Abstract
Previous studies have indicated that proapoptotic stresses downregulate the phosphatidylinositol 3-kinase [PI(3)K]/Akt survival pathway via the activation of acid-sphingomyelinase (A-SMase) and ceramide production. Ceramide induces apoptosis and inhibits PI(3)K activity without altering expression, association, or phosphorylation of receptors, adapter proteins, or PI(3)K subunits. PI(3)K inhibition by ceramide is associated with recruitment of caveolin 1 to PI(3)K-associated receptor complexes within lipid raft microdomains. Overexpression of caveolin 1 alone is sufficient to alter PI(3)K activity and sensitizes fibroblasts to ceramide-induced cell death. Most importantly, antisense expression of caveolin 1 dramatically reduces ceramide-induced PI(3)K deregulation and results in a loss-of-function stress response similar to that in A-SMase-deficient cells. Stress-induced recruitment of caveolin 1 to receptor complexes was found to be dependent on A-SMase since cell lines deficient in A-SMase did not exhibit caveolin 1 association with PI(3)K receptor complexes. Thus, a genetic link between A-SMase activation and caveolin 1-induced inhibition of PI(3)K activity exists. These results led us to propose that stress-induced changes in raft microdomains lead to altered receptor tyrosine kinase signal transduction through the modulation of caveolin 1 by ceramide.
Normally, cells require a variety of stimuli such as growth factors or integrin-mediated adhesion to prevent induction of apoptosis (31, 63). Apoptosis can occur in spite of the presence of these survival signals if the cell is subjected to adverse stimuli such as inflammatory or immunomodulatory agents (e.g., tumor necrosis factor alpha and Fas), microenvironmental cues (e.g., UV irradiation and hyperosmolarity), or anticancer therapies (e.g., gamma irradiation and daunorubicin) (3, 26, 36, 40, 42, 46, 65). Previous studies have suggested that the effects of apoptotic stimuli can deregulate a major survival signaling pathway, the phosphatidylinositol 3-kinase [PI(3)K]/Akt pathway and that this deregulation is dependent on the generation of the lipid secondary messenger ceramide (59, 65).
PI(3)K is particularly important in cellular transformation and tumor progression due to its involvement in cell cycle transitions (14, 32), cell motility (30, 54, 61), apoptotic sensitivity (28, 30, 34, 62, 64), and angiogenic capacity (1, 41, 44). PI(3)K activity has been found elevated in many cancer types (25, 49), and this has been attributed to gene amplification (56), gain-of-function translocations (26), or viral oncoprotein expression (17, 23, 59). Overexpression of PI(3)K is sufficient for oncogenic transformation (9) and is required for oncogenic transformation by various oncogenes (57, 60). Since deregulated expression of PI(3)K leads to transformation, negative regulators of PI(3)K such as the phosphatase PTEN, which dephosphorylates the D3 position of phosphoinositides, act as tumor suppressors and have been found to be deleted in a variety of cancers (7). Therefore, understanding how PI(3)K is regulated under normal physiological conditions and how this control is lost or deregulated during tumorigenesis is necessary for discerning the pathogenesis of a variety of cancers.
Studies indicate that there exists a family of PI(3)Ks, with each PI(3)K consisting of an adapter subunit and a catalytic subunit (16). Class 1a PI(3)Ks that are associated with receptor tyrosine kinases (RTKs) generally consist of the p85 (α,β) and p55γ adapter subunits and the p110 (α,β,δ) catalytic subunits (16). In response to ligand binding and RTK autophosphorylation, the PI(3)K adapter subunit is recruited via its SH2 domain to a phosphotyrosine residue (i.e., platelet-derived growth factor receptor [PDGFR]) (16). Alternatively, an intermediary adapter (i.e., IRS-1) can bind the activated receptor (i.e., insulin receptor [IR]) and recruit the PI(3)K adapter subunit to the complex (16). Activated PI(3)K then phosphorylates PIP(4) and PIP(4,5) phosphoinositides at the D3 position (16). Proteins containing plekstrin homology (PH) motifs can then bind PIP(3,4) or PIP(3,4,5), which is thought to localize these proteins to the plasma membrane and/or to potentially generate allosteric changes required for the function of the protein (4, 16). Although many proteins contain PH domains, few have been characterized in relation to PI(3)K in any detail.
The most thoroughly studied effector of PI(3)K is the Ser/Thr protein kinase Akt. Akt is a viral oncoprotein and has been shown to negatively regulate the proapoptotic effectors caspase-9, Bad, GSK-3β, and members of the forkhead family of transcription factors (5, 8, 10, 11, 34, 35, 47). Akt is also utilized for hypoxia-induced induction of hypoxia-inducible factor 1α (Hif-1α) and vascular endothelial growth factor transactivation in some tumor cell lines (41). Akt may also play a role in cell cycle progression via GSK-3β regulation of cyclin D1 (12). Therefore, stringent regulation of PI(3)K and its downstream effectors is essential for maintenance of cell proliferation and viability.
RTKs, including many of those that stimulate PI(3)K, have been reported to be localized in discrete microdomains of the plasma membrane that contain a distinct population of lipids (53). These lipid microdomains are highly enriched in sphingolipids and cholesterol (53). Studies suggest that these lipids possess unique physical characteristics, notably, decreased fluidity relative to the enriched phospholipid bilayer (15). Hypothetically, the sphingomyelin-cholesterol-enriched microdomains form a more stable lipid matrix, which in turn can act as an ordered support for receptor-mediated signaling events. This is an attractive hypothesis since the majority of receptors form complex aggregations of effector molecules. How these receptor complexes form while maximizing spatial requirements by selectively localizing various components from a densely packed cytosolic milieu to satisfy the temporal requirements of cell signaling is still poorly understood.
In response to a diverse array of cellular insults and apoptosis-related cytokines, sphingomyelin is hydrolyzed to generate ceramide (48). This results in the catalysis of up to one-half of total cellular sphingomyelin, the majority of which would be presumably associated with the plasma membrane (33). We therefore postulated that degradation of sphingomyelin to ceramide within lipid microdomains or exogenous administration of ceramide would have profound effects on receptor-mediated events within these microdomains. For this reason, we examined the effect of ceramide on receptor-activated PI(3)K activity and its modulation by a key member of the lipid rafts, caveolin 1.
MATERIALS AND METHODS
Cell lines, plasmids, and reagents.
