Abstract
High-throughput experimentation (HTE) methods are central to modern medicinal chemistry. While many HTE approaches to C-N and Csp2-Csp2 bonds are available, options for Csp2-Csp3 bonds are limited. We report here how the adaptation of nickel-catalyzed cross-electrophile coupling of aryl bromides with alkyl halides to HTE is enabled by AbbVie ChemBeads technology. Using this approach, we were able to quickly map out the reactivity space at a global level using a challenging array of 3 × 222 micromolar reactions. The observed hit rate (56%) is competitive with other often-used HTE reactions and the results are scalable. A key to this level of success was the finding that bipyridine 6-carboxamidine (BpyCam), a ligand that had not previously been shown to be optimal in any reaction, is as general as the best-known ligands with complementary reactivity. Such “cryptic” catalysts may be common and modern HTE methods should facilitate the process of finding these catalysts.
Keywords: nickel, cross-electrophile coupling, high-throughput experimentation, medicinal chemistry, carbon-carbon bond formation
Graphical Abstract
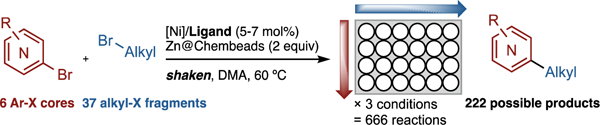
High-Throughput Experimentation, shaken, not stirred. Coating reductants onto glass beads simplifies µmol-scale cross-electrophile coupling, enabling the rapid survey of 222 different reactions and the discovery of a new, useful catalyst. Although the reactions are hetereogeneous, the glass beads enable the use of shakers instead of complex micro stirrers.
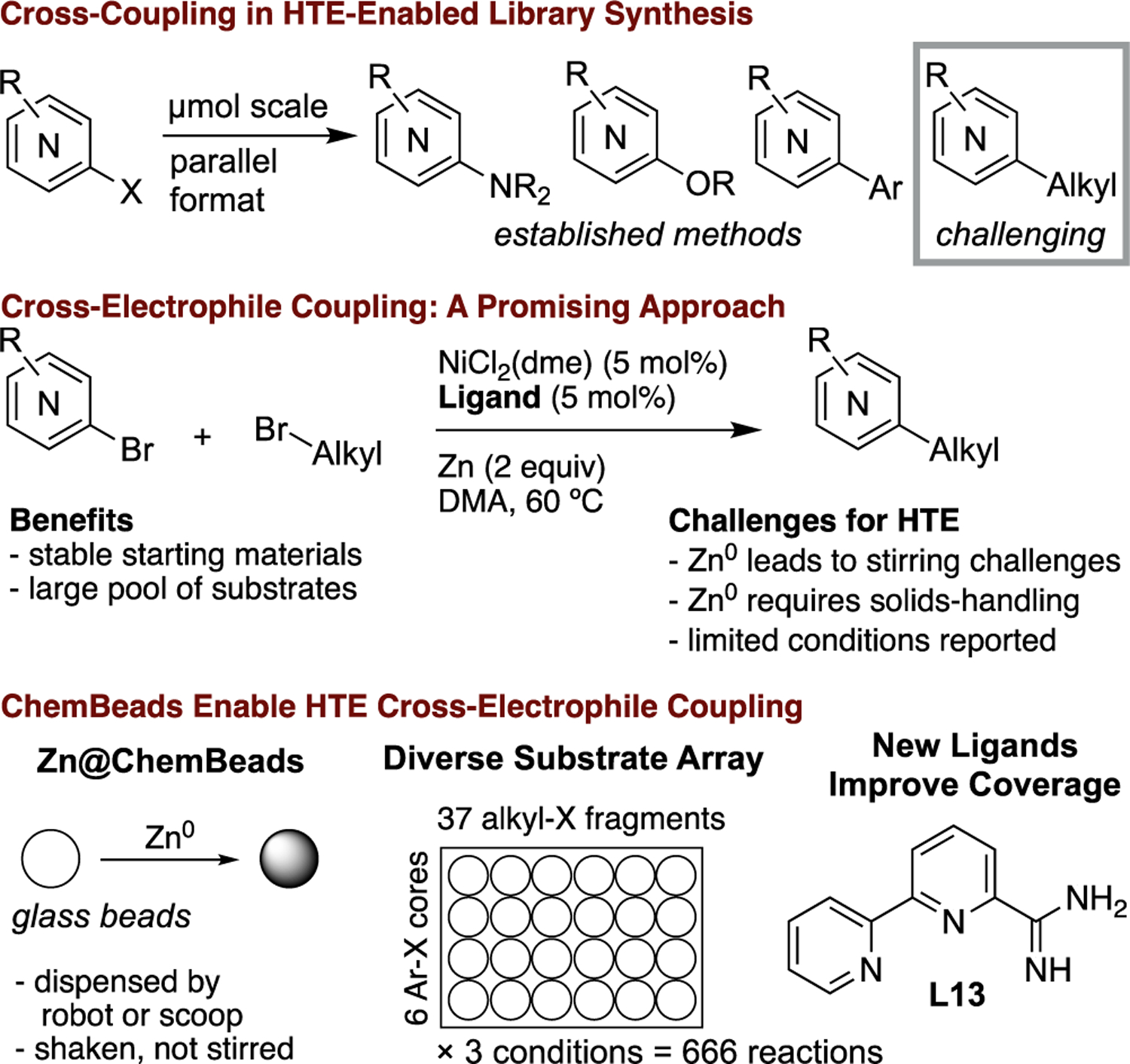
High-throughput experimentation (HTE) methods have become a key component of drug development,[1] facilitating the rapid exploration of structure-activity relationships (SAR) in medicinal chemistry and the rapid optimization of reactions in process development.[2] In industrial and academic labs, HTE methods are increasingly used in reaction optimization,[3] reaction discovery,[4] and the discovery of new ligands.[5] Translation of methods from academic labs to medicinal chemistry can be accelerated by HTE assessments using arrays of representative substrates tested against arrays of the best available catalysts and conditions.[6] To date, most of the methods adapted to HTE at AbbVie for medicinal chemistry have been C–N, C–O, and Csp2–Csp2 bond-forming reactions (Figure 1).[7] HTE methods to explore SAR while increasing Csp3 character in molecules would be valuable because increased saturation generally improves parameters important to drug discovery.[8]
Figure 1.
