TF, Bubacco L, Bisaglia M. Diabetes mellitus as a risk factor for Parkinson’s disease: a molecular point of view. Mol Neurobiol. 2018;55:8754–8763. doi: 10.1007/s12035-018-1025-9. [DOI] [PubMed] [Google Scholar]
- 25.Bonato JM, Bassani TB, Milani H, Vital MABF, de Oliveira RMW. Pioglitazone reduces mortality, prevents depressive-like behavior, and impacts hippocampal neurogenesis in the 6-OHDA model of Parkinson’s disease in rats. Exp Neurol. 2018;300:188–200. doi: 10.1016/j.expneurol.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Borelli MI, Rubio M, García ME, Flores LE, Gagliardino JJ. Tyrosine hydroxylase activity in the endocrine pancreas: changes induced by short-term dietary manipulation. BMC Endocr Disord. 2003;3:2. doi: 10.1186/1472-6823-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Born J, Lange T, Kern W, Mc Gregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 28.Bosco D, Plastino M, Cristiano D, Colica C, Ermio C, De Bartolo M, Mungari P, Fonte G, Consoli D, Consoli A, Fava A. Dementia is associated with insulin resistance in patients with Parkinson’s disease. J Neurol Sci. 2012;315:39–43. doi: 10.1016/j.jns.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Boyd A, Lebovitz HE, Feldman JM. Endocrine function and glucose metabolism in patients with Parkinson’s Disease and their alteration by L-Dopa. J Clin Endocrinol Metab. 1971;33:829–837. doi: 10.1210/jcem-33-5-829. [DOI] [PubMed] [Google Scholar]
- 30.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Dis. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 31.Brakedal B, Flønes I, Reiter FS, Torkildsen Ø, Dölle C, Assmus J, Haurgarvoll K, Tzoulis C. Glitazone use associated with reduced risk of Parkinson’s disease. Mov Disord. 2017;32:1564–1599. doi: 10.1002/mds.27128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brauer R, Bhaskaran K, Chaturvedi N, Dexter DT, Smeeth L, Douglas I. Glitazone treatment and incidence of Parkinson’s disease among people with diabetes: a retrospective cohort study. PLoS Med. 2015;12:e1001854. doi: 10.1371/journal.pmed.1001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bronwlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 34.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 35.Carta AR, Pisanu A, Carboni E. Do PPAR-gamma agonists have a future in Parkinson’s disease therapy? Parkinson Dis. 2011 doi: 10.4061/2011/689181. Dis doi:10.4061/2011/689181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cegrell L. The occurrence of biogenic monoamines in the mammalian endocrine pancreas. Acta Physiol Scand Supplementum. 1968;314:5–60. [PubMed] [Google Scholar]
- 37.Cereda E, Barichella M, Cassani E, Caccialanza R, Pezzoli G. Clinical features of Parkinson’s disease when onset of diabetes came first. Neurology. 2012;78:1507–1511. doi: 10.1212/WNL.0b013e3182553cc9. [DOI] [PubMed] [Google Scholar]
- 38.Cereda E, Barichella M, Pedrolli C, Klersyc C, Cassani E, Caccialanza R, Pezzoli G. Diabetes and risk of Parkinson’s disease: a systematic review and meta-analysis. Diabetes Care. 2011;34:2614–2623. doi: 10.2337/dc11-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu PC, Lin MT, Shian LR, Leu SY. Alterations in physiologic functions and in brain monoamine content in streptozocin-diabetic rats. Diabetes. 1986;35:481–485. doi: 10.2337/diab.35.4.481. [DOI] [PubMed] [Google Scholar]
- 40.Chung JY, Lee SJ, Lee SH, Jung YS, Ha NC, Seol W, Park BJ. Direct interaction of α-synuclein and AKT regulates IGF-1 signaling: implication of Parkinson disease. Neurosignals. 2011;19:86–96. doi: 10.1159/000325028. [DOI] [PubMed] [Google Scholar]
- 41.Chung SJ, Jeon S, Yoo HS, Kim G, Oh JS, Kim JS, Evans AC, Sohn YH, Lee PH. Detrimental effect of type 2 diabetes mellitus in a large case series of Parkinson’s disease. Parkinsonism Relat Disord. 2019;64:54–59. doi: 10.1016/j.parkreldis.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 42.Cimini A, Benedetti E, Cristiano L, Sebastiani P, D’Amico MA, D’Angelo B, Di Loreto S. Expression of peroxisome proliferator-activated receptors (PPARs) and retinoic acid receptors (RXRs) in rat cortical neurons. Neuroscience. 2005;130:325–337. doi: 10.1016/j.neuroscience.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 43.Cincotta AH, Meier AH. Bromocriptine (Ergoset) reduces body weight and improves glucose tolerance in obese subjects. Diabetes Care. 1996;19:667–670. doi: 10.2337/diacare.19.6.667. [DOI] [PubMed] [Google Scholar]
- 44.Clarke DW, Mudd L, Boyd FT jr, Fields M, Raizada MK. Insulin is released from rat brain neuronal cells in culture. J Neurochem. 1986;47:831–836. doi: 10.1111/j.1471-4159.1986.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 45.Connolly JG, Bykov K, Gagne J. Thiazolidinediones and Parkinson disease: a cohort study. Am J Epidemiol. 2015;182:936–944. doi: 10.1093/aje/kwv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dalfo E, Portero-Otín M, Ayala V, Martinez A, Pamplona R, Ferrer I. Evidence of oxidative stress in the neocortex in incidental Lewy body disease. J Neuropathol Exp Neurol. 2005;64:816–830. doi: 10.1097/01.jnen.0000179050.54522.5a. [DOI] [PubMed] [Google Scholar]
