Abstract
Context:
Limited data are available to guide cardiovascular screening in adult or masters athletes (≥35 years old). This review provides recommendations and the rationale for the cardiovascular risk assessment of older athletes.
Evidence Acquisition:
Review of available clinical guidelines, original investigations, and additional searches across PubMed for articles relevant to cardiovascular screening, risk assessment, and prevention in adult athletes (1990-2020).
Study Design:
Clinical review.
Level of Evidence:
Level 3.
Results:
Atherosclerotic coronary artery disease (CAD) is the leading cause of exercise-induced acute coronary syndromes, myocardial infarction, and sudden cardiac death in older athletes. Approximately 50% of adult patients who experience acute coronary syndromes and sudden cardiac arrest do not have prodromal symptoms of myocardial ischemia. The risk of atherosclerotic cardiovascular disease (ASCVD) can be estimated by using existing risk calculators. ASCVD 10-year risk is stratified into 3 categories: low-risk (≤10%), intermediate-risk (between 10% and 20%), and high-risk (≥20%). Coronary artery calcium (CAC) scoring with noncontrast computed tomography provides a noninvasive measure of subclinical CAD. Evidence supports a significant association between elevated CAC and the risk of future cardiovascular events, independent of traditional risk factors or symptoms. Statin therapy is recommended for primary prevention if 10-year ASCVD risk is ≥10% (intermediate- or high-risk patients) or if the Agatston score is >100 or >75th percentile for age and sex. Routine stress testing in asymptomatic, low-risk patients is not recommended.
Conclusion:
We propose a comprehensive risk assessment for older athletes that combines conventional and novel risk factors for ASCVD, a 12-lead resting electrocardiogram, and a CAC score. Available risk calculators provide a 10-year estimate of ASCVD risk allowing for risk stratification and targeted management strategies. CAC scoring can refine risk estimates to improve the selection of patients for initiation or avoidance of pharmacological therapy.
Keywords: prevention, cardiovascular disease, coronary artery disease
Higher levels of physical activity and fitness are associated with lower all-cause mortality, cardiovascular disease (CVD), and prevalence of several known malignancies.33,36,37,40,43,62,65,74,75,80 Despite the substantial health benefits provided by regular physical activity, intense exercise may paradoxically act as a trigger for life-threatening ventricular arrhythmias in the presence of underlying CVD. Sudden cardiac death (SCD) is the leading cause of sport- and exercise-related mortality in athletes.10,29,47
Preparticipation cardiovascular screening aimed at the detection of disorders associated with SCD is universally supported by major medical societies.3,11,15,48,49 However, limited data are available to guide recommendations for the cardiovascular risk assessment of older or masters athletes (≥35 years old). We present our program and rationale for the cardiovascular risk assessment of older athletes.
SCD in Older Athletes
Screening strategies must be tailored to the target population and the specific disorders at highest risk. SCD in young athletes is caused by a variety of structural and electrical disorders of the heart, including cardiomyopathies, ion channel disorders, coronary anomalies, and acquired cardiac conditions.23,29,61 In adult and senior athletes, atherosclerotic coronary artery disease (CAD) is the primary condition leading to major adverse cardiovascular events.17,23
Exercise-induced acute coronary syndromes result from atherosclerotic plaque disruption and coronary thrombosis in most athletes.26,77 Approximately 50% of patients who experience acute myocardial infarction and sudden cardiac arrest (SCA) do not have preexisting symptoms or a known history of CAD.46,56 In long-term endurance athletes, SCA and myocardial ischemia also can occur from “demand” ischemia because of an imbalance between oxygen supply and demand resulting from stable calcified plaque and a fixed coronary stenosis. 39 In a study of US marathon and half-marathon races, 39 none of the runners with SCA with serious coronary atherosclerosis had angiographic evidence of acute plaque rupture or thrombus. In the US military, the annual risk of SCD was 1 in 7300 for active personnel ≥35 years old and increased with age, approaching 1 in 1000 around age 50 years. 17
In athletes aged ≥35 years, more than 80% of all SCD is due to atheromatous CAD, and vigorous physical exertion is associated with an increased risk of acute myocardial infarction and SCD.26,45,53,57,76,78,83 The athletes at greatest risk are those with little or no background in systematic training, and only 50% of middle-aged athletes who die suddenly from atherosclerotic CAD have prodromal symptoms suggestive of myocardial ischemia.2,53,76 Thus, a cardiovascular risk assessment aimed at detecting occult CAD is necessary to effectively identify and mitigate CVD risk in older athletes.