ChemBeads-Enabled High-Throughput Cross-Electrophile Coupling.
Nickel-catalyzed cross-electrophile coupling of alkyl electrophiles with aryl electrophiles[9] has become an increasingly used approach to the formation of Csp2–Csp3 bonds in the past decade[10,11] because it is compatible with many functional groups and the pool of available substrates is large (Figure 1).[12] The stability and availability of organic electrophiles is especially attractive for HTE in medicinal chemistry, where many analogs must be generated quickly. A recently published survey of available methods for Csp3-Csp2 cross-coupling in medicinal chemistry demonstrated the potential of cross-electrophile coupling in library synthesis and its complementarity to other approaches.[13] In that study, however, the scope of the survey was limited by the format – standard parallel library synthesis in 4 mL vials at 0.1 mmol scale. HTE library generation is generally conducted on orders of magnitude smaller scale (micromole to nanomole) because only small amounts of material are needed for initial screening and, in early stages of a project, available starting materials may be limited.
The primary challenge to implementing cross-electrophile coupling chemistry in an HTE format suitable for medicinal chemistry applications at AbbVie was the heterogeneous nature of these reactions – dispensing the solid metal reductants (Zn or Mn powders) in parallel at micromolar scale[14] and efficiently stirring the reactions in multiwell plates.[15,16] The challenges of heterogeneity have motivated the development of homogeneous conditions that employ organic terminal reductants,[17] sometimes using photoredox co-catalysis[13,18] or electrochemistry[19] to help drive the reaction. While avoiding some of the challenges of dispensing and stirring, these approaches often have different scope than metal-reductant conditions and some could be a challenge to adapt to parallel plates.[13] We report here the successful application of the AbbVie ChemBeads HTE platform[20] to cross-electrophile coupling, providing a general solution to Csp2–Csp3 bond formation and facilitating the discovery of a new, general ligand (Scheme 1).
Scheme 1.
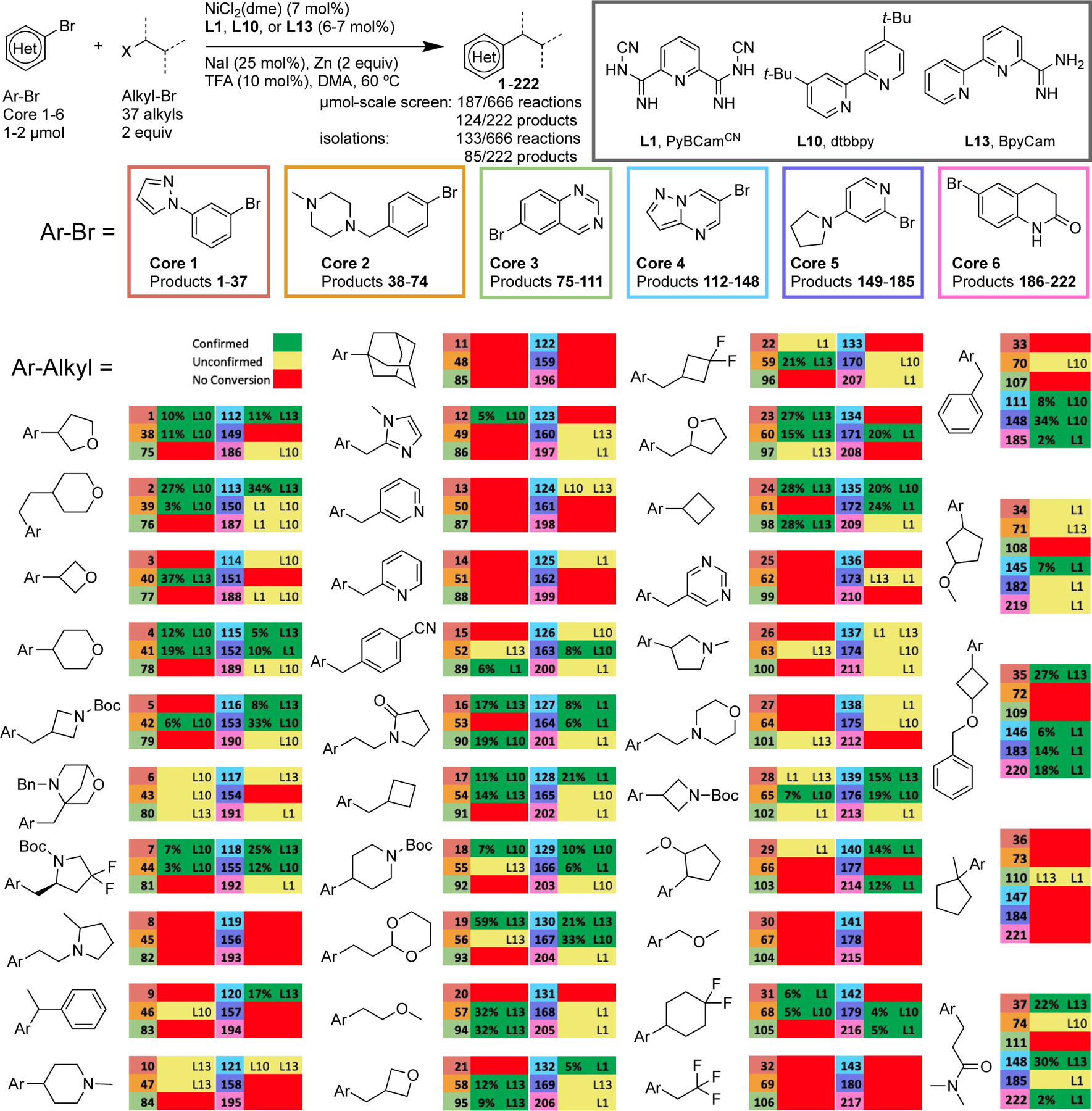
