Abstract
Objective
To compare the effectiveness of single, multiple, and multifactorial interventions to prevent falls and fall‐related fractures in community‐dwelling older persons.
Methods
MEDLINE, Embase, and Cochrane Central Register of Controlled Trials were systematically searched for randomized controlled trials (RCTs) evaluating the effectiveness of fall prevention interventions in community‐dwelling adults aged ≥65 years, from inception until February 27, 2019. Two large RCTs (published in 2020 after the search closed) were included in post hoc analyses. Pairwise meta‐analysis and network meta‐analysis (NMA) were conducted.
Results
NMA including 192 studies revealed that the following single interventions, compared with usual care, were associated with reductions in number of fallers: exercise (risk ratio [RR] 0.83; 95% confidence interval [CI] 0.77–0.89) and quality improvement strategies (e.g., patient education) (RR 0.90; 95% CI 0.83–0.98). Exercise as a single intervention was associated with a reduction in falls rate (RR 0.79; 95% CI 0.73–0.86). Common components of multiple interventions significantly associated with a reduction in number of fallers and falls rate were exercise, assistive technology, environmental assessment and modifications, quality improvement strategies, and basic falls risk assessment (e.g., medication review). Multifactorial interventions were associated with a reduction in falls rate (RR 0.87; 95% CI 0.80–0.95), but not with a reduction in number of fallers (RR 0.95; 95% CI 0.89–1.01). The following single interventions, compared with usual care, were associated with reductions in number of fall‐related fractures: basic falls risk assessment (RR 0.60; 95% CI 0.39–0.94) and exercise (RR 0.62; 95% CI 0.42–0.90).
Conclusions
In keeping with Tricco et al. (2017), several single and multiple fall prevention interventions are associated with fewer falls. In addition to Tricco, we observe a benefit at the NMA‐level of some single interventions on preventing fall‐related fractures.
Keywords: community‐dwelling, fall‐related fractures, falls, older adults
1. Key Points
The single intervention exercise is most strongly associated with a reduction in falls rate.
The single intervention basic falls risk assessment is most strongly associated with a reduction in number of fall‐related fractures.
Common components of multiple interventions significantly associated with a reduction in number of fallers and falls rate are exercise, assistive technology, environmental assessment and modifications, quality improvement strategies, and basic falls risk assessment.
2. Why Does this Paper Matter?
Previous reviews were restricted to analyzing fixed combinations of interventions, rather than disentangling the effect of the individual components from the entire combination. Yet, this is vital in order to offer a targeted/personalized fall prevention strategy, especially in high‐risk groups.
INTRODUCTION
Falls in older adults are a highly prevalent problem. Falls occur in one‐third of community‐dwelling people aged ≥65 years at least once a year. 1 Twenty percent of these falls lead to a fall‐related injury. 2 , 3
Many intrinsic and extrinsic risk factors for falling have been identified. 4 Suffering from multiple chronic conditions, for example, rheumatic disease, vertigo, may pose an even higher risk of falling; these medical conditions are prevalent in older people. 4
Fall prevention interventions target risk factors that are modifiable and can be divided into three main groups: (1) single interventions (participants receive one type of intervention), (2) multiple interventions (participants receive the same, fixed combination of two or more types of interventions), and (3) multifactorial interventions (participants receive a personalized selection out of two or more types of interventions, according to the results of a pre‐executed, personal falls risk assessment). 5 Until Tricco, 6 previous systematic reviews (SRs) and meta‐analyses were restricted to looking at combinations of multifactorial/multiple interventions on fall prevention as a whole, rather than being able to disentangle the effect of the individual components from the entire combination. 5 , 7 It is, however, important to determine which particular components are most effective, as this can result in a more accurate prevention strategy. Network meta‐analysis (NMA) enables the evaluation of individual components from multiple comparisons estimating the relative effectiveness between any pair of interventions, even if these interventions have never been compared directly. 8 , 9
Furthermore, previous reviews did not focus on multimorbid older (age ≥ 75) adults. 10 As this population have a high risk of falling, it is essential to gain more insight into which particular fall prevention interventions are most beneficial in this high risk group.
Therefore, the aim of this SR and NMA was to update the Tricco et al. search on the effectiveness of single, multiple, and multifactorial interventions and their individual components for preventing falls and fall‐related fractures in community‐dwelling older persons, with a particular focus on multimorbidity and age > 75 years.
METHODS
Protocol
The protocol for this SR and NMA was registered online with PROSPERO (PROSPERO 2019 CRD42019137466) and was developed in accordance with the preferred reporting items for systematic review and meta‐analyses (PRISMA) statement.
Study identification
We updated Tricco et al.'s 6 SR and NMA of fall prevention interventions in older adults. We applied the same search terms as used in the original Tricco et al.'s review and updated the search from December 1, 2015, until February 27, 2019. The following electronic databases were searched: MEDLINE (via PubMed), Embase (OVID), and Cochrane Central Register of Controlled Trials. The search strategies with limitations are included in Appendix S11. The electronic search was supplemented with manual searches for additional randomized controlled trials (RCTs), by reviewing the reference lists of previous reviews, 5 , 7 a recommendations statement, 11 and NMA. 12 We extended Tricco et al.'s search by searching for additional interventions (management of urinary incontinence, management of orthostatic hypotension, walking aids, and chiropractic care) from database inception to February 2019. As a NMA is time‐consuming, new papers might be published after the search period. To check whether the findings of the current NMA are consistent with most recent literature, the outcomes from two large RCTs published after the search date 13 , 14 were incorporated into a post hoc analysis.