- 47.D’Amelio M, Ragonese P, Callari G, Di Benedetto N, Palmeri B, Terruso V, Salemi G, Famoso G, Aridon P, Savettieri G. Diabetes preceding Parkinson’s disease onset. A case-control study. Parkinsonism Relat Disord. 2009;15:660–664. doi: 10.1016/j.parkreldis.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 48.De Fronzo RA. Bromocriptine: a sympatholytic, D2-dopamine agonist for the treatment of type 2 diabetes. Diabetes Care. 2011;34:789–794. doi: 10.2337/dc11-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Leeuw van Weenen JE, Parlevliet ET, Maechler P, Havekes LM, Romijn JA, Ouwens DM, Pijl H, Guigas B. The dopamine receptor D2 agonist bromocriptine inhibits glucose-stimulated insulin secretion by direct activation of the alpha2-adrenergic receptors in beta cells. Biochem Pharmacol. 2010;79:1827–1836. doi: 10.1016/j.bcp.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 50.De Pablo-Fernandez E, Goldacre R, Pakpoor J, Noyce AJ, Warner T. Association between diabetes and subsequent Parkinson disease. Neurology. 2018;91:139–142. doi: 10.1212/WNL.0000000000005771. [DOI] [PubMed] [Google Scholar]
- 51.De Pablo-Fernandez E, Sierra-Hidalgo F, Benito-Leon J, Bermejo-Pareja F. Association between Parkinson’s disease and diabetes: Data from NEDICES study. Acta Neurol Scand. 2017;136:732–736. doi: 10.1111/ane.12793. [DOI] [PubMed] [Google Scholar]
- 52.Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol. 2001;169:453–459. doi: 10.1677/joe.0.1690453. [DOI] [PubMed] [Google Scholar]
- 53.Devaskar SU, Giddings SJ, Rajakumar PA, Carnaghi LR, Menon RK, Zahm DS. Insulin gene expression and insulin synthesis in mammalian neuronal cells. J Biol Chem. 1994;269:8445–8454. [PubMed] [Google Scholar]
- 54.Dorn A, Rinne A, Hahn HJ, Bernstein HG, Ziegler M. C-peptide immunoreactive neurons in human brain. Acta Histochem. 1982;70:326–330. doi: 10.1016/S0065-1281(82)80080-9. [DOI] [PubMed] [Google Scholar]
- 55.Driver JA, Smith A, Buring JE, Gaziano JM, Kurth T, Logroscino G. Prospective cohort study of type 2 diabetes and the risk of Parkinson disease. Diabetes Care. 2008;31:2003–2005. doi: 10.2337/dc08-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 57.Duarte AI, Santos P, Oliveira CR, Santos MS, Rego CA. Insulin neuroprotection against oxidative stress is mediated by Akt and GSK-3β signaling pathways and changes in protein expression. BBA Mol Res. 2008;1783:994–1002. doi: 10.1016/j.bbamcr.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 58.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev. 1998;19:608–624. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 59.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 60.Dyer AH, Vahdatpour C, Sanfeliu A, Tropea D. The role of insulin-like growth factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience. 2016;325:89–99. doi: 10.1016/j.neuroscience.2016.03.056. [DOI] [PubMed] [Google Scholar]
- 61.Eisenhofer G, Aneman A, Friberg P, Hooper D, Fåndriks L, Lonroth H, Hunyady B, Mezey E. Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab. 1997;82:3864–3871. doi: 10.1210/jcem.82.11.4339. [DOI] [PubMed] [Google Scholar]
- 62.Ericson LE, Häkanson R, Lundquist I. Accumulation of dopamine in mouse pancreatic B-cells following injection of L-Dopa. Localization to secretory granules and inhibition of insulin secretion. Diabetologia. 1977;13:117–124. doi: 10.1007/BF00745138. [DOI] [PubMed] [Google Scholar]
- 63.Farino ZJ, Morgenstern TJ, Maffei A, Quick M, De Solis AJ, Wiriyasermkul P, Freyberg RJ, Aslanoglou D, Sorisio D, Inbar BP, Free RB, Donthamsetti P, Mosharov EV, Kellendonk C, Schwartz GJ, Sibley DR, Schmauss C, Zeltser LM, Moore H, Harris PE, Javitch JA, Frey-berg Z. New roles for dopamine D2 and D3 receptors in pancreatic beta cell insulin secretion. Mol Psychiatry. 2020;25:2070–2085. doi: 10.1038/s41380-018-0344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Falck B, Hellmann B. Evidence for the presence of biogenic amines in pancreatic islets. Experientia. 1963;19:139–140. [Google Scholar]
- 65.Feldman JM, Boyd AE, Lebovitz HE. Structural determinants of catecholamine action on in vitro insulin release. J Pharmacol Exper Ther. 1970;176:611–621. [PubMed] [Google Scholar]
- 66.Feng P, Zhang X, Li D, Ji C, Yuan Z, Wang R, Xue G, Li G, Hölscher C. Two novel dual GLP-1/GIP receptor agonists are neuroprotective in the MPTP mouse model of Parkinson’s disease. Neuropharmacology. 2018;133:385–394. doi: 10.1016/j.neuropharm.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 67.Fernandez AM, Hernandez-Garzón E, Perez-Domper P, Perez-Alvarez A, Mederos S, Matsui T, Santi A, Trueba-Saiz A, García-Guerra L, Pose-Utrilla J, Fielitz J, Olson EN, Fernandez de la Rosa R, Garcia Garcia L, Pozo MA, Iglesias T, Araque A, Soya H, Perea G, Martin ED, et al. Insulin regulates astrocytic glucose handling through cooperation with IGF-I. Diabetes. 2017;66:64–74. doi: 10.2337/db16-0861. [DOI] [PubMed] [Google Scholar]
- 68.Fernandez AM, Torres-Alemán I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13:225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- 69.Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav. 2006;89:611–616. doi: 10.1016/j.physbeh.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 70.Fitzgerald JC, Zimprich A, Carvajal Berrio DA, Schindler KM, Maurer B, Schulte C, Bus C, Hauser AK, Kübler M, Lewin R, Bobbili DR, Schwarz LM, Vartholomaiou E, Brockmann K, Wüst R, Madlung J, Nordheim A, Riess O, Martins LM, Glaab E, et al. Metformin reverses TRAP1 mutation-associated alterations in mitochondrial function in Parkinson’s disease. Brain. 2017;140:2444–2459. doi: 10.1093/brain/awx202. [DOI] [PubMed] [Google Scholar]