Results of HTE Library Survey of 222 Different Products Utilizing ChemBeads and three different cross-electrophile coupling catalysts (L1, L10, L13). Green denotes molecular ion for product observed by LC-MS at 10 µmol scale and verified by isolation at 100 µmol scale; Yellow denotes molecular ion observed at 10 µmol scale, but isolation at 100 µmol scale did not provide >95% pure product; Red denotes no ion observed. Yields reported are isolated yields after isolation by mass-directed HPLC. Ligands only noted if a large difference was noted or if the product was isolated. No noted ligand means that all three ligands worked well. See Supporting Information for experimental details.
To overcome the challenges associated with weighing solids and stirring heterogeneous reactions, we turned to our AbbVie ChemBeads technology. While we had not previously coated beads with malleable metals,[21] we found that Zn coats on glass beads well (4.8 mass%, “Zn@ChemBeads”), as long as activated Zn powder is used. While an acoustic mixer is generally required for reliable coating, Zn coated well even using a simple vortexer. In addition, we found that shaking plates on a modified orbital shaker (Torrey Pines SC20) with glass beads is a general replacement for the use of specialized stirrers and micro stir bars (Scheme 2A). Pre-coated ChemBeads are still advantageous because they simplify working on µmol scale and with solid-handling robots or calibrated scoops.
Scheme 2.
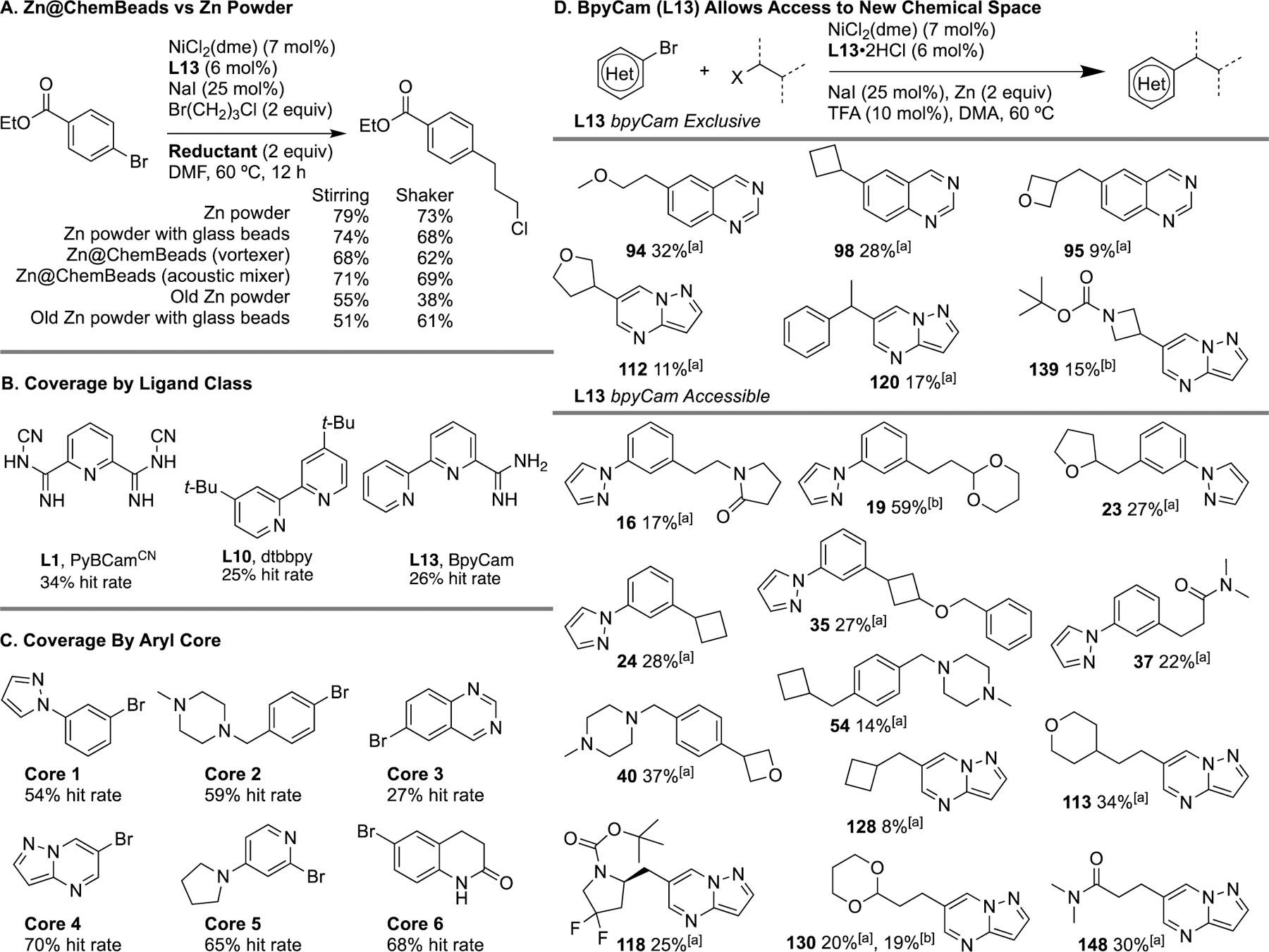
Analysis of HTE Library Results. A. Comparison of Zn powder vs Zn@ChemBeads. Yields are GC corrected against a 1,3,5-trimethoxybenzene internal standard. B. Library coverage by ligand class. C. Library coverage by aryl bromide core. D. New chemical space accessible with L13. Yields are isolated from scaled up reactions after observing a hit on HTE screens. [a] Isolated yield on 0.1 mmol scale using ChemBeads. [b] Isolated on 0.5 mmol scale using Zn powder and stir bars.
In order to examine the suitability of nickel-catalyzed coupling of aryl halides with alkyl halides for medicinal chemistry applications, an array of 222 different products was chosen to represent the diversity of aryl and alkyl coupling partners of interest to medicinal chemistry: 6 aryl halides × 37 alkyl halides (Scheme 1). While a wide array of heteroaryl halides have been explored recently in cross-electrophile coupling,[22] alkyl halides have been more limited: even in our recent study,[13] 40% of the 20 alkyl bromides tested were simple hydrocarbons. In this study, we emphasized alkyl bromides of interest to medicinal chemistry that we expected to be particularly challenging, including: neopentyl substrates,[23] tertiary alkyl substrates,[24] substrates with β-leaving groups,[25] substrates prone to methylcyclobutane radical rearrangement,[26] reactive heteroarylmethyl chlorides,[27] basic tertiary amines,[28] and precursors to valuable four-membered rings.