Rat-1 fibroblasts were seeded and maintained in Dulbecco modified Eagle medium (DMEM) containing 10% (vol/vol) fetal bovine serum (GIBCO-BRL) until reaching 90 to 100%. The MS-1418 Niemann-Pick acid-sphingomyelinase (A-SMase)-deficient and the JY wild-type cells are Epstein-Barr virus (EBV)-transformed human lymphoblasts and were a generous gift from R. Kolesnick (52). These cell lines were maintained in a 1:1 mixture of DMEM and RPMI 1640 containing 15% (vol/vol) fetal bovine serum. Lipid (Biomol or Matreya) stocks were dissolved in dimethyl sulfoxide or double-distilled water (ddH2O) in accordance with the manufacturer's recommendation. PDGF (GIBCO-BRL) and insulin (Fisher) stocks were dissolved in ddH2O. Gamma irradiation was performed in a Shepherd Mark I 137Cs irradiator at a dose rate of 543 rads/min. Caveolin 1 was excised from pCl-neo-caveolin-1 (a kind gift from E. J. Smart) by EcoRI digestion and ligated in frame by using an EcoRI-hemagglutinin (HA) tag-SalI linker into pBabe-puro. An AccI caveolin 1 cDNA digest was ligated in the antisense orientation into pBabe-puro to generate antisense caveolin 1–pBabe-puro. A PCR product containing EcoRI–caveolin 1(1-80)–EcoRI was ligated in frame to pCl-neo to generate caveolin 1Δ. All constructs were confirmed by restriction enzyme digestion and DNA sequencing. EGFP (Clontech) was used in some cotransfections. Transient transfections were performed by using Lipofectamine Plus (Gibco BRL) in accordance with the manufacturer's instructions. The Phoenix packaging cell line (a kind gift of G. Nolan) was transfected by using Lipofectamine Plus (Gibco BRL) in accordance with the manufacturer's instructions. Retroviral supernatant was collected 48 to 72 h posttransfection and either used immediately or frozen at −80°C. For retroviral infection, 2 ml of retroviral supernatant was added per 10-cm dish at 50% confluency in 6 ml of DMEM–10% fetal calf serum (FCS)–5 μg of Polybrene per ml. The cells were incubated at 32°C, and the viral supernatant-medium-Polybrene mixture was replaced every 8 h. At 24 h postinfection, the medium was replaced with fresh DMEM–10% FCS and the cells were incubated at 37°C. The cells were assayed at 36 to 48 h postinfection.
PI(3)K activity quantitation.
PI(3)kinase assays were performed as previously described (62). Briefly, cells were exposed to prolonged PI(3)K stimulation with 10% FCS followed by treatment with the indicated reagents. Cells were washed in cold phosphate-buffered saline (PBS) containing 1 mM CaCl2, 1 mM MgCl2, and 100 μM sodium orthovanadate and then lysed in cold PBS containing 1 mM CaCl2, 1 mM MgCl2, 1% (vol/vol) Nonidet P-40, 1 μg of leupeptin per ml, 1 μg of aprotinin per ml, 1 μM phenylmethylsulfonyl fluoride (PMSF), and 100 μM sodium orthovanadate. Lysates were assayed for protein concentration by the bicinchoninic acid technique (Pierce Biochemicals). Equal amounts of protein from control or treated cells were incubated with 5 μl of anti-p85 (06-195; Upstate Biotechnology Inc. [UBI]) or antiphosphotyrosine antibody (UBI) and immunoprecipitated with protein A-Sepharose beads (Sigma). Immunoprecipitants were washed three times with lysis buffer, once with 100 mM Tris (pH 7.4) containing 5 mM LiCl and 100 μM sodium orthovanadate, and once with TNE (10 mM Tris [pH 7.4] containing 150 mM NaCl, 5 mM EDTA, and 100 μM sodium orthovanadate). The immunoprecipitants were resuspended in 25 μl of TNE containing 20 μg of l-α-phosphatidylinositol-4-monophosphate (Sigma) and 5 μl of 100 mM MgCl2. Kinase reactions were carried out by adding 30 μCi of [γ-32P]ATP in 2.5 μl of 0.88 mM ATP to each reaction mixture. Thin-layer chromatography was performed with a CHCl3-methanol (MeOH)-H2O-NH4OH (40:48:10:5) solvent system. Results were visualized with a Storm 860 Phosphorimager (Molecular Dynamics).
[32P]orthophosphate labeling, immunoprecipitations, and Western blotting.
Cells were labeled with 100 μCi of [32P]orthophosphate per ml for 3 h and treated as indicated. Radiolabeled cells were washed four times in ice-cold PBS–1 mM Na3VO4–1 mM PMSF followed by lysis in immunoprecipitation buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM Na3VO4, 1 mg of leupeptin per ml, 1 mM PMSF, 10 μg of aprotinin per ml, 0.7 μg of pepstatin per ml) and immunoprecipitated as described below. Lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the gel was dried and then visualized with a phosphoimager (Molecular Dynamics). For other immunoprecipitations and immunoblotting, cell pellets were lysed in immunoprecipitation buffer, immunoprecipitated with anti-p85 (06-195; UBI), anti-PDGFR (sc-432; Santa Cruz), anti-IR (I16630; Transduction Laboratories), and anti-caveolin 1 (C13620; Transduction Laboratories), separated by electrophoresis, and transferred to polyvinylidene difluoride paper. Immunoblots were probed with anti-p85α (sc-423; Santa Cruz), anti-p110α (sc1331; Santa Cruz), anti-PDGFR (sc-432; Santa Cruz), anti-IR (I16620; Transduction Laboratories), anti-IRS-1 (I17820; Transduction Laboratories), anti-caveolin 1 (C37120; Transduction Laboratories), anti-p-Tyr (P11230; Transduction Laboratories), anti-HA (sc-805; Santa Cruz), anti-syntaxin 6 (S55420; Transduction Laboratories), anti-BiP/GRP78 (G73320; Transduction Laboratories), anti-cathepsin B (E-19) (sc-6492; Santa Cruz), anti-cytochrome oxidase I (COX I) antibody 1D6-E1-A8 (Molecular Probes), or anti-E-cadherin (C37020; Transduction Laboratories), detected with a Vistra Western ECF blotting kit (Amersham L.S.), and visualized with a Storm 860 fluorimager (Molecular Dynamics).
Detergent-insoluble buoyant membrane separation.
