Abstract
Background: Severe alcohol withdrawal syndrome (SAWS) is highly morbid, costly, and common among hospitalized patients, yet minimal evidence exists to guide inpatient management. Research needs in this field are broad, spanning the translational science spectrum.
Goals: This research statement aims to describe what is known about SAWS, identify knowledge gaps, and offer recommendations for research in each domain of the Institute of Medicine T0–T4 continuum to advance the care of hospitalized patients who experience SAWS.
Methods: Clinicians and researchers with unique and complementary expertise in basic, clinical, and implementation research related to unhealthy alcohol consumption and alcohol withdrawal were invited to participate in a workshop at the American Thoracic Society 2019 International Conference. The committee was subdivided into four groups on the basis of interest and expertise: T0–T1 (basic science research with translation to humans), T2 (research translating to patients), T3 (research translating to clinical practice), and T4 (research translating to communities). A medical librarian conducted a pragmatic literature search to facilitate this work, and committee members reviewed and supplemented the resulting evidence, identifying key knowledge gaps.
Results: The committee identified several investigative opportunities to advance the care of patients with SAWS in each domain of the translational science spectrum. Major themes included 1) the need to investigate non–γ-aminobutyric acid pathways for alcohol withdrawal syndrome treatment; 2) harnessing retrospective and electronic health record data to identify risk factors and create objective severity scoring systems, particularly for acutely ill patients with SAWS; 3) the need for more robust comparative-effectiveness data to identify optimal SAWS treatment strategies; and 4) recommendations to accelerate implementation of effective treatments into practice.
Conclusions: The dearth of evidence supporting management decisions for hospitalized patients with SAWS, many of whom require critical care, represents both a call to action and an opportunity for the American Thoracic Society and larger scientific communities to improve care for a vulnerable patient population. This report highlights basic, clinical, and implementation research that diverse experts agree will have the greatest impact on improving care for hospitalized patients with SAWS.
Keywords: alcohol withdrawal delirium, critical care, translational medical research, clinical studies, quality improvement
Contents
-
Overview
T0–T1 SAWS Research
T2 SAWS Research
T3 SAWS Research
T4 SAWS Research
-
Introduction
A Syndrome Lacking Clear Definitions
Existing Evidence Does Not Generalize to Hospitalized Patients
Research Needs Span the Translational Science Spectrum
-
Methods
Committee Composition
Conceptual Definition of SAWS
Literature Search and Evaluation
Knowledge Gaps
Document Development
-
Section 1: Pathophysiology of SAWS and Development of Novel Therapeutics (T0–T1 Research)
GABA and Glutamate Neuroadaptation
Kindling
Other Neuromodulatory Systems
Sex Differences in SAWS Pathophysiology
Challenges
Recommendations for Future Basic Science Research
-
Section 2: Translation of Research to Patients (T2 Research)
Risk Factors and Predictors of SAWS
Diagnosis and Disease Severity
Challenges
Recommendations for Research to Improve Patient Care
-
Section 3: Establishing Best Practices to Improve Clinical Outcomes (T3 Research)
Choice of Medication for Treatment of SAWS Benzodiazepine Dosing Strategies
Benzodiazepine Resistance
Benzodiazepine-Alternative GABAergic and Antiglutamatergic Medications
Antiepileptic Medications as Treatment for SAWS
Additional Pharmacologic Mechanisms Targeting SAWS
Challenges
Recommendations for Research to Improve Clinical Practice and Patient Outcomes
-
Section 4: Implementation of Research Findings for SAWS (T4 Research)
Recommendations to Promote Implementation of Evidence-based Practices
Discussion
Overview
Severe alcohol withdrawal syndrome (SAWS) is a highly morbid condition characterized by brain hyperexcitation that occurs among patients who discontinue heavy alcohol use. The definition of heavy alcohol use varies. Daily consumption in the range of four or more drinks for men, or three or more drinks for women, has been used to define heavy alcohol use, as well as binge drinking on 5 or more days per month (1). However, central nervous system alterations can occur at levels of alcohol consumption differing from these quantitative definitions, culminating in SAWS. SAWS is commonly encountered by inpatient providers of various disciplines but most patients with SAWS are managed in the ICU. Minimal evidence exists to guide inpatient management of SAWS, underscoring broad research questions that span the translational science spectrum. This research statement summarizes existing literature, identifies knowledge gaps, and offers recommendations for high-impact research related to SAWS in each domain of the Institute of Medicine (IOM) T0–T4 research continuum, in which T0–T1 includes basic science research with translation to humans, and T2, T3, and T4 include research with translation to improved patient care, clinical practice, and community health, respectively (2).
T0–T1 SAWS Research
-
•
SAWS is predominantly driven by counterregulatory neuroadaptations in γ-aminobutyric acid (GABA) and glutamate signaling that develop with exposure to alcohol over time; however, multiple hormonal and neuromodulatory systems act as higher-level regulators of the excitatory and inhibitory neurosignaling that is relevant to SAWS. Examples of such regulators include CRF (corticotropin-releasing factor), IL-6, CCL2 (chemokine ligand 2; also known as monocyte chemoattractant protein 1), ligand- and voltage-gated channels, and second messenger systems.
-
•
Repeated cycles of intoxication and withdrawal directly damage cortical neurons (especially in the frontal cortex) contributing to progressively severe episodes of withdrawal and possible loss of executive function (i.e., “kindling”).
-
•
The importance of identifying therapeutic targets beyond GABA agonism with benzodiazepines is underscored by evidence of cross-tolerance between benzodiazepines and alcohol at the GABAA receptor.
-
•
T0–T1 Recommendation 1: Neuromodulatory systems beyond GABA and glutamate should be explored to develop new diagnostic and therapeutic strategies for SAWS, including repurposing existing medications.
-
•
T0–T1 Recommendation 2: Preclinical animal models should be expanded and refined to recapitulate the full symptomatology of patients with SAWS. Important areas include developing models of co-intoxication and/or concomitant withdrawal from other substances of abuse and modeling of SAWS cooccurring with common conditions such as sepsis, trauma, and organ failure.
T2 SAWS Research
-
•
Given the high prevalence, morbidity, and costs associated with alcohol-related conditions in hospitalized patients, universal screening for alcohol use and assessment of risk for development of SAWS should be standard practices. Unfortunately, few studies have prospectively evaluated risk factors for SAWS in hospitalized patients. Existing data from small retrospective studies (most lacking validation cohorts) suggest that alcohol use disorder (AUD), history of prior withdrawal, and heavy alcohol consumption before an alcohol-related hospitalization are strong risk factors for development of SAWS.
-
•
The Clinical Institute Withdrawal Assessment for Alcohol (CIWA)–Revised (CIWA-Ar) is the most commonly used scale for grading severity of alcohol withdrawal; however, CIWA-Ar scores are heavily weighted by subjective patient-reported data that are often unreliable in acutely and critically ill patients.
-
•
Electronic health record (EHR)-based phenotypes and direct alcohol biomarkers may be useful for proactively and objectively identifying hospitalized patients with unhealthy alcohol consumption who are at risk for SAWS.
-
•
T2 Recommendation 1: Readily available EHR data should be used to create computable phenotypes and an operational definition of SAWS. This committee proposes an operational definition supported by prior literature that has face validity but requires further evaluation in health systems with EHRs.
-
•
T2 Recommendation 2: Available and objective tools should be evaluated to risk stratify hospitalized patients for the likelihood of SAWS and to grade SAWS severity. Ethanol biomarkers should be evaluated for early identification of patients at risk for SAWS. The Richmond Agitation–Sedation Scale (RASS), and other commonly used ICU agitation–sedation scales, should be compared with existing alcohol-specific tools (e.g., the CIWA-Ar or the Brief Alcohol Withdrawal Scale [BAWS]) for grading SAWS severity and titrating medications.
T3 SAWS Research
-
•
No multicenter randomized controlled trials (RCTs) have evaluated the impact of different SAWS treatments on the clinical outcomes of hospitalized patients. Existing treatment strategies for SAWS are extrapolated and modified from small studies conducted predominantly in detoxification units that have excluded patients with acute comorbidities and/or severe manifestations of alcohol withdrawal.
-
•
There are insufficient data to guide the initial choice of pharmacotherapy in hospitalized patients with SAWS.
-
•
Benzodiazepines are commonly used as the initial treatment for SAWS; however, no prospective comparisons of benzodiazepine dosing strategies in patients with acute or critical illness have been published. Preimplementation–postimplementation studies of protocols used in ICU settings support front-loading strategies and early adjunctive therapy with phenobarbital, but more rigorous study designs in diverse patient populations are needed to establish the safety and effectiveness of these approaches.
-
•
Increasing recognition of benzodiazepine-resistant SAWS suggests the need for alternative first-line and/or adjunctive therapies. Data describing benzodiazepine-alternative treatments for patients with SAWS are limited. The majority of literature focuses on use of phenobarbital as monotherapy or adjunctive therapy to benzodiazepines.
-
•
T3 Recommendation 1: Short-term, long-term, and patient-centered outcomes for clinical trials of SAWS need to be defined with input from stakeholders.
-
•
T3 Recommendation 2: A clinical trial network should be established to create a platform for conducting hybrid efficacy–effectiveness trials that can address the inherent challenges of clinical research for SAWS.
-
•
T3 Recommendation 3: Three clinical questions should be prioritized for immediate study. 1) What is the optimal first-line medication for patients with SAWS to improve outcomes such as symptom progression and death? 2) What is the most effective medication administration strategy for SAWS (e.g., symptom-triggered vs. front-loading, enteral vs. intravenous)? 3) Is protocolized and/or bundled care superior to usual care for patients with SAWS?
T4 SAWS Research
-
•
High-quality evidence-based treatments for hospitalized patients with SAWS do not yet exist; thus, no published data are available regarding how to best implement guideline-recommended care or monitor outcomes at the population level. Early consideration of implementation frameworks (e.g., Reach, Effectiveness, Adoption, Implementation, and Maintenance) and outcomes will help minimize the gaps between efficacious therapies for SAWS and their effective delivery to patients.
-
•
T4 Recommendation 1: A wide array of stakeholders (e.g., patients, caregivers, advocacy groups, community members, interdisciplinary clinicians, purchasers, payers, administrators, policy makers, and researchers) should be engaged, and nontraditional partnerships (e.g., among critical care, medical toxicology, and addiction specialists) should be established before examining an innovation’s effectiveness to accelerate the transfer of innovations into practice.
-
•
T4 Recommendation 2: Knowledge and infrastructure from existing critical care research networks should be harnessed to establish systems for real-time data collection and feedback spanning multiple hospitals. Through describing processes of patient care, feedback regarding performance, and interventions such as guideline distribution and progress updates, best practices can be refined and reinforced alongside clinical research activities for SAWS to homogenize care.
Introduction
Alcohol withdrawal syndrome (AWS) is common among hospitalized patients and can be fatal without appropriate pharmacologic management (3–5). Nevertheless, high-quality evidence to guide treatment decisions for inpatient AWS is lacking (6). Studies examining treatments for inpatient AWS are limited by small sample sizes and often exclude patients with serious medical and/or surgical comorbidities (6–10), although such conditions frequently coexist with AWS in acute care and especially critical care settings.
AWS is a continuum of neurophysiologic signs and symptoms, influenced by the severity of underlying AUD and other medical conditions that can alter brain signaling pathways (11–13). AWS has been associated with increased ICU and hospital lengths of stay, hospital-acquired infections, the risk of sepsis, and in-hospital mortality (14–17).
A Syndrome Lacking Clear Definitions
SAWS is regularly encountered and managed by ICU providers but has been inconsistently defined (18). Most clinicians and researchers would agree that SAWS is a progressive manifestation of alcohol withdrawal that often requires admission to intensive care settings for close monitoring and frequent administration of medications to address hyperautonomia (including but not limited to pyrexia, tachycardia, and/or hypertension), agitation, and/or delirium. These clinical features represent the hallmarks of SAWS, also called alcohol withdrawal delirium, delirium tremens, and “DTs” in the literature (18–20). Clear metrics and goals for pharmacologic treatment, addressing the clinical features found in hospitalized patients with SAWS, do not exist. Instead, acute management of SAWS has generally emphasized short-term outcomes, including prevention of seizures, improvement of autonomic instability, and relief of agitation (21). Longer-term treatment outcomes after episodes of SAWS such as cognitive function, engagement in care for AUD, and abstinence from alcohol remain inadequately studied.
Existing Evidence Does Not Generalize to Hospitalized Patients
Symptom-triggered dosing schedules, sometimes known as “CIWA protocols,” are widely used to guide administration of benzodiazepines in patients with presumed SAWS (8, 22); however, these strategies are not always appropriately applied and may pose risks to acutely ill hospitalized patients (23, 24). For example, CIWA protocols can be implemented in hospitalized patients with symptoms mimicking alcohol withdrawal, such as other forms of delirium. Hospitalized patients are also inappropriate candidates for symptom-driven pharmacotherapy if unable to verbally communicate, including individuals who already have SAWS or another incapacitating illness or individuals who require intubation (25). Alternative strategies to CIWA protocols for treating SAWS include fixed-dose or front-loading benzodiazepines (7, 26) and/or other classes of medications such as antiepileptics and antisympathomimetics (10, 27, 28). However, the safety and effectiveness of these different approaches to managing alcohol withdrawal in hospitalized patients are poorly understood.
Benzodiazepines are considered the first-line therapy for alcohol withdrawal but must be approached with caution in acutely ill hospitalized patients, given dose-dependent associations with somnolence, respiratory depression, delirium, and mortality (29–32). Patients with AWS may be particularly vulnerable to adverse effects from benzodiazepines, given an established relationship between chronic heavy alcohol use and delirium (33–37). Tolerance to benzodiazepines among patients with heavy alcohol use is also common (12, 38) and can reduce the effectiveness of benzodiazepines as treatment. In the setting of tolerance, attempts to use of benzodiazepines (i.e., GABA agonism) at high doses to overcome glutamate-mediated withdrawal physiology (i.e., brain hyperexcitation) can initiate a sequence of supratherapeutic benzodiazepine dosing, treatment toxicity, increased ICU admissions, and prolonged hospitalizations (39). Although the benefits of benzodiazepine alternatives for alcohol withdrawal have not been established in RCTs, studies of ICU patients in general suggest better clinical outcomes with use of nonbenzodiazepine sedatives (40–45). Studies of alcohol withdrawal treatment protocols commonly use a reduction in benzodiazepine exposure as a primary outcome (46–48), and there is mounting interest in using adjuvant medications such as dexmedetomidine for benzodiazepine-sparing effects (49–52). Given the reports of patients with SAWS who appear “resistant” to escalating doses of benzodiazepines (e.g., requiring ⩾40 mg of diazepam-equivalent benzodiazepines in 1 h) (39, 53), medications such as phenobarbital and propofol have gained attention as possible alternatives (28, 54–57). However, comparative-effectiveness studies of these medications in hospital settings have not been performed.
In hospital settings, no universally accepted method exists for tailoring SAWS treatments to individual patients or patient populations. Strategies vary by treating provider and/or inpatient context (e.g., emergency department vs. ICU) and patient characteristics (e.g., mechanically ventilated vs. not). Such heterogeneity highlights the need for clinical practice guidelines to improve both recognition and management of SAWS in acutely ill patients. Previous efforts to guide best practices for SAWS, and updated guidelines from the American Society of Addiction Medicine, do not offer specific recommendations for treatment of hospitalized patients with cooccurring medical diseases (21, 58). Instead, a consultative, multidisciplinary approach is recommended for assistance in selecting medications and/or treatment protocols for alcohol withdrawal. Although such an approach can be helpful, it raises concerns for treatment delays and misapplication of therapies that may have adverse effects. Therefore, increased understanding of the unique needs of hospitalized patients with SAWS, together with successful implementation of evidence-based practices, requires additional research.
Research Needs Span the Translational Science Spectrum
Gaps in SAWS research span the translational spectrum—from use of animal models to approximate the complexities of human disease and support biomarker development, to predictive and prognostic enrichment strategies, rigorous clinical trials to evaluate therapies, comparative-effectiveness research, and implementation studies. Recognizing a broad array of unanswered questions affecting clinical management of SAWS, the American Thoracic Society (ATS) formed an interdisciplinary working group to identify research priorities for SAWS in each domain of the IOM T0–T4 translational science spectrum (2). This report aims to 1) summarize what is known about the pathophysiology and clinical management of patients with SAWS, 2) identify key research gaps, and 3) make recommendations for high-impact research in each domain of the translational spectrum to advance the science and care of patients who experience SAWS. The overarching goal of this research statement is to propose a pragmatic research agenda that points the way forward for basic and clinical investigators of various disciplines to collaborate on investigations that will accelerate care and improve outcomes for patients with SAWS.
Methods
ATS members initiated this project after determining the topic of study was important and relevant given the common requirement for critical care among many patients with SAWS. The project was approved by the ATS Program Review Subcommittee and cosponsored by the Critical Care, Behavioral Science and Health Services Research, and Nursing Assemblies of the ATS.
Committee Composition
Two co-chairs (T.L.S. and E.L.B.), who are members of the ATS, organized the ad hoc committee. The co-chairs sought to bring together a committee with unique but complementary research expertise related to unhealthy alcohol consumption and alcohol withdrawal with research proficiency across the translational spectrum. Invitations to participate were based in part on the publication record of potential participants. Given the interdisciplinary nature of care for patients with SAWS, committee members with diverse laboratory and clinical backgrounds were invited, including basic scientists, pulmonary and critical care physicians, psychiatrists, addiction medicine specialists, emergency medicine physicians, medical toxicologists, surgeons, anesthesiologists, nurses, and pharmacists from both U.S. and international research communities, including members and nonmembers of the ATS. Not all who were invited could ultimately participate, and the total number of committee members was limited by funding for the project. The co-chairs continued to extend invitations until a sufficiently diverse cohort had been assembled. The final assembled committee was charged with addressing specific questions posed a priori relating to management of SAWS (Box 1).
Box 1.
A priori research questions.
What is the relevant pathophysiology underlying SAWS?
What clinical endpoints should be targeted through treatment?
What are the limitations of current strategies for diagnosing, grading, and treating SAWS?
What patient factors warrant consideration in the management of SAWS?
What are the methodological challenges of research involving patients with SAWS, and how can these challenges be addressed?
How can existing clinical, research, and community infrastructure and partnerships be harnessed for the advancement of SAWS care?
What strategies will ensure effective dissemination and implementation of important research findings for treatment of SAWS?
SAWS = severe alcohol withdrawal syndrome.
The committee was subdivided into four groups to address research gaps across the translational spectrum, using the IOM classification system: T0–T1 (basic research with translation to humans), T2 (research translating to patients), T3 (research translating to clinical practice), and T4 (research translating to communities) (2). The co-chairs also solicited input from the National Institute on Alcoholism and Alcohol Abuse (NIAAA). Participants disclosed potential conflicts of interest, which were vetted and managed in accordance with ATS policies and procedures.
Conceptual Definition of SAWS
Given the diverse descriptions of SAWS in the literature, the committee first developed a conceptual definition of SAWS through consensus by using a modified Delphi approach (59). Committee members were queried regarding potential SAWS definitions by using two rounds of anonymous, online surveys. Using a modified approach, without anonymity, survey responses were then summarized during a teleconference meeting in early spring of 2019 and discussed by the committee in a broad, open-ended fashion to reach verbal consensus on a working conceptual definition of SAWS. The working definition was voted on by committee members by electronic mail immediately after this meeting (approve, approve with suggested modifications, or disapprove). During a second teleconference meeting several weeks later, committee members reviewed and discussed the revised conceptual definition, followed again by electronic mail voting. Finally, T.L.S. and E.L.B. presented a summary of approved changes, facilitated additional committee discussion, and conducted a final vote regarding the conceptual definition at the in-person meeting during the ATS 2019 International Conference in Dallas, Texas.
The finalized conceptual definition (Box 2) was used to focus the content of the research statement and explicitly highlights the severity of symptoms, making the need for inpatient management likely among patients who meet the definition. The conceptual definition also stresses the pathologic mechanisms of severe withdrawal physiology (i.e., aberrant central nervous system signaling) and focuses on objective, quantifiable AWS manifestations (hyperautonomia and hyperactive delirium) rather than on patient-reported symptoms included in Diagnostic and Statistical Manual classifications, as per recommendations from the National Institute of Mental Health Research Domain Criteria (60). The literature review and committee discussions leading to recommendations in this report also emphasized acute care and ICU hospital settings.
Box 2.
Conceptual definition of severe alcohol withdrawal syndrome.
A progressive state of central nervous system hyperexcitation due to reduction or cessation of alcohol use resulting in severe signs and symptoms of hyperautonomia and hyperactive delirium.