[29] For the HTE experiments run at AbbVie, catalysts and additives were coated onto beads, substrates were dispensed as solutions, and plates were set up using a robotic solid handling system (Chemspeed Technologies). Even without a robot, Zn@ChemBeads simplify small-scale reaction setup in parallel because a calibrated scoop can be used in place of individual weighing or inaccurate zinc slurries. Initially, we chose our published optimal conditions for the coupling of aryl bromides with alkyl bromides (L = 4,4´-di-tert-butyl-2,2´-bipyridine, dtbbpy, L10)[9] and heteroaryl bromides with alkyl bromides (L = 2,6-bis(N-cyanocarboxamidine)pyridine, PyBCamCN, L1).[22b] Preliminary examination of optimal ligands from published reports (phenantholines, bipyridines, pyridine carboxamidines, terpyridines) and exchanging Mn for Zn did not provide substantial improvements in the observed hit rate beyond 46%. A major breakthrough was achieved when we examined non-optimal ligands derived from our Pfizer collaboration.[5a] We found that the ligand bipyridine-6-carboxamidine (BpyCam, L13) proved to be general and, in some cases, complementary to dtbbpy and PyBCamCN (Scheme 2B and 2D).
The success of L13 was surprising because, while pyridine carboxamidines and pyridine bis(carboxamidine) ligands have proven to be useful in several cross-coupling reactions,[5a,10d,22b] the value of (BpyCam)NiCl2 was only evident when tested against a diverse, challenging library. In analogy to similar situations in biology, we term this a “cryptic” catalyst.[30]
The overall hit rate for the 222-member µmol-scale library with the addition of L13 rose from 46% to 56% (124/222 product ions detected) (Schemes 1 and 2B). This hit rate is higher than what we had found previously using micro stir bars and a less diverse substrate set,[13] suggesting that some of the improvement is due to better mixing/activation with ChemBeads. This number is also impressive when considered in context: even methods considered reliable can give moderate hit rates in diverse medicinal chemistry libraries. For example, Merck noted only 45% of metal-catalyzed C-N bond forming reactions on complex, polar substrates succeeded.[1b] Similarly, internal AbbVie data for Pd-catalyzed amine arylation[31] gave a 55% hit rate.