Cardiovascular Risk Assessment
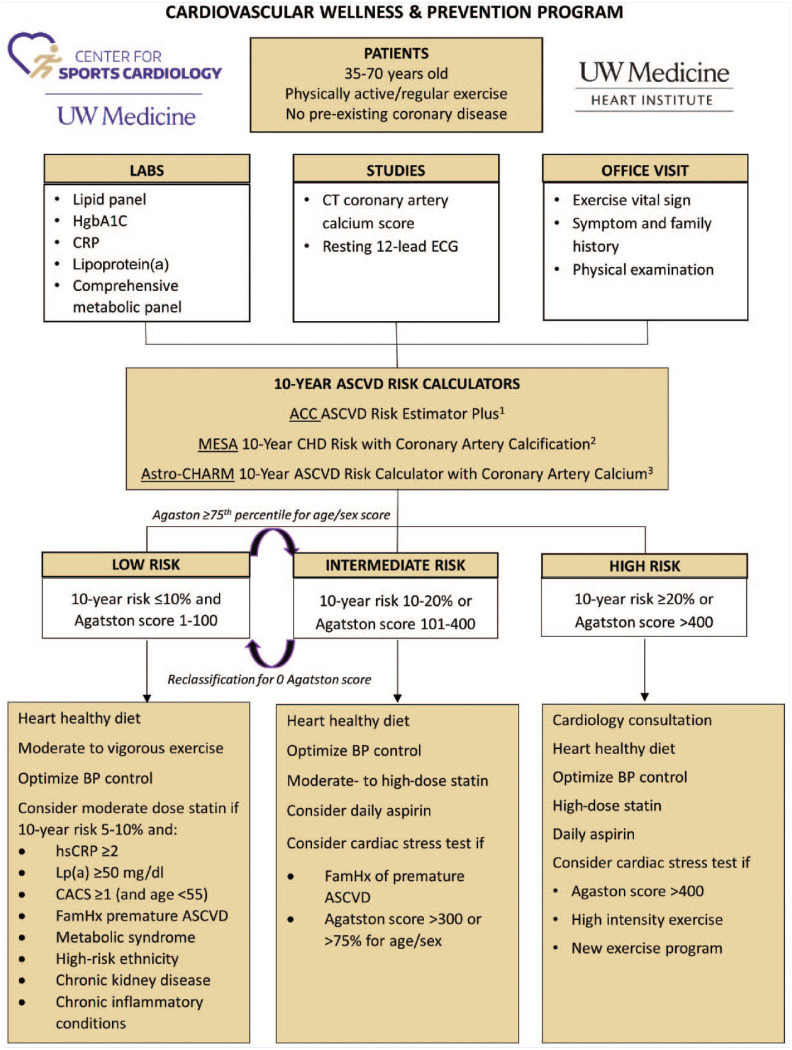
The patient’s risk of atherosclerotic cardiovascular disease (ASCVD) should be stratified into high-, intermediate-, or low-risk categories by estimating the 10-year risk of heart attack or stroke using existing risk calculators (Figure 1). A 10-year risk ≥20% is high risk, ≤10% is low risk, and between 10% and 20% is intermediate risk. The most comprehensive risk assessment combines a coronary artery calcium (CAC) score with conventional risk markers for ASCVD. CAC scoring with noncontrast computed tomography provides a noninvasive measure of subclinical CAD and offers a promising method to assess ASCVD risk in older athletes.
Figure 1.
Cardiovascular wellness and prevention program. ACC, American College of Cardiology; ASCVD, atherosclerotic cardiovascular disease; Astro-CHARM, Astronaut Cardiovascular Health and Risk Modification; BP, blood pressure; CACS, coronary artery calcium score; CHD, coronary heart disease; CRP, C-reactive protein; CT, computed tomography; ECG, electrocardiogram; FamHx, family history; HgbA1C, hemoglobin A1C; hsCRP, high-sensitivity C-reactive protein; Lp(a), lipoprotein(a); MESA, Multi-Ethnic Study of Atherosclerosis.
1 http://tools.acc.org/ASCVD-Risk-Estimator-Plus/#!/calculate/estimate/
2 https://www.mesa-nhlbi.org/MESACHDRisk/MesaRiskScore/RiskScore.aspx
The conventional cardiovascular risk assessment was derived from the Framingham study and subsequently refined with other large cohort studies. Standard elements in a risk assessment include age, sex, race, diabetes status, blood pressure (BP) or treated hypertension, lipid values, and tobacco use. It is recommended that 10-year ASCVD risk is calculated for all patients. The Framingham risk calculator is a well-validated tool, but only predicts the risk of heart attack and was derived from a less diverse cohort and so is less generalizable. 85 The American College of Cardiology/American Heart Association (ACC/AHA) Pooled Cohort Risk Calculator was developed utilizing multiple and more diverse cohorts, and incorporates stroke as an end point in addition to myocardial infarction. 27 The ACC/AHA risk calculator has been shown to overestimate risk, to variable degrees depending on the cohort studied.13,54,55,66 Other available risk calculators include Astronaut Cardiovascular Health and Risk Modification (Astro-CHARM) and Multi-Ethnic Study of Atherosclerosis (MESA), each of which incorporates the CAC score in addition to traditional risk factors.38,50 The Astro-CHARM calculator includes stroke, whereas the MESA calculator only predicts coronary heart disease risk. When a coronary calcium scan is available, the Astro-CHARM and MESA calculators provide the most comprehensive estimate of ASCVD risk. If a coronary calcium scan is not available and only conventional risk factors are known, the ACC/AHA risk estimator should be used.
Risk Enhancers
Standard risk assessment can be modified by identifying risk-enhancing factors. This is most advantageous for intermediate-risk patients who may be reclassified in a way that influences management decisions (eg, initiating statin therapy). Commonly used ancillary laboratory tests include high-sensitivity C-reactive protein (hsCRP), lipoprotein(a) (Lp(a)), and advanced lipoprotein analysis (cholesterol particle size and number). Risk-enhancing factors according to the ACC/AHA guidelines are shown in Table 1. 28
Table 1.