Literature Search and Evaluation
Existing systematic reviews did not fully address the a priori research questions. As such, a broad literature search strategy was used to identify studies that evaluated pathophysiology, diagnostics, and therapeutics for SAWS. This literature search was not intended to be a systematic review but was rather intended to be a comprehensive review to provide structure for the committee’s subsequent activities. A research librarian at the University of Colorado Anschutz Medical Campus performed a detailed search for articles relevant to SAWS published from January 1960 to March 2019 in MEDLINE (Ovid interface) by using keywords and Medical Subject Heading terms developed in conjunction with the committee (Box 3; see Tables E1 and E2 in the online supplement). Articles were excluded if they were unavailable in English or if the full text was unavailable online, given cost and time constraints. Ultimately, 251 records were retrieved. The committee co-chairs reviewed all identified publications and subdivided them into the four translational groups (i.e., T0–T1, T2, T3, and T4) on the basis of content. These initial articles formed the foundation of SAWS literature for the committee to build on. Subsequently, committee members were tasked with identifying additional studies of relevance, adding 36 additional references pertaining to SAWS pathophysiology, rating scales, risk stratification, and health services in April 2019. The committee members reviewed all references pertaining to their unique section and supplemented these references with their own search strategies. Finally, additional references were added through the spring of 2021 as new articles were published over time (adding 23 additional references).
Box 3.
Features of the literature review used to construct the research statement.
Inclusions
General—alcohol, alcohol withdrawal, alcohol dependence, alcohol use disorder, delirium tremens, withdrawal delirium, withdrawal seizure, intensive care, critical care, hospital, inpatient, Clinical Institute Withdrawal Assessment for Alcohol, Richmond Agitation–Sedation Scale, delirium, detox*, refractory, complicated, severe, resistant*
Drugs—benzodiazepine, chlordiazepoxide, lorazepam, diazepam, midazolam, phenobarbital, dexmedetomidine, ketamine, propofol, clonidine, carbamazepine
Neurobiology—γ-aminobutyric acid, glutamate, N-methyl-d-aspartate, hyperexcit*
Exclusions
Non-English, not full text, data prior to 1960, outpatient setting
Asterisks are truncation operators.
Each of the four sections, led by a section leader (S.E., M.A., S.E.J., and C.T.), met separately via teleconference in the spring of 2019 to review and synthesize the literature regarding the a priori research questions for SAWS assigned to their translational domain. Each section generated a current “state of the research” for the four translational domains, delineating notable gaps in the literature for discussion at the in-person meeting.
Knowledge Gaps
At the in-person meeting in May 2019, each of the four section leaders briefly provided a synopsis of the available literature regarding research questions assigned to their section/translational domain. The section leaders identified preliminary knowledge gaps, which were vetted and expanded on by the full committee through discussion and consensus. The four sections then convened separately in breakout sessions to further define and delineate urgent research priorities. Finally, the entire committee reconvened to present refined concepts of needed research for SAWS. The meeting was recorded, and the co-chairs and section leaders took notes, which were later used in document development.
Document Development
One chairperson (T.L.S.) drafted an outline of the research statement and circulated the outline to section leaders, who were tasked with drafting their sections of the manuscript. Notes from the in-person meeting held in May 2019 were available as needed. The co-chairs drafted all additional content with input and editing by committee members. The full committee reviewed the final draft of the manuscript and provided iterative feedback and revisions. After additional review and revision by the co-chairs and approval of the manuscript by committee members, a final draft was submitted to the ATS executive committee.
Section 1: Pathophysiology of SAWS and Development of Novel Therapeutics (T0–T1 Research)
Basic science research, classified within the T0 and T1 research domains, has driven numerous advances in the understanding of alcohol-related pathophysiology (61). Investigation of nervous system dysfunction after chronic alcohol consumption has grown exponentially in neuroscience research (62–65). Over the past decade, preclinical work has successfully promoted new translational strategies aimed at treatment of pathologic craving and escalated drinking patterns in patients with AUD (66–69). SAWS is a highly morbid manifestation of AUD that could be successfully examined by using a similar translational strategy.
At the preclinical level, the biological mediators and consequences of alcohol withdrawal have been delineated by using multiple approaches, ranging from ex vivo brain-slice recordings to whole-animal behavior (70–72). Fortunately, substantial construct and translational validity exists in animal modeling of SAWS for the human condition (73, 74). This is particularly true for objective symptoms like seizures and tachycardia (75, 76) but may also be true for subjective alterations in negative affective states such as increased irritability and anxiety-like behavior mimicking delirium, which manifest in later stages of SAWS (77, 78).
GABA and Glutamate Neuroadaptation
SAWS symptomatology is intricately tied to the neuropharmacologic effects of alcohol and opponent physiologic processes that manifest as withdrawal during periods of abstinence (72). Research in this area is generally framed in terms of allostatic adaptation to chronic alcohol consumption, defined as a neuroadaptive process of maintaining stability in brain reward systems in the face of challenge by alcohol (79, 80). Alcohol functions as a dose-dependent central nervous system depressant through its ability to alter neurotransmission across multiple brain regions, including the amygdala, hippocampus, frontal cortex, and brain stem nuclei (81). Acute alcohol intoxication principally facilitates GABAergic (inhibitory) signaling and reduces glutamatergic (excitatory) activity, producing sedation, anxiolysis, and behavioral disinhibition (82). Among individuals with chronic and heavy alcohol use, counterregulatory neuroadaptations in GABA and glutamate signaling become manifest during periods of abstinence, driving SAWS-related clinical effects in an allostatic fashion (80, 83). Across several preclinical animal models, chronic alcohol exposure is associated with increased N-methyl-d-aspartate receptor subunits and function (84–89) as well as with complementary decreases in GABA receptor subunits and function (90, 91). These neuroadaptations generate brain hyperexcitability during alcohol withdrawal, measured in rodents via EEG and electrophysiologic recordings (75, 92), which can be mitigated by medications with GABAergic and/or antiglutamatergic activity (93–96).
Kindling
Repeated cycles of intoxication and withdrawal directly damage frontal cortex neurons through glutamate-mediated excitotoxicity. The resulting brain injury is incompletely described but may result in loss of executive function and sensitization to further episodes of alcohol withdrawal (97). Ballenger and Post (98) called this phenomenon a “kindling effect.” Supporting their original hypothesis, animal studies have since demonstrated progressive EEG abnormalities in recurrent episodes of withdrawal, which are responsive to proactive treatment in the early stages but are later characterized by increasing resistance to pharmacotherapy (99, 100). This relationship may also explain continuous drinking patterns among individuals with AUD as a reinforcement mechanism and self-medication strategy (101).
Other Neuromodulatory Systems
Despite a strong focus on GABA and glutamate systems in the existing literature, neuroadaptations beyond these neurotransmitters are essential to the pathophysiology of SAWS and warrant further investigation to improve on current treatment strategies, which largely rely on benzodiazepines (28). The importance of identifying therapeutic targets beyond benzodiazepines is underscored by adverse effects associated with use of benzodiazepines in hospitalized patients (29–32). In addition, tolerance may render this class of medication ineffective for SAWS in certain patients (102), mediated by alcohol’s interaction with the GABAA/benzodiazepine receptor complex (103).
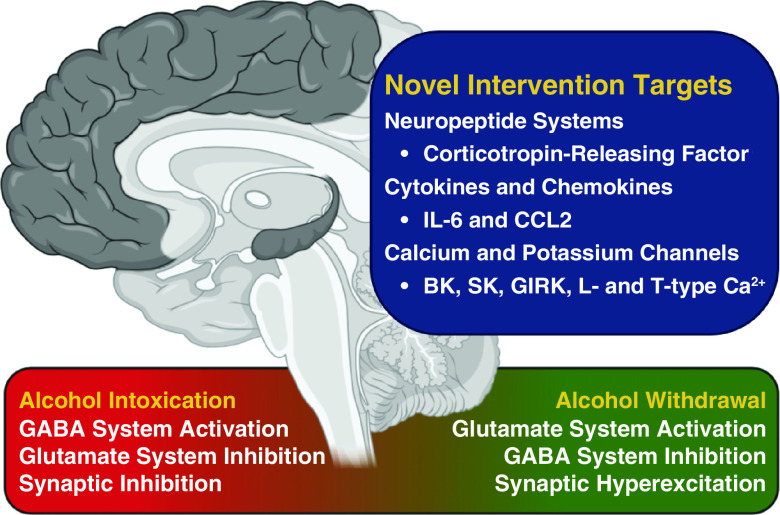
Apart from mechanisms involving GABA and glutamate, multiple hormonal and neuromodulatory systems act as higher-level regulators of excitatory and inhibitory neurosignaling during SAWS (104–106). Alcohol dysregulates several major neuropeptide systems in the brain, including CRF (107). CRF receptor signaling mediates both increased pain sensitivity and irritability-like behavior observed during withdrawal in alcohol-dependent rodents (77, 108). CRF modulation of glutamatergic and GABAergic signaling is altered by chronic alcohol exposure and subsequent withdrawal (109, 110). CRF also potentiates neuroimmune signaling (111). Furthermore, the cytokine/chemokine factors IL-6 and CCL2 modify neuronal excitability during alcohol withdrawal (78, 112, 113). Therefore, mechanisms of neuropeptide and neuroimmune dysregulation during alcohol withdrawal remain worthy of additional investigation (114, 115).
Alcohol has significant effects on ligand- and voltage-gated channels in the brain (i.e., potassium, calcium, and hyperpolarization-activated cyclic nucleotide–gated channels) (116–119). Ongoing studies are determining how these channels are altered at both transcriptional and posttranslational levels during SAWS. Second messenger systems such as PKA (protein kinase A) and PKC (protein kinase C) play important roles in posttranslational modulation of ion channel proteins, affecting channel function and/or surface expression (120). The regulation of channel expression and function that potentiates neuronal hyperexcitability during SAWS likely depends on the regions of the brain where they are expressed (121–126). These region-specific alterations underscore the need for high-resolution electrophysiologic and pharmacologic studies of neurocircuits vulnerable to SAWS-related hyperexcitation. Targeting diverse channel types and cellular messaging pathways affected by alcohol using specific pharmacologic strategies may complement existing therapeutics for SAWS and/or lead to the development of novel medications (126). Although long-term modulation of some molecular messengers implicated in SAWS may have deleterious effects, selective or short-term modulation during the vulnerable period of SAWS may ultimately prove safe and beneficial.
Sex Differences in SAWS Pathophysiology
A body of literature derived primarily from rodent model studies suggests that sex is an important biological factor influencing disease manifestations in SAWS (127–129). Male rodents exhibit more severe symptoms of alcohol withdrawal than female rodents, including greater seizure susceptibility (i.e., kindling) and slower recovery from seizure (130–132). Sex differences in alcohol withdrawal–related anxiety-like behavior—greater in male rodents and more pronounced in adults than in adolescents—may relate to differences in steroid hormone signaling (120, 133), with increased cortisol levels during alcohol withdrawal demonstrated in male versus female rodents (134) and protective effects of progesterone and endogenous neurosteroid activity demonstrated in female versus male rodents (135, 136). Female rodents also display increased levels of glutamate transporters during alcohol withdrawal that confer protection against excitotoxicity (137). In contrast, male rodents exhibit persistently increased glutamate channel subunits during withdrawal that correlate with greater seizure susceptibility (90, 130). Research examining sex differences in the relationship between seizure liability and the neurotoxic and neurodegenerative effects of chronic alcohol exposure is ongoing (138). Sex as an effect modifier of SAWS pharmacotherapy requires further investigation to determine whether these rodent observations translate to human pathophysiology.
Challenges
The primary obstacles to advancing T0–T1 SAWS research include the lack of a precise definition of SAWS and delineation of time points associated with clinical progression of SAWS. The distinct features of AUD, physical/somatic dependence, withdrawal, and SAWS do not have specific biological correlates or animal models that clearly recapitulate these conditions. In this regard, a particular challenge for basic scientists is the lack of readily accessible biomarkers to support early identification and prevention of SAWS. Greater progress and further insight might be achieved by following recommendations from the National Institute of Mental Health, which launched its Research Domain Criteria framework in 2009 to better organize diagnostic and research efforts toward valid objective criteria and away from homogeneous constructs and classifications associated with Diagnostic and Statistical Manual classifications (60).
Another challenge for application of preclinical research to patients with SAWS is the preponderance of comorbidities in this population, including acute medical and surgical illnesses and polysubstance use (139, 140). The common occurrence of polysubstance use in the setting of SAWS has been partially addressed by recent efforts from the Collaborative Research on Addiction at NIH, which aims to support integrative investigations across used substances. As a result, additional research funding is now available to understand how diverse substances such as nicotine and opioids interact with alcohol to modify SAWS vulnerability, including neurobiological mechanisms of tolerance across substances (141–143); however, animal models of other clinically relevant cooccurring conditions, such as sepsis, trauma, and organ failure, that are complicated by SAWS, have not been developed.
Recommendations for Future Basic Science Research
Recommendations for research to advance understanding of SAWS at the basic science level (T0–T1 domains) include improving the pathophysiologic understanding of SAWS and the development of preclinical models to promote clinically relevant mechanistic research.
1) Broaden the scope of SAWS pathophysiology
The first charge is to explore neuromodulatory systems beyond GABA and glutamate to improve diagnostic capabilities and to develop novel therapeutic options. In addition to GABA and glutamate, other factors are capable of regulating the balance of excitatory and inhibitory signaling in the context of alcohol dependence and withdrawal, including neuropeptide, cytokine/chemokine, and alternative ion channel mechanisms (Figure 1). Future experimental strategies should focus on examining cellular communication within and between distinct brain regions dysregulated in SAWS, in a strategy similar to those used to investigate epilepsy (144, 145). As one recent example, Lee and colleagues (125) used Designer Receptors Exclusively Activated by Designer Drugs technology to activate and inhibit hippocampal cells, leading to increased and decreased epileptiform activity, respectively, during alcohol withdrawal. Similar circuit-based approaches are being used to examine hyperalgesia and anxiety-like behavior during alcohol withdrawal, which may generate novel circuit-based avenues for treatment (146, 147).
Figure 1.
Novel intervention targets for severe alcohol withdrawal syndrome beyond GABA and glutamate. BK = large conductance calcium-activated potassium channel, CCL2 = C-C motif chemokine ligand 2; GABA = γ-aminobutyric acid; GIRK = G protein–coupled inwardly-rectifying potassium channel; L-type Ca2+ = high voltage–activated calcium channel; SK = small conductance calcium-activated potassium channel; T-type Ca2+ = low voltage–activated calcium channel.
2) Tailor preclinical models to the patient experience
The second major recommendation is to refine preclinical animal models to recapitulate the full symptomatology of patients with SAWS by using stratification by sex to understand the contexts in which sex differences matter most. The search for reliable methods to produce blood alcohol concentrations that are sufficient to mimic intoxication and relevant comorbidities observed in AUD and SAWS has been challenging. One potentially valuable method is the chronic intermittent alcohol vapor procedure (148, 149), an exposure protocol that can be paired with complementary investigative measures, including volitional alcohol self-administration (150), somatic withdrawal (151), autonomic system potentiation (76, 152), traumatic brain injury and neuroinflammation (153), and cognitive deficits (154). In the future, such exposure paradigms could be used to model comorbid conditions (e.g., sepsis, trauma, organ failure) and important clinical outcomes (e.g., long-term cognitive function) in the setting of SAWS (155). Although the vapor model may not completely recapitulate all organ-specific alterations accompanying oral alcohol intake (e.g., gastrointestinal effects) (156–159), it allows for cyclic periods of intoxication interspersed with various lengths of forced abstinence and, as such, can experimentally recreate human drinking patterns that increase SAWS susceptibility (160). Automated vapor exposures are also relatively straightforward to sustain over a period of weeks or months and may therefore support investigations to inform the timing and efficacy of prophylactic interventions for SAWS. The potential for experiments using extended alcohol exposure is notable because early detection and treatment of SAWS can reduce morbidity and mortality (19, 161).
Section 2: Translation of Research to Patients (T2 Research)
Experts agree that early identification and treatment of patients at risk for SAWS would improve patient outcomes, but there is little consensus regarding the optimal approach for risk stratifying hospitalized patients according to their likelihood of developing SAWS. An operational definition could support early identification of patients at risk for SAWS for both clinical and research purposes. Internally and externally valid operational definitions created for a variety of syndromes in critically ill patients (e.g., sepsis, acute respiratory distress syndrome) have facilitated research and advanced patient care (162–164). SAWS investigations would similarly benefit from an operational definition that could be used to identify cases with high interrater reliability, reduce study heterogeneity, and allow clinical trials to build off one another, ultimately improving care for patients.
Numerous studies have sought to understand and develop reliable predictors and assessment tools for SAWS (18, 53, 165–179). Unfortunately, few have been validated and subsequently employed in clinical studies. Interpretation of study outcomes remains limited by varying definitions of SAWS, as well as small sample sizes and single-center study designs (18, 179). To date, no diagnostic tools for SAWS have been validated in general hospital and/or ICU settings. Although early identification and prediction of disease manifestations are being increasingly applied to other ICU conditions (180–182) and are likely applicable to SAWS, appropriate resource allocation and management strategies for SAWS remain challenging without reliable algorithms to predict which patients are at risk for symptom progression.
Risk Factors and Predictors of SAWS
Between 15% and 30% of hospitalized patients have an alcohol-related condition (183–185). For many, treatment of the primary diagnosis necessitating hospitalization (e.g., infection, trauma, organ failure, etc.) becomes the focus of inpatient care, whereas addressing the underlying AUD is not prioritized. Comprehensive validated screening and triage tools are needed to identify patients with AUD who are at risk for SAWS and are likely to require high levels of care (e.g., ICU care). Current literature indicates that patients with a known AUD, patients with a history of prior AWS, or those with heavy alcohol consumption before an alcohol-related hospitalization are at highest risk for SAWS (186, 187).
Few studies have prospectively evaluated risk factors for SAWS, and heterogeneous inclusion criteria across published studies likely contribute to their inconsistent findings (18, 179). A recent meta-analysis assessing predictors of SAWS highlighted the Prediction of Alcohol Withdrawal Severity Scale (PAWSS) as a useful screening tool (179). The PAWSS is the only alcohol withdrawal prediction tool that has been developed and tested in hospitalized medical and surgical patients (177). In a cohort of hospitalized patients with a 5% prevalence of AWS, the PAWSS demonstrated positive and negative predictive values above 90% for alcohol withdrawal requiring pharmacotherapy (178). However, prospective validation of the PAWSS excluded patients with relatively severe alcohol withdrawal, including those with a revised CIWA-Ar score ⩾20 and patients who were unable to communicate, representing many if not most ICU patients with SAWS. In addition, predictive metrics of the PAWSS may have been biased because the reference group of those with true-positive results included patients with alcohol withdrawal severe enough for providers to treat.
Other risk factors for SAWS have been inferred from small, retrospective studies lacking separate validation cohorts. Importantly, the strongest identified predictors of SAWS include previous hospitalizations complicated by SAWS (e.g., prior episodes of severe withdrawal), diagnosis of AUD, and heavy alcohol consumption as measured by using the Alcohol Use Disorders Identification Test (AUDIT) (170, 172, 176, 188–190). In one meta-analysis, a history of delirium tremens had a likelihood ratio of 2.9 for the development of SAWS and was a stronger predictor than a history of withdrawal seizures (179). Other variables, including demographic characteristics, vital signs, laboratory values, and comorbidities, have been reported, but findings were mainly from small retrospective studies. Among this list of reported variables are an elevated systolic blood pressure, a blood alcohol concentration above 200 mg/dl, elevated blood urea nitrogen, hypokalemia, and thrombocytopenia (18, 170, 172, 175, 191–194). Few studies have examined how differences in patient characteristics (e.g., demographic data, laboratory values, vital signs, mental status, medical history) influence the utility of existing tools for predicting SAWS. Measurable inpatient variables may be confounded by the effects of comorbid conditions that commonly coexist in acutely ill patients, diminishing their potential utility for the assessment of SAWS risk. The potential for misclassification should also be considered a limitation in applying these findings to critically ill patients, and further studies are needed to demonstrate their added value.
Diagnosis and Disease Severity
Early and aggressive titration of pharmacotherapy guided by clinical effects is necessary to improve treatment outcomes, but among hospitalized patients, psychometric evaluation of SAWS is complicated. Hospitalized patients often have physical ailments and/or barriers precluding verbal communication (e.g., mechanical ventilation). To achieve the clinical benefits of symptom-triggered management, objective scales must be used that do not rely on patient-reported symptoms (183).
The CIWA-Ar is the most commonly described tool for grading the severity of AWS. The original CIWA scale was designed for alcohol withdrawal research (as opposed to clinical practice) and was validated in select cohorts of patients with mild-to-moderate alcohol withdrawal and no acute comorbidities (including seizures) (195). The CIWA was not designed to diagnose or grade disease severity in hospitalized patients with SAWS. Nevertheless, the CIWA-Ar scale has been used in ICU patients and continues to rely on patient self-report of gastrointestinal symptoms, tactile and auditory disturbances, anxiety, and headache (22, 25, 186, 196, 197).