Examination of the array reveals the differences and similarities between the three catalysts. For example, reactions conducted with each ligand had a similar level of success: 79/222 (36%) for PyBCamCN (L1), 61/222 (27%) for dtbbpy (L10), 62/222 (28%) for BpyCam (L13) (Scheme 2B). There was also considerable heterogeneity in what substrate combinations were successful with each ligand. For example, reactions of Core 2 did not work at all with L1, but L13 had a reasonable hit rate. On the other hand, L13 was a poor choice for Core 6, but reactions with L1 worked well. Finally, we note that the hit rate of this approach could be further improved by the use of additional modified conditions to accommodate the alkyl bromides in this study that provided no product (e.g., for adamantyl bromide,[24,32] 2,2,2-trifluoroethyl bromide,[33] MOM-Br,[34] and (1-(2-bromoethyl)-2-methylpyrrolidine)[35]). These alkyl coupling partners accounted for 24% of the reactions that failed to show any product in HTE screening. The use of even ten different sets of conditions is routine and not a barrier in HTE approaches.
Cross-couplings conducted at 10 µmol scale translated to larger scale (10× and 50× scale) as well as a normal vial/stir bar format. A subset of these reactions were performed at 100 µmol scale followed by mass-directed purification resulted in the isolation of 72/124 products with >95% purity and an additional 14 products with <95% purity. The other 37 products were not isolated because the reactions had low conversion and/or isolation was hindered by overlapping peaks. We further scaled three reactions to 500 µM scale using standard lab techniques (4 mL vials with stirbars, isolations by standard flash chromatography (19, 130, 139)). Of the 87 isolated products, 27 products were fully characterized (see Supporting Information for additional details). As the main purpose of this study was to obtain a global overview of currently accessible scope, we did not focus on reaction optimization. In our study, the reactions were carried out in parallel, with fixed concentration, temperature and reaction time, and purification was optimized for purity and speed over yield. Further optimization would presumably improve on these yields, as would the inclusion of additional conditions for specialized substrates.
These results demonstrate the power of using µmol scale high throughput experimentation to quickly identify workable conditions and map out the reactivity space of the substrates of interest. The sensitivity of the analysis tool (UPLC-MS) ensured even a trace amount of product peak signal could be detected, thus greatly eliminating the possibility for false negative findings on the micromole scale. This workflow, which is accessible with a minimal investment, allows researchers to get a global understanding of gaps in scope while using minimal amounts of material (for 666 reactions, 1.11 mmol of each core, 0.36 mmol of each alkyl halide, and 0.16 mmol of each ligand) and time (the screens were conducted over about two weeks).
We anticipate that the use of Zn@ChemBeads for cross-electrophile coupling will be broadly useful in HTE. Indeed, based on this study and the promising results we obtained, this methodology has become one of the few methods we use in screening aryl-alkyl coupling conditions for complex med-chem substrates. In addition, this study suggests that HTE libraries could be used to find catalysts that are general, but whose value is not evident with relatively simple substrate pairs. A corollary to this suggestion is that collections of ligands should be focused on diversity as much as performance in one or two test reactions. Ligands that might appear to be poor choices, and are thus not routinely screened, might in fact be just as useful as optimal ligands. These “cryptic catalysts” only show their value when challenged with the correct prompts, a task that is now possible with modern HTE.
Supplementary Material
Acknowledgements
This work was supported by the NIH (R01GM097243) and AbbVie. We thank Dr. Phil Cox (AbbVie) for assistance with data analysis, Dr. Gashaw Goshu (AbbVie) for assistance with monitoring and assembling plates, and Dr. Noah Tu (AbbVie) for assistance with ChemBeads and instrument programming.
Footnotes
Supporting information for this article is given via a link at the end of the document.