- 71.Funk N, Munz M, Ott T, Brockmann K, Wenninger-Weinzierl A, Kühn R, Vogt-Weisenhorn , Giesert F, Wurst W, Gasser T, Biskup S. The Parkinson’s disease-linked Leucine-rich repeat kinase 2 (LRRK2) is required for insulin stimulate translocation of GLUT4. Sci Rep. 2019;9:4515–4531. doi: 10.1038/s41598-019-40808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao S, Duan C, Gao G, Wang X, Yang H. Alpha-synuclein expression negatively regulates insulin receptor substrate 1 by activating mTORC1/S6K1 signaling. Int J Biochem Cell. 2015;64:25–33. doi: 10.1016/j.biocel.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 73.Garcia Barrado MJ, Iglesias Osma MC, Blanco EJ, Carretero Hernández M, Sánchez Robledo V, Catalano Iniesta L, Carrero S, Carretero J. Dopamine modulates insulin release and is involved in the survival of rat pancreatic Beta cells. PLoS One. 2015;10:e0123197. doi: 10.1371/journal.pone.0123197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gates EW, Hyman I. Use of tolbutamide in paralysis agitans. J Am Med Assoc. 1960;172:1351–1354. doi: 10.1001/jama.1960.03020130009003. [DOI] [PubMed] [Google Scholar]
- 75.Gault VA, Hölscher C. GLP-1 agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta-amyloid. Eur J Pharmacol. 2008;587:112–117. doi: 10.1016/j.ejphar.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 76.Geng X, Haiyan L, Wang J, Lehong L, Swanson AL, Sun M, Beers-Stolz D, Watkins S, Perez RG, Drain P. α-Synuclein binds the KATP channel at insulin-secretory granules and inhibits insulin secretion. Am J Physiol Endocrinol Metab. 2011;300:E276–286. doi: 10.1152/ajpendo.00262.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giguere R, Hardy J. Experience clinique avec le tolbutamide dans la malladie de Parkinson. Union Med Can. 1961;90:147–151. [PubMed] [Google Scholar]
- 79.Giuntini M, Baldacci F, Del Prete E, Bonuccelli U, Ceravolo R. Diabetes is associated with postural and cognitive domains in Parkinson’s disease. Results from a single-center study. Parkinsonism Relat Disord. 2014;20:671–672. doi: 10.1016/j.parkreldis.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 80.Godau J, Herfurth M, Kattner B, Gasser T, Berg D. Increased serum insulin-like growth factor 1 in early idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2010;81:536–538. doi: 10.1136/jnnp.2009.175752. [DOI] [PubMed] [Google Scholar]
- 81.Godau J, Knauel K, Weber K, Brockmann K, Maetzler W, Binder G, Berg D. Serum insulin-like growth factor 1 as possible marker for risk and early diagnosis of Parkinson disease. Arch Neurol. 2011;68:925–931. doi: 10.1001/archneurol.2011.129. [DOI] [PubMed] [Google Scholar]
- 82.Goldestein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharmacol Exp Ther. 2003;305:800–811. doi: 10.1124/jpet.103.049270. [DOI] [PubMed] [Google Scholar]
- 83.Goldestein DS, Swoboda KJ, Miles JM, Coppack SW, Aneman A, Holems C, Lamensdorf I, Eisenhofer G. Sources and physiological significance of plasma dopamine sulfate. J Clin Endocrinol Metab. 1999;84:2523–2531. doi: 10.1210/jcem.84.7.5864. [DOI] [PubMed] [Google Scholar]
- 84.Grillo CA, Piroli GG, Hendry RM, Reagan LP. Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Res. 2009;1296:35–45. doi: 10.1016/j.brainres.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guthoff M, Grichish Y, Canova C, Tschritter O, Veit R, Hallschmid M, Häring HU, Preissl H, Hennige Am, Fritsche A. Insulin modulates food-related activity in the central nervous system. J Clin Endocrinol Metab. 2010;95:748–755. doi: 10.1210/jc.2009-1677. [DOI] [PubMed] [Google Scholar]
- 86.Hakanson R, Lundquist I, Rerup C. On the hyperglycemic effect of dopa and dopamine. Eur J Pharmacol. 1967;1:114–119. doi: 10.1016/0014-2999(67)90047-7. [DOI] [PubMed] [Google Scholar]
- 87.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- 88.Heneka MT, Klockgether T, Feinstein DL. Peroxisome proliferator-activated receptor-γ ligands reduce neuronal inducible nitric oxide synthase expression and cell death in vivo. J Neurosci. 2000;20:6862–6867. doi: 10.1523/JNEUROSCI.20-18-06862.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heni M, Kullmann S, Ketterer C, Guthoff M, Linder K, Wagner R, Stingl KT, Veit R, Staiger H, Häring HU, Preissl H, Fritsch A. Nasal insulin changes peripheral insulin sensitivity simultaneously with altered activity in homeostatic and reward-related human brain regions. Diabetologia. 2012;55:1773–1782. doi: 10.1007/s00125-012-2528-y. [DOI] [PubMed] [Google Scholar]
- 90.Heni M, Wagner R, Kullmann S, Veit R, Husin Hm, Linder K, Benkedorff C, Peter A, Stefan N, Häring HU, Preissl H, Fritsch A. Central insulin administration improves whole-body insulin sensitivity via hypothalamus and parasympathetic outputs in men. Diabetes. 2014;63:4083–4088. doi: 10.2337/db14-0477. [DOI] [PubMed] [Google Scholar]
- 91.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 92.Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J. Type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care. 2007;30:842–846. doi: 10.2337/dc06-2011. [DOI] [PubMed] [Google Scholar]
- 93.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ishola IO, Akataobi OE, Alade AA, Adeyem OO. Glimepiride prevents paraquat-induced Parkinsonism in mice: involvement of oxidative stress and neuroinflammation. Fundam Clin Pharmacol. 2019;33:277–285. doi: 10.1111/fcp.12434. [DOI] [PubMed] [Google Scholar]
- 95.Iturriza FC, Thibault J. Immunohistochemical investigation of tyrosine-hydroxylase in the islets of Langerhans of adult mice, rats and guinea pigs. Neuroendocrinology. 1993;57:476–480. doi: 10.1159/000126394. [DOI] [PubMed] [Google Scholar]
- 96.Jalewa J, Sharma MK, Gengler S, Hölscher C. A novel GLP-1/GLP dual receptor agonist protects from 6-OHDA lesion. Neuropharmacology. 2017;117:238–248. doi: 10.1016/j.neuropharm.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 97.Jankovic J. Parkinson disease: exenatide - a drug for diabetes and Parkinson disease? Nat Rev Neurol. 2017;13:643. doi: 10.1038/nrneurol.2017.140. [DOI] [PubMed] [Google Scholar]
