In this study, Shao et al. demonstrate that the protein–protein interaction between the transcriptional coregulator ZFP423 and brown fat determination factor EBF2 is essential for restraining the thermogenic phenotype of white adipose tissue (WAT). Using CRISPR–Cas9 gene editing to disrupt the ZFP423–EBF2 protein interaction triggers widespread “browning” of WAT in adult mice, and overall their data indicate that ZFP423 controls EBF2 coactivator recruitment and PPARγ occupancy to determine the thermogenic plasticity of adipocytes.
Keywords: EBF2, PPARγ, ZFP423, beige adipocytes, rosiglitazone, white adipocytes
Abstract
Energy-storing white adipocytes maintain their identity by suppressing the energy-burning thermogenic gene program of brown and beige adipocytes. Here, we reveal that the protein–protein interaction between the transcriptional coregulator ZFP423 and brown fat determination factor EBF2 is essential for restraining the thermogenic phenotype of white adipose tissue (WAT). Disruption of the ZFP423–EBF2 protein interaction through CRISPR–Cas9 gene editing triggers widespread “browning” of WAT in adult mice. Mechanistically, ZFP423 recruits the NuRD corepressor complex to EBF2-bound thermogenic gene enhancers. Loss of adipocyte Zfp423 induces an EBF2 NuRD-to-BAF coregulator switch and a shift in PPARγ occupancy to thermogenic genes. This shift in PPARγ occupancy increases the antidiabetic efficacy of the PPARγ agonist rosiglitazone in obesity while diminishing the unwanted weight-gaining effect of the drug. These data indicate that ZFP423 controls EBF2 coactivator recruitment and PPARγ occupancy to determine the thermogenic plasticity of adipocytes and highlight the potential of therapeutically targeting transcriptional brakes to induce beige adipocyte biogenesis in obesity.
Eutherian mammals harbor distinct types of adipocytes that differentially impact energy balance and nutrient homeostasis (Rosen and Spiegelman 2014). White adipocytes contain large, unilocular fat droplets and represent the principal site for long-term energy storage in the form of triglyceride. Brown adipocytes function to catabolize stored lipids and produce heat, thus contributing to energy expenditure. Brown adipocytes are distinguished by their multilocular lipid droplet appearance, high mitochondrial content, and expression of uncoupling protein 1 (Ucp1).
Remarkably, certain WAT depots possess the capacity to adopt a catabolic thermogenic phenotype reminiscent of brown adipose tissue (BAT) (Lončar 1991). This “browning” of WAT is characterized by the emergence of fundamentally distinct UCP1+ beige adipocytes (Wu et al. 2012). Upon exposure to cold temperatures or other settings involving activation of the β-adrenergic signaling cascade, dormant unilocular beige adipocytes become active and new beige adipocytes emerge from resident adipocyte progenitors (Barbatelli et al. 2010; Rosenwald et al. 2013; Wang et al. 2013; Lee et al. 2015; Vishvanath et al. 2016; Roh et al. 2018; Shao et al. 2019). Rodent studies indicate that thermogenic remodeling of WAT can regulate glucose and lipid homeostasis and increase energy expenditure (Seale et al. 2011; Harms and Seale 2013; Kajimura et al. 2015; Shao et al. 2016). In humans, the presence of active thermogenic adipose tissue is similarly associated with cardiometabolic benefits (Becher et al. 2021). As such, driving the production and activity of thermogenic adipocytes may be a promising strategy to improve cardiometabolic health in obesity.
The transcriptional networks controlling the establishment and maintenance of the distinct adipocyte lineages in vivo are still being defined. PPARγ, along with other differentiation-linked transcription factors, represents a core transcriptional network critical for the development and maintenance of adipocytes (Mota de Sa et al. 2017); however, additional factors operate alongside PPARγ to specify an energy-storing versus energy-burning adipocyte fate (Shao and Gupta 2019; Shapira and Seale 2019). In particular, the transcription factor early B-cell factor 2 (EBF2) activates many key thermogenic genes in adipocytes (Rajakumari et al. 2013; Wang et al. 2014; Stine et al. 2016). EBF2 recruits a tissue-selective BAF chromatin remodeling complex to thermogenic gene enhancers, thereby regulating chromatin accessibility and PPARγ occupancy (Shapira et al. 2017).
White adipocytes harbor mechanisms to actively suppress the thermogenic gene program (Villanueva et al. 2013; Pearson et al. 2019; Shao and Gupta 2019). Our group has previously identified the multi-C2H2 zinc finger transcriptional coregulator ZFP423 as a potent suppressor of the thermogenic phenotype in white adipocytes (Shao et al. 2016; Hepler et al. 2017). Inducible deletion of Zfp423 in adipocytes of adult mice housed at room temperature leads to a widespread accumulation of UCP1+ multilocular beige adipocytes within the inguinal WAT (iWAT) depot. Lineage tracing reveals that beige adipocytes in this model arise from mature Adiponectin-expressing Zfp423-deficient adipocytes. This thermogenic remodeling confers resistance to diet-induced obesity and insulin resistance. Moreover, deletion of adipocyte Zfp423 in mice with established obesity unlocks the thermogenic capacity of white adipocytes and allows pharmacological β-adrenergic receptor activation to drive weight loss (Shao et al. 2016). ZFP423 interacts with EBF2 to inhibit its ability to activate the thermogenic gene program in adipocytes (Shao et al. 2016); however, the physiological importance of this protein–protein interaction in controlling the thermogenic capacity of white adipocytes, along with the molecular mechanism by which ZFP423 regulates EBF2 transcriptional activity, has remained unclear.
Here, we demonstrate that genetic disruption of the ZFP423–EBF protein interaction through CRISPR/Cas9 gene editing is sufficient to trigger widespread thermogenic remodeling of WAT in adult mice. Biochemical and molecular approaches, including mass spectrometry, genome-wide chromatin immunoprecipitation assays (ChIP-seq), and chromatin affinity purification (ChAP-seq) of endogenous genetically tagged ZFP423, reveal that ZFP423 recruits the NuRD corepressor components to EBF2 target enhancers to suppress thermogenic gene expression in white adipocytes. Remarkably, inducible deletion of Zfp423 in mature adipocytes induces a NuRD-to-BAF complex coregulator switch onto EBF2 along with a notable shift in PPARγ occupancy to thermogenic gene enhancers. This shift in PPARγ occupancy to thermogenic genes increases the antidiabetic capacity of rosiglitazone in obesity while diminishing the weight-gaining side effects of the drug. Altogether, these data reveal that the ZFP423–EBF protein interaction is critical for suppression of the thermogenic gene program in white adipocytes via limiting EBF2 coactivator engagement and its ability to promote PPARγ occupancy at thermogenic genes. These data highlight the potential for targeting thermogenic transcriptional brakes in adipocytes as part of a therapeutic strategy to induce thermogenic WAT remodeling and improve metabolic health in obesity.
Results
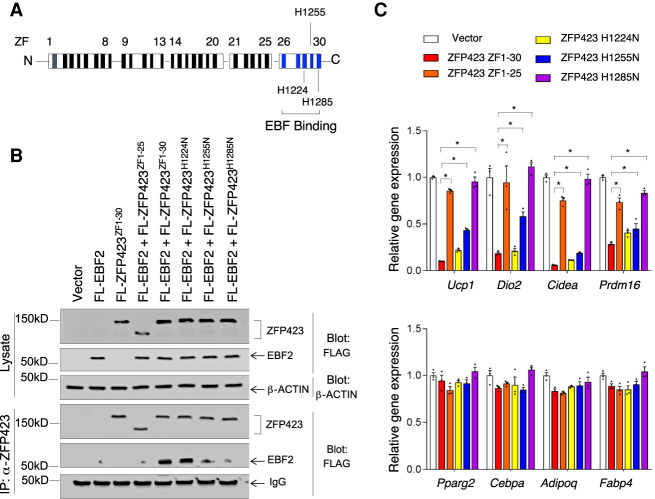
ZFs 29–30 of ZFP423 mediate its EBF2 interaction and antithermogenic function
ZFP423 physically interacts with EBF2 and inhibits its ability to transactivate thermogenic genes in adipocytes (Shao et al. 2016). The last four C-terminal C2H2 zinc fingers (ZFs) of ZFP423 (ZFs 26–30) mediate its direct interaction with the EBF family of transcription factors (Fig. 1A; Hata et al. 2000; Shao et al. 2016). We mapped the ZF(s) within the C-terminal region of ZFP423 that are essential for the ZFP423–EBF2 interaction. We engineered mutant forms of Zfp423 designed to disrupt individual C2H2 ZF motifs through histidine-to-asparagine substitutions (Fig. 1A). We used retroviral vectors to express either full-length ZFP423 (ZFs 1–30), a previously described variant lacking the C-terminal zinc fingers (ZFs 1–25) (Gupta et al. 2010), or variants with disruptions in either ZF 28 (H1224N), ZF 29 (H1255N), or ZF 30 (H1285N) in immortalized brown stromal vascular cells. Coimmunoprecipitation assays revealed that disruption of ZF 28 did not impact the ability of EBF2 to interact with ZFP423, whereas disruption of ZF 29 or 30 attenuated this interaction without affecting ZFP423 protein expression (Fig. 1B). Notably, variants of ZFP423 that did not interact with EBF2 failed to suppress thermogenic gene expression in adipocytes (Fig. 1C). In particular, the mutation of ZF 30 completely abrogated the ability of ZFP423 to suppress thermogenic genes while having no effect on adipocyte differentiation per se (Fig. 1C). Disruption of ZF 29 (H1255N) partially blocked the ability of ZFP423 to interact with EBF2 and partially attenuated the capacity for ZFP423 to suppress thermogenic gene expression (Fig. 1C). Importantly, disrupting ZF 30 did not impact other functions of ZFP423. ZFP423 promotes preadipocyte lineage commitment by interacting with SMADs through a well-characterized SMAD-binding domain (SBD) and amplifying bone morphogenic protein (BMP) signaling (Supplemental Fig. S1A; Hata et al. 2000; Gupta et al. 2010). ZFP423 H1285N maintained its ability to drive Pparg expression in NIH 3T3 cells and amplified the effects of BMP4 in activating Pparg expression (Supplemental Fig. S1B).