Risk-enhancing factors
| • Family history of premature ASCVD (male <55 years, female <65 years) |
| • Primary hypercholesterolemia (LDL-C 160-189 mg/dL, non-HDL-C 190-219 mg/dL) |
| • Metabolic syndrome |
| • Chronic kidney disease (eGFR 15-59 mL/min/1.73 m2, not on dialysis) |
| • Chronic inflammatory conditions such as psoriasis, RA, or HIV |
| • History of premature menopause and pregnancy-associated conditions such as preeclampsia |
| • High-risk race/ethnicities (eg, South Asian) |
| • Lipid/biomarkers |
| ○ Persistently elevated triglycerides ≥175 mg/dL |
| ○ Elevated hsCRP ≥2 mg/dL |
| ○ Elevated Lp(a) ≥50 mg/dL or ≥125 nmol/L |
| ○ Elevated ApoB ≥130 mg/dL |
| ○ ABI <0.9 |
ABI, ankle-brachial index; ApoB, apolipoprotein B; ASCVD, atherosclerotic cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); RA, rheumatoid arthritis.
High-Sensitivity C-Reactive Protein
hsCRP is a nonspecific serum marker of inflammation that is associated with increased risk of ASCVD events. 67 hsCRP is associated with several other CV risk factors and inflammatory markers; and, after adjustment for conventional risk factors, hsCRP is modestly associated with coronary heart disease and ischemic stroke.20,21 The Reynolds risk score is a validated CV risk calculator that incorporates hsCRP and has been shown to have better calibration and discrimination when compared with the Framingham model.9,68,72 The JUPITER trial tested the hypothesis that rosuvastatin would reduce CV events in patients with nonelevated low-density lipoprotein (LDL; <130 mg/dL) and hsCRP >2 mg/L. 69 The study population was intermediate risk (event rate ~1% per year, or estimated 10% event rate over 10 years). The trial was stopped early because of a statistically significant relative risk reduction of the composite primary end point (HR 0.56, 95% CI 0.46-0.69, P < 0.001) and all-cause mortality (HR 0.8, 95% CI 0.67-0.97, P = 0.02). 71 For the hard end point of myocardial infarction, stroke, or death, the 5-year number needed to treat was 29. 71 The CANTOS trial of canikinumab, an interleukin inhibitor, also showed that treatment significantly decreased ASCVD events in patients with prior myocardial infarction and high hsCRP. 70 Because hsCRP is a nonspecific marker of inflammation, interpretation can be confounded by concurrent illness.
Lipoprotein(a)
Lp(a) is an atherogenic, pro-inflammatory LDL-like lipoprotein composed of apolipoprotein B-100 (ApoB) covalently bound to apolipoprotein(a). 79 Levels are genetically determined, with minimal dietary or environmental influences. 79 Genetic and epidemiologic studies have identified Lp(a) as an independent risk factor for ASCVD, and strongly suggest causality. When assessed as a continuous variable, there is a 16% relative risk increase per standard deviation above the mean. 19 American and European lipid guidelines define a level ≥50 mg/dL as abnormal, while the Canadian guidelines consider a level>30 mg/dL a risk factor. 79 There are no Food and Drug Administration–approved therapies to specifically lower Lp(a), but niacin and PCSK9 inhibitors have been shown to lower levels by 20% to 30%.34,64 Statins increase Lp(a), but are still recommended for net benefit of LDL cholesterol (LDL-C) lowering. 79 Utilization of Lp(a) is not well defined in routine clinical practice, but can be useful to inform treatment decisions in patients with a strong family history of premature atherosclerosis but who otherwise have low or intermediate risk based on conventional risk markers. In an exploratory analysis of the FOURIER trial of evolocumab in secondary prevention, there was a significant association between achieved Lp(a) level and major CV events. 58 Elevated Lp(a) was not required for enrollment in the PCSK9 inhibitor outcome trials, and targeted studies for patients with high Lp(a) are underway. 73
Advanced Lipoprotein Testing
Advanced lipoprotein panels report a panel of lipid subfractions, including LDL particle size, particle number, and ApoB. LDL particle number is independently associated with atherosclerosis, and some athletes have discordant lipid profiles where LDL-C concentration is relatively normal but LDL particle number is high.12,59 Studies31,44 have failed to show increased risk discrimination when added to a conventional risk assessment. Neither the ACC/AHA prevention guidelines nor the cholesterol treatment guidelines recommend testing LDL particle size or number.4,28 Non-HDL-C levels derived from a standard lipid panel have nearly identical risk prediction compared with ApoB levels, and the National Lipid Association endorse either as an optional secondary target after LDL-C.18,35 The European Society of Cardiology lipid guidelines recommend ApoB testing, particularly in people with high triglycerides, diabetes, obesity, metabolic syndrome, or very low LDL-C. 42
CAC Scan
CAC scoring quantifies the “burden” of calcified atherosclerotic plaque and provides prognostic value. 32 CAC is quantified as the Agatston score; score >400 is severe, 101 to 400 is moderate, 11 to 100 is mild, and 1 to 10 is minimal. Robust evidence supports a significant association between elevated CAC and the risk of future ASCVD, independent of traditional risk factors or symptoms. 30 Thus, establishing the presence and severity of CAC may help identify athletes most likely to benefit from medical therapy for the primary prevention of adverse cardiovascular outcomes. CAC scoring can also reclassify risk estimates and improve selection of patients for initiation or avoidance of statin therapy.41,82 CAC score is most useful when used in intermediate risk patients, for whom a low score would reclassify them as low risk or a high score would reclassify them as high risk.