Eligibility criteria
We included (cluster) randomized and quasi‐RCTs published in any language that evaluated the effectiveness of interventions for preventing falls in community‐dwelling persons aged ≥65 years. For details of the eligible study population, interventions, comparators, and outcomes, as well as the exclusion criteria, see Table S11. We excluded studies on specific conditions (e.g., stroke, Parkinson's disease), where the effects of the interventions cannot be generalized to most community‐dwelling older people.
Study selection
Two authors each reviewed half of the study titles and abstracts that resulted from the search, and then both independently reviewed the full text of all studies that were retained. Any disagreement was resolved by consensus with a third author. To ensure consistency of the eligibility criteria applied, the authors performed a pilot‐test screening beforehand.
Data extraction and outcome definition
We created a data extraction sheet for the following variables: study characteristics; participant characteristics; and primary and secondary outcome information. We categorized the interventions into the same intervention components as used by Tricco et al, 6 and added additional intervention components (Table 1). Primary outcomes were number of fallers and number of fall‐related fractures. Secondary outcomes were number of repeated fallers, number of hip fractures, falls rate, and fracture rate.
TABLE 1.
Interventions to prevent falls categorized into 14 components
| Intervention component (abbreviation) | Description |
|---|---|
| 1. Exercise (exerc) | Including gait‐, balance‐, and functional training, strength/resistance training, flexibility, 3D training (e.g., Tai Chi, Qigong, dance and square stepping), general physical activity (e.g., walking groups), endurance training, and other |
| 2. Medication (med) | Vitamin D (cholecalciferol, alphacalcidol, sunlight, calcitriol, and ergocalciferol) |
| 3. Surgery (surg) | E.g., pacemaker implantation, hip prosthesis, or cataract removal surgery |
| 4. Management of urinary incontinence a (incont) | Assisted toileting, bladder retraining, medication (e.g., tamsulosin, finasteride, botox injections), surgery (e.g., colposuspension surgery, sling procedures) |
| 5. Fluid or nutrition therapy (nutr) | Changes in diet, provision of supplements, nutritional therapy, protein drinks |
| 6. Psychological interventions (psych) | Cognitive behavioral therapy |
| 7. Environmental assessment and modifications (envir) | Assessment and correction of home environment (e.g., flooring, home check, home safety devices, home visits by occupational therapist, home furnishings and adaptations) |
| 8. Assistive technology (assist) | Provision of aids for personal protection (e.g., hip protector) or personal mobility (e.g., walking aids a , comprehensive podiatry assessment and treatment, orthosis), aids for communication/information/signaling (e.g., vision assessment and correction with glasses, personal alarm systems, hearing aids) |
| 9. Social engagement (social) | Social group activities (watching films, leisure reading, singing, conversation), community activities, peer support (from peers or caregivers), seminars on non–health‐related topics of general interest to older adults. |
| 10. Quality improvement strategies (qualt) |
‐ Patient‐level quality improvement strategies: promotion of self‐management, patient education, patient reminders, and motivational interviewing ‐ Clinic‐level or care team level quality improvement strategies: case management, team changes, electronic patient registry, facilitated relay of information to clinicians, audit and feedback, staff education, and clinician reminders ‐ Health system‐level quality improvement strategies: Interventions with positive or negative financial incentives directed at clinicians (e.g., linked to adherence to some process of care or achievement of some target outcome). This strategy also includes positive or negative financial incentives directed at patients or system‐wide changes in reimbursement systems |
| 11. Management of orthostatic hypotension a (hypot) | Wearing elastic stockings, rising slowly, sleeping in a bed with head raised, pharmacological interventions |
| 12. Basic falls risk assessment (brisk) | Cardiovascular assessment (vital signs, ECG, loop recorder, pacemaker interrogation), medication review (review, modification, withdrawal/deprescribing), fracture risk screening (bone mineral density) |
| 13. Whole‐body vibration (vibr) | Transferring vibration of any frequency to the human body |
| 14. Chiropractic care a (chiro) | Improving sensorimotor function associated with fall risk |
Note: In general, we categorized interventions into similar components as used by Tricco et al. (2017) in order to assist with later merging of data extraction results. We also categorized the multifactorial interventions into the 14 interventions components. In order to be able to carry out analyses, we had to assume that all participants received these multifactorial intervention components.
Additional fall prevention interventions not previously investigated by Tricco et al.
Risk of bias assessment
To assess risk of bias, we used the Effective Practice and Organisation of Care (EPOC) version of Cochrane's Risk of Bias tool. 15 This EPOC version fully overlaps with the original tool, yet adds the following criteria: contamination, similar baseline values of the outcome measures, and similarity of baseline characteristics. Risk of bias assessment was performed by two authors independently and any disagreement resolved by consensus with a third author. The authors first performed a pilot test to ensure consistency in applying the risk of bias criteria.
Measures of treatment effect
For dichotomous outcomes, we calculated risk ratios (RRs) accompanied by their 95% confidence intervals (CI). For rates, whereby each participant may experience the event of interest more than once, we extracted the number of events and total participant‐time (e.g., number of person‐weeks of follow‐up) and calculated rate ratios with 95% CIs, assuming that the risk of the event occurring is constant across participants and over time.
Synthesis of results
For a detailed description of the meta‐analysis methods see Appendix S11. The primary analysis followed the standard approach whereby each distinct combination of intervention components is treated as a separate intervention, for example, assistive technology + exercise versus usual care. We employed additional statistical models to disentangle the effect (i.e., determine effect sizes) of each separate intervention component, for example, assistive technology versus usual care, and exercise versus usual care (component‐NMA [C‐NMA]). A nontechnical review of C‐NMA is previously given. 16 A basic assumption of the C‐NMA is the additivity assumption, in which the total effect of a multiple/multifactorial intervention is derived from the sum of the relevant components (Interventiona + b = Interventiona + Interventionb), thus the effect size of each individual intervention component can be determined. 17 , 18 We used statistically significant effect estimates with the highest P‐scores to rank interventions 19 and estimate the average probability of a treatment being superior to other competing treatments.
