Abstract
Gα15 activates phospholipase Cβ in response to the greatest variety of agonist-stimulated heptahelical receptors among the four Gq class G-protein α subunits expressed in mammals. Gα15 is primarily expressed in hematopoietic cells in fetal and adult mice. We disrupted the Gα15 gene by homologous recombination in embryonic stem cells to identify its biological functions. Surprisingly, hematopoiesis was normal in Gα15−/− mice, Gα15−/− Gαq−/− double-knockout mice (which express only Gα11 in most hematopoietic cells), and Gα11−/− mice, suggesting functional redundancy in Gq class signaling. Inflammatory challenges, including thioglycolate-induced peritonitis and infection with Trichinella spiralis, stimulated similar responses in Gα15−/− adults and wild-type siblings. Agonist-stimulated Ca2+ release from intracellular stores was assayed to identify signaling defects in primary cultures of thioglycolate-elicited macrophages isolated from Gα15−/− mice. C5a-stimulated phosphoinositide accumulation and Ca2+ release was significantly reduced in Gα15−/− macrophages. Ca2+ signaling was abolished only in mutant cells pretreated with pertussis toxin, suggesting that the C5a receptor couples to both Gα15 and Gαi in vivo. Signaling evoked by other receptors coupled by Gq class α subunits appeared normal in Gα15−/− macrophages. Despite discrete signaling defects, compensation by coexpressed Gq and/or Gi class α subunits may suppress abnormalities in Gα15-deficient mice.
Heterotrimeric G proteins transduce signals from ligand-activated seven-transmembrane domain receptors to effector proteins that regulate the release of intracellular second messengers, such as Ca2+ and cyclic AMP. A diverse family of G-protein-coupled receptors bind numerous hormones and neurotransmitters, peptides, small proteins, and lipid molecules. The biological functions mediated by G proteins are equally diverse, including behavioral and sensory functions, appetite control, arousal, metabolism, development, inflammation, and chemotaxis.
Heterotrimeric G proteins are composed of α and βγ subunits that can independently regulate effector proteins. Mammals express 16 distinct Gα subunit genes that are grouped in four classes, Gq, Gi, Gs, and G12, according to sequence similarity, effector regulation, and responsiveness to RGS (regulators of G-protein signaling) proteins, a recently identified family of GTPase-activating proteins (GAPs) for Gα subunits (30). The Gq class α subunits activate all isoforms of phospholipase Cβ (PLCβ), which hydrolyze the membrane lipid phosphatidylinositol-4,5-bisphosphate to produce inositol trisphosphate and diacylglycerol. PLCβ2 and PLCβ3 are also activated by Gβγ subunits, primarily released from Gi class G proteins (31), which is the basis for pertussis toxin inhibition of PLCβ activity and Ca2+ signaling evoked by Gi-coupled receptors (2, 20). Inositol trisphosphate produced by the activity of PLCβ evokes calcium release from intracellular stores, and diacylglycerol activates several isoforms of protein kinase C (PKC). Thus, Gq class α subunits regulate signaling pathways that are implicated in cellular proliferation and differentiation.
There are four Gq class α subunits in mice and humans; Gαq, Gα11, Gα14, and Gα15 or Gα16 (mouse or human ortholog, respectively [13, 33]). Gα15 and Gα11 are encoded by the tandemly duplicated Gna15 and Gna11 genes and colocalize to mouse chromosome 10, while Gαq and Gα14, encoded by the Gnaq and Gna14 genes, colocalize to mouse chromosome 19 (28). The two widely expressed Gq class α subunits, Gαq and Gα11, are 89% identical in amino acid sequence, and they couple an identical repertoire of receptors to PLCβ activation with similar efficiencies in vitro, in cultured cells, and in primary cells isolated from animals (28, 35). Analysis of single- and double-knockout (KO) mice with deficiencies in Gαq and/or Gα11 suggests that gene dosage may be a key factor in dissecting Gq class signaling pathways and their biological functions (28). The only apparent phenotypes of the single-KO Gαq−/− mice involve cell types or tissues, such as platelets and cerebellum, where Gα11, Gα14, and Gα15 expression is weak or absent (24, 27). Although mice with homozygous disruption of either the Gαq or Gα11 gene are viable and fertile, with only discrete phenotypic defects (24, 27), deletion of both genes (Gαq−/− Gα11−/−) results in embryonic lethality during midgestation (embryonic day 10.5) from a defect in cardiomyocyte proliferation (28). Cultured embryonic cardiomyocytes express Gαq and Gα11 but little or no Gα14 and Gα15, and in the absence of Gq/11, Ca2+ signaling is no longer stimulated by the mitogenic factor angiotensin II (28).
The requirement of Gq class signaling in cell proliferation suggested a possible function of Gα15 in hematopoiesis. Gα15 is the most divergent of the Gq class α subunits (55% amino acid identity to Gαq, Gα11, and Gα14) and has the most restricted expression pattern, being principally confined to hematopoietic cells (Fig. 2 and reference 33). Though Gα15 activates PLCβ isoforms similarly to other family members, it possesses different pharmacological and biochemical properties in vitro (21). Gα15 couples to many receptors that are not activators of Gαq and Gα11 (26, 37) and therefore was proposed for high-throughput screens to identify ligands and orphan receptors (12). The receptor promiscuity of Gα15 suggested that it may have unique functions in hematopoietic cells not performed by other Gq class proteins. However, the receptor promiscuity of Gα15 is less apparent when cotransfected with some C-C chemokine receptors which are coexpressed with Gα15 (3, 22). Signaling specificity in hematopoietic cells may also be controlled by other factors, such as RGS proteins, that could further limit the range of receptors which are functionally coupled by Gα15.
FIG. 2.
Expression of the Gq class α subunits Gα15, Gαq, and Gα11 in wild-type and Gα15 KO mice. (A) Expression of Gα15 in mouse hematopoietic cells. The Gα15 C-terminal antibody B861-7 was used to detect Gα15 in membranes obtained from murine resident peritoneal exudate leukocytes (PEL), peritoneal exudate macrophages collected and purified by adherence 5 days after thioglycolate injection (PEM), and total bone marrow (BM) or mouse bone marrow separated into fractions by synchrony elutriation. Fraction 1 (Fr.1) is enriched in lymphocytes and may contain erythrocytes; fraction 2 (Fr.2) contains lymphocytes and blasts; fraction 3 (Fr.3) is enriched in blasts and mature neutrophils, fraction 4 (Fr.4) contains mostly mature neutrophils. rGα15, recombinant Gα15. (B) Expression of Gα15, Gαq, and Gα11 in membranes from hematopoietic tissues of Gα15 wild-type and Gα15−/− mice, using antibodies B861-7, W082, and B825. The liver was used as a negative control for Gα15 and as a positive control for Gαq and Gα11. +/+, Gα15 wild-type mice; −/−, Gα15 KO mice; rGα15, membranes from Gα15 expressed in Sf9 cells; rGα, recombinant Gα15, Gαq, or Gα11 on their respective blots. Five micrograms of membrane protein was loaded per lane except in day 5 peritoneal exudate macrophages (3 μg).