When interpreting a CAC score in an athlete, it is important to consider the differences that have been observed in athletes compared with nonathletic cohorts. Studies1,51 of endurance athletes not only demonstrate a higher prevalence of CAC compared with matched nonathletes with similar risk profiles but also suggest coronary plaque composition in athletes is more benign and comprised of more calcified and stable plaque. In a study 51 of masters endurance athletes with a low Framingham risk score, 11% of subjects had a high CAC score (≥300), and 7.5% had luminal stenosis ≥50%. In a large study 14 of 21,758 healthy men from the Cooper Clinic preventive medicine facility, higher levels of self-reported physical activity were associated with higher CAC, but in the high-activity cohort (≥3000 MET-min/week), CAC score was not associated with all-cause or CVD mortality. In addition, the study found that for every level of CAC, higher cardiovascular fitness was associated with lower CVD mortality. The converse was also true; for any level of cardiovascular fitness, a lower CAC score was associated with a lower risk of cardiovascular events. 14 Exercise appears to influence plaque composition, and this may be 1 of the mechanisms for attenuated cardiovascular risk from exercise. It may be that CAC is a useful risk assessment tool for sedentary patients and low-volume exercisers, but has reduced discrimination for high-volume exercisers and competitive endurance athletes.
Management
Key points for the cardiovascular risk assessment and management of older athletes are summarized in Table 2.
Table 2.
Key points in the cardiovascular risk assessment and management of older athletes
| • In adult athletes ≥35 years old, CAD is the primary cause of major adverse cardiovascular events. |
| • A CAC scan quantifies the burden of calcified atherosclerotic plaque and provides important prognostic value. |
| • A comprehensive risk assessment for CVD in older athletes includes a review of conventional risk markers, physical examination, 12-lead ECG, and CAC score. |
| • 10-year risk of ASCVD should be estimated for adult athletes using a validated risk calculator such as the ACC/AHA, Astro-CHARM, or MESA calculators. |
| • CAC scoring or risk-enhancing factors such as hsCRP and Lp(a) may be used to reclassify or revise the risk assessment. |
| • Studies of endurance athletes not only demonstrate higher prevalence of CAC compared with nonathletes but also suggest coronary plaque composition in athletes is more stable and may not confer the same risk. |
| • Lifestyle interventions are recommended to optimize cardiovascular health. |
| • Blood pressure should be optimally controlled to reduce CVD risk. |
| • Statin therapy is recommended if 10-year risk of ASCVD is ≥10% and considered if risk is ≥7.5%. |
| • Low-dose aspirin 75-100 mg daily can be considered for athletes with high ASCVD risk and low bleeding risk. |
| • Stress testing is recommended for athletes with symptoms concerning for ischemic heart disease. |
| • Stress testing may be considered for asymptomatic athletes with high ASCVD risk (eg, markedly elevated CAC score ≥400). |
| • Echocardiography or cardiac MRI is recommended for athletes with physical examination or ECG abnormalities concerning for valvular heart disease or cardiomyopathy. |
ACC/AHA, American College of Cardiology/American Heart Association; ASCVD, atherosclerotic cardiovascular disease; Astro-CHARM, Astronaut Cardiovascular Health and Risk Modification; CAC, coronary artery calcium; CAD, coronary artery disease; CVD, cardiovascular disease; ECG, electrocardiogram; hsCRP, high-sensitivity C-reactive protein; Lp(a), lipoprotein(a); MESA, Multi-Ethnic Study of Atherosclerosis; MRI, magnetic resonance imaging.
Lifestyle Interventions
Adult athletes should be counseled on lifestyle interventions to improve their cardiovascular health. Dietary recommendations can be tailored to the specific athlete, but there is strong evidence that a Mediterranean or plant-based diet low in saturated fats reduces ASCVD risk.7,22 A low-salt Dietary Approaches to Stop Hypertension dietary pattern is recommended for those with high BP. 4 The relationship between exercise volume and intensity and cardiovascular risk is complex, and an in-depth discussion is outside the scope of this review. ACC/AHA guidelines recommend at least 150 minutes of moderate-intensity, or 75 minutes of vigorous-intensity exercise per week for optimal cardiovascular health. 4 Assisting patients to safely improve cardiorespiratory fitness may have additional benefit as higher levels of cardiorespiratory fitness are associated with longevity and lower all-cause mortality. 43 All adults should be screened for tobacco use, and tobacco status should be reported as a vital sign. 4 Tobacco avoidance is urged, and a combination of behavior therapy and pharmacotherapy is indicated to maximize quit rates (Class I, LOE [level of evidence] A). 4
Medical Therapy
Statin therapy is recommended for primary prevention if 10-year ASCVD risk is ≥10% (intermediate- or high-risk patients). The ACC/AHA lipid guidelines advocate for statin therapy if 10-year risk is ≥7.5%, but this lower threshold is controversial and only given a C grade by the US Preventive Services Task Force. 8 If not already performed, the ACC/AHA lipid guidelines suggest CAC scoring be considered if uncertainty remains about statin therapy after standard risk assessment (Class IIa, LOE B-NR recommendation). 4 A large cohort study of patients without known ASCVD demonstrated that patients with a moderate- (101-400) or high-risk (≥400) CAC score who received statin therapy had a significant reduction of major adverse cardiac events during the study period, although there was no risk reduction for statin-treated patients who had a CAC score of zero. 52 The ACC/AHA cholesterol treatment guidelines recommend statin therapy if the Agatston score is >100 or >75th percentile for age and sex, and to consider statin therapy for an Agatston score of 1 to 99 and age <55 years (Class IIa, LOE B-NR). 28 These recommendations are based on sparse nonrandomized data, but studies of patients with calcium scores in this range have shown cardiovascular event rates that would justify statin therapy for primary prevention.