Institute and/or researcher Twitter usernames: @weixgroup
References
- [1].a) Cooper TWJ, Campbell IB, Macdonald SJF, Angew. Chem., Int. Ed 2010, 49, 8082–8091; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2010, 122, 8258–8267. [Google Scholar]; b) Perera D, Tucker JW, Brahmbhatt S, Helal CJ, Chong A, Farrell W, Richardson P, Sach NW, Science 2018, 359, 429. [DOI] [PubMed] [Google Scholar]; b) Buitrago Santanilla A, Regalado EL, Pereira T, Shevlin M, Bateman K, Campeau L-C, Schneeweis J, Berritt S, Shi Z-C, Nantermet P, Liu Y, Helmy R, Welch CJ, Vachal P, Davies IW, Cernak T, Dreher SD, Science 2015, 347, 49–53. [DOI] [PubMed] [Google Scholar]
- [2].a) Krska SW, DiRocco DA, Dreher SD, Shevlin M, Acc. Chem. Res 2017, 50, 2976–2985. [DOI] [PubMed] [Google Scholar]; b) Mennen SM, Alhambra C, Allen CL, Barberis M, Berritt S, Brandt TA, Campbell AD, Castañón J, Cherney AH, Christensen M, Damon DB, Eugenio de Diego J, García-Cerrada S, García-Losada P, Haro R, Janey J, Leitch DC, Li L, Liu F, Lobben PC, MacMillan DWC, Magano J, McInturff E, Monfette S, Post RJ, Schultz D, Sitter BJ, Stevens JM, Strambeanu II, Twilton J, Wang K, Zajac MA, Org. Process Res. Dev 2019, 23, 1213–1242. [Google Scholar]
- [3].a) Shevlin M, ACS Med. Chem. Lett 2017, 8, 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bédard A-C, Adamo A, Aroh KC, Russell MG, Bedermann AA, Torosian J, Yue B, Jensen KF, Jamison TF, Science 2018, 361, 1220. [DOI] [PubMed] [Google Scholar]
- [4].a) Kanan MW, Rozenman MM, Sakurai K, Snyder TM, Liu DR, Nature 2004, 431, 545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) McNally A, Prier CK, MacMillan DWC, Science 2011, 334, 1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Robbins DW, Hartwig JF, Science 2011, 333, 1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Hansen EC, Pedro DJ, Wotal AC, Gower NJ, Nelson JD, Caron S, Weix DJ, Nat. Chem 2016, 8, 1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chan VS, Krabbe SW, Li C, Sun L, Liu Y, Nett AJ, ChemCatChem 2019, 11, 5748–5753. [Google Scholar]; c) Modak A, Nett AJ, Swift EC, Haibach MC, Chan VS, Franczyk TS, Shekhar S, Cook SP, ACS Catalysis 2020, 10, 10495–10499. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Biswas S, Qu B, Desrosiers J-N, Choi Y, Haddad N, Yee NK, Song JJ, Senanayake CH, J. Org. Chem 2020, 85, 8214–8220. [DOI] [PubMed] [Google Scholar]
- [6].a) Nadin A, Hattotuwagama C, Churcher I, Angew. Chem., Int. Ed 2012, 51, 1114–1122; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2012, 124, 1140–1149. [Google Scholar]; b) Kutchukian PS, Dropinski JF, Dykstra KD, Li B, DiRocco DA, Streckfuss EC, Campeau L-C, Cernak T, Vachal P, Davies IW, Krska SW, Dreher SD, Chem. Sci 2016, 7, 2604–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].a) Roughley SD, Jordan AM, J. Med. Chem 2011, 54, 3451–3479. [DOI] [PubMed] [Google Scholar]; b) Boström J, Brown DG, Young RJ, Keserü GM, Nat. Rev. Drug Discovery 2018, 17, 709–727. [DOI] [PubMed] [Google Scholar]
- [8].a) Ishikawa M, Hashimoto Y, J. Med. Chem 2011, 54, 1539–1554. [DOI] [PubMed] [Google Scholar]; b) Lovering F, Bikker J, Humblet C, J. Med. Chem 2009, 52, 6752–6756. [DOI] [PubMed] [Google Scholar]; c) Lovering F, MedChemComm 2013, 4, 515–519. [Google Scholar]; d) Yang Y, Engkvist O, Llinàs A, Chen H, J. Med. Chem 2012, 55, 3667–3677. [DOI] [PubMed] [Google Scholar]; e) Leeson PD, St-Gallay SA, Wenlock MC, MedChemComm 2011, 2, 91–105. [Google Scholar]; f) Meanwell NA, Chem. Res. Toxicol 2016, 29, 564–616. [DOI] [PubMed] [Google Scholar]
- [9].a) Goldfogel MJ, Huang L, Weix DJ, in Nickel Catalysis in Organic Synthesis (Ed.: Ogoshi S), Wiley-VCH, Weinheim, 2020, pp. 183–222. [Google Scholar]; b) Everson DA, Shrestha R, Weix DJ, J. Am. Chem. Soc 2010, 132, 920–921. [DOI] [PubMed] [Google Scholar]; c) Everson DA, Jones BA, Weix DJ, J. Am. Chem. Soc 2012, 134, 6146–6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Medicinal Chemistry analog synthesis.; a) Fakhouri L, Cook CD, Al-Huniti MH, Console-Bram LM, Hurst DP, Spano MBS, Nasrallah DJ, Caron MG, Barak LS, Reggio PH, Abood ME, Croatt MP, Bioorg. Med. Chem 2017, 25, 4355–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dumas A, Garsi J-B, Poissonnet G, Hanessian S, ACS Omega 2020, 5, 27591–27606. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Mennie KM, Vara BA, Levi SM, Org. Lett 2020, 22, 556–559. [DOI] [PubMed] [Google Scholar]; d) Hughes JME, Fier PS, Org. Lett 2019, 21, 5650–5654. [DOI] [PubMed] [Google Scholar]
- [11].Total Synthesis:; a) Boeckman RK, Niziol JM, Biegasiewicz KF, Org. Lett 2018, 20, 5062–5065. [DOI] [PubMed] [Google Scholar]; b) Fegheh-Hassanpour Y, Arif T, Sintim HO, Al Mamari HH, Hodgson DM, Org. Lett 2017, 19, 3540–3543. [DOI] [PubMed] [Google Scholar]; c) Menger M, Lentz D, Christmann M, J. Org. Chem 2018, 83, 6793–6797. [DOI] [PubMed] [Google Scholar]; d) Girvin ZC, Andrews MK, Liu X, Gellman SH, Science 2019, 366, 1528. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Cheng H-G, Yang Z, Chen R, Cao L, Tong W-Y, Wei Q, Wang Q, Wu C, Qu S, Zhou Q, Angew. Chem., Int. Ed 2021, 60, 5141–5146; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2021, 133, 5201–5206. [Google Scholar]
- [12].While there are only hundreds of readily available alkylzinc reagents, thousands of alkyl halides are readily commercially available. Numbers from eMolecules: 443 alkylzinc reagents vs 200760 alkyl bromides (April, 2019). In general, the stringent storage requirement and lack of diverse functional groups of the alkylzinc reagents also render them much less desirable than alkyl halides.