Certainty of the evidence
We used the Confidence in Network Meta‐Analysis (CINeMA) approach, a quality assessment tool, to determine the degree of confidence in NMA effect estimates (see Appendix S11). 20 , 21 CINeMA rates six domains: within‐study bias, reporting bias, indirectness, imprecision, heterogeneity, and incoherence. Specifically important for NMAs, CINeMA helpfully considers the degree of incoherence, that is, the disagreement between direct and indirect evidence.
Subgroup and sensitivity analyses
A priori subgroup NMAs were planned if sufficient available data: participants aged ≥75 years (subgroup age 75+) and participants with ≥3 co‐existent chronic conditions (subgroup multimorbidity). 22
A planned sensitivity NMA was to exclude studies with one or more domains considered high risk of bias, (caveat: with the exception of the domain for “blinding,” because most studies were unable to conceal the intervention from participants).
Post hoc, we performed a sensitivity analysis comparing multifactorial interventions with usual care to determine whether multifactorial interventions as a whole were associated with a lower risk of falls. Taking power into account, we performed this analysis for the two outcomes with the largest networks: number of fallers and falls rate.
RESULTS
Study selection
Figure 1 presents an overview of the study selection. For a complete list of included references see Appendix S12.
FIGURE 1.
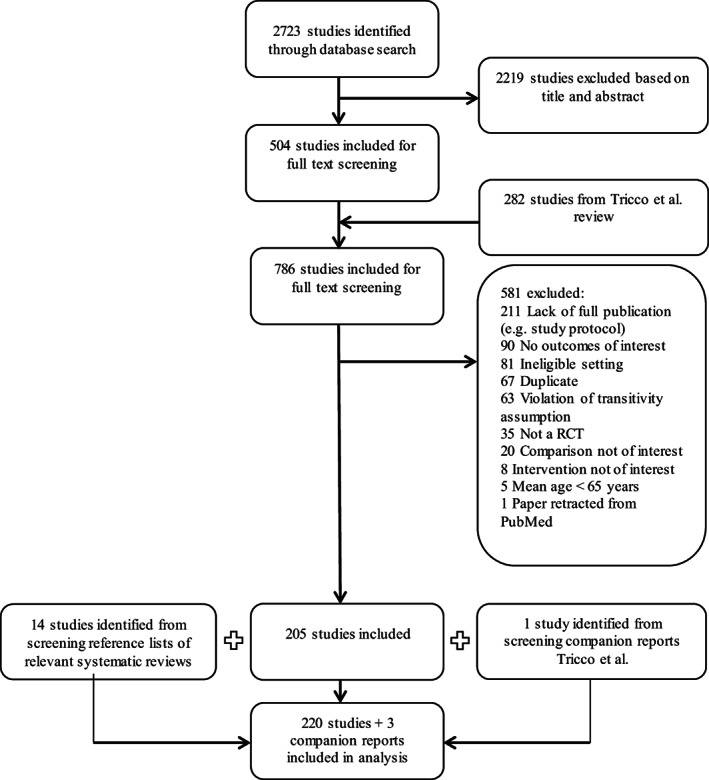
Flow diagram of study selection. RCT, randomized controlled trial
Study and participant characteristics
The study and participant characteristics of the original 220 studies identified in our search are presented in Appendix S1 and summarized in Table 2.
TABLE 2.
Summary of participant and study characteristics of the 220 randomized controlled trials (n = 104,638) identified in our original search
| Participant and study characteristics | Number of studies (%) |
|---|---|
| Mean age (years) | |
| 65–74 | 68 (30.9) |
| 75–84 | 128 (58.2) |
| ≥85 | 11 (5.0) |
| Not reported | 13 (5.9) |
| Female (%) | |
| 0–49 | 18 (8.2) |
| 50–100 | 198 (90.0) |
| Not reported | 4 (1.8) |
| History of falls in the last 12 months | |
| Fallers only | 33 (15.0) |
| Mixed | 103 (46.8) |
| Non‐fallers only | 0 |
| Not reported | 84 (38.2) |
| Year of publication | |
| 1990–2002 | 36 (16.4) |
| 2003–2007 | 45 (20.5) |
| 2008–2012 | 54 (24.5) |
| 2013–2017 | 67 (30.5) |
| 2018–2019 | 18 (8.2) |
| Continent | |
| Europe | 87 (39.5) |
| Australia/New Zealand | 49 (22.3) |
| North America | 48 (21.8) |
| Asia | 29 (13.2) |
| South America | 5 (2.3) |
| Multicontinent | 2 (0.9) |
| Study design | |
| Parallel | 192 (87.3) |
| Cluster | 27 (12.3) |
| Both | 1 (0.5) |
| Site | |
| Multicenter | 91 (41.4) |
| Single center | 129 (58.6) |
| Sample size | |
| <100 | 54 (24.5) |
| 100–299 | 78 (35.5) |
| 300–999 | 71 (32.3) |
| ≥1000 | 17 (7.7) |
| Duration of intervention (weeks) | |
| 0–26 | 114 (51.8) |
| 27–52 | 53 (24.1) |
| ≥52 | 32 (14.5) |
| Not reported | 21 (9.6) |
| Duration of follow‐up (weeks) | |
| 0–26 | 62 (28.2) |
| 27–52 | 111 (50.5) |
| ≥52 | 46 (20.9) |
| Not available/reported | 1 (0.5) |
| Number of components | |
| Single intervention | 99 (45.0) |
| Multiple intervention a | 75 (34.1) |
| Multifactorial intervention b | 46 (20.9) |
Participants received the same, fixed combination of two or more types of interventions.