When statin therapy is indicated for primary prevention, the ACC/AHA guidelines recommend initiating moderate-intensity statin with a goal to lower LDL ≥30%. 28 The European Society of Cardiology lipid guidelines do not make a distinction between primary and secondary prevention, and recommend statin therapy with LDL goal <70 mg/dL for high-risk patients, and LDL goal <55 mg/dL for very high-risk patients. 42 Coenzyme Q 10 (Co-q10) supplementation is not recommended for routine use (class III recommendation), but may be helpful to ameliorate muscle-related symptoms in statin users. Small placebo-controlled trials of Co-q10 have had equivocal results, but recent systematic reviews suggests benefit.6,63
BP control is recommended if BP >130/80 mm Hg, with a focus on lifestyle interventions for stage 1 hypertension (systolic BP 130-139 mm Hg or diastolic BP 80-89 mm Hg) and addition of pharmacotherapy for stage 2 hypertension (systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg). 84 We favor angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or dihydropyridine calcium channel blockers (eg, amlodipine) as first-line agents since they do not affect heart rate or negatively affect cardiac output. Beta blockers are generally avoided for first-line antihypertensive therapy because of negative chronotropic and inotropic effects, and studies suggesting better CV risk reduction with other antihypertensive classes. In the absence of compelling indications such as systolic heart failure or ischemic heart disease with prior myocardial infarction or angina, beta blockers are no longer recommended as first-line BP agents by the ACC/AHA. 84
There is declining enthusiasm for the use of aspirin in primary prevention. The ASCEND trial of primary prevention aspirin use in patients with diabetes demonstrated that reduction in vascular events was offset by increased major bleeding, with minimal net clinical benefit. 5 The ARRIVE trial of primary prevention aspirin use in patients without diabetes also showed no reduction in vascular events compared with placebo. 25 ARRIVE investigators intended to enroll intermediate risk patients, but in actuality enrolled a low risk population and the trial was limited by low event rates. Thus, primary prevention aspirin use is not recommended for patients at low or intermediate risk. According to ACC/AHA prevention guidelines, a daily low-dose aspirin may be considered for primary prevention in select patients at higher ASCVD risk and who are not at increased risk of bleeding. 4
Resting 12-Lead Electrocardiogram
A resting 12-lead electrocardiogram (ECG) is recommended in the cardiovascular risk assessment of older athletes. Cardiomyopathies and primary electrical diseases such as ion channel disorders account for approximately 20% of SCD cases in athletes ≥35 years old. ECG screening may also identify atrioventricular blocks and occult supraventricular arrhythmias such as atrial fibrillation that are more common in older athletes and require additional investigation. Contemporary ECG interpretation guidelines can be applied to older athletes to distinguish physiological cardiac adaptations from ECG findings suggestive of a pathologic cardiac disorder, although a lower threshold to pursue additional testing for changes suggesting ischemic heart disease should be used. 16
Stress Testing and Cardiac Imaging
Routine stress testing in asymptomatic, low-risk adults is not recommended for the general population and rarely considered an appropriate screening tool for ischemic heart disease in athletes.60,86 Stress testing in asymptomatic adults has a low positive predictive value and a high false-positive rate given most acute coronary events are attributable to nonobstructive stenosis that is unlikely to be identified by exercise testing. 24 In 1 systematic review 81 of preparticipation exercise testing, the prevalence of an abnormal stress test was 5% in athletes aged 35 to 60 years, and 8.5% in athletes >60 years old. We consider stress testing in asymptomatic athletes if the 10-year ASCVD risk estimate or CAC score is high risk and the athlete is engaging in strenuous sports or exercise.
For an athlete with symptoms concerning for ischemic heart disease, stress testing is strongly recommended. If the 12-lead ECG is normal, an exercise treadmill test (without imaging) is appropriate. 86 If the patient has left bundle branch block or other ECG abnormalities that would reduce the sensitivity or specificity of the exercise ECG such as resting T wave inversion or ST segment depression, then stress imaging is recommended (eg, treadmill echocardiogram or treadmill nuclear study). 86
Cardiac imaging with an echocardiogram and possibly cardiac MRI is recommended if the history, physical examination, or ECG are concerning for structural heart disease such as hypertrophic cardiomyopathy, dilated cardiomyopathy, arrhythmogenic cardiomyopathy, or valvular heart disease.