Treatment studies use various CIWA-Ar thresholds (of 8–20) to initiate pharmacotherapy and monitoring for patients at evaluation intervals ranging from every 10 minutes to four times daily (177, 178, 185). No study has documented a relationship between the frequency of assessments and patient outcomes. Patients receiving mechanical ventilation have been excluded from clinical investigations apart from three studies: two included patients intubated after the onset of SAWS (7, 172), and a third study considered mechanical ventilation a complication of SAWS pharmacotherapy (198).
The Sedation–Agitation Scale (SAS) is an alternative tool for grading alcohol withdrawal severity that is not reliant on patient self-report and has been used in ICU settings (199–201). Two studies used the SAS to titrate pharmacologic therapy as part of an alcohol withdrawal prevention protocol (7, 202). A score ⩾5 triggered pharmacologic intervention with a goal therapeutic score of 3–4. The Alcohol Withdrawal Scale, adapted from the SAS for use in medical ICU patients, contains six physical examination findings on a 0–3 scale (186). The BAWS further modified the Alcohol Withdrawal Scale for brevity and improved objectivity (203, 204), removing pulse and adapting the definition of agitation from the RASS (205). A BAWS score of 3 or more predicted a CIWA-Ar score ⩾8 with a sensitivity and specificity of 85% and 66%, respectively (203). Like other assessment tools, the BAWS was mainly developed and tested in patients with mild-to-moderate alcohol withdrawal (only 2.1% of the study sample had SAWS) (204), although a recent treatment study used a BAWS score ⩾6 to define cases of SAWS (206). Other withdrawal scales have been developed and reported but remain unvalidated in patients with SAWS (173, 189, 201, 207, 208).
Challenges
Several challenges that preclude accurate and reliable identification of patients with SAWS exist. In over 95% of cases, alcohol withdrawal is a secondary reason for hospitalization (177, 178, 209), resulting in possible misclassification, and vital signs, laboratory findings, and other objective data that are potentially confounded by concurrent illness. For example, delirium is common among hospitalized patients. Distinguishing SAWS-related delirium from other etiologies (including multifactorial delirium) is difficult. This complicates traditional teaching and understanding regarding descriptions of delirium tremens as the sine qua non of SAWS. In the context of delirium, the patient history and subjective data can be unreliable, limiting the utility of predictors and rating scales that are reliant on patient self-report (e.g., PAWSS and CIWA-Ar). Within cohorts of patients with SAWS, different phenotypes may benefit from alternative treatment pathways. For example, a younger patient with concurrent use of opioids may require unique pharmacotherapy compared with an older patient with decompensated cirrhosis and hepatic encephalopathy.
Recommendations for Research to Improve Patient Care
Reliable identification of hospitalized patients at risk for SAWS is an important precursor to both proactive medical management and clinical research. Clinical tools for SAWS should achieve the following: 1) reliable diagnosis, 2) anticipation of escalating care needs, and 3) guidance for tailoring pharmacotherapy. Prior experience in critical care applications of triage tools like the quick Sepsis-related Organ Failure Assessment (qSOFA) can be informative. The qSOFA was derived from retrospective data and underperformed in external validation studies, highlighting the need for independent validation of prediction models for SAWS (210). Diagnostic and prognostic tools should follow the guidelines set forth by the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis Initiative before dissemination and implementation (211). Because the prevalence of at-risk patients may vary greatly across clinical settings, future studies should focus on hospitalized populations in which the risk of SAWS is measurably high. Researchers should also test and validate predictive models of SAWS in multicenter clinical trials and observational studies, emphasizing inclusion of patients with diverse demographic characteristics to enhance generalizability. Overall, research that clarifies the following two areas of inquiry will provide an important foundation for subsequent clinical trials.
1) Use the EHR to create an operational definition of SAWS
EHR-based phenotypes offer a way to overcome the challenges associated with early identification of patients at risk for SAWS (212). A “computable phenotype” is a clinical condition that can be determined solely by using EHR data. With validated computable phenotypes, health systems could have an unprecedented ability to monitor and surveil patients at risk for SAWS in real time. Furthermore, researchers could identify representative samples of patients for inclusion in clinical trials.
As of 2017, over 95% of hospitals in the United States had adopted an EHR and over 80% had incorporated electronic clinical notes (213–215). Clinical decision support and intelligent data-driven alerts are now part of federal incentive programs promoting interoperability (216), although the quality and practice of EHR coding for AUD and AWS continue to vary (5). With increasing capacity for EHR data and financial incentives to improve the quality of care, health care is entering a digital age with more advanced computational methods to improve case identification and care throughput for SAWS. Further exploration of baseline (outpatient) characteristics available in the EHR that predict SAWS should be prioritized. Giving inpatient providers easy access to existing information (e.g., drinking history documented by primary care providers) could facilitate comprehensive and efficient inpatient care.
There are no existing recommendations regarding how to examine and prioritize SAWS phenotypes derived from readily available data within the EHR. Box 4 contains two rule-based, consensus-derived computable phenotypes generated by the committee to support identification of 1) patients at risk for SAWS and 2) patients in whom SAWS is likely present. These operational definitions incorporate clinical and therapeutic data that are readily available in the EHR and previously studied in the literature, offering face validity. Testing, refinement, and external validation are important next steps (38, 39, 217).
Box 4.
SAWS Operational Definitions: At Risk for SAWS and SAWS Is Likely.
Definition of patient at risk for SAWS
(+) Blood alcohol concentration OR ICD code for alcohol-related conditions (prehospital or at admission) OR evident heavy alcohol use in the past 30 days OR EHR order for CIWA-Ar/withdrawal assessment tool/order for similar scale indicating provider concern for alcohol withdrawal* OR PAWSS ⩾ 4.
Definition of patient in whom SAWS is likely
At risk for SAWS AND CIWA-Ar ⩾ 15† OR i.v. diazepam-equivalent benzodiazepine ⩾ 40 mg in 1 hour‡ AND exclusion of encephalopathy due solely to other causes (cirrhosis, sepsis, metabolic derangement, etc.).
*An order for patient monitoring using an alcohol withdrawal severity scale suggests a heightened clinical concern for alcohol withdrawal but is not a diagnostic criterion for SAWS. †Brief Alcohol Withdrawal Scale score of 6 OR Alcohol Withdrawal Scale score of 10 OR Richmond Agitation–Sedation Scale score of +2. Other scales also exist. ‡Forty milligrams of diazepam-equivalent benzodiazepine in 1 hour is considered a reasonable threshold to detect SAWS. The optimal dose threshold requires additional validation. CIWA-Ar = Clinical Institute Withdrawal Assessment for Alcohol–Revised; EHR = electronic health record; ICD = International Classification of Diseases; PAWSS = Prediction of Alcohol Withdrawal Severity Scale; SAWS = severe alcohol withdrawal syndrome.
The ability to accurately and efficiently identify patients at risk for or presenting with SAWS represents a critical need in screening and enrollment for clinical investigations. In retrospective observational studies, patients with an initial CIWA-Ar score >10 had a five- to sixfold increased risk of developing SAWS (171, 218). In a small prospective study of 19 hospitalized patients who were at risk for alcohol withdrawal, 10 developed delirium as measured by using the Confusion Assessment Method for the ICU (CAM-ICU), and CIWA-Ar scores were between 10 and 15 by the second day of hospitalization (219, 220).
Importantly, evidence-based guidelines supporting the best strategy for identifying patients with SAWS do not exist. Review of the available literature by this committee suggests that a threshold of >15 on the CIWA-Ar scale is appropriate. Nevertheless, employing CIWA-Ar scoring or other strategies for proactive SAWS identification will benefit from further research to refine and optimize their use. Box 4 highlights comparable scores across a selection of alcohol withdrawal severity scales for further consideration. In addition to available scoring systems, a 40-mg diazepam-equivalent cumulative dose of benzodiazepines in 1 hour is included in the operational definition of SAWS on the basis of a multicenter study in hospitalized patients (39). Other studies suggest that higher diazepam-equivalent doses may indicate severe withdrawal physiology; however, the operational definition is intended to be sensitive rather than specific. Further research will be critical to validate this approach.
2) Establish simple and objective tools for risk stratifying and grading the severity of alcohol withdrawal
A growing body of literature suggests that the use of ethanol biomarkers could be expanded for early identification of patients at risk for SAWS. Direct ethanol biomarkers such as phosphatidylethanol (PEth), ethyl glucuronide (EtG), and ethyl sulfate (EtS) demonstrate better testing characteristics than previously studied indirect biomarkers such as CDT (carbohydrate-deficient transferrin), GGT (γ-glutamyltransferase), the mean corpuscular volume, and liver aminotransferases (AST/ALT) (221–226). PEth, in particular, has been shown to identify patients with heavy alcohol use (221, 223, 227) and can be used to discriminate between severe and nonsevere AUD in ICU patients (224). There is limited research examining the use of PEth, EtG, and EtS to identify AUD in hospital settings, where patients at risk for SAWS are relatively common. The predictive validity of direct ethanol biomarkers for defining the risk of SAWS has not been reported, and evidence for using indirect biomarkers for risk stratification is sparse (228). Therefore, additional research to expand the use of direct ethanol biomarkers for SAWS identification is recommended. PEth, EtG, and EtS should be examined alone and in combination with other serum biomarkers. Direct ethanol biomarkers could facilitate point-of-care triage if proven to reliably identify patients who will require higher levels of care (e.g., ICU care), ultimately circumventing the need for patient self-reporting.
A simple and objective assessment tool for grading the severity of SAWS in hospitalized patients represents a critical need for future research and clinical advancement. The RASS may be a particularly advantageous option given its bidirectionality (i.e., ability to identify under- and overtreatment) and established widespread use in ICU settings for titrating sedating medications (229). Other agitation–sedation scales used in the ICU, such as the Riker scale, have been used in alcohol withdrawal treatment studies but have not been directly examined as part of an operational definition of SAWS (230). Future studies should compare the utility of commonly used ICU agitation–sedation scales to existing tools that are specific for alcohol withdrawal. Consideration of novel strategies that can adequately address the complex comorbidities of acutely ill hospitalized patients will also be important.
Universally accepted diagnostic and prognostic tools are needed to facilitate comparisons between new and existing pharmacotherapies for SAWS. Tools that facilitate consistent and accurate measurement of treatment responses would enable clinicians to tailor medications to individual patients. In most clinical studies of SAWS, research has focused on treatment modalities rather than on clinical assessment tools. If measures of SAWS are to improve, they must be subjected to more rigorous research that is designed specifically to assess their reliability and validity in studies that are sufficiently powered. Meeting these scientific standards will lend necessary rigor to future therapeutic trials.
Section 3: Establishing Best Practices to Improve Clinical Outcomes (T3 Research)
No multicenter RCTs have evaluated the impact of different treatments for SAWS on clinical outcomes in hospitalized patients. Commonly used treatment strategies are extrapolated from small studies of patients admitted to detoxification units without acute comorbidities or severe manifestations of alcohol withdrawal. In this section, the existing literature on clinical therapies for SAWS is examined, methodologic challenges of performing clinical trials in patients with SAWS are described, and recommendations for T3 research are outlined with the goal of establishing evidence-based practices for management of SAWS in hospitalized patients.
Choice of Medication for Treatment of SAWS
Insufficient data exist to guide the initial choice of medication for treatment of SAWS (6). Studies of patients with uncomplicated alcohol withdrawal suggest that multiple classes of medications, including benzodiazepines and barbiturates, may be reasonable first-line strategies (21). Extrapolation of data derived from patients enrolled in detoxification units provides insufficient guidance for treating hospitalized patients with more severe clinical signs and symptoms and/or active comorbid illnesses. Efficacy trials are therefore needed to clarify the preferred first-line therapy within different clinical phenotypes commonly represented among hospitalized patients with SAWS (e.g., differing illness trajectories and acute organ dysfunctions).
Benzodiazepine Dosing Strategies
Benzodiazepine dosing strategies for treatment of alcohol withdrawal include fixed-dose, symptom-triggered, and front-loading regimens (7, 8, 26, 46, 58, 231–233). Fixed-dose regimens consist of a predetermined dose of a benzodiazepine administered at regular intervals and gradually tapered over a period of days. Symptom-triggered dosing employs a more reactive approach, with the dose and frequency of benzodiazepine administration tailored to the severity of withdrawal as determined by using an alcohol withdrawal clinical assessment tool (e.g., the CIWA-Ar). Front-loading regimens use a proactive, concentrated dosing strategy, including several escalating doses or continuous infusion of a benzodiazepine (often with adjunctive medications like phenobarbital) over a short period of time.
Fixed-dose and symptom-triggered regimens have been studied in patients with AUD enrolled from detoxification units. In this specific setting, randomization to symptom-triggered therapy rather than to fixed-dose therapy resulted in shorter treatment duration and reduced cumulative exposure to benzodiazepines (8, 46); however, prospective comparisons of benzodiazepine dosing strategies have not been examined in patients with acute or critical illness. In ICU settings, the majority of evidence guiding treatment derives from preimplementation–postimplementation studies of front-loading protocols for management of SAWS. These studies suggest that front-loading strategies are associated with faster control of alcohol withdrawal symptoms, shorter ICU stays, and lower rates of intubation than usual care (7, 234, 235).
Three single-center retrospective studies have addressed outcomes related to standardized benzodiazepine protocol implementation in ICU patients with SAWS (7, 234, 235). Two studies employed escalating doses of benzodiazepines and used phenobarbital as an adjunctive therapy (7, 235). The third study evaluated outcomes after transitioning from continuous infusion of benzodiazepines (usual care at the study site) to a front-loading protocol tailored to the severity of withdrawal (234). All three studies observed clear advantages in the protocol intervention group, including improved withdrawal management and reduced cumulative benzodiazepine exposure. In the two studies that used phenobarbital adjuvant therapy, patients had a reduced need for mechanical ventilation (7, 235). Overall, these studies support the use of benzodiazepine front-loading strategies guided by withdrawal severity and early adjunctive therapy with phenobarbital for management of SAWS. However, more rigorous study designs in diverse patient populations are needed to establish the safety, and effectiveness of these approaches.
Benzodiazepines differ in terms of onset and duration of action, dosing, metabolism, and available formulations, all of which should be considered when selecting a benzodiazepine for the treatment of SAWS in a given patient. Diazepam is a commonly used and studied benzodiazepine for treating alcohol withdrawal, having both enteral and intravenous formulations and a rapid onset of action. However, some patients with AUD have underlying liver dysfunction. Diazepam oxidation and metabolism of active metabolites occurs via the liver, and thus liver dysfunction may prolong drug effects. An alternative medication is lorazepam, which is also well studied, metabolized via hepatic glucuronidation (less commonly impaired), and does not have active metabolites. Chlordiazepoxide, a long-acting benzodiazepine commonly used and studied in specialized addiction settings, allows patients to “self-taper” during the course of treatment, but its use is limited in hospital settings by the lack of an intravenous formulation.
Benzodiazepine Resistance
Despite receiving escalating doses of benzodiazepines, some patients with SAWS do not experience adequate symptom control. Mechanisms of cross-tolerance with alcohol at the GABAA receptor (see Other Neuromodulatory Systems in Section 1) have been implicated to explain this observation (38). The suboptimal response is often termed “benzodiazepine resistance.” In this context, administration of alternative medications as a first-line or adjunctive therapy may achieve faster symptom control than further escalation of benzodiazepine dosing (236, 237). Although there is no consistent definition of benzodiazepine-resistant SAWS, data suggest a high cumulative dose of a benzodiazepine administered over a short period of time (e.g., 40 mg diazepam-equivalents within 1 h) without resolution of SAWS symptoms characterizes benzodiazepine-resistant physiology (38, 39). Treatment choices for patients with SAWS that is refractory to benzodiazepines vary widely (238). Evaluation of benzodiazepine-alternative medications for benzodiazepine-resistant SAWS will be critical to establish whether morbidity associated with standard management approaches (e.g., intubation, prolonged ICU care) can be improved. Specific alternative therapies are detailed in Table 1, including the advantages and disadvantages of each medication and studied doses. Of note, antipsychotics are not included in Table 1, as they have been shown to precipitate or exacerbate seizures and are not considered an alternative therapy (58).
Table 1.
Benzodiazepine-Alternative Therapies for Management of SAWS
| Medication | Mechanism | Studied Doses | Studied in ICU Patients | Adjunct or Primary | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| Medications targeting GABA and glutamate | ||||||
| Ethanol (187, 223, 224) | • GABAA agonist | • 200 ml of 100% alcohol (half oral, half i.v.), titrated to maximum of 600 ml | No | Primary | • Replaces cause of AWS | • Poorly tolerated |
| • NMDA antagonist | • 10% ethanol infusion initiated at 50 ml/h, titrated to maximum of 30% ethanol at 50 ml/h × 48 h | • Highly variable kinetics | ||||
| • Difficult titration to effect with risk of oversedation | ||||||
| • Studies in prevention rather than treatment | ||||||
| • Lack of efficacy in treatment of SAWS | ||||||
| Ketamine (220, 282, 283) | • NMDA antagonist | • 0.3–1.6 mg/kg/h with optional loading dose | Yes | Adjunct | • Targets alternative to GABA | • Side effects of ketamine (hypertension, tachycardia) mimic SAWS |
| • Does not result in prolonged sedation | • Administered as continuous infusion (often requiring ICU care) | |||||
| • Low potential for respiratory depression and does not require mechanical ventilation | ||||||
| Phenobarbital (6, 51, 222, 226, 227, 241) | • GABAA agonist | • Loading with 6–15 mg/kg i.v. infusion | Yes | Both | • Targets glutamate in addition to GABA | • Inconsistent effects on respiratory depression |
| • NMDA antagonist | • Escalating i.v. bolus doses of 65 mg, 130 mg, and 260 mg | • Synergistic effects with BZDs | • Drug interactions due to induction of CYP metabolism | |||
| • Data suggest decreased need for ICU admission and mechanical ventilation, reduced BZD requirements, and shorter ICU LOS | ||||||
| Propofol (217, 218, 221, 225, 233) | • GABAA agonist | • 10–100 μg/kg/min | Yes | Both | • Targets alternative to GABA | • Bradycardia and tachycardia |
| • NMDA antagonist | • Fast onset and short duration of action | • Propofol-induced hypertriglyceridemia | ||||
| • Has been shown to reduce BZD requirements | • Propofol-related infusion syndrome | |||||
| • Respiratory depression requiring mechanical ventilation | ||||||
| • Administered as continuous infusion | ||||||
| Medications not targeting GABA and glutamate | ||||||
| Baclofen (240, 284, 285) | • GABAB agonist | • 10 mg orally three times daily | No | Adjunct | • Short duration of action | • Only available in enteral formulation |
| • Requires multiple daily dosing (compliance) | ||||||
| Carbamazepine (183, 286–291) | • Stabilizes neuronal membranes by inhibiting voltage-sensitive sodium channels and/or calcium channels | • 400 mg/d, up to 4,725 mg/d orally | No | Both | • Small studies suggest similar symptom control to BZD and barbiturates | • Drug interactions due to induction of CYP metabolism |
| • Sustained release form: 200 mg three times daily; 400 mg twice daily | ||||||
| Clonidine (182, 183, 292, 293) | • α2-agonist | • i.v.: 0.5–2.8 μg/kg/h | Yes | Adjunct | • Available in multiple formulations (enteral, i.v., transdermal) | • Studied formulation (i.v.) not commercially available in United States |
| • Enteral: up to 0.6 mg daily | • May be beneficial in patients withdrawing from substances other than alcohol | • Bradycardia | ||||
| • Hypotension | ||||||
| • Masks autonomic abnormalities but does not address primary pathophysiology | ||||||
| Dexmedetomidine (47, 218, 231–237) | • Selective α2-agonist | • 0.2–1.5 μg/kg/h, with optional loading dose | Yes | Both | • Has been shown to reduce BZD requirements | • Risk of hemodynamic instability, especially with loading dose (i.e., bradycardia, hypotension) |
| • May result in shorter hospital/ICU LOS | • Masks autonomic abnormalities but does not address primary pathophysiology | |||||
| • May be beneficial in patients withdrawing from substances other than alcohol | • Seizures reported in patients who did not receive GABA agonism | |||||
| • Administered as continuous infusion (often requiring ICU care) | ||||||
| Gabapentin (241–243, 294, 295) | • Stabilizes neuronal membranes by inhibiting voltage-gated calcium channels | • 600 mg every 8 h, with optional loading dose (800–1,200 mg × 1), with potential taper over 5 d | Yes | Both | • May reduce BZD requirements and decrease ICU/hospital LOS | • Only available in enteral formulation |
| • Structurally related to GABA but does not appear to bind receptor | • Potential drug of abuse | |||||
| • Oversedation | ||||||
| • Accumulation in renal impairment | ||||||
| Levetiracetam (296) | • Unclear | • 500 mg twice daily | No | Adjunct | • Generally well tolerated | • Has not shown clinical benefit (e.g., reduced BZD requirement) |
| • May indirectly modulate GABA signaling | ||||||
| Oxcarbazepine (297) | • Stabilizes neuronal membranes by inhibiting voltage-sensitive sodium channels and/or calcium channels | • 600 mg daily × 72 h (divided into three daily doses), then 300 mg daily | No | Adjunct | • Appears to be equally as effective as clomethiazole (when used in conjunction with tiapride) | • Only available in enteral formulation |
| • Fewer drug interactions than carbamazepine | • Higher incidence of hyponatremia than carbamazepine | |||||
| Pregabalin (244) | • Stabilizes neuronal membranes by inhibiting voltage-gated calcium channels | • 300 mg daily × 48 h (divided into two daily doses), then tapered 100 mg every other day | No | Primary | • No hepatic metabolism | • Only available in enteral formulation |
| • Has not shown clinical benefit (e.g., BZD requirement reduction) | ||||||
| Valproic acid (26, 179, 228, 230, 290, 298, 299) | • Stabilizes neuronal membranes by inhibiting voltage-gated sodium channels | • Sustained release form,* 300 mg three to four times daily | Yes | Both | • Associated with decreased duration of AWS treatment and hospital LOS | • Caution in patients with hepatic impairment |
| • May bind to presynaptic GABAB receptors, increasing release of GABA | • Immediate release,† 400 mg every 8 h | • Hyperammonemia | ||||
| • Increases GABA synthesis by activating glutamic acid decarboxylase | • Sustained release form, 500 mg three times daily | • Thrombocytopenia | ||||
| • Transaminitis (typically asymptomatic) | ||||||
| • Cannot be used concurrently with carbapenems |
Definition of abbreviations: AWS = alcohol withdrawal syndrome; BZD = benzodiazepine; CYP = cytochrome P450 enzymes; GABA = γ-aminobutyric acid; LOS = length of stay; NMDA = N-methyl-d-aspartate; SAWS = severe AWS.