Figure 1.
Identification of zinc fingers (ZFs) mediating ZFP423 interaction with EBF2. (A) Schematic representation of 30 zinc finger (ZF) motifs within murine ZFP423. The previously identified EBF family interaction domain is highlighted in blue. ZFs 28–30 were individually mutated through histidine-to-asparagine substitutions at the indicated histidine residues. (B) Western blot analysis of the indicated proteins in whole-cell lysates (top) and ZFP423 immunoprecipitates (bottom) from differentiated immortalized brown stromal vascular cells following sequential transduction with retrovirus carrying Ebf2 and then retrovirus expressing FLAG-tagged (FL) ZFP423 variants. (C) Relative mRNA levels of thermogenic genes (top) and common adipocyte-selective genes (bottom) in cultures of in vitro differentiated immortalized brown stromal vascular cells expressing the indicated ZFP423 variants. Bars represent mean + SEM. (*) P < 0.05 by one-way ANOVA. n = 3 biological replicates.
Genetic disruption of the adipocyte ZFP423–EBF2 interaction leads to thermogenic WAT remodeling
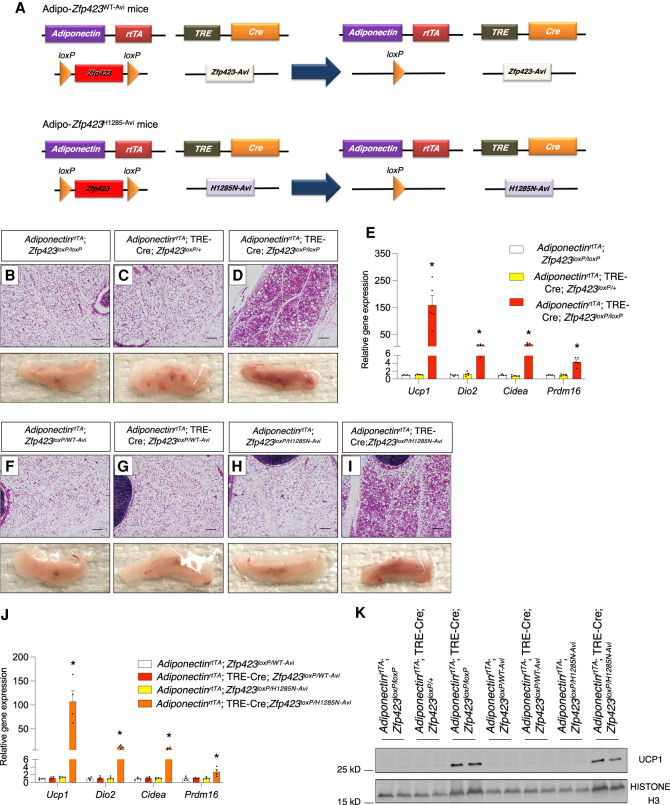
To determine the physiological importance of the adipocyte ZFP423–EBF2 interaction we used CRISPR–Cas9 gene editing to derive a mouse model in which the H1285N mutation is encoded into the endogenous Zfp423 locus. We simultaneously engineered the mutant allele to encode a 15-amino-acid sequence (GLNDIFEAQKIEWHE) at the C terminus that can be specifically biotinylated by the E. coli enzyme BirA (termed “Avi tag”) (Supplemental Fig. S2A; Beckett et al. 1999). In a parallel strain, we also engineered the wild-type Zfp423 allele to encode Avi-tagged ZFP423 (Supplemental Fig. S2A). Global homozygous Zfp423 knockout mice die shortly after birth and exhibit severe defects in the development of midline structures of the brain (Alcaraz et al. 2006; Warming et al. 2006). Mice heterozygous (Zfp423WT-Avi/+) or homozygous (Zfp423WT-Avi/ WT-Avi) for the Zfp423WT-Avi allele mice were viable and fertile and did not display any gross physiological abnormalities in comparison with wild-type littermates (Zfp423+/+). Thus, insertion of the Avi tag did not create a dominant-negative or null allele. Likewise, mice heterozygous (Zfp423H1285N-Avi/+) for the Zfp423H1285N-Avi allele were indistinguishable from wild-type littermates. These data imply that ZFP423 H1285N is not acting as a dominant negative. In contrast, animals homozygous for the H1285N mutation (Zfp423H1285N-Avi/ H1285N-Avi) did not survive beyond postnatal day 1 or 2. This is in line with prior studies indicating the importance of the ZFP423–EBF interaction in the embryonic development of the CNS (Roby et al. 2012).
To confirm the expression of ZFP423WT-Avi and ZFP423H1285N-Avi in adipocytes we crossed Zfp423WT-Avi/+ mice or Zfp423H1285N-Avi/+ mice to a model in which BirA is expressed in mature adipocytes in a CRE- and doxycycline (Dox)-dependent manner (Supplemental Fig. S2B). This new model consists of one transgene that expresses the reverse tetracycline transactivator (rtTA) under the control of a 5.4-kb promoter fragment of the adiponectin locus (Wang et al. 2010), another transgene in which CRE recombinase is expressed from a promoter containing the Tet response element (TRE-Cre), and modified Rosa26R alleles expressing E. coli BirA in a CRE-dependent manner (Rosa26Rlox-STOP-lox-BirA) (Johnson et al. 2017). Feeding of Dox-containing chow diet (Dox-chow) to adult quadruple-transgenic (AdiponectinrtTA; TRE-Cre; Rosa26Rlox-STOP-lox-BirA; Zfp423WT-Avi/+ or AdiponectinrtTA; TRE-Cre; Rosa26Rlox-STOP-lox-BirA; Zfp423H1285N-Avi/+) mice triggers CRE-mediated activation of BirA expression specifically in terminally differentiated adipocytes and subsequent biotinylation of Avi-tagged ZFP423. Upon 7 d of Dox-chow feeding, we readily detected both genetically modified ZFP423 proteins in iWAT lysates using fluorescence-conjugated streptavidin (Supplemental Fig. S2C). Importantly, both ZFP423WT-Avi and ZFP423H1285N-Avi proteins were detectable at comparable levels (Supplemental Fig. S2C).
We asked whether ZFP423 H1285N can suppress the thermogenic phenotype of adipose tissue in vivo. We derived mice in which the addition of Dox-chow results in the inactivation of one Zfp423 allele in adipocytes, with the remaining allele expressing either ZFP423WT-Avi (termed “Adipo-Zfp423WT-Avi” mice) or ZFP423H1285N-Avi (termed “Adipo-Zfp423H1285N-Avi” mice) (Fig. 2A). We placed 8-wk-old male animals on Dox-chow for 4 wk while housed at room temperature (22°C). Consistent with our prior studies, we observed widespread morphological and molecular browning of iWAT depots and increased thermogenic gene expression in epididymal WAT (eWAT) from animals lacking adipocyte Zfp423 (Zfp423-iAKO mice) (Fig. 2B–D; Supplemental Fig. S3). These phenotypes were not observed in animals heterozygous for either the Zfp423-null allele (AdiponectinrtTA; TRE-Cre; Zfp423+/loxP animals) or carrying the Zfp423WT-Avi allele, indicating that one active wild-type allele of Zfp423 is sufficient to maintain the white adipocyte phenotype and suppress UCP1 expression. Importantly, iWAT depots from mice carrying one functionally wild-type Zfp423 allele and one Zfp423H1285N-Avi allele are morphologically indistinguishable from control animals (Fig. 2F–H). This further indicates that Zfp423H1285N-Avi is not acting as a dominant negative. Remarkably, iWAT depots of Adipo-Zfp423H1285N-Avi mice, which only express Zfp423H1285N-Avi in adipocytes, resemble the iWAT depots of the adipocyte Zfp423 knockout animals. iWAT depots of Adipo-Zfp423H1285N-Avi mice contain large regions of multilocular adipocytes and express key thermogenic genes and UCP1 protein (Fig. 2I–K). These data indicate that ZFP423H1285N-Avi cannot maintain the inguinal white adipocyte phenotype. Moreover, we also observed an increase in thermogenic gene expression in eWAT depots of Adipo-Zfp423H1285N-Avi mice maintained at room temperature (Supplemental Fig. S3). Collectively, these data further implicate the ZFP423–EBF interaction as a key protein–protein interaction responsible for suppressing the thermogenic phenotype of white adipose tissue in mice.
Figure 2.