Conclusion
CAD is the leading cause of SCD in older athletes. A conventional risk assessment combined with CAC scoring provides the most accurate assessment of ASCVD risk and can be refined by risk-enhancing factors or ancillary testing as indicated. Available risk calculators provide a 10-year estimate of CVD risk allowing for risk stratification and targeted management strategies. A CAC score may be used to reclassify risk and inform treatment decisions. Lifestyle interventions and optimal BP can improve cardiovascular health in all patients, and statin therapy is recommended in patients with intermediate or high 10-year ASCVD risk. Stress testing is recommended for symptomatic athletes and may be considered in high-risk athletes or among those engaged in strenuous exercise.
Footnotes
The following author declared potential conflicts of interest: E.Y. received grants from Amgen.
References
- 1. Aengevaeren VL, Mosterd A, Braber TL, et al. Relationship between lifelong exercise volume and coronary atherosclerosis in athletes. Circulation. 2017;136:138-148. [DOI] [PubMed] [Google Scholar]
- 2. Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343:1355-1361. [DOI] [PubMed] [Google Scholar]
- 3. American Academy of Family Physicians, American Academy of Pediatrics, American College of Sports Medicine, et al. Preparticipation Physical Evaluation. 4th ed. American Academy of Pediatrics; 2010. [Google Scholar]
- 4. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:1376-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539. [DOI] [PubMed] [Google Scholar]
- 6. Banach M, Serban C, Sahebkar A, et al. ; Lipid and Blood Pressure Meta-analysis Collaboration Group. Effects of coenzyme Q10 on statin-induced myopathy: a meta-analysis of randomized controlled trials. Mayo Clin Proc. 2015;90:24-34. [DOI] [PubMed] [Google Scholar]
- 7. Chiavaroli L, Nishi SK, Khan TA, et al. Portfolio dietary pattern and cardiovascular disease: a systematic review and meta-analysis of controlled trials. Prog Cardiovasc Dis. 2018;61:43-53. [DOI] [PubMed] [Google Scholar]
- 8. Chou R, Dana T, Blazina I, et al. Statin Use for the Prevention of Cardiovascular Disease in Adults: A Systematic Review for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality; 2016. [PubMed] [Google Scholar]
- 9. Cook NR, Paynter NP, Eaton CB, et al. Comparison of the Framingham and Reynolds risk scores for global cardiovascular risk prediction in the multiethnic Women’s Health Initiative. Circulation. 2012;125:1748-1756, S1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003;42:1959-1963. [DOI] [PubMed] [Google Scholar]
- 11. Corrado D, Pelliccia A, Bjørnstad HH, et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26:516-524. [DOI] [PubMed] [Google Scholar]
- 12. Cromwell WC, Otvos JD, Keyes MJ, et al. LDL particle number and risk of future cardiovascular disease in the Framingham Offspring Study—implications for LDL management. J Clin Lipidol. 2007;1:583-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162:266-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeFina LF, Radford NB, Barlow CE, et al. Association of all-cause and cardiovascular mortality with high levels of physical activity and concurrent coronary artery calcification. JAMA Cardiol. 2019;4:174-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drezner JA, O’Connor FG, Harmon KG, et al. AMSSM Position Statement on Cardiovascular Preparticipation Screening in Athletes: current evidence, knowledge gaps, recommendations and future directions. Br J Sports Med. 2017;51:153-167. [DOI] [PubMed] [Google Scholar]
- 16. Drezner JA, Sharma S, Baggish A, et al. International criteria for electrocardiographic interpretation in athletes: consensus statement. Br J Sports Med. 2017;51:704-731. [DOI] [PubMed] [Google Scholar]
- 17. Eckart RE, Shry EA, Burke AP, et al. ; Department of Defense Cardiovascular Death Registry Group. Sudden death in young adults an autopsy-based series of a population undergoing active surveillance. J Am Coll Cardiol. 2011;58:1254-1261. [DOI] [PubMed] [Google Scholar]
- 18. Emerging Risk Factors Collaboration, Di Angelantonio E, Sarwar N, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Emerging Risk Factors Collaboration, Erqou S, Kaptoge S, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302412-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl JMed. 2018;378:e34. [DOI] [PubMed] [Google Scholar]
- 23. Finocchiaro G, Papadakis M, Robertus JL, et al. Etiology of sudden death in sports: insights from a United Kingdom regional registry. J Am Coll Cardiol. 2016;67:2108-2115. [DOI] [PubMed] [Google Scholar]
- 24. Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873-934. [DOI] [PubMed] [Google Scholar]
- 25. Gaziano JM, Brotons C, Coppolecchia R, et al. ; ARRIVE Executive Committee. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giri S, Thompson PD, Kiernan FJ, et al. Clinical and angiographic characteristics of exertion-related acute myocardial infarction. JAMA. 1999;282:1731-1736. [DOI] [PubMed] [Google Scholar]