- 98.Jurcovicova J. Glucose transport in brain-effect in inflammation. Endocr Regul. 2014;48:35–48. doi: 10.4149/endo_2014_01_35. [DOI] [PubMed] [Google Scholar]
- 99.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 100.Kamath V, Jones CN, Yip JC, Varasteh BB, Cincotta AH, Reaven GM, Chen YD. Effects of a quick-release form of bromocriptine (Ergoset) on fasting and postprandial plasma glucose, insulin , lipid, and lipoprotein concentrations in obese nondiabetic hyperinsulinemic women. Diabetes Care. 1997;20:1697–1701. doi: 10.2337/diacare.20.11.1697. [DOI] [PubMed] [Google Scholar]
- 101.Kang H, Khang R, Ham S, Jeong GR, Kim H, Jo M, Lee BD, Lee YI, Jo A, Park C, Kim H, Seo J, Paek SH, Lee YS, Choi JY, Lee Y, Shin JH. Activation of the ATF2/CREB-PGC-1α pathway by metformin leads to dopaminergic neuroprotection. Oncotarget. 2017;8:48603–48618. doi: 10.18632/oncotarget.18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kern W, Peters A, Fruehwald- Schultes B, Deininger E, Born J, Fehm HL. Improving influence of insulin on cognitive functions in humans. Neuroendocrinology. 2001;74:270–280. doi: 10.1159/000054694. [DOI] [PubMed] [Google Scholar]
- 103.Kitamura T, Feng Y, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 104.Kleinridders A, Ferris HA, Cai W, Kahn R. Insulin Action in brain regulates systemic metabolism and brain function. Diabetes. 2014;63:2232–2243. doi: 10.2337/db14-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Konner AC, Hess S, Tovar S, Mesaros A, Sànchez-Lasheras C, Evers N, Verhagen LAW, Brönneke HS, Kleinridders A, Hampel B, Kloppenburg P, Brüning HS. Role for insulin signaling in catecholaminergic neurons in control of energy homeostasis. Cell Metab. 2011;13:720–728. doi: 10.1016/j.cmet.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 106.Kotagal V, Albin RL, Müller MLTM, Koeppe RA, Frey KA, Bohnen NI. Diabetes is associated with postural instability and gait difficulty in Parkinson disease. Parkinsonism Relat Disord. 2013;19:522–526. doi: 10.1016/j.parkreldis.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kuan YC, Huang KW, Lin CL, Hu CJ, Kao CH. Effects of metformin exposure on neurodegenerative diseases in elderly patients with type 2 diabetes mellitus. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79(Pt B):77–83. doi: 10.1016/j.pnpbp.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 108.Kuwabara T, Kagawala MN, Onuma Y, Ito Y, Warashina M, Terashima K, Sanosaka T, Nakashima K, Gage FH, Asashima M. Insulin biosynthesis in neuronal progenitors derived from adult hippocampus and the olfactory bulb. Embo Mol Biol. 2011;3:742–754. doi: 10.1002/emmm.201100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lacković Z, Salković M, Kuci Z, Relja M. Effect of long-lasting diabetes mellitus on rat and human brain monoamines. J Neurochem. 1990;54:143–147. doi: 10.1111/j.1471-4159.1990.tb13294.x. [DOI] [PubMed] [Google Scholar]
- 110.Lakomy M, Chodkowska D. Cholinergic innervation of pig pancreas. Acta Histochemica. 1984;75:63–68. doi: 10.1016/S0065-1281(84)80072-0. [DOI] [PubMed] [Google Scholar]
- 111.Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N Engl J Med. 1999;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 112.Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagone-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138:4445–4455. doi: 10.1210/endo.138.10.5270. [DOI] [PubMed] [Google Scholar]
- 113.Leclerc I, Sun G, Morris C, Fernandez-Millan E, Nyirenda M, Rutter GA. AMP-activated protein kinase regulates glucagon secretion from mouse pancreatic alpha cells. Diabetologia. 2011;54:125–134. doi: 10.1007/s00125-010-1929-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Levine Y, Li GK, Michel T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. J Biol Chem. 2007;282:20351–20364. doi: 10.1074/jbc.M702182200. [DOI] [PubMed] [Google Scholar]
- 115.Lieberthal W, Zhang L, Patel VA, Levine JS. AMPK protects proximal tubular cells from stress-induced apoptosis by an ATP-independent mechanism: potential role of Akt activation. Am J Physiol Renal Physiol. 2011;301:F1177–1192. doi: 10.1152/ajprenal.00034.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.LiLi Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin HV, Ren H, Samuel VT, Lee HY, Lu TY, Shulman GI, Accili D. Diabetes in mice with selective impairment of insulin action in Glut4-expressing tissues. Diabetes. 2011;60:700–709. doi: 10.2337/db10-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lipman IJ, Boykin ME, Flora RE. Glucose intolerance in Parkinson’s disease. J Chronic Dis. 1974;27:573–579. doi: 10.1016/0021-9681(74)90031-9. [DOI] [PubMed] [Google Scholar]
- 119.Long-Smith CM, Sullivan AM, Nolan YM. The influence of microglia on the pathogenesis of Parkinson’s disease. Prog Neurobiol. 2009;89:277–287. doi: 10.1016/j.pneurobio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 120.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–269. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 121.Lu L, Fu DL, Li HQ, Liu AJ, Li JH, Zheng GQ. Diabetes and risk of Parkinson’s disease: an updated meta-analysis of case-control studies. PLoS One. 2014;9:e85781. doi: 10.1371/journal.pone.0085781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luo S, Liang Y, Cincotta AH. Intracerebroventricular administration of bromocriptine ameliorates the insulin-resistant/glucose intolerant state in hamsters. Neuroendocrinology. 1999;69:160–166. doi: 10.1159/000054415. [DOI] [PubMed] [Google Scholar]