Genetic disruption of the ZFP423–EBF2 interaction in adipocytes leads to thermogenic WAT remodeling. (A) Components of the Adipo-Zfp423WT-Avi and Adipo-Zfp423H1285N-Avi mouse models. Quadruple transgenic mice consist of AdiponectinrtTA and TRE-Cre transgenes, one floxed Zfp423 allele (Zfp423lopx/+), and one allele engineered to express either ZFP423WT-Avi (Zfp423WT-Avi/+) or ZFP423H1285N-Avi (Zfp423H1285N-Avi/+). Dox-chow diet feeding results in the inactivation of one Zfp423 allele in adiponectin-expressing mature adipocytes, with the other allele expressing either ZFP423WT-Avi in Adipo-Zfp423WT-Avi mice or ZFP423H1285N-Avi in Adipo-Zfp423H1285N-Avi mice. (B–D) Representative bright-field images of H&E-stained sections of iWAT depots obtained from adult mice with the indicated genotypes after 4 wk of Dox-chow diet feeding at room temperature. Scale bar, 200 μm. Representative photographs of dissected whole iWAT depots are shown. (E) Relative mRNA levels of thermogenic genes in iWAT from adult mice with the indicated genotypes after 4 wk of Dox-chow diet feeding at room temperature. Bars represent mean ± SEM. (*) P < 0.05 for AdiponectinrtTA; Zfp423loxp/loxP versus AdiponectinrtTA; TRE-Cre; Zfp423loxP/loxP by one-way ANOVA. n = 5 biological replicates. (F–I) Representative bright-field images of H&E-stained sections of iWAT depots obtained from adult mice with the indicated genotypes after 4 wk of DOX-chow diet feeding at room temperature. Scale bar, 200 μm. Representative photographs of dissected whole iWAT depots are shown. (J) Relative mRNA levels of thermogenic genes in iWAT from adult mice with the indicated genotypes after 4 wk of Dox-chow diet feeding at room temperature. Bars represent mean ± SEM. (*) P < 0.05 for AdiponectinrtTA; Zfp423loxp/WT-Avi versus AdiponectinrtTA; TRE-Cre; Zfp423loxP/H1285N-Avi by one-way ANOVA. n = 4 biological replicates. (K) Western blot analysis of UPC1 protein levels in whole iWAT depots isolated from adult mice with the indicated genotypes after 4 wk of Dox-chow diet feeding at room temperature.
Endogenous ZFP423 co-occupies EBF2 target genes in adipocytes
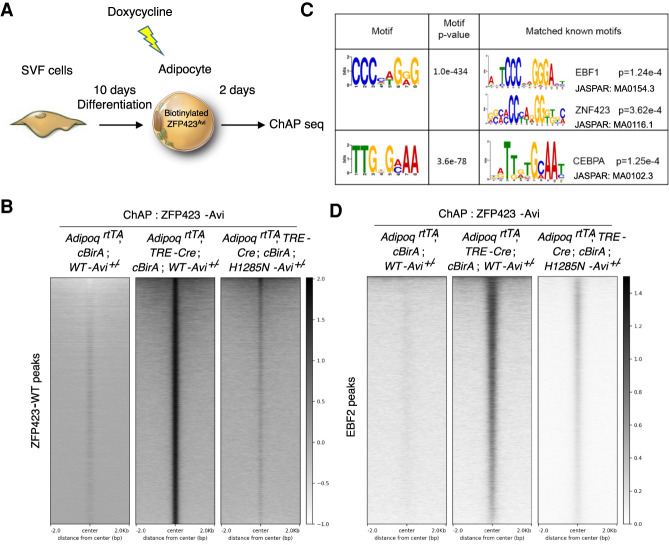
The precise mechanisms by which ZFP423 inhibits EBF2 activity are unclear. To begin to address this, we asked whether ZFP423 localizes to EBF2 target genes in adipocytes. We performed chromatin affinity purification (ChAP) of the endogenous Avi-tagged ZFP423WT-Avi and ZFP423H1285N-Avi protein using streptavidin beads. We used iWAT stromal vascular cells from mice heterozygous for either the Zfp423WT-Avi allele or Zfp423H1285N-Avi allele and capable of inducible BirA expression in an adipocyte-specific manner. Following adipocyte differentiation, we induced BirA expression in adipocytes with Dox and then isolated chromatin for ChAP sequencing analysis (Fig. 3A). ChAP-seq identified 46,146 peaks occupied by ZFP423WT-Avi in mature adipocytes (Fig. 3B; Supplemental Data Set S1). Importantly, very few of these peaks were identified in cells lacking BirA, suggesting specificity toward targets occupied by Avi-tagged ZFP423 (Fig. 3B). Motif analysis identified the well-characterized EBF response element as the most enriched motif among genomic elements occupied by ZFP423WT-Avi (Fig. 3C; Supplemental Table S1). An annotated ZNF423 (human ortholog of ZFP423) binding motif bearing close resemblance to the EBF binding motif was also identified (Fig. 3C; Supplemental Table S1). Gene ontology analysis indicated that the genes occupied by ZFP423WT-Avi are linked to several biological processes related to fatty acid metabolism, glucose metabolism, and brown adipocyte differentiation (Supplemental Table S2). In parallel, we performed EBF2 ChIP-seq analysis to identify EBF2 targets in adipocytes (Supplemental Fig. S4A,B). ZFP423WT-Avi indeed co-occupied EBF2 targets in these cells (Fig. 3D). We did not observe a significant degree of enrichment of ZFP423H1285-Avi at EBF2 target genes, suggesting that the ability of ZFP423 to occupy EBF2 targets appears dependent on its ability to interact with EBF2 (Fig. 3D). It is important to note that ZFP423H1285-Avi retained its ability to occupy DNA, including non-EBF2 targets (Fig. 3B; Supplemental Data Set S2). In fact, motif analysis revealed an enrichment of specific binding motifs among targets occupied by ZFP423H1285-Avi, including potential STAT response elements (Supplemental Table S3). Gene ontology analysis of ZFP423H1285-Avi-occupied genes indicated an enrichment in pathways/genes related to extracellular matrix organization (Supplemental Table S4). Altogether, these data indicate that endogenous ZFP423 co-occupies EBF2 target genes in adipocytes, and that this association is dependent on the ZFP423–EBF2 protein interaction.
Figure 3.
ZFP423 occupies EBF2 DNA binding sites in mature adipocytes. (A) The stromal vascular fraction (SVF) of iWAT from adult mice with the indicated genotypes was induced to differentiate in vitro. Differentiated adipocytes were treated with 5 µM Dox for 12 h to activate BirA expression and subsequent biotinylation of genetically Avi-tagged ZFP423 proteins. Forty-eight hours after Dox treatment, cells were harvested for the chromatin affinity purification (ChAP) sequencing analysis. (B) Heat map illustrating ZFP423WT-Avi- and ZFP423H1285N-Avi-occupied regions in differentiated adipocytes. (C) Characterized DNA-binding motifs enriched in ZFP423WT-Avi-precipitated DNA versus ZFP423H1285N-Avi-precipitated DNA. (D) Heat map illustrating ZFP423WT-Avi and ZFP423H1285N-Avi occupancy at EBF2 target sites in differentiated adipocytes.
Loss of ZFP423 drives an EBF2 coregulator switch at target thermogenic genes
We next investigated whether the loss of Zfp423 can impact EBF2 occupancy at its target genes. We used iWAT stromal vascular cells from control and Zfp423-iAKO mice, which allowed for Dox-inducible inactivation of Zfp423 upon their differentiation into adipocytes. As previously reported, inducible deletion of Zfp423 in differentiated adipocyte cultures leads to cell-autonomous activation of the thermogenic gene program within 48 h of doxycycline treatment. ChIP-seq analysis for EBF2 revealed that overall occupancy of EBF2 at its target genes was not altered in the absence of Zfp423 (Supplemental Fig. S4A,B). This suggests that ZFP423 does not influence EBF2 chromatin binding activity, but rather its ability to activate target gene expression.
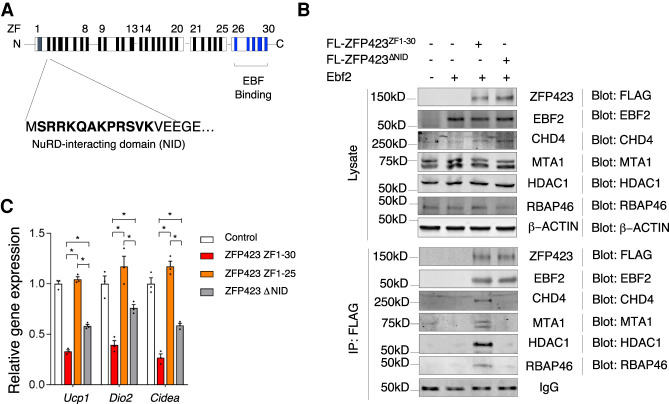
Upon DNA binding, EBF2 recruits the BRG1-containing BAF chromatin remodeling complex to brown fat-selective genes, leading ultimately to chromatin remodeling (Shapira et al. 2017). We observed that ZFP423 overexpression in brown adipocytes leads to a dissociation of the EBF2–BRG1 interaction, indicating that ZFP423 blocks EBF2 coactivator recruitment (Supplemental Fig. S4C). Transcriptional corepressors, such as ZFP423, often recruit corepressor complexes containing chromatin-modifying enzymes. Thus, we sought to identify adipocyte ZFP423-interacting partners in an unbiased manner by using affinity purification of ZFP423 complexes and mass spectrometry analysis. LC-MS/MS analysis revealed the presence of all core components of the NuRD (nucleosome remodeling deacetylase) corepressor complex in association with ZFP423 (Supplemental Data Set S3). This complex includes several chromatin remodeling factors (CHD4, MTA1/2, RBAP46/48, and MBD3), along with histone deacetylases 1 and 2 (HDAC1 and HDAC2) (Torchy et al. 2015). The presence of this complex is notable given that ZFP423 harbors a consensus “NuRD interaction domain” (NID) (Fig. 4A), which consists of 12 amino acids that directly interact with the RBAP48 subunit of the NuRD complex (Lauberth and Rauchman 2006). Our recent work on the role of ZFP423 in adipose tissue stromal cells revealed the importance of the ZFP423–NuRD complex in the regulation of stromal cell NFκB activity and inflammatory responses (Shan et al. 2020). These data suggest that the ZFP423–NuRD interaction may suppress the expression of thermogenic genes in adipocytes. All core components of the NuRD complex were readily detected by Western blot analysis of differentiated brown adipocytes (Fig. 4B). Endogenous NuRD components associate with virally expressed FLAG-ZFP423 in brown adipocyte cultures, but not with a FLAG-tagged variant of ZFP423 that is expressed but lacks the 12-amino-acid NID (FLAG-ZFP423ΔNID) (Fig. 4B). Notably, the NID of ZFP423 is essential for its full capacity to suppress thermogenic genes. Expression of FLAG-ZFP423ΔNID in brown adipocytes cannot suppress Ucp1, Cidea, and Dio2 as effectively as FLAG-ZFP423 (Fig. 4C). Together, these data support a role for the NuRD complex in the mechanism by which ZFP423 suppresses thermogenic gene expression.