Participants received a combination of two or more types of interventions, which were personalized according to the results of a pre‐executed falls risk assessment.
In 128 studies (58.2%), the mean age of participants was between 75 and 84 years and in 11 studies (5.0%) ≥85 years.
Risk of bias assessment
The risk of bias assessment was performed at the study level (see Appendix S2). Most studies had a low risk of bias for random sequence generation, similar baseline characteristics, similar baseline outcome measures, incomplete outcome data, and other bias. Over half of the studies had an unclear risk of bias for allocation concealment (i.e., concealment method not described or insufficient detail to allow judgment), contamination, and selective outcome reporting. Most studies had a high risk of bias for blinding and one in five studies a high risk of bias for incomplete outcome data. Given the methodological shortcomings emphasized here, one must interpret the findings from these studies with caution. Post hoc inclusion of two RCTs 13 , 14 did not alter these conclusions, namely a high risk of bias for blinding.
Number of fallers
The NMA for this primary outcome included 192 studies (98,388 participants), and 63 different interventions all compared with usual care. These numbers also reflect the inclusion of two RCTs published after our search period was closed, in order to present most up‐to‐date results. One study was not connected to the network, because the combinations of components reported (exerc+nutr+envir+brisk vs. exerc+nutr+envir) were not investigated by any of the other included RCTs. Therefore, this study (152 participants) was excluded from the primary analysis, but was included in the C‐NMA (Figure S2). Appendix S3 reports the RRs and P‐scores for every intervention versus usual care, in which each existing combination of components was analyzed as a distinct intervention (primary analysis). The interventions with significant associations are presented in Table 3, together with rating confidence in the results using CINeMA.
TABLE 3.
Risk ratios and rate ratios with 95% confidence interval (CI), P‐scores, and Confidence in Network Meta‐Analysis (CINeMA) confidence ratings for the interventions with a statistically significant association
| Intervention | Studies (N) | Participants (N) | Effect size (95% CI) | P‐score | CINeMA all domains | CINeMA four domains c |
|---|---|---|---|---|---|---|
| Number of fallersa | ||||||
| assist+brisk | 1 | 96 | 0.52 (0.30–0.90) | 0.89 | Low | High |
| assist+qualt | 3 | 366 | 0.58 (0.41–0.81) | 0.89 | Low | High |
| vibr | 3 | 798 | 0.61 (0.42–0.89) | 0.86 | Low | High |
| envir+assist+qualt+hypot+brisk | 1 | 397 | 0.62 (0.43–0.88) | 0.86 | Low | Moderate d |
| exerc+envir+qualt | 3 | 3646 | 0.74 (0.57–0.97) | 0.75 | Low | High |
| exerc+assist | 3 | 1338 | 0.77 (0.62–0.95) | 0.73 | Low | High |
| exerc | 56 | 14,825 | 0.83 (0.77–0.90) | 0.65 | Low | High |
| qualt+brisk | 10 | 9230 | 0.84 (0.73–0.96) | 0.62 | Low | High |
| exerc+envir+assist+qualt+brisk | 5 | 5391 | 0.85 (0.74–0.98) | 0.60 | Low | High |
| exerc+qualt | 30 | 8064 | 0.87 (0.80–0.96) | 0.56 | Low | High |
| qualt | 50 | 22,374 | 0.90 (0.83–0.99) | 0.49 | Low | High |
| qualt | 5 | 12,904 | 0.90 (0.83–0.98) | 0.49 | Low | High |
| exerc+incont+envir+assist+qualt+brisk | 1 | 552 | 1.58 (1.01–2.48) | 0.05 | Low | High |
| Number of repeated fallersa | ||||||
| vibr | 1 | 710 | 0.33 (0.12–0.91) | 0.94 | Low | High |
| exerc+assist | 1 | 1107 | 0.48 (0.25–0.93) | 0.88 | Low | High |
| exerc | 19 | 5590 | 0.71 (0.53–0.95) | 0.71 | Low | Moderate e |
| Falls rateb | ||||||
| envir+assist+qualt+hypot+brisk | 1 | 397 | 0.42 (0.30–0.58) | 0.99 | ΝΑ | ΝΑ |
| exerc+assist | 2 | 1188 | 0.68 (0.54–0.86) | 0.85 | ΝΑ | ΝΑ |
| exerc+med | 2 | 616 | 0.68 (0.47–0.98) | 0.81 | ΝΑ | ΝΑ |
| exerc+envir+assist+hypot+brisk | 4 | 973 | 0.73 (0.59–0.92) | 0.78 | ΝΑ | ΝΑ |
| exerc | 27 | 7485 | 0.79 (0.73–0.87) | 0.70 | ΝΑ | ΝΑ |
| exerc+qualt+hypot+brisk | 1 | 298 | 2.08 (1.34–3.25) | 0.01 | ΝΑ | ΝΑ |
| exerc+nutr+envir+assist+brisk | 1 | 328 | 1.84 (1.14–2.97) | 0.03 | ΝΑ | ΝΑ |
| Number of fall‐related fracturesa | ||||||
| brisk | 2 | 3046 | 0.60 (0.39–0.94) | 0.72 | Low | Moderate d |
| exerc | 10 | 5678 | 0.62 (0.42–0.90) | 0.71 | Low | High |
| Fracture rateb | ||||||
| exerc | 5 | 2511 | 0.49 (0.27–0.89) | 0.80 | NA | ΝΑ |
| exerc+qualt | 2 | 1975 | 0.52 (0.28–0.96) | 0.70 | NA | ΝΑ |
Note: Characterization not applicable (NA) because CINeMA cannot address rate outcomes.