Caveolae were isolated by a modification of the method of Liu and Anderson (38). Five 150-mm-diameter dishes of confluent Rat-1 fibroblasts were chilled on ice, washed two times with ice-cold buffer A (Tris-buffered saline plus 1 mM Na3VO4, 1 mg of leupeptin per ml, 1 mM PMSF, 10 μg of aprotinin per ml, 0.7 μg of pepstatin per ml), and pelleted at 4°C. The pellet was mixed with 1 ml of ice-cold 1% Triton X-100 in buffer A, subjected to Dounce homogenization 20 times, mixed with 1 ml of 80% sucrose in buffer B (150 mM NaCl, 25 mM Tris-HCl [pH 7.5]), and loaded onto the bottom of a 10-ml ultracentrifuge tube at 4°C. The sample was overlaid with a 10 to 30% sucrose gradient in buffer B and centrifuged at 29,000 × g for 21 h at 4°C in an SW-41 rotor. One-thousand-microliter fractions were collected in Eppendorf tubes and maintained on ice until the indicated analysis.
Caveolin 1 consensus binding motif search.
A consensus caveolin 1 binding motif was derived by using known receptor binding sites (45) as the input for E-MOTIF and subjecting the consensus sequence to the SCAN program (43). Abbreviations for the amino acid residues are as follows: A, Ala; C, Cys; D, Asp; E, Glu; F, Phe; G, Gly; H, His; I, Ile; K, Lys; L, Leu; M, Met; N, Asn; P, Pro; Q, Gln; R, Arg; S, Ser; T, Thr; V, Val; W, Trp; Y, Tyr; and a period for any amino acid.
Apoptosis determinations.
Apoptosis was quantified on a morphological basis as previously described (64). Briefly, following treatment, cells were incubated with 2 μg each of bis-benzamide (Hoechst stain no. 33342; Sigma) and propidium iodide (Sigma) per ml for 15 min. Viability ratios (number of apoptotic cells/total number of cells) were determined by scoring low-magnification fields of randomly selected fields for cells with condensed and fragmented nuclei and loss of membrane integrity. Low-magnification fields of cells expressing EGFP and caveolin 1 were compared with Hoechst or propidium iodide staining of the same field as a reference.
RESULTS
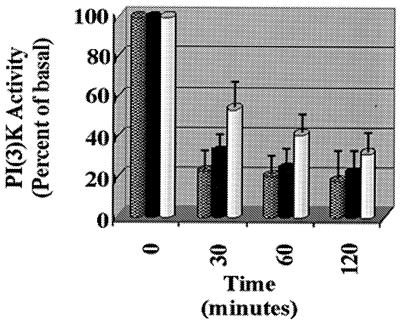
Past studies have indicated that either exogenously added ceramides or stress-induced ceramides decrease PI(3)K activity (65). To determine if this effect could be reproduced by exogenously added bacterial sphingomyelinase, we challenged Rat-1 fibroblasts with C2-ceramide, exogenous sphingomyelinase, and gamma irradiation (a well-documented ceramide-generating stress). Figure 1 demonstrates that ceramide can downregulate PI(3)K with similar kinetics irrespective of the method by which ceramide is generated.
FIG. 1.
Inhibition of PI(3)K by ceramide and ceramide-generating conditions. Rat-1 cells were exposed to 50 μM C2-ceramide (checked bars), 10 Gy of gamma irradiation (solid bars), or 600 mU of bacterial sphingomyelinase (Biomol) per ml (white bars) for the indicated times. The cells were lysed and immunoprecipitated with anti-p85; this was followed by a PI(3)K assay and a phosphorimage scan and quantitation (Molecular Dynamics).
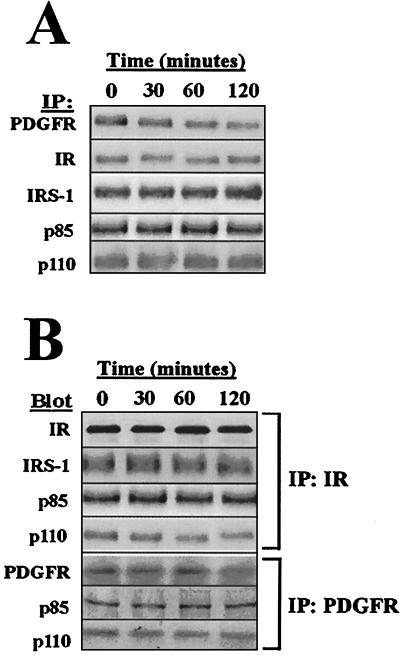
Since ceramide is a lipid secondary messenger that is thought to mediate its effects via phosphatase and kinase effectors (48), we evaluated the basal phosphorylation status of the PDGFR and IR, the IRS-1 adapter protein, and the p85α and p110α subunits of the PI(3)K heterodimer (Fig. 2A). We chose to study the p85α and p110α subunits because these PI(3)K subunits are relevant to both IR and PDGFR signaling and are the most ubiquitously expressed isoforms (2, 17, 16). The p85α and p110α subunits are also the only PI(3)K subunits implicated thus far as being mutated or amplified in carcinogenesis (9, 27, 56). The overall phosphorylation status of these proteins was not significantly altered in response to ceramide treatment. Although the PDGFR did exhibit approximately 60% lower overall phosphorylation at 2 h, this inhibition occured significantly later than the PI(3)K inhibition that was observed at 30 min. In addition, ceramide treatment did not generate altered binding of p85 or IRS-1 to the PDGFR or to the IR (Fig. 2B). The PI(3)K p110 catalytic subunit was found to be modestly dissociated from the PDGFR and IR complexes, suggesting that the effects of ceramide on PI(3)K could be due, at later times, to a dissociation of the receptor complex.
FIG. 2.
Ceramide does not alter phosphorylation or composition of PDGFR or IR complexes. (A) Cells were 32P labeled in vivo prior to 50 μM C2-ceramide treatment for the indicated times, lysis, immunoprecipitation as shown, SDS-PAGE, and phosphorimage scan. (B) Cells were treated with 50 μM C2-ceramide for the indicated times followed by lysis, immunoprecipitation as indicated, SDS-PAGE, immunoblotting as indicated, and fluorimage scan.