Figure 4.
ZFP423 recruitment of the NuRD complex is required for its suppression of thermogenic gene expression in mature adipocytes. (A) Schematic representation of 30 zinc finger (ZF) motifs within murine ZFP423 with highlighted location of the N-terminal NuRD-interacting domain (NID) and C-terminal EBF binding domain. (B) Western blot of the indicated protein expression in whole-cell lysates (top) and ZFP423 immunoprecipitates (bottom) from differentiated transduced immortalized brown stromal vascular cells expressing EBF2 and either FLAG-tagged (FL) ZFP423ZF1-30 or ZFP423ΔNID. (C) Relative mRNA levels of the indicated thermogenic genes in differentiated immortalized brown stromal vascular cells expressing EBF2 and either FLAG-tagged (FL) ZFP423ZF1-30 or ZFP423ΔNID. Bars represent mean + SEM. (*) P < 0.05 by one-way ANOVA. n = 3 biological replicates.
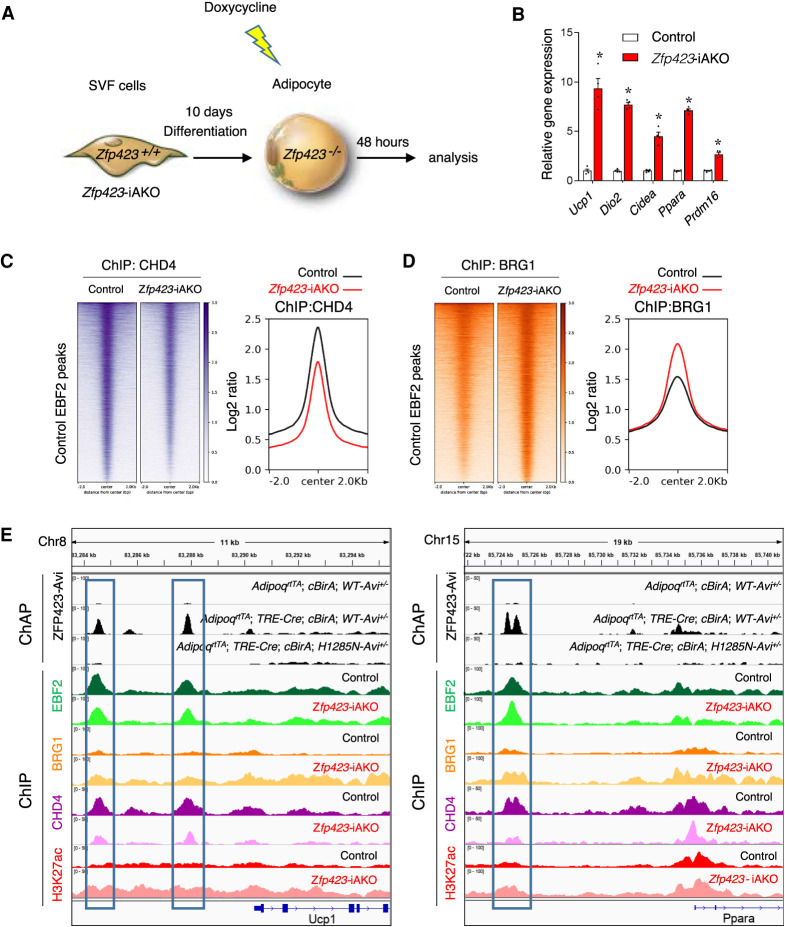
The data above raise the possibility of an EBF2 coregulator switch occurring at target loci upon inactivation of Zfp423. We performed ChIP-seq analyses for BRG1 as well as the obligate NuRD component CHD4 from control and Zfp423-deficient adipocyte cultures 48 h after the addition of Dox (Fig. 5A). At this time point following Dox exposure, levels of thermogenic genes are sharply elevated in Zfp423-deficient adipocyte cultures (Fig. 5B). Consistent with the biochemical data described above, CHD4 occupies EBF2 targets in control cells expressing Zfp423 (Fig. 5C,E). Upon inactivation of Zfp423, CHD4 occupancy at EBF2 sites is diminished (Fig. 5C,E). Notably, this occurs alongside an increase in the occupancy of BRG1 at EBF2 targets when Zfp423 is removed (Fig. 5D,E). These alterations in coregulator recruitment also coincide with increases in activating histone H3K27 acetylation (Fig. 5E). It is important to note that these changes are observed within only 48 h of inducing Zfp423 deletion in fully differentiated adipocyte cultures. Together, these data provide evidence that the loss of Zfp423 in adipocytes activates thermogenic gene expression by inducing an EBF2 coregulator switch at target genes.
Figure 5.
ZFP423 regulates EBF2 coregulator recruitment at EBF2 target genes. (A) Experimental design: iWAT SVF isolated from control or Zfp423-iAKO mice were induced to differentiate in vitro. Differentiated adipocytes were treated with 5 µM Dox for 12 h to inactivate Zfp423. Forty-eight hours after Dox treatment, cells were harvested for gene expression analysis or chromatin immunoprecipitation (ChIP) sequencing analyses. (B) Relative mRNA levels of the indicated thermogenic genes 48 h after Dox treatment of the differentiated adipocytes described in A. Bars represent mean + SEM. (*) P < 0.05 by Student's t-test. n = 4 biological replicates. (C) Heat map (left) and profile plot (right) illustrating CHD4 occupancy at EBF2-occupied regions in control and Zfp423-iAKO adipocytes. (D) Heat map (left) and profile plot (right) illustrating BRG1 occupancy at EBF2-occupied regions in control and Zfp423-iAKO adipocytes. (E) ZFP423 ChAP-seq signals and ChIP-seq signals for EBF2, BRG1, CHD4, and H3K27ac at the Ucp1 and Ppara loci in control and Zfp423-iAKO adipocytes.
Zfp423 deficiency triggers a shift in genome-wide PPARγ occupancy in adipocytes
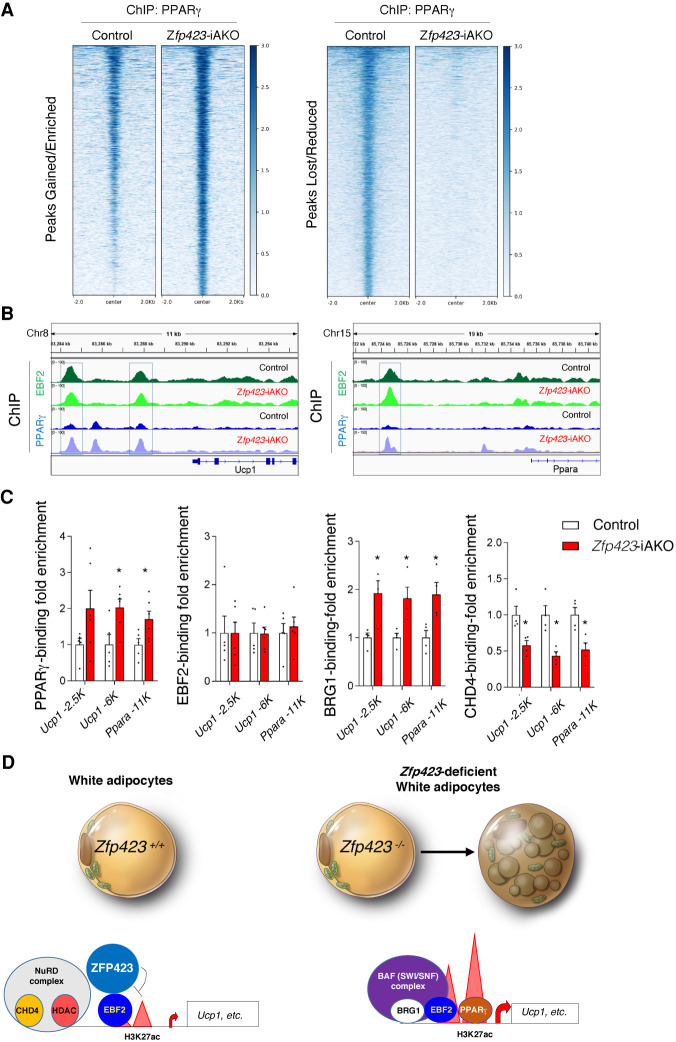
EBF2-dependent recruitment of the BAF chromatin remodeling complex to thermogenic gene enhancers increases chromatin accessibility and PPARγ occupancy (Shapira et al. 2017). Thus, we asked whether Zfp423 deficiency triggers a shift in PPARγ occupancy. We performed PPARγ ChIP-seq assays in parallel to the aforementioned EBF2, CHD4, and BRG1 ChIP-seq experiments. In contrast to EBF2, PPARγ undergoes a remarkable shift in genomic occupancy upon induction of Zfp423 deletion. Overall, there is a strong reduction in global PPARγ occupancy and binding at verified PPARγ target sites (Supplemental Fig. S5A,B; Supplemental Data Set S4). However, we also identified >2000 sites with increased PPARγ binding upon Zfp423 deletion, including sites bound by EBF2 at the Ucp1 and Ppara loci, indicative of a shift of PPARγ binding toward key thermogenic genes (Fig. 6B; Supplemental Data Set S5). Binding at target genes common to white and brown adipocytes (e.g., Fabp4, Lpl, Plin1, and Plin2) was not substantially altered by Zfp423 inactivation (Supplemental Fig. S5C–F). ChIP qPCR assays confirmed our ChIP-seq results. EBF2 binding at the Ucp1 and Ppara genes was not impacted by Zfp423 deletion, whereas PPARγ occupancy at these sites was enhanced (Fig. 6C). This was associated with enhanced BRG1 occupancy and diminished CHD4 binding (Fig. 6C). Collectively, these data suggest that ZFP423 regulates the thermogenic plasticity of white adipocytes by controlling EBF2-dependent coactivator assembly and PPARγ occupancy at key thermogenic genes (Fig. 6D).