Commercially available only as 125-, 250-, and 500-mg strength.
Dosing commercially available only as oral solution.
Benzodiazepine-Alternative GABAergic and Antiglutamatergic Medications
Nonbenzodiazepine medications targeting the main pathophysiologic mechanisms of alcohol withdrawal (GABAergic and/or antiglutamatergic effects) include ethanol, ketamine, propofol, and phenobarbital. These medications could theoretically serve as monotherapy alternatives to benzodiazepines for the initial management of SAWS (7, 238–243). Treatment of alcohol withdrawal by using ethanol is controversial. Data comparing its use (enteral or intravenous) to benzodiazepines in early/mild withdrawal have shown no clear differences in short-term clinical outcomes (202, 244, 245). The American Society of Addiction Medicine specifically recommends against the use of ethanol for the treatment of alcohol withdrawal in any context (58). There are few studies of ketamine for management of SAWS. Existing data suggest an association between the use of ketamine and decreased ICU and hospital length of stay (241). Data regarding propofol therapy for SAWS are limited to retrospective cohort studies. One study suggested that propofol may be effective for SAWS when used as a monotherapy (246). Multiple case reports describe successful use of propofol for management of benzodiazepine-resistant SAWS (55, 237). In other studies, however, when propofol was used as an adjuvant to benzodiazepines, the time to resolution of symptoms, duration of mechanical ventilation, and hospital and ICU length of stay were all increased in comparison with when benzodiazepine was used as a monotherapy (7, 238, 239). Importantly, these findings may be confounded by the severity of illness in patients who require propofol during management of SAWS, and may not be a direct consequence of the medication. Unlike patients receiving other pharmacologic therapies for SAWS, patients receiving propofol generally require mechanical ventilation.
A growing body of research supports phenobarbital as a monotherapy or adjunctive therapy to benzodiazepines for management of SAWS (247, 248). In addition to being a GABA agonist, phenobarbital is an N-methyl-d-aspartate receptor antagonist, which may overcome the pathophysiology of benzodiazepine resistance (249, 250). Studies conducted in emergency department settings suggest that phenobarbital used as a monotherapy improved the control of withdrawal and resulted in an equal or reduced need for inpatient and/or ICU admission compared with use of benzodiazepines (54, 240, 251). In the surgical–trauma ICU setting, phenobarbital-based protocols appear to be effective in preventing withdrawal-related complications, including delirium and clinically significant respiratory depression (252, 253). One study in patients with trauma reported statistically significant decreases in the rates of progression to SAWS and medication adverse effects with phenobarbital compared to a fixed-dose benzodiazepine protocol (252). A retrospective study of general medical patients treated with phenobarbital demonstrated equivalent outcomes to a fixed-dose benzodiazepine protocol (i.e., no difference in the incidence of seizures, hallucinosis and/or delirium, ICU transfer, leaving against medical advice, mortality, length of stay, or medical adverse events), despite a more prevalent history of complicated alcohol withdrawal in the phenobarbital group (254). A retrospective study in medical ICU patients that compared fixed-dose phenobarbital monotherapy to symptom-triggered lorazepam using the CIWA-Ar showed that patients treated with phenobarbital had significantly shorter ICU and hospital stays, lower incidence of mechanical ventilation, and reduced need for adjunctive medications (243). Studies of phenobarbital coupled with benzodiazepine front-loading strategies in ICU settings suggest that adjunctive phenobarbital results in lower rates of mechanical ventilation, fewer ventilator days, decreased ICU and hospital length of stay, and a variable impact on benzodiazepine requirements (7, 235).
As with benzodiazepine therapies discussed above, the quality of data describing benzodiazepine-alternative medications (including phenobarbital) for the treatment of SAWS is limited. Practical barriers may also constrain the utility of benzodiazepine-alternative pharmacotherapies; for example, ketamine infusions often require ICU admission, and propofol generally requires mechanical ventilation.
Antiepileptic Medications as Treatment for SAWS
Meta-analyses examining the effects of antiepileptics in aggregate (e.g., carbamazepine/oxcarbazepine, levetiracetam, valproic acid) for SAWS have reported no differences in clinical outcomes compared with benzodiazepines (6, 10); however, given their various pharmacologic mechanisms, evaluating antiepileptics as a single class of medication may bias the findings of these analyses toward the null. Although not specifically examined for use in SAWS, valproic acid is used for management of inpatient alcohol withdrawal at some centers based on extrapolation of data from detoxification units (28). Small studies suggest that use of valproic acid during alcohol withdrawal is associated with improved symptom management compared with benzodiazepines, but these data are limited by a lack of blinding and/or retrospective cohort designs (255, 256). Other studies suggest that use of valproic acid for alcohol withdrawal has effectiveness similar to use of phenobarbital (257) and results in reduced symptom duration, lower incidence of ICU transfer, and fewer withdrawal seizures and side effects than use of carbamazepine (198).
Additional Pharmacologic Mechanisms Targeting SAWS
Medications that do not directly target GABA and/or glutamate signaling may attenuate certain signs and symptoms of SAWS (e.g., abnormal hemodynamics or behavioral aggression) that are used to gauge disease severity. As a result, these medications can mask SAWS while having unclear effects on brain hyperexcitation. One such medication is dexmedetomidine, which has been proposed as an adjunctive agent for alcohol withdrawal. In small studies, dexmedetomidine treatment is associated with improved symptom management and reduced need for benzodiazepines and mechanical ventilation (49, 239, 258–263). Safety data, however, raise concern for increased risk of bradycardia and hypotension with use of dexmedetomidine, especially compared with benzodiazepine monotherapy (49, 258, 263). Furthermore, dexmedetomidine may be associated with alcohol withdrawal seizures, as it does not address the primary pathophysiology of alcohol withdrawal (259, 264).
Small studies of other medications in hospitalized patients with milder alcohol withdrawal highlight the need for careful evaluation of medication benefits, weighed against the risks of polypharmacy (265, 266). For example, the use of baclofen may reduce the incidence of alcohol craving when compared with placebo treatment (267), but its utility for SAWS is uncertain. Similarly, studies evaluating gabapentin as a monotherapy or adjunctive therapy to benzodiazepines have shown inconsistent benefits (268–270). In one retrospective study, the use of high-dose gabapentin was associated with reduced hospital length of stay compared to standard-of-care treatment (269). Data on the use of pregabalin suggest no difference in clinical outcomes compared to placebo (271).
Challenges
Multicenter RCTs are needed to improve treatment for SAWS, but challenges related to study design must first be addressed. Given the documented harms of placebo therapy for SAWS, it is unethical to conduct placebo-controlled trials to evaluate treatment efficacy (272). Clinical trials for SAWS will require innovative methods that can test and implement models of care in a setting where patients are often unable to provide consent. Prior experience from rigorous RCTs performed in well-established trial networks, including the National Institute on Drug Abuse Clinical Trials Network, should be employed to inform methods for ensuring trial enrollment, retention, and fidelity. The capacity of potential research participants to participate in the informed consent process should be assessed carefully, recognizing that active withdrawal can result in reduced judgment while respecting patient autonomy regarding participation in research (273, 274). A thoughtful approach to the design of clinical trials is needed, to reduce the likelihood that study participation contributes to further social marginalization or stigma (275–278) while ensuring opportunities to participate in research. Assuming that wide-ranging practice variation for SAWS exists, various existing “standards of care” could theoretically be tested against one another by using pragmatic effectiveness trial designs with a waiver of consent; however, data delineating this practice variation (e.g., describing the relative prevalence of different treatment strategies) do not yet exist.
Inpatient populations at risk for SAWS are characterized by unique factors that complicate trial participation and fidelity. Beyond impaired decision-making capacity, such factors include a wide range of underlying medical comorbidities, a high frequency of leaving against medical advice, the absence of surrogates or legally authorized representatives, and social instability (279). Creative strategies for facilitating equitable access to clinical studies among patients with SAWS will be required to move the needle in this area of research. Many patients at risk for SAWS are socioeconomically disadvantaged (280). Significant research personnel investment (e.g., social workers, peer recovery mentors, clinical research coordinators) may be needed to overcome these complex barriers. Ultimately, understanding whether patient diversity (e.g., across the socioeconomic spectrum) contributes to variability in treatment (e.g., due to disparate access to care) and/or the effectiveness of specific treatments (e.g., housing vouchers) will only be achieved through intentional enrollment of subjects reflective of all patients at risk for SAWS.
Recommendations for Research to Improve Clinical Practice and Patient Outcomes
Quality RCTs to evaluate treatment practices for SAWS will first depend on the T2 research recommendations described in Section 2—a validated operational definition and objective tools for risk stratifying and grading the severity of SAWS in potential study participants. Subsequent high-priority research aims are to delineate and standardize appropriate clinical outcomes for assessment in patients with SAWS (i.e., RCT endpoints) and to build a multicenter research collaborative to take on the challenges inherent to this patient population. This upfront research and creation of infrastructure will ultimately enable important clinical questions to be addressed regarding which medication, dosing strategy, and/or bundle of care should be prioritized for management of SAWS.
1) Determine optimal short-term, long-term, and patient- and provider-centered outcomes for clinical trials
Defining appropriate outcomes to include in RCTs for SAWS will require dedicated research. Self-reported patient measures are unreliable in patients with SAWS and are frequently inapplicable to hospitalized patients with acute and critical illness (e.g., due to communication barriers such as altered cognition or mechanical ventilation). Standard physiologic endpoints may be confounded by concurrent medical conditions (e.g., pain, cardiac disease, sepsis) and are thus potentially less robust for evaluation of SAWS. Although short-term measures of delirium, agitation, and/or coma obtained by using the CAM-ICU and RASS are well-established measures of acute brain dysfunction (219, 229, 281), these measures have yet to be explored in RCTs for SAWS. Long-term outcomes that may be more meaningful to patients (e.g., cognitive function) are complicated by the challenge of establishing a “normal baseline” and by the potential for loss to follow-up after hospital discharge.
Traditionally, studies of SAWS have focused on short-term clinical outcomes (e.g., the need for intubation, hospital length of stay, and cumulative benzodiazepine exposure) (7, 46, 235). Such outcomes are of interest to clinicians, researchers, and hospital administrators but are not necessarily patient-centric, unless associated with acute discomfort or morbidity. However, the ability of short-term clinical outcomes to predict long-term psychologic and cognitive effects or functioning could be explored, including their relationship with subsequent alcohol cravings, persistent sobriety, and cognition. Simultaneous collection of data on short-term and long-term outcomes allows early events (e.g., delirium) to be evaluated as predictors of later events (e.g., cognitive impairment at 3 mo after hospital discharge). If strongly predictive, future investigations could rely on short-term endpoints as proxies for long-term endpoints, answering important clinical questions that are meaningful to patients in shorter-duration, more cost-effective clinical trials.
High-priority short-term outcomes of treatment for SAWS include: the time to control of agitation, duration of delirium, and prevention of adverse events (e.g., the need for mechanical ventilation). During SAWS, time to control of agitation is believed to be associated with clinically important adverse events (21), although this relationship needs further elaboration to affirm its importance for treatment trials. In survivors of critical illness, duration of delirium is associated with short- and long-term mortality, long-term cognition, and functional outcomes (29, 37, 282, 283). These effects of delirium are expected to exist in patients with SAWS (35, 37, 284); thus, studies that include delirium as an outcome of treatment in this population are indicated.
Patient- and provider-centered outcomes require further exploration with stakeholders, including patients, patient advocates, and providers who care for patients with SAWS (285). Potentially important outcomes for patients include 1) cognitive function (both acutely and over time), 2) alcohol craving, 3) engagement in AUD treatment, 4) abstinence from alcohol, 5) readmission to the hospital for SAWS, 6) readmission to the hospital for other illnesses, and 7) the financial impacts of SAWS and AUD (e.g., money spent on treatment, including rehospitalizations over time and lost wages).
The concept of provider-centered outcomes in clinical research has received attention in the form of user-centered design and system approaches to promote safe and effective clinical workflows (i.e., avoiding human error) (286, 287). However, attention to provider-centered outcomes may augment the acceptability and sustainability of treatment strategies found to be of benefit in clinical trials. The impact of SAWS treatment protocols on healthcare provider mental health and well-being should be explored. Caring for patients with SAWS can be stressful, time-intensive, and exhausting, contributing to compassion fatigue, a common symptom among individuals with burnout syndrome (BOS). Importantly, studies of healthcare provider well-being and ICU patient outcomes have demonstrated a link between provider BOS and reduced quality of care, lower patient satisfaction, increased medical errors, higher rates of health care–associated infections, and even 30-day mortality (288–290). Lessons learned from evaluation of provider-centered outcomes in SAWS research could potentially inform other opportunities to address BOS in critical care settings at large.
2) Establish a clinical trial network to conduct clinical efficacy–effectiveness trials
As discussed in Section 2, foundational epidemiologic work is needed to define the ideal at-risk population for large-scale clinical trials in SAWS. A multicenter clinical trial network should be established to convene participating centers. Working groups should be assigned to establish study criteria and define clinical outcomes that are mindful of the challenges of conducting clinical research in hospitalized patients with SAWS (e.g., inadequate sample size). A network of centers with high prevalence of SAWS could facilitate successful patient recruitment, optimize statistical power, and promote sharing of biological specimens and clinical data to accelerate epidemiologic, mechanistic, genetic, and biomarker studies that clarify disease heterogeneity and the effect modification of various SAWS treatments (291–293). Financial support from funding agencies such as the NIAAA, Department of Veterans Affairs, and Patient-centered Outcomes Research Institute will be vital to establishing such a network, given the associated complexities and costs. Patient advocates will also be essential partners, ensuring that research methods and outcomes remain patient centered in this stigmatized population (276–278).
Within the structure of a clinical trial network for SAWS, pragmatic and adaptive trial designs offer important strategies for maximizing the recruitment and retention of patients, as well as providing opportunities for efficiently testing multiple interventions (294, 295). Pragmatic designs—in which comparator treatment strategies are provided to patients in “real-world” settings (without the extensive inclusion/exclusion criteria typical of traditional RCTs)—could be considered under a waiver of consent, particularly in settings where consent is impeded by patients lacking capacity because of comorbid illnesses (296, 297). Studies focused on protocol implementation may benefit from unit-level, cluster-randomized designs (i.e., each hospital ward or unit using a specific protocol for all patients) rather than from traditional patient-level randomization (i.e., patients within a given ward or unit receiving different protocols). Adaptive platform trials that incorporate periodic assessments for safety and effectiveness and protocol modifications could allow continuous comparison of treatments with prognostic enrichment strategies across different patient phenotypes (e.g., stratified by comorbid conditions—trauma, sepsis, neurosurgery, etc.), testing multiple interventions within a single hospitalized cohort (293, 295, 298). The proposed multicenter trial network could thus create a foundation for answering iterative questions regarding treatment strategies that have unclear benefit in current practice and testing new interventions that emerge amid an evolving understanding of the basic science and pharmacology of SAWS.
3) Prioritize three clinical questions for immediate study
Three clinical questions should be top priorities for clinical research focused on SAWS. First, what is the optimal first-line medication for SAWS (e.g., benzodiazepines vs. nonbenzodiazepines)? Second, what is the ideal medication dosing strategy for SAWS (e.g., symptom-triggered vs. front-loading)? The answers to these first two questions would have an immediate impact on patient care and clinical practice. Third, is protocolized and/or bundled care superior to usual care for SAWS? Testing the impact of a standardized best practice “bundle,” including items such as electrolyte replacement, intravenous thiamine, addiction counseling, and medications to treat AUD, could be specifically explored. Implementation research including protocolized and/or bundled care should be conducted simultaneously with medication trials by using the broader infrastructure of the proposed clinical trial network to expedite the transfer of knowledge into practice.
Section 4: Implementation of Research Findings for SAWS (T4 Research)
The T4 research domain involves the translation of research findings to communities, including population-level outcomes research and monitoring (e.g., morbidity, mortality, and impacts of policy changes). As previously discussed, evidence-based, validated treatment approaches for hospitalized patients with SAWS do not exist. As such approaches become available, the knowledge gap regarding how best to implement guideline-recommended care will likely become more evident. The dissemination of guidelines for treatment of SAWS will almost certainly face the same challenges as the dissemination of critical care guidelines more broadly. Concerningly, a growing body of literature suggests that even when critical care guidelines are available, patients often do not receive care consistent with the recommendations (299, 300). This section explores research strategies for anticipating and mitigating the gaps that may develop between establishing efficacious therapies for SAWS (through clinical trials) and successful delivery of these treatments to patients in real-world clinical settings.
Recommendations to Promote Implementation of Evidence-based Practices
1) Engage stakeholders
Stakeholder involvement will be important for successful implementation of best practices for SAWS. In the area of addiction, one study of stakeholders found that a lack of interprogram cooperation and communication was a deterrent to addiction treatment after detoxification (301). Barriers to effective care can be identified and modified through broad stakeholder engagement that includes a wide range of individuals: patients, their caregivers, advocacy groups, community members, providers (e.g., nurses and clinicians across disciplines and care delivery systems), purchasers, payers, administrators, policy makers, and researchers. Although implementation and stakeholder engagement are often viewed as later steps in the research translation process, incorporating these elements before establishing a treatment’s efficacy will accelerate the transfer of innovations into practice (302). Input from patients recovering from SAWS will be particularly important in delineating the needs and realities of those living with AUD. To this end, interdisciplinary collaboration will be essential. Although many existing relationships can be harnessed to improve the workflow of caring for patients with SAWS (e.g., natural collaborations among clinicians, nurses, pharmacists, and therapists working in the ICU), less traditional partnerships are also needed (e.g., bridging critical care with addiction-related specialties and ambulatory services).
2) Harness the knowledge and infrastructure of existing critical care networks
Implementation of best practices within critical care has proven challenging and requires dedicated attention and resources (303). Critical illness and organ failure often result from heterogenous syndromes rather than from distinct disease entities with well-defined pathologic boundaries, including sepsis and acute respiratory distress syndrome in addition to SAWS. Diagnosis of these conditions is complex, and treatment requires multimodal therapy more often than it requires a single targeted medication. In this context, treatment practices in ICU settings vary widely (304–306); however, in recent years, collaborative critical care networks have successfully worked to address this variation in clinical practice to improve patient outcomes (294).
The backbone of this work has involved real-time data collection and feedback—describing processes of patient care, providing feedback to centers regarding their performance benchmarked against other ICUs, and subsequent interventions, including guideline distribution and progress updates to homogenize care (307). By using lessons learned from these past experiences and the infrastructure of existing trial networks (e.g., the International Forum for Acute Care Trialists), the clinical research network proposed in Section 3 could serve a pivotal role in building a foundation for clinical trials and also in implementing treatment guidelines for SAWS (294). An exemplary model of collaborative multiinstitutional (and multinational) clinical research supporting simultaneous implementation of best practices is the REMAP-CAP (Randomized, Embedded, Multifactorial, Adaptive Platform Trial for Community-acquired Pneumonia) program (308). This hybrid model of clinical research and quality improvement (i.e., platform trial) is evaluating multiple interventions (treatments and treatment implementation strategies) for severe community-acquired pneumonia by using a continuously learning healthcare network focused on a disease rather than on testing a single intervention for that disease.