Abbreviations: assist, assistive technology; brisk, basic falls risk assessment; envir, environmental assessment and modifications; exerc, exercise; hypot, management of orthostatic hypotension; incont, management of urinary incontinence; med, medication; nutr, fluid or nutrition therapy; psych, psychological interventions; qualt, quality improvement strategies; social, social engagement; surg, surgery; vibr, whole‐body vibration.
aRisk ratios.
bRate ratios.
For the domains “within‐study bias” and “reporting bias” there were major concerns for all comparisons. In order to still maintain distinctiveness, the evaluation of the confidence in the results of the NMA was based on the remaining four domains.
Reason for downgrading CINeMA confidence rating: indirectness.
Reason for downgrading CINeMA confidence rating: heterogeneity.
Based on statistically significant effect estimates and high P‐scores, the following single and multiple interventions were most strongly associated with reductions in number of fallers: (a) combination of assistive technology (e.g., provision of aids for mobility) and basic falls risk assessment (e.g., medication review), (b) combination of assistive technology and quality improvement strategies (e.g., patient education), (c) standing on a whole‐body vibration platform to improve muscle strength and balance, and (d) combination of home modification, assistive technology, quality improvement strategies, management of orthostatic hypotension, and basic falls risk assessment (Table 3).
Post hoc inclusion of data from two RCTs 13 , 14 had little effect on our conclusions, except in one small aspect where the intervention “quality improvement” rose to statistical significance. 13
There were no concerns about inconsistency as evaluated by the node‐splitting method, overall test for inconsistency, and net‐heat plot.
In the C‐NMA, in which the relative effects of each individual intervention component can be disentangled, the following were associated with a decrease in number of fallers, compared with usual care: (a) whole‐body vibration (RR 0.61; 95% CI 0.42–0.90) and (b) exercise (RR 0.92; 95% CI 0.88–0.97). Management of urinary incontinence was associated with an increase in number of fallers (RR 1.39; 95% CI 1.08–1.79) (Table S3).
We performed an additional analysis in which all multifactorial interventions were considered as one intervention type. A multifactorial intervention was not significantly associated with a reduction in number of fallers (RR 0.95; 95% CI 0.89–1.01, P‐score 0.33; 188 studies, 91,137 participants).
We performed a sensitivity NMA excluding studies at high risk of bias, for the outcome number of fallers; the results were largely similar to the main analysis including all studies.
Subgroup analyses number of fallers
The NMA for subgroup age 75+ included 19 studies (28,945 participants, mean age 79.8 years standard deviation = 4.9) and 14 interventions that were all compared with usual care (Appendix S4). Two studies were excluded from the primary analysis, as they were unconnected to the network (Figure S4). Both studies compared vitamin D with placebo and were later included as an additional pairwise meta‐analysis. Compared with placebo, vitamin D was not associated with a reduction in falls nor fractures.
The RRs and P‐scores for every intervention versus usual care are reported in Figure S5, whereas the five interventions with a statistically significant association in Table 4. The interventions with a statistically significant association in the subgroups were consistent with the findings from the main analysis, yet fewer were observed in subgroups likely due to the smaller size of the subgroup analysis. Based on statistically significant effect estimates and high P‐scores, the single intervention exercise was most strongly associated with a reduction in number of fallers in subgroup analysis age 75+. In the C‐NMA, none of the intervention components was associated with a significant change in the number of fallers (Table S4).
TABLE 4.
Risk ratios with 95% confidence interval (CI), P‐scores, and Confidence in Network Meta‐Analysis (CINeMA) confidence ratings for the interventions with a statistically significant association versus usual care for the outcome number of fallers, subgroup age 75+
| Intervention | Studies (N) | Participants (N) | Effect size (95% CI) | P‐score | CINeMA all domains a | CINeMA four domains a |
|---|---|---|---|---|---|---|
| exerc | 3 | 1954 | 0.65 (0.50–0.85) | 0.91 | Low | High |
| qualt+brisk | 2 | 5771 | 0.75 (0.64–0.87) | 0.80 | Low | High |
| exerc+qualt | 4 | 1481 | 0.75 (0.67–0.83) | 0.81 | Low | High |
| exerc+envir+qualt | 1 | 3182 | 0.76 (0.64–0.89) | 0.78 | Low | High |
| qualt | 5 | 9681 | 0.85 (0.74–0.99) | 0.59 | Low | High |
Abbreviations: brisk, basic falls risk assessment; envir, environmental assessment and modifications; exerc, exercise; qualt, quality improvement strategies.
For the domains “within‐study bias” and “reporting bias” there were major concerns for all comparisons. In order to still maintain distinctiveness, the evaluation of the confidence in the results of the NMA was based on the remaining four domains.
The NMA for the subgroup multimorbidity included 14 studies (7879 participants), and 11 interventions that were all compared with usual care (Appendix S5). For this subgroup, there were no statistically significant effects on number of fallers resulting from the primary analysis or C‐NMA. For number of fall‐related fractures and for the secondary outcomes, only a few studies reported on subgroups age 75+ and multimorbidity, thus data were insufficient for further subgroup analysis.