Figure 6.
Zfp423 deficiency leads to increased PPARγ occupancy at EBF2 target thermogenic genes. (A) Heat maps illustrating PPARγ occupancy in control and Zfp423-iAKO adipocytes. Genomic elements with quantitatively increased (left) or reduced (right) PPARγ occupancy in control and Zfp423-iAKO adipocytes are shown. (B) ChIP-seq signals for EBF2 and PPARγ at the Ucp1 (left) and Ppara (right) loci in control and Zfp423-iAKO adipocytes. (C) ChIP-qPCR analysis of PPARγ, EBF2, BRG1, and CHD4 occupancy at the indicated EBF2-occupied regions at Ucp1 and Ppara loci in control and Zfp423-iAKO adipocytes. Bars represent mean + SEM. (*) P < 0.05 by Student's t-test. n = 5 per genotype for PPARγ and EBF2 ChIP-qPCR analysis; n = 4 per genotype for BRG1 and CHD4 ChIP-qPCR analysis. (D) Proposed model: ZFP423 acts to limit PPARγ occupancy at thermogenic genes in energy-storing white adipocytes by preventing EBF2-dependent chromatin remodeling (H3K27 acetylation). When Zfp423 is lost from white adipocytes, EBF2 engages its transcriptional coactivators and facilitates chromatin remodeling and PPARγ occupancy in a manner similar to how it operates in the brown adipocyte lineage. This facilitates the conversion of white adipocytes to a thermogenic phenotype.
Adipocyte Zfp423 inactivation increases the antidiabetic capacity of rosiglitazone
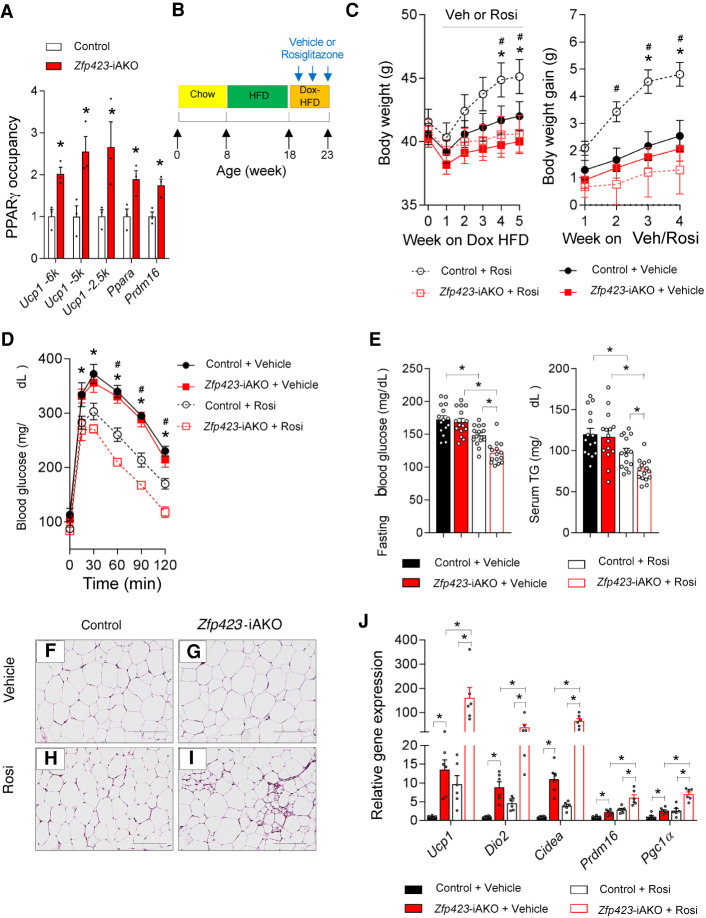
The shift in PPARγ occupancy toward thermogenic genes in the absence of Zfp423 raises the question of whether the ability of PPARγ agonists to promote thermogenic remodeling could be amplified in Zfp423-iAKO mice. First, we confirmed by ChIP-PCR analysis that the PPARγ occupancy at key thermogenic genes (Ucp1, Ppara, and Prdm16) is increased in whole iWAT depots of Zfp423-iAKO mice maintained at room temperature on Dox-chow diet for 4 wk (Fig. 7A). Next, we placed 8-wk-old control and Zfp423-iAKO mice on a high-fat diet (HFD) for 10 wk. After this initial period of HFD feeding, we switched animals to a Dox-containing HFD (Dox-HFD) for 5 wk while treating animals with vehicle or the PPARγ agonist, rosiglitazone. Thus, Zfp423 is inactivated in mature adipocytes of obese mice at the onset of drug treatment (Fig. 7B). During the 5-wk treatment period, rosiglitazone-treated Zfp423-iAKO mice gained much less weight than rosiglitazone-treated control animals (Fig. 7C). By the end of the 5-wk treatment period, rosiglitazone improved glucose tolerance, fasting glucose levels, and serum triglyceride levels to a much greater degree in obese Zfp423-iAKO mice than in control animals (Fig. 7D–E). Thus, adipocyte Zfp423 deficiency amplifies the beneficial metabolic effects of antidiabetic rosiglitazone while blocking the weight-gaining effects of the drug.
Figure 7.
Adipocyte ZFP423 inactivation enhances the beneficial effects of thiazolidinediones in diet-induced obesity. (A) ChIP-qPCR analysis of PPARγ occupancy at the indicated EBF2-occupied regions of the Ucp1, Ppara, and Prdm16 loci in whole iWAT isolated from control and Zfp423-iAKO mice after 4 wk of Dox-chow diet feeding. Bars represent mean + SEM. (*) P < 0.05 by Student's t-test. n = 3 biological replicates per genotype. (B) Experimental design: Eight-week-old control and Zfp423-iAKO mice were maintained on a 60% high-fat diet (HFD) for 10 wk before switching to Dox-HFD for another 5 wk. Vehicle or rosiglitazone (10 mg/kg/d) was delivered during the 5 wk of Dox-HFD feeding. (C) Average body weights and body weight gain of mice during Dox-HFD feeding. (*) P < 0.05 control + vehicle versus control + Rosi by two-way ANOVA, (#) P < 0.05 control + Rosi versus Zfp423-iAKO + Rosi by two-way ANOVA. n = 15 mice per group. (D) Glucose tolerance tests of control and Zfp423-iAKO mice after 4 wk of vehicle or rosiglitazone administration. (*) P < 0.05 control + vehicle versus control + Rosi by two-way ANOVA, (#) P < 0.05 for control + Rosi versus Zfp423-iAKO + Rosi by two-way ANOVA. n = 7 mice for vehicle groups; n = 8 mice for Rosi-treated groups. (E) Levels of fasting (6 h) blood glucose and serum triglycerides (TG) in control and Zfp423-iAKO mice after 4 wk of vehicle or rosiglitazone administration. Bars represent mean + SEM. (*) P < 0.05 by two-way ANOVA. n = 15 mice per group. (F–I) Representative bright-field images of H&E-stained iWAT sections from control and Zfp423-iAKO mice after 4 wk of vehicle or rosiglitazone administration. Scale bar, 200 μm. (J) Relative mRNA levels of the indicated thermogenic genes in iWAT from control and Zfp423-iAKO mice after 4 wk of vehicle or rosiglitazone administration. Bars represent mean + SEM. (*) P < 0.05 by two-way ANOVA. n = 6 per group.
Histological analysis reveals an increased frequency of multilocular adipocytes in iWAT depots of rosiglitazone-treated Zfp423-iAKO mice as compared with rosiglitazone-treated control animals (Fig. 7F–I). In fact, we observed very few iWAT multilocular adipocytes in obese control animals treated with rosiglitazone, whereas these beige-like adipocytes were readily detected in the treated Zfp423-iAKO mice (Fig. 7F–I). Quantitative gene expression analysis of the iWAT depots revealed a synergistic effect of rosiglitazone treatment and adipocyte Zfp423 deficiency on the induction of the thermogenic gene program. In obese control mice (∼40 g at the time of treatment), rosiglitazone stimulated the induction of Ucp1 ∼10-fold over baseline (vehicle) (Fig. 7J). Adipocyte Zfp423 deletion alone results in induction in Ucp1 mRNA levels also by ∼10-fold (Fig. 7J). Notably, the induction of Ucp1 expression in rosiglitazone-treated Zfp423-iAKO mice occurs by >100-fold (Fig. 7J). This robust effect of rosiglitazone on Ucp1 expression in Zfp423-iAKO mice is in line with the biochemical observations that Zfp423 deficiency leads to increased PPARγ occupancy at thermogenic genes and heightened transcriptional activity.