In the coming era of research to improve the management of SAWS, implementation frameworks such as Reach, Effectiveness, Adoption, Implementation, and Maintenance and outcomes such as those proposed by Proctor and colleagues (309) (i.e., treatment acceptability, adoption, appropriateness, feasibility, cost, penetration, and sustainability) will enable rigorous comparative-effectiveness work and enhance the efficiency of research translation to clinical practice (310). Treatments for SAWS will not be effective if not well implemented. Likewise, treatment “failures” must be understood in terms of intervention failure, implementation failure, or both. These distinctions can only be identified through early consideration and measurement of implementation outcomes, which are therefore essential to the advancement of care for patients with SAWS.
Discussion
Amid impressive research collaborations and noteworthy advances in critical care medicine, certain conditions have received less attention than others. This inequality in the application of science to practice has not clearly tracked with disease prevalence, morbidity, or mortality. Addiction research and treatment is underresourced compared with other medical conditions (275, 311, 312). Although the overarching reasons for these inequalities are likely multifactorial, in the case of SAWS, stigma and stereotypes pose challenges that have arguably blunted the standards of research and acceptable practices in this field (275–278). At the same time that opioid use disorder and overdose have captured the public’s attention and are garnering appropriate research support (313), treatment standards for the more common manifestations of AUD, including alcohol withdrawal, remain underdeveloped. This report takes a step toward addressing this imbalance by identifying several investigative opportunities for improving management of SAWS. However, subsequent steps will be the true catalysts for change. Scientists from various disciplines will need to direct their attention to the problem of SAWS, pool their energies and expertise, and convince funding agencies to support the research efforts described in this report.
Providers from various disciplines care for patients with SAWS, making it a broadly relevant problem, but this diversity may also contribute to a historical lack of ownership. Through this report, the ATS and its critical care community assume responsibility for elevating the standards of care and research affecting the vulnerable group of patients who experience SAWS. The ATS community is in fact uniquely positioned to address the gaps that currently exist between existing research on alcohol withdrawal and the complex realities of inpatient practice. As an interdisciplinary body whose members perform both clinical management and research in patients with the most extreme manifestations of SAWS—often amid organ failure, sepsis, and other forms of critical illness—the ATS provides a natural forum for diverse content experts to identify and contextualize the full range of considerations brought forward by this topic. A future goal will be to partner these efforts with other national and international specialty groups with diverse backgrounds and expertise. These groups could include the Society of Critical Care Medicine, the American College of Medical Toxicology, the American College of Emergency Physicians, the American Society of Addiction Medicine, the American Society of Health System Pharmacists, the American Academy of Clinical Toxicologists, and the Research Society on Alcoholism, although this list is by no means exhaustive. Soliciting input from individuals across the spectrum of healthcare and biomedical research will undoubtedly broaden the impact and scope of this important work.
Acknowledgments
This official research statement was prepared by an ad hoc subcommittee on severe alcohol withdrawal syndrome of the ATS Assembly on Critical Care, Assembly on Behavioral Science and Health Services Research, and Assembly on Nursing.
Members of the subcommittee are as follows:
Tessa L. Steel, M.D., M.P.H. (Co-Chair)1,2
Ellen L. Burnham, M.D., M.S.C.R. (Co-Chair)3
Majid Afshar, M.D., M.S.C.R.4*
Brendan J. Clark, M.D.5
Ivor S. Douglas, M.D.6
Amy L. Dzierba, Pharm.D.7
Scott Edwards, Ph.D.8*
Hayley B. Gershengorn, M.D.9
Nicholas W. Gilpin, Ph.D.8
Dwayne W. Godwin, Ph.D.10
Catherine L. Hough, M.D., M.Sc.11
Sarah E. Jolley, M.D., M.Sc.3*
José R. Maldonado, M.D.12
Anuj B. Mehta, M.D.13
Lewis S. Nelson, M.D.14
Mayur B. Patel, M.D., M.Sc.15
Darius A. Rastegar, M.D.16
Joanna L. Stollings, Pharm.D.15
Boris Tabakoff, Ph.D.3
Judith A. Tate, Ph.D., R.N.17
Christine Timko, Ph.D.18*
Adrian Wong, Pharm.D., M.P.H.19
*Section Leader.
1Seattle–Denver Center of Innovation, Veterans Affairs Puget Sound Health Care System, Seattle Division, Seattle, Washington; 2Division of Pulmonary, Critical Care, and Sleep Medicine, School of Medicine, University of Washington, Seattle, Washington; 3Anschutz Medical Campus, University of Colorado, Aurora, Colorado; 4University of Wisconsin–Madison, Madison, Wisconsin; 5St. Anthony Hospital and Medical Campus, Lakewood, Colorado; 6Denver Health Medical Center, Denver, Colorado; 7New York–Presbyterian Hospital, New York, New York; 8Louisiana State University Health Sciences Center, New Orleans, Louisiana; 9Miller School of Medicine, University of Miami, Miami, Florida; 10Wake Forest Baptist Medical Center, Winston-Salem, North Carolina; 11Oregon Health and Science University, Portland, Oregon; 12Stanford Health Care, Stanford, California; 13National Jewish Health, Denver, Colorado; 14Rutgers New Jersey Medical School, Rutgers, The State University of New Jersey, New Brunswick, New Jersey; 15Vanderbilt University Medical Center, Vanderbilt University, Nashville, Tennessee; 16Johns Hopkins Bayview Medical Center, Baltimore, Maryland; 17The Ohio State University, Columbus, Ohio; 18Veterans Affairs Palo Alto Health Care System, Palo Alto, California; and 19Massachusetts College of Pharmacy and Health Sciences University, Boston, Massachusetts
Acknowledgment
The authors thank Ms. Lilian Hoffecker, research librarian at the University of Colorado Anschutz Medical Campus, for assisting with the literature review. The authors also thank Ms. Kimberly Lawrence and Dr. Kevin Wilson for their contributions to this project. Without their assistance and methodologic guidance, group efforts through the American Thoracic Society like this would not be possible.
Footnotes
This official research statement of the American Thoracic Society was approved May 2021
An Executive Summary of this document is available at http://www.atsjournals.org/doi/suppl/10.1164/rccm.202108-1845ST.
This document has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author Disclosures: E.L.B. received research support from Humanetics and Quantum Leap Healthcare. I.S.D. received research support from Cheetah Medical. H.B.G. served on an advisory committee for Gilead. N.W.G. has ownership or investment interests with Glauser Life Sciences, Inc. D.W.G. served on an advisory committee for Lexaria Bioscience; and received research support from the National Institute on Alcohol Abuse and Alcoholism and National Institute of Neurological Disorders and Stroke. M.B.P. served on an advisory committee for Liberate Medical; received research support from Haemonetics and the National Institutes of Health; and received honorarium from the National Institutes of Health Center for Scientific Review. B.T. was an employee of Lohocla Research Corporation. A.W. served as a consultant for Wolters Kluwer. T.L.S., M.A., B.J.C., A.L.D., S.E., C.L.H., S.E.J., J.R.M., A.B.M., L.S.N., D.A.R., J.L.S., J.A.T., and C.T. reported no commercial or relevant noncommercial interests.
Contributor Information
Collaborators: on behalf of the American Thoracic Society Assembly on Critical Care, Assembly on Behavioral Science and Health Services Research, and Assembly on Nursing
References
- 1.Drinking levels defined. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2019https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking [Google Scholar]
- 2.Leshner AI, Terry SF, Schultz AM, Liverman CTCommittee to Review the Clinical and Translational Science Awards Program, National Center for Advancing Translational Sciences Board on Health Sciences Policy Institute of Medicine Washington, DC: National Academies Press; 2013https://www.ncbi.nlm.nih.gov/books/NBK169203/#sec_017. [PubMed] [Google Scholar]
- 3. McKeon A, Frye MA, Delanty N. The alcohol withdrawal syndrome. J Neurol Neurosurg Psychiatry. 2008;79:854–862. doi: 10.1136/jnnp.2007.128322. [DOI] [PubMed] [Google Scholar]
- 4. Monte R, Rabuñal R, Casariego E, López-Agreda H, Mateos A, Pértega S. Analysis of the factors determining survival of alcoholic withdrawal syndrome patients in a general hospital. Alcohol Alcohol. 2010;45:151–158. doi: 10.1093/alcalc/agp087. [DOI] [PubMed] [Google Scholar]
- 5. Steel TL, Malte CA, Bradley KA, Lokhandwala S, Hough CL, Hawkins EJ. Prevalence and variation of clinically recognized inpatient alcohol withdrawal syndrome in the Veterans Health Administration. J Addict Med. 2020;14:300–304. doi: 10.1097/ADM.0000000000000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the alcohol withdrawal syndrome. Cochrane Database Syst Rev. 2011;6:CD008537. doi: 10.1002/14651858.CD008537.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gold JA, Rimal B, Nolan A, Nelson LS. A strategy of escalating doses of benzodiazepines and phenobarbital administration reduces the need for mechanical ventilation in delirium tremens. Crit Care Med. 2007;35:724–730. doi: 10.1097/01.CCM.0000256841.28351.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saitz R, Mayo-Smith MF, Roberts MS, Redmond HA, Bernard DR, Calkins DR. Individualized treatment for alcohol withdrawal: a randomized double-blind controlled trial. JAMA. 1994;272:519–523. [PubMed] [Google Scholar]
- 9. Amato L, Minozzi S, Vecchi S, Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;3:CD005063. doi: 10.1002/14651858.CD005063.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Minozzi S, Amato L, Vecchi S, Davoli M. Anticonvulsants for alcohol withdrawal. Cochrane Database Syst Rev. 2010;3:CD005064. doi: 10.1002/14651858.CD005064.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turner RC, Lichstein PR, Peden JG, Jr, Busher JT, Waivers LE. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4:432–444. doi: 10.1007/BF02599697. [DOI] [PubMed] [Google Scholar]
- 12. Clapp P, Bhave SV, Hoffman PL. How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health. 2008;31:310–339. [PMC free article] [PubMed] [Google Scholar]
- 13.Nejad SH, Dubovsky A, Irwin K, Fagan S, Goverman J. In: 3rd ed. Fogel BS, Greenberg DS, editors. New York, NY: Oxford University Press; 2015. Burns; pp. 1455–1472. [Google Scholar]
- 14. Moss M, Burnham EL. Alcohol abuse in the critically ill patient. Lancet. 2006;368:2231–2242. doi: 10.1016/S0140-6736(06)69490-7. [DOI] [PubMed] [Google Scholar]
- 15. O’Brien JM, Jr, Lu B, Ali NA, Martin GS, Aberegg SK, Marsh CB, et al. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med. 2007;35:345–350. doi: 10.1097/01.CCM.0000254340.91644.B2. [DOI] [PubMed] [Google Scholar]
- 16. de Wit M, Jones DG, Sessler CN, Zilberberg MD, Weaver MF. Alcohol-use disorders in the critically ill patient. Chest. 2010;138:994–1003. doi: 10.1378/chest.09-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta NM, Lindenauer PK, Yu P-C, Imrey PB, Haessler S, Deshpande A. et al. Association between alcohol use disorders and outcomes of patients hospitalized with community-acquired pneumonia. JAMA Netw Open. 2019;2:e195172. doi: 10.1001/jamanetworkopen.2019.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goodson CM, Clark BJ, Douglas IS. Predictors of severe alcohol withdrawal syndrome: a systematic review and meta-analysis. Alcohol Clin Exp Res. 2014;38:2664–2677. doi: 10.1111/acer.12529. [DOI] [PubMed] [Google Scholar]
- 19. Sarff M, Gold JA. Alcohol withdrawal syndromes in the intensive care unit. Crit Care Med. 2010;38:S494–S501. doi: 10.1097/CCM.0b013e3181ec5412. [DOI] [PubMed] [Google Scholar]
- 20. Schuckit MA. Recognition and management of withdrawal delirium (delirium tremens) N Engl J Med. 2014;371:2109–2113. doi: 10.1056/NEJMra1407298. [DOI] [PubMed] [Google Scholar]
- 21. Mayo-Smith MF, Beecher LH, Fischer TL, Gorelick DA, Guillaume JL, Hill A, et al. Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium: an evidence-based practice guideline. Arch Intern Med. 2004;164:1405–1412. doi: 10.1001/archinte.164.13.1405. [DOI] [PubMed] [Google Scholar]
- 22. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 23. Hecksel KA, Bostwick JM, Jaeger TM, Cha SS. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc. 2008;83:274–279. doi: 10.4065/83.3.274. [DOI] [PubMed] [Google Scholar]
- 24. Eloma AS, Tucciarone JM, Hayes EM, Bronson BD. Evaluation of the appropriate use of a CIWA-Ar alcohol withdrawal protocol in the general hospital setting. Am J Drug Alcohol Abuse. 2018;44:418–425. doi: 10.1080/00952990.2017.1362418. [DOI] [PubMed] [Google Scholar]
- 25. Steel TL, Giovanni SP, Katsandres SC, Cohen SM, Stephenson KB, Murray B, et al. Should the CIWA-Ar be the standard monitoring strategy for alcohol withdrawal syndrome in the intensive care unit? Addict Sci Clin Pract. 2021;16:21. doi: 10.1186/s13722-021-00226-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muzyk AJ, Leung JG, Nelson S, Embury ER, Jones SR. The role of diazepam loading for the treatment of alcohol withdrawal syndrome in hospitalized patients. Am J Addict. 2013;22:113–118. doi: 10.1111/j.1521-0391.2013.00307.x. [DOI] [PubMed] [Google Scholar]
- 27. Muzyk AJ, Fowler JA, Norwood DK, Chilipko A. Role of α2-agonists in the treatment of acute alcohol withdrawal. Ann Pharmacother. 2011;45:649–657. doi: 10.1345/aph.1P575. [DOI] [PubMed] [Google Scholar]
- 28. Maldonado JR. Novel algorithms for the prophylaxis and management of alcohol withdrawal syndromes: beyond benzodiazepines. Crit Care Clin. 2017;33:559–599. doi: 10.1016/j.ccc.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 29. Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 30. Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 31. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24:789–856, ix. doi: 10.1016/j.ccc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 32. Ely EW, Dittus RS, Girard TD. Point: should benzodiazepines be avoided in mechanically ventilated patients? Yes. Chest. 2012;142:281–284. doi: 10.1378/chest.12-1189. [DOI] [PubMed] [Google Scholar]
- 33. Elie M, Cole MG, Primeau FJ, Bellavance F. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13:204–212. doi: 10.1046/j.1525-1497.1998.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33:66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 35. Van Rompaey B, Elseviers MM, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care. 2009;13:R77. doi: 10.1186/cc7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vasilevskis EE, Han JH, Hughes CG, Ely EW. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26:277–287. doi: 10.1016/j.bpa.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mehta S, Cook D, Devlin JW, Skrobik Y, Meade M, Fergusson D, et al. SLEAP Investigators; Canadian Critical Care Trials Group. Prevalence, risk factors, and outcomes of delirium in mechanically ventilated adults. Crit Care Med. 2015;43:557–566. doi: 10.1097/CCM.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 38. Hack JB, Hoffmann RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol. 2006;2:55–60. doi: 10.1007/BF03161171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wong A, Benedict NJ, Kane-Gill SL. Multicenter evaluation of pharmacologic management and outcomes associated with severe resistant alcohol withdrawal. J Crit Care. 2015;30:405–409. doi: 10.1016/j.jcrc.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 40. Breen D, Karabinis A, Malbrain M, Morais R, Albrecht S, Jarnvig I-L, et al. Decreased duration of mechanical ventilation when comparing analgesia-based sedation using remifentanil with standard hypnotic-based sedation for up to 10 days in intensive care unit patients: a randomised trial [ISRCTN47583497] Crit Care. 2005;9:R200–R210. doi: 10.1186/cc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carson SS, Kress JP, Rodgers JE, Vinayak A, Campbell-Bright S, Levitt J, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med. 2006;34:1326–1332. doi: 10.1097/01.CCM.0000215513.63207.7F. [DOI] [PubMed] [Google Scholar]
- 42. Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 43. Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 44. Patel SB, Kress JP. Sedation and analgesia in the mechanically ventilated patient. Am J Respir Crit Care Med. 2012;185:486–497. doi: 10.1164/rccm.201102-0273CI. [DOI] [PubMed] [Google Scholar]
- 45. Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, et al. Dexmedetomidine for Long-Term Sedation Investigators. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307:1151–1160. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 46. Daeppen J-B, Gache P, Landry U, Sekera E, Schweizer V, Gloor S, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162:1117–1121. doi: 10.1001/archinte.162.10.1117. [DOI] [PubMed] [Google Scholar]
- 47. Maldonado JR, Nguyen LH, Schader EM, Brooks JO., III Benzodiazepine loading versus symptom-triggered treatment of alcohol withdrawal: a prospective, randomized clinical trial. Gen Hosp Psychiatry. 2012;34:611–617. doi: 10.1016/j.genhosppsych.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 48. Saitz R. Medications for alcohol use disorder and predicting severe withdrawal. JAMA. 2018;320:766–768. doi: 10.1001/jama.2018.10061. [DOI] [PubMed] [Google Scholar]
- 49. Rayner SG, Weinert CR, Peng H, Jepsen S, Broccard AF. Study Institution. Dexmedetomidine as adjunct treatment for severe alcohol withdrawal in the ICU. Ann Intensive Care. 2012;2:12. doi: 10.1186/2110-5820-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Muzyk AJ, Kerns S, Brudney S, Gagliardi JP. Dexmedetomidine for the treatment of alcohol withdrawal syndrome: rationale and current status of research. CNS Drugs. 2013;27:913–920. doi: 10.1007/s40263-013-0106-6. [DOI] [PubMed] [Google Scholar]
- 51. Beg M, Fisher S, Siu D, Rajan S, Troxell L, Liu VX. Treatment of alcohol withdrawal syndrome with and without dexmedetomidine. Perm J. 2016;20:49–53. doi: 10.7812/TPP/15-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Douglas IS, Overdier K, Alapat P, Raines R, DeBoisblanc B. The Severe Alcohol Withdrawal Dexmedetomidine Utilization Study (SAWDUST) [abstract] Am J Respir Crit Care Med. 2017;194:A2899. [Google Scholar]
- 53. Benedict NJ, Wong A, Cassidy E, Lohr BR, Pizon AF, Smithburger PL, et al. Predictors of resistant alcohol withdrawal (RAW): a retrospective case-control study. Drug Alcohol Depend. 2018;192:303–308. doi: 10.1016/j.drugalcdep.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 54. Rosenson J, Clements C, Simon B, Vieaux J, Graffman S, Vahidnia F, et al. Phenobarbital for acute alcohol withdrawal: a prospective randomized double-blind placebo-controlled study. J Emerg Med. 2013;44:592–598, e2. doi: 10.1016/j.jemermed.2012.07.056. [DOI] [PubMed] [Google Scholar]
- 55. Brotherton AL, Hamilton EP, Kloss HG, Hammond DA. Propofol for treatment of refractory alcohol withdrawal syndrome: a review of the literature. Pharmacotherapy. 2016;36:433–442. doi: 10.1002/phar.1726. [DOI] [PubMed] [Google Scholar]
- 56. Hammond DA, Rowe JM, Wong A, Wiley TL, Lee KC, Kane-Gill SL. Patient outcomes associated with phenobarbital use with or without benzodiazepines for alcohol withdrawal syndrome: a systematic review. Hosp Pharm. 2017;52:607–616. doi: 10.1177/0018578717720310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oks M, Cleven KL, Healy L, Wei M, Narasimhan M, Mayo PH, et al. The safety and utility of phenobarbital use for the treatment of severe alcohol withdrawal syndrome in the medical intensive care unit. J Intensive Care Med. 2020;35:844–850. doi: 10.1177/0885066618783947. [DOI] [PubMed] [Google Scholar]