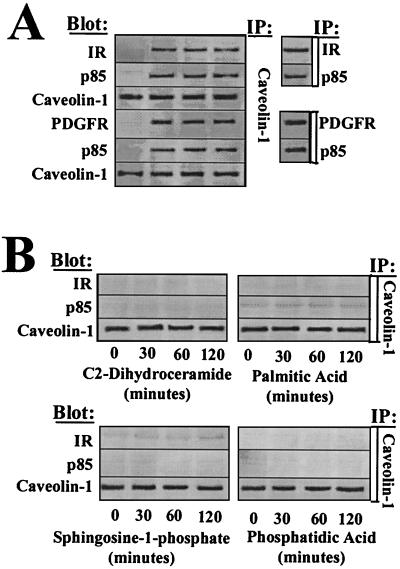
Lipid microdomains (referred to as rafts) composed of sphingolpids and cholesterol have been found to be highly enriched in signaling proteins and lipid secondary messengers. These microdomains are hypothesized to act as platforms whereby receptor complexes can be regulated spatially and temporally (45, 53) and have been found to be evolutionarily conserved from mammals to Drosophila melanogaster (20). Importantly, recent studies have suggested that perturbations in the cholesterol content of rafts can lead to altered or impaired signal transduction (22, 50). A protein originally identified as a target of v-src and later characterized as a putative tumor suppressor, caveolin 1, is often used as a marker for raft fractionation and can directly alter various signaling processes involving RTKs, nonreceptor tyrosine kinases, and the mitogen-activated protein kinase pathway (39, 45). To address whether ceramide could alter caveolin 1 association with IR or PDGFR complexes, we immunoprecipitated caveolin 1 following ceramide treatment and immunoblotted for associated receptors and the PI(3)K adapter subunit p85α. We found that caveolin 1 associates rapidly with PI(3)K receptor complexes following exogenous ceramide treatment with kinetics similar to those seen for PI(3)K inhibition (Fig. 3A). Approximately 72% of IR, 79% of PDGFR, and 82% of p85α became associated with caveolin 1 following ceramide treatment. Neither protein A beads nor cyclin E immunoprecipitates, used as controls, had IR, PDGFR, or PI(3)K associated with them nor associated PI(3)K activity (data not shown). Caveolin 1 association with receptor complexes was induced specifically by ceramide since structurally similar sphingolipids such as dihydroceramide and sphingosine 1-phosphate, sphingolipid precursors such as palmitic acid, or other structurally dissimiliar lipid secondary messengers such as phosphatidic acid did not induce a caveolin 1–receptor complex (Fig. 3B).
FIG. 3.
Caveolin 1 immunoprecipitates with PI(3)K-associated PDGFR and IR. Rat-1 cells were treated with 50 μM C2-ceramide (A) or with sphingosine-1-phosphate, palmitic acid, or phosphatidic acid (B) for the indicated times, followed by lysis, immunoprecipitation as indicated, SDS-PAGE, immunoblotting as indicated, and fluorimage scan.
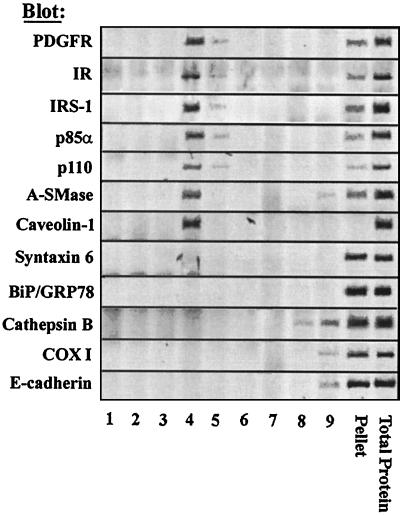
To confirm that IR and PDGFR complexes as well as A-SMase were localized to the same fraction of the plasma membrane, we utilized previously established protocols based on raft low-temperature detergent insolubility and density centrifugation. Fractionation of protein components revealed that the receptors (PDGFR, 79% ± 19%; IR, 63% ± 12% [mean ± standard deviation]), adapter proteins (IRS-I, 65% ± 18%), and PI(3)K subunits (p85α, 80% ± 12%; p110, 61% ± 8%) are clearly associated with caveolin 1 in the raft microdomain fraction, as indicated by colocalization in fractions 4 and 5 (Fig. 4A) of a 5 to 40% sucrose gradient. A-SMase, one enzyme implicated in stress-induced ceramide accumulation and previously implicated in inhibition of PI(3)K (65), is also notably enriched in these fractions (51% ± 12%) (Fig. 4A). Other resident proteins of the endoplasmic reticulum (BiP/GRP78), Golgi body (syntaxin 6), lysosomes (cathepsin B), mitochondria (COX I), and noncaveolar plasma membrane (E-cadherin) were notably absent from the raft fraction. Ceramide generation could conceivably destabilize the ordered lipid matrix of the raft, resulting in dispersion of protein components. Distribution of PI(3)K to plasmalemmal fractions other than raft microdomains in response to ceramide does not significantly occur (data not shown). Thus, ceramide induces association of caveolin 1 with PI(3)K-associated receptors within raft microdomains without generating a dispersion of raft components.
FIG. 4.
Caveolin 1, A-SMase, and receptor complexes cofractionate within raft microdomains. Cells were extracted in cold Triton X-100, and buoyant membrane complexes (detergent-resistant membranes) were isolated in isopycnic density gradients. Fractions were subjected to SDS-PAGE, immunoblotted as indicated, and subjected to fluorimage scan. Total protein immunoprecipitations are shown in lane 11. A representative experiment of three to five individual experiments is shown.
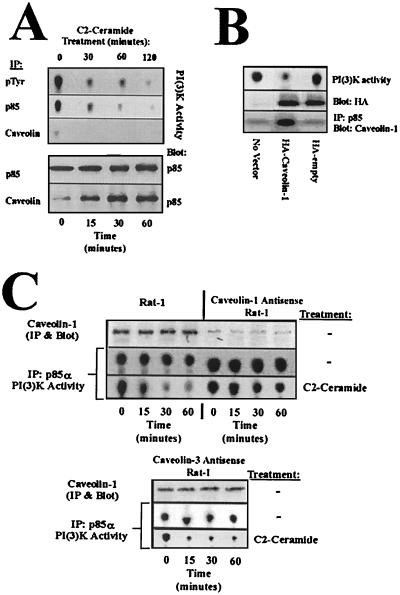
To determine to what extent PI(3)K was associated with caveolin 1 and the activity of the bound complex in response to ceramide, we assayed phosphotyrosine-associated PI(3)K activity (Fig. 5A, first subpanel), p85-α-associated PI(3)K activity (second subpanel), and caveolin 1-associated PI(3)K activity (third subpanel). PI(3)K immunoprecipitated with phosphotyrosine should account for the total PI(3)K activity associated with activated RTKs. Therefore, the majority of basal RTK-associated PI(3)K activity prior to ceramide treatment is associated with p85α, as shown by a comparison of pTyr and p85α PI(3)K activity measurements (Fig. 5A, first and second subpanels). Caveolin 1 association with p85α (Fig. 5A, fifth subpanel) is tightly correlated with PI(3)K inhibition in response to ceramide (second subpanel). Aapproximately 78% of p85α is associated with caveolin 1 in response to ceramide (Fig. 5A, fourth and fifth subpanels), and this complex is inactive (third subpanel). Thus, the majority of RTK-associated PI(3)K activity is p85α associated and is complexed to caveolin 1 in response to ceramide. The formation of p85α-caveolin 1 complexes biochemically links caveolin 1 with RTK-associated PI(3)K and suggests that caveolin 1 plays an effector role in ceramide-mediated PI(3)K deregulation.