Discussion
Great progress has been made in identifying the transcriptional machinery that drives the thermogenic program of brown/beige adipocytes (Wang and Seale 2016). The data presented here highlight the key mechanisms that white adipocytes use to suppress this thermogenic gene program. Zfp423 expression is enriched in white adipocytes as compared with thermogenic fat cells and is suppressed in adipocytes upon cold exposure or direct β-adrenergic stimulation (Shao et al. 2016). Zfp423 expression then increases in beige cells upon cessation of cold stress as they return to a dormant/inactive state (Roh et al. 2018). As such, the modulation of Zfp423 expression appears to represent a physiological switch that allows adipocytes to control their thermogenic plasticity. Collectively, our new data here point to a model in which ZFP423 acts to limit PPARγ occupancy at thermogenic genes in energy-storing white adipocytes by preventing EBF2-dependent chromatin remodeling. When Zfp423 is lost from white adipocytes, EBF2 engages its transcriptional coactivators and facilitates chromatin remodeling and PPARγ occupancy in a manner akin to how it operates in the brown adipocyte lineage. In this study, we did not perform lineage tracing analysis of adipocytes in Adipo-Zfp423H1285N-Avi mice; however, our prior lineage tracing studies of Zfp423-iAKO mice indeed demonstrate that mature unilocular adiponectin-expressing adipocytes convert to multilocular UCP1-expressing cells when ZFP423 function is lost (Shao et al. 2016).
There is considerable interest in identifying strategies to promote brown adipose tissue activity to enhance weight loss and/or improve cardiometabolic health. Nevertheless, enthusiasm for targeting brown adipose tissue in obese individuals is tempered by numerous challenges. The amount of existing thermogenic adipose in adults appears quite variable, and obesity is associated with a significant reduction in the mass and activity of this tissue (Cypess et al. 2009; van Marken Lichtenbelt et al. 2009). Thus, fully activated classical brown adipose tissue in obese individuals may not exert a significant clinical effect, particularly in the face of counterregulatory mechanisms that help maintain body weight (e.g. increased food intake or WAT catecholamine resistance). Under physiological conditions, the β-adrenergic system represents the strongest activator of adipose thermogenesis. In rodents, agonists of the β3 adrenergic receptor robustly stimulate adipocyte thermogenesis. In human adipocytes, the β2 adrenergic receptor appears to be the key driver (Blondin et al. 2020). Use of the FDA-approved adrenergic receptor agonist, mirabegron (targeting both β2 and β3 adrenergic receptors at high doses), has shown promise as an activator of BAT in healthy humans (Cypess et al. 2015); however, the high doses of mirabegron required also come with a potential for unwanted side effects. The data presented here, along with our prior work, highlight the ability of mature white adipocytes to be converted into thermogenic adipocytes when transcriptional brakes are removed. It is tempting to speculate that efforts to release the “brakes” on thermogenic gene transcription while applying β-adrenergic receptor agonists or other “accelerators” of thermogenesis may thus represent a combinatorial strategy to enhance adipose thermogenic remodeling in obesity at lower doses of agonist. As case in point, we demonstrated that the loss of adipocyte ZFP423 results in a shift in PPARγ occupancy to thermogenic gene promoters in adipocytes and enables rosiglitazone to exert a stronger effect on the thermogenic gene program of WAT in vivo. Notably, this shift in PPARγ occupancy appears to amplify the beneficial metabolic effects of antidiabetic rosiglitazone while blocking the weight-gaining effects of the drug. Clinically, there is considerable variability in the response to thiazolidinedione treatment (Sears et al. 2009). Remarkably, genetic variation that impacts PPARγ occupancy can predict the antidiabetic response to these drugs (Soccio et al. 2015; Hu et al. 2019). Although not directly tested here, it is possible that the antidiabetic effects of PPARγ agonists can be observed at lower doses with fewer side effects when their ability to drive WAT browning can be enhanced.
Targeting nonreceptor transcriptional components in a cell type-specific manner through conventional pharmacological strategies remains a tremendous challenge. Zfp423 is also expressed in the adult brain, proliferating muscle satellite cells, and endothelial cells (Warming et al. 2006; Cheng et al. 2007; Cheng and Reed 2007; Gupta et al. 2012; Addison et al. 2019). As such, efforts to manipulate ZFP423 expression or activity itself could depend on the ability to selectively target adipocytes. Nevertheless, several recent studies have highlighted the promise of cell-based therapeutic approaches to increase thermogenic adipocyte mass in vivo. Patient-derived mesenchymal progenitors can be engineered to differentiate into a thermogenic adipocyte phenotype in vitro and then transplanted into animals to induce metabolic benefits (Min et al. 2016; Wang et al. 2020). Such approaches are still in their infancy; however, the promise of cell-based therapies highlights the possibility of using the knowledge of transcriptional mechanisms governing white and brown adipocyte differentiation to devise strategies to promote the expansion of thermogenic adipose tissue in humans.
Materials and methods
Animals
AdiponenctinrtTA, TRE-Cre, and Zfp423loxP/loxP have been described previously (Shao et al. 2016). Rosa26Rlox-STOP-lox-BirA mice were obtained from Jackson Laboratories (stock 030420) and were previously described (Johnson et al. 2017). Zfp423WT-Avi and Zfp423H1285-Avi mice were generated by the Children's Research Institute Mouse Genome Engineering Core at University of Texas Southwestern using CRISPR–Cas9 gene editing. Sequences of the gRNA and repair templates used are in the Supplemental Material. All animals used in this study were male and on a pure C57BL/6 background. Mice were maintained with a 12-h light/dark cycle and free access to food and water. All animal experiments were performed according to procedures approved by the University of Texas Southwestern Institutional Animal Care and Use Committee. Details regarding rodent diets are in the Supplemental Material.
Isolation of adipose stromal vascular fraction and in vitro adipogenesis
The stromal vascular fraction (SVF) of WAT was isolated by collagenase digestion as previously described (Shao et al. 2018). Freshly isolated inguinal WAT SVF cells were cultured in DMEM/F12 plus 10% FBS, pen/strep, and gentamicin (growth media). For in vitro differentiation, iWAT SVF cells were cultured in 10% CO2 at 37°C until confluency. Confluent cultures were stimulated with adipogenic induction media (growth media supplemented with 5 µg mL−1 insulin, 1 µM dexamethasone, 0.5 mM isobutylmethyxanthine, 1 µM rosiglitazone) for 48 h. After induction, cells were maintained in growth media supplemented with 5 µg mL−1 insulin (maintenance media) for another 8 d until used for experiments.
Glucose tolerance tests and serum measurements
For glucose tolerance tests, mice were injected i.p. with glucose (1 g/kg body weight; Sigma) after an overnight fast. Blood was collected by venous bleeding from the tail vein at 0, 15, 30, 60, 90, and 120 min after injection. For measurements of fasting blood glucose, mice were fasted from 9:00 a.m. to 3:00 p.m. (6 h). All glucose concentrations were measured using Bayer Contour glucometers. Serum levels of triglycerides were determined using a triglyceride determination kit (triglyceride reagent T2449 and free glycerol reagent F6428; Sigma).
Retroviral production and transduction
Retrovirus was packaged in Phoenix cells as previously described (Gupta et al. 2010). Briefly, Phoenix packaging cells were cotransfected with 10 μg of the indicated pMSCV overexpression plasmids and 5 μg of gag-pol (Addgene 8449) and 5 μg of VSV (Addgene 8454) plasmids using Lipofectamine LTX (Thermo Fisher Scientific 15338100). Media containing viral particles were harvested 48 h after transfection and centrifuged at 600g for 5 min to clear debris. iWAT SVF cells isolated from control and Zfp423-iAKO mice were transduced with diluted virus-containing supernatants (1:1 ratio) in DMEM/F12 media containing 8 μg/mL polybrene (Sigma TR-1003) for 24 h. Following transduction, cells were maintained in fresh media for >48 h and then used for experiments.
Affinity purification and proteomics analysis
A stable cell line expressing Zfp423 was generated by drug selection of immortalized brown SVF cells transduced with pMSCV-Puro-FLAG-ZFP423 plasmids. Zfp423-overexpressing cells were lysed in Pierce IP lysis buffer (Thermo Fisher Scientific 87787) supplemented with protease inhibitor cocktail (Sigma P8340). Cell lysate was incubated with anti-FLAG (Sigma, F1804) or normal IgG (Cell Signaling Technology 2729) overnight at 4°C. Lysates were then incubated with Protein G Sepharose 4 Fast Flow (GE Healthcare Biosciences 17-0618-01) for 2 h at 4°C to capture immune complexes. Purified immune complexes were washed sequentially three times with washing buffer (50 mM HEPES at pH 7.4, 10% glycerol, 0.05% NP40, 1 mM DTT, 0.25 mM PMSF, 150 mM KCl) supplemented with protease inhibitor cocktail (Sigma P8340). Proteins were eluted with Pierce IgG elution buffer (Thermo Fisher Scientific 21004) and briefly resolved by SDS-PAGE prior to the submission to the University of Texas Southwestern Proteomics Core. Details regarding proteomics analysis are in the Supplemental Material.
Coimmunoprecipitation assay
Coimmunoprecipitation was performed as previously described (Shao et al. 2016). In brief, cells were lysed in Pierce IP lysis buffer (Thermo Fisher Scientific 87787) supplemented with 1% protease inhibitor cocktail (Sigma 8340). Following the overnight incubation with the indicated antibodies at 4°C, the cell lysates were mixed with Protein G Sepharose 4 Fast Flow (GE Healthcare Biosciences 17-0618-01) for 1 h at room temperature to capture immune complexes. After three sequential washes with Pierce IP lysis buffer, samples were eluted by boiling in 2× SDS loading buffer and resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting.