- 123.Luo S, Luo J, Meier AH, Cincotta AH. Dopaminergic neurotoxin administration to the area of the suprachiasmatic nuclei induces insulin resistance. Neuroreport. 1997;8:3495–3499. doi: 10.1097/00001756-199711100-00016. [DOI] [PubMed] [Google Scholar]
- 124.Maas JW, Yang J, Edwards RH. Endogenous leucine-rich repeat kinase 2 slows synaptic vesicle recycling in striatal neurons. Front Synaptic Neurosci. 2017;9:5. doi: 10.3389/fnsyn.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maffei A, Segal AM, Alvarez-Perez JC, Garcia Ocaňa A, Harris PE. Anti-incretin, anti-proliferative action of dopamine on β-cells. Mol Endocrinol. 2015;29:542–557. doi: 10.1210/me.2014-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Malaisse W, Malaisse-Lagae F, Wright PH, Ashmore J. Effects of adrenergic and cholinergic agents upon insulin secretion in vitro. Endocrinology. 1967;80:975–978. doi: 10.1210/endo-80-5-975. [DOI] [PubMed] [Google Scholar]
- 127.Mc Ewen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol. 2004;490:13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 128.McNay EC, Pearson-Leary J. GluT4: A central player in hippocampal memory and brain insulin resistance. Exp Neurol. 2020;323:113076. doi: 10.1016/j.expneurol.2019.113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mebel DM, Wong JC, Dong YJ, Borgland SL. Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake. Eur J Neurosci. 2012;36:2336–2346. doi: 10.1111/j.1460-9568.2012.08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mezey E, Eisenhofer G, Harta G, Hansson S, Gould L, Hunyady B, Hoffman BJ. A novel nonneuronal catecholaminergic system: exocrine pancreas synthesizes and releases dopamine. Proc Natl Acad Sci U S A. 1996;93:10377–10382. doi: 10.1073/pnas.93.19.10377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mitok KA, Freiberg EC, Schueler KL, Rbaglia ME, Stapleton DS, Kwiecien NW, Malec PA, Hebert AS, Broman AT, Kennedy RT, Keller MP, Coon JJ, Attie AD. Islet proteomics reveals genetic variation in dopamine production resulting in altered insulin secretion. J Biol Chem. 2018;293:5860–5877. doi: 10.1074/jbc.RA117.001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miyake Y, Tanaka K, Fukushima W, et Sasaki S, Kiyohara C, Tsuboi T, Yamada T, Oeda T, Miki T, Kawamura N, Sakae N, Fukuyama H, Hirota Y, Nagai M. Case-control study of risk of Parkinson’s disease in relation to hypertension, hypercholesterolemia , and diabetes in Japan. J Neurol Sci. 2010;293:82–86. doi: 10.1016/j.jns.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 133.Molnár G, Faraho N, Kocsis AS, Rozsa M, Lovas S, Boldog E, Baldi R, Csajbok E, Gardi J, Puskas G, Tamas G. GABAergic neurogliaform cells represent local sources of insulin in the cerebral cortex. J Neurosci. 2014;34:1133–1137. doi: 10.1523/JNEUROSCI.4082-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moreno S, Farioli-Vecchioli S, Ceru’ MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid x receptors in the adult rat CNS. Neuroscience. 2004;123:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 135.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 136.Mukherjee A, Morale-Scheihing D, Moreno-Gonzalez I, Gonzalez C, Taylor-Presse K, Mendez Nm Shahnawaz M, Gaber AO, Sabek OM, Fraga DW, Soto C. Induction of IAPP amyloid deposition and associated diabetic abnormalities by a prion-like mechanism. J Exp Med. 2017;214:2591–2610. doi: 10.1084/jem.20161134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mulherin AJ, Oh AH, Kim H, Grieco A, Lauffer LM, Brubaker PL. Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell. Endocrinology. 2011;152:4610–4619. doi: 10.1210/en.2011-1485. [DOI] [PubMed] [Google Scholar]
- 138.Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, Holst JJ, Langhans W, Meier JJ, Nauck MA, Perez-Tilve D, Pocai A, Reimann F, Sandoval DA, Schwartz TW, Seeley RJ, et al. Glucagon-like peptide 1 (GLP-1) Mol Metab. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators (2015) Pioglitazone in early Parkinson’s disease: a phase 2, multicentre , double-blind, randomised trial. Lancet Neurol. 14:795–803. doi: 10.1016/S1474-4422(15)00144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Engl J Med. 1994;331:1188–1193. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- 141.Obici S, Zhang BB, Kakanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 142.Offen D, Shtaif B, Hadad D, Weizman A, Melamed E, Gil-Ad I. Protective effect of insulin-like-growth-factor-1 against dopamine-induced neurotoxicity in human and rodent neuronal cultures: possible implications for Parkinson’s disease. Neurosci Lett. 2001;316:129–132. doi: 10.1016/s0304-3940(01)02344-8. [DOI] [PubMed] [Google Scholar]
- 143.Olanow WC, Prusiner SB. Is Parkinson’s disease a prion disorder? Proc Natl Acad Sci U S A. 2009;31:12571–12572. doi: 10.1073/pnas.0906759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Oltmans GA. Norepinephrine and dopamine levels in hypothalamic nuclei of the genetically obese mouse (ob/ob) Brain Res. 1983;273:394–373. doi: 10.1016/0006-8993(83)90865-x. [DOI] [PubMed] [Google Scholar]
- 145.Ong M, Foo H, Chander RJ, Wen J-C, Sitoh , Tan L, Kandiah N. Influence of diabetes mellitus on longitudinal atrophy and cognition in Parkinson’s disease. J Neurol Sci. 2017;377:122–126. doi: 10.1016/j.jns.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 146.Pagano G, Polychronis S, Wilson H, Giordano B, Ferrara N, Niccolini F, Politis M. Diabetes mellitus and Parkinson disease. Neurology. 2018;90:3549. doi: 10.1212/WNL.0000000000005475. [DOI] [PubMed] [Google Scholar]