We created Gα15-deficient mice to identify the putative biological roles of Gα15 in development, hematopoiesis, and immune function. The hypothesis was that Gα15, due to its unique characteristics within the Gq class of α subunits, may have evolved specific functions that cannot be compensated by the coexpressed family members Gαq and Gα11. The Gα15 gene was disrupted by homologous recombination in embryonic stem (ES) cells. Surprisingly, hematopoiesis and responses to several inflammatory challenges were normal in Gα15−/− mice. This suggested that either the differences in signaling were too small to provide a biological consequence or that Gα15 deficiency was compensated for by other G proteins. We used primary cultures of macrophages derived from the Gα15−/− mice and their Gα15+/+ littermates to show that the phosphoinositide (PI) and Ca2+ responses to the anaphylatoxin C5a were greatly diminished in the KO macrophages. We show that three receptors involved in the inflammatory response (C5a, P2Y2, and platelet-activating factor [PAF]) each have different G-protein coupling specificity and utilize at least two distinct pathways to induce PI release, which may provide intrinsic compensatory mechanisms in G-protein signaling pathways.
MATERIALS AND METHODS
Knockout of Gna15.
ES cells were maintained essentially as described previously (28). R1 ES cells from 129SV embryos were grown on primary embryonic fibroblasts rendered mitotically inactive by treatment with mitomycin. The Gna15 KO vector was linearized at a unique NotI site (Fig. 1) and electroporated into ES cells. Twelve heterozygous clones were obtained of 241 neomycin-resistant colonies. Four independent Gna15 heterozygous ES cell clones were injected into blastocysts, and one chimera gave germ line transmission of the mutant allele. Southern blots and 32P radiolabeling of probes were performed exactly as described elsewhere (13). Following extensive characterization of the ES clones and founder mice by Southern blot analyses to confirm homologous recombination and single integration of the targeting vector, genotyping was done by PCR. Oligonucleotide primers used were CT133 (CAGCACGCCAGCCTAGTGATG) and CT115 (CTTCACGGAGAAGCAGTACTC), to amplify a 550-bp fragment in the wild-type allele, and TW30 (AGATGCGCATCATTCACGGT) and TW144 (GATCAGCAGCCTCTGTTCCAC), to amplify a 720-bp fragment in the KO allele (Fig. 1).
FIG. 1.
KO vector and strategies for characterizing homologous recombination by Southern blotting and genotyping by PCR after electroporation of the Gα15 replacement vector. (A) Wild-type (wt) allele and the targeting vector. The restriction sites are shown to scale. tk, thymidine kinase. (B) The KO allele. The pgk::neo insertion cassette is not drawn to scale. The SpeI, EcoRV, and HindIII sites used in characterizing the wild-type and KO alleles by Southern blot are shown on each allele. The inserted pgk::neo cassette is 2.1 kb in size and contains both EcoRV and SpeI restriction sites. The 5′ probe hybridizes to a 14-kb fragment in the wild-type allele and 9.5 kb in the KO allele when DNA is digested with EcoRV. The 3′ probe recognizes 9.2- and 7-kb fragments in wild-type and KO mice after digest with SpeI and HindIII. The Neo probe hybridizes to a 14-kb SpeI fragment in the KO allele. The relative positions of PCR products obtained from amplification of the wild-type allele with primers CT115 and CT133 (550 bp) and the KO allele with primers TW30 and TW144 (720 bp) are indicated. (C) Results obtained from genotyping Gα15+/+, Gα15+/−, and Gα15−/− tail DNA by Southern blot using the 5′ probe after digest with EcoRV; ethidium bromide-stained 2.5% agarose gel of PCR products obtained from amplifying the same tail DNA with the primers described above.
Preparation of membrane proteins for Western blot analysis of G-protein α subunits.
Tissues were collected, and homogenates were prepared with a Dounce homogenizer in HMED (20 mM HEPES, 2 mM MgCl2, 1 mM EDTA, and 1 mM dithiothreitol containing protease inhibitors (0.01 mg each of leupeptin and lima bean trypsin inhibitor per ml and 0.016 mg each of phenylmethyl sulfonyl fluoride, Nα-p-tosyl-l-lysine chloromethyl ketone, and tosylsulfonyl phenylalanyl chloromethyl ketone per ml. Homogenates were centrifuged at 500 × g to remove unbroken cells and nuclei, and supernatants were centrifuged at 100,000 × g. Membranes were resuspended in HMED, and protein concentrations were determined by Bradford assay using the Bio-Rad protein assay dye reagent concentrate prior to storage at −80°C. For sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), 5 μg of membrane protein was loaded per lane unless specified. Antibody B861 recognizes specifically the C-terminal end of Gα15, and antibodies W082 and B825 recognize specifically Gαq and Gα11, respectively.
Fluorescence-activated cell sorting.
Peripheral blood was collected from the tail vein into Alsever's solution (Gibco-BRL, Grand Island, N.Y.), and single-cell suspensions were prepared from hematopoietic tissues for antibody analysis. Conjugated anti-mouse antibodies used were B220-biotin, CD4-fluorescein isothiocyanate, CD8-biotin, Gr-1-biotin, Mac-1-biotin, and Mel-14-fluorescein isothiocyanate; streptavidin-phycoerythrin (Pharmingen, San Diego, Calif.) was used when applicable; 5,000 events were collected per sample on a FACScan analytical instrument (Becton Dickinson Co., San Jose, Calif.).
Bone marrow transfer and growth.
Single-cell suspensions were prepared from bone marrow of wild-type and KO donor mice and was used for growth studies in MethoCult M-3430 (StemCell Technologies Inc., Vancouver, British Columbia, Canada) or for bone marrow transfer. Recipient mice (BALB/c) were exposed to two doses of irradiation, 500 and 400 rads, in a Gamma Cell 40 small-animal irradiator containing two 139Cs sources (Atomic Energy Ltd., Ottawa, Ontario, Canada). Irradiated recipient mice were injected with 5 × 104 donor cells in the lateral tail vein. Spleens were collected from recipient mice 9 days later and fixed in Bouin's fixative. Spleen CFU (CFU-S) were counted in a Zeiss dissection microscope. In a first experiment, four recipient mice were injected with medium alone, eight were injected with wild-type cells, and eight were injected with Gα15−/− cells. In a second, independent experiment, 4 recipient mice were injected with medium 10 were injected with wild-type cells, and 10 were injected with Gαq−/− Gα15−/− cells.