Gene expression analysis
Total RNA from tissue or cells was extracted and purified using the TRIzol reagent (Invitrogen) and the RNeasy mini kit (Qiagen). Total RNA from FACS-sorted cells was extracted using RNAqueous microRNA isolation kit (Thermo Fisher Scientific). cDNA was synthesized with M-MLV reverse transcriptase (Invitrogen) and random hexamer primers (Invitrogen). Relative expression of mRNAs was determined by quantitative PCR using SYBR Green PCR system (Applied Biosystems), and values were normalized to levels of Rps18 using the ΔΔ-Ct method. All qPCR primer sequences are listed in Supplemental Table S5.
Histological analysis
Dissected tissues were fixed in 4% paraformaldehyde overnight. Paraffin embedding, sectioning, and H&E staining were performed at the Molecular Pathology Core Facility at University of Texas Southwestern. Bright-field images were acquired using a Keyence BZ-X710 microscope.
Immunoblotting
Protein extracts from cells or tissues were prepared by homogenization in RIPA lysis buffer (Santa Cruz Biotechnology) supplemented with protease inhibitor cocktail (Sigma P8340), and phosphatase inhibitor cocktails (Sigma P5726 and P0044). Protein extracts were separated by SDS-PAGE electrophoresis and transferred onto PVDF membrane (Millipore IPFL00010). After incubation with the indicated primary antibodies overnight at 4°C, the blots were incubated with IR dye- coupled secondary antibodies (LI-COR) and visualized by the LI-COR Odyssey infrared imaging system. Details regarding antibodies and working concentrations are in the Supplemental Material.
Cross-linking
Cross-linking for ChIP or ChAP was performed as described (Shan et al. 2020). iWAT SVF isolated from the indicated animals were induced to differentiate in vitro. Differentiated adipocytes were treated with 5 µM Dox for 12 h to induce rtTA/CRE activity. Forty-eight hours after Dox treatment, cells were cross-linked with 1% formaldehyde in PBS for 10 min at 37°C and quenched in 125 mM glycine in PBS for 5 min at 4°C. For tissue ChIP, iWAT depots isolated from control or Zfp423-iAKO mice after 4 wk of Dox-chow diet feeding were diced into small pieces (∼5 mm3), fixed with 1% formaldehyde in PBS for 10 min at 37°C, and quenched in 125 mM glycine in PBS for 5 min at 4°C. Details regarding ChIP assays and library production are in the Supplemental Material.
Chromatin ffinity purifications (ChAPs)
Cross-linked cells and tissues were lysed in Farnham lysis buffer (5 mM PIPES at pH 8.0, 85 mM KCl, 0.5% NP-40, 1 mM DTT, protease inhibitor cocktail [Sigma P8340]) to obtain nuclear material. Crude nuclear pellets were collected by centrifugation and then lysed by incubation in lysis buffer containing 5 mM Tris-HCl (pH 7.9), 1% SDS, 10 mM EDTA, 1 mM DTT, and protease inhibitor cocktail (Sigma P8340). Chromatin fragmentation (200- to 500-bp length) was performed at 4°C by a Bioruptor 300 using the setting of 10 cycles of 30 sec on and 60 sec off. Soluble chromatin was diluted 1:10 with dilution buffer (20 mM Tris-HCl at pH 7.9, 1% Triton X-100, 2 mM EDTA, 150 mM NaCl, 0.1% SDS, protease inhibitor cocktail [Sigma P8340]) and precleared using Streptavidin Sepharose high-performance beads (GE Healthcare Biosciences 17-5113-01) for 1 h at 4°C. Precleared samples were incubated with the streptavidin sepharose beads for 2 h at 4°C. Affinity-purified material was consecutively washed with SDS wash buffer (2% SDS in 50 mM Tris-HCl at pH 7.9, protease inhibitor cocktail [Sigma P8340]) twice, high-salt wash buffer (50 mM Tris-HCl at pH 7.9, 1 mM EDTA, 500 mM NaCl, 0.1% sodium deoxycholate, 1% Triton X-100, protease inhibitor cocktail [Sigma P8340]), LiCl wash buffer (10 mM Tris-HCl at pH 7.9, 1 mM EDTA, 250 mM LiCl, 0.5% NP-40, 0.5% sodium deoxycholate, protease inhibitor cocktail [Sigma P8340]), and 1× Tris-EDTA (TE). After elution at SDS elution buffer (50 mM Tris-Hcl at pH 7.9, 1% SDS, 10 mM EDTA) overnight at 70°C, the affinity-purified material was digested with RNase (Roche 11119915001) and proteinase K (Thermo Fisher Scientific EO0491) prior to the purification and concentration of the affinity-purified genomic DNA by ChIP DNA Clean & Concentrator kit (Zymo Research D5201). ChAP-isolated DNA was subjected to library production (ChAP-seq) using NEBnext NGS DNA library preparation for Illumina kit (New England Biolabs E7645). Sequencing was performed with Illumina NextSeq 500 mid output (130M) by the University of Texas Southwestern McDermott Center Next-Generation Sequencing Core. ChIP/ChAP-seq analysis was performed as previously described (Shan et al. 2020). Details regarding these analyses are in the Supplemental Material.
Quantification and statistical analysis
All data were expressed as the mean + SEM. We used GraphPad Prism 7.0 (GraphPad Software, Inc.) to perform the statistical analyses. For comparisons between two independent groups, a Student's t-test was used and P < 0.05 was considered statistically significant. For in vitro studies, we estimated the approximate effect size based on independent preliminary studies. Studies designed to characterize an in vitro difference in gene expression were estimated to have a slightly larger effect size of 30% with assumed 15% standard deviation of group means. To detect this difference at a power of 80% and an α of 0.05, we predicted we would need four independent replicates per group. We estimated this effect size based on independent preliminary studies. Statistical information, including P-values, samples sizes, and repetitions, for all data sets are in Supplemental Data Set S6.
Material and data availability
The ChIP and ChAP sequencing data sets are available at GEO accession viewer (https://www.ncbi.nlm.nih.gov/geo) under the accession numbers GSE175653 and GSE175654. The mass spectrometry data sets are available at MassIVE (https://massive.ucsd.edu) under the accession number MSV000087563.
Supplementary Material
Acknowledgments
We are grateful to members of the University of Texas Southwestern Touchstone Diabetes Center for useful discussions. We thank Charlotte Lee, the University of Texas Southwestern Animal Resource Center, Metabolic Phenotyping Core, Pathology Core, Live-Cell Imaging Core, Flow Cytometry Core, and McDermott Sequencing Center for excellent guidance and assistance with experiments performed here. We thank the University of Texas Southwestern Children's Research Institute Mouse Genome Engineering Core for generating the Zfp423WT-Avi and Zfp423H1285-Avi genetic strains. This study and/or personnel were supported in part by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01 DK104789, R01 DK119163, and RC2 DK118620 to R.K.G.; American Heart Association postdoctoral fellowship 16POST26420136 and Career Development Award 19CDA34670007 from the American Heart Association and the Harry S. Moss Heart Trust to M.S.; and NIDDK R01 DK121801 and DK123356 to P.S.
Author contributions: M.S. and R.K.G. conceived the study and wrote the manuscript. M.S. and R.K.G. designed experiments. L.L. and M.S. designed genetic targeting strategies. M.S., Q.Z., A.T. B.S., and L.V. performed experiments. P.S. contributed essential reagents and edited the manuscript. All authors analyzed the data.
Footnotes
Supplemental material is available for this article.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.348780.121.
Competing interest statement
The authors declare no competing interests.