- 27. Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2935-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harmon KG, Asif IM, Maleszewski JJ, et al. Incidence, cause, and comparative frequency of sudden cardiac death in National Collegiate Athletic Association athletes: a decade in review. Circulation. 2015;132:10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hecht HS, Cronin P, Blaha MJ, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Thorac Imaging. 2017;32:W54-W66. [DOI] [PubMed] [Google Scholar]
- 31. Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hou ZH, Lu B, Gao Y, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990-999. [DOI] [PubMed] [Google Scholar]
- 33. Hussain N, Gersh BJ, Gonzalez Carta K, et al. Impact of cardiorespiratory fitness on frequency of atrial fibrillation, stroke, and all-cause mortality. Am J Cardiol. 2018;121:41-49. [DOI] [PubMed] [Google Scholar]
- 34. Jacobson TA. Lipoprotein(a), cardiovascular disease, and contemporary management. Mayo Clin Proc. 2013;88:1294-1311. [DOI] [PubMed] [Google Scholar]
- 35. Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 1—full report. J Clin Lipidol. 2015;9:129-169. [DOI] [PubMed] [Google Scholar]
- 36. Juraschek SP, Blaha MJ, Blumenthal RS, et al. Cardiorespiratory fitness and incident diabetes: the FIT (Henry Ford ExercIse Testing) project. Diabetes Care. 2015;38:1075-1081. [DOI] [PubMed] [Google Scholar]
- 37. Juraschek SP, Blaha MJ, Whelton SP, et al. Physical fitness and hypertension in a population at risk for cardiovascular disease: the Henry Ford ExercIse Testing (FIT) project. J Am Heart Assoc. 2014;3:e001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khera A, Budoff MJ, O’Donnell CJ, et al. Astronaut Cardiovascular Health and Risk Modification (Astro-CHARM) coronary calcium atherosclerotic cardiovascular disease risk calculator. Circulation. 2018;138:1819-1827. [DOI] [PubMed] [Google Scholar]
- 39. Kim JH, Malhotra R, Chiampas G, et al. Cardiac arrest during long-distance running races. N Engl J Med. 2012;366:130-140. [DOI] [PubMed] [Google Scholar]
- 40. Kyu HH, Bachman VF, Alexander LT, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lloyd-Jones DM, Braun LT, Ndumele CE, et al. Use of risk assessment tools to guide decision-making in the primary prevention of atherosclerotic cardiovascular disease: a special report from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2019;73:3153-3167. [DOI] [PubMed] [Google Scholar]
- 42. Mach F, Baigent C, Catapano AL, et al. ; ESC Scientific Document Group. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. [DOI] [PubMed] [Google Scholar]
- 43. Mandsager K, Harb S, Cremer P, Phelan D, Nissen SE, Jaber W. Association of cardiorespiratory fitness with long-term mortality among adults undergoing exercise treadmill testing. JAMA Netw Open. 2018;1:e183605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Manickam P, Rathod A, Panaich S, et al. Comparative prognostic utility of conventional and novel lipid parameters for cardiovascular disease risk prediction: do novel lipid parameters offer an advantage? J Clin Lipidol. 2011;5:82-90. [DOI] [PubMed] [Google Scholar]
- 45. Marijon E, Tafflet M, Celermajer DS, et al. Sports-related sudden death in the general population. Circulation. 2011;124:672-681. [DOI] [PubMed] [Google Scholar]
- 46. Marijon E, Uy-Evanado A, Dumas F, et al. Warning symptoms are associated with survival from sudden cardiac arrest. Ann Intern Med. 2016;164:23-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009;119:1085-1092. [DOI] [PubMed] [Google Scholar]
- 48. Maron BJ, Friedman RA, Kligfield P, et al. Assessment of the 12-lead electrocardiogram as a screening test for detection of cardiovascular disease in healthy general populations of young people (12-25 years of age): a scientific statement from the American Heart Association and the American College of Cardiology. J Am Coll Cardiol. 2014;64:1479-1514. [DOI] [PubMed] [Google Scholar]
- 49. Maron BJ, Levine BD, Washington RL, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 2: preparticipation screening for cardiovascular disease in competitive athletes: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66:2356-2361. [DOI] [PubMed] [Google Scholar]
- 50. McClelland RL, Jorgensen NW, Budoff M, et al. 10-Year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J Am Coll Cardiol. 2015;66:1643-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Merghani A, Maestrini V, Rosmini S, et al. Prevalence of subclinical coronary artery disease in masters endurance athletes with a low atherosclerotic risk profile. Circulation. 2017;136:126-137. [DOI] [PubMed] [Google Scholar]
- 52. Mitchell JD, Fergestrom N, Gage BF, et al. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J Am Coll Cardiol. 2018;72:3233-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N Engl J Med. 1993;329:1677-1683. [DOI] [PubMed] [Google Scholar]
- 54. Mora S, Wenger NK, Cook NR, et al. Evaluation of the pooled cohort risk equations for cardiovascular risk prediction in a multiethnic cohort from the Women’s Health Initiative. JAMA Intern Med. 2018;178:1231-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease pooled cohort risk equations. JAMA. 2014;311:1406-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nehme Z, Bernard S, Andrew E, Cameron P, Bray JE, Smith K. Warning symptoms preceding out-of-hospital cardiac arrest: do patient delays matter? Resuscitation. 2018;123:65-70. [DOI] [PubMed] [Google Scholar]