Number of fall‐related fractures
The number of fall‐related fractures NMA included 46 studies (43,811 participants) and 27 interventions compared with usual care (Figure S8). In 60% of studies, fractures were verified radiologically or through review of hospital records. Appendix S6 reports the RRs and P‐scores for every intervention versus usual care. Based on statistically significant effect estimates with the highest P‐scores, the single interventions basic falls risk assessment and exercise were most strongly associated with a reduction in number of fall‐related fractures; the latter with higher CINeMA confidence rating (Table 3). However, these significant reductions were lost at the C‐NMA level. Strangely, the intervention component assistive technology was significantly associated with an increase in the number of fall‐related fractures (RR 1.66; 95% CI 1.07–2.59) (Table S6).
Secondary outcomes
The results of the primary analysis (excluding post‐hoc analyses) and C‐NMA comparing all intervention components with usual care for the outcomes number of repeated fallers, falls rate, number of hip fractures, and fracture rate are presented in Appendix S7 to S10. Table 3 reports the effect sizes and P‐scores of interventions (including post‐hoc analyses) with a statistically significant association and the corresponding CINeMA confidence rating.
For falls rate, we performed an additional analysis in which all multifactorial interventions were considered as one intervention type. Compared with usual care, multifactorial interventions were significantly associated with a reduced fall frequency (RR 0.88; 95% CI 0.81–0.96, P‐score 0.54; 111 studies, 53,923 participants).
CINeMA confidence rating
Tables 3 and 4 present the CINeMA confidence ratings for interventions that were statistically significant associated with a lower risk of falls and fall‐related fractures. Appendix S11 provides detailed results from the CINeMA approach.
DISCUSSION
In this SR and NMA, we updated current evidence on prevention of falls and fall‐related fractures in older persons, with a focus on high‐risk subgroups of multimorbid older adults and aged ≥75 years. Compared with previous NMA by Tricco et al., we considered 57 new studies and added 4 interventions previously not considered.
Several single and multiple interventions were associated with a lower risk of falls (i.e., in keeping with Tricco et al.) and also with fall‐related fractures (divergent from Tricco). Exercise (single intervention) was often investigated in included studies and associated with a lower risk of all primary and secondary outcomes in the primary analysis. This was no longer evident once disentangled down to the C‐NMA level, where no effect of exercise on fracture outcomes was observed. The same applied to basic falls risk assessment. These findings did not alter after post hoc inclusion of data from two recent major RCTs. 13 , 14
Common components seen in significant multiple interventions were exercise, assistive technology, environmental assessment and modifications, quality improvement strategies, and basic falls risk assessment. In agreement with a recent Cochrane review, multifactorial interventions were associated with a reduction in falls rate, but not in number of fallers. 5 One possible explanation is that falls rate may measure falls risk more accurately than number of fallers. Although the latter counts persons who fall once or fall repetitively as one outcome event, the outcome falls rate counts each fall as a separate outcome event. Contrary to our findings above, Tricco et al. found that multifactorial intervention (comprised of exercise and quality improvement strategies) was associated with a reduction in number of fallers (OR 0.68; 95% CI 0.49–0.94). 6
We performed a meta‐analysis on vitamin D supplementation versus placebo. We can corroborate previously published literature, which showed no association of vitamin D with the risk of falls or fall‐related fractures. 23 , 24 Although Tricco et al. found an effect on fallers and injurious falls when vitamin D is combined with calcium supplementation and other intervention components. 6
Unexpectedly, considering that it is not widely used in clinical practice, whole‐body vibration was associated with a lower risk of falls. This intervention was investigated in few studies (with small study populations and high summary risk of bias), so the clinical value is still unclear. The benefit we observed may be subject to publication bias.
This study has several strengths. (1) SR and NMA were performed in accordance with the EPOC tool and CINeMA approach. (2) Based on statistically significant effect estimates combined with high P‐scores, we ranked interventions to draw conclusions. (3) By extracting information on the components forming the multifactorial interventions, we could also address which combination of components is most effective. (4) We investigated community‐dwelling older adults, applied few exclusion criteria, and included interventions that complied with the transitivity assumption; thus our results are widely generalizable. Moreover, although severe dementia was an exclusion criterion, we did allow studies with mild to moderate dementia participants as this reflects real life and the increasing prevalence within the community‐dwelling older population. (5) The large population size enabled subgroup analyses (aged 75+, multimorbidity).
This study has some limitations. (1) In contrast to Tricco and colleagues, we assigned a high risk of bias for domain blinding when falls and fractures were self‐reported in a patient‐diary, as it was often not possible to blind participants to their intervention. This may explain our larger percentage of studies deemed at high risk of bias for blinding, but is difficult to prevent due to the nature of the interventions. Furthermore, blinding participants to their assigned intervention could affect their willingness/probability of engaging with the intervention and their reporting of fall incidents. (2) Allocation concealment was unclear in half of the studies and this might affect the trust we can place in the estimates of intervention effect sizes. Although baseline characteristics and fall history were reasonably balanced (similar between the study arms in 85% and 77% of trials, respectively), it is possible that other influencing factors (e.g., willingness/probability of engaging with the interventions) were less balanced across the study arms. (3) Categorization of interventions into components allowed us to make inferences about the effect of these components as a whole (e.g., exercise), but not about specific subcategories within these components (e.g., strength training or tai chi). Where a component showed no significant effect, it could still be that subcategories within this component are effective, particularly so in cases with high “within‐component heterogeneity.” Many different interventions for fall prevention were evaluated, and working with clustered intervention components was necessary to maintain sufficient power for the NMA. (4) Similarly, to avoid insufficient power, we were unable to distinguish between different intervention dosages, durations of treatment, or between different lengths of follow‐up durations in the NMAs. However, we expect the effect of the interventions to decrease with a longer follow‐up duration, possibly reducing the overall effect estimates. Only 20% of included studies reported a follow‐up longer than 1 year. Differences in dosage and length of interventions may also lead to “within‐component heterogeneity.” (5) CINeMA software cannot address rate outcomes. However, most studies reporting rates also provided data on dichotomous outcomes, for which CINeMA assessment was possible. Due to this overlap, the overall certainty in the evidence is expected to be similar across the dichotomous and rate outcomes. (6) Finally, most studies have similar baseline risk (e.g., falls rate) across interventions. When this is violated and large discrepancies are present, this limits our ability to draw indirect comparisons across the (C‐)NMA. With few studies per comparison arm, we cannot test with certainty whether baseline risk or other factors differ across intervention comparisons. However, we attempted to mitigate this risk with clinical (− compliance with transitivity assumption) and statistical (− heterogeneity assessments) judgments.