FIG. 5.
Caveolin 1 is sufficient and required to alter PI(3)K activity in response to ceramide. (A) Rat-1 fibroblasts were treated with 50 μM C2-ceramide for the indicated times, followed by immunoprecipitation as indicated. Immunoprecipitations were split, and half were used for PI(3)K assays (top three panels) and the other half were subjected to SDS-PAGE, immunoblotting as indicated, and fluorimage scan (bottom two panels). (B) Rat-1 fibroblasts were either mock transfected or transfected with HA-caveolin or an empty HA vector. After selection, cells were lysed and split for PI(3)K assay (top panel), α-HA Western blotting (middle panel), or immunoprecipitation and blotting as indicated (bottom panel). (C) Rat-1 fibroblasts were infected with an antisense caveolin 1 construct or antisense caveolin 3 followed by C2-ceramide or null treatments and lysis. Lysates were split, and half were used for PI(3)K assays (bottom two panels of each box), and the other half were subjected to SDS-PAGE, immunoblotting as indicated, and fluorimage scan (top two panels of each box).
To substantiate the protein associations presented thus far, we transiently overexpressed either an HA-tagged caveolin 1 or an HA-tagged empty vector and assayed for PI(3)K activity and caveolin 1-p85α association in the absence of ceramide (Fig. 5B). Caveolin 1 overexpression is sufficient to bind p85α and inhibit PI(3)K activity in the absence of ceramide generation, genetically implicating caveolin 1 in the suppression of PI(3)K in response to stress. To establish whether caveolin 1 was required for inhibition of PI(3)K in response to ceramide, we utilized antisense expression of caveolin 1 followed by ceramide challenge (Fig. 5C). Antisense expression of caveolin 3, which these cells do not normally express, was used as a negative control. Antisense caveolin 1 reduced caveolin 1 expression to approximately 12% of that of control. The loss of caveolin 1 expression severely attenuates the inhibitory effects of ceramide on PI(3)K activity [60% of basal PI(3)K in antisense caveolin 1 versus 10% of basal PI(3)K in uninfected control], whereas antisense caveolin 3 expression was without significant effect [21% of basal PI(3)K in antisense caveolin 3]. These results provide strong genetic evidence that ceramide-mediated inhibition of PI(3)K is dependent on caveolin 1 expression and that caveolin 1 is sufficient for PI(3)K inhibition.
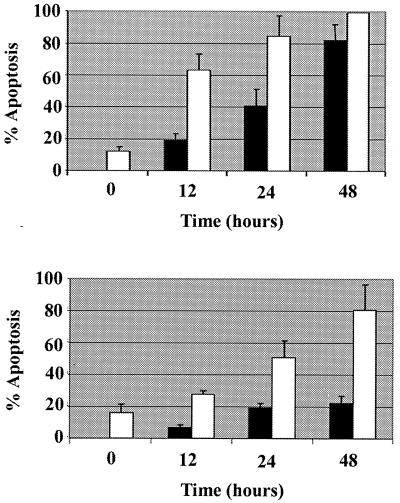
Due to the established role of PI(3)K in antiapoptotic regulation, we investigated whether caveolin 1 expression could have an effect on chromatin condensation and plasma membrane permeability, which are hallmarks of apoptosis, induced by ceramide or gamma irradiation. Wild-type caveolin 1 expression, but not a C-terminal deletion mutant lacking the caveolin scaffolding domain, increased the kinetics of Hoescht or propidium iodide staining in response to both of the cytotoxic stimuli and greatly potentiated gamma irradiation-induced cell death. Thus, caveolin 1 sensitizes fibroblasts to apoptotic stimuli, thus supporting its function as a regulator of cell survival (Fig. 6).
FIG. 6.
Caveolin 1 sensitizes fibroblasts to cell death. Rat-1 fibroblasts transiently coexpressing caveolin 1 and green fluorescent protein (GFP; as a transfection marker) or a caveolin 1 mutant lacking the scaffolding domain and C terminus (Cav-1Δ) and GFP were treated with 50 μM C2-ceramide or 10 Gy of gamma irradiation and assayed for apoptosis by Hoescht 33342 and propidium iodide staining at the indicated times. (Upper panel) Symbols: black bars, C2-ceramide plus Cav-1Δ–GFP; white bars, C2-ceramide plus caveolin 1-GFP. (Lower panel) Symbols: black bars, gamma irradiation plus Cav-1Δ–GFP; white bars, gamma irradiation plus caveolin 1-GFP).
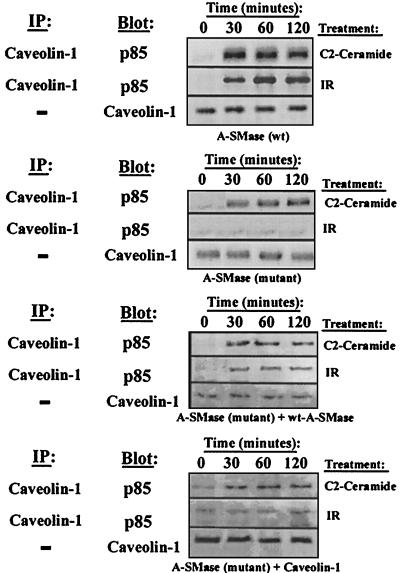
To investigate the genetic link between ceramide and caveolin 1, we utilized a lymphoblastic cell line containing a homozygous mutation in the A-SMase gene. These cells display virtually undetectable sphingomyelinase hydrolysis in response to stress and are resistant to gamma irradiation-induced apoptosis (53). Most importantly, these cells are also refractory to ceramide-mediated PI(3)K inhibition. Definitive proof linking A-SMase and caveolin 1 is exhibited in the lack of gamma irradiation-induced caveolin 1-p85α association in cells lacking functional A-SMase (Fig. 7). Treatment of these cells with ceramide elicited the association of p85α with caveolin 1, which is consistent with exogenous reconstitution of this stress response, and thus establishes the existence of this stress response mechanism in various tissue types. The A-SMase mutant cells also exhibit lower expression of caveolin 1 than do their wild-type counterparts. Transfection of the wild-type A-SMase gene into the A-SMase null cell line partially restored the sensitivity of these cells to gamma irradiation-mediated p85-caveolin 1 association. Expression of caveolin 1 in the A-SMase null cell line resulted in increased basal p85-caveolin 1 complex formation but had no effect on restoration of gamma irradiation-induced p85-caveolin 1 association. Taken together, these results strongly link ceramide generated by A-SMase as a stress-responsive lipid that controls caveolin 1 regulation of receptor-associated PI(3)K activity.