- [13].Dombrowski AW, Gesmundo NJ, Aguirre AL, Sarris KA, Young JM, Bogdan AR, Martin MC, Gedeon S, Wang Y, ACS Med. Chem. Lett 2020, 11, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].The low MW of Zn and Mn mean that sub-milligram quantities are often required and handling of solids in parallel remains a challenge. See:; a) Shevlin M, ACS Med. Chem. Lett 2017, 8, 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bahr MN, Damon DB, Yates SD, Chin AS, Christopher JD, Cromer S, Perrotto N, Quiroz J, Rosso V, Org. Process Res. Dev 2018, 22, 1500–1508. [Google Scholar]
- [15].Stir rate and method have been shown to be important in the cross-electrophile coupling of aryl halides with alkyl halides. See:; Everson DA, George DT, Weix DJ, Buergler JF, Wood JL, Org. Synth 2013, 90, 200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].There are reports of success from Merck[10c,d] with syringing slurries of zinc powder on 2.5 – 50 µmol scale, but we found that careful optimization of the apparatus is required, reproducibility can be a challenge, and reproducibility across a large plate can be challenging.
- [17].a) Anka-Lufford LL, Huihui KMM, Gower NJ, Ackerman LKG, Weix DJ, Chem.–Eur. J 2016, 22, 11564–11567. [DOI] [PubMed] [Google Scholar]; b) Suzuki N, Hofstra JL, Poremba KE, Reisman SE, Org. Lett 2017, 19, 2150–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) García-Domínguez A, Li Z, Nevado C, J. Am. Chem. Soc 2017, 139, 6835–6838. [DOI] [PubMed] [Google Scholar]; d) Flood DT, Asai S, Zhang X, Wang J, Yoon L, Adams ZC, Dillingham BC, Sanchez BB, Vantourout JC, Flanagan ME, Piotrowski DW, Richardson P, Green SA, Shenvi RA, Chen JS, Baran PS, Dawson PE, J. Am. Chem. Soc 2019, 141, 9998–10006. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Charboneau DJ, Barth EL, Hazari N, Uehling MR, Zultanski SL, ACS Catalysis 2020, 10, 12642–12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].a) Duan Z, Li W, Lei A, Org. Lett 2016, 18, 4012–4015. [DOI] [PubMed] [Google Scholar]; b) Zhang P, Le CC, MacMillan DWC, J. Am. Chem. Soc 2016, 138, 8084–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Paul A, Smith MD, Vannucci AK, J. Org. Chem 2017, 82, 1996–2003. [DOI] [PubMed] [Google Scholar]; d) Badir SO, Sim J, Billings K, Csakai A, Zhang X, Dong W, Molander GA, Org. Lett 2020, 22, 1046–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].a) Perkins RJ, Pedro DJ, Hansen EC, Org. Lett 2017, 19, 3755–3758. [DOI] [PubMed] [Google Scholar]; b) Li H, Breen CP, Seo H, Jamison TF, Fang Y-Q, Bio MM, Org. Lett 2018, 20, 1338–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Koyanagi T, Herath A, Chong A, Ratnikov M, Valiere A, Chang J, Molteni V, Loren J, Org. Lett 2019, 21, 816–820. [DOI] [PubMed] [Google Scholar]; d) Perkins RJ, Hughes AJ, Weix DJ, Hansen EC, Org. Process Res. Dev 2019, 23, 1746–1751. [Google Scholar]; e) Truesdell BL, Hamby TB, Sevov CS, J. Am. Chem. Soc 2020, 142, 5884–5893. [DOI] [PubMed] [Google Scholar]
- [20].Tu NP, Dombrowski AW, Goshu GM, Vasudevan A, Djuric SW, Wang Y, Angew. Chem., Int. Ed 2019, 58, 7987–7991; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2019, 131, 8071–8075. [Google Scholar]
- [21].Although most reagents coated well, some metal salts coated glass beads poorly.[20] In general, an acoustic mixer (Resodyn) is required for consistent coating.