This NMA provides an extensive overview of current evidence for effective fall prevention interventions in older persons. Yet some questions remain unanswered.
More research is needed on fall prevention interventions in multimorbid older persons, because this subgroup analysis lacked sufficient power for the NMA. Additional studies are needed to clarify and confirm the effect of whole‐body vibration, given the potential publication bias identified.
Further research is needed to evaluate the effects of specific subcategories within the intervention components. For example, two recent studies performed by the research group of Tricco et al. explored the effects of different quality improvement strategies and exercise interventions on falls. 25 , 26
CONCLUSION
Exercise is associated with a lower risk of falls and fall‐related fractures. Common components of significant multiple interventions are exercise, assistive technology, environmental assessment and modifications, quality improvement strategies, and basic falls risk assessment. A multifactorial intervention is associated with a reduction in falls rate, but not with a reduction in number of fallers. Over half of the studies included had methodological short comings (lack of allocation concealment and high risk of blinding). This points to a greater issue within the evidence base and highlights the need for more robust study procedures/reporting in which future policy can be based on. Few studies have investigated the effect of fall prevention interventions in multimorbid older people, which is highly recommended for future research.
CONFLICT OF INTEREST
Dr Tricco reports receiving a Tier 2 Canada Research Chair in Knowledge Synthesis grant. Dr Straus reports receiving a Tier 1 Canada Research Chair in Knowledge Translation grant. No other financial or personal conflicts were reported.
AUTHOR CONTRIBUTIONS
The study protocol was created by Lauren Dautzenberg, Shanthi Beglinger, Sofia Tsokani, Stella Zevgiti, Nicolas Rodondi, Rob J. P. M. Scholten, Anne W. S. Rutjes, Marcello Di Nisio, Wilma Knol, Dimitris Mavridis, and Huiberdina L. Koek. Lauren Dautzenberg executed the literature search. Study selection was performed by Lauren Dautzenberg and Shanthi Beglinger under supervision of Rob J. P. M. Scholten, Marielle Emmelot‐Vonk, Wilma Knol, and Huiberdina L. Koek. Data extraction was performed by Renee C. M. A. Raijmann, Shanthi Beglinger, Lisa Bretagne, and Lauren Dautzenberg. A part of the data included in this study was provided by Andrea C. Tricco, Sharon E. Straus, and Sonia Thomas. Data analysis was performed by Sofia Tsokani, Stella Zevgiti, and Dimitris Mavridis. The manuscript was drafted by Lauren Dautzenberg, Shanthi Beglinger, Rob J. P. M. Scholten, Wilma Knol, and Huiberdina L. Koek. The manuscript was revised by Sofia Tsokani, Stella Zevgiti, Renee C. M. A. Raijmann, Nicolas Rodondi, Anne W. S. Rutjes, Marcello Di Nisio, Marielle Emmelot‐Vonk, Andrea C. Tricco, Sharon E. Straus, Sonia Thomas, Lisa Bretagne, and Dimitris Mavridis. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
SPONSOR'S ROLE
The funding had no input on the study design, data collection, data analysis or interpretation, report writing, approval of the manuscript, or the decision to submit the paper for publication.
Supporting information
Data S1. Supplementary information.
ACKNOWLEDGMENTS
This work is part of the project “OPERAM: OPtimising thERapy to prevent Avoidable hospital admissions in the Multimorbid elderly” supported by the European Union‘s Horizon 2020 research and innovation programme under the grant agreement No 6342388, and by the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract number 15.0137. The opinions expressed and arguments employed herein are those of the authors and do not necessarily reflect the official views of the EC and the Swiss government. A. C. T. is funded by a Tier 2 Canada Research Chair in Knowledge Synthesis. S. E. S. is funded by a Tier 1 Canada Research Chair in Knowledge Translation.
Dautzenberg L, Beglinger S, Tsokani S, et al. Interventions for preventing falls and fall‐related fractures in community‐dwelling older adults: A systematic review and network meta‐analysis. J Am Geriatr Soc. 2021;69(10):2973-2984. 10.1111/jgs.17375
Lauren Dautzenberg and Shanthi Beglinger contributed equally to this work.
The abstract of this paper has been presented at the European Geriatric Medicine Society (EUGMS) E‐Congress 2020.