FIG. 7.
Stress-induced recruitment of caveolin 1 is dependent on A-SMase JY Niemann-Pick EBV-transformed lymphoblasts [A-SMase (wt)], MS-1418 Niemann-Pick EBV-transformed lymphoblasts [A-SMase (mutant)], MS-1418 transiently expressing wild-type A-SMase [A-SMase (mutant) + wt-A-SMase], and MS-1418 transiently expressing wild-type caveolin 1 [A-SMase (mutant) + caveolin 1] were treated with 50 μM C2-ceramide or 10 Gy of gamma irradiation for the indicated times, followed by lysis, immunoprecipitation as indicated, SDS-PAGE, immunoblotting as indicated, and fluorimage scan.
DISCUSSION
This report describes mechanistically how ceramide generation can result in inactivation of PI(3)K. Deregulation of PI(3)K by ceramide is one mechanism of inhibiting negative regulation of Akt on proapoptotic effectors, such as Bad, GSK-3β, forkhead transcription factors, and caspase-9, thereby facilitating apoptosis under suboptimal growth conditions (5, 8, 10, 11, 34, 48). We have shown genetically and biochemically that PI(3)K can be inactivated by caveolin 1 and that caveolin 1 is in turn regulated by A-SMase-generated ceramide in response to stress. These events occur within lipid raft microdomains, allowing for specificity in signaling and increased temporal control. Since the raft microdomains consist predominantly of sphingolipids and cholesterol (53) and ceramide is generated within these microdomains (38), it is possible that changes in raft lipid composition such as alterations in the sphingomyelin/ceramide ratio could alter the raft's physical characteristics. In support of this hypothesis, changes in cholesterol concentrations within the rafts have previously been shown to alter cellular signaling (19, 50). Furthermore, caveolin 1 binds cholesterol, and cholesterol is thought to intercalate under the sphingolipid headgroups, thus facilitating tighter packing and decreased entropy (51). If changes in either cholesterol or sphingomyelin occur within these rafts, a mechanism for the dynamic regulation of growth factor receptor signaling through the function of caveolin 1 and other resident raft proteins could be envisioned.
Caveolin 1 binds to a D{ILMV}WS{FY}G{IV}.{FILMY}WE{ILY}.{ST}{FLY} consensus motif (45) which many PI(3)K-activating receptors possess, including EGF receptors, PDGFRs, fibroblast growth factor (FGF) receptors, TRK receptors, VEGF receptors, TIE receptors, EPH receptors, IRs and IRS-1 receptors. Since caveolin 1 expression is known to modulate receptor-mediated mitogen-activated protein kinase activity in some cell types (13), it is highly consistent that PI(3)K inactivation is also mediated via receptor complexes. Significantly, this study strongly suggests that changes in lipid raft composition in response to adverse extracellular conditions result in altered receptor-mediated signaling, thus adding an additional level of regulatory complexity to signaling cascades.
Several studies had previously reported ceramide-mediated inhibition of Akt activation (64, 65). These studies differed in that one report described PI(3)K inhibition (65) and the other described no change in PI(3)K activity (64). The apparently disparate results could be attributed to the experimental approaches used in the studies. The authors of the report that described PI(3)K inhibition observed these results under basal activation of PI(3)K, whereas the authors of the report that did not observe significant changes found PI(3)K activity to be in a system that utilized saturating stimulation by insulin. The possibility exists that hyperactivation of the IR could result in phosphorylation of caveolin 1 and a subsequent transient decrease in its inhibitory effects on PI(3)K. Indeed, we have observed caveolin 1 phosphorylation following insulin treatment and an inability of ceramide to alter PI(3)K activity under these conditions (W. Zundel, unpublished results). However, this would also suggest that there are other effects of ceramide downstream of PI(3)K that culminate in Akt inactivation (58). To place this in perspective, it should be noted that sphingomyelin hydrolysis and subsequent ceramide accumulation are observed predominantly under conditions of cellular stress, particularly in a tumor microenvironment. Under these conditions, growth factors are often present at low concentrations and therefore PI(3)K activity is basal.
Significantly, many proteins that directly regulate apoptosis, such as caspases and tumor necrosis factor receptor family, infrequently possess loss-of-function mutations or loss of expression due to deletion or transcriptional silencing in solid tumors (6, 18). In contrast, proteins involved in the response of cells to apoptotic stress, e.g., p53, are frequently found deleted in a large number of cancers (24). Interestingly, caveolin 1 expression has been reported to be absent in variety of tumor types (46). Therefore, it is significant that certain proteins, i.e., p53 and caveolin 1, act as switches that sensitize cells to apoptosis, and it is these proteins, rather than apoptotic effectors, that are most often downregulated during tumorigenesis.
Since upstream activators of PI(3)K such as HGFR, IGF-1, PDGFR, and Src, potentiating cofactors of PI(3)K such as Ras, downstream effectors of PI(3)K such as Akt or protein kinase B, Rac, and PI(3)K itself are all amplified, possess gain-of-function mutations, or exist as viral oncogenes in a majority of cancers, deregulation of PI(3)K is now considered an essential component of oncogenesis (7). Supporting this view is the observation that negative regulators of this pathway (PTEN and caveolin 1) are known or putative tumor suppressors (37, 45). Regulation of PI(3)K by ceramide-induced caveolin 1 association with growth factor receptor complexes mechanistically links an early apoptotic response pathway to deregulation of a pathway implicated in controlling pleiotropic cellular responses which are vital for oncogenic progression and normal cell growth (30–32, 54, 55, 63). Interestingly, it has also been reported that caveolin 1 expression is extremely low in some cancer types (45) and that overexpression of caveolin 1 can reverse oncogenic phenotypes (45). Significantly, antisense expression of caveolin 1 alone is sufficient to transform fibroblasts (21). All of these factors strongly implicate A-SMase and caveolin 1 as being essential for stress-responsive control of survival within normal tissues and help elucidate why these control mechanisms are impaired during tumorigenesis.