- 57. Noakes TD, Opie LH, Rose AG, Kleynhans PH, Schepers NJ, Dowdeswell R. Autopsy-proved coronary atherosclerosis in marathon runners. N Engl J Med. 1979;301:86-89. [DOI] [PubMed] [Google Scholar]
- 58. O’Donoghue ML, Fazio S, Giugliano RP, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139:1483-1492. [DOI] [PubMed] [Google Scholar]
- 59. Otvos JD, Mora S, Shalaurova I, Greenland P, Mackey RH, Goff DC., Jr. Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J Clin Lipidol. 2011;5:105-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Peritz DC, Chung EH, Ryan JJ. The role of stress testing in the older athlete. Accessed December 2, 2017. http://www.acc.org/latest-in-cardiology/articles/2017/11/06/10/32/the-role-of-stress-testing-in-the-older-athlete
- 61. Peterson DF, Siebert DM, Kucera KL, et al. Etiology of sudden cardiac arrest and death in US competitive athletes: a 2-year prospective surveillance study. Clin J Sport Med. 2020;30:305-314. [DOI] [PubMed] [Google Scholar]
- 62. Powell KE, King AC, Buchner DM, et al. The scientific foundation for the physical activity guidelines for Americans, 2nd edition. J Phys Act Health. 2018:1-11. [DOI] [PubMed] [Google Scholar]
- 63. Qu H, Guo M, Chai H, Wang WT, Gao ZY, Shi DZ. Effects of coenzyme Q10 on statin-induced myopathy: an updated meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7:e009835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Raal FJ, Giugliano RP, Sabatine MS, et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol. 2014;63:1278-1288. [DOI] [PubMed] [Google Scholar]
- 65. Radford NB, DeFina LF, Leonard D, et al. Cardiorespiratory fitness, coronary artery calcium, and cardiovascular disease events in a cohort of generally healthy middle-age men: results from the Cooper Center Longitudinal Study. Circulation. 2018;137:1888-1895. [DOI] [PubMed] [Google Scholar]
- 66. Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67:2118-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363-369. [DOI] [PubMed] [Google Scholar]
- 68. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611-619. [DOI] [PubMed] [Google Scholar]
- 69. Ridker PM, Danielson E, Fonseca FA, et al. ; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195-2207. [DOI] [PubMed] [Google Scholar]
- 70. Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119-1131. [DOI] [PubMed] [Google Scholar]
- 71. Ridker PM, MacFadyen JG, Fonseca FA, et al. ; JUPITER Study Group. Number needed to treat with rosuvastatin to prevent first cardiovascular events and death among men and women with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (JUPITER). Circ Cardiovasc Qual Outcomes. 2009;2:616-623. [DOI] [PubMed] [Google Scholar]
- 72. Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rosenson RS, Hegele RA, Koenig W. Cholesterol-lowering agents. Circ Res. 2019;124:364-385. [DOI] [PubMed] [Google Scholar]
- 74. Shah RV, Murthy VL, Colangelo LA, et al. Association of fitness in young adulthood with survival and cardiovascular risk: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA Intern Med. 2016;176:87-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shiroma EJ, Lee IM. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation. 2010;122:743-752. [DOI] [PubMed] [Google Scholar]
- 76. Siscovick DS, Weiss NS, Fletcher RH, Lasky T. The incidence of primary cardiac arrest during vigorous exercise. N Engl J Med. 1984;311:874-877. [DOI] [PubMed] [Google Scholar]
- 77. Thompson PD. Exercise prescription and proscription for patients with coronary artery disease. Circulation. 2005;112:2354-2363. [DOI] [PubMed] [Google Scholar]
- 78. Thompson PD, Funk EJ, Carleton RA, Sturner WQ. Incidence of death during jogging in Rhode Island from 1975 through 1980. JAMA. 1982;247: 2535-2538. [PubMed] [Google Scholar]
- 79. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692-711. [DOI] [PubMed] [Google Scholar]
- 80. Vainshelboim B, Muller J, Lima RM, et al. Cardiorespiratory fitness and cancer incidence in men. Ann Epidemiol. 2017;27:442-447. [DOI] [PubMed] [Google Scholar]
- 81. van de Sande DA, Breuer MA, Kemps HM. Utility of exercise electrocardiography in pre-participation screening in asymptomatic athletes: a systematic review. Sports Med. 2016;46:1155-1164. [DOI] [PubMed] [Google Scholar]
- 82. van der Aalst CM, Denissen SJAM, Vonder M, et al. Screening for cardiovascular disease risk using traditional risk factor assessment or coronary artery calcium scoring: the ROBINSCA trial. Eur Heart J Cardiovasc Imaging. 2020. [DOI] [PubMed] [Google Scholar]
- 83. Waller BF, Roberts WC. Sudden death while running in conditioned runners aged 40 years or over. Am J Cardiol. 1980;45:1292-1300. [DOI] [PubMed] [Google Scholar]
- 84. Whelton PK, Carey RM, Aronow WS, et al. 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269-1324. [DOI] [PubMed] [Google Scholar]
- 85. Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837-1847. [DOI] [PubMed] [Google Scholar]
- 86. Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63:380-406. [DOI] [PubMed] [Google Scholar]