Funding information Horizon 2020 Framework Programme, Grant/Award Number: 6342388; the Swiss State Secretariat for Education, Research and Innovation (SERI), Grant/Award Number: 15.0137; Tier 1 Canada Research Chair in Knowledge Translation; Tier 2 Canada Research Chair in Knowledge Synthesis
REFERENCES
- 1. National Institute for Health and Care Excell . Falls in older people: assessing risk and prevention. NICE Clin Guidel. Manchester: National Institute for Health and Care Excellence; 2013:1‐33 https://www.nice.org.uk/guidance/cg161/resources/falls-in-older-people-assessing-risk-and-prevention-35109686728645. Accessed November 4, 2019. [PubMed] [Google Scholar]
- 2. Tinetti M, Speechley M, Ginter S. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701‐1707. [DOI] [PubMed] [Google Scholar]
- 3. Campbell A, Borrie M, Spears G, Jackson S, Brown J, Fitzgerald J. Circumstances and consequences of falls experienced by a community population 70 years and over during a prospective study. Age Ageing. 1990;19:136‐141. [DOI] [PubMed] [Google Scholar]
- 4. Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negria E. Risk factors for falls in community‐dwelling older people a systematic review and meta‐analysis. Epidemiology. 2010;21(5):658‐668. [DOI] [PubMed] [Google Scholar]
- 5. Hopewell S, Adedire O, Copsey BJ, et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community (review). Cochrane Database Syst Rev. 2018;7(7):1‐307. 10.1002/14651858.CD012221.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tricco A, Thomas S, Veroniki A, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta‐analysis. JAMA. 2017;318(17):1687‐1699. 10.1001/jama.2017.15006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gillespie L, Robertson M, Gillespie W, et al. Interventions for preventing falls in older people living in the community (review). Cochrane Database Syst Rev. 2012;9:1‐416. 10.1201/b12752-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80‐97. 10.1002/jrsm.1037 [DOI] [PubMed] [Google Scholar]
- 9. Mavridis D, Giannatsi M, Cipriani A, Salanti G. A primer on network meta‐analysis with emphasis on mental health. Evid Based Ment Health. 2015;18(2):40‐46. 10.1136/eb-2015-102088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Streit S, Da Costa BR, Bauer DC, et al. Multimorbidity and quality of preventive care in Swiss University primary care cohorts. PLoS One. 2014;9(4):e96142. 10.1371/journal.pone.0096142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community‐dwelling older adults us Preventive Services Task Force recommendation statement. JAMA. 2018;319(16):1696‐1704. 10.1001/jama.2018.3097 [DOI] [PubMed] [Google Scholar]
- 12. Cheng P, Tan L, Ning P, et al. Comparative effectiveness of published interventions for elderly fall prevention: a systematic review and network meta‐analysis. Int J Environ Res Public Health. 2018;15(3):498‐511. 10.3390/ijerph15030498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamb SE, Bruce J, Hossain A, et al. Screening and intervention to prevent falls and fractures in older people. N Engl J Med. 2020;383(19):1848‐1859. 10.1056/nejmoa2001500 [DOI] [PubMed] [Google Scholar]
- 14. Bhasin S, Gill TM, Reuben DB, et al.; STRIDE Trial Investigators. A randomized trial of a multifactorial strategy to prevent serious fall injuries. N Engl J Med. 2020;383(2):129‐140. 10.1056/nejmoa2002183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cochrane Effective Practice and Organisation of Care (EPOC) . Suggested Risk of Bias Criteria for EPOC Reviews. EPOC Resources Fo r Review Authors. United Kingdom: The Cochrane Effective Practice and Organisation of Care (EPOC) group; 2017. http://Epoc.Cochrane.Org/Resources/Epoc-Resources-Review-Authors. Accessed April 8, 2019. [Google Scholar]
- 16. Caldwell D, Welton N. Approaches for synthesising complex mental health interventions in meta‐analysis. Evid Based Ment Health. 2016;19(1):16‐21. 10.1136/eb-2015-102275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rücker G, Petropoulou M, Schwarzer G. Network meta‐analysis of multicomponent interventions. Biom J. 2020;62:808‐821. 10.1002/bimj.201800167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Welton N, Caldwell D, Adamopoulos E, Vedhara K. Mixed treatment comparison meta‐analysis of complex interventions: psychological interventions in coronary heart disease. Am J Epidemiol. 2009;169(9):1158‐1165. 10.1093/aje/kwp014 [DOI] [PubMed] [Google Scholar]
- 19. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta‐analysis works without resampling methods. BMC Med Res Methodol. 2015;15(1):58. 10.1186/s12874-015-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. Cinema: an approach for assessing confidence in the results of a network meta‐analysis. PLoS Med. 2020;17(4):e1003082. 10.1371/JOURNAL.PMED.1003082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salanti G, del Giovane C, Chaimani A, Caldwell DM, Higgins JPT. Evaluating the quality of evidence from a network meta‐analysis. PLoS One. 2014;9(7):e99682. 10.1371/journal.pone.0099682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jadad AR, To MJ, Emara M, Jones J. Consideration of multiple chronic diseases in randomized controlled trials. JAMA. 2011;306(24):2670‐2672. [DOI] [PubMed] [Google Scholar]
- 23. Yao P, Bennett D, Mafham M, et al. Vitamin D and calcium for the prevention of fracture: a systematic review and meta‐analysis. JAMA Netw Open. 2019;2(12):e1917789. 10.1001/jamanetworkopen.2019.17789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bolland M, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta‐analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6(11):847‐858. 10.1016/S2213-8587(18)30265-1 [DOI] [PubMed] [Google Scholar]
- 25. Tricco A, Thomas S, Veroniki AA, et al. Quality improvement strategies to prevent falls in older adults: a systematic review and network meta‐analysis. Age Ageing. 2019;48(3):337‐346. 10.1093/ageing/afy219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sibley KM, Thomas SM, Veroniki AA, et al. Comparative effectiveness of exercise interventions for preventing falls in older adults: a secondary analysis of a systematic review with network meta‐analysis. Exp Gerontol. 2021;143:111151. 10.1016/j.exger.2020.111151 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary information.