Thioglycolate-induced peritonitis.
Mice between 8 and 12 weeks of age were injected with 1 ml of 3% thioglycolate. Peritoneal cavities were lavaged at 0 (uninjected), 18, 48, or 96 h following intraperitoneal (i.p.) injection of thioglycolate. Cells from the peritoneal lavage fluid were spun onto a slide with a cytospin. Slides were stained with Diff-Quik (Fisher Diagnostic). Two to seven mice of each genotype were analyzed at each time point. Differential counts of neutrophils, eosinophils, and mononuclear cells were determined with a 100× oil immersion lens. A minimum of 100 cells were counted three times per slide.
Trichinella spiralis infection.
Twelve-week-old mice were administered 500 infective larvae orally. Six mice of each genotype (Gα15+/+ and Gα15−/−) were analyzed. Blood smears were taken from the tail vein in duplicates at days 0, 6, 9, 11, 13, 15, 17, 21, 23, 25, 27, and 30 after infection and stained with Diff-Quik. The proportion of eosinophils in 100 to 200 leukocytes was determined for each smear.
Thioglycolate-elicited macrophages for ex vivo experiments and macrophage culture.
Six- to ten-week-old mice were injected i.p. with 2 ml of 4% aged brewer's thioglycolate. Peritoneal exudates were collected with Dulbecco modified Eagle medium (DMEM) containing 5% fetal bovine serum (FBS) 4 days after injection. Cells from two to three mice were pooled per genotype. Macrophages were purified by adherence to the tissue culture dish.
Fura-2 Ca2+ imaging.
Cells were plated on coverslips and processed as described elsewhere (36). Cells were loaded with Fura-2/AM in the presence of Pluronic for 20 min and then set on ice. Coverslips were mounted onto perfusion chambers, and cells were continuously perfused during fluorescence recordings. Fluorescence was recorded with the Delta Scan I imaging system and an IC-200 camera. The images were captured with the Image Master program and analyzed with Felix.
PI assay.
Cells were distributed to 12-well tissue culture dishes at 106 cells per well and metabolically labeled 48 h with 8 μCi of [3H]myo-inositol (Dupont) per ml in labeling medium (inositol-free DMEM, 5% FBS, penicillin-streptomycin, glutamine). Labeling medium was added to each well in non-pertussis toxin-treated cells. The assay was performed essentially as described elsewhere (17). The KD for binding of 125I-C5a to the purified receptor is approximately 1.7 to 20 nM (6), and maximum PI release was determined to be obtained between 50 and 120 nM (1). The PI assays were performed with 100 nM C5a in the Gα15+/+ and Gα15−/− thioglycolate-elicited peritoneal macrophages. For UTP and PAF, dose-response curves were performed to obtain a 50% effective concentration. The 50% effective concentration for UTP stimulation of PI release was determined to be 5 to 7 μM, whereas half-maximal activity was obtained with 10 ng of PAF per ml. Subsequent assays were therefore performed with 10 μM UTP and 30 ng of PAF per ml. For treatment with pertussis toxin, pertussis toxin (List Biological Laboratories) was added to each well in labeling medium to a final concentration of 100 ng/ml, 24 h before the start of the assay.
ERK activation.
Day 4 thioglycolate-elicited peritoneal macrophages were plate purified on 100-mm-diameter dishes. Cells were incubated in starvation medium (DMEM [Cellgro], 0.5% FBS, 4% penicillin-streptomycin, glutamine) for 14 to 18 h prior to stimulation. When needed, pertussis toxin was added to 100 ng/ml with the starvation medium. Cells were stimulated with 100 nM C5a for 3 min, then rapidly rinsed, and lysed (50 mM HEPES [pH 7.5], 150 mM NaCl, 10% glycerol, 1% Triton X-100, 100 mM NaF, 0.2 mM sodium orthovanadate, 10 μg of aprotinin per ml, 1 mM phenylmethylsulfonyl fluoride). Protein concentrations were determined by Bradford analysis, and 20 μg of whole-cell lysate was loaded per lane on a 10% polyacrylamide gel for SDS-PAGE. Following transfer to a nitrocellulose membrane, the Western blots were probed with antibody V6671 (Promega).
RESULTS
Knockout of Gna15.
The Gα15 gene (Gna15) was disrupted in mice by homologous recombination in ES cells. The replacement vector (Fig. 1A) contained a neomycin resistance gene under the control of the PGK promoter (pgk::neo) flanked by 5′ (5-kb) and 3′ (3.4-kb) segments of Gna15. A null allele was created by homologous recombination of the replacement vector to delete 8 kb that included exons 3 through 6 of Gna15. The deleted exons contained three of the five amino acid motifs (7) which are essential for guanine nucleotide binding to the α subunit. The targeting vector was electroporated into ES cells, and heterozygous clones were obtained under selection with Geneticin and ganciclovir. Heterozygous ES cell clones were identified with the 5′ probe (Fig. 1B) and confirmed with the 3′ probe and the Neo probe. Nine chimeric mice were obtained, and one transmitted the Gα15 null allele to offspring. Intercrossing heterozygous Gα15+/− mice produced viable and fertile homozygous null (Gα15−/−) progeny (Fig. 1C).
Gα15−/− mice did not exhibit obvious phenotypic defects. Western blotting using an antibody directed at the C-terminal residues of Gα15 confirmed that a null allele was created (Fig. 2B). Gα15 was detected in membrane proteins from cell types and tissues that normally express Gα15 in wild-type mice but not in those isolated from Gα15−/− mice. To assess whether expression of other Gq class α subunits was altered in the absence of Gα15, we also assayed expression of Gαq, Gα11, and Gα14 in the same tissues by Western blot analysis (Fig. 2B and data not shown). The relative levels of expression of each of these G-protein α subunits were similar in wild-type and Gα15−/− mice.
Hematopoiesis appears normal in single and double Gq class KO mice.