- 58. Wong J, Saver B, Scanlan JM, Gianutsos LP, Bhakta Y, Walsh J, et al. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14:1–72. doi: 10.1097/ADM.0000000000000668. [DOI] [PubMed] [Google Scholar]
- 59. Nair R, Aggarwal R, Khanna D. Methods of formal consensus in classification/diagnostic criteria and guideline development. Semin Arthritis Rheum. 2011;41:95–105. doi: 10.1016/j.semarthrit.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Molina PE, Gardner JD, Souza-Smith FM, Whitaker AM. Alcohol abuse: critical pathophysiological processes and contribution to disease burden. Physiology (Bethesda) 2014;29:203–215. doi: 10.1152/physiol.00055.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36:2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tabakoff B, Hoffman PL. The neurobiology of alcohol consumption and alcoholism: an integrative history. Pharmacol Biochem Behav. 2013;113:20–37. doi: 10.1016/j.pbb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hendler RA, Ramchandani VA, Gilman J, Hommer DW. Stimulant and sedative effects of alcohol. Curr Top Behav Neurosci. 2013;13:489–509. doi: 10.1007/7854_2011_135. [DOI] [PubMed] [Google Scholar]
- 65. Koob GF. Neurocircuitry of alcohol addiction: synthesis from animal models. Handb Clin Neurol. 2014;125:33–54. doi: 10.1016/B978-0-444-62619-6.00003-3. [DOI] [PubMed] [Google Scholar]
- 66. Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Edwards S, Guerrero M, Ghoneim OM, Roberts E, Koob GF. Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addict Biol. 2012;17:76–85. doi: 10.1111/j.1369-1600.2010.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, et al. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest. 2015;125:3193–3197. doi: 10.1172/JCI79828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ryan ML, Falk DE, Fertig JB, Rendenbach-Mueller B, Katz DA, Tracy KA, et al. A phase 2, double-blind, placebo-controlled randomized trial assessing the efficacy of ABT-436, a novel V1b receptor antagonist, for alcohol dependence. Neuropsychopharmacology. 2017;42:1012–1023. doi: 10.1038/npp.2016.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Abrahao KP, Salinas AG, Lovinger DM. Alcohol and the brain: neuronal molecular targets, synapses, and circuits. Neuron. 2017;96:1223–1238. doi: 10.1016/j.neuron.2017.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 72. Becker HC, Mulholland PJ. Neurochemical mechanisms of alcohol withdrawal. Handb Clin Neurol. 2014;125:133–156. doi: 10.1016/B978-0-444-62619-6.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Becker HC. Animal models of alcohol withdrawal. Alcohol Res Health. 2000;24:105–113. [PMC free article] [PubMed] [Google Scholar]
- 74. Faingold CL. The Majchrowicz binge alcohol protocol: an intubation technique to study alcohol dependence in rats. Curr Protoc Neurosci. 2008;44:9.28.1–9.28.12. doi: 10.1002/0471142301.ns0928s44. [DOI] [PubMed] [Google Scholar]
- 75. Veatch LM, Becker HC. Electrographic and behavioral indices of ethanol withdrawal sensitization. Brain Res. 2002;946:272–282. doi: 10.1016/s0006-8993(02)02895-0. [DOI] [PubMed] [Google Scholar]
- 76. Ristuccia RC, Spear LP. Sensitivity and tolerance to autonomic effects of ethanol in adolescent and adult rats during repeated vapor inhalation sessions. Alcohol Clin Exp Res. 2005;29:1809–1820. doi: 10.1097/01.alc.0000183010.72764.cd. [DOI] [PubMed] [Google Scholar]
- 77. Kimbrough A, de Guglielmo G, Kononoff J, Kallupi M, Zorrilla EP, George O. CRF1 receptor-dependent increases in irritability-like behavior during abstinence from chronic intermittent ethanol vapor exposure. Alcohol Clin Exp Res. 2017;41:1886–1895. doi: 10.1111/acer.13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Roberts AJ, Khom S, Bajo M, Vlkolinsky R, Polis I, Cates-Gatto C, et al. Increased IL-6 expression in astrocytes is associated with emotionality, alterations in central amygdala GABAergic transmission, and excitability during alcohol withdrawal. Brain Behav Immun. 2019;82:188–202. doi: 10.1016/j.bbi.2019.08.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 80. Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- 81. Edwards S, Koob GF. Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol. 2010;5:393–401. doi: 10.2217/fnl.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lovinger DM, Roberto M. Synaptic effects induced by alcohol. Curr Top Behav Neurosci. 2013;13:31–86. doi: 10.1007/7854_2011_143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. N’Gouemo P, Caspary DM, Faingold CL. Decreased GABA effectiveness in the inferior colliculus neurons during ethanol withdrawal in rats susceptible to audiogenic seizures. Brain Res. 1996;724:200–204. doi: 10.1016/0006-8993(96)00304-6. [DOI] [PubMed] [Google Scholar]
- 84. Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol. 1990;176:289–296. doi: 10.1016/0014-2999(90)90022-x. [DOI] [PubMed] [Google Scholar]
- 85. Gulya K, Grant KA, Valverius P, Hoffman PL, Tabakoff B. Brain regional specificity and time-course of changes in the NMDA receptor-ionophore complex during ethanol withdrawal. Brain Res. 1991;547:129–134. [PubMed] [Google Scholar]
- 86. Snell LD, Nunley KR, Lickteig RL, Browning MD, Tabakoff B, Hoffman PL. Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Brain Res Mol Brain Res. 1996;40:71–78. doi: 10.1016/0169-328x(96)00038-1. [DOI] [PubMed] [Google Scholar]
- 87. Kalluri HS, Mehta AK, Ticku MK. Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment. Brain Res Mol Brain Res. 1998;58:221–224. doi: 10.1016/s0169-328x(98)00112-0. [DOI] [PubMed] [Google Scholar]
- 88. Haugbøl SR, Ebert B, Ulrichsen J. Upregulation of glutamate receptor subtypes during alcohol withdrawal in rats. Alcohol Alcohol. 2005;40:89–95. doi: 10.1093/alcalc/agh117. [DOI] [PubMed] [Google Scholar]
- 89. Sheela Rani CS, Ticku MK. Comparison of chronic ethanol and chronic intermittent ethanol treatments on the expression of GABAA and NMDA receptor subunits. Alcohol. 2006;38:89–97. doi: 10.1016/j.alcohol.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 90. Devaud LL, Alele P. Differential effects of chronic ethanol administration and withdrawal on gamma-aminobutyric acid type A and NMDA receptor subunit proteins in male and female rat brain. Alcohol Clin Exp Res. 2004;28:957–965. doi: 10.1097/01.alc.0000128225.83916.40. [DOI] [PubMed] [Google Scholar]
- 91. Olsen RW, Liang J. Role of GABAA receptors in alcohol use disorders suggested by chronic intermittent ethanol (CIE) rodent model. Mol Brain. 2017;10:45. doi: 10.1186/s13041-017-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Thomas MP, Monaghan DT, Morrisett RA. Evidence for a causative role of N-methyl-d-aspartate receptors in an in vitro model of alcohol withdrawal hyperexcitability. J Pharmacol Exp Ther. 1998;287:87–97. [PubMed] [Google Scholar]
- 93. Colombo G, Agabio R, Carai MAM, Lobina C, Pani M, Reali R, et al. Ability of baclofen in reducing alcohol intake and withdrawal severity: I. preclinical evidence. Alcohol Clin Exp Res. 2000;24:58–66. [PubMed] [Google Scholar]
- 94. Veatch LM, Becker HC. Lorazepam and MK-801 effects on behavioral and electrographic indices of alcohol withdrawal sensitization. Brain Res. 2005;1065:92–106. doi: 10.1016/j.brainres.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 95. Becker HC, Myrick H, Veatch LM. Pregabalin is effective against behavioral and electrographic seizures during alcohol withdrawal. Alcohol Alcohol. 2006;41:399–406. doi: 10.1093/alcalc/agl029. [DOI] [PubMed] [Google Scholar]
- 96. Ehlers CL, Sanchez-Alavez M, Wills D. Effect of gabapentin on sleep and delta and theta EEG power in adult rats exposed to chronic intermittent ethanol vapor and protracted withdrawal during adolescence. Psychopharmacology (Berl) 2018;235:1783–1791. doi: 10.1007/s00213-018-4888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. De Witte P, Pinto E, Ansseau M, Verbanck P. Alcohol and withdrawal: from animal research to clinical issues. Neurosci Biobehav Rev. 2003;27:189–197. doi: 10.1016/s0149-7634(03)00030-7. [DOI] [PubMed] [Google Scholar]
- 98. Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- 99. Ulrichsen J, Clemmesen L, Hemmingsen R. Convulsive behaviour during alcohol dependence: discrimination between the role of intoxication and withdrawal. Psychopharmacology (Berl) 1992;107:97–102. doi: 10.1007/BF02244972. [DOI] [PubMed] [Google Scholar]
- 100. Ulrichsen J, Bech B, Allerup P, Hemmingsen R. Diazepam prevents progression of kindled alcohol withdrawal behaviour. Psychopharmacology (Berl) 1995;121:451–460. doi: 10.1007/BF02246493. [DOI] [PubMed] [Google Scholar]
- 101. Edwards S. Reinforcement principles for addiction medicine: from recreational drug use to psychiatric disorder. Prog Brain Res. 2016;223:63–76. doi: 10.1016/bs.pbr.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 102. Vinkers CH, Olivier B. Mechanisms underlying tolerance after long-term benzodiazepine use: a future for subtype-selective GABAA receptor modulators? Adv Pharmacol Sci. 2012;2012:416864. doi: 10.1155/2012/416864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Liang J, Spigelman I, Olsen RW. Tolerance to sedative/hypnotic actions of GABAergic drugs correlates with tolerance to potentiation of extrasynaptic tonic currents of alcohol-dependent rats. J Neurophysiol. 2009;102:224–233. doi: 10.1152/jn.90484.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Roberto M, Gilpin NW, Siggins GR. The central amygdala and alcohol: role of γ-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb Perspect Med. 2012;2:a012195. doi: 10.1101/cshperspect.a012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gilpin NW, Roberto M. Neuropeptide modulation of central amygdala neuroplasticity is a key mediator of alcohol dependence. Neurosci Biobehav Rev. 2012;36:873–888. doi: 10.1016/j.neubiorev.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, et al. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF1 receptor antagonism. Neuropharmacology. 2012;62:1142–1151. doi: 10.1016/j.neuropharm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gilpin NW, Herman MA, Roberto M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry. 2015;77:859–869. doi: 10.1016/j.biopsych.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Varodayan FP, Correia D, Kirson D, Khom S, Oleata CS, Luu G, et al. CRF modulates glutamate transmission in the central amygdala of naïve and ethanol-dependent rats. Neuropharmacology. 2017;125:418–428. doi: 10.1016/j.neuropharm.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Breese GR, Knapp DJ. Persistent adaptation by chronic alcohol is facilitated by neuroimmune activation linked to stress and CRF. Alcohol. 2016;52:9–23. doi: 10.1016/j.alcohol.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hernandez RV, Puro AC, Manos JC, Huitron-Resendiz S, Reyes KC, Liu K, et al. Transgenic mice with increased astrocyte expression of IL-6 show altered effects of acute ethanol on synaptic function. Neuropharmacology. 2016;103:27–43. doi: 10.1016/j.neuropharm.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bray JG, Reyes KC, Roberts AJ, Gruol DL. Altered hippocampal synaptic function in transgenic mice with increased astrocyte expression of CCL2 after withdrawal from chronic alcohol. Neuropharmacology. 2018;135:113–125. doi: 10.1016/j.neuropharm.2018.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Knapp DJ, Harper KM, Whitman BA, Zimomra Z, Breese GR. Stress and withdrawal from chronic ethanol induce selective changes in neuroimmune mRNAs in differing brain sites. Brain Sci. 2016;6:25. doi: 10.3390/brainsci6030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Delery EC, Edwards S. Neuropeptide and cytokine regulation of pain in the context of substance use disorders. Neuropharmacology. 2020;174:108153. doi: 10.1016/j.neuropharm.2020.108153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Walter HJ, Messing RO. Regulation of neuronal voltage-gated calcium channels by ethanol. Neurochem Int. 1999;35:95–101. doi: 10.1016/s0197-0186(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 117. Mulholland PJK. KCa2 channels: novel therapeutic targets for treating alcohol withdrawal and escalation of alcohol consumption. Alcohol. 2012;46:309–315. doi: 10.1016/j.alcohol.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shan HQ, Hammarback JA, Godwin DW. Ethanol inhibition of a T-type Ca2+ channel through activity of protein kinase C. Alcohol Clin Exp Res. 2013;37:1333–1342. doi: 10.1111/acer.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Abrahao KP, Chancey JH, Chan CS, Lovinger DM. Ethanol-sensitive pacemaker neurons in the mouse external globus pallidus. Neuropsychopharmacology. 2017;42:1070–1081. doi: 10.1038/npp.2016.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004;28:40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- 121. Graef JD, Huitt TW, Nordskog BK, Hammarback JH, Godwin DW. Disrupted thalamic T-type Ca2+ channel expression and function during ethanol exposure and withdrawal. J Neurophysiol. 2011;105:528–540. doi: 10.1152/jn.00424.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. N’Gouemo P, Morad M. Alcohol withdrawal is associated with a downregulation of large-conductance Ca2+-activated K+ channels in rat inferior colliculus neurons. Psychopharmacology (Berl) 2014;231:2009–2018. doi: 10.1007/s00213-013-3346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. N’Gouemo P. Altered voltage-gated calcium channels in rat inferior colliculus neurons contribute to alcohol withdrawal seizures. Eur Neuropsychopharmacol. 2015;25:1342–1352. doi: 10.1016/j.euroneuro.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Akinfiresoye LR, Miranda C, Lovinger DM, N’Gouemo P. Alcohol withdrawal increases protein kinase A activity in the rat inferior colliculus. Alcohol Clin Exp Res. 2016;40:2359–2367. doi: 10.1111/acer.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lee D, Krishnan B, Zhang H, Park HR, Ro EJ, Jung YN, et al. Activity of hippocampal adult-born neurons regulates alcohol withdrawal seizures. JCI Insight. 2019;4:e128770. doi: 10.1172/jci.insight.128770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 127. Becker JB, Koob GF. Sex differences in animal models: focus on addiction. Pharmacol Rev. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Flores-Bonilla A, Richardson HN. Sex differences in the neurobiology of alcohol use disorder. Alcohol Res. 2020;40:04. doi: 10.35946/arcr.v40.2.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rath M, Guergues J, Pinho JPC, Zhang P, Nguyen TG, MacFadyen KA, et al. Chronic voluntary binge ethanol consumption causes sex-specific differences in microglial signaling pathways and withdrawal-associated behaviors in mice. Alcohol Clin Exp Res. 2020;44:1791–1806. doi: 10.1111/acer.14420. [DOI] [PubMed] [Google Scholar]
- 130. Devaud LL, Chadda R. Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcohol Clin Exp Res. 2001;25:1689–1696. [PubMed] [Google Scholar]
- 131. Veatch LM, Wright TM, Randall CL. Only male mice show sensitization of handling-induced convulsions across repeated ethanol withdrawal cycles. Alcohol Clin Exp Res. 2007;31:477–485. doi: 10.1111/j.1530-0277.2006.00328.x. [DOI] [PubMed] [Google Scholar]
- 132. Reilly W, Koirala B, Devaud LL. Sex differences in acoustic startle responses and seizure thresholds between ethanol-withdrawn male and female rats. Alcohol Alcohol. 2009;44:561–566. doi: 10.1093/alcalc/agp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Anker JJ, Carroll ME. The role of progestins in the behavioral effects of cocaine and other drugs of abuse: human and animal research. Neurosci Biobehav Rev. 2010;35:315–333. doi: 10.1016/j.neubiorev.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one in male and female rats. Alcohol Clin Exp Res. 1998;22:2055–2061. [PubMed] [Google Scholar]
- 135. Finn DA, Beckley EH, Kaufman KR, Ford MM. Manipulation of GABAergic steroids: sex differences in the effects on alcohol drinking- and withdrawal-related behaviors. Horm Behav. 2010;57:12–22. doi: 10.1016/j.yhbeh.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Tanchuck-Nipper MA, Ford MM, Hertzberg A, Beadles-Bohling A, Cozzoli DK, Finn DA. Sex differences in ethanol’s anxiolytic effect and chronic ethanol withdrawal severity in mice with a null mutation of the 5α-reductase type 1 gene. Behav Genet. 2015;45:354–367. doi: 10.1007/s10519-014-9691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Alele PE, Devaud LL. Differential adaptations in GABAergic and glutamatergic systems during ethanol withdrawal in male and female rats. Alcohol Clin Exp Res. 2005;29:1027–1034. doi: 10.1097/01.alc.0000167743.96121.40. [DOI] [PubMed] [Google Scholar]
- 138. Sharrett-Field L, Butler TR, Reynolds AR, Berry JN, Prendergast MA. Sex differences in neuroadaptation to alcohol and withdrawal neurotoxicity. Pflugers Arch. 2013;465:643–654. doi: 10.1007/s00424-013-1266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Pennings EJ, Leccese AP, Wolff FA. Effects of concurrent use of alcohol and cocaine. Addiction. 2002;97:773–783. doi: 10.1046/j.1360-0443.2002.00158.x. [DOI] [PubMed] [Google Scholar]
- 140. Martin CS. Timing of alcohol and other drug use. Alcohol Res Health. 2008;31:96–99. [PMC free article] [PubMed] [Google Scholar]
- 141. Perez E, Quijano-Cardé N, De Biasi M. Nicotinic mechanisms modulate ethanol withdrawal and modify time course and symptoms severity of simultaneous withdrawal from alcohol and nicotine. Neuropsychopharmacology. 2015;40:2327–2336. doi: 10.1038/npp.2015.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hill R, Lyndon A, Withey S, Roberts J, Kershaw Y, MacLachlan J, et al. Ethanol reversal of tolerance to the respiratory depressant effects of morphine. Neuropsychopharmacology. 2016;41:762–773. doi: 10.1038/npp.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Jacob JC, Poklis JL, Akbarali HI, Henderson G, Dewey WL. Ethanol reversal of tolerance to the antinociceptive effects of oxycodone and hydrocodone. J Pharmacol Exp Ther. 2017;362:45–52. doi: 10.1124/jpet.117.241083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Walker MC, Kullmann DM. Optogenetic and chemogenetic therapies for epilepsy. Neuropharmacology. 2020;168:107751. doi: 10.1016/j.neuropharm.2019.107751. [DOI] [PubMed] [Google Scholar]