- 147.Paisán-Ruíz C, Jain S, Evans EW, Gilks WP, Simón J, van der Brug M, López de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Martí Carrera I, Pena AS, de Silva R, Lees A, Martí-Massó JF, Pérez-Tur J, Wood NW, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 148.Palacios N, Gao X, McCullough M, Jacobs EJ, Patel AV, Mayo T, Schwarzschild MA, Ascherio A. Obesity, diabetes, and risk of Parkinson’s disease. Mov Disord. 2012;26:2252–2259. doi: 10.1002/mds.23855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pang Y, Lin S, Wright C, Shen J, Carter K, Bhatt A, Fan LW. Intranasal insulin protects against substantia nigra dopaminergic neuronal loss and alleviates motor deficits induced by 6-OHDA in rats. Neuroscience. 2016;318:157–165. doi: 10.1016/j.neuroscience.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Patil SP, Jain PD, Ghumatkar PJ, Tambe R, Sathaye S. Neuroprotective effect of metformin in MPTP-induced Parkinson disease in mice. Neuroscience. 2014;277:747–754. doi: 10.1016/j.neuroscience.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 151.Pearson-Leary J, Jahagirdar V, Sage J, McNay EC. Insulin modulates hippocampally-mediated spatial working memory via glucose transporter-4 Behav. Brain Res. 2018;338:32–39. doi: 10.1016/j.bbr.2017.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Perry TA, Haughey NJ, Mattson MP, Egan JM, Greig NH. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther J. 2008;302:881–888. doi: 10.1124/jpet.102.037481. [DOI] [PubMed] [Google Scholar]
- 153.Picillo M, Erro R, Santangelo G, Pivonello R, Longo K, Pivonello C, Vitale C, Amboni M, Moccia M, Colao A, Barone P, Pellecchia MT. Insulin-like growth factor-1 and progression of motor symptoms in early, drug-naïve Parkinson’s disease. J Neurol. 2013;260:1724–1730. doi: 10.1007/s00415-013-6851-0. [DOI] [PubMed] [Google Scholar]
- 154.Pinto M, Nissanka N, Peralta S, Brambilla R, Diaz F, Moraes CT. Pioglitazone ameliorates the phenotype of a novel Parkinson’s disease mouse model by reducing neuroinflammation. Mol Neurodegener. 2016;11:25–40. doi: 10.1186/s13024-016-0090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pitt J, Wilcoxa KC, Tortelli V, Diniz L, Oliveira MS, Dobbins C, Yu XW, Nandamuri S, Gomes F, Di Nunno N, Viola K, De Felice FD, Ferreira St, Klein W. Neuroprotective astrocyte-derived insulin/ insulin-like growth factor 1 stimulates endocytic processing and extracellular release of neuron-bound Aβ oligomers. Mol Biol Cell. 2017;28:2623–2636. doi: 10.1091/mbc.E17-06-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 157.Porceddu PF, Ismail Ogunbayode Ishola IO, Contu L, Morelli M. Metformin prevented dopaminergic neurotoxicity induced by 3, 4-methylenedioxymethamphetamine administration. Neurotox Res. 2016;30:101–109. doi: 10.1007/s12640-016-9633-5. [DOI] [PubMed] [Google Scholar]
- 158.Porte D, Jr, Graber Al, Takeshi K, Williams RH. The effect of epinephrine on immunoreactive insulin levels in man. J Clin Invest. 1966;45:228–236. doi: 10.1172/JCI105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ramalingam M, Kim SJ. The role of insulin against hydrogen peroxide-induced oxidative damages in differentiated SH-SY5Y cells. J Recept Signal Transduct Res. 2014;34:212–220. doi: 10.3109/10799893.2013.876043. [DOI] [PubMed] [Google Scholar]
- 160.Ramalingam M, Kim SJ. Insulin involved Akt/ERK and Bcl-2/Bax pathways against oxidative damages in C6 glial cells. J Recept Signal Transduct Res. 2016a;36:14–20. doi: 10.3109/10799893.2014.970276. [DOI] [PubMed] [Google Scholar]
- 161.Ramalingam M, Kim SJ. The neuroprotective role of insulin against MPP(+) -induced Parkinson’s disease in differentiated SH-SY5Y Cells. J Cell Biochem. 2016b;117:917–926. doi: 10.1002/jcb.25376. [DOI] [PubMed] [Google Scholar]
- 162.Rassu M, Del Giudice MG, Sanna S, Taymans JM, Morari M, Brugnoli A, Frassineti M, Masala A, Esposito S, Galioto M, Valle C, Carri MT, Biosa A, Greggio E, Crosio C, Iaccarino C. Role of LRRK2 in the regulation of dopamine receptor trafficking. PLoS One. 2017;12:e0179082. doi: 10.1371/journal.pone.0179082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rayner DV, Thomas MEA, Trayhurn P. Glucose transporters (GLUTs 1-4) and their mRNA in regions of the rat brain: insulin sensitive transporter expression in the cerebellum. Can J Physiol Pharm. 1994;72:476-479. doi: 10.1139/y94-069. [DOI] [PubMed] [Google Scholar]
- 164.Ren H, Yan S, Zhang B, Lu TY, Arancio O, Accili D. Glut-4 expression defines an insulin-sensitive hypothalamic neuronal population. Mol Metab. 2014;3:452–459. doi: 10.1016/j.molmet.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rodríguez MJ, Saura J, Finch CC, Mahy N, Billett EE. Localization of monoamine oxidase A and B in human pancreas, thyroid , and adrenal glands. J Histochem Cytochem. 2000;48:147–151. doi: 10.1177/002215540004800115. [DOI] [PubMed] [Google Scholar]