Differential expression of Gα15/Gα16 in hematopoietic cells according to their maturational stage (2, 16, 32, 33) suggested that hematopoiesis might be altered in the Gα15−/− mice. Therefore, we characterized the cellular composition of hematopoietic tissues of Gα15−/− mice by FACScan analysis. Single-cell suspensions were prepared for analysis from peripheral blood, spleen, thymus, peripheral and mesenteric lymph nodes, Peyer's patches, and bone marrow isolated from wild-type or mutant mice. No differences between Gα15−/− and wild-type mice were observed in the tissue composition of B cells, CD4+ and/or CD8+ T cells, neutrophils, and monocytes. The architecture of the major lymphoid organs, including thymus, spleen, peripheral lymph nodes, and Peyer's patches, was likewise intact. The proportion of plasma erythrocytes was normal as determined by hematocrit (wild type, 49.8 ± 0.003%; Gα15−/−, 50.9 ± 0.013).
The integrity of the erythroid and myeloid precursors of the Gα15−/− bone marrow was assessed by bone marrow transfer to lethally irradiated recipient mice (erythroid lineage) and by bone marrow culture in methylcellulose (myeloid lineages). The numbers of CFU-S present on the spleens of lethally irradiated recipient mice 9 days after injection with bone marrow cells were similar in recipients rescued with Gα15+/+ (11.4 ± 4.4) or Gα15−/− (8.6 ± 3.5) cells. Growth in methylcellulose also indicated that there was no difference in the number and ability of myeloid precursors to proliferate and differentiate in response to the growth factors provided in the methylcellulose (data not shown). Our findings that Gα15−/− mice produce normal numbers and ratios of B, T, and myeloid cells suggest that Gα15 is not required during hematopoiesis.
To address the potential compensatory role of other Gq family members, mice deficient in both Gαq and Gα15 were assessed for hematopoietic competence. Double-KO Gαq−/− Gα15−/− mice were obtained by crossing single-KO mice with null mutations in the Gαq and Gα15 genes. The double KO mice have all the features of the Gαq−/− mice single-KO mice (24, 27); they are smaller than their littermates and exhibit the same bleeding disorder and ataxia. These defects are not altered by the simultaneous absence of Gα15 and Gαq. As had been observed in the Gα15−/− mice, FACScan analysis showed that proportions of B cells, T cells, granulocytes, and monocytes in all tissues tested were not significantly different from those observed in wild-type animals. In a bone marrow transfer experiment, the erythroid precursors of the Gαq−/− Gα15−/− bone marrow were fully competent to rescue lethally irradiated recipient mice. The number of colonies on the spleens of recipient mice injected with Gαq−/− Gα15−/− bone marrow (13.5 ± 1.5) was not significantly different from the number of CFU-S found in recipients of wild-type cells (11.3 ± 3.8). No obvious hematopoietic deficiencies were detected in any of the Gq class single-KO mice, possibly indicating that Gq class signaling is not required in steady-state hematopoiesis. However, Gα15 is coexpressed with both Gαq and Gα11 in most hematopoietic cell types that have been analyzed (33). Thus, the activity of Gα11, for example, may be sufficient to support hematopoiesis in the absence of Gα15 and/or Gαq. Unfortunately, the double-KO Gα11−/− Gα15−/− mice cannot be obtained efficiently through meiotic recombination in compound heterozygous mice because of the proximity (6 kb) of the tandemly duplicated Gα11 and Gα15 genes on mouse chromosome 10 (13).
The Gα15−/− mice perform adequately in in vivo models of inflammation and infection.
Gα15 (and Gα16) couple many receptors for chemokines and other chemotactic molecules to the activation of PLCβ in cotransfection systems (1, 22, 26, 34, 37). These receptors, including the receptors for interleukin-8 (IL-8), C5a, PAF, thrombin, formylmethionyl-leucyl-phenylalanine, and purine nucleotides, are involved in chemotaxis and inflammation and are present on neutrophils and/or monocyte lineages that express Gα15 abundantly. We therefore tested the role of Gα15 in thioglycolate-induced peritonitis to assay the mobilization and recruitment of neutrophils (18 h following i.p. injection of thioglycolate) and macrophages (48 to 96 h following injection). The resident peritoneal cell populations, as well as recruited neutrophils and macrophages, were comparable between wild-type and KO mice at all time points studied (data not shown). Population of the peritoneal cavity with eosinophils was larger in wild-type than in Gα15−/− mice at 48 h following thioglycolate injection but not significantly different at 96 h.
These results indicate that Gα15 is not required for the recruitment of neutrophils and macrophages in a nonantigenic specific type of inflammatory challenge. Although eosinophil recruitment is not described as a characteristic response to thioglycolate-induced peritonitis, a difference between wild-type and mutant mice was observed 48 h following thioglycolate injection, suggesting that eosinophil recruitment might be deficient or altered in the Gα15−/− mice. We therefore challenged the Gα15−/− mice to a parasitic infection with T. spiralis, in which eosinophilia is a characteristic response. The percentage of peripheral blood eosinophils was determined in blood smears collected from 12 time points at 0 to 30 days after infection of wild-type and Gα15−/− mice with 350 T. spiralis infective larvae. The eosinophil response to infection was not significantly different between the Gα15+/+ and the Gα15−/− mice (data not shown), and there was no difference in the degree of infection by T. spiralis between the groups as determined by the number of infective larvae extracted from the muscle and counted approximately 80 days postinfection (data not shown).
C5a-induced signaling is defective in Gα15−/− macrophage.
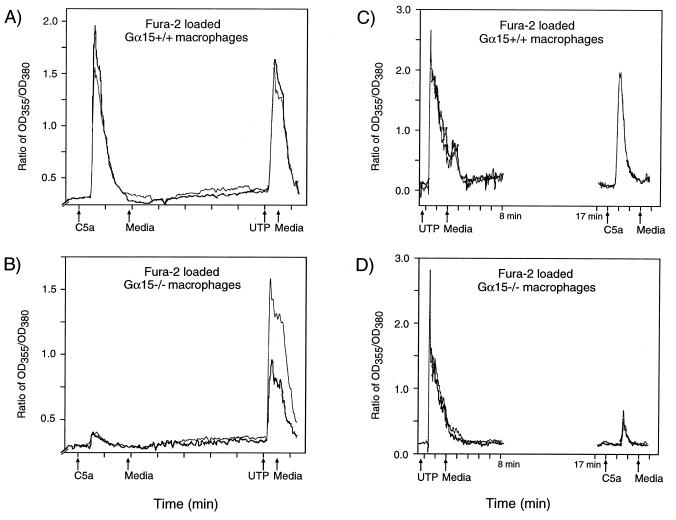
To address the possibility that biological functions of Gα15 may be compensated for by other signaling pathways in the Gα15 KO mice, we measured the biochemical responses to stimulation by various agonists in cultured primary cells. Thioglycolate-elicited macrophages, which express Gα15 abundantly (Fig. 2), were collected by peritoneal lavage and purified by adherence. In an initial screen for agonists that may utilize Gα15 during the inflammatory response, we used fluorescence microscopy to measure Ca2+ release in Fura-2-loaded macrophages. Ca2+ responses were evoked by 100 μM ATP, 100 μM UTP, 100 nM C5a, and 1 μg of PAF per ml. The Ca2+ responses to ATP and to UTP were similar in wild-type and Gα15−/− macrophages (Fig. 3 and data not shown). By contrast, the response to C5a was significantly reduced in the Gα15−/− macrophages (Fig. 3). To corroborate these findings and characterize these signaling events in populations of cells, we measured PLCβ activation in response to C5a, UTP, and PAF in wild-type and Gα15−/− macrophages metabolically labeled with [3H]myo-inositol in culture. Because ATP can also activate purinergic receptors on macrophages that are not G-protein coupled, we did not characterize its activity further.