ACKNOWLEDGMENTS
We thank R. Kolesnick, G. Nolan, and E. J. Smart for cell lines, antibodies, and plasmid constructs.
W.Z. was supported by a Markey Trust Fellowship and U.S. Public Health Service grant CA09302. L.M.S. was supported by NIH Reproductive Development Program grant 5K12HD00849. This work was supported by a Howard Hughes Young Investigator Award, an ACS Junior Faculty Research Award, and NIH grants CA 64489 and CA 67166 to A.J.G.
REFERENCES
- 1.Arbiser J L, Moses M A, Fernandez C A, Ghiso N, Cao Y, Klauber N, Frank D, Brownlee M, Flynn E, Parangi S, Byers H R, Folkman J. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci USA. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bornfeldt K E, Raines E W, Graves L M, Skinner M P, Krebs E G, Ross R. Platelet-derived growth factor. Distinct signal transduction pathways associated with migration versus proliferation. Ann N Y Acad Sci. 1995;766:416–430. doi: 10.1111/j.1749-6632.1995.tb26691.x. [DOI] [PubMed] [Google Scholar]
- 3.Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- 4.Bottomley M J, Salim K, Panayotou G. Phospholipid-binding protein domains. Biochim Biophys Acta. 1998;1436:165–183. doi: 10.1016/s0005-2760(98)00141-6. [DOI] [PubMed] [Google Scholar]
- 5.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 6.Butler L M, Hewett P J, Butler W J, Cowled P A. Down-regulation of Fas gene expression in colon cancer is not a result of allelic loss or gene rearrangement. Br J Cancer. 1998;77:1454–1459. doi: 10.1038/bjc.1998.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantley L C, Neel B G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 9.Chang H W, Aoki M, Fruman D, Auger K R, Bellacosa A, Tsichlis P N, Cantley L C, Roberts T M, Vogt P K. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 10.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 11.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 12.Diehl J A, Cheng M, Roussel M F, Sherr C J. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelman J A, Chu C, Lin A, Jo H, Ikezu T, Okamoto T, Kohtz D S, Lisanti M P. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett. 1998;428:205–211. doi: 10.1016/s0014-5793(98)00470-0. [DOI] [PubMed] [Google Scholar]
- 14.Fantl W J, Escobedo J A, Martin G A, Turck C W, del Rosario M, McCormick F, Williams L T. Distinct phosphotyrosines on a growth factor receptor bind to specific molecules that mediate different signaling pathways. Cell. 1992;69:413–423. doi: 10.1016/0092-8674(92)90444-h. [DOI] [PubMed] [Google Scholar]
- 15.Ferraretto A, Pitto M, Palestini P, Masserini M. Lipid domains in the membrane: thermotropic properties of sphingomyelin vesicles containing GM1 ganglioside and cholesterol. Biochemistry. 1997;36:9232–9236. doi: 10.1021/bi970428j. [DOI] [PubMed] [Google Scholar]
- 16.Fruman D A, Meyers R E, Cantley L C. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 17.Fukui Y, Saltiel A R, Hanafusa H. Phosphatidylinositol-3 kinase is activated in v-src, v-yes, and v-fps transformed chicken embryo fibroblasts. Oncogene. 1991;6:407–411. [PubMed] [Google Scholar]
- 18.Fults D, Pedone C A, Thompson G E, Uchiyama C M, Gumpper K L, Iliev D, Vinson V L, Tavtigian S V, Perry W L., III Microsatellite deletion mapping on chromosome 10q and mutation analysis of MMAC1, FAS, and MXI1 in human glioblastoma multiforme. Int J Oncol. 1998;12:905–910. doi: 10.3892/ijo.12.4.905. [DOI] [PubMed] [Google Scholar]
- 19.Furuchi T, Anderson R G. Cholesterol depletion of caveolae causes hyperactivation of extracellular signal-related kinase (ERK) J Biol Chem. 1998;273:21099–21104. doi: 10.1074/jbc.273.33.21099. [DOI] [PubMed] [Google Scholar]
- 20.Galbiati F, Volonte D, Goltz J S, Steele Z, Sen J, Jurcsak J, Stein D, Stevens L, Lisanti M P. Identification, sequence and developmental expression of invertebrate flotillins from Drosophila melanogaster. Gene. 1998;210:229–237. doi: 10.1016/s0378-1119(98)00064-x. [DOI] [PubMed] [Google Scholar]
- 21.Galbiati F, Volonte D, Engelman J A, Watanabe G, Burk R, Pestell R G, Lisanti M P. Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J. 1998;17:6633–6648. doi: 10.1093/emboj/17.22.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garver W S, Hossain G S, Winscott M M, Heidenreich R A. The Npc1 mutation causes an altered expression of caveolin-1, annexin II and protein kinases and phosphorylation of caveolin-1 and annexin II in murine livers. Biochim Biophys Acta. 1999;1453:193–206. doi: 10.1016/s0925-4439(98)00101-x. [DOI] [PubMed] [Google Scholar]
- 23.Ghai J, Ostrow R S, Tolar J, McGlennen R C, Lemke T D, Tobolt D, Liu Z, Faras A J. The E5 gene product of rhesus papillomavirus is an activator of endogenous Ras and phosphatidylinositol-3′-kinase in NIH 3T3 cells. Proc Natl Acad Sci USA. 1996;93:12879–12884. doi: 10.1073/pnas.93.23.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giaccia A J, Kastan M B. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 25.Harrison-Findik D, Susa M, Varticovski L. Association of phosphatidylinositol 3-kinase with SHC in chronic myelogeneous leukemia cells. Oncogene. 1995;10:1385–1391. [PubMed] [Google Scholar]
- 26.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 27.Jimenez C, Jones D R, Rodriguez-Viciana P, Gonzalez-Garcia A, Leonardo E, Wennstrom S, von Kobbe C, Toran J L, Borlado L R-, Calvo V, Copin S G, Albar J P, Gaspar M L, Diez E, Marcos M A, Downward J, Martinez-A C, Merida I, Carrera A C. Identification and characterization of a new oncogene derived from the regulatory subunit of phosphoinositide 3-kinase. EMBO J. 1998;17:743–753. doi: 10.1093/emboj/17.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan D R, Whitman M, Schaffhausen B, Pallas D C, White M, Cantley L, Roberts T M. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987;50:1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- 29.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 30.Keely P J, Westwick J K, Whitehead I P, Der C J, Parise L V. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 31.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klippel A, Escobedo M A, Wachowicz M S, Apell G, Brown T W, Giedlin M A, Kavanaugh W M, Williams L T. Activation of phosphatidylinositol 3-kinase is sufficient for cell cycle entry and promotes cellular changes characteristic of oncogenic transformation. Mol Cell Biol. 1998;18:5699–5711. doi: 10.1128/mcb.18.10.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolesnick R N, Kronke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- 34.Kops G J, de Ruiter N D, De Vries-Smits A M, Powell D R, Bos J L, Burgering B M. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 35.Kulik G, Klippel A, Weber M J. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laster S M, Wood J G, Gooding L R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988;141:2629–2634. [PubMed] [Google Scholar]