- [22].a) Kadunce NT, Reisman SE, J. Am. Chem. Soc 2015, 137, 10480–10483. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hansen EC, Li C, Yang S, Pedro D, Weix DJ, J. Org. Chem 2017, 82, 7085–7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Neopentyl substrates usually require modified conditions for high yields.[5d]
- [24].Tertiary alkyl halides have been coupled successfully, but generally have required different conditions and have been less explored with heteroaryl halides.; a) Wang X, Wang S, Xue W, Gong H, J. Am. Chem. Soc 2015, 137, 11562–11565. [DOI] [PubMed] [Google Scholar]; b) Wang X, Ma G, Peng Y, Pitsch CE, Moll BJ, Ly TD, Wang X, Gong H, J. Am. Chem. Soc 2018, 140, 14490–14497. [DOI] [PubMed] [Google Scholar]
- [25].Generally, β-leaving groups have been tolerated, but at somewhat lower yield. This has not been explored systematically with variation of the geometry and leaving group ability.[9]
- [26].Methylcyclopropyl radical (krearrangement ~ 6.7 × 107 s−1) has repeatedly been shown to rearrange faster than the coupling reaction, leading to homoallylation instead of cyclopropylmethylation. The slower 5-hexenyl radical (krearrangement ~ 2.3 × 105 s−1) tends to give mixtures of products. Methylcyclobutane radicals rearrange more slowly (krearrangement ~ 5 × 103 s−1) and could possibly work reliably in cross-electrophile coupling.; Newcomb M, in Radicals in Organic Synthesis, Vol. 1, 1st ed. (Eds.: Renaud P, Sibi MP), Wiley-VCH, Weinheim, 2001, pp. 317–336. [Google Scholar]
- [27].While coupling of benzylic chlorides and benzylic mesylates has been reported, only one example of a heteroaryl iodide (5-iodopyrimidine) and no examples of heteroarylmethyl chloride/mesylate substrates had been reported before last year.; a) Ackerman LKG, Anka-Lufford LL, Naodovic M, Weix DJ, Chem. Sci 2015, 6, 1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Anka-Lufford LL, Huihui KMM, Gower NJ, Ackerman LKG, Weix DJ, Chem.–Eur. J 2016, 22, 11564–11567. [DOI] [PubMed] [Google Scholar]; c) Brill ZG, Ritts CB, Mansoor UF, Sciammetta N, Org. Lett 2020, 22, 410–416. [DOI] [PubMed] [Google Scholar]
- [28].Tertiary amines can coordinate to metals and are easily oxidized, making them challenging in many cross-coupling reactions. See reference 13.
- [29].Radicals in strained rings can be more difficult to form and react differently than unstrained secondary radicals. Success in cross-electrophile and metallaphotoredox coupling has been mixed, with simple oxetanes and azetidines generally working well.[22]; Kolahdouzan K, Khalaf R, Grandner JM, Chen Y, Terrett JA, Huestis MP, ACS Catalysis 2020, 10, 405–411. b) [Google Scholar]
- [30].Cryptic binding, gene clusters, and natural products are only found when challenged with the correct stimulus. In the absence of the correct environment, they appear to have no useful function. We propose the term cryptic catalysts for catalysts that appear to have little value until challenged with the right conditions (types of substrates, additives).; a) Beglov D, Hall DR, Wakefield AE, Luo L, Allen KN, Kozakov D, Whitty A, Vajda S, Proc. Natl. Acad. Sci., U.S.A 2018, 115, E3416. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Scherlach K, Hertweck C, Org. Biomol. Chem 2009, 7, 1753–1760. [DOI] [PubMed] [Google Scholar]
- [31].a) Dorel R, Grugel CP, Haydl AM, Angew. Chem., Int. Ed 2019, 58, 17118–17129; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2019, 131, 17276–17287. [Google Scholar]; b) Forero-Cortés PA, Haydl AM, Org. Process Res. Dev 2019, 23, 1478–1483. [Google Scholar]; c) Ruiz-Castillo P, Buchwald SL, Chem. Rev 2016, 116, 12564–12649. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Okano K, Tokuyama H, Fukuyama T, Chem. Commun 2014, 50, 13650–13663. [DOI] [PubMed] [Google Scholar]; e) Bhunia S, Pawar GG, Kumar SV, Jiang Y, Ma D, Angew. Chem., Int. Ed 2017, 56, 16136–16179; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2017, 129, 16352–16397. [Google Scholar]
- [32].Other approaches to adamantyl coupling with aryl halides.; a) Zhang P, Le CC, MacMillan DWC, J. Am. Chem. Soc 2016, 138, 8084–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sato Y, Nakamura K, Sumida Y, Hashizume D, Hosoya T, Ohmiya H, J. Am. Chem. Soc 2020, 142, 9938–9943. [DOI] [PubMed] [Google Scholar]
- [33].Li H, Sheng J, Liao G-X, Wu B-B, Ni H-Q, Li Y, Wang X-S, Adv. Synth. Catal 2020, 362, 5363–5367. [Google Scholar]
- [34].Best current approach is using MOM-BF3K.; Molander GA, Canturk B, Org. Lett 2008, 10, 2135–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].A search of the Beilstein database via REAXYS turned up only a few examples of nucleophilic substitution and no transition-metal catalyzed coupling reactions of alkyl bromide 8.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