FIG. 3.
Ca2+ release in response to C5a and UTP in wild-type and Gα15−/− macrophages. Changes in fluorescence over time in Fura-2-loaded wild-type and Gα15−/− macrophages that responded to C5a were analyzed with the Felix program and reported as a ratio of optical density at 355 nm to optical density at 380 nm (OD355/OD380). Each tracing represents the average fluorescence changes of at least five individual cells. (A and B) Cells were exposed to C5a for 2 min and allowed to recover in the presence of Ca2+-free medium for 5 min. The cells were then exposed to UTP. (A) Wild-type cells; (B) Gα15−/− cells; (C and D) wild-type (C) and Gα15−/− (D) cells exposed to UTP for 2 min and washed with regular medium for a total of 15 min. Recording was stopped at 8 min to avoid photobleaching and restarted at 17 min prior to stimulation with C5a. Ca2+ release in the Gα15−/− macrophages was blunted in response to C5a in both protocols, whereas the response to UTP was unchanged.
PI release in response to C5a is rapid, near maximal 1 min following addition of agonist, and stable for at least 5 min (Fig. 4C). This response was at least 60% weaker in the Gα15−/− macrophages, in agreement with the results obtained in the Ca2+ fluorescence assay. In contrast, the responses to UTP and PAF (Fig. 4A and B) increased for at least 5 min after stimulation and were similar in macrophages from wild-type and Gα15−/− mice. The macrophages were treated with pertussis toxin for 24 h at 100 ng/ml to determine if the remaining activity observed in the Gα15−/− cells could be attributed to Gi coupling. The PI response to C5a was completely eliminated in Gα15−/− macrophages in the presence of pertussis toxin (Fig. 4C). PI release in response to C5a in the wild-type cells was reduced minimally by 50% in the presence of pertussis toxin. These results suggest that the C5a receptor (C5aR) is normally coupled to both Gα15 and Gi class G proteins and that the signal observed in the macrophages in response to C5a is therefore probably due to remnant Gi stimulation. The response to PAF was diminished approximately 40% by pertussis toxin in both wild-type and Gα15−/− cells, while the response to UTP was reduced 30% by pertussis toxin in wild-type and Gα15−/− mice (Fig. 4B). These results suggest that as with the C5aR, the UTP and PAF receptors couple to Gi G proteins, but in contrast to the C5aR, Gα15 does not exclusively mediate the Gq component of Ca2+ signaling in macrophages. The different patterns of G-protein coupling by these receptors suggests that the normal expression of Gi and/or Gq proteins may compensate for Gα15 deficiency and thus explain the absence of a more obvious biological phenotype in the Gα15 KO mice.
FIG. 4.
Signaling response of wild-type and Gα15−/− peritoneal macrophages to agonist stimulation and effect of pertussis toxin. (A-C) PI release in response to PAF, UTP, and C5a. Thioglycolate-elicited peritoneal macrophages from wild-type (wt) and Gα15−/− mice were metabolically labeled with [3H]myo-inositol, and PI release was measured in 5-min time courses in response to PAF (30 ng/ml; A) UTP (10 μM; B), and C5a (100 nM; C). When indicated, cells were treated for 24 h with 100 ng of pertussis toxin (Ptx; islet-activating protein) per ml prior to stimulation with agonist. The data represents average ± standard error of the mean of a total of four experiments, three with pertussis toxin (A and B), and a total of seven experiments, four with pertussis toxin (C). The data are presented as counts per minute after subtraction of the background (bkg; counts per minute when cells are treated with buffer control). (D) ERK activation in response to stimulation with C5a. Macrophages were exposed to C5a (or buffer) for 3 min and lysed in buffer containing protease and phosphatase inhibitors; 20 mg of cell lysate was loaded per lane. When indicated, the cells were exposed to pertussis toxin (100 ng/ml) overnight prior to C5a stimulation. Western blot analysis was performed with antibody V6671 (Promega), which recognizes the dually phosphorylated ERKs. p44, ERK1; p42, ERK2; Ptx, pertussis toxin.
ERK activation by C5a is normal in Gα15−/− macrophages.
Agonist stimulation of the C5a receptor was shown to activate the mitogen-activated protein (MAP) kinase pathway in a pertussis toxin-sensitive manner in human neutrophils (8) and in transfected cells (9). Because we observed a synergistic effect of Gi and Gα15 on PI release in response to C5a in wild-type and Gα15−/− macrophages, we addressed the possibility of a similar effect on ERK activation. Wild-type and Gα15−/− macrophages treated with or without pertussis toxin were stimulated with 100 nM C5a for 3 min. The activation of ERK1 and ERK2 was assessed by Western blotting. The V667A antibody (Promega) recognizes only the active, dually phosphorylated ERKs. The activation of ERKs in response to C5a was rapid, equivalent in wild-type and Gα15−/− cells, and completely inhibited by pertussis toxin treatment in both wild-type and Gα15−/− macrophages (Fig. 4D). These data indicate that C5a-induced ERK activation is entirely dependent on Gi signaling in murine macrophages, as it is in mouse and human neutrophils (8).
DISCUSSION
G proteins mediate responses to a multitude of signaling molecules that evoke neuronal, hormonal, and sensory signal transduction as well as inflammatory and immune responses. The inflammatory response to destroy foreign particles and pathogens requires an intimate interaction between numerous cell types that stimulate cell proliferation and differentiation and activate leukocyte motility and chemotaxis to the site of infection. Chemokines and classical chemoattractants stimulate these processes and are implicated in autoimmune diseases (reviewed in reference 29). Many of these agonists, including IL-8, C5a, and formylmethionyl-leucyl-phenylalanine, bind heptahelical receptors to active PLCβ and evoke Ca2+ release. A number of these chemokine receptors are coupled by pertussis toxin-sensitive Gi class proteins and Gα15, the pertussis toxin-insensitive Gq class α subunit that is predominantly expressed in hematopoietic cells (3, 22).