- 37.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner S H, Giovanella B C, Ittmann M, Tycko B, Hibshoosh H, Wigler M H, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 38.Liu P, Anderson R G. Compartmentalized production of ceramide at the cell surface. J Biol Chem. 1995;270:27179–27185. doi: 10.1074/jbc.270.45.27179. [DOI] [PubMed] [Google Scholar]
- 39.Liu P, Ying Y, Ko Y G, Anderson R G. Localization of platelet-derived growth factor-stimulated phosphorylation cascade to caveolae. J Biol Chem. 1996;271:10299–10303. doi: 10.1074/jbc.271.17.10299. [DOI] [PubMed] [Google Scholar]
- 40.Lowe S W, Ruley H E, Jacks T, Housman D E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 41.Mazure N M, Chen E Y, Laderoute K R, Giaccia A J. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997;90:3322–3331. [PubMed] [Google Scholar]
- 42.Neal J V, Potten C S. Effect of low dose ionizing radiation on the murine pericryptal fibroblast sheath: radiation damage in a mesenchymal system in vivo. Int J Radiat Biol Relat Stud Phys Chem Med. 1981;39:175–183. doi: 10.1080/09553008114550191. [DOI] [PubMed] [Google Scholar]
- 43.Nevill-Manning C G, Wu T D, Brutlag D L. Highly specific protein sequence motifs for genome analysis. Proc Natl Acad Sci USA. 1998;95:5865–5871. doi: 10.1073/pnas.95.11.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oikawa T, Shimamura M. Potent inhibition of angiogenesis by wortmannin, a fungal metabolite. Eur J Pharmacol. 1996;318:93–96. doi: 10.1016/s0014-2999(96)00864-3. [DOI] [PubMed] [Google Scholar]
- 45.Okamoto T, Schlegel A, Scherer P E, Lisanti M P. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 46.Olson R L, Everett M A. Epidermal apoptosis: cell deletion by phagocytosis. J Cutan Pathol. 1975;2:53–57. doi: 10.1111/j.1600-0560.1975.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 47.Pap M, Cooper G M. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 48.Perry D K, Hannun Y A. The role of ceramide in cell signaling. Biochem Biophys Acta. 1998;1436:233–243. doi: 10.1016/s0005-2760(98)00145-3. [DOI] [PubMed] [Google Scholar]
- 49.Phillips W A, St. Clair F, Munday A D, Thomas R J, Mitchell C A. Increased levels of phosphatidylinositol 3-kinase activity in colorectal tumors. Cancer. 1998;83:41–47. doi: 10.1002/(sici)1097-0142(19980701)83:1<41::aid-cncr6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 50.Pike L J, Miller J M. Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J Biol Chem. 1998;273:22298–22304. doi: 10.1074/jbc.273.35.22298. [DOI] [PubMed] [Google Scholar]
- 51.Sankaram M B, Thompson T E. Interaction of cholesterol with various glycerophospholipids and sphingomyelin. Biochemistry. 1990;29:10670–10675. doi: 10.1021/bi00499a014. [DOI] [PubMed] [Google Scholar]
- 52.Santana P, Pena L A, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman E H, Fuks Z, Kolesnick R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 53.Shaul P W, Anderson R G. Role of plasmalemmal caveolae in signal transduction. Am J Physiol. 1998;275:L843–L851. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- 54.Shaw L M, Rabinovitz I, Wang H H, Toker A, Mercurio A M. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- 55.Shepherd P R, Withers D J, Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J. 1998;333:471–490. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shayesteh L, Lu Y, Kuo W L, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills G B, Gray J W. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 57.Skorski T, Bellacosa A, Nieborowska-Skorska M, Majewski M, Martinez R, Choi J K, Trotta R, Wlodarski P, Perrotti D, Chan T O, Wasik M A, Tsichlis P N, Calabretta B. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. EMBO J. 1997;16:6151–6161. doi: 10.1093/emboj/16.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Summers S A, Garza L A, Zhou H, Birnbaum M J. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol. 1998;18:5457–5464. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Talmage D A, Freund R, Young A T, Dahl J, Dawe C J, Benjamin T L. Phosphorylation of middle T by pp60c-src: a switch for binding of phosphatidylinositol 3-kinase and optimal tumorigenesis. Cell. 1989;59:55–65. doi: 10.1016/0092-8674(89)90869-6. [DOI] [PubMed] [Google Scholar]
- 60.Webster M A, Hutchinson J N, Rauh M J, Muthuswamy S K, Anton M, Tortorice C G, Cardiff R D, Graham F L, Hassell J A, Muller W J. Requirement for both Shc and phosphatidylinositol 3′ kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol Cell Biol. 1998;18:2344–2359. doi: 10.1128/mcb.18.4.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wennstrom S, Siegbahn A, Yokote K, Arvidsson A K, Heldin C H, Mori S, Claesson-Welsh L. Membrane ruffling and chemotaxis transduced by the PDGF beta-receptor require the binding site for phosphatidylinositol 3′ kinase. Oncogene. 1994;9:651–660. [PubMed] [Google Scholar]
- 62.Whitman M, Kaplan D, Roberts T, Cantley L. Evidence for two distinct phosphatidylinositol kinases in fibroblasts. Implications for cellular regulation. Biochem J. 1987;247:165–174. doi: 10.1042/bj2470165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao R, Cooper G M. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 64.Zhou H, Summers S A, Birnbaum M J, Pittman R N. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J Biol Chem. 1998;273:16568–16575. doi: 10.1074/jbc.273.26.16568. [DOI] [PubMed] [Google Scholar]
- 65.Zundel W, Giaccia A. Inhibition of the anti-apoptotic PI(3)K/Akt/Bad pathway by stress. Genes Dev. 1998;12:1941–1946. doi: 10.1101/gad.12.13.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]