References
- Addison WN, Hall KC, Kokabu S, Matsubara T, Fu MM, Gori F, Baron R. 2019. Zfp423 regulates skeletal muscle regeneration and proliferation. Mol Cell Biol 39: e00447-18. 10.1128/MCB.00447-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz WA, Gold DA, Raponi E, Gent PM, Concepcion D, Hamilton BA. 2006. Zfp423 controls proliferation and differentiation of neural precursors in cerebellar vermis formation. Proc Natl Acad Sci 103: 19424–19429. 10.1073/pnas.0609184103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. 2010. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 298: E1244–E1253. 10.1152/ajpendo.00600.2009 [DOI] [PubMed] [Google Scholar]
- Becher T, Palanisamy S, Kramer DJ, Eljalby M, Marx SJ, Wibmer AG, Butler SD, Jiang CS, Vaughan R, Schöder H, et al. 2021. Brown adipose tissue is associated with cardiometabolic health. Nat Med 27: 58–65. 10.1038/s41591-020-1126-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett D, Kovaleva E, Schatz PJ. 1999. A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci 8: 921–929. 10.1110/ps.8.4.921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondin DP, Nielsen S, Kuipers EN, Severinsen MC, Jensen VH, Miard S, Jespersen NZ, Kooijman S, Boon MR, Fortin M, et al. 2020. Human brown adipocyte thermogenesis is driven by β2-AR stimulation. Cell Metab 32: 287–300.e7. 10.1016/j.cmet.2020.07.005 [DOI] [PubMed] [Google Scholar]
- Cheng LE, Reed RR. 2007. Zfp423/OAZ participates in a developmental switch during olfactory neurogenesis. Neuron 54: 547–557. 10.1016/j.neuron.2007.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LE, Zhang J, Reed RR. 2007. The transcription factor Zfp423/OAZ is required for cerebellar development and CNS midline patterning. Dev Biol 307: 43–52. 10.1016/j.ydbio.2007.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. 2009. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517. 10.1056/NEJMoa0810780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elía E, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, et al. 2015. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21: 33–38. 10.1016/j.cmet.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. 2010. Transcriptional control of preadipocyte determination by Zfp423. Nature 464: 619–623. 10.1038/nature08816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, Frontini A, Bhowmick DC, Ye L, Cinti S, et al. 2012. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab 15: 230–239. 10.1016/j.cmet.2012.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M, Seale P. 2013. Brown and beige fat: development, function and therapeutic potential. Nat Med 19: 1252–1263. 10.1038/nm.3361 [DOI] [PubMed] [Google Scholar]
- Hata A, Seoane J, Lagna G, Montalvo E, Hemmati-Brivanlou A, Massagué J. 2000. OAZ uses distinct DNA- and protein-binding zinc fingers in separate BMP-Smad and Olf signaling pathways. Cell 100: 229–240. 10.1016/S0092-8674(00)81561-5 [DOI] [PubMed] [Google Scholar]
- Hepler C, Shao M, Xia JY, Ghaben AL, Pearson MJ, Vishvanath L, Sharma AX, Morley TS, Holland WL, Gupta RK. 2017. Directing visceral white adipocyte precursors to a thermogenic adipocyte fate improves insulin sensitivity in obese mice. Elife 6: e27669. 10.7554/eLife.27669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Jiang C, Guan D, Dierickx P, Zhang R, Moscati A, Nadkarni GN, Steger DJ, Loos RJF, Hu C, et al. 2019. Patient adipose stem cell-derived adipocytes reveal genetic variation that predicts antidiabetic drug response. Cell Stem Cell 24: 299–308.e6. 10.1016/j.stem.2018.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BS, Zhao YT, Fasolino M, Lamonica JM, Kim YJ, Georgakilas G, Wood KH, Bu D, Cui Y, Goffin D, et al. 2017. Biotin tagging of MeCP2 in mice reveals contextual insights into the Rett syndrome transcriptome. Nat Med 23: 1203–1214. 10.1038/nm.4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Spiegelman BM, Seale P. 2015. Brown and beige fat: physiological roles beyond heat generation. Cell Metab 22: 546–559. 10.1016/j.cmet.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauberth SM, Rauchman M. 2006. A conserved 12-amino acid motif in Sall1 recruits the nucleosome remodeling and deacetylase corepressor complex. J Biol Chem 281: 23922–23931. 10.1074/jbc.M513461200 [DOI] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Konkar AA, Granneman JG. 2015. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J 29: 286–299. 10.1096/fj.14-263038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lončar D. 1991. Convertible adipose tissue in mice. Cell Tissue Res 266: 149–161. 10.1007/BF00678721 [DOI] [PubMed] [Google Scholar]
- Min SY, Kady J, Nam M, Rojas-Rodriguez R, Berkenwald A, Kim JH, Noh HL, Kim JK, Cooper MP, Fitzgibbons T, et al. 2016. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med 22: 312–318. 10.1038/nm.4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota de Sa P, Richard AJ, Hang H, Stephens JM. 2017. Transcriptional regulation of adipogenesis. Compr Physiol 7: 635–674. [DOI] [PubMed] [Google Scholar]
- Pearson S, Loft A, Rajbhandari P, Simcox J, Lee S, Tontonoz P, Mandrup S, Villanueva CJ. 2019. Loss of TLE3 promotes the mitochondrial program in beige adipocytes and improves glucose metabolism. Genes Dev 33: 747–762. 10.1101/gad.321059.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumari S, Wu J, Ishibashi J, Lim HW, Giang AH, Won KJ, Reed RR, Seale P. 2013. EBF2 determines and maintains brown adipocyte identity. Cell Metab 17: 562–574. 10.1016/j.cmet.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby YA, Bushey MA, Cheng LE, Kulaga HM, Lee SJ, Reed RR. 2012. Zfp423/OAZ mutation reveals the importance of Olf/EBF transcription activity in olfactory neuronal maturation. J Neurosci 32: 13679–13688a. 10.1523/JNEUROSCI.6190-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh HC, Tsai LTY, Shao M, Tenen D, Shen Y, Kumari M, Lyubetskaya A, Jacobs C, Dawes B, Gupta RK, et al. 2018. Warming induces significant reprogramming of beige, but not brown, adipocyte cellular identity. Cell Metab 27: 1121–1137.e5. 10.1016/j.cmet.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. 2014. What we talk about when we talk about fat. Cell 156: 20–44. 10.1016/j.cell.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald M, Perdikari A, Rülicke T, Wolfrum C. 2013. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 15: 659–667. 10.1038/ncb2740 [DOI] [PubMed] [Google Scholar]
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. 2011. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 121: 96–105. 10.1172/JCI44271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears DD, Hsiao G, Hsiao A, Yu JG, Courtney CH, Ofrecio JM, Chapman J, Subramaniam S. 2009. Mechanisms of human insulin resistance and thiazolidinedione-mediated insulin sensitization. Proc Natl Acad Sci 106: 18745–18750. 10.1073/pnas.0903032106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan B, Shao M, Zhang Q, Hepler C, Paschoal VA, Barnes SD, Vishvanath L, An YA, Jia L, Malladi VS, et al. 2020. Perivascular mesenchymal cells control adipose-tissue macrophage accrual in obesity. Nat Metab 2: 1332–1349. 10.1038/s42255-020-00301-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M, Gupta RK. 2019. Transcriptional brakes on the road to adipocyte thermogenesis. Biochim Biophys Acta Mol Cell Biol Lipids 1864: 20–28. 10.1016/j.bbalip.2018.05.010 [DOI] [PubMed] [Google Scholar]
- Shao M, Ishibashi J, Kusminski CM, Wang QA, Hepler C, Vishvanath L, MacPherson KA, Spurgin SB, Sun K, Holland WL, et al. 2016. Zfp423 maintains white adipocyte identity through suppression of the beige cell thermogenic gene program. Cell Metab 23: 1167.– . 10.1016/j.cmet.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M, Vishvanath L, Busbuso NC, Hepler C, Shan B, Sharma AX, Chen S, Yu X, An YA, Zhu Y, et al. 2018. De novo adipocyte differentiation from Pdgfrβ+ preadipocytes protects against pathologic visceral adipose expansion in obesity. Nat Commun 9: 890. 10.1038/s41467-018-03196-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M, Wang QA, Song A, Vishvanath L, Busbuso NC, Scherer PE, Gupta RK. 2019. Cellular origins of beige Fat cells revisited. Diabetes 68: 1874–1885. 10.2337/db19-0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira SN, Seale P. 2019. Transcriptional control of brown and beige fat development and function. Obesity 27: 13–21. 10.1002/oby.22334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira SN, Lim HW, Rajakumari S, Sakers AP, Ishibashi J, Harms MJ, Won KJ, Seale P. 2017. EBF2 transcriptionally regulates brown adipogenesis via the histone reader DPF3 and the BAF chromatin remodeling complex. Genes Dev 31: 660–673. 10.1101/gad.294405.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soccio RE, Chen ER, Rajapurkar SR, Safabakhsh P, Marinis JM, Dispirito JR, Emmett MJ, Briggs ER, Fang B, Everett LJ, et al. 2015. Genetic variation determines PPARγ function and anti-diabetic drug response in vivo. Cell 162: 33–44. 10.1016/j.cell.2015.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine RR, Shapira SN, Lim HW, Ishibashi J, Harms M, Won KJ, Seale P. 2016. EBF2 promotes the recruitment of beige adipocytes in white adipose tissue. Mol Metab 5: 57–65. 10.1016/j.molmet.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchy MP, Hamiche A, Klaholz BP. 2015. Structure and function insights into the NuRD chromatin remodeling complex. Cell Mol Life Sci 72: 2491–2507. 10.1007/s00018-015-1880-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. 2009. Cold-activated brown adipose tissue in healthy men. New England Journal of Medicine 360: 1500–1508. 10.1056/NEJMoa0808718 [DOI] [PubMed] [Google Scholar]
- Villanueva CJ, Vergnes L, Wang J, Drew BG, Hong C, Tu Y, Hu Y, Peng X, Xu F, Saez E, et al. 2013. Adipose subtype-selective recruitment of TLE3 or Prdm16 by PPARγ specifies lipid storage versus thermogenic gene programs. Cell Metab 17: 423–435. 10.1016/j.cmet.2013.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishvanath L, MacPherson KA, Hepler C, Wang QA, Shao M, Spurgin SB, Wang MY, Kusminski CM, Morley TS, Gupta RK. 2016. Pdgfrβ+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab 23: 350–359. 10.1016/j.cmet.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Seale P. 2016. Control of brown and beige fat development. Nat Rev Mol Cell Biol 17: 691–702. 10.1038/nrm.2016.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZV, Deng Y, Wang QA, Sun K, Scherer PE. 2010. Identification and characterization of a promoter cassette conferring adipocyte-specific gene expression. Endocrinology 151: 2933–2939. 10.1210/en.2010-0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. 2013. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 19: 1338–1344. 10.1038/nm.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Kissig M, Rajakumari S, Huang L, Lim HW, Won KJ, Seale P. 2014. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci 111: 14466–14471. 10.1073/pnas.1412685111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CH, Lundh M, Fu A, Kriszt R, Huang TL, Lynes MD, Leiria LO, Shamsi F, Darcy J, Greenwood BP, et al. 2020. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Sci Transl Med 12: eaaz8664. 10.1126/scitranslmed.aaz8664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warming S, Rachel RA, Jenkins NA, Copeland NG. 2006. Zfp423 is required for normal cerebellar development. Mol Cell Biol 26: 6913–6922. 10.1128/MCB.02255-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. 2012. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366–376. 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.