Several features of Gα15 made it an interesting target for mutational analysis in mice. A wide variety of heptahelical receptors which were initially found to activate Gs or Gi class α subunits were later found to also couple Gα15 and Gα16 (mouse and human orthologs, respectively), but not Gαq/11, to the activation of PLCβ. This suggested that Gα15 may have unique functions, independent of Gq/11, in hematopoietic tissues where it is normally expressed. Gα15 exhibits the most restricted expression pattern of any G-protein subunit, with the exception of those expressed only in specialized sensory transduction cells. Gα15 also has evolved at an accelerated rate relative to all other mammalian α subunit genes (13). Many genes whose primary function serves hematopoietic cell types have been noted to diverge at an accelerated rate relative to other members of the same gene family (23). This may reflect selection pressures on Gα15 and other genes which are specifically expressed in the immune cells that defend against pathogens (23). Therefore, although Gα15 is always coexpressed with Gαq and Gα11, we anticipated that it would have unique functions in hematopoiesis or immune cell function.
Not only are the Gα15−/− mice viable and fertile, but Gα15 does not appear to be required for hematopoiesis. This result was surprising since Gα15 is abundantly expressed in murine erythroleukemia cells (33), and antisense expression of Gα16 reportedly inhibited cellular growth rates of erythroleukemia cells in culture (15). By contrast, we observed normal production of plasma erythrocytes in Gα15−/− adult mice, and we did not observe an increase in fetal mortality which could have been indicative of a deficiency in fetal erythropoiesis. Furthermore, Gα15 is abundantly expressed in pre- and pro-B-cell lines and in the bone marrow (16, 33), which is rich in B cells, myeloid and lymphoid precursors, and mature neutrophils. However, all hematopoietic lineages, including B cells, T cells, neutrophils, and monocytes, appeared to be normal in the Gα15−/− mice, as did the architecture of the major lymphoid organs. Minimally, we anticipated a perturbation in the ratios of these cell-types in Gα15−/− mice, as was observed in the IL-8 receptor (IL-8R) knockout mice, because IL-8R is coupled by Gα15 (but not Gαq or Gα11) to the activation of PLCβ (34). The IL-8R−/− mice exhibited neutrophil and B-cell expansion resulting in lymphadenopathy, splenomegaly, and foci of hematopoiesis in the liver (11). Our analysis of Gα15−/− mice indicates either that Gα15 does not couple the IL-8R in vivo or that compensatory mechanisms allow IL-8 signaling in the absence of Gα15.
Analysis of Gq class KO mice indicates that Gq/11 signaling is subject to gene dosage effects (28), consistent with their similarities in receptor coupling and effector activation (35). Gαq and Gα11 are widely expressed throughout development and in adult mice. Homozygous deficiency of either gene can be tolerated, and the phenotypic abnormalities in Gαq−/− and Gα11−/− mice are relatively mild. By contrast, double-KO (Gαq−/− Gα11−/−) mice die during midgestation (embryonic day 11) due to cardiomyocyte hypoplasia and subsequent heart failure (28). Addition of a single active allele of either Gαq or Gα11 allows fetuses to survive until shortly after birth, and addition of two active gene copies, one of each or two of either gene, allows mice to survive to adulthood and to reproduce. The most obvious phenotypes in Gαq−/− mice, ataxia and a bleeding disorder (24, 27), are not apparent in either Gα11−/− or Gαq+/− Gα11+/− mice. These phenotypic differences may result from the fact that Gαq is more widely expressed and is more abundant than Gα11 in the affected tissues, although molecular mechanisms of signaling specificity have not been rigorously tested. We reasoned that gene dosage effects may be revealed in Gα15−/− Gαq−/− mice. The ataxia and bleeding disorder previously found in Gαq−/− mice were not enhanced in the double-KO mice, nor were additional defects in hematopoiesis detected. Gα11 is the only Gq class α subunit remaining in most hematopoietic cells in Gα15−/− Gαq−/− mice. To explain the absence of phenotypic defects in the double-KO mice, and assuming that Gq class signaling is important in hematopoietic cells, either Gα11 conveys all Gq class activity during hematopoiesis in normal mice or these Gq proteins are functionally redundant and Gα11 compensates for the absence of Gα15 and/or Gαq in the double-KO mice. The former possibility appears unlikely because the Gα11−/− single-KO mice have no apparent hematopoietic defect (data not shown). Unfortunately, the Gα11−/− Gα15−/− double-KO mice cannot be obtained by crossing the single-KO strains due to their chromosomal colocalization and analysis of triple mutants will require conditional KO technology because the Gαq−/− Gα11−/− mice die in utero before initiation of hematopoiesis in the fetal liver. It is therefore not yet possible to determine the function of Gq class signaling in hematopoiesis.
We next tested the possibility that Gα15 mediates signaling during immune challenge. The chemokine receptors expressed on leukocytes mediate inflammatory responses and the riddance of pathogens, and these receptors have been shown in transfection assays to couple to Gi class α subunits and Gα15 but not Gαq or Gα11 (14, 22, 34). We used thioglycolate-induced peritonitis to survey the response of neutrophils and mononuclear cells to a non-antigen-specific, T-cell-independent agent (thioglycolate). Infection with T. spiralis was used to monitor the eosinophil response to a challenge that required recognition of specific antigens. The Gα15−/− mice performed normally in these and several other challenges not described, such as ovalbumin-induced eosinophilia, croton oil-induced dermatitis, and turpentine-induced fever. A normal response to all tests was observed in Gα15−/− mice despite the fact that defective responses to an inflammatory challenge with thioglycolate occurred in chemokine receptor (e.g., IL-8R and CCR2) KO mice as well as in the PLCβ2 KO mice (5, 11, 19). Thus, the approach of using systemic readouts such as hematopoiesis or immune challenges, which are regulated by multiple signaling mechanisms, failed to detect a deficiency in Gα15−/− mice.