- 166.Rodriguez-Araujo G, Nagakami H, Hayashi H, Mori M. Alpha-synuclein elicits glucose uptake and utilization in adipocytes through the Gab1/PI3K/Akt transduction pathway. Cell Mol Life Sci. 2013;70:1123–1133. doi: 10.1007/s00018-012-1198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rodriguez-Araujo G, Nakagami H, Takami Y, Katsuya T, Akasaka H, Saitoh S, Shimamoto K, Morishita R, Rakugi H, Kaneda Y. Low alpha-synuclein levels in the blood are associated with insulin resistance. Sci Rep. 2015;5:12081. doi: 10.1038/srep12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rothemund Y, Preuschhof C, Bohner G, Bauknecht H-C, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 169.Rothman SM, Griffioen KJ, Fishbein KW, Spencer RG, Makrogiannis S, Cong W, Martin B, Mattson MP. Metabolic abnormalities and hypoleptinemia in α-synuclein A53T mutant mice. Neurobiol Aging. 2014;35:1153–1161. doi: 10.1016/j.neurobiolaging.2013.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rotte M, Barecke C, Pottag G, Klose S, Kanneberg E, Heinze HJ, Lenhert H. Insulin affects the neuronal response in the medial temporal lobe in humans. Neuroendocrinology. 2005;81:49–55. doi: 10.1159/000084874. [DOI] [PubMed] [Google Scholar]
- 171.Rubí B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, Bartley C, Maechler P. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem. 2005;280:36824–36832. doi: 10.1074/jbc.M505560200. [DOI] [PubMed] [Google Scholar]
- 172.Ryu YK, Park HY, Go J, Choi DH, Kim YH, Hwang JH, Noh JR, Lee TG, Lee CH, Kim KS. Metformin inhibits the development of L-DOPA-induced dyskinesia in a murine model of parkinson’s disease. Mol Neurobiol. 2018;55:5715–5726. doi: 10.1007/s12035-017-0752-7. [DOI] [PubMed] [Google Scholar]
- 173.Saisho Y, Harris PE, Butler AE, Galasso R, Gurlo T, Rizza RA, Butler PC. Relationship between pancreatic vesicular monoamine transporter 2 (VMAT2) and insulin expression in human pancreas. J Mol His. 2008;39:543–551. doi: 10.1007/s10735-008-9195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sandyk R. The relationship between diabetes mellitus and Parkinson’s disease. Intern J Neuroscience. 1993;69:123–130. doi: 10.3109/00207459309003322. [DOI] [PubMed] [Google Scholar]
- 175.Sarruf DA, Yu F, Nguyen HT, Williams DL, Printz RL, Niswender KD, Schwartz MW. Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology. 2009;150:707–712. doi: 10.1210/en.2008-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Savica R, Grossardt BR, Ahlskog JE, Rocca WA. Metabolic markers or conditions preceding Parkinson’s disease: a case control study. Mov Disord. 2012;27:974–979. doi: 10.1002/mds.25016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schechter R, Holtzclaw L, Sadiq F, Kahn A, Devaskar S. Insulin synthesis by isolated rabbit neurons. Endocrinology. 1988;123:505–513. doi: 10.1210/endo-123-1-505. [DOI] [PubMed] [Google Scholar]
- 178.Schernhammer E, Hansen J, Rugbjerg K, Wermuth L, Ritz B. Diabetes and risk to developing Parkinson’s disease in Denmark. Diabetes Care. 2011;34:1102–1108. doi: 10.2337/dc10-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Scheuing N, Best F, Dapp A, Dreyhaupt , Filz HP, Krakow D, Lang W, Siegelh E, Zeyfang A, Holl RH. Multicentre analysis of 178, 992 type 2 diabetes patients revealed better metabolic control despite higher rates of hypertension, stroke, dementia and repeated inpatient care in patients with comorbid Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:687–692. doi: 10.1016/j.parkreldis.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 180.Schwartz MW, Sipols AJ, Marks JL, Sanacora G, White JD, Scheurink A, Kahn SE, Baskin DG, Woods SC, Figlewicz DP. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology. 1992;130:3608–3616. doi: 10.1210/endo.130.6.1597158. [DOI] [PubMed] [Google Scholar]
- 181.Schwartz S. Bromocriptine (Ergoset) improves glycemic control in type 2 diabetes on insulin. Diabetes. 1999;48(Suppl 1):A99. [Google Scholar]
- 182.Shao SY. Rescue of alpha-synuclein cytoxicity by insulin-like growth factors (2009) Biochem Biophys Res Commun. 385:434–438. doi: 10.1016/j.bbrc.2009.05.089. [DOI] [PubMed] [Google Scholar]
- 183.Sharma SK, Chorell E, Steneberg P, Vernersson-Lindahl E, Edlund H, Wittung-Stafshede P. Insulin-degrading enzyme prevents α-synuclein fibril formation in a nonproteolytical manner. Sci Rep. 2015;5:12531. doi: 10.1038/srep12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shi Q, Liu S, Fonseca VA, Thethi TK, Shi L. Effect of metformin on neurodegenerative disease among elderly adult US veterans with type 2 diabetes mellitus. BMJ Open. 2019;9:e 024954. doi: 10.1136/bmjopen-2018-024954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev. 2000;24:855–872. doi: 10.1016/s0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- 186.Simpson N, Maffei A, Freeby M, Burroughs S, Freyberg Z, Leibel RL, Harris PE. Dopamine-mediated autocrine inhibitory circuit regulating human insulin secretion in vitro. Mol Endocrinol. 2012;26:1757–1772. doi: 10.1210/me.2012-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Simuni T, Brundin P. Is exenatide the next big thing in Parkinson’s disease? J Parkinson Dis. 2014;4:345–347. doi: 10.3233/JPD-149001. [DOI] [PubMed] [Google Scholar]
- 188.Sipols AJ, Stuber Gd, Klein Sn, Higgins MS, Figlewicz DP. Insulin and raclopride combine to decrease short-term intake of sucrose solutions. Peptides. 2000;21:1361–1367. doi: 10.1016/s0196-9781(00)00279-5. [DOI] [PubMed] [Google Scholar]
- 189.Sirtori CR, Boime P, Azarnoff DL. Metabolic response to acute and chronic L-dopa administration in patients with Parkinson’s disease. New Engl J Med. 1972;287:729–733. doi: 10.1056/NEJM197210122871501. [DOI] [PubMed] [Google Scholar]
- 190.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 191.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci. 2000;3:757–758. doi: 10.1038/77660. [DOI] [PubMed] [Google Scholar]