- 145. Klorig DC, Alberto GE, Smith T, Godwin DW. Optogenetically-induced population discharge threshold as a sensitive measure of network excitability. eNeuro. 2019;6:ENEURO.0229-18.2019. doi: 10.1523/ENEURO.0229-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Avegno EM, Lobell TD, Itoga CA, Baynes BB, Whitaker AM, Weera MM, et al. Central amygdala circuits mediate hyperalgesia in alcohol-dependent rats. J Neurosci. 2018;38:7761–7773. doi: 10.1523/JNEUROSCI.0483-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. McGinnis MM, Parrish BC, Chappell AM, Alexander NJ, McCool BA. Chronic ethanol differentially modulates glutamate release from dorsal and ventral prefrontal cortical inputs onto rat basolateral amygdala principal neurons. eNeuro. 2019;7:ENEURO.0132-19.2019. doi: 10.1523/ENEURO.0132-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Becker HC, Lopez MF. An animal model of alcohol dependence to screen medications for treating alcoholism. Int Rev Neurobiol. 2016;126:157–177. doi: 10.1016/bs.irn.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Avegno EM, Gilpin NW. Inducing alcohol dependence in rats using chronic intermittent exposure to alcohol vapor. Bio Protoc. 2019;9:e3222. doi: 10.21769/BioProtoc.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Vendruscolo LF, Roberts AJ. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol. 2014;48:277–286. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci. 2008;44:9.29.1–9.29.19. doi: 10.1002/0471142301.ns0929s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Mouton AJ, Maxi JK, Souza-Smith F, Bagby GJ, Gilpin NW, Molina PE, et al. Alcohol vapor inhalation as a model of alcohol-induced organ disease. Alcohol Clin Exp Res. 2016;40:1671–1678. doi: 10.1111/acer.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Teng SX, Katz PS, Maxi JK, Mayeux JP, Gilpin NW, Molina PE. Alcohol exposure after mild focal traumatic brain injury impairs neurological recovery and exacerbates localized neuroinflammation. Brain Behav Immun. 2015;45:145–156. doi: 10.1016/j.bbi.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Natividad LA, Steinman MQ, Laredo SA, Irimia C, Polis IY, Lintz R, et al. Phosphorylation of calcium/calmodulin-dependent protein kinase II in the rat dorsal medial prefrontal cortex is associated with alcohol-induced cognitive inflexibility. Addict Biol. 2018;23:1117–1129. doi: 10.1111/adb.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Brust JC. Acute withdrawal: diagnosis and treatment. Handb Clin Neurol. 2014;125:123–131. doi: 10.1016/B978-0-444-62619-6.00008-2. [DOI] [PubMed] [Google Scholar]
- 156. Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Stärkel P, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci USA. 2014;111:E4485–E4493. doi: 10.1073/pnas.1415174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Donnadieu-Rigole H, Pansu N, Mura T, Pelletier S, Alarcon R, Gamon L, et al. Beneficial effect of alcohol withdrawal on gut permeability and microbial translocation in patients with alcohol use disorder. Alcohol Clin Exp Res. 2018;42:32–40. doi: 10.1111/acer.13527. [DOI] [PubMed] [Google Scholar]
- 158. Xiao HW, Ge C, Feng GX, Li Y, Luo D, Dong JL, et al. Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicol Lett. 2018;287:23–30. doi: 10.1016/j.toxlet.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Tanabe J, Yamamoto DJ, Sutton B, Brown MS, Hoffman PL, Burnham EL, et al. Effects of alcohol and acetate on cerebral blood flow: a pilot study. Alcohol Clin Exp Res. 2019;43:2070–2078. doi: 10.1111/acer.14173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Knapp DJ, Breese GR. Models of chronic alcohol exposure and dependence. Methods Mol Biol. 2012;829:205–230. doi: 10.1007/978-1-61779-458-2_13. [DOI] [PubMed] [Google Scholar]
- 161. Mainerova B, Prasko J, Latalova K, Axmann K, Cerna M, Horacek R, et al. Alcohol withdrawal delirium - diagnosis, course and treatment. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:44–52. doi: 10.5507/bp.2013.089. [DOI] [PubMed] [Google Scholar]
- 162. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 163. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Kurmani S, Squire I. Acute heart failure: definition, classification and epidemiology. Curr Heart Fail Rep. 2017;14:385–392. doi: 10.1007/s11897-017-0351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Schuckit MA, Tipp JE, Reich T, Hesselbrock VM, Bucholz KK. The histories of withdrawal convulsions and delirium tremens in 1648 alcohol dependent subjects. Addiction. 1995;90:1335–1347. doi: 10.1046/j.1360-0443.1995.901013355.x. [DOI] [PubMed] [Google Scholar]
- 166. Ferguson JA, Suelzer CJ, Eckert GJ, Zhou X-H, Dittus RS. Risk factors for delirium tremens development. J Gen Intern Med. 1996;11:410–414. doi: 10.1007/BF02600188. [DOI] [PubMed] [Google Scholar]
- 167. Wetterling T, Kanitz R-D, Besters B, Fischer D, Zerfass B, John U, et al. A new rating scale for the assessment of the alcohol-withdrawal syndrome (AWS scale) Alcohol Alcohol. 1997;32:753–760. doi: 10.1093/oxfordjournals.alcalc.a008326. [DOI] [PubMed] [Google Scholar]
- 168. Shaw GK, Waller S, Latham CJ, Dunn G, Thomson AD. The detoxification experience of alcoholic in-patients and predictors of outcome. Alcohol Alcohol. 1998;33:291–303. doi: 10.1093/oxfordjournals.alcalc.a008393. [DOI] [PubMed] [Google Scholar]
- 169. Reoux JP, Malte CA, Kivlahan DR, Saxon AJ. The Alcohol Use Disorders Identification Test (AUDIT) predicts alcohol withdrawal symptoms during inpatient detoxification. J Addict Dis. 2002;21:81–91. doi: 10.1300/J069v21n04_08. [DOI] [PubMed] [Google Scholar]
- 170. Fiellin DA, O’Connor PG, Holmboe ES, Horwitz RI. Risk for delirium tremens in patients with alcohol withdrawal syndrome. Subst Abus. 2002;23:83–94. doi: 10.1080/08897070209511478. [DOI] [PubMed] [Google Scholar]
- 171. Kraemer KL, Mayo-Smith MF, Calkins DR. Independent clinical correlates of severe alcohol withdrawal. Subst Abus. 2003;24:197–209. doi: 10.1023/a:1026099426602. [DOI] [PubMed] [Google Scholar]
- 172. Lee JH, Jang MK, Lee JY, Kim SM, Kim KH, Park JY, et al. Clinical predictors for delirium tremens in alcohol dependence. J Gastroenterol Hepatol. 2005;20:1833–1837. doi: 10.1111/j.1440-1746.2005.03932.x. [DOI] [PubMed] [Google Scholar]
- 173. Wetterling T, Weber B, Depfenhart M, Schneider B, Junghanns K. Development of a rating scale to predict the severity of alcohol withdrawal syndrome. Alcohol Alcohol. 2006;41:611–615. doi: 10.1093/alcalc/agl068. [DOI] [PubMed] [Google Scholar]
- 174. Wright T, Myrick H, Henderson S, Peters H, Malcolm R. Risk factors for delirium tremens: a retrospective chart review. Am J Addict. 2006;15:213–219. doi: 10.1080/10550490600625798. [DOI] [PubMed] [Google Scholar]
- 175. Monte R, Rabuñal R, Casariego E, Bal M, Pértega S. Risk factors for delirium tremens in patients with alcohol withdrawal syndrome in a hospital setting. Eur J Intern Med. 2009;20:690–694. doi: 10.1016/j.ejim.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 176. Berggren U, Fahlke C, Berglund KJ, Blennow K, Zetterberg H, Balldin J. Thrombocytopenia in early alcohol withdrawal is associated with development of delirium tremens or seizures. Alcohol Alcohol. 2009;44:382–386. doi: 10.1093/alcalc/agp012. [DOI] [PubMed] [Google Scholar]
- 177. Maldonado JR, Sher Y, Ashouri JF, Hills-Evans K, Swendsen H, Lolak S, et al. The “Prediction of Alcohol Withdrawal Severity Scale” (PAWSS): systematic literature review and pilot study of a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol. 2014;48:375–390. doi: 10.1016/j.alcohol.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 178. Maldonado JR, Sher Y, Das S, Hills-Evans K, Frenklach A, Lolak S, et al. Prospective validation study of the Prediction of Alcohol Withdrawal Severity Scale (PAWSS) in medically ill inpatients: a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol Alcohol. 2015;50:509–518. doi: 10.1093/alcalc/agv043. [DOI] [PubMed] [Google Scholar]
- 179. Wood E, Albarqouni L, Tkachuk S, Green CJ, Ahamad K, Nolan S, et al. Will this hospitalized patient develop severe alcohol withdrawal syndrome? The rational clinical examination systematic review. JAMA. 2018;320:825–833. doi: 10.1001/jama.2018.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, et al. ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Amato MBP, Meade MO, Slutsky AS, Brochard L, Costa ELV, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 182. Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376:2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Roche AM, Freeman T, Skinner N. From data to evidence, to action: findings from a systematic review of hospital screening studies for high risk alcohol consumption. Drug Alcohol Depend. 2006;83:1–14. doi: 10.1016/j.drugalcdep.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 184. Doering-Silveira J, Fidalgo TM, Nascimento CLES, Alves JB, Seito CL, Saita MC, et al. Assessing alcohol dependence in hospitalized patients. Int J Environ Res Public Health. 2014;11:5783–5791. doi: 10.3390/ijerph110605783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Rosón B, Corbella X, Perney P, Santos A, Stauber R, Lember M, et al. ALCHIMIE Study Group. Prevalence, clinical characteristics, and risk factors for non-recording of alcohol use in hospitals across Europe: the ALCHIMIE study. Alcohol Alcohol. 2016;51:457–464. doi: 10.1093/alcalc/agv142. [DOI] [PubMed] [Google Scholar]
- 186. Watling SM, Fleming C, Casey P, Yanos J. Nursing-based protocol for treatment of alcohol withdrawal in the intensive care unit. Am J Crit Care. 1995;4:66–70. [PubMed] [Google Scholar]
- 187. Awissi D-K, Lebrun G, Coursin DB, Riker RR, Skrobik Y. Alcohol withdrawal and delirium tremens in the critically ill: a systematic review and commentary. Intensive Care Med. 2013;39:16–30. doi: 10.1007/s00134-012-2758-y. [DOI] [PubMed] [Google Scholar]
- 188. Dolman JM, Hawkes ND. Combining the audit questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients. Alcohol Alcohol. 2005;40:515–519. doi: 10.1093/alcalc/agh189. [DOI] [PubMed] [Google Scholar]
- 189. Pecoraro A, Ewen E, Horton T, Mooney R, Kolm P, McGraw P, et al. Using the AUDIT-PC to predict alcohol withdrawal in hospitalized patients. J Gen Intern Med. 2014;29:34–40. doi: 10.1007/s11606-013-2551-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Steel TL, Malte CA, Bradley KA, Hawkins EJ.Use of electronic health record data to estimate the probability of alcohol withdrawal syndrome in a national cohort of hospitalized veterans. J Addict Med. [online ahead of print] 2020 Dec 14; DOI: 10.1097/ADM.0000000000000782. [DOI] [PMC free article] [PubMed]
- 191. Lukan JK, Reed DN, Jr, Looney SW, Spain DA, Blondell RD. Risk factors for delirium tremens in trauma patients. J Trauma. 2002;53:901–906. doi: 10.1097/00005373-200211000-00015. [DOI] [PubMed] [Google Scholar]
- 192. Kapur JH, Rajamanickam V, Fleming MF. Can the blood alcohol concentration be a predictor for increased hospital complications in trauma patients involved in motor vehicle crashes? Int J Environ Res Public Health. 2010;7:1174–1185. doi: 10.3390/ijerph7031174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.The National Clinical Guideline Centre, Royal College of Physicians. London, UK: Royal College of Physicians; 2010. https://www.ncbi.nlm.nih.gov/books/NBK65581/ [Google Scholar]
- 194. Eyer F, Schuster T, Felgenhauer N, Pfab R, Strubel T, Saugel B, et al. Risk assessment of moderate to severe alcohol withdrawal: predictors for seizures and delirium tremens in the course of withdrawal. Alcohol Alcohol. 2011;46:427–433. doi: 10.1093/alcalc/agr053. [DOI] [PubMed] [Google Scholar]
- 195. Shaw JM, Kolesar GS, Sellers EM, Kaplan HL, Sandor P. Development of optimal treatment tactics for alcohol withdrawal: I. assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1:382–389. doi: 10.1097/00004714-198111000-00006. [DOI] [PubMed] [Google Scholar]
- 196. Spies CDM, Dubisz N, Neumann T, Blum S, Müller C, Rommelspacher H, et al. Therapy of alcohol withdrawal syndrome in intensive care unit patients following trauma: results of a prospective, randomized trial. Crit Care Med. 1996;24:414–422. doi: 10.1097/00003246-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 197. Spies CD, Otter HE, Hüske B, Sinha P, Neumann T, Rettig J, et al. Alcohol withdrawal severity is decreased by symptom-orientated adjusted bolus therapy in the ICU. Intensive Care Med. 2003;29:2230–2238. doi: 10.1007/s00134-003-2033-3. [DOI] [PubMed] [Google Scholar]
- 198. Eyer F, Schreckenberg M, Hecht D, Adorjan K, Schuster T, Felgenhauer N, et al. Carbamazepine and valproate as adjuncts in the treatment of alcohol withdrawal syndrome: a retrospective cohort study. Alcohol Alcohol. 2011;46:177–184. doi: 10.1093/alcalc/agr005. [DOI] [PubMed] [Google Scholar]
- 199. Sander W. Protocol for intervention and treatment of alcohol withdrawal. Axone. 1997;19:10–13. [PubMed] [Google Scholar]
- 200. Reoux JP, Oreskovich MR. A comparison of two versions of the Clinical Institute Withdrawal Assessment for Alcohol: the CIWA-Ar and CIWA-AD. Am J Addict. 2006;15:85–93. doi: 10.1080/10550490500419136. [DOI] [PubMed] [Google Scholar]
- 201. Beresford T, Anderson M, Pitts B, Learned B, Thumm B, Maravilla F, et al. The Severity of Ethanol Withdrawal Scale in scale-driven alcohol withdrawal treatment: a quality assurance study. Alcohol Treat Q. 2017;35:232–242. [Google Scholar]
- 202. Weinberg JA, Magnotti LJ, Fischer PE, Edwards NM, Schroeppel T, Fabian TC, et al. Comparison of intravenous ethanol versus diazepam for alcohol withdrawal prophylaxis in the trauma ICU: results of a randomized trial. J Trauma. 2008;64:99–104. doi: 10.1097/TA.0b013e31815eb12a. [DOI] [PubMed] [Google Scholar]
- 203. Rastegar DA, Applewhite D, Alvanzo AAH, Welsh C, Niessen T, Chen ES. Development and implementation of an alcohol withdrawal protocol using a 5-item scale, the Brief Alcohol Withdrawal Scale (BAWS) Subst Abus. 2017;38:394–400. doi: 10.1080/08897077.2017.1354119. [DOI] [PubMed] [Google Scholar]
- 204. Lindner BK, Gilmore VT, Kruer RM, Alvanzo AAH, Chen ES, Murray P, et al. Evaluation of the Brief Alcohol Withdrawal Scale protocol at an academic medical center. J Addict Med. 2019;13:379–384. doi: 10.1097/ADM.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 205. Ely EW, Truman B, Shintani A, Thomason JWW, Wheeler AP, Gordon S, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 206. Rastegar DA, Jarrell AS, Chen ES. Implementation of a protocol using the 5-item Brief Alcohol Withdrawal Scale for treatment of severe alcohol withdrawal in intensive care units. J Intensive Care Med. doi: 10.1177/0885066620952762. [online ahead of print] 2020 Aug 27; DOI: 10.1177/0885066620952762. [DOI] [PubMed] [Google Scholar]
- 207. Metcalfe P, Sobers M, Dewey M. The Windsor Clinic Alcohol Withdrawal Assessment Scale (WCAWAS): investigation of factors associated with complicated withdrawals. Alcohol Alcohol. 1995;30:367–372. [PubMed] [Google Scholar]
- 208. Pittman B, Gueorguieva R, Krupitsky E, Rudenko AA, Flannery BA, Krystal JH. Multidimensionality of the Alcohol Withdrawal Symptom Checklist: a factor analysis of the Alcohol Withdrawal Symptom Checklist and CIWA-Ar. Alcohol Clin Exp Res. 2007;31:612–618. doi: 10.1111/j.1530-0277.2007.00345.x. [DOI] [PubMed] [Google Scholar]
- 209. Foy A, Kay J. The incidence of alcohol-related problems and the risk of alcohol withdrawal in a general hospital population. Drug Alcohol Rev. 1995;14:49–54. doi: 10.1080/09595239500185051. [DOI] [PubMed] [Google Scholar]
- 210. Churpek MM, Snyder A, Han X, Sokol S, Pettit N, Howell MD, et al. Quick Sepsis-related Organ Failure Assessment, systemic inflammatory response syndrome, and early warning scores for detecting clinical deterioration in infected patients outside the intensive care unit. Am J Respir Crit Care Med. 2017;195:906–911. doi: 10.1164/rccm.201604-0854OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162:55–63. doi: 10.7326/M14-0697. [DOI] [PubMed] [Google Scholar]
- 212. Afshar M, Phillips A, Karnik N, Mueller J, To D, Gonzalez R, et al. Natural language processing and machine learning to identify alcohol misuse from the electronic health record in trauma patients: development and internal validation. J Am Med Inform Assoc. 2019;26:254–261. doi: 10.1093/jamia/ocy166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Meystre SM, Savova GK, Kipper-Schuler KC, Hurdle JF. Extracting information from textual documents in the electronic health record: a review of recent research. Yearb Med Inform. 2008;17:128–144. [PubMed] [Google Scholar]
- 214. Ford E, Carroll JA, Smith HE, Scott D, Cassell JA. Extracting information from the text of electronic medical records to improve case detection: a systematic review. J Am Med Inform Assoc. 2016;23:1007–1015. doi: 10.1093/jamia/ocv180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Public health data interoperability. Atlanta, GA: Centers for Disease Control and Prevention; 2019https://www.cdc.gov/datainteroperability/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fehrmeaningfuluse%2Fintroduction.html [Google Scholar]
- 216. Marjoua Y, Bozic KJ. Brief history of quality movement in US healthcare. Curr Rev Musculoskelet Med. 2012;5:265–273. doi: 10.1007/s12178-012-9137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Langlois H, Cormier M, Villeneuve E, Hoffman RS, Longo C, Gosselin S. Benzodiazepine resistant alcohol withdrawal: what is the clinician’s preferred definition? CJEM. 2020;22:165–169. doi: 10.1017/cem.2019.421. [DOI] [PubMed] [Google Scholar]
- 218. Salottolo K, McGuire E, Mains CW, van Doorn EC, Bar-Or D. Occurrence, predictors, and prognosis of alcohol withdrawal syndrome and delirium tremens following traumatic injury. Crit Care Med. 2017;45:867–874. doi: 10.1097/CCM.0000000000002371. [DOI] [PubMed] [Google Scholar]
- 219. Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 220. Burapakajornpong N, Maneeton B, Srisurapanont M. Pattern and risk factors of alcohol withdrawal delirium. J Med Assoc Thai. 2011;94:991–997. [PubMed] [Google Scholar]
- 221. Stewart SH, Koch DG, Willner IR, Anton RF, Reuben A. Validation of blood phosphatidylethanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res. 2014;38:1706–1711. doi: 10.1111/acer.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Wurst FM, Thon N, Yegles M, Schrück A, Preuss UW, Weinmann W. Ethanol metabolites: their role in the assessment of alcohol intake. Alcohol Clin Exp Res. 2015;39:2060–2072. doi: 10.1111/acer.12851. [DOI] [PubMed] [Google Scholar]
- 223. Walther L, de Bejczy A, Löf E, Hansson T, Andersson A, Guterstam J, et al. Phosphatidylethanol is superior to carbohydrate-deficient transferrin and γ-glutamyltransferase as an alcohol marker and is a reliable estimate of alcohol consumption level. Alcohol Clin Exp Res. 2015;39:2200–2208. doi: 10.1111/acer.12883. [DOI] [PubMed] [Google Scholar]
- 224. Afshar M, Burnham EL, Joyce C, Clark BJ, Yong M, Gaydos J, et al. Cut-point levels of phosphatidylethanol to identify alcohol misuse in a mixed cohort including critically ill patients. Alcohol Clin Exp Res. 2017;41:1745–1753. doi: 10.1111/acer.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Afshar M, Burnham EL, Joyce C, Kovacs EJ, Lowery EM. Optimal cut-points for phosphatidylethanol vary by clinical setting: response to Nguyen and Seth’s (2018) letter to the editor. Alcohol Clin Exp Res. 2018;42:2064–2065. doi: 10.1111/acer.13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Luginbühl M, Weinmann W, Butzke I, Pfeifer P. Monitoring of direct alcohol markers in alcohol use disorder patients during withdrawal treatment and successive rehabilitation. Drug Test Anal. 2019;11:859–869. doi: 10.1002/dta.2567. [DOI] [PubMed] [Google Scholar]
- 227. Viel G, Boscolo-Berto R, Cecchetto G, Fais P, Nalesso A, Ferrara SD. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13:14788–14812. doi: 10.3390/ijms131114788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Bråthen G, Bjerve KS, Brodtkorb E, Bovim G. Validity of carbohydrate deficient transferrin and other markers as diagnostic aids in the detection of alcohol related seizures. J Neurol Neurosurg Psychiatry. 2000;68:342–348. doi: 10.1136/jnnp.68.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 230. Riker RR, Fraser GL, Simmons LE, Wilkins ML. Validating the Sedation-Agitation Scale with the Bispectral Index and Visual Analog Scale in adult ICU patients after cardiac surgery. Intensive Care Med. 2001;27:853–858. doi: 10.1007/s001340100912. [DOI] [PubMed] [Google Scholar]
- 231. Mayo-Smith MF. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. Pharmacological management of alcohol withdrawal: a meta-analysis and evidence-based practice guideline. JAMA. 1997;278:144–151. doi: 10.1001/jama.278.2.144. [DOI] [PubMed] [Google Scholar]
- 232. Kosten TR, O’Connor PG. Management of drug and alcohol withdrawal. N Engl J Med. 2003;348:1786–1795. doi: 10.1056/NEJMra020617. [DOI] [PubMed] [Google Scholar]
- 233. Lee JA, Duby JJ, Cocanour CS. Effect of early and focused benzodiazepine therapy on length of stay in severe alcohol withdrawal syndrome. Clin Toxicol (Phila) 2019;57:624–627. doi: 10.1080/15563650.2018.1542701. [DOI] [PubMed] [Google Scholar]