Many cell types and signaling pathways which may mask a deficiency in Gα15 are engaged during inflammation. We reasoned that signaling defects in isolated cells might identify physiologically relevant pathways coupled by Gα15. Therefore, we challenged purified thioglycolate-elicited macrophages of wild-type and Gα15−/− mice with different G-protein-coupled agonists and measured activation of PLCβ in single cells. We found three agonists, the anaphylatoxin C5a, UTP, and PAF, that stimulated G-protein-dependent Ca2+ signaling (Fig. 3 and 4). Gα15−/− macrophage stimulated with C5a exhibited diminished inositol phosphate production and Ca2+ release compared with wild-type cells (Fig. 3 and 4C). By contrast, UTP and PAF stimulated similar responses in mutant and wild-type macrophage. Our studies are in agreement with previous analysis of the C5a response in cultured cells cotransfected with C5aR and Gα16, which suggested that C5aR-evoked Ca2+ signaling was mediated by Gα15 but not Gαq/11 (1, 9). However, pertussis toxin inhibition of PLCβ was more pronounced in the macrophage from Gα15−/− mice than in either study. Additionally, transfected cells apparently required both Gi and Gα15 coupling to the C5aR for full activation of the MAP kinase (10). By contrast, activation of MAP kinase was completely dependent on Gi-mediated signaling in both mouse macrophages (Fig. 4) and human neutrophils (8).
C5a is an 8.6-kDa terminal by-product of complement activation with inflammatory properties. Mice with an homozygous disruption of the C5aR (C5aR−/−) have several inflammatory phenotypes. They are sensitive to pulmonary infection with Pseudomonas aeruginosa and are resistant to the reverse-passive Arthus reaction (immunocomplex-induced granuloma formation) in the lung, skin, and peritoneum (18). Gα15 may be involved in mediating these effects. However, due to the ability of the receptor to induce a PI signal and Ca2+ release in the absence of Gα15, the remaining signal observed may be sufficient to produce a full biological response.
In a previous study, the Ca2+ response evoked by UTP was entirely and specifically blocked by the expression of antisense Gα16 RNA in the HEL human erythroleukemia cell line (4). In the same study, pertussis toxin partially inhibited UTP-mediated signaling in the parental cells, suggesting a synergistic activity between Gα16 and βγ released from Gi. However, we found that UTP-evoked PI production and Ca2+ release appeared normal in macrophages isolated from Gα15−/− mice (Fig. 4). Thus, the UTP responsive P2Y2 receptor expressed in murine macrophages can apparently be coupled by Gi and Gq/11. The PAF receptor is similarly coupled by Gi and Gq/11 in Gα15−/− macrophages.
The ex vivo experiments with peritoneal macrophages suggest that the absence of an apparent phenotype in Gα15−/−, Gα11−/−, and Gα15−/− Gαq−/− mice may be explained by the ability of the receptors that mediate hematopoiesis and inflammatory responses to couple to multiple G proteins, including those of both the Gi and the Gq class.
ACKNOWLEDGMENTS
ES cell lines (R1) were graciously provided by J. Rossant. We thank M. J. Bennett, G. Spangrude, and S. Muallem for insightful comments and for help with bone marrow transfers and Ca2+ imaging. Antibodies were kindly given to us by P. Sternweis, S. Mumby, and M. Cobb.
This work was supported by Pharmacological Sciences Training Grant 5-T32-GM07062 (I.D.) and by National Institutes of Health grant DK47890, March of Dimes, Leukemia Association of North Central Texas, Texas Advanced Research Program, and an American Heart Association Established Investigator Award (T.M.W.).
REFERENCES
- 1.Amatruda T T, III, Gerard N P, Gerard C, Simon M I. Specific interactions of chemoattractant factor receptors with G-proteins. J Biol Chem. 1993;268:10139–10144. [PubMed] [Google Scholar]
- 2.Amatruda T T, III, Steele D A, Slepak V Z, Simon M I. Gα16, a G-protein α subunit specifically expressed in hematopoietic cells. Proc Natl Acad Sci USA. 1991;88:5587–5591. doi: 10.1073/pnas.88.13.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai H, Charo I F. Differential regulation of G-protein-mediated signaling by chemokine receptors. J Biol Chem. 1996;271:21814–21819. doi: 10.1074/jbc.271.36.21814. [DOI] [PubMed] [Google Scholar]
- 4.Baltensperger K, Porzig H. The P2U purinoceptor obligatorily engages the heterotrimeric G-protein G16 to mobilize intracellular Ca2+ in human erythroleukemia cells. J Biol Chem. 1997;272:10151–10159. doi: 10.1074/jbc.272.15.10151. [DOI] [PubMed] [Google Scholar]
- 5.Boring L, Gosling J, Chensue S W, Kunkel S L, Farese R V, Jr, Broxmeyer H E, Charo I F. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Investig. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulay F, Mery L, Tardif M, Brouchon L, Vignais P. Expression cloning of a receptor for C5a anaphylatoxin on differentiated HL-60 cells. Biochemistry. 1991;30:2993–2999. doi: 10.1021/bi00226a002. [DOI] [PubMed] [Google Scholar]
- 7.Bourne H R, Sanders D A, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 8.Buhl A M, Avdi N, Worthen G S, Johnson G L. Mapping of the C5a receptor signal transduction network in human neutrophils. Proc Natl Acad Sci USA. 1994;91:9190–9194. doi: 10.1073/pnas.91.19.9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buhl A M, Eisfelder B J, Worthen G S, Johnson G L, Russell M. Selective coupling of the human anaphylatoxin C5a receptor and α16 in human kidney 293 cells. FEBS Lett. 1993;323:132–134. doi: 10.1016/0014-5793(93)81464-b. [DOI] [PubMed] [Google Scholar]
- 10.Buhl A M, Osawa S, Johnson G L. Mitogen-activated protein kinase activation requires two signal inputs from the human anaphylatoxin C5a receptor. J Biol Chem. 1995;270:19828–19832. doi: 10.1074/jbc.270.34.19828. [DOI] [PubMed] [Google Scholar]
- 11.Cacalano G, Lee J, Kikly K, Ryan A M, Pitts-Meek S, Hultgren B, Wood W I, Moore M W. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 12.Coward P, Chan S D, Wada H G, Humphries G M, Conklin B R. Chimeric G-proteins allow a high-throughput signaling assay of Gi-coupled receptors. Anal Biochem. 1999;270:242–248. doi: 10.1006/abio.1999.4061. [DOI] [PubMed] [Google Scholar]