- 192.Spielman LJ, Gibson DL, Klegeris A. Incretin hormones regulate microglia oxidative stress, survival and expression of trophic factors. Eur J Cell Biol. 2017;95:240–253. doi: 10.1016/j.ejcb.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 193.Stouffer M, Woods CA, Patel J, Lee CR, Witkovsky P, Bao L, Machold RP, Jones KT, Cabeza de Vaca S, Reith MEA, Carr KD, Rice ME. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signal reward. Nat Commun. 2015;6:8543. doi: 10.1038/ncomms9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sun Y, Chang YH, Chen HF, Su YH, Su HF, Li CY. Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diabetes Care. 2012;35:1047–1049. doi: 10.2337/dc11-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Svenningsson P, Wirdefeldt K, Yin L, Fan F, Markaki I, Efendic S, Ludvigsson JF. Reduced incidence of Parkinson’s disease after dipeptidyl peptidase-4 inhibitors-A nationwide case-control study. Mov Disord. 2016;3:1422–1423. doi: 10.1002/mds.26734. [DOI] [PubMed] [Google Scholar]
- 196.Szczypka MS, Kwok K, Brot MD, Marck BT, Matsumoto AM, Donahue BA, Palmiter RC. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 197.Takano K, Koarashi K, Kawabe K, Itakura M, Nakajima H, Moriyama M, Nakamura Y. Insulin expression in cultured astrocytes and the decrease by amyloid β. Neurochem Int. 2018;119:171–177. doi: 10.1016/j.neuint.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 198.Trulson ME, Jacoby JH, MacKenzie RG. Streptozocin-induced diabetes reduces brain serotonin synthesis in rats. J Neurochem. 1986;46:1068–1072. doi: 10.1111/j.1471-4159.1986.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 199.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 200.Uéda K, Saitoh T, Mori H. Tissue-dependent alternative splicing of mRNA for NACP, the precursor of non-A beta component of Alzheimer’s disease amyloid. Biochem Biophys Res Commun. 1994;205:1366–1372. doi: 10.1006/bbrc.1994.2816. [DOI] [PubMed] [Google Scholar]
- 201.Ulusoy GK, Celik T, Kayir H, Gürsoy M, Isik AT, Uzbay TI. Effects of pioglitazone and retinoic acid in a rotenone model of Parkinson’s disease. Brain Res Bul. 2011;85:380–384. doi: 10.1016/j.brainresbull.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 202.Unger JW, Livingston JN, Moss AM. Insulin receptors in the central nervous system: Localization, signaling mechanism and functional aspects. Prog Neurobiol. 1991;36:343–362. doi: 10.1016/0301-0082(91)90015-s. [DOI] [PubMed] [Google Scholar]
- 203.Ustione A, Piston DW. Dopamine synthesis and D3 receptor activation in pancreatic β-cells regulates insulin secretion and intracellular [Ca(2+)] oscillations. Mol Endocrinol. 2012;26:1928–1940. doi: 10.1210/me.2012-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Van der Heide LP, Ramarkers G MJ, Schmidt MP. Insulin signaling in the central nervous system: Learning to survive. Prog Neurobiol. 2006;79:205–211. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 205.Van Woert MH, Mueller PS. Glucose, insulin and free fatty acid metabolism in Parkinson’s disease treated with levodopa. Clin Pharmacol Ther. 1971;12:360–367. doi: 10.1002/cpt1971122part2360. [DOI] [PubMed] [Google Scholar]
- 206.Van Woert MH, Mueller PS, Ambani LM, Rathey U. Abnormal insulin secretion in Parkinson’s disease before and during L-dihydroxyphenylalanine (L-dopa) therapy. J Endocrinol. 1973;59:523–534. doi: 10.1677/joe.0.0590523. [DOI] [PubMed] [Google Scholar]
- 207.Vicente Miranda H, Szego ÉM, Oliveira LMA, Breda C, Darendelioglu E, de Oliveira RM, Ferreira DG, Gomes MA, Rott R, Oliveira M, Munari F, Enguita FJ, Simões T, Rodrigues EF, Heinrich M, Martins IC, Zamolo I, Riess O, Cordeiro C, Ponces-Freire A, et al Glycation potentiates α-synuclein-associated neurodegeneration in synucleinopathies. Brain. 2017;140:1399–1419. doi: 10.1093/brain/awx056. [DOI] [PubMed] [Google Scholar]
- 208.Vogt MC, Brüning JC. CNS insulin signaling in the control of energy homeostasis and glucose metabolism-from embryo to old age. Trends Endocrinol Metab. 2013;24:76–84. doi: 10.1016/j.tem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 209.Wahlqvist ML, Lee MS, Chuang SY, Hsu CC, Tsai HN. Metformin-inclusive sulfonylurea therapy reduces the risk of Parkinson’s disease occurring with type 2 diabetes in Taiwanese population color. Parkinsonism Relat Disord. 2012;18:753–758. doi: 10.1016/j.parkreldis.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 210.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 211.White MF, Kahn CR. The insulin signaling system. J Biol Chem. 1994;269:1–4. [PubMed] [Google Scholar]
- 212.Wilson JP, Downs RW, Jr, Feldman JM, Lebovitz HE. Beta cells monoamines: further evidence for their role in modulating insulin secretion. Am J Physiol. 1974;227:305–312. doi: 10.1152/ajplegacy.1974.227.2.305. [DOI] [PubMed] [Google Scholar]
- 213.Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xu Q, Park Y, Huang X, Hollembeck A, Blair A, Schatzkin A, Chen H. Diabetes and the risk of Parkinson’s disease. Diabetes Care. 2011;34:910–915-. doi: 10.2337/dc10-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yang YW, Hsieh TF, Li CI, Liu CS, Lin WY, Chiang JH, Li TC. Increased risk of Parkinson disease with diabetes mellitus in a population-based study. Medicine (Baltimore) 2017;96:e5921. doi: 10.1097/MD.0000000000005921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yue X, Li H, Yan H, Zhang P, Chang L, Li T. Risk of Parkinson disease in diabetes mellitus: an updated meta-analysis of population-based cohort studies. Medicine (Baltimore) 2016;95:e3549. doi: 10.1097/MD.0000000000003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zeng Y, Zhang L, Hu Z. Cerebral insulin, insulin signaling pathway, and brain angiogenesis. Neurol Sci. 2016;37:9–16. doi: 10.1007/s10072-015-2386-8. [DOI] [PubMed] [Google Scholar]