- 234. DeCarolis DD, Rice KL, Ho L, Willenbring ML, Cassaro S. Symptom-driven lorazepam protocol for treatment of severe alcohol withdrawal delirium in the intensive care unit. Pharmacotherapy. 2007;27:510–518. doi: 10.1592/phco.27.4.510. [DOI] [PubMed] [Google Scholar]
- 235. Duby JJ, Berry AJ, Ghayyem P, Wilson MD, Cocanour CS. Alcohol withdrawal syndrome in critically ill patients: protocolized versus nonprotocolized management. J Trauma Acute Care Surg. 2014;77:938–943. doi: 10.1097/TA.0000000000000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Hayner CE, Wuestefeld NL, Bolton PJ. Phenobarbital treatment in a patient with resistant alcohol withdrawal syndrome. Pharmacotherapy. 2009;29:875–878. doi: 10.1592/phco.29.7.875. [DOI] [PubMed] [Google Scholar]
- 237. McCowan C, Marik P. Refractory delirium tremens treated with propofol: a case series. Crit Care Med. 2000;28:1781–1784. doi: 10.1097/00003246-200006000-00014. [DOI] [PubMed] [Google Scholar]
- 238. Wong A, Benedict NJ, Lohr BR, Pizon AF, Kane-Gill SL. Management of benzodiazepine-resistant alcohol withdrawal across a healthcare system: benzodiazepine dose-escalation with or without propofol. Drug Alcohol Depend. 2015;154:296–299. doi: 10.1016/j.drugalcdep.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 239. Ludtke KA, Stanley KS, Yount NL, Gerkin RD. Retrospective review of critically ill patients experiencing alcohol withdrawal: dexmedetomidine versus propofol and/or lorazepam continuous infusions. Hosp Pharm. 2015;50:208–213. doi: 10.1310/hpj5003-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Nelson AC, Kehoe J, Sankoff J, Mintzer D, Taub J, Kaucher KA. Benzodiazepines vs barbiturates for alcohol withdrawal: analysis of 3 different treatment protocols. Am J Emerg Med. 2019;37:733–736. doi: 10.1016/j.ajem.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 241. Pizon AF, Lynch MJ, Benedict NJ, Yanta JH, Frisch A, Menke NB, et al. Adjunct ketamine use in the management of severe ethanol withdrawal. Crit Care Med. 2018;46:e768–e771. doi: 10.1097/CCM.0000000000003204. [DOI] [PubMed] [Google Scholar]
- 242. Sohraby R, Attridge RL, Hughes DW. Use of propofol-containing versus benzodiazepine regimens for alcohol withdrawal requiring mechanical ventilation. Ann Pharmacother. 2014;48:456–461. doi: 10.1177/1060028013515846. [DOI] [PubMed] [Google Scholar]
- 243. Tidwell WP, Thomas TL, Pouliot JD, Canonico AE, Webber AJ. Treatment of alcohol withdrawal syndrome: phenobarbital vs CIWA-Ar protocol. Am J Crit Care. 2018;27:454–460. doi: 10.4037/ajcc2018745. [DOI] [PubMed] [Google Scholar]
- 244. Fullwood JE, Mostaghimi Z, Granger CB, Washam JB, Bride W, Zhao Y, et al. Alcohol withdrawal prevention: a randomized evaluation of lorazepam and ethanol: a pilot study. Am J Crit Care. 2013;22:398–406. doi: 10.4037/ajcc2013283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Gipson G, Tran K, Hoang C, Treggiari M. Comparison of enteral ethanol and benzodiazepines for alcohol withdrawal in neurocritical care patients. J Clin Neurosci. 2016;31:88–91. doi: 10.1016/j.jocn.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 246. Lorentzen K, Lauritsen AØ, Bendtsen AO. Use of propofol infusion in alcohol withdrawal-induced refractory delirium tremens. Dan Med J. 2014;61:A4807. [PubMed] [Google Scholar]
- 247. Ives TJ, Mooney AJ III, Gwyther RE. Pharmacokinetic dosing of phenobarbital in the treatment of alcohol withdrawal syndrome. South Med J. 1991;84:18–21. doi: 10.1097/00007611-199101000-00006. [DOI] [PubMed] [Google Scholar]
- 248. Harman K, Philip A. Evaluation of effectiveness of phenobarbital in treatment of alcohol withdrawal syndrome in a medical intensive care unit [abstract] Chest. 2016;146:226A. [Google Scholar]
- 249. Silva Brum LF, Elisabetsky E. Antiepileptogenic properties of phenobarbital: behavior and neurochemical analysis. Pharmacol Biochem Behav. 2000;67:411–416. doi: 10.1016/s0091-3057(00)00400-7. [DOI] [PubMed] [Google Scholar]
- 250. Fukushima K, Hatanaka K, Sagane K, Ido K. Inhibitory effect of anti-seizure medications on ionotropic glutamate receptors: special focus on AMPA receptor subunits. Epilepsy Res. 2020;167:106452. doi: 10.1016/j.eplepsyres.2020.106452. [DOI] [PubMed] [Google Scholar]
- 251. Hendey GW, Dery RA, Barnes RL, Snowden B, Mentler P. A prospective, randomized, trial of phenobarbital versus benzodiazepines for acute alcohol withdrawal. Am J Emerg Med. 2011;29:382–385. doi: 10.1016/j.ajem.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 252. Nejad S, Nisavic M, Larentzakis A, Dijkink S, Chang Y, Levine AR, et al. Phenobarbital for acute alcohol withdrawal management in surgical trauma patients: a retrospective comparison study. Psychosomatics. 2020;61:327–335. doi: 10.1016/j.psym.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 253. Ammar MA, Ammar AA, Rosen J, Kassab HS, Becher RD. Phenobarbital monotherapy for the management of alcohol withdrawal syndrome in surgical-trauma patients. Ann Pharmacother. 2021;55:294–302. doi: 10.1177/1060028020949137. [DOI] [PubMed] [Google Scholar]
- 254. Nisavic M, Nejad SH, Isenberg BM, Bajwa EK, Currier P, Wallace PM, et al. Use of phenobarbital in alcohol withdrawal management: a retrospective comparison study of phenobarbital and benzodiazepines for acute alcohol withdrawal management in general medical patients. Psychosomatics. 2019;60:458–467. doi: 10.1016/j.psym.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 255. Myrick H, Brady KT, Malcolm R. Divalproex in the treatment of alcohol withdrawal. Am J Drug Alcohol Abuse. 2000;26:155–160. doi: 10.1081/ada-100100597. [DOI] [PubMed] [Google Scholar]
- 256. Longo LP, Campbell T, Hubatch S. Divalproex sodium (Depakote) for alcohol withdrawal and relapse prevention. J Addict Dis. 2002;21:55–64. doi: 10.1300/J069v21n02_05. [DOI] [PubMed] [Google Scholar]
- 257. Rosenthal RN, Perkel C, Singh P, Anand O, Miner CR. A pilot open randomized trial of valproate and phenobarbital in the treatment of acute alcohol withdrawal. Am J Addict. 1998;7:189–197. doi: 10.3109/10550499808998350. [DOI] [PubMed] [Google Scholar]
- 258. Bielka K, Kuchyn I, Glumcher F. Addition of dexmedetomidine to benzodiazepines for patients with alcohol withdrawal syndrome in the intensive care unit: a randomized controlled study. Ann Intensive Care. 2015;5:33. doi: 10.1186/s13613-015-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Crispo AL, Daley MJ, Pepin JL, Harford PH, Brown CVR. Comparison of clinical outcomes in nonintubated patients with severe alcohol withdrawal syndrome treated with continuous-infusion sedatives: dexmedetomidine versus benzodiazepines. Pharmacotherapy. 2014;34:910–917. doi: 10.1002/phar.1448. [DOI] [PubMed] [Google Scholar]
- 260. Lizotte RJ, Kappes JA, Bartel BJ, Hayes KM, Lesselyoung VL. Evaluating the effects of dexmedetomidine compared to propofol as adjunctive therapy in patients with alcohol withdrawal. Clin Pharmacol. 2014;6:171–177. doi: 10.2147/CPAA.S70490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Mueller SW, Preslaski CR, Kiser TH, Fish DN, Lavelle JC, Malkoski SP, et al. A randomized, double-blind, placebo-controlled dose range study of dexmedetomidine as adjunctive therapy for alcohol withdrawal. Crit Care Med. 2014;42:1131–1139. doi: 10.1097/CCM.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 262. Tolonen J, Rossinen J, Alho H, Harjola V-P. Dexmedetomidine in addition to benzodiazepine-based sedation in patients with alcohol withdrawal delirium. Eur J Emerg Med. 2013;20:425–427. doi: 10.1097/MEJ.0b013e32835c53b3. [DOI] [PubMed] [Google Scholar]
- 263. VanderWeide LA, Foster CJ, MacLaren R, Kiser TH, Fish DN, Mueller SW. Evaluation of early dexmedetomidine addition to the standard of care for severe alcohol withdrawal in the ICU: a retrospective controlled cohort study. J Intensive Care Med. 2016;31:198–204. doi: 10.1177/0885066614554908. [DOI] [PubMed] [Google Scholar]
- 264. Frazee EN, Personett HA, Leung JG, Nelson S, Dierkhising RA, Bauer PR. Influence of dexmedetomidine therapy on the management of severe alcohol withdrawal syndrome in critically ill patients. J Crit Care. 2014;29:298–302. doi: 10.1016/j.jcrc.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 265. Bates DW, Miller EB, Cullen DJ, Burdick L, Williams L, Laird N, et al. ADE Prevention Study Group. Patient risk factors for adverse drug events in hospitalized patients. Arch Intern Med. 1999;159:2553–2560. doi: 10.1001/archinte.159.21.2553. [DOI] [PubMed] [Google Scholar]
- 266. Wu C, Bell CM, Wodchis WP. Incidence and economic burden of adverse drug reactions among elderly patients in Ontario emergency departments: a retrospective study. Drug Saf. 2012;35:769–781. doi: 10.1007/BF03261973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Lyon JE, Khan RA, Gessert CE, Larson PM, Renier CM. Treating alcohol withdrawal with oral baclofen: a randomized, double-blind, placebo-controlled trial. J Hosp Med. 2011;6:469–474. doi: 10.1002/jhm.928. [DOI] [PubMed] [Google Scholar]
- 268. Mariani JJ, Rosenthal RN, Tross S, Singh P, Anand OPA. A randomized, open-label, controlled trial of gabapentin and phenobarbital in the treatment of alcohol withdrawal. Am J Addict. 2006;15:76–84. doi: 10.1080/10550490500419110. [DOI] [PubMed] [Google Scholar]
- 269. Levine AR, Carrasquillo L, Mueller J, Nounou MI, Naut ER, Ibrahim D. High-dose gabapentin for the treatment of severe alcohol withdrawal syndrome: a retrospective cohort analysis. Pharmacotherapy. 2019;39:881–888. doi: 10.1002/phar.2309. [DOI] [PubMed] [Google Scholar]
- 270. Nichols TA, Robert S, Taber DJ, Cluver J. Alcohol withdrawal-related outcomes associated with gabapentin use in an inpatient psychiatric facility. Ment Health Clin. 2019;9:1–5. doi: 10.9740/mhc.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Förg A, Hein J, Volkmar K, Winter M, Richter C, Heinz A, et al. Efficacy and safety of pregabalin in the treatment of alcohol withdrawal syndrome: a randomized placebo-controlled trial. Alcohol Alcohol. 2012;47:149–155. doi: 10.1093/alcalc/agr153. [DOI] [PubMed] [Google Scholar]
- 272. Millum J, Grady C. The ethics of placebo-controlled trials: methodological justifications. Contemp Clin Trials. 2013;36:510–514. doi: 10.1016/j.cct.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Carter A, Hall WD. Addiction neuroethics: the promises and perils of neuroscience research on addiction. Cambridge, UK: Cambridge University Press; 2012. [Google Scholar]
- 274.Ethical challenges in drug epidemiology: issues, principles and guidelines. Global Assessment Programme on Drug Abuse toolkit module 7. Vienna, Austria: United Nations Office on Drugs and Crime; 2004https://www.unodc.org/pdf/gap_toolkit_module7.pdf [Google Scholar]
- 275. Barchas JD, Elliott GR, Berger PA, Barchas PR, Solomon F. The ultimate stigma: inadequate funding for research on mental illness and addictive disorders. Am J Psychiatry. 1985;142:838–839. doi: 10.1176/ajp.142.7.838. [DOI] [PubMed] [Google Scholar]
- 276. Corrigan P, Schomerus G, Shuman V, Kraus D, Perlick D, Harnish A, et al. Developing a research agenda for understanding the stigma of addictions part I: lessons from the mental health stigma literature. Am J Addict. 2017;26:59–66. doi: 10.1111/ajad.12458. [DOI] [PubMed] [Google Scholar]
- 277. Corrigan PW, Schomerus G, Shuman V, Kraus D, Perlick D, Harnish A, et al. Developing a research agenda for reducing the stigma of addictions, part II: lessons from the mental health stigma literature. Am J Addict. 2017;26:67–74. doi: 10.1111/ajad.12436. [DOI] [PubMed] [Google Scholar]
- 278. Seear K. Addressing alcohol and other drug stigma: where to next? Drug Alcohol Rev. 2020;39:109–113. doi: 10.1111/dar.13028. [DOI] [PubMed] [Google Scholar]
- 279. Witkiewitz K, Finney JW, Harris AHS, Kivlahan DR, Kranzler HR. Recommendations for the design and analysis of treatment trials for alcohol use disorders. Alcohol Clin Exp Res. 2015;39:1557–1570. doi: 10.1111/acer.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Calling S, Ohlsson H, Sundquist J, Sundquist K, Kendler KS. Socioeconomic status and alcohol use disorders across the lifespan: a co-relative control study. PLoS One. 2019;14:e0224127. doi: 10.1371/journal.pone.0224127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Sessler CN, Grap MJ, Brophy GM. Multidisciplinary management of sedation and analgesia in critical care. Semin Respir Crit Care Med. 2001;22:211–226. doi: 10.1055/s-2001-13834. [DOI] [PubMed] [Google Scholar]
- 282. Witlox J, Eurelings LSM, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 283. Brummel NE, Jackson JC, Pandharipande PP, Thompson JL, Shintani AK, Dittus RS, et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42:369–377. doi: 10.1097/CCM.0b013e3182a645bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Sousa G, Pinho C, Santos A, Abelha FJ. Postoperative delirium in patients with history of alcohol abuse. Rev Esp Anestesiol Reanim. 2017;64:214–222. doi: 10.1016/j.redar.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 285. Frank L, Basch E, Selby JV. Patient-Centered Outcomes Research Institute. The PCORI perspective on patient-centered outcomes research. JAMA. 2014;312:1513–1514. doi: 10.1001/jama.2014.11100. [DOI] [PubMed] [Google Scholar]
- 286. Dopp AR, Parisi KE, Munson SA, Lyon AR. Aligning implementation and user-centered design strategies to enhance the impact of health services: results from a concept mapping study. Implement Sci Commun. 2020;1:17. doi: 10.1186/s43058-020-00020-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Bates DW, Singh H. Two decades since to err is human: an assessment of progress and emerging priorities in patient safety. Health Aff (Millwood) 2018;37:1736–1743. doi: 10.1377/hlthaff.2018.0738. [DOI] [PubMed] [Google Scholar]
- 288. Moss M, Good VS, Gozal D, Kleinpell R, Sessler CN. A critical care societies collaborative statement: burnout syndrome in critical care health-care professionals. A call for action. Am J Respir Crit Care Med. 2016;194:106–113. doi: 10.1164/rccm.201604-0708ST. [DOI] [PubMed] [Google Scholar]
- 289. Poghosyan L, Clarke SP, Finlayson M, Aiken LH. Nurse burnout and quality of care: cross-national investigation in six countries. Res Nurs Health. 2010;33:288–298. doi: 10.1002/nur.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Cimiotti JP, Aiken LH, Sloane DM, Wu ES. Nurse staffing, burnout, and health care-associated infection. Am J Infect Control. 2012;40:486–490. doi: 10.1016/j.ajic.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Blum JM, Morris PE, Martin GS, Gong MN, Bhagwanjee S, Cairns CB, et al. United States Critical Illness and Injury Trials Group. United States Critical Illness and Injury Trials Group. Chest. 2013;143:808–813. doi: 10.1378/chest.12-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. François B, Clavel M, Vignon P, Laterre P-F. Perspective on optimizing clinical trials in critical care: how to puzzle out recurrent failures. J Intensive Care. 2016;4:67. doi: 10.1186/s40560-016-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Talisa VB, Yende S, Seymour CW, Angus DC. Arguing for adaptive clinical trials in sepsis. Front Immunol. 2018;9:1502. doi: 10.3389/fimmu.2018.01502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Marshall JC. Global collaboration in acute care clinical research: opportunities, challenges, and needs. Crit Care Med. 2017;45:311–320. doi: 10.1097/CCM.0000000000002211. [DOI] [PubMed] [Google Scholar]
- 295. Berry SM, Connor JT, Lewis RJ. The platform trial: an efficient strategy for evaluating multiple treatments. JAMA. 2015;313:1619–1620. doi: 10.1001/jama.2015.2316. [DOI] [PubMed] [Google Scholar]
- 296. Luce JM, Cook DJ, Martin TR, Angus DC, Boushey HA, Curtis JR, et al. American Thoracic Society. The ethical conduct of clinical research involving critically ill patients in the United States and Canada: principles and recommendations. Am J Respir Crit Care Med. 2004;170:1375–1384. doi: 10.1164/rccm.200406-726ST. [DOI] [PubMed] [Google Scholar]
- 297. Rebers S, Aaronson NK, van Leeuwen FE, Schmidt MK. Exceptions to the rule of informed consent for research with an intervention. BMC Med Ethics. 2016;17:9. doi: 10.1186/s12910-016-0092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Park JJ, Thorlund K, Mills EJ. Critical concepts in adaptive clinical trials. Clin Epidemiol. 2018;10:343–351. doi: 10.2147/CLEP.S156708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Weiss CH, Baker DW, Weiner S, Bechel M, Ragland M, Rademaker A, et al. Low tidal volume ventilation use in acute respiratory distress syndrome. Crit Care Med. 2016;44:1515–1522. doi: 10.1097/CCM.0000000000001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Mukherjee V, Evans L. Implementation of the Surviving Sepsis Campaign guidelines. Curr Opin Crit Care. 2017;23:412–416. doi: 10.1097/MCC.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 301. Pullen E, Oser C. Barriers to substance abuse treatment in rural and urban communities: counselor perspectives. Subst Use Misuse. 2014;49:891–901. doi: 10.3109/10826084.2014.891615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Ramsey AT, Proctor EK, Chambers DA, Garbutt JM, Malone S, Powderly WG, et al. Designing for Accelerated Translation (DART) of emerging innovations in health. J Clin Transl Sci. 2019;3:53–58. doi: 10.1017/cts.2019.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Weiss CH, Krishnan JA, Au DH, Bender BG, Carson SS, Cattamanchi A, et al. ATS Ad Hoc Committee on Implementation Science. An Official American Thoracic Society research statement: implementation science in pulmonary, critical care, and sleep medicine. Am J Respir Crit Care Med. 2016;194:1015–1025. doi: 10.1164/rccm.201608-1690ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Meade MO, Jacka MJ, Cook DJ, Dodek P, Griffith L, Guyatt GH. Canadian Critical Care Trials Group. Survey of interventions for the prevention and treatment of acute respiratory distress syndrome. Crit Care Med. 2004;32:946–954. doi: 10.1097/01.ccm.0000120056.76356.ad. [DOI] [PubMed] [Google Scholar]
- 305. Cnossen MC, Polinder S, Andriessen TM, van der Naalt J, Haitsma I, Horn J, et al. Causes and consequences of treatment variation in moderate and severe traumatic brain injury: a multicenter study. Crit Care Med. 2017;45:660–669. doi: 10.1097/CCM.0000000000002263. [DOI] [PubMed] [Google Scholar]
- 306. Barbash IJ, Davis B, Kahn JM. National performance on the Medicare SEP-1 sepsis quality measure. Crit Care Med. 2019;47:1026–1032. doi: 10.1097/CCM.0000000000003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Keenan SP, Martin CM, Kossuth JD, Eberhard J, Sibbald WJ. The critical care research network: a partnership in community-based research and research transfer. J Eval Clin Pract. 2000;6:15–22. doi: 10.1046/j.1365-2753.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 308.Webb S, McArthur C, Bonten M, Derde L. Bethesda, MD: U.S. National Library of Medicine; 2016. https://clinicaltrials.gov/ct2/show/NCT02735707 [Google Scholar]
- 309. Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Pincus HA, Fine T. The ‘anatomy’ of research funding of mental illness and addictive disorders. Arch Gen Psychiatry. 1992;49:573–579. doi: 10.1001/archpsyc.1992.01820070067010. [DOI] [PubMed] [Google Scholar]
- 312. Chevreul K, McDaid D, Farmer CM, Prigent A, Park A-L, Leboyer M, et al. Public and nonprofit funding for research on mental disorders in France, the United Kingdom, and the United States. J Clin Psychiatry. 2012;73:e906–e912. doi: 10.4088/JCP.11r07418. [DOI] [PubMed] [Google Scholar]
- 313. Collins FS, Koroshetz WJ, Volkow ND. Helping to end addiction over the long-term: the research plan for the NIH HEAL initiative. JAMA. 2018;320:129–130. doi: 10.1001/jama.2018.8826. [DOI] [PMC free article] [PubMed] [Google Scholar]