- 13.Davignon I, Barnard M, Gavrilova O, Sweet K, Wilkie T M. Gene structure of murine Gna11 and Gna15: tandemly duplicated Gq class G-protein α subunit genes. Genomics. 1996;31:359–366. doi: 10.1006/geno.1996.0059. [DOI] [PubMed] [Google Scholar]
- 14.Gaudreau R, Le Gouill C, Metaoui S, Lemire S, Stankova J, Rola-Pleszczynski M. Signalling through the leukotriene B4 receptor involves both αi and α16, but not αq or α11 G-protein subunits. Biochem J. 1998;335:15–18. doi: 10.1042/bj3350015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghose S, Porzig H, Baltensperger K. Induction of erythroid differentiation by altered Gα16 activity as detected by a reporter gene assay in MB-02 cells. J Biol Chem. 1999;274:12848–12854. doi: 10.1074/jbc.274.18.12848. [DOI] [PubMed] [Google Scholar]
- 16.Grant K R, Harnett W, Milligan G, Harnett M M. Differential G-protein expression during B- and T-cell development. Immunology. 1997;90:564–571. doi: 10.1046/j.1365-2567.1997.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harden T K, Stephens L, Hawkins P T, Downes C P. Turkey erythrocyte membranes as a model for regulation of phospholipase C by guanine nucleotides. J Biol Chem. 1987;262:9057–9061. [PubMed] [Google Scholar]
- 18.Hopken U E, Lu B, Gerard N P, Gerard C. Impaired inflammatory responses in the reverse arthus reaction through genetic deletion of the C5a receptor. J Exp Med. 1997;186:749–756. doi: 10.1084/jem.186.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang H, Kuang Y, Wu Y, Xie W, Simon M I, Wu D. Roles of phospholipase C β2 in chemoattractant-elicited responses. Proc Natl Acad Sci USA. 1997;94:7971–7975. doi: 10.1073/pnas.94.15.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang M, Boulay G, Spicher K, Peyton M J, Brabet P, Birnbaumer L, Rudolph U. Inactivation of the Gαi2 and Gαo genes by homologous recombination. Receptors Channels. 1997;5:187–192. [PubMed] [Google Scholar]
- 21.Kozasa T, Hepler J R, Smrcka A V, Simon M I, Rhee S G, Sternweis P C, Gilman A G. Purification and characterization of recombinant G16α from Sf9 cells: activation of purified phospholipase C isozymes by G-protein α subunits. Proc Natl Acad Sci USA. 1993;90:9176–9180. doi: 10.1073/pnas.90.19.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuang Y, Wu Y, Jiang H, Wu D. Selective G-protein coupling by C-C chemokine receptors. J Biol Chem. 1996;271:3975–3978. doi: 10.1074/jbc.271.8.3975. [DOI] [PubMed] [Google Scholar]
- 23.Murphy P M. Molecular mimicry and the generation of host defense protein diversity. Cell. 1993;72:823–826. doi: 10.1016/0092-8674(93)90571-7. [DOI] [PubMed] [Google Scholar]
- 24.Offermanns S, Hashimoto K, Watanabe M, Sun W, Kurihara H, Thompson R F, Inoue Y, Kano M, Simon M I. Impaired motor coordination and persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking Gαq. Proc Natl Acad Sci USA. 1997c;94:14089–14094. doi: 10.1073/pnas.94.25.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Offermanns S, Mancino V, Revel J P, Simon M I. Vascular system defects and impaired cell chemokinesis as a result of Gα13 deficiency. Science. 1997a;275:533–536. doi: 10.1126/science.275.5299.533. [DOI] [PubMed] [Google Scholar]
- 26.Offermanns S, Simon M I. Gα15 and Gα16 couple a wide variety of receptors to phospholipase C. J Biol Chem. 1995;270:15175–15180. doi: 10.1074/jbc.270.25.15175. [DOI] [PubMed] [Google Scholar]
- 27.Offermanns S, Toombs C F, Hu Y H, Simon M I. Defective platelet activation in Gα(q)-deficient mice. Nature. 1997b;389:183–186. doi: 10.1038/38284. [DOI] [PubMed] [Google Scholar]
- 28.Offermanns S, Zhao L P, Gohla A, Sarosi I, Simon M I, Wilkie T M. Embryonic cardiomyocyte hypoplasia and craniofacial defects in Gαq/Gα11-mutant mice. EMBO J. 1998;17:4304–4312. doi: 10.1093/emboj/17.15.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Proost P, Wuyts A, van Damme J. The role of chemokines in inflammation. Int J Clin Lab Res. 1996;26:211–223. doi: 10.1007/BF02602952. [DOI] [PubMed] [Google Scholar]
- 30.Ross, E. M., and T. M. Wilkie. GTPase-activating proteins (GAPs) for heterotrimeric G proteins: regulators of G protein signaling (RGS proteins) and their relatives. Annu. Rev. Biochem., in press. [DOI] [PubMed]
- 31.Smrcka A V, Sternweis P C. Regulation of purified subtypes of phosphatidylinositol-specific phospholipase Cβ by G-protein α and βγ subunits. J Biol Chem. 1993;268:9667–9674. [PubMed] [Google Scholar]
- 32.van Willigen G, Donath J, Lapetina E G, Akkerman J W. Identification of α-subunits of trimeric GTP-binding proteins in human platelets by RT-PCR. Biochem Biophys Res Commun. 1995;214:254–262. doi: 10.1006/bbrc.1995.2282. [DOI] [PubMed] [Google Scholar]
- 33.Wilkie T M, Scherle P A, Strathmann M P, Slepak V Z, Simon M I. Characterization of G-protein α subunits in the Gq class: expression in murine tissues and in stromal and hematopoietic cell lines. Proc Natl Acad Sci USA. 1991;88:10049–10053. doi: 10.1073/pnas.88.22.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu D, LaRosa G J, Simon M I. G-protein-coupled signal transduction pathways for interleukin-8. Science. 1993;261:101–103. doi: 10.1126/science.8316840. [DOI] [PubMed] [Google Scholar]
- 35.Xu X, Croy J T, Zeng W, Zhao L P, Davignon I, Popov S, Yu K, Jiang H, Offermanns S, Muallem S, Wilkie T M. Promiscuous coupling of receptors to Gq class α subunits and effector proteins in pancreatic and submandibular gland cells. J Biol Chem. 1998;273:27275–27279. doi: 10.1074/jbc.273.42.27275. [DOI] [PubMed] [Google Scholar]
- 36.Xu X, Zeng W, Diaz J, Muallem S. Spacial compartmentalization of Ca2+ signaling complexes in pancreatic acini. J Biol Chem. 1996;271:24684–24690. doi: 10.1074/jbc.271.40.24684. [DOI] [PubMed] [Google Scholar]
- 37.Zhu X, Birnbaumer L. G-protein subunits and the stimulation of phospholipase C by Gs- and Gi-coupled receptors: lack of receptor selectivity of Gα(16) and evidence for a synergic interaction between Gβγ and the α subunit of a receptor activated G-protein. Proc Natl Acad Sci USA. 1996;93:2827–2831. doi: 10.1073/pnas.93.7.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]